Preventive Materials
Ever since fluoride was documented as a chemotherapeutic measure providing resistance in tooth enamel to in vivo demineralization and the development of active carious lesions, prevention has been the foundation for clinical restorative dentistry. The first major advance came with the administration of low-level fluorides into urban water supplies to ensure systemic ingestion during early life, when tooth structure is forming. Fluoride can also be provided systemically as a dietary supplement to inhibit caries where drinking water is not intentionally fluoridated. For patients who are at high risk for the development of caries in spite of systemic fluoride administration, various means of topical application have been developed to increase caries protection, such as toothpastes, mouth rinses, gels, and varnishes.
For effective application of the fluoride ion and uptake by the enamel surface, a vehicle material must be used to carry the active ingredient in the right concentration and place it in apposition to the tooth surface. It must then be held there for a sufficient, yet clinically practical period, to allow a high absorption rate. The vehicle must be nontoxic and easily removed from the oral cavity after the therapy is completed.
With the combination of systemic and topical fluoride applications, the prevalence of smooth surface caries has greatly diminished over the past 50 years. Pits and fissures on the occlusal surfaces of posterior teeth, however, are more resistant to fluoride uptake because the morphology of the surface structure is irregular and there is opportunity for food retention and caries initiation. These surfaces can be dealt with by applying an adhesive resin coating to fill in the irregularities and create a nonretentive smooth surface that is less likely to decay.
CHEMOTHERAPEUTIC AGENTS
The major function of toothpaste (Fig. 8-1) is to enhance cleaning of the exposed tooth surfaces and removal of pellicle, plaque, and debris left from salivary deposits and the mastication of food. As a secondary function, toothpaste can be used as a carrier for fluorides, detergents, abrasives, and whitening agents to improve the quality and esthetics of erupted teeth. The use of toothpaste is a continually growing, vital part of home health care throughout the world, resulting in a multibillion dollar industry. A practicing dentist should have a sound knowledge of the ingredients in most toothpastes and the therapeutic value, if any, in recommending them to patients for general and specific needs. Dentifrices are listed in the Guide to Dental Therapeutics published annually by the American Dental Association (ADA). Products can receive the ADA Seal of Acceptance by meeting the appropriate guidelines, as set by the Council on Scientific Affairs under the category for “fluoride-containing dentifrices.” It would appear that the ADA classifies dentifrices as both a material and a therapeutic agent.

FIGURE 8-1 A selection of toothpastes with a variety of active ingredients, including whitening agents, tartar control, total protection, and baking soda.
The general composition of most toothpastes includes the following:
• Colloidal binding agent. This agent acts as a carrier for the more active ingredients. Sodium alginate or methylcellulose will thicken the vehicle and prevent separation of the components in the tube during storage.
• Humectants. An example is glycerin, which is used to stabilize the composition and reduce water loss by evaporation.
• Preservatives. Preservatives are used to inhibit bacterial growth within the material.
• Flavoring agents. Peppermint, wintergreen, and cinnamon are added to enhance consumer appeal and to combat oral malodors.
• Abrasives. Abrasives are incorporated into all pastes to aid in the removal of heavy plaque, adhered stains, and calculus deposits. Calcium pyrophosphate, dicalcium phosphate, calcium carbonate, hydrated silica, and sodium bicarbonate are used in varying amounts to obtain this effect (Fig. 8-2).

FIGURE 8-2 Variety of toothpaste labels highlighting the abrasive material in each paste: hydrated silica (top), dicalcium phosphate (center), sodium bicarbonate (bottom).
• Detergents. An example is sodium lauryl sulfate, which is used to reduce surface tension and enhance the removal of debris from the tooth surface.
• Therapeutic agents. Therapeutic agents are added to most toothpastes marketed in North America and Europe. The use of stannous fluorides has been demonstrated to be effective in the uptake of the fluoride ion and improved resistance of fluorapatite to acid demineralization in the initiation of carious lesions.
• Other chemicals. Minor miscellaneous ingredients are included to reduce tube corrosion, stabilize viscosity, and provide pleasing coloration. Minor amounts of peroxides are included in some pastes, with marketing claims that they will remove innate discolorations and improve esthetics.
Abrasivity is one of the most important characteristics of toothpaste. Abrasion is a very important functional property that can have widespread destructive effects in the oral environment. Toothbrushing introduces three-body abrasion in areas of the mouth that are not normally subject to that type of stress. The toothbrush bristle is one factor, as it is moved across the softer dentin surface of the root in teeth with gingival recession, and the paste becomes the third body, with its abrasive particles interposed.
Abrasivity of toothpaste has been measured with two different methods. One method is to use a radioactive surface as the substrate and to measure the loss of substance after brushing by measuring radioactivity in the abraded material. The second method uses a profilometer to measure the substrate before and after a brushing experiment; the loss of contour is measured and compared. Studies have generally confirmed that the radiotracer methods are more reliable. The ADA, British Standards Institute, and ISO have standardized tests for abrasivity that differ slightly in methodology. The factors that have been associated with increased abrasion are larger particle sizes, more irregular particle shapes, harder mineral composition of the particles, amount of particles in a given volume of the paste, and toothbrush bristle stiffness. Fig. 8-3 illustrates the various abrasive particle sizes and shapes incorporated into common commercial toothpastes. Patients have used commercial powders, such as baking soda (sodium bicarbonate), directly to remove heavy stains, but this technique is contraindicated on a routine basis owing to the heavy abrasive action on exposed root surfaces.
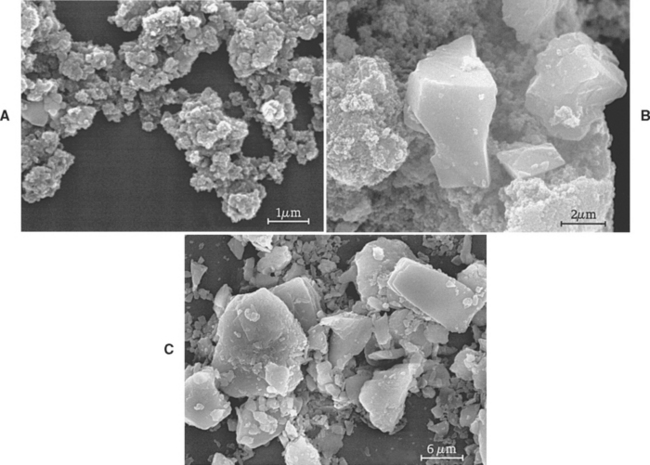
FIGURE 8-3 Scanning electron micrographs of abrasive particles taken from commercial toothpastes. A, hydrated silica; B, calcium carbonate; C, dicalcium phosphate.
Chemical agents have been placed in various toothpastes to control tartar formation, reduce caries risk, and whiten tooth surfaces. A new group of pastes have been marketed in recent years to control calculus deposition (Fig. 8-4). These pastes incorporate tetrasodium or tetrapotassium pyrophosphates, which act as inhibitors to hydroxyapatite crystal growth. Their efficacy in reducing calculus formation and the control of periodontal problems is well documented, but they do have some side effects that prohibit their use in certain individuals. They create a slightly more alkaline environment as part of the active prevention mechanism, and this can lead to soft-tissue sensitivity reactions such as burning sensations, tissue sloughing, erythema, ulceration, or migratory glossitis. Because the pyrophosphates have a bitter taste, such pastes also have an increased concentration of flavoring agents and detergents, both of which can increase the possibility of an adverse tissue response.

FIGURE 8-4 Typical antitartar toothpaste with contents noted on the label, including several pyrophosphates.
Fluoride release from over-the-counter dentifrices is one of the most effective public health measures in reducing tooth decay among both children and adults. The integrity of the tooth surface that is exposed to the oral environment depends on a balance in the dynamic interaction between demineralization and remineralization, which is an ongoing continuous process. As long as the reaction is kept in balance, the tooth surface will maintain its natural physical characteristics (composition, crystal structure, microhardness, contour). However, numerous biochemical changes can occur in the oral environment that will upset this balance, and these changes can often lead to a shift toward demineralization, with eventual loss of tooth structure. Dental plaque is normally pH neutral and contains a significant amount of calcium and phosphate ions as a supersaturated reservoir to support the remineralization process. When salivary or plaque pH is reduced below 4.5, the normal mobility of calcium and phosphate ions is restricted and they are not available to contribute to the remineralization process. This low pH occurs frequently in response to the aciduric byproducts of bacterial metabolism, especially when it is associated with plaque accumulation that contains sugars and carbohydrates after dietary ingestion. If there is a continuous loss of calcium and phosphate beyond that which can be replaced with concurrent remineralization, cavitation occurs and restorative intervention is usually indicated.
The concentrations of fluoride in over-the-counter dentifrices range from 0.025% to 0.15%, with great variations among brands. The effectiveness of fluoride-containing toothpastes in preventing caries is related directly to two variables—the concentration of fluoride available at the tooth surface to participate in the remineralization reaction and the exposure time of the fluoride ions to the tooth surface. When remineralization takes place in the presence of an increase in fluoride ions from either sodium fluoride or monofluorophosphate, the mineral structure is deposited as a fluorhydroxyapatite, which is more resistant to solubility, ion release, and demineralization. This low-level exposure of the remineralizing tooth surface to fluoride ions from dentifrices has a significant effect on both the kinetics of remineralization and the quality of the remineralized surface. Calcium fluoride that is often formed as an intermediary compound on the tooth surface in contact with a dentifrice can act as a fluoride reservoir that continues the slow release process well beyond the brushing experience. Prescription pastes and gels are also available with concentrations around 0.5% to 1.0% for application in professionally managed programs when patients are at higher risk for caries (Fig. 8-5). One precaution needs to be noted when using high fluoride-containing toothpastes with children; enamel fluorosis can develop in response to excessive ingestion.
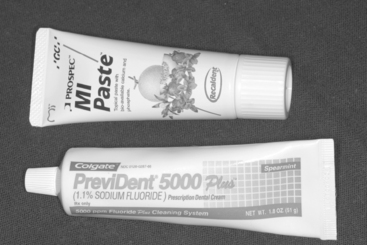
FIGURE 8-5 A high-fluoride content toothpaste (1.1%) currently under prescription drug regulation and a topical prophylaxis paste with bioavailable calcium and phosphate.
As an alternative approach to enhancing the remineralization process in normal tooth structure, efforts have been made to supply supplemental bioavailable calcium and phosphate ions as an enriched resource for mineral replacement. The additional calcium ions further supersaturate the plaque and biofilm on the tooth surface, thus promoting remineralization. Such products include over-the-counter partitioned toothpastes and professionally supplied prophylactic pastes. One of the newest products is based upon Recaldent (casein phosphopeptide–amorphous calcium phosphate), which is applied as a dental prophylaxis.
Bleaching toothpastes based on low-level peroxide formulations are also available to whiten teeth for esthetic purposes. They are somewhat effective in whitening teeth when used daily.
MOUTHWASHES
Another vehicle for delivering active agents with desirable effects to the surfaces of the teeth and gingivae is mouthwash. Mouthwash is a liquid solution that is applied as a rinse on a regular basis to enhance oral health, esthetics, and breath freshness (Fig. 8-6). Mouthwashes are most effective when applied in the morning or the evening following mechanical cleansing of the tooth surfaces with a brush and toothpaste. The usual purpose is to deliver an active ingredient to the clean surfaces of teeth or tissues in a manner that will produce the greatest treatment effect.
Mouthwashes are composed of three main ingredients. An active agent is selected for a specific health care benefit, such as anticaries activity, antimicrobial effect, fluoride delivery, or reduction of plaque adhesion. The active agent is then delivered in a solution of water or alcohol. Alcohol is used to dissolve some active ingredients, enhance flavor, and act as a preservative to prolong shelf life. Surfactants are also added to most mouthwashes to help remove debris from the teeth and dissolve other ingredients. Surfactants can be nonionic block copolymers, anionic chemicals such as sodium lauryl sulfate, or cetyl pyridinium chloride, which is cationic with antibacterial properties. Flavoring agents added for breath freshening include eucalyptol, menthol, thymol, and methyl salicylate. In formulating a mouthwash, it is extremely critical to guard against the use of additives that diminish the main effect of the active ingredient.
Two factors that should be considered in evaluating a mouthwash are its acidity and the ethanol content of the final solution. Among 12 commercial mouthwashes in the United Kingdom, most were acidic, ranging from a pH of 3.4 to 6.6, one was almost neutral (pH 6.9), and one was basic (pH 8.3). For the same mouthwashes, the ethanol content ranged from a high of 27% to 0%, with little correlation between acidity and ethanol content. Among a similar group of rinses on the U.S. market, the ethanol content showed a similar range from 27% to 0% (Fig. 8-7). To compare with alcoholic beverages, beer contains about 4% and wine about 11% ethanol. Although mouthwashes are not ingested as alcoholic beverages, there are topical effects to be avoided by using solutions with such high ethanol content on an excessive basis.

FIGURE 8-7 Labels for the mouthwashes shown in Fig. 8-6, highlighting the alcohol content of each material: Listerine (top), 21.6%; Scope (center), 15%; ACT (bottom), 0%.
The two main active ingredients in mouthwashes with a positive treatment effect are chlorhexidine and fluoride. Chlorhexidine is a strong antibacterial agent that is used primarily in patients with soft-tissue or gum infections, such as gingivitis or pericoronitis (Fig. 8-8). Acceptable concentrations are between 0.1% and 0.2%. Chlorhexidine gluconate has been shown to reduce the aerosol associated with dental operations when it is used as a preoperative rinse. It is also effective in reducing soft-tissue inflammation associated with periodontal disease, but patient acceptance is compromised by a rather bitter taste and a tendency to stain tooth surfaces.
The anticaries effect of fluoride mouthwashes is also well documented. It would appear to result from a two-stage reaction. Initially, a layer of calcium fluoride–like material is deposited on the surfaces of exposed teeth. In time, this surface layer is absorbed and the underlying mineral structure is converted from hydroxyapatite to fluorapatite, which is harder and more resistant to demineralization. The fluoride uptake was found to be dependent on concentration, with 0.2% NaF having a greater uptake than 0.05%. Uptake was also time dependent, with longer exposure times producing a greater treatment effect.
Mouthwashes can also have an effect upon restorative materials. Those with higher ethanol content can produce softening of the surfaces of resin materials, such as resin composites, compomers, and sealants. Although more significant in light-cured resin materials, the softening effect, as demonstrated by an increased water sorption rate, was also found in laboratory-processed composites. A residual staining effect was also found using one popular rinsing material that also contained eugenol. The staining effect of chlorhexidine is dependent on concentration, so it is imperative to find an effective midrange concentration that will produce only minimal staining.
There is also a question about toxicity or biocompatibility in prescribing the routine use of mouthwashes, particularly those with high ethanol content. Carcinogenic risks seem to go up with increased duration of exposure and frequency of use. The risk factor is similar to that resulting from increased ingestion of alcoholic beverages. Although there are mixed results among the various clinical studies, the association seems to be present only when the ethanol content of the rinse is high and the use is excessive.
FLUORIDE VARNISHES
Fluoride-containing varnishes provide an additional means of delivering fluoride topically to the surfaces of teeth in patients at risk for caries. Research has established their routine use in Europe for this purpose; however, the FDA has refused to approve them as anticariogenic agents. They are approved as cavity varnishes to be used under restorations and along the root surfaces of sensitive teeth with gingival recession. There are three products used routinely for topical application, usually after a prophylaxis. Two of these products contain 5% sodium fluoride (2.26% F− or 22,600 ppm) and one contains 1% difluorsilane (0.1% F− or 1000 ppm) (Fig. 8-9). The fluoride is dissolved in an organic solvent that evaporates when applied or sets when exposed to moisture, leaving a thin film of material covering all exposed tooth surfaces. The mechanism of action for a fluoride varnish is similar to that described for a fluoride mouthwash; calcium fluoride is deposited on the tooth surface and later converted through a remineralization reaction to fluorapatite.

FIGURE 8-9 Fluoride-containing cavity varnishes for professional application, supplied in a tube (Duraphat) or as a solution in unit doses (Fluor Protector).
One advantage of fluoride varnish is the extended time of exposure for the active fluoride ingredient against the tooth surface. Instead of seconds, as with a mouthwash, it may be hours before a varnish wears off. Clinical trials have documented the efficacy of varnish in treating young children at risk for caries, with reductions reported as high as 70%. Another potential use for this type of material is in the prevention of root caries in an older population, which has exposed root surfaces with increasing risk as aging occurs. Semiannual application of fluoride varnishes seems to provide optimum efficacy. The only negative aspect of using cavity varnishes is a slightly bitter taste, which is transient, and tooth discoloration, which lasts less than 24 hours. More research is necessary to fully document the value of using these materials in specific clinical situations with moderate to high caries risk.
PIT AND FISSURE SEALANTS
Pits and fissures in the occlusal surfaces of permanent teeth are particularly susceptible to decay, and fluoride treatments have been least effective in preventing caries in these areas. The susceptibility of occlusal pits and fissures to caries is related to the physical size and morphology of the individual pit or fissure, which can provide shelter for organisms and limit the effectiveness of oral hygiene procedures. Cross-sectional views of typical fissure morphology, varying from a wide V shape to a bottleneck shape, are shown in Fig. 8-10.
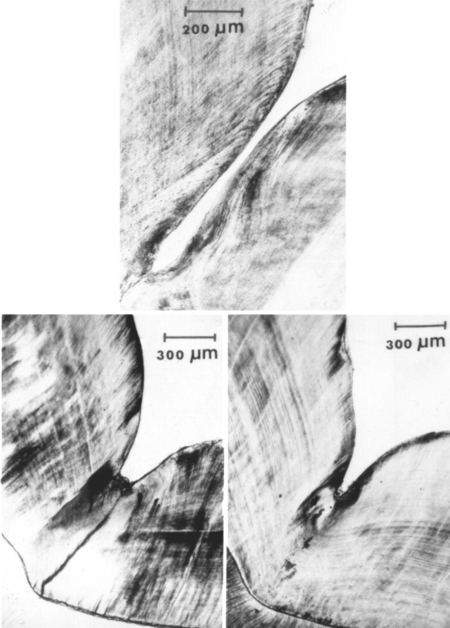
FIGURE 8-10 Sections of teeth illustrating shapes of fissures. (From Gwinnett AJ: J Am Soc Prevent Dent 3:21, 1973.)
A technique termed occlusal sealing was introduced in 1965. This procedure involved the use of methyl-2-cyanoacrylate, which was mixed with poly(methyl methacrylate) and inorganic powder and then placed in the pits and fissures. The cyanoacrylate polymerized on exposure to moisture. Since that time, sealant systems have included the Bis-GMA resins (polymerized either by chemical means or by visible light), a polyurethane sealant containing inorganic fluoride compounds, and glass ionomers. The use of sealant materials that exhibit a slow release of the fluoride ion has been advocated as a way to maintain a high surface concentration of fluoride for a longer period of time than is possible with the usual topical gel treatments.
RESIN SEALANTS
The most common sealants are based on Bis-GMA resin and are light cured, although some self-cured products are still available. The full name for Bis-GMA is 2,2-bis[4(2-hydroxy-3-methacryloyloxy-propyloxy)-phenyl] propane. The chemistry of Bis-GMA sealants is the same as that described for resin composites in Chapter 9. The principal difference is that the Bis-GMA sealants must be much more fluid to penetrate into the pits, fissures, and etched areas produced on the enamel, which provide for retention of the sealant. Three parts of the viscous Bis-GMA are mixed with one part of diluent, such as methyl methacrylate or triethylene glycol dimethacrylate, to obtain a reasonably low-viscosity sealant. An alternative but similar oligomer base is urethane dimethacrylate; some materials are formulated from a combination of the two base resins. To provide stiffness to the material and improve wear resistance, filler particles of fumed silica or silanated inorganic glasses can be added to form low-viscosity composites.
Light-Cured Sealants
Today, most sealants are light cured, activated by a diketone and an aliphatic amine. The complete reactions for resin composites are given in Chapter 9. Bis-GMA-light-cured sealant is supplied in a light-tight container and should be usable for a 12-month period. The sealant is applied to the pit and fissure area with an appropriate applicator and, when polymerization is desired, the end of the light source is held 1 to 2 mm from the surface and the sealant is exposed to light for 20 seconds. Sealants are applied in such thin sections that depth of cure should be adequate with minimal exposure times, even for opaque materials. The advantage in using a light-cured sealant is that the working time can be completely controlled by the operator and integrated with patient behavior. This control is particularly valuable when sealant is applied to very young patients or when cooperation is a problem.
Self-Cured Sealants
The first generation of chemically initiated Bis-GMA sealants was polymerized by an organic amine accelerator; commercial self-cured sealants are still available. The material is supplied as a two-component system: one component contains Bis-GMA resin and benzoyl peroxide initiator, and the other contains Bis-GMA resin with 5% organic amine accelerator. The two components are dispensed as viscous drops onto a suitable mixing surface (e.g., dappen dish, paper pad), and after adequate mixing, they are applied directly to the tooth surface. The polymerization is an addition reaction in response to the formation of radicals, although somewhat less cross-linked than those of the composite restorative materials. The reaction is exothermic, but the clinical effect is minimal because the material is placed in limited bulk. The rate of reaction for all materials is sensitive to temperature, and the material sets more quickly in the mouth (typically 3 to 5 minutes) than on the mixing surface. Because quantities are usually small, use caution to include all material in the mixing and use a gentle motion to minimize air incorporation. Air inclusions during mixing and insertion can be manifested clinically as surface voids, which can discolor and retain plaque. To ensure optimum penetration, apply the self-cured sealant quickly after mixing. Manipulation late in the setting reaction can disrupt the polymerization and induce bond failure.
Air Inhibition of Polymerization
During polymerization there is a surface layer of air inhibition that varies in depth with different commercial products. Sufficient material must be applied to completely coat all pits and fissures with a layer thick enough to ensure complete polymerization after removal of the tacky surface layer. This chemically active surface layer of unpolymerized resin is considered a source of potential biotoxicity. One of the components in the raw resin reaction is bisphenol A (BPA), which has recently been related to estrogenic activity because of the similarity of its chemical structure to estrogen. The measurement of BPA in saliva is time dependent and material specific. For some materials, it has been detected in small quantities immediately after sealant placement but not after 1 or 24 hours post placement. Most of the clinical research monitoring the presence and concentration of BPA and similar leachable monomers has been done following sealant placement or by analyzing the products. This is an important aspect of biotoxicity because these materials are used extensively on young adults and children at a most sensitive time in physical development.
To remove this uncured layer as soon as possible after curing, use an abrasive slurry of pumice, applied on a cotton pellet or with a prophylaxis cup in a rotary handpiece; this method is more effective than wiping or rinsing procedures. Premature contamination with moisture during insertion and the early application of biting forces can disrupt the setting and affect its strength and clinical durability.
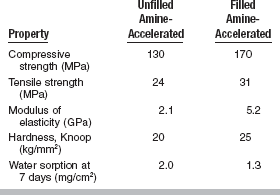
Properties of Sealants
Reports of the physical properties of sealants have been scarce because specimen preparation with such low-viscosity materials is difficult. The relationship between properties and clinical service is even more speculative than for most restorative materials. Typical properties obtained for an unfilled sealant and a filled sealant are given in Table 8-1. By adding up to 40% by weight of finely divided filler particles, as in the restorative composites, all properties except tensile strength show improvement. The specimens are usually tested for tensile failure by the diametral compression method, and the high deformation before fracture affects the reliability of the data. The modulus of elasticity shows the most dramatic improvement, and the increased rigidity makes the filled material less subject to deflection under occlusal stress. Filler is also added with the hope of improving wear resistance and making the material more visible on clinical inspection (Fig. 8-11).
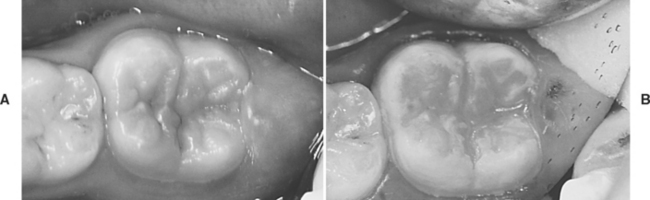
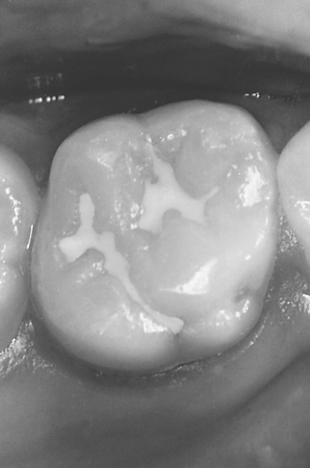
FIGURE 8-11 Typical molar with stained fissures and no diagnosable caries. A, Before sealing, and B, after sealing with a natural-colored sealant material.
Penetration studies on closed capillary tubes, which are somewhat analogous to pits and fissures, have indicated that a sealant will adapt more closely to the enamel surface if it possesses a high coefficient of penetration. Optimal penetration will occur when the sealant has a high surface tension, good wetting, and a low viscosity, thus permitting it to flow readily along the enamel surface. The surface wettability is demonstrated by the contact angle of a drop of liquid on the enamel surface. A drop that spreads readily has a low contact angle and is indicative of a highly wetted surface that is most conducive to a strong bond. Polymer tags that form in direct apposition to the surface irregularities created by acid etching are responsible for the mechanical bond that retains the sealant to enamel. Functional durability of the sealant bond can be related to stresses induced by initial polymer shrinkage, thermal cycling, deflection under occlusal forces, water sorption, and abrasion, with total failure manifested by the clinical loss of material.
Sealant materials have a variety of features that must be selected carefully by the health care provider. As previously stated, there are filled sealants that behave more like resin composites, and unfilled materials that are pure resins. Most current materials are light-cured rather than self-cured because of the ease and speed of application. Tooth-colored or clear resins are available that are very natural looking on the tooth surface, but they are also available in opaque or tinted materials to make the recall examination process easier (Fig. 8-12). A new class of color-reversible, photosensitive sealants has recently been developed. They are very similar to the light-cured sealants in resin composition and filler loading. In addition, photosensitive pigments are added that are normally colorless but change to a green or pink hue when exposed to the beam of a dental curing light. The color change lasts for about 5 to 10 minutes after exposure, while a diagnostic examination is being conducted. The change from tooth-colored to dye-colored then can be implemented as needed for visual sealant inspection at every recall visit.
An increasing number of sealants are marketed as vehicles for the slow release of fluoride, which has been documented in vitro in water solutions for a number of products. The release is highest in the first 24 hours after placement and then tapers to a low maintenance level, which may or may not be sufficient to provide extended clinical protection against caries.
Clinical Studies
Many clinical studies have been reported using the Bis-GMA systems. In earlier studies on effectiveness of treatment with sealant in newly erupting teeth, a light-cured sealant demonstrated a retention rate of 42% and an effectiveness of 35% in caries reduction after 5 years. In a similar study, a filled resin sealant showed a retention rate of 53% and a clinical effectiveness of 54% after 4 years. Results involving a quicker-setting unfilled resin sealant with very good penetration showed a retention rate of 80% and an effectiveness of 69% after 3 years. The longest published study on sealant effectiveness is a 15-year evaluation of a self-cured unfilled material, which showed 27.6% complete retention and 35.4% partial retention.
In pair-wise comparisons, the treated first molars had 31.3 decayed and filled primary tooth surfaces (dfs) and the untreated controls had 82.8 dfs. In a more current 4-year study comparing a fluoride-releasing sealant with one that did not have fluoride, retention rates were 91% for the fluoride material (77% complete and 14% partial) and 95% for the nonfluoride sealant (89% complete and 6% partial). Although the retention was somewhat lower in the fluoride-containing sealant, the caries incidence for both groups was identical (10%). In a study conducted in private practice, the 2-year retention rates for two newer fluoride-containing resins were greater than 90%, and no caries was detected on the test teeth. In a continuing study with re-treatment of all defective sealant surfaces at 6-month recalls, the teeth were maintained caries-free for a 5-year period. The re-treatment rate was highest (18%) at 6 months, and then diminished as time progressed, but at each recall period at least two teeth (about 4%) required reapplication.
Almost all studies show a direct correlation between sealant retention and caries protection. Therefore, it is important to develop materials that are retentive to enamel, resistant against occlusal wear, and easily applied with minimal opportunities for surface contamination. Current evidence indicates that sealants are most effective on occlusal surfaces where pits and fissures are well defined and retentive to food and in patients with a demonstrated risk for pit and fissure caries.
Manipulation of Sealants
The handling characteristics of a sealant are dependent on the composition of the material and the nature of the surface to which it is applied. Optimal preparation of both aspects will lead to close adaptation of the sealant to the tooth enamel, a strong seal against the ingress of oral fluids and debris, and long-term material retention.
Enamel Surface Preparation: The penetration of any of the sealants to the bottom of the pit is important. The wettability of the enamel by the sealant is improved by etching, and some advocate pretreatment with silanes in a volatile solvent. The problem of filling the fissure is real; air is often trapped in the bottom of the fissure, or the accumulation of debris at the base of the fissure prevents it from being completely filled, as shown in Fig. 8-13. Control of the viscosity of the sealant is important to obtain optimum results. The viscosity determines the penetration of resin into the etched areas of enamel to provide adequate retention of the sealant. Penetration of sealant, forming tags, to a depth of 25 to 50 μm is shown in Fig. 8-14.
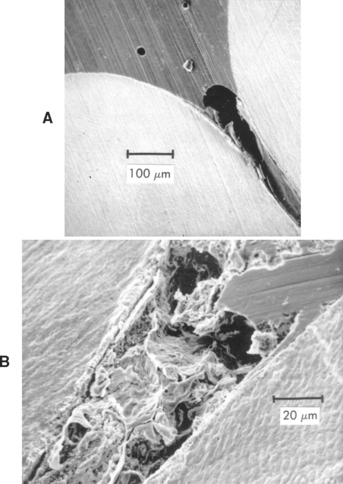
FIGURE 8-13 Section showing a fissure incompletely filled with sealant as a result of air (A) and debris (B). (From Gwinnett AJ: J Am Soc Prevent Dent 3:21, 1973.)
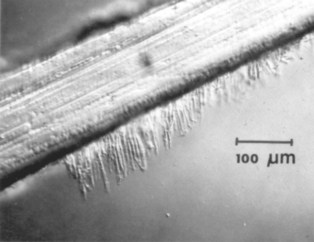
FIGURE 8-14 Penetration of sealant into etched enamel; these tags are responsible for the bonding to enamel. (From Gwinnett AJ: J Am Soc Prevent Dent 3:21, 1973.)
Etching the pit and fissure surface for a specified time (15 to 30 seconds is adequate for enamel with a normal mineral and fluoride content) with a solution or a gel of 35% to 40% phosphoric acid is recommended. Flush the acid thoroughly with water, and dry the area with warm air. Inadequate rinsing permits phosphate salts to remain on the surface as a contaminant, interfering with bond formation. Avoid rubbing the etched surface during etching and drying because the roughness developed can easily be destroyed. Isolation of the site is imperative throughout the procedure to achieve optimum tag formation and clinical success. If salivary contamination occurs during the treatment, rinse and dry the surface and repeat the acid etching. On clinical inspection, acid-etched enamel should appear white and dull with an obviously rough texture. If this appearance is not uniform, perform an additional 30 seconds of etching. The etched area should extend beyond the anticipated area for sealant application to secure optimum bonding along the margin and reduce the potential for early leakage. Clinical studies have shown that the use of a light-cured bonding agent (see Chapter 10) against the freshly etched enamel before placing the sealant will improve retention, especially when there appears to be minor moisture or salivary contamination.
A series of sixth- and seventh-generation bonding agents have been developed to simplify the technology and reduce technique sensitivity. In these systems, an acidic adhesive is placed directly onto the tooth in a single application. Because the acid component of the material is lower in concentration, the effectiveness of surface preparation is in question. In vitro bond strength studies generally indicate that the materials do not form as strong a bond to uncut enamel as to cut enamel walls and bevels. Therefore, the use of these materials for sealant placement should be guarded.
Sealant Application: Depending on its viscosity and setting time, the sealant may best be applied with a thin brush, a ball applicator, or a syringe. Take care to avoid the buildup of excess material that could interfere with developing occlusion, but apply sufficient material to completely cover all exposed pits and provide a smooth transition along the inclines of the enamel cusps. Overworking of even the light-cured sealants on the tooth surface during application can introduce air voids that appear later as surface defects.
The air-inhibited surface layer should be wiped away immediately after curing and the coating inspected carefully for voids or areas of incomplete coverage. Defects can be covered at this time by repeating the entire reapplication procedure, including the acid etch, and applying fresh sealant only to those areas with insufficient coverage. After the sealant is applied and fully cured, check and adjust the occlusion, if necessary, to eliminate functional prematurities that can result in hypersensitivity.
GLASS IONOMER SEALANTS
Because of their demonstrated ability to release fluoride and provide some caries protection on tooth surfaces at risk, glass ionomers have been suggested and tested for their ability to function as a fissure sealant. Glass ionomers are generally viscous, and it is difficult to gain penetration to the depth of a fissure. Their lack of penetration also makes it difficult to obtain mechanical retention to the enamel surface to the same degree as Bis-GMA resins. They are also more brittle and less resistant to occlusal wear. Clinical studies using various formulations of glass ionomers have shown significantly lower retention rates than resin sealants but greater fluoride deposition in the enamel surfaces. Thus there is a greater potential for latent caries protection after sealant loss.
In areas where high-risk children cannot afford dental care, a conservative holding technique has been advocated to seal remaining caries in a fluoride-rich environment and establish some degree of remineralization. Atraumatic restorative treatments (ART) involve opening a lesion, removing soft surface decay, and filling or sealing the surface with high-filled glass ionomer with a fast setting time. Future studies in this area may produce a new generation of sealant materials noted for their fluoride deposition rather than their mechanical obturation.
FLOWABLE COMPOSITE SEALANTS
Low-viscosity composites marketed as flowable composites are advocated for a wide variety of applications, such as preventive resin restorations, cavity liners, restoration repairs, and cervical restorations. These applications are not well supported with data, but their clinical use is widespread. The properties of flowable composites are described in Chapter 9.
Flowable composites are usually packaged in syringes or in compules (Fig. 8-15). These can be used for direct application to the cavity or the tooth surface. These composites can also be deposited from the syringe to a paper pad surface and then applied to the tooth using a ball-tipped applicator or a microbrush. In dispensing the material, care must be exercised to avoid introducing air bubbles, which eventually become surface voids. Depth of cure after light activation should be slightly better than that of highly filled composites.
Flowable composites provide an advantage when used as the sealant portion of a preventive resin restoration. A bonding agent should be applied to the etched enamel and light-cured to provide the basic adhesion for retention. The flowable composite can then be used to cover the restored area and the exposed pits and fissures on the occlusal surface of molars and premolars. The high flow aids in restoring minimal cavity preparations involving fissures that are cleaned or prepared with air abrasion techniques (Fig. 8-16). Because they have higher filler content than most resin sealants, flowable composites should have better wear resistance in this clinical situation. A current clinical study has verified good retention and caries resistance after 24 months. Long-term clinical efficacy of preventive resin restorations using flowable composite is yet to be established.
GLASS IONOMERS
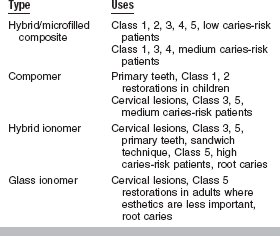
The final materials that need to be considered for caries prevention are glass ionomers and hybrid ionomers (resin-modified glass ionomers). Because of their documented slow release of fluoride, glass ionomers are used in cervical restorations in adults when esthetics is not critical. They are specifically recommended for patients with high caries risk (Table 8-2).
COMPOSITION AND REACTION
Glass ionomers are supplied as powders of various shades and a liquid. The powder is an ion-leachable aluminosilicate glass, and the liquid is a water solution of polymers and copolymers of acrylic acid. The material sets as a result of the metallic salt bridges between the Al+++ and Ca++ ions leached from the glass and the acid groups on the polymers. The reaction goes to completion slowly, with the formation of a cross-linked gel matrix in the initial set and an aluminum ion exchange strengthening the cross-linking in the final set. A chelation effect takes place with the calcium on the exposed tooth surface, creating an adhesive bond. The surfaces of new restorations should be protected from saliva during the initial set with a heavy varnish or light-cured bonding agent.
PROPERTIES
The handling characteristics and physical properties of glass ionomers can be varied to suit various clinical applications by altering the glass composition and or polyacid formulation. The properties of glass ionomers are compared qualitatively with other restorative materials in Table 8-3. Properties especially noteworthy are a modulus that is similar to dentin, a bond strength to dentin of 2 to 3 MPa, an expansion coefficient comparable to tooth structure, low solubility, and fairly high opacity. The flux used in fusion of the glass contains fluoride that is released slowly to provide an anticariogenic effect on adjacent dental plaque and tooth structure.
TABLE 8-3
Ranking of Selected Properties of Hybrid Ionomers and Glass Ionomers
| Property | Hybrid Ionomer | Glass Ionomer |
| Compressive strength | Med | Low-Med |
| Flexural strength | Med | Low-Med |
| Flexural modulus | Med | Med-High |
| Wear resistance | Med | Low |
| Fluoride release | Med-High | High |
| Fluoride rechargability | Med-High | High |
| Esthetics | Good | Acceptable |
Although the bond strength of glass ionomers to dentin is lower than that of resin composites, clinical studies have shown that the retention of glass ionomers in areas of cervical erosion are considerably better than for composites. When the dentin is conditioned (etched) using a dilute solution (15% to 25%) of polyacrylic acid, the glass ionomer may be applied without a cavity preparation. Four-year clinical data showed a retention rate for glass ionomer cervical restorations of 75%. The surfaces of the restorations seen in the studies were noticeably rough, and some shade mismatches were present. Pulp reaction to glass ionomers is mild; if the thickness of dentin is less than 1 mm, use of a calcium hydroxide liner is recommended. Although the surface remains slightly rough, cervical restorations did not contribute to inflammation of gingival tissues. Fewer Streptococcus mutans organisms exist in plaque adjacent to glass ionomer restorations.
MANIPULATION
Glass ionomers are packaged in bottles and in vacuum capsules for mechanical mixing in a mechanical mixer. In bulk dispensing, the powder and liquid are dispensed in proper amounts on the paper pad, and half the powder is incorporated to produce a homogeneous milky consistency. The remainder of the powder is added, and a total mixing time of 30 to 40 seconds is used with a typical initial setting time of 4 minutes. After placing the restorative and developing the correct contour, protect the surface from saliva by applying varnish or bonding agent. Trimming and finishing are done, if possible, after 24 hours.
The liquid in the unit-dose capsule is forced into the powder by a press and is mixed by a mechanical mixer. The mixture is injected directly into the cavity preparation with a special syringe. Working time is short and critical, so it is imperative to place the material with a minimum of manipulation. If the gel stage of the reaction is disrupted during the early phase of the reaction, the physical properties will be very low and adhesion can be lost.
Adhere rigidly to clinical techniques for glass ionomers by maintaining isolation, using adequate etching procedures, protecting the restoration from saliva after placement, and delaying final finishing for 1 day or longer if possible.
HYBRID IONOMERS
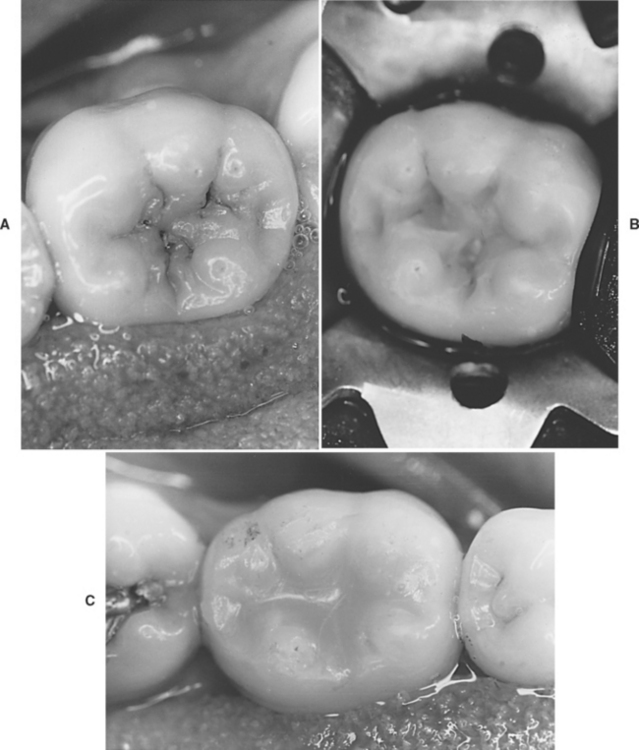
Hybrid ionomers or resin-modified glass ionomers are used for restorations in low stress-bearing areas and are recommended for patients with high caries risk (see Table 8-3). These restorations are more esthetic than glass ionomers because of their resin content. Examples of cervically eroded teeth and hybrid ionomer restorations are shown in Fig. 8-17.
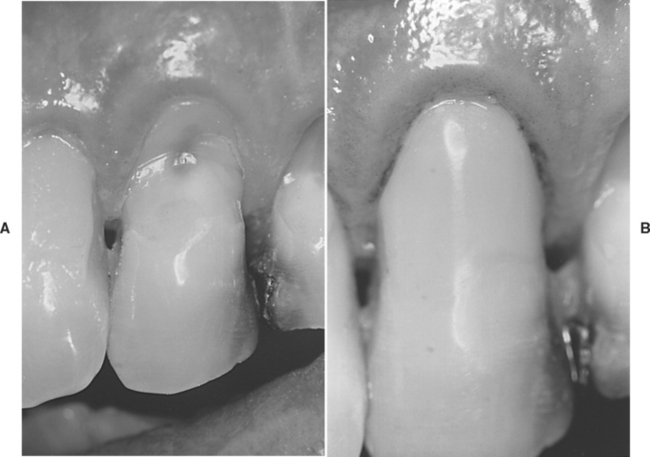
FIGURE 8-17 Restoration of a root surface lesion. A, Abrasion/erosion lesion on the facial surface of a maxillary cuspid. B, Restoration with a resin-modified glass ionomer cement.
COMPOSITION AND REACTION
The powder of hybrid ionomers is similar to that of glass ionomers. The liquid contains monomers, polyacids, and water. Hybrid ionomers set by a combined acid-base ionomer reaction and light-cured resin polymerization of 2-hydroxyethyl methacrylate. Placing a bonding agent before inserting a hybrid ionomer is contraindicated, because it decreases fluoride uptake.
PROPERTIES
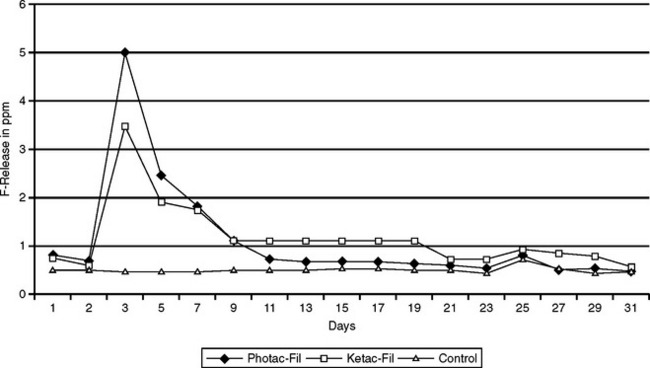
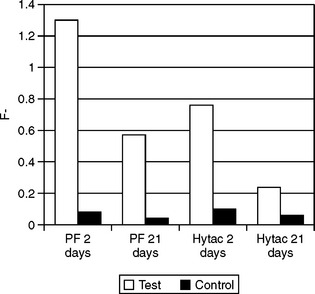
Hybrid ionomers bond to tooth structure without the use of a bonding agent. Typically, the tooth is conditioned (etched) with polyacrylic acid or a primer before placing the hybrid ionomer. The transverse strength of a hybrid ionomer is almost double that of a standard glass ionomer. Hybrid ionomers release more fluoride than compomers and composites but almost the same as glass ionomers. Fig. 8-18 illustrates the release of fluoride ions from a standard glass ionomer and resin-modified glass ionomer over a 30-day period. There is an early period of high release, which tapers after about 10 days to 1 ppm. Glass ionomers and hybrid ionomers recharge when exposed to fluoride treatments or fluoride dentifrices. Fig. 8-19 illustrates this recharge capability with a similar time-dependent release curve. In evaluating the effectiveness of this release, fluoride has been measured in plaque samples immediately adjacent to glass ionomer-based restorations (Fig. 8-20). For these two materials from the same manufacturer, plaque adjacent to the resin-modified glass ionomer had a significantly higher fluoride content than plaque adjacent to compomer restorations at 2 days and 21 days after insertion of the restorations.
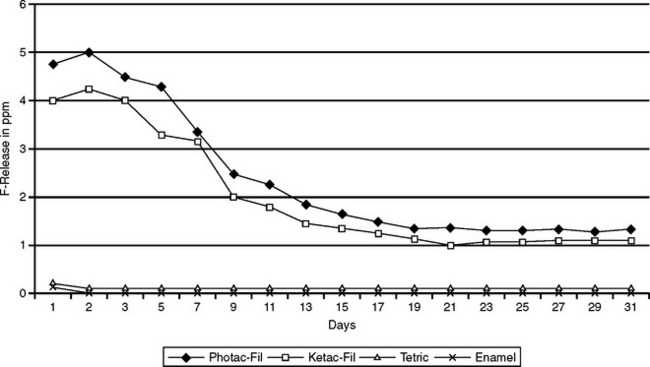
FIGURE 8-18 Fluoride release from glass ionomer cements and composite resin in distilled water over 30 days. (Adapted from Strothers JM, Kohn DH, Dennison JB et al: Dent Mater 14:129, 1998.)
MANIPULATION
An example of a hybrid ionomer packaged in capsules is shown in Fig. 8-21. For hybrid ionomers packaged in bulk as powder-liquids, manipulation is like that of standard glass ionomers. Mechanical mixing of the unit-dose capsules provides a uniform mix that has much fewer of the larger air voids than can be introduced during hand spatulation. Optimum powder/liquid ratio is critical to the long-term maintenance of physical properties and the clinical success of restorations. Unlike glass ionomer restorations, hybrid ionomers set immediately when light-cured and can be finished 5 to 10 minutes after initial set. Color can be maintained and surface texture improved by finishing the hybrid ionomer restorations in a wet environment (water spray or water-soluble lubricant) and then recoating with a protective varnish or bonding agent. Glass ionomers are an increasingly important part of operative dentistry for both an aging population with high incidence of root caries and children who have minimal dental care but high caries risk factors.
ATHLETIC MOUTH PROTECTORS
The use of athletic mouth protectors in contact sports has increased rapidly; they are routinely used in football, soccer, ice hockey, basketball, wrestling, field hockey, softball, and other sports. This increased use is justified by studies that showed that 38% of participants in sports sustained orofacial injuries and only 15% of those injured were wearing a mouth protector at the time of injury. A survey found that 62% of injuries occurred in unorganized sports. The incidence of cerebral concussions among National Collegiate Athletic Association (NCAA) Division I-A football players was observed in a clinical study to be 0.73 per 1000 exposures.
Injuries to teeth from trauma caused by athletic activity have involved pulpitis, pulpal necrosis, resorption, replacement resorption, internal hemorrhage, pulp canal obliteration, and inflammatory resorption.
As a result of the possibilities of orofacial injury, high school athletes are required to wear internal mouth protectors, and the NCAA has adopted a mouth-protector rule. As a result of these actions, more professional athletes are wearing mouth protectors. Because of the increased use of mouth protectors, it is estimated that 50,000 orofacial injuries are prevented each year.
The rationale for the use of mouth protectors is that the mouth protector acts like a shock absorber when the athlete receives a blow to the mouth or chin. The mouth protector absorbs 80% to 90% of the energy of the blow and distributes the remaining energy uniformly to the entire arch, resulting in less trauma to the oral structures.
Stock, mouth-formed (boil-and-bite), and custom mouth protectors are the three types available and all provide some protection to the athlete. A few examples of stock and mouth-formed protectors are shown in Fig. 8-22. Custom-made mouth protectors are usually vacuum-formed from sheets of flexible, thermoplastic polymers about 14 cm2 in area and 1.6 to 3 mm in thickness; they may be clear or colored. Examples of these sheets and fabricated mouth protectors are shown in Fig. 8-23. In most instances the sheets are of a single material, but they may be laminates of two or more thermoplastic polymers. Laminated mouth protectors are fabricated so that the softer of the two layers contacts the teeth and soft tissue.

FIGURE 8-22 Examples of stock and mouth-formed mouth protectors. Note the protector on the top is a mouth and lip protector and those on the left and right center have had the straps cut off to allow testing of the percentage of impact absorbed.
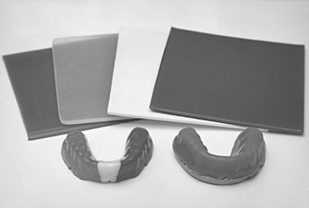
FIGURE 8-23 Thermoplastic sheets used for fabricating mouth protectors (top) and the resultant fabricated mouth protectors. On the right is a dental stone model; the protector on the left was fabricated from a tricolored sheet.
Most sheets for custom mouth protectors are vinyl acetate-ethylene copolymers (PVAc-PE). Manufacturers use several different hardnesses of the material, with copolymers containing more polyethylene being harder.
The advantages of custom-made mouth protectors are (1) excellent fit, (2) comfort, (3) ease of speaking, and (4) durability; these qualities are poor for stock protectors and poor to good for mouth-formed protectors. In spite of the advantages of custom-made protectors, they are not as common as stock or mouth-formed protectors because of their higher cost.
PROPERTIES
ANSI/ADA Specification No. 99 for Athletic Mouth Protectors and Materials defines requirements for hardness, tear strength, impact adsorption and rebound, and water sorption as listed in Box 8-1. The specification defines types and classes of mouth protectors as follows:
Type I. Thermoplastic type: Class 1, vacuum-formed; Class 2, mouth-formed
Selected properties of thermoplastic sheets and fabricated custom-made, mouth-formed, and stock protectors are listed in Table 8-4. The Shore “A” hardness of the materials for all types of mouth protectors ranges from 70 to 80, and all have low values of water sorption and solubility. The tear strength of the laminate is somewhat lower than single material sheets; it is not possible to test the values of the fabricated mouth protectors. With an impact of 113 N/cm, the percentage absorbed by thermoplastic sheets and custom mouth protectors placed on high-strength stone models in the area of the central incisors is essentially the same, with values of 80% to 90%. A wider range of impact absorption, 76% to 93%, is found for mouth-formed and stock protectors.
TABLE 8-4
Properties of PVAc-PE Sheets and Custom-Made, Mouth-Formed, and Stock Mouth Protectors at Mouth Temperature
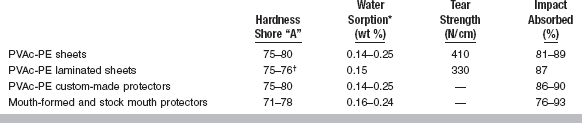
*Water solubility was an average of 0.003 wt %.
†Tongue side was softer (75).
These in vitro results confirm clinical studies that have demonstrated no difference in the incidence of oral injuries of athletes wearing custom-made, mouth-formed, or stock protectors. Thus, the main advantages of the custom-made mouth protectors are those previously mentioned—fit, comfort, and ease of speaking—which should increase the probability that the protector will be worn.
FABRICATION OF CUSTOM-MADE PROTECTORS
The fabrication of a custom-made mouth protector involves the following general steps: (1) making an alginate impression of the maxillary arch; (2) pouring a dental stone or high-strength stone model into the impression, minus the palate; (3) vacuum- or pressure-forming a heated sheet of PVAc-PE over the model; (4) trimming the excess PVAc-PE around the model; and (5) smoothing the edges of the mouth protector.
A commercial dental vacuum machine used to fabricate mouth protectors is sketched in Fig. 8-24. The procedure involves clamping the PVAc-PE sheet in the frame and raising the frame to the top position and centering the model on the perforated metal platform. Turn the heater on and heat the sheet until it sags about 3 cm at the center, at which point turn the vacuum on and push the frame down to its lower position using the plastic handles. Swing the heater out of the way and turn it off, but leave the vacuum on for 30 seconds. Allow the thermoplastic sheet to cool to room temperature before removing it from the model. A thermoplastic sheet vacuum-formed over a model is shown in Fig. 8-25. Trim the excess of the thermoplastic sheet 3 mm short of the labial fold using a curved pair of scissors, making sure to provide clearance for the buccal and labial frenum. Smooth the edges with a wheel (such as a Moore’s Satin Buff Wheel) or with the flame from an alcohol torch, followed by adaptation with wet fingers.
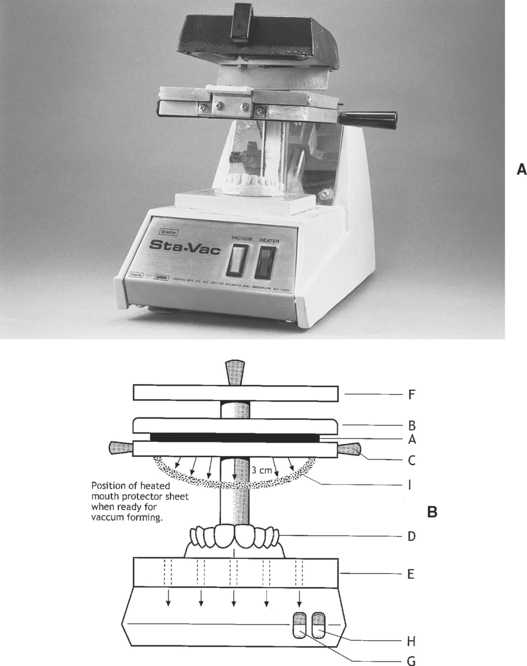
FIGURE 8-24 A, Typical vacuum-forming machine. B, Illustration showing the sheet of mouth protector material, A, which is held between the upper clamp, B, and lower clamp, C. The model, D, is centered on the perforated support plate, E, and the heater, F, is turned on by switch H. Heating continues until the sheet sags about 3 cm (shown as I), and then the vacuum switch, G, is turned on. The heated sheet is quickly lowered over the model using the attached plastic handles attached to the lower clamp, C. The sheet is vacuum-sealed to the support plate via the perforations and is then vacuum-formed over the model. The heater is turned off and swung away 90 degrees using the attached handles; the vacuum is turned off after 30 to 60 seconds. The vacuum-formed mouth protector remains on the model until cool and then trimming and finishing can begin.

FIGURE 8-25 A mouth protector sheet vacuum-formed over a model is shown on the left, and a trimmed and finished protector is shown on a model at the right.
An alternative to the vacuum machine is the pressure-lamination machine (for example, Dreve Druformat, Westone Laboratories, Colorado Springs, CO). This machine allows layers of several thermoplastic materials with different properties to be formed together. In addition, the thickness of the layers can be varied in different parts of the arch to provide extra protection for certain sports. The benefits of laminated protectors have not been verified by controlled clinical studies.
Several considerations should be taken into account related to making impressions and preparing models used to fabricate mouth protectors. Remove removable appliances before making the alginate impression. If the athlete is wearing a fixed orthodontic appliance, block it out on the model with dental stone before fabricating the mouth protector. Also, if permanent teeth are still erupting, block out that space on the model.
In the absence of commercial vacuum or pressure machines, soften the thermoplastic sheet in hot water and adapt to the model using a wet sponge and wet fingers. Significantly better adaptation results from vacuum- or pressure-forming the protector.
If it is found necessary to equalize the occlusion when the mouth protector is worn, make the following adjustment. Gently heat the contacting surfaces of the mouth protector using an alcohol torch, just enough to barely soften the material. Dip the mouth protector in warm water and place it in the athlete’s mouth. Ask the athlete to close until all the opposing teeth contact the mouth protector.
PREPARING A MOUTH-FORMED MOUTH PROTECTOR
Manufacturers supply instructions for boil-and-bite mouth protectors; in general, the procedure is as follows. Place the protector in a pan of boiling water, just removed from the heat, for 10 to 35 seconds, based on the manufacturer’s directions. Remove the protector from the hot water, immerse it in a pan of cold water for 1 second and then place it in the mouth, centering it around the maxillary teeth. Ask the athlete to bite down gently and suck out any air and water by pressing the tongue against the back of the maxillary teeth. Leave the protector in the mouth for 30 seconds before removal. If a good fit is not obtained, the procedure can be repeated. Also, if the mouth protector is too large, cut off the ends to the appropriate length before placing it in the hot water.
CARE OF ATHLETIC MOUTH PROTECTORS
Give the following recommendations to the athlete:
1. After each use, rinse the mouth protector under cold water.
2. Periodically clean the mouth protector using a solution of soap in cold water.
3. Don’t use abrasive dentifrices to clean the protector.
4. Don’t use alcohol solutions or denture cleaners to clean the protector.
5. Store the mouth protector stress-free in the container provided, or better yet, on the model on which it was fabricated. Also, retain the model to allow a replacement to be made in case the mouth protector is lost or damaged.
Allen, CE, Nunez, LJ. A look at toothpaste ingredients. Gen Dent. 1985;33:58.
De Boer, P, Duinkerke, ASH, Arends, J. Influence of tooth paste particle size and tooth brush stiffness on dentine abrasion in vitro. Caries Res. 1985;19:232.
DeLattre, VF. Factors contributing to adverse soft tissue reactions due to the use of tartar control toothpastes: report of a case and literature review. J Periodontol. 1999;70:803.
Dyer, D, Addy, M, Newcombe, RG. Studies in vitro of abrasion by different manual toothbrush heads and a standard toothpaste. J Clin Periodontol. 2000;27:99.
Hefferren, JJ, Kingman, A, Stookey, GK, et al. An international collaborative study of laboratory methods for assessing abrasivity to dentin. J Dent Res. 1984;63:1176.
Hicks, MJ, Flaitz, CM. Enamel caries formation and lesion progression with a fluoride dentifrice and a calcium-phosphate containing fluoride dentifrice: a polarized light microscope study. J Dent Child. 2000;67:21.
Ogaard, B. The cariostatic mechanism of fluoride. Compend Continuing Ed Dent. 1999;20(1Suppl):10.
Sainio, EL, Kanerva, L. Contact allergens in toothpastes and a review of their hypersensitivity. Contact Derm. 1995;33:100.
Silva, MF, Melo, EV, Stewart, B, et al. The enhanced anticaries efficacy of a sodium fluoride and dicalcium phosphate dihydrate dentifrice in a dual-chambered tube. A 2 year caries clinical study on children in Brazil. Am J Dent. 2001;14(Spec Iss):19A.
Williams, MI, Cummins, D. The technology behind Colgate Total Advanced Fresh. Compend Cont Ed Dent. 2003;24(Suppl 9):4.
Addy, M, Wade, W, Goodfield, S. Staining and antimicrobial properties in vitro of some chlorhexidine formulations. Clin Prevent Dent. 1991;12:13.
Bhatti, SA, Walsh, TF, Douglas, CWI. Ethanol and pH levels of proprietary mouthrinses. Community Dent Health. 1994;11:71.
Cruz, R, Rolla, G, Ogaard, B. Formation of fluoride on enamel in vitro after exposure to fluoridated mouthrinses. Acta Odontol Scand. 1991;49:329.
Chow, LC, Takagi, S, Frukhtbeyn, S, et al. Remineralization effect of a low-concentration fluoride rinse in an intraoral model. Caries Res. 2002;36:136.
Cole, P, Rodu, B, Mathisen, A. Alcohol-containing mouthwash and oropharyngeal cancer. J Am Dent Assoc. 2003;134:1079.
Forward, GC, James, AH, Barnett, P, et al. Gum health product formulations: what is in them and why? Periodontol. 2000;15:32. 1997
Gurgan, S, Onen, A, Koprulu, H. In vitro effects of alcohol-containing and alcohol-free mouthrinses on microhardness of some restorative materials. J Oral Rehabil. 1997;24:244.
Penugonda, B, Settembrini, L, Scherer, W, et al. Alcohol-containing mouthwashes: effect on composite hardness. J Clin Dent. 1994;5:60.
Settembrini, L, Penugonda, B, Scherer, W, et al. Alcohol-containing mouthwashes: effect on composite color. Oper Dent. 1995;20:14.
Weiner, R, Millstein, P, Hoang, E, et al. The effect of alcoholic and nonalcoholic mouthwashes on heat-treated composite resin. Oper Dent. 1997;22:249.
Winn, DM, Blot, WJ, McLaughlin, JK, et al. Mouthwash use and oral conditions in the risk of oral and pharyngeal cancer. Cancer Res. 1991;51:3044.
Banting, DW, Papas, A, Clark, DC, et al. The effectiveness of 10% chlorhexidine varnish treatment on dental caries incidence in adults with dry mouth. Gerodontology. 2000;17:67.
Beltran-Aguilar, ED, Goldstein, JW. Fluoride varnishes: a review of their clinical use, cariostatic mechanism, efficacy and safety. J Am Dent Assoc. 2000;131:589.
Petersson, LG, Twetman, S, Pakhomov, GN. The efficiency of semiannual silane fluoride varnish applications: a two-year clinical study in preschool children. J Public Health Dent. 1998;58:57.
Skold, L, Sundquist, B, Eriksson, B, et al. Four-year study of caries inhibition of intensive Duraphat application in 11–15-year-old children. Community Dent Oral Epidemiol. 1994;22:8.
Arenholt-Bindslev, D, Breinholt, V, Preiss, A, et al. Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clinical Oral Invest. 1999;3:120.
Boksman, L, Carson, B. Two-year retention and caries rates of UltraSeal XT and FluoroShield light-cured pit and fissure sealants. General Dent. 1998;46:184.
Buonocore, MG. Adhesive sealing of pits and fissures for caries prevention, with use of ultraviolet light. J Am Dent Assoc. 1970;80:324.
Charbeneau, GT, Dennison, JB. Clinical success and potential failure after single application of a pit and fissure sealant: a four-year report. J Am Dent Assoc. 1979;98:559.
Dennison, JB, Powers, JM. Physical properties of pit and fissure sealants (annot). J Dent Res. 1979;58:1430.
Dennison, JB, Straffon, LH. Clinical evaluation comparing sealant and amalgam after 7 years: final report. J Am Dent Assoc. 1988;117:751.
Feigal, RJ, Hitt, J, Splieth, C. Retaining sealant on salivary contaminated enamel. J Am Dent Assoc. 1993;124:88.
Fiegal, RJ, Quelhas, I. Clinical trial of a self-etching adhesive for sealant application: success at 24 months with Prompt-L-Pop. Am J Dent. 2003;16:249.
Folke, BD, Walton, JL, Feigal, RJ. Occlusal sealant success over ten years in a private practice: Comparing longevity of sealants placed by dentists, hygienists and assistants. Pediatr Dent. 2004;26:426.
Frencken, JE, Makoni, F, Sithole, WD. Atraumatic restorative treatment and glass-ionomer sealants in a school oral health programme in Zimbabwe. Caries Res. 1996;30:429.
Garcia-Gordoy, F, Abarzua, I, De Goes, MF, et al. Fluoride release from fissure sealants. J Clin Pediatr Dent. 1997;22:45.
Gungor, HC, Altay, N, Alpar, R. Clinical evaluation of a polyacid-modified resin composite-based fissure sealant: two year results. Oper Dent. 2004;29:254.
Handleman, SL, Buonocore, MG, Heseck, DJ. A preliminary report on the effect of fissure sealant on bacteria in dental caries. J Prosthet Dent. 1972;27:390.
Hannig, M, Grafe, A, Atalay, S, et al. Microleakage and SEM evaluation of fissure sealants placed by use of self-etching priming agents. J Dent. 2004;32:75.
Hori, M, Yoshida, E, Hashimoto, M, et al. In vitro testing of all-in-one adhesives as sealants. Am J Dent. 2004;17:177.
Horowitz, HS, Heifetz, SB, Poulsen, S. Retention and effectiveness of a single application of an adhesive sealant in preventing occlusal caries: final report after five years of a study in Kalispell, Montana. J Am Dent Assoc. 1977;95:1133.
Lygidakis, NA, Oulis, KI. A comparison of Fluroshield with Delton fissure sealant: four year results. Pediatr Dent. 1999;21:429.
Manabe, A, Kaneko, S, Numazawa, S, et al. Detection of Bisphenol-A in dental materials by gas chromatography-mass spectrometry. Dent Mater. 2000;19:75.
Myers, CL, Rossi, F, Cartz, F. Adhesive taglike extensions into acid-etched tooth enamel. J Dent Res. 1974;53:435.
O’Brien, WJ, Fan, PL, Apostolidis, A. Penetrativity of sealants and glazes. Oper Dent. 1978;3:51.
Pahlavan, A, Dennison, JB, Charbeneau, GT. Penetration of restorative resins into acid-etched human enamel. J Am Dent Assoc. 1976;93:1170.
Pardi, V, Pereira, AC, Mialhe, FL, et al. Six-year clinical evaluation of polyacid-modified composite resin used as fissure sealant. J Clin Pediatr Dent. 2004;28:257.
Pereira, AC, Pardi, V, Mialhe, FL, et al. A 3-year clinical evaluation of glass ionomer cements used as fissure sealants. Am J Dent. 2003;16:23.
Rueggeberg, FA, Dlugokinski, M, Ergle, JW. Minimizing patient’s exposure to uncured components in a dental sealant. J Am Dent Assoc. 1999;130:1751.
Simonsen, RJ. Retention and effectiveness of dental sealant after 15 years. J Am Dent Assoc. 1991;122:34.
Steinmetz, MJ, Pruhs, RJ, Brooks, JC, et al. Rechargeability of fluoride releasing pit and fissure sealants and restorative resin composites. Am J Dent. 1997;10:36.
Straffon, LH, Dennison, JB, More, FG. Three-year evaluation of sealant: effect of isolation on efficacy. J Am Dent Assoc. 1985;110:714.
Symons, AL, Chu, CY, Meyers, IA. The effect of fissure morphology and pretreatment of the enamel surface on penetration and adhesion of fissure sealants. J Oral Rehabil. 1996;23:791.
Tarumi, H, Imazato, S, Narimatsu, M, et al. Estrogenicity of fissure sealants and adhesive resins determined by reporter gene assay. J Dent Res. 2000;79:1838.
Taylor, CL, Gwinnett, AJ. A study of the penetration of sealants into pits and fissures. J Am Dent Assoc. 1973;87:1181.
Williams, B, Laxton, L, Holt, RD, et al. Tissue sealants: a 4-year clinical trial comparing an experimental glass polyalkenoate cement with a bis glycidyl methacrylate resin used as fissure sealants. Br Dent J. 1996;180:104.
Behle, C. Flowable composites: properties and applications. Pract Periodont Aesthet Dent. 1998;10:347.
Fortin, D, Vargas, MA. The spectrum of composites: new techniques and materials. J Am Dent Assoc. 2000;131:26S.
Houpt, M, Fuks, A, Eidelman, E. The preventive resin (composite resin/sealant) restoration: nine-year results. Quint Int. 1994;25:155.
Unterbrink, GL, Liebenberg, WH. Flowable resin composites as “filled adhesives”: literature review and clinical recommendations. Quint Int. 1999;30:249.
Glass Ionomers and Hybrid Ionomers
Bapna, MS, Mueller, HJ. Leaching from glass ionomer cements. J Oral Rehabil. 1994;21:577.
Berry, EA, III., Powers, JM. Bond strength of glass ionomers to coronal and radicular dentin. Oper Dent. 1994;19:122.
Braundau, HE, Ziemiecki, TZ, Charbeneau, GT. Restoration of cervical contours on nonprepared teeth using glass ionomer cement: a 4½ year report. J Am Dent Assoc. 1984;104:782.
Cattani-Lorente, MA, Dupuis, V, Moya, F, et al. Comparative study of the physical properties of a polyacid-modified composite resin and a resin-modified glass ionomer. Dent Mater. 1999;15:21.
Council on Dental Materials, Instruments, and Equipment. Using glass ionomers. J Am Dent Assoc. 1990;121:181.
Croll, TP. Glass ionomers in esthetic dentistry. J Am Dent Assoc. 1992;123:51.
Farah, JW, Powers, JM. Fluoride-releasing restorative materials. Dent Advis. 1998;15:2.
Fleming, GJ, Faroog, AA, Barralet, JE. Influence of powder/liquid mixing ratio on the performance of a restorative glass-ionomer dental cement. Biomaterials. 2003;24:4173.
Forss, H. Release of fluoride and other elements from light-cured glass ionomer in neutral and acidic conditions. J Dent Res. 1993;72:1257.
Garcia, R, Caffesse, RG, Charbeneau, GT. Gingival tissue response to restoration of deficient cervical contours using a glass-ionomer material, a 12-month report. J Prosthet Dent. 1981;46:393.
Heys, RJ, Fitzgerald, M, Heys, DR, et al. An evaluation of a glass ionomer luting agent: pulpal histological response. J Am Dent Assoc. 1987;114:607.
Hotta, M, Hirukawa, H, Aono, M. The effect of glaze on restorative glass-ionomer cements: evaluation of environmental durability in lactic acid solution. J Oral Rehabil. 1995;22:685.
Kent, BE, Lewis, BG, Wilson, AD. The properties of a glass ionomer cement. Br Dent J. 1973;135:322.
Maldonado, A, Swartz, ML, Phillips, RW. An in vitro study of certain properties of a glass ionomer cement. J Am Dent Assoc. 1978;96:785.
McLean, JW, Wilson, AD. The clinical development of the glass-ionomer cements. Aust Dent J. 1977;22:31.
Mitchell, CA, Douglas, WH. Comparison of the porosity of hand-mixed and capsulated glass-ionomer luting cements. Biomaterials. 1997;18:1127.
Mount, GJ. The role of glass ionomer cements in esthetic dentistry: a review. Esthet Dent Update. 1993;4:7.
Müller, J, Brucker, G, Kraft, E, et al. Reaction of cultured pulp cells to eight different cements based on glass ionomers. Dent Mater. 1990;6:172.
Quackenbush, BM, Donly, KJ, Croll, TP. Solubility of a resin-modified glass ionomer cement. J Dent Child. 1998;65:310.
Strother, JM, Kohn, DH, Dennison, JB, et al. Fluoride release and re-uptake in direct tooth colored restorative materials. Dent Mater. 1998;14:129.
Ribeiro, AP, Serra, MC, Paulillo, LA, et al. Effectiveness of surface protection for resin-modified glass-ionomer materials. Quint Int. 1999;30:427.
Sidhu, S, Watson, TF. Resin-modified glass ionomer materials: a status report for the American Journal of Dentistry. Am J Dent. 1995;8:59.
Weidlich, P, Miranda, LA, Maltz, M, et al. Fluoride release and uptake from glass ionomer cements and composite resins. Braz Dent J. 2000;11:89.
Wellbury, RR, Shaw, AJ, Murray, JJ, et al. Clinical evaluation of paired compomer and glass ionomer restorations in primary teeth. Br Dent J. 2000;189:93.
Wilder, AD, Boghosian, AA, Bayne, SC, et al. Effect of powder/liquid ratio on the clinical and laboratory performance of resin-modified glass ionomers. J Dent. 1998;26:369.
Ylp, HK, Smales, RJ. Fluoride release and uptake by aged resin-modified glass ionomers and a polyacid-modified resin composite. Int Dent J. 1999;49:217.
Craig, RG, Godwin, WC. Properties of athletic mouth protectors and materials. J Oral Rehabil. 2002;29:146.
DeYoung, AK, Robinson, E, Godwin, WC. Comparing comfort and wearability: custom-made vs. self-adapted mouth-guards. J Am Dent Assoc. 1994;125:1112.
Farah, JW, Powers, JM. Mouth protectors and laboratory handpieces. Dent Advis. 2000;17(6):2.
Godwin, WC, Craig, RG, Koran, A, et al. Mouth protectors in junior football players. Phys Sportsmed. 1982;10:41.
Padilla, RR, Lee, TK. Pressure-laminated athletic mouth guards: a step-by-step process. J Calif Dent Assoc. 1999;27:200.
Soporowski, NJ. Fabricating custom athletic mouthguards. Mass Dent Soc J. 1994;43:25.
Takeda, T, Ishigami, K, Ogawa, T, et al. Are all mouthguards the same and safe to use? The influence of occlusal supporting mouthguards in decreasing bone distortion and fractures. Dent Traumatol. 2004;20:150.
Westerman, B, Stringfellow, PM, Eccleston, JA. The effect on energy absorption of hard inserts in laminated EVA mouthguards. Aust Dent J. 2000;45:1.
Wilkinson, EE, Powers, JM. Properties of custom-made mouth protector materials. Phys Sportsmed. 1986;14:77.
Wilkinson, EE, Powers, JM. Properties of stock and mouth-formed mouth protectors. J Mich Dent Assoc. 1986;68:83.
Wisniewski, JF, Guskiewicz, K, Trope, M, et al. Incidence of cerebral concussions associated with type of mouthguard used in college football. Dent Traumatol. 2004;20:143.