15 The Temporomandibular Joints, Teeth, and Muscles, and Their Functions
The masticatory system is comprised of joints, muscles, teeth, and nerves that are integrated in the act of mastication. However, that is not to say that other functions and parafunctions are not performed by the components of this system (e.g., speech, yawning, singing, bruxism, clenching, and so on). In addition, when disorders occur in any component of the system, symptoms may be reflected to adjacent and associated structures (e.g., temporomandibular joint and muscle disorders) with pain (myalgia). Dysfunction of one or more of the masticatory muscles (e.g., lateral pterygoid muscle) can lead to pain and dysfunction of muscles not usually considered to be masticatory muscles (e.g., neck muscles), and such conditions are sometimes referred to as craniomandibular disorders. Also to be considered in the diagnosis of orofacial pain are possible associations between the symptoms of toothache, headache, and joint and muscle disorders. In addition, the role of mandibular movements and movements related to yawning and opening of the eustachian tubes in subjective hearing problems (stuffiness of the ears) is considered.1 This chapter provides the necessary anatomical and functional basis for further study into disorders of the masticatory system.
Temporomandibular Articulation
The temporomandibular joint (TMJ) is an example of ginglymoarthrodial articulation, and its movements are a combination of gliding movements and a loose hinge movement. The osseous portions of the joint are the anterior portion of the mandibular (glenoid) fossa and articular eminence of the temporal bone, and the condyloid process of the mandible (Figure 15-1). The functional surfaces of both the condyle and the eminence, along with the anterior aspects of the condyle, are the functional articular surfaces, not the mandibular fossa. Interposed between the condyle and temporal bone is the articular disk. It consists of dense collagenous connective tissue that, in the central area, is relatively avascular, hyalinized, and devoid of nerves (Figure 15-2). The disk is not seen on radiographs, but the bony structures in one plane can be viewed by a transcranial projection (Figure 15-3).
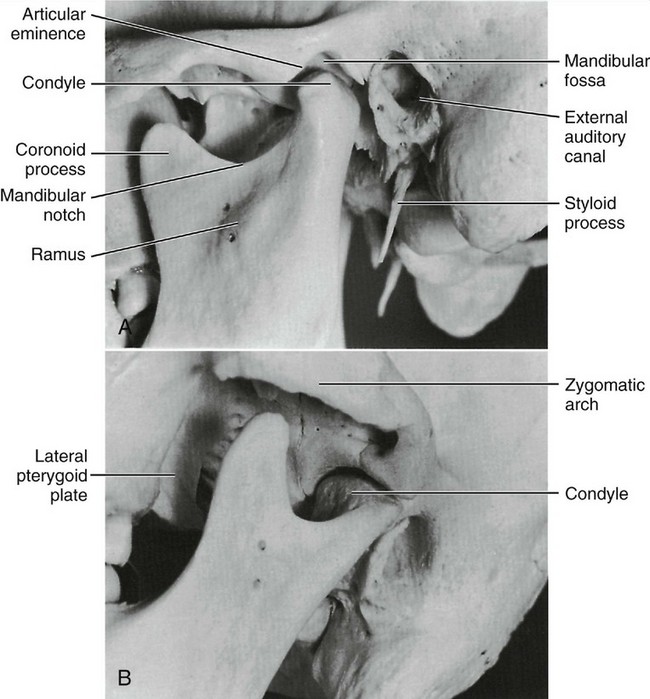
Figure 15-1 A, Relation of the condyle of the mandible to the glenoid fossa and the articular eminence of the temporal bone with the teeth in the intercuspal position. B, View of mandibular fossa and infratemporal fossa.
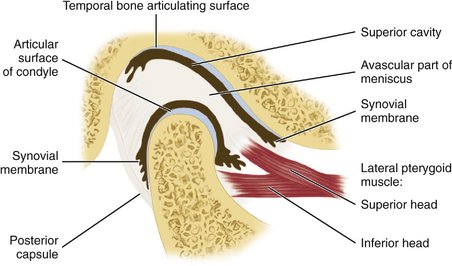
Figure 15-2 Schematic representation of the temporomandibular joint. The well-defined division of the lateral pterygoid muscle is for illustrative purposes only. A significant number of fibers of the upper part of the lateral pterygoid muscle attach to the neck of the condyle along with the inferior head of the lateral pterygoid muscle.
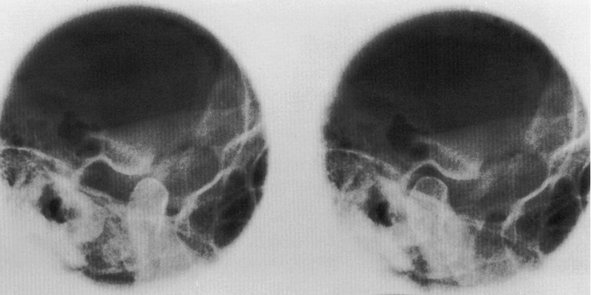
Figure 15-3 Radiograph showing the temporomandibular joint in open (left) and closed (right) position of mandible.
MANDIBULAR FOSSA
The mandibular fossa is an oval or oblong depression in the temporal bone just anterior to the auditory canal (Figure 15-4). It is bounded anteriorly by the eminentia articularis (articular eminence), externally by the middle root of the zygoma and the auditory process, and posteriorly by the tympanic plate of the petrous portion of this bone (see Figure 14-1). The shape of the mandibular fossa conforms to some extent, although not exactly, to the posterior and superior surfaces of the condyloid process of the mandible.
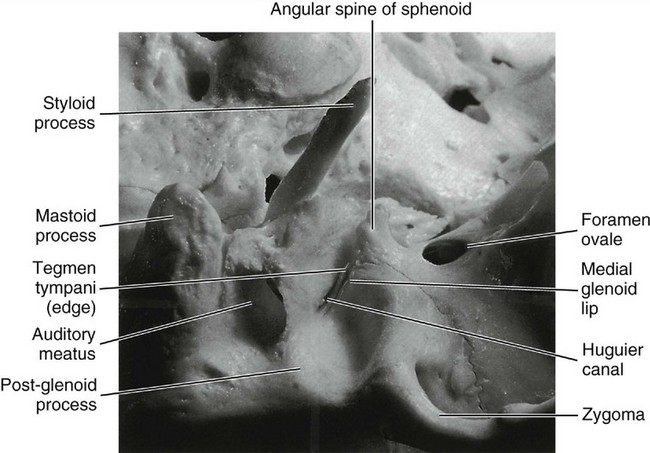
Figure 15-4 Exterior of the base of the skull showing the mandibular fossa in the inferior surface of the squamous part of the temporal bone at the base of the zygomatic process. It is divided into two halves by the petrotympanic fissure. The anterior half is included in the temporomandibular articulation.
CONDYLOID PROCESS
The condyloid process of the mandible is convex on all bearing surfaces, although somewhat flattened posteriorly, and its knoblike form is wider lateromedially than anteroposteriorly (Figure 15-5). It is perhaps two and one half times as wide in one direction as in the other. Although the development of the condyle differs in individuals, the functional design remains the same. The long axes of the condyles are in a lateral plane, and at first sight, they seem to be out of alignment, because the long axes, if the lines were prolonged, would meet at a point anterior to the foramen magnum at an angle of approximately 135 degrees. The condyle is perpendicular to the ascending ramus of the mandible (see Figure 15-5).
JOINT CAPSULE
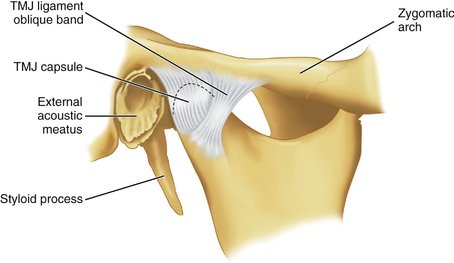
The TMJ is enclosed in a capsule (Figure 15-6) that is attached at the borders of the articulating surfaces of the mandibular fossa and eminence of the temporal bone and to the neck of the mandible. The anterolateral side of the capsule may be thickened to form a band referred to as the temporomandibular ligament. It is not always so thickened, but when clearly distinguishable as a ligament, it appears to originate on the zygomatic arch and to pass backward to attach on the lateral and/or distal surfaces of the neck of the mandible.
The capsule consists of an internal synovial layer and an outer fibrous layer containing veins, nerves, and collagen fibers. The innervation for the capsule arises from the trigeminal nerve, and several kinds of receptors have been described, including free nerve endings.2 The vascular supply arises from the maxillary, temporal, and masseteric arteries.
MANDIBULAR LIGAMENTS
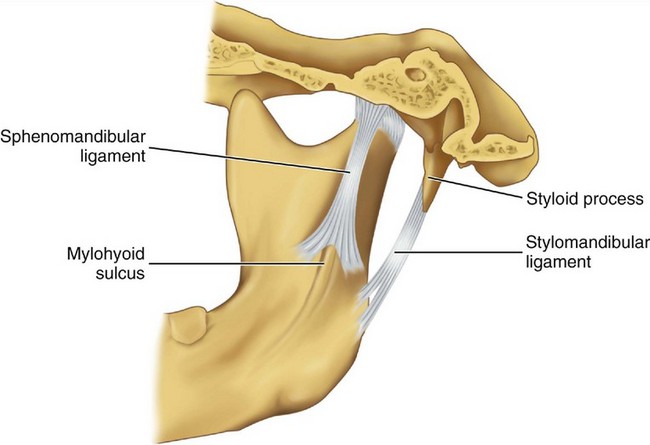
Accessory ligaments, including the stylomandibular and sphenomandibular ligaments, are considered a part of the masticatory apparatus (Figure 15-7). These ligaments do not have a direct relationship with mandibular articulation, although they may stabilize the articular system during jaw movements.
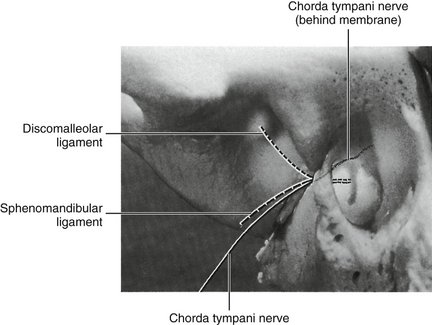
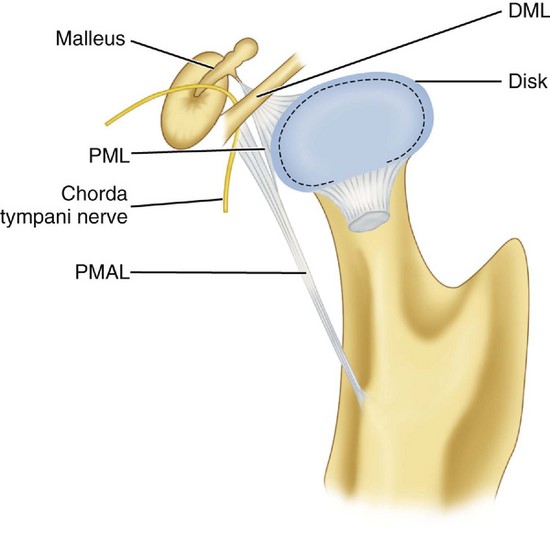
The sphenomandibular ligament arises from the angular spine of the sphenoid bone and from the petrotympanic fissures and ends broadly at the lingula of the mandible. In some instances, a continuation of ligament fibers is evident through the petrotympanic fissure via the Huguier canal (see Figure 15-4) to the middle ear, where they attach to the malleus.
Otomandibular ligaments connect the middle ear and the TMJ. These small ligaments, the discomalleolar and tympanomandibular (sphenomandibular), have been described as connecting the malleus to the TMJ disk and to the sphenomandibular ligaments (Figures 15-8 and 15-9). The role of these ligaments as causal factors in subjective TMJ-mediated auditory symptoms remains to be substantiated.3
ARTICULAR DISK
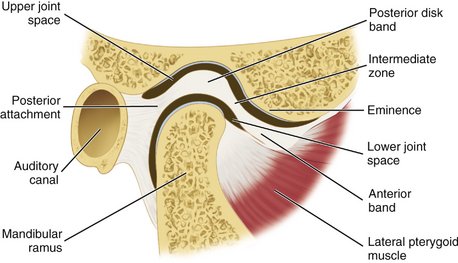
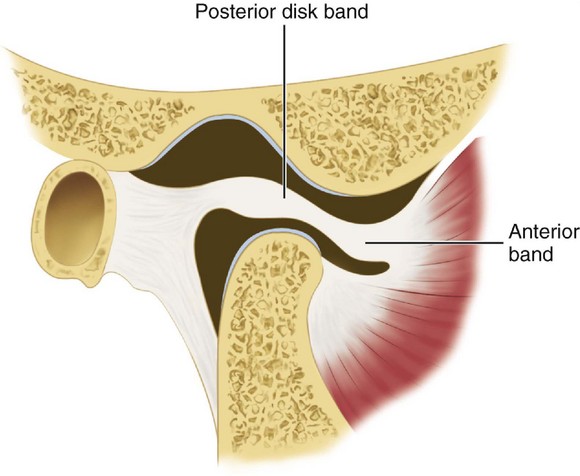
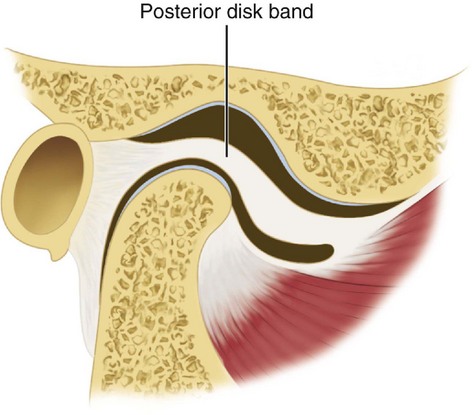
The interarticular disk (see Figure 15-2) consists of fibrous tissue shaped to accommodate the shape of the condyle and concavity of the mandibular fossa. Thicker anterior and posterior bands and a thin central zone are evident4 (Figure 15-10). The superior and inferior heads of the lateral pterygoid muscle both insert into the pterygoid fovea of the mandible with a part of the superior head inserting into the disk and capsule. The disk divides the articulating surfaces into upper and lower compartments that provide for smooth gliding function. As the jaw opens and moves forward, the intermediate zone of the disk is interposed between the anterior slope of the articular eminence and the condyle, and the bilaminar region of the disk fills in the mandibular fossa (Figure 15-11). The upper head of the lateral pterygoid muscle, which does not appear to be active during mandibular opening movement, stabilizes the relationship of the disk to the eminence. Continued anterior displacement of the disk (Figure 15-12) with the posterior band in an anterior position with the jaw closed can prevent the jaw from opening normally (i.e., locking). The cause of disk derangement is multifactorial but may include acute and chronic trauma.
MANDIBULAR POSITIONS
Basic jaw positions are usually described as centric occlusion, intercuspal position, centric relation, retruded contact position, and rest position of the mandible.5 Centric occlusion or intercuspal position is defined as maximum intercuspation of the teeth. Centric relation is a position of the mandible (or path of opening and closing without translation of the condyles) in which the condyles are in their uppermost position in the mandibular fossae and related anteriorly to the distal slope of the articular eminence (see Figure 16-44). Because the mandible appears to rotate around a transverse axis through the condyle in centric relation movement, guidance of the jaw by the clinician (see Figure 16-42) in opening and closing movements that do not have translation is referred to as hinge axis movement (Figure 15-13). In this position, the condyles are considered to be in the terminal hinge position. Under physiological conditions of the masticatory system, centric relation is used to transfer the position of the mandible (in relation to the maxilla) to an articulator.
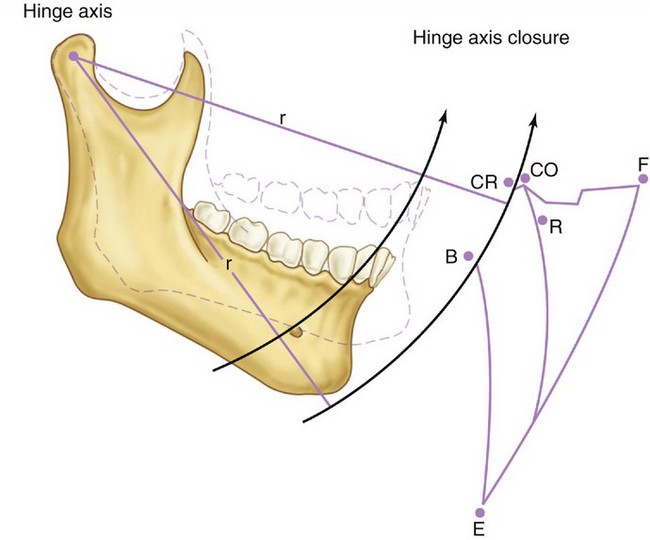
Figure 15-13 Schematic representation of mandibular movement envelope in the sagittal plane. CR, Centric relation; CO, centric occlusion; F, maximum protrusion; R, rest position; E, maximum opening; B to CR, opening and closing on hinge axis with no change in radius (r).
In the natural dentition, centric occlusion is, in the majority of people, anterior to centric relation contact on the average by approximately 1 mm.6 Centric occlusion (or acquired or habitual centric as it is sometimes called) is a tooth-determined position, whereas centric relation is a jaw-to-jaw relation determined by the condyles in the fossae. Closure into occlusion occurs usually anterior to centric relation; however, a coincidence of centric relation contact and the intercuspal position is evident in about 10% of the population.
Rest position is a postural position of the mandible determined largely by neuromuscular activity and to a lesser degree by the viscoelastic properties of the muscles. Thus, because tonicity of muscles may be influenced by the central nervous system as a result of factors such as emotional stress and by local peripheral factors such as a sore tooth, the rest position of the mandible is not consistent. The interocclusal space with the mandible in rest position and head in upright position is about 1 to 3 mm at the incisors but has considerable normal variance even up to 8 to 10 mm without evidence of dysfunction.
MANDIBULAR MOVEMENTS
In lateral movements (Figure 15-14), the condyle appears to rotate with a slight lateral shift in the direction of the movement. This movement is called the Bennett movement and may have both immediate and progressive components. By the use of recording equipment such as a pantograph or kinesiograph, it is possible to record mandibular movements in relation to a particular plane of reference (e.g., sagittal, horizontal, or frontal planes). If a point (the incisive point) located between the incisal edges of the two mandibular central incisors is tracked during maximal lateral, protrusive, retrusive, and wide opening movements, such movements are seen to take place within a border or envelope of movements.6 Functional and parafunctional movements occur within these borders. However, most functional movements such as those associated with mastication occur chiefly around centric. Border movements in the horizontal plane are shown in Figure 15-14.
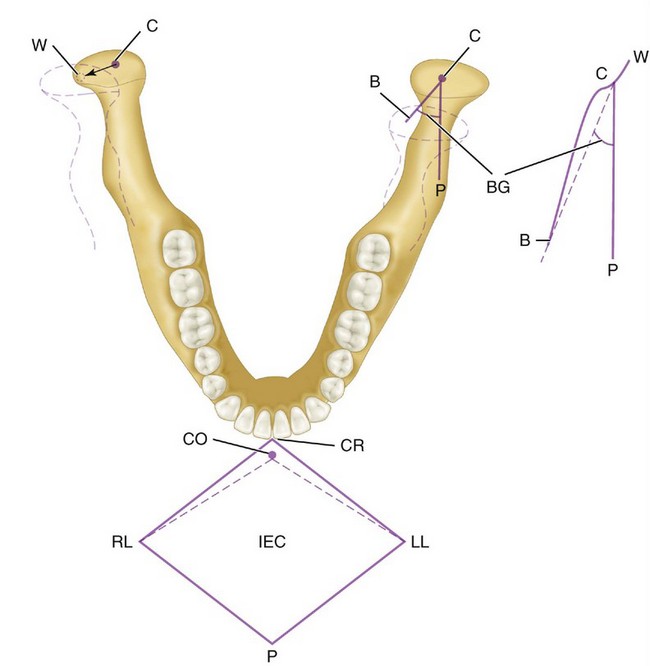
Figure 15-14 Right mandibular movement with schematic representation of movement at the incisal point in the horizontal plane (CR, LL, P, RL) and at the condyle (W, C, B, P) made by a pantograph. Teeth are not in occlusion. CR, Centric relation; LL, left lateral; P, protrusive; RL, right lateral; CO, centric occlusion; IEC, incisal edge contact. On the right side, the condyle moves from C (centric) to right working (W). On the balancing side, the left condyle moves from C along line B and makes an angle BG, called the Bennett angle. C to P, Straight protrusive movement.
The maximum opening movement is 50 to 60 mm, depending on the age and size of the individual. An arbitrary lower limit for normal of 40 mm may be in error, inasmuch as some individuals may have no difficulty incising a large apple and have no history of TMJ muscle dysfunction. The maximum lateral movement in the absence of TMJ muscle dysfunction, including pain, is about 10 to 12 mm. The maximum protrusive movement is approximately 8 to 11 mm, again depending on the size of the subject and skull morphology. The retrusive range for adults and children is about 1 mm, although 2 to 3 mm may be observed infrequently.7 The retrusive range, as measured from centric occlusion to centric relation, is considered a discrepancy between centric occlusion and centric relation. Border movements in the sagittal plane are shown in Figure 15-13.
All values for border movements must be related to function—that is, a maximum lateral movement of 7 to 8 mm to the right (10 to 12 mm to the left) must be related to the occlusion and to whether translation of the left condyle occurs, because the latter may be “fixed” because of dysfunction or pain. Such values should also be related to other functions such as incising, chewing, swallowing, and speaking. However, if such values are made a part of every patient’s dental record, any change can be evaluated in terms of dysfunction.
Muscles
Masticatory functions, speaking, yawning, and swallowing, involve reflex contraction and relaxation of the muscles of mastication whose activity is initiated voluntarily. It is impossible to determine clinically if a particular muscle is participating in a particular movement solely from its origin and insertion. Patterns of muscle contraction are complex and even in the same areas may have different functions.
The complex movements of the TMJ suggest that muscles of mastication exhibit differential regional action and regional differences in their histochemical profiles. Thus, to consider a “muscle” as a contracting entity is an oversimplification. In reality, each muscle is a collection of motor units with different properties located in different parts of a single muscle and exhibiting different activities. However, for obvious reasons, the action of the various muscles will be given as a contracting entity.
The masticatory muscles concerned with mandibular movements include the lateral pterygoid, digastric, masseter, medial pterygoid, and temporalis muscles. Also, the mylohyoid and geniohyoid muscles are involved in masticatory functions.
Several muscles associated with the ear, throat, and neck are of interest to the dentist, including tensors tympani and palatini, because the latter two may relate to subjective hearing disorders such as stuffiness, some forms of tinnitus, and noise.1,8-10
LATERAL PTERYGOID MUSCLE
The lateral pterygoid muscle has two origins: one head originates on the outer surface of the lateral pterygoid plate, and an upper or superior head originates on the greater sphenoid wing (Figures 15-15, 15-16, and 15-17, C). The insertion is on the anterior surface of the neck of the condyle. In addition, an insertion is evident of some fibers to the capsule of the joint and anterior aspect of the articular disk (Figure 15-17, C).
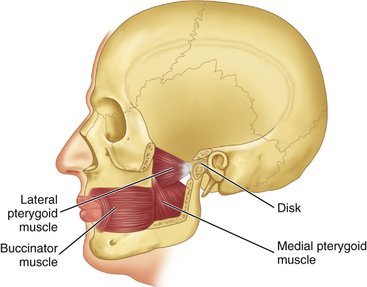
Figure 15-15 Positions of the lateral and medial pterygoid muscles shown with cutaway sections of bone.
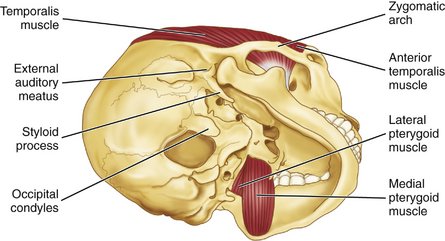
Figure 15-16 View of the medial and lateral pterygoid muscles. Note the insertion of the temporalis muscle also in Figure 15-17, B.
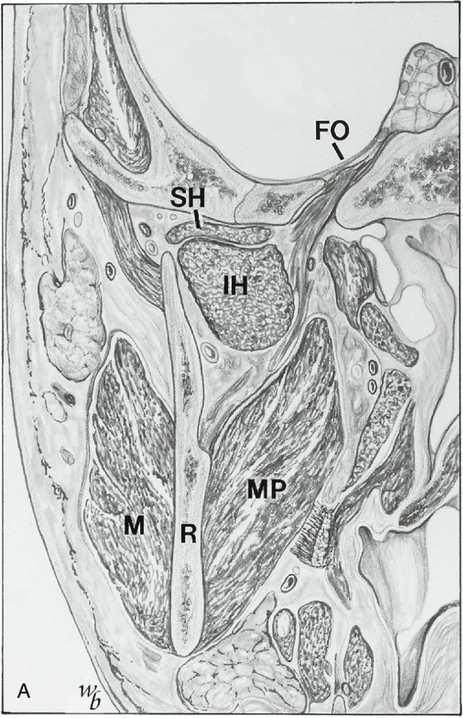
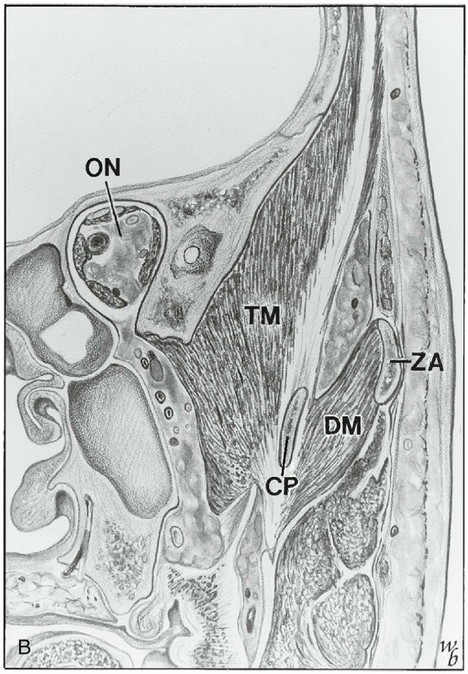
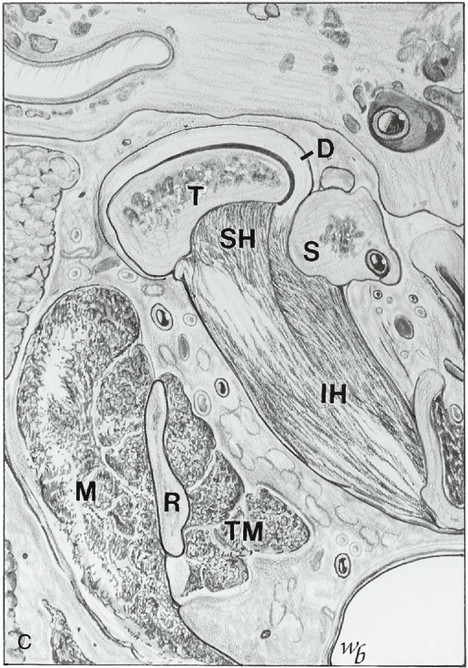
Figure 15-17 A, Coronal section showing the mandibular nerve exiting the foramen ovale (FO), the superior (SH) and inferior (IH) heads of the lateral pterygoid muscle, the ascending ramus (R), and the masseter (M) and medial pterygoid (MP) muscle. B, Optic nerve (ON), temporalis muscle (TM), deep masseter (DM) or zygomaticomandibular muscle, and coronoid process (CP). ZA, Zygomatic arch. C, Horizontal section at the level of the temporomandibular joint, showing the condyle (T), superior head of lateral pterygoid muscle (SH), inferior head (IH), ascending ramus (R), masseter muscle (M), and temporalis muscle (TM). D, Disk; S, styloid.
(Redrawn from Widmalm SE, Lillie JH, Ash MM: Anatomical and electromyographic studies of the lateral pterygoid muscle, J Oral Rehabil 14:429, 1987.)
The superior head is active during various jaw-closing movements only, whereas the inferior head is active during jaw-opening movements and protrusion only.11 The lateral pterygoid is anatomically suited for protraction, depression, and contralateral abduction. It may also be active during other movements for joint stabilization. The superior head is active during such closing movements as chewing and clenching of the teeth and during swallowing. Presumably, the superior head positions or stabilizes the condylar head and disk against the articular eminence during mandibular closing. The inferior head assists in the translation of the condyle downward, anteriorly, and contralaterally during jaw opening. The lateral pterygoid muscle is innervated by the trigeminal nerve (V) (see Figure 14-37).
MASSETER MUSCLE
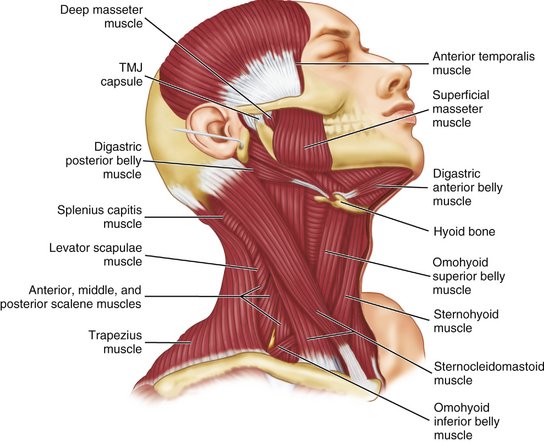
The masseter muscle extends from the zygomatic arch to the ramus and body of the mandible. The insertion of this muscle is broad, extending from the region of the second molar on the lateral surface of the mandible to the posterior lateral surface of the ramus (Figure 15-18; see also Figure 15-19). The masseter muscle is covered partly by the platysma muscle (Figure 15-19) and by the risorius muscle. The platysma is activated during firm clenching in some individuals and, having some insertion in the orbicular muscle (orbicularis oris), is sometimes active in facial expression. The risorius is affected by emotion and is active in facial expression.
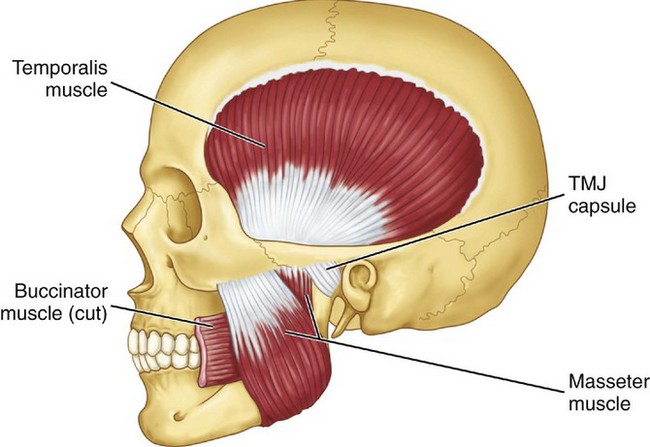
Figure 15-18 Masticatory muscles shown include the temporalis and masseter muscles. The deep masseter is attached to the zygoma (see Figure 15-17, C). TMJ, Temporomandibular joint.
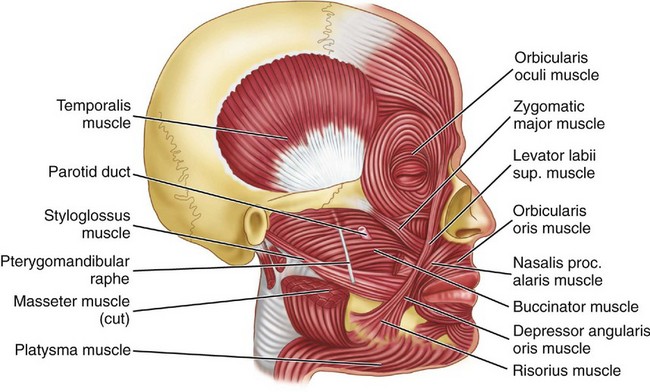
Figure 15-19 Muscles of facial expression and accessory muscles of mastication. Sup., Superior; proc., procerus.
The superficial part of the masseter muscle is separated distinctly only from the deeper layer of the muscle at the posterior upper part of the muscle. The masseter muscle is covered partly and to a variable degree with the parotid gland tissue. The center of the lower third of the masseter muscle is about 2 to 3 cm from the anterior border of the sternocleidomastoid muscle, which contracts during clenching in some individuals. The masseter muscle is active during forceful jaw closing and may assist in protrusion of the mandible. The masseter muscle is innervated by the fifth nerve (masseter nerve). The zygomaticomandibular muscle (deep masseter muscle) inserts at the coronoid process and originates on the inner surface of the zygomatic arch (see Figure 15-17, B). It may be an antagonist to the posterior temporalis and a synergist for the lateral pterygoid muscle.
MEDIAL PTERYGOID MUSCLE
The medial pterygoid muscle arises from the medial surface of the lateral pterygoid plate and from the palatine bone (see Figures 15-15 and 15-16). It inserts on the medial surface of the angle of the mandible and on the ramus up to the mandibular foramen. The principal functions of the medial pterygoid muscle are elevation and lateral positioning of the mandible. It is active during protrusion. The innervation is a branch of the mandibular division of the fifth nerve.
TEMPORALIS MUSCLE
The temporalis muscle is fan-shaped and originates in the temporal fossa (see Figures 15-16; 15-17, B; 15-18; and 15-20). On passing to the zygomatic arch, it forms a tendon that inserts into the anterior border and mesial surface of the coronoid process of the mandible and along the anterior border of the ascending ramus of the mandible (see Figure 15-16). The anterior fibers extend along the anterior border of the ramus almost to the third molar. The muscle has three component parts and appears to behave as if it consisted of three distinct parts. The temporal muscle is the principal positioner of the mandible during elevation. The posterior part is active in retruding the mandible, and the anterior part is active in clenching. The anterior part may act as a synergist with the masseter in clenching, whereas the posterior part acts as an antagonist to the masseter in retruding the jaw. The temporalis muscle is innervated by temporal branches of the mandibular division of the fifth nerve.
DIGASTRIC MUSCLE
The attachment of the anterior digastric muscle is at or near the lower border of the mandible and near the midline (see Figure 15-20). A tendon is between the anterior and posterior digastric muscles that is attached by a looplike strip of fascia to the hyoid bone. The anterior digastric muscle is covered by the platysma muscle, and beneath lie the mylohyoid and the geniohyoid muscles. All of these muscles are considered to be active during various phases of jaw opening. A mylohyoid branch of the mandibular division of the fifth nerve innervates the anterior digastric muscle (see Figure 14-37); the digastric branch of the facial nerve innervates the posterior digastric muscle.
GENIOHYOID MUSCLE
The geniohyoid muscle lies superior to the mylohyoid muscle and adjacent to the midline. It arises from the mental spine on the posterior aspect of the symphysis menti of the mandible. It inserts on the anterior surface of the hyoid bone. When the mandible is fixed, the hyoid bone is drawn forward and upward; when the hyoid bone is fixed, the lower jaw is depressed. Innervation is from C1, C2, and possibly the hypoglossal nerve (see Figure 14-37).
TENSOR TYMPANI AND PALATINI MUSCLES
The tensor tympani, tensor veli palatini, and levator veli palatini muscles (Figures 15-21 and 15-22) may have clinical significance for subjective auditory symptoms associated with temporomandibular and muscle disorders (TMDs).1,8,9 These muscles are innervated by the trigeminal nerve and therefore may respond to similar kinds of ascending information from joints, skin, and muscles and from descending inputs from higher centers that converge on interneurons and the trigeminal motor nucleus (Figure 15-23). Disturbances of auditory tube opening during swallowing that may occur with TMJ and TMDs appear to be consistent with restricted function and anatomy of the tensor palatini and levator veli palatini muscles.10 If a causal connection between subjective hearing symptoms and TMDs is to be established, additional evidence-based research is needed.
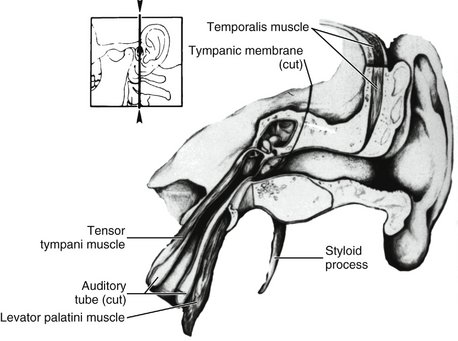
Figure 15-21 Section through the ear showing inner ear structures and the tensor tympani muscle, which is active in stretching the tympanic membrane.
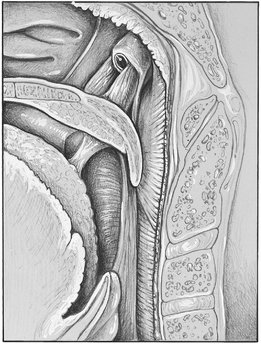
Figure 15-22 Muscles of the throat. Eustachian tube and tensor palatinus muscle, which is active in opening the auditory tube.
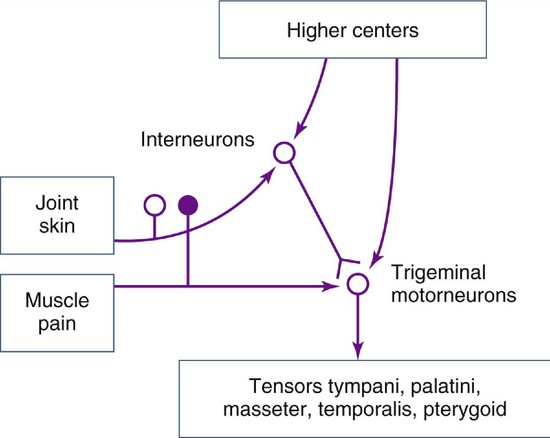
Figure 15-23 Information from joints, skin, muscles, and higher centers converge on the interneurons and trigeminal motor nucleus.
Restricted jaw opening and prevention of yawning seen in TMJ and TMDs can lead to otological symptoms such as subjective hearing loss associated with sensations of stuffiness in the ear.1 Thus, pain in and around the joints and muscles may influence the otomandibular muscles via interneurons in the intertrigeminal area and might be responsible for a few cases of tinnitus associated with restricted jaw opening due to TMJ and muscle disorders.
HEAD AND NECK MUSCLES
The muscles of the head and neck have been of interest to the dentist because of a potential relationship among the occlusion, TMJs, and pain in these muscles and/or muscle contraction headache. The possible role of the epicranius muscles in tension headache has yet to be clarified. The sternocleidomastoid muscle (see Figure 15-20) is often affected in patients with TMJ muscle dysfunction and often co-contracts with jaw clenching. The functions of the orbicularis oris and buccinator muscles (see Figure 15-19) appear to have a significant role for optimal function of complete dentures. The seventh (VII) nerve innervates all the muscles of facial expression.
Other muscles that are of interest to the dentist because of TMJ and muscle disorders are the scalenus, splenius, iliocostalis cervicis, and omohyoideus. However, the association between TMDs and myalgia of the muscles has not been established. A number of problems in oral motor function, such as lip posture in the aged, may be related to a generalized deterioration in performance or to a specific motor function rather than the dental state or prescription medication. Most reports on motor disorders and aging have focused on disturbances in control originating in the nervous system.
Mandibular Movements and Muscle Activity
Mandibular movement during normal function and during parafunction (e.g., bruxism) involve complex neuromuscular patterns originating in part in a pattern generator in the brainstem and modified by influences from higher centers (see Figure 15-23), namely, the cerebral cortex and basal ganglia, and from peripheral influences (e.g., the periodontium, muscles, and so on). However, a detailed discussion of such movements is beyond the scope of this text. Rather, the discussion relates to muscle activity as seen in electromyography for jaw opening and closing, protrusion, retrusion, and lateral movements.
MANDIBULAR OPENING
The digastric, mylohyoid, and geniohyoid muscles are active during jaw opening, either slowly or maximally against resistance. No activity occurs in the temporalis and masseter muscles when the mouth is opened slowly and the jaw is opened maximally, although some activity may occur in the medial pterygoid muscle. When the jaw is opened against resistance, the temporalis muscle remains silent. During opening movements, the lateral pterygoid muscles show initial and sustained activity. In forced depression, the digastric muscle is activated almost as soon as the lateral pterygoid muscle is. Generally, the activity of the anterior digastric muscle follows that of the lateral pterygoid muscle.
MANDIBULAR CLOSING
While the mandible is being elevated slowly, without contact of the teeth, no activity is evident in any portion of the temporalis muscle. Elevation without contact or resistance is brought about by contraction of the masseter and medial pterygoid muscle. The temporalis, masseter, and medial pterygoid muscles affect elevation against resistance. The suprahyoid muscles act as an antagonist of the elevator muscles. Closure into maximal intercuspation (centric occlusion) may involve contraction of facial and neck muscles.
RETRUSION
Voluntary mandibular retrusion with the mouth closed is brought about by contraction of the posterior fibers of the temporalis muscle and by the suprahyoid and infrahyoid muscles. Retraction of the mandible from protrusion and without occlusal contact is effected by the contraction of the posterior and middle fibers of the temporalis muscles. Slight activity of the suprahyoid may be the result of slight jaw opening to allow the teeth to glide over each other from centric occlusion to centric relation.
PROTRUSION
Protrusion of the mandible without occlusal contact results from contraction of the lateral and medial pterygoid muscles and also masseter muscles. Protraction against resistance is brought about by contraction of the lateral and medial pterygoid muscles and of the masseter and suprahyoid muscle group. Protrusion with the teeth in occlusion is achieved by contraction of the pterygoid and masseter muscles. Only slight activity occurs in the suprahyoid muscles. In combined protraction and opening, activity is evident in the medial and lateral pterygoid muscles, the masseter muscles, and sometimes the anterior fibers of the temporalis muscles.
LATERAL MOVEMENTS
Lateral movement of the mandible to the right side (without occlusal contact) is achieved by ipsilateral contraction of primarily the posterior fibers of the temporalis muscle. The suprahyoid muscles are active in maintaining the jaw slightly depressed and protruded. Movement to the left side without occlusal contact is brought about by the contralateral contraction of the medial pterygoid and masseter muscles. Lateral movement to the right side against resistance is achieved by the ipsilateral contraction of the temporalis muscle and by some activity in the ipsilateral masseter and medial pterygoid muscles. Movement to the left side against resistance is achieved by the contralateral contraction of the medial pterygoid and masseter muscles. Lateral movement to the right with occlusal contact is achieved by ipsilateral contraction of the temporalis muscle. Movement to the left with occlusal contact is brought about by contralateral contraction of the medial pterygoid and masseter muscles. Both lateral pterygoid muscles initiate depression of the mandible, and the contralateral muscle initiates a lateral transversion. Lateral movements of the jaw are achieved by ipsilateral contraction of the posterior and middle fibers of the temporalis muscle and by contralateral contraction of the lateral and medial pterygoid muscles and the anterior fibers of the temporalis muscle. Parts of the temporal and masseter muscles may act as antagonists or synergists during horizontal movements and minimum separation of the teeth.
CHEWING
Chewing is highly complex oral motor behavior usually seen in the frontal plane in simple form (Figure 15-24). No archetypal chewing cycle exists. The means of the dimensions of the chewing cycle are between 16 and 20 mm for vertical movements and between 3 and 5 mm for lateral movements. The duration of the cycle varies from 0.6 to 1 second depending on the type of food. The speed of masticatory movement varies within each cycle, both according to the type of food and among individuals. Speed, duration, and form of the chewing cycle vary with the type of occlusion, kind of food, and presence of dysfunction.
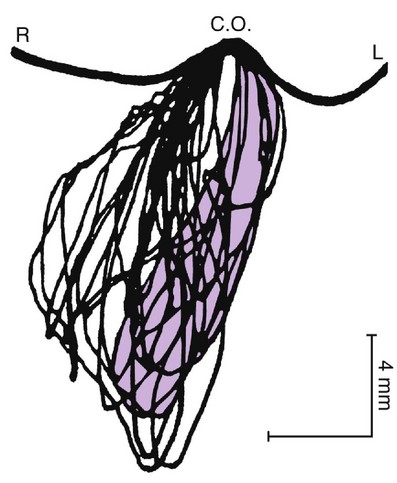
Figure 15-24 Mandibular movements during the process of chewing naturally. Incisor point movement is seen in the frontal plane. L, Left; C.O., centric occlusion; R, right.
Occlusal contacts occur in centric occlusion in at least 80% to 90% of all chewing cycles, especially near complete trituration of the bolus. With closing and opening movements, contact gliding is seen. In the closing phase, the contact glide depends on the type of occlusion and type of food. Where tough food is the normal diet (e.g., as with Australian aborigines) with corresponding occlusal wear, the chewing movement shows a long contact glide (2.8 ± 0.35 mm)12 compared with the short contact glide (0.90 ± 0.36 mm) of Europeans living on a modern diet of easily triturated food.13 The chewing force reaches a maximum in centric occlusion and lasts for 40 to 170 milliseconds, and the peak electromyographic activity of the temporal and masseter muscles lasts for a mean of 41 ± 26 milliseconds. Some chewing force is maintained for gliding tooth contact on the opening phase. In the intercuspal position, the jaw is stationary, or it pauses for approximately 100 milliseconds before the next cycle begins.
SWALLOWING
Swallowing involves most of the tongue muscles and buccal musculature.14 In the initial stage of swallowing, the bolus moves from the mouth to the fauces (Figure 15-25). The bolus is then moved from the fauces to the esophagus and finally through the esophagus to the stomach. When saliva is swallowed, total participation of the suprahyoid muscles occurs, with marked activity of the digastric and mylohyoid muscles, followed by moderate activity in the geniohyoid muscles. The medial pterygoid muscle is often active; less often, the temporalis and masseter muscles are active with occlusal contact.
ORAL MOTOR BEHAVIOR
Oral motor behavior refers to function and parafunction of the mouth and associated structures. More generally, behavior includes observable actions ranging from simple movements such as retrusion or protrusion to more complex movements such as chewing. To accomplish complex behavior, sensorimotor systems consisting of muscles and neural processes are required for the initiation, programming, and execution of motor functions.
Chewing movements depend on complex integrative neural processes of the central nervous system that may be initiated by either internal or external influences, including innate drives, emotional states, and instructions to patients. During chewing, a large amount of proprioceptive (i.e., muscle sense) and exteroceptive (i.e., tactile sense) information is fed to the central nervous system (e.g., cerebral cortex, brainstem, basal ganglia, spinal cord). Rhythmic movements such as chewing are largely programmed or preprogrammed and involve learning, which reduces the need for peripheral sensory input. However, inputs from muscle, joint, tendon, and periodontal receptors still have important functions, especially in relation to learning, new experiences, and protective reflexes. Neuronal mechanisms must be present to provide for modification of reflexes and continued updating of masticatory movements by information about such factors as occlusal forces and the state and location of the bolus.
OVERVIEW OF MASTICATION
The act of mastication begins with “setting the system” by sight, tactile sense, and smell to receive the food. Involvement of the tactile sense may range from picking up the food to grasping the food with the incisor teeth. When the food is taken into the mouth, the lips, tongue, and periodontium function to estimate size, hardness, and other characteristics that must be correlated with previous behavior required for chewing. This information sets the chewing program in the pattern generator, including subsequences that relate to central and peripheral influences already in progress. Orofacial receptors such as periodontal mechanoreceptors may monitor occlusal forces and control the jaw-closing muscles. The chewing program may be altered according to stages of chewing or in response to information from receptors in specific areas such as the palatal mucosa and tongue. The chewing rhythm may be stopped because of noxious stimuli. It is probable that rhythmic and repetitive chewing behavior is disturbed by dysfunctional states of the occlusion and/or the TMJs through which the bolus is shifted and the jaw moved to another position for the next forceful tooth-food contact. Thus chewing cycles may occur without the development of an actual power stroke in dysfunction states.
1. Ash MM, et al. Current concepts of the relationship and management of temporo-mandibular disorders and auditory symptoms. J Mich Dent Assoc. 1990;72:550.
2. Thilander B. Innervation of the temporomandibular joint capsule in man. Trans R Sci Dent. 1961;7:9.
3. Ash CM, Pinto OF. The TMJ and the middle ear: structural and functional correlates for aural symptoms associated with temporomandibular joint dysfunction. Int J Prosthodont. 1991;4:51.
4. Dolwick MF, Sanders B. TMJ internal derangement and arthrosis. St Louis: Mosby; 1985.
5. Ash MM. Philosophy of occlusion. Dent Clin North Am. 1995;39:233.
6. Posselt U. Studies in the mobility of the human mandible. Acta Odont Scand. 1952;10(suppl):3.
7. Ash MM, Ramfjord SP. Occlusion, ed 4. Philadelphia: Saunders; 1995.
8. Rubinstein B, et al. Prevalence of signs and symptoms of craniomandibular disorders in tinnitus patients. Cranio. 1990;4:186.
9. Myrhaug H. The theory of otosclerosis and morbus meniere (labyrinthine vertigo) being caused by the same mechanism: physical irritants, an otognathic syndrome. Bergen: Bergmanns Boklrykkeri A/S; 1981.
10. Misurya VK. Functional anatomy of tensor palatini and levator palatini muscles. Arch Otolaryngol. 1976;102:265.
11. Widmalm SE, Lillie JH, Ash MMJr. Anatomical and electromyographic studies of the lateral pterygoid muscle. J Oral Rehabil. 1987;14:429.
12. Beyron H. Occlusal relations and mastication in Australian aborigines. Acta Odont Scand. 1964;22:597.
13. Ahlgren J. Masticatory movements in man. In: Anderson DJ, Matthews B, editors. Mastication. Bristol, England: John Wright and Sons, 1976.
14. Anson JB. An atlas of human anatomy, ed 2. Philadelphia: Saunders; 1962.
Aarstad T. The capsular ligament of the temporomandibular joint and retrusion facets of the dentition in relation to mandibular movements. Oslo: Acad Forlag; 1954. (Translated by H Christie)
Ahlgren J. Mechanisms of mastication. Acta Odont Scand. 1966;24((suppl)44):1.
Ash MM. Paradigmatic shifts in TMD and occlusion. J Oral Rehabil. 2001;28:1.
Buxbaum JD, et al. A comparison of centric relation with maximum intercuspation based upon quantitative electromyography. J Oral Rehabil. 1982;9:45.
Callander CL. Surgical anatomy, ed 2. Philadelphia: Saunders; 1939.
Carlsoo S. Nervous coordination and mechanical function of the mandibular elevators. Acta Odont Scand. 1952;10((suppl)11):1.
Deaver JB. Surgical anatomy of the human body, ed 2. Philadelphia: Blakiston; 1926.
Duthie N, Yemm R. Muscles involved in voluntary mandibular retrusion in man. J Oral Rehabil. 1982;9:155.
Eagle WW. Asymptomatic styloid process. Arch Otolaryngol. 1949;49:490.
Eriksson P-O, et al. Special histochemical muscle-fibre characteristics of the human lateral pterygoid muscle. Arch Oral Biol. 1981;26:495.
Gandevia SC, Mahutte CK. Joint mechanics as a determinant of motor unit organization in man. Med Hypotheses. 1980;6:527.
Gibbs CH, et al. Functional movements of the mandible. J Prosthet Dent. 1971;26:604.
Gosen AJ. Mandibular leverage and occlusion. J Prosthet Dent. 1974;31:369.
Grant PG. Lateral pterygoid: two muscles? Am J Anat. 1973;138:1.
Hansson T, et al. Thickness of the soft tissue layers and the articular disk in the temporomandibular joint. Acta Odont Scand. 1977;35:77.
Hickey JC. Mandibular movements in three dimensions. J Prosthet Dent. 1963;13:72.
Hjortsjo CH. The mechanism in the temporomandibular joint. Acta Odont Scand. 1953;11:5.
Hylander WL. The human mandible: lever or link? Am J Phys Anthropol. 1975;43:227.
Kawumura Y, Nobuhara M. Studies on masticatory function. II. The swallowing threshold of persons with normal occlusion and malocclusion. Med J Osaka Univ. 1957;8:241.
Lehr RP, Owens SE. An electromyographic study of the human lateral pterygoid muscles. Anat Rec. 1980;196:441.
McNamara JA. The independent functions of the two heads of the lateral pterygoid muscle. Am J Anat. 1973;138:197.
Moiler E. The chewing apparatus. Acta Physiol Scand. 1966;69((suppl)280):1.
Owall B, Moller E. Tactile sensibility during chewing and biting. Odontol Revy. 1974;25:327.
Smith RJ. Mandibular biomechanics and temporomandibular joint function in primates. Am J Phys Anthropol. 1978;49:341.
Stohler CS, Ash MM. Mandibular displacement in complete chewing sequence. J Dent Res. 1982;61:273. (abstract)
Storey AT. Joint and tooth articulation in disorders of jaw movement. In: Kawamura Y, Dubner R, editors. Oral-facial sensory and motor function. Tokyo: Quintessence, 1981.
Takahashi T, et al. The role of oral kinesthesia in the determination of the swallowing threshold. J Dent Res. 1983;62:327.
Toller PA. The synovial apparatus and temporomandibular joint function. Br Dent J. 1961;111:355.
Vitti M, Basmajian JV. Integrated actions of masticatory muscles: simultaneous EMG from eight intramuscular electrodes. Anat Rec. 1977;187:173.
Wompler HW, et al. Scanning electron microscopic and radiographic correlation of articular surfaces and supporting bone in the mandibular condyle. J Dent Res. 1980;59:754.
Wyke BD. Neuromuscular mechanisms influencing mandibular posture: a neurologist’s view of current concepts. J Dent. 1974;2:111.
Yurkstas AA. The masticatory act. J Prosthet Dent. 1965;15:248.
 site for additional study resources.
site for additional study resources.