Disorders of the male genitalia
Disorders of the scrotal contents
Introduction
Abnormalities of the scrotal contents include disorders of the testis or its coverings, the spermatic cord and inguino-scrotal hernias (see Ch. 32). Distinguishing between them usually requires only clinical examination. Diagnoses that must not be missed are testicular tumours and testicular torsion. Other problems include inflammation, hydrocoeles and cysts, maldescent and testicular trauma, as well as varicocoele. Male sterilisation and disorders of the penis are also covered in this chapter.
Clinical examination of scrotal lumps and swellings
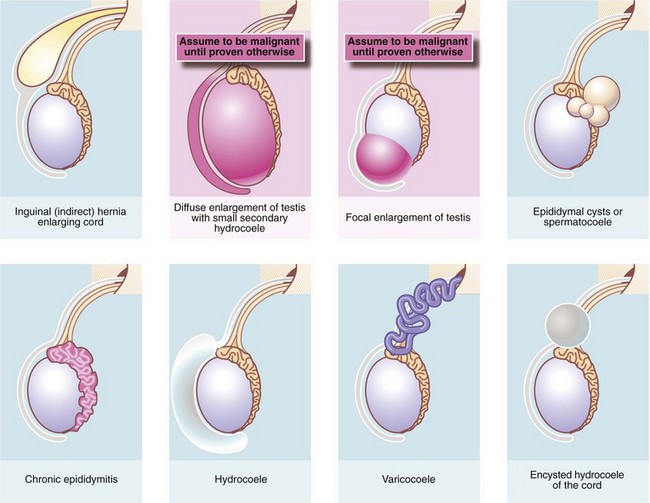
A lump or swelling in the scrotum may be:
• A solid or cystic mass arising from a component of scrotal contents or spermatic cord. These include testis, epididymis, epididymal appendage, vas deferens and pampiniform venous plexus
• A collection of fluid in the tunica or processus vaginalis (hydrocoele)
• An indirect inguinal hernia extending along the embryological path of testicular descent into the scrotum
The important disorders of the scrotum and contents are summarised in Table 33.1, with their anatomical and clinical significance.
The origin of a scrotal lump: The first objective is to determine if the swelling arises in the groin, the spermatic cord or the scrotum and is achieved by palpating the cord at the scrotal neck. In a hernia, the cord is broader than normal and the hernia can be shown to communicate with the abdominal cavity by a cough impulse or by reducing the hernia. Spermatic cord swellings (varicocoele or cyst) are usually easily recognised. In purely scrotal lumps, the spermatic cord is a normal diameter.
Testicular and epididymal lumps: With a scrotal abnormality, an attempt should be made to palpate testis and epididymis separately, and to determine their relationship to the lump. If the testis is enlarged or has a lump within it, this is a tumour until proven otherwise. Testicular swellings due to lymphoma, leukaemia or granulomatous infections (e.g. tuberculosis or syphilitic gumma) may be softer but this is unreliable. Any testicular pathology may cause a little fluid to accumulate in the tunica vaginalis producing a small secondary hydrocoele but this rarely interferes with testicular palpation.
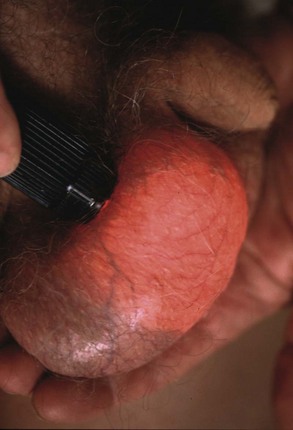
Lumps in the epididymis (cysts, chronic epididymitis or, rarely, tuberculous granulomata) are discrete from, but attached to, an otherwise normal testis. Tiny focal lumps in the epididymis are rarely clinically important. Infective lesions cause diffuse and usually painful thickening of the epididymis, whereas epididymal cysts are almost always located at the upper pole. Epididymal cysts are filled with clear fluid and therefore transilluminate. Transillumination (Fig. 33.1) is demonstrated by shining a strong beam of light through the scrotum in a partly darkened room. If the lesion is fluid-filled, it will glow (except in the case of blood). About 10% of cysts in the epididymis, and most in the cord, are filled with an opalescent fluid containing spermatozoa (spermatocoeles) which can also transilluminate.
Scrotal pain
Acute pain (Box 33.1)
In acute scrotal pain, testicular torsion must be excluded since the torted testis can be saved if operation is performed promptly; an exploratory operation is mandatory if torsion cannot be confidently excluded. Torsion occurs mainly in adolescents but occasionally in young adults. Recurrent, incomplete torsion may cause transient episodes of severe pain or poorly defined lower abdominal pain. In these cases, the anatomical relationship of the testis to the tunica vaginalis is often abnormal so the testes lie horizontally rather than vertically when standing. These ‘bell-clapper’ testes are susceptible to torsion. The main differential diagnosis at all ages is acute epididymitis. Torsion of an epididymal appendage (hydatid of Morgagni) produces symptoms similar to testicular torsion in children but less severe; surgical exploration is usually still required to exclude it. A traumatic haematocoele also causes acute pain but the trauma or surgery that preceded it points to the likely diagnosis.
Chronic pain
Chronic scrotal pain is most often due to inflammation. It can often be traced to a vasectomy, although the cause is usually obscure and treatment often ineffective. Patients present weeks or months after the operation, complaining of localised tenderness at the operation site or a general ache in one side of the scrotum. If there is a small tender lump due to a stitch granuloma, this is usually cured by excision. Sperm leakage (sperm granuloma) following vasectomy may also cause chronic pain, Pain is a feature of chronic bacterial epididymitis, which usually follows an acute episode.
Inflammation of the epididymis and testis
Bacterial epididymitis is the most common inflammatory disorder of scrotal contents. It is usually secondary to urethral infection that has ascended via the vas deferens. The source is a urinary tract infection with coliforms (in the 50–65 age group) or a sexually transmitted infection with Chlamydia or Neisseria gonorrhoeae (common in the 15–30 age group). Epididymitis is often incorrectly called orchitis or epididymo-orchitis. The testis is rarely infected, although the inflammation surrounding epididymitis may cause testicular tenderness.
In epididymitis, pain usually begins acutely. It may present as a surgical emergency and be clinically indistinguishable from testicular torsion. On examination, the affected side of the scrotum and its contents are swollen, oedematous and tender, and the scrotal skin can be red and warm. It may be difficult to palpate testis and epididymis separately once infection is established. In a boy under 15, epididymitis must never be diagnosed in the absence of urinary symptoms, a proven urinary infection or urethritis. Such an ‘acute scrotum’ must be explored to exclude torsion (see p. 428).
Treatment of acute epididymitis is initially with bed rest for pain relief and at least a month of an appropriate broad-spectrum antibiotic. The infecting organism is often not identified but attempts should be made to do so using urine cultures, blood cultures or culture of urethral discharge after prostatic massage. Ofloxacin is often favoured on a ‘best-guess’ basis as it covers Chlamydia and Gram-negative organisms. Persistent or chronic epididymitis may cause the patient to suffer chronic scrotal tenderness. Chronic epididymitis may also result from inadequate antibiotic treatment of an acute episode.
Tuberculous epididymitis: Tuberculosis may involve the epididymis via bloodstream spread from a pulmonary or other focus. A tuberculous urinary tract infection can spread to the epididymis, with swelling as the presenting complaint. Typically, the whole length of the epididymis is thickened, non-tender and ‘cold’. In contrast to bacterial epididymitis, the epididymis can be readily distinguished from the testis on palpation. If untreated, the testis may also become involved.
Diagnosis requires analysis of serial early morning urine specimens (EMUs) for mycobacteria or, more reliably, histological examination of percutaneous needle biopsies. If tuberculosis is confirmed, a search must be made for pulmonary and urinary tract disease (see Ch. 38).
Orchitis
Primary bacterial orchitis is rare and may result from pyogenic infection in the genital tract or elsewhere. Tertiary gummatous syphilis may involve the testis, producing diffuse non-tender enlargement. This is now rare and there is usually a history of primary and secondary lesions. Sometimes a gumma is found unexpectedly during investigation of a suspected testicular tumour.
Viral orchitis is most often caused by mumps. In post-pubertal males, bilateral mumps orchitis produces infertility in 50%; elevated follicle-stimulating hormone (FSH) blood levels following orchitis usually indicate the patient is infertile. Mumps orchitis manifests 4–6 days after the onset of parotitis with extreme testicular tenderness and an inflammatory hydrocoele. Treatment is directed at symptomatic relief. Mumps has reappeared since the spurious MMR vaccine scare. Other viruses affecting the testis include Coxsackie, human immunodeficiency virus (HIV), Epstein–Barr, varicella and, in earlier times, smallpox.
Hydrocoele
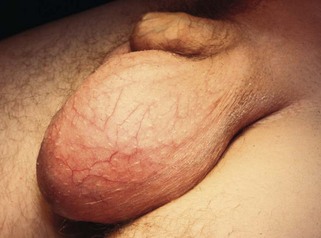
A hydrocoele is an excessive collection of fluid within the tunica vaginalis, i.e. in the serous space surrounding the testis. Like the peritoneal cavity, the tunica normally contains a little serous fluid which is produced and reabsorbed at the same rate (Fig. 33.2).
In infants and children, a hydrocoele is usually an expression of a patent processus vaginalis (PPV). In some, the scrotal swelling disappears overnight, and is known as a communicating hydrocoele. Provided there is no hernia, hydrocoeles below the age of 1 year usually resolve spontaneously. For older children, ligation of the PPV is required.
Primary hydrocoeles may develop in adulthood, particularly in the elderly, by slow accumulation of serous fluid, presumably by impaired reabsorption. These can reach a huge size, containing several hundred millilitres of fluid but the lesions are otherwise asymptomatic. The swelling is soft and non-tender and the testis cannot usually be palpated. The presence of fluid is confirmed by transillumination.
Note that a secondary hydrocoele may develop in response to testicular tumour or inflammation. In most, the hydrocoele is small and the testis can easily be palpated to reveal the primary abnormality.
Management: For symptomatic patients, a hydrocoele operation can be performed by everting the sac and oversewing the edges (Jaboulay procedure) or plicating the sac (Lord's method). If the sac is thick, it is best excised. Alternatives include observation alone or periodic aspiration (rarely performed) if the patient is unsuitable for surgery. If a testicular tumour is a possibility, a hydrocoele must not be aspirated as malignant cells can be disseminated via scrotal skin to its lymphatic field.
Hydrocoele of the cord
Rarely, a hydrocoele develops in a remnant of the processus vaginalis somewhere along the course of the spermatic cord. This hydrocoele also transilluminates, and is known as an encysted hydrocoele of the cord (see Fig. 33.3). In females, a multicystic hydrocoele of the canal of Nuck sometimes presents as a swelling in the groin. It probably results from cystic degeneration of the round ligament.
Fournier's scrotal gangrene
Fournier's scrotal gangrene is a form of necrotising fasciitis of genitalia and perineum and does not involve the testes. There is often consequent systemic sepsis. The underlying causes are varied and include pre-existing primary hydrocoele, genitourinary trauma, either accidental or iatrogenic, perirectal abscess and urethral stricture. Predisposing factors include diabetes mellitus, corticosteroids, chemotherapy and alcohol abuse. The infecting organism is principally an anaerobe but there is often synergistic aerobic infection. Treatment is urgent and includes intravenous antibiotics and surgical excision of all necrotic tissue, with the wound left open to heal by secondary intention.
Epididymal cyst and spermatocoele
Multiple cysts can develop in the upper pole of the epididymis and present as a painless scrotal swelling (see Fig. 33.3). Epididymal cysts affect a slightly younger age group than hydrocoeles. The testis can be palpated separately from the cysts, which transilluminate.
Less common is a spermatocoele, a single cyst containing spermatozoa. Spermatocoeles usually occur in the head of the epididymis and may present like a third testis. They are clinically similar to epididymal cysts but may or may not transilluminate. Occasionally they occur in the spermatic cord and surgical excision may cause obstruction to passage of sperm. If a patient wishes to remain fertile and the cysts are bilateral, excision may be contraindicated.
Varicocoele
A varicocoele (see Figs 33.3 and 33.4) represents dilatation and tortuosity of the pampiniform plexus of the spermatic vein in the cord. The condition is much more common on the left (90%), so it may result from the different venous drainage of the two sides: on the left, the testicular vein drains into the high-pressure renal vein, whereas the right testicular vein drains directly into the inferior vena cava.
Varicocoele is common, affecting about 10% of young adult males. It is usually asymptomatic but is often discovered during examination for infertility. Varicocoele increases scrotal temperature, which may inhibit sperm function and cause possible loss of testicular volume. When lying flat, the distended veins often collapse and become impalpable. Varicocoele is best diagnosed if the patient is examined standing, when the varicocoele feels like ‘a bag of worms’.
Rarely, a varicocoele may be caused by an invading renal cell carcinoma obstructing the left renal vein. Such varicocoeles do not collapse when the patient lies flat.
In adults, surgical treatment of varicocoele is only indicated for relief of pain or treatment of low sperm count (oligospermia). In the child or adolescent, treatment may be advised to preserve spermatogenesis. The treatment of choice is percutaneous embolisation. Alternatively, surgical ligation can be performed laparoscopically or via an open approach.
Testicular tumours
Testicular tumours are relatively uncommon, making up about 1.5% of male cancers, but they are the most common cancer in men in their third and fourth decades. They are important because curative treatment is now available for most of them. Testicular lymphatic drainage and the route of lymphatic metastases is towards intra-abdominal nodes and is determined by the embryology of testicular descent (see Fig. 33.5). Note that this is different from scrotal skin, which drains towards inguinal nodes.
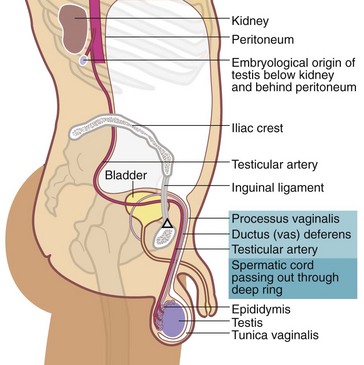
Fig. 33.5 Embryological descent of the testis
The testicular artery marks the line of descent of testis towards the scrotum. In the embryo, the arterial supply is direct from the aorta and this persists even when the testis has reached the scrotum
There is roughly one new case of testicular malignancy in 20 000 males per annum, and half have already metastasised by the time of presentation. Undescended testes are at least 30 times more likely to turn malignant, although the individual risk is still low. These tumours are usually seminomas.
More than 90% of primary testicular tumours are derived from germ cells, the rest being classified by the World Health Organization as sex cord/gonadal stromal tumours. A detailed classification of malignant testicular tumours is shown in Box 33.2 and the WHO histopathological classification is shown in Box 33.3. Germ cell testicular tumours are generally fast-growing, aggressive tumours that metastasise to intra-abdominal lymph nodes and later via the bloodstream to the lungs.
Non-germ cell Leydig cell tumours, derived from the gonadal stroma, often present with excess hormone secretion rather than a testicular lump. This causes precocious puberty in a child and testicular feminisation in an adult.
Testes may be secondarily involved in more widespread malignancy such as lymphoma, chronic lymphocytic leukaemia or, in children, acute lymphoblastic leukaemia. These rarely present as lumps but the surgeon may be asked to perform testicular biopsy as part of monitoring.
Pathology of testicular tumours
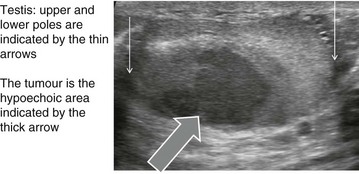
Seminomas: More than half of malignant testicular tumours are seminomas, derived from spermatocytes (Fig. 33.6). They occur predominantly between the ages of 20 and 45 with a peak incidence at 35 years. The cut surface of a seminoma is typically pale, creamy-white and homogeneous. Histologically, tumour cells are uniform and tightly packed. A distinct but rare form of seminoma, spermatocytic seminoma, occurs between the ages of 50 and 70 years and almost never metastasises.
Teratomas: Teratomas are slightly less common than seminomas and their peak incidence is a decade earlier. Since they are derived from multipotent cells, teratomas may contain tissue from all germ cell layers: ectoderm, mesoderm and endoderm. Teratomas exhibit a wide range of differentiation. Differentiated and intermediate tumours contain a collection of tissues resembling mature adult tissues: in particular, squamous epithelium (ectodermal), cartilage and smooth muscle (mesodermal), and respiratory epithelium (endodermal). Consequently, the cut surface often appears variegated, with cystic areas and patches of necrosis and haemorrhage; this is easily distinguished from seminoma with the naked eye.
Clinical features of testicular tumours
A malignant testicular tumour usually presents as a painless, progressively enlarging testicular lump. If the testicular capsule becomes involved, a secondary hydrocoele may appear but this is usually small and does not hinder palpation.
Seminoma and teratoma both spread via lymphatics to para-aortic nodes at about the level of L1/2. Spread is then proximally along the lymphatic chain then thoracic duct to supraclavicular nodes and the systemic circulation. Lung secondaries are particularly common in teratomas. Poorly differentiated testicular tumours metastasise early and may present as enlarged abdominal or cervical lymph nodes, or with symptoms of lung metastases. The primary testicular lesion may be very small or even impalpable.
A solid testicular lump must be assumed to be a tumour until proven otherwise. The history is rarely helpful but may include an episode of trauma which merely drew attention to the lump. On examination, the testis is either diffusely enlarged or contains a discrete lump which is firm and non-tender. Systemic examination may reveal evidence of metastases. Malignant cervical nodes may be palpable but inguinal nodes are not involved unless the tumour has spread to scrotal skin, which is rare except when biopsy or orchidectomy has been performed through a scrotal incision. Orchidectomy for suspected tumour should always be carried out via an inguinal approach.
Investigation and treatment of testicular tumours
The outlook for treated testicular tumours, even with metastases, is often good. Cure rates have improved dramatically over the past 35 years with the evolution of scientifically based treatment protocols and availability of CT scanning and tumour markers for staging and monitoring the disease. Investigation and treatment usually take place in parallel. The aims are to confirm the diagnosis, detect any metastases and stage the disease, then to treat according to the stage.
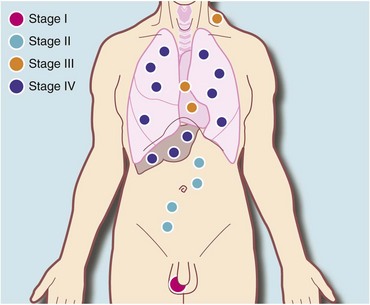
The first investigation is scrotal ultrasonography. If this confirms a solid testicular mass, surgical exploration is required. Preliminary staging investigations are usually performed next, including chest X-ray or CT (to look for hilar node involvement and lung secondaries), CT abdomen and pelvis to assess retroperitoneal nodes and blood levels of tumour markers (which must be measured before treatment). A standard method of staging testicular tumours is shown in Figure 33.7 and Box 33.4. Tumour markers contribute to diagnosis and staging and are useful for tracking residual or recurrent metastases because blood levels correlate with tumour bulk. Serial postoperative measurements help monitor disease progress and the impact of therapy.
Tumour markers: Human chorionic gonadotrophin (beta-hCG) is secreted by syncytiotrophoblastic cells and levels may rise in any tumour type, particularly poorly differentiated germ cell tumours. Alpha-fetoprotein (AFP) is produced by yolk sac elements. About 75% of patients with metastatic teratoma have elevated AFP levels but this marker is not expressed in seminoma. Lactic dehydrogenase (LDH) is elevated in more than half the patients with metastatic seminoma.
Surgical exploration: Orchidectomy is the only appropriate treatment for the primary tumour and is usually performed as part of the diagnostic process. The surgical approach is via an inguinal incision to avoid involving scrotal skin. The spermatic cord is temporarily clamped to prevent venous spread of tumour cells and the testis is brought out for inspection and palpation. If the testis is obviously malignant, orchidectomy is performed, dividing the cord at the internal inguinal ring. If there is diagnostic doubt, a testicular biopsy is taken and examined immediately by frozen section before proceeding. The other testis is usually unaffected and can be preserved. Further treatment is planned according to tumour type and stage.
Management of seminoma: For stage 1 seminoma, i.e. disease confined to the testis, some oncologists advise no further treatment after orchidectomy, although carboplatin-based chemotherapy is occasionally recommended. Seminoma is very radiosensitive and radiotherapy can also play a role for the primary, although it is not usually recommended by the European Association of Urologists in view of the high cure rate from standard treatment. For stages IIa and b (i.e. abdominal lymphadenopathy up to 5 cm diameter), radical radiotherapy to the ipsilateral (same side) para-aortic and iliac nodes gives a cure rate of about 95%. Oligospermia of the contralateral testis may occur even if it is lead-shielded but is usually transient. In stage IIb, chemotherapy is an alternative to radiotherapy using etoposide and cisplatin (EP), or cisplatin, eposide, bleomycin (PEB).
Management of teratomas and other non-seminomatous germ cell tumours: Up to 25% of patients with stage I disease would relapse within a year of orchidectomy without further treatment. Radiotherapy has no curative role in these types of tumour. There are three options for further treatment for stage I disease:
In the USA, lymph node dissection is often employed and provides a good cure rate but risks ejaculatory failure from autonomic nerve damage. In the UK, meticulous surveillance is the preferred option. Chemotherapy for relapse is virtually always successful and thus the 75% of patients that do not relapse are spared additional treatment.
Chemotherapy is indicated for all patients with known metastatic disease, including those in whom the only evidence of metastatic disease is elevated tumour markers. Results of chemotherapy for metastatic disease were transformed in the 1970s by the use of cisplatin. The Einhorn regimen, originating from Indiana, combined this with vinblastine and bleomycin and gave even better cure rates of 70%. Later, etoposide replaced vinblastine (bleomycin, etoposide, cisplatin—‘BEP’) increasing cure rates to 85%. Three-quarters of patients with teratomas and other non-seminomatous germ cell tumours have low-volume disease, and in these, cure can be expected in 95%.
Long-term surveillance: After chemotherapy, surgical debulking (‘salvage’) of residual lymph node masses is occasionally indicated. In the long term, tumour markers and sequential CT scans are used to monitor the success of treatment. Recurrent disease can nearly always be successfully treated by radiotherapy, chemotherapy or surgery.
Fertility: Many patients with testicular tumours are already subfertile at presentation, and chemotherapy is likely to further adversely affect fertility. Patients need to be counselled carefully and, if required, semen can be collected and stored before treatment so that artificial insemination or in vitro fertilisation may be performed later. However, the success rate is poor.
Treatment of testicular tumours is summarised in Box 33.5.
Absent scrotal testis (cryptorchidism)
Failure of testicular descent or cryptorchidism occurs in 3–4% of male infants at birth and this falls to about 1% by 12 months. It can be classified as:
• Retractile—intermittent active cremasteric reflex draws the testis out of scrotum. It can be gently ‘milked’ back into the scrotum
• Incomplete descent—testis lies along the normal line of descent; intra-abdominal, inguinal or pre-scrotal (Fig. 33.5)
• Ectopic—abnormal line of testicular descent outside the external ring; testis may be palpable in the perineum, femoral region or base of penis
• Atrophic—secondary to trauma, iatrogenic (hernia operation or scrotal surgery) or androgen deprivation as part of prostate cancer treatment. Occasionally, an adult testis may become displaced upwards towards the inguinal canal following trauma or hernia surgery; this is known as a trapped testis. The testis is normal sized but is fixed in position by adhesions. If trauma was the cause and there were other major injuries, the scrotal injury may have been overlooked
• Absent—antenatal intra-abdominal torsion, orchidectomy after delayed diagnosis of torsion or bilateral orchidectomy for treatment of prostate cancer
Management of maldescent of the testis
In developed countries, maldescent is usually identified at screening during early childhood and surgically corrected by orchidopexy at a young age (see Ch. 51); to best preserve spermatogenesis, the testis should be surgically placed in the scrotum between 6 months and 1 year. There is some evidence that orchidopexy before the age of 10 decreases the risk of testicular cancer.
Torsion of the testis or epididymal appendage
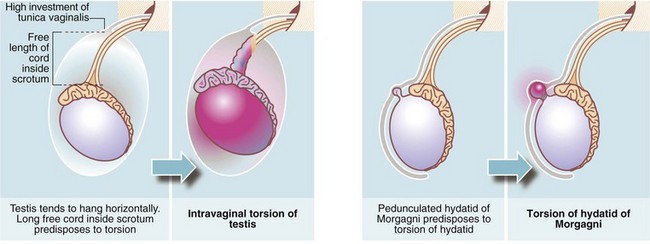
Testicular torsion (Figs. 33.8 and 33.9)
In infants, the newly descended testis and its investing tunica vaginalis are mobile within the scrotum. These testes may undergo extravaginal torsion which presents as a hard, swollen testis. Later in childhood, the testis becomes suspended in a near vertical position, anchored by the spermatic cord and by attachments to the posterior scrotal wall. This attachment prevents rotation. Minor anatomical variations can produce a narrow-based pedicle with a horizontal (‘bell-clapper’) testicular lie, that allows the testis to twist about its axis within the tunica (intravaginal torsion). When this occurs, pampiniform plexus veins become compressed causing venous congestion. After a few hours, venous infarction occurs unless torsion is corrected. Trauma during sport may sometimes initiate the process, and there have been reports of successful manual untwisting on the sports field. In general, however, torsion is an emergency requiring prompt diagnosis and urgent surgical treatment to save the testis.
Torsion presents with a sudden onset of severe testicular pain often with poorly localised central abdominal pain (because the testis retains its embryological nerve supply) and sometimes vomiting. In the early stages, the affected testis is tender, slightly swollen and drawn up into the neck of the scrotum where the cord may be palpably thickened. With these features, the diagnosis is seldom in doubt. At a later stage, the scrotal skin becomes red and oedematous, making accurate palpation difficult. At this point, torsion may be difficult to distinguish from acute epididymitis and the scrotum must be explored surgically.
Torsion of the epididymal appendage (hydatid of morgagni)
The hydatid of Morgagni is a small embryological remnant at the upper pole of the testis (see Fig. 33.9). This may undergo torsion and produce symptoms similar to testicular torsion, out of proportion to the small size of the infarcted tissue. Infarction of the hydatid is of no consequence except that it must be distinguished from testicular torsion.
Management of suspected testicular torsion
Differentiating between acute epididymitis and torsion can be difficult. If a firm diagnosis cannot be reached, surgical exploration is mandatory. Investigations are of little value: radionuclide studies and Doppler ultrasound examination may be employed to show testicular blood flow but results can be misleading.
Urgent operation is usually imperative as delay leads to testicular necrosis after about 8 hours. A scrotal incision is made and the testis is examined and untwisted. If the testis is black and fails to recover its colour, it is necrotic and should be removed to prevent it inducing a sympathetic contralateral orchiopathy. If some colour is restored, the testis is best left, although it may later atrophy. After untwisting, the testis is sutured to the tunica vaginalis or placed in a surgically created dartos pouch to prevent recurrence. Both testes should be secured as predisposition to torsion is usually bilateral.
Trauma to the testis
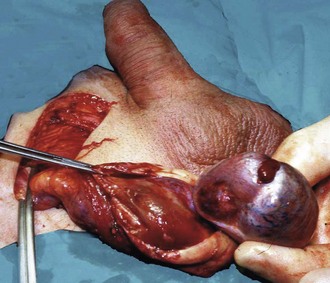
The testes may be injured during contact sports or fights. The dense fibrous capsule investing the testis (tunica albuginea) may remain intact or it may split, but the testis is extremely painful in either case. If the tunica remains intact, a testicular haematoma results; if it splits, the testicular parenchyma bursts and bleeds into the tunica vaginalis cavity, resulting in a haematocoele.
If pain is severe and persistent, the scrotum can be explored surgically. Pain from a haematoma can be relieved by incising the tunica albuginea. Evacuating a haematocoele, however, may be impossible because blood infiltrates the tissues. If a haematocoele can be evacuated, the tunica albuginea rupture is best repaired.
Male sterilisation
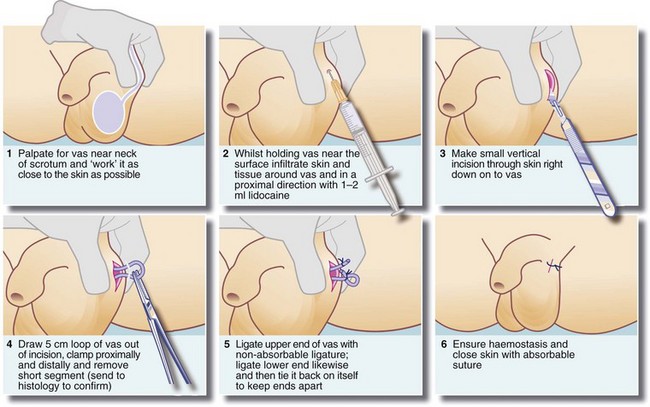
Male sterilisation by vasectomy is a simple, effective method of birth control. It can be performed under local anaesthesia at little cost and requires no special equipment. The essential prerequisite is that the couple involved should have completed their family, since reversal is technically difficult and unreliable. A technique of vasectomy is illustrated in Figure 33.10.
Most procedures involve removing a section of the vas (ductus) deferens and ligating or cauterising the cut ends. For medicolegal reasons, the nature of the excised portion can be confirmed histologically. Spermatozoa remain in the proximal duct system for several months after vasectomy, so the operation cannot be considered a success until at least two successive sperm counts are negative. These need to be performed about 1 month apart, after 20–25 ejaculations. Despite correct operative technique and negative sperm counts, there is still a late failure rate of about 1 case in every 500. Failure suspected of resulting from spontaneous vas reconnection can be proved by a positive sperm count. This is because of the problem of determining paternity in the case of unexpected pregnancy!
Early complications of vasectomy include postoperative scrotal haematoma (usually operator error) and wound infection. Later, failure of sterilisation may become apparent, or a sperm granuloma may present as a tender scrotal swelling near the cut end of the vas for which further excision is usually required. Chronic debilitating pain can occur in the testis and the patient must be warned of this possibility at the time of consent. The patient must also be warned of the possibility of spontaneous reversal.
Disorders of the penis
Problems with the foreskin (prepuce) are common and form most penile surgical disorders. Other disorders are uncommon in adults but the most serious is carcinoma of the penis. In children, penile disorders are either developmental or minor inflammatory conditions, discussed in Chapter 51. Disorders of the foreskin include balano-posthitis (inflammation of the glans and foreskin), phimosis (stricture of the preputial meatus), paraphimosis (acute constriction of the glans by a tight retracted foreskin) and balanitis xerotica obliterans (idiopathic sclerosis of the foreskin). Peyronie's disease (idiopathic fibrosis of the corpora cavernosa) is now a frequent presentation in urological clinics (see below). Exclusion of cancer is usually the first step.
Foreskin problems in adults
A common complaint is that the foreskin will not fully retract, causing pain on intercourse. Such phimosis is usually caused by fibrosis of the foreskin and may be due to chronic or recurrent low-grade Candida infection. Phimosis is aggravated by attempts at retraction which cause minor tears of the inner epithelial lining, inducing further inflammation and fibrosis. The condition may be accompanied by stenosis of the urethral meatus, also due to recurrent inflammation and fibrosis. Treatment usually involves circumcision but a preputioplasty or reshaping operation is sometimes successful.
Balano-posthitis (balanitis)
The term balano-posthitis refers to overt inflammation of the glans penis and foreskin (Greek: balanos gland, posthe foreskin); it is the correct term but the short form ‘balanitis’ has passed into common usage. The condition occurs most commonly in children. Inflammation alone is most often caused by Candida or faecal bacteria, but this problem rarely reaches the surgeon.
Balanitis xerotica obliterans is a fibrotic condition of the foreskin of unknown aetiology and is probably analogous to lichen sclerosus of the vulva in females. It produces a thickened, stenosed, often depigmented foreskin which is often adherent to the glans. Circumcision is usually curative. As many as 25% of children with phimosis have this condition. The process can involve the urethral meatus and may cause stenosis, sometimes requiring meatotomy or meatoplasty at the same time. More rarely it results in a urethral stricture likely to require urethroplasty.
Paraphimosis
If a phimotic foreskin is forcibly retracted, the tight meatal band may lodge in the coronal sulcus making reduction impossible. This is known as paraphimosis. Progressive oedema of the glans penis and foreskin then exacerbates the difficulty of reduction. It may occur at any age, but is particularly common in elderly men in whom the foreskin is not correctly pulled forwards after retraction for catheterisation or washing the glans (nurses and junior doctors are the main culprits). Paraphimosis also occurs in children and adolescents experimenting with foreskin retraction or cleaning beneath it. In most cases, the foreskin can be reduced by firm manual compression of the glans and foreskin. Local anaesthetic jelly is applied first for lubrication and pain relief. Sometimes, incising the tight ring may be necessary under local or general anaesthesia to effect reduction. Preputioplasty, in which the band is incised longitudinally and the skin sutured transversely, can often be performed at the same time to solve the problem in the long term. If not, circumcision or preputioplasty is usually performed at a later date when the oedema and inflammation have resolved.
Circumcision
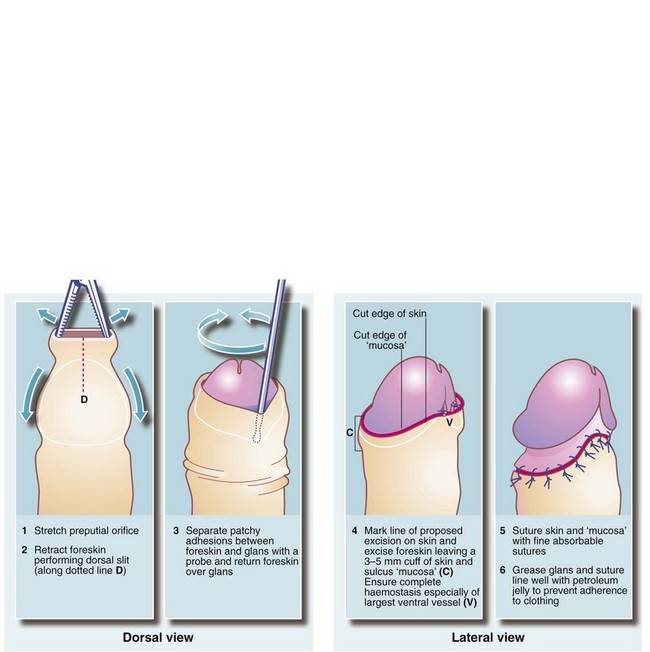
Circumcision is or has been a widespread practice in many communities for religious or cultural reasons. From a strictly scientific or medical standpoint, however, it is difficult to support as a general health measure, although there is evidence that circumcision can reduce the risk of recurrent urinary tract infections, and definitely reduces the risk of transferring HIV. It also reduces the frequency of carcinoma of the penis. Routine circumcision for recurrent balanitis, phimosis and even paraphimosis in children has largely gone out of favour as most cases respond to local treatments or to preputioplasty. Misguided circumcision in babies may predispose to ammoniacal nappy rash and meatal stenosis.
Circumcision should be reserved for unresolved phimosis, recurrent balanitis and sclerosis from balanitis xerotica obliterans. A surgical technique is shown in Figure 33.11. During the operation, the urethral meatus should be checked for stenosis. If present, a meatotomy may be required. An occasional early postoperative complication is haemorrhage which usually requires surgical re-exploration. Postoperative bleeding can best be prevented by meticulous haemostasis at operation.
Peyronie's disease
This disease of unknown aetiology occurs in young to middle-aged adults. Some cases are thought to result from penile trauma during sexual activity and others are associated with Dupuytren's contracture of the palmar fascia. Slowly progressive asymmetrical fibrotic plaques develop in the fascia surrounding the corpora cavernosa. The corpus spongiosum, including the glans, is spared. The plaques may become calcified and visible on X-ray. The condition causes the penis to bend towards the affected side on erection, making intercourse difficult and painful. There may be spontaneous partial resolution with time.
Severe cases require surgery. Nesbit's operation involves creating pleats in the corpus on the contralateral side. Another approach involves excision of the plaques, which are replaced by a patch of tunica vaginalis. Either procedure may restore symmetrical erection, although Nesbit's procedure results in a shorter penis. Penile prosthetic implants may be required if erection is inadequate for satisfactory sexual intercourse. ‘Medical treatments’ of Peyronie's disease, i.e. steroid injections or radiation, are of no benefit.
Carcinoma of the penis
Carcinoma of the penis is rare in developed countries and almost unknown in circumcised males. Poor hygiene and accumulation of smegma are suspected aetiological factors but there is growing evidence of a viral aetiology linked to that of carcinoma of the uterine cervix in females (human papilloma virus).
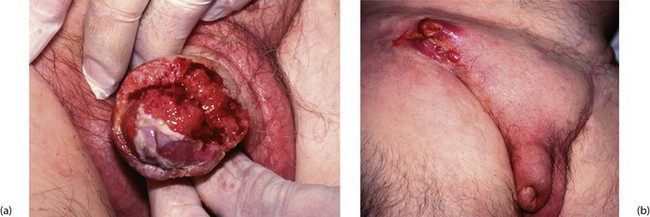
Histologically, the tumours are squamous cell carcinomas, usually well differentiated, which arise from the inner surface of the foreskin or the glans penis near the coronal sulcus. The tumour invades locally and tends to penetrate the distal urethra (Fig. 33.12a). Metastatic spread is to inguinal lymph nodes (see Fig. 33.12b). Erythroplasia of Queyrat is the term given to severe dysplasia and carcinoma-in-situ of the glans that may represent a precursor of invasive carcinoma.
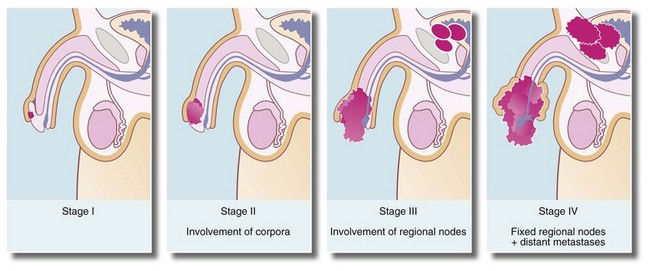
Most cases of carcinoma of the penis are found in the elderly. The disease is usually well advanced before an irregular lump, bleeding or discharge is noticed. In uncircumcised males, the lesion may be hidden by the foreskin. Figure 33.13 illustrates staging of the disease.
Local surgical excision or ‘glansectomy’ is often performed, with reconstructive surgery if feasible. In advanced cases, ‘penectomy’, and block dissection of inguinal lymph nodes if they are involved, may be required. Radiotherapy can be used in stage I disease if the urethra is not involved and for palliation in stage IV disease.
Priapism
Priapism is an abnormally prolonged penile erection lasting 4 hours or more. It is usually painful and can occur without sexual stimulation, and does not resolve after ejaculation. The abnormality affects only the corpora cavernosa and is caused by a disturbance of mechanisms that control penile detumescence. The corpus spongiosum surrounding the urethra and forming the glans penis is not affected and the glans remains flaccid.
There are two main types of priapism. High-flow (arterial) priapism occurs when an artery ruptures into the lacunar spaces of the corpora cavernosa. This is often associated with trauma and is relatively uncommon and painless. Compared with venous priapism, there is less tumescence and it is less painful. Low-flow (venous) priapism is due to prolonged corporeal venous stasis caused by occlusion of the venous outlet mechanism (veno-occlusive priapism). This eventually results in penile ischaemia. It is very painful and, unless treated, results in long-term fibrosis and impotence (loss of the ability to achieve an erection). In veno-occlusive priapism, tissue changes that will lead to fibrosis are already evident within 24 hours, whereas there is no such change in arterial priapism.
The most common cause of priapism is a side-effect of injected intracavernosal drugs used to treat erectile dysfunction.
Treatment of veno-occlusive priapism must be prompt to avoid long-term erectile problems. Ice packs and external compression are a useful temporising measure but usually will not give long-term relief. Aspiration of blood from the cavernosa using a butterfly needle is the next step. Intracavernous injection of an alpha-adrenergic agonist (phenylephrine) or oral terbutaline, a beta-2-adrenergic receptor agonist, usually produces detumescence. If these measures are unsuccessful, operative management by means of a surgical shunt may be necessary but results are sometimes disappointing.