Prosthetic Applications of Polymers
PROPERTIES OF DENTURE BASE MATERIALS
Poly(methyl methacrylate) polymers were introduced as denture base materials in 1937. Previously, materials such as vulcanite, nitrocellulose, phenol formaldehyde, vinyl plastics, and porcelain were used for denture bases. The acrylic resins were so well received by the dental profession that by 1946, 98% of all denture bases were constructed from methyl methacrylate polymers or copolymers. Other polymers developed since that time include vinyl acrylic, polystyrene, epoxy, nylon, vinyl styrene, polycarbonate, polysulfone-unsaturated polyester, polyurethane, polyvinylacetate-ethylene, hydrophilic polyacrylate, silicones, light-activated urethane dimethacrylate, rubber-reinforced acrylics, and butadiene-reinforced acrylic.
Acrylic polymers have a wide variety of applications in restorative dentistry as denture bases, artificial teeth, denture repair materials, impression trays, provisional restorations, and maxillofacial appliances for skeletal defects. Provisional restorations are discussed in Chapter 9, and impression trays are discussed in Chapter 12.
PROPERTIES OF DENTURE BASE MATERIALS
The following list indicates the requirements for a clinically acceptable denture base material:
2. Satisfactory thermal properties
3. Processing accuracy and dimensional stability
4. Chemical stability (unprocessed as well as processed material)
5. Insolubility in and low sorption of oral fluids
10. Adhesion to plastics, metals, and porcelain
Many commercial materials meet these requirements. The vast majority of dentures made today are fabricated from heat-cured poly(methyl methacrylate) and rubber reinforced poly(methyl methacrylate). Fractures of dentures still occur, but are usually associated with carelessness or unreasonable use by the patient. Considering functional stresses, the oral environment, and expected service life, denture base materials perform remarkably well.
PHYSICAL FORM AND COMPOSITION
Denture base plastics are commonly supplied in a powder-liquid or a gel form. The powder-liquid type may contain the materials listed in Box 21-1.
Powder
Most commercial materials contain poly(methyl methacrylate), modified with small amounts of ethyl, butyl, or other alkyl methacrylates to produce a polymer somewhat more resistant to fracture by impact. The powder also contains an initiator such as benzoyl peroxide (see formula below) or diisobutylazonitrile to initiate the polymerization of the monomer liquid after being added to the powder.

The peroxide initiator may be added to the polymer or be present as a residual from the polymerization reaction and is present in amounts from 0.5% to 1.5%.
Pure polymers, such as poly(methyl methacrylate), are clear and are adaptable to a wide range of pigmentation. The pigments used to obtain the various tissue-like shades are compounds such as mercuric sulfide, cadmium sulfide, cadmium selenide, ferric oxide, or carbon black, although the use of cadmium salts is suspect because of demonstrated toxicity. These pigments may be locked into the polymer beads by addition during the commercial polymerization, or mechanically mixed with the polymer beads after polymerization, as shown in Fig. 21-1. Generally, the latter method is used, and the uneven distribution of the pigment in the final denture provides a mottled, natural appearance. In addition to coloring agents, zinc or titanium oxides are used as opacifiers, with titanium dioxide being most effective. Dyed synthetic fibers made from nylon or acrylic are usually added to simulate the small capillaries of the oral mucosa.
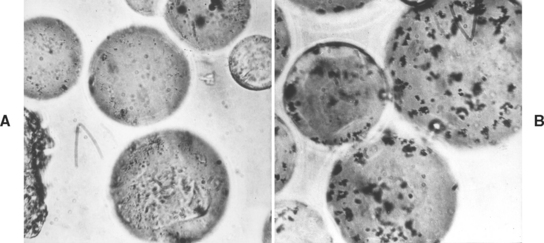
FIGURE 21-1 Polymer beads with the pigment locked into the polymer, A, and mechanically mixed with the polymer, B (×450). (Courtesy Dentsply International, York, PA.)
Plasticizers such as dibutyl phthalate may be incorporated in the powder or the monomer. Inorganic particles such as glass fibers and beads or zirconium silicate have been added to plastics. The particles are usually treated with a coupling agent such as an unsaturated triethoxysilane to improve the wetting and bonding of the inorganic particles and the plastic. Studies have reported on the addition of whiskers of alumina, silicon carbide, boron nitride, and carbon fibers to dental plastics. Adding glass fibers and alumina (sapphire) whiskers increases the stiffness, decreases the thermal coefficient of expansion, and increases thermal diffusivity. Polyethylene-woven yarn and polyaramid fabric have also been used to reinforce acrylic polymers.
Most denture bases are radiolucent. Pieces of fractured dentures or temporary acrylic crowns have been aspirated by patients during traumatic injury and are difficult if not impossible to locate. A few denture base materials contain heavy metal compounds of elements such as barium or radiopaque glass fillers added to improve the radiopacity. It is necessary to add up to 20% by weight of these compounds to give sufficient radiopacity, and this results in a reduction in the strength of the material and a change in the appearance of the denture. A 3-year clinical study of a commercial radiopaque polymer, however, showed the dentures performed well and remained radiopaque. Other additives that provide radiopacity include bismuth or uranyl salts at concentrations of 10% to 15% and zirconyl dimethacrylate at 35%. Recently a new radiopaque terpolymer has been synthesized containing [2′3′5′-triodobenzyoyl]-ethyl methacrylate, methyl methacrylate and 2-hydroxyethyl methacrylate. This methacrylate terpolymer may find use as a radiopaque denture base material. It has been shown that esthetically pleasing radiopaque plastics can be made that do not demonstrate cytotoxicity or mutagenicity and have reasonable properties for prosthetic applications. In the future, efforts must be made to improve the handling properties, flexural deflection, and water sorption of radiopaque denture base materials.
Liquid
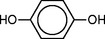
The liquid component of the powder-liquid type acrylic resin is methyl methacrylate, but it may be modified by the addition of other monomers. Because these monomers may be polymerized by heat, light, or traces of oxygen, inhibitors are added to give the liquid adequate shelf life. The inhibitor most commonly used to prevent premature polymerization is hydroquinone, shown here, which may be present in concentrations of 0.003% to 0.1%.
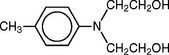
When a chemical accelerator rather than heat is used to speed up the peroxide decomposition and enable the polymerization of the monomer at room temperature, an accelerator is included in the liquid. These accelerators are tertiary amines, sulfinic acids, or the more stable salts of sulfinic acid. Commonly used amines are N,N-dimethyl-para-toluidine, and N,N-dihydroxyethyl-para-toluidine. These systems are referred to as self-curing, cold-curing, or autopolymerizing resins. The pour-type of denture resin is included in this category.
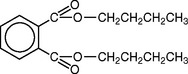
Plasticizers are sometimes added to produce a softer, more resilient polymer. They are generally relatively low-molecular-weight esters, such as dibutyl phthalate. Plasticizer molecules do not enter the polymerization reaction but do interfere with the interaction between polymer molecules. This makes the plasticized polymer softer than the pure polymer. One disadvantage in using plasticizers is that they gradually leach out of the plastic into oral fluids, resulting in hardening of the denture base. A polymer also may be plasticized by the addition of some higher ester such as butyl or octyl methacrylate to methyl methacrylate. The esters polymerize and form a more flexible plastic. This type of internal plasticizing does not leach out in the oral fluids, and the material remains flexible.
If a cross-linked polymer is desired, organic compounds such as glycol dimethacrylate are added to the monomer. Cross-linking compounds are characterized by reactive —CR=CH— groups at opposite ends of the molecules and serve to link long polymer molecules together. Using cross-linking agents provides greater resistance to minute surface cracking, termed crazing, and may decrease solubility and water sorption. Cross-linking materials may be present in amounts of 2% to 14%, but have little effect on the tensile strength, flexural properties, or hardness of acrylic plastics, although recovery from an indentation by a metal ball such as a Rockwell Superficial Hardness indenter is somewhat improved.
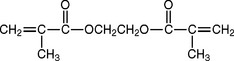
OTHER DENTURE MATERIALS
Several modified poly(methyl methacrylate) materials have been used for denture base applications. These include pour-type denture resins, hydrophilic polyacrylates, high-impact strength resins, rapid heat-polymerized acrylics, and light-activated materials.
Pour-Type Acrylics
The chemical composition of the pour-type denture resins is similar to poly(methyl methacrylate) materials that are polymerized at room temperature. The principal difference is in the size of the polymer powder or beads. The pour-type denture resins, commonly referred to as fluid resins, have much smaller powder particles; when mixed with monomer, the resulting slurry is very fluid. The mix is quickly poured into an agar-hydrocolloid or modified plaster mold and allowed to polymerize under pressure at 0.14 MPa. Centrifugal casting and injection molding are techniques used to inject the slurry into the mold.
High Impact Strength Acrylics
Denture base materials that have greater impact strength have been introduced. These polymers are reinforced with butadiene-styrene rubber. The rubber particles are grafted to methyl methacrylate to bond to the acrylic matrix. These materials are supplied in a powder-liquid form and are processed in the same way as other heat-accelerated methyl methacrylate materials.
Rapid Heat–Polymerized Acrylics
These hybrid acrylics are polymerized in boiling water immediately after being packed into a denture flask. The initiator is formulated from both chemical- and heat-activated initiators to allow rapid polymerization without the porosity one might expect. After placing the denture in boiling water, the water is brought back to a full boil for 20 minutes. After bench cooling to room temperature, the denture is deflasked, trimmed, and polished in the conventional manner.
Light-Activated Resins
This denture base material consists of a urethane dimethacrylate matrix with an acrylic copolymer, microfine silica fillers, and a photo-initiator system. It is supplied in premixed sheets having a claylike consistency. The denture base material is adapted to the cast while it is still pliable. The denture base can be polymerized in a light chamber without teeth and used as a record base. The teeth are processed to the base with additional material and the anatomy is sculptured while the material is still plastic. The acrylic is polymerized in a light chamber with blue light of 400 to 500 nm. The denture rotates in the chamber to provide uniform exposure to the light source. Various formulations of light-activated acrylic are used for many prosthetic applications.
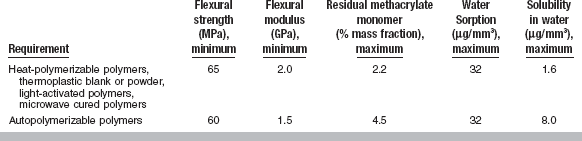
ANSI/ADA SPECIFICATION NO. 12 (ISO 1567) FOR DENTURE BASE RESINS
The scope, requirements, and procedures for evaluating denture base plastics are listed in ANSI/ADA Specification No. 12. The plastics covered by the specification includes acetal, acrylic, carbonate, dimethacrylate acid ester, styrene, sulfone, and vinyl polymers, or mixtures of any of these polymers, as well as copolymers. Categories include the following types and classes:
Type 1—Heat-polymerizable polymers (Class 1, powder and liquid; Class 2, plastic cake)
Type 2—Autopolymerizable polymers (Class 1, powder and liquid; Class 2, powder and liquid pour-type resins)
Type 3—Thermoplastic blank or powder
The specification lists a number of general requirements for the nonprocessed materials. The liquid should be as clear as water and free of extraneous material, and the powder, plastic cake, or precured blank should be free of impurities such as dirt and lint. The specification further states that (1) a satisfactory denture shall result when the manufacturer’s instructions are followed; (2) the denture base should be nonporous and free from surface defects; (3) the cured plastic should take a high gloss when polished; (4) the processed denture should not be toxic to a normal, healthy person; (5) the color should be as specified; (6) the plastic should be translucent; and (7) the cured plastic should not show any bubbles or voids.
The specific requirements are that (1) within 5 minutes after reaching the proper consistency, indicated by clean separation from the walls of a glass mixing jar, the material shall have adequate flow properties so it will intrude to a depth of at least 0.5 mm into a 0.75-mm-diameter hole when a load of 50 N is placed on a plate 5 mm thick and 50 mm2 in area (this test is modified for pour-type acrylics); (2) water sorption shall not be more than 32 μg/mm3 after immersion for 7 days at 37° C; (3) the solubility shall not be more than 1.6 μg/mm3 for Types 1, 3, 4, and 5 and 8.0 μg/mm3 for Type 2 after the water sorption specimen is dried to constant weight; (4) the plastic shall show no more than a slight color change when exposed 24 hours to a specified ultraviolet lamp test; (5) the flexural strength shall be a minimum of 65 MPa for Types 1, 3, 4, and 5 and 60 MPa for Type 2; (6) the flexural modulus shall be a minimum of 2.0 GPa for Types 1, 3, 4, and 5 and 1.5 GPa for Type 2; and (7) the residual methyl methacrylate monomer shall be a maximum of 2.2% for Types 1, 3, 4, and 5 and 4.5% for Type 2. These properties are summarized in Table 21-1.
PROPERTIES OF DENTAL PLASTICS
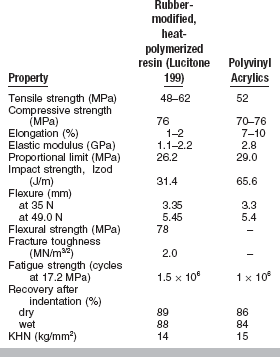
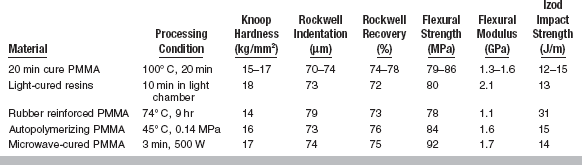
Conventional heat-accelerated acrylic resins are still the predominant denture base materials in use. These materials are typically low in strength, soft and fairly flexible, brittle on impact, and fairly resistant to fatigue failure. The properties of poly(methyl methacrylate) and polyvinyl acrylic are listed in Table 21-2. Several properties of newer denture base materials are listed in Table 21-3.
TABLE 21-2
Properties of Denture Base Polymers
Adapted from Smith LT, Powers JM, Ladd D: Int J Prosthodont 5:315, 1992; Stafford GD, Bates JF, Huggett R et al: J Dent 8:292, 1980.
TABLE 21-3
Mechanical Properties of New Denture Resins
Adapted from Smith LT, Powers JM, Ladd D: Int J Prosthodont 5:315, 1992.
Tensile and Compressive Strength
Table 21-2 reveals small differences between poly(methyl methacrylate) and polyvinyl acrylic. The two acrylics have adequate tensile and compressive strength for complete or removable partial denture applications. Fractures in these materials are usually caused by accidental dropping of a denture or by faulty fabrication. Fractures may also be caused by flexure fatigue from cyclic stresses of low magnitude in service.
Elongation
Elongation, in combination with the ultimate strength, is an indication of the toughness of the plastic. The larger the area under the stress-strain curve, the tougher the material is. Materials having a combination of reasonable tensile strength and elongation will be tough materials, and those with low elongation will be brittle. Examples of tough materials are polyvinylchloride or polyethylene, whereas poly(methyl methacrylate) is more brittle.
Values for percent elongation of polyvinyl acrylics are considerably higher than for poly(methyl methacrylate) and, as expected, the polyvinyl acrylics are tougher and permit larger deformation before fracture.
Proportional Limit
There is some question as to whether dental plastics possess a true proportional limit, because they may be permanently deformed at low stresses, and, as a result, the proportional limit obtained is a function of the rate of stress application. Despite this characteristic, the proportional limit determined under standard conditions is important. A plastic with a low proportional limit will begin to deform permanently at a low stress. If the percent elongation is relatively high, it may deform permanently to a considerable extent before rupture. If the proportional limit is high, considerable stress is required before permanent deformation will occur. A denture material should have a proportional limit sufficiently high that permanent deformation does not result from the stress applied during mastication. Permanent deformation may result in loss of retention or loosening of the teeth embedded in the denture base. The values reported in Table 21-2 for the proportional limit of poly(methyl methacrylates) and polyvinyl acrylics are approximately the same and the dimensional stability of dentures made of these materials would be expected to be similar.
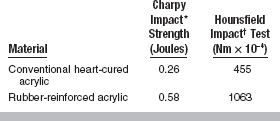
Impact Strength
Impact strength is a measure of the energy absorbed by a material when it is broken by a sudden blow. The impact strength for polyvinyl acrylics is about twice that of poly(methyl methacrylates) (see Table 21-2), which indicates that polyvinyl acrylic absorbs more energy on impact and is more resistant to fracture.
Although the addition of plasticizers may increase the impact strength of plastics, the increases are accompanied by decreases in hardness, proportional limit, elastic modulus, and compressive strength. Ideally, a denture base plastic should have sufficiently high impact strength to prevent breakage on accidental dropping, but not at the expense of the other properties.
The impact strength of several denture base materials is listed in Tables 21-3 and 21-4. There are differences in relative impact strength with all tests. However, the impact strength of rubber-reinforced acrylic is considerably higher in all instances. When surface defects are present, this improved impact resistance is significantly reduced.
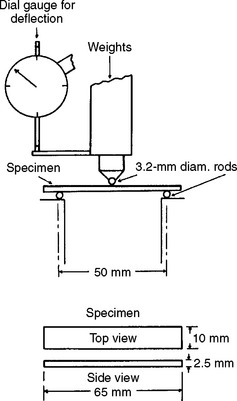
Flexural Strength and Modulus
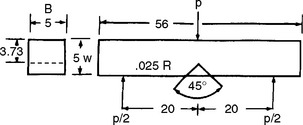
In the evaluation of denture plastics, flexural strength measurements are used to a greater extent than either tensile or compressive strength, because this test more closely represents the type of loading in vivo. Flexural strength is determined by applying an increasing load until fracture at the center of a test specimen, as shown in Fig. 21-2. The deflection in millimeters at the middle of the plastic specimen is recorded, allowing the flexural modulus to be calculated. As shown in Table 21-3, the flexural strength varies from 78 to 92 MPa and the flexural modulus varies from 1.1 to 2.1 GPa for various denture base resins. When compared with metals used as denture bases, the elastic moduli of all plastics are quite low.
A transverse deflection test was used until the late 1990s to evaluate denture base resins. The requirements were that the deflection in the center of the specimen should be no more than 2.5 mm for a load of 15 to 35 N, and between 2.0 and 5.5 mm for a load of 15 to 50 N. All specimens were tested in water at 37° C after storage in water for 2 days at 37° C.
A comparison of the transverse deflection of several types of materials is seen in Table 21-2. Note that the pour-type acrylic fractured in this test and the rapid heat-cured acrylic was slightly less flexible than the other materials.
Fatigue Strength
Dentures are subjected to a large number of smaller cyclic stresses during mastication. For this reason, the fatigue properties of denture plastics are important. Fatigue strength represents the number of cycles before failure at a certain stress. Values of the fatigue strength at a stress of 17.2 MPa for poly(methyl methacrylates) and polyvinyl acrylic plastics are 1.5 × 106 cycles and 1 × 106 cycles, respectively. Because the current denture base plastics hold up well in service, a value of 1 × 106 cycles at 17.2 MPa is apparently an adequate fatigue strength value.
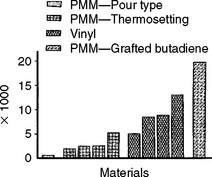
There are many flexural fatigue tests reported in the literature for denture base resins. Results from one of these tests are shown in Fig. 21-3. The specimens were repeatedly flexed under a load of 35.8 N at 342 flexures per minute in a fatigue-testing machine. The flexural fatigue strength of the rubber reinforced acrylic was superior to the other materials, and the pour-type acrylic had the lowest value.
Fracture Toughness
Because the geometry of denture bases is complex and stresses can be concentrated in flaws on the surface or in frenum notches, cracks can occur in the denture base. Several tests are available for determining fracture toughness. One method for testing fracture toughness is to bend a notched specimen and record the force required for crack propagation (Fig. 21-4). Several denture base materials are compared in Table 21-5. The fracture toughness of the high-impact acrylic appears to be no better than that of vinyl or conventional acrylic resin. The rapid heat-cured acrylic had the highest fracture toughness, and the pour-type acrylic the lowest toughness. The fracture toughness of acrylic is greater when specimens are saturated with water rather than dry.
Compressive Creep
When denture base resins are placed under a load they will deform (creep) with time. The lowest compressive creep rates are found for heat-polymerized materials. Chemically accelerated acrylics, both dough and pour-type, have higher values for compressive creep. At low stress levels, the type and quantity of cross-linking agents have no major affect on creep. However, at higher stress levels, creep decreases with increasing quantities of cross-linking agents. For heat-polymerized materials, when the temperature is increased from 37° to 50° C the mode of failure changes from brittle to ductile. For autopolymerizing acrylics, the materials fail in a ductile manner at both temperatures.
Recovery after Indentation
Indentation recovery of plastics in equilibrium with water is generally lower than when dry. The recovery from indentation of a 1.27-cm diameter steel ball loaded for 10 minutes at 30 kg is given in Table 21-2. The time allowed for recovery was 10 minutes. Recovery was 86% to 89% for dry specimens and 84% to 88% for wet specimens. The results for several new products are seen in Table 21-3.
A modified Wallace hardness tester has been used to measure the hardness, creep, and recovery of denture base polymers and the effects of cyclic loading. Results demonstrate the viscoelastic nature of denture base polymers. A torsional pendulum also may be used to evaluate the viscoelastic properties of denture base resins. These tests are useful in evaluating the effect of free monomer, plasticizers, and degree of cross-linking.
Hardness
The low Knoop hardness number of the denture base plastics (see Tables 21-2 and 21-3) indicates these materials may be scratched easily and abraded. Cross-linked poly(methyl methacrylate) is only slightly harder than regular poly(methyl methacrylate) (about 15 kg/mm2). The incorporation of fillers in plastics may alter the resistance to abrasion, but the hardness of the plastic matrix remains unchanged. Polishing, shell blasting, and cleaning denture bases by brushing should be carried out with this in mind.
Abrasion Resistance
Abrasion resistance of denture base resins has been evaluated by abrading specimens against 600-grit silicon carbide paper for 1 hour under a stress of 0.26 MPa in water at 37° C and measuring loss of material (Table 21-6). All materials had similar wear characteristics. However, the vinyl acrylic had the best and the pour-type acrylic had the least wear resistance.
THERMAL CHARACTERISTICS
The thermal properties of acrylics are important because they are usually processed at 74° C and in service are in contact with hot and cold foods and beverages. If a chemical accelerator is used rather than heat, the acrylic is still subjected to exothermic heat resulting from the polymerization reaction.
Thermal Conductivity
Dental plastics are poor thermal and electrical conductors. Compared with gold, cobalt alloys, or even human dentin, which have thermal conductivities of 0.7, 0.16, and 1.3 × 10−3 cal/sec/cm2 (° C/cm), respectively, the values for the various types of plastics listed in Table 21-7 are low. Low thermal conductivity allows plastic denture bases to serve as an insulator between the oral tissues and hot or cold materials placed in the mouth. It has been shown that the inclusion of sapphire whiskers in poly(methyl methacrylate) increases heat conductivity.
Specific Heat
Specific heat, or the heat required to raise the temperature of a gram of plastic 1° C, is a thermal property closely related to thermal conductivity. Although it is not obvious, it may be shown that the ratio of thermal conductivity to the product of specific heat and density is a constant for a particular material and represents the velocity of temperature disturbance in a plastic. The higher this ratio, termed diffusivity, the greater the velocity of heat transfer through a material. The specific heats for poly(methyl methacrylates) and polyvinyl acrylics are similar, and the respective thermal conductivities are not greatly different; therefore, the diffusivity of plastics will be roughly equivalent, 0.123 mm2/sec.
Thermal Coefficient of Expansion
The temperature change from processing temperature to room temperature or mouth temperature indicates the importance of the thermal coefficient of expansion. Plastics have relatively high thermal coefficients of expansion (71 to 81 × 10−6/° C) compared with other dental materials. Gold, amalgam, and tooth structure have values of 14.4 × 10−6/° C, 22 to 28 × 10−6/° C, and 11.4 × 10−6/° C, respectively. The addition of fillers such as glass reduces the thermal coefficient of expansion, although the reduction is not a linear function of the amount of the filler present. Thermal expansion is important in the fit of denture bases. It is apparent that a denture that fits a cast accurately at room temperature will not fit exactly the same at mouth temperature.
Heat Distortion Temperature
Heat distortion temperature is a measure of the ability of a plastic to resist dimensional distortion by heat. This is the temperature at which a specimen flexurally loaded at 1.8 MPa stress deflects a distance of 0.25 mm. These temperatures are sufficiently high that they are of little concern except in the repair of dentures. The heat distortion temperature for polyvinyl acrylic is 54° to 77° C and for poly(methyl methacrylates) 71° to 91° C. These values suggest the need for keeping repair temperatures low by using chemically or light-polymerized materials for this purpose.
Plastics or polymers, when heated, are transformed from a glassy or brittle condition to a rubbery condition. The temperature of this transition is called the glass transition, Tg. Molecular motions are allowed above this temperature that are not per-mitted at lower temperatures, thus plastics are easier to deform above Tg.
OTHER PROPERTIES OF DENTURE POLYMERS
The density, or the weight in grams of a cubic centimeter (g/cm3) of material, varies in plastics because of variations in molecular weight. Denture base plastics have densities ranging from 1.16 to 1.36 g/cm3 (Table 21-8), or slightly greater than the density of water.
TABLE 21-8
Miscellaneous Properties of Poly (Methyl Methacrylate) Denture Base Polymers
| Property | Poly(methyl methacrylates) |
| Density (g/cm3) | 1.16–1.18 |
| Polymerization shrinkage (% by volume) | 6* |
| Dimensional stability | Good |
| Water sorption (mg/cm2; ADA Test) | 0.69 |
| Water solubility (mg/cm2) | 0.02 |
| Resistance to weak acids | Good |
| Resistance to weak bases | Good |
| Effect of organic solvents | Soluble in ketones, esters, and aromatic and chlorinated hydrocarbons |
| Processing ease | Good |
| Adhesion to metal and porcelain | Poor |
| Adhesion to acrylics | Good |
| Colorability | Good |
| Color stability | Yellows very slightly |
| Taste or odor | None |
| Tissue compatibility | Good |
| Shelf life | Powder and liquid, good; gel, fair |
*Monomer shrinkage in mixes with polymer/monomer ratios of approximately 3:1.
Polymerization Shrinkage
The density of methyl methacrylate monomer is only 0.945 g/cm3 at 20° C, compared with 1.16 to 1.18 g/cm3 for poly(methyl methacrylate). This increase in density is mainly accounted for by an approximate 21% decrease in volume of monomer during polymerization. Because the ratio of polymer to monomer used in the preparation of dental poly(methyl methacrylates) and polyvinyl acrylics is usually 3 : 1, the free volumetric shrinkage amounts to about 6%. The light-activated denture base material has low polymerization shrinkage of 3% because higher-molecular-weight oligomers are used.
It should be pointed out that the linear shrinkage values reported in the literature are generally much less than would be expected (Table 21-9) on the basis of the free volumetric shrinkage, because a portion of the polymerization takes place after the plastic has attained a solid condition, resulting in residual stresses in the plastic rather than additional shrinkage. An ideal plastic would be one that had no polymerization shrinkage, but thermal dimensional change would still result from cooling the plastic from molding temperature to room temperature.
Dimensional Stability and Accuracy
The dimensional stability of the denture during processing and in service is important in the fit of the denture and the satisfaction of the patient. In general, if the denture is properly processed, the original fit and the dimensional stability of the various denture base plastics are good. However, excess heat generated during finishing of the denture can easily distort a denture base by releasing residual stresses.
It has been shown that chemically activated denture bases processed by dough molding with a dimensional accuracy of −0.1% were more accurate than heat-activated denture bases at −0.4%. The most accurate dentures were produced using either a chemically activated pour resin processed under pressure at 45° C or a microwave-activated resin. A visible light-activated resin was more accurate than a conventional heat-activated resin. In the past, injection-molded dentures were less accurate than compression-molded materials. Recent studies demonstrate that dentures processed by new injection molding methods are more accurate than standard compression molding. The increase in vertical dimension of occlusion was very small for the injection-molded acrylic when compared with the conventional compression-molded acrylic.
In another report, six different denture base materials were processed by heat, light, or microwave energy. The denture bases were removed from the casts, finished, and polished. After storage in distilled water for 42 days to allow for water sorption, the dentures were placed back on the stone casts on which they were made and ranked for accuracy of fit by five evaluators. Denture bases processed by microwave energy, low heat at 45° C (autopolymerizing acrylics), or visible light fit better than resins processed at either 74° C (conventional acrylics) or 100° C (rapid heat-cured acrylics).
When evaluated in two dimensions, the dimensional stability of denture bases is usually reported at less than 1%. In one study the accuracy of maxillary dentures was measured at six locations from anterior to posterior. For all materials studied, the accuracy was better from the anterior of the denture to the middle of the palate (generally <100 μm) and became worse toward the posterior of the denture. In a recent study of three-dimensional stability of several products, changes ranged from 0.2% to 8.1% in the frontal dimension, and 0.2% to 9% in the lateral dimension. Changes were greatest in the cross-arch dimension.
There are numerous articles in the literature that report conflicting results for accuracy of denture base resins. However, it is encouraging that emphasis is being placed on dimensional accuracy and some of the newer materials appear superior to older products.
Water Sorption and Solubility
The sorption of water also alters the dimensions of acrylic dentures. This change in dimension is, for the most part, reversible and the plastic may go through numerous expansions and contractions when alternately soaked in water and dried. However, repeated wetting and drying of dentures should be avoided by the patient, because irreversible warpage of the denture bases may result. Denture plastics of the same type may vary considerably in water sorption because of the presence of additives. The thickness of the plastic specimen and the type of polymer influence whether equilibrium water sorption will be attained in 24 hours.
Temperature also affects the rate at which water is absorbed, because the diffusion coefficient is increased by a factor of two between room and oral temperature and the equilibrium absorption value does not change.
Denture plastics can be tested for water sorption: a dried plastic disk 50 mm in diameter and 0.5 mm thick is stored in distilled water at 37° C for 7 days, after which the increase in water is determined and the sorption recorded in micrograms per cubic millimeter. In addition, the solubility of the plastic is measured on the same specimen by redrying to constant weight in a desiccator and reweighing to determine the loss in weight in micrograms per cubic millimeter. A denture plastic should have water sorption not more than 32 μg/mm3 after immersion for 7 days at 37° C and solubility not more than 1.6 μg/mm3 for Types 1, 3, 4, and 5 plastics and 8.0 μg/mm3 for Type 2 plastics (see Table 21-1).
Resistance to Acids, Bases, and Organic Solvents
The resistance of denture plastics to water solutions containing weak acids or bases is good to excellent. Denture plastics are quite resistant to organic solvents, with poly(methyl methacrylate) being more resistant than polyvinyl acrylic. Both are soluble in aromatic hydrocarbons, ketones, and esters. Alcohol will cause crazing in certain denture plastics. Ethanol also functions as a plasticizer and can reduce the glass transition temperature. Therefore, solutions containing alcohol should not be used for cleaning or storing dentures. Incorporating ethylene glycol dimethacrylate as a cross-linking agent in denture base resins has little effect on water sorption, but significantly improves solvent resistance.
Adhesion Properties
The adhesion of denture plastics to untreated porcelain or metals is generally poor. As a result, porcelain teeth or combined metal and plastic bases should be designed so that the porcelain or metal is held by mechanical retention. It has been shown that the lack of adhesion between a plastic base and porcelain teeth provides an area in which microorganisms present in the oral fluids may incubate, and this makes maintenance of a clean denture more difficult. This problem can be avoided by the use of plastic teeth, which form a bond with denture base materials or by organosilane treatment of porcelain teeth (although this treatment is not routinely used). The silane coupling agent, γ-methacryloxypropyltrimethoxysilane, provides a bond between the porcelain and the plastic surface. Many studies have demonstrated the effectiveness of bonding denture base resins to partial denture alloys or titanium using the following ingredients alone or in combination: silica blasting, metal etching, silane coupling agents, 4-META adhesive resin, other organic primers such as 10-methacryloxydecyl dihydrogen phosphate.
Esthetics
The esthetic qualities of denture base plastics include such properties as colorability, color stability, taste, and odor. The ability of the plastics to be colored and their compatibility with dyed synthetic fibers for characterization are both good. Color-measuring systems have been used to compare the actual color of denture base resins with the color of gingival tissues. Results indicate that few commercial products actually match the color of the tissue they are replacing. A color stability test requires that a specimen exposed for 24 hours to an ultraviolet light source shall not show more than a slight change in color when compared with an original specimen. One study questioned the stain resistance of the light-activated denture acrylic. Current denture products have no taste or odor when properly processed.
Tissue Compatibility
The tissue compatibility or allergic sensitization of the skin to the components of denture plastics or to processed plastics has been a subject of considerable contention. It may be concluded that completely polymerized poly(methyl methacrylate) or polyvinyl acrylics rarely cause allergic reactions but that methyl methacrylate monomer or other trace components in the monomer may produce an allergic reaction. Dentures prepared by polymerization of methyl methacrylate with a chemical accelerator may have sufficient residual methyl methacrylate monomer present in the finished denture to cause an allergic reaction in patients who are sensitive to methyl methacrylate monomer. This reaction diminishes as residual monomer leaches out of the plastic. Allergic reactions to heat-processed denture base plastics also occur but less often than with chemically accelerated plastics. Again, residual monomer is considered the allergen, and strict adherence to processing instructions recommended by the manufacturer can keep the residual monomer to a minimum. When patients are known to have suffered from an allergic reaction, processing the denture for extended periods (such as 24 versus 8 hours) may be helpful. Residual monomer levels can also be reduced dramatically by processing heat-polymerized poly(methyl methacrylate) in a water bath for 7 hours at 70° C, followed by boiling for 1 hour. Boiling has only a slight effect on the dimensional accuracy of the processed dentures. Vinyl acrylic or light-activated denture base materials are an alternative for those patients who are sensitive to methyl methacrylate monomer.
In one study, 53 patients wearing dentures and suffering from “burning-mouth syndrome” were evaluated for allergies related to various components of denture base plastics. Epicutaneous patch tests were used. Approximately 15 patients demonstrated a positive skin test to one or more of the following: N,N-dimethyl-para-toluidine, hydroquinone, formaldehyde, methyl methacrylate, and p phenylenediamine, as well as several metallic compounds. Pigments also may be toxic. There is one case reported of a patient who had an extensive systemic reaction to dentures. Patch testing was used and the patient had a positive reaction to the pure dye supplied by the manufacturer. The patient had no reaction to the unpigmented acrylic.
Plasticizers are commonly used in fairly high concentrations to lower the glass transition temperature and to decrease brittleness in soft denture liners. Unfortunately, some plasticizers, particularly phthalates, are known toxins; products that contain these materials should be evaluated for biocompatibility. With the demand for safety from health-related products, greater attention will be focused on the tissue compatibility of denture base materials.
The growth of Candida albicans on the surface of dentures is a concern for many denture patients. This organism is associated with denture stomatitis. An in vitro study has demonstrated the effectiveness of chlorhexidine gluconate in eliminating this organism. The chlorhexidine apparently can bind to acrylic surfaces for at least 2 weeks. Treating acrylic with Nystatin, followed by drying, produces similar results. The use of phenolic disinfectants can cause surface damage to denture base resins and is not recommended.
In addition to Candida albicans, many other microorganisms can adhere to denture base acrylics, such as Streptococcus oralis, Bacteroides gingivalis, B. intermedius, and S. sanguis. It is not surprising that many organisms adhere more to rougher surfaces than to those that are highly polished.
Shelf Life
The shelf life, or useful storage time at room temperature, for denture base plastics varies considerably. Acrylics packaged in the powder-liquid form have excellent shelf life, because the powder is almost indefinitely stable and the liquid usually is adequately protected from polymerization during storage by a hydroquinone inhibitor. Vinyl acrylics, packaged as a gel in which the monomer is in contact with the polymer, must be stored at refrigerator temperature (about 2° C) to have a reasonable storage life of 1 to 2 years. The shelf life of light-activated denture base materials has not been reported.
MANIPULATION AND PROCESSING OF DENTURE BASE POLYMERS
As mentioned previously, denture base polymers are supplied in several forms. The techniques used to process these materials into a finished complete or partial denture are briefly described subsequently, as are the justifications for the various procedures presented.
Texts on prosthetic dentistry describe impression techniques, pouring master stone casts, setting denture teeth, preparing the waxed denture, investing in a denture flask, removing the wax, and coating the stone mold to prevent adhesion of the polymer. A set of waxed dentures on stone casts with the teeth in position is shown in Fig. 21-5, and a flask that contains maxillary porcelain teeth ready for packing the acrylic dough is shown in Fig. 21-6.
HEAT-ACCELERATED ACRYLIC DENTURE POLYMERS
The general method for processing a heataccelerated acrylic denture base material consists of proportioning and mixing the polymer powder and the liquid monomer and allowing the monomer to react physically with the polymer in a sealed jar until a doughy consistency is reached. Before packing, all stone surfaces of the mold are coated with an alginate separator and allowed to dry. The dough is then packed into the treated denture mold containing the artificial teeth and “trial packed” by repeated application of slow pressure with a flask press until no excess flash remains and the material has a glossy surface. Polymerization is accomplished by applying heat and pressure, which are maintained until polymerization is complete. The flask is then bench cooled to room temperature, and the denture is deflasked, finished, and polished.

The reaction involved in the heat-accelerated powder-liquid type of acrylic is outlined in a simplified equation here. The powder, consisting of the polymer plus the initiator, and the liquid monomer, containing the inhibitor, are proportioned in the ratio of approximately 3:1 by volume.
Proportioning
There must be sufficient liquid to completely wet the polymer powder. The powder and liquid are mixed with a stainless steel spatula and then kept in the sealed jar during the initial stages of reaction to avoid loss of monomer by evaporation. Incompletely wetted portions can result in a streaked or blanched appearance in the denture because of incomplete polymerization of the polymer during processing. Care should be taken to avoid breathing the monomer vapor. Animal studies have shown that the monomer can affect respiration, cardiac function, and blood pressure.
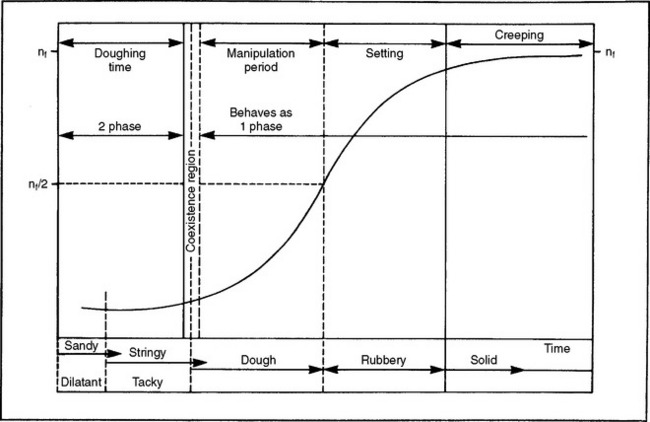
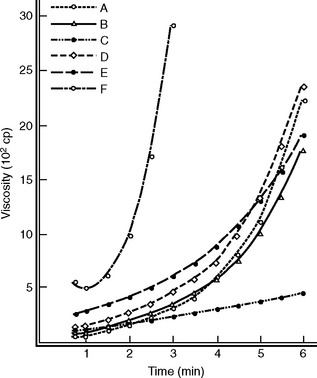
The polymer-monomer mixture, on standing, goes through several distinct consistencies, which may be qualitatively described as (1) sandy, (2) stringy or sticky, (3) doughy or puttylike, (4) rubbery or elastic, and (5) stiff. When the mixture is doughy it has desirable qualities for packing into a denture flask. Different products vary considerably in the time required to reach the doughy condition and the length of time they remain at the packing consistency. These stages or consistencies are represented by a model of the viscosity of the polymer/monomer mix (Fig. 21-7). The symbol nf represents the final viscosity and nf/2 is one half of this value. The powder-liquid mixture of a denture base polymer should be at the packing consistency when the mixture separates cleanly from the walls of the glass mixing jar. This consistency will be attained less than 40 minutes from the start of mixing. The consistency, determined 5 minutes after the packing consistency is reached, should be such that when 5 g of the material are placed over 0.75-mm diameter holes in a brass plate and loaded with a 50-N weight, the dough intrudes into the holes to a depth of not less than 0.5 mm. If the material passes this test, adequate time should be available for trial packing of the denture mold and for final closure. During the various consistency stages, little polymerization is taking place and the reaction occurring is physical in nature. This reaction includes some solution of the polymer in the monomer and some absorption of the monomer by the polymer, as well as wetting of the polymer particles. For conventional acrylic polymers, no substantial polymerization occurs until the denture flask is heated to above 70° C. If too much monomer is used in the mixture, polymerization shrinkage will be greater, additional time will be required to reach the packing consistency, and there will be a tendency for porosity to occur in the denture. If too little monomer is used, the polymer will be insufficiently wetted, the dough will be difficult to manage, and the quality of the final denture will be compromised.
Packing
The powder-liquid mixture should be packed into the flask at the doughy stage (Fig. 21-8, A) for several reasons. If it is packed at the sandy or stringy stages, too much monomer will be present between the polymer particles, the material will be of too low a viscosity to pack well, and it will flow out of the flask too easily. Packing too early may also result in porosity in the final denture base. If packed at the rubbery-to-stiff stage, the material will be too viscous to flow well under the pressure of the flask press, and metal-to-metal contact of the flask halves will not be obtained. Delayed packing will result in loss of detail in the denture, movement or fracture of the teeth, and an increase in the contact vertical dimension of the denture. Some polymers now have increased working time and remain in the doughy stage for periods approaching 1 hour. This permits the packing of several dentures at the same time. Other products, particularly the rubber-reinforced materials, have shorter working times, and in some instances only one or two dentures should be packed with one mix. The polymer dough should not be manipulated excessively with bare hands. The monomer is a good solvent for body oils and may pick up dirt from the hands, resulting in a nonesthetic denture. Monomer may also enter the blood stream through the skin.
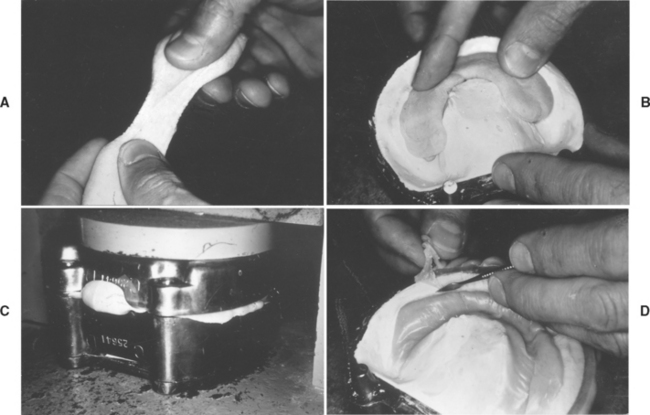
FIGURE 21-8 A, Powder-liquid acrylic denture base material in the dough consistency. B, An excess being placed in the mold. C, The excess being forced out between the two portions of the flask. D, The flash being trimmed away. (Adapted from Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 7, St. Louis, 2000, Mosby.)
The acrylic dough is packed into the flask in slight excess by use of a hydraulic, pneumatic, or mechanical press (Fig. 21-8, B), and this excess is removed by trial packing procedures, with a damp cellophane or polyethylene film used as a separator for the upper half of the flask. The film separator allows easy separation of the flask halves during trial packing procedures. The closing force of the press is applied slowly during the trial packing to allow the excess or flash to flow out between the halves of the flask (Fig. 21-8, C). The flask is opened at intervals and the flash trimmed away (Fig. 21-8, D). Before final closure, the separating film is removed and discarded. During final closure of the flask, metal-to-metal contact of the flask halves is completed in the press, the flasks are placed in a flask press that maintains pressure, and the denture is processed.
Processing
For heat-polymerized polymers the curing temperature must be maintained close to 74° C, because the polymerization reaction is strongly exothermic. The heat of reaction will be added to the heat used to raise the material to the polymerization temperature. The temperature rise at various positions in a denture flask during a curing cycle is illustrated in Fig. 21-9. The initial temperature increases are in the following order—flask, plaster or stone, tinfoil, and polymer—because the outside of the flask is in contact with the water bath. The temperatures in the different areas increase at approximately the same rate until the temperature of the polymer dough reaches about 70° C. At this point the material becomes quite fluid, and the decomposition rate of the benzoyl peroxide initiator is rapid enough for a substantial amount of polymerization to take place. As the polymerization reaction proceeds, the exothermic heat of reaction increases the temperature of the polymer to values considerably above the surrounding materials and above the boiling point of the methyl methacrylate monomer. This occurs because both the polymer and stone are poor thermal conductors and the heat of reaction is dissipated slowly. Also, the larger the mass or bulk of material, the higher the peak temperature attained in the polymer. In thick sections of a denture the temperature rise will be greater than in thin sections. It has been demonstrated that the strength of thin sections of a denture may be less than that of the thick sections because of a lower degree of polymerization. Because of the excessive temperature rise, porosity will more likely occur in thick sections of the denture.
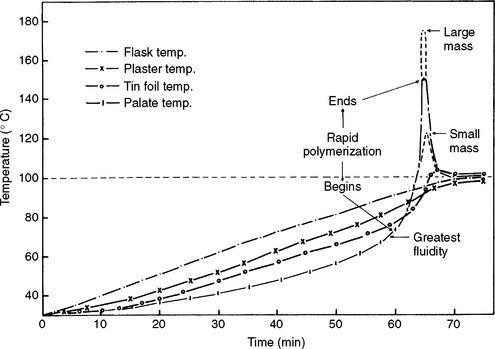
FIGURE 21-9 Temperature observed at various positions in a denture flask during processing. (Adapted from Tylman SD: J Am Dent Assoc 29:1845, 1942.)
The porosity that developed in two polymers cured at 74°, 82°, and 100° C is shown in Fig. 21-10. Product A showed no porosity at 74° C, a negligible amount at 82° C, and moderate porosity at 100° C. Product B, however, evidenced no porosity at 74° C, a moderate amount at 82° C, and severe porosity at 100° C. On the basis of a large number of studies, a satisfactory processing temperature for most products is between 71° and 77° C, although, as indicated in Fig. 21-10, some products can be processed at higher temperatures without serious difficulty. A satisfactory processing procedure is to cure the polymer in a constant temperature water bath at 74° C for 8 hours or longer. Longer curing times, such as overnight, will not result in any degradation of properties. Another satisfactory processing method, which permits curing in a shorter time, is to heat at 74° C for 1.5 hours and then increase the temperature of the water bath to boiling for an additional hour.

FIGURE 21-10 Effect of the processing temperature on the porosity of two acrylic dental plastics. (From Peyton FA: J Am Dent Assoc 40:525, 1950.)
The porosity shown in the specimens in Fig. 21-10 is in the center. The absence of porosity in the periphery of the specimens results from lower temperatures in these areas because the heat can be dissipated to the surrounding dental stone. The center of the specimens experience higher temperatures because poor thermal conductivity of the polymer prevents the dissipation of heat. Porosity in thick sections of dentures therefore may exist below the surface of the polymer, and in pigmented materials it may not be noticed until grinding or polishing exposes the deeper layers.
Porosity also results when insufficient pressure is maintained on the flask during processing, but the distribution of the porosity is different from that shown in Fig. 21-10. Porosity resulting from insufficient pressure is distributed uniformly throughout the material, rather than concentrated in the center.
Other problems associated with rapid initial heating of the acrylic dough above 74° C are production of internal stresses, warpage of the denture after deflasking, and checking or crazing around the necks of the artificial teeth. High internal stresses resulting from rapid heating combined with the heat of polymerization may be released later and cause distortion and misfit of the denture base.
A variety of other methods of supplying the necessary heat to accelerate the polymerization reaction have been used. They include steam, dry heat supplied by electric platens, dry-air oven, infrared heating, induction or dielectric heating, and microwave radiation. The results of various processing studies have shown that equally satisfactory clinical results may be obtained with any of these methods compared with the water bath method if adequate temperature control and pressure are maintained.
Deflasking and Finishing
After polymerization, the flask is removed from the water bath and allowed to cool to room temperature. If the flask is opened prematurely while the polymer is still warm, warpage is likely to occur. Rapid cooling also tends to increase stresses in the denture, which may be released at a later time. During the cooling process, thermal shrinkage occurs. The thermal shrinkage takes place as a result of the relatively high thermal coefficient of expansion of the polymer. The magnitude of this thermal shrinkage will depend on the difference between the temperature at which the polymer hardens and room or mouth temperature, whichever is the final reference point. It is apparent that as long as the polymer is soft, it will shrink with the stone cast, which has a different thermal coefficient. When the polymer hardens, stresses are induced as cooling continues, because the polymer is forced to follow the shape of the cast despite the differences in thermal coefficients. Deflasking of the denture after cooling, however, allows some of the stresses in the denture to be released, and warpage occurs. Numerous studies dealing with the linear shrinkage of denture bases, measured across the posterior region, have shown that for normal processing conditions, the linear shrinkage is 0.3% to 0.5%. Usually more shrinkage is observed in mandibular than in maxillary dentures because of their shape.
A change in the contact vertical dimension of a denture during processing, as measured on an articulator, is also important. These changes are caused by variations in flask pressure, flask temperature, consistency of the dough, and strength of the stone mold. The pressure developed during closure of the flask is possibly the most important factor. If proper precautions are taken, the vertical opening may be held to 0.5 mm rather than reported variations of 2 to 5 mm.
After cooling, the denture is ejected from the flask and the stone is removed. Stone adhering to the polymer may be removed by shell blasting, a process whereby ground walnut shells are used to abrade the stone with little or no effect on the polymer. After deflasking, the denture is trimmed with an arbor band and acrylic burs and polished. A wet polishing wheel and a slurry of pumice and water should be used to avoid heating the acrylic, which can cause measurable warpage. Tin oxide has been used to produce the final polish. However, tin oxide has been identified as a biological hazard and should be used with caution. After finishing, the denture should be stored in water.
Residual Monomer
During the polymerization process the amount of residual monomer decreases rapidly at first, then more slowly. The amount of residual monomer in a denture polymer processed at 70° C and at 100° C is shown as a function of the time and processing in Fig. 21-11. At zero time the monomer content was 26.2%. After 1 hour at 70° C it decreased to 6.6%, and at 100° C to 0.31%. After 4 hours the residual monomer was 4.0% and 0.29%, respectively. It required 168 hours at 70° C for the residual monomer to approximate the value obtained after 1 hour at 100° C. These data support the use of a 1-hour terminal boil for processing dentures, as described earlier. However, the processing temperature should not be raised to boiling until most of the polymerization is completed, or porosity may result.
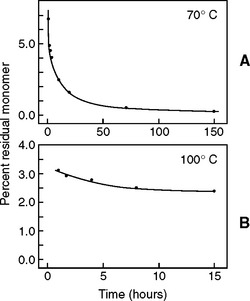
FIGURE 21-11 Residual monomer concentration in the polymerization of methyl methacrylate in dental plastics at 70° C, A, and 100° C, B, as a function of the time of processing. (Adapted from Smith DC: Br Dent J 105:86, 1958.)
The highest residual monomer level is observed with chemically accelerated denture base polymers at 1% to 4% shortly after processing. Storing the denture for several days at elevated temperatures (up to 50° C) and excluding oxygen can significantly reduce the monomer level. However, this may be impractical. The rapid heat-cured denture base materials have significant residual monomer levels from 1% to 3% when they are processed in less than 1 hour in boiling water. If they are processed for 7 hours at 70° C and then boiled for 3 hours, the residual monomer content may be less than 0.4%.
If heat-processed materials are to be used for patients sensitive to residual monomer, processing for longer times in boiling water should reduce the monomer to an acceptable level. Because there is evidence that poly(methyl methacrylate) monomer has poor biocompatibility, every effort should be made to eliminate residual monomer or reduce it to very low levels.
Dimensional Changes
Dimensional changes take place when dentures are stored in water or are in contact with oral fluids. A considerable number of studies have reported on dimensional changes occurring during processing and storage of dentures. The area described with the most uniform results has been the posterior region. In general, heat-cured dentures stored in water show a linear expansion in this region of 0.1% to 0.2%, which partially but not completely compensates for the processing shrinkage of 0.3% to 0.5%. The net linear change can vary from a shrinkage of 0.1% to 0.4%. The major portion of the expansion in water takes place during the first month, and changes are insignificant after 2 months.
In a study comparing the fit of maxillary dentures made from five different commercial products, denture bases were placed on their respective casts after being stored in water at 37° C for 42 days to ensure equilibrium. The materials were ranked from best fit to worst fit by a panel of trained observers. The relative fit was determined by the space between the base and the cast in the palatal region. One rubber-modified acrylic had the worst fit, whereas another had a fit that was similar to the conventional acrylic. The best fit was observed with the vinyl-modified and the hydroxyethyl acrylics. There was no correlation with water sorption values, and therefore the fit of the denture bases may be related more to the glass transition temperature (glassy to brittle transition) than to water sorption for these materials.
There is always concern regarding what a net shrinkage of 0.1% to 0.4% represents clinically. A number of other steps in the preparation of a complete denture may cause dimensional inaccuracies, such as making the impression, pouring the stone cast, and preparing and investing the waxed denture. The oral tissues apparently accommodate small dimensional changes in dentures.
Injection Molding Denture Base Resins
Several manufacturers have reintroduced injection molding materials and equipment and the process is gaining in popularity. The flasking and boiling out of the waxed denture is similar to that used for compression molding. For injection molding, a hollow sprue connects the mold cavity created by wax boilout to an external opening on the flask and a high-pressure injection cylinder is connected to the opening. The denture base resin is mixed and placed in the cylinder. When the material reaches the proper consistency it is injected into the mold cavity under high pressure. The pressure is maintained during the polymerization cycle, and as polymerization shrinkage occurs additional material enters the flask. Several studies have noted increased dimensional accuracy with this technique. Injection molding is also used for microwavable and pour-type acrylics.
CHEMICALLY ACCELERATED ACRYLIC DENTURE POLYMERS—COMPRESSION MOLDING
Chemically accelerated dental polymers, often called chemically curing resins, self-curing resins, cold-curing resins, or autopolymerizing resins, are similar to heat-accelerated dental polymers. The principal difference is that the polymerization reaction is accelerated by a chemical, such as N,N-dihydroxyethyl -para-toluidine, rather than by heat, as indicated by the simplified equation below (compare with the equation given in the earlier section on heat-accelerated acrylic denture polymers).
The amine accelerator reacts with the peroxide initiator at room temperature and sufficient free radicals are produced to initiate the polymerization reaction. Except for initiation, the remainder of the polymerization reaction is the same as for the heat-accelerated type. The reaction is exothermic and polymerization still results in volumetric shrinkage, but the polymer does not reach as high a peak temperature.
Manipulation and Processing
The general procedure for compression molding a chemically accelerated polymer is much the same as for the heat-accelerated type, except after final flask closure the dough is allowed to polymerize at room temperature or in a warm water bath in a pressure vessel.
The denture mold is packed when the polymer-monomer mixture reaches the doughy stage. Several trial closures are made and the flask removed. Care must be taken to make both the trial and final closure before the dough becomes so stiff that final closure is not possible. The chemically accelerated materials start to polymerize soon after the powder and liquid are mixed and proceed more rapidly through the various consistency stages than the heat-accelerated types. The average time needed to reach packing consistency is only 5 minutes for the chemically accelerated type, compared with 15 minutes for the heat-polymerized acrylic. It is more difficult to pack a number of denture flasks from one mix and still obtain complete flask closure. Additional working time may be obtained when the ingredients and the mixing jar are cooled in a refrigerator.
Properties
After the flask is packed it should remain closed and under clamp pressure for a minimum of 2.5 hours to ensure polymerization. Compared with heat-cured polymers, chemically cured types do not reach the same degree of polymerization. The higher residual monomer acts as a plasticizer, which results in higher flexural deflection values and lower flexural strengths. After 15 days in water, however, chemically cured acrylics are nearly as hard as heat-cured. If, after 2.5 hours of curing at room temperature, the flask is boiled for 0.5 to 1 hour, properties comparable to the heat-cured type are obtained, and the residual monomer content is considerably reduced.
Peak temperatures observed at various positions in a denture cured by chemical acceleration are not as high as for heat-polymerized types. The linear shrinkage across the posterior region of a chemically cured maxillary denture after deflasking is about 0.3% compared with 0.5% for a heat-cured maxillary denture. As in the case of heat-cured dentures, mandibular dentures prepared with chemically accelerated denture polymers show more dimensional change than corresponding maxillary dentures. The lower dimensional change of the chemically cured type results because less residual stress is produced in the denture during the processing cycle. This is substantiated by the fact that the chemically accelerated denture polymers produce less shrinkage when processed at 20° to 25° C rather than at 37° C. When chemically cured dentures are placed in water, an increase in the molar-to-molar distance is observed. After 1 month this expansion amounts to about 0.3%, which approximately compensates for the processing shrinkage of 0.3%. Additional storage up to 9 months results in a total expansion of 0.4%, and the net dimensional change at this time is 0.1%, compared with the master model. It should be noted that water sorption of the chemically cured acrylics is about the same as the heat-cured type; however, the solubility is higher for the chemically cured. This higher solubility is caused by the loss of residual monomer from the chemically cured acrylic.
On the basis of these observations, it can be concluded that chemically cured acrylic dentures are generally about 0.1% oversize after several months of service and heat-cured acrylic dentures are 0.3% to 0.4% undersize.
The presence of some amine accelerators may cause problems in color stability. These amines produce colored products on oxidation, and therefore the color stability of chemically cured acrylics may not be as good as that of heat-cured acrylics, although definite improvements have been made since their introduction. Several products are now available that comply with the ANSI/ADA Specification No. 12 color stability test. Activators such as organic sulfinic acids can be used to improve color stability, but these compounds have certain disadvantages, such as chemical instability.
FLUID RESIN ACRYLIC DENTURE POLYMERS
The fluid resin technique takes advantage of the flow properties of polymer-monomer mixtures in the early consistency stage and the smaller size of the polymer powder particles. A very fluid mix also results from a much higher monomer-polymer ratio of about 1:2.5. The polymer and monomer are mixed and then poured into the mold in the denture flask, and no trial packing is required. This procedure is advantageous in the preparation of the saddles for partial dentures, in which trial packing is difficult and flow of polymer around the metal framework is required. It also requires less expensive equipment than the heat-accelerated acrylic. Manufacturers claim that a denture can be produced in much less time using this procedure. This was not substantiated in a laboratory survey.
This technique involves the use of agar or alginate hydrocolloid or, less commonly, a soft stone or silicone mold. The presence of water in the hydrocolloid does not interfere with the polymerization of the slurry of polymer and monomer. The technique involves preparation of a hydrocolloid gel mold of the waxed-up denture, as shown in Fig. 21-12, A. After the gel is formed, the model, including the waxed denture, is removed. The wax is removed from the model and teeth, and the teeth are reinserted into the mold (Fig. 21-12, B). The model is repositioned in the mold after an alginate separator has been applied and allowed to dry. The denture flask is constructed so that two or three circular holes can be cut from the side of the flask through the gel to the posterior portion of the model. A slurry of chemically activated acrylic is poured through one of these holes into the mold space provided by the loss of the wax (Fig. 21-12, C). The remaining hole or holes provide vents for the excess acrylic slurry, thus ensuring adequate filling of the mold space. After pouring the acrylic the flask is placed in a pressure vessel containing warm water, and air pressure of 0.1 to 0.2 MPa is applied. Only 30 to 45 minutes are necessary for polymerization. After processing, the gel is easily broken away from the denture and the denture looks as clean as the one shown in Fig. 21-12, D. Because of higher polymerization shrinkage, dentures fabricated by this technique are slightly less accurate than heat-activated dentures processed in a stone mold. When compared with heat-cured resins, pour-type acrylics are characterized by lower impact and fatigue strengths, higher creep values, lower flexural bend strength, lower water sorption values, and higher solubility. The technique is interesting, because the hydrocolloid mold is easy to prepare, processing time is shortened considerably, the agar hydrocolloid may be reused, problems of broken teeth are eliminated, and deflasking is simplified.
FIGURE 21-12 Slurry casting of chemically accelerated acrylic denture base material. A, Pouring of agar mold. B, Agar mold with denture teeth in position. C, Pouring of powder-liquid slurry into the agar mold. D, Appearance of denture after processing.
Commercial fluid resins may vary considerably in apparent viscosity. Fig. 21-13 demonstrates the dramatic increase in apparent viscosity of six different fluid resins as a function of time and at a constant shear rate (rotational speed). It illustrates the importance of pouring the fluid resins into the mold soon after the powder and liquid phases are mixed if lower viscosities are to be obtained. The apparent viscosity of fluid resins appears to be an important factor when fluid resin denture materials are evaluated.
LIGHT-CURED DENTURE POLYMERS
Light curing of a denture is a novel method compared with other processing methods. After the try-in of the waxed-up trial denture is completed, a roll of light-activated acrylic is placed over the occlusal surfaces of the teeth to form a template having three reference areas on the master cast (Fig. 21-14, A). The template is cured in the light chamber for 10 minutes, then the teeth are removed from the trial denture. Removal is simple because the wax softens under the heat of the high-intensity light bulbs. The teeth, the attached template, and the cast are placed in boiling water to remove all traces of wax (Fig. 21-14, B).
FIGURE 21-14 Processing of a light-activated denture base. A, Pick-up of the denture teeth from the waxed trial denture using a rope of the light-activated acrylic. B, Removal of all traces of wax from the teeth and master cast. C, Adaptation of light-activated denture base material to the master cast. D, Reseating the teeth on the master cast and beginning the shaping of the anatomical portion of the denture. E, Final adaptation of the anatomical portion of the denture, just before polymerization in the light chamber. F, Completed light-activated acrylic denture. (Courtesy Dentsply International, York, PA.)
After coating the master cast with a release agent, a sheet of the light-activated denture base material is adapted to the cast and trimmed to the boxing edge (Fig. 21-14, C). The base is then polymerized in the light chamber.
A strip of the light-activated acrylic is placed on the underside of the teeth after they have been coated with a bonding agent. The teeth are then repositioned in the original position on the denture base using the template (Fig. 21-14, D). The teeth are held in position by polymerization in the light chamber.
The anatomical portion of the denture is completed using more of the base material to sculpt the surface and develop the final shape of the denture (Fig. 21-14, E). After contouring, final polymerization is accomplished in the light chamber and the denture is removed from the cast and finished in a conventional manner (Fig. 21-14, F).
FACTORS INVOLVED IN DENTURE RETENTION
Adaptation of the denture base to the oral mucosa has been cited as an important factor in the retention of dentures. Other factors are (1) capillary forces involving the liquid film between the oral tissues and the denture base; (2) surface forces controlling the wetting of the polymer denture base by the saliva; (3) the thickness of the salivary film between the denture and the oral tissues; (4) the surface tension of the saliva; (5) the viscosity of the saliva; and (6) atmospheric pressure. High surface tension, area, and wetting increase retention, as does a thin film of saliva (see Chapter 2). A technical discussion of all factors involved in the retention of dentures is not within the scope of this text.
EFFECT OF AUXILIARY MATERIALS ON DENTURE POLYMERS
A number of materials are used in making and maintaining a denture. These materials may affect the final properties and function of the denture. Examples of such materials are (1) dental plaster and stone, (2) impression materials, (3) wax, (4) mold separators, (5) artificial teeth, (6) characterization materials, (7) repair and reline materials, and (8) denture cleansers.
Plaster and Stone
The strength of the plaster or stone used to invest the wax denture is of concern, because a weak investment resulting from a thin mix or incomplete mixing will not adequately support the artificial teeth during packing of the denture mold. As a result of teeth shifting, the finished dentures may have faulty occlusion. Another problem involved in the use of a stone mold is that the thermal coefficient of expansion, or contraction, is different from the thermal coefficients of the teeth and the polymer used to form the denture. After the polymerization reaction, the polymer and investment cool to room temperature and attempt to contract according to their individual thermal coefficients. The stone cast with a different thermal coefficient creates residual stresses in the acrylic as it cools. These stresses may be released after the processed denture is deflasked and deformation or crazing may result. One study suggests that using a high expansion dental stone in the final impression will compensate for the polymerization shrinkage of the denture base resin and provide greater accuracy in the denture base.
Impression Materials
When alginate materials are used to make an impression, it is important that the cast be poured as soon as possible. If dimensional changes occur in the impression, these inaccuracies will be reflected in the final fit of the denture base.
Waxes
Baseplate wax that is not removed from the crown portion of the teeth before the denture is flasked will cause problems. During the removal of the wax in boiling water, the wax on the coronal area of the teeth is also removed and will result in shifting of the teeth, poor articulation, or broken teeth when the denture is packed. A more common problem is the presence of a residual wax film on the gingival portions of the artificial teeth after the boilout of the wax, which prevents the adherence of the denture base to the teeth. This may be avoided by the addition of detergent to the water used in the boilout procedure, followed by rinsing with clear boiling water.
Mold Separators
Materials such as aqueous solutions of sodium silicate, calcium oleate, or sodium or ammonium alginate are used to facilitate removal of the processed denture from the gypsum cast. The common alginate separators contain about 2% sodium alginate in water with small amounts of glycerin, alcohol, sodium phosphate, and preservatives. Care must be exercised to avoid coating acrylic teeth with these release agents because this will interfere with the bond between the denture base and the teeth.
Characterization Materials
A variety of materials are used for the characterization of dentures. Included in this group of materials are dyed synthetic fibers and pigments. Addition of dyed acrylic fibers to simulate the blood vessels of the oral mucosa does not alter the water sorption or strength of the dental polymer significantly.
Denture Cleansers
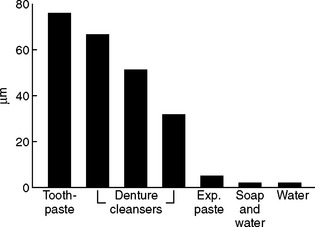
Denture cleansers and cleaning methods may scratch and wear dentures. Denture acrylic has been tested for wear using various commercially available denture cleaning pastes, an experimental paste, soap and water, and water against a reciprocating soft toothbrush. The results shown in Fig. 21-15 are dramatic. The use of water, or soap and water, produced little or no wear compared with the commercial denture cleansers and toothpaste. Daily brushing with a very soft brush (similar to the toothbrushes used in periodontal therapy) is very effective in keeping dentures clean and will not abrade the denture or teeth appreciably if abrasive cleansers are not used. Most immersion denture cleansers are effective in the removal of mucin, stains, and loosely attached food debris. Some immersion cleaners have been demonstrated to be effective sanitizing agents. A water solution containing a hypochlorite and a glassy phosphate (Calgon) is an effective denture cleanser that does not cause discoloration of the dental polymer or the metal retainer pins in porcelain artificial teeth when used occasionally. A solution consisting of 1 tsp of a hypochlorite, such as Clorox, and 2 tsp of Calgon in half a glass of water has been recommended for occasional overnight immersion of acrylic dentures in a glass container. Dentures soaked in this solution must be thoroughly rinsed before use. This cleanser is not recommended for use on prostheses containing cobalt-chromium or nickel-chromium alloys because chlorine solutions tend to darken these metals.
REPAIR MATERIALS
An important application of acrylics in prosthetic dentistry is in the repair of broken dentures. Acrylic repair materials are usually the powder-liquid type, similar to those used for denture bases, and are usually heat-accelerated or chemically accelerated. Light-activated and microwave-cured acrylics have also been shown to be a fast and effective repair materials. The material of choice will depend on the following factors: (1) length of time required for making the repair, (2) flexural strength obtainable with the repair material, and (3) degree to which dimensional accuracy is maintained during repair.
In most cases, when dentures fracture, the pieces can be easily approximated because brittle failure has occurred. If the pieces cannot be realigned and permanent deformation has taken place, it is important to carefully check the adaptation of the denture to the soft tissues. One technique for making a repair requires holding, or luting, the broken pieces together with sticky wax, pouring a stone model on the inside of the denture, and investing the model and denture in a flask. Then the wax is removed, the fracture line is opened with a bur to allow for a reasonable amount of repair acrylic, and the ground surfaces are painted with monomer or a 4 : 1 mixture of monomer and polymer. Acrylic dough is packed into the area being repaired, and the material is cured, cooled, deflasked, and finished.
If a heat-accelerated acrylic is used, the denture should be completely flasked, and curing of the repair material preferably should be carried out at temperatures no greater than 74° to 77° C for 8 hours or longer. This procedure minimizes the dimensional change of the denture base.
In a similar manner, the repair may be done without flasking, by use of a chemically accelerated acrylic. After a cast is poured, the fracture line is opened and the acrylic is painted into the defect. The denture is then placed in a pressure vessel under air pressure until polymerization is complete. Repairs using light-activated acrylic are done in a similar manner. After the acrylic dough is packed into the defect, curing is done in the light chamber. A bonding agent is used to increase the strength of the repair.
The use of chemically accelerated and lightactivated acrylic resins for repairs does not require flasking. The procedure is rapid and may be done while the patient waits. The dimensional accuracy is maintained because not enough heat is present during polymerization to cause warpage from the release of stresses. The chemically accelerated acrylic resins, however, have the disadvantage of lower flexural strength in the repaired area than a heat-cured acrylic. In general, the flexural strength of a heat-cured repair is about 80% of that of the original polymer, and the flexural strength of a chemically cured repair is about 60% of the original material. Light-activated acrylic is affected by water storage, resulting in lower flexural strength than the self-cured repair resin. Regardless of the type of polymer used for repair, the shape of the edges in the repair area should be tapered and all corners rounded to avoid areas of high stress concentration. These ground edges are painted with monomer to soften the polymer to obtain a chemical bond between the repair material and the denture.
The use of chemically accelerated repair material without flasking at room temperature and pressure may result in a repair area containing porosity. Polymerization in the center of the repair proceeds rapidly, but polymerization at the surface is slowed by the inhibiting effect of the oxygen. When the repair is done in water, under air pressure of 0.2 MPa and a temperature of 30° C, the porosity is greatly reduced. The residual monomer content of specimens processed either in air or under pressure is about 3%.
The reported net dimensional change across the molar region of a maxillary denture for a heat-cured repair conducted at 73.5° C is −0.3%, whereas for a chemically cured repair it is 0.2%. It also has been reported that a denture repaired with chemically accelerated polymer will fit the model better. This may be the result of less warpage or because the denture is slightly oversized. Studies have been done on the contour of denture bases before they were broken and after repair. The results indicate that better reproduction of dimensions results when chemically accelerated repair materials are used to repair dentures originally prepared from heat-cured or self-cured acrylic polymers. Superior results also are obtained when a heat-curing acrylic repair material is used to repair a denture prepared from a chemically accelerated acrylic polymer. Less satisfactory reproduction of dimensions is obtained when a heat-curing repair material is used to repair a heat-cured denture. If the cause of breakage is not corrected, such as faulty occlusion, fit, or both, repairs usually will fail regardless of which repair material is used.
ANSI/ADA Specification No. 13 for Denture Self-Curing Repair Resins
The requirements of the chemically accelerated repair polymers or the self-curing repair resins are listed in ANSI/ADA Specification No. 13. The specification includes self-curing powder-liquid polymers, which may be either pink or clear. These materials must satisfy the requirements for thermal stability toxicity, porosity, polish, color, translucency, water sorption and solubility, transverse deflection, and color stability. The water sorption shall be less than 0.8 mg/cm2 of surface area and the solubility shall be less than 0.04 mg/cm2 of surface area. The flexural deflection between a 14.7- and 24.4-N load shall be less than 1.5 mm, and the deflection between a 14.7- and 39.2-N load shall be less than 4.5 mm. In general, the color stability test is difficult for chemically accelerated repair materials to pass.
RELINING AND REBASING DENTURES
The adaptation of a denture to the soft tissues and bearing areas may change over time because of alteration in the contour of the soft tissues and resorption of underlying bone. Generally, when retention of the denture becomes unsatisfactory, replacement of the denture is indicated. In some cases in which the denture is an intermediate prosthesis or rapid changes to supporting bone are taking place such as during bone remodeling after tooth extraction, the surface of the denture base or the complete denture base can be replaced. This process is called relining or rebasing.
Relining
Relining is a process in which a film of polymer is added to the inside of the denture to obtain an improved adaptation with the denture-bearing mucosa. This is accomplished by (1) making an impression of the denture-bearing mucosa using the denture as a tray, reflasking the denture, removing the impression material, and packing and curing the new liner; or (2) making a chairside reline in which the reline material is used to make the impression.
Two different types of reline materials may be used—one permanent, the other temporary. The former may be either a heat-accelerated, chemically accelerated, or light-activated acrylic. The relining process is carried out either by a flasking procedure or curing in a light chamber, depending on the type of material. Permanent reline materials may be identical to those from which permanent denture bases are made.
The problems involved in relining a denture are much the same as those encountered in repairing a denture: (1) a good chemical bond is desired between the reline polymer and the denture polymer; (2) satisfactory strength of the relined denture is necessary; (3) no warpage or dimensional change should result in the denture because of the relining procedure; (4) no change in occlusion and occlusal vertical dimension should take place; and (5) relining should take as short a time as possible for patient convenience. For heat-polymerized reline materials the area to be relined is softened by monomer and the acrylic is packed soon after reaching the doughy consistency to achieve a chemical bond between the two materials. The polymerization should occur at temperatures between 74° and 77° C to prevent warpage. Chemically accelerated reline materials have an advantage because lower peak temperatures during polymerization may minimize warpage.
The light-activated material is used directly in the denture to record the tissue surface. A bonding agent is used before placing the material in the denture. Polymerization is achieved in a light chamber. The light-activated acrylic allows the entire reline procedure to be completed in 30 to 45 minutes.
Temporary relining polymers are used directly in the mouth, and a chemically accelerated curing system is used. These materials are considered temporary because they are porous and stain easily or foul with microorganisms. In addition, most of these products are not color stable. Temporary reline materials polymerize at mouth temperature and the reaction is exothermic, with peak polymerization temperatures of 59° to 79° C and peak temperature times of 6 to 11 minutes. The lower temperatures generally correspond to longer times. Peak temperatures of 79° C can cause tissue injury. To avoid burning of the tissues, the denture is usually taken from the mouth after a few minutes, chilled in cool water, and returned to the mouth. In addition to the exothermic heat, direct contact of monomer on the oral tissues may elicit a burning sensation. Temporary reline polymers, however, do not cause any clinically significant warpage of the denture.
ANSI/ADA Specification No. 17 for Denture Base Temporary Relining Resin
ANSI/ADA Specification No. 17 for temporary relining resin is for hard-setting, self-curing polymers of the powder-liquid type. This specification contains requirements for the consistency, peak temperature, hardening time, polish, porosity, hardness, water sorption and solubility, and color stability. The peak temperature reached during processing should not be more than 75° C and should occur between 6 and 15 minutes after mixing. The processed polymer should have a smooth glossy surface when polished by usual methods and should be free of large numbers or sizes of bubbles. The Knoop hardness shall be not less than 10 kg/mm2. The water solubility and sorption shall be less than 0.2% and 2.0%, respectively, when stored for 24 hours at 37° C. The requirements for hardness and solubility in water therefore are less demanding for temporary relining materials than for denture base polymers. Temporary relining polymers, however, must pass a color stability test, which requires that the specimen show no more than a slight color change after an exposure of 24 hours. This requirement may be difficult to satisfy, because many products show noticeable color changes when exposed to ultraviolet light.
Rebasing
Rebasing refers to a technique in which the dimensional relations of the teeth are maintained and the entire denture base is replaced. This technique is generally as time consuming and costly as fabricating a completely new denture. The method is sometimes used when there are many discrepancies in the adaptation of the denture-bearing area to the oral mucosa that cannot be resolved with the reline, and the denture is relatively new. A cast is prepared from an impression made in the denture. After flasking, the old denture base is removed except around the teeth. The denture is then processed according to standard procedures with new acrylic. The end result is a new denture base retaining the original teeth in the same position as in the original denture. As in the case of reline procedures, extreme care must be given to avoid changes in the occlusion or occlusal vertical dimension.
Tissue Conditioning Liners
Tissue conditioners are soft elastomers used to treat an irritated mucosa supporting a denture. They are mixed at chairside, placed in the denture, and seated in the patient’s mouth. These materials will conform to the anatomy of the residual ridge, gel in that position, and continue to flow slowly after application. Tissue conditioning is used to provide time for healing of the soft tissues or as a diagnostic tool to assess patient’s tolerance of new occlusal schemes or occlusal vertical dimension. They are used only for short-term applications and should be replaced every 3 to 5 days. Inhibition of the growth of oral bacterial flora is associated with some materials, and this should promote healing of inflamed tissues.
Tissue conditioners are composed of a powder containing poly(ethyl methacrylate) and a liquid containing an aromatic ester-ethyl alcohol (up to 30%) mixture. Tissue conditioners are very soft elastomers with a hardness from 13 to 49 Shore A hardness units 24 hours after mixing. They will show a weight loss from 4.9% to 9.3% after 24 hours as a result of the loss of alcohol. These materials deform easily, and with a stress of 200 g/cm2 applied 15 minutes after the set of the material, the compression will range from 60% to 83% of the original length. When the stress is removed, there will be a recovery from 22% to 48%. When unstrained specimens are left in a humidifier for 24 hours, they will tend to “slump,” or shorten under their own weight. Within a few days, tissue conditioners become stiffer as a result of the loss of alcohol.
Tissue conditioners are formulated to have specific viscoelastic properties. The viscosities of a number of tissue conditioners are listed in Table 21-7. The values were obtained 2 hours after mixing and under prolonged static loads. Under cyclic loading, which parallels cyclic masticatory forces, the materials demonstrate elastic behavior, particularly when the frequency is more than 1 Hz.
The properties that make tissue conditioners effective are (1) viscous behavior, which allows adaptation to the irritated denture-bearing mucosa over a period of several days; and (2) viscoelastic and elastic behavior, which cushions the cyclic forces of mastication and bruxism. The viscoelastic properties are influenced by the molecular weight of the polymer powders and the powder/liquid ratio. Dentures with elastomeric relines generally have improved adaptation to the soft tissues, which help distribute occlusal forces more evenly across bearing areas. Retention and comfort of the denture are improved.
TABLE 21-10
Viscosity of Tissue Conditioners 2 Hours after Mixing
Adapted from Braden M: J Dent Res 49:496, 1970.
It has been suggested that the initial flow depends on the time of loading, volume of the material, and load applied when seating the denture. A rheologic study has shown that different products must be seated in the mouth at different times from the start of mixing because they may differ both in viscosity or gelation time. The gelation time is related to the molecular weight and particle size of the polymer powder, the ethanol content, and the plasticizer used.
Soft or Resilient Denture Liners
Soft or plasticized acrylic, vinyl polymers, and copolymers, as well as natural and silicone rubber products, are used as denture liners for patients with irritation of the denture-bearing mucosa, areas of severe undercuts, or congenital or acquired defects of the palate. Some of these materials are designed to be cured in situ, and others are designed for laboratory processing. Some products reportedly have an inhibitory effect on the growth of Candida albicans, whereas others appear to support the growth of microorganisms. Desirable properties are (1) high bond strength to the denture base, (2) dimensional stability of the liner during and after processing, (3) long-term resilience, (4) low water sorption, (5) color stability, (6) ease of processing, and (7) biocompatibility.
Mouth-Cured Soft Liners
Conventional mouth-cured soft liners are used to improve the comfort and retention of dentures that are used as interim prostheses. After several weeks they may begin to foul and debond from the denture. They are mixed chairside, placed in the denture, and seated in the patient’s mouth until polymerized, which generally takes a few minutes. Three types of materials are generally used:
1. Powder—poly(ethyl methacrylate) and peroxide initiator; liquid—aromatic esters, ethanol, and tertiary amines
2. Powder—poly(ethyl methacrylate), plasticizers such as ethyl glycolate, and a peroxide initiator; liquid—methyl methacrylate and tertiary amines
Biocompatibility of these materials is of interest because it has been demonstrated both in vitro and in vivo that both tissue conditioners and chairside soft liners leach out significant amounts of alcohol and phthalate esters.
Processed Soft Liners
Processed soft liners are used with denture patients who experience chronic discomfort from their dentures because of anatomic or occlusal challenges. The cushioning effect of soft liners has been evaluated using an impact test with an accelerometer. Of four soft liners tested, all were found to reduce impact forces compared with denture base resin. Although these materials fulfill a need, they generally are not permanent solutions. A year is considered good service. These materials tend to pull away from the denture base or become porous and foul smelling. They are processed in the laboratory in a manner similar to processing of a denture base. Several addition-silicone elastomers can be processed either intraorally or in the laboratory. Finishing of soft denture liners is often difficult because the materials are soft. Several types of processed soft liners are available, including plasticized acrylics, plasticized vinyl acrylics, heat- and room temperature–cured silicones, hydrophilic acrylates, and polyphosphazine. The compositions of various commercial products of plasticized acrylic and silicones are shown in Tables 21-11 and 21-12.
ANSI/ADA Specification No. 75 (ISO 10139) for Resilient Lining Materials for Removable Dentures
ANSI/ADA Specification No. 75 (ISO 10139) for Resilient Lining Materials for Removable Dentures has two parts. Part 1 covers materials for short-term use and specifies requirements for development of elastic recovery and change in compliance with age. Part 2 covers materials for long-term use and specifies requirements for depth of penetration and depth of penetration ratio.
Several physical properties of four categories of laboratory processed soft liners, determined 24 hours after processing, are presented in Table 21-11. The properties of these materials show a wide range of values. For example, a plasticized acrylic may have very high tear resistance and a high Shore A hardness value. Because softer materials are generally considered kinder to the tissues, the gain in tear resistance is offset by hardness. Conversely, a silicone soft liner may have a low Shore A hardness value and low tear resistance. Many of these materials tend to increase in hardness and stiffness with time, and their physical and mechanical properties are also affected by prolonged storage in water or in the oral environment.
The water solubility and sorption of soft liners is complex. When placed in water, plasticizers and other components may leach out over extended periods while water is absorbed until equilibrium is reached. Absorbed water can have a detrimental effect on the adhesion of soft liners to acrylic denture bases, particularly if the rate of diffusion is rapid. At 1 week, water sorption will be from 0.2 to 5.6 mg/cm2 and solubility from 0.03 to 0.40 mg/cm2 for various commercial products. An ideal processed soft liner would have no soluble components and low water sorption.
Processed soft liners are intended to be used for extended periods. In some patients it has been observed that a yeast, Candida albicans, and other microorganisms may grow on and within the liner, resulting in a rough and hardened surface. Antimicrobial agents have been proposed to eliminate this problem. Color changes have also been demonstrated for some liners subjected to accelerated aging.
The bond strengths for the materials listed in Table 21-13 are seen in Table 21-14. The materials were tested in a two-phase tensile test. Two test conditions were used; the soft liners were processed against both polymerized and unpolymerized acrylic resin. Again the results were variable. For both test conditions the polyphosphazine material demonstrated higher bond strength. It also was surprising that for three of the four groups, the bond strength was higher to polymerized acrylic than to unpolymerized acrylic. Most manufacturers recommend processing the soft liner and the denture base acrylic at the same time to improve the bond strength. Only the plasticized vinyl acrylic had higher bond strength when processed against unpolymerized acrylic. Recently, bonding agents have been introduced that significantly increase the bond strength of several addition silicones to denture base resin.
TABLE 21-13
Properties of Laboratory-Processed Soft Liners
Adapted from Dootz ER, Koran A, Craig RG: J Prosthet Dent 67:707, 1992.
TABLE 21-14
Bond Strength of Laboratory-Processed Soft Denture Liners
Adapted from Kawano F, Dootz ER, Koran A, Craig RG: J Prosthet Dent 68:367, 1992.
Dynamic viscoelastic measurements are effective for evaluating the elastic behavior of elastomers over time. In one study evaluating a plasticized acrylic, a fluoroelastomer, a heat-cured silicone, and a self-curing addition silicone, the acrylic and fluoroelastomer demonstrated viscoelastic behavior, whereas the silicones exhibited elastic behavior. The silicones were more stable over time than the other materials.
Growth of Candida albicans and other microorganisms continues to challenge the use of short- and long-term soft denture liners. Unfortunately, researchers and manufacturers have yet to develop materials that will not support the growth of microorganisms. At this time, only excellent oral and denture hygiene and the use of antimicrobial agents are effective in minimizing this problem.
Unfortunately, many of the short- and long-term resilient denture liners contain significant amounts of plasticizers, many of which have questionable biocompatibility. Phthalate esters have caused epithelial changes when polymer disks containing these esters have been implanted in the hamster cheek pouch. The potentially premalignant changes are a cause for concern because the amount of this plasticizer leached from a typical soft liner may be between 10 and 40 times greater than environmental and food uptake. Although even this amount of plasticizer is low, demonstrated biocompatibility of soft denture liners should be considered.
Soft liners have an important place in denture prosthetics, but require improved strength, improved adhesion to the denture base, and the ability to inhibit the growth of microorganisms before they will be considered permanent.
DENTURE TEETH
Denture teeth are often prepared from acrylic and modified acrylic materials similar to denture polymers. Different pigments are used to produce the various tooth shades, and usually a cross-linking agent is used to improve strength and prevent crazing. Polymer teeth are prepared in layers of different colors so the shade is gradually lightened toward the incisal and occlusal portions to give these areas a translucent appearance. The gingival or body portion may not be as highly cross-linked as the incisal or occlusal portion. This is done to improve the chemical bond between the teeth and the denture base. Fillers may be added to increase resistance to wear. Recently, the use of composite materials as denture tooth materials has received some interest and new, modified tooth materials that appear to have increased wear resistance have been introduced.
As indicated in the discussion on the physical properties of dental polymers, poly(methyl methacrylate) has satisfactory chemical properties for use as polymer teeth. It is nontoxic and insoluble in oral fluids but is soluble to some extent in ketones and aromatic hydrocarbons. The mechanical properties of compressive strength (76 MPa), abrasion resistance, elastic modulus (2700 MPa), elastic limit (55 MPa), and hardness (18 to 20 kg/mm2) are low when compared with other restorative materials or with human enamel and dentin.
Of necessity, a discussion of the merits of polymer teeth includes a general comparison of the properties of polymer and porcelain teeth, a number of which are listed in Table 21-15. Certain properties may be listed as a disadvantage or an advantage, depending on the particular purpose and point of view involved. For example, the softness and low abrasion resistance of polymer teeth may be cited as a disadvantage, because the teeth are more easily abraded and the occlusion and vertical dimension of the denture may be altered. On the other hand, low wear resistance also has been cited as an advantage, because polymer teeth are easy to grind and polish and tend to be self-adjusting in service. The hardness of porcelain teeth compared with polymer teeth is an advantage, whereas the resulting brittleness of porcelain compared with the toughness of polymer teeth is a disadvantage.
TABLE 21-15
Comparison of Properties of Plastic and Porcelain Teeth
| Plastic Teeth | Porcelain Teeth |
| High resilience | Very brittle |
| Tough | Friable |
| Soft—low abrasion resistance | Hard—high abrasion resistance |
| Insoluble in mouth fluids—some dimensional change | Inert in mouth fluids—no dimensional change |
| Low heat-distortion temperature, cold flow under pressure | High heat-distortion temperature; no permanent deformation under forces of mastication |
| Bond to denture base plastic | Poor bond-to-denture base plastic*; mechanical retention provided in tooth design |
| Natural appearance | Natural appearance |
| Natural feel—silent | Possible clicking sound in use |
| Easy to grind and polish | Grinding removes surface glaze |
| Crazing and blanching—if non-cross linked | Occasional cracking |
The main difference between porcelain and polymer teeth is that polymer teeth are softer but tougher than porcelain teeth. Other differences are the low elastic modulus, low resistance to cold flow, low abrasion resistance, and high impact strength of polymer teeth. Both polymer and porcelain teeth are insoluble in oral fluids. Porcelain teeth are resistant to organic solvents, such as ketones and aromatic hydrocarbons, which will react with non-cross-linked polymer teeth. Polymer teeth composed of poly(methyl methacrylate) show small dimensional changes when placed in water. As expected, vinyl acrylic teeth do not show as much dimensional change as the totally acrylic teeth. Porcelain teeth show no dimensional change when stored in water and exhibit no permanent deformation from forces exerted on them in the mouth.
For denture teeth, the coefficient of friction of acrylic against acrylic in the presence of water or saliva is lower than the coefficient for porcelain against porcelain. The lowest coefficients of friction, however, are obtained when porcelain and acrylic specimens oppose each other.
In Fig. 21-16, the frictional behavior of acrylic denture teeth is shown for central incisors from two manufacturers. The tangential force on a diamond slider being drawn across the teeth was measured under various loads in distilled water. The width of the wear track was also measured. In general, low values of track width occurred with low tangential force values, and higher track widths and more severe surface failure were associated with higher values of tangential force. The “enamel” surfaces, which were the labial surfaces of the teeth, were more resistant to penetration and surface damage than the “dentin” surfaces, which were prepared by removing 1 mm of acrylic from the ridge lap area of the tooth.
FIGURE 21-16 Tangential force versus normal load for a diamond slider on “enamel” and “dentin” surfaces of two manufacturers’ acrylic teeth (A and B) in water. (Adapted from Raptis CM, Powers JM, Fan PL: J Dent Res 60:908, 1981.)
Polymer teeth possess low heat-distortion temperatures, although the use of cross-linked polymers has improved this property considerably. Care should be taken not to flame polymer teeth during the preparation of a waxed denture. Porcelain teeth have high heat-distortion temperatures; however, sudden temperature changes may cause cracking or fracture. As indicated previously, polymer teeth exhibit cold flow, or permanent deformation, under stresses below their elastic limit, and their dimensions may be altered slightly during use. Polymer teeth are of a similar material to the denture base and may be chemically bonded to the base. The bond strength between chemically accelerated acrylics and polymer teeth is lower, and the use of mechanical retention on the underside of the teeth may prove useful. Highly cross-linked acrylic denture teeth may be treated with 4-methacryloxyethyltrimellitic anhydride to improve the bond strength to denture base resins. An adhesive containing this compound is also available for bonding acrylic to nickel-chromium and cobalt-chromium denture bases or partial dentures. This allows acrylic posterior palatal seals to be added to metal bases and metal base dentures to be relined, when necessary.
Porcelain teeth are usually fabricated with mechanical retention for bonding to the denture base. It has been shown that treating porcelain teeth with a silane coupling agent, such as γ-methacryloxypropyl-trimethoxy silane, provides a surface treatment that allows the teeth to be chemically bonded to the denture base material. When the bond was tested in tension, the porcelain ruptured rather than the bond. Because the use of cross-linked acrylic teeth has made the chemical bonding to the denture base more difficult, mechanical retention is often provided for polymer teeth. The chemical bond produced between polymer and porcelain teeth and the denture base has the distinct advantage of preventing capillary spaces around the teeth, which are difficult to clean and in which microorganisms may grow.
Both types of artificial teeth have a realistic appearance, but the noise or clicking of porcelain teeth against each other is about three times greater than polymer against porcelain or polymer against polymer. The processing of a single tooth replacement and the characterization of polymer teeth are considerably easier than for porcelain teeth. In addition, polymer teeth may be ground and polished with ease, whereas grinding of porcelain teeth removes the surface glaze, making repolishing more difficult. Porcelain teeth should not be used opposing natural teeth or gold restorations because excessive wear will occur.
ANSI/ADA SPECIFICATION NO. 15 (ISO 22112) FOR SYNTHETIC DENTURE TEETH
ANSI/ADA Specification No. 15 presents the desired properties for polymeric denture teeth. Only an abstract of this specification is included here, because a large number of requirements are listed.
1. Dimensions of teeth: The dimensions shall be within 5% of those stated by the manufacturer.
2. Color and blend: Sets of anterior and posterior teeth, representing each shade from the same manufacturer, shall exhibit no perceptible color differences between each other and the manufacturer’s shade guide.
3. Freedom from biologic hazard: Refer to ISO 10993-1, Biological Evaluation of Medical Devices.
4. Surface finish: No stain will occur in service.
5. Retention of finish: After processing and reprocessing, the teeth shall be capable of being polished to restore the original finish.
6. Re-polishing: The teeth shall be capable of being ground and re-polished to a finish equivalent to their original appearance.
7. Quality of bonding to denture-base polymers: The teeth shall be capable of being bonded to heat-polymerized denture-base materials.
8. Color stability: There shall be no perceptible color change in the exposed teeth.
9. Resistance to blanching, distortion and crazing: When tested as outlined, no tooth shall exhibit blanching or distortion.
10. Dimensional stability: When tested as outlined, the dimensional change of a tooth shall be within ±2% of its original mesial-distal dimension.
MAXILLOFACIAL MATERIALS
Maxillofacial materials are used to correct facial defects resulting from cancer surgery, accidents, or even congenital deformities. Noses, ears, eyes and orbits, or any other part of the head and neck may be replaced by these prostheses (Fig. 21-17).
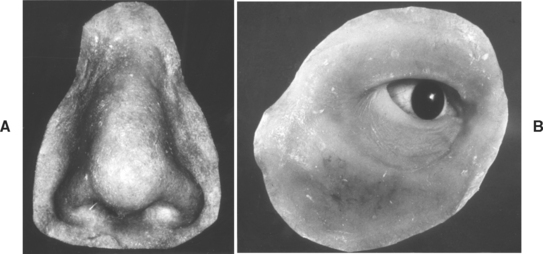
FIGURE 21-17 Maxillofacial appliances. A, Nose. B, Eye and orbit. (From Craig RG, editor: Dental materials: a problem-oriented approach, St. Louis, 1978, Mosby.)
Maxillofacial prostheses are difficult to fabricate and have a relatively short life of 6 months to several years in service. They fail as a result of inherent problems with static and dynamic properties over varying periods and because of color degradation. The prostheses are expensive to fabricate, and many patients are unable to replace the prostheses as often as recommended.
The several types of materials for maxillofacial prostheses vary considerably in ease of preparation and physical properties.
POLY(METHYL METHACRYLATE)
Poly(methyl methacrylate) was once commonly used for maxillofacial prostheses, and is still used occasionally to make artificial facial parts. When properly pigmented, these prostheses can look quite realistic. The main disadvantages are that the acrylic is hard and stiff, does not flex when the face moves, and does not have the feel of skin. Stone molds are generally used with poly(methyl methacrylate), and these are sacrificed when the prosthesis is deflasked after processing.
POLYURETHANE
Polyurethanes are used as maxillofacial materials. The formation of polyurethane is the result of the direct addition of diisocyanate to a polyol in the presence of an initiator, as shown in the following equation. Isophorone polyurethane has also been used as a maxillofacial material.
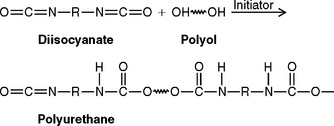
The reaction must be carried out in a dry atmosphere, or carbon dioxide will be produced and a porous elastomer will result. The diisocyanates are very toxic and must be handled with extreme care. The processing temperature of 100° C is reasonable, and stone molds can be used.
ROOM TEMPERATURE-VULCANIZED SILICONES
Because of their good physical properties and favorable processing characteristics, room temperature–vulcanized (RTV) silicones have become popular as maxillofacial materials, and are now used more often than any other material. They have good physical and mechanical properties, are easy to color and process, and allow the use of stone molds. There has been a steady improvement in the physical and mechanical properties of these materials. They are affected by accelerated aging, but not to a degree that compromises their usefulness as maxillofacial materials. These RTV silicones are similar to addition silicone impression materials in that they consist of vinyl- and hydride-containing siloxanes and are polymerized with a chloroplatinic acid catalyst.
OTHER ELASTOMERS
Several elastomers have been investigated for use as maxillofacial materials, including aliphatic polyurethanes, chlorinated polyethylene, silphenylene polymers, organophosphazenes, butadiene-styrene, butadiene-acrylonitrile, and silicone-poly(methyl methacrylate) block copolymers.
FABRICATION OF THE PROSTHESES
The method for fabricating a prosthesis is similar for most materials. An impression is made of the affected area with alginate. A master cast is poured, duplicating the defect on the patient. The artificial part (such as a nose) is then modeled in wax or clay on the master cast and tried on the patient to see if it fulfills the esthetic requirements for form.
The pattern is then invested in a manner similar to that used for complete dentures. Denture flasks are often used for this purpose. When the prosthesis is quite complex (such as an eye and orbit), three- or four-part molds are made. With some materials, metal molds are required because of high processing temperatures. After the pattern is invested, it is removed from the mold by use of a boiling water bath.
The mold is now ready to make the prosthesis. The patient should be present so pigments may be added to the elastomer to give a realistic appearance and match the patient’s skin color. Generally, dry mineral earth pigments or artist’s oil-based pigments are used. Color matching is done by mixing small amounts of the pigments into the elastomer. Some clinicians use color tabs and predetermined pigment formulations to match skin color. When a color match is achieved, the elastomer is compression molded and processed according to the manufacturer’s instructions.
After processing, the prosthesis is removed from the mold and the excess flash is removed. The prosthesis is then delivered to the patient. Surface pigmentation is generally done to give the prosthesis a more lifelike appearance. Medical grade silicone adhesives are often used to carry and bond surface colorants to the prosthesis. When mechanical undercuts are not present for retention, the patient may use adhesives to keep the prosthesis in place.
PHYSICAL PROPERTIES
The static and dynamic properties of the more popular maxillofacial materials are shown in Table 21-16. Values are representative of commercial products in each category. Tensile strength is important, because when the patient removes the prosthesis, high tensile forces are applied, particularly in thin areas.
Three of the materials are similar in maximum percent elongation with values from 422% to 445%. Knowing the percent elongation is helpful, because different parts of the face have different requirements in terms of how far the elastomer must stretch to accommodate facial movement.
Tear resistance is important for maxillofacial materials because prostheses may be torn when patients remove them. In the pants tear test, a thin sheet of the elastomer is made in the shape of a pair of pants. The legs of the pants are then pulled slowly apart and the energy required to propagate a tear is measured. RTV silicone has excellent tear resistance, because the samples do not tear but stretch, as in tensile elongation. Polyurethane has good tear resistance at 6.7 × 106 dynes/cm.
The evaluation of maxillofacial materials should involve not only the static but also the dynamic physical properties, because the stress-strain curves for elastomers are nonlinear. Because of the nonlinearity of the stress-strain properties of these materials, they function differently at high and low rates of loading. The dynamic modulus is the ratio of stress to strain applied for small cyclic deformations at a given frequency at a specified point on the normal stress-strain curve, or it is the slope of the stress-strain curve at a given point corresponding to a given frequency.
In practical terms, elastomers with a high dynamic modulus are rather rigid materials, whereas materials with a low dynamic modulus are more flexible. In Table 21-16, the RTV silicone has the lowest dynamic elastic modulus at 2.12 MPa.
During the last few years there have been many significant studies on the physical properties of unpigmented and pigmented maxillofacial elastomers as a function of accelerated aging. New elastomers are also being evaluated for maxillofacial applications. It is gratifying to see renewed interest in an area of biomaterials that is often overlooked.
Efforts have been made to modify the stress-strain properties of maxillofacial materials to match living facial tissues. Medical-grade silicone adhesive has been combined with an RTV silicone base in various ratios to control the elastic properties. The stress-strain profiles of human facial tissues and various ratios of the silicone elastomers are shown in Fig. 21-18. The curves demonstrate that it is possible to formulate silicones that will match the elastic properties of facial tissues.
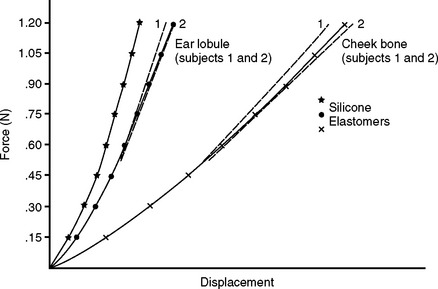
FIGURE 21-18 Stress-strain properties of human facial tissues and various silicone elastomers. (Adapted from Farah JW, Robinson JC, Hood JAA et al: J Oral Rehabil 15:277, 1988.)
Major advances have been made in the last few years in the area of maxillofacial materials. New materials have made it possible to make lifelike maxillofacial prostheses that last longer in service.
Andreopoulos, AG, Polyzois, GL, Demetriou, PP. Repairs with visible light-curing denture base materials. Quint Int. 1991;22:703.
Arab, J, Newton, JP, Lloyd, CH. The effect of an elevated level of residual monomer on the whitening of a denture base and its physical properties. J Dent. 1989;17:189.
Arima, T, Murata, H, Hamada, T. The effects of cross-linking agents on the water sorption and solubility characteristics of denture base resin. J Oral Rehabil. 1996;23:476.
Barclay, SC, Forsyth, A, Felix, DH, et al. Case report—hypersensitivity to denture materials. Br Dent J. 1999;187:350.
Berg, E, Gjerdet, NR. The effects of pressure and curing temperature on porosity of two chemically activated acrylics. Dent Mater. 1985;1:204.
Chandler, HH, Bowen, RL, Paffenbarger, GC. Physical properties of a radiopaque denture base material. J Biomed Mater Res. 1971;5:335.
Clark, RL. Dynamic mechanical thermal analysis of dental polymers. I. Heat cured poly(methyl methacrylate)-based materials. Biomaterials. 1989;10:494.
Consani, RL, Domitti, SS, Mesquita, MF, et al. Effect of packing types on the dimensional accuracy of denture base resin cured by the conventional cycle in relation to post-pressing times. Braz Dent J. 2004;15:63.
Craig, RG. Denture materials and acrylic base materials. Curr Opin Dent. 1991;1:235.
Dar-Odeh, NS, Harrison, A, Abu-Hammad, O. An evaluation of self-cured and visible light-cured denture base materials when used as a denture base repair material. J Oral Rehabil. 1997;24:755.
Darvell, BW, Clark, RK. The physical mechanisms of complete denture retention. Br Dent J. 2000;189:248.
Donovan, TE, Hurst, RG, Campagni, WV. Physical properties of acrylic resin polymerized by four different techniques. J Prosthet Dent. 1985;54:522.
Ellsworth, K. Fatigue failure in denture base polymers. J Prosthet Dent. 1969;21:257.
Firtell, DN, Harman, LC. Porosity in boilable acrylic resin. J Prosthet Dent. 1983;49:133.
Fletcher, AM, Purnaveja, S, Amin, WM, et al. The level of residual monomer in self-curing denture-base materials. J Dent Res. 1983;62:118.
Grant, AA, Atkinson, HF. Comparison between dimensional accuracy of dentures produced with pour-type resin and with heat-processed materials. J Prosthet Dent. 1971;26:296.
Hargreaves, AS. Equilibrium water uptake and denture base resin behavior. J Dent. 1978;6:342.
Hargreaves, AS. The effects of cyclic stress on dental polymethylmethacrylate. J Oral Rehabil. 1983;10:137.
Harrison, WM, Stansbury, BE. The effect of joint surface contours on the transverse strength of repaired acrylic resin. J Prosthet Dent. 1970;23:464.
Heath, JR, Davenport, JC, Jones, PA. The abrasion of acrylic resin by cleaning pastes. J Oral Rehabil. 1983;10:159.
Hill, RG. The crosslinking agent ethylene glycol dimethacrylate content of the currently available denture base resins. J Dent Res. 1981;60:725.
Hill, RG, Bates, JF, Lewis, TT, et al. Fracture toughness of acrylic denture base. Biomaterials. 1983;4:112.
Huggett, R, Brooks, SC, Bates, JF. The effect of different curing cycles on the dimensional accuracy of acrylic resin denture base materials. Quint Dent Technol. 1984;8:81.
Jagger, RG, Huggett, R. The effect of crosslinking on sorption properties of a denture base material. Dent Mater. 1990;6:276.
Jagger, DC, Harrison, A, Jandt, KD. The reinforcement of dentures. J Oral Rehabil. 1999;26:185.
Kalachandra, S, Turner, DT. Water sorption of poly(methyl methacrylate). III. Effects of plasticizers. Polymer. 1987;28:1749.
Kalachandra, S, Turner, DT. Water sorption of plasticized denture acrylic lining materials. Dent Mater. 1989;5:161.
Khan, Z, von-Fraunhofer, JA, Razavi, R. The staining characteristics, transverse strength, and microhardness of a visible light-cured denture base material. J Prosthet Dent. 1987;57:384.
Koda, T, Tsuchiya, H, Yamauchi, M, et al. High-performance liquid chromatographic estimation of eluates from denture base polymers. J Dent. 1989;17:84.
Lafuente JD: Accuracy of injected and poured denture base acrylic resins. Master’s thesis, University of Texas Health Science Center at Houston Dental Branch, Houston, 1997.
Lamb, DJ, Ellis, B, Priestly, D. The effects of processing variables on levels of residual monomer in autopolymerizing dental acrylic resin. J Dent. 1983;11:80.
Latta, GH, Jr., Bowles, WF, Conkin, JE. Three-dimensional stability of new denture base resin systems. J Prosthet Dent. 1990;63:654.
Leggat, PA, Kedjarune, U. Toxicity of methyl methacrylate in dentistry. Int Dent J. 2003;53:126.
Lorton, L, Phillips, RW. Heat-released stress in acrylic dentures. J Prosthet Dent. 1979;42:23.
Ma, T, Johnson, GH, Gordon, GE. Effects of chemical disinfectants on the surface characteristics and color of denture resins. J Prosthet Dent. 1997;77:197.
Messersmith, PB, Obrez, A, Lindberg, S. New acrylic resin composite with improved thermal diffusivity. J Prosthet Dent. 1998;79:278.
Mutlu, G, Huggett, R, Harrison, A, et al. Rheology of acrylic denture base polymers. Dent Mater. 1990;6:288.
NaBadalung, DP, Powers, JM, Connelly, ME. Comparison of bond strengths of three denture base resins to treated nickel-chromium-beryllium alloy. J Prosthet Dent. 1998;80:354.
NaBadalung, DP, Powers, JM. Effectiveness of adhesive systems for a Co-Cr removable partial denture alloy. J Prosthodont. 1998;7:17.
Nogueira, SS, Ogle, RE, Davis, EL. Comparison of accuracy between compression- and injection-molded complete dentures. J Prosthet Dent. 1999;82:291.
Ohkubo, C, Watanabe, I, Hosoi, T, et al. Shear bond strengths of polymethyl methacrylate to cast titanium and cobalt-chromium frameworks using five metal primers. J Prosthet Dent. 2000;83:50.
Oysaed, H, Ruyter, IE. Creep studies of multiphase acrylic systems. J Biomed Mater Res. 1989;23:719.
Parvizi, A, Lindquist, T, Schneider, R, et al. Comparison of the dimensional accuracy of injection-molded denture base materials to that of conventional pressure-pack acrylic resin. J Prosthodont. 2004;13:83.
Phoenix, RD. Introduction of a denture injection system for use with microwaveable acrylic resins. J Prosthodont. 1997;6:286.
Polyzois, GL, Karkazis, HC, Zissis, AJ. Dimensional stability of dentures processed in boilable acrylic resins: a comparative study. J Prosthet Dent. 1987;57:639.
Powers, JM, Koran, A. Color of denture resins. J Dent Res. 1977;56:754.
Rached, RN, Powers, JM, Del Bel Cury, AA. Efficacy of conventional and experimental techniques for denture repair. J Oral Rehabil. 2004;31:1130–1138.
Rached, RN, Powers, JM, Del Bel Cury, AA. Repair strength of autopolymerizing, microwave, and conventional heat-polymerized acrylic resins. J Prosthet Dent. 2004;92:79–82.
Rawls, HR, Granier, RJ, Smid, J, et al. Thermomechanical investigation of poly(methylmethacryate) containing an organobismuth radiopacifying additive. J Biomed Mater Res. 1996;31:339.
Rawls, HR, Starr, J, Kasten, FH, et al. I. Radiopaque acrylic resins containing miscible heavy-metal compounds. Dent Mater. 1990;6:250.
Robinson, JG, McCabe, JF. Impact strength of acrylic resin denture base materials with surface defects. Dent Mater. 1993;9:355.
Rodford, RA. Further development and evaluation of high-impact-strength denture base materials. J Dent. 1990;18:151.
Ruyter, IE, Espevik, S. Compressive creep of denture base polymers. Acta Odont Scand. 1980;38:169.
Ruyter, IE, Svendsen, SA. Flexural properties of denture base polymers. J Prosthet Dent. 1980;43:95.
Smith, LT, Powers, JM. Relative fit of new denture resins polymerized by heat, light and microwave energy. Am J Dent. 1992;5:140.
Smith, LT, Powers, JM, Ladd, D. Mechanical properties of new denture resins polymerized by visible light, heat and microwave energy. Int J Prosthodont. 1992;5:315.
Soni, PM, Powers, JM, Craig, RG. Physical and mechanical properties of acrylic and modified acrylic denture resins. J Mich Dent Assoc. 1977;59:418.
Stafford, GD, Bates, JF, Huggett, R, et al. A review of the properties of some denture base polymers. J Dent. 1980;8:292–306.
Stafford, GD, Huggett, R, Causton, BE. Fracture toughness of denture base acrylics. J Biomed Mater Res. 1980;14:359.
Strohaver, RA. Comparison of changes in vertical dimension between compression and injection molded complete dentures. J Prosthet Dent. 1989;62:716.
Teraoka, F, Takahashi, J. Controlled polymerization system for fabricating precise dentures. J Prosthet Dent. 2000;83:514.
Vallittu, PK. A review of fiber-reinforced denture base resins. J Prosthodont. 1996;5:270.
Vallittu, PK. Some aspects of the tensile strength of unidirectional glass fibre-polymethyl methacrylate composite used in dentures. J Oral Rehabil. 1998;25:100.
Vermilyea, SG, Powers, JM, Koran, A. The rheological properties of fluid denture base resins. J Dent Res. 1978;57:227.
Wang, X, Powers, JM, Connelly, ME. Color stability of heat activated and chemically activated fluid resin acrylics. J Prosthodont. 1996;5:266.
Wollff, EM. The effect of cross-linking agents on acrylic resins. Aust Dent J. 1962;7:439.
Amin, WM, Fletcher, AM, Ritchie, GM. The nature of the interface between polymethylmethacrylate denture base materials and soft lining materials. J Dent. 1981;9:336.
Braden, M. Tissue conditioners. I. Composition and structure. J Dent Res. 1970;49:145.
Braden, M. Tissue conditioners. II. Rheologic properties. J Dent Res. 1970;49:496.
Braden, M, Wright, PS. Water sorption and water solubility of soft lining materials for acrylic dentures. J Dent Res. 1983;62:764.
Dootz, ER, Koran, A, Craig, RG. Comparison of the physical properties of eleven soft dental liners. J Prosthet Dent. 1992;67:707.
Duran, RL, Powers, JM, Craig, RG. Visco-elastic and dynamic properties of soft liners and tissue conditioners. J Dent Res. 1979;58:1801.
El-Hadary, A, Drummond, JL. Comparative study of water sorption, solubility, and tensile bond strength of two soft lining materials. J Prosthet Dent. 2000;83:356.
Graham, BS, Jones, DW, Sutow, EJ. An in vivo and in vitro study of the loss of plasticizer from soft polymer-gel materials. J Dent Res. 1991;70:870.
Graham, BS, Jones, DW, Sutow, EJ. Clinical implications of resilient denture lining material research. II. Gelation and flow properties of tissue conditioners. J Prosthet Dent. 1991;65:413.
Hayakawa, I, Hirano, S, Kobayashi, S, et al. The creep behavior of denture supporting tissues and soft lining materials. Int J Prosthodont. 1994;7:339.
Iwanaga, H, Murakami, S, Murata, H, et al. Factors influencing gelation time of tissue conditioners. J Oral Rehabil. 1995;22:225.
Jones, DW, Sutow, EJ, Hall, GC, et al. Dental soft polymers: plasticizer composition and leachability. Dent Mater. 1988;4:1.
Kawano, F, Dootz, ER, Koran, A, et al. Bond strength of soft denture liners to denture base resin. J Prosthet Dent. 1992;68:368.
Kawano, F, Dootz, ER, Koran, A, et al. Sorption and solubility of 12 soft denture liners. J Prosthet Dent. 1994;72:393.
Kawano, F, Dootz, ER, Koran, A, et al. Bond strength of six soft denture liners processed against polymerized and unpolymerized poly(methyl methacrylate). Int J Prosthodont. 1997;10:178.
Kawano, F, Ohguri, T, Koran, A, et al. Influence of lining design of three processed soft denture liners on cushioning effect. J Oral Rehabil. 1999;26:962.
Kimoto, S, Kitamura, M, Kodaira, M, et al. Randomized controlled clinical trial on satisfaction with resilient denture liners among edentulous patients. Int J Prosthodont. 2004;17:236.
McCabe, JF. A polyvinylsiloxane denture soft lining material. J Dent. 1998;26:521.
Minami, H, Suzuki, S, Ohashi, H, et al. Effect of surface treatment on the bonding of an autopolymerizing soft denture liner to a denture base resin. Int J Prosthodont. 2004;17:297.
Murata, H, Hamada, T, Taguchi, N, et al. Viscoelastic properties of tissue conditioners—influence of molecular weight of polymer powders and powder/liquid ratio and the clinical implications. J Oral Rehabil. 1998;25:621.
Murata, H, Shigeto, N, Hamada, T. Viscoelastic properties of tissue conditioners: stress relaxation test Maxwell model analogy. J Oral Rehabil. 1990;17:365.
Murata, H, Taguchi, N, Hamada, T, et al. Dynamic viscoelasticity of soft liners and masticatory function. J Dent Res. 2002;81:123.
Murata, H, Taguchi, N, Hamada, T, et al. Dynamic viscoelastic properties and the age changes of long-term soft denture liners. Biomaterials. 2000;21:1422.
Nikawa, H, Jin, C, Hamada, T, Murata, H. Interactions between thermal cycled resilient denture lining materials, salivary and serum pellicles and Candida albicans in vitro. Part I. Effects on fungal growth. J Oral Rehabil. 2000;27:41.
Nikawa, H, Yamamoto, T, Hamada, T. Effect of components of resilient denture-lining materials on the growth, acid production and colonization of Candida albicans. J Oral Rehabil. 1995;22:817.
Nazhat, SN, Parker, S, Braden, M. Silica-filled elastomer/methacrylate systems as soft liners. J Biomater Sci Polym Ed. 2004;15:727.
Razavi, R, Kahn, Z, von Fraunhoffer, JA. The bond strength of a visible light-cured reline resin to acrylic resin denture base material. J Prosthet Dent. 1990;63:485.
Yilmaz, H, Aydin, C, Bal, BT, et al. Effects of different disinfectants on physical properties of four temporary soft denture-liner materials. Quint Int. 2004;35:826.
Cunningham, JL, Benington, IC. An investigation of the variables which may affect the bond between plastic teeth and denture base resin. J Dent. 1999;27:129.
Ekfeldt, WA, Ivanhoe, JR, Adrian, ED. Wear mechanism of resin and porcelain denture teeth. Acta Odont Scand. 1989;47:391.
Huggett, R, John, G, Jagger, RG, et al. Strength of the acrylic denture base tooth bond. Br Dent J. 1982;153:187.
Koran, A, Craig, RG, Tillitson, EW. Coefficient of friction of prosthetic tooth materials. J Prosthet Dent. 1972;27:269.
Ogle, RE, Davis, EL. Clinical wear study of three commercially available artificial tooth materials: Thirty month results. J Prosthet Dent. 1998;79:145.
Raptis, CM, Powers, JM, Fan, PL. Frictional behavior and surface failure of acrylic denture teeth. J Dent Res. 1981;60:908.
Suzuki, S, Sakoh, M, Shiba, A. Adhesive bonding of denture base to plastic denture teeth. J Biomed Mater Res. 1990;24:1094.
Umekawa, Y, Nagai, E, Ohtani, K, et al. In vitro wear of composite denture teeth. J Dent Res. 2003;82:428.
Chalian, VA, Drane, JB, Standish, SM. Maxillofacial prosthetics. Baltimore: Williams & Wilkins, 1971.
Craig, RG, Koran, A, Yu, R. Color stability of elastomers for maxillofacial appliances. J Dent Res. 1978;57:866.
Craig, RG, Koran, A, Yu, R. Elastomers for maxillofacial applications. Biomaterials. 1980;1:112.
Dootz, ER, Koran, A, Craig, RG. Physical properties of three maxillofacial materials as a function of accelerated aging. J Prosthet Dent. 1994;71:379.
Haug, SP, Moore, BK, Andres, CJ. Color stability and colorant effect on maxillofacial elastomers. Part II: weathering effect on physical properties. J Prosthet Dent. 1999;81:423.
Hulterstrom, AK, Ruyter, IE. Changes in appearance of silicone elastomers for maxillofacial prostheses as a result of aging. Int J Prosthodont. 1999;12:498.
Kiat-amnuay, S, Lemon, JC, Powers, JM. Effects of opacifiers on color stability of pigmented maxillofacial silicone A-2186 subjected to artificial aging. J Prosthodont. 2002;11:109.
Koran, A, Craig, RG. Dynamic properties of maxillofacial prostheses. J Dent Res. 1975;54:1216.
Kouyoumdjian, J, Chalian, VA, Moore, BK. A comparison of the physical properties of a room temperature vulcanizing silicone modified and unmodified. J Prosthet Dent. 1985;53:388.
Lai, JH, Hodges, JS. Effects of processing parameters on physical properties of the silicone maxillofacial prosthetic materials. Dent Mater. 1999;15:450.
Parker, S, Braden, M. Soft prosthesis materials based on powdered elastomers. Biomaterials. 1990;11:482.
Polyzois, GL. Color stability of facial silicone prosthetic polymers after outdoor weathering. J Prosthet Dent. 1999;82:447.
Polyzois, GL. Mechanical properties of 2 new addition-vulcanizing silicone prosthetic elastomers. Int J Prosthodont. 1999;12:359.
Turner, GE, Fisher, TE, Castleberry, DJ, et al. Intrinsic color of isophorone polyurethane for maxillofacial prosthetics. J Prosthet Dent. 1984;51:673.