Chapter 36 The Endocrine Pancreas and Management of Diabetes Mellitus
The pancreas is a gland with both exocrine and endocrine functions; its major hormones are insulin and glucagon. Their main physiological effects are the regulation of nutrient storage and blood glucose levels. Inadequate production of insulin causes diabetes mellitus, a common disorder of carbohydrate metabolism that affects about 2% of the population and has serious long-term complications due to vascular disease, impaired circulation and damage to kidneys, eyes and feet. Pharmacological treatment of diabetes depends on the type: patients with type 1 diabetes are dependent on injections of insulin; many formulations of human and bovine insulin are available. People with type 2 diabetes may be treated with improved diet and exercise and/or maintained on oral hypoglycaemic agents, often with additional insulin. Hypoglycaemia is a potential adverse effect of all hypoglycaemic agents; it can also occur in other conditions, and can be treated with hyperglycaemic medications such as glucose or glucagon.
Key abbreviations
DCCT Diabetes Control and Complications Trial
HbA1C glycosylated haemoglobin
IDDM insulin-dependent diabetes mellitus
NIDDM non-insulin-dependent diabetes mellitus
Key background: the endocrine pancreas
Hormones
The pancreas is a gland that lies transversely across the abdomen, in close contact with the duodenum. It secretes into the duodenum, via the pancreatic duct, a clear, colourless fluid (about 1200–1500 mL/day) containing mainly water, sodium bicarbonate, salts and enzymes that digest proteins, fats, carbohydrates and nucleic acids—these are the exocrine functions of the gland (see Unit 9).
Scattered among the clusters of exocrine cells are small pockets of endocrine tissue known as the pancreatic islets, or islets of Langerhans, making up about 2% of the weight of the pancreas. These cells have endocrine functions: they produce hormones that are secreted into the bloodstream and are involved in nutrient balance, particularly blood glucose levels, and gastrointestinal (GI) functions. Different cell types produce specific hormones: insulin is produced by beta cells, glucagon by alpha, or A, cells, and somatostatin (GHRIF) by delta, or D, cells. (Somatostatin is considered in Chapter 33; it has significant effects in inhibiting not only the release of growth hormone but also of insulin and glucagon.)
Insulin
Insulin can be considered as the body’s main fuel storage hormone; it is secreted by the pancreatic beta cells in response to raised levels of glucose in the blood (see Clinical Interest Box 36-1). Insulin’s overall functions are to ensure that tissues have sufficient chemical substrates for energy, storage, anabolism and repair.
Clinical Interest Box 36-1 History of diabetes and insulin
The condition has been known for thousands of years; early Egyptian references describe flesh melting into urine, unquenchable thirst and inevitable early death.
The involvement of the pancreas was described
Minkowski and von Mering demonstrated that a pancreatectomised dog produced large volumes of urine with a high sugar content
Diabetic patients were shown to have pancreatic lesions in the islets of Langerhans
The concept of hormones secreted from a gland into the bloodstream developed
Toronto scientist Banting and medical student Best extracted insulin from islet tissue and successfully treated pancreatectomised dogs; this was such a major breakthrough in the treatment of diabetes that Banting and the Professor of Physiology, McLeod, very soon received the 1923 Nobel Prize for Medicine for their work
Insulin was first available clinically
As people with diabetes could be treated and hence live longer, the long-term complications were noted—especially renal and vascular problems and retinopathy
The amino acid sequence and structure of the 2 polypeptide chains of insulin were determined by English biochemist Frederick Sanger, for which he was awarded the 1958 Nobel Prize (he won a second Nobel Prize in 1980 for his work determining the base sequences in nucleic acids)
The first oral hypoglycaemic agent became available
Recombinant human insulin was produced by genetic engineering techniques
Currently, the primary cause of type 1 diabetes is still not definite—a viral infection leading to autoimmune destruction of the pancreatic islet cells is postulated.
Insulin is a protein hormone consisting of two polypeptide chains joined by disulfide bridges; the chains contain 51 amino acids, the exact sequence of which is known. It is synthesised in the beta cells from a larger protein known as proinsulin, which acts as the storage form of the hormone. Insulin’s importance in medicine is indicated by the fact that it was the first protein for which the amino acid sequence was determined, and the first which was synthesised by genetic engineering technologies (see Clinical Interest Box 36-1).
Release and circulation of insulin
The most important physiological stimulus is raised blood glucose level: within 30–60 seconds of absorption of glucose after a meal, there is increased release of insulin. This occurs via an excitation–secretion coupling process with depolarisation of cell membranes and calcium influx, then exocytosis of insulin-containing secretory vesicles, with a rapid initial rise due to release of stored insulin, then a slower delayed phase over 60–90 minutes when both stored and newly synthesised insulin are released to deal with the raised sugar level in the blood. A fall in blood glucose directly inhibits insulin secretion, which shifts metabolism to the post-absorptive pattern levels such as occur during fasting.
Other stimuli to insulin secretion include:
Insulin is released via capillaries into the portal circulation to the liver. There is a low basal level of release in pulses every 15–30 minutes, with increased release in response to stimuli.
Insulin release is inhibited by somatostatin and by adrenaline (via α2 receptors). Deficiencies of release occur in pancreatic disorders (diabetes mellitus, pancreatitis, tumours) and other endocrine disorders (Cushing’s disease, acromegaly), and can be caused by drugs, including alloxan (of mainly experimental interest) and the thiazide diuretics.
Insulin is circulated bound to β-globulin. As a protein, it is rapidly digested in the gut if given orally, with a half-life of only a few minutes (this explains why the hormone must be administered parenterally to treat diabetes). It has much longer biological duration of action (2–4 hours), however, as it is taken up and bound to receptors in the tissues where it acts.
Actions of insulin
Overall, insulin facilitates removal of glucose from the blood into muscle and fat cells and promotes storage of nutrients. After binding of insulin to specific receptors on membranes of target cells, glucose transporters are recruited from intracellular storage pools to the cell membrane, where they facilitate the active uptake of glucose into the cell where it is ‘trapped’ as glucose-6-phosphate. A great variety of biochemical reactions and processes are mediated by insulin: in summary, it affects uptake, utilisation and storage of carbohydrates, fats and amino acids in liver, adipose and muscle cells, so that nutrients are stored as glycogen, triglycerides and fatty acids, and proteins. It thus controls intermediary metabolism, promotes the anabolic state (building up) and has long-term effects on cell proliferation and growth regulation. Glycogenolysis, lipolysis and proteolysis are inhibited.
The actions of insulin are physiologically antagonised by the catabolic hormones, i.e. adrenocorticotrophic hormone (ACTH), glucocorticoids, adrenaline, growth hormone (GH) and thyroxine. Hence, low insulin levels (and diabetes) can occur secondary to other endocrine disorders, including acromegaly and Cushing’s disease.
Mechanism of action
The mechanism of action of insulin is via binding to membrane receptors on target cells and activation of a tyrosine kinase enzyme. This initiates cascades of phosphorylation reactions leading to many kinase and phosphatase activities, as well as DNA transcription and cell replication. Intracellular vesicles containing a glucose transporter (GLUT-4) fuse with the plasma membrane and the transporter is inserted, leading to a rapid 10-to 30-fold increase in glucose uptake by the cell. Cells in the brain, exercising muscle and liver are not dependent on insulinmediated glucose uptake.
Glucagon
Glucagon is a 29-amino-acid polypeptide hormone secreted by the alpha cells of the islets of Langerhans in response to hypoglycaemia. It was discovered in 1923 as a contaminant of insulin preparations. It can be considered a fuel-mobilising hormone, in contrast with the fuelstorage functions of insulin; it has been called an ‘antiinsulin’. Glucagon acts primarily by stimulating hepatic glycogenolysis and gluconeogenesis (the conversion of glycerol and amino acids to glucose) and by inhibition of glycogen synthesis, which produces an elevation in the concentration of glucose in the blood. The effects of glucagon are accelerated by stimulation of the synthesis of cyclic 3́,5́-adenosine monophosphate (cyclic AMP). Hepatic and adipose tissue lipolysis is enhanced, producing free fatty acids and glycerol, which stimulate ketogenesis. Glucagon does not mobilise muscle glycogen. It stimulates release of catecholamines and hence inhibits tone and motility in GIT smooth muscle, and may have other sympathomimetic effects.
Secretion of glucagon is stimulated by low blood sugar levels (hypoglycaemia) and high-protein meals, and by exercise and stress, including infections. Secretion is inhibited by insulin and hyperglycaemia. (In diabetes, the lack of insulin leads to increased release of glucagon, which contributes to the markedly raised blood sugar levels and eventually to the state of ketosis.)
Glucagon also increases release of GH and ACTH, and (paradoxically) of insulin. It is used clinically to treat insulininduced hypoglycaemia (see Drug Monograph 36-1).
Glucagon is released from pancreatic alpha cells to maintain plasma levels of glucose by decreasing glycogen synthesis, and promoting glycogenolysis and gluconeogenesis. It also enhances fat breakdown and degradation of liver protein.
Indications
Glucagon is indicated for the treatment of severe hypoglycaemia in people with diabetes, and to terminate insulin coma. It is useful in hypoglycaemia only if liver glycogen is available; thus it is ineffective for chronic hypoglycaemia, starvation and adrenal insufficiency. It is also used as an adjunct for GI radiography, as it produces relaxation and decreases peristalsis, improving the outcome of GIT examination.
Pharmacokinetics
As glucagon is a protein, it must be parenterally administered (IM, IV or SC). It has a half-life of 5-10 minutes and an onset of action (hyperglycaemic) according to route of administration: IV, 5-20 minutes; IM, 15 minutes; and SC, 30-45 minutes. Its duration of action is 1.5 hours. It is bound in the liver, kidneys and other organs, and is metabolised in the blood and organs.
Clinical aspects
No significant drug interactions are reported. Adverse effects are mild and may include nausea or vomiting and an allergic reaction. It is safe in pregnancy and lactation, but is contraindicated in people with glucagon hypersensitivity, phaeochromocytoma or a history of insulinoma. The adolescent and adult dose for hypoglycaemia is 0.5-1 mg IM, IV or SC, repeated in 20 minutes when necessary.
Control of blood glucose
Insulin and glucagon
Both the primary hormones released by the pancreas have major roles in control of blood glucose levels, as described above. Carbohydrate metabolism is controlled by a finely balanced interaction of several endocrine factors, in the adrenal, anterior pituitary and thyroid glands; these processes are summarised in Figure 36-1.
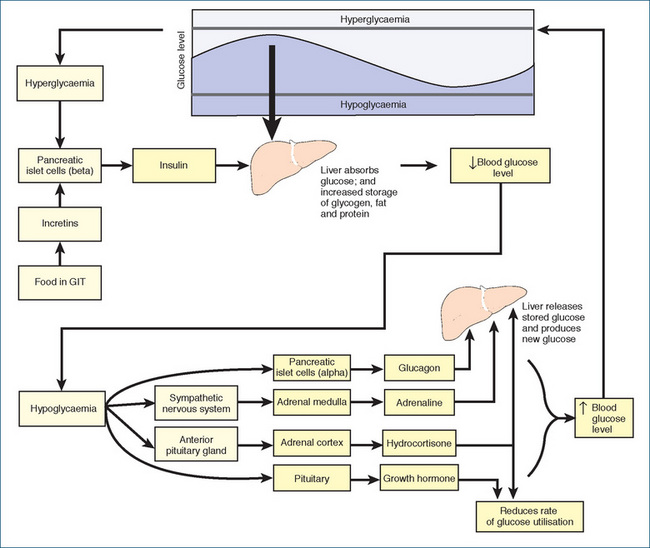
Figure 36-1 Control of blood glucose levels. Raised blood glucose levels and the presence of food in the GIT (via incretins) cause the pancreas to release insulin, which causes the liver to absorb excess blood glucose and leads to storage of glycogen, fat and protein. When blood glucose levels are low, the alpha cells in the islets of Langerhans secrete glucagon, which stimulates liver glycogenolysis and gluconeogenesis. The sympathetic nervous system signals the adrenal medulla to secrete adrenaline, while the anterior pituitary gland signals the adrenal cortex to release hydrocortisone. Both substances enhance gluconeogenesis, while adrenaline also increases glycogenolysis, and hydrocortisone slows down the rate of glucose utilisation and raises the plasma level of amino acids available for glucose production. The pituitary secretes growth hormone, which decreases cellular glucose utilisation and promotes glycogenolysis. The hypothalamus (not shown) is also involved, by sensing high levels of nutrients and suppressing glucose production.
When plasma blood glucose declines, glucagon is released and increases blood glucose levels by the mechanisms described above. The release of glucagon (and raised blood glucose level) stimulates insulin secretion, which then inhibits further release of glucagon. This feedback mechanism keeps the plasma glucose level around the optimum.
When blood glucose increases, glycogenesis (the conversion of excess glucose to glycogen for storage in skeletal muscle and the liver) occurs, gluconeogenesis is slowed and glucose uptake into cells is facilitated, thus lowering blood glucose levels.
Central nervous system influences
It is now apparent that hypothalamic centres control both energy balance and glucose homeostasis (see review by Prody & Obici [2006]). The availability of peripheral nutrients such as glucose and fatty acids is sensed in the arcuate nucleus of the hypothalamus, where insulin receptors play an important role in suppressing endogenous glucose production, via alterations in release and biosynthesis of various neuropeptides and activation of ATP-sensitive potassium channels. Neural signals are relayed via efferent vagal fibres to the liver, where glucose production is inhibited. The anti-obesity hormone leptin improves insulin sensitivity via actions in the arcuate nucleus, partly by activating neurones producing melanocortins with anorectic (appetite suppressant) actions (see Chapter 50). In diabetes type 2 and obesity, the brain fails to correctly perceive and respond to peripheral signals of nutrient availability.
Incretins
Incretins are newly-discovered peptide hormones secreted from the GIT into the circulation in the presence of food. They thus ‘alert’ the pancreatic β-cells to impending rise in blood glucose levels and cause increased insulin secretion, via raised cAMP levels and calcium-induced exocytosis. The incretins currently known are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulotropic polypeptide (GIP); both stimulate glucose-dependent insulin release, enhance β-cell proliferation and delay gastric emptying. Response to incretins is reduced in patients with type 2 diabetes.
Pathologies
Diabetes mellitus
The most important disease involving the endocrine pancreas is diabetes mellitus, which is characterised by polyuria associated with a chronic disorder of carbohydrate and lipid metabolism, and an inappropriate rise in glucose level in the blood, due to a relative or absolute lack of insulin.
The term ‘diabetes mellitus’ refers to the ‘copious urine, sweet or honey-tasting’, distinguishing it in earlier times (when doctors diagnosed by tasting the patient’s urine) from diabetes insipidus, in which the copious urine was dilute and tasteless. There are many causes and forms of diabetes mellitus and impaired glucose tolerance, but all lead to hyperglycaemia and a wide range of metabolic and cardiovascular problems (see Clinical Interest Box 36-1 for a brief outline of the history of diabetes).
Epidemiology
The self-reported prevalence of diabetes mellitus in the adult Australian population is around 3.5%; the prevalence increases with age, so in those over 65 years of age the prevalence is more like 14%. However, there may be as many people again in whom the disorder has not been diagnosed. Of people with diabetes, approximately 83% report having type 2. Diabetes is the ninth leading cause of death in Australia (Australian Bureau of Statistics 2007); it is the main underlying cause in 2.8% of registered deaths and is a contributing factor in almost 10% of all deaths. The cost to the Australian community of treating diabetes in 2000/01 was estimated to be about $784 million. It is recommended that all people over 55 years be screened for glucose intolerance, plus younger people with added risk factors.
Worldwide, the incidence of type 2 diabetes is increasing; risk factors include disadvantaged socioeconomic status, overweight and obesity, lack of exercise and hypertension. Increasing incidence is occurring in the Australian Aboriginal and Torres Strait Islander population (see Clinical Interest Box 36-2) and in the Polynesian population in New Zealand (Clinical Interest Box 36-3). In developing countries, the mortality rate is unacceptably high, often due to inadequate, expensive and irregular supplies of insulin. In many Pacific Island countries, prevalence is increasing rapidly, due partly to a shift away from traditional diet (vegetables and fruit) to Western-type diet, fatty meats and ‘junk foods’, with the consequent rise in obesity.
Clinical Interest Box 36-2 Diabetes in the australian population
The National Health Survey carried out by the Australian Bureau of Statistics in 2004/05 showed that Aboriginal and Torres Strait Islander Australians have a 2-4 times higher prevalence of type 2 diabetes and coronary heart disease than do Australians of European descent. Diabetes was the underlying cause of death in 6.7% of deaths of people registered as Indigenous; this is thought to be an underestimate. Median age at death from diabetes was 62.8 years, compared with 81.2 for non-Indigenous Australians. Renal disease and infections (lungs, skin and urinary tract) are the most common complications.
An earlier follow-up study in two remote Australian Aboriginal communities showed that, over a period of 8 years, the population diabetes incidence rate was 20.3 cases per 1000 person-years. Risk of developing diabetes was closely associated with body mass index (BMI), with the BMI-associated diabetes incidence among the highest in the world. The risk of diabetes in the lowest BMI category was 2-5 times greater than in corresponding non-Aboriginal people.
The rapid rise in prevalence of diabetes mirrors that of other nutrition-related ’lifestyle’ disorders, including obesity, cardiovascular disease and chronic renal diseases, in many other countries as well. It has been suggested that, whereas the high level of insulin resistance among Aboriginal and Torres Strait Islander Australians would have protected them on a traditional low-fat/high-fibre diet requiring physical energy to obtain it, this insulin resistance is unsuited to a Westernised high-carbohydrate diet. Poor access to nutritious affordable food and quality health services and inadequate understanding of the role of diet and obesity in diabetes are barriers to optimal diabetes management in isolated communities. Other risk factors include low birth weight, children and adults being overweight, cigarette smoking, excessive alcohol drinking and social factors such as poverty, overcrowding in inferior housing, poor standards of hygiene and poor understanding of health and nutrition.
Hyperglycaemia in type 2 diabetes is best controlled with weight loss, adequate diet and exercise, reduced alcohol consumption and use of oral hypoglycaemic agents. Establishment of local registers of people with diabetes and regular assessment and screening are recommended, along with health, exercise and nutrition education programs, more affordable healthy food supplies, strong community-based involvement in public health programs and quality use of medicines to treat hypertension, high cholesterol levels and type 2 diabetes.
Based on: Australian Bureau of Statistics 2007; Gracey 2007; O’Dea et al 2007.
Clinical Interest Box 36-3 Diabetes in new zealand
Diabetes mellitus is a major and increasing health problem in New Zealand. According to the New Zealand Health Survey 2006/07,7.5% of adults have doctor-diagnosed diabetes. The Ministry of Health estimates that as many as 50% of New Zealanders with diabetes remain undiagnosed. Although the ethnic make-up of New Zealand is predominantly European (Pakeha), the Polynesian population, consisting mainly of Indigenous Maori and more recent immigrants from other Pacific Islands, is increasing rapidly. Maori and Pacific Island people were three times more likely to have been diagnosed with diabetes than European/Pakeha people. The prevalence of obesity in these Polynesians is also high. These factors plus an ageing population are increasing the incidence and prevalence of type 2 diabetes in New Zealand. For reasons unknown the incidence of type 1 diabetes is also rising. Studies of diabetes in inner urban communities in Auckland (New Zealand’s largest city) have shown high prevalence among Maori, Pacific Islander and some Asian ethnic groups there. Adults in the most deprived areas were twice as likely to be diagnosed with diabetes compared with those living in the least deprived areas.
Diabetic nephropathy is the most common cause of end-stage renal failure in New Zealand. Polynesian people with diabetes, particularly Maori, have a very high rate of diabetic nephropathy and develop renal failure at a more rapid rate than European/ Pakeha patients. This may relate to a genetic susceptibility to nephropathy, the younger age at which they develop diabetes and socioeconomic or cultural factors leading to less adequate medical care. A study carried out in South Auckland among people with diabetes who had poor blood glucose control showed that presenting medications in blister pack forms led to significant improvements in glycaemic control and systolic blood pressure measurements.
Metformin, the biguanide oral hypoglycaemic agent used in type 2 diabetes, remains a major cause of drug-associated mortality in New Zealand. Of 12 cases of lactic acidosis associated with metformin reported to the New Zealand Centre for Adverse Reactions Monitoring, eight had a fatal outcome, mostly in patients with pre-disposing factors such as renal insufficiency, chronic hepatic disease and conditions associated with hypoxia, including surgery, sepsis, increasing age, dehydration and alcoholism.
Sources: Simmons et al 1999; New Zealand 2002/03 and 2006/07 Health Survey, Ministry of Health 2008; Joshy & Simmons 2006.
Pathology
Uncontrolled diabetes is a devastating disease that is a major cause of blindness, end-stage renal disease, neuropathies, accelerated atherosclerosis and lower limb amputations. The two general classifications for diabetes mellitus are type 1 diabetes (formerly known as insulindependent diabetes mellitus, IDDM, or juvenile-onset) and type 2 diabetes (non-insulin-dependent diabetes mellitus, NIDDM, maturity-onset). The features of the two types are summarised in Table 36-1.
Table 36-1 Features of type 1 and type 2 diabetes
| FEATURE | TYPE 1 | TYPE 2 |
| Synonyms (former) | IDDM, juvenile-onset | NIDDM, maturity-onset |
| Age of onset | Usually <20 years | Usually >35 years |
| Onset of symptoms | Sudden (symptomatic) | Gradual (usually asymptomatic) |
| Body weight | Usually non-obese | Obese (80%) |
| Family history | Usually negative | Often positive |
| Incidence (% all diabetes, approximate) | 15% | 85% |
| Insulin levels | Low, then absent | May be low, normal or high (insulin resistance) |
| Insulin-dependent | Yes | Usually not (may progress to be) |
| Insulin resistance | No | Yes |
| Insulin receptors | Normal | Usually low or defective |
| Complications | Frequent | Frequent |
| Ketoacidosis | Prone | to Rare |
| Dietary modifications | Mandatory | Mandatory |
| Treatment | Insulin essential | Diet, oral hypoglycaemic agents, possibly insulin |
The complex disorder of carbohydrate, fat and protein metabolism is primarily a result of a complete lack of insulin (type 1) or a relative lack of insulin or defects of the insulin receptors (type 2). Glucose cannot be readily taken up into cells and glycogen fails to store in the liver, although the conversion of glycogen back to glucose and the formation of glucose from other substances (gluconeogenesis) are not necessarily impaired. As a result, the blood glucose level rises rapidly. When it exceeds a critical level (the renal threshold), the excess is secreted by the kidney (glycosuria). Symptoms include increased appetite (polyphagia), thirst (polydipsia), weight loss, increased urine output (polyuria), weakness (fatigue) and itching (e.g. pruritus vulvae).
Diagnosis is by the signs and symptoms described above and by measurement of high blood glucose levels (casual 11.1 mmol/L, fasting 7.0 mmol/L). Glucose tolerance testing is a more stringent criterion, in which a standard dose (75 g) of glucose is administered after overnight fasting, and the level of glucose is measured in a venous blood sample 2 hours later (11.1 mmol/L or more indicates diabetes).
Type 1 diabetes
This type of diabetes usually occurs before the age of 20 and was previously called juvenile-onset diabetes; it accounts for about 15% of all diabetes. It is thought that a viral infection (possibly unnoticed) sets up an autoimmune response by antibodies against islet beta-cells, causing pathological changes and fibrosis in the tissue, which leads to a critical lack of insulin and abrupt onset of symptoms. There is some evidence of an inherited predisposition to type 1 diabetes; however, the exact triggering events are not understood.
The derangement of carbohydrate metabolism results in an abnormally high breakdown of proteins and fats. The ketone bodies (acetoacetic acid, acetone and β-hydroxybutyric acid) that result from oxidation of fatty acids accumulate faster than they can be oxidised, resulting in ketosis and acidosis; they can often be smelt (sweet and fruity) on the breath of people with diabetes. Diabetic ketoacidosis (DKA) is a medical emergency requiring specialist care; treatment includes rehydration, insulin replacement, potassium replacement and sometimes bicarbonate to reverse acidosis. Patients are also prone to muscle cramps, faintness, cardiac arrhythmias and infections. Regular 2–3 times daily injections of exogenous insulin are required lifelong for survival.
Type 2 diabetes
Type 2 diabetes was previously known as maturity-onset diabetes because the onset is usually after age 35. About 85% of the diabetic population has type 2 diabetes. Generally, people with this type of diabetes have some functioning islet cells, so they are not fully dependent on insulin for survival. There is impaired insulin secretion (especially the early phase after glucose load) and/or insulin resistance because of receptor and post-receptor defects. Hypokalaemia following use of thiazide diuretics in hypertension appears to contribute to glucose intolerance and type 2 diabetes in some patients.
Type 2 diabetic patients were typically middle-aged to elderly; however, with increasing obesity and physical inactivity in populations, younger people are developing type 2 diabetes, even children; other risk factors include positive family history. Diabetes affects males and females equally. The condition comes on gradually, with glucose intolerance often associated with hypertension and hyperlipidaemia. Although older at the time of diagnosis, patients with type 2 diabetes are still at risk of long-term complications and of hyperosmolar coma, but DKA is rare.
Gestational diabetes
This category includes women in whom diabetes or impaired glucose tolerance is detected first during pregnancy (but excludes women with diabetes who become pregnant). Gestational diabetes develops in approximately 10% of pregnancies. Insulin resistance often develops during the second and third trimesters. Hyperinsulinaemia may cause increased fetal growth, organomegaly and neonatal hypoglycaemia. Strict dietary measures and optimum blood glucose control with insulin are essential to reduce the risk of fetal abnormality and perinatal morbidity. Following childbirth, the maternal impaired glucose tolerance or diabetes may resolve.
Course and complications
The course of untreated diabetes mellitus is progressive. The symptoms of diabetic coma and acidosis are directly or indirectly the result of the accumulation of ketone bodies. Respiration becomes rapid and deep, the breath has an odour of acetone, the blood glucose level is elevated, the patient becomes dehydrated, and stupor and coma develop unless treatment is prompt.
The long-term complications of diabetes mellitus can lead to an increase in morbidity and mortality, despite treatment with insulin (type 1) or diet modification and oral hypoglycaemic agents (type 2). Most of the complications have been shown to be due to thickening of the basement membrane of small blood vessels (microvascular disease), leading to ischaemia, neuropathies, nephropathy and diabetic retinopathy, which can include vitreal haemorrhage, retinal detachment and blindness. Macrovascular disease (atherosclerosis and thrombosis of larger vessels) may result in coronary artery disease, strokes, gangrene leading to amputations and cardiomyopathy leading to heart failure. There is increased risk of infections (due to poor circulation and high blood glucose levels) and impaired wound healing; foot infections may lead to osteomyelitis, gangrene and amputation (see review by Bowen [2007]).
Comorbidities with diabetes are very common, partly because the risk factors for diabetes are the same risk factors for many cardiovascular disorders. Elderly patients with diabetes frequently also suffer from hypertension, cerebrovascular disease, arthritis, asthma or mental health problems. Thus there is a high likelihood of polypharmacy for the many pathologies and of escalating drug interactions.
Metabolic syndrome
The ‘metabolic syndrome’ was first defined by the World Health Organization in 1998; subsequent definitions have attempted to refine the definition, which now details risk factors such as high triglycerides, low high-density lipoprotein cholesterol level, hypertension, elevated fasting blood glucose and increased waist circumference. Measures of inflammation, endothelial dysfunction and coagulation may also be abnormal, which emphasises that metabolic syndrome is considered an insidious inflammatory state that predisposes individuals to cardiovascular disease. It is believed that obesity and inactivity promote insulin resistance and excessive insulin secretion, exacerbated by accumulation of adipose tissue via various mediators and cytokines.
While the concept of the metabolic syndrome remains controversial, it is gaining acceptance and appears to be increasing in prevalence in the developed world largely as a result of increased obesity; the prevalence of metabolic syndrome in American society has been estimated to be as high as one-third of the population. Rapid increases in prevalence of obesity and metabolic syndrome in developing countries in both adults and children is leading to increased morbidity and mortality due to type 2 diabetes and cardiovascular disease. Two lifestyle behaviours in adolescents particularly linked with obesity, insulin resistance and metabolic syndrome are high intake of sugar-sweetened drinks and low levels of physical activity.
Treatment
It is recommended that large-scale community intervention programs focus on increased physical activity and healthier food options (fruit, vegetables, whole grains, dairy products and unsaturated fats), especially for children. Weight-reduction surgery is very effective in treatment, suggesting the central role of obesity in the syndrome. Some dietary supplements and alternative therapies that have been shown to have some benefit include eicosapentaenoic acid and docosahexaenoic acid (in fish oils), soy proteins, dietary fibre, polyphenolic compounds, modest wine intake and green tea. However, overall ‘there is no substitute for therapeutic lifestyle changes, including healthful eating and increased physical activity’ (Hollander & Mechanick 2008).
Hypoglycaemia
Hypoglycaemia can occur in starvation, when meals are missed or diet is inadequate, and as an adverse effect of various drugs (see Table 36-2). It is also a common consequence of many diabetes treatments, as use of hypoglycaemic agents inevitably (almost by definition) leads to swings in blood glucose levels around the normal values. The risks and benefits of hypo- and hyperglycaemia must be balanced. The DCCT and UKPDS trials have proved that maintaining euglycaemia is important in minimising diabetic complications, so patients need education with respect to treatment of hypoglycaemia without getting into vicious cycles of swinging blood glucose levels. The symptoms (see Clinical Interest Box 36-4) are similar to those due to sympathetic nervous system overactivity or impaired CNS activity.
Table 36-2 Some drugs reported to cause hyperglycaemia or hypoglycaemia
| HYPERGLYCAEMIA (hence insulin requirements may be increased) | HYPOGLYCAEMIA (hence insulin requirements may be reduced) |
| Antipsychotics (some) | ACE inhibitors |
| Calcineurin inhibitors | Alpha-blockers |
| Glucagon | Anabolic steroids (some) |
| Glucocorticosteroids | Aspirin (analgesic doses) |
| Growth hormones | Beta-blockers (non-selective) |
| Loop diuretics | Disopyramide |
| Phenytoin | Ethanol (alcohol) |
| Progestogens (oral contraceptives) | Growth hormones |
| Protease inhibitors | Insulins |
| Quinolone antibiotics (floxacins) | MAO inhibitors |
| Sympathomimetics (adrenaline, high-dose salbutamol) | Oral hypoglycaemics |
| Thiazide diuretics | Quinine, quinidine |
| Thyroid hormones | Quinolone antibiotics (floxacins) |
| Tricyclic antidepressants | Sulfonamides |
Note that growth hormone and its analogues can either increase or decrease blood glucose concentrations; beta-blockers may mask the symptoms of hypoglycaemia.
Source: AMH 2010; MIMS Annual OnLine.
Clinical Interest Box 36-4 Symptoms of hypoglycaemia and hyperglycaemia
People administering insulin or other hypoglycaemic agents, and family and friends of patients with diabetes, should be aware of the signs and symptoms of hypoglycaemia and hyperglycaemia and know what action to take if they occur.
Treatment with hyperglycaemic agents
In mild to moderate hypoglycaemia, a readily available sugar source should be taken, such as jelly beans, honey or a sweet drink. This is followed with a complex carbohydrate that is more slowly absorbed, such as bread or dried fruit. In severe hypoglycaemia, in which the patient is unconscious or cannot take oral glucose, glucagon 1 mg (adult) or 0.5 mg (children <5 years) is administered SC or IM (see Drug Monograph 36-1). It is recommended that all patients at risk of developing hypoglycaemia carry a glucagon injection kit with them, and their families or carers should know how to administer it. In the hospital or clinic situation, glucose 50% or 10% solution is administered IV. Unconscious patients with hypoglycaemia should wake within 4–6 minutes of these therapies.
Treatment with glucose
Glucose is a monosaccharide that is absorbed from the intestine and then either used or stored by the body. Medically, it is indicated to treat or manage hypoglycaemia and is administered orally or parenterally. It is also present in many oral and parenteral electrolyte solutions, peritoneal dialysis solutions, formulations for rehydration, food supplements and blood glucose monitoring kits. In adults, about 10–20 g is administered orally and repeated in 10 minutes if necessary. The only adverse effects have been some reports of nausea. No significant drug interactions are reported.
Management of diabetes mellitus
General management plans
The general rationale for treatment is to replace insulin to physiological levels; to obtain metabolic control with insulin, oral hypoglycaemic drugs or exercise and dietary regimens; and to avoid or delay acute symptoms and long-term complications. Type 2 diabetes occurs often in association with hypertension and hyperlipidaemia; both can compound the risks of coronary heart disease and cerebrovascular disease. For all diabetes patients, vascular risk factors need to be assessed, lifestyle measures implemented and treatment with antihypertensives and lipid-lowering drugs optimised. Diabetes Australia has published targets for treatment, including specified maximum levels for blood glucose, cholesterol, blood pressure, body mass index (BMI), protein excretion and alcohol intake. Patients are strongly advised to give up cigarette smoking, as the combined effects of cigarettes and diabetes on the vascular system are potentially disastrous.
The treatment plan for diabetes mellitus usually involves a multidisciplinary team, including an endocrinologist, specialist nurse, podiatrist, diabetes educator and dietician, with referrals to specialists (e.g. for ophthalmic care) and specialised care during pregnancy, concurrent illness, surgery, travel and other stressful times. Patients need to be taught to self-administer insulin or an oral hypoglycaemic agent as necessary to help control blood glucose levels.
Standard pharmacological treatment plans are, for type 1: daily insulin, with doses determined by monitored blood glucose levels (BGLs); and for type 2: dietary and weight control and/or oral hypoglycaemic agent (OHA) and insulin as necessary. Recent advances in diabetic therapy include:
Blood glucose monitoring
Large-scale trials were carried out from the 1970s to the 1990s to assess the importance of control of blood glucose levels in delaying development of complications of diabetes. The Diabetes Control and Complications Trial (DCCT) in type 1 diabetes proved conclusively that tight control of blood glucose reduces the microvascular risks of retinopathy, nephropathy and neuropathy (Keen 1994). The United Kingdom Prospective Diabetes Study (UKPDS) in type 2 diabetes revealed that intensive therapy with oral hypoglycaemic agents and insulin and good blood pressure control reduce the risk of cardiovascular complications (UKPDS Group 1998). Overall, these trials showed that tight control of blood sugar levels in diabetes markedly decreases the progression to severe complications, so the aim of therapy for diabetes is to avoid frequent large swings in blood sugar levels. More recent large randomised controlled trials (such as the Action to Control Cardiovascular Risk in Diabetes, ACCORD, and the Veterans Affairs Diabetes Trial, VADT) have confirmed that good glycaemic control lowers the risk of microvascular events, but benefits in macrovascular events (myocardial infarction, stroke and death) are less clear.
To maintain euglycaemia (defined as normal level of glucose in the blood: 3.5–8 mmol/L), blood sugar levels are regularly determined by blood glucose monitoring (self-monitored blood glucose [SMBG]), which has been simplified by the availability of both visual test strips and strips used in blood glucose meters. Such devices allow patients to make the necessary adjustments with medication, diet and exercise. Urine tests are simpler and less invasive, but less reliable, and only detect levels that exceed the renal threshold for glucose, after a time-lag, so they are now used mainly for detection of ketone bodies in the urine.
Patients need to become aware of impending hypoglycaemia, which can occur rapidly from excess insulin or OHA dosage, unexpectedly high levels of exercise, inadequate food intake or other factors that impair blood glucose control. Common early symptoms of a ‘hypo’ are faintness, sweating and tremor; if untreated with an oral rapidly absorbed glucose source, this can lead to coma and death (see Clinical Interest Box 36-4). Hyperglycaemia, on the other hand, can lead to diabetic ketoacidosis and hyperosmolar coma; treatment is with rehydration and insulin. Other drugs, both prescribed and over-thecounter, and social drugs can all affect blood glucose levels and hence alter diabetic control. Table 36-2 and Clinical Interest Box 36-5 describe some of these effects.
Clinical Interest Box 36-5 Effects of commonly abused drugs on diabetes management
Many drugs can raise or lower blood glucose levels, including the commonly used and abused drugs shown below.
Glycosylated haemoglobin
A better indicator of long-term management of diabetes is that of glycosylated (glycated) haemoglobin (HbA1C), a form of haemoglobin that has a glucose molecule attached. This form of haemoglobin (normally about 7%) is increased in the red blood cells of people with poorly controlled diabetes; the glucose remains attached to haemoglobin for the life of the red blood cell, so the level of glycosylated haemoglobin reflects the average blood glucose concen tration over the past 3 months. It is commonly recommended that glycosylated haemoglobin be measured every 3 to 6 months in diabetes; levels of HbA1C above 9% show poor control.
Sugar intake
Chronic hypoglycaemia is particularly dangerous for young people with diabetes, as the developing brain is dependent on glucose as an energy source; management of children with diabetes thus errs on the side of hyperglycaemia. Parents and carers need to be aware of the sugar content of medicines as well as that of foods and drinks; many medicines formulated for children have a high sugar content to encourage compliance (as Mary Poppins sang so engag ingly, ‘A spoonful of sugar helps the medicine go down’). Syrup, elixir and suspension formulations frequently have 50%–70% w/v (g/100 mL) sucrose and may contain other calorigenic sweetening agents such as sorbitol or honey. The carbohydrate content may have to be considered in the diet, particularly if the medicines are for another chronic condition, such as epilepsy or asthma. The sugars can also have deleterious effects on teeth if taken chronically. Other sweeteners may be more appropriate for people with diabetes or those on a strict diet. Lists are sometimes published of the sugar content of medicines, or of sugar-free formulations; see Clinical Interest Box 36-6 for some ‘sugar-free’ liquid formulations.
Clinical Interest Box 36-6 ‘sugar-free’ oral formulations
The sugar and calorigenic contents of medications may significantly add to the glucose load in patients with diabetes or other glucose intolerant conditions; formulations are changed often by the manufacturers, so the best advice is for such patients to check the list of contents every time a medication is purchased. The following is a listing of some medications that are currently sugar-free (active ingredients are given in brackets).
Amoxil Syrup SF powder (amoxycillin)
Cilamox SF Syrup (amoxycillin)
Fungilin Lozenges (amphotericin)
Septrin SF Suspension (trimethoprim/sulfamethoxazole)
Dymadon Drops and Suspensions (paracetamol)
Epilim SF Liquid (sodium valproate)
Various cough lozenges: Difflam SF, Duro-Tuss SF, Strepsils SF
Ventolin Syrup SF (salbutamol)
SF = sugar-free. Although these formulations are claimed to be ‘sugar-free’, they may contain significant amounts of sorbitol, which is a poorly-absorbed polyhydric alcohol with calorific value that can disturb diabetic control (and in itself is an osmotic laxative). Another sweetener added to some products is saccharine; some products are also noted to be gluten-free or colour-free.
Source: MIMS Annual OnLine [19 March 2010].
Insulin replacement
By the time type 1 diabetes is diagnosed there is usually no functioning islet tissue remaining in the pancreas, and patients are thus totally dependent on an exogenous source of insulin as life-saving, lifelong therapy (hence the term ‘insulin-dependent diabetes mellitus’ IDDM). Oral hypoglycaemic agents (OHAs) cannot be used in these patients, as the oral agents all depend for their actions on there being some remaining islet tissue that secretes some insulin.
Insulin formulations
Sources
Insulin preparations available in Australia now are either human insulin (synthesised in the laboratory by chemical alteration of pork insulin or by recombinant DNA technology) or bovine insulin (derived from beef pancreas)—rarely used now. Beef insulin differs from human insulin by three amino acids, whereas the pork insulin differs from human insulin by a single amino acid. The human (or recombinant) insulin is identical to the insulin produced by the human pancreas; see Drug Monograph 36-2. Insulins produced by recombinant DNA technology are identified by the suffixes rys, recombinant yeast Saccharomyces cerevisiae, or rbe, recombinant bacteria Escherichia coli.
Drug Monograph 36-2 Human insulin
Human insulin has all the properties and actions of the natural hormone.
Indications
Insulin is indicated for the treatment of type 1 diabetes and for treatment of type 2 diabetes during emergencies, in stress situations, during pregnancy or as an adjunct to treatment with oral hypoglycaemic agents.
Pharmacokinetics
The wide variety of insulins available (including combination mixtures) allows titration of dose for tight blood glucose control depending on the patient’s needs and lifestyle. The onset, time to peak and duration of action depend on the particular type and proportions of insulin used. Insulin injected SC will be gradually leached from the injection site into the bloodstream and will circulate to tissues where it acts, especially in the liver, muscles and fat. Insulin is metabolised and inactivated rapidly in most tissues of the body; the disulf ide bonds are cleaved, then the peptide chains are broken down into amino acids. However, the biological activity continues much longer.
Adverse reactions
Rare with human insulin; allergic reactions and lipodystrophy can occur. Overdose is indicated by symptoms of hypoglycaemia: faintness, sweating and tremor.
Drug interactions
Many prescribed drugs can affect blood glucose concentrations and thus interact with insulin and impair diabetes control, in particular corticosteroids, β-blockers, thiazide diuretics and ACE inhibitors (see Table 36-2); dosage adjustments of insulin may be necessary. Beta-blockers (including eye preparations) can mask the symptoms of hypoglycaemia, such as increased pulse rate and lowered blood pressure, and may prolong hypoglycaemia by blocking gluconeogenesis. Cardioselective β-blockers in low dosages, such as metoprolol and atenolol, cause fewer problems than do non-selective blockers.
Many other medications, or the sugar content in them, and socially-taken drugs can also cause hyperglycaemia or hypoglycaemia or interfere with diabetes management, so the patient’s medication regimen should always be closely monitored (see Clinical Interest Boxes 36-5 and 36-6).
Warnings and contraindications
Insulin should be used with caution in patients with liver or kidney disease, high fever, severe infection, hyperthyroidism, inadequately controlled adrenal or pituitary disorders, diarrhoea, intestinal obstruction or vomiting, and in patients who have had recent surgery or trauma. Insulin is contraindicated in patients with hypoglycaemia or with hypersensitivity to human insulin solutions. Changes in type, brand or species of insulin should be made cautiously.
Many diabetic patients continue to be effectively treated with beef insulin if they have not developed insulin resistance, insulin allergies or lipodystrophy (a breakdown of subcutaneous fat occurring after repeated injections) at the insulin injection sites. There is a higher degree of immunogenicity reported with beef insulin than with human insulin. Subcutaneously administered human insulin may be absorbed faster and have a shorter duration of action than the animal insulins. It is standard practice now to prescribe human insulin whenever possible. Patients switched from animal to human insulin should be closely monitored initially because a dosage adjustment may be necessary. When human insulin was introduced, there was some suggestion that patients found it harder to predict when a ‘hypo’ (i.e. a hypoglycaemic state) was coming on; however, this has not been proven to be a problem clinically.
Time-course of formulations
Insulins, whether human or bovine, have been formulated in many different ways to alter the pharmacokinetic properties of the mixture; the formulations are described as ultra-short-acting, short-acting, intermediate-acting or long-acting (all contain 100 IU/mL; see Clinical Interest Box 36-7). The wide range of formulations available allows careful titration of dosage to maximise blood glucose control and minimise fluctuations.
Clinical Interest Box 36-7 Units of insulin activity
All early preparations of insulin were purified extracts of animal pancreas (beef or pig), but the content could not be guaranteed to be 100% pure insulin. Hence the only way to quantify the insulin level in the extract was by means of a bioassay, i.e. an experiment in which the activity of the extract in lowering blood sugar levels of an experimental animal (such as a rabbit) was assayed and compared with that of a known standard preparation of insulin.
A standard ’unit of insulin activity’ had to be defined and used, rather than an absolute unit such as milligrams. The International Unit (IU) of insulin activity was, for example, at one stage defined as ’the hypoglycaemic activity present in 0.04167 mg of the 4th International Standard Preparation’ (of ox and pig pancreas extract). Calculation will show that this highly purified extract contained about 24 IU/mg. Currently, 1 IU is equivalent to 0.035 mg of anhydrous recombinant human insulin (i.e. approximately 28.6 IU/mg).
Using these units, it can be calculated that the human pancreas normally contains about 200 IU insulin and that about 50 IU of insulin are secreted each day. This maintains the fasting glucose concentration at about 6 mmol/L (0.8-1.2 mg/mL).
Insulin can now be prepared synthetically or by genetic engineering techniques so that we know it is 100% pure, hence amounts and doses could be expressed in milligram terms. The IU of activity is, however, so well known and accepted that it remains with us. Thus, all insulin preparations in Australia are standardised to contain 100 IU/mL. This facilitates dosing and minimises errors.
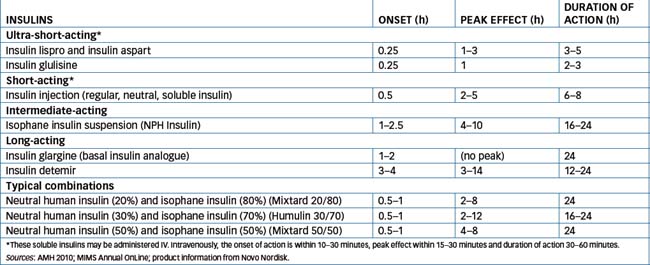
The chemical nature of the protein is not changed (except in the ‘bolus’ and ‘basal’ analogues in which minor changes are made in the amino acid sequences), only the rate at which it acts and is inactivated. Table 36-3 lists some types of insulin formulations, their approved names and pharmacokinetic characteristics; the formulations are also compared graphically in Figure 36-2. (Note that the time-course of action may vary among individuals, or at different times in the same individual, and is dependent on the site of injection, blood supply, body temperature, physical activity etc.) Premixed formulations are also available, i.e. 20/80, 30/70 and 50/50 mixes of short- and intermediate-acting types, giving a wide range of possible insulins.
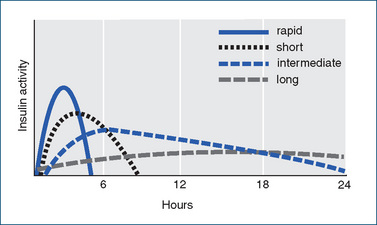
Figure 36-2 Insulin formulations’ durations of action (see also Table 36-3). Curves indicate approximate times of onset, peak and duration of hypoglycaemic activity of the main types of insulin formulations. The ultra-short formulation, insulin glulisine, has peak action at about 1 hour after injection and duration 2–3 hours.
Insulin stocks not in use are normally stored in the refrigerator, but the vial or cartridge currently in use is stable at room temperature. Freezing the solution denatures and inactivates the protein. Note that, whereas the oldertype insulin formulations were all chemically compatible and could be mixed together, some newer forms cannot, especially insulin glargine.
New, ultra-short-acting synthetic insulin analogues, insulin lispro, insulin aspart and insulin glulisine, have been developed. In these insulins two or three amino acids in human insulin have been changed. The primary advantage of the new insulins is that they have a more rapid onset of action than regular insulin; therefore they are administered as a ‘bolus’ immediately before a meal. They also have an earlier peak effect and shorter duration of action, so people with type 1 diabetes will usually require concurrent use of an intermediate- or long-acting insulin product as well to provide basal insulin activity. Insulin lispro and insulin aspart are also formulated crystallised with protamine, which prolongs the release and biological actions.
Recently, two new insulin analogues have been approved for use as ‘basal’ insulins, as their long, flat absorption profiles give them a long-acting effect more reproducible than those of the older long-acting formulations (insulin zinc suspension [IZS] and NPH1). Insulin glargine has a slightly different amino acid sequence, which makes it soluble in the acidic solution in the vial but, after injection, microcrystals form in the tissues, from which insulin is slowly released. This formulation may cause pain on injection and must not be mixed with other insulins. Insulin detemir is a form in which a fatty acid compound is attached to the insulin molecule; after administration, the complex binds to albumin in the plasma and tissues, and insulin is slowly released. The detemir formulation may cause local reactions, but is less likely to cause weight gain than other insulins.
Insulin administration
Routes of administration
Insulin is usually administered ‘ac, SC’, i.e. before meals (15–30 minutes before), subcutaneously. Injection sites are alternated, rotating around the abdomen and thighs, to minimise adverse local effects from the injections. In an emergency (diabetic ketoacidosis), short-acting insulins can be administered IV by infusion or IM.
Insulins are supplied in vials, all in the strength 100 IU/ mL. Many formulations are also available prepacked in cartridge ‘pens’, which facilitate injection and improve convenience and compliance with therapy. Vials or cartridges of insoluble preparations (i.e. all except regular insulin) should be rotated between the hands and inverted gently before a dose is withdrawn, to resuspend the protein; the container should not be shaken vigorously because this could denature the protein.
Portable insulin pumps have improved the metabolic state of some type 1 patients who did not have adequate diabetic control after intensive dietary restrictions and multiple daily injections of insulin. The insulin pump is battery-operated and computer-programmed to release small amounts of ultra-short-acting insulin per hour. It does not analyse the BGL, but is programmed based on the individual’s estimated daily insulin needs, diet and physical exercise. The patient can also push a button that releases a bolus dose to cover each meal consumed. Pumps are expensive, subject to mechanical failure and require careful maintenance. However, continuous insulin infusion has been shown to reduce slightly the glycosylated haemoglobin level in adults with type 1 diabetes; effects in type 2 are less clear.
Clinical trials of oral, nasal and inhaled insulin formulations are continuing; an effective non-injected form would be a wonderful improvement for people with diabetes.
Insulin dosage
There is no standard dose of insulin for the diabetic person; requirements depend on many factors, including lifestyle, weight, diet, exercise levels, stress, illness and pregnancy. (During pregnancy, insulin is the drug of choice to control diabetes; insulin requirements may drop for 24–72 hours after delivery and slowly return to pre-pregnancy levels in about 6 weeks.) Each patient’s needs must be determined individually to avoid hypoglycaemia and hyperglycaemia. A typical daily dose might be in the order of 0.7 IU/kg per day (reflecting the normal pancreatic production of about 50 IU per day) split into 2–4 injections and possibly two or three different types of insulin.
Insulin dosages should not be considered to be a fixed regimen; the dosage may need adjustment as a result of physical growth (child growing into adolescent then adult), illness, the development of anti-insulin antibodies, concomitant administration of certain medi cations, changes in lifestyle, missing a meal or doing unexpected exercise. Specific instructions may be required regarding insulin administration for a preoperative patient because of the alteration in the patient’s dietary patterns and metabolic requirements as the result of the surgical procedure. Treatment programs need to be reviewed regularly and adjusted as necessary, with the prescriber, other health-care professionals and patient working closely together to manage hypoglycaemia and hyperglycaemia and, if possible, avoid long-term complications.
Dosage regimens
As described earlier, the DCCT and UKPDS trials showed the importance of tight control of BGL, and hence techniques (‘insulin algorithms’) were developed to help determine the exact amount of insulin required at any time. Patients are taught to carry out self-monitoring of blood glucose (SMBG), and to adjust their insulin doses if the SMBG values are too high or low.
‘Basal–bolus’ regimen
A dose of short-acting insulin is given before each meal, plus some intermediate- or long-acting insulin at bedtime. This regimen is demanding, but it mimics well the body’s natural rhythms of insulin release, i.e. a low basal level, with peaks in response to carbohydrate loads from meals.
New techniques
Inhalation is a potentially viable route for administration of insulin, as it is for some other protein hormones (e.g. vasopressin, given as a snuff; see Drug Monograph 33-3). This method is being tested and it has been shown that some insulin is absorbed into the systemic circulation if delivered to the alveoli via an aerosol formulation. Further studies of safety and efficacy are ongoing.
Transplantation of pancreatic islet tissue from cadaver donors has been investigated extensively for some years; there are problems, however, both with supply and with rejection episodes. An alternative is to use stem cells with the ability to differentiate into islet cells; such cells have been shown to be effective in animal models of diabetes, and may become useful in human medicine.
Management of type 2 diabetes
Lifestyle factors
Weight reduction with diet control and increased exercise are important in therapy and may be all that is required to prevent progression from the condition of impaired glucose tolerance to overt type 2 diabetes. If symptoms persist after weight reduction, patients may also require OHAs and, when necessary, insulin; the target is a blood glucose reading below 10 mmol/L. The UKPDS trial showed that patients with type 2 diabetes benefited from tight control of both blood glucose and blood pressure, and from attention to other cardiovascular risk factors such as hyperlipi daemia, sedentary lifestyle and smoking.
Drugs
Metformin is recommended as first-line therapy unless contraindicated (as in renal, hepatic or cardiac impairment and in the very elderly). A sulfonylurea is alternative or additional therapy, and insulin is added or substituted if OHAs do not adequately control hyperglycaemia. It is now recognised that the introduction of insulin in type 2 diabetes should not be a last resort but may be implemented early in management, especially in young people, to minimise development of long-term complications. A regimen of daytime oral drug(s) with bedtime intermediateacting insulin is recommended as simple, acceptable and resulting in rapid improvement in glycaemic control.
Cardiovascular risk factors, obesity and insulin resistance are so prevalent with type 2 diabetes that this combination has been called ‘diabesity’ or the ‘metabolic syndrome’ (discussed above). A high proportion of such patients will not only be on metformin, with or without insulin, but also are likely to be taking antihypertensive agents (especially an ACE inhibitor), aspirin (for its antiplatelet effects) and a statin (to treat hyper cholesterolaemia). As the beneficial effects of all these medications are additive, it has been suggested that a ‘type 2 tablet’ could be produced containing metformin, aspirin, a statin and an ACE inhibitor. However, such a multi-drug formulation would suffer the disadvantages common to all combinations: that the dose of an individual agent cannot be adjusted relative to the others and that the drugs with the longest half-lives may accumulate. Equally, lifestyle changes suitable for preventing type 2 diabetes, such as healthy eating, regular exercise, healthy body weight, limited alcohol intake and ceasing cigarette smoking, will bring about major improvements in control of most cardiovascular diseases—a ‘type 2 lifestyle plan’.
Oral hypoglycaemic agents
The oral hypoglycaemic agents are useful only in type 2 diabetes, as they generally depend for their action on some residual insulin secretion from the pancreas. The OHAs act by various mechanisms, summarised in Figure 36-3; they may stimulate further insulin release, lower insulin resistance, sensitise cells to the actions of insulin, reduce glucose load, enhance secretion of incretins or alter absorption of carbohydrates.
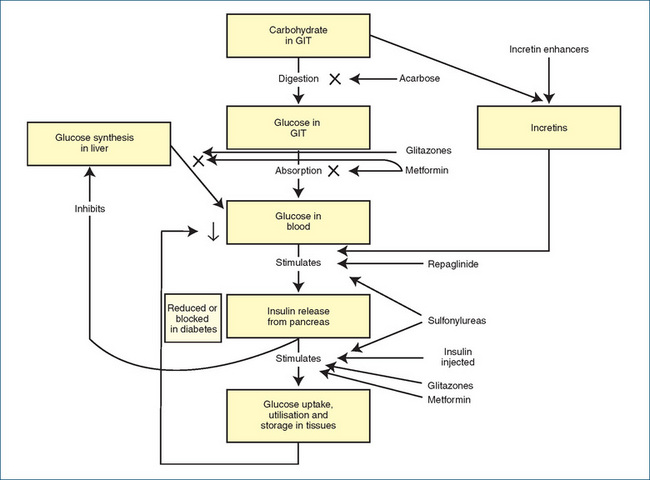
Figure 36-3 Mechanisms of action of oral hypoglycaemic agents (and injected insulin). GIT = gastrointestinal tract; X = process is reduced by drug.
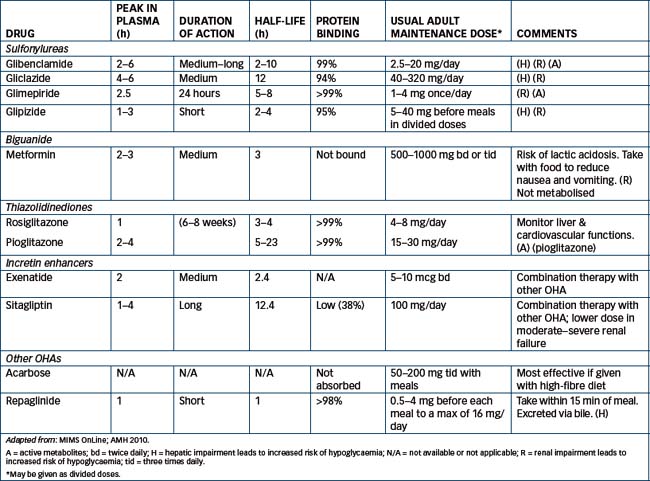
Until the 1990s, there were only two main groups of OHAs: the sulfonylureas and the biguanides. Recently, new types of agents, including acarbose, thiazolidindiones (glitazones), repaglinide and incretin-enhancers have been trialled and used clinically; however, the definitive place of the new agents in the management of type 2 diabetes has not yet been clearly established (see Table 36-4 for a summary of the OHAs, their pharmacokinetic parameters and usual adult doses). One agent, troglitazone, looked useful but had only a brief clinical life: it was approved in Australia in 1998 for restricted use in type 2 diabetes, was closely monitored due to concern about adverse hepatic effects and withdrawn in 2000. Related drugs are now being monitored closely for similar effects.
The choice of drug depends on several factors, including the patient’s weight, pancreatic, renal and liver functions and response to trialled drugs. It is estimated that 50% of type 2 diabetics will require insulin within 10 years of diagnosis; typically, the patient is stabilised on an OHA, and an intermediate- or long-acting insulin is administered at night.
Biguanides: metformin
A biguanide OHA for the treatment of type 2 diabetes is metformin (Drug Monograph 36-3). Its mechanism of action is not completely understood; it is known to:
Metformin is a biguanide oral hypoglycaemic agent; it increases the efficacy of available insulin and sensitivity of cells to insulin, enhances peripheral glucose uptake and utilisation and decreases gluconeogenesis in the liver; it rarely causes hypoglycaemia.
Indications
Metformin is indicated for the treatment of uncomplicated type 2 diabetes in adults and children over 10 years old, when diabetes is not controlled by diet and exercise; it may be given as adjunct therapy with insulin or another OHA. It reduces the risk of diabetes-associated complications or mortality.
Pharmacokinetics
Metformin is absorbed after an oral dose along the length of the GIT. It has a short half-life (5-10 hours) and is excreted unchanged in the urine (see Table 36-4).
Adverse reactions
Adverse reactions include GI upsets such as nausea, vomiting, anorexia and diarrhoea (common) and, rarely, lactic acidosis, acute hepatitis, vitamin B12 anaemia or hypoglycaemia.
Warnings and contraindications
Use with caution in patients with GIT problems and conditions affecting blood glucose levels. Use is avoided in people with metformin hypersensitivity, severe liver or kidney disease, lactic acidosis, cardiac disorders, severe burns, dehydration or severe infections, in people in diabetic coma or with ketoacidosis and in those who have recently had major surgery or trauma.
Australian Pregnancy Safety classification is C: however, it is recommended that pregnant and lactating women requiring hypoglycaemic therapy use insulin. Recent studies of women with polycystic ovary syndrome prescribed metformin to treat insulin resistance have shown some benefits, with fewer diabetes complications in women who became pregnant.
Drug interactions
Drug interactions occur with alcohol (increased risk of lactic acidosis), drugs that impair glucose tolerance (diuretics including thiazides), drugs that cause hyperglycaemia (thyroid products, glucocorticosteroids, β2 agonists), drugs that cause hypoglycaemia (other OHAs, β-blockers) and drugs that compete for renal transport mechanisms and reduce clearance of metformin (including cimetidine, calcium channel blockers, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim and vancomycin). Vitamin K antagonists (e.g. warfarin) may reduce metformin clearance. Blood glucose levels should be monitored whenever another drug is introduced, as dosage adjustment may be necessary (see also Table 36-2 and Clinical Interest Boxes 36-5 and 36-6).
It does not affect the pancreatic beta cells; therefore it does not increase insulin release and is unlikely to cause hypoglycaemia. It is preferred to sulfonylureas in overweight patients as it is less likely to cause weight gain and improves the plasma lipid profile (see review by Bosi [2009]). Metformin is now available in combination tablet formulations with glibenclamide or with rosiglitazone.
The first drug released in this chemical category was phenformin, but it was withdrawn from the market due to its association with potentially life-threatening lactic acidosis. Metformin subsequently fell from favour temporarily, but it has been associated only rarely with this complication and is now the drug of first choice in type 2 diabetes. However, metformin is contraindicated in patients at risk of lactic acidosis, i.e. those with liver or kidney disease, the elderly and those taking alcohol or drugs that raise metformin levels.
Sulfonylureas
The sulfonylureas were developed as a spin-off from the sulfonamide antibacterial agents: it was noticed that some patients taking sulfonamides had lowered blood sugar levels. The sulfonylureas enhance the release of insulin from the beta cells in the pancreas and increase the cellular sensitivity to insulin in body tissues. Their mechanism of action is to bind to receptors and block ATP-sensitive potassium channels (K-ATP) that normally open when [K] is low, causing increased potassium conductance and hence inhibition of insulin secretion. Modulation by sulfonylureas closes the channels, thus blocking potassium efflux and causing cell depolarisation, calcium entry and insulin secretion. Hence they decrease glycogenolysis and gluconeogenesis and reduce blood glucose concentration in people with a functioning pancreas.
The first-generation sulfonylureas included chlorpropamide and tolbutamide, both of which have now been withdrawn. The second-generation agents include glibenclamide (see Drug Monograph 36-4), glipizide, gliclazide and glimepiride; they are more potent than the earlier drugs. All are taken with meals, to minimise risk of hypoglycaemia. Choice is made on the basis of pharmacokinetic parameters, as those with long half-lives are more risky in elderly patients, those that are excreted as active drug or metabolites are more risky in patients with renal disease, and those that are eliminated mainly by hepatic inactivation are more risky in people with liver disease.
Drug Monograph 36-4 Glibenclamide
Glibenclamide is a sulfonylurea oral hypoglycaemic agent. It increases pancreatic insulin secretion and possibly lowers insulin resistance; hence it increases storage of carbohydrates, fats and proteins and decreases blood glucose.
Indications
Glibenclamide is indicated for the treatment of uncomplicated type 2 diabetes in patients whose diabetes cannot be controlled by diet alone.
Pharmacokinetics
Glibenclamide is inactivated in the liver and has a variable elimination half-life (2-10 hours) (see Table 36-4).
Adverse reactions
The most serious adverse effects are hypoglycaemia and weight gain; GI effects (nausea, vomiting, abdominal distress) and rashes are also common.
Warnings and contraindications
Use with caution in patients with concurrent illness and in elderly patients and those with hepatic or renal impairment.
Avoid use in people with hypersensitivity to antidiabetic drugs, sulfonamides or thiazide-type diuretics, with any conditions that may cause hyperglycaemia (e.g. high fevers and severe infection), or severe liver function impairment. Avoid use in patients in diabetic coma (or with ketoacidosis), in those undergoing surgery and in pregnancy or lactation (insulin administration is substituted).
Dosage and administration
Average dosage is 2.5-20 mg/day before breakfast, but for doses over 10 mg daily the remainder is taken before the evening meal. Dosage is individualised on the basis of blood glucose determinations. Australian Pregnancy Safety classification is C: it is recommended that pregnant women requiring hypoglycaemic therapy use insulin.
Common adverse effects include hypoglycaemia, weight gain, GIT and taste disturbances and rashes. Serious blood disorders and allergic reactions occur rarely. Interactions are common with drugs that are metabolised by similar pathways, including sulfonamides, non-steroidal antiinflammatory drugs, coumarins, alcohol and monoamine oxidase inhibitors, and with drugs that compete for protein-binding sites.
Thiazolidinediones (‘glitazones’)
Two new agents for use in treatment of type 2 diabetes are pioglitazone and rosiglitazone. These drugs enhance the sensitivity of peripheral tissues and the liver to insulin and thus reduce insulin resistance, which is common in type 2 diabetes. Their mechanism of action is via activation of the peroxisome proliferator-activated (PPAR)-gamma receptor, a nuclear receptor that regulates gene transcription especially in adipocytes, where it regulates glucose and lipid metabolism via proteins including GLUT-4 (the princi pal glucose transporter protein), lipoprotein lipase and transporter and binding proteins for fatty acids. Circulating free fatty acid levels are thus reduced.
Reductions in BGLs occur soon after starting treatment, but the full effect on insulin sensitivity is not seen for several weeks. The drugs can be used in combination with metformin and sulfonylureas and are recommended in type 2 diabetes uncontrolled by diet alone.
Adverse effects include anaemia, peripheral oedema, weight gain and increased risk of heart failure and peripheral limb fractures. Pioglitazone appears to be safer with respect to patients with increased cardiovascular risk factors; rosigliatazone is more likely to increase total triglyceride levels and LDL particle size, so its prescribing is restricted. An earlier drug in this class (troglitazone) was withdrawn due to hepatic toxicity, so patients taking the newer glitazones are monitored carefully for signs of hepatic or heart failure. If no significant improvement in glycaemic control occurs after 8 weeks of therapy, the glitazone should be stopped and insulin therapy started.
Incretin enhancers
As described earlier, incretins are peptides that are released from the digestive tract in response to food; those currently known are glucose-dependent insulinotropic (poly)peptide (GIP) and glucagon-like peptide-1 (GLP-1). They stimulate glucose-dependent insulin release, enhance β-cell proliferation, reduce post-prandial glucose levels especially, delay gastric emptying and reduce appetite; people with type 2 diabetes have a markedly lowered response to incretins. Thus new drugs that enhance incretin actions are useful in type 2 diabetes, and provide a new group of drugs with different modes of action. The long-term effects of these drugs are as yet unknown; they are currently used as add-on therapy, usually with metformin (see reviews by Prins [2008], Reutens [2008] and McGill [2009]).
Exenatide is called an incretin-mimetic, as it is a peptide with potent agonist activity at GLP-1 receptors; as a peptide, it must be given by injection. Liraglutide (not yet available in Australia or New Zealand), another peptide GLP-1 receptor agonist, has a longer half-life and is proving safe and effective in recent clinical trials in patients unable to maintain glycaemic control with diet, exercise and standard OHAs. The GLP-1 agonists induce weight loss but frequently cause transient nausea, dyspepsia and diarrhoea; hypoglycaemia can occur, and immune reactions against the peptides. Pancreatitis is a serious potential adverse event that is being monitored.
Sitagliptin and vildagliptin inhibit dipeptidyl peptidase 4, the enzyme that inactivates GLP-1 and GIP; they have long half-lives and can be administered orally once daily. They increase incretin levels and β-cell glucose sensitivity in patients with type 2 diabetes to normal levels, and also reduce glucagon secretion and preserve β-cell mass. They appear to have few adverse effects (mainly gastrointestinal and musculoskeletal complaints) and low incidence of hypoglycaemia unless used with a sulfonylurea. Serious hypersensitivity reactions have been reported.
Other oral hypoglycaemic agents
Miscellaneous OHAs include an α-glucosidase inhibitor (acarbose), repaglinide and orlistat. These drugs will be discussed briefly; their pharmacokinetic characteristics and mechanisms of action are summarised in Table 36-4 and Figure 36-3, respectively. Complementary and alternative medicine methods relevant to the treatment of diabetes are discussed in Clinical Interest Box 36-8.
Clinical Interest Box 36-8 Complementary and alternative therapies in diabetes mellitus
Patients with type 1 diabetes are dependent on insulin and must continue taking adequate doses. Various CAM therapies, however, are effective in helping to lower blood glucose levels or to improve glucose tolerance, allowing reduced doses of insulin or oral hypoglycaemic agents, or improving quality of life. Effective techniques include:
Acupuncture has generally been shown to be ineffective in the treatment of diabetes.
Patients should also be warned about taking products when they do not know the active ingredients. Several brands of Chinese ’herbal remedies’ sold as anti-diabetic treatments have been found to be adulterated with synthetic prescription hypoglycaemic agents such as glibenclamide and phenformin. Taking these remedies as adjuncts to prescribed drugs could lead to a dangerous drop in blood glucose levels.
Adapted from: Spencer & Jacobs 1999; Braun & Cohen 2007.
Alpha-glucosidase inhibitor: acarbose
Acarbose is an oral α-glucosidase inhibitor that delays digestion and absorption of carbohydrates in the small intestine; therefore, after a meal a smaller increase in blood glucose is noted. Thus any insulin produced by the pancreas in type 2 diabetes has a smaller glucose load to handle. It is indicated as an adjunct to diet for the treatment of type 2 diabetes; it may be given alone or in combination with a sulfonylurea to lower blood glucose. It does not increase insulin secretion or cause lactic acidosis or weight gain.
Absorption of acarbose is intentionally minimal (around 2%) as its actions are confined in the gut; later, metabolites (35%) may be absorbed from the GIT (for half-life, peak effect and duration of action, see Table 36-4). Drug interactions occur particularly with drugs that affect absorption in the intestine, such as digestive enzymes and cholestyramine. The most frequent adverse reactions are disturbed gut functions. If hypoglycaemia occurs, it should be treated with glucose rather than sucrose, as the absorption of sucrose will be impaired by the drug. As the drug is relatively new, use in pregnancy, lactation and renal impairment is not advised.
Repaglinide
Repaglinide is a novel non-sulfonylurea hypoglycaemic agent of the glitinide class, introduced in Australia in 2000. It stimulates the beta cells of the pancreas to produce insulin and improves insulin secretion in response to raised glucose levels, acting by a mechanism similar to that of the sulfonylureas (affecting the ATP-sensitive K+ channels) but at a different binding site. It is short-acting and is given with meals; it can cause hypoglycaemia and other GIT disturbances. Dosage may need to be decreased in liver impairment. Safety in pregnancy and lactation has not yet been established. New related agents are mitiglinide and nateglinide.
Orlistat
Orlistat is not itself an OHA, but is a potent, irreversible inhibitor of gastric and pancreatic lipase, and thus is useful in treatment of obesity (see Figure 50-3). It prevents absorption of approximately 30% of dietary fat and thus helps reduce weight as adjunctive therapy in type 2 diabetes.
New approaches to treatment of diabetes
Oral drugs that effectively and safely control hyperglycaemia would revolutionise treatment of diabetes and obviate the discomfort and adverse effects of regular injections of insulin. New types of small molecules are being tested as novel OHAs; potential biological targets include:
Immunosuppressant drugs are also being trialled, to delay the onset of diabetes, and blockers of the renin– angiotensin–aldosterone system to confer protection of the kidneys and reduce diabetic nephropathy.
Transplantation of autologous (patient’s own) haematopoietic stem cells in patients with newly-diagnosed type I diabetes is an exciting technique showing some promise for long-term improvement of β-cell function and reduction in dependence on insulin. (Experiments in mice have shown that embryonic stem cells can be induced to differentiate into cells resembling pancreatic β-cells in their sensitivity to glucose and production of insulin, and aggregate into structures resembling pancreatic tissue).
Key points
 The primary hormones of the pancreas are insulin and glucagon; both are involved in regulation of blood glucose levels and storage of nutrients as fuels.
The primary hormones of the pancreas are insulin and glucagon; both are involved in regulation of blood glucose levels and storage of nutrients as fuels. Normally, when blood glucose levels fall, glucagon release is increased, which facilitates the breakdown of glycogen stored in the liver to raise and restore blood glucose levels. In addition, the release of glucagon stimulates insulin secretion, inhibits further release of glucagon and maintains the homeostasis of carbohydrate metabolism.
Normally, when blood glucose levels fall, glucagon release is increased, which facilitates the breakdown of glycogen stored in the liver to raise and restore blood glucose levels. In addition, the release of glucagon stimulates insulin secretion, inhibits further release of glucagon and maintains the homeostasis of carbohydrate metabolism. Insulin facilitates the uptake of glucose into cells and promotes the storage of glycogen, lipids and protein.
Insulin facilitates the uptake of glucose into cells and promotes the storage of glycogen, lipids and protein. Diabetes mellitus is a disorder of carbohydrate metabolism that results from a relative or absolute insulin deficiency or insulin resistance. Diabetes mellitus is classified as type 1 (insulin-dependent diabetes mellitus or juvenile-onset diabetes) and type 2 (non-insulin-dependent diabetes mellitus or maturity-onset diabetes). Long-term complications can be severe and are minimised by tight control of blood glucose levels.
Diabetes mellitus is a disorder of carbohydrate metabolism that results from a relative or absolute insulin deficiency or insulin resistance. Diabetes mellitus is classified as type 1 (insulin-dependent diabetes mellitus or juvenile-onset diabetes) and type 2 (non-insulin-dependent diabetes mellitus or maturity-onset diabetes). Long-term complications can be severe and are minimised by tight control of blood glucose levels. People with type 1 diabetes require lifelong insulin replacement; diet and lifestyle factors must also be controlled, and a multidisciplinary clinical team is usually involved.
People with type 1 diabetes require lifelong insulin replacement; diet and lifestyle factors must also be controlled, and a multidisciplinary clinical team is usually involved. Various formulations of human or beef insulins are available, with differing pharmacokinetic characteristics; dosage regimen is determined by results of self-monitored blood glucose estimations.
Various formulations of human or beef insulins are available, with differing pharmacokinetic characteristics; dosage regimen is determined by results of self-monitored blood glucose estimations.Review exercises
References and Further reading
Australian Bureau of Statistics. Causes of Death Australia 2007. Canberra: ABS; 2009. ABS Cat. No. 3303. 0
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Bosi E. Metformin—the gold standard in type 2 diabetes: what does the evidence tell us? Diabetes. Obesity and Metabolism. 2009;11(Suppl 2):3-8.
Bowen K. Managing foot infections in patients with diabetes. Australian Prescriber. 2007;30(1):21-24.
Braun L., Cohen M. Herbs & Natural Supplements: An Evidence-Based Guide, 2nd edn. Sydney: Elsevier; 2007.
Daniel M., Rowley K.G., McDermott R., Mylvaganam A., O’Dea K. Diabetes incidence in an Australian aboriginal population: an 8-year follow-up study. Diabetes Care. 1999;22:1993-1998.
Endocrinology Expert Group. Therapeutic Guidelines: Endocrinology. Melbourne: Therapeutic Guidelines Limited; 2009. version 4
Gracey M.S. Nutrition-related disorders in Indigenous Australians: how things have changed. Medical Journal of Australia. 2007;186(1):15-17.
Greenfield J.R., Chisholm D.J. Thiazolidinediones: mechanisms of action. Australian Prescriber. 2004;27(3):67-70.
Hague W.M. Metformin in pregnancy and lactation. Australian Prescriber. 2007;30(3):68-69.
Hollander J.M., Mechanick J.I. Complementary and alternative medicine and the management of the metabolic syndrome. Journal of the American Dietetic Association. 2008;108(3):495-509.
Inzucchi S.E., McGuire D.K. New drugs for the treatment of diabetes. Part II: Incretin-based therapy and beyond. Circulation. 2008;117:574-584.
Joshy G., Simmons D. Epidemiology of diabetes in New Zealand: revisit to a changing landscape. New Zealand Medical Journal. 2006;119(1235):1-15.
Keen H. The Diabetes Control and Complications Trial (DCCT). Health Trends. 1994;26:41-43.
MacIsaac R.J., Jerums G. Clinical indications for thiazolidinediones. Australian Prescriber. 2004;27(3):70-74.
McGill J.B. Insights from the liraglutide clinical development program—the Liraglutide Effect and Action in Diabetes (LEAD) studies. Postgraduate Medicine. 2009;121(3):16-25.
McGuire D.K., Inzucchi S.E. New drugs for the treatment of diabetes. Part I: Thiazolidinediones and their evolving cardiovascular complications. Circulation. 2008;117:440-449.
Ministry of Health. A Portrait of Health: Key Results of the 2006/07 New Zealand Health Survey. Auckland: Ministry of Health; 2008.
National Prescribing Service. NPS Newsletter 39: Reducing risk in type 2 diabetes. Sydney: NPS; 2005.
O’Dea K., Rowley K.G., Brown A. Diabetes in Indigenous Australians: possible ways forward. Medical Journal of Australia. 2007;186(10):494-495.
Prins J.B. Incretin mimetics and enhancers: mechanisms of action. Australian Prescriber. 2008;31(4):102-104.
Prody E., Obici S. Minireview: the brain as a molecular target for diabetic therapy. Endocrinology. 2006;147:2664-2669.
Reutens A.T., Shaw J.E. Incretin mimetics and enhancers: clinical applications. Australian Prescriber. 2008;31(4):104-108.
Scheen A.J. The endocannabinoid system: a promising target for the management of type 2 diabetes. Current Protein and Peptide Science. 2009;10(1):56-74.
Simmons D., Harry T., Gatland B. Prevalence of known diabetes in different ethnic groups in inner urban South Auckland. New Zealand Medical Journal. 1999;112:316-319.
Spencer J.W., Jacobs J.J. Complementary/Alternative Medicine: An Evidence-Based Approach. St Louis: Mosby; 1999.
UK Prospective Diabetes Study Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
Wong J. Starting insulin treatment in type 2 diabetes. Australian Prescriber. 2004;27(4):93-96.
Australian Institute of Health and Welfare: www.aihw.gov.au/diabetes/index.cfm
Diabetes Australia: www.diabetesaustralia.com.au
New Zealand Medicines and Medical Devices Safety Authority: www.medsafe.govt.nz
United Kingdom Prospective Diabetes Study, post-trial monitoring: www.dtu.ox.ac.uk/index.php?maindoc=/ukpds/
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology