Chapter 34 The Thyroid Gland and Antithyroid Drugs
The thyroid hormones thyroxine and tri-iodothyronine increase oxygen consumption and basal metabolic rate; accelerate carbohydrate, lipid and protein metabolism; increase sensitivity to sympathetic stimulation and promote growth; and are required for normal development of the central nervous system. Disorders of thyroid function thus have major effects on virtually all aspects of bodily functions, including growth and development, energy levels and nervous and reproductive systems.
In this chapter, the synthesis, actions and control of thyroid hormones and the pathologies associated with hypothyroidism and hyperthyroidism are briefl y reviewed, and the uses of thyroid hormones in tests for assessment of thyroid function are described. Replacement thyroid hormones are useful in treating hypothyroidism, and iodine (iodide ion), radioactive iodine and thiourea (thionamide) drugs in treating hyperthyroidism.
Key abbreviations
T3 tri-iodothyronine (liothyronine)
T4 tetra-iodothyronine (thyroxine)
Key background: the thyroid gland
Anatomy and functions
THE thyroid gland, one of the most richly vascularised tissues of the body, is located in the throat region, in front of the trachea (see Figure 33-1). It has two lateral lobes, linked by a narrow central section. (The small parathyroid glands (usually four) are located on the posterior surface of the thyroid lobes.) The thyroid lobules contain follicles full of a viscid colloid secretion, enclosed by follicular cells, which produce thyroxine (tetra-iodothyronine, T4) and tri-iodothyronine (T3).
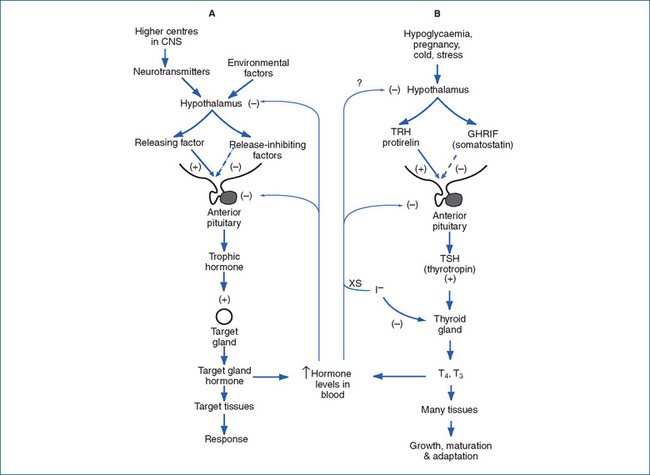
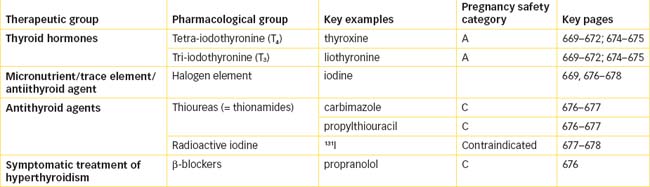
Figure 34-1 Secretion and control of thyroid hormones. A General control mechanisms for hormone secretion. B Control mechanisms for thyroid hormone secretion. Environmental factors influence secretion of the hypothalamic factors thyrotrophin-releasing hormone (TRH) and growth hormone release-inhibiting factor (GHRIF, somatostatin) to increase or decrease release from the anterior pituitary of thyrotrophin (TSH), which stimulates production in the thyroid glands of thyroxine (T4) and tri-iodothyronine (T3).
The gland also contains parafollicular cells, the source of calcitonin. Because of its role in calcium metabolism, calcitonin is discussed in the chapter on the parathyroid gland and calcium balance (Chapter 37). In this chapter, however, we generally use the term ‘thyroid hormones’ to refer only to T4 and T3 and not to calcitonin.
The history of discovery of thyroid gland functions and hormones is interesting. In 1883, it was found that cretinism and myxoedema might result from loss of thyroid function. (These conditions are described later in the section on hypothyroidism.) Very soon afterwards, experiments showed that extracts of thyroid glands could reverse the effects of thyroidectomy, i.e. that active substances found in the gland could be circulated and act elsewhere in the body and affect many aspects of homeostasis, growth and metabolism. In 1914, the main thyroid hormone, T4, was purified and crystallised, allowing detailed studies of its actions and uses. It was not until 1952, however, that T3 was discovered, and not until 1961 that the actions of calcitonin were demonstrated. The roles and clinical uses of the hypothalamic factors, including thyrotrophin-releasing hormone (TRH), are still being studied and clarified.
Thyroid hormones
Synthesis, release and control
Thyroxine (T4) and tri-iodothyronine (T3) are amino acid hormones, being iodinated derivatives of tyrosine. They are usually stored in the thyroid gland and circulated in the bloodstream bound to proteins. Control of thyroid hormone levels is complicated, involving a complex negative-feedback mechanism between the thyroid gland and the hypothalamic–pituitary axis. Hormone levels also depend on iodine levels and body temperature (see Figure 34-1). Selenium is required at trace elements levels as a cofactor in the biosynthesis and function of a number of seleno-proteins involved in thyroid hormone metabolism and gland functions.
Negative feedback control
High levels of circulating thyroid hormones activate the typical negative-feedback loops, by inhibiting the synthesis of genes for both protirelin (thyrotrophin-releasing hormone, TRH) and anterior pituitary thyroid-stimulating hormone (TSH, thyrotrophin) at the transcriptional level. This inhibits synthesis and release of TSH, thus overall reducing production of thyroid hormones.
Role of thyrotrophin (TSH)
Low levels of circulating thyroid hormones increase the release of TSH from the pituitary gland and appear to influence the secretion of TRH from the hypothalamus.
TSH binds to thyroid cells (and cells of thyroid tumours) and, via activation of adenylate cyclase and phosphorylation of enzymes, then stimulates many aspects of thyroid gland function, including:
In the long term, an increase in TSH leads to both thyroid hypertrophy (greater size of cells) and hyperplasia (greater number of cells). Thyrotrophin alfa, a recombinant form of human TSH, is used in testing for remnants of thyroid cancers after thyroidectomy surgery.
Role of iodine
The negative-feedback mechanism responds only slowly to changes in iodide levels: low levels make it difficult for sufficient hormones to be synthesised, while excessively high levels switch off production, via negative feedback and by acting directly at the organification step in hormone synthesis.
Iodine is a non-metallic element in the halogen group, with an atomic mass of 127; it is essential for thyroid hormone synthesis. Around 1 mg iodine is required by an adult each week; most of this is ingested in food, water and iodised table salt (see Clinical Interest Box 34-1). An iodide pump takes up iodide from the extracellular fluid, traps it and concentrates it to many times the level found in plasma, thus the thyroid gland normally contains virtually all the iodide in the body. The ratio of iodide in the thyroid gland to that in the plasma (serum) is expressed as the T/S ratio; normally this ratio ranges from 20:1 to 39:1. (In hypoactivity of the gland the ratio may be 10:1; in hyperactivity it may be as great as 250:1.)
Clinical interest box 34-1 Disorders due to iodine deficiency
If iodide intake is low, thyroid hormones cannot be synthesised in sufficient quantities, and thyroid functions are impaired.
Adapted from: Hetzel 2000; Delange & Lecomte 2000; Zimmerman 2009.
Synthesis of T4 and T3
The synthesis, storage, release, secretion and circulation of the hormones are complicated—during the process, the scene of action moves from the bloodstream into the follicle cell, thence into the follicle lumen, back into the cell and finally into the blood again. A summary of the processes involved is given below and in Figure 34-2, and the control mechanisms are shown diagrammatically in Figure 34-1.
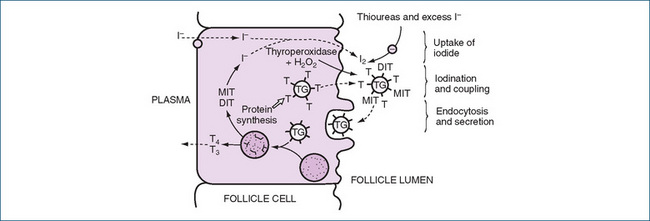
Figure 34-2 Synthesis of thyroid hormones. Iodide is taken up from the bloodstream into the thyroid cell, where it is bound to tyrosine residues (T), then coupled to form thyroid hormones, which are stored as thyroglobulin (TG) in the follicle lumen before release into the circulation (see text for details and abbreviations).
Adapted from: Rang et al 2003, Figure 25-1; used with permission.
Mechanism of thyroid hormone actions
Tri-iodothyronine, the main active intracellular hormone, enters the nucleus of target cells and binds to specific receptors that act as transcription factors to activate or repress various genes. (There are at least three forms of the thyroid hormone receptor.) Production of mRNA is modified, and hence synthesis of specific proteins, e.g. Na+–K+-ATPase, is altered. Thyroxine, T4, has the same mechanism and physiological actions, although T3 is more potent than T4.
Physiological effects of thyroid hormones
The thyroid hormones T4 and T3 have a diffuse effect and do not have one specific target organ; no special cells or tissues appear to be particularly affected by the thyroid hormones. They are not necessary for survival under normal conditions, although reduced levels can affect quality of life.
Overall, T4 and T3 increase oxygen consumption and basal metabolic rate; accelerate carbohydrate, lipid and protein metabolism; promote normal gastrointestinal tract, cardiovascular, reproductive and temperature regulation functions; increase sensitivity to sympathetic stimulation; promote growth and development; and are required for normal development and functioning of the CNS. The growth-promoting actions of thyroid hormones are said to be ‘permissive’, i.e. a normal thyroxine level permits the cells of the body to function properly. Children who develop hypothyroidism after birth have increasingly slow bodily growth and delayed maturity (see Figure 34-3).
Figure 34-3 Effect of thyroxine on growth: response of a 6-year-old hypothyroid girl treated with thyroxine. Open circles indicate bone age. The short stature caused by hypothyroidism starting insidiously in childhood is readily reversed by regular exogenous administration of thyroxine, and the catch-up growth is usually marked and complete, as shown here.
Source: Tanner 1990. Reproduced with permission from Castlemead.
The long delay in onset of action of thyroid hormones and their prolonged duration of action rule them out as minute-to-minute regulators of physiological function. Instead, their role is more likely to be that of establishing and maintaining long-term functions such as growth, maturation and adaptation.
Pharmacological treatment of hypothyroidism
Hypothyroidism and goitre
Simple goitre
Prolonged iodine deficiency in the diet results in increased TRH and TSH, and hence enlargement of the thyroid gland, known as a simple goitre. The enlarged thyroid scavenges residual traces of iodine from the blood. This type of goitre (simple or non-toxic) can be prevented by providing an adequate supply of iodine. Iodine is not abundant in most foods, except fish and seafood, so iodised salt is frequently the primary source of iodine in areas where seafood is expensive or not readily available.
Note that the presence of goitre is not diagnostic, as an enlarged thyroid gland may also be due to excessive stimulation of the gland in thyrotoxicosis.
Hypothyroidism
Pathology
Hypothyroidism is common, occurring in about 2% of the population, especially in middle-aged and elderly women; it is associated with autoimmune disorders, previous Graves’ disease (and antithyroid therapy) and Down syndrome. Aetiological factors include post-thyroidectomy or radiation therapy, iodine deficiencies (simple non-toxic goitre), Hashimoto’s disease (autoantibodies to thyroid antigens), antithyroid drugs and also lithium, amiodarone, iodine-containing contrast media and kelp products. Patients with primary hypothyroidism have low T3 and T4 levels despite an elevated TSH level. The condition can easily be missed, as it has very variable and non-specific presentations and development is usually insidious; the TSH stimulation test is diagnostic (see Clinical Interest Box 34-2).
Clinical interest box 34-2 Diagnostic testing for hypothyroidism
TRH and TSH are used as diagnostic agents for assessing thyroid function. The TSH stimulation test is a very sensitive test used to diagnose and differentiate types of hypothyroidism. TRH is administered to assess the pituitary’s response to TRH.
Thus the TSH stimulation test can differentiate a primary from a secondary hypothyroidism, and differentiate hypopituitary from hypothalamic hypothyroidism. See Figure 34-1 for an illustration of this thyroid feedback mechanism.
There are several other thyroid function tests, including the free T4 index (FTI), T3 resin uptake (T3RU) test and total or free (unbound) T4 levels; the tests are used to determine the exact site of the dysfunction and hence to optimise therapy.
Drug interactions 34-1 Thyroid hormone preparations
| Drug | Possible effects and management |
| Anticoagulants, oral (warfarin or phenindione) | Thyroid hormones can enhance the therapeutic effects of the oral anticoagulant: a decrease in anticoagulant oral dosage may be required. Monitor coagulation time closely, using the international normalised ratio (INR) |
| Cholestyramine, ciprofloxacin, colestipol, alumin ium hydroxide, calcium carbonate, ferrous sulfate, raloxifene and sucralfate | These drugs reduce the absorption of thyroxine from the gastrointestinal tract. A 2-hour interval is recommended between administration of these drugs and thyroxine |
| Sympathomimetics and tricyclic antidepressants | The effects of one or both medications may be increased; cardiovascular adverse reactions can result. Monitor closely, as dosage adjustments may be necessary |
| Imatinib, rifampicin, oestrogen-containing contraceptives | May increase metabolism of thyroxine; dose may need to be increased |
Clinical manifestations
Clinical manifestations are seen in most systems of the body and include bradycardia, infertility, muscle pain, cold intolerance, lethargy, husky voice, ‘non-toxic goitre’, hair loss and neurological and psychiatric problems. There is decreased clearance of many drugs and increased sensitivity to digoxin. Severe hypothyroidism in the adult is called myxoedema, referring to the thickened skin caused by acid mucopolysaccharide accumulation. In the last stage of longstanding, inadequately treated or untreated hypothyroidism, coma sets in accompanied by hypotension, hypoventilation, hypothermia, hyponatraemia and hypoglycaemia.
In children
Hypothyroidism in a young child (formerly known as cretinism) is characterised by slowed physical and mental development, which leads to dwarfism and mental retardation. The condition may result from faulty development or atrophy of the thyroid gland during fetal life and may be caused by lack of iodine in the mother (see Clinical Interest Box 34-1). In congenital hypothyroidism, thyroid hormone levels equal to or above those required for the adult must be established immediately after birth to prevent permanent mental and physical retardation.
In elderly patients
In elderly people, hypothyroidism is the second most common endocrine disease; it is often missed or misdiagnosed. Only one-third of the geriatric patients exhibit the typical signs and symptoms of cold intolerance and weight gain. Most often, the symptoms are non-specific, such as failing to thrive, stumbling and falling episodes and incontinence. If neurological involvement has occurred, a misdiagnosis of dementia, depression or a psychotic episode may be made. Laboratory tests for plasma T4 and TSH are used to confirm hypothyroidism.
Treatment
Therapy is simple: for life-long thyroid replacement the thyroid hormones are safe, stable, cheap and available orally; dosage regimens can be adjusted in response to thyroid function test results.
Thyroxine is the usual drug of choice, 100–200 mcg daily, with dosage adjusted upwards in pregnancy and possibly decreased in the elderly and before surgery. In myxoedema coma, a medical emergency, treatment is with T3, the more potent and rapidly acting hormone.
In children who develop hypothyroidism, the delay in growth and maturity can be reversed by administration of T4. There is a rapid catch-up growth spurt, and eventually the expected adult height is attained (see Figure 34-3, and compare with Figure 33-5).
Elderly patients are usually more sensitive to, and experience more adverse reactions (particularly cardiovascular effects) to, thyroid hormones than other age groups, so it is recommended that thyroid replacement doses be individualised, with lower doses than usual and slower dosage adjustments.
Thyroid hormone preparations
For many years, natural extract or desiccated thyroid tissue was used for replacement therapy, but the synthetic thyroid hormones available today are better standardised and more stable formulations, and so are generally prescribed. The preparations available in Australia are: thyroxine (levothyroxine, T4; Drug Monograph 34-1) and liothyronine (tri-iodothyronine, T3); the latter hormone is more potent and has a shorter half-life, so is preferred for emergency and short-term use. Table 34-1 illustrates the usual adult dosing schedules for thyroid products.
Drug monograph 34-1 Thyroxine sodium
Levo-thyroxine (thyroxine) is the thyroid T4 hormone given exogenously as a drug; it has all the chemical and pharmacological properties of the natural hormone.
Indications
Thyroid supplements are indicated for the treatment of hypothyroidism, treatment and prevention of goitre, replacement therapy after thyroid block in hyperthyroidism and treatment of thyroiditis and thyroid carcinoma (high doses for suppressive effects).
Pharmacokinetics
Thyroxine is adequately absorbed from the gastrointestinal tract (50%–75%) and is highly protein-bound in the circulation; there is some enterohepatic recycling. The plasma half-life is about 6–7 days in euthyroid people, and the duration of biological effect is much longer, so steady state may not be reached for 3–4 weeks; response to altered dosage is slow. It is metabolised in the same way as endogenous thyroid hormone—some in peripheral tissues and smaller amounts in the liver—and metabolites are excreted in bile and urine. Thyroxine can be dosed once daily and is given on an empty stomach, usually before breakfast.
Adverse reactions
Adverse effects generally correspond to symptoms of hyperthyroidism: tachycardia, elevated temperature, diarrhoea, hand tremors, increased irritability, weight loss and insomnia. A rare adverse reaction is an allergic skin rash. Adverse effects are dose-related and may occur more rapidly with T3 than with T4, mainly because the former has a faster onset of action.
The general signs of underdosage are those of hypothyroidism: coldness, dry skin, constipation, lethargy, headaches, drowsiness, tiredness, weight gain and muscle aching. During the early period of treatment, hair loss may occur in children.
Warnings and contraindications
Use with caution in patients with diabetes mellitus, adrenocortical or pituitary insufficiency, cardiac disease and malabsorption problems. Avoid use in people with hyperthyroidism, thyrotoxicosis or thyroid hypersensitivity. Requirements increase during pregnancy, and dosage should be adjusted depending on TSH levels.
Dosage and administration
See Table 34-1. Thyroxine tablets are available in a range of doses (50, 75, 100 and 200 mcg), which allows easy adjustment of doses. The stability of thyroxine tablets is limited; patients need to watch the ‘use by’ dates on their packs (see Roberts 2004). Storage at 2–8°C is generally recommended (currently tablets can be stored at 25°C for up to 21 days).
Table 34-1 Thyroid preparations: adult dosing schedules
| DRUG | AVERAGE DAILY DOSE | ADULT DOSAGE SCHEDULES |
| Thyroxine | 100–200 mcg (0.1–0.2 mg) | Orally: initially 50–100 mcg daily. Dose changes should be considered only every 3 to 4 weeks, based on TSH tests. Maintenance dose is lower in elderly people. Fine dose adjustments can be achieved with alternating doses of 50, 100 or 200 mcg tablets. |
| Liothyronine | 20–60 mcg (0.02–0.06 mg) | Orally: 20–60 mcg daily, in 2–3 divided doses. For myxoedema coma, treatment may be initiated with 60 mcg by stomach tube (under specialist supervision). For maintenance therapy of myxoedema, thyroxine is preferred. |
Pregnancy safety for both thyroid products has been established as category A.
The goal of treatment of patients with hypothyroidism or myxoedema is to eliminate their symptoms and restore them to a normal physical and emotional state (i.e. render them euthyroid). Clinical response is more important than blood hormone levels; laboratory assay of TSH is used to assess adequacy of therapy and compliance. Because of the long half-life and slow response time for T4, plasma TSH concentrations are measured 2, 4 and 10 months after initiation of therapy, and annually thereafter for adults.
Thyroid hormones are also used as replacement therapy after thyroidectomy (near-total or total) as treatment for thyroid cancers: T4 not only replaces missing hormone, but also activates the negative-feedback loop and thus suppresses pituitary release of TSH, which would stimulate any remaining thyroid cancer cells.
Pharmacological treatment of hyperthyroidism
Hyperthyroidism (thyrotoxicosis)
Pathology
Excessive formation of the thyroid hormones and their release into the circulation result in thyrotoxicosis; this occurs in conditions including toxic multi-nodular goitre, toxic hot nodule, exophthalmic goitre (Graves’ disease), subacute thyroiditis and as an adverse reaction to some drugs (iatrogenic causes), including thyroid hormones, excess iodine and amiodarone. (Amiodarone is an interesting drug: it is an antiarrhythmic agent with two atoms of iodine per drug molecule. It can cause either hypothyroidism, by blocking release of T3 and T4, or hyperthyroidism by causing focal thyroiditis.)
Clinical manifestations
Primary hyperthyroidism is characterised by elevated levels of T3 and T4 despite a decreased level of TSH. In pituitary (secondary) hyperthyroidism, levels of TSH, T3 and T4 all rise. Hyperthyroidism leads to symptoms the opposite of those seen in myxoedema (see Clinical Interest Box 34-3). The metabolic rate is increased, sometimes as much as 60% or more. The body temperature is frequently above normal, the pulse rate is fast and the patient complains of feeling too warm. Other symptoms include restlessness, anxiety, emotional instability, muscle tremor and weakness, sweating and exophthalmos (bulging of the eyes). The raised T4 levels can cause cardiomegaly, tachycardia, congestive heart failure and hepatic damage. Drug clearances may be increased, so doses of other drugs might need to be increased. In thyroid storm, a sudden onset of exaggerated hyperthyroid symptoms occurs, especially those affecting the nervous and cardiovascular systems, because of elevated T4 levels. Thyroid storm is a life-threatening condition, potentially leading to heart failure and coma.
Clinical interest box 34-3 Hyperthyroidism and hypothyroidism: clinical features
| Hyperthyroidism | Hypothyroidism | |
| Eyes | Prominent | Eyelids oedematous; ptosis |
| Temperature | Intolerance to heat | Intolerance to cold |
| Weight | Appetite increases, weight loss | Appetite decreases, weight gain |
| Emotional | Increased nervousness, irritability, insomnia | Lethargic, depressed, increase in sleeping needs |
| Gastrointestinal | Diarrhoea | Constipation |
| Neuromuscular | Fast deep tendon reflexes; tremor | Slow or delayed deep tendon reflexes; myalgia |
| Extremities | Hot, moist skin; sweating | Cold, dry skin; myxoedema |
| Cardiovascular | Arrhythmias; heart failure | Bradycardia; ischaemic heart disease |
| Drug clearances | Increased | Decreased |
Graves’ disease
Graves’ disease, ‘exophthalmic goitre’, is the commonest cause of hyperthyroidism in patients under 40 years old, affecting about 0.4% of the population; it is an autoimmune condition affecting women more often than men. There are two main types of autoantibodies against thyroid antigens: thyroid-stimulating antibodies, which lead to the signs of hyperthyroidism, and thyroid-growth antibodies, which stimulate growth and hence lead to goitre. (In Hashimoto’s thyroiditis, conversely, the autoantibodies are destructive and produce hypothyroidism.) The first symptoms noticed may be fullness in the neck, difficulty in doing up the collar button and grittiness in the eyes; other signs and symptoms are as described in the text under ‘Hyperthyroidism (thyrotoxicosis)’. The classic sign, exophthalmos (i.e. protruding eyes), is due to fat deposition behind the eyeballs and oedema of the muscles controlling eye movements, leading to excessive fibrosis and eyelid retraction; corneal ulceration can occur. As well as therapy of the thyroid dysfunction to render the patient euthyroid, immunosuppressants are required to minimise the autoimmune processes, and moisturising eye-drops are helpful.
Treatment
The aims of treatment are to decrease thyroid hormone overproduction and block peripheral effects of excess T4; before the advent of the thiourea antithyroid drugs (carbimazole and propylthiouracil), treatment was surgical, by subtotal resection of the hyperactive gland. Typically, the stages of treatment now are:
Relapse is common, as is weight gain; hypothyroidism is treated as necessary with thyroxine. Treatment in pregnancy is difficult because thioureas cross the placenta and can cause goitre and cretinism, and 131I is contraindicated; careful monitoring is required.
Beta-adrenoceptor antagonists (e.g. propranolol) are frequently used as adjunctive therapy to provide relief of hyperthyroid symptoms due to the peripheral effects of excess T4, including tachycardia, tremor and sweating. Both cardioselective and non-selective ß-blockers are effective; they should be used with caution in cardiovascular disease and are contraindicated in asthma. (These drugs are covered in more detail in Chapter 12.)
Antithyroid agents
Antithyroid drugs lower the basal metabolic rate by interfering with the formation, release or action of thyroid hormones; some occur naturally and are known as goitrogens, e.g. in cabbages, turnips and celery seeds. Those used clinically are the thiourea (thionamide) derivatives, iodine (iodide ion) and radioactive iodine (Drug Monographs 34-2 and 34-3). Note that accurate diagnosis and optimal treatment of hyperthyroidism require careful monitoring with thyroid function tests.
Drug monograph 34-2 Carbimazole and propylthiouracil
Actions
The thioureas act as antithyroid drugs by inhibiting synthesis of thyroid hormones; they concentrate in the thyroid gland and inhibit organic binding of I2.
Indications
These agents are indicated for the treatment of hyperthyroidism, either in a short course in thyroid storm or before surgery or radiotherapy, or in a long course as adjunct therapy for treatment of thyrotoxicosis.
Pharmacokinetics
Thioureas are readily absorbed from the GIT. Carbimazole is a prodrug; it is rapidly converted in the body to the active metabolite, methimazole. The half-life of each thiourea drug is relatively short (2–6 hours); however, the effects may take some weeks to be maximal, as the body may already have large stores of preformed thyroid hormones. Thus, the peak effect occurs in about 7 weeks with carbimazole and 17 weeks with propylthiouracil. They are metabolised in the liver and excreted by the kidneys; they cross the placenta and can cause fetal hypothyroidism and goitre, and are excreted in breast milk (Pregnancy Category C, and contraindicated during breastfeeding).
Adverse reactions
These include rash, pruritus, dizziness, loss of taste, nausea, vomiting, leucopenia, paraesthesias and stomach pain; fever, mouth ulcers and sore throat may be early indications of serious agranulocytosis. Overall, signs of thyrotoxicosis indicate inadequate dosing, and signs of hypothyroidism indicate possible overdosage (see Clinical Interest Box 34-3). Propylthiouracil is more likely to cause liver damage.
Warnings and contraindications
Use with caution in patients with a low leucocyte count; lowest effective dose should be used during pregnancy, with regular monitoring. Avoid use in people with a history of carbimazole or propylthiouracil hypersensitivity or liver impairment. Regular blood tests and liver and thyroid function tests are recommended.
Dosage and administration
Dosage depends on usage: after initial 3–4 weeks of high-dose antithyroid therapy, either dosage is regularly adjusted to maintain euthyroid status or high dosage is maintained and thyroxine added to restore thyroid function to normal (‘block and replace’ regimen).
The carbimazole oral adult dosage is initially 20–60 mg daily, reducing to a maintenance dose of 5–15 mg daily. In the ‘block and replace regimen’ the initial dose is continued with the addition of thyroxine 100–150 mcg as necessary. The propylthiouracil initial oral adult dosage is 200–400 mg daily in divided doses. The maintenance dose is 25–300 mg daily in divided doses.
In each case, treatment is continued with monitoring for about 2 years, as remissions do occur. Relapse, however, is frequent.
Drug interactions 34-2 Thiourea antithyroid drugs
| Drug | Possible effects and management |
| Amiodarone, iodinated glycerol, lithium or potassium iodide | Increased or excess amounts of amiodarone, iodide or iodine can result in a decreased response to antithyroid drugs. Iodine defi ciency, however, may result in an increased response to the anti thyroid medications. Monitor closely |
| Anticoagulants (warfarin or phenindione) | As thyroid status approaches normal, the response to anticoagulants may decrease or, if the thiourea produces a drug-induced hypoprothrombinaemia, the anticoagulant response may increase. Monitor closely because anticoagulant doses are adjusted based on INR test results |
| Digitalis glycosides | As thyroid status and basal metabolic rate approach normal, plasma levels of digoxin and digitoxin may increase. Monitor closely, as dosage adjustments might be necessary |
| Sodium iodide 131I | Thyroid uptake of 131I may be decreased by antithyroid agents. Antithyroid drug should be stopped at least 4 days before and for 3 days after 131I therapy |
| Theophylline | May be metabolised faster by hyperthyroid patient; theophylline concentration and effects should be monitored when antithyroid drugs are commenced, and dose adjusted if necessary |
Thiourea antithyroid drugs
The thioureas (also known as thionamide or thioureylene derivatives) carbimazole and propylthiouracil inhibit thyroid hormone synthesis by inhibiting the iodination of tyrosine residues in thyroglobulin. Propylthiouracil (but not carbimazole) also inhibits the conversion of T4 to T3 in peripheral tissues, which may make it more effective for treatment of thyroid crisis or storm. These drugs all contain a sulfur–carbon–nitrogen linkage; they are closely related chemically to the sulfonamide antibacterials and the sulfonylurea hypoglycaemic agents, and both of these drug groups may also interfere with thyroid function.
Iodine and iodides
Iodine
Iodine is the oldest of the antithyroid drugs. Although a small amount of iodine is necessary for normal thyroid function and to synthesise thyroid hormones, large amounts of iodine depress TRH and TSH release (see Figure 34-2). In a patient with thyrotoxicosis high-dose iodine administration thus causes inhibition of thyroid hormone synthesis and release from the hyperfunctioning thyroid gland. High doses of iodides such as in Lugol’s solution are generally used for 7–14 days before thyroid surgery to decrease the gland’s size and vascularity, resulting in diminished blood loss and a less complicated surgical procedure (see Clinical Interest Box 34-4).
Clinical interest box 34-4 Lugol’s solution
Lugol’s solution, or Aqueous Iodine Solution BP, was first made and documented in 1829. It is a mixture of 5% iodine and 10% potassium iodide in water; the total iodine content is about 130 mg/mL. It has been used in the past as an antiseptic, to disinfect drinking water, as a stain for cells, in marine aquariums and as a reagent in assay of starch. After oral administration, the iodine is converted to iodide in the gastrointestinal tract before systemic absorption. Iodine solution is indicated to protect the thyroid gland from radiation before and after the administration of radioactive isotopes of iodine or in radiation emergencies, and in patients with hyperthyroidism, to suppress thyroid function and vascularity prior to thyroidectomy. The adult dose of Lugol’s solution before thyroid surgery is 1 mL/day (in divided doses, administered in a full glass of water, fruit juice or milk) for 10–14 days to depress thyroid function.
It is used with caution in patients with tuberculosis, iodine or potassium iodide hypersensitivity, bronchitis, hyperkalaemia or kidney impairment, and in pregnancy. Adverse reactions include diarrhoea, nausea, vomiting, stomach pain, rash, swelling of the salivary gland and a metallic taste in the mouth.
Lugol’s solution used to be a favourite ‘extemporaneous product’ for examiners at pharmacy colleges to include in practical dispensing examinations: the carelessness of students could be readily judged by the amount of brown staining of students’ fingers and laboratory coats.
Iodine is also used in medicine for its bactericidal, fungicidal and viricidal actions; solutions such as povidone–iodine are used as antiseptics, especially in podiatry to reduce fungal foot infections. Potassium iodide is present in many cough mixtures as an expectorant. Small amounts of iodine are included in dozens of OTC multivitamin/mineral preparations and supplements.
Many people believe they have an ‘iodine allergy’; however, iodine itself is far too small a molecule to be allergenic. The allergy would be to another component of an iodine-containing preparation, such as an iodinated contrast medium (see below), povidone–iodine antiseptic or an iodine-containing drug such as amiodarone. Similarly, seafood allergies are not due to any supposed content of iodine, but to particular proteins in the seafood (see review by Katelaris [2009]).
Radioactive iodine (131I)
Radioactive iodine is preferred for people who are poor surgical risks, such as debilitated or elderly patients and those with advanced cardiac disease. It is also used for patients who have not responded adequately to drug therapy or who have had recurrent hyperthyroidism after surgery. The 131I radioactive isotope of iodine is chemically identical to iodine, so it has the same pharmacokinetic parameters. After oral administration, it is taken up actively by thyroid cells and accumulates in thyroid tissue, where the ionising beta-radiation emitted selectively damages thyroid cells (see Drug Monograph 34-3). It is an interesting example of radiopharmaceuticals, where dosage is in units of radioactivity rather than mg of active drug.
Drug monograph 34-3 Radioactive iodine
Indications
Radioactive iodine (131I) is indicated for the treatment of hyperthyroidism and thyroid carcinoma, and is also used in diagnostic thyroid function tests.
Pharmacokinetics
Administered orally (usually as sodium iodide in a capsule), it has an onset of effect within 2–4 weeks; the peak therapeutic effect occurs between 2 and 4 months; and it is mainly excreted by the kidneys, 50% within 24 hours. It has a radionuclide half-life of about 8 days; principal types of radiation are beta and gamma rays.
Adverse reactions
These include sore throat, neck swelling or pain, temporary loss of taste, nausea, vomiting, gastritis and painful salivary glands. After treatment for hyperthyroidism, the patient may experience increased or unusual irritability or tiredness. There is a small increased risk of subsequent thyroid cancer. After treatment for thyroid carcinoma, the patient may experience fever, sore throat, chills (due to leucopenia) and increased bleeding episodes (thrombocytopenia).
If hypothyroidism occurs after treatment, symptoms should be monitored and thyroid function tests carried out for replacement therapy.
Warnings and contraindications
Use with caution in patients with diarrhoea, vomiting, kidney function impairment or severe thyrotoxic cardiac disease, especially the elderly. Avoid use in people with hypersensitivity to radiopharmaceutical preparations, and in pregnancy and breastfeeding.
Precautions for radioactivity safety must be observed; after high doses, patients’ excretions are collected for safe disposal.
Dosage
Dosage depends on the size and activity of the gland, and the indications for which it is being administered; dosage is in millicuries (mCi) or in the SI units megabecquerels (MBq, where 1 Bq = 2.7 × 10−11 Ci). For example, 5–15 mCi may be prescribed for hyperthyroidism, whereas 50–100 mCi is required for thyroid carcinoma.
The primary disadvantage of using surgery or radioactive iodine therapy, in addition to the risks involved with surgery and radiation, is the induction of hypothyroidism. However, it is now recognised that, in the long term, definitive therapy that produces hypothyroidism, followed by replacement with adequate thyroxine, is an easier regimen for maintaining euthyroidism than frequent changes of antithyroid drug doses.
Iodinated contrast media
Contrast media used in radiographic tests often contain tri-iodinated benzoic acid derivatives and a small amount of free iodine in solution. Hypersensitivity reactions to contrast media can occur, such as immediate reactions (pruritis, urticaria, anaphylaxis) and delayed reactions (urticaria, angioedema, rash or, rarely, Stevens–Johnson syndrome). Modern solutions are close to iso-osmotic and are non-ionic, and only rarely cause hypersensitivity reactions. If patients have had previous severe anaphylactic reactions to contrast media, the use of such media is contraindicated (see Katelaris [2009]).
Key points
 The thyroid gland has important homeostatic and controlling actions in growth and development, metabolism and energy balance, and cardiovascular and nervous system functions.
The thyroid gland has important homeostatic and controlling actions in growth and development, metabolism and energy balance, and cardiovascular and nervous system functions. The main thyroid hormones are thyroxine (T4) and liothyronine (tri-iodothyronine, T3); calcitonin is also produced and is involved in calcium balance.
The main thyroid hormones are thyroxine (T4) and liothyronine (tri-iodothyronine, T3); calcitonin is also produced and is involved in calcium balance. Iodine is actively taken up from the circulation by the thyroid gland and incorporated in T3 and T4, which are stored in the thyroid follicles, bound in thyroglobulin.
Iodine is actively taken up from the circulation by the thyroid gland and incorporated in T3 and T4, which are stored in the thyroid follicles, bound in thyroglobulin. Hypothyroidism is manifest generally as slowed body activities; severe hypofunctioning in the adult leads to myxoedema and in the infant to dwarfism and mental retardation. Replacement therapy with thyroid hormones is effective and safe.
Hypothyroidism is manifest generally as slowed body activities; severe hypofunctioning in the adult leads to myxoedema and in the infant to dwarfism and mental retardation. Replacement therapy with thyroid hormones is effective and safe. Hyperthyroidism (thyrotoxicosis) leads to speeding up of body functions, with potential damage particularly to the cardiovascular system and eyes.
Hyperthyroidism (thyrotoxicosis) leads to speeding up of body functions, with potential damage particularly to the cardiovascular system and eyes.Review exercises
References and further reading
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Ceccarelli C., Bencivelli W., Vitti P., et al. Outcome of radioiodine-131 therapy in hyperfunctioning thyroid nodules: a 20 years’ retrospective study. Clinical Endocrinology. 2005;62(3):331-335.
Chiamolera M.I., Wondisford F.E. Minireview: thyrotropinreleasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;150(3):1091-1096.
Davoren P. Modern management of thyroid replacement therapy. Australian Prescriber. 2008;31(6):159-161.
Delange F., Lecomte P. Iodine supplementation: benefits outweigh risks. Drug Safety. 2000;22:89-95.
Endocrinology Expert Group. Therapeutic Guidelines: Endocrinology, version 4. Melbourne: Therapeutic Guidelines Limited; 2009.
Hetzel B.S. Iodine and neuropsychological development. Journal of Nutrition. 2000;130(2S Suppl):493S-495S.
Katelaris C.H. “Iodine allergy” label is misleading. Australian Prescriber. 2009;32(5):125-128.
Rang H.P., Dale M.M., Ritter J.M., Moore P.K. Pharmacology, 5th edn. Edinburgh: Churchill Livingstone; 2003. [ch 28]
Roberts G.W. Taking care of thyroxine. Australian Prescriber. 2004;27(3):75-76.
Tanner J.M. Foetus into Man: Physical Growth from Conception to Maturity, 2nd edn. Ware: Castlemead; 1990.
Zimmermann M.B. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. American Journal of Clinical Nutrition. 2009;89(2):668S-672S.
New Zealand Medicines and Medical Devices Safety Authority: www.medsafe.govt.nz
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology