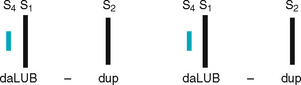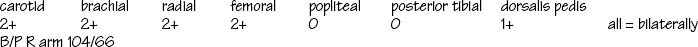Chapter Twelve Heart and neck vessels
INTRODUCTION
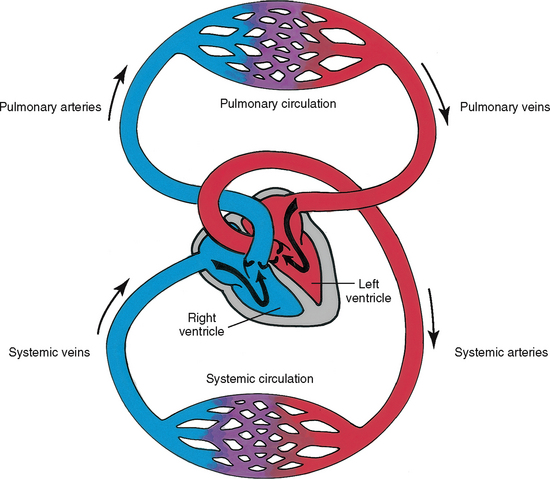
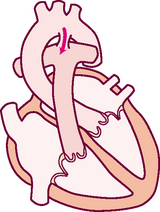
The cardiovascular system consists of the heart, a muscular pump, and the blood vessels. The blood vessels are arranged in two continuous loops, the pulmonary circulation and the systemic circulation (Fig 12.1). When the heart contracts, it pumps blood simultaneously into both loops. In order to appreciate the impact of disease and trauma to this complex and dynamic system you are advised to first review the structure and function of the cardiovascular system.
POSITION AND SURFACE LANDMARKS
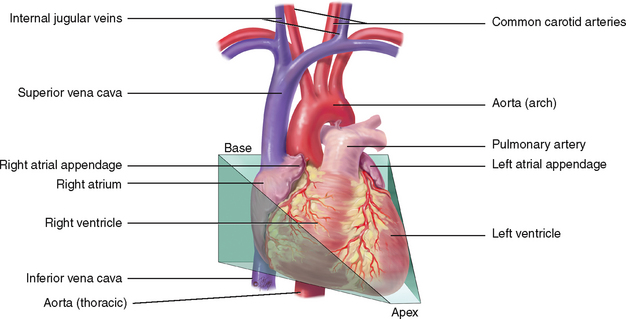
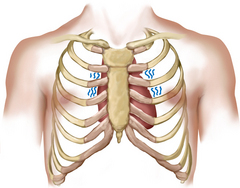
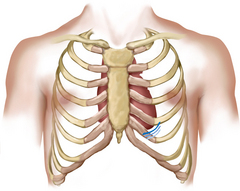
The praecordium is the area on the anterior chest overlying the heart and great vessels (Fig 12.2). The great vessels are the major arteries and veins connected to the heart. The heart and the great vessels are located between the lungs in the middle third of the thoracic cage, called the mediastinum. The heart extends from the second to the fifth intercostal space and from the right border of the sternum to the left midclavicular line.
Think of the heart as an upside-down triangle in the chest. The ‘top’ of the heart is the broader base, and the ‘bottom’ is the apex, which points down and to the left (Fig 12.3). During contraction, the apex beats against the chest wall, producing an apical impulse. This is palpable in most people, normally at the fifth intercostal space, 7 to 9 cm from the midsternal line on the left side.
Inside the body, the heart is rotated so that its right side is anterior and its left side is mostly posterior. Of the heart’s four chambers, the right ventricle forms the greatest area of anterior cardiac surface. The left ventricle lies behind the right ventricle and forms the apex and slender area of the left border. The right atrium lies to the right and above the right ventricle and forms the right border. The left atrium is located posteriorly, with only a small portion, the left atrial appendage, showing anteriorly.
The great vessels lie bunched above the base of the heart. The superior and inferior vena cava return deoxygenated venous blood to the right side of the heart. The pulmonary artery leaves the right ventricle, bifurcates and carries the venous blood to the lungs. The pulmonary veins return the freshly oxygenated blood to the left side of the heart, and the aorta carries it out to the body. The aorta ascends from the left ventricle, arches back at the level of the sternal angle and descends behind the heart.
HEART WALL, CHAMBERS AND VALVES
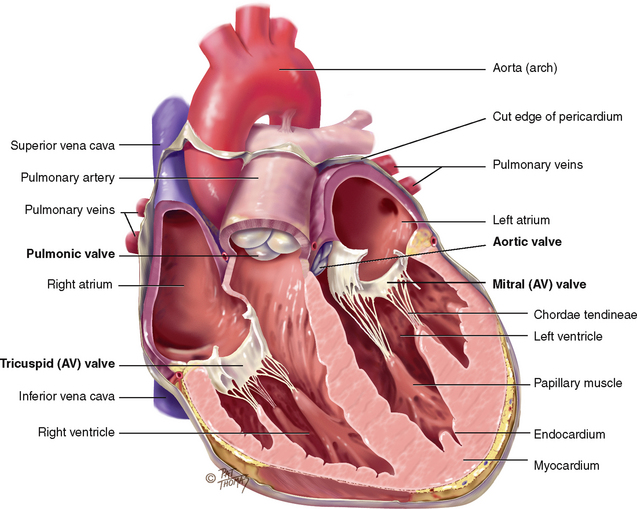
The heart wall has three layers. The pericardium is a tough, fibrous, double-walled sac that surrounds and protects the heart (see its cut edge in Fig 12.4). It has two layers that contain 10–30 mL of serous pericardial fluid. This ensures smooth, friction-free movement of the heart muscle. The pericardium is adherent to the great vessels, oesophagus, sternum and pleurae and is anchored to the diaphragm. The myocardium is the muscular wall of the heart; it does the pumping. The endocardium is the thin layer of endothelial tissue that lines the inner surface of the heart chambers and valves.
The common metaphor is to think of the heart as a pump. But consider that the heart is actually two pumps; the right side of the heart pumps blood into the lungs, and the left side of the heart simultaneously pumps blood into the body. The two pumps are separated by an impermeable wall, the septum. Each side has an atrium and a ventricle. The atrium (Latin for ‘anteroom’) is a thin-walled reservoir for holding blood, and the thick-walled ventricle is the muscular pumping chamber. (It is common to use the following abbreviations to refer to the chambers: RA, right atrium; RV, right ventricle; LA, left atrium; and LV, left ventricle.)
The four chambers are separated by swinging-door-like structures, called valves, whose main purpose is to prevent backflow of blood. The valves are unidirectional; they can only open one way. The valves open and close passively in response to pressure gradients in the moving blood.
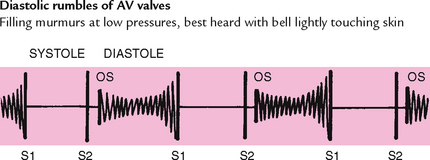
There are four valves in the heart (see Fig 12.4). The two atrioventricular (AV) valves separate the atria and the ventricles. The right AV valve is the tricuspid, and the left AV valve is the bicuspid or mitral valve (so named because it resembles a bishop’s mitred cap). The valves are thin leaflets that are anchored by collagenous fibres (chordae tendineae) to papillary muscles embedded in the ventricle floor. The AV valves open during the heart’s filling phase, or diastole, to allow the ventricles to fill with blood. During the pumping phase, or systole, the AV valves close to prevent regurgitation of blood back up into the atria. The papillary muscles contract at this time, so that the valve leaflets meet and unite to form a perfect seal without turning themselves inside out.
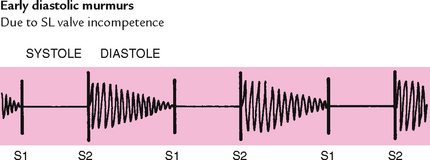
The semilunar (SL) valves are set between the ventricles and the arteries. Each valve has three cusps that look like half moons. The SL valves are the pulmonic valve in the right side of the heart and the aortic valve in the left side of the heart. They open during pumping, or systole, to allow blood to be ejected from the heart.
Note that no valves are present between the vena cava and the right atrium, nor between the pulmonary veins and the left atrium. For this reason, abnormally high pressure in the left side of the heart gives a person symptoms of pulmonary congestion, and abnormally high pressure in the right side of the heart shows in the neck veins and abdomen.
DIRECTION OF BLOOD FLOW
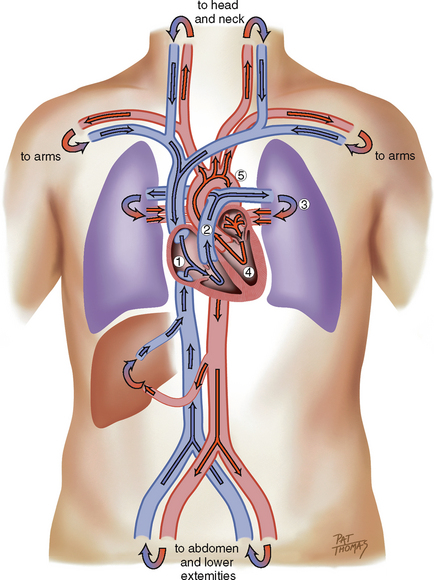
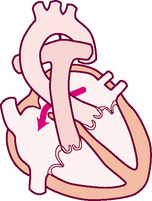
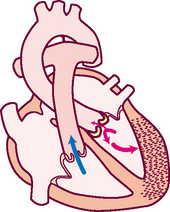
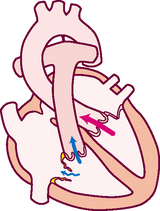
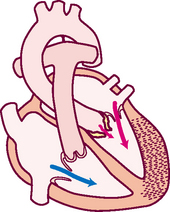
Think of a deoxygenated red blood cell being drained downstream into the vena cava. It is swept along with the flow of venous blood and follows the route illustrated in Fig 12.5.
1. From liver to right atrium (RA) through inferior vena cava
Superior vena cava drains venous blood from the head and upper extremities
From RA, venous blood travels through tricuspid valve to right ventricle (RV)
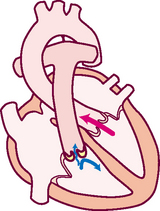
2. From RV, venous blood flows through pulmonic valve to pulmonary artery
Pulmonary artery delivers deoxygenated blood to lungs
Pulmonary veins return oxygenated blood to LA
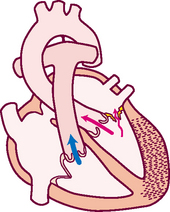
4. From LA, arterial blood travels through mitral valve to LV
Remember that the circulation is a continuous loop. The blood is kept moving along by continually shifting pressure gradients. The blood flows from an area of higher pressure to one of lower pressure.
CARDIAC CYCLE
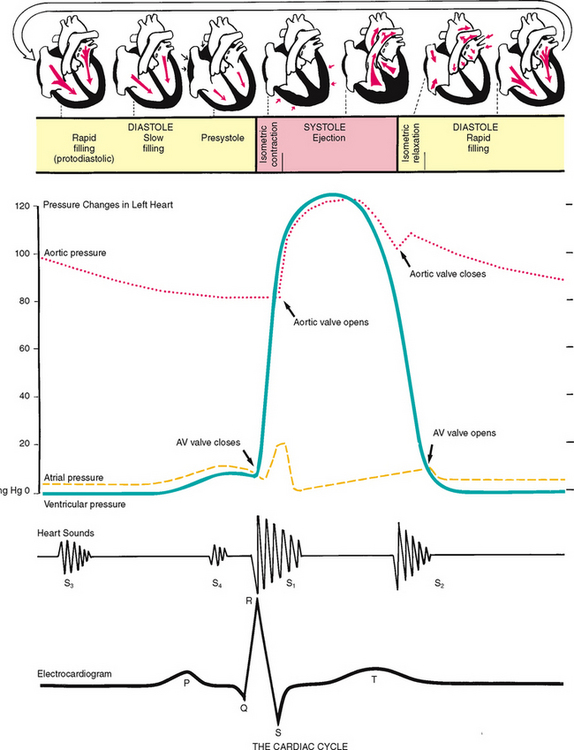
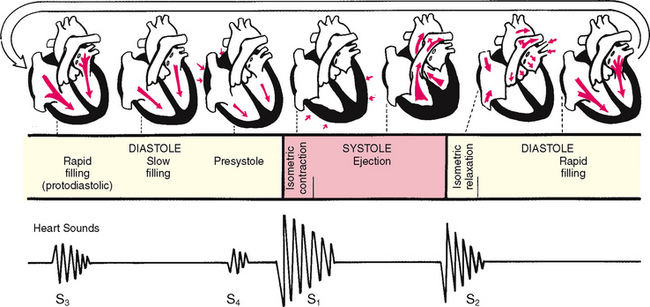
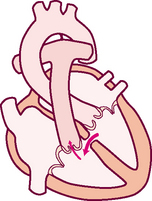
The rhythmic movement of blood through the heart is the cardiac cycle. It has two phases, diastole and systole. In diastole, the ventricles relax and fill with blood. This takes up two thirds of the cardiac cycle. The heart’s contraction is systole. During systole, blood is pumped from the ventricles and fills the pulmonary and systemic arteries. This is one-third of the cardiac cycle.
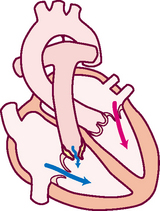
Diastole.
In diastole, the ventricles are relaxed, and the AV valves (i.e. the tricuspid and mitral) are open (Fig 12.6). (Opening of the normal valve is acoustically silent.) The pressure in the atria is higher than that in the ventricles, so blood pours rapidly into the ventricles. This first passive filling phase is called early or protodiastolic filling.
Towards the end of diastole, the atria contract and push the last amount of blood (about 25% of stroke volume) into the ventricles. This active filling phase is called presystole, or atrial systole, or sometimes the ‘atrial kick’. It causes a small rise in left ventricular pressure. (Note that atrial systole occurs during ventricular diastole, a confusing but important point.)
Systole.
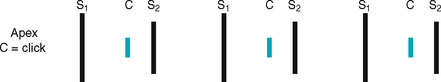
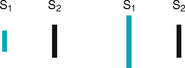
Now so much blood has been pumped into the ventricles that ventricular pressure is finally higher than that in the atria, so the mitral and tricuspid valves swing shut. The closure of the AV valves contributes to the first heart sound (S1) and signals the beginning of systole. The AV valves close to prevent any regurgitation of blood back up into the atria during contraction.
For a very brief moment, all four valves are closed. The ventricular walls contract. This contraction against a closed system works to build pressure inside the ventricles to a high level (isometric contraction). Consider first the left side of the heart. When the pressure in the ventricle finally exceeds pressure in the aorta, the aortic valve opens and blood is ejected rapidly.
After the ventricle’s contents are ejected, its pressure falls. When pressure falls below pressure in the aorta, some blood flows backwards towards the ventricle, causing the aortic valve to swing shut. This closure of the semilunar valves causes the second heart sound (S2) and signals the end of systole.
Diastole again.
Now all four valves are closed and the ventricles relax (called isometric or isovolumic relaxation). Meanwhile, the atria have been filling with blood delivered from the lungs. Atrial pressure is now higher than the relaxed ventricular pressure. The mitral valve drifts open and diastolic filling begins again.
Events in the right and left sides.
The same events are happening in the right side of the heart, but pressures in the right side of the heart are much lower than those of the left side because less energy is needed to pump blood to its destination, the pulmonary circulation. Also, events occur just slightly later in the right side of the heart because of the route of myocardial depolarisation. As a result, two distinct components to each of the heart sounds exist, and sometimes you can hear them separately. In the first heart sound, the mitral component (M1) closes just before the tricuspid component (T1). And with S2, aortic closure (A2) occurs slightly before pulmonic closure (P2).
HEART SOUNDS
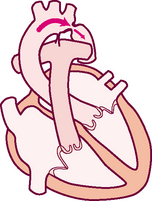
Events in the cardiac cycle generate sounds that can be heard through a stethoscope over the chest wall. These include normal heart sounds and, occasionally, extra heart sounds and murmurs (Fig 12.7).
Normal heart sounds
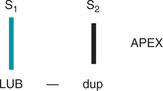
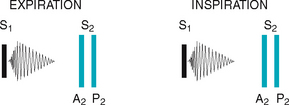
The first heart sound (S1) occurs with closure of the AV valves and thus signals the beginning of systole. The mitral component of the first sound (M1) slightly precedes the tricuspid component (T1), but you usually hear these two components fused as one sound. You can hear S1 over all the praecordium, but usually it is loudest at the apex.
The second heart sound (S2) occurs with closure of the semilunar valves and signals the end of systole. The aortic component of the second sound (A2) slightly precedes the pulmonic component (P2). Although it is heard over all the praecordium, S2 is loudest at the base.
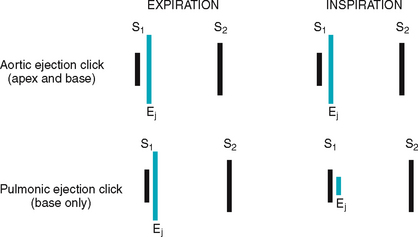
Effect of respiration.
The volume of right and left ventricular systole is just about equal, but this can be affected by respiration. To learn this, consider the phrase:
That means that during inspiration, intrathoracic pressure is decreased. This pushes more blood into the vena cava, increasing venous return to the right side of the heart, which increases right ventricular stroke volume. The increased volume prolongs right ventricular systole and delays pulmonic valve closure.
Meanwhile on the left side, a greater amount of blood is sequestered in the lungs during inspiration. This momentarily decreases the amount returned to the left side of the heart, decreasing left ventricular stroke volume. The decreased volume shortens left ventricular systole and allows the aortic valve to close a bit earlier. When the aortic valve closes significantly earlier than the pulmonic valve, you can hear the two components separately. This is a split S2.
Extra heart sounds
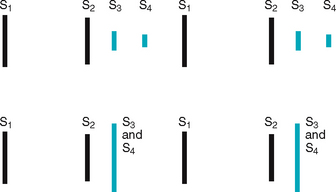
Third heart sound (S3).
Normally diastole is a silent event. However, in some conditions, ventricular filling creates vibrations that can be heard over the chest. These vibrations are S3. The S3 occurs when the ventricles are resistant to filling during the early rapid filling phase (protodiastole). This occurs immediately after S2, when the AV valves open and atrial blood first pours into the ventricles. (See a complete discussion of S3 in Table 12.7.)
TABLE 12.7 Diastolic extra sounds
Murmurs
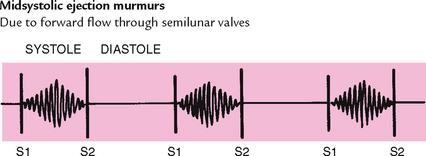
Blood circulating through normal cardiac chambers and valves usually makes no noise. However, some conditions create turbulent blood flow and collision currents. These result in a murmur, much like a pile of stones or a sharp turn in a stream creates a noisy water flow. A murmur is a gentle, blowing, swooshing sound that can be heard on the chest wall. Conditions resulting in a murmur are as follows:
Characteristics of sound
All heart sounds are described by:
1. Frequency (pitch)—heart sounds are described as high pitched or low pitched, although these terms are relative because all are low-frequency sounds, and you need a good stethoscope to hear them
2. Intensity (loudness)—loud or soft
3. Duration—very short for heart sounds; silent periods are longer
CONDUCTION
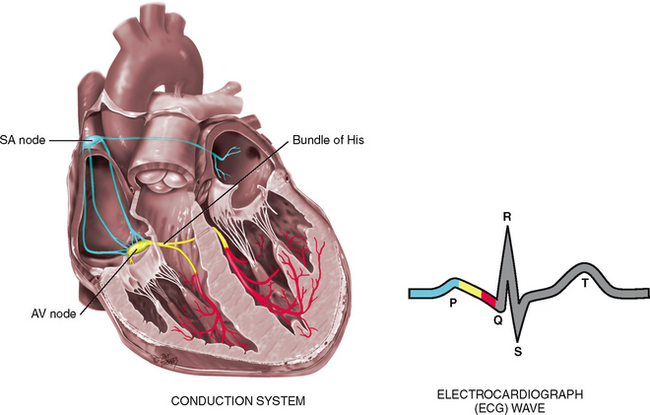
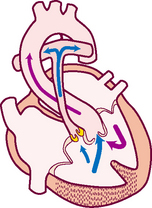
Of all organs, the heart has a unique ability—automaticity. The heart can contract by itself, independent of any signals or stimulation from the body. The heart contracts in response to an electrical current conveyed by a conduction system (Fig 12.8). Specialised cells in the sinoatrial (SA) node near the superior vena cava initiate an electrical impulse. (Because the SA node has an intrinsic rhythm, it is the ‘pacemaker’.) The current flows in an orderly sequence, first across the atria to the AV node low in the atrial septum. There, it is delayed slightly so that the atria have time to contract before the ventricles are stimulated. Then, the impulse travels to the bundle of His, the right and left bundle branches, then through the ventricles.
The electrical impulse stimulates the heart to do its work, which is to contract. A small amount of electricity spreads to the body surface, where it can be measured and recorded on the electrocardiograph (ECG). The ECG waves are arbitrarily labelled PQRST, which stand for the following elements:
P wave—depolarisation of the atria
PR interval—from the beginning of the P wave to the beginning of the QRS complex (the time necessary for atrial depolarisation plus time for the impulse to travel through the AV node to the ventricles)
Electrical events slightly precede the mechanical events in the heart. The ECG juxtaposed on the cardiac cycle is illustrated in Figure 12.6.
PUMPING ABILITY
In the resting adult, the heart normally pumps between 4 and 6 L of blood per minute throughout the body. This cardiac output equals the volume of blood in each systole (called the stroke volume) times the number of beats per minute (heart rate). This is described as:
CO = SV × HR
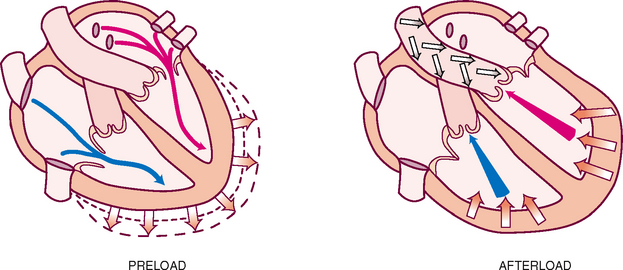
The heart can alter its cardiac output to adapt to the metabolic needs of the body. Preload and afterload affect the heart’s ability to increase cardiac output.
Preload is the venous return that builds during diastole. It is the length to which the ventricular muscle is stretched at the end of diastole just before contraction (Fig 12.9).
When the volume of blood returned to the ventricles is increased (as when exercise stimulates skeletal muscles to contract and force more blood back to the heart), the muscle bundles are stretched beyond their normal resting state to accommodate. The force of this switch is the preload. According to the Frank-Starling law, the greater the stretch, the stronger is the heart’s contraction. This increased contractility results in an increased volume of blood ejected (increased stroke volume).
Afterload is the opposing pressure the ventricle must generate to open the aortic valve against the higher aortic pressure. It is the resistance against which the ventricle must pump its blood. Once the ventricle is filled with blood, the ventricular end diastolic pressure is 5 to 10 mmHg, whereas that in the aorta is 70 to 80 mmHg. To overcome this difference, the ventricular muscle tenses (isovolumic contraction). After the aortic valve opens, rapid ejection occurs.
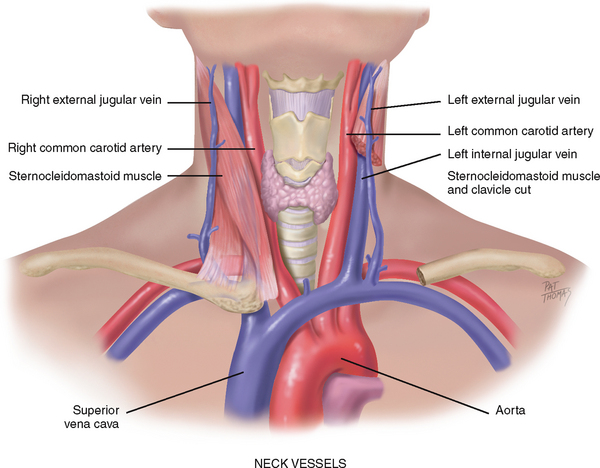
THE NECK VESSELS
Cardiovascular assessment includes the survey of vascular structures in the neck—the carotid artery and the jugular veins (Fig 12.10). These vessels reflect the efficiency of cardiac function.
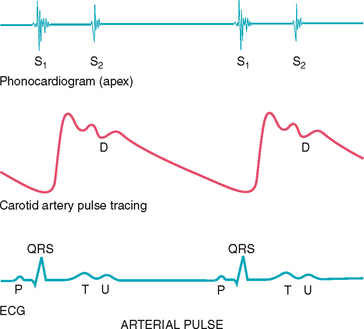
The carotid artery pulse
The pulse can be described as a pressure wave generated by each systole pumping blood into the aorta. The carotid artery is a central artery—that is, it is close to the heart. Its timing closely coincides with ventricular systole. (Assessment of the peripheral pulses is found in Chapter 11, and blood pressure assessment is found in Chapter 9.)
The carotid artery is located in the groove between the trachea and the sternocleidomastoid muscle, medial to and alongside that muscle. Note the characteristics of its waveform (Fig 12.11): a smooth rapid upstroke, a summit that is rounded and smooth and a downstroke that is more gradual and that has a dicrotic notch caused by closure of the aortic valve (marked D in the figure).
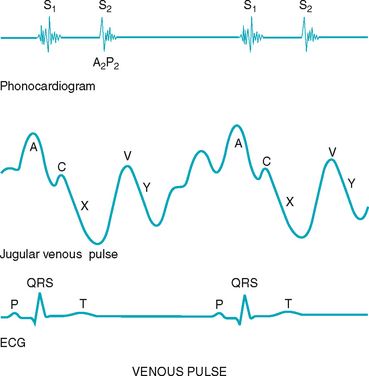
Jugular venous pulse and pressure
The jugular veins empty deoxygenated blood directly into the superior vena cava. Because no cardiac valve exists to separate the superior vena cava from the right atrium, the jugular veins give information about activity on the right side of the heart. Specifically, they reflect filling pressure and volume changes. Because volume and pressure increase when the right side of the heart fails to pump efficiently, the jugular veins expose this.
Two jugular veins are present in each side of the neck (see Fig 12.10). The larger internal jugular lies deep and medial to the sternocleidomastoid muscle. It is usually not visible, although its diffuse pulsations may be seen in the sternal notch when the person is supine. The external jugular vein is more superficial; it lies lateral to the sternocleidomastoid muscle, above the clavicle.
Although an arterial pulse is caused by a forward propulsion of blood, the jugular pulse is different. The jugular pulse results from a backwash, a waveform moving backwards caused by events upstream. The jugular pulse has five components, as shown in Figure 12.12.
The five components of the jugular venous pulse occur because of events in the right side of the heart. The A wave reflects atrial contraction because some blood flows backwards to the vena cava during right atrial contraction. The C wave, or ventricular contraction, is backflow from the bulging upwards of the tricuspid valve when it closes at the beginning of ventricular systole (not from the neighbouring carotid artery pulsation). Next, the X descent shows atrial relaxation when the right ventricle contracts during systole and pulls the bottom of the atria downwards. The V wave occurs with passive atrial filling because of the increasing volume in the right atria and increased pressure. Finally, the Y descent reflects passive ventricular filling when the tricuspid valve opens and blood flows from the RA to the RV.
DEVELOPMENTAL CONSIDERATIONS
Infants and children
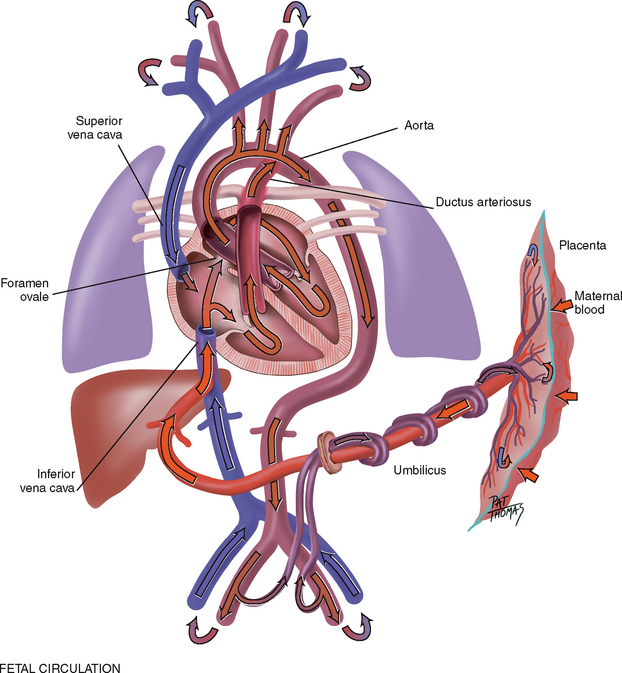
The fetal heart functions early; it begins to beat at the end of 3 weeks gestation. The lungs are nonfunctional, but the fetal circulation compensates for this (Fig 12.13). Oxygenation takes place at the placenta, and the arterial blood is returned to the right side of the heart. There is no point in pumping all this freshly oxygenated blood through the lungs, so it is rerouted in two ways. First, about two-thirds of it is shunted through an opening in the atrial septum, the foramen ovale, into the left side of the heart, where it is pumped out through the aorta. Second, the rest of the oxygenated blood is pumped by the right side of the heart out through the pulmonary artery, but it is detoured through the ductus arteriosus to the aorta. Because they are both pumping into the systemic circulation, the right and left ventricles are equal in weight and muscle wall thickness.
Inflation and aeration of the lungs at birth produces circulatory changes. Now the blood is oxygenated through the lungs rather than through the placenta. The foramen ovale closes within the first hour because of the new lower pressure in the right side of the heart than in the left side. The ductus arteriosus closes later, usually within 10 to 15 hours of birth. Now, the left ventricle has the greater workload of pumping into the systemic circulation, so that when the baby has reached 1 year of age, the left ventricle’s mass increases to reach the adult ratio of 2:1, left ventricle to right ventricle.
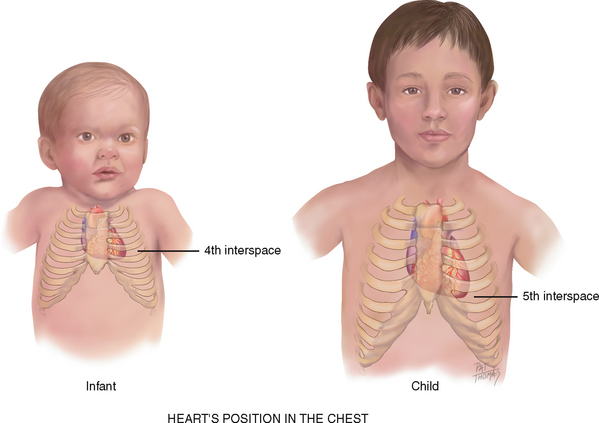
The heart’s position in the chest is more horizontal in the infant than in the adult; thus the apex is higher, located at the fourth left intercostal space (Fig 12.14). It reaches the adult position when the child reaches age 7 years.
The pregnant female
Blood volume increases by 30% to 40% during pregnancy, with the most rapid expansion occurring during the second trimester. This creates an increase in stroke volume and cardiac output and an increased pulse rate of 10 to 15 beats per minute. Despite the increased cardiac output, arterial blood pressure decreases in pregnancy as a result of peripheral vasodilatation. The blood pressure drops to its lowest point during the second trimester, and then rises after that. The blood pressure varies with the person’s position, as described in the Objective data section below.
Late adulthood (65+ years)
It is difficult to isolate the ‘ageing process’ of the cardiovascular system per se because it is so closely interrelated with lifestyle, habits and diseases. We now know that lifestyle is a modifying factor in the development of cardiovascular disease; smoking, diet, alcohol use, exercise patterns and stress have an influence on coronary artery disease. Lifestyle also affects the ageing process; cardiac changes once thought to be due to ageing are partially due to the sedentary lifestyle accompanying ageing (Fig 12.15). What is left to be attributed to the ageing process alone?
Haemodynamic changes with ageing
• From age 20 to 60 years, systolic blood pressure increases by about 20 mmHg, and by another 20 mmHg between ages 60 and 80 years (Wei, 1992). This is due to stiffening of the large arteries which, in turn, is due to calcification of vessel walls (arteriosclerosis). This stiffening creates an increase in pulse wave velocity because the less compliant arteries cannot store the volume ejected.
• The overall size of the heart does not increase with age, but left ventricular wall thickness increases. This is an adaptive mechanism to accommodate the vascular stiffening mentioned earlier that creates an increased workload on the heart.
• No significant change in diastolic pressure occurs with age. A rising systolic pressure with a relatively constant diastolic pressure increases the pulse pressure (the difference between the two).
• No change in resting heart rate occurs with ageing.
• Cardiac output at rest is not changed with ageing.
• There is a decreased ability of the heart to augment cardiac output with exercise. This is shown by a decreased maximum heart rate with exercise and diminished sympathetic response. Noncardiac factors also cause a decrease in maximum work performance with ageing: decrease in skeletal muscle performance, increase in muscle fatigue, increased sense of dyspnoea. Chronic exercise conditioning will modify many of the ageing changes in cardiovascular function (Zipes et al, 2005).
Arrhythmias.
The presence of supraventricular and ventricular arrhythmias increases with age. Ectopic beats are common in ageing people; although these are usually asymptomatic in healthy older people, they may compromise cardiac output and blood pressure when disease is present.
Tachyarrhythmias may not be tolerated as well in older people. The myocardium is thicker and less compliant, and early diastolic filling is impaired at rest (Wei, 1992). Thus, it may not tolerate a tachycardia as well because of shortened diastole. Also, tachyarrhythmias may further compromise a vital organ whose function has already been affected by ageing or disease. For example, a ventricular tachycardia produces a 40% to 70% decrease in cerebral blood flow. Although a younger person may tolerate this, an older person with cerebrovascular disease may experience syncope (Cheitlin and Zipes, 2001).
ECG.
Age-related changes in the ECG occur as a result of histological changes in the conduction system. These changes include:
• Prolonged PR interval (first-degree AV block) and prolonged Q-T interval, but the QRS interval is unchanged
• Left axis deviation from age-related mild LV hypertrophy and fibrosis in left bundle branch
Although the haemodynamic changes associated with ageing alone do not seem severe or portentous, the fact remains that the incidence of cardiovascular disease increases with age. The incidence of coronary artery disease increases sharply with advancing age, and accounts for about half of the deaths of people over 65. Hypertension (systolic >140 mmHg and diastolic >90 mmHg) and heart failure also increase with age. Certainly, lifestyle habits (smoking, chronic alcohol use, lack of exercise, diet) play a significant role in the acquisition of heart disease. Also, increasing the physical activity of older adults—even at a moderate level—is associated with a reduced risk of death from cardiovascular diseases and respiratory illnesses. Both points underscore the need for health teaching as an important treatment parameter.
Risk factors
The major modifiable risk factors for heart disease and stroke are high blood pressure, smoking, high cholesterol levels, obesity, physical inactivity and diabetes. In addition, for some women, the use of oral contraceptives and postmenopausal hormones is a risk factor.
Hypertension.
It is estimated that 11% of Australians have hypertension (AIHW, 2008). Untreated hypertension risks organ damage to heart, main blood vessels, brain and kidneys. More Aboriginal and Torres Strait Islander people experience high blood pressure from a younger age than other Australians (AIHW, 2008). The most accurate means of BP monitoring is 24-hour ambulatory monitoring (Clement et al, 2003).
Smoking.
In Australia, 20% of adults smoke daily (AIHW, 2004). There are mixed reports about the incidence by age, gender and ethnicity. Regardless of these variables all clients need to be reminded to quit smoking for the sake of their health on each presentation to a health service. ‘Quit’ services are government funded and printed information is available in most languages.
High cholesterol.
In a study characterising the lipid profile of Indigenous Australians and Torres Strait Islanders, a significantly lower high density lipoprotein (HDL) level was recorded when compared to that of non-Indigenous Australians, independent of age, sex and diabetic status (O’Neal et al, 2008). This observational data supports the need for ongoing attention to reduce the deleterious effects of dyslipidaemia on heart health, with intensive efforts required to assist those most susceptible.
Obesity.
A body mass index (BMI) of 25 kg/m2 or higher is considered to be overweight, and a BMI of 30 kg/m2 or higher is considered to be obese. The World Health Organization (WHO) has reported that obesity is reaching epidemic proportions. Obesity compounds any health problem. Almost 60% of the Australian population is considered to be obese (ASSO, 2009). Census data has identified that excess weight is directly attributed to some 7% of cardiac deaths (AIHW, 2002).
Diabetes.
The incidence of diabetes is rising internationally. This is attributed to increasing rates of obesity, physical inactivity and an ageing population (AIHW, 2009). It is known that there is a higher incidence of diabetes in Indigenous populations, those living in lower socioeconomic areas, those born overseas or living in regional or remote areas of Australia. However, national data sets are often not reported by each of these subsets (AIHW, 2009). Type 2 diabetes increases the risk of heart disease two- to four-fold and abolishes the protectiveness of female sex in the non-diabetic population (Bates and Jerums, 2003). The most recent Australian self-reported data set indicates that 4% of Australians had diabetes in 2007–08 (ABS, 2009). The complications of diabetes are well recognised and include cardiovascular and renal disease and blindness (Bate and Jerums, 2003).
SUBJECTIVE DATA
An accurate assessment requires the nurse to obtain a careful history from the patient of the symptoms they experience and to then undertake the clinical examination that articulates with what the patient has reported. These have been grouped under the following headings:
| Assessment guidelines | Clinical significance and clinical alerts |
|---|---|
Angina, an important cardiac symptom, occurs when the heart’s vascular supply cannot keep up with metabolic demand. Chest pain also may also be of pulmonary, musculoskeletal or gastrointestinal origin; it is important to differentiate. A squeezing ‘clenched fst’ sign is characteristic of angina, but the symptoms below may be anginal equivalents in the absence of chest pain (Reigle, 2005). |
|
| • Pain brought on by: activity—what type; rest; emotional upset; after eating; during sexual intercourse; with cold weather? | |
| • Any associated symptoms: sweating, ashen grey or pale skin, heart skips beat, shortness of breath, nausea or vomiting, racing of heart, extreme fatigue? | Note that the presence of chest pain and/or a squeezing sensation in the chest and the associated symptoms described here are typical of serious cardiac circulatory insufficiency. |
| • Pain made worse by moving the arms or neck, breathing, lying flat? | Try to differentiate pain of cardiac versus noncardiac origin. |
| • Pain relieved by rest or glyceryl trinitrate? How many tablets/sprays? | Clinical alert: if the person has taken more than 3 glyceryl trinitrate tablets/sprays in 30 minutes without relief they must be urgently referred for further assessment and treatment by a medical practitioner. |
Dyspnoea (shortness of breath (SOB)) or dyspnoea on exertion (shortness of breath on exertion (SOBOE))–quantify exactly (e.g. after walking two level blocks) Paroxysmal nocturnal dyspnoea (PND) occurs with heart failure. Lying down increases volume of intrathoracic blood, and the weakened heart cannot accommodate the increased load. Classically, the person awakens after 2 hours of sleep with the perception of needing fresh air. |
|
| • Does the shortness of breath interfere with activities of daily living? If so, what does your shortness of breath prevent you from doing? | |
| 3 Orthopnoea. How many pillows do you use when sleeping or lying down? | Orthopnoea is the need to assume a more upright position to breathe. Note the exact number of pillows used. |
| Sputum production, mucoid or purulent. Haemoptysis is often a pulmonary disorder but also occurs with mitral stenosis. | |
| Fatigue from decreased cardiac output is worse in the evening, whereas fatigue from anxiety or depression occurs all day or is worse in the morning. | |
| • How have these symptoms (chest pain, fatigue, breathlessness) affected your life? | |
| 6 Cyanosis or pallor. Ever noted that your facial skin turns blue or ashen? | Cyanosis or pallor occurs with myocardial infarction or low cardiac output states as a result of decreased tissue perfusion. |
| 8 Nocturia. Do you awaken at night with an urgent need to urinate? How long has this been occurring? Any recent change? | Nocturia–recumbency at night promotes fluid reabsorption and excretion; this occurs with heart failure in the person who is ambulatory during the day. |
9 Cardiac history. Any history of: hypertension, elevated cholesterol, heart murmur, congenital heart disease, rheumatic fever or unexplained joint pains as a child or youth, recurrent tonsillitis, anaemia? |
|
| 10 Family cardiac history. Any family history of: hypertension, obesity, diabetes, coronary artery disease (CAD), sudden death at younger age, i.e. <60 years? | |
11 Personal habits (cardiac risk factors). • Nutrition: Please describe your usual daily diet. (Note if this diet is representative of the basic food groups, the amount of calories, cholesterol and any additives such as salt.) What is your usual weight? Has there been any recent change?
• Smoking: Do you smoke cigarettes or other tobacco? At what age did you start? How many packs per day? For how many years have you smoked this amount? Have you ever tried to quit? If so, how did this go?
• Alcohol: How much alcohol do you usually drink each week, or each day? Beer/wine/spirits? When was your last drink? What was the number of drinks that episode? Have you ever been told you had a drinking problem?
|
|
| Additional history for infants | |
| To screen for heart disease in infants, note fatigue during feeding. An infant with heart failure takes small amounts at each feed; becomes dyspnoeic with sucking; may be diaphoretic, then falls into exhausted sleep; awakens after a short time hungry again. | |
| 3 Growth: Has the baby grown as expected by growth charts and about the same as siblings or peers? | Poor weight gain is an indicator of heart abnormalities. |
| 4 Activity: Were this baby’s developmental milestones achieved as expected? Is the baby able to play without tiring? How many sleeps does the baby take each day? How long does a sleep last? | |
| Additional history for children | |
| 1 Growth: Has the child grown as expected by growth charts? | |
| 2 Activity: Is this child able to keep up with siblings or age mates? Is the child willing or reluctant to go out to play? Is the child able to climb stairs, ride a bike? Does the child squat to rest during play or to watch television, or assume a knee–chest position while sleeping? Have you noted ‘blue spells’ during daily activities? | Fatigue. Record specifc limitations. Cyanosis is a serious indication of heart or lung dysfunction. |
| 3 Has the child had any unexplained joint pains or unexplained fever? | |
| 4 Does the child have frequent headaches, nosebleeds? | |
| 5 Does the child have frequent respiratory infections? How many per year? How are they treated? Have any of these proved to be streptococcal infections? | |
| 6 Family history: Does the child have a sibling with a heart defect? Is anyone in the child’s family known to have chromosomal abnormalities, such as Down syndrome? | |
| Additional history for the pregnant female | |
| Hypertension, proteinuria and oedema are signs of pre-eclampsia, a serious condition that can occur during pregnancy, placing the mother and fetus at risk. Those pregnant with these symptoms should be referred to their medical practitioner or midwife. | |
| 2 Have you had any faintness or dizziness with this pregnancy? | |
| Additional history for the adult over 65 years | |
| 2 Do you take any medications for your illness such as digitalis, beta-blockers? Aware of side effects? Have you recently stopped taking your medication? Why? | Poor adherence may be related to side effects or lack of understanding of the chronic nature of heart disease. |
| 3 Environment: Does your home have any stairs? How often do you need to climb them? Does this have any effect on activities of daily living? |
OBJECTIVE DATA
The focus of the clinical examination is to examine the patient for clinical signs that support or are in addition to the patient’s report of symptoms. In particular, assessment of the cardiovascular system is aimed at finding out risk factors for skin breakdown, pain, falling. In addition, the objective assessment is aimed at finding out the severity of symptoms and impact on daily function.
| Preparation | Equipment needed |
|---|---|
| When performing an assessment of the carotid arteries, the person can be sitting up. To assess the jugular veins and the praecordium, the person should be supine with the head and chest slightly elevated. | |
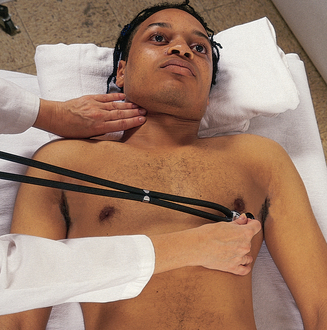
Stand on the person’s right side; this will facilitate your hand placement and auscultation of the praecordium.
The room must be warm—chilling makes the person uncomfortable, and shivering interferes with heart sounds. Take scrupulous care to ensure quiet; heart sounds are very soft, and any ambient room noise masks them.
Ensure the female’s privacy by keeping her breasts draped. The female’s left breast overrides part of the area you will need to examine. Gently displace the breast upwards, or ask the woman to hold it out of the way.
1 Pulse and blood pressure (Ch 9) 2 Extremities (Ch 11) |
| Procedures and normal findings | Abnormal findings and clinical alerts |
|---|---|
| THE NECK VESSELS | |
Palpate each carotid artery medial to the sternocleidomastoid muscle in the neck (Fig 12.16). Avoid excessive pressure on the carotid sinus area higher in the neck; excessive vagal stimulation here could slow down the heart rate, especially in adults over 65. Take care to palpate gently. Palpate only one carotid artery at a time to avoid compromising arterial blood to the brain. |
Carotid sinus hypersensitivity is the condition in which pressure over the carotid sinus leads to a decreased heart rate, decreased BP and cerebral ischaemia with syncope. This may occur in older adults with hypertension or occlusion of the carotid artery. |
| Feel the contour and amplitude of the pulse. Normally the contour is smooth with a rapid upstroke and slower downstroke, and the normal strength is 2+ or moderate (Ch 11). Your findings should be the same bilaterally. | Diminished pulse feels small and weak (decreased stroke volume). Increased pulse feels full and strong (hyperkinetic states) (see Table 11.1). |
| Inspect the jugular venous pulse | |
From the jugular veins you can assess the central venous pressure (CVP) and thus judge the heart’s efficiency as a pump. Although the external jugular vein is easier to see, the internal (especially the right) jugular vein is attached more directly to the superior vena cava and is thus more reliable for assessment. You cannot see the internal jugular vein itself, but you can see its pulsation. The person should be placed in the supine position anywhere from a 30 to a 45 degree angle, wherever you can best see the pulsations. In general, the higher the venous pressure is, the higher the position you need. Remove the pillow to avoid fexing the neck; the head should be in the same plane as the trunk. Turn the person’s head slightly away from the examined side, and direct a strong light tangentially onto the neck to highlight pulsations and shadows. |
|
| Note the external jugular veins overlying the sternocleidomastoid muscle. In some persons, the veins are not visible at all, whereas in others they are full in the supine position. As the person is raised to a sitting position, these external jugulars fatten and disappear, usually at 45 degrees. | |
| Now look for pulsations of the internal jugular veins in the area of the suprasternal notch or around the origin of the sternocleidomastoid muscle around the clavicle. You must be able to distinguish internal jugular vein pulsation from that of the carotid artery. It is easy to confuse them because they lie close together. Use the guidelines shown in Table 12.1. | |
| THE PRAECORDIUM | |
| Inspect the anterior chest | |
Arrange tangential lighting to accentuate any flicker of movement. Pulsations. You may or may not see the apical impulse, the pulsation created as the left ventricle rotates against the chest wall during systole. When visible, it occupies the fourth or fifth intercostal space, at or inside the midclavicular line. It is easier to see in children and in those with thinner chest walls. |
A heave or lift is a sustained forceful thrusting of the ventricle during systole. It occurs with ventricular hypertrophy as a result of increased workload. A right ventricular heave is seen at the sternal border; a left ventricular heave is seen at the apex (see Table 12.8). |
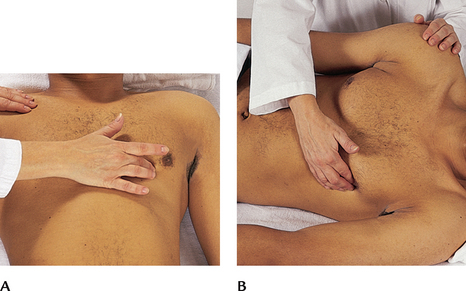
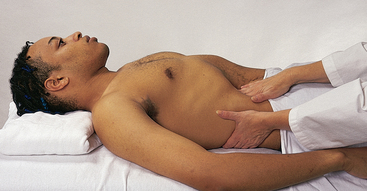
| Palpate the apical impulse | |
| Localise the apical impulse precisely by using one finger pad (Fig 12.17, A). Asking the person to ‘exhale and then hold it’ aids the examiner in locating the pulsation. You may need to roll the person midway to the left to find it; note that this also displaces the apical impulse further to the left (Fig 12.17, B). | The point at which you can palpate the apical impulse is the point at which you place your stethoscope to auscultate the apical heart sounds. |
| Note: | Cardiac enlargement:
• Left ventricular dilatation (volume overload) displaces impulse down and to left, and increases size more than one space. • Increased force and duration but no change in location occurs with left ventricular hypertrophy and no dilatation (pressure overload) (see Table 12.8). |
| The apical impulse is easily palpated in most adults. It is not palpable in obese persons or in persons with thick chest walls. With high cardiac output states (anxiety, fever, hyperthyroidism, anaemia), the apical impulse increases in amplitude and duration. | Not palpable with chronic obstructive pulmonary disease due to hyperinflated lungs. |
| Auscultation of the apical (mitral) area | |
In most situations the generalist registered nurse will only need to perform the skill of auscultation of the two heart sounds at the apex of the heart. To do this you will use both endpieces of your stethoscope when auscultating heart sounds. Although all heart sounds are low frequency, the diaphragm is for relatively higher pitched sounds, and the bell is for relatively lower pitched ones. Locate the fifth intercostal space at the left midclavicular line—mitral valve area. Before you begin, alert the person: ‘I always listen to the heart in a number of places on the chest. Just because I am listening a long time does not necessarily mean that something is wrong’. After you place the stethoscope, try closing your eyes briefly to tune out any distractions. Concentrate, and listen selectively to one sound at a time. Consider that at least two, and perhaps three or four, sounds may be happening in less than 1 second. You cannot process everything at once. Begin with the diaphragm endpiece and use the following routine: (1) note the rate and rhythm, (2) identify S1 and S2 and (3) assess S1 and S2 separately. |
|
| Note the rate and rhythm. The rate ranges normally from 60 to 100 beats per minute. (Review the full discussion of the pulse in Ch 9 and the normal rates across age groups.) The rhythm should be regular, although sinus arrhythmia occurs normally in young adults and children. With sinus arrhythmia, the rhythm varies with the person’s breathing, increasing at the peak of inspiration and slowing with expiration. Note any other irregular rhythm. If one occurs, check if it has any pattern, or if it is totally irregular. | |
| When you notice any irregularity, check for a pulse deficit by auscultating the apical beat while simultaneously palpating the radial pulse. Count a serial measurement (one after the other) of apical beat and radial pulse. Normally, every beat you hear at the apex should perfuse to the periphery and be palpable. The two counts should be identical. When different, subtract the radial rate from the apical and record the remainder as the pulse deficit. | A pulse deficit signals a weak contraction of the ventricles; it occurs with atrial fbrillation, premature beats and heart failure. |
| • S1 is louder than S2 at the apex; S2 is louder than S1 at the base. | |
| • S1 coincides with the carotid artery pulse. Feel the carotid gently as you auscultate at the apex; the sound you hear as you feel each pulse is S1 (Fig 12.18). | |
| • S1 coincides with the R wave (the upstroke of the QRS complex) if the person is on an ECG monitor. | |
| Listen to S1 and S2 separately. Note whether each heart sound is normal, accentuated, diminished or split. Inch your diaphragm across the chest as you do this. | |
First heart sound (S1). Caused by closure of the AV valves, S1 signals the beginning of systole. You can hear it over the entire praecordium, although it is loudest at the apex (Fig 12.19). (Sometimes the two sounds are equally loud at the apex, because S1 is lower pitched than S2.) |
Causes of accentuated or diminished S1 (see Table 12.3). Both heart sounds are diminished with conditions that place an increased amount of tissue between the heart and your stethoscope: chronic obstructive pulmonary disease (hyperinflated lungs), obesity, pericardial fuid. |
| You can hear S1 with the diaphragm with the person in any position and equally well in inspiration and expiration. | |
| Second heart sound (S2). The S2 is associated with closure of the semilunar valves. You can hear it with the diaphragm, over the entire praecordium, although S2 is loudest at the base (Fig 12.20). | Accentuated or diminished S2 (see Table 12.5). |
| FURTHER OBJECTIVE ASSESSMENT FOR ADVANCED PRACTICE | |
| The assessments that are described in the following section require advanced skill and scope of practice. Nurses working in specialist cardiac care units, intensive care units as well as community health centres may need to develop these skills. Advanced assessment for infants and children is performed by specialist neonatal and paediatric nurses, some midwives and maternal and child health nurses. | |
| Estimate the jugular venous pressure | |
| Follow on from inspecting the jugular venous pulse, think of the jugular veins as a CVP manometer attached directly to the right atrium. You can ‘read’ the CVP at the highest level of pulsations (Fig 12.21). Use the angle of Louis (sternal angle) as an arbitrary reference point, and compare it with the highest level of venous pulsation. Hold a vertical ruler on the sternal angle. Align a straight edge on the ruler like a T-square, and adjust the level of the horizontal straight edge to the level of pulsation. Read the level of intersection on the vertical ruler; normal jugular venous pulsation is 2 cm or less above the sternal angle. Also state the person’s position, for example, ‘internal jugular vein pulsations 3 cm above sternal angle when elevated 30 degrees’. | Elevated pressure is a level of pulsation that is more than 3 cm above the sternal angle while at 45 degrees. This occurs with heart failure. |
| If you cannot find the internal jugular veins, use the external jugular veins and note the point where they look collapsed. Be aware that the technique of estimating venous pressure is difficult and is not always a reliable predictor of CVP. Consistency in grading among examiners is difficult to achieve. | |
| If venous pressure is elevated, or if you suspect heart failure, perform hepa-tojugular reflux (Fig 12.22). Position the person comfortably supine and instruct them to breathe quietly through an open mouth. Hold your right hand on the right upper quadrant of the person’s abdomen just below the rib cage. Watch the level of jugular pulsation as you push in with your hand. Exert firm sustained pressure for 30 seconds. This empties venous blood out of the liver sinusoids and adds its volume to the venous system. If the heart is able to pump this additional volume (i.e. if no elevated CVP is present), the jugular veins will rise for a few seconds, then recede to the previous level. | If heart failure is present, the jugular veins will elevate and stay elevated as long as you push. |
| Percussion is used to outline the heart’s borders, but often it has been displaced by the chest x-ray or echocardiogram. These are much more accurate in detecting heart enlargement. When the right ventricle enlarges, it does so in the anteroposterior diameter, which is better seen on x-ray flm. Also, percussion is of limited usefulness with the female breast tissue or in an obese person or a person with a muscular chest wall. | |
| However, there are times when your percussing hands are the only tools you have with you, such as in an outpatient setting, extended care facility, or the person’s home. When you need to search for cardiac enlargement, place your stationary finger in the person’s fifth intercostal space over on the left side of the chest near the anterior axillary line. Slide your stationary hand towards yourself, percussing as you go, and note the change of sound from resonance over the lung to dull (over the heart). Normally, the left border of cardiac dullness is at the midclavicular line in the fifth interspace and slopes in towards the sternum as you progress upwards, so that by the second interspace the border of dullness coincides with the left sternal border. The right border of dullness normally matches the sternal border. | Cardiac enlargement is due to increased ventricular volume or wall thickness; it occurs with hypertension, CAD, heart failure and cardiomyopathy. |
| Auscultation | |
| The neck vessels | |
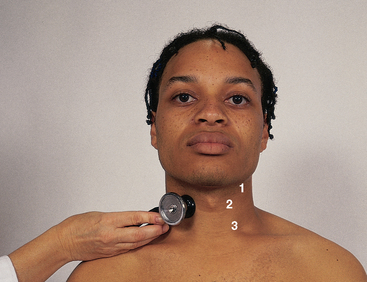
For persons middle-aged or older, or who show symptoms or signs of cardiovascular disease, follow on from inspection of the neck vessels and auscultate each carotid artery for the presence of a bruit (pronounced bru’-ee) (Fig 12.23). This is a blowing, swishing sound indicating blood flow turbulence; normally none is present. |
A bruit indicates turbulence due to a local vascular cause, such as atherosclerotic narrowing. |
| Keep the neck in a neutral position. Lightly apply the bell of the stethoscope over the carotid artery at three levels: (1) the angle of the jaw, (2) the midcervical area and (3) the base of the neck (see Fig 12.23). Avoid compressing the artery because this could create an artifcial bruit, and it could compromise circulation if the carotid artery is already narrowed by atherosclerosis. Ask the person to take a breath, exhale and hold it briefly while you listen so that tracheal breath sounds do not mask or mimic a carotid artery bruit. (Holding the breath on inhalation will also tense the levator scapulae muscles, which makes it hard to hear the carotids.) Sometimes you can hear normal heart sounds transmitted to the neck; do not confuse these with a bruit. | A carotid bruit is audible when the lumen is occluded by ½ to ⅔. Bruit loudness increases as the atherosclerosis worsens until the lumen is occluded by ⅔. After that, bruit loudness decreases. When the lumen is completely occluded, the bruit disappears. Thus absence of a bruit does not ensure absence of a carotid lesion. A murmur sounds much the same but is caused by a cardiac disorder. Some aortic valve murmurs (aortic stenosis) radiate to the neck and must be distinguished from a local bruit. |
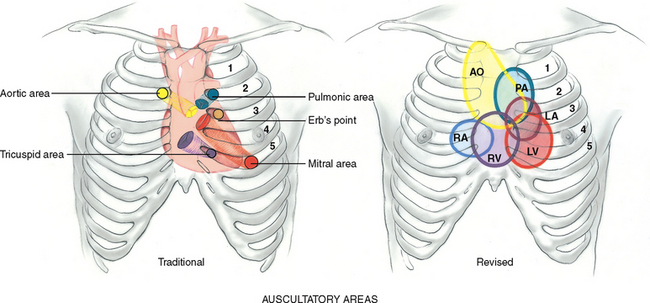
| In addition to auscultation at the apical area, some advanced practice nurses need to develop skills in auscultation across the praecordium. First you need to identify the auscultatory areas where you will listen. These include the four traditional valve ‘areas’ (Fig 12.24). The valve areas are not over the actual anatomical locations of the valves but are the sites on the chest wall where sounds produced by the valves are best heard. The sound radiates with the direction of blood flow. The valve areas are: | |
| Do not limit your auscultation to only four locations. Sounds produced by the valves may be heard all over the praecordium. (For this reason, many experts even discourage the naming of the valve areas.) Thus, learn to inch your stethoscope in a rough Z pattern, from the base of the heart across and down, then over to the apex. Or start at the apex and work your way up. Include the sites shown in Figure 12.24. | |
| After you place the stethoscope, try closing your eyes briefly to tune out any distractions. Concentrate, and listen selectively to one sound at a time. Consider that at least two, and perhaps three or four, sounds may be happening in less than 1 second. You cannot process everything at once. Begin with the diaphragm endpiece and use the following routine: (1) note the rate and rhythm, (2) identify S1 and S2, (3) assess S1 and S2 separately, (4) listen for extra heart sounds and (5) listen for murmurs. | |
| A split S1 is normal, but it occurs rarely. A split S1 means you are hearing the mitral and tricuspid components separately. It is audible in the tricuspid valve area, the left lower sternal border. The split is very rapid, with the two components only 0.03 second apart. | |
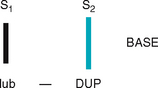
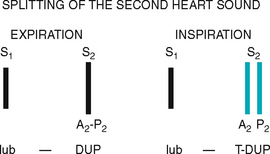
| Splitting of S2. A split S2 is a normal phenomenon that occurs towards the end of inspiration in some people. Recall that closure of the aortic and pulmonic valves is nearly synchronous. Because of the effects of respiration on the heart described earlier, inspiration separates the timing of the two valves’ closure, and the aortic valve closes 0.06 second before the pulmonic valve. Instead of one DUP, you hear a split sound—T-DUP (Fig 12.25). During expiration, synchrony returns, and the aortic and pulmonic components fuse together. A split S2 is heard only in the pulmonic valve area, the second left interspace. | |
| When you first hear the split S2, do not be tempted to ask the person to hold their breath so that you can concentrate on the sounds. Breath holding will only equalise ejection times in the right and left sides of the heart and cause the split to go away. Instead, concentrate on the split as you watch the person’s chest rise up and down with breathing. The split S2 occurs about every fourth heartbeat, fading in with inhalation and fading out with exhalation. | A fixed split is unaffected by respiration; the split is always there. A paradoxical split is the opposite of what you would expect; the sounds fuse on inspiration and split on expiration (see Table 12.5). |
Focus on systole, then on diastole, and listen for any extra heart sounds. Listen with the diaphragm, then switch to the bell, covering all auscultatory areas (Fig 12.26). Usually, these are silent periods. When you do detect an extra heart sound, listen carefully to note its timing and characteristics. During systole, the midsystolic click (which is associated with mitral valve prolapse) is the most common extra sound (see Table 12.6). The third and fourth heart sounds occur in diastole; either may be normal or abnormal (see Table 12.7). |
A pathological S3 (ventricular gallop) occurs with heart failure and volume overload; a pathological S4 (atrial gallop) occurs with CAD (see Table 12.7 for a full description). |
| Listen for murmurs. A murmur is a blowing, swooshing sound that occurs with turbulent blood flow in the heart or great vessels. Except for the innocent murmurs described, murmurs are abnormal. If you hear a murmur, describe it by indicating these following characteristics: | Murmurs may be due to congenital defects and acquired valvular defects. Study Tables 12.9 and 12.10 for a complete description. |
| Timing. It is crucial to define the murmur by its occurrence in systole or diastole. You must be able to identify S1 and S2 accurately to do this. Try to further describe the murmur as being in early, mid- or late systole or diastole; throughout the cardiac event (termed pansystolic or holosystolic/pandiastolic or holodiastolic); and whether it obscures or muffes the heart sounds. | A systolic murmur may occur with a normal heart or with heart disease; a diastolic murmur always indicates heart disease. |
Loudness. Describe the intensity in terms of six ‘grades’. For example, record a grade ii murmur as ‘ii/vi’. Grade i—Barely audible, heard only in a quiet room and then with difficulty Grade ii—Clearly audible, but faint Grade iii—Moderately loud, easy to hear Grade iv—Loud, associated with a thrill palpable on the chest wall Grade v—Very loud, heard with one corner of the stethoscope lifted off the chest wall Grade vi—Loudest, still heard with entire stethoscope lifted just off the chest wall |
|
| Pitch. Describe the pitch as high, medium or low. The pitch depends on the pressure and the rate of blood flow producing the murmur. | |
Pattern. The intensity may follow a pattern during the cardiac phase, growing louder (crescendo), tapering off (decrescendo) or increasing to a peak and then decreasing (crescendo–decrescendo, or diamond shaped). Because the whole murmur is just milliseconds long, it takes practice to diagnose any pattern. Quality. Describe the quality as musical, blowing, harsh or rumbling. |
The murmur of mitral stenosis is rumbling, whereas that of aortic stenosis is harsh (see Table 12.10). |
| Location. Describe the area of maximum intensity of the murmur (where it is best heard) by noting the valve area or intercostal spaces. | |
| Radiation. The murmur may be transmitted downstream in the direction of blood flow and may be heard in another place on the praecordium, the neck, the back or the axilla. | |
Posture. Some murmurs disappear or are enhanced by a change in position. Some murmurs are common in healthy children or adolescents and are termed innocent or functional. Innocent indicates having no valvular or other pathological cause; functional is due to increased blood flow in the heart (e.g. in anaemia, fever, pregnancy, hyperthyroidism). The contractile force of the heart is greater in children. This increases blood flow velocity. The increased velocity plus a smaller chest measurement makes an audible murmur. The innocent murmur is generally soft (grade ii), midsystolic, short, crescendo– decrescendo and with a vibratory or musical quality (‘vooot’ sound like fddle strings). Also, the innocent murmur is heard at the second or third left intercostal space and disappears with sitting, and the young person has no associated signs of cardiac dysfunction. Although it is important to distinguish innocent murmurs from pathological ones, it is best to suspect all murmurs as pathological until they are proved otherwise. Diagnostic tests such as ECG, phonocardiogram and echocardiogram are needed to establish an accurate diagnosis. |
|
| Change position. After auscultating in the supine position, roll the person towards their left side. Listen with the bell at the apex for the presence of any diastolic filling sounds (i.e. the S3 or S4) (Fig 12.27). | S3 and S4 and the murmur of mitral stenosis sometimes may be heard only when on the left side. |
| Ask the person to sit up, lean forward slightly and exhale. Listen with the diaphragm firmly pressed at the base, right and left sides. Check for the soft, high-pitched, early diastolic murmur of aortic or pulmonic regurgitation (Fig 12.28). | Murmur of aortic regurgitation sometimes may be heard only when the person is leaning forwards in the sitting position. |
| DevelOPmenTAl COnSiDerATiOnS | |
| Infants | |
| The transition from fetal to pulmonic circulation occurs in the immediate newborn period. Fetal shunts normally close within 10 to 15 hours but may take up to 48 hours. Thus, you should assess the cardiovascular system during the first 24 hours and again in 2 to 3 days. | Failure of shunts to close (e.g. patent ductus arteriosus (PDA), atrial septal defect (ASD)): see Table 12.9. |
| Note any extra cardiac signs that may refect cardiac status particularly in the skin, liver size and respiratory status. The skin colour should be pink to pinkish brown, depending on the infant’s genetic heritage. If cyanosis occurs, determine its first appearance—at or shortly after birth versus after the neonatal period. Normally, the liver is not enlarged, and the respirations are not laboured. Also, note the expected parameters of weight gain throughout infancy. | Persistent cyanosis at or just after birth signals oxygen desaturation of congenital heart disease (Table 12.9). The most important signs of heart failure in an infant are persistent tachycardia, tachypnoea and liver enlargement. Engorged veins, gallop rhythm and pulsus alternans are also signs. Respiratory crackles are an important sign in adults but not in infants. |
| Palpate the apical impulse to determine the size and position of the heart. Because the infant’s heart has a more horizontal placement, expect to palpate the apical impulse at the fourth intercostal space just lateral to the midclavicular line. It may or may not be visible. | The apex is displaced with: |
| The heart rate is best auscultated because radial pulses are hard to count accurately. Use the small (paediatric size) diaphragm and bell (Fig 12.29). The heart rate may range from 100 to 180 per minute immediately after birth, then stabilise to an average of 120 to 140 per minute. Infants normally have wide fuctuations with activity, from 170 per minute or more with crying or being active to 70 to 90 per minute with sleeping. Variations are greatest at birth and are even more so with premature babies (see Table 9.2). | Persistent tachycardia is >200 beats per minute in newborns, or >150 beats per minute in infants. Bradycardia is <90 beats per minute in newborns or <60 beats per minute in older infants or children. This causes a serious drop in cardiac output because the small muscle mass of their hearts cannot increase stroke volume significantly. |
| Expect the heart rhythm to have sinus arrhythmia, the phasic speeding up or slowing down with the respiratory cycle. | Investigate any irregularity except sinus arrhythmia. |
| Rapid rates make it more challenging to evaluate heart sounds. Expect heart sounds to be louder in infants than in adults because of the infant’s thinner chest wall. Also, S2 has a higher pitch and is sharper than S1. Splitting of S2 just after the height of inspiration is common, not at birth, but beginning a few hours after birth. | Fixed split S2 indicates atrial septal defect (see Table 12.9). |
| Murmurs in the immediate newborn period do not necessarily indicate congenital heart disease. Murmurs are relatively common in the first 2 to 3 days because of fetal shunt closure. These murmurs are usually grade i or ii, are systolic, accompany no other signs of cardiac disease and disappear in 2 to 3 days. The murmur of PDA is a continuous machinery murmur, which disappears by 2 to 3 days. On the other hand, absence of a murmur in the immediate newborn period does not ensure a perfect heart; congenital defects can be present that are not signalled by an early murmur. It is best to listen frequently and to note and describe any murmur according to the characteristics listed above. | Persistent murmur after 2 to 3 days, holosystolic murmurs or those that last into diastole, and those that are loud–all warrant further evaluation. |
| Children | |
| Note any extracardiac or cardiac signs that may indicate heart disease: poor weight gain, developmental delay, persistent tachycardia, tachypnoea, dyspnoea on exertion, cyanosis and clubbing. Note that clubbing of fingers and toes usually does not appear until late in the first year, even with severe cyanotic defects. | |
| The apical impulse is sometimes visible in children with thin chest walls. Note any obvious bulge or any heave—these are not normal. | A praecordial bulge to the left of the sternum with a hyperdynamic praecordium signals cardiac enlargement. The bulge occurs because the cartilaginous rib cage is more compliant. A substernal heave occurs with right ventricular enlargement, and an apical heave occurs with left ventricular hypertrophy. |
Palpate the apical impulse: in the fourth intercostal space to the left of the midclavicular line until age 4 years; at the fourth interspace at the midclavicular line from age 4 to 6 years; and in the fifth interspace to the right of the midclavicular line at age 7 years (Fig 12.30). |
|
| The average heart rate slows as the child grows older, although it is still variable with rest or activity (see Table 9.2). | |
| The heart rhythm remains characterised by sinus arrhythmia. Physiologic S3 is common in children (see Table 12.7). It occurs in early diastole, just after S2, and is a dull soft sound that is best heard at the apex. | |
| A venous hum—due to turbulence of blood flow in the jugular venous system—is common in healthy children and has no pathological significance. It is a continuous, low-pitched, soft hum that is heard throughout the cycle, although it is loudest in diastole. Listen with the bell over the supraclavicular fossa at the medial third of the clavicle, especially on the right, or over the upper anterior chest. | |
| The venous hum is not usually affected by respiration, may sound louder when the child stands and is easily obliterated by occluding the jugular veins in the neck with your fingers. | This latter manoeuvre helps differentiate the venous hum from other cardiac murmurs (e.g. PDA). |
| Heart murmurs that are innocent (or functional) in origin are very common through childhood. Some authors say they have a 30% occurrence, and some authors say nearly all children may demonstrate a murmur at some time. Most innocent murmurs have these characteristics: soft, relatively short systolic ejection murmur; medium pitch; vibratory; best heard at the left lower sternal or midsternal border, with no radiation to the apex, base or back. | Distinguish innocent murmurs from pathological ones. This may involve referral to another examiner or the performance of diagnostic tests such as the ECG or ultrasonography. |
| For the child whose murmur has been shown to be innocent, it is very important that the parents understand this completely. They need to believe that this murmur is just a ‘noise’ and has no pathological significance. Otherwise, the parents may become overprotective and limit activity for the child, which may result in the child developing a negative self-concept. | |
| The pregnant female | |
| The vital signs usually yield an increase in resting pulse rate of 10 to 15 beats per minute and a drop in blood pressure from the normal pre-pregnancy level. The BP decreases to its lowest point during the second trimester and then slowly rises during the third trimester. The BP varies with position. It is usually lowest in left lateral recumbent position, a bit higher when supine and highest when sitting (Cunningham et al, 2005). | Suspect pregnancy-induced hypertension with a sustained rise of 30 mmHg systolic or 15 mmHg diastolic under basal conditions. |
| Inspection of the skin often shows a mild hyperaemia in light-skinned women because the increased cutaneous blood flow tries to eliminate the excess heat generated by the increased metabolism. Palpation of the apical impulse is higher and lateral compared with the normal position, as the enlarging uterus elevates the diaphragm and displaces the heart up and to the left and rotates it on its long axis. | |
| Auscultation of the heart sounds shows changes caused by the increased blood volume and workload: | |
| The last-mentioned murmur is termed a mammary souffe (pronounced soof’ f’l), which occurs near term or when the mother is lactating; it is due to increased blood flow through the internal mammary artery. The murmur is heard in the 2nd, 3rd or 4th intercostal space; it is continuous, although it is accented in systole. You can obliterate it by pressure with the stethoscope or one finger lateral to the murmur. | Murmurs of aortic valve disease cannot be obliterated. |
| The ECG has no changes except for a slight left axis deviation due to the change in the heart’s position. | |
| Use caution in palpating and auscultating the carotid artery. Avoid pressure in the carotid sinus area, which could cause a refex slowing of the heart rate. Also, pressure on the carotid artery could compromise circulation if the artery is already narrowed by atherosclerosis. | |
| When measuring jugular venous pressure, view the right internal jugular vein. The aorta stiffens, dilates and elongates with ageing, which may compress the left neck veins and obscure pulsations on the left side. | |
| The chest often increases in anteroposterior diameter with ageing. This makes it more difficult to palpate the apical impulse and to hear the splitting of S2. The S4 often occurs in people over 65 with no known cardiac disease. Systolic murmurs are common, occurring in over 50% of such people. | The S3 is associated with heart failure and is always abnormal over age 35 years (see Table 12.7). |
| Occasional premature ectopic beats are common and do not necessarily indicate underlying heart disease. When in doubt, obtain an ECG. However, consider that the ECG only records for one isolated minute in time and may need to be supplemented by a test of 24-hour ambulatory heart monitoring. | |
| The intensity of S1 depends on three factors: (1) position of AV valve at the start of systole, (2) structure of the valve leafets and (3) how quickly pressure rises in the ventricle. |
| Factor | Examples | |
|---|---|---|
| Loud (Accentuated) S1 | ||
 |
1 Position of AV valve at start of systole–wide open and no time to drift together | Hyperkinetic states where blood velocity is increased: exercise, fever, anaemia, hyperthyroidism |
| 2 Change in valve structure–calcification of valve, needs increasing ventricular pressure to close the valve against increased atrial pressure | Mitral stenosis with leafets still mobile | |
| Faint (Diminished) S1 | ||
 |
1 Position of AV valve–delayed conduction from atria to ventricles. Mitral valve drifts shut before ventricular contraction closes it | First-degree heart block (prolonged PR interval) |
| 2 Change in valve structure–extreme calcification, which limits mobility | Mitral insufficiency | |
| Severe hypertension–systemic or pulmonary | ||
| Varying intensity of S1 | ||
 |
1 Position of AV valve varies before closing from beat to beat | Atrial fibrillation—irregularly-irregular rhythm |
| 2 Atria and ventricles beat independently | Complete heart block with changing PR interval | |
| Split S1 | ||
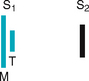 |
Mitral and tricuspid components are heard separately | Normal but uncommon |
TABLE 12.6 Systolic extra sounds
TABLE 12.8 Abnormal pulsations on the praecordium
 |
 |
| Base | Left sternal border |
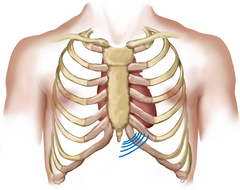
| A lift (heave) occurs with right ventricular hypertrophy, as found in pulmonic valve disease, pulmonic hypertension and chronic lung disease. You feel a diffuse lifting impulse during systole at the left lower sternal border. It may be associated with retraction at the apex because the left ventricle is rotated posteriorly by the enlarged right ventricle. | |
 |
 |
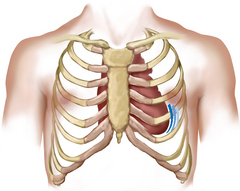
| Apex | Apex |
| Cardiac enlargement displaces the apical impulse laterally and over a wider area when left ventricular hypertrophy and dilatation are present. This is volume overload, as in mitral regurgitation, aortic regurgitation and left-to-right shunts. | The apical impulse is increased in force and duration but is not necessarily displaced to the left when left ventricular hypertrophy occurs alone without dilatation. This is pressure overload, as found in aortic stenosis or systemic hypertension. |
Images © Pat Thomas, 2006.
TABLE 12.1 Characteristics of jugular versus carotid pulsations
| Internal jugular pulse | Carotid pulse | |
|---|---|---|
| 1 location | Lower, more lateral, under or behind the sternocleidomastoid muscle | Higher and medial to this muscle |
| 2 Quality | Undulant and diffuse, two visible waves per cycle | Brisk and localised, one wave per cycle |
| 3 Respiration | Varies with respiration; its level descends during inspiration when intrathoracic pressure is decreased | Does not vary |
| 4 Palpable | No | Yes |
| 5 Pressure | Light pressure at the base of the neck easily obliterates | No change |
| 6 Position of person | Level of pulse drops and disappears as the person is brought to a sitting position | Unaffected |
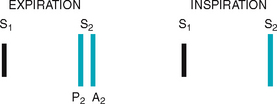
TABLE 12.5 variations in split S2
| Normal splitting | ||
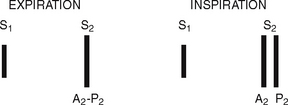 |
| Condition | Example | |
|---|---|---|
| Fixed split | ||
 |
A fixed split is unaffected by respiration; the split is always there. | Atrial septal defect Right ventricular failure |
| Paradoxical split | ||
 |
Conditions that delay aortic valve closure cause the opposite of a normal split. In inspiration, P2 is normally delayed so with a paradoxical split, the sounds fuse. In expiration you hear the split, in the order of P2A2. | |
| Wide split | ||
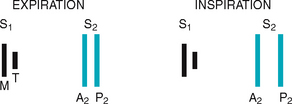 |
When the right ventricle has delayed electrical activation, the split is very wide on inspiration and is still there on expiration. | Right bundle branch block (which delays P2) |
TABLE 12.9 Congenital heart defects
Images © Pat Thomas, 2006.
TABLE 12.10 Murmurs due to valvular defects
 |
S, Subjective data; O, objective data.
Regurgitation Images © Pat Thomas, 2006.
Promoting a healthy lifestyle Women and heart attack
The Heart Truth: women and heart attacks
When someone complains of chest pain or pain radiating down the left arm, almost everyone thinks heart attack. After all, these are the symptoms that typically occur. Aren’t they? Well, yes and no. They are the most ‘typical’ symptoms men get, but not women. For women, the symptoms can be quite different or atypical. These ‘atypical’ symptoms may be one of the reasons that more women are dying from heart disease than men these days.
Women are more likely to feel angina as a hot or cold burning sensation, or even as a tenderness to touch, in their back, shoulders, arms or jaw. They often have no chest discomfort or pain at all. Women’s symptoms of heart attacks frequently include nausea, vomiting, indigestion, shortness of breath or extreme fatigue but, again, no chest pain. All of these symptoms are easy to attribute to something else other than the heart, and are often ignored. Scientific evidence now shows that women tend to minimise their symptoms of cardiac disease. However, part of this may be due to a lack of awareness.
The Heart Truth is a national awareness and prevention campaign about heart disease in women in the United States of America sponsored by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI). It is The Heart Truth awareness and prevention campaign that first introduced the Red Dress as the national symbol for women and heart disease awareness. This campaign is also run in Australia and New Zealand by their national Heart Foundations and is known as Go Red for Women. Since its introduction, many women have taken ownership of the symbol.
Women’s not so ‘atypical’ symptoms of cardiovascular disease or a heart attack include:
• Pain, discomfort, deep ache, pressure or throbbing ‘above the waist’—including the back, shoulders, arms, stomach, jaw and, sometimes, the chest
• Breathlessness or inability to catch the breath—with or without exertion or when waking up
• Clammy sweating and dizziness—what some refer to as a ‘cold sweat’
• Anxiety—feelings of impending doom or unusual nervousness
Cardiovascular disease is the number one cause of death in women. Women should be made aware of and pay particular attention to the symptoms and seek immediate care if they occur.
Australian National Heart Foundation: www.heartfoundation.org.au; www.goredforwomen.com.au
National Institutes of Health National Heart, Lung, and Blood Institute: www.nhlbi.nih.gov
New Zealand National Heart Foundation: www.nhf.org.nz; www.goredforwomen.org.nz
The Heart Truth: Awareness and Prevention: www.4women.gov/hearttruth
DOCUMENTATION AND CRITICAL THINKING
FOCUSED ASSESSMENT: CLINICAL CASE STUDY
Mr NV is a 53-year-old male manual labourer admitted to the CCU with chest pain.
Subjective
1 year ago NV admitted to hospital with crushing substernal chest pain, radiating to L shoulder, accompanied by nausea, vomiting, diaphoresis.
Diagnosed as an AMI (anterior myocardial infarction), hospitalised 7 days, discharged with glyceryl trinitrate (Anginine) prn for anginal pain.
Did not return to work. Activity included walking 1 km/day and gardening. Had occasional episodes of chest pain with exercise, relieved by rest.
1 day ago—had increasing frequency of chest pain, about every 2 hours, lasting few minutes, saw pain as warning to visit local doctor.
Day of admission—severe substernal chest pain (‘like someone sitting on my chest’) unrelieved by rest. Saw local doctor, while in office had episode of chest pain same as last year’s, accompanied by diaphoresis, no nausea and vomiting or shortness or breath, relieved by 2 glyceryl trinitrate sprays. Transferred to hospital by ambulance. No further pain since admission 2 hours ago.
Family hx—mother died of AMI at age 57.
Personal habits—smokes 1½ pack cigarettes daily × 34 years (25.5 pack years), no alcohol, diet—trying to limit fat and fried food, still high in added salt.
Objective
Extremities: Skin pink, no cyanosis. Upper extrem.—capillary refill sluggish, no clubbing. Lower extrem.—no oedema, no hair growth 10 cm below knee bilaterally.
Neck: External jugulars flat. Internal jugular pulsations present when supine and absent when elevated to 45 degrees.
Praecordium: Inspection. Apical impulse visible 5th ics, 7 cm left of midsternal line, no heave.
Palpation: Apical impulse palpable in 5th and 6th ics.
Auscultation: Apical rate 92 regular, S1-S2 are normal, not diminished or accentuated.
ABNORMAL FINDINGS
TABLE 12.2 Clinical portrait of heart failure
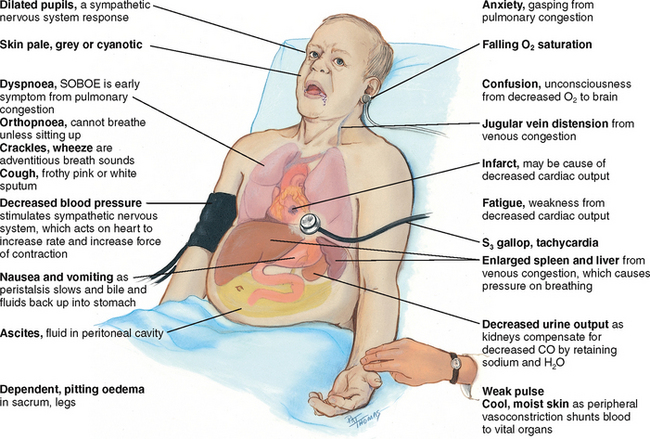 |
| Decreased cardiac output occurs when the heart fails as a pump, and the circulation becomes backed up and congested. |
| Signs and symptoms of heart failure come from two basic mechanisms: (1) the heart’s inability to pump enough blood to meet the metabolic demands of the body, and (2) the kidney’s compensatory mechanisms of abnormal retention of sodium and water to compensate for the decreased cardiac output. This increases blood volume and venous return, which causes further congestion. |
| Onset of heart failure may be: (1) acute, as following a myocardial infarction when direct damage to the heart’s contracting ability has occurred; or (2) chronic, as with hypertension, when the ventricles must pump against chronically increased pressure. |
ABNORMAL FINDINGS FOR ADVANCED PRACTICE
| Condition | Example | |
|---|---|---|
| Accentuated S2 | ||
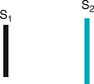 |
1 Higher closing pressure | Systemic hypertension, ringing or booming S2 |
| 2 Exercise and excitement increase pressure in aorta | ||
| 3 Pulmonary hypertension | Mitral stenosis, heart failure | |
| 4 Semilunar valves calcified but still mobile | Aortic or pulmonic stenosis | |
| Diminished S2 | ||
 |
1 A fall in systemic blood pressure causes a decrease in valve strength | Shock |
| 2 Semilunar valves thickened and calcifed, with decreased mobility | Aortic or pulmonic stenosis |
Australasian Society for the Study of Obesity (ASSO). 2009: Fast facts—statistics. Available from: http://www.asso.org.au/home/obesityinfo/stats/fastfacts.
Australian Bureau of Statistics (ABS). National health survey: users’ guide—electronic publication, 2007–08, Cat. no. 4363.0.55.001. Available at http://www.abs.gov.au/ausstats/abs@.nsf/mf/4363.0.55.001, 2009.
Australian Institute of Health and Welfare (AIHW): Diabetes prevalence in Australia: an assessment of national data sources. Diabetes series no. 12. Cat. no. CVD 46, Canberra, 2009, AIHW.
Australian Institute of Health and Welfare (AIHW). Australia’s Health. Canberra: AIHW, 2008. Cat. no. AUS 99
Australian Institute of Health and Welfare (AIHW). Chronic disease & associated risk factors in Australia 2001. Canberra: AIHW, 2002.
Australian Institute of Health and Welfare (AIHW). Heart, stroke & vascular diseases–Australian facts 2004. Canberra: AIHW, 2004. Cat. no. 27 CVD
Bate KL, Jerums G. Preventing complications of diabetes. Med J Aust. 2003;179(9):498–503.
Boie E. Initial evaluation of chest pain. Emerg Med Clin North Am. 2005;23:937–957.
Bonow R, Mann D, Zipes D, et al. Braunwald’s heart disease: a textbook of cardiovascular medicine, 9th edn. Philadelphia: WB Saunders, 2011.
Carrol M, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294:1773–1781.
Carter T, Brooks C. Pericarditis: inflammation or infarction? J Cardiovasc Nurs. 2005;20:239–244.
Clement DL, De Buyzere ML, De Bacquer DA, et alfor the Office versus Ambulatory Pressure Study Investigators. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. New Engl J Med. 2003;348:2407–2415.
Cunningham FG, et al. Williams’ obstetrics, 23rd edn. New York: McGraw-Hill, 2010.
DeVon HA, Ryan CJ. Chest pain and associated symptoms of acute coronary syndromes. J Cardiovasc Nurs. 2005;20:232–238.
Fink A. Endocarditis after valve replacement surgery. Am J Nurs. 2006;106:40–52.
Hayek E, Gring C, Griffin B. Mitral valve prolapse. Lancet. 2005;365:507–518.
Holcomb S. Recognizing and managing anemia. Nurse Pract. 2005;30:16–33.
Holman J. Preoperative evaluation: Priorities and pointers, part 1: Cardiac assessment. Consultant. 2005;45:1296–1301.
Holman J. Preoperative evaluation: Priorities and pointers, part 2. Consultant. 2005;45:1307–1314.
Kliegman RM, et al. Nelson textbook of pediatrics, 19th edn. Philadelphia: Saunders, 2010.
Klieman L, Hyde S, Berra K. Cardiovascular disease risk reduction in older adults. J Cardiovasc Nurs. 2006;21:527–539.
O’Neal DN, Piers LS, Iser DM, et al. Australian Aboriginal people and Torres Strait Islanders have an atherogenic lipid profile that is characterised by low HDL-cholesterol level and small LDL particles. Journal of Atherosclerosis. 2008;201(2):368–377.
Patel D, Wasserman A. Chest pain: Is it life-threatening or benign? Consultant. 2005;45:21–28.
Perloff JK. Physical examination of the heart and circulation, 4th edn. Shelton, CT: People’s Publishing House, 2009.
Rasmusson K. Heart failure epidemic boiling on the surface. Nurse Pract. 2006;31:12–23.
Reigle J. Evaluating the patient with chest pain: the value of a comprehensive history. J Cardiovasc Nurs. 2005;20:226–231.
Scheetz L. Aortic dissection. Am J Nurs. 2006;106:55–59.
Tilkian AG, Conover MB. Understanding heart sounds and murmurs with an introduction to lung sounds, 4th edn. Philadelphia: WB Saunders, 2001.
Yusuf S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:937–952.