Chapter 29 Metabolic diseases
INTRODUCTION 1613
PRODUCTION DISEASES 1618
Among domestic farm animals, the metabolic diseases achieve their greatest importance in dairy cows and pregnant ewes. In the other species, these diseases occur only sporadically. The high-producing dairy cow always verges on abnormal homeostasis and the breeding and feeding of dairy cattle for high milk yields is etiologically related to metabolic disease so common in these animals.
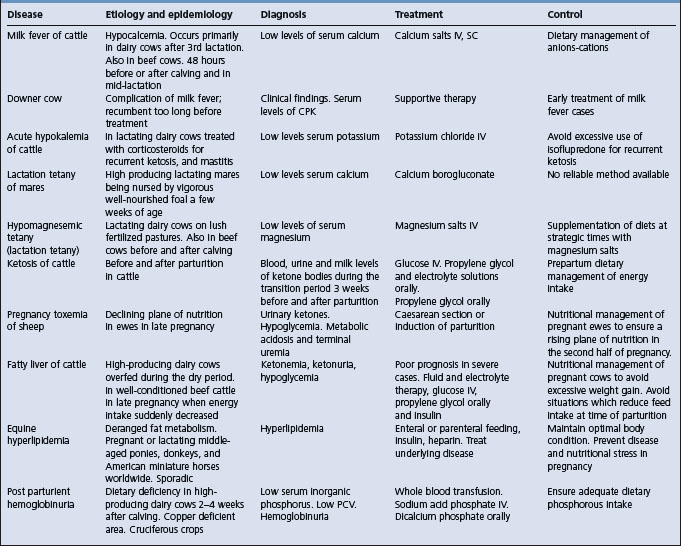
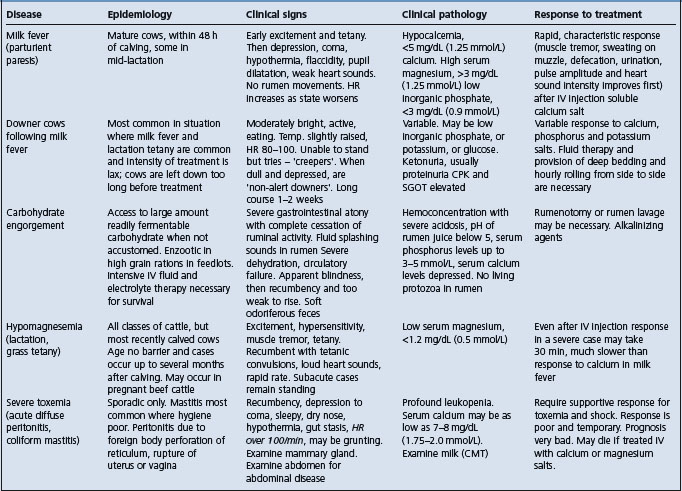
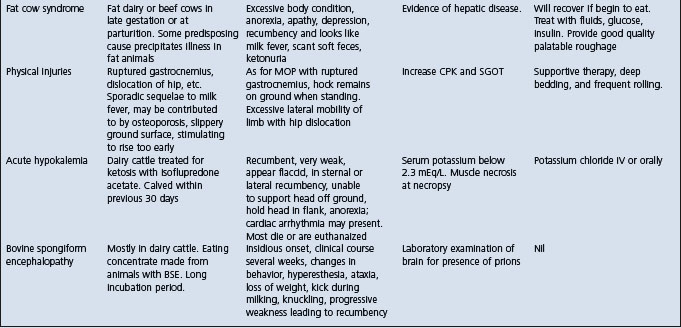
The salient features of the common metabolic diseases of farm animals are summarized in Table 29.1.
Periparturient period in cattle and sheep
As milk production in dairy cows increases and as herds become larger, the incidence of metabolic disease increases. In dairy cows, the incidence of metabolic diseases is highest in the period commencing at calving and extending until the peak of lactation is reached and their susceptibility appears to be related to the extremely high turnover of fluids, salts and soluble organic materials during the early part of lactation. With this rapid rate of exchange of water, sodium, calcium, magnesium, chlorides and phosphates, a sudden variation in their excretion or secretion in the milk or by other routes, or a sudden variation in their intake because of changes in ingestion, digestion or absorption, may cause abrupt, damaging changes in the internal environment of the animal. It is the volume of the changes in intake and secretion and the rapidity with which they can occur that affects the metabolic stability of the cow. In addition, if the continued nutritional demands of pregnancy are exacerbated by an inadequate diet in the dry period, the incidence of metabolic disease will increase. The effect of pregnancy is particularly important in ewes, especially those carrying more than one lamb.
Transition period in dairy cows
The literature on managing the transition period of the cow from 3 weeks before parturition to 3 weeks after parturition to optimize health and productivity has been reviewed.1 It is a crucial stage in the production cycle of the dairy cow; no other period can affect subsequent production, health, and reproductive performance so greatly.1,2 The success of the transition period effectively determines the profitability of the cow during that lactation. Nutritional or management limitations during this time may impede the ability of the cow to reach maximal milk production. The primary challenge faced by cows is a sudden and marked increase of nutrient requirements for milk production, at a time when dry matter intake and thus nutrient supply, lags far behind. Dry matter intake typically declines during the final week before parturition. This decline and changes in endocrine profiles contribute to elevated blood nonesterified fatty acids which have been related to the occurrence of lipid-related metabolic diseases such as fatty liver and ketosis. The magnitude of the decline in intake as parturition approaches may be a better indicator of metabolic health of post partum cows than level of intake. Diet, body condition score and parity influence dry matter intake and energy balance. The occurrence of diseases during the transition period results in lost milk production during the time of illness and often for the entire lactation.
A key area of the biology of transition cows is lipid metabolism.3 Excessive lipid metabolism from adipose tissue is linked with greater incidences of periparturient diseases. Fatty livers have been described in ketotic cows in the 1950s. Hepatic fat accumulation was then noted in normal cows during early lactation. This was followed by a description of a ‘fat mobilization syndrome’ in early lactation, in which cows mobilized body lipids from adipose tissue and deposited lipids in the liver, muscle, and other tissues. This was followed by descriptions of elevated non esterified fatty acid concentrations during the last 7 days before calving being associated with a greater incidence of ketosis, displaced abomasum and retained fetal membranes but not of milk fever. Understanding the metabolism of NEFA by the liver is a critical component of understanding the biology of the transition cow. Extreme rates of lipid mobilization lead to increased uptake of NEFA by the liver and increases triglyceride accumulation. If this lipid infiltration becomes severe, the syndrome of hepatic lipidosis or fatty liver may result, which can result in a prolonged recovery from other diseases, increased incidence of other diseases, and increased susceptibility to induction of ketosis.
It is now known that lipid metabolism in the prepartum dairy cow is important in the occurrence of displaced abomasum.4,5 Significant risk factors for displaced abomasum included a negative energy balance prepartum (as estimated from plasma NEFAs), a high body condition score, suboptimal feed bunk management prepartum, prepartum diets containing >1.65 Mcal of net energy for lactation/kg of dry matter, winter and summer seasons, high genetic merit and low parity.5
Metabolic tests can now be used to predict displaced abomasum in dairy cattle.4 In cows which develop a left-side displacement of the abomasum, mean NEFA concentrations begin to diverge from the mean in cows without LDA 14 days before calving, whereas mean serum β-hydroxybutyrate (BHBA) concentrations did not diverge until the day of calving.4 Prepartum, only NEFA concentration was associated with risk of subsequent LDA. Between 0 and 6 days before calving, cows with NEFA concentrations ≥0.5 mEq/L were 3.6 times more likely to develop a LDA after calving. For prospective application, among samples taken 4–10 days before expected calving, the optimum NEFA cutpoint remained 0.5 mEq/L. The sensitivity, specificity and likelihood ratio were 46%, 82%, and 2.6, respectively. Between 1 and 7 days post partum, retained placenta, metritis and increasing serum concentrations of BHBA and NEFA were associated with increased risk of subsequent LDA. The odds of LDA were eight times greater in cows with serum BHBA ≥1200 μmol/L. Cows with BHBA concentrations ≥1200 μmol/L were 3.4 times more likely to develop LDA. Serum calcium concentrations were not associated with LDA. In summary, strategic use of metabolic tests to monitor transition dairy cows should focus on NEFA in the last week prepartum and BHBA in the first week post partum.
The nutritional management strategies to optimize the metabolic health of transition cows has been reviewed.6 During the transition period, dairy cows undergo large metabolic adaptations in glucose, fatty acid, and mineral metabolism.6 The practical goal of nutritional management during this period is to support these metabolic adaptations. A 2-group nutritional strategy for dry cows to minimize overfeeding of nutrients during the early dry period but increase nutrient supply during the late dry period is now being recommended.6 Increasing the amount of energy supplied through dietary carbohydrate during the prepartum period results in generally positive effects on metabolism and performance of transition cows. But the form of that carbohydrate (whether starch or highly digestible neutral detergent fiber) may be of lesser importance. Attempts to increase energy supply by feeding dietary fat sources or decrease energy expenditure by supplying specific fatty acids such as trans-10, cis-12 conjugated linoleic acid to decrease milk fat output during early lactation do not decrease the release of nonesterified fatty acids (NEFA) from adipose tissue.
In addition to nutritional management strategies to optimize health of the transition cow, certain feed additives are in use to reduce subclinical ketosis and reduce the incidence of displaced abomasum.7 Monensin is a carboxylic polyether ionophore produced by a naturally occurring strain of Streptomyces cinnamonesis. Monensin exerts its many effects by shifting the microbial populations in the rumen. It changes the ratio of volatile fatty acids in the rumen, increasing propionic acid and reducing the molar percentages of butyric acid and acetic acid. Improved rumen propionic acid improves gluconeogenesis. When administered in controlled release capsule (CRC) 2–4 weeks before calving, monensin reduced the incidence of both clinical ketosis and displaced abomasum postcalving.8 In a large dataset, monensin showed a trend for a 25% reduction in the incidence of retained placenta. Monensin improves energy metabolism which reduces the incidence of all three ‘energy associated diseases’, retained placenta, displaced abomasum and clinical ketosis. In Canada, the monensin-controlled release capsule (CRC) is approved as an aid in the prevention of subclinical ketosis in lactating dairy cattle. The capsule delivers 335 mg of monensin daily for 95 days.9 Cows treated with the monensin CRC 3 weeks before calving had decreased NEFA and BHBA and increased concentrations of serum cholesterol and urea in the week immediately pre-calving. Monensin has no effect on calcium, phosphorus, or glucose in the pre-calving period. After calving, concentrations of phosphorus were lower and BHBA lower and cholesterol and urea higher in monensin-treated cows. The lower NEFA values indicate less fat mobilization and the higher cholesterol suggest greater lipoprotein export from the liver. The higher urea levels are thought to be due to a protein-sparing effect in the rumen, resulting in an increased supply of amino acids in the small intestine. There was no effect of treatment on NEFA, glucose, or calcium in the first week post-calving. Monensin treatment administered pre-calving significantly improved indicators of energy balance in both the immediate pre-calving and post-calving periods.
Abnormalities of the blood levels of the four macrominerals, calcium, phosphorus, magnesium, and potassium in the cow during the transition period are involved in subclinical hypocalcemia, clinical milk fever, hypomagnesemia, and acute hypokalemia.10
Knowledge of the complex behavioral needs of the dairy cow is essential in order to provide adequate housing during the transition period. In North American dairy herds, the flow of cows through the transition period often necessitates many changes of pens, which are disruptive to the social organization of cow groups. Stocking rates that exceed stall and feed bunk capacity place even greater challenges on the dairy cow at this time. Alternative strategies for cow grouping and improvements in pen and stall design which provide greater behavioral freedom for the dairy cow and improvements in health and productivity have been described.11
Management of the dairy cow at calving time is a major topic which requires that the veterinarian educate the animal attendants and owners.12 The objective is to ensure the delivery of a viable, live calf and smooth transition of the cow without complications, from the dry period to the lactation period. The two major problems encountered at calving time are dystocia and perinatal mortality.
The diagnosis and treatment of dairy cows with periparturient diseases requires a program suited to the particular herd.13 Particularly in large herds, there is a need for collaboration between the veterinarian, nutritionist, manager of the herd, and the animal attendants. Specific procedures should be developed for each herd based on past experience with the problems of recently-calved cows, the facilities, the skills of the workers, the priorities of management, and the flow patterns of cows in the herd. Every effort must be made to prevent periparturient diseases in the cows. In general, diseases in the early post partum period originate in the feeding and management of the dry cow. Important principles include a protocol of grouping parturient cows according to the feeding program and handling facilities on the farm. Groups of cows can be screened for mastitis, visual evidence of illness, daily milk yield, body temperature, urine pH, palpated for evidence of metritis. Individual cows which have been identified by a screening method must be examined individually to make a diagnosis and decide on a treatment protocol based on the particular diagnosis.
The use of reliable records to monitor the health and production of dairy cows during the transition period is essential to evaluate the efficacy of programs at the farm level.14 Monitors of transition cow management programs will assist in determining how well cows are prepared for milk production and good health in the coming lactation. Appropriate monitoring will focus on three areas: cows that die or are culled in early lactation, the productivity of the surviving cows in early lactation, and the rates of disease in the periparturient period.
Cows which leave the herd in the first 60 days of lactation are usually culled because of disease or injury.14 Removal rates and their causes can be a critical monitor of the efficacy of transition cow management programs. Measuring productivity and health of cows in early lactation involves monitoring peak milk, daily milk yields, first test mature equivalent 305-day projected milk, milk components at first Dairy Herd Improvement Association (DHIA) test day, milk fat percentage, ratios of test-day components, somatic cell count at first DHIA test day.14 DHIA records also allow comparison of the performance of each cow in early lactation to her performance in the prior lactation. Comparisons can be made of the changes in somatic cell count between the last test of the prior lactation and the first test of the current lactation and mature equivalent 305-day difference from the prior lactation to the first test of the current lactation
Health and production records in dairy herds have traditionally emphasized reproductive events and treatments given. The records should capture the information about the common diseases which occur in most dairy herds.14 The record system should be set up to:
• Monitor rates of well-defined disease events as a measure of the effectiveness of health and production programs and to aid problem solving
• Determine the clinical efficacy of treatments by monitoring retreatment rates for specific diseases
• Maintain an individual cow history record for cow-side use to enhance treatment decisions
• Measure compliance and consistency of implementation of the health program being used
• Reconcile pharmaceutical purchases with treatment protocol entries and to meet regulatory requirements on the use of pharmaceuticals in food animals
• Determine the costs of certain disease rates over achievable targets. The costs of specific diseases are compelling to most dairy herd producers. Good records can generate an incidence rate of common diseases. These costs include the immediate cost of treatment, the cost of the veterinarian’s and herdsman’s time and the cost of milk withheld from the market. For the majority of diseases of recently-calved cows, the cost per disease in the USA is about US$320.00 with a range from $150 to $450 as from the year 2001.
An adequate record system will allow producers and veterinarians to determine the differences between actual performance and benchmark performance and then determine the causes of the shortfall. The most important determinants of profitability on dairy farms are milk income and feed cost and the difference between milk income and feed costs is the return over feed index (ROF).15 Many factors affect the ROF index. These include: three times daily milking, component percentages in the herd milk test, milk fat and protein percentages, use of an E. coli mastitis vaccine and use of monensin in the lactating cow diet.15 One of the most important factors associated with profitability is milk production. From 80% to 95% of the income on dairy farms is derived from milk sales. Thus, it is critical that the producer, the veterinarian and other advisors collaborate to plan the animal health and production program which will result in the optimum ROF.
Other recent developments indicate that environmental and management factors can be manipulated to ease the transition into lactation.16 The photoperiod, defined as the duration of light exposure an animal receives within a day, can be adjusted to produce dramatic effects on periparturient health and subsequent lactational efficiency. Increasing the frequency of milking in the immediate post partum period also produces persistent increases in milk yield and improvements in mammary health. In both techniques, evidence is emerging to support the concept that alteration of prolactin sensitivity is the mechanism underlying health and production responses.
The prospect of 0 days for dry periods are being explored as a possible alternative management scheme in dairy herds.17 In high-producing dairy cows, the dry period is influenced by parity and management practice. Multiparous cows that were continuously milked and treated with bovine somatotrophin (bST) demonstrated negligible production losses in the next lactation. First-calf heifers, however, demonstrated large reductions in milk yield.
Voluntary dry matter intake in periparturient dairy cattle
The factors affecting voluntary dry matter intake (VMDI) of lactating cattle are extremely important and have received much attention for many decades. The literature on the integration of metabolism and intake regulation in animals has been reviewed.18 A substantial dip in VMDI is initiated in late pregnancy and continues into early lactation.18 Pregnancy in dairy heifers has been shown to reduce VMDI from week 26 of pregnancy by 1.53% (approximately 0.17 kg) per week until 3 weeks before calving. In one study, in which the energy density of the diet remained constant during the last 168 days of pregnancy, a similar decline in energy intake during the last trimester of pregnancy both in heifers and lactating cows when diet energy was high (11.6 MJ of metabolizable energy/kg of DM), while the decline was much smaller or insignificant at lower energy densities (10.3 or 8.3 MJ of metabolizable energy/kg of DM).18 The lowest VMDI occurs at calving. Post partum VDMI increases, but the rate varies widely. In cows given diets of constant composition, the milk yield typically peaks at 5–7 weeks post partum, while the maximum intake is reached between 8 and 22 weeks after calving. The increase in intake from week 1 post partum to time of peak intake varies from between 2% and 111%. These differences in intake are affected by the diet fed during lactation but may also depend on prepartum feeding by way of, at least in part, the influence and the degree of fatness and or body condition score (BCS) of the animal. Voluntary dry matter intake is considerably higher in multiparous cows compared with primiparous cows. The intake capacity of primiparous cows calving at an age of 2 years is only about 80% of that of multiparous cows in the first part of lactation. The normal pattern of intake may be severely influenced by disease states. Both clinical and subclinical infections are known to substantially reduce appetite and performance.
The dip in VMDI has traditionally been attributed to physical constraints such as the enlarging uterus but this role may be overemphasized. The dip coincides with changes in reproductive status, fat mass and metabolic changes in support of lactation. A number of metabolic signals may have a role in intake regulation. These signals include nutrients, metabolites, reproductive hormones, stress hormones, leptin, insulin, gut peptides, cytokines and neuropeptides such as neuropeptide Y, galanin and corticotrophin-releasing factor.18
During late pregnancy and lactation, energy requirements increase considerably. Fetal energy requirements on day 250 of pregnancy have been calculated at 2.3 Mcal/d for Holstein cows. During lactation, energy requirement is increased to 26 Mcal net energy in cows producing 30 kg milk/day. Major changes in metabolism occur to cope with this increase in nutrient requirements. On a diet high in energy density, pregnant heifers will have a relatively high plasma concentration of glucose and a relatively low concentration of NEFA. Post partum, the concentration of NEFA is high while glucose is reduced. These changes reflect the large need for glucose and nutrients by the mammary gland and that dairy cows increase the use of lipid as a source of energy to support lactation. The NEFA begins to rise 2–3 weeks before calving and peaks at calving or during the first week of lactation. Glucose increases during the last week before calving and drops abruptly post partum to reach a minimum of 1–3 weeks into lactation. Post partum changes in the plasma concentration of BHBA are generally opposite those of glucose.
Immunosuppression during the transition period
In addition to the adaptations in classical metabolism which occurs during the transition period, cows during this period undergo a period of reduced immunological capacity during the periparturient period.19 The immune dysfunction is broad in scope, affects multiple functions of various cell types and lasts about 3 weeks prior to calving until about 3 weeks after calving. Cows during this period are more susceptible to mastitis. The etiology of periparturient immunosuppression is multifactorial and not well understood but seems to be related to physiologic changes associate with parturition and the initiation of lactation and to metabolic factors related to these events. Glucocorticoids are immunosuppressants, are elevated at parturition and have been postulated to have a role in periparturient immunosuppression.
The effects of parturition on cytoplasmic glucocorticoid expression (GR) in neutrophils and the correlation of the expression with serum cortisol concentration and total leukocyte count and neutrophil counts in periparturient cows has been examined.20 Neutrophils from periparturient cows had a 49% reduction in GR expression at calving, compared with GR expression 2–4 weeks before calving and 39% reduction, compared with neutrophils from cows in mid-pregnancy. The reduction in neutrophil GR expression is detectable 1 week before calving and most severe at calving and 24 h after calving. Multiparous cows have prolonged GR down-regulation of their neutrophils compared with primiparous cows which may be associated with a higher incidence of mastitis in older cows. Serum cortisol concentrations and total leukocyte and neutrophil counts were significantly increased at calving and returned to baseline value by 24 h after calving. Thus, a cortisol-induced neutrophil GR down-regulation and neutrophil migration dysfunctions occur in periparturient dairy cows. There is impaired expression of adhesin molecules and decreased migration capacity of blood neutrophils. Rapid recruitment of neutrophils into newly infected mammary tissue is the key immunologic defense against mastitis-causing pathogens in ruminants.
Experimentally, nonesterified fatty acids in vitro significantly reduces immunosuppressiveness of mononuclear cells of ewes which may be associated with the impairment of cell-mediated and humoral immunity in sheep and cattle with ketosis.21
Because vitamin E is a fat-soluble membrane antioxidant which enhances the functional efficiency of neutrophils by protecting them from oxidative damage following intracellular killing of ingested bacteria, the parenteral administration of vitamin E has been explored for the prevention of peripartum diseases such as retained placenta, metritis, and clinical mastitis. Only cows with marginal vitamin E status (serum α-tocopherol <2.5×10−3) 1 week before calving will have a reduction in the risk of retained placenta following a subcutaneous injection of 3000 IU of vitamin E.22 In cows with an adequate level of serum vitamin E there was no reduction and primiparous animals were most likely to benefit from vitamin E 1 week before parturition. The associations between peripartum serum vitamin E, retinol and β-carotene in dairy cattle and disease risk indicated that an increase in α-tocopherol of 1 μg/ML in the last week prepartum reduced the risk of retained placenta by 20%, whereas serum NEFA concentrations ≥0.5 mEq/L tended to increase the risk of retained placenta by 80%. In the last week prepartum, a 100 ng/mL increase in serum retinol was associated with a 60% decrease in the risk of early lactation clinical mastitis.23
Diseases of lactation
In the next phase of the production cycle, parturition is followed by the sudden onset of a profuse lactation which, if the nutrient reserves have already been seriously depleted, may further reduce them to below critical levels and clinical metabolic disease then occurs. The essential metabolite which is reduced below the critical level determines the clinical syndrome which will occur. Most attention has been paid to variations in balances of calcium and inorganic phosphates relative to parturient paresis; magnesium relative to lactation tetany; blood glucose and ketones and hepatic glycogen relative to ketosis; and, potassium relative to hyperkalemia on cereal grazing, but it is probable that other imbalances are important in the production of as yet unidentified syndromes.
The vast majority of production diseases of dairy cows occur very early in lactation. At this time, the cow is producing milk at a rate which is substantially less than her maximum. In terms of rate, high and low milk yielding cows are producing rather similar amounts at this time. However, in terms of acceleration, the change in milk yield per day, it is highest immediately after calving.
During the succeeding period of lactation, particularly in cows on test schedules and under the strain of producing large quantities of milk, there is often a variable food intake, especially when pasture is the sole source of food and instability of the internal environment inevitably follows. The period of early lactation is an unstable one in all species. Hormonal stimulation at this stage is so strong that nutritional deficiency often does not limit milk production and a serious drain on reserves of metabolites may occur.
Recombinant bovine somatotrophin.
Recombinant bovine somatotrophin (rBST) is a synthetically derived hormone that may be identical to naturally occurring bovine growth hormone, or slightly modified by the addition of extra amino acids. The product was approved in the USA in 1993 and its use began commercially in 1994 in dairy herds to increase milk production.24 A meta-analysis of the effects of rBST on milk production, animal health, reproductive performance and culling has been done in Canada and the drug was not approved for use. Recombinant bovine somatotrophin was found to increase milk production by 11.3% in primiparous cows and 15.3% in multiparous cows; although there was considerable variation between studies. While some statistically significant effects on milk composition (percentage of butterfat, protein, and lactose) were found, they were all very small. Treatment increased dry matter intake by an average of 1.5 kg/d during the treatment period and dry matter intake remained elevated on into the first 60 days of the subsequent lactation. Despite the increase in dry matter intake, treated animals had lower body condition scores at the end of the treatment period and the reduced scores persisted through until the start of the subsequent lactation. Recombinant bovine somatotrophin increased the risk of clinical mastitis by approximately 25% during the treatment period but there was insufficient data to draw firm conclusions about the effects of the drug on the prevalence of subclinical intramammary infections. Use of rBST increased the risk of a cow failing to conceive by approximately 40%. For cows which did conceive, there was no effect on services per conception and only a small increase in average days open. Use of the drug had no effect on gestation length, but the information about a possible effect on twinning was equivocal. Cows treated with rBST had an estimated 55% increase in the risk of developing clinical signs of lameness. There appeared to be an increased risk of culling in multiparous cows. Use of the drug in one lactation period appeared to reduce the risk of metabolic diseases (particularly ketosis) in the early period of the subsequent lactation. The reproductive effects of the drug could be controlled by delaying its use until the cows were confirmed pregnant.
In 1998, an expert panel appointed by the Canadian Veterinary Medical Association at the request of Health Canada, found a number of legitimate animal welfare concerns associated with the use of rBST. In 1999, Health Canada announced that it would not approve the use of rBST for sale in Canada. The Royal College of Physicians and Surgeons of Canada Expert Panel on Human Safety of rBST found no biologically plausible reason for concern about human safety if rBST were to be approved for sale in Canada.
In 1999, a working group from within the Scientific Committee on Animal Health and Animal Welfare of the European Commission presented a more extensive report which summarized similar results and engaged substantive discussion of animal welfare from the points of view of physiologists and epidemiologists. It concluded that rBST should not be used in dairy cattle. In October 1999, the European Commission banned the use of and marketing of rBST in the European Union as of 1 January, 2000.25 The animal welfare aspects of the use of rBST and the laws and ethical issues, data analysis, epidemiologic evaluation, and public policies involved for the different reasons made in the USA, Canada, and Europe with regard to the use of rBST in dairy cattle have been reviewed and discussed elsewhere.25,26
A comprehensive econometric model was developed to evaluate the potential effects of rBST approval on the Japanese dairy industry.27 Simulation results indicate that rBST approval would accelerate structural change in Japan’s dairy industry toward fewer, larger farms. Negative effects of rBST on farm income are projected to be more severe for smaller farms, because of higher costs, lower profit-earning ability, lower milk yields, and lower adoption rates of rBST. Larger farms would benefit from rBST adoption if milk demand is maintained. However, if public health concerns about rBST induce significant milk demand decreases, even the largest farms’ income and cow numbers would decrease. Thus, Japan’s dairy industry could be caught in a double downward spiral of declining milk prices and production.
Breed susceptibility
The fact that some dams are affected much more by these variations than others is probably explainable on the basis of variations in internal metabolism and degree of milk production between species and between individuals. Between groups of cows, variations in susceptibility appear to depend on either genetic or management factors. Certainly, Jersey cows are more susceptible to parturient paresis than cows of other breeds and Guernseys seem to be more susceptible to ketosis. Even within breeds, considerable variation is evident in susceptibility between families. Under these circumstances, it seems necessary to invoke genetic factors, at least as predisposing causes.
Management practices
The management practices of most importance are housing and nutrition. In those sections of North America where cattle are housed during the winter and in poor pasture areas, ketosis is prevalent. In the Channel Islands, local cattle are unaffected by lactation tetany, whereas the disease is prevalent in the UK. In New Zealand, metabolic diseases are complex and the incidence is high, both probably related to the practice of having the cows calve in late winter when feed is poor and to the practice of depending entirely on pasture for feed and to the high proportion of Jerseys in the cattle population.
A knowledge of these various factors is essential before any reasonable scheme of prevention can be undertaken. It should also indicate that although the more common disease entities are presented in this chapter, there is high probability that a disturbance of more than one of the metabolites mentioned may occur simultaneously in the one animal and give rise to complex syndromes which are not described here. The disease entities dealt with must be considered as arbitrary points in a long scale of metabolic disturbances.
Occurrence and incidence of metabolic diseases
A knowledge of the etiological and epidemiological factors involved will help in understanding the occurrence and incidence of the various metabolic diseases.28 Largely because of variations in climate, the occurrence of metabolic disease varies from season to season and from year to year. In the same manner, variations in the types of disease occur. For example, in some seasons, most cases of parturient paresis will be tetanic; in others, most cases of ketosis will be complicated by hypocalcemia. Further, the incidence of metabolic disease and the incidence of the different syndromes will vary from region to region. Ketosis may be common in areas of low rainfall and on poor pasture. Lactation tetany may be common in colder areas and where natural shelter is poor. Recognition of these factors can make it possible to devise a means whereby the incidence of the diseases can be reduced.
The metabolic diseases, because of high prevalence and high mortality rate, are of major importance in some countries, so much so that predictive systems are being set up. Rapid analysis of stored feed samples, pasture and soil is commonly used in Europe and North America but the interesting development has been the recognition of ‘production diseases’ and the consequent development of metabolic profile tests, particularly in the UK and in Europe.
Production diseases
The term ‘production disease’ includes those diseases previously known as ‘metabolic diseases’, such as parturient paresis (milk fever), hypomagnesemia, acetonemia, and perhaps some other conditions, all of which are attributable to an imbalance between the rates of input of dietary nutrients and the output of production. When the imbalance is maintained, it may lead to a change in the amount of the body’s reserves of certain metabolites, or their ‘throughput’ and sufficiently large changes in throughput. The generalization applies principally to the hypoglycemias (ketosis) and hypomagnesemias and partly to the hypocalcemias. In these diseases, output is greater than input either because of the selection of cattle which produce so heavily that no naturally occurring diet can maintain the cow in nutritional balance, or because the diet is insufficient in nutrient density or unevenly balanced. For example, a ration may contain sufficient protein for milk production but contains insufficient precursors of glucose to replace the energy excreted in the milk. While agreeing with the generalization on which the term ‘production disease’ is based, we propose to continue to use the expression ‘metabolic disease’ because of common usage.
Relationship between lactational performance and health of dairy cattle
The literature on the relationship between lactational performance and health in dairy cattle has been reviewed.29 Based on a review of 11 epidemiological and 14 genetic studies there was little evidence that high yielding cows have increased risk of dystocia, retained placenta, metritis, and left-side displacement of the abomasum. The results for periparturient diseases were inconsistent. While no phenotypical relationship between milk yield and the risk of ketosis and lameness was found, selection for higher milk yield will probably increase the lactational incidence risk for these diseases. Mastitis was the only disease where there was a clear relationship between milk yield and risk of infection. Continued selection for high milk yield will worsen this situation.
However, some authors claim that ‘Reviewing existing literature, even with structured literature selection, is inadequate to the task of elucidating the relationship between the lactational performance and risk of production diseases’.29 The most notable feature of the literature evaluation is the large variability that exists between studies. This strongly suggests that there are important factors that need to be considered before meaningful conclusions concerning the relationship between lactational performance and risk of disease can be drawn.
COMPTON METABOLIC PROFILE TEST
Because of the emphasis on health management beginning in the 1970s, it became popular to explore methods of predicting the occurrence of metabolic disease in advance, so that control strategies could be considered and put into place. It was thought possible to predict the occurrence of production disease in a dairy herd by monitoring certain components of the blood on a regular basis. If the blood level fell below ‘normal’, it was assumed that intake needed to be increased to compensate for the negative balance created by excessive output.
The Compton metabolic profile is based on the concept that the laboratory measurement of certain components of the blood will reflect the nutritional status of the animal, with or without the presence of clinical abnormalities. For example, a lower than normal mean blood glucose in a group of dairy cows in early lactation may indicate an insufficient intake of energy which may or may not be detectable clinically. On a theoretical basis, the ability of the laboratory to make an objective assessment of the input–output (nutrient–productivity) relationships is an attractive tool for the veterinarian engaged in providing a complete health management service to a herd. The test would theoretically be able to detect the qualitative and quantitative adequacy of the diet of cows expected to produce a certain quantity of milk or return to estrus within a desirable length of time following parturition. A reliable test for the early diagnosis of nutritional deficiency or metabolic disease would be a major step forward in attempting to optimize livestock production and obtain maximum yields at minimum costs.
Some of the literature on metabolic profile testing in dairy cows has been reviewed.30-32 The use and interpretation of metabolic profiles in dairy herds in the Dairy Herd Health and Productivity Service (DHHPS) have been reviewed.33
Methods are needed to monitor nutritional and metabolic status of dairy herds. The most valuable methods will be those which are sensitive enough to detect change before clinical or economic consequences are manifested. A major challenge in the application of metabolic profile testing is dealing with extraneous sources of variation. Successful management of extraneous variation requires sampling strategies based on animal grouping and testing of multiple animals. Larger herds may be more suitable because they are able to better design sampling strategies and to spread the costs of testing across more animals. In addition, the cost–benefit may be greater in larger herds because of the high cost of inadequate feeds and feeding programs. Statistical Process Control methods offer a unique approach to interpretation which may increase the usefulness of metabolic profiles.30
There was considerable interest in the test following its earlier descriptions which stimulated considerable field research. The results of the research have thus far indicated that the test may be useful only as an aid in the diagnosis of nutritional imbalance and production diseases. The results of the test are usually difficult to interpret without a careful conventional assessment of the nutritional status and reproductive performance of the herd and it appears doubtful that the test would reveal significant abnormalities which could not be detected using conventional clinical methods. There was considerable controversy about the practicality of the test. Because of costs of the test, the profile testing must be carefully planned with specific objectives. A regional diagnostic laboratory with automated analytical equipment should be available and this is often a major limiting factor. The test should not be undertaken unless normal values for each laboratory measurement are available from the population within the area. The results from the groups within the herd are compared with local population means. Metabolic profiles have also been suggested as an aid in the selection of superior individuals.
METABOLIC PROFILES FOR INDIVIDUAL COWS
The prediction of whether an individual cow is metabolically within normal range to undergo a stressful lactation at a high level of production would seem to be a useful undertaking. This could be particularly important under management conditions of heavy concentrate feeding, lead feeding, or zero grazing or even indoor housing. There are no well-established protocols for conducting such profile tests. The ‘parturition syndrome’, dealt with later under the ‘fat cow syndrome’ is considered to be predictable by the estimation of blood levels of total cholesterol and glutamic oxalate transaminase. In pastured cattle in New Zealand, the test has been found to be ineffective. Similar tests conducted on individual cows using many serum enzymes and electrolytes as indicators have not proved to be useful if used on only one occasion.
Usefulness of metabolic profile testing
Metabolic profiles in dairy cows were used initially in Britain in the 1960s. Success was limited primarily by the unjustified expectation that all biochemical concentrations in the blood of cows would reflect nutritional intake and status at all times.34 However, the practical value was found in the approach as an aid to nutritional management. Later, in the 1970s the approach was reassessed and reinstituted, culminating in a program for farmers evaluating health and productivity using metabolic profile testing as an integral part of a health management program involving a multidisciplinary approach. The system now depends on a team approach involving farmer, veterinarian, and agricultural adviser. The blood testing part, if useful information is to be obtained, depends critically on following a set of firm criteria for selection of small groups of typical cows within each herd, the timing of testing in relation to concentrate feeds, feed changes, and stage of lactation and the collection of other data about the cows such as body weight and condition, productivity and feeding. The successful approach has been to look, following specific times of nutritional change, at metabolite levels in strictly defined small representative groups of cows within each herd in conjunction with information on body condition and weight, milk performance, and feeding. Comparison with optimum values, the degree of variation from them and comparisons between groups within herds have allowed information about nutritional constraints on productivity to be made available to farmers more quickly and more specifically than by other means.
Most metabolic profile testing has been used in temperate climates. The effectiveness of the technique for identifying constraints on productivity in small herds in tropical and sub-tropical countries has been examined.34 The study involved 13 projects with 80 cows in each, done in six Latin American, six Asian, and one southern European countries. Data were also collected on feeding, body condition score and weight change, parasitism, and reproduction. In Chile, Mexico, Paraguay, Philippines, Uruguay, and Venezuela, globulin levels were high in >17% of cows samples on each occasion. In Paraguay, 49% of cows had high globulin levels at 2–3 months after calving. This suggests that inflammatory disease was present although this was not always investigated. In all countries except Mexico and Venezuela, high β-hydroxybutyrate levels before calving in many cows highlighted the presence of body condition loss in late pregnancy, an important potential constraint on productivity and fertility. Fewer cows had high BHB levels in lactation, whereas change in BCS and weight was more sensitive for measuring negative energy balance. Urea concentrations were low in only small numbers of cows suggesting that dietary protein shortages were not common. Albumin levels were low mainly in cows where globulin values were high and therefore did not provide additional information. In China, pregnant yaks over winter had high BHB and low albumin values, suggesting that they were seriously underfed. This resulted in a successful nutritional intervention in the following winter. Inorganic phosphorus values were within the reference range in most countries most of the time, suggesting, contrary to expectation, that this mineral was not commonly a constraint. In summary, the use of metabolic profile testing proved valuable in drawing attention to important potential constraints on productivity in dairy cows in tropical and subtropical environments and in confirming those which were not.34
Metabolic profile testing has been used for the prevention of periparturient diseases in dairy cows in Japan.35 In herds with a high incidence of periparturient disease, low blood values of hematocrit, albumin, glucose, cholesterol, calcium, and magnesium were observed in the dry period. The values correctly diagnose malnutrition as the cause of the periparturient diseases. Following feeding management changes, there was a low incidence of these diseases and the metabolic profiles were normal indicating that feeding management had improved. Because the traditional metabolic profile test is difficult to apply to peripartum cows because they are in state of physiological abnormality and the results are difficult to interpret, doing a test every 10 days during the dry and lactation periods has been evaluated.36 The criteria were interpreted by the deviations from the reference mean values of metabolites rather than the actual values. The body condition score, albumin, blood urea nitrogen, glucose, total cholesterol, non-esterified fatty acids, γ-glutamyl transpeptidase, and aspartate aminotransferase, fluctuated during the dry and early lactation periods and there were large changes in the hematocrit, blood urea nitrogen, total cholesterol, and magnesium and high nonesterfied fatty acids in herds with a high incidence of peripartum diseases.
The values of the variables which deviated from the reference values for the metabolic profile components were able to assess milk production and feeding which is a practical tool for auxiliary feeding evaluation.37
The Dairy Herd Health and Productivity Service (DHHPS) in the UK provides the opportunity for veterinarians to lead a multidisciplinary team which can monitor health, fertility, and production and can plan, when necessary, corrective action.38 Metabolic profiling and body condition scoring found that at least a third of the cows sampled were mobilizing excessive fat during the transition from the dry period to early lactation. Improving both health and nutrition, before and after calving, would improve reproductive performance in many herds. A team approach, with farmers, veterinarians, nutritionists, and other advisors working together with well defined goals and objectives, is necessary if progress is to be made in improving reproductive performance. High milk yields cannot always be the excuse for suboptimal fertility.38
Biological and statistical basis for herd testing
The interpretation of herd-based tests for metabolic diseases is different from interpreting laboratory tests for metabolites from individual cows.39 Test results from individual cows are interpreted by comparing the value to a normal reference range established by the laboratory that did the testing. Normal ranges are often derived by calculating a 95% confidence interval (or a similar statistic) of test results from 100 or more clinically normal animals.
Herd test results for metabolic diseases can be interpreted as either the mean test result of the subgroup sampled or as the proportion of animals above or below a certain cut point within the subsample. If a metabolite is associated with disease when it is above or below a biologic threshold (cut point) then it should be evaluated as a proportional outcome. For example, subclinical ketosis in dairy herds can be monitored by testing for β-hydroxybutyrate (BHBA) or other ketone bodies in blood or milk. Subclinical ketosis is a threshold disease and cows are affected only when ketone concentrations are elevated. Blood BHBA concentrations above 1400 μmol/L is the most commonly used cut point for subclinical ketosis. Early lactation cows with BHB concentrations above this cut point are a threefold greater risk to develop either clinical ketosis or displaced abomasum. Non-esterified fatty acids (NEFA) concentrations in blood are an indicator of negative energy balance in prepartum cows. Elevated NEFAs before calving are associated with increased risk for displaced abomasum after calving. A threshold above 0.400 mEq/L for cows between 2 and 14 days of calving is suggested as an appropriate cut point.
It is also necessary to determine the alarm level for the proportion of animals above or below the described cut point. The alarm level is determined from research results or clinical experience. The suggested alarm level proportions for BHBA with a cut point of =1400 μmol/L is >10% and for NEFAs with a cut point of =0.400 mEq/L is >10%.
Herd-based testing is useful only when a sufficient number of cows within the herd are tested, which gives reasonable confidence that the results truly represent the entire population of eligible cows in the herd. The minimum sample size for herd-based tests with proportional outcomes is 12 cows. Cows to be sampled need to be selected from the appropriate eligible or at risk group.
The ‘proper’ use of metabolic profiles depends on care with the timing of blood tests, the selection of cows to be included and the collection and use of background information about the farm, feeding, and feeding system and physical state and performance of the cows.40
As of 2005, for 5 years the Dairy Herd Health and Productivity Service (DHHPS) in the UK have been using the metabolic profile approach as an aid to the management of dairy cow nutrition. Effectively the approach has been to ‘ask the cows’ what they think of their nutrition – by following a set of ‘rules’ on timing, cow selection and the use of background information.33 The involvement of a team approach at the farm, including private veterinarian and nutritional adviser, to put the findings in the correct context and to identify the appropriate responses has been vital. Data on health and fertility from many of these farms has also been collected.
Variables in dairy-herd metabolic profile testing
Energy balance
Non-esterified fatty acids (NEFAs)
Non-esterified fatty acids are a sensitive indicator of energy balance. They are useful for monitoring energy status of dry cows in the last month of gestation, when rapid changes in energy balance status may not be detectable from changes in body condition score.31 High values of NEFAs indicate negative energy balance which occurs in animals which are inappetent for any illness.
The serum levels of NEFAs have been monitored in dairy cows as predictors of displaced abomasum.41 In cows with LDA, mean NEFA concentration began to diverge from the mean in cows without LDA 14 d before calving, whereas mean serum β-hydroxybutyrate (BHBA) concentrations did not diverge until the day of calving. Prepartum, only NEFA concentration was associated with risk of LDA. Between Day 0 and 6 days after calving, cows with NEFA concentration of ≥0.5 mEq/L were 3.6 times more likely to develop LDA after calving. Strategic use of metabolic tests to monitor transition dairy cows should center on NEFA. In the last week prepartum and BHBA in the first week post partum.41 In another study, cows with plasma NEFA >0.3 mEq/L between 3 and 35 days before calving were twice as likely to subsequently have an LDA.42 In cows with serum BHBA ≥1200 or 1400 μmol/L in the first week post partum the odds of LDA were three and four times greater, respectively, than in cows with BHBA below the cut points.43
Serum β-hydroxybutyric acid (BHBA)
Serum BHBA concentrations are affected by energy and glucose balance and are a less specific indicator of energy balance than plasma NEFA. High values are associated with reduced milk production, increased clinical ketosis and LDA and reduced fertility.30 The gold standard test for subclinical ketosis is blood BHBA which is more stable ketone body than acetone or acetoacetate. Subclinical ketosis may start at serum concentrations above 1000 μmol/L. The alarm level for the proportion of cows above the cut-point of 1400 μmol/L has not been determined but it is suggested that no more than 10% subclinical ketosis should be tolerated in early lactation cows.32 Serum concentrations of 1400 μmol/L or greater in the first 2 weeks post-calving was found to cause a three-fold greater risk for cows to subsequently develop either clinical ketosis or LDA.44
Between 1 and 7 days post partum, retained placenta, metritis and increasing serum concentrations of BHBA and NEFA were associated with increased risk of subsequent LDA. The odds of LDA were eight times greater in cows with serum BHBA = 1200 μmol/L were 3.4 times more likely to develop LDA.41
Blood glucose
Blood glucose concentrations are usually lower in early lactation and during the winter months; in early lactation, there is a heavy demand for glucose and during the winter the energy intake is likely to be lower than necessary to meet requirements. One major cause of variation in blood glucose may be the major fluctuations in daily feed intake. Investigations of feed intake of dairy cows on commercial farms have shown that concentrate dispensers are commonly incorrectly adjusted and errors of more than 50% in feed intake are sometimes found. In situations of marginal energy imbalance, blood glucose concentration levels may be unreliable as an index of the adequacy of energy intake. Several factors may cause short-term changes in blood glucose. Blood glucose decreases at the time of milk secretion, which makes sampling time critical. Blood glucose may also be influenced by the chemical nature of the carbohydrate and physical form of the feed and the roughage content of the feed. In addition, elevation of blood glucose has been associated with excitement and low environmental temperature.
There is some conflicting evidence about the relationship between mean values of blood glucose of a lactational group and insufficient energy intake and reproductive inefficiency. In some work, there is an expected relationship between low blood glucose and an increased incidence of ketosis. In others, the relationship is not clear, however there was a more consistent relationship between the actual energy intake as a percentage of requirement and the plasma non-esterified fatty acids, but this finding was not sufficiently reliable to be useful. The mean plasma glucose concentrations within 3 days before or after first service of cows which conceived on first service was higher than that of cows which returned, but the difference was only approaching significance at the 5% level and it is doubtful whether this could be of practical value. Although free fatty acids are more sensitive than blood glucose as an indicator of energy status of the lactating cow, the excessive variability of this relationship during early lactation limits its usefulness. Free fatty acids begin to increase several weeks prepartum, peak at parturition and decrease gradually to normal levels after several weeks of lactation. Blood glucose levels follow a similar pattern; however, there may be a period in early lactation when blood metabolite levels and particularly free fatty acids, are not entirely responsive to energy intake, but are perhaps under additional hormonal regulation.
Protein nutrition and metabolism
Urea nitrogen testing to evaluate protein
Milk urea N (MUN) can be used as a management aid to improve dairy herd nutrition and monitor the nutritional status of lactating dairy cows. Urinary N (UN) excretion has been shown to have a positive linear relationship with MUN. Elevated MUN indicates excess protein has been fed to the dairy cow for her given level of production. When adequate energy is in the diet of ruminants, both blood urea nitrogen and milk urea nitrogen have long been known to be indicators of their protein status. Increases in plasma urea concentration and ammonia occur primarily as a result of inefficient nitrogen utilization. An excess of rumen degradable protein results in an increase in the concentration of rumen ammonia, which is absorbed through the rumen wall and transported to the liver, where it is converted to urea. The catabolism of body protein for gluconeogenesis can also result in the production of ammonia, which is also converted to urea in the liver. Plasma urea has been the most commonly used blood constituent for monitoring protein status and intake. Urea moves passively from the blood into the milk and there is a close relationship between its concentrations in the two fluids. Thus, milk urea has been used as a non-invasive substitute for the measurement of the protein status and protein intake of ruminants. There is also a relationship between urinary nitrogen excretion (UN) and milk nitrogen (MUN). It has been estimated that when dietary energy remained unchanged, milk urea concentration increased by 12–18 mg/L for each additional 60 g of digestible crude protein fed to cows already receiving adequate protein.45 Milk samples should be submitted to an accredited diagnostic laboratory for MUN analysis. The Azotest Strip, an on-farm dipstick test, lacks accuracy and is not recommended.46
The milk urea nitrogen target concentrations for lactating dairy cows fed according to National Research Council Recommendations have been evaluated.47 Target N ranges from approximately 150 to 200 g/d. The target MUN concentrations are now 8.5–11.5 mg/dL for most dairy herds compared with the previous target concentrations of 12–16 mg/dL.47,48 Milk urea, together with percentage milk protein is being used increasingly as an indicator of the dietary protein–energy balance. In many European countries and North American states and provinces, UREA analyses are available in Dairy Herd Improvement (DHI) programs. The somatic cell count did not lower UREA concentrations in quarters with elevated SCC.49 The time of sampling can have a significant effect on UREA concentrations; the highest in the morning and the diurnal pattern was not influenced by intrinsic factors like parity, days post partum or daily milk yield.49 The levels were significantly increased after refrigeration for 1 week.
Providing dairy farmers with information regarding their herd’s MUN should result in more accurate feed management and change toward target values. A survey of dairy farmers in a region in Virginia and Maryland indicated that 89.5% did not routinely use MUN prior to participating in the project, but most (88%) extension agents and nutritionists in the region recommended it. Providing MUN results and interpretive information to farmers changed feeding practices and subsequent MUN results.50
High milk yield in dairy cows is dependent on high intakes of dietary protein (17–19% crude protein) and energy. However, in the ruminant, high protein diets may be associated with reduced reproductive performance. In the UK, dairy cattle are housed over the winter months and turned out to graze in the spring. Spring turnout coincides with the first flush of new pasture growth which can contain very high levels of rapidly degradable protein. Many dairy herds in the UK experience a short-term fall in pregnancy rates at spring turnout. It has been suggested that the problem at turnout is worst on pastures heavily fertilized with nitrogenous fertilizers.
Several reviews of the literature have examined the effect of protein nutrition on reproduction in dairy cows. The reported effect of high nitrogen intake on fertility is inconsistent. Experimentally, the ingestion of a high level of degradable protein commencing 10 days before insemination in lactating dairy cows had no effect on reproductive performance of the lactating high yielding dairy cow.51 The relationship between milk urea concentration and the fertility of dairy cows from 250 herds in the UK found no relationship between bulk milk urea concentration and fertility, or between changes in bulk milk urea concentrations and fertility.45 Also, the relationship between the milk urea concentration 5 days after service and the fertility of individual cows was examined. There was no significant difference between the milk urea concentration of the cows which became pregnant and those that did not.
A meta-analysis of the literature evaluated the associations between dietary requirements for protein for dairy cattle, the metabolism of protein in cattle, factors influencing the degradability of protein in ruminant feeds, and factors influencing milk urea concentrations.52 There are good correlations between dietary protein intake and rumen ammonia, blood urea, and milk urea concentrations. The effect of increasing dietary protein on milk production has been defined through feeding trials and modeling methods used to provide feeding standards. Ryegrass clover pastures provide feed in many of the temperate dairy regions of the world and for much of the year pasture crude protein may exceed 30%, of which a high proportion is rapidly degradable. High dietary protein intakes may have a negative effect on reproductive performance in lactating dairy cows, but the role of milk urea as a predictor of fertility needs further definition given the high conception rates in many Australasian dairy herds.52 High intakes of dietary protein may induce adaptations in urea metabolism and the negative relationship identified between high intakes of dietary protein and fertility for Northern Hemisphere dairy herds may not necessarily apply in Australasian dairy herds. Because of the potential for cows to adapt to high protein diets, the use of single milk urea determination on a herd will have limited value as an indicator of nutritional status and little value as a predictor of fertility.52 The differing observations between various production systems indicate the need for careful consideration in applying recommendations for dietary protein management based on milk urea concentrations. Milk urea determinations may, however, have value, particularly when used in conjunction with other herd and nutritional data to assess the protein nutrition of dairy herds. It is highly unlikely that single or even serial determinations of milk urea in single cows or bulk tank milk will have a high predictive value for determining the risk of conception in the cow or herd.52
Serum albumin
Serum albumin is related to protein status of the animal. Lactation stage has a substantial effect on serum albumin. Animals should be grouped into dry cows, early lactation (1–10 weeks) and later lactation. Minimal values for dry cow means are from 2.9 to 3.1 g/dL, for recently calved cows from 2.7 to 2.9 g/dL and 3.0 to 3.2 g/dL for cows in later lactation.
Hematology
Hematocrit (packed cell volume)
The hematocrit can be used as a general reflection of health. In most dairy herds, a low hematocrit may be a reflection of suboptimal energy and protein nutrition. Mean values of packed cell volume (PCV), hemoglobin and serum iron are consistently higher in non-lactating cows than in lactating cows. Parasitism causing blood loss will result in a low hematocrit. The hematocrit varies with lactation stage, being highest in dry cows and lowest in early lactation. Cows should be grouped by lactation stage.
Mineral nutrition
Serum inorganic phosphorus
Serum inorganic phosphate levels tend to fall following long-term insufficient dietary intake and hyperphosphatemia may occur in cattle grazing on highly fertilized pasture.
Serum calcium
Serum calcium levels vary only within narrow limits and are not sensitive indicators of input–output balance. However, abnormally low levels in late pregnancy indicate a dangerous situation.
Serum magnesium
Serum magnesium levels are usually low during the winter months and subclinical hypomagnesemia exists in many herds, especially pregnant beef cattle. This can be converted into clinical hypomagnesemia with a sudden deprivation of feed or a sudden fall in environmental temperature. Supplementation of the diet with magnesium salts is protective.
Serum sodium
Low levels of serum sodium occur in early lactation in cows grazing on summer pastures without supplementation with salt. Levels down to 135 mmol/L may be associated with depraved appetite and polydipsia and polyuria.
Serum potassium
Serum potassium levels have been difficult to interpret because the levels of the electrolyte in serum are not necessarily indicative of potassium deficiency. Its normal serum concentration is much more variable than sodium and its average concentration in roughages of all kinds is nearly always in excess of requirements; any abnormalities are usually in the direction of excess.
Timing of blood tests
In relation to feed changes
As changes in the diet of ruminants require changes in the character of rumen activity, blood samples for metabolic profiles should not be done until 2 weeks after a major change and activity has had time to adapt. Minor changes such as an increase in the quantity of an existing component or in access to the same ration do not require a wait of more than 7–10 days. Changes in forage type, such as turnout to pasture, housing, or the introduction of silage require the full 2 weeks. The same applies for introduction of concentrates or of a new type of concentrate.
In relation to feeding
There can be changes in biochemical values in blood associated with feeding. These are most marked in cows receiving all their concentrate ration at milking time. In such cases, 2 h should be allowed to elapse after milking before blood sampling. In circumstances where the major part of the concentrate input is mixed with the forages and is available for most of each 24 h, the timing of tests in relation to feeding is less critical. If lower yielding mid lactation cows are included (see later), their results can be used as a check to see if there is an effect of feeding on the biochemical values in the blood samples. Cows should not be separated at milking time and confined for hours without access to food waiting for blood sampling as this can also affect the results.
In relation to calving pattern and seasonal feeding changes
The cow in early lactation is the most important because what happens to her in the first few weeks after calving has the major influence on her subsequent productivity, including her future fertility efficiency. Therefore, blood sampling for metabolic profiles should be carried out at the beginning of each new calving season, with the first cows checked so that the majority can benefit from the information derived.
Of equal importance is the need to test as soon as possible after the introduction of a new ration, so that evaluation of the cows’ biochemistry can be made available as quickly as possible, i.e. what the cows, the end users, think of the ration.
Therefore planning of metabolic profile tests needs to be done in advance and should take in to account both expected calving pattern and feed changes. Without planning along these lines, time may be lost and productivity with it.
Selection of cows
Picking appropriate cows for blood sampling is very important. This is because some of the metabolites looked at, particularly those relating to energy balance, can quickly return to the optimum range as cows adapt themselves, including their productivity, to a nutritional constraint. It is possible for cows to experience a significant energy deficit in the first 2–3 weeks of lactation because of intake problems, lose excessive body condition, perhaps modify their milk yield, and have their subsequent fertility efficiency suppressed but yet still arrive at 4 weeks calved with all biochemical measurements within the optimum ranges. This is because the common appetite constraint of the new calved has worked its way out and there is plenty of food available for lower performance than anticipated. If blood is sampled at 4 weeks calved or longer, a farmer could see thin, under-producing cows with poor fertility but with nothing abnormal about their biochemistry. Thus, the farmer would be entitled to feel the metabolic profile test was of no value. However, if those cows had been blood sampled at 14 days calved instead of 27, the blood results would have been quite different and would have identified the nutritional constraint on productivity.53
The ‘Rules’ for metabolic profiling of dairy cattle recommend sampling from the following groups:
• Early lactation (EL): between 10 and 20 days of lactation
Individual variations in biochemical values are such that single cows should not be tested. Groups of no less than five should be sampled. They should not be picked at random but rather should be typical, average cows of their stage of lactation. Cows with extremes of performance – either very high or very low – should not be selected. Cows with problems should also not be included because the type of analysis carried out is not designed to clarify individual problems. It is important to make all this clear to farmers in advance because they cannot be expected to appreciate the limitations of the analyses made. Experience in the Dairy Herd Health and Productivity Service in the UK33 suggests that selecting cows for metabolic profiles may be best done by the veterinarian in advance of the test after looking at the calving and production records. If there is a specific concern such as a poor conception rate, farmers may expect only cows which have failed to conceive to be sampled. This hardly ever delivers helpful information as any nutritional constraints have by then been compensated for and blood biochemical values are usually within optimum ranges. The best approach may be to include such cows as the mid-lactation group.
Early lactation group (EL)
The definition used for this group is most critical for the reasons given in the previous paragraph. Since the original Compton metabolic profile where high yielding cow was used as the definition, the importance of this group has become increasingly apparent. The definition also has had to be changed to take into account changes in farm practice. The way cows are fed now – total mixed rations, increased out of-parlor concentrate feeding – has reduced the time after calving by when they can adapt themselves to an unsatisfactory diet. To be sure of detecting the presence of an energy constraint in particular, blood sampling should be carried out between 10 and 20 days calved– less than 10 days and the yield is still too far below peak for the test to be a realistic one for early lactation performance; more than 20 days and some cows will be thin, unproductive and subfertile but have compensated for their nutrition and they may have normal blood metabolite values.
Mid-lactation group (ML)
Some cows which are passed the period of peak yield and so passed the greatest period of potential nutritional stress should always be included. They should be between 50 and 120 days calved so that they are still relatively high yielding. This group provides a within-herd comparison with the early lactation cows. Without this it is very difficult to distinguish between problems caused by constraints on intake of food or protein and energy content; to identify changes in biochemical values caused by mistiming of tests in relation to feeding or by oddities in the diet such as silage with a high butyric acid content; and to make judgments on concentrate/forage usage within the herd.
Dry cow group (D)
As the dry period is so important to the success of the following lactation, blood sampling to make sure nutrition is adequate is essential. However, the nature of the measurements which can be made means that primarily cows in the last 7–10 days of pregnancy should be sampled. Cows tested with longer to go than that tend to have normal measurements of energy balance even though they can still get in to difficulty. This is because the period of greatest risk is when the volume of the pregnant uterus increases to the point that it can seriously inhibit food intake. It follows that, in a seasonal calving herd, the first dry cows which come in to these last 7–10 days ought to be blood sampled, so that the information can be used for the benefit of the others still to come in to the maximum risk period.
Blood sampling a group of dry cows with 1 month or longer to go to calving at the same time can sometimes provide a useful within herd comparison with respect to energy balance. It may also identify the presence of dietary protein inadequacy – specifically rumen degradable – in the early part of the dry period.
In the DHHPS program, a majority of farms do metabolic profiles 3–4 times a year at critical times as a check ‘ask-the-cows-what-they-think’ exercise. Thus metabolic profiles as part of a pro-active preventive health and productivity programme. Some of the larger farms may do more than 10 tests a year to cover feed changes and to check on the success of any corrective action.
In the DHHPS program, a standard DHHPS metabolic profile includes analysis on blood plasma for β-hydroxybutyrate (BHB), glucose, non-esterified fatty acid (NEFA), urea-nitrogen (urea N), albumin, globulin, magnesium, and inorganic phosphate.33 Analyses for copper and glutathione peroxidase (GSHPx) are done on approximately one-third of samples received and thyroxine T4 on even fewer. Biochemical analysis is performed using two Bayer Opera auto-analysers, with standard internal controls. It also employs an independent, external quality control system. Derivation of optimum metabolite values are summarized in Table 29.2. They are BHB <1.0 mmol/L in cows in milk, <0.6 mmol/L in dry cows; glucose >3.0 mmol/L; NEFA <0.7 mmol/L in cows in milk and <0.5 mmol/L in dry cows; ureaN >1.7 mmol/L; albumin >30 g/L; globulin <50 g/L; magnesium >0.7 mmol/L; inorganic phosphate >1.3 mmol/L; copper >9.2 μmol/L; glutathione peroxidase (GSHPx) >50 U/g HB; thyroxine T4 >20 nmol/L.
Table 29.2 Metabolic profile parameters in cattle. Optimum values
| Parameter | SI units | |
|---|---|---|
| Butyrate Milkers | Below 1.00 mmol/L | |
| Dry cows | Below 0.60 mmol/L | |
| Plasma glucose | Over 3.00 mmol/L | |
| NEFA Milkers | Below 0.70 mmol/L | |
| Dry cows | Below 0.40 mmol/L | |
| UreaN | 1.70–5.00 mmol/L | |
| Albumin | Over 30.00 g/L | |
| Globulin | Under 50.0 g/L | |
| Magnesium | 0.80–1.30 mmol/L | |
| Phosphate (inorganic) | 1.40–2.50 mmol/L | |
| Copper | 9.40–19.00 μmol/L | |
| Thyroxine T4 (iodine) | Over 20.00 nmol/L | |
| GSHPx (selenium) | Over 50units/g Hb | |
The data in Table 29.3 uses only the cows fitting precisely the definitions of EL, ML, and D. It shows that, overall, an average of 30% EL cows had metabolite results reflecting satisfactory energy status as did 61% of ML and 43% of D. In both EL and ML groups, glucose is the metabolite most commonly outside its optimum range, followed by BHB and NEFA. The percentage of NEFA values above optimum is low in ML cows. The most common finding is high BHB and low glucose in the same cow. In tests showing most cows in an EL group with results like that, there is usually one or two with high NEFAs as well. Some EL cows show only low glucose or only high NEFA. Where low glucose only predominates in EL cows, ML cows often show the same picture.
Table 29.3 Annual (April–March) percentages outside optimum ranges of metabolite results in blood plasma in adult dairy cows33
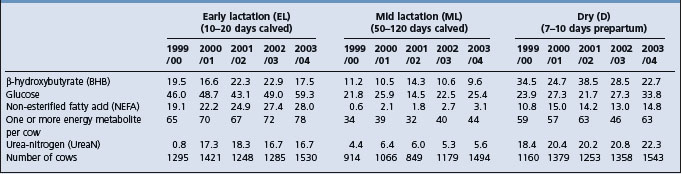
UreaN results in Table 29.3 show that the EL stage is more vulnerable to low values than later in lactation, even though the cows would have been on the same diets in virtually every case. In fact an even greater average percentage51 in 1361 cows blood sampled between 0 and 9 days after calving over the 5 years showed low ureaN.
The proportion of low ureaN results in D cows is high (Table 29.3). In addition to the category shown of 10 days or less before calving, 4335 cows were sampled at more than 10 days prepartum over the 5 years and 22% of them had low ureaN values too.
Results outside the optimum ranges for albumin (0.6%), magnesium (2.5%), inorganic phosphate (1.0%), copper (10%), GSHPx (3%) are relatively uncommon. Thyroxine T4 analysis was carried out in 836 samples on specific request and only 3% were below optimum.
Background information
So that full value can be obtained by the farmer from the metabolic profile approach, information about the cows and the farm should accompany the blood samples to the laboratory. This should include cow identification; last calving date for milkers/expected for dry cows; body weight – by calculation from heart-girth measurement with a weighband pulled to a constant 5 kg tension is the best, because it is not affected by gutfill and usually most practical, because no mechanical weighing device/crush is required; body condition score by a palpation method; current daily milk yield; expected current daily milk yield; lactation number; daily supplementary feed intakes; daily estimated forage intakes; analytical description of feeds and current herd milk solids percentages. It is useful to have information on herd size, breed, feeding systems, and health and fertility. A note of what concerns the farmer has, if any, should also be made.
Interpretation of results at the farm
Circumstances where the diagnosis of a nutritional constraint from blood samples is clearly correct, but the cause(s) are unclear from a distance and could be many, are common. Therefore it is very important that a final interpretation of what is not working and what are the best and most economic solutions ought to be made at the farm with the information from the laboratory to hand. Farm advisory visits should be made as soon as the results are available and discussions made, including farm staff and any other advisers involved. Experience in the DHHPS suggests that such a team approach produces a more balanced strategy and is more beneficial than each party working in isolation.
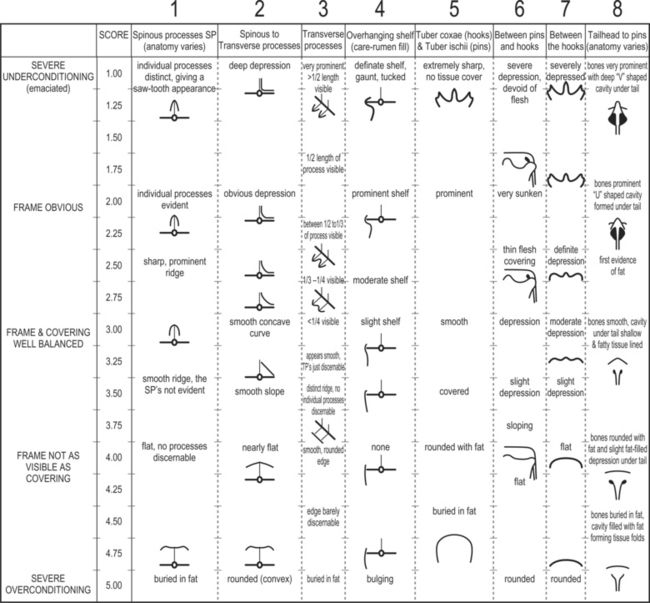
Body condition score (BCS)
Managing body reserves is critical for successful cow management and requires an accurate assessment of the cow’s ‘condition’. Body condition scoring is an important aspect of metabolic diseases of farm animals. Body weight alone is not a valid indicator of body reserves, as cows of a specific weight may be tall and thin or short and fat. The energy stores may vary by as much as 40% in cows of similar body weight, which emphasizes the futility and inaccuracy of relying on body weight alone as an index of cow condition. In addition, because tissue mobilization in early lactation occurs as feed intake is increasing, decreases in body tissue weight can be masked by enhanced fill of the gastrointestinal tract, so that body weight changes do not reflect changes in adipose tissue and lean tissue weight.54
There is a strong positive relationship (r2 = 0.86) between body condition score (BCS) and the proportion of physically dissected fat in Friesian cows. Therefore, the visual or tactile (palpation) appraisal of the cow’s body condition score provides a good assessment of body fat reserves, ignoring, or minimizing the effect of, frame size and intestinal contents. Most animal and dairy scientists acknowledge successfully manipulating BCS as an important management factor, influencing or having a relationship to animal health, milk production, and reproduction in the modern dairy cow. For example, cows which lost 0.5–1.0 point in BCS between parturition and first service achieved pregnancy rate to first service of 53%, while those losing >1.0 point achieved a rate of 17%.55 In a seasonal pasture-based system for Holstein–Friesian cows, it is necessary to maintain a BCS at ≥2.75 during the breeding season. Body condition score is important in achieving good reproductive performance. Loss of body condition between calving and first service should be restricted to 0.5 BCS to avoid a detrimental effect on reproductive performance.55
BCS is a subjective method of assessing the amount of metabolizable energy stored in fat and muscle (body reserves) on a live animal. BCS in dairy cows is done using a variety of scales and systems. This method involves palpating the cow to assess the amount of tissue under the skin. Scoring body condition and assessing changes in the body condition of dairy cattle have become strategic tools in both farm management and research. BCS is being researched worldwide. But international sharing, comparing, and use of data generated are limited because different BCS systems are used. There is difficulty in interpreting the literature because of variability in the way authors apply scoring methods. In the USA, Canada, and Ireland a 5-point BCS system is used for dairy cows, whereas Australia and New Zealand use 8- and 10-point scales, respectively.56 The following scoring method is recommended for the 0–5 scale. The BCS chart adapted from Edmonson et al. appears in Figure 29.1.54
Relationships among international body condition scoring
The New Zealand 10-point scale was compared with the scoring systems in the USA, Ireland, and Australia by trained assessors.35 Cows were assessed visually in the USA and Australia and in Ireland, cows were assessed by palpating key areas of the cow’s body. Significant positive linear relationships were found between the New Zealand 10-point scale and the other scoring systems. The relationship between the 10-point BCS scale used in New Zealand and Ireland and the USA are summarized in Table 29.4.
Dohoo IR, et al. A meta-analysis review of the effects of recombinant bovine somatotrophin. 1. Methodology and effects on production. Can J Vet Res. 2003;67:241-251.
Dohoo IR, et al. A meta-analysis review of the effects of recombinant bovine somatotrophin. 2. Effects on animal health, reproductive performance and culling. Can J Vet Res. 2003;67:252-264.
Westwood CT, Lean IJ, Kellaway RC. Indications and implications for testing of milk urea in dairy cattle: A quantitative review. Part 1. Dietary protein sources and metabolism. N Z Vet J. 1998;46:87-96.
Westwood CT, Lean IJ, Kellaway RC. Indications and implications for testing of milk urea in dairy cattle: A quantitative review. Part 2. Effect of dietary protein on reproductive performance. N Z Vet J. 1998;46:123-130.
Overton TR, Waldron MR. Nutritional management of transition dairy cows: strategies to optimize metabolic health. J Dairy Sci. 2004;87:E105-E119.
Cook NB, Nordlund KV. Managing the transition cow to optimize health and productivity. Vet Clin North Am Food Anim Pract. 2004;20:447-701.
Herdt TH. Metabolic disorders in ruminants. Vet Clin North Am Food Anim Pract. 2000;16:215-403.
Grummer RR, Mashek DG, Hayirli A. Dry matter intake and energy balance in the transition period. Vet Clin North Am Food Anim Pract. 2004;20:447-470.
Goff JP. Macromineral disorders of the transition cow. Vet Clin North Am Food Anim Pract. 2004;20:471-494.
Cook NB, Nordlund KV. Behavioral needs of the transition cow and considerations for special needs facility design. Vet Clin North Am Food Anim Pract. 2004;20:495-520.
Mee JF. Managing the dairy cow at calving time. Vet Clin North Am Food Anim Pract. 2004;20:521-546.
Guterbock WM. Diagnosis and treatment programs for fresh cows. Vet Clin North Am Food Anim Pract. 2004;20:605-626.
Nordlund KV, Cook NB. Using herd records to monitor transition cow survival, productivity and health. Vet Clin North Am Food Anim Pract. 2004;20:627-649.
Oetzel GR. Monitoring and testing dairy herds for metabolic disease. Vet Clin North Am Food Anim Pract. 2004;20:651-674.
Dahl GE, Auchtung TL, Reid ED. Manipulating milk production in early lactation through photoperiod changes and milking frequency. Vet Clin North Am Food Anim Pract. 2004;20:675-685.
Collier RJ, Annen EL, Fitzgerald AC. Prospects for zero days dry. Vet Clin North Am Food Anim Pract. 2004;20:687-701.
Herdt TH. Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Vet Clin North Am Food Anim Pract. 2000;16:215-230.
Duffield T. Subclinical ketosis in lactating dairy cattle. Vet Clin North Am Food Anim Pract. 2000;16:231-253.
Geishauser T, Leslie K. Duffield T. Metabolic aspects in the etiology of displaced abomasum. Vet Clin North Am Food Anim Pract. 2000;16:255.
Gerloff BJ. Dry cow management for the prevention of ketosis and fatty liver in dairy cows Vet Clin North Am Food Anim Pract. 2000;16:283-292.
Rook JS. Pregnancy toxemia of ewes, does and beef cows. Vet Clin North Am Food Anim Pract. 2000;16:293. 231
Goff JP. Pathophysiology of calcium and phosphorus disorders. Vet Clin North Am Food Anim Pract. 2000;16:319-337.
Martens H, Schweigel M. Pathophysiology of grass tetany and other hypomagnesemias. Implications for clinical management. Vet Clin North Am Food Anim Pract. 2000;16:339-368.
Oetzel GR. Management of dry cows for the prevention of milk fever and other mineral disorders. Vet Clin North Am Food Anim Pract. 2000;16:369-386.
Herdt TH. Variability characteristics and test selection in herd-level nutritional and metabolic profile testing. Vet Clin North Am Food Anim Pract. 2000;16:387-403.
Herdt TH, Dart B, Neuder L. Will large dairy herds lead to revival of metabolic profile testing? Proc Am Assoc Bov Pract. 2001;34:27-34.
Drackley JK. Biology of dairy cows during the transition period: the Final Frontier? J Dairy Sci. 1999;82:2259-2273.
Ingvartsen KL, Dewhurst RJ, Friggens NC. On the relationship between lactational performance and health: is it yield or metabolic imbalance that cause production diseases in dairy cattle? A position paper. J Dairy Sci. 2003;83:277-308.
Ingvartsen KL, Andersen JB. Integration of metabolism and intake regulation: A review focusing on periparturient animals. J Dairy Sci. 2000;83:1573-1597.
1 Cook NB, Nordlund KV. Vet Clin North Am Food Anim Pract. 2004;20:447-701.
2 Grummer RR, Mashek DG, Hayirli A. Vet Clin North Am Food Anim Pract. 2004;20:447-470.
3 Drackley JK. J Dairy Sci. 1999;82:2259.
4 LeBlanc SJ, et al. J Dairy Sci. 2005;88:159.
5 Cameron REB, et al. J Dairy Sci. 1998;81:132.
6 Overton TR, Waldron MR. J Dairy Sci. 2004;87:E105-E119.
7 Duffield TF, Bagg RN. Aust Vet J. 2000;41:388.
8 Duffield T, et al. J. Dairy Sci. 2002;85:397.
9 Duffield TF, et al. J Dairy Sci. 2003;86:1171.
10 Goff JP. Vet Clin North Am Food Anim Pract. 2004;20:471-494.
11 Cook NB, Nordlund KV. Vet Clin North Am Food Anim Pract. 2004;20:495-520.
12 Mee JF. Vet Clin North Am Food Anim Pract. 2004;20:521-546.
13 Guterbock WM. Vet Clin North Am Food Anim Pract. 2004;20:605-626.
14 Nordlund KV, Cook NB. Vet Clin North Am Food Anim Pract. 2004;20:627-649.
15 McLaren CJ. J Dairy Sci. 2005;88:419.
16 Dahl GE, Auchtung TL, Reid ED. Vet Clin North Am Food Anim Pract. 2004;20:675-685.
17 Collier RJ, Annen EL, Fitzgerald AC. Prospects for zero days dry. Vet Clin North Am Food Anim Pract. 2004;20:687-701.
18 Ingvartsen KL, Andersen JB. J Dairy Sci. 2000;83:1573-1597.
19 Mallard BA, et al. J Dairy Sci. 1998;81:585.
20 Preisler MT, et al. Am J Vet Res. 2000;61:14.
21 Lacetera N, et al. Am J Vet Res. 2002;63:414.
22 LeBlanc SJ, et al. J Dairy Sci. 2002;85:1416.
23 LeBlanc SJ. J Dairy Sci. 2004;87:609.
24 Dohoo IR, et al. Can J Vet Res. 2003;67:241. 252
25 Kronfeld DS. J Am Vet Med Assoc. 2000;216:1719.
26 Fetrow JP. J Am Vet Med Assoc. 2001;218:1886.
27 Kinoshita J, et al. J Dairy Sci. 2004;87:1565.
28 Herdt TH. Vet Clin North Am Food Anim Pract. 2000;16:215-403.
29 Ingvartsen KL, et al. Prev Vet Med. 2003;83:277.
30 Herdt TH, Dart B, Neuder L. Will large dairy herds lead to revival of metabolic profile testing? Proc Am Assoc Bov Pract. 2001;34:27-34.
31 Herdt TH. Vet Clin North Am Food Anim Pract. 2000;16:387-403.
32 Oetzel GR. Herd-based biological testing for metabolic disorders. Preconvention Seminar 7. Am Assoc Bov Pract. 2003;36:1-16.
33 Whitaker DA, et al. Cattle Practice. 2005;13:27.
34 Whitaker DA, et al. Prev Vet Med. 1999;38:119.
35 Kida KJ. Vet Med Sci. 2002;64:557.
36 Kida KJ. Vet Med Sci. 2002;64:1003.
37 Kida KJ. Vet Med Sci. 2003;65:677.
38 Kelly JM, Whitaker DA. Br Soc Anim Sci. 2000;26:209-222.
39 Oetzel GR. Monitoring and testing dairy herds for metabolic disease. Vet Clin North Am Food Anim Pract. 2004;20:651-674.
40 Whitaker DA. Use and interpretation of metabolic profiles. In: Andrews AH, editor. The health of dairy cattle. Oxford, Malden, MA: Blackwell Science; 2000:89-107.
41 LeBlanc SJ, et al. J Dairy Sci. 2005;88:159.
42 Cameron REB, et al. J Dairy Sci. 1998;81:132.
43 Geishauser T, et al. Vet Clin North Am Food Anim Pract. 2000;16:255.
44 Duffield T. Vet Clin North Am Food Anim Pract. 2000;16:231-253.
45 Cottrill B, et al. Vet Rec. 2002;151:413.
46 Godden S, et al. Bov Pract. 2003;37:36.
47 Jonker JS, et al. J Dairy Sci. 1999;82:1261.
48 Kohn RA, et al. J Dairy Sci. 2002;85:227.
49 Eicher R, et al. Can Vet J. 1999;40:487.
50 Jonker JS, et al. J Dairy Sci. 2002;85:939.
51 Dawuda PM, et al. Anim Reprod Sci. 2004;81:195.
52 Westwood CT, et al. N Z Vet J. 1998;46:87. 123
53 Whitaker D. J Vet Postgrad Study. 2005;27:43.
54 National Research Council. Nutrient requirements of dairy cattle, 7th revised ed., Washington DC: National Academy Press; 2001:13-27.
PARTURIENT PARESIS (MILK FEVER)
A disease of cattle, sheep, and goats occurring around the time of parturition and caused by hypocalcemia and characterized by weakness, recumbency, and ultimately shock and death.
Etiology Hypocalcemia just before or after parturition.
Epidemiology Adult dairy cows in third parity and older; 4–9% with low case fatality. Most commonly within 48 h after calving but also occurs several weeks before or after. Occurs in beef cattle in epidemics. Occurs in sheep and goats in epidemics usually following stressors. Prepartum diets high in calcium.
Signs Three progressively worse stages including the following signs:
• General muscular weakness leading to sternal recumbency with lateral kink of neck
Clinical pathology Hypocalcemia, hypophosphatemia, variable serum magnesium. Increased creatine phosphokinase (CPK) and aminotransferase (AST) due to ischemic muscle necrosis.
Necropsy findings No specific lesions. Ischemic muscle necrosis of large muscles of pelvic limbs because of prolonged recumbency.
Metabolic and nutritional disease
Injuries to pelvis and pelvic limbs
Diagnostic confirmation Hypocalcemia and response to treatment with calcium borogluconate.
Treatment Calcium borogluconate IV. Calcium chloride in oral gel.
Control Dietary management to reduce prepartum intake of calcium. Dietary cation–anion difference program. Calcium gel oral dosing before calving, at calving and 12 and 24 h after calving. Parenteral vitamin D and analogs.
ETIOLOGY
A depression of the levels of ionized calcium in tissue fluids is the basic biochemical defect in milk fever. A transient period of subclinical hypocalcemia (total plasma calcium <1.9 mmol/L) occurs at the onset of lactation caused by an imbalance between calcium output in the colostrum and influx of calcium to the extracellular pool from intestine and bone. The onset of lactation results in a sudden large demand on the calcium homeostasis. A cow producing 10 kg of colostrum (2.3 g of Ca/kg) will lose 23 g of calcium in a single milking. This is about nine times as much calcium as that present in the entire plasma calcium pool of the cow.1 Calcium lost from the plasma pool must be replaced by increasing intestinal absorption and bone resorption of calcium. During the dry period, calcium requirements are minimal at about 10–12 g/d. At parturition, the cow must mobilize about 30 g or more of calcium into the calcium pool per day. Hypocalcemia occurs in spite of apparently adequate function of the parathyroid and vitamin D endocrine system and most cows adapt within 48 h after calving by increases in plasma concentrations of parathyroid hormone and 1,25-(OH)2D vitamin at the onset of the hypocalcemia and mobilize calcium by increasing intestinal absorption and bone resorption.
About 5–20% of adult cows are unable to maintain plasma calcium and consequently develop severe hypocalcemia (total plasma calcium, 1.0–1.4 mmol/L) or clinical milk fever which requires treatment.2
EPIDEMIOLOGY
Occurrence
Cattle
The disease occurs most commonly in high-producing adult lactating dairy cattle. Lactating beef cows are affected but less commonly.
Mature dairy cows are most commonly affected in the 5–10-year age group, although rare cases have been observed at the first and second calvings. The hypocalcemia at calving is also age related and most marked in cows at their 3rd to 7th parturition; it is infrequent at the first parturition.
There are differences in susceptibility between the breeds but the differences are small.2 Field observations have for many years suggested that Jersey’s are most susceptible but the reported 33% was observed in a sample compared with 9.6% incidence in other breeds may be associated with the older age of many Jersey cows. The disease in beef cattle breeds occurs either in individual cows or in herd outbreaks.3
Individual cows and to some extent families of cows, are more susceptible than others; the disease tends to recur at successive parturitions. The heritability of susceptibility to milk fever and hypocalcemia has been assessed as insignificant; in several breeds examined it was of the order of 6–12%. Complete milking in the first 48 h after calving, as opposed to normal sucking by a calf, appears to be a precipitating factor. Several studies have reported that the incidence of milk fever is positively associated with the level of milk production.2
In cattle, milk fever occurs at three main stages in the lactation cycle. Most prepartum cases occur in the last few days of pregnancy and during parturition but rare cases occur several weeks before calving. Some cases will occur a few hours before parturition or at the time of parturition when the attendant expects the cow to calve and the second stage of parturition does not occur because of uterine inertia due to hypocalcemia. Most cases occur within the first 48 h after calving and the danger period extends up to about the 10th postpartum day. Up to 20% of cases can occur subsequent to the 8th day after calving. In such cases the declines in serum calcium and phosphorus levels are smaller and the increases in serum magnesium levels are greater than in parturient cows. The clinical signs are also less severe and there are fewer relapses after treatment. Occasional cases occur 6–8 weeks after parturition (mid-lactation). Such cases are most often recurrences of the disease in highly susceptible cows which were affected at calving. Undue fatigue and excitement may precipitate such attacks and there is a special susceptibility at estrus. In the latter case, the depression of appetite by the elevation of blood estrogen levels may be a significant factor.
The plasma levels of phosphorus also decrease and the plasma levels of magnesium increase as occurs in cows at the time of parturition. Hypocalcemic episodes lasting 1–2 days may occur two or three times with a periodicity of about 9 days. These cows are referred to as ‘calcium cyclers’ and the magnitude of the cycling was increased by feeding cows 200 g/d of 1,25-dihydroxyvitamin D for 5 days around the time of parturition. Fluctuations in the intestinal absorption of calcium during this period may be the cause of calcium cycling. Subclinical hypocalcemia is of major significance because it inhibits reticulorumen motility, which affects appetite and exacerbates the negative energy balance already existing in the cow in 1st month of lactation.
Episodes of subclinical hypocalcemia occur in up to 50% of adult cows during the first few weeks of lactation. It is suggested that these calcium cyclers are animals whose calcium homeostatic mechanisms have not adapted well enough.
Starvation for 48 h also causes severe depression of serum calcium levels and this may be of importance in the production of hypocalcemic paresis in this species at times other than in the postparturient period. Pregnant beef cattle may develop hypocalcemic paresis during the winter months when they are fed on poor-quality roughage; within a group of such cows the less aggressive ones may suffer selective malnutrition. The disease has also occurred in beef cows affected with diarrhea of undetermined etiology. As another explanation of the heightened susceptibility of cows at estrus, a possible depression of the degree of ionization of calcium under the influence of increased serum estrogens is suggested. However, there were no significant differences in total serum calcium or plasma ionized calcium values in cows from 48 h before and after estrus.
Hypocalcemic syndromes in ruminants are also observed at times other than related to parturition. Thus, it can be part of an early or mild overeating of fermentable carbohydrate. The IV administration of certain aminoglycosides, especially neomycin, elihydrostreptomycin and gentamicin, may cause a reduction in the degree of ionization of serum calcium and a syndrome similar to milk fever. Oral dosing with zinc oxide (40 or 120 mg Zn/kg BW) as a prophylaxis against facial eczema in ewes causes a serious fall in serum calcium levels 24 h later. Caution is recommended with the use of these drugs in parturient cows.
Sheep and goats
In sheep, the disease commonly occurs in outbreaks in groups of ewes exposed to forced exercise, long-distance transport, sudden deprivation of food and grazing on oxalate-containing plants or green cereal crops. These circumstances commonly precipitate outbreaks of hypocalcemic paresis in sheep, mature ewes are the most susceptible, particularly in the period from 6 weeks before to 10 weeks after lambing. Up to 25% of the flock may be affected at one time. The disease also occurs in young sheep up to about 1 year old, especially when they graze green oats, but also when pasture is short in winter and spring, as in southeast Australia. The disease is manifested by paresis but in the rest of the flock poor growth, lameness and bone fragility can be detected. A sudden deprivation of feed or forced exercise of ewes can cause marked depression of the serum calcium levels. However, ewes are in a susceptible state in early lactation because they are in negative calcium balance. In late lactation a state of positive balance is due to a low rate of bone resorption. There is an unexplained occurrence of hypocalcemia in sheep fed on hay when they are supplemented with an energy-rich concentrate which increases their calcium intake. Some of the concentrates fed to ewes in feedlots contain supplementary magnesium as a prevention against hypomagnesemia, which may affect calcium absorption and precipitate hypocalcemia in susceptible ewes. Another occurrence in ewes is at the end of a drought when the pasture growth is lush and very low in calcium content. The incidence may be as high as 10% and the case-fatality rate 20% in ewe flocks in late pregnancy or early lactation.
Hypocalcemia in sheep depresses endogenous glucose production and in late pregnancy in combination with hyperketonemia, facilitates the development of pregnancy toxemia.4
In goats, a depression in serum levels of calcium and phosphorus occurs similar to that in cows but in ewes no such depression occurs at lambing and the intervention of a precipitating factor appears to be necessary to further reduce the serum calcium level below a critical point.
Milking goats become affected mostly during the 4–6-year age group. Cases occur before and after kidding, some later than 3 weeks after parturition. Clinical syndromes are identical to those in cows, including the two stages of ataxia and recumbency. Serum calcium levels are reduced from normal levels for parturient does of 9.4–3.6 mg/dL (2.35–0.9 mmol/L).
Morbidity and case fatality
Several epidemiological studies of milk fever have shown an incidence risk of 5–10%, calculated either as the lactational incidence or incidence per cow year.2 Generally the disease is sporadic but on individual farms the incidence may rarely reach 25–30% of high-risk cows. In Victoria, Australia, 85% of dairy herds calve in the spring and the incidence of milk fever ranges from 2% to 5%.5 With early treatment relatively few deaths occur in uncomplicated cases but incidental losses due to aspiration pneumonia, mastitis, and limb injuries may occur. From 75% to 85% of uncomplicated cases respond to calcium therapy alone. A proportion of these animals require more than one treatment, either because complete recovery is delayed, or because relapse occurs. The remaining 15–25% are either complicated by other conditions or incorrectly diagnosed.
Subclinical hypocalcemia (total plasma calcium <1.9 mmol/L) occurs in dairy cattle during the first few weeks of lactation. The incidence of subclinical hypocalcemia based on measurement of blood calcium around the time of calving and later in lactation ranges from 23% to 39%.2 Up to 50% of aged cows may be unable to maintain plasma calcium above the lower normal limit (2.18 mmol/L) as defined by the 99% confidence interval of plasma calcium concentrations in cows outside the first month of lactation. In New Zealand, up to 40% of apparently normal cows may have subclinical hypocalcemia during the first 12 days of lactation.6 Up to 33% of cows grazing pasture in New Zealand may have subclinical hypocalcemia on the day of calving.
In the USDA National Animal Health Monitoring System Dairy study of 2002, 1446 cows from 480 dairy herds in 21 states were sampled within 48 h of parturition. Subclinical hypocalcemia increased with advancing age and represented 25.3%, 43.9%, and 57.8% of first, second, and third lactation cows, respectively.7 Of the groups studied, 38.7% were on a DCAD program and the incidence of hypocalcemia was significantly less in those animals on the DCAD program. Also, with normal calcium levels had lower serum NEFAs indicating that their energy status was better than those with hypocalcemia.
Risk factors
The literature on the risk factors associated with milk fever has been reviewed.2
Animal risk factors
Serum calcium levels decline in all adult cows at calving due to the onset of lactation. Serum calcium levels decline to lower levels in some cows than in others and it is this difference which results in the varying susceptibility of animals to parturient paresis.6 First-calf heifers rarely develop milk fever because while some degree of hypocalcemia occurs during the first few days of lactation, they are able to adapt rapidly to the high demands of calcium for lactation. With increasing age, this adaptation process is decreased and results in moderate-to-severe hypocalcemia in most adult cows. The adaptation mechanism is directly related to the efficiency of intestinal absorption of calcium, which decreases with increasing age.
Three factors affect calcium homeostasis and variations in one or more of them may be important in causing the disease in any individual:
1. Excessive loss of calcium in the colostrum beyond the capacity of absorption from the intestines and mobilization from the bones to replace. Variations in susceptibility between cows could be due to variations in the concentration of calcium in the milk and the volume of milk secreted.
2. Impairment of absorption of calcium from the intestine at parturition.
3. Mobilization of calcium from storage in the skeleton may not be sufficiently rapid to maintain normal serum levels. The calcium mobilization rate and the immediately available calcium reserves are sufficiently reduced in cows in later pregnancy to render them incapable of withstanding the expected loss of calcium in the milk. In older cows, bone resorption makes only a minor contribution to the total rate of calcium mobilization at parturition and is therefore of minor importance for the prevention of periparturient hypocalcemia. Osteoblasts are the only type of bone cell to express the 1,25-(OH)2D receptor protein and the decrease in the numbers of osteoblasts with increasing age could delay the ability of bone to contribute calcium to the plasma calcium pool.1
It was once postulated that failure to secrete sufficient levels of parathyroid hormone or 1,25-dihydroxyvitamin D was the primary defect in cows which developed milk fever. While it is accepted that the calcium homeostatic mechanisms, regulated by parathyroid hormone and 1,25-dihydroxyvitamin D, fail to maintain normal blood calcium concentrations resulting in severe hypocalcemia, the nature of the endocrine defect is not well understood. It was also once thought that calcitonin, a hormone which inhibits bone calcium resorption was a cause of milk fever but this has not been demonstrated in cows with milk fever.1 Recent studies have shown that the secretion of parathyroid hormone and the production of 1,25-dihydroxyvitamin D is similar in most cows with or without milk fever.1 However, about 20% of cows treated for parturient paresis experience relapsing episodes of hypocalcemia which require further treatment. These cows fail to produce adequate levels of 1,25-dihydroxyvitamin D at the onset of lactation.6 Both relapsing and non-relapsing cows develop the same degree of hypocalcemia and secondary hyperparathyroidism, but production of 1,25-dihydroxyvitamin D is about two-fold greater in non-relapsing cows than relapsing cows. Following treatment of parturient hypocalcemia with calcium salts IV and restoration of ruminal and intestinal motility, non-relapsing cows establish calcium homeostasis over the next 3–4 days by increasing intestinal absorption of calcium which is activated by a sufficient level of 1,25-dihydroxyvitamin D. In relapsing cows, even when rumen and intestinal motility are restored after treatment, hypocalcemia and paresis are likely to occur because of insufficient plasma 1,25-dihydroxyvitamin D. These cows may remain in this stage of prolonged hypocalcemia for several days and only after a few days and several repeated treatments with calcium will the plasma levels of 1,25-dihydroxyvitamin D increase to an adequate level to maintain calcium homeostasis. It is also unlikely that the parathyroid hormone-related protein in the colostrum of milk fever cows is involved in the disease.6
Tissue 1,25-dihydroxyvitamin D receptor concentrations decline with age, which renders older cows less able to respond to 1,25-dihydroxyvitamin D.1 The intestinal 1,25-(OH)2D receptor numbers decline with age in the cow and thus the older cow is less able to respond to the hormone and will take longer to adapt intestinal calcium absorption mechanisms to meet lactational demands for calcium.6
A perplexing situation in dairy practice is the recently calved cow with peracute coliform mastitis which may also be mildly hypocalcemic and have some of the clinical signs of milk fever. The Escherichia coli endotoxin given IV depresses serum calcium and phosphate levels so that coliform mastitis may contribute to a degree of hypocalcemia in individual cows. However, there is no evidence that cows with peracute coliform mastitis require calcium therapy similar to that used in typical milk fever.
A high BCS increases the risk of milk fever. The odds ratio of milk fever with a BCS >4/5 on the first milk recording day after calving was 4.32 and cows with milk fever had a postpartum pre-disease 12 kg higher body weight compared with healthy cows indicating an increased risk of milk fever due to higher body weight. Cows with subclinical hypocalcemia in the winter period had significantly higher mean body weight over the 60 days post partum than normocalcemic cows but the effect was not significant in cows calving during the summer months.
Dietary and environmental risk factors
Several dietary factors of the pregnant cow during the prepartum period (last 4 weeks) can influence the incidence of milk fever in cattle.
Feeding more than 100 g of calcium daily during the dry period is associated with an increased incidence of milk fever.2 A 500 kg cow requires only about 31 g of calcium to meet daily maintenance and fetal demands in late gestation. When a cow is fed a high calcium diet (>100 g Ca/d), its daily requirement for calcium can be met almost entirely by passive absorption of dietary calcium. The active transport of calcium from the diet and bone calcium resorption mechanisms are homeostatically depressed and become quiescent. As a consequence, at calving, the cow is unable to use bone calcium stores or intestinal calcium absorption mechanisms and is susceptible to severe hypocalcemia until these mechanisms can be activated, which may take several days.
Feeding prepartum diets containing a low concentration of calcium prevents milk fever by activating calcium transport mechanisms in the intestine and bone prior to parturition, thus allowing the animal to adapt more rapidly to the lactational drain of calcium. Feeding diets high in calcium just before parturition may also lower the incidence of milk fever by increasing the absorption of calcium. This will provide sufficient calcium to overcome the relative lack of calcium from bone resorption which results from a high calcium intake.9
In sheep, hypocalcemia may occur in pregnant ewes fed a calcium-deficient diet over a prolonged period. A high dietary level of magnesium in late pregnancy may also predispose to hypocalcemia in pregnant ewes but this has not been documented. Ewes fed a diet with a fixed cation excess (82.3 mEq/100 g DM) had higher urine pH and lower urine calcium concentrations, lower blood ionized calcium concentrations after an overnight fast and tended to develop hypocalcemia more rapidly after an ethylenediamine tetra-acetate (NaEDTA) infusion. This suggests that a dietary fixed cation–anion balance may be a risk factor for hypocalcemia in pregnant ewes.
Prepartum diets high in phosphorus (>80 g P/d) also increases the incidence of milk fever and the severity of hypocalcemia.10 High dietary levels of phosphorus increase the serum level of phosphorus which is inhibitory to the renal enzymes that catalyze production of 1,25-(OH)2D, which when decreased reduce the intestinal calcium absorption mechanisms prepartum.
Dietary cation-anion difference (DCAD).
The anion–cation dietary difference exerts a strong, linear effect on the incidence of milk fever. The dietary cation– anion balance in the prepartum diet may be more important than the level of dietary calcium as a risk factor for milk fever.2 Prepartum diets high in cations such as sodium and potassium are associated with an increased incidence of milk fever, while diets high in anions, especially chloride and sulfur, are associated with a decrease in the incidence of the disease. Alkaline diets containing an excessive concentration of sodium and potassium can result in an increased incidence of the disease. Most forages such as legumes and grasses are high in potassium and are alkaline. Metabolic alkalosis predisposes cows to milk fever. Under most circumstances the alkalosis is induced by the potassium in the diet.11 The addition of anions to the diet of dairy cows prior to parturition effectively reduced the incidence of milk fever by inducing a metabolic acidosis which facilitates bone resorption of calcium.
The quantitative relationship between feed cations and anions predict the alkylogenic or acidogenic response in the animal or the DCAD. The difference between the number of cation and anion particles absorbed from the diet determines the pH of the blood. The cation–anion difference of a diet is commonly described in milliequivalents (mEq) per kg (dry matter) DM (mEq/kg DM) feed. This value is calculated as: DCAD = (Na+ + K+) – (Cl−+S−).10 Calcium, magnesium and phosphorus content of feeds will also affect acid–base status but are considered to have minor importance in the calculation of the DCAD. The DCAD equation describes how the ions contained in the feeds affect the metabolic processes in the body by the absorption and further metabolism and thereby causing changes in the systemic acid–base status. Systemic acidification induced by anionic supplementation affects the function of the parathyroid (PTH) hormone. The major effect of systemic acidification is to cause an increased response to PTH which results in increased retention of calcium and enhanced mobilization of calcium from bone. A total DCAD in the range of –100 to –200 mEq/kg feed DM is effective in controlling milk fever. Most natural feedstuffs fed to dairy cows will, on a molar basis, have a surplus of sodium and potassium compared with chloride and sulfur. A composite ration, with a negative DCAD value, is difficult to achieve in commercial dairy herd conditions.
A meta-analysis of 75 feeding trials designed to study the nutritional risk factors for milk fever in dairy cattle found that the prepartum dietary concentrations of S and dietary anion–cation balance [(Na + K) – (Cl + S)] were the two nutritional factors most strongly correlated to the incidence of milk fever.2 Dietary S acts as a strong anion and reduces the risk of milk fever and increasing the dietary S concentrations lowers the odds ratio of developing milk fever. Increasing dietary Na and crude protein increased the odds ratios, but to a lesser extent.
The incidence of milk fever has been decreased by the addition of chloride and sulfur in excess relative to sodium and potassium in the prepartum diet of Holstein cows. High anion diets increase the plasma levels of 1,25-(OH)2D prior to parturition, activating intestinal calcium absorption and possibly bone calcium resorption mechanisms prior to onset of lactation.10 Monitoring the pH of urine is a sensitive method for assessing the risk of milk fever.12 The urine pH within 48 h prior to parturition has a significant negative correlation with serum calcium and inorganic phosphorus. The sensitivity, specificity, positive and negative predictive values of urine pH test prior to parturition, using a cut off level of above pH 8.25, were 100%, 81%, 55%, and 100%, respectively.12
ECONOMIC IMPORTANCE
Unlike many years ago, the economic losses from milk fever have decreased because calcium borogluconate is an effective treatment which many owners can administer. Significant costs are associated with veterinary intervention and losses due to complications. However, while veterinarians now treat fewer cases of uncomplicated milk fever, there may be an increase in cases which are complicated by factors other than hypocalcemia.
The literature on the effects of clinical milk fever and subclinical hypocalcemia is difficult to interpret because of the complex relationships between milk production, parity of lactation, breed of cattle, epidemiological methods used, management systems being used, and the reproducibility of the clinical observations and the accuracy of the recording systems used.2 In general, there is insufficient information available to document the consequences of milk fever and subclinical hypocalcemia. A summary of several consequences which have been examined follows here.
The downer cow syndrome associated with milk fever cases which fail to respond within a few hours and remain recumbent for several hours or several days before subsequently standing, or die, or are euthanized represents an important cause of economic loss because of treatment costs, long-term care costs, loss of milk production and loss of the value of the animal. Acute mastitis due to environmental pathogens is a common complication of prolonged recumbency in downer cows associated with milk fever. Some studies have found downer cows had a probability of 35% of being culled in the first 150 days of lactation and a relative risk of being culled of 29.2.2
Dystocia and reproductive disease.
Hypocalcemia at the time of parturition can result in uterine inertia which may cause dystocia and uterine prolapse. In general, there is an increased risk of dystocia associated with milk fever whether the farmer or the veterinarian attends to the dystocia.2
Several studies have found an increased risk of retained placenta following milk fever.2
A few studies have found an indirect relationship between milk fever and subsequent metritis.2
There is no reliable evidence that the occurrence of milk fever or subclinical hypocalcemia in cows which recover following treatment affects milk production in the subsequent lactation. Some studies have found a limited effect, no effect or even positive effect of milk fever on milk production.
An odds ratio of 8.1 or mastitis has been estimated; for coliform mastitis an odds ratio of 9.0 and for acute clinical mastitis a relative risk of 1.5 following milk fever have been found.2
Odds ratios ranging from 2.3 to 3.4 for left side displacement of the abomasum occurring in dairy cows with hypocalcemia at parturition have been estimated.2
Studies on the occurrence of ketosis following milk fever have found relative risks or odds ratios ranging from 1.3 to 8.9 and using all the confidence intervals the relative risks/odds ratios range from 1.1 to 15.3.2
A temporary drop in body weight occurs in cows with milk fever but there is no long-term effect. In cows with subclinical hypocalcemia in early lactation there may be some weight loss compared with cows with normal levels of calcium.2
There may be an increased probability of culling cows which have had milk fever because of the complications or direct or indirect consequences associated with the disease.2 There is some evidence of culling cows in early lactation because of milk fever but not in late lactation.
PATHOGENESIS
The literature on the role of acid–base physiology on the pathogenesis of parturient hypocalcemia and the application of the DCAD in theory and practice has been reviewed.11
Hypocalcemia
Plasma calcium concentration is normally maintained between 2.1 and 2.6 mmol/L (8.5 –10.4 mg/dL). Almost all dairy cows will experience subclinical hypocalcemia, <1.8 mmol/L (7.5 mg/dL) within 24 h after calving. In some cows, the hypocalcemia is more severe, <1.25 mmol/L (5 mg/dL) causing neuromuscular dysfunction resulting in clinical milk fever. Without treatment, levels may continue to decline to about 0.5 mmol/L (2 mg/dL) which is usually incompatible with life.13
Hypocalcemia is the cause of the signs of typical ‘milk fever’. Atony of skeletal muscle and plain muscle are well-known physiological effects of hypocalcemia. Hypophosphatemia and variations in levels of serum magnesium also occur and have secondary roles. In experimental hypocalcemia in cattle, there is:
• A marked reduction in the stroke volume and cardiac output
Total plasma calcium concentration at calving is not significantly related to fat and protein-corrected milk yield at any lactation period.14
In rare cases, pathological changes in the myocardium of hypocalcemic parturient cows have been reported.9 The cows were hypocalcemic, recumbent, with tachycardia, arrhythmia, and dyspnea and failed to respond to calcium therapy, however, the cause of the lesions was not determined. The plasma natriuretic peptide concentrations were elevated indicating myocardial injury.15
Experimental hypocalcemia
The literature of Na2 EDTA induced hypocalcemia has been reviewed.16 A standardized flow rate of 1.2 mL/kg per hour of a 5% solution of Na2 EDTA until recumbency, results in changes in plasma ionized calcium, total calcium, inorganic phosphate and magnesium comparable with spontaneous milk fever.17 Induced hypocalcemia in cows results in a depression of frequency and amplitude of rumen contractions as early as 1.0 mmol/L of ionized serum calcium well before any clinical signs of hypocalcemia are detectable and while feeding behavior and rumination are still normal.18 The induction of subclinical hypocalcemia in cows results in a linear decrease in feed intake and chewing activity as the plasma ionized calcium decreases.18 The reduced feed intake was observable at ionized calcium below 0.9 mmol/L before the cows had developed other signs of hypocalcemia.18 Feed intake approached zero when ionized calcium declined to 0.6 mmol/L. This suggests that hypocalcemia may contribute to the reduction in feed intake prepartum and depresses the rumination process ultimately leading to anorexia. Hypercalcemia induced with calcium borogluconate depresses the frequency of rumen contractions but not the amplitude.19
In experimental hypocalcemia in sheep, blood flow is reduced by about 60% to all tissues except kidney, heart, lung, and bladder in which the reduction is not as high. During periods of prolonged hypocalcemia in cows and ewes, blood flow to skeletal muscles and the alimentary tract may be reduced to 60–70% of normal for a long period and predispose to the downer cow syndrome. In both cows and sheep there is a significant increase in PO2 causing an impairment of oxygen uptake by the pulmonary blood flow and an impairment of peripheral tissue uptake of oxygen during hypocalcemia in cows and sheep. Serum calcium and serum phosphate levels are significantly lower in clinical cases than in normal, comparable cows and there is some relationship between the severity of the signs and the degree of biochemical change. The complete response to the parenteral administration of calcium salts in most cases and the occurrence of tetany coincident with hypocalcemia after the IV administration of NaEDTA is further evidence of the importance of hypocalcemia. In addition, some signs indicative of parathyroprivical tetany in other species are observed in the initial stages of milk fever:
• Tetany, particularly of the hindlimbs
• Hypersensitivity and convulsive movements of the head and limbs.
The IV infusion of EDTA into cows over a period of 4–8 h results in severe hypocalcemia and paresis which is a reliable model for the reproduction of the disease. In the experimental disease, there are additional signs such as excessive salivation, excessive lip and tongue actions and tail lifting. The serum muscle enzyme levels of creatine phosphokinase (CPK) and aminotransferase (AST) increase due to muscle injury associated with prolonged recumbency. Blood glucose levels increase and serum phosphorus and potassium levels decrease. A prolongation of the ST interval of the electrocardiogram (ECG) occurs, which may be useful as a diagnostic aid if suitable mini-ECG recorders could be made available for field use.
The prolonged infusion of EDTA in sheep over 18 h at a rate to induce hypocalcemia and maintain recumbency resulted in prolonged periods of recumbency ranging from 36 to 64 h before the animals were able to stand. There are also decreases in plasma sodium, plasma potassium, and erythrocyte potassium and prolonged increases in packed cell volumes, which suggests that fluid replacement therapy may be indicated in cattle with prolonged recumbency associated with hypocalcemia. A 4-h IV infusion of EDTA in high erythrocyte potassium and low erythrocyte potassium dairy cows causes decreases in plasma inorganic phosphorus and plasma potassium which are still below normal 24 h later.20 The AST(SGOT), CPK and packed cell volumes (PCVs) and white blood cell (WBC) counts are also elevated 24 h later. Plasma magnesium and erythrocyte sodium and potassium were decreased but this action was delayed. The increase in PCV was most pronounced in the low erythrocyte potassium cows, which may provide some clues about the pathogenesis of the downer cow syndrome. Some cows may have a more precipitate increase in PCVs due to loss of plasma volume and an inability to mobilize calcium. A 200 mL solution of 10 g of sodium chloride and 0.5 g of potassium chloride can be given IV to sheep safely over a period of 4–8 min to study the effects of administering such hypertonic solutions in downer animals.20
Hypomagnesemia
When hypomagnesemia coexists with hypocalcemia the clinical signs continue but with normal or higher than normal levels, relaxation, muscle weakness, depression, and coma supervene. It is likely that the hypocalcemic tetany is overcome by the relative hypermagnesemia (the ratio of Ca:Mg may change from 6:1 to 2:1) approximating the ratio at which magnesium narcosis develops. There is normally a rise in serum magnesium levels at calving but in those cases of parturient paresis in which tetany is a feature serum magnesium levels are low. These low levels are in many cases expressions of a seasonal hypomagnesemia.
Hypophosphatemia
Low serum phosphorus levels occur in milk fever and contribute to the clinical signs. Some cases of milk fever may not respond to calcium injectins even though the serum calcium levels return to normal but may appear to recover when the udder is inflated and serum phosphorus levels rise. Field observations indicate sodium acid phosphate given orally or IV may result in recovery of cases not responding initially to calcium salts. However, it is difficult to reconcile the biochemical and clinical findings with low serum phosphorus levels because of the absence of recumbency in other animals with profound hypophosphatemia for long periods. A possible explanation is that the hypophosphatemia which occurs in milk fever is secondary to the hypocalcemia and recumbency rather than being a concurrent event. There is experimental evidence to support this and it also seems probable that the hypophosphatemia could prolong the duration of recumbency.
CLINICAL FINDINGS
Cattle
Three stages of milk fever in cattle are commonly recognized and described.
Stage 1
In the first stage, the cow is still standing. This is also the brief stage of excitement and tetany with hypersensitivity and muscle tremor of the head and limbs. The animal is disinclined to move and does not eat. There may be a slight shaking of the head, protrusion of the tongue, and grinding of the teeth. The rectal temperature is usually normal to slightly above normal. Stiffness of the hindlegs is apparent, the animal is ataxic and falls easily and, on going down, the hindlegs are stuck out stiffly.
Careful observations by owners and veterinarians have revealed an even earlier stage than the first one. It is characterized by anorexia, agalactia, rumen stasis, scant feces and a normal temperature, heart rate and respirations. There are no obvious signs of excitement and hypersensitivity characteristic of the first stage. Affected cows may remain in this prodromal stage for several hours; they are perplexing diagnostically and respond quickly to calcium therapy. Cows with this form of hypocalcemia may be the ‘calcium cyclers’ described earlier.
Stage 2
The second stage is prolonged sternal recumbency. Consciousness is usually depressed; the cow has a drowsy appearance in sternal recumbency, usually with a lateral kink in the neck or the head turned into the flank. When approached, some of these cows will open their mouths, extend their head and neck and protrude their tongues, which may be an expression of apprehension and fear in an animal unable to stand. The tetany of the limbs present in the first stage is not present and the cow is unable to stand. The muzzle is dry, the skin and extremities cool, and the rectal temperature subnormal (36–38°C, 97–101°F). There is a marked decrease in the absolute intensity of the heart sounds and an increase in rate (about 80 bpm). The arterial pulse is weak and the venous pressure is also low, making it difficult to raise the jugular veins. The respirations are not markedly affected, although a mild forced expiratory grunt or groan is sometimes audible.
The eyes are usually dry and staring. The pupillary light reflex is incomplete or absent and the diameter of the pupil varies from normal to maximum dilatation. A detailed examination of the pupils of cows with parturient paresis, non-paretic disorders, and non-parturient paresis found that the mean sizes of the pupils were not significantly different from one another.21 Rather, disparity of the size of the pupils was common. Ruminal stasis and secondary bloat are common and constipation is characteristic. There is also relaxation of the anus and loss of the anal reflex.
In cows which develop hypocalcemia a few hours before or at the time of parturition, the second stage of parturition may be delayed, which is unexpected in a mature cow. Examination of the reproductive tract usually reveals a fully dilated cervix and normal presentation of the fetus. The cow may be in any stage of milk fever and administration of calcium borogluconate IV will usually result in a rapid beneficial response and normal parturition.
Prolapse of the uterus is a common complication of milk fever and often the calcium levels are lower than in parturient cows without uterine prolapse. Thus it is standard practice to treat cases of uterine prolapse with calcium salts IV.
Stage 3
The third stage is lateral recumbency. The cow is almost comatose and although the limbs may be stuck out there is complete flaccidity on passive movement and the cow cannot assume sternal recumbency on its own. In general, the depression of temperature and the cardiovascular system are more marked. The heart sounds are almost inaudible and the rate increased up to 120 bpm; the pulse is almost impalpable and it may be impossible to raise the jugular veins. Bloat is usual because of lateral recumbency. Without treatment, a few animals remain unchanged for several hours but most become progressively worse during a period of several hours and dye quietly from shock in a state of complete collapse.
Mild to moderate tetany and hyperesthesia persisting beyond the first stage suggests a concurrent hypomagnesemia. There is excitement and fibrillary twitching of the eyelids and tetanic convulsions are readily precipitated by sound or touch. Trismus may be present. The heart and respiratory rates are increased and the heart sounds are much louder than normal. Without treatment death occurs during a convulsion.
Sheep and goats
The disease in pastured ewes is similar to that in cattle. The early signs include a stilty, proppy gait and tremor of the shoulder muscles. Recumbency follows, sometimes with tetany of the limbs but the proportion of ewes with hypocalcemia which are recumbent in the early stages is much less than in cattle. A similar generalization applies to female goats. The characteristic posture is sternal recumbency, with the legs under the body or stretched out behind. The head is rested on the ground, there may be an accumulation of mucus exudate in the nostrils. The venous blood pressure is low and the pulse impalpable. Mental depression is evidenced by a drowsy appearance and depression of the corneal reflex. There is loss of anal reflex, constipation, tachycardia, hyposensitivity, ruminal stasis and tympany, salivation and tachypnea.22 Response to parenteral treatment with calcium salts is rapid, the ewe is normal 30 min after a SC injection. Death often occurs within 6–12 h if treatment is not administered. The syndrome is usually more severe in pregnant than in lactating ewes, possibly because of the simultaneous occurrence of pregnancy toxemia or hypomagnesemia. Fat late pregnant ewes on high grain diets indoors or in feedlots show a similar syndrome accompanied by prolapses of the vagina and intestine.
CLINICAL PATHOLOGY
Total serum calcium levels are reduced to below 8 mg/dL (2.0 mmol/L), usually to below 5 mg (1.2 mmol/L) and sometimes to as low as 2 mg (0.5 mmol/L). The reduction is usually, but not always, proportional to the severity of the clinical syndrome. Average figures for total serum calcium levels in the three species are cows 5.2± 1.2 mg/dL (1.30± 0.30 mmol/L), ewes 4.6± 1.5 mg/dL (1.15± 0.37 mmol/L), goat does 3.8± 0.6 mg/dL (0.94± 0.15 mmol/L).
Total serum calcium levels are a basis for comparison between species. Blood levels of ionized calcium are a better indicator of calcium status but their estimation has been too difficult until recently. Although total serum calcium levels are used to express the animals’ status with regard to calcium, it is possible that differences between the ionized and non-ionized compartments of total calcium may be more important than the total level. The development of a reliable calcium ion-selective electrode now makes it possible quickly and directly to determine the biologically active portion of calcium in plasma or serum. However, the correlation between ionized and total calcium is excellent. Equine, bovine, and ovine blood may be stored for up to 48 h without any clinically relevant alteration of blood calcium ion concentration.
Normal levels of ionized calcium (as CaF) in venous whole blood of cows are 4.3–5.1 mg/dL (1.06–1.26 mmol/L) serum, slight hypocalcemia 4.2–3.2 mg/dL (1.05–0.80 mmol/L), moderate 3.2–2.0 mg/dL (0.79–0.50 mmol/L) and severe hypocalcemia <2.0 mg/dL (<0.50 mmol/L) serum. Total serum calcium levels are reduced below normal in all cows at calving whether they have milk fever or not, but not in ewes.
A commercially available water hardness test kit can be used as a rapid, inexpensive method of estimating serum calcium concentrations for the diagnosis of hypocalcemia in dairy cattle.23 There is a high correlation and linear relationship between the test kit results and a standard laboratory based method. However, the blood sample must be centrifuged to obtain serum for use in the test kit.
Serum magnesium levels are usually moderately elevated to 4–5 mg/dL (1.65–2.06 mmol/L) but in some areas low levels may be encountered, especially in cows at pasture.
Serum inorganic phosphorus levels are usually depressed to 1.5–3.0 mg/dL (0.48–0.97 mmol/L).
Blood glucose levels are usually normal, although they may be depressed if ketosis occurs concurrently. Higher than normal blood glucose levels are likely to occur in cases of long duration and are therefore an indication of a poorer than normal prognosis.
Serum muscle enzymes
Prolonged recumbency results in ischemic muscle necrosis and increases in the serum muscle enzymes creatine phosphokinase (CPK) and aspartate aminotransferase (AST) or SGOT. During prolonged recumbency following treatment for milk fever, the levels of CPK will remain elevated if muscle necrosis is progressive in animals which are not rolled from side to side every few hours to reduce the effects of compression on the large muscle groups of the pelvic limbs (see Downer cow syndrome).
Hemogram
Changes in the leukocyte count include an eosinopenia, a neutrophilia, and a lymphopenia suggestive of adrenal cortical hyperactivity, but similar changes occur at calving in cows which do not develop parturient paresis. High plasma cortisol levels and packed cell volumes occur in cows with milk fever and are higher still in cows that do not respond to treatment. They are expressions of stress and dehydration. Clinicopathological findings in the other species are not described in detail except with regard to depression of total serum calcium levels.
DIFFERENTIAL DIAGNOSIS
A diagnosis of milk fever is based on the occurrence of paresis and depression of consciousness in animals following parturition. The diagnosis is supported by a favorable response to treatment with parenteral injections of calcium solutions and by biochemical examination of the blood. In ewes, the history usually contains some reference to recent physical stress and the disease is more common in the period preceding lambing.
In the immediate postpartum period, there are several diseases which cause recumbency in cows and their differentiation is summarized in Table 29.5.
Several diseases which occur at the time of parturition must be differentiated from milk fever in cattle. These are grouped here according to:
Metabolic diseases
Hypomagnesemia may occur as the sole cause of recumbency or it may accompany a primary hypocalcemia so that the case presented is one of parturient paresis complicated by lactation tetany. Hyperesthesia, tetany, tachycardia, and convulsions are common instead of the typical findings of depression and paresis in milk fever.
Hypophosphatemia, which commonly accompanies milk fever, is suggested as a cause of continued recumbency in cows after partial response to calcium therapy; serum inorganic phosphorus levels are low and return to normal if the cow stands or following treatment with phosphate salts. A sudden onset of recumbency in dairy cows associated with a marginal deficiency of phosphorus has been reported.24
Hypokalemia in dairy cows is characterized by extreme weakness or recumbency, especially after treatment for ketosis with isoflupredone. Hypokalemia is marked as ranging from 1.4 to 2.3 mEq/L. The case-fatality rate is high in spite of therapy with potassium. Hypokalemic myopathy is present at necropsy.
Ketosis may complicate milk fever, in which case the animal responds to calcium therapy by standing but continues to manifest the clinical signs of ketosis, including in some cases the nervous signs of licking, circling, and abnormal voice.
Diseases associated with toxemia and shock
During the immediate postparturient period, several diseases occur commonly and are characterized by toxemia.
Peracute coliform mastitis is characterized by:
• Fever initially followed by hypothermia
• Enlarged mammary gland(s) with watery and serous-like secretions with small particles barely visible.
Aspiration pneumonia secondary to regurgitation and aspiration of rumen contents may occur as a complication of thirdstage milk fever. Fever, dyspnea, expiratory grunt, severe depression and anxiety are common. Auscultation of the lungs reveals the presence of abnormal lung sounds. Aspiration pneumonia should be suspected if the animal has been lying on its side, especially if there is evidence of regurgitation of ruminal contents from the nostrils, no matter how small the amount, or if there is a history of the animal having been drenched. Abnormal auscultatory findings may not be detectable until the second day. Early diagnosis is imperative if the animal is to be saved and the mortality rate is always high.
Acute diffuse peritonitis resulting from traumatic perforation of the reticulum or uterus is characterized by:
• Grunting or groaning with each respiration
• Fluid splashing sounds on ballottement of the abdomen (paralytic ileus).
Carbohydrate engorgement results in:
Many cases resemble second-stage milk fever.
Toxemic septic metritis occurs most commonly within a few days after parturition and is characterized by:
The fetal placenta may be retained. Some affected cows are weak and prefer recumbency, which resembles milk fever. Prolapse and rupture of uterus causes varying degrees of:
A history of difficult parturition or assisted dystocia with fetotomy may be associated with rupture of the uterus. The administration of calcium salts may cause ventricular fibrillation and sudden death.
Although some elevation of the temperature may be observed in these severe toxemic states, it is more usual to find a subnormal temperature. The response to calcium therapy is usually a marked increase in heart rate and death during the injection is common. Every case of recumbency must be carefully examined as these conditions may occur either independently or as complications of parturient paresis. In our experience, about 25% of cases of postparturient recumbency in cows are due primarily to toxemia or injury rather than to hypocalcemia.
Injuries to the pelvis and pelvic limbs
Injuries to the pelvis and pelvic limbs are common at parturition because of the marked relaxation of the ligaments of the pelvic girdle. Seven types of leg abnormality have been described in this group at an incidence level of 8.5% in 400 consecutive cases of parturient paresis. The abnormalities included radial paralysis, dislocation of the hips and rupture of gastrocnemius muscle. In most instances the affected animals are down and unable to stand but they eat, drink, urinate and defecate normally, have a normal temperature and heart rate and make strong efforts to stand, particularly with the forelimbs.
Maternal obstetrical paralysis is the most common injury. Although this occurs most frequently in heifers after a difficult parturition, it may also occur in adult animals following an easy birth and occasionally before parturition, especially in cows in poor body condition. The mildest form is evidenced by a frequent kicking movement of a hindleg, as though something was stuck between the claws. All degrees of severity from this, through knuckling and weakness of one or both hindlegs, to complete inability to rise may occur, but sensation in the affected limb is usually normal. There is traumatic injury to the pelvic nerves during passage of the calf. There are often gross hemorrhages, both deep and superficial and histopathological degeneration of the sciatic nerves. In individual animals, injury to the obturator nerves is common and results in defective adduction of the hindlimbs. The position of the hindlimbs may be normal but in severe cases, especially those with extensive hematoma along the sciatic nerve trunk, the leg may be held extended with the toe reaching the elbow as in dislocation of the hip; however in the latter case there is exaggerated lateral mobility of the limb. Additional injuries causing recumbency near parturition include those associated with degenerative myopathy, dislocation of the hip and ventral hernia.
Dislocation of the coxofemoral joint can cause recumbency and inability to stand in some cows, while others can stand and move around. Recumbent cows are usually in sternal recumbency and the affected limb is abducted excessively. In standing cows, the affected limb is usually extended, often difficult to flex and often rotated about its long axis. The diagnostic criteria are:
• Sudden onset of lameness with the affected limb extended and possibly rotated
• Displacement of the greater trochanter of the femur from its normal position relative to the ischiatic tuber and coxal tuber of the pelvis
• Ability to abduct the limb manually beyond its normal range
• Crepitus in the hip on abduction and rotation of the limb
• Ability to palpate the femoral head per rectum or per vaginum against the cranial border of the ilium or pubis in cases of cranioventral dislocation, or in the obturator foramen in cases of caudoventral dislocation.
Manual replacement by closed reduction is successful in 80% of craniodorsal dislocation and 65% in caudodorsal dislocation. The ability to stand before reduction is the most useful prognostic aid.
Degenerative myopathy (ischemic muscle necrosis)
Degenerative myopathy affecting primarily the large muscles of the thighs, occurs commonly in cattle which have been recumbent for more than several hours. At necropsy, large masses of pale muscle are present surrounded by muscle of normal color. Clinically it is indistinguishable from sciatic nerve paralysis. Markedly increased serum levels of CPK occur in cows recumbent for several hours following the initial episode of milk fever due to ischemic necrosis. Persistent elevation of CPK indicates progressive ischemic muscle necrosis due to continued compression of large muscle masses of the pelvic limbs. Rupture of the gastrocnemius muscle or separation of its tendon from either the muscle or the tuber calcis may also cause myopathy.
Downer cow syndrome
Downer cow syndrome is a common sequel to milk fever in which the cow was in sternal recumbency for several hours before being treated with calcium. Following treatment, most of the clinical findings associated with milk fever resolved except the animal was unable to stand. Clinically, the animal may be normal except for recumbency and will commonly recover and stand normally within several hours or a few days. Most downer cows eat and drink normally, their vital signs are within the normal range and their alimentary tract function is normal. However, some are anorexic, may not drink, exhibit bizarre movements of lying in lateral recumbency, and dorsally extend their head and neck frequently, moan and groan frequently, assume a frog-legged posture with their pelvic limbs and crawl or creep around the stall and may die or are euthanized for humane reasons in a few days. The diagnostic dilemma with these cows is that they resemble milk fever and whether or not to treat them with additional amounts of calcium salts is questionable.
Non-parturient hypocalcemia
Paresis with mental depression and associated with low total serum calcium levels can occur in cows at times other than at parturition. The cause is largely unexplained but the syndrome occurs rarely in animals other than ruminants. Hypocalcemia may occur after gorging on grain and may be a significant factor in particular cases. Sudden rumen stasis due to traumatic reticulitis may rarely cause hypocalcemic paresis. Diarrhea, particularly when cattle or sheep are placed on new lush pasture, may also precipitate an attack. Access to plants rich in oxalates may have a similar effect, particularly if the animals are unaccustomed to the plants. Affected animals respond well to calcium therapy but relapse is likely unless the primary cause is corrected. The differential diagnosis of diseases of non-parturient cows manifested principally by recumbency is also summarized in Table 29.5.
Hypocalcemic paresis in sheep and goats
Hypocalcemia in sheep must be differentiated from pregnancy toxemia in which the course is much longer, the signs indicate cerebral involvement and the disease is restricted to pregnant ewes. There is no response to calcium therapy and a positive test for ketonuria is almost diagnostic of the disease. At parturition, goats are susceptible to enterotoxemia and hypoglycemia (rarely), both of which present clinical signs similar to parturient paresis.
Hypocalcemia in sows
Hypocalcemia is rare in sows. The disease must be differentiated from the mastitis, metritis and agalactia complex, which is characterized by:
Treatment
Every effort should be made to treat affected cows as soon as possible after clinical signs are obvious. Treatment during the first stage of the disease, before the cow is recumbent, is the ideal situation. The longer the interval between the time the cow first becomes recumbent and treatment, the greater the incidence of the downer cow syndrome due to ischemic muscle necrosis from prolonged recumbency. Complications of milk fever occur when cows have been in sternal recumbency for more than 4 h. Farmers must be educated to appreciate the importance of early treatment. Cows found in lateral recumbency (third stage) should be placed in sternal recumbency until treatment is available. This will reduce the chances of aspiration if the cow regurgitates. Cows that have difficulty finding solid, non-slip footing beneath them, for example, a slippery barn floor or slippery mud, will often not try to stand and may develop ischemic myonecrosis. Avoidance of this complication necessitates the placement of rubber or other mats under the cow or transportation of the cow to a piece of pasture with a dense sward on it. A temperature of greater than 39°C (102°F) is an indication of a higher than average mortality rate due to pre-existing complications.
Standard treatment
Calcium borogluconate at 10–200 g is the treatment of choice. The solutions available vary from 18 to 40% calcium borogluconate. Most cows with milk fever can be treated successfully with 8–10 g of calcium (calcium borogluconate is 8.3% calcium). For cattle, 400–800 mL of a 25% solution is the usual dose. The dose rate of calcium is frequently under discussion. There is a general tendency for veterinarians to underdose with calcium salts, largely because of toxic effects which tend to occur when all of the calcium is given IV. As an initial dose a large cow (540–590 kg) requires 800–1000 mL of a 25% solution and a small cow (320–360 kg) 400–500 mL. Underdosing increases the chances of incomplete response, with inability of the cow to rise, or of relapse. In general, 12 g of calcium is superior to 8 g, which in turn is superior to 6 g.
The standard rate of administration is a rapid intravenous administration of the calculated dose of calcium borogluconate (often supplemented with phosphorus, magnesium, and glucose) over a period of 15 min. Hypercalcemia (up to 22 mg/dL) occurs following IV administration of calcium borogluconate over a period of 12–15 min. The plasma calcium concentration will gradually decline over a period of several hours by which time calcium homeostasis should begin to occur and levels return to normal by 12–24 h following treatment. However, in some cows calcium homeostasis is ineffective and subclinical or clinical hypocalcemia may recur.
Because up to 50% of cows may not respond to the initial rapid administration, it has been postulated that the increases in electrolytes are only transient and that slower IV infusion would be more effective.25 The slow infusion of a calcium solution via an IV indwelling catheter over 6 h was compared with the conventional single IV administration of 600 mL of a 40% calcium borogluconate solution containing 18.78 g calcium gluconate and borogluconate with 6% magnesium hypophosphite (11.82 g magnesium hypophosphite) over 15 min in cows recumbent with milk fever.25 Cows receiving the rapid infusion responded more quickly and stood sooner and their demeanor returned to normal more quickly. The slow infusion consisted of 200 mL IV over a 10 min period and the remaining 400 mL added to 10 L of a solution of 90 g sodium chloride and 500 g glucose and given via IV drip over a 6 h period at a rate of 1.7 L/h. In cows treated rapidly, the serum calcium and magnesium levels increased rapidly compared with the infused cows.26 In both groups, the serum inorganic phosphorus increased slowly with the mean concentration reaching a maximum at 3 h and then decreasing slightly. Some cows are still hypophosphatemic several hours later.
In sheep and goats, the recommended amount is 15–20 g IV with an optional 5–10 g SC. Sows should receive 100–150 mL of a similar solution IV or SC.
Routes of administration
IV and SC routes
The intravenous (IV) route is preferred because the response is rapid and obvious. The heart should be auscultated throughout the intravenous administration for evidence of gross arrhythmia, bradycardia, and tachycardia. If any of these occurs, the intravenous administration should be interrupted and continued only after the heart sounds return to normal. If the cardiac irregularity continues, the remainder of the solution can be given subcutaneously. The best recommendation is to give as much of the solution as possible intravenously and the remainder subcutaneously. The common practice of giving half the dose intravenously and half subcutaneously is a reasonable compromise because with this method there are fewer relapses. If a cow has been previously treated subcutaneously by the farmer, additional calcium given intravenously may cause toxicity if the improved circulation enhances the absorption of the subcutaneous calcium.
SC route
The subcutaneous (SC) route is commonly used by farmers who treat affected cows at the first sign of hypocalcemia, preferably during the first stage when the cow is still standing or as prophylaxis to all high-risk cows immediately after calving. The SC route has also been used by veterinarians when the effects of IV administration of calcium are uncertain or if an unusual response occurs during IV administration. There are limitations to the effectiveness of SC calcium solutions given to cows with milk fever.27 Cows given 300 mL of 33.3% or 40% calcium borogluconate SC had serum calcium levels of 1.4 mmol/L; those receiving 600 mL had serum calcium levels of 2.1 mmol/L, at mean intervals of 4.8 and 12.0 h between treatments by the herdsman and veterinary attention.27 At the time of sampling, 48% of cows receiving 600 mL of calcium borogluconate had a serum calcium level below 2.0 mmol/L. If the veterinarian is unable to treat the cow within 1 h, a dose of 600 mL of 40% calcium borogluconate should be given SC in two sites and massaged well to promote absorption. Waiting for more than 1 h to assess the effect of one treatment SC is regarded as conducive to development of the downer cow syndrome. The cow should then be placed in a dry area with her limbs positioned to minimize ischemic necrosis and covered with straw and tarpaulins until the veterinarian arrives.
Toxemic cows are very susceptible to the IV administration of calcium borogluconate and death may occur. In such cases the heart rate increases markedly (up to 160 bpm), there is respiratory distress, trembling and collapse and the cow dies within a few minutes. SC or IP administration is preferred in cows with severe toxemia due to aspiration pneumonia, metritis, and mastitis.
Typical response to calcium borogluconate
Cows with milk fever exhibit a typical pattern of response to calcium borogluconate IV if the response is favorable, including:
• Muscle tremor, particularly of the flanks and often extending to the whole body
• Slowing and improvement in the amplitude and pressures of the pulse
The feces are in the form of a firm fecal ball with a firm crust and covered with mucus, occasionally with a few flecks of blood. Urination usually does not follow until the cow stands. A slight transitory tetany of the limbs may also be observed. Many cows will eat and drink within minutes following successful treatment if offered feed and water.
The rate of response to treatment is affected by many factors as set out below and it is unwise to quote what might be expected as an acceptable rate of recovery after treatment. This is particularly true if cows are treated by the farmer and only difficult cases are presented to the veterinarian. In general, if all cases are considered and there are no exceptional circumstances, recovery can be expected immediately after treatment in about 60% of cases and in a further 15% after 2 h; 10% have recoveries complicated by one of the diseases discussed earlier and 15% can be expected either to die or to require disposal. Of those which recover after one treatment, 25–30% can be expected to relapse and require further treatment.
Unfavorable response to calcium borogluconate
An unfavorable response is characterized by a marked increase in heart rate in cows affected with toxemia and acute heart block in apparently normal animals especially with overdosage, with too rapid injection and in cases in which treatment has been unduly prolonged. In the latter, the maximum tolerated dose of calcium borogluconate by IV administration is about 250 mL of a 25% solution. Overdosage may occur when farmers treat cases unsuccessfully by multiple SC injections and these are followed by an IV dose. When the peripheral circulation is poor, it is probable that the calcium administered SC is not absorbed until the circulation improves following the IV injection and the large doses of calcium then absorbed cause acute toxicity. In all cases of IV injection, the circulation must be monitored closely. Some degree of arrhythmia occurs in most cases but if there is gross arrhythmia or a sudden increase in heart rate, the injection should be stopped temporarily or continued with great caution. In normal circumstances at least 10 min should be taken to administer the standard dose. The acute toxic effect of calcium salts seems to be exerted specifically on heart muscle with a great variety of defects occurring in cardiac action; the defect type depends on the specific calcium salt used and the speed of injection. ECG changes after induced hypercalcemia show increased ventricular activity and reduced atrial activity. Atropine is capable of abolishing the resulting arrhythmia.
Sudden death may also occur after calcium injections if the cow is excited or frightened, which may be due to an increased sensitivity to epinephrine. When affected cows are exposed to the sun or a hot, humid atmosphere, heatstroke may be a complicating factor. In such cases an attempt should be made to reduce the temperature to below 39.5°C (103°F) before the calcium is administered. The incidence of cardiac arrhythmia and other abnormalities as detected by ECG during treatment with calcium salts IV is so high that there are doubts expressed about the suitability of this form of treatment.
Chronic toxicity may also occur. In laboratory animals, severe uremia due to extensive calcium deposits in the kidney occur after the SC injection of calcium chloride and borogluconate and similar deposits are often seen at necropsy in cows dying after multiple injections of calcium salts administered at short intervals.
Failure to respond to treatment
A failure to respond favorably to treatment may be due to an incorrect or incomplete diagnosis, or inadequate treatment. A poor response to treatment includes: (1) no observable changes in the clinical findings immediately following the calcium administration or (2) the animal may respond to the calcium in all respects with the exception of being unable to stand for varying periods of time following treatment. An inadequate response also includes relapses after successful recovery, which usually occur within 48 h of the previous treatment. Relapses are more common in certain individual cows such as mature Jersey cows, which may experience as many as five or six episodes around one calving. Also, the incidence of relapse is much higher in cases which occur just before calving than in those which occur afterwards. The needs of individual animals for calcium replacement vary widely, depending on their body weight and the degree of hypocalcemia. Incomplete responses may be more common in older cows and may be associated with diminished skeletal reserves of calcium and inability of the normal mechanisms to maintain serum calcium levels during the period of excessive demands of lactation. The duration of the illness and the posture of the cow also affect the response. In an extensive field study, there were no downer cows or deaths in cows still standing when first treated, 13% of downers and 2% of deaths occurred in cows in sternal recumbency and 37% of downers and 12% of deaths occurred in cows in lateral recumbency when first treated.28 Therefore, in general, the longer the period from onset of milk fever to treatment, the longer the period of post-treatment recumbency and the higher the case-fatality rate. In another study, 67% of cows recovered after a single treatment, 90% after two treatments, and 92–99% after three treatments. After routine treatment, 37% of cases rose unassisted within 10 min, 23% required some assistance, 26% recovered after longer periods of recumbency, and 14% died or were destroyed or sold for slaughter. The best procedure to follow if response does not occur is to revisit the animal at 12-hourly intervals and check the diagnosis. If no other cause of the recumbency can be determined, the initial treatment can be repeated on a maximum of three occasions. Beyond this point, further calcium therapy is seldom effective. A low body temperature, due probably to exposure to low environmental temperature and increased wind velocities, is positively correlated with a high proportion of deaths and poor responses.
At the second visit, solutions containing either phosphorus, magnesium, or dextrose may be administered, depending upon the clinical signs presented and the results of available biochemical tests. Glucose is usually administered as 500 mL of a 40% solution, sodium acid phosphate as 200 mL of a 15% solution, and magnesium sulfate as 200–400 mL of a 15% solution. Composite solutions containing calcium, magnesium, phosphorus and glucose are also in common use as initial treatments. There is controversy about these socalled ‘polypharmacy’ preparations. They have no advantage, but are likely to remain popular when milk fever cases are complicated by metabolic disorders other than hypocalcemia. They have no effect on the relapse rate when compared with calcium salts alone.
GENERAL MANAGEMENT AND CLINICAL CARE PROCEDURES
The care of the cow and the calf following milk fever is important. The calf should be removed from the cow and for the first 48 h only sufficient milk should be drawn for the calf’s maintenance. A gradual return to full milking can then be permitted. If the cow is recumbent for any length of time, she must be kept propped up in sternal recumbency and not left in lateral recumbency, which may result in regurgitation and aspiration pneumonia. The cow should be rolled from side to side every few hours and provided with adequate bedding or moved to a suitable non-slip ground surface. In extreme climatic conditions, erection of a shelter over the cow is advisable if she cannot be moved to permanent shelter. If a cow is recumbent for more than 48 h, assisted lifting using appropriate cow lifters several times daily should be considered. However, heroic measures to get cows to stand should be avoided. Gentle nudging in the ribs or the use of an electric prod are the maximum stimulants advised. The best assistance that can be given to a cow attempting to stand is a good heave at the base of the tail when she is halfway up.
CONTROL
Various methods for the control of milk fever in ruminants, especially dairy cows, are available. They include dietary management during the transition period before and after calving, administration of calcium gels orally at the time of parturition and administration of vitamin D and its metabolites and analogs immediately before parturition to enhance the mobilization of calcium. When the incidence of milk fever increases to above 10% of high-risk cows (third or later lactations), a specific control program is necessary. When the incidence is low, a specific control program may not be economical and the alternative is to monitor cows carefully at the time of parturition and for 48 h after parturition and treat affected animals during the first stage of the disease if possible.
Various aspects of the literature on the principles of control of milk fever in cattle, under different circumstances have been reviewed.29-31 The different strategies for the control of milk fever in dairy cows has been examined by expert opinions.32 The two control strategies predicted to be most relevant were calcium gel orally peripartum used alone and in combination with a low-calcium diet. Several control strategies for milk fever in dairy herds have been evaluated by stochastic simulation in order to analyze the technical and economic effects at the herd level and to assess frameworks for decision support system development.33 The simulated technical and economic effects indicated a complex interaction between herd and control strategy. In general, the most comprehensive control strategies were economically inferior to similar but less comprehensive control strategies.
Dietary management during prepartum period
For purposes of optimal nutritional management of dairy cows which are fed prepared feeds (not pasture-based), the dry period is divided into at least two distinct categories – cows in the early and middle portion of the dry period (far-off or regular dry cow group) and cows in the final 3 weeks prior to their calving date (pre-fresh, transition, close-up, near, lead feeding, or steam-up group).31 Large herds may have additional subgroups of dry cows depending on management circumstances and facilities available. Special attention must be given to the mineral nutrition of the close-up group. Minerals should be provided to close-up cows in known quantities, either as part of a grain mixture or total mixed ration (TMR).
Calcium and phosphorus nutrition
Level of calcium in diet
Diets high in calcium during the prepartum period can result in a high incidence of milk fever and diets low in calcium will reduce the incidence of milk fever in dairy cows. Feeding more than 100 g of calcium daily during the dry period is associated with an increased incidence of milk fever. A cow weighing 500 kg requires only about 33 g/daily of calcium to meet maintenance and fetal calcium demands in the last 2 months of late gestation.30 Low calcium diets (20 g Ca/d) fed during the last 2 weeks before parturition are highly reliable and effective. The low levels of dietary calcium activate the calcium homeostatic mechanisms before calving and the cow is more able to absorb calcium from the digestive tract and to mobilize calcium from bone reserves. At least 14 days of a low calcium diet are required to be effective in minimizing the incidence of milk fever. Feeding dry cows rations low in calcium results in activation of the calcium homeostatic mechanisms before calving making the cow able to mobilize the large quantities of calcium for the final stages of prenatal growth and colostrum production. When dietary calcium availability is decreased below calcium requirements, the cow is brought into negative calcium balance. This leads to a secretion of parathyroid (PTH) which increases renal reabsorption of calcium within minutes, stimulates calcium resorption from bone within hours to days, and stimulates renal vitamin D metabolism to towards production of 1,25-dihydroxyvitamin D (1,25-(OH)2D) within hours or days. The (1,25-(OH)2D) stimulates the active transport of calcium across the intestinal epithelial cells. During bone resorption, urinary excretion of pyridinoline and deoxypyridinoline, derived from collagen breakdown, is increased.
Practicality of feeding diets low in calcium
There are practical problems with the implementation of the recommendation to feed diets low in calcium. It is difficult to reduce the amounts of calcium and phosphorus fed to cows for several reasons:
• Inability to grow sufficient quantities of feeds, such as corn silage, for the entire herd
• Suitability of land for legume crops which are high in calcium
• Inability to add sufficient phosphorus to lower the ratio of calcium to phosphorus to palatability when quantities of phosphorus are added to the ration.
Most farms utilizing home-grown forages, especially alfalfa, find it difficult to obtain forages which are low in calcium. A low calcium diet can be achieved by replacing some or all alfalfa hay in the dry cow diet with grass hay and using additional corn silage and concentrates. While feeding diets low in calcium during the prepartum period is very effective, the very low calcium intake required necessitates that the cow be in negative calcium balance and in a state of withdrawal of calcium from bone.
Binding dietary calcium
It is possible to prevent milk fever and subclinical hypocalcemia by adding a substance to the feed capable of binding dietary calcium and making it unavailable for absorption. The oral administration of sodium aluminum silicate or zinc oxide to cows in late lactation results in a decrease in total serum calcium.34 Supplementing the dry cow ration with sodium aluminium silicate (zeolite A) at the rate of 1.4 kg of zeolite pellets per day (0.7 kg of pure zeolite) for the last 2 weeks of pregnancy results in an increase in plasma calcium around calving.35 Plasma magnesium and inorganic phosphate levels were decreased and serum 1,25-(OH)2D was significantly increased.35 Feed intake was decreased among zeolite-treated cows during the last 2 weeks of pregnancy but there was no effect on milk yield, milk fat, and milk protein in the subsequent lactation. The addition of zeolite to the daily ration during the last month of pregnancy prevented parturient paresis and subclinical hypocalcemia in Jersey cows.36 Feeding a vegetable oil supplement (soya bean oil) to pregnant pastured dairy cattle during the last 2–3 weeks of pregnancy is effective in preventing milk fever and increases milk solids production in early lactation.37 The same supplement has been used to stimulate calcium absorption and reduction in susceptibility to fasting-induced hypocalcemia in pregnant ewes.38,39 Following supplementation, the ewes are fasted overnight to challenge calcium homeostasis. Following fasting, there is a greatly increased capacity to absorb calcium.
Level of phosphorus in diet
Increased levels of dietary phosphorus, >80 g/head per day, can also increase the incidence of milk fever. The increased intake increases the serum level of phosphorus which has an inhibitory effect on renal enzymes. These enzymes catalyze the production of 1,25-(OH)2 D, which when lowered will reduce intestinal calcium absorption. If the reduction of calcium is impractical, the lowering of phosphorus to below requirements may be beneficial.
Calcium and phosphorus ratio in diet
If the ration is low in calcium, the resulting negative balance of calcium can be expected to stimulate activity of the parathyroid gland. Early researchers made use of this physiological mechanism by feeding a high phosphorus/low calcium ration to cows during the last month of pregnancy. With a Ca:P ratio of 6:1, 30% of cows developed parturient paresis; at a Ca:P ratio of 1:1, 15% developed the disease; and at a ratio of 1:3.3, no cases occurred. Although there is no apparent effect on the subsequent lactation there is the possibility, if the negative balance of calcium is prolonged or repeated frequently, that such a ration may contribute to the development of osteoporosis. Dietary phosphorus concentrations can have an influence on calcium homeostasis.
Acidifying rations in the prepartum diet: Cation–anion difference (DCAD)
A more reliable method of controlling milk fever in dairy cows when the calcium intake exceeds NRC requirements is to manipulate the dietary cation–anion difference (DCAD) during the prepartum period.11,40 Diets high in cations, especially sodium and potassium, tend to induce milk fever compared with those high in anions, primarily chloride and sulfur, which can reduce the incidence. Because most legumes and grasses are high in potassium, many of the commonly used prepartum diets are alkaline resulting in metabolic alkalosis. The feeding of diets which are high in ratio of calcium to phosphorus and containing an excess of anions relative to cations will result in mild metabolic acidosis completely compensated by nonrespiratory mechanisms (decreased blood bicarbonate and base excess: Pco2 and pH are unaffected).41 There is increased concentration of serum calcium due to an increase in the intestinal absorption of calcium. Two parathyroid hormone (PTH) dependent functions, bone resorption, and renal production of 1,25-dihydroxyvitamin D, are enhanced in cows fed diets with added anions which increase their resistance to milk fever and hypocalcemia.11
The DCAD is expressed using the equation DCAD in mEq/kg DM = (Na + K) – (Cl + S). The equation does not include other dietary cations and anions such as Ca2, Mg2, and PO4 which have a minor role. Most studies indicate that a DCAD of – 50 to 100 mEq/kg DM is optimal for the prevention of milk fever.1 Supplementation of diets in the last 3 weeks prepartum with anionic salts at a rate sufficient to decrease DCAD to –15 mEq/100 g of dietary DM and urine pH to 6.0 prevented most cases of parturient hypocalcemia.42 Except for cows pregnant with twins, that rate of supplementation did not affect DM intake and energy balance. A moderate rate of supplementation to reduce the DCAD to 0 mEq/100 g dietary DM and urine pH to 7.3 also did not decrease feed intake or energy status but was less effective in preventing parturient hypocalcemia. Monitoring urine pH can be a useful aid to find the effective intermediate inclusion rate and it is suggested that a urine pH of about 6.5 is ideal.42 Commercial anionic products fed to non-lactating dairy cows in a total mixed ration, after 4 days reduced urine pH below the desired threshold of 6.5.43
The equation assigns the same acidification potency to each mEq of Cl and SO4, but Cl is absorbed to a greater extent than SO4. Calculation of the DCAD of a diet requires use of the equivalent weights of the electrolytes. The equivalent weight is equal to the molecular weight divided by the valence. A milliequivalent (mEq) is used to express equivalent weights: 1 mEq equals 1/1000 of an equivalent. Table 29.6 provides reference values for calculating equivalent weights of important electrolytes and converting from percent diet dry matter (DM) to mEq/kg. Once mEq are calculated, the DCAD can then be determined by subtracting the anions from the cations.31
Table 29.6 Molecular weights, equivalent weights, and conversions from percent to milliequivalents (%–mEq) of anions and cations used in calculating dietary cation–anion difference
The DCAD is calculated from the percent element in the diet dry matter. The equation is as follows: mEq/100 g DM = [(% Na ÷ 0.023) + (% K ÷ 0.039)] – [(% Cl ÷ 0.0355) + (% S ÷ 0.016)]. Based on current evidence, the range which achieves the lowest incidence of milk fever is a DCAD of – 10 to – 15 mEq/100 g DM or – 100 to 150 mEq/kg DM.
Most typical diets fed to dry cows have DCADs of about 100–250 mEq/kg DM. Addition of a cationic salt such as sodium bicarbonate to the dry cow diets increases the DCAD and increases the incidence rate of milk fever. Adding an anion source or a mixture of anionic salts containing Cl and S relative to Na and K to the diet lowers the DCAD and reduces the incidence of milk fever. Commonly used sources of anion salts include the Cl and SO4 salts of calcium, ammonium and magnesium. The phosphate salts have not been used because they are only weakly acidifying.
The addition of anions to the diet to reduce dietary DCAD is limited because of problems with palatability of the anionic salt sources commonly used. If the diet DCAD is >250 mEq/kg, it is difficult to add enough anionic salts to lower the DCAD to the recommended –100 mEq/kg of the diet without affecting palatability.
In one study, the incidence of milk fever was 47% when prepartum cows were fed a ration with a DCAD of +330.5 mEq/kg dietary DM and 0% when the prepartum ration had a balance of –128.5 mEq/kg dietary DM.40 The incidence of milk fever was reduced by the addition of chloride and sulfur in excess relative to sodium and potassium in the diet.40 Because anions are considered acidogenic and cations alkylogenic, an excess of acid-forming elements in periods of calcium stress will increase the concentration of calcium in the blood, either by intestinal absorption or bone mobilization.31 Cows fed prepartum diets containing alfalfa haylage with added chlorides of magnesium, ammonia, and calcium tended to have higher plasma calcium concentrations of calcium and a lower incidence of milk fever than did cows fed either of two cationic diets. Plasma hydroxyproline, an index of bone resorption, also increases prior to parturition in cows fed a high anion diet. The plasma levels of 1,25-(OH)2D also increase prior to parturition in cows fed a high anion diet, which increases calcium absorption and bone resorption.31 Feeding rations with reduced mEq of dietary ([Na+ + K+] – [CI− + SO4=]) to – 4 mEq/kg dietary DM to dry cows significantly affected some of the parameters of bone formation but did not enhance the rate of bone resorption. Feeding acid diets to pregnant cows during the last 28 days of pregnancy increased the mobilization of calcium by 13% 14 days before parturition and 28% by the time of parturition, whereas it had declined by 14% at 14 days before parturition in alkali-fed cows. The increased concentrations of 1,25-(OH)2D were responsible for the stimulation of both intestinal calcium absorption and bone resorption, which helped to prevent severe parturient hypocalcemia.
Anion salts for acidification of prepartum diets for dairy cows
Several anion salts are available for addition to the ration of prepartum dairy cows to prevent milk fever.44 Generally, acidification of the cows occurs in approximately 36 h following addition of the anionic salts to the ration; it also takes less than 36 h for the cow to return to an alkaline state following removal of the salts from the diet. The relative acidifying activity of anionic salts commonly used to prevent milk fever has been evaluated.44 Salts of chloride have about 1.6 times the acidifying activity of sulfate. Calcium and magnesium, which are usually not included in the DCAD equation, have a small but significant alkalinizing effect when accompanied by chloride or sulfate. The ranking of the anion sources tested at a dose of 2 Eq/day, from most to least potent urine acidifier was hydrochloric acid, ammonium chloride, calcium chloride, calcium sulfate, magnesium sulfate, and sulfur. Magnesium sulfate is the most palatable of the anionic salts commonly supplemented and calcium chloride is the least palatable. Sulfates are poor acidifiers and should be limited in use. It is best to add the anionic salts to a total mixed ration. Because of the low incidence of milk fever in heifers there is no need to feed anionic salts to heifers.42
Anionic salts can reduce dry matter intake when more than 300 mEq of anions/kg diet DM are supplemented in the diet.31 The reductions in dry matter intake are commonly ascribed to decreased palatability but may represent a response to the metabolic acidosis induced by the salts.41 The duration of feeding anion salts ranges from 21 to 45 days before expected parturition. At least 5 days of consumption are necessary for maximal benefit.
Ammonium chloride is more effective than most other salts as an acidifier. The addition of ammonium chloride salts to prepartum diets offers considerable promise as a practical and reliable method of control of milk fever. Experimentally, the addition of ammonium chloride and ammonium sulfate, each at 100 g/head per day, to the prepartum diets 21 days prior to parturition, decreased the incidence of milk fever from 17% in the unsupplemented group to 4% in the supplemented group.
Strategies for supplementing anion sources
A systematic protocol for the addition of anions to a prepartum diet and monitoring its effects is as follows:
1. Macromineral analysis of all available forages for prepartum cows.
2. Select feed ingredients with a low DCAD especially those low in potassium.
3. Calculate the DCAD of the diet without any supplemental anion sources. If the DCAD is more than 250 mEq/kg, then it is too high. Replace some of the forage with a lower DCAD forage.
4. Balance dietary magnesium at 0.40%, DM by adding additional magnesium chloride or magnesium sulfate. Magnesium chloride is preferred.
5. Evaluate the feeding management of the prepartum cows. Ensure adequate feeding space and quality of feed.
6. Add supplemental chloride to the prepartum cow diet to lower DCAD to about –150 mEq/kg DM.
7. Evaluate dietary non-protein nitrogen (NPN) and degradable intake protein (DIP) of the diet. If NPN is more than 0.50% of the diet DM or DIP is more than 70% of crude protein, then reduce the amount of ammonium salts or other NPN or DIP sources in the diet.
8. Elevate the dietary calcium to 1.5–1.8% DM or provide daily intake of about 150 g/head per day. Negative DCAD diets increase urinary calcium excretion and thus more dietary calcium is necessary to meet requirements.
9. Monitor dry matter intake of the prepartum cow group. Consider more palatable anion sources or a reduced dose of anion sources if dry matter intake is depressed. Low dry matter intakes in prepartum cows increases the risk for fatty liver and ketosis after calving.
10. After 1 week of feeding anionic salts, monitor the pH of close-up dry cows. Urinary pH is an accurate indication of optimal dietary acidification. Collect urine from at least six cows at one time and average the urinary results. Adjust the dose of supplemental anions to achieve an average urinary pH of between 6.0 and 7.0.
DCAD and acid–base balance of dairy cows on pasture-based diets
The literature on the nutritional strategies for the prevention of hypocalcemia for dairy cows in pasture-based systems has been reviewed.6 The dairy industries of southern Australia and New Zealand are based largely on fresh pasture and pasture silage and grazed pasture is the key determinant of the DCAD. The concentration of potassium is often in excess of 4% and the DCAD >500 mEq/kg DM, in pasture-based diets yet the incidence risk of milk fever is not higher than those in other countries where dietary potassium is much lower. For a considerable part of spring and early summer, the DCAD of pasture in those countries may be in excess of +500–700 mEq/kg DM.5,29 The variation in the DCAD of pasture and the difficulty in accurately assessing dry matter intake makes an accurate reduction in DCAD difficult to achieve practically.5,45 Pasture cation–anion difference in those conditions is not greatly influenced by stocking rate or associated management practices. The urine pH of grazing dairy cows in south-eastern Australia remains relatively constant throughout the year despite changes in stage of lactation, management practices, season, weather, and large changes in DCAD. It appears that a very low DCAD (< + 150 mEq/kg DM is required to alter systemic pH substantially. The DCAD of pasture throughout the year in south-eastern Australia ranges from 0 to 800 mEq/kg DM and is often outside the levels previously recommended for optimal performance of lactating cows. A high DCAD at the time of parturition, for spring-calving herds on pasture, presents practical problems in administering the large amounts of anionic salts required to lower urine pH and to decrease the incidence of hypocalcemia.5,29
The dietary cation–anion difference and the health and production of pasture-fed dairy cows in early lactation in southeastern Australia has been examined in an indoor feeding experiment.46 The dairy industry there is largely perennial ryegrass-based and supplemented with cereal grains, pasture hay, and pasture silage. The DCAD can range from 0 to + 76 mEq/100 g. Blood pH and urine pH are reduced when a low DCAD ration is fed, but there is a threshold level, between + 52 and + 102 mEq/100 g, above which little change in systemic pH occurs.46 As DCAD increased from + 21 to +127 mEq/100 g, DMI intake and milk yield decreased. In nonlactating periparturient cows fed freshly cut pasture supplemented with varying levels of salts to alter the DCAD which ranged from – 12 to + 69 mEq/100 g.47 With decreasing DCAD, the pH of the blood and urine decreased resulting in a nonrespiratory systemic acidosis. When the DCAD was negative, the urinary output of calcium increased. No differences in milk production due to alteration of the DCAD. Due to the high DCAD in the base diet offered to pasture-fed dairy cows and the requirement for a negative DCAD to increase calcium absorption, it is unlikely that the DCAD concept is a practical means of preventing milk fever in pasture-fed cows.
In these pasture-based systems, sulfur (S) is considered a more important dietary constituent in determining the risk of hypocalcemia than either chloride or potassium.45 The absorption efficiency of S is less than either Cl or K and would not be expected to incur the same change in systemic pH. Thus its importance in hypocalcemia prevention, does not fit with the current understanding of how manipulation of DCAD influences calcium homeostasis. Studies indicate that pre-calving dietary S is more important in the control of hypocalcemia than either K or Cl concentration. Although the effects of a systemic acidosis on Ca absorption is accepted, the effect of S on periparturient Ca homeostasis when absorption of S is low in comparison to Cl, Na, or K suggest that there are mechanisms involved that are not related to acid-base balance. Dietary S concentration was more important in the control of hypocalcemia than either dietary K or Cl concentration.
An increased incidence of milk fever may occur in pastured-based dairy when the diet is supplemented with Cl and S, even though calcium absorption, as indicated by urine calcium concentration increases. The increased incidence may be due to a greater demand for dietary calcium after calving following a reduction in the pH of body fluids pre-calving and the fact that pasture-based diets, as opposed to total mixed rations, are generally low in calcium. Supplementation of cows with calcium after calving increased plasma calcium concentration on the day of calving and during the subsequent 14 days.48 Milk production was not affected by pre- or post-calving treatments.
Experimentally, the application of potassium fertilizer on pasture resulted in a DCAD ranging from 350 to 535 mEq/kg DM but calcium homeostasis in pasture-based dairy cows was not changed.45 Plasma concentrations were increased and the risk of clinical periparturient hypocalcemia was reduced by MgCl2 and MgSO4 delivered by 150 g MgCl2, 200 g MgSO4, and 35 g MgO/head daily for 21 days prepartum.45 After calving cows were supplemented with 150 g CaCO3/head per day for 4 days. Improvements in calcium homeostasis were not due to an altered systemic pH.
The optimum DCAD for lactating cows grazing fresh pasture and the effect of deviating from the optimum on milk production has been examined under experimental manipulating the dietary DCAD using a drench in early-lactation dairy cows in New Zealand.49 Dietary cation-anion differences ranged from + 23 to + 88 mEq/100 g of DM. As DCAD increased, there was a linear increase in blood pH and HCO3 concentration and blood base excess. Plasma concentrations of Mg, K, Cl declined as DCAD increased and Na increased. Urinary excretion of Ca decreased as DCAD increased. Increasing DCAD did not significantly affect milk yield or milk protein but the concentration and yield of milk fat increased linearly. Milk production results suggest that DCAD for optimal production on pasture diets may be higher than the +20 mEq/100 g DM previously identified for total mixed rations.
Summary of macromineral nutritional strategies for the prevention of hypocalcemia in the soon-to-calve, or transition dairy cow in pasture-based systems. (Transition period is defined as 3–4 weeks prepartum and 3–4 weeks postpartum)
• When dairy cows are dried off, they are commonly moved onto nonirrigated pastures until calving. In the summer, dry cows would be put onto actively growing tropical pasture, whereas in autumn, winter and spring, the pasture is most likely to be tropical pasture carried over from the previous summer. This carryover pasture is likely to be supplemented with medium quality hay, silage and grain or molasses 2–3 weeks before calving. Anionic salts have been added to these diets
• The DCAD on a yearly basis ranges from 0 to 80 mEq/100 g DM5
• The incidence of milk fever in Australia ranges from 1.6 to 5.4% but in some years, the incidence in individual herds may reach 20%. The incidence of subclinical hypocalcemia can range widely; up to 40% of apparently normal cows had subclinical hypocalcemia (total plasma calcium <1.9 mmol/L during the first 12 days of lactation)
• The concentration of ionized calcium in blood plasma is under elaborate homeostatic control. This provides for the maintenance of a pool of readily available calcium which can be drawn on for the deposition of maternal and fetal bone and for colostrum and milk
• The traditional method of preventing hypocalcemia has been to restrict calcium intake during the dry period which stimulates renal synthesis of 1,25-dihydroxyvitamin D3 prior to calving. The cow must be in negative calcium balance
• In temperate climates, reducing dietary calcium to recommended low levels can be difficult to achieve but in tropical pastures the levels are already low
• Excessive levels of potassium may be the most important dietary risk factor for milk fever in Australian feeding systems. Potassium contents of pastures may be as high as 4–5% of DM. The use of potassium fertilizers exacerbates the problem. Potassium and dietary DCAD peak in winter and are lowest in autumn. The majority of cows in Victoria, Australia, calve in winter to early spring when the potassium levels are high. Excess potassium results in alkalosis which reduces the sensitivity of bone and renal tissue to PTH
• Hypomagnesemia influences calcium homeostasis and diets high in potassium reduce the concentration of plasma magnesium. Magnesium supplementation of the transition diet should be done to ensure that magnesium requirements are met (0.2–0.4% of DMI)
• Excessive dietary phosphorus increases the concentration of phosphorus in plasma which can induce hypocalcemia and increase the incidence of milk fever at calving. Supplements likely to increase the dietary intake of phosphorus above 35 g/day should not be fed to cows in the weeks prior to calving
• Excessive intakes of sodium can contribute to the development of metabolic alkalosis and should be avoided.
Options for reducing the risk of hypocalcemia in pasture-fed dairy cows:
A low calcium diet in the prepartum period will activate calcium homeostasis at calving. An alternative is to feed calcium binding agents for 1–3 weeks prepartum.
Hypomagnesemia influences calcium homeostasis, potentially predisposing the cow to milk fever at calving. Diets high in potassium can reduce the concentration of plasma magnesium and may be a mechanism linking high dietary potassium to hypocalcemia. Recommended concentrations for dietary magnesium levels are within the range of 0.2–0.4% of total DM intake. Plasma levels are readily elevated when magnesium is added to the diet.
Oral administration of gels of calcium chloride or calcium propionate immediately after calving is effective in preventing milk fever. Feeding cows an excess of calcium in the form of calcium carbonate during the first few weeks of lactation is beneficial. Increasing dietary concentration of calcium from 0.68% to 1.02% DM within 8 h of calving has an immediate effect on plasma calcium.6
Manipulating dietary DCAD with anion feeds
The concept of manipulating the DCAD to reduce hypocalcemia under North American and European circumstances is well documented.6 Addition of anionic salts to the diet of transition dry cows to achieve a metabolic acidosis improves the ability of the cow to mobilize calcium at parturition. The pH of both blood and urine consistently fall in response to an appropriate decline in dietary cation excess. For the transition dry cow, the DCAD must fall to between – 10 and – 15 mEq/100 g DM to prevent milk fever.
In pasture-fed systems, such as in Australia and New Zealand, the pasture diets are high in DCAD (+ 50 mEq/100 g DM). The more positive the pasture DCAD, the more difficult it is to balance it with anionic salts. Because anionic preparations are relatively unpalatable, cows will not eat enough for total DCAD to fall below 0 mEq/100 g DM.
An alternative to anionic salts is to select feedstuffs with low to moderate DCAD. Lower quality hays and straws have a lower DCAD than high quality forages. However, the feedstuffs must be analyzed before making such a recommendation. Lower quality roughages are also low in calcium. Concentrates with high negative DCAD include molasses and brewer’s grain. Molasses is high in potassium but also high in sulfur.
Measurement of urine pH as a method of monitoring the efficacy of DCAD manipulations and its on-farm use should be encouraged. Urine pH is unlikely to fall to any degree until DCAD of the diet drops below approximately 10–20 mEq/100 g DM. For protection against milk fever, the urine pH needs to be maintained between 5.5 and 6.2 in the last weeks before calving.1 This could be reduced to a cut-off in the range of pH 6.5–7.0.13,31
Calcium gel dosing
The oral administration of easily absorbed calcium salts such as calcium chloride providing 40–50 g calcium per dose as a bolus, a gel, a paste or a liquid, given in 3–4 doses beginning 12–24 h before calving, to 24 h after calving will prevent a significant proportion of milk fever cases.30 The oral administration of one or two doses as a supplement to intravenous calcium borogluconate is also effective in reducing the incidence of relapses. Calcium chloride is caustic and can cause oral lesions. The use of calcium formate (350 mL of 48.6% aqueous suspension), four times at approximately 12 h intervals, had no adverse effects on cows and is considered a safe form of calcium supplementation on adult dairy cows.50 Oropharyngeal abscesses secondary to trauma and laceration caused by the administration of the boluses may occur.
Vitamin D and its metabolites or analogs
Vitamin D3 (cholecalciferol) administered parenterally was historically, a popular prophylactic against milk fever. However, because of the potential for toxicity of vitamin D injections and expense of its analogs, these are no longer used. The literature on the role of vitamin D in calcium homeostasis and its use in the prevention of periparturient paresis in cattle has been reviewed.6 Their uses are summarized here and advantages and disadvantages outlined.
In an attempt to reverse the negative calcium balance of susceptible cows the administration of vitamin D and its analogs have been used to increase intestinal absorption of calcium. Vitamin D3 is hydroxylated in the liver and the resulting metabolite is 25-hydroxycholecalciferol. This is metabolized in the kidney to 1,25-dihydroxycholecalciferol, which has an active hypercalcemic effect but is difficult to synthesize. One of its analogs, 1-α-hydroxycholecalciferol is as active, is easy to prepare, and is used pharmacologically.
Oral dosing with vitamin D2 and the parenteral administration of vitamin D3 and dihydrotachysterol all have their proponents. Oral dosing with 20 million IU of vitamin D2/d for 5 days to cows immediately prior to calving can markedly reduce the expected incidence of milk fever. The exact date of calving is often difficult to determine and if the administration is discontinued for up to 4 days before calving, an unusually high incidence of the disease may follow, probably because of the depression of parathyroid activity which follows the administration. The danger of causing metastatic calcification also exists as this has been produced with smaller doses (10–20 million IU daily for 10 days). Pregnant cows are more susceptible to calcification than non-pregnant animals. Treatment with larger doses or for longer periods than those recommended earlier should be avoided because of the danger of toxic effects. Smaller doses reduce the risk of calcification but also reduce the degree of calcium retention.
A single dose of 10 million IU of vitamin D3 IM given 2–8 days before parturition has been considered as optimal. A dose of 1 million units per 45 kg BW has given consistently better results. This may explain the variable results and why results have been more favorable in Jersey cattle. If the cow fails to calve after the 8th day, another 10 million units may be administered and repeated every 8 days until the cow calves. Subclinical calcification may occur in vessel walls but this is unlikely if dietary calcium and phosphorus intake is adequate. Single doses of 40 million units of vitamin D can be lethal. One of the disadvantages of this method is the likelihood that cows which do not calve at the anticipated time can be more seriously affected than if they receive no treatment. The hypercalcemic effect of cholecalciferol by injection is very much longer when it is administered by IM injection (up to 25 days) than by IV injection (up to 3 days) for this reason and because occasional cases of shock occur after the IV administration, especially if more than one injection is given, the IM route is preferred. The injection of vitamin D is preferred to feeding it and a protection rate of up to 80% can be anticipated. It is estimated that 95% of Jersey cattle are protected.
Other compounds with vitamin D activity but which avoid the possibility of causing hypervitaminosis D and are therefore useful in the prevention of milk fever are:
• 25-Hydroxycholecalciferol injected IM at 8 mg 3–10 days before calving and repeated at weekly intervals. Single doses of 4 mg are not effective in reducing the occurrence of parturient hypocalcemia or milk fever.
• 1,25-Dihydroxyvitamin D35 given at 200 μg daily, orally, to calving cows reduces the development of hypocalcemia but does not completely prevent milk fever. When 1,25-(OH)2D is given IM between 1 and 4 days of calving, it is effective in preventing milk fever. When administered less than 24 h or more than 4 days before calving, parturient paresis is not effectively prevented. Repeated injections at 4–7 day intervals until calving can be used, but toxicity can be a problem. A third problem with this metabolite is that the IM injection can result in milk fever 1–2 weeks after parturition because the exogenous metabolite may inhibit the endogenous production in some cows and when the exogenous product is cleared from the body, the cow is unable to produce sufficient 1,25-(OH)2D to maintain enhanced intestinal absorption of calcium.
• 24-F-1,25-dihydroxyvitamin D3 given at 100–150 μg 5 days before the expected date of parturition was effective.23 Cows which did not calve within 7 days were given a second dose. The incidence of milk fever in untreated controls, those receiving 100 and 150 mg, was 85%, 43%, and 29%, respectively. The use of SC-released product implanted 7 days before parturition and repeated at 7 day intervals until calving resulted in milk fever in 80% of controls and 9% in treated cows. The SC pellet maintained levels of the vitamin D metabolite for about 10 days, compared with the IM injection, which results in very high concentrations in the plasma in the first 48 h after injection.
• 1-α hydroxyvitamin D3 at 350 μg IM is effective as a preventive to milk fever if given 72 and 24 h before parturition. If calving has not occurred naturally within 72 h, a second injection is given. Parturition is induced if calving has not occurred 2 days after the second injection. The preferred site of injection is the serratus muscle of the neck, which results in a more effective response. To avoid the problems created by cows not calving at the predicted time, a combined regimen including induction of parturition by the administration of corticosteroid with the injection of 1-α-hydroxycholecalciferol is reported to be successful. Injection of cows with the same vitamin D analog plus a prostaglandin (cloprostenol) was unsuccessful in preventing milk fever. A dose of 700 μg given 6–8 days before calving is also recommended. Another recommendation suggests 500 μg at 2–5 days prior to parturition. An evaluation of routine use of 1-α-hydroxyvitamin D3 indicated that cows which developed retained placenta and metritis may be at greater risk of not conceiving within 150 days from calving. There is some transfer of the metabolite from the maternal to fetal plasma. Injection within 24 h of the onset of milk fever is ineffective, but if it is given more than 24 h and less than 1 week before the onset of the disease, the protection is excellent.
General management practices
The following management practices are suggested:
• Avoid overfattening by either reducing the energy concentration of the ration or restricting the intake during the prepartum period. This also appears to stimulate appetite, thus keeping cows on feed
• Avoid stresses at the time of parturition
• Provide a clean well-bedded box stall with conditions conducive to cow comfort and allow the animal to exercise
• Make frequent observations of cows prone to milk fever from 48 h before to 48 h after parturition for evidence of milk fever and immediate treatment will reduce the incidence of the downer cow syndrome associated with milk fever
• At calving the cow should receive an oral dose of a calcium salt in a gel, as set out later, followed by a diet with a high calcium content (over 1% of dry matter). The critical day is the day of calving and a sharp increase in calcium intake on this day can significantly reduce the occurrence of milk fever
• If hypomagnesemia is a likely concomitant, the diet should be supplemented with 60 g magnesium oxide daily.
Enemark JMD, Thilsing T, Jorgensen RS. Proceedings of the Abildgaard Symposium on hypocalcemia, acidosis and calcium homeostasis. Acta Vet Scand. 2003;97:1-160.
Nielsen K. On the founder of the Danish Veterinary School: Peter Christian Abildgaard and the inventor of the first efficient treatment for milk fever: Jorgen Jorgensen Schmidt. Acta Vet Scand. 2003;97:7-8.
Block E. Manipulation of dietary cation-anion difference on nutritionally related production diseases, productivity and metabolic responses of dairy cows. J Dairy Sci. 1994;77:1437-1450.
Horst RL, Goff JP, Reinhardt TA. Calcium and vitamin D metabolism in the dairy cow. J Dairy Sci. 1994;77:1936-1951.
Horst RL, et al. Strategies for preventing milk fever in dairy cattle. J Dairy Sci. 1997;80:1269-1280.
Jorgensen RJ, et al. Induced hypocalcemia by Na EDTA infusion. A review. J Vet Med. 1999;46:389-407.
Houe H, et al. Milk fever and subclinical hypocalcemia-An evaluation of parameters on incidence risk, diagnosis, risk factors and biological effects as input for a decision support system for disease control. Acta Vet Scand. 2001;42:1-29.
Horst RL, Goff JP, Reinhardt TA. Role of vitamin D in calcium homeostasis and its use in prevention of bovine periparturient paresis. Acta Vet Scand. 2003;97:35-50.
Goff JP, Horst RL. Role of acid-base physiology on the pathogenesis of parturient hypocalcemia (milk fever) — the DCAD theory in principle and practice. Acta Vet Scand. 2003;97:51-56.
Roche JR. Hypocalcemia and DCAD for the pasture-based transition cow — a review. Acta Vet Scand. 2003;97:65-74.
Goff JP. Pathophysiology of calcium and phosphorus disorders. Vet Clin North Am Food Anim Pract. 2000;16:319-337.
Goff JP. Treatment of calcium, phosphorus, and mineral balance disorders. Vet Clin North Am Food Anim Pract. 2000;16:619-639.
Thilsing-Hansen T, Jorgensen RJ, Ostergard S. Milk fever control principles: A review. Acta Vet Scand. 2002;43:1-19.
Oetzel GR. Management of dry cows for the prevention of milk fever and other mineral disorders. Vet Clin North Am Food Anim Pract. 2000;16:369-386.
1 Horst RL, et al. J Dairy Sci. 1997;80:1269-1280.
2 Houe H, et al. Acta Vet Scand. 2001;42:1-29.
3 Moisan PG. Can Vet J. 1994;35:714.
4 Schlumbohm C, Harmeyer J. J Dairy Sci. 2003;86:1953.
5 Roche JR, et al. Grass Forage Sci. 2000;55:26.
6 McNeill DM, et al. Aust J Argic Res. 2002;53:755.
7 Horst RL, et al. Int Conf Prod Dis Farm Animals. 2004.
8 Horst RL, et al. Acta Vet Scand Suppl. 2003;97:35-50.
9 Yamagishi N, et al. Vet Rec. 1999;144:67.
10 Goff JP. Vet Clin North Am Food Anim Pract. 2000;16:319-337.
11 Goff JP, Horst RL. Acta Vet Scand. 2003;97:51-56.
12 Seifi HA, et al. Vet J. 2004;167:281.
13 Goff JP. Vet Clin North Am Food Animal Pract. 2000;15:619.
14 Ostergaard S, Larsen T. J Dairy Sci. 2000;83:2438.
15 Yamagishi N, et al. Vet Rec. 2001;148:628.
16 Jorgensen RJ, et al. J Vet Med. 1999;46:389.
17 Mellau LSB, et al. Acta Vet Scand. 2000;42:251.
18 Hansen SS, et al. Vet Res Comm. 2003;27:193.
19 Jorgensen RJ, et al. Acta Vet Scand. 1998;39:331.
20 Fenwick DC. Br Vet J. 1992;148:413.
21 Fenwick DC. Can Vet J. 1989;30:426.
22 Cockcroft PD, Whiteley P. Vet Rec. 1999;144:529.
23 Matsas DJ, et al. J Am Vet Med Assoc. 1999;214:826.
24 Gerloff BJ, Swenson EP. J Am Vet Med Assoc. 1996;208:716.
25 Braun U, et al. Vet Rec. 2004;154:336.
26 Braun U, et al. Vet Rec. 2004;154:666.
27 Fenwick DC. Vet Rec. 1994;134:446.
28 Fenwick DC. Aust Vet J. 1969;45:111. 454
29 Roche JR. Acta Vet Scand. 2003;97:65-74.
30 Thilsing-Hansen T, et al. Acta Vet Scand. 2002;43:1.
31 Oetzel GR. Vet Clin North Am Food Anim Pract. 2000;16:369.
32 Sorensen JT, et al. Prev Vet Med. 2002;55:69.
33 Ostergaard S, et al. Prev Vet Med. 2004;86:209.
34 Jorgensen RJ, et al. J Dairy Sci. 2001;84:609.
35 Thilsing-Hansen T, et al. J Dairy Sci. 2002;85:1855.
36 Thilsing-Hansen T, et al. J Dairy Sci. 2001;84:691.
37 Wilson GF. N Z Vet J. 2001;49:78.
38 Wilson GF. J Dairy Sci. 2001;49:115.
39 Wilson GF. Acta Vet Scand. 2003;97:77.
40 Block E. J Dairy Sci. 1994;77:1437.
41 Vagnoni DB, et al. J Dairy Sci. 1998;81:1643.
42 Moore SJ, et al. J Dairy Sci. 2000;83:2095.
43 Bowman GR, et al. Can J Anim Sci. 2003;83:319.
44 Goff JP, et al. J Dairy Sci. 2004;87:1245.
45 Roche JR, et al. J Dairy Sci. 2002;85:3444.
46 Roche JR, et al. J Dairy Sci. 2003;86:970.
47 Roche JR, et al. J Dairy Sci. 2003;86:979.
48 Roche JR, et al. J Dairy Sci. 2003;86:2658.