Diseases associated with Leptospira spp.
LEPTOSPIROSIS
Etiology
Leptospira interrogans (many distinct serovars). Leptospira borgpetersenii (many distinct serovars)
Epidemiology
Worldwide distribution, most commonly in warm, wet climates. Occurs in cattle, sheep and goats, pigs, and horses. Host-adapted (maintenance or reservoir) and non-host-adapted (accidental or incidental) leptospirosis dependent on response of each species to particular serovars. Prevalence of infection greater than incidence of clinical disease. Transmission by urine of infected animals; some wildlife species may transmit tocattle. Ground surface moisture most important factor for persistence of organism. Major zoonosis
Signs
Acute, subacute, and chronic forms. Fever, acute hemolytic anemia, changes in milk, stillbirths, abortion in all species (especially pigs), weak neonates, infertility, milk drop syndrome, periodic ophthalmia (recurrent uveitis in horse)
Clinical pathology
Demonstration and/or culture of organism in blood, urine, cervico-vaginal mucus, body fluids, and tissues; serological tests, primarily macroscopic agglutination test. ELISA and DNA probes
Lesions
Anemia, jaundice, hemoglobinuria, serous hemorrhages, autolysis of aborted fetuses, fetal hepatitis, and nephritis.
Diagnostic confirmation
Culture or demonstrate organism in body fluids or tissues; high serum titers
Cattle
• Acute leptospirosis: anaplasmosis; rape and kale poisoning; postparturient hemoglobinuria; bacillary hemoglobinuria
• Chronic leptospirosis: all other causes of abortion in cattle (Table 18.6). Milk drop syndrome
Horses
Abortion, stillbirths, perinatal deaths of foals due to: Streptococcus zooepidemicus; Salmonella abortivoequina; Escherichia coli; Actinobacillus equuli (Shigella equuli). Equine herpes virus; equine viral arteritis. Weak neonatal foals due to isoimmunization hemolytic anemia. Periodic ophthalmia from other causes of iridocyclitis of horses, and conjunctivitis, keratitis and hypopyon
ETIOLOGY
The pathogenic leptospires are classified into one species of Leptospira interrogans containing over 212 serovars arranged into 23 serogroups,1 for example, L. interrogans serovar pomona. Differentiation between serovars, formerly serotypes, belonging to a particular serogroup is by cross-agglutination tests. Two strains are considered different if, after cross-absorption with adequate amounts of heterologous antigen, 10% or more of the heterologous titer regularly remains in either of the two antisera.1 Because this system is subjective, the restriction endonuclease analysis (REA) of leptospiral DNA is used as a genotyping taxonomic tool2 which is less time and labor consuming than cross-agglutination absorption and gives highly reproducible results. This method allows observations between strains of the same serovar which can be correlated with differences in the epidemiology of the strains and, possibly, the pathogenicity of the strains. It has also found instances in which strains have been incorrectly identified by conventional typing methods.
Serological typing has revealed many groups, some of which share antigenic configuration. The serovar is the serologically least divisible recognized type. It is accepted as the basis of taxonomy as the subspecific level. The serovar name is spelled with a lower case initial letter, e.g. serovar pomona. The name of the serovar was previously italicized but this is no longer recommended.
Serovars which share antigens can be identified after controlled absorption and agglutination. They are grouped into serogroups, which have no taxonomic status, but are convenient for application such as diagnosis and epidemiology. The name of the serogroup is spelled with an initial capital letter, e.g. serogroup Pomona. Within some serovars further subgroups can be identified by genomic analysis. These groups are types of the serovar, and are not serologically distinguishable from one another (e.g. serovar hardjo, types hardjoprajitno and hardjobovis).
EPIDEMIOLOGY
Animal risk factors
Serovars and species susceptibility. The epidemiology of leptospirosis is most easily understood by classifying the disease into two broad categories: host-adapted and non-host-adapted leptospirosis. An animal infected with a host-adapted serovar of the organism, is a ‘maintenance’ or ‘reservoir’ host. Exposure of susceptible animals to non-host-adapted serovars results in accidental or incidental disease. Each serovar is adapted to a particular maintenance host, although they may cause disease in any mammalian species.
A serovar behaves differently within its maintenance host species than it does in other, incidental or accidental hosts. A maintenance host is characterized by:
1. A high susceptibility to infection
2. Endemic transmission within the host species
3. Relatively low pathogenicity for its host
4. A tendency to cause chronic rather than acute disease, producing insidious economic loss through reproductive losses
5. Persistence of the serovar in the kidney and sometimes the genital tract
6. A low antibody response to infection, with difficulties in diagnosis
Examples of this relationship are serovar bratislava in swine, and serovar hardjo type hardjo-bovis in cattle. In contrast, an incidental host is characterized by:
1. Relatively low susceptibility to infection but high pathogenicity for the host
2. A tendency to cause acute, severe rather than chronic disease
3. Sporadic transmission within the host species and acquisition of infection from other species, sometimes in epidemic form
5. A marked antibody response to infection, making for ease of diagnosis
An example of this relationship is serovar pomona (kennewicki) infection in cattle.
Some common leptospiral serovars and their maintenance hosts are as follows:
| Serovar | Maintenance hosts |
|---|---|
| hardjo-bovis (North America): | cattle |
| hardjo-prajitno (Europe): | cattle, pig, |
| bratislava: | horse |
| pomona (kennewicki): | pig, skunk, racoon, opossum |
| grippotyphosa: | racoon, opossum, squirrel |
| icterohemorrhagiae: | brown rat |
Some common leptospiral serovars and their accidental hosts are as follows:
| Serovar | Accidental hosts |
|---|---|
| hardjo: | sheep, man |
| pomona: | sheep, cattle |
| grippotyphosa: | sheep, cattle |
| icterohemorrhagiae: | cattle, pig |
Calves and lambs are highly susceptible to infection and septicemia is likely to occur.
Occurrence and prevalence of infection
Most leptospiral infections are subclinical3 and infection is more common than clinical disease. L. pomona is the commonest infection in all farm animals but its international distribution is unpredictable; it had not been present in the United Kingdom until recent years and then only sporadically. The number of serovars of concern in domestic animals has been increasing and serovars and their antibodies have been detected which were thought previously to be exotic.
L. canicola infection has been recorded in cattle and in pigs and specific antibodies have been detected in horses. L. icterohaemorrhagiae is a rare isolation in large animals but has been reported in cattle and pigs, and serological evidence of infection has been found in the horse. L. hyos (L. mitis) has been isolated from cattle and pigs, L. grippotyphosa from cattle and goats, and positive serological tests have been obtained in horses. L. sejroe, L. hebdomadis and L. australis infection have been observed in cattle. L. szwajizak is thought to be the predominant serovar in Israel.
Serological surveys of cattle in the African continent reveal evidence of antibodies against numerous leptospiral serovars4 and some previously not described strains of serovars. In West Africa, serosurveys of dairy herds revealed 45% of cattle were positive to one or more serovars, which probably represented natural infection because vaccination had not been practiced.4
Leptospirosis is common in farm animals in Portugal.5 Outbreaks of clinical disease have been recorded in cattle and pigs, and also in sheep and goats, and in horses to a lesser extent. In Italy, serological surveys indicate that sheep, horses, pigs, and dogs have the highest number of positive responses.6
CHARACTERISTICS OF LEPTOSPIROSIS IN SPECIES OF ANIMAL
Leptospirosis affects all farm animal species and the epidemiological characteristics of the infection, some of which are unique with a species and important in the diagnosis, treatment and control strategies, are outlined here.
Cattle
Leptospira interrogans is divided into seven genospecies of leptospires, two of which are L. interrogans and L. borgpetersenii.7 L. borgpetersenii serovar genotype hardjo-bovis formerly L. interrogans serovar hardjo-bovis occurs worldwide3 and in many areas outranks L. pomona in cattle. It is now the commonest serovar in cattle in some parts of Australia, New Zealand, the United States, and Canada. L. interrogans serovar genotype hardjo-prajitno so far has been recovered only in the United Kingdom, Nigeria, India, Malaysia, Brazil, Mexico, and the United States. L. hardjo is a common cause of abortion in dairy cattle in Brazil.8 New serovars occur occasionally, as for example, serovar ngavi, in the serogroup Tarassovi isolated from oxen in Zimbabwe.9L. interrogans serovar hardjo (type hardjoprajitno) and L. borgopetersenii (type hardjo-bovis) are serologically indistinguishable but genetically distinct.10 The former has been isolated primarily from cattle in the UK, while the latter is common in cattle populations throughout the world.
L. hardjo-prajitno has not yet been identified as a pathogen of North American cattle.11 Because this genotype, instead of hardjo-bovis is used in vaccines it may explain the lack of complete protection of animals against hardjo-bovis and the difficulties in laboratory diagnosis. In addition, there are at least two variations of the hardjo-prajitno genotype. These differences may be associated with differences in pathogenicity of hardjo strains, and although the cross-agglutination absorption test cannot distinguish these strains as separate serotypes, the degree of cross-protection conferred by these heterologous genotypes in vaccines is not known.
Cattle are the maintenance host for L. hardjo and are the only reservoir. L. hardjo is an important cause of bovine abortion3 and is the commonest leptospiral infection in man. It is a common infection in Australian sheep and may affect up to 40% of the population.
Seroprevalence surveys found 34–65% of sera obtained from cows at slaughter in parts of the United States had antibodies to leptospira serovars.3 Approximately 30% of all sera were positive when tested for L. hardjo, the host-adapted serovar of cattle. This seroprevalence is similar to reports from Europe, Australasia, and South America, in which 25–65% of all cows tested for L. hardjo were positive. The morbidity rate for clinical disease may vary from 10–30%, depending on the clinical manifestation of infection, and the case–fatality rate is usually low at about 5%. The case–fatality rate in calves is much higher than in adult cattle. A high rate of abortions (up to 30%) and loss of milk production are the major causes of loss but deaths in calves may also be significant.
Serovar hardjo-bovis is the most common serovar of cattle in the UK,3 Australia, New Zealand, and North America. Abattoir surveys of sera and kidneys of beef cattle in Quebec found a high prevalence of infection and nephritis associated with hardjo-bovis in contrast to pomona, which occurs more frequently in dairy cattle in that area. It is also possible that hardjo-bovis infection may be more common in feedlot cattle because they originated from Alberta, where the prevalence of infection with that serovar is prominent.
In serological survey of dairy cows in herds with suboptimal reproductive efficiency in a region in Spain. L. bratislava and L. grippotyphosa were the most prevalent serovars.12 The risk of seroconversion against L. grippotyphosa was higher during the spring season while L. bratislava did not differ among seasons. The prevalence of L. hardjo was low which indicates that the reproductive inefficiency was unassociated with hardjo. In surveys of dairy and beef cattle in Spain, L. bratislava is the most frequently detected serovar while hardjo is at a relatively low seroprevalence compared with similar studies in western European countries.13 In Spain, serovars grippotyphosa, tarassovi and copenhagi are more frequent in dairy herds, probably related to management practices and geographical location of these herds which facilitate the contact with maintenance hosts for these serovars.
A serological sampling of adult dairy cows in Brazil, not vaccinated for leptospirosis, and from herds with lowered fertility, 47% were positive for L. hardjo.14 The major risk factor associated with seropositivity was co-grazing with other species, mainly pigs.
L. pomona and L. hardjo-bovis are responsible for most bovine leptospirosis in Australia.11 Serovar pomona is a pig pathogen for which cattle are an accidental host and is a cause of bovine abortion, and fatal hemolytic disease in calves. Serovar hardjo-bovis is adapted to cattle as a maintenance host, is maintained in the bovine population but has a relatively low pathogenicity.11 It is responsible for epidemics of agalactia, the milk drop syndrome, and a major cause of infertility. However, diagnostic surveys in Australia suggest that hardjo-bovis is not responsible for any substantial proportion of bovine abortion in contrast to the situation in Northern Ireland, where the genotype hardjo-prajitno is present. In beef cattle in Queensland, Australia, the major serovars in order of decreasing crude seroprevalence were hardjo (15.8%), tarassovi (13.9%), pomona (4.0%), and szwajizak (2%).15 Vaccinates were not included in the hardjo and pomona seroprevalence; and the seroprevalence for hardjo and pomona tended to increase with age of the animals. The data indicate that serovars other than hardjo, pomona, and tarassovi, are unlikely to have a significant role in bovine infertility, and cattle are unlikely to be a source of human infection in central Queensland.
Seroprevalence surveys in Ontario, found hardjo-bovis was most commonly found in beef cattle, whereas pomona was most commonly found in dairy cattle. In Prince Edward Island, 14% of dairy cows were serologically positive for serovar hardjo. Serological surveys of cattle farms in Alberta found infection with hardjo-bovis was widespread across the province and the prevalence has increased. In contrast, pomona reactors were found usually on single premises within a locality as compared to the clustering of hardjo reactor herds. This is an expression of the difference between host–parasite relationships in which cattle are well-adapted reservoir hosts for hardjo, generally inducing a weak agglutinin response to natural infection and remaining capable of transmitting infection for months or years.
With pomona infection, cattle tend to develop high agglutinin titers with or without clinical disease, and not remain long-term carriers. Thus, pomona infections can remain limited to a single herd unless cattle are dispersed to other herds at the height of infection. In addition, reservoir hosts such as skunks may become infected and contaminate other premises, or a water supply common to a number of premises becomes contaminated when the water is near neutral pH (about 15–25°C) and of appropriate volume to deliver infectious numbers of organisms to cattle downstream.
As a measure of the loss associated with L. hardjo-bovis in beef cattle, the percentage of cows which are serologically positive has been related to the proportion of the herd affected with lactation failure, and there is a greater wastage in reactor cows.
Seroprevalence surveys of mature cattle in the United States found antibodies of hardjo-bovis were most common, followed by pomona and copenhagi.16 This confirmed earlier observations that the nationwide seroprevalence of hardjo-bovis was greater than pomona. The isolation rates were also higher in beef cattle than in dairy cattle and higher in bulls than in cows. Combined culture and immunofluorescence results found that 2% of mature cattle were renal carriers of leptospires.16
In Greece, the seroprevalence in pigs, goats, sheep, cattle, and dogs is 17.8%, 16.2%, 5.7%, 12.6%, and 11.4%, respectively.17 The overall prevalence is high, and against multiple serovars, indicating a considerable level of infection in each animal species, and that the zoonotic risk is considerable. In Turkey, L. hardjo is the dominant serovar identified in serological surveys of cattle, but L. grippotyphosa is the dominant serovar causing clinical disease in cattle while the disease in sheep is uncommon.18
The distribution of isolates and prevalence of serovars varies according to regions of the country.16 Both isolation rate and seroprevalence are higher in the south eastern, south central, and Pacific coast regions than for other regions of the United States.16 The isolation rate is related more to regional temperature than to amount of precipitation. This suggests that high levels of precipitation are not required for transmission of leptospirosis; oases in arid lands and deserts may become well-defined endemic zones by introduction of carrier animals.
Farmed deer
Leptospirosis is a well-established clinical disease in farmed deer in New Zealand.19 Slaughterhouse surveys of farmed deer in New Zealand found serological evidence of serovar hardjo in 73.6%, pomona in 41.5%, copenhagi in 11.3%, and tarassovi in 15.1% of farms. Antibodies to serovars australis, ballum, and balonica were present in three, one, and four of six herds studied, respectively. The titer prevalence to hardjo was higher than that of pomona and other serovars within farms. Renal lesions were characteristic of subclinical leptospirosis, and spirochetes were present in renal tubules. Cultures were positive in 10 stags from six lines with similar prevalence across age groups. On-farm surveys found a 10 to 30% within-herd prevalence of pomona and hardjo titers in 56% of 3-month-old deer herds; by 11 months of age, 100% of herds were titer-positive with high prevalences to one or both serovars.
Sheep and goats
The disease in sheep has been reported in many countries and in goats in Israel. Leptospirosis in sheep can cause lamb losses due to congenital infections, starvation of lambs because of the acute agalactia of hardjo infection of the ewes, and fatal infection of feedlot lambs infected with grippotyphosa. Deaths of animals and loss of condition in mildly affected animals are the main causes of loss.
Although few outbreaks are recorded, an infection rate of 75% is not uncommon in sheep and case–fatality rates usually average about 20% in this species and up to 45% in goats. L. pomona is the common infection and the cause of most clinical leptospirosis in sheep. Infection with L. hardjo occurs but is unlikely to be a source of infection for cattle herds. Sheep are not natural maintenance hosts for pomona or hardjo and are likely to have infections of relatively short duration, producing severe pathological effects. However, persistent leptospiruria due to hardjo in sheep where no contact with cattle has occurred suggests that sheep may be a maintenance host for this serovar.20 This could complicate control of hardjo infection in cattle which are free of this serovar, and infected sheep are a potential zoonotic risk to abattoir workers, sheep farmers, and shearers which previously had not been considered. Infection with serovar hardjo is widespread in Merino stud rams in South Australia.21
Pigs
In infected herds the prevalence of positive serological reactors is high, and in large infected pig populations is about 20%. Economic losses are about equally divided between abortions and deaths of weak and unthrifty newborn pigs. In Iowa, 38% of sera from National Animal Health Monitoring System herds were positive to one or more of 12 serovars. Infection of pigs at slaughter is associated with multifocal interstitial nephritis, which results in condemnation of kidneys.2 L. pomona (genotype kennewicki) has been the predominant infection in pigs but with the widespread use of bacterins against it, other infections are assuming importance. L. tarassovi, copenhageni, ballum, bratislava, muenchen, and hardjo are now isolated more frequently.
Swine are affected by several leptospiral serovars and the clinical signs often associated with these infections include poor reproductive performance.22-24 Seropositive sows have a greater risk of weak newborn pigs and having more weak newborn piglets per litter.23 In some areas suboptimal reproductive performance was associated with certain serovars such a grippotyphosa and not others such as autumnalis, bratislava, pomona, and icterohemorrhagiae.24
The most common serovar antibodies found in pigs in Prince Edward Island swine herds were L. icterohemorrhagiae, L. bratislava, L. autumnalis, and L. pomona.25 Only herds with a higher prevalence of L. bratislava had more infertility as measured by non-productive sow days per parity.
Pigs in intensive housing present a different problem from those in more conventional housing or at pasture. In large pig units the possibility for cross-infection is high because of high population density. The movement of pigs from pen to pen, and access to effluent from other pens are the critical means of spread in these circumstances. The spread of infection within piggeries is encouraged by mixing infected with uninfected pigs, which results in epidemics within the pens. Transmission from infected to susceptible grower pigs occurs continuously in grower houses, with a constant proportion of pigs becoming infected each week. Introduction onto a farm may be via an imported boar who frequently is found to harbor leptospires in the genital tract. Lepospira were found commonly in the kidneys of slaughter fattening pigs in Vietnam but are not considered to be the cause of the white spotted kidneys of pigs.26
The Australis serogroup of leptospires is now important because of an increasing awareness that antibodies to bratislava are widespread in the pig populations of many countries, the recovery of lora, muenchen, and bratislava from pigs, and the involvement of bratislava and muenchen in reproductive problems of swine herds. All of the pig isolates of the Australis serogroup have been identified as either bratislava or muenchen, and there are also differences at the subserovar level which may be important in understanding the epidemiology of the Australis serogroup, the development of efficacious vaccines, and the pathogenesis of disease. Certain genotypes are associated with abortion and stillbirths in pigs, while another genotype may be responsible for meningitis in piglets.
House mice on swine farms may be serologically positive to L. bratislava, which suggests a possible source of the organism.
Horses
• Recurrent uveitis. Leptospirosis is relatively mild in the horse, except for blindness due to recurrent uveitis or periodic ophthalmia. When groups of horses are infected, up to 30% of the adult horses may be serologically positive with a higher prevalence in tropical areas. The dominant serovar varies widely between localities. A high seroprevalence of bratislava occurs in healthy horses in Ireland and in horses in Ohio. The high seroprevalence to bratislava suggests that the horse may be a maintenance host for this serovar.
• Nonulcerative keratouveitis.A case of nonulcerative uveitis associated with leptospira infection in a 2-year-old horse in Japan has been described.27 The horse was seropositive to three serovars of L. interrogans (pyrogenes, canicola, and icterohemorrhagiae
• Neonatal foal disease. A severe rapidly fatal illness in foals characterized by massive pulmonary hemorrhage and kidney disease has been associated with serological evidence of bratislava and serogroup Australis serovar lora
• Abortion and stillbirths. Leptospirosis is an important cause of abortions and stillbirths in the horse population of central Kentucky in the United States. L. kennewicki, grippotyphosa, pomona, and bratislava were isolated from aborted fetuses.28 Leptospirosis has also been diagnosed as a cause of abortions, stillbirths, neonatal illness, and neonatal death on a horse farm involved in a flooding incident.29 Serological surveys of thoroughbred and standardbred horses in Ontario revealed a higher prevalence of bratislava, which increased with age. In a survey of horses in Alberta, titers to L. icterohemorrhagiae, bratislava, copenhagi, and autumnalis were common (94.6%, 56.6%, 46.5%, and 43.5%, respectively). The prevalence to other serovars ranged from 0.8–27.2%.30 The probability of being seropositive increased by approximately 10% with each year of life. Horses managed as individuals (e.g. racetrack horses) were about half as likely to be seropositive as those managed in groups (e.g. rodeo horses). A giant cell hepatitis in four aborted foals has been associated with the presence of pomona (kennewicki).
The risk factors associated with the likelihood of seropositivity to L. pomona, L. autumnalis, and L. bratislava in horses in New York State have been quantified.31
Rodent exposure was associated with risk of exposure to all serovars. Management was associated positively with the risk of exposure to serovars pomona and bratislava, but not with risk of exposure to autumnalis. Soil and water had a positive association with risk of exposure to pomona and autumnalis but not to bratislava. The wildlife index value and the population density of horses turned out together were associated with risk of exposure to autumnalis.
A bacteriological survey of kidneys from abattoir horses in Portugal found serogroups L. australis and L. pomona, which were identified as L. bratislava, and L. kirschneri serovar Tsaratsovo, respectively.32 Leptospiral antibodies were more than 1:10 in 37% of the horses.
Methods of transmission
The source of infection is an infected animal which contaminates pasture, drinking water, and feed by infective urine, aborted fetuses, and uterine discharges. All of the leptospiral types are transmitted within and between species in this way. A viable infected neonate can harbor the infection for several weeks after birth. The semen of an infected bull may contain leptospirae and transmission by natural breeding or artificial insemination can occur but is uncommon. In rams, the semen is likely to be infective for only a few days during the period of leptospiremia; in boars there is no evidence of coital transmission. L. interrogans serovar hardjo is excreted from the genital tract of aborting cows for as long as 8 days after abortion or calving and is detectable in the oviducts and uterus for up to 90 d after experimental infection and in naturally infected cows. It may also be present in the genital tract of bulls and venereal spread of the infection is possible. Young pigs may act as carriers for 1 year and adult sows for 2 months. Because of the high intensity and long duration of the infection in pigs, they play an important role in the epidemiology of leptospirosis.
Leptospiruria
Urine is the chief source of contamination because animals, even after clinical recovery, may shed leptospirae in the urine for long periods. All animals which have recovered from infection may intermittently shed organisms in the urine and act as ‘carriers’. In cattle, leptospiruria may persist for a mean period of 36 d (10–118 d) with the highest excretion rate in the first half of this period. Sheep and horses are not common sources of infection because of low grade and intermittent leptospiruria. In any species, the leptospirae may persist in the kidney for much longer periods than they can be recovered from the urine by routine laboratory methods. Urine drinking by calves is not an uncommon form of pica in some dairy herds and is a means of transmission.
Wildlife as source of infection
Although surveys of the incidence of leptospirosis in wildlife have been conducted and the pathogenic effects of L. pomona on some species, particularly deer and skunks have been determined, the significance of wildlife as a source of infection for domestic animals is uncertain. Variable rates of seroprevalence to leptospires have been documented in white-tailed deer, mule deer, pronghorns, moose, red deer, and elk.33 There is a high prevalence of infection in feral pigs, and in wild brown rats trapped on farms in the United Kingdom the prevalence of L. icterohaemorrhagiae and bratislava was about 14%.34 L. canicola is known to spread from domestic dogs and jackals to cattle and, when hygiene is poor, even from humans to cattle. The serovar bratislava has been associated with severe interstitial nephritis in raccoons in a recreational area in Quebec which were also serologically positive to pomona, hardjo, and grippotyphosa.35
Portal of entry of organism
Entrance of the organism into the body occurs most probably through cutaneous or mucosal abrasions. Transplacental transmission is uncommon but neonatal infection in utero has occurred. Oral dosing is an unsatisfactory method for experimental transmission as compared to injection and installation into the nasal cavities, conjunctival sac, and vagina.
Environmental and management risk factors
Survival of the organism in the environment depends largely upon variations in soil and water conditions in the contaminated area. The organism is susceptible to drying, and a pH lower than 6 or greater than 8 is inhibitory. Low urinary pH in cattle fed with brewer’s grains may inactivate leptospires in animals with leptospiruria.36 Ambient temperatures lower than 7–10°C (44.6–50°F) or higher than 34–36°C (93–96°F) are detrimental to its survival.
Ground surface moisture and water is the most important factor governing the persistence of the organism in bedding or soil; it can persist for as long as 183 days in water-saturated soil but survives for only 30 min when the soil is air-dried. In soil under average conditions, survival is likely to be at least 42 d for L. pomona. It survives in free, surface water for long periods; the survival period is longer in stagnant than in flowing water although persistence in the latter for as long as 15 d has been recorded. Contamination of the environment and capacity of the organism to survive for long periods under favorable conditions of dampness may result in a high incidence of the disease on heavily irrigated pastures, in areas with high rainfall and temperate climate, in fields with drinking water supplies in the form of easily contaminated surface ponds, and in marshy fields and muddy paddocks or feedlots. Because of the importance of water as a means of spreading infection, new cases are most likely to occur in wet seasons and low lying areas, especially when contamination and susceptibility are high. A differential distribution has been observed in the prevalence of seropositives in cattle in Australia. L. hardjo antibodies have a high prevalence through all rainfall areas, but L. pomona is much more common in low rainfall areas.
Certain management factors have been identified which pose risks of L. hardjo infection being introduced into dairy herds:37
Economic importance
Leptospirosis is a major cause of economic loss in farm animals. The majority of leptospiral infections are subclinical and associated with fetal infections causing abortions, stillbirths, and the birth of weak neonates with a high death rate in cattle, sheep, horses, and pigs. In cattle, epidemics of abortions, infertility, and increased culling rate cause major economic losses. Epidemics of agalactia in dairy herds, the milk drop syndrome, are associated with infection with L. hardjo.
Zoonotic implications
In the past decade, leptospirosis has emerged as a globally important infectious disease in human medicine.38,39 It is uncommon in developed countries but the incidence is increasing in travelers returning from endemic countries. The epidemiology has undergone major changes, with a shift away from the traditional occupational disease in developed countries, to a disease associated with recreational exposures.39 It is now recognized as an emerging, potentially epidemic disease associated with excess rainfall in tropical settings, representing a significant public health hazard. Mortality in humans with leptospirosis remains significant because of delays in diagnosis due to lack of diagnostic infrastructure and adequate clinical suspicion when patients are presented for medical diagnosis and care. The differential diagnosis of febrile illness in returning travelers should include leptospirosis. Pulmonary hemorrhage is increasingly being recognized as a major, often lethal, manifestation of leptospirosis in humans, the pathogenesis of which is unclear. Treatment consists of tetracyclines and beta-lactam/cephalosporins. No vaccine is available for the prevention of leptospirosis in humans.
Leptospirosis is an important zoonosis and is an occupational hazard to butchers, farmers, and veterinarians.40 The high prevalence of leptospiral infection (serovars pomona and hardjo) in Texas cattle represents potential threats to human health and agricultural economics.41 The incidence of positive agglutination tests in humans in contact with infected cattle is surprisingly low and clinical cases in humans in which the infection is acquired from animals are not common. Human infection is most likely to occur by contamination with infected urine or uterine contents. Veterinarians may become infected by handling the tissues and urine of sows which have aborted from pomona infection. Although leptospirae may be present in cow’s milk for a few days at the peak of fever in acute cases, the bacteria do not survive for long in the milk and are destroyed by pasteurization. However, farm workers who milk cows are highly susceptible to L. interrogans serovar hardjo infection and one New Zealand survey found 34% of milkers were seropositive, mostly to L. interrogans serovar hardjo, but a high proportion also to L. interrogans serovar pomona. This has aroused alarm and leptospirosis became known as ‘New Zealand’s No. 1 dairy occupational disease’. A campaign of vaccination of dairy cattle across the country resulted in a marked decrease in the incidence of the disease in humans. In most situations, dogs, cats, and horses are unlikely to contribute to human infection.40
Leptospirosis is New Zealand’s most common occupationally acquired infectious disease, and the incidence of the disease is high compared with other temperate developed countries.42 The epidemiology of leptospirosis in New Zealand has been changing. The annual incidence of human leptospirosis in New Zealand from 1990–98 was 4.4 per 100 000. Incidence was highest among meat processing workers (163/100 000), livestock farm workers (91/100 000) and forestry-related workers (24/100 00). The most commonly detected serovars were L. borgpetersenii sv. ballum (11.9%). The annual incidence of leptospirosis declined from 5.7/100 000 in 1990–92 to 2.9/100 000 in 1995–98. The incidence of L. borgpetersenii sv. hardjo and L. interrogans sv. pomona infection declined, while the incidence of L. borgpetersenii sv. ballum infection increased. The increasing incidence of L. borgpetersenii sv. ballum suggests changing transmission patterns via direct or indirect exposure to contaminated water. The risk of transmission of leptospirosis from dairy cattle infected with L. hardjo to dairy workers in Israel was low.43
Leptospirosis is a risk factor for swine producers and slaughterhouse workers. An epidemiological investigation of people exposed to infected pigs from a university-owned swine herd found 8% of the workers were confirmed to have leptospirosis.44 Risk factors included smoking and drinking beverages while working with infected pigs. Washing hands after work was protective. Leptospirosis is infrequently diagnosed by human physicians in the United States and veterinarians have often informed physicians of the potential of leptospirosis in humans. The disease can be prevented through appropriate hygiene, sanitation, and animal husbandry.44 It is essential to educate people working with animals or animal tissues about measures for reducing the risk of exposure to such zoonotic pathogens as leptospira.
Veterinary students may be exposed to leptospirosis by taking courses in food inspection and technology, on-farm clinical work experiences, contact with pets especially carnivores, and contact with animal traders.45 In a one-year period, the seroprevalence of leptospirosis in veterinary students in a veterinary school in Spain increased from 8.1% to 11.4%. The incidence of the disease during the study was 0.0394.
PATHOGENESIS
Leptospirosis manifests itself as a disease in several different ways. Leptospires invade the host across mucosal surfaces or softened skin. They have the ability to bind to epithelial cells and attach to the constituents of the extracellular matrix through an active process involving surface proteins. Pathogenic leptospires are found extracellularly between cells of the liver and kidney. Release of lymphokines such as tumor necrosis factor (TNF-alpha) from monocytes through the endotoxic activity of the leptospiral LOS may be an important virulence mechanism. Induction of TNF-alpha release may help explain the damage to endothelial cells with resultant hemorrhage seen in severe leptospirosis.
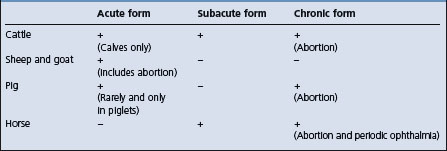
Leptospirosis can occur as an acute and severe disease due to septicemia with evidence of endotoxemia such as hemorrhages, hepatitis, nephritis, meningitis; as a subacute moderately severe disease with nephritis, hepatitis, agalactia, and meningitis, or as a chronic disease characterized by abortion, stillbirth, and infertility. In the occult form there is no clinical illness. The form of the disease that occurs depends largely on the species of the host as set out in Table 20.3. Variations between serotypes of L. interrogans in their pathogenicity also affect the nature of the signs which appear. For example, in L. pomona infections, intravascular hemolysis and interstitial nephritis are important parts of the disease. However, L. hardjo does not produce hemolysin and does not cause interstitial nephritis. But it does cause clinical infection in sexually mature, lactating or pregnant females. Thus infection occurs in the pregnant uterus and lactating mammary gland resulting in septicemia, abortion, and mastitis. The pathogenesis of the disease associated with L. pomona is set out as follows.
Acute form
After penetration of the skin or mucosa, the organisms multiply in the liver and migrate to, and can be isolated from, the peripheral blood for several days until the accompanying fever subsides. At this time serum antibodies begin to appear and organisms can be found in the urine.
Septicemia, capillary damage, hemolysis, and interstitial nephritis
During the early period of septicemia, sufficient hemolysin may be produced to cause overt hemoglobinuria as a result of extensive intravascular hemolysis. This is an unlikely event in adult cattle, but is common in young calves. If the animal survives this phase of the disease, localization of the infection may occur in the kidney. Hemolysis depends on the presence of a serovar which produces hemolysin. Capillary damage is common to all serovars and during the septicemic phase, petechial hemorrhages in mucosae are common. Vascular injury also occurs in the kidney and if the hemolysis is severe, anemic anoxia and hemoglobinuric nephrosis may occur. There is some evidence that leptospires produce a lipopolysaccharide endotoxin which may exacerbate the vascular lesions. The infection localizes in the renal parenchyma, causing an interstitial nephritis and persistence of the leptospirae in these lesions results in prolonged leptospiruria. The renal lesion develops because the infection persists there long after it has been cleared from other tissue sites. In the acute phase of the disease, the animal may die from septicemia or hemolytic anemia or both. Subsequently, the animal may die of uremia caused by interstitial nephritis.
Focal chronic interstitial nephritis, also called ‘white spotted kidney’ is a common finding in clinically healthy cattle at slaughter and has frequently been assumed to be related to current of prior infection with Leptospira spp. However, studies of ‘white spotted kidney’ in cattle at the abattoir indicate that neither Leptospira spp. nor active infection by other bacteria are associated with the lesions.46
Abortion
Following systemic invasion, abortion may occur due to fetal death, with or without placental degeneration. Abortion usually occurs several weeks after septicemia because of the time required to produce the changes in the fetus, which is usually autolyzed at birth. Abortion occurs most commonly in the second half of pregnancy, due probably to the greater ease of invasion of the placenta at this stage, but may occur at any time from 4 months on. Although abortion occurs commonly in both cattle and horses after either the acute or the subacute form of the disease, abortion without prior clinical illness is also common. This is particularly the case in sows and occurs to a less extent in cows and mares; this may be due to degenerative changes in the placental epithelium. Leptospirae are rarely present in the aborted fetuses, however if the aborted fetus has survived the infection long enough to produce antibodies, these may be detectable.
Experimental infection of serologically negative pregnant cattle with a north-Queensland strain of L. borgpetersenii serovar hardjo resulted in seroconversion and shedding of the organism in the urine.47 Elective cesarean sections were done 6 weeks after challenge. No evidence of L. hardjo infection of the fetuses occurred. Some of the fetuses had histopathological lesions consistent with Neospora sp. infection.
Encephalitis
Localization of leptospirae in nervous tissue is common in sheep and goats and may result in the appearance of signs of encephalitis.
Subacute and occult forms
In the subacute form, the pathogenesis is similar to that of the acute septicemic form, except that the reaction is less severe. It occurs in all species, but is the common form in adult cattle and horses. Occult cases, with no clinical illness but with rising antibody titers, are common in all animals. These are difficult to explain but may be associated with strains of varying pathogenicity. But with leptospirosis, characteristically, differences between groups may be associated with prior immune status, environmental conditions, or number of carriers in relation to severity of exposure.
Periodic ophthalmia (recurrent uveitis) in the horse
There is some evidence of a causal relationship between leptospiral infection and periodic ophthalmia in the horse.48 The incidence of serologically positive reactors is higher in groups of horses affected with periodic ophthalmia than in normal animals. Agglutinins are present in the aqueous humor in greater concentration than in the serum. Serological surveys indicate that leptospira infection is not a major factor in the etiology of equine anterior uveitis in the United Kingdom, but serological evidence of pomona is associated with uveitis in horses in the United States. The opacity in both cornea and lens is a consequence of the antigenic relationship between leptospires and components of the ocular tissues and does not require the presence of living bacteria. A 52-kDa protein appears to be involved in the antigenic relationship between the leptospires and equine ocular tissues and is located inside the bacterium. The uveitis alters the composition of the aqueous humor and impedes the nutrition of the ocular structures, leaving sequelae such as iris atrophy, synechiae, and corneal opacity.
Retinal immunopathology in horses with uveitis has been described and may be a primary immunological event in equine uveitis, providing evidence that leptospira-associated uveitis may be a distinct subset of equine uveitides.48
Immune mechanisms
Following infection, specific antibodies are induced which opsonize leptospires, facilitating their elimination from most parts of the body. However, leptospires which reach the proximal renal tubules, genital tract and mammary gland appear to be protected from circulating antibodies. They persist and multiply in these sites, and may be excreted and transmitted to susceptible, in-contact animals, primarily by urine. Furthermore, and of major importance, the level of serum antibody commonly declines to undetectable levels in animals which are persistently infected.
The first serological response with L. hardjo infection is the production of immunoglobulin M (IgM) antibodies. These rise rapidly but commonly decline to undetectable concentrations by 4 weeks after infection. Within 1–2 weeks of infection, IgG1 antibodies appear, and at 3 months they represent 80% of antibodies detected in the microscopic agglutination test (MAT). The MAT titer peaks 11–21 d after infection but may vary from 3200 to an undetectable concentration. The MAT titer declines gradually over 11 months but the persistence is variable. Vaccination induces antibodies that are mainly of the IgG class with levels peaking at 2 weeks after a two-dose vaccination but decreasing rapidly to levels lower than those after natural infection. Approximately 95% of vaccinated heifers do not have MAT antibodies 20 weeks after the second of two vaccinations given 4 weeks apart and the absence of titers is not necessarily an indication that protection has waned. Vaccinated animals are protected from natural challenge for many months after their MAT titers become undetectable. The serological response of calves vaccinated at 3 months of age is lower than those vaccinated at 6 months of age because of the presence of maternal antibody. Transfer of passive immunity antibodies to newborn calves occurs via the colostrum and the antibodies persist in the calves for 2–6 months.
While antibodies against leptospiral lipopolysaccharides (LPS) give passive protection in some animal models, cattle vaccinated against serovar hardjo with pentavalent vaccines are vulnerable to infection with serovar hardjo despite the presence of high titers of anti-LPS antibody. It is now known that peripheral blood mononuclear cells (PBMC) from cattle vaccinated with an L. interrogans serovar hardjo vaccine which protects against serovar hardjo proliferated in vitro in response to hardjo antigens. Thus a cell-mediated immune response to serovar hardjo may be necessary for protection.10 A protective killed vaccine against serovar hardjo induces a strong antigen-specific proliferative response by PBMC from vaccinated cattle 2 months after the first dose of vaccine. This response was absent from unvaccinated cattle. The mean response peaked by 2 months after completion of the two-dose vaccination regimen, and substantial proliferation was measurable in vitro cultures throughout 7 months of the study period. Up to one-third of the PBMC from vaccinated animals produced gamma interferon (IFN-γ after 7 days in culture with antigen. One-third of the interferon gamma producing cells were (gamma delta lymphocytes, with the remainder cells being CD4+ T-cells.10 Thus a very potent Th1-type immune response was induced and sustained following vaccination with a killed bacterial vaccine adjuvanted with aluminum hydroxide and the involvement of gamma delta T-cells in the response. The induction of this Th1-type cellular immune response is associated with the protection afforded by the bovine leptospiral vaccine against L. borgpetersenii serovar hardjo.10
The immune response of naïve and vaccinated cattle following challenge with a virulent strain of L. borgpetersenii serovar hardjo has been examined.49 Beginning at 2 weeks after challenge, gamma interferon was measured in antigen-stimulated PBMC cultures from nonvaccinated animals, although the amount produced was always less than that in cultures of PBMC from vaccinated animals. IFN-γ+ cells were also evident in antigen-stimulated cultures of PBMC from vaccinated but not from nonvaccinated animals throughout the post-challenge period. Naïve and vaccinated animals had similar levels of antigen-specific immunoglobulin G1 (IgG1) following challenge; vaccinated animals had two-fold more IgG2. It is evident that while infection may induce a type 1 response, it is too weak to prevent establishment of chronic infection.
CLINICAL FINDINGS
The clinical findings in leptospirosis are similar in each animal species and do not vary greatly with the species of Leptospira except that infection with icterohaemorrhagiae usually causes a severe septicemia. For convenience the various forms of the disease are described as they occur in cattle, and comparisons are made with the disease in other species. In all animals the incubation period is 3–7 d.
Cattle
Leptospirosis in cattle may be acute, subacute or chronic and is usually associated with pomona or hardjo.
Acute leptospirosis associated with pomona
Calves up to 1 month old are most susceptible to the acute leptospirosis. The disease is manifested by septicemia, with high fever (40.5–41.5°C; 105–107°F), anorexia, petechiation of mucosae, depression, and acute hemolytic anemia with hemoglobinuria, jaundice and pallor of the mucosae. Because of the anemia, tachycardia, loud heart sounds and a more readily palpable apex beat are present: dyspnea is also prominent. The case–fatality rate is high and if recovery occurs, convalescence is prolonged. In adult cattle, abortion due to the systemic reaction may occur at the acute stage of the disease. Milk production is markedly decreased and the secretion is red-colored or contains blood clots, and the udder is limp and soft. Mastitis as part of leptospirosis has often been described in cattle and a high somatic cell count in grossly abnormal milk suggests mastitis, but these changes are due to a general vascular lesion rather than local injury to mammary tissue. Severe lameness due to synovitis is recorded in some animals and a necrotic dermatitis, probably due to photosensitization, in others.
Subacute leptospirosis associated with L. pomona
The subacute form of leptospirosis differs from the acute form only in degree. Similar clinical findings are observed in a number of affected animals but not all of the findings are present in the same animal. The fever is milder (39–40.5°C; 102–105°F), and depression, anorexia, dyspnea and hemoglobinuria are common but jaundice may or may not be present. Abortion usually occurs 3–4 weeks later. One of the characteristic findings is the marked drop in milk production and the appearance of blood-stained or yellow-orange, thick milk in all four quarters without apparent physical change in the udder.
Chronic leptospirosis associated with L. pomona
The clinical findings in the chronic form of leptospirosis are mild and may be restricted to abortion. Severe ‘storms’ of abortions occur most commonly in groups of cattle which are at the same stage of pregnancy when they are exposed to infection. The abortions usually occur during the last trimester of pregnancy. Apart from the abortion, there is no depression of reproductive efficiency in cattle affected by leptospirosis. Many animals in the group develop positive agglutination titers without clinical illness.
There are occasional reports of leptospiral meningitis in cattle. In coordination, excessive salivation, conjunctivitis and muscular rigidity are the common signs.
Leptospirosis associated with L. hardjo
Infertility and milk drop syndrome occurs only in pregnant or lactating cows because the organism is restricted to proliferation in the pregnant uterus and the lactating mammary gland. There is a sudden onset of fever, anorexia, immobility and agalactia. The milk is yellow to orange and may contain clots. The udder is flabby, there is no heat or pain, and all four quarters are equally affected. The sudden drop in milk production may affect up to 50% of cows at one time and cause a precipitate fall in the herd’s milk yield. The decline may last for up to 8 weeks but in individual cows milk production will return to normal within 10–14 days. The milk may have a high leukocyte count which subsides over a period of about 14 days as milk production returns. In some cases, there is no evidence of mastitis, no change in the consistency of the milk and no changes in the udders of affected cows, but leptospiruria may be present in up to 30% of affected cows. In endemically infected dairy herds, there may be no relationship between seropositive and seronegative cows in different lactations, nor at different stages of lactation and total lactation milk yield.50
The herd fertility status incorporating the first service conception rate, the number of services per conception for cows conceiving, the calving-to-conception interval, and the culling rate usually reveals a low reproductive performance, especially during the year of the diagnosis.51 The effect is also temporary and not easily detected. Exposure of nonvaccinated dairy cows to L. hardjo can be associated with a subsequent reduction in fertility, as indicated by a greater time from calving to conception and a higher number of breedings per conception.52Abortion may occur several weeks after the initial infection and may also occur as the only evidence of the disease; in some areas or circumstances it is the principal clinical manifestation of leptospirosis due to hardjo, and the principal cause of abortion in cattle. In others it is thought to be an uncommon cause of abortion. This may be related to different strains of the serotype, or to the degree to which the disease has become enzootic. Thus outbreaks of milk yield drop and systemic illness appears to be the characteristic clinical picture when the disease first appears in an area. However, as natural immunity develops in adult cows, only heifers become newly infected, and the only sign is abortion. Furthermore, many cows have subclinical infections with hardjo in which only a fall in milk yield may be detectable.
Leptospirosis associated with szwajizak, produced experimentally, is characterized by a short bout of fever, listlessness and anorexia, and diarrhea in some. The illness lasts for 24 h.
Pigs
Pomona is the common infection, tarassovi is the other common infection, and chronic leptospirosis is the commonest form of the disease in pigs. It is characterized by abortion and a high incidence of stillbirths. Failure to conceive is not usually observed in leptospirosis but has been reported in infections with canicola. In an infected herd the rearing rate may fall as low as 10–30%. An abortion ‘storm’ may occur when the disease first appears in a herd but abortions diminish as herd immunity develops. Most abortions occur 2–4 weeks before term. Piglets produced at term may be dead or weak and die soon after birth. Hardjo may be a sporadic cause of reproductive disease and muenchen and bratislava are occasional isolations during investigations of porcine abortion and stillbirths. In a survey of swine farms in Canada, those herds with a high serological prevalence of serovars bratislava and pomona had more infertility as measured by non-productive sow days per parity.25 There was no association between infertility and antibodies to serovars autumnalis and icterohemorrhagiae.25
Rarely the acute form as it occurs in calves also occurs in piglets in both natural field outbreaks and in experimentally produced cases. Icterohaemorrhagiae infection causes septicemic leptospirosis with a high mortality rate.
Sheep and goats
The disease is rare in these species so that good descriptions of the naturally occurring disease in them are lacking; most affected animals are found dead, apparently from septicemia. Affected animals are febrile, dyspneic, snuffle, and hang their heads down. Some have hemoglobinuria, pallor of mucosae and jaundice, and die within 12 h. Lambs, especially those in poor condition, are most susceptible. The chronic form may occur and is manifested by loss of bodily condition, but abortion seems to be almost entirely a manifestation of the acute form when the infection is pomona. With hardjo, abortion has been recorded as the only clinical sign, and oligolactia and agalactia, similar to the bovine milk drop syndrome, have been observed in lactating ewes.
Horses
Abortion
Leptospira interrogans serovar pomona type kennewicki is a major cause of abortions and stillbirths in the equine population in Kentucky in the United States;53,54 other serovars also occur. The gestational ages range from 140 d to full-term mean (250 d).55 Horses in central Kentucky are exposed to multiple Leptospira serovars including bratislava, icterohemorrhagiae, grippotyphosa, pomona genotype kennewicki, hardjo and canicola.56 In some years in Kentucky, leptospiral infection is a leading cause of abortion in which fetoplacental infections account for one-third of the abortions, stillbirths and perinatal deaths and in which 75% are due to bacterial infection.57
Periodic ophthalmia
Recurrent uveitis in horses (periodic ophthalmia, moon blindness, recurrent iridocyclitis) is a late complication of systemic leptospirosis in horses with signs beginning months to years after naturally acquired or experimentally induced infection. It is often associated with infection with L. interrogans serovar pomona. Clinically there are recurrent episodes of ocular disease including photophobia, lacrimation, conjunctivitis, keratitis, a pericorneal corona of blood vessels, hypopyon and iridocyclitis. Recurrent attacks usually terminate in blindness in both eyes. There is a strong relationship between uveitis and leptospiral seroactivity in horses. Seropositive horses with uveitis are at increased risk of losing vision, compared with seronegative horses with uveitis, and Appaloosas are at an increased risk of developing uveitis and associated blindness, compared with that in non-Appaloosas. The disease has been produced experimentally by producing infection with pomona. Infection with pomona in foals has been observed in association with Rhodococcus equi to cause a very heavy mortality. The foals died of a combination of interstitial nephritis and uremia, and pulmonary abscessation and chronic enteritis. Leptospirosis has been suspected as a cause of renal dysfunction in a horse58 and hematuria and leptospiruria described in a foal.59
Nonulcerative keratouveitis associated with leptospiral infection has been described in horses.27 Photophobia, epiphora, and blepharospasm are common. Hyperemia of the bulbar conjunctiva, edema of the paralimbal cornea, pupillary block and iris bombe are also present. As the disease progresses, there may be hyphema, hypopyon, and organized fibrin in the anterior chamber, myosis, dyscoria due to posterior synechiae and the cornea may become opaque and vascularized.27 The cornea retains no fluorescein dye.
CLINICAL PATHOLOGY
General considerations
Laboratory procedures used in the diagnosis include culture or detection of leptospires in blood or body fluids, and detection and measurement of antibody in blood and body fluids such as urine, cerebrospinal fluid and cervico-vaginal mucus.60 Culture of leptospires is laborious and can take up to 2 months. Serological and microbiological detection of chronically infected animals is difficult, as is the confirmation of leptospirosis as a direct cause of reproductive losses in a herd. A positive diagnosis of leptospirosis in individual animals is often difficult because of the variation in the nature of the disease, the rapidity with which the organism dies in specimens once they are collected and their transient appearance in various tissues. During the septicemic stage, leptospirae are present only in the blood and there may be laboratory evidence of acute hemolytic anemia and increased erythrocyte fragility and often hemoglobinuria. A leukopenia has been observed in cattle while in other species there is a mild leukocytosis. However, the only positive diagnostic measure at this stage of the disease is culture of the blood. If abortion occurs, the kidney, lung and pleural fluid of the aborted fetuses should be examined for the presence of the organism. Serological testing at the time of abortion is often unreliable because the acute titers have already peaked and are declining. In the stage immediately after the subsidence of the fever, antibodies begin to develop and the leptospirae disappear from the blood and appear in the urine. The leptospiruria is accompanied by albuminuria of varying degrees and persists for varying lengths of time in the different species.
The diagnosis of leptospirosis is much easier on a herd basis than in a single animal because in an infected herd, some animals are certain to have high titers and the chances of demonstrating or isolating the organism in urine or milk are increased with samples being taken from many animals. On the other hand, in a single animal, depending on when the infection occurred, the titer may have declined to a low level and be difficult to interpret. This becomes particularly important for the clinician confronted with a diagnosis of abortion due to leptospirosis in which the infection may have occurred several weeks previously and the serum may be negative or the titers too low for an accurate interpretation. Examination of the urine may be useful in these cases.
Serological and related tests
Acute and convalescent sera taken 7–10 d apart should be submitted from each clinically affected animal, or from those with a history of abortion, and sera should also be taken from 15–25% of apparently normal animals. Ten blood samples should be taken from each of the yearlings, the first-calf dams, the second-calf dams, and the mature age group in order to determine the infection status across the herd. If possible, wildlife or rodents which are known to inhabit the farm and use nearby water supplies should be captured and laboratory examinations of their tissues and blood carried out and the results compared with those obtained in the farm animals.
The Microscopic Agglutination Test (MAT) is the most commonly used serological test for the diagnosis of leptospirosis. In animals which survive infection, acute leptospirosis can readily be diagnosed on the basis of demonstrating a rising antibody titer in acute and convalescent sera.60 Although paired sera are normally considered necessary so that a rise in titer can be detected, in cases of bovine abortion or stillbirth, infection may have occurred 1–4 weeks before the abortion by which time the MAT titers may be declining. Following infection, the IgM class of antibodies are first to appear followed by IgG antibodies, which persist for longer than IgM antibodies. The MAT detects both IgM and IgG antibodies. The MAT is particularly useful in diagnosis of disease associated with incidental, non-host-adapted serovars or acute disease associated with host-adapted serovars. It is less useful in diagnosis of chronic disease in maintenance hosts since antibody response to infection may be negligible in chronic infections or may persist from subclinical infections. In pigs, MAT has an adequate sensitivity for some serovars, such as pomona, but is insensitive to infection associated with bratislava. The herd serological response to infection is often more helpful than the individual’s response in chronic infections in maintenance hosts. Because agglutinating antibodies wane, the sensitivity of the MAT in detecting animals infected for more than 2 years is low, probably less than 50%. A major concern is the failure of the MAT to differentiate between titers after vaccination and those after natural infection since the titers may be of similar magnitude; however, titers after infection are in general, higher and persist longer than vaccination titers. Vaccinated cattle which subsequently become infected, may not mount an agglutinating antibody response.
A MAT titer of ≥100 is considered positive but there are several considerations in evaluating the MAT response. The MAT is a serogroup-specific test and serovars representative of all suspected serogroups should be tested. The test is a more sensitive detector of IgM antibody than IgG. It has a low sensitivity in chronic leptospirosis for detecting maintenance hosts. The test is inadequate for the detection of the carrier state in maintenance hosts, because titers ≥100 against host-adapted serovars have a low sensitivity but high specificity. The MAT is not a measure of immunity to infection because vaccination results primarily in an IgG response, with low (100–400) and transient (1–4 months) titers but immunity commonly persists in vaccinated animals long after MAT titers are negative. In cattle, titers of ≥100 are considered significant and a four-fold rise in titer on a paired sample taken 2 weeks apart is diagnostic. In abortion associated with incidental serovars, MAT titers against pomona and other incidental serovars are high, often ≥3000.
Paired sera are of limited value in chronic infections because abortion occurs after infection and titers are static or declining. In chronic hardjo infections, a recently aborting cow with a titer of ≥300 has about a 60%, of ≥1000 an 80%, and ≥3000 a 90% chance of fetal infection. If several aborting cows have high titers (≥300), this is evidence for the diagnosis of leptospirosis in unvaccinated herds. A semiautomated complement fixation test is available and is comparable in efficiency with the MAT.
The ELISA test is much more accurate than the others and has many advantages from the point of view of laboratory practice. It can be specific for IgM antibodies or IgG antibodies. A positive IgM-specific ELISA result can therefore indicate that infection occurred within the previous month. It has excellent diagnostic specificity and sensitivity, convenient technical features including automation, and can be used efficiently as a screening test for large numbers of serum samples. Some difficulty is encountered in interpreting the significance of titers of antibody in serum. For a diagnosis of leptospiral abortion in cattle, a reciprocal titer of 3000 is proposed as the threshold for pomona but no similar critical figure is available for hardjo. For a herd diagnosis of leptospirosis due to hardjo 10 animals from each of the yearling, first-calf heifer, second-calf heifer and adult cow groups should be tested.
An indirect ELISA has been developed for the detection of bovine antibodies to multiple Leptospira serovars including canicola, copenhagi, grippotyphosa, hardjo-bovis pomona, and sejroe.61
An antibody capture ELISA is available to detect antibodies to a protective lipopolysaccharide fraction of Leptospira borgpetersenii serovar hardjo in cattle.62
A commercially available ELISA and the Immunocomb Leptospirosis Kit which detect L. hardjo antibodies have been compared with the MAT.63 The Immunocomb and ELISA tests both exceeded the positive results obtained with the MAT.63 The Immunocomb is very simple, and quick, requiring no sophisticated equipment.
Aqueous humor antibody. Measurement of aqueous humor antibody titers against leptospires in horses offers a more accurate means of establishing a diagnosis of leptospiral-associated uveitis than serology alone.
Serology in pigs. A comparison of diagnostic procedures for the diagnosis of porcine leptospirosis reveals that the apparent (maximum) sensitivities of diagnostic procedures for detecting infection were as follows: MAT (at a titer of 64 or 1024) 95%; IgM enzyme immunoassay 82%; culture of kidneys 61%; presence of white spots 55%; immunogold staining 52%; presence of large white spots 30%; and, Warthin–Starry silver staining 20%. An axial filament ELISA is a sensitive and specific test for the detection of antibodies against L. interrogans on a species rather than serovar level and has advantages over the MAT.
Demonstration or culture of organism or antibody
A number of tests are available to detect leptospires or leptospiral DNA in tissues or body fluids.64
Culture of urine. Of all the laboratory diagnostic tests for leptospirosis, the examination of urine samples for the organism probably offers the best opportunity to demonstrate the presence of infection. Following natural infection with L. hardjo, cattle may shed leptospires in the urine for between 28 and 40 weeks;65 following experimental infection, shedding occurs for about 26–32 weeks. After the initial infection, large numbers of leptospires are shed in the urine for several weeks and thereafter there is a progressive decline in the numbers shed, which may be associated with a sharp increase in urinary anti-leptospiral IgG and IgA antibody levels.66 Urine samples should be obtained from a cross-section of affected and non-affected (in-contact) animals. Furosemide can be given IV at 0.5–0.8 mg/kg BW and the second voiding of urine collected for culture. For maximum efficiency, one-half of each urine sample should be submitted with added formalin (1 drop to 20–30 mL of urine) and the other half submitted in the fresh state. The formalin prevents bacterial overgrowth and the fresh urine sample may be used for culture. Examination of urine using dark-field microscopy or fluorescent antibody test are useful tests. The fluorescent antibody test is more sensitive than dark field microscopy, detects degenerated as well as intact leptospires and may be serovar specific. Standard techniques for culture of leptospires have been described.65
Detection of organism in urine
Fluorescent staining of antibody in urine. A fast and accurate diagnostic method for detecting the presence of leptospirae and for identifying serotypes. Antibodies also appear in urine and milk and their measurement may have some significance in special circumstances.
Antibody in cervico-vaginal mucus. An ELISA has been used to detect specific antibody to L. hardjo in the cervico-vaginal mucus as early as 2 weeks after natural or experimental infection and may reach high levels after 8 weeks.67 This may show some promise in diagnosis but has not yet been evaluated.
A comparison of a PCR assay with bacteriologic culture, immunofluorescence, and nucleic acid hybridization for detection of L. borgpetersenii serovar hardjo in urine of cattle found all were sensitive but a single technique was not the most sensitive for each animal tested.68 Two techniques in combination are recommended for maximal sensitivity.
Detection of organism in tissues
DNA probes and PCR. Leptospires can be detected in tissues using a DNA genomic probe and DNA-based techniques will probably provide rapid and sensitive diagnostic techniques that are serovar- and genotype-specific. Nucleic acid hybridization is a sensitive and rapid test for the detection of leptospires in the urine of cattle which become infected subsequent to vaccination, and is superior to bacteriological culture and fluorescent antibody testing. The polymerase chain reaction is also a promising test for the rapid detection of small numbers of leptospires in the urine of cattle infected with L. hardjo-bovis. A multiplex PCR is highly sensitive for detection of the organism in aborted bovine fetuses.69
Using a leptospira PCR assay, L. kirschneri has been identified as a potential cause of abortion in an fetal foal born on a farm with a history of repeated abortions.70 Further confirmation of L. kirschneri was done by DNA sequence analyses of the PCR amplified DNA fragment.
Detection of organism in semen
In some countries, bulls destined for artificial insemination centers must be free of antibody to hardjo, grippotyphosa, canicola, pomona, sejroe, and icterohaemorrhagiae at a final serum dilution of 1:100 in the MAT. However, since animals with leptospirosis may not have a serum titer, it is possible that the semen of serologically negative but infected bulls may contain leptospires. Culture of leptospires is difficult, costly and time consuming. A polymerase chain reaction assay has been developed to detect pathogenic leptospires in the semen and urine of infected bulls.71,72 PCR is a method of great potential for the detection of Leptospira spp. at bovine artificial insemination units.54
Detection in aqueous humor of horses with uveitis
Using PCR to detect the presence of Leptospira DNA, 70% of horses with uveitis were positive for Leptospira DNA, and 28% were culture positive for leptospires from the aqueous humor;73 only 6% of horses free of uveitis used as controls were positive. The serologic results did not correlate well with the presence of Leptospira DNA or organisms in the aqueous humor.
NECROPSY FINDINGS
Acute bovine leptospirosis is characterized by anemia, jaundice, hemoglobinuria and subserosal hemorrhages. There may be ulcers and hemorrhages in the abomasal mucosa. Pulmonary edema and emphysema are also common in this species. Histologically, there is focal or diffuse interstitial nephritis, centrilobular hepatic necrosis and in some cases, vascular lesions in the meninges and brain in subacute to chronic infections. Leptospirae may be visible in silver-stained sections, especially in the proximal convoluted tubules ofthe kidney. In acute infections there may be minimal inflammation, with only hemoglobin-filled renal tubules and centrilobular hepatic necrosis evident microscopically.
In the later stages, the characteristic finding is a progressive interstitial nephritis manifested by small, white, cortical foci which are initially raised but become slightly depressed as the lesion ages. Many clinically normal cattle presented to abattoirs have these lesions, which may represent sequela to episodes of bacteremia from a variety of pathogens and should not be considered pathognomonic for leptospirosis.74,75Aborted bovine fetuses are usually autolyzed to the point where no lesions or bacteria can be demonstrated. Even in a fresh fetus the positive identification of leptospirae in lesions is not an easy task. Culture of these organisms is difficult – L. interrogans serovar hardjo is particularly fastidious in its cultural requirements. The use of a fluorescent antibody technique assists in the demonstration of organisms but false-positives are common unless the test is interpreted by an experienced diagnostician. Dark-field microscopy may be attempted but is not well-suited to tissues collected at necropsy. PCR techniques show considerable promise, although sample processing requirements are stringent and the use of multiple primer sequences may be required in some cases.46,76 Immunoperoxidase techniques are highly useful in the demonstration of leptospirae in formalin-fixed tissues, although this test is not serovar specific. Traditional silver-based staining of fixed material is also successful in a few cases. Antibodies to leptospirae are detectable in the serum of some aborted fetuses.3
Gross placental lesions in cases of equine abortion and stillbirth associated with leptospirosis include nodular cystic allantoic masses, diffuse edema, and areas of necrosis with a mucoid exudate on the chorionic surface. The liver is enlarged, mottled and pale-red to yellow. The kidneys are swollen and edematous with pale, radiating streaks in both cortex and medulla. Microscopic changes may include a suppurative and non-suppurative nephritis, dissociation of hepatocytes, a mixed leucocytic infiltration of portal triads, a giant cell hepatopathy, pneumonia and myocarditis. Thrombosis, vasculitis and a mixed population of inflammatory cells are evident in the placenta.55 A variety of tests, as described for cattle, are available to try to confirm the diagnosis.
Aborted piglets are usually severely autolytic, with blood-stained fluid in the subcutis and filling the body cavities. Multiple necrotic foci, 1–4 mm in diameter and irregular in outline, are found in the liver of approximately 40% of aborted fetuses. Microscopic inflammatory changes may also be found in the kidneys. The fetal membranes are thick and edematous. Leptospirae can be demonstrated utilizing the battery of tests already mentioned for cattle.
Samples for confirmation of diagnosis
• Bacteriology – chilled kidney, liver, placenta (CULT (has special growth requirements), FAT, PCR)
• Histology – formalin-fixed kidney, liver, brain, heart, lung, placenta (LM, IHC)
• Serology – heart-blood serum or pericardial fluid from fetus (MAT).
The zoonotic potential of this organism should be noted when handling carcasses and submitting specimens.
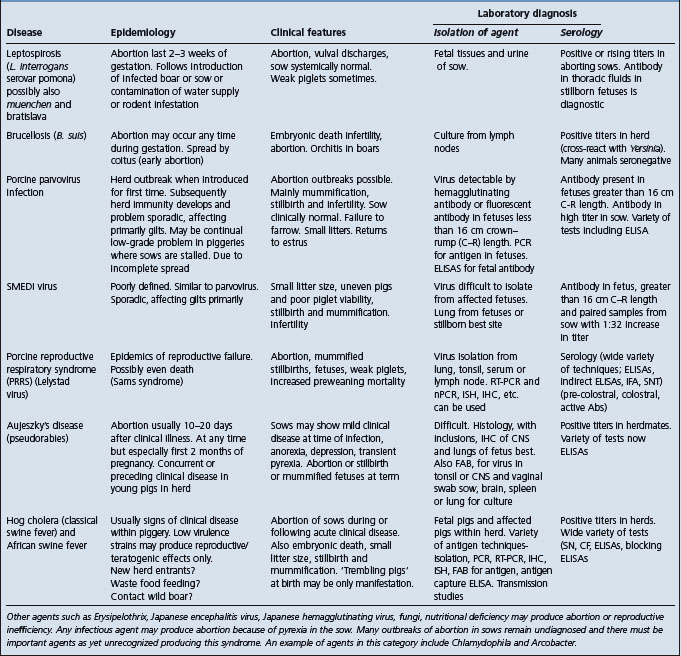
The differential clinical diagnosis of the common forms of leptospirosis in each species is as follows:
Cattle
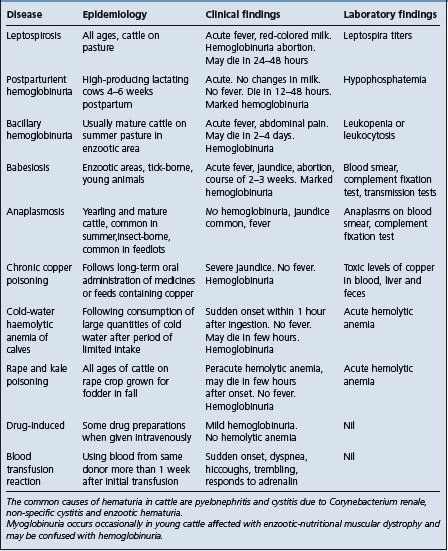
• Acute leptospirosis – must be differentiated from those diseases causing hemolytic anemia with or without hemoglobinuria (Table 20.4). They include: Babesiosis; anaplasmosis; rape and kale poisoning postparturient hemoglobinuria); bacillary hemoglobinuria
• Chronic leptospirosis causing abortion – must be differentiated from all other causes of abortion in cattle (Table 18.7). Most diagnostic surveys reveal that a specific cause is identifiable in only about 30% of fetuses submitted to a diagnostic laboratory. The vaccination history of the aborting cattle is a crucial part of the history since, for example, outbreaks of abortions due to infectious bovine rhinotracheitis occur primarily in unvaccinated cows. The specific causes of abortion in cattle vary depending on geographical location. Other common causes of abortion in cattle include: infectious bovine rhinotracheitis; protozoal abortion (Sarcocytis sp., Toxoplasma gondii and Neospora caninum). Less common causes are: brucellosis; bovine virus diarrhea; pine needle abortion; mycotic placentitis; campylobacter; Actinomyces pyogenes, ureaplasma
• Milk drop syndrome – characterized by a sudden drop in milk yield in up to 30–50% of the cows within several days. Must be differentiated from other causes of a decline in milk production of the herd including: (i) change of feed; (ii) change of management; and (iii) epidemic of infectious disease such as bovine respiratory disease.
Pigs
• Abortion in the last trimester – the common manifestation of leptospirosis in pigs and must be differentiated from all other causes of abortion, mummification and stillbirths in swine (Table 20.2). Other common causes of abortion in swine are: parvovirus; porcine reproductive respiratory syndrome. Less common causes are: brucellosis; pseudorabies; Stillbirth, Mummification, Embryonic death, Infertility (SMEDI) virus.
Sheep and goats
Chronic copper poisoning and poisoning caused by rape in sheep may present a clinical picture similar to that in leptospirosis but there will be no febrile reaction. Anaplasmosis associated with Anaplasma ovis may be accompanied by fever and hemoglobinuria but is more commonly a chronic, emaciating disease.
Horses
• Abortion, stillbirths, perinatal deaths of foals – Streptococcus zooepidemicus; Salmonella abortivoequina; Escherichia coli; Actinobacillus equuli. Other bacterial infections: Equine herpes virus; Equine viral arthritis; fungal infections. Diagnosis depends on laboratory examination of fetal tissues and fluids including bacterial culture, direct fluorescent antibody test for equine herpes virus and leptospires, serologic examination of fetal fluids for leptospiral antibodies using the MAT and special stains to demonstrate leptospires in fetal tissues
• Isoimmune hemolytic anemia – within 36 hours after birth, there is weakness, hemoglobinuria, pallor, failure to suck, tachycardia, high case–fatality rate and cross-matching blood tests
• Infectious equine anemia – chronic relapsing fever, anemia, weakness, jaundice, edema, oral mucous membrane hemorrhages, Coggins serological test
• Exertional rhabdomyolysis – acute onset of stiff gait, weakness, sweating, distress, myoglobinuria, creatine kinase test
• Periodic ophthalmia differentiate from other causes of iridocyclitis of horses, and conjunctivitis, keratitis and hypopyon which may occur in equine viral arthritis.
TREATMENT
The primary aim of treatment is to control the infection before irreparable damage to the liver and kidneys occurs. Treatment with dihydrostreptomycin preferably, or one of the tetracyclines, as soon as possible after signs appear is recommended. The results of treatment are often disappointing because in most instances animals are presented for treatment only when the septicemia has subsided. The secondary aim of treatment is to control the leptospiruria of ‘carrier’ animals and render them safe to remain in the group.
For infections due to pomona, dihydrostreptomycin (12 mg/kg BW IM twice daily for 3 d) is effective in the treatment of the systemic infection. For the elimination of leptospiruria in cattle and pigs, a single dose of dihydrostreptomycin (25 mg/kg BW IM) is recommended.77,78 In an outbreaks in cattle the simultaneous treatment of all animals with dihydrostreptomycin at 25 mg/kg BW IM and vaccination has been successful in preventing new cases and abortion when pregnant cattle are involved. When the participation rate of dairy farmers in an area in the Netherlands was high, the combination of antimicrobial and vaccination was economical. A similar approach is recommended for outbreaks in swine. Annual revaccination and regular serological testing for new infections, combined with controlling the source of new infections, will usually successfully control further outbreaks. A surveillance system in the area is necessary, however, to detect the introduction of new serotypes. Streptomycin is no longer available for use in the United States and long-acting oxytetracycline at 20 mg/kg BW is an alternative.64 Oxytetracycline, tilmicosin, and ceftiofur are also effective for resolving leptospirosis in cattle.79 For the treatment of L. hardjo, amoxycillin is also an alternative to streptomycin.80
Dihydrostreptomycin G (25 mg/kg BW IM for 1, 3, or 5 d), or oxytetracycline (40 mg/kg BW IM daily for 3 or 5 d), tylosin (44 mg/kg BW IM daily for 5 d), or erythromycin (25 mg/kg BW IM daily for 5 d) are all effective for treatment of persistent leptospirosis due to pomona in swine.81
In vitro studies indicate that leptospires are highly susceptible to ampicillin, amoxycillin, penicillin G, cefotaxime, erythromycin, fluoroquinoline ciprofloxacin and have a good susceptibility to streptomycin, tylosin and tetracyclines. Doxcycline and penicillin G are effective in treating acute leptospirosis.
For outbreaks of leptospiral abortion in horses, treatment of pregnant mares with dihydrostreptomycin (50 mg/kg BW) IM daily for 3–5 d can minimize further abortions10; this treatment regimen however has not been extensively evaluated.
For equine periodic ophthalmia, most recommended treatments have little effect on the course of the disease. A course of a suitable antibiotic systemically, and the administration of a corticosteroid, either parenterally in an acute episode, or subconjunctivally in a chronic case, is most likely to be satisfactory. Nonulcerative keratouveitis require long-term and intensive medication, and recur with tapering of treatment.27 Topical and subconjunctival corticosteroids are recommended in controlling nonulcerative keratouveitis. Intravitreal implantation of cyclosperine is effective.53 Atropine eye ointment is also usually applied three times daily to maintain dilatation of the pupil.
In groups of pigs, the feeding of oxytetracycline (800 g/t of feed for 8–11 d) is claimed to eliminate carriers. Antimicrobial feeding should begin 1 month before farrowing to avoid the occurrence of abortion. Other experimental trials using long-acting tetracycline by injection or tetracycline in the feed have been inconclusive and the use of mass feeding techniques as control programs should not be recommended lightly. Antimicrobial feeding has also been suggested as a preventive measure in calves. The feeding of small amounts of tetracyclines (3 mg/kg/d BW) for 7 d before and 14 d after exposure, prevents the appearance of clinical signs but not infection as measured by the agglutination-lysis reaction.
CONTROL
Biosecurity and biocontainment
On an individual farm, leptospirosis can be eradicated or controlled by vaccination. With the diagnostic tests available, the success of antimicrobial therapy in eliminating the carrier state, and the vaccines, it is now reasonable to attempt eradication of the disease from individual herds, and possibly from areas. The major risk is the introduction of carrier animals of any species, or by reintroduction of the infection by rodents or other wildlife. It is because of this risk that most programs aim at containment rather than eradication. In these circumstances where only sporadic cases occur, it might be more profitable to attempt to dispose of reactors or treat them to insure that they no longer act as carriers.
The first step in control is to identify the source of the original source of infection and to interrupt transmission.2 Sources of infection include clinically affected animals, aborted fetuses, placentas, carrier animals, wildlife, dogs and cats, and environmental sources such as water supplies. Education about leptospirosis is an effective method for reducing its incidence and its effects. Intensive well directed education and publicity campaigns in New Zealand, used in conjunction with a campaign for immunization of cattle, reduced the incidence of leptospirosis.2 Groups to which educational efforts should be directed include professionals in human and veterinary medicine and public health, primary human and animal health care practitioners, wildlife and conservation scientists, water and sewage engineers and planners, health administrators and educators, and not least, the public at risk.
Risk-assessment and computer simulation models of the possible costs of infections due to L. hardjo have been developed to explore the risks and likely consequences to producers of the disease in dairy herds in the United Kingdom. Three main considerations are important when assessing the risks and likely financial implications of the disease to dairy producers:
1. Likelihood of a herd being infected
2. Likely effects of the disease on the dairy enterprise, both physically and financially, following the initial infection compared to a leptospirosis-free herd
3. Likely longer-term effects of the disease.37
The probability of infection of cattle by L. hardjo is increased by four factors:
1. Purchase of infected cattle
2. Co-grazing or common grazing with infected cattle or sheep
3. Use of natural service with an infected bull
4. Access of cattle to contaminated water such as streams, rivers, flood or drainage water.
Assessment of the risks facing different types of herds suffering losses from L. hardjo can then be used to help support decisions concerning control of the disease.
The major consequences of L. hardjo infection are milk loss and abortion in the dairy herd, and the risk of illness in man. The financial losses associated with disease in a herd according to the presence of different risk factors can be estimated using a static risk model.37 The cost of various control strategies can then be considered in the light of these expected costs which result from doing nothing to control the disease. A dynamic simulation model can be used to consider how the disease might develop in a dairy herd following initial infection and over the next few years.
Producers with one or more of the main risk factors should consider strategies which (i) directly remove or diminish those risk factors or (ii) indirectly diminish their importance for the herd, for example, by vaccination. Strategies which successfully diminish one or more of the risk factors, but leave one other will yield little benefit due to the importance of each of the identified risk factors.
Vaccination is one strategy which can diminish all of the risk factors and provide some degree of assurance against potentially high and costly disease losses. Producers with high-risk herds are likely to choose vaccination. The dynamic simulation model estimates that the cost of endemic disease can be relatively high and that some form of vaccination strategy would be cost-effective. If a herd continues to have any of the risk factors, then whole-herd vaccination is likely to be the preferred option, since the disease could otherwise easily be reintroduced. Decision tree analysis of leptospirosis vaccination in beef cattle in Australia indicates that the beneficial economic effects of vaccination depend on the value of the calf and the probability of calf loss due to leptospirosis.
Artificial insemination centers.
Bulls destined for artificial insemination centers must be free of antibody to hardjo, grippotyphosa, canicola, pomona, sejroe, and icterohaemorrhagiae at a final serum dilution of 1:100 in the MAT. However, since animals with leptospirosis may not have a serum titer, it is possible that the semen of serologically negative but infected bulls may contain leptospires. Culture of leptospires is difficult, costly and time consuming. A PCR assay has been developed to detect pathogenic leptospires in the semen and urine of infected bulls71 (see Clinical pathology).
Eradication
Detection and elimination of carrier animals presents some difficulties
Positive reactors to the MAT do not necessarily void infective urine and to determine their status as carriers necessitates repeated examination of the urine for the organism. For practical purposes, serologically suspicious and positive reactors should be considered carriers and culled or treated as described above. In groups of pigs, it should be assumed that infection is herd-wide and all pigs should be treated as though they were carriers. In these circumstances, the feeding of antimicrobials provides some protection, although it is not guaranteed to eliminate the carrier state. Leptospirosis has been eradicated from commercial pig herds by treating all pigs with dihydrostreptomycin at 25 mg/kg BW IM at one time. However, if the pigs have been exposed to heavy infection, not all of them are completely cleared of leptospiruria, and further treatment will be necessary.
In cattle herds, if the bulls are infected they should not be used naturally or for artificial insemination even though the antimicrobials in the semen diluent is sufficient to insure that no spread occurs. Elimination of infection can be difficult, especially in large commercial herds in an endemic area in which replacement cows and bulls are introduced from sales yards and cattle mingle with other herds on range. Eradication of hardjo is a possibility in purebred herds in which intensive measures are economically feasible and owners should be urged to undertake a program to eliminate leptospirosis from the herd and to prevent its entry. The following measures can be taken to eliminate hardjo infection:
1. Judicious combination of group serological testing
4. Possibly artificial insemination
Bulls suspected of spreading infection should be treated to reduce the level of urinary shedding regardless of subsequent vaccination. Exposure of cattle to herds, heavily infected with leptospirosis, for example, on communal grazing pastures should be avoided. The herd should be monitored periodically, coincident with other serological testing. In endemic areas, all cattle over 6–9 months of age should be vaccinated, and vaccination should be continued for up to 5 years to minimize the number of susceptible cattle until no long-term shedders remain in the herd.
Simple management procedures to limit the infection in beef cows until their second calves are born, and the culling of older carriers can greatly decrease and possibly eradicate the infection from a herd. Virgin yearling bulls are used on virgin yearling heifers, and young cows are segregated from older cows until 38–39 months of age, when they go to pasture after being bred, with their second calf. This delays direct exposure of heifers to infected cattle until their third breeding. These practices must be combined by monitoring infection by serological and other laboratory methods.
If eradication is attempted and completed, introduced animals should be required to pass a serological test on two occasions at least 2 weeks apart before allowing them to enter the herd. Urine examination for leptospirae should be carried out if practicable.
Occupational hygiene
Occupational hygiene is important for prevention of leptospirosis wherever the disease is known to occur predominantly in certain occupational groups. Exposure to risk is unavoidable in people whose livelihoods depend on rice planting or farming, sugar cane cutting, work in tropical forests, keeping peridomestic pigs, building or maintaining drains or sewers, milking cows, and slaughtering or herding pigs or cattle. Occupational hygiene is concerned with means of minimizing the risks, by employing measures to reduce direct or indirect contact with animals which might be infected.
Control of the source of the organism is achieved by appropriate hygienic strategies. If the environmental sources of infection are identifiable, in the form of yards, marshes and damp calf pens, every attempt must be made to avoid animal contact with these infective surroundings. In dairy farming areas where human leptospirosis comes from cattle, milkers and transporters should be protected. The main measures available are immunization of cattle and pigs, education, and modification of work practices, including protective clothing. It is important to avoid urine splash. Cows should be handled calmly to minimize urination in the milking sheds. Sheds and yards should be hosed out so as to avoid aerosol and splash from urine on the floors. Herdspeople and milkers must be made aware of the risks of infection from mud contaminated with urine. Wet areas should be drained or fenced and pens disinfected after use by infected animals. The possibility that rats and other wild animals may act as a source of infection suggests that contact between them and farm animals should be controlled.
Meat industry workers are at high risk during the examination and dressing of carcasses. The dangerous parts of carcasses are the bladder, urine and kidneys. Handling bovine or porcine kidneys with bare hands is especially risky and rubber gloves should be worn. Wearing protective clothing in abattoirs is not well accepted because speed is paramount and gloves are an impediment. Nevertheless it offers protection.
Control of clinical disease by immunization
A control program to limit the occurrence of clinical disease is achieved by vaccination to maintain a high level of immunity in herd.
Vaccination
Vaccination against leptospirosis in cattle and swine is in general use and an effective method for control of the disease. In New Zealand, a publicity campaign to promote the widespread vaccination of cattle resulted in a marked reduction in the incidence of human leptospirosis. Most of the vaccines are formalin-inactivated bacterins containing one or more serotypes. Vaccines containing Freund’s complete adjuvant induce higher serological responses but not necessarily superior protection. The immune response is serotype-specific and protection is dependent on the use of bacterins containing serotypes prevalent in the area. The bacterins induce a low titer to the MAT which appears early and declines after several weeks; however, protective immunity against the disease and renal infection persists for at least 12 months in cattle. Regular serological testing in herds vaccinated annually can be used to monitor new infections since these will induce a titer to the MAT. However, neither the CFT nor the MAT can reliably differentiate serological responses in cattle following leptospiral vaccination from those following natural infections.
Cattle
The difficulty of keeping purebred herds free of hardjo infection increases as the reservoir of infection increases. Several control measures can be applied especially in large herds which are at high risk. In endemic areas, transmission in commercial herds can be suppressed by annual vaccination of bulls, replacement heifers, and 2- and 3-year-old females a few weeks before release of the bulls. A cow which fails to carry a calf to term, or that produces a dead or weak calf, should be culled. Potential replacement heifer calves should be handled and raised in segregation from the adult herd after weaning and vaccinated a month before exposure to older cattle. Herd sires should be purchased from uninfected herds or at least purchased subject to a negative serological test.
Vaccination as part of a herd health program should start with the calves at 4–6 months of age, followed by revaccination annually. Such programs should provide significant rises in calving rates, but have little or no effect on perinatal or postnatal losses.
Bovine leptospiral vaccines and their efficacy
Many vaccines are available but there is conflicting evidence about their efficacy. A pentavalent leptospiral vaccine containing hardjo-bovis did not protect cows from experimental infection with hardjo-bovis. Vaccination of cattle with a pentavalent leptospiral vaccine containing either hardjo-bovis or hardjo-prajitno failed to protect cattle from experimental infection with hardjo-bovis 6 months after vaccination.10 The hardjo-bovis vaccine is more antigenic than the hardjo-prajitno as measured by higher antibody titers in vaccinated animals. Calves as young as 4 weeks of age, vaccinated in the presence of maternally derived antibody can be fully protected against homologous virulent challenge.
Most bovine leptospiral bacterins contain the reference strain hardjo-prajitno. However, North American cattle are predominantly infected with hardjo-bovis. DNA probe techniques can be used to identify animals infected with hardjo-bovis which enables diagnosticians to correctly diagnose infection in animals which have been previously vaccinated. Serovar hardjo-prajitno is used to prepare the hardjo component of pentavalent, whole-cell, leptospiral vaccines. The hardjo component of whole cell leptospiral vaccines is a weak immunogen, as evidenced by low-serum agglutinating antibody titers in vaccinated cattle and short duration immunity. Restriction endonuclease analysis of Australian and New Zealand isolates of L. pomona reveal that most closely resemble the serovar kennewicki reference strain, and all differed from the reference strain of serovar pomona. This suggests that vaccine manufactures should consider using the genotypes which are most prevalent in cattle and pigs. In New Zealand, a trivalent vaccine containing inactivated L. borgpetersenii serovar hardjo Hardjobovis, L. pomona and copenhagi has been developed and tested for efficacy and potency in cattle.
Leptospiral vaccines used in cattle in the United States are inactivated whole-cell vaccines containing L. interrogans serovar hardjo (type hardjoprajitno), canicola, pomona, and icterohemorrhagiae and L. kirschneri serovar grippotyphosa. These pentavalent vaccines provide adequate protection against disease associated with each of the serovars in the vaccine except serovar hardjo. They have failed to prevent abortion, stillbirth, and vertical transmission of infection when vaccinated cows were challenged with L. borgpetersenii serovar hardjo during pregnancy, and the infection rates for control and vaccinated cattle did not differ. Attempts to improve the protection against L. borgpetersenii serovar hardjo by including L. borgpetersenii serovar hardjo in a pentavalent vaccine or by increasing the quantity of serovar hardjo antigen in a monovalent serovar hardjo vaccine failed. However, monovalent vaccines with a field isolate of L. borgpetersenii serovar hardjo and another with L. interrogans serovar hardjo found these vaccines prevented infection and colonization following challenge with L. borgpetersenii serovar hardjo strains from the United States and Europe.10
A protective killed vaccine against serovar hardjo induced a strong, sustained Th1 or cell-mediated response.10 The vaccine is composed of a whole-cell bovine isolate of L. borgpetersenii serovar hardjo and aluminum hydroxide and is given as two doses subcutaneously 4 weeks apart.49 Following vaccination, a type 1 (Th1) cell-mediated response occurred characterized by the production of gamma interferon cells including CD4+ and WC1 gamma delta T-cells. Vaccinated animals had twofold-more IgG2.
A monovalent L. borgpetersenii serovar hardjo (type hardjo-bovis) vaccine commercially available in Australia, New Zealand, Ireland, and the UK, given as two doses, 4 weeks apart, protected heifers against renal colonization and urinary shedding when challenged with L. borgpetersenii serovar hardjo strain 203 four months after vaccination.82 None of the animals shed leptospires in their urine, or kidneys at necropsy. In contrast, all nonvaccinated control heifers became infected with serovar hardjo and shed organisms in their urine. A monovalent US reference hardjo vaccine failed to prevent infection, renal colonization, and urinary shedding in cattle challenged with L. borgpetersenii serovar hardjo.82 The reference vaccine was prepared with L. borgpetersenii serovar hardjo (type hardjo-bovis) rather than L. interrogans serovar hardjo (type hardjoprajitno), which is used in many cattle leptospiral vaccines available in the US, because L. borgpetersenii serovar hardjo is the organism which infects cattle in the US.82
Two monovalent Hardjo vaccines provided protection from infection against L. borgpetersenii serovar hardjo while a pentavalent vaccine containing the Hardjo organisms did not.76 The protective monovalent vaccines produced strong cell-mediated immune responses in vaccinated cattle as demonstrated by proliferation of lymphocytes and production of IFN-gamma by their peripheral blood mononuclear cells in response to culture with serovar Hardjo antigens. This response is generally much lower or absent in antigen-stimulated cultures of PBMC from cattle vaccinated with the pentavalent vaccine and nonvaccinated cattle.
In conclusion, protective immunity to serovar Hardjo correlates with induction of a substantial immune response that is characterized by antigen-specific IFNgamma-producing T-cells, IgG1 and IgG2 antibodies which react with antigens common between serovars as well as antibodies that are largely serovar-specific and agglutinate leptospires through reactivity with surface lipopolysaccharide. These results contrast with those induced by pentavalent vaccines that have a superior ability to induce antibodies that agglutinate all the serovars of leptospires included in their formulation with the exception of serovar hardjo.
There is no cross-immunity between L. pomona and hardjo, and in areas where both diseases occur, a bivalent vaccine is used routinely. If separate vaccines are used the L. pomona vaccine should be administered at least once annually, but the L. hardjo vaccine provides some protection against L. szwajizak.
Vaccination of calves less than 3 months of age is unlikely to be effective and is not recommended, but vaccination of cows in late pregnancy gives effective immunity to their calves.
Vaccination of sows and gilts before breeding with a bivalent vaccine, containing pomona and tarassovi, protects them against infection and the development of leptospiruria and is widely practiced, especially in large intensive piggeries. In the United-States, vaccination of gilts and sows with two doses of a bacterin containing five or six leptospiral serovars, one of which contained bratislava, before the first breeding and thereafter before each breeding improves reproductive performance. L. bratislava is an important cause of abortions in sows in North America and Europe and vaccination is effective. Vaccination of pregnant gilts and sows can provide protection to the piglets for the first several weeks after birth.
Vaccination and antimicrobial strategies.
Whether or not to vaccinate depends upon the cost of the procedure relative to the losses which can be anticipated. If the disease is spreading rapidly, as evidenced by the frequent appearance of clinical cases, a high range of titers or rising titers in a number of animals, (i) all clinical cases and positive reactors should be treated; (ii) the negative animals vaccinated; and (iii) the herd moved on the first day of treatment to a clean field. Retesting a group to determine the rate of spread would be an informative procedure but active measures must usually be commenced before this information is available. Another variation of this program, and a highly practical one, is the vaccination of all cattle in the herd, and the treatment with one dose of dihydrostreptomycin (25 mg/kg BW IM) of all pregnant cows to eliminate renal infection and leptospiruria. However, antimicrobial therapy is not highly efficacious, especially in cattle infected with hardjo.
A successful control strategy has been described for hardjo infection in a large, closed beef herd. All animals were treated with dihydrostreptomycin at 25 mg/kg BW IM once followed by removal to a clean pasture to prevent new cases, and annual vaccination of the whole herd for 5 years. All cattle introduced into the herd were treated with the antimicrobial and quarantined; at the end of the trial the entire herd was treated prophylactically with the antimicrobial to minimize the risk of residual infection. By the end of the trial all young stock entering the breeding program were seronegative. There was serological evidence of a high level of control and bacteriological monitoring at the end of the trial indicated that hardjo had been eliminated from the herd.
Vaccination is also recommended to protect animals continuously exposed to infection from wildlife, other domestic species, and rodents. The serological status of these groups can also be determined as necessary before a decision is made to vaccinate.
If only sporadic cases occur, it may be more profitable to attempt to dispose of reactors or treat them to insure that they no longer act as carriers. A degree of immunity is likely to occur in pigs after natural infection and when the disease is endemic, ‘herd immunity’ may significantly decrease incidence of clinical disease.
One of the theoretical disadvantages of vaccination is the possible development of renal carrier animals which are sufficiently immune to resist systemic invasion but not colonization of the kidney, which leads to the development of a carrier animal with transient leptospiruria. This may occur but not sufficiently frequently to invalidate vaccination.
Ellis WA. Leptospirosis as a cause of reproductive failure. In: Diagnosis of abortion. Vet Clin North Am Food Anim Pract. 1994;10:463-478.
Smith CR, Ketterer PJ, McGowan MR, Corney BGA. Review of laboratory techniques and their use in the diagnosis of Leptospira interrogans serovar hardjo infection in cattle. Aust Vet J. 1994;71:290-294.
Faine SB, Adler CA, Bolin CA, Perolat P. Leptospira and leptospirosis, 2nd edn. Melbourne: MedSci Press, 1999.
Marshall RB, Manktelow BW. Fifty years of leptospirosis research in New Zealand: A perspective. NZ Vet J. 2002;50:61-63.
O’Keefe JS. A brief review on the laboratory diagnosis of leptospirosis. NZ Vet J. 2002;50:9-13.
Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757-771.
Adler B. Pena-Moctezuma de la A. Leptospira. In: Gyles CL, Prescott JF, Songer JG, Thoen CO, editors. Pathogenesis of bacterial infections in animals. 3rd edn. Oxford: Blackwell Publications; 2004:385-396.
Levett PN. Leptospirosis: A forgotten zoonosis? Clin Appl Immunol Rev. 2004;4:435-448.
1 Kmety E, Dikken H. Classification of the species Leptospira interrogans and history of its serovars. Groningen, The Netherlands: University Press, 1993.
2 Faine SB, et al. Leptospira and leptospirosis, 2nd edn. Melbourne: MedSci Press, 1999.
3 Ellis WA. Vet Clin North Am Food Anim Pract. 1994;10:463.
4 Niang M, et al. Prev Vet Med. 1994;18:259.
5 Rocha T. Rev Sci Tech. 1998;17:699.
6 Cerri D, et al. Microbiologica. 2003;26:383.
7 Adler B, Pena-Moctezuma de la A. Leptospira. In: Gyles CL, Prescott JF, Songer JG, Thoen CO, editors. Pathogenesis of bacterial infections in animals. 3rd edn. Oxford: Blackwell Publications; 2004:385-396.
8 Langoni H, et al. Prev Vet Med. 1999;40:271.
9 Feresu SB, et al. Int J Syst Bact. 1998;48:207.
10 Naiman BM, et al. Infect Immun. 2001;69:7550.
11 Corney G.B., et al. Aust Vet J. 1996;73:75.
12 Guitian FJ, et al. Vet Microbiol. 2001;80:275.
13 Alonso-Andicoberry C, et al. Prev Vet Med. 2001;52:109.
14 Lilenbaum W, Souza GN. Res Vet Sci. 2003;75:249.
15 Black PF, et al. Aust Vet J. 2001;79:344.
16 Miller DA, et al. Am J Vet Res. 1991;52:1761. 1766
17 Burriel AR, et al. Vet Rec. 2003;153:146.
18 Ozdemir V, Erol E. Vet Rec. 2002;150:248.
19 Wilson PR, et al. NZ Vet J. 1998;46:131.
20 Gerritsen MJ, et al. Am J Vet Res. 1994;55:1232.
21 Ellis GR, et al. Aust Vet J. 1994;71:203.
22 Choi C, et al. Vet Rec. 2001;148:416.
23 Neto JSF, et al. Prev Vet Med. 1997;31:87.
24 Boqvist S, et al. Theriogenology. 2002;58:1327.
25 Van Til LD, Dohoo IR. Am J Vet Res. 1991;55:352.
26 Boqvist S, et al. Vet Microbiol. 2003;93:361.
27 Wada S, et al. Vet Ophthalmol. 2003;6:191.
28 Donahue JM, Williams NM. Vet Clin North Am Equine Pract. 2000;16:443.
29 Kinde H, et al. Equine Vet J. 1996;28:327.
30 Lees VW, Gale SP. Can. Vet J. 1994;35:635.
31 Barwick RS, et al. Prev Vet Med. 1998;36:153.
32 Rocha T, et al. Res Vet Sci. 2004;76:199.
33 Bender LC, Hall PB. J Wildlife Dis. 1996;32:121.
34 Webster JP, et al. Epidemiol Infect. 1995;114:195.
35 Mikaelian I, et al. Can Vet J. 1997;38:440.
36 Leonard F, et al. Vet Rec. 1992;131:53.
37 Bennett RM. Vet Rec. 1993;132:59.
38 Bharti AR, et al. Lancet Infect Dis. 2003;3:757.
39 Levett PN. Clin Appl Immunol Rev. 2004;4:435.
40 Marshall RB, Manktelow BW. Aust Vet J. 2002;50:61.
41 Talapada MD, et al. Vector Borne Zoonotic Dis. 2003;3:141.
42 Thornley CN, et al. Epidemiol Infect. 2002;128:29.
43 Belmaker I, et al. Isr Med Assoc J. 2004;6:24.
44 Campagnolo ER, et al. J Am Vet Med Assoc. 2000;216:676.
45 Simon MC, et al. Vet Rec. 1999;144:287.
46 Uzal FA, et al. Vet Microbiol. 2002;86:369.
47 Smith CR, et al. Aust Vet J. 1997;75:822.
48 Kalsow CM, et al. Ocul Immunol Inflamm. 1998;6:239.
49 Naiman BM, et al. Infect Immun. 2002;70:6147.
50 Dhaliwal GS, et al. Vet Rec. 1996;139:110. 139
51 Dhaliwal GS, et al. Vet Rec. 1996;138:272.
52 Guitian J, et al. J Am Vet Med Assoc. 1999;215:515.
53 Donahue JM, et al. J Vet Diag Invest. 1995;7:87.
54 Heineman MB, et al. Vet Microbiol. 2000;73:261.
55 Poonacha KB, et al. Vet Pathol. 1993;30:362. 369
56 Williams DM, et al. Equine Vet J. 1994;26:105.
57 Giles RC, et al. J Am Vet Med Assoc. 1993;203:1170.
58 Divers TJ, et al. J Am Vet Med Assoc. 1992;201:1391.
59 Bernard WV, et al. J Am Vet Med Assoc. 1993;203:276.
60 O’Keefe JS. NZ Vet J. 2002;50:9-13.
61 Surujballi O, Mallory M. Am J Vet Res. 2004;68:1.
62 Yan KT, et al. Vet Microbiol. 1999;69:173.
63 Woodward MJ, et al. Vet Rec. 1997;141:603.
64 Bolin CA, Alte DP. Bov Pract. 1999;33:50.
65 Leonard FC, et al. Vet Rec. 1995;131:435.
66 Leonard FC, et al. Res Vet Sci. 1993;55:195.
67 Dhaliwal GS, et al. Res Vet Sci. 1996;60:157. 163
68 Wagenaar J, et al. Am J Vet Res. 2000;61:316.
69 Richtzenheim LS, et al. Vet Microbiol. 2002;87:139.
70 Vemulapalli R, et al. J Vet Diag Invest. 2005;17:67.
71 Masri SA, et al. Can J Vet Res. 1997;61:15.
72 Heinemann MB, et al. Aust Vet J. 1999;77:32.
73 Faber NA, et al. J Clin Microbiol. 2000;38:2731.
74 Yener Z, Keles H. J Vet Med A Physiol Pathol Clin Med. 2001;48:441.
75 Uzal A, et al. Vet Microbiol. 2002;24:369.
76 Brown RA, et al. Vaccine. 2001;21:4448.
77 Parma AE, et al. Vet J. 1997;153:75.
78 Gerrisen MJ, et al. Am J Vet Res. 1994;55:339.
79 Alt DP, et al. J Am Vet Med Assoc. 2001;219:636.
80 Smith CR, et al. Aust Vet J. 1997;75:818.
81 Alt DP, Bolin CA. Am J Vet Res. 1996;57:59.