Diseases of Bone Manifested in the Jaws
This chapter discusses disorders of bone that do not easily fit into well-defined categories of disease.
Bone Dysplasias
Bone dysplasias constitute a group of conditions in which normal bone is replaced with fibrous tissue containing abnormal bone or cementum. These lesions must be differentiated from tumors because the treatment is very different. Fibro-osseous lesion, originally a histopathologic term, is a commonly used term that includes the following bone dysplasias and neoplasms and other lesions of bone.
FIBROUS DYSPLASIA
Fibrous dysplasia results from a localized change in normal bone metabolism that results in the replacement of all the components of cancellous bone by fibrous tissue containing varying amounts of abnormal-appearing bone. This may be the result of a sporadic gene mutation. At the histologic level, the result is the appearance of numerous short, irregularly shaped trabeculae of woven bone. These trabeculae are not aligned in response to stress, but rather have a random orientation. This histologic appearance is responsible for the abnormal internal trabecular pattern seen in radiographs. Fibrous dysplasias may be solitary or multiple (Jaffe type) or may occur in another multiple form associated with McCune-Albright syndrome, which usually comprises polyostotic fibrous dysplasia, cutaneous pigmentation (café au lait spots), and hyperfunction of one or more of the endocrine glands.
Clinical Features
The solitary (monostotic) form of fibrous dysplasia, which accounts for 70% of all cases, is the type that most often involves the jaws. The most common sites (in order) are the ribs, femur, tibia, maxilla, and mandible. The multiple (polyostotic) form usually is found in children younger than 10 years, whereas monostotic disease typically is discovered in a slightly older age group. The lesions usually become static when skeletal growth stops, but proliferation may continue, particularly in the polyostotic form. The lesions may become active in pregnant women or with the use of oral contraceptives; and abnormal growth may occur after surgical intervention in young patients. Studies of the sex distribution of fibrous dysplasia show no sexual predilection except for McCune-Albright syndrome, which affects females almost exclusively. Symptoms of the disease may be mild or absent. Monostotic fibrous dysplasia often is discovered as an incidental radiographic finding. Patients with jaw involvement first may complain of unilateral facial swelling or an enlarging deformity of the alveolar process. Pain and pathologic fractures are rare. If extensive craniofacial lesions have impinged on nerve foramina, neurologic symptoms such as anosmia (loss of the sense of smell), deafness, or blindness may develop.
Radiographic Features
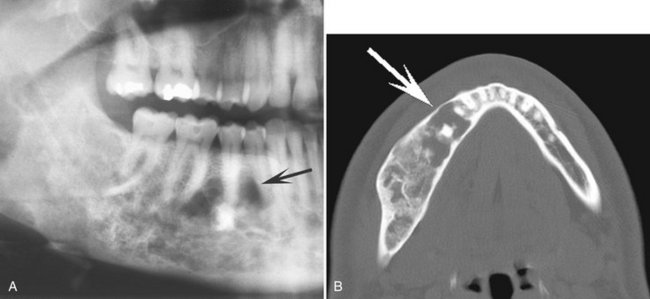
Location.: Fibrous dysplasia involves the maxilla almost twice as often as the mandible and occurs more frequently in the posterior aspect. Lesions more commonly are unilateral (Fig. 24-1) except for very rare extensive lesions of the maxillofacial region that are bilateral.
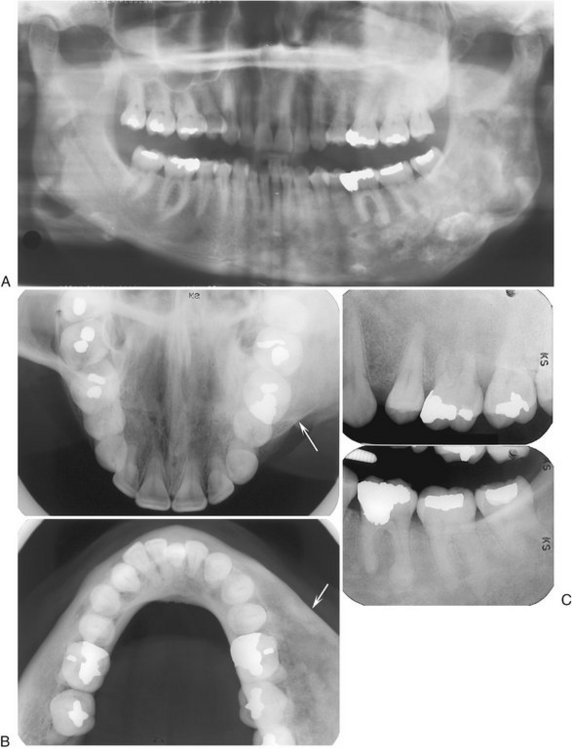
FIG. 24-1 A, Unilateral fibrous dysplasia involving the left maxilla and mandible. B, Note the expansion of the lateral aspect of the maxilla and mandible (arrow) and the increased bone density caused by an increase in the number of internal trabeculae. C, Periapical films show a mixed radiolucent-radiopaque internal structure; however, the overall radiopacity is greater than on the right side of the jaws.
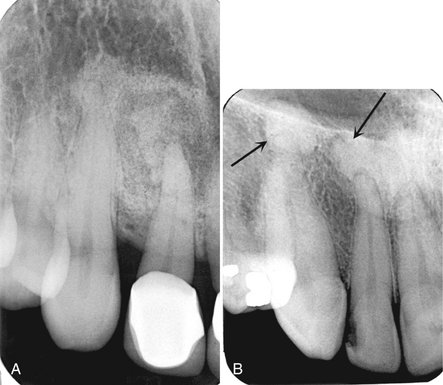
Periphery.: The periphery of fibrous dysplasia lesions most commonly is ill defined, with a gradual blending of normal trabecular bone into an abnormal trabecular pattern. Occasionally the boundary between normal bone and the lesion can appear sharp and even corticated, especially in young lesions (Fig. 24-2).
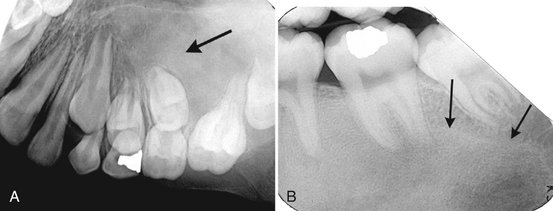
FIG. 24-2 A, Fibrous dysplasia in the posterior maxilla, with an ill-defined anterior margin that blends into the normal bone pattern in the region of the unerupted cuspid. The internal pattern is granular (arrow). B, In contrast, the margin of this case of mandibular fibrous dysplasia has a well-defined, almost corticated margin (arrows).
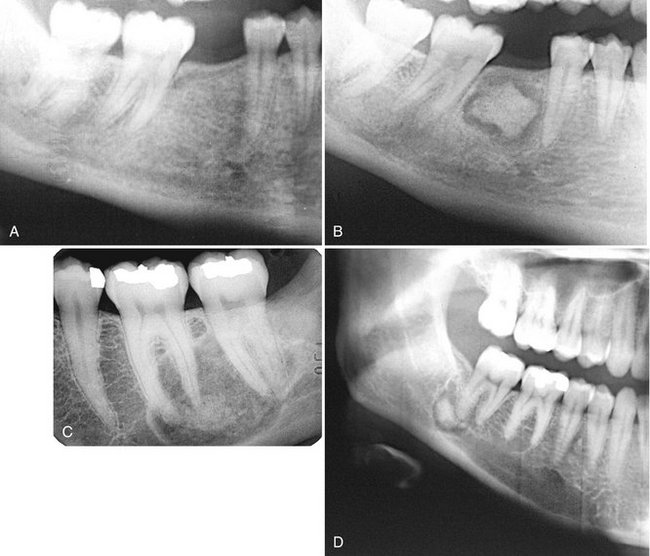
Internal Structure.: The density and trabecular pattern of fibrous dysplasia lesions vary considerably. The variation is more pronounced in the mandible and more homogeneous in the maxilla. The internal aspect of bone may be more radiolucent, more radiopaque, or a mixture of these two variations compared with normal bone (see Fig. 24-1). The internal density is more radiopaque in the maxilla and the base of the skull. Early lesions may be more radiolucent (Fig. 24-3) than are mature lesions and in rare cases may appear to have granular internal septa, giving the internal aspect a multilocular appearance.
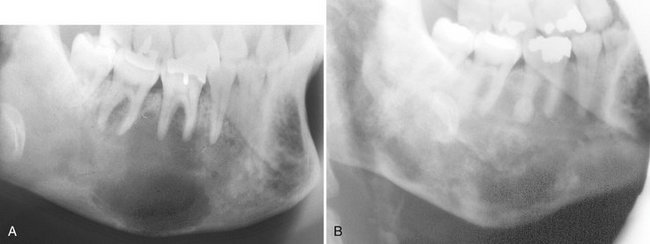
FIG. 24-3 Fibrous dysplasia in the mandible. A, An early radiolucent stage. B, The same case 18 years later shows a more mature radiopaque appearance.
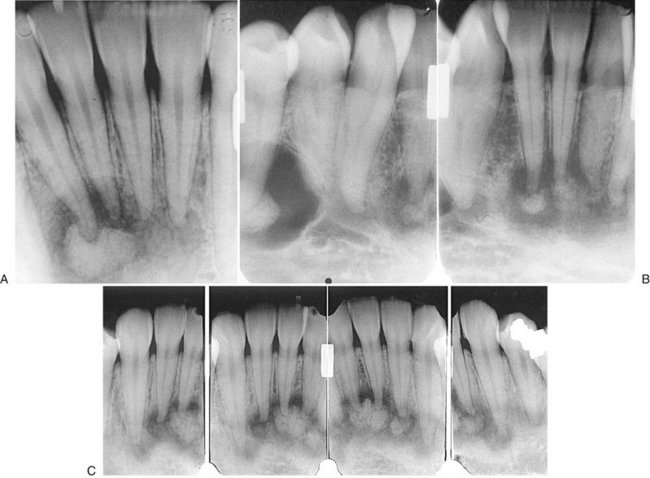
The abnormal trabeculae usually are shorter, thinner, irregularly shaped, and more numerous than normal trabeculae are. This creates a radiopaque pattern that can vary; it may have a granular appearance (or “ground-glass” appearance, resembling the small fragments of a shattered windshield), a pattern resembling the surface of an orange (peau d’orange), a wispy arrangement (cotton wool), or an amorphous, dense pattern (Fig. 24-4). A distinctive characteristic is the organization of the abnormal trabeculae into a swirling pattern similar to a fingerprint (Fig. 24-5). Occasionally, radiolucent regions resembling cysts may occur in mature lesions of fibrous dysplasia. These are bone cavities that are analogous to simple bone cysts (Fig. 24-6).
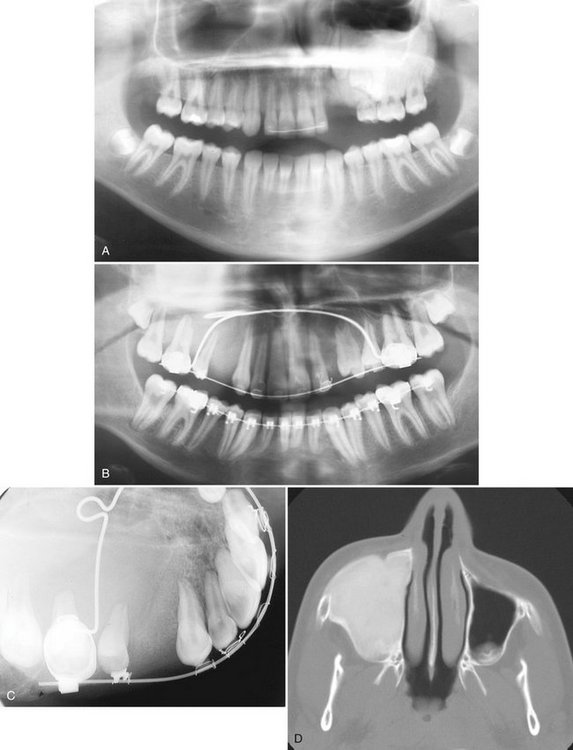
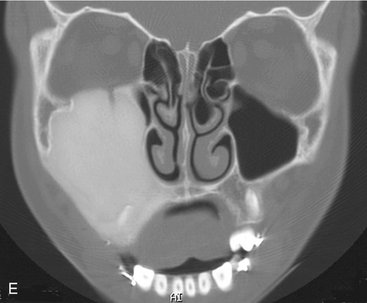
FIG. 24-4 A, Very dense amorphous pattern is seen in a lesion of fibrous dysplasia involving the left maxilla and preventing the normal eruption of the cuspid and the bicuspids. B through E, Panoramic, occlusal, axial, and coronal computed tomographic images of an example of fibrous dysplasia with a homogeneous, dense pattern that occupies most of the right maxillary sinus.
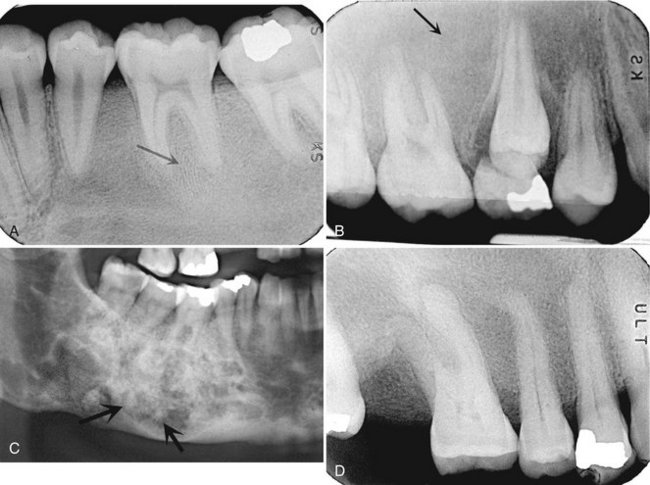
FIG. 24-5 A series of films showing a variety of internal patterns of fibrous dysplasia. A, A fingerprint pattern around the roots of the first molar (arrow). Note the change in the lamina dura around the molars into the abnormal bone pattern. B, A granular, or ground-glass, pattern (arrow). C, A cotton wool pattern. Note the almost circular radiopaque regions (arrows). D, An orange-peel pattern.
Effects on Surrounding Structures.: If the fibrous dysplasia lesion is small, it may have no effect on surrounding structures (subclinical variety). The effects on the involved bone may include expansion with maintenance of a thinned outer cortex (Fig. 24-7). Fibrous dysplasia may expand into the antrum by displacing its cortical boundary and subsequently occupying part or most of the maxillary sinus. Extension into the maxillary antrum usually occurs from the lateral wall, and the last section of the sinus to be involved usually is the most posterosuperior portion. Often the extension into the sinuses appears as a parallel thickening of the outer cortical border, resulting in a residual antral air space that still has approximately the normal anatomic shape of a an antrum (Fig. 24-8). Cortical boundaries such as the floor of the antrum may be changed into the abnormal bone pattern. Often the bone surrounding the teeth is altered without affecting the dentition, and a distinct lamina dura disappears because this bone also is changed into the abnormal bone pattern (see Fig. 24-5). If the fibrous dysplasia increases the bone density, the periodontal ligament space may appear to be very narrow. Fibrous dysplasia can displace teeth or interfere with normal eruption, complicating orthodontic therapy. In rare cases, some root resorption may occur. Involved teeth may have hypercementosis. Fibrous dysplasia appears to be unique in its ability to displace the inferior alveolar nerve canal in a superior direction (Fig. 24-8).
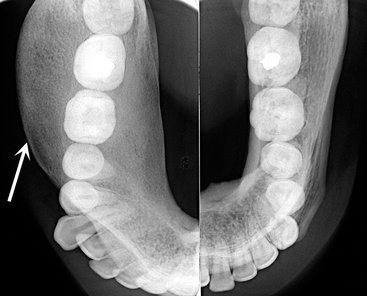
FIG. 24-7 Occlusal views of both sides of the mandible of the same patient. Note the expansion of the right side of the mandible caused by fibrous dysplasia. The outer cortical plates have been displaced and thinned but are still intact (arrow).
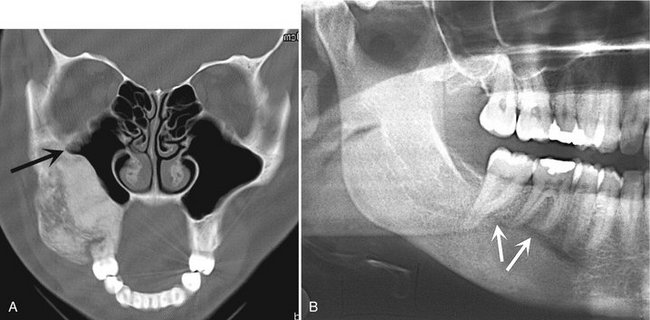
FIG. 24-8 A, A coronal CT scan with bone algorithm of a maxillary lesion of fibrous dysplasia. The lesion has caused the lateral wall of the maxilla to expand into the maxillary antrum. The shape of the lateral wall of the sinus has maintained the zygomatic recess (arrow). B, Mandibular fibrous dysplasia that has displaced the inferior alveolar nerve canal in a superior direction (arrows).
Differential Diagnosis
Other diseases can alter the bone pattern in a similar fashion. Metabolic bone diseases such as hyperparathyroidism may produce a similar pattern. However, these diseases are polyostotic; bilateral; and, unlike fibrous dysplasia, do not cause bone expansion. Paget’s disease may produce a similar pattern and may cause expansion, but it occurs in an older age group, and when it involves the mandible, the whole mandible is involved, unlike the unilateral tendency of fibrous dysplasia. Occasionally periapical cemental dysplasia may show a similar bone pattern, but the distribution is different in that it often is bilateral, with an epicenter in the periapical region. Furthermore, periapical cemental dysplasia also occurs in an older age group. With spontaneous healing of a simple bone cyst, the radiographic and histologic appearance of the new bone may be very similar to that of fibrous dysplasia.
Of paramount importance is the differentiation of osteomyelitis and osteogenic sarcoma because of both radiologic and histologic similarities. Osteomyelitis may result in enlargement of the jaws, but the additional bone is generated by the periosteum; therefore the new bone is laid down on the surface of the outer cortex, and close examination may reveal evidence of the original cortex within the expanded portion of the jaw. Fibrous dysplasia, in contrast, expands the internal aspect of bone, displacing and thinning the outer cortex so that the remaining cortex maintains its position at the outer surface of the bone. The identification of sequestra aids in the identification of osteomyelitis. Osteogenic sarcoma may produce a similar pattern but should show malignant radiologic features (see Chapter 23).
Some difficulty may arise in differentiating cemento-ossifying fibroma of the maxilla, especially the juvenile ossifying fibroma type. If the bone pattern is altered around the teeth without displacement of the teeth from one specific epicenter, the lesion probably is fibrous dysplasia. The shape of the bone expansion of fibrous dysplasia into the antrum reflects the original outer contour of the antral wall, which is different from the more convex extension of a neoplasm.
Management
In most cases the radiographic characteristics of fibrous dysplasia and the clinical information are sufficient to allow the practitioner to make a diagnosis without a biopsy. There are reports of exaggerated growth from stimulation of a lesion during surgical intervention in young patients. A consultation with a dental radiologist is advisable. The radiologist may supplement the examination with computed tomography (CT), which can give a more accurate, three-dimensional representation of the extent of the lesion and can serve as a precise baseline study for future comparisons. It is reasonable to continue occasional monitoring of the lesion or ask the patient to report any changes. With most lesions, growth is complete at skeletal maturation; therefore orthodontic treatment and cosmetic surgery may be delayed until this time. Sarcomatous changes are unusual but have been reported, especially if therapeutic radiation has been given. In the case of female patients, hormonal changes from pregnancy or the use of oral contraceptives may stimulate growth or result in the development of lesions within the area of fibrous dysplasia, such as aneurysmal bone cysts or giant cell granulomas.
CEMENTO-OSSEOUS DYSPLASIAS
Periapical cemental dysplasia and florid osseous dysplasia are essentially the same process but are separated on the basis of the extent of involvement of the jaws.
Synonyms
Cementoma, fibrocementoma, sclerosing cementoma, periapical osteofibrosis, periapical fibrous dysplasia, and periapical fibro-osteoma
Definition
Periapical cemental dysplasia (PCD) is a localized change in normal bone metabolism that results in the replacement of the components of normal cancellous bone with fibrous tissue and cementum-like material, abnormal bone (similar to that seen in fibrous dysplasia), or a mixture of the two. By definition the lesion is located near the apex of a tooth.
Clinical Features
PCD is a common bone dysplasia that typically occurs in middle age; the mean age is 39 years. It occurs nine times more often in females than in males and almost three times more often in blacks than in whites. It also frequently is seen in Asians. The involved teeth are vital, and the patient usually has no history of pain or sensitivity. The lesions usually come to light as an incidental finding during a periapical or panoramic radiographic examination made for other purposes. The lesions can become quite large, causing a notable expansion of the alveolar process, and may continue to enlarge slowly.
Radiographic Features
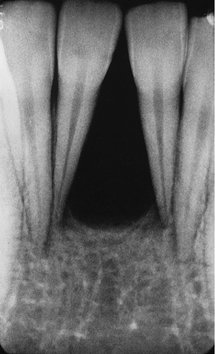
Location.: The epicenter of a PCD lesion usually lies at the apex of a tooth (Fig. 24-9). In rare cases the epicenter is slightly higher and over the apical third of the root. The condition has a predilection for the periapical bone of the mandibular anterior teeth, although any tooth can be involved, and in rare cases the maxillary teeth may be involved (Fig. 24-10). In most cases the lesion is multiple and bilateral, but occasionally a solitary lesion arises. If the involved teeth have been extracted, this lesion can still develop but the periapical location is less evident (Fig. 24-11). In these cases the term cemental dysplasia may be more appropriate.
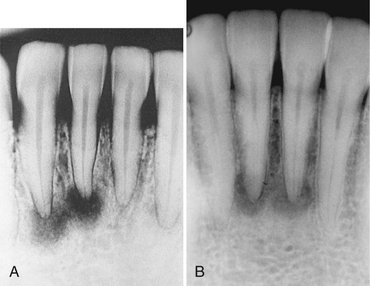
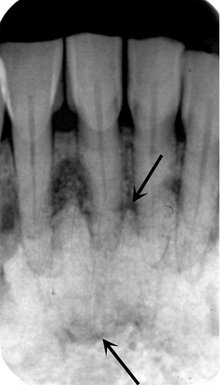
FIG. 24-9 Periapical cemental Dysplasia: Radiolucent Stage.
Two periapical films showing loss of lamina dura; however, the periodontal membrane space can still be seen around some of the teeth.
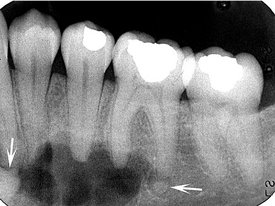
Periphery and Shape.: In most cases the periphery of a PCD lesion is well defined. Often a radiolucent border of varying width is present, surrounded by a band of sclerotic bone that also can vary in width (Fig. 24-12). The sclerotic bone represents a reaction of the immediate surrounding bone. The lesion may be irregularly shaped or may have an overall round or oval shape centered over the apex of the tooth.
Internal Structure.: The internal structure varies, depending on the maturity of the lesion. In the early stage, normal bone is resorbed and replaced with fibrous tissue that usually is continuous with the periodontal ligament (causing loss of the lamina dura). Radiographically, this appears as a radiolucency at the apex of the involved tooth (see Fig. 24-9).
In the mixed stage, radiopaque tissue appears in the radiolucent structure. This material usually is amorphous; has a round, oval, or irregular shape; and is composed of cementum or abnormal bone (Fig. 24-12). Sometimes the cementum-like material forms a swirling pattern (Fig. 24-13). These structures sometimes are called cementicles; however, this is a radiographic term that does not necessarily represent the histologic appearance. In rare cases the radiopaque material resembles the abnormal trabecular patterns seen in fibrous dysplasia (Fig. 24-13).
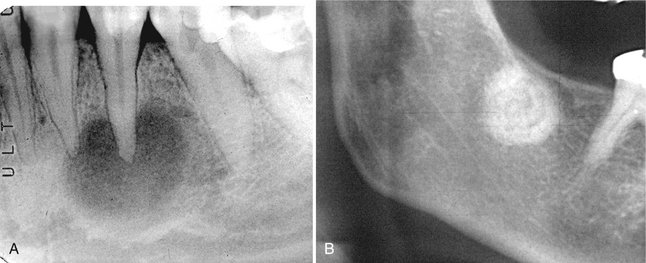
FIG. 24-13 A, Periapical cemental dysplasia with a fibrous dysplasia type of internal bone pattern. B, A swirling internal pattern of cemental dysplasia.
In the mature stage, the internal aspect may be totally radiopaque without any obvious pattern. Usually, a thin radiolucent margin can be seen at the periphery because this lesion matures from the center outward (Fig. 24-14). Occasionally, this radiolucent margin is not apparent, which makes the differential diagnosis more difficult. The internal structure may appear dramatically radiolucent if cavities resembling simple bone cysts form within the cemental lesions (Fig. 24-15). In some cases the simple bone cyst extends beyond the original margin of the cemental lesion.
Effects on Surrounding Structures.: The normal lamina dura of the teeth involved with the lesion is lost, making the periodontal ligament space either less apparent or giving it a wider appearance (see Fig. 24-9). The tooth structure usually is not affected, although in rare cases some root resorption may occur. Also, occasionally hypercementosis occurs on the root of a tooth positioned within the lesion. Some lesions stimulate a sclerotic bone reaction from the surrounding bone. Small lesions do not cause expansion of the involved jaw. However, larger lesions may cause expansion of the jaw, an area that is always bordered by a thin, intact outer cortex similar to that seen in fibrous dysplasia. The expansion is usually undulating in shape. This lesion may elevate the floor of the maxillary antrum.
Differential Diagnosis
In early (radiolucent) PCD lesions, the most important differential diagnosis is periapical rarefying osteitis. Occasionally PCD cannot be distinguished from this inflammatory lesion by radiographic characteristics alone. In these cases the final diagnosis must rely on clinical information such as testing of the vitality of the involved tooth.
In the case of a solitary mature form of PCD, the differential diagnosis may include a benign cementoblastoma, especially when the lesion is periapical to the mandibular first molar. This tumor is usually is attached to the surface of the root, which may be partly resorbed. Also, the peripheral soft tissue capsule is better defined and there may be a unique pattern to the internal structure, such as a radiating pattern. Expansion caused by the tumor is more concentric and less undulating than in PCD. The presence or absence of clinical symptoms may help distinguish PCD from benign cementoblastoma. Another lesion to consider is an odontoma. Odontomas often start occlusal to a tooth and prevent its eruption, but some odontomas may have a periapical position. The organization of the internal aspect into toothlike structures and the identification of enamel (very radiopaque) can help in the differential diagnosis. Also, the peripheral cortex and soft tissue capsule of an odontoma are more uniform in width and better defined than is the periphery of PCD. In mature PCD lesions, the appearance may resemble that of a dense bone island. The finding of a radiolucent periphery, even if very slight, indicates a diagnosis of PCD. Solitary lesions may be difficult to differentiate from a cemento-ossifying fibroma. Cases that have been quiescent and then suddenly start to grow aggressively suggest that there may be a continuum between the category of cemento-osseous dysplasia and cemento-osseous tumors.
Management
The diagnosis of PCD can be made on the basis of the appropriate radiologic and clinical characteristics. In fact, a possible complication of biopsy is secondary infection, which may occur in lesions that have abundant cementum formation and poor vascularity. Normally treatment is not required. However, if the teeth have been removed and if considerable atrophy of the alveolar ridge has occurred, these segments of cementum may reach the mucosal surface, much in the same way as stones become exposed in old, worn concrete. These pieces of cementum can perforate the mucosa when positioned under a denture, and the result is secondary infection. If this occurs, the pieces of cementum may have to be removed surgically because they can act as sequestra in osteomyelitis.
Definition
Florid osseous dysplasia (FOD) is a widespread form of PCD. Normal cancellous bone is replaced with dense acellular cemento-osseous tissue in a background of fibrous connective tissue. The lesion has a poor vascular supply, a condition that likely contributes to its susceptibility to infection. In some cases a familial trend can be seen. No clear definition indicates when multiple regions of PCD should be termed FOD. However, if PCD is identified in three or four quadrants or is extensive throughout one jaw, it usually is considered to be FOD.
Clinical Features
Several key similarities exist between FOD and PCD, including the age, sex, and racial profiles of patients and comparable radiographic and histologic appearances. Most patients with FOD are female and middle aged (mean age, 42 years), although the age range is broad. The condition shows a marked predilection for blacks and Asians. A few documented cases appear to have a familial pattern. Often FOD produces no symptoms and is found incidentally during a radiographic examination. Occasionally patients complain of low-grade, intermittent, poorly localized pain in the affected bone, especially when a simple bone cyst has developed within the lesion. Extensive lesions often have an associated bony swelling. If the lesions become secondarily infected, features of osteomyelitis may develop, including mucosal ulceration, fistulous tracts with suppuration, and pain. In fact, historically, FOD that was secondarily infected was diagnosed as chronic sclerosing osteomyelitis without the identification of the underlying bone dysplasia. A CT examination should be ordered to determine the extent of involvement with osteomyelitis. Teeth in the involved bone are vital unless other dental disease coincidentally affects them.
Radiographic Features
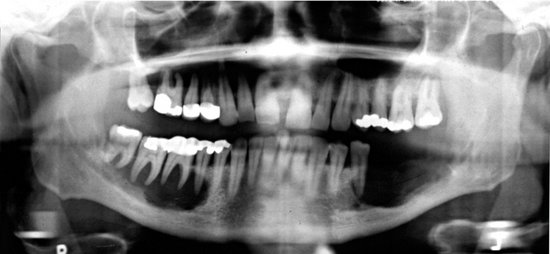
Location.: FOD lesions usually are bilateral and present in both jaws (Fig. 24-16). However, when they are present in only one jaw, the mandible is the more common location. The epicenter is apical to the teeth, within the alveolar process and usually posterior to the cuspid. In the mandible, lesions occur above the inferior alveolar canal.
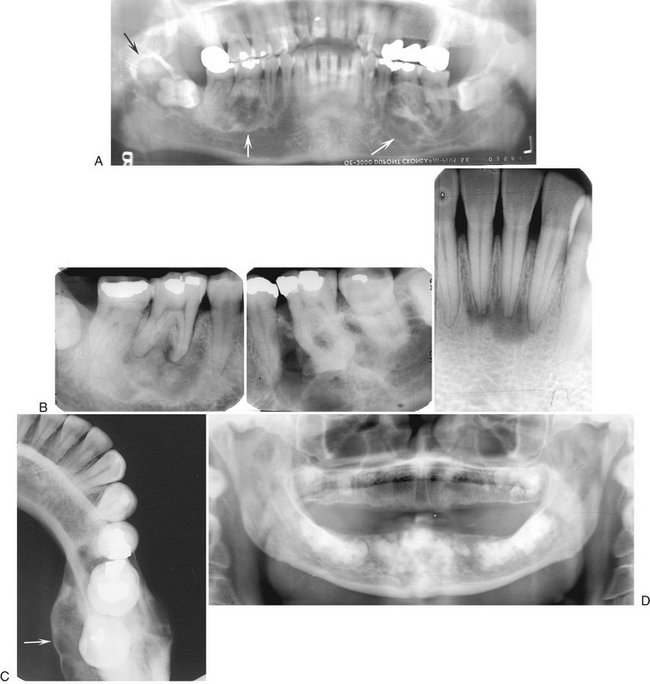
FIG. 24-16 Florid Osseous Dysplasia.
A, Three mixed radiopaque-radiolucent lesions in the periapical regions throughout the jaws (arrows); although the right third molar is horizontally impacted, the lesion still has a periapical relationship (black arrow). B, Composite of periapical films of the same case; note the appearance of the lesions involving the mandibular incisors (not apparent in the panoramic film), which are identical to periapical cemental dysplasia. C, Occlusal film of the left mandibular lesion showing undulating expansion of the medial cortical plate (arrow). D, Panoramic film of a different case showing multiple, very mature, almost totally radiopaque lesions in edentulous jaws. The epicenter of all lesions is above the inferior alveolar canal.
Periphery.: The periphery usually is well defined and has a sclerotic border that can vary in width, very similar to PCD. The soft tissue capsule may not be apparent in mature lesions.
Internal Structure.: The density of the internal structure can vary from an equal mixture of radiolucent and radiopaque regions to almost complete radiopacity. Some prominent radiolucent regions, which usually represent the development of a simple bone cyst, may be present (Fig. 24-17). These cysts may enlarge with time, even beyond the boundary of the lesion into the surrounding normal bone, or may fill in with abnormal dysplastic cemento-osseous tissue. The radiopaque regions can vary from small oval and circular regions (cotton-wool appearance) to large, irregular, amorphous areas of calcification. These calcified masses are similar in appearance to those seen in mature PCD lesions.
Effects on Surrounding Structures.: Large FOD lesions can displace the inferior alveolar nerve canal in an inferior direction. FOD also can displace the floor of the antrum in a superior direction and can cause enlargement of the alveolar bone by displacement of the buccal and lingual cortical plates. The roots of associated teeth may have a considerable amount of hypercementosis, which may fuse with the abnormal surrounding cemental tissue of the lesion. Extraction of these teeth may be difficult.
Differential Diagnosis
The fact that FOD is bilateral and centered in the alveolar process helps in the differentiation from other lesions. Paget’s disease of bone may also show cotton wool–type radiopaque regions with associated hypercementosis. However, Paget’s disease affects the bone of the entire mandible, whereas FOD is centered above the inferior alveolar canal. Furthermore, Paget’s disease often is polyostotic, involving other bones as well as the jaws. The well-defined nature of FOD, with its radiolucent periphery and surrounding sclerotic border, also is useful in making the differential diagnosis.
Another disease that may resemble FOD is chronic sclerosing osteomyelitis. Regions of cementum-like masses may appear similar to the sequestrum seen in osteomyelitis. This is not to be confused with a situation where FOD has become secondarily infected, resulting in osteomyelitis. The cemental-like masses that are secondarily infected have a wider and more profound radiolucent border (Fig. 24-18). CT imaging is essential for the diagnosis and to determine the extent of the osteomyelitis within the FOD.
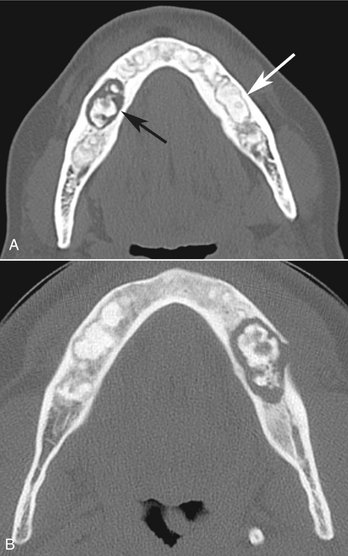
FIG. 24-18 A, Axial CT image with bone algorithm of a case of florid osseous dysplasia (FOD); multiple foci of cemental dysplasia are bordered by a soft tissue capsule (white arrow) and a cemental mass has become secondarily infected with a wider and more pronounced radiolucent border (black arrow). B, Axial CT image of a different case of osteomyelitis from secondarily infected FOD; note the break in the outer cortex where the lesion is draining into the surrounding soft tissues.
Management
Under normal circumstances, FOD does not require treatment, although there is value in obtaining a panoramic film to establish the extent of the disease. Unlike with fibrous dysplasia, no age limit is apparent for the cessation of growth of FOD. Because of the propensity for development of secondary infections in FOD, the patient should be encouraged to maintain an effective oral hygiene program to avoid odontogenic infections. Also, if the teeth are extracted and severe atrophy of the alveolar process occurs, as in PCD, the cementum-like masses emerge and the pressure of the overlying denture may cause dehiscence in the mucosa, resulting in osteomyelitis. If this occurs, the avascular cementum-like masses become large sequestra. The osteomyelitis may spread slowly throughout the jaw from one region of FOD to another. It may be necessary to remove large areas of cementum-like tissue, leaving very little residual bone for prosthetic treatment.
Other Lesions of Bone
Definition
Cemento-ossifying fibroma (COF) is classified as and behaves like a benign bone neoplasm. However, it appears in this chapter because it often is considered to be a type of fibro-osseous lesion. This bone tumor consists of highly cellular, fibrous tissue that contains varying amounts of abnormal bone or cementum-like tissue. In the past this lesion was classified as two different entities depending on whether bone or cementum was the predominant calcified product. When the histologic appearance of most of the calcified tissue was of irregular trabeculae of woven bone, the term ossifying fibroma was used. The resulting internal pattern may be very similar to or indistinguishable from that of fibrous dysplasia. One distinguishing feature that may be present is a soft tissue capsule at the periphery, not seen in fibrous dysplasia. When the predominant calcified component was cementum, the term cementifying fibroma was used. However, the microscopic appearance of an ossifying fibroma and a cementifying fibroma can be very similar, and the two are now thought to represent a spectrum of one disease and are combined under the name cemento-ossifying fibroma.
Juvenile ossifying fibroma is a very aggressive form of COF that occurs in the first two decades of life. Although the histopathologic definition of this entity is controversial, the radiologic appearance has similarities to that of COF.
Clinical Features
The clinical features of COF can vary from indolent to aggressive behavior. The characteristics are more like those of a tumor than a bone dysplasia. COF can occur at any age but usually is found in young adults. Females are affected more often than males. The disease usually is asymptomatic at the time of discovery. Occasionally facial asymmetry develops. Displacement of the teeth may be an early clinical feature, although most lesions are discovered during routine dental examinations. In cases of juvenile ossifying fibroma, rapid growth may occur in a young patient, resulting in deformity of the involved jaw.
Radiographic Features
Location.: COF appears almost exclusively in the facial bones and most commonly in the mandible, typically inferior to the premolars and molars and superior to the inferior alveolar canal. In the maxilla it occurs most often in the canine fossa and zygomatic arch area.
Periphery.: The borders of COF lesions usually are well defined. A thin, radiolucent line, representing a fibrous capsule, may separate it from surrounding bone (Fig. 24-19). Sometimes the bone next to the lesion develops a sclerotic border.
Internal Structure.: The internal structure of a COF lesion is a mixed radiolucent-radiopaque density with a pattern that depends on the amount and form of the manufactured calcified material. In some instances the internal structure may appear almost totally radiolucent with just a hint of calcified material. In the type that contains mainly abnormal bone, the pattern may be similar to that seen in fibrous dysplasia, or a wispy (similar to stretched tufts of cotton) or flocculent pattern (similar to large, heavy snowflakes) may be seen (Fig. 24-20). Lesions that produce more cementum-like material may contain solid, amorphous radiopacities (cementicles) similar to those seen in cemental dysplasia (see Fig. 24-19).
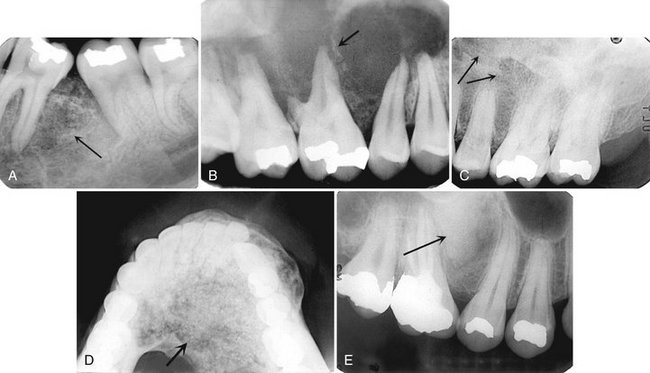
FIG. 24-20 Various Bone Patterns Seen in Cemento-Ossifying Fibromas.
A, A wispy trabecular pattern (arrow). B, Most of this pattern is radiolucent with a few wispy trabeculae (arrow). C, A fibrous dysplasia, granular-like pattern (arrows). D, A flocculent pattern with larger tufts of bone formation (arrow). E, A solid, radiopaque, cementum-like pattern (arrow).
Effects on Surrounding Structures.: COF can be distinguished from the previously mentioned bone dysplasias by its tumorlike behavior. This is reflected in the growth of the lesion, which tends to be concentric within the medullary part of the bone with outward expansion approximately equal in all directions. This can result in displacement of teeth or of the inferior alveolar canal and expansion of the outer cortical plates of bone. A significant point is that the outer cortical plate, although displaced and thinned, remains intact. The COF lesion can grow into and occupy the entire maxillary sinus (Fig. 24-21), expanding its walls outward; however, a bony partition always exists between the internal aspect of the remaining sinus and the tumor. The lamina dura of involved teeth usually is missing, and resorption of teeth may occur.
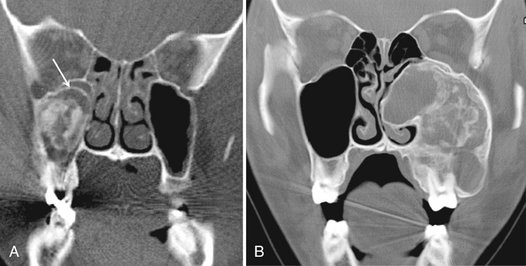
FIG. 24-21 Large cemento-ossifying fibromas involving the maxilla. A, A coronal CT scan of a lesion invaginating the maxillary antrum. Unlike in fibrous dysplasia, the peripheral border of the lesion (arrow) does not parallel the original shape of the antrum. B, A coronal CT scan of a larger lesion expanding the maxilla, occupying all the maxillary antrum and extending into the nasal fossa.
Differential Diagnosis
The differential diagnosis of COF includes lesions with a mixed radiolucent-radiopaque internal structure. The differentiation from fibrous dysplasia can be very difficult. The boundaries of a COF lesion usually are better defined, and these lesions occasionally have a soft tissue capsule and cortex, whereas fibrous dysplasia usually blends in with surrounding bone. The internal structure of fibrous dysplasia lesions in the maxilla may be more homogeneous and may show less variation. Both types of lesions can displace teeth, but COF displaces from a specific point or epicenter. Fibrous dysplasia rarely resorbs teeth. The expansion of the jaws associated with COF is more concentric about a definite epicenter but fibrous dysplasia enlarges the bone while distorting the overall shape to a smaller degree; in other words, the expanded bone still resembles normal morphology.
Great difficulty may arise in differentiating ossifying fibroma from fibrous dysplasia when the lesion involves the maxillary antrum. Fibrous dysplasia usually displaces the lateral wall of the maxilla into the maxillary antrum, maintaining the outer shape of the wall, whereas an ossifying fibroma has a more convex shape because it extends into the maxillary antrum (see Figs. 24-8 and 24-21). Also, fibrous dysplasia may change the bone around the teeth without displacing them from an obvious epicenter of a concentrically growing benign tumor. The importance of this differentiation lies in the treatment, which is resection for an ossifying fibroma and observation for fibrous dysplasia.
The differential diagnosis of the type of COF that produces mainly cementum-like material from PCD may be difficult, especially with large single lesions of PCD. However, cemental dysplasia usually is multifocal where as COF is not. Also, the presence of a simple bone cyst is a characteristic of cemental dysplasia. COF behaves in a more tumorlike fashion, with the displacement of teeth and concentric expansion. A wide sclerotic border and undulating expansion are more characteristic of the slow-growing cemental dysplasia.
Other lesions to be considered include those that may have internal calcifications similar to the pattern seen in COF. These include giant cell granuloma, calcifying odontogenic cysts, calcifying epithelial odontogenic (Pindborg) tumors, and adenomatoid odontogenic tumors.
Occasionally, the diagnosis of osteogenic sarcoma is considered. However, characteristics suggesting a malignant lesion should be seen, such as cortical bone destruction and invasion into the surrounding soft tissues and along the periodontal ligament space.
Management
The prognosis of COF is favorable with surgical enucleation or resection. Large lesions require a detailed determination of the extent of the lesion, which can be obtained with CT imaging. Even if the lesion has reached appreciable size, it usually can be separated from the surrounding tissue and completely removed. Recurrence after removal is unlikely.
Definition
Central giant cell granuloma (CGCG) is thought to be a reactive lesion to an as-yet-unknown stimulus and not a neoplastic lesion. However, radiographically the characteristics of the lesion are similar to those of a benign tumor and occasionally maxillary lesions may have some malignant-type characteristics. The histologic appearance consists primarily of fibroblasts, numerous vascular channels, multinucleated giant cells, and macrophages. The relationship of the benign giant cell tumor to the giant cell granuloma is controversial and unclear.
Clinical Features
CGCG is a common lesion in the jaws that affects mostly adolescents and young adults; at least 60% of cases occur in individuals younger than 20 years. The most common presenting sign of CGCG is painless swelling. Palpation of the suspect bone area may elicit tenderness, although in a minority of cases the patient may complain of pain. The overlying mucosa may have a purple color. Some of these lesions cause no symptoms and are found only on routine examination. The lesion usually grows slowly, although it may grow rapidly, creating the suspicion of a malignancy.
Radiographic Features
Location.: Lesions develop in the mandible twice as often as in the maxilla. In the fist two decades there is a tendency for the epicenter of the lesion to be anterior to the first molar in the mandible and anterior to the cuspid in the maxilla. However, in older individuals this lesion can occur in greater frequency in the posterior aspect of the jaws.
Periphery.: Because this neoplasm grows relatively slowly, it usually produces a well-defined radiographic margin in the mandible. In most cases the periphery shows no evidence of cortication. Lesions in the maxilla may have ill-defined, almost malignant-appearing, borders.
Internal Structure.: Some CGCG lesions show no evidence of internal structure (Fig. 24-22), especially small lesions. Other cases have a subtle granular pattern of calcification that may require a bright light source behind the film to enable visualization. Occasionally this granular bone is organized into ill-defined, wispy septa (Fig. 24-23). If present, these granular septa are characteristic of this lesion, especially if they emanate at right angles from the periphery of the lesion. This characteristic is even stronger if a small indentation of the expanded cortical margin is seen at the point where this right-angle septum originates (Fig. 24-24). In some instances the septa are better defined and divide the internal aspect into compartments, creating a multilocular appearance.
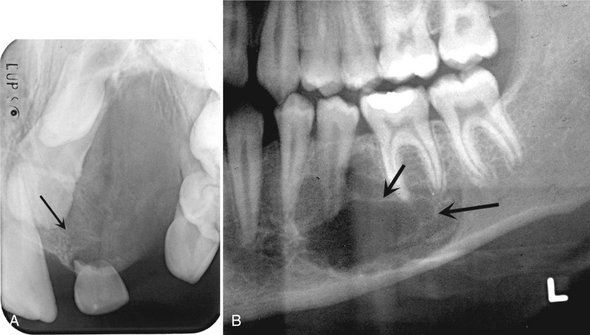
FIG. 24-23 Various internal patterns seen in giant cell granulomas. A, A lesion in the anterior maxilla with a very fine granular pattern (arrow). B, A portion of a panoramic film showing wispy, ill-defined internal septa (arrows).
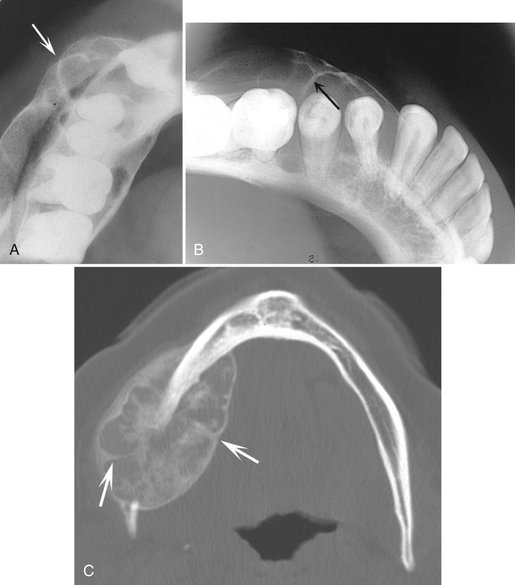
FIG. 24-24 Characteristic expansion of the outer cortical plates caused by giant cell granulomas. Note the uneven expansion in A (arrow) and the indentation of the expansion with a right-angled septum in B (arrow). C, Axial CT scan with bone algorithm revealing a giant cell granuloma within the mandible causing undulating expansion and containing two right-angled septa (arrows).
Effects on Surrounding Structures.: Giant cell granulomas often displace and resorb teeth. The resorption of tooth roots is not a constant feature, but when it occurs, it may be profound and irregular in outline. The lamina dura of teeth within the lesion usually is missing. The inferior alveolar canal may be displaced in an inferior direction. This lesion has a strong propensity to expand the cortical boundaries of the mandible and maxilla. The expansion usually is uneven or undulating in nature, which may give the appearance of a double boundary when the expansion is viewed by use of occlusal film. The bone forming the border of the expanded mandible often has a granular texture compared with cortical bone (see Fig. 24-23, C). In some instances the outer cortical plate of bone is destroyed instead of expanded; this occurs more often in the maxilla, where the cortical bone destruction may give the lesion a malignant appearance.
Differential Diagnosis
If the internal structure of the CGCG contains septa, the differential diagnosis may include ameloblastoma, odontogenic myxoma, and aneurysmal bone cyst. If a granular internal structure is present, COF may be considered. Useful characteristics for differentiating an ameloblastoma include the following: ameloblastomas tend to occur in an older age group and more often in the posterior mandible, and ameloblastomas have coarse, curved, well-defined trabeculae, whereas giant cell granulomas have wispy, ill-defined trabeculae, some of which are at right angles to the periphery. Odontogenic myxomas occur in an older age group, may have sharper and straighter septa, and do not have the same propensity to expand as do giant cell granulomas. Interestingly, aneurysmal bone cysts can appear identical radiographically to giant cell granulomas, especially in the appearance of the internal septa. However, aneurysmal bone cysts are comparatively rare lesions that occur more often in the posterior aspect of the jaws and usually cause profound expansion.
A small CGCG lesion with a totally radiolucent internal structure may be similar in appearance to a cyst, especially a simple bone cyst. Evidence of displacement or resorption of the adjacent teeth or expansion of the outer cortical bone is more characteristic of a giant cell granuloma. The radiographic image and histologic appearance of brown tumors of hyperparathyroidism may be identical to those of CGCG. Also, the appearance may be identical to that seen in cherubism; however, the lesions in cherubism or multiple and have epicenters that are located in the most posterior aspect of the mandible and maxilla.
Management
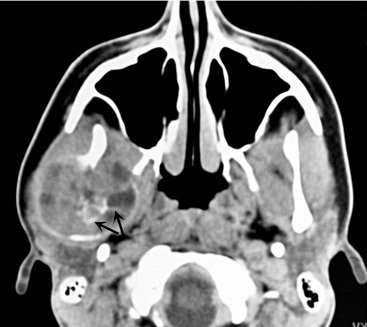
If the lesion is in the maxilla, CT scans can be used to establish the exact extent and involvement of surrounding structures, such as the maxillary antrum or nasal cavity. Also, CT imaging is required for large lesions, which pose the possibility of destruction of the outer cortical bone, to determine whether the adjacent soft tissue has been invaded. Occasionally this lesion behaves very aggressively. If CGCG occurs after the second decade of life, hyperparathyroidism should be considered and serum testing for elevated calcium or parathormone levels or full-body technetium bone scans can be ordered.
Treatment may include enucleation and curettage and in some instances resection of the jaw. The patient should be followed up carefully to rule out recurrence, especially if conservative treatment is used. Recurrences are rare and are more common in the maxilla.
Definition
An aneurysmal bone cyst (ABC) usually is considered to be a reactive lesion of bone rather than a cyst or true neoplasm. Some believe that it represents an exaggerated localized proliferative response of vascular tissue in bone. This lesion may be related to the CGCG because of similarities in both the radiographic and histologic appearance (presence of giant cells). ABCs occasionally develop in association with other primary lesions such as fibrous dysplasia, central hemangioma, giant cell granuloma, and osteosarcoma. Its etiology remains unclear.
Clinical Features
More than 90% of reported jaw lesions have occurred in individuals younger than 30 years. The condition appears to have a predilection for females. An ABC in the jaw usually manifests as a fairly rapid bony swelling (usually buccal or labial). Pain is an occasional complaint, and the involved area may be tender on palpation.
Radiographic Features
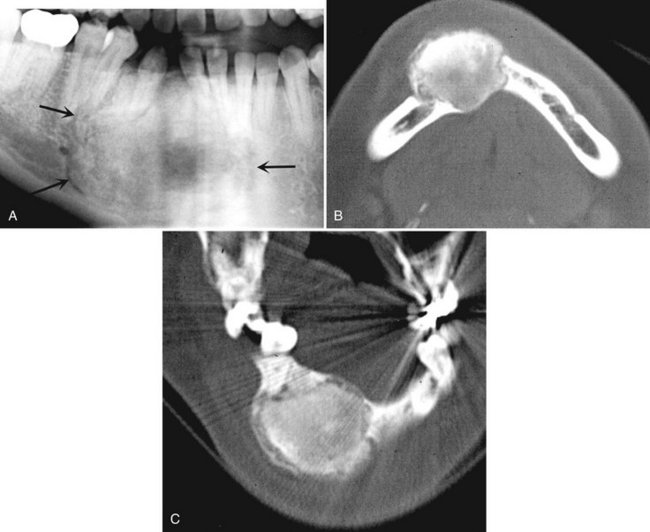
Location.: The mandible is involved more often than the maxilla (ratio of 3 : 2), and the molar and ramus regions are more involved than the anterior region (Fig. 24-25).
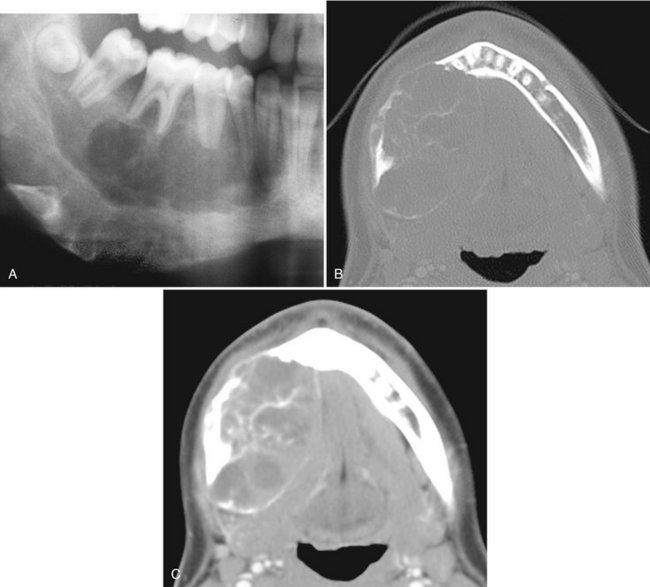
FIG. 24-25 A, A cropped panoramic image of aneurysmal bone cyst occupying the body of the right mandible. Two axial CT images at the same level of this case with bone algorithm (B); note the wispy, faint septa and soft tissue algorithm (C) and the low-attenuation regions of the internal structure representing fluid density.
Periphery and Shape.: The periphery usually is well defined, and the shape is circular or “hydraulic.”
Internal Structure.: Small initial lesions may show no evidence of an internal structure. Often the internal aspect has a multilocular appearance. The septa bear a striking resemblance to the wispy, ill-defined septa seen in giant cell granulomas (Figs. 24-25 and 24-26). Another similar finding is septa positioned at right angles to the outer expanded border. In CT soft tissue algorithm images, there may be more radiolucent regions, some of which have a roughly circular shape. These likely represent large vascular spaces.
Effects on Surrounding Structures.: After an ABC becomes large, there is a strong propensity for extreme expansion of the outer cortical plates (see Figs. 24-25 and 24-26). This characteristic is more dramatic in these cysts than in most other lesions. ABCs can displace and resorb teeth.
Differential Diagnosis
The multilocular appearance of ABCs most resembles that of giant cell granulomas; in fact, the radiographic appearance of the two lesions may be identical. However, ABCs may expand to a greater degree, and they are more common in the posterior parts of the mandible. Ameloblastoma may be considered, but this lesion usually occurs in an older age group. ABCs may show a similarity to cherubism, which interestingly has giant cell–like features, but cherubism is a multifocal bilateral disease.
The diagnosis is based on biopsy results. A hemorrhagic aspirate favors the diagnosis of ABC. A CT scan also is recommended to better determine the extent of the lesion.
Management
Surgical curettage and partial resection are the primary means of treatment. The recurrence rate is fairly high, ranging from 19% to about 50% after curettage and approximately 11% after resection. This indicates a need for careful follow-up.
Definition
Cherubism is a rare inherited autosomal dominant disease that causes bilateral enlargement of the jaws, giving the child a cherubic facial appearance. Rare unilateral lesions have been reported. The term familial fibrous dysplasia was an unfortunate choice of early terminology because this lesion is not a bone dysplasia. It is composed of giant cell granuloma–like tissue and does not form a bone matrix. These lesions regress with age.
Clinical Features
Cherubism develops in early childhood between 2 and 6 years of age. The most common presenting sign is a painless, firm, bilateral enlargement of the lower face. Enlargement of the submandibular lymph nodes may occur, but no systemic abnormalities are involved. Because children’s faces are rather chubby, mild cases may go undetected until the second decade. Profound swelling of the maxilla may result in stretching of the skin of the cheeks, which depresses the lower eyelids, exposing a thin line of sclera and causing an “eyes raised to heaven” appearance.
Radiographic Features
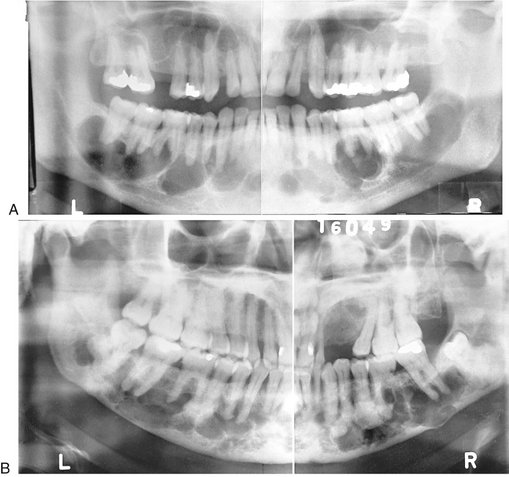
Location.: This lesion is bilateral and often affects both jaws. When it is present in only one jaw, the mandible is the most common location. The epicenter is always in the posterior aspect of the jaws, in the ramus of the mandible or the tuberosity of the maxilla (Fig. 24-27). The lesion grows in an anterior direction and in severe cases can extend almost to the midline.
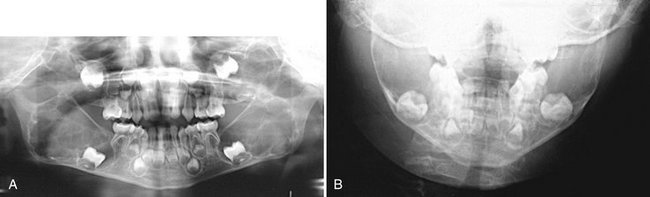
FIG. 24-27 A case of cherubism. A, A panoramic image showing four lesions in the maxilla and mandible. The epicenters of the lesions are in the maxillary tuberosity and mandibular ramus; note the anterior displacement of the unerupted maxillary first molars. The internal structure contains ill-defined septa. B, A portion of the posteroanterior skull view showing expansion of the mandible.
Internal Structure.: The internal structure resembles that of CGCG, with fine, granular bone and wispy trabeculae forming a prominent multilocular pattern.
Effects on Surrounding Structures.: Expansion of the cortical boundaries of the maxilla and mandible by cherubism can result in severe enlargement of the jaws. Maxillary lesions enlarge into the maxillary sinuses. Because the epicenter is in the posterior aspect of the jaws, the teeth are displaced in an anterior direction. The degree of displacement can be severe, and with some lesions the tooth buds are destroyed.
Differential Diagnosis
Although the radiographic appearance of cherubism may be similar to that of giant cell granuloma, the fact that cherubism is bilateral with an epicenter in the ramus should provide a clear differentiation. The differentiation of cherubism from fibrous dysplasia should not present any difficulties because fibrous dysplasia is more commonly a unilateral disease; also, the multilocular appearance and anterior displacement of teeth are more characteristic of cherubism. Cherubism may bear some similarity to multiple odontogenic keratocysts in basal cell nevus syndrome. The bilateral symmetry of cherubism, along with the anterior displacement of teeth and multilocular appearance, are characteristics that will help with the differential diagnosis.
Management
The distinctive radiographic features of cherubism may be more diagnostic than the histopathologic findings; therefore the diagnosis can rely on the radiologic findings alone. Treatment can be delayed because the cystlike lesions usually become static and fill in with granular bone during adolescence and at the end of skeletal growth. After skeletal growth has stopped, conservative surgical procedures, if required, may be done for cosmetic problems. Surgery also may be required to uncover displaced teeth, and orthodontic treatment may be needed.
Definition
Paget’s disease is a skeletal disorder and essentially a disease involving osteoclasts, resulting in abnormal resorption and apposition of osseous tissue in one or more bones. The disease may involve many bones simultaneously, but it is not a generalized skeletal disease. It is initiated by an intense wave of osteoclastic activity, with resorption of normal bone resulting in irregularly shaped resorption cavities. After a period of time, vigorous osteoblastic activity ensues, forming woven bone. Paget’s disease is seen most frequently in Britain and Australia and somewhat less often in North America.
Clinical Features
Paget’s disease is primarily a disease of later middle and old age, having an incidence of about 3.5% in individuals older than 40 years. The incidence of involvement in males is approximately twice that of females at age 65 years.
Affected bone is enlarged and commonly deformed, resulting in bowing of the legs, curvature of the spine, and enlargement of the skull. The jaws also enlarge when affected. Separation and movement of teeth may occur, causing malocclusion. Dentures may be tight or may fit poorly in edentulous patients.
Bone pain is an inconsistent symptom, most often directed toward the weight-bearing bones; facial or jaw pain is uncommon. Patients with Paget’s disease may also have ill-defined neurologic pain as the result of bone impingement on foramina and nerve canals. Patients with Paget’s disease often have severely elevated levels of serum alkaline phosphatase (greater than with any other disorder) during osteoblastic phases of the disease. These patients also often have high levels of hydroxyproline in the urine.
Radiographic Features
Location.: Paget’s disease occurs most often in the pelvis, femur, skull, and vertebrae and infrequently in the jaws (Fig. 24-28). It affects the maxilla about twice as often as the mandible. Whenever the jaws are involved, it is important to note that the entire mandible or maxilla is affected. Although this disease is bilateral, occasionally it affects only one maxilla or the involvement may be significantly greater on one side.
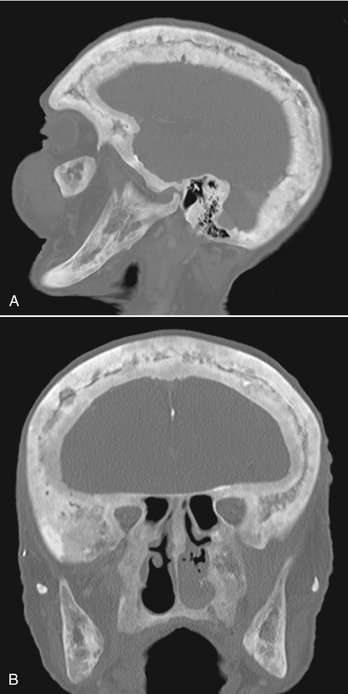
FIG. 24-28 A, Sagittal and, B, coronal bone algorithm CT images of a case of Paget’s disease involving all the cranial bones and the maxilla and mandible; note the increase in bone density and dimension between the internal and outer cortex of the skull. The coronal CT image demonstrates enlargement of the mandibular ramus.
Internal Structure.: Generally the appearance of the internal structure depends on the developmental stage of the disease. Paget’s disease has three radiographic stages, although these often overlap in the clinical setting: an early radiolucent resorptive stage, a granular or ground glass–appearing second stage, and a denser, more radiopaque appositional late stage. These stages are less apparent in the jaws.
The trabeculae are altered in number and shape. Most often they increase in number, but in the early stage they may decrease. The trabeculae may be long and may align themselves in a linear pattern (Fig. 24-29), which is more common in the mandible. They also may be short, with random orientation, and may have a granular pattern similar to that of fibrous dysplasia. A third pattern occurs when the trabeculae may be organized into rounded, radiopaque patches of abnormal bone, creating a cotton-wool appearance (Fig. 24-30).
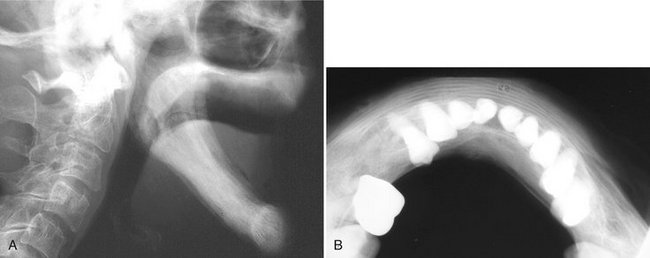
FIG. 24-29 A, An edentulous mandible involved with Paget’s disease. B, an occlusal film of another case; note the loss of normal outer cortex and the linear alignment of trabeculae. (B courtesy Dr. Ross Macdonald, Adelaide, Australia.)
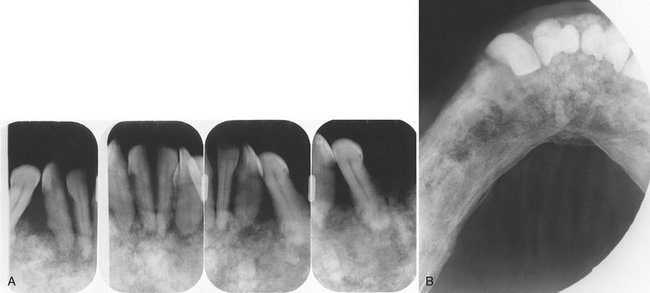
FIG. 24-30 Paget’s Disease.
A, Multiple radiopaque masses in the mandible that have a cotton-wool appearance. B, Note the expansion of the mandible and the maintenance of a thin outer cortical plate.
The overall density of the jaws may decrease or increase, depending on the number of trabeculae. Often the disease produces areas of bone that appear radiolucent (commonly the alveolar process) and regions of increased density in one bone.
Effects on Surrounding Structures.: Paget’s disease always enlarges an affected bone to some extent, even in the early stage. Often the bone enlargement is impressive. Prominent pagetoid skull bones may swell to three or four times their normal thickness. In enlarged jaws the outer cortex may be thinned but remains intact. The outer cortex may appear to be laminated in occlusal projections (see Fig. 24-29). When the maxilla is involved, the disease invariably involves the sinus floor. However, the air space usually is not diminished to a great extent. Cortical boundaries such as the sinus floor may be more granular and less apparent as sharp boundaries. The lamina dura may become less evident and may be altered into the abnormal bone pattern. Often hypercementosis develops on a few or most of the teeth in the involved jaw. This hypercementosis may be exuberant and irregular, which is characteristic of Paget’s disease (Fig. 24-31). As previously mentioned, the teeth may become spaced or displaced in the enlarging jaw.
Differential Diagnosis
Paget’s disease may appear similar to fibrous dysplasia. However, Paget’s disease occurs in an older age group and is almost always bilateral. In the maxilla, fibrous dysplasia has a tendency to encroach on the antral air space, whereas Paget’s disease does not. The linear trabeculae and cotton-wool appearance of Paget’s disease are distinctive. FOD may have a cotton-wool pattern, but these lesions are centered above the inferior alveolar nerve canal and most commonly have a radiolucent capsule. The changes seen in FOD do not affect all of the jaw, unlike with Paget’s disease. The bone pattern in Paget’s disease may show some similarities to the bone pattern in metabolic bone diseases, and both conditions may be bilateral. However, Paget’s disease enlarges bone, and metabolic diseases do not.
The specific bone pattern changes, the late age of onset, the enlargement of the involved bone, and the extreme elevation of serum alkaline phosphatase aid in the differential diagnosis.
Management
Currently Paget’s disease usually is managed medically, with calcitonin, sodium etidronate, or lately bisphosophonates. Medication relieves pain and reduces the serum alkaline phosphatase levels and osteoclastic activity. Surgery may be required to correct deformities of the long bones and to treat fractures.
There are complications of this disease that are of concern. Extraction sites heal slowly. The incidence of jaw osteomyelitis is higher than for nonaffected individuals. Osteogenic sarcoma develops in about 10% of patients with polyostotic disease. Characteristics such as invasion and bone destruction, as described in Chapter 22, indicate the presence of a malignant neoplasm.
Definition
The disorders included in the category of Langerhans’ cell histiocytosis (LCH) are abnormalities that result from the abnormal proliferation of Langerhans’ cells or their precursors. Langerhans’ cells are specialized cells of the histiocytic cell line that normally are found in the skin. The abnormal proliferation of Langerhans’ cells and eosinophils results in a spectrum of clinical diseases. Historically, histiocytosis X was classified into three distinct clinical forms: eosinophilic granuloma (solitary), Hand-Schüller-Christian disease (chronic disseminated), and Letterer-Siwe disease (acute disseminated).
A newly proposed LCH classification creates two categories: nonmalignant disorders, such as unifocal or multifocal eosinophilic granuloma, and malignant disorders, including Letterer-Siwe disease and variants of histiocytic lymphoma. Research has shown that all forms of LCH are clonal and therefore have a neoplastic nature.
Clinical Features
Head and neck lesions are common at initial presentation, and approximately 10% of all patients with LCH have oral lesions. Often the oral changes are the first clinical signs of the disease.
Eosinophilic granuloma (EG) usually appears in the skeleton (ribs, pelvis, long bones, skull, and jaws) and in rare cases in soft tissue. This condition occurs most often in older children and young adults but may develop later in life. The lesions often form quickly and may cause a dull, steady pain. In the jaws the disease may cause bony swelling, a soft tissue mass, gingivitis, bleeding gingiva, pain, and ulceration. Loosening or sloughing of the teeth often occurs after destruction of alveolar bone by one or more foci of EG. The sockets of teeth lost to the disease generally do not heal normally. EG may have a single focus or may develop into a multifocal, aggressive disease. The disseminated form may involve multiple bone lesions, diabetes insipidus, and exophthalmos, a condition previously defined as Hand-Schüller-Christian disease.
Letterer-Siwe disease is a malignant form of LCH that most often occurs in infants less than 3 years of age. Soft tissue and bony granulomatous reactions disseminate throughout the body, and the condition is marked by intermittent fever, hepatosplenomegaly, anemia, lymphadenopathy, hemorrhage, and failure to thrive. Lesions in bone are rare. Death usually occurs within several weeks of the onset of the disease.
Radiographic Features
For ease of discussion, this chapter divides LCH jaw lesions into two groups: those that occur in the alveolar process and intraosseous lesions that occur elsewhere in the jaws. The radiographic features of this condition generally are similar to those of malignant neoplasms.
Location.: The alveolar type of LCH lesions are commonly multiple, whereas the intraosseous type usually is solitary. The mandible is a more common site than the maxilla, and the posterior regions are more involved than are the anterior regions (Fig. 24-32). The mandibular ramus is a common site of intraosseous lesions. Solitary lesions of the jaws may be accompanied by lesions in other bones.
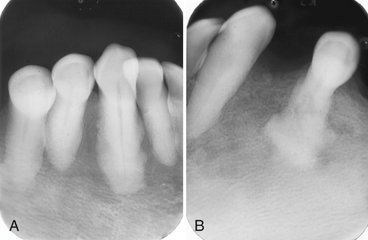
Periphery and Shape.: The periphery of EG lesions varies from moderately to well defined but without cortication; the periphery sometimes appears punched out (Fig. 24-33). The margins may be smooth or somewhat irregular. The alveolar lesions commonly start in the midroot region of the teeth. The bone destruction progresses in a circular shape, and after it includes a portion of the superior border of the alveolar process, it may give the impression that a section of the alveolar process has been scooped out (see Figs. 24-32 and 24-34). The shape of intraosseous lesions may be irregular, oval, or round.
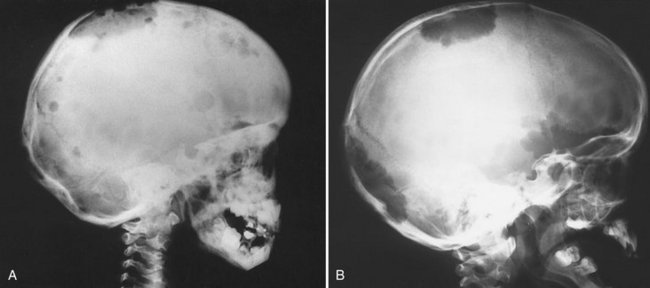
FIG. 24-33 Two lateral skull films of lesions of Langerhans’ cell histiocytosis showing well-defined, punched-out lesions. (Courtesy Dr. H. G. Poyton, Toronto, Ontario.)
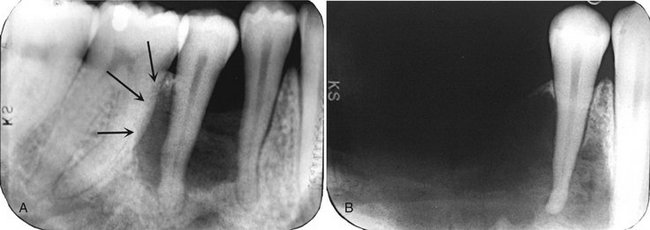
FIG. 24-34 Two periapical films of the same area of the mandible taken approximately 1 year apart in a patient with Langerhans’ cell histiocytosis. A, The earlier phase of the disease produces a scooped out shape (arrows) that shows that the epicenter of the lesion is in the midroot area of the involved teeth, unlike in periodontal disease. B, One year later, bone destruction is extensive, resulting in loss of teeth. (Courtesy Dr. D. Stoneman, Toronto, Ontario, Canada.)
Effects on Surrounding Structures.: LCH destroys bone. In alveolar lesions the bone around teeth, including the lamina dura, is destroyed, and as a result the teeth appear to be standing in space. The lesion does not displace teeth, although teeth may move because they are bereft of bone support (Fig. 24-35). Only minor root resorption has been reported. Of note is the ability of these lesions to stimulate periosteal new bone formation; this occurs more commonly with the intraosseous type of lesion (Fig. 24-36). The periosteal new bone formation is indistinguishable from the appearance seen in inflammatory lesions of the jaws. This lesion can destroy the outer cortical plate and in rare cases it extends into the surrounding soft tissues on CT examination.
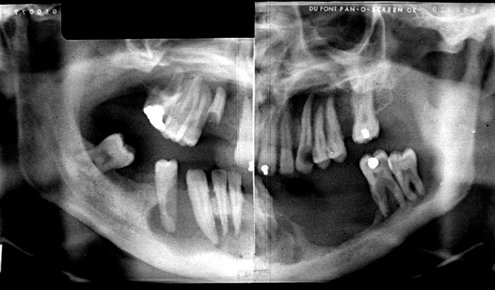
FIG. 24-35 A panoramic film showing the bone destruction that can occur with Langerhans’ cell histiocytosis. The bone around many of the remaining mandibular teeth has been destroyed, leaving the teeth apparently unsupported. (Courtesy Dr. D. Stoneman, Toronto, Ontario, Canada.)
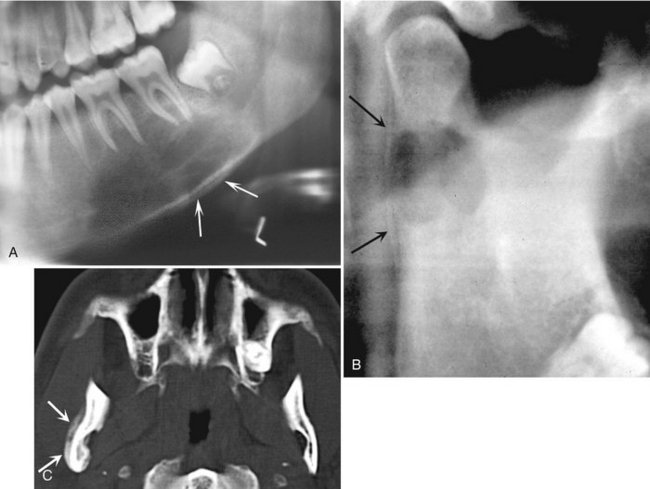
FIG. 24-36 Examples of Langerhans’ cell histiocytosis with periosteal reaction. A, A large region of bone destruction in the body of the mandible with periosteal reaction along the inferior border (arrows). B, A lesion in the condylar neck with a faint periosteal reaction along the posterior border of the ramus (arrows). C, An axial CT scan of the lesion in B showing a periosteal reaction (arrows) that extends beyond the area of bone destruction.
Differential Diagnosis
The major differential diagnosis of alveolar type lesions is periodontal disease and squamous cell carcinoma. An important characteristic in differentiation of periodontal disease is the fact that the epicenter of the bone destruction in LCH is approximately in the midroot region, resulting in a scooped out appearance. In contrast, the bone destruction in periodontal disease starts at the alveolar crest and extends apically down the root surface. Differentiation of a squamous cell carcinoma may not be possible by radiographic characteristics alone, although the borders of an LCH lesion typically are better defined. Multiple lesions in a younger age group (usually in the first three decades) are more likely to be LCH than squamous cell carcinoma, which typically appears as a single lesion in middle or old age. LCH may bear a superficial resemblance to simple bone cysts, but the alveolar crest is maintained in simple bone cysts and a partial cortex may be present.
The differential diagnosis of solitary intraosseous lesions includes metastatic malignant neoplasia and malignant tumors from adjacent soft tissues. However, the well-defined borders and the periosteal reaction seen in histiocytosis help in the differential diagnosis.
Patients suspected of having LCH should be referred to an oral and maxillofacial radiologist for a complete workup; this may include nuclear imaging to detect other possible bone lesions. The radiologic workup should be followed by a biopsy. The histologic appearance of histiocytosis may be hidden by changes caused by secondary infection from the oral cavity in alveolar lesions. Therefore it is important to correlate the radiographic findings with the histologic appearance of the biopsy.
Management
Treatment of localized lesions usually consists of surgical curettage or limited radiation therapy. Surgical management of jaw lesions usually is preferable because it has a low recurrence rate. The earlier EG of the mandible is diagnosed and controlled, the fewer teeth are lost to bone destruction. Disseminated disease is treated with chemotherapy.
Cohen, MM, Howell, RE. Etiology of fibrous dysplasia and McCune-Albright syndrome. Int J Oral Maxillofac Surg. 1999;28:366–371.
Ebata, K, Usami, T, Tohnai, I, et al. Chondrosarcoma and osteosarcoma arising in polyostotic fibrous dysplasia. J Oral Maxillofac Surg. 1992;50:761–764.
MacDonald-Jankowski, DS, Yeung, R, Li, TK, et al. Computed tomography of fibrous dysplasia. Dentomaxillofac Radiol. 2004;33:114–118.
Ozek, C, Gundogan, H, Bilkay, U, et al. Craniomaxillofacial fibrous dysplasia. J Craniofac Surg. 2002;13:382–389.
Petrikowski, CG, Pharoah, MJ, Lee, L, et al. Radiographic differentiation of osteogenic sarcoma, osteomyelitis and fibrous dysplasia of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:744–750.
Tokano, H, Sugimoto, T, Noguchi, Y, et al. Sequential computed tomography images demonstrating characteristic changes in fibrous dysplasia. J Laryngol Otol. 2001;115:757–759.
Waldron, C, Giansanti, J. Benign fibroosseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases. Oral Surg Oral Med Oral Pathol. 1973;35:190–201.
Waldron, C, Giansanti, J. Benign fibro-osseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases, II: benign fibro-osseous lesions of periodontal ligament origin. Oral Surg Oral Med Oral Pathol. 1973;35:340–350.
Loh, FL, Yeo, JF. Florid osseous dysplasia in Orientals. Oral Surg Oral Med Oral Pathol. 1989;68:748–753.
MacDonald-Jankowski, DS. Florid cemento-osseous dysplasia: a systematic review. Dentomaxillofac Radiol. 2003;32:141–149.
Mahomed, F, Altini, M, Meer, S, et al. Cemento-osseous dysplasia with associated simple bone cysts. J Oral Maxillofac Surg. 2005;63:1549–1554.
Melrose, RJ, Abrams, AM, Mills, BG. Florid osseous dysplasia: a clinical-pathologic study of 34 cases. Oral Surg. 1976;41:62–82.
Toffanin, A, Bennett, R, Manconi, R. Familial florid cemento-osseous dysplasia: a case report. J Oral Maxillofac Surg. 2000;58:1440–1446.
Eversole, L, Merrell, PW, Strub, D. Radiographic characteristics of central ossifying fibroma. Oral Surg. 1985;59:522–527.
Sciubba, JJ, Younai, F. Ossifying fibroma of the mandible and maxilla: review of 18 cases. J Oral Pathol Med. 1989;18:315–321.
Williams, HK, Mangham, C, Speight, PM. Juvenile ossifying fibroma: an analysis of eight cases and a comparison with other fibro-osseous lesions. J Oral Pathol Med. 2000;29:13–18.
De Lange, J, Van den Akker, HP. Clinical and radiological features of central giant-cell lesions of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:464–470.
Kaffe, I, Ardekian, L, Taicher, S, et al. Radiologic features of central giant cell granuloma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:720–726.
Waldron, CA, Shafer, WG. The central giant cell reparative granuloma of the jaws. An analysis of 38 cases. Am J Clin Pathol. 1966;45:437–447.
Buraczewski, J, Dabska, M. Pathogenesis of aneurysmal bone cyst. Relationship between the aneurysmal bone cyst and fibrous dysplasia of bone. Cancer. 1971;28:597–604.
Kaffe, I, Naor, H, Calderon, S, et al. Radiological and clinical features of aneurysmal bone cyst of the jaws. Dentomaxillofac Radiol. 1999;28:167–172.
Struthers, P, Shear, M. Aneurysmal bone cyst of the jaws, I: clinicopathological features. Int J Oral Surg. 1984;13:85–91.
Struthers, P, Shear, M. Aneurysmal bone cyst of the jaws, II: pathogenesis. Int J Oral Surg. 1984;13:92–100.
Bianchi, SD, Boccardi, A, Mela, F, et al. The computed tomographic appearances of cherubism. Skeletal Radiol. 1987;16:6–10.
Hyckel, P, Berndt, A, Schleier, P, et al. Cherubism—new hypotheses on pathogenesis and therapeutic consequences. J Craniomaxillofac Surg. 2005;33:61–68.
Von Wowern, N. Cherubism: a 36-year long-term follow-up of 2 generations in different families and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:765–772.
Rao, VM, Karasick, D. Hypercementosis—an important clue to Paget disease of the maxilla. Skeletal Radiol. 1982;9:126–128.
Reddy, SV. Etiology of Paget’s disease and osteoclast abnormalities. J Cell Biochem. 2004;93:688–696.
Sofaer, J. Dental extractions in Paget’s disease of bone. Int J Oral Surg. 1984;13:79–84.
Van Staa, TP, Selby, P, Leufkens, FG, et al. Incidence and natural history of Paget’s disease in bone in England and Wales. J Bone Miner Res. 2002;17:465–471.
LANGERHANS’ CELL HISTIOCYTOSIS
Cline, MJ. Histiocytes and histiocytosis. Blood. 1996;84:2840–2853.
Coppes-Zantinga, A, Egeler, RM. The Langerhans cell histiocystosis X files revealed. Br J Haematol. 2002;116:3–9.
Dagenais, M, Pharoah, MJ, Sikorski, PA. The radiographic characteristics of histiocytosis X. A study of 29 cases that involve the jaws. Oral Surg Oral Med Oral Pathol. 1992;74:230–236.
Hicks, J, Flaitz, CM. Langerhans cell histiocytosis: current insights in a molecular age with emphasis on clinical oral and maxillofacial pathology practice. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(2 suppl):S42–S66.
Wong, GB, Pharoah, MJ, Weinberg, S, et al. Eosinophilic granuloma of the mandibular condyle: report of three cases and review of the literature. J Oral Maxillofac Surg. 1997;55:870–878.