Blood, haematopoiesis and bone marrow
Introduction
Blood is a suspension of cells in fluid. It is circulated around the body by the heart and, as a result of this circulation, blood serves as the transport vehicle for gases, nutrients, waste products, cells and hormones.
The fluid is known as plasma and a typical sample is composed of 90% water, 8% protein, 1% inorganic salts, 0.5% lipids, 0.1% glucose and other minor components. The proteins are numerous and diverse, including albumin, blood coagulation factors, anti-proteases, transport proteins and antibodies (immunoglobulins). Collectively, these proteins exert a water-binding effect known as colloidal osmotic pressure which helps regulate the distribution of fluid between the plasma and the extracellular space, serving to keep the fluid in the circulation.
Plasma components, including hormones, lipids, salts, water molecules and small proteins, are constantly exchanged with the extracellular fluid of body tissues in accordance with the blood's transport functions. Proteins and plasma are not demonstrated by light microscopy except as a background colour.
Blood Cell Types
The cellular components of the blood are:
• Red blood cells (erythrocytes) are specialised cells containing the red pigment haemoglobin. They provide most of the oxygen transport from the lungs and much of the return carbon dioxide carriage. They are immotile and serve their function only as the result of being passively circulated around the vascular system. The fraction of blood (by volume) occupied by erythrocytes is called the haematocrit and is in the range of 0.35 to 0.50 in adults.
• White blood cells (leucocytes) constitute an important part of the defence and immune systems of the body but undertake these functions mainly in the tissues; leucocytes in circulating blood are in transit or are simply waiting in reserve.
• Platelets (thrombocytes) are specialised cells which bind to and coat damaged vessel walls, plug small defects in blood vessel walls and help activate the blood-clotting cascade. They are essential for haemostasis, the system that controls bleeding.
In adults these cells are formed in the bone marrow, a process known as haemopoiesis or haematopoiesis.
Methods Used to Study Blood and Bone Marrow Cells
Blood cell numbers are now usually counted by sophisticated laboratory analysers. Blood cell morphology is examined by microscopy using cytological methods. A standard method is to place a drop of blood on a glass slide and spread (smear) it into a very thin layer one cell thick. This smear is then air dried, which results in the cells spreading like fried eggs, making the cells appear larger and giving a clear view into the thinned cytoplasm. These smears are alcohol fixed and stained with Romanowsky-type stains which use polychromatic dyes containing multiple molecular variants to give subtle complexity in the staining. These are the best stains for morphology of blood and bone marrow; common examples are Giemsa and Wright. Distinctive staining characteristics are easily identified and reflect the affinity of the various cellular organelles for different stain components:
• Basophilia (deep blue) - affinity for the basic dye methylene blue; DNA in nuclei and RNA in ribosomes.
• Azurophilia (purple) - affinity for azure dyes; typically lysosomes, one of the granule types in leucocytes.
• Eosinophilia (pink/red) - affinity for the acidic dye eosin, thus also described as acidophilia.
• Neutrophilia (salmon pink/lilac) - affinity for a dye once erroneously believed to be of neutral pH; characteristic of the specific cytoplasmic granules of neutrophil leucocytes.
Examination of haematopoietic bone marrow in adults involves sampling from the axial skeleton, usually the iliac crest in the pelvis, with an aspiration sample and often a bone core. Aspiration samples, but not usually bone cores, can also be obtained from the sternum. Aspirated tissue fragments are smeared and stained like blood. Larger tissue pieces and cores of bone are examined as histology preparations, often stained with haematoxylin and eosin (H&E).
White Cell Series
Five types of leucocytes are normally present in human blood and are classified into two groups:
Granulocytes
Granulocyte types are named for the staining characteristics of their prominent type-specific cytoplasmic granules: neutrophil (lilac), eosinophil (red) and basophil (blue). Granulocytes have a single nucleus segmented into multiple lobes, assuming variable shapes that led to the use of the term polymorphonuclear leucocytes or polymorphs, mainly because early microscopists mistook the multi-lobed nuclei for multiple nuclei. The term polymorph is sometimes used as a synonym for granulocyte. To confound matters further, polymorph is often used specifically for neutrophils as they are the commonest granulocyte. Granulocytes are also referred to as myeloid cells due to their origin from bone marrow, but they are not the only white blood cells to be formed in bone marrow.
Granulocytes are important components of the innate (non-learned) defences against infection (see Ch. 11); however, this role usually takes place in the tissues, not in blood. All leucocytes carry surface proteins which can bind to receptors on the endothelial cells of blood vessels (see Ch. 8) and, via this binding, granulocytes actively adhere to vessel walls and then migrate into the tissues using pseudopodial movement.
With the exception of eosinophils entering intestinal mucosa, granulocytes do not normally enter tissues in any number; they just circulate. They enter tissues in response to chemotactic signals and due to changes in the expression of endothelial cell surface receptors, induced by mediators of acute inflammation.
Neutrophils are highly phagocytic. They engulf and kill microorganisms and ingest cell debris and particulate matter. These cells release multiple important pro-inflammatory chemical signals and regulators contributing to the overall inflammatory process. Granulocytes have a short ‘one-shot’ functional life; having left the circulation they die in the tissues and do not re-enter the blood.
Lymphocytes and monocytes
Lymphocytes and monocytes have non-lobulated nuclei and were described as mononuclear leucocytes by early microscopists to distinguish them from the polymorphs.
Lymphocytes play a key role in all immune responses, facilitating and regulating inflammation. In contrast to other leucocytes, their activity is directed toward specific foreign agents (antigens), providing a learned and targeted response, both antibody- and cell-mediated. Lymphocytes routinely traffic through tissues, then through lymphatics and lymph nodes and finally re-enter the circulation, providing routine surveillance against foreign antigens. They have an indefinite lifespan and are capable of proliferation.
Monocytes are highly phagocytic cells, ingesting micro-organisms, cell debris and particulate matter. They routinely enter some tissues, can mature into macrophages (including becoming resident tissue macrophages) and can have extended tissue survival.
Monocytes and lymphoid cells produce, secrete and have receptors for a large number of inflammation-related chemical mediators.
Haematopoiesis
Haematopoiesis is the process of production of mature blood cells from precursors. This is a major task as an adult produces 100 × 109, i.e. a hundred billion, granulocytes each day. This production is ultimately derived from the pluripotential stem cells and their more differentiated offspring, the haematopoietic stem cells. Stem cells are the cells which provide the lifelong reserve; they actively maintain their own population (self-renewing). They also proliferate and differentiate, contributing to the maintenance of the next level of more mature differentiated populations of stem cells or progenitors. As we move through the process, the emphasis for these cells progressively shifts from self-renewal to proliferation and differentiation.
These cells have been extensively studied in animal transplantation models and in laboratories, in short and long-term culture systems. Many of the identified precursors have been named colony forming units (CFU) or burst forming units (BFU) and the regulatory molecules have been named colony-stimulating factors (CSF), reflecting this laboratory background. Other regulatory molecules have been called interleukins, cytokines or growth factors, depending on their laboratory histories.
The stem cells, progenitors and the later differentiated forms all depend on a complex microenvironment for growth, proliferation and differentiation. This microenvironment is physical, with cell-cell attachments, signalling by these attachments and local secretion of growth factors. The analogy of seed (stem cell) and soil (microenvironment) has often been used.
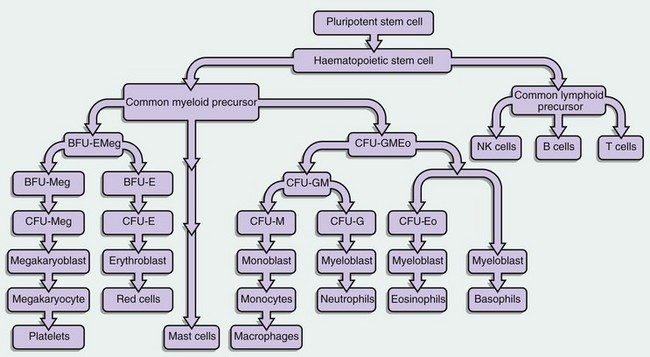
FIG. 3.1 Haematopoietic stem cells and progenitors
This diagram illustrates some of the identified stem cells and precursors in haematopoiesis. The haematopoietic stem cell (HSC) differentiates into a common lymphoid precursor and a common myeloid precursor (CMP) also known as the CFU-GEMM (CFU–granulocyte, erythroid, monocyte, megakaryocyte).
This common myeloid precursor differentiates into a common erythroid and megakaryocyte progenitor, the BFU-EMeg (BFU–erythroid megakaryocyte) and into a combined granulocyte and monocyte precursor, the CFU-GMEo (CFU–granulocyte, monocyte, eosinophil).
These precursors differentiate as shown in the diagram into a committed precursor type for each individual lineage. BFU- and CFU-GM precursors produce large numbers of progeny (hundreds to thousands), while the individual lineage CFU- precursors may only have 20 to 50 progeny.
Each recognisable blast in a bone marrow smear (other than megakaryocyte lineage) might average 16 offspring when counted as the final differentiated cells.
The mast cell derivation pathway is unclear but is believed to be of early myeloid lineage.
Major haematopoietic growth factors
Haematopoiesis is tightly controlled by growth factors and the microenvironment. Growth factors, with the possible exception of erythropoietin, are multifunctional. A single growth factor will influence several different stages of several lineages and promote proliferation and/or differentiation, maturation, egress from bone marrow and survival in tissue. The differences in effect are due to the developmental stage of the cell on which the growth factor is acting, combined with its differentiation, surface receptors and the multitude of other signals it is receiving (see Table 3.1).
The haematopoietic stem cell (HSC) is particularly influenced by stem cell factor (SCF), Flt-3 ligand and vascular endothelial growth factor (VEGF), while the supporting stromal cells of the microenvironment are responsive to interleukin-1 (IL-1) and tumour necrosis factor (TNF). These stem cells are not geographically constrained; they normally circulate in blood in small numbers. Granulocyte-CSF (G-CSF) will mobilise stem cells into the circulation in large numbers. These circulating stem cells then home to sources of stromal derived factor-1 (SDF-1), a product of the haematopoietic microenvironment.
Major generic drivers for haematopoiesis in general are interleukin 3 (IL-3), granulocyte-monocyte CSF (GM-CSF) and stem cell factor (SFC); these promote most lineages at most stages. Thrombopoietin, produced in the kidney and liver, is important for megakaryocyte and platelet production and also promotes the early stages of red cell production. Erythropoietin, a protein hormone produced mostly in the kidneys, drives the later part of red cell production (from CFU-E) but has little effect on the early red cell progenitors. Granulocyte CSF (G-CSF) and monocyte CSF (M-CSF) promote granulocyte and monocyte lineages while interleukin-5 (IL-5) helps drive eosinophil production.
These stem cells and progenitor cells are not recognisable by microscopy. Many resemble lymphocytes and are only identifiable by their expression of different combinations of cell surface molecules. The earliest committed cells recognised in marrow smears are the blasts e.g. myeloblasts, proerythroblasts, monoblasts, etc. These are quite late in the process, about to undertake final proliferation and differentiation. A BFU-E takes about 7 days to become very many CFU-E; each CFU-E takes about 7 days to become many recognisable proerythroblasts and each proerythroblast will form about 16 mature red cells, taking 6 to 7 days. Note that the adult requirement is for about 2.5 billion red cells/kg body weight each day. Many of these growth factors are now available and are used therapeutically.
TABLE 3.1
Major haematopoietic growth factors
| Factor | Abbreviation | Cells affected |
| Stem cell factor | SCF | All |
| Granulocyte-monocyte colony-stimulating factor (CSF) | GM-CSF | Most |
| Interleukin-3 | IL-3 | All |
| Interleukin-11 | IL-11 | Megakaryocyte production |
| Interleukin-5 | IL-5 | Eosinophil production |
| Granulocyte CSF | G-CSF | Granulocyte production |
| Monocyte CSF | M-CSF | Monocyte production |
| Thrombopoietin | TBO | Megakaryocyte and red cell production |
| Erythropoietin | EPO | Red cell production |
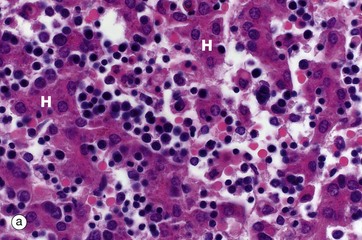
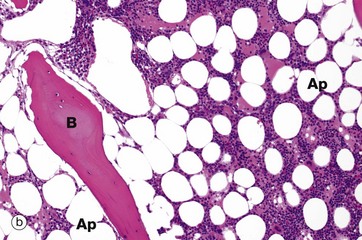
FIG. 3.2 Haematopoiesis in liver and bone marrow
(a) H&E (MP) (b) H&E (LP)
Haematopoiesis begins in early intrauterine life in an embryonic organ, the yolk sac. It soon becomes established in the liver sinusoids. These are the vascular spaces between the hepatocytes H, in micrograph (a), where numerous small, darkly staining haematopoietic cells are seen. This is the dominant site of haematopoiesis from 3 to 7 months gestation. As bones develop, haematopoiesis establishes in the spaces between bone trabeculae B in all bones and, by birth, this provides sufficient space for all the haematopoiesis so that extramedullary haematopoiesis comes to an end. With growth through childhood, bone marrow space increases faster than total body growth and, increasingly, the marrow become occupied by adipocytes Ap (fat cells). Haematopoietic marrow has a macroscopic red colour, while adipocyte-dominated marrow is yellow. By early adulthood, most of the marrow in the limb bones is yellow marrow, while the axial skeleton remains red and haematopoietic, although usually with 30% to 60% of the volume being admixed adipocytes. Micrograph (b) shows adult vertebral marrow with moderate numbers of adipocytes.
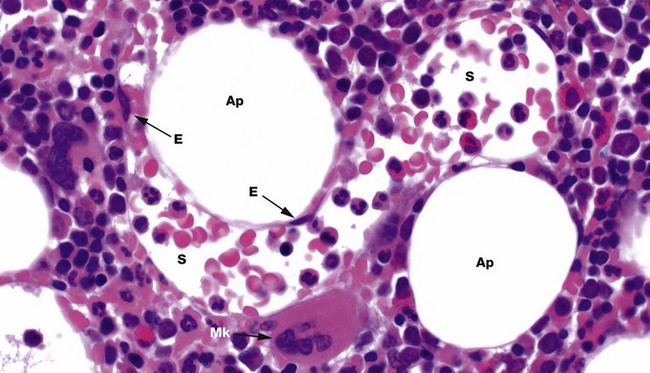
FIG. 3.3 Bone marrow sinusoids
H&E (HP)
Bone marrow has a framework of vascular sinusoids lined by endothelial cells E and intervening spaces called marrow cords, supported by a meshwork of fibroblastic cells with long, branched cytoplasmic processes (reticular cells) and reticulin fibres (type III collagen). Macrophages are numerous and may also have long, branched processes. Adipocytes Ap and plasma cells are present in significant numbers. The result is a microenvironment which supports haematopoiesis and the marrow cords are correspondingly cellular. Megakaryocytes Mk sit next to the sinusoids S so their cytoplasmic processes, proplatelets, can be released into the circulation. Erythroblastic islands also sit next to sinusoids, with red cell precursors adhering to long macrophage processes that direct red cells to, and control egress into, the sinusoids. Granulocyte precursors tend to sit away from the sinusoids, but these are motile cells which can migrate into sinusoids.
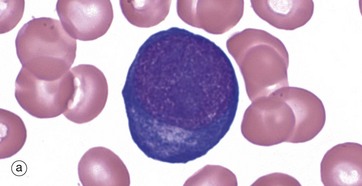
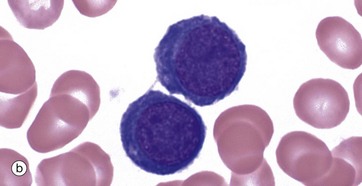
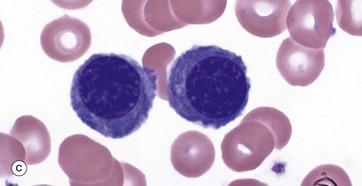
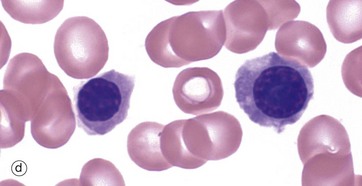
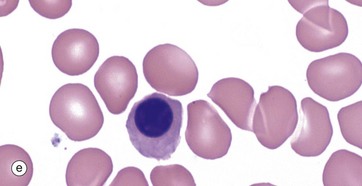
FIG. 3.4 Erythropoiesis
(a–e) Giemsa (HP)
These micrographs from a bone marrow smear illustrate the stages of erythropoiesis. The process of erythropoiesis involves:
• Progressive reduction in size and cytoplasmic organelles, helped by cell divisions diluting cytoplasmic contents in the early stages.
The proerythroblast, micrograph (a), is the first recognisable erythrocyte precursor; the cell is large with fine, granular nuclear chromatin containing one or more paler nucleoli. The relatively sparse cytoplasm is strongly basophilic due to its high content of RNA and ribosomes. A narrow, pale zone of cytoplasm close to the nucleus represents the Golgi apparatus. Proerythroblasts divide and differentiate, producing smaller cells called basophilic erythroblasts or early normoblasts, micrograph (b). These are smaller cells with some condensation and clumping of chromatin.
The next morphological forms are called polychromatic erythroblasts (intermediate normoblasts). In these cells the cytoplasm develops a grey colouration due to increasing cytoplasmic haemoglobin. As this is a mixture of basophilia and eosinophilia, the term polychromasia is applied. The nuclear chromatin becomes increasingly condensed. These cells are no longer capable of division. Micrograph (c) shows an early example and parts of several white cell precursors while micrograph (d) shows two late examples.
The final nucleated form, micrograph (e), is the orthochromatic erythroblast (late normoblast). The cytoplasm is rich in haemoglobin but still contains ribosomes with continuing haemoglobin synthesis. Cytoplasmic organelles are degenerate. The nuclear chromatin, and nucleus, becomes extremely condensed; the nucleus is then extruded. The result is an anucleate early red cell, the reticulocyte (Fig. 3.5).
The process of nuclear condensation and extrusion may be incomplete, leaving small, spherical, condensed nuclear remnants in the red cells. These are known as Howell-Jolly bodies; they are normally pinched off from red cells in a small envelope of plasma membrane by splenic macrophages and are not found in blood. However, in persons who have had a splenectomy, it is normal to find small numbers of red cells containing Howell-Jolly bodies in a blood smear.
Red cells share a common progenitor with megakaryocytes. Proliferation and differentiation to produce red blood cells is stimulated by the growth factor erythropoietin, produced mostly in kidneys, although early stages are also supported by thrombopoietin and some interleukins.
In bone marrow histology, red cell precursors are found in small scattered clusters called erythroblastic islands. Each of these is centered on a macrophage with long cytoplasmic processes and membrane folds which accommodate the precursors. Precursors move outward along the processes as they mature, reaching the sinusoidal endothelium whence they enter the circulation. These macrophages provide cell-cell contact and signalling, and control the release of maturing red cells into sinusoids.
Each proerythroblast will generally become 16 red blood cells over 5 to 7 days. Each of these micrographs shows numerous mature red blood cells in the smears, with absence of nuclei and often central pallor.
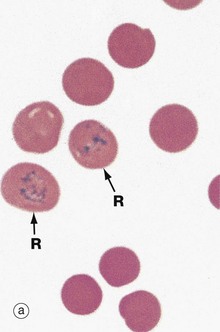
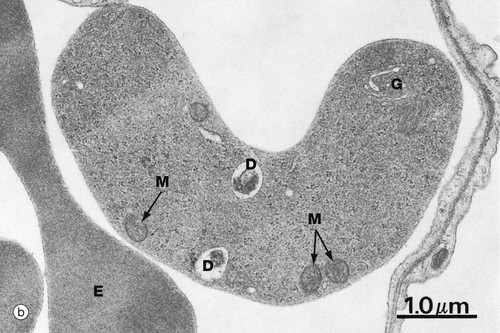
FIG. 3.5 Reticulocytes
(a) Cresyl blue/eosin (HP) (b) EM ×16 000
Reticulocytes are immature red blood cells and are the form in which erythrocytes are released from the bone marrow. They still contain mitochondria, ribosomes and Golgi elements and continue to synthesise haemoglobin. Final maturation into erythrocytes occurs within 48 hours of release. The rate of release of reticulocytes into the circulation generally equals the rate of removal of spent erythrocytes by spleen and liver. Since the lifespan of circulating erythrocytes is about 120 days, reticulocytes constitute slightly less than 1% of circulating red blood cells. Reticulocytes are slightly larger than mature erythrocytes and their staining is slightly basophilic due to the ribosomes and RNA. Increased reticulocytes can be suspected from a blood film by the varying basophilia of the cells on a smear, a finding called polychromasia (many colours).
Reliable reticulocyte identification and counting requires special techniques such as supravital staining, illustrated in micrograph (a). A fresh blood sample is incubated with the basic dye, brilliant cresyl blue, resulting in a blue-stained reticular precipitate R in reticulocytes, due to the interaction of the dye with RNA. Diagnostic identification and counting of reticulocytes is now usually done using automated laboratory systems such as flow cytometry.
Micrograph (b) shows the ultrastructure of a reticulocyte, with part of an adjacent mature erythrocyte E for comparison. Overall, the cytoplasmic density is lower due to a lower concentration of haemoglobin. Scattered ribosomes can still be seen, as well as a few mitochondria M, occasional degenerating mitochondria D and a small Golgi remnant G.
When severe erythrocyte loss occurs, such as after haemorrhage or haemolysis, the rate of erythrocyte production in the bone marrow increases and the proportion of reticulocytes in circulating blood rises (reticulocytosis). Clinically, an elevated reticulocyte count indicates functional marrow, while a decreased count may mean impaired production. This is important in the investigation of anaemia.
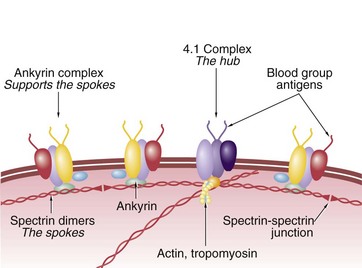
FIG. 3.6 Red cell cytoskeleton
Diagram
The erythrocyte plasma membrane is composed of a lipid bilayer, stabilised by various proteins. Blood group substances are carbohydrate and protein antigens on the surface. Spectrin is a long, dimeric, springy protein which forms a meshwork like a geodesic dome just inside the plasma membrane. Spectrins form the spokes and attach to membrane-bound hubs containing protein 4.1, actin and tropomyosin proteins among others. Each spoke is further supported along its length by a second membrane protein complex containing ankyrin. At the end away from the hubs, the spectrin spokes form non-covalent spectrin-spectrin junctions. As the red cell membrane bends, the spectrin molecules stretch. When the deformation becomes sufficiently severe, the spectrin-spectrin dimeric junctions separate, allowing the hub and spoke complexes to separate and rearrange, dynamically reshaping the cytoskeleton. The actin is part of a contractile segment, possibly applying tension, to the spectrins.
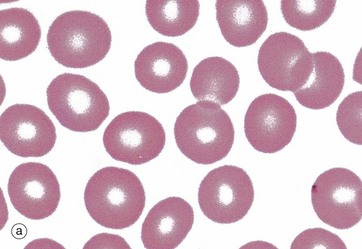
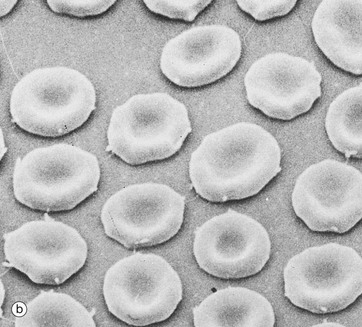
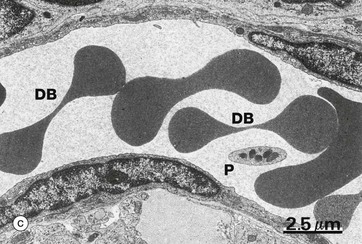
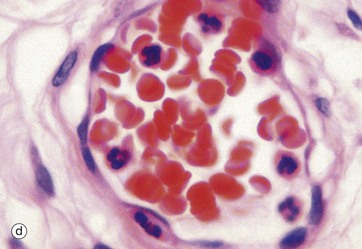
FIG. 3.7 Erythrocytes
(a) Giemsa (HP) (b) Scanning EM ×2400 (c) EM ×6000 (d) H&E (HP)
The erythrocyte is highly adapted for its principal function of oxygen and carbon dioxide transport. It simply consists of an outer plasma membrane with a supporting protein cytoskeleton, enclosing concentrated haemoglobin molecules and a limited number of enzymes for cell maintenance. Haemoglobin is an iron-containing protein which binds and releases oxygen and provides most of the oxygen transport capability in blood.
Micrograph (a) demonstrates the characteristic appearance of erythrocytes in a stained spread (smear) of peripheral blood. The pale staining of the central region is a result of a biconcave disc shape, better seen in scanning electron microscopy (SEM), micrograph (b). The biconcave disc shape provides a 20% to 30% greater surface area than a sphere relative to cell volume, thus facilitating gaseous exchange. This shape, along with the flexibility of the cytoskeleton (see Fig. 3.6 opposite) allows the erythrocyte to deform readily, and erythrocytes of average diameter (7.2 µm) are able to squeeze through small capillaries 3 to 4 µm in diameter. The biconcave shape is determined not only by the cytoskeleton but also by its electrolyte and water content and the lipid composition of the membrane.
The transmission EM (c), illustrates erythrocytes within a capillary. The classic dumbbell shape DB is seen when the plane of section is through the thin central zone in the middle of the cell. Note the absence of internal organelles and the high electron density due to the iron atoms in the haemoglobin. There is also a platelet P in this image.
Red cells have a high affinity for eosin and appear intensely orange-red in H&E-stained tissue sections, micrograph (d). In smears the staining varies with the type of Romanowsky stain used but is generally red-brown, red or grey, micrograph (a).
The transport of oxygen by haemoglobin is not dependent on erythrocyte metabolism. However, erythrocytes use energy to maintain electrolyte gradients across the plasma membrane and to protect from and reverse oxidative injury. The energy required is derived from anaerobic metabolism of glucose; they have no mitochondria.
The lifespan of an erythrocyte averages 120 days. Without the appropriate organelles, erythrocytes are unable to replace deteriorating enzymes and membrane proteins and repair damage. Misshapen red cells and cells with inflexible cytoskeletons are removed from the circulation by spleen and liver macrophages.
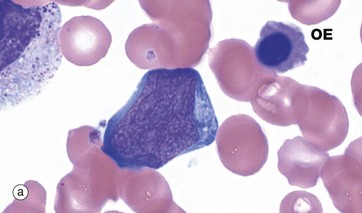
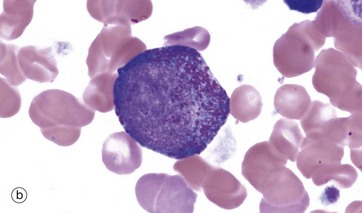
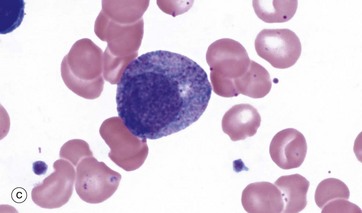
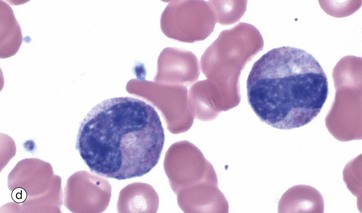
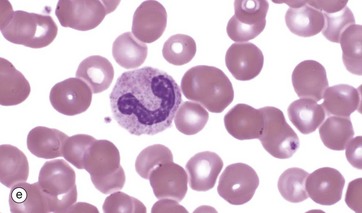
FIG. 3.8 Granulopoiesis
(a–e) Giemsa (HP)
This series of photographs illustrates the development of neutrophil granulocytes. The stages for eosinophils and basophils are similar.
The myeloblast, micrograph (a), is the earliest recognisable stage in granulopoiesis. This name is inappropriate and was derived from an incorrect view that granulocytes were the only white cells formed in myeloid tissue (bone marrow). Myeloblasts are large cells with open chromatin, several large nucleoli and basophilic cytoplasm. More differentiated myeloblasts (myeloblasts II) have small numbers of primary azurophilic (purple) granules. While the ultimate differentiation of these cells has been biologically determined, this is not visible until the myelocyte stage (see below), when production of secondary (specific) granules enables identification. An orthochromatic erythroblast OE is noted in this image.
Promyelocytes, micrograph (b), are the next stage and have abundant primary (azurophilic) granules. They may show slight chromatin condensation in an otherwise fine, open chromatin pattern.
Myelocytes, micrograph (c), are identified by the development of secondary or specific granules and progressive chromatin condensation; this process continues through several further cell divisions. The number and proportion of primary granules is progressively decreased, diluted by the repeated divisions of the cytoplasm, whilst specific granules are progressively produced. The myelocyte illustrated is a neutrophilic myelocyte and shows pink cytoplasmic colouration due to the neutrophil secondary granules. Eosinophil myelocytes will have eosinophil-specific granules and so on.
The metamyelocyte, micrograph (d), is now an end cell, incapable of cell division. It begins nuclear segmentation with an increasingly indented nucleus and shows progressive cytoplasmic maturation. Immediate precursors of mature granulocytes tend to have an irregular horseshoe nucleus and are termed stab cells or band forms, micrograph (e).
Immature neutrophils enter a functional reserve pool in the marrow which is equivalent to about 5 days of neutrophil production. Then, on entry to the circulation, about half these band forms circulate whilst the rest adhere to the endothelial walls of small vessels, forming a marginated pool. These pools provide sizeable reserve resources and are mobilised on demand (e.g. by chemotaxins).
If the demand is sufficiently extreme, metamyelocytes and myelocytes are also mobilised into the circulation and hence into the tissues; this has been called left shift. The alternative of increased maturation is called right shift but is not commonly seen.
The normal development process in marrow from myeloblast to myelocyte takes 6 days and from myelocyte to release of neutrophil into blood another 7 days. Production is driven by a range of growth factors and cytokines, including G-CSF, GM-CSF, IL-3, and IL-5.
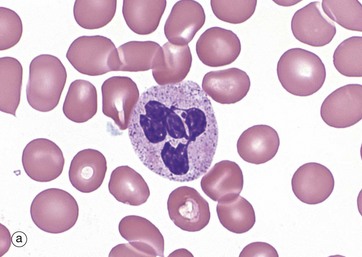
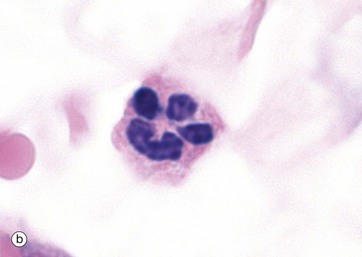
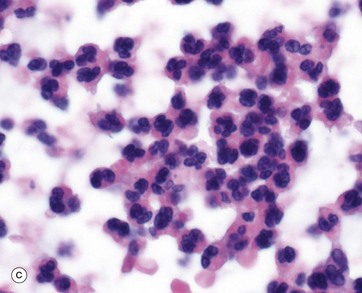
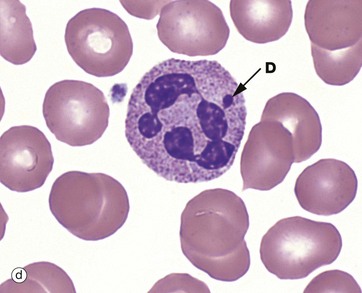
FIG. 3.9 Neutrophils
(a) Giemsa (HP) (b) H&E (HP) (c) H&E (MP) (d) Giemsa (HP)
Neutrophils account for 40% to 60% of the leucocytes in the circulating blood, with 1.0 to 5.0 × 109/L. They are 12 to 14 µm in diameter. The lifespan of a neutrophil is a few days and they are rarely found in normal tissue.
Neutrophils exhibit progressive segmentation of their nucleus, with a young cell having 2 lobes, the average cell 3 to 4 lobes and older cells 5 lobes. They have a lightly stippled granular pink cytoplasmic appearance due to numerous small membrane-bound granules (0.2 to 0.8 µm in diameter), micrograph (a). These granules include the azurophilic primary granules (purple) and the specific secondary granules (pink/lilac), tertiary granules and secretory granules. In H&E stains, they have pink or pale red cytoplasm, micrograph (b).
Neutrophils leave the vascular space in response to chemotactic signals generated by inflammation. They are highly motile, phagocytose bacteria and kill them by fusing the phagosome with neutrophil primary granules and producing activated oxygen derivatives. Under certain conditions, they degranulate, releasing granule contents including inflammatory mediators, antibacterial enzymes and tissue matrix breakdown enzymes. Massed neutrophils and their debris in tissue are visually recognised as pus, micrograph (c). Neutrophils do not re-enter the blood stream from tissue but undergo lysis or apoptosis in tissues.
As an incidental finding the inactivated X chromosome in females is seen as a small drumstick-shaped appendage D in a few (3%) percent of neutrophils, micrograph (d).
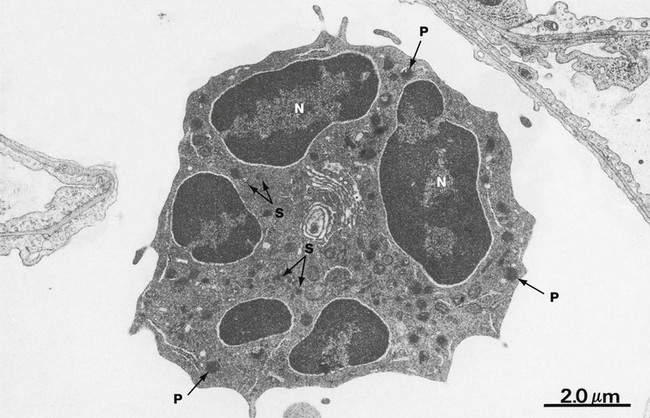
FIG. 3.10 Neutrophil
EM ×10 000
With electron microscopy, neutrophils have three distinguishing features. Firstly, multiple nuclear lobes N with condensed chromatin; these lobes are seen as separate in the thin EM sections. Secondly, the cytoplasm contains many membrane-bound granules. The primary granules P are large, spheroidal and electron-dense. The secondary or specific granules S are more numerous, small and often rod-like and are of variable density and shape. Tertiary and secretory granules cannot be readily distinguished from other membrane-bound vesicles on ultrastructure. The third feature is that other cytoplasmic organelles are scarce. Additionally, the cytoplasm is particularly rich in dispersed glycogen.
The mature neutrophil has few organelles for protein synthesis and has a limited capacity to regenerate secreted proteins; it tends to degenerate after a single burst of activity. The paucity of mitochondria and the abundance of glycogen in neutrophils reflect the importance of the anaerobic mode of metabolism. Energy production via glycolysis permits neutrophils to function in the poorly oxygenated environment of damaged tissues. Neutrophils are highly motile cells, moving through the extracellular spaces in a crawling fashion with an undulating pseudopodium typically thrust out in the line of advance. Motility and endocytotic (phagocytic) activity are reflected in a large content of the contractile proteins, actin and myosin, as well as tubulin and microtubule-associated proteins.
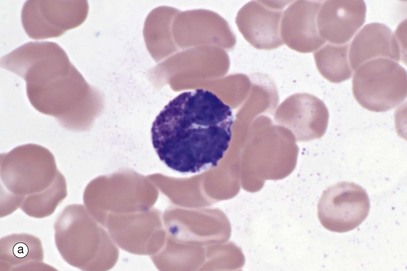
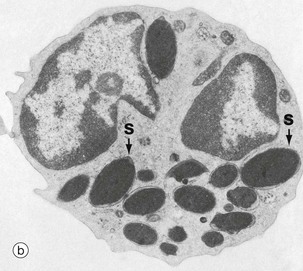
FIG. 3.11 Basophils
(a) Giemsa (HP) (b) EM ×10 500
Basophils, micrograph (a), are the least common leucocyte, constituting <0.5% of leucocytes in blood; they are intermediate in size (14-16 µm in diameter) between neutrophils and eosinophils. A mature basophil has a bilobed nucleus, but this is usually obscured by numerous large, densely basophilic (deep blue) specific granules which are larger, but fewer in number, than those of eosinophils. The granule contents are highly soluble in water and may be dissolved away during preparation. Basophils are formed in the bone marrow, sharing a common precursor with eosinophils and develop through stages analogous to neutrophils and eosinophils. Basophils enter tissue in response to inflammation and chemotaxins. They are not believed to re-enter the blood. Their tissue half-life is uncertain.
When stained with the basic dye toluidine blue, the granules of basophils bind the blue dye but stain a purple colour. The phenomenon of staining a different colour to that of the dye is called metachromasia (see Fig. 4.20).
With electron microscopy, micrograph (b), the basophils show their large, slightly variable, oval specific (secondary) granules filled with electron-dense material. Crystalloids, lipid whorls and dense inclusions can be found in the granules (not illustrated).
The granules have a core of sulfated glycosaminoglycans, specifically chondroitin sulfate and some heparan sulfate; this is responsible for their staining characteristics. Functionally, the granules contain histamine, other vasoactive chemicals and enzymes (see Table 3.3).
Mast cells are tissue cells with many similarities to basophils, also derived from bone marrow, while remaining a different and distinct cell type (see Fig. 4.20.)
TABLE 3.3
Functional products of basophils
| Products | Actions |
| Major basic protein | Same protein as eosinophils; toxic to parasites |
| Charcot-Leyden protein | As per eosinophils, but in small amounts |
| Histamine and other vasoactive amines | Vasoactive; congestion and oedema |
| Tryptase | Enzyme, useful blood marker for basophils and mast cell activity |
| Carboxypeptidase | Enzyme |
| Leukotrienes and prostaglandins | Lipid mediators |
| Interleukin (IL)-4, IL-13, exotaxins | Cytokine release |
| Vascular endothelial growth factor (VEGF) | Growth factor release |
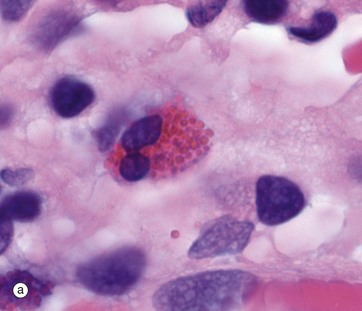
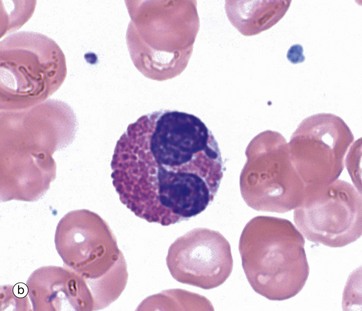
FIG. 3.12 Eosinophils
(a) H&E tissue (HP) (b) Giemsa blood (HP)
Eosinophils account for 1% to 6% of leucocytes in circulating blood; their numbers exhibit diurnal variation, being greatest in the morning and least in the afternoon. The production of eosinophils by the bone marrow is controlled by the cytokine interleukin 5 (IL-5) and to a lesser extent, interleukin 3 (IL-3) and granulocyte-monocyte colony-stimulating factor (GM-CSF). Eosinophils circulate in the blood for approximately 18 hours and exit from capillaries to enter the tissues, where the majority (>95%) of eosinophils reside.
Under normal conditions, tissue-based eosinophils are found in the mucosa of the gastrointestinal tract, mainly the intestine. Small numbers, probably in transit, are found in the spleen and lymph nodes.
Eosinophils enter other tissues in response to chemotactic signals generated by mucosal inflammatory or allergic responses; these signals include chemokines eotaxin-1 and eotaxin-2, IL-5 and some leukotrienes. The process of egress from vessels partially activates the eosinophils and they are further activated by mediators released as part of T helper cell type 2 immune responses (TH2), including IL-5, IL-3 and GM-CSF. Appropriate stimuli cause granule and mediator release. Unlike neutrophils, they are not phagocytic cells.
Eosinophils are believed to survive in tissues for extended periods (8-12 days and longer), but experimental data are limited. Eosinophils do not generally recirculate; from the intestine they exit into the bowel lumen or otherwise undergo lysis.
The eosinophil (12-17 µm in diameter) is larger than the neutrophil and is easily recognised by its numerous large specific granules, which stain bright red with eosin, micrograph (a), and a more brick-red with Romanowsky methods, micrograph (b). Most cells have a bilobed nucleus, but as cells mature in tissue, the nucleus can further segment. The densely packed cytoplasmic granules may partially obscure the nucleus.
The specific granules are membrane bound, of uniform size, with a matrix and a crystalloid cubic lattice structure (see Fig. 3.13 opposite). They contain extremely alkaline (i.e. basic) proteins, especially the major basic protein. Other proteins include eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN) and an eosinophil peroxidase (EPO). These proteins are toxic to parasites, some RNA viruses and, in certain circumstances, tissues.
TABLE 3.4
Functional products of eosinophils
| Product | Function |
| Major basic protein | Toxic to parasites; degranulates mast cells and basophils |
| Eosinophil-derived neurotoxin (EDN) | Ribonuclease with antiviral activity |
| Eosinophil cationic protein (ECP) | Cell membrane injury; mast cell degranulation |
| Eosinophil peroxidase (EPO) | Generation of reactive oxygen species, including superoxide, and hypobromic acid (from bromide ions) |
| Histaminase, phospholipase, acid phosphatase, arylsulphatase, cathepsin | Enzyme |
| Leukotrienes and prostaglandins | Lipid mediators |
| Interleukin (IL)-1, IL-2, IL-4, IL-5, IL-8, IL-13, tumour necrosis factor (TNF)-α | Cytokines |
| Transforming growth factor beta (TGF-β), TGF-α, vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) | Growth factors |
| Charcot-Leyden crystal protein (galectin-10) | Unknown, but can form crystals in tissue |
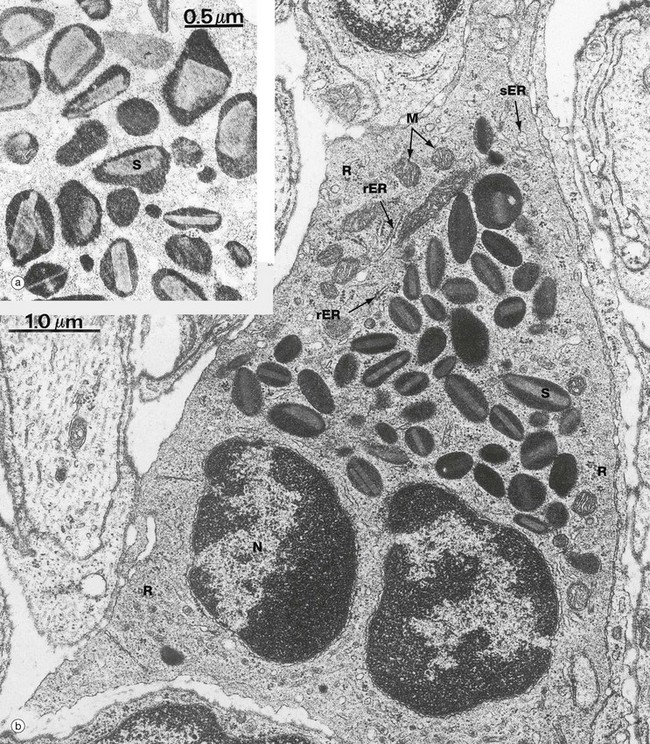
FIG. 3.13 Eosinophil
(a) EM ×25 000, human (b) EM ×20 000, mouse
On electron microscopy, these cells are dominated by the large, ovoid, specific granules S, each containing an elongated crystalloid. In humans, as illustrated in micrograph (a), the crystalloids are relatively electron-lucent and irregular in form, but in many other mammals they have a more regular discoid shape. Micrograph (b) shows an eosinophil within the tissues of a mouse; in this species the crystalloids are also relatively electron-lucent. Eosinophils have only small numbers of mitochondria and extensive smooth and some rough endoplasmic reticulum sER and rER. Note also the free ribosomes R and the bilobed nucleus N.
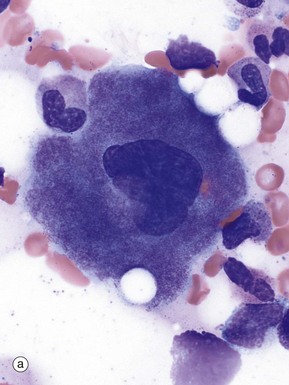
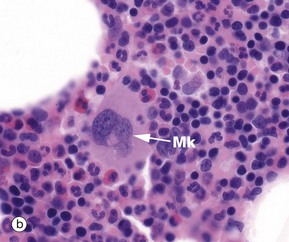
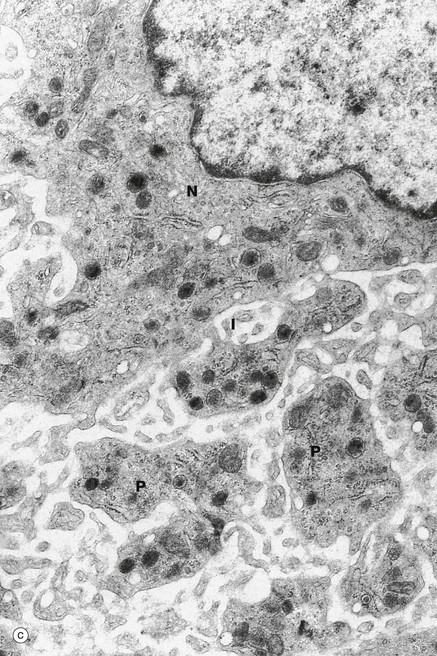
FIG. 3.14 Platelets and megakaryocytes
(a) Giemsa (HP) (b) H&E (MP) (c) EM ×6000
Megakaryocytes are responsible for platelet production and are the largest cells found in the bone marrow (30-100 µm). In smears, micrograph (a), they have large polylobated nuclei containing clumped dispersed chromatin, inconspicuous nucleoli and abundant cytoplasm filled with fine basophilic granules. In H&E-stained histology preparations, micrograph (b), megakaryocytes Mk are easily recognised by their size, lobulated nuclei and abundant pale eosinophilic cytoplasm.
The development and maturation of megakaryocytes is complex and the early precursors are not reliably recognisable on light microscopy. The precursor of the megakaryocyte in the bone marrow is called a megakaryoblast.
The mature cells are polyploid, having undergone repeated nuclear replication without cell division (endomitosis). A mature cell may have undergone as many as seven reduplications of nuclear and cytoplasmic constituents without cell division; hence the huge cell size and multilobed nucleus.
As they mature, the extensive cytoplasm becomes filled with fine basophilic granules, reflecting a profusion of cytoplasmic organelles, granules, vesicles and tubules. There is also an extensive system of demarcation membranes, complex invaginations of the plasma membrane, which forms the basis for ultimate fragmentation into individual platelets.
Megakaryocytes sit adjacent to bone marrow sinusoids and, when mature, extrude pseudopodia known as proplatelets into the sinusoid lumens. These pseudopodia fragment, releasing platelets. Whole megakaryocytes are known to enter the sinusoid lumens, as they are occasionally found in pulmonary capillaries, where some platelets are presumably also released. The proportions released from these two sites are not known although marrow is believed to be dominant.
Each megakaryocyte will release 2000 to 4000 platelets. Each day about 100 million megakaryocytes (108) are needed to supply 2 × 1011 platelets for the average adult.
On electron microscopy of mature cells, micrograph (c), there is a perinuclear zone N with usual organelles (Golgi apparatus, rough and smooth endoplasmic reticulum, developing granules, centrioles), an intermediate zone I with an extensive system of interconnected demarcation membranes and an outer zone P of yet more extensive membranes and cytoskeletal filaments.
Megakaryocytes and red cells have a common precursor. Megakaryocyte production, differentiation and maturation is partially driven by thrombopoietin, a growth factor produced mainly in the liver, together with interleukins (IL-3 and IL-11) and other growth factors.
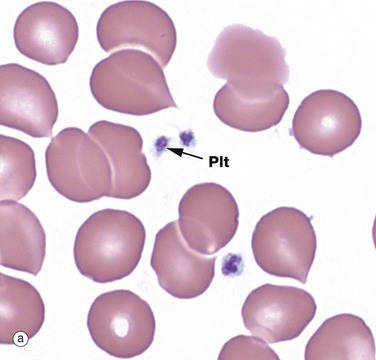
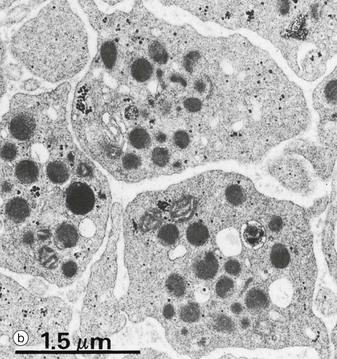
FIG. 3.15 Platelets
(a) Giemsa (HP) (b) EM ×18 000
Micrograph (a) shows several platelets Plt. Platelets (thrombocytes) are small, non-nucleated fragments of cytoplasm released from megakaryocytes. Platelets are small, round or oval, biconvex cytoplasmic discs, varying in size from 1.5 to 3.5 µm diameter. In blood smears, they have a central purple-stained granular appearance, due to their numerous organelles and a poorly seen pale-staining periphery. Platelet numbers in circulating blood range from 150 to 500 × 109/L, and they survive there for 5 to 10 days.
Platelets have most of the organelles of other cells except nuclei. The conspicuous granules/organelles when seen on EM (b) can be classified into several types:
• Alpha granules are variable in size and shape. These contain many proteins related to adhesion, blood clotting and growth factors for repair.
• Dense granules are very electron-dense. They contain serotonin, ADP, ATP, Ca2+ and Mg2+.
• Lysosomes are membrane-bound vesicles as found elsewhere, containing the usual enzymes (see Ch. 1).
Platelets are surprisingly complex. They have a marginal band of microtubules in their peripheral cytoplasm, associated with abundant contractile proteins, actin and myosin; this is a contractile system.
Located deep to the marginal band of microtubules and scattered throughout the cytoplasm is the dense tubular system (DTS), consisting of narrow membranous tubules with homogeneous electron-dense contents; these contain Ca2+ and enzymes related to the synthesis of lipid mediators of platelet activation, specifically cyclooxygenase and thromboxane synthetase.
Platelets also contain a system of interconnected membrane channels, the surface-connected canalicular system (SCCS), which is in continuity with the external environment via external pits; granules fuse with this system to release their contents to the exterior.
Platelets are functionally complex. They have over 50 different types of surface receptors. They respond to vessel injury to prevent bleeding and are active in blood clotting and tissue repair. On exposure to damaged tissue, platelets adhere to exposed collagen and other basement membrane proteins via their surface membrane receptors. Activation leads to contraction of the microtubule system and degranulation with release of granule contents, serotonin and ADP. Activated platelets also produce the lipid mediator thromboxane. These signals recruit adherence of additional platelets, with formation of a platelet plug.
Degranulation leads to transfer of membrane proteins from storage in the granule membranes, via the membrane fusion, onto the platelet surface. This includes the glycoprotein (GP)Ib-IX-V complex and platelet integrin αIIbβ3 (GPIIb-IIIa), both important receptors. Release of coagulation factors and Ca2+, plus the exposure of negatively charged platelet lipids, provides a surface for assembly of coagulation factor enzyme complexes. This facilitates the coagulation cascade with production of fibrin fibrils which bind the platelets together, strengthening the platelet plug. On a larger scale, this fibrin entraps red cells and results in a blood clot.
Activated platelets take on a stellate shape, with long pseudopodia. Subsequent contraction of the cytofilaments pulls the plug or clot tighter, a process known as clot retraction.
Platelets also release an array of growth factors to simulate repair; platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-β) amongst others. Many of the platelet functional products are inherited from the parent megakaryocyte, but some are obtained from plasma via membrane receptors, subsequent endocytosis and then storage in granules e.g. 5-HT (serotonin).
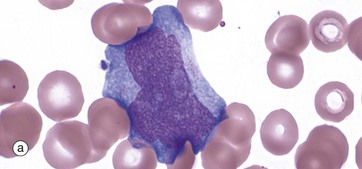
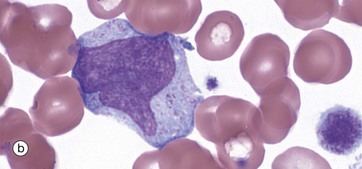
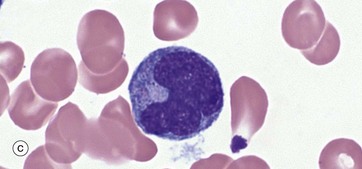
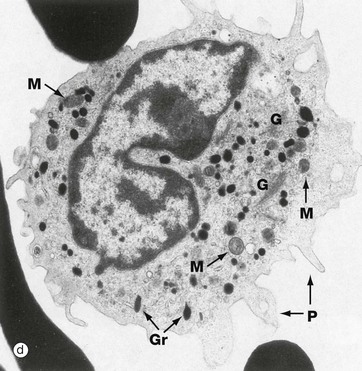
FIG. 3.16 Monocytes
(a–c) Giemsa (HP) (d) EM ×20 000
Monopoiesis, the formation of monocytes, is described as having three morphological stages. The first is the monoblast, micrograph (a). These mature with development of cytoplasmic granules and the start of a ‘frosted glass’ character to the cytoplasm; they are then called promonocytes, micrograph (b). These proliferate and mature into monocytes, micrograph (c). A typical promonocyte will undertake two serial cell divisions to produce 4 monocytes in a process taking about 60 hours.
Monocytes are the largest of the white cells (up to 20 µm in diameter) and constitute from 2% to 10% of leucocytes in peripheral blood. They circulate for 3 to 4 days on average before migrating into tissues. These cells are motile, highly phagocytic and may mature in tissues into tissue resident macrophages of varying kinds with extended lifespans.
Monocytes, micrograph (c), are characterised by a large, eccentrically placed nucleus which stains less intensely with more open chromatin than other leucocytes. Nuclear shape is variable but often with a deep indentation in the nucleus near to the centre of the cell, giving a horseshoe shape. Two or more nucleoli may be visible. Cytoplasm is abundant and stains pale greyish-blue with Romanowsky methods. There are numerous small, purple-stained lysosomal granules and cytoplasmic vacuoles which confer a ‘frosted-glass’ appearance.
With the electron microscope, micrograph (d), the cytoplasm is seen to contain a variable number of ribosomes, polyribosomes and little rough endoplasmic reticulum. The Golgi apparatus G is well developed and is located with the centrosome in the vicinity of the nuclear indentation. Small elongated mitochondria M are prolific. Small pseudopodia P extend from the cell, reflecting phagocytic ability and amoeboid movement.
The cytoplasmic granules Gr of monocytes are electron-dense and homogeneous. Half resemble primary (azurophilic) granules of neutrophils and these contain myeloperoxidase, acid phosphatase, elastase and cathepsin G. The other half are secretory granules containing plasma proteins, membrane adhesion proteins and tumour necrosis factor alpha (TNF-α).
Monocytes are capable of continuous lysosomal activity and regeneration and utilise aerobic and anaerobic metabolic pathways, depending on the availability of oxygen in the tissues.
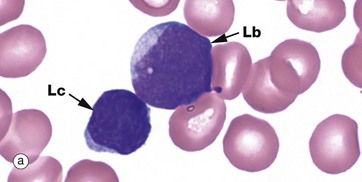
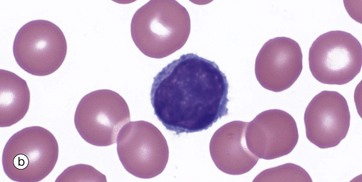
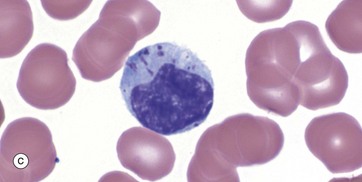
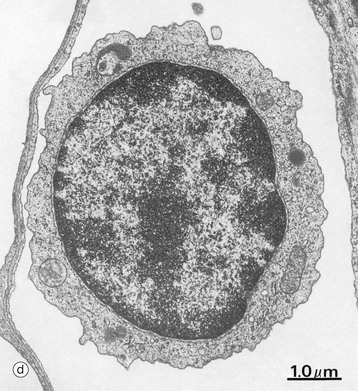
FIG. 3.17 Lymphocytes
(a–c) Giemsa (HP) (d) EM ×15 000
Lymphocytes have a central role in immunological defence mechanisms and are described in detail in Ch. 11. Lymphocyte production from haematopoietic stem cells occurs in marrow. An early recognisable form is the lymphoblast Lb, illustrated in micrograph (a), with an adjacent lymphocyte Lc. The lymphoblast is larger than a lymphocyte, with fine, open nuclear chromatin, a few pale inconspicuous nucleoli and scant cytoplasm. Some lymphoblasts mature in bone marrow into B cells, others mature via the thymus into T cells, while yet others differentiate into natural killer cells (NK cells) in bone marrow.
The lymphocytes circulate between various lymphoid tissues and other tissues of the body via the blood and lymphatic vessels. They continuously transit through tissues and back into the circulation as part of immune surveillance. Lymphoid cells have a variable lifespan ranging from weeks to an indefinite lifespan and, unlike granulocytes, are not end cells; they can proliferate, with most proliferation occurring in tissues. Lymphopoiesis is a constant but relatively inconspicuous activity in bone marrow.
Lymphocytes constitute 20% to 40% of the circulating leucocytes, with 1.0 to 4.5 × 109/L. Lymphocytes are the smallest of the white cells, being only slightly larger than erythrocytes. They generally have a round or oval, densely stained nucleus with clumped chromatin and a relatively small amount of pale, basophilic, non-granular cytoplasm Lc, micrograph (a). These small lymphocytes are ‘inactive’ forms.
A proportion of the normal lymphoid cells are larger with bigger nuclei, more cytoplasm and small numbers of cytoplasmic granules. These are known as large granular lymphocytes (LGL) and represent natural killer cells or cytotoxic T lymphocytes, micrograph (b).
The state of the nucleus and the amount of cytoplasm depends upon the activity of the cell. When activated or proliferating, the lymphocytes increase is size with larger nuclei, open chromatin, visible large nucleoli and more cytoplasm. Some of these reactive cells circulate in the blood, particularly during inflammatory processes; activated B cells may settle in tissue, maturing into long-lived antibody-secreting plasma cells.
Micrograph (d) is an electron micrograph of a small circulating lymphocyte in a pulmonary capillary. The nucleus is rounded but slightly indented and the chromatin moderately condensed and clumped; nucleoli are not present. The sparse cytoplasm contains a few mitochondria, a rudimentary Golgi apparatus, minimal endoplasmic reticulum and a comparatively large number of free ribosomes. The plasma membrane exhibits small cytoplasmic projections which appear as short microvilli.
Review
Clinical blood cell tests (the full blood count) usually include counts of cells in blood and size information about red cells and platelets. These vary. Neonatal normal values vary with the time since birth, differing from one day of life to the next. Normal values in children differ from normal values in adults, and male and female values differ after adolescence until at least menopause. There are also population-based differences, subtle differences with the exact counting technology used and differences due to the individual machine's calibration. For all these reasons the blood count numbers in Table 3.5 are indicative only. To facilitate interpretation of full blood counts, the testing laboratory will provide appropriate normal ranges for their tests for the patient's demographic.
Information on cell dynamics such half life and production are not part of clinical blood counts.
TABLE 3.6
Review of blood cell functions
| Cell type | Function | Other details |
| Red blood cells (erythrocytes) | Oxygen and carbon dioxide carriage | End anucleate cells; specialised for mechanical flexibility; 120-day lifespan; contain concentrated haemoglobin |
| Neutrophils | Inflammation and defence against bacteria | End cells, cannot re-enter blood; numerous granules with proinflammatory and antibacterial products; phagocytose and kill bacteria |
| Eosinophils | Inflammation, allergy and defence against parasites | End cells, cannot re-enter blood; numerous granules with proinflammatory and anti-parasite products |
| Basophils | Inflammation, allergy and defence against parasites | End cells, cannot re-enter blood; numerous granules with proinflammatory products |
| Monocytes | Inflammation and defence against infections | Can mature into macrophages, including becoming long-term tissue macrophages; ingest organisms and debris; major cytokine producers |
| Lymphocytes | Adaptive immune system | After formation can proliferate in tissues and lymph nodes and recirculate through blood |
| Platelets (thrombocytes) | Blood clotting (haemostasis) | Cell fragments produced by fragmentation of megakaryocyte cytoplasm; major source of growth factors at sites of injury |