Immune system
Introduction
All living tissues are subject to the constant threat of invasion by disease-producing foreign agents and microorganisms (pathogens) i.e. bacteria, viruses, fungi, protozoa and multicellular parasites such as worms. These organisms may invade the body, multiply and destroy functional tissue, causing illness and potentially death. Three main lines of defense have consequently evolved:
Protective surface mechanisms
These provide the first line of defense and, while intact, provide excellent protection from many disease-causing organisms. However, pathogens may enter the body via breaches in the skin or mucosal linings of the gut, respiratory and genitourinary tracts. The skin, with its surface layer of keratin, constitutes an impenetrable barrier to most microorganisms, unless breached by injury such as abrasion or burning. The mucous surfaces of the body, such as the conjunctiva and oral cavity, are protected by a variety of antibacterial substances including defensins, short antimicrobial peptides that are found in surface mucus, and the enzyme lysozyme, which is secreted in tears and saliva. The respiratory tract is protected by a layer of surface mucus that is continuously removed by ciliary action and replaced by goblet cells. Maintenance of an acidic environment in the stomach, vagina and, to a lesser extent, the skin, inhibits the growth of pathogens in these sites. When such defenses fail and an infection takes hold, the two other main types of defense mechanism are activated.
The innate immune system
The innate immune response provides a rapid reaction to infections and, characteristically, the same magnitude of response each time the same pathogen is encountered (i.e. there is no learning in the innate system). The cells, proteins and peptides involved circulate in the blood of healthy individuals in sufficient amounts to overcome many trivial infections and contain more serious infections until an adaptive immune response can develop. The cellular components include neutrophils, eosinophils, basophils and macrophages, as well as tissue resident cells such as histiocytes and mast cells. The proteins and peptides of the innate response include complement, acute-phase proteins, chemokines and interleukins. The major functions of the most important components of the innate immune system are outlined in Table 11.1. The innate immune response causes a pathological condition known as inflammation, familiar to anyone who has ever had a cut finger. Acute inflammation is characterised by vascular changes including dilatation, enhanced permeability of capillaries and increased blood flow, resulting in the production of a fibrin-rich inflammatory exudate, thus bringing the proteins and cells required for early defence to the site of infection. Many of the cells and signalling molecules of the innate immune system are vital to the functioning of the adaptive immune system.
TABLE 11.1
Major components of innate immunity
| Component | Actions |
| Neutrophil polymorphs | Phagocytosis and killing of pathogenic organisms Secretion of cytokines and extracellular antimicrobial molecules, including neutrophil extracellular traps (NETs) and pattern recognition molecules (PRMs) |
| Macrophages | Phagocytosis and killing of pathogenic organisms, removal of foreign material and dead cells Secretion of a wide range of cytokines and interleukins Present antigen to lymphocytes (adaptive immune system) |
| Eosinophils | Destroy larger multicellular pathogens Modulate allergic responses |
| Natural killer (NK) cells | Recognise and kill virus-infected and cancerous cells |
| Complement | Opsonises organisms to facilitate phagocytosis Chemoattractant for various cells Membrane attack complex (MAC) kills cells by puncturing plasma membrane |
| Acute-phase proteins | Plasma proteins which are increased during inflammation e.g. C-reactive protein (CRP) Wide range of actions that promote defense against pathogens |
| Chemokines | Recruit cells to specific sites and activate cells of innate and adaptive immune systems Induce differentiation of cells to more active and effective subtypes Wound healing and angiogenesis |
| Interleukins | Signalling molecules produced by many cell types, including macrophages, dendritic cells, lymphocytes Regulate the immune system |
The adaptive immune system
The adaptive immune system is characterised by the ability to learn, so that second and subsequent encounters with a pathogen elicit a greater, more specific and faster response. This is the basis of lifelong immunity to certain infections after an initial infection or vaccination. The adaptive system builds on and is intimately associated with the innate immune system. Adaptive immunity depends on cell division to produce large numbers of lymphocytes with specificity for a particular pathogen (or antigen) and thus takes 3 to 5 days to develop a significant response. Lymphocytes are able to kill or disable pathogens either by a cellular response (T lymphocytes or T cells) or a humoral response (B lymphocytes or B cells) or, commonly, a combination of both. Adaptive immunity amplifies some of the mechanisms of the innate response. For instance, antibody, produced by B cells, coats bacteria (opsonisation) to facilitate phagocytosis by neutrophils and also directly activates the complement cascade. The adaptive immune response is also controlled by the innate response, as T lymphocytes require the services of antigen presenting cells (APCs) such as macrophages and dendritic cells for activation.
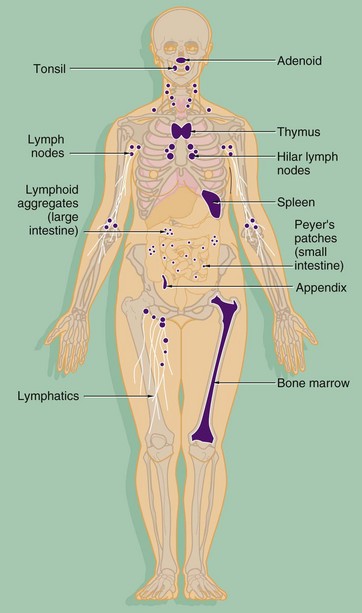
FIG. 11.1 The organs of the immune system
The components of both the innate and adaptive systems are found throughout the body. The lymphocytes of the adaptive immune system are produced in the bone marrow from haematopoietic stem cells along with the cells of the innate system (see Ch. 3). As well as circulating in the blood, the cells of the adaptive immune system form specialised lymphoid tissues and also constitute a significant component of other tissues such as the gastrointestinal tract. The major lymphoid organs include:
• The thymus, situated in the anterior mediastinum, is the site of maturation of immature T lymphocytes.
• The bone marrow is not only the home of lymphocyte stem cells but is also the site of B lymphocyte maturation.
• The lymph nodes, found at the junctions of major lymphatic vessels, are the sites where both T and B lymphocytes may interact with antigen and APCs from the circulating lymph, leading to lymphocyte activation and cell division.
• The spleen, situated in the left upper quadrant of the abdomen, is the location where T and B lymphocytes may interact with blood-borne antigen and undergo stimulation and cell division.
• Mucosa-associated lymphoid tissue (MALT) includes the tonsils and adenoids in the oropharynx, Peyer's patches and lymphoid aggregates of the small and large intestines, respectively, and a diffuse population of lymphocytes and plasma cells in the mucosae of the gastrointestinal, respiratory and genitourinary tracts. These specialised lymphoid tissues respond to antigens entering the body through these mucosae.
The thymus and bone marrow, where immature lymphocytes acquire the receptors to recognise antigen, are known as primary lymphoid organs. The spleen, lymph nodes and organised lymphoid tissues of MALT, where lymphocytes are activated in response to antigen, are the secondary lymphoid organs.
Lymphocytes
Lymphocytes comprise some 20% to 50% of white cells in the circulation. Most circulating lymphocytes measure 6 to 9 µm (i.e. about the same size as erythrocytes) and are called small lymphocytes. About 3% are large lymphocytes, measuring 9 to 20 µm. The light and electron microscopic features of lymphocytes are described in Fig. 3.17. Briefly, small lymphocytes have a round to ovoid nucleus occupying about 90% of the cell volume, with a thin rim of basophilic (bluish) cytoplasm.
Lymphocytes constantly patrol the body, circulating in the blood, lymph and other extracellular fluids and pausing in the organised lymphoid tissues. Secondary lymphoid organs are arranged to optimise the chances of an antigen meeting a potentially reactive lymphocyte and facilitating lymphocyte activation. If an antigen binds to a lymphocyte surface receptor, the lymphocyte will be activated and a specific response to that antigen is triggered. Obviously, the immune response must be tightly controlled so as to be active when there is a potentially serious infection, but not react against harmless components of everyday life such as food proteins or even against normal components of the body (autoimmunity).
The effectiveness of the adaptive immune system in recognising the huge range of pathogenic organisms found in nature depends upon the unique ability of lymphocytes to produce an equally huge range of antigen receptors i.e. the B cell receptor (BCR), comprising surface immunoglobulin (sIg) plus accessory molecules for B cells and the T cell receptor (TCR) for T cells. The ability of antibody to bind to antigen is determined by the physico-chemical properties of the antibody. Put simply, the shape and electrical charge of the binding site of the antibody must be complementary to the antigen, and the closer the fit of binding site to antigen, the stronger the bond formed and the greater the likelihood of the lymphocyte being stimulated. The TCR binds to antigen by similar reciprocity of shape and charge but it must also bind to the major histocompatibility complex (MHC) (see Figs 11.2 and 11.3). During maturation of lymphocytes, alternate components of the antigen-binding part of the antigen receptor genes are spliced together (rearranged) in a random fashion. Thus a huge range of possible antigen specificities are generated before the lymphocytes have a chance to meet external antigen.
The role of T lymphocytes
T cells have a number of effector and regulatory functions. Immature T lymphocytes migrate from the bone marrow to the thymus where they develop into mature T lymphocytes. The process of maturation includes proliferation, rearrangement of TCR genes, and acquisition of the surface receptors and accessory molecules of the mature T cell. At this stage, T cells with the ability to react with ‘self-antigens’ (normal body components) are removed by apoptosis, creating a state of self-tolerance. Mature T cells then populate the secondary lymphoid organs and, from there, continuously recirculate via the bloodstream in the quest for antigen.
T lymphocytes may develop into one of the functional subsets detailed below. These subsets develop from naïve T cells, depending on the mixture of cytokines and interleukins to which they are exposed, and can be identified in the laboratory by means of their surface receptors and accessory molecules.
The best known subsets of T cells include:
• T helper cells (TH cells). These T lymphocytes ‘help’ other cells to perform their effector functions by secreting a variety of mediators known as interleukins. TH cells thus provide ‘help’ to B cells, cytotoxic T cells (see below) and macrophages. TH cells can be subdivided into subgroups with different functions. TH1 cells tend to promote a cell-mediated reaction, important for defence against viruses and intracellular pathogens. TH2 cells are important for humoral (antibody mediated) responses and TH17 modify and augment certain types of acute inflammation. TH cells express the surface markers CD2, CD3 and CD4.
• Cytotoxic T cells (TC cells). These lymphocytes are able to kill virus-infected and some cancer cells. They require interaction with TH cells to become activated and proliferate to form clones of effector cells. TC cells express CD2, CD3 and CD8.
• Regulatory T cells (TREG). These cells suppress immune responsiveness to self-antigens (autoimmunity) and switch off the response when antigen is removed. These cells usually express CD4 and FOXP3.
• Memory T cells develop from activated T cells to provide a ‘rapid reaction force’ for a subsequent encounter with the same antigen. This is the basis of persisting immunity after infection with some organisms and also the basis of vaccination.
• γδ T cells are a subset of T cells where the TCR is a heterodimer consisting of one γ chain and one δ chain, rather than the usual heterodimer of one α and one β chain. These cells populate the epithelium of the gastrointestinal tract and are CD8 positive.
The role of B lymphocytes
B lymphocytes are derived from precursors in the bone marrow and also mature there. Stimulated B cells mature into plasma cells that synthesise large amounts of antibody (immunoglobulin). Immunoglobulins fall into five different structural classes (IgG, IgA, IgD, IgM and IgE) and are secreted into and circulate in the blood. Immunoglobulin molecules are also anchored in the plasma membrane of B cells, with the antigen-binding region exposed to the external environment. This surface immunoglobulin is the antigen receptor for B lymphocytes (part of the BCR), and when it binds antigen the B cell is activated, generally with the ‘help’ of a TH cell responding to the same antigen.
Once activated, the B cell undergoes mitotic division to produce a clone of cells able to synthesise immunoglobulin of the same antigen specificity. Most of the B cells of such a clone mature into plasma cells. When an antigen is encountered for the first time, this is described as the primary immune response. A few cells from the same clone mature to become memory B cells, small long-lived circulating lymphocytes that are able to respond quickly to any subsequent challenge with the same antigen. Antibody production during this secondary immune response occurs much more rapidly, is of much greater magnitude and produces IgG rather than IgM. This phenomenon explains the lifetime immunity that follows many common infections; it is also the general principle on which vaccination is based. Antibodies neutralise or destroy invading organisms by a number of methods (see Fig. 11.2).
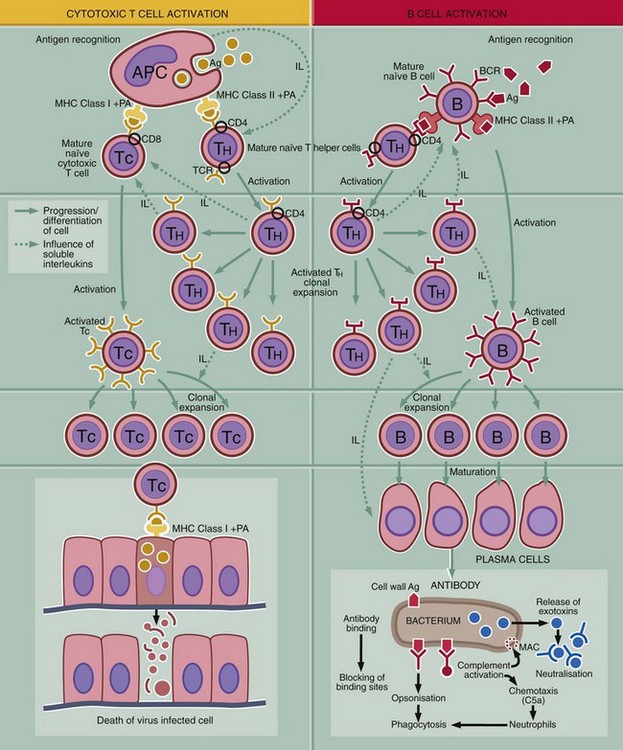
FIG. 11.2 The basics of the immune response (illustration opposite)
This diagram outlines the key steps in the adaptive immune response, i.e. recognition of antigen, activation of the response, generation of effector mechanism and destruction or inactivation of the antigen.
Recognition of antigen
T and B cells carry antigen receptors on their surface, the T cell receptor (TCR) and B cell receptor (BCR). The BRC consists of surface immunoglobulin plus certain accessory molecules. Random rearrangement of the genes for the variable region of the receptor molecules gives rise to receptors with a truly staggering range of antigen binding sites. Each individual T or B cell has specificity for only one antigen, but the entire population is very varied.
Activation of the immune system
Initiation of an immune response first requires contact between antigen Ag and surface receptors on mature lymphocytes. There are several mechanisms of activation:
1. Activation of T cells is dependent on antigen presenting cells APC. The antigen is taken up by an APC (e.g. macrophage, B lymphocyte, dendritic cell, Langerhans cell of skin) and broken down to short peptides (see Fig. 11.3). Processed antigen PA is then bound to a major histocompatibility complex molecule MHC, and the MHC-peptide complex is incorporated into the cell membrane so that the bound antigenic peptide is exposed to the extracellular fluid. Contact with a mature T cell bearing a T cell receptor with appropriate specificity activates the T cell. The type of response depends on whether the peptide is presented bound to MHC class I or II. Antigenic peptides bound to class II MHC molecules induce a T helper cell TH response needed to activate B cells B and cytotoxic T cells TC. B cell receptors (sIg) or TC receptor must also bind to the antigen for activation to occur. TH cells secrete a variety of interleukins IL that mediate activation, clonal expansion and maturation of the B or cytotoxic T cell response.
2. Antigen synthesised within a body cell (e.g. tumour cell, virus-infected cell) is presented on the APC plasma membrane bound to a class I MHC protein where it is recognised by cytotoxic T cells TC. Cytotoxic T cells are able to kill the abnormal cells directly. TH activation is also required for a TC response to be mounted.
3. B lymphocytes interact with unprocessed antigens. They recognise antigen by means of the BCR (surface immunoglobulin, sIg). In most cases, the unprocessed antigen is presented to the B cell on the surface of an APC such as a follicular dendritic cell in a lymphoid follicle. The majority of antigens can only activate a B cell if there is ‘help’ from an activated T helper cell TH. Activation without T cell help will occur if sIg binds to a protein or polysaccharide antigen with a repeating chemical structure (e.g. the polysaccharide coat of the bacterium Pneumococcus). Such antigens are often known as T cell–independent antigens. Few naturally occurring antigens are of this type (not illustrated).
Generation of effector mechanisms
1. Production of antibodies by plasma cells. Mechanisms of antibody-mediated antigen elimination are as follows:
• Antibody blocks the entry of organisms (such as viruses) into cells by binding to viral surface antigens.
• Antigen-antibody complexes (immune complexes) activate complement to produce (among other factors) the membrane attack complex MAC, which punctures the outer membrane of the attacking organism.
• Bound antibody with or without complement opsonises organisms and facilitates phagocytosis by neutrophils and macrophages.
• Antibody is essential for antibody-dependent cell cytotoxicity (ADCC) (see below).
• Antibody bound to toxins inactivates them and facilitates their removal by phagocytic cells.
2. Cell-mediated cytotoxicity is the destruction by apoptosis of abnormal cells by cytotoxic T cells, natural killer (NK) cells or antibody dependent cytotoxic cells.
3. Certain types of organism, such as Mycobacterium tuberculosis, the cause of tuberculosis, activate T helper cells (TH1) to secrete cytokines that in turn activate macrophages. Activated macrophages are more effective at killing phagocytosed organisms. This is the mechanism of type IV hypersensitivity (chronic granulomatous inflammation) (not illustrated).
Termination of the immune response
There are a number of mechanisms for switching off the immune response when the need for it has been removed. These include removal of antigen, the short life span of plasma cells, the activities of regulatory T cells and a variety of other mechanisms that downregulate the activity of T and B cells. It is vital that the immune response is terminated when no longer needed to prevent damage to normal tissue from an overenthusiastic immune response. These mechanisms are also important in the prevention of autoimmunity.
Immunological memory
When activated lymphocytes undergo clonal expansion during an immune response, some of the cells so generated mature to become memory T and B cells. These lymphocytes have a similar appearance to naïve lymphocytes but are able to produce a faster and more effective response to a smaller quantity of antigen. This is known as a secondary immune response and is the basis of lifelong immunity after certain infections and of vaccination.
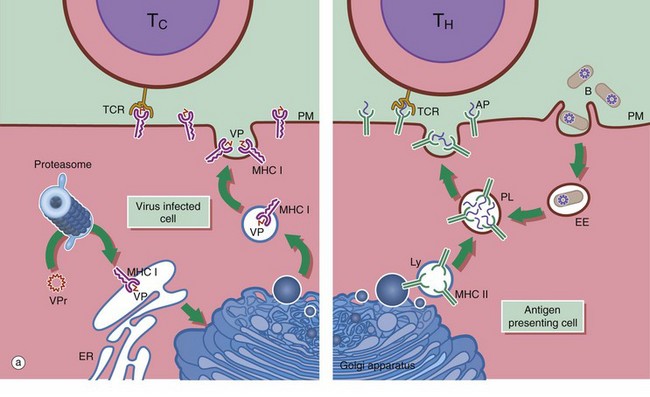
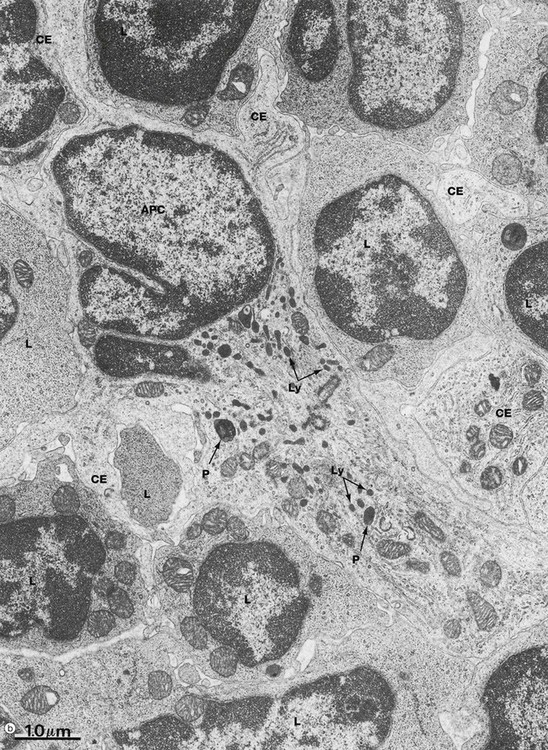
FIG. 11.3 Lymphocytes and antigen presenting cells (illustration (b) opposite)
(a) Schematic diagram (b) EM ×18 000
Antigen presenting cells APC are vital for the activation of lymphocytes to produce an adaptive immune response. They include macrophages, dendritic cells and B lymphocytes. Dendritic cells patrol the body surfaces and phagocytose invading pathogens. Dendritic cells are versatile and potent APCs. Some appear to be resident in the lymph node while others, carrying antigen from peripheral tissues, migrate in the lymph to the regional lymph nodes. Dendritic cells are found in the paracortical area of lymph nodes. This group of cells also includes interdigitating cells of the thymus and Langerhans cells of the skin. Follicular dendritic cells are accessible to B cells in the germinal centres of lymph nodes. They are similar cells which are able to bind antibody-antigen complexes to their surface without prior processing.
APC function is shown on the right side of diagram (a). Antigen (e.g. a bacterium B) is taken up by APCs into an early endosome EE that fuses with a lysosome containing major histocompatibility complex class II molecules MHC II. The antigen is broken down into short antigenic peptides AP that bind to MHC II and the peptide-MHC II complex is transported to the plasma membrane. After fusion of the phagolysosome PL with the plasma membrane PM, the MHC II-peptide complex is exposed on the cell surface where it may come into contact with helper T cells TH. If the T cell receptor TCR on the TH cell can bind to that particular peptide-MHC II complex, activation will occur and the adaptive immune response will proceed. Obviously, processing of a bacterium will generate many different antigenic peptides, but only one peptide and one TH cell is shown here for simplicity.
In general, TH cells recognise peptide bound to MHC II and cytotoxic T cells TC recognise antigen bound to MHC class I MHC I. On the left of diagram (a), processing of intrinsic viral antigen in a virus-infected cell is shown. The viral protein VPr is chopped into short peptides VP by a proteasome (an organelle that breaks down abnormal proteins). The peptides bind to MHC I and are presented on the cell surface for interaction with a TC. Almost all body cells express MHC I but usually only APCs express MHC II.
Micrograph (b) illustrates several lymphocytes and an APC in a lymph node. Lymphocytes and APCs exhibit similar features in other lymphoid tissues. The lymphocytes L are relatively small with round nuclei and condensed chromatin that tends to be clumped around the periphery of the nucleus. Cell outlines are fairly regular with occasional surface projections. The scanty cytoplasm contains plentiful free ribosomes and a few mitochondria but little endoplasmic reticulum, lysosomes or secretory granules.
The centre of the field is occupied by the large cell body of an antigen presenting cell APC, in this case a dendritic cell. These have numerous long branched cytoplasmic extensions CE reaching out between the surrounding lymphocytes so that a single dendritic cell can be in contact with many different lymphocytes. Its nucleus is deeply indented with dispersed chromatin; in this example, the plane of section has resulted in a small nuclear extension appearing to be separate from the main part of the nucleus. Typically, the APC cytoplasm contains numerous small lysosomes Ly and larger phagosomes P.
Thymus
The thymus is a flattened lymphoid organ located in the upper anterior mediastinum and lower part of the neck. The thymus is most active during childhood, reaching a weight of about 30 to 40 g at puberty, after which it undergoes slow involution so that in the middle-aged or older adult it may be difficult to differentiate from adipose tissue macroscopically.
In the embryo, the thymus originates from epithelial outgrowths of the ventral wing of the third pharyngeal pouch on each side. These merge in the midline, forming a single organ subdivided into numerous fine lobules. The epithelium develops into a sponge-like structure containing a labyrinth of interconnecting spaces that become colonised by immature T lymphocytes derived from haematopoietic tissue elsewhere in the developing embryo. Towards the centre of the organ, the epithelial framework has a coarser structure with smaller interstices and a much smaller lymphocyte population, so that on microscopic examination, the gland has a highly cellular outer cortex and a less cellular central medulla.
The epithelial cells of the thymus provide a mechanical supporting framework for the lymphocyte population. Cortical epithelial cells also promote T cell differentiation and proliferation. Furthermore, the epithelial cells secrete a number of different hormones that regulate T cell maturation and proliferation within the thymus and in other lymphoid organs and tissues. The inner surfaces of the thymic capsule and septa are invested by a continuous layer of thymic epithelial cells resting on a basement membrane. The epithelium also forms sheaths around the blood vessels, creating a barrier to the entry of antigenic material into the thymic parenchyma. This is known as the blood-thymus barrier.
The functions of the thymus include:
• Development of immunocompetent T lymphocytes from bone marrow–derived T cell precursors to produce mature TH and TC cells.
• Proliferation of clones of mature naïve T cells to supply the circulating lymphocyte pool and peripheral tissues.
• Development of immunological self-tolerance. More than 98% of maturing cells die by apoptosis within the thymus, and many of these are self-reactive.
• The thymus secretes various polypeptides with hormonal characteristics, including thymulin, thymopoietin and various thymosins. These hormones regulate T cell maturation, proliferation and function within the thymus and peripheral lymphoid tissues. They also interact with other endocrine systems in the regulation of inflammation.
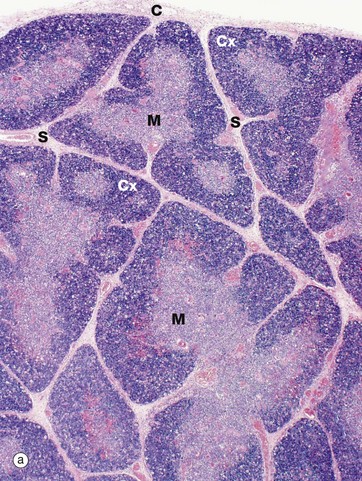
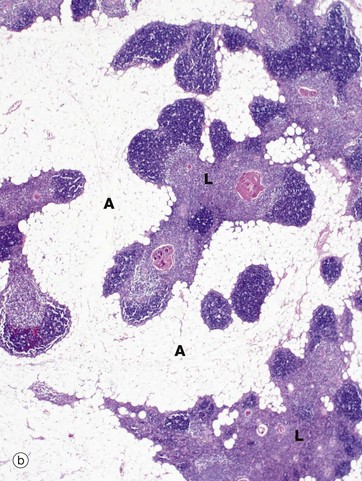
FIG. 11.4 Thymus
(a) Infant, H&E (LP) (b) Adult, H&E (LP)
The infant thymus (a) is a lobulated organ invested by a loose collagenous capsule C from which interlobular septa S containing blood vessels radiate into the substance of the organ. The thymic tissue is divided into two distinct zones, a deeply basophilic outer cortex Cx and an inner eosinophilic medulla M; distinction between the two is most marked in early childhood, as in this specimen.
In the adult (mid-30s in this case), the thymus (b) is already well into the process of involution, which involves two distinct processes, fatty infiltration and lymphocyte depletion. Fat cells (adipocytes) first begin to appear at birth, their numbers slowly rising until puberty when the rate of fatty infiltration increases markedly. Fatty infiltration of the interlobular septa occurs first, spreading out into the cortex and later the medulla. Thus, in the mature thymus islands of lymphoid tissue L are separated by areas of adipose tissue A. At this age, the cortex and medulla can still be differentiated. In the elderly, the thymus can be very difficult to detect both macroscopically and microscopically, with only small islands of lymphoid tissue lost in a sea of adipose tissue. Lymphocyte numbers begin to fall from about 1 year of age, the process continuing thereafter at a constant rate. Despite this, the thymus continues to provide a supply of mature T lymphocytes to the circulating pool and peripheral tissues. Lymphocyte depletion results in collapse of the epithelial framework. However, cords of epithelial cells persist and continue to secrete thymic hormones throughout life.
The normal process of slow thymic involution associated with aging should be distinguished from acute thymic involution, which may occur in response to severe disease and metabolic stress associated with pregnancy, lactation, infection, surgery, malnutrition, malignancy and other systemic insults. Stress involution is characterised by greatly increased lymphocyte death and is probably mediated by high levels of corticosteroids; thus the size and activity of the adult thymus are often underestimated if examined after prolonged illness.
Numerous small branches of the internal thoracic and inferior thyroid arteries enter the thymus via the interlobular septa, branching at the corticomedullary junction to supply the cortex and medulla. Postcapillary venules in the corticomedullary region have a specialised cuboidal endothelium similar to that of the high endothelial venules of the lymph node (see Fig. 11.11), which allows passage of lymphocytes into and out of the thymus. The venous and lymphatic drainage follow the course of the arterial supply; there are no afferent lymphatics. Sympathetic and parasympathetic nerve fibres derived from the sympathetic chain and phrenic nerves, respectively, accompany the blood vessels into the thymus.
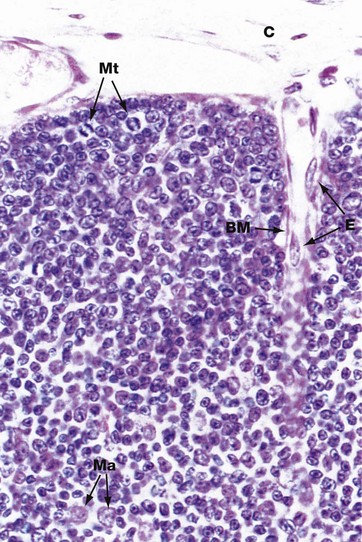
FIG. 11.5 Thymic cortex
H&E (HP)
Prothymocytes migrate in the blood from the bone marrow and enter the thymus at the corticomedullary junction. They move to the subcapsular area of the cortex to begin their maturation into mature naïve T cells. The thymic cortex is thus packed with immature and maturing T cells, (thymocytes). In the outer cortex, large lymphocytes (lymphoblasts) divide by mitosis to produce clones of smaller mature T cells. These undergo further maturation as they move deeper into the cortex towards the medulla. This differentiation is promoted by interaction with the specialised epithelial cells known as thymic nurse cells, which are found in the outer cortex. Each nurse cell envelops multiple lymphocytes and supports their progression through the early stages of maturation. It is during this process that the T cell receptor genes are rearranged and the cells acquire the surface markers or phenotype of mature helper and cytotoxic T cells. T cells in the cortex begin to express the TCR-CD3 complex and the co-receptors CD4 and CD8. Cells failing to make these adjustments successfully die by apoptosis and are taken up by pale-stained macrophages Ma. Several mitotic figures Mt can be seen in the outer cortex in this micrograph.
Note also in this micrograph a small capillary lined by flattened endothelial cells E, entering the cortex from the capsule C. Around the capillary, the basement membrane BM of epithelial cells can be discerned at the interface between the thymic framework and supporting tissue elements. The epithelial framework of the cortex is more delicate and finely branched than that of the medulla and the cells cannot be distinguished in this H&E-stained micrograph, being obscured by the mass of lymphocytes. However, immunostaining techniques can demonstrate these cells (see Fig 11.7).
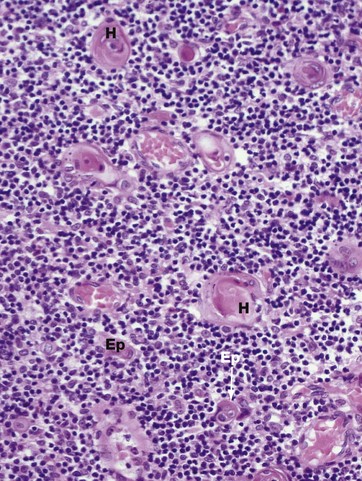
FIG. 11.6 Thymic medulla
(a) H&E (HP)
Maturing thymocytes migrate from the cortex to the medulla. The dominant histological feature of the thymic medulla is the robust epithelial component Ep. The epithelial cells have large pale-stained nuclei, eosinophilic cytoplasm and prominent basement membranes. A particular feature in the medulla are the lamellated Hassall corpuscles H that first appear in fetal life and increase in number and size thereafter. These are formed from groups of keratinised epithelial cells, often with fragments of debris at their centre, and probably represent a degenerative phenomenon.
Also found in the medulla are dendritic cells, also known as a thymic interdigitating cells, which express high levels of both class I and II MHC proteins. It appears that these cells present normal self-components, self-antigens, to maturing T cells. Any self-reactive T cells that identify themselves by becoming activated are obliterated by apoptosis. This is known as clonal deletion or negative selection. Thus the thymus is the organ where self-reactive T cells are removed, preventing the development of autoimmunity.
At the end of their journey through the thymus, the mature T cells enter the blood vessels and lymphatics to join the pool of circulating T lymphocytes and populate the T lymphocyte domains of other lymphoid organs.
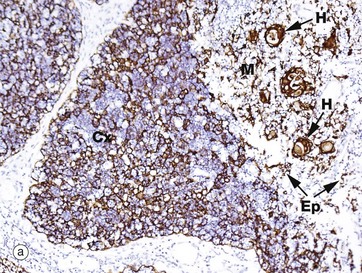
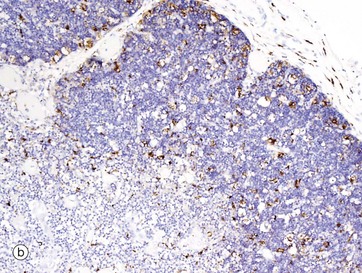
FIG 11.7 Thymus
(a) Immunohistochemical stain for cytokeratin (AE1/3) (MP) (b) Immunohistochemical stain for CD68 (MP)
The epithelial cells and macrophages of the thymus are generally difficult to visualise in a standard H&E section, as they are obscured by the dense population of lymphocytes. However, they may be highlighted by immunohistochemical techniques (see Appendix 2). Micrograph (a) shows infant thymus tissue that has been stained using an antibody to cytokeratin. Many different cytokeratin antibodies are commercially available and are widely used in routine histopathology practice. This micrograph demonstrates the extensive delicate network of epithelial cells in the cortex Cx, as well as the more rugged epithelial framework of the medulla M. Individual epithelial cells Ep as well as Hassall corpuscles H are stained brown. In micrograph (b) the tissue has been stained with an antibody to CD68, which is strongly expressed by macrophages. Some dendritic cells also express CD68, so the brown-stained cells visible in both the cortex and medulla will include a mixture of both cell types, although predominantly macrophages. Macrophages phagocytose lymphocytes that have died by apoptosis, either because they have failed to go through the sequential steps of maturation properly or because having done so they have shown themselves to be self-reactive. Dendritic cells (or thymic interdigitating cells) present self-antigens to developing T cells. Those T cells that declare themselves to be self-reactive are culled (clonal deletion) to maintain a state of self-tolerance.
Lymph Nodes
Lymph nodes are bean-shaped, encapsulated, highly organised structures that are interposed along the larger regional vessels of the lymph vascular system. The human body has about 450 lymph nodes, grouped mainly in areas where the lymphatics converge to form larger trunks as in the neck, axillae, groins, lung hila, mesentery of the bowel and para-aortic areas. Lymph nodes process antigen from the interstitial fluid that arrives at the node in the lymph.
Lymph nodes are the primary site to stimulate an immune response to antigens in the lymph. They are organised to bring together antigen, potentially reactive lymphocytes and APCs and to provide the best environment to stimulate an adaptive immune response. As described earlier, antigen-loaded dendritic cells from skin and mucosal sites, along with free antigen and cytokines, migrate in the lymph to the regional lymph nodes. The acute inflammatory process increases the flow of lymph by flooding the infected or damaged tissue with extracellular fluid. Mature naïve lymphocytes constantly traffic between the periphery and organised lymphoid tissues via the blood and lymph circulation to maximise their chances of encouraging appropriate antigen.
Thus within the lymph node, the necessary elements for stimulation of the adaptive immune response are brought together, and T and B cells undergo clonal expansion and maturation. B cells mature into antibody-secreting plasma cells. Effector T lymphocytes and plasma cells leave the nodes in the efferent lymph and recirculate to the damaged or infected tissue. In addition, water and electrolytes from the lymph that percolate through the node are also returned to the blood circulation via the high endothelial venules.
Lymph nodes can be considered as three functional compartments:
• The stromal compartment which is packed with lymphocytes and APCs
• The lymphatic/sinus compartment which acts as a sieve for antigen
• The vascular compartment which delivers lymphocytes to the lymph node along with the usual nutrients
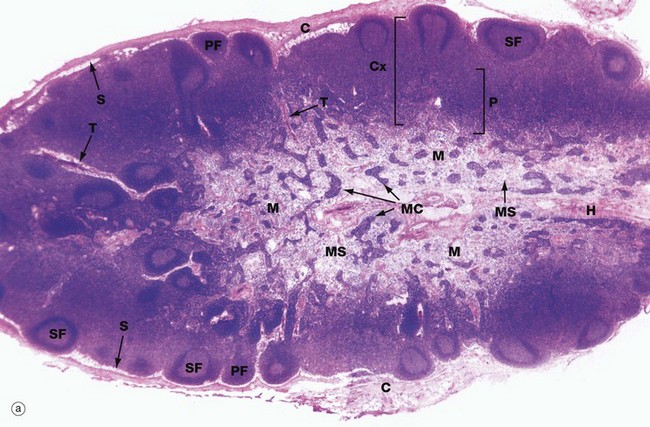
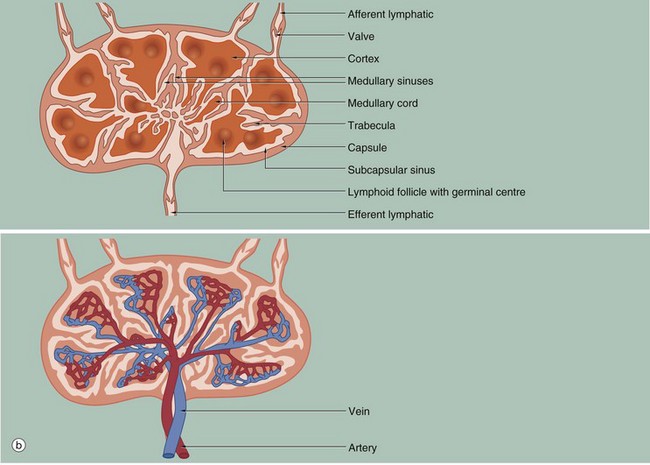
FIG. 11.8 Lymph node structure and vascular organisation (illustrations (a) and (b) opposite)
(a) H&E (LP) (b) Schematic diagram
Lymph nodes are small, bean-shaped organs situated in the course of lymphatic vessels such that lymph draining back to the bloodstream first passes through one or more lymph nodes. Inactive nodes are only a few millimetres long but may increase greatly in size when mounting an active immunological response. Most lymph nodes in the body show some degree of ‘reactive change’ in response to the constant barrage of antigen to which they are exposed. As shown in micrograph (a), the outer part of the lymph node is highly cellular and is known as the cortex Cx, whilst the central area, the medulla M, is less cellular. At the hilum H, the efferent lymphatic drains efferent lymph from the lymph node. The hilum is also the site of entry of the artery bringing blood to the lymph node and the vein leaving the node. The lymph node is surrounded by a collagenous capsule C from which trabeculae T extend for a variable distance into the substance of the node.
Afferent lymphatic vessels, as shown in diagram (b), divide into several branches outside the node then pierce the capsule to drain into a narrow space called the subcapsular sinus S that encircles the node beneath the capsule. From here, a labyrinth of channels called cortical sinuses passes towards the medulla through the cortical cell mass; sinuses adjacent to the trabeculae (trabecular sinuses) pursue a more direct course towards the medulla, but nevertheless form part of the cortical sinus system. The cortical sinuses are generally difficult to visualise because of their highly convoluted shape and numerous fine extensions that penetrate the cellular mass of the cortex (see Fig. 11.9). The superficial cortex contains a number of dense cellular aggregations, the follicles. Most of these in this particular example are secondary follicles SF with a pale-stained germinal centre; others are inactive primary follicles PF. B cells respond to antigen in the cortex and undergo stimulation, clonal expansion and maturation in the follicles, the presence of germinal centres indicating that an active immune response is underway.
The deeper cortex or paracortex P is also densely cellular but has a more homogeneous staining appearance. T lymphocytes interact with antigen presenting cells in the paracortex and undergo a similar process of activation and clonal expansion. T helper cells migrate towards the cortex to provide ‘help’ to B cells while activated cytotoxic T cells leave the node to perform their functions in the periphery.
At the left of the field, some lymphoid follicles appear to be located deep in the paracortex; this is not the case but is a product of the plane of section, which passes at that point through the superficial cortex.
The dominant feature of the medulla is the network of broad interconnected lymphatic channels called medullary sinuses MS that converge upon the hilum in the concavity of the node. Lymph drains from the hilum in the efferent lymphatic into one or more additional nodes, which in turn drain into more proximal nodes before eventually joining the blood stream via the thoracic duct or right lymphatic duct. Thus the lymph is filtered through a number of lymph nodes to facilitate the exposure of large numbers of lymphocytes to antigens in the lymph. Extensions of the cortical cell mass extend into the medulla as medullary cords MC.
The blood supply of the lymph node, as shown in diagram (b), is derived from one or more small arteries which enter at the hilum and branch in the medulla, giving rise to extensive capillary networks supplying the cortical follicles, paracortical zone and medullary cords. The vascular system provides the main route of entry of lymphocytes into the node, as well as supplying its metabolic requirements. Within the paracortex, the postcapillary high endothelial venules (HEV) have a cuboidal endothelium specialised for the exit of lymphocytes. Recognition by lymphocytes of these exit sites requires the presence of specific complementary adhesion molecules on the surface of both the endothelial cells and lymphocytes. Different groups of lymphocytes home to different tissues. Thus lymphocytes from the mucosa of the gut migrate to mesenteric lymph nodes, then to the spleen and back to mucosal tissues. Lymphocytes from the skin travel to their regional lymph nodes and then return to the skin. This is made possible by the different adhesion molecules or vascular addressins in the HEV of the different lymph node groups and the corresponding binding molecules on the lymphocytes. The HEV drain into small veins that leave the node via the hilum.
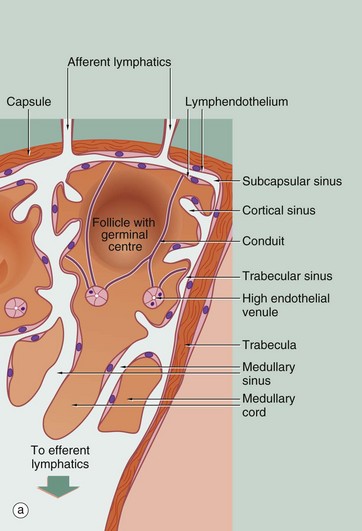
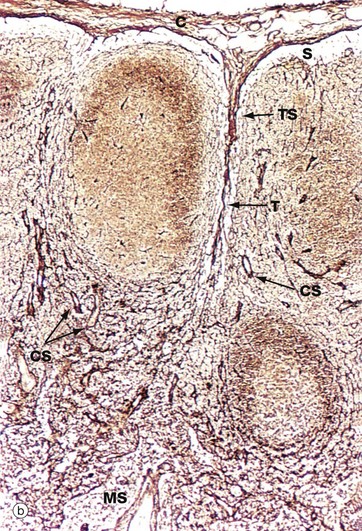
FIG. 11.9 Structure of the lymph node
(a) Schematic diagram (b) Reticulin method (LP)
Diagram (a) illustrates the three functional compartments within the lymph node: the lymphatic sinuses, the blood vessels and the stromal compartment. A network of lymphatic sinuses permeates the node and is continuous with the lumen of the afferent and efferent lymphatic vessels. The sinuses are lined by lymphatic endothelial cells and contain a population of macrophages (see Fig. 11.14). Sinuses carry lymph, lymphocytes, antigen, dendritic cells and macrophages into the node. Blood vessels form a microvascular network in the node; of particular note are the high endothelial venules (HEV) that are the major site of entry of circulating lymphocytes into the node. The interstitial compartment is packed with lymphocytes. Lymphocytes that do not recognise antigen while in the node leave in approximately 12 to 18 hours in the efferent lymph to circulate through other lymph nodes. The lymphatic and blood vessel endothelia thus define the boundaries of the three compartments and control passage of cells and molecules between the different compartments.
Micrograph (b) shows the fine reticular architecture of the lymph node; reticulin fibres are stained blackish-brown and lymphocyte nuclei appear lighter brown. The main structural support for the lymph node is derived from the collagenous capsule C and trabeculae T, which extend into the node. From these, a fine meshwork of reticulin fibres extends throughout the node, providing a supporting framework for the mass of lymphocytes and accessory cells within the stroma. The reticular network is particularly dense in the cortex, except for the follicular areas where it is relatively sparse. This network is draped in specialised stromal cells, fibroblastic reticular cells (FRC), which form a structural skeleton similar to a sponge. The spaces of the sponge are filled with lymphocytes and dendritic cells. The subcapsular sinus S, trabecular sinuses TS, other cortical sinuses CS and medullary sinuses MS are kept patent by a fine skeleton of reticulin fibres which traverse the sinuses.
Another component of the collagenous skeleton of the paracortex is the conduit system. Conduits are bundles of specialised collagen fibres that run from the subcapsular sinus to the HEV; they are too small to be identified in micrograph (b). The conduits are wrapped in a basement membrane which is in turn covered by fibroblastic reticular cells. FRC are the cells that produce the collagen framework of the node but also have a role in bringing together dendritic cells and T cells to facilitate antigen presentation. Conduits are thought to carry soluble antigens (small molecules) and cytokines into the lymph node parenchyma. They also carry fluid from the lymph back to the blood stream, i.e. from the subcapsular sinus to the HEV, so that the lymph leaving the node is more concentrated than the lymph entering it.
Antigen may enter the subcapsular sinus either as soluble or particulate antigen or as antigen carried by dendritic cells. Soluble antigen that is smaller than 70 kDa is able to pass into the stromal compartment via pores in the floor of the subcapsular sinus. These small antigens are transported in the conduits into the stroma where they may interact with APCs and lymphocytes. Larger particles are phagocytosed by the sinus macrophages and processed before presentation to T cells; thus intact bacteria and virus cannot pass rapidly through the lymph node to enter the blood.
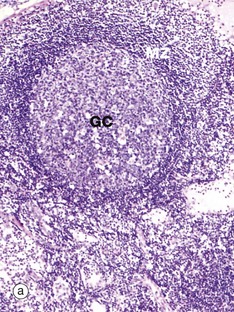
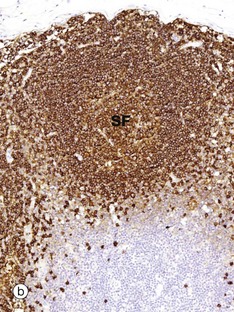
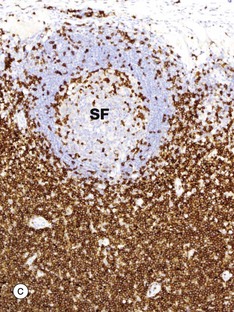
FIG. 11.10 Lymph node distribution of T and B lymphocytes
(a) H&E (MP) (b) Immunohistochemical method CD20 (MP) (c) Immunohistochemical method CD3 (MP)
The outer part of the lymph node cortex is characterised by lymphoid follicles. These are aggregates of B cells which may be in a resting state as in a primary follicle. However, once B cells have encountered a suitable antigen and become stimulated, the follicle undergoes a change as shown in the secondary follicle in micrograph (a). Secondary follicles consist of a pale centre, the germinal centre GC surrounded by a darker zone known as the mantle zone MZ. The mantle zone is made up of small resting B cells, the condensed nuclear chromatin giving the dark blue colour. The mantle zone is usually asymmetrical, with the wider side towards the capsule. The germinal centre is the site of B cell activation, clonal expansion and differentiation and consists of dividing B cells which are larger and paler than the small inactive lymphocytes of the marginal zone.
Micrographs (b) and (c) employ the immunohistochemical method with markers for B lymphocytes (CD20) and T lymphocytes (CD3), respectively. It is obvious that the bulk of the cells in the secondary follicle SF illustrated are B lymphocytes, which are stained dark brown in micrograph (b), while the paracortex contains very rare B cells. However, using antibody to CD3 shows that the paracortex is densely packed with T cells, with only a few T cells found within the secondary follicle. CD3 is found on both TH and TC cells, but use of other markers such as CD4, characteristic of TH cells, would demonstrate that these are TH which are present in the germinal centre to provide ‘help’ for B cells undergoing activation.
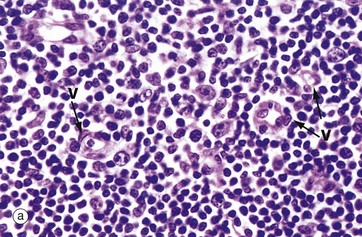
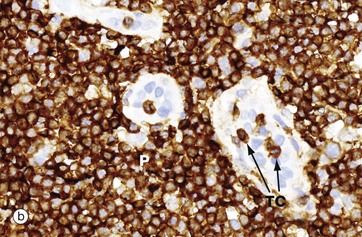
FIG. 11.11 Paracortical zone
(a) H&E (HP) (b) Immunohistochemical method CD3 (HP)
T lymphocytes are the main cell type in the paracortical zone. Circulating T cells enter the lymph node through the walls of high endothelial venules V into the paracortical zone; they rejoin the circulation some 12 to 18 hours later in the efferent lymph.
When activated, T lymphocytes enlarge to form immunoblasts, histologically similar to their B cell counterparts, and divide to produce clones of activated T lymphocytes. Indeed, in a T cell–dominated immunological response, the paracortical zone may be greatly expanded, a pattern known as the paracortical reaction. Activated T cells are then disseminated via the circulation to peripheral sites where much of their activity occurs.
The main antigen presenting cells in the paracortex are the dendritic cells, which are in close contact with the naïve T cells circulating through this zone. These cells are derived from macrophage precursors including the Langerhans cells of the skin. Micrograph (b) illustrates two high endothelial venules V which are lined by tall cuboidal rather than the usual flattened endothelial cells. The endothelial cell nuclei are stained pale blue. T cells TC are seen passing through the endothelium to enter the paracortex P, which is packed with further T cells. These endothelial cells express on their surface specific lymphocyte-binding molecules known as addressins that allow lymphocytes to bind to the endothelium as the first step of migration into the tissue.
Approximately 90% of lymphocytes enter the parenchyma of the node via the HEV, while the rest arrive in the afferent lymph. While in the lymph node, lymphocytes are highly motile, moving through the parenchyma to come into contact with a large number of APCs to optimise their chances to encounter cognate antigen.
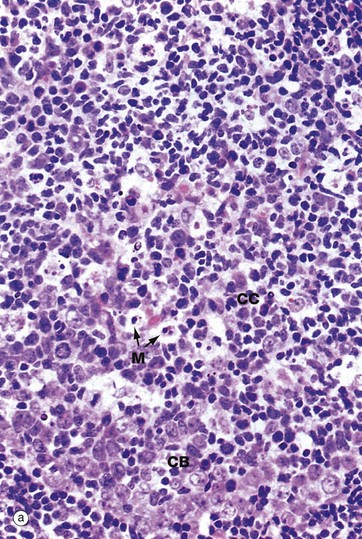
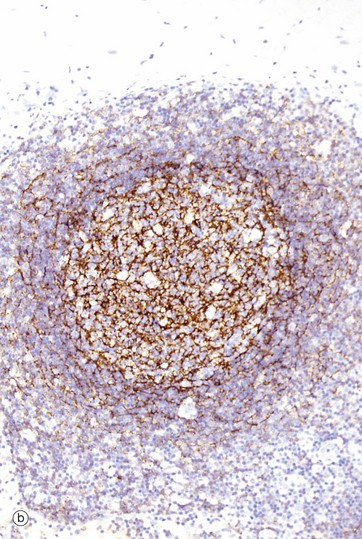
FIG. 11.12 Lymphoid follicle and germinal centre
(a) H&E (HP) (b) Immunohistochemical method CD21 (HP)
Micrograph (a) shows the germinal centre of a secondary lymphoid follicle. Primary follicles which are unstimulated consist entirely of the same cell types as the mantle zone.
The cells of the germinal centre are mainly actively dividing B cells. The germinal centre is not uniform in colour but is darker towards the medulla, reflecting the organisation of the different cell types within it. Resting B cells enter the lymph node via the HEV and, if they encounter an antigen with which they can react, enter the cycle of blast transformation to produce clones of plasma cells and B memory cells. The first step is activation to give rise to centroblasts CB, large mitotically active cells with round nuclei that are found in the darker zone of the germinal centre closer to the medulla. These differentiate into centrocytes CC, found in the paler zone of the germinal centre towards the lymph node capsule. These cells are of variable size and have folded, irregular (‘cleaved’) nuclei. Mitotic figures are absent in this area. Centrocytes migrate towards the paler capsular zone of the germinal centre where they go through further cycles of division to produce either immunoblasts or memory B cells. Immunoblasts move to the medullary cords where they complete their differentiation into plasma cells, capable of secreting large amounts of antibody. In the germinal centre, a further ingenious device ensures even greater diversity of antibody specificity. Centroblasts undergo increased mutation of the immunoglobulin genes (somatic hypermutation), thus creating further variations in immunoglobulin structure. Those centroblasts with the antibody structure that binds most tightly to the antigen (high-affinity antibody) are then stimulated to differentiate into plasma cells and memory cells. At this stage, class switch recombination also occurs so that the plasma cells produced by the germinal centres secrete IgG or IgA antibody rather than IgM, which is characteristic of the early immune response. Memory cells, which resemble small lymphocytes, take up residence in the mantle zone of the follicle or may join the recirculating pool of small lymphocytes.
Other cells found in the germinal centres include:
• Follicular dendritic cells (FDC) are the major antigen-presenting cells of the follicles and are thought to be of mesenchymal cell origin. These are difficult to see in routine H&E stains, but their dendritic processes can be demonstrated (stained brown) as in micrograph (b) using an antibody to CD21. These cells are found in all areas of the germinal centre and also form a meshwork in the mantle zone and in primary follicles. They can retain antigen on their surface for many months and present this unprocessed antigen to B cells. FDCs may have a role in maintaining the activity of memory cells, as well as stimulating a primary immune response.
• The interestingly named tingible body macrophages M are easily seen in routine sections in active germinal centres. They contain within their cytoplasm numerous apoptotic bodies derived from B lymphocytes that have not been successful in generating a high-affinity antibody.
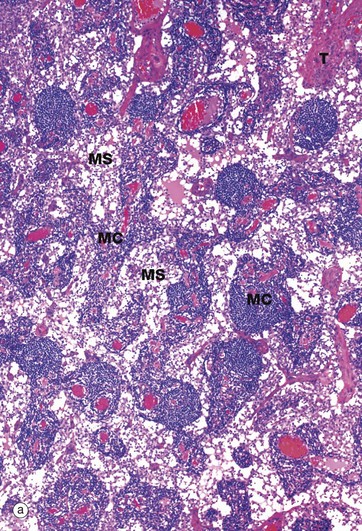
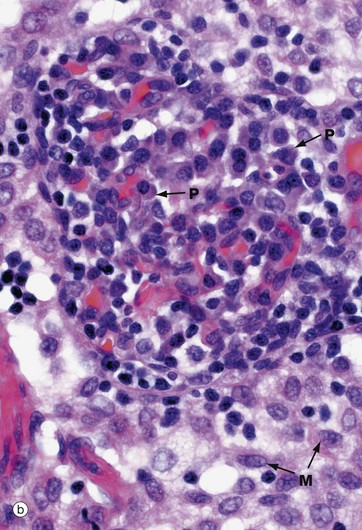
FIG. 11.13 Medullary cords and sinuses
(a) H&E (LP) (b) H&E (HP)
Micrograph (a) illustrates the structure of the lymph node medulla, with branching medullary cords MC separated by irregular medullary sinuses MS. Throughout the medulla are trabeculae T extending from the collagenous supporting tissue of the capsule. Plasma cells and their precursors, plasmablasts which have migrated from the germinal centres, are the major cell types in the medullary cords. Here, B lymphocytes complete the final stages of maturation to form plasma cells. Plasma cells synthesise antibody that is carried to the general circulation in efferent lymph; some plasmablasts also migrate from the node in efferent lymph to take up residence in peripheral tissues.
Micrograph (b) shows a higher magnification view of the medullary cords and sinuses. In the right and central part of the micrograph, there is a medullary cord packed with plasmablasts and plasma cells P. In contrast, the sinus, which contains mainly sinus macrophages M, is paler stained. As in the subcapsular and trabecular sinuses, fine reticular strands traverse the medullary sinuses, providing support for sinus macrophages.
Plasma cells are differentiated B lymphocytes which are specialised for the production of large quantities of antibody. Plasma cells are not usually detectable in the circulating blood but are found in the tissues, in particular the medullary cords of lymph nodes, the white pulp of the spleen, the supporting tissues of mucosal surfaces (e.g. lamina propria of intestine) and the bone marrow.
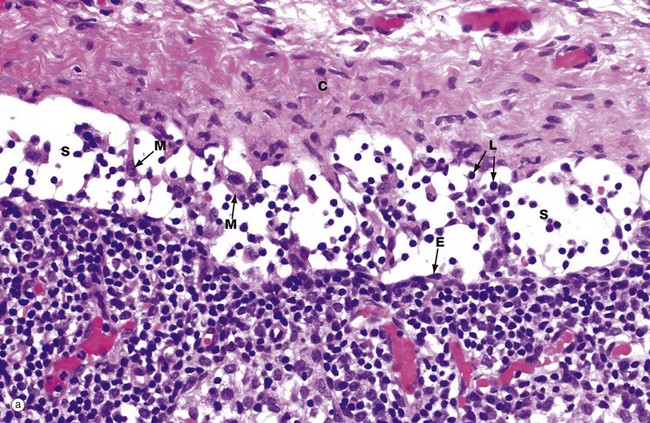
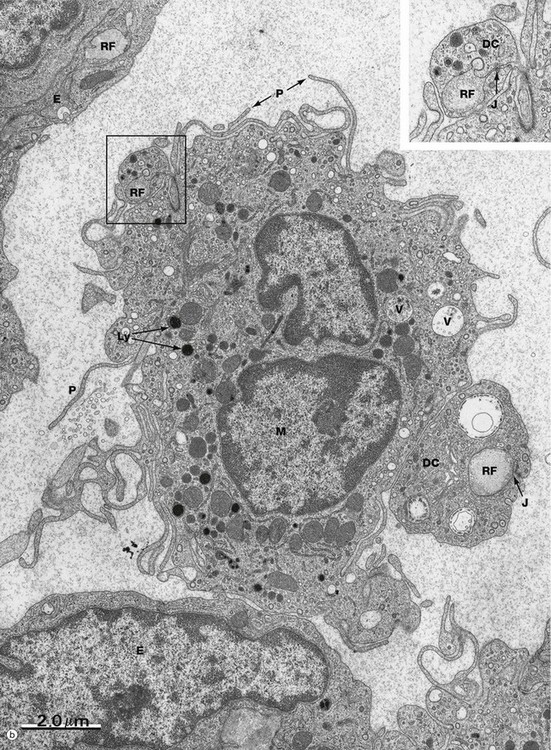
FIG. 11.14 Capsule and subcapsular sinus (illustration (b) opposite)
(a) H&E (HP) (b) EM ×11 000, inset ×20 000
The fibrous capsule C of the lymph node is pierced by branches of afferent lymphatic vessels with valves to ensure one-way flow. The afferent lymphatics bring lymphocytes, antigen-carrying dendritic cells, macrophages and particulate antigen into the node. Micrograph (a) illustrates the subcapsular sinus S at high magnification. Endothelial cells E lining the sinus can just be identified at this magnification but are much better seen in micrograph (b). The lymph node sinuses are traversed by fine reticulin strands that provide support for large eosinophilic sinus macrophages M. These macrophages filter antigen and other debris from afferent lymph. Large molecules and particulate antigen are retained within the sinus, but small antigenic molecules percolate through the parenchyma of the lymph node via the conduit system, a system of bundles of collagenous fibres that form a transport route for fluid and small molecules between the sinus system and the high endothelial venules via the paracortex. The macrophages and dendritic cells in the sinus process larger antigens and carry them into the node to present them to lymphocytes within the node. Lymphocytes L arriving in the afferent lymph also are found within the subcapsular sinus.
In micrograph (b), the structures of the subcapsular sinus are seen in much more detail. Endothelial cells E line the sinus. Reticular fibres RF are surrounded by the cytoplasmic projections (or dendrites) of dendritic cells DC that wrap all the way around the reticular fibres and form junctions J with themselves (see inset, which is an enlargement of the area outlined). A sinus macrophage M is draped between the two reticular fibres and, within its cytoplasm, the machinery for antigen processing is readily apparent i.e. plentiful lysosomes Ly and endocytotic vacuoles V. The macrophage also has plentiful cell processes P to increase the surface area. Thus the subcapsular sinus of the lymph node acts as a ‘strainer’ for antigen entering the node.
Mucosa-Associated Lymphoid Tissue (MALT)
Lymphoid tissue is distributed at many mucosal surfaces throughout the body, important sites being the gastrointestinal tract (gut-associated lymphoid tissue, GALT), bronchial tree (bronchial-associated lymphoid tissue, BALT), and oropharynx (Waldeyer ring). The lymphoid tissue may be arranged either as a diffuse population or as non-encapsulated organised aggregations, such as the tonsils or the Peyer's patches of the small bowel. Follicles with germinal centres, similar to those of lymph nodes, are found in the organised lymphoid tissues. The breast also contains a population of lymphocytes and plasma cells.
The total mass of lymphoid tissue in the gastrointestinal, respiratory and genitourinary tracts is enormous and is collectively known as mucosa-associated lymphoid tissue (MALT). The larger aggregations function in a manner analogous to lymph nodes, sampling antigenic material entering the tracts and initiating both antibody-mediated and cytotoxic immune responses where appropriate; they contain discrete B and T cell zones as well as antigen-processing accessory cells.
The diffusely scattered lymphocytes seen in the lamina propria of the gut and respiratory tree are mainly T lymphocytes. Smaller numbers of B cells are also present, as well as plasma cells. All classes of antibody are produced, with IgA predominating. IgA is secreted into the gut lumen bound to a carbohydrate moiety, secretory piece, which is synthesised in the epithelium and renders IgA resistant to proteolytic enzymes. This secretory IgA protects against pathogens in the gut lumen before they breach the tissues. IgA also reaches the gut in bile, being taken up from blood and secreted into bile in a similar fashion. IgG and IgM are secreted into the lamina propria to deal with organisms that elude the surface protective mechanisms. IgE is also produced and triggers release of histamine from mast cells that are present in large numbers in the lamina propria.
Considerable numbers of lymphocytes are found within the epithelium of the small and large intestines and are present in particularly large numbers in the epithelium overlying Peyer's patches. These lymphocytes are almost exclusively CD8 positive γδ T cells.
The epithelium overlying all MALT aggregations is specialised for the sampling of luminal contents for antigen and acts as the equivalent of the afferent lymphatics of the lymph node. The lymphatics associated with MALT are all efferent from the MALT and pass to regional lymph nodes (e.g. cervical, mesenteric, hilar).
MALT acts as an integrated unit with a separate route of lymphocyte circulation in parallel with the peripheral lymphoid circulation. When antigen is encountered, it is carried to local MALT tissue. Stimulated lymphocytes migrate to regional lymph nodes where clonal expansion takes place. Effector cells then pass via the thoracic duct and general circulation to the gastrointestinal and respiratory mucosae. MALT lymphocytes carry surface binding molecules that attach to the addressins on high endothelial venules in MALT tissue but not in peripheral tissue.
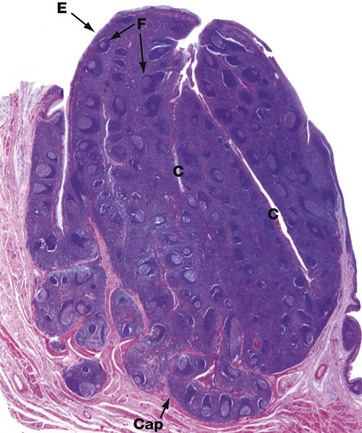
FIG. 11.15 Palatine tonsil
H&E (LP)
The palatine tonsils are organised masses of lymphoid tissue which along with the lingual, pharyngeal and tubal tonsils (adenoids) form Waldeyer ring.
The luminal surface is covered by stratified squamous epithelium E that deeply invaginates the tonsil, forming blind-ended tonsillar crypts C. The base of the tonsil is separated from underlying muscle by a dense collagenous hemicapsule Cap. The tonsillar parenchyma contains numerous lymphoid follicles F with germinal centres similar to those found in lymph nodes. Particulate matter or bacteria entering the crypts from the oropharynx are passed to the follicles by transcytosis by the epithelial cells of the crypt lining and an immune response is initiated. Efferent lymphatics pass to the deep cervical chain of lymph nodes, and activated lymphocytes migrate to the lamina propria of the oral mucosa and nasopharynx and other mucosae.
Antigen uptake occurs in a similar manner in the lingual, pharyngeal and tubal tonsils, the latter being covered with respiratory-type epithelium rather than stratified squamous epithelium.
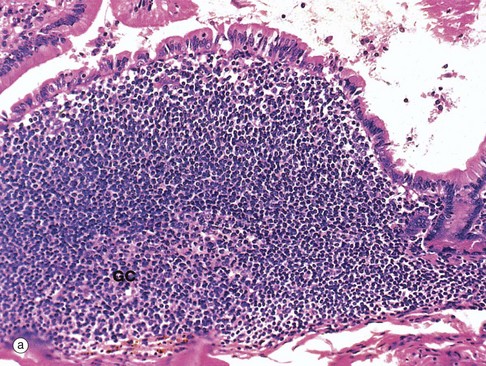
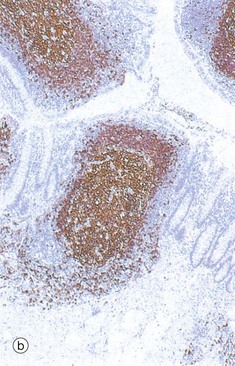
FIG. 11.16 Gut-associated lymphoid tissue
(a) Peyer's patch, H&E (MP) (b) The appendix, immunohistochemical stain for CD20 (LP)
Organised lymphoid tissue is found in all parts of the normal gastrointestinal system except the stomach. This is often called gut-associated lymphoid tissue (GALT). The largest lymphoid aggregates are the Peyer's patches of the small intestine, which are groups of lymphoid follicles located in the mucosa, where they bulge dome-like into the gut lumen. Usually there are few villi overlying Peyer's patches. They are least numerous in the duodenum and most prominent in the terminal ileum. Micrograph (a) illustrates part of a Peyer's patch in the ileum, showing only a single lymphoid follicle. The follicle is similar to those in lymph nodes, consisting of a germinal centre GC composed of proliferating and maturing B cells (centroblasts and centrocytes) surrounded by a mantle of small, resting lymphocytes. Immediately beneath the epithelium is a zone of mixed lymphocytes and macrophages. The area between follicles is occupied by T lymphocytes and, like its lymph node equivalent the paracortex, contains high endothelial venules.
The epithelium overlying these dome areas is specialised for antigen uptake. Scattered among the epithelial cells are low cuboidal M cells, epithelial cells with numerous surface microfolds instead of the usual microvilli. These cells are specialised for transcytosis and take up antigen from the lumen of the gut and transport it into the underlying Peyer's patch. Goblet cells are scanty in these areas.
Antigen entering the Peyer's patch is taken up by antigen-presenting cells and presented to T lymphocytes. IgA-committed B cells responding to the antigen migrate via afferent lymphatics to mesenteric lymph nodes where the immunological response is greatly amplified. Activated lymphocytes enter the circulation via the thoracic duct and home to the lamina propria of the gut where they undergo final maturation into plasma cells. During lactation, GALT B cells migrate to the breast, mature into plasma cells and secrete IgA into the milk to protect the newborn.
Micrograph (b) shows lymphoid tissue in the wall of the appendix. The immunohistochemical method used here stains the B cells brown and confirms that, as in lymph nodes, lymphoid follicles consist mainly of B cells with intervening T cell areas.
Spleen
The spleen is a large lymphoid organ situated in the left upper part of the abdomen. It receives a rich blood supply via a single artery, the splenic artery, and is drained by the splenic vein into the hepatic portal system. The splenic parenchyma is basically dark red, the red pulp, with small macroscopically visible white nodules, the white pulp, scattered throughout its substance.
In humans, the spleen has four main functions:
• Production of immunological responses against blood-borne antigens
• Removal of particulate matter and aged or defective blood cells, particularly erythrocytes, from the circulation
• Recycling iron to the bone marrow
• Haematopoiesis in the normal fetus and in adults with certain diseases
Removal of the spleen in childhood or adolescence renders the individual susceptible to infection by certain pyogenic bacteria, but in adults splenectomy has less effect. Presumably adults have been naturally immunised against these organisms.
The spleen performs the same function for blood that lymph nodes perform for lymph. The structure of the spleen allows intimate contacts to be made between blood and lymphocytes, just as the structure of the lymph node facilitates the interaction of afferent lymph and lymphocytes. The histology of the spleen varies according to the animal models used. This description is specific to the human spleen.
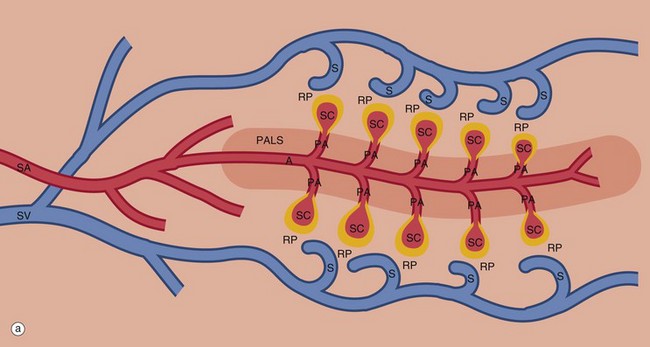
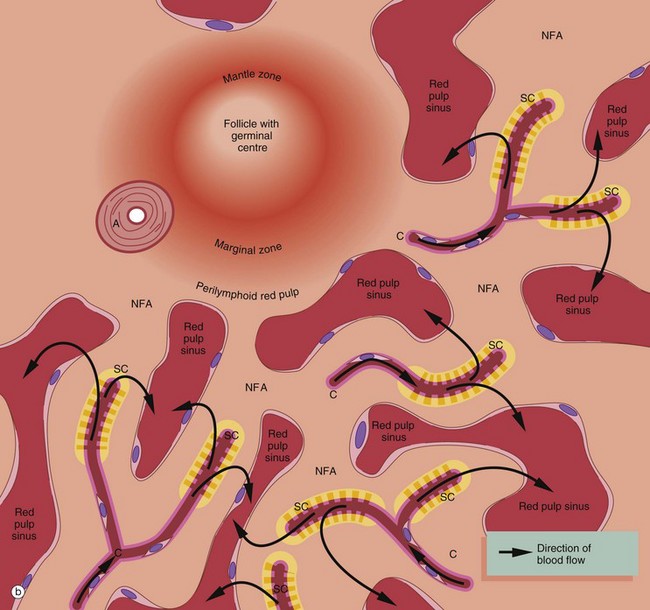
FIG. 11.17 Splenic vasculature and red pulp (illustrations opposite)
An overview of the splenic circulation is shown in diagram (a) and a more detailed view of the red pulp in diagram (b). Blood enters the spleen in the splenic artery SA, which branches repeatedly within the parenchyma (only a few branches are shown for simplicity). The larger arteries are surrounded by a fibrocollagenous sheath that disappears in the smaller branches. These central arteries A are so named because they have a cylindrical cuff of lymphoid tissue around them, the periarteriolar lymphoid sheath PALS, consisting mainly of TH cells. The central artery gives off a number of short branches at right angles, the penicilliary arteries PA, and these terminate in two to three sheathed capillaries SC (only one is shown for each penicilliary artery). These unique vessels are small blind-ending capillaries with no endothelial lining but surrounded instead by an aggregate of macrophages. Thus the blood arriving in a sheathed capillary must traverse this wall of macrophages before entering the red pulp RP. The sheathed capillaries therefore form the first part of the filtering mechanism of the spleen.
Splenic red pulp
The splenic parenchyma is permeated by an interconnected network of sinuses S that drain in turn into larger sinuses, tributaries of the splenic vein SV and finally the hepatic portal vein. The sinuses are lined by endothelial cells resting upon a basement membrane with numerous narrow slits. The reticulin fibres of the sinusoidal basement membrane are arranged in a circular fashion and are continuous with the reticulin meshwork of the parenchyma (see Fig. 11.19b).
Blood cells entering the parenchyma from the sheathed capillaries squeeze through the walls of the sinuses to drain out of the organ via the splenic vein, an arrangement known as the open circulation. The rate of flow in this system approximates the rate through capillaries elsewhere in the body.
Most of the red pulp parenchyma in diagram (b) consists of loose tissue supported by reticulin fibres permeated by capillaries C, terminating as sheathed capillaries SC. The parenchyma removes particulate matter and aged or abnormal erythrocytes from the blood, the defective cells being less deformable and thus unable to negotiate the narrow slits in the sinusoidal basement membrane. Trapped cells are removed by the macrophages of the sheathed capillaries and the parenchyma. The mechanism of recognition of effete red cells is probably based on diminished deformability, but immunological mechanisms may also be involved.
Numerous small patches of the red pulp parenchyma (comprising in total a volume comparable to that of the white pulp) are devoid of capillaries and contain mainly T and B lymphocytes and macrophages. Adjacent sinuses are blind-ended and bulb-shaped and their endothelial lining cells have been shown to have characteristics similar to high endothelial venules of lymph nodes. Lymphocytes probably exit these sinuses to enter these non-filtering areas NFA of the red pulp parenchyma, and these areas should be considered as a functional part of the splenic lymphoid tissue.
Perilymphoid (perifollicular) zones
The zone of red pulp immediately surrounding the white pulp differs from the rest of the red pulp, being devoid of sinuses, having only a sparse reticulin meshwork and containing a large number of red and white blood cells in the same proportion as that of blood. About 10% of blood entering the spleen is believed to pass into this perilymphoid parenchyma, from which it passes much more slowly into the surrounding more widely spaced sinuses than in the rest of the red pulp. The function of these perilymphoid (perifollicular) zones is unclear, but the sluggish blood flow may be a means of enhancing the interaction of blood cells, antigens and antibodies.
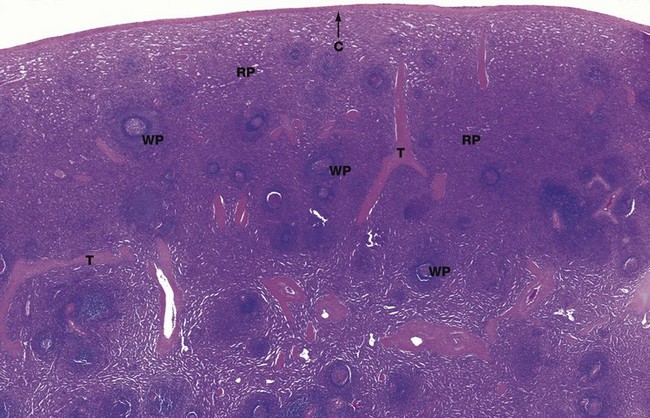
FIG. 11.18 Spleen
H&E (LP)
Macroscopically the spleen appears to consist of discrete 0.5 to 1.0 mm white nodules called the white pulp, embedded in a red matrix called the red pulp. Microscopically, as shown here, the white pulp WP consists of lymphoid aggregations and the red pulp RP, making up the bulk of the organ, is a highly vascular tissue.
The spleen has a thin fibroelastic capsule C which has an outer surface covering of mesothelium (the peritoneum) from which short trabeculae T extend into the parenchyma. The capsule is thickened at the hilum and is continuous with supporting tissues that sheath the larger blood vessels entering and leaving the organ. The spleen has no afferent lymphatics, but efferent lymphatics also exit the spleen at the hilum.
In dogs and horses the spleen is also a reservoir of blood, and these supporting tissues contain smooth muscle to pump blood out; in humans only a few smooth muscle cells persist. The splenic artery divides into several major branches which enter the hilum and branch to form numerous arterioles.
In the white pulp, the T cell areas surround the central arteries, forming the periarteriolar lymphoid sheath (PALS). In humans this lymphoid tissue is less well organised than in other animals, but the term PALS persists.
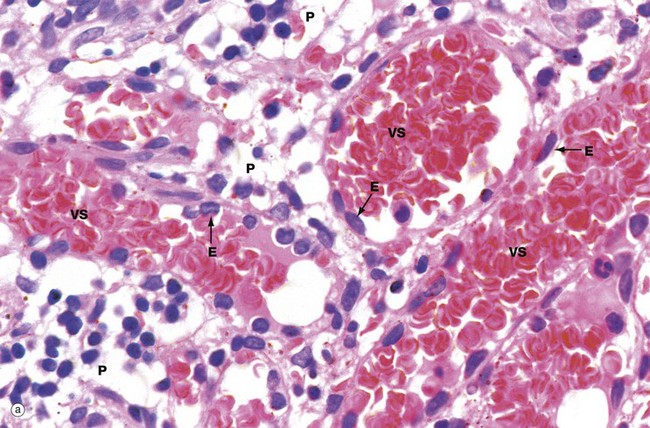
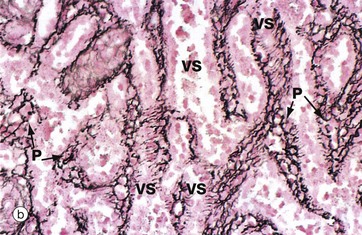
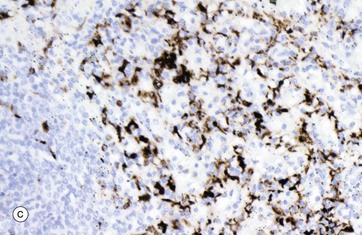
FIG. 11.19 Red pulp
(a) H&E (HP) (b) Reticulin method (MP) (c) Immunohistochemistry for CD68 (MP)
Micrograph (a) illustrates the red pulp, consisting of the parenchyma P permeated by broad interconnected venous sinuses VS. Seen in section, the parenchymal tissue between the sinusoids is considerably narrower than the diameter of the sinusoids and the area occupied by sinuses is greater than that of the parenchyma; in three-dimensional terms, however, the parenchyma makes up 70% of the volume and the sinuses only 30%. The two-dimensional view gave rise to the misleading term cords (of Billroth) to describe the parenchymal tissue. The three-dimensional structure of the red pulp is analogous to a Swiss cheese, with the holes representing the sinuses and the cheese representing the parenchyma.
The parenchyma is composed of the macrophages of sheathed capillaries, other macrophages and blood cells in transit. Non-filtering areas are devoid of sheathed capillaries and contain a greater proportion of lymphocytes. The macrophages are responsible for destruction of aged or damaged blood cells. The different nucleated cell types of the parenchyma cannot be reliably distinguished in this type of preparation.
The venous sinuses are lined by elongated, spindle-shaped endothelial cells E lying parallel to the long axes of the sinuses. The venous sinuses have thus been likened to tall wooden barrels with both ends open, with the endothelial cells represented by the wooden staves and hence described as stave cells. Slits occur between the endothelial cells, the endothelial basement membrane being discontinuous over the slits. Blood cells, particularly viable erythrocytes, squeeze between the stave cells to reach the venous sinuses; these drain into progressively larger vessels that converge to form the splenic vein. Micrograph (b) shows red pulp stained by the reticulin method to demonstrate the supporting framework of the parenchyma P. The basement membranes of the venous sinuses VS show the greatest concentration of reticulin fibres, encircling the endothelium in a manner reminiscent of the steel bands holding together a wooden barrel. Fine reticular strands traverse the parenchyma, linking the whole structure together and providing support for parenchymal macrophages and a small number of fibroblasts responsible for elaboration of the reticulin. In some sinuses, the plane of section is such that the parallel bands of reticulin can be seen encircling the sinuses. Other sinuses are cut in such a way that only the erythrocytes in the lumina are visible. The parenchymal macrophages are demonstrated in micrograph (c), stained brown by the immunohistochemical method. The meshwork structure of the splenic parenchyma is easily seen in this micrograph.
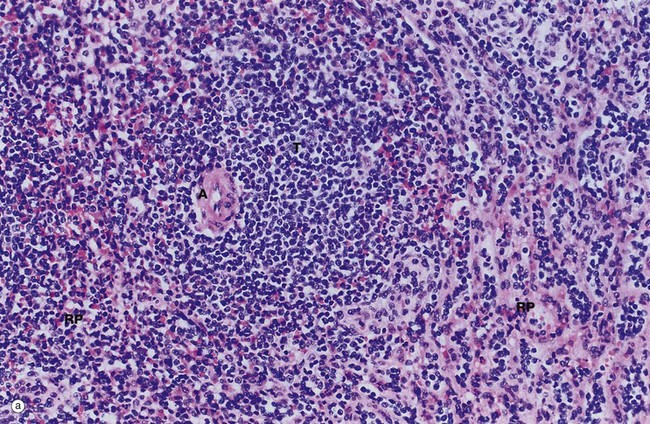
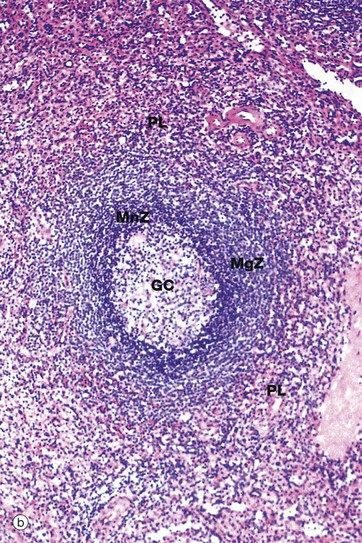
FIG. 11.20 Splenic lymphoid tissue
(a) H&E (HP) (b) H&E (HP)
The splenic white pulp is of two types, T cell and B cell, together making up 5% to 20% of the total mass of the spleen. The functions of these areas appear to be similar to those of the paracortex and superficial cortex of lymph nodes, respectively. The non-filtering areas of red pulp parenchyma (see Fig. 11.17) should probably be considered part of the splenic lymphoid tissue mass also, but its immunological function remains to be elucidated.
Micrograph (a) shows a T cell area typically forming an eccentric cylindrical sheath T around a central artery A and containing small lymphocytes, mainly of the T helper subset. This is equivalent to the periarteriolar lymphoid sheath (PALS) in animals. Note the way the T cell mass merges with the surrounding red pulp parenchyma RP. Small lymphatics arise in the T lymphocyte areas, forming a network around the arterioles and then continuing with the larger arteries to the hilum to drain into a group of adjacent lymph nodes.
B cells form follicles, usually located in at the edge of the PALS, as illustrated in micrograph (b). In young people, many of the follicles exhibit germinal centres GC similar to those of the lymph node, although the proportion of follicles with germinal centres diminishes with age. At the follicle periphery is a narrow zone of small lymphocytes called the mantle zone MnZ beyond which is a broader marginal zone MgZ of less densely packed medium-sized lymphocytes, supported by a framework of reticulin fibres. The marginal zone contains unique subsets of B cells and macrophages. The red pulp around the marginal zone, the perilymphoid red pulp PL, also contains lymphocytes which may simply be migrating from the sinuses to the white pulp.
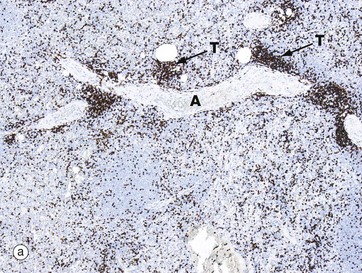

FIG 11.21 Splenic lymphoid tissue
(a) Immunohistochemistry for CD3 (MP) (b) Immunohistochemistry for CD20 (MP)
The distribution of T and B cells in the white pulp of the spleen can be demonstrated using immunostains for T and B cell markers. The section of spleen in micrograph (a) is stained using the T cell marker CD3. Thus the congregation of T cells T around the central artery A is easily identified. As mentioned above, in humans the periarteriolar lymphoid sheath is much less prominent than it is in many other species. A largely unstained primary lymphoid follicle is also noted.
In micrograph (b) the antibody CD20 highlights the B cell follicles. As in lymph nodes, unstimulated follicles consist of homogeneous lymphoid aggregates as shown here PF with no germinal centers, in contrast to the stimulated lymphoid follicle in Fig. 11.20b. The unstained T cell zone T is also easily identified.