CHAPTER 9 Neurogenic bladder disorders
INTRODUCTION
Neurogenic bladder dysfunction describes abnormal function of the bladder and urethra due to lesions affecting their innervation, either within the central nervous system or in the peripheral nerves of the lower urinary tract.
Neurological conditions can alter bladder and urethral function by altering:
PATTERNS OF NEUROGENIC DYSFUNCTION
Although each individual patient will have a unique pattern of lower urinary tract dysfunction and require an individual management plan, the site of the lesion gives an indication of the likely pattern of the dysfunction.
Neurological lesions can be divided into four broad anatomical areas:
Suprapontine
Causes
Typically suprapontine disorders are due to cerebro-vascular accidents (CVA), brain tumours, head injury, dementia and cerebral palsy.
Pattern of dysfunction
Neurogenic detrusor overactivity (NDO) commonly occurs due to reduced cortical inhibitory control of the micturition reflex. As the pontine co-ordination centres are unaffected by this lesion, detrusor and sphincter function co-ordination is preserved; for this reason, patients with suprapontine lesions usually do not develop high pressure neurogenic bladders. However many patients appropriately increase sphincter activity during DO to avoid urgency incontinence, and this increase in EMG activity has been coined pseudodyssynergia. In addition, some patients with cortical lesions lose the ability to voluntarily void and others lose the sensation of bladder fullness and urgency.
Pontine
Causes
Typically pontine disorders are due to Parkinson’s disease, multiple system atrophy (MSA) and multiple sclerosis (MS).
Pattern of dysfunction
The brainstem contains both the pontine micturition centre (PMC) and the pontine storage centre (PSC); therefore a lesion in this area can cause of variety of storage and voiding dysfunctions to occur simultaneously. These include NDO, detrusor underactivity, detrusor sphincter dyssynergia (DSD) and external sphincter relaxation.
Suprasacral spinal cord
Pattern of dysfunction
Due to the loss of higher detrusor inhibition and co-ordination of voiding these patients typically have DO associated with DSD. Bladder filling sensation will also be lost in a complete SCI. Spontaneous reflex voiding can occur; however it is uncontrolled and associated with incontinence. DSD may lead to high voiding pressure and upper tract damage and this is a particularly ‘dangerous’ pattern of urological dysfunction; it is classically seen in high level (cervical cord) lesions.
Sacral and subsacral
Causes
Typically from myelomeningocoele, spina bifida, MS, diabetes mellitus and iatrogenic from surgical injury.
Pattern of dysfunction
A variety of patterns of dysfunction are seen depending on the level of the lesion and the extent of the denervation. A complete sacral or subsacral lesion will lead to an acontractile detrusor, incompetent urethra and loss of bladder sensation. A complete lesion around the conus may demonstrate an acontractile detrusor with a normal or overactive urethra. A complete lumbo-sacral lesion may cause an overactive detrusor with an incompetent urethra. Most lesions are however incomplete and depending on the pathways disrupted can give a varying pattern of dysfunction, e.g. injury to the pudendal nerves may lead to an incompetent urethra whereas an injury to the pelvic nerves may lead to an underactive detrusor with impaired bladder sensation but with a normally functioning urethra. Injury to the afferent axons and pathways may lead to diminished bladder sensation. In addition, incomplete lesions can result in neurogenic detrusor overactivity and high pressure neurogenic bladders.
URODYNAMICS FOR NEUROGENIC BLADDER DISORDERS
In the management of neurogenic lower urinary tract function:
Urodynamic investigations can characterize the nature of the detrusor and sphincteric abnormality to:
Many patients with neurological disorders don’t display symptoms or signs until significant damage has occurred to the upper tracts or to detrusor function. Frequently urodynamic findings in this group of patients do not correlate well with the neurological examination findings and therefore the examination findings should not be used to plan the urological management. Instead all patients with neurological dysfunction that affects or is likely to affect lower urinary tract function should receive a thorough urodynamic assessment at an early stage to fully characterize the function and to plan appropriate management; patients may require subsequent follow-up urodynamic investigations to determine the success of any intervention and the extent of any change in lower urinary tract function.
Assessment of lower urinary tract dysfunction is no different from that in neurologically normal patients, consisting of history, examination, voiding diaries, uroflowmetry and pressure flow studies (± EMG). However the interpretation is often more complex and there are specific hazards such as autonomic dysreflexia; therefore the investigations are best performed in specialist centres using video urodynamics. It must be remembered that patients with neurogenic bladder dysfunction also suffer from the same lower urinary tract disorders as the rest of the population, e.g. BOO related to the prostate, and these conditions may further complicate assessment and management. Management should be tailored for each patient depending on the pattern of dysfunction and a plan for follow-up, other relevant investigations (renal function, ultrasound) and repeat urodynamic assessments should be carried out.
NDO AND SPHINCTER OVERACTIVITY (DSD)
The presence of both NDO and sphincter overactivity is a particularly dangerous urodynamic situation and as a rule of thumb those patients with a competent bladder outflow and a pre-micturition pressure of 40 cm H2O or higher are at particular risk of developing upper urinary tract problems due to the high backpressure, although this is by no means an absolute value and damage to the upper tracts can occur at lower pressures (Figure 9.1). If this pattern of dysfunction is present then the preservation of renal function is of the utmost importance; this requires control of both the NDO and the sphincter overactivity.
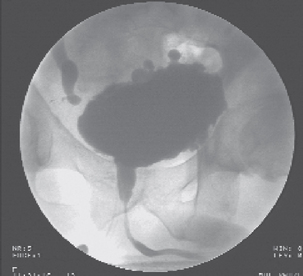
Figure 9.1 Video urodynamic screening in a spinal cord injured patient, showing obstruction at the level of the sphincter (DSD), right vesico-ureteric reflux and hydronephrosis and also multiple bladder diverticuli.
Treatment strategies for the NDO include:
Treatment strategies for the overactive sphincter are to ensure adequate drainage; these include:
Incontinence that occurs as a result of a sphincterotomy or stent insertion can be controlled by implanting an artificial urinary sphincter.
The patient should at the least have annual urodynamic assessments, PVRs and kidney function tests performed and the patient should be followed up according to recognized guidelines such as those produced by the International Consultation on Incontinence (ICI).
SPINAL SHOCK
Patients who have lesions above the sacral micturition centre initially go through a period of spinal shock in which there is loss of neurological (reflex) activity below the level of the injury. This usually results in complete urinary retention due to an acontractile detrusor and also to the maintenance of some residual sphincteric competence. Until there is recovery of some neurological activity the patient will require either indwelling or intermittent catheterization.
URODYNAMICS IN PRACTICE: NEUROGENIC BLADDER DYSFUNCTION
Technique
PRACTICAL POINTS IN PERFORMING PRESSURE/FLOW CYSTOMETRY
Performing urodynamics in patients who have neurogenic bladder dysfunction can be challenging and technical modifications may be necessary to obtain the maximum amount of information. Specific issues include:
Detrusor leak point pressure
In neurogenic patients who have a high filling pressure the upper tracts have an increased risk of being damaged if there is a subtracted detrusor pressure of more than 30–40 cm H2O documented during filling cystometry. It is important to realize that neurogenic patients can have dangerously high DLLPs even though they have stress urinary incontinence and low abdominal leak point pressures (ALPP) secondary to intrinsic sphincter deficiency (see Chapter 4).
Autonomic dysreflexia
Autonomic dysreflexia is the exaggerated sympathetic output that occurs in response to a noxious autonomic stimulus below the level of injury in patients who have spinal cord injuries above T6. This manifests as hypertension associated with bradycardia, headache, profuse sweating and flushing above the level of the injury. Spontaneous bladder overdistension or high detrusor pressures may precipitate episodes of autonomic dysreflexia, and may be due to a blocked catheter, inadequate drainage, bladder stones or UTI. Similarly, pressure/flow cystometry may also precipitate autonomic dysreflexia during filling or in relation to DSD and should be prevented by slow filling rates and the maintenance of low volumes and detrusor pressures. Autonomic dysreflexia is a life threatening event and must be treated immediately. All units performing urodynamics on spinal cord injured patients should have a protocol in place for the emergency treatment of the condition.
If autonomic dysreflexia occurs during pressure/flow urodynamics:
ELECTROMYOGRAPHY
Electromyography (see Chapter 3) is the study of the bio-electric potentials generated by depolarization of skeletal muscle. The primary value of EMG is for identifying neuropathy. The functional unit in EMG is the motor unit, which is composed of:
An excitatory impulse in the motor neurone causes each of its muscle fibres to contract and the sum of this activity is called the motor unit action potential (MUAP). The waveform of the MUAP is generally biphasic or triphasic and may be detected by electrodes and displayed on an oscilloscope screen, strip chart or on a urodynamic monitor if performed synchronously with pressure/flow cystometry.
Individually recorded on an oscilloscope, the MUAP has its own amplitude, duration and firing frequency. When a motor neurone is damaged the muscle fibres that have lost their nerve supply become re-innervated by adjacent healthy nerve fibres resulting in fewer but larger motor units. This results in MUAPs of larger amplitude and increased complexity (polyphasic) and duration. These changes in EMG may be used to infer the presence of neurological disease.
Types of electrode
Surface electrodes
Surface electrodes (skin, anal plug and catheter) record total electrical output from the muscles of the pelvic floor and are the standard electrodes used in clinical practice. They cannot record individual MUAPs, but allow assessment of overall muscle behaviour. Surface electrodes should be applied to an area as close to the muscle under investigation as possible. They can be difficult to secure and provide less reproducible results than needle electrodes.
Needle electrodes
Individual MUAPs may be detected by needle electrodes placed directly into or near the muscle to be studied. Most commonly the needle is placed directly into the peri-urethral sphincter. A variety of different types of needle electrodes exist including concentric, bipolar, monopolar and single fibre. They permit a more precise recording of EMG activity and analysis of individual MUAPs than surface electrodes. The technique is, however, user dependent and requires considerable expertise; it is also uncomfortable for patients. Its use is therefore generally limited to research and highly specialized centres.
Recording site
EMGs are commonly recorded from three muscles:
The easiest muscle to use is the external anal sphincter because placement is simple and dislodgement is less common. In most patients the EMG recorded from the three sites is similar, but in some neurological disorders, particularly demyelinating disease and partial cauda equina lesions, there may be significant differences between the recordings. It is therefore recommended by many to always record from the peri-urethral musculature.
Interpretation
Volitional control of the urinary sphincter is demonstrated by an increase and decrease in EMG activity associated with active contraction and relaxation of the pelvic floor musculature respectively.
During filling there is a normal progressive increase in EMG activity referred to as recruitment. Just prior to voiding the urethral musculature should relax and electrical silence during EMG monitoring is seen (Appendix 3, example trace 1). This relaxation should persist throughout the detrusor contraction.
Abnormal persistent EMG activity during voiding can be due to:
In contrast, pseudodyssynergia is the normal voluntary contraction of the external sphincter and pelvic floor muscles in response to an involuntory detrusor contraction (in the filling phase of the study) in an attempt to prevent urgency incontinence.
True DSD occurs only in patients who have neurological disorders. Several patterns of DSD have been described on EMG:
OTHER SPECIALIZED NEUROPHYSIOLOGICAL STUDIES
Nerve conduction studies
These are performed by stimulating a peripheral nerve and monitoring the time for a response to occur in its innervated muscle. The time until the first measurable muscle response is termed the motor latency. These studies test the integrity of nerve pathways, demonstrating prolonged latencies when there is injury to the nerve with associated demyelination. The most commonly tested latency used to assess neuropathic bladder dysfunction is the bulbocavernosus reflex.
Nerve conduction studies have been found to be beneficial in diagnosing neurological disease, but require elaborate instrumentation and expert interpretation.
Evoked responses
Evoked responses are potential changes in neural tissue resulting from distant stimulation, usually electrical. They are used to test the integrity of peripheral, spinal and central nervous pathways. As with nerve conduction studies, their usage is confined to specialized neurophysiology centres.
Bethanechol supersensitivity test
When an organ is deprived of its nerve supply it will develop hypersensitivity to its own neurotransmitter (Cannon’s law of denervation). The bethanechol supersensitivity test is based on this theory; during the test 2.5 mg of bethanechol chloride, an acetylcholine like agent, is administered subcutaneously following an initial pressure/flow study. During a repeat pressure/flow study a rise in detrusor pressure of more than 15 cm H2O compared to the first study at 100 ml filling is a positive result and implies that the detrusor is denervated. A negative result suggests that the detrusor failure is due to a non-neurological cause.
False negative and false positive results are common and bethanechol is contraindicated for patients who have cardiac disease, hypertension, asthma, peptic ulcer or bladder outlet obstruction. Use of this technique remains contentious.
Ice water test
Cold temperature provokes the bladder and increases detrusor activity. The ice water test is performed by instilling 90 ml of sterile ice-cold water (4°C) into an empty bladder through a 16 Fr catheter without filling the catheter balloon. The test is positive if the catheter is ejected together with a significant amount of water within 1 min of installation (in the absence of straining). A positive result implies that the patient has NDO and may be of some use in diagnosing the cause of incontinence in patients with spinal cord injury (Figure 9.2).
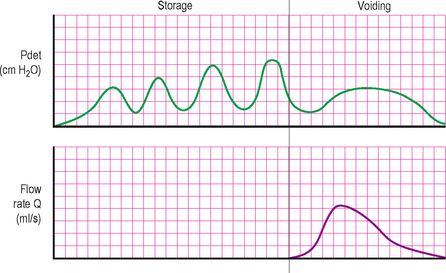
Figure 9.2 Urodynamic trace, showing neurogenic detrusor overactivity (NDO) associated with an incompetent urethral mechanism. Note the relatively low voiding pressure and ‘normal’ flow rate.
NEUROGENIC BLADDER DYSFUNCTION: PRACTICAL POINTS
 Detrusor overactivity in a ‘stepping pattern’ (consecutive detrusor contractions probably superimposed on loss of compliance) (Figure 9.3b).
Detrusor overactivity in a ‘stepping pattern’ (consecutive detrusor contractions probably superimposed on loss of compliance) (Figure 9.3b).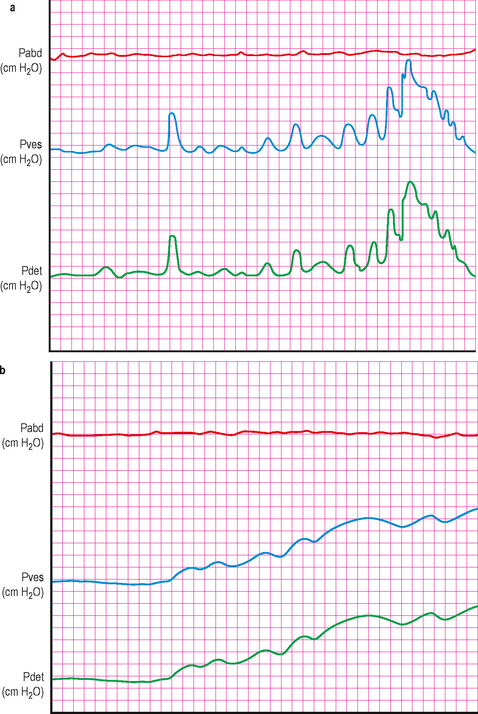
Figure 9.3 (a) and (b) Patterns of detrusor overactivity frequently seen in neurogenic dysfunction. (a) Shows repetitive phasic detrusor contractions with a crescendo and stepping pattern as the bladder volume increases. (b) Shows a stepping pattern with consecutive detrusor contractions. It is probable that there is also an underlying decrease in bladder compliance, with the detrusor contractions ‘superimposed’ upon this.