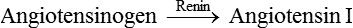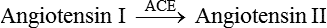Cardiovascular Regulation
Introduction
Cardiovascular control makes circulatory adjustments which are essential to cope up with the timely needs of each and every organ of the body, and is thus of fundamental importance for survival.
Need for cardiovascular control
Functions served by cardiovascular control are:
• To increase the blood supply to active tissues, e.g. during exercise to skeletal and cardiac muscles.
• Redistribution of blood to increase or decrease the heat loss from the body as per requirements.
• Circulatory adjustments during routine cardiovascular stresses like change in posture, hours of excitement, fear, anxiety, meals and sleep, etc.
• Maintenance of adequate flow to vital organs, such as brain, heart and kidney, all the times including emergencies such as shock and haemorrhage, even at the expense of the circulation to the rest of the body, as the vital organs may develop irreversible changes in no time. For example, the brain is irreversibly damaged within 3 min of ischaemia, while skin, skeletal muscle and gastrointestinal tract can tolerate reduction of blood for a longer duration.
Circulatory adjustments
Circulatory adjustments which ensure that all of the organs receive sufficient blood flow are: (1) control of blood volume and (2) control of arterial pressure. These circulatory adjustments are made by the cardiovascular control mechanisms primarily by regulating following parameters.
A Regulation of cardiac performance, i.e. alterations in the activities of heart which include the following.
1. Chronotropic action, i.e. effect on heart rate which may be in the form of:
• Increased heart rate (tachycardia) or positive chronotropic effect.
• Decreased heart rate (bradycardia) or negative chronotropic effect.
2. Inotropic action, i.e. effect on force of contraction which may be in the form of:
B Regulation of performance of blood vessels, which primarily includes the following.
Neural control mechanisms
Neural regulation of circulation is of fundamental importance since it responds within seconds. The nervous regulation mainly controls systemic functions of circulatory system (whenever required) such as:
• Redistribution of blood flow to different parts of the body.
Components of neural control mechanism
The neural cardiovascular regulating mechanism consists of the following.
A. Medullary cardiovascular control centres. These are the prime centres concerned with neural control of circulation. These include:
• Medullary sympathetic centre [vasomotor centre (VMC)].
• Medullary parasympathetic centre (nucleus ambiguus).
• Medullary relay centre for cardiorespiratory and afferents [nucleus of tractus solitarius (NTS)].
B. Autonomic nervous system supplying the heart and blood vessels. The regulation of circulation by medullary control centres is exerted almost entirely through the autonomic nervous system (ANS). The sympathetic component of ANS is most important for controlling circulation and the parasympathetic component mainly contributes to the regulation of heart functions.
C. Afferent impulses to medullary centres. The VMC is influenced by afferent impulses from the higher centres and a large number of other areas.
D. Role of skeletal nerves and muscles in controlling blood pressure.
Medullary cardiovascular control centres
1. Vasomotor centre
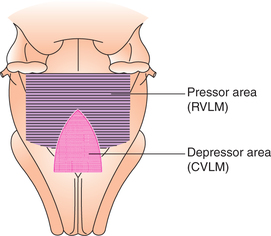
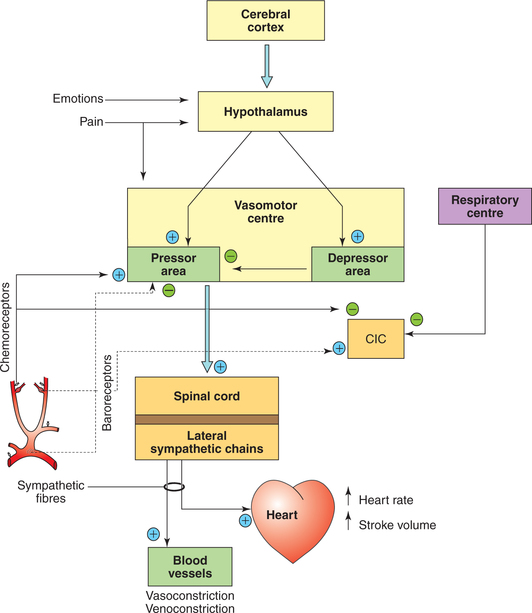
Though popularly known as VMC, more appropriately it should be called as medullary sympathetic centre. It is the primary cardiovascular regulatory centre located in the medulla oblongata of brainstem. It consists of groups of neurons situated bilaterally in the reticular substance of medulla at the floor of fourth ventricle. The medullary cardiovascular centre is constituted by following different areas (Fig. 4.5-1).
Pressor area
Pressor area is located in the rostral ventrolateral medulla (RVLM). It contains glutaminergic neurons which exert excitatory effect on the thoracolumbar spinal sympathetic neurons.
Continuous sympathetic vasoconstrictor tone. Normally, the neurons forming pressor area of the VMC show inherent tonic activity, i.e. they discharge rhythmically (at a rate of about 1 impulse per second) in a tonic fashion to excite sympathetic preganglionic neurons present in the intermediolateral grey column of the spinal cord. In this way, the continuous signals are passed to the sympathetic vasoconstrictor nerves fibres over the entire body. Sympathetic vasoconstrictor tone.
Depressor area
Depressor area is situated bilaterally in the caudal ventrolateral medulla (CVLM). Stimulation of neurons forming depressor area produces decrease in the sympathetic activity due to inhibition of the tonically discharging impulses of the pressor area causing:
• Arteriolar dilation, which decreases systemic blood pressure.
• Venodilatation, which increases storage of blood in the venous reservoir and decreases venous return and cardiac output.
• Decrease in heart rate or negative chronotropic effect.
• Decrease in force contraction or negative inotropic effect.
2. Medullary parasympathetic centre
Medullary parasympathetic centre or cardiac vagal centre (earlier also called cardioinhibitory centre) is now called by its specific name, i.e. the nucleus ambiguus. The neurons located in this centre are not tonically active. Nucleus ambiguus receives afferents via NTS and in turn sends inhibitory pathway in the form of vagal fibres to: heart to decrease the heart rate and force of cardiac contraction.
3. Medullary relay station for cardiorespiratory afferents
NTS of the vagus nerve forms the so-called medullary relay station for the cardiorespiratory afferents. It receives afferents from most of the baroreceptors and chemoreceptors. Cells of the NTS, in turn, relay the information to VMC and cardiac vagal centre (nucleus ambiguus) that control sympathetic and parasympathetic outputs, respectively.
Autonomic nerve supply to heart and blood vessels
Autonomic nerve supply to heart
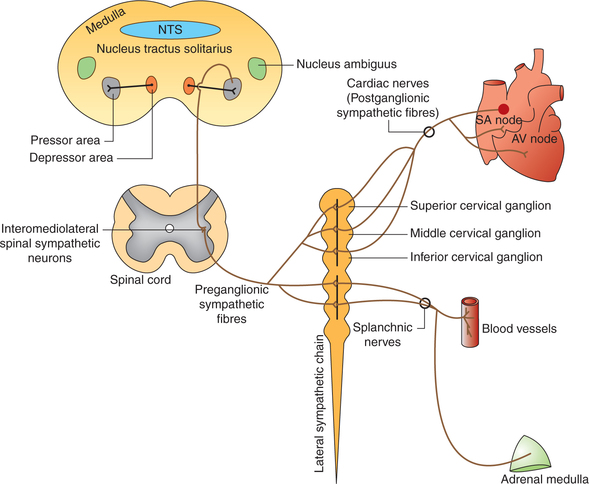
Sympathetic supply
Spinal sympathetic centre is formed by the neurons located in the intermediolateral horns of the spinal cord extending from T1 to L2 spinal segments.
• Preganglionic sympathetic fibres (small, myelinated) supplying the heart arise from the neurons lying in the intermediolateral horns of the T1–T5 spinal segments and pass into the sympathetic trunk to superior, middle and inferior cervical ganglia, and upper thoracic ganglia where they synapse (Fig. 4.5-2).
• Postganglionic fibres (long, unmyelinated) leave the ganglia and pass via superior, middle and inferior cardiac sympathetic nerves, and supply to the nodal tissues [sinoatrial (SA) node and atrioventricular (AV) node] and the cardiac muscles of atria and ventricles (Fig. 4.5-2). It is important to note that:
Stimulation of cardiac sympathetic nerves causes:
• Increased heart rate (positive chronotropic effect),
• Increase in the conduction of impulse through heart (positive dromotropic action),
• Increase in the excitability of myocardium (positive bathmotropic effect) and
• Increase in the force of contraction of myocardium (positive inotropic effect).
Parasympathetic supply
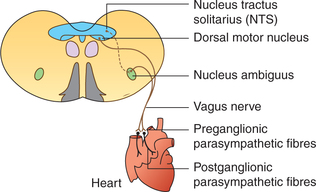
Parasympathetic fibres to the heart are carried through two vagii (Fig. 4.5-3).
Preganglionic fibres (long, myelinated) arise from the nucleus ambiguus located in the medulla and travel along the vagii to reach the heart through their cardiac branches to synapse in the ganglia located within the superficial and deep cardiac plexuses and also in the walls of atria.
Postganglionic fibres (small, unmyelinated) are distributed to the atria, SA node, AV node and AV bundle. It is important to note that:
• The right vagus is distributed mainly to SA node,
• The left vagus is distributed mainly to AV node,
• No vagal motor fibres are distributed to the ventricles and
Stimulation of parasympathetic fibres to heart causes:
• Decrease in heart rate (negative chronotropic effect).
• Decrease in conduction of impulse through the conduction tissue (negative dromotropic effect).
• Decrease in the excitability of atria only (negative bathmotropic effect).
• Decrease in the force of contraction of atria only (negative inotropic effect). There is no effect on the force of contraction of ventricles.
Vagal tone. There is a good deal of tonic vagal discharge, called the vagal tone, in humans and other large animals. Therefore, when vagii are cut in the experimental animals, the heart rate rises. Similarly, in adult humans, the resting heart rate which is about 72 beats/min rises to 150–180 beats/min after the administration of vagolytic drugs, such as atropine, because of the unopposed sympathetic tone. When both adrenergic and cholinergic systems are blocked in humans, the heart rate is approximately 100 beats/min. Since the resting heart rate is about 72/min, it confirms that at rest the vagal tone is greater than the sympathetic tone.
Autonomic nerve supply to blood vessels
The autonomic efferents supplying the blood vessels produce two types of effects.
Vasoconstriction effect
Vasoconstriction effect is produced by the sympathetic fibres supplying the blood vessels which originate from the intermediolateral horns in T1–L2 spinal segments.
Vasoconstrictor fibres have norepinephrine and sometimes neuropeptide Y as neurotransmitter, and are called noradrenergic fibres.
Sympathetic vasoconstrictor fibres show tonic (i.e. continuous) discharge at the rate of about 1 impulse/s. Therefore, when the sympathetic nerves are cut (sympathectomy) there occurs:
• Vasodilation – which leads decreased peripheral resistance → decreased diastolic blood pressure.
• Venodilatation – increased venous capacity → decreased venous return → decreased end-diastolic volume → decreased stroke volume → decreased cardiac output → decreased systolic blood pressure.
Stimulation of sympathetic fibres produces:
• Constriction of arterioles – increased peripheral resistance → increased diastolic blood pressure.
• Venoconstriction – decreased venous capacity → increased venous return → increased end-diastolic volume → increased stroke volume → increased cardiac output → increased systolic blood pressure.
Both these mechanisms are responsible for the regional redistribution of blood and at the time of need the blood is diverted from the skin, skeletal muscles and splanchnic area to the heart and brain.
Vasodilation effect
Neural vasodilation effect on the blood vessels is produced by following mechanisms.
1. Decrease in discharge of noradrenergic vasoconstrictor nerves.
2. Sympathetic cholinergic vasodilator nerves. Some of the organs of the body, such as skeletal muscles, heart, lungs, liver, kidney and uterus in addition to adrenergic vasoconstrictor sympathetic fibres also receive innervation by cholinergic vasodilator sympathetic fibres having acetylcholine and vasoinhibitory peptide (VIP) as neurotransmitter. These fibres originate from the cerebral cortex, relay in the hypothalamus and midbrain, and pass through the medulla (without relay in the VMC) to the sympathetic neurons located in the intermediolateral grey column of the spinal cord (Fig. 4.5-4). These fibres are not tonically active and get activated only in biological stresses, for example during exercise, child birth, etc. and help in increasing the blood flow.
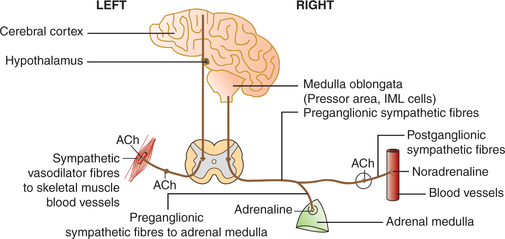
Fig. 4.5-4 Pathway of sympathetic vasodilator fibres (on left side) and sympathetic vasoconstrictor system (on right side).
3. Parasympathetic vasodilator nerves
• Blood vessels, in general, do not have parasympathetic innervation with following exceptions:
– Sacral outflow parasympathetic fibres represented by nervi erigentes, which supplies sexual erectile tissue and is responsible for vasodilation in external genitalia during sexual excitement.
– Cranial outflow of parasympathetic fibres along chorda tympani branch of facial nerve to salivary glands.
– The postganglionic cholinergic neurons on the blood vessels contain acetylcholine, VIP and PHM-27 as neurotransmitters.
It is important to note that parasympathetic vasodilator fibres play little role in the control of general circulation. Activation of such nerves only contributes to pleasure and fulfilling important biological functions.
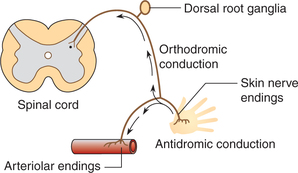
4. Vasodilation by axon reflex. Conduction of normal sensory afferent impulses from the skin to spinal cord is called orthodromic conduction. However, in certain situations, for example, when a firm stroke is applied across the skin the afferent impulses in the sensory nerves from the skin are relayed antidromically down branches of the sensory nerves that innervate blood vessels (Fig. 4.5-5). The antidromic conduction of impulses causes release of substance P from the nerve endings, which produces vasodilation and increases capillary permeability. This local neural mechanism (which does not involve the CNS) is called axon reflex. It is responsible for the local vasodilation and does not contribute in systemic control of circulation.
Afferent impulses to medullary cardiovascular control centres
The medullary control centres are influenced by afferent control impulses from the higher centres and a large number of other areas (Fig. 4.5-6). These include:
• Afferent impulses from higher centres controlling VMC.
• Afferent impulses from respiratory centres.
• Cardiovascular reflex mechanisms operating through medullary control centres.
Afferent impulses from higher centres controlling vasomotor centre and cardiac vagal centre
Cerebral cortex
There are descending tracts to the vasomotor area from the cerebral cortex (particularly the limbic cortex) that relay in the hypothalamus. Some examples of the influence of limbic system on the VMC are:
Hypothalamus
The hypothalamus serves to integrate many somatic and autonomic responses. Examples are:
• Temperature regulation. The effect of temperature changes on the hypothalamic centres is relayed to the medulla, which causes the vessels of skin to constrict (heat conservation) or to dilate (heat dissipation).
• Emotional stresses influence heart rate and blood pressure by impulses relayed from the hypothalamus to stimulate or inhibit the medullary centres.
Afferent impulses from respiratory centres
Impulses arising from the respiratory centres affect the heart rate by changing the vagal tone, and the alterations produced are known as sinus arrhythmia which occurs during forced breathing.
• During inspiration, the impulses arising from the respiratory centres during inspiration inhibit the cardiac vagal centre causing reduced vagal tone and sinus tachycardia.
• During expiration, the respiratory centres stop sending inhibitory impulses to the cardiac vagal centre causing increased vagal tone and sinus bradycardia.
Cardiovascular reflex mechanisms affecting medullary control centres
Cardiovascular reflex mechanisms are multiple subconscious special nervous control mechanisms that operate through medullary control centres all the time to maintain the arterial pressure within normal range. These include:
Baroreceptor reflex mechanisms
Baroreceptors, also known as mechanoreceptors or pressure receptors, are the stretch receptors located in the walls of heart and large blood vessels. These are spray-type nerve endings, i.e. they are extensively branched, knobby, coiled and intertwined ends of myelinated nerve fibres. These are stimulated by distension of the structures in which they are located and so they discharge at an increased rate when the pressure in these structures rises. The increased baroreceptor discharge leads to inhibition of tonic discharge of vasoconstrictor nerves and excitation of vagal innervation of heart, and thereby produces vasodilation, venodilation, bradycardia, decrease in cardiac output and decrease in blood pressure.With this definition of the baroreceptor reflex mechanism the baroreceptors will be discussed in detail as under following headings.
Classification and location of baroreceptors
Functional classification
Functionally baroreceptors can be grouped as follows.
1. High-pressure baroreceptors, which monitor the arterial circulation. These include the baroreceptors located at:
2. Low-pressure baroreceptors are located in the low-pressure area of circulation and are collectively referred to as cardiopulmonary receptors. These include:
• Atrial receptors scattered in the wall of right and left atrium.
• Baroreceptors located in the right atrium at the entrance of the superior and inferior vena cavae and in the left atrium at the entrance of pulmonary veins.
• Pulmonary receptors located in the wall of pulmonary trunk and its divisions into the right and left pulmonary artery.
Carotid and aortic arch baroreceptors
Location of carotid and aortic arch baroreceptors
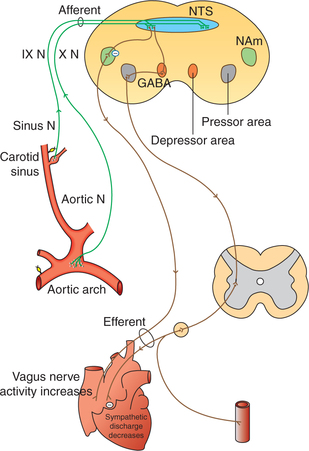
Carotid baroreceptors are located in the carotid sinus, which is a small dilatation of the internal carotid artery just above the bifurcation of the common carotid artery into external and internal carotid branches (Fig. 4.5-7).
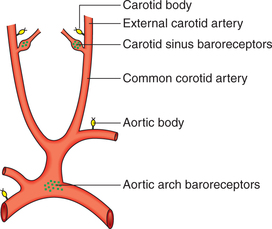
Fig. 4.5-7 Location of baroreceptors (carotid sinus and aortic arch) and chemoreceptors (carotid bodies and aortic bodies).
Aortic arch baroreceptors are located in the wall of arch of aorta (Fig. 4.5-7).
Other systemic arterial baroreceptors (similar to carotid and aortic baroreceptors) are also found at the root of right subclavian artery and junction of thyroid artery, and in the common carotid artery.
Innervation of baroreceptors (Fig. 4.5-8)
Carotid sinus baroreceptors are innervated by the carotid sinus nerve (Hering's nerve), which is a branch of glossopharyngeal nerve.
All other baroreceptors are supplied by the vagus nerve.
Afferent fibres from the baroreceptors pass via the glossopharyngeal and vagus nerves to the medulla. Most of them end in the NTS, where they secrete an excitatory transmitter, presumably, glutamate.
Buffer nerves. The carotid sinus nerve and vagal fibres from the carotid sinus and aortic arch baroreceptors, respectively, are commonly called buffer nerves because these are involved in buffering the blood pressure, i.e. preventing sudden rise and fall in the blood pressure.
Projections from NTS (excitatory glutaminergic projections) terminate on to the:
Response of carotid and aortic baroreceptors to pressure
Response from carotid baroreceptors have been studied in detail. Salient features of these receptor's responses to pressure are given below.
Baroreceptor response. At normal blood pressure levels the fibres of the buffer nerves discharge at a low rate which increases when the pressure in the carotid sinus and aortic arch rises, and declines when the pressure falls (Figs. 4.5-9 and 4.5-10).
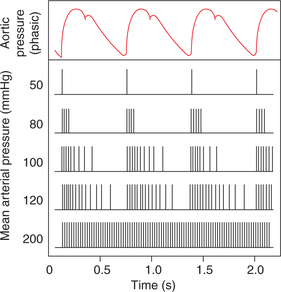
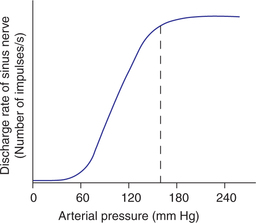
Fig. 4.5-9 Discharges (vertical lines) in a single afferent nerve fibre from carotid sinus at various levels of mean arterial pressure, plotted against changes in aortic pressure with time.
The effect of different arterial pressure levels on the discharge rate in carotid sinus nerve shown in Fig. 4.5-10 depicts that:
• The minimum pressure of about 60 mm Hg at which carotid baroreceptors are stimulated is called threshold of baroreceptor reflex.
• Above threshold level, the baroreceptors respond progressively more rapidly till the discharge rate reaches a plateau, at 150–160 mm Hg, i.e. there is no further increase in response. Thus, the carotid baroreceptors exhibit a great sensitivity as they respond to pressure that varies from approximately 50–160 mm Hg.
• In the normal operating rate at 95–100 mm Hg, even a slight change in pressure causes a strong change in the baroreceptor reflex signals to readjust the arterial pressure back towards the normal.
• When pressure decreases below normal levels, the baroreceptor discharge decreases and reflexly brings the pressure to normal. Conversely, when pressure increases above normal, the baroreceptor discharge also increases and reflexly brings the pressure to normal.
The carotid baroreceptors respond both to the mean pressure and the pulse pressure. Thus, the baroreceptor discharge would increase:
• When the mean pressure rises and the pulse pressure remains unchanged or
• When the pulse pressure rises and the mean pressure remains unchanged.
Carotid baroreceptors respond much more to a rapidly changing pressure than to a stationary pressure.
Pressure–buffer system of baroreceptors. From the above description it is clear that baroreceptor system opposes both increase as well as decrease in the arterial pressure. Therefore, it is called a pressure–buffer system and the nerves from the baroreceptors are called buffer nerves.
Baroreceptors resetting. Baroreceptors possess a property to reset themselves in 1–2 days to whatever pressure they are exposed. Therefore, in chronic hypertension the baroreceptor reflex mechanism resets to maintain an elevated rather than a normal arterial pressure. Because of this property the baroreceptor system has no role to play for long-term regulation of the mean arterial pressure. Thus, the baroreceptor reflex mechanism plays an important role only in preventing the extreme variations in blood pressure which occur for a short term.
Cardiac baroreceptors
Cardiac baroreceptors are located in the walls of heart subendocardially. All cardiac receptors are innervated by the vagus nerve. These include the following.
Atrial stretch receptors
Atrial stretch receptors present in the walls of atria are also called low-pressure receptors.
Atrial stretch receptors have been studied in detail by Prof. AS Paintal (an Indian scientist) in 1953.
Role of atrial stretch receptors
The atrial stretch receptors have been associated with following roles in the cardiovascular control.
1. As low-pressure receptors. The atrial stretch receptors along with pulmonary receptors play an important role to minimize arterial pressure changes in response to change in blood volume. Low-pressure receptors cannot detect the systemic arterial pressure, they do provide information about the circulating blood volume, i.e. greater the venous return, greater will be the discharge from the receptor fibres.
2. Atrial reflex control of heart rate (Bainbridge reflex). Bainbridge noted that sudden rise in the atrial pressure after rapid infusion of saline or blood in anaesthetised animals produced tachycardia, if the initial heart rate was low. This effect is known as Bainbridge reflex. Atrial stretch receptors may be responsible for this reflex. The afferent signals from these receptors pass through the vagus nerves to the medulla of brain. The efferent signals are transmitted back through both the vagal and the sympathetic nerves to increase the heart rate and force of contraction. Thus, this reflex helps to prevent damming of blood in the veins, atria and pulmonary circulation.
3. Atrial reflex control of blood volume (volume reflex). When there is volume overload the atrial stretch receptors help to return the blood volume back towards normal by following mechanisms, which collectively are called volume reflex:
(i) Stretch of the atria causes very significant reflex dilatation of the afferent arterioles in the kidney leading to rise in glomerular capillary pressure with resultant increase in filtration of fluid into the kidney tubules.
(ii) Stretch of the atria also transmits signals to the hypothalamus to decrease the secretion of antidiuretic hormone (ADH), which diminishes the reabsorption of water from tubules.
(iii) Stretch of the atria also causes release of a chemical called atrial natriuretic peptide (ANP), which causes powerful diuresis and thus brings blood volume back to normal.
The above described mechanisms (i–iii), which collectively constitute volume reflex, act as a volume controller and thus indirectly act as a pressure controller as well. Because excess volume increases the cardiac output and thus the arterial pressure as well.
Ventricular receptors
The ventricular baroreceptors are scattered throughout the left ventricle and interventricular septum. They discharge irregularly and no physiological significance can be attached to these receptors.
Bezold–Jarisch reflex or coronary chemoreflex refers to the reflex apnoea followed by rapid breathing, hypotension and bradycardia, which occur following injection of certain drugs like serotonin, veratridine or nicotine into the coronary arteries supplying the left ventricle (injection into the right coronary artery is ineffective) in experimental animals. This reflex is probably produced by the chemical stimulation of the left ventricular stretch receptors.
Pulmonary baroreceptors
Pulmonary baroreceptors are located in the walls of pulmonary trunk and its divisions, the right and left pulmonary artery. The pulmonary receptors along with the atrial receptors constitute the so-called low-pressure receptors or cardiopulmonary receptors, and play an important role to minimize arterial pressure changes in response to change in blood volume as discussed above (see page 183).
Role of chemoreceptor reflexes in cardiovascular control
Chemoreceptors are chemosensitive cells that respond to following changes in blood:
Location of chemoreceptor. The chemoreceptors are present in (Fig. 4.5-7):
1. Carotid bodies. These are 1–2 mm in size and are located in the bifurcation of each common carotid artery. These are innervated by carotid sinus nerve, which is a branch of glossopharyngeal nerve.
2. Aortic bodies are one to three in number, located adjacent to arch of aorta. These are innervated by aortic nerve (branch of vagus nerve).
Functions of chemoreceptors
1. Respiratory control. Chemoreceptors are primarily concerned with the regulation of pulmonary ventilation and are discussed in much more detail in Chapter 5.6, page 245.
2. Cardiovascular control. The chemoreceptors exert their role in cardiovascular regulation in following conditions:
• In hypoxia, there occurs increased chemoreceptor discharge, which not only produces hyperventilation but also excites the VMC leading to peripheral vasoconstriction and increase in the arterial blood pressure. Thus, unlike the inhibitory action of arterial baroreceptors, the chemoreceptors have an excitatory effect on the VMC.
• In hypotension due to severe haemorrhage, the increased chemoreceptor discharge may help to raise the arterial blood pressure.
Note. It is important to note that the chemoreceptors are not stimulated strongly until the arterial pressure falls below 60 mm Hg. Therefore, it is at lower pressures that this reflex becomes important and helps to prevent still further fall in pressure.
Direct effects on vasomotor area
The VMC is directly affected by locally produced hypoxia and hypercapnia. Examples of direct effects are CNS ischaemic response and Cushing reflex.
1. Central nervous system ischaemic response
• When blood pressure falls below 60 mm Hg, the blood flow to the vasomotor area in the brainstem is decreased enough to cause CNS ischaemia.
• As a result of CNS ischaemia, the CO2/lactic acid are accumulated locally near the VMC and excite the neurons of VMC strongly.
• Excitation of VMC causes strong sympathetic stimulation leading to vasoconstriction. There occurs immediate increase in the blood pressure. This most powerful response that activates sympathetic vasoconstrictor system strongly is called CNS ischaemic response. This acts as an emergency arterial pressure control system.
2. Cushing reflex
When intracranial pressure is increased and becomes equal to the arterial pressure, it compresses the arteries in the brain, and blood supply to the vasomotor area is compromised. The hypoxia and hypercapnia produced locally increase the discharge from VMC. The resultant rise in the systemic pressure tends to restore the blood supply to medulla. This effect is called Cushing reflex. The resultant increase in blood pressure also causes reflex bradycardia via baroreceptor response. Thus, bradycardia is an important feature of raised intracranial pressure.
Afferents from nociceptive stimuli
Afferents carrying pain sensations also affect VMC and evoke either pressor or depressor reflex effect as:
Pressor effect in the form of an increase in the blood pressure and tachycardia is caused due to the sympathetic activity by somatic pain afferents, i.e. unmyelinated C-fibres which stimulate the pressor area of VMC.
Depressor effect in the form of hypotension and bradycardia is produced by visceral pain afferents, i.e. thin myelinated fibres which synapse with depressor area of VMC and cause inhibition of sympathetic activity.
Humoral control mechanisms
Humoral regulation of circulation refers to the regulation by substances secreted into or absorbed into body fluids, e.g. hormones, ions, etc. Most important humoral factors affecting circulation are:
Circulating vasodilators
The circulating vasodilators include the following.
KININS
Kinins are peptides which cause vasodilation. Two forms of kinins with similar action found are:
• Bradykinin is nonapeptide found in the plasma and
• Lysyl-bradykinin or kallidin is a decapeptide found in body tissues.
Functions of kinins
• They cause vasodilation by relaxing vascular smooth muscle (VSM) via nitric oxide (NO) and increase capillary permeability.
• Kinins play role in regulating blood flow especially to skin, salivary glands and GIT glands. Therefore, they are formed during active secretion in sweat glands, salivary glands and in exocrine portion of pancreas.
• By regulating blood flow to skin the kinins probably play a role in thermoregulatory vascular adjustments.
Circulating vasoconstrictors
The circulating vasoconstrictors include catecholamines, angiotensin II and vasopressin.
Catecholamines
Catecholamines are released on the sympathetic stimulation and include the following.
Epinephrine. It stimulates both α and β adrenergic receptors:
• Stimulation of α-receptors results in vasoconstriction in skin and splanchnic areas.
• Stimulation of β-receptors results in dilation of the vessels in the skeletal muscles, liver and coronary arteries.
• The β-receptor-induced vasodilation is more dominant than α-receptors-induced vasoconstriction. So, the net effect is slight lowering of peripheral resistance producing slight fall in diastolic blood pressure.
Norepinephrine. It has a generalized vasoconstrictor action as it has much greater effect on α- than on β-receptors. Therefore, it increases peripheral resistance and raises the diastolic blood pressure.
Renin–angiotensin system
The renin–angiotensin system has important roles in the regulation of blood pressure and extracellular fluid volume.
Renin secretion and angiotensin formation
• Renin, a protease enzyme is secreted by juxtaglomerular cells of the kidney into the blood. Its secretion is stimulated by a decrease in the blood pressure.
• Renin catalyzes the conversion of angiotensinogen (α2-globulin substrate present in the plasma) to angiotensin I.
• Angiotensin I is converted into angiotensin II by the action of angiotensin converting enzyme (ACE) present in the endothelium of blood vessels throughout the body, especially in the lungs and kidneys.
Effects of angiotensin II
Angiotensin II has three principal effects by which it can elevate the arterial pressure.
1. Vasoconstriction. Angiotensin II is the most potent pressor substance being 4–8 times more potent than norepinephrine. This effect of angiotensin II is important in the intermediate blood pressure control during circumstances, such as acute haemorrhage.
2. Decrease in salt and water excretion by kidney. Angiotensin II causes salt and water retention by the kidney. This action slowly increases extracellular fluid volume, which increases arterial pressure over a period of hours and days. Thus, this effect of angiotensin II plays an important role in the long-term control of arterial pressure.
3. Stimulation of thirst. Angiotensin II is a powerful stimulator of thirst. It leads to consumption of large volumes of water, leading to a rise in blood volume. This mechanism also plays some role in long-term control of blood pressure.
Ions and other chemical factors
The increased concentration of many different ions and chemical factors can also alter local blood flow by causing vasodilation or vasoconstriction.
• Calcium ions cause vasoconstriction.
• Potassium ions cause vasodilation.
• Hydrogen ions (decreased pH) cause vasodilation.
• Carbon dioxide causes vasodilation in most tissues and marked vasodilation in the brain.
• Glucose or other vasoactive substances, when increased in quantities, raise the osmolarity of blood and cause vasodilation.
Local control mechanisms
Local cardiovascular control mechanisms are primarily concerned with the control of blood flow to the tissues locally.
The mechanisms that are present in most tissues of the body are:
1. Autoregulation, i.e. control of flow during changes in the arterial pressure,
2. Role of local vasodilator metabolites and factors,
1. Autoregulation (control of flow during changes in arterial pressure)
Autoregulation is the ability of an organ or tissue to adjust its vascular resistance and maintain a relatively constant blood flow over a wide range of arterial pressure. For details see page 167.
2. Role of local vasodilator metabolites
The accumulation of local vasodilator metabolites increases local blood flow. The greater the rate of metabolism in the tissue, the greater is the rate of production of tissue metabolites. These include:
• Decrease in O2 tension and pH,
• Increase in pCO2 and osmolality,
Local vasodilator metabolites increase blood flow during following conditions.
Active hyperaemia refers to the vasodilation which occurs when the tissue metabolic rate increases. The dilation of local blood vessels helps the tissues to receive the additional nutrients required to sustain its new level.
Metabolic theory of autoregulation states that any vasodilator metabolites which accumulate in the tissues during active metabolism will produce autoregulation. When blood flow decreases, they accumulate and the vessels dilate; when blood flow increases, they are washed away.
Reactive hyperaemia is a phenomenon by which the local blood flow to the organ is controlled after a period of ischaemia.
3. Role of localized vasoconstrictors
Serotonin released from platelets in the injured tissue is responsible in part for the vasoconstriction, which occurs in haemostasis.
Decrease in tissue temperature causes vasoconstriction and this local response to cold plays a part in temperature regulation.
4. Role of substances released by endothelium
Vascular endothelial cells make up a large and important organ. These cells secrete many growth factors and vasoactive substances, which play an important role in the local control of blood flow. The vasoactive substances include:
(i) Prostaglandins and thromboxane A2
• Prostacyclin is prostaglandin produced by the endothelial cells from arachidonic acid via cyclooxygenase pathway. It inhibits platelet aggregation and promotes vasodilation.
• Thromboxane A2 is produced by platelets and also from arachidonic acid. It promotes platelet aggregation and vasoconstriction.
(ii) Endothelium-derived relaxing factor
EDRF is the name given to a substance which is released by vascular endothelial cells and produces vasodilation. Later on, it was identified to be nitric oxide (NO) in chemical structure.
Mechanism of vasodilation by NO. The NO that is synthesized in the endothelium diffuses to smooth muscle cells, where it activates soluble guanylyl cyclase, producing cyclic GMP, which in turn mediates the relaxation of VSM by decreasing intracellular Ca2+ concentration.
 Medullary cardiovascular control centres
Medullary cardiovascular control centres