Regulation of Respiration
Neural regulation of respiration
Afferent impulses to respiratory centres
 Afferent impulses from higher centres
Afferent impulses from higher centres
Introduction
Respiration is regulated by a complex integration of neural control mechanisms which are modified by certain respiratory reflexes and chemical control mechanisms
Neural control mechanisms include:
• A system for automatic control of respiration as an involuntary function. The involuntary control system of respiration is located in the medullary and pontine centres of the brainstem.
• A system for voluntary control of respiration is located in the cerebral cortex
Thus, respiration enjoys the distinction of being an involuntary function which can be influenced voluntarily.
Respiratory reflexes which modify the effects of neural mechanisms are those initiated by stimulation of stretch receptors, irritant receptors, J-receptors and chest wall receptors.
Chemical control mechanisms are influenced by alterations in arterial pO2, pCO2 and H+ concentration.
Functions of respiratory regulatory mechanisms include:
• Genesis of normal respiratory spontaneous rhythm. This is the function of medullary and pontine centres of neural mechanism.
• Control of rate and depth of respiration, i.e. adjustment of total ventilation to match metabolic needs of the body so that arterial oxygen tension (pO2), carbon dioxide tension (pCO2) and H+ concentration (pH) are almost maintained constant. This function is accomplished by all the respiratory control mechanisms acting in unison (Fig. 5.6-1).
Neural regulation of respiration
The neural mechanisms regulating respiration can be described under two headings:
Automatic control system
Neural genesis of respiratory rhythm
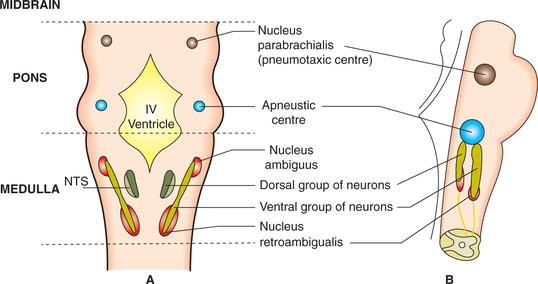
The involuntary neural control system regulates respiration by several groups of neurons situated bilaterally in medulla and pons which include medullary respiratory centres, pontine respiratory centres and reticular activating system (RAS).
I Medullary respiratory centres
The medullary respiratory centres include two groups of neurons: the dorsal respiratory group (DRG) and ventral respiratory group (VRG), which generate the basic respiratory rhythm.
1 Dorsal respiratory group neurons
Most of the neurons are located within the nucleus of tractus solitarius (NTS) and some in the adjacent reticular substance (Fig. 5.6-2). The DRG mainly contains inspiratory cells called I-neurons that discharge during inspiration only. Depending upon the functional activity performed the I-neurons forming DRG are of three types.
(i) Inspiratory neurons. The DRG mainly contains inspiratory cells called I-neurons that discharge during inspiration only. The axons I-neurons cross the midline and descend on the contralateral side of spinal cord to make contact with the spinal motor neurons of the inspiratory muscles, namely the diaphragm (supplied by phrenic nerve arising from C3–C5) and the external intercostal muscles (supplied by intercostal nerves). In other words the I-neurons in the DRG are the upper motor neurons of respiratory muscles. These cells are thought to be responsible for generating basic respiratory rhythm activity.
(ii) Inspiratory off-switch (IOS) neurons. Inspiratory off-switch neurons refer to a groups of neurons that are responsible for terminating the activity of I-neurons and causes turning off of excitation of muscles of inspiration (diaphragm and external intercostal muscles). These muscles therefore relax allowing elastic recoil of the chest wall and the lungs to cause expiration. After expiration again there is signal for starting another cycle.
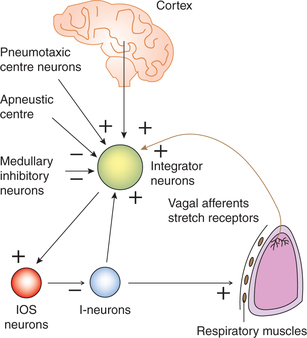
(iii) Integrator neurons. Integrator neurons are another type of DRG neurons. They subserve following integrating functions.
• The integrator neurons when depolarize to a critical level lead to firing of the so-called IOS neurons which are responsible for terminating the inspiratory ramp. The I-neurons trigger the IOS neurons indirectly through integrators and thereby bring about the termination of their own discharge. This forms the basic circuitry of the generation respiratory rhythm (Fig. 5.6-3). In this way cycle of inspiration/expiration goes on continuously to cause tidal respiration.
• The integrator neurons receive both excitatory and inhibitory inputs (Fig. 5.6-3) and thus integrate the activity of I-neurons and IOS neurons accordingly.
• The excitatory inputs to integrator neurons come from: – Cerebral cortex
• The inhibitory inputs to integrator neurons come from the medullary inhibitory neurons which form the so-called apneustic centre.
2 Ventral respiratory group neurons
Ventral respiratory group neurons contain both inspiratory cells called I-neurons and expiratory cells called E-neurons (c.f. DRG which contains only I-neurons). Their axons cross the midline and descend on the contralateral side to make contact with the motor neuron pool for the muscles of expiration, i.e. internal intercostal muscles and abdominal muscles.
Interaction of I- and E-neurons
The I- and E-neurons have inhibitory connections to each other, i.e. there exists reciprocal innervation between the two. Therefore, the motor neurons to the expiratory muscles are inhibited when those supplying the inspiratory muscles are active and vice versa (Fig. 5.6-4).
II Pontine respiratory centres
The pontine centres include the apneustic centre (APN) and pneumotaxic centre (PNC), both of which modify the activity of medullary respiratory centres.
1 Apneustic centre
Apneustic centre refers to a group of inhibitory neurons located bilaterally in the lower part of pons (Fig. 5.6-2). It sends signals to the integrator neurons of DRG that affects the IOS neurons and prevent the switch-off of the inspiratory ramp signals from the I-neurons (Fig. 5.6-3). This increases the tidal volume and duration of inspiration, resulting in a deeper and more prolonged inspiratory effort termed as apneusis. However, normally the APN is inhibited by impulses carried by the vagus nerves and also by the activity of the PNC.
2 Pneumotaxic centre
The pneumotaxic centres are located bilaterally in the upper pons.
Functions. The PNC inhibits the APN. Therefore, stimulation of PNC shortens inspiration, leading to shallow and more rapid respiratory pattern.
Thus, though rhythm of respiration resides in the DRG neurons in medulla, PNC and APN control these neurons to regulate the depth and rate of respiration.
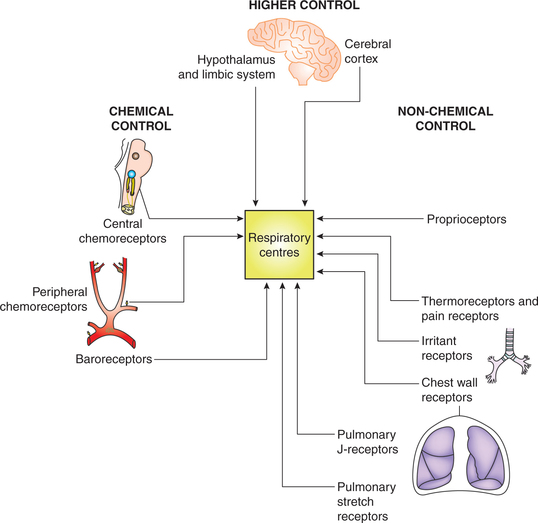
Afferent impulses to respiratory centres
The respiratory centres generate the respiratory rhythm and execute their effects through the efferent nerves supplying the respiratory muscles. The activity of respiratory centres in turn is influenced by the afferent impulses from the lungs and various other parts of the body.
Various afferent impulses to the respiratory centres can be grouped as (Fig. 5.6-5):
• Afferent impulses from higher centres.
• Afferent impulses from non-chemical receptors, which may constitute non-chemical regulation of respiration.
• Afferent impulses from the chemical receptors, which constitute the ‘chemical regulation of respiration’, and hence has been described separately.
Afferent impulses from higher centres
The afferent impulses from the higher centres which influence the involuntary activity of respiratory centres mainly include voluntary control system and limbic control system.
1 Voluntary control system
The voluntary control of respiration is mediated by a pathway which originates from the neocortex and bypasses the medullary respiratory centres to project directly on the spinal respiratory neurons. The voluntary control of breathing is exercised during the following.
Activities like talking, singing and swimming: and breath-holding, i.e. breathing can be stopped voluntarily for about 50–60 s (breath-holding time). But after this time the chemical drive overrides the voluntary inhibition and the person has uncontrollable desire to breathe and ultimately breathing is resumed involuntarily. That is why it is impossible to commit suicide by holding the breath voluntarily.
Voluntary hyperventilation, i.e. voluntary over-breathing can be done for sometime only similar to breath-holding. Effects of hyperventilation are discussed in chemical control of respiration.
Afferent impulses from non-chemical receptors
Afferent impulses from the receptors other than the chemoreceptors, i.e. from non-chemical receptors include the following.
1 Afferent impulses from pulmonary stretch receptors (Hering–Breuer reflex)
The Hering–Breuer inspiratory inhibitory reflex is initiated when the stretch receptors located in the smooth muscles of the bronchi and bronchioles are stimulated by inflation of the lungs. The impulses are then sent through vagii nerves to pontomedullary respiratory centres to inhibit respiration. This reflex is weakest in human. It does not play any regulatory role in tidal respiration. This reflex is initiated only when the tidal volume is more than 1–1.5 L. Thus, the reflex tends to limit the tidal volume.
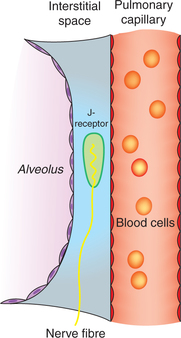
2 Afferent impulses from J-receptors
Afferent impulses from J-receptors constitute the J-reflex. The J-receptors were discovered by an Indian physiologist AS Paintal in 1954. The name J-receptors (juxtapulmonary capillary receptors) was given to them because of their location very close to the pulmonary capillaries (Fig. 5.6-6).
• The J-reflex response is characterized by apnoea followed by hyperventilation, bradycardia, hypotension and weakness of skeletal muscles.
• Physiological role of J-receptors has been postulated during exercise, especially at high altitude when some fluid is entrapped in the alveolar interstitial space which stimulates the J-receptors producing dyspnoea and reduction of the skeletal muscle tone. This effect would discourage exercise, thereby taking away the trigger for pulmonary congestion.
3 Afferent impulses from the irritant receptors in the respiratory tract
Irritant receptors are located below the mucosa of whole respiratory tract. These are stimulated by smoke, noxious gases, particulate matter in the inspired air and in a number of other conditions. These receptors initiate following reflexes.
(i) Cough reflex. This is a protective reflex caused by stimulation of irritant receptors in the pharynx, larynx, trachea and bronchi (conducting zone of respiratory tract). By this endeavour the irritants may be expelled out of the respiratory tract.
(ii) Sneezing reflex is also a protective reflex produced on stimulation of irritant receptors of the nasal mucosa.
(iii) Hering–Breuer deflation reflex is produced on stimulation of irritant receptors located in the bronchial epithelium due to distortion of bronchial epithelium caused by large deflations of the lungs as seen in pneumothorax and lung collapses (atelectasis). This reflex may also be responsible for the sighs or yawning. The reflex helps in opening up the collapsed alveoli again.
(iv) Deglutition reflex refers to temporary apnoea produced during pharyngeal phase of swallowing of food. It is a protective reflex which prevents the entry of food particles into the respiratory tract.
4 Afferent impulses from proprioceptors
Proprioceptors are the receptors present in the muscles, tendons and joints, and are stimulated during change in the position of different parts of the body. This reflex helps in increasing ventilation during exercise.
5 Afferent impulses from chest wall stretch receptors
Chest wall stretch receptors are nothing but the muscle spindles present in the intercostal muscles. Stretching of the intercostal muscles produce a stretch reflex due to the stimulation of muscle spindles that is characterized by contraction of intercostal muscles. The muscle spindles present in the respiratory muscles help to co-ordinate breathing during change in posture or during speech.
6 Afferent impulses from baroreceptors
Baroreceptors or pressure receptors located in the carotid sinus and aortic arch (see details on page 183) are stimulated by increase in the arterial blood pressure. Though they play a primary role in regulation of blood pressure, but the impulses do travel to respiratory centres and cause inhibition of respiration; in physiological conditions the baroreceptors play an insignificant role in regulation of respiration. The adrenaline apnoea observed on injection of high doses of adrenaline causes a large rise in the arterial pressure which in turn inhibits respiration by afferent impulses from the baroreceptors to the respiratory centres.
7 Afferent impulses from thermoreceptors
Thermoreceptors are those receptors which are stimulated by a change in the body temperature. When warm receptors are stimulated the impulses are conveyed to cerebral cortex via somatic afferent nerves. Cerebral cortex in turn stimulates the respiratory centres to produce hyperventilation.
Respiration helps to maintain body temperature, as some amount of heat is lost in the expired air. In dogs panting is one of the major mechanisms of thermoregulation.
Chemical regulation of respiration
The chemical factors regulating respiration are pCO2, pO2 and pH of blood. These factors influence respiration in such a way that their own blood levels are maintained constant. The chemical mechanism of regulation operates through the chemoreceptors.
Chemoreceptors
Chemoreceptors are the sensory nerve endings which are highly sensitive to changes in pCO2, pO2 and pH of blood. These are of three types:
Peripheral chemoreceptors
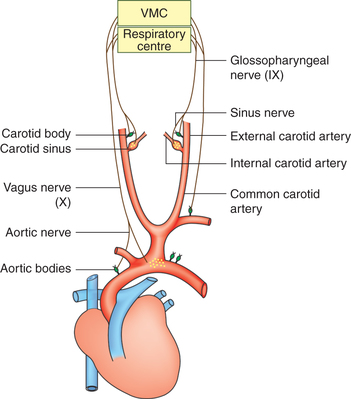
Location. Peripheral chemoreceptors include the carotid and aortic bodies (Fig. 5.6-7).
• Carotid body is located on either side near the bifurcation of common carotid artery.
• Aortic bodies, two or more in number, are located near the arch of aorta.
Structure. Each carotid and aortic body consists of:
Capsule surrounding each carotid and aortic body is very thin.
Sinusoidal large capillaries present below the capsule surround the main mass of each body.
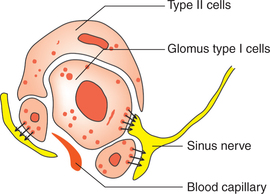
Epithelial cells. The main mass of the body consists of islands of epithelial cells which are of two types: type I and type II (Fig. 5.6-8).
• Type I or glomus cells. These cells have dense-core granules containing catecholamines (probably dopamine). Unmyelinated nerve endings are closely applied to these cells, these nerve endings are cup shaped and have dopamine receptors (D2) on them. When exposed to hypoxia the type 1 cells release catecholamine which stimulates the D2 receptors.
• Type II cells which are probably glial cells are also closely applied to the type 1 cells.
• Nerve fibres. Afferent fibres from the carotid body join the sinus nerve, a branch of glossopharyngeal (IX) nerve and ultimately ascends to the medulla. Those from the aortic body join the aortic nerve branch of vagus (Xth cranial) nerve and ascend to the medulla.
Blood flow to each carotid and aortic body is highest in the body (2000 mL/100 g/min). Therefore, the O2 needs of these cells can be met largely by dissolved O2 only.
Functions. The peripheral chemoreceptors respond to lowered pO2, increased pCO2 and increased H+ concentration in the arterial blood. The afferent impulses from the chemoreceptors stimulate the DRG neurons, which lead to an increased rate and depth of respiration called hyperventilation.
Factors affecting peripheral chemoreceptors stimulation
(i) O2 tension versus O2 content. The peripheral chemoreceptors monitor the dissolved O2, i.e. pO2, rather than its total content. They are stimulated when pO2 falls below 60 mm Hg.
(ii) Elevated pCO2. Elevated pCO2 (by 10 mm Hg) also stimulates the peripheral chemoreceptors, but the major effect of CO2 is on the central chemoreceptors.
(iii) H+ concentration when increased in the blood (decreased pH by 0.1 unit) stimulates the peripheral chemoreceptors.
(iv) Increase in plasma K+ levels may stimulate the peripheral chemoreceptors even in the absence of hypoxia. Increase in plasma K+ levels during exercise contributes to exercise-induced hyperventilation.
(v) Asphyxia, i.e. combination of O2 lack plus CO2 excess in the blood stimulates the peripheral chemoreceptors.
Effects of stimulation of peripheral chemoreceptors
• They regulate the respiration from breath to breath and their stimulation increases the rate and depth of respiration.
• They also cause increase in the blood pressure and tachycardia.
• About 15%−20% of resting respiratory drive is due to the stimulatory effect of CO2 on the peripheral chemoreceptors.
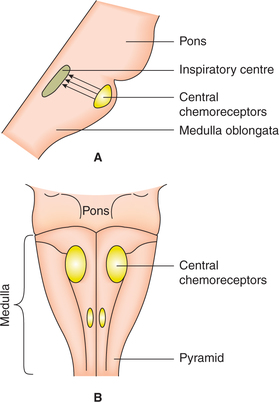
Central chemoreceptors
Location. Central chemoreceptors are the cells (neurons) that lie just beneath the ventral surface of the medulla oblongata and are therefore also called medullary receptors (Fig. 5.6-9).
The neurons forming central chemoreceptors project directly over to the respiratory centres which are located slightly deeper to the central chemoreceptors.
Stimulation characteristics of central chemoreceptors are the following.
• They respond to H+ concentration in the surrounding interstitial fluid and cerebrospinal fluid (CSF).
• The magnitude of stimulation is directly proportional to the local H+ concentration, which in turn parallels arterial pCO2.
• Mechanism by which an increase in CO2 concentration affects central chemoreceptors. CO2 readily crosses the blood–brain barrier because it is a small, very soluble, uncharged molecule. In the CSF, CO2 combines with water to form H2CO3, which dissociates into H+ and 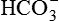 ions. The increase in H+ concentration of CSF and interstitial fluid stimulates the central chemoreceptors, whereas a decrease in the H+ concentration inhibits respiration. It is important to note that the blood–brain barrier does not allow the charged ions (e.g. H+,
ions. The increase in H+ concentration of CSF and interstitial fluid stimulates the central chemoreceptors, whereas a decrease in the H+ concentration inhibits respiration. It is important to note that the blood–brain barrier does not allow the charged ions (e.g. H+, 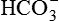 , etc.) to cross through readily. Because of this reason if the arterial pCO2 is kept constant experimentally a decrease in the arterial pH (raised H+ concentration) fails to stimulate central chemoreceptors.
, etc.) to cross through readily. Because of this reason if the arterial pCO2 is kept constant experimentally a decrease in the arterial pH (raised H+ concentration) fails to stimulate central chemoreceptors.
• Central chemoreceptors are not stimulated by hypoxia, rather like any other cell they are depressed by hypoxia.
• Central chemoreceptors are also inhibited by anaesthesia, cyanide and during sleep.
Effects of stimulation of central chemoreceptors are to regulate the respiration from minute-to-minute. Their stimulation leads to an increase in rate and depth of respiration.
Effect of pO2, pCO2 and h+ concentration on respiration
Effect of hypoxia on respiration
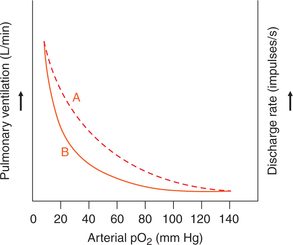
• The normal arterial pO2 is 100 mm Hg, which may fall in many conditions (see page 252), producing the socalled hypoxic hypoxia.
• When the arterial pO2 levels falls to between 100 and 60 mm Hg, not much effect is produced on ventilation. However, a marked increase in the pulmonary ventilation occurs when the pO2 falls below 60 mm Hg (Fig. 5.6-10).
Effect of hypercapnia on respiration
Normal arterial pCO2 is 40 mm Hg, which is kept constant by chemical regulation of respiration.
Hypercapnia, i.e. rise in pCO2 rarely occurs due to an increase in CO2 production. Clinically, it may occur in restrictive lung disorders (see page 216).
• An increase in arterial pCO2 causes a prompt increase in the pulmonary ventilation mainly by stimulating the central chemoreceptors resulting in CO2 washout and a near restoration of arterial pCO2 to normal level (40 mm Hg). There exists a linear relation between increase in arterial pCO2 and increase in pulmonary ventilation.
• When central chemoreceptors are depressed by anaesthesia, then CO2 increases respiration through stimulation of peripheral chemoreceptors. Thus, CO2 acts as a main regulator of respiration.
Carbon dioxide narcosis. It develops when arterial pCO2 increases above 50 mm Hg. Accumulation of such a large amount of CO2 (hypercapnia) in the body depresses the central nervous system, including respiratory centres producing headache, confusion, convulsions, and finally coma and death may occur.
Effect of arterial ph on respiration
(i) Increased H+ concentration (metabolic acidosis) produces prolonged respiratory centre stimulation via peripheral chemoreceptors, leading to a decrease in the arterial pCO2 by elimination of larger amounts of CO2, thus producing compensatory fall in the blood H+ concentration. The related aspects of renal correction of acid–base balance are discussed in Chapter 6.3, page 292.
(ii) Decreased H+ concentration (metabolic alkalosis) depresses respiratory centre via peripheral chemoreceptors, leading to retention of CO2 and an increase in the arterial pCO2. The secondary changes in the arterial pCO2 compensate for the primary metabolic defects and help to restore H+ concentration of blood.
Common causes of metabolic alkalosis, i.e. increase in 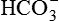 concentration in blood secondary to decreased H+ concentration in blood: excessive vomiting with loss of HCl from the body.
concentration in blood secondary to decreased H+ concentration in blood: excessive vomiting with loss of HCl from the body.
Respiratory acidosis and alkalosis. It may be added here that primary changes in the pulmonary ventilation also effect the pH of blood causing respiratory acidosis or alkalosis.
(iii) Primary pulmonary hypoventilation may lead to elevation of arterial pCO2 (hypercapnia) producing the socalled respiratory acidosis.
(iv) Primary pulmonary hyperventilation may cause a decrease in the arterial pCO2 producing the so-called respiratory alkalosis.
Interaction of pO2, pCO2 and ph in regulation of respiration
In many physiological or clinical situations more than one factor may be present. Their interaction is summarized here.
1 Interaction of pCO2 and pO2
Hypoxia sensitizes the respiratory mechanism to excess of CO2 or H+ concentration, therefore increased pCO2 and H+ concentration produce a much greater effect.
Some other aspects related to chemical regulation of respiration
Effects of hyperventilation
Effect of short-lasting severe hyperventilation
The voluntary hyperventilation may show following effects (Fig. 5.6-11).
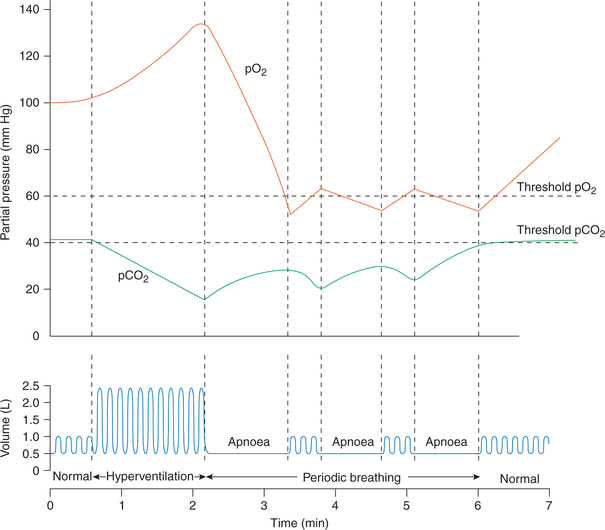
Fig. 5.6-11 Effect of hyperventilation on arterial pO2, pCO2 and respiration. Note the correlation between pCO2, pO2 and periods of hyperventilation, apnoea, and periodic breathing and normal breathing.
(i) Effects on respiration. After a period of hyperventilation any of the following pattern of respiration may be seen for a small period before normal respiration is restored:
• Hypoventilation for a prolonged period is seen in most of the individuals.
• Apnoea, i.e. complete cessation of breathing for 1−2 min may occur in some individuals.
• Periodic breathing (Cheyne–Stokes breathing), i.e. alternate phases of apnoea and breathing may occur for sometime in a few individuals.
(ii) Effects on arterial pCO2 and pO2, and their correlation with effect on respiration (Fig. 5.6-11)
• Arterial pO2 may go as high as 150 mm Hg and pCO2 as low as 15 mm Hg after a period of voluntary hyperventilation.
• Apnoea occurring in some individuals at the end of hyperventilation seems to be related to lack of CO2.
• During phase of apnoea, due to metabolism of body, there occurs a decline in the arterial pO2 and an increase in pCO2. Depending upon the interaction between levels of pO2 and pCO2 attained following effects can occur on respiration:
– If the arterial pCO2 is reached at threshold level (40 mm Hg) then a normal breathing is resumed.
– If the hypoxic stimulus (decreased pO2) becomes strong before the pCO2 reaches a threshold level then periodic breathing (Cheyne–Stokes breathing) may result, i.e. a few breaths eliminate hypoxic drive, then breathing stops and restarts when again hypoxic drive stimulates it. Such cycles are repeated till pCO2 reaches threshold level to normalize breathing.
Effects of prolonged moderate hyperventilation
Prolonged moderate hyperventilation, i.e. twofold to fivefold increase in the pulmonary ventilation may occur under following circumstances:
The prolonged moderate hyperventilation is associated with a decrease in alveolar and arterial pCO2. The low arterial pCO2, which lasts for many days, may result in the following complication in the body.
Neurological changes include tetany, dizziness and light headache. The consciousness may be dulled or even lost.
 Dorsal respiratory group neurons
Dorsal respiratory group neurons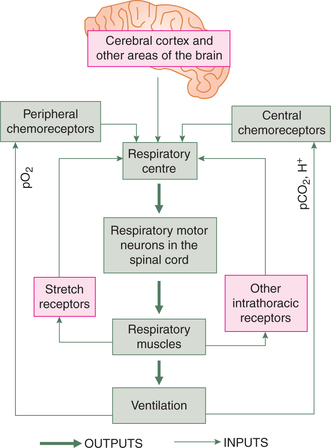
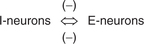
 Important Note
Important Note