Immunology
Every living organism is confronted by continual assaults from its environment and, therefore, must develop some defense system to survive. The defense ranges from physical barriers, such as skin, to highly sophisticated specific immune system. The latter is a remarkably adaptive system in vertebrates, which protects against invasions by an apparently limitless variety of environmental agents that are foreign to the body (antigens) and that may disturb the body-homeostasis. These agents may be micro-organisms or their products, foods, chemicals, drugs, animal hair, etc. Immune system has a highly complex organization, comprising of cells, tissues, and molecules, which are involved in specific-recognition and interaction with the antigen followed by its inactivation/elimination from body. The immune system offers protection against apparently limitless types of antigens, especially proteins and polysaccharides, but not directly against small molecules (Haptens), although such molecules may elicit an immune response if they are bound to a protein of the body.
Two essential functional elements of the immune system are recognition and response. The system effectively recognizes subtle chemical changes that discriminate foreign cells (nonself) from body’s own cells and proteins (self) and mounts an effective response against the nonself by enlisting participation of a large number of cells, soluble factors and other molecules. The response, known as the effector function of immune system, eliminates the activating antigen with incredible precision by either cellular or humoral mechanisms. In the cellular mechanism, lymphocytes (called T cells) sensitized to specific antigen, target the antigen directly in a process called cell-mediated response. In addition to causing cytotoxic destruction of virus infected cells and bacteria in this manner, the T cells also cause activation of other cells of the immune system. In the humoral mechanism, a different type of lymphocytes (called B cells) manufacture specialized proteins (i.e. immunoglobulins or antibodies), which protect against organisms by a variety of mechanisms. These include neutralization of toxins, lysis of bacteria in the presence of complement proteins, opsonization of bacteria to facilitate phagocytosis, and preventing adherence of bacteria and viruses to cell surfaces.
In the present chapter an account is given of molecular and cellular biology of the immune system and the current concepts about the mechanisms by which it protects the body against the invading pathogens. Details have been simplified to reveal the essential structure and physiology of the immune system, in order that the clinical application of immunology may be appreciated.
By the end of this chapter the student should learn.
Organization of immune system: the organs, cells and molecules involved in generating innate and specific immune responses.
Antigens: Epitopes, haptens; antigen processing and presentation.
Immune responses: humoral and cell mediated, role of B cells, accessory cells, and various populations of T cells in the generated immune responses.
Antibodies, complement, hybridoma and monoclonal antibodies.
Vaccines, immunological techniques, immunodysregulation disorders.
I Historical Milestones
Immunity is the body’s ability to resist certain harmful substances; the Latin term immunis meaning “exempt” being the source of the English word immunity. The discipline of immunology, the science of man’s resistance to disease, grew out of the observation that a person could catch certain infectious diseases only once in lifetime. It had been a common knowledge that the individuals who had recovered from certain infectious diseases were thereafter protected from the same disease. The concept of immunity was expressed in folklores dating back to 430 B.C., as evidenced by Thucydides’ account of the Peloponnesian War. In this he noted that those who got infected with plague and survived, could safely nurse the sick without the risk of contracting the disease again. Even if they caught the disease a second time, the second attack was never fatal. In ancient times, Indians and Chinese were known to obtain protection against smallpox by inoculating live organisms from the diseased pustules.
However, such observations and concepts were translated successfully into a medically effective practice only in recent times. In 1718, Lady Wortley Montagu, a renowned English author and wife of the British Ambassador to Turkey, reported that the Turks induced immunity against smallpox by administering the crusts derived from smallpox pustules through small cuts in skin or into nostrils (the technique termed variolation). She tried to introduce this technique into England in 1718, but the method was considered too dangerous. Edward Jenner (1798), a British physician, improved this technique and discovered means of preventing smallpox. Jenner intentionally caused a mild cowpox infection in James Phipps, a healthy 8-year-old boy, in order to protect him against smallpox. Phipps developed cowpox, but when Jenner intentionally infected him with the smallpox matter, the boy did not develop smallpox. Thus, inoculation with the cow-pox matter protected against smallpox.
Nearly a hundred years later, Louis Pasteur appreciated the role of vaccination (as the Frenchman called the procedure) in protecting against infectious diseases. He recognized that aging impairs virulence of pathogens, and that such an avirulent strain might be administered to protect against the disease. This followed his chance observation that the infectious agent in cholera of chickens (now known as Pasteurella antiseptica) became avirulent on ageing, but this avirulent form protected the chickens against subsequent exposure to fresh virulent preparations. This led Pasteur to realize that it was possible to immunize individuals by using inactivated organisms. Such organisms retained their ability to provoke an immune response (they were immunogenic) while failing to induce the disease (they were not pathogenic). He called this avirulent strain of organisms a vaccine (from Latin vacca, which means cow) in honour of Jenner’s pioneering contribution with cowpox inoculation. These findings were subsequently extended to other diseases and demonstrated that introduction of dead or weakened (i.e. attenuated) viruses or bacteria, or their toxins, into the body developed resistance to diseases.
Discovery of Two Types of Immune Responses
Humoral and cellular
The first insight into the mechanism of immunity was provided by Emil von Behring (Nobel Prize in 1890) who recognized antibodies to diphtheria toxin in serum. The immune response mediated by the soluble antibodies present in serum was called the humoral response (the body fluids used to be called humours). The other type of immune response, the cell-mediated response, was elucidated around this time only from discovery of Elie Metchnikoff in 1883 who hypothesized that cells, rather than antibodies, were responsible for the immune response. These cells attacked immunogens directly rather than synthesizing special protective proteins (i.e. immunoglobulins).
Subsequently it was shown that interrelated roles of both types—humoral and cellular activities—were essential for development of effective immune response. Lymphocytes were identified as the cells playing a major role in both types of immune responses.
Selective and instructional theories
Several theories were proposed to account for the distinct specificity of antibody molecule against the non-self. The Selective theory (Paul Ehrlich, 1900) proposed that the antigen is selected and bound to preformed cell surface antibody having a complementary structure. This in turn would enhance the cellular production of this antibody. The selective theory further suggested that specificity of the antibody had been determined prior to antigen exposure; the role of antigen appeared merely to select the appropriate antibody and induce its proliferation.
The instructional theory suggested that the major role in determining the specificity of the antibody molecule was played by antigens. A portion of a particular antigen was suggested to serve as a template, and an antibody complementary to the antigen was formed. Thus, this theory envisages antigen to be instructing synthesis of the antibody. In the 1930s and the 1940s various forms of instructional theories were proposed. However, in the 1950s attention was once again reverted to selection theories and currently a refined form of it, called Clonal Selection theory, is widely accepted in modern immunology. This theory will be dealt with in more details later in the chapter.
II Non-specific and specific Immune Responses
The immune responses include specific and non-specific components. Non-specific immunity, sometimes referred to as innate, inborn, unchanging or natural derives from all those elements with which an individual is born and that are always present and available at short notice. The specific immunity (also called acquired or adaptive) occurs after exposure to an agent and is mediated by antibodies and by lymphoid cells. It came late in evolutionary terms and is present only in vertebrates.
A joint operation of innate and adaptive responses provides an excellent all round protection against a variety of challenges from the environment.
A Non-specific (Innate) Immune Response
It is considered more primitive than the specific immune response and can be envisioned as the basic state of resistance or the first-line of defense that a species possesses. It is innate in that it is not affected by any prior contact with the infectious agent although its activity can be up-and down-regulated by a number of factors. It mainly uses mechanical barriers to infectious agents, e.g. skin and associated secreted products—mucus, tears, etc. It also involves physiological barriers, e.g. temperature and pH. These purely physical barriers to infection are supplemented by certain cells (e.g. natural killer cells), certain proteins (e.g. the complement cascade and interferons) and other processes such as phagocytosis and inflammation (Table 33.1).
Table 33.1
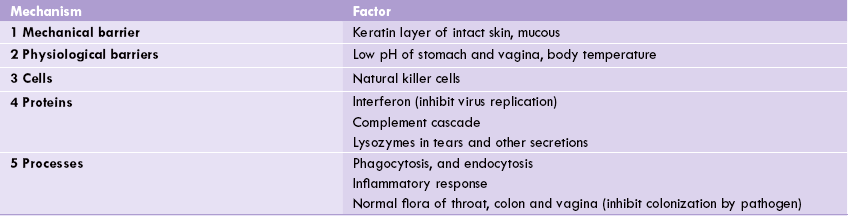
1 and 2 restrict entry of pathogen, whereas 3, 4 and 5 inhibit growth and survival of pathogen.
Some of the important factors enlisted in Table 33.1 are discussed here.
1. Temperature and pH: Many species display innate immunity against certain infections simply because their body temperature inhibits survival of the invading pathogen. Body temperature of chickens, for example, is too high to permit growth of anthrax bacteria, thereby providing an innate physiologic barrier to this infection. Low pH in our stomach is also highly unfavourable for pathogen growth.
2. Lysozyme, an enzyme first described by Alexander Fleming and found in tears and mucous secretions, is capable of attacking and catalyzing the hydrolytic destruction of the peptidoglycan layer of the bacterial cell wall. Thus, Fleming is credited with discovery of both endogenous and exogenous antibiotics (lysozyme and penicillin respectively).
3. Phagocytosis and endocytosis: In phagocytosis the plasmamembrane of the phagocytotic cell expands around the particulate material; the particulate material includes the whole pathogenic microorganisms. Intracellularly, the ingested particle is entrapped in phagocytic vacuole into which lysosomes release their enzymes, which then digest the particle into small breakdown products, e.g. peptides, nucleotides and sugars. Phagocytosis differs from endocytosis in that the expansion of membrane requires participation of microfilaments, which do not take part in endocytosis. More-over, only specialized cells are capable of phagocytosis, whereas endocytosis is carried out by virtually all cells.
Phagocytosis is enhanced by a variety of factors that make the foreign cell an easy target. These factors, collectively called opsonins, consist of antibodies and components of complement system.
Specialized phagocytotic cells include tissue macrophages and granulocytes, which make up 60% of the leukocytes in the blood. In addition to using lysosomal enzymes, they kill the internalized invading pathogen by use of cytotoxic free radical attack also. In the case of granulocytes, the oxidative attack on invading organisms is so vigorous that the cells themselves rarely survive, whereas macrophages can carry out many rounds of killing.
4. Inflammatory response: Inflammation is the body’s response to injury or tissue damage, which is provoked by presence of invading pathogen. Its purpose is to limit and then repair the damage wrought by the injurious agent. The cardinal signs of inflammation are redness or rubor, swelling or tumour, heat or calor and pain or dolor. These signs are due to increased blood flow, increased capillary permeability, and the escape of fluid and cells (macrophages and lymphocytes) from the engorged capillaries into the tissue spaces. The increased permeability is due to several chemical mediators, of which histamine, prostaglandins and leukotrienes are the most important. Bradykinin is an important mediator of pain.
The fluid that accumulates in tissues, called exudate, has high content of serum proteins which possess antibacterial properties. As part of the inflammatory process, neutrophils and macrophages, both of which arrive early, supplement the antibacterial action by engulfing the bacterial cells. Certain proteins, known collectively as the acute-phase reactants, are also produced early in acute inflammation (within 1–2 days). The best known of these is C-reactive protein, which is synthesized in the liver and is thought to play a role in activating the alternative pathway of complement activation by binding to the surface of bacteria.
It is clear from the foregoing discussion that the so called more ancient and primitive innate response is still an important aspect of protection against infection in humans. Its impairment can lead to life-threatening infections (Case 33.1). Moreover, it freely interacts with the more recent, more specific adaptive immune response (described below) to provide an excellent protection against a variety of invading pathogens.
B Specific Immune Response
If the non-specific defenses are unsuccessful, e.g. due to persistence of the triggering agent, the specific immune response is brought into play, which is capable of specifically recognizing and selectively eliminating foreign microorganisms and molecules. Unlike innate immunity, which is inborn, the acquired immunity is a consequence of an encounter with a foreign substance. The antigenicity (ability of the foreign substance to induce an immune response) is determined by one or more specific molecular groups on its structures, known as antigenic determinants (or epitopes). When lymphocytes (B- or T-cells) recognize a foreign substance (antigen), they respond by transforming into lymphoblasts and then into antibody secreting plasma cells; or into cytokine secreting helper-cells or activated cytotoxic cells, as depicted in Figure 33.1.
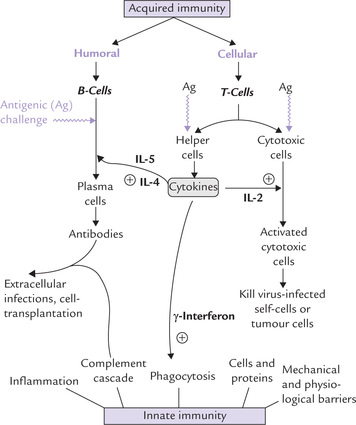
Fig. 33.1 Introduction to the interactions and functions of major components of immune system (IL = Interleukin).
The second distinctive feature of specific immunity is that (unlike innate immunity) it displays specificity, diversity, memory and self/non-self recognition. These four features characterize all immune responses:
1. Specificity: The immune system can distinguish subtle differences among antigens. A single base mutation, resulting in a single amino acid substitution, may be recognized effectively.
2. Diversity: The immune system is capable of generating a vast array of structurally different recognition molecules of almost any specificity, and therefore can respond to billions of antigens.
Memory: The immune system employs a mechanism to remember the specific encounter so that if the same foreign (or nonselff) antigen is encountered again it can be dealt with more quickly and effectively. This is achieved by induction of a heightened state of immune reactivity in secondary encounter.
3. Self/nonselfrecognition: The immune system responds only to those antigens that are not “self “. Because of this attribute, self-antigens are spared, which is essential because response to self-antigens can result in fatal auto-immune diseases.
The specific immune responses are mediated by humoral and cellular elements, which complement each other. As indicated earlier, (a) if the response to an immunogen is the production of soluble antibodies in the body fluids (i.e. humours), it is called humoral immunity, and (b) if the response is through cytotoxic or killer cells, then the immunity is known as cell-mediated. The cells responsible are lymphocytes, that develop and reside in the lymphoid tissues and are of two major types:
1. B-lymphocytes: They are independent of the thymus but dependent on the bursa of Fabricius in birds; its equivalent in man is bone marrow. B lymphocytes are responsible for the humoral immunity. The antibodies produced in this type recognize and destroy specific foreign antigens, either alone or along with complement.
2. T-lymphocytes: They are dependent upon thymus for becoming immunologically competent from non-competent predecessor originating in the bone marrow, and are responsible for cell-mediated immunity. The activated T lymphocytes (cytotoxic cells) act on self-cells infected by virus, destroying the self-cell as well as the viral antigen. A subset of T-helper cells lie at the heart of all immune responses: they release a group of proteins (cytokines), which activates different arms of immune system (Fig. 33.1).
A coordinated interplay of the innate and the specific immune response successfully defends against most invading pathogens. However, these pathogens develop various means of evading the host defenses, thereby intensifying the challenge (Box 33.1).
Though in the foregoing discussion, the two arms of immune response—innate and specific—have been discussed separately, they do not operate independently of each other. They interact with each other, as evidenced by the following examples:
• Cells of phagocytic systems, most notably macrophages, capture the foreign antigen and present them (antigens) to cells of specific immune response, leading to activation of the latter.
• The soluble factors (cytokines), produced during a specific immune response, augment the activity of these phagocytic cells.
A well-orchestrated and carefully regulated interplay of the two arms of immune system, as outlined in Figure 33.1, effectively eliminates the foreign invaders. Detailed mechanisms of these cooperative interactions are presented throughout the rest of this chapter.
III Antigens and Immunogens
Antigens are molecules that react with antibodies, whereas immunogens are molecules that induce an immune response. In most cases antigens are immunogens and the terms are used interchangeably. The features of molecules that determine immunogenicity are as follows:
1. Foreignness: The molecule should be recognized as nonself by the immune system
2. Molecular size: Macromolecules having molecular weight over 100,000 are the most potent immunogens; those below 10,000 are weakly immunogenic, whereas small ones, e.g. an amino acid are non-immunogenic. Exceptions are seen in haptens.
3. Structural/chemical complexity: A certain amount of complexity is required for immunogenicity. For example, homopolymers are less immunogenic than heteropolymers.
4. Antigenic determinants (Epitopes): Epitopes are small chemical groups on the antigen molecule that can elicit immune response and react with antibody. An antigen can have one (monovalent) or more (multivalent) antigenic determinants.
5. Others: Dosage, route and timing of antigen administration and the genetic constitution of the host also affect immunogenicity.
Haptens
Generally, antigens are immunogens but there are certain important exceptions, e.g. haptens which are not able to provoke immune response but which can bind to antibodies. Haptens are usually small molecules, but some high molecular weight nucleic acids are haptens as well. They cannot induce the immune response by themselves but do become immunogenic when bound to a “carrier” protein. The carrier protein may activate T-helper cell, while the hapten interacts with the B cell, bearing surface receptor antibody (i.e. IgM) specific for the hapten. The activated T-helper cell stimulates the B cell to produce antibodies to the hapten.
Adjuvants enhance immune response to an immunogen though they are unrelated to the latter. Some human vaccines contain adjuvants.
IV Role of Lymphocytes
Lymphocytes are the cells primarily involved in generation of an immune response. There are approximately 1012 lymphocytes in the human body, the total cellular-mass of which is equivalent to that of the liver. The four features which characterize all immune responses—specificity, diversity, memory and self/nonself recognition—are mediated by lymphocytes.
Lymphocytes are produced from haematopoietic stem cells in the bone marrow and differentiate in the central lymphoid organs: B cells in bone marrow and T cells in the thymus. Through this process, called maturation, lymphocytes become immunocompetent (Fig. 33.2). From central lymphoid organs, the immunocompetent lymphocytes migrate through the bloodstream to the peripheral lymphoid tissues (lymph nodes, spleen, tonsils, etc.) which are sites of lymphocyte activation by antigen.
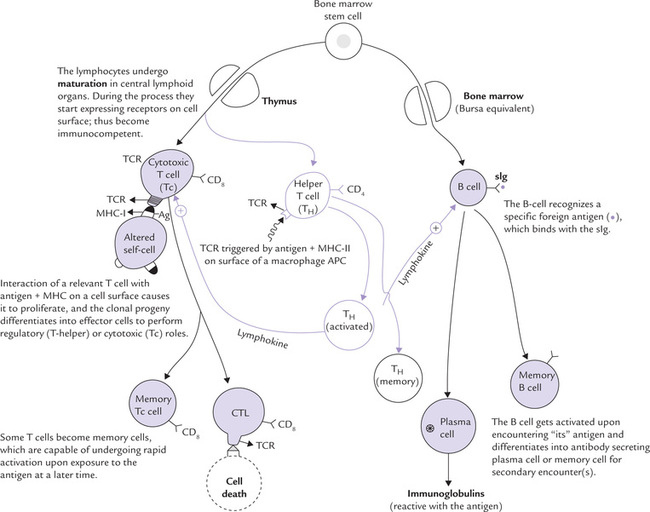
Fig. 33.2 Origin of B and T cells and their role in induction of humoral and cell-mediated immunity (APC = antigen-presenting cell, TCR = T cell receptor, CTL = cytotoxic T lymphocyte, sIg = surface immunoglobulin, Ag = antigen).
B cells and T cells comprise respectively 50–70% and 10–20% of the total lymphocyte population. Both recognize antigens by means of membrane receptors: specificity of the immune response is through the recognition of the antigen by such receptors.
A third population, termed natural killer (NK) cells, make up the remainder of the lymphocytes. NK cells are so called because they demonstrate the ability to kill without prior exposure or sensitization. Despite their different functions, discussed below, it is not possible to discern any morphologic features that can be used to distinguish B cells and T cells, and NK cells from one another. Instead, their identification is based on immunophenotypic or functional studies.
A The B Lymphocytes
B cells, the mediators of humoral immune response, undergo their early development in the bone marrow. This process, termed maturation, is antigen independent, leading to appearance of unique antigen binding membrane receptors. The B-cell receptor is a membrane bound immunoglobulin (IgM, in its monomeric form), termed surface immunoglobulin (sIg). There are approximately 105 sIg molecules on the membrane of a single B cell. Each sIg molecule has an identical antigen binding specificity, which enables the B cell to recognize specifically “its” antigen (Fig. 33.2).
Structure of a slg
It consists of two identical heavy poly-peptide chains and two identical light chains, held together by disulphide bonds. The amino terminal ends of each heavy and light chain fold together to form the antigen-binding cleft (Chapter 5). The sIg differs from the secreted immunoglobulin in the amino acid sequences at the carboxy end of the heavy chain: the sIg contains a transmembrane helix that is lacking in the secreted immunoglobulin. This structural difference results from the optional use of a small exon for the membrane attachment region (Chapter 24).
Activation of B-cell
Upon encountering an antigen, for which its surface immunoglobulin is specific, the B cell gets activated in a peripheral lymphoid tissue. It undergoes rapid cell division and its clonal progeny differentiates into two cell types: (a) the antibody secreting plasma cells, and (b) the memory cells (Fig. 33.2).
• Plasma cells are the effector cells, which produce an enormous amount of one of the five classes of antibodies in a short lifespan of a few days. A single plasma cell can secrete more than 2000 antibody molecules per second; all clonal progeny from a given B cell being of the same antigen-binding specificity. The secreted antibodies are the major effector molecules of humoral immunity (discussed later).
• Memory cells, on the other hand, have a longer lifespan (circulate for several years) and continue to express membrane bound immunoglobulins with the same specificity as the original parent cell. They remain in the body to respond to the same antigen during a subsequent encounter.
B The T Lymphocytes
T cells derive their name from the site of maturation in the thymus. Roles of T cells are three-fold:
• They kill host cells infected with pathogens, which, because of their intracellular locations, are sequestered from antibodies.
• Where infection persists, they help in maintaining an inflammatory response.
• Finally, they secrete cytokines which are regulators of various immune effector cells.
Like B cells, the T lymphocytes are also produced in the bone marrow, but unlike B cells which mature within the bone marrow, T cells migrate to the thymus gland to mature (Table 33.2). The term maturation implies acquiring immunocompetence versus antigen of a given specificity. During maturation, the T cell starts expressing a unique membrane receptor for antigen, called T cell receptor (TCR).
Table 33.2
Comparative features of T and B cells
| Feature | T cells | B cells |
| Site of maturation | Thymus | Bone marrow |
| Mediator | Cellular immunity | Humoral immunity |
| Immunoglobulin synthesis | No | Yes |
| Synthesis of cytokines | Yes | No |
| Antigen receptor on surface | Heterodimer | IgM (monomer) |
| CD3rirrttAÏn rtn çurfnrA | Yes | No |
Structure of TCR
The receptor is a heterodimer, comprising two non-identical polypeptide chains, linked by disulphide bonds.
These chains are termed the TCR-α, β, γ, and δ chains and the functional combinations are αβ and γ δ chains. Each chain comprises two domains—one constant and one variable. The amino terminals of the two chains pair to form a cleft within which antigen binds. Although TCR is structurally distinct from immunoglobulin, it is in some respects the counterpart of the latter, most notably with regards to structure of its antigen-binding site.
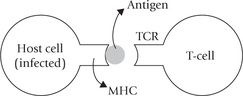
What distinguishes the TCR from membrane-bound antibody on B cells (i.e. the sIg) is that it recognizes antigen only when antigen is complexed with a cell-membrane protein, i.e. the major histocompatibility complex (MHC) molecule. It is as if MHC is a signpost, guiding the T-cytotoxic cell to its target.
This points to the fundamental difference between the humoral and the cell-mediated branches of the immune system. Whereas the B cell is capable of binding soluble antigen, the T-cell system is restricted to binding antigen displayed on self-cells with a background of self-molecules, in the form of MHC. The antigen in association with MHC may be presented on the surface of the antigen-presenting cells or on virus infected cells, cancer cells and grafts (Box 33.2).
T cell subpopulations
Two major subpopulations are known:
• immunoregulatory T-helper cells, and
• cytotoxic T cells (Fig. 33.2).
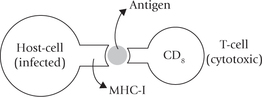
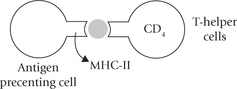
These two subpopulations of T cells can be distinguished by their display of either the one or the other of the two membrane glycoproteins, CD4 or CD8. The T-helper cells display CD4 and recognize antigens associated with MHC molecules of class II. The cytotoxic T cells display CD8 and recognize antigens associated with class I-MHC molecules. Thus, the T-helper cells (Tc) are class II restricted and T-cytotoxic cells are class I restricted.
Activation of T cells
Interaction of TH cells with antigen + MHC-II results in activation of the former, so that it proliferates extensively and starts secreting various growth factors, known collectively as cytokines, or more specifically lymphokines. Secretion of lymphokines is a crucial event which brings about activation of B cells, Tc cells, phagocytic cells and various other cells that participate in the immune response (e.g. macrophages).
The Tc cell on the other hand is activated by interaction with an antigen-MHC-I complex on the surface of an altered self cell in the presence of appropriate lymphokines. The activated Tc cells proliferate and differentiate into effector cells, called cytotoxic T lymphocytes (CTL), which mediate the killing of the altered self cells (Fig. 33.2).
In addition to TH and Tc, another type of T cell called T-suppressor cell has been postulated, but it is still not clear whether its lineage is distinct from the TH and Tc subpopulations. Unlike the TH and Tc, they do not display any CD surface markers (Box 33.3).
C Natural Killer Cells
These are large granular cells, important in resistance to virus infections and probably also malignancies. When a cell becomes infected by a virus or undergoes malignant transformation, its surface molecules are altered in that it starts expressing high molecular weight glycoproteins on the membrane. The latter are recognized by NK cells. These cells bear NK cell-receptors which recognize these glycoproteins on the surface of the altered self-cells, at an early stage before the virus has had a chance to reproduce. The NK cells are activated by the cytokine, inter-feron-7, as discussed later. When activated, they release their granular contents into the space between the target and the NK cell, including a molecule called perforin, which acts, as its name implies, by punching a hole in the cell membrane of the target cell leading to its death.
NK cells also have receptors for immunoglobulin, the latter links the NK cell and the target cell closely. This phenomenon called antibody-dependent cell-mediated cytolysis (ADCC) has been described earlier (Chapter 5).
V Organs of the Immune System
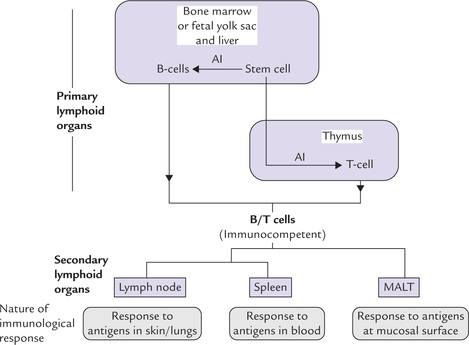
Organs of the immune system, or the lymphoid organs, are classified as primary or secondary on the basis of their respective functions. The primary lymphoid organs provide appropriate environment wherein maturation of naive lymphocytes into antigen-recognizing immune-competent lymphocytes takes place, the most notable of them are T and B cells. The secondary lymphoid organs trap antigen from defined tissues or vascular spaces and provide sites where these antigens interact with the immuno-competent lymphocytes. An antigen-driven proliferation and differentiation of the mature lymphocytes follows to generate an immune response.
The organization of primary and secondary lymphoid organs is illustrated in Figure 33.3.
A Primary Lymphoid Organs
These include the fetal yolk sac, the liver in the embryo, and the bone marrow and thymus after birth. Lymphocytes originate and undergo early development and differentiation in the primary lymphoid tissues. Their location alters during gestation: in early embryo the main site of lymphocyte production is the fetal yolk sac but later in embryonic life this shifts to the liver. Still later this shifts to the bone marrow and the thymus where it will remain throughout the rest of an individual’s life.
Stem cells in the bone marrow either differentiate to give rise to B cells or they migrate to the thymus where their development into various types of T cells is fostered (Fig. 33.3). The B cell progenitors initially formed in the marrow pass through a maturation process to become mature B cells; the process results in changing a (virgin) B cell into an immunocompetent B cell (i.e. capable of mounting an immune response). An immunocompetent cell is committed to a particular antigenic specificity. The cells that migrate to the thymus gland (i.e. the immature thymocytes) mature there to become antigen-committed, immunocompetent T cells. Maturation of both B and T cells are antigen-independent processes, occurring prior to the antigenic challenge.
Thymus
The thymus is a bilobed structure found in the anterior chest or mediastinum above the heart wherein the T-cell progenitors enter as immature thymocytes, and mature into functional T cells. The maturation involves:
• Expression of T-cell receptor (TCR) on cell surface.
• Commitment of such TCR expressing cell to recognize and respond to a given antigen.
Structure of thymus
Thymus has epithelial and mesenchymal (supporting tissue) components in addition to lymphoid contributions. At the microscopic level, each lobule is organized into two compartments: an outer cortex which is densely packed with thymocytes, and an inner medullary area which is sparsely populated with thymocytes.
Though the actual maturation sequence is not known, the T-cell maturation appears to progress as the immature thymocytes migrate from the cortex to the medulla. During their migration, these cells interact with three-dimensional network of the thymic stromal cells (composed of epithelial cells, interdigitating dendritic cells and macrophages) which make up the framework of the thymus and contribute to the thymocyte maturation. Many of these cells physically interact with the developing thymocytes and also secrete hormonal factors (e.g. thymosin, thymopoietin and thymulin) necessary for the differentiation and maturation of the T lymphocytes. The thymic stromal cells secrete a cytokine, IL-7, which also plays a role in T-cell maturation within thymus.
Selection processes during maturation
The mature, immunocompetent T cells so formed would be subsequently interacting with foreign antigens. However, T cell is capable of interacting with an antigen only when the latter is displayed in association with a self-MHC molecule (Fig. 33.2). Therefore, it is subjected to a rigorous selection process so that only those T cells which (a) can recognize antigenic peptide in the context of self-MHC, and (b) which are self-tolerant (non-reactive with self antigen) are released from the thymus. Thymic stromal cells play an important role in the selection process, which has both positive and negative elements:
1. Positive selection: The thymic stromal cells express high levels of class I and class II MHC molecules to which the developing thymocyte is exposed all through the maturation process. Only the T cells bearing receptors that recognize foreign peptides in association with self-MHC will be selected and allowed to mature further. Any developing thymocyte, incapable of recognizing self-MHC plus foreign-antigen will not be selected and eliminated by apoptosis or programmed cell death.
2. Negative selection: Among the positively selected cells, some will be potentially self-reactive, i.e. reacting with self-antigen. These cells must be eliminated. Negative selection targets for destruction of any such cell bearing a high affinity receptor for self-antigen (or self-antigen associated with self MHC).
So rigorous and unsparing are these processes that 95–99% of all thymocyte progeny dies within the thymus, without ever fully maturing. Rest of the < 5% cells, which are self tolerant with diverse TCRs on their surface, appear in circulation. Subsequently, these cells would migrate to secondary lymphoid organs where they form various effector population of T cells (Fig. 33.3).
Bone Marrow
In birds, a lymphoid organ called the bursa of Fabricius is the primary site of B-cell maturation, whereas in humans bone marrow serves as the “bursa equivalent” where the B cell matures. The process is antigen-independent, leading to the production of immunocompetent (and self-tolerant cells) with diverse monomeric IgM and IgD molecules expressed on their surface. These molecules constitute the B-cell receptors or surface immunoglobulins (sIg).
Subsequently, when B cells reach the secondary lymphoid tissue and encounter foreign antigen, they are stimulated to proliferate to yield two types of cells: plasma cells and memory cells (Fig. 33.2). Plasma cells secrete the soluble antibody corresponding to the sIg found on the stimulated parent cell, and memory cells persist until a second encounter with that antigen occurs, when they mediate the secondary immune response (Chapter 5).
B Secondary Lymphoid Organs
The adaptive immune response occurs mainly in the secondary lymphoid organs: the lymph nodes, the spleen, and the mucosal-associated lymphoid tissues (MALT) (Fig. 33.3). These tissues are uniquely suited to trap pathogens and their secreted antigens, and to present these to the naive lymphocytes that constantly pass through.
• Lymph nodes: Microorganisms that enter the body through the skin or the lungs drain to regional lymph nodes where they stimulate an immune response.
• Spleen: Microbes that enter the bloodstream stimulate an immune response in the spleen.
• MALT: Microorganisms and food antigens that enter the gastrointestinal tract are collected in the MALT.
Common to all lymphoid tissues is the degree of compartmentalization, with specific areas for T cells and B cells, and areas of overlap where they interact.
Lymph Nodes
Structurally, the lymph node is divided in three concentric regions called cortex, paracortex and medulla. The outermost cortex contains follicular structures of two types: the unstimulated primary follicles and the stimulated secondary follicles. The latter are characterized by the presence of germinal centre—the sites of intense B-cell activation and differentiation into plasma and memory cells. The paracortex, which lies inner, to the cortex, contains primarily T cells. The innermost medulla contains several antibody-secreting plasma cells.
Spleen
The spleen contains non-lymphoid tissue populated by macrophages and erythrocytes, where the old and defective red blood cells are destroyed and removed (the red pulp); as well as lymphoid areas, termed the white pulp. The latter contains T cells and several interdigitating dendritic cells. The blood-borne antigens are trapped by their interdigitating dendritic cells, which present these antigens to TH cells, thereby initiating immune response.
MALT
It comprises lymphoid elements found at various locations along mucous membrane surfaces where they trap antigen and provide localized sites for lymphocyte interaction with that antigen. Structurally these tissues range from loose clusters of lymphoid cells with little organization in the lamina propria mucosa of the intestinal villi to organized structures such as the tonsils, adenoids, appendix and Peyer’s patches. Lymphocytes are also found singly throughout the mucosal epithelium.
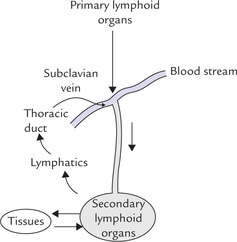
Lymphocyte traffic around lymphoid tissue
There is a one way traffic of T and B cells from the primary lymphoid organs into the bloodstream and continuous re-circulation of these cells between the secondary lymphoid organs, tissues and bloodstream. Initially, these cells migrate via blood from the primary to the secondary lymphoid organs (lymph nodes, spleen and MALT), where they are stimulated by antigens. Lymphatics drain extracellular fluid as lymph through the lymph nodes and into the thoracic duct, which returns the lymph to the bloodstream by emptying into the left subclavian vein (Fig. 33.4). Thus, there is a continuous re-circulation.
As the lymphocytes re-circulate, they make contact with antigens presented on the surface of antigen-presenting cells in the secondary lymphoid organs. However, only a very small percentage (about one in 103 to 106) of the lymphocytes can recognize a particular antigen within a relatively short period of time in order to generate specific immune response. The odd of small percentage of immunocompetent lymphocytes actually making contact with the antigen is greatly overcome by the extensive lymphocytes traffic into and out of lymphoid tissues, especially after an antigenic challenge and by cell adhesion molecules (Box 33.4).
VI Cells of the Immune System
A wide range of cells participate in non-specific and specific immunity. As described earlier, lymphocytes are the pivotal and critically important cellular components of the immune system; all other cells play only accessory/supportive roles, such as serving either to activate lymphocytes or to increase effectiveness of antigen clearance by phagocytosis, or secretion of cytokines.
The cells of immune system can be divided in two major groups:
(a) the antigen-presenting cells: macrophages, interdigitating cells, Langerhans cells, follicular dendritic cells, etc. and
(b) white blood cells or leucocytes, divided into five categories: neutrophils, eosinophils, basophils, lymphocytes and monocytes (the first three cell types listed are referred to under the general name of granulocytes).
Many of the cells involved in immunity fulfil more than one function; for example, macrophages, as the name (“larger eater”) suggests are the cells chiefly involved in engulfment of microorganisms (i.e. phagocytosis). In addition, they are also known to play a role in induction of immune response by presenting antigens on their surface to specific T lymphocytes.
A Lymphocytes
As discussed earlier lymphocytes are the motile, non-phagocytic cells which play a central role in immune response, being responsible for attributes of specificity, diversity, memory and self/nonself recognition—hallmarks of an immune response. The constantly circulating lymphocytes between tissues, lymphoid organs, blood and lymph provide a high degree of cellular integration to the immune system as a whole. Different lineages of lymphocytes and their functions have been already dealt with, in detail.
B Macrophages
The macrophages in tissues, together with the circulating monocytes in the blood constitute the mononuclear phagocytic system. Macrophages derive from bone marrow promonocytes which, after differentiation to blood monocytes, finally settle in tissues as mature macrophages. Monocytes remain for about 8 hours in bloodstream, and then enlarge and migrate to tissues as they differentiate into macrophages.
Many organs contain characteristic population of macrophages, e.g. liver Kupffer cells, lung alveolar macrophages, brain microglial cells, splenic lymphoid macrophages, and kidney mesangial cells.
Functions
Macrophages serve dual role being involved in phagocytosis and in presenting antigens on their surface to specific T-cells.
Phagocytosis
Cells of the mono-nuclear phagocytic system—macrophages and monocytes—with their impressive anti-microbial potential, present formidable weaponry against the invading pathogen. In fact, macrophages are one of the two cell types involved in engulfment and digestion of exogenous antigens, other ones being micro-phages (small eaters) or polymorphonuclear neutrophils, discussed later.
In addition to the whole pathogenic microorganisms, the antigens engulfed by these phagocytic cells include cellular debris, insoluble particles, injured and dead host cells, and activated clotting factors. Engulfed by cytoplasmic processes, the antigen initially comes to lie in a vacuole termed a phagosome, and subsequently digested by lysosomal contents. The latter include hydrogen peroxide, active oxygen metabolites, peroxidase, lysozyme, lactoferrin, cationic proteins and a rich variety of proteolytic and other hydrolytic enzymes. The digested material is then extruded from the cell by exocytosis.
Note
Aggregates of macrophages, granulomas, are characteristic of many chronic infectious and idiopathic inflammatory diseases such as tuberculosis, leprosy, and sarcoidosis.
Antigen presentation and processing
Following phagocytosis, the macrophages process the ingested antigen and then present it appropriately in association with MHC molecules. In general, when antigen is taken up by macrophages, a proportion is degraded by phagocytic digestion while part is fixed to the cell surface where it is thought to be in a strongly immunogenic state in association with MHC class II molecules. Antigen presentation in association with MHC-II is an essential requirement for activation of TH cells, which cannot recognize an antigen alone. As discussed earlier, this presentation is a crucial event in the development of humoral and cell-mediated immune responses.
Secretory role
Following phagocytosis, the macrophages release a number of protein factors, which are central to the development of the immune response. Interleukin 1 (IL-I) is the first one to be released after macrophage phagocytose antigen. IL-I is not only required for activation of a variety of cells (e.g. T and B cells, neutrophils, fibroblasts), but it also effects vascular endothelium to influence the inflammatory response and acts as endogenous pyrogen: it acts on thermo-regulatory centre in the hypothalamus, leading to fever response. The other factors released by macrophages and their function in immune response are as below:
1. Interferon alpha: Activates cellular genes resulting in the production of proteins that confer an antiviral state on cell.
2. Tumour necrosis factors (TNF): Cause necrosis of a variety of cells including tumour cells. Along with IL-I, the TNFs play a critical role in initiation of inflammatory response. TNF-alpha, also known as cachectin, has profound effects on general cellular metabolism; it causes weight loss, fever, acute phase reaction, etc.
3. Hydrolytic enzymes: Can be secreted by activated macrophages in tissues where they promote inflammatory response.
4. Complement proteins: Assist in elimination of foreign pathogens and in the amplifying inflammatory reaction.
Finally, activated macrophages secrete a number of cytokines (Interleukin-6, 7-interferon, GM-CSF, G-CSF and M-CSF), which are considered in detail subsequently in this chapter.
C Granulocytes
These cells have granulated cytoplasm that stains with acid or basic dyes. On the basis of cytoplasmic staining characteristics and cellular morphology, they have been divided in three major types: eosinophils staining with acidic dye (eosin Y); basophils staining with basic dye (methylene blue); and neutrophils staining with both acidic and basic dyes. Both neutrophils and eosinophils are phagocytic, whereas basophils have a major role in allergic responses.
Functions
Neutrophils, which are much more numerous than other granulocytes, constitute 50–70% of the circulating white blood cells (basophils 1–3% and eosinophils < 0.1%). They are produced in the bone marrow during haematopoiesis and released into the peripheral blood where they circulate for 7–10 days, and then migrate into the tissues where they have a 3-days-lifespan. Process of phagocytosis by neutrophils is same as that for macrophages, except that the lytic enzymes contained in the cytoplasmic granules mediate killing of pathogen mainly by a cytotoxic free radical attack. The attack is so vigorous that the granulocyte cells themselves rarely survive, whereas in case of macrophages several rounds of killing can be carried out by the same cell. Failure of polymorphs to fulfill the above task leads to severe infections, though clinical features may be minimal (Case 33.2).
Eosinophils are also motile phagocytic cells like neutrophils but their phagocytic role is mainly to aid human host defenses against parasites and worms. They are also implicated in allergic diseases such as asthma. Their presence in tissues, and a high count of eosinophils in the blood (eosinophilia), is therefore, often a marker of an allergic disease or parasitic infection.
Basophils are not phagocytic as mentioned earlier. They have a large number of cytoplasmic granules containing pharmacologically active substances, which play important role in development of allergic responses.
D Dendritic Cells
These cells have peculiar structural features, such as an unusual shape resembling dendrites of a nerve cell and the cell surface being covered with a maze of long membrane processes. They express high level of MHC molecules on their surface, therefore suited to function as important antigen-presenting cells for T-cell activation. After capturing antigen in tissues, dendritic cells migrate to various lymphoid organs where they present the antigen to T cells. They are widely distributed in non-lymphoid organs and tissues, in lymphoid organs (e.g. interdigitating dendritic cells and follicular dendritic cells) and in blood and lymph (e.g. veiled cells).
Though the dendritic cells at these different locations have several morphologic and functional differences, they appear to have arisen from a common progenitor cell and may represent various stages of a single lineage.
VII Molecules of Immune Response
A Proteins Encoded by Major Histocompatibility Complex (MHC)
The MHC complex is a large complex of genes, with multiple loci, present on the short arm of chromosome 6. Although the MHC was originally identified by its role in transplant rejection, it is now recognized that proteins encoded by this gene cluster are involved in many aspects of immunological recognition.
General organization
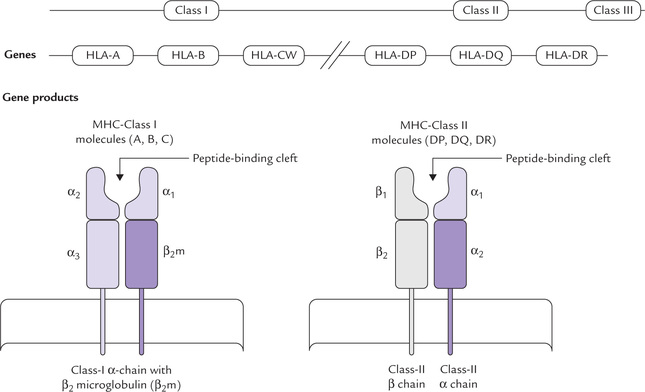
The MHC gene cluster is divided into three regions termed class I (also designated HLA-ABC), class II (also designated HLA-D) and class III. The same nomenclature is applied to the respective polypeptide products: the polypeptide products of class I, II, and III genes are termed respectively MHC class I, II and III molecules (Fig. 33.5). The first two (i.e. class I and II molecules) are collectively known as human leucocyte antigens (HLA antigens) which are found on many cells, not only leucocytes. They determine the tissue type of an individual.
Class I molecules
These are glycoproteins found on the membrane of nearly all cells of the body except red blood cells. They comprise one peptide chain, encoded by the MHC called α-chain, associated with a different polypeptide, β2-microglobulin (the latter is encoded elsewhere in the genome). The class I molecules are involved in immune recognition by binding the antigenic peptides synthesized by intracellular pathogens (bacteria or viruses). The peptides bound to MHC-I molecule is presented on the cell surface, and is recognized by receptors on CD8 T cells.
Class II molecules
These polypeptide products of the class II MHC genes have more limited tissue distribution, being constitutively expressed on antigen-presenting cells, including macrophages, dendritic cells, and B lymphocytes. They perform the same function as the class I molecules in immune recognition (and have similar structural motif), but the peptides presented by MHC class II molecules can only be recognized by receptors on CD4 T-helper cells and not by those of CD8 T-cells.
Class III molecules
These are involved with the inflammatory response by virtue of coding for soluble mediators, including complement components and TNF.
MHC loci and allelic variants
The class I genes encoding the α-chains are organized in several loci, the most important of which in humans are HLA-A, HLA-B and HLA-CW (there are two loci in mice-K and D). These genes are highly polymorphic and so there are numerous variants of HLA-A, HLA-B and HAL-C within the population. Each locus has a large number of different alleles (different forms of the same gene), which are transmitted and expressed in a co-dominant fashion. Because of their closeness on the chromosome, they are inherited ‘enbloc’ as parts of a haplotype. Since a person inherits one allele from each parent for each locus, a multiple class I MHC molecules are expressed on each of his nucleated cells.
The principal class II molecules are designated DP, DQ, and DR, and like the MHC class I molecules and they are highly polymorphic. As in the case of the class I MHC, there are large number of different alleles for each class II locus. Owing to inheritance of one allele from each parent for each locus, the surface of the antigen-presenting cell has multiple class II MHC molecules.
Intensely polymorphic nature of MHC system is illustrated in Box 33.5.
Subunit structure of MHC molecules
Schematic representation of a human class I molecule (Fig. 33.5) shows that it is a heterodimer composed of one transmembrane α-chain bound non-covalently to β2 microglobulin. The α-chain is folded into three protein domains (α1, α2 and α3), the first two of which form, a cleft into which the peptide antigen binds.
The class II molecule is composed of two transmembrane glycoproteins chains, α and β, each folded into two-protein domains. The antigen peptide binds in a cleft between the two chains.
B Cytokines
Cytokines are the proteins secreted from a variety of cells and tissues that act as soluble mediators of the inflammatory and immune responses. They orchestrate the immune response by transmitting signals to and between cells engaged in the response (Table 33.3). They also regulate several biological processes, such as cell growth, tissue repair, fibrosis and morphogenesis.
Table 33.3
Source and action of some cytokines
| Cytokine | Major source | Important functions |
| Interleukin-1 | Activated macrophages and other antigen-presenting cells | Activates T-helper cells leading to secretion of interleukin-2, causes fever. |
| Interleukin-2 | Activated T-helper cells | Activates helper and cytotoxic T cells. |
| Interleukin-4 | Activated T-helper cells | Stimulates B-cell growth and promotes Ig isotype switching. |
| Interleukin-5,6 | Activated T-helper cells | Stimulates B-cell differentiation into antibody-secreting plasma cells. |
| Gamma interferon | Activated T-helper cells | Stimulates phagocytes, especially macrophages. |
| Tumour necrosis factor | Activated macrophages | Activates neutrophils mediated septic shock, causes necrosis of tumours, cytotoxic to normal tissue also (but have short half-life). |
General characteristics
A number of cytokines originating from various sources are known, which are grouped into different families, but all have several common features and characteristics.
1. They are peptide or glycoprotein in nature, are antigenic non-specific, transiently secreted and have short half-lives. Their orbit of influence is also of limited range.
2. Cytokines bring about their effects by interacting with receptors on surfaces of their target cells. They are active at concentrations between 10-9 and 10-15 moles, and the majority act within short distances of the site of their production (paracrine) or on the same cell that produced them (autocrine). A few are capable of acting on cells distant from the site of their production.
3. The cell types on which they act may be multiple (pleiotropy). They show significant overlap in their functions (redundancy) and have potential for interaction via the effects they mediate. As will be discussed later, the specific immune response is driven by direct cellular interactions together with the effects of cytokines.
Biological effects
The cytokines are given special names to indicate their source or actions:
• Interleukins (IL): ILs are secreted by one leukocyte to influence another. Currently, 18 interleukins have been recognized. They play important role in regulation of activities of various cells of specific and innate immune responses. Source and functions of common interleukins (1, 2, 4–6) are outlined in Table 33.3, and a detailed account of their roles in making an immune response is given subsequently (see cellular cooperation in immune response).
• Interferons (IFNs): IFNα and IFNβ are produced altruistically by virus-infected cells. They bind to nearby cells and induce a generalized antiviral state by inhibiting viral replication. Interferon 7 is secreted by certain activated TH cells and promotes killing by macrophages and natural killer cells. It also plays important part in regulating the specific immune response.
• Tumour necrosis factor: These chemicals released from activated macrophages, are toxic to tumour cells, as discussed earlier. Two major types: TNF-α (MW 17 kDa) and TNF-β (MW 25 kDa) are known. Though they are cytotoxic to normal tissues also, their effect is limited due to their short half-life.
• Chemokines: These are relatively recently discovered family of mediators that bring about chemokinesis (i.e. movement in response to chemical stimuli). IL-8 is emerging as an extremely important neutrophil chemotactic agent in vivo. Receptors for these mediators appear to act as co-receptors for the infection of lymphocytes by human immunodeficiency virus.
Cytokines are more practically classified based on the principal effect or role they mediate:
1. Pro-inflammatory cytokines: Tumour necrosis factor(TNF)-a, IL-1, IL-6, IL-8 and other chemokines, IL-12 and IL-15.
2. Anti-inflammatory: Transforming growth factor (TGFβ) and IL-10.
3. Immuno-stimulatory cytokines: For cellular responses IL-2 and IFN7; and for humoral responses IL-4, IL-13, TGFβ and IL-10.
C Molecules Involved in Antigen Recognition
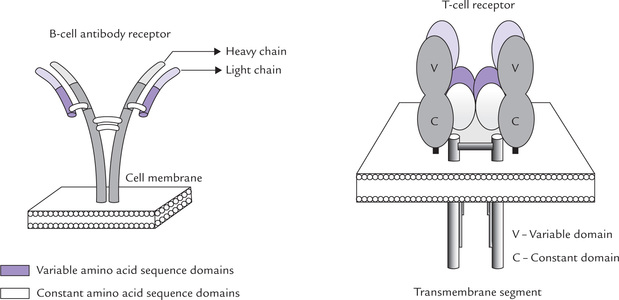
Specific receptors present on T and B cells are involved in recognition of the antigen. As indicated, the T-cell receptors (TCR) are heterodimeric polypeptide chains and the B-cell receptors are membrane bound antibodies or surface immuno-globulins (IgM in monomeric form) (Fig. 33.6). These receptors are too small to recognize antigens in their entirety, which are generally very large and complex. They recognize discrete sites on antigens (i.e. antigenic determinants, or epitopes), which are the immunologically active regions on a complex antigen.
Although both T and B-cell receptors belong to the same immunoglobulin super-gene family and exhibit several structural similarities in terms of folds and domains, there are some significant differences also.
The important similarities are: (a) both have hand-in-glove relationship with the corresponding antigenic element, (b) both have a limited number of genes that rearrange to form them, and (c) both show marked variability in the sequence of amino acids that actually come into contact with the epitope (the rest of the molecule being relatively constant in their amino acid sequence).
The important differences between the two types are that (a) TCR has two chains rather than four, and (b) TCR recognizes an epitope only when it is displayed together with molecules of the major histocompatibility complexes on self cell, whereas B-cell receptors recognize free antigen.
T-cell receptors
Four types of polypeptide chains (α, β, γ and δ) make up the T-cell receptors (TCR) in two functional combinations (αβ and γδ). Each chain comprises one constant and one variable domain and a C-terminal transmembrane region. The domains in each chain are organized into folds. The chains are joined by a disulphide bridge. There are gene-families encoding the two chains; these are segmented in the germ line and undergo developmental diversification.
The antigen-binding site of the TCR is in the cleft formed by the adjoining single variable domains of the constituent alpha (Vα, beta (Vβ), gamma (Vγ, or delta (Vδ) chains.
B-cell receptors
It is the membrane form of the immunoglobulin serving as receptor for antigen. These surface immunoglobulins (sIgs) are Y-shaped molecules made up of four polypeptide chains—a pair of heavy chains, each of approximate molecular weight of 50 kDa and a pair of light chains each of approximate molecular weight of 23 kDa. There are areas of constant and variable sequences of amino acids in both the heavy and the light chains. The variably sequenced amino terminal domains of both the heavy (VH-variable heavy) and the light (VL-variable light) chains form a pocket. The latter constitutes the antigen binding site, termed the fragment antigen binding (Fab) portion sites, at the end of the Fab sites. The remaining relatively constant amino acid sequence domains of the chains, termed constant heavy (CH) and constant light (CL), form the stem that provides transduction effects.
Note
Unlike B cells which secrete immunoglobulins (the secreted form are B-cell receptors), no secreted version of the TCR is made.
MHC I and MHC II molecules
These are expressed respectively on all nucleated cells and on antigen-presenting cells, and are encoded by different loci within the MHC complex. The T-cell receptors can interact with antigen only in association of MHC molecules. The T-helper cells generally recognize antigen expressed together with the class II MHC molecule (class II restricted) and the T-cytotoxic cells antigen together with class I MHC molecules.
VIII Immunoglobulins
Immunoglobulins are the secreted forms of the B cell receptors, which react specifically with the antigen that stimulated their production. They are directed at extracellular infections, especially bacteria and their products, the extracellular phase of viral infection and individual cell transplantation. There are three ways by which they participate in host defense:
1. Neutralization: Immunoglobulins bind to and neutralize a bacterial toxin, preventing it from interacting with host cells and causing harmful effect. Unbound toxin reacts with receptors on the host cell, whereas the toxin- immunoglobulin complex cannot. Immuno-globulins also neutralize complete viral particles and bacterial cells by binding to them and inactivating them.
2. Opsonization: Antibodies can coat a foreign antigen, and this makes the latter an easier target for phagocytes (macrophages and polymorphonuclear leukocytes). In this process, called opsonization, the antibodies coating an antigen render it recognizable as foreign by phagocytes (Chapter 5).
3. Complement activation: Antibodies coating a bacterial cell form a receptor for the complement system. The latter eventually forms a protein complex on the surface of the bacterium that favours its uptake and destruction by phagocytes and can, in some cases, directly kill the bacterium. Thus antibodies target pathogens and their products for disposal by phagocytes.
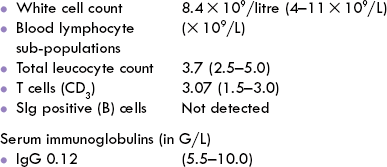
In view of pivotal role played by antibodies in body’s defense, it is evident that primary antibody deficiency results in hazardous consequences (Case 33.3).
Functions of individuals classes of antibodies and their structural features has been dealt with in detail in Chapter 5. Some additional information about immunoglobulins is presented here.
Antibodies illustrate excellent diversity of the immune system
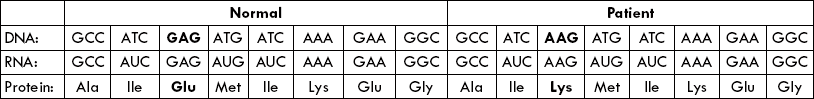
An animal can synthesize a limitless number of antibodies, each one with the ability to interact with a specific antigen, and together these millions of antibodies have the ability to recognize any and all nonself elements they come into contact with. How can the genes for some millions of different antibodies find space in the human genome. The mechanism by which such diversity arises from a basic template presents a good example of special genetic mechanisms, like DNA rearrangement and RNA splicing, being used for diversity (Chapter 24).
For each type of immunoglobulin chain, i.e. kappa light chain (kL), lambda light chain (λL) and the five heavy chains (αH, γH, μH and εH), there is a separate pool of gene segments in germline located on different chromosomes (genes for kL, λL and five heavy chains are on chromosomes 2, 22 and 14 respectively). Each pool contains a set of different genes for the variable and constant domains. During development of the B lymphocyte, these separate genes are combined into a single transcription unit that codes for a complete L or H chain.
Assembly of light chain (kL) gene
(Fig. 33.7): Most of the variable domain (reaching from amino acid 1 to 95) is encoded by V genes(V for variable), and the rest of the variable domain (amino acids 96–108) is encoded by J genes (J for joining). The constant region (starting from amino acid 109) is encoded by a single C gene (Cκ). The germline contains a large repertoire of V and J genes: about 50 V genes and 5 J genes are present.
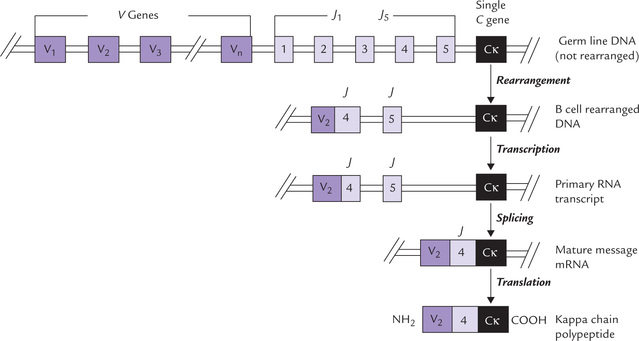
Fig. 33.7 Genetic events leading to assembly of a light chain gene. The kappa light chain (κL) shown here. It is encoded by separate gene clusters. During development of B-cell, these separate genes are combined into a single transcription unit, that codes for a complete light chain.
In course of B-cell differentiation, one of the V genes is selected at random from the library of V genes and spliced to the J genes to form a complete variable domain gene (V + J). Thus, two different segments are united into one functional gene by DNA rearrangements. This variable domain gene is then transcribed together with the constant domain gene. Transcription of the entire sequence of V + J + CR—one V gene spliced to one J sequence, together with the single Ck gene—yields a large primary transcript. These events, occur in the nucleus, as does the splicing of the RNA transcript, from which all the introns and the unused J chains are excised, and the remaining coding sequences are then joined into a continuous strand of mRNA. The latter is then transported into the cytoplasm where it is translated into the κ-chain polypeptide.
Assembly of the heavy chain gene(Fig. 24.12)
The heavy chain genes are assembled in the same way but the heavy chain gene cluster contains a set of approximately 30 D genes (D for diversity), in addition to the V J and Ch genes. During B-cell differentiation, the first translocation brings a Vregion close to a D segment and J segments to form a complete variable domain gene of the heavy chain (VH): V + D + J. This VH is then assembled with the C gene and transcribed.
Thus, it is observed that L and H genes of virtually unique structure are constructed by a recombination process that randomly selects one out of each set of gene segments and assembles them with C gene.
Immunoglobulin class switching (isotype switching)
During its development, the B cell is able to change the class of its antibody without changing the antigen-binding specificity. Initially all B cells carry IgM specific for an antigen and produce IgM antibody in response to exposure to that antigen. IgM often is followed by IgD and eventually IgG, IgA or IgE may appear. This phenomenon is known as class switching and takes place either before or (more commonly) after exposure to antigen. In class switching, same assembled VH gene can sequentially associate with different Ch genes so that the immunoglobulins produced later (IgG, IgA, IgE) have the same specificity as the original IgM but have different characteristics. The process is stimulated by lymphokines.
Another example of ‘switching’ is seen in haemoglobin synthesis, as described in Chapter 24.
Monoclonal antibodies and hybridoma technology
Administration of a protein or polysaccharide to an animal (that is of a species unrelated to the source of the immunogen) results in production of heterogenous antibodies. This is because the antibodies are formed by several different clones of cells, i.e. they are polyclonal. A large protein immunogen usually has several antigenic sites (i.e. epitopes), each of which gives rise to a distinct antibody, hence the name polyclonal antibody.
In contrast to polyclonal antibodies, a monoclonal antibody reacts with only a single epitope. The monoclonal antibodies arise from a single clone of cells, e.g. in a plasma cell tumour (myeloma) and are homogenous. The monoclonal antibodies can be synthesized in the laboratory by fusing a myeloma cell with an antibody-producing cell. Such hybridoma cell produces virtually unlimited quantities of monoclonal antibodies that are useful in diagnostic tests and in research.
Steps in synthesis: The monoclonal antibodies-synthesizing-hybrid-cells are made in the following manner:
1. An animal, e.g. mouse is injected with the antigen of interest. The antigen bears more than one epitope.
2. The spleen of the mouse is removed and the spleen cells are grown in a culture dish.
3. The mouse myeloma cells are added to the culture dish. These cells have an important attribute of immortality: they have unlimited lifespan in culture. They lack the salvage enzyme, HGPRT.
4. The myeloma cells are fused with the antibody-secreting (spleen) cells. The fusion is promoted by adding certain chemicals, e.g. polyethylene glycol. The hybrid cells now contain the gene of normal mouse as well as of myeloma cells.
5. Selection of hybrid cells is now carried out in HAT medium. By culturing in this medium (HAT = hypoxanthine-amethopterin-thymidine), the hybrid cells are separated from the other two cell types, i.e. the unfused myeloma cells and the antibody-producing cells.
Thus, after sometime only the hybrid cells are left in the medium. They have acquired the property of immortality from myeloma cells and the property of secreting a particular antibody from the antibody-secreting cell.
The resulting clones are screened for the production of antibody to the antigen of interest.
Monoclonal antibodies were first produced by Georges Kohler and Cesal Milstein in 1975, and they were awarded Nobel Prize in 1984.
Technically, production of human monoclonal antibodies is also possible. This is accomplished by fusion of human lymphocytes with human plasmacytoma cell lines.
Uses of monoclonal antibodies
Being more specific, the monoclonal antibodies bind antigen with increased avidity. Therefore, smaller quantity of monoclonal antibody is required. They target an antigen specifically, and so the reaction is more specific.
Chimeric monoclonal antibodies consisting of mouse variable regions and human constant regions are being made for use in treating human diseases such as leukaemia. Presence of the human constant region gives following advantages:
1. Activation of complement is possible with the human-derived constant region (whereas it is not if the constant region is mouse-derived).
2. Antibodies are not formed against the chimeric monoclonal antibody (whereas antibodies are formed if the constant region is mouse-derived).
Lately, chimeric antibodies have been found useful for eliminating tumour cells by complement mediated cytotoxicity.
IX More about Complement Cascade
The complement cascade is an amplifying cascade, similar to those responsible for blood clotting and fibrinolysis. As discussed earlier in Chapter 5, the complement system comprises a group of about 20 proteins, some of which are enzymes. The complement cascade involves stepwise activation, via proteolysis of these proteins till the formation of a lytic complex. Further information about complement components, activation pathway, general principles of reactions, and their clinical significance will be discussed in the present section. Generally, the term “complement” refers to the ability of this effector mechanism to complement, i.e. augment the effects of other components of the immune system, e.g. antibody. In fact, activation of complement system (a component of non-specific immune response) is one of the most important antibody effector functions of the specific immune response.
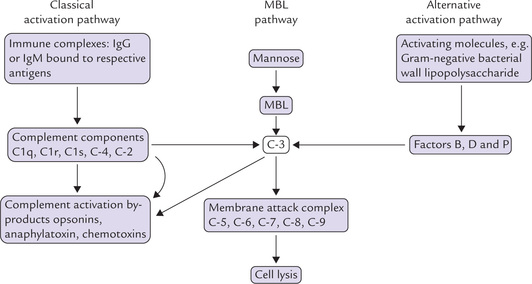
Activation of the complement cascade
This can be triggered in two ways: (a) by antigen-antibody complexes (i.e. the classical pathway), or (b) by bacteria in combination with several serum factors. (i.e. the alternative pathway). Both these lead to a common sequence of events, which result in formation of a lytic complex called membrane attack complex. The latter attacks the membrane with the help of tiny tubules, which results in appearance of holes in the cell membrane, and cell death ensues. In addition to the lytic effect, the complement system initiates specific cellular functions that mediate inflammatory response. These functions include attracting neutrophils and macrophages to an area where the foreign material is located, promoting phagocytosis and opsonization, etc.
Note
In addition to the classical and the alternative pathways, a mannose-binding ligand (MBL) pathway to complement activation has also been defined, which, like the alternative pathway, is triggered directly by man-nose, found in cell walls of fungi, bacteria and viruses.
Complement components
Approximately 20 proteins, that are present in normal human serum, comprise the complement system. They act in concert, and in an orderly sequence, to generate biologically active molecules, such as enzymes, opsonins, anaphylatoxins and chemotaxins. The complement components are designated as C1, C2, C3, C4, etc. but they do not act in the same sequence as their identification numbers. Most components are β-globulins made in liver, and have proteinase activity when activated.
Sequence of events in complement activation
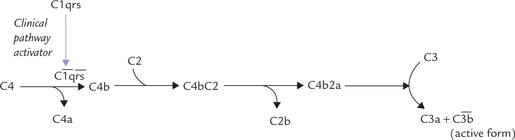
An outline of the important stages of complement activation is shown in the Figure 33.8. The activation of C3, the pivotal and critically important component, is an absolute requirement for full complement activation. There are three possible pathways to this activation: classical, alternative and MBL.
The classical pathway is triggered when immune complexes (IgG or IgM bound to its specific antigen) attracts the complement component C1 (made up of C1q, Clr and Cls). The latter binds to the constant region of the immunoglobulin and gets activated. The activated form is depicted as 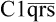 : the horizontal bar denotes activated form of a complement fragment. The activated C1 acquires proteolytic activity. It in turn cleaves and activates the next component, C4, splitting it into C4a and C4b. The latter then binds with C2 and cleaves it into C2a and C2b. C4b and C2a then act together on C3 and split it into C3a and C3b. The latter is the activated form of C3. These events are illustrated in Figure 33.9.
: the horizontal bar denotes activated form of a complement fragment. The activated C1 acquires proteolytic activity. It in turn cleaves and activates the next component, C4, splitting it into C4a and C4b. The latter then binds with C2 and cleaves it into C2a and C2b. C4b and C2a then act together on C3 and split it into C3a and C3b. The latter is the activated form of C3. These events are illustrated in Figure 33.9.
In the alternative pathway (and the MBL pathway), activation of C3 is achieved by materials such as bacterial cell walls and endotoxins. In their presence, C3 is slowly hydrolyzed to C3a and C3b (other proteins, e.g. factor B, P and D also participate in the activation). The alternative pathway may therefore, be particularly relevant before a primary immune response has been mounted.
Once activation of C3 is achieved, the terminal membrane attack complex, which comprises the components C5, C6, C7, C8 and C9 is generated. This complex eventually generates the polymeric ring structure that inserts into the cell membrane of bacteria and is responsible for cell lysis.
Biological activities of complement activation by-products
A number of biologically active molecules are generated during complement activation. Some of the important biological activities of these are as below:
1. C3a: Smooth muscle contraction, increase in vascular permeability, platelet aggregation.
2. C3b: Opsonization and phagocytosis.
3. C4a: Smooth muscle contraction and vascular permeability increase.
4. C5a: Smooth muscle contraction, vascular permeability increase, platelet aggregation, polymorph and monocyte chemotaxis, neutrophil hydrolytic enzyme release.
X The Recognition of and Response to Nonself
The challenge for the immune system is to be able to provide protection against the antigenic elements that arise as a result of the presence of an immunogen, or against foreign cells (both known as nonself) that invade the body. It must not react in this way with the body’s own proteins (known as self). Thus, a nonself structure must be recognized effectively for mounting an immune response against it.
Generation of specificity and diversity
Attributes of specificity and diversity that characterize all immune responses, apply to both B and T cells. In a given B-cell, for example, a unique specificity is generated by a combinatorial process, which involves random rearrangements of a series of gene segments encoding the sIg molecules. As a result, the B cell comes to express a single gene arrangement for the antibody’s heavy and light chains (Fig. 33.7), and so each B lymphocyte synthesizes antibody molecules of a single specificity, some of which are displayed as receptors on its membrane. Fine specificity of the antibody molecule is reflected in the incredible precision by which it can discriminate the protein antigens that may differ by only a single amino acid.
The distinct specificity of the antibody molecule is coupled to an enormous diversity—estimated to exceed 10 different antibody specificities. Diversity is also accounted by gene rearrangements, discussed above.
Clonal selection
How do antibodies arise? Does the antigen “instruct the B cell to make an antibody, or does the antigen “select” a B cell endowed with the pre-existing capacity to make the antibody. It appears that the latter alternative, i.e. clonal selection accounts for antibody formation. As discussed, the specificity of each T and B cell is determined prior to contact with the antigen: each lymphocyte expresses a unique receptor specificity, which is determined prior to the appearance of an antigen.
The antigen comes into picture only when it interacts with a (B or T) cell, which carries a matching receptor on its surface. After the antigen binds, cell is stimulated to proliferate and form a clone of cells, each with the same immunologic specificity as the original parent cell and the process is termed clonal selection.
Clonal selection occurs within both the humoral and cell-mediated branches of the immune system. In the humoral branch, antigen induces clonal proliferation of the antigen-reactive B cells into a clone of memory B cells and effector cells, called plasma cells. The plasma cells secrete antibodies reactive with the activating antigen.
Similar process occurs in T-lymphocyte population where antigen-MHC complex induces clonal proliferation into T-memory and effector cells (Fig. 33.2).
XI Cellular Cooperation in the Immune Response
For eliciting an effective immune response, interaction between various cell types is required during various events discussed below.
Antigen Processing and Presentation
Antigen is too large to be recognized in its entirety by either T- or B-cell receptors, therefore it is essential that antigen is processed and presented with a class II MHC molecule on the membrane of an antigen-presenting cell. Macrophages, interdigitating cells, dendritic cells and Langerhans cells are the major types of antigen presenting cells.
Activation and Proliferation of T-helper Cells
The antigen-dependent activation of T-helper (TH) cells is the central event in both cellular and humoral immune response. In fact, activation of the naive TH requires two signals, (i) binding with antigen-MHC complex, presented on surface of antigen-presenting cell (APC), and (ii) stimulation by IL-1.
As shown in Figure 33.10, binding of an antigen-MHC complex to a naive TH causes the APC to secrete interleukin-1 (IL-1). The TH cell is then induced (by IL-1) to undergo transition from G0 to G1 of the mitotic cycle and to secrete another cytokine, interleukin-2 (IL-2). The activated TH cell now starts expressing surface receptors of IL-2. The IL-2 binds to the receptor protein and induces further proliferation of TH. Interestingly, expression of IL-2 receptors is induced by IL-2 itself, which thereby acts in an autocrine fashion. A positive feedback loop is set up, which leads to a burst of proliferative activity of the TH cells. Pivotal role of IL-2 in this process has important clinical applications (Box 33.6).
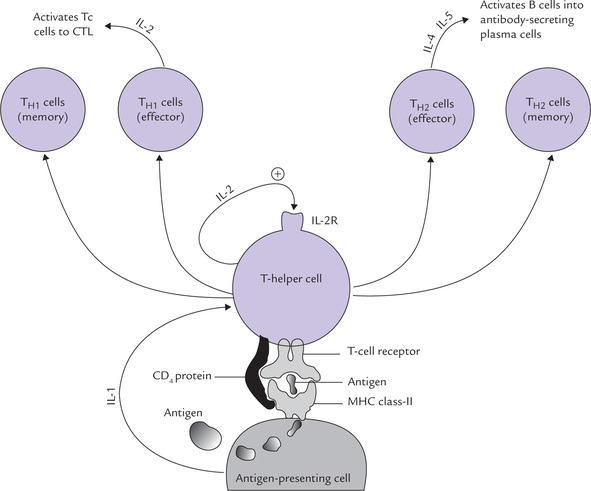
Fig. 33.10 Activation and subsequent effector roles of T-helper cell (TH) (IL-1 = interleukin, IL-2R = receptor for interleukin-2, CTL = cytotoxic T-lymphocyte).
The expanded population of the TH cells develops one of the two phenotypes (TH1 and TH2) characterized by the cytokines produced. The TH1 cells continue to secrete IL-2, and also begin to release interferon gamma, while TH2 cells switch to the production of IL-4 and IL-5. These changes relate to different functions as:
Generation of the Humoral Response
The naive B cell captures antigen with its surface immuno-globulin (sIg) as discussed earlier (Fig. 33.2). This is important for the activation of the B cell. In addition, IL-4 and IL-5 secreted by TH2 cells are also required for activation. The following series of events are known to occur:
1. The antigen bound to its surface immunoglobulinreceptor (sIg) is endocytosed by the B cell. Intracellularly, it is chopped into peptides, which are then re-presented on the cell membrane together with a class II MHC molecule (Fig. 33.11).
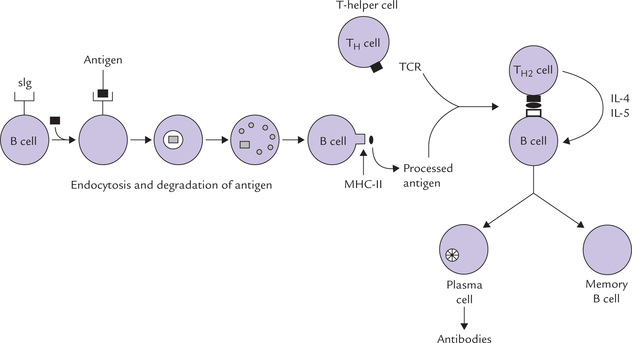
Fig. 33.11 Steps in conversion of naive B-cell into the antibody secreting plasma cell and the memory B cell (sIg = surface immunoglobulin, IL = interleukin, MHC = major histocompatibility complex, TCR = T-cell receptor).
2. The antigen/MHC complex is then recognized by the antigen-specific TH cells (in this way, the B cells themselves serve as antigen-presenting cells) and the interaction prompts the clonal expansion and activation of this TH into cytokine-secreting TH2 cells.
3. The cytokines IL-4 and IL-5 secreted by TH2 then activate the B cell. (The IL-1 secreted by macrophages also serves as a growth factor for B cell).
4. The activated B cell then undergoes a series of cell divisions over approximately a 5-day period, ultimately resulting in its transformation into a population of both antibody-secreting plasma cells and memory cells.
The requirement of lymphokines-induced B-cell proliferation helps explain why it takes 5 days for a primary immune response to be established. In secondary immune response there is advantage of having memory TH cells, which may initiate the response straight off.
Generation of the Cell-mediated Response
As in case of humoral response, an activated and clonally expanded population of antigen-activated, T-helper cells is crucial for generation of the cell-mediated response. Macrophages, which express the processed antigen in association with MHC II on their cell surface, serve as antigen-presenting cells (APCs) in the cell-mediated response. The T-helper cells recognize the processed antigen + MHC II, get transformed into effector TH1 cells, which secrete interleukin-2 (Fig. 33.10). The latter acts on the cytotoxic T cells (Tc) and transforms them into cytotoxic T lymphocytes (CTLs), which attack the infected self cell, e.g. virus infected cell (recall that such infected cell would present viral antigen along with MHC class I molecule on its cell surface).
The cell-mediated response to a virus infected cell is shown in Figure 33.12.
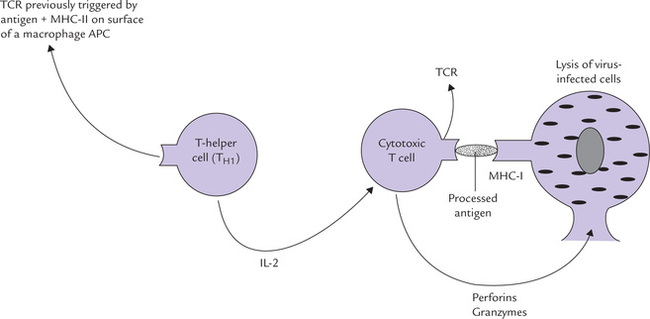
Fig. 33.12 Cooperation between T-helper cell and cytotoxic T cell in causing lysis of virus-infected cell (IL = interleukin, MHC = major histocompatibility complex, TCR = T-cell receptor).
The targeted cell is then killed by the secretion of several destructive proteins from CTL, the chief of which are the perforins and granzymes. The former undergo calcium-dependent polymerization in the extracellular fluid and this allows them to form channels in the plasma membrane of target cells. This in turn provides a route by which the proteolytic granzymes gain access to cells and destroy them.
XII Non-specific Effector Cells are influenced by TH Cells and Immunoglobulins
TH Influences activity of various non-specific effector cells
Activities of various non-specific effector cells, such as macrophages and natural killer cells, are enhanced by cytokines secreted by TH cells. These cells in turn play important roles in cell-mediated responses. Following examples will make this point clear.
1. Cytokines like interleukin-2 and interferon gamma, secreted by TH have been shown to activate macrophages, which in response exhibit enhanced ability to phagocytose the invading pathogens.
2. The natural killer cell is another non-specific effector cell whose activity is enhanced by interleukin-2 and gamma interferon. Natural killer cells, the large lymphocytes that lack the specific antigen-binding receptors of either B or T cells, is shown to kill tumour cells also.
Immunoglobulins provide specificity to non-specific effector cells
Both macrophages and NK cells have membrane receptors that can bind the Fc portion of the immunoglobin molecule. The Fab portion of the immunoglobulin can bind with the foreign cell. Thus an antibody can bind both to the foreign cell and the macrophage/NK, thereby acting as a bridge.
Simply stated, the C-terminal provides specificity and the N-terminal engages a non-specific cell which mediates the actual killing. For example, if immunoglobulin forms a complex with the corresponding antigen on surface of a solid particle (e.g. a virus or a bacterium) the antibody-coated particle can be phagocytosed by macrophages (or neutrophils). Such stimulation of phagocytosis, called opsonization has been discussed earlier in chapter 5.
Likewise, in antibody-dependent cell-mediated cytolysis (ADCC), the immunoglobulin bound to target cell acts as a tag for the natural killer cell. ADCC and opsonization show that the innate and the adaptive immune responses interact in a cooperative manner. More examples of such cooperation are illustrated in Box 33.7.
XIII Specific Immune Response to Some Infections
An interplay of both arms of specific immune system is often required for defense against the invading pathogen. However, the relative importance of humoral versus cell-mediated immunity varies with the type of infection. In infections with pyogenic bacteria, humoral immunity is more important; whereas protection against viral and intracellular bacterial infections is dependent on cellmediated immunity. Individuals with antibody deficiencies are therefore prone to recurrent infections with pyogenic bacteria and those with defective T-cell functions suffer from severe limitations in controlling viral infections. They also show increased susceptibility to mycobacteria. Since it is impossible to discuss immune response to all pathogens, two illustrative examples to discuss immune responses to mycobacteria and HIV are given, and immunopathogenesis of these infections outlined. (The undergraduates may skip rest of this section.)
A Mycobacterial Infection
Mycobacterium tuberculosis is an obligate intracellular, slow growing bacteria, which produces no toxins and causes disease only by challenging body’s immune defense mechanisms coupled with an inexorable increase in number. Around one third of the world’s population is infected with this pathogen, which is responsible for causing 3 million deaths worldwide per year. Infection commonly occurs by inhalation. On entry into the body, mycobacteria are promptly taken up by macrophages, processed and presented to the T cells (CD4+ and CD8+).
Presentation of mycobacterial antigens to T cells triggers clonal expansion and release of TH1 cytokines, e.g. Y-IF, TNF and IL-2. This leads to macrophage activation and granuloma formation (granuloma is made up largely of macrophages, which have fused to form giant cells and also differentiated to epithelioid cells). These changes enable the immunocompetent individuals to contain and effectively eliminate the organism.
Mycobacteria have evolved clever strategies to evade the host immune response and survive inside macrophages, which thereby shelter bacteria and act as reservoirs of infection. Sheltered within macrophages, the mycobacteria can survive for several years. Development of active disease (tuberculosis) occurs either when the host response is attenuated or when exposure to certain risk factors occurs, or both. Some important risk factors are advanced age, alcoholism, drug abuse, malnutrition or impaired cellular immunity (e.g. by immunosuppressive therapy or HIV virus infection). The T-cell response is inadequate for the infection caused by the reactivated pathogen, and the gran-ulomatous response is only partially able to contain it. An area of progressive granulomatous inflammation develops which slowly distorts the surrounding structures by fibrosis. In severe cases, macrophages are induced (by mycobacteria and by ineffective T-cell response) to persistently secrete cytokines, resulting in systemic ill health.
B Human Immunodeficiency Virus (HIV) Infection
Disease associated with retroviruses HIV types 1 and 2 infection is characterized by major defects in immunity, and selective elimination of CD4+ lymphocytes and macrophages. Profound weakening of immune system because of depletion of the CD4+ cells leaves the patient vulnerable to a spectrum of disorders from a transient, acute glandular fever-like illness to life-threatening vascularized neoplasms, and severe, disseminated, opportunistic infections. Acquired immune deficiency syndrome (AIDS) is the final stage in the progression of infectious disease caused by HIV. It has been suggested that as many as 8-10 million persons have been infected with HIV, 70% of which are accounted by heterosexual transmission, with severe implications for the infants born to HIV-positive mothers.
HIV viruses enter susceptible cells though binding of viral envelope glycoprotein to specific receptors on the cell surface. Any cell bearing the CD4+ antigen is capable of being infected by HIV; typically these are T-helper cells, but some B cells, macrophages and glial cells of the central nervous system also express CD4, even in low amount. HIV can also enter through binding of betachemokine receptor (CCR5) or by fusion between the viral lipid envelope and the target cell membrane. The RNA viral genome is copied into a DNA form by a viral enzyme termed reverse transcriptase and the latter is integrated into the genome of the infected cell, where it may remain latent. Once reactivated, the DNA is used as a template for the RNA required for virus production. New virions are produced at exceedingly high rate of 109-1010 virions per day. The host immune response finds itself unequal to the challenge posed by HIV because of the following reasons:
1. Precipitate fall in the absolute number of the TH cells follows the HIV infection. This results in profound immunosuppression.
2. HIV has intrinsically unstable epitopes for antibody to bind to. It makes the production of functionally neutralizing antibody by B cell extremely difficult. The same applies to the T cells as well.
3. Exceedingly fast rate of replication of HIV is coupled with as many as 108 mutations per day.
Finally, laboratory evaluation of AIDS patients typically demonstrates lymphopenia, selective depletion of T lymphocytes, and marked reduction in T- to B-cell ratio. Serum immunoglobulin levels are often elevated, but specific antibody production in response to antigen challenge is deficient. Once AIDS has developed, prognosis is very poor but median survival varies with viral load (i.e. the HIV-RNA in the blood) at presentation.
XIV Vaccines
Since the first use of vaccine by Jenner against small pox two centuries ago, deliberate stimulation of an immune response by immunization or vaccination has led to virtual elimination of several diseases like small pox, and has been one of the most effective measures in controlling infectious diseases. Yet suitable vaccines for a large number of infectious diseases like malaria, tuberculosis, AIDS, etc. are still to develop. Rapid advances in immunology and recombinant DNA technology are brought into use for the development of safe and effective vaccines and important breakthrough towards these goal have been made in recent years.
Vaccines are attenuated or killed organism, antigenic component of disease causing microbe, toxins produced by bacteria or DNA coding for an antigen which are deliberately administered to an individual to protect him from a host of infectious disease. The process of deliberate administration of a vaccine is known as vaccination. An ideal vaccine should mimic the immunological stimulus associated with natural infection, have no side effects, be readily available, cheap, easily administered and should provide long lasting immunity. There are certain immunological requirements of an ideal vaccine. It should activate antigen-presenting cells to initiate antigen processing followed by cytokines production and activation of both T and B cells, leading to formation of neutralizing antibody and development of memory cells responsible for resisting subsequent infections. Generation of T cells to several epitopes in order to overcome antigenic variation of pathogen and persistence of antigen on follicular dendritic cells in lymphoid tissue where memory B cells are recruited are the other characteristics of an ideal vaccine.
Types of Vaccines
Inactivated or killed vaccine
Killed or inactivated microbes have been widely used as vaccines. Vaccines for cholera, pertussis, typhoid, polio (Salk), etc. are made out of killed bacteria or virus. Although these vaccines are safe, very often they fail to evoke immune response as observed during natural infection. Killed microbes cannot produce proteins in the cytosol and so antigen processing does not take place and the cytotoxic T cells are not generated by these vaccines.
Live attenuated vaccine: Organisms attenuated to decrease virulency either by growing in culture medium so that they replicate poorly in human cells or by recombinant DNA technology where the segment of gene for virulency is either deleted or mutated. These attenuated microbes on administration do not cause any disease, yet they are highly immunogenic because the replication of the living microbes provides the host with larger dose of antigen required for developing cytotoxic T cell memory through antigen presentation. BCG, polio (Salk), MMR (measles, mumps and rubella) vaccines are made out of live attenuated microorganism. In spite of the general acceptance of attenuated vaccine, it has some demerits as well. It may induce disease to immune compromised hosts and also has the possibility of the virus strain to reacquire virulence.
Toxoid vaccine: Toxins released by microbes like C. diphtheria, B. pertussis, C. tetani are mostly responsible for the disease stage. Purified inactivated toxins are not very effective as vaccines. Mixed with suitable adjuvant (e.g. tetanus toxoid along with aluminium salt) they serve as effective vaccines. DPT triple vaccine is a mixture of diphtheria, tetanus and pertussis toxins where the latter also serves as an adjuvant.
Subunit vaccine
Vaccination with whole organism at times may elicit poor immune response and even hyper-sensitivity due to the presence of large number of antigens which are not associated with protective immunity. Identification and isolation of protective antigen from an organism and the use of these immunogenic sub-units as vaccines is known as subunit vaccine. When such an immunogenic peptide is formed artificially by the use of recombinant DNA technique they are called recombinant vaccines. These vaccines are safe, and protective. Commercially available recombinant vaccines have been developed for hepatitis B.
DNA vaccine
Immune response can be induced by injecting DNA encoding a protective antigen and is known as DNA vaccine. A viral gene coding for a core or capsid protein is inserted into a plasmid. The plasmids are injected into the host cells from where the they migrate to the nucleus. Viral proteins are expressed in the cells producing both cell-mediated and humoral immune response.
XV Immunological Techniques
A Precipitin Reaction
When an antigen solution is mixed with a specific antiserum, they form an antigen-antibody complex which may precipitate out. The amount of precipitation depends on (a) relative concentrations of the antigen and the antibody, and (b) valency of the antigen. The concentration at which maximum precipitation occurs is known as equivalence point. The two common techniques which are based on precipitin reaction are immunodiffusion and immunoelectrophoresis.
In immunodiffusion antibody, mixed with agar is layered on a glass slide. A number of wells were cut on the gel and antigen at different concentration are added to it. Slowly, antigen diffuses from the well, its concentration falls and when it reaches the equivalence point it combines with antibody and forms precipitin ring. The diameter of the precipitin ring is proportional to the antigen concentration. This method is used routinely in clinical immunology for quantitation of immunoglobulins, complement components, Transferrin, C-reactive protein, α-fetoprotein, etc.
Electrophoresis of a mixture of antigen in gel and their identification by the use of specific antisera is the principle behind immunoelectrophoresis. In this technique, sample containing mixture of proteins is subjected to electrophoresis in agar gel. Proteins migrate to various positions in the gel. Now antisera to a specific protein is added into a horizontal trough made in agar gel. Development of precipitin arc identifies the presence of the protein of interest. A modified version of immunoelectrophoresis known as rocket immunoelectrophoresis is a quantitative technique which involves electrophoresis of antigen into a gel containing the antibody. The precipitin arc has the appearance of a rocket, the length of which is related to antigen concentration.
Antigen concentration can also be assayed by nephelometry. When an antigen is added to a solution of excess antibody, small aggregates of antigen-antibody complex (not precipitation which is formed when antigen and antibody are present at equivalence) is formed which creates a cloudiness or turbidity. This can be measured by forward scattering of a monochromatic incident light with the help of a nephelometer. Nowadays, nephelometry is replacing immunodiffusion for the measurement of immunoglobulins, CRP, C3, etc.
B Agglutination
When an antigen is displayed of the surface of a large particle such as bacteria, RBC, etc., addition of antibodies cause antigen to clump or agglutinate. This phenomenon is known as agglutination and, is used in most qualitative serological assays. When RBC is the antigen, the term haemagglutination is used. Haemagglutination is the basis of determining blood groups.
C Radioimmunoassay (RIA)
RIA is commonly used to measure the level of hormones in blood and tissue fluids. For estimation by RIA one should have a pure preparation of antigen or antibody. In RIA for antigen, pure antibody against it is radiolabelled usually with I125. The unlabelled antigen present in the sample is allowed to attach to a solid support such as plastic tube/wells of a microtiter plate. Now labelled antibody is added and allowed to react with the antigen attached to the solid surface. Any unbound antibody is removed by washing. The amount of radioactivity retained in the tube/wells gives a measure of the amount of the antigen present in the sample.
D Enzyme Linked Immunosorbent Assay (ELISA)
ELISA is used in clinical diagnostics for assaying hormones, cytokines, viral antigen/antibody, etc. In ELISA, an enzyme (usually peroxidase, phosphatase, etc.) is linked chemically to the antibody. Antigenic component to be assayed is allowed to attach to the solid surface of an ELISA plate. Now enzyme linked antibodies are allowed to bind to the antigen followed by washing of excess antibody. Addition of a suitable substrate is converted to a colour reaction product which can be read in an ELISA reader.
A modified form of ELISA known as sandwich ELISA is used to detect cytokines, etc. Instead of antigen being directly attached to ELISA plate, antigen specific antibodies are bound to the plate. These are able to bind antigen (component to be assayed) with high affinity. A separate enzyme linked antibody that recognizes a different epitope of immobilized first antibody is then used to detect bound antigen.
XVI Immunologic Dysfunction
Immune system is mostly beneficial but some abnormalities in quality, quantity or direction of the response in certain situations may lead immunodysregulation, which may have hazardous consequences:
A Immunodeficiency Disorders
These are an important group of immunologic dysfunctions where a component of immune system, specific or nonspecific, may be absent. This makes the affected individual more susceptible to infections. The immunodeficiency may be primary (inherited) or secondary (acquired).
The primary disorder may deplete humoral or cellular immunity, or both may be affected. In congenital agammaglobulinaemia, an X-linked disease, the humoral immune response is impaired but the cell-mediated response is unaffected. An absence of B cells is compatible with a normal lifestyle so long as infusions of immunoglobulin G are maintained.
In DiGeorge syndrome, cell-mediated response is impaired because the thymic processing of the T cells does not occur due lack of thymus. Severe combined immunodeficiency disease (SCID) severely impairs both humoral and cell-mediated responses because of absence of functional T cells. It is severe because it is fatal and it is combined because in humans B cells cannot function without help from T cells, so that even if the B cells are not primarily affected by the defect, humoral response is impaired.
B Allergy
When immune response results in exaggerated or in appropriate reactions harmful to the host, the term hyper-sensitivity or allergy is used. Clinical manifestations of these reactions are typical in a given individual and occur on contact with the specific antigen to which the individual is hypersensitive. The first contact of the individual with the antigen sensitizes, i.e. induces the antibody, and then subsequent contacts elicit the allergic response.
Tendency to develop allergic response is inherited by some individuals. They have high level of IgE (also termed reaginic antibody) in their blood. This antibody mediates allergic reactions. It binds avidly to the surface of basophils and mast cells, where it acts as an antigen receptor. The binding of an allergen (antigen capable of generating allergic response) to surface IgE induces the release of stored histamine from the cell. Most of the allergic symptoms are mediated by the released histamine, and most antiallergic drugs act by blocking the effects of histamine on its target cells.
Hypersensitivity reactions can be subdivided into four major types: type I, II and III are antibody-mediated, whereas type IV is cell-mediated. These diseases are included in the discipline of clinical immunology and immunopathology. More information on these is beyond the scope of this book.
C Autoimmunity
One particular aspect of immunodysregulatory disorders is that of self-reactivity (i.e. autoimmunity). Normally this is avoided because of intricate controlling and suppressor mechanisms. Included among such mechanisms is the process of thymic education by positive and negative selections. The other processes which eliminate the self-reactive clones are clonal selection and anergy (the T cells recognizing a self-antigen is likely to be rendered incapable of further response; the process is referred to as anergy and constitutes an extension of self-tolerance initiated in the thymus). It is quite likely that an error may occur in one of these complex processes, which help a meticulous discrimination between the self and the non-self. The error may creep in spontaneously or be induced by some exogenous factor such as viral infection, often in a genetically predisposed individuals. The result will be an immune response directed against “self” and the inflammatory damage, and the ensuing condition is referred to as autoimmunity.
A wide spectrum of autoimmune diseases is known to range from the organ-specific to non-organ-specific. The reaction against ubiquitous antigens lead to non-organ-specific autoimmune diseases, whereas the reactions to unique components of individual tissues and organs result in organ-specific diseases. The former are best exemplified by systemic lupus erythematosus (SLE), in which the apparent target antigens are common to all nuclei. Consequently, damage is seen in a variety of tissues. The organ-specific disorders are exemplified by autoimmune thyroiditis, where the apparent target is thyroid peroxidase. Consequences may vary in severity from trivial to fatal.
There are a number of diseases associated with particular MHC antigens. For example, presence of DR2 is associated with increased frequency of multiple sclerosis, and DR3 or B8 are associated with myasthenia gravis, or thyrotoxicosis. Occasionally, the MHC status appears to be protective rather than associated with increased frequency of a disease. These associations are of great interest scientifically, but rarely of value in clinical practice.
Exercises
Essay type questions
1. Enumerate various types of lymphocyte populations and describe their roles in invoking specific immune response. Why are the T-helper cells said to occupy a central place in all immune responses.
2. Discuss the steps in synthesis of immunoglobulins by naive B cell following an antigenic challenge. Mention briefly the disorders related to immunoglobulins.
3. Discuss three ways by which immunoglobulins participate in the host defense. Explain the mechanisms by which human genome can direct synthesis of limitless variety of immunoglobulin molecules.
4. Explain with the help of suitable examples as to how innate and adaptive immune responses interact with each other. Why are both arms of immune responses— humoral as well as cell-mediated—affected in severe combined immunodeficiency disease?
5. What is a subunit vaccine? Discuss strategies involved in production of a bacterial vaccine.
 Immune response to nonself elements, the antigens, involves a number of components. Some of these show unique specificity for the particular stimulating antigen(s) and comprise the adaptive immune response.
Immune response to nonself elements, the antigens, involves a number of components. Some of these show unique specificity for the particular stimulating antigen(s) and comprise the adaptive immune response.