Benign Tumors of the Jaws
Definition
Benign tumors represent a new uncoordinated growth that generally has the following characteristics. Benign tumors are slowly growing and spread by direct extension and not by metastases. Histologically they tend to resemble the tissue of origin. For example, an ameloblastoma, a tumor thought to be derived from odontogenic epithelium, is often composed of cells that resemble ameloblasts. It is thought that benign tumors have unlimited growth potential. Often hamartomas are included in the category of benign tumors. However, hamartomas are overgrowths of disorganized normal tissue that have a limited growth potential. For example, an odontoma is a hamartoma of dental tissue (disorganized enamel, dentine, and pulp tissues) derived from the dental follicle that stops growing at approximately the same time as other normal dental tissues. Included in this chapter are hyperplasias. Hyperplasia refers to a growth formed by an increase in the number of cells of a tissue but differs from hamartomas in that the tissue is in a normal arrangement. Hyperplasia is generally thought to be a reaction to a stimulus such as inflammation. Therefore they have limited growth potential and tend to regress when the stimulus is removed.
Clinical Features
Benign tumors typically have an insidious onset and grow slowly. These tumors usually are painless, do not metastasize, and are not life threatening unless they interfere with a vital organ by direct extension.
Benign tumors are usually detected clinically by enlargement of the jaws or are found during a radiographic examination. Sometimes the radiologic examination is to try to discover the reason for the lack of development of a tooth.
Radiographic Examination
Once the clinician has made a preliminary diagnosis of the presence of a tumor, a full radiologic examination should be made to fully document the extent and characteristics of the lesion. This may entail further films such as intraoral and occlusal radiographs or a panoramic film. For central bone lesions the addition of computed tomography is essential for assessing the effects on the surrounding osseous structures. If the lesion originates in soft tissue or has extended from bone into the surrounding soft tissue, then magnetic resonance imaging may be required.
A thorough radiologic examination will provide information regarding the extent of the lesion, and sometimes the characteristics are so specific that a preliminary diagnosis of the type of benign tumor can be made. On the other hand, the imaging characteristics of the lesion may fail to indicate the type of tumor. A thorough workup will also indicate the most favorable biopsy site. The radiologic examination should always be completed before the biopsy procedure.
Radiographic Features
The following general features suggest the presence of a benign neoplasm.
LOCATION
Because many tumors have a specific anatomic predilection, the location of a particular neoplasm is important in establishing the differential diagnosis. For example, odontogenic lesions occur in the alveolar processes above the inferior alveolar nerve canal, where tooth formation occurs. Vascular and neural lesions may originate inside the mandibular canal, arising from the neurovascular tissues. Cartilaginous tumors occur in jaw locations where residual cartilaginous cells lie, such as around the mandibular condyle.
PERIPHERY AND SHAPE
Benign tumors enlarge slowly by formation of additional internal tissue. Because of this, the radiographic borders of benign tumors appear relatively smooth, well defined, and sometimes corticated. If the tumor produces a calcified product, for example, abnormal tooth material or abnormal bone, the most mature part of the tumor will be in the central region with the most immature aspect at the periphery. This sometimes results in a radiolucent band of soft tissue or capsule at the periphery where the calcified product has not yet formed; this band separates the more mature internal radiopaque portion from the surrounding normal bone.
INTERNAL STRUCTURE
The internal structure may be completely radiolucent or radiopaque or may be a mixture of radiolucent and radiopaque tissues. If the lesion contains radiopaque elements, these structures usually represent either residual bone or a calcified material that is being produced by the tumor. For instance, curved septa that are characteristic in ameloblastoma represent residual bone trapped inside the tumor that has remodeled into curved septa. The ameloblastoma does not produce bone. On the other hand, the osteoblastoma often has an internal granular radiopaque pattern produced by the abnormal bone that is actually being manufactured by the tumor. Often the internal pattern is characteristic for specific types of tumors and may help with the diagnosis. A totally radiolucent internal structure is not as useful as an aid to the diagnosis.
EFFECTS ON SURROUNDING STRUCTURES
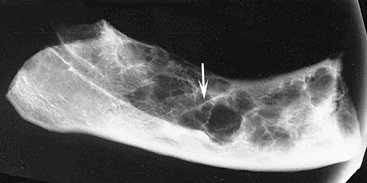
The manner in which a tumor affects adjacent tissues may suggest a benign behavior. For example, a benign tumor exerts pressure on neighboring structures, resulting in the displacement of teeth or bony cortices. If the growth is slow enough, there will be adequate time for the outer cortex to remodel in response to the pressure, resulting in an appearance that the cortex has been displaced by the tumor (Fig. 22-1). This is caused by simultaneous resorption of bone along the inner surface (endosteal) of the cortex and deposition of bone along the outer cortical surface by the periosteum (Fig. 22-2). Through this remodeling process, the cortex maintains its integrity and resists perforation, although faster growing tumors may exceed this process, resulting in perforation of the cortex. Benign tumors may also cause bodily displacement of nearby teeth (Fig. 22-3). The movement of teeth adjacent to benign tumors is slow because these lesions grow slowly.
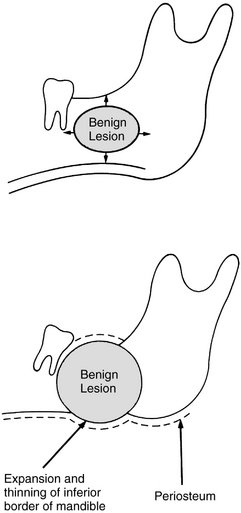
FIG. 22-1 Benign lesions growing in bone tend to be round or oval. They grow by displacing adjacent tissues. (From Matteson SR, Tyndall DA, Burkes EJ et al: The radiology of benign and malignant lesion, Dent Radiogr Photogr 57:35-52, 78-84, 1985.)
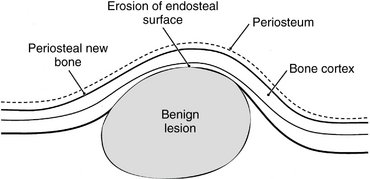
FIG. 22-2 The host bone of a benign tumor may expand as a result of outward remodeling of its cortical borders. As the benign tumor extends toward the periphery of the bone, the periosteum lays down new bone along the outer cortex, thereby maintaining the integrity of the cortex.
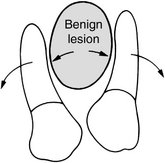
FIG. 22-3 A benign lesion usually grows slowly, causing displacement of adjacent teeth. (From Matteson SR, Tyndall DA, Burkes EJ et al: The radiology of benign and malignant lesions, Dent Radiogr Photogr 57:35-52, 78-84, 1985.)
The roots of teeth may be resorbed by either benign or malignant tumors, but root resorption is more commonly associated with benign processes. The benign tumors especially likely to resorb roots are ameloblastomas, ossifying fibromas, and central giant cell granulomas. Benign tumors tend to resorb the adjacent root surfaces in a smooth fashion. Bone dysplasias such as fibrous dysplasia do not usually resorb teeth. When root resorption is associated with malignant tumors, the resorption is usually in smaller quantities, causing thinning of the root into a “spiked” shape.
Hyperplasias
Bony hyperplasias are included in this chapter but are not considered tumors because of the normal arrangement of the tissue and the limited growth potential; in some cases this growth is in response to a stimulus. Bony hyperplasias are growths of normal new bone that sometimes occur in characteristic locations. In dentistry the terms exostosis and hyperostosis are both used to describe a bony growth that occurs on the surface of normal bone. It should be noted that in medicine the term exostosis often is used for a surface bony growth with a cartilage cap (osteochondroma). Therefore the term hyperostosis may be preferred to avoid confusion.
Definition
Torus palatinus is a bony protuberance (hyperostosis) that occurs in the middle third of the midline of the hard palate.
Clinical Features
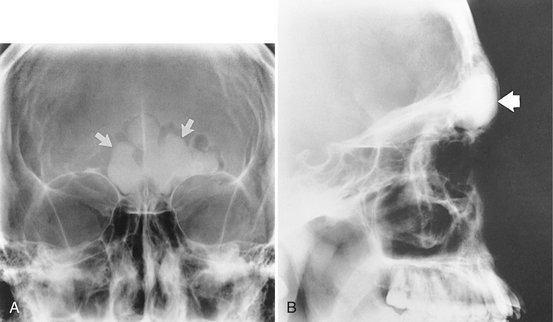
Torus palatinus, the most common exostoses, occurs in about 20% of the population, although various studies have shown marked differences in racial groups. It develops about twice as often in women as in men and more often in Native Americans, Eskimos, and Norwegians. Although it may be discovered at any age, it is rare in children. It usually begins developing in young adults before 30 years of age and is thought to arise through interplay of genetic and environmental factors. The base of the bony nodule extends along the central portion of the hard palate, and the bulk reaches downward into the oral cavity. The size and shape of a torus palatinus can vary, and these lesions have been described as flat, lobulated, nodular, or mushroomlike (Fig. 22-4, A). Normal mucosa covers the bony mass and may appear pale and sometimes ulcerated when traumatized. Patients often are unaware of this hyperplasia, and those who do discover it may insist that it occurred suddenly and has been growing rapidly.
Radiographic Features
Location.: On maxillary periapical or panoramic radiographs, a torus palatinus appears as a dense radiopaque shadow below and attached to the hard palate. It may be superimposed over the apical areas of the maxillary teeth, especially if the torus has developed in the middle or anterior regions of the palate. The image of a palatal torus may project over the roots of the maxillary molars (Fig. 22-4, B), but the shadow will usually move in its position relative to the roots of the teeth if another film is taken with a different horizontal or vertical angulation of the central ray (Fig. 22-5).
Periphery and Shape.: The border of the radiopaque shadow usually is well defined and may have a convex or a lobulated outline (Fig. 22-6).
Treatment
A torus palatinus usually does not require treatment, although removal may be necessary if a maxillary denture is to be made.
Definition
Torus mandibularis is a hyperostosis that protrudes from the lingual aspect of the mandibular alveolar process, usually near the premolar teeth.
Clinical Features
Tori occur less often on the lingual surface of the mandible than on the palate, with the former occurring in about 8% of the population. These tori develop singly or multiply, unilaterally or bilaterally (usually bilaterally), and most often in the premolar region. The size also varies, ranging from an outgrowth that is just palpable to one that contacts a torus on the opposite side. In contrast to torus palatinus, torus mandibularis develops later, being first discovered in middle-aged adults. However, it has the same sex predilection as torus palatinus. In women the occurrence of torus mandibularis correlates with that of torus palatinus, but this apparently is not the case in men. As with torus palatinus, torus mandibularis may occur more often in those of East Asian ancestry.
Genetic and environmental factors seem to be involved in the development of torus mandibularis, but masticatory stress is reported as an essential factor underlying its formation. The high prevalence among Eskimos and other subarctic peoples who make extraordinary chewing demands on their teeth seems to support this suggestion. Also, a patient with a torus mandibularis has, on average, more teeth present than a patient without a torus.
Radiographic Features
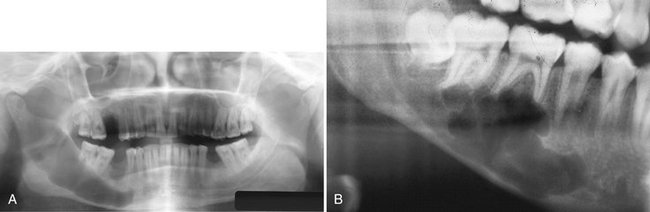
Location.: Recognition of mandibular tori relies on their appearance and location. Their presence bilaterally reinforces this impression, although they can occur unilaterally. On mandibular periapical radiographs, a torus mandibularis appears as a radiopaque shadow, usually superimposed on the roots of premolars and molars and occasionally over a canine or incisor. It usually is superimposed over about three teeth.
Periphery.: Mandibular tori are sharply demarcated anteriorly on periapical films and are less dense and less well defined as they extend posteriorly (Fig. 22-7). There is no margin between the periphery of the torus and the surface of the mandible because the torus is continuous with the mandibular cortex.
Internal Structure.: On occlusal radiographs a mandibular torus appears as a radiopaque and homogeneous (Fig. 22-8).
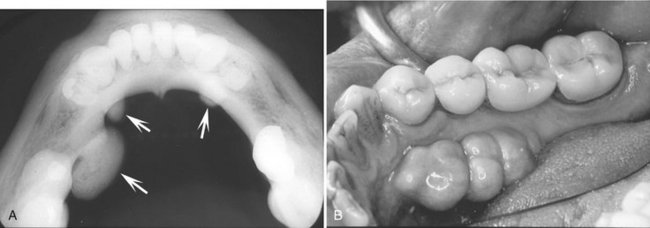
FIG. 22-8 A, An occlusal radiograph shows an unusual case of mandibular tori (arrows) where the number and size are not symmetric between the sides. B, Clinical picture of a different case; the tori extend from the region of the cuspid to the first molar. (B courtesy Dr. Bernard Friedland, Harvard University.)
OTHER EXOSTOSES
Definition
In addition to the torus, other exostoses may occur at other sites in the jaws. These are usually small regions of osseous hyperplasia of cortical bone and occasionally internal cancellous bone that usually occur on the surface of the alveolar process.
Clinical Features
Exostoses may develop most commonly on the buccal surface of the maxillary alveolar process, usually in the canine or molar area. They may also occur on the palatal surface or crest and less commonly on the mandibular alveolar process. Occasionally they will grow on the crest under a pontic of a fixed bridge. They are less common than mandibular or palatine tori, may attain a large size, and may be solitary or multiple. They are nodular, pedunculated, or flat prominences on the surface of the bone. They are covered with a normal mucosa and are bony hard on palpation. No published data indicate their actual incidence or whether they occur more often in one sex. As with the exostoses described previously, they appear to be more prevalent in Native Americans.
Radiographic Features
Location.: The maxillary alveolar process is the most common location and usually the image overlaps the roots of the adjacent teeth.
Periphery.: The periphery of an exostosis is usually well defined and smoothly contoured with a curved border (Fig. 22-9). However, some may have poorly defined borders that blend radiographically into the surrounding normal bone.
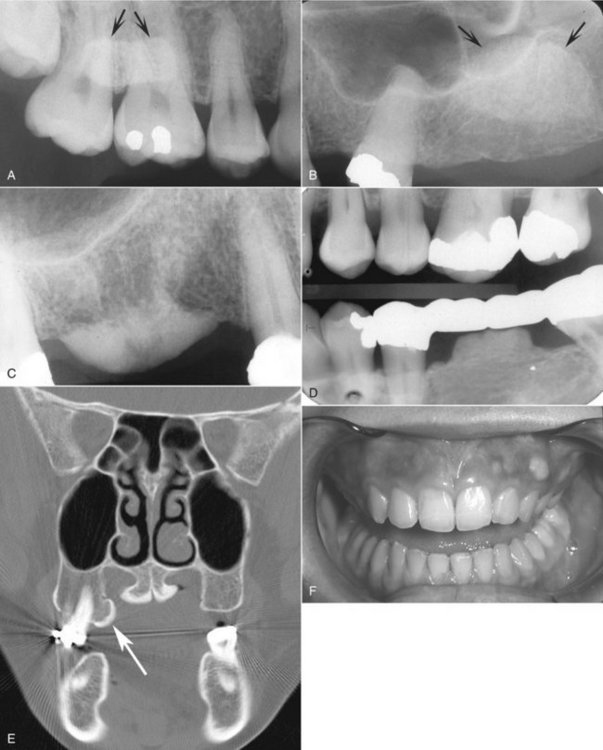
FIG. 22-9 A, A periapical film of a region of hyperostosis on the buccal aspect of the maxillary alveolar process, seen as an region of slight increase in radiopacity overlapping the roots of the molars (arrows). B, Another example overlapping an edentulous ridge. C, An example occurring on the crest of the alveolar ridge. D, An example occurring under a bridge pontic. E, A coronal CT image of hyperostosis located on the palatal aspect of the right maxillary alveolar process; note the presence of a maxillary torus. F, A clinical photograph of a small hyperostosis occurring on the labial surface of the maxillary alveolar ridge.
Definition
Dense bone islands (DBIs) are the internal counterparts of exostoses. They are localized growths of compact bone that develop within the cancellous bone.
Radiographic Features
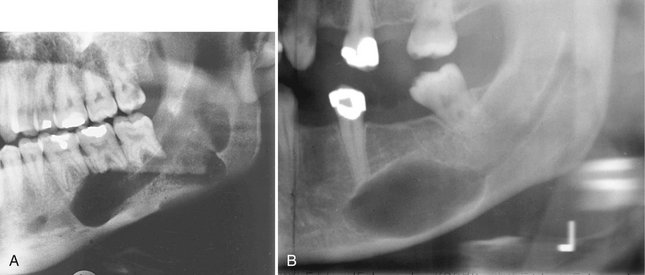
Location.: DBIs are more common in the mandible than in the maxilla. They occur most often in the premolar-molar area (Fig. 22-10), although their existence does not correlate with the presence or absence of teeth.
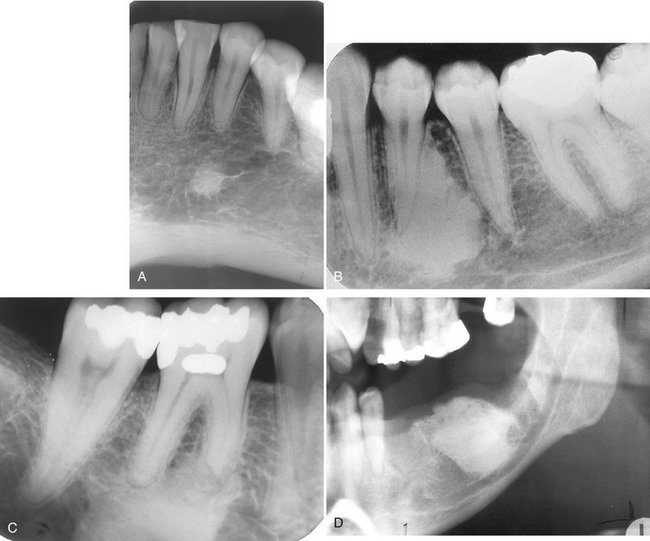
FIG. 22-10 A, A small dense bone island apical to the first bicuspid; note the lack of a soft tissue capsule and that some of the surrounding trabeculae appear to merge into the radiopaque mass. B, A larger dense bone island between the bicuspids; note the normal-appearing periodontal membrane space. C, Another example apical to the first molar causing external root resorption of the mesial root. D, A large dense bone island occupying the body of the left mandible.
Periphery.: The periphery is usually well defined but occasionally blends with the trabeculae of the surrounding bone. There is no trace of a radiolucent margin or capsule; the radiopaque dense bone island abuts directly against normal bone.
Internal Structure.: The internal aspect of DBIs usually is uniformly radiopaque without any characteristic pattern, but sometimes, depending on its form and thickness, there may be patches of more radiolucent areas.
Effects on Surrounding Structures.: In rare instances a DBI is located periapical to a tooth root and is associated with external root resorption (see Fig. 22-10, C). The tooth most often involved is the mandibular first molar. In all circumstances the tooth is vital and the root resorption appears to be self-limiting. In very rare cases DBIs can inhibit the eruption of a tooth and even displace a tooth.
Differential Diagnosis
Several radiopaque lesions must be considered in forming a differential diagnosis. Periapical cemental dysplasia can be differentiated by the presence of its radiolucent periphery. When a DBI is located at the root apex, it may resemble periapical sclerosing osteitis. However, in periapical osteitis there is an associated widening of the periapical portion of the periodontal membrane space. Also, periapical osteitis should be centered on the root apex of the tooth and extend in a more symmetric form in every direction. Finally, an inflammatory lesion may have an apparent etiology such as a large restoration or carious lesion. There may be some similarity to hypercementosis or a benign cementoblastoma, but in both cases there should be a soft tissue (radiolucent) capsule at the periphery. DBIs are often static but may increase in size, especially when there is active growth of the jaws. If several DBIs (five or more) are present, multiple polyposis syndromes (e.g., Gardner’s syndrome) should be considered.
Benign Tumors
The benign neoplasias are separated into two major groups, odontogenic tumors and nonodontogenic tumors.
ODONTOGENIC TUMORS
Odontogenic tumors arise from the tissues of the odontogenic apparatus. According to the World Health Organization (WHO), these tumors can be classified into three categories depending on the type of tissue that comprises each tumor. The categories are tumors of odontogenic epithelium, mixed tumors of both odontogenic epithelium and odontogenic ectomesenchyme (connective tissue), and tumors composed of primarily ectomesenchyme. Odontogenic tumors comprise 1.3% to 15% of all oral tumors. The following text presents benign jaw tumors according to their tissues of origin. This format should assist the reader in learning to correlate the radiographic appearance of tumors with the underlying pathologic basis of the disease process.
ODONTOGENIC EPITHELIAL TUMORS
Definition
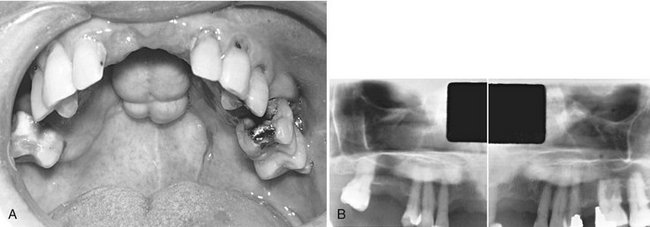
The ameloblastoma, a true neoplasm of odontogenic epithelium, is a persistent and locally invasive tumor; it has aggressive but benign growth characteristics. The ameloblastoma represents about 1% of all oral odontogenic epithelial tumors and 11% of all odontogenic tumors. It is an aggressive neoplasm that arises from remnants of the dental lamina and dental organ (odontogenic epithelium). Malignant forms of this neoplasm do exist and are discussed in Chapter 23. Ameloblastomas may be divided into the solid/multicystic type, the unicystic type, and the desmoplastic type. The unicystic variant may develop as a single entity or may form from the epithelial lining of a dentigerous cyst, called a mural (within the wall) ameloblastoma. The existence of peripheral (soft tissue location) forms of this neoplasm is well documented.
Clinical Features.: There is a slight predilection for this lesion to occur in men, and it develops more often in blacks. Although it may be found in the young (age 3 years) and in individuals older than 80 years, most patients are between 20 and 50 years, with the average age at discovery about 40 years.
Ameloblastomas grow slowly, and few, if any, symptoms occur in the early stages. The tumor is frequently discovered during a routine dental examination. Usually the patient eventually notices gradually increasing facial asymmetry. Swelling of the cheek, gingiva, or hard palate has been reported as the chief complaint in 95% of untreated maxillary ameloblastomas. The mucosa over the mass is normal, but teeth in the involved region may be displaced and become mobile. In most cases patients with ameloblastomas do not have pain, paresthesia, fistula, ulcer formation, or tooth mobility. As the tumor enlarges, palpation may elicit a bony hard sensation or crepitus as the bone thins. If the lesion destroys overlying bone, the swelling may feel firm or fluctuant. As it grows, this tumor can cause bony expansion and sometimes erosion through the adjacent cortical plate with subsequent invasion of the adjacent soft tissues.
An untreated tumor may grow to great size and is more of a concern in the maxilla, where it can extend into vital structures and reach into the cranial base. Tumors that develop in the maxilla may extend into the paranasal sinuses, orbit, nasopharynx, or vital structures at the base of the skull. Recurrence rates are higher in older patients and in those with multilocular lesions. As seen with other jaw tumors, local recurrence, whether detected radiographically or histologically, may have a more aggressive character than the original tumor.
Radiographic Features
Location.: Most ameloblastomas (80%) develop in the molar-ramus region of the mandible, but they may extend to the symphyseal area. Most lesions that occur in the maxilla are in the third molar area and extend into the maxillary sinus and nasal floor. In either jaw this tumor can originate in an occlusal position to a developing tooth (Fig. 22-11).
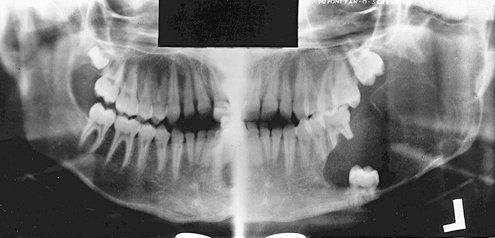
FIG. 22-11 A unicystic ameloblastoma developing occlusal to the left second mandibular molar causing expansion of the mandibular body and ramus to the sigmoid notch and condylar neck and inferior displacement of the mandibular second molar and root resorption of the alveolar left first molar. (Courtesy E. J. Burkes, DDS, Chapel Hill, N.C.)
Periphery.: The ameloblastoma is usually well defined and frequently delineated by a cortical border. The border is often curved, and in small lesions the border and shape may be indistinguishable from a cyst (Fig. 22-12). The periphery of lesions in the maxilla is usually more ill defined.
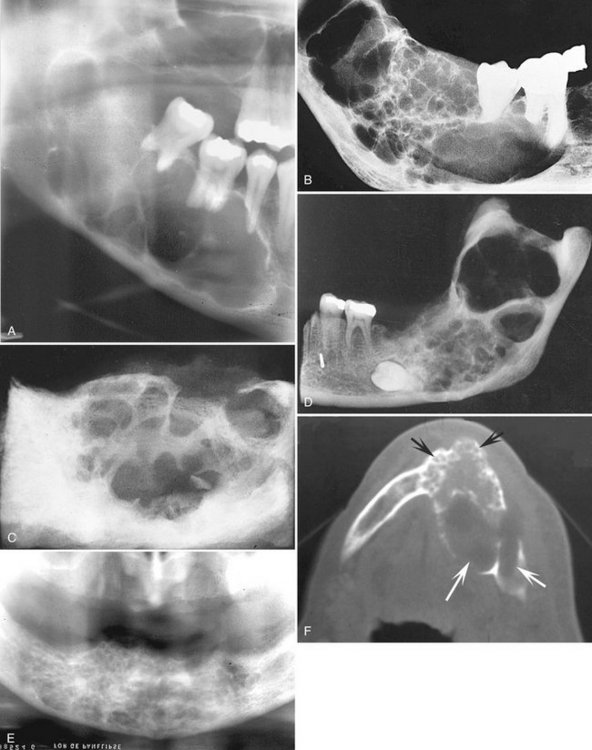
FIG. 22-12 Multilocular Ameloblastomas.
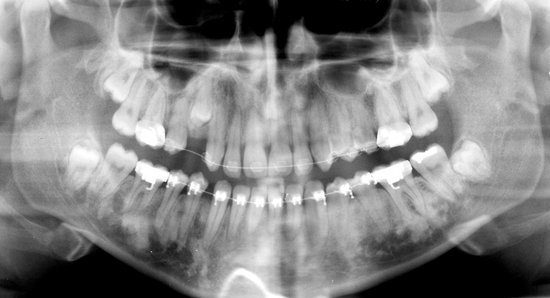
A, A large lesion in the mandibular body and ramus shows only a few rather straight septa. B, Lateral radiograph of a resected mandibular specimen containing a multilocular ameloblastoma; note the coarse curved septa. C, Another surgical specimen of an ameloblastoma. D, A large multilocular lesion in the right mandibular ramus. E, A cropped panoramic image showing small loculations that are more common in the anterior mandible. F, An axial CT image with bone algorithm showing a large ameloblastoma; note the smaller loculations in the anterior mandible (black arrows) and the larger loculations in the posterior mandible (white arrows).
Internal Structure.: The internal structure varies from totally radiolucent (see Fig. 22-11) to mixed with the presence of bony septa creating internal compartments. These septa can be straight but are more commonly coarse and curved and originate from normal bone that has been trapped within the tumor. Because this tumor frequently has internal cystic components, these septa are often remodeled into curved shapes providing a honeycomb (numerous small compartments or loculations) or soap bubble (larger compartments of variable size) patterns (see Fig. 22-12). Generally the loculations are larger in the posterior mandible and smaller in the anterior mandible. In the desmoplastic variety the internal structure can be composed of very irregular sclerotic bone resembling a bone dysplasia or bone-forming tumor (Fig. 22-13).
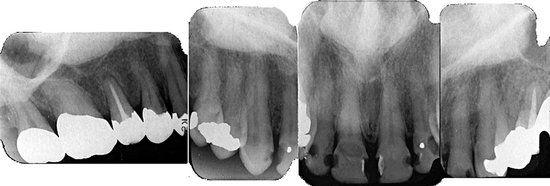
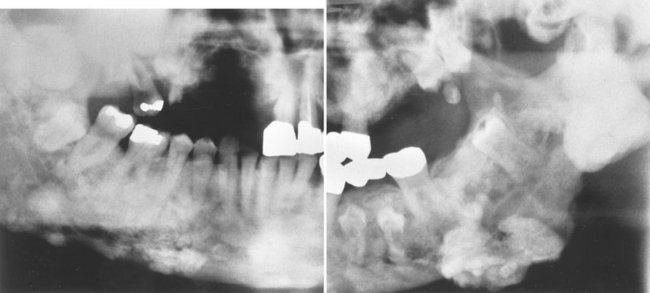
Effects on Surrounding Structures.: There is a pronounced tendency for ameloblastomas to cause extensive root resorption (Fig. 22-14). Tooth displacement is common. Because a common point of origin is occlusal to a tooth, some teeth may be displaced apically. An occlusal radiograph may demonstrate cystlike expansion and thinning of an adjacent cortical plate leaving a thin “eggshell” of bone (Fig. 22-15). Computed tomographic (CT) images often reveal regions of perforation of the expanded cortical plate as a result of the inability of the production of periosteal new bone to keep up with the rate of growth of the expanding ameloblastoma (Fig. 22-16). Unicystic types of ameloblastoma may cause extreme expansion of the mandibular ramus, and often the anterior border of the ramus is no longer visible in the panoramic image (Fig. 22-17).
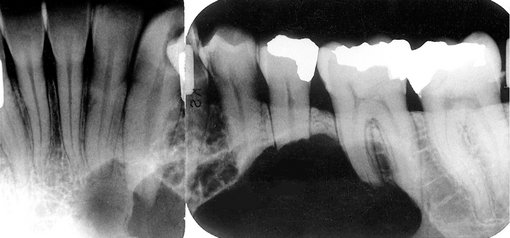
FIG. 22-14 Root resorption of the premolars and canine adjacent to a radiolucent ameloblastoma in the left mandible.
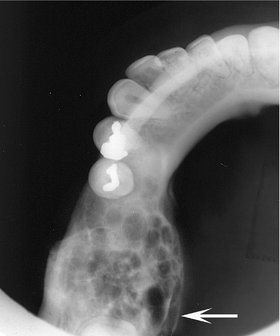
FIG. 22-15 An occlusal film demonstrating expansion of the lingual cortex with maintenance of a thin outer shell of bone (arrow).
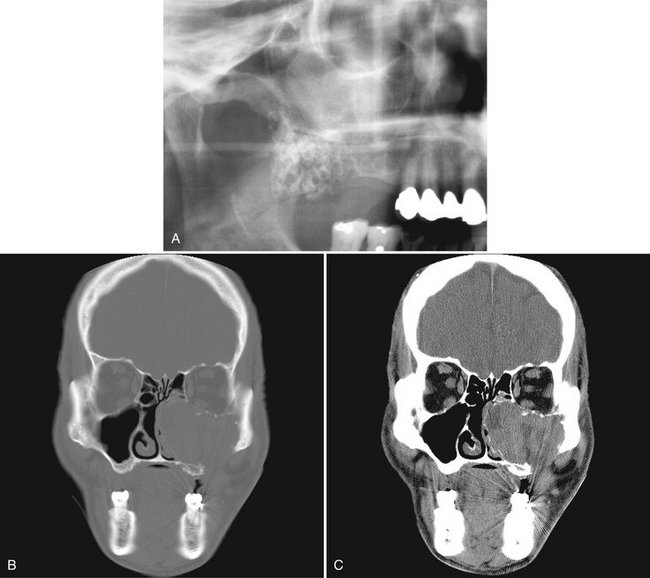
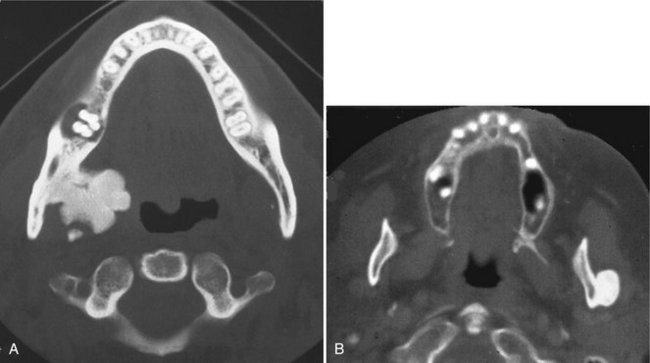
FIG. 22-16 A cropped panoramic image of an ameloblastoma involving the left maxilla; note the multilocular appearance in the tuberosity region. It is not possible to determine the extent of the lesion with the panoramic film. B and C, The same coronal CT image slices with both bone and soft tissue algorithms of the same case; note the aggressive nature of the tumor as it has grown into the sinus and nasal fossa and perforated the lateral cortical plate of the maxilla.
RECURRENT AMELOBLASTOMA
Ameloblastomas may recur when the initial surgical procedure inadequately removes the entire tumor. Recurrent tumor has a characteristic appearance of multiple small cystlike structures with very coarse sclerotic cortical margins (Fig. 22-18) sometimes separated by normal bone.
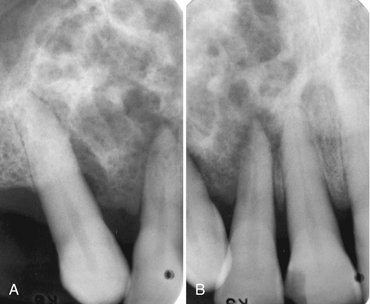
FIG. 22-18 Periapical films of a recurrent ameloblastoma of the right maxilla. Note the sclerotic margins of the small cystic lesions.
Additional Imaging
If a preliminary diagnosis of ameloblastoma is made, then CT imaging is highly recommended. CT imaging cannot only confirm the diagnosis but also will accurately demonstrate the anatomic extent of the tumor (see Fig. 22-16). Of importance is the ability of CT imaging to detect perforation of the outer cortex and invasion into the surrounding soft tissues. If soft tissue invasion is extensive, magnetic resonance imaging (MRI) will provide superior images of the nature and extent of the invasion. CT examination is essential in the postsurgical follow-up assessment of ameloblastoma.
Differential Diagnosis
Small unilocular ameloblastomas that are located around the crown of an unerupted tooth often cannot be differentiated from a dentigerous cyst. Because the appearance of the internal bony septa is important for the identification of ameloblastoma, other types of lesions that also have internal septa such as the odontogenic keratocyst, giant cell granuloma, odontogenic myxoma, and ossifying fibroma may have a similar appearance. The odontogenic keratocyst may contain curved septa, but usually the keratocyst tends to grow along the bone without marked expansion, which is characteristic of ameloblastomas. Giant cell granulomas occur in a younger age group and have more granular or wispy ill-defined septa. Odontogenic myxomas may have similar-appearing septa; however, there are usually one or two thin sharp, straight septa, which is characteristic of the myxoma. Even the presence of one such septum may indicate a myxoma. Also, myxomas are not as expansile as ameloblastomas and tend to grow along the bone. The septa in ossifying fibroma are usually wide, granular, and ill defined and often there are small irregular trabeculae.
Treatment
The most common treatment is surgical resection. The surgical procedure should take into account the tendency of the neoplasm to invade adjacent bone beyond its apparent radiographic margins. CT and MRI are useful in determining the exact extent of the tumor. If the ameloblastoma is relatively small, it may be removed completely by an intraoral approach, but larger lesions may require resection of the jaw. The maxilla is usually treated more aggressively because of the tendency of ameloblastoma to invade adjacent vital structures. Radiation therapy may be used for inoperable tumors, especially those in the posterior maxilla.
Definition
Calcifying epithelial odontogenic tumors (CEOTs) are rare neoplasms. They account for about 1% of odontogenic tumors. These tumors usually are located within bone and produce a mineralized substance within amyloid-like material. CEOTs have a distinctive microscopic appearance with epithelium that resembles the stratum intermedium of the enamel organ.
Clinical Features
A CEOT is less aggressive than the ameloblastoma and is found in about the same age group. Rarely this tumor may have an extraosseous location. The neoplasm is somewhat more common in men, and patients range in age from 8 to 92 years, with an average age of about 42 years (considerably younger in men and somewhat older in women). Jaw expansion is a regular feature and usually the only symptom. Palpation of the swelling reveals a hard tumor.
Radiographic Features
Location.: As with ameloblastomas, CEOTs have a definite predilection for the mandible, with a ratio of at least 2 : 1, and most develop in the premolar-molar area, with a 52% association with an unerupted or impacted tooth. In about half of cases, radiographs taken early in the development of these tumors reveal a radiolucent area around the crown of a mature, unerupted tooth.
Periphery.: The border may have a well defined cystlike cortex. In some tumors the boundary may be irregular and ill defined.
Internal Structure.: The internal aspect may appear unilocular or multilocular with numerous scattered, radiopaque foci of varying size and density. The most characteristic and diagnostic finding is the appearance of radiopacities close to the crown of the embedded tooth (Fig. 22-19). In addition, small, thin, opaque trabeculae may cross the radiolucency in many directions.
Differential Diagnosis
Lesions with completely radiolucent internal structures may mimic dentigerous cysts or even ameloblastomas. Other lesions with radiopaque foci, including adenomatoid odontogenic tumor, ameloblastic fibro-odontoma, and calcifying odontogenic cyst may have similar appearances. However, the prominent location of the CEOT and the age of the patient will help in the differential diagnosis.
MIXED TUMORS (OF ODONTOGENIC EPITHELIUM AND ODONTOGENIC ECTOMESENCHYME)
Synonyms
Compound odontoma, compound composite odontoma, complex odontoma, complex composite odontoma, odontogenic hamartoma, calcified mixed odontoma, and cystic odontoma
Definition
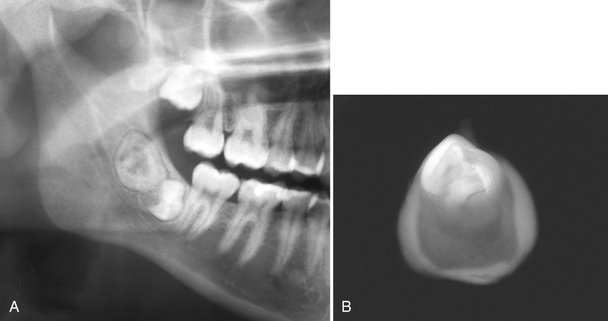
The term odontoma is used to identify a tumor that is radiographically and histologically characterized by the production of mature enamel, dentin, cementum, and pulp tissue. These components are seen in various states of histodifferentiation and morphodifferentiation. Because of its limited and slow growth and well-differentiated tooth tissue, this lesion is considered to be a hamartoma and not a true tumor.
The structural relationship of the component tissues may vary from nondescript masses of dental tissue referred to as a complex odontoma to multiple well-formed teeth (denticles) of a compound odontoma. A dilated odontoma has been described as another type of odontoma; however, this is a single structure that actually may be the most severe expression of a dens in dente.
Clinical Features
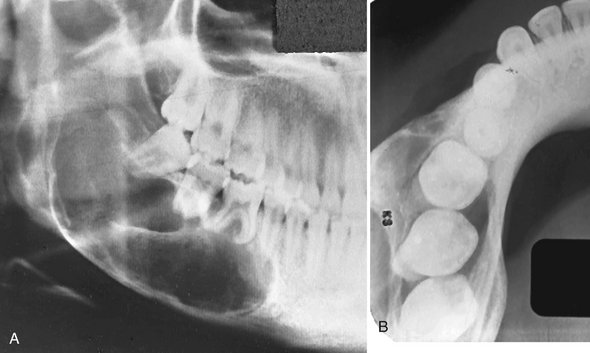
Odontomas are the most common odontogenic tumor. They often interfere with the eruption of permanent teeth (Fig. 22-20). The lesion shows no sex predilection, and most begin forming while the normal dentition is developing. Odontomas develop and mature while the corresponding teeth are forming and cease development when the associated teeth complete development. Most odontomas occur in the second decade of life and many times are found during investigation of delayed eruption of adjacent teeth or retained primary teeth. In rare cases odontomas are associated with primary teeth. They persist if left untreated, although they do not continue to increase in size and so may be detected later in life. Compound odontomas are about twice as common as the complex type. Although the compound variety forms equally between men and women, 60% of complex odontomas occur in women. In rare circumstances a compound odontoma may erupt into the oral cavity of a child.
Radiographic Features
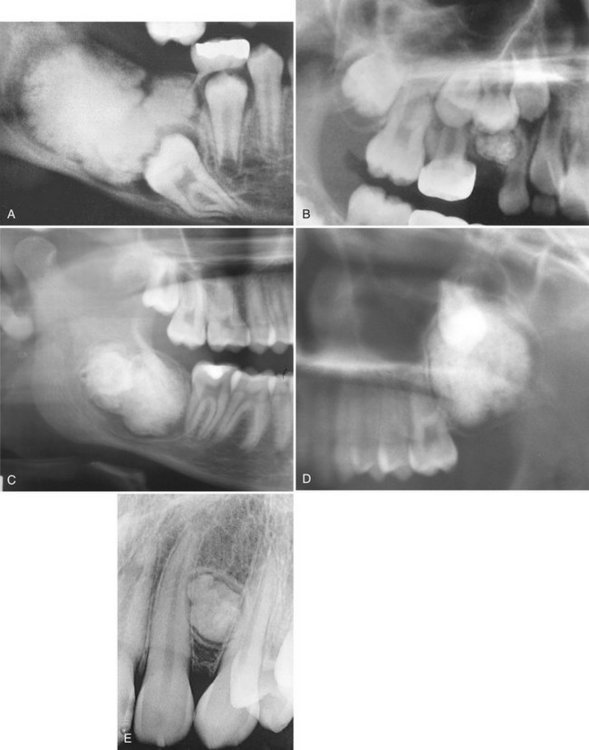
Location.: More of the compound type (62%) occur in the anterior maxilla in association with the crown of an unerupted canine. In contrast, 70% of complex odontomas are found in the mandibular first and second molar area.
Periphery.: The borders of odontomas are well defined and may be smooth or irregular. These lesions have a cortical border, and immediately inside and adjacent to the cortical border is a soft tissue capsule.
Internal Structure.: The contents of these lesions are largely radiopaque. Compound odontomas have a number of toothlike structures or denticles that look like deformed teeth (Fig. 22-21). Complex odontomas contain an irregular mass of calcified tissue (see Fig. 22-20). The degree of radiopacity is equivalent to or exceeds that of adjacent tooth structure and may vary in the degree of radiopacity from one region to another, reflecting variations in amount and type of hard tissue that has been formed. A dilated odontoma has a single calcified structure with a more radiolucent central portion that has an overall form like a donut (Fig. 22-22).
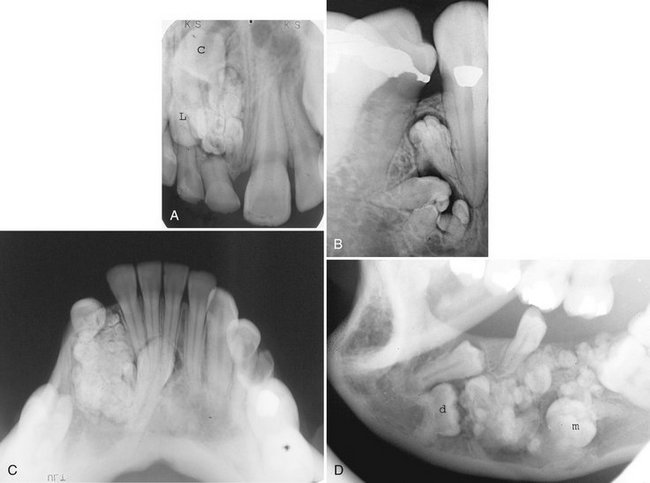
FIG. 22-21 Several examples of compound odontomas; note the numerous internal components and the radiolucent capsule. A, An example in the anterior maxilla that has interfered with the eruption of the central incisor (C) and the lateral incisor (L). B, Within the mandible. C, Within the anterior mandible interfering with the eruption of the cuspid. D, Within the mandible interfering with the eruption of the first premolar, deciduous molar (d), and the first molar (m).
Effects on Surrounding Structures.: Odontomas can interfere with the normal eruption of teeth. Most odontomas (70%) are associated with abnormalities such as impaction, malpositioning, diastema, aplasia, malformation, and devitalization of adjacent teeth. Large complex odontomas may cause expansion of the jaw with maintenance of the cortical boundary.
Differential Diagnosis
A toothlike appearance of the radiopaque structures within a well-defined lesion leads to easy recognition of a compound odontoma. Complex odontomas differ from cemento-ossifying fibromas by their tendency to associate with unerupted molar teeth and because they usually are more radiopaque than cemento-ossifying fibromas. Odontomas may also develop in much younger patients than do cemento-ossifying fibromas. Periapical cemental dysplasia may resemble complex odontomas but lesions are usually multiple and centered on the periapical region of teeth. However, if the cemental dysplastic lesion is solitary and located in an edentulous region of the jaws, the differential diagnosis may be more difficult. The periphery of cemental dysplasia usually has a wider uneven sclerotic border, whereas odontomas have a well-defined cortical border and usually the soft tissue capsule is more uniform and better defined with odontomas than in cemental dysplasia. Dense bone islands, although radiopaque, do not have a soft tissue capsule, as is seen with odontomas.
Treatment
Complex and compound odontomas are usually removed by simple excision. They do not recur and are not locally invasive.
Synonyms
Soft odontoma, soft mixed odontoma, mixed odontogenic tumor, fibroadamantoblastoma, and granular cell ameloblastic fibroma
Definition
Ameloblastic fibromas are benign mixed odontogenic tumors. They are characterized by neoplastic proliferation of epithelium resembling dental lamina and the primitive mesenchymal components resembling the dental papilla. Enamel, dentin, and cementum are not formed in this tumor.
Clinical Features
The behavior of ameloblastic fibromas is completely benign. Complete agreement has not been reached regarding sex predilection. Most of these tumors occur between 5 and 20 years of age, during the period of tooth formation, with an average age of about 15 years. They usually produce a painless, slow-growing expansion and displacement of the involved teeth (Fig. 22-23). Although the most common symptom is swelling or occlusal pain, the tumor may be discovered on a routine dental radiograph. It may be associated with a missing tooth.
Radiographic Features
Location.: Ameloblastic fibromas usually develop in the premolar-molar area of the mandible. In some cases the tumor may involve the ramus and extend forward to the premolar-molar area. A common location is near the crest of the alveolar process (Fig. 22-24) or in a follicular relationship with an unerupted tooth (located occlusal to the tooth), or it may arise in an area where a tooth failed to develop.
Periphery.: The borders of an ameloblastic fibroma are well defined and often corticated in a manner similar to that of a cyst.
Internal Structure.: An ameloblastic fibroma is more commonly unilocular (totally radiolucent) (Fig. 22-25) but may be multilocular with indistinct curved septa (see Fig. 22-23).
Differential Diagnosis
A common difficulty will occur in differentiating a small tumor with a follicular relationship to an unerupted tooth from a small dentigerous cyst or a hyperplastic follicle. In fact, the radiologic features may not allow differentiation among these three entities. This tumor may have similar features to an ameloblastoma; however, the ameloblastic fibroma occurs at an earlier age and the septa in an ameloblastoma are more defined and coarse. The septa in ameloblastic fibroma are infrequent and often very fine. Giant cell granulomas may appear multilocular, but these tumors usually have an epicenter anterior to the first molar in young patients and the septa are characteristically granular and ill defined. Odontogenic myxomas can appear multilocular, but usually a few sharp straight septa can be identified, which are not characteristic of ameloblastic fibromas and myxomas, and usually occur in an older age group.
Treatment
Ameloblastic fibromas are benign, and the rate of recurrence is low. A conservative surgical approach, including enucleation and mechanical curettage of the surrounding bone, is reported to be successful for these cases.
Definition
An ameloblastic fibro-odontoma is a mixed tumor with all the elements of an ameloblastic fibroma but with scattered collections of enamel and dentine. Some authorities consider the ameloblastic fibro-odontoma to be an early stage of a developing odontoma; however, there is compelling evidence that the ameloblastic fibro-odontoma is a separate entity that has a more neoplastic behavior than does the odontoma. On the other hand, there are probably some lesions that are incorrectly identified as an ameloblastic fibro-odontoma that are really developing odontomas.
Clinical Features
The clinical features are similar to those of odontomas, often associated with a missing tooth or a tooth that has failed to erupt. Occasionally, this tumor takes the position of a missing tooth. This tumor appears during the same age as odontomas and ameloblastic fibromas with no particular sex predilection.
Radiographic Features
Location.: Most cases occur in the posterior aspect of the mandible. The epicenter of the lesion is usually occlusal to a developing tooth or toward the alveolar crest.
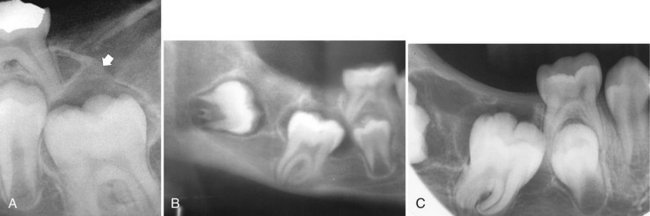
Internal Structure.: The internal structure is mixed, with the majority of the lesion being radiolucent. Small lesions may appear as enlarged follicles with only one or two small discrete radiopacities. Larger lesions may have a more extensive calcified internal structure (Fig. 22-26). In some cases these small calcifications have a round shape with a radiopaque enamel-like margin, giving a shape similar to that of a small doughnut. Most often an associated impacted tooth is present.
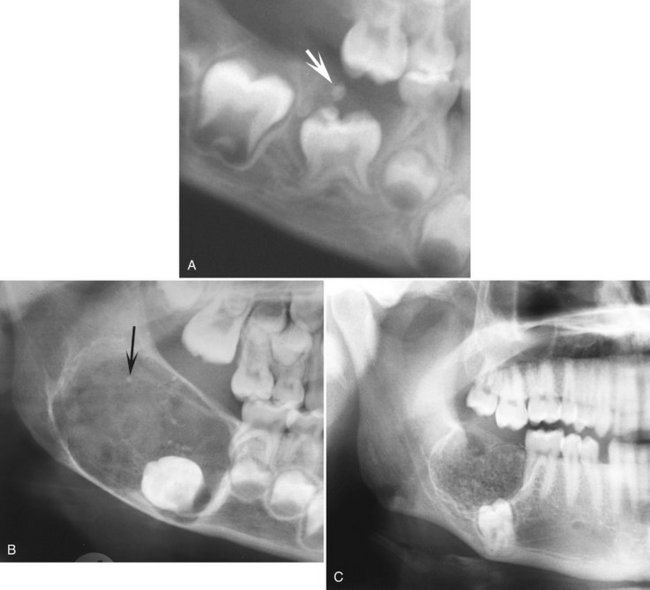
FIG. 22-26 Three examples of ameloblastic fibro-odontoma. A, A cropped panoramic film with a lesion occlusal to a second deciduous molar. The lesion is ill defined and radiolucent except for two small radiopacities (arrow). B, A cropped panoramic image of a well-defined radiolucent lesion with only a few scattered radiopacities. C, A cropped panoramic image of a lesion with a larger number of radiopacities.
Differential Diagnosis
If calcification is not detected, this tumor cannot be differentiated from an ameloblastic fibroma. Differentiation from a developing odontoma may be difficult, but generally these tumors have a greater soft tissue component (radiolucent) than does an odontoma. It may be argued that, given time, the amount of hard tissue will increase; however, the distribution of hard tissue is different. A complex odontoma, which shares a common location, usually has one mass of disorganized tissue in the center, whereas the ameloblastic fibro-odontoma will usually have multiple scattered mature small pieces of dental hard tissue. Although the compound odontoma has multiple denticles, the posterior mandible is a rare location and the organization of the tooth material in ameloblastic fibro-odontomes is never organized enough to resemble a tooth. Last, the ameloblastic fibro-odontomas do not occur early enough, compared with odontomas, to be considered a precursor.
Definition
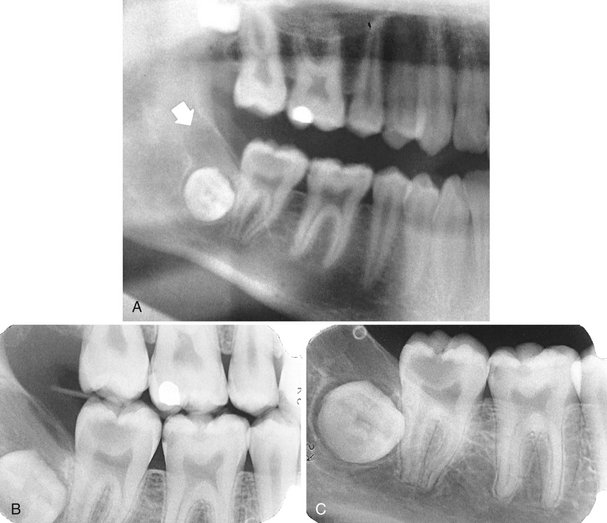
Adenomatoid odontogenic tumors are uncommon nonaggressive tumors of odontogenic epithelium in variety of patterns mixed with mature connective tissue stroma. The origin of adenomatoid odontogenic tumors may be from enamel organ epithelium, and it is classified as a mixed tumor because it contains connective tissue stroma and sometimes dentinoid material. Adenomatoid odontogenic tumors comprise 3% of all oral tumors. Both central and peripheral tumors occur. The central tumors are divided into the follicular type (those associated with the crown of an embedded tooth) and the extrafollicular type (those with no embedded tooth). Approximately 73% of central lesions are of the follicular type.
Clinical Features
Adenomatoid odontogenic tumors appear in the age range of 5 to 50 years; however, about 70% occur in the second decade, with an average age of 16 years. The tumor has a 2 : 1 female predilection. The follicular type is diagnosed somewhat earlier than the extrafollicular type, probably because the failure of the associated tooth to erupt is noted. The tumor is slow growing and presents as a gradually enlarging, painless swelling or asymmetry, often associated with a missing tooth.
Radiographic Features
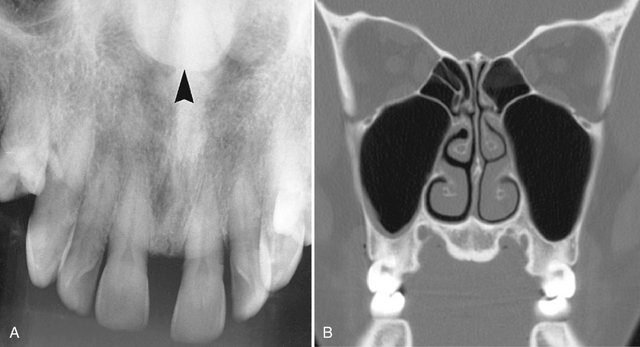
Location.: At least 75% of adenomatoid odontogenic tumors occur in the maxilla (Fig. 22-27). The incisor- canine-premolar region, especially the cuspid region, is the usual area involved in both jaws. It occurs more commonly in the maxilla. This tumor may have a follicular relationship with an impacted tooth; however, often it does not attach at the cementoenamel junction but surrounds a greater part of the tooth, most often a canine (Fig. 22-28).
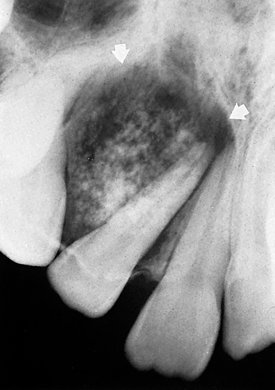
FIG. 22-27 An adenomatoid odontogenic tumor in the region of the right maxillary canine and lateral incisor. Calcification is present within the tumor mass, and the canine and lateral incisor have been displaced by the lesion. (Courtesy R. Howell, DDS, Morgantown, W.V.)
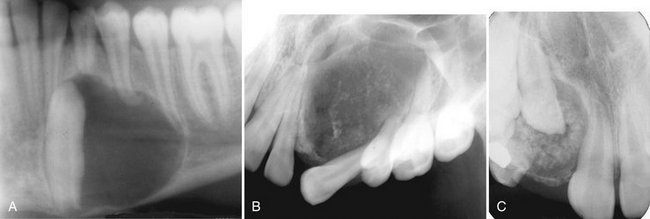
FIG. 22-28 Examples of adenomatoid odontogenic tumor with various amount of internal calcification. A, A cropped panoramic film with a totally radiolucent lesion associated with a mandibular cuspid. B, A lesion with sparse pebblelike calcifications associated with a maxillary cuspid. C, A lesion related to a maxillary lateral incisor with abundant calcification.
Internal Structure.: Radiographically, radiopacities develop in about two thirds of cases. One tumor may be completely radiolucent, another may contain faint radiopaque foci (Fig. 22-28), and some may show dense clusters of ill-defined radiopacities; occasionally the calcifications are small with well-defined borders, like a cluster of small pebbles (see Fig. 22-28, B). Intraoral radiographs may be required to demonstrate the calcifications within the lesion, which may not be seen on panoramic radiographs. Microscopic studies have verified that the size, number, and density of small radiopacities in the central radiolucency of the lesion vary from tumor to tumor and seem to increase with age.
Differential Diagnosis
When this tumor is completely radiolucent and has a follicular relationship with an impacted tooth, differentiation from a follicular cyst or a pericoronal odontogenic keratocyst may be difficult. If the attachment of the radiolucent lesion is more apical than the cementoenamel junction, a follicular cyst can be discounted. However, this would not exclude an odontogenic keratocyst. If there is a calcified product (radiopacities) in this tumor, then other lesions with calcifications might be entertained in the differential diagnosis. The maxillary and mandibular anterior regions are also common sites for calcifying odontogenic cysts. It may not be possible to differentiate the extrafollicular type of adenomatoid odontogenic tumor from the calcifying odontogenic cyst. The ameloblastic fibro-odontoma and the CEOT occur more commonly in the posterior mandible.
Treatment
Conservative surgical excision is adequate because the tumor is not locally invasive, is well encapsulated, and is separated easily from the bone. The theory that adenomatoid odontogenic tumors are hamartomas is supported by the innocuous behavior of the lesion because, as with odontomas, adenomatoid odontogenic tumors stop developing about the time tooth structures complete their growth. The recurrence rate is 0.2%.
MESENCHYMAL TUMORS (ODONTOGENIC ECTOMESENCHYME)
Definition
Odontogenic myxomas are uncommon, accounting for only 3% to 6% of odontogenic tumors. They are benign, intraosseous neoplasms that arise from odontogenic ectomesenchyme and resemble the mesenchymal portion of the dental papilla. These myxomas are not encapsulated and tend to infiltrate the surrounding cancellous bone but do not metastasize. They have a loose, gelatinous consistency and show microscopic characteristics similar to those of soft tissue myxomas of the extremities. Odontogenic myxomas develop only in the bones of the facial skeleton. The theory that this lesion develops from odontogenic rather than nonodontogenic ectomesenchyme is supported by the fact that it appears only in the jaws, it affects young people, it occasionally is related to a tooth that failed to erupt or is missing, and in some cases odontogenic epithelium can be detected microscopically.
Clinical Features
If odontogenic myxomas have a sex predilection, they slightly favor females. Although the lesion can occur at any age, more than half arise in individuals between 10 and 30 years old; it rarely occurs before age 10 years or after age 50 years. It grows slowly and may or may not cause pain. Eventually it causes swelling and may grow quite large if left untreated. It may also invade the maxillary sinus. Recurrence rates as high as 25% have been reported. This high rate may be explained by the lack of encapsulation of the tumor, its poorly defined boundaries, and the extension of nests or pockets of myxoid (jellylike) tumor into trabecular spaces, where they are difficult to detect and remove surgically.
Radiographic Features
Location.: Myxomas more commonly affect the mandible by a margin of 3 : 1. In the mandible these tumors occur in the premolar and molar areas and only rarely in the ramus and condyle (non–tooth-bearing areas). Myxomas in the maxilla usually involve the alveolar process in the premolar and molar regions and the zygomatic process.
Periphery.: The lesion usually is well defined, and it may have a corticated margin but most often is poorly defined, especially in the maxilla.
Internal Structure.: When it occurs pericoronally with an impacted tooth, an odontogenic myxoma may have a cystlike unilocular outline, although the majority has a mixed radiolucent-radiopaque internal pattern. Residual bone trapped within the tumor will remodel into curved and straight, coarse or fine septa. The presence of these septa gives the tumor a multilocular appearance. A characteristic septa identified with this tumor is a straight, thin-etched septa (Fig. 22-29). These have been described as making a tennis racket–like or stepladder-like pattern, but this pattern is rarely seen. In reality, the majority of the septa are curved and coarse but the finding of one or two of these straight septa will help in the identification of this tumor (Fig. 22-30).
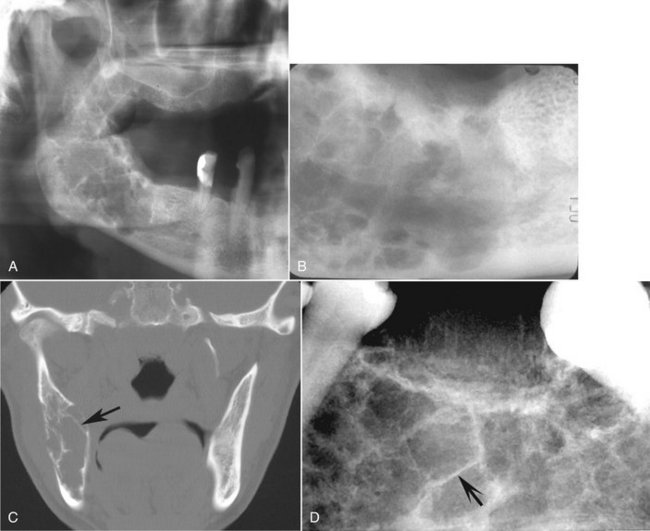
FIG. 22-29 An odontogenic myxoma. A, A panoramic film of a large myxoma in the body and ramus of the right mandible. B, A periapical view. C, A coronal CT image of the same case; note the presence of a few straight septa, especially visible in the CT image (arrow). The CT image also shows some modest expansion considering the overall size of the tumor. D, A periapical view of a different lesion; note the one straight sharp septa (arrow).
Effects on Surrounding Structures.: When growing in a tooth-bearing area, the tumor displaces and loosens teeth but rarely causes resorption of teeth. The lesion also frequently scallops between the roots of adjacent teeth, similar to a simple bone cyst. This tumor has a tendency to grow along the involved bone without the same amount of expansion seen with other benign tumors; however, when a large size is achieved, there may be considerable expansion.
Additional Imaging.: CT and in particular MRI can help in establishing the intraosseous extent of the tumor and thus guide the surgeon in planning the resection margins. The high tissue signal characteristic of this tumor in T2-weighted magnetic resonance images is particularly useful in establishing tumor extent and the presence of a recurrent tumor (Fig. 22-31).
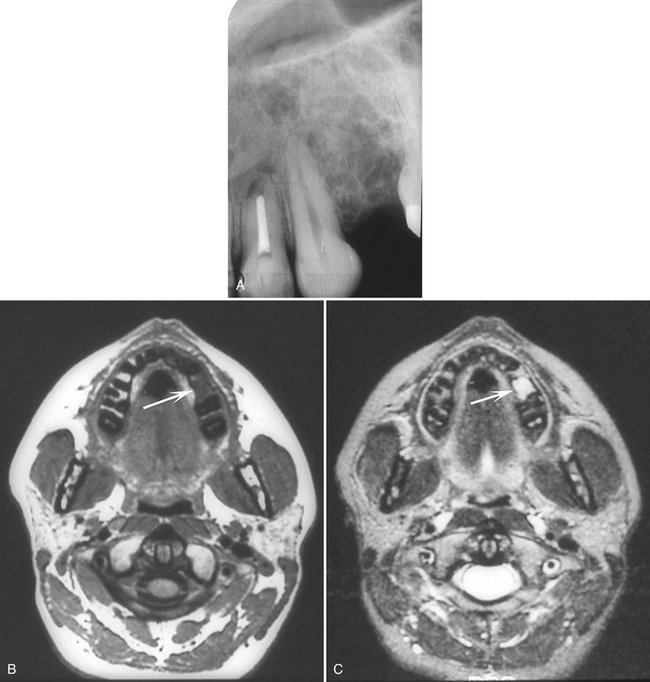
FIG. 22-31 A, Periapical film taken to investigate a possible recurrence of an odontogenic myxoma in the alveolar process between the cuspid and the first molar after treatment by surgical curettage. B, Magnetic resonance axial image with T1 weighting showing a low signal (black) from the segment of the alveolar process between the cuspid and molar. C, Magnetic resonance axial image of the same image slice as B but with T2 weighting resulting in a high signal (white) from the same alveolar segment, which is characteristic of an odontogenic myxoma and confirming the presence of a recurrence.
Differential Diagnosis
Because odontogenic myxomas most often have a multilocular internal pattern, the differential diagnosis should include other multilocular lesions such as ameloblastomas, central giant cell granulomas, and central hemangiomas. The finding of characteristic thin straight septa with less-than-expected bone expansion is very useful in the differential diagnosis. On occasion, a small area of expansion with straight septa may be projected over an intact outer bony cortex and give a spiculated appearance seen in osteogenic sarcomas (see Fig. 22-29, D). Careful inspection of this area of expansion will reveal a thin but intact outer cortex that would not be seen in osteogenic sarcoma. On occasion, the odontogenic fibroma will have the same radiographic characteristics as, and cannot be reliably differentiated from, the myxoma.
Treatment
Odontogenic myxomas are treated by resection with a generous amount of surrounding bone to ensure removal of myxomatous tumor that infiltrates the adjacent marrow spaces. With appropriate treatment, the prognosis is good.
Definition
Benign cementoblastomas are slow-growing mesenchymal neoplasms composed principally of cementum-like tissue. The tumor manifests as a bulbous growth around and attached to the apex of a tooth root. Its histologic characteristics are similar to those of osteoblastomas, and some authors consider cementoblastomas to be osteoblastomas. Putative cementoblasts that compose this tumor produce cementum-like material and abnormal bone. The tumor most often develops with permanent teeth but in rare cases occurs with primary teeth.
Clinical Features
Although statistical data suggest that benign cementoblastomas are uncommon, many believe that they occur more often than published accounts indicate. The lesion is more common in males than in females, and the ages of reported patients range from 12 to 65 years, although most patients are relatively young. There is no racial predilection. The tumor usually is a solitary lesion that is slow growing but that may eventually displace teeth. The involved tooth is vital and often painful. The pain seems to vary from patient to patient and can be relieved by anti-inflammatory drugs.
Radiographic Features
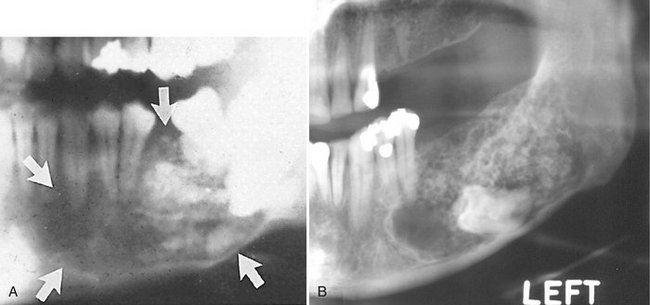
Location.: Benign cementoblastomas occur more often in the mandible (78%) and form most commonly on a premolar or first molar (90%).
Periphery.: The lesion is a well-defined radiopacity with a cortical border and then a well-defined radiolucent band just inside the cortical border.
Internal Structure.: Benign cementoblastomas are mixed radiolucent-radiopaque lesions where the majority of the internal structure is radiopaque. The resulting pattern may be amorphous or may have a wheel spoke pattern (Fig. 22-32). The density of the cemental mass usually obscures the outline of the enveloped root. This central radiopaque mass as mentioned is surrounded by a radiolucent band, indicating that the tumor is maturing from the central aspect to the periphery.
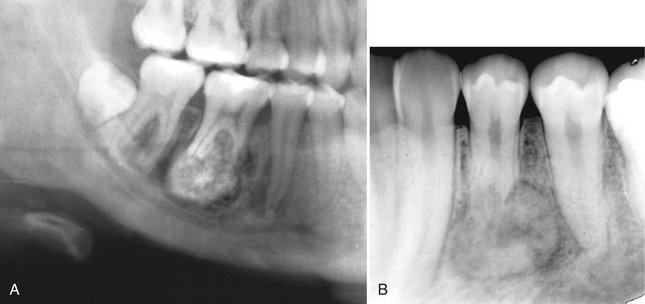
FIG. 22-32 Cementoblastoma.
A, A portion of a panoramic radiograph showing a large, bulbous, radiopaque mass attached to the roots of the mandibular right first molar. A radiolucent band can be seen surrounding the mass, and root resorption of the molar roots has occurred. B, A periapical radiograph of a lesion associated with a bicuspid. (Courtesy B. Pynn, Canada.)
Differential Diagnosis
The most common lesion to simulate this appearance is a solitary lesion of periapical cemental dysplasia. The differential diagnosis may be difficult in some cases and the presence or absence of symptoms or observation of the lesion over a period of time may be required. In general, the radiolucent band around the benign cementoblastoma is usually better defined and uniform than with cemental dysplasia. Also, the pattern of growth of the cementoblastoma results in a more uniform circular shape than the more irregular undulating outline of cemental dysplasia. Other lesions that may be included in the differential diagnosis would be periapical sclerosing osteitis, dense bone island, and hypercementosis. However, periapical sclerosing osteitis and dense bone islands do not have a soft tissue capsule, as does the benign cementoblastoma. Hypercementosis should be surrounded by a periodontal membrane space, which is usually thinner than the soft tissue capsule of the benign cementoblastoma, and there is no root resorption or jaw expansion with hypercementosis.
Definition
Central odontogenic fibromas are rare neoplasms that sometimes are divided into two types according to histologic appearance: the simple type contains mature fibrous tissue with sparsely scattered odontogenic epithelial rests and the WHO type, which is more cellular, has more epithelial rests and may contain calcifications that resemble dysplastic dentin, cementum, or osteoid. One theory is that these types merely represent a spectrum and that odontogenic myxoma may be a part of this range.
Clinical Features
Most cases of central odontogenic fibromas occur between the ages of 11 and 39 years. The neoplasm shows a definite female preponderance, with a reported ratio of 2.2 : 1. Affected patients may be asymptomatic or may have swelling and mobility of the teeth.
Radiographic Features
Location.: Central odontogenic fibromas occur slightly more often in the mandible. The prevalent site in the mandible is the molar-premolar region and in the maxilla anterior to the first molar.
Internal Structure.: Smaller lesions usually are unilocular, and larger lesions have a multilocular pattern. The internal septa may be fine and straight, as in odontogenic myxomas, or it may be granular, resembling those seen in giant cell granulomas. Some lesions are totally radiolucent, whereas unorganized internal calcification has been reported in others.
Differential Diagnosis
The histologic features may resemble those of a central (originating in bone) desmoplastic fibroma if no epithelial rests are apparent. Desmoplastic fibromas are more aggressive and tend to break through the peripheral cortex and invade surrounding soft tissue. The septa in desmoplastic fibroma will be very thick, straight, and angular. If thin, straight septa are present in the odontogenic fibroma, it may not be possible to differentiate this neoplasm from an odontogenic myxoma on radiographic criteria alone. If granular septa are present, the radiographic appearance may be identical to that of a giant cell granuloma.
Nonodontogenic Tumors
BENIGN TUMORS OF NEURAL ORIGIN
Definition
A central neurilemmoma is a tumor of neuroectodermal origin, arising from the Schwann cells that make up the inner layer covering the peripheral nerves. Although rare, it is the most common intraosseous nerve tumor. This tumor has practically no potential for malignant transformation.
Clinical Features
Neurilemmomas grow slowly, can occur at any age (but most commonly arise in the second and third decades), and occur with equal frequency in both males and females. The mandible and sacrum are the most common sites. These lesions cause few symptoms other than those related to the location and size of the tumor. The usual complaint is a swelling. Although pain is uncommon unless the tumor encroaches on adjacent nerves, paresthesia may arise, especially with lesions originating in the inferior alveolar canal. Pain, when present, usually develops at the site of the tumor; if paresthesia occurs, it is felt anterior to the tumor.
Radiographic Features
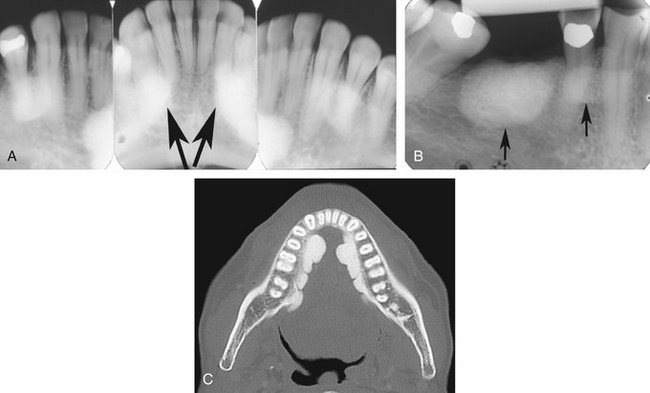
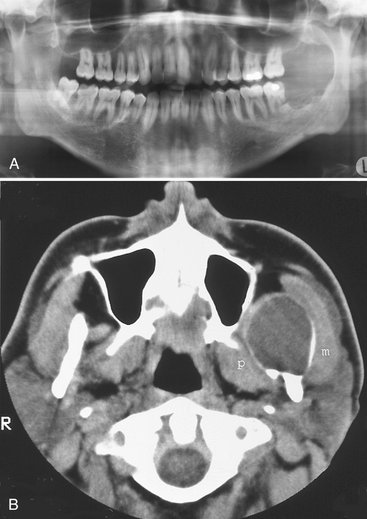
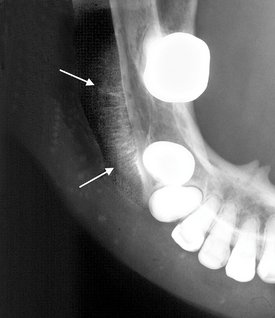
Location.: Neurilemmomas most often involve the mandible, with fewer than 1 in 10 cases occurring in the maxilla. The tumor most often is located within an expanded inferior alveolar nerve canal posterior to the mental foramen (Fig. 22-33).
Periphery.: In keeping with its slow growth rate, the margins of this tumor are well defined and usually corticated as it expands the cortical walls of the inferior alveolar canal. Small lesions may appear cystlike but more commonly are fusiform in shape as the tumor expands the canal.
Internal Structure.: The internal structure is uniformly radiolucent. When lesions have a scalloping outline, this may give a false impression of a multilocular pattern.
Effects on Surrounding Structures.: If the tumor reaches either the mandibular foramen or mental foramen, it can cause enlargement of the foramen. Expansion of the inferior alveolar canal is slow and thus the outer cortex of the canal is maintained; the expansion of the canal is usually localized with a definite epicenter, unless the lesion is large. The expanding tumor may cause root resorption of adjacent teeth (see Fig. 22-33).
Differential Diagnosis
Because neurilemmomas most commonly originate within the inferior alveolar canal, vascular lesions such as a hemangioma or arteriovenous fistula should be considered. However, neurilemmomas have a distinct epicenter, whereas vascular lesions will usually cause a more uniform widening of the whole canal; they do not have an obvious epicenter and usually change the course of the canal, most commonly to a serpiginous shape. Only neural tumors and vascular lesions originate within the inferior alveolar canal, but malignant lesions that grow down and enlarge the canal should be in the differential diagnosis. When this happens, the appearance is different, with an irregular widening and destruction of the cortical boundaries of the canal.
Treatment
Excision is usually the treatment of choice. These lesions generally do not recur if they are completely removed. A capsule usually is present, facilitating surgical removal, although occasionally preservation of the nerve may not be possible. However, periodic examination is indicated to check for recurrence.
Definition
Despite its name, a neuroma is not a neoplasm. Rather, it is an overgrowth of severed nerve fibers attempting to regenerate with abnormal proliferation of scar tissue after a fracture involving a peripheral nerve. As a result, the proliferating nerve forms a disorganized collection of nerve fibers composed of varying proportions of axons, perineural connective tissue, Schwann cells, and scar tissue. The original nerve damage may be the result of mechanical or chemical irritation of the nerve caused by fracture, orthognathic surgery, removal of a tumor or cyst, extrusion of endodontic cement, dental implants, or tooth extraction.
Clinical Features
Central neuromas are slow-growing reactive hyperplasias that seldom become large, rarely exceeding 1 cm in diameter. They may cause a variety of symptoms, including severe pain resulting from pressure applied as the tangled mass enlarges in its bony cavity or as the result of external trauma. The patient may have reflex neuralgia, with pain referred to the eyes, face, and head.
Radiographic Features
The radiographic features of a neuroma relate to the extent and shape of the proliferating mass of neural tissue.
Location.: The most common location is the mental foramen, then the anterior maxilla and the posterior mandible.
Differential Diagnosis
It is not possible to differentiate this lesion from other benign neural tumors.
Treatment
Treatment is recommended because neuromas tend to continue to enlarge. They also may cause pain. Regardless of the type of injury that precipitates development of the neuroma, recurrence is uncommon after simple excision.
Definition
Neurofibromas are moderately firm, benign, well-circumscribed tumors caused by proliferation of Schwann cells in a disorderly pattern that includes portions of nerve fibers, such as peripheral nerves, axons, and connective tissue of the sheath of Schwann. As neurofibromas grow, they incorporate axons. In contrast, neurilemomas are composed entirely of Schwann cells and grow by displacing axons.
Clinical Features
The central lesion of a neurofibroma may be the same as the multiple lesions that develop in von Recklinghausen disease. Central lesions also may occur in that syndrome but are rare. Neurofibromas can occur at any age but usually are found in young patients. Neurofibromas associated with the mandibular nerve may produce pain or paresthesia. Neurofibromas also may expand and perforate the cortex; causing swelling that is hard or firm to palpation.
Radiographic Features
Location.: Central neurofibromas may occur in the mandibular canal, in the cancellous bone, and below the periosteum.
Periphery.: As with neurilemomas, the margins of the radiolucency in neurofibromas usually are sharply defined and may be corticated. However, despite the benign nature and slow growth of the neurofibroma, some of these lesions have indistinct margins.
Internal Structure.: The tumors usually appear unilocular but on occasion may have a multilocular appearance.
Effects on Surrounding Structures.: A neurofibroma of the inferior dental nerve shows a fusiform enlargement of the canal (Fig. 22-34).
Differential Diagnosis
Differentiation from other types of neural lesions may not be possible. This tumor can be differentiated from vascular lesions because the expansion of the canal is in a fusiform shape, whereas vascular lesions enlarge the whole canal and alter its path.
Treatment
Solitary central lesions that have been excised seldom recur. However, it is wise to re-examine the area periodically because these tumors are not encapsulated and some undergo malignant change.
Definition
Neurofibromatosis is a syndrome consisting of café-au-lait spots on the skin, multiple peripheral nerve tumors, and a variety of other dysplastic abnormalities of the skin, nervous system, bones, endocrine organs, and blood vessels. The two major classifications are NF-1, a generalized form, and NF-2, a central form. Oral lesions may occur as part of NF-1 or may be solitary and are called segmental or forme fruste manifestations (Fig. 22-35). Recent observations of abnormal fat tissue in close association with changes in the osseous structure of the mandible support the theory that a mesodermal dysplasia is part of the spectrum of changes that may be observed in NF-1 lesions.
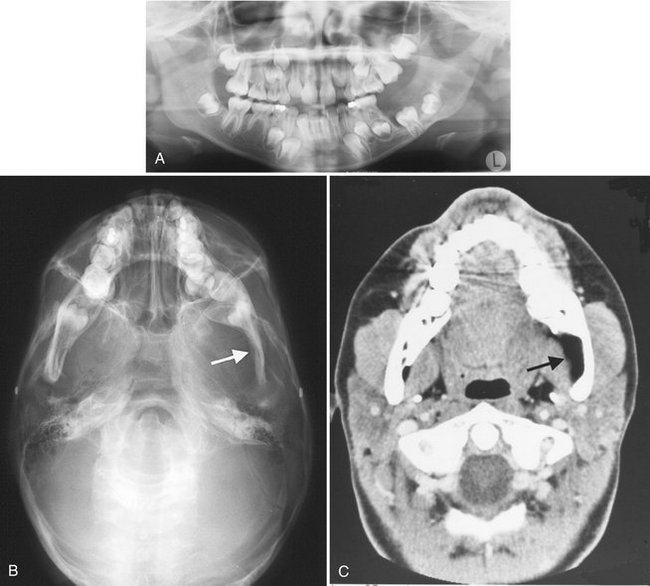
FIG. 22-35 An example of segmental neurofibromatosis involving the mandible. A, Panoramic film demonstrating enlargement of the left coronoid notch, enlargement of the mandibular foramen, and interference of the eruption of the first and second molars. B, Basal skull view of the same case revealing thinning and bowing of the ramus in a lateral direction (arrow). C, CT axial image with soft tissue algorithm showing fatty tissue adjacent to the abnormal ramus (arrow).
Clinical Features
Neurofibromatosis is one of the most common genetic diseases, occurring in 1 in every 3000 births and present in about 30 people per 10,000 population. The peripheral nerve tumors are of two types, schwannomas and neurofibromas. Some manifestations are congenital, but most appear gradually during childhood and adult life. Café-au-lait spots become larger and more numerous with age; most patients eventually have more than six spots larger than 1.5 cm in diameter. Other skin lesions include freckles, soft pedunculated cutaneous neurofibromas, and firm subcutaneous neurofibromas.
Radiographic Features
The radiographic changes in the jaws with neurofibromatosis can be characteristic. These changes include the following alterations in the shape of the mandible: enlargement of the coronoid notch in either or both the horizontal and vertical dimensions, an obtuse angle between the body and the ramus, deformity of the condylar head, lengthening of the condylar neck, and lateral bowing and thinning of the ramus, as seen in basal skull views (see Fig. 22-35). Changes in mandibular morphology can continue to increase in severity through the second decade. Other radiographic changes include enlargement of the mandibular canal and mental and mandibular foramina and an increased incidence of branched mandibular canal. Erosive changes to the outer contour of the mandible and interference with normal eruption of the molars also may occur. Abnormal accumulations of fatty tissue within deformities of the mandible have been observed in images produced by CT (see Fig. 22-35, C).
MESODERMAL TUMORS
Definition
Osteomas can form from membranous bones of the skull and face. The cause of the slowly growing osteoma is obscure, but the tumor may arise from cartilage or embryonal periosteum. It is not clear whether osteomas are benign neoplasms or hamartomas. This lesion may be solitary or multiple, occurring on a single bone or on numerous bones. Osteomas originate from the periosteum and may occur either externally or within the paranasal sinuses (Fig. 22-36). It is more common in the frontal and ethmoid sinuses than in the maxillary sinuses (see Chapter 27). Structurally, osteomas can be divided into three types: those composed of compact bone (ivory), those composed of cancellous bone, and those composed of a combination of compact and cancellous bone.
Clinical Features
Osteomas can occur at any age but most frequently are found in individuals older than 40 years. The only symptom of a developing osteoma is the asymmetry caused by a bony, hard swelling on the jaw. The swelling is painless until its size or position interferes with function. Osteomas are attached to the cortex of the jaw by a pedicle or along a wide base. The mucosa covering the tumor is normal in color and freely movable. Cortical-type osteomas develop more often in men, whereas women have the highest incidence of the cancellous type. Although most osteomas are small, some may become large enough to cause severe damage, especially those that develop in the frontoethmoid region.
Radiographic Features
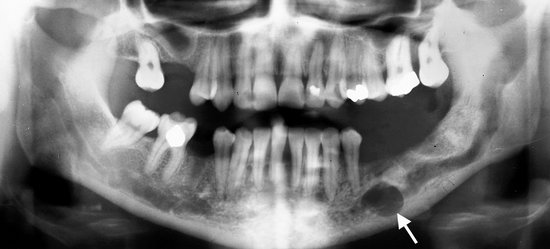
Location.: The mandible is more commonly involved than the maxilla. Osteomas are found most frequently on the posterior aspect of the mandible commonly on the lingual side of the ramus or on the inferior mandibular border below the molars (Fig. 22-37). Other locations include the condylar and coronoid regions. The mandibular lesion may be exophytic, extending outward into adjacent soft tissues (Fig. 22-38). The lesions also occur in the paranasal sinuses, especially the frontal sinus.
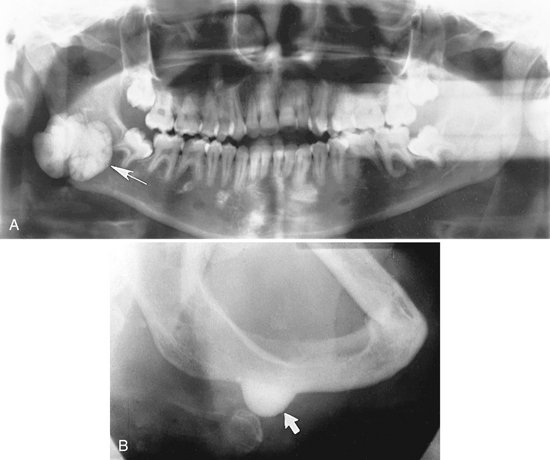
FIG. 22-37 An osteoma. A, A panoramic radiograph shows an osteoma in the right mandibular angle region (arrow). B, An oblique lateral jaw radiograph shows a solid radiopaque osteoma attached to the inferior border of the mandible (arrow). (A from Matteson SR et al: Semin Adv Oral Radiol Dent Radiol Photogr 57:1, 1985.)
Differential Diagnosis
Usually the appearance is characteristic and does not present a problem with diagnosis. However, osteomas involving the condylar head can be difficult to differentiate from osteochondromas, osteophytes, or condylar hyperplasia; those involving the coronoid process may be similar to osteochondromas. A small osteoma may be similar in appearance to a torus or a large hyperostosis (exostosis).
Treatment
Unless the osteoma interferes with normal function or presents a cosmetic problem, this lesion may not require treatment. In such cases the osteoma should be kept under observation. Resection of osteomas is possible and may be difficult if the osteoma is of the cortical (ivory) type.
Definition
Gardner’s syndrome is a type of familial multiple polyposis where there is an associated neoplasm. This syndrome is a hereditary condition characterized by multiple osteomas, multiple dense bone islands (enostosis), epidermoid cysts, subcutaneous desmoid tumors, and multiple polyps of the small and large intestine. The associated osteomas appear during the second decade. They are most common in the frontal bone, mandible, maxilla, and sphenoid bones (Fig. 22-39). A significant feature of familial multiple polyposis is the strong predilection of the intestinal polyps to undergo malignant conversion, making early detection of the syndrome important. Because the osteomas and enostosis often develop before the intestinal polyps, early recognition of the syndrome may be a lifesaving event. Occasionally osteomas may not be present, but the presence of five or more dense bone islands may indicate the presence of a familial multiple polyposis syndrome (Fig. 22-40). Multiple unerupted supernumerary and permanent teeth in both jaws also occur with Gardner’s syndrome. Multiple osteomas may occur as isolated findings in the absence of the diseases associated with Gardner’s syndrome.
Treatment
The removal of osteomas is not generally necessary unless the tumors interfere with normal function or present a cosmetic concern. It is most important to recognize the relationship of multiple osteomas and multiple dense bone islands with familial multiple polyposis syndromes for early diagnosis. A family history of intestinal cancer may also help. These patients should be referred to their family physicians for examination for intestinal polyposis and treatment.
Definition
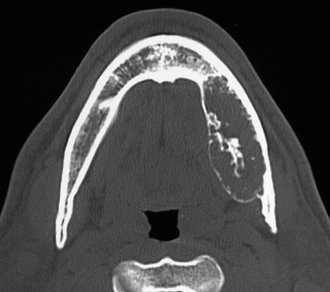
A hemangioma is a proliferation of blood vessels creating a mass that resembles a neoplasm, although in many cases it is actually a hamartoma. Hemangiomas can occur anywhere in the body but are most frequently noticed in the skin and subcutaneous tissues. The central (intraosseous) type most often is found in the vertebrae and skull. It rarely develops in the jaws, and an even smaller number of maxillary lesions have been reported. The lesions may be developmental or traumatic in origin.
Clinical Features
Hemangiomas are more prevalent in females than males, at a ratio of 2 : 1. This tumor occurs most commonly in the first decade but may occur later in life. Enlargement is slow, producing a nontender expansion of the jaw that occurs over several months or years. The swelling may or may not be painful, is not tender, and usually is bony hard. Pain, if present, probably is the throbbing type. Some tumors may be compressible or pulsate, and a bruit may be detected on auscultation. Anesthesia of the skin supplied by the mental nerve may occur. The lesion may cause loosening and migration of teeth in the affected area. Bleeding may occur from the gingiva around the neck of the affected teeth. These teeth may demonstrate rebound mobility; that is, when depressed into their sockets, they rebound to their original position within several minutes because of the pressure of the vascular network around the tooth. Aspiration with a syringe produces arterial blood that may be under considerable pressure.
Radiographic Features
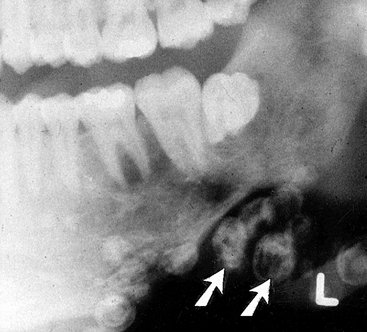
Location.: Hemangiomas affect the mandible about twice as often as the maxilla. In the mandible the most common site is the posterior body and ramus and within the inferior alveolar canal.
Periphery.: In some instances the periphery is well defined and corticated, and in other cases it may be ill defined and even simulate the appearance of a malignant tumor. This variation probably is related to the amount of residual bone present around the blood vessels. The formation of linear spicules of bone emanating from the surface of the bone in a sunraylike appearance can occur when the hemangioma breaks through the outer cortex and displaces the periosteum (Fig. 22-41).
Internal Structure.: When residual bone is trapped around the blood vessels, the result may be a multilocular appearance. Small radiolucent locules may resemble enlarged marrow spaces surrounded by coarse, dense, and well-defined trabeculae (Fig. 22-42). These internal trabeculae may produce a honeycomb pattern composed of small circular radiolucent spaces that represent blood vessels that are oriented in the same direction of the x-ray beam. When the inferior alveolar canal is involved, the whole canal is increased in width, and often the normal path of the canal is altered into a serpiginous shape sometimes creating a multilocular appearance (Fig. 22-43). Some lesions may be totally radiolucent. When the hemangioma involves soft tissue, the formation of phlebolith (small areas of calcification or concretions found in a vein with slow blood flow) may occur within surrounding soft tissues (Fig. 22-44). They develop from thrombi that become organized and mineralized and consist of calcium phosphate and calcium carbonate.
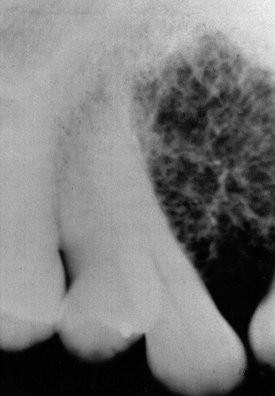
FIG. 22-42 A hemangioma in the anterior maxilla shows a coarse trabecular pattern. (Courtesy E. J. Burkes, DDS, Chapel Hill, N.C.)
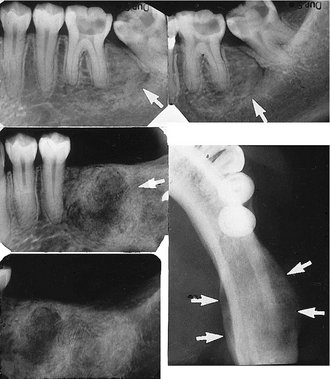
Effects on Surrounding Structures.: The roots of teeth in the region of the vascular lesion are often resorbed or displaced. When the lesion involves the inferior alveolar nerve canal, the canal can be enlarged along its entire length and its shape may be changed to a serpiginous path. The mandibular and mental foramen may be enlarged. Hemangiomas can influence the growth of bone and teeth. The involved bone may be enlarged and have coarse internal trabeculae. Also, developing teeth may be larger and erupt earlier when in intimate relationship with a hemangioma (Fig. 22-45).
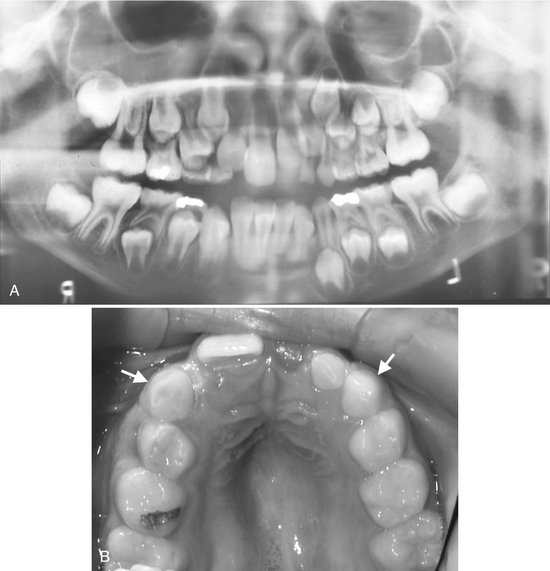
FIG. 22-45 A, A panoramic film demonstrating the effect of a soft tissue hemangioma on the developing dentition. The root development and eruption of the right cuspids and bicuspids are significantly advanced compared with the left side. B, An occlusal photograph from the same case; note the difference in size of the maxillary deciduous cuspids.
Further diagnostic imaging to better document the distribution and degree of involvement of the osseous and soft tissues of the maxillofacial region should include modalities such as conventional angiography and magnetic resonance angiography.
Differential Diagnosis
Hemangiomas should be considered in the differential diagnosis of multilocular lesions involving the body of the ramus and body of the mandible. Demonstration of involvement of the inferior alveolar canal is an important indicator of a vascular lesion. In most cases soft tissue changes suggest a vascular lesion. When a hemangioma produces a sunray spiculated bone pattern at its periphery, the appearance may be difficult to differentiate from an osteogenic sarcoma (see Fig. 22-41).
Treatment
Central hemangiomas should be treated without delay because trauma that disrupts the integrity of the affected jaw may result in lethal exsanguination. Specifically, embolization (introduction of inert materials into the lesion by a vascular route), surgery (en bloc resection with ligation of the external carotid artery), and sclerosing techniques have been used singly or together.
Definition
An A-V fistula, an uncommon lesion, is a direct communication between an artery and a vein that bypasses the intervening capillary bed. It usually results from trauma but in rare instances may be a developmental anomaly. An A-V fistula can occur anywhere in the body, in soft tissue, in the alveolar process, and centrally in the jaw. The head and neck are the most common sites.
Clinical Features
The clinical appearance of a central A-V aneurysm can vary considerably, depending on the extent of bone or soft tissue involvement. The lesion may expand bone, and a mass may be present in the extraosseous soft tissue. The soft tissue swelling may have a purple discoloration. Palpation or auscultation of the swelling may reveal a pulse. On the other hand, neither the bone nor the soft tissue may be expanded, and no pulse may be clinically apparent. Aspiration produces blood. Recognition of the hemorrhagic nature of these lesions is of utmost importance because extraction of an associated tooth may be immediately followed by life-threatening bleeding.
Radiographic Features
Location.: These lesions most commonly develop in the ramus and retromolar area of the mandible and involve the mandibular canal.
Internal Structure.: A tortuous path of an enlarged vessel in bone may give a multilocular appearance. Otherwise the lesion is radiolucent.
Effects on Surrounding Structures.: Both central lesions and those in adjacent soft tissue can erode bone resulting in well-defined (cyst like) lesions in the bone. Changes in the inferior alveolar canal may occur, as described in the preceding section on hemangiomas.
Additional Imaging.: CT with contrast injection is a useful method for aiding the differential diagnosis of any vascular lesion and other neoplasms of the jaws (Fig. 22-46). An imaging modality called magnetic resonance angiography is now being used routinely to document the size and extent and the vessels involved with the vascular lesion. Angiography, a radiographic procedure performed by injecting a radiopaque contrast agent into the blood vessels and making radiographs, will provide the same information and is usually used when interventional therapy is planned in conjunction with the angiography (Fig. 22-47).
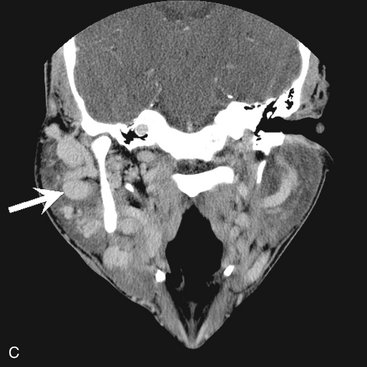
FIG. 22-46 A, A panoramic film of a patient with an A-V malformation; note the enlarged and irregular inferior alveolar canal. B and C, Axial and coronal CT images with soft tissue algorithm and after intravascular contrast was administered, which will cause the blood vessels to be more radiopaque (arrows).
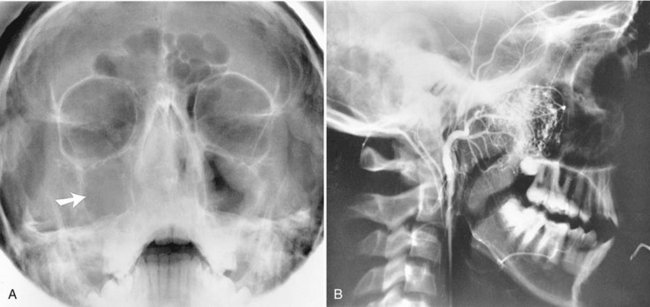
FIG. 22-47 A vascular lesion in the right maxillary sinus. A, A Waters’ radiograph shows the opacified maxillary antrum (arrow). B, Note the tumor vascularization in this angiogram. A radiopaque dye has been injected into the vasculature to enhance visualization. (Courtesy G. Himadi, DDS, Chapel Hill, N.C.)
Differential Diagnosis
Occasionally the radiographic appearance is not specific for the A-V aneurysm. The differential diagnosis is similar to that for hemangiomas and includes multilocular lesions. However, association with the inferior alveolar canal is important in the differentiation.
Definition
An osteoblastoma is an uncommon, benign tumor of osteoblasts with areas of osteoid and calcific tissue. This tumor occurs most often in the spine of a young person. Agreement apparently is increasing that, if osteoblastomas and osteoid osteomas are different lesions, they differ only in size and morphologic and histologic features. For example, the osteoid trabeculae in an osteoblastoma generally are larger (broader and longer, with wider trabecular spaces than those in an osteoid osteoma). An osteoblastoma is usually less painful, and it has more osteoclasts. In addition, benign osteoblastomas are considered more aggressive lesions. On the level of their ultrastructures, the two lesions essentially are similar or at least closely related.
Clinical Features
Both osteoblastomas and osteoid osteomas are rare in the jaws. The male-to-female ratio is 2 : 1, although some studies indicate a higher female occurrence and the average age is 17 years, with most lesions occurring in the second and third decades of life. Clinically, patients often report pain and swelling of the affected region; however, the pain is more severe in osteoid osteomas and is often relieved by salicylates.
Radiographic Features
Location.: Osteoblastomas are found both in the tooth-bearing regions and commonly around the temporomandibular joint (within the condyle or the temporal bone).
Periphery.: The borders may be diffuse or may show some sign of a cortex. Lesions often have a soft tissue capsule around the periphery, indicating that this tumor is more mature in the central regions where there is evidence of abnormal bone (Fig. 22-48).
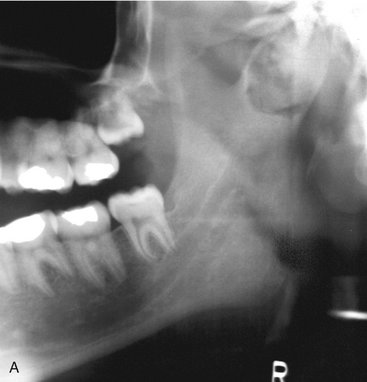
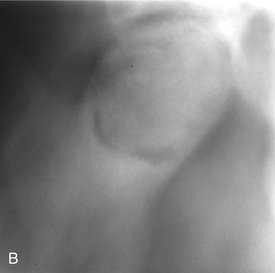
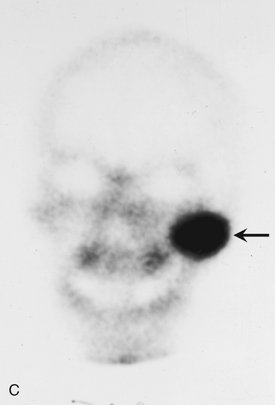
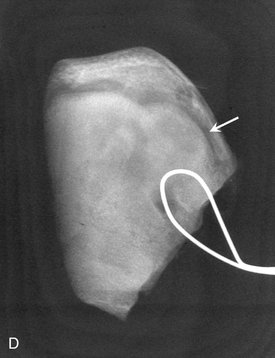
FIG. 22-48 A, A cropped panoramic film of an osteoblastoma occupying the left condyle; note the enlargement of the condyle and the presence of a soft tissue capsule surrounding an internal structure of granular bone. B, A tomograph of the left condyle.C, A technetium bone scan demonstrating increased bone activity in the left condyle (arrow). D, A radiograph of the surgical specimen; note the internal granular bone surrounded by a soft tissue capsule (arrow).
Differential Diagnosis
An important differential diagnosis may be a well-defined osteogenic sarcoma because the histologic appearance may be very similar. The differentiation may rely on the benign features of the osteoblastoma as revealed in the radiographic images. Osteoblastomas do not normally break through cortical boundaries and invade surrounding soft tissue. Osteoid osteomas are usually smaller and have an associated sclerotic bone reaction at the periphery. Sometimes the appearance of an osteoblastoma may be similar to a large area of cemental dysplasia. Both have a soft tissue capsule, but the osteoblastoma behaves more aggressively, like a tumor.
Treatment
Osteoblastomas are treated with curettage or local excision. Recurrences have been described, and in a few cases the differentiation from a low-grade osteosarcoma may be difficult.
Definition
An osteoid osteoma is a benign tumor that is extremely rare in the jaws. Its true nature is not known, but some investigators think it is a variant of osteoblastoma. The tumor has an oval or round, tumorlike core usually only about 1 cm in diameter, although some may reach 5 or 6 cm. This core consists of osteoid and newly formed trabeculae within highly vascularized, osteogenic connective tissue. The tumor usually develops within the outer cortex but may form within the cancellous bone. There is a sclerotic bone reaction around the periphery, often thinner in lesions within the cancellous bone.
Clinical Features
Osteoid osteomas occur most often in young people, usually males between the ages of 10 and 25 years. They seldom occur before 4 years or after 40 years. This condition affects at least twice as many males as females. Most of the lesions occur in the femur and tibia; the jaws are rarely involved. Severe pain in the bone that can be relieved by anti-inflammatory drugs is characteristic. In addition, the soft tissue over the involved bony area may be swollen and tender.
Radiographic Features
Location.: The lesion is most common in the cortex of the limb bones. In those that do occur in the jaws, somewhat more develop in the body of the mandible.
Periphery.: The margins are well defined by a rim of sclerotic bone (Fig. 22-49).
Differential Diagnosis
Osteoid osteomas are extremely rare in the jaws. A clinician who suspects that a sclerotic lesion is an osteoid osteoma should also consider sclerosing osteitis, cemento-ossifying fibroma, benign cementoblastoma, and cemental dysplasia. The presence of a central radiolucency usually eliminates enostosis or osteosclerosis. Scintigraphy by a bone scan will help in the differential diagnosis by revealing considerable vascularity in the blood pool phase and a very high comparative bone metabolism.
Treatment
Complete excision is currently the recommended treatment because it often relieves the pain and cures the disease. Although spontaneous remission can occur in some cases, the data are insufficient for identifying such cases in advance.
Definition
A desmoplastic fibroma of bone is an aggressive, infiltrative neoplasm that produces abundant collagen fibers. It is composed of fibroblast-like cells that have ovoid or elongated nuclei in abundant collagen fibers. The lack of pleomorphism of the cells is important.
Clinical Features
Patients usually complain of facial swelling, pain (in rare cases), and sometimes dysfunction, especially when the neoplasm is close to the joint. The lesion occurs most often in the first two decades of life, with a mean reported age of 14 years. Although it originates in bone, the tumor may invade the surrounding soft tissue extensively. It also may occur as part of Gardner’s syndrome.
Radiographic Features
Location.: Desmoplastic fibromas of bone may occur in the mandible or maxilla, but the most common site is the ramus and posterior mandible.
Periphery.: The periphery is most often ill defined and has an invasive characteristic commonly seen in malignant tumors.
Internal Structure.: The internal aspect may be totally radiolucent, especially when the lesion is small. Larger lesions appear to be multilocular with very coarse, thick septa. These wide septa may be straight or have an irregular shape (Fig. 22-50). In T1-weighted MRI scans the internal structure has a low signal, which helps in determining intraosseous extent because of the contrast with the high signal from the bone marrow.
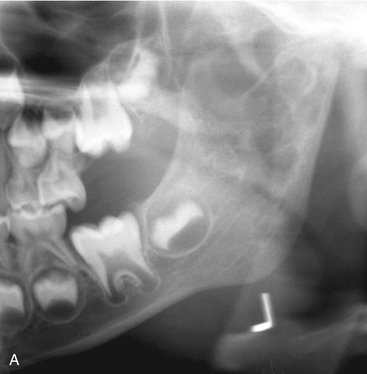
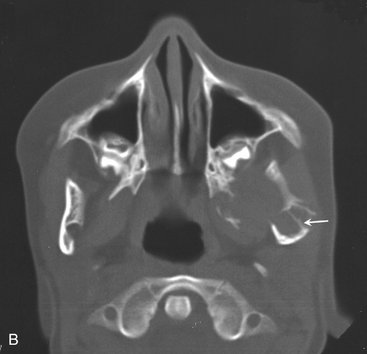
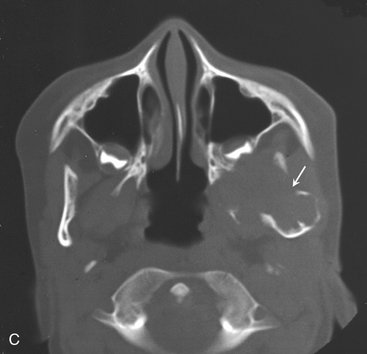
FIG. 22-50 A, A cropped panoramic film of a case of a central desmoplastic fibroma centered within the left condyle and ramus. B, Axial CT image with bone algorithm revealing thick, straight septa (arrow). C, Another CT image at a higher level revealing that the tumor has broken through the anterior cortex of the condylar head.
Differential Diagnosis
Distinguishing this neoplasm from a fibrosarcoma may be difficult during the histologic examination. The radiographic appearance may not be helpful because a desmoplastic fibroma often has the appearance of a malignant neoplasm. However, the presence of coarse, irregular, and sometimes straight septa may help support the correct diagnosis. The appearance of these septa also helps differentiate the lesion from other multilocular tumors. Very small lesions may resemble simple bone cysts.
Barnes, L, Eveson, JW, Reichart, P, et al. Odontogenic tumors. In: World Health Organization Classification of Tumours, ed. Pathology and genetics of head and neck tumours. Lyon: IARC Press, 2005.
Daley, TD, Wysocki, GP, Pringle, GA. Relative incidence of odontogenic tumors and oral and jaw cysts in a Canadian population. Oral Surg Oral Med Oral Pathol. 1994;77:276–280.
Hoffman S, Jacoway JR, Krolls SO: Intraosseous and parosteal tumors of the jaws, atlas of tumor pathology, series 2, fascicle 24, Washington, DC, 1987, Armed Forces Institute of Pathology.
Krishnan Unni, K. Dahlin’s bone tumors: general aspects and data on 11,087 cases, ed 5. Philadelphia: Lippincott-Raven; 1996.
Regezi, JA, Kerr, DA, Courtney, RM. Odontogenic tumors: an analysis of 706 cases. J Oral Surg. 1978;36:771–778.
Eggan, S, Natvig, B, Gåsemyr, J. Variation in torus palatinus prevalence in Norway. Scand J Dent Res. 1994;102:54–59.
Gorsky, M, Raviv, M, Kfir, E, et al. Prevalence of torus palatinus in a population of young and old Israelis. Arch Oral Biol. 1996;41:623–625.
Haugen, LK. Palatine and mandibular tori; a morphologic study in the current Norwegian population. Acta Odontol Scand. 1992;50:65–77.
Eggen, S, Natvig, B. Concurrence of torus mandibularis and torus palatinus. Scand J Dent Res. 1994;102:60–63.
McDonnel, D. Dense bone island: a review of 107 patients. Oral Surg Oral Med Oral Pathol. 1993;76:124–128.
Petrikowski, GC, Peters, E. Longitudinal radiographic assessment of dense bone islands of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:627–634.
Atkinson, CH, Harwood, AR, Cummings, BJ. Ameloblastoma of the jaw: a reappraisal of the role of megavoltage irradiation. Cancer. 1984;53:869–873.
Heffez, L, Mafee, MF, Vaiana, J. The role of magnetic resonance imaging in the diagnosis and management of ameloblastoma. Oral Surg Oral Med Oral Pathol. 1988;65:2–12.
Ueta, E, Yoneda, K, Ohno, A, et al. Intraosseous carcinoma arising from mandibular ameloblastoma with progressive invasion and pulmonary metastasis. Int J Oral Maxillofac Surg. 1996;25:370–372.
Weissman, JL, Snyderman, CH, Yousem, SA, et al. Ameloblastoma of the maxilla: CT and MR appearance. AJNR Am J Neuroradiol. 1993;14:223–226.
Dare, A, Yamaguchi, A, Yoshiki, S, et al. Limitation of panoramic radiography in diagnosing adenomatoid odontogenic tumors. Oral Surg Oral Med Oral Pathol. 1994;77:662–668.
Hicks, MJ, Flaitz, CM, Batsakis, JG. Pathology consultation: adenomatoid and calcifying epithelial odontogenic tumors. Ann Otol Rhinol Laryngol. 1993;102:159.
Philipsen, HP, Reichart, PA, Zhang, KH, et al. Adenomatoid odontogenic tumor: biologic profile based on 499 cases. J Oral Pathol Med. 1991;20:149–158.
CALCIFYING EPITHELIAL ODONTOGENIC TUMOR
Franklin, CD, Pindborg, JJ. The calcifying epithelial odontogenic tumor: a review and analysis of 113 cases. Oral Surg Oral Med Oral Pathol. 1976;42:753–765.
Kaplan, I, Buckner, A, Calderon, S, et al. Radiological and clinical features of calcifying epithelial odontogenic tumor. Dentomaxillofac Radiol. 2001;30:22–28.
Patiño, B, Fernández-Alba, J, Garcia-Rosado, A, et al. Calcifying epithelial odontogenic (Pindborg) tumor: a series of 4 distinctive cases and review of the literature. J Oral Maxillofac Surg. 2005;63:1361–1368.
Haishima, K, Haishima, H, Yamada, Y, et al. Compound odontomes associated with impacted maxillary primary central incisors: report of 2 cases. Int J Paediatr Dent. 1994;4:251–256.
Kaugars, GE, Miller, ME, Abbey, LM. Odontomas. Oral Surg Oral Med Oral Pathol. 1989;67:172–176.
Nik-Hussein, NN, Majid, ZA. Erupted compound odontoma. Ann Dent. 1993;52:9–11.
Dallera, P, Bertoni, F, Marchetti, C, et al. Ameloblastic fibroma: a follow-up of six cases. Int J Oral Maxillofac Surg. 1996;25:199–202.
Trodahl, JN. Ameloblastic fibroma: a survey of cases from the Armed Forces Institute of Pathology. Oral Surg Oral Med Oral Pathol. 1972;33:547–558.
Cohen, MA, Mendelsohn, DB. CT and MR imaging of myxofibroma of the jaws. J Comput Assist Tomogr. 1990;14:281–285.
Peltola, J, Magnusson, B, Happonen, RP, et al. Odontogenic myxoma: a radiographic study of 21 tumours. Br J Oral Maxillofac Surg. 1994;32:298–302.
Simon, ENM, Merkx, MA, Vuhahula, E, et al. Odontogenic myxoma: a clinicopathological study of 33 cases. Int J Oral Maxillofac Surg. 2004;33:333–337.
Sumi, Y, Miyaishi, O, Ito, K, et al. Magnetic resonance imaging of myxoma in the mandible: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:671–676.
Brannon, RB, Fowler, CB, Carpenter, WM, et al. Cementoblastoma: An innocuous neoplasm? A clinicopathological study of 44 cases and review of the literature with special emphasis on recurrence. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:311–320.
Jelic, JS, Loftus, MJ, Miller, AS, et al. Benign cementoblastoma: report of an unusual case and analysis of 14 additional cases. J Oral Maxillofac Surg. 1993;51:1033–1037.
Ruprecht, A, Ross, AS. Benign cementoblastoma (true cementoblastoma). Dentomaxillofac Radiol. 1983;12:31–33.
Gardner, DG. Central odontogenic fibroma: current concepts. J Oral Pathol Med. 1996;25:556–561.
Handlers, JP, Abrams, AM, Melrose, RJ, et al. Central odontogenic fibroma: clinicopathologic features of 19 cases and review of the literature. J Oral Maxillofac Surg. 1991;49:46–54.
Kaffe, I, Buchner, A. Radiologic features of central odontogenic fibroma. Oral Surg Oral Med Oral Pathol. 1994;78:811–818.
Chi, AC, Carey, J, Muller, S, et al. Intraosseous schwannoma of the mandible; case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:54.
Minowa, K, Sakakibara, N, Yoshikawa, K, et al. CT and MRI findings of intraosseous schwannoma of the mandible: a case report. Dentomaxillofac Radiol. 2007;36:113–116.
Fan, X, Qiu, W, Zhang, Z, et al. Comparative study of clinical manifestation, plain-film radiography and computed tomographic scan in arteriovenous malformations of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:503–509.
Lund, BA, Dahlin, DC. Hemangiomas of the mandible and maxilla. J Oral Surg. 1964;22:234–242.
Zlotogorski, A, Buchner, A, Kaffe, I, et al. Radiological features of central haemangioma of the jaws. Dentomaxillofac Radiol. 2005;34:292–296.
Bilkay, U, Erdem, O, Ozek, C, et al. Benign osteoma with Gardner syndrome: review of the literature and report of a case. J Craniofac Surg. 2004;15:506–509.
Earwaker, J. Paranasal osteomas: a review of 46 cases. Skeletal Radiol. 1993;22:417–423.
Matteson, S, Deahl, ST, Alder, ME, et al. Advanced imaging methods. Crit Rev Oral Biol Med. 1996;7:346–395.
Thakker, NS, Evans, DG, Horner, K, et al. Florid oral manifestations in an atypical familial adenomatous polyposis family with late presentation of colorectal polyps. J Oral Pathol Med. 1996;25:459–462.
D’Ambrosio, JA, Langlais, RP, Young, RS. Jaw and skull changes in neurofibromatosis. Oral Surg Oral Med Oral Pathol. 1988;66:391.
Lee, L, Yan, YH, Pharaoah, MJ. Radiographic features of the mandible in neurofibromatosis: a report of 10 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:361–367.
Alvares Capelozza, AL, Gião Dezotti, MS, Casati Alvares, L, et al. Osteoblastoma of the mandible: systematic review of the literature and report of a case. Dentomaxillofac Radiol. 2005;34:1–8.
Jones, AC, Prihoda, TJ, Kacher, JE, et al. Osteoblastoma of the maxilla and mandible: a report of 24 cases, review of the literature, and discussion of its relationship to osteoid osteoma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:639–650.
Lucas, DR, Unni, KK, McLeod, RA, et al. Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol. 1994;25:117–134.
Hopkins, KM, Huttula, CS, Kahn, MA, et al. Desmoplastic fibroma of the mandible: review and report of two cases. J Oral Maxillofac Surg. 1996;54:1249–1254.
Said-Al-Naief, N, Fernandes, R, Louis, P, et al. Desmoplastic fibroma of the jaw: a case report and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:82–94.