Inflammatory Lesions of the Jaws
Inflammatory lesions are by far the most common pathologic condition of the jaws. The jaws are unique from other bones of the body in that the presence of teeth creates a direct pathway for infectious and inflammatory agents to invade bone by means of caries and periodontal disease. The body responds to chemical, physical, or microbiologic injury with inflammation. The inflammatory response destroys or walls off the injurious stimulus and sets up an environment for repair of the damaged tissue.
Under normal conditions, bone metabolism represents a balance of osteoclastic bone resorption and osteoblastic bone production. This is a complex, interdependent relationship in which osteoblasts mediate the resorptive activity of the osteoclasts. Mediators of inflammation (cytokines, prostaglandins, and many growth factors) tip this balance to favor either bone resorption or bone formation. For the purposes of this chapter, all inflammatory conditions of bone, regardless of the specific etiology, are considered to represent a spectrum or continuum of conditions with different clinical features (e.g., site, severity, duration).
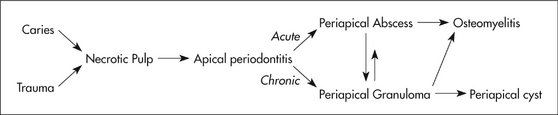
When the initial source of inflammation is a necrotic pulp and the bony lesion is restricted to the region of the tooth, the condition is called a periapical inflammatory lesion. When the infection spreads in the bone marrow and is no longer contained to the vicinity of the tooth root apex, it is called osteomyelitis. Another source of inflammatory lesion in bone is extension of inflammation into bone from the overlying soft tissues; this type of lesion includes periodontal lesions (see Chapter 18) and pericoronitis, an inflammation that arises in the tissues surrounding the crown of a partially erupted tooth. It must be emphasized that the names of the various inflammatory lesions tend to describe their clinical and radiologic presentations and behavior; however, all have the same underlying disease mechanism.
General Clinical Features
The four cardinal signs of inflammation—redness, swelling, heat, and pain—may be observed in varying degrees with inflammation of the jaws. Acute lesions are those of recent onset. The onset typically is rapid, and these lesions cause pronounced pain, often accompanied by fever and swelling. Chronic lesions have a prolonged course with a longer insidious onset and pain that is less intense. Fever may be intermittent and low grade, and swelling may occur gradually. In fact, some chronic, low-grade infections may not produce any significant clinical symptoms.
General Radiographic Features
With periapical inflammatory lesions, which are pathologic conditions of the pulp, the epicenter typically is located at the apex of a tooth. However, lesions of pulpal origin also may be located anywhere along the root surface because of accessory canals or perforations caused by root canal therapy or root fractures. Periodontal lesions have an epicenter that is located at the alveolar crest. If periodontal bone loss is severe, the bone inflammatory changes may extend to the root furcation level or even to the root apex. Osteomyelitis, a diffuse, uncontained inflammation of the bone, most commonly is found in the posterior mandible. The maxilla rarely is involved.
PERIPHERY
Most often the periphery is ill defined, with a gradual blending of normal trabecular pattern into a sclerotic pattern, or the normal trabecular pattern may gradually fade into a radiolucent region of bone loss.
INTERNAL STRUCTURE
The internal structure of inflammatory lesions presents a spectrum of appearances. Cancellous bone may respond to an insult by tipping the bone metabolic balance either in favor of resorption (giving the area a radiolucent appearance) or toward bone formation (resulting in a radiopaque or sclerotic appearance). Usually there is a combination of these two reactions. The radiolucent regions may show no evidence of previous trabeculation or a very faint pattern of trabeculation. The increased radiopacity is caused by an increase in bone formation on existing trabeculae. Radiographically these trabeculae appear thicker and more numerous, replacing marrow spaces. In acute disease, resorption typically predominates; with chronic disease, excessive bone formation leads to an overall radiopaque, sclerotic appearance. In cases of osteomyelitis, careful examination of the x-ray films may reveal sequestra, which appear as ill-defined areas of radiolucency containing a radiopaque island of nonvital bone.
EFFECTS ON SURROUNDING STRUCTURES
The effects of inflammation on surrounding cancellous bone include stimulation of bone formation, resulting in a sclerotic pattern, or bone resorption, resulting in radiolucency. The periodontal ligament space involved in the lesion will be widened; this widening is greatest at the source of the inflammation. For example, with periapical lesions the widening is greatest around the apical region of the root and in periodontal disease the widening is greatest at the alveolar crest. With chronic infections, root resorption may occur and cortical boundaries may be resorbed. The periosteal component of bone, whether on the surface of the jaws or lining the floor of the maxillary sinus, also responds to inflammation. The periosteum contains a layer of pluripotential lining cells that, under the right conditions, differentiate into osteoblasts and lay down new bone. Inflammatory exudate from infection within the bone can penetrate the cortex, lift up the periosteum from the surface of the bone, and stimulate the periosteum to produce new bone. Because inflammatory exudate is a fluid, the periosteum is lifted from the surface of bone in a manner that positions the periosteum almost parallel to the surface of the bone; thus the layer of new bone is almost parallel to the bone surface.
PERIAPICAL INFLAMMATORY LESIONS
Periapical inflammatory lesions have been called acute apical periodontitis, chronic apical periodontitis, periapical abscess, and periapical granuloma. Radiolucent presentations have been called rarefying osteitis, whereas radiopaque presentations have been called sclerosing osteitis, condensing osteitis, and focal sclerosing osteitis. Chapter 21 presents a discussion of periapical cysts of inflammatory origin (radicular cysts).
Definition
A periapical inflammatory lesion is defined as a local response of the bone around the apex of a tooth that occurs as a result of necrosis of the pulp or through destruction of the periapical tissues by extensive periodontal disease (Fig. 20-1). The pulpal necrosis may occur as a result of pulpal invasion of bacteria through caries or trauma. In Figure 20-1, the periapical inflammatory lesion is characterized by apical periodontitis, an inflammatory process that may histologically represent either a periapical abscess or a periapical granuloma. Toxic metabolites from the necrotic pulp exit the root apex to incite an inflammatory reaction in the periapical periodontal ligament and surrounding bone (apical periodontitis). This reaction is characterized histologically by an inflammatory infiltrate composed predominantly of lymphocytes mixed with polymorphonuclear neutrophils. Depending on the severity of the response, the neutrophils may collect to form pus, resulting in an apical abscess. This result is categorized as acute inflammation. Alternatively, in an attempt to heal from apical periodontitis, the body stimulates the formation of granulation tissue mixed with a chronic inflammatory infiltrate composed predominantly of lymphocytes, plasma cells, and histiocytes, giving rise to periapical granuloma. Entrapped epithelium (the rests of Malassez) may proliferate to form a radicular or apical cyst. Acute exacerbations of the chronic lesions may occur intermittently.
If the surrounding bone marrow becomes involved with the inflammatory reaction through the spread of pyogenic organisms, the localized periapical abscess may transform into osteomyelitis. The exact point at which a periapical inflammatory lesion becomes osteomyelitis is not easily determined or defined. The size of the area of inflammation is not as important as the severity of the reaction. However, considering the size of the lesion as one factor, periapical inflammatory lesions usually involve only the local bone adjacent to the apex of the tooth, and osteomyelitis involves a larger area of bone. Periapical lesions occasionally may be large, but the epicenter of the lesion remains in the vicinity of the tooth apex. If the periapical lesion extends farther, so that the lesion no longer is centered on the tooth apex, osteomyelitis may be considered as a possible diagnosis. The distinction between periapical inflammation and osteomyelitis can be made if sequestra are detected radiographically. Progression from periapical inflammation to osteomyelitis is relatively rare, and other factors play a role in its development, such as a decrease in the host defenses and an increase in the virulence of pathogenic microorganisms.
Clinical Features
The symptoms of periapical inflammatory lesions can range across a broad spectrum, from being asymptomatic to an occasional toothache to severe pain with or without facial swelling, fever, and lymphadenopathy. A periapical abscess usually manifests with severe pain, mobility and sometimes elevation of the involved tooth, swelling, and tenderness to percussion. Palpation of the apical region elicits pain. Spontaneous drainage into the oral cavity through a fistula (parulis) may relieve the acute pain. In rare cases a dental abscess may manifest with systemic symptoms (e.g., fever, facial swelling, lymphadenopathy) along with the pain. The acute lesion may evolve into a chronic one (periapical granuloma or cyst), which may be asymptomatic except for intermittent flare-ups of “toothache” pain, which mark the acute exacerbation of the chronic lesion. Patients often give a history of intermittent pain. The associated tooth may be asymptomatic, or it may be sensitive to percussion and mobile. More often, however, the periapical lesion arises in the chronic form de novo; in this case it may be asymptomatic. It is important to understand that the clinical presentation does not necessarily correlate with the histologic or radiographic findings.
Radiographic Features
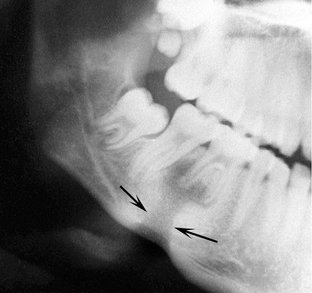
The radiographic features of periapical inflammatory lesions vary depending on the time course of the lesion. Because very early lesions may not show any radiographic changes, diagnosis of these lesions relies solely on the clinical symptoms (Fig. 20-2). More chronic lesions may show lytic (radiolucent) or sclerotic (radiopaque) changes, or both.
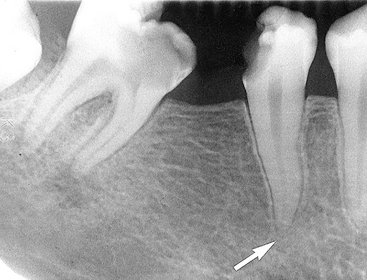
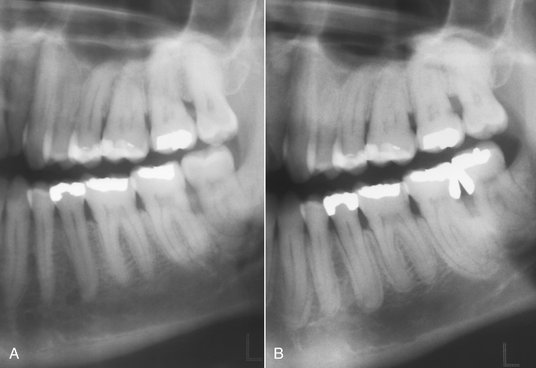
FIG. 20-2 A very early lesion involving the pulp of the second bicuspid without significant change in the periapical bone (arrow). In contrast, note the loss of the lamina dura and periapical bone at the apex of the mesial root of the second molar. Also note the subtle halo of sclerotic bone reaction around this apical radiolucency.
Location.: In most cases the epicenter of periapical inflammatory lesions is found at the apex of the involved tooth (Fig. 20-3). The lesion usually starts within the apical portion of the periodontal ligament space. Less often, such lesions are centered about another region of the tooth root. This may occur because of accessory pulpal canals, perforation of the root structure from instrumentation of the pulp canal, and root fracture.
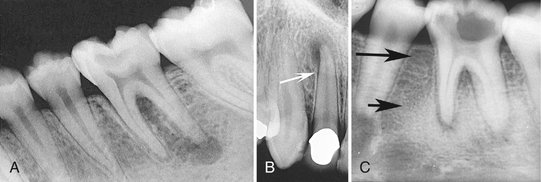
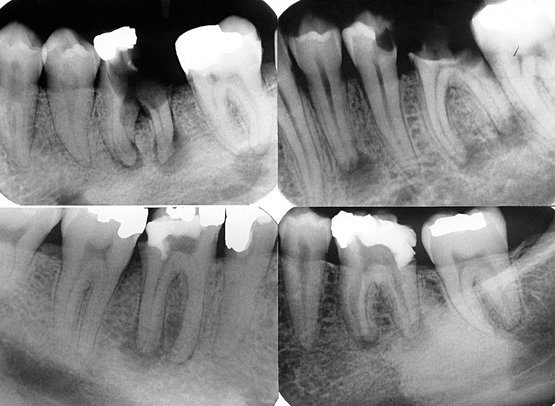
FIG. 20-3 Periapical inflammatory lesions associated with a mandibular first molar (A) and a maxillary lateral incisor (B). Note that in both cases the epicenter of bone destruction is located at the apex of the root. Also, note gradual widening of the periodontal membrane space (arrow) characteristic of an inflammatory lesion. C, This periapical image of sclerosing osteitis related to the first molar shows a gradual transition from thick and numerous trabeculae (short arrow) to a normal trabecular pattern (long arrow).
Periphery.: In most instances the periphery of periapical inflammatory lesions is ill defined, showing a gradual transition from the surrounding normal trabecular pattern into the abnormal bone pattern of the lesion (Figs. 20-3, C, and 20-4). Rarely the periphery may be well defined, with a sharp transition zone and an appearance suggesting a cortical boundary.
Internal Structure.: Early periapical inflammatory lesions may show no radiographic change in the normal bone pattern. The earliest detectable change is loss of bone density, which usually results in widening of the periodontal ligament space at the apex of the tooth and later involves a larger diameter of surrounding bone. At this early stage no evidence may be seen of a sclerotic bone reaction (see Fig. 20-2). Later in the evolution of the disease, a mixture of sclerosis and rarefaction (loss of bone giving a radiolucent appearance) of normal bone occurs (see Fig. 20-4). The percentage of these two bone reactions varies. When most of the lesion consists of increased bone formation, the term periapical sclerosing osteitis is used (Fig. 20-5), and when most of the lesion is undergoing bone resorption, the term periapical rarefying osteitis is used (see Fig. 20-3). The area of greatest bone destruction usually is centered on the apex of the tooth, with the sclerotic pattern located at the periphery. The radiolucent regions may be bereft of any bone structure or may have a faint outline of trabeculae. Close inspection of sclerotic regions reveals thicker than normal trabeculae and sometimes an increase in the number of trabeculae per unit area. In chronic cases the new bone formation may result in a very dense sclerotic region of bone, obscuring individual trabeculae. Occasionally the lesion may appear to be composed entirely of sclerotic bone (sclerosing osteitis), but usually some evidence exists of widening of the apical portion of the periodontal membrane space (see Fig. 20-5).
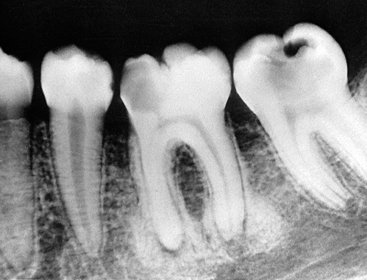
FIG. 20-5 Periapical sclerosing osteitis associated with the first molar. This is called a sclerosing lesion because most of the lesion is bone formation, resulting in a very radiopaque density. Note, however, the small region of bone loss next to the root apex and the widening of the periodontal membrane space.
Effects on Surrounding Structures.: As mentioned previously, periapical inflammatory lesions may stimulate either the resorption of bone or the manufacture of new bone. The lamina dura around the apex of the tooth usually is lost. The sclerotic reaction of the cancellous bone may be limited to a small region around the tooth apex or in some cases may be extensive. In rare instances in the mandible the sclerotic reaction may extend to the inferior cortex. In chronic cases external resorption of the apical region of the root may occur. If the lesion is long standing, the pulp canal may appear wider than adjacent teeth. This is a result of the death of odontoblasts and subsequent cessation of the formation of secondary dentin, which occurs naturally with time to diminish the caliber of the pulp canal slowly.
Nearby cortical boundaries may be destroyed, such as a segment of the floor of the maxillary antrum, the floor of the nasal fossa, or the buccal or lingual plates of the alveolar process immediately adjacent to the root apex. These lesions are capable of producing an inflammatory periosteal reaction, most notably in the adjacent floor of the maxillary antrum. This usually results in a thin layer of new bone produced by the inflamed periosteum within the maxillary antrum, sometimes referred to as a “halo shadow” (Fig. 20-6). A regional mucositis may be present within the adjacent segment of the maxillary antrum. Periosteal reaction may also occur on the buccal or lingual surfaces of the alveolar process and in rare cases on the inferior aspect of the mandible.
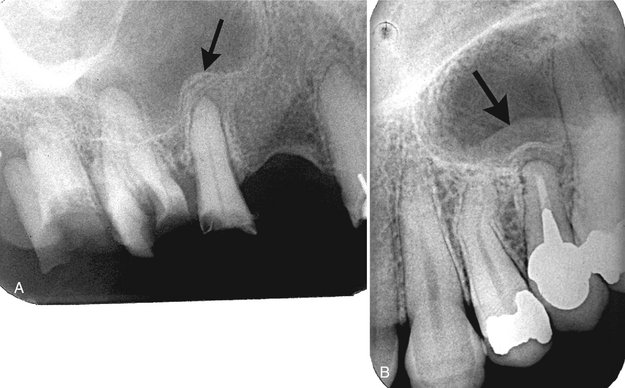
FIG. 20-6 Periostitis resulting in bone formation emanating from the floor of the maxillary antrum that arises from apical inflammatory lesions. A, Laminated type of periosteal bone formation (arrow). B, Periostitis and mucositis. The mucositis is characterized by a slight radiopaque band (arrow) next to the periosteal bone formation.
Differential Diagnosis
The two types of lesions that most often must be differentiated from periapical inflammatory lesions are periapical cemental dysplasia (PCD) and an enostosis (dense bone island, osteosclerosis) at the apex of a tooth. In the early radiolucent phase of PCD, the radiographic characteristics may not reliably differentiate this lesion from a periapical inflammatory lesion (Fig. 20-7). The diagnosis may rely solely on the clinical examination, including a test of tooth vitality. With long-standing periapical inflammatory lesions, the pulp chamber of the involved tooth may be wider than the adjacent teeth. More mature PCD lesions may show evidence of a dense, radiopaque structure within the radiolucency, which helps in the differential diagnosis. Also, a common site for PCD is associated with the apical region of the mandibular anterior teeth. External root resorption is more common with inflammatory lesions than with PCD. When enostosis is centered on the root apex, it may mimic an inflammatory lesion. However, the periodontal ligament space around the apex of the tooth has a normal uniform width (Fig. 20-8). Also, the periphery of an enostosis usually is well defined and does not blend gradually with surrounding trabeculae.
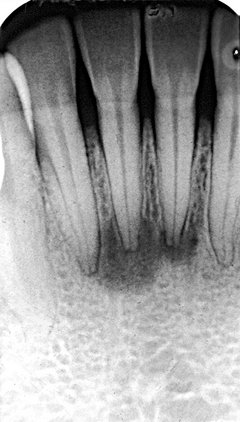
FIG. 20-7 Two early lesions of periapical cemental dysplasia related to the apical region of the mandibular central incisors; note the similarity to apical rarefying osteitis.
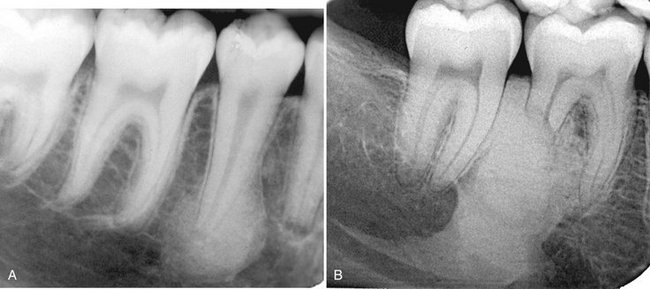
FIG. 20-8 Enostosis (dense bone island) in periapical positions. A, Enostosis around the apex of a second bicuspid. Note that the periodontal membrane space is uniform in width. B, Enostosis associated with apical root resorption of a vital tooth. The most common site of enostosis and root resorption is the mesial or distal root of mandibular first molars.
Small, radiolucent periapical lesions with a well-defined periphery simulating a cortex may be either periapical granulomas or cysts (radicular cysts). Differentiation may not be possible unless other characteristics of a cyst, such as displacement of adjacent structures and expansion of the outer cortical boundaries of the jaw, are present. Lesions larger than 1 cm in diameter usually are radicular cysts. If the patient has had endodontic treatment or apical surgery, a periapical radiolucency may remain that may look like periapical rarefying osteitis (Fig. 20-9). In either case the destroyed bone may not be replaced with normal-appearing bone but with dense fibrous scar tissue. The differential diagnosis cannot be made on radiologic grounds alone; thus the clinical signs and symptoms must take precedence.
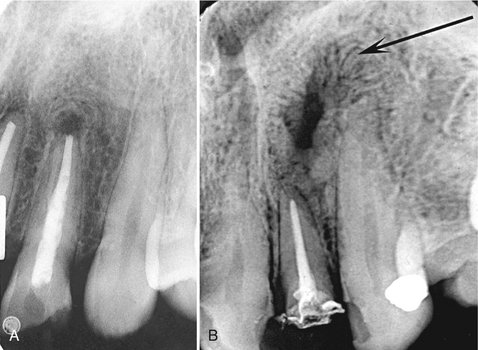
FIG. 20-9 A, Radiolucent apical scar left after successful endodontic treatment. B, Healing periapical inflammatory lesion associated with the apical region of a maxillary lateral incisor. Note the radiating, spokelike pattern of new bone forming from the periphery of the lesion.
In rare cases metastatic lesions and malignancies such as leukemia may grow in the periapical segment of the periodontal membrane space. Close inspection of the surrounding bone may reveal other small regions of malignant bone destruction.
Management
Standard dental treatment of periapical lesions includes root canal therapy or extraction with the intention of eliminating the necrotic material in the root canal and hence the source of inflammation. If left untreated, the tooth may become asymptomatic because of drainage established through the carious lesion or a parulis. However, the possibility always exists that the lesion will spread to involve a larger area of bone, resulting in osteomyelitis or into the surrounding soft tissue, which may result in a space infection or cellulitis.
PERICORONITIS
Definition
The term pericoronitis refers to inflammation of the tissues surrounding the crown of a partially erupted tooth. It is most often seen in association with the mandibular third molars in young adults. The gingiva surrounding the erupted portion of the crown becomes inflamed when food or microbial debris becomes trapped under the soft tissue. The gingiva subsequently becomes swollen and may become secondarily traumatized by the opposing occlusion. This inflammation may extend into the bone surrounding the crown of the tooth.
Clinical Features
Patients with pericoronitis typically complain of pain and swelling. Trismus is a common presentation when the partially erupted tooth is a lower third molar, and usually pain is felt on occlusion. An ulcerated operculum is usually the source of the pain. Pericoronitis can affect patients of any age or sex but is most commonly seen during the time of eruption of the third molars in young adults.
Radiographic Features
The radiologic signs of pericoronitis can range from no changes when the inflammatory lesion is confined to the soft tissues to localized rarefaction and sclerosis to osteomyelitis in the most severe cases.
Location.: When bone changes are associated with pericoronitis, they are centered on the follicular space or the portion of the crown still embedded in bone or in close proximity to bone. The mandibular third molar region is the most common location.
Periphery.: The periphery of pericoronitis is ill defined, with a gradual transition of the normal trabecular pattern into a sclerotic region.
Internal Structure.: The internal structure of bone adjacent to the pericoronitis most often is sclerotic with thick trabeculae. An area of bone loss or radiolucency immediately adjacent to the crown that enlarges the follicular space may be seen (Fig. 20-10). If this lesion spreads considerably, the internal pattern becomes consistent with osteomyelitis (see the next section).
Effects on Surrounding Structures.: As with the periapical inflammatory lesions, pericoronitis may cause the typical changes of sclerosis and rarefaction of surrounding bone. In extensive cases, evidence of periosteal new bone formation may be seen at the inferior cortex, the posterior border of the ramus, and along the coronoid notch of the mandible.
Differential Diagnosis
The differential diagnosis of pericoronitis includes other mixed density or sclerotic lesions that can exist adjacent to the crown of a partially erupted third molar. These include enostosis and fibrous dysplasia. The clinical symptoms indicative of an inflammatory lesion usually exclude these conditions. Neoplasms to be considered include the sclerotic form of osteosarcoma and, in older patients, squamous cell carcinoma. The occurrence of squamous cell carcinoma in the midst of a preexisting inflammatory lesion may be difficult to identify. Features characteristic of malignant neoplasia, such as profound cortical bone destruction and invasion, aids with the diagnosis.
OSTEOMYELITIS
Osteomyelitis is an inflammation of bone. The inflammatory process may spread through the bone to involve the marrow, cortex, cancellous portion, and periosteum. In the jaws pyogenic organisms that reach the bone marrow from abscessed teeth or postsurgical infection usually cause osteomyelitis. However, in some instances no source of infection can be identified, and hematogenous spread is presumed to be the origin. In some patients no infectious organisms can be identified, possibly because of previous antibiotic therapy or inadequate methods of bacterial isolation. Bacterial colonies also may be present in small, isolated pockets of bone that may be missed during sampling.
In patients with osteomyelitis, the bacteria and their products stimulate an inflammatory reaction in bone, causing destruction of the endosteal surface of the cortical bone. This destruction may progress through the cortical bone to the outer periosteum. In young patients, in whom the periosteum is more loosely attached to the outer cortex of bone than it is in adults, the periosteum is lifted up by inflammatory exudate, and new bone is laid down. This periosteal reaction is a characteristic but not pathognomonic feature of osteomyelitis. The hallmark of osteomyelitis is the development of sequestra. A sequestrum is a segment of bone that has become necrotic because of ischemic injury caused by the inflammatory process.
Numerous forms of osteomyelitis have been described. For the sake of simplicity, we group them into two major phases, acute and chronic, recognizing that these represent two ends of a continuum without a definite separating boundary in the process of bone inflammation. Other forms of osteomyelitis have been described as separate and distinct clinicopathologic entities with unique radiographic features. These are Garré’s osteomyelitis and diffuse sclerosing osteomyelitis. We consider them as part of the same continuum. Garré’s osteomyelitis is an exuberant periosteal response to inflammation. Diffuse sclerosing osteomyelitis is a chronic form of osteomyelitis with a pronounced sclerotic response. It is important to understand that all these variations of osteomyelitis have the same underlying process of the bone’s response to inflammation. The features expressed by each subtype represent only variations in the type and degree of bone reaction.
Osteomyelitis may resolve spontaneously or with appropriate antibiotic intervention. However, if the condition is not treated or is treated inadequately, the infection may persist and continue to spread and become chronic in about 20% of patients. Some chronic systemic diseases, immunosuppressive states, and disorders of decreased vascularity may predispose an individual to the development of osteomyelitis. For example, osteopetrosis, sickle cell anemia, and acquired immunodeficiency syndrome have been documented as underlying factors in the development of osteomyelitis.
Synonyms
Acute suppurative osteomyelitis, pyogenic osteomyelitis, subacute suppurative osteomyelitis, Garré’s osteomyelitis, proliferative periostitis, and periostitis ossificans are synonyms for the acute phase of osteomyelitis.
Definition
The acute phase of osteomyelitis is caused by infection that has spread to the bone marrow. With this condition, the medullary spaces of the bone contain an inflammatory infiltrate consisting predominantly of neutrophils and, to a lesser extent, mononuclear cells. In the jaws the most common source of infection is a periapical lesion from a nonvital tooth. Infection also can occur as a result of trauma or hematogenous spread.
The changes described by Garré may accompany acute osteomyelitis. It is thought that the inflammatory exudate spreads subperiosteally, elevating the periosteum and stimulating formation of new bone. This condition is more common in younger people because in these individuals the periosteum is loosely attached to the bone surface and has greater osteogenic potential.
Clinical Features
The acute phase of osteomyelitis can affect people of all ages, and it has a strong male predilection. It is much more common in the mandible than in the maxilla, possibly because of the poorer vascular supply to the mandible. The typical signs and symptoms of acute osteomyelitis are rapid onset, pain, swelling of the adjacent soft tissues, fever, lymphadenopathy, and leukocytosis. The associated teeth may be mobile and sensitive to percussion. Purulent drainage also may be present. Paresthesia of the lower lip in the third division of the fifth cranial nerve distribution is not uncommon.
Radiologic Examination
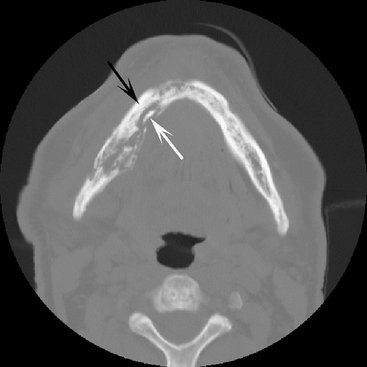
In addition to a complete examination with plain films (panoramic, intraoral periapical and occlusal films), the following additional modalities may be used. A two-phase nuclear medicine study composed of a technetium bone scan followed by a gallium citrate scan may help to confirm the diagnosis. With inflammatory lesions, a positive result on the technetium scan indicates increased bone metabolic activity, and a positive result on the gallium scan in the same location indicates an inflammatory cell infiltrate. Computed tomography (CT) is the imaging method of choice. CT reveals more bone surface for detecting periosteal new bone and is the best imaging method for detecting sequestra (Fig. 20-11). Magnetic resonance imaging (MRI) with T2 weighted images to display abnormal bone marrow edema has been used.
Radiographic Features
Very early in the disease, no radiographic changes may be identifiable. The bone may be filled with inflammatory exudate and inflammatory cells and may show no radiographic change.
Location.: The most common location is the posterior body of the mandible. The maxilla is a rare site.
Periphery.: Acute osteomyelitis most often presents an ill-defined periphery with a gradual transition to normal trabeculae.
Internal Structure.: The first radiographic evidence of the acute form of osteomyelitis is a slight decrease in the density of the involved bone, with a loss of sharpness of the existing trabeculae. In time the bone destruction becomes more profound, resulting in an area of radiolucency in one focal area or in scattered regions throughout the involved bone (Fig. 20-12). Later, the appearance of sclerotic regions becomes apparent. Sequestra may be present but usually are more apparent and numerous in chronic forms (Fig. 20-13). Sequestra can be identified by closely inspecting a region of bone destruction (radiolucency) for an island of bone. This island of nonvital bone may vary in size from a small dot (smaller sequestra usually are seen in young patients) to larger segments of radiopaque bone.
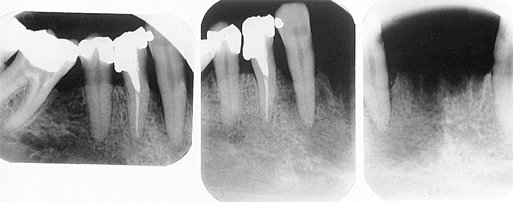
FIG. 20-12 Acute osteomyelitis involving the body of the right mandible, with initial blurring of bony trabeculae. (Courtesy Lars Hollender, DDS, PhD, Seattle, Wash.)
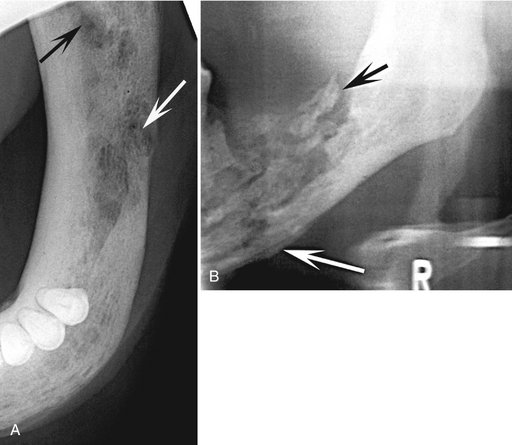
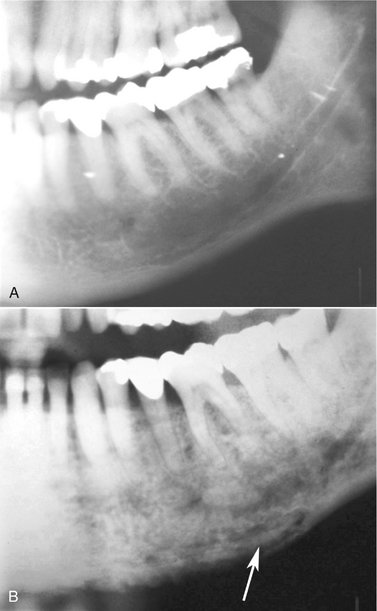
FIG. 20-13 Examples of sequestra.
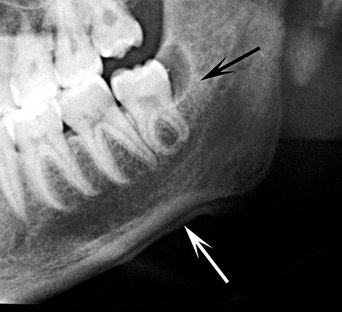
A, Occlusal film demonstrates small sequestra as radiopaque islands of bone in radiolucent regions in the chronic phase of osteomyelitis (arrows). B, Panoramic film reveals large sequestra (black arrow) and a periosteal reaction at the inferior border of the mandible in a case of chronic osteomyelitis (white arrow).
Effects on Surrounding Structures.: Acute osteomyelitis can stimulate either bone resorption or bone formation. Portions of cortical bone may be resorbed. An inflammatory exudate can lift the periosteum and stimulate bone formation. Radiographically, this appears as a thin, faint, radiopaque line adjacent to and almost parallel or slightly convex to the surface of the bone. A radiolucent band separates this periosteal new bone from the bone surface (Fig. 20-14). As the lesion develops into a more chronic phase, cyclic and periodic acute exacerbations may produce more inflammatory exudate, which again lifts the periosteum from the bone surface and stimulates the periosteum to form a second layer of bone. This is detected radiographically as a second radiopaque line almost parallel to the first and separated from it by a radiolucent band. This process may continue and may result in several lines (an onion-skin appearance), and eventually a massive amount of new bone may be formed. This is referred to as proliferative periostitis and is seen more often in children (Fig. 20-15). The effects on the teeth and lamina dura may be the same as those described for periapical inflammatory lesions.
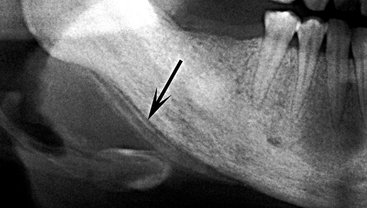
FIG. 20-14 Osteomyelitis of the mandible with a periosteal reaction located at the inferior cortex. Note the radiolucent line (arrow) between the inferior cortex of the mandible and the first layer of periosteal new bone. A second radiolucent line separates the second layer of new bone from the first layer.
Differential Diagnosis
The differential diagnosis of the acute phase of osteomyelitis may include fibrous dysplasia, especially in children. Aside from the clinical signs of acute infection, the most useful radiographic characteristic to distinguish osteomyelitis from fibrous dysplasia is the way the enlargement of the bone occurs. The new bone that enlarges the jaws in osteomyelitis is laid down by the periosteum and therefore is on the outside of the outer cortical plate. In fibrous dysplasia the new bone is manufactured on the inside of the mandible; thus the outer cortex, which may be thinned, is on the outside and contains the lesion. This point of differentiation is important because the histologic appearance of a biopsy of new periosteal bone in osteomyelitis may be similar to that of fibrous dysplasia, and the condition may be reported as such.
Malignant neoplasia (e.g., osteosarcoma, squamous cell carcinoma) that invades the mandible at times may be difficult to differentiate from the acute phase of osteomyelitis, especially if the malignancy has been secondarily infected via an oral ulcer; this may result in a mixture of inflammatory and malignant radiographic characteristics. If part of the inflammatory periosteal bone has been destroyed, the possibility of a malignant neoplasm should be considered. The differential diagnosis may include other lesions that can cause bone destruction and may stimulate a periosteal reaction that is similar to that seen in inflammatory lesions. Langerhans’ cell histiocytosis causes lytic ill-defined bone destruction and often results in the formation of periosteal reactive new bone. This lesion rarely stimulates a sclerotic bone reaction such as that seen in osteomyelitis. Leukemia and lymphoma may stimulate a similar periosteal reaction.
Management
As with all inflammatory lesions of the jaws, removal of the source of inflammation is the primary goal of therapy. Antimicrobial treatment is the mainstay of treatment of acute osteomyelitis, along with establishing drainage. This may entail removal of a tooth, root canal therapy, or surgical incision and drainage.
Synonyms
Chronic diffuse sclerosing osteomyelitis, chronic nonsuppurative osteomyelitis, chronic osteomyelitis with proliferative periostitis, and Garré’s chronic nonsuppurative sclerosing osteitis
Definition
The chronic phase of osteomyelitis may be a sequela of inadequately treated acute osteomyelitis, or it may arise de novo. Diffuse sclerosing osteomyelitis refers to chronic osteomyelitis in which the balance in bone metabolism is tipped toward increased bone formation, producing a subsequent sclerotic radiographic appearance. The symptoms of the chronic form generally are less severe and have a longer history than those of the acute form. They include intermittent, recurrent episodes of swelling, pain, fever, and lymphadenopathy. As with the acute form, paresthesia and drainage with sinus formation also may occur. In some cases pain may be limited to the advancing front of the osteomyelitis, or the patient may have little or no pain. Histologically, a chronic inflammatory infiltrate may be seen within the medullary spaces of bone; however, this may be quite sparse, with only fibrosis of the marrow seen with scattered regions of inflammation. At this stage of the disease, the offending etiologic agent rarely is found because culture results usually are negative. If left untreated, osteomyelitis can spread and involve both sides of the mandible. Further spread into the temporomandibular joint may cause a septic arthritis, and ear infections and infection of the mastoid air cells also may develop.
Chronic osteomyelitis as illustrated here is similar to the bone lesions described in chronic recurrent multifocal osteomyelitis (CRMO) and osteomyelitis of the SAPHO syndrome (synovitis [inflammatory arthritis], acne [pustulosa], pustulosis [psoriasis, palmoplantar pustulosis], hyperostosis [acquired], and osteitis [osteomyelitis]) with respect to the radiographic findings, lack of microbiologic findings, and clinical features such as intermittent recurrent pain and swelling of the involved bone. CRMO is a condition that often occurs symmetrically in the long bones in children. It is characterized by pain of the affected bone with or without swelling and has been described as a nonpurulent osteomyelitis with negative microbiologic cultures. The radiographic features are identical to chronic osteomyelitis as described here. Of interest is that treatment has consisted of systemic steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and bisphosphonates therapy because antibiotic and surgical therapy has not been effective treatment. It may be that chronic osteomyelitis of the jaw in children is a unifocal variant of CRMO.
The radiographic features of the bone lesions of SAPHO are similar if not identical to those of chronic osteomyelitis, and these lesions are refractory to antibiotic therapy, responding to anti-inflammatory agents such as steroids and NSAIDs.
It is possible that the pathophysiologic features of the jaw lesions of chronic osteomyelitis are identical to those of these two conditions.
Radiologic Examination
If chronic osteomyelitis is suspected from the clinical examination, in addition to a complete series of plain films, CT is the imaging method of choice. CT is important for a correct diagnosis with the ability to demonstrate sequestra (see Fig. 20-11) and periosteal new bone and allows accurate staging of the disease, which is important for future assessment of healing. MRI is not as useful because of the lack of bone marrow edema in the chronic phase; however, it may be of use during acute exacerbation of the disease. Scintigraphy by use of bone scans, gallium, or labeled white blood cells is not particularly useful for differential diagnosis. Bone scans indicate increased bone formation, which is nonspecific, and often gallium scans (which highlight inflammatory cells) are not positive because of a very low population of inflammatory cells. The amount of bone activity assessed with bone scans with SPECT (single-photon emission CT) has been used to monitor healing. There are also reports of the use of positron emission tomography to detect a high cellular metabolic rate in tissues, but this type of imaging is nonspecific.
Radiographic Features
Periphery.: The periphery may be better defined than in the acute phase, but it is still difficult to determine the exact extent of chronic osteomyelitis. Usually a gradual transition is seen between the normal surrounding trabecular pattern and the dense granular pattern characteristic of this disease. When the disease is active and is spreading through bone, the periphery may be more radiolucent and have poorly defined borders.
Internal Structure.: The internal structure comprises regions of greater and lesser radiopacity compared with surrounding normal bone. Most of the lesion usually is composed of the more radiopaque or sclerotic bone pattern (Fig. 20-16). In older, more chronic lesions the internal bone density can be exceedingly radiopaque and equivalent to cortical bone. In these cases no obvious regions of radiolucency may be seen. In other cases, small regions of radiolucency may be scattered throughout the radiopaque bone. A close inspection of the radiolucent regions may reveal an island of bone or sequestrum within the center (Fig. 20-17). Often the sequestrum appears more radiopaque than the surrounding bone. Detection may require illumination of the radiolucent regions of the film with an intense light source. CT is superior for revealing the internal structure and sequestra, especially in cases with very dense sclerotic bone. The bone pattern usually is very granular, obscuring individual bone trabeculae.
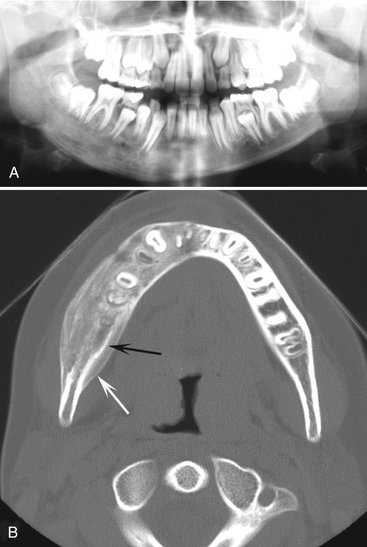
FIG. 20-16 Chronic osteomyelitis. A, This panoramic film demonstrates chronic osteomyelitis of the patient’s right mandible; note the increase in density and size of the right mandible compared with the left side. B, An axial CT image using bone window of the mandible of the same case. Note the increase in bone density, width of the mandible, and the new periosteal bone formation (white arrow) and evidence of the original cortex (black arrow).
Effects on Surrounding Structures.: Chronic osteomyelitis often stimulates the formation of periosteal new bone, which is seen radiographically as a single radiopaque line or a series of radiopaque lines (similar to onion skin) parallel to the surface of the cortical bone. Over time the radiolucent strip that separates this new bone from the outer cortical bone surface may be filled in with granular sclerotic bone. When this occurs, it may not be possible to identify the original cortex, which makes it difficult to determine whether the new bone is derived from the periosteum. After a considerable amount of time the outer contour of the mandible also may be altered, assuming an abnormal shape, and the girth of the mandible may be much larger than on the unaffected side. The roots of teeth may undergo external resorption, and the lamina dura may become less apparent as it blends with the surrounding granular sclerotic bone. If a tooth is nonvital, the periodontal ligament space usually is enlarged in the apical region. In patients with extensive chronic osteomyelitis, the disease may slowly spread to the mandibular condyle and into the joint, resulting in a septic arthritis. Further spread may involve the inner ear and mastoid air cells. Chronic lesions may develop a draining fistula, which may appear as a well-defined break in the outer cortex or in the periosteal new bone (Fig. 20-18).
Differential Diagnosis
Very sclerotic, radiopaque chronic lesions of osteomyelitis may be difficult to differentiate from fibrous dysplasia, Paget’s disease, and osteosarcoma. In children, osteomyelitis with a proliferative periosteal response may be misinterpreted as fibrous dysplasia (see the section Differential Diagnosis under Acute Osteomyelitis). Differentiation of the chronic form of osteomyelitis may be even more difficult if considerable remodeling and loss of a distinct original cortex have occurred. In these cases, inspection of the bone surface at the most peripheral part of the lesion may reveal subtle evidence of periosteal new bone formation. The presence of sequestra indicates osteomyelitis. Paget’s disease affects the entire mandible, which is rare in osteomyelitis. Periosteal new bone formation and sequestra are not seen in Paget’s disease. Dense, granular bone may be seen in some forms of osteosarcoma, but usually evidence of bone destruction is found. A characteristic spiculated (sunraylike) periosteal response also may be seen. As mentioned in the section on acute osteomyelitis, other entities such as Langerhans’ cell histiocytosis, leukemia, and lymphoma may stimulate a similar periosteal response, but these usually produce evidence of bone destruction characteristic of malignant tumors.
The imaging method of choice for aiding in the differential diagnosis is CT because of its ability to reveal sequestra and periosteal new bone.
Management
Chronic osteomyelitis tends to be more difficult to eradicate than the acute form. In cases involving an extreme osteoblastic response (very sclerotic mandible), the subsequent lack of a good blood supply may work against healing. Hyperbaric oxygen therapy and creative modes of long-term antibiotic delivery have been used with limited success. Surgical intervention, which may include sequestrectomy, decortication, or resection, often is necessary. The probability of successful treatment, especially when using long-term antibiotic therapy with decortication, is greater in the first two decades of life. If cultures are negative, antibiotic therapy is not effective. It may be that the inflammatory response has become the main disease process and anti-inflammatory agents such as steroids and NSAIDs are more effective. More recently, the use of bisphosphonate therapy has provided some therapeutic success.
Diagnostic Imaging of Soft Tissue Infections
Diagnostic imaging may be used to confirm the presence and extent of soft tissue infections. MRI and CT may be used to differentiate soft tissue neoplasia from inflammatory lesions. MRI can be used in the T2 or TI with gadolinium and fat suppression modes to detect the presence of soft tissue edema. CT usually is used with intravenous contrast. The CT image characteristics that suggest the presence of a soft tissue inflammation include abnormal fascial planes, thickening of the overlying skin and adjacent muscles, streaking of the fat planes, and abnormal collections of gas in the soft tissue (Fig. 20-19). Over time the contrast between soft tissue planes may disappear, and the presence of an abscess may become evident as a well-defined region of low density surrounded by a wide border of contrast-enhanced (more radiopaque) tissue. Lymphadenopathy resulting from infections such as tuberculosis of the head and neck may be visualized on magnetic resonance and CT images (Fig. 20-20).
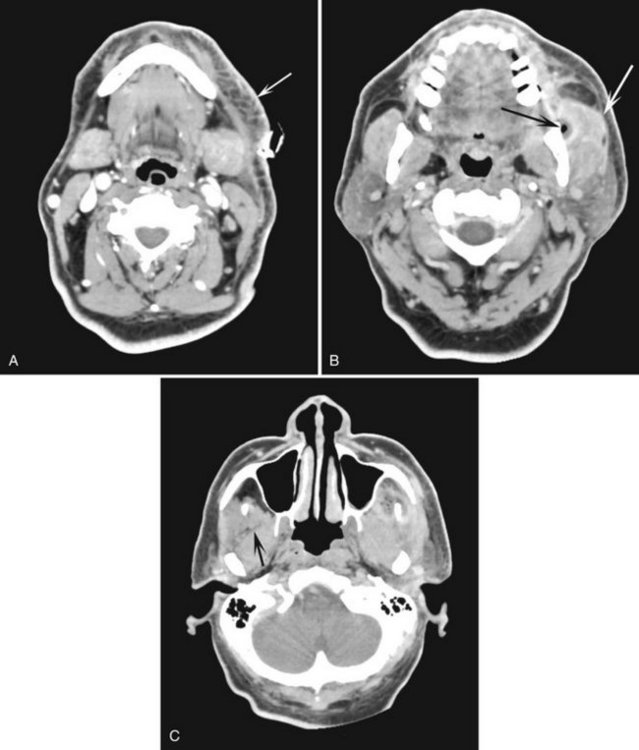
FIG. 20-19 Three axial CT images, using a contrast medium, of a soft tissue infection demonstrating streaking (reticulation pattern) of the fat planes and thickening of the skin (arrow) (A); thickening of the masseter muscle (white arrow) and a radiolucent pocket of gas (black arrow) (B); and loss of distinctive soft tissue planes for example individual muscles defined by fat planes (the lateral border of the normal lateral pterygoid muscle [arrow] is not apparent on the opposite affected side) (C). (Courtesy Stuart White, DDS, Los Angeles, Calif.)
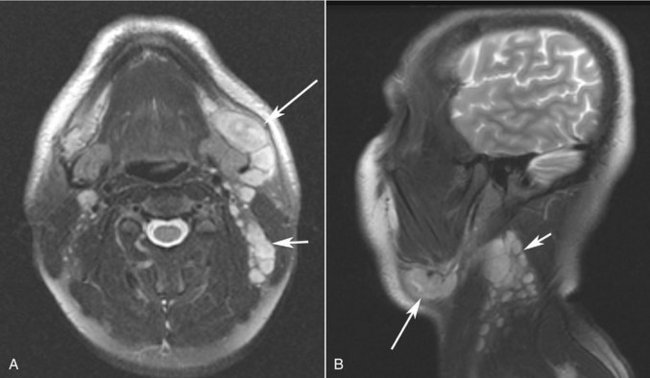
FIG. 20-20 A, Axial T2-weighted and B, sagittal T2-weighted magnetic resonance images of a case of tuberculosis with significant lymphadenopathy involving the submandibular lymph nodes (long arrows) and level II nodes (short arrows).
RADIATION-INDUCED CHANGES TO BONE
Therapeutic radiation damages the cellular elements of bone tissue by immediate or delayed cell death, cellular injury with recovery, arrested cellular division, or abnormal repair with neoplasia. The maturity and type of bone and the dose of radiation are factors that affect how the bone responds to this injury. When immature bone is irradiated, growth retardation occurs; the amount is related to the radiation dose and the stage of bone growth: the earlier the stage the greater the effect. Radiation damage to mature bone affects the osteoblasts, resulting in a decrease in matrix formation and damage to the fine blood vessels. The following discussion is limited to the mature bone of the maxillofacial region.
As with osteomyelitis, there is a spectrum of radiographic appearances of radiation damage to bone. These range from sclerosis with patchy radiolucency to osteoradionecrosis. When radiation damage to bone progresses to osteoradionecrosis, it is often first diagnosed clinically as an exposed bone sequestrum in the oral cavity before there are significant radiographic changes.
OSTEORADIONECROSIS
Osteoradionecrosis refers to an inflammatory condition of bone (osteomyelitis) that occurs after the bone has been exposed to therapeutic doses of radiation usually given for a malignancy of the head and neck region. It is characterized by the presence of exposed bone for a period of at least 3 months occurring at any time after the delivery of the radiation therapy. Doses above 50 Gy usually are required to cause this irreversible damage. Bone that has been irradiated is hypocellular and hypovascular. The lack of sufficient vascularity results in a hypoxic environment in which adequate healing of bone is compromised. Although infection may be a contributing factor, it is not necessarily the primary insult after the radiation damage has occurred. In many cases dental extraction and denture trauma after radiation therapy have been implicated as etiologic factors. Secondary infection is common, further fomenting the inflammatory reaction. Because of the difficulty of management, this serious complication of radiation therapy carries a high morbidity rate.
Clinical Features
The mandible is much more commonly affected than the maxilla is. This is likely due to the microanatomy and comparatively less vasculature of this bone. The posterior mandible is affected more often than the anterior portion. The posterior body of the mandible is more frequently in the direct field of the radiation treatment because primary tumors and metastatic lesions in lymph nodes being treated are commonly adjacent to this part of the mandible. Loss of mucosal covering and exposure of bone is the hallmark of osteoradionecrosis. Pathologic fracture also may occur. The exposed bone becomes necrotic as a result of loss of vascularity from the periosteum and subsequently sequestrates, often leading to exposure of more bone. Pain may or may not be present. Intense pain may occur, with intermittent swelling and drainage extraorally. However, many patients feel no pain with bone exposure.
Radiologic Examination
The prescription of diagnostic imaging would be the same as used for chronic-phase osteomyelitis with CT being the imaging modality of choice.
Radiographic Features
The radiographic features of osteoradionecrosis have many similarities to those of chronic osteomyelitis, and the reader is referred to that section for a detailed description. The following is a description of the radiographic changes seen in bone that has received a considerable amount of therapeutic radiation. An early characteristic change is a well-defined area of bone resorption within the outer cortical plate of the mandible (Fig. 20-21). Later changes are quite variable and may be predominantly lytic or sclerotic or a mixture (Fig. 20-22). However, the presence of osteoradionecrosis cannot always be diagnosed radiographically and often clinically obvious signs of exposed necrotic bone may not be accompanied by significant radiologic changes.
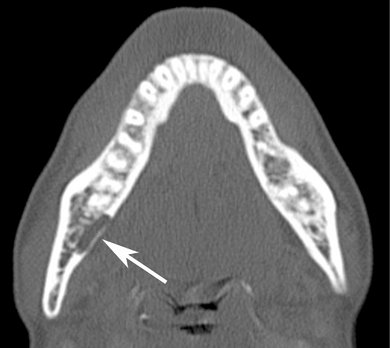
FIG. 20-21 An axial CT image showing a well-defined region of cortical bone resorption (arrow), an early change in therapeutic radiation exposure.
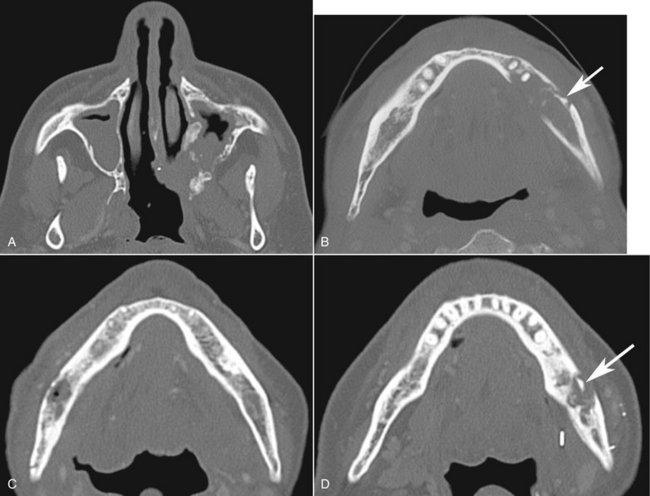
FIG. 20-22 A series of axial CT images of different patients with various reactions to therapeutic levels of radiation exposure, including irregular resorption of the maxilla in A, mostly a resorptive reaction in the mandible with a sequestra (arrow) in B, a mixture of sclerosis and resorption in the mandible in C and D; note the sequestra (arrow) and slight periosteal response in D.
Location.: The mandible, especially the posterior mandible, is the most common location for osteoradionecrosis. The maxilla may be involved in some cases.
Periphery.: The periphery is ill defined and similar to that in osteomyelitis. If the lesion reaches the inferior border of the mandible, irregular resorption of this bony cortex often occurs.
Internal Structure.: A range of bone formation to bone destruction occurs, often with the balance heavily toward more bone formation, giving the affected bone an overall sclerotic or radiopaque appearance. This is very similar to chronic osteomyelitis. The bone pattern is granular. Scattered regions of radiolucency may be seen, with and without central sequestra. The affected maxillary bone may also be very sclerotic and have areas of bone resorption (see Fig. 20-22).
Effects on Surrounding Structures.: Inflammatory periosteal new bone formation is uncommon, possibly because of the deleterious effects of radiation on potential osteoblasts in the periosteum. In very rare cases the periosteum appears to have been stimulated to produce bone, resulting in new bone formation on the outer cortex in an unusual shape. Radiation exposure may also stimulate the resorption of bone, especially in the maxilla, which may be similar in appearance to bone destruction caused by a malignant neoplasm. The most common effect on the surrounding bone is the stimulation of sclerosis. In the alveolar process of the maxilla and mandible, there may be irregular widening of the periodontal membrane space similar to that seen in malignant neoplasia or it may simulate periapical rarefying osteitis. Also, there may be bone resorption, very similar to periodontal disease (Fig. 20-23).
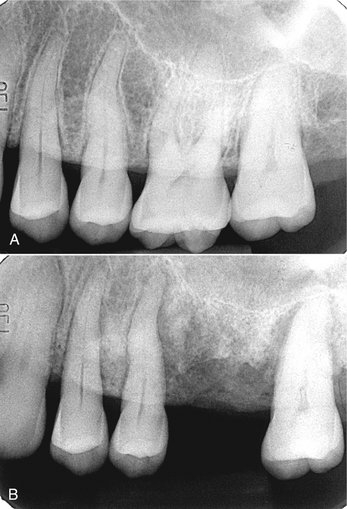
FIG. 20-23 Osteoradionecrosis of the maxilla. These periapical films were taken before radiotherapy (A) and within 6 months of receiving the radiation (B). Note the combination of bone sclerosis and profound bone destruction around the teeth and alveolar crest and widening of the periodontal membrane space.
Differential Diagnosis
Bone resorption, stimulated by high levels of irradiation, may simulate bone destruction from a malignant neoplasm, especially in the maxilla. For this reason, the detection of a recurrence of the malignant neoplasm (usually squamous cell carcinoma) in the presence of osteoradionecrosis may be very difficult. If recurrence is suspected, CT and MRI may be used to detect an associated soft tissue mass. Differentiation from other sclerotic lesions, as in chronic osteomyelitis, is less difficult because of the history of radiation therapy.
Management
The treatment of osteoradionecrosis currently is unsatisfactory. Decortication with sequestrectomy and hyperbaric oxygen with antibiotics have been used with limited success because of poor healing after surgery. Conservative approaches with the aim of therapy to maintain the integrity of the lower border of the mandible, keeping the site free of infection and the patient free of pain, may in the long term prove more successful. Fortunately, the incidence of osteoradionecrosis has declined because preventive therapy has proved quite effective. Removal of teeth that have significant periodontal disease or have a poor prognosis before radiation treatment and excellent oral and denture hygiene are the mainstays of preventive treatment.
BISPHOSPHONATE-RELATED OSTEONECROSIS OF THE JAWS
Bisphosphonates are potent synthetic analogs of pyrophosphates that act to inhibit osteoclasts and reduce bone metabolism. These drugs have become important in the treatment of bone lesions of multiple myeloma, hypercalcemia of malignancy, metastatic bone tumors, and osteoporosis. In recent years a complication of intraoral exposure of necrotic bone has been described in patients receiving these medications. The bone exposure occurs more commonly in patients receiving the more potent aminobisphosphonates intravenously and after an invasive dental surgical procedure such as extraction, periodontal or endodontic surgery, or implant placement. Bisphosphonate-related osteonecrosis has now been well documented, although the pathogenesis remains unclear.
Clinical Features
Clinically, patients typically have an area of exposed bone after an invasive dental surgical procedure. However, denture trauma and spontaneous cases have been known to occur. Ulceration of palatal tori resulting in bone exposure is most likely the result of trauma. The most common areas affected are the posterior mandible (60%) and the maxilla (40%) and both (9%). The incidence of bone exposure is difficult to determine, but recent studies suggest that approximately 3% of patients receiving these drugs will have exposed bone. The areas may be asymptomatic or present with pain and swelling.
Radiographic Features
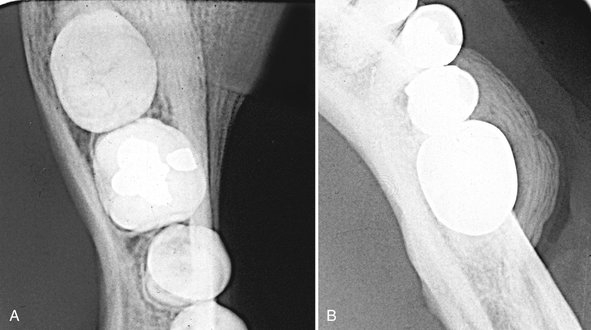
There is a spectrum of radiographic findings that may or may not correlate well with the clinical symptoms. More often than not, there are no specific radiographic findings with the clinically exposed bone. In other cases the radiographic changes are not dissimilar to osteoradionecrosis or chronic osteomyelitis with the presence of sequestra (Fig. 20-24). Other reported findings include an increase in bone sclerosis (Fig. 20-25), widening of the periodontal membrane space, and thickening of the lamina dura (Fig. 20-26).
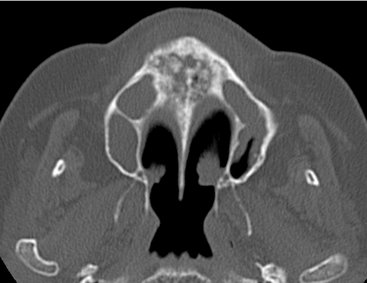
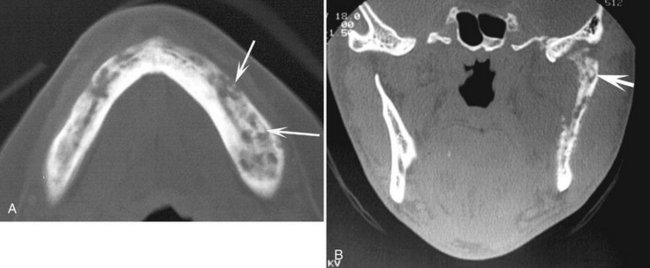
FIG. 20-24 An axial CT image of a patient with bisphosphonate-related osteonecrosis; note the several sequestra in the anterior palate.
Management
Unfortunately, treatment of bisphosphonate-related bone exposure is not satisfactory. Surgical intervention and hyperbaric oxygen therapy have not been consistently successful. The mainstay of therapy is preventive in nature. Patients who will be administered the potent aminobisphosphonates should have a dental examination to remove potential and real sources of infection to obviate the need for invasive dental procedures in the future. This is further complicated by the fact that the half-life of these drugs in bone can be quite lengthy (estimated at ≈12 years). Once bone is exposed, treatment is aimed at controlling the symptoms of pain and infection with antibiotic mouthrinses and systemic antibiotic therapy.
PERIAPICAL INFLAMMATORY LESIONS
Heersche, JNM. Bone cells and bone turnover: the basis for pathogenesis. In: Tam CS, Heersche JNM, Murray TM, eds. Metabolic bone disease: cellular and tissue mechanisms. Boca Raton, FL: CRC Press, 1989.
Stern, MH, Dreizen, S, Mackler, BF, et al. Quantitative analysis of cellular composition of human periapical granuloma. J Endocrinol. 1981;7:117–122.
Blakey, GH, White, RP, Jr., Offenbacher, S, et al. Clinical/biological outcomes of treatment for pericoronitis. J Oral Maxillofac Surg. 1996;54:1150–1160.
Becker, W. Imaging osteomyelitis and the diabetic foot. Q J Nucl Med. 1999;43:9–20.
Compeyrot-Lacassagne, S, Rosenberg, AM, Babyn, P, et al. Pamidronate treatment of chronic noninfectious inflammatory lesions of the mandible in children. J Rheumatol. 2007;34:1585–1589.
Guhlmann, A, Brecht-Krauss, D, Suger, G, et al. Chronic osteomyelitis: detection with FDG PET and correlation with histopathologic findings. Radiology. 2006;2006:749–754.
Ledermann, HP, Kaim, A, Bongartz, G, et al. Pitfalls and limitations of magnetic resonance imaging in chronic posttraumatic osteomyelitis. Eur Radiol. 2000;10:1815–1823.
Morrison, WB, Schweitzer, ME, Batte, WG, et al. Osteomyelitis of the foot: relative importance of primary and secondary MR imaging signs. Radiology. 1998;207:625–632.
Nordin, U, Wannfors, K, Colque-Navarro, P, et al. Antibody response in patients with osteomyelitis of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:429.
Orpe, EC, Lee, L, Pharoah, MJ. A radiological analysis of chronic sclerosing osteomyelitis of the mandible. Dentomaxillofac Radiol. 1996;25:125–129.
Petrikowski, CG, Pharoah, MJ, Lee, L, et al. Radiographic differentiation of osteogenic sarcoma, osteomyelitis, and fibrous dysplasia of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:744–750.
Suei, Y, Taguchi, A, Tanimoto, K. Diagnostic points and possible origin of osteomyelitis in synovitis, acne, pustulosis, hyperostosis and osteitis (SAPHO) syndrome: a gradiographic study of 77 mandibular osteomyelitis cases. Rheumatology. 2003;42:1398–1403.
Suei, Y, Tanimoto, K, Taguchi, A, et al. Possible identity of diffuse sclerosing osteomyelitis and chronic recurrent multifocal osteomyelitis: one entity or two. Oral Med Oral Pathol Oral Radiol Endod. 1995;80:401–408.
Van Merkesteyn, JP, Groot, RH, Bras, J, et al. Diffuse sclerosing osteomyelitis of the mandible: clinical radiographic and histologic findings in twenty-seven patients. J Oral Maxillofac Surg. 1988;46:825–829.
Wannfors, K, Hammarström, L. Infectious foci in chronic osteomyelitis of the jaws. Int J Oral Surg. 1985;14:493–503.
Wood, RE, Nortjé, CJ, Grotepass, F, et al. Periostitis ossificans versus Garré’s osteomyelitis, Part I: what did Garré really say? Oral Surg Oral Med Oral Pathol. 1988;65:773–777.
RADIATION-INDUCED CHANGES TO BONE
Becker, M, Schroth, G, Zbären, P, et al. Long-term changes induced by high-dose irradiation of the head and neck region: imaging findings. Radiographics. 1997;17:5–26.
Williams, HJ, Davies, AM. The effect of x-rays on bone: a pictorial review. Eur Radiol. 2006;16:619–633.
Curi, MM, Dib, LL. Osteoradionecrosis of the jaws: a retrospective study of the background factors and treatment in 104 cases. J Oral Maxillofac Surg. 1997;55:540–544.
Hermans, R, Fossion, E, Ioannides, C, et al. CT findings in osteoradionecrosis of the mandible. Skeletal Radiol. 1996;25:31–36.
Hutchison, IL, Cullum, ID, Langford, JA, et al. The investigation of osteoradionecrosis of the mandible by 99mTc-methylene diphosphonate radionuclide bone scans. Br J Oral Maxillofac Surg. 1990;28:143–149.
Marx, RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–288.
Wong, JK, Wood, RE, McLean, M. Conservative management of osteoradionecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:16–21.
BISPHOSPHONATE-RELATED OSTEONECROSIS
Jadu, F, Lee, L, Pharoah, M, et al. A retrospective study assessing the incidence, risk factors and comorbidities of pamidronate-related necrosis of the jaws in multiple myeloma patients. Ann Oncol. 2007;18:2015–2019.
Woo, SB, Hellstein, J, Kalmar, JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761.