Nervous tissues
Introduction
The nervous system provides rapid and precise communication between different parts of the body via the action of specialised nerve cells called neurones. These highly specialised cells are interconnected and function to gather and process information and then generate appropriate response signals. The nervous system is divided into two main parts:
• The central nervous system (CNS) comprising the brain and spinal cord
• The peripheral nervous system (PNS) comprising the nerves which run between the CNS and other tissues, together with nerve ‘relay stations’ termed ganglia
Histologically, the entire nervous system merely consists of variations in the arrangement of neurones and their supporting tissues. Functionally, the nervous system can be divided into the somatic nervous system, which is involved in voluntary functions, and the autonomic nervous system which exerts control over many involuntary functions. The autonomic system can be further divided into sympathetic and parasympathetic systems.
The functions of the nervous system depend on a fundamental property of neurones called excitability. As in all cells, the resting neurone maintains an ionic gradient across its plasma membrane, thereby creating an electrical potential. Excitability involves transiently changing the membrane permeability in response to appropriate stimuli so that the ionic gradient is partially reversed and the plasma membrane loses its electrical potential (i.e. becomes depolarised). Depolarisation itself simulates adjacent plasma membrane to depolarise, causing a wave of depolarisation called an action potential to spread along the plasma membrane. This is followed by rapid repolarisation in which the membrane re-establishes its resting potential.
At sites of intercommunication between neurones called synapses, transfer of the action potential (the message) is via chemicals. Depolarisation of the neurone causes the release of chemical transmitter substances, neurotransmitters, which stimulate receptors in the adjacent neurone, modulating the potential for or triggering an action potential. Neurones are arranged in pathways, often with many interconnecting neurones, for the conduction of action potentials from receptors to effector (acting) organs. Neurotransmitters serve not only to mediate neurone-to-neurone transmission but also act as chemical signals from neurones to receptors on the effector organs which also exhibit excitability.
The effector organs of the voluntary somatic pathways are generally skeletal muscle causing movement, while those of involuntary pathways are usually smooth muscle, cardiac muscle and muscle-like epithelial cells (myoepithelial cells) within exocrine glands.
This chapter encompasses nerve cells and their supporting cells, focused on the histological structure of the peripheral nervous system and simple types of sensory receptors. Details of the supporting cells and arrangement of nervous tissue in the central nervous system are the subject of Ch. 20, while the structure of the highly specialised organs of sensory reception, eyes and ears, is presented in Ch. 21.
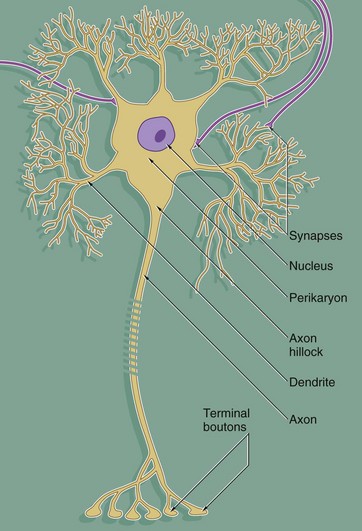
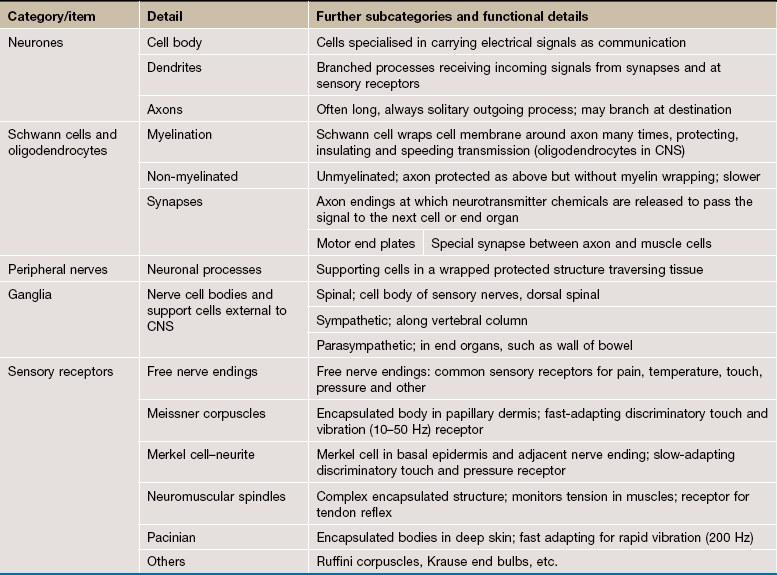
FIG. 7.1 The neurone
Despite great variation in size and shape in different parts of the nervous system, all neurones have the same basic structure as shown in this idealised diagram. The neurone consists of a large cell body containing the nucleus surrounded by cytoplasm known as the perikaryon. Processes of two types extend from the cell body, namely a single axon and one or more dendrites. Dendrites are highly branched tapering processes which either end in specialised sensory receptors (as in primary sensory neurones) or form synapses with neighbouring neurones from which they receive stimuli. In general, dendrites function as the major sites of information input into the neurone.
Each neurone has a single axon arising from a cone-shaped portion of the cell body called the axon hillock. The axon is a cylindrical process up to 1 metre in length, terminating on other neurones or effector organs by way of a variable number of small branches which end in small swellings called terminal boutons. Action potentials arise in the cell body as a result of integration of afferent (incoming) stimuli; action potentials are then conducted along the axon to influence other neurones or effector organs by release of neurotransmitter chemicals. Axons are commonly referred to as nerve fibres.
In general, the cell bodies of all neurones are located in the central nervous system; exceptions are the cell bodies of most primary sensory neurones and the terminal effector neurones of the autonomic nervous system where, in both cases, the cell bodies lie in aggregations called ganglia in peripheral sites.
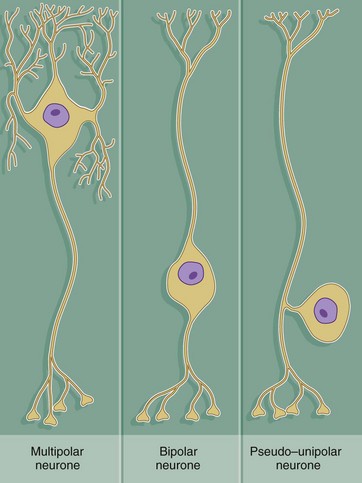
FIG. 7.2 Basic neurone types
Throughout the nervous system, neurones have a wide variety of shapes which fall into three main patterns according to the arrangement of the axon and dendrites with respect to the cell body.
The most common form is the multipolar neurone in which numerous dendrites project from the cell body; the dendrites may all arise from one pole of the cell body or may extend from all areas of the cell body surface. In general, intermediate, integratory and motor neurones conform to this pattern.
Bipolar neurones have only a single dendrite which arises from the pole of the cell body opposite to the origin of the axon. These unusual neurones act as receptor neurones for the senses of smell, sight and balance. Most other primary sensory neurones are described as pseudo-unipolar neurones, since a single dendrite and the axon arise from a common stem of the cell body; this stem is formed by the fusion of the first part of the dendrite and axon of a bipolar type of neurone during embryological development.
As a general rule, neurone impulses are conveyed along dendrites towards the nerve cell body (afferent) while axons usually convey impulses away from the nerve cell body (efferent).
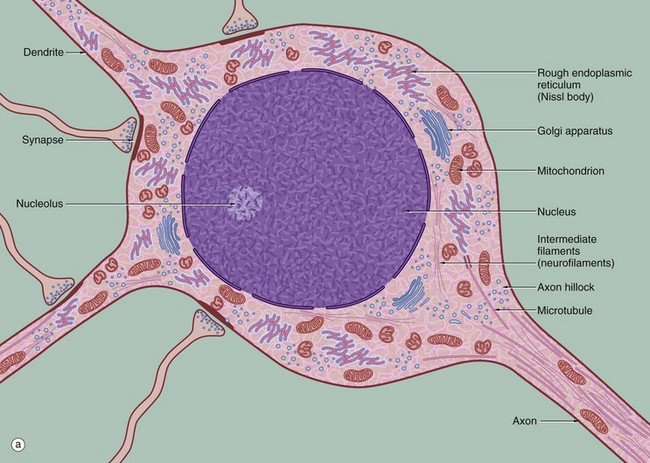
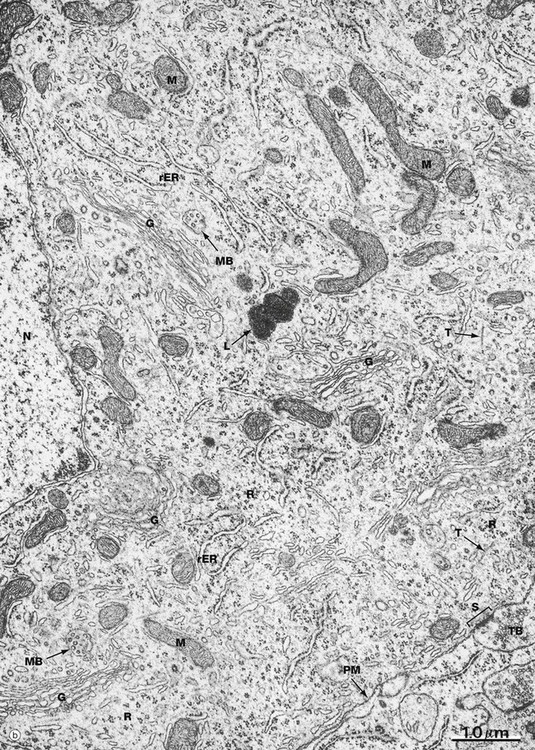
FIG. 7.3 Ultrastructure of the neurone (illustration (b) opposite)
(a) Schematic diagram (b) EM ×19 000
The diagram (a) illustrates the main ultrastructural features of the neurone, in this case a multipolar neurone with an axon and two dendrites. The nucleus is large, round or ovoid and usually centrally located within the perikaryon. Reflecting the intense metabolic activity of the neurone (and consequent need to replace proteins which are rapidly turned over), the chromatin is completely dispersed and the nucleolus is a conspicuous feature.
The cytoplasm of the cell body contains large aggregations of rough endoplasmic reticulum which correspond to the Nissl substance of light microscopy (see Fig. 7.4); the rough endoplasmic reticulum extends into the dendrites but not into the axon hillock or axon. Rough endoplasmic reticulum is a much more prominent feature in large neurones, such as somatic motor neurones, than in smaller neurones such as those of the autonomic nervous system. A diffuse Golgi apparatus is found adjacent to the nucleus. Smooth endoplasmic reticulum is not a prominent feature of the perikaryon, but tubules, cisternae and vesicles are prominent in the axon and dendrites. The mitochondria of the perikaryon are numerous and have the usual rod-like appearances; those of the axon are extremely slender and elongated.
Neurones are very metabolically active and expend much energy in maintaining ionic gradients across the plasma membrane. Neurones synthesise neurotransmitter substances or their precursors in the perikaryon from where they are transported along the axon to the synapse to be released when appropriately stimulated.
Numerous intermediate filaments (neurofilaments) and microtubules are arranged in parallel bundles throughout the perikaryon and along the length of the axon and dendrites. The electron micrograph (b) shows part of the cell body of a neurone and includes a portion of the nucleus N. At the lower right, part of the neuronal plasma membrane PM, is seen, including a synapse S with the terminal bouton TB of an adjacent neurone. Features of the cytoplasm of the perikaryon are areas of rough endoplasmic reticulum rER, free ribosomes R and scattered mitochondria M. An extensive Golgi apparatus G is represented by several stacks of flattened membranous cisternae. Associated with the Golgi are several multivesicular bodies MB, which are involved in transport to other organelles including lysosomes L. Microtubules T can be identified in oblique section, but neurofilaments are not readily identifiable.
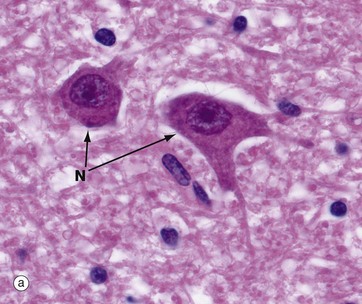
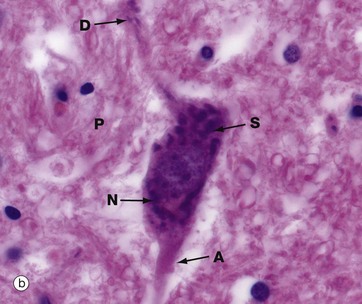
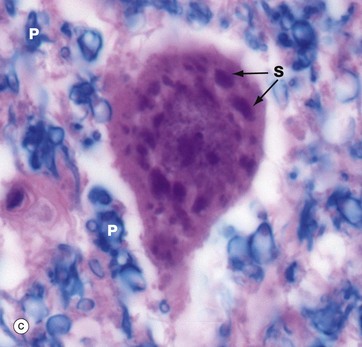
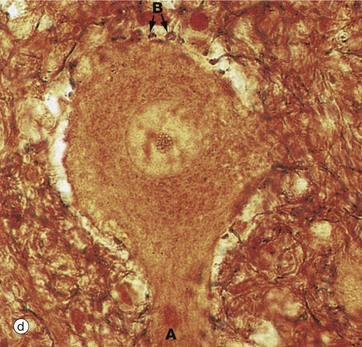
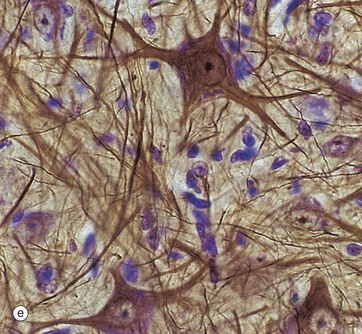
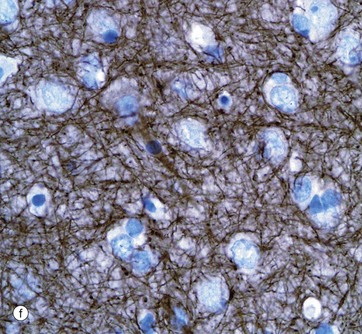
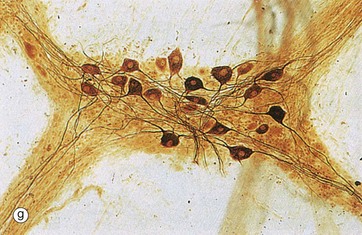
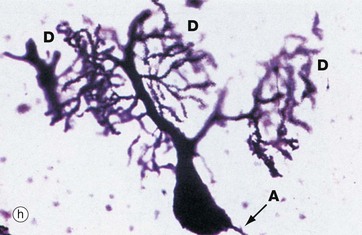
FIG. 7.4 Neurones and methods of study with light microscopy (illustrations (a–f) opposite)
(a, b) H&E (HP) (c) Luxol fast blue, H&E (HP) (d) Gold method (HP) (e) Gold/toluidine blue (HP) (f) Immunohistochemistry for neurofilament protein (HP) (g) Spread preparation, gold method (MP) (h) Golgi-Cox (HP)
Special staining techniques are required to show the rich structural detail of the nervous system. Heavy metal impregnation techniques with gold and silver are valuable in the study of neurone morphology and were widely employed by the pioneers of neuroanatomy such as Cajal and Golgi, from whom they take their names. Likewise, spread preparations often permit the examination of complete neurones and their cytoplasmic processes. Immunohistochemistry can also be used to identify neurone-specific proteins (e.g. neurofilament protein).
Micrographs (a) and (b), stained with H&E, show neurones N in the central nervous system; the nuclei are huge in comparison with those of surrounding support cells. Dispersed chromatin and prominent nucleoli reflect a high level of protein synthesis. Neurones have extensive cytoplasm which is basophilic (blue stained) due to extensive ribosomal RNA. Many of the ribosomes are associated with rough endoplasmic reticulum, found gathered in patches called Nissl substance S, which extends into dendrites D but not the axon A. In H&E preparations, virtually no detail can be seen of cytoplasmic processes, which merge into a fibrillary background termed the neuropil P composed of both nerve cell and support cell processes.
In micrograph (c), the Nissl substance is seen as dark purple material giving the neuronal cytoplasm a mottled appearance. Here the myelin (see below) is stained blue, demonstrating the structure of the neuropil P. A very similar neurone is shown in micrograph (d) using a heavy metal impregnation technique that highlights small axons. Numerous axons from other neurones with tiny terminal boutons B can be seen forming synapses with the cell body.
Micrograph (e) employs another gold method providing excellent detail of neuronal shape and showing the cytoskeleton in the dendrites and axons; the blue counter-stain demonstrates the nuclei of surrounding support cells. Note that detail in the neuronal processes is lost as they pass out of the plane of focus.
Micrograph (f) shows an area of the brain stained with an antibody to neurofilament protein. This highlights the complex network of axons within the neuropil between neurones. Neurones are seen as clear spaces containing a nucleus in this preparation.
Spread preparations, as shown in micrograph (g), also outline the complexity of nerve cell processes, both axons and dendrites. Here, neurones in a small peripheral ganglion and their main cytoplasmic processes are clearly delineated.
Micrograph (h) illustrates a very thick section stained by a silver impregnation method and shows a Purkinje cell in the cerebellar cortex. These cells have a single small axon A at one pole and an extraordinary finely branching dendritic tree D at the other pole. Note that the base of the dendritic system is in this case much larger than that of the axon.
Myelinated and Non-Myelinated Nerve Fibres
In the peripheral nervous system, all axons are enveloped by highly specialised cells called Schwann cells, which provide structural and metabolic support. In general, small-diameter axons (e.g. those of the autonomic nervous system and small pain fibres) are simply enveloped by the cytoplasm of Schwann cells; these nerve fibres are said to be non-myelinated. Large-diameter fibres are wrapped by a variable number of concentric layers of the Schwann cell plasma membrane forming a myelin sheath; such nerve fibres are said to be myelinated. Within the central nervous system, myelination is similar to that in the peripheral nervous system except that the myelin sheaths are formed by cells called oligodendrocytes (see Fig. 20.3). There are distinct chemical differences between central and peripheral myelin. In all nerve fibres, the rate of conduction of action potentials is proportional to the diameter of the axon; however, myelination greatly increases axon conduction velocity compared with that of a non-myelinated fibre of the same diameter.
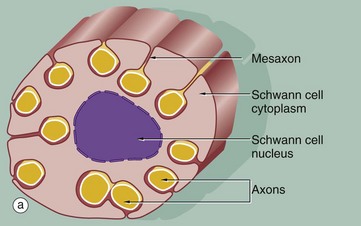
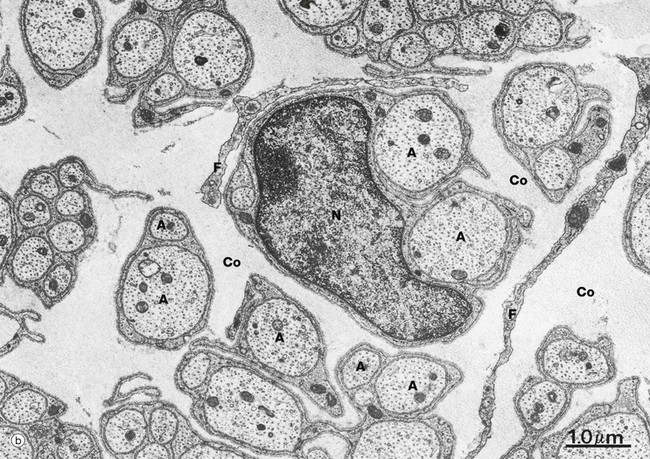
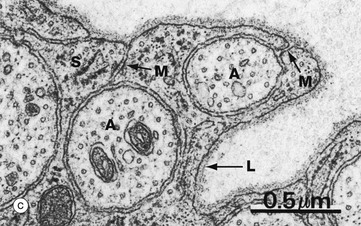
FIG. 7.5 Non-myelinated nerve fibres
(a) Diagram (b) EM ×15 000 (c) EM ×36 000
The relationship of non-myelinated axons with their supporting Schwann cell is illustrated in diagram (a). One or more axons become longitudinally invaginated into the Schwann cell so that each axon is embedded in a channel, invested by the Schwann cell plasma membrane and cytoplasm. The Schwann cell plasma membrane becomes apposed to itself along the opening of the channel, thus effectively sealing the axon within an extracellular compartment bounded by the Schwann cell. The zone of apposition of the Schwann cell membrane is called the mesaxon. Note that more than one axon may occupy a single channel within the Schwann cell. Each Schwann cell extends for only a short distance along the nerve tract, and at its termination the ensheathment is continued by another Schwann cell with which it interdigitates closely end to end.
At low magnification in micrograph (b), non-myelinated axons A of various sizes are seen ensheathed by Schwann cells S; one of the Schwann cells has been sectioned transversely through its nucleus N. Note the variable number of axons enclosed by each Schwann cell. Delicate cytoplasmic extensions of fibroblasts F can be seen in the endoneurium.
At high magnification in micrograph (c), part of the cytoplasm of a Schwann cell S is shown ensheathing several axons A; axons are readily identified by their content of smooth endoplasmic reticulum and microtubules, seen in cross-section. Several mesaxons M can be seen. The external surface of the Schwann cell is bounded by an external lamina L, equivalent to lamina densa in epithelia.
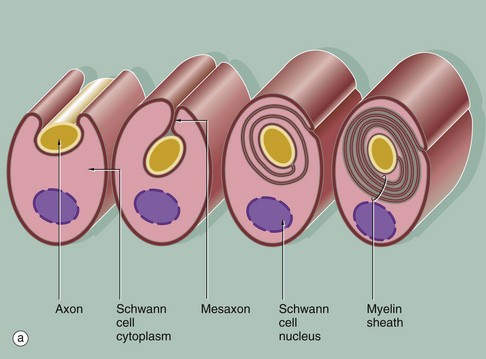
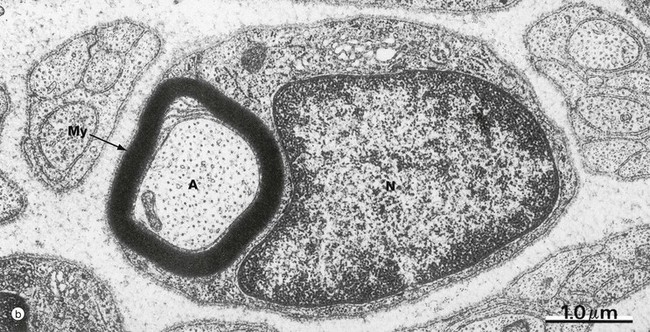
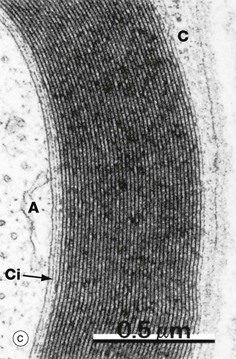
FIG. 7.6 Myelinated nerve fibre
(a) Diagram (b) EM ×20 000 (c) EM ×46 000
In peripheral nerves, myelination begins with the invagination of a single nerve axon into a Schwann cell; a mesaxon is then formed. As myelination proceeds, the mesaxon rotates around the axon thereby enveloping the axon in concentric layers of Schwann cell cytoplasm and plasma membrane. The cytoplasm is then excluded so that the inner leaflets of plasma membrane fuse with each other and the axon becomes surrounded by multiple layers of membrane which together constitute the myelin sheath. The single segment of myelin produced by each Schwann cell is termed an internode; this ensheaths the axon between one node of Ranvier and the next (see Fig. 7.7).
In micrograph (b), a myelinated nerve fibre from the PNS is sectioned transversely at the level of the nucleus of an ensheathing Schwann cell N. The single axon A is enveloped by many layers of fused Schwann cell plasma membrane forming the myelin sheath My.
Micrograph (c) shows that the compact myelin sheath consists of many regular layers of membrane. The darker lines, termed the major dense lines, arise by fusion of cytoplasmic leaflets. The intervening intraperiod lines represent closely apposed external membrane leaflets. The substantial lipid content of these modified membrane layers insulate the underlying axon A, preventing ion fluxes across the axonal plasma membrane except at the nodes of Ranvier. The main bulk of the Schwann cell cytoplasm C encircles the myelin sheath. However, a thin layer of Schwann cell cytoplasm also persists immediately surrounding the axon Ci.
In the CNS, oligodendrocytes are responsible for myelination; a single oligodendrocyte, however, forms multiple myelin internodes which contribute to the ensheathment of as many as 50 individual axons (see Fig. 20.3).
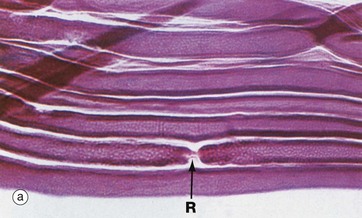
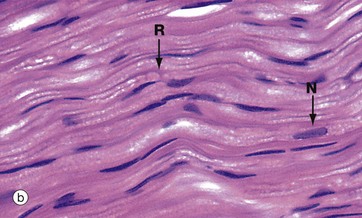
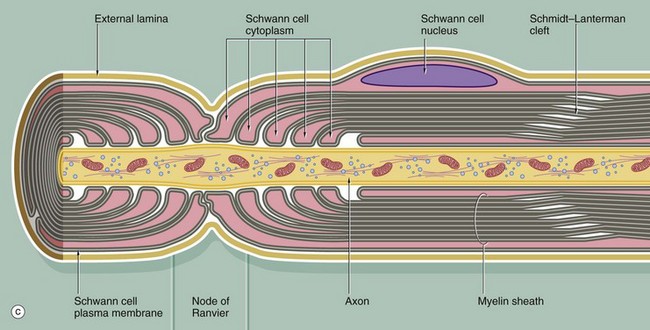
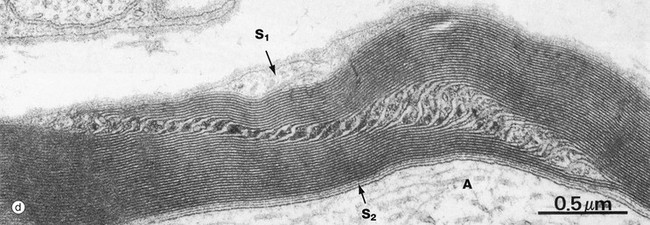
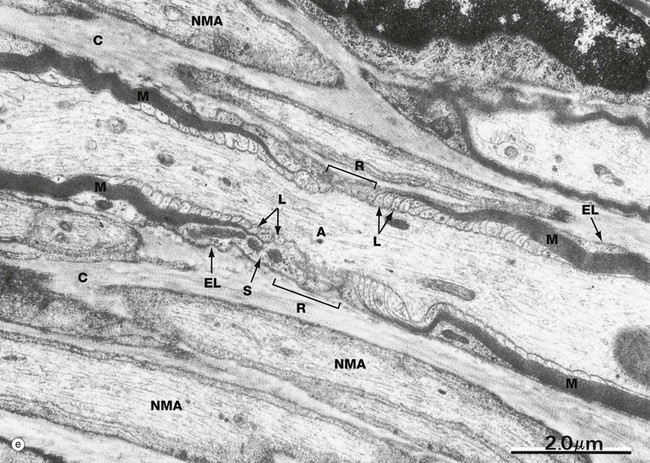
FIG. 7.7 Nodes of Ranvier and Schmidt-Lanterman incisures (illustrations (a–d) opposite)
(a) Teased preparation, Sudan black (MP) (b) H&E (MP) (c) Schematic diagram (d) EM ×42 000 (e) EM ×14 000
The myelin sheath of an individual axon is provided by many Schwann cells (oligodendrocytes in the CNS), each Schwann cell covering only a segment of the axon. Between the Schwann cells, there are short intervals where the axon is not covered by a myelin sheath; these points are known as nodes of Ranvier.
Micrograph (a) shows a node of Ranvier R in a teased preparation of myelinated axons. With this method, only the lipid of the myelin has been stained and thus Schwann cell nuclei are not seen.
Micrograph (b) shows axons in longitudinal section stained with H&E. Due to a fixation artifact, myelin sheaths appear ‘bubbly’; the lipid is mostly dissolved out during preparation and is therefore unstained. A node of Ranvier R is identifiable in the large axon in midfield. These are very difficult to see in routine preparations. Most of the elongated nuclei N are those of Schwann cells.
Diagram (c) illustrates the manner in which Schwann cells terminate at the node of Ranvier, so exposing the axon to the surrounding environment. Note the manner in which cytoplasmic processes of adjacent Schwann cells interdigitate at the node; also note the continuation of the Schwann cell basement membrane (external lamina) across the node. The myelin sheath prevents the nerve action potential from being propagated continuously along the axon, and the action potential travels by jumping from node to node. This mode of conduction, known as saltatory conduction, greatly enhances the conduction velocity of axons. The internodal length is related to the diameter of the axon and may be up to 1.5 mm in the largest fibres.
Micrograph (e) illustrates the ultrastructure of a node of Ranvier R. The axon A is characterised by numerous neurofilaments, microtubules and elongated mitochondria. A myelin sheath M can be identified at each end of the field, the myelin becoming progressively thinner as it approaches the node. This is because, as it approaches the node, each compact major dense line expands to form a small membrane loop L containing Schwann cell cytoplasm, the loops directly abutting the axonal plasma membrane. Externally, a broader layer of Schwann cell cytoplasm S containing mitochondria envelops the nodal area. Note the external lamina EL of the Schwann cell and collagen fibrils C in surrounding endoneurium. Several non-myelinated axons NMA are seen nearby.
At certain points within the internodal myelin sheath, narrow channels of cytoplasm are retained and connect the main bulk of the Schwann cell cytoplasm peripherally to the narrow zone of Schwann cell cytoplasm adjacent to the axon. These uncompacted regions are known as Schmidt-Lanterman incisures or clefts; in longitudinal section, as in electron micrograph (d), the incisure passes obliquely across the width of the compact sheath. The axon is marked A, the peripheral Schwann cell cytoplasm S1 and the periaxonal Schwann cell cytoplasm S2.
The layers of cell membrane that form myelin are bound together by special proteins that differ between the central and peripheral nervous systems (CNS and PNS). In the CNS, proteolipid protein links the exoplasmic surfaces, while cytoplasmic surfaces are linked by myelin basic protein. In the PNS, P0 protein associates with myelin basic protein to form the major dense line. PNS myelin also contains peripheral myelin protein-22.
Synapses and Neuromuscular Junctions
Synapses are highly specialised intercellular junctions which allow communication by linking neurones to neurones. Individual neurones communicate via a widely variable number of synapses, depending on their location and function. Classically, the axon of one neurone synapses with the dendrite of another neurone (axodendritic synapse), but axons may synapse with the cell bodies of other neurones (axosomatic synapses) or with other axons (axoaxonic synapses); dendrite-to-dendrite and cell body–to–cell body synapses have also been described.
The mechanism of conduction of the nerve impulse involves the release from one neurone of a chemical neurotransmitter which then diffuses across the narrow intercellular space in the synapse to induce excitation or inhibition in the target neurone or effector cell of that synapse. This is achieved via specific receptors for the neurotransmitter incorporated in the opposing plasma membrane. Neurotransmitter is cleared from the synaptic cleft by specific enzymatic degradation or reuptake by the axon or both, freeing the synapse for further signals.
The chemical nature of neurotransmitters varies and the morphology of synapses are highly variable in different parts of the nervous system, but the principle of synaptic transmission and the basic structure of synapses are similar throughout. For a given synapse, the signal transmission is unidirectional, but the response on the target cell may be either excitatory or inhibitory, depending on the specific synapse and its location.
Similar intercellular junctions link neurones to their effector cells, such as muscle fibres; where neurones synapse with skeletal muscle, the specialised synapses are referred to as neuromuscular junctions or motor end plates.
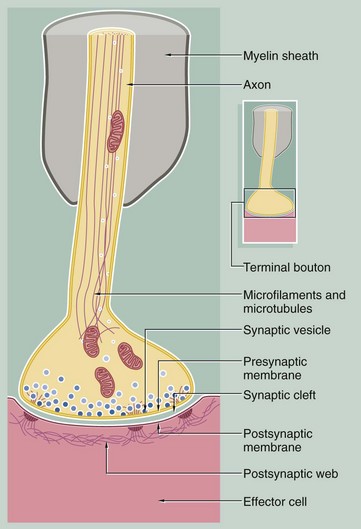
FIG. 7.8 Synapse
This diagram illustrates the general structure of the synapse. The axon responsible for propagating the stimulus terminates at a bulbous swelling or terminal bouton; this is separated from the plasma membrane of the opposed neurone or effector cell by a narrow intercellular gap of uniform width (20–30 nm) called the synaptic cleft. The terminal boutons are not myelinated. The boutons contain mitochondria and membrane-bound vesicles of neurotransmitter substance known as synaptic vesicles which are approximately 50 nm in diameter.
There are many different types of neurotransmitter substance which are different in CNS and PNS e.g. acetylcholine, noradrenaline (norepinephrine), glutamate or dopamine. Synaptic vesicles are transported into the synaptic bouton down the axon from the cell body. Vesicles can also be formed in the synaptic bouton by recycling of vesicle membrane. Protein synthesis can also occur in the synaptic bouton.
Synaptic vesicles aggregate towards the presynaptic membrane and, on arrival of an action potential, dock with the membrane and release their contents into the synaptic cleft by exocytosis (see Ch. 1). The neurotransmitter diffuses across the synaptic cleft to stimulate receptors in the postsynaptic membrane. Associated with synapses are a variety of biochemical mechanisms such as hydrolytic and oxidative enzymes which inactivate the released neurotransmitter between successive nerve impulses. Transmitter may also be taken up back into the terminal bouton and be recycled into new synaptic vesicles. The cytoplasm beneath the postsynaptic membrane often contains a feltwork of fine fibrils, the postsynaptic web, which may be associated with desmosome-like structures in maintaining the integrity of the synapse.
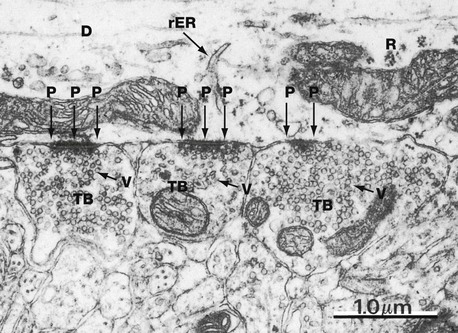
FIG. 7.9 Axodendritic synapse
EM ×22 000
This micrograph from the CNS illustrates three terminal boutons TB (probably from different axons) forming synapses with a dendrite D. The dendrite can be identified as such by its content of ribosomes R and rough endoplasmic reticulum rER (which are not present in axons). Note the presence of numerous uniform-sized synaptic vesicles V and a few mitochondria M within the terminal boutons. The postsynaptic density P contributes to the structural stability of the closely apposed pre- and postsynaptic membranes.
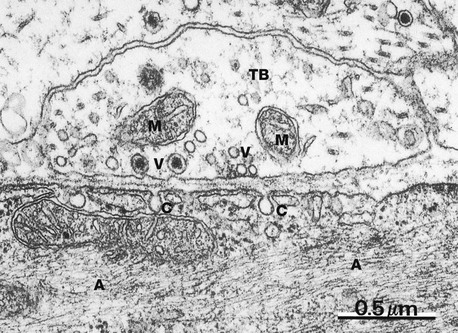
FIG. 7.10 Autonomic synapse
EM ×42 000
This micrograph illustrates a synapse between an axon of the autonomic nervous system and a smooth muscle cell in the intestine. The terminal bouton TB contains mitochondria M and a number of synaptic vesicles V, some of which contain a dense central core, probably representing an electron-dense carrier protein; such dense core vesicles are a feature of autonomic synapses. Frequently more than one neurotransmitter substance is present in individual autonomic neurones.
The postsynaptic membrane exhibits flask-like invaginations C which may represent caveolae. Note the uniform width of the synaptic cleft between the pre- and postsynaptic membranes. The smooth muscle cell contains numerous fine actin microfilaments A.
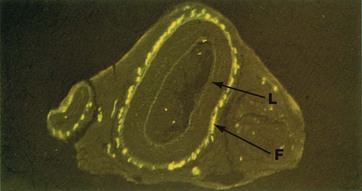
FIG. 7.11 Sympathetic nerve endings
Formalin-induced fluorescence (MP)
Noradrenaline (norepinephrine) is the main postganglionic neurotransmitter in the sympathetic nervous system. When noradrenaline combines with formalin (and some other compounds) it becomes fluorescent and can be visualised by fluorescence microscopy.
This micrograph illustrates formalin-induced fluorescence F in the outer layer of large and small arteries, corresponding to the presence of sympathetic noradrenergic nerve endings. Weak background autofluorescence outlines the general structure; note that the internal elastic lamina L (see Fig. 8.10) of the large artery in midfield is particularly autofluorescent.
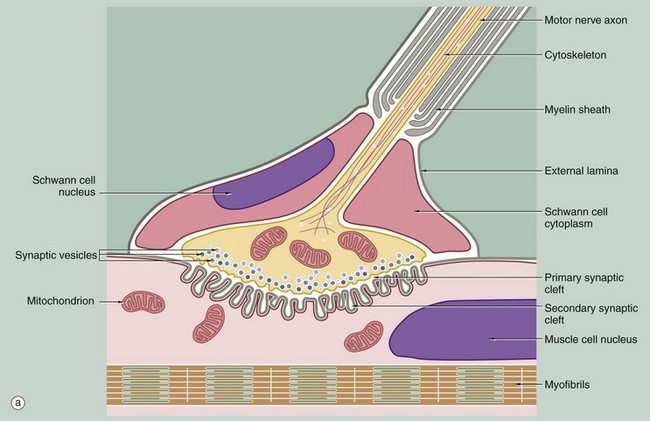
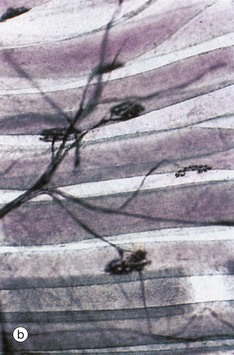

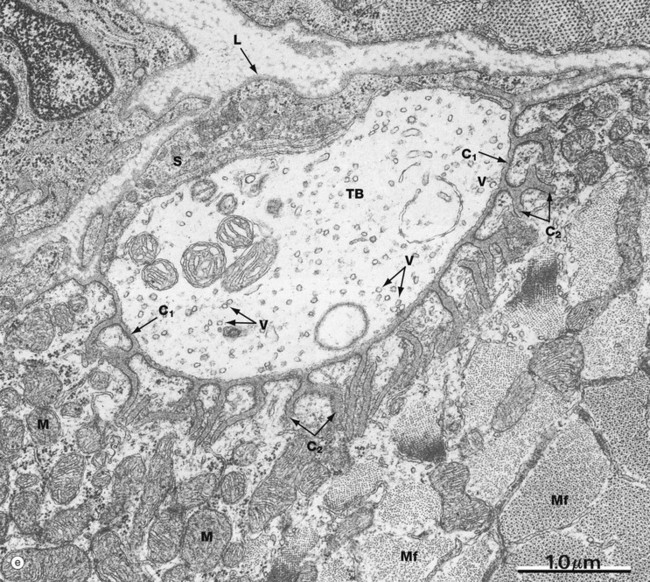
FIG. 7.12 Motor end plates (illustrations (b–e) opposite)
(a) Schematic diagram (b) Teased preparation, gold method (MP) (c) Teased preparation, gold method (HP) (d) Histochemical method for acetylcholinesterase (MP) (e) EM ×26 000
The motor end plates of skeletal muscle have the same basic structure as other synapses, with the addition of several important features. Firstly, one motor neurone may innervate from a few to more than a thousand muscle fibres depending on the precision of movement of the muscle; the motor neurone and the muscle fibres which it supplies together constitute a motor unit.
At low magnification in micrograph (b), the terminal part of the axon of a motor neurone is seen dividing into several branches, each terminating as a motor end plate on a different skeletal muscle fibre near to its midpoint. Micrograph (c) shows the lowermost of these motor end plates at higher magnification. The axonal branch is seen to lose its myelin sheath and divides to form a cluster of small bulbous swellings (terminal boutons) on the muscle fibre surface.
As seen in the diagram, the motor end plate occupies a recess in the muscle cell surface, described as the sole plate, and is covered by an extension of the cytoplasm of the last Schwann cell surrounding the axon. The external lamina (basement membrane) of the Schwann cell merges with that of the muscle fibre and the delicate collagenous tissue investing the nerve (endoneurium) becomes continuous with the endomysium of the muscle fibre (not illustrated).
Each of the terminal swellings of the cluster making up the motor end plate has the same basic structure as the synapse shown in Fig. 7.8, but the postsynaptic membrane of the neuromuscular junction is deeply folded to form secondary synaptic clefts perpendicular to the primary synaptic cleft. The overlying presynaptic membrane is also irregular, and the cytoplasm immediately adjacent contains numerous synaptic vesicles. The remaining cytoplasm of the terminal bulb contains many mitochondria and a membrane compartment for recycling secretory vesicles. The sole plate of the muscle fibre also contains a concentration of mitochondria and an aggregation of muscle cell nuclei.
The neurotransmitter of somatic neuromuscular junctions is acetylcholine, the receptors for which are concentrated at the margins of the secondary synaptic clefts. The hydrolytic enzyme acetylcholinesterase is present deeper in the clefts, associated with the external lamina, and is involved in deactivation of the neurotransmitter between successive nerve impulses. The histochemical technique illustrated in micrograph (d) defines the location of motor end plates by an insoluble brown deposit produced by the enzyme.
Micrograph (e) demonstrates the ultrastructure of a motor end plate, the terminal bouton TB typically lying in a depression in the skeletal muscle surface and invested externally by Schwann cell cytoplasm S and its external lamina L. Note the uniform width of the primary synaptic cleft C1 and the branching nature of the numerous secondary synaptic clefts C2. The underlying cytoplasm is packed with mitochondria M. Myofibrils Mf are seen in transverse section at the lower right of the field. The terminal bouton contains numerous synaptic vesicles V of uniform size; other membranous elements represent parts of the endoplasmic reticulum and a few mitochondria.
Peripheral Nervous Tissues
Peripheral nerves are anatomical structures which may contain any combination of afferent or efferent nerve fibres, of either the somatic or autonomic nervous systems. Each peripheral nerve is composed of one or more bundles (fascicles) of nerve fibres. Within the fascicles, each individual nerve fibre with its investing Schwann cell is surrounded by a delicate packing of loose vascular supporting tissue called endoneurium. Each fascicle is surrounded by a condensed layer of robust collagenous tissue invested by a layer of flat epithelial cells called the perineurium. In peripheral nerves consisting of more than one fascicle, a further layer of loose collagenous tissue called the epineurium binds the fascicles together and is condensed peripherally to form a strong cylindrical sheath. Peripheral nerves receive a blood supply via numerous penetrating vessels from surrounding tissues and accompanying arteries. Larger vessels course longitudinally within the epineurium, with a capillary network penetrating the perineurium into endoneurium.
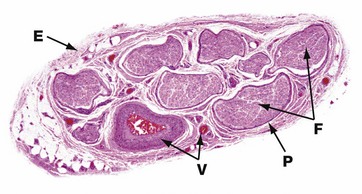
FIG. 7.13 Peripheral nerve
H&E (LP)
This micrograph illustrates the typical appearance of a medium-sized peripheral nerve in transverse section. This specimen consists of eight fascicles F, each of which contains many nerve fibres. Each fascicle is invested by perineurium P and the nerve as a whole is encased in a loose collagenous tissue sheath, the epineurium E, which is condensed at its outermost aspect. Blood vessels V of various sizes can be seen in the epineurial connective tissue.
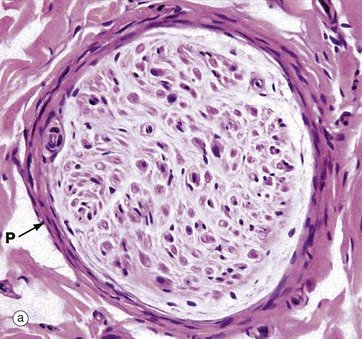
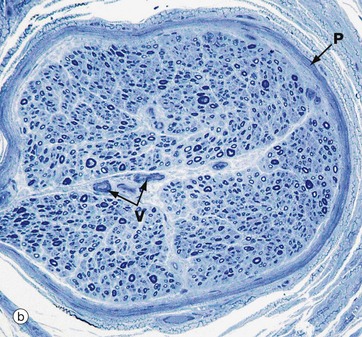
FIG. 7.14 Peripheral nerve
(a) H&E (MP) (b) Resin toluidine blue (MP)
The peripheral nerves shown in transverse section in micrographs (a) and (b) each consists of a single fascicle, invested by the perineurium P composed of several layers of flattened cells with elongated nuclei.
In micrograph (a) individual myelin sheaths are just visible as small circular structures, formed of myelin sheath proteins left after removal of lipid by tissue processing. Most of the nuclei seen within the fascicle are those of Schwann cells which mark the course of individual axons. Fibroblasts of the endoneurium are scattered amongst the much more numerous Schwann cells. It is possible to distinguish a minority of the large myelinated axons in this paraffin-embedded material stained with H&E. Around the outside of the perineurium are bundles of pink-staining epineurial collagen.
Micrograph (b) is a preparation of nerve embedded in epoxy resin and stained with toluidine blue. The myelin sheaths are stained dark blue and can be seen as small circular structures. Axons run down the centre of each myelin sheath but are not resolved at this magnification. In the centre of the fascicle are small endoneurial blood vessels V. The perineurium P runs around the fascicle.
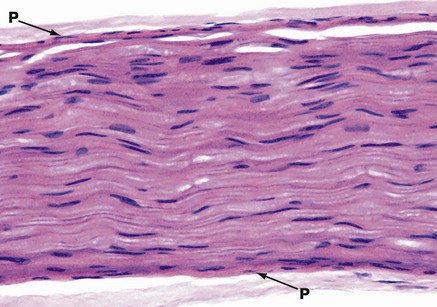
FIG. 7.15 Peripheral nerve
H&E (HP)
This micrograph illustrates the typical appearance of a single nerve fascicle in longitudinal section. It contains many nerve fibres. The perineurium P is seen on each side. The elongated nuclei are mainly those of Schwann cells, but some will also be those of endoneurial fibroblasts. It is not easy to discriminate between these cells in this type of preparation. Nerve fibres often follow an undulating or zigzag pattern in longitudinal section, as shown here.
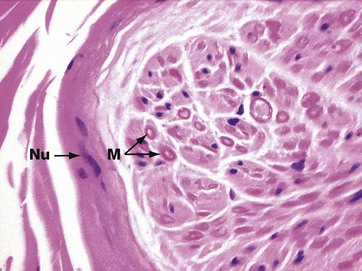
FIG. 7.16 Peripheral nerve
H&E (HP)
In routinely fixed and stained preparations, myelin is poorly preserved because it is largely composed of lipid material. Schwann cell cytoplasm and structural proteins in the myelin are, however, well-preserved and have eosinophilic staining properties. This is the edge of a peripheral nerve cut transversely; the nerve contains axons of different types and calibre, some of which are myelinated. Heavily myelinated fibres M can be identified by a pink ring formed by the protein remnants of the myelin sheath, with a pale centrally located axon. Small non-myelinated fibres cannot easily be identified. Several flattened nuclei Nu of perineurial cells are also seen in the perineurium.
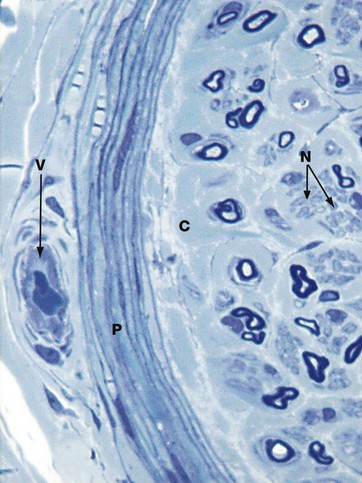
FIG. 7.17 Peripheral nerves in section
Toluidine blue (HP)
In tissue which has been fixed in glutaraldehyde and embedded in epoxy resin, myelin is well preserved and stains darkly with toluidine blue. A mixture of large and small myelinated fibres can be seen as dark-staining ring-like structures. These are often collapsed or elliptical in profile in sections, as here. The axon contained within each myelin sheath is seen as a pale structure, but no detail can be resolved at this magnification.
Schwann cell cytoplasm stains a paler shade of blue than the myelin and can be seen surrounding small clusters of small, non-myelinated axons N. In between bundles of nerve fibres is the paler-staining endoneurial collagen C.
The perineurium P is well shown in this type of preparation and resolves into several layers of flattened cells separated by thin layers of collagen. The elongated nuclei of perineurial cells are well seen. Outside the perineurium are bundles of epineurial collagen and a small blood vessel V.
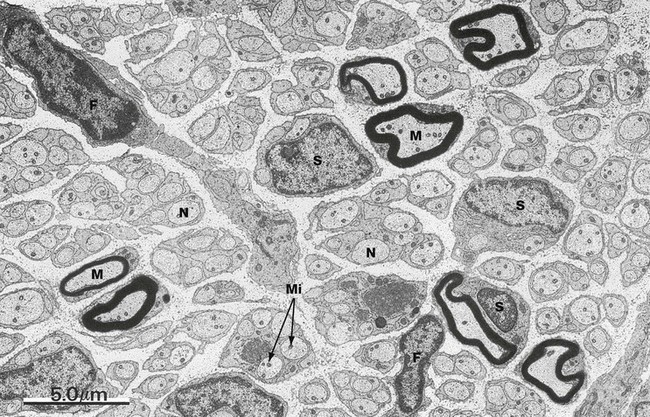
FIG. 7.18 Peripheral nerve
EM ×5000
The ultrastructural features of a typical peripheral nerve are shown in this micrograph. Both myelinated axons M and more numerous non-myelinated axons N are present, both ensheathed by Schwann cells S. The axons contain dot-like structures which are mitochondria Mi. The endoneurium mainly consists of loosely arranged collagen fibrils (difficult to identify at this magnification) lying parallel to the nerve fibres. The nuclei of two fibroblasts F can be identified and fibroblast processes extend through the endoneurium.
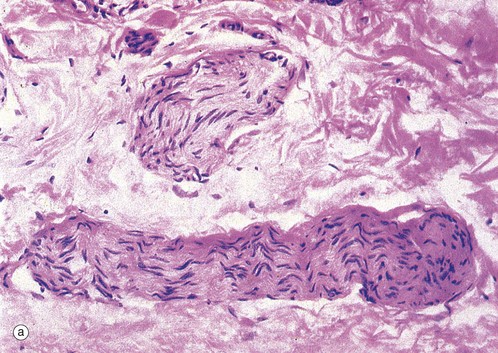
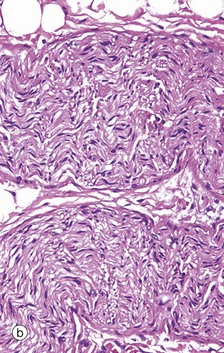
FIG. 7.19 Small peripheral nerves
(a) H&E (HP) (b) H&E (HP)
These micrographs illustrate the appearance of small peripheral nerves in the tissues. Micrograph (a) shows two small nerves in the dermis of the skin, each nerve consisting of a single fascicle of fibres. The nerve at the bottom of the field is cut in longitudinal section; the wavy shape of the Schwann cell nuclei reflects the course of the axons, which are thereby protected from damage when the skin is stretched. The nerve in the upper part of the field is cut in oblique section. Note the dense irregular collagenous dermal tissue surrounding the nerves in this specimen. Micrograph (b) shows a small peripheral nerve in the submucosa of the large bowel. This nerve runs a zigzag course in the tissue and the plane of section has cut it in the long axis as it folds. This allows the nerve to stretch as the bowel moves with peristalsis.
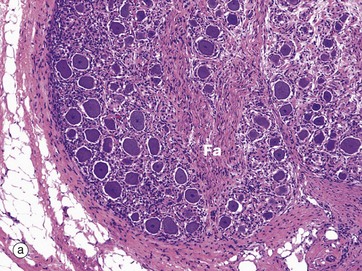
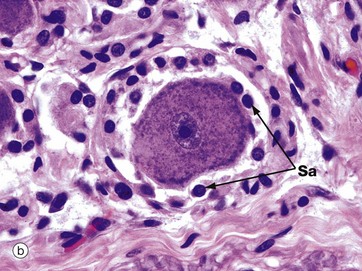
FIG. 7.20 Spinal ganglion
(a) H&E (MP) (b) H&E (HP)
Ganglia are discrete aggregations of neurone cell bodies located outside the CNS. The spinal ganglia are located on the posterior nerve roots of the spinal cord as they pass through the intervertebral foramina; they contain the cell bodies of the primary sensory neurones which are of the pseudo-unipolar form (see Fig. 7.2).
At low magnification in micrograph (a), note the fascicle Fa of nerve fibres passing to the centre of the ganglion, the ganglion cells being located peripherally. At high magnification in micrograph (b), a nerve cell body is seen to be surrounded by a layer of rounded satellite cells Sa which provide structural and metabolic support and have similar embryological origin to the Schwann cells (neural crest). The whole ganglion is encapsulated by condensed supporting tissue which is continuous with the perineurial and epineurial sheaths of the associated peripheral nerve.
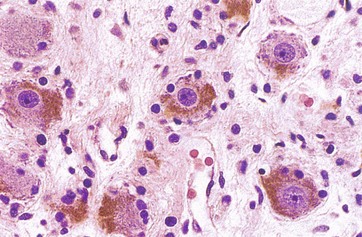
FIG. 7.21 Sympathetic ganglion
H&E (HP)
Sympathetic ganglia are found adjacent to the vertebral column. They have a similar structure to that of somatic sensory ganglia, with a few minor differences. The ganglion cells are multipolar and thus more widely spaced, being separated by numerous axons and dendrites, many of which pass through the ganglion without being involved in synapses. As seen in this micrograph, the nuclei of the ganglion cells tend to be eccentrically located and the peripheral cytoplasm contains a variable quantity of brown-stained lipofuscin granules, representing cellular debris sequestered in residual bodies. The satellite cells are smaller in number and irregularly placed due to the numerous dendritic processes of the ganglion cells.
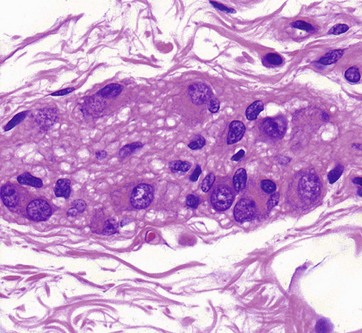
FIG. 7.22 Parasympathetic ganglion
H&E (HP)
In the parasympathetic nervous system, the cell bodies of the terminal effector neurones are usually located within or near the organ concerned. Commonly, a few neurone cell bodies are clumped together with supporting cells to form tiny ganglia scattered in the supporting tissue.
This micrograph shows a minute ganglion from the wall of the gastrointestinal tract. Like all neurones, the ganglion cells are recognised by their large nuclei, dispersed chromatin, prominent nucleoli, and extensive basophilic cytoplasm. As in other ganglia, the neurones are surrounded by small Schwann cells and afferent and efferent nerve fibres.
Sensory Receptors
Sensory receptors are nerve endings or specialised cells which convert (transduce) stimuli from the environment into afferent nerve impulses; the impulses pass into the CNS where they initiate appropriate voluntary or involuntary responses. Muscle spindles, along with the Golgi tendon apparatus (not described), are part of the system of proprioception which provides conscious and unconscious information about orientation, skeletal position, muscle tension and movement. The receptors can be generically called proprioceptors. Receptors which respond to external stimuli including touch, pressure, cutaneous pain, temperature, smell, taste, sight and hearing have been called exteroceptors. By tradition, the eye, ear and receptors for the senses of smell and taste are described as organs of special sense; they are the subject of Ch. 21.
There are also receptors sensing aspects of body physiology and the functioning of viscera, such as blood chemoreceptors, vascular (pressure) baroreceptors, and receptors for the distension of hollow viscera such as the urinary bladder. These have been called interoceptors.
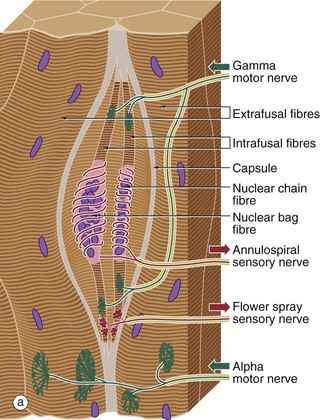
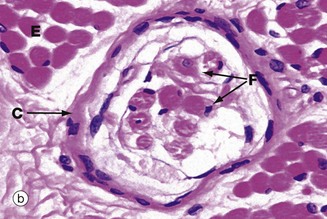
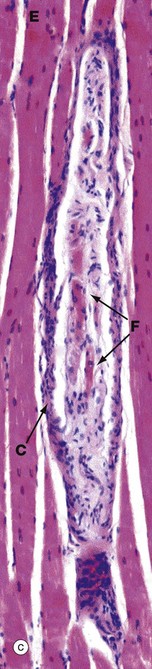
FIG. 7.23 Neuromuscular spindle
(a) Schematic diagram (b) H&E, TS (MP) (c) H&E, LS (MP)
Neuromuscular spindles are stretch receptor organs within skeletal muscles which are responsible for the regulation of muscle tone via the spinal stretch reflex. Neuromuscular spindles are encapsulated fusiform structures up to 6 mm long but less than 1 mm in diameter. They lie parallel to the muscle fibres, embedded in endomysium or perimysium. Each spindle contains 2 to 10 modified skeletal muscle fibres called intrafusal fibres F, which are much smaller than skeletal muscle fibres proper E (extrafusal). The intrafusal fibres have a central non-striated area in which their nuclei tend to be concentrated. Two types of intrafusal fibres are recognised. In one type, the central nuclear area is dilated, these fibres being known as nuclear bag fibres. In the other type, there is no dilatation and the nuclei are arranged in a single row, giving rise to the name nuclear chain fibres.
Associated with the intrafusal fibres are branched non-myelinated endings of large myelinated sensory fibres which wrap around the central non-striated area, forming annulospiral endings. Additionally, flower-spray endings of smaller myelinated sensory nerves are located on the striated portions of the intrafusal fibres. These sensory receptors are stimulated by stretching of the intrafusal fibres, which occurs when the (extrafusal) muscle mass is stretched. This stimulus evokes a simple two-neurone spinal cord reflex, causing contraction of the extrafusal muscle mass. This removes the stretch stimulus from the spindle and equilibrium is restored (tap a tendon and see).
The sensitivity of the neuromuscular spindle is modulated via small (gamma) motor neurones controlled by the extra-pyramidal motor system. These gamma motor neurones innervate the striated portions of the intrafusal fibres; contraction of the intrafusal fibres increases the stretch on the fibres and thus the sensitivity of the receptors to stretching of the extrafusal muscle mass. Many of the features of the spindle organ are shown in these micrographs. The most easily recognisable features are the discrete capsule C, which is continuous with the endomysium of the surrounding muscle, and the small size of the intrafusal muscle fibres F, best seen in longitudinal section in micrograph (c).
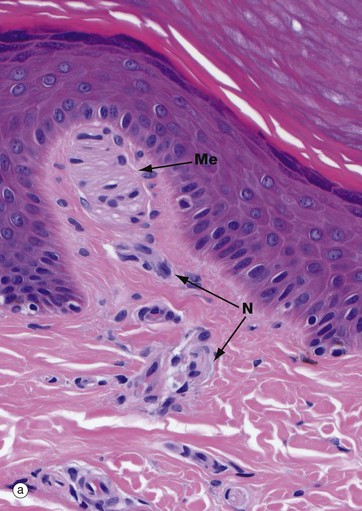
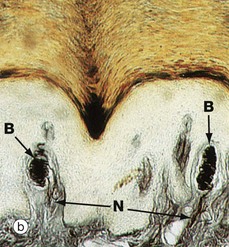
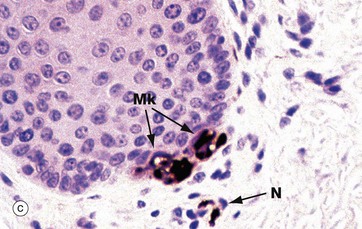
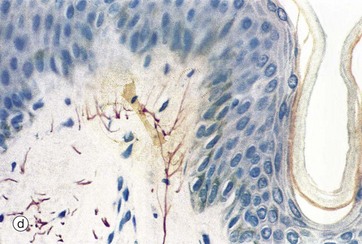
FIG. 7.24 Skin receptors
(a) Meissner corpuscle, H&E (HP) (b) Meissner corpuscle, silver/haematoxylin (HP) (c) Merkel cells, immunohistochemical stain (HP) (d) Free nerve endings, silver/haematoxylin (HP)
Image (a) shows a Meissner corpuscle Me. These are small, encapsulated receptors found in the papillary dermis of fingertips, soles of the feet, nipples, eyelids, lips and genitalia. They are fast-adapting mechanoreceptors which detect changes in texture and vibration (10–50 Hz). They are ovoid with a delicate collagenous tissue capsule surrounding a mass of plump oval cells arranged transversely; these are probably specialised Schwann cells. Non-myelinated branches from a large myelinated sensory nerve N ramify throughout the cell mass of the Meissner corpuscle as seen in micrograph (b) in which the nerve processes B are stained black.
Merkel cells Mk are located in the basal layers of the epidermis and are illustrated in micrograph (c) (see also Fig. 9.8). Merkel cell cytoplasm contains dense core vesicles with ultrastructural features similar to those found in synapses. An adjacent free nerve ending, served by large-diameter myelinated fibres N, forms a Merkel cell–neurite complex. These are slow-adapting mechanoreceptors, sensitive to sustained touch and pressure. Merkel cell–based receptor arrays provide information about prolonged pressure, while Meissner corpuscle–based arrays provide information about changes in pressure and vibrations; together these provide the sense of fine discriminatory touch as in finger tips, lips, etc. Free nerve endings, micrograph (d), provide mainly general light touch, stretching, temperature and pain sensations.
There are other skin receptors of note. These include the free nerve endings associated with hair follicles which sense hair position or movement (think cats' whiskers!). Ruffini corpuscles are robust spindle-shaped structures found in deep skin, particularly in the soles of the feet; these detect tension. Krause end bulbs are delicate encapsulated receptors found in the lining of the oropharynx and in the conjunctiva of the eye.
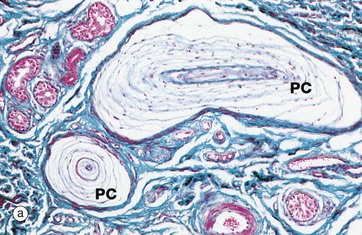
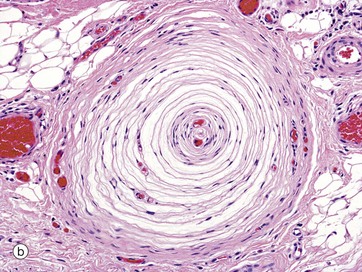
FIG. 7.25 Pacinian corpuscles
(a) Masson trichrome (MP) (b) H&E (MP)
Pacinian corpuscles are large, encapsulated sensory receptors responsive to pressure, coarse touch and rapid vibration (200–300 Hz); they are found in the deeper layers of the skin, ligaments and joint capsules, in some serous membranes, mesenteries, and viscera.
Pacinian corpuscles range from 1 to 4 mm in length and in section have the appearance of an onion. These organs consist of a delicate capsule enclosing many concentric lamellae of flattened cells (probably modified Schwann cells) separated by interstitial fluid spaces and delicate collagen fibres. Towards the centre of the corpuscle the lamellae become closely packed and the core contains a single, large, unbranched, non-myelinated nerve fibre with several club-like terminals. The nerve fibre becomes myelinated on leaving the corpuscle. Distortion of the Pacinian corpuscle produces an amplified mechanical stimulus in its core which is transduced into an action potential in the sensory neurone. It is a rapidly adapting mechanoreceptor.