Amalgam
An amalgam is an alloy of mercury and one or more other metals. Dental amalgam is produced by mixing liquid mercury with solid particles of an alloy of silver, tin, copper, and sometimes gold, indium, palladium, platinum, selenium, and zinc. This combination of solid metals is known as amalgam alloy. It is important to differentiate between dental amalgam and the amalgam alloy that is commercially produced and marketed as small filings, spheroid particles, or a combination of these, suitable for mixing with liquid mercury to produce the dental amalgam.
Once amalgam alloy is freshly mixed with liquid mercury, it has the plasticity that permits it to be conveniently packed or condensed into a prepared tooth cavity. After condensing, the dental amalgam is carved to generate the required anatomical features and then hardens with time. Amalgam is used most commonly for direct, permanent, posterior restorations and for large foundation restorations, or cores, which are precursors to placing crowns. Dental amalgam restorations are reasonably easy to insert, are not overly technique sensitive, maintain anatomical form, have reasonably adequate resistance to fracture, prevent marginal leakage after a period of time in the mouth, can be used in stress-bearing areas, and have a relatively long service life.
The principal disadvantage of dental amalgam is that the silver color does not match tooth structure. In addition, amalgam restorations are somewhat brittle; are subject to corrosion and galvanic action; may demonstrate a degree of breakdown at the margins of tooth and amalgam; and do not help retain weakened tooth structure. Finally, there are regulatory concerns about amalgam being disposed in the wastewater. In summary, dental amalgam is a highly successful material clinically and is very cost effective, but alternatives such as cast gold and esthetic restorative materials are now very competitive in terms of frequency of use. Many argue, however, that the use of amalgam must be strongly supported given its large public health benefit in the United States and many other countries.
In this chapter, the composition and morphology of the different dental amalgams are presented, followed by a discussion of low- and high-copper amalgams, the chemical reactions occurring during amalgamation, and the resultant microstructures. Various physical and mechanical properties are covered in the next section, as well as the factors related to the manipulation of amalgam. Finally, biological effects of amalgam and mercury are presented.
DENTAL AMALGAM ALLOYS
ANSI/ADA Specification No. 1 for amalgam alloy (ISO 24234) includes a requirement for composition. This specification does not state precisely what the composition of alloys shall be; rather, it permits some variation in composition. The chemical composition must consist essentially of silver and tin. Copper, zinc, gold, palladium, platinum, indium, selenium, or mercury may be included in lesser amounts. Metals such as palladium, platinum, gold, and indium in smaller quantities and copper in larger quantities have been included to alter the corrosion resistance and certain mechanical properties of the finished amalgam mass. These and other elements may be included, provided the manufacturer submits the alloy’s composition and adequate clinical and biological data to the ADA’s Council on Scientific Affairs to show that the alloy is safe to use as directed.
Alloys with more than 0.01% zinc are classified as zinc containing, and those with less than 0.01% as non-zinc alloys. Zinc has been included in amalgam alloys as an aid in manufacturing by helping to produce clean, sound castings of the ingots used for cut particle alloys. Although improved manufacturing procedures have resulted in the elimination of zinc in most alloys, recent studies have shown that small amounts of zinc in high-copper dental amalgams improve clinical performance, presumably by reducing brittleness.
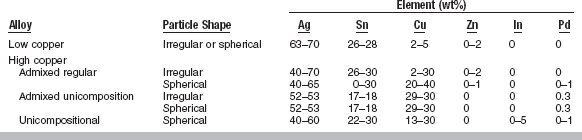
The approximate composition of commercial amalgam alloys is shown in Table 11-1, along with the shape of the particles. The alloys are broadly classified as low-copper (5% or less copper) and high-copper alloys (13% to 30% copper). Particles are irregularly shaped; microspheres of various sizes; or a combination of the two. Scanning electron micrographs of the particles are shown in Fig. 11-1. The low-copper alloys are either irregular or spherical. Both morphologic types contain silver and tin in a ratio approximating the intermetallic compound Ag3Sn. High-copper alloys contain either spherical particles of the same composition (unicompositional) or a mixture of irregular and spherical particles of different or the same composition (admixed).
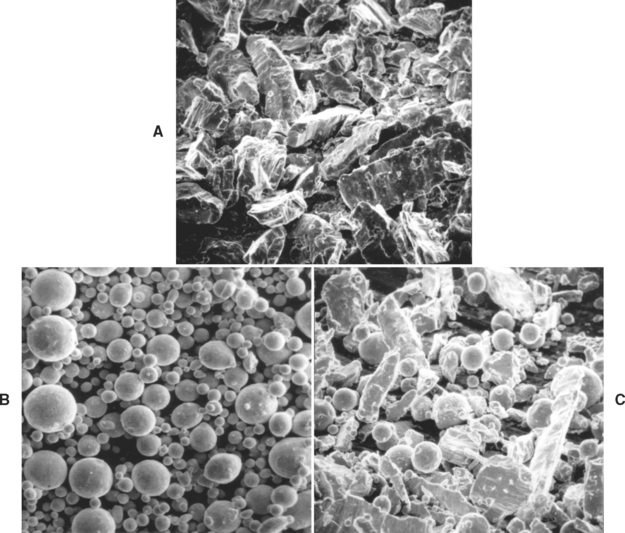
FIGURE 11-1 Scanning electron micrographs. A, Lathe-cut; B, spherical; and C, admixed amalgam alloys.
When the particles have different compositions, the admixed alloys are made by mixing particles of silver and tin with particles of silver and copper. The silver-tin particle is usually irregular in shape, whereas the silver-copper particle is usually spherical. The composition of the silver-tin particles in most commercial alloys is the same as that of the low-copper alloys. Different manufacturers, however, have somewhat different compositions for the silver-copper particle. The compositional ranges of the spherical silver-copper particles are shown in Table 11-1. The admixed regular alloy contains 33% to 60% spherical particles that have a composition close to the eutectic composition of Ag3Cu2 (see Fig. 6-9); the balance is irregular particles.
Like the admixed alloy, the unicompositional alloys have higher copper contents than the conventional lathe-cut or spherical low-copper alloys, but all the particles are spherical, as seen in Fig. 11-1. The silver content of the unicompositional alloys varies from 40% to 60%, copper content varies from 13% to 30%, and tin content varies only slightly.
A high-copper admixed alloy is also available, in which both spherical and irregular particles have the same composition and the copper content is between 29% and 30%. High-copper alloys are less commonly supplied as unicompositional, irregular particles. The lathe-cut, high-copper alloys contain more than 23% copper.
Interest has increased in admixed amalgams containing 10% to 15% indium (In) in the mercury. The addition of In to Hg decreases the amount of Hg needed, decreases the Hg vapor during and after setting, and increases the wetting. These amalgams have low creep and lower early-compressive strengths, but higher final strengths than comparable amalgams without indium. It is proposed that the lower levels of Hg vapor are due to oxides of In formed at the surface or the lower amount of Hg used in the mix.
It is estimated that more than 90% of the dental amalgams currently placed are high-copper alloys. Of the high-copper alloys, admixed are used more often than spherical types, and fewer irregularly shaped or lathe-cut types are selected. A high-copper alloy is selected to obtain a restoration with high early strength, low creep, good corrosion resistance, and good resistance to marginal fracture.
In general, alloy composition; particle size, shape, and distribution; and heat treatment control the characteristic properties of the amalgam.
PRODUCTION
To produce lathe-cut alloys, the metal ingredients are heated and protected from oxidation until melted, then poured into a mold to form an ingot. The ingot is cooled relatively slowly, leading to the formation of mainly Ag3Sn (γ) and some Cu3Sn (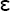 ), Cu6Sn5 (η′), and Ag4Sn (β). After the ingot is completely cooled, it is heated for various periods of time (often 6 to 8 hours) at 400° C to produce a more homogeneous distribution of Ag3Sn. The ingot is then reduced to filings by being cut on a lathe and ball milled. The particles are passed through a fine sieve and then ball milled to form the proper particle size. The particles are typically 60 to 120 μm in length, 10 to 70 μm in width, and 10 to 35 μm in thickness. Most products are labeled as fine-cut. The particle size and shape of lathe-cut amalgam alloys are shown in Fig. 11-1, A.
), Cu6Sn5 (η′), and Ag4Sn (β). After the ingot is completely cooled, it is heated for various periods of time (often 6 to 8 hours) at 400° C to produce a more homogeneous distribution of Ag3Sn. The ingot is then reduced to filings by being cut on a lathe and ball milled. The particles are passed through a fine sieve and then ball milled to form the proper particle size. The particles are typically 60 to 120 μm in length, 10 to 70 μm in width, and 10 to 35 μm in thickness. Most products are labeled as fine-cut. The particle size and shape of lathe-cut amalgam alloys are shown in Fig. 11-1, A.
In general, freshly cut alloys amalgamate and set more promptly than aged particles, but some aging of the alloy is desirable to improve the shelf life of the product. The aging is related to relief of stress in the particles produced during the cutting of the ingot. The alloy particles are aged by subjecting them to a controlled temperature of 60° to 100° C for 1 to 6 hours. Irregularly shaped high-copper particles are made by spraying the molten alloy into water under high pressure.
Spherical Particles
Spherical particles of low- or high-copper alloys are produced when all the desired elements are melted together. In the molten stage the metallic ingredients form the desired alloy. The liquid alloy is then sprayed, under high pressure of an inert gas, through a fine crack in a crucible into a large chamber. Depending on the difference in surface energy of the molten alloy and the gas used in the spraying process, the shape of the sprayed particles may be spherical or somewhat irregular, as shown in Fig. 11-1, B. The diameter of the spheres varies from 2 to 43 μm.
SILVER-TIN ALLOY
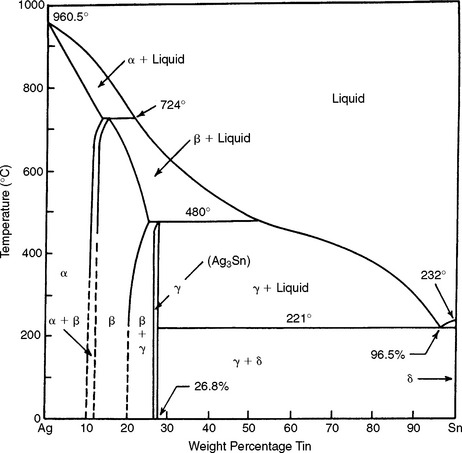
Because two of the principal ingredients in the amalgam alloy are silver and tin, it is appropriate to consider the binary system and the equilibrium phase diagram for these two metals, as shown in Fig. 11-2.
The most important feature in this diagram concerning the silver-tin alloy is that, when an alloy containing approximately 27% tin is slowly cooled below a temperature of 480° C, an intermetallic compound (Ag3Sn) known also as the gamma (γ) phase is produced. This Ag3Sn compound is an important ingredient in the silver amalgam alloy and combines with mercury to produce a dental amalgam of desired mechanical properties and handling characteristics. This silver-tin compound is formed only over a narrow composition range. The silver content for such an alloy would be approximately 73%. Practically, the tin content is held between 26% and 30%, and the remainder of the alloy consists of silver, copper, and zinc. If the concentration of tin is less than 26%, the beta (β) phase, which is a solid solution of silver and tin, forms. In one product, 5% tin is replaced by 5% indium, whereas another product contains less than 1% palladium. Adding this small amount of palladium enhances the mechanical properties and corrosion resistance. The replacement of silver by an equal amount of copper produces a copper-tin compound (Cu3Sn).
In general, larger (>30%) or smaller (<26%) quantities of tin in the alloy are detrimental to the final properties of the amalgam. The reason for this unfavorable shift in properties is generally considered related to the fact that the amount of Ag3Sn is reduced as the percentage of tin is altered beyond the indicated limits. This is the basis for the rather narrow limits of the alloy compositions of current products with acceptable properties.
Silver-tin amalgam alloys compounded to produce largely Ag3Sn react favorably with mercury to produce only slight dimensional setting changes when properly manipulated. The strength of the amalgam mass is greater from the Ag3Sn compound than from an excess of tin. In addition, the setting time is shortened by increasing silver content. Creep resistance is also superior when an alloy of Ag3Sn is used rather than one with higher tin content.
AMALGAMATION PROCESSES
The amalgam alloy is intimately mixed with liquid mercury to wet the surface of the particles so the reaction between liquid mercury and alloy can proceed at a reasonable rate. This mixing is called trituration. During this process, mercury diffuses into the γ phase of the alloy particles and begins to react with the silver and tin portions of the particles, forming various compounds, predominantly silver-mercury and tin-mercury compounds, which depend on the exact composition of the alloy. The silver-mercury compound is Ag2Hg3 and is known as the gamma one (γ1) phase, and the tin-mercury compound is Sn7–8Hg and is known as the gamma two (γ2) phase. However, the silver-tin, silver-mercury, and tin-mercury phases are not pure. For example, Ag3Sn always contains some copper and occasionally small amounts of zinc. The Ag2Hg3 dissolves small amounts (1% to 3%) of tin and Cu6Sn5 (η′), and similarly, Cu6Sn5 could dissolve various elements present. Therefore γ, γ1, and γ2 are better descriptive terms of these three phases formed in dental amalgam than are the pure compounds.
While crystals of the γ1 and γ2 phases are being formed, the amalgam is relatively soft and easily condensable and carvable. As time progresses, more crystals of γ1 and γ2 are formed; the amalgam becomes harder and stronger, and is no longer condensable or carvable. The lapse of time between the end of the trituration and when the amalgam hardens and is no longer workable is called working time.
The amount of liquid mercury used to amalgamate the alloy particles is not sufficient to react with the particles completely. Therefore, the set mass of amalgam contains unreacted particles. About 27% of the original Ag3Sn compound remains as unreacted particles. A simplified reaction of a low-copper amalgam alloy with mercury can be summarized in the following manner:
γ(Ag3 Sn)+Hg→γ1(Ag2 Hg3)+γ2(Sn7–8 Hg)+unreactedγ(Ag3Sn)
The dominating phase in a well-condensed, low-copper dental amalgam is the Ag2Hg3 (γ1) phase, which is about 54% to 56% by volume. The percentages of the γ and γ 2 phases are 27% to 35% and 11% to 13%, respectively.
HIGH-COPPER ALLOYS
The main difference between the low- and high-copper amalgam alloys is not merely the percentage of copper but the effect that the higher copper content has on the amalgam reaction. The copper in these alloys is in either the silver-copper eutectic or Cu3Sn (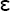 ) form. The proper amount of copper causes most, if not all, of the γ2 phase to be eliminated within a few hours after its formation or prevents its formation entirely. The γ2 phase in amalgam is the weakest and is the most susceptible to corrosion; therefore, restorations using amalgam made with insufficient copper tend to have inferior physical and mechanical properties and a shorter period of clinical serviceability than restorations made from the high-copper amalgams.
) form. The proper amount of copper causes most, if not all, of the γ2 phase to be eliminated within a few hours after its formation or prevents its formation entirely. The γ2 phase in amalgam is the weakest and is the most susceptible to corrosion; therefore, restorations using amalgam made with insufficient copper tend to have inferior physical and mechanical properties and a shorter period of clinical serviceability than restorations made from the high-copper amalgams.
Reaction of Mercury in an Admixed High-Copper Amalgam Alloy
During trituration, mercury diffuses into the amalgam particles and dissolves. The solubility of mercury in silver, tin, and copper differs considerably. Whereas 1 mg of mercury dissolves in copper, 10 mg can dissolve in silver and 170 mg in tin, all at the same temperature. Therefore, particles composed mainly of silver and tin dissolve almost all the mercury, whereas very little mercury is dissolved by the silver-copper eutectic particles. The mercury dissolved in the silver-tin particles reacts as in low-copper alloys and forms the γ1 and γ2 phases, leaving some silver-tin particles unreacted. In a relatively short time, however, the newly formed γ2 phase (Sn7–8Hg) around the silver-tin particles reacts with silver-copper particles, forming Cu6Sn5, the eta prime (η′) phase of the copper-tin system, along with some of the γ1 phase (Ag2Hg3) around the silver-copper particles. The amalgamation reaction may be simplified as follows:
The initial reaction is the same as for low-copper dental amalgam,
γ(Ag3 Sn)+Ag-Cu eutectic)+Hg→γ1(Ag2Hg3)+γ2(Sn7–8Hg)+unreactedγ(Ag3Sn)+unreacted Ag-Cu(eutectic)
and the secondary, slow solid-state reaction is
γ2(Sn7–8Hg)+Ag-Cu(eutectic)→η′(Cu6Sn5)+γ1(Ag2Hg3)+unreacted Ag-Cu(eutectic)
Reaction of Mercury in a Unicompositional Alloy
In high-copper unicompositional alloys, the alloy particles contain both γ(Ag3Sn) and 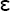 (Cu3Sn) similar to the low-copper lathe cut alloys, but the amount of the
(Cu3Sn) similar to the low-copper lathe cut alloys, but the amount of the 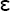 (Cu3Sn) phase is much greater to accommodate the additional copper. These alloys are usually spherical in nature and because spherical particles cool very rapidly, the
(Cu3Sn) phase is much greater to accommodate the additional copper. These alloys are usually spherical in nature and because spherical particles cool very rapidly, the 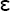 (Cu3Sn) phase is finely dispersed throughout the γ(Ag3Sn) phase. When liquid mercury is mixed with these alloys, it diffuses into the surface of these particles and γ1(Ag3Hg3) as well as η′(Cu6Sn5) are formed. The reaction occurs in a ring around the spherical particles and consists of γ and η′ with no remaining γ or
(Cu3Sn) phase is finely dispersed throughout the γ(Ag3Sn) phase. When liquid mercury is mixed with these alloys, it diffuses into the surface of these particles and γ1(Ag3Hg3) as well as η′(Cu6Sn5) are formed. The reaction occurs in a ring around the spherical particles and consists of γ and η′ with no remaining γ or 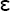 in this ring. The reaction can be summarized as follows:
in this ring. The reaction can be summarized as follows:
[γ(Ag3Sn)+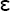 (Cu3Sn)]+Hgγ1(Ag3Hg3)andη(Cu6Sn5)+unreacted[γ(Ag3Sn)+
(Cu3Sn)]+Hgγ1(Ag3Hg3)andη(Cu6Sn5)+unreacted[γ(Ag3Sn)+ (Cu3Sn)]
(Cu3Sn)]
Thus the reaction of mercury with either the high-copper admixed or the unicompositional alloys results in a final reaction, with Cu6Sn5 (η′) being produced rather than Sn7–8Hg (γ2).
In some high-copper alloys, there may be residual γ2 of less than 1%. Note that there is no definitive proof that the γ2 phase ever forms, even temporarily. By the time electron microprobe analyses can be performed, the reaction will have reached equilibrium, and the final reaction products of η′ and γ1 will have already formed.
MICROSTRUCTURE OF AMALGAM
In dental applications the amount of liquid mercury used to amalgamate with the alloy particles is less than that required to complete the reaction. Thus the set amalgam mass consists of unreacted particles surrounded by a matrix of the reaction products. The reaction is principally a surface reaction, and the matrix bonds the unreacted particles together. The initial diffusion and reaction of mercury and alloy are relatively rapid, and the mass changes rapidly from a plastic consistency to a hard mass. Completion of the reaction may take several days to several weeks, which is reflected by the change in mechanical properties over this time.
The microstructures of set amalgam of the low-copper, lathe-cut, and spherical types are shown in Fig. 11-3. The outlines of the unreacted alloy particles (γ) are visible (A). The γ1 and γ2 phases in the matrix are identified by the letters B and C, respectively. Voids in each of the two specimens are identified by the letter D. After the completion of the solid-state reaction in the high-copper admixed and unicompositional alloys, the microstructures show no γ2 phase (Fig. 11-4).
FIGURE 11-3 Microstructure of set dental amalgam, etched with iodine etch. A, Lathe-cut particles: A, unreacted original particle, γ; B, γ1; C, γ2; D, void. B, Spherical alloy particles: A, original particle; B, γ1; C, γ2; D, void. (From Allen FC, Asgar K, Peyton FA: J Dent Res 44:1002, 1965.)
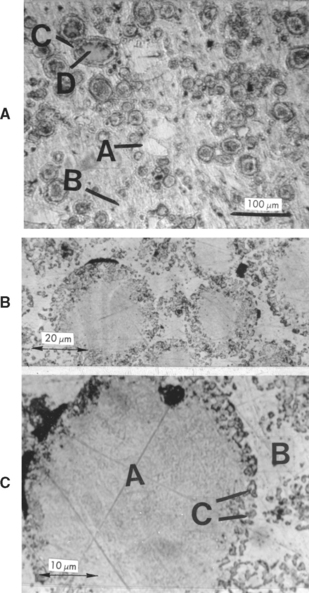
FIGURE 11-4 Microstructure of high-copper admixed (A) and spherical unicompositional (B, C) alloys. A, A is an unreacted portion of γ; B is the γ1 phase; C is the reaction zone around the Ag-Cu eutectic particle; D is an unreacted portion of an Ag-Cu particle. C, A is an unreacted portion of a spherical unicompositional Ag-Sn-Cu particle; B is the γ1 phase; C is the reaction zone around an original particle.
PROPERTIES OF AMALGAM
Important properties for dental amalgam include dimensional change, compressive strength, creep, and corrosion resistance. These properties may be explained in part by the composition, microstructure, and manipulation of the amalgam.
ANSI/ADA SPECIFICATION NO. 1 FOR AMALGAM ALLOY
ANSI/ADA Specification No. 1 (ISO 24234) for amalgam alloy contains requirements that help significantly control the qualities of dental amalgam. The specification lists three physical properties as a measure of amalgam quality: creep, compressive strength, and dimensional change. When a cylindrical specimen is 7 days old, a 36-MPa stress is applied in a 37° C environment. Creep is measured between 1 and 4 hours of stressing. The maximum allowable creep is 1%. The minimum allowable compressive strength 1 hour after setting, when a cylindrical specimen is compressed at a rate of 0.25 mm/min, is 80 MPa and is 300 MPa at 24 hours after setting. The dimensional change between 5 minutes and 24 hours must fall within the range of −15 to +20 μm/cm.
PHYSICAL AND MECHANICAL PROPERTIES
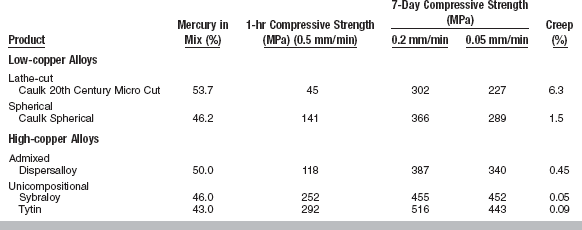
Resistance to compression forces is the most favorable strength characteristic of amalgam. Because amalgam is strongest in compression and much weaker in tension and shear, the prepared cavity design should maximize compressive stresses in service and minimize tension or shear stresses. The early-compressive strengths (after 1 hour of setting) for several low- and high-copper alloys are listed in Table 11-2. The percentage of mercury used in preparing the specimens is also listed; the lathe-cut alloy requires the greatest amount of mercury, and the unicompositional alloy the least. Notice that amalgams are viscoelastic and the compressive strength is a function of the rate of loading. In general, the higher the rate of loading, the higher the compressive strength, although some studies have shown that compressive strength may decrease at very high strain rates. As a result, when comparing the compressive strength of amalgam specimens, it is imperative that they be tested at the same rate of loading.
TABLE 11-2
Compressive Strength and Creep of Amalgams
Adapted from Malhotra ML, Asgar K: J Am Dent Assoc 96:446, 1978.
When subjected to a rapid application of stress either in tension or in compression, a dental amalgam does not exhibit significant deformation or elongation and, as a result, functions as a brittle material. Therefore, a sudden application of excessive force to amalgam tends to fracture the amalgam restoration.
The high-copper unicompositional materials have the highest early-compressive strengths of more than 250 MPa at 1 hour. The compressive strength at 1 hour is lowest for the low-copper lathe-cut alloy (45 MPa). These data indicate that only some of the older lathe-cut alloys would meet the requirement for compressive strength at 1 hour of ANSI/ADA Specification No. 1. High values for early-compressive strength are an advantage for an amalgam, because they reduce the possibility of fracture by the application of prematurely high occlusal forces by the patient before the final strength is reached. The compressive strengths at 7 days and the final strengths are again highest for the high-copper unicompositional alloys, with only modest differences in the other alloys.
Tensile Strength
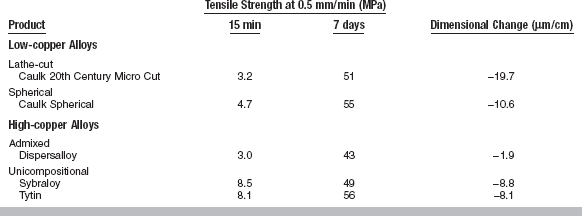
The tensile strengths of various amalgams after 15 minutes and 7 days are listed in Table 11-3. The tensile strengths at 7 days for both non-γ2 and γ2-containing alloys are about the same. The tensile strengths are only a fraction of their compressive strengths; therefore, cavity designs should be constructed to reduce tensile stresses resulting from biting forces.
TABLE 11-3
Tensile Strength and Dimensional Change of Amalgams
Adapted from Malhotra ML, Asgar K: J Am Dent Assoc 96:447, 1978.
The tensile strengths at 15 minutes for the high-copper unicompositional alloys are significantly higher than for the other alloys. High early tensile strengths are important, because they serve to resist fracture by prematurely applied biting forces.
Transverse Strength
Transverse strength is determined by the application of load to cause bending in a rectangular specimen of amalgam. It is correlated to tensile strength and is much easier to conduct. This property is sometimes referred to as modulus of rupture.
Strength of Various Phases
The relative strengths of the different amalgam phases are important. By studying the initiation and propagation of a crack in a set amalgam, the relative strength of the different phases can be observed. Fig. 11-5 shows the propagation of a crack in a dental amalgam specimen. It is possible to view the crack initiation and propagation of an amalgam specimen under a conventional metallographical microscope with a strain viewer. The propagation of the crack can be halted and the specimen etched to identify the various phases. Results of such studies have led to the following ranking of the different phases of a set low-copper amalgam from strongest to weakest: Ag3Sn (γ), the silver-mercury phase (γ1), the tin-mercury phase (γ2), and the voids.
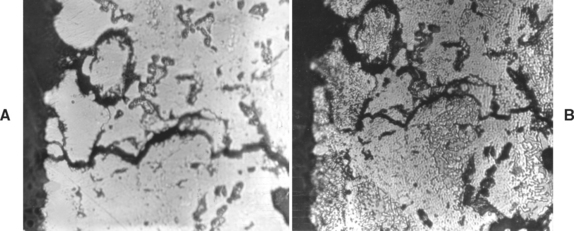
FIGURE 11-5 Propagation of a crack in a dental amalgam. A, Unetched. B, After etching. (From Asgar K, Sutfin L: J Dent Res 44:985, 1965.)
Silver-mercury and tin-mercury act as a matrix to hold the unreacted amalgam alloy together. When relatively smaller amounts of silver-mercury and tin-mercury phases form, up to a certain minimum required for bonding the unreacted particles, a set amalgam is stronger. When a higher percentage of mercury is left in the final mass, it reacts with more of the amalgam alloy, producing larger amounts of silver-mercury and tin-mercury phases and leaving relatively smaller amounts of unreacted particles. The result is a weaker mass. Therefore, the effect of various manipulative conditions can be explained in this manner. In high-copper amalgams, there is preferential crack propagation through the γ1 phase and around copper-containing particles.
Elastic Modulus
When the elastic modulus is determined at low rates of loading, such as 0.025 to 0.125 mm/min, values in the range of 11 to 20 GPa are obtained. High-copper alloys tend to be stiffer than low-copper alloys. If the rate of loading is increased so the viscoelastic property does not significantly influence the elastic modulus, values of approximately 62 GPa have been obtained.
Creep
The viscoelastic properties of amalgam are also reflected by the creep or permanent deformation under static loads. Under continued application of a compressive force, an amalgam shows a continued deformation, even after the mass has completely set. Amalgam has no tendency for work hardening or for resisting deformation more effectively after the mass has been deformed, as may be experienced with the cast gold alloys.
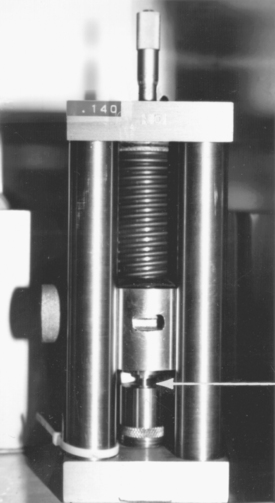
Values for creep are determined in an instrument similar to that shown in Fig. 11-6. A cylindrical specimen is placed in the position indicated by the arrow 7 days after preparation. A static stress of 36 MPa is applied by the spring. The change in length of the specimen is determined at 37 ± 0.3° C by a calibrated differential transformer, the output of which is recorded on a chart. The change in length between 1 hour and 4 hours after placing the static stress is used to calculate the percentage creep.
Creep values for various amalgams are listed in Table 11-2. The highest value of 6.3% was found for the low-copper cut alloy, and the lowest values (0.05% to 0.09%) were determined for the high-copper unicompositional spherical alloys. The high-copper admixed alloy and one of the low-copper alloys had slightly higher creep values of 0.45% to 0.50%, and 1.5%, respectively.
It has been shown by numerous studies that clinical performance as measured by the extent of marginal fracture is improved in amalgam restorations that do not contain the tin-mercury phase (γ2); these amalgams exhibit lower creep than the γ2-containing amalgams as shown in Table 11-2. Even though creep varies among the γ2-free amalgams, significant differences in their clinical performance have not been shown.
Note that the low creep values of high-copper amalgams increase the brittleness of the amalgam and decrease the relief of stresses at contact areas under load. As a result, a high-modulus base under a high-copper amalgam is essential to minimize deformation and the development of tensile stresses at the amalgam-cement base interface.
Dimensional Change
The dimensional change during the setting of amalgam is one of its most significant properties. Modern amalgams mixed with mechanical amalgamators usually have negative dimensional changes. The initial contraction after a short time (the first 20 minutes) is believed to be associated with the solution of mercury in the alloy particles. After this period an expansion occurs, although the total change remains negative, which is believed to be a result of the reaction of mercury with silver and tin and the formation of the intermetallic compounds. The dimensions become nearly constant after 6 to 8 hours, and thus the values after 24 hours are final values. The only exception to this statement is the excessive delayed dimensional change resulting from contamination of a zinc-containing alloy with water during trituration or condensation.
The dimensional change may be determined with an instrument such as the one shown in Fig. 11-7. The amalgam specimens identified by the arrows are placed in position 5 minutes after setting, and the probe is placed on top of them. The probe is mechanically attached to a differential transformer, and the electrical output is used to determine expansion or contraction. The change in length can be determined continuously, although ANSI/ADA Specification No. 1 requires only the value at 24 hours.
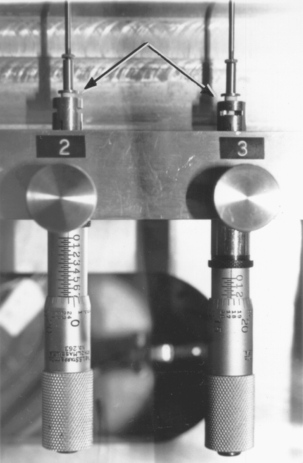
FIGURE 11-7 An instrument for measuring dimensional change of amalgam. Arrows point to amalgam specimens.
The dimensional changes in micrometers per centimeter for the various alloys are listed in Table 11-3. The largest dimensional change of −19.7 μm/cm occurred with the low-copper, lathe-cut alloy, and the lowest change of −1.9 μm/cm was for the high-copper admixed alloy. All the amalgams meet the requirements of ANSI/ADA Specification No. 1 of −15 to +20 μm/cm. Notice that the ranking of the dimensional change does not correlate with any of the other mechanical properties. The dimensional change is susceptible to influence from various manipulative factors, especially final mercury content. Higher mercury content results in less shrinkage but also in lower mechanical strength.
The clinical significance of dimensional change is related to the occasional occurrence of postoperative sensitivity associated with newly placed amalgam restorations. Amalgam does not adhere to tooth structure; therefore, a negative dimensional change would result in an interfacial gap between the amalgam restoration and tooth structure. When a cavity is prepared that cuts through dentin in a tooth requiring restoration, pulpal fluid in the tubules can flow outward. When an amalgam that has a negative dimensional change is placed, the interfacial gap fills with pulpal fluid. Changes in pressure of this fluid are considered to be one of the major causes of postoperative sensitivity. Apparently, the size of the interfacial gap is a key factor in determining whether sensitivity will occur, with larger gaps being particularly prone in this regard.
Alloys that pass ADA Specification No. 1 for negative dimensional changes of −15 μ/cm or less have not been shown by scientific studies to have an uncommon amount of postoperative sensitivity. Some of the newer high-copper amalgams consisting of only spherical particles have been reported to show a greater propensity for this sensitivity. The reason for this anomaly was found by in vitro microleakage studies using air pressure through the marginal gaps of simulated Class I restorations. These studies showed that spherical particle alloys leaked more than lathe-cut alloys, even though their respective dimensional changes were not significantly different. Examination showed that the surfaces of these amalgams next to the cavity walls exhibited a relatively uneven texture for the spherical particle alloys compared to a smoother texture for the lathe-cut alloys. Thus, the interfacial space filled by pulpal fluid was greater for the spherical particle alloys but was not measured by the dimensional change test.
The use of dentin bonding agents to seal the dentinal tubules before placement of an amalgam restoration has proven to be an effective solution to the problem of postoperative sensitivity of spherical particle amalgams.
Corrosion
In general, corrosion is the progressive destruction of a metal by chemical or electrochemical reaction with its environment. Excessive corrosion can lead to increased porosity, reduced marginal integrity, loss of strength, and the release of metallic products into the oral environment.
The following compounds have been identified on dental amalgams in patients: SnO, SnO2, Sn4(OH)6Cl2, Cu2O, CuCl2 · 3Cu(OH)2, CuCl, CuSCN, and AgSCN.
Because of their different chemical compositions, the different phases of an amalgam have different corrosion potentials. Electrochemical measurements on pure phases have shown that the Ag2Hg3 (γ1) phase has the highest corrosion resistance, followed by Ag3Sn (γ), Ag3Cu2, Cu3Sn (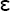 ), Cu6Sn5 (η′), and Sn7–8Hg (γ2). However, the order of corrosion resistance assigned is true only if these phases are pure and they are not in the pure state in dental amalgam.
), Cu6Sn5 (η′), and Sn7–8Hg (γ2). However, the order of corrosion resistance assigned is true only if these phases are pure and they are not in the pure state in dental amalgam.
The presence of small amounts of tin, silver, and copper that may dissolve in various amalgam phases has a great influence on their corrosion resistance. The γ1 phase has a composition close to Ag2Hg3 with 1% to 3% of dissolved tin. The higher the tin concentration of Ag2Hg3 (γ1), the lower its corrosion resistance. In general, the tin content of the γ1 phase is higher for low-copper alloys than for high-copper alloys. The presence of a relatively high percentage of tin in low-copper alloys reduces the corrosion resistance of their γ1 phase so it is lower than their γ phase. This is not true for high-copper alloys. The average depth of corrosion for most amalgam alloys is 100 to 500 μm.
In the low-copper amalgam system, the most corrodible phase is the Sn7–8Hg or γ2 phase. Although a relatively small portion (11% to 13%) of the amalgam mass consists of the γ2 phase, in time and in an oral environment the structure of such an amalgam will contain a higher percentage of corroded phase. On the other hand, neither the γ nor the γ1 phase is corroded as easily. Studies have shown that corrosion of the γ2 phase occurs throughout the restoration, because it is a network structure. Corrosion results in the formation of tin oxychloride from the tin in the γ2, and also liberates mercury, as shown in the following equation:
Sn7–8Hg+½O2+H2O+Cl−→Sn4(OH)6Cl2+Hg
The reaction of the liberated mercury with unreacted γ can produce additional γ1 and γ2. It has been proposed that the dissolution of the tin oxide or tin chloride and the production of additional γ1 and γ2 result in porosity and lower strength.
The high-copper admixed and unicompositional alloys do not have any γ2 phase in the final set mass. The Cu6Sn5 or η′ phase formed with high-copper alloys is not an interconnected phase such as the γ2 phase, and it has better corrosion resistance. However, η′ is the least corrosion-resistant phase in high-copper amalgams; and a corrosion product, CuCl2·3Cu(OH)2, has been associated with storage of amalgams in synthetic saliva, as shown here:
Cu6Sn5+½O2+H2O+Cl−→CuCl2·3Cu(OH)2+SnO
Phosphate buffer solutions inhibit the corrosion process; thus saliva may provide some protection of dental amalgams from corrosion.
A study of amalgams that had been in service for 2 to 25 years revealed that the bulk elemental compositions were similar to newly prepared amalgams, except for the presence of a small amount of chloride and other contaminants. The compositions of the phases were also similar to new amalgams, except for internal amalgamation of the γ particles. The distribution of phases in the clinically aged amalgams, however, differed from that of new amalgams. The low-copper amalgams had decreased amounts of γ, γ1, and γ2 and increased β1 and tin-chloride. High-copper admixed amalgams had decreased γ1, increased β1, and enlarged reaction rings of γ1 and η′. There was also evidence of a conversion of γ1 to β1 and γ2 to η′.
Note that the processes of corrosion and wear are frequently coupled and that wear can lower the corrosion potential and increase the corrosion rate by an order of magnitude.
Fig. 11-8 compares an amalgam restoration on the distal portion of a tooth prepared from a low-copper spherical alloy with one on the mesial portion prepared from high-copper admixed alloy. The restorations have been in service for 3 years, and the higher marginal fracture, presumably resulting from the corrosion of the γ2 phase of the low-copper amalgam, is readily apparent.
FIGURE 11-8 Amalgam restoration from a low-copper spherical alloy (left) and an amalgam from a high-copper admixed alloy (right) after 3 years of service. (Courtesy GT Charbeneau, Ann Arbor, University of Michigan School of Dentistry.)
Surface tarnish of low-copper amalgams is more associated with γ than γ1, whereas in high-copper amalgams surface tarnish is related to the copper-rich phases, η′ and silver-copper eutectic.
PROPERTIES OF MERCURY
ANSI/ADA Specification No. 6 (ISO 24234) for dental mercury requires that mercury have a clean reflecting surface that is free from surface film when agitated in air. It should have no visible evidence of surface contamination and contain less than 0.02% nonvolatile residue. Mercury that complies with the requirements of the United States Pharmacopoeia also meets requirements for purity in ANSI/ADA Specification No. 6. Mercury amalgamates with small amounts of many metals and is contaminated by sulfur gases in the atmosphere, which combine with mercury to form sulfides. Small quantities of these foreign materials in mercury destroy its bright, mirror-like surface and can be readily detected by visual inspection.
Mercury, which has a freezing point of −38.87° C, is the only metal that remains in the liquid state at room temperatures. It combines readily to form an amalgam with several metals such as gold, silver, copper, tin, and zinc, but does not combine under ordinary conditions with such metals as nickel, chromium, molybdenum, cobalt, and iron.
Mercury boils at 356.9° C, and, if pure, has a significant vapor pressure at room temperature. Extended inhalation can result in mercury poisoning. Globules dropped on a surface roll about freely without leaving a tail and retain their globular form. This tendency to form globules is related to the high surface tension of liquid mercury, which is 465 dynes/cm at 20° C, as compared with 72.8 dynes/cm for water. Mercury with a very high degree of purity exhibits a slight tarnish after a short time because impurities contaminate the metal and produce a dull surface appearance. Impurities in mercury can reduce the rate at which it combines with the silver alloy.
MANIPULATION OF AMALGAM
The selection of an alloy involves a number of factors, including setting time, particle size and shape, and composition, particularly as it relates to the elimination of the γ2 phase and the presence or absence of zinc. It is estimated that more than 90% of the dental amalgams currently placed are high-copper alloys. The majority of the alloys selected are high-copper unicompositional (spherical) and admixed types, with the admixed being favored slightly. A high-copper alloy is favored because the result is a restoration with no γ2, high early strength, low creep, good corrosion resistance, and good resistance to marginal fracture.
Finer particle sizes are used for low-copper, irregular alloys because of improved properties and enhanced clinical convenience. Finer particles produce a smoother surface during carving and finishing. The clinical manipulation of dental amalgam alloys is influenced to a modest extent by the shape of the particles. Lathe-cut alloys exhibit rough, irregular surfaces having a large area/volume ratio to react with mercury, and generally require nearly 50% or more mercury to obtain adequate plasticity during trituration. Spherical alloys are smoother; consist of various sizes of spheres (2 to 43 μm), which are important in packing; have more regular surfaces with a lower area/volume ratio; and generally require less mercury for trituration and development of suitable plasticity. Mercury concentrations as low as 42% permit acceptable handling characteristics with certain products.
Lathe-cut and spherical alloys react differently to condensation forces. These differences result from frictional forces within the amalgam mass that offer higher resistance to the face of the condenser in lathe-cut alloys than in spherical alloys. Carving the excess amalgam from the overfilled cavity to restore morphological and functional anatomy presents further differences.
Because of improved manufacturing, few products contain zinc because the contamination of a zinc-containing alloy by moisture may result in excessive dimensional change. If an alloy contains more than 0.01% zinc, the package must carry a printed precaution that the amalgam made from the material will show excessive corrosion and expansion if moisture is introduced during mixing and condensation.
PROPORTIONS OF ALLOY TO MERCURY
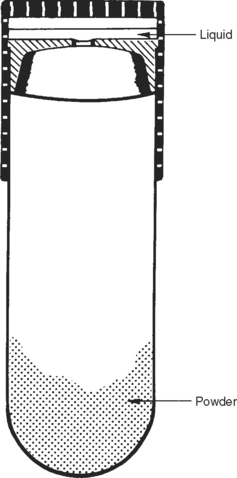
Correct proportioning of alloy and mercury is essential for forming a suitable mass of amalgam for placement in a prepared cavity. Some alloys require mercury-alloy ratios in excess of 1:1, whereas others use ratios of less than 1:1; the percentage of mercury varies from 43% to 54%. Automatic mechanical dispensers for alloy and mercury have been used in the past and are described in previous editions of this textbook. With the recommendation for “no touch” procedures for handling mercury and amalgam, capsules with preproportioned amounts of alloy and mercury have been substituted for mercury and alloy dispensers. The correct amounts of alloy and mercury are kept separated in the capsule by a membrane, as shown in the sketch in Fig. 11-9. Just before the mix is triturated the membrane is ruptured by compression of the capsule, or it is automatically activated during trituration. Various manufacturers’ amalgam alloys with their corresponding capsules are shown in Fig. 11-10. Some capsules contain a plastic pestle in the shape of a disk or rod, as illustrated in the disassembled capsules in Fig. 11-11. To prevent any escape of mercury from the friction-fitted capsule during trituration, some capsules are hermetically sealed; the mercury is contained in a small plastic film packet that ruptures during mixing.
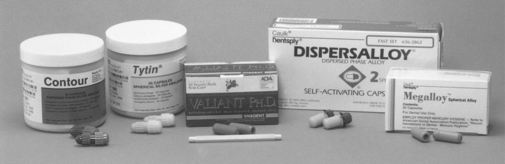
FIGURE 11-10 Examples of spherical and admixed dental amalgam alloys in capsules. (From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St. Louis, 2004, Mosby.)
Size of Mix
Manufacturers commonly supply capsules containing 400, 600, or 800 mg of alloy and the appropriate amount of Hg, color coded for ease of identification. Clinical consensus is that these amounts are sufficient for most restorations. It is usually suggested that if larger amounts are required that several smaller mixes be made at staggered times so that the consistency of the mixed amalgam remains reasonably constant during the preparation of the restoration. However, capsules containing 1200 mg of alloy are available if a large amount of amalgam is needed to produce an amalgam core on a severely broken down tooth.
MIXING OF AMALGAM
Trituration of amalgam alloy and mercury is done with a mechanical mixing device called an amalgamator or triturator. The amalgamator shown in Fig. 11-12 has controls for the speed and duration of trituration. The amalgamator has a housing that is placed over the capsule area during trituration to confine any mercury lost from the capsule during mixing.
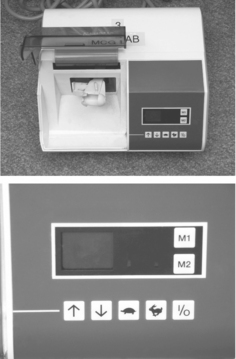
FIGURE 11-12 Variable-speed amalgamator for triturating amalgam. The control panel (bottom photograph) has controls for on and off (I/O), arrows to increase or decrease the time of trituration, and buttons to specify high (rabbit) or slow (turtle) trituration speeds. The M1 and M2 buttons are for preprogrammed trituration. (From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St. Louis, 2004, Mosby.)
The capsule holder is attached to a motor that rotates the holder and capsule eccentrically. The trituration may be accomplished simply by the agitation of the alloy particles and mercury, or the manufacturer may have included a plastic pestle to aid in the mixing.
Spherical or irregular low-copper alloys may be triturated at low speed (low energy), but most high-copper alloys require high speed (high energy). Effective trituration depends on a combination of the duration and speed of mixing. Duration of amalgamation is the easiest factor to vary; however, it should be emphasized that variations of 2 to 3 seconds of mixing time may be enough to produce an amalgam that is undermixed or overmixed. Mechanical amalgamators allow some variation in speed to adjust to differing amounts of alloy and mercury in the capsules.
Low-, medium-, and high-speed amalgamators operate at about 3200 to 3400, 3700 to 3800, and 4000 to 4400 cycles per minute, respectively, at correct line voltage. However, an amalgamator set at a speed of 3300 cpm may actually be operating at 3000 cpm with a decrease in line voltage from 120 to 100 volts, and undermixed amalgams may result. This problem can be avoided by installing a voltage regulator between the line plug and the amalgamator. Using a parameter called the coherence time (t c), defined as the minimum mixing time required for an amalgam to form a single coherent pellet, it has been found that the compressive strength, dimensional change, and creep are optimized if mixing is carried out for a time of 5t c. The value of t c can be determined experimentally for a particular amalgam alloy, size of mix, and speed of the amalgamator. However, most packages of amalgam alloys will contain recommendations for times and speeds for a variety of amalgamators, and these guidelines should be followed.
With the introduction of disposable capsules containing premeasured amounts of amalgam alloy and mercury, mercury and alloy dispensers have become obsolete, as have reusable capsules; however, the discussion of their selection and may be found in the 9th and earlier editions of this textbook.
Mixing Variables
Undermixing, normal mixing, or overmixing can result from variations in the condition of trituration of the alloy and mercury. The three mixes have a different appearance and respond differently to subsequent manipulation. The undermixed amalgam appears dull and is crumbly, the normal mix appears shiny and separates in a single mass from the capsule, and the overmixed amalgam appears soupy and tends to stick to the inside of the capsule. Examples of these mixes are shown in Fig. 11-13. The three types of mixes have characteristically different mechanical properties of dimensional change, strength, and creep. These three conditions can be developed from variations in the mixing variables described earlier. Therefore, the type of mix contributes to the success or failure of the amalgam restoration.
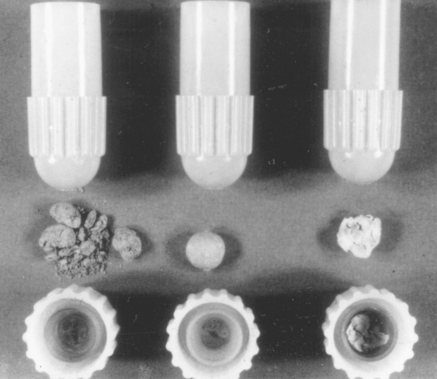
FIGURE 11-13 Amalgam. Left, undermixed; center, normal mixed; right, overmixed. (Courtesy Dr. K Asgar, University of Michigan School of Dentistry, Ann Arbor, Mich.)
Not all types of alloys respond in the same manner to overtrituration and undertrituration. Spherical and lathe-cut alloys respond differently. The effects of overtrituration and undertrituration of amalgam on working time, dimensional change, compressive and tensile strengths, and creep are summarized as follows.
Working Time and Dimensional Change: Working time of all types of amalgam, spherical or irregular, decreases with overtrituration. High- or low-copper alloys respond alike. Overtrituration results in slightly higher contraction for all types of alloys. High- and low-copper alloys show the same effect.
Compressive and Tensile Strengths: Both compressive and tensile strengths of irregularly shaped alloys increase by overtrituration. However, this is not true for spherical alloys. Compressive and tensile strengths of spherical alloys are greatest at normal trituration time. Both overtrituration and undertrituration reduce compressive and tensile strengths. The admixed high-copper alloys consist of both shapes of particles and behave like spherical alloys; normal trituration times produce the highest strength values, whereas overtrituration results in significant decreases in strength.
Creep: Overtrituration increases creep, and undertrituration lowers it. As mentioned earlier in this chapter, two properties that are closely related to the clinical behavior of alloys are low creep and high compressive strength. By overtriturating irregular amalgams, a higher compressive strength can be obtained, which is beneficial. However, the amalgam has a higher creep, a property that is not desirable. If there is doubt about the correct trituration time, a slightly overtriturated amalgam is better than a slightly undertriturated one. This suggestion is particularly true for high-copper alloys.
Some manufacturers recommend altering the trituration time to obtain a longer or shorter working time. Altering the trituration time does change the working time of amalgam, but it also affects other properties. When amalgam is triturated for shorter than normal times, mercury does not completely wet the outer surface of amalgam particles. As a result, mercury does not react with the amalgam alloy over the entire surface of the particle. The mass remains soft for a longer period of time, producing an amalgam with a longer working time. Such an amalgam mass contains excessive amounts of porosity, has lower strength, and possesses poorer corrosion resistance.
Overtrituration reduces working time, causing the reaction rate to increase because the amalgamated mass becomes hot. When amalgams with longer or shorter working times are desired, one should use amalgam alloys that are designed to react faster or slower and not attempt to achieve the change by altering the trituration time.
CONDENSATION OF AMALGAM
During condensation, adaptation of the amalgam mass to the cavity walls is accomplished and the operator controls the amount of mercury that will remain in the finished restoration, which in turn influences the dimensional change, creep, and compressive strength. In general, the more mercury left in the mass after condensation, the weaker the alloy. With irregularly shaped alloys, in which a higher percentage of mercury is used initially, the operator should remove as much mercury as possible during condensation by using as great a force as possible on the condenser. With spherical alloys, the amount of mercury supplied in the capsules is lower, and it is not necessary to remove as much mercury as for the irregularly shaped alloys; however, increasing the condensation pressure from 3 to 7 MPa results in a significant increase in compressive strength. Further increase in condensation pressure to 14 MPa does not result in additional compressive strength.
Hand or Mechanical Condensation
A large number of instruments designed for hand condensation of amalgam have been available to the dental profession for many years. The instruments and the techniques for their use have been described in textbooks of operative dentistry.
In general, a suitable instrument for hand condensation of amalgam would be shaped so that the operator could readily grasp it and exert a force of condensation by appropriately placing one finger on a finger rest of the instrument. Hand instruments that do not permit a convenient grasping may inhibit proper condensation practices and mercury removal. In many instances, circular condenser tips may prove adequate, whereas in other cavity areas and designs, the triangular, oval, crescent, or other shape of tip may be effective. In general, a condenser tip that is too small in cross-section tends to be ineffective in condensing a reasonable quantity of amalgam. The size of the condenser tip and the direction and magnitude of the force placed on the condenser also depend on the type of amalgam alloy selected.
With irregularly shaped alloys, one should use condensers with a relatively small tip, 1 to 2 mm, and apply high condensation forces in a vertical direction. During condensation, as much mercury-rich mass as possible should be removed from the restoration.
When condensers with small tips are used with high condensation forces on spherical amalgams, the particles tend to roll over one another, the tip penetrates the amalgam, and the mass does not adapt well to the cavity walls. With spherical alloys one should use condensers with larger tips, almost as large as the cavity permits. For example, at the cervical margin of a Class 2 preparation with a small opening, a condenser with a very small tip should be used. As the cavity is filled and the opening toward the occlusal surface becomes larger, condensers with larger tips should be used. Because of the spherical shape of the particles, a lateral direction of condensation provides better adaptation of amalgam to cavity walls than of condensation toward the pulpal floor. With high-copper spherical amalgams, a vertical and lateral direction of condensation with vibration is recommended.
Small- to medium-diameter condensers are advocated with admixed high-copper alloys with a medium to high force and vertical and lateral directions of condensation.
Many mechanical devices are available for condensing amalgam. These devices are more popular and more useful for condensing irregularly shaped alloys when high condensation forces are required. With the development of spherical alloys, the need for mechanical condensers was eliminated. Ultrasonic condensers are not recommended because during condensation they increase the mercury vapor level to values above the safety standards for mercury in the dental office.
Effect of Delay in Condensation
It is important that an amalgam be condensed into the tooth cavity promptly after the mercury and alloy are suitably mixed. Delay of the condensation operation permits the amalgam to set partially before being transferred to the cavity. A delay in the condensation operation with a partial reaction of the mercury and alloy makes it impossible to remove the mercury effectively during condensation. As a result, an amalgam mass that has remained uncondensed for any period of time will contain more mercury than one that is condensed promptly. The resulting amalgam with the additional mercury content will show less strength in compression and higher creep. Delay in the condensation operation reduces the plasticity of the mix, and amalgams with reduced plasticity do not adapt well to the cavity walls. In a large restoration involving considerable time to place the amalgam mass, condensation of the final portions of amalgam becomes a problem. In such cases, it is preferable to make two smaller mixes of amalgam rather than one excessively large mix and not to use the amalgam if more than 3 or 4 minutes have elapsed from the time of initial mixing.
Mercury Content of Amalgam Restorations
Amalgam restorations containing greater amounts of mercury in the set mass demonstrate less favorable clinical characteristics. Having more mercury in the set amalgam produces a greater amount of Ag2Hg3 and Sn7–8Hg, the γ1 and γ2 phases, thereby leaving less unreacted Ag3Sn, the γ phase. As discussed earlier, both γ1 and γ2 have lower strength than the γ phase. Therefore, when amalgam specimens are subjected to compressive stress, those containing increasing quantities of mercury exhibit decreasing strength values. The compressive strength decreases 1% for each 1% increase in mercury above 60%.
The mercury content of an amalgam restoration is not uniform throughout. Higher concentrations of mercury are located around the margins of the restoration. As a result, cavities should be overfilled and then carved back to minimize this problem. When using alloys that require higher mercury/alloy ratios, as much mercury as possible should be removed from the amalgamated mass. Note that the maximum allowable amount of mercury remaining in a hardened amalgam mass depends on the original mercury/alloy ratio. In other words, for alloys requiring high mercury/alloy ratios for trituration, 50% mercury in the hardened amalgam might be acceptable; however, for alloys needing low mercury/alloy ratios for trituration, 50% mercury in the set amalgam would be detrimental.
Although the lower mercury/alloy ratios currently being used are favorable regarding the total quantity of mercury in the set mass, remember that condensation forces alter mercury content within the restoration. Because condensation brings mercury to the surface of the amalgam mass, such “plashy” material should be periodically removed when filling the cavity to prevent trapping high mercury concentrations within the restoration. Overfilling of the cavity is carried out for the same reason, that is, to remove the amalgam that contains higher mercury content from the restoration contour.
When alloys that permit lower mercury/alloy ratios are used to obtain a plastic mass suitable for condensation, the operator should expect a lesser volume of excess mercury to be brought to the surface for removal than was observed with older materials.
Moisture Contamination During Insertion
Moisture contamination during the mixing and condensing operations is the factor that may produce excessive expansion. There is no evidence, however, that the presence of moisture on the surface will cause any serious damage once the condensation operation is completed and the restoration is finished, except for trimming and polishing.
Because moisture in the saliva is a potential source of contamination for the amalgam, the tooth cavity must remain dry and the amalgam must be free from saliva contamination. Techniques and procedures in operative dentistry provide for an isolated field of operation, and these techniques should be followed to gain the best properties of the set amalgam.
With zinc-containing amalgam, the presence of saliva on the amalgam during condensation probably was a principal source of excessive delayed expansion and other poor qualities in the restoration. Moisture contamination of a zinc-containing amalgam mass from any source results in an excessive delayed expansion of several hundred micrometers per centimeter after the restoration has been placed in the tooth for several hours or days. This excessive expansion results from the decomposition of moisture. The trapped hydrogen gas in the amalgam restoration continues to be developed until sufficient force is produced to cause the excessive expansion. This decomposition of moisture results from the presence of zinc in the amalgam alloy and can be overcome by the use of non-zinc alloys.
FACTORS RELATED TO FINISHING AMALGAM RESTORATIONS
When an amalgam restoration has been properly placed, with adequate condensation, and the excess mercury has been removed from the final surface layer of the restoration, it will be sufficiently hardened within a few minutes to permit careful carving. If the restoration is not well condensed, it will not harden promptly, and the carving operation must be delayed. Usually the amalgam is sufficiently well set and hardened that carving with sharp instruments can be started almost immediately after condensation.
Burnishing, or rubbing the newly condensed amalgam with a metal instrument having a broad surface contact, can be employed to smooth the surface, thereby making the amalgam more susceptible to finishing and polishing. Burnishing can produce a tenfold reduction in surface roughness.
If final finishing and polishing are to be done at a second appointment, the restoration should be left undisturbed for a period of at least 24 hours. The patient should be cautioned that the freshly inserted restoration is relatively weak and that heavy biting forces should be avoided for a few hours after the time of insertion. Occlusal contacts must be carefully established. However, current all-spherical high-copper alloys have a much higher early strength than other types and can withstand biting forces sooner than earlier amalgams. One-hour compressive strengths of spherical high-copper alloys are about twice as high as high-copper admixed types and are comparable with those of low-copper alloys at 6 to 7 hours.
High-copper unicompositional amalgams with high early strengths can be finished at the first appointment. After condensation the surface is burnished and carved for clear definition of the margins, and all excess amalgam is removed. A creamy paste of triple-x silex and water is applied gently with an unwebbed rubber cup and a slow-speed handpiece. Light pressure should be applied for no more than 30 seconds per surface, and polishing should be directed from the center toward the margins of the restoration.
This early finishing begins 8 to 10 minutes after the start of trituration, depending on the particular alloy. Results of a 3-year clinical study have shown that restorations polished 8 minutes after trituration and those polished after 24 hours had no difference in longevity. Also, as time in the mouth increased, it became difficult to determine which method had been used to finish the restoration. The 24-hour polishing procedure used in the study was that normally used for polishing amalgam restorations. The procedure used for the 8-minute polish was different; no polishing bur was used, and the amalgam was carved carefully. Because the 24-hour polishing technique requires a second appointment, many restorations go without polishing. The main advantage of the 8-minute polishing technique is the elimination of the second appointment. This technique is limited to those amalgams that have high early-compressive strengths.
A well-finished and well-polished restoration will retain its surface appearance and be easier to keep clean than one that is poorly finished, because a rough surface on the restoration contains microscopic pits in which acids and small food particles from the mouth accumulate. These pits tend to encourage galvanic action on the surface of the restoration, leading to tarnish and perhaps even a corroded appearance.
The final polish at a second appointment is developed through a series of final finishing and polishing steps after a careful carving operation. This final polish is accomplished through a sequence of operations that includes the use of fine stones and abrasive disks or strips. To develop the final polish, a rotating soft brush is used to apply a suitable polishing agent, such as extrafine silex, followed by a thin slurry of tin oxide.
During the final polishing operation, the restoration should remain moist to avoid overheating from the use of dry polishing surfaces. Because the amalgam is weak in tension and shear resistance, it should not be drawn over the margin by burnishing or drawing operations that tend to produce extensions that subsequently will be fractured from the amalgam mass. To avoid such overextensions, all recommended operative practices should be followed faithfully.
BONDING OF AMALGAM
Although amalgam has been a highly successful restorative material when used as an intracoronal restoration, it does not restore the strength of the clinical crown to its original strength. Additional features, such as pins, slots, holes, and grooves to increase retention of the restoration, must be supplied with the preparations for large amalgam restorations, but they do not reinforce the amalgam or increase its strength.
With the development of adhesive systems for dental composites came the opportunity to attempt to bond amalgams to tooth structure. Bonding agents containing 4-META, an acronym for 4-methacryloxyethyl trimellitic anhydride (see Chapter 10), have been the most successful products. Shear bond strengths of amalgam to dentin as high as 10 MPa have been reported using these adhesives. Comparable values for the shear bond strength of microfilled composites to dentin using these same adhesives have been 20 to 22 MPa. The fracture resistance of teeth restored with amalgam-bonded MOD restorations was more than twice that of restorations containing unbonded amalgams. Also, in spite of the lower shear strength of amalgam-bonded-to-dentin test specimens compared with composites, the fracture strength of MODs in teeth restored with bonded amalgams was as high as for composites, although neither were as high (45% to 80%) as values for the intact tooth. As expected, amalgam-bonded MODs with narrow preparations had higher strengths than those with wide preparations. Other studies showed the retention of amalgam-bonded MODs with proximal boxes was as great as pin-retained amalgams. In addition, amalgam-bonded restorations decreased marginal leakage in Class 5 restorations compared with unbonded amalgams. Finally, the bonding agents for amalgam have not been successful in increasing the amalgam-to-amalgam bond strength in the repair of amalgam restorations. Thus at this stage of development, adhesive bonding of amalgam restorations to tooth structures is an improvement over nonbonded amalgams.
MERCURY AND BIOCOMPATIBILITY ISSUES
Amalgams have been used for 150 years; about 200 million amalgams are inserted each year in the United States and Europe. In spite of its substantial history, however, periodically concern arises about the biocompatibility of amalgam. Allergic reactions to mercury in amalgam restorations do occur, albeit infrequently. This is not surprising, because there is no material that 100% of the population is immune to 100% of the time. However, such allergic responses usually disappear in a few days or, if not, on removal of the amalgam. Aside from varying reports of mercury accumulation, no other local or systemic effects from mercury contained in dental amalgam have been demonstrated. If amalgam is used correctly, biocompatibility should not be a problem.
Even in their passive condition, metals are not inert. In vitro and in vivo experiments have established that there is a passive dissolution from all metals. The following eight questions are linked to the issues of dissolution, corrosion, and potential allergic response and toxicity:
1. Is any material released into the mouth?
3. What is the form of the released material?
4. How much material is released?
5. In what subsequent reactions do the released products get involved?
6. What percentage of the released products is excreted and what percentage is retained?
7. Where does the retained percentage accumulate?
8. What biological responses will result from the retained fraction?
Therefore, any analysis of the literature and discussion of mercury toxicity in amalgams must continually refer to these eight questions, particularly questions 3 and 4, relating to the dosage and form of the mercury to which the body is exposed.
SOURCES OF MERCURY
In addressing these eight questions, the sources of the potential toxins must be evaluated. Exposure to mercury can occur from many different sources, including diet, water, air, and occupational exposure (Table 11-4). The World Health Organization (WHO) has estimated that eating seafood once a week raises urine mercury levels to 5 to 20 μg/L, two to eight times the level of exposure from amalgam (1 μg/L = 1 mg/m3 = 1 part per billion [ppb]). Thus, the amount of mercury vapor released from amalgam is less than that received from eating many common fish. It has been estimated that a patient with nine amalgam occlusal surfaces will inhale daily only about 1% of the amount the Occupational Safety and Health Administration (OSHA) allows to be inhaled in the workplace. Blood and urine mercury levels are easily influenced by other factors and cannot often be directly linked to amalgam. In general, elemental mercury from amalgam seems to make only a small contribution to the total body burden of mercury. On the basis of epidemiological studies, blood and serum mercury levels correlate highly with occupational exposure and diet, whereas urine mercury relates to amalgam burden. Urine mercury levels relate to methods of condensation and ventilation more than to the amalgam per se.
FORMS OF MERCURY
Mercury has many forms, including organic and inorganic compounds. The most toxic organic compounds are methyl and ethyl mercury, and the next most toxic form is mercury vapor. The least toxic forms of mercury are the inorganic compounds. Liquid mercury reacts with silver to form an inorganic silver-mercury compound via a metallic bond. Reports of people and animals being poisoned by eating food high in mercury are traced to the contamination of these foods by methyl mercury.
Mercury vapor is released, in minute quantities, during all procedures involving amalgam, including mixing, setting, polishing, and removal. Mercury vapor has also been reported to be released during mastication and drinking of hot beverages. The amount of mercury on amalgam surfaces has correlated with the quantity of mercury used during trituration. However, measuring the flow and flow rate is difficult and not precise, especially when working with a small area such as the mouth. Furthermore, ambient mercury must be considered, especially if such readings are taken in a dentist’s office. With good ventilation, mercury levels return to background levels 10 to 20 minutes after placing an amalgam, and a charcoal filter system decreases levels 25% during the operative procedure. Fresh amalgams release more mercury than 2-year-old amalgams even with a Streptococcus mutans biofilm, and it has been shown that most oral organisms can grow in dental plaque containing 2 μg of mercury. Under normal conditions amalgam is covered by saliva, tending to reduce vapor pressure. Amalgams can also be constrained with a sealant resin for the first several days after insertion. Adding indium (8% to 14%) also decreases the vapor pressure.
CONCENTRATIONS OF MERCURY
OSHA has set a Threshold Limit Value (TLV) of 0.05 mg/m3 as the maximum amount of mercury vapor allowed in the workplace. Nearly all dental offices worldwide comply with this standard. As an example of the factor of safety in this boundary, the fetuses of pregnant rats exposed to atmospheres with mercury concentrations of 2 mg/m3 showed no ill effects. Fetuses exposed to mercury concentrations of 5 mg/m3, or 40 times the allowable concentration, were stillborn. The lowest dose of mercury that elicits a toxic reaction is 3 to 7 μg/kg body weight. Paresthesia (tingling of extremities) occurs at about 500 μg/kg of body weight, followed by ataxia at 1000 μg/kg of body weight, joint pain at 2000 μg/kg of body weight, and hearing loss and death at 4000 μg/kg of body weight. Therefore these values are much greater in magnitude than the exposure to mercury from amalgam or from a normal diet.
Mercury in Urine
The body cannot retain metallic mercury, but passes it through the urine. By using radioactive mercury in amalgams, it is possible to monitor the mercury levels in urine caused only by dental amalgams. One study showed that urine mercury levels peak at 2.54 μg/L 4 days after placing amalgams and, after 7 days return to zero. On removal of amalgam, urine mercury levels reach a maximum value of 4 μg/L and return to zero after a week. Although mercury is readily cleared in both cases, peak urine levels of mercury are nearly twice as great when amalgam is removed rather than inserted. The same is true for mercury vapor, with higher levels recorded on removal of an amalgam than on insertion. Other studies, using more sensitive techniques such as atomic absorption spectroscopy, show conflicting findings. There are reports demonstrating no increase in urine mercury levels and reports showing higher levels. Even in those cases in which urine mercury is elevated, the concentrations are still less than 1 μg/L.
As a comparison, consider the WHO estimate that eating seafood once a week will raise urine mercury to 5 to 20 μg/L, or two to eight times the level of exposure from amalgam determined in the study just cited. Neurological changes are not detected until urine mercury levels exceed 500 μg/L, nearly 170 times the peak levels found on insertion of an amalgam.
Mercury in Blood
The maximum allowable level of mercury in the blood is 3 μg/L. Several studies have shown that freshly placed amalgam restorations elevate blood mercury levels to 1 to 2 μg/L. Removal of amalgam decreases blood mercury levels, with a half-time of approximately 1 to 2 months for elimination of mercury. However, as with urine mercury levels, there is first an increase of around 1.5 μg/L, which decreases in about 3 days. One study monitored blood mercury levels for a year and showed that patients with amalgams had a lower than average blood mercury level (0.6 μg/L) than patients without amalgams (0.8 μg/L). Presumably the blood mercury level is easily influenced by other factors and therefore cannot be explicitly related to amalgam. Evidently, a relationship exists between plasma and urine mercury levels.
Another study showed that patients with and without amalgams do not differ in the mean number or percentage of lymphocytes. Some studies have shown the blood mercury levels of dentists to be normal, whereas others report an increase. For those studies that indicate higher blood mercury levels in dentists, results have varied regarding any correlation between mercury concentration and number of amalgams placed. Elevated blood mercury levels may relate to mercury spills in the office, a factor that can easily be controlled. Both blood and serum mercury levels seem to correlate best with occupational exposure and not with the number of amalgams or length of time with amalgams in place.
Release of Corrosion Products
Mercury release into various media, including water, saline, buffered citric and phosphoric acid, and synthetic saliva, has been measured by a number of techniques, such as atomic emission spectroscopy and atomic absorption spectroscopy. Ion release tends to be greatest in the first 1 to 24 hours after trituration. Once the amalgam is fully set, ionic dissolution is very low. This reduction in ion flux with time probably results from a combination of the chemical reaction progressing further and the formation of a passive surface film. In general, low-copper alloys release more ions than high-copper alloys, because of their inferior corrosion resistance. Greater amounts of mercury and silver are released from unpolished specimens than from polished specimens.
The effect of electrolytic concentration on corrosion has been compared for conventional and high-copper admixed alloys following storage for 4 months. The main corrosion products were tin compounds at the surface of the amalgams. Low-copper amalgam showed surface corrosion only, whereas subsurface corrosion occurred with high-copper amalgam, especially following immersion in an NaCl solution without phosphate. For low-copper amalgam, the release of elements decreased with time, possibly indicating passivation. For high-copper amalgam, the release of elements increased with time, except for copper and tin in a solution with a high concentration of phosphate, indicating that phosphate inhibits corrosion of the copper-tin phases. Other studies have revealed a tendency for tin and copper to be preferentially released from amalgam. Presumably, tin release originates from surface corrosion, whereas copper release results from subsurface corrosion. Stronger galvanic influences enhance copper release and, to a lesser extent, zinc release. Tin tends to provide a passive layer and to suppress the dissolution of mercury. It is suspected that indium functions similarly. In zinc-free alloys, the tin oxide is mercury depleted.
Another recent study has shown that following 1 week of aging in 0.9% NaCl solution at 37° C, the amount of mercury released from γ1 was 14 to 60 times that released from amalgam and 5 times that released from β1. The γ2 phase released the least amount of mercury.
ALLERGIC REACTIONS AND DISEASE
Allergic reactions to mercury in amalgam restorations are rare, although there are case reports of allergic contact dermatitis, gingivitis, stomatitis, and remote cutaneous reactions. Such responses usually disappear on removal of the amalgam. Other local or systemic effects from mercury contained in dental amalgam have not been demonstrated. No wellconducted scientific study has conclusively shown that dental amalgam produces any ill effects.
Random reports of various diseases, such as multiple sclerosis, cannot unequivocally link the diseases to amalgams and therefore must be interpreted with caution. Reports of multiple sclerosis patients being instantly cured when amalgam is removed cannot be upheld scientifically. Because a week must pass for all mercury to be cleared by the body, an instantaneous recovery after removing the potential source of the mercury is unlikely.
Local Reactions
In patients with oral lesions near amalgam sites, positive patch tests have been reported. However, the appropriate patch test has still not been determined, and many of the materials used for patch testing contain excessive concentrations of mercury. There are also reports of inflammatory reactions of the dentin and pulp, similar to the reactions to many other restorative materials. Mercury has been found in the lysosomes of macrophages and fibroblasts in some patients with lesions. Inflammation can usually be alleviated with a cavity liner. With the increased use of more corrosion-resistant amalgams, the volume of corrosion products and subsequent reactions are reduced.
Macrophages play an important role in the removal of foreign particulate matter from tissue. A number of cell culture studies have assessed the potential cytotoxicity of amalgam and its constituents. Unreacted mercury or copper leaching out from high-copper alloys has usually been the constituent leading to adverse responses. An in vitro study of the effects of particulate amalgams and their individual phases on macrophages showed that all particles except the γ2 are effectively phagocytized by macrophages. Cell damage was seen in treated cultures exposed to particulate γ1.
Systemic Reactions
Implantation studies have shown that amalgam is reasonably well tolerated by soft and hard tissue. In a rabbit muscle implantation model, biological reactions to amalgams were found to depend on the time of implantation. All amalgams were strongly toxic 1 hour after setting. After 7 days, only high-copper amalgam showed any reaction.
In another series of studies, low- and high-copper amalgam powders and various phases of amalgam were implanted subcutaneously in guinea pigs. The result was a mild, early inflammatory response, in which particles were taken up by macrophages and giant cells. After 1.5 to 3 months, chronic granulomas developed. With low-copper amalgam, early changes occurred in the intracellular material, associated with the rapid degradation of the γ2 phase. Later, intracellular particles from both low- and high-copper amalgam underwent progressive degradation, producing fine secondary particles containing silver and tin, which were distributed throughout the lesions and gave rise to macroscopic tattooing of the skin. Secondary material and small, degrading, primary particles from both types of amalgam were detected in the submandibular lymph nodes.
Elevated mercury levels were detected in the blood, bile, kidneys, liver, spleen, and lungs, with the highest concentrations found in the renal cortex. Mercury was excreted in the urine and feces. Mercury levels in the blood, liver, renal cortex, and feces were lower with the high-copper amalgam.
Black, refractile particulate deposits approximately 1 to 3 μm in diameter were found in the cytoplasm and nuclei of kidney cells. The ratio of nuclear to cytoplasmic deposits was higher in animals receiving high-copper amalgam. The cytoplasmic deposits consisted of collections of fine particles within lysosomes. Both lysosomal and nuclear deposits contained mercury and selenium, which were present in the animals’ diet at low levels. Neither this study nor others have demonstrated any changes in biochemical function of any of the laden organs.
Subcutaneous implantation of only the powdered γ2 phase led to a limited initial release of mercury from extracellular material. Thereafter, chronic granulomas developed around the implants, and particles degraded slowly in macrophages and giant cells. Fine secondary particles containing tin were produced. Subcutaneous implantation of only the powdered γ1 phase induced a severe initial tissue response, and the majority of the material was extruded from the healing wounds. This process was accompanied by the release of significant amounts of mercury, which appeared in the body organs and excreta. The small numbers of particles remaining in the tissues underwent a slow degradation in macrophages and giant cells in chronic granulomas. Minute secondary particles containing silver and sulfur were deposited in the tissues and gave rise to macroscopic tattooing of the skin above the implants.
In another study, primates received occlusal amalgam fillings or maxillary bone implants of amalgam for 1 year. Amalgam fillings caused deposition of mercury in the spinal ganglia, anterior pituitary, adrenal, medulla, liver, kidneys, lungs, and intestinal lymph glands. Maxillary amalgam implants released mercury into the same organs, except for the liver, lungs, and intestinal lymph glands. Organs from control animals were devoid of precipitate.
Note that studies on powders probably overestimate the amount of breakdown products, and therefore biological response, because the surface area of powders can be 5 to 10 times the surface area of a solid component. It must also be emphasized that any reaction to amalgam, whether in cell culture, local tissue response, or systemic response, does not necessarily imply a reaction to mercury. Such reactions could be in response to some other constituent of the amalgam or corrosion product. For example, in vitro cell culture testing that measured fibroblasts affected by various elements and phases of amalgams has shown that pure copper and zinc show greater cytotoxicity than pure silver and mercury. Pure tin has not been shown to be cytotoxic (Fig. 11-14). The γ1 phase is moderately cytotoxic. Cytotoxicity is decreased by the addition of 1.5% and 5% tin (Fig. 11-15). However, the addition of 1.5% zinc to γ1 containing 1.5% tin increases cytotoxicity to the same level as that of pure zinc. Whenever zinc is present, higher cytotoxicity is revealed. High-copper amalgams show the same cytotoxicity as a zinc-free, low-copper amalgam. The addition of selenium does not reduce amalgam cytotoxicity, and excessive additions of selenium increase cytotoxicity. The cytotoxicity of amalgams decreases after 24 hours, possibly from the combined effects of surface oxidation and further amalgamation. The results of this study suggest that the major contributor to the cytotoxicity of amalgam alloy powders is probably copper, whereas that for amalgam is zinc.
RISKS TO DENTISTS AND OFFICE PERSONNEL
Of the two groups of people (i.e., patients and dental office personnel) potentially at risk to mercury exposure with dental amalgam, the dental office personnel are at greater risk because of frequent handling of the freshly mixed material. The concern with the potential for mercury toxicity therefore centers primarily on dental office personnel. The blood mercury levels of dentists have been shown to be normal in several studies, especially when the following recommendations in mercury hygiene are followed:
1. Store mercury in unbreakable, tightly sealed containers.
2. To confine and facilitate the recovery of spilled mercury or amalgam, perform all operations involving mercury over areas that have impervious and suitably lipped surfaces.
3. Clean up any spilled mercury immediately. Droplets may be picked up with narrow-bore tubing connected (via a wash-bottle trap) to the low-volume aspirator of the dental unit.
4. Use tightly closed capsules during amalgamation.
5. Use a no-touch technique for handling the amalgam.
6. Salvage all amalgam scrap and store it under water that contains sodium thiosulfate (photographic fixer is convenient).
7. Work in well-ventilated spaces.
8. Avoid carpeting dental operatories; decontamination of carpeting is very difficult.
9. Eliminate the use of mercury-containing solutions.
10. Avoid heating mercury or amalgam.
11. Use water spray and suction when grinding dental amalgam.
12. Use conventional dental amalgam condensing procedures, manual and mechanical, but do not use ultrasonic amalgam condensers.
13. Perform yearly mercury determinations on all personnel regularly employed in dental offices.
14. Determine mercury vapor levels in operatories periodically.
15. Alert all personnel who handle mercury, especially during training or indoctrination periods, of the potential hazard of mercury vapor and the necessity for observing good mercury and amalgam hygiene practices.
Allen, EP, Bayne, SC, Becker, IM, et al. Annual review of selected dental literature: report of the committee on scientific investigation of the American Academy of Restorative Dentistry. J Prosthet Dent. 1999;82:54.
Allen, EP, Bayne, SC, Donovan, TE, et al. Annual review of selected dental literature. J Prosthet Dent. 1996;76:82.
Bryant, RW. γ2 Phase in conventional dental amalgams—discrete clumps or continuous network? A review. Aust Dent J. 1984;29:163.
De Rossi, SS, Greenberg, MS. Intraoral contact allergy: a literature review and case reports. J Am Dent Assoc. 1998;129:1435.
Halbach, S. Amalgam tooth fillings and man’s mercury burden [Review]. Human Exper Toxicol. 1994;13:496.
Jendresen, MD, Allen, EP, Bayne, SC, et al. Annual review of selected dental literature: report of the committee on scientific investigation of the American Academy of Restorative Dentistry. J Prosthet Dent. 1998;80:109.
Jendresen, MD, Allen, EP, Bayne, SC, et al. Annual review of selected dental literature: report of the committee on scientific investigation of the American Academy of Restorative Dentistry. J Prosthet Dent. 1997;78:82.
Jendresen, MD, Allen, EP, Bayne, SC, et al. Annual review of selected dental literature: report of the committee on scientific investigation of the American Academy of Restorative Dentistry. J Prosthet Dent. 1995;74:83.
Lloyd, CH, Scrimgeour, SN. Dental materials: 1994 literature review. J Dent. 1996;24:159.
Lloyd, CH, Scrimgeour, SN. Dental materials: 1995 literature review. J Dent. 1997;25:180.
Mitchell, RJ, Okabe, T. Setting reactions in dental amalgam: part 1. Phases and microstructures between one hour and one week [Review]. Crit Rev Oral Biol and Med. 1996;7:12.
Okabe, T, Mitchell, RJ. Setting reactions in dental amalgam: part 2. The kinetics of amalgamation [Review]. Crit Rev Oral Biol Med. 1996;7:23.
Strang, R, Whitters, CJ, Brown, D, et al. Dental materials: 1996 literature review. J Dent. 1998;26:196.
Whitters, CJ, Strang, R, Brown, D, et al. Dental materials: 1997 literature review. J Dent. 1999;27:407.
Allan, FC, Asgar, K, Peyton, FA. Micro-structure of dental amalgam. J Dent Res. 1965;44:1002.
Asgar, K. Amalgam alloy with a single composition behavior similar to Dispersalloy. J Dent Res. 1974;53:60.
Asgar, K, Sutfin, L. Brittle fracture of dental amalgam. J Dent Res. 1965;44:977.
Baran, G, O’Brien, WJ. Wetting of amalgam alloys by mercury. J Am Dent Assoc. 1977;94:898.
Boyer, DB, Edie, JW. Composition of clinically aged amalgam restorations. Dent Mater. 1990;6:146.
Branstromm, M, Astrom, A. The hydrodynamics of the dentine: Its possible relationship to dentinal pain. Int Dent J. 1972;22:219.
Brockhurst, PJ, Culnane, JT. Organization of the mixing time of dental amalgam using coherence time. Aust Dent J. 1987;32:28.
Brown, IH, Maiolo, C, Miller, DR. Variation in condensation pressure during clinical packing of amalgam restorations. Am J Dent. 1993;6:255.
Brown, IH, Miller, DR. Alloy particle shape and sensitivity of high-copper amalgams to manipulative variables. Am J Dent. 1993;6:248.
Corpron, R, Straffon, L, Dennison, J, et al. Clinical evaluation of amalgams polished immediately after insertion: 5-year results. J Dent Res. 1984;63:178.
Dunne, SM, Gainsford, ID, Wilson, NH. Current materials and techniques for direct restorations in posterior teeth. Part 1: silver amalgam. Int Dent J. 1997;47:123.
Farah, JW, Hood, JAA, Craig, RG. Effects of cement bases on the stresses in amalgam restorations. J Dent Res. 1975;54:10.
Farah, JW, Powers, JM. High copper amalgams. Dent Advis. 1987;4(2):1.
Farah, JW, Powers, JM. Dental amalgam and mercury. Dent Advis. 1991;8(2):1.
Gottlieb, EW, Retief, DH, Bradley, EL. Microleakage of conventional and high copper amalgam restorations. J Prosthet Dent. 1985;53:355.
Hero, H. On creep mechanisms in amalgam. J Dent Res. 1983;62:44.
Jensen, SJ, Jørgensen, KD. Dimensional and phase changes of dental amalgam. Scand J Dent Res. 1985;93:351.
Johnson, GH, Bales, DJ, Powell, LV. Clinical evaluation of high-copper dental amalgams with and without admixed indium. Am J Dent. 1992;5:39.
Johnson, GH, Powell, LV. Effect of admixed indium on properties of a dispersed phase high-copper dental amalgam. Dent Mater. 1992;8:366.
Jørgensen, KD. The mechanism of marginal fracture of amalgam fillings. Acta Odont Scand. 1965;23:347.
Jørgensen, KD, Esbensen, AL, Borring-Moller, G. The effect of porosity and mercury content upon the strength of silver amalgam. Acta Odont Scand. 1966;24:535.
Jørgensen, KD, Wakumoto, S. Occlusal amalgam fillings; marginal defects and secondary caries. Odont Tskr. 1968;76:43.
Katz, JL, Grenoble, DE. A composite model of the elastic behavior of dental amalgam. J Biomed Mater Res. 1971;5:515.
Kawakami, M, Staninec, M, Imazato, S, et al. Shear bond strength of amalgam adhesives to dentin. Am J Dent. 1994;7:53.
Leinfelder, KF. Dental amalgam alloys. Curr Opin Dent. 1991;1:214.
Letzel, H, Van’t Hof, MA, Marshall, GW, et al. The influence of the amalgam alloy on the survival of amalgam restorations: a secondary analysis of multiple controlled clinical trials. J Dent Res. 1997;76:1787.
Lloyd, CH, Adamson, M. Fracture toughness (KlC) of amalgam. J Oral Rehabil. 1985;12:59.
Mahler DB: Amalgam, International State-of-the-Art Conference on Restorative Dental Materials, Bethesda, Md, 1986.
Mahler, DB. Slow compressive strength of amalgam. J Dent Res. 1972;51:1394.
Mahler, DB. The high-copper dental amalgam alloys. J Dent Res. 1997;76:537.
Mahler, DB, Adey, JD. Factors influencing the creep of dental amalgam. J Dent Res. 1991;70:1394.
Mahler, DB, Adey, JD, Marantz, RL. Creep versus microstructure of gamma 2 containing amalgams. J Dent Res. 1977;56:1493.
Mahler, DB, Adey, JD, Marek, M. Creep and corrosion of amalgam. J Dent Res. 1982;61:33.
Mahler, DB, Adey, JD, Marshall, SJ. Effect of time at 37 degrees C on the creep and metallurgical characteristics of amalgam. J Dent Res. 1987;66:1146.
Mahler, DB, Marantz, RL, Engle, JH. A predictive model for the clinical marginal fracture of amalgam. J Dent Res. 1980;59:1420.
Mahler, DB, Nelson, LW. Factors affecting the marginal leakage of amalgam. J Am Dent Assoc. 1984;108:50.
Mahler, DB, Nelson, LW. Sensitivity answers sought in amalgam alloy microleakage study. J Am Dent Assoc. 1984;125:282.
Mahler, DB, Terkla, LG, van Eysden, J, et al. Marginal fracture vs mechanical properties of amalgam. J Dent Res. 1970;49:1452.
Mahler, DB, van Eysden, J, Terkla, LG. Relationship of creep to marginal fracture of amalgam. J Dent Res. 1975;54:183.
Malhotra, ML, Asgar, K. Physical properties of dental silver-tin amalgams with high and low copper contents. J Am Dent Assoc. 1978;96:444.
Martin, JA, Bader, JD. Five-year treatment outcomes for teeth with large amalgams and crowns. Oper Dent. 1997;22:72.
McCabe, JF, Carrick, TE. Dynamic creep of dental amalgam as a function of stress and number of applied cycles. J Dent Res. 1987;66:1346.
Meletis, EI, Gibbs, CA, Lian, K. New dynamic corrosion test for dental materials. Dent Mater. 1989;5:411.
O’Brien, WJ, Greener, EH, Mahler, DB. Dental amalgam. In: Reese JA, Valega TM, eds. Restorative dental materials: an overview. London: Quintessence, 1985.
Ogura, H, Miyagawa, Y, Nakamura, K. Creep and rupture of dental amalgam under bending stress. Dent Mater J. 1989;8:65.
Osborne, JW, Gale, EN. Failure at the margin of amalgams as affected by cavity width, tooth position, and alloy selection. J Dent Res. 1981;60:681.
Papathanasiou, AG, Curzon, ME, Fairpo, CG. The influence of restorative material on the survival rate of restorations in primary molars. Paediatr Dent. 1994;16:282.
Powers, JM, Farah, JW. Apparent modulus of elasticity of dental amalgams. J Dent Res. 1975;54:902.
Ryge, G, Telford, RF, Fairhurst, CW. Strength and phase formation of dental amalgam. J Dent Res. 1957;36:986.
Sarkar, NK, Eyer, CS. The microstructural basis of creep of gamma 1 in dental amalgam. J Oral Rehabil. 1987;14:27.
Smales, RJ, Hawthorne, WS. Long-term survival of extensive amalgams and posterior crowns. J Dent. 1997;25:225.
Watkins, JH, Nakajima, H, Hanaoka, K, et al. Effect of zinc on strength and fatigue resistance of amalgam. Dent Mater. 1995;11:24.
Wilson, NH, Wastell, DC, Norman, RD. Five-year performance of high-copper content amalgam restorations in a multiclinical trial of a posterior composite. J Dent. 1996;24:203.
Wing, G, Ryge, G. Setting reactions of spherical-particle amalgam. J Dent Res. 1965;44:1325.
Young, FA, Jr., Johnson, LB. Strength of mercury-tin phase in dental amalgam. J Dent Res. 1967;46:457.
Zardiackas, LD, Anderson, L, Jr. Crack propagation in conventional and high copper dental amalgam as a function of strain rate. Biomaterials. 1986;7:259.
Barkmeier, WW, Gendusa, NJ, Thurmond, JW, et al. Laboratory evaluation of Amalgambond and Amalgambond Plus. Am J Dent. 1994;7:239.
Ben-Amar, A, Liberman, R, Rothkoff, Z, et al. Long term sealing properties of Amalgambond under amalgam restorations. Am J Dent. 1994;7:141.
Boyer, DB, Roth, L. Fracture resistance of teeth with bonded amalgams. Am J Dent. 1994;7:91.
Eakle, WS, Staninec, M, Yip, RL, et al. Mechanical retention versus bonding of amalgam and gallium alloy restorations. J Prosthet Dent. 1994;72:351.
Edgren, BN, Denehy, GE. Microleakage of amalgam restorations using Amalgambond and Copalite. Am J Dent. 1992;5:296.
Fischer, GM, Stewart, GP, Panelli, J. Amalgam retention using pins, boxes, and Amalgambond. Am J Dent. 1993;6:173.
Hadavi, R, Hey, JH, Strasdin, RB, et al. Bonding amalgam to dentin by different methods. J Prosthet Dent. 1994;72:250.
Ianzano, JA, Mastrodomenico, J, Gwinnett, AJ. Strength of amalgam restorations bonded with Amalgambond. Am J Dent. 1993;6:10.
Nuckles, DB, Draughn, RA, Smith, TI. Evaluation of an adhesive system for amalgam repair: bond strength and porosity. Quint Int. 1994;25:829.
Santos, AC, Meiers, JC. Fracture resistance of premolars with MOD amalgam restorations lined with Amalgambond. Oper Dent. 1994;19:2.
Staninec, M, Holt, M. Bonding of amalgam to tooth structure: tensile, adhesion and microleakage tests. J Prosthet Dent. 1988;59:397.
Staninec, M. Retention of amalgam restorations: undercuts versus bonding. Quint Int. 1989;20:347.
Mercury and Biocompatibility Issues
Abraham, JE, Svare, EW. The effect of dental amalgam restorations on blood mercury levels. J Dent Res. 1984;63:71.
American Dental Association Council on Scientific Affairs. Dental amalgam—update on safety concerns. J Am Dent Assoc. 1998;129:494.
Arenholt-Bindslev, D. Environmental aspects of dental filling materials. Eur J Oral Sci. 1998;106:713.
Bakir, F, Damluji, SF, Amin-Zaki, L, et al. Methyl mercury poisoning in Iraq. Science. 1973;181:230.
Berglund, A. Estimation of the daily dose of intra-oral mercury vapor inhaled after release from dental amalgam. J Dent Res. 1990;69:1646.
Berglund, A, Bergdahl, J, Hansson Mild, K. Influence of low frequency magnetic fields on the intra-oral release of mercury vapor from amalgam restorations. Eur J Oral Sci. 1998;106:671.
Berlin, MH, Clarkston, TW, Friberg, LT, et al. Maximum allowable concentrations of mercury vapor in air. Lakartidningen. 1967;64:3628.
Berry, TG, Nicholson, J, Troendle, K. Almost two centuries with amalgam: where are we today? J Am Dent Assoc. 1994;125:392.
Berry, TG, Summitt, JB, Chung, AKH, et al. Amalgam at the new millennium. J Am Dent Assoc. 1998;129:1547.
Birke, G, Johnels, AG, Plantin, L-O, et al. Hg i livsmedel (3): Metylkvicksilverförgiftning genom förtaring av fisk? [Hg in food (3): Methyl mercury poisoning through eating fish?]. Lakartidningen. 1967;64:3628.
Bolewska, J, Holmstrup, P, Moller-Madsen, B, et al. Amalgam-associated mercury accumulations in normal oral mucosa, oral mucosal lesions of lichen planus and contact lesions associated with amalgam. J Oral Pathol Med. 1990;19:19.
Brune, D. Corrosion of amalgams. Scand J Dent Res. 1981;89:506.
Burrows, D. Hypersensitivity to mercury, nickel and chromium in relation to dental materials. Int Dent J. 1986;36:30.
Chang, SB, Siew, C, Gruninger, SE. Factors affecting blood mercury concentrations in practicing dentists. J Dent Res. 1992;71:66.
Chew, CL, Soh, G, Lee, AS, et al. Comparison of release of mercury from three dental amalgams. Dent Mater. 1989;5:244.
Clarkson, TW. The toxicology of mercury. Crit Rev Clinical Lab Sci. 1997;34:369.
Consumer Reports: The mercury in your mouth 56(5): 316, 1991.
Council on Dental Materials and Devices. Recommendations in mercury hygiene. J Am Dent Assoc. 1976;92:1217.
Council on Dental Materials, Instruments, and Equipment. Safety of dental amalgam. J Am Dent Assoc. 1983;106:519.
Corbin, SB, Kohn, WG. The benefits and risks of dental amalgam: current findings reviewed. J Am Dent Assoc. 1994;125:381.
Cox, SW, Eley, BM. Further investigations of the soft tissue reaction to the gamma 1 phase (Ag2Hg3) of dental amalgam, including measurements of mercury release and redistribution. Biomaterials. 1987;8:296.
Cox, SW, Eley, BM. Further investigations of the soft tissue reaction to the gamma 2 phase (Sn7–8Hg) of dental amalgam, including measurements of mercury release and redistribution. Biomaterials. 1987;8:301.
Cox, SW, Eley, BM. Mercury release, distribution and excretion from subcutaneously implanted conventional and high-copper amalgam powders in the guinea pig. Arch Oral Biol. 1987;32:257.
Cox, SW, Eley, BM. Microscopy and x-ray microanalysis of subcutaneously implanted conventional and high-copper dental amalgam powders in the guinea pig. Arch Oral Biol. 1987;32:265.
Cox, SW, Eley, BM. The release, tissue distribution and excretion of mercury from experimental amalgam tattoos. Br J Exp Pathol. 1986;67:925.
Craig, RG. Biocompatibility of mercury derivatives. Dent Mater. 1986;2:91.
Danscher, G, Horsted-Bindslev, P, Rungby, J. Traces of mercury in organs from primates with amalgam fillings. Exp Mol Pathol. 1990;52:291.
Ekstrand, J, Bjorkman, L, Edlund, C, et al. Toxicological aspects on the release and systemic uptake of mercury from dental amalgam. Eur J Oral Sci. 1998;106:678.
Eley, BM, Cox, SW. Renal cortical mercury levels associated with experimental amalgam tattoos: effects of particle size and amount of implanted material. Biomaterials. 1987;8:401.
Eley, BM, Cox, SW. The development of mercury- and selenium-containing deposits in the kidneys following implantation of dental amalgams in guinea pigs. Br J Exp Pathol. 1986;67:937.
Ferracane, JL, Adey, JD, Nakajima, H, et al. Mercury vaporization from amalgams with varied alloy compositions. J Dent Res. 1995;74:1414.
Ferracane, JL, Engle, JH, Okabe, T, et al. Reduction in operatory mercury levels after contamination or amalgam removal. Am J Dent. 1994;7:103.
FDI Commission. Environmental issues in dentistry—mercury. Int Dent J. 1997;47:105.
Forsell, M, Larsson, B, Ljungqvist, A, et al. Mercury content in amalgam tattoos of human oral mucosa and its relations to local tissue reactions. Eur J Oral Sci. 1998;106:582.
Gronka, PA, Bobkoskie, RL, Tomchick, GJ, et al. Mercury vapor exposures in dental offices. J Am Dent Assoc. 1970;81:923.
Guthrow, CE, Johnson, CB, Lawless, KB. Corrosion of dental amalgam and its component phases. J Dent Res. 1967;46:1372.
Haikel, Y, Gasser, P, Salek, P, et al. Exposure to mercury vapor during setting, removing, and polishing amalgam restorations. J Biomed Mater Res. 1990;24:1551.
Heintze, U, Edwardsson, S, Derand, T, et al. Methylation of mercury from dental amalgam and mercuric chloride by oral streptococci in vitro. Scand J Dent Res. 1983;91:150.
Holland, GA, Asgar, K. Some effects of the phases of amalgam induced by corrosion. J Dent Res. 1974;53:1245.
Johansson, C, Moberg, LE. Area ratio effects on metal ion release from amalgam in contact with gold. Scand J Dent Res. 1991;99:246.
Kaaber, S. Allergy to dental materials with special reference to the use of amalgam and polymethylmethacrylate. Int Dent J. 1990;40:359.
Kaga, M, Seale, NS, Hanawa, T, et al. Cytotoxicity of amalgams. J Dent Res. 1988;67:1221.
Kaga, M, Seale, NS, Hanawa, T, et al. Cytotoxicity of amalgams, alloys, and their elements and phases. Dent Mater. 1991;7:68.
Kingman, A, Albertini, T, Brown, LJ. Mercury concentrations in urine and whole blood associated with amalgam exposure in a US military population. J Dent Res. 1998;77:60.
Kuntz, WD. Maternal and cord blood background mercury level. Am J Obstet Gynecol. 1982;143:440.
Kurland, LT, Faro, SN, Siedler, H. Minamata disease. World Neurol. 1960;1:370.
Laine, J, Kalimo, K, Forssell, H, et al. Resolution of oral lichenoid lesions after replacement of amalgam restorations in patients allergic to mercury compounds. Br J Dermatol. 1992;126:10.
Langolf, GD, Chaffin, DB, Henderson, R, et al. Evaluation of workers exposed to elemental mercury using quantitative test of tremor and neuromuscular function. Am Ind Hyg Assoc J. 1978;39:976.
Langworth, S, Elinder, CG, Gothe, CJ, et al. Biological monitoring of environmental and occupational exposure to mercury. Int Arch Occup Environ Health. 1991;63:161.
Lyttle, HA, Bowden, GH. The level of mercury in human dental plaque an interaction in vitro between biofilms of Streptococcus mutans and dental amalgam. J Dent Res. 1993;72:1320.
Lyttle, HA, Bowden, GH. The resistance and adaptation of selected oral bacteria to mercury and its impact on their growth. J Dent Res. 1993;72:1325.
Mackert, JR, Jr. Dental amalgam and mercury. J Am Dent Assoc. 1991;122:54.
Mackert, JR, Jr., Berglund, A. Mercury exposure from dental amalgam fillings: absorbed dose and the potential for adverse health effects. Crit Rev Oral Biol Med. 1997;8:410.
Mackert, JR, Jr., Leffell, MS, Wagner, DA, et al. Lymphocyte levels in subjects with and without amalgam restorations. J Am Dent Assoc. 1991;122:49.
Mandel, ID. Amalgam hazards: an assessment of research. J Am Dent Assoc. 1991;122:62.
Marek, M. Acceleration of corrosion of dental amalgam by abrasion. J Dent Res. 1984;63:1010.
Marek, M. Corrosion test for dental amalgam. J Dent Res. 1980;59:63.
Marek, M. The effect of the electrode potential on the release of mercury from dental amalgam. J Dent Res. 1993;72:1315.
Marek, M. The release of mercury from dental amalgam: the mechanism and in vitro testing. J Dent Res. 1990;69:1167.
Marek, M, Hockman, RF, Okabe, T. In vitro corrosion of dental amalgam phases. J Biomed Mater Res. 1976;10:789.
Marshall, SJ, Lin, JHC, Marshall, GW. Cu2O and CuCl2 · 3Cu(OH)2 corrosion products on copper rich dental amalgams. J Biomed Mater Res. 1982;16:81.
Martin, MD, Naleway, C, Chou, H-N. Factors contributing to mercury exposure in dentists. J Am Dent Assoc. 1995;126:1502.
Mateer, RS, Reitz, CD. Galvanic degradation of amalgam restorations. J Dent Res. 1972;51:1546.
Miller, JM, Chaffin, DB, Smith, RG. Subclinical psychomotor and neuromuscular changes exposed to inorganic mercury. Am Ind Hyg Assoc J. 1975;36(10):725.
Moberg, LE, Johansson, C. Release of corrosion products from amalgam in phosphate containing solutions. Scand J Dent Res. 1991;99:431.
Molin, M, Marklund, S, Bergman, B, et al. Plasma-selenium, glutathione peroxidase in erythrocytes and mercury in plasma in patients allegedly subject to oral galvanism. Scand J Dent Res. 1987;95:328.
Molin, M, Marklund, SL, Bergman, B, et al. Mercury, selenium, and glutathione peroxidase in dental personnel. Acta Odontol Scand. 1989;47:383.
Mueller, HJ, Bapna, MS. Copper-, indium-, tin-, and calcium-fluoride admixed amalgams: release rates and selected properties. Dent Mater. 1990;6:256.
Okabe, T, Ferracane, J, Cooper, C, et al. Dissolution of mercury from amalgam into saline solution. J Dent Res. 1987;66:33.
Okabe, T, Yomashita, T, Nakajima, H, et al. Reduced mercury vapor release from dental amalgams prepared with binary Hg-In liquid alloys. J Dent Res. 1994;73:1711.
Olsson, S, Bergman, M. Daily dose calculations from measurements of intra-oral mercury vapor. J Dent Res. 1992;71:414.
Olsson, S, Berhlund, A, Bergman, M. Release of elements due to electrochemical corrosion of dental amalgam. J Dent Res. 1994;73:33.
Olstad, ML, Holland, RI, Pettersen, AH. Effect of placement of amalgam restorations on urinary mercury concentration. J Dent Res. 1990;69:1607.
Ott, KH, Vogler, J, Kroncke, A, et al. Mercury concentrations in blood and urine before and after placement of non-gamma 2 amalgam fillings. Dtsch Zahnarztl Z. 1989;44:551.
Palaghias, G. The role of phosphate and carbonic acid-bicarbonate buffers in the corrosion processes of the oral cavity. Dent Mater. 1985;1:139.
Pierce, P, Thompson, JF, Likosky, WH, et al. Alkyl mercury poisoning in humans. J Am Med Assoc. 1972;220:1439.
Pohl, L, Bergman, M. The dentist’s exposure to elemental mercury vapour during clinical work wit amalgam. Act Odont Scand. 1995;53:1023.
Powell, LV, Johnson, GH, Bales, DJ. Effect of admixed indium on mercury vapor release from dental amalgam. J Dent Res. 1989;68:1231.
Powell, LV, Johnson, GH, Yashar, N, et al. Mercury vapor release during insertion and removal of dental amalgam. Oper Dent. 1994;19:70.
Sandborough-Englund, G, Elinder, C-G, Landworth, S, et al. Mercury in biological fluids after mercury removal. J Dent Res. 1998;77:615.
Sarkar, NK, Park, JR. Mechanism of improved corrosion resistance of Zn-containing dental amalgams. J Dent Res. 1988;67:1312.
Saxe, SR, Snowdon, DA, Wekstein, MW, et al. Dental amalgam and cognitive function in older women: findings from the nun study. J Am Dent Assoc. 1995;126:1495.
Scarlett, JM, Gutenmann, WH, Lisk, DJ. A study of mercury in the hair of dentists and dental-related professionals in 1985 and subcohort comparison of 1972 and 1985 mercury hair levels. J Toxicol Environ Health. 1988;25:373.
Schmalz, G, Schmalz, C. Toxicity tests on dental filling materials. Int Dent J. 1981;31:185.
Skare, I, Engqvist, A. Urinary mercury clearance of dental personnel after a long-term intermission in occupational exposure. Swed Dent J. 1990;14:255.
Snapp, KR, Boyer, DB, Peterson, LC, et al. The contribution of dental amalgam to mercury in blood. J Dent Res. 1989;68:780.
Syrjanen, S, Hensten-Pettersen, A, Nilner, K. In vitro testing of dental materials by means of macrophage cultures. II. Effects of particulate dental amalgams and their constituent phases on cultured macrophages. J Biomed Mater Res. 1986;20:1125.
Takaku, S. Studies of mercury concentration in saliva with particular reference to mercury dissolution from dental amalgam into saliva. Gakho Shikwa. 1982;82:285.
Veron, C, Hildebrand, HF, Martin, P. Dental amalgams and allergy. J Biol Buccale. 1986;14:83.
von Mayenburg, J, Rakoski, J, Szliska, C. Patch testing with amalgam at various concentrations. Contact Dermatitis. 1991;24:266.