Chapter 43 Overview of Antimicrobial Chemotherapy and Antibiotic Resistance
Infections have always been a concern to those caring for the ill and injured. There is no doubt that the development of antimicrobial drugs throughout the 20th century has had an enormous effect on the health and wellbeing of the global population. Since the mass production of penicillin in 1943, the number of antibiotics has continued to grow. Unfortunately, the use of antimicrobial drugs has been accompanied by the emergence of resistant microorganisms. The problems facing health-care professionals now include not only the prudent use of antimicrobial drugs but the need to develop new agents and to curtail the continued emergence of resistant ‘superbugs’.
Key abbreviations
ESBL extended spectrum β-lactamases
MIC minimum inhibitory concentration
MRSA methicillin-resistant Staphylococcus aureus
VRSA vancomycin-resistant Staphylococcus aureus
VRE vancomycin-resistant Enterococcus
hVISA heterogenous-vancomycin intermediate resistant Staphylococcus aureus
Key background
Infections
INFECTIOUS diseases comprise a wide spectrum of illnesses caused by pathogenic microorganisms. Some common pathogens and their likely sites of infection in the body are listed in Table 43-1. These pathogens can cause pneumonia, urinary tract infections, upper respiratory tract infections, gastroenteritis, venereal disease, vaginitis, tuberculosis and candidiasis, to name but a few.
Table 43-1 Primary organisms and common sites of infection
| Organism | Infection site |
| Gram-positive cocci | |
| Staphylococcus aureus | |
| Staphylococcus epidermidis | Burns, skin infections, decubital and surgical wounds, paranasal sinus and middle ear (chronic sinusitis and otitis), lungs, lung abscess, pleura, endocardium, bone (osteomyelitis), joints |
| Streptococcus pneumoniae | Paranasal sinus and middle ear, lungs, pleura |
| Streptococcus pyogenes (group A β-haemolytic) | Burns, skin infections, decubital and surgical wounds, paranasal sinus and middle ear, throat, bone (osteomyelitis), joints |
| Streptococcus, viridans group | Endocardium |
| Gram-positive bacilli | |
| Clostridium tetani (anaerobe) | Puncture wounds, lacerations and crush injuries; toxins affecting nervous system |
| Corynebacterium diphtheriae | Throat, upper part of the respiratory tract |
| Gram-negative cocci | |
| Neisseria gonorrhoeae | Urethra, prostate, epididymis and testes, joints |
| Neisseria meningitidis | Meninges |
| Enteric Gram-negative bacilli | |
| As a group (Bacteroides, Enterobacter, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, other Proteus, Salmonella, Serratia, Shigella) | Peritoneum, biliary tract, kidney and bladder, prostate, decubital and surgical wounds, bone |
| Bacteroides | Brain abscess, lung abscess, throat, peritoneum |
| Enterobacter | Peritoneum, biliary tract, kidney and bladder, endocardium |
| Escherichia coli | Peritoneum, biliary tract, kidney and bladder |
| Klebsiella pneumoniae | Lungs, lung abscess |
| Other Gram-negative bacilli | |
| Burkholderia cepacia, Burkholderia gladiol, Burkholderia pseudomallei | Respiratory tract; sputum |
| Haemophilus influenzae | Meninges, paranasal sinus and middle ear, lungs, pleura |
| Pseudomonas aeruginosa | Burns, paranasal sinus and middle ear (chronic otitis media), decubital and surgical wounds, lungs, joints |
| Acid-fast bacilli | |
| Mycobacterium tuberculosis, Mycobacterium avium | Lungs, pleura, peritoneum, meninges, kidney and bladder, testes, bone, joints |
| Miscellaneous | |
| Chlamydia trachomatis | Genitalia, endocervical, rectum, eyes (neonates) |
| Mycoplasma pneumoniae | Lungs |
| Spirochaetes | |
| Treponema pallidum (syphilis) | Any tissue or vascular organ of the body |
| Fungi | |
| Aspergillus | Paranasal sinus and middle ear, lungs |
| Candida species | Skin infections, throat, lungs, endocardium, kidney and bladder, vagina |
| Cryptococcus | Lungs |
| Pneumocystis jiroveci | Lungs |
| Viruses | |
| Cytomegalovirus (CMV) | Saliva, urine or other body fluids |
| Enterovirus, mumps virus, and others | Meninges, epididymis, testes |
| Herpes virus or varicella-zoster virus | Skin infections (herpes simplex or zoster) |
| Respiratory viruses (including Epstein–Barr virus) | Throat, lungs |
| Anaerobes | |
| Gram-positive | |
| Clostridium difficile | |
| Clostridium perfringens | |
| Peptococcus species | Deep wounds, gut |
| Peptostreptococcus species | |
| Gram-negative | |
| Bacteroides fragilis | |
| Fusobacterium species | |
Infection, the invasion and multiplication of pathogenic microorganisms in body tissues, causing disease by local cellular injury, secretion of a toxin or by antigen–antibody reaction in the host, is classified primarily as local or systemic. A localised infection involving the skin or internal organs may progress to a systemic infection. A systemic infection involves the whole body rather than a localised area of the body. Several terms describe the degree of local or systemic infection.
Colonisation is the localised presence of microorganisms in body tissues or organs; these microorganisms can be pathogenic or part of the normal flora. Colonisation alone is not necessarily an infection: it signifies the potential for infection, depending on the multiplication of the microorganisms or an alteration in the individual’s host defence mechanisms. When flora at their normal colonisation site are altered (e.g. by the administration of an antibiotic that affects pathogens and some but not all of the normal microorganisms), the unaffected microorganisms within that environment may grow in an uninhibited manner, causing a secondary infection.
Inflammation is a protective mechanism of body tissues in response to invasion or toxins produced by colonising microorganisms (see Chapter 47). This reaction consists of cytological and histological tissue responses for the localisation of phagocytic activity and destruction or removal of injurious material, leading to repair and healing.
Bacteraemia is the presence of viable bacteria in the circulatory system. Septicaemia refers to a systemic infection caused by microorganism multiplication in the circulation. Although bacteraemia can lead to septicaemia in an immunocompromised host, it is (depending on the pathogen) usually a short-lived, self-limited process. In an immunocompromised host, bacteraemia can rapidly produce an overwhelming systemic disease. Sepsis is a syndrome with multiple organ involvement that is a result of microorganisms or their toxins circulating in the blood.
For non-pathogenic organisms colonising humans or causing transient bacteraemia without tissue invasion, antibiotic therapy is rarely required in immunocompetent people, whereas prophylactic antibiotic therapy might be required in immunocompromised individuals. In most cases of localised inflammation, such as wound infections, pneumonia or urinary tract infections, antimicrobial drugs reduce the number of viable pathogens. This permits the immune system to eliminate microorganisms. Antimicrobial drugs are also an essential part of the treatment of septicaemia and sepsis.
Microorganisms are divided into several groups: bacteria, mycoplasma, spirochaetes, fungi and viruses. Bacteria are classified according to their shape, such as bacilli, spirilla and cocci, and their capacity to be stained. Specific identification of bacteria requires a Gram stain and culture with chemical testing. A Gram stain is a sequential procedure involving crystal violet and iodine solutions followed by alcohol that allows the rapid classification of organisms into groups such as Gram-positive or Gramnegative bacilli (rods) or cocci. The culture procedures identify specific organisms, but they require 24–48 hours for completion.
Often, antibiotic selection is empirical and is based on the prescriber’s clinical impression. Once culture and sensitivity results are available, the antibiotic might be changed. This is referred to as directed therapy.
Antimicrobial therapy
The treatment of an infectious disease depends on the microorganism responsible, and different groups of antimicrobial drugs are used to treat different groups of microorganisms. Antimicrobial drugs can help cure or control most infections caused by microorganisms but they alone do not necessarily produce the cure. They are often adjuncts to methods such as surgical incision and drainage, and wound debridement for removal of nonviable infected tissue.
The first major antimicrobial drug group was the sulfonamides, which were introduced into clinical practice in 1933. So successful were these drugs in treating staphylococcal septicaemia that Gerhard Domagk was awarded the Nobel Prize for Medicine in 1939 for his discovery of the antibacterial effects of the sulfonamide drug prontosil. The authorities in Germany at that time forced him to decline the award but he later received the diploma and the medal. The second major group of antimicrobial drugs was the penicillins (introduced in the 1940s).
Antibiotics are natural substances derived from certain organisms (bacteria, fungi and others) that can suppress growth or destroy microorganisms (see Clinical Interest Box 43-1). There are now thousands of natural, synthetic and semisynthetic antibacterial drugs that are all commonly referred to as antibiotics. Other antimicrobial agents include the antimycobacterial, antifungal and antiviral agents. These drugs differ markedly in their physicochemical characteristics, mechanisms of action, pharmacological properties and antimicrobial spectra.
Clinical Interest Box 43-1 Australian medicinal plants
It has been suggested that more than 100 species of Australian plants are used by Aborigines to treat sores, cuts and wounds. Many exhibit promising properties for treating bacterial and fungal infections. Plants exhibiting astringent properties can be used to staunch bleeding and to provide a natural protective bandage. Tinctures of the inner bark of river red gum (Eucalyptus camaldulensis) and cocky apple (Planchonia careya) have been applied to sores for this reason. The stalks of the caustic bush, or caustic vine (Sarcostemma australe), and milkwood tree (Alstonia actinophylla) ooze a milky sap that also provides a protective bandage. This sap might possess antibacterial activity, as the proteolytic enzymes are reported to digest bacteria in infected tissue. Antibacterial activity has also been reported in root decoctions of hopwood (Dodonea viscosa). These have been used for cuts and open wounds and the leaves chewed as a remedy for toothache. This plant is widespread throughout eastern Australia and the presence of numerous active constituents (perhaps flavonoids) might provide the noted antibacterial and anti-inflammatory effects.
Wounds can be disinfected by infusions from plants of the Myrtaceae families known for their essential or volatile oils (tea tree and eucalypt). The active ingredients are thought to be cineoles. The yellow box (Eucalyptus melliodora) is one of the few eucalypts containing greater than 70% cineoles and which exhibits antimicrobial activity. Studies at the University of Western Sydney have determined moderate antimicrobial activity against Staphylococcus aureus, Escherichia coli and Candida albicans. Extracts of the lemon-scented gum (Eucalyptus citriodora) exhibit bacteriostatic activity; the active ingredients are thought to be citronellal, citriodorol and kinos. A well-known antiseptic available for sale is tea-tree oil, obtained from distillation of the leaves of Melaleuca alterniflora. It contains the substance terpinen-4-ol, which is thought to be bacteriostatic.
Bathing the eyes in an infusion of sneezeweeds (Centipeda minima or Centipeda thespidioides) may treat ocular infections such as sandy blight. Although the active ingredient is un known, the leaves, stems and flowers may contain alkaloids. Similarly the inner bark and sapwood of the green plum (Buchanania obovata) are used for treating oral and eye infections.
Antibiotic pharmacodynamics
The goal of antimicrobial therapy is to destroy or suppress the growth of infecting microorganisms so that normal host defences and other supporting mechanisms can control the infection, resulting in a cure. With regard to antibiotics, pharmacodynamics relates the concentration of the antibiotic to its antimicrobial efficacy. This relationship is influenced by the drug’s pharmacokinetic profile; the latter includes absorption, distribution, metabolism and excretion. Ultimately the success of antimicrobial therapy depends on:
In pharmacokinetic terms this translates to:
The varying relationships between T, Cmax, MIC and AUC0-24 have been shown to correlate with antimicrobial efficacy, bacterial eradication and a reduction in the development of resistance. In simple terms antimicrobial drugs can display concentration-dependent kill characteristics and/or time-dependent effects. For example, depending on the bacteria a high ciprofloxacin Cmax:MIC ratio (∼10) assists bacterial killing while a high AUC0–24/ MIC (>125) predicts better clinical outcomes for infections caused by Gram-negative organisms. Similarly, aminoglycosides exhibit concentration-dependent kill characteristics and suboptimal dosing may lead to adaptive resistance because of reduced drug uptake by the bacteria. In contrast the β-lactam antibiotics exhibit time-dependent killing; that is, their efficacy is related to the time that the concentration of the β-lactam exceeds the MIC of the bacteria, that is, T > MIC. Current data suggest that for penicillin the plasma drug concentration should be above the MIC for ∼50% of the dosing interval, 60%–70% for cephalosporins and 40% for carbapenems (Drusano 2004). It is apparent that achieving the pharmacodynamic target for antibiotic exposure is essential for both clinical efficacy and for reducing the emergence of resistant microbial strains.
Mechanism of action
To exert its effects, an antimicrobial agent must first gain access to the target site. Specific antibiotics or antimicrobial agents in certain circumstances (e.g. changes in permeability of the blood–brain barrier with meningitis) are capable of penetrating the site of infection and exerting a pharmacological effect on the bacteria. Sometimes, as in the case of infections of the skin and eyes, local application to the infected area is necessary. Once the drug has reached its site of action, it can exert either bactericidal or bacteriostatic effects, depending on its mechanism of action.
Bacteriostatic agents inhibit bacterial growth, allowing intact and active host defence systems time to remove the invading microorganisms. Bactericidal agents, on the other hand, cause bacterial cell death and lysis and eradicate the infection, which is important especially in situations of impaired host defences. Antimicrobial agents can be divided into bacteriostatic and bactericidal categories; the sulfonamides are an example of the former and the penicillins of the latter. Such categorisation is not always valid or reliable because the same antimicrobial agent might have either effect depending on the dose administered and the concentration achieved at its site of action. Tetracycline, for example, is generally bacteriostatic but may be bactericidal in high concentrations. Chloramphenicol, which is often listed as a bacteriostatic drug, has bactericidal effects against Streptococcus pneumoniae and Haemophilus influenzae in the cerebrospinal fluid.
Antimicrobial agents exert their bacteriostatic or bactericidal effects in one of four major ways (see Chapter 44):
Antibiotic resistance
The emergence of antibiotic-resistant organisms continues to be a major problem in high-dependency health-care settings (e.g. intensive care units), in hospitals in general and in other health-care arenas (e.g. aged care facilities). Once useful antibiotics are no longer effective and the ability to treat some infections has been lost leading to increased health-care expenditure related to morbidity and mortality. Overwhelming evidence indicates that the main driver of acquired resistance is the prescribing of antimicrobial drugs, and resistance is most prevalent in countries where antibiotic use is considered heavy, e.g. Spain, Portugal, Luxemborg and Italy (Livermore 2005). It is estimated that ∼70% of the bacteria implicated in hospital-acquired infections in the USA are resistant to one or more antibiotics. A major concern for all health professionals is that, in this era of increasing drug-resistant pathogens, the development of antimicrobial drugs has slowed dramatically as pharmaceutical companies pursue more lucrative areas of drug development (e.g. cardiovascular drugs, antiretroviral drugs, drugs for obesity). Indeed between 1998 and 2002 the FDA approved 225 new drugs of which 7 were antibiotics, and during 2003/04 only a further 2 antibiotics were approved (Scheetz et al 2006).
In general, when using antibiotics if the concentration of the antibiotic at the site of infection inhibits the pathogen and is below the concentration that is toxic to human cells, the pathogen is considered ‘susceptible’. If, however, the concentration of the antibiotic exceeds what is considered ‘safe’ to human cells, the pathogen is considered to be ‘resistant’ to the drug. Resistance can be either intrinsic or acquired. Intrinsic resistance refers to the organism’s ‘innate chromosomal (genetic) makeup that predictably specifies the resistance. For example, all streptococci are intrinsically resistant to aminoglycosides because their inherent genetic composition results in a cell wall that does not permit penetration of this class of antibiotics to its site of action at the ribosome (Mulvey et al 2009). In contrast acquired resistance ‘arises in an organism because of a change (mutation) in its genetic makeup or because of the acquisition of new genetic information (new DNA) specifying a new mechanism of resistance that the organism did not previously possess’ (Mulvey et al 2009). Mutations occur infrequently but invariably result in development of resistant bacteria that were susceptible initially to an antibiotic. From a historical perspective, with each cycle of introduction of new antibiotics, there has been an accompanying cycle of emerging resistance to the drugs. This is a worldwide problem but it is not a new one. Resistant microorganisms were present in our environment before the introduction of antibiotics into clinical medicine. In terms of ‘bacterial warfare’, many bacteria produce antibiotics to protect themselves against the inhibitory effects of antibiotics produced by a neighbouring bacterial species. This ‘strong selective pressure consistently resulted in the survival and spread of resistant bacteria’ (Barbosa & Levy 2000). Within 2 years of the introduction of penicillin, resistance began to be noted. The emergence of microorganisms resistant to antibiotics has continued (Table 43-2).
Table 43-2 Examples of emergence of antibiotic resistance
| Year | Antibiotic resistance |
| 1947 | Resistance to penicillin |
| 1960s | Streptomycin-resistant Enterococcus |
| 1961 | Methicillin-resistant Staphylococcus aureus (MRSA) |
| 1968 | Multi-drug resistant Shigella |
| 1970s | Ampicillin-resistant Neisseria gonorrhoeae, Haemophilus influenzae and Escherichia coli |
| Penicillin-resistant Streptococcus pneumoniae | |
| 1979 | Gentamicin-resistant Enterococcus faecalis |
| 1980s | Cephalosporin-resistant Klebsiella pneumoniae |
| Vancomycin-resistant Staphylococcus aureus (VRSA) | |
| 1983 | Extended spectrum β-lactamase (ESBL) resistance in Klebsiella pneumoniae and Serratia marcescens |
| 1986 | Vancomycin-resistant Enterococcus faecium (VRE) |
| 1987 | Ciprofloxacin-resistant Escherichia coli |
| 1994 | Vancomycin-resistant Enterococcus faecium, reported in Melbourne |
| 1996 | Extended spectrum β-lactamase (ESBL) resistance in Enterobacteriaceae |
| 1997 | Vancomycin-resistant Staphylococcus aureus, reported in Japan |
| 2001 | Heterogeneous-vancomycin intermediate resistant Staphylococcus aureus (hVISA) reported in Australia |
| 2002 | High-level vancomycin resistant Staphylococcus aureus reported in USA |
Sources: Swartz 2000, Paterson & Bonomo 2005, Mulvey et al 2009.
Mechanisms of antimicrobial drug resistance
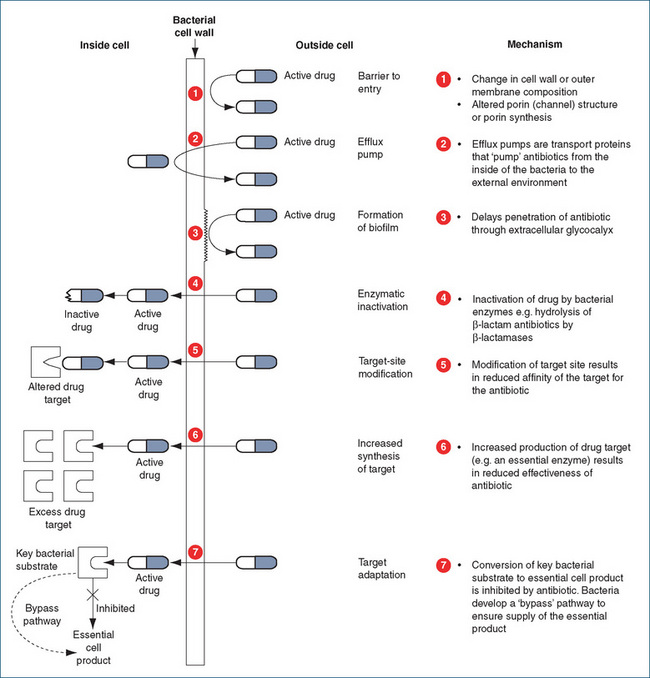
Resistance can occur in one of several ways (Figure 43-1), for example:
Combating antimicrobial drug resistance
Many resistant strains of bacteria exist in Australasia, and there are on-going problems with methicillin-resistant Staphylococcus aureus in outpatients and inpatients in Australia. In 1994, the first case of vancomycin-resistant Enterococcus faecium was reported in Melbourne. The appearance and spread of antimicrobial resistance is, however, not limited to bacteria. Resistance has now been reported among fungi, e.g. Candida albicans, and helminths (Geerts & Gryseels 2000). Of increasing importance worldwide is the emergence and spread of multi-drugresistant HIV-1 (Omrani & Pillay 2000) and Mycobacterium tuberculosis (Schluger 2000). The latter is of particular concern with evidence of multi-drug-resistant tuberculosis in provinces in China and countries of the former Soviet Union (Wright et al 2009).
The adverse consequences of increasing antimicrobial drug resistance will inevitably be increases in rates of hospitalisation, duration of hospital stay and rates of mortality. To assist in combating these problems cooperative relationships have developed between health professionals. Variously called antibiotic management programs, anti biotic vigilance programs and antibiotic management strategies, they are more frequently referred to as antimicrobial stewardship programs. These programs focus on:
Such programs require long-term commitment and a willingness to invest resources and a process of evaluation to determine their benefit.
Other strategies to combat antimicrobial drug resistance include:
Superinfection
Superinfection is an infection that occurs during antimicrobial therapy delivered for therapeutic or prophylactic reasons. Most antibiotics reduce or eradicate the normal microbial flora of the body, which are then replaced by resistant exogenous or endogenous bacteria. If the number of these replacement organisms is large and the host conditions favourable, clinical superinfection can occur.
Around 2% of people treated with antibiotics contract superinfections. The risk is greater when large doses of antibiotics are given, when more than one antibiotic is administered concurrently and when broad-spectrum drugs are used. Some specific antimicrobials are more commonly associated with superinfection than others. For example, Pseudomonas organisms frequently colonise and infect individuals taking cephalosporins. In a similar manner, people taking tetracyclines may become infected with Candida albicans. Generally, superinfections are caused by microorganisms that are resistant to the drug the individual is receiving. In the past, penicillinase-producing staphylococci were the most common cause of superinfection. Staphylococcus aureus and Staphylococcus epidermidis super infections, especially with methicillin-resistant strains, are again on the rise. Gram-negative enteric bacilli and fungi are the most common offenders. The proper management of superinfections includes discontinuing the drug being given or replacing it with another drug to which the organism is sensitive, culturing the suspected infected area and administering an antimicrobial agent effective against the new offending organism.
General guidelines for use of antibiotics
Perhaps the first question that should be posed is whether an antimicrobial drug is needed (see Clinical Interest Box 43-2). Several important principles, however, guide the judicious and optimal use of the antibiotics (AMH 2010; Ferguson 2004; Antibiotic Expert Group 2006). These include:
Clinical Interest Box 43-2 Don’t take the bite out of antibiotic
Doctors around New Zealand are urging people to make sure antibiotics don’t lose their effectiveness. The Wise Use of Antibiotics Campaign launched in May 2001 aimed to educate people to use antibiotics only when required because of rising concern over the number of cases of methicillinresistant Staphylococcus aureus.
In 2000, only 20% of New Zealanders understood that antibiotics were not an effective way to treat colds and flu. By the end of winter 2003, research showed that nearly half the people who visited their doctor understood this role of antibiotics. Chicken soup, fluids, simple analgesics and rest were promoted as suitable management for uncomplicated viral respiratory conditions. PHARMAC (the Pharmaceutical Management Agency) announced an almost 15% drop in antibiotic prescribing in the year after the campaign had been launched. However, the 2008/09 PHARMAC annual report indicated that antibiotics were currently listed 14th in terms of expenditure. In 2004 expenditure on antibiotics was $13.06 million and this had risen to $16.37 million in 2008/09.
Adapted from: www.pharmac.govt.nz [4 March 2010].
As most antimicrobial agents have a specific effect on a limited range of microorganisms, the prescriber must formulate a specific diagnosis about the potential pathogens or organisms most likely causing the infection. The drug most likely to be specifically effective against the suspected microorganism can then be selected (empirical therapy). It is known, for example, that microorganisms commonly isolated in acute adult infections of the lung include pneumococci, Haemophilus-strain streptococci and staphylococci. Antimicrobial agents specifically toxic to those organisms may be administered temporarily. The drugs can then be changed, if necessary, after laboratory reports have been received.
Identification of the microorganism is most reliably accomplished by obtaining specimens from the infected area if possible (e.g. urine, sputum or wound drainage) or by obtaining venous blood specimens and sending them to the laboratory for culture and identification of the causative organism. It is desirable to receive culture and sensitivity reports before initiating antimicrobial therapy. Once the organism has been identified, the appropriate drug can be administered (directed therapy).
In some situations, however, it is not practical to wait for laboratory results. For example, antimicrobial therapy must be initiated without delay in acute, life-threatening situations, such as peritonitis, septicaemia or pneumonia. In such situations, the choice of antimicrobial agent for initial use may be based on a tentative identification of the pathogen.
Some infections are most effectively treated with only one antibiotic. In other situations, combined antimicrobial drug therapy may be indicated. Indications for the simultaneous use of two or more antimicrobial agents include:
Indiscriminate use of combined antimicrobial drug therapy should be avoided because of expense, toxicity and higher incidence of superinfections and resistance.
Dosage and duration of therapy
Administering antimicrobial drugs for therapeutic purposes in adequate dosage and for long enough periods is an important principle of infectious disease therapy. Fortunately, plasma concentrations of some of the more potent antibiotics (e.g. aminoglycosides) can be monitored to prevent or minimise the risk of toxicity. Failure of antimicrobial therapy is frequently the result of drug doses being too small or being given for too short a period. Follow-up cultures should be obtained to assess the effectiveness of therapy.
Inadequate drug therapy may lead to remissions and exacerbations of the infectious process and can contribute to the development of resistance. When antibiotics are used prophylactically, they are usually given for short periods to enhance host defence mechanisms. For example, with perioperative antibiotics, a loading dose is given immediately before surgery and continued for 48 hours after surgery.
Many antimicrobial agents are currently in use, and health-care professionals should be familiar with the general characteristics of each drug group or category and with one or two prototype drugs in each group. Because the dosage for any given antibiotic varies with the type of infection, the site of infection and the age and health status of the individual, only general dosages or dose ranges are given in this text. The manufacturer’s package insert or a hospital formulary or pharmacy should be consulted for specific dosages.
Paediatric implications
In assessing the appropriateness of antibiotic therapy in children, the following criteria are generally accepted:
Role of host defence mechanisms
No antimicrobial agent will cure an infectious process if host defence mechanisms are inadequate. Such drugs act only on the causative organisms of infectious disease and have no effect on the defence mechanisms of the body, which need to be assessed and supported. Many infections do not require drug therapy and are adequately combated by the individual’s defence mechanisms, including antibody production, phagocytosis, interferon produc tion, fibrosis or gastrointestinal rejection (vomiting and diarrhoea). Host defence mechanisms may, however, be diminished, for example in people with diabetes mellitus or neoplastic disease and in immunocompromised individuals (e.g. those with HIV).
The status of the host’s defence mechanisms will also influence the choice of therapy, route of administration and dosage. If an infection is fulminating, for example, parenteral (preferably intravenous) administration of a bactericidal drug will be selected rather than oral administration of a bacteriostatic drug. Large loading doses of antimicrobial agents are often administered at the beginning of treatment of severe infections to achieve maximum blood concentrations rapidly. Factors influencing drug dosage are, however, often related to the status of a patient’s renal function. Because many antimicrobial agents are excreted by the kidneys, a major management problem exists with individuals who have compromised renal function. Drug dosages are then generally reduced in parallel with the person’s creatinine clearance. Haemodialysis can further alter the therapeutic regimen. In some disease states (such as burns), antibiotic dosage may need to be increased to achieve therapeutic levels. In short, the administration of an antimicrobial agent specifically toxic to the isolated microorganism is not the only important measure in antimicrobial therapy. An additional and very important determinant of the effectiveness of an antimicrobial agent is the functional state of the host’s defence mechanisms.
Key points
 Antimicrobial therapy may include either bacteriostatic (inhibiting bacterial growth) or bactericidal (causing bacterial cell death and lysis) drugs or drugs that have both effects, depending on the concentration at the site of action.
Antimicrobial therapy may include either bacteriostatic (inhibiting bacterial growth) or bactericidal (causing bacterial cell death and lysis) drugs or drugs that have both effects, depending on the concentration at the site of action. The goal of antimicrobial therapy is to destroy or to suppress the growth of infecting microorganisms so that normal host defences and other supporting mechanisms can control the infection, resulting in its cure.
The goal of antimicrobial therapy is to destroy or to suppress the growth of infecting microorganisms so that normal host defences and other supporting mechanisms can control the infection, resulting in its cure. Resistance can be either intrinsic or acquired. From a historical perspective, with each cycle of introduction of new antibiotics, there has been an accompanying cycle of emerging resistance to the drugs.
Resistance can be either intrinsic or acquired. From a historical perspective, with each cycle of introduction of new antibiotics, there has been an accompanying cycle of emerging resistance to the drugs. Resistance is a worldwide problem but it is not a new one, as resistant microorganisms were present in our environment before the introduction of antibiotics into clinical medicine.
Resistance is a worldwide problem but it is not a new one, as resistant microorganisms were present in our environment before the introduction of antibiotics into clinical medicine. Resistance can occur in one of several ways: (1) inability of the antimicrobial drug to reach the potential site of its action; (2) the production of an enzyme that modifies or destroys the structure of the antibiotic, which renders it inactive; (3)alteration of the target site for the drug so it can no longer bind to the target; (4) the drug being pumped out by an efflux pump; or (5) the development of bypass pathways that compensate for the loss of function due to the antimicrobial drug.
Resistance can occur in one of several ways: (1) inability of the antimicrobial drug to reach the potential site of its action; (2) the production of an enzyme that modifies or destroys the structure of the antibiotic, which renders it inactive; (3)alteration of the target site for the drug so it can no longer bind to the target; (4) the drug being pumped out by an efflux pump; or (5) the development of bypass pathways that compensate for the loss of function due to the antimicrobial drug. Strategies to combat antimicrobial drug resistance include encouraging optimal use of antimicrobials; selective control, restriction or removal of antimicrobial agents or classes; use of antimicrobial drugs in rotation or cyclic patterns; and use of combinations of antimicrobial drugs to prevent emergence of resistance.
Strategies to combat antimicrobial drug resistance include encouraging optimal use of antimicrobials; selective control, restriction or removal of antimicrobial agents or classes; use of antimicrobial drugs in rotation or cyclic patterns; and use of combinations of antimicrobial drugs to prevent emergence of resistance. Superinfection is an infection that occurs during antimicrobial therapy delivered for therapeutic or prophylactic reasons.
Superinfection is an infection that occurs during antimicrobial therapy delivered for therapeutic or prophylactic reasons. Guidelines have been developed for the prudent use of antibiotics; these include using an antibacterial drug only when indicated, identifying the infecting microorganism, determining the susceptibility of the microorganism, using a drug with the narrowest spectrum of activity for the known or likely organism, using a single drug unless combination therapy is specifically indicated to ensure efficacy or reduce the emergence of resistance, using a dose of drug that is high enough to ensure efficacy with minimal toxicity and reduces the likelihood of resistance and using a short duration of treatment (e.g. 1 week) unless evidence indicates that a longer duration is required.
Guidelines have been developed for the prudent use of antibiotics; these include using an antibacterial drug only when indicated, identifying the infecting microorganism, determining the susceptibility of the microorganism, using a drug with the narrowest spectrum of activity for the known or likely organism, using a single drug unless combination therapy is specifically indicated to ensure efficacy or reduce the emergence of resistance, using a dose of drug that is high enough to ensure efficacy with minimal toxicity and reduces the likelihood of resistance and using a short duration of treatment (e.g. 1 week) unless evidence indicates that a longer duration is required.Antibiotic Expert Group. Therapeutic Guidelines: Antibiotic, version 13. Melbourne: Therapeutic Guidelines; 2006.
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Barbosa T.M., Levy S.B. The impact of antibiotic use on resistance development and persistence. Drug Resistance Update. 2000;3:303-311.
Drusano G.L. Antimicrobial pharmacodynamics: critical interactions of “bug” and “drug”. Nature Reviews of Microbiology. 2004;2:289-300.
Ferguson J. Antibiotic prescribing: how can emergence of antibiotic resistance be delayed? Australian Prescriber. 2004;27:39-42.
Chambers H.F. General considerations of antimicrobial therapy. In Brunton L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th edn, New York: McGraw-Hill, 2006. [ch 42]
Geerts S., Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clinical Microbiology Reviews. 2000;13:207-222.
Gottesman B.S., Carmeli Y., Shitrit P., Chowers M. Impact of quinolone restriction on resistance patterns of Escherichi coli isolated from urine by culture in a community setting. Clinical Infectious Diseases. 2009;49:869-875.
Livermore D.M. Minimising antibiotic resistance. Lancet. 2005;5:450-459.
Mulvey M.R., Simor A.E. Antimicrobial resistance in hospitals : how concerned should we be? Canadian Medical Association Journal. 2009;180:408-415.
Omrani A.S., Pillay D. Multi-drug resistant HIV-1. Journal of Infection. 2000;41:5-11.
Roberts J.A., Kruger P., Paterson D.L., et al. Antibiotic resistance—what’s dosing got to do with it? Critical Care Medicine. 2008;36:2433-2440.
Scheetz M.H., Hurt K.M., Noskin G.A., Oliphant C.M. Applying antimicrobial pharmacodynamics to resistant gram-negative pathogens. American Journal of Health-System Pharmacy. 2006;63:1346-1360.
Schluger N.W. The impact of drug resistance on the global tuberculosis epidemic. International Journal of Tuberculosis and Lung Disease. 2000;4:571-575.
Swartz M.N. Impact of antimicrobial agents and chemotherapy from 1972 to 1998. Antimicrobial Agents and Chemotherapy. 2000;44:2009-2016.
Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775-781.
Wright A., Zignol M., Van Duen A., et al. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug resistance Surveillance. Lancet. 2009;373:1861-1873.