Chapter 41 Plant description, morphology and anatomy
Plant form ranges from unicellular plants—for example, yeasts and some green algae—to the strongly differentiated higher plants. Examples of pharmaceutical interest may be found in most of the larger groups and a quick perusal of the families involved in Chapter 5 of this textbook illustrates this point.
Characteristically the higher plants consist in the vegetative phase of roots, stems and leaves with flowers, fruits and seeds forming stages in the reproductive cycle. Modifications of the above structures are frequently present—rhizomes (underground stems), stolons (runners with a stem structure), stipules, bracts (modified leaves), tendrils (modified stems), etc. Certain organs may appear to be missing or much reduced—for example, the reduction of leaves in some xerophytic plants.
It is most important that students acquire the ability to interpret morphological and anatomical descriptions of crude drugs as found in pharmacopoeias and allied works and also to record adequately the features of whole or powdered drugs and adulterants of commercial significance.
As indicated in Chapter 2, for convenience of study, drugs may be arranged not only according to families and chemical constituents, but also into such morphological groups as barks, roots, leaves, seeds, etc. Some drugs constitute more than one morphological part—for example, whole herbs and commercial ‘roots’, which may consist of both rhizomes and roots.
LEAVES AND TOPS (′HERBS′)
These consist of stems (often limited in their girth by ‘official’ requirements) and leaves often associated with flowers and young fruits. All portions of such drugs need to be described.
Aerial stem
Note dimensions, shape, colour, whether herbaceous or woody, upright or creeping, smooth or ridged, hairs present or not and if so whether of the glandular or covering form. Note arrangement of tissues as seen in transverse section.
Position and arrangement of leaves
Radical (arising from the crown of the root) or cauline (arising from the aerial stem). In the Solanaceae note adnation (the fusion of part of the leaf with the stem). The arrangement may be alternate (e.g. lobelia), opposite, decussate (in pairs alternately at right angles; e.g. peppermint) or whorled.
Structure of the aerial stem
The primary stem (Fig. 41.1A) shows the following structure: epidermis, cortex, medullary rays, medulla and a vascular system taking the form of a dictyostele. The epidermis is composed of a single layer of compactly arranged cells and bears stomata. The cortex is usually parenchymatous, the outer layers of cells in aerial stems containing chloroplasts. The layers of cortex cells immediately underlying the epidermis may be collenchymatous, constituting a hypodermis. The endodermis is usually not well-differentiated in aerial stems, although a layer of cells containing starch (starch sheath) and corresponding in position to the endodermis may be defined. Underground stems often resemble roots in showing a more or less well-differentiated endodermis with characteristic Casparian strips (thickenings).
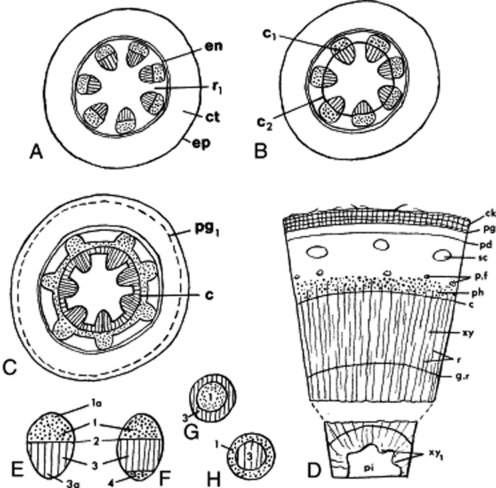
Fig. 41.1 Stem structure of dicotyledons (transverse section). A, primary structure showing seven vascular bundles; B, development of a complete cambial ring by formation of the interfascicular cambium; C, beginning of a secondary growth; D, stem after a number of seasons of growth, outer cork now present. E–H, types of vascular bundle: E, collateral; F, bicollateral; G, amphivasal; H, amphicribal. c, Cambium; c1, fascicular cambium; c2, interfascicular cambium; ck, cork; ct, cortex; en, endodermis; ep, epidermis; g.r, growth ring; pd, phelloderm; p.f, pericyclic fibres; pg, phellogen; pg1, developing phellogen; pi, pith; r, rays; r1, primary medullary ray; sc, sclerenchyma; xy, xylem; xy1, primary xylem; 1, phloem; 1a, protophloem; 2, fascicular cambium; 3, xylem; 3a, protoxylem.
The pericycle may take the form of a complete or a discontinuous ring of fibres or may be parenchymatous and ill-defined. Pericycle fibres may form a cap outside each primary phloem group.
The vascular bundles of the dictyostele are usually collateral, but are in some cases bicollateral (Cucurbitaceae, Solanaceae, Convolvulaceae) (Fig. 41.1E–H). The xylem is differentiated centrifugally and the protoxylem is endarch; the phloem is differentiated centripetally and the protophloem is exarch (cf. the root). The differentiation, in dicotyledons, is usually incomplete, so that a zone of meristematic cells (the intrafascicular cambium) separates the primary vascular tissues. Such a bundle is described as open, in contrast to the closed bundle typical of monocotyledons. In the bicollateral bundle the intrafascicular cambium occurs between the xylem and the outer phloem group.
Secondary thickening is initiated by tangential divisions in the intrafascicular cambium. The daughter cells cut off on the inner side differentiate into xylem and those cut off to the outside into phloem. The amount of secondary xylem produced in both stems and roots, in general, exceeds the amount of secondary phloem. As the process of secondary thickening of the stem proceeds, its dictyostele is converted into a solid cylinder of secondary tissues. The intrafascicular cambia become linked to form a continuous cambial cylinder by the development of interfascicular cambia in the ray tissue (Fig. 41.1B, C). The cambial activity may spread out from the intrafascicular cambia across the rays, or in other cases cambial activity may originate at a median point in the ray and then by lateral extension from both intrafascicular and interfascicular cambia the cambial cylinder may be completed.
In woody perennials the cambial divisions are arrested during the winter but are renewed each spring. The xylem produced at different seasons varies in texture. The spring wood is characterized by abundance of relatively thin-walled large conducting elements; the autumn wood, by a high proportion of thick-walled mechanical elements such as wood fibres. A similar alteration between sieve tissue and phloem fibres may occur in the secondary phloem. With increase in girth the central core of xylem may become non-functional, dark in colour and packed with metabolic byproducts forming a heartwood or duramen. Sandalwood is the heartwood of Santalum album and is packed with volatile oil. The blocking of the vessels in the formation of heartwood occurs by the development of tyloses (see Fig. 42.6P).
The secondary increase in diameter of the vascular cylinder is accompanied by changes in the outer tissues. The epidermis and part or all of the primary cortex may be shed. A phellogen may arise in the epidermis, cortex or pericycle and give rise externally to cork and internally to a variable amount of phelloderm (Fig. 41.1D).
For the investigation of the anatomy of stems, transverse sections and radial and tangential longitudinal sections should be prepared from the drug previously moistened or soaked. For a study of the individual elements, disintegrated material should be used (see Chapter 43).
The following structures are constantly present in powdered stems: cork and vascular tissues in varying amount; abundant parenchyma often containing starch. Calcium oxalate and other cell inclusions may be present. Aleurone grains are absent.
The relative amounts, size, shape and form of the structural elements are of first importance in identification. The xylem elements, which are well-preserved in dry drugs, are of particular importance.
BARKS
As understood in commerce, barks consist of all tissues outside the cambium. In botany the term ‘bark’ is sometimes restricted to the ‘outer bark’—that is, the periderm and all tissues lying outside it.
A young bark (Fig. 41.1) is composed of the following tissues.
In commercial barks the above structures have been modified by the activity of the cambium and the cork cambium or phellogen. Growth of the new tissues produced by the cambium causes the tissues of the primary bark to be tangentially stretched, compressed or torn. As these cells are stretched tangentially they may be divided by radial walls—for example, in the medullary rays. During this dilatation groups of parenchymatous cells in the cortex and phloem may be thickened into sclerenchymatous cells. The cambium produces secondary phloem, which often consists of alternating zones of sieve elements and phloem fibres. The pericycle is frequently ruptured, and parenchymatous cells which grow into the spaces may develop into sclerenchyma.
The cork cambium or phellogen may arise in the epidermis (e.g. willow), primary cortex or pericycle. The phellogen produces on its outer side cork, and on its inner side chlorophyll containing suberized cells which form the secondary cortex or phelloderm. These three layers are known as the periderm. If the cork cambium develops in or near the pericycle, a part of the whole of the primary cortex will lie outside the cork and will be gradually thrown off. Lenticels replace stomata for purposes of gaseous exchange; and as the cork increases, the amount of chlorophyll-containing tissue decreases.
The natural curvature of the bark increases when the bark is removed from the tree and dried. Large pieces of trunk bark, especially if subjected to pressure, may be nearly flat. Terms used to describe the curvature are illustrated in Fig. 41.2. Some commercial barks (e.g. cinnamon and quillaia) consist of the inner bark only. In quillaia the dark patches often found on the outer surface are known as rhytidome (literally, ‘a wrinkle’). This term is applied to plates of tissue formed in the inner bark by successive development of cork cambia.
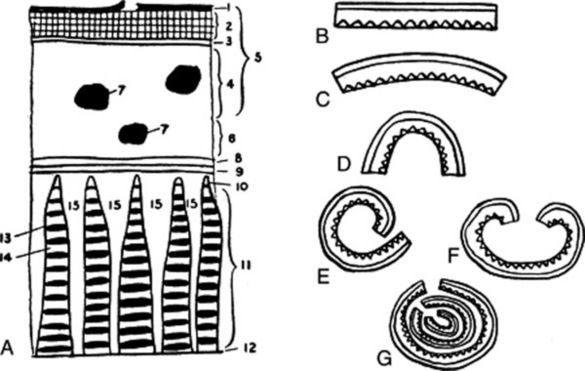
Fig. 41.2 Barks. A, diagram showing a typical arrangement of the tissues: 1, outer surface frequently showing lichens, lenticels and remains of primary tissues cut off by the cork; 2, cork; 3, cork cambium or phellogen; 4, phelloderm or secondary cortex; 5, periderm; 6, inner part of primary cortex; 7, groups of cortical sclerenchyma; 8, endodermis; 9, pericycle; 10, primary phloem; 11, secondary phloem; 12, cambium; 13, band of lignified fibres; 14, sieve elements; 15, medullary rays. B–G, shapes of barks: B, flat; C, curved; D, channelled; E, single quill; F, double quill; G, compound quill.
Barks may be described under the following headings.
Size and shape
Fracture
Short, fibrous, splintery, granular, etc. The fracture depends largely on the number and distribution of sclereids and fibres. A bark frequently breaks with a short fracture in the outer part and a fibrous fracture in the phloem.
Transverse surface
A smoothed transverse surface, especially if stained with phloroglucinol and hydrochloric acid, will usually show the general arrangement of the lignified elements, medullary rays and cork. Sections, however, are more satisfactory and can be used for a microscopical examination of calcium oxalate.
Anatomy
The cork cells in transverse section are often tangentially elongated and arranged in regular radial rows. In surface view they are frequently polygonal. The cell walls give a suberin reaction; the cell contents frequently give a positive tannin reaction. The cortex is usually composed of a ground mass of parenchyma. An outer band of collenchyma often occurs. Secretion cells, sclereids and pericyclic fibres may occur scattered or in groups in the cortex. The cortical cells often contain starch or other typical cell inclusions such as calcium oxalate.
Sieve tubes, companion cells, phloem parenchyma and medullary ray cells are always present in the phloem, but these soft tissues may not be well-preserved in medicinal barks. The sieve tubes, unless well-developed, are observed only after special treatment. Secretion cells, phloem fibres and sclereids may or may not be present in the phloem.
Xylem tissue is usually absent but may be present in small amounts on the inner surface of the bark.
The following should all be carefully noted in the anatomical examination of barks: the presence or absence of outer bark (cork, phellogen, phelloderm); the structure, amount and site of origin of the cork; the extent, cell structure and cell contents of the cortex; the presence or absence and, if present, the distribution, size and form of sclereids, phloem fibres and secretion cells; and the width, height, distribution and cell structure and contents of the medullary rays. When calcium oxalate is present, its crystalline forms and their distribution should be studied.
Transverse and longitudinal sections should be prepared. The size and form of sclereids and phloem fibres are best studied in disintegrated material. Preparations treated with cellulose, lignin, starch, callus, oil, suberin and tannin stains should be examined.
The cell types mentioned above are discussed in Chapter 42.
WOODS
Although few drugs consist solely of wood, no description of a stem or root is complete without an account of its wood. Wood consists of the secondary tissues produced by the cambium on its inner surface. The cells composing these tissues—the vessels, tracheids, wood fibresand parenchyma—are not necessarily all lignified. In some cases (e.g. the wood of belladonna root) non-lignified elements predominate. The distribution of the lignified elements may be ascertained by treating smoothed transverse, radial and tangential surfaces or sections with phloroglucinol and hydrochloric acid. In trees, the cells of the old wood frequently become coloured as they fill with waste products such as resins, tannins and colouring matters. This central region is called the heartwood, while the outer wood, which still retains its normal appearance and functions, is called the sapwood. Commercial guaiacum wood and logwood consist of heartwood.
In transverse section woods usually show annual rings each of which normally represents a season’s growth. In some tropical species the annual rings are not well-marked, owing to the absence of a seasonal interruption in growth. The so-called false annual rings found in, for example, quassia are irregular rings formed by alternating zones of wood parenchyma and fibres. The width and height of medullary rays are of diagnostic importance in the case of Jamaica and Surinam quassias and rhubarbs. The grain of wood primarily results from the arrangement of the annual rings and medullary rays, but is modified by the wavy course of the wood elements which causes the wood to split irregularly. Irregular splitting is largely dependent on the number of lateral branches which cause knots in the wood.
Woods may be described under the following headings.
Hardness and behaviour when split
LEAVES OR LEAFLETS
The following features can be used to describe leaves.
Lamina
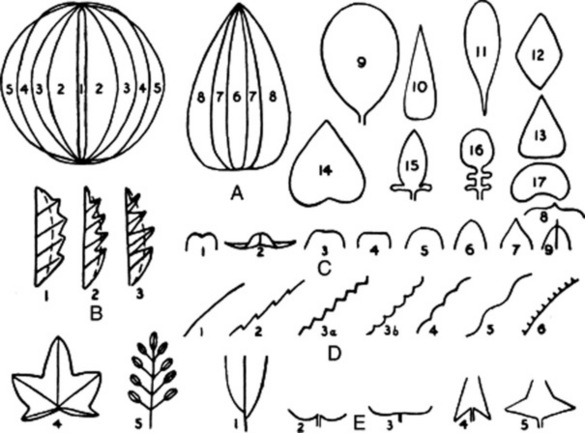
Fig. 41.3 Terms applied to leaves. A, Shape: 1, acicular; 2, elliptical; 3, oval; 4, oblong; 5, round; 6, linear; 7, lanceolate; 8, ovate; 9, obovate; 10, subulate; 11, spatulate; 12, diamond-shaped; 13, cuneate; 14, cordate; 15, auriculate; 16, lyrate; 17, reniform. B, Composition and incision: 1, pinnatifid; 2, pinnatipartite; 3, pinnatisect; 4, palmatifid; 5, imparipinnate. C, Apex: 1, emarginate; 2, recurved; 3, retuse; 4, truncate; 5, obtuse; 6, acute; 7, acuminate; 8, mucronate; 9, apiculate. D, Margin: 1, entire; 2, serrate; 3a and 3b, dentate; 4, crenate; 5, sinuate; 6, ciliate; E, Base: 1, asymmetric; 2, cordate; 3, reniform; 4, sagittate; 5, hastate.
Anatomy
A study of the anatomy of the leaf reveals that there is a basic structural pattern yielding characters that enable the presence of a leaf to be detected in a powder. Other less general characters will make possible such distinctions as that between monocotyledonous and dicotyledonous leaves, and between xerophytic and mesophytic leaves. The more detailed anatomical characters will, when taken together, allow of the identification of the genus and ultimately of the species of leaf. A knowledge of the diagnostic characters of any leaf permits of the detection of contaminants and substitutes.
The leaf (Fig. 41.4) is built up of a protective epidermis, a parenchymatous mesophyll and a vascular system. The shape, size and wall structure of the epidermal cells; the form, distribution and relation to the epidermal cells of the stomata; the form, distribution and abundance of epidermal trichomes are all of diagnostic importance.
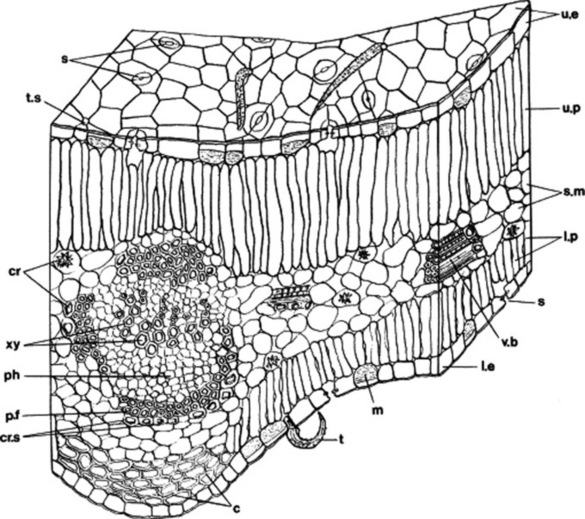
Fig. 41.4 Transverse section of senna leaflet: c, collenchyma; cr, calcium oxalate crystals; cr.s, crystal sheath; l.e, lower epidermis; l.p, lower palisade; m, mucilage cell; ph, phloem; p.f, pericyclic fibre; s, stomata; s.m, spongy mesophyll; t, trichome; t.s, trichome scar; u.e, upper epidermis; u.p, upper palisade; v.b, vascular bundle; xy, xylem vessels.
The mesophyll may or may not be differentiated into spongy mesophyll and palisade tissue. Palisade tissue may be present below both surfaces or occur only below the upper epidermis. In all green leaves the mesophyll cells are rich in chloroplasts. The mesophyll, although typically parenchymatous, may contain groups of collenchyma or sclerenchyma, secretion ducts or latex tissue, oil or mucilage cells, or hydathodes (water pores). Cells may contain inclusions such as crystals or calcium oxalate, the form, size and distribution of which may have importance.
The vascular systems of leaves fall into two main classes: the reticulate venation typical of dicotyledons and the parallel venation of monocotyledons. The structure of the individual veins is subject to considerable variation. The midrib bundle of the dicotyledonous leaf may be poorly or markedly differentiated. In leaves with a well-differentiated midrib the palisade tissue is usually interrupted in the midrib region and collenchyma frequently occurs above and below the midrib bundle. The main veins, in dicotyledonous leaves, are open and usually collateral (Fig. 41.4); less commonly they are bicollateral. The xylem faces towards the upper surface. Various degrees of secondary thickening of the midrib bundle are seen. The lateral veins are almost entirely collateral even in cases where the midrib bundle is bicollateral. The smallest veins often consist of xylem only. The veins of monocotyledonous leaves are closed bundles.
The midrib bundle is often, as in the Solanaceae, enclosed in an endodermis which may take the form of a starch sheath. The development of the pericycle is variable, in some cases being parenchymatous and containing secretion cells, in some cases consisting of a sheath of pericyclic fibres with their long axes parallel to the vein.
For the investigation of the structure of a leaf it is necessary to examine transverse sections of the lamina and midrib; portions of the whole leaf, including leaf margin, cleared in chloral hydrate; and surface preparations of both epidermi. Sections should be cleared, if necessary, and stained for cellulose and lignin. In individual cases it may be necessary to apply microchemical tests for mucilage, tannin, cutin, volatile oil, calcium oxalate or carbonate.
Powdered leaves
The following are consistently present: epidermis with stomata; cellulose parenchyma; not very abundant small-sized vascular elements and chlorophyll (except in bulb leaves). Structures frequently present are epidermal trichomes, glands, palisade cells, crystals of calcium oxalate, collenchyma and pericyclic fibres (see also Chapter 42).
For the differentiations of closely allied leaves it may be necessary to make determinations of such differential characters as vein-islet number, stomatal number, stomatal index and palisade ratio (q.v.).
INFLORESCENCES AND FLOWERS
The following features serve to describe the complex structure of flowers.
Axis or receptacle of inflorescence
The main axis of an inflorescence is called the rachis, while the branches bearing flower clusters and individual flowers are termed peduncles and pedicels, respectively. The term receptacle of the inflorescence must not be confused with the receptacle of the flower (see below). In the Roman chamomile the receptacle of the inflorescence is conical and solid, a membranous palea subtends each floret and the capitulum is surrounded by an involucre of bracts.
Type of flower
Monocotyledon or dicotyledon. Unisexual or hermaphrodite. Regular or zygomorphic. Hypogynous, perigynous or epigynous (Fig. 41.5).
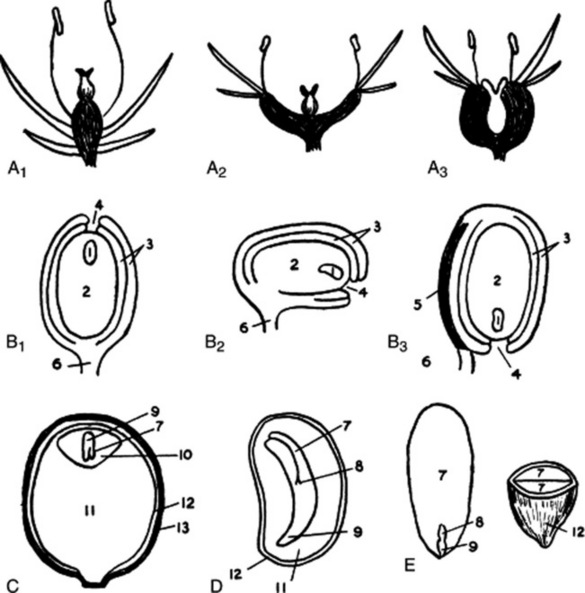
Fig. 41.5 A1, A2 and A3, hypogynous, perigynous and epigynous flowers; B1, B2 and B3, orthotropous, campylotropous, and anatropous ovules; C, fruit of Piper with single albuminous seed; D, albuminous seed of Papaver, E, exalbuminous seed of almond. 1, embryo sac; 2, nucellus; 3, integuments; 4, micropyle; 5, raphe; 6, funicle; 7, cotyledon; 8, plumule; 9, radicle; 10, endosperm; 11, perisperm; 12, testa; 13, pericarp.
Receptacle of the flower (thalamus or torus) is the extremity of the peduncle on which the calyx, corolla, etc. are inserted. When the receptacle is elongated below the calyx, it is called a hypanthium, or if below the ovary, a gynophore or stalk of the ovary (cf. clove).
Calyx
Note number of sepals if polysepalous or divisions if gamosepalous. Caducous (e.g. poppy) or persistent (e.g. belladonna). Describe colour, shape, hairs, etc., as for a leaf.
Corolla
Note number of petals if polypetalous or divisions if gamopetalous. Observe any special characteristics such as venation (henbane) and oil glands (clove petals).
Androecium
Note number of stamens; whether free or joined (monadelphous, diadelphous, etc.), didynamous or tetradynamous, epipetalous, etc. Dehiscence of anthers (valves, pores or slits).
Anatomy
The flower stalk or pedicel has a stem structure and in the powdered form exhibits the appropriate elements. The bracts, calyx and, to a lesser extent, corolla have a leaf structure and will yield such elements as epidermis with stomata, glandular and covering hairs, mesophyll cells, oil glands and crystals. The epidermal cells of the corolla often have a papillose or striated cuticle. Delicate coloured fragments of the corolla can often be distinguished in coarsely powdered drugs. A characteristic papillose epidermis may sometimes be present on the stigmas of the gynaecium. Characteristic fragments of the anther wall are diagnostic of the presence of flowers. Of first importance is the occurrence, size, shape and wall structure of pollen grains.
With powdered flowers the pollen grains, portions of the fibrous layer of the anther wall and the papillose epidermis of the stigmas are obvious features.
FRUITS
The following classification shows the principal types of fruit met with in pharmacognosy.
1 Simple, dry, indehiscent fruits
2 Simple, dry, dehiscent fruits
3 Schizocarpic or splitting fruits
A familiar example of this group is the cremocarp, the bicarpellary fruit of the Umbelliferae, which splits into two mericarps.
4 Succulent fruits
Examples of fruits of the Solanaceae are shown in Fig. 41.6.
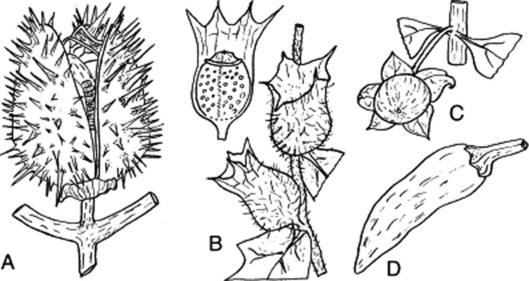
Fig. 41.6 Fruits of the Solanaceae. A and B, capsules: A, ripe fruit of Datura stramonium; B, pyxidia of Hyoscyamus niger with upper fruit showing calyx partly removed. C and D, berries of Atropa belladonna and Capsicum sp., respectively. All four fruits are basically bilocular but stramonium fruit becomes almost completely four-celled by the development of a false septum.
Shape and dimensions
Adhesion
Superior or inferior. Fruits from inferior ovaries usually show floral remains at the apex (e.g. cardamom, fennel, unpeeled colocynth and lobelia).
Dehiscence
Dehiscent or indehiscent. Different types of dehiscence are shown by the legume, follicle, siliqua and the pyxidium and other capsules. Most capsules split longitudinally into valves which are usually equal in number to or double those of the loculi or placentae. Dehiscence is termed septicidal if the valves separate at the line of junction of the carpels or loculicidal if the valves separate between the placentae or dissepiment. In the latter case the placentae or dissepiment may remain attached either to the axis or to the valves.
Anatomy
The pericarp is bounded by inner and outer epidermi which, in general, resemble those of leaves. The outer epidermis may bear stomata and hairs. In fleshy fruits the internal tissue is mainly parenchymatous, resembling the mesophyll of leaves. In dry fruits and fleshy dry fruits it usually contains fibres or sclereids. Secretory tissues such as vittae, oil ducts or cells, and latex tissue are commonly present in the pericarp of medicinal fruits. Husk of cardamoms can be detected by the presence of pitted fibres, spiral vessels and abundant empty parenchymatous cells. The endocarp of almond, sometimes used as an adulterant, consists mainly of sclereids.
Portions of receptacle (e.g. the rind of colocynth), persistent sepals and flower stalk may be present.
SEEDS
Seeds may be produced from orthotropous, campylotropous or anatropous ovules (Fig. 41.5). Care must be taken to distinguish seeds from fruits or parts of fruits containing a single seed (e.g. cereals and the mericarps of the Umbelliferae). The seed consists of a kernel surrounded by one, two or three seed coats. Most seeds have two seed coats, an outer testa and an inner tegmen. The seed is attached to the placenta by a stalk or funicle. The hilum is the scar left on the seed where it separates from the funicle. The raphe is a ridge of fibrovascular tissue formed in more or less anatropous ovules by the adhesion of funicle and testa. The micropyle is the opening in the seed coats which usually marks the position of the radicle. An expansion of the funicle or placenta extending over the surface of the seed like a bag is known as an aril or arillus. A false aril or arillode resembles an aril, but is a seed coat. A caruncle or strophiole is a protuberance arising from the testa near the hilum.
The kernel may consist of the embryo plant only (exalbuminous seeds), or of the embryo surrounded by endosperm or perisperm or both (albuminous seeds) (Fig. 41.5). Endosperm and perisperm are tissues containing food reserves and are formed, respectively, inside and outside the embryo sac.
The description of a seed may be arranged as follows.
Size, shape and colour
Anatomy
The testas of seeds often yield highly diagnostic characters. A highly diagnostic sclerenchymatous layer is often present (Fig. 41.7K, I). The number of cell layers, and their structure, arrangement, colour and cell contents are subject to characteristic variations. The epidermis of the testa is often composed of highly characteristic, thick-walled cells (Fig. 41.7A, B, E, J, L). It may bear characteristic hairs (Fig. 41.7M).
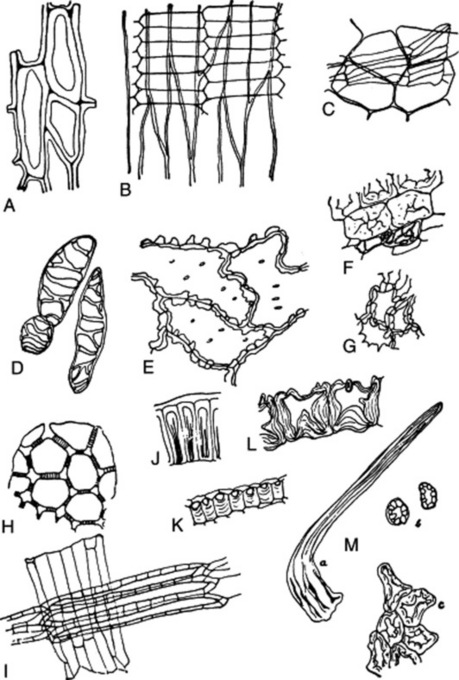
Fig. 41.7 Some diagnostic structures of fruits and seeds. A, lignified epidermal cells of testa of seed of Lobelia inflata; B, epidermis of testa of cardamom seed, with fragment of the underlying parenchymatous layer attached; K, sclerenchymatous layer of testa of cardamom seed in transverse section; C, ‘parqueting cells’ of inner epidermis of the pericarp of fennel, with parenchyma of mesocarp attached; D, lignified reticulate ‘parenchyma’ cells of the mesocarp of fennel. E, epidermis of capsicum seed in surface view; F, sclereids and reticulate cells of testa of colocynth seed; G, sclereids of same in surface view; J, epidermis of testa of colocynth seed in transverse section; H, pigment layer of testa of linseed in surface view; I, sclerenchymatous layer of testa of linseed seen in surface view and with hyaline layer adherent; L, epidermis of testa of stramonium seed in transverse section; M, lignified hair of nux vomica: a, whole hair; b, transverse sections of limb of hair; c, periclinal section through the bases of several hairs.
The storage tissues perisperm and endosperm, and in other cases cotyledons, are composed of uniform cells often containing characteristic cell contents (e.g. aleurone, starch, calcium oxalate, fixed oil, volatile oil). The cell walls are often considerably thickened (e.g. nux vomica).
The radicle, plumule and leaf-like cotyledons yield little of diagnostic significance to the powdered drug.
Transverse and longitudinal sections of fruit and seeds should be prepared. Disintegration makes possible a study of the structure of the individual layers and elements and of structures such as vittae.
The variation in structure between different fruits and seeds is considerable. Aleurone grains, carbohydrate reserves and a little vascular tissue are constantly present in seeds. Fruits yield similar characters, except that the amount of vascular tissue is greater and lignified elements of the pericarp are often present.
SUBTERRANEAN ORGANS
Under this heading it is convenient to discuss: (1) stem structures such as corms, bulbs, stem-tubers and rhizomes and (2) root structures such as true and adventitious roots and root-tubers. Many drugs which are commonly spoken of as roots consist wholly or partly of rhizomes (e.g. rhubarb and gentian) and in many cases the gradual transition from stem to root makes an accurate differentiation of the two parts impossible.
Monocotyledonous rhizomes can be distinguished from dicotyledonous rhizomes by the scattered arrangement of their vascular bundles. Stem structures may usually be distinguished from roots by the fact that they bear buds and possess a well-marked pith. In underground organs chlorophyll is absent, and starch, when present, is usually abundant and in the form of large grains of reserve starch.
The following scheme may be used with suitable modifications for the description of most subterranean organs.
Subterranean stems
Food reserves and chemical tests
Odour and taste
Anatomy
Most of the important drugs derived from roots are those of dicotyledons and the following brief description of their fundamental structural pattern is restricted to that of the typical dicotyledonous type.
The primary root (Fig. 41.8A) shows the following structures: a piliferous layer composed of a single layer of thin-walled cells, devoid of cuticle and bearing root hairs formed as lateral outgrowths of the cells; a parenchymatous cortex, the innermost layer of which is differentiated into an endodermis; and a vascular cylinder or stele taking the form of a radial protostele or less frequently of a medullated protostele. The vascular tissues of the stele are enclosed in a singleor many-layered pericycle. The protostele is composed of a central mass of xylem tissue with two or more radiating arms and of phloem groups located between the xylem arms. The xylem is differentiated in a centripetal direction, so that the protoxylem groups occupy the ends of the xylem arms and the metaxylem makes up the inner xylem mass. The number of protoxylem groups is usually fairly constant for a given species, but some variation is not uncommon (e.g. valerian). The xylem is described as diarch (Solanum spp.), triarch (alfalfa), tetrarch (liquorice, Ipomoea spp.) or polyarch, according to the number of protoxylem groups present. The central xylem cylinder is medullated in some cases (e.g. valerian). The phloem groups are usually separated from the xylem cylinder by a narrow zone of parenchyma (‘fundamental parenchyma’).
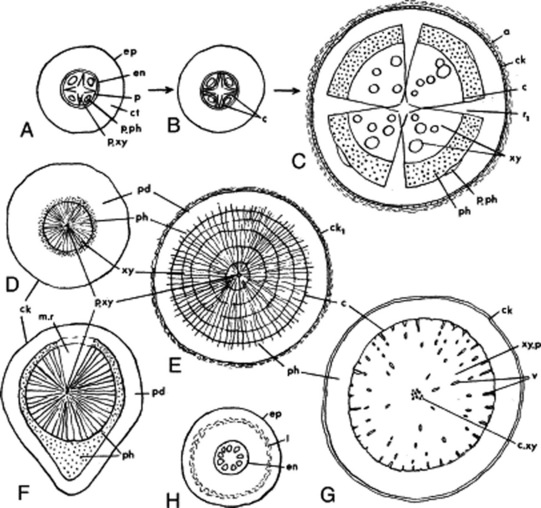
Fig. 41.8 Root strucutures in transverse section. A–C, initiation of secondary growth in liquorice root: A, primary structure; B, development of cambium; C, formation of secondary phloem and xylem and of the cork cambium. D–G, variations in root structure: D, ipecacuanha; E, Rauwolfia serpentina; F, senega; G, belladonna; H, veratrum, a monocotyledon wih no secondary thickening. a, Degenerative cortex; c, cambium; ck, cork; ck1, stratified cork; ct, cortex; c.xy, central xylem; en, endodermis; ep, epidermis; I, lacuna; m.r, medullary ray; p, pericycle; pd, phelloderm; ph, phloem; p.ph, primary phloem, p.xy, primary xylem; r1, primary medullary ray; v, xylem vessels, xy, xylem; xy.p. xylem parenchyma.
In many roots increase in diameter of the axis is accomplished by secondary thickening. Secondary thickening is initiated in the zone of ‘fundamental parenchyma’, the whole or part of which becomes meristematic. The derived cells mature as secondary phloem centrifugally and as secondary xylem centripetally. From the point of initial cambial activity there is a progressive tangential development, the cambia extending laterally until they reach the points where the protoxylem groups abut on to the pericycle. The pericycle opposite the protoxylem groups becomes meristematic and thus a continuous cambial cylinder is formed (Fig. 41.8B). The activity of the cambium opposite the protoxylem groups gives rise to the broad primary rays (Fig. 41.8C).
The cylinder of secondary tissues is composed of xylem and phloem elements which at first tend to be arranged in regular radial rows. This arrangement often becomes less regular, owing to irregular growth of the individual elements and further division and growth of the xylem and phloem parenchyma cells. The structure of the secondary xylem and phloem is described in Chapter 42.
Coincident with the development of the secondary vascular tissues other changes take place (Fig. 41.8C). The primary phloem groups are forced outwards and gradually obliterated. Divisions take place in the pericycle, so that it increases in diameter with the expansion of the vascular cylinder. Often the pericycle also increases in thickness, becoming many layers, and forms a ‘secondary cortex’. The piliferous layer, cortex and endodermis become fractured and are cut off by the formation of a phellogen in the outermost layer of cells derived from the pericycle. At a still later stage a new phellogen may arise in the secondary phloem, with a consequent disintegration of the pericycle.
The structures of the primary and secondary roots of dicotyledons show many deviations from the general plan described above. Figure 41.8D–G indicates variations in root structure arising from the preferential development of certain tissues. Monocotyledons characteristically exhibit no secondary thickening (Fig. 41.8H). Jalap shows anomalous secondary thickening.
UNORGANIZED DRUGS
Many types of unorganized drugs are discussed in Part 5, namely: fixed oils, fats and waxes; volatile oils; resins, oleoresins, oleo-gum-resins, balsams and gums. To these must be added dried juices (e.g. aloes), latices (e.g. opium) and extracts (e.g. agar and catechu).
The following scheme may be used in their examination.