Chapter 33 The Neuroendocrine System and Pituitary Gland
The endocrine system comprises glands that produce hormones necessary for a variety of vital functions in the body. The hormones are secreted directly into the bloodstream, which carries the hormones to other organs or tissues that they control or regulate. Neuroendocrine interactions between the brain (hypothalamus) and the endocrine system (at the pituitary gland), via secretion of hypothalamic releasing factors and release-inhibiting factors, help control the functions of the pituitary gland and hence of many other endocrine glands. Negative feedback control via raised levels of target gland hormones also maintains homeostasis.
Disorders of the pituitary gland commonly manifest as hyper- or hyposecretion of target gland hormones. Hypothalamic factors may be used in treatment, along with surgery and irradiation of tumours.
Although the pituitary gland secretes many hormones, detailed discussion in this chapter is limited to two anterior pituitary hormones—growth hormone (and its release-inhibiting factor, somatostatin) and prolactin—and the posterior pituitary hormones vasopressin and oxytocin. Other hormones will be discussed in the appropriate chapters more directly involved with the endocrine glands that are targets for pituitary trophic hormones.
Key abbreviations
ACTH adrenocorticotrophic hormone (corticotrophin)
ADH antidiuretic hormone (vasopressin)
CRF corticotrophin-releasing factor
FSH follicle-stimulating hormone
GH growth hormone (somatotropin)
GHRIF growth hormone release-inhibiting factor
GnRH gonadotrophin-releasing hormone
HPA hypothalamic–pituitary–adrenal
IGF-1 insulin-like growth factor 1
Key background: endocrine glands and hormones
Major endocrine glands
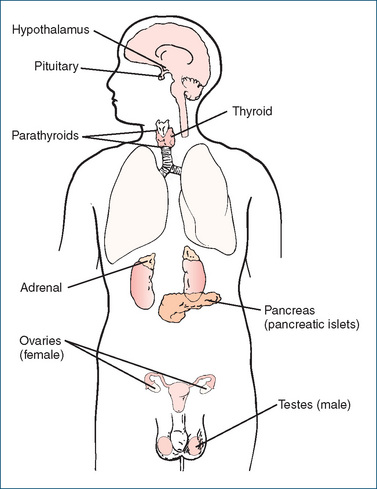
ENDOCRINE glands are groups of cells that produce and secrete hormones into the bloodstream; they are usually highly vascular, and the circulating blood collects and distributes the hormones to virtually all other cells in the body. The major endocrine glands are the pituitary gland, which can be considered the central controlling gland, and its ‘target glands’: the thyroid and adrenal glands, ovaries and testes; the parathyroids and pancreas are not controlled by the pituitary gland. (Endocrine glands were originally known as ‘ductless glands’, to distinguish them from exocrine glands such as sweat glands, which secrete their products into ducts; see Clinical Interest Box 33-1.) The target glands may themselves release hormones that are transported via the blood to other tissues (e.g. the adrenal cortex responds to pituitary corticotrophin and produces various steroid hormones), or they may respond with generalised effects, such as bone and muscle growth in response to stimulation by growth hormone (see Table 33-1).
Clinical interest box 33-1 Death from ductless glands—or was it from digitalis?
The mystery story opens in a gentlemen’s club in post-World War I London, England. An elderly retired general is found to have died quietly in his chair by the fire. His doctor declares the death to be from ‘natural causes’ after heart failure. The doctor explains that the general had been taking digitalis to ‘relieve the feebleness of the heart’s action’, and had succumbed despite having taken a powerful dose not long before his death. Detective Lord Peter Wimsey, however, is not satisfied with the diagnosis…
Soon after, Lord Peter is present at a literary cocktail party, at which the hostess explains to a guest that ‘a new young man is going to read a paper on ductless glands [which] will be “news” in next to no time—ever so much more up-to-date than vitamins… So very wonderful about glands, isn’t it?… such a hope for us all. What young criminals really needed was a little bit of rabbit-gland or something… all pineal or pituitary, and they come right again’.
It seems that the guest speaker will be the general’s doctor who is researching into endocrinology and is planning to establish a new clinic ‘to make everybody good by glands. It’s the science of the future; it puts biology in quite a new light. We’re on the verge of some really interesting discoveries… anything does for these women as long as it’s new—especially if it’s sexual’. A reporter notes that ‘Glands are news, you know. He’ll be one of these fashionable practitioners. Shrewd man—knows there’s money in glands. If only he could start one of these clinics for rejuvenating people, he could be a millionaire’.
Eventually, it transpires that the doctor had given his patient a lethal dose of digitalis in capsules, hoping thereby to inherit sufficient wealth to establish his endocrine clinic. The morals of the story appear to be:
Source: Dorothy L Sayers, The Unpleasantness at the Bellona Club, first published by Victor Gollancz, London, 1921; New England Library, London, 1977.
Table 33-1 The major endocrine glands (excluding the pituitary), their hormones and main functions
| GLAND | HORMONES | FUNCTIONS |
| Adrenal cortex | Glucocorticoids, mineralocorticoids and some sex hormones | Regulates carbohydrate and protein metabolism and fluid balance; also involved in inflammatory and immune responses |
| Corpus luteum, placenta | Progesterone | Menstrual cycle, pregnancy |
| Ovary, placenta | Oestradiol | Female sex organs and characteristics; menstrual cycle, pregnancy |
| Pancreas | Insulin; glucagon | Glucose uptake, fat synthesis; gluconeogenesis |
| Parathyroid | Parathyroid hormone | Calcium balance |
| Testes | Testosterone | Male sex organs, characteristics and behaviour |
| Thyroid | Thyroxine, tri-iodothyronine; calcitonin | Metabolism, growth, protein synthesis; calcium balance and bone resorption |
In this Unit we concentrate on the ‘classical’ endocrine glands and their hormones, as shown in Figures 33-1 and 33-4. The main functions of these hormones are summarised in this chapter to provide an overview of endocrine function. Details of control of the gland, individual hormone actions, and monographs on the hormones, their analogues and antagonists when used as drugs are discussed in subsequent chapters in this unit and in Unit XII.
Other endocrine tissues
Many organs not usually considered as endocrine glands do secrete into the bloodstream ‘hormones’ that act on distant tissues. For example, cells in the gastrointestinal tract secrete gastrin and cholecystokinin, helping regulate digestion; the pineal gland secretes melatonin, involved in sleep–waking cycles; the thymus gland secretes factors involved in immunity; and the kidneys secrete erythropoietin, involved in red blood cell production. During pregnancy, the placenta also has endocrine functions.
Control of endocrine gland functions
Control by the neuroendocrine system
To maintain balance in the internal environment (homeostasis), physiological functions must be able to be regulated; in the endocrine system there are multiple levels of control (see Figure 33-2, showing control of growth hormone secretion). At the highest level, environmental, cognitive and emotional factors may influence hormone concentrations; this interaction between the brain and endocrine glands is known as the neuroendocrine system. The hypothalamus secretes into the bloodstream (the hypothalamic–hypophyseal portal system) active proteins known as hypothalamic factors (or hormones) that either stimulate or inhibit release of hormones from the anterior pituitary gland. The hypothalamic control of the posterior pituitary gland (neurohypophysis), however, is not hormonal but neuronal; its hormones are released from nerve endings in response to neural stimuli from the hypothalamus.
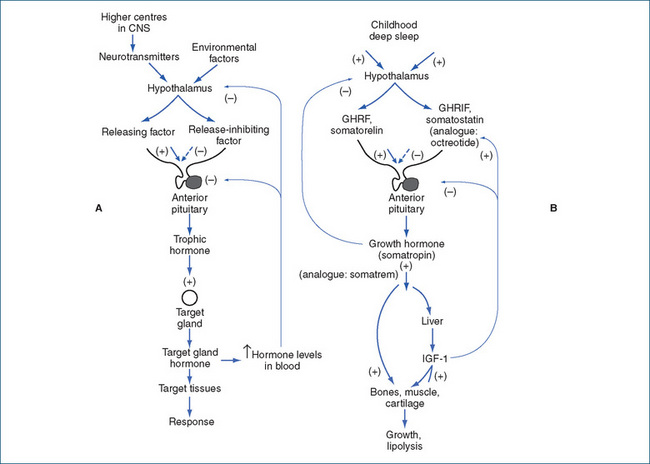
Figure 33-2 Levels of control of growth hormone secretion. A General negative feedback control systems. B Growth hormone controls. (+) indicates stimulation or increase, and (−) inhibition or decrease. GHRF = growth hormone-releasing factor; GHRIFF = growth hormone release-inhibiting factor; IGF-1F = insulin-like growth factor 1.
Hypothalamic factors
Several hypothalamic factors have been identified and many have been synthesised, including the following: growth hormone-releasing factor (GHRF), growth hormone release-inhibiting factor (GHRIF, somatostatin; see Drug Monograph 33-1), thyrotrophin-releasing hormone (TRH), corticotrophin-releasing factor (CRF), gonadotrophinreleasing hormone (GnRH, gonadorelin), leutinising hormone releasing hormone (LHRH) and prolactin releaseinhibit ing factor (PRIF, dopamine). The hypothalamic releasing factors are all peptides, ranging in size from a tripeptide (TRH) to large proteins. Their specificity of action is not absolute: for example, TRH can increase the release of prolactin as well as thyrotrophin, while GHRIF inhibits the release of GH, TSH, insulin, glucagon and various gastrointestinal tract hormones and autacoids.
Drug monograph 33-1 Octreotide
Octreotide is a synthetic octapeptide analogue of GHRIF (somatostatin). It is a potent agent that also inhibits secretion of many gastrointestinal hormones, including insulin, glucagon, gastrin and VIP (vasoactive intestinal peptide). (Lanreotide is an analogue with much longer-lasting activity, given by IM injection every 2 weeks. Another analogue, pasireotide, is more selective for suppression of ACTH secretion and is used in treatment of Cushing’s disease.)
Indications
Octreotide is indicated for lowering blood levels of growth hormone and IGF-1 to normal in persons with acromegaly who are unable to have, or have not responded to, other therapies such as surgery or radiotherapy. It is also used to treat the symptoms associated with carcinoid tumours, such as flushing and severe diarrhoea; to prevent complications following pancreatic surgery; and for treating bleeding oesophageal varices and hypoglycaemia.
Pharmacokinetics
It is rapidly absorbed after SC injection, with peak levels reached after 0.4 hour, duration of action up to 12 hours and an elimination half-life of about 1.5 hours; about 32% is excreted in the urine unchanged. A modified release long-acting form is available for monthly IM injections.
Drug interactions
Due to its effects on fluid, electrolyte and glucose balance, octreotide can interact with many drugs; glucose, fluid and electrolyte levels should be monitored. Absorption of cyclosporine or cimetidine may be reduced or delayed, and clearance of CYP3A4 substrates (e.g. quinidine) may be reduced.
Adverse reactions
These include local injection-site reactions and gastrointestinal disorders including nausea and vomiting, abdominal pain and steatorrhoea, also headache and thyroid dysfunction. Severe gallstone formation may necessitate cholecystectomy.
Warnings and contraindications
Use with caution in patients with diabetes mellitus, gastro intestinal tract tumours or severe kidney impairment, and in pregnancy; contraindicated during breastfeeding. Thyroid function requires monitoring during long-term treatment. Avoid use in persons with octreotide hypersensitivity and gallbladder disease.
The neuroendocrine control process can be summarised as follows:
This has been described as a ‘cascading amplifier’ process, as at each stage the response (e.g. release of hormone or growth of tissues) is magnified many thousandfold. Thus minute amounts of monoamine neurotransmitter may eventually lead to dramatic changes in behaviour or growth.
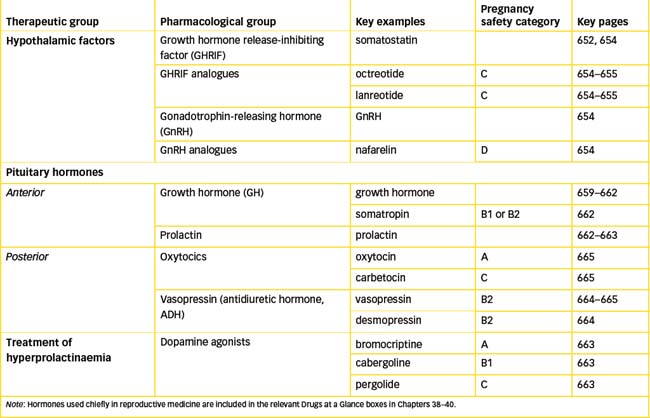
The medical uses of some hypothalamic factors are summarised in Table 33-2. Other releasing factors are sometimes used by specialist endocrinologists in diagnostic tests of pituitary or target gland functions. Antagonists to some releasing factors have been developed, for example cetrorelix (a LHRH antagonist) and ganirelix (a GnRH antagonist), both of which are used to control ovulation in women in assisted reproductive technology programs such as in vitro fertilisation (IVF). These are discussed in Unit 12, ‘Drugs affecting the reproductive systems’.
Table 33-2 Hypothalamic factors in medical use
| HYPOTHALAMIC FACTOR | CHARACTERISTICS | CLINICAL USES |
| GHRIF (somatostatin); also octreotide and lanreotide, analogues with longer half-lives | 14-Amino-acid peptide, inhibits release of GH; also inhibits release of TSH, insulin, glucagon and gastrointestinal hormones | Used in acromegaly and in therapy of various endocrine tumours |
| GnRH; also nafarelin, leuprorelin analogues | 10-Amino-acid peptide, causes release of FSH and LH | Used in diagnosis, and in infertility, uterine disorders, prostate and breast cancers |
| LHRH analogues (goserelin, triptorelin) | Synthetic analogues of GnRH with selectivity for LH release | Treatment of advanced and metastatic prostate cancer |
Control by negative feedback
The anterior pituitary and some of the target glands have a negative feedback relationship, as shown in Figure 33-2. As the level of the target-gland hormone builds up in the bloodstream, it inhibits further secretion of both the specific hypothalamic releasing factor and of the trophic hormone by the pituitary (the long negative feedback loops), thereby preventing excessive hormone effects. Exogenous hormones given as drugs can also activate this negative feedback effect; thus corticosteroids administered chronically for asthma can switch off the hypothalamic–pituitary–adrenal axis (i.e. the linked functions of the hypothalamus, the anterior pituitary gland and the adrenal cortex) and leave the body less able to respond to stress or infections.
Other controls
The negative feedback concept alone, however, is not enough to account for changes in serum levels of target-gland hormones; environmental, emotional and psychological factors are also involved in the neuroendocrine system. Stress, for example, can induce release of corticotrophin (ACTH—see Clinical Interest Box 33-2), and emotional factors can delay menstruation in women. An anterior pituitary hormone may also inhibit secretion of the specific hypothalamic factor that stimulates its release (the short negative feedback loop). The central nervous system thus plays a decisive role in regulating pituitary function to meet environmental demands.
Clinical interest box 33-2 Responses to stress
The integrated responses of the body to stress are a good example of the complex relationships between the nervous systems and endocrine systems. Some of the processes involved in response to stress include:
Thus, overall the body is ‘fired up’ to overcome stress, meet emotional crises, perform strenuous tasks and resist blood loss. If excessively prolonged, however, the responses can lead to exhaustion, negative feedback effects and inability to respond to infection or immune challenge (see de Kloet et al [2005]; Lightman [2008]).
Less commonly, there may be a positive feedback effect. An example is in childbirth, when uterine contractions stimulate the posterior pituitary gland to release oxytocin, which stimulates increased uterine contractions; the cycle is ended by the birth of the baby. Hormone concentrations are also regulated by other hormones, by changes in plasma concentrations of ions and nutrients and by nervous system effects. Secretion may thus be episodic, pulsatile or follow a daily or monthly rhythm.
Hypo-and hyper-functioning of glands
Alterations in control mechanisms, gland functions and hormone secretion may culminate in endocrine disease states. Hormone concentrations may be increased above normal (e.g. hyperpituitarism), often as a result of hormonesecreting tumours (adenomas), or may be decreased (e.g. hypothyroidism), due to gland atrophy or impairment of hormone synthesis. (The situation in which hormone concentrations are normal is given the prefix ‘eu-’, e.g. euthyroid.) Conditions involving hypersecretion of hormones may be treated by surgery or with anti-hormones (or irradiation of a tumour). Hyposecretion can usually be treated simply by replacing the missing hormone with exogenous natural or synthetic hormone.
Certain cell-surface receptors can become antigenic and stimulate formation of antibodies that accelerate receptor destruction, block receptor function or mimic the action of the hormone. Among the receptor disorders are Graves’ disease and insulin-resistant diabetes mellitus.
Hormones
Hormones are natural, active chemical substances that are secreted into the bloodstream from endocrine glands and initiate or regulate the activity of an organ or group of cells in another part of the body. They have specific, well-defined physiological effects on metabolism, growth, homeostasis and integration of bodily functions. Some of the major developments of the 20th century in biology and medicine were the recognition, isolation, purification and chemical and cellular investigation of most known hormones. Once their chemical structure was known, duplicating and mimicking hormones by chemical synthesis and/or genetic engineering techniques became possible.
The list of major hormones includes the hypothalamic factors, which stimulate or inhibit release of anterior pituitary hormones, the hormones from the anterior and posterior pituitary glands, the thyroid hormones, parathyroid hormone, pancreatic insulin and glucagon, several potent steroids from the adrenal cortex and the gonadal hormones of both sexes. These hormones and their functions are summarised in Tables 33-1 to 33-3; each will be discussed in much greater detail, and its uses in endocrine medicine described, in subsequent chapters.
Table 33-3 The hormones secreted by the anterior pituitary gland, their functions and related pathological conditions
| ANTERIOR PITUITARY HORMONE | FUNCTIONS | RELATED PATHOLOGIES |
| Thyroid-stimulating hormone (TSH, thyrotrophic hormone, thyrotrophin) | Stimulates the thyroid gland to produce thyroid hormones, hence regulates metabolic rate, growth and maturation; also affects central nervous system and cardiovascular functions; and calcium metabolism | Graves’ disease, hyperthyroidism |
| Adrenocorticotrophic hormone (ACTH, corticotrophin) | Stimulates the cortex of the adrenal gland to produce glucocorticoids, mineralocorticoids and precursors to sex hormones, hence regulates metabolism and fluid balance | Cushing’s disease, Addison’s disease |
| Growth hormone (GH, somatotrophin) | Promotes growth in most tissues; regulates metabolism | Pituitary adenomas, acromegaly and gigantism, dwarfism |
| Follicle-stimulating hormone (FSH) | Stimulates the growth and maturation of the ovarian follicle, regulates menstruation or spermatogenesis | Dysmenorrhoea, infertility |
| Luteinising hormone (LH), also known (in the male) as interstitial cell-stimulating hormone (ICSH) | Regulates reproduction (ovulation, formation of the corpus luteum, or spermatogenesis; secretion of sex hormones) | Dysmenorrhoea, infertility |
| Prolactin | Proliferation and secretion of the mammary glands | Pituitary adenomas, galactorrhoea, gynaecomastia |
| Melanocyte-stimulating hormone (MSH) | Functions in humans are not defined; does darken skin |
Chemical classes of hormones
The major types of hormones are the steroid hormones, amino-acid-derived hormones and polypeptides and simple proteins. The main clinical significance of knowing the chemical class of a hormone is that it affects how the hormone is administered: peptide and protein hormones cannot be given orally, as they would be digested in the gastrointestinal tract, so they are administered by injection (parenterally) or sometimes as nasal sprays.
Steroids
Steroid hormones are secreted by the adrenal cortex and the sex glands (testes and ovaries). They are lipid-soluble cholesterol derivatives (see Figure 33-3); their physiological effects begin when the steroid enters the cell nucleus, with subsequent binding to the specific steroid receptor. Steroid hormones are usually secreted as they are synthesised, rather than being stored.
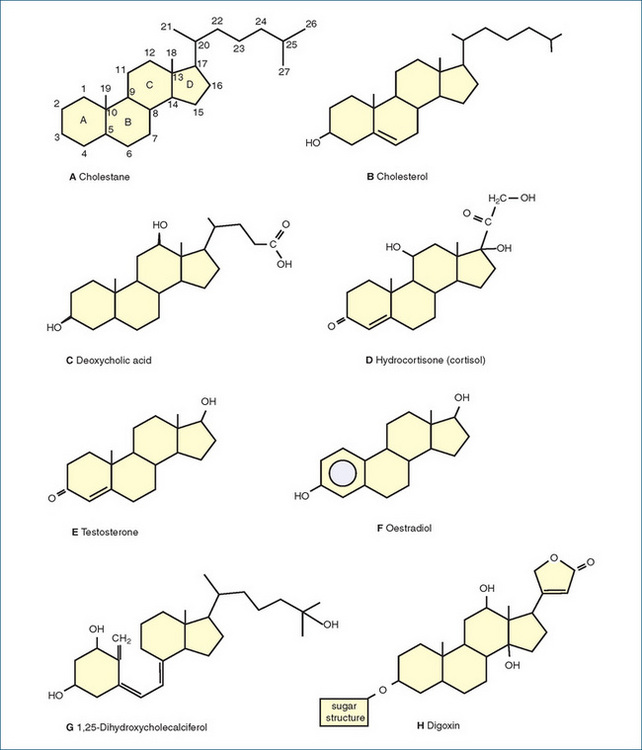
Figure 33-3 Chemical structures of some naturally occurring steroids. A A typical steroid (cholestane), showing the conventional ring lettering and carbon atom numbering pattern. B Cholesterol, a component of cell membranes and precursor to other steroids. C Deoxycholic acid, a bile acid. D Hydrocortisone, a glucocorticoid. E Testosterone, a male sex hormone. F Oestradiol, a female sex hormone. G 1,25-dihydroxycholecalciferol, an active form of vitamin D. H Digoxin, a cardiac glycoside.
Amino acid derivatives
Amino acid derivatives include the thyroid hormones, iodinated derivatives of the amino acid tyrosine. The catecholamines secreted from the adrenal medulla, adrenaline and noradrenaline (sometimes considered hormones), are also tyrosine derivatives (see Figure 12-1).
Peptides
Polypeptide hormones (<20 amino acid residues) include the posterior pituitary hormones, oxytocin and vasopressin, and some of the hypothalamic releasing factors. Protein hormones (>20 amino acids) include the classic hormones insulin, growth hormone and parathyroid hormone, and other releasing factors. Peptide and protein hormones are generally stored in cells in membrane-bound vesicles and are released by exocytosis.
Other hormones
By our general definition, a multitude of endogenous active chemicals released into the bloodstream would be classified as hormones: the list has been suggested to range from ions such as sodium and calcium, through the neurotransmitters adrenaline and noradrenaline, to steroids such as vitamin D. Local hormones (paracrines, or autacoids) such as prostaglandins, histamine and nitric oxide, which are secreted and released to act in the same or nearby cells and tissues, could also be included.
General functions of hormones
Hormones from the various endocrine glands function together to regulate vital processes, including:
Interactions among hormones account for homeostasis of physiological functions such as blood pressure con trol, responses to stress, and conception, development and breastfeeding of a baby. Other complicated effects seen in endocrine physiology include the thyroid hormones having a permissive effect on the lipolytic action of adrenaline, increasing adrenaline actions. In other situations, two hormones can have opposing effects; thus glucagon can be considered to have anti-insulin actions.
Duration of actions
Some hormones exert their physiological effects immediately, while others require minutes or hours before their effects occur. Some effects end immediately when the hormone disappears from the circulation, while other responses persist for hours, days or weeks after hormone concentrations have returned to basal levels. The steroid hormones typically have slow and prolonged actions because they induce synthesis of new proteins, and long half-lives because they are lipid-soluble and tend to be retained in the enterohepatic circulation.
Hormones are not ‘used up’ in exerting their physiological effects, but must be inactivated or excreted if the internal environment is to remain stable. Inactivation occurs enzymatically in the liver, kidney, blood or target tissues. Excretion of hormone metabolites is primarily via the urine and, for steroids, the bile. Most hormones are destroyed rapidly, having a half-life in blood of 10–30 minutes. Some, however, such as the catecholamines, have half-lives of seconds, whereas thyroid hormones have half-lives measured in days. This wide range in times of onset and duration of hormonal activity contributes to the flexibility of the endocrine system.
Receptor mechanisms of actions
Receptors specific for particular hormones are situated in target organs in the cell membranes or inside nuclei. A hormone has affinity for and binds to its receptor, leading to transduction events inside the cell that mediate the hormone’s actions; the hormone has no effect on tissues that do not carry its specific receptors. For example, steroid hormones act intracellularly on specific steroid recep tors in the nucleus; activation of the receptor upor downregulates gene expression, leading to altered transcription of DNA and hence protein synthesis; the newly synthesised proteins ultimately bring about the actions of the hormone.
Water-soluble hormones, such as the peptides, proteins and catecholamines, cannot enter cells but act through receptors located in the cell membranes. The general mechanisms are similar to those for neurotransmitters and may involve second messenger systems such as adenylate cyclase and cyclic adenosine monophosphate, or cyclic guanine monophosphate, diacylglycerol or inositol triphosphate (see Chapter 5), which then activate protein kinases and phosphorylate other enzymes, leading to the physiological responses attributed to the hormone.
Transport of hormones
Transport of a hormone in blood is usually in a bound form, and specific binding proteins exist, such as thyroxine-binding globulin. The process is closely analogous to that of protein binding of drugs; in each case, binding increases the transportability of the drug or hormone in blood, decreases its movement across membranes in the kidney or across the blood–brain barrier and acts as a reserve depot in the blood. Only the free, unbound hormone or drug is available to act at receptors or to cross membranes.
Use of hormones as drugs
In medicine, hormones are generally used in three ways:
The endogenous hormones themselves may be used as drugs; or if they have very short half-lives, are expensive or difficult to extract, synthetic analogues with similar activities but better pharmacokinetic properties may be administered.
Dosing: International Units or milligrams?
In the past, hormones produced for therapeutic use were extracted from animal (or human) cadaver tissues, then purified and tested biologically for pharmacological activity, so that the activity of the extracts could be quantified. As it was not possible to be sure that the natural preparation was 100% pure, the activity of such an extract was compared in biological assays with international standard preparations, and the strength of the new preparation was quoted in terms of ‘International Units’ (IU) of activity, rather than in milligrams of active extract. Thus insulin preparations, for example, were standardised to contain 100 IU hypoglycaemic activity per millilitre of solution (see the section in Chapter 4 on ‘Bioassays’).
Although many hormone preparations are now prepared purely synthetically or by recombinant DNA technology, and it is possible to ensure that they are 100% pure, such preparations may still have the doses quoted in IU rather than in absolute amounts (mg).
The pituitary gland
FTHE pituitary gland exerts important effects in regulating the function of other endocrine glands and hormones. The pituitary body in an adult human is about the size of a pea and occupies a niche in the sella turcica of the sphenoid bone (see Figure 28-6, showing the anatomical relationship of the pituitary gland to the hypothalamus, the nose and the sphenoidal sinus). The pituitary gland consists of an anterior lobe (adenohypophysis), a posterior lobe (neurohypophysis) and the smaller pars intermedia, composed of secreting cells, the function of which is not well understood. The two main lobes develop separately in the embryo and remain histologically and functionally distinct. The anterior lobe consists of ectodermal tissue derived from the roof of the buccal cavity, whereas the posterior lobe consists of neural tissue derived by downward projection from the floor of the third ventricle in the brain.
The variety of hormone preparations available that affect or are secreted by the pituitary gland are generally used as replacement therapy for hormone deficiency or as diagnostic aids to elucidate hypofunctional or hyperfunctional gland disorders.
Anterior pituitary hormones
The secretion of anterior pituitary hormones is regulated by hypothalamic releasing and release-inhibiting factors, and by negative feedback control from target gland hormones (Figure 33-2). The hormones of the anterior part of the pituitary gland exert important effects in regulating the secretion of other hormones; Figure 33-4 shows anterior pituitary hormones and their principal target organs. Note that four of the hormones, adrenocorticotrophic hormone (ACTH; corticotrophin), thyroid-stimulating hormone (TSH; thyrotrophin) and the gonadotrophins, FSH and LH, regulate the functions of other endocrine glands and are referred to as trophic hormones, as they nourish or change the functions of the target glands where they act.1 The other three (growth hormone, melanocyte-stimulating hormone and prolactin) act directly on target organs. The main functions of the hormones are listed in Table 33-3; common pathological conditions related to gland or hormone dysfunction are also indicated. The pharmacology of growth hormone (GH) and prolactin will be discussed in detail in this section; TSH, ACTH and the gonadotrophins are considered in subsequent chapters.
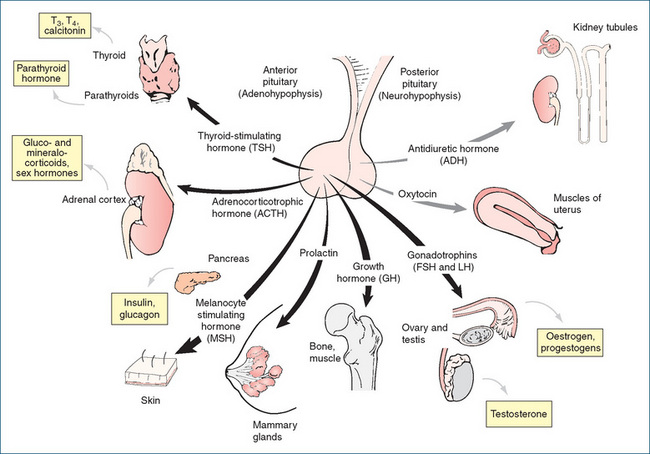
Figure 33-4 Pituitary hormones. Major hormones of the adenohypophysis and neurohypophysis and their principal target organs; hormones produced by target glands are shown in boxes. Note that there are no pituitary trophic hormones for the pancreas or parathyroid glands, and that some target glands do not produce further hormones. FSHF = follicle-stimulating hormone; LHF = luteinising hormone; T3F = tri-iodothyronine; T4F = tetra-iodothyronine.
Growth hormone
Growth hormone (and its recombinant form, somatropin) is the main growth factor influencing the development of the body. A 191-amino-acid protein, it promotes skeletal, visceral and general growth. Acromegaly, gigantism and dwarfism are associated with pathological conditions of this protein hormone (see Clinical Interest Box 33-3); its use is banned in sport. The anterior lobe of the pituitary gland in the average adult usually contains about 5–10 mg GH, the greatest amount of all pituitary hormones.
Clinical interest box 33-3 Gigantism, dwarfism and short stature
Chronic GH hyper secretion causes excessive production of IGF-1, with over growth of bone and soft tissues and generalised systemic disorders. GH-secreting pituitary adenomas cause the classical clinical syndromes of acromegaly and gigantism.
If this occurs in adults (in whom the epiphyses of long bones have already fused), the manifestations include enlarge ment of the hands and feet and coarsening of facial features. Arthritis, hypertension, organomegaly (excessive growth of organs) and diabetes are common.
GH hypersecre tion in childhood and adolescence leads to gigantism, with striking acceleration of linear growth, plus the features of acromegaly. Despite being enormously tall, pitui tary giants are not abnormally strong because of thyroid, cardiovascular, joint and vision problems. (Thus the Biblical giant Goliath would have been very susceptible to young David’s slingshot.)
Treatment is with trans-sphenoidal surgery, radiation therapy and lanreotide or octreo tide, both somatostatin analogues (Drug Monograph 33-1). If there is insufficient shrinkage of tumour and reduction in GH secretion, other drugs can be added, such as pegvisomant, an antagonist at the GH receptor (not readily available in Australia).
Congenital GH deficiency leads to hypopituitary dwarfism, with early-onset growth failure and delayed onset of puberty. Treatment is with GH and appropriate gonadotrophins.
The use of GH to increase height in short children is controversial (unless there are medical reasons for the short stature)—the short height usually concerns the parents more than the child. In Australia, supplies of somatropin as a subsidised drug are restricted by the Growth Hormone Program of the Pharmaceutical Benefits Branch of the Department of Health and Ageing to permanent Australian residents who are eligible for Medicare benefits. Guidelines for GH prescription include that the child be in lower than the first centile for height and lower than 25th centile for growth rate over a period of at least one year. Tests for ‘biochemical growth hormone deficiency’ may be required. There are special criteria for children with growth retardation secondary to brain tumours or irradiation for brain tumours, and for infants with multiple pituitary insufficiency. Treatment is with GH injections SC 6–7 per week, with individualised dose, e.g. 0.1 IU/kg/day. GH is contraindicated after closure of the epiphyses (see www.health.gov.au/hGH).
Secretory system
The amount of GH secreted decreases during the lifespan: it is very high in the newborn and decreases progressively throughout childhood, puberty and adulthood. The levels of control of GH secretion are shown in Figure 33-2B, and the sites of action of hormones and analogues used in pharmacological treatment of disorders of GH secretion are indicated. Normally, release of GH is pulsatile during the 24-hour cycle: levels can vary by factors of 10–100. Secretion is increased by GHRF and during deep sleep in children, and decreased by GHRIF.
Physiological and pharmacological actions
The anabolic (growth-increasing) effects of growth hormone are indirect, being due to the effects of another mediator (somatomedin), now identified as insulin-like growth factor 1 (IGF-1). This is produced in the liver and is directly responsible for skeletal and soft tissue growth and increased protein synthesis in cartilage and bone. It is also involved in tissue hypertrophy and wound healing. A major pharmacological consequence of GH use is therefore an increase in growth, whereas a deficiency in growth hormone usually results in dwarfism. Growth hormone is used to treat children deficient in GH (see Drug Monograph 33-2 and Clinical Interest Box 33-4). When it is injected, children grow at a normal or faster-than-normal rate, and ‘catch-up’ growth brings them close to the stature they would be expected to attain naturally, as shown in Figure 33-5.
Drug monograph 33-2 Somatropin, recombinant
Somatropin products are all synthesised by recombinant DNA technology and are identical in amino-acid sequence to the human growth hormone. Somatropin is used to stimulate linear growth in patients who lack sufficient endogenous GH. The size of organs and size and number of muscle cells and red cell mass are also increased. An increase in cellular protein synthesis and lipid mobilisation with lipolysis resulting in a decrease in body fat stores has also been reported. A diabetogenic effect may follow insulin overproduction, due to insulin resistance.
Indications
Somatropin is indicated for the treat ment of growth failure in children caused by a pituitary growth hormone deficiency, or in Turner’s syndrome, Prader-Willi syndrome or chronic renal insufficiency; also in severe GH deficiency in adults. It is sometimes abused by athletes seeking increased size and strength.
Pharmacokinetics
As it is a protein, GH can only be administered parenterally. The maximum serum level occurs at about 5 hours and the elimination half-life of parenteral (SC) somatropin is about 4 hours.
Drug interactions
When somatropin is given concurrently with glucocorticoids or ACTH, the growth-promoting effects of GH may be impaired. Doses of other replacement hormones require careful adjust ment and monitoring. There may be interactions with other substrates of CYP3A4, including anticonvulsants and cyclosporin; reference texts should be consulted for individual combinations.
Adverse reactions
Antibodies to GH have been reported, but it is rare for a patient not to respond to therapy. An allergictype reaction (rash and itching) and lipodystrophy have been reported at the site of injection. Hypothyroidism, arthralgia, ‘growing pains’, fluid retention and intracranial hypertension can occur. Excessive doses may produce gigantism and acromegaly.
Warnings and contraindications
Use with caution in patients with hypothyroidism, diabetes or cancer. Avoid use in persons with GH hypersensitivity, intracranial tumour or closed epiphyses, and during pregnancy or lactation.
Dosage and administration
The dosage of somatropin for children is individualised and administered as SC injections. The growth rate response is monitored after 3–6 months to determine whether dosage adjustment is necessary. Therapy is usually con tinued until epiphyseal closure occurs or there is no further response. (1 mg somatropin is equivalent to 3 IU.)
Clinical interest box 33-4 Creutzfeldt–jakob disease and cows
Growth hormone is unusual in that it is species-specific; thus, whereas beef or pork insulin can be used in human medicine, and ACTH from animals works in humans, animal GH does not. Hence GH for use in medicine originally had to be extracted from human cadavers. Some hypothalamic and pituitary factors to be used in medicine, including GHRIF, were also obtained from human cadaver material.
However, brain extracts were found to be able to transmit Creutzfeldt–Jakob disease, a slowly progressive fatal disease of the central nervous system. Use of human pituitary extracts resulted in the deaths of some patients many years after receiving these products.
Synthetic analogues, or human hormones prepared by recombinant DNA technology, are now available for use, including several formulations of somatropin (recombinant human GH, hGH [Drug Monograph 33-2]), and tetracosactrin (a synthetic analogue of ACTH), while octreotide, a synthetic analogue of somatostatin, is used to inhibit the release of growth hormone.
Other human tissues (i.e. not from the central nervous system) cannot transmit Creutzfeldt–Jakob disease, so human gonadotrophins can be safely prepared from the urine of pregnant or menopausal women, provided that precautions are taken against other transmissible agents such as HIV.
An Argentinian biotechnology company, BioSidus, has genetically engineered transgenic cows, into which have been inserted the human gene for growth hormone production. The cows now produce milk rich in hGH. It has been claimed that with just 20 such cows, BioSidus can produce enough hGH to supply all developing countries.
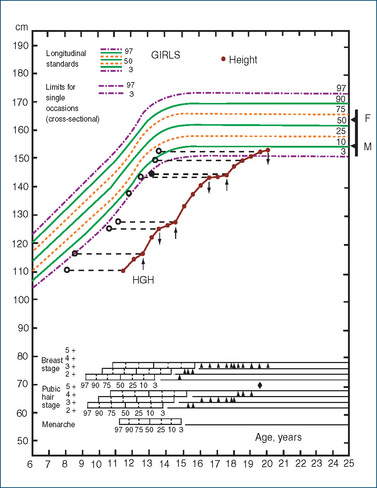
Figure 33-5 Effects of growth hormone (HGH) treatment in a girl with isolated GH deficiency, showing catch-up growth following treatment given over three periods indicated by arrows. Open circles represent bone age. F and M are father’s and mother’s height centiles and the bar represents predicted height centile range for their children. Puberty ratings at each age are shown by arrowheads, lower section.
Source:Tanner 1989. Reproduced with permission from Castlemead Publications.
Growth hormone has many metabolic effects. It:
There are still questions related to the best age of starting treatment and duration of treatment, and ethical issues related to patient selection, risk of off-label use and diversion to illicit use such as attempted enhancement of performance in sports persons (see review by Weise and Nahata [2004]).
Inhibitors of GH action
The natural inhibitor of GH secretion is the hypo thalamic factor GHRIF. Acromegaly, caused by excessive secretion of GH, can be treated with GHRIF (somatostatin) or its analogues such as octreotide (see Drug Mono graph 33-1).
Dopamine agonists such as bromocriptine and cabergoline are used in acromegaly, but appear to be effective in only 25% of patients. Dopamine agonists bind to D2 receptors on GH-producing cells in the anterior pituitary gland and decrease GH production. However, there are serious adverse effects in other body regions, especially nausea and orthostatic hypotension.
New drugs not yet available in Australia (in 2010) are pegvisomant, an antagonist at GH receptors, and pasireotide, an agonist at somatostatin receptors. Pegvisomant is a protein that is administered SC; it effectively normalises IGF-1 levels in up to 97% of patients; it may also have a role in treatment of diabetes. Further clinical trials will determine what the long-term positions of these drugs are in treatment of acromegaly; surgery remains the first-line treatment (see review by Feelders et al [2009]).
Prolactin
A lactogenic factor (prolactin or mammotrophin) functions in the proliferation and secretion of the mammary glands of mammals. Human prolactin is a protein hormone (198 amino acids in a single peptide chain), closely related chemically to GH and to the placental hormone human chorionic gonadotrophin (human placental lactogen). All three hormones appear to have evolved from a single ancestral gene. Females have about 1.5 times the male concentration of prolactin. The functions of the hormone in males and in non-lactating females are not clearly established.
Secretory system
The main hypothalamic control over prolactin release is inhibitory, as the main hypothalamic factor is prolactin release-inhibiting factor (PRIF). There is good evidence that PRIF is in fact the neurotransmitter dopamine. Stimuli for release of prolactin include oestrogens, suckling by a baby, dopamine antagonists (notably the neuroleptic agents used in schizophrenia) and thyrotrophin-releasing hormone. Secretion is decreased by dopamine agonists such as bromocriptine and cabergoline (used in Parkinson’s disease). A new specific dopamine D2-receptor agonist, quinagolide, is used in hyperprolactinaemia for its selective inhibition of prolactin secretion.
Physiological actions (in females)
Prolactin causes an increase in the amount of breast tissue during pregnancy (via actions of oestrogens), and in milk production, and possibly ‘nest-building behaviour’. Gonadotrophin release and ovulation are suppressed, which tends to have natural contraceptive effects, decreasing the likelihood of conception during breastfeeding.2
Anterior pituitary disorders
Hyperpituitarism
Hypersecretion of anterior pituitary hormones is most commonly due to a pituitary adenoma (a hormonesecreting tumour). The clinical manifestations are both those of the ‘space-occupying lesion’ effects (raised intracranial pressure, compression of the brainstem and optic nerves) and those of excess hormone levels (pituitary and/ or target-gland hormones). Thus prolactin-secreting adenomas manifest as gynaecomastia, galactorrhoea and infertility; GH-secreting adenomas as gigantism or acromegaly; and ACTH-secreting adenomas as Cushing’s syndrome.
First-line treatment is usually surgical removal of the tumour by a trans-sphenoidal approach. Prolactinsecreting tumours are effectively suppressed by a dopamine agonist acting as a PRIF analogue; those available are bromocriptine, cabergoline and quinagolide (see Clinical Interest Box 33-5). Acromegaly is treated with surgery, somatostatin analogues such as octreotide (Drug Monograph 33-1) or pegvisomant, a GH antagonist (see review by Lim [2009]).
Clinical interest box 33-5 Dopamine and lactation
Galactorrhoea (excessive production of milk other than after pregnancy) can occur in both men and women, and is due to hyperprolactinaemia. High prolactin levels are usually due to low levels of the hypothalamic inhibitory factor PRIF (dopa mine). This can occur in hypothalamic lesions and tumours, and also after use of dopamine-blocking agents such as antipsychotic drugs.
Treatment is with dopamine agonists, to mimic PRIF and stimulate hypothalamic dopamine receptors and hence decrease synthesis and release of prolactin in the anterior pituitary gland. Dopamine agonists used include apomorphine, levo dopa and especially bromocriptine, cabergoline or quinagolide. They are useful in pituitary adenomas and in preventing lactation (also in Parkinson’s disease; see Chapter 20, Clinical Interest Box 20-3 and Drug Monograph 20-3).
Because dopamine is a neurotransmitter in many pathways in both the central and peripheral nervous systems, there are many adverse reactions and adverse drug inter actions whenever dopamine agonists or antagonists are used. Adverse effects occur particularly in the central nervous sys tem, motor nervous system, cardiovascular system, endocrine glands and gastrointestinal tract.
Hypopituitarism
Deficiencies of pituitary hormones are most commonly due to non-hormone-secreting tumours or to an adenoma damaging particular cell types in the pituitary; combination deficiencies are common. For diagnosis, levels of both pituitary hormones and target-gland hormones are measured to distinguish between primary pituitary hyposecretion and target-gland hypofunction or negative feedback effects. Treatment is usually lifelong and requires replacing all target-gland hormones; imbalance in adrenal cortex hormones is corrected first, as this can be lifethreatening.
Posterior pituitary hormones
The neural-type tissue in the posterior pituitary gland secretes two hormones: oxytocin (a hormone that stimulates the smooth muscle of the uterus to contract) and vasopressin (antidiuretic hormone [ADH], with antidiuretic and vasopressor actions). As described earlier, the posterior lobe of the pituitary gland, the neurohypophysis, consists almost entirely of glial cells and neurons, with their cell bodies in the paraventricular and supraoptic nuclei of the hypothalamus. The hormones are synthesised in the hypothalamus and stored in secretory granules that are transported down the axons to the nerve endings in the neurohypophysis, from where they are released in response to neural stimuli.
Availability of these hormones in pure form has clarified their structures, actions and mechanisms of action, and has allowed better control of their therapeutic use. They are both nonapeptides (9-amino-acid residues), with very similar chemical structures. Their effects are not specific; for example, a certain overlap of pharmacological actions exists even in the pure preparations: pure oxytocin has some vasopressin activity and vice versa. The antidiuretic potency of vasopressin is much greater than its pressor (causing an increase in blood pressure) potency. The hormones are released together3 into the circulation but in varying proportions depending on the stimulus: thus during uterine contractions in the process of childbirth, and in response to suckling by the infant, mainly oxytocin is released, whereas in response to fluid loss mainly ADH is released.
Vasopressin (antidiuretic hormone, ADH)
Vasopressin is released in response to raised plasma osmotic pressure. This may occur after haemorrhage, water deprivation or other factors that cause diuresis or decrease the circulating blood volume. Obtained from natural sources and originally named because of its effects in raising the blood pressure, vasopressin itself is not often used in medicine now that potent, more specific synthetic analogues have been developed. Derivatives such as felypressin (mainly vasoconstrictor) and desmopressin (mainly antidiuretic; Drug Monograph 33-3) have very little, if any, oxytocic activity.
Drug monograph 33-3 Desmopressin
Vasopressin analogues include lypressin (lys-vasopressin, i.e. one of the original amino acids substituted with lysine) and felypressin (phe-lys-vasopressin), which are predominantly vasoconstrictors, and desmopressin (1-desamino-8-D-argvasopressin; DDAVP), a specific V2-receptor agonist with potent ADH activity. Desmopressin has a longer duration of activity than the other agents, as it is more resistant to metabolic inactivation.
Indications
Desmopressin is used to treat pituitary diabetes insipidus. It is not effective for polyuria induced by renal impairment or for nephrogenic or drug-induced diabetes insipidus. Desmopressin is also used intranasally for primary nocturnal enuresis, and the parenteral dosage form is used to treat haemorrhage in patients with haemophilia A or von Willebrand’s disease.
Pharmacokinetics
About 10% of an intranasal dose becomes bioavailable, thus the nasal spray dose is 10 times the parenteral dose. Desmopressin admin istered IM or SC has a half-life of 8–75 minutes; the duration of effect is 8–20 hours. The intranasal effect lasts 10–12 hours. The drug is excreted by the kidneys.
Adverse reactions
Headache, nausea, mild stomach cramps, pain and swelling at the injection site; rare: allergic reaction, water retention, intoxi cation and cardiac failure, hyponatraemia, convulsions. No significant drug interactions have been reported.
Dosage and administration
The adult injection dosage is 1–4 mcg daily IM or SC when needed to treat central diabetes insipidus or haemorrhage. The adult nasal spray dose is 10–40 mcg (1–2 sprays to one or both nostrils) when urination frequen cy increases or a significant thirst sensation occurs. Desmopressin is also available in oral or sublingual tablet form; because of its low bioavailability the oral dose is approximately 10–30 times that for the nasal spray, i.e. for children 100–200 mcg three times daily (tablets) or 60–120 mcg three times daily (sublingual wafers), compared to 2.5–20 mcg daily by nasal spray.
Mechanisms and actions
Vasopressin has been shown to act by activation of vasopressin V1 receptors (via inositol phosphate production) and V2 receptors (via adenylate cyclase). It increases the permeability of renal distal tubule walls to water and hence causes resorption of water, resulting in decreased urine volume with a higher osmolarity (i.e. antidiuresis). The mechanism for this effect is via an aquaporin (a water channel) in the epithelial cells; in the resting state the aquaporin 2 is localised to intracellular vesicle membranes, but when vasopressin binds to its cell-surface receptor it activates a signalling pathway that causes these vesicles to fuse with the plasma membrane, increasing the resorption of water and reducing urine production. Inactivating mutations in either the VP gene or the aquaporin 2 gene can lead to diabetes insipidus, in which large volumes of dilute urine are excreted.
In 100-fold higher doses, vasoconstrictor effects occur via V1 receptors, useful in treating haemorrhage but causing raised blood pressure and potentially angina. Vasopressin also has many non-renal actions, including smooth muscle contraction, platelet aggregation, raised factor VIII levels (hence its use in haemorrhage and haemophilia) and increased release of ACTH and hydrocortisone. It also has neuromodulator actions and may be involved in learning and memory.
Clinical aspects
Vasopressin and its analogues are used as replacement therapy in pituitary diabetes insipidus and in bleeding conditions. They have been used for their antidiuretic effects in nocturnal enuresis (bed-wetting) and as vasoconstrictors in formulations of local anaesthetics. Because they are peptides, these hormones are rapidly metabolised by peptidases. They are best administered intranasally as a finely divided powder (a snuff) or as a nasal spray.
There are many drug interactions; for example, vasopressin sensitivity is increased by carbamazepine, while it is decreased by lithium and methoxyflurane. Note that demeclocycline, a tetracycline antibiotic, is also a specific ADH antagonist and is used to produce diuresis in the syndrome of inappropriate secretion of ADH (SIADH).
Oxytocin
Oxytocin means ‘rapid birth’, a term derived from the hormone’s ability to contract the pregnant uterus. When released during childbirth, it causes physiological-type contractions, i.e. regular and coordinated towards the cervix, with relaxation in between. The non-pregnant uterus is relatively insensitive to oxytocin but during pregnancy, uterine sensitivity to oxytocin gradually increases, with the uterus being most sensitive at term. Large amounts of oxytocin have been detected in the blood during the expulsive phase of delivery. A positive feedback mechanism may be operating: more forceful contractions of uterine muscle and greater stretching of the cervix and vagina result in more oxytocin release. Oxytocin acts directly on the myometrium, having a stronger effect on the fundus than on the cervix, and is used clinically to induce or enhance labour (see Drug Monograph 33-4).
Indications
Oxytocin is administered IM or IV to induce, augment or manage labour when uterine muscle function is inadequate, to treat postpartum haemorrhage and to stimulate lactation. Uterine motility and fetal heart rate must be monitored. It is contraindicated if there is fetal distress or if vaginal delivery is contraindicated.
Pharmacokinetics
This product is a peptide so is inactivated rapidly in the liver with a half-life of 1–6 minutes. Hence it is usually given by IV infusion, when its onset of action is immediate, although uterine contractions increase gradually over 15–60 minutes before they stabilise. Duration of action is until about 1 hour after the infusion is stopped.
Drug interactions
When used concurrently with sodium chloride or urea for intra-amniotic induction of labour or with other oxytocics, uterine rupture or severe cervical laceration may occur. Prostaglandins and inhalational anaesthetics may enhance the actions of oxytocin. Whenever such combinations are used, the mother and fetus should be closely monitored.
Adverse reactions
These include nausea, vomiting, hypotension, tachycardia and irregular heart rate with the parenteral drug. Prolonged therapy may result in water intoxication. Oxytocin may occasionally cause fetal bradycardia, dysrhythmias and neonatal jaundice. Careful monitored use of oxytocin has contributed significantly to the safety of childbirth; it has an Australian pregnancy safety classification of A.
Dosage and administration
The dose to induce labour is 2 mU/min by IV infusion, increased at intervals of >30 min until a contraction pattern is established that simulates normal labour (up to a maximum of 32 mU/min). It is administered IM to manage the third stage of labour, sometimes in conjunction with ergometrine (see Chapter 38).
Oxytocin also transiently impedes uterine blood flow and stimulates the mammary glands to increase milk excretion from the breast, although it does not increase the production of milk. Release of oxytocin during suckling by the infant helps reduce the uterus to pre-pregnancy size. Because of its close similarity to ADH, oxytocin also has weak ADH-like actions but may have transient vasodilator (not vasoconstrictor) action. There is no distinct clinical syndrome related to oxytocin deficiency. (Oxytocin has in the past been administered by nasal spray to stimu late milk excretion and breastfeeding; however, there was little evidence of efficacy and this formulation has been withdrawn.)
Carbetocin, a new synthetic analogue of oxytocin, is an octapeptide with similar therapeutic actions (stimulating uterine contractions and milk excretion) but a prolonged duration of action, for up to 1 hour. It is given by single IV injection after caesarean section, to prevent excessive postpartum haemorrhage. Adverse effects are similar to those of oxytocin; however, carbetocin has a pregnancy safety category C, as it is only indicated for use postdelivery.
Key points
 The system is composed of specialised glands, which secrete into the bloodstream hormones that act on specific target cells to produce complex responses.
The system is composed of specialised glands, which secrete into the bloodstream hormones that act on specific target cells to produce complex responses. Pathological conditions in this system usually involve the overproduction or underproduction of hormones, and are treated by surgery, anti-hormones or replacement hormone therapy.
Pathological conditions in this system usually involve the overproduction or underproduction of hormones, and are treated by surgery, anti-hormones or replacement hormone therapy. The neuroendocrine system (the interactions between the hypothalamus in the brain and the pituitary gland) helps coordinate CNS and endocrine functions.
The neuroendocrine system (the interactions between the hypothalamus in the brain and the pituitary gland) helps coordinate CNS and endocrine functions. The pituitary gland consists of two main parts: the anterior lobe, which produces seven hormones, and the posterior lobe, producing two hormones.
The pituitary gland consists of two main parts: the anterior lobe, which produces seven hormones, and the posterior lobe, producing two hormones. The pituitary hormones are secreted into the bloodstream and act on target glands or organs, which respond by secreting other hormones or regulating growth or metabolism.
The pituitary hormones are secreted into the bloodstream and act on target glands or organs, which respond by secreting other hormones or regulating growth or metabolism. The functions of the anterior pituitary gland (adenohypophysis) are controlled by hypothalamic factors, which may stimulate or inhibit release of anterior pituitary hormones, and by negative feedback loops from targetgland hormones.
The functions of the anterior pituitary gland (adenohypophysis) are controlled by hypothalamic factors, which may stimulate or inhibit release of anterior pituitary hormones, and by negative feedback loops from targetgland hormones. Various hypothalamic factors are used in medicine to diagnose endocrine disorders or treat dysfunction of the target glands.
Various hypothalamic factors are used in medicine to diagnose endocrine disorders or treat dysfunction of the target glands. The pituitary hormones are usually used for replacement therapy in hormone deficiencies, such as drug therapy for a specific pituitary or target-gland disorder.
The pituitary hormones are usually used for replacement therapy in hormone deficiencies, such as drug therapy for a specific pituitary or target-gland disorder. Octreotide is similar to somatostatin, the growthhormone-inhibiting agent, so it is used in acromegaly and in gastrointestinal tract tumours.
Octreotide is similar to somatostatin, the growthhormone-inhibiting agent, so it is used in acromegaly and in gastrointestinal tract tumours. Prolactin is hypersecreted in pituitary adenomas and as an adverse effect of antidopamine drugs. It can be suppressed by dopamine agonist drugs that mimic the prolactinrelease-inhibiting factor.
Prolactin is hypersecreted in pituitary adenomas and as an adverse effect of antidopamine drugs. It can be suppressed by dopamine agonist drugs that mimic the prolactinrelease-inhibiting factor. The posterior lobe (neurohypophysis) secretes two hormones synthesised in the hypothalamus, under neural control.
The posterior lobe (neurohypophysis) secretes two hormones synthesised in the hypothalamus, under neural control.Review exercises
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Bolander F.F. Molecular Endocrinology, 3rd edn. Amsterdam: Elsevier Academic; 2004.
de Kloet E.R., Joels M., Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6(6):463-475.
Endocrinology Expert Group. Therapeutic Guidelines: Endocrinology, version 4. Melbourne: Therapeutic Guidelines Limited; 2009.
Feelders R.A., Hofland L.J., van Aken L.J., et al. Medical therapy of acromegaly: efficacy and safety of somatostatin analogues. Drugs. 2009;69(16):2207-2226.
Lightman S.L. The neuroendocrinology of stress: a never ending story. Journal of Neuroendocrinology. 2008;20(6):880-884.
Lim E.M. Drug treatment of pituitary tumours. Australian Prescriber. 2009;32(1):19-21.
Melmed S., Conn P.M., editors. Endocrinology: Basic and Clinical Principles. Totowa NJ: Humana Press, 2005.
Tanner J.M. Foetus into Man: Physical Growth from Conception to Maturity. Ware: Castlemead; 1989.
Weise K.L., Nahata M.C. Growth hormone use in children with idiopathic short stature. Annals of Pharmacotherapy. 2004;38(9):1460-1468.
Growth Hormone: restrictions on prescribing: www.health.gov.au/hGH
New Zealand Medicines and Medical Devices Safety Authority: www.medsafe.govt.nz
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology