Supporting/connective tissues
Introduction
Supporting/connective tissue is the term applied to tissues which provide general structure, mechanical strength, space filling (sculpting body shape), and physical and metabolic support for more specialised tissues.
Connective tissues usually have three structural properties with corresponding construction materials:
• Tensile strength to resist pulling, stretching and tearing. This is provided by strong fibres of structural proteins from the collagen family.
• Elasticity to facilitate return to original shape after mechanical distortion. This is provided mostly by specialised elastin fibrils which function like rubber.
• Volume (i.e. bulk/substance). This is provided by glycoproteins and complex carbohydrates with profound water-binding ability, forming a wet gel known as ground substance.
The combined mix of fibres and ground substance is called extracellular matrix and this determines the physical properties of the tissue. Matrix is produced and assembled under the control of support cells, most commonly fibroblasts. The cells of supporting tissue are derived from precursor cells in primitive (fetal) supporting tissue called mesenchyme.
Supporting tissues occur with diverse physical properties. In most organs, loose connective tissue (also known as areolar tissue) acts as a biological packing and wrapping material. Tissue with a greater density of fibres provides a structural framework. Dense forms of supporting tissue provide tough physical support in the dermis of the skin, comprise the robust capsules of organs such as the liver and spleen, and the specialised high–tensile strength ligaments and tendons. Cartilage and bone, both major skeletal components, are specialised forms of connective tissue that are considered separately in Ch. 10.
Specialised fat storage is a further function, with adipose tissues having important metabolic roles. White adipose tissue also provides a structural fill and forms part of shock-absorbing padding. Highly metabolically active brown adipose tissue helps in the regulation of body temperature and body weight.
In addition, supporting tissues usually contain blood vessels, lymphatic vessels and associated nerves. Repair of tissue damage, especially wound closure and scar formation, is also largely a function of supporting tissues, involving both the support cells and blood and lymphatic vessels.
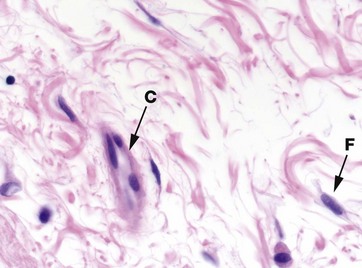
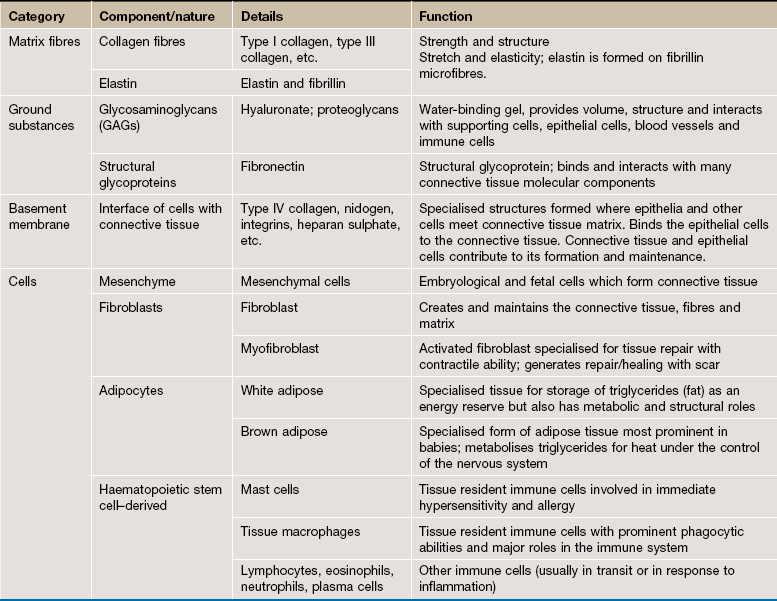
FIG. 4.1 Components of connective tissue
H&E (HP)
The components of connective tissue are seen in this micrograph of tissue from the submucosa of the bowel wall. The main component is extracellular matrix material which is largely composed of organized bundles of fibrous proteins, seen as wavy bundles of pink-stained material. Ground substance is unstained and is seen as the pale spaces between the pink-staining fibrous proteins.
The cell density of support tissues is generally low, reflected by the scattered cell nuclei seen in this type of tissue. The cells seen here are fibroblasts F with a few cells of the immune defence system. In the centre of this micrograph is a blood vessel C.
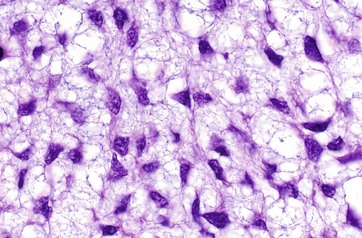
FIG. 4.2 Mesenchyme
H&E (HP)
Mesenchyme is the embryological tissue from which all types of supporting/connective tissue are derived. Mesenchymal cells are relatively unspecialised and are capable of differentiation into all supporting tissue cell types. Some mesenchymal cells remain in mature supporting tissue and act as stem cells (see Ch. 2).
Mesenchymal cells have an irregular, star (stellate) or spindle (fusiform) shape, with delicate branching cytoplasmic extensions which form an interlacing network throughout the tissue. The nuclei have dispersed chromatin and visible nucleoli. The matrix consists almost exclusively of blue-staining ground substance without mature fibres, facilitating diffusion of metabolites to and from developing tissues.
Fibres of Connective Tissue
The fibrous components of connective tissues are of two main types: collagen (including reticulin, which was formerly considered a separate fibre type) and elastin.
Collagen
Collagen is the main fibre type found in most supporting tissues and is the most abundant protein in the human body. Its notable function is the provision of tensile strength to resist pulling, stretching and tearing.
Collagen is secreted into the extracellular matrix by connective tissue cells (e.g. fibroblasts) in the form of a tropocollagen monomer. This consists of three polypeptide chains (each called an alpha chain and not necessarily all identical), bound together to form a helical protein structure 300 nm long and 1.5 nm in diameter. In the extracellular matrix, these tropocollagen molecules polymerise longitudinally and also side-to-side, forming collagen fibrils which are cross-linked by the enzyme lysyl oxidase.
At least 28 different types of collagen (designated by Roman numerals I to XXVIII) have now been delineated in the collagen super-family on the basis of morphology, amino acid composition and physical properties. Collagens can be fibre forming, mesh/network forming or cell membrane–associated proteins
• Type I collagen is the main structural collagen and is found in fibrous supporting tissue, skin (dermis), tendons, ligaments and bone. The tropocollagen molecules polymerise longitudinally and also side-to-side to form fibrils, and these are strengthened by numerous intermolecular bonds. Parallel collagen fibrils are further arranged into strong fibre bundles 2 to 10 µm in diameter, which confer great tensile strength to the tissue. These collagen fibres are visible with the light microscope, staining pink with H&E, with fibres in varying patterns of orientation, size and density according to the mechanical support required in the tissue.
• Type II collagen is the main structural collagen of hyaline cartilage and consists of fibrils in the cartilage ground substance.
• Type III collagen forms the delicate branched ‘reticular’ supporting meshwork which is prominent in highly cellular tissues such as the liver, bone marrow and lymphoid organs. This fibre was initially recognised by its affinity for silver salts and was (and often still is) called reticulin.
• Type IV collagen is a network/mesh-forming collagen and is an important constituent of basement membranes.
• Type VII collagen forms special anchoring fibrils that link extracellular matrix to basement membranes.
The remaining collagen types are present in various specialised situations.

FIG. 4.3 Collagen
(a) EM ×32 000 (b) SEM ×32 000, teased preparation
The typical appearance of type I collagen is shown here. The fibres are seen in transverse T and longitudinal L sections. A characteristic feature is the cross-banding, with a periodicity of about 64 nm which results from the polymerisation of the tropocollagen molecules (300 nm long) each overlapping the next by about a quarter of their length.
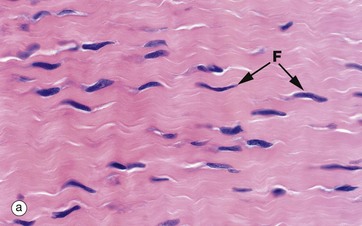
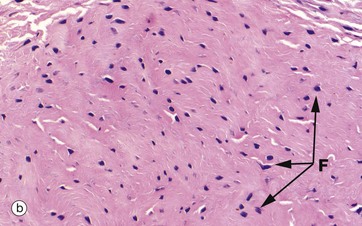
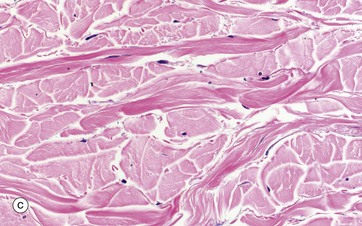
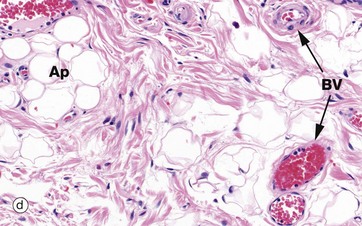
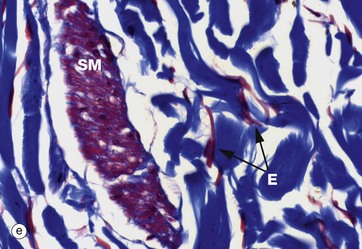
FIG. 4.4 Collagen
(a) H&E (HP) (b-d) H&E (MP) (e) Trichrome (HP)
These micrographs show variations in the size and packing of collagen fibres (type I collagen) and demonstrates fibroblasts F. Micrographs (a) and (b) are from a fascia in the hand, with (a) longitudinal and (b) transverse; in these the collagen fibres are large, tightly packed and oriented in one direction for maximal tensile strength. Micrograph (c) is from the dermis of the skin, with less tightly packed collagen fibres running perpendicular to each other (longitudinal and transverse) to give strength in both directions. Image (d) is fibroadipose tissue from a finger, with fine collagen fibres coursing between adipocytes Ap and blood vessels BV. Image (e) is a trichrome stain of skin; collagen stains blue, smooth muscle SM red, and elastin fibres E red.
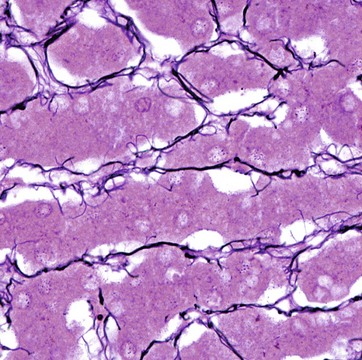
FIG. 4.5 Reticulin fibres (type III collagen)
Silver impregnation method/neutral red (HP)
Reticulin fibres form a delicate supporting framework for many cellular organs such as endocrine glands, lymph nodes, bone marrow and liver. A fine network of branching fibres ramifies throughout the parenchyma, often anchored to collagenous septa which traverse the tissue. Reticulin is a non-banded form of collagen, type III collagen.
Reticulin fibres stain poorly in H&E preparations but are able to absorb metallic silver, staining them black. Reticulin is the earliest type of collagen fibre to be produced during the development of all supporting tissues. It is found in varying quantities in most mature supporting tissues.
This micrograph shows the fine reticulin scaffolding of the liver; the framework supports the hepatocytes (the purple-stained plates of cells) and the sinusoids through which blood flows.
Elastin
Elastin is arranged as fibres and/or discontinuous sheets in the extracellular matrix where it confers the properties of stretching and elastic recoil. Elastin is a protein synthesised by fibroblasts in the form of a precursor monomer known as tropoelastin. The monomers are polymerised in the extracellular matrix by the enzyme lysyl oxidase, with extensive cross-linking of lysine amino acid side chains. Deposition of elastin in the form of fibres requires the presence of a template of microfibrils of the structural glycoprotein fibrillin and associated glycoproteins. These become incorporated around and within the ultimate elastic fibre.
Elastin is the name of both the fibre and the polymerised protein. There are also two named related fibres, oxytalan and elaunin, which have more fibrillin and less polymerised tropoelastin than generic elastin.
Elastin is found in varying proportions in most supporting tissues, conferring elasticity to enable recovery of tissue shape following normal physiological deformation. Elastin is present in large amounts in tissues such as lung, skin and urinary bladder. It is an important constituent of the wall of blood vessels; in arteries, elastin provides the stretch and recoil to smooth and transmit the pulse pressure generated by each heartbeat. In the lung, the stretch and recoil of the elastin is basic to that organ's function.
Elastic fibres are eosinophilic and when large they are slightly refractile, meaning they bend light differently to other tissue components. This may enable their recognition; however, special elastin stains are usually needed.
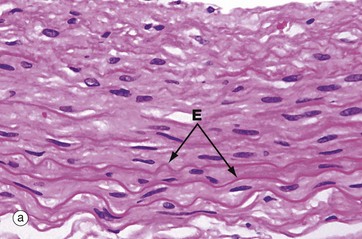

FIG. 4.6 Elastin fibres
(a) H&E (HP) (b) Elastin stain (HP)
Micrograph (a) shows the wall of an elastic artery, made up mainly of alternating smooth muscle cells and thick sheets of elastin admixed with collagen (see Ch. 8 for blood vessels). Like collagen and smooth muscle cytoplasm, elastin E is eosinophilic; it is recognisable here because the elastin sheets are thick and slightly refractile, slightly more eosinophilic than the other components and have a wave-like conformation due to relaxation of the vessel wall.
Micrograph (b) shows a histological section of an elastic artery stained specifically for elastin; with this method, elastin is stained black and collagen red. The functional properties of large arteries are mainly determined by the amount of elastin in their walls, which allows stretching and recoil with the pulse pressure generated by the heart.
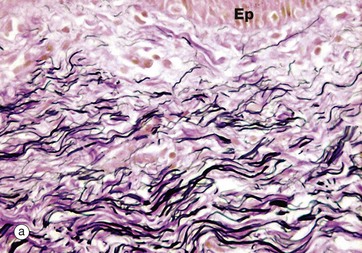
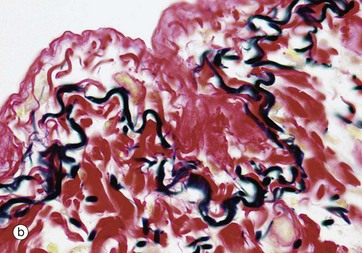
FIG. 4.7 Elastin fibres
(a) Elastin stain (MP) (b) Elastin stain (HP)
In micrograph (a) of skin, the pink-stained, coarse, closely packed bundles of collagen in the dermis are interwoven by elastic fibres, stained black. Elastic fibres in the dermis allow the skin to stretch and recoil, keeping it wrinkle-free. The epidermis Ep is just visible.
Micrograph (b) shows pleura (see Ch. 12) where a layer of elastic fibres, stained black, is woven into the collagen supporting tissues (stained red). The lung contains abundant elastic fibres which help to expel air in expiration.
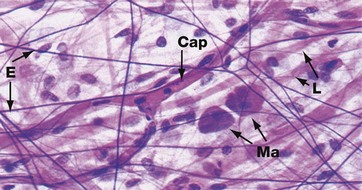
FIG. 4.8 Elastin fibres (spread preparation)
Elastin H&E (HP)
In most tissues, elastin occurs as short, branching fibres which form an irregular network throughout the tissue. This is not easily seen in tissue sections. It can be better demonstrated in spread preparations such as in this micrograph in which elastin fibres E are stained dark purple, collagen fibres L are stained pink and nuclei are stained blue. A branched capillary Cap crosses the field and two densely stained mast cells Ma are also seen (see Fig. 4.20).
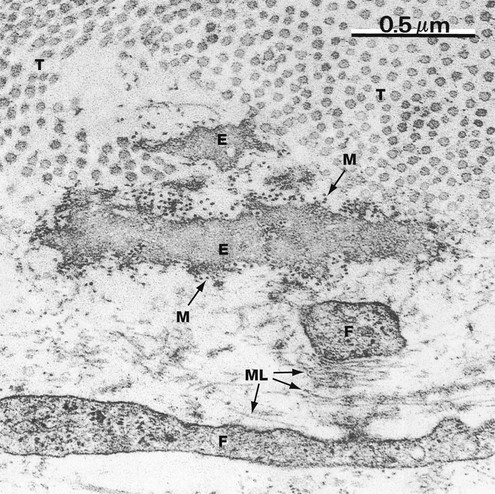
FIG. 4.9 Elastin
EM ×50 000
This micrograph shows elastin E in the delicate supporting tissue underlying the epithelium of mouse trachea. The field also contains collagen fibrils T (cut in transverse section) and the fine cytoplasmic extensions of fibroblasts F, responsible for elaboration of the extracellular constituents.
The elastin is mostly an amorphous mass of polymerised tropoelastin. Microfibrils M of the structural glycoprotein fibrillin, which is involved in the process of elastin deposition, can just be discerned at this magnification as dots (in transverse section) lying within and around the elastin. Microfibrils can also be seen in the lower part of the field cut in longitudinal section ML in association with small amounts of elastin protein.
Ground Substance
Ground substance derived its name from being an amorphous transparent material with the physical character of semi-solid gel. It is a mixture of glycoproteins and complex carbohydrates with profound water-binding ability. Extracellular fluid, both water and salts (particularly sodium), are bound to these molecules, providing volume and compression resistance to the tissue and its tissue turgor (i.e. the internal pressure). They form the physical milieu and indirectly control the passage of both molecules and cells through the tissue and the exchange of metabolites with the circulatory system.
The carbohydrates are long, unbranched polysaccharide chains of seven different types, each composed of repeating units of two sugar derivatives, usually a uronic acid and an amino sugar such as N-acetyl glucosamine. This gives rise to the term glycosaminoglycan (GAG).
Hyaluronate, also known as hyaluronic acid, consists of repeating D-glucuronate (β1,3)-N-acetyl-D-glucosamine units and is the predominant GAG, forming huge unbranching linear molecules of 100,000 to 10,000,000 molecular weight.
The other GAGs include chondroitin-4-sulphate, chondroitin-6-sulphate, dermatan sulphate, keratan sulphate, heparan sulphate and heparin sulphate. Each of these molecules contains sulphated N-acetyl groups substituted onto galactosamine sugars in the repeating carbohydrate units, making them highly negatively charged (acidic). These charged groups prevent the carbohydrate chains from folding into globular aggregates, causing them to remain in an expanded linear form, thereby occupying a large volume for a small mass. The charged side groups also render them extremely hydrophilic, attracting a large volume of water and positive ions, particularly sodium.
These GAGs (other than hyaluronate) exist as carbohydrate chains covalently linked to various protein molecules, forming a range of molecular structures containing up to 90% to 95% carbohydrate. These are called proteoglycans. There are numerous specific proteins, including perlecan, syndecan, decorin, lumican and aggrecan. Proteoglycans have various specific functions. Some bind to hyaluronic acid producing massive quaternary structures, others interact with collagens or bind to various other matrix molecules including remodelling enzymes, enzyme inhibitors, growth factors, cytokines and cell surface receptors.
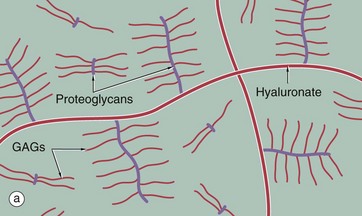
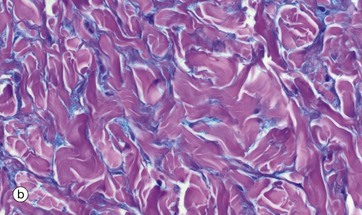
FIG. 4.10 Ground substance
(a) Diagram (b) Alcian blue PAS (HP)
The diagram (a) represents long, linear hyaluronate molecules and proteoglycans with their covalently attached glycosaminoglycans (GAGs) in a gel. Micrograph (b) shows ground substance in the form of the wispy blue-staining material between pink collagen fibres in a micrograph of skin. Ground substance can be sometimes seen in H&E sections of connective tissue as a background pale blue colour between collagen fibres, but can be seen more clearly with appropriate special stains as here. It was, and sometimes still is, referred to as tissue mucin (historically proteoglycans were called mucoproteins and GAGs mucopolysaccharides).
Structural glycoproteins
In addition to the proteoglycans, there are further glycoprotein molecules important in ground substance. These include two fibril-forming molecules, fibrillin (discussed in the section on elastin) and fibronectin.
Fibronectin has binding sites for many connective tissue components and plays a part in controlling the deposition and orientation of collagen in extracellular matrix. Fibronectin molecules bind to collagens, to heparan sulphate (a GAG) and to specific membrane receptors on cells.
Cell membranes incorporate a group of transmembrane protein complexes called integrins, which act as cell adhesion molecules. One of these acts as a fibronectin receptor; it binds internally within the cytoplasm to actin filaments of the cytoskeleton and externally binds with the fibronectin. This interaction forms part of a specialised layer of extracellular matrix called basement membrane where cells meet matrix. Here other non-filamentous glycoproteins also play a structural role (see opposite).
Basement Membranes
Basement membranes are sheet-like arrangements of extracellular matrix proteins which act as an interface between the support tissues and epithelial or parenchymal cells. Basement membranes are also associated with blood vessels and muscle cells and form a limiting membrane around the central nervous system. The term derives from the initial recognition of membranes lying beneath the basal cells of epithelia. In the context of muscle and nervous tissue, the term external lamina is often applied.
Basement membranes have several functions:
• Provide physical binding of the epithelium to the underlying tissue and physical support
• Control of epithelial growth and differentiation, they form a barrier to downward epithelial growth; this is only breached if epithelia undergo malignant transformation (cancer).
• Permit the flow of nutrients, metabolites and other molecules to and from an epithelium, as epithelium is devoid of blood vessels
• Where a cell layer acts as a selective barrier to the passage of molecules from one compartment to another (e.g. between the lumen of blood vessels and adjacent tissues), the basement membrane assumes a critical role in regulating permeability. This role of forming a selective barrier reaches an extreme level of sophistication in the kidney, where the glomerular basement membrane is part of the highly selective filter for molecules passing from the bloodstream into the urine.
The main components of basement membranes and external laminae are the glycosaminoglycan heparan sulphate, type IV collagen, and the structural glycoproteins fibronectin, laminins and nidogen-1. While fibronectin appears to be produced by fibroblasts of the supporting tissue, the rest are at least partly elaborated by the tissues being supported.
The structural framework is a fine meshwork of type IV collagen, a mesh/network-forming collagen found exclusively in basement membranes. The glycoprotein laminin, in concert with nidogen (also called entactin), binds the type IV collagen and links to other basement membrane constituents and to laminin receptors on the basal plasma membranes of the epithelial cells.
Type III collagen (reticulin) is bound via the fibrillar glycoprotein fibronectin to integrins in the epithelial basal plasma membrane. Fibronectin also binds the GAG heparan sulphate. Basement membranes vary in their molecular details between sites and between types of epithelia.
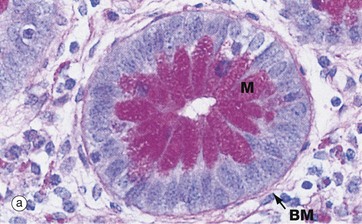
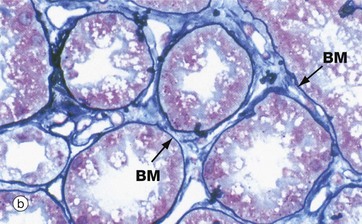
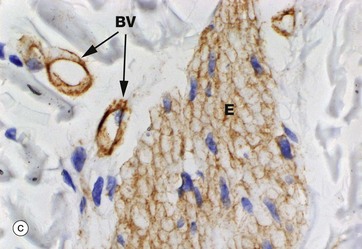
FIG. 4.11 Basement membrane
(a) PAS haematoxylin (HP) (b) Jones methenamine silver (MP) (c) Immunoshistochemical stain for type IV collagen (MP)
Basement membranes can be seen beneath epithelia when the membrane is relatively thick or when histochemical or other methods for basement membrane components are used.
Micrograph (a) is of a duodenal crypt lined by mucus-secreting cells stained with PAS. The PAS reacts with the complex carbohydrates in the proteoglycans of the basement membrane BM and with the mucin M in secretory vacuoles in the apical cytoplasm of the epithelial cells. In (b), a silver impregnation method with affinity for reticulin highlights the basement membrane BM of renal tubules. In (c), an immunohistochemical stain using an antibody to type IV collagen shows the basement membrane around blood vessels BV and external laminae E around individual smooth muscle cells in a hair-related muscle in skin; the muscle cell cytoplasm is unstained, although the muscle cell nuclei can be seen.
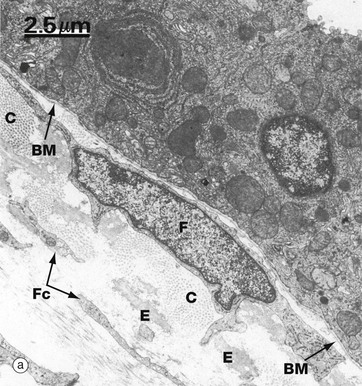
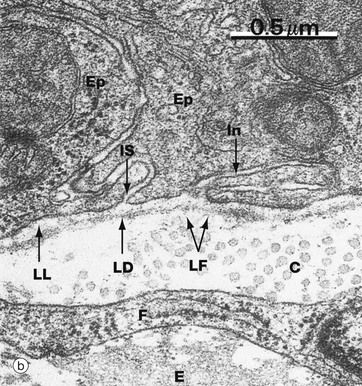
FIG. 4.12 Basement membrane
(a) EM ×5000 (b) EM ×45 000
Micrograph (a) shows the epithelial lining of mouse trachea. A basement membrane BM can be seen separating it from the underlying supporting tissue which contains the cell body and nucleus of a fibroblast F, numerous fine fibroblast cytoplasmic processes Fc, bundles of collagen fibrils C (cut in transverse section) and elastin fibres E.
At higher magnification in micrograph (b), the basement membrane can be seen to have three layers. The relatively electron-lucent lamina lucida LL (ranging from 10-50 nm in width) abuts the basal epithelial cell plasma membrane. The intermediate layer is electron-dense and is called the lamina densa LD (20-300 nm thick). Beyond the lamina densa is the broad, relatively electron-lucent lamina fibroreticularis LF, which merges with the fibrous and fibrillary (reticular) components of the underlying supporting tissue. In this field, note collagen fibrils C, part of a fibroblast F, and elastin E. The basement membrane typically passes uninterrupted beneath the intercellular space IS between two epithelial cells Ep and beneath a basal invagination In in of one of these cells.
The lamina densa was formerly known as the basal lamina. The terms basal lamina and basement membrane were used interchangeably until it was realised that the single layer seen with the light microscope corresponded to the combination of all three layers seen with the electron microscope. If used, the term basal lamina should be confined to meaning lamina densa.
The Cells of Supporting/Connective Tissue
The cells of supporting tissue are derived from precursor cells in primitive (fetal) supporting tissue called mesenchyme. Their dominant common function is synthesis, maintenance and recycling of extracellular matrix material.
• Fibroblasts secrete, maintain and recycle the matrix in most tissues.
• Myofibroblasts are an activated form of fibroblast associated with repair. They have a contractile function as well as a role in secretion of matrix.
• Adipocytes are modified support cells specialised in the storage and metabolism of fat; collectively they form adipose tissue.
• Chondrocytes, osteoblasts and osteocytes are responsible for secreting and maintaining the matrix in cartilage and bone, respectively (see Ch. 10).
With the important exception of cartilage, supporting tissues are vascularised, containing arteries, capillaries, veins and lymphatics with associated cells of the innate immune system, such as mast cells and tissue macrophages. Cells of the adaptive immune system access the tissues via the blood vessels.
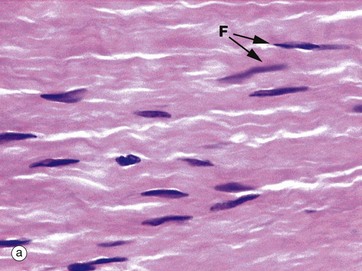
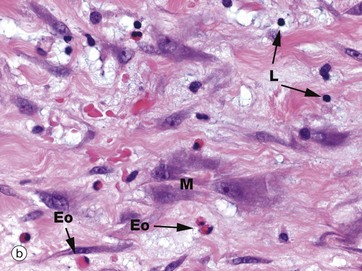
FIG. 4.13 Fibroblasts and myofibroblasts
(a, b) H&E (MP)
Micrograph (a) demonstrates the typical histological appearance of mature fibroblasts in collagenous tissue; collagen fibres are stained pink. The fibroblast nuclei F are condensed and elongated in the direction of the collagen fibres. The cytoplasm is relatively scanty and barely visible. The cell is long and thin with fine cytoplasmic processes extending into the matrix. The function of fibroblasts is to maintain the integrity of supporting tissue, including continuous turnover of its constituents.
Micrograph (b) demonstrates active fibroblasts called myofibroblasts M in repair of damaged tissue with early fibrosis/scarring. The nuclei are large with prominent nucleoli and the cytoplasm is extensive and basophilic. The basophilic extracellular matrix is prominent. A few eosinophils Eo and lymphocytes L are present. These myofibroblasts have a contractile function and play an important role in the contraction and shrinkage of the resultant scar tissue.
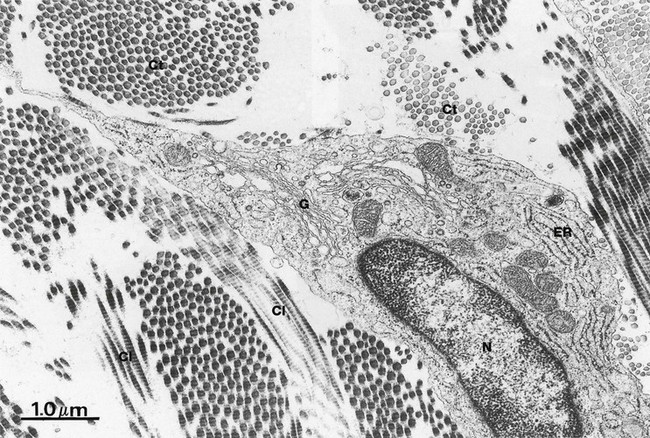
FIG. 4.14 Fibroblasts
EM ×18 500
This micrograph illustrates the body of a mature fibroblast within supporting tissue. The nucleus N is moderately condensed and nucleoli are not seen here. The small quantity of cytoplasm is mostly occupied by rough endoplasmic reticulum ER, reflecting the dominant protein-secreting function of this type of cell. The Golgi apparatus G is visible and a few mitochondria are present. Bundles of collagen fibrils are seen in transverse Ct and longitudinal section Cl in the extracellular matrix.
During active synthesis of extracellular fibres, fibroblast cytoplasm becomes markedly expanded, with the rough ER and Golgi apparatus becoming prominent features. Fibroblasts synthesise and secrete the glycosaminoglycans, collagen, elastin and all other extracellular constituents; however, in mature fibroblasts, few secretory vesicles are found.
Adipose Tissue
Many supporting tissues contain cells specialised for the storage of fat; these cells are called adipocytes. They are ultimately derived from mesenchyme. The recognisable precursors to adipocytes are called lipoblasts. Adipocytes are found in isolation or in small clusters throughout loose supporting tissues and are found as the main cell type in adipose tissues.
The fat in adipocytes is stored as triglycerides and is derived from three main sources: dietary fats circulating in the bloodstream as chylomicrons, triglycerides synthesised in the liver and transported in blood in lipoproteins, and triglycerides synthesised from glucose within adipocytes. Adipose tissue was regarded as an inactive energy store, but it is an extremely important participant in general metabolic processes, acting as a store of substrate for the energy-generating processes of almost all tissues.
Reflecting this metabolic importance, adipose tissue generally has a rich blood supply, although the capillaries can be collapsed and inconspicuous in tissue sections. The rate of fat deposition and utilisation within adipose tissue is largely determined by dietary intake and energy expenditure, but a number of hormones profoundly influence metabolism. Adipocytes have receptors for insulin, glucocorticoids, growth hormone and noradrenalin (norepinephrine) that modulate uptake and release of fat.
In addition, adipocytes have an endocrine role. Adipocytes modulate energy metabolism and influence general metabolism through secretion of several protein messengers and in coordination with hormones such as insulin, contribute to regulation of body mass. The protein messengers from adipose tissue have been collectively called adipocytokines and include leptin, adipsin, resistin, adiponectin, tumour necrosis factor (TNF)-α, and plasminogen activator inhibitor type 1. Note that this includes proteins from both adipocytes and tissue macrophages. The protein hormone leptin is involved in the regulation of appetite.
There are two main types of adipose tissue, which have been called white and brown. White adipose tissue is often macroscopically a pale yellow, while brown adipose tissue is so named as it has a darker brown tint. Triglycerides are liquid at body temperature and the mobility and feel of adipose tissue to palpation is determined by the amount of fibrous supporting tissue components at the individual sites.
White adipose tissue
This type of adipose tissue comprises up to 20% of total body weight in normal (target weight) well-nourished male adults and up to 25% in females but can reach more than 50% in obesity. It is distributed throughout the body, particularly in the deep layers of the skin. Its roles include:
• Triglyceride storage and mobilisation
• Structural fill; fills in spaces such as in pelvic and perirectal areas and axilla. Contributes to sculpting body shape and outline.
• Acts as a thermal insulator under the skin
• Forms part of shock-absorbing padding e.g. around kidneys. Fat divided into lobules, each surrounded by fibrous tissue, forms a flexible and deformable cushion against compression, essentially a biological bubble wrap. The skin generally uses this architecture, but it is particularly prominent in finger pulps and the soles of feet.
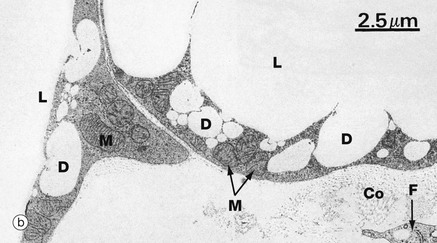
FIG. 4.15 White adipose tissue
(a) H&E (HP) (b) EM ×6000
The typical appearance of white adipose tissue is illustrated in micrograph (a). Adipose tissue is pale staining because virtually all the cell is occupied by lipid, which is dissolved out in paraffin-embedded tissue preparations. The cell membrane, a thin rim of peripheral cytoplasm and the external lamina collectively give a ‘chicken-wire’ appearance.
Fat stored in adipocytes accumulates as lipid droplets that fuse to form a single large droplet which distends and occupies most of the cytoplasm. The adipocyte nucleus N is compressed and displaced to one side of the stored lipid droplet and the cytoplasm is reduced to a small rim around the periphery. In some cells, tangential slicing of the top or bottom of a cell is seen as a sheet of pink-stained cytoplasm P. Note the minute-appearing blood capillaries C compared with the size of the surrounding adipocytes.
The EM (b) shows the periphery of two adjacent adipocytes. Contrary to the impression given by light microscopy, the main lipid droplet L in each cell has an irregular outline with numerous tiny droplets D at the periphery in the process of fusion with the main droplet. The lipid is not bounded by a membrane. The thin rim of cytoplasm contains the usual organelles, most notably mitochondria M. Each adipocyte is surrounded by an external lamina. In the adjacent extracellular tissue, a fibroblast cytoplasmic process F and collagen fibrils Co can be seen.
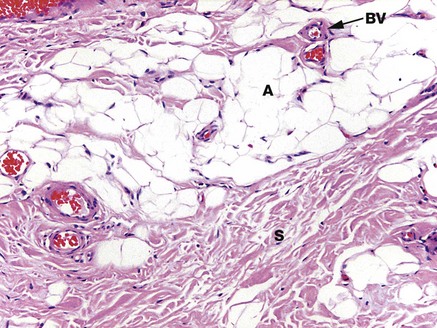
FIG. 4.16 Fibroadipose tissue
H&E (MP)
This micrograph demonstrates the typical appearance of adipocytes A scattered within collagenous supporting tissue. Adipocytes occur here either singly or in small groups. There are several small blood vessels BV with thin walls, and abundant collagen fibres both as fine fibres dividing the adipocytes into small groups and in broad arrays called septa S which provide structural strength.
Brown adipose tissue
This variant of adipose tissue is found particularly in newborn mammals and some hibernating animals. Only small amounts of brown adipose tissue are found in human adults, mainly around the adrenals. This tissue is rich in mitochondria and specialised for generation of heat; it plays a part in body temperature regulation.
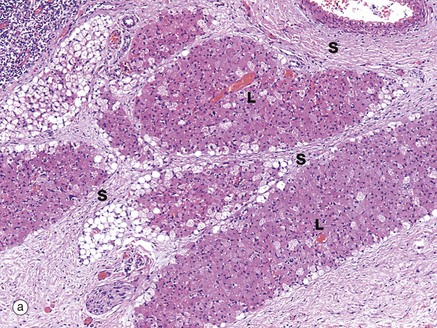
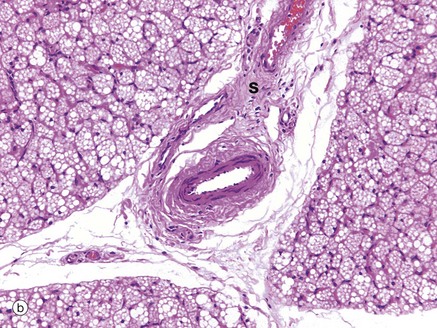
FIG. 4.17 Brown adipose tissue
(a) H&E (LP) (b) H&E (MP)
These micrographs demonstrate the typical histological appearance of brown adipose tissue. As seen in micrographs (a) and (b), brown adipose tissue is arranged in lobules Lo separated by fibrous septa S which convey blood vessels and sympathetic nerve fibres.
At low magnification, two cell morphologies (appearances) can be seen. Many cells, and especially cells at the centre of lobules, are pink-stained due to cytoplasm packed with mitochondria. Others, especially those at the periphery of lobules, have pale-stained cytoplasm due to the presence of multiple vesicles containing lipid.
Brown adipose tissue is involved in non-shivering thermogenesis, an increase in metabolic activity producing heat and induced by cold stress. Brown adipose tissue is characterised by expression of a unique uncoupling protein, UCP1 (uncoupling protein 1, previously called thermogenin). This protein, in association with several other modulating factors, serves to uncouple mitochondrial metabolism from production of ATP to produce heat.
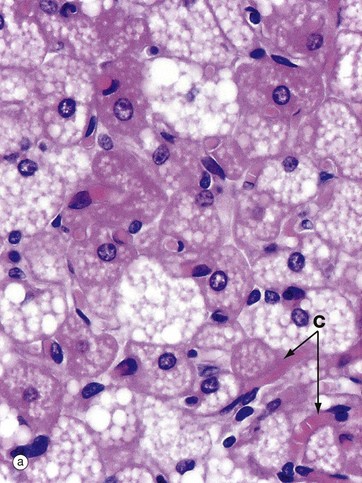
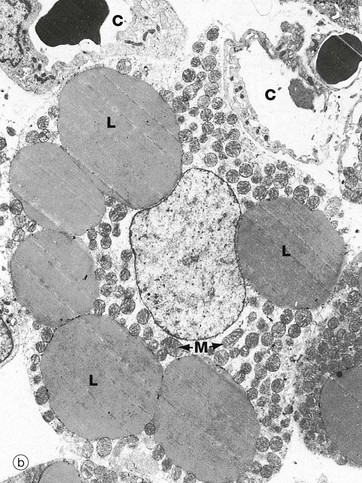
FIG. 4.18 Brown adipose tissue
(a) H&E (HP) (b) EM ×4070, rabbit
At high magnification in micrograph (a), the nuclei of brown adipocytes are seen to be eccentrically located within the cell but, unlike those of white adipocytes, the nuclei are large and surrounded by a significant quantity of strongly eosinophilic cytoplasm. The stored lipid is contained within multiple droplets, all of which have been dissolved away during tissue processing. Note the rich network of capillaries C between the brown adipocytes.
The electron micrograph (b) shows brown adipose tissue taken from a newborn rabbit and readily demonstrates the multilocular nature of stored lipid L. The cytoplasm of brown adipocytes is crammed with mitochondria M which have numerous closely packed cristae. These mitochondria are extremely rich in cytochromes, part of the electron transport chain involved in oxidative energy production; this accounts for the brown colour of brown adipose tissue when examined macroscopically.
Unlike the metabolism of other tissues, in brown adipose cells the process of electron transport is readily uncoupled from the phosphorylation of ADP to form ATP. The energy derived from oxidation of lipids, and energy released by electron transport in the uncoupled state, is dissipated as heat which is conducted to the rest of the body by the rich vascular network. Note the intimate association of capillaries C with the brown adipocyte in this electron micrograph.
Human neonates and other baby mammals utilise brown adipose tissue to generate body heat during the vulnerable period after birth. Brown adipose tissue undergoes involution in early infancy and in adult humans is found only in restricted sites such as around the adrenal gland and great vessels in retroperitoneal fat. The production of heat by brown adipose tissue is controlled directly by the sympathetic nervous system.
The Defence Cells in Supporting Tissue
The supporting tissues not only contain cells responsible for their synthesis, maintenance and metabolic activity, but also a variety of cells with defence and immune functions. Traditionally, these cells have been divided into two categories: fixed (intrinsic) cells and wandering (extrinsic) cells.
The intrinsic defence cells of supporting tissue are the tissue-fixed macrophages (histiocytes) and mast cells. Tissue-fixed macrophages are now generally believed to be derived from circulating monocytes (see Fig. 3.16) which have become at least temporarily resident in supporting tissues. Mast cells are similar to basophils in structure and function (see Fig. 3.11), but there are structural differences which show that mast cells are not merely basophils resident in the tissues.
The wandering category of defence and immune cells includes all the remaining members of the white blood cell series (see Ch. 3). Although leucocytes (white blood cells) are usually considered as a constituent of blood, their principal site of activity is outside the blood circulation, within tissues. Leucocytes are normally found only in relatively small numbers but, in response to tissue injury and other disease processes, their numbers increase greatly. The supporting tissues of those regions of the body which are subject to the constant threat of pathogenic invasion, such as the gastrointestinal and respiratory tracts, contain a larger population of leucocytes, maintaining constant surveillance.
Macrophage phagocyte system
Particulate matter injected into circulation and normal constituents such as old red blood cells are cleared from the circulation by cells of the bone marrow, liver, spleen and lymph nodes. The common function of the responsible cells is their ability to phagocytose particulate matter; they can be called phagocytes. These cells are derived from haematopoietic stem cells in the marrow and are also called tissue macrophages (see Fig. 4.19). It is convenient to refer to them and their functions as a group, the macrophage phagocyte system (MPS).
Historically, these were referred to as the reticuloendothelial system (RES). Phagocytic cells are found associated with vascular and lymphatic spaces in liver, bone marrow and spleen and were incorrectly thought to have features in common with the endothelial cells (see Ch. 8). These organs also tend to be rich in a supporting framework of reticulin fibres (see Fig. 4.5) upon which are draped cells with long cytoplasmic processes described as reticulum or reticular cells. Given these associations they were thought to represent a single functional ‘reticuloendothelial’ system. The term is still commonly used to refer to the MPS.
Dendritic cells and histiocytes
Amongst the cell types found in tissues, particularly those in contact with the external environment (e.g. skin, nose, lung, stomach, intestines) are cells which may have long, branched cytoplasmic processes called dendrites, hence dendritic cells, but with a specific function as antigen-presenting cells (APCs). These cells phagocytose, partially digest and present antigen to T cells with coexpressed membrane T cell–activating molecules, thereby inducing immune responses to antigens (see Ch. 11). They migrate to lymph nodes when stimulated.
Dendritic cells of skin are named Langerhans cells. The APCs have an origin from haematopoietic stem cells in the bone marrow, many with a monocyte lineage (myeloid dendritic cells), while some have a plasma cell–like appearance (plasmacytoid dendritic cell). Dendritic cells express Toll-like receptors (TLRs), a type of receptor that recognizes molecules common to several bacterial species.
Other cells such as reticular cells may have long dendritic processes but are mesenchymal supporting cells, not APCs. There is thus a potential difficulty with terminology due to overlap between morphology (dendritic shape) and function (APC). Because APCs are phagocytic, they have historically been called histiocytes, a term also used for macrophages.
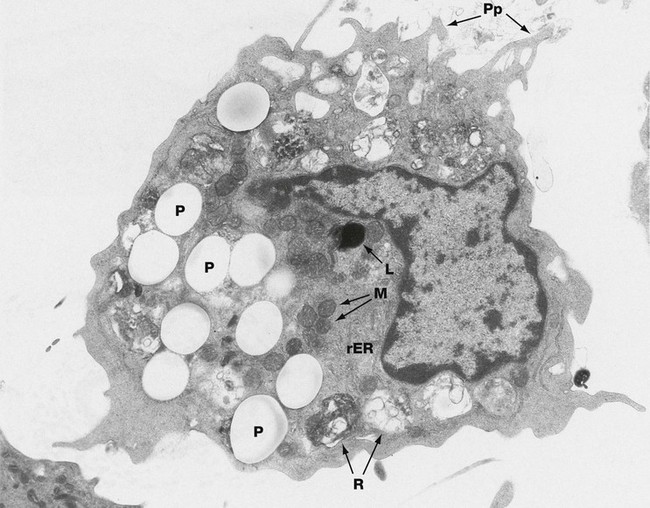
FIG. 4.19 Macrophage
EM ×11 600
The ultrastructural features of macrophages vary widely according to their state of activity and tissue location. This micrograph shows an active macrophage obtained from the peritoneum of a rat which had previously been injected intraperitoneally with latex particles; a number of particles P have been engulfed by the macrophage.
The macrophage nucleus is irregular, with heterochromatin typically clumped around the nuclear envelope. The cytoplasm contains a few mitochondria M and a variable amount of free ribosomes and rough endoplasmic reticulum rER. In quiescent macrophages, lysosomes L are abundant, but their number is much reduced in actively phagocytic cells; additional lysosomes are later produced via the Golgi apparatus. The macrophage cytoplasm contains an assortment of phagosomes and residual bodies R. Residual material may be released from the macrophage by exocytosis or may remain sequestered in the tissues, as occurs with the dyes used in tattooing of the skin. Actively phagocytic cells exhibit irregular cytoplasmic projections or pseudopodia Pp which are involved in amoeboid movement and phagocytosis.
In addition to their role as tissue scavengers, macrophages play an important role in the adaptive immune system (see Ch. 11). Macrophages process antigenic material before presenting it to memory lymphocytes, thus acting as APCs; lymphocytes are then stimulated to undergo specific immune responses. As a result of various immune mechanisms, antigenic material may become combined or coated with substances such as antibodies and complement, which are then collectively known as opsonins, a process known as opsonisation. Opsonins are recognised by surface receptors on the macrophage surface and this greatly enhances the phagocytic ability of macrophages and other phagocytes such as neutrophils (see Chs 3 and 11). Cytokines released during the immune response, especially cytokines from activated T cells, act directly on macrophages to increase greatly their metabolic and phagocytic activity. Macrophages also secrete a variety of cytokines that act to enhance local and systemic immune responses.
In some disease states, under cytokine stimulation macrophages may develop an enhanced phenotype for killing microorganisms, the epithelioid macrophage, particularly seen in diseases such as tuberculosis and leprosy. Macrophages can also fuse to form multinucleated histiocytes/giant cells.
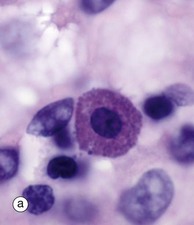
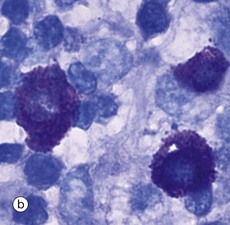
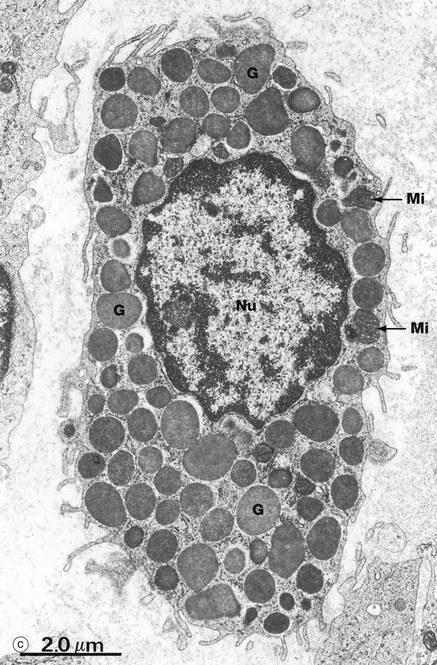
FIG. 4.20 Mast cells
(a) H&E (HP) (b) Toluidine blue (HP) (c) EM ×12 000
Mast cells are found in all supporting tissues but are particularly prevalent in the skin, gastrointestinal lining, the serosal lining of the peritoneal and pleural cavities and around blood vessels. Their major constituents and functions are very similar to those of basophils, to which they are related (Fig. 3.11). Mast cells are long-lived with the ability to proliferate in the tissues. Mast cell degranulation results in the release of histamine and other vasoactive mediators which induce the immediate hypersensitivity (anaphylactoid) response characteristic of urticaria, allergic rhinitis, asthma and anaphylactic shock.
Mast cells may be inconspicuous in routine histological sections due to the water solubility of their densely basophilic granules, which tend to be lost during preparation. Special techniques of fixation, embedding and staining may be employed. With suitable preparation, micrograph (a), however, the characteristic feature of mast cells is an extensive cytoplasm packed with large granules; these are smaller in size, though more numerous, than those of basophils. When stained with certain blue basic dyes such as toluidine blue, the granules bind to the dye, changing its colour. This property of staining a different colour to the dye is known as metachromasia, micrograph (b).
In the electron micrograph (c), mast cell granules G are seen to be membrane bound and to contain a dense amorphous material. The granules are liberated from the cell by exocytosis when stimulated during an inflammatory or allergic response. The cytoplasm contains a few rounded mitochondria Mi and a little rough endoplasmic reticulum. The non-segmented nucleus Nu has less condensed chromatin than that of basophils. Other differences from basophils include a more uniform distribution of their thin surface processes, a greater number of cytoplasmic filaments and a lack of glycogen granules.
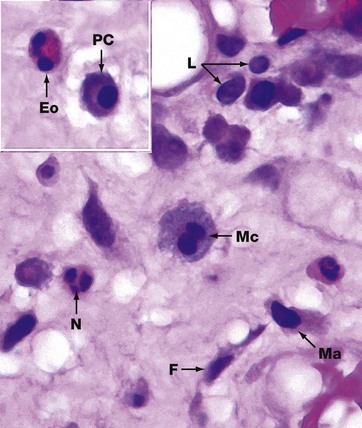
FIG. 4.21 Leucocytes in loose connective tissue
H&E (HP)
The appearance of leucocytes in tissue sections differs from their appearance in blood smears (see Ch. 3). In this micrograph, a variety of leucocytes are seen in the loose supporting tissue beneath the nasal epithelium. Fibroblasts F are identified by their elongated nuclei. Neutrophils N have multilobed nuclei and pale-staining cytoplasm. Eosinophils Eo are recognised by their bilobed nuclei and strongly eosinophilic cytoplasmic granules. Mast cells Ma show finely granular cytoplasm and often an eccentric rounded nucleus.
Lymphocytes L have small, densely stained nuclei and a thin halo of poorly stained cytoplasm. Lymphocyte nuclei are approximately 7 to 8 µm in diameter and provide a useful reference for the size of other cells in tissue sections. Plasma cells PC have eccentric round nuclei, abundant amphophilic cytoplasm and a pale-stained perinuclear area (hof) which represents a well-developed Golgi apparatus.
When they have been actively phagocytic, macrophages may be recognised by their large size and cytoplasmic content of engulfed material. Note, however, that active macrophages have an extremely variable appearance depending on the nature of their phagocytic activity. The macrophage shown in this tissue Mc has a granular pale purple cytoplasm, possibly representing mucous material phagocytosed from the local environment.