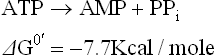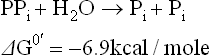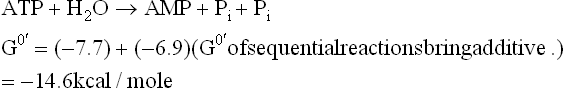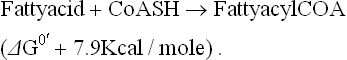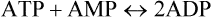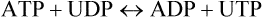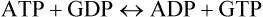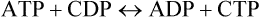Overview of Metabolism and Cell Bioenergetics
Metabolism is defined as the sum total of all the chemical reactions that are taking place in the body. Metabolism is derived from a Greek word, metabellein, which means “to change”. It includes the process by which cells use food material to obtain energy, store excess calories for future use and build up various substances. Metabolism also includes degradation and excretion of unnecessary compounds. In short, metabolism is sum total of all those processes that turn food into flesh.
Bioenergetics is the field of biochemistry that deals with transformation and utilization of energy in biological systems. It concerns only with the initial and the final energy states of reaction components, and predicts the energetic feasibility of chemical reaction. However, it provides no information about the mechanism or the rate of reaction. These aspects are measured in kinetics.
In this chapter, general aspects about metabolism and cellular bioenergetics are discussed. After going through this chapter, the student should be able to understand:
• Fundamental design of metabolic network; interdependence of metabolic reactions.
• Regulation of metabolic pathways and various techniques used to study the details of metabolism.
• Principles of bioenergetics: laws of thermodynamics; free energy, entropy and enthalpy; equilibrium constant.
• Role of ATP as energy carrier; concept of high-energy and super high-energy compounds and substrate level phosphorylation; other energy rich nucleoside triphosphates.
I Overview of Metabolism
A General Considerations
Metabolism serves two important purposes:
1. To release energy from the ingested food material through catabolic degradation, and to convert this energy into a form that can be used for cellular work.
2. To transform small organic compounds into macromolecules. This aspect of metabolism also includes transformation of one group of organic compounds into another.
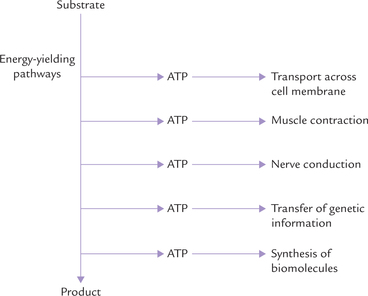
During catabolic degradation, the energy inherent in the organic molecules (particularly carbohydrates and lipids) is released. It is then trapped and stored as adenosine triphosphate (ATP). The stored energy can be released from ATP when needed and used to perform cellular work (Fig. 8.1 ). The major cellular works are:
• Transport of organic molecules and inorganic ions across the cell membrane.
• Mechanical work, such as muscle contraction.
The second major purpose of metabolism is to synthesize a vast array of macromolecules, which include carbohydrates, proteins, lipids and nucleic acids. It is amazing that so many diverse biomolecules are intracellularly synthesized from a limited number of organic compounds. Evidently, thousands of reactions are involved in the processes which split, join and rearrange the atoms of organic compounds, thus resulting in generation of complex biomolecules. Each of these reactions are catalyzed by a specific enzyme.
A bird’s eye view of the scheme of metabolism is presented in this chapter with a focus on fundamental principles underlying metabolism in the cell. The details of reactions of individual metabolic pathways are given in subsequent chapters.
B Metabolic Reactions are Interdependent and Interconnected
Metabolic reactions do not occur in isolation, or in a random or haphazard manner. Rather they are organized into multi-step sequences called metabolic pathways. Each reaction forms just one step in a metabolic pathway and is part of a larger scheme that involves several other interrelated reactions.
In metabolic pathways, the product of one reaction serves as a substrate for the next one; the product of second reaction is substrate for the third reaction, and so on.
Such series of consecutive reactions allows the cell to carry out highly complex molecular conversions intracellularly. It is noteworthy that these conversions occur in mild conditions of temperature and pH that prevail within the cell.
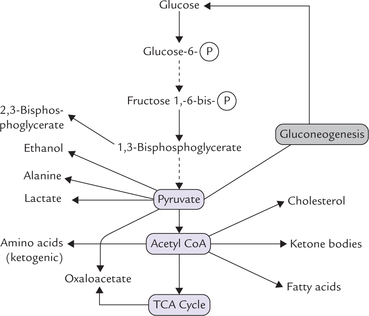
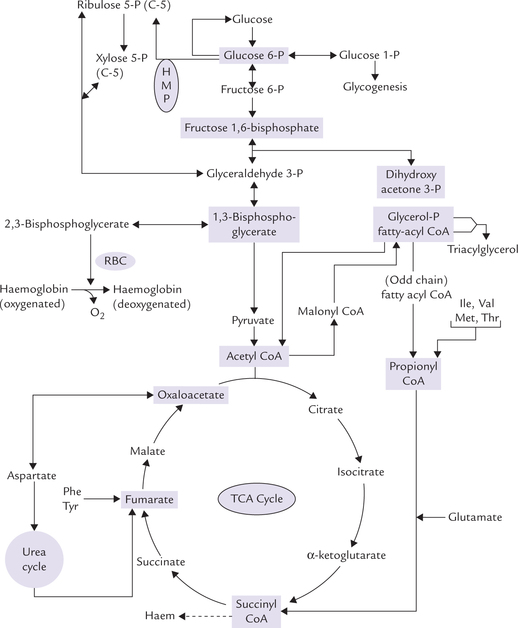
An important example that illustrates this design is the sequence of reactions that converts glucose to pyruvate (i.e. glycolytic sequence). As soon as the glucose enters the cell, a phosphate group from ATP is added to it to form glucose 6-phosphate. Glucose 6-phosphate becomes the substrate for the next reaction, in which an isomerase converts it into fructose 6-phosphate. The latter then serves as substrate for another enzyme-catalyzed reaction, and the sequence continues through six more reactions until glucose is converted to pyruvate. The reactions of this metabolic pathway are summarized in Figure 8.2 .
The energy inherent in the substrate glucose is released in small packets in a stepwise fashion and is effectively captured. If the glucose to pyruvate conversion occurred in a single step, the energy inherent in the glucose molecule could not have been trapped as ATP so effectively. a stepwise transformation ensures efficient and effective trapping of the energy. Further, some of the intermediates of this pathway are channeled into other pathways; for example, glucose 6-phosphate can enter glycogenesis or the pentose phosphate pathway, and 1,3-bisphosphoglycerate can form 2,3-bisphosphoglycerate.
Metabolic pathway appears like an intricate and integrated web of chemical reactions wherein the individual threads are interconnected at several points. In subsequent chapters, individual metabolic pathways would be discussed separately with an aim to simplify and categorize. This might give an erroneous impression that each pathway is self-contained and isolated. However, in reality, all the pathways are interdependent. In fact, if any reaction sequence is followed through the metabolic network sufficiently, we can find it to be connected to every other. For example, the end product of glycolytic sequence is pyruvate which serves as the starting point for gluconeogenesis. It can also be converted to lactate (i.e. anaerobic glycolysis), oxaloacetate (by carboxylation), or acetyl CoA by pyruvate dehydrogenase complex (Fig. 8.2). Acetyl CoA can serve as a precursor for fatty acids, cholesterol, ketone bodies, etc.
Similarly, various other metabolic intermediates, such as glucose 6-phosphate, dihydroxyacetone phosphate, succinyl CoA, etc. also serve as common links between different pathways. Such metabolites are referred to as the branch point compounds.
Figure 8.3 shows important interconnections through branch point compounds (other than pyruvate and acetyl CoA), e.g. oxaloacetate, succinyl CoA and glucose 6-phosphate.
C Regulation of Metabolism
How are metabolic pathways regulated and why is it necessary to regulate them? As noted earlier, all cells are capable of carrying out the central metabolic pathways (such as glycolysis, critic acid cycle, gluconeogenesis, β-oxidation, lipogenesis, etc.). However, it is not desirable to run them all at the same time. For example, when the cell is adequately supplied with energy, the energy-yielding pathways like β-oxidation and TCA must be impeded; and at the same time, the available energy should be used to drive forward the energy requiring pathways, such as lipogenesis and gluconeogenesis.
Moreover, regulation of the metabolic pathways ensures economical use of nutrients. Imagine a cell present in a glucose rich medium. Glucose serves as its major carbon source in this environment. If this cell is shifted to a medium containing alanine as well, it would be a wasteful expenditure if the cell continued to produce alanine from glucose. An efficient cell would turn off its own alanine producing machinery and instead obtain alanine from the medium.
Control of metabolic reactions is accomplished through regulation of enzyme activity. Within a cell, the enzyme activity can be regulated in two ways.
1. By increasing or decreasing the catalytic activity of the enzyme molecule. This is accomplished through (i) allosteric modulation, or by (ii) covalent modulation.
Both the mechanisms induce change in conformation of the enzyme protein, thereby altering its activity.
2. By increasing or decreasing the total number of enzyme molecules. This is accomplished by inducing changes in the enzyme synthesis (by alteration in either transcription or translation), or the enzyme degradation. Hormones play an important role in regulating the enzymes by this mechanism, termed induction-repression. (For details on regulation of enzyme activity, see Chapter 6.)
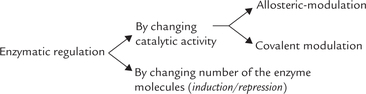
The most economical way to regulate a pathway is to change the activity of the first enzyme of the pathway. Inhibition of the first enzyme stops the production of the end product without wasting any energy of nutrients in the production of unnecessary early intermediates. Thus, the enzymes catalyzing the production of early substrates in a pathway are prime target of metabolic regulation.
D Study of Metabolic Pathways
A number of methods are employed for detailed study of a metabolic pathway so as to elucidate its sequence and its relation with other metabolic pathways. One of the earliest methods, though elementary in nature, involved determination of nature and amount of various compounds present intracellularly. This information was then used to discern the metabolic pathway. However, such analysis yielded a long list of chemicals present intracellularly, but did not provide much information about the metabolic interrelations.
Studies with isotopic tracers and metabolic blocks have been invaluable in this regard. Using these aids it has become possible to know the exact sequence of metabolic pathways. In addition, examination of cell-free extract has revealed finer details of metabolic processes and their regulation.
Isotopic Tracers
Metabolic fate of a compound can be traced by putting a label (tag) on it. Follow up of the tag then serves as a useful indicator of the metabolic route taken by the labelled compound. This strategy is analogous to putting a flag atop a car which helps to identify it in busy traffic.
Earlier, certain dyes were used for the purpose of labelling (i.e. chemical tagging). However, it was found that these dyes might interfere with the normal metabolism of the compound. The chemical tagging is, therefore, of a limited value. Thereafter, search for tags that would not alter metabolism continued. Isotopes were found to be ideal tags.
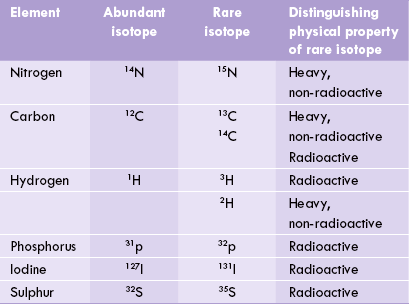
Isotopes are alternative forms of the naturally occurring elements. Chemical properties of an isotope are identical to that of the more abundant form of the element. Its atomic number is also the same, but atomic weight is different from that of the natural element. For example, atomic number of all isotopes of carbon is 6, but molecular weight may be 12, 13 or 14 (Table 8.1 ). The chemical properties of the isotopes are identical. Therefore, cells cannot distinguish between isotopes of the same element. Cells cannot distinguish glucose that contains carbon with an atomic weight of 14 (14C-glucose) from the natural one having an atomic weight of 12 (12C-glucose). However, fate of 12C-glucose can be followed distinctly from the 14C-glucose, lending support to the statement, “isotopes are the compounds that cells cannot see but a scientist can see.”
Isotopes have unusual physical properties which help their identification. For example, heavy isotopes of nitrogen (15N) when incorporated in DNA, gives the latter a higher density. The DNA molecule produced from heavy nitrogen settles at a lower position than its 14N counterpart on performing centrifugation. This strategy was employed by Stahl and co-workers to elucidate the semi-conservative nature of DNA replication (Chapter 21). Some isotopes of carbon are radioactive in nature and emit ionizing radiations. The latter can be detected on radioactivity counter. As a result, the labelled compounds can be easily identified in the complex metabolic maze. For instance, where 14C-acetyl CoA is fed to experimental rabbits, the label appears in tissue fatty acids and tissue cholesterol. Therefore, it can be concluded that acetyl CoA serves as a precursor for fatty acids and cholesterol.
Metabolic Blocks
Metabolic pathways can be blocked by using inhibitors of specific enzymes or by use of the mutant cells which lack one of the other enzyme of the pathway. As a result of the block, the substrate of the blocked reaction tends to accumulate. Sometimes the earlier intermediates of the pathways (i.e. the ones lying a few steps prior to the block) also accumulate. Characterization of the accumulated compounds helps in understanding of the pathway.
If the first step is blocked, the first intermediate of the pathway (V) accumulates. Blocking the second step results in accumulation of first two intermediates (V & W). This information can be used for elucidating the pathway.
Metabolic blocks provide useful information about the sequence of events, i.e. the order in which the pathway intermediates are produced. Suppose, in the above pathway a metabolic block leads to production of V and W, but not of X, Y and Z. This may lead to the conclusion that V and W are produced earlier than X, Y and Z. If a different block allows the cell to produce V, W, X and Y, but not Z, one may logically conclude that Z is the final product of the pathway. In the same manner different metabolic blocks are employed and the results are summed up. This permits placement of intermediates in a defined order.
Cell-free Extracts
The most elaborate metabolic analysis is conducted in cellfree systems. These systems contain all the enzymes and coenzymes that are present intracellularly and which are necessary for the reaction to proceed. In this way, the reactions taking place within the cell are made to occur in vitro.
The information gained by isotopes and metabolic block, when combined with information from studies with the cellfree extract, makes it possible to reconstruct the pathway. For example, to elucidate the first step of a given pathway, the initial substrate is mixed with the cell-free extract. Characterization of the product formed helps us to be sure of correct elucidation of the first step. For example, when phenylalanine (labelled) is mixed with cell-free extract obtained from hepatocytes, the lable appears in the tyrosine molecule after some time. It may, therefore, be concluded that the first reaction of phenylalanine metabolism is:
If, however, the labelled tyrosine is not produced in this system, the obvious conclusion is that there is a metabolic block, i.e. phenylalanine to tyrosine conversion is blocked. Mostly likely, the enzyme catalyzing this reaction (phenylalanine hydroxylase) is deficient, or the coenzyme for the reaction is not available (Case 4.3). Study of various reactions in this manner help in correct identification and placement of the metabolic intermediates.
Studies with cell-free extract also permit detailed study of a given metabolic pathway, separately from the others. Further, effect of various extraneous conditions, such as pH, temperature and substrate concentration can be studied by changing these factors. In this manner not only the sequence of the pathway but also its regulation can be studied.
Steps of Cell-free Extracts Preparation from the Isolated Cells
In a number of clinical cases in this book, diagnostic utility of studies with cell-free extracts is expressed.
Step I: Rupture of the cell membrane, i.e. cell lysis, as described in Chapter 7. The intracellular contents are thereby obtained.
Step II: Separation of the intracellular contents from the solids, such as cell membrane and cellular debris by ultracentrifugation. The supernatant represents the cell-free extract.
II Bioenergetics: Principles
Energy is defined as capacity to do work; it is required for performing various activities. The living cells need energy for performing various activities, discussed on page 146.
Billions of years of evolution has taught the living cells to use energy very economically and efficiently.
Energy transformations, both by the living systems as well as by the artificial devices, take place according to the laws of thermodynamics. Thermodynamics is a branch of physical science that deals with energy changes.
A Laws of Thermodynamics
The First Law
The first law of thermodynamics, also referred to as the law of conservation of energy, states that energy can neither be created nor destroyed. In course of any physical or chemical reaction, one form of energy may change to some other form, but the total amount of energy in the universe always remains constant.
For example, electric energy changes to heat energy in room heater, heat energy changes to mechanical energy in rail engine and mechanical energy changes to electric energy in hydroelectric plants. These examples show that various forms of energy are interconvertible. However, none of these transformations brings about any net generation or loss of energy.
The Second Law
All physical and chemical reactions tend to proceed in such a direction that useful energy of the reacting system is irreversibly converted to a randomized and useless form, known as entropy. The reactions proceed in this direction till entropy reaches maximum possible under the prevailing circumstances. At this point, called the equilibrium point, no further progress of the spontaneous reaction is possible.
Thus in all reacting systems, disorder or randomness is favoured at the cost of orderliness. To be more explicit, fall in useful energy content of the system occurs with a concomitant rise in the randomized energy of the universe. The reacting system implies collection of matter undergoing a reaction, and the universe includes both, the system and its surroundings. This literally includes the whole of earth, or even the outer space.
B Free Energy, Entropy and Enthalpy
Useful energy is the form of energy that is capable of performing work. It is broadly classified in two major types:
1. Heat energy, which is capable of performing work through change of temperature.
2. Free energy which is capable of performing work at a constant temperature.
Since human body maintains a constant temperature (i.e. isothermic), it cannot utilize the heat energy. Free energy is the useful form of energy in humans because of its ability to function at constant temperature. It performs various functions (mentioned on page 146) and, in the process, gets converted to the randomized form, i.e. entropy. These relations can be expressed in the equation form as below:
• ΔG is change in the free energy content of the reacting system. It equals the difference in energy level between the substrates and products.
• ΔS is change in entropy of the universe.
• T is absolute temperature measured in Kelvin.
• ΔH implies change in heat content of the system, which is termed as enthaply (the word means “warming within”).
In course of any ongoing reaction, entropy of the universe (system + surroundings) always increases. The value of ΔS, therefore, has a positive sign. Since rise in entropy is accompanied by a corresponding fall in free energy of the system, the value of ΔG is negative. The enthalpy change may be positive or negative, depending on whether the system absorbs or releases heat. It is positive when the heat absorption occurs and is negative when heat release occurs. However, in the isothermic state prevailing in the body, its value is zero.
Finally, it must be emphasized that entropy or disorder is not an entirely useless activity. Since increase in entropy is an irreversible process, it gives direction to all biological activities.
C Standard Free Energy Change
The free energy change, ΔG is the most important thermodynamic function because it is the driving force of the reactions. Like ΔH, it is measured in kilocalories per mole (kcal/mol) or kilojoules per mole (KJ/mol). a negative sign of ΔG indicates an exergonic reaction, the one that proceeds spontaneously and forms product from subratate. a positive sign of ΔG indicates an endergonic reaction which can proceed only in the backward direction. At equilibrium, ΔG equals zero. ΔG is not a property of the reaction as such but is affected by relative reactant concentrations. To obtain a convenient energetic expression that predicts the equilibrium of the reaction, we have to define the standard free energy change (ΔG0). The standard conditions are as below:
• Temperature of 25°C (298°K).
• Pressure of 1.0 atm (760 mmHg).
• The reactants and products are all present in the concentration of 1.0 M.
The standard free energy change of a given reaction is an immutable constant and it equals the difference in energy level between the substrates and products, under standard conditions, and is shown in Table 8.2 . It predicts equilibrium of a reaction.
The standard free energy change (G0) must be differentiated from the actual free energy change (ΔG) of a reaction:
1. ΔG0 is measured under standard conditions, stated above and has a constant value for a given reaction. In contrast, the actual free energy change is measured at the conditions prevailing during the reaction. It is a function of the conditions of concentration, pH and temperature actually prevailing during the reaction, which are not necessarily the standard conditions.
2. Further, the value of the actual free energy change keeps changing as the conditions change. It has negative value in the beginning of the reaction, which becomes less and less negative as the reaction progresses towards equilibrium. At equilibrium its value falls to zero.
ΔG0 has a definite relation with ΔG and with equilibrium constant of the reaction, as illustrated in Boxes 8.1 and 8.2, respectively.
Standard Free Energy Changes of Sequential Reactions
Total standard free energy change of a reaction sequence equals the sum of the standard free energy changes of the individual reactions of the sequence.
Glucose + ATP → Glucose 6-phosphate + ADP
Glucose 6-phosphate + H2O → Glucose + Pi
The sum of these two reactions is
It has a standard free energy change of (–4.0) + (–3.3) = –7.3 kcal/mole.
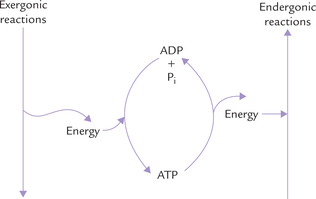
D Role of ATP in Cell Bioenergetics
ATP (adenosine triphosphate) serves as the mediator of biological energy transfers. It acts as an energy carrier in biological systems. It links the energy-yielding (i.e. exergonic) and the energy-requiring (i.e. endergonic) processes.
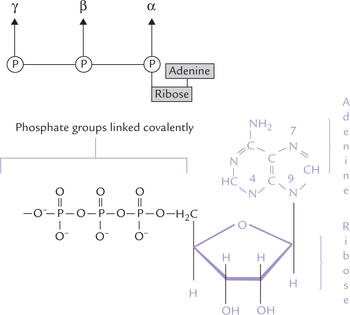
Composition
ATP is a nucleotide consisting of the following three components (Fig. 8.4 ):
1. Purine base which is adenine.
2. Ribose sugar which is a 5-carbon sugar (i.e. a pentose). The first carbon of ribose is linked to N-9 of adenine through N-glycosidic linkage.
3. Phosphate groups, three in number, are designated α-, β- and γ-phosphates. The α-phosphate is linked to C-5 of the ribose.
Functions
ATP serves as a link between the energy-yielding (exergonic) pathways and the energy-requiring (endergonic) processes, as mentioned earlier.
Normally, the exergonic processes are thermodynamically favoured and therefore, occur spontaneously. The endergonic processes, on the other hand, are thermodynamically unfavoured, and hence, require input of energy to be driven forward. These two types of processes are coupled, so that the energy of exergonic reaction can be used to drive progression of the endergonic reaction.
In most biological transformations, ATP is generated during exergonic reactions, using the free energy released (Fig. 8.5 ). Thus each molecule of ATP represents stored free energy.
Some of the energy requiring processes include biosynthesis, active transport, transfer of genetic information and movement (Fig. 8.1). Thus, ATP serves as a primary and universal carrier of free energy. It is also referred to as the currency of free energy in the body.
Standard free energy (DG0 )
The standard free energy of hydrolysis of ATP is –7.3 kcal/mole. It implies that 7.3 kcal of free energy is released, under standard conditions, from 1.0 mole of ATP by the following reactions.
Conversely, reformation of ATP occurs by attachment of a terminal phosphate group to ADP. The process requires input of the same amount of free energy. Reasons for high value of the standard free energy of hydrolysis of ATP are given in Box 8.3.
The energy obtained from various catabolic pathways is used for the ATP generation: 7.3 kcal of energy is required for the synthesis of 1.0 M of ATP (from 1.0 M each of ADP and Pi). Therefore, each mole of ATP represents 7.3 kcal of stored energy. During the energy-requiring processes, hydrolysis of ATP occurs, which liberates the required energy. In this way, generation of ATP is followed by its hydrolysis, which is ΔGain followed by regeneration, and so on. Thus the γ-phosphate group of ATP
E Low-Energy, High-Energy and Super High-Energy Compounds
ATP is high-energy compound placed higher than several compounds on the thermodynamic scale (Table 8.2). Such compounds having ΔG0’ less than that of ATP are termed the low-energy compounds. On the other side of the thermodynamic scale lie certain compounds, ΔG0’ of which is higher than that of ATP. Such compounds are referred to as the super high-energy compounds; phosphoenol pyruvate (PEP) and creatine phosphate having ΔG0’ of –14.8 kcal/mole and –10.3 kcal/mole respectively are some examples.
Phosphoenol pyruvate
Phosphoenol pyruvate (PEP) is an intermediate in glycolysis. Its conversion to the next glycolytic intermediate, pyruvate, under standard conditions, yields 14.8 kcal/mole energy. This is more than sufficient for the formation of ATP, which needs 7.3 kcal/ mole. Thus, ATP, formation (i.e. phosphorylation) is coupled with transformation of a substrate (i.e. PEP in this case). This process is called substrate level phosphorylation.
Creatine phosphate
It is stored in the skeletal muscles, where it helps in quick in generation of ATP. During muscle exercise, ATP is rapidly hydrolyzed to meet the energy needs of the exercising muscle. Consequently, the ATP levels within the muscular tissue tend to get depleted. Creatine phosphate replenishes the ATP under these circumstances. Conversion of this compound to creatine and phosphate releases energy, which is used for ATP generation.
This is also an example of the substrate level phosphorylation. It permits the skeletal muscles to perform the intermittent strenuous work by maintaining the intracellular ATP levels normal. Since ATP lies midway in the thermodynamic scale, i.e. between the super high energy compounds and the low energy compounds (Table 8.2), it is a suitable compound to serve as a currency of free energy in the body.
F Cleavage of ATP to AMP and Pyrophosphate
In course of most endergonic reactions, ATP is converted to ADP by removal of the terminal γ-phosphate group (orthophosphate cleavage). However, in certain other endergonic reactions, the β-and the γ-phosphate groups are simultaneously removed in one piece to form AMP and pyrophosphate (pyrophosphate cleavage).
This followed by cleavage of the pyrophosphate, which yields two phosphate groups.
Sum of these two reactions and its G0’ is
This is double than the usual 7.3 kcal/mole yield by orthophosphate cleavage of ATP. Release of a larger amount of energy in this manner can drive certain complex biosynthetic reactions forward. For example, 7.3 kcal/ mole, obtained by orthophosphate cleavage of ATP would be insufficient to drive the activation of fatty acid to completion, which requires an input of about 7.9 kcal/mole.
Release of 14.6 kcal/mole of energy by the pyrophosphate cleavage of ATP can drive this reaction to completion.
Amount of energy provided by pyrophosphate cleavage of ATP is evidently far in excess of that required for the fatty acid activation. The surplus (i.e. 14.6 – 7.9 = 6.7 kcal/ mole) dissipates as entropy. However, dissipation is not a wasteful expenditure, since it helps to drive the reaction forward and further ensures its irreversibility.
In contrast to ADP, which returns to the ATP cycle by directly accepting a phosphate group, AMP requires participation of an additional enzyme, adenylate kinase, to form ATP. This enzyme catalyzes reversible phosphorylation of AMP to yield two ADPs:
The ADPs so formed directly accept phosphate group(s) to form ATP. Acting in the reverse direction, the adenylate kinase has another important role: it helps to maintain a constant intracellular ATP level by catalyzing the transfer of the β-phosphate of one ADP to another ADP molecule. Thus it supplements the ATP generation by creatine kinase in the contracting muscle.
G Other Energy Rich Nucleoside Triphosphates
There exist several other nucleoside 5’-triphosphates which are analogous in structure to ATP, and which are also energy-rich like ATP. Some examples are guanosine triphosphate (GTP), cytidine triphosphate (CTP), and uridine triphosphate (UTP). They are present in all cells but in a much lower concentration than ATP. Although ATP is the major carrier of the phosphate group, the other types of nucleoside triphosphates are specialized to serve in certain biosynthetic pathways. For example, CTP is used in phospholipid biosynthesis and UTP in glycogen synthesis. Synthesis of the nucleoside triphosphates is catalyzed by the enzymes called nucleoside diphosphokinases: ATP mostly serves as the donor of the terminal phosphate groups.
Low concentration of corresponding 2’-deoxyribonucleoside 5’-triphosphates is also present in cells. These are 2’-deoxyadenosine 5’-triphosphate (dATP) and 2’- deoxyguanosine 5’-triphosphate (dGTP), 2’-deoxycytidine 5’-triphosphate (dCTP), and 2’-deoxythymidine 5’- triphosphate (dTTP).
 Metabolism comprises a highly integrated network of chemical reactions, which can be subdivided into catabolism and anabolism. Catabolic reactions are used to extract energy from fuels, and anabolism comprises reactions that use this energy for biosynthesis.
Metabolism comprises a highly integrated network of chemical reactions, which can be subdivided into catabolism and anabolism. Catabolic reactions are used to extract energy from fuels, and anabolism comprises reactions that use this energy for biosynthesis.
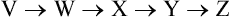
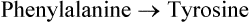
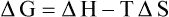
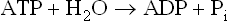
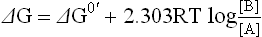
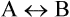 ) equilibrium describes a state when rate of the forward reaction equals that of the reverse reaction. In this state no net chemical transformation occurs, so that equilibrium concentrations of both a and B are invariable. Their ratio is termed as equilibrium constant (
) equilibrium describes a state when rate of the forward reaction equals that of the reverse reaction. In this state no net chemical transformation occurs, so that equilibrium concentrations of both a and B are invariable. Their ratio is termed as equilibrium constant (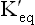 of the reaction)
of the reaction)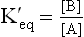
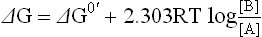
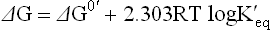
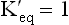 , value of log
, value of log 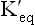 is zero. Hence, ΔG0 = 0, meaning that product contains the same amount of free energy as the reactant, and therefore the reaction stands at equilibrium. Likewise, if the Keq > 1.0, its G0’ is negative and if Keq < 1.0, its G0’ is positive and the reaction respectively proceeds in the forward and the reverse direction.
is zero. Hence, ΔG0 = 0, meaning that product contains the same amount of free energy as the reactant, and therefore the reaction stands at equilibrium. Likewise, if the Keq > 1.0, its G0’ is negative and if Keq < 1.0, its G0’ is positive and the reaction respectively proceeds in the forward and the reverse direction. 
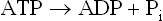
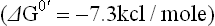
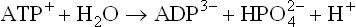
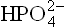 are negatively charged and therefore, have little tendency to approach one another and recombine to form ATP.
are negatively charged and therefore, have little tendency to approach one another and recombine to form ATP.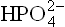 is much lower than that of ATP.
is much lower than that of ATP.