Dental caries
David J Manton and Linda Hayes-Cameron

Factors influencing dental caries
Dentists spend most of their time treating dental caries, and yet many clinicians have a poor understanding of the mechanisms by which caries is initiated, how to identify patients at risk and how to put management plans in place to ensure that the disease does not progress. Too often, only the outcomes of the carious process are treated and not the cause of the disease itself.
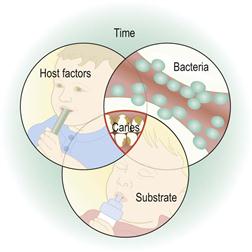
Dental caries involves a complex process of enamel demineralization and remineralization that occurs due to the action of organic acids produced by microorganisms within the dental plaque. Dental caries is a multifactorial disease, resulting from the interplay between environmental, behavioural and genetic factors. The four factors that influence its progression are shown in Figure 4.1.
Dental plaque biofilm
Increasingly, dental plaque is viewed as a dynamic biofilm (Figure 4.2). This implies that plaque maintains its own microenvironment and has actions that influence oral health. While the plaque biofilm is usually viewed as undesirable, the presence of a healthy biofilm may be positive, e.g. in acting as a fluoride reservoir or as a protective barrier to erosion.
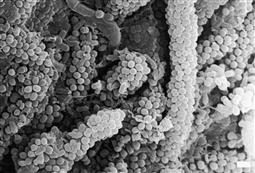
Figure 4.2 Scanning electron micrograph of dental plaque (×4555 magnification). This image shows the typical ‘corncob’ arrangement of streptococci held by an extracellular polysaccharide matrix on a web of central filamentous microorganisms. (Courtesy, Institute of Dental Research, SEM Unit, Westmead.)
Dental plaque contains bacteria that are both acidogenic and aciduric. Although many bacterial subspecies have been shown to be associated with caries, Streptococcus mutans is still believed to be the most important bacterium in the initiation and progress of this disease in combination with lactobacilli. In the caries process, once the pH of the plaque drops below a critical level (around 5.5), it causes desaturation with respect to tooth mineral, the enamel demineralizes and there is net mineral loss. This desaturation will last for 20 min or longer, depending on the availability of fermentable substrate and the effect of the saliva, fluoride and calcium and phosphate.
Mutans streptococci (incl. S. mutans and S. sobrinus) is the major group of bacteria involved in the initiation of enamel demineralization. Normally, an infant is inoculated vertically with S. mutans by the mother/primary caregiver or horizontally by peers at a playgroup or childcare centre. The initial inoculation was thought to be dependent on the presence of a hard surface, and therefore the eruption of the first tooth, however recent research has shown the presence of this organism in newborns. In general, the earlier the detection of significant levels of mutans streptococci, the greater the caries risk of an infant. Repeated consumption of fermentable carbohydrates leads to the proportional overgrowth of mutans streptococci and other aciduric and acidogenic organisms, and the subsequent production of organic acids (lactic, formic, acetic), an increase in the extracellular polysaccharide matrix and a change in the relative components of the microflora leading to increased risk of dental caries.
Substrates
Bacteria can use fermentable carbohydrates as a ready source of energy and the end-products of the glycolytic pathway in bacterial metabolism are acids. Sucrose is the fermentable carbohydrate most frequently implicated, but it is important to remember that the bacteria can use all fermentable carbohydrates, including cooked starches. Although any carbohydrate may cause the production of acids, it is the availability of glucose that drives bacterial metabolism to produce lactate rather than weaker by-products such as formate, acetoacetate and alcohols. The lactate is subsequently excreted from the cell as lactic acid. Furthermore, the amount of fermentable carbohydrate is relatively unimportant, as even minute amounts of fermentable carbohydrate will be used immediately – the frequency of exposure is the important factor.
Host factors
The traditional triad of host factors – the teeth, the microbes and their diet – is a simplistic representation of the complex interrelationships in the oral cavity. With regard to the caries process, the quality of tooth structure and the saliva are the major host factors that should be considered. Poor tooth quality, such as hypomineralized enamel, is associated with increased rates of caries, and changes in salivary quantity and/or quality has a profound effect on the whole oral environment, affecting caries rates, oral comfort, periodontal health and resistance to infection.
Saliva
The importance of saliva is often over-looked, however, it has several critical roles in the caries process. Saliva is excreted from the major and minor glands at different rates and with different constituents depending on the presence or absence of stimulatory factors. Saliva stimulated by chewing has increased calcium and phosphate ion concentrations. A gustatory effect, such as that induced by some food acids, has been shown to stimulate a higher flow rate of saliva than stimulation by mechanical chewing. By removing substrate and buffering plaque acid, saliva helps to balance the caries process and has a critical role in remineralization as it provides a stabilized supersaturated solution of calcium and phosphate ions as well as fluoride ions from extrinsic sources. The major constituent of saliva is water (~99.5%), with a wide range of other inorganic and organic components, the most relevant being the salivary proteins, especially the histatins, mucins and statherins, which provide:
• antibacterial and antifungal and antiviral activity.
• lubrication, which also assists in bolus formation.
• inhibition of demineralization and stabilization of calcium and phosphate ions, which assists remineralization.
Therefore, a decrease in the amount or quality of saliva can significantly increase the caries risk.
Time
When acid challenges occur repeatedly, the eventual collapse of enough enamel crystals and subsequently rods will result in surface breakdown. This may take from months to years, depending on the intensity and frequency of the acid attack. This means that in all mouths (as most mouths will contain some cariogenic bacteria) there is continual demineralization and remineralization of enamel; therefore, an individual is never free of dental caries. The process of enamel demineralization and remineralization is constantly cycling between net loss and gain of mineral. It is only when the balance leans towards net loss that clinically identifiable signs of the process become apparent. The long-term outcome of this cycling is determined by:
• The composition and amount of plaque.
• Sugar consumption – especially sucrose (frequency and timing).
Thus the term ‘caries-free’ often used to describe a child with no visible decay is best changed to the term ‘caries-inactive’ to more accurately reflect this clinical reality. For the balance to be maintained, there should be sufficient time between cariogenic challenges for the remineralization process to take place. When these challenges become too frequent, or occur when salivary flow is reduced, the rate of demineralization and subsequent tooth breakdown will increase.
The caries process
Dental enamel demineralization is a chemical process. The dissolution of hydroxyapatite can be described simply:
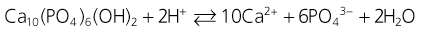
with enamel demineralization summarized as a net loss of enamel mineral due to the action of either intrinsic or extrinsic acids, leading to dental caries or erosion. Dental caries is primarily caused by lactic and acetic acids which diffuse through the plaque and into the enamel pores between the rods as neutral ion species, where they dissociate and decrease the pH of the fluid surrounding the enamel crystals. Once dissociated, the protons dissolve the hydroxyapatite crystal surface depending on the degree of saturation of the specific apatite and the inter-rod fluid calcium and phosphate ion concentration increases.
The buffering of calcium and phosphate at the enamel surface and in the plaque biofilm leads to the development of a subsurface (or white spot lesion) with a proportionately hypermineralized surface layer. The optical changes occur due to the increased pore spaces between the thinned rods and the effect this has on the refractive qualities of the enamel. The continuation of this process eventually undermines the support for the surface layer and surface breakdown occurs – the development of a physical cavity.
Caries detection
With the significant reduction in the prevalence, incidence and severity of caries in a great proportion of Western society over the past three decades, notwithstanding some disadvantaged communities and individuals who remain at high risk, the sensitivity of many diagnostic tests for caries has been reduced. Occlusal caries detection is complicated clinically by surface morphology, fluoride exposure, anatomical fissure topography and the presence of plaque and stain. The current methods used commonly for caries detection are:
Newer methods of caries detection
In the past two decades, laser and light-induced fluorescence methods have been developed to detect and quantify enamel mineral content. These methods rely on the different fluorescence characteristics (loss of fluorescence) of demineralized enamel or dentine due to the scattering of light or excitation of materials in the carious lesion. There is a strong correlation between mineral loss and fluorescence in white spot (demineralized) lesions of enamel, however these results can be confounded by stains, calculus and poor operating technique. Over-diagnosis of caries (false positive results) is the main problem.
The recent commercial development of detection systems such as Diagnodent™, QLF-D™, Canary™, Soprolife™ and CarieScan™ have the potential to increase the accuracy of detection of enamel and dentinal caries. This is because:
Approximal caries
The detection of approximal caries at an early stage is important in paediatric dentistry due to the large proportional size of the pulp in deciduous teeth. New detection methods, such as the Diagnodent pen, have been developed for this task, however, results of research indicate that these should only be used as adjuncts to traditional methods, such as bitewing radiographs and visual and tactile examination (Chawla et al. 2012).
Preventing dental caries
Preventing, reversing or at least slowing down dental caries generally consists of altering one or more of the factors described above.
Diet modification
Although often given minimal attention by dental practitioners, diet is probably the single most important factor in caries risk. Although some dietary habits have changed, the overall consumption of sugar has increased over the past 50 years in most Western countries, especially related to increasing consumption of carbonated beverages. Many foods, although not obviously cariogenic, contain hidden sugars and fermentable carbohydrates. Dietary histories may be useful in identifying those children at high risk. Achieving changes in dietary habits is extremely difficult and therefore advice must be individual, practical and realistic.
• Frequency of intake is more important than overall quantity.
• ‘Grazing’ between meals should be discouraged.
• The frequent consumption of soft drinks (including fruit juices and sports drinks) should be avoided. Not only are they cariogenic but also extremely erosive and highly caloric.
• Sweets are useful rewards, but should be limited to mealtimes.
• Many foods labelled ‘No added sugar’ contain high levels of natural sugars.
• Dietary advice should not be all negative. Positive alternatives should be identified.
• The chewing of pH-neutral sugar-free gum increases salivary flow and assists in remineralization and the prevention of demineralization.
• Probably the best dietary advice of all is to ‘give teeth a rest’ for at least 2 hours between every meal or snack.
Fluorides
The principal mode of action of all fluoridated modalities (toothpastes, rinses, gels and community water fluoridation) is the topical effect at the enamel surface. Even low concentrations of fluoride in the micro-environment around the teeth inhibit demineralization and promote remineralization of the tooth surface. The incorporation of fluoride (as fluoroapatite) into the enamel will decrease its solubility (and increase its resistance to caries). However, it is now recognized that the incorporation of systemically administered fluoride into developing (unerupted) enamel has a lesser role in increasing enamel resistance (see Chapter 4).
Calcium and phosphate
The ability for net remineralization to occur is limited by available calcium and phosphate ions, intrinsically provided by saliva; therefore remineralization is ‘saliva limited’. Attempts have been made over past decades to provide supersaturated ionic calcium and phosphate solutions to increase remineralization. However, these attempts have been limited by the low solubility of these ions. Recently, it has been reported that milk-derived casein phosphopeptides stabilize calcium and phosphate in an amorphous form (casein phosphopeptide-amorphous calcium phosphate; CPP-ACP), providing a supersaturated environment that drives remineralization and limits demineralization. CPP-ACP has been added to topical pastes, chewing gums and mouthrinses to increase remineralization and decrease demineralization, and to sports drinks to decrease erosivity.
Fissure sealants
Even in communities with a low incidence of caries, the pits and fissures are still susceptible to caries. The most effective way to prevent pit and fissure caries is by fissure sealing (see Chapter 5).
Plaque removal
Tooth brushing
In communities with water fluoridation, caries mostly occurs in pits and fissures and interproximally. If all plaque could be removed from the tooth surfaces, dental caries would not occur. However, this is not physically and behaviourally possible.
• Besides removing plaque, tooth brushing should be regarded also as vehicle for topical fluoride application.
• The mechanical action of tooth brushing alone will not prevent caries, as it does not effectively remove plaque from the areas mentioned above.
• Children should be encouraged to adopt good brushing habits. Brushing should commence when teeth first erupt, as a part of everyday hygiene. Gauze or a facecloth on a finger, or a small very soft toothbrush may be used to remove the plaque in infants.
• It is beneficial for adults to continue to assist with tooth brushing until children are around 8–10 years old and have developed the dexterity to remove plaque effectively by themselves. Ideally, tooth brushing should be carried out twice a day with fluoridated toothpaste but parents should understand that at least once a day is essential to decrease the risk of dental caries (see Chapter 4).
Flossing
In preschool years, and in early mixed dentition, the interproximal surfaces of the primary molars become more at risk of caries. Parents can be shown how to floss these areas when the teeth are in contact and especially if there are signs of demineralization. Older children should be taught to floss themselves. They may find it easier to use one of the commercial floss holders.
Disclosing of plaque
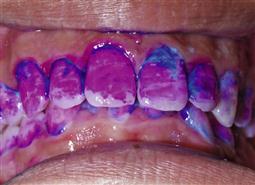
Children, their parents and older patients find it difficult to know when they have effectively removed plaque from their teeth. Disclosing solutions and tablets are very useful for helping patients and parents to see and remove plaque more effectively (Figure 4.3).
Antimicrobials
Antibacterial mouthwashes have become part of the preventive dentistry regimen in the past few years. They do have a role for some patients in caries prevention. In particular, chlorhexidine- and triclosan-containing rinses, gels, toothpastes or varnishes may be used for patients with high risk of caries to help with plaque and microbial control. Their main role is as one part of the multifactorial management of high caries individuals and especially for those who are medically compromised. Systemic antimicrobials (antibiotics) cause significant alterations in oral microflora and have no use in caries prevention.
Determining patients at risk of dental caries
The development of a treatment strategy for a patient that is based on risk factors pertinent to that individual is the gold standard of minimally invasive treatment. That is, before deciding the appropriate methods and preventive products to recommend, the patient’s caries risk should be determined. This may be achieved by considering several aspects:
• Presence of white spot lesions and their activity status.
• Individual and familial past caries history.
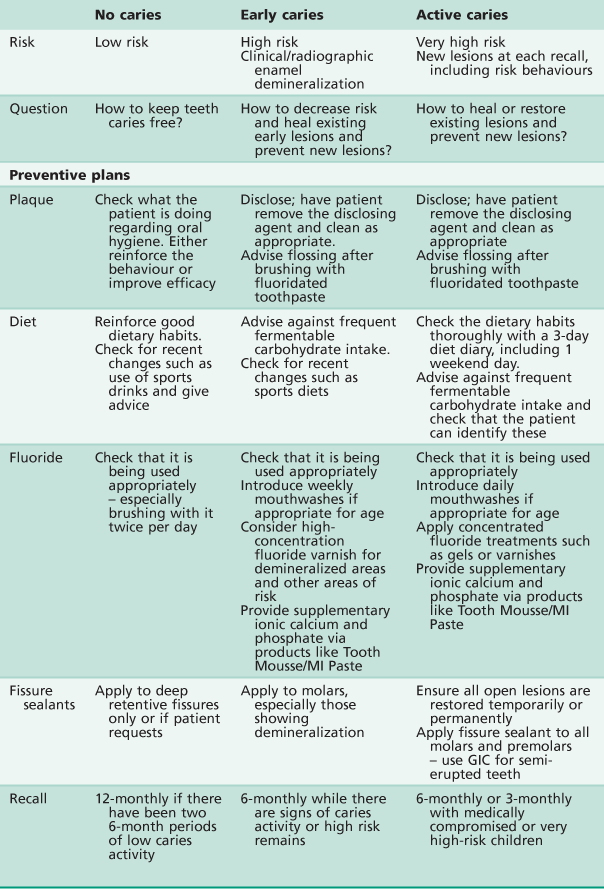
These factors – especially the background factors – can only be a guide, but they are important to consider when deciding what preventive measures to put in place for individual patients. It has been reported that the dentist’s ‘hunch or gut feeling’ is the most reliable predictor of future caries risk. When the risk has been determined, a preventive programme, incorporating the appropriate methods, can be used. A suggested approach is shown in Table 4.1.
Individual characteristics
• Different teeth sites are at risk at different ages.
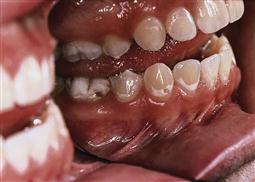
• Teeth may be at particular risk in association with orthodontic treatment (Figure 4.4), those with developmental defects of enamel (Figure 4.5) or in those children with a medical comorbidity.
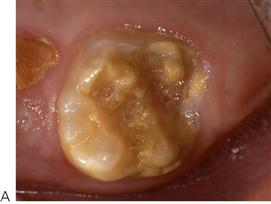
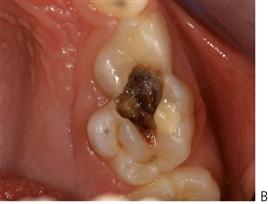
Figure 4.4 (A) Hypomineralized and/or hypoplastic teeth will be predisposed to caries, particularly if the enamel deficiencies are plaque retentive. If the enamel is completely absent and dentine is exposed, then these teeth can deteriorate rapidly. (B) Interproximal lesions that are cavitated and unable to be thoroughly cleaned do not arrest.
Diet
• Fermentable carbohydrate frequency?
• What is the parent’s/patient’s knowledge of foods with sugars?
• Protective foods (i.e. dairy foods)?
• Are there risk-associated habits?
• Nursing bottle in bed or at-will breast-feeding (esp. as it relates to other social factors)?
Family history of caries
Cariogenic bacteria are transmitted vertically from the parents and possibly horizontally from other caregivers and close associates such as siblings and playgroup members. Most cariogenic bacteria are commensal oral microorganisms, and require the appropriate environment to thrive, so trying to avoid inoculation will not prevent caries long term in an otherwise susceptible individual.
Early childhood caries
One of the causes of early childhood caries or rampant caries in young children is allowing infants and toddlers to sleep with a bottle containing fermentable carbohydrates (Figure 4.6). The reported prevalence ranges from 2.5% to 15%.
Characteristics of feeding bottle induced early childhood caries
• Rampant caries affecting the maxillary anterior teeth (Figure 4.7).
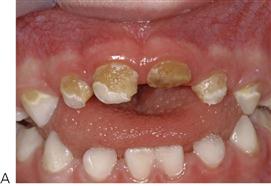
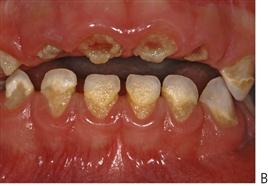
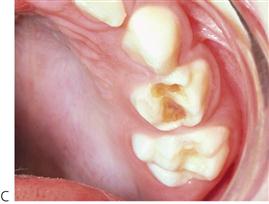
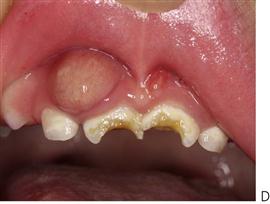
Figure 4.7 Early childhood caries (ECC). (A) ECC showing the characteristic pattern of decay. The upper anterior teeth and the molars are affected but the lower anterior teeth are spared. (B) A particularly rampant case of ECC where a pacifier had been dipped in honey (or any other cariogenic agent). Both the upper and lower anterior teeth may be affected. (C) The first primary molars are carious due to a bottle habit at night. There is no interproximal decay (due to open contact points) and the canines that have erupted later, are unaffected. (D) An abscess following pulp necrosis in carious upper incisors. Extraction is required and the abscess will resolve following removal of the tooth and drainage of the pus. It is important to note that the teeth are not fractured due to trauma, but are carious.
• Lesions appear as teeth erupt – later on posterior teeth, both the maxillary and mandibular first primary molars.
• Canines are affected less than first molars because of later eruption.
• Mandibular anterior teeth are usually unaffected. This is thought to be because of salivary flow and the position of the tongue. However, if they are affected, this would indicate extremely high risk.
• The bottle is often used as a pacifier to get the infant to sleep.
• Early childhood caries occurs in all socioeconomic groups and as such, often reflects the social dynamics of the family. Children who are difficult sleepers or have colic are often pacified with a bottle. The bottle can contain any liquid with fermentable carbohydrate, even milk. Commonly, drinks and juices containing vitamin C are used.
• This pattern of caries may also occur with prolonged at-will breast-feeding in conjunction with a cariogenic solids diet. The at-will breast-feeding may be an associated factor with other sociodemographic variables that influences caries risk.
• Once the habit has ceased and/or the areas of decay become self-cleansing, the carious process may arrest and the lesions will appear darker (Figure 4.8).
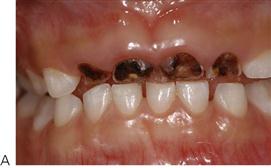
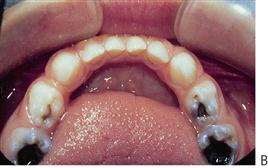
Figure 4.8 (A) ECC in a child showing arrested caries. Removal of the cause of caries has allowed the process of demineralization to slow down. (B) When the carious process has resulted in an open lesion, it is possible to slow and arrest the process. These lesions are quite different to those shown in Figure 4.4B and while they appear quite extensive, there was no pulp exposure.
Aetiological factors of early childhood caries
• Long periods of exposure to cariogenic substrate. In infants, if this is from a feeding bottle, exposure to cariogenic fluids can be for up to 8 hours. However, other habits such as ‘grazing’ (snacking on cariogenic food constantly) also puts many children at risk, as does the use of feeding cups and sipper bottles filled with sugary fluids that toddlers walk around with.
• Low salivary flow rate at night, and therefore reduced time for remineralization and acid buffering.
• Parental history of active and untreated caries – particularly in the mother.
Social aspects of night-waking and feeding
It is important to understand normal feeding routines or when these may be associated with habits contributing to early childhood caries. Breast-feeding is encouraged, however, some mothers are unable to breast-feed and there may be circumstances when babies have medical interventions that restrict their ability to breast-feed and so take bottle-feeds (cleft palate or early days of the neonatal intensive care admission). It is NOT recommended that children fall asleep with a bottle at night.
General recommendations for infant feeding routines

All infants wake at least once overnight and often resettle themselves. This may happen without the knowledge of the parents. Infants wake more often when they are unwell; there are changes in their social and physical environment or when learning a new developmental skill, such as pulling themselves up on the side of the cot to stand. If the parent offers milk or another feed to assist with resettling, then this night-waking behaviour will be reinforced and the infant will learn that the reward is the means to resettle. Unfortunately, this sets up conditioning for having feeds overnight that are not required developmentally (i.e. after 10–12 months of age). Babies that fill up with milk overnight may refuse their solid meals during the day. Children are best settled with comforts such as patting, cuddling and verbal reassurance. Unnecessary additional overnight feeds may reflect the parent(s) inability to cope with a wakeful, crying infant. Some reasons for offering feeds overnight include:
In these cases, used as a quick fix to try to settle the infant.
It is important to give appropriate advice to the family about early childhood caries. Blame should never be attributed; in many situations, the condition may have arisen out of ignorance, misinformation or tiredness and frustration of coping with an infant with poor sleeping habits. Elimination of an ‘at risk’ bottle habit can be achieved by gradually reducing the amount of sugar in the bottle by diluting with water, which may be done over several weeks. Alternatively, some parents find it easier to remove the bottle, immediately offering sips of water only. The only dentally safe fluid in a feeding bottle is water.
Understanding developmentally appropriate feed, sleep and play patterns will help both the parents and the dentist come to the conclusion that the family may require extra assistance with the infant’s routine. Providing empathy and support for the challenges they are experiencing with night waking is essential before offering education regarding early childhood caries. Referral to community health nurses, lactation consultants or secondary parenting services is recommended to assist with the physical and psychological support necessary to change these behavioural patterns.
Management
• Dietary advice and modification.
• Fluoride and/or CPP-ACP application.
• Use of biofilm moderating/antimicrobial products.
• Restoration of teeth. This may consist of intracoronal tooth-coloured restorations in small lesions. For more extensive lesions, composite resin-strip crowns for anterior teeth and stainless steel crowns for posterior teeth may be required (Chapter 6).
• Extractions if required. Loss of the upper anterior teeth will not result in space loss if the canines have erupted. Speech development should not be affected. If posterior teeth have to be extracted, the parents will need to be informed about possible space-loss, and an assessment should be carried out to determine if a space maintainer is appropriate.
Treatment under general anaesthesia is often required for small children. Unfortunately, a large proportion of children continue to get new lesions even after extensive treatment, as the risk factors have not been altered. The use of motivational interviewing methodology is seen as a more effective way of gaining positive behavioural change.
Further reading
Dental caries
1. Armfield JW. High caries children in Australia: a tail of caries distribution. Australian Dental Journal. 2005;50:204–206.
2. Chawla N, Messer LB, Adams GG, et al. An in vitro comparison of detection methods for approximal carious lesions in primary molars. Caries Research 2012. 2012;46:161–169.
3. Cochrane NJ, Cai F, Huq NL, et al. New approaches to enhanced remineralization of tooth enamel. Journal of Dental Research. 2011;89:1187–1197.
4. Kidd EAM. How ‘clean’ must a cavity be before restoration? Caries Research. 2004;38:305–313.
5. Featherstone JD. Remineralization, the natural caries repair process – the need for new approaches. Advances in Dental Research. 2009;21:4–7.
6. Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral healthcare. Caries Research. 2004;38:182–191.
7. Kühnisch J, Dietz W, Stößer L, et al. Effects of dental probing on occlusal surfaces – a scanning electron microscopy evaluation. Caries Research. 2007;41:43–48.
8. Law V, Seow WK, Townsend G. Factors influencing oral colonization of mutans streptococci in young children. Australian Dental Journal. 2007;52:93–100.
9. Manton DJ. Dental caries: Where to from here? Annals of the Royal Australasian College of Dental Surgeons. 2008;19:73–76.
10. Marsh P. Dental plaque as a biofilm and a microbial community – implications for health and disease. BMC Oral Health. 2006;6(Suppl 1):S14.
11. Marsh P. Contemporary perspective on plaque control. British Dental Journal. 2012;212:601–606.
12. Ruby J, Goldner M. Nature of symbiosis in oral disease. Journal of Dental Research. 2007;86:8–11.
13. Ismail AI, Sohn W, Lim S, et al. Predictors of dental caries progression in primary teeth. Journal of Dental Research. 2009;88:270–275.
14. Van Nieuw Amerongen A, Bolscher JG, Veerman ECI. Salivary proteins: protective and diagnostic value in cariology? Caries Research. 2004;38:247–253.
Early childhood caries
1. Alm A, Wendt LK, Koch G, et al. Caries in adolescence – influence from early childhood. Community Dentistry Oral Epidemiology. 2012;40:125–133.
2. Berkowitz RJ, Amante A, Kopycka-Kedzierawski DT, et al. Dental caries recurrence following clinical treatment for severe early childhood caries. Pediatric Dentistry. 2011;33:510–514.
3. D’Mello G, Chia L, Hamilton SD, et al. Childhood obesity and dental caries among paediatric dental clinic attenders. International Journal of Paediatric Dentistry. 2011;21:217–222.
4. Elfrink MEC, ten Cate JM, Jaddoe VWV, et al. Deciduous molar hypomineralization and molar incisor hypomineralization. Journal of Dental Research. 2012;91:551–555.
5. Ghanim AM, Morgan MV, Mariño RV, et al. Risk factors of hypomineralised second primary molars in a group of Iraqi schoolchildren. European Archives of Paediatric Dentistry. 2012;13:111–118.
6. Isong IA, Luff D, Perrin JM, et al. Parental perspectives of early childhood caries. Clinical Pediatrics. 2012;51:77–85.
7. Karki AJ, Thomas DR, Chestnutt IG. Why has oral health promotion and prevention failed children requiring general anaesthesia for dental extractions? Community Dental Health. 2011;28:255–258.
8. Kilpatrick NM, Neumann A, Lucas N, et al. Oral health inequalities in a national sample of Australian children aged 2–3 and 6–7 years. Australian Dental Journal. 2012;57:38–44.
9. Kramer MS, Vanilovich I, Matush L, et al. The effect of prolonged and exclusive breast-feeding on dental caries in early school-age children. Caries Research. 2007;41:484–488.
10. Marchant S, Brailsford SR, Twomey AC, et al. The predominant microflora of nursing caries lesions. Caries Research. 2001;35:397–406.
11. O’Mullane D, Parnell C. Early childhood caries: a complex problem requiring a complex intervention. Community Dental Health. 2011;28:254.
12. Palmer CA, Kent Jr R, Loo CY, et al. Diet and caries-associated bacteria in severe early childhood caries. Journal of Dental Research. 2010;89:1224–1229.
13. Ramos-Gomez FJ, Gansky SA, Featherstone JD, et al. Mother and youth access (MAYA) maternal chlorhexidine, counselling and paediatric fluoride varnish randomized clinical trial to prevent early childhood caries. International Journal of Paediatric Dentistry. 2012;22:169–179.
14. Nagarkar SR, Kumar JV, Moss ME. Early childhood caries-related visits to emergency departments and ambulatory surgery facilities and associated charges in New York state. Journal of the American Dental Association 2012. 2012;143:59–65.
15. Seow WK. Environmental, maternal, and child factors which contribute to early childhood caries: a unifying conceptual model. International Journal of Paediatric Dentistry. 2012;22:157–168.
16. Sheiham A. Dental caries affects body weight, growth and quality of life in pre-school children. British Dental Journal. 2006;201:625–626.
17. Weintraub JA, Prakash P, Shain SG, et al. Mothers’ caries increases odds of children’s caries. Journal of Dental Research. 2010;89:954–958.