Neonatal infection
Etiology Common infections for each animal species are listed under etiology below. Most are bacterial.
Epidemiology Commonly predisposed by management and environmental factors that increase the exposure risk and load and decrease the resistance of the neonate.
Clinical findings Septicemia or bacteremia with localization is most common but signs can be specific for the infecting agent.
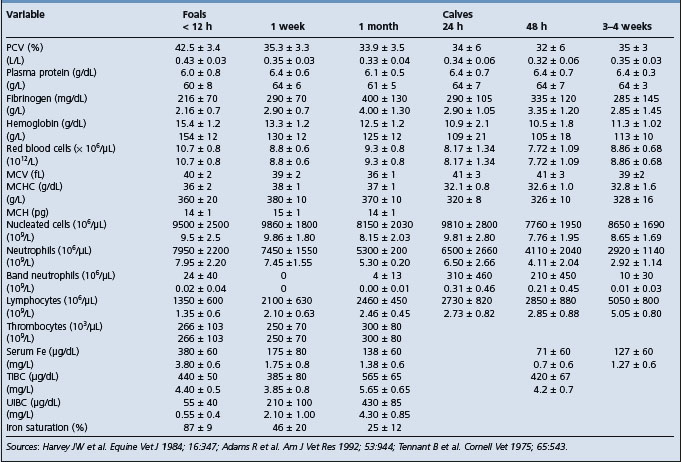
Clinical pathology White blood cell and differential counts, toxic change, serum immunoglobulin concentrations, arterial oxygen concentrations, metabolic acidosis, fibrinogen levels, blood culture.
Necropsy findings Specific to disease.
Diagnostic confirmation Specific to disease.
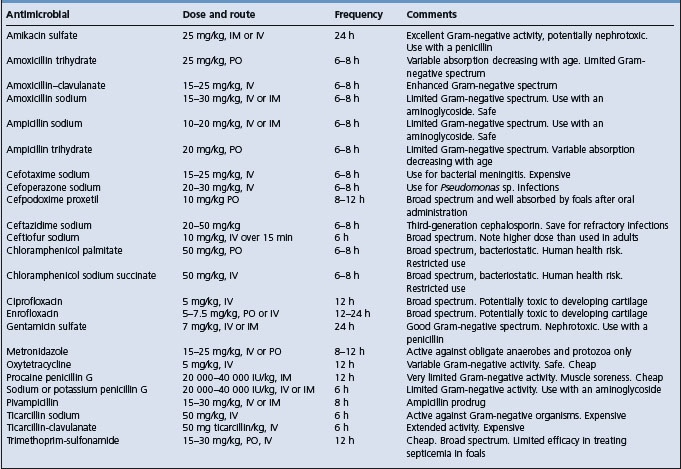
Treatment General therapy may include antibacterial therapy, blood or plasma transfusion, correction of acid–base disturbance, fluid and electrolyte therapy, and supportive treatment.
Infection is a common cause of morbidity and mortality in neonates. There are a number of specific infectious pathogens that can cause disease. Other infectious agents, normally considered to have low virulence, can also cause disease if the immunological status of the neonate is not at an optimum level. Maternal immunoglobulins are not transferred transplacentally in ungulates and the newborns are at particular risk for infectious disease during the neonatal period because they rely on the acquisition of immunoglobulins from colostrum for passive antibody protection.
ETIOLOGY
In domestic farm animals the common infections that can produce disease during the neonatal period are as follows. (Relative importance and prevalence statistics are not given, as these vary from area to area and with differing management systems.)
Calves
• Bacteremia and septicemia associated with Escherichia coli, Listeria monocytogenes, Pasteurella spp., streptococci or Salmonella spp.
• Enteritis associated with enterotoxigenic E. coli, Salmonella spp., rotavirus and coronavirus, Cryptosporidium parvum and Clostridium perfringens types A, B and C; and occasionally by the virus of infectious bovine rhinotracheitis and bovine virus diarrhea.
Pigs
• Septicemia with or without localization in joints, endocardium and meninges associated with Streptococcus suis, Streptococcus equisimilis, Streptococcus zooepidemicus and L. monocytogenes
• Bacteremia, septicemia and enteritis associated with E. coli
• Transmissible gastroenteritis, Aujeszky’s disease, swine pox, enterovirus infections, and vomiting and wasting disease are associated with viruses
• Enteritis associated with C. perfringens, Campylobacter spp., rotavirus and Coccidia spp.
• Arthritis and septicemia associated with Erysipelothrix rhusiopathiae.
Foals
• Septicemia with localization associated with E. coli, Actinobacillus equuli, Klebsiella pneumoniae, α-hemolytic streptococci, S. zooepidemicus, L. monocytogenes, Rhodococcus equi and Salmonella typhimurium
• Enteritis associated with C. perfringens types A, B, and C., Clostridium difficile, R. equi, Salmonella spp., Strongyloides westeri, C. parvum and rotavirus.
Lambs
• Septicemia or bacteremia with localization in joints and/or synovia and/or leptomeninges associated with E. coli, L. monocytogenes, streptococci, micrococci, E. rhusiopathiae and Chlamyodophila spp.
• Enteritis associated with enterotoxigenic E. coli, Salmonella spp., rotavirus and coronavirus and C. parvum
• Lamb dysentery associated with C. perfringens type B and C
• Gas gangrene of the navel associated with Clostridium septicum, Clostridium novyi and Clostridium chauvoei
• Pyemia associated with Staphylococcus aureus, Fusobacterium necrophorum and Arcanobacterium pyogenes
• Pneumonia, polyserositis and peritonitis associated with Pasteurella multocida and Mannheimia haemolytica.
The following agents are recorded as causing neonatal infections but are less common than those listed above and not of as great importance.
Calves
Pseudomonas aeruginosa, Streptococcus pyogenes, Streptococcus faecalis, S. zooepidemicus, Pneumococcus spp.; enteritis due to Providencia stuartii, Chlamydophila spp., A. equuli.
Lambs
S. aureus (tick pyemia); enteritis due to E. coli, rotavirus; pneumonia due to Salmonella abortus–ovis.
EPIDEMIOLOGY
The occurrence of neonatal disease is broadly influenced by two main factors: the exposure or infection pressure of the infectious agent to the neonate and the ability of the neonate to modulate the infection so that disease does not occur. With some agents the organism is sufficiently virulent in its own right that an exposure can lead to disease. With others, the majority, the defenses of the host must be compromised or the infection challenge must be very high before clinical disease occurs. Management of the neonate has a great influence on both these factors and the recognition and correction of these risks is the key to the prevention of neonatal disease in both the individual and the group.
Sources of infection
Postnatal infection
The vast majority of infections are acquired by the neonate after birth from the enteric or respiratory tract flora of the dam, from the environment or from close contact with other infected neonates. Depending upon the specific agent, the reservoir of infection may be in a carrier animal or in the environment. Details for the common neonatal diseases are given under the individual disease headings in the chapters on special medicine.
Prenatal infection
Some bacterial infections that manifest with neonatal disease are acquired in utero. The majority of these are agents that cause abortion, and neonatal septicemia is only part of the spectrum of abortion and perinatal death associated with these agents. Examples would be many of the agents producing abortion in sheep.
Some septicemic infections in foals, particularly those associated with A. equuli, S. zooepidemicus, Salmonella abortivoequina and possibly some E. coli septicemic infections, are acquired by prenatal infection. If the disease is intrauterine in origin it must gain entrance via the placenta, and probably by means of a placentitis due to a blood-borne infection or an existing endometritis. In the latter case, disinfecting the uterus before mating becomes an important hygienic precaution; disinfecting the environment may have little effect on the incidence of the disease.
Viral infections that are acquired in utero are listed in the section on congenital disease.
Routes of transmission
The portal of infection is commonly by ingestion but may occur via aerosol infection of the respiratory tract. Organisms capable of invading to produce a bacteremia and septicemia invade through the nasopharynx or through the intestinal epithelium. An alternate route of infection and invasion is via the umbilicus. Routes of excretion are via the feces in enteric disease and the nasal secretions, urine and sometimes the feces in septicemic disease to result in contamination of the neonatal environment.
Where neonates are in groups or in close contact, direct transmission by fecal, respiratory secretion and urine aerosols can also occur. Neonatal bull calves that are group-housed and that suck each other’s navels can transmit infection by this activity.
Risk factors and modulation of infection
Immunity
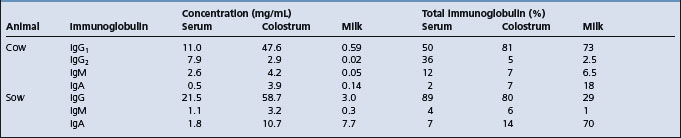
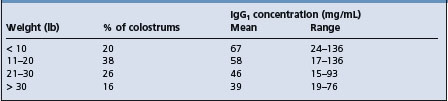
All newborn farm animals are more susceptible to infection than their adult counterparts. The calf, lamb, piglet and foal are born without significant levels of immunoglobulins and possess almost no resistance to certain diseases until after they have ingested colostrum and absorbed sufficient quantities of immunoglobulins from the colostrum. Failure of transfer of colostral immunoglobulins is a major determinant and is discussed under that heading later in this chapter.
Immune responsiveness
All components of the immune system are present in foals and calves at birth but the immune system of the newborn animal is less mature than its adult counterpart, at least for the first 30 days of life, and does not respond as effectively to many antigens.
Immune responsiveness is age-dependent but also varies with the antigen.1 In colostrum-fed animals part of the inefficiency of the newborn to produce humoral antibody following infection of antigen is the interference from circulating colostral antibody and the downregulation by colostrum of endogenous immunoglobulin production.2-4
Colostrum-deprived calves respond actively to injected antigens and are believed to be immunologically competent at birth with respect to most antigens. Immune competence begins during fetal life and the age of gestation at which this occurs varies according to the nature of the antigen. The bovine fetus will produce antibody to some viruses, beginning at 90–120 days, and by the third trimester of gestation it will respond to a variety of viruses and bacteria.5 The lamb will respond to some antigens beginning as early as 41 days and not until 120 days for others. The piglet at 55 days and the fetal foal also respond to injected antigens.
The presence of high levels of antibody in the precolostral serum of newborn animals suggests that an in-utero infection was present, which is useful for diagnostic purposes. The detection of immunoglobulins and specific antibodies in aborted fetuses is a useful aid in the diagnosis of abortion in cattle.
Exposure pressure
The exposure pressure is a factor of the cleanliness of the environment of the neonate. The phenomenon of a ‘buildup of infection’ in continual-throughput housing for neonatal animals has been recognized for decades and has been translated to many observations of risk for neonatal disease associated with suboptimal hygiene and stocking density in both pen and paddock birthing areas. Details for the individual species are provided in the section on perinatal disease.
Age at exposure
With several agents that produce neonatal disease, the age of the neonate at infection and the infecting dose have a significant influence on the outcome. Examples are the importance of age with respect to susceptibility to disease associated with some enteric infections. Disease associated with enteropathogenic E. coli and with C. perfringens type B and C occurs only in young animals and if infection can be avoided by hygiene in this critical period disease will not occur regardless of subsequent exposure. Colostrum-deprived calves show significant resistance to challenge at 7 days of age with strains of E. coli that invariably produce septicemic disease if challenged at the time of birth and isolation of an immunocompromised neonate is an important factor in its survival. Thus the management of the neonate and its environment is a critical determinant of its health. Age at exposure also varies with the epidemiology of the pathogen and segregated early weaning is used to reduce transmission of and infection with certain pathogens in pigs.
PATHOGENESIS
The pathogenesis varies with the neonatal infectious disease under consideration and is given for each of these in the special medicine section.
Following invasion via the nasopharynx and the gastrointestinal tract, the usual pattern of development is a bacteremia followed by septicemia with severe systemic signs; or a bacteremia with few or no systemic signs, followed by localization in various organs. If the portal of entry is the navel, local inflammation occurs – ‘navel ill’ – which can be easily overlooked if clinical examination is not thorough. From the local infection at the navel, extension may occur to the liver or via the urachus to the bladder and result in chronic ill-health. Extension systemically may produce septicemia.6
Localization is most common in the joints, producing a suppurative or nonsuppurative arthritis. Less commonly there is localization in the eye to produce a panophthalmitis, in the heart valves to cause valvular endocarditis, or in the meninges to produce a meningitis.
In some cases these secondary lesions take time to develop and signs usually appear at 1–2 weeks of age. This is especially true with some of the streptococcal infections, where bacteremia may be present for several days before localization in the joints and meninges produces clinical signs. Bacterial meningitis in newborn ungulates is preceded by a bacteremia followed by a fibrinopurulent inflammation of the leptomeninges, choroid plexuses and ventricle walls but does not affect the neuraxial parenchyma. It is proposed that the bacteria are transported in monocytes, which do not normally invade the neuraxial parenchyma.
Dehydration, acid–base and electrolyte imbalance can occur very quickly in newborn animals, whether diarrhea and vomiting (pigs) are present or not, but obviously are more severe where there is fluid loss into the gastrointestinal tract. In Gram-negative sepsis the prominent signs are those of endotoxemia.
CLINICAL FINDINGS
The clinical findings depend on the rapidity of growth of the organism, its propensity to localize and its potential to produce toxemia. With organisms that have a low propensity for toxemia there is fever, depression, anorexia and signs referable to localization. These include endocarditis with a heart murmur; panophthalmitis with pus in the anterior chamber of the eye; meningitis with rigidity, pain and convulsions; and polyarthritis with lameness and swollen joints. With more virulent organisms there are clinical signs of toxemia as well as bacteremia, including fever, severe depression, prostration, coma, petechiation of mucosae, dehydration, acidosis and rapid death.7,8
The clinical and clinicopathological characteristics of the septicemic foal have been detailed in an outbreak of septicemia in colostrum-deprived foals9 and on the clinical records of 38 septicemic foals admitted to a referral clinic,10 where the survival rate of septicemic foals, 26%, was markedly less than the rate for all other foal admissions. The major clinical findings included lethargy, unwillingness to suck, inability to stand without assistance but remaining conscious, unawareness of environment and thrashing or convulsing, diarrhea, respiratory distress, joint distension, central nervous system abnormalities, uveitis and colic. Fever was not a consistent finding.
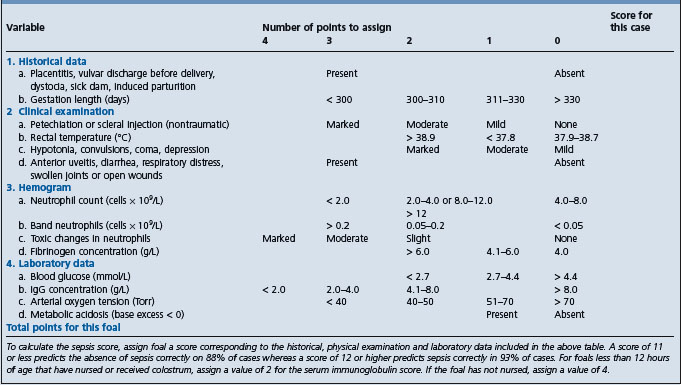
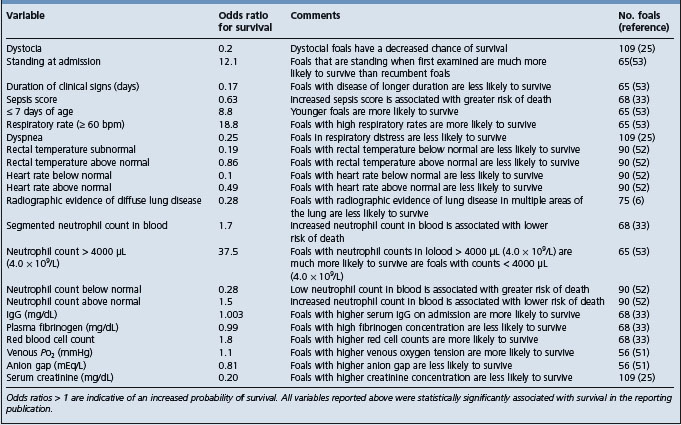
A sepsis score has been developed for foals based on 14 measures related to historical, clinical and laboratory data (Table 3.6). The score derived from the collective differential scoring of these data has been found to be more sensitive and specific for infection than any parameter taken individually.7 However, a subsequent study of 168 foals presented to a university hospital found that the sepsis score correctly predicted sepsis in 58 out of 86 foals and nonsepsis in 24 out of 45 foals resulting in a sensitivity of 67%, a specificity of 75%, a positive predictive value of 84% and a negative predictive value of 55%, and it was suggested that the score system should be used with care as the low negative predictive value limited its clinical utility.11
A sepsis score, based on fecal consistency, hydration, behavior, ability to stand, state of the umbilicus and degree of injection of scleral vessels, is described for calves and has reasonable predictive value.12
The clinical findings specific to individual etiological agents are given under their specific headings in the special medicine section of this book.
CLINICAL PATHOLOGY
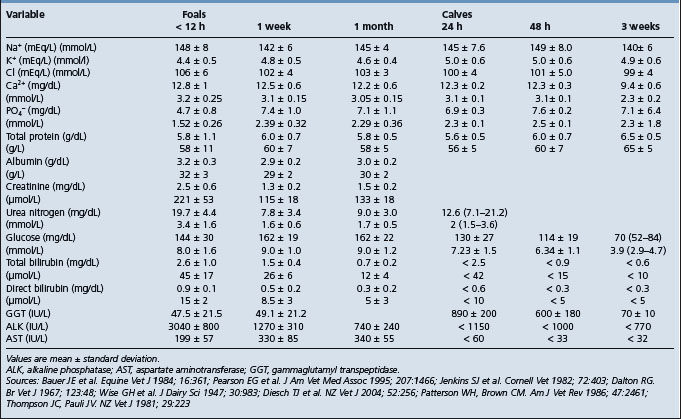
Clinical pathology is used as an integral part of the evaluation of a sick neonate and to help formulate a treatment plan. A major evaluation is to attempt to confirm the presence or absence of sepsis and this type of evaluation has been developed most successfully in the foal. Blood culture is part of this examination but the time for a positive result limits its value in the acutely ill neonate. Laboratory findings in foals with neonatal sepsis are variable and depend upon the severity, stage and site of infection.8 Serial examinations are commonly used. In examinations relating to the possible presence of septicemia, particular emphasis is placed on the results of the white blood cell and differential counts, the presence of toxic change, serum immunoglobulin concentrations, arterial oxygen concentrations, presence of metabolic acidosis and fibrinogen levels.7,8,12
• The principles of diagnosis of infectious disease in newborn animals are the same as for older animals. However, in outbreaks of suspected infectious disease in young animals there is usually a need for more diagnostic microbiology and pathology
• With outbreaks, owners should be encouraged to submit all dead neonates as soon as possible for a meaningful necropsy examination
• In addition to postmortem examination it is necessary to identify the factors that may have contributed to an outbreak of disease in newborn calves, piglets or lambs and only detailed epidemiological investigation will reveal these
TREATMENT
The first principle is to obtain an etiological diagnosis if possible. Ideally a drug sensitivity of the causative bacteria should be obtained before treatment is given, but this is not always possible. It may be necessary to choose an antibacterial based on the tentative diagnosis and previous experience with treatment of similar cases.
Outbreaks of infectious disease are common in litters of piglets and groups of calves and lambs, and individual treatment is often necessary to maximize survival rate. There is usually no simple method of mass-medicating the feed and water supply of sucking animals and each animal should be dosed individually as necessary. Supportive fluid and electrolyte therapy and correction of acid–base disturbances are described in detail in Chapter 2.
The provision of antibodies to sick and weak newborn animals through the use of blood transfusions or serum is often practiced, especially in newborn calves in which the immunoglobulin status is unknown. Whole blood given at the rate of 10–20 mL/kg body weight, preferably by the intravenous route, will often save a calf that appears to be in shock associated with neonatal diarrhea. The blood is usually followed by fluid therapy. Serum or plasma can also be given at half the dose rate. The blood should not be taken from a cow near parturition as the circulating immunoglobulins will be low from the transfer into the mammary gland.
Plasma is often incorporated into the therapeutic regimen in foals, both for its immunoglobulin content and for its effect on blood volume and osmotic pressure. Stored plasma can be used. A dose of 20 mL plasma/kg body weight given slowly intravenously is often used, but significantly higher doses are required to elevate circulating immunoglobulins by an appreciable amount.8 Blood may be collected, the red blood cells allowed to settle and the plasma removed and stored frozen. The donor plasma should be prescreened for compatibility. Lyophilized hyperimmune equine serum as a source of antibodies may also be fed to foals within 4 hours after birth. Good nursing care is also essential.
Further information on treatment is given in the section on critical care for the newborn later in this chapter.
1 Watson DL, et al. Res Vet Sci. 1994;57:152.
2 Kitching RP, Salt JS. Aust Vet J. 1995;151:379.
3 Ellis JA, et al. J Am Vet Med Assoc. 1996;208:393.
4 Aldridge BM, et al. Vet Immunol Immunopathol. 1998;62:51.
5 Tierney TJ, et al. Vet Immunol Immunopathol. 1997;57:229.
6 Staller GS, et al. J Am Vet Med Assoc. 1995;206:77.
7 Brewer BD, Koterba AM. Equine Vet J. 1988;20:18.
8 Carter GK. Compend Contin Educ Pract Vet. 1986;8:S256.
9 Robinson JA, et al. Equine Vet J. 1993;25:214.
10 Koterba AM, et al. Equine Vet J. 1984;16:376.