Salivary Gland Radiology
Definition of Salivary Gland Disease
Dental diagnosticians have responsibility for detecting disorders of the salivary glands. A familiarity with salivary gland disorders and applicable current imaging techniques is an essential element of the clinician’s armamentarium. Both major and minor salivary glands may be involved pathologically; however, this chapter deals only with the major glands. Salivary gland disease processes may be divided into the following clinical categories: inflammatory disorders, noninflammatory disorders, and space-occupying masses. Inflammatory disorders are acute or chronic and may be secondary to ductal obstruction by sialoliths, trauma, infection, or space-occupying lesions such as neoplasia. Noninflammatory disorders are metabolic and secretory abnormalities associated with diseases of nearly all the endocrine glands, malnutrition, and neurologic disorders. Space-occupying masses are cystic or neoplastic; the neoplasms are benign or malignant.
Clinical Signs and Symptoms
Diseases of the major salivary glands may have single or multiple clinical features. Swellings in the areas of the parotid and submandibular glands should create a clinical suspicion of salivary gland disease. Pain and altered salivary flow may be present. Because the periodicity and longevity of these symptoms are important in the differential diagnosis, a review of the medical history and physical condition of the patient may provide important information. A history of skin, endocrine, or swallowing abnormalities may suggest a systemic collagen disease or metabolic disorder.
Differential Diagnosis of Salivary Enlargements
ENLARGEMENTS OF THE PAROTID AREA
Unilateral enlargements of the parotid area are categorized by the presence of a discrete, palpable mass or a diffuse swelling. If no mass is apparent, sialadenitis should be considered. Sialadenitis may be primary or secondary to ductal obstruction (retrograde). A mass superficial to the gland may represent lymphadenitis, infected preauricular cyst, infected sebaceous cyst, benign lymphoid hyperplasia, or extraparotid tumor. A mass intrinsic to the gland suggests a neoplasm (benign or malignant), intraglandular lymph node, or hamartoma. Rapid growth, facial nerve paralysis, rock-hard texture, pain, and older age of occurrence are clinically suggestive of malignant neoplasms.
The differential diagnosis of asymptomatic bilateral enlargements of the parotid area may include benign lymphoepithelial lesion, Sjögren syndrome, alcoholism, medication (iodine and certain heavy metals), and Warthin tumor. Painful bilateral enlargement may occur after radiation treatment or as a result of bacterial or viral sialadenitis (including mumps) when accompanied by systemic symptoms.
A differential diagnosis of diffuse facial swelling in the parotid region, but not related to abnormalities of the gland, includes hypertrophy of the masseter muscle, accessory parotid gland, lesions related to the temporomandibular joint, and osteomyelitis of the ramus of the mandible. A palpable mass superficial to the gland suggests lymphadenitis, an infected preauricular or sebaceous cyst, benign lymphoid hyperplasia, or extraparotid tumor (Box 31-1).
ENLARGEMENTS OF THE SUBMANDIBULAR AREA
Unilateral enlargement of the submandibular area associated with tender lymph nodes is suggestive of sialadenitis, which may be primary or secondary to ductal obstruction or decreased salivary flow (retrograde). Unilateral enlargement without tender lymph nodes suggests a neoplasm, cyst, lymphoepithelial lesion, or fibrosis. An intraglandular mass may be neoplastic or cystic. Neoplasms of the submandibular gland have a greater chance of being malignant than do those of the parotid gland. In turn, sublingual gland neoplasms have a still greater chance of being malignant than do those of the submandibular glands. Rapid growth, rock-hard texture, pain, and older age of occurrence are clinically suggestive of malignancy. Masses superficial or adjacent to the submandibular gland may be lymph nodes or extraglandular neoplasms.
Bilateral enlargement of the submandibular gland area suggests bacterial or viral sialadenitis. Although mumps is primarily a viral infection of the parotid glands, it may also occur in the submandibular glands. Other causes of swelling in the submandibular region include Sjögren syndrome, enlarged lymph nodes, submandibular space infection, and branchial cleft cyst (see Box 31-1).
Applied Diagnostic Imaging of the Salivary Glands
Diagnostic imaging of salivary gland disease may be undertaken to differentiate inflammatory processes from neoplastic disease, distinguish diffuse disease from focal suppurative disease, identify and localize sialoliths, and demonstrate ductal morphology. In addition, diagnostic imaging attempts to determine the anatomic location of a tumor, differentiate benign from malignant disease, demonstrate the relationship between a mass and adjacent anatomic structures, and aid in the selection of biopsy sites.
ALGORITHM FOR DIAGNOSTIC IMAGING
Plain film radiography is an appropriate starting point for imaging the major salivary glands from a cost-benefit point of view. It can demonstrate sialoliths and the possible involvement of adjacent osseous structures. Because obstructive and associated inflammatory conditions are the most common disorders and primarily involve the ductal system, conventional sialography is commonly the most appropriate next imaging modality. If the patient is allergic to the iodine contrast agent used in sialography, magnetic resonance imaging (MRI), computed tomography (CT), or ultrasonography (US) may be selected as alternative imaging modalities. Recent studies comparing the diagnostic yield of MRI with that of sialography suggest that MRI might replace sialography in the future as the imaging modality of choice for ductal pathosis. Sialography or CT is the best imaging modality for the detection of sialoliths (sialolithiasis). If sialography eliminates inflammatory disorders or suggests the presence of a space-occupying mass (either cystic or solid), then contrast-enhanced CT or MRI is appropriate for evaluation. US is an alternative technique to differentiate cystic lesions from solid masses and to identify advanced autoimmune lesions. Functional disorders such as xerostomia are appropriately imaged with sialography or scintigraphy. Scintigraphy can provide important physiologic information that may be helpful in forming the differential diagnosis.
PLAIN FILM RADIOGRAPHY
Plain film radiography is a fundamental part of the examination of the salivary glands and may provide sufficient information to preclude the use of more sophisticated and expensive imaging techniques. It has the potential to identify unrelated pathoses in the areas of the salivary glands that may be mistakenly identified as salivary gland disease, such as resorptive or osteoblastic changes in adjacent bone causing periauricular swelling mimicking a parotid tumor. Panoramic and conventional posteroanterior (PA) skull radiographs may demonstrate bony lesions, thus eliminating salivary pathosis from the differential diagnosis. Unilateral or bilateral functional or congenital hypertrophy of the masseter muscle may clinically mimic a salivary tumor. A plain film extraoral radiograph may demonstrate a deep antegonial notch, overdeveloped mandibular angle, and exostosis on the outer surface of the angle in cases of masseter hypertrophy.
Plain film radiographs are useful when the clinical impression, supported by a compatible history, suggests the presence of sialoliths (stones or calculi). Such an examination should include both intraoral and extraoral images to demonstrate the entire region of the gland. Sialoliths may be multiple at different locations. It is expedient to decrease the usual exposure by about half to avoid overexposure of the sialoliths. However, this technique is limited by the fact that 20% of the sialoliths of the submandibular gland and 40% of those of the parotid gland are not well calcified, rendering them radiolucent and not visible in plain films. Radiolucent sialoliths are rarely found in the sublingual glands.
INTRAORAL RADIOGRAPHY
Sialoliths in the anterior two thirds of the submandibular duct are typically imaged with a cross-sectional mandibular occlusal projection as described in Chapter 8 (Fig. 31-1). The posterior part of the duct is demonstrated with an over-the-shoulder occlusal projection view, where the directing cone is placed on the shoulder and central ray directed in an anterior direction through the angle of the mandible, with the patient’s head tilted to the unaffected side and rotated back (Fig. 31-2).
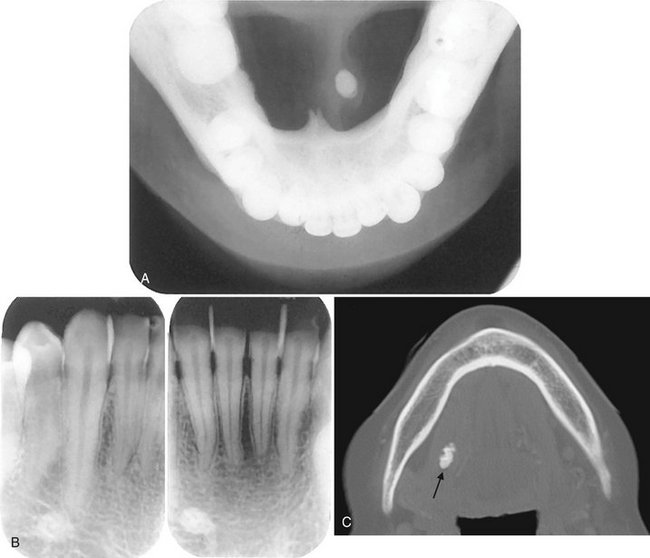
FIG. 31-1 A, Underexposed mandibular occlusal radiograph demonstrating radiopaque sialolith in Wharton duct. Note the classic laminated appearance. B, Periapical radiographs of the same case. The radiopaque calculus can be localized lingual to the teeth by applying appropriate object localization rules. C, An axial bone algorithm CT image showing a sialolith in the submandibular duct (arrow).
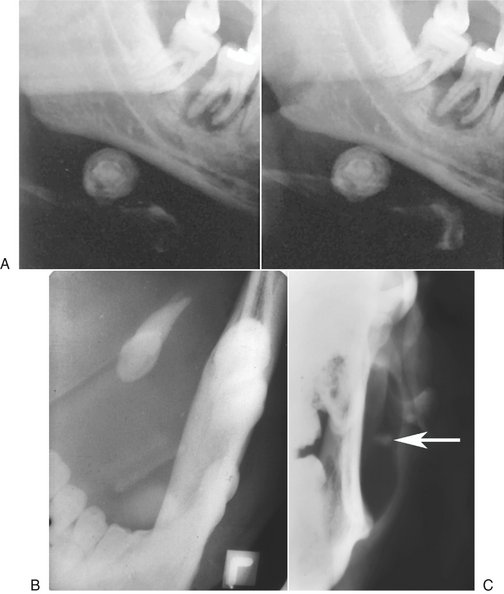
FIG. 31-2 A, Stereoscopic panoramic plain film projection. Note the laminated appearance of this sialolith in the submandibular gland. The image of the sialolith is magnified because of its relatively lingual placement in the image layer. Taken from slightly different horizontal angles, a three-dimensional appearance can be obtained when viewed with stereobinoculars. B, Over-the-shoulder occlusal projection revealing a sialolith. C, Anteroposterior skull view with cheek blown out to provide air contrast to reveal a parotid sialolith (arrow).
Parotid sialoliths are more difficult to demonstrate than the submandibular variety as a result of the tortuous course of Stensen duct around the anterior border of the masseter and through the buccinator muscle. As a rule, only sialoliths anterior to the masseter muscle can be imaged on an intraoral film. To demonstrate sialoliths in the anterior part of the duct, an intraoral film packet is held with a hemostat inside the cheek, as high as possible in the buccal sulcus and over the parotid papilla. The central ray is directed perpendicular to the center of the film.
EXTRAORAL RADIOGRAPHY
A panoramic projection frequently demonstrates sialoliths in the posterior duct or reveals intraglandular sialoliths in the submandibular gland if they are within the image layer (see Fig. 31-2). The image of most parotid sialoliths is superimposed over the ramus and body of the mandible, making oblique lateral radiographs of the mandible of limited value. To demonstrate sialoliths in the submandibular gland, the lateral projection is modified by opening the mouth, extending the chin, and depressing the tongue with the index finger. This improves the image of the sialolith by moving it inferior to the mandibular border.
Sialoliths in the distal portion of Stensen duct or in the parotid gland are difficult to demonstrate by intraoral or lateral extraoral views. However, a PA skull projection with the cheeks puffed out may move the image of the sialolith free of the bone, rendering it visible on the projected image (see Fig. 31-2). This technique may also demonstrate interglandular sialoliths that may be obscured during sialography.
CONVENTIONAL SIALOGRAPHY
First performed in 1902, sialography is a radiographic technique where a radiopaque contrast agent is infused into the ductal system of a salivary gland before imaging with plain films, fluoroscopy, panoramic radiography, conventional tomography, or CT. Sialography remains the most detailed way to image the ductal system (Fig. 31-3). The parotid and submandibular glands are more readily studied with this technique. Although the sublingual gland is difficult to infuse intentionally, it may be fortuitously opacified while the Wharton duct is infused to image the submandibular gland.
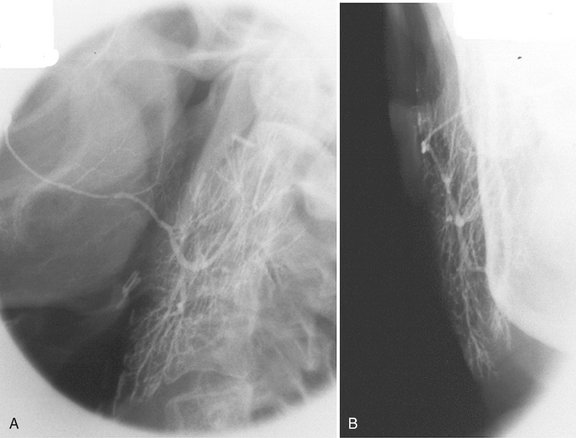
FIG. 31-3 Sialography.
A, Lateral projection of the parotid demonstrating opacification all the way to the terminal ducts and acini. B, Anteroposterior projection of the same gland demonstrating “parenchymal blushing” from acinar opacification. (Courtesy Oral and Maxillofacial Imaging Center, Baylor College of Dentistry, Dallas, Tex.)
A survey or “scout” film is usually made before the infusion of the contrast solution into the ductal system as an aid in verifying the optimal exposure factors and patient positioning parameters and for the detection of radiopaque sialoliths or extraglandular pathosis.
With this technique, a lacrimal or periodontal probe is used to dilate the sphincter at the ductal orifice before the passage of a cannula (blunt needle or catheter) connected by extension tubing to a syringe containing contrast agent. Lipid-soluble (e.g., Ethiodol) or non–lipid-soluble (e.g., Sinografin) contrast solution is then slowly infused until the patient feels discomfort (usually between 0.2 and 1.5 ml, depending on the gland being studied). These iodine-containing agents render the ductal system radiopaque. The filling phase can be monitored by fluoroscopy or with static films. The intent is to opacify the ductal system all the way to the acini. The image of the ductal system appears as “tree limbs,” with no area of the gland devoid of ducts. With acinar filling, the “tree” comes into “bloom,” which is the typical appearance of the parenchymal opacification phase (Fig. 31-4). The gland is allowed to empty for 5 minutes without stimulation. If postevacuation images suggest contrast retention, a sialogog such as lemon juice or 2% citric acid may be administered to augment evacuation by stimulating secretion. Non–lipid-soluble contrast agents are preferred because of reports of inflammatory reactions subsequent to inadvertent extravasation of lipid-soluble agents.
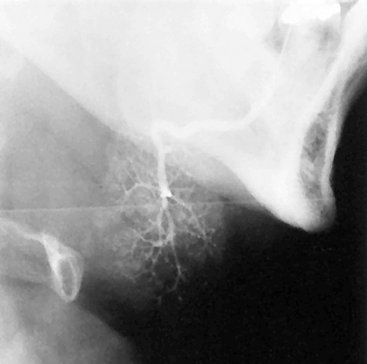
FIG. 31-4 Sialogram of Normal Submandibular Gland. This lateral view demonstrates parenchymal blushing. Normal fine branching is visible. Lack of parenchymal blushing at the anteroinferior margin is caused by radiographic burnout.
Sialography is indicated for the evaluation of chronic inflammatory diseases and ductal pathoses. Contraindications include acute infection, known sensitivity to iodine-containing compounds, and immediately anticipated thyroid function tests.
COMPUTED TOMOGRAPHY
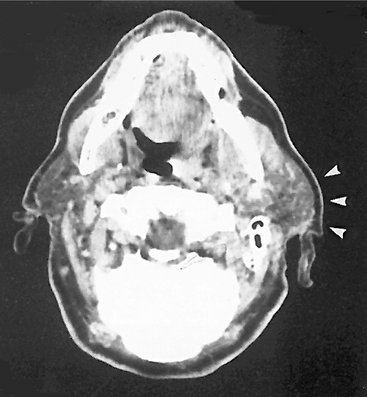
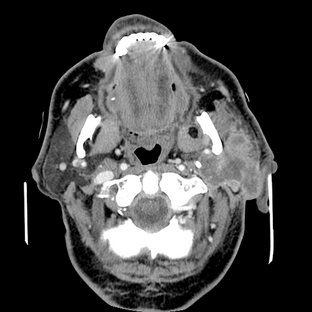
CT is useful in evaluating structures in and adjacent to salivary glands; it displays both soft and hard tissues and minute differences in soft tissue densities. Thin axial and coronal images with soft tissue algorithm commonly after intravenous administration of a contrast agent are typically acquired (Fig. 31-5). (See Chapter 14 for a description of the CT process.) Glandular tissues are usually easily discernible from surrounding fat and muscle. The parotid glands are more radiopaque than the surrounding fat but less opaque than adjacent muscles. Although the submandibular and sublingual glands are similar in density to adjacent muscles, they are readily identified on the basis of shape and location. The submandibular and sublingual glands are most easily identified on directly acquired contrast-enhanced coronal CT scans. CT is useful in assessing acute inflammatory processes and abscesses as well as cysts, mucoceles, and neoplasia. Calcifications such as sialoliths are also well depicted with CT.
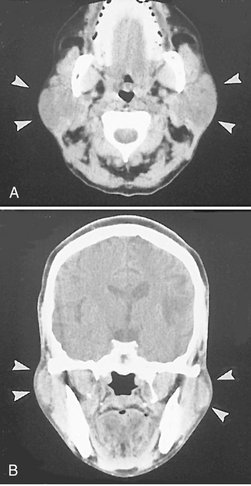
FIG. 31-5 CT Images with Soft Tissue Algorithm.
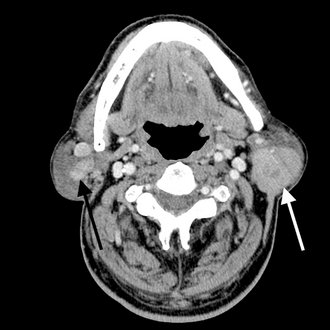
A, Axial view demonstrating bilateral enlargement of the parotid glands (arrowheads). B, Coronal view of the same patient. The clinical/histopathologic diagnosis was autoimmune parotitis. (Courtesy Department of Radiology, Baylor University Medical Center, Dallas, Tex.)
MAGNETIC RESONANCE IMAGING
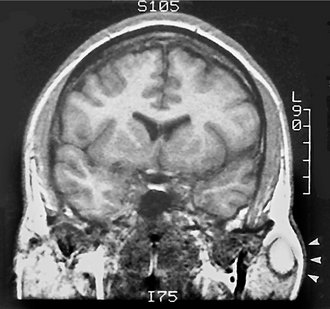
MRI typically provides a different and better soft tissue contrast resolution than does CT; it also results in fewer problems with streak artifacts from metallic dental restorative materials (Fig. 31-6). (See Chapter 14 for a description of the basic concepts and principles of MRI.) Axial views are acquired for all sequences, with coronal and sagittal views as needed. Noncontrast T1- and T2-weighted sequences are obtained, followed by T1-weighted postcontrast, fat-suppressed images. Fast-spin echo T2-weighted images may also require fat suppression.
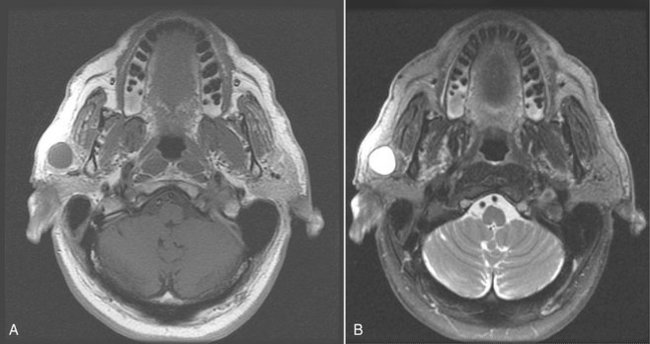
FIG. 31-6 These magnetic resonance images reveal a lymphoepithelial cyst involving the right parotid gland. A, This axial T1-weighted image reveals a well-defined circular lesion involving the right parotid gland with an internal signal isointense to muscle, and the matching T2-weighted image (B) reveals that the lesion has a high internal signal because of the fluid content.
Although indications for CT and MRI occasionally overlap, MRI is usually the imaging method of choice because of superior display of salivary gland masses, internal structures, and regional extension of the lesions into adjacent tissues or spaces, especially in examining the submandibular glands. The use of intravenous contrast (most commonly gadolinium) is helpful in distinguishing between cystic and solid masses and in the evaluation of perineural spread of malignant tumors. Studies have shown MRI with evoked salivation as a natural contrast medium to accurately reveal ductal morphology and to identify sialoliths.
SCINTIGRAPHY (NUCLEAR MEDICINE, POSITRON EMISSION COMPUTED TOMOGRAPHY)
Nuclear medicine, or scintigraphy, provides a functional study of the salivary glands, taking advantage of the selective concentration of specific radiopharmaceuticals in the glands. (See Chapter 14 for a description of the nuclear medicine procedures used to acquire images.) When 99mTc-pertechnetate is injected intravenously, it is concentrated in and excreted by glandular structures, including the salivary, thyroid, and mammary glands. The radionuclide appears in the ducts of the salivary glands within minutes and reaches maximal concentration within 30 to 45 minutes. A sialogog is then administered to evaluate secretory capacity. All major salivary glands can be studied at once.
Although this technique has high diagnostic sensitivity, it lacks specificity and demonstrates little morphology. Pathosis may be demonstrated by an increased, decreased, or absent radionuclide uptake (Fig. 31-7). Lesions that concentrate 99mTc-pertechnetate are Warthin tumors and oncocytomas. The diagnosis of salivary gland tumors from nuclear medicine scans is not completely reliable. Because of relatively low image resolution, CT and MRI are preferred for the evaluation of salivary masses. A recent advance in nuclear medicine is positron emission computed tomography (PET). In spite of a much greater resolution than scintigraphy, PET has not been useful classifying salivary tumors as benign or malignant.
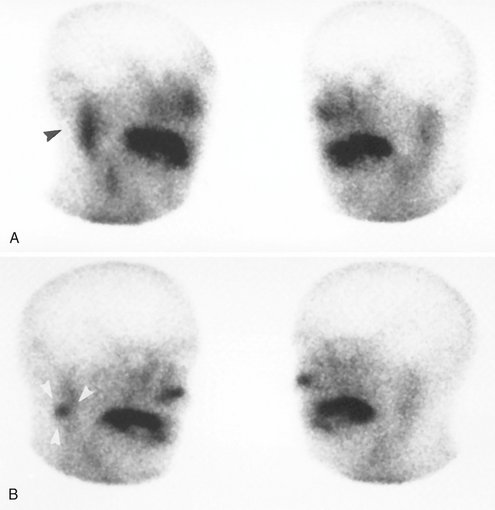
FIG. 31-7 Scintigraphy.
A,99mTc-pertechnetate scan of the salivary glands (right and left anterior oblique views) demonstrates increased uptake of radioisotope in the right parotid gland (black arrowhead). B, Scintigram taken after administration of a sialogog (lemon juice) demonstrates retention of isotope in right parotid gland (white arrowheads). This is a typical presentation of salivary stasis, Warthin tumor, or oncocytoma.
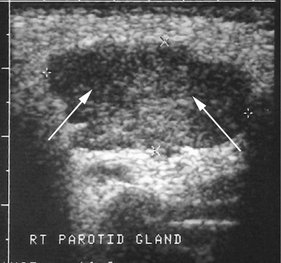
ULTRASONOGRAPHY
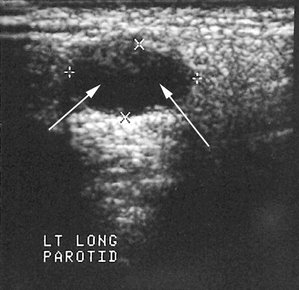
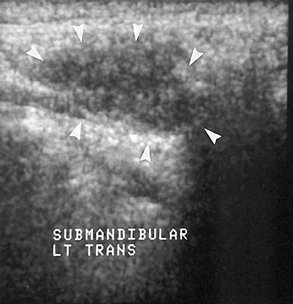
Compared with CT and MRI, US has the advantages of being relatively inexpensive, widely available, painless, easy to perform, and noninvasive. (For a full description of US, see Chapter 14.) The primary application of US is the differentiation of solid masses from cystic ones (Fig. 31-8). Recent studies suggest that this technique may also be helpful in detecting sialoliths and diagnosing advanced autoimmune lesions (Sjögren syndrome).
Image Interpretation of Salivary Gland Disorders
OBSTRUCTIVE AND INFLAMMATORY DISORDERS
Clinical Features
Sialoliths can obstruct the secretory ducts, resulting in chronic retrograde infections because of a decrease in salivary flow. Clinical symptoms include intermittent swelling and pain with eating and signs of infection. Sialoliths may form in any of the major or minor salivary glands or their ducts, but usually only one gland is involved. The submandibular gland and Wharton duct are by far the most frequently involved (83% of cases). If one stone is found, at least a one in four chance exists that others are present.
Radiographic Features
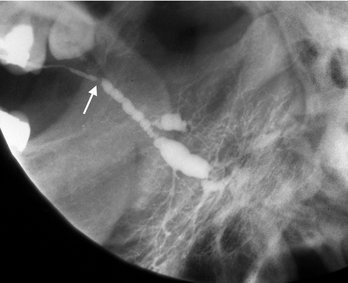
Depending on their degree of calcification, sialoliths may appear either radiopaque or radiolucent on radiographic examinations (20% to 40% of cases may not be calcified enough to be radiopaque and are sometimes referred to as “mucous plugs”) (see Fig. 31-1). Sialoliths vary in shape from long cigar shapes to oval or round shapes. When visible, they usually have a homogeneous radiopaque internal structure. Sialography is helpful in locating obstructions that are undetectable with plain radiography, especially if the sialoliths are radiolucent. The contrast agent usually flows around the sialolith, filling the duct proximal to the obstruction (Fig. 31-9 and 31-10). The ductal system is frequently dilated proximal to the obstruction and implies the presence of an obstruction even when it is not visible. The contrast agent that flows around the sialolith is more radiopaque and may obscure small sialoliths. Radiolucent sialoliths appear as ductal filling defects (see Figs. 31-9 and 31-12). Sialography should not be performed if a radiopaque stone has been shown by plain radiography to be in the distal portion of the duct because the procedure may displace it proximally into the ductal system, complicating subsequent removal. CT may also detect minimally calcified sialoliths not visible on plain films.
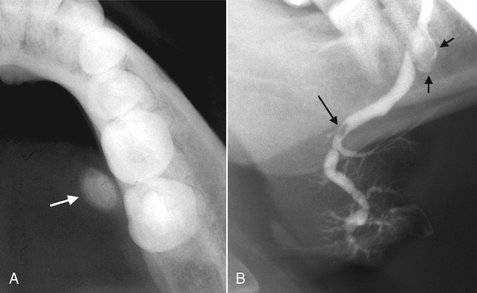
FIG. 31-9 A, This partial image of a standard mandibular occlusal film reveals the presence of a sialolith (arrow). B, Sialograph of the same patient demonstrating flow of contrast past the stone (short arrows) and a negative filling defect (long arrow) from a smaller radiolucent sialolith. The proximal secondary ducts within the gland show abnormal irregular widening indicating sialodochitis.
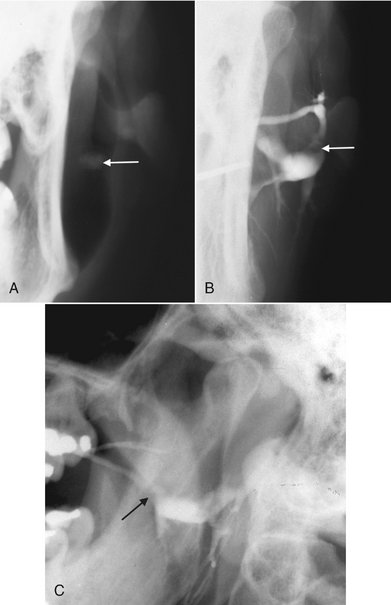
FIG. 31-10 A, Cropped view of a posteroanterior skull view as part of a parotid investigation; the cheek has been puffed out, providing air contrast and revealing a poorly calcified sialolith (arrow). B, Cropped view of a posteroanterior skull view of the sialograph of the same patient with the negative filling defect representing the sialolith (arrow) seen in A. C, Lateral view of the same patient revealing the filling defect (arrow) and abnormal dilation of the proximal ducts.
US is of limited value in the diagnosis of inflammatory and obstructive diseases, but recent studies indicate it is fairly reliable in demonstrating sialoliths. More than 90% of stones larger than 2 mm are detected as echo-dense spots with a characteristic acoustic shadow.
Sialoliths must be differentiated from phleboliths and dystrophic calcification of lymph nodes. Phleboliths typically demonstrate a radiolucent center. Calcified lymph nodes usually appear to be “cauliflower” shaped. In the panoramic image palatine tonsilliths have a similar location as parotid sialoliths, superimposed over the ramus, but can be differentiated in that they are typically multiple and punctate.
Treatment
Treatment of sialolithiasis may consist of encouragement of spontaneous discharge through the use of sialogogs to stimulate secretion. Sialography may also stimulate discharge, especially if an oil-based contrast agent is used. If discharge does not occur, the sialolith may be removed by surgery or by more conservative retrieval methods, and as a last resort the involved salivary gland may be removed.
Definition
Bacterial sialadenitis is an acute or chronic bacterial infection of the terminal acini or parenchyma of the salivary glands.
Clinical Features
Acute bacterial infections most commonly affect the parotid gland, but the submandibular gland may also be involved. Most cases are unilateral and may occur at any age. The typical clinical presentation is swelling, redness, tenderness, and malaise. Enlarged regional lymph nodes and suppuration may also be noted. Those most commonly afflicted are elderly persons, postoperative patients, and debilitated patients who have poor hygiene as a result of reduced salivary secretion and retrograde infection by the oral flora (usually Staphylococcus aureus and Streptococcus viridans). Reduced salivary secretion may also be drug related or the result of occlusion of a major duct. Untreated acute suppurative infections typically form abscesses. Diagnosis is based on clinical observation, systemic symptoms, and the expression of pus from the duct.
Chronic inflammation may affect any of the major salivary glands, causing extensive swelling and culminating in fibrosis. This may be a consequence of an untreated acute sialadenitis or associated with some type of obstruction resulting from sialolithiasis, noncalcified organic debris, or stricture (scar or fibrosis) formation in the excretory ducts. Bacteria or viruses may not be detected in the gland or saliva. The parotid is most often involved. During periods of painful swelling, pus may be expressed from the ductal orifice and salivary stimulation may cause pain. Episodic in nature, signs of generalized sepsis are seldom present. The obstruction may be congenital or caused by sialolithiasis, trauma, infection, or neoplasia. Typical clinical symptoms are intermittent swelling, pain when eating, and superimposed infection resulting from salivary stasis.
Radiographic Features
Sialography is contraindicated in acute infections because disrupted ductal epithelium may allow extravasation of contrast agent, resulting in a foreign body reaction and severe pain. This technique is appropriate for use in cases of suspected chronic infections. Epithelial flattening may lead to mildly dilated terminal ducts and saclike acini, which is demonstrable with sialography. The saclike acinar areas are referred to as sialectasia. An even distribution throughout the gland is seen in recurrent parotitis and autoimmune disorders. If connected to the ductal system, abscess cavities may fill with contrast media during sialography. Abscess cavities appear on CT as walled-off areas of lower attenuation within an enlarged gland. US may distinguish between diffuse inflammation (echo-free, light image) and suppuration (less echo-free, darker image) and detect sialoliths greater than 2 mm in diameter. US examination may also demonstrate abscess cavities, if present and may be the study of choice for recurrent parotitis, especially in children. Contrast-enhanced CT may demonstrate glandular enlargement (Fig. 31-11). However, MRI is an appropriate alternative examination in cases in which sialography is contraindicated or not technically possible. On MRI, inflamed glands are usually enlarged and demonstrate a lower tissue signal on T1-weighted images and a higher signal on T2-weighted images than that of the surrounding muscle. Advanced sialadenitis may present in combination with sialolithiasis, sialodochitis, abscess formation, and fistulas.
Treatment
Treatment of bacterial sialadenitis typically begins conservatively with attention to oral hygiene, local massage, increased fluid intake, and the use of oral sialogogs (sour citrus fruit wedges or salivary stimulants). An appropriate antibiotic regimen may also be indicated. If symptoms continue, surgical remedies ranging from partial to total excision of the gland may be considered.
Clinical Features
Sialectasia or dilation of the ductal system is a prominent sialographic presentation of sialodochitis. It is common in both the submandibular (Fig. 31-12) and parotid glands. If interstitial fibrosis develops, it is apparent in sialograms as a sausage-string appearance of the main duct and its major branches produced by alternate strictures and dilations. Recently these changes have been seen with the use of thin-section MRI. Scintigraphy and CT are not typically indicated in the diagnosis of inflammatory ductal diseases of the salivary glands. They are costly and nonspecific and typically do not provide any more useful information than sialography does.
Synonyms
Myoepithelial sialadenitis, Sjögren syndrome, benign lymphoepithelial lesion, Mikulicz disease, sicca syndrome, dacryosialoadenopathia atrophicans, and autoimmune sialosis
Definition
Autoimmune sialadenitis represents a group of disorders that affect the salivary glands and share an autosensitivity. The range of clinical and histopathologic manifestations suggests that these disorders represent different developmental stages of the same immunologic mechanisms, differing only in the extent and intensity of tissue reaction. Different forms may share a common etiology.
Clinical Features
The clinical manifestations range from recurrent painless swelling of the salivary glands (usually the parotid gland) to a stage that includes enlargement of the lacrimal glands. Glandular swelling may be accompanied by xerostomia and xerophthalmia (primary Sjögren syndrome), and subsequently by a connective tissue disease such as rheumatoid arthritis, progressive systemic sclerosis, systemic lupus erythematosus, or polymyositis (secondary Sjögren syndrome). The process may progress to benign lymphoepithelial lesions that can assume the proportions of a tumor. A presumptive diagnosis can be made on the basis of any two of the following three features: dry mouth, dry eyes, and rheumatoid disease. The disease is most common in adults, primarily in the 40- to 60-year-old age group with a 90% to 95% female prevalence. The childhood form is only one-tenth as common as the adult form and there is much less chance of advanced parotid disease. Studies have shown a 44 times greater risk for development of non-Hodgkin lymphoma than in control subjects. Mikulicz disease has been included within the diagnosis of primary Sjögren syndrome but represents a unique condition consisting of enlargement of the lacrimal and salivary glands and characterized by few autoimmune reactions.
Radiographic Features
Sialography is helpful in the diagnosis and staging of autoimmune disorders. The early stages of disease are witness to the initiation of punctate (less than 1 mm) and globular (1 to 2 mm) spheric collections of contrast agent evenly distributed throughout the glands. These collections are referred to as sialectases (Fig. 31-13). At this stage, the main duct may appear normal, but the intraglandular ducts may be narrowed or not even evident. Sialectasia typically remains after the administration of a sialogog, which is an indication that contrast agent is pooled extraductally.
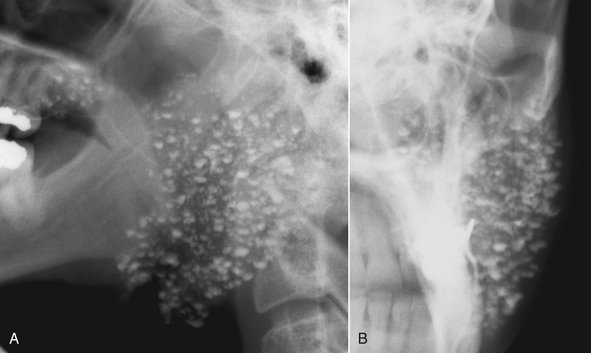
FIG. 31-13 Conventional Sialography of Left Parotid.
A, Lateral projection demonstrates punctate sialectases distributed throughout the gland, which is suggestive of autoimmune sialadenitis. Clinical/histopathologic diagnosis was Sjögren syndrome. B, Anteroposterior projection of the same gland.
As the disease progresses, the collections of contrast agent increase in size (greater than 2 mm in diameter) and are irregular in shape. These pools of contrast agent are termed cavitary sialectases. These larger sialectases are fewer in number and less uniformly distributed throughout the glands than are punctate or globular sialectases (Fig. 31-14). Progressively larger cavities of contrast agent and dilation of the main ductal system may also be present. At the end point of this disorder, complete destruction of the gland occurs. Cavitation and glandular fibrosis are the result of recurrent inflammation. The differential diagnosis of this appearance would include chronic bacterial or granulomatous infections and multiple parotid cysts associated with human immunodeficiency virus (HIV) infection. However, diffuse cervical lymphadenopathy is common in HIV disease and uncommon in Sjögren syndrome. Thin-section MRI has been shown to be reliable in depicting sialodochitis and sialectasia, especially when globular changes are present.
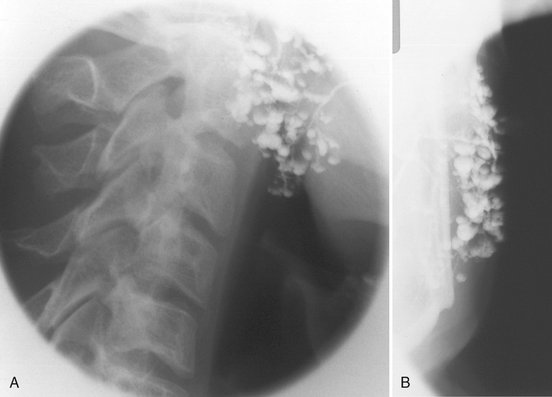
FIG. 31-14 Sialography of the Left Parotid.
Punctate (small spheric), globular (larger spheric), and cavitary (larger, irregular) sialectases with some dilation of the main duct are suggestive of advanced autoimmune disease with parenchymal destruction with retrograde infection in lateral (A) and anteroposterior (B) projections. Clinical/histopathologic diagnosis was Sjögren syndrome. (Courtesy Oral and Maxillofacial Imaging Center, Baylor College of Dentistry, Dallas, Tex.)
Treatment
The management of autoimmune disorders of the salivary glands is directed toward relief of symptoms. Underlying systemic rheumatoid conditions are typically treated with anti-inflammatory agents, corticosteroids, and immunosuppressive therapeutic agents. Salivary stimulants, increased fluid intake, and artificial saliva and tears are symptomatic treatment regimens for the eyes and mouth. More advanced inflammatory changes may be treated surgically by local or total excision of the symptomatic gland.
NONINFLAMMATORY DISORDERS
Definition
Sialadenosis is a nonneoplastic, noninflammatory enlargement of primarily the parotid salivary glands. It is usually related to metabolic and secretory disorders of the parenchyma associated with diseases of nearly all the endocrine glands (hormonal sialadenoses), protein deficiencies, malnutrition in alcoholics (dystrophic-metabolic sialadenoses), vitamin deficiencies, and neurologic disorders (neurogenic sialadenoses).
Radiographic Features
Sialography may demonstrate enlargement of the affected glands or a normal appearance. In enlarged glands, the ducts will be splayed. CT and MRI provide a more straightforward depiction of the glands but are nonspecific and require correlation with the clinical findings and history.
Treatment
The management of sialadenosis hinges on identifying the cause of the metabolic or secretory disorder. Conservative treatment, including local massage, increased fluid intake, and the use of oral sialogogs (sour citrus fruit wedges or salivary stimulants), is appropriate.
Definition
Cysts of the salivary glands are rare (less than 5% of all salivary gland masses) and most commonly occur unilaterally in the parotid gland (see Fig. 31-6). They may be congenital (branchial), lymphoepithelial, dermoid, or acquired, including mucous retention cysts (obstructions from any etiology). Cystic salivary lesions may be intraglandular or extraglandular in nature and may progress to such proportions that they are clinically palpable and must be distinguished from neoplasia. Cystic neoplasms do occur, but they are discussed separately in this chapter. Mucous extravasation pseudocysts lack an epithelial lining and result from ductal rupture. Ranulas are retention cysts that usually occur as a result of obstruction of the sublingual duct. Benign lymphoepithelial cysts are thought to be sequelae of cystic degeneration of salivary inclusions within lymph nodes. Multicentric parotid cysts associated with HIV have been reported and are termed benign lymphoepithelial lesions of human immunodeficiency syndrome. These lesions are accompanied by cervical lymphadenopathy, occur bilaterally, and are usually in the superficial portion of the parotid gland (Fig. 31-15). Secondary parotitis may develop.
Radiographic Features
On sialographic examination, cystic masses may be indirectly visualized only by the displacement of the ducts arching around them. Cystic lesions typically appear as well-circumscribed, nonenhancing (with contrast), low-density areas when examined on CT. Cysts appear as well-circumscribed, high-signal areas on T2-weighted MRI, but they do not enhance after gadolinium contrast, as do benign mixed tumors. When imaged with US, cysts are sharply marginated and echo free (represented as a dark area) (Fig. 31-16).
BENIGN TUMORS
Salivary gland tumors are relatively uncommon and occur in less than 0.003% of the population. They account for about 3% of all tumors. Some 80% of the salivary tumors arise in the parotid, 5% in the submandibular, 1% in the sublingual, and 10% to 15% in the minor salivary glands. The majority (70% to 80%) of these tumors occur in the superficial lobe of the parotid gland. Most are benign or low-grade malignancies. High-grade malignancies are uncommon. The chance of neoplasms of major salivary glands being benign varies directly with the size of the gland.
Radiographic Features
Benign tumors and low-grade malignancies may have a similar appearance, with well-defined margins, which are most apparent on CT or MRI examinations. Because of the higher density of the submandibular gland, which can equal that of the neoplasm and obscure the tumor, intravenous contrast enhancement is required during the CT examination. This causes the tumor to appear more radiopaque because the vascularity of the tumor is greater than that of the adjacent salivary gland tissue. MRI is a preferential modality for salivary gland neoplasia, especially for the submandibular gland, because of its superior soft tissue contrast resolution. On US examination, benign masses are typically less echogenic than parenchyma, sharply defined, and of essentially homogeneous echo strength and density. Benign tumors may present as low-intensity (dark) or high-intensity (light) tissue signals on MRI, although the relative intensity of the signal may indicate the presence of lipid, vascular, or fibrous tissues. Sialography may suggest a space-occupying mass when the ducts are compressed or smoothly displaced around the lesion (the “ball-in-hand” appearance) (Fig. 31-17).
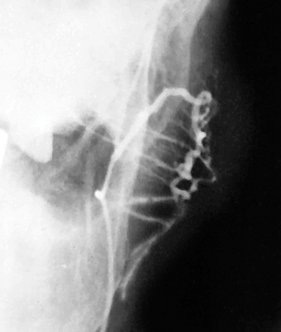
FIG. 31-17 Sialogram of Left Parotid (Anteroposterior View).
A mass within the gland is inferred by the appearance of the ducts displaced around the lesion. This is referred to as the “ball-in-hand” appearance, which is suggestive of a space-occupying mass. (Courtesy Department of Radiology, Baylor University Medical Center, Dallas, Tex.)
Treatment
The management of benign tumors of the major salivary glands is typically surgical. Benign tumors of the parotid gland may be either partially or totally excised. Submandibular and sublingual glands are invariably totally excised.
Definition
The benign mixed tumor is a neoplasm arising from the ductal epithelium of major and minor salivary glands exhibiting epithelial and mesenchymal components.
Clinical Features
The benign mixed tumor accounts for 75% of all salivary gland tumors; 80% are found in the parotid gland, 4% in the submandibular gland, 1% in the sublingual gland, and 10% in the minor salivary glands. This tumor typically occurs in the fifth decade of life as a slow-growing, unilateral, encapsulated, asymptomatic mass. A slight female predilection exists. Recurrence occurs in 50% of cases after excision. Malignant transformation is reported in up to 15% of untreated cases.
Radiographic Features
The CT presentation of the benign mixed tumor is a sharply circumscribed infrequently lobulated and essentially round homogeneous lesion that has a higher density than the adjacent glandular tissue (Fig. 31-18, A). Calcifications within the tumor are commonly seen and are well depicted on CT. This tumor has various tissue signals in different MRI techniques such as relatively low (dark) in T1-weighted, intermediate on proton density-weighted, and homogeneous high-intensity (bright) on T2-weighted images (Fig. 31-18). Foci of low signal intensity (dark areas) usually represent areas of fibrosis or dystrophic calcifications. If a calcification is present (signal void) the diagnosis favors a benign mixed tumor; otherwise, it is difficult to differentiate this tumor from other parotid masses.
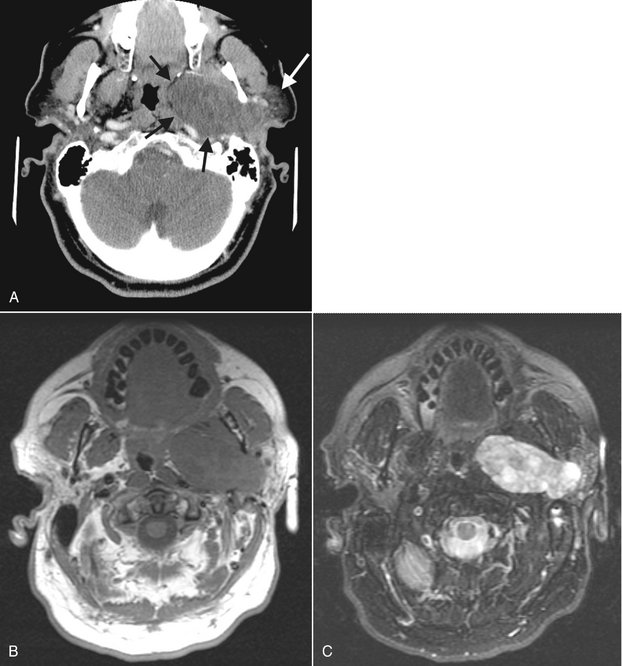
FIG. 31-18 CT and MRI Images of a Pleomorphic Adenoma.
A, In the axial CT soft tissue algorithm image, note the well-defined periphery (black arrows) and the internal density that is less than surrounding muscles. The remaining parotid gland (white arrow) is displaced laterally. B, In the axial MRI T1-weighted image, the tissue signal of the tumor is isointense with muscle. C, In the T2-weighted image, note the increased signal of the tumor, which is now hyperintense to muscle.
Benign mixed tumor does not usually concentrate 99mTc-pertechnetate. Therefore the tumor appears as a cold spot when examined by scintigraphy. Solid tumors larger than 5 mm are usually well visualized.
Definition
Warthin tumor is a benign tumor arising from proliferating salivary ducts trapped in lymph nodes during embryogenesis of the salivary glands.
Clinical Features
Warthin tumor is the second most common benign neoplasm of the salivary glands, accounting for 2% to 6% of the parotid tumors. In the parotid, it is usually found in the inferior lobe of the gland. This unusual type of tumor is a slow-growing, painless, round-to-ovoid mass. In 20% of cases the tumors are multiple. Warthin tumor typically afflicts males older than 40 years and may be unilateral or bilateral (Fig. 31-19).
Radiographic Features
CT and MRI are the preferred techniques for imaging Warthin tumor. The CT and MRI appearance of this tumor is not specific and is typical of benign salivary tumors as described for the benign mixed tumor. On CT, this tumor may be of either soft tissue or cystic density. On MRI, it is heterogeneous and may demonstrate hemorrhagic foci. Warthin tumor is characteristically intensely hot on 99mTc-pertechnetate scans. Oncocytoma (oxyphilic adenoma) may also accumulate the 99mTc-pertechnetate but is uncommon and more likely to be bilateral (see Fig. 31-7). Oncocytoma has been reported to be present in essentially everyone older than 70 years. The US presentation of Warthin tumor is that of a solid mass (anechoic), if the mass is not cystic, as some are (see Fig. 31-8).
Definition
Hemangioma is a benign neoplasm of proliferating endothelial cells (congenital hemangioma) and vascular malformations, including lesions resulting from abnormal vessel morphogenesis.
Clinical Features
Hemangioma is the most frequently occurring nonepithelial salivary neoplasm, accounting for 50% of the cases. As many as 85% arise in the parotid gland. It is the most common salivary gland tumor during infancy and childhood. The average age at diagnosis is 10 years, with 65% occurring in the first two decades of life. They are frequently unilateral and asymptomatic. A 2:1 female-to-male predilection exists. Treatment is by local excision for those who do not undergo spontaneous remission.
Radiographic Features
Phleboliths are common in this tumor. They appear as discrete soft tissue calcifications with a radiolucent center and are best identified on plain films and CT. Displaced ducts curving about the mass may also be apparent on sialography. The CT presentation of hemangioma is a soft tissue mass that is well distinguished from surrounding tissue, especially when intravenous contrast enhancement is used. On MRI, the tumor has a signal similar to that of adjacent muscle on T1-weighted images and a very high signal on T2-weighted images. Although US usually demonstrates well-defined margins in the hemangioma, ill-defined margins may also be noted. Strongly hypoechoic hemangioma may have a complex US appearance resulting from the multiple interfaces in the lesion. Phleboliths image as multiple hyperechoic areas within the body of the gland itself.
MALIGNANT TUMORS
About 20% of tumors in the parotid are malignant compared with 50% to 60% of submandibular tumors, 90% of sublingual tumors, and 60% to 75% of minor salivary gland tumors.
Radiographic Features
The radiographic presentation of malignant tumors is variable and is related to the grade, aggressiveness, location, and type of tumor. In many cases it is not possible to determine whether a tumor is malignant or benign (Fig. 31-20). However, features such as ill-defined margins (Fig. 31-21), invasion of adjacent soft tissues (such as fat spaces), and destruction of adjacent osseous structures are considered to be typical indicators of malignancy.
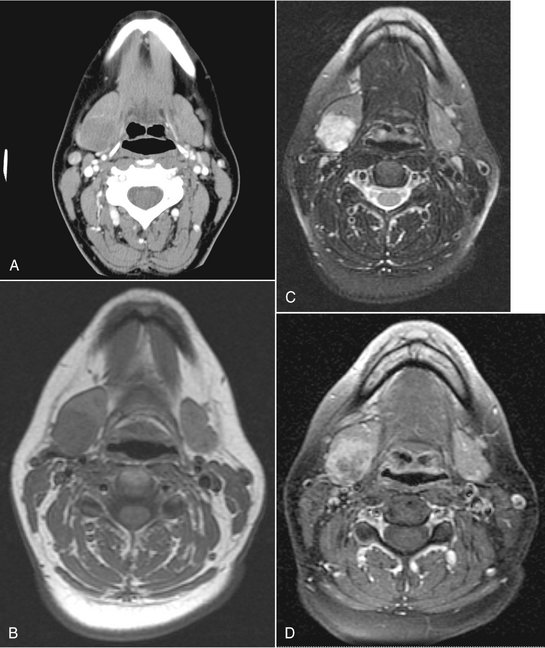
FIG. 31-20 These four axial CT and magnetic resonance images depict an adenoid cystic carcinoma of the right submandibular gland. Note the well-defined periphery, making it difficult to differentiate from a benign tumor. A, The internal density of the tumor in this soft tissue algorithm CT image is almost equal to the remaining gland. B, The tissue signal in this T1-weighted magnetic resonance image is very slightly less than the remaining gland. However, in C, a T2-weighted magnetic resonance image, the high signal of the tumor contrasts with the remaining gland. Similarly, in D, a T1-weighted postgadolinium, fat-saturation image, the tumor has a higher signal than in the remaining gland.
Treatment
The management of malignant tumors of the major salivary glands is typically surgical. Low-grade malignant tumors of the parotid gland may be either partially or totally excised. Submandibular and sublingual glands are invariably totally excised. High-grade tumors may require radical neck dissection. Combinations of surgery, therapeutic radiation, and chemotherapy may also be used.
Definition
Mucoepidermoid carcinoma is a malignant tumor composed of a variable admixture of epidermoid and mucous cells arising from the ductal epithelium of the salivary glands.
Clinical Features
This is the most common malignant salivary gland tumor (35%). Just over half occur in the major salivary glands, most commonly the parotid gland; the rest are found in the minor glands, with the palate being the most frequent location. The aggressiveness of the lesion varies with its histologic grade. A wide age range exists, with the highest prevalence in the fifth decade of life. A slight predilection for females exists. The low-grade variety rarely metastasizes. Clinically, this tumor appears as a movable, slowly growing, painless nodule not unlike a benign mixed tumor. It is usually only 1 to 4 cm in diameter. The prognosis is good; the 5-year survival rate is greater than 95%.
In contrast to low-grade mucoepidermoid carcinomas, high-grade tumors often cause facial pain and paralysis, have ill-defined margins, and are relatively immobile. Metastasis by blood and lymph are common, with recurrence in half the patients after excision. The prognosis is poor and varies with the histologic grade; the 5-year survival rate may be as low as 25%.
Radiographic Features
Low-grade mucoepidermoid carcinomas are typically not apparent on plain films unless destructive changes to adjacent osseous structures have occurred. The sialographic, CT, MRI, US, and scintigraphic presentations of this tumor are similar to those previously described for benign salivary tumors. However, low-grade mucoepidermoid carcinoma may present a lobulated or irregularly sharply circumscribed appearance on contrast enhanced CT or MRI (Fig. 31-22). Cystic area may present and, rarely, calcifications may be seen.
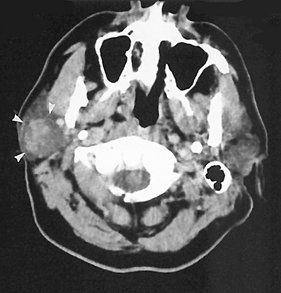
FIG. 31-22 Contrast-enhanced axial soft tissue algorithm CT image demonstrating a mass in right parotid gland with a poorly marginated, heterogeneous, slightly lobulated appearance (white arrows). Poorly defined margins suggest a low-grade malignancy rather than a benign tumor, although the CT appearance of both is similar. Histopathologic diagnosis was low-grade mucoepidermoid carcinoma. (Courtesy Department of Radiology, Baylor University Medical Center, Dallas, Tex.)
The radiographic diagnosis of high-grade mucoepidermoid carcinoma typically relies on the appearance of irregular margins and ill-defined form when the mass is examined with CT or MRI. In CT images, the tumor as an irregular homogeneous mass, slightly denser than the gland parenchyma. If intravenous contrast is added to the CT study, the result is a sharply defined homogeneous mass that is considerably more opaque. CT is also a reliable technique for the detection of invasion of adjacent osseous structures.
In contrast to low-grade malignancies and benign neoplasms, high-grade mucoepidermoid carcinoma, like most high-grade malignancies, has homogeneous low signal intensity (dark) on T1-weighted images, but T2-weighted images are more heterogeneous and intense (brighter) than T1-weighted images but still slightly darker (low signal) relative to the surrounding tissues. Regardless of clinical presentation and margins, low signal intensity is suggestive of a high-grade malignancy. Cavitary sialectasia and ductal displacement may be noted on sialographic images of this tumor.
Synonyms
Carcinoma ex mixed tumor, carcinoma ex pleomorphic adenoma, and malignant pleomorphic adenoma
Definition
The malignant mixed tumor is composed of three distinct types of tumors. The most common is carcinoma ex mixed tumor, which arises from the epithelial components of a preexisting benign mixed tumor. The other two, which are extremely rare, are a true malignant mixed tumor (from both epithelial and mesenchymal components of a mixed tumor) and the metastasizing mixed tumor, which appears histologically benign but behaves in a malignant fashion.
OTHER MALIGNANT AND METASTATIC TUMORS
Although the incidence of other malignant tumors of the major salivary glands is low, a significant variety exists in their histogenesis. Of all malignant salivary gland tumors, 23% are adenoid cystic carcinomas; however, the majority of these neoplasms develop in the minor salivary glands.
Adenocarcinoma accounts for 6.4% of all salivary gland malignancies, with acinic cell carcinoma, primary lymphoma, and squamous cell carcinoma occurring with even less frequency. Pain, paresthesia, and even paralysis may be present, especially in high-grade tumors. Interestingly, the pain associated with acinic cell carcinoma is not considered to be as grave a sign as in other malignant salivary tumors. Tumor spread may be by direct invasion or metastasis. Adenoid cystic carcinoma also spreads along nerve sheaths and is best demonstrated on postcontrast MRI where nerve enhancement and enlargement is present. Metastasis of tumors of the salivary glands is not unusual. Metastatic lesions in the parotid gland are more common than in the other salivary glands because of the extensive lymphatic and circulatory components of the parotid gland. Most metastatic lesions of the parotid gland occur through the lymphatic system and include squamous cell carcinoma, lymphoma, and melanoma. Although considerably fewer lesions are the result of hematogenous dissemination, metastasis from the lung, breast, kidney, and gastrointestinal tract has been reported.
Radiographic Features
The presentation of these tumors is nonspecific and similar to that of the high-grade mucoepidermoid carcinoma previously described. US may demonstrate echo-free cystic areas in adenoid cystic carcinomas (Fig. 31-23).
Del Balso, AM, Ellis, GE, Hartman, KS, et al. Del Balso AM, ed. Diagnostic imaging of the salivary glands and periglandular regions. Philadelphia: WB Saunders, 1990.
Freling, NJM. Imaging of salivary gland disease. Semin Roentgenol. 2000;35:12–20.
Harnsberger, HR, Hudgins, PA, Wiggins, RW, III., et al. Pocket radiologist, head and neck 100 top diagnoses. Salt Lake City: Amirsys; 2002.
Harnsberger, HR, Hudgins, R, Wiggins, P, et al. Diagnostic imaging head and neck. Salt Lake City: Amirsys; 2004.
Lufkin, RB, Hanafee, WN. MRI of the head and neck. New York: Raven Press; 1992.
Rabinov, K, Weber, AL. Radiology of the salivary glands. Boston: GK Hall Medical Publishers; 1985.
Rankow, RM, Polayes, IM. Diseases of the salivary glands. Philadelphia: WB Saunders; 1976.
Rice, DH, Becker, TS. The salivary glands. In: Hanafee WN, Ward PH, eds. Clinical correlations in the head and neck. New York: Thieme Medical Publishers, 1994.
Seifert, G, Miehlke, A, Hanbrich, J, et al. Diseases of the salivary glands. Stuttgart, Germany: George Thieme Verlag; 1986.
Van den Akker, HP. Diagnostic imaging in salivary gland disease. Oral Surg. 1988;66:625–637.
Watson, MG. Investigation of salivary gland disease. Ear Nose Throat J. 1989;68(84):87–93.
Ollerenshaw, R, Ross, SS. Radiological diagnosis of salivary gland disease. Br J Radiol. 1951;24:538–548.
Silvers, AR, Som, PM. Salivary glands. Radiol Clin North Am. 1998;36:941–966.
Weissman, JL. Imaging of the salivary glands. Semin Ultrasound CT MR. 1995;16:546–568.
Yousem, DM, Kraut, MA, Chalian, AA. Major salivary gland imaging. Radiology. 2000;216:19–29.
Drag, NA, Brown, JE, Wilson, RF. Pain and swelling after sialography: is it a significant problem? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:385–388.
Eisenbud, L, Cranin, N. The role of sialography in the diagnosis and therapy of chronic obstructive sialadenitis. Oral Surg Oral Med Oral Pathol. 1963;16:1181–1199.
Kalk, WW, Vissink, A, Spijkervet, HK, et al. Parotid sialography for diagnosing Sjögren syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:131–137.
Manashil, GB. Clinical sialography. Springfield: Charles C Thomas; 1978.
Whaley, K, Blair, S, Low, PS, et al. Sialographic abnormalities in Sjögren’s syndrome, rheumatoid arthritis, and other arthritides and connective tissue diseases: a clinical and radiological investigation using hydrostatic sialography. Clin Radiol. 1972;23:474–482.
Varghese, JC, Thornton, F, Lucey, BC, et al. A prospective comparative study of MR sialography and conventional sialography of salivary duct disease. AJR Am J Roentgenol. 1999;173:1497–1503.
Yune, HY, Klatte, EC. Current status of sialography. Am J Roentgenol Radiumt Ther Nucl Med. 1972;115:420–428.
COMPUTED TOMOGRAPHY OF THE MAJOR SALIVARY GLANDS
Bryan, RN, Miller, RH, Ferreyro, RI, et al. Computed tomography of the major salivary glands. AJR Am J Roentgenol. 1982;139:547–554.
Casselman, JW, Mancuso, AA. Major salivary gland masses: comparison of MR imaging and CT. Radiology. 1987;165:183–189.
Kosaka, M, Kamiishi, H. New strategy for the diagnosis of parotid gland lesions utilizing three-dimensional sialography. Comput Aided Surg. 2000;5:42–45.
Lloyd, RE, Ho, KH. Combined CT scanning and sialography in the management of parotid tumors. Oral Surg Oral Med Oral Pathol. 1988;65:142–144.
MAGNETIC RESONANCE IMAGING OF THE MAJOR SALIVARY GLANDS
Browne, RFJ, Golding, SJ, Watt-Smith, SR. The role of MRI in facial swelling due to presumed salivary gland disease. Br J Radiol. 2001;74:127–133.
Jäger, L, Menauer, F, Holzknecht, N, et al. MR sialography of the submandibular duct—an alternative to conventional sialography and US? Radiology. 2000;216:665–671.
Jungehulsing, M, Fischbach, R, Schroder, U, et al. Magnetic resonance sialography. Otolaryngol Head Neck Surg. 1999;121:488–494.
Kaneda, T, Minami, M, Ozawa, K, et al. MR of the submandibular gland: normal and pathologic states. AJNR Am J Neuroradiol. 1996;17:1575–1581.
Mandelblatt, S, Braun, IF, Davis, PC, et al. Parotid masses: MR imaging. Radiology. 1987;163:411–414.
Som, PM, Biller, HF. High-grade malignancies of the parotid gland; identification with MR imaging. Radiology. 1989;173:823–826.
Swartz, JD, Rothman, MI, Marlowe, FI, et al. MR imaging of parotid mass lesions: attempts at histopathologic differentiation. J Comput Assist Tomogr. 1989;13:789–796.
NUCLEAR MEDICINE (SCINTIGRAPHY) OF THE MAJOR SALIVARY GLANDS
Chaudhuri, TK, Stadalnik, RC. Salivary gland imaging. Semin Nucl Med. 1980;10:400–401.
Garcia, RR. Differential diagnosis of tumors of the salivary glands with radioactive isotopes. Int J Oral Surg. 1974;3:330–334.
Greyson, ND, Noyek, AM. Radionuclide salivary scanning. J Otolaryngol Suppl. 1982;10:1–47.
Keyes, JW, Jr., Harkness, BA, Greven, KM, et al. Salivary gland tumors: pretherapy evaluation with PET. Radiology. 1994;192:99–102.
Mishkin, FS. Radionuclide salivary gland imaging. Semin Nucl Med. 1981;11:258–265.
Van den Akker, HP, Busemann-Sokole, E. Absolute indications for salivary gland scintigraphy with 99mTc-pertechnetate. Oral Surg. 1985;60:440–447.
ULTRASONOGRAPHY OF THE MAJOR SALIVARY GLANDS
Gooding, GAW. Gray scale ultrasound of the parotid gland. AJR Am J Roentgenol. 1980;134:469–472.
Gritzmann, G. Sonography of the salivary glands. AJR Am J Roentgenol. 1989;153:161–166.
Mandel, LK. Ultrasound findings in HIV-positive patients with parotid swellings. J Oral Maxillofac Surg. 2001;59:283–286.
Martinoli, C, Derchi, LE, Solbiati, L, et al. Color doppler sonography of salivary glands. AJR Am J Roentgenol. 1994;163:933–941.
Rothberg, R, Noyek, AM, Goldfinger, M, et al. Diagnostic ultrasound imaging of parotid disease-a contemporary clinical perspective. J Otolaryngol. 1984;13:232–240.
Shimizu, M, Ussmüller, J, Donath, K, et al. Sonographic analysis of recurrent parotitis in children: a comparative study with sialographic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:606–615.
OBSTRUCTIVE AND INFLAMMATORY DISORDERS
Aung, W, Yamada, I, Umehara, I, et al. Sjögren’s syndrome: comparison of assessments with quantitative salivary gland scintigraphy and contrast sialography. J Nucl Med. 2000;41:257–262.
Brook, I. Acute bacterial suppurative parotitis: microbiology and management. J Craniofac Surg. 2003;14:37–40.
Gonzales, L, Mackenzie, AH, Tarar, RA. Parotid sialography in Sjögren’s syndrome. Radiology. 1970;97:91–93.
Hughes, M, Carson, K, Hill, J. Scintigraphic evaluation of sialadenitis. Br J Radiol. 1994;67:328–331.
Kassan, SS, Moutsopoulos, HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164:1275–1284.
Langlais, RP, Kasle, MJ. Sialolithiasis: the radiolucent stones. Oral Surg Oral Med Oral Pathol. 1975;40:686–690.
Lemon, SI, Imbesi, SG, Shikhman, AR. Salivary gland imaging in Sjögren syndrome. Future Rheumatol. 2007;2:83–92.
Scully, C. Sjögren’s syndrome: clinical and laboratory features, immunopathogenesis, and management. Oral Surg Oral Med Oral Pathol. 1986;62:510–523.
Som, PM, Shugar, JM, Train, JS, et al. Manifestations of parotid gland enlargement: radiographic, pathologic, and clinical correlation—part 1, the autoimmune pseudosialectasias. Radiology. 1981;141:415–419.
Yamamoto, Y, Harada, S, Ohara, M, et al. Clinical and pathological differences between Mikulicz’s disease and Sjögren’s syndrome. Rheumatology (Oxford). 2005;44:227–234.
Chilla, R. Sialadenosis of the salivary glands of the head. Studies on the physiology and pathophysiology of parotid secretion. Adv Otorhinolaryngol. 1981;26:1–38.
Boahene, DKO, Olsen, KD, Lewis, JE, et al. Mucoepidermoid carcinoma of the parotid gland—the Mayo Clinic experience. Arch Otolaryngol Head Neck Surg. 2004;130:849–856.
Boles, R, Raines, J, Lebovits, M, et al. Malignant tumors of salivary glands: a university experience. Laryngoscope. 1980;90:729–736.
Byrne, MN, Spector, JG, Garvin, CF, et al. Preoperative assessment of parotid masses: a comparative evaluation of radiographic techniques to histologic diagnosis. Laryngoscope. 1989;99:284–292.
Day, TA, Deveikis, J, Gillespie, MB, et al. Salivary gland neoplasms. Curr Treat Options Oncol. 2004;5:11–26.
Del Balso, AM, Williams, E, Tane, TT. Parotid masses: current modes of diagnostic imaging. Oral Surg. 1982;54:360–364.
Eneroth, CM. Salivary gland tumors in the parotid gland and the palate region. Cancer. 1971;27:1415–1418.
Gottesman, RI, Som, PM, Mester, J, et al. Observations on two cases of apparent submandibular gland cysts in HIV positive patients: MR and CT findings. J Comput Assist Tomogr. 1996;20:444–447.
Mirich, DR, McArdle, CB, Kulkarni, MV. Benign pleomorphic adenomas of the salivary glands: surface coil MR imaging versus CT. J Comput Assist Tomogr. 1987;11:620–623.
Thawley, SE, Panbje, WR. Comprehensive management of head and neck tumors. Philadelphia: WB Saunders; 1987.
Tsai, SC, Hsu, HT. Parotid neoplasms: diagnosis, treatment, and intraparotid facial nerve anatomy. J Laryngol Otol. 2002;116:359–362.