KETOSIS, SUBCLINICAL KETOSIS, ACETONEMIA
Etiology A multifactorial disorder of energy metabolism. Negative energy results in hypoglycemia and ketonemia (the accumulation in blood of acetoacetate, β-hydroxybutyrate and their decarboxylation products acetone and isopropanol).
Epidemiology Primary ketosis and subclinical ketosis occurs predominantly in well-conditioned cows with high lactation potential, principally in the first month of lactation with a higher prevalence in cows with a higher lactation number. Loss of body condition in the dry period and immediately post partum. Secondary ketosis occurs where other disease reduces feed intake.
Clinical findings Cattle show wasting with decrease in appetite, fall in body condition and milk production. Some have short periods of bizarre neurological and behavioral abnormality. Response to treatment is good. Subclinical ketosis is detected by tests for ketones, usually in milk or urine.
Clinical pathology Hypoglycemia, ketonemia, ketonuria, or elevated ketones in milk.
Necropsy findings None specific.
Diagnostic confirmation Ketonemia, ketonuria or elevated ketones in milk.
Treatment In cattle, parenteral glucose with corticosteroid and oral glucose precursors such as propylene glycol, occasionally insulin. In cattle, the disease responds readily to treatment and is self-limiting.
Control Correction of energy imbalance. Herd biochemical monitoring coupled with condition scoring.
ETIOLOGY
Glucose metabolism in ruminants
The maintenance of adequate concentrations of glucose in the blood is critical to the regulation of energy metabolism. The ruminant absorbs very little dietary carbohydrate as hexose sugar because dietary carbohydrates are fermented in the rumen to short chain fatty acids, principally acetate (70%), propionate (20%) and butyrate (10%). Consequently, glucose needs in ruminants must largely be met by gluconeogenesis. Propionate and amino acids are the major precursors for gluconeogenesis with glycerol and lactate of lesser importance.1
Propionate is produced in the rumen from starch, fiber, and proteins. It enters the portal circulation and is efficiently removed by the liver, which is the primary glucose-producing organ. Propionate is the most important glucose precursor; an increased availability can spare the hepatic utilization of other glucose precursors,2 and production of propionate is favored by a high grain inclusion in the diet.3
The majority of amino acids are glucogenic and are also important precursors for gluconeogenesis. Dietary protein is the most important quantitative source but the labile pool of body protein is also an important source; together they contribute to energy synthesis and milk lactose synthesis as well as milk protein synthesis.1
Dietary acetate is transported to peripheral tissues and to the mammary gland and metabolized to long chain fatty acids for storage as lipids or secretion as milk fat.
Energy balance
In high-producing dairy cows there is often a negative energy balance in the first few weeks of lactation. The highest dry matter intake does not occur until 8–10 weeks after calving but peak milk production is at 4–6 weeks and energy intake may not keep up with demand. In response to a negative energy balance and low serum concentrations of glucose and insulin, cows will mobilize adipose tissue with consequent increases in serum concentrations of non-esterified fatty acids (NEFA) and subsequently BHBA. The hepatic mitochondrial metabolism of fatty acids promotes both gluconeogenesis and ketogenesis. Cows partition nutrients during pregnancy and lactation and are in a lipolytic stage in early lactation and at risk for ketosis during this period.
Hepatic insufficiency in ketosis
Hepatic insufficiency has been shown to occur in bovine ketosis but it does not occur in all cases.3,4 It has been suggested that ketosis can be divided into two types.3,5 In Type I, or ‘spontaneous’ ketosis, it is proposed that the gluconeogenic pathways are maximally stimulated and ketosis occurs when the demand for glucose outstrips the capacity of the liver for gluconeogenesis because of an insufficient supply of glucose precursors. Rapid entry of non-esterified fatty acids (NEFA) into hepatic mitochondria occurs and results in high rates of ketogenesis and high blood ketones. There is little conversion of NEFA to triglycerides resulting in little fat accumulation in the liver. In Type II ketosis, manifest with fatty liver, gluconeogenic pathways are not maximally stimulated and consequently mitochondrial uptake of NEFA is not as active and NEFA become esterified in the cytosol, forming triglyceride. The capacity of cattle to transport triglyceride from the liver is low, resulting in accumulation and fatty liver.3 The occurrence of a fatty liver can further suppress hepatic gluconeogenic capacity. Hepatic insufficiency may occur more commonly in those cows predisposed to ketosis by overfeeding in the dry period.5
Ketone formation
Ketones arise from two major sources: butyrate in the rumen and mobilization of fat. A large proportion of butyrate produced by rumen fermentation of the diet is converted to β-hydroxybutyrate (BHBA) in the rumen epithelium and is absorbed as such. Free fatty acids produced from the mobilization of fat are transported to the liver and oxidized to produce acetyl-CoA and NADH.
Acetyl-CoA may be oxidized via the TCA cycle or metabolized to acetoacetyl-CoA. Its oxidation via the TCA cycle depends upon adequate supply of oxaloacetate from the precursor propionate. If propionate, and consequently oxaloacetate, is deficient, oxidation of acetyl-CoA via the TCA cycle is limited and it is metabolized to acetoacetyl CoA and subsequently to acetoacetate and BHBA.1
The ketones BHBA and acetoacetate can be utilized as an energy source. They are normally present in blood and their concentration is a result of the balance between production in the liver and utilization by the peripheral tissues.
Role of insulin and glucagon
The regulation of energy metabolism in ruminants is primarily governed by insulin and glucagon. Insulin acts as a glucoregulatory hormone stimulating glucose use by tissues and decreasing hepatic gluconeogenesis. Blood insulin concentrations decrease with decreasing blood concentrations of glucose and propionic acid. Insulin also acts as a liporegulatory hormone stimulating lipogenesis and inhibiting lipolysis. Glucagon is the primary counter-regulatory hormone to insulin. Their counteracting effects play a central role in the homeostatic control of glucose. A low insulin: glucagon ratio stimulates lipolysis in adipose tissue and ketogenesis in the liver. Cows in early lactation have low insulin: glucagon ratios because of low blood insulin and are in a catabolic state.5 Elevated ketones may stimulate insulin production and may act as a negative feedback.5,6 Regulation is also indirectly governed by somatotropin, which is the most important determinant of milk yield in cattle and is also lipolytic. Factors that decrease the energy supply to ruminants, that increase the demand for glucose, or that increase the utilization of body fat as an energy source are likely to increase ketone production and ketonemia. There is however considerable cow-to-cow variation in risk for clinical ketosis.
ETIOLOGY OF BOVINE KETOSIS
It is not unreasonable to view clinical ketosis as the top end of a spectrum of a metabolic state that is common in heavily producing cows in the post-calving period. This is because high-yielding cows in early lactation are in negative energy balance and are subclinically ketotic as a result. This can be predisposed by nutrition inadequacies during the dry period.
Ruminants are particularly vulnerable to ketosis because, although very little carbohydrate is absorbed as such, a direct supply of glucose is essential for tissue metabolism, particularly the formation of lactose. The utilization of volatile fatty acids for energy purposes is also dependent upon a supply of available glucose. This vulnerability is further exacerbated, particularly in the cow, by the tremendous rate of turnover of glucose.
In the period between calving and peak lactation, the demand for glucose is increased and cannot be completely restrained. Cows will reduce milk production in response to a reduction of energy intake, but this does not follow automatically nor proportionately in early lactation because hormonal stimuli for milk production overcome the effects of reduced food intake. Under these circumstances, lowered blood glucose levels result in a lowered blood insulin. Long chain fatty acids are released from fat stores under the influence of both a low blood insulin:glucagon ratio and the influence of high somatotropin concentration, and this leads to increased ketogenesis.
Individual cow variation
The rate of occurrence of negative energy status, and therefore the frequency of clinical cases, has undoubtedly increased sharply in the recent past because of the steep increase in the lactation potential of the modern dairy cow. Because of the mammary gland’s metabolic precedence in the partitioning of nutrients, especially glucose, milk production continues at a high rate, causing an energy drain. In many individual cows, the need for energy is beyond their capacity for dry matter intake but there is between-cow variation in risk under similar nutritional stress.1-35 Clinical ketosis has been produced in recently calved dairy cows by reducing the daily feed intake by 15–20% ad libitum and supplementing it with 1,3-butanediol, a ketogenic substrate. The biochemical characteristics of ketosis including depletion of hepatic glycogen and major increases in hepatic stores of triglycerides and ketone bodies were produced but ketosis was only produced in those cows that had a predisposition to the disease.7,8
Types of bovine ketosis
There are many theories on the cause, biochemical and hormonal pathogenesis of ketosis, and the importance of predisposing factors. Reviews of these studies are cited at the end of this disease section. In general, it can be stated that clinical ketosis occurs in ruminants when they are subjected to demands on their resources of glucose and glycogen that cannot be met by their digestive and metabolic activity.
Lean1 has presented a classification of the disease based on its natural presentation in intensively and extensively managed dairy herds, and one that accounts for the early lactational demand for glucose, a limited supply of propionate precursors and preformed ketones or mobilized lipids in the pathogenesis. Such a classification includes the following geneses of ketosis, which will be discussed in turn:
Primary ketosis (production ketosis)
This is the ketosis of most herds, the so-called estate acetonemia. It occurs in cows in good to excessive body condition that have high lactation potential and are being fed good-quality rations but that are in a negative energy balance. There is a tendency for the disease to recur in individual animals, which is probably a reflection of variation between cows in digestive capacity or metabolic efficiency. A proportion of cases appear as clinical ketosis but a much greater proportion occur as cases of subclinical ketosis in which there are increased levels of circulating ketone bodies but no overt clinical signs.
Secondary ketosis
This occurs where other disease results in a decreased food intake. The cause of the reduction in food intake is commonly the result of abomasal displacement, traumatic reticulitis, metritis, mastitis, or other diseases common to the postparturient period. A high incidence of ketosis has also been observed in herds affected with fluorosis. An unusual occurrence reported was an outbreak of acetonemia in a dairy herd fed on a ration contaminated by a low level (9.5 ppm) of lincomycin, which caused ruminal microbial dysfunction.9 The proportion of cases of acetonemia which are secondary, and their diagnosis as such, are both matters of great interest as a significant proportion of cases of ketosis are secondary to other disease.
Alimentary ketosis
This form is due to excessive amounts of butyrate in silage and possibly also due to decreased food intake resulting from poor palatability of high butyrate silage. Silage made from succulent material may be more highly ketogenic than other types of ensilage because of its higher content of preformed butyric acid.10 Spoiled silage is also a cause and toxic biogenic amines in silage, such as putrescine, may also contribute.11 This type of ketosis is commonly subclinical but it may predispose to the development of production or primary ketosis.
Starvation ketosis
This occurs in cattle that are in poor body condition and that are fed poor-quality feedstuffs. There is a deficiency of propionate and protein from the diet and a limited capacity of gluconeogenesis from body reserves. Affected cattle recover with correct feeding.
Ketosis due to specific nutritional deficiency
Specific dietary deficiencies of cobalt and possibly phosphorus may also lead to a high incidence of ketosis. This may be due in part to a reduction in the intake of total digestible nutrients (TDN), but in cobalt deficiency, the essential defect is a failure to metabolize propionic acid into the tricarboxylic acid (TCA) cycle. The problem is restricted to the cobalt deficient areas of the world, although the occurrence of cobalt deficiency in high-producing dairy cows in non-deficient areas has been described.12
There is a marked nadir in food intake around calving, followed by a gradual increase. This increase is quite variable between cows, but in the great majority of cases does not keep pace with milk yield. The net result is that high-yielding dairy cows are almost certain to be in negative energy balance for the first 2 months of lactation.13
EPIDEMIOLOGY
Occurrence
Ketosis is a disease of dairy cattle and is prevalent in most countries where intensive farming is practiced. It occurs mainly in animals housed during the winter and spring months and is rare in cows that calve on pasture. In housed or free-stalled cattle it occurs year around. The occurrence of the disease is very much dependent upon management and nutrition and varies between herds. As might be expected, lactational incidence rates vary between herds and a recent review of eleven epidemiological studies showed a lactation incidence rate for ketosis that varied from 0.2–10.0%.14
Rates of subclinical ketosis are influenced by the cut-point of plasma BHBA used for definition but are much higher, especially in undernourished herds, and can approach 40%.2,15-19
Animal and management risk factors
There are conflicting reports on the significance of risk factors for ketosis and subclinical ketosis which probably reflect that the disease can be a cause or effect of interacting factors. The disease occurs in the immediate postparturient period with 90% of cases occurring in the first 60 days of lactation.15-20 Regardless of specific etiology, it occurs most commonly during the first month of lactation, less commonly in the second month, and only occasionally in late pregnancy. In different studies, the median time to onset following calving has varied from 10 to 28 days,20,21 with some recent studies showing a peak prevalence of subclinical ketosis in the first 2 weeks post-calving.2,15 A prolonged previous inter-calving interval increases risk.2
Cows of any age may be affected but the disease increases from a low prevalence at the first calving to a peak at the fourth. Lactational incidence rates of clinical ketosis of 1.5% and 9%, respectively were found in a study of 2415 primiparous and 4360 multiparous cows.22 Clinical ketosis can also recur in the same lactation.
Herd differences in prevalence are very evident in clinical practice, and in the literature, with some herds having negligible occurrence. Although apparent differences in breed incidence are reported, evidence for an heritable predisposition within breeds is minimal.17,20,23 Feeding frequency has an effect with the prevalence much lower in herds that feed TMR ad libitum compared with herds that fed roughage and concentrate separately of that feed twice a day.
There are conflicting reports on the relation between BCS at calving and ketosis but it is suggested that studies that have found no relationship have not had many fat cows in the herds examined.24,25 Fat body condition post partum was observed to be associated with a higher first test day milk yield, milk fat to protein ratio of >1.5, increased body condition loss and a higher risk for ketosis.25 In another study, cows with a BCS >3.25 at parturition and that lost 0.75 BCS in the first 2 months of lactation developed subclinical ketosis.26 Body condition loss during the dry period also increases risk for ketosis in the following lactation.2,27,28
There is no clear association with season. In some but not all summer grazing areas, a higher risk is generally observed in cattle during the winter housing period.2,29 Higher prevalence has been observed in the late summer and early winter in Scandinavian countries.30
There is a greater risk for the development of ketosis in cows that have an extended long dry period, that develop milk fever, retained placenta, lameness or hypomagnesemia.21,25,28,31-35 Cows with twins are also at risk for ketosis in the terminal stages of pregnancy.36,37 There is a bidirectional relation between risk for displaced abomasum and risk for ketosis, but in a field study of 1000 cows in 25 herds, cows that had a serum BHBA greater than 1400 μmol/L in the first 2 weeks of lactation had odds of 4:1 that displaced abomasums would be diagnosed 1–3 weeks later.38 In another study of 1010 cows a serum concentration of 1500 μmol/L or greater in the first 2 weeks of lactation was found to be associated with a threefold increase in ketosis or displaced abomasums.2
Economic significance
Clinical and subclinical ketosis are major causes of loss to the dairy farmer.2,19,39 In rare instances the disease is irreversible and the affected animal dies but the main economic loss is due to the loss of production while the disease is present, the possible failure to return to full production after recovery and the increased occurrence of periparturient disease.1,2 Both clinical and subclinical ketosis are accompanied by decreased milk yields and lower milk protein and milk lactose1,2,16,35,40 and increased risk for delayed estrus and lower first service conception rates, increased inter-calving intervals10,41 and increased risk of cystic ovarian disease, metritis and mastitis and increased involuntary culling.11,35,42 A year 2001 report has estimated the loss from a single case of subclinical ketosis at US$145.19
PATHOGENESIS
Bovine ketosis
The principal metabolic disturbances observed, hypoglycemia and ketonemia, may both exert an effect on the clinical syndrome. However, in the experimental disease in cattle, it is not always clear what determines the development of the clinical signs in cases that convert from subclinical to clinical ketosis.43 In many cases, the severity of the clinical syndrome is proportional to the degree of hypoglycemia and this, together with the rapid response to parenterally administered glucose in cattle, suggests hypoglycemia as the predominant factor. This hypothesis is supported by the development of prolonged hypoglycemia and a similar clinical syndrome to that of ketosis, after the experimental, IV or SC injection of insulin (2 units/kg BW).
However, in most field cases the severity of the clinical syndrome is also roughly proportional to the degree of ketonemia. This is an understandable relationship as ketone bodies are produced in larger quantities as the deficiency of glucose increases. However, the ketone bodies may exert an additional influence on the signs observed. Acetoacetic acid is known to be toxic and probably contributes to the terminal coma in diabetes mellitus in man.
The nervous signs which occur in some cases of bovine ketosis are thought to be caused by the production of isopropyl alcohol, a breakdown product of acetoacetic acid in the rumen, although the requirement of nervous tissue for glucose to maintain normal function may also be a factor in these cases.
Spontaneous ketosis in cattle is usually readily reversible by treatment; incomplete or temporary response is usually due to the existence of a primary disease with ketosis present only as a secondary development, although fatty degeneration of the liver in protracted cases may prolong the recovery period. Changes in ruminal flora after a long period of anorexia may also cause continued impairment of digestion.
Immunosuppression has been demonstrated with energy deficiency and ketosis.44,45 The higher susceptibility of ketotic postpartum cows to local and systemic infections may be related to impairment of the respiratory burst of neutrophils which occurs with elevated levels of BHBA.46
CLINICAL FINDINGS
Two major clinical forms of bovine ketosis are described – wasting and nervous – but these are the two extremes of a range of syndromes in which wasting and nervous signs are present in varying degrees of prominence.
The wasting form is the most common of the two and is manifest with a gradual but moderate decrease in appetite and milk yield over 2–4 days. In herds that feed components separately, the pattern of appetite loss is often unusual in that the cow first refuses to eat grain, then ensilage but may continue to eat hay. The appetite may also be depraved.
Body weight is lost rapidly, usually at a greater rate than one would expect from the decrease in appetite. Farmers usually describe affected cows as having a ‘woody’ appearance due to the apparent wasting and loss of cutaneous elasticity due presumably to disappearance of subcutaneous fat. The feces are firm and dry but serious constipation does not occur. The cow is moderately depressed and the hangdog appearance and disinclination to move and to eat may suggest the presence of mild abdominal pain.
The temperature and the pulse and respiratory rates are normal and although the ruminal movements may be decreased in amplitude and number, they are within the normal range unless the course is of long duration when they may virtually disappear. A characteristic odor of ketones is detectable on the breath and often in the milk.
Very few affected animals die, but without treatment the milk yield falls and although spontaneous recovery usually occurs over about a month, as equilibrium between the drain of lactation and food intake is established, the milk yield is never fully regained. The fall in milk yield in the wasting form may be as much as 25% and there is an accompanying sharp drop in the solids-not-fat content of the milk. In the wasting form, nervous signs may occur in a few cases but rarely comprise more than transient bouts of staggering and partial blindness.
Signs are usually bizarre and begin quite suddenly. The syndrome is suggestive of delirium rather than of frenzy and the characteristic signs include:
• Straddling or crossing of the legs
• Head pushing or leaning into the stanchion
• Aimless movements and wandering
Hyperesthesia may be evident, the animal bellowing on being pinched or stroked. Moderate tremor and tetany may be present and there is usually an incoordinate gait. The nervous signs usually occur in short episodes which last for 1 or 2 h and may recur at intervals of about 8–12 h. Affected cows may injure themselves during the nervous episodes.
Subclinical ketosis
Many cows that are in negative energy balance in early pregnancy will have ketonuria without showing clinical signs, but will have diminished productivity including depression of milk yield and a reduction in fertility. Clinical diagnosis is not effective and in one study,22 diagnosis by routine urine testing at 5–12 days post partum was considerably more efficient (15.6% detected) than diagnosis by the herdsman (4.35% detected). In a British study of 219 herds the annual mean rate of reported clinical ketosis was 0.5 per 100 adult cows but the rate of subclinical ketosis, as defined by high blood concentrations of BHBA and non-esterified fatty acids, was substantially higher.47,48
Potential milk production is reduced by 1–9%.17,20 Surveys of large populations show a declining prevalence of ketosis-positive cows after a peak in the period immediately after calving, and a positive relationship between hyperketonemia and high milk yield.15,49 Infertility may appear as an ovarian abnormality, delayed onset of estrus or as endometritis resulting in an increase in calving to conception interval and reduced conception rate at first insemination.
CLINICAL PATHOLOGY
Hypoglycemia, ketonemia and ketonuria are characteristic of the disease.
Glucose
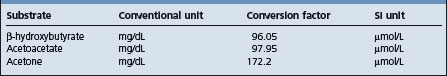
Blood glucose levels are reduced from the normal of approximately 50 mg/dL to 20–40 mg/dL. Ketosis secondary to other diseases is usually accompanied by blood glucose levels above 40 mg/dL and often above normal. Conversion factors are shown in Table 29.7.
Blood ketones
Most commonly, plasma or serum β-hydroxybutyrate (BHBA) measured in SI units is used for analysis of ketonemia. BHBA is the predominant circulating ketone body. Plasma concentrations of BHBA significantly correlate with plasma concentrations of acetoacetate but acetoacetate is unstable in samples whereas BHBA is relatively stable.2 Normal cows have plasma BHBA concentrations less than 1000 μmol/L, cows with sub-clinical ketosis have concentrations greater than 1400 μmol/L, and cows with clinical ketosis have concentrations often in excess of 2500 μmol/L. Plasma BHBA shows some diurnal variation in cows fed twice daily with peak concentrations occurring approximately 4 h after feeding and higher concentrations in the morning than in the afternoon. This is not seen in cows fed a total mixed ration ad libitum.50,51
Plasma BHBA is not a cost effective or convenient analysis for routine analysis and cow side monitoring and the content of acetoacetate or BHBA in urine and milk are used for these purposes. Concentrations of BHBA and acetoacetate in urine and milk are less than those in blood and the correlation coefficients for blood and milk BHBA and blood and milk acetoacetate are 0.66 and 0.62, respectively.52
Milk and urine cowside tests
Cowside tests have the advantage of being inexpensive, giving immediate results, and they can be used as frequently as necessary. A minor source of error is that the concentration of ketone bodies in these fluids will depend not only on the ketone level of the blood but also on the amount of urine excreted or on the milk yield. Milk is less variable, easier to collect and may give fewer false negatives with subclinical ketosis.
Milk and urine ketone levels have been traditionally detected by the reaction of acetone and acetoacetate with sodium nitroprusside and can be interpreted in a semi-quantitative manner based on the intensity of the reaction. Several products are available commercially as test powders or strips are commonly accompanied by a color chart that allows a classification in grades such as negative, trace, small, moderate, large, based on the intensity of the color of the reaction.
Conventional wisdom is that milk powder tests are not sensitive for detection of subclinical ketosis (report too many false negatives) and urine tests are not sufficiently specific (report too many false positives).53
The sensitivity and specificity of the nitroprusside powder test with milk in various studies is reported as 28–90% and 96–100%, respectively.16,53,54 More recently, a milk strip test detecting the presence of BHBA in milk is available and is graded on the concentration of BHBA in μmol/L. In different studies it has a reported sensitivity and specificity of 73–96% and 69–96%, respectively.53-57 These variations are, in part, due to different plasma BHBA reference values (1200 and 1400 μmol/L) for designation of subclinical ketosis and different cut points used in urine BHBA. Somatic cell counts greater than 1 million cells/mL will cause an elevation in reading of both the BHBA strip test and the nitroprusside tests.
A nitroprusside tablet has a reported sensitivity and specificity of 100% and 59%, respectively, compared with serum BHBA concentrations above 1400 μmol/L16 and a nitroprusside strip test a reported sensitivity and specificity of 78% and 96% with a urine cut point corresponding to ‘small’ on the color chart or 49% and 99% with a urine cut point corresponding to ‘moderate’ on the color chart.53 BHBA test strips when used with urine has a reported sensitivity and specificity of 73% and 96%, respectively at a urine cut point of 100 μmol/L BHBA and 27% and 99% at a urine cut point of 200 μmol/L BHBA.53
One author has suggested that the nitroprusside urine strip test or the BHBA milk strip test are best for screening individual cows for ketosis in herds with average prevalence but that the nitroprusside powder test would have limited application.53
Milk fat concentration tends to increase and milk protein concentration tends to decrease during postpartum negative energy balance. A fat to protein ratio >1.5 in first day teat milk is indicative of a lack of energy supply in the feed and of risk for ketosis.25
Clinical chemistry and hematology.
White and differential cell counts are variable and not of diagnostic value for ketosis.
There are usually elevations of liver enzymes but liver function tests are within the normal range. Liver biopsy is the only accurate method to determine the degree of liver damage.58 Plasma concentrations of non-esterified fatty acids are elevated as are cholesterol concentrations and bilirubin. Bilirubin is not a sufficiently sensitive indicator to asses the extent of fat mobilization and liver function.26,27 Liver glycogen levels are low and the glucose tolerance curve may be normal. Volatile fatty acid levels in the rumen are much higher in ketotic than in normal cows and the ruminal levels of butyric acid are markedly increased relative to acetic and propionic acids. There is a small but significant fall in serum calcium levels (down to about 9 mg/dL (2.25 mmol/L)), due probably to increased loss of base in the urine to compensate for the acidosis.
NECROPSY FINDINGS
The disease is not usually fatal in cattle but fatty degeneration of the liver and secondary changes in the anterior pituitary gland and adrenal cortex may be present.
Cattle
The clinical picture is usually too indefinite, especially in cattle, to enable a diagnosis to be made solely on clinical grounds. General consideration of the history, with particular reference to the time of calving, the duration of pregnancy in ewes and the feeding program, and biochemical examination to detect the presence of hypoglycemia, ketonemia, and ketonuria are necessary to establish a diagnosis.
TREATMENT
In cattle, a number of effective treatments are available but in some affected animals, the response is only transient; in rare cases, the disease may persist and cause death or necessitate slaughter of the animals. Most of these cases are secondary and failure to respond satisfactorily to treatment is due to the primary disease.
The rational treatment in ketosis is to relieve the need for glucose formation from tissues and allow ketone body utilization to continue normally. Theoretically, the simplest means of doing this is by the administration of glucose replacement therapy. The effect of the administration of glucose is complex but it allows the reversal of ketogenesis and the establishment of normal patterns of energy metabolism.12 Ideally, treatment should be at an early stage of the disease to minimize loss and with subclinical ketosis this requires biochemical testing.52
Replacement therapy
Glucose (dextrose)
The IV injection of 500 mL of a 50% solution of glucose results in transient hyperglycemia, increased insulin and decreased glucagon secretion, and reduced plasma concentration of non-esterified fatty acids. It effects a marked improvement in most cows but relapses occur commonly unless repeated treatments are used. This is probably due to the transience of the hyperglycemia or insufficient dosing – the dose required varies directly with the amount of lactose being lost in the milk. A significant proportion of the administered glucose is lost to urinary excretion. SC injections prolong the response but are not recommended as they cause discomfort, and large unsightly swellings, which often become infected, may result. IP injections of 20% solution of dextrose may be used alternatively but are also accompanied by risk of infection.
Other sugars
Other sugars, especially fructose, either alone or as a mixture of glucose and fructose (invert sugar), and xylitol, have been used in an effort to prolong the response but idiosyncrasies to some preparations, in the form of polypnea, muscle tremor, weakness and collapse, can occur while the injection is being given.
Propylene glycol and glycerine/glycerol
To overcome the necessity for repeated injections, propylene glycol can be administered as a drench. The traditional does is 225 g twice daily for 2 days, followed by 110 g daily for 2 days to cattle, but higher volumes are also used. Propylene glycol (200–700 g daily), or salts of propionic acid, can be administered in the feed and give good results. Administration in feed is preferred by some because this method avoids dangers of aspiration with drenching; however, cows not used to its inclusion in the feed may show feed refusal. It is recommended that for best results, dosing with these preparations be preceded by an IV injection of glucose.
Parenteral infusions of glucose solutions and the feeding of glycerol depress the fat content of milk, and the net saving in energy may favorably influence response to these drugs. Glycerol and propylene glycol are not as efficient as glucose because conversion to glucose does utilize oxaloacetate. Propylene glycol is absorbed directly from the rumen and acts to reduce ketogenesis by increasing mitochondrial citrate concentrations; its metabolism to glucose occurs via conversion to pyruvate with subsequent production of oxaloacetate via pyruvate carboxylase.12
Other glucose precursors
Because of its glucogenic effect, sodium propionate is theoretically a suitable treatment but when administered in 110–225 g doses daily, the response in cattle is often very slow. Lactates are also highly glucogenic but both calcium and sodium lactate (1 kg initially, followed by 0.5 kg for 7 days) and sodium acetate (110–500 g/d) have given less satisfactory results than those obtained with sodium propionate. Ammonium lactate (200 g for 5 days) has however, been used extensively with reported good results.
Lactose, in whey, or in granular form in the diet, can increase dry matter intake but increases ruminal butyrate and plasma BBHA concentrations.59
Hormonal therapy
The efficiency of glucocorticoids in the treatment of bovine ketosis has been demonstrated in both experimental and field cases. Hyperglycemia occurs within 24 h of administration and appears to result from a repartitioning of glucose in the body rather than from gluconeogenesis.7 Historically, many preparations have been used successfully but current drugs are more potent, require lower dosage, and have fewer side-effects. A hyperglycemic state is produced for 4–6 days in ketotic cows given 10 mg of dexamethasone 21-isonicotinate and other preparations such as dexamethasone sodium phosphate (40 mg) and flumethasone (5 mg) are also used. Label regulations vary between countries and in general, the recommendations of the manufacturer with regard to use and dosage should be followed. Profound hypokalemia with high case fatality is a potential sequel to prolonged repeated therapy of ketosis with isoflupredone acetate.60 Response of cows with primary ketosis to treatment with corticosteroids and IV glucose is superior, with fewer relapses, than therapy with corticosteroids or glucose alone.61
Insulin facilitates cellular uptake of glucose, suppresses fatty acid metabolism and stimulates hepatic gluconeogenesis. It is administered in conjunction with either glucose or a glucocorticoid and may be of particular value in early-onset cases of ketosis that are unresponsive to glucose or corticosteroid therapy6 but is not commonly used. The dose of protamine zinc insulin is 200–300 IU per animal administered SC every 24–48 h as required.
have also been used for treatment of lactational ketosis and ketosis in late pregnant cows that are overfat, stressed, or have twin fetuses. Experimentally, 60 mg and 120 mg of trenbolone acetate are effective as single injections but no extensive field trials are recorded and the drug is banned for use in food animals in most countries.
Vitamin B12 and cobalt are indicated in regions where cobalt deficiency is a risk factor for ketosis. They are sometimes administered to cattle with ketosis in regions where cobalt deficiency does not occur but their therapeutic value is not proven. Cysteamine (a biological precursor of coenzyme A) and also sodium fumarate have been used to treat cases of the disease. Reported results were initially good but the treatment has not been generally adopted. The recommended dose rate of cysteamine is 750 mg IV for three doses at 1–3 day intervals.
Glucagon although ketogenic is strongly gluconeogenic and glycogenolytic and glucagon concentrations are decreased in the blood of fat cows at calving and cows with ketonemia. It could be of value in prevention and therapy but it would require a prolonged delivery system as it has a very short physiologic half life and its effects following a single injection are short-lived.62
CONTROL
The control of clinical ketosis is integrally related to the adequate nutrition of the cow in the dry and lactating period. This encompasses details such as:
It is difficult to make general recommendations for the control of the disease because of the many conditions under which it occurs, its probable multiple etiology, and feeding systems that vary from those that feed components separately to those that feed total mixed rations. Cows should neither have been starved nor be overfat at calving. Careful estimation of diets by reference to feed value tables is recommended and detailed recommendations on diet and management are available with the caveat that planned rations can deviate from feed bunk rations and feed bunk dry matter and actual dry matter intake may not be the same. Too low a feeding frequency and the feeding of concentrates separate from roughage rather than as a total mixed ration can lead to an increase in rates of ketosis.
In the USA, dry cows are typically divided into two groups; ‘far off’ and ‘close up’ cows. ‘Far off’ cows are generally fed to National Research Council (NRC) dry cow feeding guidelines and ‘close up’ cows are given a ration that is halfway between the dry cow and early lactation ration starting 3 weeks before estimated calving and aiming to maximize dry matter intake and provide adequate energy.2,63-65 Practical recommendations based on British feeding standards and units are also available.66,67
In high-producing cows being fed stored feeds, poor quality roughage commonly leads to acetonemia. Wet ensilage containing much butyrate, and moldy or old and dusty hay, are the main offenders. In concentrates, it is the change of source which creates off-feed effects and precipitates attacks of acetonemia.
Cows that are housed should get some exercise each day and in herds where the disease is a particular problem during the stabling period, the cattle should be turned out to pasture as soon as possible in the spring.
The ration should contain adequate amounts of cobalt, phosphorus and iodine.
If there is a high incidence in a herd receiving large quantities of ensilage, reduction of the amount fed for a trial period is indicated.
Energy supplements
Propylene glycol is used for the prevention of clinical and subclinical ketosis. Traditionally, propylene glycol has been drenched to cattle in early lactation at doses varying from 350 to 1000 mL daily for 10 days after calving. There is a linear effect of dose on plasma glucose.68 Propylene glycol can also added to feed and is frequently present in commercial feed product but a bolus dose of propylene glycol is more effective in raising blood glucose than incorporation in feed.2 A dose of 1 L per day given as an oral drench for 9 days prior to parturition has also been shown efficacious.69 At doses above 500 mL administered by drench or present in feed some cows may develop rapid and shallow respiration, ataxia, salivation, and somnolence.
Glycerol can be substituted for propylene glycol at equivalent dose rates. A preliminary report of a small experimental study with larger doses of glycerol showed that glycerol given orally at a dose of 1, 2, or 3 L elevated blood glucose concentrations to 16, 20, and 25% of pre-treatment values at 0.5 h after treatment and that these concentrations remained elevated for 8 h. Staggering, depression and diuresis were observed in some cows given the 2 or 3 L dose but this could be prevented by administering the glycerol in a large (37 L) volume of water. It concluded that a dose of 1 L was effective in increasing milk production and reducing urinary letones.70 Glycerol, fed as a constant component in the transition dairy cow diet is not effective, and possibly may be ketogenic when fed continually.71 Glycerol should only be used as drench in hypoglycemic cows and not fed as a component of the diet.
Propionic acid and its salts
Propionic acid absorbed across the rumen wall is transported to the liver where it is converted to glucose via gluconeogenesis to result in an increase in serum blood glucose levels. Older literature reports that 110 g/d fed daily for 6 weeks, commencing at calving, has given good results in reducing the incidence of clinical bovine ketosis and improving production, but is not palatable and has the risk of reducing feed intake. In controlled trials, feeding energy supplements containing propionic acid and/or its salts for 3 weeks prepartum and 3 weeks post partum had a beneficial effect on milk production but a variable effect on reducing subclinical ketosis.72,73
Ionophores
Ionophores alter bacterial flora of the rumen, leading to decreases in Gram-positive bacteria, protozoa, and fungi and increases in Gram-negative bacteria. The net effect of these changes in bacterial flora is increased propionate production and a decrease in acetate and butyrate production providing increased gluconeogenic precursors. Field trials with monensin have demonstrated a reduction in plasma BHBA and a reduced prevalence of clinical ketosis.29,74,75 It can be administered as a slow release capsule to cattle 2–4 weeks before calving. The capsule contains 32 g of monensin and releases approximately 335 mg monensin a day for 95 days. Ionophores are not labeled for inclusion in lactating cow rations in some countries.
Niacin
Niacin is antilipolytic and induces increases in blood glucose and insulin but there is conflicting evidence that niacin given in the feed has a beneficial effect on subclinical ketosis in cattle.1,20,76 It has been suggested that it should be supplemented from 2 weeks prior to parturition to 12 weeks post partum.77
General control
Biochemical monitoring of herds for subclinical ketosis and adequacy of periparturient feeding can be conducted using blood glucose estimations on a sample of cows in their second week of lactation.55 Blood glucose levels of below 35 mg/dL (1.9 mmol/L) suggest subclinical ketosis. For individual cows, blood glucose estimations should be done at about 14 days after calving. This method of monitoring is expensive.
More commonly, testing for ketones in urine or milk of cows in their first or second week of lactation is recommended for early detection of ketosis and early treatment to prevent milk loss and ketosis-associated diseases. One recommendation is to routinely test such cows on a specific day each week.19 This should be coupled with body condition scoring to monitor the efficacy of the nutritional program. Condition scoring at dry off, mid dry period, calving, calving plus 20–50 days, and two to three subsequent periods in lactation have been suggested.28,67 Plasma glucose coupled with plasma BHBA are the best predictive model for monitoring energy balance of cattle on a pasture diet with milk acetone the best ‘on-farm’ predictor. However, the variation in milk acetone is high and frequent sampling is required for accurate estimation.78
Automated monitoring by in-line measurements of ketone bodies in milk have been studied and may be of particular value in large dairies. BHBA is proposed as the candidate as it is the more robust in milk, and where cows are fed a TMR, is not subject to significant diurnal variation. It can be measured with a fluorometric method that requires no pretreatment of the milk.79,80
Littledike ET, Young JW, Beitz DC. Common metabolic diseases of cattle: ketosis, milk fever, grass tetany and downer cow complex. J Dairy Sci. 1981;64:1465-1482.
Kronfield DS. Major metabolic determinants of milk volume, mammary efficiency, and spontaneous ketosis in dairy cows. J Dairy Sci. 1982;65:2204-2212.
Baird GD. Primary ketosis in the high-producing dairy cow: clinical and subclinical disorders, treatment, prevention and outlook. J Dairy Sci. 1982;65:1-10.
Kelly JM, Whitaker DA. Subclinical ketosis and dairy cows. Vet Ann. 1984;24:83-93.
Brockman RP, Loorveld B. Hormonal regulation of metabolism in ruminants; a review. Livestock Prod Sci. 1986;14:313-334.
Herdt TH, Emery RS. Therapy of diseases of ruminant intermediary metabolism. Vet Clin North Am Food Anim Pract. 1991;8:91-106.
Lean IJ, et al. Bovine ketosis: a review. I Epidemiology and pathogenesis. Vet Bull. 1991;61:1209-1218.
Lean, et al. Bovine ketosis: a review. II Biochemistry and prevention. Vet Bull. 1992;62:1-13.
Gerloff BJ. Dry cow management for the prevention of ketosis and fatty liver in dairy cows. Vet Clin North Am Food Anim Pract. 2000;16:283-292.
Duffield T. Subclinical ketosis in lactating dairy cattle. Vet Clin North Am Food Anim Pract. 2000;16:231-253.
Herdt TH. Ruminant adaption to negative energy balance influences on the etiology of ketosis and fatty liver. Vet Clin North Am Food Anim Pract. 2000;16:215-230.
Geishauser T, et al. Monitoring for subclinical ketosis in dairy herds. Comp Cont Educ Pract Vet: Food Anim Pract. 2001;23:S65-S71.
Nielsen NI, Ingvartsen KL. Propylene glycol for dairy cows. A review of the metabolism of propylene glycol and its effects on physiological parameters, feed intake, milk production and risk of ketosis. Anim Feed Sci Technol. 2004;115:191-213.
Huxley J. Optimising health, productivity and welfare of dairy cattle: on-farm nutrition. In Practice. 2004;26:466-475.
Hayirli A, Grummer RR. Factors affecting the dry matter intake prepartum in relationship to etiology of peripartum lipid-related metabolic disorders: a review. Can J Anim Sci. 2004;84:337-347.
Oerzel GR. Monitoring and testing herds for metabolic disease Vet Clin North Am Food Anim Pract. 2004;20:651-674.
1 Lean IJ, et al. Vet Bull. 1992;62:1.
2 Duffield T. Vet Clin North Am Food Anim Pract. 2000;16:231.
3 Herdt TH. Vet Clin North Am Food Anim Pract. 2000;16:215.
4 Steen A, et al. J Vet Med Assoc. 1997;44:521.
5 Holtenuis P, Holtenuis K. J Vet Med Assoc. 1996;43:579.
6 Herdt TH, Emery RS. Vet Clin North Am Food Anim Pract. 1992;81:91.
7 Mills SE, et al. J Dairy Sci. 1986;69:362.
8 Drackley JK, et al. J Dairy Sci. 1992;75:1622.
9 Rice DA, et al. Vet Rec. 1983;113:495.
10 Andersson L, Lyndstrom K. Zentralbl Veterinarmed. 1985;32:15.
11 Tveil B, et al. J Dairy Sci. 1992;75:2421-2433.
12 Sanders DE. Comp Cont Educ Pract Vet. 1989;11:757.
13 Knight CH. Proc Nutr Soc. 2001;60:527-537.
14 Ingvartsen KL, et al. Livestock Product Sci. 2003;83:277.
15 Duffield TF, et al. Can Vet J. 1997;38:713.
16 Nielen M, et al. Can Vet J. 1994;35:229.
17 Dohoo IR, Martin SW. Am J Comp Med. 1984;48:1.
18 Carrier J, et al. J Dairy Sci. 2004;87:3725.
19 Geishauser T, et al. Comp Cont Educ Pract Vet: Food Anim Pract. 2001;23:S65.
20 Lean IJ, et al. Vet Bull. 1991;61:1209.
21 Bigras-Poulin M, et al. Prev Vet Med. 1992;10:79.
22 Nir O. Acta Vet Scand Suppl. 2003;98:21.
23 Uribe HA, et al. J Dairy Sci. 1995;78:421.
24 Gillund P, et al. J Dairy Sci. 2001;84:1390.
25 Heuer C, et al. J Dairy Sci. 1999;82:295.
26 Busatol A, et al. J Vet Med A. 2002;49:455.
27 Ill-Hwa K, Gook-Hyun S. Theriogenol. 2003;60:1445.
28 Markusfeld O, et al. Vet Rec. 1997;141:67.
29 Duffield TF, et al. J Dairy Sci. 1998;81:2866.
30 Tweit B, et al. J Dairy Sci. 1992;75:2421.
31 Dohoo IR, et al. Prev Vet Med. 1984;2:665-671.
32 Eriksson JA, Wretler E. World Rev Anim Prod. 1990;25:29.
33 Grohn YT, et al. J Dairy Sci. 1989;72:1876.
34 Smith TR, et al. J Dairy Sci. 1997;80:1569.
35 Lean IJ, et al. Res Vet Sci. 1994;57:200.
36 Morris CA, et al. Proc N Z Soc Anim Prod. 1992;52:21.
37 Tyler JW, et al. J Am Vet Med Assoc. 1994;204:1665.
38 Geishauser T, et al. Vet Clin North Am Food Anim Pract. 2000;16:255.
39 Vagirholm I, et al. Prev Vet Med. 1991;10:195.
40 Miettinen PVA, Setala JJ. Prev Vet Med. 1993;17:1.
41 Harman JL, et al. Am J Vet Res. 1996;57:640.
42 Miettinen PVA. J Vet Med Assoc. 1994;41:102.
43 Veenhuizen JJ, et al. J Dairy Sci. 1991;74:4238.
44 Goff JP, et al. J Dairy Sci. 1997;80:1260.
45 Lacetera N, et al. Am J Vet Res. 2002;63:958.
46 Hoeben D, et al. Vet Immunol Immunopath. 1997;58:165.
47 Whitaker DA, et al. Vet Rec. 2004;155:43.
48 Laven R, Howe M. Vet Rec. 2004;155:63.
49 Anderson L, Emanuelson LI. Prev Vet Med. 1985;3:449.
50 Eicher R, et al. Acta Vet Scand. 1999;60:1493.
51 Nielsen N, et al. Acta Vet Scand. 2003;98:305.
52 Enjalbert FMC, et al. J Dairy Sci. 2001;84:583.
53 Carrier J, et al. J Dairy Sci. 2004;87:3725.
54 Geishauser TK, et al. J Dairy Sci. 1988;81:438.
55 Geishauser TK, et al. J Dairy Sci. 2000;83:296.
56 Osborne TM, et al. Proc Am Assoc Bov Practit. 2002;35:188.
57 Dirksen G, Breitner WJ. Vet Med Assoc. 1993;40:779.
58 Grohn Y, et al. J Dairy Sci. 1983;66:2320.
59 DeFrain JM, et al. J Dairy Sci. 2004;87:2486.
60 Sielman ES, et al. J Am Vet Med Assoc. 1997;210:240.
61 Shpigel NY. J Am Vet Med Assoc. 1996;208:1702.
62 Hippen AR. Vet Clin North Am Food Anim Pract. 2000;16:267.
63 Oetzel GR. Comp Cont Educ Pract Vet: Food Anim Pract. 1998;20:391.
64 Gerloff B. J Vet Clin North Am Food Anim Pract. 2000;16:283.
65 Sniffen CJ, Herdt TH. Vet Clin North Am Food Anim Pract. 1992;81:1.
66 Baird GD, et al. Br Vet J. 1974;130:214-318.
67 Huxley J. In Practice. 2004;26:466.
68 Nielsen NI, Ingvartsen KL. Anim Feed Sci Technol. 2004;115:191.
69 Studer VA, et al. J Dairy Sci. 1993;76:2931.
70 Goff JP, Horst RL. Acta Vet Scand Suppl. 2003;98:214.
71 DeFrain JM, et al. J Dairy Sci. 2004;87:4195.
72 Ballard C, et al. Anim Feed Sci Technol. 2001;93:55.
73 Mandebvu P, et al. Anim Feed Sci Technol. 2003;105:81.
74 Heuer YH, et al. J Dairy Sci. 2001;84:1085.
75 Duffield TF, Bagg RN. Aust Vet J. 2000;41:388.
76 Neilsen N, Ingvartsen KL. Acta Vet Scand Suppl. 2003;98:306.
77 Hutjens MF. Vet Clin North Am Food Anim Pract. 1992;81:525.
78 Clark CE, et al. Livestock Prod Sci. 2005;94:199.