Chapter 42 Cell differentiation and ergastic cell contents
Modifications to the basic structure of the living plant cell involving composition of the cell wall, cell shape and cell contents, are found in the various plant tissues and furnish those microscopical characters of drug plants which are of value in identification and in the detection of adulteration.
THE CELL WALL
The original cell wall may, during the differentiation of the cell, undergo various chemical modifications that profoundly change its physical properties. Principal among these are the deposition of further cellulose or hemicellulose and incrustation of the wall by lignin, cutin or suberin. Algal cell walls, which commonly contain pectin mixed with cellulose, xylose, mannose or silica, may contain also hemicellulose, alginic acid, fucoidin and fucin (Phaeophyta), geloses (Rhodophyta) and chitin.
Cellulose walls
Certain colour reactions can be applied for the recognition of cellulose cell walls. The colour reactions vary with differences in the relative proportions of cellulose, hemicellulose and pectin present.
Lignified walls
Lignin is a strengthening material which impregnates the cell walls of tracheids, vessels, fibres and sclereids of vascular plants; it constitutes 22–34% of woods. Chemically, it is a complex phenylpropanoid (C6–C3) polymer which differs according to its source, lignin from dicotyledons being different from that of the conifers (Fig. 21.1). In the wall, it appears to occur chemically combined with hemicellulose and is built up in greatest concentration in the middle lamellae and in the primary walls. Lignified cell walls after treatment with Schultze’s macerating fluid will show cellulose reactions.
For the identification of lignified walls the following tests are available:
Suberized and cutinized walls
Suberin and cutin consist of mixtures of substances, chiefly highly polymerized fatty acids such as suberic acid, COOH[CH2]6COOH, although the acids present in the two substances are not identical. These materials waterproof cells in which they occur. Suberin thickenings, such as are found in cork cells and endodermal cells, usually consist of carbohydrate-free suberin lamellae. Cutin forms a secondary deposit on or in a cellulose wall. Leaves are frequently covered with a deposit of cutin which may show characteristic papillae, ridges or striations. Beneath the cuticle, the cellulose wall may also be impregnated with cutin (cutinized), so that these walls may show a gradation from pure cellulose on the inside, through layers of cellulose impregnated with pectin compounds and fatty substances, to the outer cuticle, which is free of cellulose. Waxes (largely esters of higher monohydric alcohols and fatty acids) occur with suberin and cutin. Unlike the latter, they readily melt on warming and are extractable with fat solvents. Such waxes in the form of minute rods or particles give a glaucous effect to the structures which they cover and are responsible for the ‘bloom’ of many fruits, stems, etc. Wax is found in larger amounts on the leaves of Myrica, and in the wax palms, Copernicia, it coats the leaves heavily (Carnauba wax).
The reactions of suberin and cutin are almost identical.
Mucilaginous cell walls
Certain cell walls may be converted into gums and mucilages. This gummosis (gummous degeneration) may be observed in the stems of species of Prunus, Citrus and Astragalus, in testas of many seeds (e.g. linseed and mustard) and in the outer layers of many aquatic plants. In the case of gum-yielding species of Astragalus, gummosis commences near the centre of the pith and spreads outwards through the primary medullary rays. The polysaccharide walls, excepting the primary membranes, swell and are converted into gum, the lumen, which frequently contains starch, becoming very small. When the stem is incised, whole tissues are pushed out by the pressure set up by the swelling of the gum. The commercial gum has a definite cell structure. The reaction of gums and mucilages is described below under ‘Cell Contents’.
Chitinous walls
Chitin (C8H13O5N)n, a polyacetylamino-hexose, forms the major part of the cell walls of crustaceans, insects and many fungi (e.g. ergot). It gives no reactions for cellulose or lignin. When heated with 50% potash at 160–170°C for 1 h, it is converted into chitosan, C14H26O16N2, ammonia and acids such as acetic and oxalic. The mass may be dissolved in 3% acetic acid and the chitosan reprecipitated by the addition of a slight excess of alkali. Chitosan gives a violet colour when treated first with a 0.5% solution of iodine in potassium iodide, and then with 1% sulphuric acid. The test may be applied to shrimp scales, first freed from carbonate by means of 5% hydrochloric acid, to the elytra of beetles or to defatted ergot.
PARENCHYMATOUS TISSUE
Meristematic tissue is usually composed of cells characterized by isodiametric form (except in the case of the provascular tissues), by possessing a protoplast capable of division and a primary cell wall composed of cellulose. The fundamental parenchyma occurring in various parts of the plant is potentially meristematic, and such cells achieve maturity without further differentiation except for an increase in cell size and wall thickness and a restricted change of form. The pith, cortex and rays of the plant axis and the mesophyll of the leaves are composed, at least in part, of such parenchyma. The mesophyll cells often contain abundant chloroplasts, and may be differentiated into palisade and spongy mesophyll. An early stage of differentiation may be seen in the lignified pitted parenchyma constituting the pith of the stems of Lobelia inflata and Cephaelis ipecacuanha, and the pitted cellulose parenchyma of the pulp of Citrullus colocynthis.
THE EPIDERMIS
The epidermis consists of a single layer of cells covering the whole plant. The epidermis of the root constitutes the piliferous layer and that of the shoot is a highly differentiated and compact layer of cells. The epidermal cells, in contrast to the stomatal guard cells, are often devoid of chloroplasts. Epidermal cells show great variety in form, giving characteristic patterns when seen in surface view. In transection they are often flattened parallel to the surface, and square or rectangular in shape. The outer walls are often convex and the most markedly thickened.
The epidermis of the stems of trees and shrubs is usually obliterated early by the development of a cork cambium, but on the stems of herbaceous plants and in leaves, fruits and seeds the epidermis persists and often yields highly diagnostic characters.
For leaves in particular, the shape of the epidermal cells in surface view and in section (Fig. 42.1A–D), the nature and distribution of the wall thickening, the presence or absence of cuticle and its form, the distribution and structure of the stomata, the presence or absence of well-differentiated subsidiary cells to the stomata, the presence of characteristic cell inclusions such as cystoliths, the presence or absence and form, size and distribution of epidermal trichomes and the presence and distribution of water-pores should all be carefully noted in describing the characters of an epidermis.
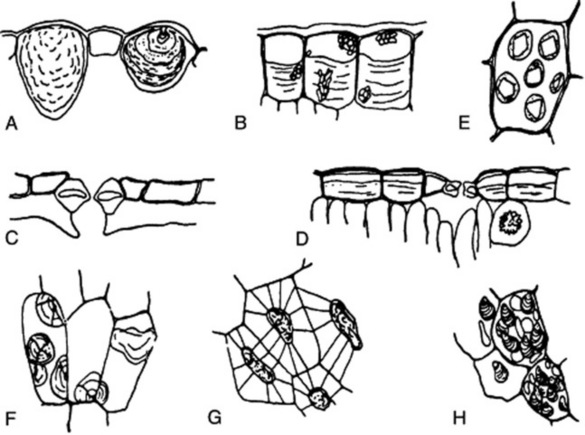
Fig. 42.1 A, Epidermal cells of Urtica dioica containing cystoliths of calcium carbonate. B, Epidermal cells of the leaf of Barosma betulina showing sphaero-crystalline masses of diosmin and a thick deposit of mucilage on the inner tangential walls. C, Cells of the lower epidermis of Arctostaphylos uva-ursi showing thick cuticle and sunken stomata. D, Upper epidermis of Cassia angustifolia showing mucilage cells and stomata; a cell of the underlying mesophyll contains a cluster crystal of calcium oxalate. E, Aleurone grains showing crystalloid and globoid, from the endosperm of the seed of Ricinus communis. F, Sphaerocrystalline masses of inulin in dahlia tuber. G, Cells of the endosperm of the seed of Strychnos nux-vomica showing walls of reserve cellulose, traversed by plasmodesmata. H, Parenchymatous cells from the rhizome of Zingiber officinale containing starch grains.
The structures of the epidermis and stomata are of first importance in the microscopical identification of leaves (see Fig. 42.2). Straight-walled epidermal cells are seen in, for example, jaborandi, coca and senna leaves; wavy-walled epidermal cells in stramonium, hyoscyamus and belladonna; beaded walls in Lobelia inflata and Digitalis lanata; a papillose epidermis in coca leaf. A thick cuticle is developed in Aloe leaf and bearberry leaf; a striated cuticle in belladonna, jaborandi, Digitalis lutea and D. thapsi. Mucilage is present in the epidermis of senna and buchu leaves. Cystoliths of calcium carbonate occur in the epidermal cells of Urticaceae and Cannabinaceae; sphaero-crystals of diosmin occur in buchu epidermis (Fig. 42.1B).
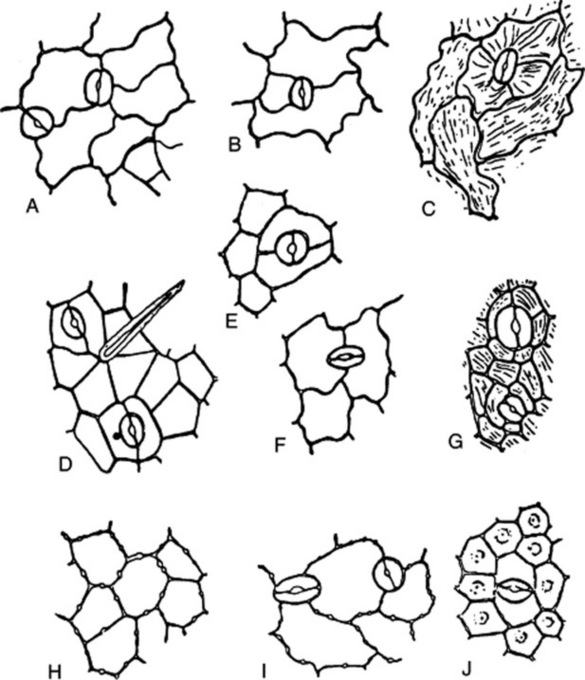
Fig. 42.2 Epidermis of leaves: A, lower epidermis of Digitalis purpurea; B, lower epidermis of Hyoscyamus niger, C, upper epidermis of Atropa belladonna; D, lower epidermis of Cassia angustifolia; E, lower epidermis of Rosmarinus officinalis; F, lower epidermis of Mentha piperita; G, lower epidermis of Pilocarpus jaborandi; H, upper epidermis of Lobelia inflata; I, lower epidermis of Digitalis lanata; J, lower epidermis of Erythroxylum coca. A, Anomocytic type of stomata; B and C, anisocytic type of stomata; D, paracytic type of stomata; E and F, diacytic type of stomata; G, actinocytic type of stomata.
The stomata may be surrounded by cells resembling the other epidermal cells (anomocytic, formerly ranunculaceous, type), but in other cases definite subsidiary cells may be distinguished. Three main types are distinguishable: the anisocytic (formerly cruciferous) type, with the stoma surrounded by three or four subsidiary cells, one of which is markedly smaller than the others; the paracytic (formerly rubiaceous) type, with two subsidiary cells with their long axes parallel to the pore; and the diacytic (formerly caryophyllaceous) type with two subsidiary cells, with their long axis at right angles to the pore of the stomata (Fig. 42.2). There are variations among these types (e.g. the actinocytic type, in which the subsidiary cells are arranged along the radii of a circle) and altogether some 31 types have been recognized. (For a survey of the classification of morphological types of stomata see M. Baranova, Bot. Rev., 1992, 58, 49).
Often, when viewed under the light microscope as cleared preparations, the outlines of the epidermal cells and stomata do not appear as definite as the line drawings (Fig. 42.2) might suggest. This is due tothe convoluted arrangements of cells on the leaf surface and is illustrated by the scanning electron micrographs included in the digitalis and Solanaceae descriptions in Part 5.
The distribution of stomata between the upper and lower epidermis shows great variation. The stomata may be entirely confined to the lower epidermis, as in Ficus species, bearberry, boldo, buchu, coca, jaborandi and maté leaves. The leaves of savin show stomata confined to two localized areas of the lower surface. The floating leaves of aquatics have stomata confined to the upper epidermis. Sometimes they are evenly distributed on both surfaces; most commonly they are more numerous on the lower surface. For ‘stomatal number’ and ‘stomatal index’ see Chapter 43.
The epidermis of fruits and seeds may yield characters of diagnostic value (see Fig. 41.7). The outer and inner epidermi of the pericarp of the umbelliferous fruits are highly characteristic structures. Characteristic cells with thickened pitted walls form the outer epidermis of the pericarp in vanilla, juniper and capsicum. The outer epidermi of the pericarp of coriander and vanilla contain prisms of calcium oxalate. A striated cuticle is seen in aniseed, caraway and star anise fruits. Thickened palisade-like cells form the epidermis of the testa of colocynth and fenugreek seeds. Characteristic elongated tapering cells form the epidermis of cardamoms. Thickened lignified cells form the epidermis of lobelia seed, and mucilage cells that of linseed and of white and black mustard.
EPIDERMAL TRICHOMES
Most leaves and many herbaceous stems, flowers, fruits and seeds possess hairs or trichomes of one kind or another. Many show hairs of more than one type. Hairs may be grouped into non-glandular or clothing hairs, and glandular hairs. Clothing hairs may be unicellular or multicellular. Unicellular hairs vary from small papillose outgrowths to large robust structures (Figs 42.3, 42.4). Multicellular hairs may be uniseriate, biseriate or multiseriate or complicated branched structures (Fig. 42.4). The chemical nature of the cell wall, and the presence of pits or protuberances or of cell inclusions, such as cystoliths, should be noted.
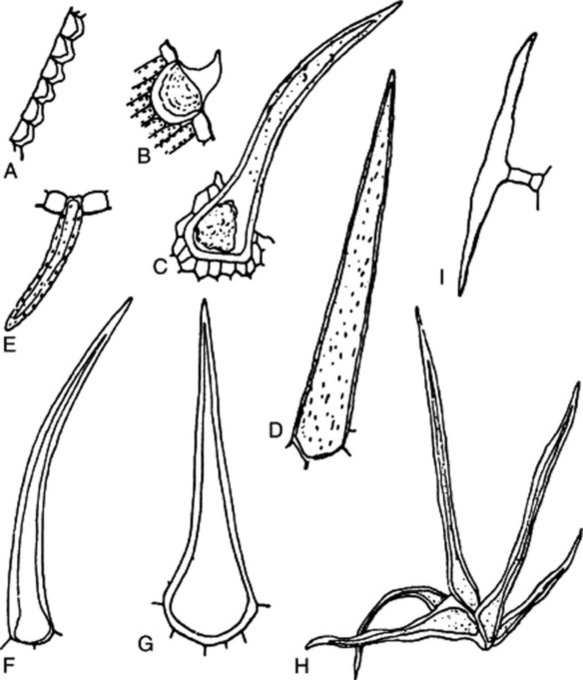
Fig. 42.3 Epidermal trichomes. A, Papillae of lower epidermis of Coca leaf. B–G, Unicellular hairs; B, papillose epidermal cell with cystolith from leaf of Cannabis; C, cystolith clothing hair from floral bract of Cannabis; D, Lobelia inflata leaf; E, senna leaf; F, lignified hair of Ailanthus; G, comfrey. H, Group of unicellular hairs from Hamamelis leaf. I, T-shaped hair of Artemisia absinthium.
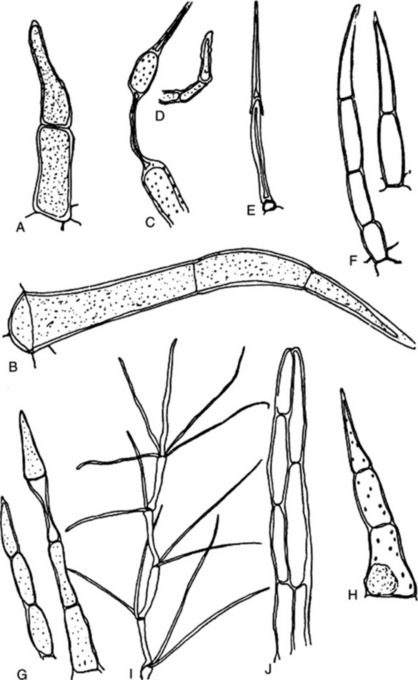
Fig. 42.4 Epidermal trichomes. A–H, Uniseriate clothing hairs; I, multicellular branched hair; J, biseriate hair. A, Datura metel; B, Datura stramonium; C, Mentha piperita; D, Thymus vulgaris; E, Plantago lanceolata; F, Hyoscyamus niger; G, Digitalis purpurea; H, Xanthium strumarium; l, Verbascum thapsus; J, Calendula officinalis.
Glandular hairs may have a unicellular or a multiseriate stalk; the glandular head may be unicellular or multicellular (Fig. 42.5). The cuticle of the gland may be raised by the secretion (Fig. 42.5E and F). In peppermint the oil secretion beneath the cuticle contains crystals of menthol. A particular type of hair is often characteristic of a plant family or genus—for example, biseriate hairs of the form shown in Fig. 42.4J are common in the Compositae, while glandular hairs such as Fig. 42.5A, B and C are found in the Solanaceae, and such as Fig. 42.5E in the Labiatae. For types of hairs found on seeds, see sections on cotton, strophanthus seeds and nux vomica seeds.
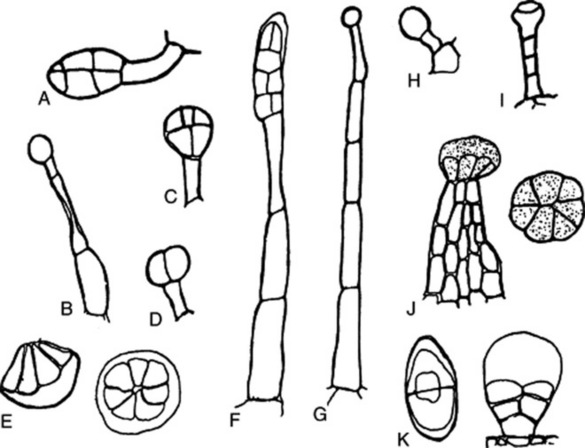
Fig. 42.5 Glandular hairs: A and B, Atropa belladonna; C, Datura stramonium; D, Digitalis purpurea; E, multicellular labiate glandular hair; F, Hyoscyamus niger; G and H, Primula vulgaris; I, Digitalis lutea; J, Cannabis sativa; K, Artemisia maritima.
Trichomes serve a number of functions, which include physical and chemical protection for the leaf against microbial organisms, aphids and insects, and the maintenance of a layer of still air on the leaf surface, thus combating excess water loss by transpiration. The secretions of glandular trichomes of certain genera constitute important materials for the perfumery, food and pharmaceutical industries; some secretions contain narcotic resins and others give rise to skin allergies. The sesquiterpenes of the capitate and non-capitate glandular trichomes of Helianthus annuus are antimicrobial and the glandular trichomes of some Solanum species contain sucrose esters of carboxylic acids such as 2-methyl-propanoic and 2-methylbutyric acid, which are aphid deterrents. The isolated secretory cells of the pellate glandular trichomes of Mentha piperita can carry out the de novo synthesis of monoterpenes. These studies have been facilitated by improved methods of trichome microsampling.
THE ENDODERMIS
The endodermis is a specialized layer of cells marking the inner limit of the cortex. A typical endodermis is usually present in roots, in aquatic and subterranean stems and in the aerial stems of certain families (e.g. Labiatae and Cucurbitaceae). Leaves and aerial stems often show a starch sheath, probably representing a modified endodermis.
The cells of the endodermis appear in transverse section four-sided, oval or elliptical and often extended in the tangential direction. The cells are longitudinally elongated, with the end walls often transverse. A primary endodermis, such as can be studied in lobelia stem, is characterized by the deposition, in the radial walls, of special modified material (resembling cutin) in the form of a Casparian strip. Subsequently, a suberin lamella may be laid down within the primary wall, giving a secondary endodermis. This may be followed by the deposition of a secondary wall of lignocellulose, giving a tertiary endodermis, as in Aletris and Smilax. The structure of the endodermis is of value in differentiating between the commercial species of Smilax.
CORK TISSUE
As the plant axis increases in diameter, a cork cambium or phellogen usually arises which, by its activity, produces new protective tissues, known collectively as periderm, which replace the epidermis and part or all of the primary cortex. The cells of the cork cambium undergo tangential divisions giving rise externally to phellem or cork tissue and internally to phelloderm or secondary cortex. Usually, only a limited production of phelloderm occurs, so that the number of cork layers greatly exceeds the number of phelloderm layers. However, wide secondary cortex is seen in ipecacuanha root (Fig. 41.8) and taraxacum.
In roots the cork cambium arises in the pericyle; in stems it may arise in the epidermis or the subepidermal layer or be deep-seated. The first-formed cork cambium may be functional throughout the life of the plant and may itself keep pace with the increase in girth, giving rise to an even smooth bark. A persistent cork cambium, failing to increase in diameter, gives rise to the fissured bark of the cork oak and cork elm. Often, however, the first-formed cork cambium has only a limited period of activity and is replaced by secondary cambia of more deep-seated origin; this process may be repeated again and again.
Cork tissue is built up of a compact mass of cells, usually rectangular in transverse sections (Fig. 21.13C) five- or six-sided in surface view (see Fig. 21.13E) and often arranged in regular radial rows. The cell wall is composed of inner and outer cellulose layers and a median suberin lamella, or of a suberin lamella laid down upon the primary cellulose wall. The cellulose layers may be lignified, as in cassia bark. The mature cork cell is dead, impermeable to water and often filled with dark reddish-brown contents rich in tannins and related substances.The presence of cork cells in powdered drugs may show adulteration or use of low-quality or improperly peeled drug (e.g. cinnamon, ginger and liquorice).
The formation of cork puts out of action the stomatal apparatus, and involves the formation of special breathing pores or lenticels. The lenticels are larger in size and smaller in number than the stomata they replace. The simplest form of lenticel consists of a mass of unsuberized thin-walled cells which become rounded off and are known as complementary tissue. Often, however, in the lenticel area, the cork cambium gives rise not only to complementary tissue, but also, alternating with it, to diaphragms of suberized cells, with well-marked intercellular air spaces.
COLLENCHYMA
Collenchyma is a living tissue, directly derived from parenchyma, but having greater mechanical strength. The walls are thickened, the thickening being composed of cellulose and being laid down in longitudinal strips commonly located at the angles of the cells. The cells are usually four- to six-sided in transverse section, axially elongated when seen in longitudinal section. Their walls, being composed of cellulose, have considerable plasticity, and, hence, collenchyma constitutes the typical mechanical tissue of herbaceous stems and of the petioles and midribs of leaves. Collenchyma is present above and below the midrib bundle in many leaves (e.g. senna, stramonium, hyoscyamus, belladonna, digitalis and lobelia); in the wings of lobelia stem; in the cortex of cascara bark; and in the pericarp of colocynth and capsicum.
SCLEREIDS
Sclereids or stone cells are sclerenchymatous cells approximately isodiametrical in shape. The walls of the typical sclereid are thick, lignified, often showing well-marked stratification and traversed by pit-canals which are often funnel-shaped or branched. The cell lumen is usually small, sometimes almost completely obliterated. Cell contents of diagnostic significance may be present (e.g. prisms of calcium oxalate in calumba, starch grains in cinnamon).
Sclereids commonly occur in the hard outer coats of seeds and fruits and in the bark and pericyclic regions of woody stems. They occur isolated or in small groups in quillaia and calumba, in larger groups in cascara (Fig. 21.13) and wild cherry bark (Fig. 25.3) or in definite sclereid layers, as in cinnamon (Fig. 22.10) and cassia bark. The absence of sclereids from frangula and cinchona barks aids in their microscopical identification. The presence of elongated sclereids in powdered ipecacuanha is diagnostic of the presence of stem; lignified sclereids, which are present in clove stalk (Fig. 22.12), should be almost absent from powdered cloves. Characteristic sclereids are present in the rind and seed coat of colocynth (Fig. 41.7F and G).
Attention can appropriately be called, in this section, to the sclerenchymatous layer of the testas of linseed (Fig. 41.7I) and cardamoms (Fig. 41.7K); to the pitted fusiform sclerenchymatous cells ofthe mesocarp of coriander (Fig. 22.6); to the lignified reticulate cells occurring in the mesocarp of fennel (Fig. 41.7D) and dill, and in the inner part of the testa of colocynth (Fig. 41.7F); and to the lignified idioblasts seen in the lamina of hamamelis leaf.
FIBRES
Tissue composed of spindle-shaped or elongated cells with pointed ends is known as prosenchyma. When cells of this kind are thick-walled, they are known as fibres. The cell wall may be composed of almost pure cellulose or may show various degrees of lignification in the form of sclerotic or sclerenchymatous fibres.
Fibres are developed from a single cell, the fibre initial, which during its development grows rapidly in the axial direction. During this period of growth the tips of the elongating cells may push past one another, a process known as ‘gliding growth’ and made possible by a modification in the state of the middle lamella. Most mature fibres are unicellular, but occasionally transverse septa develop (e.g. ginger). Fibres are best differentiated on the basis of the tissue in which they occur (i.e. as cortical fibres, pericyclic fibres, xylem fibres or phloem fibres).
Frequently, fibres are differentiated in the pericycle; thus flax consists of the pericyclic fibres of Linum usitatissimum and hemp of the pericyclic fibres of Cannabis sativa. The cell wall of the flax fibre is composed of almost pure cellulose; in hemp some lignification has taken place.
Isolated groups of pericyclic fibres occur in lobelia stem and in cinnamon bark. The meristeles of clove hypanthium are enclosed in an incomplete sheath of pericyclic fibres. The lignified, moderately thick-walled pericyclic fibres, accompanied by a parenchymatous sheath of cells containing prisms of calcium oxalate, constitute an important diagnostic character of senna leaf (Fig. 21.10). The presence of pericyclic fibres in the midrib of the leaves of Digitalis lutea and D. thapsi contrasts with their absence in D. purpurea and D. lanata.
Xylem fibres may be regarded as being directly derived from tracheids, and intermediate forms, having a limited conducting function and known as fibre-tracheids occur (Fig. 42.6A–C). The fibre-tracheid has smaller pits, thicker walls and usually more tapering ends than the typical tracheid. Wood fibres have thicker walls and pits reduced to minute canals. Occasionally, wood fibres are septate (Fig. 42.6D). Cells having a fibre-like form with living contents and simple pits but which are really fusiform xylem parenchyma cells are termed ‘substitute fibres’. The mature wood fibre is a dead lignified element. The autumn wood is usually characterized by containing a higher proportion of wood fibres than the spring wood. The ground mass of secondary xylem of Picroena excelsa is built up of compactly arranged thick-walled wood fibres and the secondary xylem of liquorice (Fig. 23.11) contains wood fibres arranged in bundles, which alternate with the small groups of vessels and are enclosed in a sheath of xylem parenchyma containing prisms of calcium oxalate. The secondary xylem in gentian, rhubarb and jalap is free from fibres.
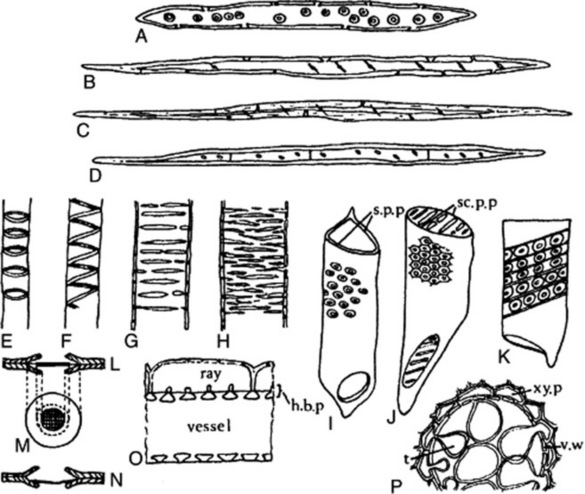
Fig. 42.6 Xylem components. A, Tracheid; B, fibre-tracheid; C, xylem fibre; D, septate fibre; E, annular vessel; F, spiral vessel; G, scalariform vessel; H, reticulate vessel; I, vessel segment with round bordered pits and simple perforation plate (s.p.p.) at either end; J, vessel segment with hexagonal pits caused by crowding, and scalariform perforation plate (sc.p.p) at either end; K, vessel segment with bordered pits and band of tertiary thickening. L, M, N, structure of the bordered pit; L and N, sections show overarched secondary wall, pit membrane and central torus, the latter, in N, closing the pit mouth; M, surface view of same; O, half-bordered pit pairs (h.b.p.) connecting a vessel and ray cells; P, vessel in transverse section showing development of tyloses. t, Tylosis; v.w, vessel wall; xy.p, xylem parenchyma.
Phloem fibres may occur in both primary and secondary phloem; they may or may not be lignified. Their thickened walls are traversed by simple pits, in contrast to the fine-bordered pits of the wood fibres. Phloem fibres constitute the ‘hard bast’ of earlier writers. Jute consists of the phloem fibres from the stems of various species of Corchorus. The phloem fibres of liquorice resemble those of the xylem in being enclosed in a crystal sheath. The distribution, abundance, size and shape of the phloem fibres constitute important characters for the differentiation of medicinal barks. Phloem fibres occur isolated or in irregular rows in the barks of cinnamon (Fig. 22.10), cassia and cinchona (Fig. 26.33). The phloem fibres of cinnamon can be differentiated from those of cassia by their smaller diameter. In barks, in which fibres occur isolated or in rows, the area of fibres per gram of powdered bark can be made a criterion for determining the amount present in mixtures. The phloem fibres of cinchona constitute a prominent feature of the powder; they are large (80–90 μm in diameter), are fusiform in shape and have very thick walls, conspicuously striated and traversed by funnel-shaped pits (Fig. 26.33). The secondary phloem of cascara, frangula and quillaia is composed of alternating zones of hard and soft phloem. The phloem fibres of cascara are accompanied by a crystal sheath (Fig. 21.13); those of quillaia are characterized by their tortuous, irregular outline and often exhibit enlarged and forked apices. Fibres are absent from the phloem of gentian and ipecacuanha.
XYLEM
The primary xylem is composed of protoxylem and metaxylem. Secondary growth in thickness of the stem and root of gymnosperms and dicotyledons is accompanied by the formation of secondary xylem. The structural elements of xylem are tracheids, vessels or tracheae, xylem fibres, xylem parenchyma and rays.
The tracheid is derived from a single cell and can be regarded as the basic cell type of xylem tissue. It takes the form of an elongated water-conducting cell, with a lignified and variously thickened and pitted cell wall (Fig. 42.6A). At maturity it is a dead element. The pits are bordered (Fig. 42.6L–N), although in some cases the borders are so narrow that the pits appear simple. In gymnosperms the pits are confined to the radial walls.
The character of the secondary wall thickening enables us to distinguish annular, spiral, scalariform and reticulate tracheids. Transition forms between these types are not uncommon. Annular and spiral tracheids occur most frequently in protoxylem; scalariform and reticulate tracheids most frequently in metaxylem and secondary xylem. True vessels are absent in gymnosperms, in which the secondary xylem consists of a homogeneous tracheidal system only broken by narrow medullary rays and in some cases by a slight development of xylem parenchyma. Cellulose wadding is made from high grade sulphite pulp usually prepared from coniferous wood, and when examined microscopically shows typical coniferous tracheids, with bordered pits and a small amount of wood parenchyma. The tissues are completely delignified in the preparation of the pulp. Tracheids occur in the secondary xylem of some angiosperms (e.g. ipecacuanha).
Vessels or tracheae constitute the fundamental conducting elements of the xylem of the angiosperms. The vessel is derived from a vertical series of cells, in which increase in diameter and dissolution of the end-walls occurs so that a continuous tube is formed. The most primitive type of vessel consists of a vertical series of tracheid-like segments in which some of the scalariform pits of the adjacent end-walls have broken down to give slit-like openings; the most advanced type of vessel shows complete dissolution of the end-walls of the constituent segments (see Fig. 42.6I–K). The vessels of the protoxylem show annular or spiral thickening, those of the later-formed xylem scalariform and reticulate thickening (Fig. 42.6E–H). The secondary wall thickening is composed of lignocellulose. Larger vessels may have a complete secondary wall perforated only by pits. These pits are subject to considerable variation in size, form and crowding and sometimes bands of tertiary thickening are laid down within the secondary wall (Fig. 42.6K).
Spiral and annular vessels are typical of protoxylem, and usually occur in the protoxylem of stems and roots, in small vascular bundles and in the veins of leaves. Thus, small amounts of such vessels are seen in gentian, clove, squill and most leaves (e.g. senna, belladonna, hyoscyamus and stramonium). Spiral and scalariform vessels occur in lobelia stem. Reticulate vessels occur in gentian, ginger and rhubarb, those of the last two drugs being almost non-lignified. Vessels showing numerous bordered pits occur in quassia, jalap, sandalwood, hydrastis and the stems of belladonna and aconite.
The living meshwork of the secondary xylem is made up of rays and xylem parenchyma which permeate the dead mass of mature vessels, tracheids and wood fibres. The xylem parenchyma cells are often axially elongated, sometimes thin-walled but often with walls showing thickening and lignification. The walls are traversed by simple pits or, where the cells abut on vessels or tracheids, by half-bordered pits. Xylem parenchyma may function as a storage tissue, the cells becoming blocked with starch (as in ipecacuanha). The xylem parenchyma cells may grow into the vessel cavities and form tyloses which block up the vessel and render it non-functional, a process which occurs in the development of heartwood (Fig. 42.6P). The distribution of xylem parenchyma may be diffuse, vasicentric when it forms sheaths around the larger vessels, or terminal when a zone of xylem parenchyma is formed towards the end of each year’s growth. The formation of concentric zones of xylem parenchyma may give rise to ‘false annual rings’, as in quassia. In transverse sections the medullary rays appear radially arranged and where the ray cells about on to vessels they may possess half-bordered pits (Fig. 42.6O).
PHLOEM
The structural elements of phloem include sieve tubes, companion cells, phloem parenchyma and secretory cells. The sieve tube is the conducting element of the phloem. It is formed from a vertical series of elongated cells, interconnected by perforations in their walls in areas known as sieve plates. The perforations may be restricted to smaller areas, sieve fields, several of which are contained in each sieve plate. The sieve plates may occur in the end-walls or lateral walls of the sieve tube (Fig. 42.7). The mature sieve plate is coated with a film of callus, which may increase in amount and form a callus pad completely blocking the sieve plate (Fig. 42.7D). The development of the callus pad may render the sieve tube permanently functionless; in other cases the callus pad formed in the autumn is redissolved in the spring. The mature sieve tube lacks a nucleus, but while functional contains cytoplasm. Sieve tubes may often be detected by recognition of the callus pads, which show typical staining reactions.
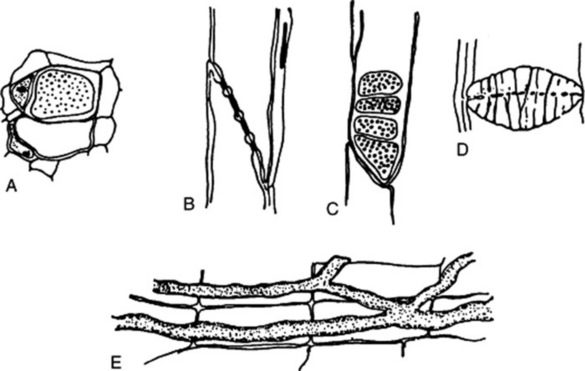
Fig. 42.7 Phloem elements. A, Sieve tubes and companion cells in transverse section, one of the sieve tubes showing a transverse sieve plate in surface view; B and C, respectively, tangential and radial longitudinal views of a sieve tube, showing an oblique sieve plate with four sieve fields; D, sieve plate in winter condition, showing deposit of callus; E, radial longitudinal view of laticifers in the root of Taraxacum officinale.
In view of their delicate structure and lack of lignification, sieve tubes are difficult to observe in commercial drugs. The sieve tubes of cascara bark can often be detected, even in the powdered drug, when stained with corallin soda. They are sometimes also to be observed in powdered gentian.
The companion cells are intimately associated with the sieve tubes both structurally and functionally. The sieve tube and the companion cells are derived from a common mother cell of the procambial strand in primary phloem or from a phloem mother cell derived from the cambium in secondary phloem. The phloem mother cell undergoes longitudinal division into two daughter cells of unequal size, the smaller of which becomes the companion cell. The companion cell is characterized by its dense protoplast and well-developed nucleus, and by possessing a thin cellulose wall.
The cells of the phloem parenchyma are usually axially elongated, although they may remain isodiametric and be arranged in linear series. They remain typically thin-walled.
The phloem often contains secretory cells (e.g. ginger, cinnamon, cassia and jalap). Laticiferous tissue may also occur in the phloem (e.g. lobelia and taraxacum) (Fig. 42.7E).
SECRETORY TISSUES
Secretory tissues include secretory cells, secretory cavities or sacs, secretory ducts or canals and latex tissue.
Oil cells occur in ginger (Fig. 42.8D), pepper, mace, cardamoms, cinnamon (Fig. 42.8G) and cassia. Large oil cells form an important diagnostic character of powdered sassafras root bark. Cells containing resins (Fig. 42.8H), oleoresins and mucilage are common. Enzyme storage cells occur in many endospermic seeds (e.g. the myrosin cells of the Cruciferae). Storage cells, crystal cells and tannin cells may also be considered under this heading.
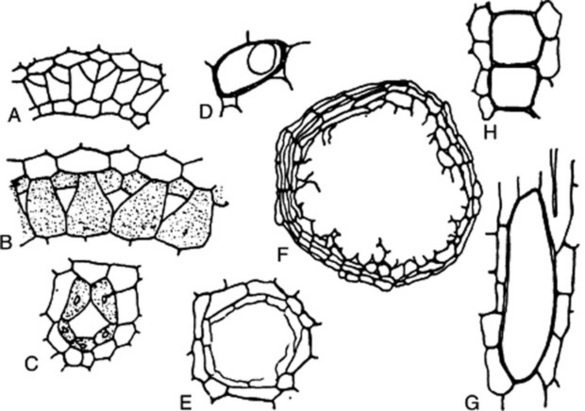
Fig. 42.8 Secretory cells and ducts. A, B, C, Stages in development of a typical schizogenous oil duct in the Umbelliferae (after Hayward); D, oil cell of ginger; E and F, schizolysigenous oil glands in Barosma and Citrus; G, oil cell of cinnamon seen in longitudinal section; H, resin cells of jalap.
Secretory cavities or sacs may arise by separation of the cells and subsequent formation of a secretory epithelium (schizogenously) or by breakdown of the cells forming a cavity not bounded by a definite epithelium (lysigenously). Schizogenous oil cavities occur in eucalyptus, lysigenous oil cavities in Gossypium species. Secretory products may appear in cells before the latter break down to give a lysigenous cavity. Schizolysigenous oil cavities occur in the Rutaceae and the Burseraceae. The oil cavity develops from a mother cell, which undergoes division to give daughter cells which separate, leaving a schizogenous central cavity. The walls of the cells surrounding this central cavity then break down, forming an oily secretion, and the cavity continues to increase in size lysigenously (Fig. 42.8E, F).
The vittae of the Umbelliferae are schizogenous oleoresin canals (Fig. 42.8A–C) and they occur in the stem, roots and leaves.The oleoresin ducts of Pinus species are also of schizogenous origin. Schizogenous oleoresin ducts which enlarge lysigenously occur in some members of the Leguminosae (e.g. Copaifera).
Latex (Laticiferous) tissue consists of either cells or tubes which contain a fluid with a milky appearance arising from the suspension of small particles in a liquid dispersion medium with a very different refractive index. The suspended particles vary in nature, and may be hydrocarbons composed of essential oils, resins and rubber. Alkaloids are present in the latex of Papaveraceae, the proteolytic enzyme papain in the latex of Carica (pawpaw) and vitamin B1 in that of Euphorbia. Latex cells are typical of the Euphorbiaceae, Moraceae, Cannabinaceae, Apocynaceae and Asclepiadaceae. In the Euphorbiaceae the cells destined to form the latex systems are differentiated in the embryo. From these embryonic initials the branched tubular latex cells of the mature plant are developed. The latex cells have thickened walls and numerous nuclei, and contain latex in which characteristic dumb-bell-shaped starch grains may be present. The long, sinuous latex cells of Cannabis sativa are unbranched.
Laticifers are also formed by the partial or complete fusion of a longitudinal series of cells. They occur in Convolvulaceae, Campanulaceae and the suborder Liguliflorae of the Compositae. The Papaveraceae possess latex elements intermediate in structure between latex cells and vessels. The laticifers of Ipomoea consist of longitudinal rows of cells which retain their transverse walls. A similar condition is seen in Sanguinaria. In Chelidonium the marginal parts of the transverse walls persist; in Papaver and Argemone there is only slight evidence of the original transverse walls. The laticifers in the Liguliflorae take the form of a continuous non-septate series of passages usually occurring in the primary and secondary phloem. Taraxacum officinale (Fig. 42.7E) shows concentric zones of anastomosing latex vessels in the phloem of both rhizome and root.
It is often difficult or impossible to determine the mode of origin of the laticifers except by following their development from the embryonic or seedling stages. A further difficulty in delimiting elements of a laticiferous nature arises from their association in some plants with idioblasts containing tannins, mucilage, etc., and from the fact that latex material may also occur in schizogenous canals.
ERGASTIC CELL CONTENTS
The cell contents with which we are concerned in pharmacognosy are those which can be identified in vegetable drugs by microscopical examination or by chemical and physical tests. These cell contents represent either food-storage products or by-products of metabolism, and include carbohydrates, proteins, fixed oils and fats, alkaloids and purines, glycosides, volatile oils, gums and mucilages, resins, tannins, calcium oxalate, calcium carbonate and silica; being non-living, they are referred to as ergastic.
Starch
Starch occurs in granules of varying sizes in almost all organs of plants; it is found most abundantly in roots, rhizomes, fruits and seeds, where it usually occurs in larger grains than are to be found in the chlorophyll-containing tissues of the same plant. The small granules formed in chloroplasts by the condensation of sugars are afterwards hydrolysed into sugars so that they may pass in solution to storage organs where, under the influence of leucoplasts, large grains of reserve starch are formed. Starch is of considerable pharmaceutical importance and is fully discussed in Chapter 20.
Proteins
Storage protein occurs in the form of aleurone grains which are particularly well seen in oily seeds (e.g. castor seed (see Fig. 42.1E) and linseed). The simplest aleurone grain consists of a mass of protein surrounded by a thin membrane. Often, however, the ground mass of protein encloses one or more rounded bodies or globoids and an angular body known as the crystalloid. Aleurone grains are best observed after defatting and removal of starch, if these are present in large amount. Sections being examined for aleurone should be treated with the following reagents:
The endosperm cells of nutmeg each contain one large and several smaller aleurone grains. The large aleurone grains are 12–20 μm in diameter, and contain a large well-defined crystalloid. Aleurone grains, containing globoids, are present in the endosperm and cotyledons of linseed. Some of the aleurone grains of the endosperm of fennel contain a minute cluster crystal of calcium oxalate; others contain one or more globoids.
Fixed oils and fats
Fixed oils and fats are widely distributed and occur in both vegetative and reproductive structures. They often occur in seeds, where they may replace the carbohydrates as a reserve food material, and are not uncommonly associated with protein reserves. As lipids, fats form an essential component of biological membranes.
Reserve fats occur in solid, frequently coloured or crystalline masses which melt on warming. Feathery crystalline masses of fat occur in the endosperm of nutmeg. Fixed oils occur as small highly refractive drops. Oil globules, associated with aleurone grains, can be well seen in the cotyledons of linseed and colocynth and in the endosperm of nux vomica and umbelliferous fruits. Oils and fats are soluble in ether-alcohol, but, with a few exceptions, such as castor oil, are sparingly soluble in alcohol. They are coloured brown or black with a 1% solution of osmic acid, and red with a diluted tincture of alkanna. The latter stains rather slowly and should be allowed to act for at least 30 min. A cold mixture of equal parts of a saturated solution of potash and strong solution of ammonia slowly saponifies fixed oils and fats. After some hours, characteristic soap crystals may be observed. For a full discussion of fixed oils and fats, see Chapter 19.
Gums and mucilages
Gums, mucilages and pectins are polysaccharide complexes formed from sugar and uronic acid units. They are insoluble in alcohol but dissolve or swell in water. They are usually formed from the cell wall (e.g. tragacanth) or deposited on it in successive layers. When such cells are mounted in alcohol and irrigated with water, the stratification may often be seen (e.g. mustard and linseed).
Specific tests for these substances are at present lacking, but the following are useful. The official Solution of Ruthenium Red stains the mucilage of senna and buchu leaves, althaea, linseed and mustard. It also stains sterculia gum but has less action on tragacanth. A lead acetate medium can be used to prevent undue swelling or solution of the substance being tested. Some forms of mucilage are stained by the BP Alkaline Solution of Corallin, e.g. that found in squill. Others are stained by chlor-zinc-iodine or methylene blue dissolved in alcohol and glycerin.
The pharmaceutical gums are described in Chapter 20.
Volatile oils and resins
Volatile oils occur as droplets in the cell. They are sparingly soluble in water but dissolve in alcohol (cf. fixed oils). They resemble fixed oils (q.v.) in their behaviour towards osmic acid and tincture of alkanna, but they are not saponified when treated with ammoniacal potash.
Resins may be associated with volatile oil or gum, or may be found in irregular masses which are insoluble in water but soluble in alcohol. Resins, oleoresins and gum resins are usually secreted into secretory cavities or ducts. They stain slowly with diluted tincture of alkanna. For details of volatile oil and resin-containing drugs see Chapter 22.
Tannins
Tannins are widely distributed in plants and occur in solution in the cell sap, often in distinct vacuoles. If it is desired to study the distribution of the tannins in the plant, the sections must be cut dry, since tannins are soluble in water and alcohol. If sections of galls are so cut and mounted in clove oil, plates of tannin may be observed. Sections containing tannins acquire a bluish-black or greenish colour when mounted in a dilute solution of ferric chloride. For tannin- containing drugs see Chapter 21.
Alkaloids and glycosides
These important secondary metabolites are rarely visible in plant cells without the application of specific chemical tests.
Crystals
Various crystalline deposits may occur in plant cells.
Calcium oxalate
Oxalic acid rarely occurs in the free state in plants but is extremely common as its calcium salt in the form of crystals. It is dimorphous and is found either as the trihydrate, belonging to the tetragonal system of crystals, or as the monohydrate, belonging to the monoclinic system.
Crystals of the tetragonal system form as a result of supersaturation of the cell sap with calcium oxalate. They have all three axes at right angles to one another; two of the axes are equal in length and the third, or principal axis, may be either shorter or longer. They are illustrated in Fig. 42.9A–D; and in addition to these forms, the tiny sandy crystals or microcrystals found in the Solanaceae (Fig. 26.8) and other families probably belong to this system. In the monoclinic system the crystals have such forms as shown in Fig. 42.9E–I, and result from an excess of oxalic acid in the cell sap. They have three unequal axes with the two lateral axes at right angles to one another, but one only of these is at right angles to the third axis. These crystals shine more brightly when viewed in polarized light than do the trihydrate crystals.
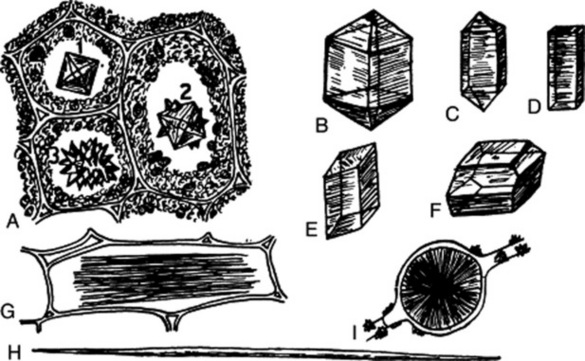
Fig. 42.9 Calcium oxalate. A–D, Crystals of the tetragonal system; E–I, crystals of the monoclinic system. A3, A rosette crystal formed of tetragonal crystals as seen in A1 and A2; D, a tetragonal prism; E, a monoclinic prism; G, raphides; H, a single needle crystal; I, a sphaerocrystal. (After Thoms’ Handbuch der Pharmazie.)
Usually it is sufficient to describe the general form and size of the crystals, without reference to a crystallographical class. The most common forms encountered are prisms (senna, hyoscyamus, quassia, liquorice, cascara, quillaia, rauwolfia, calumba); rosettes (rhubarb, stramonium, cascara, senna, clove, jalap); single acicular crystals (ipecacuanha, gentian, cinnamon); bundles of acicular crystals (squill); microsphenoidal or sandy crystals (belladonna).
When calcium oxalate is present, it is important that the types of crystal, their size and distribution be recorded. Cascara shows cluster crystals generally distributed in the ground mass of parenchyma and prisms confined to the rows of parenchymatous cells forming a sheath round the fibres (Fig. 21.13). The prisms of calcium oxalate in calumba are contained in the sclereids.
The cells containing calcium oxalate may differ from those surrounding them in size, form or contents, and are often referred to as idioblasts.
Calcium oxalate is usually present to the extent of about 1% in plants but in some structures such as the rhizome of rhubarb it may exceed 20% of the dry weight. It often forms a character of considerable diagnostic importance. The solanaceous leaves may be distinguished from one another, belladonna by its sandy crystals, stramonium by its cluster crystals, and henbane by its single and twin prisms. Similarly, phytolacca leaves and roots, which both possess acicular crystals, are distinguished from belladonna leaves and roots, which have sandy crystals. Other instances of the diagnostic importance of calcium oxalate are given under the individual drugs and no attempt is made to give here more than a few selected examples, which will be extended by the student in further reading.
Sections to be examined for calcium oxalate may be cleared with chloral hydrate or caustic alkali, as these reagents only very slowly dissolve the crystals. The polarizing microscope will often assist in the detection of small crystals. Crystals may be identified ascalcium oxalate if they are insoluble in acetic acid and caustic alkali, soluble in hydrochloric and sulphuric acids without effervescence and show, after solution in 50% sulphuric acid, a gradual separation of needle-like crystals of calcium sulphate at the site of the original crystals.
Calcium carbonate
This may be found embedded in or incrusted in the cell walls. Concretions of calcium carbonate formed on outgrowths of the cell wall are termed cystoliths. They occur in the orders Urticaceae, Moraceae, Cannabinaceae and Acanthaceae, and in some of the Combretaceae and Boraginaceae. Well-formed cystoliths are seen in the enlarged upper epidermal cells and in the clothing hairs of the lower epidermis of the leaf of Cannabis sativa (Fig. 42.3). When the mineral substance of the cystolith is dissolved out in dilute acid, there remains a small, often stratified, basis composed of cellulose. Calcium carbonate can be identified by the fact that it dissolves with effervescence in acetic, hydrochloric or sulphuric acid. If 50% sulphuric acid is used, needle-shaped crystals of calcium sulphate gradually separate.
Hesperidin and diosmin
These occur as feathery-like aggregates or sphaerocrystalline masses in the cells of many of the Rutaceae and in isolated plants of other families. Crystalline masses of diosmin are present in the upper epidermal cells of buchu leaves (Fig. 42.1B). These crystals are insoluble in organic solvents but soluble in potassium hydroxide.
Silica
This substance forms the skeletons of diatoms (see ‘agar’ and ‘kieselguhr’), and occurs as an incrustation on cell walls or as masses in the interior of cells (e.g. in the cells of the sclerenchymatous layer of cardamom seeds). Silica is insoluble in all acids except hydrofluoric. It may be examined by igniting the material and treating the ash with hydrochloric acid, the silica remaining unaltered.