Notes on staining techniques
Haematoxylin and eosin (H&E)
This is the most commonly used technique in animal histology and routine pathology. The basic dye, haematoxylin, stains acidic structures a purplish blue. Nuclei, ribosomes and rough endoplasmic reticulum have a strong affinity for this dye owing to their high content of DNA and RNA, respectively. In contrast, eosin is an acidic dye which stains basic structures red or pink. Most cytoplasmic proteins are basic and hence cytoplasm usually stains pink or pinkish red. In general, when the H&E staining technique is applied to animal cells, nuclei stain blue and cytoplasm stains pink or red.
Periodic acid–schiff reaction (PAS)
Staining methods that specifically stain components of cells and tissues are called histochemical staining techniques. Such techniques are invaluable for the understanding of cell and tissue structure and function, and for making a diagnosis on diseased tissues. The PAS reaction stains complex carbohydrates a deep red colour, traditionally described as magenta. The mucin produced by goblet cells of the gastrointestinal and respiratory tracts stains magenta with this technique (and is therefore termed PAS-positive). Basement membranes and the brush borders of kidney tubules and the small and large intestines are also PAS-positive, as is cartilage and to some extent collagen. Glycogen, the intracellular storage form of carbohydrate found in cells such as hepatocytes and muscle cells, is also PAS-positive.
Masson trichrome
This technique is a so-called connective tissue technique since it is used to demonstrate supporting tissue elements, principally collagen. As its name implies, the staining technique produces three colours: nuclei and other basophilic structures are stained blue; collagen is stained green or blue depending on which variant of the technique is used; and cytoplasm, muscle, erythrocytes and keratin are stained bright red.
Alcian blue
Alcian blue is a mucin stain which may be used in conjunction with other staining methods such as H&E or van Gieson (see below). Certain types of mucin, but not all, are stained blue by the Alcian blue method, as is cartilage. When the technique is combined with van Gieson, the Alcian blue colour becomes green.
van Gieson
This is another connective tissue method in which collagen is stained red, nuclei are blue and erythrocytes and cytoplasm yellow. When used in combination with an elastic stain, elastin is stained blue/black in addition to the results described above. This staining technique is particularly useful for blood vessels and skin.
Reticulin stain
This method demonstrates the reticulin fibres of supporting tissue, which are stained blue/black by this technique. Nuclei may be counterstained blue with haematoxylin, or red with the dye neutral red.
Azan
This technique is traditionally classed as a connective tissue method but is excellent for demonstrating fine cytological detail, especially in epithelium. Nuclei are stained bright red; collagen, basement membrane and mucin are stained blue; muscle and red blood cells are stained orange to red.
Giemsa
This technique is a standard method for staining blood cells and other smears of cells (e.g. bone marrow). Nuclei are stained dark blue to violet, background cytoplasm pale blue and erythrocytes pale pink.
Toluidine blue
This stain is one of the few stains which will differentially stain tissues in very thin epoxy resin sections (see Appendix 3) and is particularly used in the high-resolution investigation of the structure of the glomerulus in health and disease, as well as for high-resolution light microscopy of nerves. This dye also stains mast cell granules reddish purple in paraffin sections, a property called metachromasia.
Goldner's trichrome stain
This method is applied to acrylic resin sections (see Appendix 3) of undecalcified bone to distinguish between mineralised bone and unmineralised osteoid, and has a haematoxylin component which also stains the nuclei of osteoblasts, osteocytes, osteoclasts and marrow cells. The von Kossa stain (a silver method) also distinguishes between mineralised bone and osteoid but shows no cellular detail.
Silver and gold methods
These methods were extremely popular at the end of the nineteenth century and are occasionally used today to demonstrate such fine structures as cell processes (e.g. in neurones, motor end-plates and intercellular junctions). Depending on the method used, the end product is black, brown or golden.
Nissl and methylene blue methods
These techniques use a basic dye to stain the rough endoplasmic reticulum found in neurones; when this is seen as clumps it is called Nissl substance.
Sudan black and osmium
These dyes stain lipid-containing structures such as myelin a brownish-black colour for light microscopy. Osmium is also used as the staining agent that provides contrast in electron microscopy. Grids prepared for electron microscopy are stained with osmium tetroxide in solution. Electron-dense structures are those that have affinity for osmium staining.
Immunohistological techniques
A variety of immunohistochemical techniques are vital for diagnostic purposes as well as for research. Micrographs employing this method are used in several chapters of this book to highlight specific histological features (e.g. see Figs 1.14, 11.10, and 17.21). For this reason the basic technique is described here in some detail.
Immunohistochemical techniques depend on the exquisite specificity of antibodies for their antigen. Thus any substance (antigen) can be specifically identified, provided antibodies for it are available. To demonstrate a particular substance (e.g. insulin) in sections of tissue, antibodies must be produced. This is done by injecting human insulin into a laboratory animal, which obligingly recognises the human peptide as foreign and produces antibodies to it. A virtually inexhaustible source of antibody can then be created using monoclonal technology. (In actual practice you order a vial of antibody from a recognised supplier.)
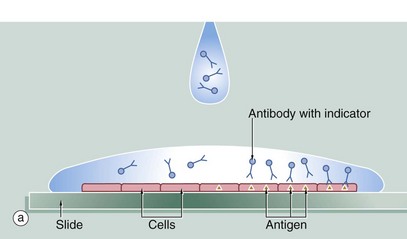
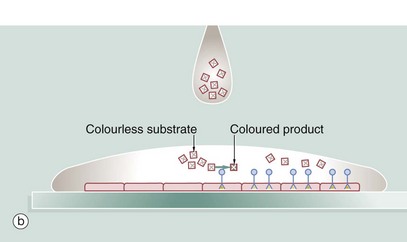
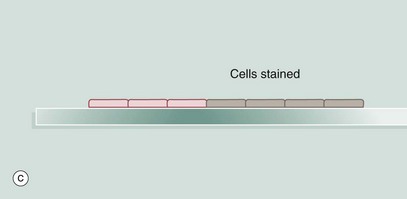
A section of tissue is placed on a glass slide (a), and a solution of antibody is laid over the tissue. The antibody binds to the antigen and excess antibody is washed away so that only cells containing the particular antigen have antibody bound to them. To demonstrate the position of antibody, the antibody is prelinked to an indicator substance. This may be a fluorescent substance such as fluorescein, in which case the technique is known as immunofluorescence, and the position of antibody can be visualised using a fluorescence microscope. It is more useful to bind to the antibody an enzyme that is able to convert a colourless substrate to a coloured product. A solution of the substrate is laid over the tissue section (b) with the bound antibody. After a period of incubation, the coloured product can be seen on the section using an ordinary light microscope (c). An enzyme commonly used for this purpose is horseradish peroxidase and the technique is then called the immunoperoxidase technique. A further modification of this principle for electron microscopy uses antibodies linked to gold which, as gold is electron-dense, can be detected by electron microscopy (immunogold labelling). Many other variants of this technique are available.