Principles of cancer management
Introduction
Malignant disease afflicts about a third of all people at some time and about one in four die from it. Cancer patients are a major part of the general surgical workload and use as much as 40% of surgical bed occupancy. They place disproportionate demands on services, as operations are often extensive and patients are usually older, slower to recover and more prone to complications. Although rates of many malignancies are falling, the total numbers are rising because of increasing life expectancy. In younger age groups, surgical workload from cancer is growing because of earlier detection, increasing technical capabilities and greater patient expectations. The incidence of common malignancies in Western countries is shown in Table 13.1.
Table 13.1
The 10 most commonly diagnosed cancers in males and females in the UK (from Cancer Research UK, 2008)*; figures have been rounded
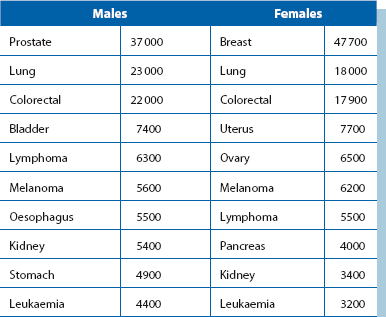
*See also Table 27.1 (p. 354)
Screening for malignancy (see Ch. 6), notably cervical, breast and colorectal cancer, has so far produced only a modest impact on mortality, because of poor patient compliance (in cervical and colorectal cancer) and because detecting biologically early disease (in breast cancer) is elusive.
Whether curative treatment can be offered depends on location and nature of the primary cancer (i.e. tissue type, metastatic potential) and the extent of spread. Management of particular cancers is becoming more protocol-driven as good randomised trials have identified the most effective treatments. These treatments increasingly involve surgery combined with other treatments, particularly radiotherapy, chemotherapy, and for certain cancers, hormone manipulation. Targeted therapies are also starting to play a role, in particular trastuzumab (Herceptin®) in HER2-positive breast cancer.
Whilst the detection and treatment of many types of cancer is improving, a universal preventative or cure for ‘cancer’ is unlikely ever to appear.
Neoplasia
The main characteristic of all neoplasms is uncontrolled growth that persists even after the initiating stimulus has been removed. The p53 protein is a vital cell cycle regulator and has been called the ‘guardian of the genome’ for its role in preventing genome mutation and stabilising cells in the face of pathological onslaughts. Abnormalities of p53 have been found in over 50% of all cancers and strenuous research efforts are continuing to modify its behaviour.
Most neoplasms can readily be categorised as benign or malignant according to histological pattern, and for most malignancies, it is possible to predict the likelihood of invasion or of metastasis. Sometimes this is difficult; for example, distinguishing between leiomyoma and leiomyosarcoma may only be achieved by following its long-term behaviour. Neoplasms may also be difficult to distinguish clinically from other tumour-like disorders such as hyperplasia (e.g. parathyroid adenoma from hyperplasia) or a hamartoma (a benign growth composed of a mixture of tissues normally found in that area of the body).
Benign neoplasms
Benign neoplasms usually grow slowly. They are typically well demarcated and often encapsulated, with a histological appearance closely reminiscent of the tissue of origin. Benign tumours present to the surgeon in a variety of ways summarised in Box 13.1.
Malignant neoplasms
Malignant neoplasms or tumours are typically non-encapsulated with a poorly defined, irregular outline due to local invasion. They usually grow progressively and often rapidly. Histologically, the cells range from well differentiated to anaplastic (i.e. little or no resemblance to parent tissue), with the aggression of the neoplasm increasing as differentiation decreases.
The cells of malignant neoplasms as well as their nuclei often vary widely in shape and size. The extent of this pleomorphism also tends to correlate with the degree of malignancy and the future clinical behaviour of the tumour. The supporting tissue stroma of some malignancies may undergo fibrous hyperplasia, which accounts for some of the characteristic clinical features; these include hardness to palpation (induration), intestinal obstruction caused by annular carcinomas of the large bowel, and retraction and dimpling of skin overlying breast cancers. On the other hand, in highly aggressive tumours, the supporting tissue stroma may be inadequate for nutritional support, leading to necrosis and patchy haemorrhage within the tumour. This often presents as a sudden onset of pain and a mass.
Malignant tumours present in a variety of ways as summarised in Box 13.2.
Carcinogenesis
Multiple primary lesions and recurrences: Most cancers are probably caused by environmental factors plus factors intrinsic to the patient. About two-thirds of cancers can be attributed in some way to external environmental factors such as ionising radiation, virus infections and carcinogens in air, food and water. When cancer develops, it is likely that the whole of the affected organ or tissue has already been altered by the carcinogen. Consequently, new primary tumours, as distinct from local recurrences, may develop later. This is a factor in deciding follow-up after treatment, although clearly distinguishing between new primaries and recurrences can be impossible. Certain tissues, particularly those of the bladder, breast, skin, head and neck, and large bowel, are at particular risk of new primary carcinomas.
Growth and spread of malignant tumours
Malignant tumours spread by local growth and infiltration and by distant metastasis via lymphatics, the bloodstream and across coelomic cavities. Carcinogenesis, however, is not a single pathological event. Rather, both the onset of uncontrolled proliferation and the capacity for distant spread evolve in a series of mutations over successive cell divisions. Most malignancies probably arise from a single cell line (i.e. are monoclonal) and the initial event is the acquisition of the capability to grow progressively. About 30 cell division cycles are probably needed to produce a clinically detectable lesion of 1 cm diameter containing 1000 million cells. Particular cellular properties, often arising through multiple mutations, enable a tumour to invade surrounding tissues whilst other properties allow invasion of lymphatic or blood capillaries, dissemination and ‘taking root’ in regional lymph nodes or other distant tissues.
These pathophysiological factors have important clinical consequences. Firstly, the earlier the primary tumour is detected and removed (i.e. the fewer cell division cycles have occurred), the greater the chance of complete cure. Unfortunately, mutations that permit metastasis can appear very early, i.e. by about 20 cell division cycles, when the primary lesion is too small to be detected (about 1 mm diameter).
There are two conflicting theories about the significance of regional lymph node involvement in cancer. Both recognise that lymph node involvement implies a worse prognosis. Halsted believed that lymph nodes are important in halting spread, at least for a time, and that radical surgery to remove the nodes offers the best potential for cure. In more than 50% of large bowel cancers, for example, Halsted's view is probably correct and radical surgery remains the treatment of choice. In many other cancers, haematogenous spread is often occult and probably occurs early, rendering the disease incurable by surgery. Unfortunately, identifying which patients already have metastatic disease remains difficult, although better imaging, laparoscopy and biopsy techniques are improving pre-treatment staging.
An alternative view about the significance of involved nodes, expressed by Fisher, is that any metastases indicate failure of host defences and therefore denote systemic metastasis beyond the nodes. In breast cancer, systemic treatments with cytotoxic and/or hormonal therapy have been developed with the intention of eliminating or suppressing micrometastases. These have improved survival in a small proportion of patients. Nevertheless, local surgery is important in controlling loco-regional disease in the breast, chest wall and axilla.
In practice, when lymph node metastases are present, the probability of surgical cure depends on the point at which the cancer develops its capacity for haematogenous spread. This can be about the same time as lymph node metastases appear, so surgical removal of metastases would be ineffective because haematogenous metastases would already be multifocal. Occasionally blood-borne metastasis appears to be a solitary event and a cure can be achieved—for example, by partial hepatectomy for colorectal cancer or pulmonary lobectomy for renal cell carcinoma, as well as in some paediatric malignancies. The liver, lungs, bone and brain are common organs for haematogenous spread. Multiple metastases often respond temporarily to palliative chemotherapy, radiotherapy or hormone manipulation (e.g. carcinomas of breast, uterus, kidney and prostate), but complete cure is very rarely achieved.
This model of the inception of metastatic potential means that progress in cancer management has to focus on primary prevention, very early diagnosis, and research to find effective non-surgical approaches, e.g. chemotherapy, hormonal manipulation and targeted therapeutic approaches.
Treatment of malignant tumours
Basic principles of cancer management
Two broad considerations determine the approach to treatment for any cancer patient. The first is whether an attempt can and should be made to achieve a cure or whether palliation is more appropriate. This choice depends on the nature of the tumour, the extent of local spread and whether distant metastases are believed to be present. The second consideration is the prognosis. This takes further factors into account, including the natural history of the type of cancer and the patient's age and co-morbidity and consequent ability to withstand surgery. Systems of staging have been devised for each tumour type, many based on the TNM system which scores characteristics of the Tumour, the extent of regional lymph Node involvement and the presence of Metastases. Staging is used in planning treatment, as a guide to prognosis and as a standardised descriptive tool for comparing efficacy of treatment in different patients and in different centres.
Cancer often recurs some time after the primary treatment, and the success of treatment is often described in terms of survival after a given number of years rather than ‘cure’. Five-year survival is a common yardstick and in many cases can be taken to imply cure. Nevertheless, some tumours, particularly breast cancer, may recur in a disseminated form as long as 30 years after apparently successful eradication. Conversely, colorectal cancer and testicular teratoma and seminoma rarely recur after the patient has survived 7 years.
Teamworking in cancer management
There is a growing trend towards involving a range of specialists working in cooperative teams to manage patients with complex problems. Structured multidisciplinary teams are effective for managing complex cancer cases where the diagnosis needs careful consideration and where the treatment package may involve radiotherapy, chemotherapy or targeted therapy pre- or postoperatively, meticulous planning of an operation, prostheses or reconstructive surgery, stoma care and specialist nursing or physiotherapy, as well as social and psychological support.
The benefits of working in this way stem from the battery of experience brought by experts in different disciplines, particularly in cases where diagnostic and therapeutic options are not clear. Cases can be discussed at any stage in the diagnostic or treatment process and an optimum treatment plan generated; the group has joint responsibility for implementing the plan. Multidisciplinary teams (MDTs) have also been successfully introduced into other areas, for example neck lumps and rheumatoid disease. However, having such teams is expensive and takes staff away from other duties. It is a luxury where health care is less developed or poorly funded.
Treatment options
The treatment options for malignant disease include surgical excision, radiotherapy, chemotherapy, hormonal manipulation and molecular targeted therapies, with two or more of these often used in combination. Treatment offered to an individual depends on tumour type and extent, molecular profile, the patient's overall fitness and whether the aim is cure or palliation. A radical or aggressive approach may be recommended where the aim is cure, provided the scientific evidence supports this approach. Where cure is not possible, or where disease has relapsed after radical treatment, the aim is to palliate, i.e. to alleviate the symptoms.
Palliative care focuses on quality of life when cure is not possible and involves weighing up the benefits of treatment against its burdens whilst focusing on what is important to the patient and family. Specialist palliative care teams can offer invaluable outpatient support in difficult matters of symptom control, or psychological, social or spiritual problems. Some patients may need short admissions to specialist units (often called hospices) for symptom control and eventually for terminal care.
Most standard cancer treatments involve unpleasant side-effects, easily disregarded by doctor and patient in their enthusiasm for treatment. Patients need to be appropriately informed and involved in treatment decisions, though the amount of information given varies, with some wanting the whole story and others less. How much the patient and the family want to know may differ but a doctor may speak independently to family members with the patient's consent. A diagnosis of cancer should not be concealed from a competent patient as suspicion and fear of the unknown often causes more distress than a frank explanation of the diagnosis and its ramifications. A period of time should be allowed for the patient to formulate further questions and a follow-up consultation arranged in a few days. It often helps to have a friend or relative present. Patients are also helped by well-constructed printed information, by self-help groups and by specially trained cancer nurses who can spend the necessary time and act as an intermediary if necessary.
Surgery for cancer
General principles of cancer surgery (Box 13.3)
The ideal result of cancer surgery is complete eradication of the malignant disease without radically interfering with function. Nearly a third of cancer patients can be cured in this way; these are mainly patients with only primary disease (without nodal or metastatic disease). Decisions about whether to embark on major elective surgery depends on thorough assessment of the nature of the disease and its stage. Modern techniques of cross-sectional imaging, laparoscopy and intraoperative ultrasound greatly assist and may save patients from fruitless radical surgery. Evidence-based reviews of treatments for malignancies at different stages also help decide indications for surgery, with level 1 evidence from well-conducted randomised controlled trials being best.
Metastases apparently confined to local lymph nodes are usually excised along with the primary tumour or else at a second operation. In gastric cancer, familiarity with the lymphatic drainage guides excision of defined node groups and this appears to give better long-term cure rates. Even in the presence of incurable metastases, surgical excision of the primary is sometimes required to relieve local effects of the tumour, e.g. bleeding, pain or bowel obstruction. Similar results can often be obtained by radiotherapy, chemotherapy or physical means of destruction such as radiofrequency probes or lasers. Palliative surgery may be required for specific distressing symptoms from locally advanced or metastatic disease (e.g. dysphagia from oesophageal cancer, severe haemorrhage from a bladder tumour) or some emergency problem (e.g. acute bowel obstruction).
Sometimes surgery is used to debulk a tumour, usually combined with chemotherapy, to improve its efficacy (as in ovarian carcinoma) or to avoid leaving unstable tissue in abdominal nodes (as in testicular teratoma). Increasingly, chemotherapy and/or radiotherapy is used to ‘downsize’ a malignant tumour to facilitate excisional surgery, e.g. rectal cancer. This is sometimes inaccurately called ‘down-staging’, since it has no effect on metastatic disease.
Radiotherapy
General principles of radiotherapy
The value of ionising radiation in treating malignant tumours was recognised soon after the discovery of X-rays in 1895, and radiotherapy is now employed at some stage in about half of all patients with malignant disease. Orthovoltage X-rays (up to 250 kV) were the basis of radiotherapy until megavoltage irradiation in the late 1950s. Cobalt-60 machines provided more penetrating radiation, but have been superseded by linear accelerators which provide photon beams with an energy of 5–20 MeV. With increased energy, the radiation dose peaks deep to the skin, avoiding the former severe skin reactions. Radiotherapy works by the target tissue absorbing radiation, which induces highly reactive free radicals that damage DNA and cause cell death at mitosis. Cancer cells with a high rate of proliferation are particularly sensitive but normal tissues with high rates of cell turnover (e.g. gut mucosa and bone marrow) are also vulnerable. The total dose given depends on the aims of treatment, the site and volume of the tumour and its relationship to important normal tissues. Treatment is by a variable number of sessions or fractions over several weeks to allow normal tissues to recover between treatments.
The larger the volume of tumour, the greater the dosage of irradiation required. Since dosage is limited by the tolerance of normal tissues, radiotherapy is more effective for small lesions. Radiation dose is measured in Gray (Gy). A common daily dose is around 2 Gy, sufficient to destroy about 50% of viable cells in an average tumour; each subsequent dose destroys 50% of the remainder, causing a logarithmic decline in viable cell numbers as treatment proceeds. Planning radiotherapy for lesions deep within the body has improved as a result of enhanced CT and magnetic resonance imaging (MRI).
Radiation can be directed to a tumour in three ways:
• External beam irradiation—this is most commonly employed for skin lesions and deeply located tumours
• Local application of radioisotopes (brachytherapy)—this involves placing the radiation source on or in the tissue to be irradiated. Plaque sources of radiation can be employed for skin malignancies, and radioactive iridium wires or caesium needles can be implanted in the oral cavity, prostate, skin and sometimes breast, giving high-dose local irradiation. Implantation is usually performed under general anaesthesia. For cancer of the uterus and cervix, the radioactive source is placed in a sealed container within the uterine or vaginal cavity; it can be inserted without risk to staff via a flexible tube from the radiation safe using computer control
• Systemic radioisotope therapy—radioactive iodine given by mouth or intravenously is a well-established treatment for thyrotoxicosis and can be used for well-differentiated thyroid cancers even if metastases are present, provided the rest of the thyroid has been removed. Attempts are being made to direct radioisotopes precisely to cancer cells by attaching them to tumour-specific markers such as monoclonal antibodies, thus realising the dream of a ‘magic bullet’, but techniques remain largely in an experimental stage
Major applications of radiotherapy
Radiotherapy has three major applications: as a primary cure, as adjuvant treatment to surgery (and/or chemotherapy) or as palliation. The treatment objective must be clearly defined before treatment is begun.
Primary curative radiotherapy
Radiotherapy with curative intent is known as radical, and is widely used for basal cell and squamous cell carcinomas of skin. Where rates of cure are comparable to surgery, radiotherapy can be used for tumours that are technically difficult to remove or where surgery would be particularly mutilating, as in the head, neck and larynx. Radiotherapy can be directed at the primary lesion and regional lymph nodes if appropriate. In head and neck cancers without distant metastases, cure rates of 50–90% can be achieved with radiotherapy alone, with similar results in carcinoma of the cervix. Radiotherapy is employed in most common solid tumours and in early cases of Hodgkin and non-Hodgkin lymphomas. The efficacy and application of radiotherapy for various tumour types is summarised in Table 13.2. Radiotherapy can also achieve cure in up to 50% of bladder cancers, with salvage cystectomy reserved for recurrence.
Adjuvant radiotherapy
The principle underlying adjuvant therapy, whether radiological, chemical or hormonal, is to target clinically undetectable micrometastases believed to be responsible for local, regional and systemic recurrence after a primary has apparently been completely removed. Adjuvant radiotherapy can be applied to local tissue and regional nodes before surgery (neoadjuvant therapy), after surgery or both. Neoadjuvant therapy, comprising radiotherapy, chemotherapy or both may also be used to ‘downsize’ a cancer to make surgery practicable, e.g. in locally advanced rectal cancer.
Adjuvant radiotherapy is widely employed for cancer of the breast after removing the primary lesion locally or by mastectomy. If axillary nodes are involved, radiotherapy can be an alternative to radical lymph node clearance; survival rates are comparable to those achieved by surgery. However, the trend of opinion favours radical lymph node clearance for the improved diagnostic accuracy it provides and the prognostic value of knowing the number of nodes involved. The preference for surgery or radiotherapy remains one of local choice. Radiotherapy is also highly effective adjuvant therapy in seminoma of the testis.
Palliative radiotherapy
Radiotherapy for palliation is employed for local control of primary or metastatic lesions to treat symptoms whilst causing minimal side-effects. It is also employed to prevent anticipated complications, e.g. spinal cord compression. Much lower total doses are used than for attempts at cure; short courses or single high-dose fractions are tolerable and convenient for the patient. Clearly, palliative radiotherapy can only be justified if expert assessment predicts that therapeutic benefit is likely.
Radiotherapy is particularly effective in controlling metastatic deposits in bone and brain. The pain of bone metastases can often be completely relieved by radiotherapy, as can some of the neurological manifestations of brain secondaries.
Radiotherapy is often valuable in providing symptomatic relief in advanced disease. In ulcerating breast cancer, radiotherapy can shrink the primary lesion, permitting healing of overlying skin. Similarly, the distressing symptoms of cough, haemoptysis and pleuritic pain from advanced lung cancer can be eased and symptoms of local recurrence can often be controlled, e.g. pain from rectal cancer. Indications for palliative radiotherapy are summarised in Box 13.4.
Complications of radiotherapy
Despite the precise use of high-energy radiotherapy, side-effects and complications still occur; the main early effects are outlined in Table 13.3.
Long-term side-effects of radiotherapy
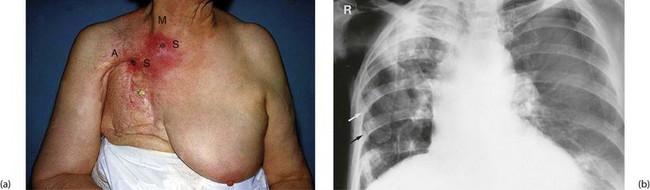
Modern radiotherapy using high-energy sources with meticulous treatment planning and delivery causes fewer side-effects than the orthovoltage treatment used in earlier days. Side-effects such as osteoradionecrosis are rare (see Fig. 13.1), but endarteritis obliterans may be a long-term complication affecting any tissue subjected to radiotherapy. The effect is progressive impairment of blood supply, loss of specialised tissues and replacement with fibrosis. In the chest, radiotherapy can cause pulmonary fibrosis and in women treated for left-sided breast cancer, there is an increased risk of cardiac events; techniques have been modified as a result. In the gastrointestinal tract, the effects of radiation on bowel (radiation enteritis) can be particularly serious, with continued bleeding (in large bowel) and stricture formation (in small bowel). The same reaction probably accounts for delayed or incomplete healing after surgery with breakdown of intestinal anastomoses or formation of internal fistulae. Radiation colitis most commonly results from treatment of uterine cervical cancer.
Chemotherapy
General principles of chemotherapy
Success with chemotherapeutic agents in curing many haematological and childhood malignancies encouraged the use of similar drugs for solid tumours previously treated only by surgery or radiotherapy.
Drugs destroy tumour cells by exploiting their increased mitotic and metabolic rates. The main types of chemotherapeutic agent are as follows:
• Antimetabolites—analogues of normal cellular nutrients, e.g. methotrexate acts as a substitute for folinic acid
• Alkylating agents—these bind to DNA, e.g. nitrogen mustards
• Drugs which cross-link DNA, e.g. cisplatin
• Drugs which disrupt the mitotic spindle, e.g. vinca alkaloids, taxanes
These cytotoxic mechanisms also affect normal tissues, usually to a lesser extent, and are responsible for most side-effects of chemotherapy. Drug are often used in combination, selected so the toxic effects of each drug impact on a different organ system; this means that each drug can be given in full tumour-toxic dose without excessive damage to normal tissues. The most effective combinations and doses for each tumour type have been established mainly by empirical trials. Very good combinations were developed in the 1970s and 1980s, e.g. CMF for breast cancer, CHOP for lymphoma and BEP for testicular cancer, but an increasing range of new chemotherapeutic drugs promise improvements. Drugs are usually given intravenously, often by infusion, in a series of four to six short courses separated by 3–4 weeks to allow recovery of normal tissues.
Experience has revealed a wide spectrum of sensitivities of different malignant tumours to cytotoxic therapy. The sensitivities of different tumour types are summarised in Box 13.5.
Major applications of chemotherapy
Chemotherapy offers substantial benefit and a chance of cure in some well-defined malignancies, as shown in Box 13.5. However, clinicians treating cancer need to be clear whether their objective is cure or palliation, and base decisions on published clinical trials. Given the potential for side-effects and the high cost, there is no place for speculative chemotherapy where evidence shows no benefit.
Primary curative treatment
This is mainly indicated for highly sensitive germ cell tumours, high-grade lymphomas and solid tumours and leukaemias of childhood. Chemotherapy may be used alone (e.g. Hodgkin's disease), or it may follow removal of the primary tumour (e.g. teratoma, Wilms' tumour), or it may be used first and then any residual tumour resected (e.g. abdominal para-aortic nodes in testicular teratoma).
Adjuvant chemotherapy
This involves systemic chemotherapy added to local treatment of the primary to destroy already disseminated but undetectable micrometastases. Adjuvant chemotherapy is often used for breast cancer with axillary lymph node involvement, where 5- and 10-year survival rates have both been improved by 5–15%. In cancer of colon and rectum, adjuvant therapy, mainly using 5-fluorouracil (5FU) combinations, has a role in extending recurrence-free survival and life expectancy. Current information on this and other trials is available from Cancer Research UK (www.cancerhelp.org.uk). Chemotherapy plus radiotherapy (chemo-radiotherapy) often replaces the need for radical surgery for anal canal, cervical and some head and neck cancers.
General palliative treatment
This is the rationale for chemotherapy in disseminated malignancy of highly sensitive and moderately sensitive tumours. Cure is rare but quality of life may be greatly improved, often through alleviation of specific symptoms (e.g. breast cancer causing lymphatic obstruction) and in some cases, life may be prolonged.
Side-effects of chemotherapy
Chemotherapy is especially toxic to cells with rapid turnover, e.g. bone marrow and gastrointestinal epithelium. Toxic effects are summarised in Box 13.6.
Hormonal manipulation
The growth of certain tumours, notably carcinoma of the prostate and some breast cancers, is dependent on sex hormones. Removal of the gonads or use of hormone blocking drugs can have a valuable inhibitory effect on tumour growth. Hormonal manipulation in the treatment of prostatic cancer is covered in detail in Chapter 35, p. 455, and breast cancer in Chapter 45, p. 555.
Targeted therapies
Oncologists have long dreamed of finding precise ways of delivering cancer-killing drugs to destroy cancer cells without damaging normal cells. This idea of a ‘magic bullet’ is now becoming real employing monoclonal antibodies. The specific antibody recognises certain protein configurations on the surface of cancer cells and ‘locks’ on to them. The intention is for cells to destroy themselves or trigger the body's immune system to recognise the marked cells as ‘non-self’ and attack them. So far, this has limited application but much work is continuing in the field.
Lymphoma
The antigen CD20 is found on the surface of normal B-cell lymphocytes (but not their precursors) and also on the surface of nearly all abnormal malignant B-cell lymphocytes found in 75% of non-Hodgkin lymphoma. The drug rituximab is a genetically engineered chimeric murine/human monoclonal IgG1 antibody directed against the CD20 antigen. Normal and abnormal B-cells are both destroyed but the body quickly replaces the normal cells, so side-effects from lack of B-cells are minimal. Rituximab combined with chemotherapy has increased response rates in B-cell lymphoma by 20% compared with chemotherapy alone and has become the desirable standard treatment. However, the drug can cause serious adverse effects including mucocutaneous reactions and the rare but fatal infusion reaction complex of organ failure.
Breast cancer
Human epidermal growth receptor 2 (HER2) is an antigenic protein involved in transmitting growth signals from outside the cell to the nucleus; a strong signal causes the cell to divide. About 20% of breast cancers have excess HER2 (known as HER2-positive tumours). Trastuzumab (trade name Herceptin) is a recombinant DNA-derived humanised monoclonal IgG1 antibody which binds selectively to the surface HER2 receptor (see Ch. 45, p. 556). Its anti-cancer effects are brought about by blocking cell growth and stimulating the immune system to destroy the tumour cells. As with similar drugs, there are potentially serious hazards including severe hypersensitivity reactions, cardiotoxicity and adverse pulmonary events. Herceptin has also recently been approved for the treatment of advance gastric cancers that are HER2-positive.
Imatinib
In chronic myeloid leukaemia, the Philadelphia chromosome leads to production of an abnormal ‘fusion protein’ enzyme which continuously activates tyrosine kinase (TK) receptors. This causes uncontrolled cellular proliferation. A similar process occurs in gastrointestinal stromal tumours (GIST), with activation of the c-kit receptor. Imatinib preferentially occupies the TK active receptor site, thereby decreasing the activity of several oncoprotein TK enzymes. As a result, the drug inhibits cellular proliferation and induces apoptosis (programmed cell death) in malignant cells.
The future
Many other monoclonal antibodies as well as other small molecules that interfere with cellular receptors are under development or are in clinical trials for a range of tumour types. Other small molecules approved for use in particular cancer types include:
• Gefitinib and erlotinib (small molecular epidermal growth factor (EGFR) inhibitors, used in EGFR mutation-positive lung cancers)
• Sunitinib, sorafenib and pazopanib (multi-targeted tyrosine kinase inhibitors, approved for renal cell carcinoma)
• Lapatinib (tyrosine kinase inhibitor of HER2 and EGFR approved for use in metastatic HER2-positive breast cancer)
It is likely such targeted therapies will improve the outcome for an escalating range of malignancies. Early stage clinical trials now emphasise the drugs' biological effects, using validated biomarkers as endpoints, and an adaptive approach for analysing results in real time.
Palliative care
The principles of palliative care are outlined in Box 13.7. The diagnosis of cancer has immense significance to the patient and the patient's family. Areas affected include:
• Psychological—adjustment reaction, anxiety, depression
• Social—loss of role: unable to care for family, no longer viewed as husband/wife/companion
• Financial—loss of job, expense of frequent hospital visits
For specialists, there is a danger of concentrating on the primary disease while failing to treat the patient as a whole. Palliative care is concerned with the problems listed, with the emphasis on the patient's priorities, during and after treatment where complete cure is not possible. As well as trying to bring positive assistance, a doctor's duty includes ‘doing no harm’ and there is no ethical dilemma in withdrawing treatment unlikely to give benefit.
Increasingly, patients are living longer with a large tumour load. This means they often experience symptoms with multiple aetiologies, resulting in treatment with numerous and often too many drugs. Diagnostic tests should be used only to identify causes likely to be remediable. Time must be made available for clear and honest explanation of proposed treatments with patients and families. Palliative care focuses on quality of life, and many clinicians, including family practitioners, are keen to look after their patients at the end of their lives, ensuring a comfortable and dignified demise. Specialist palliative care physicians and nurses can support the primary care team and the patient and family at home and in specialist palliative care units (often called hospices).
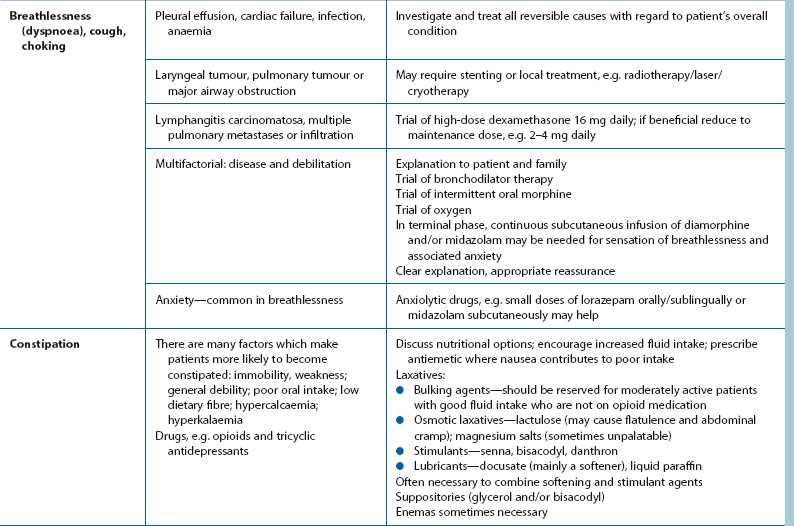
Approach to common symptoms requiring palliation other than pain
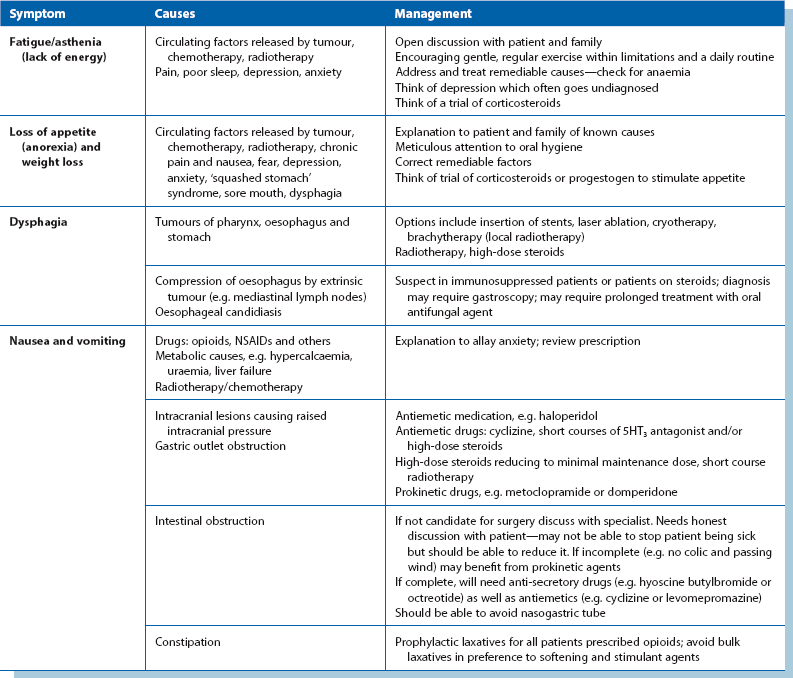
Good communication is the foundation of palliative care. Giving patients and families time to talk about their thoughts and concerns often in itself provides comfort. Palliative pain relief is covered in the next section, and management of other common symptoms is detailed in Table 13.4. Effective management of the symptoms of advancing cancer requires frequent reassessment, and frequent discussion with the patient to assess his or her own priorities.
Cancer pain
About three-quarters of patients with advanced cancer have pain at some stage. How individuals perceive pain is influenced by what it means to them (e.g. my cancer is getting worse), as well as other factors in their life, which, if deteriorating, can worsen pain. Addressing these factors is an important way of helping.
Assessment of pain: Patients frequently have more than one source of pain and each must be assessed. Pain measurement tools (e.g. visual analogue or verbal rating scales) and charts can be helpful. In assessing pain, it is useful to use a classification to help plan how to relieve it:
• Visceral pain—a constant ‘tumour ache’ caused by pressure of cancer or invasion of internal organs. Although severe, it often responds to opioids
• Neuropathic pain—caused by pressure or destruction of nerves. Often difficult to describe, this may be a clue to their aetiology. Sometimes words like ‘tingling’ or ‘shooting’ are used and pain is in the distribution of a nerve root or peripheral nerve. Such pains are usually only partially responsive to opioids and require the use of adjuvant analgesics such as tricyclic antidepressants (e.g. amitriptyline), anti-epileptics (e.g. gabapentin) or corticosteroids to reduce oedema around tumours
• Bone pain—caused by infiltration of cancer. Clinical features depend on the location, e.g. spinal lesions may cause pressure on the cord causing nerve pain, anaesthesia and weakness. Skull base lesions can lead to cranial nerve palsies
Bony metastases often lead to incident pain where pain is tolerable at rest but becomes much worse with movement. This is often difficult to treat and requires local radiotherapy plus bisphosphonate infusions (particularly in breast and prostate cancers and myeloma), together with analgesics. Incident pains are not usually responsive to opioids but adjuvants (mentioned above) may help. It is best to take medication before the activity known to lead to pain.
Principles of cancer pain management:
• The aim is to control pain as well as possible, with a balance between analgesia and the side-effects of medication
• Analgesia should be given regularly not just ‘as required’
• Analgesics should be titrated against the severity of pain using the three-step analgesic ladder developed by the World Health Organization (Fig. 13.2; see also Table 13.5)
Table 13.5
Adjuvant analgesics and other treatments for management of cancer pain
| Treatment method | Indications |
| Non-steroidal anti-inflammatory agents—via oral/rectal/transdermal routes | Reduce inflammatory component in pain. Have a role for bony and other musculoskeletal pains and may help neuropathic pains |
| Tricyclic antidepressants (usually as single evening dose) | Used in treatment of neuropathic pain. Also help patients to sleep. Can help mood as dose is increased |
| Anticonvulsants (e.g. gabapentin) | Valuable for neuropathic pain |
| Antispasmodics (e.g. hyoscine butylbromide—Buscopan) | Reduce visceral contractions in colicky visceral pain (intestinal, biliary, ureteric) and overt bowel obstruction |
| Muscle relaxants (e.g. diazepam) | Relieve muscle spasms |
| Antibiotics (e.g. metronidazole tablets or gel) | Help treat infected superficial lesions, e.g. ulcerated skin, breast or head and neck tumours (usually involve Gram-negative organisms) |
| Corticosteroids (e.g. dexamethasone 4–8 mg daily, prednisolone 30–60 mg daily) Dexamethasone causes less fluid retention |
Shrink oedema associated with tumour to relieve pressure effects, e.g. cerebral tumours, spinal cord and peripheral nerve compression, liver capsule stretching, tumour swelling causing obstruction to bowel, biliary or urinary tracts |
| Topical anti-inflammatory agents | For oral and nasal mucositis, ulcerated skin lesions, radiation proctitis |
| Local palliative radiotherapy | For bone metastases, compressive lesions of brain and spinal cord, large airway and superior vena caval obstruction, fungating or bleeding superficial lesions |
| Bisphosphonates (e.g. pamidronate, clodronate) | Predominantly used for the relief of bone pain in metastatic breast cancer, and multiple myeloma. Can be given parenterally as bolus or continuously as oral therapy |
| Radioactive isotope therapy | Used for the relief of bone pain due to metastatic disease |
| Palliative chemotherapy | Sometimes relieves pain by shrinkage of chemosensitive tumours |
| Nerve blocks (often under radiological guidance) | Very helpful in treating pain in specific areas, e.g. intercostal blocks for chest wall pain, coeliac plexus blocks for pancreatic and other ‘foregut’ pain. Often reduce the overall requirement for systemic analgesia |
| Physiotherapy and associated physical modalities (e.g. massage, TENS, hot or cold packs) | Muscle spasm, inflammatory component of pains. Massage for muscle spasms and lymphoedema. TENS may help neuropathic pain |
| Skeletal immobilisation | Elective internal fixation of long bones for incipient or actual pathological fracture of long bones |
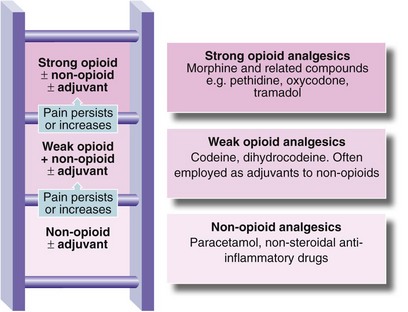
Fig. 13.2 Three-step analgesic ladder for cancer pain control (WHO 1986) (see Table 13.5 for adjuvants)
The World Health Organization has stated that pain relief should be:
Opioid analgesia: Morphine is generally the opioid of choice. Most patients' pain can be managed with oral morphine, the dose being titrated up or down according to analgesic requirements. Addiction is not a problem amongst cancer patients. If the pain abates, for example after radiotherapy, it is easy to reduce and sometimes to stop morphine. Nevertheless, this should be done gradually. Tolerance and diminishing effectiveness of morphine is uncommon, even among patients who take the drug for months. If a patient on a stable dose requires an increase, this usually reflects disease progression.
Strong opioids are administered parenterally when patients cannot take oral medication because of dysphagia, nausea, vomiting or fatigue. The intravenous route can be used in hospital but the subcutaneous route is preferred at home. This route is suitable for single ‘breakthrough’ doses and for continuous infusion. Infusions are given via an indwelling ‘butterfly’ needle, usually controlled with a battery-operated syringe driver. In the UK, diamorphine is preferred to morphine because smaller volumes provide equi-analgesic doses.
Establishing treatment: The preferred method for establishing dosage is by titration. The usual starting dose will be 5–10 mg of immediate-release morphine 4-hourly (or 2.5–5 mg for elderly patients). For those with renal or liver impairment or in the very elderly, 6–8-hourly intervals may be required. In addition to the regular dose, the same dose should be prescribed on an ‘as required’ basis to treat pain which ‘breaks through’ before the next dose is due. If morphine relieves the pain, the next day's regular dose (and p.r.n. dose) is ⅙ of the total given over the previous 24 hours, or ½ for slow-release morphine.
Other opioids are used less commonly in palliative care and usually for specific indications. These are summarised in Table 13.6.
Table 13.6
Opioid drugs used in palliative care
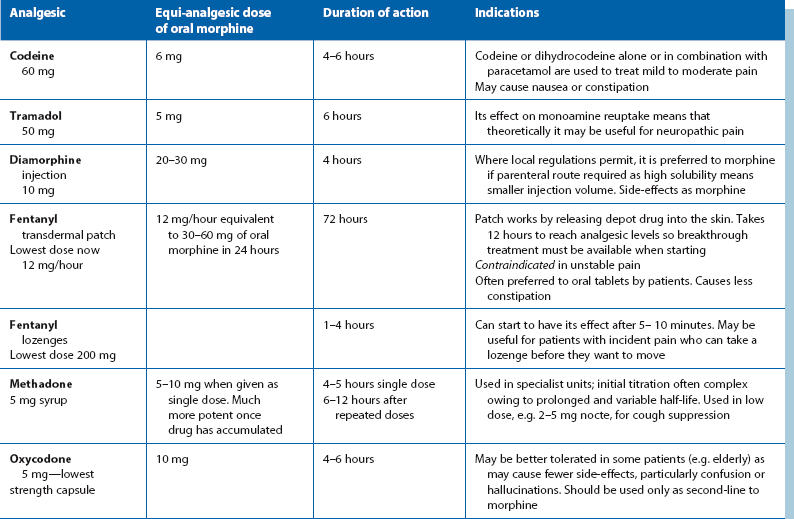
Note: there is no role for meptazinol or pethidine in the long-term treatment of cancer pain as they have limitations, e.g. analgesic ceiling, short duration of action or accumulation of toxic metabolites, which render them unsuitable for regular long-term use.