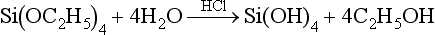Gypsum Products and Investments
CHEMICAL AND PHYSICAL NATURE OF GYPSUM PRODUCTS
CALCIUM SULFATE-BONDED INVESTMENTS
PROPERTIES OF CALCIUM SULFATE–BONDED INVESTMENTS
Gypsum products probably serve the dental profession more adequately than any other materials. Dental plaster, stone, high-strength/high-expansion stone, and casting investment constitute this group of closely related products. With slight modification, gypsum products are used for several different purposes. For example, impression plaster is used to make impressions of edentulous mouths or to mount casts, whereas dental stone is used to form a die that duplicates the oral anatomy when poured into any type of impression. Gypsum products are also used as a binder for silica in gold alloy casting investment, soldering investment, and investment for low-melting-point nickel-chromium alloys. These products are also used as a mold material for processing complete dentures. The main reason for such diversified use is that the properties of gypsum materials can be easily modified by physical and chemical means.
The dihydrate form of calcium sulfate, called gypsum, usually appears white to milky yellowish and is found in a compact mass in nature. The mineral gypsum has commercial importance as a source of plaster of paris. The term plaster of paris was given this product because it was obtained by burning the gypsum from deposits near Paris, France. Deposits of gypsum, however, are found in most countries.
CHEMICAL AND PHYSICAL NATURE OF GYPSUM PRODUCTS
Most gypsum products are obtained from natural gypsum rock. Because gypsum is the dihydrate form of calcium sulfate (CaSO4 · 2H2O), on heating, it loses 1.5 g mol of its 2 g mol of H2O and is converted to calcium sulfate hemihydrate (CaSO4 · ½H2O), sometimes written (CaSO4)2 · H2O. When calcium sulfate hemihydrate is mixed with water, the reverse reaction takes place, and the calcium sulfate hemihydrate is converted back to calcium sulfate dihydrate. Therefore, partial dehydration of gypsum rock and rehydration of calcium sulfate hemihydrate constitute a reversible reaction. Chemically, the reaction is expressed as shown below.
The reaction is exothermic, and whenever 1 g mol of calcium sulfate hemihydrate is reacted with 1.5 g mol of water, 1 g mol of calcium sulfate dihydrate is formed, and 3900 calories of heat are developed. This chemical reaction takes place regardless of whether the gypsum material is used as an impression material, a die material, or a binder in casting investment.
MANUFACTURE OF DENTAL PLASTER, STONE, AND HIGH-STRENGTH STONE
Three types of base raw materials are derived from partial dehydration of gypsum rock, depending on the nature of the dehydration process. Plasters are fluffy, porous, and least dense, whereas the hydrocal variety has a higher density and is more crystalline. Densite is the densest of the raw materials. These three types of raw materials are used to formulate the four types of relatively pure gypsum products used in dentistry. They are classified as plasters (model and laboratory), low- to moderate-strength dental stones, high-strength/low-expansion dental stones, and high-strength/high-expansion dental stones, or
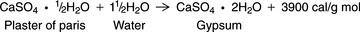
alternatively as Types 2, 3, 4, and 5 in ANSI/ADA Specification No. 25 (ISO 6873).
Although these types have identical chemical formulas of calcium sulfate hemihydrate, CaSO4 · ½H2O, they possess different physical properties, which makes each of them suitable for a different dental purpose. All four forms are derived from natural gypsum deposits, with the main difference being the manner of driving off part of the water of the calcium sulfate dihydrate. Synthetic gypsum can also be used to formulate some products, but is less popular because of higher manufacturing costs.
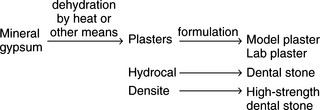
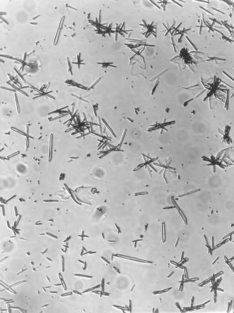
Plasters are produced when the gypsum mineral is heated in an open kettle at a temperature of about 110° to 120° C. The hemihydrate produced is called β-calcium sulfate hemihydrate. Such a powder is known to have a somewhat irregular shape and is porous in nature. These plasters are used in formulating model and lab plasters. Crystals of model plaster are shown in Fig. 13-1.
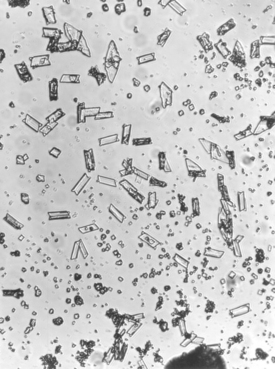
If gypsum is dehydrated under pressure and in the presence of water vapor at about 125° C, the product is called hydrocal. The powder particles of this product are more uniform in shape and denser than the particles of plaster. Crystals of a dental stone are shown in Fig. 13-2. The calcium sulfate hemihydrate produced in this manner is designated as α-calcium sulfate hemihydrate. Hydrocal is used in making low- to moderate-strength dental stones.
Types 4 and 5 high-strength dental stones are manufactured with a high-density raw material called densite. This variety is made by boiling gypsum rock in a 30% calcium chloride solution, after which the chloride is washed away with hot water (100° C) and the material is ground to the desired fineness. The powder obtained by this process is the densest of the types. These materials are generally formulated as high-strength/low-expansion dental stone or high-strength/high-expansion dental stone.
Gypsum products may be formulated with chemicals that modify their handling characteristics and properties. Potassium sulfate, K2SO4, and terra alba (set calcium sulfate dihydrate) are effective accelerators. Sodium chloride in small amounts shortens the setting reaction but increases the setting expansion of the gypsum mass. Sodium citrate is a dependable retarder. Borax, Na2B4O7, is both a retarder and accelerator. A mixture of calcium oxide (0.1%) and gum arabic (1%) reduces the amount of water necessary to mix gypsum products, resulting in improved properties. Type 4 gypsum differs from Type 5 in that Type 4 contains extra salts to reduce its setting expansion.
CHEMICAL REACTION
The chemical reaction that takes place during the setting of gypsum products determines the quantity of H2O needed for the reaction. The reaction of 1 g mol of plaster with 1.5 g mol of water produces 1 g mol of gypsum material. In other words, 145 g of plaster requires 27 g of water to react and form 172 g of gypsum or 100 g of plaster requires 18.6 g of water to form 118 g of calcium sulfate dihydrate. As seen in practice, however, model plaster cannot be mixed with such a small amount of water and still develop a mass suitable for manipulation. Table 13-1 shows the recommended mixing water, required water, and excess water for model plaster, dental stone, and high-strength dental stone.
TABLE 13-1
Required and Excess Water for Gypsum Materials*

*Water-powder ratio varies with each product.
For example, to mix 100 g of model plaster to a usable flowable consistency, use 45 g of water. Note that only 18.6 g of 45 g of water reacts with the 100 g of model plaster; the excess (45 g − 18.6 g = 26.4 g) is distributed as free water in the set mass without taking part in the chemical reaction. When the set material is dried, the excess water evaporates and leaves porosity in the structure, weakening it. Therefore, set model plaster is weaker than dental stone, which in turn is weaker than high-strength dental stone. If 100 g of model plaster is mixed with 50 g of water, the resultant mass is thinner and mixes and pours easily into a mold, but the quality of the set gypsum is inferior and weaker than when 45 g of water is used. When model plaster is mixed with a lesser amount of water, the mixed mass is thicker, is more difficult to handle, and traps air bubbles easily when it is poured into a mold, but the set gypsum is usually stronger. Thus, careful control of the proper amount of water in the mix is necessary for proper manipulation and quality of the set mass.
Water/Powder Ratio of Dental Stone and High-Strength Dental Stone
The reason for the differences among the recommended amounts of mixing water for model plaster, dental stone, and high-strength dental stone is in the shape and form of the calcium sulfate hemihydrate crystals. Some calcium sulfate hemihydrate crystals are comparatively irregular in shape and porous in nature, as are the crystals in model plaster, whereas the crystals of dental stone and the two high-strength dental stones are dense and more regular in shape, as shown in Figs. 13-1 and 13-2. This difference in the physical shape and nature of the crystals makes it possible to obtain the same consistency with less excess water with dental stone and high-strength dental stones than with model plaster.
When mixed with water, model plaster, dental stone, or high-strength dental stones set to a hard mass of gypsum. The gypsum products known as high-strength dental stones (Types 4 and 5) are the strongest, the mass produced as model plaster is the weakest, and dental stone produces an intermediate strength material. Note, however, that all gypsum products have the same chemical formula, and that the chemical nature of the masses produced by mixing them with water is also identical; the differences among them are primarily in their physical properties.
Mechanism of Setting
The most important and well-recognized theory for the mechanism of the setting is the crystalline theory. It was originated in 1887 by Henry Louis Le Chatelier, a French chemist. The theory received the full support of Jacobus Hendricus van’t Hoff, a famous Dutch chemist in Berlin at the turn of the century. According to the explanation of van’t Hoff, the setting reaction of water with calcium sulfate hemidrate to from calcium sulfate dihydrate is caused by the difference in solubility between these two components. Calcium sulfate dihydrate is less soluble than the hemihydrate form. When the hemihydrate dissolves in water, the dihydrate, being of lower solubility, is then supersaturated and precipitates out of solution from points of nucleation in the form of needle-like crystals. Bonding between contacting crystals results in the final cohesive structure.
Volumetric Contraction
Theoretically, calcium sulfate hemihydrate should contract volumetrically during the setting process. However, experiments have determined that all gypsum products expand linearly during setting. As indicated earlier, when 145.15 g of calcium sulfate hemihydrate reacts with 27.02 g of water, the result is the production of 172.17 g of calcium sulfate dihydrate. However, if the volume rather than the weight of calcium sulfate hemihydrate is added to the volume of water, the sum of the volumes will be about 7% less than the volume of calcium sulfate dihydrate. In practice about 0.2% to 0.4% linear expansion is obtained. According to the crystalline theory of Le Chatelier and van’t Hoff, the expansion results from the thrusting action of gypsum crystals, CaSO4 · 2H2O, during their growth from a supersaturated solution. The fact that the contraction of gypsum is not visible does not invalidate its existence, and when the volumetric contraction is measured by a dilatometer, it is determined to be about 7%. Because of the linear expansion of the outer dimensions, which is caused by the growth of calcium sulfate dihydrate, with a simultaneous true volumetric contraction of calcium sulfate dihydrate, these materials are porous when set.
Effect of Spatulation
The mixing process, called spatulation, has a definite effect on the setting time and setting expansion of the material. Within practical limits an increase in the amount of spatulation (either speed of spatulation or time or both) shortens the setting time. Obviously when the powder is placed in water, the chemical reaction starts, and some calcium sulfate dihydrate is formed. During spatulation the newly formed calcium sulfate dihydrate breaks down to smaller crystals and starts new centers of nucleation, from which the calcium sulfate dihydrate can be precipitated. Because an increased amount of spatulation causes more nuclei centers to be formed, the conversion of calcium sulfate hemihydrate to dihydrate is accelerated.
Effect of Temperature
The temperature of the water used for mixing, as well as the temperature of the environment, has an effect on the setting reaction of gypsum products. The setting time probably is affected more by a change in temperature than by any other physical property. Evidently the temperature has two main effects on the setting reaction of gypsum products.
The first effect of increasing temperature is a change in the relative solubilities of calcium sulfate hemihydrate and calcium sulfate dihydrate, which alters the rate of the reaction. The ratio of the solubilities of calcium sulfate dihydrate and calcium sulfate hemihydrate at 20° C is about 4.5. As the temperature increases, the solubility ratios decrease, until 100° C is reached and the ratio becomes 1. As the ratio of the solubilities becomes lower, the reaction is slowed, and the setting time is increased. The solubilities of calcium sulfate hemihydrate and calcium sulfate dihydrate are shown in Table 13-2.
TABLE 13-2
Solubility of Calcium Sulfate Hemihydrate and Calcium Sulfate Dihydrate at Different Temperatures
| Temperature (° C) | CaSO4 · ½H2O (g/100 g water) | CaSO4 · 2H2O (g/100 g water) |
| 20 | 0.90 | 0.200 |
| 25 | 0.80 | 0.205 |
| 30 | 0.72 | 0.209 |
| 40 | 0.61 | 0.210 |
| 50 | 0.50 | 0.205 |
| 100 | 0.17 | 0.170 |
Adapted from Partridge EP, White AH: J Am Chem Soc 51:360, 1929.
The second effect is the change in ion mobility with temperature. In general, as the temperature increases, the mobility of the calcium and sulfate ions increases, which tends to increase the rate of the reaction and shorten the setting time.
Practically, the effects of these two phenomena are superimposed, and the total effect is observed. Thus, by increasing the temperature from 20° to 30° C, the solubility ratio decreases from 0.90/0.200 = 4.5 to 0.72/0.209 = 3.4, which ordinarily should retard the reaction. At the same time, however, the mobility of the ions increases, which should accelerate the setting reaction. Thus, according to the solubility values, the reaction should be retarded, whereas according to the mobility of the ions, the reaction should be accelerated. Experimentation has shown that increasing the temperature from room temperature of 20° C to body temperature of 37° C increases the rate of the reaction slightly and shortens the setting time. However, as the temperature is raised over 37° C, the rate of the reaction decreases, and the setting time is lengthened. At 100° C the solubilities of dihydrate and hemihydrate are equal, in which case no reaction occurs, and plaster does not set.
Effect of Humidity
When the relative humidity increases to 70% and above, moisture in the air can cause some conversion of hemihydrate to dihydrate. Because dihydrate crystals can accelerate the reaction by providing more nuclei for crystallization, the initial result is acceleration of setting. However, further contamination by moisture can reduce the amount of hemihydrate remaining to form gypsum and retardation of setting will occur. Therefore, all gypsum products should be kept in a closed container and well protected from moisture in the air.
Effect of Colloidal Systems and pH
Colloidal systems such as agar and alginate retard the setting of gypsum products. If these materials are in contact with CaSO4 · ½H2O during setting, a soft, easily abraded surface is obtained. Accelerators such as potassium sulfate are added to improve the surface quality of the set CaSO4 · 2H2O against agar or alginate.
These colloids do not retard the setting by altering the solubility ratio of the hemihydrate and dihydrate forms, but rather by being adsorbed on the hemihydrate and dihydrate nucleation sites, thus interfering in the hydration reaction. The adsorption of these materials on the nucleating sites retards the setting reaction more effectively than adsorption on the calcium sulfate hemihydrate.
Liquids with low pH, such as saliva, retard the setting reaction. Liquids with high pH accelerate setting.
PROPERTIES
The important properties of gypsum products include quality, fluidity at pouring time, setting time, linear setting expansion, compressive strength, hardness and abrasion resistance, and reproduction of detail. Some of these property requirements, described by ANSI/ADA Specification No. 25 (ISO 6873), are summarized in Table 13-3.
TABLE 13-3
Property Requirements for Gypsum Materials
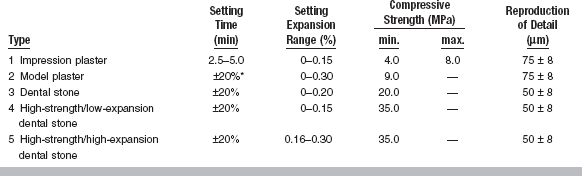
*Setting time shall be within 20% of value claimed by manufacturer.
SETTING TIME
The time required for the reaction to be completed is called the final setting time. If the rate of the reaction is too fast or the material has a short setting time, the mixed mass may harden before the operator can manipulate it properly. On the other hand, if the rate of reaction is too slow, an excessively long time is required to complete the operation. Therefore, a proper setting time is one of the most important characteristics of gypsum materials.
The chemical reaction is initiated at the moment the powder is mixed with water, but at the early stage only a small portion of the hemihydrate is converted to gypsum. The freshly mixed mass has a semifluid consistency and can be poured into a mold of any shape. As the reaction proceeds, however, more and more calcium sulfate dihydrate crystals are produced. The viscosity of the mixed mass increases, and the mass can no longer flow easily into the fine details of the mold. This time is called the working time.
The final setting time is defined as the time at which the material can be separated from the impression without distortion or fracture. The initial setting time is the time required for gypsum products to reach a certain arbitrary stage of firmness in their setting process. In the normal case, this arbitrary stage is represented by a semihard mass that has passed the working stage but is not yet completely set. At final setting, the conversion of calcium sulfate hemihydrate to calcium sulfate dihydrate is virtually completed.
Measurement
The initial setting time is usually measured arbitrarily by some form of penetration test, although occasionally other types of test methods have been designed. For example, the loss of gloss from the surface of the mixed mass of model plaster or dental stone is an indication of this stage in the chemical reaction and is sometimes used to indicate the initial set of the mass. Similarly, the setting time may be measured by the temperature rise of the mass, because the chemical reaction is exothermic.
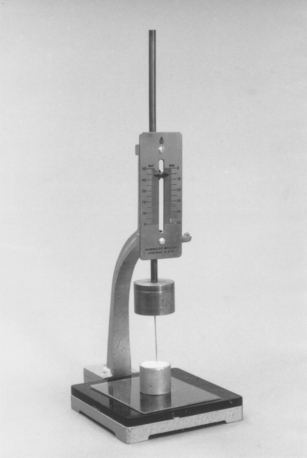
The Vicat apparatus shown in Fig. 13-3 is commonly used to measure the initial setting time of gypsum products. It consists of a rod weighing 300 g with a needle of 1-mm diameter. A ring container is filled with the mix, the setting time of which is to be measured. The rod is lowered until it contacts the surface of the material, then the needle is released and allowed to penetrate the mix. When the needle fails to penetrate to the bottom of the container, the material has reached the Vicat or the initial setting time. Other types of instruments, such as Gillmore needles, can be used to obtain the initial and final setting times of gypsum materials.
Control of Setting Time
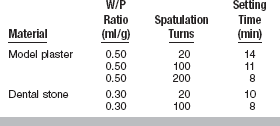
Methods for controlling setting time have been discussed previously. Initially the manufacturer can add various components that act as either accelerators or retarders. The operator can alter setting time by changing the temperature of the mix water and by changing the degree of spatulation. Water-powder (W/P) ratio can also affect setting time; using more water in the mix can prolong the setting time as shown in Table 13-4.
The easiest and most reliable way to change the setting time is to add different chemicals. Potassium sulfate, K2SO4, is known as an effective accelerator, and the use of a 2% aqueous solution of this salt rather than water reduces the setting time of model plaster from approximately 10 minutes to about 4 minutes. On the other hand, sodium citrate is a dependable retarder. The use of a 2% aqueous solution of borax to mix with the powder may prolong the setting time of some gypsum products to a few hours.
If a small amount of set calcium sulfate dihydrate is ground and mixed with model plaster, it provides nuclei of crystallization and acts as an accelerator. The set gypsum used as an accelerator is called terra alba, and it has a pronounced effect at lower concentrations. The setting time changes significantly if the amount of terra alba present in the mix is changed from 0.5% to 1%. However, terra alba concentrations above 1% have less effect on the setting time. Manufacturers usually take advantage of this fact and add about 1% terra alba to plaster. Thus, the setting time of model plaster is altered less in normal use because of opening and closing the container.
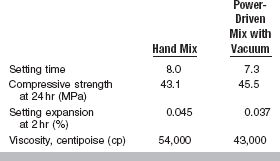
The W/P ratio has a pronounced effect on the setting time. The more water in the mix of model plaster, dental stone, or high-strength dental stone, the longer the setting time, as shown in Table 13-4. The effect of spatulation on setting time of model plaster and dental stone is shown in Table 13-5. Increased spatulation shortens the setting time. Properties of a high-strength dental stone mixed by hand and by a power-driven mixer with vacuum are shown in Table 13-6. The setting time is usually shortened for power mixing compared with hand mixing.
VISCOSITY
The viscosities of several high-strength dental stones and impression plaster are listed in Table 13-7. A range of viscosities from 21,000 to 101,000 centipoises (cp) was observed for five different high-strength stones. More voids were observed in casts made from the stones with the higher viscosities. Impression plaster is used infrequently, but it has a low viscosity, which makes it possible to take impressions with a minimum of force on the soft tissues (mucostatic technique).
TABLE 13-7
Viscosity of Several High-Strength Dental Stones and Impression Plaster

*Adapted from Garber DK, Powers JM, Brandau HE: J Mich Dent Assoc 67:133, 1985. Stones were mixed with 1% sodium citrate solution to retard setting. Viscosity was measured 4 minutes from the start of mixing.
COMPRESSIVE STRENGTH
When set, gypsum products show relatively high values of compressive strength. The compressive strength is inversely related to the W/P ratio of the mix. The more water used to make the mix, the lower the compressive strength.
Model plaster has the greatest quantity of excess water, whereas high-strength dental stone contains the least excess water. The excess water is uniformly distributed in the mix and contributes to the volume but not the strength of the material. Set model plaster is more porous than set dental stone, causing the apparent density of model plaster to be lower. Because high-strength dental stone is the densest, it shows the highest compressive strength, with model plaster being the most porous and thus the weakest.
The 1-hour compressive strength values are about 12.5 MPa for model plaster, 31 MPa for dental stone, and 45 MPa for high-strength dental stones. These values are representative for the normal mixes, but they vary as the W/P ratio is increased or decreased. The effect of the W/P ratio on the compressive strength of these materials is given in Table 13-8. As shown in Table 13-6, the compressive strength of a high-strength dental stone is improved slightly by vacuum mixing. Evidently, when stone is mixed with the same W/P ratio as model plaster, the compressive strength of dental stone is almost the same as that of model plaster. Similarly, the compressive strength of high-strength dental stone with W/P ratios of 0.3 and 0.5 is similar to the normal compressive strength of dental stone and model plaster.
TABLE 13-8
Effect of Water/Powder Ratio on the Compressive Strength of Model Plaster, Dental Stone, and High-Strength Dental Stone
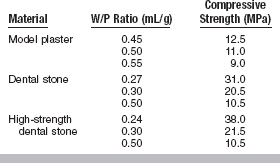
All mixes spatulated 100 turns and tested 1 hour after the start of mixing.
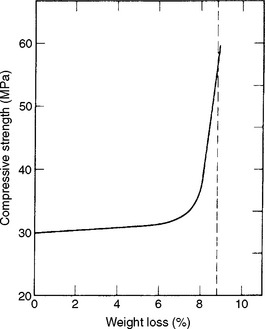
At 1 or 2 hours after the final setting time, the hardened gypsum material appears dry and seems to have reached its maximum strength. Actually, this is not the case. The wet strength is the strength of gypsum materials with some or all of the excess water present in the specimen. The dry strength is the strength of the gypsum material with all of its excess water driven out. The dry compressive strength is usually about twice that of the wet strength. Notice that as the hardened mass slowly loses its excess water, the compressive strength of the material does not increase uniformly. The effect of drying on the compressive strength of dental stone is shown in Fig. 13-4. Theoretically, about 8.8% of excess water is in the hardened mass of the stone. As the mass loses up to 7% of the water, no appreciable change develops in the compressive strength of the material. When the mass loses 7.5% of the excess water, however, the strength increases sharply, and when all of the excess (8.8%) is lost, the strength of the material is over 55 MPa.
The drying time for gypsum materials varies according to the size of the gypsum mass and the temperature and humidity of the storage atmosphere. At room temperature and average humidity, about 7 days are necessary for an average denture flask filled with gypsum materials to lose the excess water.
SURFACE HARDNESS AND ABRASION RESISTANCE
The surface hardness of unmodified gypsum materials is related in a general way to their compressive strength. High compressive strengths of the hardened mass correspond to high surface hardnesses. After the final setting occurs, the surface hardness remains practically constant until most excess water is evaporated from the surface, after which its increase is similar to the increase in compressive strength. The surface hardness increases at a faster rate than the compressive strength, because the surface of the hardened mass reaches a dry state earlier than the inner portion of the mass.
Attempts have been made to increase the hardness of gypsum products by impregnating the set gypsum with epoxy or methyl methacrylate monomer that is allowed to polymerize. Increases in hardness were obtained for model plaster but not for dental stone or high-strength dental stone. Increases in scratch resistance of 15% to 41% were observed for a high-strength dental stone impregnated with epoxy resins or a light-cured dimethacrylate resin. Generally, impregnating set gypsum with resin increases abrasion resistance, but decreases compressive strength and surface hardness.
The idea of drying molds, casts, or dies in an oven to obtain a quick, dry compressive strength and dry surface hardness of a material is not practical, because the gypsum would be dehydrated, which would reduce the strength instead of increasing it. Soaking the gypsum dies or casts in glycerin or different oils does not improve the surface hardness but rather makes the surface smoother, so that a wax carver or other instrument will not cut the stone as it slides over the surface. Mixing high-strength dental stone with a commercial hardening solution containing colloidal silica (about 30%) improves the surface hardness of the set gypsum. The Knoop hardnesses of two commercial high-strength dental stones were 54 and 77 kg/mm2 when mixed with water. When the hardening solution was used, these values increased to 62 and 79 kg/mm2, respectively. Increased surface hardness does not necessarily mean improved abrasion resistance because hardness is only one of many factors that can affect wear resistance. Two-body abrasion studies suggest that the commercial hardening solutions do not improve the abrasion resistance of high-strength dental stones. However, the clinical relevancy of the two-body abrasion test on gypsum has not been established. Further studies of abrasion resistance and methods of measurement are needed. As discussed in the chapter on impression materials, gypsum dies abrade more readily than epoxy dies, even though the gypsum dies are harder.
Although the use of disinfectant chemicals on gypsum dies effectively destroys potentially dangerous organisms, some can damage the surface of a die. Surfaces can be eroded, and the surface hardness can be adversely affected by treatment with some commonly used disinfectants. Other disinfectants, including sodium hypochlorite solutions, have very little effect on the surfaces of gypsum dies.
REPRODUCTION OF DETAIL
ANSI/ADA Specification No. 25 requires that Types 1 and 2 reproduce a groove 75 μm in width, whereas Types 3, 4, and 5 reproduce a groove 50 μm in width (see Table 13-3). Gypsum dies do not reproduce surface detail as well as electroformed or epoxy dies because the surface of the set gypsum is porous on a microscopic level (Fig. 13-5). Air bubbles are often formed at the interface of the impression and gypsum cast because freshly mixed gypsum does not wet some elastomeric impression materials (condensation silicones) well. The incorporation of nonionic surfactants in silicone impression materials improves the wetting of the impression by slurry water. The use of vibration during the pouring of a cast reduces the presence of air bubbles. Contamination of the impression in which the gypsum die is poured by saliva or blood can also affect the detail reproduction. Rinsing the impression and blowing away excess water can improve the detail recorded by the gypsum die material.
SETTING EXPANSION
When set, all gypsum products show a measurable linear expansion. The percentage of setting expansion, however, varies from one type of gypsum material to another. Under ordinary conditions, plasters have 0.2% to 0.3% setting expansion, low- to moderate-strength dental stone about 0.15% to 0.25%, and high-strength dental stone only 0.08% to 0.10%. The setting expansion of high-strength/high-expansion dental stone ranges from 0.10% to 0.20%. Typically, over 75% of the expansion observed at 24 hours occurs during the first hour of setting.
The setting expansion may be controlled by different manipulative conditions and by the addition of some chemicals. Mechanical mixing decreases setting expansion. As shown in Table 13-6, a vacuum-mixed high-strength stone expands less at 2 hours than when mixed by hand. Power mixing appears to cause a greater initial volumetric contraction than is observed for hand mixing. The W/P ratio of the mix also has an effect, with an increase in the ratio reducing the setting expansion. The addition of different chemicals affects not only the setting expansion of gypsum products, but may also change other properties. For example, the addition by the manufacturer of sodium chloride (NaCl) in a small concentration increases the setting expansion of the mass and shortens the setting time. The addition of 1% potassium sulfate, on the other hand, decreases the setting time but has no effect on the setting expansion.
If during the setting process, the gypsum materials are immersed in water, the setting expansion increases. This is called hygroscopic expansion. A typical, high-strength dental stone has a setting expansion of about 0.08%. If during the setting process the mass is immersed in water, it expands about 0.10%. Increased expansion is observed when dental stone hardens as it comes in contact with a hydrocolloid impression. A more detailed explanation of hygroscopic expansion is presented later under casting investments with a gypsum binder.
MANIPULATION
When any of the gypsum products is mixed with water, it should be spatulated properly to obtain a smooth mix. Water is dispensed into a mixing bowl of an appropriate size and design (Fig. 13-6). The powder is added and allowed to settle into the water for about 30 seconds. This technique minimizes the amount of air incorporated into the mix during initial spatulation by hand. Spatulation can be continued by hand using a metal spatula with a stiff blade (see Fig. 13-6), a hand-mechanical spatulator (Fig. 13-7), or a power-driven mechanical spatulator (Fig. 13-8). A summary of the effect of various manipulative variables on the properties of gypsum products is presented in Table 13-9.

FIGURE 13-6 Flexible rubber mixing bowl and metal spatula with a stiff blade. (From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St. Louis, 2004, Mosby.)

FIGURE 13-7 Mechanical spatulator for use with small gypsum mixes. (From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St. Louis, 2004, Mosby.)
FIGURE 13-8 Power-driven mechanical spatulator with a vacuum attachment. (From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St. Louis, 2004, Mosby.)
Spatulation by hand involves stirring the mixture vigorously while wiping the inside surfaces of the bowl with the spatula. Spatulation to wet and mix the powder uniformly with the water requires about 1 minute at 2 revolutions per second.
Spatulation with a power-driven mechanical spatulator requires that the powder initially be wet by the water as with hand mixing. The mix is then spatulated for 20 seconds on the low-speed drive of the mixer. Vacuuming during mixing reduces the air entrapped in the mix. Vibration immediately after mixing and during pouring of the gypsum minimizes air bubbles in the set mass.
Pouring an impression with gypsum requires care to avoid trapping air in critical areas. The mixed gypsum should be poured slowly or added to the impression with a small instrument such as a wax spatula. The mass should run into the rinsed impression under vibration in such a manner that it pushes air ahead of itself as it fills the impressions of the teeth. Commonly, the teeth of a cast are poured in dental stone or high-strength dental stone, whereas the base is poured in model plaster for easier trimming.
Once poured, the gypsum material should be allowed to harden for 45 to 60 minutes before the impression and cast are separated and disinfected. Models can be disinfected by immersion in 1:10 dilution of sodium hypochlorite for 30 minutes or with a spray of iodophor following manufacturer’s instructions.
CASTING INVESTMENTS
The adoption of the casting practice in dentistry for making gold alloy inlays, crowns, bridges, and other restorations represents one of the major advances in restorative dentistry. In recent years, alloys with higher melting points, the palladium and base metal alloys, have been cast into crowns, bridges, and removable partial denture restorations by using basically the same lost-wax technique used for dental gold alloys. All such casting operations involve (1) a wax pattern of the object to be reproduced; (2) a suitable mold material, known as investment, which is placed around the pattern and permitted to harden; (3) suitable furnaces for burning out the wax patterns and heating the investment mold; and (4) proper facilities to melt and cast the alloy. An investment can be described as a ceramic material that is suitable for forming a mold into which a metal or alloy is cast. The operation of forming the mold is described as investing. Details of the casting technique are described in Chapter 17.
PROPERTIES REQUIRED OF AN INVESTMENT
1. Easily manipulated: Not only should it be possible to mix and manipulate the mass readily and to paint the wax pattern easily, but the investment should also harden within a relatively short time.
2. Sufficient strength at room temperature: The investment should permit ease in handling and provide enough strength at higher temperatures to withstand the impact force of the molten metal. The inner surface of the mold should not break down at a high temperature.
3. Stability at higher temperatures: Investment must not decompose to give off gases that could damage the surface of the alloy.
4. Sufficient expansion: It must expand enough to compensate for shrinkage of the wax pattern and metal that takes place during the casting procedure.
5. Beneficial casting temperatures: Preferably the thermal expansion versus temperature curve should have a plateau of the thermal expansion over a range of casting temperatures.
6. Porosity: It should be porous enough to permit the air or other gases in the mold cavity to escape easily during the casting procedure.
7. Smooth surface: Fine detail and margins on the casting should be preserved.
8. Ease of divestment: The investment should break away readily from the surface of the metal and should not have reacted chemically with it.
These requirements describe an ideal investment. No single material is known that completely fulfills all these requirements. However, by blending different ingredients one can develop an investment that possesses most of the required qualities. These ideal qualities are the basis for considering the behavior and characteristics of casting investments.
COMPOSITION
In general, an investment is a mixture of three distinct types of materials: refractory material, binder material, and other chemicals.
Refractory Material
This material is usually a form of silicon dioxide, such as quartz, tridymite, or cristobalite, or a mixture of these. Refractory materials are contained in all dental investments, whether for casting gold or high-melting-point alloys.
Binder Material
Because the refractory materials alone do not form a coherent solid mass, some kind of binder is needed. The common binder used for dental casting gold alloy is α-calcium sulfate hemihydrate. Phosphate, ethyl silicate, and other similar materials also serve as binders for high-temperature casting investments. These latter investments are described later in conjunction with investment for casting high-melting-point alloys.
Other Chemicals
Usually a mixture of refractory materials and a binder alone is not enough to produce all the desirable properties required of an investment. Other chemicals, such as sodium chloride, boric acid, potassium sulfate, graphite, copper powder, or magnesium oxide, are often added in small quantities to modify various physical properties. For example, small amounts of chlorides or boric acid enhance the thermal expansion of investments bonded by calcium sulfate.
CALCIUM SULFATE-BONDED INVESTMENTS
The dental literature and patent references describe the quantity and purpose of each of a variety of ingredients in dental casting investments. In general, the investments suitable for casting gold alloys contain 65% to 75% quartz or cristobalite, or a blend of the two, in varying proportions; 25% to 35% of α-calcium sulfate hemihydrate; and about 2% to 3% chemical modifiers. With the proper blending of these basic ingredients, the manufacturer is able to develop an investment with an established group of physical properties that are adequate for dental gold casting practices. A list of specific compositions is of little value, however, because the final product’s properties are influenced by both the ingredients present in the investment and the manner in which the mass is manipulated and used in making the mold.
Investments with calcium sulfate hemihydrate as a binder are relatively easy to manipulate, and more information about the effect of different additives, as well as various manipulative conditions, is available for this type than for other types, such as those that use silicates or phosphates as binders. The calcium sulfate–bonded investment is usually limited to gold castings, and is not heated above 700° C. The calcium sulfate portion of the investment decomposes into sulfur dioxide and sulfur trioxide at temperatures over 700° C, tending to embrittle the casting metal. Therefore, the calcium sulfate type of binder is usually not used in investments for making castings of high-melting-point metals such as palladium or base metal alloys.
PROPERTIES OF CALCIUM SULFATE–BONDED INVESTMENTS
ANSI/ADA Specification No. 2 (ISO 7490) for gypsum-bonded casting investments applies to two different types of investments suitable for casting dental restorations of gold alloys:
Both types have calcium sulfate as a binder material. The physical properties included in this specification are appearance of powder, fluidity at working time, setting time, compressive strength, linear setting expansion, and linear thermal expansion. The values allowed for these properties are summarized in the specification. The manipulation of investments in the inlay casting procedure is discussed in detail in Chapter 17.
EFFECT OF TEMPERATURE ON INVESTMENT
In casting with the lost-wax process, the wax pattern is invested and placed in an oven at a temperature that melts and removes the pattern from the investment, thereby leaving a mold cavity into which the molten metal is cast. This oven temperature varies from one technique to another, but in no case is it lower than 550° C or higher than 700° C for calcium sulfate–bonded investments. During the heating process, the refractory material is affected differently by the thermal changes than is the gypsum binder.
Effect of Temperature on Silicon Dioxide Refractories
Each of the polymorphic forms of silica—quartz, tridymite, and cristobalite—expands when heated, but the percentage of expansion varies from one type to another. Pure cristobalite expands to 1.6% at 250° C, whereas quartz expands about 1.4% at 600° C, and the thermal expansion of tridymite at 600° C is less than 1%. The percentage of expansion of the three types of silica versus temperature is shown in Fig. 3-12. As seen in Fig. 3-12, none of the three forms of silica expands uniformly; instead they all show a break (nonlinearity) in their thermal expansion curves. In the case of cristobalite the expansion is somewhat uniform up to about 200° C. At this temperature its expansion increases sharply from 0.5% to 1.2%, and then above 250° C it again becomes more uniform. At 573° C quartz also shows a break in the expansion curve, and tridymite shows a similar break at a much lower temperature.
The breaks on the expansion versus temperature curves indicate that cristobalite and quartz each exist in two polymorphic forms, one of which is more stable at a higher temperature and the other at a lower temperature. The form that is more stable at room temperature is called the α-form, and the more stable form at higher temperatures is designated as the β-form. Tridymite has three stable polymorphic forms. Thus, the temperatures of 220° C for cristobalite, 573° C for quartz, and 105° and 160° C for tridymite are displacive transition temperatures. A displacive change involves expansion or contraction in the volume of the mass without breaking any bonds. In changing from the α-form (which is the more stable form at room temperature) to the β-form (which is stable at higher temperatures), all three forms of silica expand. The amount of expansion is highest for cristobalite and lowest for tridymite.
The quartz form of silica is found abundantly in nature, and it can be converted to cristobalite and tridymite by being heated through a reconstructive transition during which bonds are broken and a new crystal structure is formed. The α-quartz is converted to β-quartz at a temperature of 573° C. If the β-quartz is heated to 870° C and maintained at that temperature, it is converted to β-tridymite. From β-tridymite, it is possible to obtain either α-tridymite or β-cristobalite. If β-tridymite is cooled rapidly to 120° C and held at that temperature, it is changed to α-tridymite, which is stable at room temperature. On the other hand, if β-tridymite is heated to 1475° C and held at that temperature, it is converted to β-cristobalite. Further heating of β-cristobalite produces fused silica, but if it is cooled to 220° C and held at that temperature, α-cristobalite is formed. These transitions are shown in the following equation.
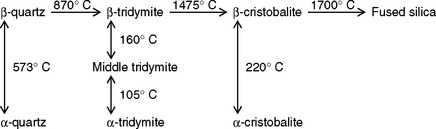
All forms of silica are in their α-forms in the investment, and during the heating process they are converted completely or in part to their corresponding β-forms. This transition involves an expansion of the mass, which helps to compensate for the casting shrinkages.
Effect of Temperature on Calcium Sulfate Binders
The binder used for gold alloy casting investments in dentistry is α-calcium sulfate hemihydrate. During the investing process, some of the water mixed with the investment reacts with the hemihydrate and is converted to calcium sulfate dihydrate, whereas the remainder of the water is uniformly distributed in the mix as excess water. During the early stages of heating, the excess water evaporates. As the temperature rises to about 105° C, calcium sulfate dihydrate starts losing water. The investment mass is then heated still further to the proper temperature for casting the metal. In this way, anhydrous calcium sulfate, silica, and certain chemical additives remain to form the mold into which the gold alloy is cast.
It has been observed experimentally that investment expands when it is first heated from room temperature to about 105° C, then contracts slightly or remains unchanged up to about 200° C, and registers varying degrees of expansion, depending on the silica composition of the investment, between 200° and 700° C. These properties are explained as follows. Up to about 105° C, ordinary thermal expansion occurs. Above 105° C, the calcium sulfate dihydrate is converted to anhydrous calcium sulfate. Dehydration of the dihydrate and a phase change of the calcium sulfate anhydrite cause a contraction. However, the α-form of tridymite (which might be present as an impurity) is expanding and compensates for the contraction of the calcium sulfate sufficiently to prevent the investment from registering a serious degree of contraction. At elevated temperatures, the α-forms of silica present in the investment are converted to the β-forms, which cause some additional expansion.
The thermal expansion curves for a currently available hygroscopic type of investment containing quartz (A) and a thermal expansion type of investment containing cristobalite (B) are shown in Fig. 13-9, which illustrates the expected degree of expansion at different temperatures. The expansion of the silica content of the investment must not only be sufficiently high to overcome all the contraction, but should also take place at temperatures close to the temperature at which contraction of the hemihydrate occurs.
Cooling of the Investment
When the investment is allowed to cool, the refractory and binder contract according to a thermal contraction curve that is different from the thermal expansion curve of the investment (Fig. 13-10). On cooling to room temperature, the investment exhibits an overall contraction compared with its dimensions before heating. On reheating to the temperature previously attained, the investment does not expand thermally to the previous level; moreover, the process of cooling and reheating causes internal cracks in the investment that can affect the quality of the casting.
SETTING EXPANSION OF CALCIUM SULFATE–BONDED INVESTMENT
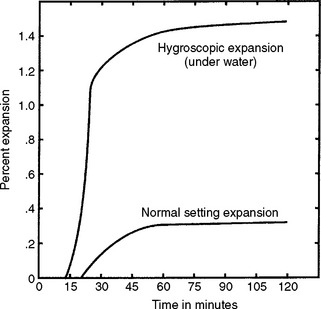
All the calcium sulfate–bonded investments currently available for casting gold alloys have both setting and thermal expansion. The sum of these two expansions results in a total dimensional change that is an essential property of dental casting investments because it provides compensation for the casting shrinkage of the casting alloys. The setting expansion of an investment, like that of other gypsum products discussed earlier in this chapter, is the linear expansion that takes place during the setting of the investment. If the investment is setting surrounded by air, the expansion is referred to as normal setting expansion. On the other hand, if the mixed investment is setting in contact with water, the expansion is substantially greater and is called hygroscopic setting expansion. Such contact with water can be achieved in the commonly used casting techniques of (1) placing a wet liner inside the casting ring in which the investment is poured, or (2) if after investing, the casting ring is placed in a water bath. The presently accepted mechanism for hygroscopic setting expansion also relates to the normal setting expansion that occurs when the investment mix sets in contact with air. The basis for this mechanism lies in the role played by the surface tension of the mix water and can be described as follows. After the investment is mixed, water surrounds the components of the setting investment. As the reaction of the calcium sulfate binder progresses, the surrounding water is reduced and growing gypsum crystals impinge on the surface of the remaining water whose surface tension inhibits outward crystal growth. When the water needed for the reaction is used up and the reaction is virtually completed, the growth of gypsum crystals stops in its inhibited form.
If the investment is poured into a casting ring having a water-filled liner, the gypsum crystals can grow further, but only until the new water surface provided by the additional water in the liner is reached; surface tension then inhibits further growth. If water is supplied to the mixed investment mass by immersing the invested ring in a water bath, no new surface is close enough to provide inhibition of crystal growth. The resulting hygroscopic setting expansion for complete immersion as measured in an unconfined trough (ANSI/ADA Specification No. 2) is more than twice that of normal expansion. However, when the investment is setting in a confined ring, hygroscopic expansion is limited by the confinement of the ring. For hygroscopic expansion, the additional water provided must be presented to the investment during setting. This is significantly different than adding more water to the premixture components (i.e., increasing the W/P ratio). Another requirement for hygroscopic expansion is that the additional water be presented before the observed “loss of gloss,” which is when the setting reaction is not complete, and the mix water can still be observed on the surface of the setting investment. This allows the additional water to join the remaining mix water and extend the water surface so that the action of surface tension is either delayed or inactive.
Particle Size of Silica
The particle size of calcium sulfate hemihydrate has little effect on hygroscopic expansion, whereas the particle size of silica has a significant effect. Finer silica produces higher setting and hygroscopic expansions.
Silica/Binder Ratio
Investments usually contain 65% to 75% silica, 25% to 35% calcium sulfate hemihydrate, and about 2% to 3% of some additive chemicals to control the different physical properties and to color the investments. If the silica/stone ratio is increased, the hygroscopic expansion of the investment also increases, but the strength of the investment decreases.
Water/Powder Ratio
As with the setting expansion of gypsum products, the more water in the mix (the thinner the mix or the higher the W/P ratio), the less the normal and hygroscopic setting expansions. Less thermal expansion is also obtained with a thinner mix.
Spatulation
The effect of spatulation on the setting and hygroscopic expansion of the investment is similar to that on the setting expansion of all gypsum products.
Age of Investment
Investments that are 2 or 3 years old do not expand as much as freshly prepared investments. For this reason, the containers of investment must be kept closed as much as possible, especially if the investment is stored in a humid atmosphere.
Water-Bath Temperature
For the water-bath immersion technique, the temperature of the water bath has a measurable effect on the wax pattern. At higher water-bath temperatures, the wax pattern expands, requiring less expansion of the investment to compensate for the total casting shrinkage. In addition, higher water-bath temperatures soften the wax. The softened wax then provides less resistance to the expansion of the investment, thus making the setting expansion more effective. The net effect is higher expansion of the mold with higher water-bath temperatures.
THERMAL AND HYGROSCOPIC CASTING INVESTMENT
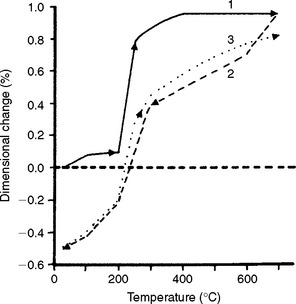
Casting techniques involving gypsum-bonded investments are often classified as thermal or hygroscopic techniques. The thermal technique directs placing the invested ring after setting into the burnout oven set for a relatively high temperature (649° C) while the hygroscopic technique directs immersing the invested ring before setting in a water bath and then, after setting, placing the ring into the burnout oven set for relatively low temperature (482° C). Although all gypsum-bonded investments exhibit both thermal and hygroscopic setting expansion, the relative proportion of these two expansions can vary. Investments used in the thermal technique usually contain crystobalite as the refractory ingredient, which has a high thermal expansion. Investments used in the hygroscopic technique usually contain quartz or tridymite, which have lower thermal expansions but higher hygroscopic setting expansions.
HYGROSCOPIC-THERMAL GOLD CASTING INVESTMENT
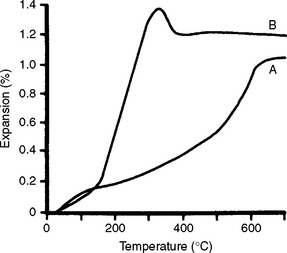
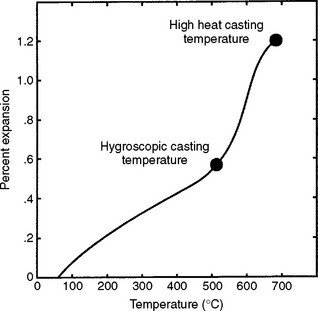
There is one gold casting investment on the market that was designed for use with either hygroscopic or thermal type of casting techniques. Fig. 13-11 shows the high thermal expansion of this investment in the range between 482° C and 649° C. This expansion is high enough to use the investment with the thermal casting technique, without water immersion. However, when immersed in a water bath, the investment expands hygroscopically (Fig. 13-12). With the hygroscopic technique, the investment needs to be heated to only 482° C to provide the appropriate expansion.
INVESTMENT FOR CASTING HIGH-MELTING-POINT ALLOYS
Most palladium and base metal alloys used for partial dentures and ceramic-metal restorations have high melting temperatures. They should be cast at a mold temperature greater than 700° C. For this reason, calcium sulfate–bonded investments are usually not used for casting these alloys. Only one base metal alloy for dental applications possesses a low enough melting point to be cast into a mold at 700° C with a calcium sulfate binder. This alloy is an exception, because base metal alloys are usually cast into molds at 850° to 110° C. To withstand these high temperatures, the molds require different types of binders, such as silicate and phosphate compounds. This type of investment usually has less than 20% binder, and the remainder of the investment is quartz or another form of silica.
Phosphate-Bonded Investment
The most common type of investment for casting high-melting point alloys is the phosphate-bonded investment. This type of investment consists of three different components. One component contains a water-soluble phosphate ion. The second component reacts with phosphate ions at room temperature. The third component is a refractory, such as silica. Different materials can be used in each component to develop different physical properties.
The binding system of a typical phosphate-bonded investment undergoes an acid-base reaction between acid monoammonium phosphate (NH4H2PO4) and basic magnesia (MgO). The soluble phosphate in water reacts with the sparingly soluble magnesia at its surface, forming a binding medium with filler particles embedded in the matrix. The chemical reaction at room temperature can be expressed simply as follows:
NH4H2PO4 + MgO + H2O → NH4MgPO4 · 6H2O + H2O
The water produced by this reaction at room temperature lowers the viscosity of the mix as spatulation continues.
As the reaction takes place, colloidal particles are formed with a strong interaction among the particles. During setting and burnout, the sequence of chemical and thermal reactions causes various phase changes, providing room-temperature strength (green strength) and high-temperature strength that enable the investment to withstand the impact of high-melting-point alloys. Phases formed at high temperatures include Mg2P2O7 and subsequently Mg3(PO4)2. To produce higher expansion, a combination of different particle sizes of silica is used.
These investments can be mixed with water or with a special liquid supplied by the manufacturer. The special liquid is a form of silica sol in water. As shown in Fig. 13-13, phosphate-bonded investments possess higher setting expansion when they are mixed with the silica sol than when mixed with water. With a mix containing silica sol, the investment mass is capable of expanding hygroscopically, whereas if the mix is only water, the hygroscopic expansion of such an investment is negligible. Not all phosphate-bonded investments, however, can expand hygroscopically. Using silica sol instead of water with phosphate-bonded investment also increases its strength considerably. Fig. 13-14 shows thermal expansion curves of two commercial phosphate-bonded investments mixed according to the manufacturers’ recommended liquid/powder ratio. Both the setting and thermal expansions must be considered in selecting these investments.
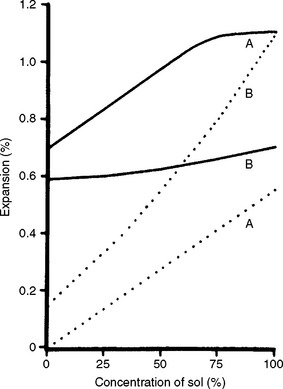
FIGURE 13-13 Effect of silica sol concentration on thermal expansion (solid lines) at 800° C and setting expansion (dotted lines) of two phosphate-bonded investments (A, thermal expansion type; B, hygroscopic expansion type). (Adapted from Zarb GA, Bergman G, Clayton JA, MacKay HF, editors: Prosthodontic treatment for partially edentulous patients, St. Louis, 1978, Mosby.)
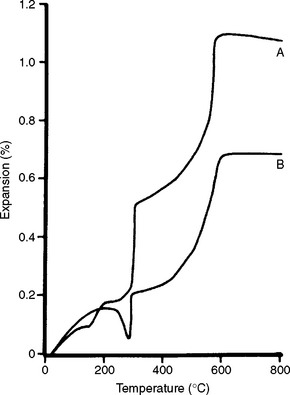
FIGURE 13-14 Thermal expansion curves of two phosphate-bonded investments mixed at recommended liquid/powder ratios (A, thermal expansion type; B, hygroscopic expansion type). (Adapted from Zarb GA, Bergman B, Clayton JA, MacKay HF, editors: Prosthodontic treatment for partially edentulous patients, St. Louis, 1978, Mosby.)
ANSI/ADA Specification No. 42 (ISO 9694) for dental phosphate-bonded casting investments specifies two types of investments for alloys having a solidus temperature above 1080° C:
Type 1: For inlays, crowns, and other fixed restorations
Type 2: For partial dentures and other cast, removable restorations
The following properties and their specified values are described by the specification: fluidity, initial setting time, compressive strength, and linear thermal expansion. The setting time must not differ by more than 30% from the time stated by the manufacturer. The compressive strength at room temperature shall not be less than 2.5 MPa for Type 1 investments and 3.0 MPa for Type 2 investments. The linear thermal expansion must not differ by more than 15% from the time stated by the manufacturer.
Silica-Bonded Investment
Another type of binding material for investments used with casting high-melting-point alloys is a silica-bonding ingredient. This type of investment may derive its silica bond from ethyl silicate, an aqueous dispersion of colloidal silica, or from sodium silicate. One such investment consists of a silica refractory, which is bonded by the hydrolysis of ethyl silicate in the presence of hydrochloric acid. The product of the hydrolysis is a colloidal solution of silicic acid and ethyl alcohol, which can be written as
In practice, however, the reaction is more complicated, and instead of tetrasilicic acid, which is converted into SiO2 · 2H2O, a polymerized compound of silicon is formed with the following structure:
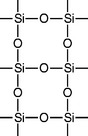
This material has an even higher silica content and better refractory properties than the SiO2 · 2H2O.
Ethyl silicate has the disadvantage of giving off flammable components during processing, and the method is expensive; thus, other techniques and methods have been developed to reduce the use of this material. Sodium silicate and colloidal silica are more common binders of the silica type.
Today this investment is usually supplied with two bottles of special liquid, instead of water, with which the investment powder should be mixed. In one of the bottles the manufacturer usually supplies a properly diluted water-soluble silicate solution. The other bottle usually contains a properly diluted acid solution, such as a solution of hydrochloric acid. The contents of each bottle can be stored almost indefinitely. Before use, mix an equal volume from each bottle and allow the mixed liquids to stand for a prescribed time, according to the manufacturer’s instructions, so hydrolysis can take place and freshly prepared silicic acid forms.
ANSI/ADA Specification No. 91 (ISO 11246) for ethyl silicate casting investments specifies setting time, compressive strength, and linear thermal expansion. The setting time must not differ by more than 30% from the time stated by the manufacturer. The compressive strength at room temperature shall not be less than 1.5 MPa. The linear thermal expansion must not differ by more than 15% from the time stated by the manufacturer.
BRAZING INVESTMENT
When brazing (soldering) the parts of a restoration, such as clasps on a removable partial denture, the parts must be surrounded with a suitable ceramic or investment material before the heating operation. The assembled parts are temporarily held together with sticky wax until they are surrounded with the appropriate investment material, after which the wax is softened and removed. The portion to be soldered is left exposed and free from investment to permit wax removal and effective heating before it is joined with solder.
ANSI/ADA Specification No. 93 (ISO 11244) for dental brazing investments defines two types of investment:
The specification specifies quality, fluidity, setting time, compressive strength, linear thermal expansion, and linear setting expansion. The setting time must not differ by more than 30% from the time stated by the manufacturer. The compressive strength shall be in the range of 2.0 to 10 MPa. The linear setting and thermal expansions must not differ by more than 15% from the time stated by the manufacturer.
The investment for soldering of low-melting-point alloys is similar to casting investments containing quartz and a calcium sulfate hemihydrate binder. For high-melting-point alloys, a phosphate-bonded investment is used.
Soldering investments are designed to have lower setting and thermal expansions than casting investments, a feature that is desirable so the assembled parts do not shift in position during the setting and heating of the investment. Soldering investments are often made of ingredients that do not have as fine a particle size as the casting investment, because the smoothness of the mass is less important. Relatively little information is available in the dental literature on the properties of soldering investments.
INVESTMENT FOR ALL-CERAMIC RESTORATIONS
Two types of investment materials have been developed recently for producing all-ceramic restorations. The first type is used for the cast glass technique. This investment is provided by the manufacturer of the glass casting equipment and is composed of phosphate-bonded refractories. The second type of investment for making all-ceramic restorations is the refractory die type of material, which is used for all-ceramic veneers, inlays, and crowns. Refractory dies are made by pouring the investment into impressions. When the investment is set, the die is removed, and is heated to remove gases that may be detrimental to the ceramic (degassing). A refractory die spacer may be added to the surface. Next, porcelain or other ceramic powders are added to the die surface and fired. These materials must accurately reproduce the impression, remain undamaged during the porcelain firing, and have a thermal expansion compatible with that of the ceramic (otherwise the ceramic could crack during cooling). These materials are also phosphate-bonded, and they generally contain fine-grained refractory fillers to allow accurate reproduction of detail. ANSI/ADA Specification No. 92 (ISO 11245) for phosphate-bonded refractory die materials describes the required properties.
Buchanan, AS, Worner, HK. Changes in the composition and setting characteristics of plaster of paris on exposure to high humidity atmospheres. J Dent Res. 1945;24:65.
Chong, JA, Chong, MP, Docking, AR. The surface of gypsum cast in alginate impression. Dent Pract. 1965;16:107.
Combe, EC, Smith, DC. Some properties of gypsum plasters. Br Dent J. 1964;117:237.
Docking, AR. Gypsum research in Australia: the setting process. Int Dent J. 1965;15:372.
Docking, AR. Some gypsum precipitates. Aust Dent J. 1965;10:428.
Earnshaw, R. The consistency of gypsum products. Aust Dent J. 1973;18:33.
Earnshaw, R, Smith, DC. The tensile and compressive strength of plaster and stone. Aust Dent J. 1966;11:415.
Fairhurst, CW. Compressive properties of dental gypsum. J Dent Res. 1960;39:812.
Fan, PL, Powers, JM, Reid, BC. Surface mechanical properties of stone, resin, and metal dies. J Am Dent Assoc. 1981;103:408.
Garber, DK, Powers, JM, Brandau, HE. Effect of spatulation on the properties of high-strength dental stones. J Mich Dent Assoc. 1985;67:133.
Hollenback, GM, Smith, DD. A further investigation of the physical properties of hard gypsum. J Calif Dent Assoc. 1967;43:221.
Jorgensen, KD. Studies on the setting of plaster of paris. Odont Tskr. 1953;61:305.
Lindquist, JT, Brennan, RE, Phillips, RW. Influence of mixing techniques on some physical properties of plaster. J Prosthet Dent. 1953;3:274.
Mahler, DB. Hardness and flow properties of gypsum materials. J Prosthet Dent. 1951;1:188.
Mahler, DB. Plasters of paris and stone materials. Int Dent J. 1955;5:241.
Mahler, DB, Asgarzadeh, K. The volumetric contraction of dental gypsum material on setting. J Dent Res. 1953;32:354.
Neville, HA. Adsorption and reaction. I. The setting of plaster of paris. J Phys Chem. 1926;30:1037.
Peyton, FA, Leibold, JP, Ridgley, GV. Surface hardness, compressive strength, and abrasion resistance of indirect die stones. J Prosthet Dent. 1952;2:381.
Phillips, RW, Ito, BY. Factors affecting the surface of stone dies poured in hydrocolloid impressions. J Prosthet Dent. 1952;2:390.
Sanad, MEE, Combe, EC, Grant, AA. The use of additives to improve the mechanical properties of gypsum products. J Dent Res. 1982;61:808.
Sarma, AC, Neiman, R. A study on the effect of disinfectant chemicals on physical properties of die stone. Quint Int. 1990;21:53.
Stern, MA, Johnson, GH, Toolson, LB. An evaluation of dental stones after repeated exposure to spray disinfectants. Part I: Abrasion and compressive strength. J Prosthet Dent. 1991;65:713.
Sweeney, WT, Taylor, DF. Dimensional changes in dental stone and plaster. J Dent Res. 1950;29:749.
Torrance, A, Darvell, BW. Effect of humidity on calcium sulphate hemihydrate. Aust Dent J. 1990;35:230.
von Fraunhofer, JA, Spiers, RR. Strength testing of dental stone: a comparison of compressive, tensile, transverse, and shear strength tests. J Biomed Mater Res. 1983;17:293.
Wiegman-Ho, L, Ketelaar, JAA. The kinetics of the hydration of calcium sulfate hemihydrate investigated by an electric conductance method. J Dent Res. 1982;61:36.
Williams, GJ, Bates, JF, Wild, S. The effect of surface treatment of dental stone with resins. Quint Dent Technol. 1983;7:41.
Worner, HK. Dental plasters. I. General, manufacture, and characteristics before mixing with water. Aust J Dent. 1942;46:1.
Worner, HK. Dental plasters. II. The setting phenomenon, properties after mixing with water, methods of testing. Aust J Dent. 1942;46:35.
Worner, HK. The effect of temperature on the rate of setting of plaster of paris. J Dent Res. 1944;23:305.
Anderson, JN. Applied dental materials, ed 5. Oxford: Blackwell Scientific, 1976.
Asgar, K, Lawrence, WN, Peyton, FA. Further investigations into the nature of hygroscopic expansion of dental casting investments. J Prosthet Dent. 1958;8:673.
Asgarzadeh, K, Mahler, DB, Peyton, FA. The behavior and measurement of hygroscopic expansion of dental casting investment. J Dent Res. 1954;33:519.
Chew, CL, Land, MF, Thomas, CC, et al. Investment strength as a function of time and temperature. J Dent. 1999;27:297.
Delgado, VP, Peyton, FA. The hygroscopic setting expansion of a dental casting investment. J Prosthet Dent. 1953;3:423.
Docking, AR. The hygroscopic setting expansion of dental casting investments. I. Aust J Dent. 1948;52:6.
Docking, AR, Chong, MP, Donnison, JA. The hygroscopic setting expansion of dental casting investments. II. Aust J Dent. 1948;52:160.
Docking, AR, Chong, MP. The hygroscopic setting expansion of dental casting investments. IV. Aust J Dent. 1949;53:261.
Docking, AR, Donnison, JA, Chong, MP. The hygroscopic setting expansion of dental casting investments. III. Aust J Dent. 1948;52:320.
Eames, WB, Edwards, CR, Jr., Buck, WH, Jr. Scraping resistance of dental die materials: a comparison of brands. Oper Dent. 1978;3:66.
Earnshaw, R. The effect of restrictive stress on the thermal expansion of gypsum-bonded investments. I. Inlay casting investments, “thermal expansion” type. Aust Dent J. 1966;11:345.
Earnshaw, R. The effects of additives on the thermal behaviour of gypsum-bonded casting investments. I. Aust Dent J. 1975;20:27.
Earnshaw, R, Morey, EF, Edelman, DC. The effect of potential investment expansion and hot strength on the fit of full crown castings made with phosphate-bonded investment. J Oral Rehabil. 1997;24:532.
Higuchi, T. Study of thermal decomposition of gypsum bonded investment. I. Gas analysis, differential thermal analysis, thermobalance analysis, x-ray diffraction. Kokubyo Gakkai Zasshi. 1967;34:217.
Jones, DW. Thermal analysis and stability of refractory investments. J Prosthet Dent. 1967;18:234.
Jones, DW, Wilson, HJ. Setting and hygroscopic expansion of investments. Br Dent J. 1970;129:22.
Lyon, HW, Dickson, G, Schoonover, IC. Effectiveness of vacuum investing in the elimination of surface defects in gold castings. J Am Dent Assoc. 1953;46:197.
Lyon, HW, Dickson, G, Schoonover, IC. The mechanism of hygroscopic expansion in dental casting investments. J Dent Res. 1955;34:44.
Luk, HW-K, Darvell, BW. Strength of phosphate-bonded investments at high temperature. Dent Mater. 1991;7:99.
Luk, HW-K, Darvell, BW. Effect of burnout temperature on strength of phosphate-bonded investments. J Dent. 1997;25:153.
Luk, HW-K, Darvell, BW. Effect of burnout temperature on strength of phosphate-bonded investments—part II. J Dent. 1997;25:423.
Mahler, DB, Ady, AB. An explanation for the hygroscopic expansion of dental gypsum products. J Dent Res. 1960;39:578.
Mahler, DB, Ady, AB. The influence of various factors on the effective setting expansion of casting investments. J Prosthet Dent. 1963;13:365.
Matsuya, S, Yamane, M. Thermal analysis of the reaction between II-CaSO4 and quartz in nitrogen flow. Gypsum Lime. 1980;164:3.
Miyaji, T, Utsumi, K, Suzuki, E, et al. Deterioration of phosphate-bonded investment on exposure to 100% relative humidity atmosphere. Bull Tokyo Med Dent Univ. 1982;29:53.
Moore TE: Method of making dental castings and composition employed in said method, U.S. Patent 1,924,874, 1933.
Mori, T. Thermal behavior of the gypsum binder in dental casting investments. J Dent Res. 1986;65:877.
Neiman, R, Sarma, AC. Setting and thermal reactions of phosphate investments. J Dent Res. 1980;59:1478.
Norling, BK, Reisbick, MH. Wetting of elastomeric impression materials modified by nonionic surfactant additions. J Dent Res. 1977;56B:148. (abstr)
O’Brien, WJ, Nielsen, JP. Decomposition of gypsum investment in the presence of carbon. J Dent Res. 1959;38:541.
Phillips, RW. Relative merits of vacuum investing of small castings as compared to conventional methods. J Dent Res. 1947;26:343.
Ryge, G, Fairhurst, CW. Hygroscopic expansion. J Dent Res. 1956;35:499.
Schilling, ER, Miller, BH, Woody, RD, et al. Marginal gap of crowns made with a phosphate-bonded investment and accelerated casting method. J Prosthet Dent. 1999;81:129.
Schnell, RJ, Mumford, G, Phillips, RW. An evaluation of phosphate bonded investments used with a high fusing gold alloy. J Prosthet Dent. 1963;13:324.
Shell, JS, Dootz, ER. Permeability of investments at the casting temperature. J Dent Res. 1961;40:999.
Shell, JS, Hollenback, GM. Setting and thermal investment expansion in longitudinal and transverse directions. J Calif Dent Assoc. 1965;41:511.
Tiara, M, Okazaki, M, Takahashi, J, et al. Effects of four mixing methods on setting expansion and compressive strength of six commercial phosphate-bonded silica investments. J Oral Rehabil. 2000;27:306.
Weinstein LJ: Composition for dental molds, U.S. Patent 1,708,436, 1929.