Characteristics of Cells and Membrane Transport
Cell is the basic functional unit of all forms of life. It is the live unit containing a number of living components that are sequestered from the external environment by a membranous barrier. The cell membrane is selectively permeable since it permits passage of only selected substances across it, being impermeable to most others.
In prokaryotes, such as bacteria, algae and rickettsia, the cell itself is the organism. In some eukaryotes, like yeast and fungi, aggregation of the cells occurs to form tissues. In the higher forms of life, such as plants and animals, the tissues are further organized to form the whole organ systems. The crucial difference between the eukaryotic and the prokaryotic cell is the presence in the former of internal membrane structures, particularly the nucleus. Such structures are not seen in prokaryotic cells.
Most eukaryotic cells are 10-30 times longer in their linear dimension than the prokaryotic cells. For example, the Escherichia coli cell is about 1 mm long, whereas the hepatocyte is about 30 mm in diameter. Thus, the relative surface to volume ratio is much larger in this microorganism compared to that in the hepatocyte but the size is much smaller.
For all cell types, it is advantageous to have membrane surfaces on which various membrane-related phenomena, such as enzyme-catalyzed reactions and cellular transport takes place. Bacteria make use of the cell membrane for this purpose, whereas eukaryotic cells have acquired internal membranes for the same. The surface area of these internal membranes account for 96-98% of the total membrane area associated with the hepatocyte. However, exceptions do occur in eukaryotes. For instance, the plasma membrane of the intestinal epithelial cell has a large surface area, a very useful adaptation which enables it to absorb nutrients (Chapter 26).
This chapter outlines the structure and function of a typical eukaryotic cell. It also describes characteristics of biological membranes and membrane transport. After going through this chapter, the student should be able to understand:
• Chemical composition and architecture of a eukaryotic cell; functions of various subcellular components.
• Biological membranes: lipid bilayer structure, fluid mosaic model, the role of phospholipids, proteins (extrinsic and intrinsic), and other biomolecules in the bilayer structure; membrane asymmetry and fluidity, specialized membrane structures.
• Mechanisms for transport of substances across the permeability barrier, i.e. passive and carrier mediated transport, exocytosis and endocytosis, various types of channels and ionophores.
I Cell Structure
Different types of cells vary enormously in their size, shape and specialized functions. Despite these outward differences, various kinds of cells are remarkably similar in their basic structural features. All cells are rendered self-contained by the surrounding plasma membrane, the basic molecular architecture of which consists of two layers of lipid molecules. A number of specialized proteins are embedded in the plasma membrane. Enclosed by the membrane there is the cytoplasm in which a vast array of chemical reactions of metabolism occurs. Also present in the cytoplasm is a nucleus or nuclear body in which genetic material is replicated and stored (as deoxyribonucleic acid). A number of organelles are also present, each of which performs a specialized task; e.g. mitochondria are power plants of eukaryotic cells, lysosomes are pockets containing lethal enzymes, peroxisomes are peroxide-destroying vesicles, Golgi bodies are secretory vesicles, ribosomes synthesize proteins and endoplasmic reticulum form a maze of membrane channels through the cytoplasm.
A Composition: Chemical Viewpoint
An average eukaryotic cell contains 70% or more water. About 10% of the weight of the cell is contributed by inorganic matter, and the rest is accounted by organic material.
Inorganic Constituents
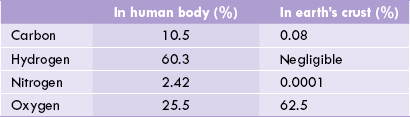
Inorganic constituents of the cell comprise mineral elements. Although more than 100 mineral elements are found in nature, only 22 are present in cells. Most abundant of these are carbon, hydrogen, nitrogen and oxygen. Together, these four elements account for about 98% of the total mineral elements present in living systems. The relative abundance of these elements in the human body and earth’s crust is shown in Table 7.1 .
These four elements make up thousands of intracel-lular compounds. Most are highly reduced and energy rich. Other cellular inorganic constituents are cations like Na+, K+, Ca2+, Fe2+, Cu2+, Mg2+ and Zn2+; and anions like C1-,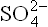 and
and 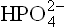 About 1.0% of the weight of the cell is accounted for by inorganic matter.
About 1.0% of the weight of the cell is accounted for by inorganic matter.
Organic Constituents
The organic molecules in the cell, about 500 in number, are of diverse types. Some of them having relatively low molecular weight (MW 50-350 D) account for about 4.0% of the wet weight of the cell, e.g. monosaccharides, amino acids, fatty acids, nitrogenous bases, etc. The high molecular weight organic compounds (MW 2-2000 kD) are mainly proteins; the latter roughly accounts for 10-20% of the wet weight of the cell. Lipids, complex carbohydrates, DNA and RNA contribute approximately 3.0%, 3.0%, 1.0%, and 5.0% of the cellular wet weight, respectively. However, per cent composition of the above-mentioned cellular components varies depending on the cell type; a given cell may be rich in one type of bio-molecule but lack in others. For example, adipocytes contain about 98% triacylglycerols, and RBCs contain about 40% proteins.
B Subcellular Organelles of Eukaryotic Cell
Ultrastructure of a cell showing various subcellular (internal) organelles is illustrated in Figure 7.1 . Each organelle is specialized for performing a specific task (as outlined later in Table 7.3). Relative proportions of different organelles vary from one cell type to another. Moreover, a given organelle may carry out a certain function in a given cell type only. For example, release of glucose from glucose 6-phosphate can occur in hepatocytes (because of the presence of the enzyme, glucose 6-phosphatase), but not in cells of other tissues.
Separation of Subcellular Organelles
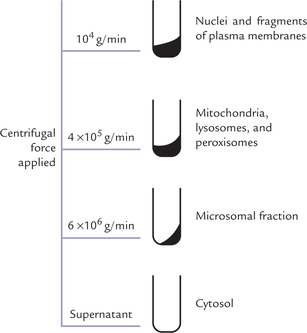
The technique for separating subcellular organelles (subcellular fractionation) involves breaking open the cell by homogenization and separating various organelles from one another by centrifugation.
Separation of organelles for biochemical studies was first studied by Schneider and Pallade in late 1940s, and Albert Claude was awarded Nobel Prize in 1974 for fractionating subcellular organelles. The latter showed that different organelles, present in homogenate of a tissue, such as rat liver, could be separated by a procedure known as differential velocity centrifugation.
The following steps are involved in subcellular fractionation:
• The tissue is suspended in 0.25 M sucrose solution and the cells are disrupted by means of the shearing forces generated in a potter homogenizer.
• The homogenate thus prepared is then subjected to high-speed centrifugation. This process brings about separation of various organelles because different organelles sediment at different speeds in a centrifugal field, depending on their density and size. More the density and the size, more readily does the sedimentation take place. To some extent, shape of the subcel-lular organelle also determines its sedimentation.
• Centrifugal forces of sufficient magnitude and duration are used to produce separation of the organelles. Nucleus being the heaviest particle, sediments most readily. Centrifugation at 104 g/min results in pelleting of nucleus.
• Centrifugal force of 4 × 105 g/min results in sedimentation of mitochondria, lysosomes and peroxisomes (Fig. 7.2 ).
Separation of other organelles is then achieved in the same way, i.e. by increasing the centrifugal force.
Some enzymes, called the marker enzymes, are preferentially located in particular organelles, as shown in Table 7.2 . They are used to identify purity of an organelle preparation: a given organelle is identified (after centrifugation) by measuring the activity of its marker enzyme in various subcellular fractions.
Table 7.2
Marker enzymes. A given marker enzyme is present within only one compartment of the cell
| Subcellular organelle | Marker enzyme |
| Mitochondria | Inner membrane: ATP synthase |
| Lysosome | Cathepsin |
| Golgi complex | Galactosyl transferase—mannosidase I (cis); Fucosyl transferase (trans) |
| Microsomes | Glucose 6-phosphatase |
| Cytoplasm | Lactate dehydrogenase |
Why do Eukaryotic Cells Subcellular Organelles?
The subcellular organelles provide several benefits to the eukaryotic cell. They create compartments within the cell. Each compartment is specialized for a specific role. Major advantages of having compartments are as below:
1. Enhances efficiency of reactions: In the metabolic pathway, the reactions are sequential, which means that product of a reaction serves as substrate for the next reaction. Such reactions work much more efficiently if they are held in close proximity within a compartment.
2. Storage: Specific substance can be stored within a distinct intracellular compartment, from where it can be released when the need arises. For example, calcium ions are stored in sarcoplasmic reticulum and released to promote muscle contraction.
3. Reciprocal regulation of opposing pathways: The pathways that carry out opposing groups of reactions (such as fatty acid synthesis and degradation) do not occur simultaneously. A mechanism for their reciprocal regulation exists by which when one of the pathways is operative, the opposite pathway is inhibited. A smooth working of this arrangement is possible only when the opposite pathways are located in different compartments. For instance, the pathway for fatty acid synthesis is located in cytosol, whereas the pathway for its degradation is located in the mitochondrial matrix.
4. The pH within an organelle can be maintained at a different level than the rest of the cell (e.g. lysosomes). Lysosomes are membrane-bound digestive vesicles of varying sizes and shapes (average diameter = 0.5 μm) that contain lytic enzymes (called hydrolase) at a relatively low pH (4.5-5.5).
The hydrolases cause intracellular digestion of mac-romolecules. Each lysosome contains over 30 hydro-lases, which can digest all types of biomolecules (e.g. proteins, nucleic acids, carbohydrates, and lipids). Therefore, lysosomes are referred to as the potential lethal bags of the cell. Since the lysosomal membrane separates these enzymes from the cytosol, the cyto-solic contents cannot be digested by them under normal circumstances. Even when the acid hydrolases leak out of the organelle because of membrane damage, they can cause only minimal damage to the cytosolic contents. This is because their optimum pH is low, and so they are readily inactivated at the relatively high pH prevailing in the cytosol.
Cytoskeleton
Electron microscopic studies have shown that a complex network of protein filaments is present in the cytoplasm. The filaments form a flexible, framework within the cell, called cytoskeleton. Pivotal role is played by the cytoskeleton in a variety of cellular functions, such as intracellular transport, maintenance of shape of the cell, motility and cell division.
Three types of filaments are usually found in the mammalian cell: (a) microfilaments of 5 nm diameter, (b) microtubules of 25 nm diameter, mainly present in the long cells of the nervous system, and (c) myosin, which is mainly present in the muscle cells.
Cytoplasm
The cellular matrix in which the subcellular organelles are embedded is known as cytoplasm. It accounts for approximately 50% of the cell volume. Cytoplasm was previously believed to be an inert jelly. However, it is now known that cytoplasm contains a variety of enzymes that participate in a number of metabolic reactions. The other components of the cytoplasm are glycogen granules and fat droplets.
Role played by various organelles is given in Table 7.3. For details the student is advised to refer to a textbook of cell biology.
Table 7.3
Functions of subcellular organelles
| Organelle | Function |
| Nucleus | Contains chromatin composed of DNA and proteins, RNA synthetic apparatus, and a fibrous matrix |
| Nucleolus | A nuclear subcompartment where most of the cell’s rRNA is synthesized |
| Endoplasmic reticulum (smooth) | Site of biosynthesis of several biomolecules, detoxifies certain hydrophobic compounds |
| Ribosome | Protein synthesis |
| Golgi apparatus | Functions in the synthesis, processing and sorting out of secreted proteins, lysosomal proteins and certain membranes |
| Mitochondrion | Principal site of oxidative pathways (e.g. TCA, β-oxidation), ATP production, urea cycle (partly) and haem synthesis (partly) |
| Lysosome | Acidic organelle, contains a battery of lytic enzymes that degrade material internalized by the cell, and worn-out cellular membranes and organelles |
| Peroxisome | Oxidation of long chain fatty acids, D-amino acids and α-hydroxy fatty acids |
| Cytoplasm | Site of several metabolic pathways |
II Biological Membranes
Biological membranes are large flexible sheets that are universal elements of cell structure. Each cell is surrounded by a plasma membrane, which forms a boundary between the cell and its environment. It plays important role in:
(a) cell-cell recognition and communication,
(b) maintenance of the shape of cell,
(d) controlling movements of molecules between the inside and the outside of the cell.
Eukaryotic, but not prokaryotic cells, possess intracel-lular membranes as well, which encompass various cell organelles, thus segregating one part of the cell from the other and also enabling each organelle to carry out its characteristic cellular function.
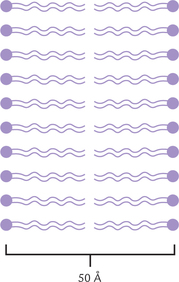
Membranes are very active metabolically and have significant influence on cell metabolism. Most membranes are around 50 Å (5 nm) thick. In general, the membranes of organelles are thinner than the plasma membrane. All membranes have similar structural organization, which may be pictured as a mosaic of globular proteins embedded in a fluid-like phospholipid bilayer.
A number of diverse functions are performed by biological membranes, as outlined below:
Membrane lipids—Diffusion barrier, controlling movement of specific molecules, maintain shape of cell.
Membrane proteins—Enzymes, carrier activities, signal transduction, link between cytoskeleton and extracellular matrix.
Carbohydrates—Cell-cell recognition, adhesion and receptor action.
A Chemical Composition of Membranes
Lipids and proteins are the two major components of all biological membranes, as mentioned earlier. Relative proportions of the two vary greatly amongst different types of membranes. For example, lipids account for about 20% of the total weight of the rat liver cell membrane to over 54% in myelin sheath. In most membranes, 50-65% of the total membrane mass is accounted by proteins. Membranes of organelles have a higher percentage of proteins because of their greater participation in enzymatic and carrier (i.e. transport) activities. Inner mitochondrial membrane (IMM) contains highest proportion of proteins (75%).
Other membrane components, present in smaller quantities (5-8%) are glycolipids and glycoproteins. Carbohydrates do not exist in free form in membranes. Small quantities of free and esterified cholesterol are also present in most membranes.
B The Lipid Bilayer
The bilayer structure of membranes is due to amphipathic nature of the major membrane lipids, i.e. the phospho-lipids. A phospholipid molecule is oriented in such a way that its polar head group is exposed on the external surface of the membrane, and the fatty acyl chain is oriented to the inside of the membrane. This forms a sheet-like phospholipid bilayer, which is two molecules thick (Fig. 7.3 ). The fatty acyl chains of the phospholipids in each layer, or leaflet, form a hydrophobic core that is 2-3 nm thick in most membranes. The diffusion barrier mentioned above is because of this hydrophobic core, which is highly impermeable to polar molecules and ions.
Understanding of the membrane structure has evolved gradually over a period of several years and still new information is being added. As a result, the proposed model for membrane structure continues to undergo modifications and refinements. It was in 1925 that Gorter and Grendel first proposed the lipid bilayer structure for the biological membranes. In 1935, Davson and Danielle suggested another model in which phospholipids were the major constituents that formed the matrix or continuous part of the membrane. Few globular proteins were also present, but only towards the polar exterior. A major question with these earlier models was as to how the membrane proteins interacted with the lipid bilayer. No satisfactory explanation was in sight, which casted doubt on general validity of these models.
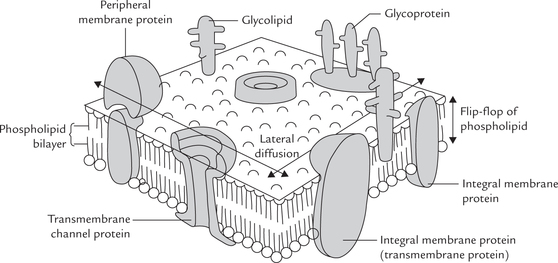
In 1972, Jonathan Singer and Garth Nicolson proposed the fluid mosaic model (Fig. 7.4 ), in which some proteins called intrinsic proteins are actually immersed in the lipid bilayer, while others are loosely attached to the surface of the membrane, i.e. extrinsic proteins. The important features of the fluid-mosaic model, the most accepted model for the overall structure of biological membranes, and supported by a wide variety of experimental observations, are excerpted as below:
• The bilayer organization of lipids in membranes can be viewed as two dimensional solutions of oriented lipids; and there are proteins embedded in it. The proteins may be intrinsic (tightly associated with hydrophobic core) or extrinsic (loosely bound to membrane), as discussed.
• The integral membrane proteins can be considered as ’icebergs’ floating in two dimensional lipid ’sea’ The bilayer organization of the lipids acts both as solvent for the amphipathic integral membrane proteins and as a permeability barrier.
• Membrane proteins are free to diffuse laterally in the plane of the bilayer unless restricted in some way, but would not be able to flip from one side of the bilayer to the other.
• Some lipids may interact with certain membrane proteins. These interactions are essential for the normal functioning of the protein.
C Membrane Components
Membrane Lipids
Phospholipids, sphingolipids, and cholesterol constitute the major membrane lipids.
Phospholipids
These are the most predominant molecular components of all membranes. The principal membrane phospholipid is phosphatidylcholine (lecithin), which accounts for 40-50% of the total phospholipid content. Other phospholipids, present in relatively small quantities, are phosphatidylethanolamine (cephalin), phos-phatidylserine, cardiolipins and inositol phosphoglycerides.
Sphingolipids
These derivatives of sphingosine, an amino alcohol, with a long hydrocarbon chain, are the second important class of lipids found in biomembranes. The sphingomyelins, which contain a phosphocholine head group, are major components. Other sphingolipids are glycolipids in which a single sugar residue or branched oligosaccharide is attached to the sphingosine backbone (Chapter 3). Glycolipids constitute 2-10% of the total lipids in plasma membranes; they are most abundant in nervous tissue.
Cholesferol
The third important class of membrane lipids is cholesterol and its derivatives. Though cholesterol is especially abundant in the plasma membrane of mammalian cells, it is absent from most prokaryotic cells. It appears almost entirely hydrophobic in composition, but it is actually amphipathic because its hydroxyl group can interact with water. It is oriented in such a way in the membrane that its hydrophilic hydroxyl group faces the exterior and the cyclopentanophenanthrene ring fits into the hydrophobic lipid phase of the membrane.
The proportion of lipid component of different membranes vary. Neutral lipids and sphingolipids occur in high concentration in plasma membranes. The inner mitochondrial membrane (IMM) is especially rich in cardiolipins and phosphatidylethanolamine High concentration of sphingolipids is present in myelin sheaths of axons of neural tissue, whereas the intracellular membranes primarily contain phospholipids with relatively smaller amount of sphingolipids or cholesterol. The cholesterol content of a given membrane may vary with the nutritional status of an individual.
Membrane Proteins
The membrane proteins are involved in a number of specific functions. While the continuity of biological membranes, and their ability to act as permeability barrier for water-soluble molecules are due to the properties of the lipid bilayer, other membrane functions can be explained by properties of the membrane proteins. The most important of these are:
1. Enzymatic activities: A number of biochemical reactions are catalyzed by the membrane proteins which possess enzymatic activity; e.g. Na+–K+ ATPase that maintains a steep gradient of Na+ and K+ across the plasma membrane.
2. Carrier activities: Several water-soluble intermediates, nutrients and waste products must be carried across the lipid bilayer. Simple diffusion of these substances is not possible due to selectively permeable nature of the membrane. Their movement across the membranous barrier is made possibly by certain transport proteins (i.e. translocases).
3. Signal transduction: The message inherent in a hormone or neurotransmitter or other such extracellular substances must cross the membranous barrier so that it may influence intracellular events. This is made possible by signal transduction by specific proteins (Chapter 29).
4. Interactions with the extracellular matrix and the cytoskel-eton: These interactions are essential for the integrity of the cell and of the tissue as a whole. Proteins such as fibronectin, ankyrin and spectrin participate in these interactions.
5. Some membrane proteins regulate permeability for inorganic ions: This forms the basis for the excitability properties of some biological membranes.
Peripheral and Integral Proteins
As mentioned earlier, the membrane proteins are divided in two classes: peripheral (or extrinsic) and integral (or intrinsic). The classification is based on the location and the ease with which they can be removed from the membrane.
The peripheral proteins can be released from the membrane by relatively mild treatment such as by salt solutions of different ionic strengths, or through alteration of the surrounding pH. These proteins usually possess enzymatic activity. They may be bound to either face of the membrane by electrostatic interactions and hydrogen bonds (Fig. 7.4). Removal of peripheral proteins causes little or no change in the integrity of the membrane.
The integral proteins are embedded deeply in the bilayer, and are attached by hydrophobic bonds or van der Waals forces. Mostly they span the whole bilayer and when they do so they are called transmembrane proteins (Fig. 7.4). They serve as important transport proteins.
It requires use of detergents or organic solvents to separate the integral proteins from the membrane. The integral proteins are usually tightly bound with lipids. Removal of the lipid component during the separation causes denaturation of the protein, and consequent loss of its activity.
Other Membrane Components
Carbohydrates are relatively minor components, forming 5-8% of the total membrane mass. Most carbohydrates occur as oligosaccharide units that form part of membrane glycolipids and glycoproteins. Glucose, galactose, mannose, fucose, N-acetylglucosamine, and sialic acid are some of the major carbohydrates present in these hybrid molecules. It is noteworthy that carbohydrates never exist in free form in the membranes.
The carbohydrates are generally located towards the exterior of plasma membrane. They play an important role in cell-cell recognition, adhesion and receptor action. However, they may be located in the inner leaflet of the membrane, e.g. in endoplasmic reticulum where they face the membrane enclosed channel.
D Membrane Asymmetry
Membranes have asymmetric structure. Proteins are mainly responsible for it because they are inserted in an asymmetric fashion (Fig. 7.4). Further contributors to asymmetry are the oligosaccharide units that always project towards the exterior. Lipid components are also distributed in an asymmetric fashion, for example, in the erythrocyte membrane, the outer leaflet of the bilayer contains mostly phosphatidylcholine and sphingolipids, whereas the inner one contains phosphatidylethanol-amine and phosphatidylserine.
E Membrane Fluidity
The fact that some membrane components can move in the lipid bilayer indicates fluid nature of biological membranes. A number of factors influence the fluidity of membranes, which in turn influences its physiological function. Temperature and composition of the membrane are the two major factors that influence the membrane fluidity. At a relatively low temperature the fluidity is less, and therefore, limited mobility of membrane components is possible. As the temperature is raised, a phase transition occurs that results in tremendous increase in fluidity. The phase transition temperature (melting temperature; Tm) provides a measure of the temperature at which the transition occurs. The Tm value itself depends on the membrane composition.
The nature and relative abundance of membrane constituents have significant effect on the membrane fluidity. For example:
• The short chain fatty acids increase the fluidity while the long chain fatty acids tend to decrease it.
• Unsaturated fatty acids with their cis-double bonds do not pack closely; this results in more fluid structures in which the Tm is lowered. More the number of double bonds, greater is the fluidity of the membrane (and hence lower is the value of Tm).
Cholesterol content of the membrane also alters the fluidity of the membrane. With its rigid ring system, it decreases the fluidity of most membranes. It can, however, also insert itself between the fatty acid chains and prevent their crystallization. In this respect, cholesterol acts like an impurity that decreases the melting point of a chemical.
F Specialized Membrane Structures
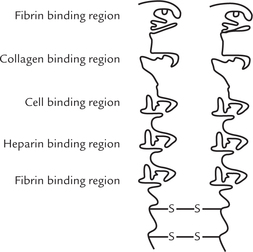
Fibronectin (fibro = fibre, nectin = connect)
This is a protein (600 Å long) that permits binding of the cell with components of the extracellular matrix. It is made up of two polypeptide chains (250 kD each), which are linked by disulphide bridges at their carboxyl terminals. It contains distinct domains, each one of which is capable of binding specific molecules, as shown in Figure 7.5 . Malignant cells are deficient in fibronectin which is responsible, at least partly, for their invasive character.
Several important functions are performed by fibronec-tin. Fibroblasts and other such cells, involved in repair mechanisms, adhere to a clot by attaching themselves through fibronectin.
Tight junctions
These are present between the two cells that lie in close approximation. They form narrow hydro-philic channel through which calcium and other small molecules pass from one cell to another. As shown in Figure 7.6, only three (and not four) layers of plasma membrane are present at the tight junctions.
G Red Blood Cell (Erythrocyte) Membrane
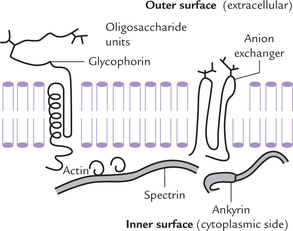
The erythrocyte membrane is ideal for studying membrane structure because it is easily isolated without any contamination with other subcellular membranes. Study of this membrane has given insight into the role of various membrane components, particularly the membrane proteins. Three major proteins—glycophorin, anion-exchanger and ankyrin are present, in addition to several other minor proteins.
Glycophorin
It is an integral glycoprotein (MW 30 kD), with several oligosaccharide units attached to the amino-terminal, which faces extracellularly (Fig. 7.7 ). These oli-gosaccharides determine the antigenic specificity of the membrane. Function of glycophorin is still not fully understood because the individuals lacking it to do not suffer from any specific abnormality.
Anion exchanger
It is an integral glycoprotein, accounting for one-third of the total membrane proteins (MW 106 kD). It facilitates extrusion of bicarbonate ions from erythrocyte in exchange for chloride ions. This transport mechanism plays an important role in acid-base homeostasis.
Ankyrin
It is a peripheral protein located towards cytoplasmic side of the erythrocyte (Fig. 7.7). It is cross linked to another peripheral protein called spectrin, which is filamentous in nature and about 1000 Å long. These two interconnected proteins, together with actin, form a mesh-work that underlies the erythrocyte membrane. This mesh-work is responsible for the resilience and stability of the erythrocyte membrane.
H Micelles and Liposomes
When a suspension of pure phospholipids or a mixture of phospholipids is mechanically dispersed in aqueous solution, the phospholipids aggregate into one of the two spherical forms: micelles or liposomes. In both the structures, the hydrophobic effect causes the fatty acyl chains to aggregate and exclude water molecules from the core. The type of structure formed depends on several factors, including the length of the fatty acyl chains, their degree of saturation, and temperature.
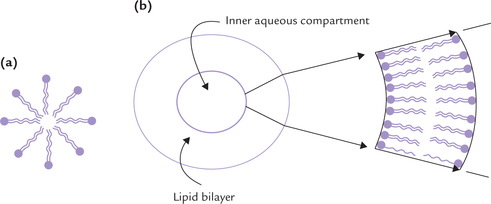
Micelles
These are small globular structures with hydrophilic surface and hydrophobic core (Fig. 7.8a ). They can be formed by all polar lipids, including ordinary detergents. However, micelles are rarely formed from the natural phosphoglycerides, whose fatty acyl chains are too bulky to fit into the interior of a micelle.
Liposomes
A lipid bilayer may close in on itself, forming a spherical vesicle separating the external environment from an internal compartment. Such vesicles are termed liposomes (Fig. 7.8b). They have diameter in the range of 501000 nm. Wall of a liposome consists of two layers of lip-ids and encloses an inner aqueous compartment in which small ions or molecules can be trapped. Liposomes can be artificially prepared by agitating a polar lipid such as lecithin suspended in water, with high frequency sound waves.
Biomedical importance of liposomes
1. Liposomes are used to study membrane properties and their role as permeability barrier. They are also used to introduce a number of impermeant substances into the cells.
2. Liposomes have important therapeutic applications because they can carry various drugs and enzymes.
Liposomes are prepared with specific drugs or enzymes encapsulated inside. They carry these substances to their target tissues. The biodegradable nature of the liposomes permits release of the encapsulated substance within the vicinity of the target tissue. Many antibiotics, antiviral, antifungal and anti-inflammatory agents have been found to be highly effective when delivered in this fashion. It may be possible to prepare liposomes with a high degree of tissue specificity.
III Transport Across Cell Membrane
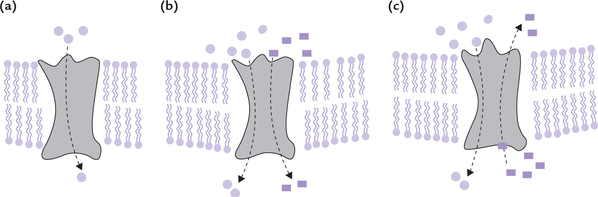
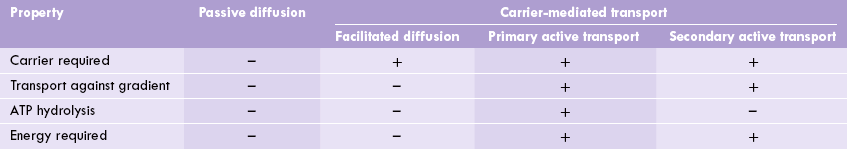
Biological membranes mediate interaction of the cell with environment. Several useful substances present extracel-lularly enter the cells through the membranous barrier. Likewise, the intracellular waste products leave the cell by moving across the membrane. During the transport across the membrane, these substances encounter varying degrees of resistance—some can freely diffuse through the membrane (passive diffusion), whereas movement of others is restricted and requires a carrier, usually integral membrane proteins (carrier-mediated transport).
Some types of carrier-mediated transport processes occur along the concentration gradient (facilitated transport); others are driven by hydrolysis of ATP (active transport) against a concentration gradient.
A Passive Diffusion
It is a spontaneous, unaided movement of the solute particles along their concentration gradient. The particles move from the higher concentration to the lower concentration, till they equilibrate. At equilibrium point, the entropy reaches a maximum possible level under the prevailing circumstances, and therefore, no further movement of the solute particle occurs.
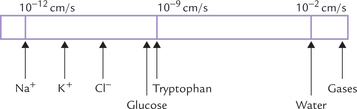
Permeability coefficient is an expression that indicates relative ease of diffusion of a given substance through the lipid bilayer. Figure 7.9 represents the permeability coefficient values of some substances in a descending order. The membrane is: (i) highly permeable to gases (e.g. CO2, NO, and O2), small hydrophobic molecules and small uncharged polar molecules (e.g. steroids, ethanol, and urea); (ii) moderately permeable to water (permeability coefficient 10-2 cm/sec); and (iii) essentially impermeable to ions and to large polar molecules (sugars, amino acids, etc.), which have extremely low permeability coefficients, and require presence of specific transport mechanisms to move across the membrane.
B Carrier-mediated Transport
It occurs through mediation of integral membrane proteins, which have been variously known as permeases, porters or translocases. These proteins are highly specific. For example, the erythrocyte glucose transporter has high affinity for D-glucose, but low affinity for the related sugars, D-mannose and D-galactose (10-20 times lower).
The carrier-mediated transport may be facilitated or active; both are specific for the solute transport and both can be inhibited by structural analogues. For example, 1,5-anhydroglucocitol can inhibit transport of glucose across the membrane. When a solute molecule is transported singly, the system is known as uniport. In some cases, transport of a molecule requires an obligatory simultaneous cotransport of another molecule, either in the same direction or in the opposite direction. When both substances move in the same direction, the process is known as symport. Conversely, when they move in opposite directions, process is called the antiport (Fig. 7.10 ).
Facilitated Diffusion
In facilitated diffusion movement of particles occurs along their concentration gradient. It requires mediation of specific integral membrane proteins for "facilitating" the movement of the solutes (glucose, other sugars, amino acids). The transport proteins serve to hasten the process so that equilibrium is attained far more rapidly than it occurs in passive diffusion.
Mechanism of action of these proteins is not clearly understood. One possible explanation is that a transport protein oscillates between two conformations. In one conformation, the solute-binding site is exposed towards the extracellular space (Fig. 7.11a ). When the solute binds to this site, a conformation change is induced in the transport-protein, which permits the solute to be transferred to the cytosolic side of the membrane (Fig. 7.11b and c). Release of the solute on this side occurs next because its affinity for the transport protein in the changed conformation is low (Fig. 7.11d). The release triggers the return of the protein to its original conformation. This whole process is reversible, meaning that the solute can be extruded from the cell by reversal of the above events. The direction of movement depends on the relative concentration of solute on two sides of the membrane.
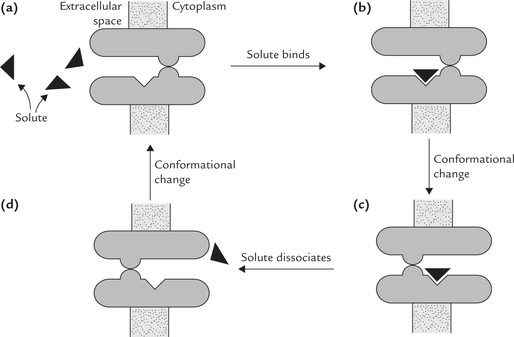
Fig. 7.11 Schematic representation of faciliated transport. There is no external energy source, so the net transport is down the concentration gradient.
Kinetics of facilitated transport obeys the Michaelis-Menten rate law. A plot of the initial rate of transport (V0) versus the concentration gradient, is hyperbolic (Fig. 7.12 ). As the concentration gradient increases, the rate of transport also rises. Rate of transport depends on the per cent saturation of the carrier protein. It rises to maximum (Vmax) when all the carrier protein molecules are fully saturated with the solute. Thus, systems subjected to facilitated diffusion are saturable and the Km value for the transport protein corresponds to the concentration of solute at which the carrier protein is half-saturated.
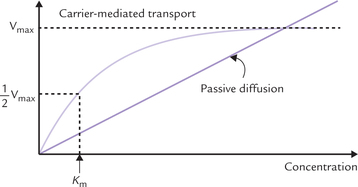
Fig. 7.12 Kinetics of facilitated diffusion by a transport protein. The protein is saturable and displays Michaelis-Menten-type binding kinetics in a similar manner to enzymes. On the other hand, in passive diffusion. The rate of transport is directly proportional to concentration gradient.
Certain well characterized transport systems are discussed below.
Glucose Transporters
Glucose is transported across the cell membrane by facilitated diffusion system. The glucose transport family comprises five members, named GLUT-1 to GLUT-5. They shuttle between two conformational states, one in which the substrate-binding site faces outward and the other in which the binding site faces inward. All transmembrane proteins are similar in size, having about 500 amino acid residues and 12 transmembrane helices. Their primary structures are remarkably similar, and they display a tissue-specific pattern of expression; for example, GLUT-1 is abundant in erythrocytes and GLUT-4 in muscles.
• GLUT-1 molecule in erythrocytes has a Km of 15-20 mmol/L; it is mostly active under fasted state.
• GLUT-2 in pancreatic cells has higher Km(10 mmol/ L), which, in response to raised blood glucose concentration, mediates an increase in the cellular uptake of glucose, leading to insulin secretion.
• GLUT-4, present in insulin sensitive tissues like muscles and adipose tissue, is translocated from intracel-lular vesicles to the plasma membrane by insulin. This facilitates glucose uptake during meals.
• GLUT-3 and -5 are found in brain and testis; and in intestinal epithelial cells, respectively. Their properties have not yet been elucidated in detail.
Chloride Transporters
1. 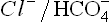 antiport: This transport system is located on the erythrocyte membrane. It moves the bicarbonate ions, generated intracellularly by action of carbonic anhydrase, out of the erythrocytes with a concomitant movement of chloride ions into the cell. In this way, electroneutrality is maintained (Chapter 1).
antiport: This transport system is located on the erythrocyte membrane. It moves the bicarbonate ions, generated intracellularly by action of carbonic anhydrase, out of the erythrocytes with a concomitant movement of chloride ions into the cell. In this way, electroneutrality is maintained (Chapter 1).
2. Cystic fibrosis transmembrane conductance regulator (CFTR): This carrier protein is located on cells of exocrine glands. It forms a cAMP regulated Cl- channel, through which the movement of chloride ions across the epithelial cells occurs. Defective action or faulty localization of CFTR in cell membrane leads to diseases (Case 7.1).
Others
Specific transport proteins for a number of other substances, including amino acids, have been identified. These transporters facilitate movement of various amino acids across renal tubular and intestinal mucosal cell membranes. Defective transport may lead to a number of clinical disorder, as exemplified in Case 13.4.
Active Transport
In active transport, the solute molecules are moved against a concentration gradient through expenditure of energy. Therefore, active transport is coupled to the energy state of the cell. The active transport is classified as primary and secondary.
The primary active transport systems use energy obtained by hydrolysis of ATP to drive transport of the molecules (Na+ K+ Ca+ or H+) across the membrane. Some examples are: the Na+–K+ ATPase, the proton-pump in stomach, etc.
In secondary active transport systems the movement of the substrate molecule across the membrane is coupled to the movement of an ion (Na+, H+) down its concentration gradient. Thus, a pre-existing ionic gradient of Na+ or H+ is used to drive the "uphill" movement of a substrate. Example: Coupled movement of glucose and Na+ across the intestinal cells.
Primary Active Transport
In this section, three transport systems involved in the primary active transport are discussed. These are: Na+–K+ dependent ATPase for antiport of sodium and potassium ions, Ca2+-dependent ATPase for moving calcium ions, and proton pump in the stomach that antiports protons and potassium.
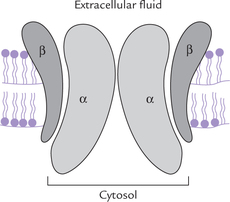
Na+–K+ dependent ATPase
The Na+–K+ dependent ATPase was first detected in the crab nerves by Jens Skou in 1957. It was named so because it requires both Na+ and K+ (apart from Mg2+), and uses ATP energy for its action. The Na+–K–dependent ATPase is an antiporter which actively pumps K+ into the cell from extracellular fluid (ECF), with a concomitant extrusion of Na+ from the cell. It is located in the plasma membrane of all cells, with highest activity in nervous tissue and muscle. It maintains the steep gradients of sodium and potassium across the plasma membrane, as shown in Figure 7.13 . It is a major consumer of metabolic energy, hydrolyzing about 100 molecules of ATP per second under optimal conditions. About a third of total energy requirement of the cell is used to drive the Na+–K+ ATPase; in electrically excitable cells, it accounts for about 70% of the cell’s energy. Thus, a significant proportion of the basal metabolic rate (20-25%) is allotted to sodium-potassium pumping.
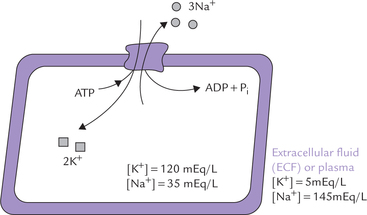
Fig. 7.13 A tentative model of Na+–K+ ATPase, also showing concentration gradients of sodium and potassium across the biomembrane.
Subunit structure: The Na+–K+ ATPase is an oligomeric integral membrane protein composed of two types of subunits; the stoichiometry is α2 β2 (Fig. 7.14 ).
The α-subunit (MW = 112,000) brings about hydrolysis of ATP. In addition, each α-subunit has binding sites for Na+ on the cytosolic side and for K+ towards the extracellular side.
The β-subunit is smaller (MW = 55,000). It is a gly-coprotein, whose function is unknown.
Sequence of ionic pumping activities: Three sodium ions are ejected for every two potassium ions pumped into the cell, and one ATP molecule is hydrolyzed in the process. The sequence of ionic pumping activities (that proceed in four steps) is represented in Figure 7.15 .
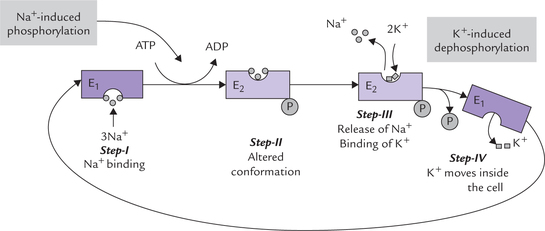
Fig. 7.15 Sequence of ionic pumping activities of the Na+—K+ ATPase. E1 and E2 refer to protein conformations in dephos-phorylated and phosphorylated forms, respectively. E1 is inside open conformation with high affinity for Na+; and E2 is outside open conformation with high affinity for K+.
Step I: The sodium ions bind with α-subunits towards cytosolic side of the latter. Binding of sodium induces phosphorylation of this subunit (termed Na+-induced phosphorylation); the phosphate group is obtained from ATP This group gets attached to a specific aspartyl residue of the α-subunit.
Step II: The phosphorylation in turn induces a change in the conformation of the pump protein (from E1 to E2 form), whereby its orientation changes in such a way that its ion-binding site is everted (Fig. 7.15). The ion-binding site now faces the exterior, and consequently the sodium ions are moved out into the extracellular fluid (ECF).
Step III: The phosphorylated protein has low affinity for sodium, so that these ions are released into ECF. Binding affinity of the phosphorylated protein is high for K+ and so it binds these ions. Two K+ ions attach with specific binding sites present on the extracellular side of the α-subunit.
Step IV: The K+ ions trigger release of the phosphate ions (termed K+-induced dephosphorylation). The dephos-phorylation favours the E1 conformation so that the pump protein reverts to the original E1 conformation. With change of conformation (E2→E1), the ion-binding site is inverted so that potassium ions move into the cytoplasm. Intracellularly they are released on account of low affinity of the E1 conformation for K+.
(a) Ouabain, a steroid extracted from plants, inhibits activity of Na+–K+ ATPase. It binds to the extracellular face of the -subunit and prevents the K+-induced dephosphorylation process. This disrupts the functions of the Na+–K+ ATPase resulting in inhibition of the ion translocation.
(b) Digitalis is another steroid of plant origin (obtained from Digitalis purpurea), which is therapeutically used for its ability to increase contractile activity of the cardiac muscle (i.e. cardiotonic effect). This action is due to the inhibitory effect of digitals on the Na+–K+ ATPase. Inhibition of the pump activity results in a raised intracellular concentration of Na+ due to decreased extrusion of this ion. Consequently, the Na+ gradient across the cell membrane falls. The diminished gradient in turn causes building up of the intracellular calcium concentration, which enhances the force of contraction.
How does the diminished sodium gradient lead to rise in cytosolic calcium concentration? The major mechanism for removal of calcium out of the myocardial cell is by a Ca2+/Na+ antiporter in the plasma membrane. This anti-porter depends on sodium gradient for effective calcium transport. With the rise in the cytosolic sodium concentration, the sodium gradient diminishes, and more calcium remains in the cytoplasm.
Digitalis is very toxic, and overdosage results in fatal arrhythmias because a normal sodium gradient is essential for the normal excitability of the myocardial cells.
Ca2+—dependent ATPase
The Ca2–dependent ATPase is present in most membranes but is most abundant in the sarcoplasmic reticulum of skeletal muscles, where it accounts for about 80% of the integral membrane proteins. It actively moves calcium from the cytosol into the sarcoplasmic reticulum against the concentration gradient. The process requires expenditure of ATP energy.
Sarcoplasmic reticulum is a repository of calcium ions in the resting muscle. A nerve impulse causes release of these ions into the cytosol. This results in about 100-fold increase in the cytosolic calcium concentration. The cytosolic calcium in turn induces muscle contraction. Subsequent muscle relaxation depends on removal of calcium from the cytosol. It is at this stage that the Ca2+-dependent ATPase comes into play. It removes calcium from the cytosol by pumping it into the sarcoplasmic reticulum.
The Ca2–dependent ATPase resembles the Na+–K–dependent ATPase in three aspects.
(i) Both can exist in two conformational states, which depends on the state of phosphorylation. Conformation of the phosphorylated form is different from that of the dephosphorylated form.
(ii) Both contain a specific aspartyl group to which attachment of the phosphoryl group occurs.
(iii) The phosphorylation-dephosphorylation cycle is responsible for the oscillation of these transport proteins between two conformational states.
Proton pump in the stomach
Is expressed in gastric parietal cells in response to stimuli such as histamine and gastrin. The pump antiports two cytoplasmic protons and two extracellular potassium ions, coupled with hydrolysis of a molecule of ATP. The protons are moved against a steep concentration gradient (cytoplasmic concentration, 10-6 M vs luminal concentration, 0.15 M). With the movement of H+, Cl- is secreted through a chloride channel, producing hydrochloric acid (HCl) in the lumen.
Secondary Active Transport
It is an ion-driven cotransport mechanism, in which movement of a substrate against its concentration gradient is coupled to movement of another ion (Na+ or H+) along its concentration gradient. An example is glucose-Na+ symport. As shown in Figure 7.16, Na+ is moving along its concentration gradient, and glucose against the gradient. Though not coupled to ATP hydrolysis, this method of transport is classified as active transport because there is energy expenditure: the energy is derived from electrochemical gradient of Na+ (Table 7.4 ). It is primarily used for absorption of glucose as also amino acids in the intestinal mucosa. It is also employed in the proximal renal tubules for the reabsorption of glucose and amino acids from the primary filtrate. Indeed, kidney and intestine often use the same sodium cotransporter, and therefore, many transport defects are expressed in both the organs.
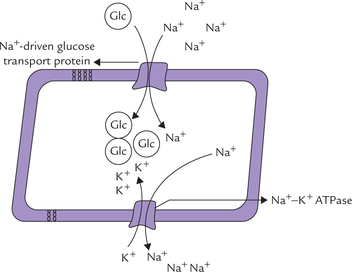
Fig. 7.16 Secondary active transport: coupled movements of Na+ and glucose. The Na+ is subsequently moved out of the all by action of a Na+/K+ ATPase on the basolateral membrane.
Knowledge of the coupled movement of Na+ and glucose forms the basis of glucose rehydration therapy in cholera. Oral administration of glucose prevents loss of Na+ in the diarrhoea fluid to a considerable extent. Glucose transport is powered by Na+ concentration gradient in renal tubules and choroid plexus as well.
Models for Action of Transporters
The transport proteins are a special class of integral membrane proteins that bind ligands on one side of the membrane with high affinity and specificity, and release them on the other side. Details about the transport processes at the molecular level are not clear. Following are the conceptual models that have been proposed to explain some of these processes.
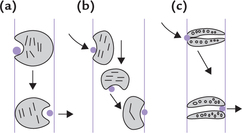
Mobile carrier system
Binding of the ligand physically alters the orientation of the transport protein, possibly by rotation through plane of the membrane. As a result, the ligand moves to the other side of the membrane (Fig. 7.17a ). This is also referred to as the ping-pong model.
Shuttle system
The transport protein, after binding with the ligand, shuttles back and forth across the lipid bilayer (Fig. 7.17b).
Transmembrane pore system
Some transport proteins (referred to as the pore ionophores) contain a pore through which specific substances are transported (Fig. 7.17c). The system is firmly embedded in the membrane because the hydrophobic side chains of the constituent amino acids intercalate with the membrane matrix. Only certain selected substances can move through the pore. This model is also visualized as being guarded because it opens only when such substances arrive.
Evidence for existence of these models comes from studies with the following antibiotics: valinomycin, a diffusion (shuttle) type ion transporter, and gramicidin, a pore type ion transporter. These antibiotics are also known as the ionophores since they permit greater mobility of ions across the membranous permeability barrier. They are important experimental tools for studying the transport phenomena, in addition to being important therapeutic agents.
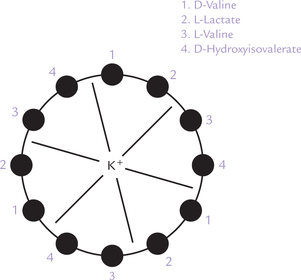
Valinomycin is, cyclic trimer, comprising a three tetra-peptide units (Fig. 7.18 ). It is obtained from Streptomyces. It permits transport of K+ across cell membranes. It has a polar interior that interacts with K+, and a non-polar exterior which enables it to interact with the membrane. K+ is held in the centre of the cyclic structure through electrostatic interactions with carbonyl oxygen of the peptide bonds. The non-polar exterior is due to the hydrophobic side chains of the non-polar amino acids that constitute this antibiotic. These side chains easily intercalate with the hydrophobic membrane matrix, which enables the valinomycin molecule to shuttle back and forth across the membrane. During the process, the K+, held in the centre of the antibiotic, is carried across the permeability barrier.
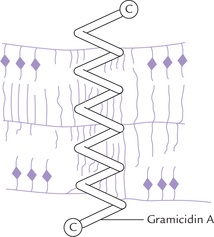
Gramicidin A is an antibiotic isolated from Bacillus brevis. It forms a tail-to-tail dimer, which makes a pore across the membrane (Fig. 7.19 ). The two helical monomers span the membrane. Length of the helix is about 3 nm, which is consistent with the hydrophobic region of a typical phospholipid bilayer. The helical pore, having a diameter of O.4 nm, can transport several cations, such as H+, Na+, K+, etc. Ion transport by gramicidin is 1OOO times faster than that by valinomycin.
Exocytosis and Endocytosis
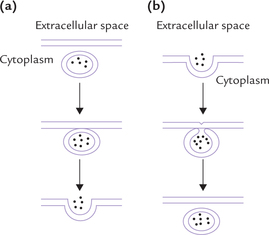
Exocytosis
Exocytosis refers to the process by which outward movement of certain intracellular substances occurs (Fig. 7.2Oa ). Intracellular enzymes, hormones, lipoproteins and a variety of other endogenously synthesized molecules move out of the cell through exocytosis. A classical example is extrusion of the lipoprotein, VLDL, from hepatocytes. The nascent VLDL particles, synthesized in the rough endoplasmic reticu-lum, undergo glycosylation in the Golgi apparatus. The glycosylated particles are then packaged into membrane-bound vesicles. The latter move towards the cell membrane, the vesicular contents (i.e. the VLDL particles) are released extracellularly.
Endocytosis
Endocytosis refers to the process of cellular uptake of certain extracellular substances (Fig. 7.20b). Uptake of variety of extracellular macromolecules, that are important for the cell, occurs through endocytosis. The process begins with invagination of a segment of the plasma membrane; the invaginated portion encloses the extracellular macro-molecule to be internalized. The invaginated segment then closes upon itself to form a vesicle. The latter pinches off next, and enters the cell. In many instances, the internalized vesicle fuses with lysosome and its contents are digested by the lysosomal hydrolases.
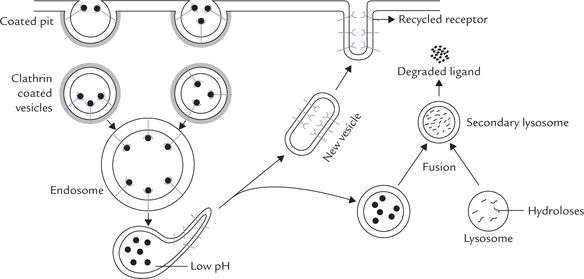
Receptor-mediated endocytosis: In case of certain extracellular macromolecules, the internalization is preceded by interaction with certain cell surface receptors. The process is called receptor-mediated endocytosis and is responsible for uptake of a variety of essential metabolites, including low density lipoprotein (LDL), vitamin B12 bound to the carrier protein (i.e. transcobalamin II), and iron bound to transferrin. Several growth factors (e.g. nerve growth factor), hormones (e.g. insulin), toxins and some viruses also enter the cell through this process.
Action of LDL receptors is presented in Figure 7.21 . The receptors are transmembrane glycoproteins. They are located in some specific indentations of the cell membrane called the coated pits. Cytoplasmic aspect of these pits have high concentration of a protein, called clathrin which forms a lattice work. Interaction of ligands with the receptors is followed by invagination of coated pits and subsequent sealing off to form coated vesicles. Clathrin helps in the process of sealing off. In cytosol, the clathrin is shed and several coated vesicles fuse together to form large structures called endosomes.
Certain important changes take place in the endo-some which permit recycling of the receptor. A proton pump builds up a high proton concentration within the endosome which drops the pH to about 5. At this pH, dissociation of the ligand and the receptor occurs. The separated receptor is then enclosed in a new vesicle and recycled back to the plasma membrane. The remainder of the endosome fuses with lysosome and its contents are degraded by the lysosomal enzymes.
Transport Through Gap Junctions
Cell-to-cell transport is made possible through passageways connecting the interiors of the contagious cell (Fig. 7.22 ). These passageways span the intervening space, or gap between the apposed cells and hence, are termed the gap junctions, or cell-to-cell channels. All polar molecules with molecular weight of 1 kD or less can be transported through gap junctions. They move along a concentration gradient by passive diffusion.
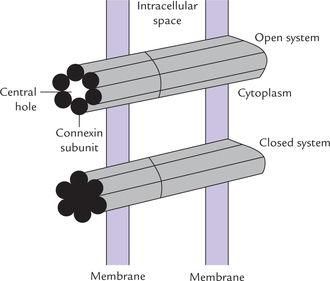
Structure
A transmembrane protein, known as con-nexin (MW 32 kD) is the major structural element of gap junction. Total of 12 connexin subunits form a single gap junction. Six of these subunits are arranged in a hexagonal array in such a manner that they enclose a central hole about 20 A wide (Fig. 7.22).
These six subunits form a half-channel called con-nexon (i.e. hemichannel). Two such hemichannels join ends in the intercellular space to form a gap junction, which encloses a functional channel between the communicating cells. These cell-to-cell channels differ from other membrane channels in two important aspects.
• They traverse two membranes rather than one. Therefore, they form connection from cytosol to cytosol; rather than cytosol to extracellular space, or cytosol to lumen of an organelle.
• They have a functional nature; a high concentration of calcium or low pH can close a channel. The sub-units of connexin slide and rotate in such a way that closure of the central hole occurs. Switching between the open and closed state is smooth because free energies of these two states do not differ markedly. As a result, passage of molecules occurs rapidly as per the cellular requirements.
Functions
Primary function of gap junctions is to serve as passage-ways for inorganic ions and a variety of metabolites, such as sugars, amino acids and nucleotides. In contrast, proteins, nucleic acids and polysaccharides are too large to traverse these channels. Evidently, gap junctions are of prime importance for intercellular communication.
Other functions of gap junctions are:
1. They are essential for nourishment of the cells that lie distant from blood vessels, such as the cells of lens and bone.
2. They ensure synchronous and rapid response of cells of excitable tissues, such as the heart muscle. This is because, in response to a stimulus, the ions rapidly flow from one cell to another, thus ensuring a coupled and quick response.
3. Current evidence suggests that the communicating channels are important for development and tissue differentiation.
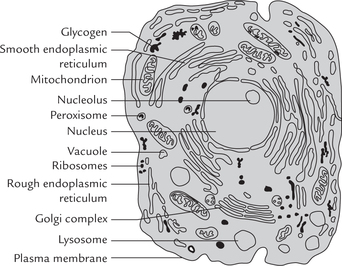
 Cells in eukaryotes have a membrane bound nucleus, and a number of other membrane-bound subcellular organelles, each of which carries out a specific function.
Cells in eukaryotes have a membrane bound nucleus, and a number of other membrane-bound subcellular organelles, each of which carries out a specific function.