Chapter 4 The biological effects and risks associated with X-rays
CLASSIFICATION OF THE BIOLOGICAL EFFECTS
The biologically damaging effects of ionizing radiation are classified into three main categories:
The somatic effects are further subdivided into:
• Acute or immediate effects — appearing shortly after exposure, e.g. as a result of large whole body doses (Table 4.1)
• Chronic or long-term effects — becoming evident after a long period of time, the so-called latent period (20 years or more), e.g. leukaemia.
Table 4.1 Summary of the main acute effects following large whole-body doses of radiation
| Dose | Whole-body effect |
|---|---|
| 0.25 Sv | Nil |
| 0.25–1.0 Sv | Slight blood changes, e.g. decrease in white blood cell count |
| 1–2 Sv | |
| 2–6 Sv | |
| 6–10 Sv | |
| >10 Sv | Brain damage, coma, death |
Somatic deterministic effects
These are the damaging effects to the body of the person exposed that will definitely result from a specific high dose of radiation. Examples include skin reddening and cataract formation. The severity of the effect is proportional to the dose received, and in most cases a threshold dose exists below which there will be no effect.
Somatic stochastic effects
Stochastic effects are those that may develop. Their development is random and depends on the laws of chance or probability. Examples of somatic stochastic effects include leukaemia and certain tumours.
These damaging effects may be induced when the body is exposed to any dose of radiation. Experimentally it has not been possible to establish a safe dose — i.e. a dose below which stochastic effects do not develop. It is therefore assumed that there is no threshold dose, and that every exposure to ionizing radiation carries with it the possibility of inducing a stochastic effect.
The lower the radiation dose, the lower the probability of cell damage. However, the severity of the damage is not related to the size of the inducing dose. This is the underlying philosophy behind present radiation protection recommendations (see Ch. 8).
Genetic stochastic effects
Mutations result from any sudden change to a gene or chromosome. They can be caused by external factors, such as radiation or may occur spontaneously.
Radiation to the reproductive organs may damage the DNA of the sperm or egg cells. This may result in a congenital abnormality in the offspring of the person irradiated. However, there is no certainty that these effects will happen, so all genetic effects are described as stochastic.
A cause-and-effect relationship is difficult, if not impossible, to prove. Although ionizing radiation has the potential to cause genetic damage, there are no human data that show convincing evidence of a direct link with radiation. Risk estimates have been based mainly on experiments with mice. It is estimated that a dose to the gonads of 0.5–1.0Sv would double the spontaneous mutation rate. Once again it is assumed that there is no threshold dose.
Effects on the unborn child
The developing fetus is particularly sensitive to the effects of radiation, especially during the period of organogenesis (2–9 weeks after conception). The major problems are:
• Congenital abnormalities or death associated with large doses of radiation
• Mental retardation associated with low doses of radiation.
As a result, the maximum permissible dose to the abdomen of a woman who is pregnant is regulated by law. This is discussed further in Chapter 8.
Harmful effects important in dental radiology
In dentistry, the size of the doses used routinely are relatively small (see Ch. 3) and well below the threshold doses required to produce the somatic deterministic effects. However, the somatic and genetic stochastic effects can develop with any dose of ionizing radiation. Dental radiology does not usually involve irradiating the reproductive organs, thus in dentistry somatic stochastic effects are the damaging effects of most concern.
HOW DO X-RAYS CAUSE DAMAGE?
The action of radiation on cells and the damaging effects are illustrated in Figure 4.1. and classified as:
• Direct action or damage as a result of ionization of macromolecules
• Indirect action or damage as a result of the free radicals produced by the ionization of water.
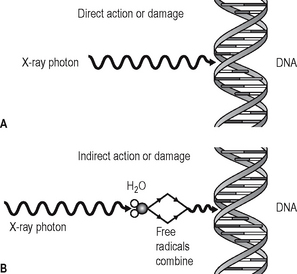
Fig. 4.1 Diagram illustrating the action and damaging effects of radiation on cells. A Direct action or damage — the X-ray photon interacts directly with the DNA. B Indirect action or damage — the X-ray photon ionizes water to produce free radicals which damage the DNA.
Direct action or damage
The X-ray photons, or high-energy ejected electrons, interact directly with, and ionize, vital biologic macromolecules such as DNA, RNA, proteins and enzymes. This ionization results in the breakage of the macromolecule’s chemical bonds, causing them to become abnormal structures, which may in turn lead to inappropriate chemical reactions. Rupture of one of the chemical bonds in a DNA macromolecule may sever one of the side chains of the ladder-like structure. This type of injury to DNA is called a point mutation. The subsequent chromosomal effects from direct damage could include:
• Inability to pass on information
• Only temporary damage — the DNA being repaired successfully before further cell division.
If the radiation directly affects somatic cells, the effects on the DNA (and hence the chromosomes) could result in a radiation-induced malignancy. If the damage is to reproductive stem cells, the result could be a radiation-induced congenital abnormality.
What actually happens in the cell depends on several factors, including:
Indirect action or damage
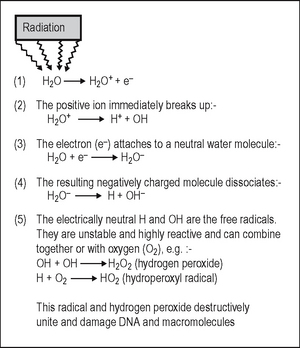
This process, which is shown in Figure 4.2, involves the breakdown of the water molecule into smaller molecules, producing both ions and free radicals in the process. The free radicals can recombine to form hydrogen peroxide, a cellular poison, and a hydroperoxyl radical, another toxic substance. Both of these substances are highly reactive and produce biologic damage. By themselves, free radicals may transfer excess energy to other molecules, thereby breaking their chemical bonds and having an even greater effect. As about 80% of the body consists of water, the vast majority of the interactions with ionizing radiation are indirect.
ESTIMATING THE MAGNITUDE OF THE RISK OF CANCER INDUCTION
Quantifying the risk of somatic stochastic effects, such as radiation-induced cancer, is complex and controversial. Data from groups exposed to high doses of radiation are analysed and the results are used to provide an estimate of the risk from the low doses of radiation encountered in diagnostic radiology. The high-dose groups studied include:
• The survivors of the atomic explosions at Hiroshima and Nagasaki
• Patients receiving radiotherapy
• Radiation workers — people exposed to radiation in the course of their work
The problem of quantifying the risk is compounded because cancer is a common disease, so in any group of individuals studied there is likely to be some incidence of cancer. In the groups listed above, that have been exposed to high doses of radiation, the incidence of cancer is likely to be increased and is referred to as the excess cancer incidence. From the data collected, it has been possible to construct dose–response curves (Fig. 4.3), showing the relationship between excess cancers and radiation dose. The graphs can be extrapolated to zero (the controversy on risk assessment revolves around exactly how this extrapolation should be done), and a risk factor for induction of cancer by low doses of radiation can be calculated.
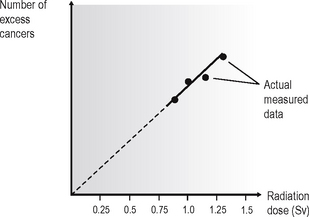
Fig. 4.3 A typical dose–response curve, showing excess cancer incidence plotted against radiation dose and a linear extrapolation of the data to zero.
A broad estimate of the magnitude of the risk of developing a fatal radiation-induced cancer, from various X-ray examinations is shown in Table 4.2. These are based broadly on the NRPB booklet Guidelines on Patient Dose to Promote the Optimisation of Protection for Diagnostic Medical Exposures published in the UK in 1999 and the European Guidelines on Radiation Protection in Dental Radiology published in 2004.
Table 4.2 Broad estimate of the risk of a standard adult patient developing a fatal radiation-induced malignancy from various X-ray examinations (NRPB 1999)
| X-ray examination | Estimated risk of fatal cancer |
|---|---|
| Traditional bitewing/periapical (50 kV, D speed film, 10 cm fsd) | 1 in 2 000 000 |
| Modern bitewing/periapical (70 kV, F speed film, 20 cm fsd) | 1 in 20 000 000 |
| Panoramic — average (estimates range from 0.21 − 1.9) | 1 in 1 000 000 |
| Skull (PA) | 1 in 670 000 |
| Skull (Lat) | 1 in 2 000 000 |
| Chest (PA) | 1 in 1 000 000 |
| Lumbar spine (AP) | 1 in 29 000 |
| Barium swallow | 1 in 13 000 |
| Barium enema | 1 in 3000 |
| CT chest | 1 in 2500 |
| CT head | 1 in 10 000 |
Risk is age-dependent, being highest for the young and lowest for the elderly. The risks shown in Table 4.2 are for a 30-year-old adult. The 2004 European Guidelines and the 2004 Selection Criteria in Dental Radiography booklet published by the Faculty of General Dental Practice (UK) of the Royal College of Surgeons in England, both recommend that these should be modified by the multiplication factors shown in Table 4.3 which represent averages for the two sexes. In fact at all ages, risks for females are slightly higher and risks for males slightly lower.
Table 4.3 The multiplication factor for risk for different age groups
| Age group (years) | Multiplication factor for risk |
|---|---|
| <10 | × 3 |
| 10–20 | × 2 |
| 20–30 | × 1.5 |
| 30–50 | × 0.5 |
| 50–80 | × 0.3 |
| 80+ | negligible risk |
This epidemiological information is being updated continually and recent reports suggest that the risk from low-dose radiation may be considerably greater than thought previously. However, the present figures at least provide an idea of the comparative order of magnitude of the risk involved from different investigations. This in turn helps keep the risks associated with dental radiology in perspective.
SUMMARY
The biological effects of ionizing radiation can be extremely damaging. Somatic deterministic effects predominate with high doses of radiation, while somatic stochastic effects predominate with low doses. Dental radiology employs low doses and the risk of stochastic effects is very small. However, the number of dental radiographs is very high — estimated at between 20–25 million intraoral and extraoral radiographs per year in the UK alone. It has been estimated that the overall risk from dental radiography in the UK is in the order of 10 fatal malignancies per year. The various important dose-reduction and dose-limitation measures that are therefore necessary to keep all exposures as low as reasonably practicable (ALARP), for both patients and for dental staff, are outlined in Chapter 8.