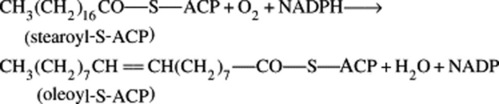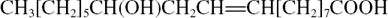Chapter 19 Hydrocarbons and derivatives
Hydrocarbons contain carbon and hydrogen only and, from these, by the addition of functional groups and by interaction, all other natural compounds can theoretically be derived. In a particular class of compounds such as volatile oils, the components of any one member may be biosynthetically related (e.g. menthol and menthone in oil of peppermint) although because of their different functional groups they may undergo different sets of chemical reactions and possess different pharmacological properties. Among the most common functional groups are carboxylic acids, alcohols, ketones, aldehydes and phenols; biochemical interactions produce esters, lactones etc.
In this book most examples of medicinal plants containing the above are considered under their respective biogenetic groupings and in this chapter the detailed description of drugs is restricted to those examples in which simple acids, alcohols and esters comprise the principal medicinal components.
HYDROCARBONS
Although not featuring strongly in the pharmaceutical armamentarium, hydrocarbons are important in nature as components of cuticular waxes. The majority of these are odd-numbered long-chain alkanes within the range C25–35 formed by decarboxylation of the next higher, even-numbered, free fatty acid. In recent years the long-chain polyenes of the Compositae have been systematically investigated in relation to their chemotaxonomic importance. Isoprene (C5H8), the unsaturated hydrocarbon moiety from which the terpenoids (isoprenoids) can be constructed (Fig. 18.17), has not to date been found free in nature. A number of cyclic terpenoid hydrocarbons including limonene, pinene, phellandrene and cadinene are components of essential oils. Rubber, gutta and the carotenes are polyunsaturated terpenoids.
MONOBASIC ACIDS
Organic acids possess one or more carboxyl groups and a monobasic acid may be represented as RCOOH. The very high frequency of the biochemical occurrence of the carboxyl group means that acids are found in all living organisms and as derivatives of all the major metabolic groups. They participate in essential metabolism and in this capacity range from the simple acids of the respiratory sequence to the complex deoxyribonucleic acids associated with the storage and transmission of hereditary characters. In the metabolic cycles they frequently function in association with coenzymes, and may accumulate as simple salts, esters and amides, or less frequently in the free state. Amino acids are discussed in Chapter 18.
C1–C6 Monocarboxylic acids
A number of these acids together with hydroxy- and keto-derivatives are intermediates in the early stages of the biosynthesis of fats, isoprenoid compounds and various amino acids (q.v.). In the free state they are not found abundantly in nature but occur scattered throughout the plant kingdom in the esterified form as a feature of some volatile oils, resins, fats, coumarin derivatives and alkaloids.
Some common acids are illustrated in Table 19.1.
Table 19.1 Examples of C1–C6 monocarboxylic acids.
| Name | Structure | Comments |
|---|---|---|
| Formic acid |
 |
Name derives from its first isolation from the ant, Formica rufa. A decomposition product of many vegetable materials. Occurs free in the hairs of the stinging nettle; combined in the gitaloxigenin series of cardioactive glycosides. N-formyl-L-methionine is involved in the initiation of protein synthesis on ribosomes |
| Acetic acid |
 |
An essential primary metabolite, particularly as acetyl-CoA. Common in the esterified form |
| Propionic acid |
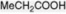
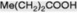 |
Produced in the fatty acid oxidative cycle when an acyl-CoA with an odd number of carbon atoms is involved. Esterified as a tropane alkaloid |
| n-Butyric acid |
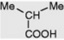 |
Occurs in traces in many fats |
| n-Valeric acid |
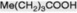 |
Not common; component of Convolvulaceous resins |
| iso-Valeric acid |
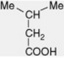 |
Free and esterified in Valeriana spp. Combined in some tropane alkaloids (e.g. valeroidine) and in the pyranocoumarin, dihydrosamidin. Intermediate in the metabolism of leucine |
| 2-Methylbutyric acid |
 |
Component of some tropane and Veratrum alkaloids, Convolvulaceous glycosides and the pyranocoumarin visnadin |
| Caproic acid |
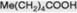 |
Occurs in traces in many fats |
| Crotonic acid (trans- butenoic acid) |
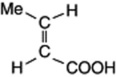 |
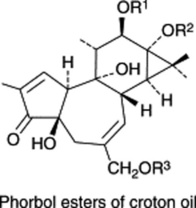
Constituent of croton oil |
| Tiglic acid |
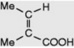 |
Occurs in croton oil (glycoside) from Croton tiglium. The acid of many minor tropane alkaloids, e.g. tigloidine. Component of Convolvulaceous resins and Symphytum alkaloids. Biosynthetically derived from isoleucine |
| Angelic acid |
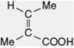 |
Occurs in the rhizome of Angelica. Esterifying acid of the Schizanthus alkaloid schizanthine X and of some volatile oils, e.g. chamomile oils. Component of the Cevadilla seed alkaloid cevadine and Symphytum alkaloids |
| Senecioic acid |
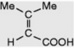 |
First isolated from a species of Senecio (Compositae). Occurs as the esterifying acid of some alkaloids of Dioscorea and Schizanthus. Component of the pyranocoumarin samidin |
Fatty acids
These acids are important as components of plant oils (acyl lipids) in which they occur as esters with the trihydric alcohol glycerol. They are also components of the resins of the Convolvulaceae and of waxes in which they are esterified with long-chain alcohols. Most are C10 to C20straight-chain monocarboxylic acids with an even number of carbon atoms. Over 200 have been isolated from natural sources but relatively few are ubiquitous in their occurrence. They may be saturated (e.g. palmitic and stearic acids) or unsaturated (e.g. oleic acid). The double bonds, with a few minor exceptions such as the seed oil of pomegranate, are cis.
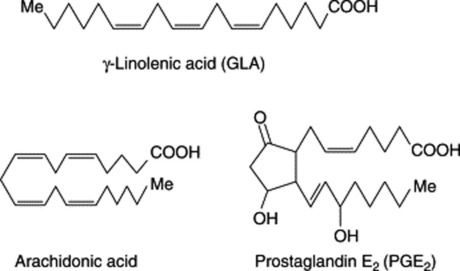
Less commonly they are cyclic compounds such as hydnocarpic acid and the prostaglandins. The latter are a group of physiologically active essential fatty acids found in most body tissues and are involved in the platelet-aggregation and inflammatory processes. They promote smooth muscle contraction making them of clinical use as effective abortifacients and for inducing labour. All the active natural prostaglandins are derivatives of prostanoic acid (see Table 19.4). A rich source of prostaglandin A2 (PGA2) is the soft coral Plexaura homomalla. Although recognized in the 1930s, and their structures determined in 1962, it was not until 1988 that prostaglandins were unequivocally established as components of some higher plants (cambial zones and buds of Larix and Populus spp.)
Table 19.4 Cyclic unsaturated acids.
| Common name | Structural formula |
|---|---|
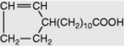
| Hydnocarpic |
 |
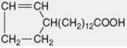
| Chaulmoogric |
 |
| Gorlic |
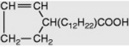 |
| Prostanoic |
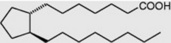 |
| PGA2 |
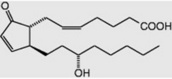 |
The characteristic acid of castor oil, ricinoleic acid (hydroxyoleic acid) has both a hydroxyl group and an unsaturated double bond. A range of acetylenic fatty acids occurs throughout the plant kingdom and some of them possess antifungal and antibacterial properties. The biogenetic relationship between these, the olefinic fatty acids and the saturated fatty acids is outlined later in this chapter.
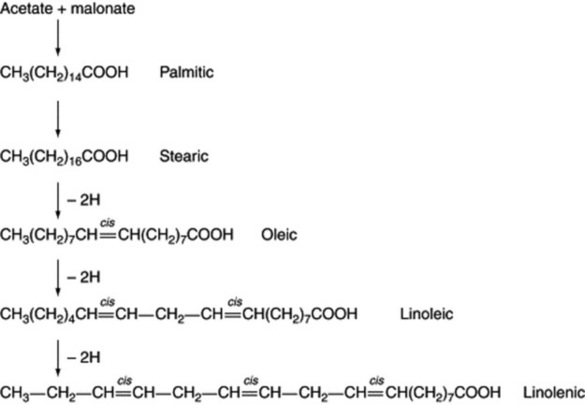
Examples of fatty acids are listed in Tables 19.2-19.4. It will be noted that some have more than one unsaturated bond, the bonds being interspersed by methylene groups. These polyunsaturated acids have received much attention in recent years both regarding their role in dietary fats and as medicinals. All the common acids have trivial names but in order to indicate more precisely their structures without recourse to the full systematic chemical name each can be represented by a symbol. Thus α-linolenic, systematic name all-cis-Δ9,12,15-octadecatrienoic acid, has 18 carbons and three double bonds which can be represented by 18:3. The position of the double bonds is then indicated by the n-x convention where n = number of carbon atoms in the molecule and x is the number of inclusive carbon atoms from the methyl (ω) end to the first carbon of the first double bond, in this case 3, so that the symbol for α-linolenic acid is 18:3(n-3). The positions of the two remaining double bonds are deduced by the fact that they will follow on from each other being separated only by one methylene (-CH2-) group. In this area students may find the literature situation somewhat confusing because in some texts the acids may be symbolized on the basis of conventional chemical systematic numbering—for fatty acids the carboxyl carbon being C1. For α-linolenic acid this is represented as 18:3(9c.12c.15c), c indicating a cis-bond. The advantage of the first system is that it indicates any bioequivalence of the double bonds in acids of different chain-length, bearing in mind that chain elongation in vivo proceeds at the carboxyl end of the molecule by the addition of 2C units. Thus it can be seen from Table 19.3 that γ-linolenic acid and arachidonic acid both fall into the biochemical ω-6 family of unsaturated fatty acids and their respective symbols 18:3(n-6) and 20:4(n-6) reflect this whereas symbols based on chemical nomenclature for these acids viz 18:3(6c,9c,12c) and 20:4(5c,8c,11c,14c) do not. A comparison of symbols for some common unsaturated acids is shown in Table 19.5.
Table 19.2 Straight-chain saturated acids.
| Common name | Systematic name | Structural formula |
|---|---|---|
| Caprylic | n-Octanoic | CH3(CH2)6COOH |
| Capric | n-Decanoic | CH3(CH2)8COOH |
| Lauric | n-Dodecanoic | CH3(CH2)10COOH |
| Myristic | n-Tetradecanoic | CH3(CH2)12COOH |
| Palmitic | n-Hexadecanoic | CH3(CH2)14COOH |
| Stearic | n-Octadecanoic | CH3(CH2)16COOH |
| Arachidic | n-Eicosanoic | CH3(CH2)18COOH |
Table 19.3 Straight-chain unsaturated acids.
| Common name | Number of unsaturated bonds | Structural formula |
|---|---|---|
| Palmitoleic | 1 | CH3(CH2)5CH=CH(CH2)7COOH |
| Oleic | 1 | CH3(CH2)7CH=CH(CH2)7COOH |
| Petroselinic | 1 | CH3(CH2)10CH=CH(CH2)4COOH |
| Ricinoleic | 1 | CH3(CH2)5CH(OH)CH2CH=CH–(CH2)7COOH |
| Erucic | 1 | CH3(CH2)7CH=CH(CH2)11COOH |
| Linolenic | 2 | CH3(CH2)4CH=CHCH2CH=CH–(CH2)7COOH |
| α-Linoleic | 3 | CH3CH2CH=CHCH2CH=CHCH2–CH=CH(CH2)7COOH |
| γ-Linolenic | 3 | CH3(CH2)4CH=CHCH2CH=CHCH2CH=CH(CH2)4COOH |
| Arachidonic | 4 | CH3(CH2)4CH=CHCH2CH=CHCH2–CH=CHCH2CH=CH(CH2)3COOH |
Table 19.5 Comparison of symbols ascribed to unsaturated fatty acids.
| Common name of acid | Symbol employing biochemical equivalence of double bonds | Symbol based on chemical nomenclature |
|---|---|---|
| Palmitoleic | 16:1 (n-7) | 16:1 (9c) |
| Oleic | 18:1 (n-9) | 18:1 (9c) |
| Petroselinic | 18:1 (n-12) | 18:1(6c) |
| Ricinoleic | 18:1 (n-9) (hydroxy at n-7) | D(+)-12h-18:1(9c) (h = hydroxy) |
| Erucic | 22:1 (n-9) | 22:1 (13c) |
| Linoleic | 18:2 (n-6) | 18:2 (9c,12c) |
| Eicosadienoic | 20:2 (n-6) | 20:2 (11c,14c) |
Under certain conditions, which are specified in pharmacopoeias, iodine or its equivalent is taken up at these double bonds and the so-called iodine value is thus a measure of unsaturatedness. The iodine value is the number of parts of iodine absorbed by 100 parts by weight of the substance. Near-infrared spectroscopy can also be used todetermine this value as it is directly related to the HC=CH stretch bands at 2130 nm in the spectrum. Iodine values are useful constants for acids, fixed oils, fats and waxes, and help to indicate the composition of complex mixtures as well as pure substances.
Biosynthesis of unsaturated fatty acids
Before the elucidation of the overall chemistry of formation of polyunsaturated fatty acids such as linoleic in the early 1960s by Bloch, knowledge concerning the biosynthesis of these compounds lagged behind that of the saturated acids. Recent progress has been much more rapid and, in general, it now appears that in aerobic organisms, monoenoic acids with the double bond in the 9,10-position arise by direct dehydrogenation of saturated acids. In higher plants, for this reaction, coenzyme A may be replaced by the acyl carrier protein (ACP), and Bloch has demonstrated that stearoyl-S-ACP is an effective enzyme substrate of the desaturase system of isolated plant leaf chloroplasts. The reduced forms of nicotinamide adenine dinucleotide (NADH) or nicotinamide adenine dinucleotide phosphate (NADPH) and molecular oxygen are cofactors.
The position of the introduced double bond in respect to the carboxyl group is governed by the enzyme; hence, chain length of the substrate acid is most important. The hydrogen elimination is specifically cis but a few unusual fatty acids such as that in the seed oil of Punica granatum with the structure 18:3 (9c,11t,13c) have trans bonds. As illustrated in Fig. 19.1, further double bonds may be similarly introduced to give linoleic and linolenic acid.
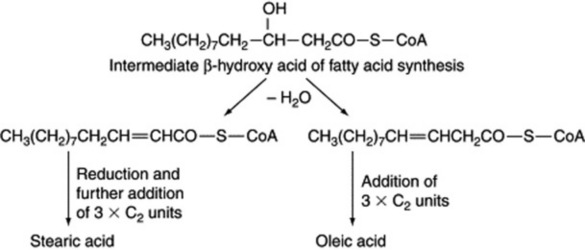
Unsaturated fatty acids can also be formed in plants by elongation of a medium-chain-length unsaturated acid. This appears to occur by the formation of an intermediate, β,γ-unsaturated acid rather than the α,β-unsaturated acid normally produced in saturated fatty acid biosynthesis; the β,γ-bond is not reduced and more C2 units are added in the usual way (Fig. 19.2).
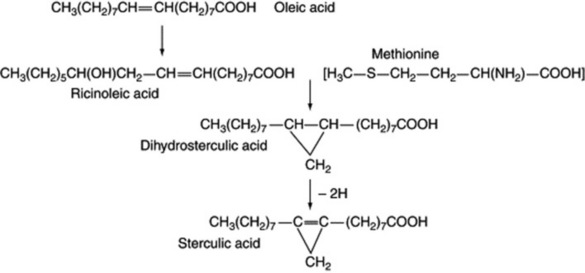
Sterculic acid, a component of seed oils of the Malvaceae and Sterculiaceae, is a cyclopropene and is also derived from oleic acid, with methionine supplying the extra carbon atom to give, first, the cyclopropane. Ricinoleic acid is a hydroxy fatty acid found in castor oil seeds and is again biosynthesized from oleic acid (Fig. 19.3).
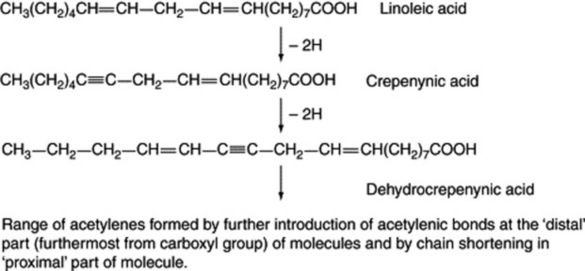
Some of the natural acetylenes and acetylenic fatty acids have obvious structural similarities to the more common fatty acids. The hypothesis that triple bonds are formed from double bonds by a mechanism analogous to that for the formation of double bonds and involving structurally and stereochemically specific enzymes has now received experimental support. By this means (Fig. 19.4) the range of acetylenes found in Basidiomycetes and in the Compositae, Araliaceae and Umbelliferae can be derived from linoleic acid via its acetylenic 12,13-dehydroderivative, crepenynic acid, an acid first isolated from seeds oils of Crepis spp.
Aromatic acids
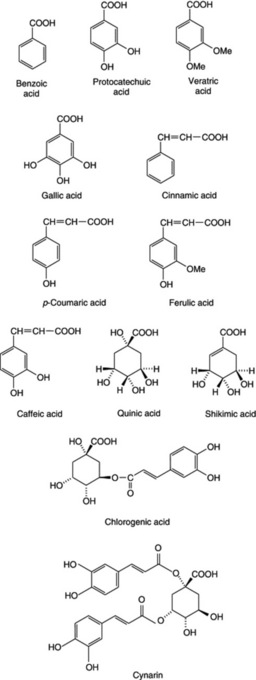
Two common aromatic acids are benzoic acid and cinnamic acid (unsaturated side-chain), which are widely distributed in nature and often occur free and combined in considerable amounts in drugs such as balsams. Truxillic acid, a polymer of cinnamic acid, occurs in coca leaves. Other related acids of fairly common occurrence are those having phenolic or other groupings in addition to a carboxyl group; such are: salicyclic acid (o-hydroxybenzoic acid), protocatechuic acid (3,4-dihydroxybenzoic acid), veratric acid (3,4-dimethoxybenzoic acid), gallic acid (3,4,5-trihydroxybenzoic acid) and 3,4,5-trimethoxybenzoic acid. Similarly, derived from cinnamic acid, one finds p-coumaric acid (p-hydroxycinnamic acid), ferulic acid (hydroxymethoxycinnamic acid), caffeic acid (hydroxycinnamic acid) and 3,4,5-trimethoxycinnamic acid. Unbelliferone, which occurs in combination in asafoetida, is the lactone of dihydroxycinnamic acid.
Acids having an alcohol group are quinic acid (tetrahydroxyhexahydrobenzoic acid), which occurs in cinchona bark and in some gymnosperms; mandelic acid, C6H5CHOHCOOH, which occurs in combination in cyanogenetic glycosides such as those of bitter almonds and other species of Prunus; and shikimic acid, an important intermediate metabolite (see Fig. 18.8). Shikimic acid has itself acquired recent pharmaceutical importance as the starting material for the semi-synthesis of the antiviral drug oseltamivir (Tamiflu®) for use against bird flu infections in humans. Its principal source has been star-anise fruits (q.v.), leading to a supply shortage of the plant material. Other natural sources rich in this acid and of potential future use are needles of the Pinaceae (S. Marshall, Pharm. J., 2007, 279, 719) and the fruits (gumballs) of the American sweet gum tree Liquidamber styraciflua (q.v.). The acid is also produced commercially by the fermentation of genetically modified Escherichia coli. Tropic acid and phenyllactic acid are two aromatic hydroxy acids that occur as esters in tropane alkaloids (q.v.). For examples of the above see Fig. 19.5.
Chlorogenic or caffeotannic acid is a condensation product of caffeic acid and quinic acid. It occurs in maté, coffee, elder flowers, lime flowers, hops and nux vomica and is converted into a green compound, which serves for its detection, when an aqueous extract is treated with ammonia and exposed to air. See also ‘Pseudotannins’ (Chapter 21).
The biogenesis of the aromatic ring has been discussed in Chapter 18.
DIBASIC AND TRIBASIC ACIDS
Oxalic acid, (COOH)2, forms the first of a series of dicarboxylic acids which includes malonic acid, CH2(COOH)2, and succinic acid, (CH2)2(COOH)2. Closely related to malonic acid is the unsaturated acid fumaric acid, COOH–CH=CH–COOH. Malic acid contains an alcohol group and has the formula COOH–CH2–CHOH–COOH. It is found in fruits such as apples and tamarinds. A high percentage of tartaric acid, COOH–(CHOH)2–COOH, and its potassium salt occurs in tamarinds and other fruits.
The tribasic acids, citric, isocitric and aconitic are closely related to one another. The Krebs’ citric acid cycle, which is discussed in Chapter 18, is very important. Citric acid is abundant in fruit juices, and aconitic acid, which occurs in Aconitum spp., is anhydrocitric acid. It forms part of the Krebs’ cycle and the glyoxalate cycle in microorganisms.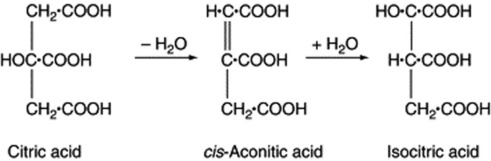
Of interest are opines, a group of substances formed by a host plant after infection with Agrobacterium spp.; a number of these compounds are di- and tri-carboxylic acids. For further details see Chapters 13 and 14.
ALCOHOLS
Alcohols possess one or more hydroxyl groups and exist naturally in either the free state or combined as esters. Like phenols they generally have common names ending in ‘ol’ (e.g. ethanol, glycerol and mannitol). They can be classed according to the number of hydroxyl groups present: monohydric alcohols-one hydroxyl, dihydric-two, trihydric-three and polyhydric-four or more. Furthermore each hydroxyl group may be classed as primary: –CH2OH (e.g. ethanol), secondary: –CHOH– (e.g. isopropanol) or tertiary: ≡COH (e.g. t-butanol). The remainder of the molecule may be saturated or unsaturated, aliphatic or aromatic. Numerous examples will be encountered throughout the text.
Monohydric aliphatic alcohols
Lower members of the series are found principally combined as esters e.g. methyl salicylate in oil of wintergreen and methyl and ethyl esters responsible for some fruit aromas. Esterified long-chain alcohols are constituents of some pharmaceutically important animal waxes and include cetyl alcohol (C16H33OH), ceryl alcohol (C26H53OH) and myricyl alcohol (C30H61OH). Such alcohols also participate in the formation of esters which are constituents of leaf cuticular waxes; an example is Carnauba Wax BP which contains myricyl cerotate.
Monohydric terpene alcohols
These are alcohols associated with that large group of compounds which arise from mevalonic acid and have isoprene as a fundamental structural unit. Pharmacognostically they are particularly evident as constituents of volatile oils namely: (1) non-cyclic terpene alcohols occur in many volatile oils—for example, geraniol in otto of rose, its isomer nerol in oils of orange and bergamot and linalol both free and combined as linalyl acetate in oils of lavender and rosemary; (2) monocyclic terpene alcohols are represented by terpineol and its acetate in oil of neroli and menthol and its acetate in oil of peppermint; (3) dicyclic terpene alcohols are particularly abundant in the Coniferae (e.g. sabinol and its acetate in Juniperus sabina). In the dicotyledons oil of rosemary contains borneol and its esters.
Monohydric aromatic alcohols
Benzyl alcohol, C6H5CH2OH, and cinnamyl alcohol, C6H5CH= CHCH2OH, occur both free and as esters of benzoic and cinnamic acids in balsams such as Tolu and Peru. The latter balsam is sometimes adulterated with cheap synthetic benzyl benzoate.
Included in this class is coniferyl alcohol, which forms an important component of the lignin molecule. Lignins are extremely complex phenylpropane polymers; they form an important strengthening material of plant cell walls and vary in composition according to their source, see Chapter 21, section on ‘Lignans and Lignin’.
Dihydric alcohols
Dihydric alcohols or glycols are compounds containing two hydroxyl groups; they are found naturally in many structural classes of compounds. The bicyclic amino alcohol 3,6-dihydroxytropane occurs free and as esters in a number of species of the Solanaceae and Erythroxylaceae, the dihydric alcohol panaxadiol is a component of some ginseng steroids, and oenanthotoxin, the poisonous constituent of the hemlock water dropworts (Oenanthe spp.), is a polyene diol.
Trihydric alcohols
As with the glycols these compounds occur in a range of structural types. An important example is glycerol (propan-1,2,3-triol), an essential component of fixed oils and fats which are discussed in more detail below.
Polyhydric aliphatic alcohols
The following are alcohols with either four or six hydroxyl groups. The meso form of erythritol, CH2OHCHOHCHOHCH2OH, is found in seaweeds and certain lichens both free and combined with lecanoric acid. The hexahydric sugar alcohols (e.g. sorbitol, mannitol and dulcitol) are formed in nature by the reduction of either an aldehyde group of an aldose or the keto groups of a ketose. Sorbitol is abundant in many rosaceous fruits, mannitol in manna and dulcitol in species of Euonymus.
ESTERS
Esters arise from the union of an alcohol and an acid with loss of water: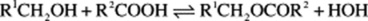 The reaction is reversible and in plants esterase enzymes control the reaction.
The reaction is reversible and in plants esterase enzymes control the reaction.
Many different types of esters are known, and those formed by an acetylation of an alcoholic group are very common and are found in many biosynthetic groups of metabolites including volatile oils, e.g. linalyl acetate in lavender. Esters which involve aromatic acids such as benzoic and cinnamic acids with corresponding alcohols are sometimes found associated with free acids, other volatile metabolites and resins, in such products as balsams (see drugs described at the end of this chapter). A number of alkaloids (e.g. atropine and reserpine) are esters.
A particularly important group of esters from the pharmaceutical viewpoint is that comprising the lipids or fatty esters. These involve a long-chain fatty acid of the type described earlier and alcohols such as glycerol and the higher monohydric alcohols.
The term ‘lipid’ includes not only fixed oils, fats and waxes (simple lipids), but also phosphatides and lecithins (complex lipids), which may contain phosphorus and nitrogen in addition to carbon, hydrogen and oxygen. These substances are widely distributed in both the vegetable and animal kingdoms, and in plants they are particularly abundant in fruits and seeds. In animals the depot fats resemble those found in plants, while the complex lipids occur mainly in the more active tissues such as the brain and liver. The latter group plays an important role in the structure of cellular membranes, the hydrophobic nature of the fatty acids being all-important to their biological role.
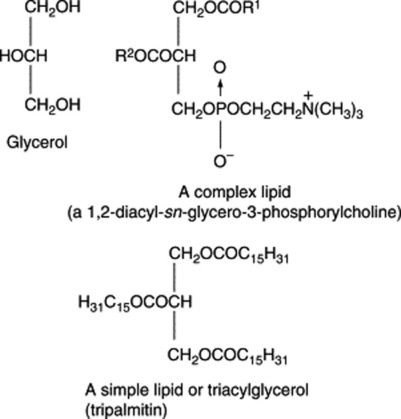
The lecithins are esters of glycerophosphoric acid in which the two free hydroxyls of the glycerol are esterified with fatty acids while one of the two remaining groups of the phosphoric acid is esterified to an alcohol (choline, ethanolamine, serine, glycerol or inositol). Because plants have no mechanism for controlling their temperature, they must possess membrane lipids that remain mobile at relatively low temperatures. This property is conferred by the methylene-linked cis double bonds of the polyunsaturated acids bound as esters with the polar lipids. Conversely, in simple lipids, all three hydroxyl groups are esterified with fatty acids and these compounds have been traditionally referred to as triglycerides, although with current nomenclature triacylglycerols is preferred. The prefix sn is now employed to denote the stereospecific numbering of the molecule.
Fats and fixed oils
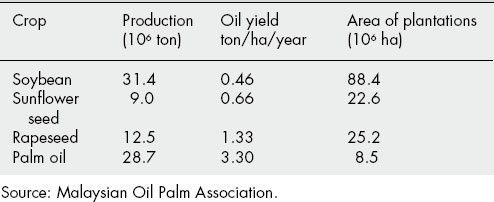
As agricultural crops, seeds used for the extraction of fixed oils rate in importance second only to cereals. Over the last 60 years the production of oils for the food industry has increased enormously, whereas consumption by industrial and other users has remained relatively static but, in the pharmaceutical industry at least, not without interesting developments. Fixed oils are also obtained from fruit pericarps and in some instances such as the palm, Elaeis guineensis (Palmae), two oils differing in properties and chemical composition are obtained—the pleasantly flavoured palm kernel oil from the endosperm and palm oil from the orange-yellow fleshy pericarp. Oil seed crops are particularly advantageous commercially as following the expression of the oil a valuable high protein cattle feed remains. Also, such crops have benefited from plant breeding both regarding the yield and nature of the oil produced, and the morphology of the plant itself (see Chapter 14).
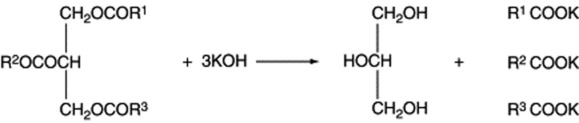
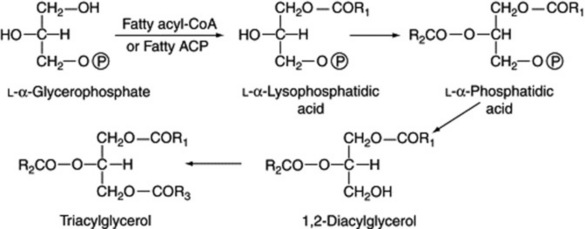
A naturally occurring mixture of lipids such as olive oil or oil of theobroma may be either liquid or solid and the terms ‘oil’ and ‘fat’ have, therefore, no very precise significance. Coconut oil and chaulmoogra oil, for example, leave the tropics as an oil and arrive in Western Europe as a solid. Even an oil such as olive oil will largely solidify in cold weather. In general, acylglycerols involving saturated fatty acids are solid and those of unsaturated acids are liquids. When both types are present, as in crude cod-liver oil, cooling results in the deposition of saturated acylglycerols such as stearin. In most medicinal cod-liver oils these solid materials are removed by freezing and filtration. Acylglycerols are represented by the general formula given below and can be hydrolysed by heating with caustic alkali to form soaps and glycerin.
If the fatty acids represented by R1, R2 and R3 are the same, the triacylglycerol is known as a simple triacylglycerol—for example, tripalmitin on hydrolysis yields three molecules of palmatic acid. In nature, however, R1, R2 and R3 are usually different and the ester is known as a mixed triacylglycerol. On hydrolysis they frequently yieldboth saturated and unsaturated acids (Fig. 19.6); there is a strong tendency for unsaturated acids, particularly the C18 olefinic acids, to be linked to the secondary hydroxyl.
Biogenesis
Acylglycerols are formed from the fatty acyl-CoA or, more probably, the fatty acyl carrier protein (ACP) and L-α-glycerophosphate, as indicated in Fig. 19.7.
Extraction
Most commercial oils are derived from either seeds or fruits and nowadays are mostly extracted by the producing country and exported as the crude oil. Sophisticated derivatizations of oils are mainly carried out by the importing countries.
The initial treatment before extraction depends on the botanical structure—for example, American cotton seeds require delinting and castor seeds and ground nuts require decorticating. Special machines are available for these purposes. Removal of the oil may take the form of cold or hot expression, centrifuging or solvent extraction, again depending on the commodity. With seeds the remaining cake usually forms a valuable cattle feed and for this reason complete removal of the oil is not always necessary. The crude oil requires refining, however, as for example with olive oil, the first expressed oils, extra virgin and virgin constitute the premium grades and require no further purification. Cold-drawn oils usually require nothing further than filtration; castor oil requires steaming to inactivate lipase; the addition of a determined amount of alkali may be required to remove free acid; and washing and decolorization may be performed. An antioxidant may be added and its nature and concentration stated on the label. Specific points concerning preparation are mentioned under the individual oils described at the end of the chapter. Where appropriate, the refined pharmaceutical oils are suitable for use in the manufacture of parenteral dosage forms.
Quantitative tests
A number of quantitative tests are commonly used to evaluate fixed oils and fats. Acid value refers to the number of mg of potassium hydroxide required to neutralize the free acids in 1 g of the oil; high acid values arise in rancidified oils. Particularly low values are officially specified for those oils to be used in parenteral dosage forms; for refined wheat-germ oil, for example, the value is  0.3, whereas for the refined oil for general use the value is
0.3, whereas for the refined oil for general use the value is  0.9 and for the unrefined oil
0.9 and for the unrefined oil  20.0. Similar figures apply to other fixed oils so used. Saponification value: the hydrolysis reaction of lipids (above) can be used to determine the saponification value of the oil and is expressed as the number of mg of potassium hydroxide required to neutralize the free acids in, and to hydrolyse the esters in, 1 g of the substance. Ester value is the difference between the saponification and acid values. Iodine value (see ‘Fatty acids’) gives a measure of the unsaturation of the oil. Oils which partially resinify on exposure to air are known as semidrying or drying oils. Such oils (e.g. linseed oil) have high iodine values. In some cases, particularly for animal fats such as butter, the determination of volatile acidity is useful, since the lower fatty acids such as butyric acid are volatile in steam and this may be used for their separation and estimation. It is frequently useful to determine unsaponifiable matter, which consists of compounds such as sterols which remain after saponification of the acylglycerols and removal of the glycerol and soaps by means of solvents. The content of brassicasterol in the sterol fraction of fixed oils is limited by the Pharmacopoeia for some oils, e.g. maximum 0.3% for evening primrose oil and borage oil.
20.0. Similar figures apply to other fixed oils so used. Saponification value: the hydrolysis reaction of lipids (above) can be used to determine the saponification value of the oil and is expressed as the number of mg of potassium hydroxide required to neutralize the free acids in, and to hydrolyse the esters in, 1 g of the substance. Ester value is the difference between the saponification and acid values. Iodine value (see ‘Fatty acids’) gives a measure of the unsaturation of the oil. Oils which partially resinify on exposure to air are known as semidrying or drying oils. Such oils (e.g. linseed oil) have high iodine values. In some cases, particularly for animal fats such as butter, the determination of volatile acidity is useful, since the lower fatty acids such as butyric acid are volatile in steam and this may be used for their separation and estimation. It is frequently useful to determine unsaponifiable matter, which consists of compounds such as sterols which remain after saponification of the acylglycerols and removal of the glycerol and soaps by means of solvents. The content of brassicasterol in the sterol fraction of fixed oils is limited by the Pharmacopoeia for some oils, e.g. maximum 0.3% for evening primrose oil and borage oil.
The acetyl value is the number of milligrams of potassium hydroxide required to neutralize the acetic acid freed by the hydrolysis of the acetylated fat or other substance. The oil is first acetylated with acetic anhydride, which combines with any free hydroxyl groups present, and the product is then isolated after thorough removal of acid resulting from the reagent; its saponification value is determined together with that of the original oil. The acetyl value is calculated from the difference between these two figures.
The hydroxyl value of a substance depends on the number of free hydroxyl groups present. It is expressed as the number of milligrams of potassium hydroxide required to neutralize the acid combined by acylation of the sample. Most fixed oils have low values, which arise from small quantities of sterols present; castor oil is an exception (minimum value 150), arising from the high proportion of ricinoleic acid present.
Under unsuitable storage conditions, such as exposure to light and air, fixed oils undergo secondary oxidation to give peroxides that then generate aldehydes and ketones. Such deterioration is detected by measurement of the peroxide value and anisidine value. The former is described by the Pharmacopoeia as ‘the number that expresses in milliequivalents of oxygen the quantity of peroxide contained in 1000 g of the substance, as determined by the prescribed methods’. These methods involve the liberation of iodine from a potassium iodide solution by the peroxides present in the sample and titration with 0.01 Msodium thiosulphate solution. For refined oils such as olive, borage, evening primrose and wheat-germ the typical value is 10; if these oils are to be used for parental dosage forms the maximum is 5. The maximum permissible value is higher for the virgin oils, e.g. olive = 20. Peroxide values are also used for fish oils, e.g. maximum value for farmed salmon oil, 5.0.
Anisidine values are determined photometrically (350 nm) and depend on the coloured complex produced by the interaction of p-anisidine (the methyl ether of p-aminophenol) with aldehydes and ketones. They are used principally for the evaluation of fish oils, including type-A cod-liver oil and farmed salmon oil (maximum 10).
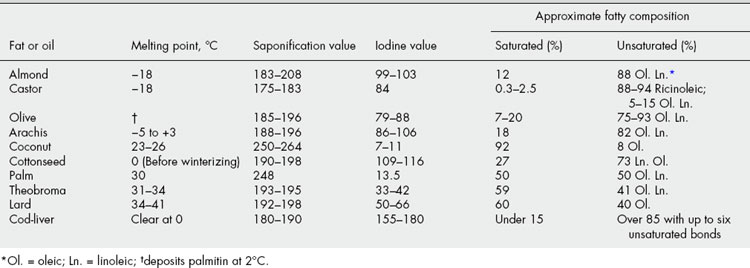
Certain physical constants of fixed oils and fats are significant: specific gravity, melting point, refractive index and sometimes optical rotation (e.g. castor oil). Table 19.6 shows how chemical standards are related to chemical composition. The gas chromatographic separation and quantification of the acids produced by the hydrolysis of specific fixed oils is an official method for their identification and quality control; type chromatograms are included in the BP/EP. Such detailed analyses often eliminate the necessity of rountinely applying all the above quantitative standards. Some examples of this application are given for the oils in Table 16.4. Comments on the detection of adulterants in the more expensive oils can be found under individual headings.
Waxes
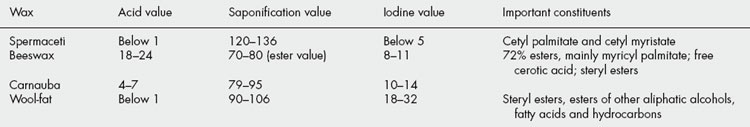
The term ‘wax’, although sometimes applied to the hydrocarbon mixture hard paraffin, is best confined to those natural mixtures containing appreciable quantities of esters derived from higher monohydric alcohols of the methyl alcohol series combined with fatty acids. In this series of alcohols the members change from liquids to solids, become less soluble in water and have higher melting points with increase in molecular weight. The first solid of the series is dodecyl alcohol, C12H25OH. Waxes include vegetable products such as carnauba wax and animal products such as spermaceti, beeswax and so-called ‘wool-fat’.
Although waxes are abundant in nature (e.g. on epidermal surfaces), a limited number only are of commercial importance; some of the best-known and their chief alcohols are given at the end of the chapter.
An important practical difference between fats and waxes is that fats may be saponified by means of either aqueous or alcoholic alkali but waxes are only saponified by alcoholic alkali. This fact is used for the detection of fats when added as adulterants to waxes (e.g. for detecting the fat ‘Japan wax’ as an adulterant in beeswax). Saponification of the wax ester cetyl palmitate may be represented as:
While fats consist almost entirely of esters, waxes, in addition to esters of the cetyl palmitate type, often contain appreciable quantities of free acids, hydrocarbons, free alcohols and sterols. The hydrocarbons and sterols are unsaponifiable and both spermaceti and wool fat, which contain considerable quantities of these, have high saponification values. If analytical data for fats and waxes are compared, it will be noted that the acid values of waxes tend to be higher—for example, beeswax contains about 15% of free cerotic acid, C26H53COOH. In most waxes, iodine values are relatively low and unsaponifiable matter is high (Table 19.7).
DRUGS CONTAINING ACIDS, ALCOHOLS AND ESTERS
ROSELLE
The dried calyces and epicalyces of Hibiscus sabdariffa L., family Malvaceae, collected during the fruiting period, constitute the drug ‘roselle’. As an ornamental, the plant is grown globally in subtropical areas and the leaves, stems and seeds also find use as colourants, flavourings and as a source of fibre (rosella hemp). Its common name, Jamaica sorrel, arose following its early introduction to that country. Commercial supplies of the drug come principally from S.E. Asia, Egypt and the Sudan.
Characters
The easily broken, crimson to violet drug consists of the flower portions comprising a pitcher-shaped calyx with five recurved teeth and, below, and attached to it, an epicalyx of up to about twelve obovate leaflets. The powder, examined microscopically, exhibits coloured parenchymatous cells containing cluster crystals of calcium oxalate, mucilage-containing cells, vascular tissue, epidermal cells, anisocytic stomata, covering trichomes, a few glandular trichomes and pollen grains with spiney exines.
Constituents
Roselle contains a considerable quantity of free acids including citric, tartaric and malic acids and the lactone of hydroxycitric acid. The BP/EP requires a minimum content of 13.5% of acids expressed as citric acid determined by potentiometric titration. Phenolic compounds include anthocyanins involving the glycosides of delphinidin and cyanidin for which the Pharmacopoeia specifies and absorbance of  0.350 for the whole drug and
0.350 for the whole drug and  0.250 for the cut drug when measured at 520 nm in a prescribed aqueous extract. A spectrophotometric assay for total anthocyanins has been reported (T. Sukwattanasinit et al., Planta Medica, 2007, 73, 1517). Various polysaccharides have been recorded; for a report on their stimulation of proliferation and differentiation of human keratinocytes see C. Brunold et al., Planta Med., 2004, 70, 373.
0.250 for the cut drug when measured at 520 nm in a prescribed aqueous extract. A spectrophotometric assay for total anthocyanins has been reported (T. Sukwattanasinit et al., Planta Medica, 2007, 73, 1517). Various polysaccharides have been recorded; for a report on their stimulation of proliferation and differentiation of human keratinocytes see C. Brunold et al., Planta Med., 2004, 70, 373.
Use
A colourant and flavouring component for herbal preparations. Traditionally, all parts of the plant have been employed as an astringent and cooling agent; it has a diuretic action. Antioxidant and hypocholesterolemic activities have been investigated (V. Hirunpanich et al., J. Ethnopharmacol., 2006, 103, 252).
Tamarind pulp
The drug consists of the fruit of the tree Tamarindus indica (Leguminosae) deprived of the brittle, outer part of the pericarp and preserved with sugar. The fruits are about 5–15 cm long. They have a brittle epicarp, a pulpy mesocarp, through which run from the stalk about five to nine branched fibres, and a leathery endocarp. The latter forms from four to twelve chambers, in each of which is a single seed.
In the West Indies the fruits ripen in June, July and August. The epicarps are removed, the fruits are packed in layers in barrels, and boiling syrup is poured over them; alternatively, each layer of fruits is sprinkled with powdered sugar.
Tamarind pulp occurs as a reddish-brown, moist, sticky mass, in which the yellowish-brown fibres mentioned above are readily seen. Odour, pleasant and fruity; taste, sweet and acid.
The seeds, each enclosed in a leathery endocarp, are obscurely four-sided or ovate and about 15 mm long. They have a rich brown testa marked with a large patch or oreole. Within the testa, which is very thick and hard, lies the embryo. The large cotyledons are composed very largely of hemicellulose which stains blue with iodine.
The pulp contains free organic acids (about 10% of tartaric, citric and malic), their salts (about 8% of potassium hydrogen tartrate), a little nicotinic acid and about 30–40% of invert sugar. It is reported that the tartaric acid is synthesized in the actively metabolizing leaves of the plant and then translocated to the fruits as they develop. The addition of sugar to the manufactured pulp, to act as a preservative, somewhat lowers the natural proportion of acids.
Flavonoid C-glycosides (vitexin, isovitexin, orientin and isoorientin) occur in the leaves. The fixed oil of the seeds contains a mixture of glycerides of saturated and unsaturated (oleic, linoleic) acids.
Tamarind pulp is a mild laxative and was formerly used in Confection of Senna; it has traditional medicinal uses in the W. Indies and in China and the leaves have been suggested as a commercial source of tartaric acid.
Manna
The name ‘manna’ is applied to a number of different plant products. The biblical manna was probably the lichen Lecanora esculenta, which can be carried long distances by wind. The only manna of commercial importance is ash manna, derived from Fraxinus ornus (Oleaceae). The drug is collected in Sicily. When the trees are about 10 years old, transverse cuts are made in the trunk. A sugary exudation takes place and when sufficiently dried is picked off (flake manna) or is collected on leaves or tiles.
Manna occurs in yellowish-white pieces up to 15 cm long and 2 cm wide or in agglutinated masses of broken flakes, with a pleasant odour and sweet taste. It contains about 55% of the hexahydric alcohol mannitol, relatively small amounts of hexose sugars but larger amounts of the more complex sugars mannotriose and mannotetrose (stachyose). The triose on hydrolysis yields glucose (1 mol) and galactose (2 mol), while the tetrose yields glucose (1 mol), fructose (1 mol) and galactose (2 mol). Manna has a mild laxative action.
SUMATRA BENZOIN
Two commercial varieties of benzoin—Sumatra benzoin and Siam benzoin—are included in the BP/EP. Sumatra benzoin (Gum Benjamin) is a balsamic resin obtained from the incised stem of Styrax benzoin Dryand, and Styrax paralleloneurus Perkins (Styracaceae). It is produced almost exclusively from cultivated trees grown in Sumatra, although the tree is also native to Java and Borneo.
History
The drug was noted by Ibn Batuta, who visited Sumatra in the fourteenth century, but was not regularly imported into Europe until the sixteenth century.
Collection and preparation
Sumatra benzoin is a purely pathological product and there is some evidence to show that its formation is brought about not only by the incisions made, but also by fungi (see ‘Stress Compounds’, Chapter 18). In Sumatra the seeds are sown in rice fields, the rice shading the young trees during their first year. After the harvesting of the rice the trees are allowed to grow until they are about 7 years old.
Tapping
The rather complicated process consists of making in each trunk three lines of incisions which are gradually lengthened.The first triangular wounds are made in a vertical row about 40 cm apart, the bark between the wounds being then scraped smooth. The first secretion is very sticky and is rejected. After making further cuts, each about 4 cm above the preceding ones, a harder secretion is obtained. Further incisions are made at 3-monthly intervals and the secretion instead of being amorphous becomes crystalline. About 6 weeks after each fresh tapping the product is scraped off, the outer layer (finest quality) being kept separate from the next layer (intermediate quality). About 2 weeks later the strip is scraped again, giving a lower quality darker in colour and containing fragments of bark. Fresh incisions are then made and the above process is repeated. After a time the line of incisions is continued further up the trunk.
Grades
The above three qualities are not sold as such but are blended in Palembang to give the benzoin grades of commerce. The best grade contains the most ‘almonds’ and the worst contains a few almonds but abundant resinous matrix. The blending is done by breaking up the drug, mixing different proportions of the three qualities and softening in the sun. It was formerly exported after stamping into tins but now the commercial drug arrives in plaited containers with a plastic wrapping.
Characters
Sumatra benzoin occurs in brittle masses consisting of opaque, whitish or reddish tears embedded in a translucent, reddish-brown or greyish-brown, resinous matrix. Odour, agreeable and balsamic but not very marked; taste, slightly acrid. Siamese benzoin occurs in tears or in blocks. The tears are of variable size and flattened; they are yellowish-brown or reddish-brown externally, but milky-white and opaque internally. The block form consists of small tears embedded in a somewhat glassy, reddish-brown, resinous matrix. It has a vanilla-like odour and a balsamic taste.
When gradually heated, benzoin evolves white fumes of cinnamic and benzoic acids which readily condense on a cool surface as a crystalline sublimate. On warming a little powdered benzoin with solution of potassium permanganate, a faint odour of benzaldehyde is noted with Sumatra benzoin but not with the Siamese. When an alcoholic solution of ferric chloride is added to an alcoholic extract of Siamese benzoin, a green colour is produced. Sumatra benzoin does not give this test. The BP/EP includes a TLC test for the absence of Dammar gum, a copal resin, used in the manufacture of varnishes and apparently derived from species of Hopea, Shorea and Vateria family Dipterocarpaceae.
Constituents
Sumatra benzoin contains free balsamic acids (cinnamic and benzoic) and esters derived from them. Also present are triterpenoid acids such as siaresinolic acid (19-hydroxyoleanolic acid) and sumaresinolic acid (6-hydroxyoleanolic acid). For the formula of oleanolic acid see under ‘Triterpenoid Saponins’. The content of total balsamic acids (calculated as cinnamic acid) is at least 20%, and the amount of cinnamic acid is usually about double that of benzoic acid. Up to about 20% of free acids may be present. High-grade material from S. paralleloneurum contains benzoic acid 3%, vanillin 0.5% and cinnamic acid 20–30%.
Allied drug
Palembang benzoin, an inferior variety produced in Sumatra, may be collected from isolated trees from which the resin has not been stripped for some time. It is easily distinguished, being very light in weight and breaking with an irregular porous fracture. It consists almost entirely of reddish-brown resin, with only a few very small tears embedded in it. Palembang benzoin is used as a source of natural benzoic acid.
Uses
Benzoin, when taken internally, acts as an expectorant and antiseptic. It is mainly used as an ingredient of friar’s balsam, or as a cosmetic lotion prepared from a simple tincture. It finds considerable use world-wide in the food, drinks, perfumery and toiletry industries; it is a component of incense.
SIAM BENZOIN
Siam benzoin BP/EP is obtained by incision of the trunk of Styrax tonkinensis (Pierre) Craib ex Hartwich; it contains 45.0–55.0% of total acids calculated as benzoic acid (dried drug). It is the traditional source of benzoin for a number of European pharmacopoeias.
The drug is produced in relatively small areas in the Thai province of Luang Prabang, northern Laos and northern Vietnam from trees growing in the wild at an altitude of between 600 and 2500 m. It seems that this height is necessary for resin production; not all trees are productive.
The method of collection appears similar to that for Sumatra benzoin, the resin being produced at the interface of the bark and wood layers only after injury. The collected tears are sorted into grades based on size and colour, the most esteemed being the largest and palest. Length of tears commonly varies from a few millimetres to 3 cm, flattened or sometimes, if large, concavo-convex as would be expected if the resin collected between the bark and the wood of the tree. They are yellowish-white to reddish on the outer surface, often in the commercial drug cemented together by the brownish resin, which increases due to oxidation as the material is stored. The fracture of the tears is waxy-white with an agreeable odour resembling vanilla.
Constituents
The combined acid content of Siam benzoin consists principally of coniferyl benzoate and a very small amount of coniferyl cinnamate (coniferyl alcohol, see Fig. 21.2, is found in the cambial sap of both gymnosperms and angiosperms). Free benzoic acid amounts to some 10% of the drug. Other constituents are triterpenoid acids and esters, and vanillin.
Tests
Compared with Sumatra benzoin, Siam benzoin is expensive and is liable to adulteration with the former, which can be detected by the BP/EP TLC test; this indicates an absence of cinnamic acid in the genuine drug. This absence also means that no odour of benzaldehyde is produced when the powdered drug is warmed with a solution of potassium permanganate cf. Sumatra benzoin. Also, Siam benzoin in ethanol, on treatment with ferric chloride solution, gives a green, not yellow, colour. The pharmacopoeial assay for total acids involves the back-titration with hydrochloric acid of a hydrolysed solution of the drug in alcoholic potassium hydroxide solution.
ASH LEAF
Ash leaf BP/EP consists of the dried leaves of Fraxinus excelsior L., the common ash, or Fraxinus oxyphylla M. Bieb., family Oleaceae, having a minimum content of 2.5% total hydroxycinnamic acid derivatives expressed as chlorogenic acid.
The common ash is found throughout temperate Europe, in Western Asia and extending northwards into Scandanavia; it is common in Britain. The leaves are up to 30 cm in length, opposite, pinnate and consisting of a rachis bearing 9–13 leaflets. It is the latter that constitute the official drug; they are about 7 cm long with short petiolules, lanceolate to ovate, apex apiculate to acuminate and a sharp, shallow forward-toothed margin. Colour dark green on the upper surface, lighter below.
Elements of the powdered drug include: upper epidermis with some striated cuticle and a lower surface with anomocytic stomata, occasional covering trichomes and, rarely, glandular trichomes.
Constituents of the leaf embrace the coumarin glycoside fraxin (formula shown in Table 21.2), various hydroxycinnamic acid derivatives, e.g. chlorogenic acid (see Fig. 19.5), tannins, the sugar alcohol mannite and bitter principles. The BP assay is based on the colour reaction of an acidified methanolic extract of the sample with solutions of sodium nitrite and sodium molybdate followed by alkali. Absorbance is measured at 525 nm.
Ash leaf has a mild laxative and diuretic action.
Allied species
Fraxinus ornus is a commercial source of manna (q.v.) and its leaflets can be substituted for the above. It may be distinguished by not affording the characteristic zones of acteoside, chlorogenic acid and rutin (see Fig. 21.15) when subjected to TLC examination. Hydroxycoumarins, secoiridoid glycosides, phenylethanoids and flavonoids have been reported in the plant. For a review, including biological activities, see I. Kostova, Fitoterapia, 2001, 72, 471; similarly for the genus (39–63 spp., depending on the classification) and featuring over 150 compounds, biological activities and 150 references, see I. Kostova and T. Iossifova, Fitoterapia, 2007, 78, 85–106.
ARTICHOKE LEAF
The leaves of the globe artichoke, Cynara scolymus L., family Asteraceae/Compositae, have been long-used in traditional medicine and are now included in the BP/EP, the BHP and the Complete German Commission E Monographs. The plant is native to the Mediterranean region and northern Africa and probably evolved from C. cardunculus at an early date (D. Bown, Encyclopedia of Herbs, 1995, Dorling Kindersley, London).
Leaves, up to ca 70 cm long and 30 cm wide, are collected and dried just before the flowering stage. The leaf lamina is deeply lobed, reaching to 1–2 cm of the midrib but becoming pinnatifid towards the petiole. The margin is coarsely toothed and trichomes cover both surfaces, being particularly dense and twisted on the lower surface. Hairs also cover the petioles, which, together with the main veins, are flat on the upper surfaces and raised and ridged on the lower.
The greyish-green to brown powder exhibits epidermi of straight- or sinuous-walled cells and many anomocytic stomata. Covering, multicellular, uniseriate trichomes occur scattered or in felted masses together with fewer glandular trichomes having brown contents in a monoseriate or biseriate head. Groups of lignified fibres and reticulately thickened vessels arise from veins of the petiole and midrib. The drug has a slight odour and a salty taste followed by bitterness.
Phenolic acids are important constituents and include chlorogenic acid, caffeic acid and cynarin (1, 5-di-O-caffeoylquinic acid) (see Fig. 19.5). The BP specifies a minimum requirement for chlorogenic acid of 0.8%, which is determined by liquid chromatography using chlorogenic acid reference solution for peak area comparison. Flavonoids include luteolin-7β-D-glucoside and the 7β-D-rutionoside. The former, together with chlorogenic acid, are used in the official TLC test for identity. Other constituents include volatile oil, sesquiperpene lactones, e.g. cynaropicrin, inulin, tannins and phytosterols.
Artichoke leaf is used for the treatment of indigestion and dyspepsia; for its use as a hepatic, see Chapter 29.
NETTLE LEAF
All parts of the nettle are used medicinally, the dried roots and herb in the BHP and the dried leaves in the BP/EP. The latter specifies two species—Urtica dioica L., the stinging nettle, and U. urens L., the small nettle. Both species are common throughout North temperate regions.
U. urens is an annual herb with the lower leaves shorter than their petioles, whereas U. dioica is a coarse hispid perennial with the lower leaves longer than their petioles.
The green powdered drug has a slight odour and bitter taste. Cells of both epidermises have sinuous anticlinal walls and the lower epidermis includes numerous anomocytic stomata. Cystoliths containing large calcium carbonate masses are present in the epidermal layers (see Fig. 42.1A). Clothing trichomes, stinging hairs and glandular trichomes are numerous. In the powder, the upper cells of the stinging trichomes are usually broken off.
The constituents of nettle have been extensively researched. They include a number of acids such as chlorogenic, caffeoylmalic, caffeic, malic and fumaric. Flavonoids include quercetin and its glycosides isoquercitrin and rutin (see Fig. 21.15). 5-Hydroxytryptamine (serotonin) is a component of the stinging hairs. Other metabolites include lignans, scopoletin (see Table 21.2) and choline acetyltransferase.
Scopoletin and chlorogenic acid are used as reference compounds in the BP TLC identification test. The assay is by liquid chromatography, requiring a minimum of 0.3% for the sum of the caffeoylmalic acid and chlorogenic acid content, expressed as chlorogenic acid. The high total ash limit for the drug (20.0%) is dictated by the considerable natural inorganic content, particularly silicic acid and calcium and potassium salts, which are present in the leaves.
Nettle has been used traditionally to treat many disorders. It is a diuretic and is employed in various rheumatic conditions and to assist micturition in cases of benign prostatic hyperplasia.
ECHINACEA SPP.
Echinacea species (coneflowers), are perennial herbs of the Compositae/Asteraceae native to the prairie regions of Ohio where they were used by the Plains tribes to treat a variety of conditions, particularly wounds. Three species are currently important. Roots of Echinacea augustifolia DC., the narrow-leaved coneflower, and E. pallida Nutt., the pale cornflower, are included in the BP/EP. The whole plant of E. purpurpea (the purple coneflower) is much used for the commercial preparation of herbal medicaments, it being the largest of the three species and easy to cultivate. All continue to receive research attention in connection with their phytochemical, pharmacological and clinical attributes.
Particular groups of compounds can be found in all three species but variations occur concerning specific metabolites. Some aspects of earlier research must also be treated with caution because it is now known that some commercial batches of so-called E. augustifolia grown in Europe were in fact E. pallida (R. Bauer et al., Planta Medica, 1988, 54, 426; Sci. Pharm., 1987, 55, 159; P. R. Bradley, Brit. Herb. Comp., 1992, 1, 81).
The roots of the two official drugs are not easily differentiated by their morphological and microscopical characteristics; in the powders, numerous sclereids and phytomelanin deposits occur in both. However, TLC can be used for the identification and also for the detection of E. purpurea in adulterated E. augustifolia, and to detect other Echinacea spp. and Parthenium integrifolium in E. pallida. A recent DNA study [sequence characterized amplified region (SCAR) analysis] has demonstrated the distinction of E. purpurea from the other two species (B. Adinolfi et al., Fitoterapia, 2007, 78, 43).
Constituents
A caffeic acid derivative, echinacoside, is present in the roots of both E. augustifolia and E. pallida. Cynarin, a quinic acid derivative, occurs only in the former. Esters involving tartaric acid, such as caftaric acid and cichoric acid, occur in small amounts in both species. Other constituents include high-molecular-weight polysaccharides, alkylamides, acetylenes, volatiles including humulene (see Fig. 21.4) and traces of pyrrolizidine alkaloids (F. Pellati et al., Phytochemistry, 2006, 67, 1359 and references cited therein).
The BP/EP requires minimum contents of echinacoside for E. augustifolia root (0.5%) and E. pallida root (0.2%) determined by liquid chromatography with spectrometric detection at 330 nm.
Actions
Echinacea is considered to have immunostimulant properties based on its alkylamide, polysaccharide and cichoric acid components. Preparations of the drug have become popular for the prevention and treatment of the common cold and other respiratory complaints. A recent meta-analysis of fourteen studies (S. A. Saah et al., The Lancet Infectious Diseases, 2007, 7, 473) indicated that Echinacea reduced both the incidence (by 58.0%) and the duration (by 1.4 days) of the common cold. For a mini-review on the role of alkamides as an active principle, see K. Woelkart and R. Bauer, Planta Medica, 2007, 73, 615; for a report on this controversial aspect, see B. Barrett, HerbalGram, 2006, 70, 36. Other traditional uses involve its anti-inflammatory and antibacterial properties.
PYGEUM BARK
Pygeum bark is obtained from the stems and branches of Prunus africana (Hook f.) Kalkm. syn. Pygeum africanum Hook f., family Rosaceae, a tree indigenous to the rain-forests of Africa. Cameroon is the principal exporter. The increased demand for the drug from Europe and the US has led, as with other trees not easily cultivated commercially, to the danger of over-collection.
The whole or cut bark is now included in the BP/EP and consists of dark- to reddish-brown pieces with an outer wrinkled cork with some lichens attached and an inner longitudinally striated surface. The powder exhibits typical bark characteristics: cork cells, sclereids in groups or singly, fibres principally in groups, pigmented cells, small starch grains and cluster crystals of calcium oxalate.
Constituents
Identified constituents include aliphatic alcohols occurring as ferulic acid esters, phytosterols, triterpenoid pentacyclic acids and a lipid fraction involving C12–C24 fatty acids. Pharmacopoeial tests include a minimum extractive value of 0.5% (continuous Soxhlet-type extraction with methylene chloride as solvent) and a TLC separation to show characteristic bands including those of β-sitosterol and ursolic acid.
PERU BALSAM
Balsam of Peru is obtained from the trunk of Myroxylon balsamum var. pereirae (Leguminosae), after it has been beaten and scorched. The drug is produced in Central America (San Salvador, Honduras and Guatemala) and is now included in the European Pharmacopoeia and the BP (2000).
History
The drug derives its name from the fact that when first imported into Spain it came via Callao in Peru. It was known to Monardes and the method of preparation was described as early as 1576, although afterwards forgotten. In 1860 the collection was described and illustrated by Dorat.
Collection and preparation
In November or December strips of bark, measuring about 30 × 15 cm, are beaten with the back of an axe or other blunt instrument. The bark soon cracks and may be pulled off after 2 weeks. As in the case of Tolu balsam, the secretion is purely pathological in origin and very little balsam can be obtained from the bark unless it is charred with a torch about 1 week after the beating. The balsam produced in the bark is obtained by boiling the bark in water and is known as tacuasonte (prepared without fire) or balsamo de cascara (balsam of the bark).
The greater part of the balsam, however, is prepared, after the removal of the bark, by the second method. The balsam which exudes is soaked up with rags, which, after some days, are cleaned by gently boiling with water and squeezing in a rope press. The balsam sinks to the bottom and, the water having been decanted, the balsam (balsamo de trapo) is poured off and strained.
Less destructive methods of preparation have been investigated and include the removal of narrow strips of bark and the replacement of scorching with the use of a hot iron. With this treatment the tree recovers in 6 months, compared with 8 years after the drastic traditional method. The drug is chiefly exported from Acajutla (San Salvador) and Belize (British Honduras) in tin canisters holding about 27 kg.
Characters
Balsam of Peru is a viscid liquid of a somewhat oily nature, but free from stickiness and stringiness. When seen in bulk, it is dark brown or nearly black in colour, but in thin layers it is reddish-brown and transparent. The original containers have a whitish scum on the surface. The balsam has a pleasant, somewhat vanilla-like odour and an acrid, slightly bitter taste.
The drug is almost insoluble in water. It is soluble in one volume of alcohol (90%), but the solution becomes turbid on the addition of further solvent. The relative density, 1.14–1.17, is a good indication of purity, and if abnormal indicates adulteration with fixed oils, alcohol, kerosene, etc. The BP/EP includes tests for the absence of artificial balsams (solubility characteristics in petroleum spirit), fixed oils (solubility in chloral hydrate solution) and turpentine (odour test).
Constituents
The official drug is required to contain not less than 45.0% w/w and not more than 70% w/w of esters, assayed gravimetrically. The chief balsamic esters present are benzyl cinnamate (cinnamein) C6H5CH=CHCOOCH2C6H5 (sap. value 234), benzyl benzoate (sap. value 264.3) and cinamyl cinnamate (styracin). The drug also contains about 28% of resin, which is said to consist of peruresinotannol combined with cinnamic and benzoic acids, alcohols (nerolidol, farnesol and benzyl alcohol) and small quantities of vanillin and free cinnamic acid.
Work on the isoflavonoids contained in the trunk-wood has indicated that considerable chemical differences characterize the various forms or species of the M. balsamum group.
Prepared storax
Prepared storax is a balsam obtained from the wounded trunk of Liquidambar orientalis (Hamamelidaceae) and subsequently purified. This is known as Levant storax and is obtained from a small tree found in the south-west of Turkey.
Collection and preparation
In the early summer the bark is injured by bruising or by making incisions. After a time the outer bark may be pared off, or the whole bark may be left until the autumn, when it is removed. The pieces of bark are pressed in horse-hair bags, first in the cold and again after steeping in hot water. Sometimes the bark is boiled with water and again pressed. The exhausted bark is used in the East for fumigation. The crude or liquid storax is exported in casks from Izmir.
Storax is obtained by dissolving the crude balsam in alcohol, filtering and recovering the solvent at as low a temperature as possible so as not to lose any of the volatile constituents. The alcohol-insoluble matter consists of vegetable debris and a resin.
Characters
Crude storax is a greyish, viscous liquid with a pleasant odour and bitter taste. It usually contains about 20–30% of water. About 82–87% is alcohol soluble.
Purified storax forms a brown, viscous, semisolid mass which loses not more than 5% of its weight when dried on a water-bath for 1 h. It is completely soluble in alcohol and partially in either. It has a characteristic balsamic odour and taste.
Constituents
Storax is very rich in free and combined cinnamic acid. After purification it yields 30–47% of total balsamic acids.
By steam distillation storax yields an oily liquid containing phenylethylene (styrene), C6H5CH=CH2, cinnamic esters, vanillin and free cinnamic acid. The resinous portion of the drug consists of resin alcohols present both free and combined with cinnamic acid. The presence of cinnamic acid in the drug is shown by the odour of benzaldehyde which is produced when the drug is mixed with sand and warmed with a solution of potassium permanganate.
Recent research carried out in Turkey has shown the presence of many compounds not previously reported; these include monoterpenes, phenylpropanes and aliphatic acids.
Allied drug
American storax obtained from L. styraciflua, a large tree found near the Atlantic coast from Central America to Connecticut, is also used in the USA. This balsam resembles the Levant storax in constituents. Thirty-six compounds have been identified in the leaf-oil of the plant and tannins and related phenolics obtained from cell cultures. American researchers have reported the fruits as a rich source of shikimic acid, starting material for the synthesis of the antiviral drug oseltamivir. The yield is greatly superior to that from star-anise fruits, the accepted source material.
TOLU BALSAM
Tolu Balsam is obtained by incision from the trunk of Myroxylon balsamum (L.) Harms. var. balsamum (Leguminosae), a large tree that differs but little from that yielding balsam of Peru. Wild trees occur in Colombia and Venezuela and in the former country large quantities of balsam were produced in the neighbourhood of the Magdalena and Cauca rivers. The trees are cultivated in the West Indies, particularly in Cuba.
History
Balsam of Tolu was described by Monardes in 1574 and its collection was observed by Weir in 1863.
Collection
The drug is collected by making V-shaped incisions in the bark, the secretion being received in a calabash placed in the angle of the V. Many such receivers are fixed on each tree, the yield per tree being 8–10 kg. Periodically, the balsam is transferred to larger containers. It is exported in tins from Cartagena, Sabanilla and Sta. Marta.
Characters
When freshly imported, tolu is a soft, yellow semisolid. On keeping it turns to a brown, brittle solid. It softens on warming, and if a little is then pressed between two glass slides, microscopical examination shows crystals of cinnamic acid, amorphous resin and vegetable debris. Odour is aromatic and fragrant; taste, aromatic; the drug forms a plastic mass when chewed.
It is almost entirely soluble in alcohol, the solution being acid to litmus, and giving a green colour with ferric chloride (the latter possibly owing to the presence of resinotannol). Like other drugs containing cinnamic acid, it yields an odour of benzaldehyde when a filtered decoction is oxidized with potassium permanganate solution.
Constituents
Tolu contains about 80% of resin derived from resin alcohols combined with cinnamic and benzoic acids. The drug is rich in free aromatic acids and contains about 12–15% of free cinnamic and about 8% of free benzoic acid (acid value from 100–160). Other constituents are esters such as benzyl benzoate and benzyl cinnamate and a little vanillin. Recent investigations have shown the presence of other esters, styrene, eugenol, vanillin, ferulic acid, 1,2-diphenylethane, mono- and sesquiterpene hydrocarbons and alcohols. The balsam also contains numerous triterpenoids. Total balsamic acids (BP/EP, 25–50%) are determined by titration following hydrolysis of the esters.
PHARMACEUTICAL FIXED OILS AND FATS
ALMOND OIL
Almond oil is a fixed oil obtained by expression from the seeds of Prunus dulcis (Miller) E. A. Webb (Rosaceae) var. dulcis (sweet almond) or P. dulcis (Miller) D. A. Webb var. amara (D.C.) Buchheim (bitter almond) or a mixture of both varieties.
The oil is mainly produced from almonds grown in the countries bordering the Mediterranean (Italy, France, Spain and North Africa).
Characters of plants and seeds
Almond trees are about 5 m in height and the varieties, except for differences in the seeds, are almost indistinguishable. The young fruits have a soft, felt-like pericarp, the inner part of which gradually becomes sclerenchymatous as the fruit ripens to form a pitted endocarp or shell. The shells, consisting mainly of sclerenchymatous cells, are sometimes ground and used to adulterate powdered drugs.
The sweet almond is 2–3 cm in length, rounded at one end and pointed at the other. The bitter almond is 1.5–2 cm in length but of similar breadth to the sweet almond. Both varieties have a thin, cinnamon-brown testa which is easily removed after soaking in warm water, a process which is known as blanching. The oily kernel consists of two large, oily planoconvex cotyledons, and a small plumule and radicle, the latter lying at the pointed end of the seed. Some almonds have cotyledons of unequal sizes and are irregularly folded. Bitter almonds are sometimes found in samples of sweet almonds, particularly those of African origin; their presence may be detected by the sodium picrate test for cyanogenetic glycosides.
Constituents
Both varieties of almond contain 40–55% of fixed oil, about 20% of proteins, mucilage and emulsin. The bitter almonds contain in addition 2.5–4.0% of the colourless, crystalline, cyanogenetic glycoside amygdalin (see Chapter 25).
Refined almond oil is obtained by grinding the seeds and expressing them in canvas bags between slightly heated iron plates. They are sometimes blanched before grinding, but this does not appear to be of any particular advantage. The oil is clarified by subsidence and filtration. It is a pale yellow liquid with a slight odour and bland, nutty taste. The BP/EP specifies oleic acid 62–86%, linoleic acid 20–30%, palmitic acid 4–9% together with lesser amounts of other acids as produced by the hydrolysis of the oil using GLC analysis. There are tests for the absence of other oils and sterols; the sterol fraction of the genuine oil consists principally of β-sitosterol (73–87%).
Virgin almond oil BP/EP conforms to similar tests as above but is not refined.
Hydrogenated almond oil is also included in the BP/EP.
Essential or volatile oil of almonds is obtained from the cake left after expressing bitter almonds. This is macerated with water for some hours to allow hydrolysis of the amygdalin to take place. The benzaldehyde and hydrocyanic acid are then separated by steam distillation.
Bitter almond oil contains benzaldehyde and 2–4% of hydrocyanic acid. Purified volatile oil of bitter almonds has had all its hydrocyanic acid removed and therefore consists mainly of benzaldehyde.
ARACHIS OIL
Arachis oil is obtained by expression from the seeds of Arachis hypogaea L. (Leguminosae) (earth-nut, ground-nut, peanut) a small annual plant cultivated throughout tropical Africa and in India, Brazil, southern USA and Australia. Various genotypes exist which show differences in the relative amounts of fatty acids contained in the oil. Enormous quantities of the fruits and seeds are shipped to Marseilles and other European ports for expression. Ground-nuts are the world’s fourth largest source of fixed oil.
Preparation
During ripening the fruits bury themselves in the sandy soil in which the plants grow. Each fruit contains from one to three reddish-brown seeds. The fruits are shelled by a machine. The kernels contain 40–50% of oil. Owing to the high oil content the seeds, when crushed, are somewhat difficult to express. After the initial ‘cooking’, part of the oil is removed in a low-pressure expeller and the cake is solvent extracted. The two oil fractions are then mixed before purification. The press cake forms an excellent cattle food. The ground pericarps have been used as an adulterant of powdered drugs.
Constituents
Arachis oil consists of the glycerides of oleic, linoleic, palmitic, arachidic, stearic, lignoceric and other acids. When saponified with alcoholic potassium hydroxide, crystals of impure potassium arachidate separate on standing. Arachis oil is one of the most likely adulterants of other fixed oils (e.g. olive oil). The BP examination of the oil is similar to that mentioned under ‘Olive Oil’ below; the temperature at which the cooling, hydrolysed oil becomes cloudy should not be below 36°C. As with olive oil more stringent standards are required for oil to be used parenterally.
Uses
Arachis oil has similar properties to olive oil. It is an ingredient of camphorated oil but is used mainly in the production of margarine, cooking fats, etc.
Hydrogenated arachis oil is produced by refining, bleaching, hydrogenating and deodorizing the above. As with other hydrogenated oils it is much thicker than the parent oil, constituting a soft, off-white mass. There are various types of the hydrogenated oil with so-called nominal drop points determined as prescribed in the Pharmacopoeia; these fall within the range 32–42°C and, within this range, the drop point should not differ by more than 3°C from the nominal value. Again, as with other hydrogenated oils there is a limit for nickel ( 1ppm) determined by atomic absorption spectrometry. The peroxide value should not exceed 5.0%.
1ppm) determined by atomic absorption spectrometry. The peroxide value should not exceed 5.0%.
COCONUT OIL
The expressed oil of the dried solid part of the endosperm of the coconut, Cocos nucifera L. (Palmae) is a semisolid, melting at about 24°C and consisting of the triglycerides of mainly lauric and myristic acids, together with smaller quantities of caproic, caprylic, oleic, palmitic and stearic acids. This constitution gives it a very low iodine value (7.0–11.0) and a high saponification value.
The particularly high proportion of medium chain-length acids means that the oil is easily absorbed from the gastrointestinal tract, which makes it of value to patients with fat absorption problems.
Fractionated coconut oil
Fractionated and purified endosperm oil of the coconut C. nucifera, or Thin Vegetable Oil of the BPC, contains triglycerides containing only the short and medium chain-length fatty acids (e.g. octanoic, decanoic; see Table 19.2). It maintains its low viscosity until near the solidification point (about 0°C) and is a useful nonaqueous medium for the oral administration of some medicaments.
Medium-chain Triglycerides BP/EP, synonymous with the above, may also be obtained from the dried endosperm of Elaeis guineensis (Palmae). The fatty acid composition of the hydrolysed oil, determined by GC, has the following specifications: caproic acid  2%, caprylic acid 50–80%, capric acid 20–50%, lauric acid
2%, caprylic acid 50–80%, capric acid 20–50%, lauric acid  3.0%, myristic acid
3.0%, myristic acid  1.0%.
1.0%.
COTTONSEED OIL
Cottonseed oil is expressed from the seeds of various species of Gossypium (Malvaceae) in America and Europe. In the UK, Egyptian and Indian cottonseed, which do not require delinting on arrival, are largely used. See under ‘Cotton’.
The preparation of cottonseed oil is one of hot expression and a pressure of about 10 000 kPa is used. The crude oil is thick and turbid and is refined in various ways, that known as ‘winter bleached’ being the best of the refined grades. Cottonseed oil is a semi-drying oil and has a fairly high iodine value. When used to adulterate other oils its presence may be detected by the test for semidrying oils described in the BP monograph for Arachis Oil.
LINSEED AND LINSEED OIL
Linseed (flaxseed) is the dried ripe seed of Linum usitatissimum L. (Linaceae), an annual herb about 0.7 m high with blue flowers and a globular capsule. The flax has long been cultivated for its pericyclic fibres and seeds. Supplies of the latter are derived from South America, India, the USA and Canada. Large quantities of oil are expressed in England, particularly at Hull, and on the Continent.
Macroscopical characters
The seeds are ovate, flattened and obliquely pointed at one end; about 4–6 mm long and 2–2.5 mm broad. The testa is brown, glossy and finely pitted. Odourless; taste, mucilaginous and oily. If cruciferous seeds are present, a pungent odour and taste may develop on crushing and moistening. A transverse section shows a narrow endosperm and two large, planoconvex cotyledons.
Microscopical characters
Microscopical examination of the testa shows a mucilage-containing outer epidermis; one or two layers of collenchyma or ‘round cells’; a single layer of longitudinally directed elongated sclerenchyma; the hyaline layers or ‘cross-cells’ composed in the ripe seed of partially or completely obliterated parenchymatous cells with their long axis at right angles to those of the sclerenchymatous layer; and an innermost layer of pigment cells. The outer epidermis is composed of cells, rectangular or five-sided in surface view, which swell up in water and become mucilaginous. The outer cell walls, when swollen in water, show an outer solid stratified layer and an inner part yielding mucilage, itself faintly stratified. The radial layers or ‘round cells’ are cylindrical in shape and show distinct triangular intercellular air spaces. The sclerenchymatous layer is composed of elongated cells, up to 250 mu;m in length, with lignified pitted walls. The hyaline layers often remain attached to portions of the sclerenchymatous layer in the powdered drug (see Fig. 41.7I). The pigment layer is composed of cells with thickened pitted walls and containing amorphous reddish-brown contents (Fig. 41.7H). The cells of the endosperm and cotyledons are polygonal with somewhat thickened walls, and contain numerous aleurone grains and globules of fixed oil. Starch is present in unripe seeds only.
Constituents
Linseed contains about 30–40% of fixed oil, 6% of mucilage (BP swelling index for whole seeds  4.0), 25% of protein and small quantities of the cyanogenetic glucosides linamarin and lotaustralin. Other constituents are phenylpropanoid glycosides (L. Luyengi et al., J. Nat. Prod., 1993, 56, 2012), flavonoids, the lignan (–)-pinoresinol diglucoside (a tetrahydrofurofuran-type lignan—see Table 21.7) and the cancer chemoprotective mammalian lignan precursor secoisolariciresinol diglucoside (S.-X. Qiu et al., Pharm. Biol., 1999, 37, 1). Recently, 22 different lignans, mainly of the aryltetralin type, have been identified from Bulgarian species of Linum, section Syllinum (N. Vasilev et al., Planta Medica, 2008, 74, 273).
4.0), 25% of protein and small quantities of the cyanogenetic glucosides linamarin and lotaustralin. Other constituents are phenylpropanoid glycosides (L. Luyengi et al., J. Nat. Prod., 1993, 56, 2012), flavonoids, the lignan (–)-pinoresinol diglucoside (a tetrahydrofurofuran-type lignan—see Table 21.7) and the cancer chemoprotective mammalian lignan precursor secoisolariciresinol diglucoside (S.-X. Qiu et al., Pharm. Biol., 1999, 37, 1). Recently, 22 different lignans, mainly of the aryltetralin type, have been identified from Bulgarian species of Linum, section Syllinum (N. Vasilev et al., Planta Medica, 2008, 74, 273).
Cell cultures of Linum album are able to synthesize and accumulate the lignans podophyllotoxin and 5-methylpodophyllotoxin (T. Smollny et al., Phytochemistry, 1998, 48, 975).
Linseed oil
The extraction of linseed oil is one of hot expression of a linseed meal and the press is adjusted to leave sufficient oil in the cake to make it suitable as a cattle food.
Linseed oil of BP quality is a yellowish-brown drying oil with a characteristic odour and bland taste; much commercial oil has a marked odour and acrid taste. On exposure to air it gradually thickens and forms a hard varnish. It has a high iodine value ( 175) as it contains considerable quantities of the glycosides of unsaturated acids. Analyses show α-linolenic acid, C17H29COOH (36–50%), linoleic acid C17H31COOH (23–24%), oleic acid C17H33COOH (10–18%), together with some saturated acids—myristic, stearic and palmitic (5–11%). For the formation of the unsaturated acids, see Figs. 19.1 and 19.2.
175) as it contains considerable quantities of the glycosides of unsaturated acids. Analyses show α-linolenic acid, C17H29COOH (36–50%), linoleic acid C17H31COOH (23–24%), oleic acid C17H33COOH (10–18%), together with some saturated acids—myristic, stearic and palmitic (5–11%). For the formation of the unsaturated acids, see Figs. 19.1 and 19.2.
For use in paint, linseed oil was boiled with ‘driers’ such as litharge or manganese resinate which, by forming metallic salts, caused the oil to dry more rapidly. Such ‘boiled oils’ must not be used for medicinal purposes.
Uses
Crushed linseed is used in the form of a poultice and whole seeds are employed to make demulcent preparations. The oil is used in liniments, and research has suggested that hydrolysed linseed oil has potentially useful antibacterial properties as a topical preparation in that it is effective against Staphylococcus aureus strains resistant to antibiotics. Linseed cake is a valuable cattle food.
OLIVE OIL
Olive oil (salad oil, sweet oil) is a fixed oil which is expressed from the ripe fruits of Olea europoea L. (Oleaceae). The olive is an evergreen tree, which lives to a great age but seldom exceeds 12 m in height. It produces drupaceous fruits about 2–3 cm in length. The var. latifolia bears larger fruits than the var. longifolia, but the latter is said to yield the best oil. The oil is expressed in all the Mediterranean countries and in California. Italy, Spain, France, Greece and Tunisia produce 90% of the world’s production.
Olive oil has been ranked sixth in the world’s production of vegetable oils (F. D. Gunstone et al., 1994, The Lipid Handbook, Chapman and Hall).
History
The olive appears to be a native of Palestine. It was known in Egypt in the seventeenth century BC, and was introduced into Spain at an early period.
Collection and preparation
The methods used for the preparation of the oil naturally vary somewhat according to local conditions. In the modern factories hydraulic presses are widely used but in the more remote districts the procedure is essentially that which has been followed for hundreds of years and is described in earlier editions of this book. The first oil to be expressed from the ground fruits is known as virgin oil; subsequently the marc may be solvent extracted to obtain the lower quality oil. The superior grades of oil are extra virgin, virgin and pure or refined. The Pharmacopoeia includes monographs for both the virgin oil and refined oil.
Characters
Olive oil is a pale yellow liquid, which sometimes has a greenish tint. The amount of colouring matter present, whether chlorophyll or carotene, appears to determine the natural fluorescence of the oil in ultraviolet light.
The oil has a slight odour and a bland taste. If the fruits used have been allowed to ferment, the acid value of the oil will be higher than is officially permitted. It should comply with the tests for absence of arachis oil, cotton-seed oil, sesame oil and tea-seed oil. The latter oil, which is obtained from China, is not from the ordinary tea plant but from a tree, Camellia sasanqua.
Constituents
Olive oils from different sources differ somewhat in composition. This may be due either to the use of the different varieties of olive or to climatic differences. Two types of oilmay be distinguished: (a) that produced in Italy, Spain, Asia Minor and California, which contains more olein and less linolein than type; and (b), produced in the Dodecanese and Tunisia. Typical analyses of these types are:
The characteristic odour of olive oil, particularly the virgin oils, arises from the presence of volatile C6 alcohols (hexanol, E-2-hexenol, Z-3-hexenol), C6 aldehydes and acetylated esters. Oils arising from different chemotypes can be distinguished by headspace gas–liquid chromatography (M. Williams et al., Phytochemistry, 1998, 47, 1253). The dehydrogenases which produce the unsaturated alcohols have been studied in the pulp of developing olive pericarps (J. J. Salas and J. Sánchez, Phytochemistry, 1998, 48, 35).
The BP/EP examination of the oil includes a TLC test for identity and, to detect foreign oils, the GC determination of the individual methyl esters of the acids produced by hydrolysis. Limits of the principal acids are given above: other percentage limits, which exclude foreign oils, include saturated fatty acids of chain length less than C16( 0.1), linolenic (
0.1), linolenic ( 1.2), arachidic (
1.2), arachidic ( 0.7), behenic (
0.7), behenic ( 0.2). There are also limits for a number of sterols in this fraction of the oil including campestrol
0.2). There are also limits for a number of sterols in this fraction of the oil including campestrol  4.0%, cholesterol
4.0%, cholesterol  0.5%, Δ7-stigmasterol 7ngt;0.5% and Δ5, 24-stigmastadienol.
0.5%, Δ7-stigmasterol 7ngt;0.5% and Δ5, 24-stigmastadienol.
For the biosynthesis of the acids, see the introduction to this chapter.
The saturated glycerides tend to separate from the oil in cold weather; at 10°C the oil begins to become cloudy and at about 0°C forms a soft mass.
Uses
Olive oil is used in the preparation of soaps, plasters, etc., and is widely employed as a salad oil. Oil for use in the manufacture of parenteral preparations is required to have a lower acid value and peroxide value than that normally required, and to be almost free of water (0.1%) as determined by the specified Karl Fischer method.
Recent research has suggested that olive oil may protect against colonic carcinogenesis by virtue of its action on prostaglandins: rats fed on a diet containing olive oil, as distinct from those receiving safflower oil, were protected (R. Bartoli et al., Gut, 2000, 46, 191).
Both fruits and oil are now widely promoted in health-food stores on account of their α-linolenic acid content.
PALM OIL AND PALM KERNEL OIL
Palm oil
The oil is obtained by steaming and expression of the mesocarp of the fruits of Elaeis guineënsis Jacq. (Palmae). This palm occurs throughout tropical Africa but over half of the world production of oil originates from Malaysia. In terms of world production palm oil had by 2003 overtaken that of sunflower and rapeseed oils (Table 19.8). It will be noted that for the oils listed, palm oil is superior regarding land use and productivity.
Palm oil is yellowish-brown in colour, of a buttery consistency (m.p. 30°C) and of agreeable odour. Palmitic and oleic acids are the principal esterifying acids.
Palm kernel oil
Palm kernel oil is obtained by heating the separated seeds for 4–6 hours to shrink the shell, which is then cracked and the kernels removed whole. The oil is then obtained by expression. It differs chemically from palm oil (above) in containing a high proportion (50%) of the triglycerides of lauric acid, a saturated, medium chain-length fatty acid (Table 19.2). The mixture of other acids resembles that found in coconut oil.
RAPESEED OIL
Refined Rapeseed Oil BP/EP is obtained by mechanical expression or by extraction from the seeds of Brassica napus L. and B. campestris L.
The crop is now extensively cultivated in Europe and oils with various properties depending on glyceride composition are commercially available. As was indicated in Chapter 14 not all varieties yield an oil suitable for medicinal purposes so that the specified pharmacopoeial limits of the various esterifying acids are important standards as are relative density (c. 0.917) and refractive index (c. 1.473). Oleic, linoleic and linolenic acids are the principal esterifying acids, with eicosenoic acid and erucic acid limited to 5.0 and 2.0% of the acid fraction, respectively.
As with some other oils, the addition of a suitable antioxidant is permitted, the name and concentration of which must be stated on the label together with a statement as to whether the oil was obtained by mechanical expression or by extraction.
SAFFLOWER OIL
Safflower oil is obtained by expression or extraction from the seeds (achenes) of Carthamus tinctorius L. (Compositae) or hybrids of this species. Commonly known as safflower, false saffron, saffron thistle, the plant is native to Mediterranean countries and Asia and has been used for colouring and medicinal purposes from Ancient Egyptian and Chinese times. The pigment from the flowers (carthamin) is yellow in water and red in alcohol and was the traditional dye for the robes of Buddhist monks. It is now cultivated largely for the seed oil in its countries of origin, as well as in the US, Australia, Africa and S.E. Asia.
As produced commercially, safflower oil is of two types—type 1 coming from the original species and type 2 from hybrid forms producing an oil rich in oleic acid; the acid fraction of the type 1 oil has oleic acid limits of 8–12% and the type 2 oil 70–84%. Other acids are palmitic, stearic and linoleic acids generally proportionately higher for the type 1 oil.
The pharmacopoeial oil is refined and may have an added antioxidant; it should comply with the usual tests for fixed oils with brassicasterol limited to 0.3% of the sterol fraction.
Safflower oil, like linseed oil, is a drying oil and has found commercial use in paints and varnishes. It is used in the food industry and is recommended for its cholesterol-lowering properties.
SESAME OIL
Sesame oil (Gingelly oil, Teel oil) is obtained by refining the expressed or extracted oil from the seeds of Sesamum indicum L. (Pedaliaceae), a herb which is widely cultivated in India, China, Japan and many tropical countries. The oil is official in the EP and BP.
The seeds contain about 50% of fixed oil which closely resembles olive oil in its properties and which it has, in some measure, replaced. It is a pale yellow, bland oil which on cooling to about −4°C solidifies to a buttery mass; it has a saponification value the same as that for olive oil and a somewhat higher iodine value (104–120).
Principal components of the oil are the glycerides of oleic and linoleic acids with small proportions of palmitic, stearic and arachidic acids. It also contains about 1% of the lignan sesamin, and the related sesamolin. The characteristic phenolic component is the basis of the BP test for identity and also the test for the detection of sesame oil in other oils. The original test involved the production of a pink colour when the oil was shaken with half its volume of concentrated hydrochloric acid containing 1% of sucrose (Baudouin’s test). However, some commercially refined oils may not give a positive Baudouin’s test. With the current BP test for the absence of sesame oil in other oils, e.g. olive oil, the reagents are acetic anhydride, a solution of furfuraldehyde and sulphuric acid; a bluish-green colour is a positive result. The composition of triglycerides in sesame oil is determined by liquid chromatography, those triglycerides having as acid radicals oleic 1 part and linoleic 2 parts, and those having oleic 2 parts and linoleic 1 part being among the most predominant.
As stated above, sesame seeds and oil also contain lignans; these are antioxidants of the tetrahydrofurofuran-type (Table 21.7) and include sesamin, sesamolinol and sesamolin. Another lignan, sesaminol, is formed during industrial bleaching of the oil. The biological activities of these compounds include reduction in serum cholesterol levels and increased vitamin E activities. For the biogenesis of such lignans see M. J. Kato et al., Phytochemistry, 1998, 47, 583.
SOYA OIL
Soya oil is obtained from the seeds of Glycine soja Sieb. and Zucc. and Glycine max (L.) Merr (G. hispida (Moench) Maxim).
Refined soya oil. Processing involves deodorization and clarification by filtration at about 0°C. It should remain bright when kept at 0°C for 16 h. The principal esterifying fatty acids are linoleic (48–58%), oleic (17–30%), linolenic (5–11%) and stearic (3–5%). There is an official limit test of not more than 0.5% for the brassicasterol content of the sterol fraction of the oil.
Hydrogenated soya oil occurs as a white mass or powder; it consists principally of the triglycerides of palmitic and stearic acid.
SUNFLOWER OIL
Sunflower oil is obtained from the seeds of Helianthus annuus L. by mechanical expression or by extraction; it is then refined. Principal components of the fatty-acid mixture produced by hydrolysis of the oil are linoleic acid (48–74%), oleic acid (14–40%), palmitic acid (4–9%) and stearic acid (1–7%).
The oil can be identified (BP/EP test) by the comparison of a thin-layer chromatogram of the sample with that of the pharmacopoeial illustration of the typical oil. Other tests include relative density (c. 0.921) and refractive index (c. 1.474).
THEOBROMA OIL
Oil of theobroma or cocoa butter may be obtained from the ground kernels of Theobroma cacao (Sterculiaceae) by hot expression. The oil is filtered and allowed to set in moulds. Much is refined in Holland. Cocoa butter, as it is commonly termed, consists of the glycerides of stearic, palmitic, arachidic, oleic and other acids. These acids are combined with glycerol partly in the usual way as triglycerides and partly as mixed glycerides in which the glycerol is attached to more than one of the acids. It is the most expensive of the commercial fixed oils and may be adulterated with waxes, stearin (e.g. coconut stearin), animal tallows or vegetable tallows (e.g. from seeds of Bassia longifolia and Stillingia sebifera). For the character and tests for purity of oil of theobroma, see the pharmacopoeias. Its melting point (31–34°) makes it ideal for the preparation of suppositories.
GERM OILS
The refined and virgin wheat-germ oils are described in the BP/EP and are obtained by cold expression or by other suitable mechanical means from the germ of the wheat grain, Triticum aestivum L. The principal fatty acids involved are linoleic (52–59%), palmitic (14–19%), oleic (12–23%) and linolenic (3–10%) with limits for ecosenoic ( 2.0%) and stearic (
2.0%) and stearic ( 2.0%). Brassicasterol is limited to 0.3% (max) in the sterol fraction. The acid value requirement for the refined oil is low (
2.0%). Brassicasterol is limited to 0.3% (max) in the sterol fraction. The acid value requirement for the refined oil is low ( 0.9%, or
0.9%, or  0.3% if the oil is to be used for parenteral purposes) but much higher (
0.3% if the oil is to be used for parenteral purposes) but much higher ( 20.0%) for the virgin oil.
20.0%) for the virgin oil.
CASTOR OIL (VIRGIN CASTOR OIL)
Castor oil (cold-drawn castor oil) is a fixed oil obtained from the seeds of Ricinus communis (Euphorbiaceae). The fruit is a three-celled thorny capsule. The castor is a native of India; the principal producing countries are Brazil, India, China, the former Soviet Union and Thailand. There are about 17 varieties, which may be roughly grouped into shrubs and trees producing large seeds, and annual herbs producing smaller seeds. It is mainly the smaller varieties that are now cultivated and these have been developed, by breeding, to give high-yielding seed plants. Mechanical harvesting is now replacing hand-picking.
Characters of seeds
The seeds show considerable differences in size and colour. They are oval, somewhat compressed, 8–18 mm long and 4–12 mm broad. The testa is very smooth, thin and brittle. The colour may be a more or less uniform grey, brown or black, or may be variously mottled with brown or black. A small, often yellowish, caruncle is usually present at one end, from which runs the raphe to terminate in a slightly raised chalaza at the opposite end of the seed. The testa is easily removed to disclose the papery remains of the nucellus surrounding a large oily endosperm. Within the latter lies the embryo, with two thin, flat cotyledons and a radicle directed towards the caruncle. Castor seeds, if in good condition, have very little odour; taste, somewhat acrid. If the testas are broken, rancidity will develop.
Preparation and characters of oil
Ninety per cent of the world’s castor oil is extracted in Brazil and India. Relatively small amounts of the whole seeds are now exported. The various processes involved in the preparation of castor oil are described in the 8th edition of this book. Briefly, the seeds are deprived of their testas and the kernels cold-expressed in suitable hydraulic presses. The oil is refined by steaming, filtration and bleaching. Cold expression yields about 33% of medicinal oil and further quantities of oil of lower quality may be obtained by other methods.
Medicinal castor oil (virgin castor oil) is a colourless or pale yellow liquid, with a slight odour and faintly acrid taste. For its chemical and physical constants, see the pharmacopoeias. The acid value increases somewhat with age and an initially high value indicates the use of damaged seeds or careless extraction or storage. Castor oil has an extremely high viscosity.
Constituents
Castor seeds contain 46–53% of fixed oil, which consists of the glycosides of ricinoleic, isoricinoleic, stearic and dihydroxystearic acids. The purgative action of the oil is said to be due to free ricinoleic acid and its stereoisomer, which are produced by hydrolysis in the duodenum. These acids have the formula,
For the biogenesis of ricinoleic acid, see Fig. 19.3. Castor oil and the oil from Ricinus zanzibarinus are remarkable for their high ricinoleic acid content, which is about 88% and 92% respectively.
The cake left after expression contains extremely poisonous toxins known as ricins, which make it unfit for use as a cattle food. In the body they produce an antitoxin (antiricin). Ricin D is a sugar protein with a strong lethal toxicity; it contains 493 amino acids and 23 sugars. Two other ricins, acidic ricin and basic ricin, have similar properties. Ricin and abrin (see ‘Abrus Seeds’ below) exhibit antitumour properties. The seeds also contain lipases and a crystalline alkaloid, ricinine, which is not markedly toxic and is structurally related to nicotinamide.
Uses
Castor oil, once widely used as a domestic purgative, is now more restricted to hospital use for administration after food poisoning and as a preliminary to intestinal examination. Owing to the presence of ricin, the seeds have a much more violent action than the oil and are not used as a purgative in the West. Non-ionic surfactants (polyethoxylated castor oils) of variable composition are produced by the reaction of castor oil with ethylene oxide and are used in certain intravenous preparations which contain drugs with low aqueous solubility. The oil and its derivatives find many non-pharmaceutical uses including the manufacture of Turkey Red Oil, soaps, paints, varnishes, plasticizers and lubricants.
The following castor oil derivatives, described as pharmaceutical aids are included in the BP/EP:
Allied drugs
Croton seeds are obtained from Croton tiglium (Euphorbiaceae), a small tree producing similar capsules to those of castor but devoid of spines. The seeds resemble castor seeds in size and shape but have a dull, cinnamon-brown colour and readily lose their caruncles. They contain about 50% of fixed oil which contains croton resin; also ‘crotin’, a mixture of croton-globulin and croton-albumin comparable with ricin. The oil also contains diesters of the tetracyclic diterpene phorbol (esterifying acid at R1 and R2, R3 = H in the formula below); acids involved are acetic as a short-chain acid, and capric, lauric and palmitic as long-chain acids. These compounds are cocarcinogens and also possess inflammatory and vesicant properties (see ‘Diterpenes’). Also present are phorbol-12,13,20-triesters (R1, R2 and R3 are all acyl groups in the formula shown). These are ‘cryptic irritants’, so called because they are not biologically active as such but become so by removal of the C-20 acyl group by hydrolysis. Rotation locular counter-current chromatography (q.v.) has been used to separate these two groups of esters. A number of the phorbol esters have been tested for anti-HIV-1 activity (S. El-Mekkawy et al., Phytochemistry, 2000, 53, 457). The plant also contains alkaloids. Croton oil should be handled with extreme caution; it is not used in Western medicine, but if taken internally, it acts as a violent cathartic.
Physic nuts or Purging nuts are the seeds of Jatropha curcas, another member of the Euphorbiaceae. The seeds are black, oval and 15–20 mm in length. They contain about 40% of fixed oil and a substance comparable with ricin, called curcin. Both seeds and oil are powerful purgatives.
Abrus seeds (prayer beads) are the attractive red and black, but poisonous, seeds of Abrus precatorius (Leguminosae). They contain a toxic glycoprotein (abrin) resembling ricin together with another non-toxic peptide having haemagglutinating properties. Various alkaloids (abrine, hyaphorine, precatorine) of the indole type have been reported, also various sterols and lectins. The seeds have been used in folklore medicine in Asia, Africa and S. America to treat many ailments, also to procure abortion and to hasten labour. In India they are employed as an oral contraceptive and as they are remarkably uniform, and each weighs about 1 carat (c. 200 mg), have been used traditionally as weights.
EVENING PRIMROSE OIL
The fixed oil obtained by extraction and/or expression from the seeds of Oenothera spp. (O. biennis L., O. lamarkiana L.) Onagraceae contains substantial mounts of esterified γ-linolenic acid (GLA), a C18 6,9,12-triene.
The principal species cultivated in the UK is O. biennis which yields an oil containing 7–9% GLA, although more recent work shows higher yields for the oils of some other species, namely O. acerviphilla nova (15.68%), O. paradoxa (14.41%) and an ecotype of O. rubricaulis (13.75%). Research has involved breeding new varieties for high yields of oil and reducing the lifecycle of the plant from 14 to 7 months (Pharm. J., 1994, 252, 189).
The sequence for the formation of such acids in the plant via cis-linoleic acid has already been indicated in Fig. 19.1. In animal tissues it appears that the prostaglandins are formed from dietary linoleic acid by conversion to GLA which undergoes C2 addition and further desaturation to give acids such as arachidonic acid, an immediate precursor of some prostaglandins.
The pharmacopoeial tests for the oil are similar to those quoted for borage oil, below.
Beneficial effects of evening primrose oil may well be related to affording a precursor of the prostaglandins for those individuals whose enzymic conversion of linoleic acid to GLA is deficient. The oil is now widely marketed as a dietary supplement, for cosmetic purposes, and more specifically for the treatment of atopic eczema and premenstrual syndrome (prostaglandin E may be depleted in this condition). Further possibilities include its use in diabetic neuropathy and rheumatoid arthritis.
BORAGE OIL
The refined fixed oil expressed from the seeds of Borago officinalis L. (starflower), family Boraginaceae contains a higher content of γ-linolenic (GLA) and somewhat less linoleic acid than evening primrose oil. Cultivation of the crop in the UK started some years ago and details of the commercial production of the seed, plant-breeding programme and husbandry are given in a general article by A. Fieldsend, Biologist, 1995, 42, 203.
Principal fatty acids involved in the oil, with BP/EP limits, are linoleic (30–41%), γ-linolenic (17–27%), oleic (12–22%), palmitic (9–12%) and smaller percentages of others. The Pharmacopoeia also states maximum limits for acid value, peroxide value, non-saponifiable matter, alkaline impurities and brassicasterol.
The oil is used for the same medicinal purposes as evening primrose oil. However, concerning its use for the treatment of atopic eczema, a clinical trial (A. Takwale et al., Br. Med. J., 2003, 327, 1385) failed to establish any benefit from the high levels of GLA contained in the oil.
SAW PALMETTO FRUIT
Saw palmetto fruit BP/EP, BHP is obtained from the palm Serenoa repens (Bartram) Small, [Sabal serrulata (Michaux) Nichols], family Arecaceae/Palmae, collected when ripe and dried. It is a low, shrubby palm with simple or branched stems usually up to about 3 m in length and bearing palmate leaves divided into many segments and having a petiole edged with sharp needles; hence the common name. The small flowers give rise to globose, one-seeded drupes turning bluish-black when ripe. It is indigenous to the sandy coastal regions of the S.E. states of the US, where it colonizes large areas; commercial supplies come mainly from Florida. The seeds were utilized both as a food and medicinally by the Native Americans.
The dried, dark brown to black fruit is semi-spherical to ovoid, up to 25 mm long, 15 mm wide with a rugged surface formed by shrinkage on drying. The remains of the three-toothed tubular calyx and style may be seen at the distal end, and the scar of the pedicel at the lower end. The hard seed is oval to spherical, measuring some 12 × 8 mm and comprising a thin testa, small perisperm and a larger, horny, pale endosperm and embryo.
A microscopical examination of the powder shows cuticularized, reddish-brown polygonal cells of the epicarp; mesocarpic parenchymatous cells, which are often oil filled or containing crystals of silica; scattered or small groups of sclereids; sclerenchymatous endocarp; and seed characteristics that include pigmented cells of the testa, thickened pitted cells of the endosperm containing fixed oil and aleurone grains.
The drug has an aromatic odour reminiscent of vanilla and a soapy, somewhat acrid, although sweetish, taste.
Constituents
Fatty acids constitute an important feature of the drug. In the mesocarp, originally the fleshy part of the fruit, they occur in the free state, and in the seeds as triacylglycerols. The principal free acids are oleic, lauric, myristic and palmitic acids, with lesser amounts of caproic, caprylic, capric, stearic, linoleic and linolenic acids; for formulae see Tables 19.1, 19.2 and 19.3. The same acids are involved in the fats of the seed endosperm, with oleic and lauric acids most commonly occurring as mixed triacylglycerols (see Fig. 19.7). Fatty acid esters involving propyl and ethyl alcohol (pricipally propyl laurate) are present in small amounts in various extracts.
Other constituents include: flavonoids such as rutin, isoquercitrin (see Fig. 21.15) and others; phytosterols—sitosterol (see Fig. 23.5), its glucosides and esters, and stigmasterol (see Fig. 23.5); immuno-stimulant polysaccharides involving galactose, arabinose, mannose, rhamnose, glucuronic acid and others; carotenoids; volatile oil.
The BP/EP gives a TLC identification test for the powdered drug using β-amyrin and β-sitosterol as reference substances. An assay for total fatty acids, minimum requirement for the dried drug 11.0%, employs gas chromatography of a dimethylformamide extract of the powder; the individual acids separated on the chromatogram are identified by comparison with the chromatogram of a reference solution of the acids mentioned above.
Actions
Most clinical and pharmacological studies on saw palmetto fruits have involved the use of hexane, supercritical CO2 (see Chapter 17) and ethanol extracts of the drug. Among the activities observed is that of the inhibition of 5α-reductase thus impeding the conversion of testosterone to dehydrotestosterone. The principal use of the drug is for the symptomatic treatment of benign prostatic hyperplasia.
For extended accounts and bibliography on the drug, see E. Bombardelli and P. Morazzoni, Fitoterapia, 1997, 68, 99–113 (58 refs); P. Bradley, British Herbal Compendium, 2006, pp. 345–352 (62 refs).
Hydnocarpus oil
This is the fixed oil obtained by cold expression from the fresh ripe seeds of Hydnocarpus wightiana, H. anthelmintica, H. heterophylla and other species of Hydnocarpus, and also of Taraktogenos kurzii. These plants are found in India, Burma, Siam and Indo-China and belong to the Flacourtiaceae.
The oil of H. wightiana contains hydnocarpic acid (about 48%), chaulmoogric acid (about 27%), gorlic and other acids (formulae, see Table 19.4); the structures of several new cyclopentenyl fatty acids have recently been elucidated. These acids do not appear to be formed from straight-chain acids and they accumulate during the last 3–4 months of maturation of the fruit. They are strongly bactericidal towards the leprosy micrococcus, but the oil has now to a large extent been replaced by the ethyl esters and salts of hydnocarpic and chaulmoogric acid. The esterified oil of H. wightiana is preferable to that of other species, in that it yields when fractionated almost pure ethyl hydnocarpate. This was included in the BPC (1965) but has now been deleted, as more effective remedies are available.
WOOL FAT
Wool fat (anhydrous lanolin) is a purified fat-like substance prepared from the wool of the sheep, Ovis aries (Bovidae).
Raw wool contains considerable quantities of ‘wool grease’ or crude lanolin, the potassium salts of fatty acids and earthy matter. Raw lanolin is separated by ‘cracking’ with sulphuric acid from the washings of the scouring process and purified to fit it for medicinal use. Purification may be done by centrifuging with water and by bleaching.
Wool fat is a pale yellow, tenacious substance with a faint but characteristic odour. It is insoluble in water and a high proportion of water may be incorporated with it by melting (m.p. 36–42°C) and stirring. Soluble in ether and chloroform. Like other waxes, it is not readily saponified by aqueous alkali, but an alcoholic solution of alkali causes saponification. Saponification value 90–105; iodine value 18–32; acid value not more than 1. Hydrous wool fat or lanolin contains 25% water.
The chief constituents of wool fat are cholesterol and isocholesterol, unsaturated monohydric alcohols of the formula C27H45OH, both free and combined with lanoceric, lanopalmitic, carnaubic and other fatty acids. Wool fat also contains aliphatic alcohols such as cetyl, ceryl and carnaubyl alcohols. Butylated hydroxytoluene, up to 200 ppm, may be added as an antioxidant.
The pharmacopoeial test for pesticide residues is complex and involves their isolation and subsequent identification. A maximum of 0.05 ppm is permitted for each organochlorine pesticide, 0.5 ppm for each other pesticide and 1ppm for the sum of all pesticides.
Wool Alcohols BP/EP are prepared by the saponification of crude lanolin and the separation of the alcohol fraction. The product consists of steroid and triterpene alcohols, including cholesterol (not less than 30%) and isocholesterol. As for wool fat, an antioxidant may be added.
To test for cholesterol dissolve 0.5 g in 5 ml of chloroform, add 1 ml acetic anhydride and two drops of sulphuric acid; a deep-green colour is produced.
Hydrogenated Wool Fat BP/EP is obtained by the high pressure/high temperature hydrogenation of anhydrous wool fat. It contains a mixture of higher aliphatic alcohols and sterols.
Wool fat is used as an emollient base for creams and ointments.
LARD
Lard (prepared lard) is the purified internal fat of the hog, Sus scrofa (order Ungulata, Suidae).
For medicinal purposes lard is prepared from the abdominal fat known as ‘flare’, from which it is obtained by treatment with hot water at a temperature not exceeding 57°C.
Lard is a soft, white fat with a non-rancid odour. Acid value not more than 1.2. Lard has a lower melting point (34–41°C) and a higher iodine value (52–66) than suet. Saponification value 192–198. It should be free from moisture, beef-fat, sesame-seed and cotton-seed oils, alkalis and chlorides.
Lard contains approximately 40% of solid glycerides such as myristin, stearin and palmitin, and 60% of mixed liquid glycerides such as olein. These fractions are somewhat separated by pressure at 0°C and sold as ‘stearin’ and ‘lard oil’ respectively. Lard is used as an ointment base but is no longer official in Britain. It is somewhat liable to become rancid, but this may be retarded by benzoination, Siamese benzoin being more effective than the Sumatra variety.
WAXES
YELLOW BEESWAX, WHITE BEESWAX
Beeswax is obtained by melting and purifying the honeycomb of Apis mellifica and other bees. The wax is imported from the West Indies, California, Chile, Africa, Madagascar and India. The EP and BP include separate monographs for the yellow and the white wax.
Preparation
Wax is secreted by worker bees in cells on the ventral surface of the last four segments of their abdomen. The wax passes out through pores in the chitinous plates of the sternum and is used, particularly by the young workers, to form the comb.
Yellow beeswax is prepared, after removal of the honey, by melting the comb under water (residual honey dissolving in the water and solid impurities sinking), straining, and allowing the wax to solidify in suitable moulds.
White beeswax is prepared from the above by treatment with charcoal, potassium permanganate, chromic acid, chlorine, etc., or by the slow bleaching action of light, air and moisture. In the latter method the melted wax is allowed to fall on a revolving cylinder which is kept moist. Ribbon-like strips of wax are thus formed which are exposed on cloths to the action of light and air, being moistened and turned at intervals until the outer surface is bleached. The whole process is repeated at least once, and the wax is finally cast into circular cakes.
Characters
Beeswax is a yellowish-brown or yellowish-white solid. It breaks with a granular fracture and has a characteristic odour. It is insoluble in water and sparingly soluble in cold alcohol, but dissolves in chloroform and in warm fixed and volatile oils (e.g. oil of turpentine).
Constituents
Beeswax is a true wax, consisting of about 80% of myricyl palmitate (myricin), C15H31COOC30H61, with possibly a little myricyl stearate. It also contains about 15% of free cerotic acid, C26H53COOH, an aromatic substance cerolein, hydrocarbons, lactones, cholesteryl esters and pollen pigments.
CARNAUBA WAX
Carnauba wax, included in the BP/EP (2000) and USP/NF (1995), is derived from the leaves of Copernicia cerifera (Palmae). It is removed from the leaves by shaking and purified to remove foreign matter.
The wax is hard, light brown to pale yellow in colour and is supplied as a moderately coarse powder, as flakes or irregular lumps; it is usually tasteless with a slight characteristic odour free from rancidity. Esters, chiefly myricyl cerotate, are the principal components, with some free alcohols and other minor constituents. The acid value is low (BP, USP/NF, 2–7), the saponification value 78–95. It has an iodine value of 7–14. The BP/EP includes a TLC test for identity. Carnauba wax is used in pharmacy as a tablet-coating agent and in other industries for the manufacture of candles and leather polish. It has been suggested as a replacement for beeswax in the preparation of phytocosmetics.