Central nervous system
The central nervous system (CNS) consists of the brain and spinal cord and is composed of neurones, neuronal processes, supporting cells of the CNS (glial cells) and blood vessels. The CNS is invested with meninges and is suspended in fluid, the cerebrospinal fluid (CSF) which is produced by specialised choroid plexus structures.
Macroscopically, all parts of the CNS are made up of grey matter and white matter. Grey matter contains most of the neurone cell bodies and their dendritic processes while the white matter contains the axons. The lipid-rich myelin sheaths around the axons accounts for the white appearance of the white matter.
Central nervous tissue consists of a vast number of neurones and their processes embedded in a mass of support cells, collectively known as neuroglia, which form almost half of the total mass of the CNS. These are highly branched cells that occupy the spaces between neurones; they have intimate functional relationships with the neurones, providing both mechanical and metabolic support.
Four principal types of neuroglia are recognised:
• Oligodendrocytes are the CNS equivalent of the Schwann cells of the peripheral nervous system and are responsible for the formation of myelin sheaths in the CNS.
• Astrocytes are highly branched cells that pack the interstices between the neurones, their processes and oligodendrocytes. They provide mechanical support as well as mediating the exchange of metabolites between neurones and the vascular system. They also form part of the blood-brain barrier. Astrocytes play an important role in repair of CNS tissue after damage
• Microglia are the CNS representatives of the monocyte-macrophage system and have defence and immunological functions.
• Ependymal cells make up a specialised epithelium which lines the ventricles and spinal canal
Central nervous tissue proper lacks collagenous supporting tissue which is confined to the immediate surrounds of penetrating blood vessels and to the meninges that invest the outer surface of the brain. The CNS also contains little extracellular material.
While the basic organisation of grey and white matter remains consistent throughout the brain, in detail they form complex arrangements of the basic components to give a rich microscopic anatomy (histology) which is highly related to the macroscopic anatomy and function. In this chapter after description of the specialised supporting cells and tissues, including CSF production (choroid plexus), we will illustrate the microanatomy of some major structures in the CNS.
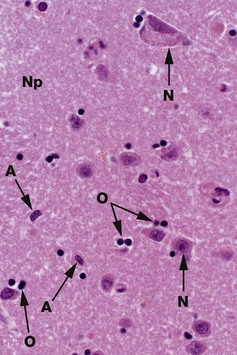
FIG. 20.1 Grey matter
H&E (HP)
Common staining methods permit neurones N to be readily distinguished from glial cells. While neurones vary greatly in different regions of the brain, they are usually recognisable by their large nuclei, prominent nucleoli and dispersed chromatin. There is usually extensive basophilic granular cytoplasm, and parts of one or more processes may be visible, often due to processing artefact.
Types of neuroglia are difficult to differentiate from each other with certainty by common staining methods. In the mature CNS, as in this specimen, oligodendrocytes O have small round condensed nuclei; their cytoplasm is unstained by routine methods, including H&E. In grey matter, oligodendrocytes are not only scattered between the nerve cell bodies along with the astrocytes but also tend to be aggregated around the neurone cell bodies. Other glial cells in the image, marked A, are probably astrocytes.
The nuclei of both neurones and neuroglia are surrounded by a dense network of branching cytoplasmic cell processes, axons and dendrites. This is seen as a fibrillar eosinophilic material on H&E and is called neuropil Np. Only some of the fibres are myelinated axons.
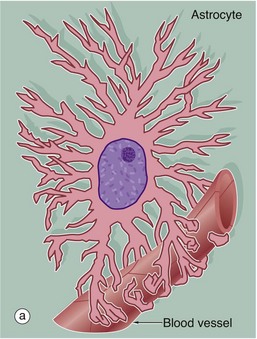
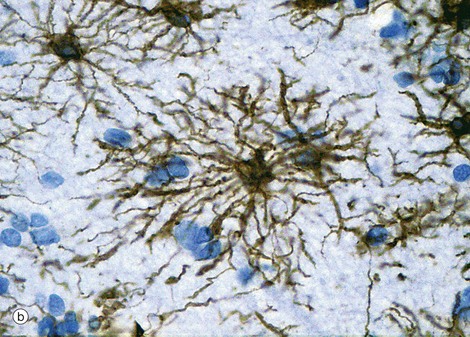
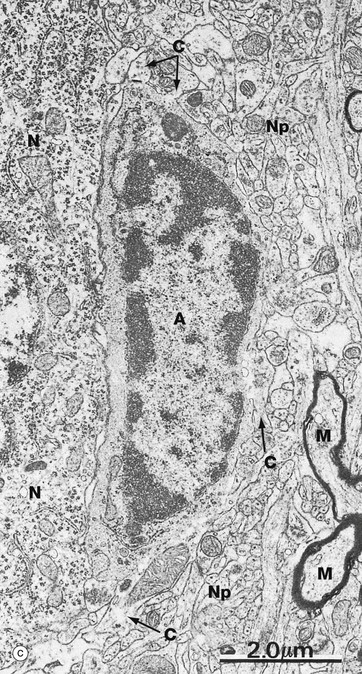
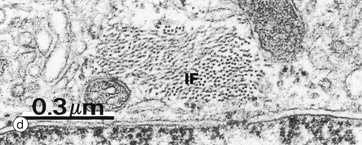
FIG. 20.2 Astrocytes
(a) Diagram (b) Immunohistochemical method for glial fibrillary acidic protein (HP)
(c) EM ×12 000 (d) EM ×57 500
Astrocytes are identified by immunohistochemical staining for a protein called glial fibrillary acidic protein (GFAP) in micrograph (b); these are the most numerous glial cells in grey matter. They have long, branched processes which occupy much of the interneuronal spaces in the neuropil. In grey matter, many of the astrocyte processes end in terminal expansions adjacent to the non-synaptic regions of neurones. Other processes of the same astrocytes terminate upon the basement membranes of capillaries; these perivascular feet cover most of the surface of the capillary basement membranes and form part of the blood-brain barrier as illustrated in the diagram (a). Similar foot processes invest the basement membrane between the CNS and the innermost layer of the meninges, the pia mater (see Fig. 20.9), forming a relatively impermeable barrier called the glia limitans. Astrocytes mediate metabolic exchange between neurones and blood and regulate the composition of the intercellular environment of the CNS.
All astrocytes contain bundles of intermediate filaments and microtubules. The intermediate filaments are formed of GFAP, which is characteristic of astrocytes. The astrocytes of grey matter have numerous short, highly branched cytoplasmic processes and are described as protoplasmic astrocytes. These are demonstrated in micrograph (b) by using immunohistochemical staining for GFAP. By contrast, the astrocytes of white matter have relatively few and straight cytoplasmic processes rich in intermediate filaments and are known as fibrous astrocytes.
Micrograph (c) shows an astrocyte A lying adjacent to a nerve cell body N in the cerebral cortex. The astrocyte cytoplasm contains many ribosomes, a little rough endoplasmic reticulum and a few small mitochondria and lysosomes. The origins of several cytoplasmic extensions C can be identified. The cytoplasm appears moderately electron-dense due to its content of intermediate filaments IF, which can be seen at higher magnification in micrograph (d). Typical of CNS grey matter, the adjacent neuropil Np contains numerous neuronal and glial processes in various planes of section; some myelinated axons M are included in the field.
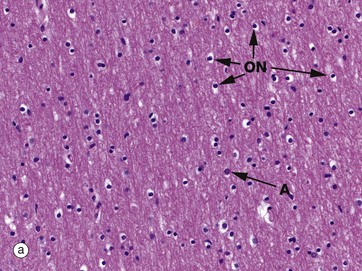
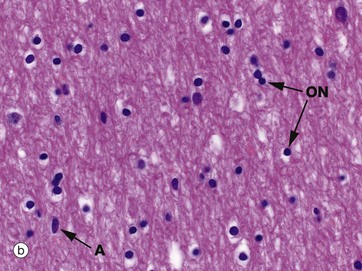
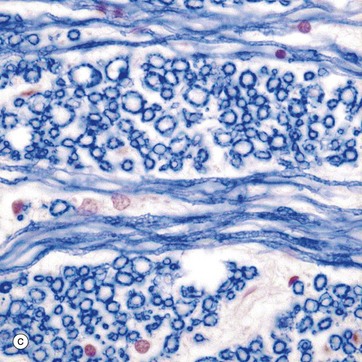
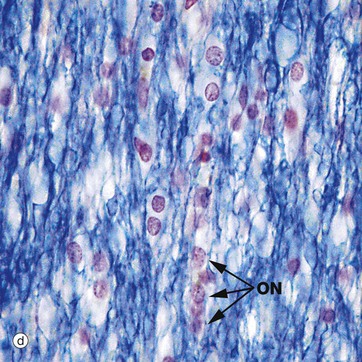
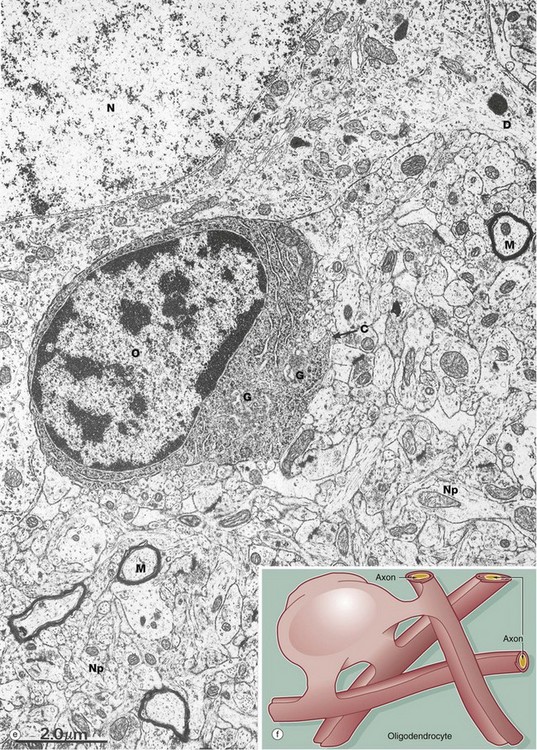
FIG. 20.3 White matter CNS myelin and oligodendrocytes (illustrations (e) and (f) opposite)
(a) H&E (MP) (b) H&E (HP) (c) TS, solochrome cyanin (HP) (d) LS, solochrome cyanin (HP) (e) EM ×13 000 (f) Schematic diagram
White matter consists of nerve fibres (axons), often myelinated by oligodendrocytes, organised in tracts, with supporting astrocytes, microglia and vessels. Oligodendrocytes were so named because original heavy metal impregnation methods showed that they only had a small number of short, branched processes (Greek: oligos = few, dendron = tree). It is now known that oligodendrocytes are responsible for myelination of axons and the processes originally described are the short pedicles that connect the cell body to the myelin sheaths.
A single oligodendrocyte can contribute to the myelination of up to 50 axons from the same or different fibre tracts, as illustrated in the diagram (f). Conversely, any one axon will require the services of numerous different oligodendrocytes because of the limited length of the myelin segments (internodes) produced by each oligodendrocyte. The mechanism of myelin sheath formation is very similar to that of Schwann cells in peripheral nerve (see Fig. 7.6). Oligodendrocytes are the predominant type of neuroglia in white matter, as well as being abundant in grey matter.
Micrographs (a) and (b) show CNS white matter, with the oligodendrocyte nuclei ON often having an artefactual perinuclear halo and a few larger astrocyte nuclei A. Micrographs (c) is of a myelin stain, with the transverse ring-shaped profiles of blue-stained myelin each surrounding an axon, unstained and not visible in this preparation. Micrograph (d) is of longitudinal fibres using the same stain. Oligodendrocyte nuclei ON can be seen as rounded red-stained profiles.
Oligodendrocytes aggregate closely around nerve cell bodies in the grey matter, where they are thought to have a support function analogous to that of the satellite cells which surround nerve cell bodies in peripheral ganglia (see Fig. 7.20).
The electron micrograph (e) shows an oligodendrocyte O lying adjacent to a nerve cell body N, with a neuronal dendrite D at the upper right. The oligodendrocyte contains prominent rough endoplasmic reticulum, ribosomes and Golgi apparatus G. The commencement of a cytoplasmic process C is seen. The remainder of the image shows the complexity of the neuropil Np, comprising glial and neuronal processes including myelinated axons M.
Myelin sheath formation begins in the CNS of the human embryo at about 4 months gestation, with the formation of most sheaths at least commenced by about the age of 1 year. From this time, successive layers continue to be laid down, with final myelin sheath thickness being achieved by the time of physical maturity.
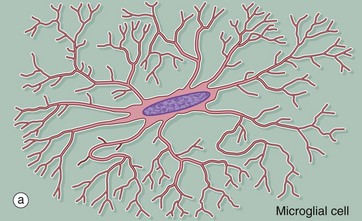
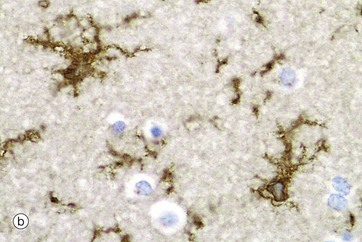
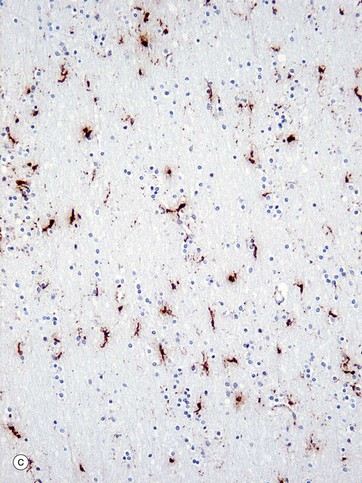
FIG. 20.4 Microglia
(a) Diagram (b) Ricinus communis agglutinin (HP) (c) Immunohistochemical method for CD68 (MP)
Microglia are small cells derived from cells of mesenchymal origin which invade the CNS at a late stage of fetal development. As shown in the diagram, microglia have elongated nuclei and relatively little cytoplasm, which forms fine highly branched processes. In consequence, they are difficult to identify in conventional preparations for light microscopy.
Immunohistochemical staining provides the best way to see microglia. Micrograph (b) shows the ramified profile of microglia cells, identified by staining using ricinus communis agglutinin which binds to sugars on the membrane of this cell type. Micrograph (c) highlights the distribution of this cell type in white matter from the cerebral hemisphere. The rounded nuclei in the background are mainly oligodendrocytes.
In response to tissue damage, microglia transform into large amoeboid phagocytic cells and are thus the CNS representatives of the macrophage-monocyte defence system (see Fig. 4.19). CD68 stains cells of macrophage lineage, including microglial cells. Other macrophages, distinct from microglia, are present in the space surrounding the CNS capillaries but are separated from the CNS compartment proper by the perivascular feet of astrocytes.
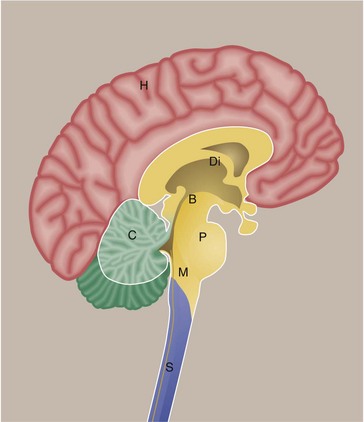
FIG. 20.5 Main anatomical divisions in the CNS
This diagram shows the brain and spinal cord viewed in a sagittal section. The main part of the brain consists of the paired cerebral hemispheres H which are invested by the cerebral cortex. Deep within the brain are large grey matter structures which form the diencephalon Di. Included in this region are the basal ganglia and thalamus. The cerebral hemispheres are connected to structures below by the midbrain B. In this region, there are important nuclei including the substantia nigra. The pons P connects to the medulla M as well as to the cerebellum C. The pons, medulla and midbrain are often collectively termed the brainstem. The spinal cord S extends from the lower end of the medulla.
Specific terms are used to describe arrangements of cells and their connections:
• The grey mater over the surface of the brain is termed the cortex.
• A discrete arrangement of neurones, often with a specific function, is termed a nucleus.
• An arrangement of neuronal cells running along the spinal cord is termed a column.
• A defined bundle of axons running in white matter is termed a tract or a fascicle.
• The crests of folds are termed gyri (singular: gyrus) while the clefts between folds are termed sulci (singular: sulcus). In the cerebellum these folds are termed folia.
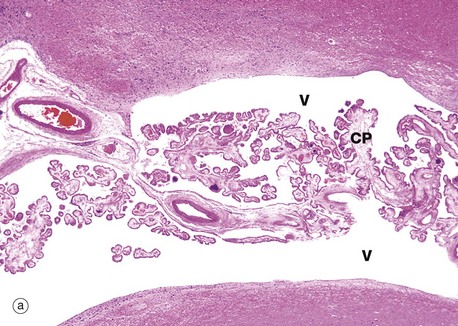
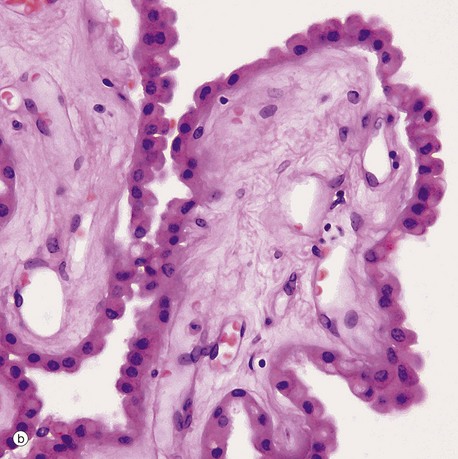
FIG. 20.6 Choroid plexus
(a) H&E (LP) (b) H&E (HP)
The choroid plexus is a vascular structure arising from the wall of each of the four ventricles of the brain and responsible for the production of cerebrospinal fluid (CSF). CSF drains from the interconnected ventricular cavities via three channels connecting the fourth ventricle with the subarachnoid space which surrounds the CNS. CSF is produced at a constant rate and is reabsorbed from the subarachnoid space into the superior sagittal venous sinus, via finger-like projections called arachnoid villi. Thus the CNS is suspended in a constantly circulating fluid medium which acts as a support and shock absorber.
Each choroid plexus consists of a branching system of blood vessels which run in fronds composed of collagenous tissue and covered by a cuboidal or columnar epithelium. The choroid plexus is therefore a villous structure. Micrograph (a) shows the choroid plexus CP within a ventricle of the brain V.
Micrograph (b) shows detail of one of the choroid plexus processes. The capillaries and vessels of the choroid plexus are large, thin-walled and sometimes fenestrated lying in a fibrous core. The epithelial cells rest on a basal lamina. At the ultrastructural level, long bulbous microvilli project from the luminal surfaces of the choroid plexus epithelial cells and their cytoplasm contains numerous mitochondria, features which suggest that the elaboration of CSF is an active process.
The mode of CSF secretion involves active secretion of sodium ions by choroid epithelial cells into the CSF, followed by passive movement of water from the local vessels. Continuous tight junctions (zonula occludens) between epithelial cells contribute to a blood-CSF barrier, preventing ingress of almost all other molecules.
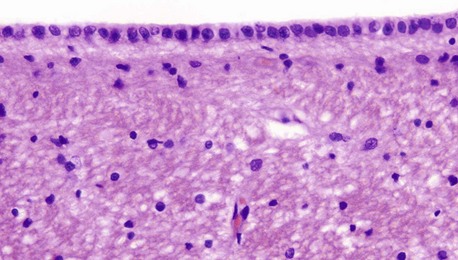
FIG. 20.7 Ependyma
H&E (HP)
Ependymal cells form the lining of the ventricles and spinal canal. Cuboidal or low columnar in shape, the cells are tightly bound together at their luminal surfaces by the usual epithelial junctional complexes. Unlike epithelia, however, ependymal cells do not rest on a basement membrane but, rather, the bases of the cells taper and then branch into fine processes which ramify within the underlying layer of processes derived from astrocytes. At the luminal surface, there are a variable number of cilia. Microvilli are also present and probably have absorptive and secretory functions. The ependymal layer tends to become incomplete with increasing age.
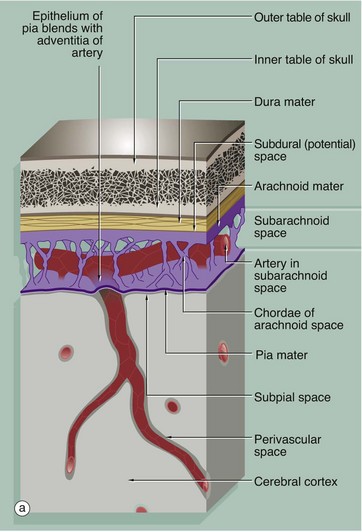
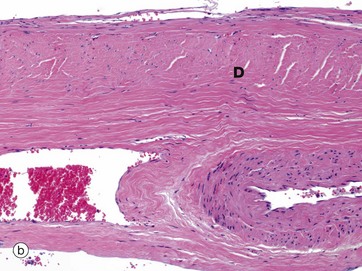
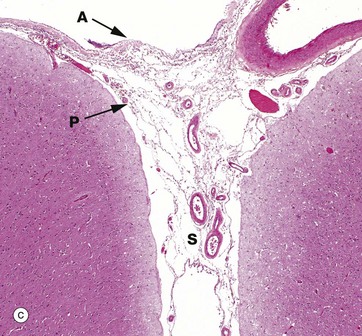
FIG. 20.8 Meninges
(a) Diagram (b) Dura mater, H&E (MP) (c) H&E (LP)
The brain and spinal cord are invested by three layers of supporting tissue, collectively called the meninges. The surface of the nervous tissue is covered by a delicate layer called the pia mater, containing collagen fibres, fine elastin fibres and occasional fibroblasts, separated from the astrocytic processes of underlying CNS parenchyma by a basement membrane. The basement membrane is completely invested by astrocytic processes, the two layers forming the impermeable glia limitans.
Overlying the pia mater is a thicker fibrous layer, the arachnoid mater, which derives its name from the presence of cobweb-like strands which connect it to the underlying pia; since the pia and arachnoid are continuous, they may be considered as a unit, the pia-arachnoid, also called the leptomeninges. The space between the pia and arachnoid is called the subarachnoid space and, in places, forms large cisterns. This subarachnoid space is connected with the ventricular system by three foramina in the fourth ventricle (in the brainstem), and CSF circulates continuously from the ventricles into the subarachnoid space.
The subarachnoid space is lined by flattened arachnoidal cells. The outer surface of the arachnoid mater is also lined by flat cells. As shown in the diagram, arteries and veins passing to and from the CNS pass in the subarachnoid space loosely attached to the pia mater and invested by subarachnoid meningothelium. As the larger vessels extend into the nervous tissue, they are surrounded by a delicate sleeve of pia mater. Between the penetrating vessels and the pia there is a perivascular space. In humans, the pia blends with the adventitia of the vessel as it penetrates the brain, thus separating the perivascular space from the subarachnoid space. This pia component is not present around the capillaries of the CNS.
External to the arachnoid mater is a dense fibroelastic layer called the dura mater D, micrograph (b), which is lined on its internal surface by flat cells. The dura is closely applied to but not connected with the arachnoid layer and there is the potential for a space, the subdural space, to develop between the two layers. In the cranium, the dura mater merges with the periosteum of the skull but also extends into the brain space as several folds. A large fold, the falx, extends along the midline from top of the skull into the space between the cerebral hemispheres while a horizontal fold, the tentorium, is attached to the posterior skull and extends into the space between the cerebral hemispheres and cerebellum. These folds help support the brain and contain venous sinuses, forming part of the brain's venous return system.
Around the spinal cord, dura is suspended from the periosteum of the spinal canal by denticulate ligaments, the intervening epidural space being filled with loose fibrofatty tissue and a venous plexus.
The pia and arachnoid layers of the brain meninges are illustrated in micrograph (c). Pia mater P is attached to the surface of the brain and continues into the suclus S and around the penetrating vessels. The arachnoid mater A appears to be a completely separate layer and bridges the sulcus. Meningeal vessels lie in the subarachnoid space.
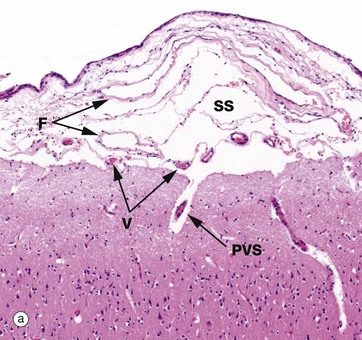
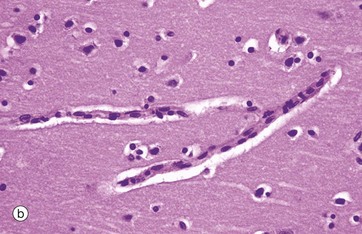
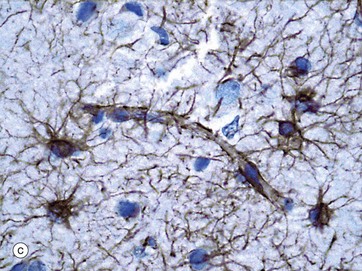
FIG. 20.9 Meninges
(a) H&E (MP) (b) H&E (HP) (c) Immunohistochemical method for GFAP (HP)
The pia and arachnoid layers of the brain meninges are illustrated in micrograph (a). In this micrograph, delicate fibrous strands F can be discerned traversing the subarachnoid space SS to connect the pia and arachnoid layers. Small vessels V can be seen in the subarachnoid space. A penetrating vessel is also seen, surrounded by a perivascular space PVS. The perivascular space is extremely narrow, although it often appears artefactually wider as in this micrograph. The CNS contains no lymphatics and interstitial fluid is thought to drain outwards from the brain substance to join the subarachnoid CSF via the perivascular spaces, contributing as much as 20% of its volume.
As seen in micrograph (b), by light microscopy the capillaries of the CNS are similar to those elsewhere in the body, with flattened endothelial cells resting on a basement membrane; however, unlike elsewhere, these endothelial cells are not fenestrated and are bound together by continuous tight intercellular junctions (zonula occludens). Externally, the basement membranes are covered by the perivascular foot processes of astrocytes, shown in micrograph (c), where brown-stained processes form a continuous layer.
Perfusion studies show that the CNS capillaries are impermeable to certain plasma constituents, especially larger molecules, forming a blood-brain barrier. The capillary endothelium plays the central role, since the junctions between endothelial cells are sealed; the endothelial cells exhibit little or no pinocytosis. Luminal surface membranes contain various enzymes which destroy neurotoxic metabolites and neuroactive humoral substances.
Maintenance of barrier-type endothelium appears to be under the control of astrocyte foot processes. The blood-brain barrier provides neurones with a relatively constant biochemical and metabolic environment, protection against endogenous and exogenous toxins and infective agents and insulates the neurones from circulating neurotransmitters and other humoral agents. The capillaries of the choroid plexus, the pituitary and pineal glands and the vomiting centre of the hypothalamus are, however, devoid of this barrier to allow for their specialised functions.
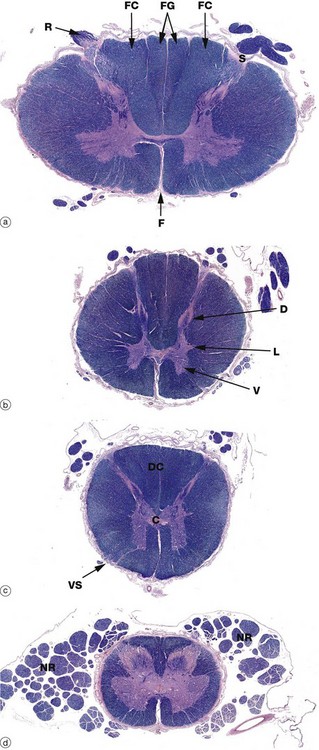
FIG. 20.10 Spinal cord transverse sections
Luxol fast blue, H&E photomontage, (LP) (a) Cervical (b) Thoracic (c) Lumbar (d) Sacral
The structure of the spinal cord is similar throughout its length, with four regions demonstrated in this series of micrographs. In transverse sections, the central mass of grey matter has the shape of a butterfly, the ventral horns V being prominent and containing the cell bodies of the large alpha lower motor neurones. The dorsal horns D contain the cell bodies of small second-order sensory neurones. These relay upwards sensory information on pain and temperature and participate in spinal reflexes. Small lateral horns L are found in the thoracic and upper lumbar regions and contain cell bodies of sympathetic nervous system efferent neurones. The volume of grey matter is more extensive in the cervical and lumbar regions and the cord is larger, corresponding to innervation of the limbs.
The central canal lies in the central commissure C of grey matter; it is lined by ependymal cells and contains CSF. The white matter consists of ascending tracts of sensory fibres and descending motor tracts; passing up the spinal cord towards the brain, more and more fibres enter and leave the cord so that the volume of white matter increases progressively from the sacral to cervical regions.
The spinal cord has a deep ventral (anterior) median fissure F but, dorsally, there is only a shallow dorsal midline sulcus. On each side, a dorsolateral sulcus S marks the line of entry of the dorsal nerve roots R, part of which can be seen in micrograph (a). The white matter between the dorsal horns represents the ascending dorsal columns DC which convey fibres for senses of vibration, proprioception and fine touch to the medulla where they synapse with second-order sensory neurones in the gracile and cuneate nuclei. In the cervical region, each dorsal column is subdivided into two fascicles: the medial fasciculus gracilis FG, conveying fibres from the lower limbs, and the lateral fasciculus cuneatus FC, conveying fibres from the upper limbs.
Ventrolateral sulci VS may be discernible, marking the line of exit of the ventral nerve roots. The ventrolateral white matter contains most notably the lateral spinothalamic tract (pain and temperature), the ventral spinothalamic tract (light touch), the spinocerebellar tracts and the corticospinal tract (motor).
The spinal cord is invested by meninges, with the dura mater being loosely connected to the periosteum of the vertebral canal by denticulate ligaments; the residual epidural space is filled by adipose tissue and a venous plexus. During development, the vertebral column lengthens more than the enclosed spinal cord and the segmental levels of the lower part of the cord therefore lie above the corresponding intervertebral foramina. Below the cervical region, the nerve roots NR pursue an increasingly oblique course in the subarachnoid space before passing through the intervertebral foramina and are thus found adjacent to the cord, particularly in the lumbar and sacral regions.
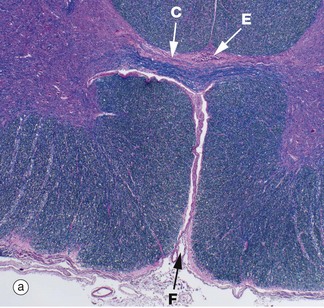
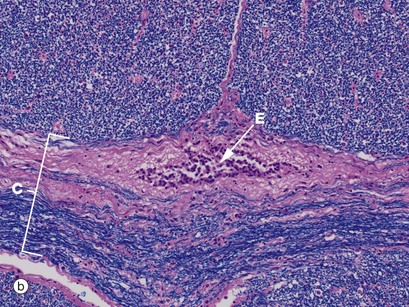
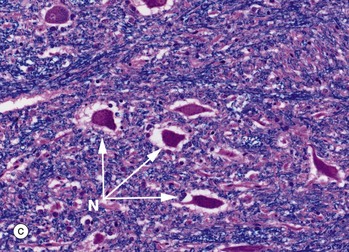
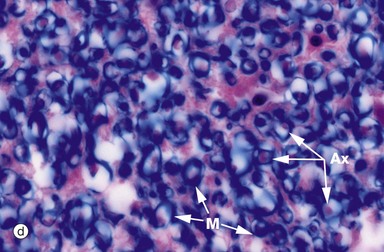
FIG 20.11 Spinal cord and central canal
Luxol fast blue, H&E (a) Ventral spinal cord (MP) (b) Commissure and spinal canal (HP) (c) Grey matter anterior horn (HP) (d) White matter (HP)
Luxol fast blue stains the myelinated sheaths of nerve fibres a dark blue while the H&E stain colours the cytoplasm of cells and their processes red. Micrograph (a) illustrates the ventral median fissure F and the anterior spinal vessels which are the cord's main blood supply. Both images (a) and (b) illustrate the central commissure C where both grey matter (pink-staining cells and their processes) and blue-staining myelinated nerved fibres cross the midline. The spinal canal E with its ependymal cell lining is identified in both images but can only be seen clearly in image (b). Micrograph (c) illustrates part of the grey matter, the anterior horn with large neurones with their purple-staining cytoplasmic Nissl substance. Parts of their dendritic processes or axons are visible as a result of shrinkage artefact. These cells are the alpha motor neurones N (lower motor neurones) responsible for voluntary skeletal muscle control and thus voluntary movement. They are situated in a background of small cells, cell processes and nerve fibres. Micrograph (d) is a high-power view of the cord white matter, formed of massed nerve fibres with axons Ax and their surrounding myelin sheaths M. Large numbers of small myelinated axons and unmyelinated axons are also present but are not visible at this power and in this kind of section.
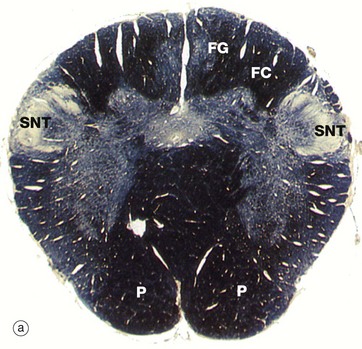
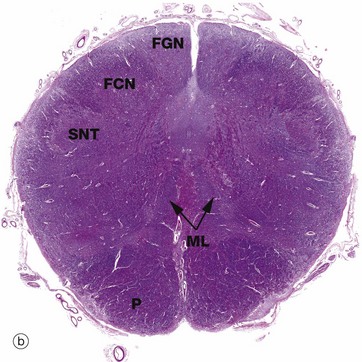
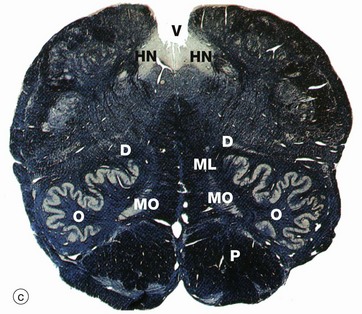
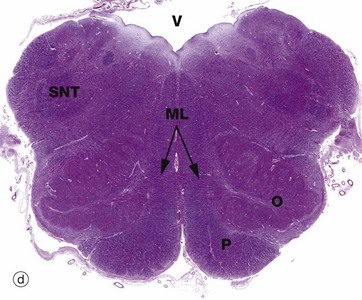
FIG. 20.12 Medulla oblongata
(a) Weigart-Pal (LP) (b) H&E (LP) (c) Weigart-Pal (LP) (d) H&E (LP); (b) and (d) are digitally enhanced photomontages
The medulla oblongata is the most caudal part of the brainstem and connects to spinal cord. The images are in order (a) to (d) from caudal (towards tail) to rostral (towards head). The Weigart-Pal used in images (a) and (c) is a myelin stain which leaves the grey matter relatively unstained.
The prominent ventral pyramid P on each side of the medulla are the corticospinal tracts which transmit voluntary motor signals from the motor cortex to alpha motor neurones in the anterior horns of the spinal cord. About 85% of fibres cross to the opposite side in the decussation of the pyramids located in the medulla (remember, the left brain cortex supplies the right side of the body).
All of the ascending (sensory) pathways in the spinal cord pass through the medulla, although their arrangement differs from the spinal cord. The easiest traced are the dorsal white matter columns of the spinal cord which convey ascending proprioceptive, vibration and discriminatory touch fibres. These axons relay with neurones in the similarly situated gracile FGN and cuneate FCN nuclei located in the dorsum of medulla, micrograph (b), at the rostral end of the gracile FG and cuneate FC fasciculi of the dorsal columns, seen in micrograph (a). The axonal fibres of the tract (after the relay) then pass upwards to the thalamus via the medial lemniscus ML which lies medially.
The medulla contains numerous other ascending and descending tracts and also includes various tracts and nuclei of the eighth to the twelfth cranial nerves. The medulla also contains a spinal nucleus which is part of the trigeminal nerve (cranial nerve V) and associated tract; this spinal nucleus of the trigeminal tract SNT is recognisable dorsolaterally throughout the medulla, with its tract of white matter lying superficially. The hypoglossal nucleus HN can also be identified in micrograph (c).
In the most caudal medulla, image (a), grey matter can be seen still roughly resembling the butterfly shape seen in sections of the spinal cord. The ventral grey matter horns contain cell bodies of lower motor neurones running in the spinal accessory and first cervical nerves.
A prominent feature of the upper medulla is the inferior olivary nucleus O, named for a gross anatomical resemblance to an olive. It has a convoluted appearance in transverse section and is associated with several smaller accessory nuclei D, including the dorsal accessory olivary nucleus N and the medial accessory olivary nucleus MO. The neurones of this inferior olivary complex relay central and spinal afferent stimuli to the cerebellar cortex. Dorsally, the spinal canal opens into the fourth ventricle V.
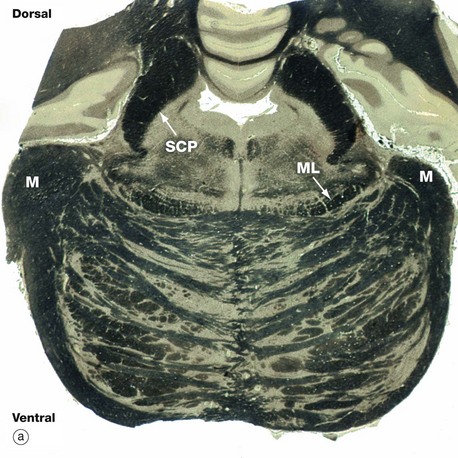
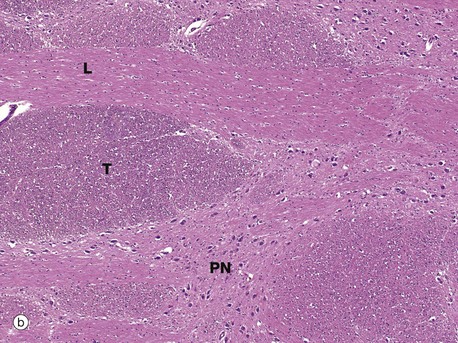
FIG. 20.13 Pons
(a) Mid-pons, Weigart-Pal (LP) (b) Basal pons, H&E (MP)
The pons is the middle portion of the brainstem, lying between the midbrain rostrally and medulla caudally. In transverse section, it comprises two parts, a bulky ventral region (basal pons) and a smaller dorsal (tegmental) region. The basal pons consists of crisscrossed bundles of longitudinal and transverse fibres between which lie collections of neurone cell bodies known as pontine nuclei.
The longitudinal fibres of the basal pons consist of descending fibres of two main types. There are axons of the corticospinal tract which transmit voluntary motor signals from the motor cortex to the alpha motor neurones of the ventral horns of the spinal cord. On traversing the pons, these axons converge to form the pyramids of the medulla.
A second group of descending fibres originate in various areas of the cortex and synapse in the pontine nuclei, from which fibres then pass in the transverse bundles, crossing the midline to enter the cerebellum via the middle peduncles M.
The dorsal tegmentum contains the ascending spinothalamic (sensory) tracts and the nuclei of the fifth, sixth and seventh cranial nerves. On each side, the medial lemniscus ML is readily identifiable; this represents the upward continuation of proprioceptive, vibration and fine touch pathways of the spinal dorsal columns, relayed via the gracile and cuneate nuclei of the medulla.
The cerebellum is located on the dorsum of the brainstem at the pons and is connected via the cerebellar peduncles, which are subdivided into superior, middle, and inferior peduncles reflecting the three main fibre tracts. Part of the middle peduncle M is present in sections through the mid-pontine level, as in micrograph (a), while the superior cerebellar peduncles SCP are very prominent. The main bulk of the superior cerebellar peduncles is made up of fibres from the central nuclei of the cerebellum passing upwards to the thalamus and then to the motor cortex.
Image (b) is a higher-power view of the basal pons and shows in transverse section T the rostral-caudal fibre bundles, including the cortical spinal motor tracts. In longitudinal section L are fibres crossing between the cerebellar hemispheres. There is also the interspersed grey matter with its neurones representing the pontine motor nuclei PN.
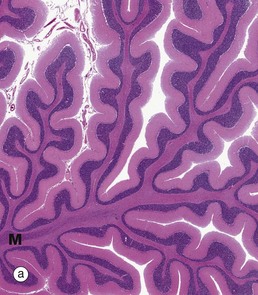
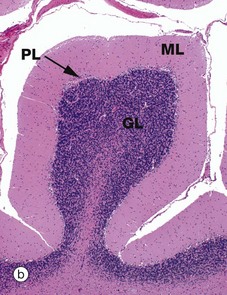
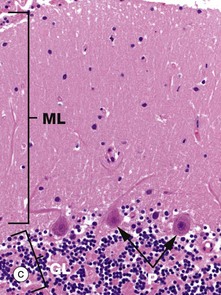
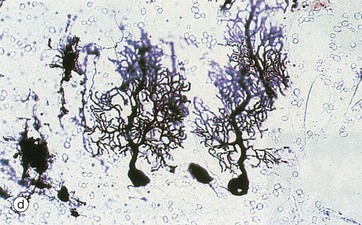
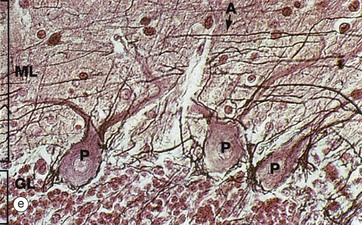
FIG. 20.14 Cerebellum
(a) H&E (LP) photomontage (b) H&E (LP) (c) H&E (MP) (d) Golgi–Cox (MP) (e) Bielschowsky/neutral red (HP)
The cerebellum coordinates muscular activity and maintains posture and equilibrium. It consists of a cortex of grey matter with a central core of white matter containing four pairs of nuclei. Afferent and efferent fibres pass to and from the brainstem via inferior, middle and superior cerebellar peduncles, linking medulla, pons and midbrain, respectively.
As seen in micrograph (a), the cerebellar cortex forms a series of deeply convoluted folds or folia supported by a branching central white matter M (sometimes called the medulla of the cerebellum, but not to be confused with the nearby medulla oblongata). At higher magnification in micrograph (b), the cortex is seen to consist of three layers. The outer molecular layer ML contains relatively few neurones and large numbers of unmyelinated fibres. The inner granular cell layer GL is extremely cellular. Between the two is a single layer of huge neurones called Purkinje cells PL. Purkinje cells P are seen at higher magnification in micrograph (c); they have very large cell bodies, a relatively fine axon extending down through the granular cell layer GL and an extensively branching dendritic system which arborises in the molecular layer ML. The extraordinary dendritic system of Purkinje cells is best demonstrated by heavy metal methods as in micrograph (d). The deep granular cell layer of the cortex contains numerous small neurones, the non-myelinated axons of which pass outwards to the molecular layer where they bifurcate to run parallel to the surface to synapse with the dendrites of Purkinje cells. Micrograph (e) demonstrates these granular cell axons in the molecular layer. The axons A are stained black with silver and their cell bodies in the granular cell layer GL are counter-stained with neutral red. In addition to the Purkinje P and granular cells already described, there are three other types of small neurones in the cerebellar cortex: stellate cells and basket cells scattered in the molecular layer ML and Golgi cells scattered in the superficial part of the granular cell layer. Note black-stained fibrils of basket cell axons surrounding the Purkinje cell bodies P in micrograph (e).
Afferent fibres enter the cerebellum from the brainstem and then pass via the central white matter to make complex connections with granular cells; these, in turn, connect with Purkinje dendrites via basket cells and other neurones of the cerebellar cortex. The efferent fibres from the cerebellar cortex are the Purkinje cell axons which traverse the granular cell layer to synapse in the central nuclei of the cerebellum.
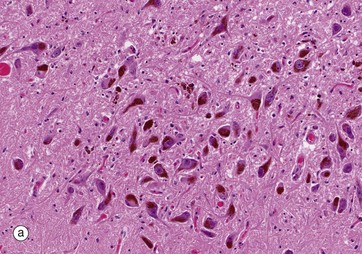
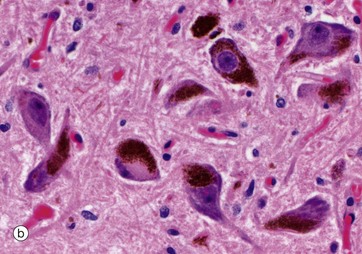
FIG. 20.15 Substantia nigra
(a) H&E (LP) (b) H&E (HP)
The substantia nigra is a large mass of grey matter extending throughout the midbrain; on each side it divides the cerebral peduncles into dorsal and ventral parts and, in sections of the midbrain as in micrograph (a), it is easily recognised by neurones containing dark pigment from which its name derives. The substantia nigra has extensive connections with the cortex, spinal cord, corpus striatum and reticular formation and appears to play an important part in the fine control of motor function.
The neurones of the substantia nigra are multipolar in form, and, in adults, the cytoplasm contains numerous granules of neuromelanin pigment as seen in micrograph (b). The pigmented neurones of the substantia nigra contain dopamine, which appears to act as a neurotransmitter causing inhibitory effects, particularly on neurones in the corpus striatum (one of the basal ganglia of the diencephalon).
Neuromelanin is contained in membrane-bound granules. Very little neuromelanin is present at birth, with the amount increasing during childhood and thereafter rising with increasing age. The origin of neuromelanin is still debated, with proposals that it is enzymatically generated or is merely an oxidation byproduct of dopamine synthesis. Functionally, it may sequester metals such as iron, as well as toxic organic compounds.
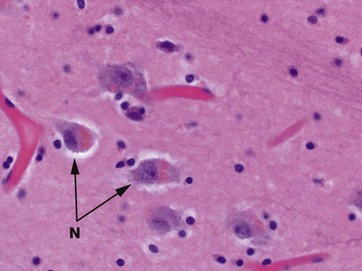
FIG. 20.16 Thalamus
H&E (HP)
The thalami are large masses of grey matter lying on each side of the third ventricle and comprising the main bulk of the diencephalon, the central core of the cerebrum. Other large basal ganglia include the corpus striatum and caudate nucleus. Functionally, the thalamus is subdivided into a large number of nuclei, including reticular and motor nuclei as well as specific sensory nuclei containing the cell bodies of neurones with axons projecting to the cerebral cortex. The thalamus constitutes an extremely complex relay and integration centre for information from almost all parts of the CNS. This micrograph shows the histological appearance of a typical basal nucleus, consisting of a loose aggregation of neurone cell bodies N in a background of neuropil. As an age-related change, these neurones have accumulated light brown lipofuscin pigment consisting of indigestible lipid byproducts.
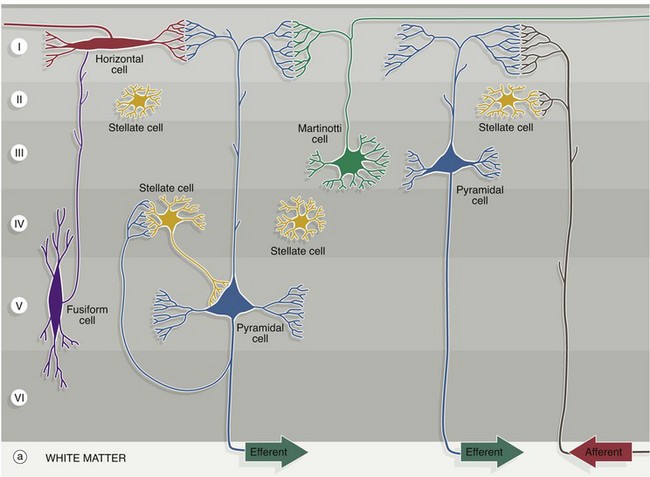
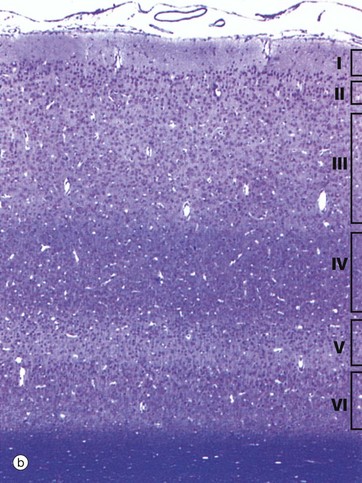
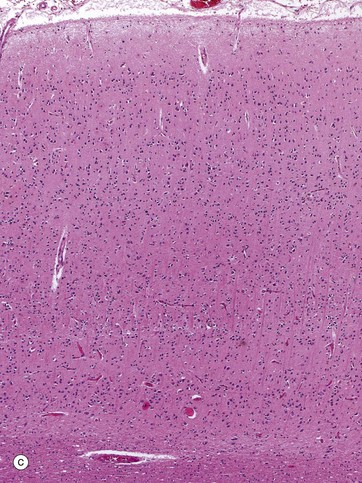
FIG. 20.17 Cerebral cortex (illustrations opposite)
(a) Neurone cell types diagram (b) Methylene blue (MP) (c) H&E (MP)
The cerebral hemispheres consist of a folded cortex of grey matter overlying white matter which conveys fibres between different parts of the cortex and to and from other parts of the CNS.
Histologically, the neurones of the cerebral cortex can be divided into five different morphological types which are arranged in several layers. The biologically oldest part of the cortex is concerned with olfaction (smell) and the neurones are arranged into three layers. In mammals, most of the cortex is called neocortex and consists of six layers. In humans, this constitutes about 90%; the three-layered pattern persists only in the olfactory cortex and in the cortical part of the limbic system in the temporal lobe.
The synaptic interconnections within the cortex are complex, with any one neurone synapsing with several hundred others. However, there are several basic principles of cortical organisation and function:
• Functional units are disposed vertically, corresponding to the general orientation of axons and major dendrites.
• Afferent fibres (incoming axons) generally synapse high in the cortex with the dendrites of efferent neurones whose cell bodies sit in deeper layers of the cortex.
• Efferent (outgoing) pathways, typically axons from pyramidal cells, tend to give off branches which pass back into more superficial layers and communicate with their own dendrites via interneuronal connections with other cell types.
The five characteristic types of cortical neurone are shown diagrammatically in (a), the pyramidal and stellate cells being by far the most common types:
• Pyramidal cells have pyramid-shaped cell bodies with the apex directed towards the cortical surface. From the apex, a thick branching dendrite passes towards the surface where it has an array of fine dendritic branches. An axon arises from the base of the cell and passes into the white matter though, in the case of small superficial cells, the axon may synapse in the deep layers of the cortex. Short dendrites also arise from the edges of the base and ramify laterally. The size of pyramidal cells varies, with the smallest tending to sit superficially. The huge upper motor neurones of the motor cortex, known as Betz cells, are the largest pyramidal cells in the cortex.
• Stellate (granule) cells are small neurones with a short vertical axon and several short branching dendrites, giving the cell body the shape of a star, although other shapes have been described. With routine histological methods, the cells look like small granules, giving rise to their alternative name.
• Cells of Martinotti are small polygonal cells with a few short dendrites; the axon extends towards the surface and bifurcates to run horizontally, usually in the most superficial layer.
• Fusiform cells are spindle-shaped cells oriented at right angles to the surface of the cerebral cortex. The axon arises from the side of the cell body and passes superficially. Dendrites extend from each end of the cell body, branching into deeper and more superficial layers.
• Horizontal cells of Cajal are small and spindle-shaped but oriented parallel to the surface. They are the least common cell type and are only found in the most superficial layer, where their axons pass laterally to synapse with the dendrites of pyramidal cells.
In addition to neurones, the cortex contains supporting neuroglial cells (astrocytes), oligodendroglia and microglia.
The neocortex is arranged into six layers, with the layers differing in neurone morphology, size and population density. They merge with one another, rather than being sharply demarcated. They also vary in thickness and detail from one region of the brain to another. Micrographs (b) and (c) illustrate the typical layered appearance of the cerebral cortex and its layers:
I Plexiform (molecular) layer. The superficial layer contains dendrites and axons of cortical neurones making synapses with one another; nuclei are sparse and are those of neuroglia and horizontal cells of Cajal.
II Outer granular layer. This contains a dense population of small pyramidal cells and stellate cells, admixed with various axons and dendritic connections from deeper layers.
III Pyramidal cell layer. Pyramidal cells of moderate size predominate in this broad layer, the cells increasing in size deeper in the layer. Martinotti cells are also present.
IV Inner granular layer. This layer consists mainly of densely packed stellate cells.
V Ganglionic layer. Large pyramidal cells and smaller numbers of stellate cells and cells of Martinotti make up this layer, its name originating from the huge pyramidal (ganglion) Betz cells of the motor cortex.
VI Multiform cell layer. This is named for the wide variety of differing morphological forms found in this layer. It contains numerous small pyramidal cells and cells of Martinotti as well as stellate cells, especially superficially, and fusiform cells in the deeper part.
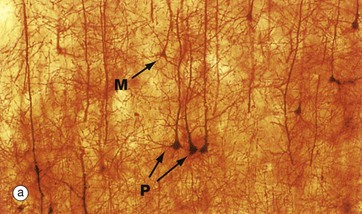
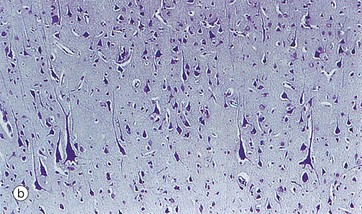
FIG. 20.18 Cortex
(a) Golgi method (MP) (b) Nissl method (MP)
Micrograph (a) shows a part of layer V; a thick section is employed, stained with a heavy metal impregnation technique which demonstrates considerable morphological detail. Several pyramidal cells P are easily identifiable, with their principal dendrites rising towards the cortical surface. Their axons are not seen as they are not in the plane of the section. A cell of Martinotti M is identified by its polygonal shape.
Micrograph (b) also shows layer V but, in this case, the section is thinner and stained by a routine histological method. At this magnification, there is little morphological detail; nevertheless, most of the cells are identifiable as pyramidal cells, increasing in size in the deeper part and including several very large cells.
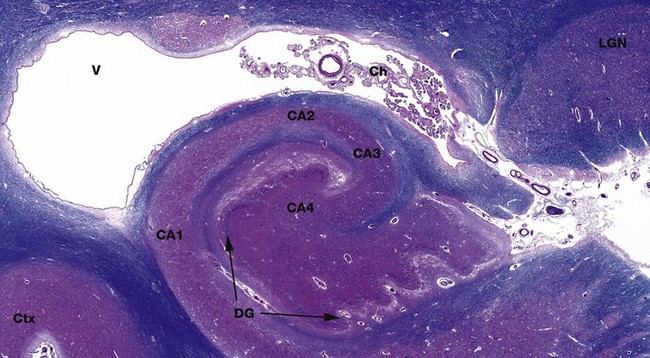
FIG. 20.19 Ammon's horn
(a) Luxol fast blue, H&E (LP)
Ammon's horn, more formally the cornus ammonis CA, is a recognisable structure in the hippocampus in the inferomedial part of the temporal lobe. Ammon's horn is named in reference to the coiled horns of sheep. Its main neurone layer has been traditionally divided into four areas CA1-CA4. The irregular folded segment is the end of the dentate gyrus DG, also part of the hippocampus. Above is the anterior part of the temporal horn of the lateral ventricle V, with CSF-producing choroid plexus Ch. The lateral geniculate nucleus LGN is a relay for visual information from the retina to the visual cortex in the occipital lobe. It has a layered architecture. The hippocampus is an important brain structure involved in long-term memory.