Chapter 37 Pharmacology of the parathyroid glands and bone
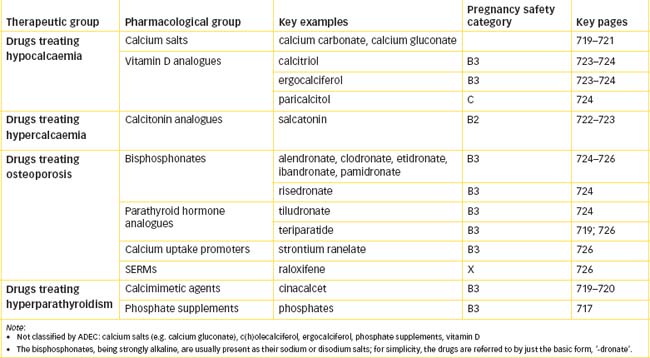
The parathyroid glands have a major role in controlling the body’s calcium and bone mineral levels via the actions of parathyroid hormone and interactions with calcitonin and vitamin D. Impaired calcium balance or bone mineral homeostasis can lead to severe bone pathologies. Various hormones and analogues (parathyroid hormone, calcitonin, oestrogens), vitamin D and bisphosphonate drugs are used to treat bone pathologies.
Key abbreviations
BMP bone morphogenetic proteins
HRT hormone replacement therapy
Key background: parathyroid glands and bone mineral balance
The parathyroid glands
Anatomy
THE parathyroid glands are small bean-shaped glands lying behind the thyroid gland; there are usually two pairs—one pair on the dorsal surface of each lobe of the thyroid gland—but the number can range from two to six, and ectopic parathyroid tissue can occur elsewhere in the body. The adult glands consist of masses of cells, between which are abundant adipocytes and vascular channels. Removal of the parathyroid glands (as frequently happened in surgery on the thyroid gland before the significance of the parathyroid glands was recognised) results in severe hypocalcaemia, leading to tetany and death.
The primary function of the parathyroids is to secrete parathyroid hormone (PTH), which maintains adequate levels of calcium in the blood and extracellular fluid. Note that the parathyroid glands are not subject to higher control from the pituitary gland or hypothalamus (see Figure 33-4) but respond directly to, and help control, blood calcium levels (analogous to the pancreas responding to and controlling blood glucose).
Calcium-sensing receptors
The cells of the parathyroid gland have in their membranes specialised calcium sensors (G-protein-coupled receptors) that are the master regulators of PTH secretion and calcium levels in the body. When calcium levels in the extracellular fluid are low, the parathyroids are stimulated to synthesise and secrete PTH, which acts to conserve calcium. When plasma calcium levels are high, binding of calcium to the receptors activates G-proteins, leading to activation of phospholipase C enzymes and eventually to inhibition of further PTH secretion (see review by Theman and Collins [2009]). Mutations in the calcium-sensing receptor gene lead to familial disorders of calcium balance: inactivating mutations cause hypercalcaemia, while acti vating mutations cause hypocalcaemia.
Calcium-sensing receptors are present in many other tissues as well, where their functions are less clear; other cations can also bind to the receptors. Complex feedback loops involving calcium, phosphorus, PTH and vitamin D all participate in controlling calcium levels. The calcimimetic compound cinacalcet (see Drug Monograph 37-2) is an allosteric modulator at the calcium-sensing receptor used to treat hyperparathyroidism.
Drug monograph 37-1 Teriparatide
Teriparatide, a recombinant form of the active fragment of human PTH, is referred to as a ‘bone formation agent’, as it activates osteoblasts via binding to specific PTH cell surface receptors.
Indications
Teriparatide is indicated for treatment of osteoporosis in men and postmenopausal women, when other agents are unsuitable and there is a high risk of fractures. Calcium and vitamin D supplements are administered concurrently. Teriparatide has been shown to increase bone mineral density in the spine and reduce the risk of new bone fractures.
Pharmacokinetics
Teriparatide is administered by SC injection; it has a high bioavailability and rapid absorption and elimination, with a half-life of approximately 1 hour. Metabolism is believed to occur in the liver and kidney.
Drug interactions
Clinical experience with teriparatide is still limited; no significant interactions have been reported.
Adverse reactions
In animal studies, high doses of teriparatide caused a higher incidence of osteosarcomas. The relevance of this finding to humans has not been determined; however, currently the lifetime maximum duration of teriparatide treatment has been set at 18 months, and all patients must have the possible risks explained and must give informed consent before treatment. Hypercalcaemia may be exacerbated. Other adverse reactions include nausea, headache, dizziness, leg cramps, arthralgia and hyperuricaemia.
Drug monograph 37-2 Cinacalcet
Cinacalcet increases the sensitivity of calcium-sensing receptors to extracellular calcium, thus reducing PTH secretion and serum calcium concentration.
Indications
Cinacalcet is indicated in some cases of primary hyperparathyroidism, in patients with chronic kidney disease on dialysis with secondary hyperparathyroidism and in hyperparathyroidism secondary to hypocalcaemia.
Pharmacokinetics
There is only low bioavailability (25%) of the drug from tablets; this is increased by taking with food. Maximum plasma concentrations are reached after 2–6 hours, and Css after about 7 days. Cinacalcet is 97% plasma-protein bound and has extensive distribution in tissues, with a consequent massive volume of distribution (>1000 L). The elimination is thus biphasic, with half-lives of 6 hours then 30–40 hours; once-daily dosing is effective. The drug is metabolised by several enzymes to inactive metabolites, which are mainly excreted by the kidneys.
Adverse drug reactions
The main adverse effects are seizure, hypocalcaemia, weakness and paraesthesias, bone disease, GI upsets, reduced testosterone concentrations, hypersensitivity and rash, hypotension and worsening cardiac failure.
Drug interactions
There are potential interactions with CYP2D6 substrates (e.g. metopropolol, flecainide, thioridazine and most tricyclic antidepressants) and strong CYP3A4 inhibitors (e.g. erythromycin, -conazole antifungals) or inducers (phenytoin, rifampicin, St John’s wort).
Warnings and contraindications
Precautions are advised in hypocalcaemia, in cardiac and hepatic impairment and in pregnancy, lactation and children; calcium and PTH levels should be monitored.
Dosage and administration
Dosage needs to be titrated according to response; typical starting doses are 30 mg twice daily in primary hyperparathyroidism or parathyroid carcinoma and 30 mg once daily in dialysis patients with secondary hyperparathyroidism; doses can be increased gradually to maximum 180 mg daily for renal disease and 90 mg four times daily for parathyroid cancer.
Pathologies
Hypoparathyroidism
Hypoparathyroidism may be surgical (after surgery on the throat), autoimmune, familial or idiopathic. The signs and symptoms of hypoparathyroidism are those of hypocalcaemia (decreased serum calcium levels) and tetany; serum phosphate levels are raised (due to loss of the phosphaturic effect of PTH). The symptoms include muscle spasms, convulsions, gradual paralysis with dyspnoea and death from exhaustion. Before death, gastrointestinal (GI) haemorrhages and haematemesis often occur and at death the intestinal mucosa is congested.
The symptoms of tetany are relieved by administration of calcium salts (see Clinical Interest Box 37-1); PTH itself has too short a half-life to be useful clinically. Usually, vitamin D levels are low, so large doses of vitamin D also help to restore the normal calcium level in the blood and relieve tetany. The patient is initially hospitalised because frequent assessment of blood calcium and phosphate levels is essential.
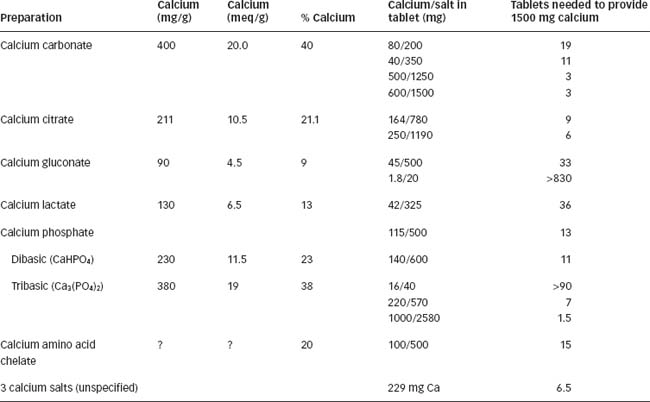
Clinical interest Box 37-1 Getting enough calcium
Good calcium intake, especially in adolescence and pregnancy, is important for building and maintaining strong bones and preventing osteoporosis. The 1997 New Zealand National Nutrition Survey (Russell et al 1999), carried out by staff of the University of Otago, con ducted home interviews with 4636 New Zealand adults, including 704 Maori, with the following findings related to calcium and dairy food intake:
In the table below, the approximate calcium contents of typical calcium supplements are listed as milligrams Ca per gram of the calcium salt, milliequivalents Ca per gram salt, or percentage of calcium in the salt. The average number of tablets providing 1.5 g calcium (RDI for postmenopausal women) is also calculated.
Hyperparathyroidism
Primary hyperparathyroidism is the third most common endocrine disorder, with highest incidence in postmenopausal women; it is hyperactivity of the parathyroid glands, with excessive secretion of PTH. Generally, it is caused by a parathyroid adenoma or by spontaneous hyperplasia. PTH elevations produce increased resorption of calcium from the skeletal system and increased absorption of calcium by the kidneys and the GI system. Elevated plasma levels of calcium with high urine phosphate levels can lead to renal stones, bone pain with skeletal lesions and pathological fractures.
Because tumours may cause this syndrome, surgery is usually the first-line treatment. High serum levels of calcium may require immediate treatment (see below under ‘Hypercalcaemia’). Other drugs used (discussed later) include calcimimetics, bisphosphonates, SERMs and oestrogen/progestogen hormone replacement therapy.
Secondary hyperparathyroidism occurs commonly in renal osteodystrophy associated with chronic renal insufficiency, and often requires parathyroidectomy. Prophylactic low doses of vitamin D and calcium may prevent the parathyroid cell hyperplasia that occurs to compensate for low plasma calcium levels resulting from inadequate renal activation of vitamin D.
Bone mineral homeostasis
Bone remodelling
Bone has three major functions:
Bone is constantly renewing itself in the process of remodelling, as old bone is resorbed by osteoclast cells and new bone deposited by osteoblasts. Until the age of about 20–25 years, bone mass increases and stabilises as the bone growth achieved during childhood and adolescence is consolidated; thereafter, during adulthood, bone is lost slowly until after menopause in women, when the rate of bone loss increases. Elderly people are likely to suffer from osteoporosis (softened bones), leaving them at higher risk of fractures.
Calcium balance
The process of bone remodelling is integrated by many endocrine and other factors, including vitamin D, PTH, calcitonin and plasma calcium levels (see Figure 37-1). The daily turnover of calcium in and out of bone is estimated to be at least 500 mg, i.e. about half of the average daily dietary requirements for calcium. Increased bone resorption (stimulated by PTH and vitamin D) leads to mobilisation of calcium from bone, i.e. calcium is released into the extracellular fluid and made available for physiological actions.
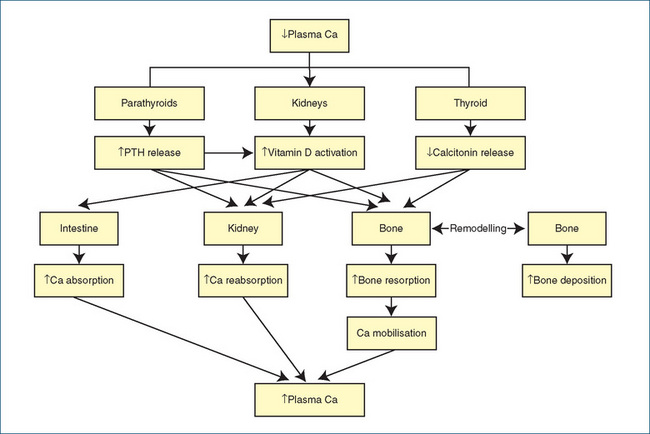
Figure 37-1 Calcium balance. Flowchart summarising the main factors regulating plasma calcium levels. Low calcium levels trigger increased release of PTH and vitamin D and inhibit release of calcitonin, leading to conservation of calcium from the intestine, kidneys and bone, hence raising plasma calcium. Other factors involved (not shown) include calcium-sensing receptors, bone morphogenetic proteins, RANKL, phosphate levels, growth hormone, glucocorticoids, oestrogens and prolactin. Ca = calcium; PTH = parathyroid hormone; RANKL = receptor activator of nuclear factor kappa B ligand.
Adapted from: Grills 2002.
Phosphate balance
Bone resorption also mobilises phosphate from the calcium phosphate present in bone mainly as hydroxyapatite. Phosphate is involved in many biochemical pathways, e.g. energy balance and phosphorylation of enzymes; as a component of nucleic acids, phospholipids and proteins; in buffering systems in body fluids and in many cell signalling reactions. Although the specific mechanisms regulating phosphate levels are not well identified, overall, vitamin D and PTH tend to increase calcium reabsorption from the kidney tubules while increasing phosphate excretion, thus conserving calcium but removing phosphate. Phosphate is present in many foods, so phosphate supplements are rarely needed.
Other chemicals with phosphaturic activity include fibroblast growth factor 23 (FGF23), a protein hormone that inhibits renal tubular phosphate transport and is involved in maintenance of the skeleton. Mutations in the FGF23 gene are responsible for disorders of phosphate metabolism such as hypophosphataemic rickets and tumour-associated osteomalacia.
Bone morphogenetic proteins
Bone morphogenetic proteins (BMPs) are cytokines that induce the formation of bone and cartilage and are important during embryonic skeletal development and bone repair after fracture. It has long been known that bone has great capacity for regeneration and repair; these factors were first described in 1965, and since then at least 20 BMPs have been discovered, several belonging to the superfamily of proteins known as transforming growth factor beta proteins. They are present in bone matrix and act via specific cell surface receptors in several types of tissue, with signal transduction via serine/threonine kinase receptors and subsequent actions in the cell nucleus involving gene transcription and protein synthesis.
BMPs are being used clinically in situations such as spinal fusion and fracture fixation operations, in cases of delayed healing and non-union, in craniofacial and periodontal applications and in chronic kidney disease. Currently, recombinant forms of BMP2 and BMP7 are approved by the FDA in America for clinical use (see review by Dean et al [2009]).
Other bone-regulating factors
Receptor activator of nuclear factor-kappa B (RANK), its ligand (RANKL) and osteoprotegerin are all members of the tumour necrosis factor receptor superfamily, with roles in bone remodelling and mineral balance. Their functions in conditions such as osteoporosis, bone loss and rheumatoid arthritis are still being elucidated. RANKL is considered the principal mediator of osteoclastic bone resorption. Denosumab, a specific monoclonal antibody to RANKL, thus slows the rate of bone resorption; it is currently undergoing clinical trials in osteoporosis where it shows promise in increasing bone mineral density (BMD) and reducing bone turnover.
Many other bone-regulating growth factors and peptides are still being identified and studied, including PTH-related protein (a paracrine regulator of bone formation), leptin (secreted by adipocytes, with negative effects on osteoblasts), osteocalcin (a bone-derived hormone with positive effects on bone mineral density that also regulates energy metabolism via effects on insulin and adiponectin), cathepsin K (a lysosomal protease expressed by osteoclasts) and vitamin K (via carboxylation of osteocalcin). Drugs affecting these mediators will no doubt in the future prove interesting and useful in various bone diseases.
Bone pathologies
General pathologies affecting bone are described in this section; more specific disorders due to lack of a particular mineral or hormone are described in a later relevant section, e.g. hypocalcaemia under ‘Calcium’ and rickets under ‘Vitamin D’.
Osteoporosis
Osteoporosis refers to increased bone fragility and consequent risk of fracture due to reduced bone density—the lower the bone density, the greater the risk of low-trauma bone fracture, especially fractures of the vertebrae, hip or forearm. Osteoporosis is an important health problem: as described earlier, bone strength is lost progressively during the adult years, so osteoporosis has an increasing prevalence in communities as the proportion of the population aged 65 and over increases. (It is estimated that, by the year 2050, people aged over 65 will comprise 22% of the Australian population; the incidence of vertebral wedge fractures in women over 70 years is 20%–25%). Fractures contribute significantly to morbidity, mortality and growing health-care costs.
There are many risk factors for osteoporosis in women: late menarche (first menstrual period), episodic amenorrhoea and early menopause. These all indicate the protective effect that oestrogens normally have on bones. Low calcium intake, excessive phosphate, no or excessive exercise, tobacco, caffeine and excessive alcohol are also contributing factors. Drugs such as long-term glucocorticoids or thyroid hormones, antacids, anticonvulsants, antidepressants and sedatives are all implicated. Malabsorption syndromes, hyperparathyroidism and other endocrine disorders can also impair calcium balance. Men also experience bone loss with ageing and declining sex steroid levels, and osteoporosis also occurs in men as they age.
Paget’s disease of bone (osteitis deformans)
Paget’s disease is a disorder of bone remodelling, with focal areas of greatly increased rates of bone turnover and disorganised remodelling, leading to soft, poorly mineralised bone, hypercalcaemia, bone pain, limb deformities, fractures, deafness,1 osteoarthritis and nerve compression problems. The aetiology is unknown; however, mutations in four genes involved have been identified, and possible triggers include a viral infection and deficiency of dietary calcium. It is estimated to affect 3%–4% of middle-aged to elderly Australians but only a small proportion (5%) of these may require treatment.
Treatment is firstly with bisphosphonates (see later), or with calcitonin and analgesics. (A cytotoxic antibiotic, plicamycin (mithramycin), which inhibits osteoclastic activity, was formerly used in Paget’s disease and hypercalcaemia but has been withdrawn because of excessive toxicity.) Serum and urine markers of bone turnover are monitored and surgery may be necessary to free entrapped nerves or in cases of spinal cord compression.
Hormones and drugs affecting bone
Parathyroid hormone
PTH is a polypeptide of 84 amino acids. The active component has a half-life of 2–4 minutes; smaller peptide fragments produced in the liver and kidneys have longer half-lives. PTH has multiple effects, ultimately culminating in raised plasma calcium levels (see Figure 37-1). It also reduces phosphate concentration, permitting more calcium to be mobilised. The main effects are:
Mechanism of action
The mechanism of PTH action in the bone or kidney is incompletely understood; in osteoblasts PTH activates Wnt signalling (Wnt proteins are signalling molecules that regulate cell-to-cell interactions) and increases osteoblast differentiation, numbers and survival. By integrated effects in various tissues, PTH acts to increase inflow of calcium into extracellular fluid and protect against hypocalcaemia. The ‘bottom line’ in calcium balance is the level of calcium in the blood, as this provides the source for all calcium functions in the body; in this context, bone acts as a depot of calcium to be mobilised.
Teriparatide
A PTH analogue, teriparatide, which consists of the active fragment (amino acids 1–34) of PTH, has recently become available for treatment of osteoporosis (see Drug Monograph 37-1).
Calcimimetics
Calcimimetics are a new class of drugs that can inter act with calcium-sensing receptors and increase their affinity for calcium, hence inhibiting parathyroid cell proliferation, PTH synthesis and secretion. Calcimimetics thus reduce calcium, PTH and phosphate levels and are useful in treating primary and secondary hyperparathyroidism. The first such agent in clinical use is cinacalcet; it can be considered an anti-PTH agent (see Drug Monograph 37-2).
Calcium
Physiological roles
Calcium serves an important central position in cellular physiology and metabolic regulation; although 99% of body calcium is in bone, the remaining 1% of body calcium has major roles in:
The level of extracellular and intracellular calcium is tightly controlled. Calcium homeostasis has to maintain exact levels of calcium, despite varying amounts of absorption in the diet and excretion via the kidneys and faeces. It must provide for calcium in bones and teeth and maintain a gradient across cell membranes, such that the level of calcium inside cells is only 1/10,000 of that outside. The hormones regulating calcium balance are PTH (see above), calcitonin and vitamin D (see Figure 37-1).
Pharmacological uses of calcium
Dietary sources
The recommended daily dietary intake (RDI) of calcium for adults (including pregnant and breastfeeding women) is 1000 mg/day; for post-menopausal women and men >70 years old, requirements are higher—1300 mg/day to prevent osteoporosis (see later discussion). The main sources of calcium in the diet are dairy produce, soybeans, spinach, yeast products, nuts and edible bones. The activity of calcium in a product depends on total calcium ion (elemental) content. Note that, as some calcium salts contain only a small proportion of calcium (e.g. calcium gluconate: 9% calcium), it is important to check the dose on bottle labels as mg calcium rather than mg calcium salt.
Many milk products now have ‘high calcium’ to encourage adequate intake; for example, while normal whole milk contains about 120 mg calcium per 100 mL (and 3.4 g fat), low-fat milk contains about 138 mg calcium and 0.1 g fat per 100 mL, and high-calcium milk about 175 mg calcium and 0.1–1.3 g fat per 100 mL.
Calcium supplements
With the general recognition of the importance of calcium in bone structure and in prevention of osteoporosis in our ageing populations, calcium has become ‘trendy’ and calcium supplements abound. Formulations available in supermarkets and health food shops, marketed as multivitamin or mineral preparations, contain wide variations in calcium (range: about 1.8 mg/tablet to 200 mg/ tablet); average amount appears to be about 40–80 mg. Tablets marketed as mineral supplements may contain much higher levels, e.g. 300–600 mg, even up to 1 g calcium/tablet. Calcium gluconate is also available as a 10% solution (10 g/100 mL) for injection, to treat acute hypocalcaemia and tetany and for use in formulation of parenteral nutrition solutions. (Clinical Interest Box 37-1 lists some calcium salts present in calcium supplement tablets or capsules.)
Treatment of hypocalcaemia
The main cause of low calcium levels is hypoparathyroidism, as explained above. Kidney problems can cause renal osteodystrophy because of low vitamin D levels. Hypocalcaemia leads to hyperexcitability of nerves, manifest as paraesthesias (‘pins and needles’), spasms (including arrhythmias, dysphonia and dysphagia), tetany and fits.
Treatment in the acute situation is with calcium. Calcium is also used to prevent and treat osteoporosis. It is given orally and also IV, but not IM or SC, as solutions are irritant. Calcium can interact adversely with other drugs, including digoxin (causing arrhythmias), bisphosphonates (decreasing their absorption), calcium channel blockers (a physiological antagonism) and tetracyclines (causing yellow discolouration of teeth and bones).
Moderate hypocalcaemia can be controlled with the vitamin D analogue calcitriol. Table 37-1 lists drugs used to treat hypocalcaemia.
Table 37-1 Treatment of hypocalcaemia
| Drug | Usual adult dose |
| Calcium gluconate | IV: 10–20 mL of 10% solution given slowly over 5–10 minutes (magnesium may also be required); then oral calcium 1.5–4 g/day in three divided doses, adjusted depending on plasma calcium concentration |
| Vitamin D analogues | |
| C(h)olecalciferol (vitamin D3) | As a single drug, available only in tablets or capsules (1000 IU, 25 mcg); daily adult dose assuming no or minimal sunlight exposure is 5 mcg for those <50 years, 10 mcg if 51–70 years, & 15 mcg if >70 years |
| Calcitriol | Oral: 0.25 mcg daily, increased every 2–4 weeks if necessary to a max of 0.5 mcg twice daily IV: 0.5 mcg three times a week at the end of haemodialysis, increased every 2–4 weeks if necessary |
Adapted from: AMH 2010.
Treatment of hypercalcaemia
The most common cause of hypercalcaemia is hyperparathyroidism, due to an adenoma of the parathyroid gland causing excessive secretion of PTH. Other aetiologies include Paget’s disease of bone; excess vitamin D causing excess calcium to be retained; and occurrence in various malignancies of osteolytic bone metastases, with increased bone turnover and hypercalcaemia. Excess calcium may also be consumed as calcium salts in other medications, including antacids. The clinical manifestations are weakness, arrhythmias, nausea and vomiting, constipation and ectopic calcification, e.g. as kidney stones. Treatment is by rehydration and with calcitonin or bisphosphonate drugs. Table 37-2 describes typical recommendations for treatment of hypercalcaemia.
Table 37-2 Treatment of hypercalcaemia
| INCREASE CALCIUM EXCRETION | |
| Saline rehydration and diuresis | Infuse normal saline (100–200 mL/h) to increase calcium excretion. Monitor fluid intake, output and electrolytes for evidence of fluid overload |
| INHIBIT BONE RESORPTION | |
| Salcatonin | Slow IV infusion/injection: 5–10 IU/kg daily. Tolerance can develop in 24–72 h, so corticosteroids may be prescribed concurrently |
| Clodronate (dose depends on indication, level of renal impairment & route of administration) | Hypercalcaemia or osteolytic bone metastases: 1.6–3.2 g daily in divided doses |
| INCREASE SENSITIVITY OF CALCIUM-SENSING RECEPTORS | |
| Cinacalcet | Initially 30 mg twice daily, with food |
Adapted from: AMH 2010.
Calcitonin
Physiological roles
Calcitonin, the third major hormone product of the thyroid gland, was discovered in 1961. It is a polypeptide secreted by thyroid C cells when there is a high calcium concentration in the blood, especially when this is due to conditions of increased bone resorption. Calcitonin has several actions:
Calcitonin can thus be considered as a natural antagonist of the actions of PTH and vitamin D (see Figure 37-1 and review by Naot and Cornish [2008]).
Mechanism of action
Calcitonin (and other peptide hormones of the calcitonin family) act via specific G-protein-coupled receptors, which form dimers with receptor activity modifying proteins (RAMPs) and have different affinities for the specific ligands. Calcitonin binds to its receptors on the cell membranes of target tissues and, via G-protein-coupled mechanisms and cyclic AMP, mediates the many effects leading to reduction in plasma calcium levels.
Pharmacological uses
Sources of calcitonin used in the past have included active extracts of porcine, human and salmon thyroid tissue. The salmon hormone is particularly potent, has a slightly longer half-life and has been given the approved name salcatonin (Drug Monograph 37-3). Porcine and human products have been discontinued in Australia. Salcatonin is 10–40 times more potent by weight than human calcitonin and has greater affinity for receptor binding sites in bone and kidney. Salcatonin is used to treat hypercalcaemia and Paget’s disease of bone (described later).
Drug monograph 37-3 Salcatonin (Salmon calcitonin)
Calcitonin is the calcium-lowering hormone. The salmon extract salcatonin has the same physiological actions as the human hormone; it is now produced synthetically.
Pharmacokinetics
Because it is a peptide, calcitonin cannot be administered orally; it is usually given SC, but also IM and IV. The elimination half-life is 60–90 minutes but the biological halflife is considerably longer. The peak effect in hypercalcaemia occurs in 2 hours and the duration of action is 6–8 hours. Tachyphylaxis develops over several days. Excretion of metabolites is via the kidneys.
Onset of the therapeutic effect in Paget’s disease may range from 6 to 24 months of regular treatment, although some improvement (measured by a decrease in serum alkaline phosphatase levels) may occur within the first few months.
Adverse reactions and drug interactions
No significant drug interactions have been reported. Adverse effects include flushing or a tingling sensation of the face and hands, increased urinary frequency, nausea, vomiting and pain or swelling at the injection site. Allergic reactions, antibody development and visual disturbances can occur.
Indications
Avoid use in people with a history of protein allergy or calcitonin hypersensitivity. Few data are available on use in children, pregnancy or lactation.
Dosage and administration
The usual salcatonin adult dosage for Paget’s disease is 80–100 IU daily, treatment for months or years is required; for hypercalcaemia it is 5–10 IU/kg daily by slow IV infusion or injection. To reduce the occurrence of nausea or flushing, bedtime administration is suggested; if necessary, an antiemetic may be administered.
Calcitonin-related peptides
Peptide hormones with structural similarities to calcitonin include calcitonin gene-related peptide (CGRP), amylin, adrenomedullin and intermedin; they are produced by various tissues but all appear to target bone. Like calcitonin, amylin and CGRP also inhibit osteoclast activity and bone resorption, while amylin, CGRP and adrenomedullin induce osteoblast proliferation and promote bone formation. Further research on these mediators of bone function will doubtless produce clinically useful drugs in the future.
Vitamin D
Sources and synthesis
‘Vitamin D’ refers to a group of molecules related to cholesterol (Figure 33-3B) derived from the diet and metabolised in the body to the active compound (1,25-dihydroxycholecalciferol, Figure 33-3G); hence they are not, strictly speaking, vitamins. Many sterol sources and tissues are involved in the production of vitamin D:
Physiological roles
Vitamin D is involved in calcium, phosphate and magnesium metabolism in bone and the GIT. Its actions are to raise the plasma calcium level by increasing calcium absorption (in the intestine), by re absorption (in the kidney distal tubule) and mobilisation (from bone)—actions similar to those of PTH.
Vitamin D also has a permissive role in PTH actions. Requirements are increased during pregnancy and lactation, as the vitamin D deficient infant is at risk of rickets, hypocalcaemic seizures, limb pain and bone fractures. Vitamin D does not distribute effectively into milk, so an exclusively breast-fed infant is particularly at risk and requires oral supplements (400 IU daily) for the first 12 months.
Mechanism of action
The mechanism of action of vitamin D is generally similar to that of the steroid hormones: it enters the nucleus, activates vitamin D receptors (present in more than 36 cell types) and sets in train a series of reactions leading to gene transcription and synthesis of calcium-binding proteins and bone matrix proteins.
Related pathologies
Deficiencies of vitamin D are characterised by demineralised bones, which are weak and soft; treatment is with vitamin D (see Clinical Interest Box 37-2).
Clinical interest Box 37-2 Re-emerging rickets
Nutritional rickets, thought to have been cured in the early part of the 20th century when vitamin D and its role in bone strength were discovered, has made an unexpected return in recent years throughout the world, even in sunny countries. The increasing numbers of case reports are thought to be due to low dietary intake of vitamin D and decreased sunshine exposure, particularly in children and women who wear long protective clothing, in children of recent immigrant families from Africa or the Middle East, in the elderly who are housebound or in residential care and also in people with dark skin and those who regularly use high-SPF sunscreens.
There are recommendations that a staple food such as flour or bread should be fortified with added vitamin D to prevent deficiencies, much as common salt is iodised to prevent deficiencies of thyroid hormones (see Wark [2003]; Munns et al [2006]; Benson and Skull [2007]).
Rickets and osteomalacia
In various pathological conditions (e.g. malabsorp tion, liver disease, kidney failure) and in countries where people are not exposed to sufficient sunlight (due to long winters or highly protective clothing), sufficient active vitamin D cannot be produced, so calcium balance is impaired and hypocalcaemia can result. This leads to the conditions rickets (in children) and osteomalacia (in adults), marked by defects in bone mineralisation, with bone weakness, bending and distortion. It is prevented or treated with a vitamin D compound (ergocalciferol or calcitriol); this is effective in relieving hypocalcaemia but cannot correct already deformed bones.
Pharmacological uses
There are various forms of vitamin D available, for example calcitriol (see Drug Monograph 37-4), colecalciferol (vitamin D3, formerly spelt cholecalciferol) and a new analogue paricalcitol. Ergocalciferol (vitamin D2) is now only available in low doses in multivitamin formulations. They are administered to prevent or treat deficiencies of vitamin D.
Drug monograph 37-4 Calcitriol
There are several forms of vitamin D available. Vitamins D2 and D3 require activation in the liver and kidney, and have a slow onset (4–8 weeks) and long duration of action (8–16 weeks). They are useful for preventing vitamin D deficiencies in people with adequate kidney function. Calcitriol is the preactivated form of the vitamin, 1,25-dihydroxycolecalciferol.
Indications
Calcitriol is indicated in vitamin D deficiencies, hypocalcaemia, hypoparathyroidism, renal osteodystrophy, chronic renal dialysis (which may remove calcium or vitamin D) and postmenopausal and corticosteroid-induced osteoporosis.
Pharmacokinetics
Calcitriol is well absorbed from the intestine, requiring bile salts for absorption. It enters the enterohepatic circulation and metabolites (some active) are excreted in the faeces and urine; some are stored in fat. The elimination half-life is about 3–6 hours but the effects of a single dose last for several days. It is transported across the placenta (ADEC Preg nancy Safety Category B3) and into breast milk; hence it is contraindicated during pregnancy and lactation.
Adverse drug reactions and interactions
The main adverse effects are those of hypercalcaemia, i.e. gastrointestinal disturbances, polyuria and ectopic calcification (see above). Significant interactions occur with digoxin (arrhythmias), cholestyramine (impaired absorption) and calcium supplements or thiazide diuretics (hypercalcaemia).
Paricalcitol
Paricalcitol is a new vitamin D analogue, approved for use in renal osteodystrophy associated with hyperparathyroidism secondary to chronic renal failure that leads to impaired activation of vitamin D. The oral form is well absorbed, and metabolites are excreted mainly via the faeces with a half-life of 4–7 hours. Adverse events include diarrhoea, oedema, allergic reactions, arthritis and dizziness.
Bisphosphonates
The bisphosphonates are relatively new drugs specifically designed to decrease bone turnover. They are analogues of pyrophosphate (basic structure P–O–P), which is a natural inhibitor of bone mineralisation.2 The bisphosphonates have the general structure P–C–P, with two phosphonate groups linked by carbon rather than oxygen, making them more resistant to enzymatic inactivation.
Mechanism of action
Bisphosphonate drugs are incorporated into bone, where they form a depot for some months. They act to inhibit normal and abnormal resorption, primarily by decreas ing the activity of osteoclasts, and inhibit pathologic cal cification. Simple, non-nitrogen-containing clodronate and etidronate are metabolically incorporated into ATP analogues, and thus inhibit ATP-dependent enzymes. The early drugs in the group, especially etidronate, in high doses reduced bone formation, making them less useful in treating disorders of bone. The nitrogen-containing analogues (alendronate [Drug Monograph 37-5], ibandronate, pamidronate, tiludronate, zoledronate and risedronate) inhibit bone resorption without also inhibiting bone formation. There are many proposed sites of action, including inhibition of a key enzyme (farnesyl pyrophosphate synthase) in the mevalonate pathway, hence inhibiting synthesis of many GTP-binding proteins important in regulation of osteoclast functions.
Drug monograph 37-5 Alendronate, a bisphosphonate
Alendronate has the actions of the bisphosphonates: it impedes bone resorption and reduces bone turnover.
Indications
Alendronate is indicated in Paget’s disease, for the prevention and treatment of post-menopausal osteoporosis and for preventing and treating corticosteroid-induced osteoporosis. Other drugs in the group may also be indicated for hypercalcaemia associated with cancer, heterotopic ossification and osteolytic metastases (bone metastases).
Pharmacokinetics
Alendronate has most unusual pharmacokinetic characteristics: it has virtually zero bioavailability, zero plasma levels and no metabolism, and a (terminal) half-life of more than 10 years! This is because the drug is taken up 50% into the bones, where it forms a stable depot for several weeks; the remaining 50% of an oral dose is excreted in the urine unchanged.
Drug interactions
Oral medications, including antacids, calcium and mineral (iron, magnesium) supplements, and food or beverages may interfere with the absorption of alendronate. Administration of the medications should be separated by at least 2 hours. Alendronate increases the risk of gastric ulceration with NSAIDs, so the combination should be avoided.
Warnings and contraindications
Use all bisphosphonates with caution in patients with oesophageal disorders, other gastrointestinal diseases and kidney impairment, or if the patient is reportedly hypersensitive to them.
Dosage and administration
Because of the risk of oesophagitis, it is recommended that bisphos phonates be taken with a full glass of liquid and that the patient remain upright for at least the next 30 minutes, to facilitate delivery of the dose to the stomach.
The usual adult dosage of alendronate for Paget’s disease is 40 mg daily (at least 30 minutes before breakfast) for 6 months; for osteoporosis, the dosage is 5–10 mg once daily or 70 mg once a week.
Clinical aspects
Indications for use
Early uses were to inhibit ectopic calcification and as agents for bone imaging. The bisphosphonates are now the major drugs used to treat disorders involving excessive bone resorption, so they are useful for:
Specific bisphosphonates are indicated for particular disorders, and the routes, doses, management, associated risks and counselling vary, so it is difficult to generalise about their clinical use in specific situations.
Formulations and administration
All bisphosphonates have low oral bioavailability, which is reduced by food, antacids and minerals such as iron and calcium. For those taken orally, the recommendation is that the tablet or capsule must be taken with a full glass of plain water at least 30 minutes before eating, and the person should remain upright until after eating (this is because early bisphosphonates including alendronate tended to cause acid regurgitation, oesophageal ulcer and oesophagitis). Ibandronic acid, pamidronate and zoledronic acid are only formulated for administration by injection.
Combination products are available with other drugs affecting bone. For example, risedronate is available in compound tablet formulations with calcium carbonate and colecalciferol, alendronate with colecalciferol, and etidronate with calcium.
Adverse drug reactions and interactions
There are many adverse effects especially in the GIT, including gas production, acid regurgitation, oesophageal ulcer, gastritis, dysphagia, constipation and diarrhoea. Hypocalcaemia, muscle pain and headaches are common, and flu-like symptoms after IV injection. Intravenous administration of zoledronate and pamidronate has led to cases of nephrotoxicity, such as tubular necrosis and glomerulosclerosis; ibandronate appears to be safer. Bisphosphonates may increase the risk of renal impairment, atrial fibrillation and heart failure.
A rare (0.1%–0.3% risk) but potentially severe adverse effect is osteonecrosis of the jaw, apparently triggered in susceptible patients by dental extractions, periodontal disease or oral trauma. Patients on bisphosphonates should have dental assessment and any required treatment before starting on therapy, and regular dental checks thereafter.
As described earlier, antacids, minerals and food reduce the oral absorption of bisphosphonates and reduce their activity; these drugs should not be taken within 2 hours of a bisphosphonate. The risk of gastric ulceration with NSAIDs is increased by alendronate; indomethacin increases tiludronate concentration and increases the risk of adverse effects.
Strontium
Strontium, an element with chemical properties very similar to those of calcium, has long been known to have ‘bone-seeking’ roles: at moderate supplementation levels strontium promotes calcium uptake into bone, whereas at higher dietary levels strontium has rachitogenic (causing rickets) actions.
Strontium ranelate
Mechanism of action
A derivative, strontium ranelate, was approved in Australia in July 2005 for use in treatment of post-menopausal osteoporosis. The ranelate radical is an unusual fivemembered ring with sulfur and cyanide substituents and four carboxyl ‘arms’, which act as carriers of calcium or strontium. The molecule has a unique mode of action, decreasing bone resorption and stimulating bone formation via increased osteoblast differentiation and replication, collagen synthesis and bone matrix mineralisation, along with inhibited osteoclast activity. After oral administration, the molecule dissociates and strontium is taken into bone; it is slowly released and eventually excreted (half-life, 60 hours) by the gut and kidney.
Clinical use
Strontium ranelate has been shown to be effective in postmenopausal women with a history of bone fractures, reducing incidence of new fractures and increasing bone mineral density. It is particularly effective is reducing non-vertebral and hip fractures in frail elderly women. Further clinical studies are needed to determine its use in prevention of post-menopausal bone loss, and in combination therapy with bisphosphonates. The usual dose is 2 g granules made up as an oral liquid, taken at bedtime; absorption is reduced by calcium. Adverse reactions include nausea, diarrhoea, increased risk of venous thromboembolism and neurological problems such as headache, seizures and memory loss. Rarely, severe lifethreatening allergic reactions can occur.
Oestrogens
During their reproductive years, women have low risk of osteoporosis. This is attributed to the protective effects of oestrogens, which may oppose the actions of PTH on bone—PTH normally increases resorption and mobilises calcium.
Selective oestrogen receptor modulators (SERMs)
The SERM3 raloxifene has partial agonist and partial antagonist oestrogenic properties. It has positive effects on bone and lipid metabolism and antagonistic effects on the uterus and breast, minimising the risk of oestrogendependent cancers. Consequently, raloxifene prevents bone resorption without stimulating breast or uterine tissue. Recent evidence is emerging that raloxifene is as effective as tamoxifen (Drug Monograph 42-3), another SERM, at preventing oestrogen-receptor positive breast cancer in women at increased risk of breast cancer. Lasofoxifene, a new SERM, is currently in clinical trials in post-menopausal osteoporosis. This group of drugs is considered in the chapter on the female reproductive system (Chapter 38).
Drug treatment of osteoporosis
General aspects
Management involves assessing risk factors and contributing conditions, reducing the risk of falls, monitoring bone density by densitometry techniques and attention to diet, especially intake of vitamin D and calcium. Calcium (1.0–1.5 g/day to maintain bone minerals) should be adjunctive therapy for all people with osteoporosis, unless contraindicated (e.g. hypercalcaemia).
Anabolic agents
Drugs used to treat osteoporosis either inhibit bone resorption (antiresorptive) or have anabolic (building up) actions—the only current anabolic agent is parathyroid hormone or teriparatide, which stimulates bone formation (Drug Monograph 37-1). It is used for the treatment of severe osteoporosis when the risk of fracture is extremely high, or if other effective therapies are unsuitable. New anabolic drugs being developed act by antagonising the calcium-sensing receptor or targeting various proteins involved in calcium balance or bone remodelling.
Antiresorptive agents
Antiresorptive agents used in post-menopausal osteoporosis include:
New antiresorptive agents in development include denosumab, an antibody to RANKL (a cytokine involved in activation of osteoclasts), and inhibitors of other enzymes or mediators involved in bone resorption (see reviews by Martin and Seeman [2007], Cole et al [2008] and Deal [2009]).
Corticosteroids
The previous groups of drugs are all useful in treatment of bone disorders; however, corticosteroids have adverse effects on bone: they are known to increase the risk of osteoporosis (see Drug Monograph 35-1). Osteoporosisrelated fractures occur in 30%–50% of patients receiving long-term glucocorticoids in daily doses of 2.5 mg prednisolone or equivalent. Several mechanisms have been proposed: glucocorticoids impair transcription of the collagenase gene and block induction of the osteocalcin (calcium-binding) gene by vitamin D, reduce intestinal calcium absorption, increase urinary excretion of calcium and inhibit functions and replenishment of osteoblasts. After short-term treatment with corticosteroids, fracture risk usually falls rapidly.
Current recommendations are that all patients on corticosteroid therapy should routinely receive concurrent calcium and vitamin D supplements, and those with higher risk of fractures (with low bone density and post-menopausal women) should also receive a bisphosphonate (see review by Romas [2008]). Teriparatide and calcitriol are also used to prevent corticosteroid-induced osteoporosis.
Complementary and alternative therapies
Nutrition is one of many factors that affect bone health; in particular, there is good evidence that adequate dietary intake through the lifespan of calcium, vitamin D and vitamin K enhances bone density and reduces risk of osteoporosis later in life. Many other minerals, vitamins and peptides have also been proposed as beneficial to bone health. Potential detrimental dietary factors include excesses of alcohol, caffeine, sodium, fluoride, phosphorus and vitamin A.
Nutraceuticals
The term nutraceutical has been coined to include extracts of foods claimed to be beneficial to health and formulated in typical pharmaceutical products; examples are flavonoid antioxidants and beta-carotene from orangeyellow plants. ‘Functional foods’ are those consumed as part of a normal diet and for which health benefits are claimed, such as probiotic yoghurts and vitamin- or mineral-enriched food products. In the area of bone health, some studies have shown benefits from dietary phytoestrogens (e.g. in soy-based foods) in increasing BMD and non-digestible polysaccharides (such as inulin-type fructans) in enhancing calcium absorption from the diet.
Key points
 The parathyroid glands synthesise and secrete parathyroid hormone (PTH), a peptide hormone that maintains appropriate calcium levels in the extracellular fluid.
The parathyroid glands synthesise and secrete parathyroid hormone (PTH), a peptide hormone that maintains appropriate calcium levels in the extracellular fluid. In hypoparathyroidism, severe hypocalcaemia and tetany can occur; the use of vitamin D and calcium supplements will usually restore the calcium and phosphorus levels to normal.
In hypoparathyroidism, severe hypocalcaemia and tetany can occur; the use of vitamin D and calcium supplements will usually restore the calcium and phosphorus levels to normal. In hyperparathyroidism, the primary approach is usually surgery; hypercalcaemia also responds to rehydration, calcitonin and bisphosphonate drugs.
In hyperparathyroidism, the primary approach is usually surgery; hypercalcaemia also responds to rehydration, calcitonin and bisphosphonate drugs. Calcium has important roles in the body both in physiological processes and in bone structure; calcium balance is tightly controlled by PTH, calcitonin and vitamin D.
Calcium has important roles in the body both in physiological processes and in bone structure; calcium balance is tightly controlled by PTH, calcitonin and vitamin D. Bone undergoes continual remodelling; bone resorption leads to mobilisation of both calcium and phosphate from bone components.
Bone undergoes continual remodelling; bone resorption leads to mobilisation of both calcium and phosphate from bone components.Review exercises
References and further reading
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Benson J., Skull S. Hiding from the sun: vitamin D deficiency in refugees. Australian Family Physician. 2007;36(5):355-357.
Cashman K.D. Diet, nutrition and bone health. Journal of Nutrition. 2007;137:2507S-2512S.
Chen D., Zhao M., Mundy G.R. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233-241.
Cole Z., Dennison E., Cooper C. Update on the treatment of postmenopausal osteoporosis. British Medical Bulletin. 2008;86:129-143.
Deal C. Potential new drug targets for osteoporosis. Nature Clinical Practice Rheumatology. 2009;5(1):20-27.
Dean D.B., Watson J.T., Moed B.R., Zhang Z. Role of bone morphogenetic proteins and their antagonists in healing of bone fracture. Frontiers in Bioscience. 2009;14:2878-2888.
Endocrinology Expert Group. Therapeutic Guidelines: Endocrinology, version 4. Melbourne: Therapeutic Guidelines Limited; 2009.
Goss A., Backhouse P. Medicinal mishap: bisphosphonates and osteonecrosis of the jaws. Australian Prescriber. 2007;30(4):96-97.
Grills B. Osseous structure and function: study guide. Melbourne: La Trobe University, 2002.[unpublished].
Khan A., Grey A., Shoback D. Medical management of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. Journal of Clinical Endocrinology and Metabolism. 2009;94(2):373-381.
Lee N.K., Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends in Endocrinology and Metabolism. 2008;19(5):161-166.
Martin T.J., Seeman E. New mechanisms and targets in the treatment of bone fragility. Clinical Science. 2007;112(2):77-91.
Munns C., Zacharin M.R., Rodda CP., et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Medical Journal of Australia. 2006;185(5):268-272.
Naot D., Cornish J. The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism. Bone. 2008;43(5):813-818.
Nguyen T.V., Center J.R., Eisman J.A. Osteoporosis: underrated, underdiagnosed and undertreated. Medical Journal of Australia. 2004;180(5 Suppl):S18-S22.
Pors Nielsen S. The biological role of strontium. Bone. 2004;35(3):583-588.
Quarles L.D. Endocrine functions of bone in mineral metabolism regulation. Journal of Clinical Investigation. 2008;118:3820-3828.
Ralston S.H., Langston A.L., Reid I.R. Pathogenesis and management of Paget’s disease of bone. Lancet. 2008;372(9633):155-163.
Roberts D.M., Singer R.F. Management of renal bone disease. Australian Prescriber. 2010;33(2):34-37.
Romas E. Corticosteroid-induced osteoporosis and fractures. Australian Prescriber. 2008;31(2):45-49.
Russell D., Parnell W., Wilson N., et al. NZ Food: NZ People. Key results of the 1997 National Nutrition Survey. Wellington: New Zealand Ministry of Health; 1999.
Russell R.G.G. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119:S150-S162.
Seeman E., Eisman J.A. Treatment of osteoporosis: why, whom, when and how to treat. Medical Journal of Australia. 2004;180(6):298-303.
Theman T.A., Collins M.T. The role of the calcium-sensing receptor in bone biology and pathophysiology. Current Pharmaceutical Biotechnology. 2009;10(3):289-301.
Vogel V.G., Constantino J.P., Wickerham D.L., et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. Journal of the American Medical Association. 2006;295:2727-2741.
Wark J.D. Calcium supplementation: the bare bones. Australian Prescriber. 2003;26(6):126-127.
Winzenberg T., Powell S., Jones G. Strontium ranelate: does it affect the management of postmenopausal osteoporosis? Australian Family Physician. 2007;36(8):631-632.
Wolf P.L. If clinical chemistry had existed then. Clinical Chemistry. 1994;40(2):328-335.
Australian and New Zealand Bone and Mineral Society: www.anzbms.org.au/
MIMS OnLine New Zealand Medicines and Medical Devices Safety Authority: www.medsafe.govt.nz
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology