Chapter 10 Diseases of the respiratory system
PRINCIPLES OF RESPIRATORY INSUFFICIENCY 471
PRINCIPAL MANIFESTATIONS OF RESPIRATORY INSUFFICIENCY 473
SPECIAL EXAMINATION OF THE RESPIRATORY SYSTEM 480
PRINCIPLES OF TREATMENT AND CONTROL OF RESPIRATORY TRACT DISEASE 493
DISEASES OF THE LUNGS 498
DISEASES OF THE PLEURA AND DIAPHRAGM 519
DISEASES OF THE UPPER RESPIRATORY TRACT 530
Principles of respiratory insufficiency
The principal function of the respiratory system is gas exchange in which oxygen is transferred from the environment to the blood and carbon dioxide is moved in the opposite direction. Other important functions include a role in thermoregulation in most species, acid–base regulation in concert with the kidney, as an endocrine organ (e.g. angiotensin-converting enzyme), in the metabolism of metabolically active substances, including eicosanoids and nitric oxide, and in the immune response to inhaled immunogens and pathogens. Capillaries in the lungs of the farm animal species and horses also possess intravascular macrophages, which are important as a reticuloendothelial organ in the processing of antigens – an action achieved by similar cells in the liver of dogs, cats, and humans. Interference with these functions can occur in a number of ways and can have a variety of manifestations that are apparent during disease. The most readily apparent failure of the respiratory system is failure of gas exchange with resultant hypoxemia and hypercapnia. However, failure of other functions of the respiratory system can also result in clinically apparent disease.
Failure of gas exchange, and the resultant hypoxia and hypercapnia, is responsible for most of the clinical signs of respiratory disease and for respiratory failure, the terminal event of fatal cases. Death due to respiratory failure is due to hypoxia. An understanding of hypoxia, hypercapnia and respiratory failure is essential to the study of clinical respiratory disease.
DEFINITIONS
A number of terms are used to describe the function of the respiratory tract, or abnormalities that arise because of a variety of diseases. Many of these terms are described in more detail in the text that follows, but a brief definition of each is provided here:
• Hypoxia is a broad term meaning diminished availability of oxygen to tissues
• Hypoxemia is deficient oxygenation of blood, usually assessed by measurement of blood oxygen tension, or by measurement of blood hemoglobin saturation and hemoglobin concentration, and subsequent calculation of blood oxygen content
• Hypercapnia is an abnormally high carbon dioxide tension in blood
• Pao2 is the oxygen tension (partial pressure) in arterial blood
• PAo2 is the oxygen partial pressure in alveolar gas
• Paco2 is the carbon dioxide tension in arterial blood
• PAco2 is the carbon dioxide partial pressure in alveolar air
• Cao2 is the arterial oxygen content (milliliters of O2 per 100 mL of blood)
• Pvo2 is the oxygen tension (partial pressure) in venous blood
• Pvco2 is the carbon dioxide tension in venous blood
• Cvo2 is the venous oxygen content (milliliters of O2per 100 mL of blood)
• Respiratory failure is the inability of an animal to maintain arterial blood oxygenation and carbon dioxide tension within the normal range
• Dyspnea refers to signs of respiratory distress in animals (in humans it describes the sensation of air hunger, which is a symptom and not a sign)
• Polypnea is an excessively high rate of breathing
• Tachypnea is an excessively high rate of breathing, with the implication that the breathing is shallow
HYPOXIA
Failure of the tissues to receive an adequate supply of oxygen occurs in a number of ways and the differences are clinically relevant, in that they are associated with failure of different organ systems, different diseases, and have fundamentally different pathophysiological mechanisms
Hypoxic (or hypoxemic) hypoxia
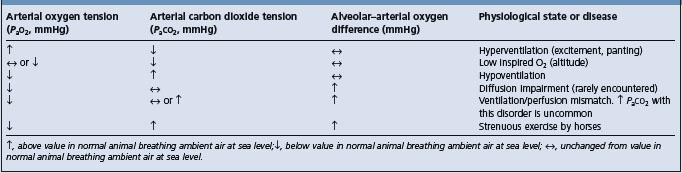
Hypoxic (or hypoxemic) hypoxia occurs when there is inadequate oxygenation of blood (hypoxemia) and is usually associated with disease of the respiratory tract or other causes of hypoventilation. Situations in which there is inadequate oxygenation of blood in the lungs include hypoventilation, ventilation/perfusion mismatches, diffusion impairment, low inspired oxygen tension and extrapulmonary right-to-left shunting.
Hypoventilation occurs in animals with depressed consciousness, such as occurs with general anesthesia and heavy sedation, or in newborns, in which the central respiratory drive is suppressed. Airway obstruction caused by the presence of foreign bodies in the airway, luminal obstruction by masses, such as retropharyngeal abscesses in horses with strangles, laryngeal spasm or bronchoconstriction can cause inadequate alveolar ventilation and hypoxemia. Diseases that prevent adequate inflation of lungs cause alveolar hypoventilation and the consequent hypoxemia. These diseases include pneumothorax, pleural effusion or respiratory muscle weakness, such as can occur with botulism, tick paralysis, tetanus, strychnine poisoning or severe white muscle disease.
Ventilation/perfusion ( /
/ ) mismatches occur when the distribution of blood flow in the lungs does not match the distribution of alveolar ventilation, with the result that areas of lung that are well ventilated are not adequately perfused and those areas that are well perfused by blood are not well ventilated. Ventilation/perfusion mismatches are the most important cause of hypoxemia in many lung diseases, including pneumonia.
) mismatches occur when the distribution of blood flow in the lungs does not match the distribution of alveolar ventilation, with the result that areas of lung that are well ventilated are not adequately perfused and those areas that are well perfused by blood are not well ventilated. Ventilation/perfusion mismatches are the most important cause of hypoxemia in many lung diseases, including pneumonia.
Diffusion impairment occurs when there is decreased transfer of oxygen from alveolar air that has a normal PAo2 to red blood cells in alveolar capillaries because of: increased distance of diffusion through the alveolar membranes, such as might occur with pulmonary edema; decreased surface area available for diffusion, such as occurs with positional atelectasis or pulmonary embolism; or decreased transit time of red cells through the alveolar capillaries, such as occurs in horses during heavy exercise.
Low inspired oxygen tension occurs naturally only in animals at high altitude. It can also occur during anesthesia if there are defects in the ventilator causing low oxygen tension in the gases delivered to the animal.
Extrapulmonary right-to-left shunting occurs most commonly as a vascular defect (see Ch. 8).
The actual cause of hypoxemia in an individual animal or disease is often multifactorial and not simply a result of one of the mechanisms described above. For instance, cows placed in dorsal recumbency during general anesthesia become hypoxemic because of compression of the thorax by the abdominal viscera, thereby causing hypoventilation and compression atelectasis with diffusion impairment, ventilation/perfusion mismatching and reduced cardiac output because of reduced venous return.1
Anemic hypoxia
Anemic hypoxia occurs when there is a deficiency of hemoglobin per unit volume of blood (anemia). The percentage saturation of the available hemoglobin and the oxygen tension of arterial blood are normal but as a result of the low hemoglobin concentration the oxygen-carrying capacity of the blood is reduced. Anemia due to any cause has these characteristics. The decrease in oxygen-carrying capacity caused by a 50% reduction in hemoglobin concentration from normal values (from 20 g/dL to 10 g/dL) is much greater than the decrease that results from a 50% reduction in arterial oxygen tension from normal (e.g. a reduction from 100 mmHg to 50 mmHg).
Alteration of hemoglobin to pigments, such as methemoglobin or carboxyhemoglobin, that are not capable of carrying oxygen has the same effect on oxygen content as anemia. Thus in poisoning caused by nitrite, in which hemoglobin is converted to methemoglobin, and in that due to carbon monoxide, when the hemoglobin is converted to carboxyhemoglobin, there is hypoxia due to inadequate oxygenation of blood.
Circulatory hypoxia
Circulatory hypoxia occurs as a result of inadequate delivery of oxygen to tissue because of inadequate perfusion of tissues by blood. The blood is usually adequately oxygenated but blood flow rate to tissues is not, and therefore the rate at which it delivers oxygen to tissue is less than the amount of oxygen required to support the metabolic function of that tissue. In other words, the rate of delivery of oxygen to tissue does not match the metabolic requirements of that tissue. A common cause of this is low cardiac output, such as occurs with congestive heart failure or hypovolemic shock. It also occurs with local interruption to arterial flow such as the thrombotic emboli of thromboembolic colic of horses or compression of vessels, such as in right displacement and torsion of the abomasum.
Histotoxic anoxia
Histotoxic anoxia occurs when oxygen delivery to tissue is adequate because both oxygen content of arterial blood and blood flow are appropriate, but the tissue is unable to utilize oxygen. Cyanide poisoning is the only common cause of this form of anoxia.
Consequences of hypoxia
Consequences of inadequate delivery of oxygen include changes in almost all body systems. The central nervous system and heart are most susceptible to the immediate and acute effects of hypoxia, whereas clinical signs related to hypoxic damage to the gastrointestinal tract and kidneys are somewhat delayed. Central nervous system hypoxia is evident as mild changes in mentation, such as depression, progressing through decreased alertness to coma and death. Cardiac changes include a reduction in the force and efficiency of contraction due to impaired myocardial contractility, and an increased susceptibility to arrhythmia. The kidney, gut and liver are all metabolically active tissues and therefore susceptible to hypoxia. Renal function is reduced during hypoxia, with the renal medulla being most sensitive to decreases in oxygen delivery. Signs of gastrointestinal dysfunction during hypoxia include ileus, abdominal pain and abdominal distension due to accumulation of gas and liquid in the gastrointestinal tract. Liver dysfunction can be evident as decreases in blood glucose concentration and increases in serum activity of liver-derived enzymes (alkaline phosphatase, gamma-glutamyl transpeptidase, sorbitol (inositol) dehydrogenase) and metabolites (bile acids, bilirubin).
Some metabolically active tissues, when deprived of oxygen, use anaerobic metabolism to sustain energy supply for short periods of time (depending on the tissue, but the brain cannot survive without oxygen for more than 2–3 min). Use of anaerobic glycolysis for energy causes metabolic acidosis. Animals in respiratory failure therefore often have a mixed acid–base disturbance characterized by metabolic and respiratory acidosis.
COMPENSATORY MECHANISMS
Compensation of respiratory insufficiency occurs as both short-term and long-term events. Short-term compensatory mechanisms for low arterial oxygen tension or oxygen delivery to tissues occur within seconds to minutes and include respiratory, cardiovascular and behavioral responses. Stimulation of respiratory centers in the medulla oblongata by low arterial oxygen tension (Pao2) and high arterial carbon dioxide tension (Paco2) causes an increase in respiratory minute volume mediated by an increase in tidal volume and respiratory frequency. Both low oxygen tension and high carbon dioxide tension in arterial blood, together or separately, are potent stimulators of these events. Inadequate tissue oxygenation also stimulates an increase in cardiac output, mainly as a result of increased heart rate and to a lesser extent by an increase in stroke volume. Splenic contraction, in those species such as the horse in which the spleen is an important reservoir of red blood cells, increases both blood volume and hemoglobin concentration, thereby increasing the oxygen-carrying capacity of blood. Hypoxemia also causes animals to attempt to decrease their oxygen requirement by decreasing physical activity, including moving and eating.
Longer-term compensatory mechanisms include an increase in erythropoietin secretion by the kidney with subsequent increases in bone marrow production of red blood cells and an increase in hemoglobin concentration in blood. This polycythemia increases the oxygen-carrying capacity of blood. Severe polycythemia, such as occurs with congenital cardiac anomalies causing chronic right-to-left shunting, increases the viscosity of blood and impairs tissue perfusion, increases the workload of the heart and the risk of thromboembolism. Longer-term compensatory mechanisms also include changes in ventilatory pattern, such as in horses with heaves, and behavior.
CARBON DIOXIDE RETENTION (HYPERCAPNIA)
Respiratory insufficiency results in decreased elimination of carbon dioxide and its accumulation in blood and tissues. Animals breathing room air that are hypercapnic are always hypoxemic. Increasing the oxygen tension of inspired air can alleviate the hypoxemia but, by reducing hypoxic stimulation of the respiratory center, can cause further increments in arterial Pco2.
Acute hypercapnia causes a respiratory acidosis that reduces both blood and cerebrospinal fluid pH.2 The clinical signs of acute hypercapnia are initial anxiety followed by central nervous system depression and eventual coma and death. These clinical abnormalities are attributable to declines in the pH of cerebrospinal fluid (CSF), a consequence of the ease with which carbon dioxide crosses the blood–brain barrier. Decreases in CSF pH are greater for respiratory acidosis than for a similar degree of metabolic acidosis. Severe hypercapnia also causes peripheral vasodilation, which can contribute to arterial hypotension, and cardiac arrhythmia. The acid–base effects of chronic hypercapnia are compensated by renal mechanisms that return the arterial and CSF pH to almost normal and therefore do not cause more than mild clinical disease in most instances. So long as oxygen delivery to tissue is maintained, animals can tolerate quite high arterial carbon dioxide tensions for a number of days or longer – this is referred to as ‘permissive hypercapnia’ and is sometimes an alternative to artificial or mechanical ventilation of animals with respiratory insufficiency.
RESPIRATORY FAILURE
Respiratory movements are involuntary and are stimulated and modified by the respiratory centers in the medulla. The centers appear, at least in some species, to have spontaneous activity that is modified by afferent impulses to higher centers, including: cerebral cortex and the heat-regulating center in the hypothalamus; from the stretch receptors in the lungs via the pulmonary vagus nerves; and from the chemoreceptors in the carotid bodies. The activity of the center is also regulated directly by the pH and oxygen and carbon dioxide tensions of the cranial arterial blood supply. Stimulation of almost all afferent nerves may also cause reflex change in respiration, stimulation of pain fibers being particularly effective.
Respiratory failure is the terminal stage of respiratory insufficiency in which the activity of the respiratory centers diminishes to the point where movements of respiratory muscles cease. Respiratory failure can be paralytic, dyspneic or asphyxial, or tachypneic, depending on the primary disease.
The respiratory failure that occurs in animals with pneumonia, pulmonary edema and upper respiratory tract obstruction is caused by combinations of hypoventilation, ventilation/perfusion mismatch and diffusion impairment, which leads to hypercapnia and hypoxemia. Hypercapnia and hypoxia stimulate the respiratory center and there is a potent respiratory drive evident as markedly increased respiratory rate and effort. As the disease progresses these changes become more marked until death occurs as a result of central nervous system or cardiac failure. Animals that die of the central nervous system effects of respiratory failure typically have dyspnea followed by periods of gasping and apnea just before death.
Paralytic respiratory failure is caused by depression of the respiratory centers or paralysis of the muscles of respiration. Depression of the respiratory center occurs with poisoning by respiratory center depressants, such as general anesthetics, or damage to the respiratory center, such as might occur with brainstem injury. Paralysis of respiratory muscles occurs in disease such as botulism, tetanus, strychnine poisoning, white muscle disease, severe hypocalcemia and tick paralysis. The signs of paralytic respiratory failure are a gradual or abrupt cessation of respiratory movements without preceding signs of increased respiratory effort or dyspnea. The animal is often unconscious, or unable to move, during the later stages of the disease.
The differentiation of these types of failure is of some importance in determining the type of treatment necessary. In the paralytic form of respiratory failure the optimal treatment is mechanical ventilation, along with removal of the inciting cause. Administration of respiratory stimulants is seldom effective as sole therapy. The more complex pathogenesis of respiratory failure in most diseases requires a therapeutic approach that removes each of the underlying defects. In most cases this is achieved by treating the inciting disease, for example administering antimicrobials to an animal with pneumonia or furosemide to an animal with pulmonary edema, in addition to supportive care including, potentially, nasal or pharyngeal insufflation with oxygen, or mechanical ventilation.
Principal manifestations of respiratory insufficiency
Respiratory disease is evident as one or more of a variety of signs detectable on clinical examination. The signs vary with the etiology of the disease and its anatomic location. Diseases that impair ventilation or gas exchange have hypoxemia and hypercapnia as prominent life-threatening abnormalities. Infectious and inflammatory diseases can cause prominent clinical abnormalities as a result of a systemic inflammatory response and toxemia. The toxemia may be so severe (e.g. in calf diphtheria, aspiration pneumonia and equine pleuritis) as to cause death even though oxygen and carbon dioxide exchange are not greatly impaired. The common signs of respiratory disease are:
ABNORMALITIES IN RATE, DEPTH AND EASE OF BREATHING
Polypnea is a rate of breathing that is faster than observed in clinically normal animals of the same species, breed, age, sex and reproductive status in a similar environment.
Tachypnea also describes an increased rate of breathing, although with the implication that breathing is shallow (i.e. of a reduced tidal volume).
Hyperpnea is an abnormal increase in the rate and depth of breathing (an abnormally high minute volume) but the breathing is not labored and is not associated with signs from which one could infer represent distress on the part of the animal (i.e. the animal is not dyspneic). This assessment requires measurement of minute ventilation or arterial blood gas tensions.
Dyspnea is a term borrowed from human medicine, in which it refers to the sensation of shortness of breath or air hunger. It is used in veterinary medicine to describe labored or difficult breathing in animals that also display some signs of distress, such as anxious expression, unusual posture or stance, or unusual behavior.
Dyspnea is a physiological occurrence after strenuous exercise and is abnormal only when it occurs at rest or with little exercise. It is usually caused by hypoxia with or without hypercapnia, arising most commonly from diseases of the respiratory tract. In pulmonary dyspnea one other factor may be of contributory importance; there may be an abnormally sensitive Hering–Breuer reflex. This is most likely to occur when there is inflammation or congestion of the lungs or pleura. Rapid, shallow breathing results.
Expiratory dyspnea is prolonged and forceful expiration, usually associated with diffuse or advanced obstructive lower airway disease. The dyspnea of pulmonary emphysema is characteristically expiratory in form and is caused by anoxic anoxia and the need for forced expiration to achieve successful expulsion of the tidal air. It is commonly accompanied by an audible expiratory grunt in ruminants but less so in pigs and almost never in horses.
Inspiratory dyspnea is prolonged and forceful inspiration due to obstruction of the extrathoracic airways, such as with laryngeal obstruction or collapse of the cervical trachea. It may also be associated with abnormalities that restrict thoracic expansion, such as restrictive lung diseases and space-occupying lesions of the thorax. It is accompanied by a stridor or loud harsh sound on inspiration when the cause is obstruction of the extrathoracic airways, such as is typical of laryngeal or tracheal disease.
Open-mouth breathing is labored breathing with the mouth held open, commonly with the tongue protruded in ruminants and most commonly associated with advanced pulmonary disease or obstruction of the nasal cavities.
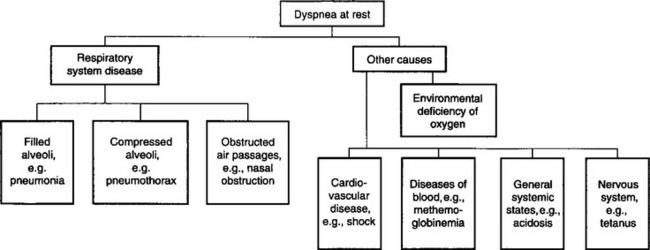
DISEASES CAUSING DYSPNEA AT REST OR LACK OF EXERCISE TOLERANCE
Dyspnea, along with hypoxemia and hypercapnia, are the clinical and laboratory findings most likely to attract attention to the possible presence of disease in the respiratory system. A brief summary of the causes of dyspnea is outlined in Figure 10.1. It is most important, when attempting to differentiate diseases that cause dyspnea, to include diseases of systems other than the respiratory system that can result in dyspnea. Dyspnea at rest is usually, but not always, caused by respiratory tract disease, whereas exercise intolerance can be caused by disease in the respiratory, cardiovascular, musculoskeletal and other body systems.
Respiratory tract disease
Respiratory tract diseases interfere with normal gas transfer, through the mechanisms discussed above. Characteristics of respiratory disease that lead to dyspnea or lack of exercise tolerance include:
• Flooding of alveoli with inflammatory cells and/or protein-rich fluid – pneumonia and pulmonary edema
• Atelectasis (collapsed alveoli and small airways) – pleural effusion, hemothorax, hydrothorax, pneumothorax, chylothorax, pyothorax, prolonged recumbency of large animals and diaphragmatic hernia
• Airway obstruction – nasal obstruction, pharyngeal/laryngeal obstruction, tracheal/bronchial obstruction, bronchoconstriction and bronchiolar obstruction.
Cardiovascular disease
This causes inadequate perfusion of tissues including the lungs. There is reduced oxygen delivery to tissues, even in the presence of normal arterial oxygenation:
• Cardiac disease. Cardiac dyspnea results from heart failure and is multifactorial. In animals with dyspnea attributable to cardiac disease there are other readily evident signs of heart failure
• Peripheral circulatory failure – usually due to hypovolemic shock, although shock associated with toxemia, including endotoxemia, can cause dyspnea. There are always other prominent signs of disease.
Diseases of the blood
These cause inadequate delivery of oxygen to tissues because of anemia or presence of hemoglobin that is unable to carry oxygen.
Nervous system diseases
Diseases of the nervous system affect respiratory function by one of several mechanisms:
• Paralysis of respiratory muscles occurs in tick paralysis or botulism. Tetanic spasm of respiratory muscles, such as in tetanus or strychnine toxicosis, also impairs or prevents alveolar ventilation. Both flaccid and tetanic paralysis cause hypercapnia and hypoxemia and, in extreme situations, death by suffocation
• Paralysis of the respiratory center, as in poisoning by nicotine sulfate, or overall central nervous system depression, causes hypoventilation because of impaired ventilatory drive
• Stimulation of the respiratory center, so-called neurogenic dyspnea, occurs as a result of stimulation of the center by a small irritative lesion, such as in animals with encephalitis, or administration of drugs, such as lobeline, that increase sensitivity of the respiratory center to hypoxemia or hypercapnia.
Musculoskeletal diseases
• Muscle diseases. Diseases of the respiratory muscles can impair ventilation. These include white muscle disease in lambs, calves, and foals, and some congenital diseases (such as glycogen branching enzyme deficiency in foals)
• Fatigue. Animals with primary severe respiratory disease can develop fatigue of the respiratory muscles (intercostal, diaphragm, accessory muscles of respiration), which can further impair ventilation
• Trauma. Fractured ribs can impair ventilation both because of the pain of breathing and because of mechanical disruption to respiration (flail chest).
General systemic states
Tachypnea can occur in a number of systemic states in which there is no lesion of the respiratory tract or nervous system. These include:
Miscellaneous poisons
A number of poisons cause dyspnea as a prominent sign, but in most cases the pathogenesis has not been identified.
• Farm chemicals, including metaldehyde and dinitrophenols (probable mechanism is stimulation of respiratory center)
• Organophosphates and carbamates (probable mechanism is alteration of pulmonary epithelium), urea (probably effective as ammonia poisoning)
• Nicotine depressing the respiratory center
• Poisonous plants, including fast-death factor of algae, the weeds Albizia, Helenium, Eupatorium, Ipomoea, Taxus spp. and Laburnum and ironwood (Erythrophleum spp.); all appear to act at least in part by central stimulation.
ABNORMAL POSTURE
Animals with respiratory disease, and especially those in respiratory distress, often adopt an unusual posture and are rarely recumbent except in the terminal stages of the disease. Animals in severe respiratory distress will stand with the head and neck held low and extended. Animals, except horses, will often have open-mouthed breathing. Horses, except in extreme and unusual circumstances, are unable to breath through the mouth because of the anatomical arrangement of the soft palate, which effectively provides an airtight barrier between the oropharynx and nasopharynx. Cattle with severe respiratory distress and open-mouthed breathing will often drool large quantities of saliva – probably a consequence of decreased frequency of swallowing as the animal labors to breath.
The positioning of the legs is often abnormal. Severely affected animals, and those with pleuritic pain (horses or cattle with pleuritis) or severe respiratory distress, will usually stand with elbows (humeroradial joint) abducted. The animals are reluctant to move but when forced to do so can react violently. They are resistant to diagnostic or therapeutic interventions that interfere even transiently with their ability to breath.
NORMAL AND ABNORMAL BREATH SOUNDS
Auscultation of the lungs and air passages is the most critical of the physical examinations made of the respiratory system. The examination should be performed in as quiet an environment as possible, though it is often difficult to achieve a silent listening environment in large animal practice. The animal should be adequately restrained so that the examiner can concentrate on the lung sounds, and should not be sedated or anesthetized because of the depression in lung sounds that can occur in these instances. To be effective and diagnostically reliable, auscultation must be systematic. Both the upper and lower parts of the respiratory tract must be examined in every case. It is preferable to begin the examination by auscultating the larynx, trachea and the area of the tracheal bifurcation in order to assess the rate of air flow and the volume of air sound to be heard over the lungs.
GENERATION OF BREATH SOUNDS
The animal must be breathing to generate lung sounds. The lung sounds are generated by movement of air in the large and mid-sized airways, including the trachea and bronchi. The greater the velocity of air in the airways, the louder the noise, explaining the loud sounds that are generated in the trachea. Air movement in the bronchioles, terminal airways and alveoli is silent because of the large combined cross-sectional area of these airways and consequent low velocity of air movement and laminar character of the airflow. Sound is generated by turbulent airflow and the degree of turbulence is affected by the velocity of airflow and the diameter of the airway. This sound is then transmitted through the lung and chest wall to the surface of the thorax, where it can be detected by use of a stethoscope.
Quiet breath sounds can be a result of low tidal volume with resultant low velocity of airflow, or impaired transmission of sounds to the surface of the chest. Sound is transmitted most readily through dense liquids such as water. Most tissue, except fat, is approximately 70% water and transmits sounds readily. Sound is reflected at the interface of two media of markedly different densities – such as air and tissue – and less sound is transmitted. Thus, in the normal lung there is marked attenuation (softening) of breath sounds because of the extensive air–tissue interfaces. This is evident by comparing the intensity of breath sounds heard over the trachea to those heard over the chest wall. However, lung sounds are more readily transmitted when areas of the lung do not contain air, such as occurs with atelectasis, pulmonary edema or infiltration of lung by inflammatory exudates. Sounds generated in the large airways are more readily transmitted through this consolidated tissue and are evident at the chest wall as louder bronchial breath sounds. The presence of bronchial breath sounds that are audible on the chest surface is dependent upon the presence of a patent bronchus with airflow to generate the lung sounds and of tissue that readily transmits the sounds generated in the bronchus. Lung sounds will not be heard if they are not generated (as a result of lack of airflow in bronchi) or are muffled by extensive accumulations of fluid or fat between the lung and the chest wall. Lung sounds are reduced in animals with airflow of low velocity in large airways, such as occurs in animals with low tidal volumes, or in which there is obliteration of the bronchial lumen by fluid or tissue. Low tidal volumes occur in animals at rest or in those in which there is rapid but shallow (low tidal volume) breathing. Obliteration of the bronchial lumen occurs in many diseases, including pneumonia.
REBREATHING (‘BAGGING’) examination
Detection of abnormal lungs sounds is optimized by increasing the animal’s tidal volume, and thereby the velocity of airflow in large airways. An expeditious means of temporarily increasing the animal’s tidal volume is to occlude the nostrils for a brief period (30–60 s). When the animal is again allowed to breath it will take several large, deep breaths, during which lung sounds can be ausculted. However, the increase in tidal volume is transient and does not permit time for detailed auscultation of the chest. A preferred technique is to place an airtight bag over the animal’s muzzle such that all the air that it inhales is contained within the bag. The volume of air in the bag should exceed the anticipated stimulated tidal volume of the animal. As a rule of thumb, the volume of the bag should be sufficient to allow the animal a tidal volume of 10–15 mL of air per kilogram of body weight (BW). A 500 kg horse or cow therefore needs a bag that contains 10 L of air. Hyperventilation is stimulated by an increase in carbon dioxide content of inspired air with subsequent hypercapnia and stimulation of the respiratory center. A more refined technique has the animal inhaling gas that is 5% carbon dioxide and 95% oxygen, thereby preventing hypoxemia due to the examination. Rebreathing examinations (or ‘bagging’) are not indicated if abnormal lung sounds are detected on initial examination as the results of the rebreathing examination will not add any additional information. Animals in respiratory distress should not be subjected to a rebreathing examination because it might worsen the hypoxemia or hypercapnia already present, and is inhumane. Rebreathing examinations are indicated when respiratory disease is suspected but initial auscultation of the thorax does not reveal abnormal lung sounds.
INTERPRETATION OF BREATH SOUNDS
Terminology used to describe normal and abnormal lung sounds is now well established and should be used consistently so that it is a useful diagnostic aid.3,4 Associations between abnormal respiratory sounds and diseases and abnormalities of respiratory function are well established. Correct identification of lung sounds, and consistency in terms used to describe them, therefore permits greater diagnostic accuracy and provides the ability to accurately and precisely describe diseases. The identification and clinical significance of respiratory sounds are summarized in Table 10.1. The clinician must carefully auscultate both the upper respiratory tract (larynx, trachea) and the entire aspects of both lung fields and interpret the sounds that are audible or not audible. The variables that must be interpreted include:
• The nature of the sounds (increased or decreased breath sounds, crackles or wheezes)
Table 10.1 Identification and clinical significance of breath sounds
The questions that should be asked are:
• Are the breath sounds of normal intensity?
• Are the breath sounds normal or abnormal?
• If abnormal sounds are present, what are they (crackles, wheezes, stridor, stertor, etc.; see Table 10.1)?
Interpretation of these variables should indicate the nature of the lesion. Examples are summarized in Table 10.1. Lung sounds can be divided into normal breath sounds and abnormal breath sounds.
Breath sounds are produced by air movement through the tracheobronchial tree. The terms ‘bronchial sounds’ and vesicular sounds are not anatomically accurate or based on physiological principles and should not be used. The term ‘breath sounds’ should be used. These are the sounds which are audible clearly over the trachea and which are attenuated over the lungs. Breath sounds are of normal, increased or decreased intensity. Abnormally loud or soft breath sounds can be attributed to either changes in sound production in the airways by changes in flow rate or altered transmission of sound through various normal or abnormal tissues or fluids in the thorax, as discussed above.
Normal breath sounds
Normal breath sounds vary in quality depending on where the stethoscope is placed over the respiratory tract. They are loudest over the trachea and base of the lung and quietest over the diaphragmatic lobes of the lung. Normal breath sounds are louder on inspiration than on expiration because inspiration is active with more rapid airflow, whereas expiration is passive in normal animals and associated with lower rates of airflow. Breath sounds may be barely audible in obese animals or in the noisy surroundings common in field conditions.
Increased loudness of breath sounds is heard in normal animals with increased respiratory rate and depth of respiration. This can occur for physiological reasons such as exercise, excitement or a high environmental temperature. They can also occur in abnormal states such as fever, acidosis or pulmonary congestion in early pneumonia or myocardial disease.
Decreased loudness or an almost complete absence of breath sounds occurs in pleural effusion or pneumothorax because of almost complete reflection of the breath sounds at the pleural surface due to the mismatching of the acoustic properties of the pleural tissues and fluids. Space-occupying masses between the lung and the thoracic wall also cause a relative absence of breath sounds over the site as do areas of lung that are not ventilated, such as a pulmonary abscess.
Increased loudness of breath sounds
The normal breath sounds heard over the trachea may sound abnormally loud over the lungs because of changes in the transmission properties of the respiratory system.5 This is because, when sound waves pass through structures of different physical properties, the amount of sound transmitted depends on the matching of acoustic properties of the different structures. Consolidation results in less reflection of sound at the thoracic wall and consequently more transmission to the stethoscope. Thus, in consolidation, the breath sounds are much louder than normal. These are harsh breath sounds that approximate those heard over the trachea. They are audible on inspiration and expiration but become louder on expiration in abnormal states such as consolidation or atelectasis. Any disease in which the bronchial lumen remains open and the surrounding lung tissue has been replaced by cells, exudate or tissues (consolidation) that transmit sound without reflection will result in increased bronchial sounds.
Abnormal breath sounds
Abnormal breath sounds include crackles and wheezes. Crackles are discontinuous sounds and wheezes are continuous sounds.6
Crackles are abnormal lung sounds described as clicking, popping or bubbling sounds. They are caused by airways that remain closed for a portion of inspiration and then suddenly open. The crackling is caused by the sudden equalization of pressure between the proximal and distal part of the airway.6 Crackles may thus be caused by the presence of exudate and secretions in the airways, and edematous bronchial mucosa. Crackling lung sounds are also audible in cattle with interstitial pulmonary emphysema. Crackling sounds may move their point of maximum intensity following coughing, presumably as a result of movement of exudate.
Wheezes are continuous whistling, squeaking sounds caused by vibrations of airways or air passing through a narrowed airway. They can be characterized as monophonic (single tone) or polyphonic (multiple tones) and by the timing of their occurrence in the respiratory cycle. Inspiratory wheezing suggests obstruction of the upper airways, usually extrathoracic. Expiratory wheezing usually indicates intrathoracic airway obstruction such as bronchoconstriction with or without distal airways that are narrowed because of tenacious exudate.
Pleuritic friction sounds are a combination of continuous and discontinuous sounds produced by the rubbing together of inflamed parietal and visceral pleura. The sound is loud, coarse and usually not influenced by coughing. Pleuritic friction sounds are not common and their absence does not preclude the presence of pleuritis, particularly in the horse. Pleuritic friction rubs may also occur in cattle with severe diffuse pulmonary emphysema as: the relatively dry parietal and visceral surfaces rub together during the respiratory cycle.
Absence of lung sounds occurs when the breath sounds are reflected at the interface between the lung and thoracic wall by the presence of a medium such as a space-occupying mass, fluid or air. The common causes of the ‘silent lung’ include pleural effusion, space-occupying masses of the thorax, large pulmonary abscess, complete destruction of a lobe of lung including the terminal airways, such as can occur with bronchial lumen occlusion by a foreign body or tumor, and diaphragmatic hernia.6
Extraneous sounds. Miscellaneous unexpected sounds that are occasionally audible over the thorax include peristaltic sounds, skin and hair sounds caused by the stethoscope, crepitating sounds due to subcutaneous emphysema and muscular contractions. Subcutaneous emphysema occurs in diseases in which there is leakage of air from the lungs or airways into the subcutaneous space. This occurs with bullous lung disease in cattle, rib fractures and pneumothorax, and after percutaneous tracheal aspirate in animals that cough. Coughing in these animals causes air to be forced out of the trachea through the hole through which the tracheal aspirate was obtained. This occurs in the period of coughing when intratracheal pressures are markedly increased just prior to the opening of the glottis.
RESPIRATORY NOISES
Respiration may be accompanied by audible noises that indicate certain normal or abnormal occurrences in the respiratory tract such as sneezing, snorting, stridor, stertor or snoring, wheezing, roaring, expiratory grunting and snuffling, bubbling and rattling sounds.
Sneezing is a sudden, involuntary, noisy expiration through the nasal cavities caused reflexly by irritation of the nasal mucosae. Sneezing occurs in rhinitis and obstruction of the nasal cavities, and digital manipulation and examination of the nasal mucosae.
Snorting is a forceful expiration of air through the nostrils as in a sneeze, but a snort is a voluntary act used by horses and cattle as a device to intimidate potential predators.
Stridor is an inspiratory stenotic sound originating from a reduction in the caliber of the larynx, as occurs in laryngeal edema and abscess.
Stertor or snoring is a deep guttural sound on inspiration originating from vibrations of pharyngeal mucosa. Snoring is often intermittent, depending on the animal’s posture. For example, a fat young bull will often snore when he is dozing half asleep, with his head hung down, but the snore will disappear when he is alert and his head is held up in a more normal position. Stertor can occur during expiration in horses with dorsal displacement of the soft palate.
Wheezing is a high-pitched sound made by air flowing through a narrow lumen, such as a stenotic or inflamed nasal cavity.
Roaring may occur during exercise and is caused by air passing through a larynx with a reduced lumen, e.g. laryngeal hemiplegia in horses.
Expiratory grunting is a clearly audible grunting noise synchronous with expiration. It is most common in cattle with diffuse pulmonary disease. A painful grunt may occur in painful diseases of the thorax such as fibrinous pleuritis and is unassociated with inspiration or expiration.
Snuffling, bubbling or rattling sounds may be audible over the trachea or base of the lungs when there is an accumulation of secretion, or exudate, in the nasal cavities, larynx or trachea. These are most clearly audible on inspiration.
COUGHING
A cough is an explosive expiration of air from the lungs. It is initiated by reflex stimulation of the cough center in the medulla oblongata by irritation of sensory receptors in one of various organs, especially the respiratory tract. The stimulus may originate in the pharynx, larynx, trachea or bronchi. Coughing may also be initiated by irritation of the esophagus, as in choking. The act of coughing consists of several stages:
• Deep inspiration followed by closure of the arytenoid cartilages (glottis)
• Compression of the air in the lungs and large increase in pressure in the thorax and airways by a forced expiratory effort against a closed glottis
• A sudden relaxation of the arytenoid adductor muscles, resulting in opening of the larynx and abrupt, vigorous and forced expiration. Coughing in horses is associated with transient dorsal displacement of the soft palate so that material in the airways caudal to the larynx is expelled through the mouth
• The sudden opening of the glottis allows an explosive expiration, during which the linear velocity attains a speed of several hundred kilometers per hour. The intrathoracic airways collapse after opening of the glottis during the forced expiration, whereas the extrathoracic airways are momentarily dilated.
The purpose of coughing is to remove the excess mucus, inflammatory products or foreign material from the respiratory tract distal to the larynx. Coughing indicates the existence of primary or secondary respiratory disease.
Coughing can be assessed according to several characteristics. Coughing is infrequent in the early stages of respiratory tract disease but can become frequent as the degree of inflammation in the larynx, trachea and bronchi becomes more severe. Assessment of the severity of coughing, at least in horses, requires prolonged observation (preferably for an hour).7 Coughing is a fairly specific but not very sensitive indicator of pulmonary inflammation.8 If coughing is detected then it is quite likely that the animal has inflammation of the airways, whereas failure to detect coughing does not reliably rule out the presence of clinically significant airway inflammation.8 The severity of coughing in horses is closely linked to the severity of inflammation and accumulation of mucopus in the airways.7,8 Race horses that cough are 10 times more likely to have more than 20% neutrophils in a tracheal aspirate, and more than 100 times more likely to have more than 80% neutrophils.8 The frequency of coughing correlates well with maximal changes in pleural pressure, extent of mucus accumulation and proportion of neutrophils in bronchoalveolar lavage fluid of horses with heaves (recurrent airway obstruction).7 Coughing is therefore a specific indicator of the presence of respiratory inflammation.
The frequency of coughing is an indicator of the severity of lung disease in horses7 and presumably in other species. Horses that cough more than four times per hour have increased likelihood of mucus accumulation and higher pleural pressure changes during breathing than do horses that cough fewer than four times per hour.7
A cough cannot be induced in normal adult cattle and horses by manual manipulation of the larynx or trachea. If a cough can be induced in an adult horse by manual manipulation of the larynx or trachea, then this indicates airway inflammation and is a reason for further examination of the respiratory tract.
The most common causes of coughing in farm animals are due to diseases of the larynx, trachea, bronchi and lungs, which are presented under the headings of diseases of those parts of the respiratory tract later in this chapter.
CYANOSIS
Cyanosis is a bluish discoloration of the skin, conjunctivae and visible mucosae caused by an increase in the absolute amount of reduced hemoglobin in the blood. It can occur only when the hemoglobin concentration of the blood is normal or nearly so, and when there is incomplete oxygenation of the hemoglobin. Cyanosis is apparent when the concentration of deoxygenated hemoglobin in blood is greater than 5 g/dL (50 g/L).9 Cyanosis does not occur in anemic animals. The bluish discoloration should disappear when pressure is exerted on the skin or mucosa. In most cases, the oral mucous membranes are examined for evidence of cyanosis, although the skin of the pinna and the urogenital mucous membranes will suffice. Examination of vaginal mucosa is preferred in horses that have severe congestion of the oral and nasal mucosa as a result of disease affecting the head, such as cellulitis or bilateral jugular thrombophlebitis. Artificial lighting and skin pigmentation affect the ability to detect cyanosis.
Methemoglobinemia is accompanied by discoloration of the skin and mucosae but the color is more brown than blue and cannot be accurately described as cyanosis.
Cyanosis is classified as central or peripheral. Central cyanosis is present when arterial oxygen saturation is below normal with concentration of deoxygenated hemoglobin exceeding 4–5 g/dL. Peripheral cyanosis occurs when there is localized desaturation of blood despite arterial oxygen saturation being normal. This usually occurs because there is diminished blood flow to tissue, with a resulting increase in oxygen extraction by the ischemic tissues and low end-capillary and venous hemoglobin saturation.
• Congenital cardiac diseases that cause right-to-left shunting
• Pulmonary diseases that cause hypoemia. Cyanosis is not usually marked in pulmonary disease unless the degree of ventilation/perfusion mismatch is severe
• Upper airway obstruction causing hypoxemia. Cyanosis is common and is a sign of life-threatening disease in severe cases of laryngeal obstruction, as occurs in severe laryngitis in calves with necrotic laryngitis or horses with bilateral laryngeal paralysis (lead poisoning, after tracheal intubation during anesthesia, idiopathic)
Peripheral causes of cyanosis include:
• Arterial obstruction, such as is seen in horses with aortoiliac thrombosis (‘saddle thrombus’) or thrombosis of distal limbs (such as can occur with severe septicemia)10
Central cyanosis is characterized by decreased arterial oxygen saturation due to right-to-left shunting of blood or impaired pulmonary function. Central cyanosis due to congenital heart disease or pulmonary disease characteristically worsens during exercise. Central cyanosis usually becomes apparent at a mean capillary concentration of 4–5 g/dL reduced hemoglobin (or 0.5 g/dL methemoglobin). Since it is the absolute quantity of reduced hemoglobin in the blood that is responsible for cyanosis, the higher the total hemoglobin content the greater the tendency toward cyanosis. Thus cyanosis is detectable in patients with marked polycythemia at higher levels of arterial oxygen saturation than in patients with normal hematocrit values, and cyanosis may be absent in patients with anemia despite marked arterial desaturation. Patients with congenital heart disease often have a history of cyanosis that is intensified during exertion because of the lower saturation of blood returning to the right side of the heart and the augmented right-to-left shunt. The inspiration of pure oxygen (100% Fio2) will not resolve central cyanosis when a right-to-left shunt is present, but can resolve when primary lung disease or polycythemia is causing the cyanosis.
Peripheral cyanosis is caused by obstruction of blood flow to an area. This can occur as a result of arterial or venous obstruction, although it is usually more severe when arterial blood flow is obstructed. Obstruction of arterial blood flow also causes the limb to be cold and muscle and nerve function in the ischemic area to be impaired. Cyanosis can also occur as a result of cutaneous vasoconstriction due to low cardiac output or exposure to cold air or water. It usually indicates stasis of blood flow in the periphery. If peripheral cyanosis is localized to an extremity, arterial or venous obstruction should be suspected. Peripheral cyanosis due to vasoconstriction is usually relieved by warming the affected area.
Heart failure can cause cyanosis that is restricted to the extremities, probably because of reduced blood flow to extremities during this disease and the consequent markedly lower end-capillary oxygen content. Blood in the venous end of the capillaries, and in the venous bed draining these tissues, is therefore deoxygenated and cyanosis is observed. While this type of cyanosis has a peripheral distribution, its underlying cause is central.
NASAL DISCHARGE
Excessive or abnormal nasal discharge is usually an indication of respiratory tract disease. Nasal discharges are common in all the farm animal species. Cattle can remove some or all of the nasal discharge by licking with their tongue, while horses do not remove any.
Origin
The nasal discharge is usually obvious but the determination of its origin and significance can be difficult and elusive. The history should determine the duration of the nasal discharge and if it has been unilateral or bilateral.
Nasal discharges may originate from lesions in the nasal cavities, congenital defects of the hard palate such as cleft palate in the newborn, paranasal sinuses, guttural pouch in the horse, pharynx, larynx, trachea and lungs. Diseases of the esophagus and stomach that cause dysphagia and regurgitation or vomition can also cause a nasal discharge stained with feed material.
The origin of a nasal discharge is sometimes determined by close inspection of the external nares and the visible aspects of the nasal cavities using a pointed source of light. Some important infectious diseases of the respiratory tract characterized by lesions of the nasal mucosae can be identified by examination of the external nares for the origin of a nasal discharge. If the source of the discharge is not apparent on this examination, then more detailed investigation is warranted.
Examination
The characteristics of the discharge are noted carefully by inspection. It may be copious, serous, mucoid, purulent, caseous, streaked with blood, foul-smelling (ozena) or contain feed particles.
• A copious bilateral serous nasal discharge is characteristic of early inflammation of the nasal cavities such as in viral rhinitis
• A bilateral mucoid discharge suggests inflammation of a few days duration
• A bilateral purulent discharge can indicate inflammation in the upper or lower respiratory tract
• A copious bilateral caseous discharge suggests an allergic or bacterial rhinitis
• Foul-smelling nasal discharges are usually associated with necrosis of tissues anywhere in the nasal cavities, the guttural pouch in the horse, or severe necrotic and gangrenous pneumonia
• A bilateral foul-smelling discharge containing feed particles suggests dysphagia, regurgitation or vomition
• In most cases, a chronic unilateral nasal discharge suggests a lesion of one nasal cavity
• A bilateral nasal discharge suggests a lesion posterior to the nasal system.
Examination of the paranasal sinuses for evidence of pain and facial deformity will assist in the diagnosis of sinusitis. Percussion is useful in identifying paranasal sinuses that are filled with fluid or tissue as sinuses affected in this way do not produce a resonant sound when the skin overlying the sinus is tapped. The pharynx and larynx of cattle can be examined through the oral cavity whereas a flexible endoscope is necessary for close examination of the upper and lower respiratory tract of horses or cattle of almost any age to determine the origin of a nasal discharge. The examination should include both nares, the region of the opening of the nasomaxillary sinus (this opening cannot be seen), the nasopharynx (in horses) or the pharynx (in other species), the guttural pouches in horses, the larynx and the trachea, preferably to the level of the carina, although this might not be possible in large animals or when short endoscopes are used.
Radiography of the structures of the head and pharynx is also useful to locate lesions of the nasal cavities and paranasal sinuses that might be the origin of a nasal discharge.
Nasal discharge and location of lesion
There is not necessarily a correlation between the characteristics of a nasal discharge and the nature of any pulmonary lesions. In exudative pneumonias in cattle, mucopus is produced and is moved up the trachea and into the pharynx by the mucociliary mechanism or by coughing. Some of it is then swallowed and some may be deposited in the nasal cavities and moved forward to the external nares by ciliary action. In the horse, with its long soft palate, most purulent material from the lungs will be deposited in the nasal cavities and appear as a nasal discharge.
Sampling of nasal discharge
When infectious disease is suspected, nasal swabs can be collected and submitted for microbiological examination. Nasal swabs are useful only when a specific etiological agent is suspected and demonstration of its presence will confirm the cause of the disease. Examples of this include strangles (Streptococcus equi), influenza (equine or porcine), infectious bovine rhinotracheitis and Mycoplasma bovis. Submission of nasal swabs for culture yields mixed flora and the results are impossible to interpret, with the exception noted above. Organisms cultured from nasal or nasopharyngeal swabs are not representative of those cultured from lungs in individual animals but might be somewhat useful in herd outbreaks of disease.11,12 Culture of transtracheal aspirates or, in cattle but not horses, bronchoalveolar lavage fluid, is representative of organisms causing pulmonary disease.12,13 Cytological examination of the nasal discharge can reveal exfoliated cells in the case of nasal tumors or eosinophils when allergic rhinitis is present.
EPISTAXIS AND HEMOPTYSIS
• Epistaxis (blood from the nostril) is in most instances a result of disease of the mucosae of the upper respiratory tract but it may originate anywhere in the upper or lower respiratory tract. Epistaxis occurring during or within several hours of intense exercise by horses is due to exercise-induced pulmonary hemorrhage
• Hemoptysis is the coughing up of blood. The blood usually originates from hemorrhage in the lower respiratory tract. The presence of hemoptysis is difficult to detect in animals. Hemoptysis occurs in horses, which is perhaps unexpected given the anatomic separation of the nasopharynx and oropharynx.
Pulmonary hemorrhage, particularly in the horse, may be manifested as epistaxis. Pulmonary hemorrhage in cattle is commonly manifested as hemoptysis and epistaxis. These are described in more detail later in this chapter.
A small amount of serosanguineous fluid in the nostrils, as occurs in equine infectious anemia and infectious equine pneumonia, does not represent epistaxis, which must also be differentiated from the passage of blood-stained froth caused by acute pulmonary edema. In this instance the bubbles in the froth are very small in size and passage of the froth is accompanied by severe dyspnea, coughing and auscultatory evidence of pulmonary edema.
THORACIC PAIN
Spontaneous pain, evidenced by grunting with each respiratory cycle, usually indicates pleural pain, such as from a fractured rib, torn intercostal muscle or traumatic injury, including hematoma of the pleura, or pleurisy. A similar grunt may be obtained by deep palpation or gentle thumping over the affected area of the thoracic wall, with a closed fist or a percussion hammer. Pain due to a chronic deep-seated lesion cannot be detected in this way. The use of a pole under the sternum, as described under traumatic reticuloperitonitis, provides a useful alternative.
Special examination of the respiratory system
In addition to the routine clinical examination of the respiratory tract, there are a number of diagnostic techniques that can be used to aid in making a specific diagnosis, providing a reliable prognosis and formulating the most rational treatment. These techniques are being used more commonly by species specialists, particularly on valuable animals. Most equine practices have flexible endoscopes for the examination of the upper respiratory tract of horses. Medical imaging using thoracic radiography and ultrasonography of animals with suspected lung disease is now common, and the laboratory evaluation of respiratory tract secretions and exudates are commonplace. All these techniques increase the costs of making a diagnosis, and it is therefore important to consider whether the additional diagnostic testing will improve the final outcome of the case. Techniques for advanced evaluation of the respiratory system include:
• Auscultation and percussion of the thorax
• Endoscopy of the upper airways, guttural pouch (in Equidae), trachea, bronchi and larger bronchioles
• Invasive endoscopic examination of the sinuses using rigid endoscopes
• Pleuroscopy using either rigid or flexible endoscopes
• Radiographic examination of the skull, pharynx, larynx, guttural pouch (in Equidae), trachea and thorax
• Computed tomographic and magnetic resonance imaging
• Scintigraphic examination of respiratory function
• Ultrasonographic examination of the soft tissue of the pharynx and larynx, and thorax
• Collection and evaluation of respiratory tract secretions:
• Pulmonary function testing, including measurement of tidal and minute volumes, pleural pressure, forced expiratory volume, flow-volume loops, forced oscillometry, and CO2breathing
• Collection and analysis of exhaled breath condensate
AUSCULTATION AND PERCUSSION
The techniques of auscultation and percussion used in examination of the thorax are discussed in Chapter 1 and references on percussion of the thorax are available.14,15 Percussion of the thorax is a useful means of determining lung margins and therefore of detecting the presence of overinflation, as occurs with heaves in horses,16 or areas of consolidation. Consolidation is evident as a loss of resonance, and detection of this abnormality can reveal the presence of excessive pleural fluid or pulmonary consolidation. There is excellent agreement in the assessment of lung margins determined by percussion and by ultrasonographic examination.16 Percussion is therefore a valuable diagnostic tool, especially when ultrasonographic examination is not available.
ENDOSCOPIC EXAMINATION OF THE AIRWAYS (RHINOLARYNGOSCOPY, TRACHEOBRONCHOSCOPY)
Horses
Flexible endoscopes allow examination of the upper respiratory tract of horses including the nasal cavities, nasopharynx, auditory tube diverticula (guttural pouches), palatal arch, epiglottis, larynx, trachea and major bronchi. For examination to the level of the rostral trachea an endoscope of 1 m in length is suitable. However, an endoscope of 1.5 m in length is useful for examining to the level of the thoracic inlet. The endoscope is usually less than 1.5 cm in diameter. Endoscopic examinations are tolerated by most horses with the minimum of restraint (application of a nose or ear twitch). Sedation should be avoided if a purpose of the examination is to determine the functional integrity of the pharynx and larynx. Sedation depresses laryngeal function and impairs assessment of the symmetry and abductor function of the arytenoid cartilages. Sedated horses are more likely to displace the soft palate and to fail to return it to its normal position.
Rhinolaryngoscopic examination of horses should include a careful examination of the ventral and middle meatuses, turbinates, region of the nasomaxillary sinus opening (this cannot be visualized directly but discharge from it can be detected), ethmoidal turbinates, nasopharynx, soft palate, guttural pouches, dorsal pharyngeal recess, epiglottis and larynx. The endoscope should be used to examine both left and right nasal cavities and ethmoid turbinates. Both guttural pouches should be examined. Passage of the endoscope into the guttural pouch is best achieved by passing the endoscope through the ipsilateral nasal cavity. The guttural pouch is then entered by first introducing a thin, stiff tube, such as an endoscopic biopsy instrument, through the biopsy port of the endoscope into the guttural pouch. The endoscope is then rotated so that the entrance to the guttural pouch is opened and the endoscope is carefully advanced into the pouch. An alternative technique involves insertion of a stiff catheter, such as a Chambers mare uterine catheter, into the guttural pouch such that the entrance is dilated to enable passage of the endoscope.
Many disorders of the equine pharynx and larynx manifest only during strenuous exercise because of the high pressures generated in the airways by the large minute ventilation of exercising horses. Pressures in the pharynx and larynx that are of similar magnitude to those occurring during intense exercise can be induced in resting horses by 60 seconds of nasal occlusion.4 The respiratory efforts of horses during nasal occlusion can therefore be used to simulate those during exercise, thereby permitting detection of disorders of the pharynx (displacement of the soft palate) and larynx (mild laryngeal hemiplegia) that would not otherwise be apparent in a resting horse. Rhinolaryngoscopic examination can also be performed on horses running on a treadmill (see Exercise testing, below).
Bronchoscopic examination requires an endoscope that is at least 2 m in length and less than 1.5 cm in diameter. Horses must be sedated for bronchoscopic examination (a combination of xylazine, 0.25–0.5 mg/kg intravenously, and butorphanol, 1 mg per 40 kg intravenously, works well). Instillation of lidocaine (20 mL of 2% lidocaine diluted with 40 mL of isotonic saline or similar) minimizes coughing. The lidocaine is instilled into the trachea through the biopsy channel of the endoscope. The airways are examined in a systematic fashion and results are recorded using a system that has been described for identifying the major airways.17,18 Lobar bronchi are identified on the basis of the side of the bronchial tree on which they are found and the order in which they originate from the primary bronchus.17 On the right side, RB1, RB2 and RB3 refer to the right cranial lobar bronchus and subsequent right bronchi, respectively. On the left side, LB1 and LB2 refer to the left cranial lobar bronchus and the left caudal lobar bronchus, respectively. Segmental bronchi are identified by consecutive numbers in the order of origin from the lobar bronchus. The direction of the segmental bronchus is denoted by the capital letters D (dorsal), V (ventral), L (lateral), M (medial), R (rostral) and C (caudal). Subsegmental bronchi are identified in the order of origin from the segmental bronchi, using lower case letters.
Cattle
The nasopharynx, pharynx and larynx of cattle can be examined by endoscopy19 and this should be done without sedation if possible.20 Xylazine is not recommended because it commonly interferes with normal laryngeal function. Acepromazine is recommended if necessary.20
The anatomy of the proximal portion of the respiratory tract of cattle differs from that of horses. The nasal septum does not completely separate the left and right aspects of the nasopharynx. In cattle, the nasal septum tapers caudodorsally, allowing both ethmoturbinates to be observed from one side. The pharyngeal septum is contiguous with the nasal septum and merges with the caudodorsal wall of the pharynx. The nasopharyngeal openings of the auditory tubes are visible. The appearance of the vocal cords is similar to that observed in the horse. Cattle do not have a laryngeal saccule and a laryngeal ventricle is not visible rostral to the vocal cords. During endoscopy, the arytenoid cartilages are maintained in fully abducted position. Constriction of the pharynx during swallowing is accompanied by rostroventral movement of the pharyngeal septum, completely occluding the nasopharynx, which differs from the situation in the horse.
ENDOSCOPY OF PARANASAL SINUSES
The paranasal sinuses of the mature horse can be examined with a 4 mm arthroscope while standing and sedated or under general anesthesia.21 The procedure is technically challenging and is usually performed by surgeons experienced in the use of arthroscopic equipment inserted through portals created by trephining holes in the sinus. The side to be examined is determined by physical, radiographic and rhinoscopic examination of the animal. Endoscopic examination is indicated in animals in which diagnosis of the disease requires collection of tissue from the sinus. Therapeutic interventions that can be performed during endoscopic examination of the paranasal sinuses include lavage, removal of accretions of inflammatory material, drainage of cysts and creation or enlargement of drainage holes.
PLEUROSCOPY
Pleuroscopy using a rigid or flexible endoscope enables direct visual inspection of the pleural cavity for the diagnosis of pleural disease.22 The technique is particularly valuable in diagnosis of diseases of the thorax that extend to the pleural surface and do not exfoliate large quantities of cells, thereby making diagnosis by examination of fluid obtained by pleurocentesis unlikely. The procedure is useful in collection of tissue samples, such as from suspected thoracic neoplasia,23 or in therapeutic procedures including relief of pleural adhesions and resection of lung sections.24
The procedure is performed in standing, sedated horses restrained in stocks. Strict aseptic technique is used. The portal for insertion of the endoscope is at the level of the eighth to 12th intercostal space with optimal examination of intrathoracic structures obtained via the 10th or 12th intercostal space. Either a rigid endoscope (10 mm diameter, 57 cm length) or flexible endoscope (10 mm diameter, 1 m length) can be used. The endoscope is inserted through a small incision in the intercostal space made under local anesthesia. The ipsilateral lung is partially collapsed by induced pneumothorax to permit visualization of intrathoracic structures. The mediastinum is intact in most horses. Inadequate collapse of the lung increases the likelihood of it being damaged during the procedure. The lung is reinflated by removal of air in the pleural space at the end of the procedure. Potential complications of the procedure include pneumothorax, hemothorax, damage to intrathoracic structures and infection.
RADIOGRAPHY
Radiography of the head, neck and thorax is valuable in the diagnosis of diseases of the respiratory tract of animals. Examination is hindered by the large size of adult horses and cattle through the need for specialized, high-capacity equipment for obtaining radiographs, and the need for adequate restraint. Radiographic examination of adult animals in the field using portable radiographic units is very limited. However, large practices with fixed radiographic units capable of generating sufficient voltage and amperage can obtain diagnostic radiographs of the thorax of adult horses and cattle. Diagnostic films of smaller animals, including adult sheep and goats and foals and calves, can be obtained using portable units capable of generating 80–100 kVp and 15–20 mA.
Examination of the thorax of large animals is restricted to lateral radiographs because the large amount of tissue prevents adequate exposure for ventrodorsal views. Multiple films are required for complete examination of the thorax, and the exposure needed for optimal quality films varies among anatomical sites. Localization of focal lesions can be achieved by examining sets of radiographs that include images collected with the horse or cow standing first with one side to the plate and then with the other side toward the plate. The lesion will appear larger in views obtained with the lesion closer to the source of X-rays.
Radiographs of calves and foals can be recorded with them standing or recumbent. Images obtained with the foal or calf in lateral recumbency with the forelimbs pulled forward permit optimal examination of the cranial thorax. However, calves or foals that are recumbent for prolonged periods of time (e.g. > 30 min) can develop atelectasis of the down lung that can mimic pneumonia radiographically. Ventrodorsal views assist with localizing lesions in foals and calves. Radiographic evidence of lung disease is common in ill neonatal foals (74% having such lesions in one study),25 and is not related to clinical evidence of respiratory disease or dyspnea.26 The characteristics of lung lesions detected in neonatal foals are associated with likelihood of survival. Guidelines for recognition of pulmonary patterns in foals have been proposed (Table 10.2)26 and these guidelines are likely to be useful aids for interpretation and description of pulmonary patterns in neonates of other species.
Table 10.2 Guidelines for radiographic pulmonary pattern recognition in foals26
| Alveolar lung pattern (Vessels not visualized. There is displacement of air from the distal air spaces of the lung leading to a relatively homogeneous increase in soft tissue opacity. Formation of air bronchograms is usually associated with the pattern but is not always present) | |
| Absent | The pulmonary vessels are easily seen |
| Minimal alveolar component (< 10%) | No visualization of vessels in < 10% of the lung field. Usually occurs in conjunction with a moderate or severe interstitial lung pattern |
| Focal (> 10% to 30%) | No visualization of vessels in 11–30% of lung fields. Air bronchograms might or might not be present within < 30% of lung fields |
| Localized (> 30% to 50%) | No visualization of vessels in 31–50% of lung fields. Air bronchograms might or might not be present within < 50% of lung fields |
| Extensive (≥ 50%) | No visualization of vessels in ≥ 50% of lung fields. Air bronchograms might or might not be present throughout the entire section of lung field |
| Interstitial lung pattern (Characterization of the non-air-containing elements of the lungs including blood vessels and bronchi) | |
| Normal | Clear visualization of vessels. Borders are well defined |
| Mild increase | The pulmonary vessels appear slightly ill defined (hazy borders with loss of visualization of the fine vascular structures). Mildly lacy appearance to lung field |
| Moderate increase | The vessels are ill defined, resulting in moderately lacy appearance and increased opacity of the lung field |
| Marked increase | Significantly increased opacity; vessel borders are barely recognizable |
| Bronchial pattern (Characterized by alterations in bronchial wall thickness and density, or in bronchial lumen diameter. Note that periobronchial cuffing is a feature of interstitial not bronchial pattern) | |
| Normal | Bronchial structures seen in cross section appear as small, thin-walled hollow rings between paired vessels. The bronchial walls are barely distinguishable when viewed side-on and are not clearly visualized at the periphery of the lung field |
| Moderate increase | A few thickened bronchial walls evident in cross section (‘doughnuts’) at the periphery of the lung fields. Longitudinal sections appear as tram lines reaching two-thirds of the way to the lung periphery |
| Marked increase | Extensive bronchial thickening might be observed, extending far into the periphery of the visible lung field |
Radiography can assist in the recognition and differentiation of atelectasis and consolidation, interstitial and exudative pneumonias, the alveolar pattern of pulmonary disease, neoplasms, pleural effusions, pneumothorax, hydropericardium and space-occupying lesions of the thorax. Cardiomegaly, abnormalities of the cranial mediastinum, fractures of ribs and diaphragmatic hernia can also be detected.
Many pulmonary diseases do not have lesions that are readily detected on radiographic examination. Failure to detect abnormalities on radiographic examination of the thorax does not eliminate pulmonary disease. Furthermore, radiographically detectable signs of lung disease can persist after the animal has clinical and clinicopathological signs of recovery or improvement.
Bronchography utilizing contrast agents is of value in determining the patency of the trachea and bronchi, but general anesthesia is required to overcome the coughing stimulated by the passage of the tracheal catheter. Using a fluoroscope to determine the location of the catheter tip, the contrast agent can be deposited in each dependent lobe in turn. This technique is used infrequently. Computed tomographic (CT) examination of the lung is very sensitive and specific for lung disease in companion animals and is technically feasible in calves, foals and small ruminants. The technique is useful in the diagnosis of mediastinal disease in foals.27
Radiographic examination of the trachea can reveal the presence of abnormalities in shape, such as occur with tracheal collapse, or the presence of foreign bodies or exudate.
Radiographic examination of the head can identify diseases of the paranasal sinuses, ethmoids and pharynx. Radiographic examination is useful in defining diseases of the guttural pouches and in detecting retropharyngeal abscesses or abnormalities, such as the presence of foreign bodies. The CT anatomy of the head of horses and foals has been described.28-30 CT imaging of the nasal cavities and paranasal sinuses of horses is useful in the detection of diseases of these structures,31,32 and of the teeth,33 pharynx, larynx and guttural pouches.34 The technique is technically feasible in ruminants and pigs, although there are few reports of its use in these species.35
Magnetic resonance (MR) imaging is useful in diagnosis of diseases of the head, and the anatomy as visualized on MR imaging of the head of horses has been reported.36 Unfortunately, the lack of units suitable for examination of large animals precludes routine use of this imaging modality.
SCINTIGRAPHY (NUCLEAR IMAGING)
The basis of pulmonary scintigraphy is detection at the body surface of radiation emitted from the lungs after injection or inhalation of radioactive substances.37 The technique has been described in both horses and calves.37,38 The technique has limited diagnostic usefulness in large animals because of the need for availability of appropriate isotopes and detection equipment. Furthermore, the large size of adult cattle and horses limits the sensitivity of the technique. The technique has been used to determine the distribution of pharmaceuticals administered by aerosolization and the presence of ventilation/perfusion mismatches. Alveolar clearance can be detected using scintigraphic examination. Currently pulmonary scintigraphy is largely a research tool.
ULTRASONOGRAPHY
Ultrasonographic examination of the thorax of farm animals and horses is a very useful diagnostic tool. Ultrasonographic examination of the thorax provides diagnostic information that is not obtained by radiographic examination. The widespread availability of portable ultrasound units and the ability to image parts of the thorax using ultrasound probes intended for examination of the reproductive tract of mares and cows makes this a potentially valuable diagnostic aid for both field and hospital-based practitioners. Furthermore, the absence of radiation exposure and the ‘real-time’ nature of images obtained by ultrasonography aid in frequent assessment and monitoring of abnormalities and performance of diagnostic or therapeutic procedures such as thoracocentesis or aspiration of masses.
There are limitations to imaging imposed by aerated lung and the bones of the ribcage. Examination of the thorax is limited by the presence of ribs and aerated lungs because the sound waves used to create ultrasound images are reflected from these surfaces. Ultrasonography cannot reveal lesions of the lungs that are not confluent with the visceral pleura. Imaging windows are restricted to the intercostal spaces but this impediment can be overcome by scanning through adjacent intercostal spaces and angling of the ultrasound beam.
Ultrasonographic examination of the thorax should be performed in a consistent manner that ensures thorough examination of the thorax. Preferences for the pattern of examination differ somewhat among examiners, but one common and successful technique is to scan each intercostal space from dorsal to ventral starting at the 17th intercostal space in horses and the 12th intercostal space in cattle. The ultrasound probe is slowly moved from dorsal to ventral while the examiner studies the images. When one scanning of one intercostal space is completed, the probe is moved to the most dorsal aspect of the next intercostal space and the examination is repeated. Each side of the chest is examined in this manner. This consistent and thorough examination ensures that no important or localized abnormalities are missed. The examination is performed in adult horses and cattle with the animal standing. The rostral thorax is scanned by pulling the ipsilateral forelimb forward. This is more readily achieved in horses than in cattle. Thorough examination of the rostral thorax might require placing the animal in lateral recumbency. Calves and foals can be examined either standing or in lateral recumbency.
Ultrasound examination of the thorax is particularly useful for detecting diseases of the pleura, pleural space or lung surface. This is in addition to the well-documented utility of ultrasonographic examination of the heart and great vessels (see Ch. 8). The normal ultrasonographic anatomy of the thorax of cattle, horses and calves has been determined.39-41 The following is a partial list of disorders or abnormalities detectable by percutaneous ultrasonographic examination of the thorax of farm animals or horses (excluding cardiac abnormalities):
• Characteristics of pleural fluid (flocculent, bubbles, fibrin)
• Extent of pleural fluid accumulation
• Localized areas of pleural fluid accumulation
• Nonaerated lung (atelectatic, consolidated)
• Pulmonary abscesses (must be confluent with visceral pleura)
• Intrathoracic masses (thymic lymphoma, cranial thoracic mass, gastric squamous cell carcinoma)
• Pleural roughening (‘comet-tail’ lesions)
• Pulmonary hematoma42
Ultrasonographic examination is more sensitive and specific than radiographic examination in detecting the presence of pleural fluid42 and is particularly useful in the diagnosis and management of pleuritis in horses and cattle43,44 and pneumonia in calves.45 The extent of pulmonary lesions detected at necropsy correlates closely with the results of ultrasonographic examination of calves with pasteurellosis.46 Ultrasonographic examination is useful in diagnosis of thoracic diseases of cattle.47 Ultrasonography can identify pulmonary lesions in horses with infectious viral pneumonia48 and is a viable alternative, though not as sensitive, to radiology in the evaluation of foals with Rhodococcus equi pneumonia.49 Ultrasonography is very useful in identifying the presence of pleural fluid and guiding thoracocentesis to sample and drain this fluid.
LABORATORY EVALUATION OF RESPIRATORY SECRETIONS
SAMPLING RESPIRATORY SECRETIONS
When an inflammatory disease process of the respiratory tract is suspected, the collection of samples of secretions and exudate for microbiological and cytological examination can be considered. The objective is to obtain a sample uncontaminated with environmental flora, which are common in the upper respiratory tract, and to isolate the pathogen(s) or demonstrate inflammatory cells which may be associated with the lesion. This can be done by:
NASAL SWAB
A swab of the nasal cavities is a reliable method for the evaluation of the secretions associated with disease of the upper respiratory tract such as infectious bovine rhinotracheitis and allergic rhinitis. However, when attempting to assess the health status of the lungs the nasal swab can be unsatisfactory because microbiological examination usually yields a large population of mixed flora, consisting of pathogens and nonpathogens, which is difficult to interpret.
NASOPHARYNGEAL SWABS
For more reliable results and to lessen the contamination that occurs with nasal cavity samples, swabs of the laryngeal–pharyngeal area can be collected. A swab in a long covered sheath, of the type used for collecting cervical swabs from mares, is easily passed through the nasal cavities to the pharyngeal area. Significant differences may exist between the microbial isolates from nasopharyngeal swabs and those from lung tissues, which makes nasal swabs unreliable for diagnosis. For example, at the individual animal level, nasopharyngeal swabs and bronchoalveolar lavage show only moderate agreement; at the group or herd level the isolation rates of various organisms are similar.50
For isolation of viruses associated with disease of the upper respiratory tract, nasal swabs are satisfactory provided a copious amount of nasal discharge is collected and the swabs are kept moist during transport to the laboratory. Nasal swabs sometimes contain an insufficient amount of secretion, and certain viral pathogens can become inactivated in transit.
NASAL LAVAGE
When larger quantities of nasal discharge are required for research purposes, nasal washings are usually collected, the simplest technique being irrigation of the nasal cavities and collection into an open dish. From these samples, it is possible to isolate bacteria and viruses, and identify immunoglobulins. The development of immunofluorescent and enzyme-linked immunosorbent assay (ELISA) tests for agents of infectious disease has provided reliable systems for the diagnosis of a variety of virus diseases in the early stages of infection. A technique and apparatus are available that obtain much better samples than the conventional cotton-wool swab provides. A vacuum pump aspirates epithelial cells and secretion from the nasal passage and pharynx. Cell smears are then prepared for microscopic examination and the mucus and cells are used for conventional microbiological isolation.
PARANASAL SINUS FLUID
Fluid can be collected from the frontal and paranasal sinuses of most of the domestic large animals. Indications for collection of fluid include the presence or suspected presence of disease of the paranasal sinus. Medications can be administered and infected sinuses lavaged using this approach.51,52 Absolute contraindications are few but include failure to be able to adequately restrain the animal. Demonstration of fluid in the paranasal sinuses is aided by radiographic examination of the skull. Fluid is collected by percutaneous centesis of the frontal or maxillary sinus and submitted for cytological and bacteriological examination (Gram stain, culture). The procedure is: restraint of the animal, which can include the induction of moderate sedation by administration of alpha-2 agonists and narcotics, or in cattle restraint in a head gate with the head secured with a halter. The area over the centesis site is prepared aseptically and the skin and subcutaneous tissues are anesthetized with local anesthetic.51 A stab incision (< 1 cm) is made in the skin and subcutaneous tissues. A hole is then drilled into the sinus using a Jacob’s chuck with a Steinmann pin (2–4 mm diameter). Only a short (5 mm) length of the Steinmann pin should be exposed by the chuck. The hole is drilled by applying steady pressure and making alternating clockwise and counterclockwise movements with the chuck. Entry into the sinus cavity is evident as a sudden release of tension and easy passage of the Steinmann pin. The pin is then withdrawn and sterile polyethylene tubing is inserted into the sinus cavity. Fluid can be aspirated at this time or, if none is forthcoming, 10–20 mL of sterile 0.9% saline or similar fluid can be instilled to the sinus cavity. Some of this fluid may run out the nostril if the animal’s muzzle is lower than the sinus. Complications include injury to adjacent structures, including the infraorbital nerve (trigeminal nerve), nasolacrimal duct or parotid salivary duct near its entrance to the oral cavity at the level of the upper cheek teeth. Hemorrhage is usually minor and self-limiting. Subcutaneous emphysema resolves within days. Cellulitis is a risk, especially for animals with septic processes in the paranasal sinuses. Prophylactic administration of antibiotics should be considered in these cases.
GUTTURAL POUCH FLUID
Indications for collection of fluid from the guttural pouches of equids include bacteriological or polymerase chain reaction (PCR) examination to determine if the horse is infected by S. equi (the etiological agent of strangles) or to investigate the suspected presence of other inflammatory or neoplastic disease. The preferred method of collection is during endoscopic examination of the guttural pouch. During this examination, fluid can be collected through a polyethylene tube inserted through the biopsy port of the endoscope. Fluid collected in this manner is potentially contaminated by organisms in the upper respiratory tract, and results of bacteriological examination should be interpreted with caution. Usually, bacteriological examination is for the presence of S. equi and demonstration of its presence is all that is required for a diagnosis of infection. Fluid can also be obtained from the guttural pouch by blind passage of a firm catheter, such as a Chambers mare catheter or 10 French dog urinary catheter, into the guttural pouch. This procedure requires some skill and there is always the uncertainty that one might not have actually manipulated the catheter into the guttural pouch. A third technique involves percutaneous puncture of the guttural pouch just posterior to the ramus of the mandible and ventral to the ear. This technique has the potential to yield fluid that is uncontaminated by organisms from the upper respiratory tract, but carries with it a high risk of injury to the important vascular and neural structures in and around the guttural pouch (internal and external carotid arteries, pharyngeal branch of the vagus nerve, hypoglossal nerve, and others). Percutaneous sampling of guttural pouch fluid should not be undertaken without careful consideration of the risks and benefits of the procedure.
TRACHEOBRONCHIAL SECRETIONS
The collection and evaluation of tracheobronchial secretions is a useful method for assessing lower airway disease and is widely used in the determination of the etiology of infectious pneumonia (viral, mycoplasmal, fungal, and parasitic) or the severity of disease (bronchoalveolar lavage fluid cytology in horses with heaves, exercise-induced pulmonary hemorrhage in athletic horses). It is also used as a tool in evaluating respiratory health of intensively housed animals, such as in piggeries.53 Cytological examination of recovered fluid can provide valuable information about the severity, extent and etiology of disease of the lower airway. There are two methods of sampling tracheobronchial secretions – aspiration of tracheal fluid or lavage of bronchioles and distal airways. Each sampling method yields fluid of differing characteristics and source and interpretation of the results of examination of these fluids depends on their source and the method of collection.
Comparison of tracheal aspirates and bronchoalveolar lavage fluid
Examination of tracheal aspirates and bronchoalveolar lavage fluid yields different, but often complementary, information about the lower respiratory tract. The differences between tracheal aspirates and bronchoalveolar lavage fluid arise because cell populations, and types of cell, differ markedly among segments of airways. There is no correlation between cytological features of tracheal aspirates and bronchoalveolar lavage fluid of horses, and this is probably the case in other species.54 Tracheal aspirates are representative of cell and bacterial populations of the large conducting airways (trachea and mainstem bronchi), which can originate in both the large and small conducting airways and the alveoli.54 Secretions of more distal airways can be modified during rostrad movement, such that fluid in a tracheal aspirate is not representative of processes deeper within the lung. Furthermore, disease localized to one region of the lung can alter tracheal fluid. Examination of tracheal aspirates is useful for detecting inflammation of the large airways and for isolation of microorganisms causing disease in these structures. There is no good evidence that findings on examination of tracheal aspirates correlate with abnormalities in pulmonary function, and tracheal aspirates do not accurately reflect lesions in the lungs of horses.55
Bronchoalveolar lavage is useful for sampling secretions in the more distal airways. It provides a sample of secretions that have not been contaminated by upper respiratory tract organisms or secretions before collection and the sample is therefore assumed to be more representative of small airway and, to a lesser extent, pulmonary parenchymal and alveolar secretions or exudates. Bronchoalveolar lavage is useful in the detection of widespread lung disease but not necessarily in the detection of localized disease. Tracheal aspirates, because they in theory represent a composite sample of secretions from all regions of the lung, are likely to be more sensitive in detecting focal disease, such as a pulmonary abscess. Bronchoalveolar lavage fluid composition correlates well with pulmonary function in horses.56,57
There is little agreement in cytological examination of tracheal aspirates and bronchoalveolar lavage fluid of sick and healthy horses, and this difference probably exists in other species. Typically, the proportion of cells that are neutrophils is much higher in tracheal aspirates than in bronchoalveolar lavage fluid of both horses with heaves and normal horses.54,58 Mast cells are detected more frequently, and eosinophils less frequently, in bronchoalveolar lavage fluid than in tracheal fluid of normal horses.59
Tracheal aspirates
Indications for collection of tracheal aspirates include the need for microbiological and cytological assessment of tracheal fluids. The primary indication is collection of samples for microbiological diagnosis of infectious respiratory disease. Other indications include detection and characterization of inflammation of the conducting airways. Contraindications include severe respiratory distress, although this is not an absolute contraindication, inability to adequately restrain the animal, and severe, spontaneous coughing. Percutaneous tracheal aspirate collection performed in animals with severe coughing can result in development of severe subcutaneous emphysema as a result of the high intratracheal pressures associated with the early phase of coughing. Most animals in which percutaneous tracheal aspirates are collected subsequently have radiographic evidence of pneumomediastinum.
Tracheal aspirates can be collected either by percutaneous puncture of the trachea or through an endoscope passed through the upper airways. The advantage of percutaneous collection of tracheal aspirates is that there is minimal risk of contamination of the sample by upper respiratory tract or oropharyngeal secretions. Microbiological examination of the samples is therefore likely to accurately reflect microbes present in tracheal fluid. Collection of tracheal aspirates through an endoscope markedly increases the risk of contamination of the sample with oropharyngeal fluids, and compromises the diagnostic utility of culture of the sample. This disadvantage is partially alleviated by the use of guarded catheters inserted through the endoscope.60,61 The disadvantage of percutaneous collection of tracheal fluid is that it is invasive and there is a risk of localized cellulitis and emphysema at the site of puncture. Endoscopic collection is relatively noninvasive and readily accomplished.5 We prefer use of the percutaneous method when accurate microbiological assessment of samples is desired.
Percutaneous transtracheal aspiration
This is a practical method that has been used extensively in the horse60 and is adaptable to cattle,62 sheep and goats. For the horse, a 60 cm no. 240–280 polyethylene tube is passed through a 9–14-gauge needle inserted into the trachea between two rings. Commercially prepared kits for performing tracheal aspirates in horses are available that include all catheters and needles required. An alternative to polyethylene tubing is to use an 8–10 French male dog urinary catheter inserted through an appropriately sized cannula. The site for insertion of the needle or cannula is at the junction of the proximal and middle one-thirds of the ventral neck. The horse is usually sedated prior to insertion of the needle or cannula. The skin site is prepared aseptically and a short stab incision is made after the area has been anesthetized. The cannula is removed to avoid cutting the tube and the tube is pushed in as far as the thoracic inlet. Fluid typically pools in the trachea at the thoracic inlet in horses with lung disease (the tracheal lake or pool) and it is this fluid that is aspirated. Thirty to 50 mL of sterile saline (not bacteriostatic saline) is rapidly infused. The catheter or tubing should be rotated until tension is felt on aspiration by a syringe. Fluid is aspirated and submitted for cytological, microbiological or other examination.
Complications such as subcutaneous emphysema, pneumomediastinum and cellulitis can occur, which necessitates care and asepsis during the procedure. Sudden movement of the cannula during insertion of the tubing may cause part of the tube to be cut off and to fall into the bronchi, but without exception this is immediately coughed up through the nose or mouth.
Endoscopic sampling of tracheal secretions
The flexible fiberoptic endoscope can be used to obtain tracheal lavage samples and at the same time visualize the state of the airways.15,60 The process is as for rhinolaryngoscopic examination with the addition of passage of a catheter through the biopsy port of the endoscope. Tracheal fluid is then visualized and aspirated through the catheter. The clinical advantages of the endoscopic collection include noninvasiveness, visual inspection of the airways, guidance of the catheter, and speed.60 The use of an endoscope with a guarded tracheal swab minimizes contamination by oropharyngeal secretions but does not eliminate it.5,61
Assessment of results
Microbiological examination can yield any one or more of a variety of bacteria, depending on the species examined, the animal’s age and its clinical condition. Tracheal aspirates of normal animals rarely yield any bacterial growth on culture. Growth of unusual organisms or known oropharyngeal commensal bacteria from samples obtained by endoscopic examination should not be given undue clinical significance as they probably result from contamination of the tracheal aspirate during collection. Pseudomonas spp. and anaerobes isolated from tracheal aspirates collected by endoscopy are almost always contaminants and of no clinical significance.5 The extent of contamination of tracheal aspirate samples by oropharyngeal bacteria can be estimated from the number of squamous epithelial cells in the sample.59 There is an apparent approximate linear relationship between the number of squamous cells per milliliter of fluid and the number of colony-forming bacterial units in tracheal aspirates. Samples containing over approximately 10 squamous epithelial cells per milliliter of tracheal aspirate had markedly greater bacterial contamination.59 Examination of Gram-stained smears of tracheal fluid is specific but not very sensitive for detection of bacteria, compared with culture.63 In other words, if examination of a Gram-stained smear of tracheal fluid reveals bacteria, then the sample is likely to yield bacteria on culture, whereas failure to detect bacteria predicts poorly the likelihood of growth of bacteria on culture of the sample. This indicates that examination of Gram-stained samples of tracheal fluid does not reliably predict bacterial isolation, and if an infectious etiology is suspected the fluid should be cultured. Results of the microbiological examination of the tracheal fluid should be consistent with the animal’s clinical condition and expected isolates.
Cytological examination of tracheal fluid is an important diagnostic tool. Various stains are available to aid identification of cell types and numbers in tracheal aspirates. Neutrophils, macrophages, lymphocytes and epithelial cells are readily identified on the basis of their classical morphology and staining using fast Romanowsky stain (Diff-Quik®), but this stain is not suitable for identifying mast cells in equine tracheal fluid and probably that of other species.59 Leishman’s stain is useful to identify mast cells.59 Clinically normal horses typically have fewer than 20–30% of cells as neutrophils with the majority of remaining cells being macrophages, lymphocytes and epithelial cells.54,61 Animals with inflammation of the airways typically have increased cell counts and proportion of neutrophils, and large amounts of mucus. Horses with inflammatory airway disease such as heaves typically have more than 20% of the cells as neutrophils (see Heaves, below), and those with infectious pneumonia often have 50–90% of cells as neutrophils. The presence of eosinophils is considered abnormal and is consistent with parasite migration (Parascaris equorum in foals, Dictyocaulus viviparus in calves). The presence of hemosiderin-laden macrophages is evidence of prior pulmonary hemorrhage.64
Bronchoalveolar lavage
Bronchoalveolar lavage provides a sample of secretions and cells of the distal airways and alveoli, referred to as bronchoalveolar lavage fluid. It is a widely used procedure in horses and, to a lesser extent, cattle and calves,65-67 sheep68 and pigs.53,69 Analyses performed on bronchoalveolar lavage fluid include measurement of cell number and type, culture (usually in pigs and cattle) and analysis of immune proteins and surfactant. It is a relatively noninvasive procedure that allows cytological and biochemical evaluations of the lower airways and alveoli, which are useful diagnostic aids when evaluating animals with lung disease. While fiberoptic bronchoscopy and tracheal aspirates permit assessment of the major bronchi and upper airways, bronchoalveolar lavage offers an extension of the diagnostic potential by sampling the terminal airways and alveolar spaces.
The primary indication for collection of bronchoalveolar lavage fluid is acute or chronic lung disease. This includes both infectious and noninfectious diseases, although interpretation of samples collected by passage of the collection tube through the nostrils or mouth is complicated by the inevitable contamination of the sample by oropharyngeal commensal bacteria. Despite this shortcoming, the technique has been used to detect pneumonia associated with Mycoplasma sp. in cattle.70 Contraindications are few, with respiratory distress being an obvious one. Complications of bronchoalveolar lavage are also few, and include a mild neutrophilia in lavaged sections of lungs and changes in phagocytic function of pulmonary macrophages, and microbial content, for several days after the procedure.31,71
A shortcoming of bronchoalveolar lavage is that it lavages only a small region of the lung, with the risk that focal lung disease is not detected. This is best exemplified in pneumonia in horses, in which bronchoalveolar lavage fluid from pneumonic horses can contain large numbers and a high proportion of neutrophils or can be normal, depending on the area of lung lavaged. Therefore, the bronchoalveolar lavage procedure is a very specific but not very sensitive test for pneumonia in horses.72 Abnormal lavage fluid is helpful diagnostically, whereas normal results do not exclude the presence of foci of pulmonary disease. The lavage samples may be normal in horses affected with pneumonia or pleuropneumonia and, because of these false-negative results, this is not the best diagnostic technique to evaluate a horse with pneumonia.13 In contrast, the tracheobronchial aspirates are more sensitive and most horses with pneumonia have cytological abnormalities.13
Endoscopic bronchoalveolar lavage
Endoscopic bronchoalveolar lavage has the advantage of permitting visual examination of the airways during the procedure and selection of the region of the lung to be lavaged. This technique does require access to sophisticated endoscopic equipment. The technique described below for horses can be modified for use in other species.73
Horses for bronchoalveolar lavage should be appropriately restrained. Sedation is usually essential and is achieved by administration of alpha-2-agonists. Coadministration of narcotics is recommended by some authorities to reduce the frequency and severity of coughing. Butorphanol tartrate 10 mg for a 400 kg horse is recommended, although this drug is not as effective as intratracheal lidocaine at reducing the frequency or severity of coughing when combined with detomidine for collection of bronchoalveolar lavage fluid.74 Effective suppression of coughing during collection of bronchoalveolar lavage fluid can be achieved by instillation of lidocaine (60 mL of a 0.7% solution – made by diluting 20 mL of 2% lidocaine solution by addition of 40 mL of isotonic saline). The lidocaine solution is administered as the endoscope enters the rostral trachea. A twitch can be applied to the nares. The endoscope must be at least 2 m in length and the external diameter should be 10–15 mm. Endoscopes of 10 mm diameter will pass to about the fifth-generation bronchi, whereas endoscopes of larger diameter will not pass quite as far into the lung. The endoscope is passed until it wedges and then 300 mL of warmed (to reduced bronchospasm) isotonic saline is introduced in 5 × 60 mL aliquots. Air is infused after the last aliquot to ensure that all fluid is instilled. After the horse has taken between one and three breaths the fluid is withdrawn and the aliquots are mixed. There is no difference in the cytological composition of the first and subsequent aliquots.75
Blind bronchoalveolar lavage
Commercial bronchoalveolar lavage tubes are available for use in horses, and are suitable for use in adult cattle and calves.67 The tubes are made of silicone and are therefore considerably more pliable than stomach tubes (which are not suitable for this procedure). The tubes are 2 m in length and have an external diameter of about 8 mm. The horse is restrained and sedated as for endoscopic bronchoalveolar lavage and the tube is passed through one nostril into the trachea. The tube is then advanced until it wedges, evident as no further insertion of the tube with mild pressure. Continued vigorous attempts to pass the tube can result in the tube flexing in the pharynx and a loop of the tube entering the mouth. After the tube wedges, the cuff on the tube is inflated to prevent leakage of fluid around it, 300 mL of warm isotonic saline is instilled, the tube is flushed with air and fluid is aspirated. The fluid should be foamy and, if cell counts are high, slightly cloudy.
Bronchoalveolar lavage can be performed in conscious sheep by insertion of 1.7 mm external diameter polyethylene tubing through a cannula inserted percutaneously in the trachea.68 The tubing is inserted until resistance is detected (about 40–45 cm in an adult sheep) and the lung is lavaged with 30 mL of sterile isotonic saline.
Laboratory assessment of tracheobronchial secretions
A problem with comparison of cell counts of bronchoalveolar lavage fluid reported by different authors is the use of inconsistent quantities of fluid to perform the lavage. The use of different volumes alters the extent of dilution of the fluid. There is a need for uniformity in technique.76 An approach to this problem has been to measure substances in the bronchoalveolar lavage fluid that can provide an indication of the extent of dilution of the sample. Both endogenous (urea, albumin) and exogenous (inulin, methylene blue) markers have been used. Dilution factors using urea concentration in plasma and in bronchoalveolar lavage fluid appear to be useful.77 The assumption is that urea concentrations in bronchial and alveolar secretions will be identical to that in plasma. The formula for correcting for dilution that occurs during collection of bronchoalveolar lavage fluid is:
Dilution factor = Urea concentration in bronchoalveolar lavage fluid/Urea concentration in plasma,
where urea concentration in bronchoalveolar lavage fluid and in plasma is expressed in the same units. The volume of the pulmonary epithelial lining fluid can then be calculated:
Pulmonary epithelial lining fluid volume = dilution factor × volume of bronchoalveolar lavage fluid.
Samples for cytology are submitted for preparation involving centrifugation of the sample to concentrate cells for preparation of slides for staining and microscopic examination. At least for samples from horses, examination of smears made directly from the sample, without centrifugation, is diagnostically useful.78 As for tracheal fluid, the proportion of mast cells in equine bronchoalveolar lavage fluid is underestimated if cells are stained with fast Romanowsky stain (Diff-Quik®).79
Diagnostic value
The aspirates from normal animals contain ciliated columnar epithelial cells, mononuclear cells and a few neutrophils with some mucus. The concentration of the cells depends on the volume of fluid infused and the disease status of the animal. Representative values for various species are listed in Table 10.3. The general pattern is that animals with inflammatory airway disease, either infectious or noninfectious, have a higher proportion of neutrophils than do disease-free animals. However, ranges of normal values vary considerably depending on the species, the age of the animal and its management (primarily housing conditions). Care should be taken not to overinterpret findings on examination of tracheal aspirates or bronchoalveolar lavage fluid. While there is good correlation between microbiological results and cell counts in bronchoalveolar lavage fluid of calves with pneumonia65 and Thoroughbred race horses with inflammatory airway disease,80 this association might not hold for all diseases or species.
Thoracocentesis (pleurocentesis)
Paracentesis of the pleural cavity is of value when the presence of pleural fluid is suspected and, in the absence of ultrasonographic examination, needs to be confirmed, and when sampling of pleural fluid for cytological and bacteriological examination is indicated. The primary indication for sampling pleural fluid is the presence of excess pleural fluid. Sampling of pleural fluid is usually accompanied by therapeutic drainage, in which case the cannula used for sampling is larger than if only collection of pleural fluid is desired. Contraindications are minimal, especially if the procedure can be performed under ultrasonographic guidance. The principal contraindication is the inability to restrain an unruly animal, as this increases the risk of laceration of the lung or a coronary vessel, or cardiac puncture. Complications include hemorrhage from lacerated intercostal or pleural vessels, pneumothorax secondary to laceration of the lung or introduction of air through the cannula, cardiac puncture and sudden death, irritation of the myocardium and ventricular arrhythmia (premature ventricular contractions), or coronary artery laceration and subsequent cardiac tamponade and death. There is a risk of cellulitis at the site of centesis, especially if indwelling cannulas are maintained for more than a day.
The procedure is performed with the animal standing. Sedation or systemic analgesia is usually not needed, unless it is medically indicated or the animal is not easily restrained. The equipment for sampling of pleural fluid from adult horses or cattle is a blunt 10–15 cm cannula of approximately 3 mm diameter (such as a bovine teat cannula) or a 7.5 cm spinal needle. The blunt-tipped cannula is preferred because use of it reduces the risk of laceration of vital structures. A three-way stopcock or similar device should be attached to the hub of the needle or cannula and closed to prevent aspiration of air when the pleural cavity is entered. The site for centesis is best identified by ultrasonographic examination of the thorax or, if that is not available, by percussion and auscultation of the chest to identify the fluid level. A commonly used site is the seventh intercostal space on the left side and the sixth intercostal space on the right side. The skin should be clipped of hair and aseptically prepared. The region can be anesthetized with approximately 10 mL of 2% lidocaine, mepiricaine or similar product. The cannula or needle should be introduced over the rib and then directed cranial to the rib (the intercostal vessels and nerves course along the caudal edge of the rib). If a cannula is used then a slight ‘popping’ sensation is felt as the cannula perforates the parietal pleura. A syringe is attached to the cannula or needle and fluid is aspirated from the pleural space.
Collected fluid should be examined visually. Normal pleural fluid, which is present in small quantities in normal animals, is clear and slightly yellow. Abnormal fluid can be bloody, thick and yellow, suggestive of purulent material, or flocculent. The material should be smelled – a foul odor is usually present when the pleural fluid is infected by anaerobic bacteria and is a sign of a poor prognosis. Cytological examination should be performed, including white cell count and measurement of total protein concentration. Ancillary measurements on pleural fluid include pH, Pco2, Po2, bicarbonate, glucose and lactate. Sterile pleural fluid has a pH, Po2 and Pco2 and lactate, glucose and bicarbonate concentrations similar to those of venous blood.81 Infected pleural fluid is acidic, hypercarbic and has an increased concentration of lactate and decreased concentrations of bicarbonate and glucose compared to venous blood.81 Pleural fluid should be cultured for aerobic and anaerobic bacteria and mycoplasmas. Antimicrobial susceptibility should be determined for isolated organisms. Fungal cultures are rarely indicated.
Ultrasound-guided needle puncture of a suspected lung abscess to determine the species of bacteria present is sometimes practiced but there is the risk that infection will be spread to the pleura by this technique. This technique is not recommended as a routine procedure as microbiological examination of tracheal aspirates will probably yield the offending bacteria.
PULMONARY FUNCTION TESTS
Pulmonary function tests provide quantitative assessment of pulmonary ventilatory function through measurement of expired and inspired gas volumes, intrathoracic pressures and derivations of these variables – sometimes referred to as pulmonary mechanics. The techniques are widely used in research into pulmonary diseases, especially heaves, in horses, and have been adapted for use in ruminants.82 A relatively simple assessment of pulmonary function is measurement of pleural pressure changes during respiration. This can be achieved by either insertion of a blunt cannula through the intercostal space or passage of a balloon catheter into the thoracic esophagus. The pressure changes during respiration are then recorded and the maximal pressure change between inspiration and expiration is calculated. The pressure change is closely correlated with airway resistance to airflow and is an excellent indicator of the severity of bronchoconstrictive diseases.
More complex measurements are made by application of an airtight face mask containing a flow meter to the animal. Combined with measures of airway pressure, air flow during tidal breathing yields measures of tidal volume, minute volume, respiratory rate, pulmonary resistance and pulmonary dynamic compliance. Measurements made with the animal at rest are relatively insensitive to small changes in pulmonary function and the sensitivity of these tests to detect heaves is low.57 The sensitivity of changes in maximal pleural pressure and resistance of the lower airways are 44% and 22%, respectively.57 The sensitivity of the test can be increased by measuring these variables during exercise. Measurement of pulmonary mechanics in horses with heaves is reproducible over both short (hours) and long (months) periods of time, indicating the usefulness of these techniques for monitoring of disease progression and response to therapy.83
Measurement of flow–volume loops has been performed for both stationary and exercising horses.84,85 A number of variables are derived from these measures and used as indicators of pulmonary function. However, the large variability in these measures in stationary horses (16–32%) severely limits the utility of this test to detect mild or subclinical respiratory disease. Similarly, flow–volume loops in exercising horses with obstructive lung disease of moderate severity do not differ markedly from those of the same horses when they do not have lung disease. Flow–volume loops have limited use in evaluation of lung function in animals.
Other tests of pulmonary function include the nitrogen dilution test and the single-breath diagram for CO2. For the nitrogen dilution test concentrations of nitrogen in exhaled air are measured while the animal breathes 100% oxygen. A number of variables are calculated from the decay curve of nitrogen concentration in exhaled air, including the functional residual capacity.86 There are clinically significant differences between animals with normal respiratory function and those with pulmonary disease. However, this test is not readily adapted for routine clinical use. Volumetric capnography is the graphic examination of expired breath CO2 concentrations versus expired volume to create a single-breath diagram for CO2.87 The results are divided into phase I, which represents the relatively carbon-dioxide-free air from the proximal or orad conducting airways, phase II, which is the transitional phase, and phase III, which is the carbon-dioxide-rich air from the alveoli. Measures of pulmonary function obtained include estimates of dead space ratio, physiological dead space volume and alveolar efficiency.87 The clinical utility of this test and its ability to detect mild or subclinical disease in animals have not been demonstrated.
Impulse oscillometry offers the potential of being a potentially clinically useful test of respiratory function in both horses and cattle.46,88,89 The test measures impedance of the respiratory system and provides estimates of respiratory resistance and reactance.46 The technique has the advantage of being more sensitive to changes in pulmonary function than measurement of pleural pressure changes,90 is minimally influenced by respiratory rate and tidal volume91 and is relatively easier to perform than more complex measures of respiratory mechanics. The test involves fitting an airtight facemask containing a pneumotachograph for measurement of respiratory volumes and tubing to a horse. The tubing is attached to a loudspeaker, which is used to generated square-wave signals containing harmonics between 0 and 10 Hz. Information from the system is analyzed using a computer program and indices of pulmonary resistance and reactance are determined. The forced oscillation technique in feedlot cattle with naturally occurring shipping fever indicates the presence of a large increase in pulmonary resistance and a decrease in dynamic compliance with obstructive lung disease located mainly at the level of large airways but also in small airways.92 The clinical utility of the technique remains to be determined.
The sensitivity of these tests can be increased by provocative tests in which animals are administered agents, such as histamine or methacholine, that cause bronchoconstriction in animals with reactive airways.90
Measurement of forced expiratory flow–volume curves and forced vital capacity in horses is a sensitive indicator of bronchoconstriction.57,93 The test involves the heavily sedated horse having a nasotracheal tube inserted. The nasotracheal tube is then attached to a large vacuum reservoir and a valve is opened abruptly. The maximum rate of forced expiratory airflow is measured and various variables indicative of pulmonary function are calculated, including forced expiratory volume in one second (FEV1).93 The clinical utility of this test of pulmonary function is limited by the extensive instrumentation of the animal and the need for sophisticated electronics.
A portable system for monitoring cardiovascular and respiratory function in large animals is available. Pulmonary function testing of cattle is also being examined and may provide some understanding of the pathophysiology of respiratory tract disease. Calves between 1 and 8 months of age with chronic respiratory disease have:82
• Significantly reduced inspiratory and expiratory times and tidal volume
• Significantly increased respiratory frequency and airway resistance
• More negative transpulmonary pressure values when compared to predicted values for the same calves.
Arterial oxygen and carbon dioxide tensions are the only variables which correlate with clinical scores.
ARTERIAL BLOOD GAS ANALYSIS
Measurement of Pao2, Paco2 and arterial oxygen content (Cao2) provides valuable information about pulmonary function and oxygen delivery to tissues. The arterial oxygen tension and arterial oxygen content are not equivalent. The arterial oxygen tension (Pao2) is a measure of the partial pressure of oxygen in arterial blood determined by the amount of oxygen dissolved in the blood (not the amount bound to hemoglobin) and the temperature of the blood – it is not a direct measure of arterial oxygen content. Arterial oxygen content is the amount of oxygen per unit of blood and includes both dissolved oxygen and that bound to hemoglobin. The oxygen tension can be viewed as the driving force for diffusion of oxygen from capillaries into mitochondria (in which the oxygen tension is about 2 mmHg), whereas the oxygen content is the amount of oxygen delivered to tissue. Both are important measures of pulmonary function and oxygen delivery to tissue.
Measurement of oxygen tension in blood is achieved by analysis of an appropriately collected sample of arterial blood using a blood gas analyzer (oxygen electrode). Instruments designed for medical or veterinary clinical use measure pH, Po2 and Pco2 at a temperature of 37°C. Depending on the software included with the instrument, various derived values are also reported, including bicarbonate concentration, base excess and oxygen saturation. It is important to understand that oxygen saturation reported by blood gas instruments is a calculated value and might not be correct. Oxygen saturation is measured by a co-oximeter, which is different from a blood gas machine, and the amount of oxygen carried by hemoglobin is then calculated from this value and the assumption that each gram of hemoglobin, when fully saturated, carries approximately 1.34–1.39 mL of oxygen. The total oxygen content of blood is calculated by adding the amount carried by hemoglobin to the amount of oxygen dissolved in the aqueous phase of the blood. The formula is:
where O2content is in mL/100 mL, Sao2 is the arterial oxygen saturation (%), 1.34 is the amount of oxygen carried by fully saturated hemoglobin (mL/g), [Hb] is the concentration of hemoglobin in blood (g/100 mL), 0.003 is the amount of oxygen dissolved in the aqueous phase of 100 mL of blood for each 1 mmHg increase in Po2, and Pao2 is the oxygen tension in arterial blood. The appropriate substitutions can be made to calculate the oxygen content of venous blood.
The oxygen content of arterial blood is the critical factor (with cardiac output) in determining oxygen delivery to tissues. However, measurement of arterial oxygen content is not as readily accomplished as measurement of arterial oxygen tension. Therefore, in animals with normal hemoglobin concentration and function the arterial oxygen tension is used as a surrogate measure of arterial oxygen content. In doing so, it must be recognized that the extent of hemoglobin saturation with oxygen is dependent on both the affinity of hemoglobin for oxygen and the oxygen tension of the blood. The oxygen tension/percentage saturation relationship is sigmoidal, with 50% saturation occurring at about 30 mmHg in most species (there are minor variations) and 80% saturation at a Po2 of 45–55 mmHg.94 The sigmoidal shape of the oxygen– hemoglobin saturation curve has important clinical consequences. Small decrements in Pao2 from normal values (usually 95–105 mmHg in animals breathing ambient air at sea level) have a minimal effect on oxygen content of blood. Many modern blood gas analyzers have software that calculates oxygen content of blood, but it must be recognized that these calculations often use an assumed, not measured, hemoglobin concentration (usually 15 g/dL) and values for the human So2–Po2 relationship. These assumed values may not be correct for animals and one should always check the assumptions used to calculate oxygen content of blood before accepting and acting on those values. Direct measurement of blood oxygen content is restricted to research laboratories – indirect estimates gained from oxygen saturation and hemoglobin concentration are usually sufficiently accurate for clinical use.
The oxygen tension in blood is proportional to the amount of oxygen dissolved in the aqueous phase of the blood and the temperature of the blood. For a given amount of oxygen dissolved in blood, the tension varies according to the temperature of the animal. Almost all blood gas analyzers measure the Po2 at 37°C. If the animal’s body temperature is markedly different from that then the reported Po2 can be erroneous. For instance, the Pao2 of a horse with a body temperature of 40°C measured using an analyzer with a temperature of 37°C would be 80 mmHg (the Pco2 would be 35 mmHg). If the Pao2 was adjusted for the difference between the horse’s body temperature and that of the analyzer, then the reported Pao2 would be 100 mmHg (and the Paco2 would be 44 mmHg). Failure to make the appropriate temperature corrections can result in errors of 6–7% per °C.95 When interpreting blood gas values, attention should be paid to the temperature of the animal and consideration given to adjusting gas tension values according to the animal’s body temperature. This is probably only clinically important when there are extreme deviations from normal temperature and oxygen tension. Most blood gas analyzers include software that makes the appropriate corrections.
The arterial oxygen tension is determined in the alveolus by the alveolar oxygen tension and the alveolar–arterial difference. The alveolar oxygen tension (PAo2) can be calculated from:
where Fio2 is the inspired oxygen fraction (21% for ambient air), PB is the barometric pressure (760 mmHg at sea level), PH2o is the partial pressure of water vapor in the alveolar air (4 mmHg at 37°C), and RQ is the respiratory quotient (usually assumed to be 0.8 for resting animals). The alveolar–arterial Po2 difference (A–a Po2) is calculated:
The A–a Po2 difference has clinical significance in that it is an indicator of pulmonary function that is somewhat independent of inspired oxygen fraction and is therefore useful in animals being supplemented with oxygen (there is a small increase in A–a difference with marked increases in Fio2). Increases in A–a Po2 difference are indicative of ventilation/perfusion mismatches, with the A–a Po2 difference increasing with worsening ventilation/perfusion abnormalities.
Normal values
Values obtained from clinically normal animals breathing room air at sea level vary slightly between species, with most animals having an arterial Pao2 of 95–105 mmHg and a Paco2 of 35–45 mmHg. Oxygen saturation in clinically normal animals breathing air at sea level is above 98% and oxygen content of arterial blood is 16–24 mL/dL of blood (this depends on the hemoglobin concentration in blood). The difference in oxygen content of arterial and mixed venous blood is usually 4–8 mL/dL of blood. Values can be influenced substantially by changes in physiological state (exercise, hyperpnea), positioning, pulmonary disease and altitude (Table 10.4). Positioning of the animal can be important, especially in neonatal foals, in which the compliant chest wall can impair ventilation in laterally recumbent foals – foals have lower arterial oxygen tension when in lateral recumbency than when in sternal recumbency.96
Collection of arterial blood gas samples
Arterial samples can be collected from any of the appropriate peripheral arteries, which vary depending on species. An arterial sample is representative of aortic blood in almost all instances. Samples can be collected from the carotid, transverse facial, metacarpal and metatarsal arteries in horses and foals, and from the carotid, radial and coccygeal arteries in cattle and calves. Minimally invasive arterial access is difficult in pigs.
Samples should be collected in glass or plastic syringes in which the dead space has been filled with heparin solution. Typically, a 3 mL plastic syringe containing approximately 0.1 mL of sodium heparin and attached to a 22–25-gauge needle is used. All air should be expelled from the syringe before collection of the sample, and care should be taken to not introduce air into the syringe until blood gas tensions are measured. Air in the syringe will increase the measured oxygen tension of blood from normal animals. The sample should be measured as soon after collection as possible (within minutes). If immediate analysis is not available, the sample should be stored in iced water until analysis to prevent consumption of oxygen, production of carbon dioxide and a decrease in pH. Storage of arterial samples in plastic syringes in iced water can increase the oxygen tension from 100 mmHg to 109 mmHg in as little as 30 minutes.97 This does not occur when samples are stored in glass syringes in iced water. The pHa and Paco2 are not affected by the type of syringe.
VENOUS BLOOD GAS ANALYSIS
Measurement of gas tensions in venous blood is of limited value in assessing pulmonary function because of the extensive and variable effects of passage through the capillary beds on gas tensions. However, measurement of venous oxygen tension, saturation or content can be useful in assessment of the adequacy of oxygen delivery to tissue. The oxygen tension, saturation and content of venous blood depends on the extent of oxygenation of arterial blood, the blood flow to the tissues, the metabolic rate of the tissues drained by the veins from which blood is sampled, and the transit time of blood through capillaries. The multiplicity of these factors means that determining the precise reasons for abnormalities in venous blood gas tensions is not possible. However, some generalizations can be made about venous oxygen tension, saturation and content.
In normal, resting animals, oxygen delivery to tissues exceeds oxygen needs (demand) of the tissue, with the result that venous blood draining these tissues is only partially desaturated. Hence, venous blood from the pulmonary artery (mixed venous blood) has oxygen tension, saturation and content of approximately 35–45 mmHg, 80–90% and 12–18 mL/100 mL (the latter depending on hemoglobin concentration in addition to hemoglobin saturation). However, in situations in which oxygen delivery to tissue is decreased to levels that only just meet or do not meet the oxygen needs of tissue, there is extraction of a greater proportion of the oxygen in blood and venous oxygen tension, saturation and content decline and the arterial–venous difference in oxygen content increases. Reasons for oxygen delivery to tissue not meeting the oxygen needs of that tissue are decreased perfusion of tissue, such as can occur with shock or circulatory failure, anemia or decreased Pao2. Additionally, tissues with a high metabolic rate, such as exercising muscle, have high oxygen demands that can outstrip delivery.
Ideally, whole body assessment of oxygen delivery by measurement of venous blood gas tensions is best achieved by examination of mixed venous blood. Mixed venous blood represents an admixture of blood draining all tissues and is collected from the pulmonary artery (although samples collected from the right ventricle or atrium are also appropriate in most instances). While this blood is optimal for assessment of oxygen delivery to tissue, collection of mixed venous samples is not routine because of the need for catheterization of the pulmonary artery. Samples from peripheral veins are therefore used, but care should be taken when interpreting these values as venous blood gas tensions can vary considerably among veins.98 For animals with normal circulatory status, blood gas tensions in jugular vein blood are likely to be reasonable estimates of mixed venous gas tensions. However, if circulatory function is not normal, then samples from peripheral veins may not be indicative of values in mixed venous blood.
Samples for venous blood gas analysis should be collected into syringes in which the dead space is filled with sodium or lithium heparin solution. The volume of heparin should not be more than 2% of the amount of blood. Samples should be processed promptly. If samples cannot be processed within an hour they should be stored in iced water. Samples stored in iced water for 24 hours have values that are minimally different from those before storage, while samples stored at 25°C change markedly in 2–3 hours.99,100
PULSE OXIMETRY
Pulse oximeters are devices for measurement of blood oxygen saturation that attach to skin or mucous membranes and sense the absorption spectrum of light by hemoglobin (the same principle is used in bench top co-oximeters) in the underlying tissues. The devices are widely used for noninvasive monitoring of oxygenation in humans and have been adopted for use in animals. However, important challenges to their use exist in animals, not least of which is the presence of hair and densely pigmented skin in most farm animals. The devices have important deficiencies when used in foals and adult horses but those applied to the ear, lip or tongue of foals have good sensitivity and specificity for detecting arterial So2 of less than 90 mmHg (12 kPa).101,102 The devices consistently underestimate arterial So2 at low saturations.101,102 Care should be taken when using these devices to monitor arterial hemoglobin saturation in animals.
BLOOD LACTATE CONCENTRATION
Measurement of blood lactate concentration is useful in assessing the adequacy of oxygen delivery to tissues. Hypoxia causes a shift to anaerobic metabolism and the production of lactate. Lactate production is related to the severity and duration of hypoxia, with more severe hypoxia resulting in greater accumulation of lactate in tissues and its subsequent diffusion or transport into blood. Hypoxia also reduces the rate of removal of lactate from blood. The combination of increased production and decreased removal causes lactate to accumulate in blood. Measurement of blood lactate concentrations (which are usually lower than plasma lactate concentrations) is gaining increasing clinical usefulness as ‘point-of-care’ analyzers become more readily available and testing more affordable.
Samples for measurement of blood lactate can be collected into syringes containing heparin solution (as used for measurement of blood gas tensions) if the sample is to be analyzed within 30 minutes.103 Samples should be stored in iced water until analysis. Prolonged storage at room temperature results in increases in blood lactate concentration. If sample collection is anticipated to be delayed, then samples should be collected into evacuated tubes containing sodium fluoride and potassium ethylenediamine tetraacetic acid (EDTA) – the sodium fluoride inhibits glycolysis. However, plasma lactate concentrations collected in these tubes are approximately 10% lower than in samples collected into tubes containing heparin – probably because of the osmotic effect of sodium fluoride/potassium EDTA on red cells. Samples for clinical analysis should be collected into syringes containing a heparin solution and analyzed within 30 minutes of collection. Measurement of blood or plasma lactate concentrations can be made using ‘point-of-care’ analyzers, although these can yield results that differ markedly from traditional analyzers, especially in animals with extreme values for hematocrit (severe anemia or polycythemia). Ideally, blood and plasma lactate concentrations should be measured only on analyzers that have been validated for the species and clinical situation being studied.104
Blood lactate and plasma lactate concentrations are not equal, with blood lactate concentration being lower because of the dilutional effect of red blood cells, which have a lower lactate concentration than plasma. However, most clinical assessments are based on blood lactate concentrations. Mixed venous or arterial blood lactate concentrations in most farm animal species are less than 2 mmol/L in normal, healthy animals. Tissue hypoxia, in addition to other conditions such as toxemia and septic shock, can increase blood lactate concentration. Blood lactate concentrations between 2 and 4 mmol/L should be interpreted with caution whereas values above 4 mmol/L are indicative of clinically important disruption of oxygen transport and cellular metabolism. Repeated measurements over time can be useful for assessing progression of disease or efficacy of treatment. For instance, plasma lactate concentrations above 4 mmol/L in cattle with pneumonia are predictive of death within 24 hours.105
COLLECTION AND ANALYSIS OF EXHALED BREATH CONDENSATE
Collection and analysis of exhaled breath condensate has use primarily in research studies at the current time. Breath condensate is collected and analyzed for markers of pulmonary or systemic disease. Induction of pneumonia in calves by infection with Pasteurella multocida causes increases in concentration of leukotriene B4 in breath condensate.106 Horses with heaves have higher concentrations of hydrogen peroxide than normal horses – probably a result of the airway neutrophilia in affected animals.107
LUNG BIOPSY
Percutaneous biopsy of the lung is useful in confirming diagnosis of lung disease by providing tissue for histological and microbiological examination. The procedure in cattle, sheep and horses is described.108-110 Indications for the procedure include the presence of diseases of the lungs in which a diagnosis cannot be arrived at through other forms of examination, including tracheal aspiration or bronchoalveolar lavage. It can also be used for assessing the severity of histological changes and response to therapy. The procedure is best suited for widespread diseases of the lung, but can be used for diseases that produce focal lesions if the biopsy is performed with ultrasonographic guidance. Contraindications include abnormalities in clotting function, pneumothorax and severe respiratory distress. The danger in performing lung biopsy in animals in severe respiratory distress is that complications of biopsy, such as pneumothorax, hemothorax or hemorrhage into airways, could further impair lung function and cause the death of the animal.
Complications include pneumothorax, hemothorax, hemorrhage into airways with subsequent hemoptysis or epistaxis, pulmonary hematoma and dissemination of infection from infected lung to the pleural space. Pneumothorax, which is usually not clinically apparent, occurs in most horses in which the procedure is performed.108 Coughing and epistaxis occur in about 20% and 10% of horses, respectively.108 Life-threatening hemorrhage occurs uncommonly (= 2% of cases). Bleeding into the airways, detected by tracheobronchoscopic examination, occurred in 16 of 50 horses after use of the manually discharged biopsy needle and in five of 50 horses after use of the automatically discharged needle.108 Two of 60 cows collapsed immediately after the procedure, but subsequently stood and recovered.109 The remaining cows had no clinical abnormalities detected after biopsy, although necropsy examination 24 hours later revealed small lesions in the pulmonary parenchyma at the site of biopsy.109 One of 10 healthy sheep had coughing and bloody nasal discharge after lung biopsy.110
The procedure is performed in adult horses and cattle using a 14-gauge biopsy needle, either manually operated or one that discharges automatically. Such instruments yield tissue in over 95% of attempts in cattle.109 The area for examination is best determined by radiographic or ultrasonographic examination of the thorax. A common site for biopsy is at the junction of the dorsal and middle thirds of the thorax at the ninth intercostal space in cattle and sheep109,110 and the 13th intercostal space in horses. The procedure is best performed with the animal standing. The skin over the area should be clipped of hair and aseptically prepared and local anesthesia induced by injection of 2% lidocaine or a similar compound into the intercostal space. A 0.5 cm incision is made through the skin and the biopsy instrument is advanced through the caudal intercostal space (intercostal vessels and nerves course along the caudal aspect of the ribs) and into the lung perpendicular to the skin surface. The instrument is advanced approximately 2 cm into the lung and tissue is collected at the end of inspiration. The procedure is repeated as necessary for collection of samples for histological and microbiological examination. The skin incision is closed with a single suture if necessary. The animal is then monitored closely for 12–24 hours for signs of coughing, epistaxis, hemoptysis, fever or respiratory distress. Hemorrhage into the airways is usually evident, often within minutes of completing the procedure, by the animal coughing. Hemorrhage into the airways is often evident as hemoptysis, even in horses. Respiratory distress can be caused by pneumothorax, hemothorax or hemorrhage into airways. Treatment includes percutaneous aspiration of pleural air, administration of oxygen by insufflation or, in extreme instances, mechanical ventilation.
RESPIRATORY SOUND SPECTRUM ANALYSIS
Analysis of respiratory sounds has utility in the diagnosis of disorders of the upper respiratory tract of horses. Respiratory sounds can be detected by a small microphone near the horse’s nostril with the recording made by a tape recorder or similar device worn on the saddle or girth strap.111,112 Studies can be performed with horses running on either a treadmill or outside over ground. Dorsal displacement of the soft palate produces broad-frequency expiratory noises with rapid periodicity (rattling), whereas dynamic unilateral collapse of the arytenoid causes an increase in inspiratory broad band high-frequency noise.111-113 The technique correctly identifies more than 90% of horses with dynamic collapse of the left arytenoid cartilage (‘roarers’).113
EXERCISE TESTING
Exercise testing for assessment of respiratory tract function is essentially limited to horses. Such tests are usually conducted on a treadmill, although some are amenable to use in the field. Tests available for use on horses running on a treadmill include endoscopic examination of the upper airway, respiratory noise analysis, blood gas analysis and measurement of respiratory mechanics. The most important of these in a clinical setting is videoendoscopy, during exercise on a treadmill, to detect dysfunction of the upper airway of horses.114 Some disorders of the upper respiratory tract, such as progressive weakness of the laryngeal abductor muscles, axial deviation of the aryepiglottic fold and epiglottic retroversion, can only be diagnosed by endoscopic examination performed during strenuous exercise.115 The interested reader is referred to texts devoted to this topic.116
Principles of treatment and control of respiratory tract disease
TREATMENT OF RESPIRATORY DISEASE
Treatment of diseases of the lower respiratory tract depends on the cause of the disease. However, the common principles are:
• Ensure adequate oxygenation of blood and excretion of carbon dioxide
• Relieve pulmonary inflammation
• Effectively treat infectious causes of respiratory disease
• Supportive care to minimize demands for respiratory gas transport.
Respiratory gas transport
Cause of acute death in animals with respiratory disease is usually failure of transport of respiratory gases with subsequent hypoxemia and hypercapnia. Treatment of failure of oxygenation of blood and excretion of carbon dioxide can be achieved through administration of supplemental oxygen or mechanical ventilation. The reasons for failure of respiratory gas transport are discussed above, and should be considered when therapy of an animal with respiratory disease and hypoxemia with or without hypercarbia is planned. Animals with hypercarbia and hypoxemia are probably hypoventilating and consideration should be given to increasing the animal’s minute ventilation through relief of airway obstruction (e.g. by foreign bodies or bronchoconstriction), improvement in function of the respiratory muscles (restore hydration, maintain normal blood concentrations of electrolytes, including calcium), and positional adjustments (foals have better respiratory function when in sternal recumbency33). Artificial ventilation should be considered, but is impractical for long-term treatment in animals other than those housed in referral centers. Ventilation/perfusion abnormalities cause hypoxemia with normal to only slightly elevated Paco2 in most affected animals. Oxygen therapy can be useful in ameliorating or attenuating the hypoxemia due to ventilation/perfusion abnormalities.
OXYGEN THERAPY
The principal treatment for hypoxemia caused by diseases of the lungs is the administration of oxygen. Oxygen therapy is not often used in large animals in field situations but the use of a portable oxygen cylinder may find a place in tiding animals over a period of critical hypoxia until inflammatory lesions of the lungs subside. It has been used most often in valuable calves and foals.117 Oxygen therapy must be given continuously, requires constant or frequent attendance on the animal, and can be expensive. Supplemental oxygen is usually administered through a nasal cannula with the tip placed in the nasopharynx, through a mask or through a cannula inserted percutaneously in the trachea. The use of an oxygen tent is impractical.
Oxygen therapy is useful only when hypoxemia is attributable to failure of oxygen transport in the respiratory system. It is of no value when the hypoxia is due to toxins that interfere with oxygen metabolism in tissues (e.g. cyanide). Oxygen therapy will only minimally increase oxygen transport in animals with anemia, abnormal hemoglobin (methemoglobinemia) or cardiovascular shock (stagnant hypoxia). Cases of pneumonia, pleurisy, and edema and congestion of the lungs are most likely to benefit from provision of supplemental oxygen.
Oxygen is often administered to newborn animals, either during resuscitation after birth or in those animals with respiratory disease. The value of supplemental oxygen in increasing Pao2 has been examined in foals, but the recommendations probably apply to newborns of other species as well. Both a facemask and nasopharyngeal tube are effective in increasing Pao2 when oxygen is administered at 10 L/min.96 The ability to elevate arterial oxygen increases with age from birth to 7 days of age because of the existence of right-to-left shunts in the newborn foal.96 Maximal changes in arterial oxygen tension occur within 2 minutes of the start of supplementation. In normal foals a flow rate of 4 L/min increases arterial oxygen tension, but responses in sick foals are often attenuated as a result of positional effects on gas exchange (recumbency) and other causes of hypoventilation. Nasal insufflation improves arterial oxygen tensions and acid–base status in mild to moderately affected foals but may not be sufficient for oxygenation of foals with severe impairment of gas exchange. Intranasal catheters are also difficult to maintain in active sucking foals and require the use of higher oxygen flow rates to achieve beneficial effects. Oxygen should be delivered through a system that includes a humidifier so the insufflated gas is humidified and therefore drying of the respiratory mucosa is minimized.
A transtracheal oxygen delivery system has been used in foals with pneumonia and rapidly progressive dyspnea and hypoxemia despite intranasal oxygen therapy.117 A catheter is inserted into the midcervical trachea and directly distally in the tracheal lumen for approximately 25 cm. The catheter is attached to about 6 m of oxygen tubing and suspended above the foal, allowing it to move around the stall and suck the mare for up to 6 days without dislodging the catheter. This system was more effective than nasal insufflation in increasing arterial oxygen tension, probably because the catheter tip is in the distal trachea and bypasses a significant length of dead space that would not be oxygenated were the oxygen delivered into the nasopharynx.
In foals with neonatal respiratory distress, signs of respiratory failure may be evident at birth or several hours after birth. Tachypnea, shallow and paradoxic respiration, an expiratory grunt with accentuated abdominal effort, and cyanosis are all common. Management of foals with respiratory distress includes oxygen therapy but, when the distress is severe, oxygen insufflation alone is insufficient to improve the Pao2, which is usually 45–60 mmHg (6.0–8.0 kPa). The atelectasis and alveolar hypoventilation worsen, resulting in progressive hypoxemia and respiratory acidosis, which requires ventilatory assistance by the use of continuous positive airway pressure.
In cattle and adult horses the nasal tube must be inserted to the nasopharynx because passage short of this causes excessive waste of oxygen. The length of tube inserted should equal the distance from the nostril to a point one-third of the way from the lateral canthus of the eye to the base of the ear. Insertion of a nebulizer in the system permits the simultaneous administration of antibiotics and moisture to prevent drying of the pharyngeal mucosa. The volume of oxygen used should be about 10–20 mL of oxygen per min per kg of body weight. Repeated measurement of arterial oxygen tension, if available, is useful for determining the flow rate. Arterial oxygen tension responds to changes in the rate of administration of oxygen within several minutes.
Oxygen toxicity is a risk in animals breathing pure oxygen for periods exceeding 1–2 days, but this rarely occurs in veterinary medicine because supplementation with oxygen does not result in the animal breathing pure oxygen (except for animals under general anesthesia).
RESPIRATORY STIMULANTS
Use of respiratory stimulants, including doxapram, picrotoxin, leptazol (Metrazol), nikethamide (Coramine), caffeine and amfetamine sulfate, which has been advocated in the past, is not useful or recommended in animals with hypoxemia due to respiratory disease. In these animals there is already maximal stimulation of the respiratory center and administration of drugs such as caffeine or doxapram is at best useless and at worst harmful, in that they can increase oxygen demand, in particular myocardial oxygen demand, thus exacerbating any oxygen deficit. The drugs might be useful in stimulating respiration in animals with pharmacological depression of the respiratory center by general anesthetics and sedatives.
MECHANICAL VENTILATION
Short-term mechanical ventilation can be achieved in neonates and small adults by use of a nasotracheal tube and a hand-operated bellows, which is usually in the form of a resilient bag equipped with a one-way valve. The animal’s trachea is intubated and the bag is connected and squeezed to supply a tidal volume of approximately 5–10 mL/kg BW at a rate of approximately 20 breaths per minute. Commercial bags (Ambubag®) are available in a variety of sizes suitable for neonates and small ruminants. There is a simple device for respiratory resuscitation of newborn calves and lambs consisting of a mouthpiece, a nonreturn valve, a flange and an oral tube.118 Ventilation of larger animals requires use of compressed gases and appropriate valving systems, including a Hudson demand valve.119 In an emergency situation, artificial ventilation of neonates and small ruminants can be achieved by mouth-to-nose ventilation by the veterinarian. This should be done only with an awareness of the risks of disease transmission (e.g. a weak newborn calf could be infected by Brucella sp. or Leptospira sp.).
Prolonged mechanical ventilation is an activity requiring special equipment and expertise. It is indicated for the treatment of diseases of neonates, and perhaps adults,120 that cause hypoxemia and hypercarbia. There is usually a significant component of hypoventilation in these diseases and this is a prime indication for use of mechanical ventilation. An excellent example is the use of mechanical ventilation to treat foals with botulism.121 In experienced hands, this technique is effective. Because of the highly technical and demanding requirements for mechanical ventilation, the interested reader is referred to more detailed sources for descriptions of the methodology.122
ANTI-INFLAMMATORY THERAPY
Many infectious and noninfectious diseases of the lower respiratory tract have inflammation as a major component of the tissue response to the initial insult. Primarily inflammatory diseases include heave and inflammatory airway disease of horses. Inflammation is an important component of pneumonia and some of the allergic or toxic lung diseases. Suppression of the inflammatory response is indicated when the inflammatory response is exacerbating clinical signs of the disease through obliteration of alveoli (inflammatory atelectasis), blockage of airways by inflammatory exudates and infiltration of bronchial walls, and bronchoconstriction as a consequence of inflammation increasing airway reactivity. Administration of anti-inflammatory drugs is indicated as the definitive therapy in noninfectious inflammatory airway diseases (with control achieved by environmental controls, see below). Care must be taken that suppression of the inflammatory response does not impair innate and adaptive immune responses to infectious agents.
Anti-inflammatory drugs used in the treatment of diseases of the respiratory tract include glucocorticoids and nonsteroidal anti-inflammatory drugs (NSAIDs), with other agents such as leukotriene antagonists, interferon and cromolyn sodium used in particular situations.
Nonsteroidal anti-inflammatory drugs are useful in the treatment of infectious respiratory disease of cattle and horses, and likely other species. The drugs act by inhibiting the inflammatory response induced by the infecting organism and tissue necrosis. Meloxicam (0.5 mg/kg subcutaneously, once), when administered with tetracycline, improves weight gain and reduces the size of lesions in lungs of cattle with bovine respiratory disease complex over those of animals treated with tetracycline alone.123 NSAIDs also improve the clinical signs of cattle with respiratory disease.124 Use of these drugs is routine in horses with pneumonia or pleuritis.
Glucocorticoids are administered for control of inflammation in a variety of inflammatory lung diseases but notably heaves of horses and interstitial pneumonia of foals. Treatment can be administered orally, by intravenous or intramuscular injection, or by inhalation. Oral, intramuscular or intravenous administration results in systemic effects of the agents. Inhalation of glucocorticoids provides therapy directed to the site of the disease and minimizes, but does not always prevent, the systemic effects of the drugs. Drugs for inhalation are usually human preparations of fluticasone, beclomethasone and flunisolide that are available as metered-dose inhalers. The compounds are administered through a mask adapted so that a large proportion of the drug is inhaled. Anti-inflammatory responses in the airways are pronounced and result in marked improvement in respiratory function in horses with heaves (see Heaves, Recurrent airway obstruction).
IMMUNOMODULATORS
Interferon is used for the treatment of inflammatory airway disease in race horses and feedlot cattle with respiratory disease.125,126 A dose of 50–150 IU of interferon-alpha administered orally once daily for 5 days reduced signs of airway inflammation in young Standardbred race horses.127 Immune stimulation by injection of a suspension of Propionibacterium acnes has been investigated for treatment of chronic inflammatory airway disease in horses. The compound enhances expression of interferon-gamma and NK-lysin in peripheral blood mononuclear cells, increases the proportion of CD4+ cells in peripheral blood and increases phagocytic activity of cells in peripheral blood.128,129 Similar changes were detected in bronchoalveolar lavage fluid.129 The effect on respiratory disease has yet to be definitively determined.
ANTIMICROBIAL THERAPY
Bacterial infections of the respiratory tract of all species are treated with antimicrobial agents given parenterally or, less commonly, orally. Individual treatment is usually necessary and the duration of treatment will depend on the causative agent and the severity when treatment was begun. In outbreaks of infectious respiratory disease the use of mass medication of the feed and water supplies may be advisable for the treatment of subacute cases and for convalescent therapy. The response to mass medication will depend on the total amount of the drug ingested by the animal and this is a reflection of the appetite or thirst of the animal, the palatability of the drug and its concentration in the feed or water. The choice of drug used will depend on its cost, previous experience on similar cases and the results of drug sensitivity tests if available. The individual treatment of all in-contact animals in an affected group may be useful in controlling an outbreak of respiratory disease such as shipping fever in feedlot cattle.
Selection of antimicrobials is based on the principles detailed in Chapter 4. Briefly, antimicrobials for treatment of bacterial respiratory disease should be active against the causative agent, should be able to achieve therapeutic concentrations in diseased lung and should be convenient to administer. The antimicrobials should be affordable and, if used in animals intended as human food, must be approved for use in such animals.
Antimicrobials for treatment of lung disease are preferably those that achieve therapeutic concentrations in diseased lung tissue after administration of conventional doses. This has been convincingly demonstrated for the macrolide (azithromycin, erythromycin, clarithromycin),130 triamilide (tulathromycin)131,132 and fluoroquinolone (danofloxacin, enrofloxacin)133,134 antimicrobials, and fluorfenicol135 in a variety of species. The beta-lactam antimicrobials (penicillin, ceftiofur) are effective in treatment of pneumonia in horses, pigs, and ruminants despite having chemical properties that do not favor their accumulation in lung tissue.
Routes of administration include oral (either individually or in medicated feed or water), parenteral (subcutaneous, intramuscular, intravenous) or by inhalation. Intratracheal administration of antimicrobials to animals with respiratory disease is not an effective means of achieving therapeutic drug concentrations in diseased tissue. Aerosolization and inhalation of antimicrobials has the theoretic advantage of targeting therapy to the lungs and minimizing systemic exposure to the drug. However, while administration by inhalation achieves good concentrations of drug in bronchial lining fluid, it does not penetrate unventilated regions of the lungs, in which case parenteral or oral administration of antimicrobials is indicated. Aerosol administration of gentamicin to horses and ceftiofur sodium to calves with pneumonia has been investigated. Aerosol administration of gentamicin to normal horses results in gentamicin concentrations in bronchial lavage fluid 12 times that achieved after intravenous administration.136 Aerosolized ceftiofur sodium (1 mg/kg) is superior to intramuscular administration in treatment of calves with Pasteurella (Mannheimia) haemolytica.137
BRONCHODILATOR DRUGS
Bronchoconstriction is an important component of the increased airway resistance present in many animals with disease of the lower respiratory tract. Administration of bronchodilators can relieve respiratory distress and improve arterial blood oxygenation. Bronchodilatory drugs are beta-2-agonists (clenbuterol, albuterol/salbutamol, terbutaline), parasympatholytic drugs (ipratropium, atropine) and methylxanthines (aminophylline, theophylline).
The indication for the use of bronchodilators is relief of bronchoconstriction. Bronchoconstriction is an important component of the pathophysiology of many diseases of the lungs and airways. Bronchodilators are used extensively in horses with heaves and inflammatory airway disease, and less so in animals with infectious diseases. Contraindications are few but caution should be exercised when using these drugs in animals that are severely hypoxemic as the beta-2-agonists can transiently worsen gas exchange by increasing perfusion of nonventilated sections of the lung, and in pregnant animals, in which the tocolytic effect of the beta-2-agonists can delay parturition. The use of beta-2-adrenergic agonist bronchodilator drugs in food animals is not permitted in most countries because of the risk of contamination of foodstuffs intended for consumption by people. This is particularly the case with clenbuterol, a drug approved in many countries for use in horses that is administered to cattle illicitly as a growth promoter. People can be poisoned by clenbuterol in tissues of treated cattle.138
The beta-2-adrenergic agonists are potent and effective bronchodilators that can be administered orally, intravenously or by inhalation. These drugs also enhance mucociliary clearance of material from the lungs. Most administration is oral or by inhalation. Use of these drugs is restricted to horses and the drugs are discussed in the section on Heaves.
Parasympatholytic (anticholinergic) drugs relieve vagally mediated bronchoconstriction. Again, their use is restricted to horses. These drugs can cause tachycardia and gastrointestinal dysfunction, including ileus.
The methylxanthines are used in horses and have been investigated for use in cattle with respiratory disease. Their use in horses is mainly of historical interest because the availability of the more efficacious beta-2-adrenergic agonists and parasympatholytic drugs has superseded the use of methylxanthines. The use of theophylline in feedlot cattle with respiratory disease in field conditions is associated with accumulation of toxic concentrations in blood and an excessive mortality rate.139
MUCOLYTICS, MUCOKINETIC AND ANTITUSSIVE DRUGS
Many groups of drugs are used in the therapy of respiratory diseases with the objective of improving mucokinesis or effective mucociliary clearance.140 Mucokinetic agents have been divided into six groups according to their mode of action.
• Diluents, surface acting agents and mucolytics are supposed to reduce the viscosity of the respiratory secretions
• Bronchomucotropic agents, formerly called expectorants, are supposed to increase the production of a less viscous mucus
• Other agents, such as beta-adrenergic agonists and methylxanthine derivatives, promote more effective clearance of mucus and act as ciliary augmentors or bronchodilators.
The aim of mucokinetic agents is to decrease the viscosity of the respiratory secretions but in some animals with respiratory disease the excessive secretions are of low viscosity and the use of a mucolytic agent in such cases would further decrease mucokinesis. There is little or no evidence that administration of mucolytic or mucokinetic agents, with the possible exception of clenbuterol and dembrexine, relieves signs of respiratory disease or hastens recovery.
Inflammation of the lower respiratory tract results in production of mucus and immigration of inflammatory cells. This accumulation of material is cleared by rostral movement into the pharynx, where it is discharged through the nostrils or swallowed. Clearance is by the mucociliary apparatus or coughing. Mucolytics are agents that alter the constituents of mucoid or purulent respiratory secretions and make them less viscous. Bromhexine (Bisolvon: Boehringer Ingelheim) is a popular mucolytic with horse owners. It is said to reduce the viscosity of airway mucus and increase mucus production, although its clinical efficacy has not been determined. It may be of some value in cattle to increase mucociliary clearance. Dembrexine (Sputolosin: Boehringer Ingelheim) alters the carbohydrate side chains of mucin and improves its flow properties and is reported to decrease coughing and hasten recovery in horses with respiratory disease.141
Hyperhydration, the administration of large quantities of fluids intravenously, has been suggested as being useful in the treatment of horses with accumulation of excessive amounts of mucus or mucopus in the lower airways. However, experimental trials have demonstrated that this approach is not effective in horses with heaves.142
Bronchomucotropic agents (expectorants) are administered with the intention of augmenting the volume of respiratory secretions by stimulating the mucus-producing cells and glands. Formerly called expectorants, they are supposed to increase the production of a less viscous mucus. These compounds include the iodides, and ammonium and glycerol guaiacolate, which are commonly found in cough mixtures. These are commonly used in farm animals, especially horses, although their efficacy is unknown.
Coughing is a common sign in animals with respiratory disease, and it is an important pulmonary defense mechanism, allowing the expulsion of mucus and foreign bodies. Antitussive (cough suppressant) drugs are infrequently used in large-animal medicine. These drugs should only be used when definitive therapy has been implemented for the underlying disease. Control of the underlying disease will in almost all instances resolve the coughing. It is not appropriate to use antitussive agents (butorphanol, codeine, diphenhydramine) to suppress a cough when the underlying cause is unknown or untreated.
SURFACTANT
Surfactant is critical to normal alveolar function and a lack of this complex phospholipid results in progressive alveolar collapse.143 Lack of surfactant is an important cause of respiratory disease in newborn animals, with those born prematurely being at increased risk. Attempts have been made to prevent acute respiratory disease in premature newborn foals, such as those delivered by cesarian section because of maternal disease, but the results have been disappointing.144
SURGERY
Many conditions of the upper respiratory tract of horses are amenable to surgical correction. Tracheostomy is often used in the emergency or urgent relief of acute upper airway obstruction, and in the removal of large amounts of tracheal debris, such as occurs in animals with smoke inhalation. Drainage of excessive or infected pleural fluid can be therapeutic in animals with pleuritis.
GENERAL NURSING CARE
Animals with respiratory disease should have minimal or no enforced activity and environmental stressors should be minimized. One of the most important aspects of the treatment of respiratory tract disease in farm animals is the provision of a comfortable, well-ventilated environment during and after the disease episode. Affected animals should be placed in a draft-free area that is adequately ventilated and supplied with an abundance of bedding for comfort and warmth, particularly during convalescence. Feed and water should be readily available and dusty feeds avoided.
CONTROL OF RESPIRATORY DISEASE
Infectious diseases of the respiratory tract of farm animals are caused by a combination of infectious agents and predisposing causes such as inclement weather, the stress of weaning or transportation and poorly ventilated housing, each of which can weaken the defense mechanisms of the animal. Prevention and control of these diseases include:
• Minimizing exposure to inciting agents (infectious or physical)
• Maximizing innate resistance by ensuring that the animals are in excellent general health through attention to nutrition, housing and animal welfare
• Maximizing adaptive resistance by the administration of effective vaccines such that maximal resistance is produced to coincide with the time of greatest risk of the disease.
IMPORTANCE OF DIAGNOSIS
For some complex respiratory diseases of food animals it is becoming increasingly more difficult to obtain a definitive etiological diagnosis because some of the common diseases appear to be caused by multiple infections rather than a single one. Most of the infective agents that cause respiratory disease are ubiquitous in the environment and are present as normal residents in the nasal cavities of normal animals. This often creates difficulty with the interpretation of the microbiological findings in outbreaks of respiratory disease because the infectious agents can commonly be isolated from both sick and well animals. Thus there may be no well-defined cause-and-effect relationship and the predisposing causes begin to assume major importance in any control program.
MANAGEMENT TECHNIQUES
Most of the common respiratory diseases occur at certain times under certain conditions and successful control will depend on the use of management techniques before the disease is likely to occur. For example, in beef cattle, pneumonic pasteurellosis can be kept to a minimum with the use of certain management procedures that minimize stress at weaning. The incidence of pneumonia can be minimized in young bulls destined for a performance testing station if they are weaned well in advance of movement to the test center. In North America, bovine respiratory disease is most common in feedlots where young cattle from several different backgrounds have been mingled after having been transported long distances. Outbreaks of equine respiratory disease occur in young horses that are assembled at the racetrack for training or at horse shows.
HOUSING FACILITIES
Cattle and pig barns that are overcrowded, damp and cold during the cold winter months and hot and stuffy during the summer months can predispose to a high incidence of pneumonia. The morbidity and mortality from pneumonia may be much higher when the ammonia concentration of the air is high or if it is dusty.
The incidence of pulmonary inflammation and coughing (heaves) in horses is much higher in those that are housed in barns that are dusty and not ventilated compared to horses kept outdoors. Bad stabling management as a major cause of coughing in horses was described almost 200 years ago but there is still a major emphasis on the clinical management of chronic coughing in housed horses using a wide spectrum of antibiotics, expectorants and other drugs. More consideration of good housing and ventilation is necessary.
In pigs, enzootic pneumonia is widespread but the effects of the pneumonia can be maintained at an insignificant level with adequate housing, ventilation and nutrition. Too much emphasis has been placed on the attempted eradication of Mycoplasma spp., which is extremely difficult, and insufficient emphasis on building design and ventilation methods.
VACCINES
Vaccines are available for the immunization of farm animals against some of the common infectious diseases of the respiratory tract. Their advantages and disadvantages are discussed under each specific disease. The general principles underlying use of vaccines for control of respiratory disease are that:
• The disease must be caused by a disease that is infectious
• There must be an effective vaccine suitable for use in the species and age group of animals at most risk of the disease. Ideally, this will be known from published, appropriately designed trials testing the vaccine in a group of animals identical to those in which the vaccine will be used in practice
• The vaccine must be administered to animals in such a manner (route, timing, frequency) as to optimize the immunization (adaptive immunity)
• The timing of the vaccination program should be such that maximal resistance to the anticipated diseases is achieved at the time of greatest risk of the disease
• Vaccination should be part of an on-going program of disease control and should not be regarded as a panacea with which to rectify other shortcomings in management of the animals.
ENVIRONMENTAL CONTROL
In effect, the principles of control and prevention of airborne respiratory disease are based largely on keeping the levels of pathogens in the air at a low level. This can be accomplished by a combination of the following practices:
• The use of filtered-air positive pressure ventilation systems
• The removal of affected animals from the group
• Increasing the ventilation rate of the building unit
• Subdivision of the unit into small units, each with its own ventilation system
• A continual disinfection system where appropriate and practicable
• The provision of supplemental heat so that during cold weather the ventilation can be maintained and animals will not huddle together to keep warm and thereby increase the exposure rate of infection
• The use of vaccines for specific diseases of the respiratory tract
Roudebush P. Lung sounds. J Am Vet Med Assoc. 1982;181:122-126.
Kotlikoff MI, Gillespie JR. Lung sounds in veterinary medicine. Part 1: Terminology and mechanisms of sound production. Compend Contin Educ Pract Vet. 1983;5:634-639.
Kotlikoff MI, Gillespie JR. Lung sounds in veterinary medicine. Part 2: Deriving clinical information from lung sounds. Compend Contin Educ Pract Vet. 1983;6:462-467.
Curtis RA, et al. Lung sounds in cattle, horses, sheep, and goats. Can Vet J. 1986;27:170-172.
McGorum BC, et al. Clinical examination of the respiratory tract. In: Radostits OM, et al, editors. Veterinary Clinical Examination and Diagnosis. Philadelphia, PA: WB Saunders; 2000:299-348.
1 Klein L, Fisher N. Am J Vet Res. 1988;49:1605.
2 Geiser DR, et al. Am J Vet Res. 1996;57:1483.
3 Curtis RA, et al. Can Vet J. 1986;27:170.
4 Holcombe SJ, et al. Am J Vet Res. 1996;57:1258.
5 Sweeney CR, et al. J Am Vet Med Assoc. 1989;195:1225.
6 Roudebush P. J Am Vet Med Assoc. 1982;181:122.
7 Robinson NE, et al. Am J Vet Res. 2003;64:550.
8 Christley RM, et al. Vet Rec. 2001;148:99.
9 Krotje KJ. Compend Contin Educ Pract Vet. 1987;9:271.
10 Brianceau P, Divers TJ. Equine Vet J. 2001;33:105.
11 Thomas A, et al. Vet Res Commun. 2002;26:333.
12 Allen JW, et al. J Vet Res. 1991;55:341.
13 Rossier Y, et al. J Am Vet Med Assoc. 1991;198:1001.
14 Tyler JW, et al. J Am Vet Med Assoc. 1990;197:52.
15 Roudebush P, Sweeney CR. J Am Vet Med Assoc. 1990;197:714.
16 Bakos Z, et al. Acta Vet Hung. 2003;51:249.
17 Smith BL, et al. Equine Vet J. 1994;26:283.
18 Sweeney CR, et al. Am J Vet Res. 1992;53:1953.
19 Anderson DE, et al. Am J Vet Res. 1994;55:901.
20 Anderson DE, et al. Am J Vet Res. 1994;55:1196.
21 Ruggles AJ, et al. Vet Surg. 1993;22:508.
22 Peroni JF, et al. Equine Vet J. 2001;33:231.
23 Fry MM, et al. Equine Vet J. 2003;35:723.
24 Lugo J, et al. Am J Vet Res. 2002;63:1232.
25 Bedenice D, et al. J Vet Intern Med. 2003;17:868.
26 Bedenice D, et al. J Vet Intern Med. 2003;17:876.
27 Wion L, et al. Equine Vet J. 2001;33:523.
28 Morrow KL, et al. Vet Radiol Ultrasound. 2000;41:491.
29 Smallwood JE, et al. Vet Radiol Ultrasound. 2002;43:99.
30 Cornelisse CJ, et al. Am J Vet Res. 2001;62:1856.
31 Probst A, et al. Vet Radiol Ultrasound. 2005;46:44.
32 Davis JL, et al. J Am Vet Med Assoc. 2002;221:1460.
33 Kosch PC, et al. Equine Vet J. 1984;16:312.
34 Tietje S, et al. Equine Vet J. 1996;28:98.
35 Frame EM, et al. Vet Rec. 2000;146:558.
36 Arencibia A, et al. Vet Radiol Ultrasound. 2000;41:313.
37 Votion D, Lekeux P. Equine Vet Educ. 1999;11:300.
38 Coghe J, et al. Vet J. 2000;160:15.
39 Braun U, et al. Am J Vet Res. 1996;57:432.
40 Reimer JM. Compend Contin Educ Pract Vet. 1990;12:1321.
41 Jung C, Bostedt H. Vet Radiol Ultrasound. 2004;45:331.
42 Braun U. Vet Rec. 2004;155:92.
43 Reef VB, et al. J Am Vet Med Assoc. 1991;198:2112.
44 Braun U, et al. Vet Rec. 1997;141:12.
45 Rabeling B, et al. Vet Rec. 1998;143:468.
46 Van Erck E, et al. Equine Vet J. 2004;36:21.
47 Flock M. Vet J. 2004;167:272.
48 Gross DK, et al. J Vet Intern Med. 2004;18:718.
49 Ramirez S, et al. Vet Radiol Ultrasound. 2004;45:172.
50 Allen JW, et al. Can J Vet Res. 1991;55:341.
51 Lindsay WA, Hedberg EB. Vet Med. January 1991:72.
52 Ward JL, Rehbun WC. J Am Vet Med Assoc. 1992;201:326.
53 Mombarg MJ, et al. J Vet Med B. 2002;49:424.
54 Dersken FJ, et al. Equine Vet J. 1989;21:23.
55 Larson VL, Busch RH. Am J Vet Res. 1985;46:144.
56 Hoffman AM, et al. Am J Vet Res. 1998;59:176.
57 Couetil LL, et al. Am J Vet Res. 2001;62:538.
58 Malikides N, et al. Aust Vet J. 2003;81:685.
59 Malikides N, et al. Aust Vet J. 2003;81:681.
60 Whitwell KE, Greet TC. Equine Vet J. 1984;16:499.
61 Christley RM, et al. Equine Vet J. 1999;31:197.
62 Pringle JK, et al. Can J Vet Res. 1988;52:239.
63 Labonville M, et al. Can Vet J. 2001;42:623.
64 McKane SA, et al. Aust Vet J. 1993;70:401.
65 Allen JW, et al. Can J Vet Res. 1992;56:122.
66 Hagberg M, et al. Parasitol Immunol. 2005;27:151.
67 Lakritz J, et al. Am J Vet Res. 2004;65:163.
68 Sheehan M, et al. Vet Rec. 2005;157:309.
69 Hensel A, et al. Am J Vet Res. 1994;55:1697.
70 Thomas A, et al. Vet Rec. 2002;151:472.
71 Traub-Dargatz JL, et al. Am J Vet Res. 1988;49:1026.
72 Hoffman AM, et al. J Am Vet Med Assoc. 1990;196:392.
73 Silflow RM, et al. Vet Immunol Immunopathol. 2005;103:129.
74 Westermann CM, et al. Am J Vet Res. 2005;66:1420.
75 Pickles K, et al. Equine Vet J. 2002;34:288.
76 Sweeney CR, et al. Am J Vet Res. 1992;53:1376.
77 Kirschvink N, et al. Vet Res. 2001;32:145.
78 Pickles K, et al. Equine Vet J. 2002;34:292.
79 Leclere M, et al. J Vet Intern Med. 2006;20:377.
80 Chaman PS, et al. Vet Rec. 2000;146:91.
81 Brumbaugh GW, Benson PA. Am J Vet Res. 1990;51:1032.
82 Collie DDS. Br Vet J. 1992;148:33.
83 Jean D, et al. Am J Vet Res. 1999;60:11.
84 Guthrie AJ, et al. Vet Res Commun. 1995;19:331.
85 Connally BA, Derksen FJ. Am J Vet Res. 1994;55:589.
86 Gallivan GJ, et al. Can Vet J. 1990;54:99.
87 Herholz C, et al. Vet Res Commun. 2001;26:467.
88 Reinhold P, et al. Vet Rec Jan. 2002;26:150. 2004; 109.
89 Uystepruyst CH, et al. Res Vet Sci. 2000;68:47.
90 Van Erck E, et al. Am J Vet Res. 2003;64:1414.
91 Van Erck E, et al. Vet J. 2004;168:259.
92 Gustin P, et al. Res Vet Sci. 1990;49:319.
93 Couetil LL, et al. J Appl Physiol. 2000;88:1870.
94 Clerbaux Th, et al. Comp Biochem Physiol. 1993;106A:687.
95 Fedde MR. Equine Vet J. 1991;23:410.
96 Stewart JH, et al. Equine Vet J. 1984;16:329.
97 Mahoney JJ, et al. Clin Chem. 1991;37:1244.
98 Bajcsy ACS, et al. J Vet Med A. 1999;46:255.
99 Szenci O, Besser T. J Am Vet Med Assoc. 1990;197:471.
100 Assal AN, et al. Nord Vet Med. 1978;30:354.
101 Koenig J, et al. Can J Vet Res. 2003;67:169.
102 Chaffin MK, et al. Equine Vet J. 1996;28:437.
103 King CM, et al. Aust Vet J.. 1994;71:382.
104 Schulman ML, et al. J S Afr Vet Assoc. 2001;72:12.
105 Goghe J, et al. Vet J. 2000;160:139.
106 Reinhold P, et al. Am J Vet Res. 2000;61:742.
107 Deaton C, et al. Free Radical Res. 2004;38:201.
108 Venner M, et al. J Vet Intern Med. 2006:20.
109 Braun U, et al. J Am Vet Med Assoc. 1999;215:679.
110 Braun U, et al. Vet Rec. 2000;146:525.
111 Derksen FJ, et al. Am J Vet Res. 2001;62:659.
112 Franklin SH, et al. Equine Vet J. 2003;35:264.
113 Cable CS, et al. Am J Vet Res. 2002;63:1707.
114 Dart AJ, et al. Aust Vet J. 2001;79:109.
115 Tan RH, et al. Vet J. 2005;170:243.
116 Hinchcliff KW, et al, editors. Equine sports medicine and surgery: basic and clinical sciences of the Equine athlete. Edinburgh: Elsevier Health Science, 2004.
117 Hoffman AM, Viel L. Equine Vet J. 1992;24:239.
118 Henninger W, et al. Vet Radiol Ultrasound. 2003;44:269.
119 Johnson CB, et al. Vet Rec. 1994;135:569.
120 Mitten LA, et al. Equine Vet J. 1994;26:420.
121 Wilkins PA, Palmer JE. J Vet Intern Med. 2003;17:708.
122 Palmer JE. Vet Clin North Am Equine Pract. 1994;10:167.
123 Friton GM, et al. Vet Rec. 2005;156:809.
124 Elitok B, Elitok OM. J Vet Pharmacol Ther. 2004;27:317.
125 Moore I, et al. Can Vet J. 2004;45:594.
126 Georgiades J. Arch Immunol Ther Exp. 1993;41:205.
127 Moore BR, et al. Equine Vet J. 1997;29:142.
128 Davis EG, et al. Vet Ther. 2003;4:5.
129 Flaminio MJ, et al. Vet Immunol Immunopathol. 1998;63:303.
130 Davis JL, et al. J Vet Pharmacol Ther. 2002;25:99.
131 Benchaaoui HA, et al. J Vet Pharmacol Ther. 2004;27:203.
132 Evans NA. Vet Ther. 2005;6:83.
133 Terhune TN, et al. Am J Vet Res. 2005;66:342.
134 Apley MD, Upson DW. Am J Vet Res. 1993;54:937.
135 Aslan V, et al. Vet Q. 2002;24:35.
136 McKenzie HC, Murray MJ. Am J Vet Res. 2000;61:1185.
137 Sustronck B, et al. Res Vet Sci. 1995;59:267.
138 Barbosa J, et al. Food Addit Contam. 2005;22:563.
139 McKenna DJ, et al. J Am Vet Med Assoc. 1989;195:603.
140 Dixon PM. Vet Rec. 1992;131:229.
141 Matthews AG, et al. Vet Rec. 1988;122:106.
142 Jean D, et al. Equine Vet J. 2004;36:628.
143 Danlois F, et al. Vet J. 2003;165:65.
144 Costa LRR, et al. Compend Contin Educ Pract Vet. 2004;26:460.
145 Ishizaki H, et al. Vet Immunol Immunopathol. 2005;105:67.
146 Holcombe SJ, et al. Equine Vet J. 2001;33:244.
147 Moore RR, et al. Am J Vet Res. 1995;56:562.
Diseases of the lungs
PULMONARY CONGESTION AND EDEMA
Pulmonary congestion is caused by an increase in the amount of blood in the lungs due to engorgement of the pulmonary vascular bed. It is sometimes followed by pulmonary edema when intravascular fluid escapes into the parenchyma and alveoli. The various stages of the vascular disturbance are characterized by respiratory compromise, the degree depending upon the amount of alveolar air space which is lost.
ETIOLOGY
Pulmonary congestion and edema is a common terminal event in many diseases but is frequently overshadowed by other disturbances. Congestion that is clinically apparent may be primary when the basic lesion is in the lungs or secondary when it is in some other organ, most commonly the heart.
Pulmonary edema occurs because of imbalances in the Starling forces across the pulmonary capillary. From a clinical perspective, the common proximate causes of pulmonary edema are injury to the endothelium of the pulmonary capillary with subsequent leakage of protein-rich fluid into the interstitial spaces, elevated blood pressure in the alveolar capillaries, or low plasma oncotic pressure. Damage to pulmonary vascular endothelium can occur in infectious diseases (e.g. African horse sickness) or intoxications (endotoxemia). Physical injury, including inhalation of excessively hot air or smoke, can damage the alveolar epithelium with secondary damage to capillary endothelium. Elevated pulmonary capillary pressure occurs in left-sided heart failure (ruptured chordae tendineae of the mitral valve) and during strenuous exercise by horses. Low plasma oncotic pressure occurs in diseases causing hypoproteinemia but is rarely a cause for pulmonary edema by itself, although it contributes to the pulmonary edema in hypoproteinemic animals administered large volumes of fluids intravenously.
Pulmonary edema
Pulmonary edema as a sequel to pulmonary capillary hypertension or pulmonary microvascular damage4 occurs in:
• Acute pneumonia – Pasteurella haemolytica produces several virulence factors that induce direct or leukocyte-mediated pulmonary endothelial cell injury4
• Gram-negative sepsis in ruminants and pigs4
• Congestive heart failure and acute heart failure, e.g. the myocardial form of enzootic muscular dystrophy in inherited myocardiopathy of Hereford calves; ruptured mitral valve or chordae tendonae
• Inhalation of smoke or manure gas1
• Transient upper airway obstruction in the horse (negative pressure pulmonary edema)5
• After general anesthesia in horses6
• Yew (Taxus sp.) intoxication2
• Exercise-induced pulmonary edema in race horses3
• Fumonisin intoxication in pigs7
• Specific diseases, including: mulberry heart disease of swine; East Coast fever in cattle; the pulmonary form of African horse sickness; Hendra virus infection of horses; poisoning with organophosphates, alpha-naphthyl thiourea (ANTU) or ionophore antibiotics (monensin, salinomycin); plant poisonings by oleander, Hymenoxis spp. and Phenosciadium spp.
• Doxycycline intoxication of calves8
• Clostridium perfringens type D epsilon toxin in calves and sheep9,10
• The Barker syndrome in young pigs
• Semen embolism.11
PATHOGENESIS
In pulmonary congestion, ventilation is reduced and oxygenation of the blood is impaired. Oxygenation is reduced by the decreased rate of blood flow through the pulmonary vascular bed. Hypoxemic anoxia develops and is the cause of most of the clinical signs that appear.
Hypoxemia occurs in pulmonary edema because of ventilation/perfusion abnormalities, diffusion abnormalities (although this is usually a minor contributor to the hypoxemia), and hypoventilation caused by the physical obstruction of airflow by fluid and foam in the airways. The edema is caused by damage to the capillary walls by toxins or anoxia or by transudation of fluid due to increased hydrostatic pressure in the capillaries. Filling of the alveoli, and in severe cases the bronchi, effectively prevents gaseous exchange.
Smoke inhalation in horses results in decreased oxygen content of inspired air and exposure of the respiratory tract tissues to various noxious gases.1 Following smoke inhalation, diffuse tracheobronchial mucosal sloughing occurs, which, if progressive, causes separation of the epithelium and development of pseudomembranous casts, which may cause partial or complete airway obstruction. Pulmonary edema is also extensive.
CLINICAL FINDINGS
All degrees of severity of pulmonary congestion and edema occur commonly in farm animals and only the most severe form is described here. The depth of respiration is increased to the point of extreme dyspnea with the head extended, the nostrils flared and mouth-breathing. Breathing movements are greatly exaggerated and can be best described as heaving; there is marked abdominal and thoracic movement during inspiration and expiration. A typical stance is usually adopted, with the front legs spread wide apart, the elbows abducted and the head hung low. The respiratory rate is usually increased especially if there is hyperthermia, which occurs in acute anaphylaxis and after violent exercise as well as in the early stages of pneumonia. The heart rate is usually elevated (up to 100/min) and the nasal mucosa is bright red or cyanotic in terminal cases.
In acute pulmonary congestion there are harsh breath sounds but no crackles are present on auscultation.
When pulmonary edema develops, loud breath sounds and crackles are audible over the ventral aspects of the lungs. In long-standing cases there may be emphysema with crackles and wheezes of the dorsal parts of the lungs, especially if the lesion is caused by anaphylaxis.
Coughing is usually present but the cough is soft and moist and is not painful. A slight to moderate serous nasal discharge occurs in the early stage of congestion but in severe pulmonary edema this increases to a voluminous, frothy nasal discharge, which is often pink-colored due to blood.
The primary importance of pulmonary congestion is as an indicator of early pathological changes in the lung or heart. Spontaneous recovery occurs quickly unless there is damage to alveolar epithelium, or myocardial asthenia develops. Severe pulmonary edema has much greater significance and usually indicates a stage of irreversibility. Death in cases of pulmonary edema is accompanied by asphyxial respiratory failure.
Smoke inhalation in horses is characterized by:
• Diffuse wheezes throughout the lungs
• A bronchointerstitial pattern radiographically
• The horse may expectorate large proteinaceous tracheobronchial casts.1
The prognosis is good if affected animals can survive the initial stages of pulmonary damage and secondary organ involvement.
CLINICAL PATHOLOGY
Laboratory examinations are of value only in differentiating the causes of the congestion or edema. Bacteriological examination of nasal swabs and a complete hematological examination, looking particularly for the presence of eosinophilia, are the standard examinations that are carried out.
NECROPSY FINDINGS
In acute pulmonary congestion the lungs are dark red in color. Excessive quantities of venous blood exude from the cut surface. Similar but less marked changes occur in milder forms of congestion but are only seen in those animals that die from intercurrent disease. Histologically the pulmonary capillaries are markedly engorged and some transudation and hemorrhage into alveoli is evident.
Macroscopic findings in pulmonary edema include swelling and loss of elasticity of the lungs, which pit on pressure. They are usually paler than normal. Excessive quantities of serous fluid exude from the cut surface of the lung. Histologically there are accumulations of fluid in the alveoli and parenchyma.
The diagnosis of pulmonary congestion and edema is always difficult unless there is a history of a precipitating cause such as an infectious disease, strenuous exercise, ingestion of toxicants, or inhalation of smoke or fumes. Pneumonia usually presents itself as an alternative diagnosis and a decision cannot be based entirely on the presence or absence of pyrexia. The best indication is usually the presence of toxemia but this again is not entirely dependable. Bacterial pneumonia is usually accompanied by some toxemia but cases of viral pneumonia are often free of it. Response to antibacterial treatment is one of the best indications, the only variable being the tendency for congestion and edema of allergic origin to recover spontaneously. In many instances there will be doubt and it is then advisable to treat the animal for both conditions.
TREATMENT
The principles of treatment of pulmonary congestion and edema are one or more of: reduction of pulmonary capillary pressure (by reduction either of pulmonary venous or pulmonary arterial pressure); alleviation of pulmonary microvascular damage; and correction of low plasma oncotic pressure. The treatment of pulmonary congestion and edema must first be directed at correction of the primary cause as listed under etiology. Affected animals should be confined at rest in a clean, dry environment and exercise avoided.
Pulmonary capillary pressure can be reduced in animals with left-sided heart failure by reduction of cardiac preload, improvement in cardiac pump function or a combination of these factors. These topics are dealt with in detail in Chapter 8. Briefly, preload can be reduced by administration of furosemide and pump function improved by administration of drugs that improve myocardial function (digoxin) or decrease afterload (arterial vasodilators). The usual first step is the administration of furosemide (1–2 mg/kg intravenously).
Alleviation of pulmonary microvascular damage is more difficult. Administration of anti-inflammatory drugs including NSAIDs or glucocorticoids is indicated in animals in which microvascular damage is suspected. These drugs are used to treat, among other diseases, smoke inhalation of horses.1
Plasma oncotic pressure can be increased by intravenous infusion of plasma (10–40 mL/kg) or synthetic colloids such as hetastarch. Administration of crystalloid solutions should be judicious and the amount of fluid administered must be monitored carefully to ensure that only sufficient fluids to meet the needs of the animal are given.
Oxygen should be administered to hypoxemic animals in conjunction with other specific treatments.
Special diseases
When edema is due to organophosphate poisoning prompt administration of atropine may reduce fluid transudation. In these cases the animal is in considerable danger and repeated injections may be necessary. Details of the recommended treatment regimen are given in the section on treatment of poisoning by organophosphorus compounds.
Epinephrine is recommended in pulmonary edema due to anaphylaxis. It will have an immediate pharmacological effect, which may be followed by the use of a corticosteroid to maintain vascular integrity and to decrease permeability of pulmonary vessels. Antihistamines are commonly used in conjunction with epinephrine for the treatment of acute pulmonary edema due to anaphylaxis. However, recent studies of experimental anaphylaxis in cattle and horses have shown that the antihistamines may be of limited value because histamine and serotonin are of relatively limited significance as mediating substances. On the other hand, the kinins, prostaglandins and slow-release substances may be more important.
Studies in cattle have found that antihistamines and 5-hydroxytryptamine (5-HT) antagonists failed to protect cattle in experimental hypersensitivity. Sodium meclofenamate has been more successful in antagonizing experimental anaphylaxis in cattle and horses. Acetylsalicylic acid was more effective than antihistamines or antiserotonin agents in providing symptomatic relief in experimental acute interstitial pneumonia of calves.
It is difficult, however, to extrapolate the results of these studies in which the drugs were usually given before or at the same time as the experimental disease was produced. There is a need for development of more effective antianaphylactic drugs for the treatment of acute anaphylaxis in farm animals, which invariably results in pulmonary edema and emphysema. Thus epinephrine is the drug of choice for the emergency treatment of pulmonary edema due to anaphylaxis.
1 Kemper T, et al. J Am Vet Med Assoc. 1993;202:91.
2 Cope RB, et al. Vet Hum Toxicol. 2004;46:279.
3 Boden LA, et al. Equine Vet J. 2005;37:269.
4 Breider MA. J Am Vet Med Assoc. 1993;203:300.
5 Kollias-Baker CA, et al. J Am Vet Med Assoc. 1993;202:1116.
6 Senior M. Vet Anesth Analg. 2005;32:193.
7 Gumprecht LA, et al. Toxicology. 2001;160:71.
8 Yeruham I, et al. J Vet Med B. 2002;49:406.
9 Uzal FA, et al. J Comp Pathol. 2002;126:71.
PULMONARY HYPERTENSION
Pulmonary hypertension is an increase in pulmonary arterial pressure above normal values due to structural or functional changes in the pulmonary vasculature. Primary pulmonary hypertension occurs in cattle with high-altitude disease. Chronic pulmonary hypertension results in right-side congestive heart failure due to right ventricular hypertrophy or cor pulmonale.
Causes
Hypoxemia is a potent stimulus of pulmonary arterial pressure through increased pulmonary vascular resistance induced by pulmonary vasoconstriction.1 Pulmonary artery pressure can also increase in response to increases in cardiac output that are not matched by pulmonary vasodilation – the most extreme example of this being the large increase in pulmonary artery pressure of strenuously exercising horses. Alveolar hypoxia causes constriction of the precapillary pulmonary vessels, resulting in pulmonary hypertension. Conditions which may induce hypoxia include:
At high altitudes, the low inspired oxygen tension causes hypoxic pulmonary vasoconstriction and hypertension that are common causes of cor pulmonale (brisket disease) in cattle. Susceptible cattle can be identified by measurement of pulmonary artery pressure before clinical disease develops. This test is used to select bulls for use in high-altitude pastures. Cattle grazing pastures that contain locoweed have an increased incidence of brisket disease but the pathogenesis is unknown. Although uncommon, right-sided congestive heart failure and pulmonary hypertension can occur in cows at low altitudes with primary lung disease.1
Pulmonary hypertension occurs in neonates and is a consequence of persistent fetal circulation (see Ch. 8). This is particularly a problem of cloned calves.2
An outbreak of pulmonary hypertension in a group of dairy calves 5–6 months of age has been described.3 Some affected calves died suddenly. Clinical findings included lethargy, anorexia, pale mucous membranes, tachypnea, tachycardia, weakness, engorged jugular veins and loss of body condition.3 Right-side cardiac catheterization revealed pulmonary hypertension. Necropsy findings revealed evidence of right-sided congestive heart failure, and periarteritis and fibrosis of the pulmonary and bronchial arteries. Lesions were characterized by variable stages of vasculitis; the airways were free of pathological changes. Ingestion of monocrotaline, a pyrrolizidine alkaloid, can cause similar pulmonary vascular lesions in rats but no evidence of such ingestion was found in affected calves.
Pulmonary hypertension occurs secondary to left-sided heart disease in horses, although the hypertension has been mistakenly identified as the primary lesion.4
ATELECTASIS
Atelectasis is collapse of the alveoli due to failure of the alveoli to inflate or because of compression of the alveoli. Atelectasis is therefore classified as obstruction (resorption), compression or contraction. Obstruction atelectasis occurs secondary to obstruction of the airways, with subsequent resorption of alveolar gases and collapse of the alveoli. This disease is usually caused by obstruction of small bronchioles by fluid and exudate. It is common in animals with pneumonia or aspiration of a foreign body. Compression atelectasis occurs when intrathoracic (intrapleural) pressure exceeds alveolar pressure, thereby deflating alveoli. This occurs when there is excessive pleural fluid or the animal has a pneumothorax. In large animals it also occurs in the dependent lung or portions of lung in recumbent animals. Compression atelectasis is the explanation for the large shunt fraction and hypoxemia that occurs in anesthetized horses.1 Compression atelectasis and secondary bronchopneumonia can occur in horses kept in flotation tanks for up to several weeks for treatment of skeletal injuries.2 Contraction atelectasis occurs when there is compression of parts of the lung by fibrotic changes in the pleura. Patchy atelectasis occurs in the absence of surfactant, such as can occur in newborns. Failure of the lung to inflate, or development of atelectasis of the lungs of the newborn, usually those born prematurely, occurs because of lack of pulmonary surfactant. The disorder can progress to hyaline membrane disease. Affected newborn animals are severely dyspneic, hypoxemic, cyanotic, weak and commonly die in a few hours.
The clinical signs of atelectasis are not apparent until there is extensive involvement of the lungs. Animals develop respiratory distress, tachypnea, tachycardia and cyanosis. Blood gas analysis reveals hypoxemia, with or without hypercapnia. Thoracic radiographs reveal pulmonary consolidation. Ultrasonographic examination of the thorax demonstrates consolidated lung.
Atelectasis is reversible if the primary obstruction or compression is relieved quickly before secondary consolidation and fibrosis occur.
ACUTE RESPIRATORY DISTRESS SYNDROME
This is a well-recognized clinical syndrome of humans characterized by acute onset of hypoxemia and pulmonary infiltrates without increases in left atrial pressure (i.e. without evidence of cardiogenic pulmonary edema). Precipitating causes include both direct and indirect lung injury, including sepsis, multiple transfusions, trauma, near-drowning, smoke inhalation, pancreatitis and more. The underlying lesion is diffuse alveolar capillary damage with secondary severe pulmonary edema. The disease occurs spontaneously in domestic animals1 and, although the spontaneous disease is not extensively documented, the disease produced experimentally as a model of the human disease is better described.2
Acute respiratory distress syndrome (ARDS) in animals occurs in newborns and in adult animals. The disease in some newborn farm animals is related to lack of surfactant but except for animals born prematurely this is more the exception than the rule. Most young animals and all adult animals with ARDS have some inciting acute lung injury that then progresses to ARDS.1,3,4 The causes can be infectious (e.g. influenza virus infection), physical (smoke inhalation) or toxic (endotoxin).
The pathophysiology of the disease involves a common final pathway that results in damage to alveolar capillaries. The initial injury can be to either the endothelium of pulmonary capillaries or to alveolar epithelium. Damage to these structures leads to extravasation of protein-rich fluid and fibrin with subsequent deposition of hyaline membranes. The capillary injury is attributed to activated leukocytes (macrophages and neutrophils) and cytokines. Accumulation of hyaline membranes and ventilation/perfusion mismatches impair respiratory gas exchange and cause hypoxemia.
The clinical signs are characteristic of acute, progressive pneumonia. Animals are anxious, tachycardic, tachypneic and have crackles and wheezes on thoracic auscultation. Severely affected animals can be cyanotic. Thoracic radiographs reveal diffuse pulmonary infiltrates. Hematologic changes are characteristic of the inciting disease but usually include leukopenia. There is arterial hypoxemia.
Treatment includes administration of anti-inflammatory drugs (NSAIDs with or without glucocorticoids), colloids, antimicrobials and oxygen. The arterial blood gas response to oxygen therapy is often minimal in severely affected animals. If it is available, mechanical ventilation can be useful, although the prognosis is grave. Inhalation of nitric oxide is beneficial in some humans with the disease, and there are anecdotal reports that it has been used to treat foals with ARDS.