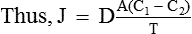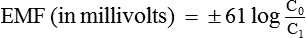Transport Through Cell Membrane
The physiological activities of a cell depend upon the substances like nutrients, oxygen and water, which must be transported into the cell, and at the same time metabolic waste must be transported out of the cell. Various processes involved in the transport of substances across the cell membrane may be grouped as under:
Passive transport
Passive transport refers to the mechanism of transport of substances along the gradient without expenditure of any energy. It depends upon the physical factors like concentration gradient, electrical gradient and pressure gradient. Since the transport of substances occurs along the gradient, this process is also called down-hill movement. The passive transport mechanisms operating at the cell membrane level are diffusion and osmosis.
Diffusion
Diffusion refers to passive transport of molecules from areas of higher concentration to areas of lower concentration. Diffusion through cell membrane is divided into two subtypes called simple diffusion and facilitated diffusion.
Simple diffusion
In simple diffusion there occurs a net flux of the molecules from the areas of high concentration to areas of low concentration due to constant random movement. Quantitatively, the net movement of the molecules across a permeable membrane is expressed by Fick's law of diffusion, which states that rate of diffusion (J) is directly proportional to the difference in the concentration of the substance in two regions [concentration gradient, i.e. (C1 – C2)] and cross-sectional area (A) and inversely proportional to the distance to be travelled, i.e. thickness of the membrane (T).
where D is the diffusion coefficient.
The diffusion of molecules across the biological membranes differs depending upon the lipid solubility, water solubility, type of electrical charge and size of the molecules. Further, selective permeability of the semipermeable cell membrane also affects the diffusion of different molecules. How the different molecules diffuse across a cell membrane are discussed below.
Simple diffusion of lipid-soluble substances through the cell membrane
The rate of diffusion through the lipid bilayer of the cell membrane is directly proportional to the solubility of a substance in lipids. Therefore, molecules of substances like oxyge n, nitrogen, carbon dioxide, alcohol, steroid hormones and weak organic acids and bases, being lipid soluble, diffuse very rapidly through the lipid bilayer of the cell membrane.
Simple diffusion of water and other lipid-insoluble molecules through the cell membrane
Astonishingly, water and other lipid-insoluble substances can also pass easily through the cell membrane. It has been shown that it is possible due to the presence of the so-called protein channels (made from transmembrane proteins) in the cell membrane.
Diffusion through protein channels
The protein channels are tube-shaped channels which extend in the cell membrane from the extracellular to the intracellular ends (Fig. 1.3-1). Therefore, even the highly lipid-insoluble substances can diffuse by simple diffusion directly through these channels of the cell membrane.
The protein channels have been equipped with following characteristics:
Selective permeability of protein channels
The protein channels are highly selective, i.e. each channel can permit only one type of ion to pass through it. This results from the characteristics of the channel itself, such as its diameter, its shape and nature of electrical charges along its inside surfaces. Examples of some selective channels are given below:
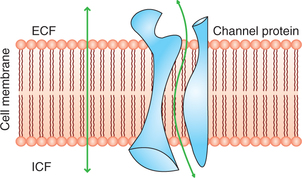
• Sodium channels are specifically selective for the passage of sodium ions. These are 0.3 x 0.5 nm in size and their inner surfaces are strongly negatively charged (Fig. 1.3-2).
• Potassium channels are specifically selective for the passage of potassium ions. These are 0.3 x 0.3 nm in size and are not negatively charged.
Gating mechanism in protein channels
Some protein channels are continuously open, whereas most others are ’gated’, i.e. they are equipped with actual gate-like extensions of the transport protein molecule which can open and close as per requirement. This gating mechanism is a means of controlling the permeability of the channels. The opening and closing of gates are controlled by three principal ways:
1. Voltage-gated channels. These respond to the electrical potential across the cell membrane. As shown in Fig. 1.3-2 in the case of sodium channels the gates are located at the outer end of the channels and remain tightly closed when there is a strong negative charge on the inside of cell membrane. When the inside of cell membrane loses its negative charge, these gates open and there occurs a tremendous inflow of sodium ions. This is the basis of occurrence of action potentials in nerves that are responsible for nerve signals.
2. Ligand-gated channels. Gates of these channels open when some other chemical molecule binds with the gate proteins that is why this is also called chemical gating. The ligand (binding molecules) may be internal or external
• The external or extracellular ligands are also called first messengers. One of the most important example of external ligand channel gating is the effect of acetylcholine on the so-called acetylcholine channels. This gate plays an important role in transmission of nerve signals from one nerve cell to another and from nerve cells to muscle cells.
• The internal or the intracellular ligands are also called second messengers. The examples include intracellular Ca2+, cAMP or G protein produced in the cells. The second messenger generally activates protein kinases.
3. Mechanical-gated channels. Some protein channels are opened by mechanical stretch. These mechanosensitive channels play an important role in cell movements.
Facilitated diffusion
The water-soluble substances having larger molecules such as glucose cannot diffuse through the protein channels by simple diffusion. Such substances diffuse through the cell membrane with the help of some carrier proteins. Therefore, this type of diffusion is called facilitated or the carrier-mediated diffusion. There are many types of carrier proteins in the cell membrane, each type having binding sites that are specific for a particular substance. Among the most important substances that cross cell membranes by facilitated diffusion are glucose and most of the amino acids.
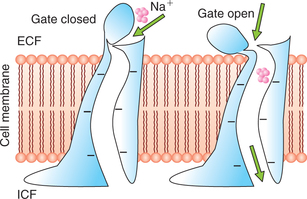
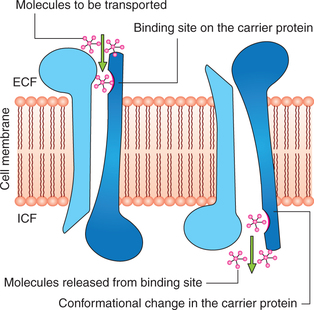
Mechanism of facilitated diffusion
Postulated mechanism for facilitated diffusion is shown in Fig. 1.3-3. As shown in the figure a conformational change occurs in the carrier protein after the molecule to be transported is bound at the receptor site. The repetitive spontaneous configurational changes allow the diffusion of the molecule.
Types of carrier protein systems
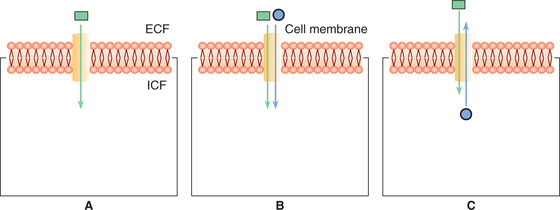
Three types of carrier protein systems are known: uniport, symport and antiport (Fig. 1.3-4). The symports and anti-port are together known as co-transport.
1. Uniport. In this system the carrier proteins transport only one type of molecules (Fig. 1.3-4A).
2. Symport. In this system transport of one substance is linked with transfer of another substance. For example, facilitated diffusion of glucose in the renal tubular cells is linked with the transport of sodium (Fig. 1.3-4B).
3. Antiport. In this system the carrier proteins exchange one substance for another. For example, Na+–H+ exchange or Na+–H+ exchange in the renal tubules (Fig. 1.3-4C).
Differences between simple and facilitated diffusion
1. Specificity. The carrier proteins are highly specific for different molecules.
2. Saturation. As shown in Fig. 1.3-5, in simple diffusion the rate of diffusion increases proportionately with the increase in the concentration of the substance and there is no limit to it. However, in facilitated diffusion the rate of diffusion increases with increase in concentration gradient to reach a limit beyond which a further increase in the diffusion cannot occur. This is called saturation point, and here all the binding sites on the carrier proteins are occupied and the system operates at its maximum capacity.
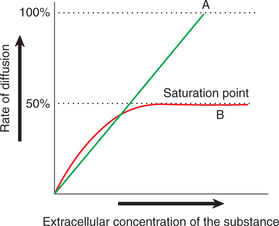
Fig. 1.3-5 Effect of concentration of substance on rate of diffusion in: A, simple diffusion and B, facilitated diffusion.
3. Competition. When two molecules, say A and B are carried by the same protein there occur a competition between the two molecules for the transport. Thus, an increase in the concentration of ‘A’ molecule will decrease the transport of molecule ’B’ and vice versa. No such competition is known to occur in simple diffusion.
Factors affecting net rate of diffusion
The diffusion of the substance can occur either way, i.e. extracellular fluid (ECF) to intracellular fluid (ICF) or vice-versa, depending upon the prevailing environment. The factors which affect the net rate of diffusion in the desired direction are given below.
1. Cell membrane permeability. Permeability of the cell membrane (P) is the major determining factor for the net diffusion, which in turn depends upon the following factors:
• Thickness of the membrane. The diffusion is inversely proportional to the thickness of the cell membrane.
• Lipid solubility. Diffusion is directly proportional to the lipid solubility of the substance.
• Distribution of protein channels in the cell membrane. The rate of diffusion of lipid-insoluble substance is directly proportional to the number of channels per unit area of the cell membrane.
• Temperature. Rate of diffusion increases with increase in the temperature. This is because of the increased motion of the molecules and ions of the solution with increase in temperature.
• Size of the molecules. Rate of simple diffusion is inversely proportional to the size of molecules.
• Area of the membrane. The net diffusion of the substance is directly proportional to the total area of the membrane.
2. Concentration gradient. The simple diffusion is directly proportional to the concentration gradient, but the facilitated diffusion, however, has certain limitations beyond certain level of concentration gradient (Fig. 1.3-5).
3. Electrical potential gradient. Electrical potential across the cell membrane is another important factor which affects the diffusion of ions across the cell membrane. Net diffusion of any ion across the cell membrane (J) will be the resultant of combined effect of concentration gradient and electrical potential gradient.
At normal body temperature (37°C), the electrical gradient that will balance the concentration gradient of a univalent ion can be determined by Nernst equation:
where
• EMF is the electromotive force between outside and inside of the membrane,
This equation is extremely important in understanding the membrane potential, hence discussed in detail in the relevant section (page 24).
4. Pressure gradient. It has been observed that the increased amount of energy is available to cause net movements of molecules from the high pressure side towards the low pressure side.
Osmosis
Osmosis refers to diffusion of water or any other solvent molecules through a semipermeable membrane (i.e. membrane permeable to solvent but not to the solute) from a solution containing lower concentration of solutes towards the solution containing higher concentration of solutes; Fig. 1.3-6 shows osmosis across a selective permeable membrane. When a sodium chloride solution is placed on one side of the membrane and water on the other side (Fig. 1.3-6A) then the net movement of water occurs from the pure water into the sodium chloride solution (Fig. 1.3-6B).
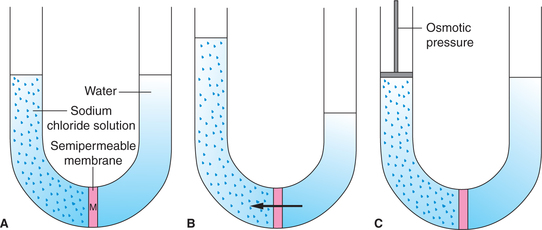
Fig. 1.3-6 Diagrammatic representation of phenomenon of osmosis: A, semipermeable membrane ’M’ separates sodium chloride solution from pure water; B, net movement of water occurs through M from pure water to the sodium chloride solution and C, demonstration of osmotic pressure (net movement of water from pure water to sodium chloride solution is prevented by applying appropriate pressure on the solution).
Osmotic pressure
Osmotic pressure refers to the minimum pressure which when applied on the side of higher solute concentration prevents the osmosis. Figure 1.3-6C shows that when appropriate pressure is applied the net diffusion of water into the sodium chloride solution is prevented
The osmotic pressure in the body fluids refers to the pressure exerted by the solutes dissolved in water or other solvents. The osmotic pressure exerted by the colloidal substances in the body is called colloidal osmotic pressure. The colloidal osmotic pressure due to plasma colloids (proteins) is called oncotic pressure.
The osmotic pressure depends upon the number of molecules or ions dissolved in a solution rather than their size, type or chemical composition. In case of non-dissociated solutes, 1 g molecular weight of any substance shall contain similar number of molecules and hence exerts similar degree of osmotic pressure, i.e. equal to 22.4 atm, whereas, in case of dissociated solutes the osmotic pressure depends upon the number of ions resulting from its dissociation.
Osmole, osmolality and osmolarity
Osmole is the unit used in place of grams to express the concentration in terms of number of osmotically active particles in a given solution. One osmole is equal to the molecular weight of a substance in grams divided by the number of freely moving particles liberated in solution by each molecule. Thus,
• A molar solution of glucose contains 1 mole and exerts osmotic pressure of 1 atm.
• A molar solution of NaCl contains 2 osmoles (1 mole of Na+ and 1 mole of Cl–) and exerts osmotic pressure of 2 atm.
• A molar solution of CaCl2 contains 3 osmoles (1 mole of Ca2+ and 2 moles of Cl– and thus exerts osmotic pressure of 3 atm.
Osmolality of a solution refers to the number of osmotically active particles (osmoles) per kilogram (kg) of a solution, whereas osmolarity refers to the number of osmoles per litre (L) of a solution. Therefore, osmolarity is affected by the volume of the various solutes in the solution and the temperature, while the osmolality is not. The osmotic pressure is determined by the osmolality and not the osmolarity. However, the quantitative differences between the osmolarity and osmolality are less than 1%. In practice, osmolarity is more frequently used in physiological studies, since it is far more easier to measure osmolarity vis-a-vis osmolality.
Normal plasma osmolality. The normal osmolality of the extracellular and intracellular fluids is 290 milliosmoles per kilogram (mOsm/kg). In the plasma of the total osmolality 270 mOsm are contributed by Na+, Cl– and 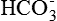 . The remaining 20 mOsm are contributed by glucose and urea. Because of the large molecular weight and hence lesser number of particles, plasma proteins (70 g/L) contribute 2 mOsm to the total plasma osmolality.
. The remaining 20 mOsm are contributed by glucose and urea. Because of the large molecular weight and hence lesser number of particles, plasma proteins (70 g/L) contribute 2 mOsm to the total plasma osmolality.
Tonicity of fluids
In clinical practice, the word tonicity always refers to tonicity of a solution with respect to that of plasma (290 mOsm). In other words, it is the red blood cell (RBC) membrane across which the tonicity is tested. Thus,
• Isotonic fluids are those which have osmolality similar to plasma. RBCs neither shrink nor swell in such solution (Fig. 1.3-7A). A solution of 0.9% NaCl is isotonic with plasma.
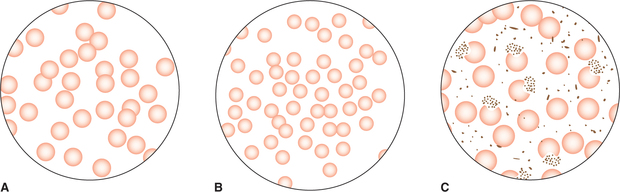
Fig. 1.3-7 Tonicity of fluids: A, isotonic fluid (0.9% NaCl) has osmolarity similar to plasma, RBCs neither shrink nor swell in it; B, hypertonic fluid (2% NaCl) has osmolarity higher than plasma, RBCs shrink in it and C, hypotonic fluid (0.3% NaCl) has osmolarity lower than plasma, RBCs swell in it.
• Hypertonic fluids have osmolality higher than the plasma. The RBCs shrink in such solutions by losing water by osmosis (Fig. 1.3-7B).
• Hypotonic fluids are those whose osmolality is lower than that of plasma. The RBCs swell up in hypotonic solutions by gaining water by osmosis (Fig. 1.3-7C).
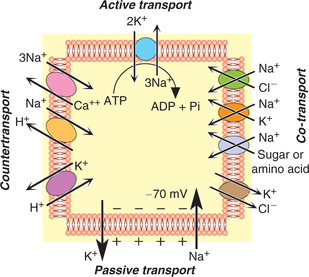
Active transport
Active transport refers to the mechanism of transport of substances against the chemical and/or electrical gradient. Active transport involves expenditure of energy which is liberated by breakdown of high-energy compounds like adenosine triphosphate (ATP). Since the transport of substances occur against the chemicoelectrical gradient; this process is also called uphill movement.
Mechanism of active transport
The active transport is also carrier-mediated, since the carrier proteins are involved in the transport mechanism; so like facilitated diffusion the active transport also shows specificity, saturation and competition.
Since the active transport mechanism involves the expenditure of energy, the transporting carrier protein system is also called the ’active pump mechanisms’.
Substances transported actively across the cell membrane include:
Types of active transport
The active transport is of two types:
A Primary active transport processes
In primary active transport process, the energy is derived directly from the breakdown of ATP or some other high-energy phosphate compound. Some of the important pumps involved in the primary active transport processes are:
1 Sodium-potassium pump
Sodium-potassium (Na+–K+) pump is present in all the cells of the body. It is involved with the active transport of sodium ions outwards through the cell membrane and potassium ions inwards simultaneously. Thus, this pump is responsible for maintaining the Na+ and K+ concentration differences across the cell membrane and for establishing a negative electrical potential inside the cells.
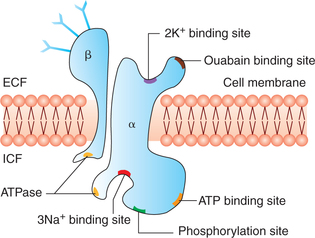
Structure of Na+–K+ pump (Fig. 1.3-8). The carrier protein involved in Na+–K+ pump is a complex consisting of two separate protein units, a larger α subunit (molecular weight approximately 100,000) and a smaller β subunit (molecular weight approximately 55,000). The α subunit is mainly concerned with Na+–K+ transport. It has got following binding sites:
• Three intracellular sites—one each for binding sodium ions (3Na+) and ATP, and one phosphorylation site.
• Two extracellular sites—one each for binding potassium ions (2K+) and ouabain.
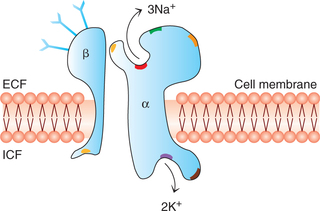
Mechanism of operation of Na+-K+ pump (Fig. 1.3-9). The functioning of Na+-K+ pump involves the use of enzyme ATPase. The enzyme ATPase is activated when three sodium ions and one ATP molecule bind to their respective binding sites. The activated ATPase catalyzes the hydrolysis of ATP to ADP and liberates a high-energy phosphate bond of energy (phosphorylation). The energy so liberated is believed to cause a conformational change in the carrier protein molecule extruding sodium into the ECF. This is followed by binding of two potassium ions to the receptor site on extracellular surface of the carrier protein and dephosphorylation of a subunit which returns to its previous conformation, releasing potassium into the cytoplasm.
Functions of Na+-K+ pump. The Na+-K+ pump subserves two main functions:
1. Controlling the cell volume. It is the most important function of the Na+-K+ pump, without which most of cells of the body will swell up until they burst. When the Na+-K+ pump fails the cells swell up and burst.
2. Electrogenic activity. Na+-K+ pump acts as an electrogenic pump since it produces a net movement of positive charge out of the cell (3Na+ out and 2K+ in); thus creating electrical potential across the cell membrane. This is basic requirement in nerves and muscles to transmit the signals.
2 Calcium pump
The calcium pump forms another important active transport mechanism. Like Na+-K+ pump, it also operates through a carrier protein which has ATPase activity. But the difference from Na+-K+ pump is that the carrier protein binds calcium ions rather than sodium and potassium ions. The calcium pump helps in maintaining extremely low concentration of calcium in the intracellular fluid (10,000 times less than the ECF).
B Secondary Active Transport Processes
In secondary active transport processes, the energy is derived secondarily from the energy which has been stored in the form of ionic concentration differences between the two sides of a membrane, created in the first place by primary active transport. At many areas in the body transport of some other substance is coupled with the active transport of Na+, i.e. the same carrier protein which is involved in the active transport of Na+ also secondarily transports some other substance. The secondary active transport of substance may occur in the form of sodium co-transport or sodium countertransport.
Sodium co-transport
The carrier protein here acts as a symport, i.e. transports some other substance along with the sodium. Substances carried by sodium co-transport include glucose, amino acids, chloride and iodine (Fig. 1.3-10).
Fig. 1.3-10 Postulated mechanism of sodium co-transport of glucose (secondary active transport): A, carrier protein has two receptor sites, one for sodium and one for glucose and B, con-formational change in carrier proteins causes transport of both glucose and sodium inside the cell simultaneously.
Sodium countertransport
The carrier protein involved here acts as an antiport, i.e. sodium ion is exchanged for some other substance. Some of the sodium countertransport mechanism occurring in the body are sodium-calcium countertransport, sodium-hydrogen countertransport, and other countertransport systems (Fig. 1.3-11).
Vesicular transport
Vesicular transport mechanisms are involved in the transport of macromolecules such as large protein molecules which can neither pass through the membrane by diffusion nor by active transport mechanisms. The vesicular transport mechanisms include endocytosis, exocytosis and transcytosis.
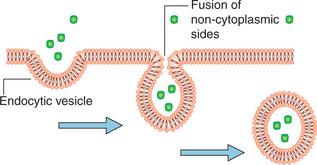
Endocytosis
Endocytosis is the process in which the substance is transported into the cell by infolding of the cell membrane around the substance and internalising it (Fig. 1.3-12). It is further categorized into three types.
1. Pinocytosis, i.e. cell drinking, refers to the process of engulfing liquid substances by the enfolding of cell membrane, e.g. reabsorption by renal tubular epithelial cells.
2. Phagocytosis, i.e. cell eating, is the process of engulfing solid particles such as bacteria, dead tissue and foreign particles by the cells. The process of phagocytosis involves three steps: (i) the attachment stage, (ii) the engulfment stage and (iii) killing or degradation stage (see page 92).
3. Receptor-mediated endocytosis. In this process the substance to be transported binds with the special receptor protein present on the cell surface. The receptor protein-substance complex is then engulfed by the cell membrane by the process of endocytosis. Transport of iron and cholesterol into the cells occurs by receptor-mediated endocytosis.
Exocytosis
Exocytosis (3) is reverse of endocytosis, i.e. by this process the substances are expelled from the cell without passing through the cell membrane. In this process the substances which are to be extruded are collected in the form of granules or vesicles which move towards the cell membrane. Their membrane then fuses to the cell membrane. The area of fusion breaks down releasing the contents to the exterior and leaving the cell membrane intact. Release of hormones and enzymes by secretory cells of the body occurs by exocytosis. The process of exocytosis requires Ca2+ and energy along with docking proteins. Excretion of specific hormones and granules by the cells is termed as emiocytosis.
Other transport processes
So far we have considered transport across the cell membrane, i.e. movement of substances between the ICF and the ECF through the cell membrane. In addition to this, there are many situations in the body where transport of substances occurs through the epithelia and the capillary endothelial cell membrane. Some of these processes discussed briefly are:
Transport across epithelia
Transport across epithelia involves movement of the substances from one side of the epithelium to the other. The transepithelial transport occurs in body cavities lined by continuous sheet of cells such as in gastrointestinal tract, renal tubules, pulmonary airways and other structures. For transepithelial transport to occur the cells need to be bound by tight junctions, and have different ion channels and transport protein in different parts of their membrane. Transport across epithelia may occur in two ways:
1. Transport through the cells proper. In gastrointestinal tract and in urinary tubules substances are transferred from the lumen into the epithelial cells at their apical borders and then from the basal borders of the cells into interstitial fluid. From the interstitial fluid the substances are finally transported into the blood through the capillaries.
2. Transport across the tight junctions. It has been identified that in the epithelia of small intestine, proximal renal tubules and gall bladder, most of the sodium chloride passes through the tight junctions (areas of tight adherence between the two adjacent cells) rather than traversing the whole length of the epithelial cells.
Ultrafiltration
When a solution of protein and salt is separated from plain water or a less concentrated salt solution by a membrane permeable to salt and water and not to the protein, there will be a net movement of water on the protein side by diffusion and a movement of salt away from the protein side. This process is called dialysis.
Ultrafiltration. Refers to occurrence of dialysis under hydrostatic pressure (Fig. 1.3-12). Ultrafiltration is occurring at the capillary level in the body. The capillary blood is under hydrostatic pressure. The pressure is 35 mm Hg near the arteriolar end and gradually declines to 12 mm Hg near the venous end of the capillary. Through the capillaries there occurs ultrafiltration of all the constituents of the plasma except the proteins into the interstitial spaces. Ultrafiltration plays important role in the formation of body fluids (see page 168) (Fig. 4.4-12).
 Diffusion
Diffusion