Chapter Fourteen Thorax and lungs
INTRODUCTION
The respiratory system is an important system responsible for the supply of oxygen, removal of carbon dioxide and maintenance of acid–base balance of arterial blood through hypoventilation and hyperventilation. In order to appreciate the impact of disease and trauma to this complex and dynamic system you are advised to first review the structure and function of the thoracic cage, the lungs and tracheobronchial tree and the respiratory centre in the brainstem.
POSITION AND SURFACE LANDMARKS
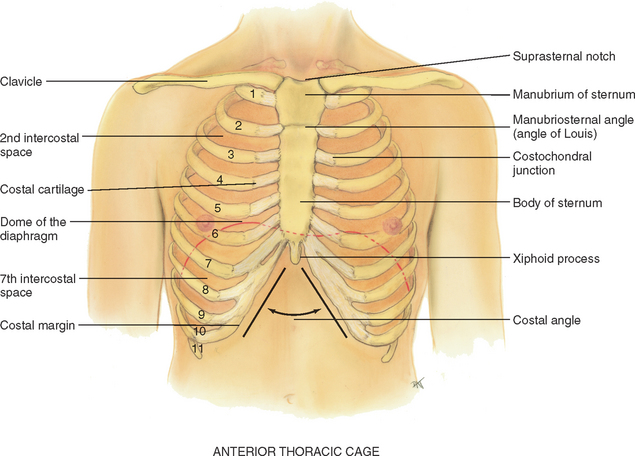
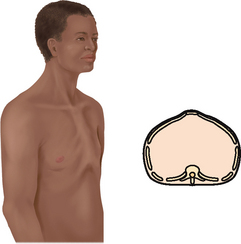
The thoracic cage is a bony structure with a conical shape, which is narrower at the top (Fig 14.1). It is defined by the sternum, 12 pairs of ribs and 12 thoracic vertebrae. Its ‘floor’ is the diaphragm, a musculotendinous septum that separates the thoracic cavity from the abdomen. The first seven ribs attach directly to the sternum via their costal cartilages: ribs 8, 9 and 10 attach to the costal cartilage above, and ribs 11 and 12 are ‘floating’, with free palpable tips. The costochondral junctions are the points at which the ribs join their cartilages. They are not palpable.
Anterior thoracic landmarks
Surface landmarks on the thorax are signposts for underlying respiratory structures. Knowledge of landmarks will help you localise a finding and will facilitate communication of your findings to others.
Suprasternal notch: feel this hollow U-shaped depression just above the sternum, in between the clavicles.
Sternum: the ‘breastbone’ has three parts: the manubrium, the body and the xiphoid process. Walk your fingers down the manubrium a few centimetres until you feel a distinct bony ridge, the manubriosternal angle.
Manubriosternal angle: often called the sternal angle or the ‘angle of Louis’, this is the articulation of the manubrium and body of the sternum, and it is continuous with the second rib. The angle of Louis is a useful place to start counting ribs, which helps localise a respiratory finding horizontally. Identify the angle of Louis, palpate lightly to the second rib and slide down to the second intercostal space. Each intercostal space is numbered by the rib above it. Continue counting down the ribs in the middle of the hemithorax, not close to the sternum where the costal cartilages lie too close together to count. You can palpate easily down to the tenth rib.
The angle of Louis also marks the site of tracheal bifurcation into the right and left main bronchi; it corresponds with the upper border of the atria of the heart, and it lies above the fourth thoracic vertebra on the back.
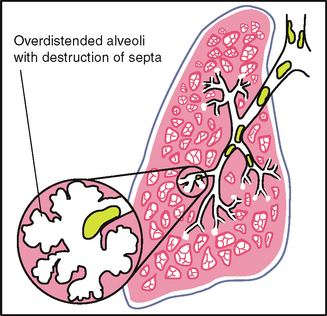
Costal angle: the right and left costal margins form an angle where they meet at the xiphoid process. Usually 90 degrees or less, this angle increases when the rib cage is chronically overinflated, as seen in patients with emphysema.
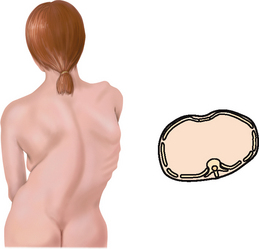
Posterior thoracic landmarks
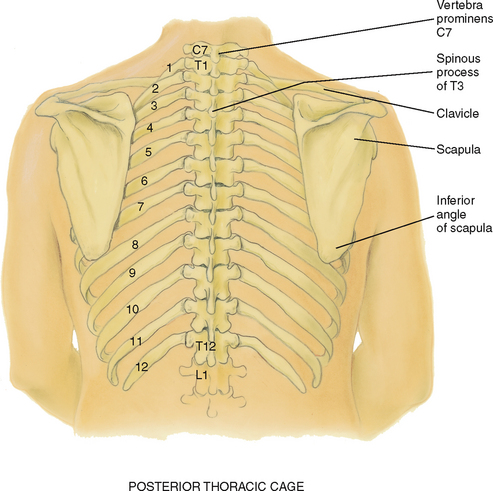
Counting ribs and intercostal spaces on the back is a bit harder due to the muscles and soft tissue surrounding the ribs and spinal column (Fig 14.2).
Vertebra prominens: start here. Flex your head and feel for the most prominent bony spur protruding at the base of the neck. This is the spinous process of C7. If two bumps seem equally prominent, the upper one is C7 and the lower one is T1.
Spinous processes: count down these knobs on the vertebrae, which stack together to form the spinal column. Note that the spinous processes align with their same numbered ribs only down to T4. After T4, the spinous processes angle downwards from their vertebral body and overlie the vertebral body and rib below.
Inferior border of the scapula: the scapulae are located symmetrically in each hemithorax. The lower tip is usually at the seventh or eighth rib.
Twelfth rib: palpate midway between the spine and the person’s side to identify its free tip.
Reference lines
Use the reference lines to pinpoint a finding vertically on the chest. On the anterior chest, note the midsternal line and the midclavicular line. The midclavicular line bisects the centre of each clavicle at a point halfway between the palpated sternoclavicular and acromioclavicular joints (Fig 14.3).
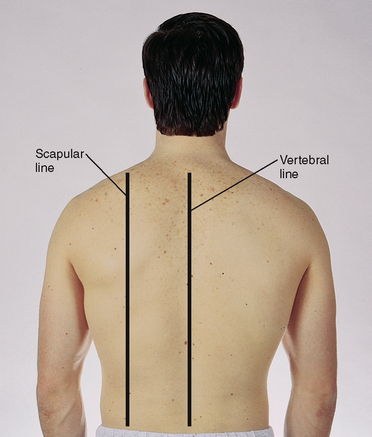
The posterior chest wall has the vertebral (or midspinal) line and the scapular line, which extends through the inferior angle of the scapula when the arms are at the sides of the body (Fig 14.4).
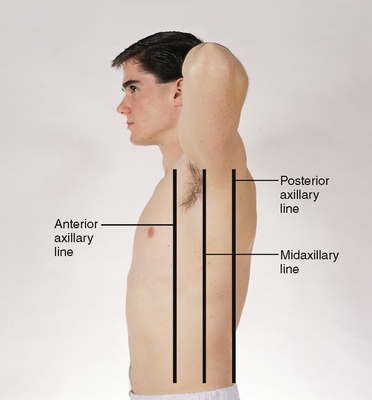
Lift up the person’s arm 90 degrees, and divide the lateral chest by three lines: the anterior axillary line extends down from the anterior axillary fold where the pectoralis major muscle inserts; the posterior axillary line continues down from the posterior axillary fold where the latissimus dorsi muscle inserts; and the midaxillary line runs down from the apex of the axilla and lies between and parallel to the other two (Fig 14.5).
THE THORACIC CAVITY
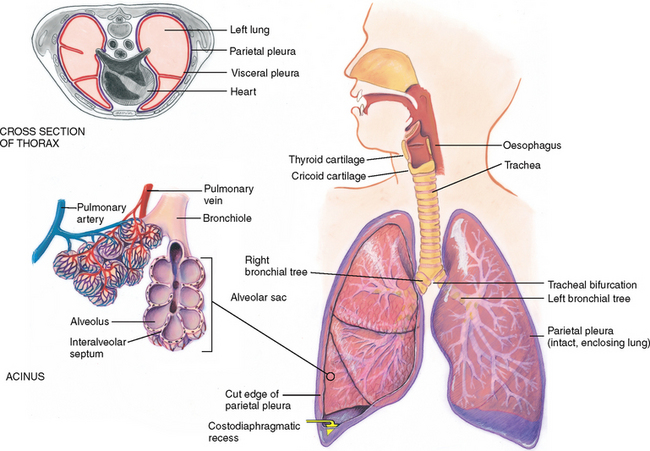
The mediastinum is the middle section of the thoracic cavity containing the oesophagus, trachea, heart and great vessels. The right and left pleural cavities, on either side of the mediastinum, contain the lungs.
Lung borders: in the anterior chest, the apex, or highest point, of lung tissue is 3 or 4 cm above the inner third of the clavicles. The base, or lower border, rests on the diaphragm at about the sixth rib in the midclavicular line. Laterally, lung tissue extends from the apex of the axilla down to the seventh or eighth rib. Posteriorly, the location of C7 marks the apex of lung tissue, and T10 usually corresponds to the base. Deep inspiration expands the lungs, and their lower border drops to the level of T12.
Lobes of the lungs
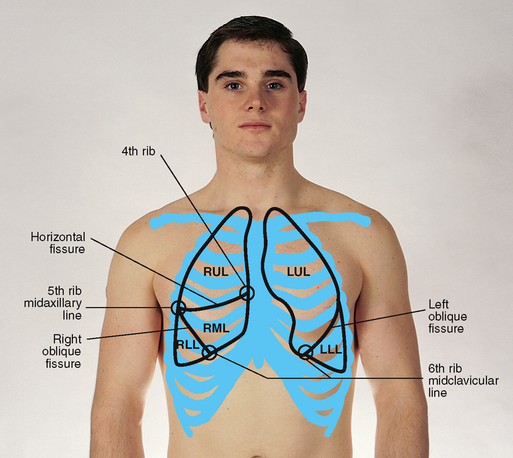
The lungs are paired but not precisely symmetrical structures (Fig 14.6). The right lung is shorter than the left lung because of the underlying liver. The left lung is narrower than the right lung because the heart bulges to the left. The right lung has three lobes, and the left lung has two lobes. These lobes are not arranged in horizontal bands like dessert layers in a parfait glass. Rather, they stack in diagonal sloping segments and are separated by fissures that run obliquely through the chest.
Anterior: on the anterior chest, the oblique (the major or diagonal) fissure crosses the fifth rib in the midaxillary line and terminates at the sixth rib in the midclavicular line. The right lung also contains the horizontal (minor) fissure, which divides the right upper and middle lobes. This fissure extends from the fifth rib in the right midaxillary line to the third intercostal space or fourth rib at the right sternal border.
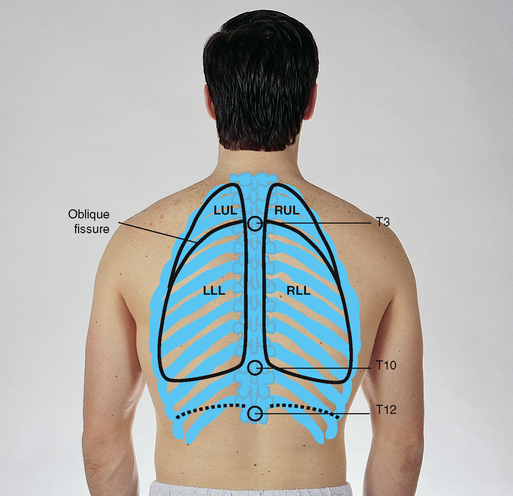
Posterior: the most remarkable point about the posterior chest is that it is almost all lower lobe (Fig 14.7). The upper lobes occupy a smaller band of tissue from their apices at T1 down to T3 or T4. At this level, the lower lobes begin, and their inferior border reaches down to the level of T10 on expiration and to T12 on inspiration. Note that the right middle lobe does not project onto the posterior chest at all. If the person abducts the arms and places the hands on the back of the head, the division between upper and lower lobes corresponds to the medial border of the scapulae.
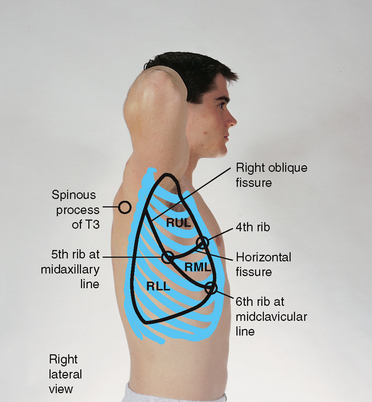
Lateral: laterally, lung tissue extends from the apex of the axilla down to the seventh or eighth rib. The right upper lobe extends from the apex of the axilla down to the horizontal fissure at the fifth rib (Fig 14.8). The right middle lobe extends from the horizontal fissure down and forwards to the sixth rib at the midclavicular line. The right lower lobe continues from the fifth rib to the eighth rib in the midaxillary line.
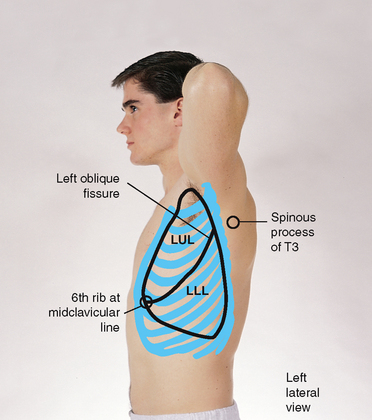
The left lung contains only two lobes, upper and lower (Fig 14.9). These are seen laterally as two triangular areas separated by the oblique fissure. The left upper lobe extends from the apex of the axilla down to the fifth rib at the midaxillary line. The left lower lobe continues down to the eighth rib in the midaxillary line.
Using these landmarks, take a marker and try tracing the outline of each lobe on a willing partner. Take special note of the three points that commonly confuse beginning examiners:
Pleurae
The thin, slippery pleurae form an envelope between the lungs and the chest wall (Fig 14.10). The visceral pleura lines the outside of the lungs, dipping down into the fissures. It is continuous with the parietal pleura lining the inside of the chest wall and diaphragm.
The inside of the envelope, the pleural cavity, is a potential space filled with only a few millilitres of lubricating fluid. It normally has a vacuum, or negative pressure, which holds the lungs tightly against the chest wall. The lungs slide smoothly and noiselessly up and down during respiration, lubricated by a few millilitres of fluid. Think of this as similar to two glass slides with a drop of water between them; although it is difficult to separate the slides, they slide smoothly back and forth. The pleurae extend about 3 cm below the level of the lungs, forming the costodiaphragmatic recess. This is a potential space: when it abnormally fills with air or fluid, it compromises lung expansion.
Trachea and bronchial tree
The trachea lies anterior to the oesophagus and is 10 to 11 cm long in the adult. It begins at the level of the cricoid cartilage in the neck and bifurcates just below the sternal angle into the right and left main bronchi. Posteriorly, tracheal bifurcation is at the level of T4 or T5. The right main bronchus is shorter, wider and more vertical than the left main bronchus.
The trachea and bronchi transport gases between the environment and the lung parenchyma. They constitute the dead space, or space that is filled with air but is not available for gaseous exchange. This is about 150 mL in the adult. The bronchial tree also protects alveoli from small particulate matter in the inhaled air. The bronchi are lined with goblet cells which secrete mucus that entraps the particles. The bronchi are lined with cilia, which sweep particles upwards where they can be swallowed or expelled.
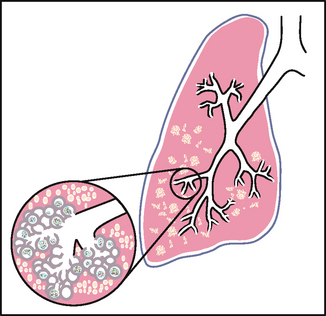
An acinus is a functional respiratory unit that consists of the bronchioles, alveolar ducts, alveolar sacs and the alveoli. Gaseous exchange occurs across the respiratory membrane in the alveolar duct and in the millions of alveoli. Note how the alveoli are clustered like grapes around each alveolar duct. This creates millions of interalveolar septa (walls) that increase tremendously the working space available for gas exchange. This bunched arrangement creates a surface area for gas exchange that is as large as a tennis court.
MECHANICS OF RESPIRATION
There are four major functions of the respiratory system: (1) supplying oxygen to the body for energy production, (2) removing carbon dioxide as a waste product of energy reactions, (3) maintaining homeostasis (acid–base balance) of arterial blood and (4) maintaining heat exchange (less important in humans).
By supplying oxygen to the blood and eliminating excess carbon dioxide, respiration maintains the pH or the acid–base balance of the blood. The body tissues are bathed by blood that normally has a narrow acceptable range of pH. Although a number of compensatory mechanisms regulate the pH, the lungs help maintain the balance by adjusting the level of carbon dioxide through respiration. That is, hypoventilation (slow, shallow breathing) causes carbon dioxide to build up in the blood, and hyperventilation (rapid, deep breathing) causes carbon dioxide to be blown off.
Control of respirations
Normally our breathing pattern changes without our awareness in response to cellular demands. This involuntary control of respirations is mediated by the respiratory centre in the brainstem (pons and medulla). The major feedback loop is humoral regulation, or the change in carbon dioxide and oxygen levels in the blood and, less importantly, the hydrogen ion level. The normal stimulus to breathe for most of us is an increase of carbon dioxide in the blood, or hypercapnia. A decrease of oxygen in the blood (hypoxaemia) also increases respirations but is less effective than hypercapnia.
Changing chest size
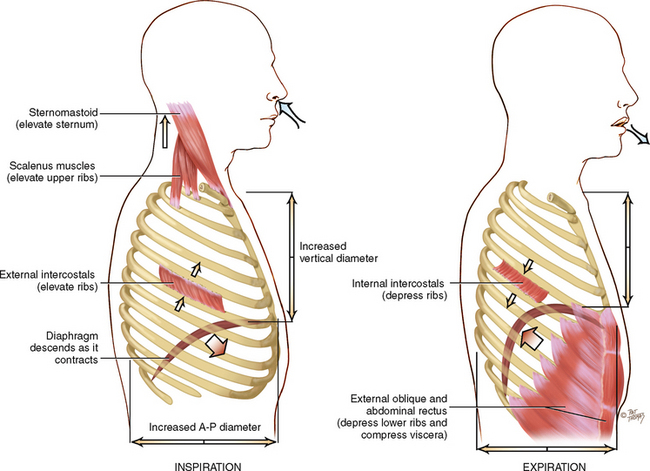
Respiration is the physical act of breathing: air rushes into the lungs as the chest size increases (inspiration) and is expelled from the lungs as the chest recoils (expiration). The mechanical expansion and contraction of the chest cavity alters the size of the thoracic container in two dimensions: (1) the vertical diameter lengthens or shortens, which is accomplished by downwards or upwards movement of the diaphragm and (2) the anteroposterior diameter increases or decreases, which is accomplished by elevation or depression of the ribs (Fig 14.11).
In inspiration, increasing the size of the thoracic container creates a slightly negative pressure in relation to the atmosphere, so air rushes in to fill the partial vacuum. The major muscle responsible for this increase is the diaphragm. During inspiration, contraction of the bell-shaped diaphragm causes it to descend and flatten. This lengthens the vertical diameter. Intercostal muscles lift the sternum and elevate the ribs, making them more horizontal. This increases the anteroposterior diameter.
Expiration is primarily passive. As the diaphragm relaxes, elastic forces within the lung, chest cage and abdomen cause it to dome up. All this squeezing creates a relatively positive pressure within the alveoli, and the air flows out.
Forced inspiration, such as that after heavy exercise or occurring pathologically with respiratory distress, commands the use of the accessory neck muscles to heave up the sternum and rib cage. These neck muscles are the sternomastoids, the scaleni and the trapezii. In forced expiration, the abdominal muscles contract powerfully to push the abdominal viscera forcefully in and up against the diaphragm, making it dome upwards and making it squeeze against the lungs.
DEVELOPMENTAL CONSIDERATIONS
Infants and children
During the first 5 weeks of fetal life, the primitive lung bud emerges; by 16 weeks, the conducting airways reach the same number as in the adult; at 32 weeks, surfactant, the complex lipid substance needed for sustained inflation of the air sacs, is present in adequate amounts; and by birth the lungs have 70 million primitive alveoli ready to start the job of respiration.
Breath is life. When the newborn inhales the first breath, the lusty cry that follows reassures straining parents that their baby is all right (Fig 14.12). The baby’s body systems all develop in utero, but the respiratory system alone does not function until birth. Birth demands its instant performance.
When the cord is cut, blood is cut off from the placenta, and it gushes into the pulmonary circulation. Relatively less resistance exists in the pulmonary arteries than in the aorta, so the foramen ovale in the heart closes just after birth. (See the discussion of fetal circulation in Ch 12.) The ductus arteriosus (linking the pulmonary artery and the aorta) contracts and closes some hours later, and pulmonary and systemic circulation are functional.
Respiratory development continues throughout childhood, with increases in diameter and length of airways, and increases in size and number of alveoli, reaching the adult range of 300 million by adolescence.
The relatively smaller size and immaturity of children’s pulmonary systems, and the presence of older caregivers who smoke, result in enormous vulnerability and increased risks to child health. Prenatal exposure results in chronic hypoxia and low birth weight. Postnatal exposure to environmental tobacco smoke (ETS) is linked to increased rates of otitis media, respiratory tract infections and childhood asthma (DiFranza et al, 2004). Other conditions associated with ETS exposure include sudden infant death syndrome (SIDS), negative behavioural and cognitive functioning and increased rates of adolescent smoking.
The pregnant female
The enlarging uterus elevates the diaphragm 4 cm during pregnancy. This decreases the vertical diameter of the thoracic cage, but this decrease is compensated for by an increase in the horizontal diameter. The increase in oestrogen level relaxes the chest cage ligaments. This allows an increase in the transverse diameter of the chest cage by 2 cm, and the costal angle widens. The total circumference of the chest cage increases by 6 cm. Although the diaphragm is elevated, it is not fixed. It moves with breathing even more during pregnancy, which results in an increase in tidal volume (Cunningham et al, 2005).
The growing fetus increases the oxygen demand on the mother’s body. This is met easily by the increasing tidal volume (deeper breathing). Little change occurs in the respiratory rate. An increased awareness of the need to breathe develops, even early in pregnancy, and some pregnant women may interpret this as dyspnoea although structurally nothing is wrong.
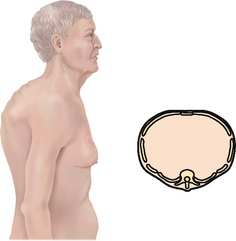
Late adulthood (65+ years)
The costal cartilages become calcified, which produces a less mobile thorax. Respiratory muscle strength declines after age 50 years and continues to decrease into the 70s. A more significant change is the decrease in elastic properties within the lungs, making them less distensible and lessening their tendency to collapse and recoil. In all, the ageing lung is a more rigid structure that is harder to inflate.
These changes result in an increase in small airway closure, and that yields a decreased vital capacity (the maximum amount of air that a person can expel from the lungs after first filling the lungs to maximum) and an increased residual volume (the amount of air remaining in the lungs even after the most forceful expiration).
With ageing, histological changes (i.e. a gradual loss of intraalveolar septa and a decreased number of alveoli) also occur, so less surface area is available for gas exchange. In addition, the lung bases become less ventilated as a result of the closing off of a number of airways. This increases the older person’s risk of dyspnoea with exertion beyond their usual workload.
The histological changes also increase the older person’s risk of postoperative pulmonary complications. That is, the older person has a greater risk of postoperative atelectasis and infection from a decreased ability to cough, a loss of protective airway reflexes and increased secretions.
CULTURAL AND SOCIAL CONSIDERATIONS
A disproportionately large number of tuberculosis (TB) cases reported in Australia up to 2005 were individuals born in countries other than Australia. During the period 1994–2005, the TB rate in foreign-born persons in Australia was approximately 7 times that of Australian persons. Among cases in foreign-born persons in Australia, more than half were reported in persons from Vietnam, India and China (Communicable Disease Intelligence, 2006).
Biocultural differences in the size of the thoracic cavity significantly influence pulmonary functioning as determined by vital capacity and forced expiratory volume. In descending order, the largest chest volumes are found in Caucasians, African-Americans, Asians and Native Americans. Even when the shorter height of Asians is considered, their chest volume remains significantly lower than that of Caucasians and African-Americans.
SUBJECTIVE DATA
Subjective data relating to the respiratory system can give important clues as to an individual’s respiratory health and function as well as indicators of risk for development of lung disorders. Gathering information about respiratory function is important in determining an individual’s ability to perform activities of living.
| Assessment guidelines | Clinical significance and clinical alerts |
|---|---|
| Some conditions have a characteristic timing of a cough: | |
| Continuous throughout day–acute illness (e.g. respiratory infection) | |
| Afternoon/evening–may reflect exposure to irritants at work or during the day | |
| Night–postnasal drip, sinusitis | |
| Early morning–chronic bronchial inflammation or smoking. | |
| • Do you cough up any phlegm or sputum? How much? What colour is it? | Chronic bronchitis is characterised by a history of productive cough for 3 months of the year for 2 years in a row. |
| Non-productive coughs are associated with upper respiratory tract infections or early congestive cardiac failure. | |
| • Cough up any blood? Does this look like streaks or frank blood? Does the sputum have a foul odour? | Some conditions have characteristic sputum production: white or clear mucoid–colds, bronchitis, viral infections; yellow or green–bacterial infections; rust coloured–tuberculosis, pneumococ-cal pneumonia; pink, frothy–pulmonary oedema, some sympathomimetic medications have a side effect of pink-tinged mucus. |
| • How would you describe your cough: hacking, dry barking, hoarse, congested, bubbling? | Some conditions have a characteristic cough: mycoplasma pneumonia–hacking; early heart failure–dry; croup–barking; colds, bronchitis, pneumonia–congested. |
| • Does the cough seem to come with anything: activity, position (lying), fever, congestion, talking, anxiety? | |
| • Does activity make it better or worse? | |
| • What treatment have you tried? Prescription or over-the-counter medications, vaporiser, rest, position change? | Consider whether the problem can be attributed to adverse medication effects; for example, angiotensin converting enzyme (ACE) inhibitors are associated with the side effect of a persistent cough. |
| • Does the cough bring on anything: chest pain, ear pain? Is it tiring? Are you concerned about it? | |
| 2 Shortness of breath: Ever had any shortness of breath or hard-breathing spells? What brings it on? How severe is it? How long does it last? | Determine how much activity precipitates the shortness of breath (SOB)–state specific number of blocks walked, number of stairs. |
| • Is it affected by position, such as lying down? | Orthopnoea is difficulty breathing when supine. State number of pillows needed to achieve comfort (e.g. ‘two-pillow orthopnoea’). |
| Changes in sleep pattern may affect activities of living due to fatigue during the day. | |
| • Occur at any specific time of day or night? | Paroxysmal nocturnal dyspnoea is awakening from sleep with SOB and needing to be upright to achieve comfort. |
| • Shortness of breath episodes associated with night sweats? | Diaphoresis. |
| • Or cough, chest pain or bluish colour around lips or nails? Wheezing sound? | Cyanosis. |
| • Episodes seem to be related to food, pollen, dust, animals, season or emotion? | Asthma attacks may be associated with a specific allergen or extreme cold, anxiety. |
| • What do you do in a hard-breathing attack? Take a special position, or use pursed-lip breathing? Use any oxygen, inhalers or medications? | Assess effect of coping strategies and the need for more teaching. |
| • How does the shortness of breath affect your work or home activities? Getting better or worse or staying about the same? | Assess effect on activities of daily living. |
| 3 Chest pain with breathing: Any chest pain with breathing? Please point to the exact location. | Chest pain with pulmonary origin may be a late sign of pulmonary disease. |
| • When did it start? Constant or does it come and go? | |
| • Describe the pain: burning, stabbing? | |
| • Brought on by respiratory infection, coughing or trauma? Is it associated with fever, deep breathing, unequal chest inflation? | |
| • What have you done to treat it? Medication or heat application? | |
| 4 History of respiratory infections: Any past history of breathing trouble or lung diseases such as bronchitis, emphysema, asthma, pneumonia? | Consider sequelae after these conditions. |
| • Any unusually frequent or unusually severe colds? | Because most people have had some colds, it is more meaningful to ask about excess number or severity. |
| • Any family history of allergies, tuberculosis or asthma? | |
| 5 Smoking history: Do you smoke cigarettes or cigars? At what age did you start? How many packs per day do you smoke now? For how long? | |
| • Have you ever tried to quit? What helped? Why do you think it did not work? What activities do you associate with smoking? | Most people already know they should quit smoking. Instead of admonishing, assess smoking behaviour and ways to modify daily smoking activities. |
| • Live with someone who smokes? | |
| 6 Environmental exposure. Are there any environmental conditions that may affect your breathing? Where do you work? At a factory, chemical plant, coal mine, farming, outdoors in a heavy traffic area? | Farmers may be at risk for grain inhalation, pesticide inhalation. |
| • Do you do anything to protect your lungs, such as wear a mask or have the ventilatory system checked at work? Do you do anything to monitor your exposure? Have periodic examinations, pulmonary function tests, x-ray examination? | |
| • Do you know what specific symptoms to note that may signal breathing problems? | General symptoms: cough, shortness of breath. Some gases produce specific symptoms: carbon monoxide–dizziness, headache, fatigue; sulfur dioxide–cough, congestion. |
| 7 Self-care behaviours: Last tuberculosis skin test (Mantoux), chest x-ray study, pneumonia or influenza immunisation? | |
| Additional history for infants and children | |
| 4 to 6 uncomplicated upper respiratory infections per year is expected in early childhood. | |
| • (For child under 2 years of age): At what age were new foods introduced? Was the child breast fed or bottle fed? | Consider new foods or formula as possible allergens. |
| Screen for onset and follow course of childhood chronic respiratory problems: asthma, bronchitis. | |
| Young child is at risk for accidental aspiration, poisoning and injury. | |
| • Has anyone taught you emergency care measures in case of accidental choking or a hard-breathing spell? | |
| Environmental smoke increases the risk of ear and respiratory infections in children (DiFranza et al, 2004). | |
| Additional history for the adult over 65 years | |
| Older adults have a less efficient res piratory system (decreased vital capacity, less surface area for gas exchange), so they have less tolerance for activity. | |
| May have reduced capacity to perform exercise because of pulmonary function deficits of ageing. | |
| Sedentary or bedridden people are at risk for respiratory dysfunction. | |
| • How about energy level? Do you tire more easily? How does your illness affect you at home? At work? | Activities may decrease because of increasing shortness of breath or pain. |
| Some older adults feel pleuritic pain less intensely than younger adults. | |
| • Any chest pain after a bout of coughing? After a fall? | Precisely localised sharp pain (points to it with one finger)–consider fractured rib or muscle injury. |
OBJECTIVE DATA
Objective data collection begins at the first contact with the client (observation) for obvious abnormalities or difficulties breathing. A thorough assessment will involve collecting data through inspection, palpation, percussion and auscultation to evaluate lung function and respiratory health.
| Preparation | Equipment needed |
|---|---|
| Ask the person to sit upright and the male to disrobe to the waist. For the female, leave the gown on and open at the back. When examining the anterior chest, lift up the gown and drape it on her shoulders rather than removing it completely. This promotes comfort by giving her the feeling of being somewhat clothed. These provisions will ensure further comfort: a warm room, a warm diaphragm endpiece, adequate lighting and a private examination time with no interruptions. | Stethoscope Alcohol wipe |
| Perform the inspection, palpation, percussion and auscultation on the posterior and lateral thorax. Then move to face the person and repeat the four manoeuvres on the anterior chest. This avoids repetitiously moving front to back around the person. | |
| Finally, clean your stethoscope endpiece with an alcohol wipe. Because your stethoscope touches many people, it could be a possible vector for both aerobic and anaerobic bacteria. Cleaning with an alcohol wipe is a very effective measure to prevent cross contamination. |
| Procedure and normal. ndings | Abnormal. ndings and clinical alerts |
|---|---|
| INSPECT THE POSTERIOR CHEST | |
| Thoracic cage | |
| Note the shape and configuration of the chest wall. The spinous processes should appear in a straight line. The thorax is symmetrical, in an elliptical shape, with downward sloping ribs, about 45 degrees relative to the spine. The scapulae are placed symmetrically in each hemithorax. | Skeletal deformities may limit thoracic cage excursion: scoliosis, kyphosis (see Table 14.3). |
| The anteroposterior diameter should be less than the transverse diameter. The ratio of anteroposterior to transverse diameter is from 1:2 to 5:7. | Anteroposterior = transverse diameter, or ‘barrel chest’. Ribs are horizontal, chest appears as if held in continuous inspiration. This occurs in chronic emphysema from hyperinflation of the lungs (see Table 14.3). |
| The neck muscles and trapezius muscles should be developed normally for age and occupation. | Neck muscles are hypertrophied in chronic obstructive pulmonary disease (COPD) from aiding in forced respirations. |
| Note the position the person takes to breathe. This includes a relaxed posture and the ability to support one’s own weight with arms comfortably at the sides or in the lap. | People with COPD often sit in a tripod position, leaning forwards with arms braced against their knees, chair or bed. This gives them leverage so that their rectus abdominis, intercostal and accessory neck muscles all can aid in expiration. |
| Assess the skin colour and condition. Colour should be consistent with person’s genetic background, with allowance for sun-exposed areas on the chest and the back. No cyanosis or pallor should be present. Note any lesions. Inquire as to any change in a naevus on the back, for example, where the person may have difficulty monitoring (see Ch 17). | Use the ABCD system when assessing lesions and naevi (A–asymmetry, B–border, C–colour, D–diameter). |
| PALPATE THE POSTERIOR CHEST | |
| Symmetrical expansion | |
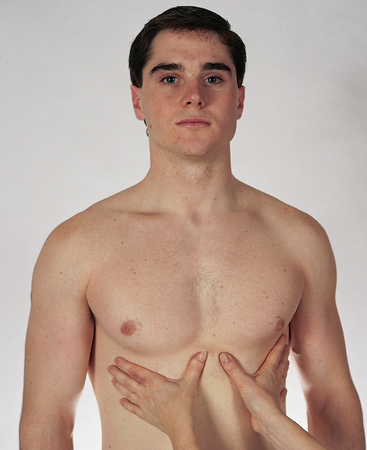
| Confirm symmetrical chest expansion by placing your warmed hands on the posterolateral chest wall with thumbs at the level of T9 or T10. Slide your hands medially to pinch up a small fold of skin between your thumbs (Fig 14.13). | |
| Ask the person to take a deep breath. Your hands serve as mechanical amplifiers; as the person inhales deeply, your thumbs should move apart symmetrically. Note any lag in expansion. | Unequal chest expansion occurs with marked atelectasis or pneumonia; with thoracic trauma, such as fractured ribs; or with pneumothorax. |
| Pain accompanies deep breathing when the pleurae are inflamed. | |
| Using the fingers, gently palpate the entire chest wall. This enables you to note any areas of tenderness, to note skin temperature and moisture, to detect any superficial lumps or masses and to explore any skin lesions noted on inspection. | Crepitus is a coarse crackling sensation palpable over the skin surface. It occurs in subcutaneous emphysema when air escapes from the lung and enters the subcutaneous tissue, as after open thoracic injury or surgery. |
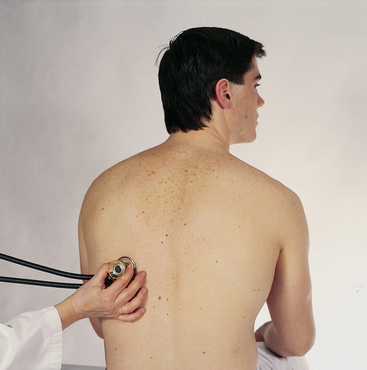
| AUSCULTATE THE POSTERIOR CHEST | |
| The passage of air through the tracheobronchial tree creates a characteristic set of noises that are audible through the chest wall. These noises may also be modified by obstruction within the respiratory passageways or by changes in the lung parenchyma, the pleura or the chest wall. | |
| Breath sounds | |
| Evaluate the presence and quality of normal breath sounds. The person is sitting, leaning forwards slightly, with arms resting comfortably across the lap. Instruct the person to breathe through the mouth, a little bit more deeply than usual, but to stop if they begin to feel dizzy. Be careful to monitor the breathing throughout the examination and offer times for the person to rest and breathe normally. The person is usually willing to comply with your instructions in an effort to please you and to be a ‘good patient’. Watch that they do not hyperventilate to the point of fainting. | |
| Use the flat diaphragm endpiece of the stethoscope and hold it firmly on the person’s chest wall. Listen to at least one full respiration in each location. Side-to-side comparison is most important. | |
Do not confuse background noise with lung sounds. Become familiar with these extraneous noises that may be confused with lung pathology if not recognised: 1 Examiner’s breathing on stethoscope tubing 2 Stethoscope tubing bumping together 4 Patient’s hairy chest; movement of hairs under stethoscope sounds like crackles (rales) (see Table 14.6)–minimise this by pressing harder or by wetting the hair with a damp cloth |
|
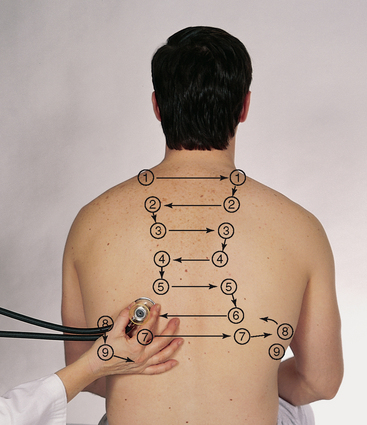
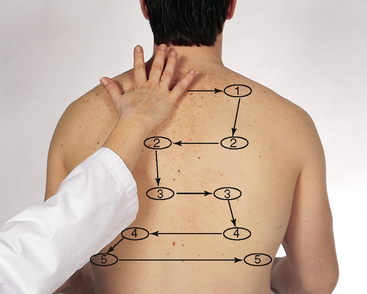
| While standing behind the person, listen to the following lung areas–posterior from the apices at C7 to the bases (around T10) and laterally from the axilla down to the seventh or eighth rib. Use the sequence illustrated in Fig 14.14. | |
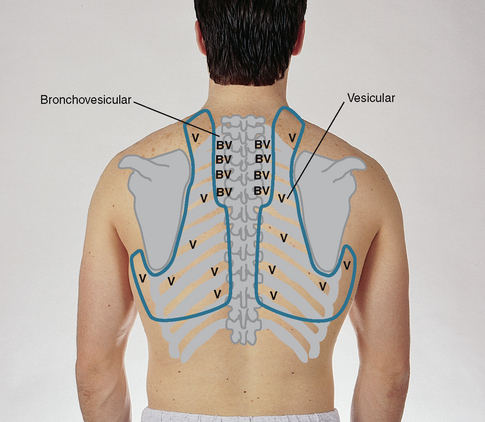
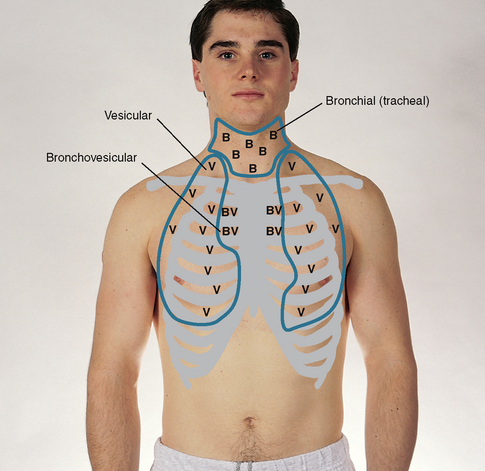
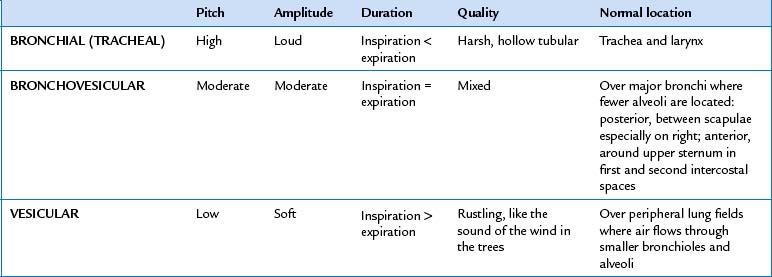
| Continue to visualise approximate locations of the lobes of each lung so that you correlate your findings to anatomical areas. As you listen, think (1) what AM I hearing over this spot? and (2) what should I EXPECT to be hearing? You should expect to hear three types of normal breath sounds in the adult and older child: bronchial (sometimes called tracheal or tubular), bronchovesicular and vesicular. Study the description of the characteristics of these normal breath sounds in Table 14.1. | |
| Note the normal location of the three types of breath sounds on the chest wall of the adult and older child (Figs 14.15 and 14.16). | Decreased or absent breath sounds occur: |
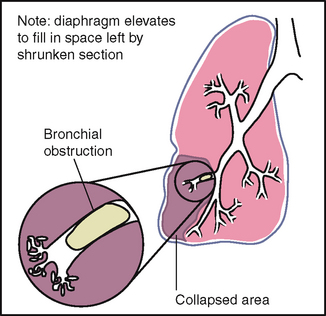
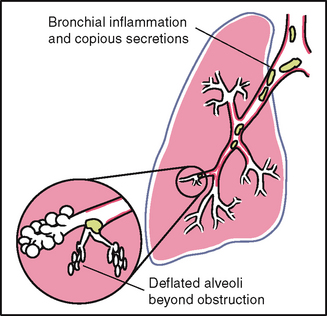
1 When the bronchial tree is obstructed at some point by secretions, mucous plug or a foreign body 2 In emphysema as a result of loss of elasticity in the lung fibres and decreased force of inspired air; also the lungs are already hyperinflated so the inhaled air does not make as much noise 3 When anything obstructs transmission of sound between the lung and your stethoscope, such as pleurisy or pleural thickening, or air (pneumothorax) or fluid (pleural effusion) in the pleural space. A silent chest means no air is moving in or out, which is an ominous sign. |
|
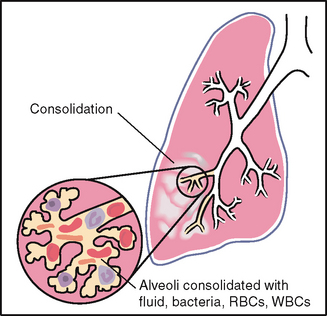
| Increased breath sounds mean that sounds are louder than they should be (e.g. bronchial sounds are abnormal when they are heard over an abnormal location, the peripheral lung fields). They have a high-pitched tubular quality, with a prolonged expiratory phase and a distinct pause between inspiration and expiration. They sound very close to your stethoscope, as if they were right in the tubing close to your ear. They occur when consolidation (e.g. pneumonia) or compression (e.g. fluid in the intrapleural space) yields a dense lung area that enhances the transmission of sound from the bronchi. When the inspired air reaches the alveoli, it hits solid lung tissue that conducts sound more efficiently to the surface. | |
| Adventitious sounds | |
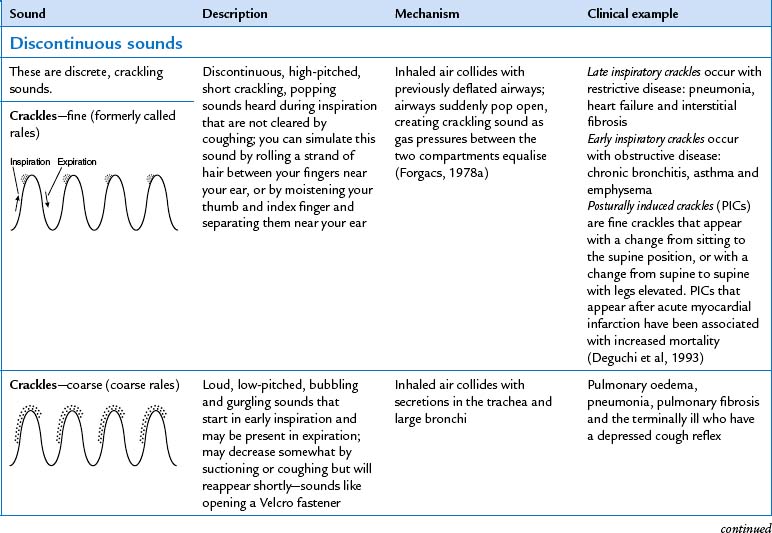
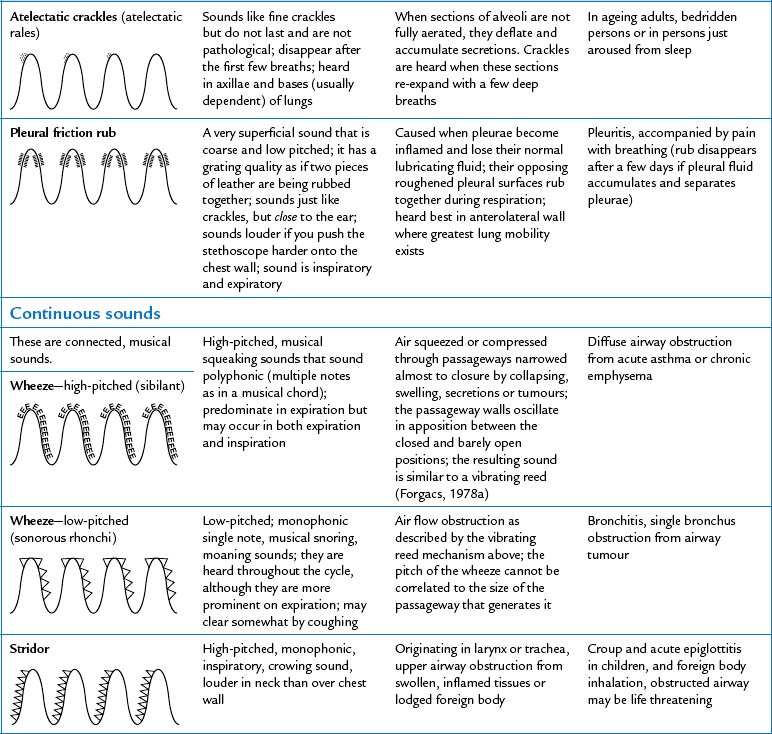
| Note the presence of any adventitious sounds. These are added sounds that are not normally heard in the lungs. If present, they are heard as being superimposed on the breath sounds. They are caused by moving air colliding with secretions in the tracheobronchial passageways or by the popping open of previously deflated airways. Sources differ as to the classification and nomenclature of these sounds (see Table 14.6), but crackles (or rales) and wheeze (or rhonchi) are terms commonly used by most examiners. | Study Table 14.6 for a complete description of these abnormal adventitious breath sounds. |
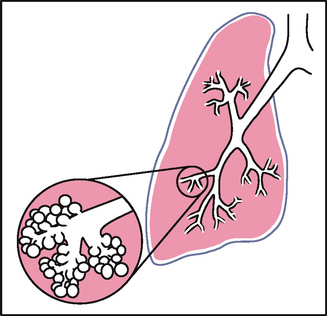
| One type of adventitious sound, atelectatic crackles, is not pathological. These crackles are short, popping, crackling sounds that sound like fine crackles but do not last beyond a few breaths. When sections of alveoli are not fully aerated (as in people who are asleep or in the elderly), they deflate slightly and accumulate secretions. Crackles are heard when these sections are expanded by a few deep breaths. Atelectatic crackles are heard only in the periphery, usually in dependent portions of the lungs, and disappear after the first few breaths or after a cough. | |
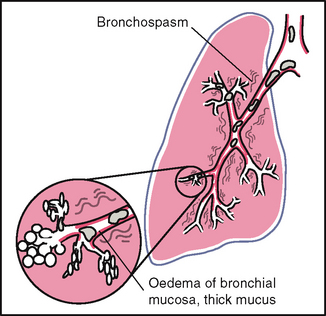
| In the past, persons were asked to ‘take a deep breath and blow it out hard’ to screen for the presence of wheezing. However, this manoeuvre is futile because slight wheezing may occur on maximal forced exhalation in healthy people. | During normal tidal flow, high-pitched wheeze occurs with asthma. |
| Voice sounds | |
| Determine the quality of voice sounds or vocal resonance. The spoken voice can be auscultated over the chest wall just as it can be felt in tactile fremitus described earlier. Ask the person to repeat a phrase such as ‘ninety-nine’ while you listen over the chest wall. Normal voice transmission is soft, muffled and indistinct; you can hear sound through the stethoscope but cannot distinguish exactly what is being said. Pathology that increases lung density enhances transmission of voice sounds. | Consolidation or compression of lung tissue will enhance the voice sounds, making the words more distinct. |
| Eliciting the voice sounds is not usually done in the routine examination. Rather, these are supplemental manoeuvres that are performed if you suspect lung pathology on the basis of earlier data. When they are performed, you are testing for possible presence of bronchophony, egophony and whispered pectoriloquy (Table 14.7). | |
| INSPECT THE ANTERIOR CHEST | |
| Note the shape and configuration of the chest wall. The ribs are sloping downwards with symmetrical interspaces. The costal angle is within 90 degrees. Development of abdominal muscles is as expected for the person’s age, weight and athletic condition. | |
| Note the person’s facial expression. The facial expression should be relaxed and benign, indicating an unconscious effort of breathing. | Tense, strained, tired facies accompany COPD. |
| The person with COPD may purse the lips in a whistling position. By exhaling slowly and against a narrow opening, the pressure in the bronchial tree remains positive and fewer airways collapse. | |
| Assess the level of consciousness. The level of consciousness should be alert and cooperative. | Cerebral hypoxia may be reflected by excessive drowsiness or by anxiety, restlessness and irritability. |
| Note skin colour and condition. The lips and nail beds are free of cyanosis or unusual pallor. The nails are of normal configuration. Explore any skin lesions. | Clubbing of distal phalanx occurs with chronic respiratory disease. |
| Cutaneous angiomas (spider naevi) associated with liver disease or portal hypertension may be evident on the chest. | |
| Assess the quality of respirations. Normal relaxed breathing is automatic and effortless, regular and even and produces no noise. The chest expands symmetrically with each inspiration. Note any localised lag on inspiration. | |
| No retraction or bulging of the interspaces should occur on inspiration. | Retraction suggests obstruction of respiratory tract or increased inspiratory effort is needed, as with atelectasis. Bulging indicates trapped air as in the forced expiration associated with emphysema or asthma. |
| Normally, accessory muscles are not used to augment respiratory effort. However, with very heavy exercise, the accessory neck muscles (scalene, sterno-mastoid, trapezius) are used momentarily to enhance inspiration. | |
| The respiratory rate is within normal limits for the person’s age (see Table 9.3) and the pattern of breathing is regular. Occasional sighs normally punctuate breathing. | Tachypnoea and hyperventilation, bradypnoea and hypoventilation, periodic breathing (see Table 14.4). |
| PALPATE THE ANTERIOR CHEST | |
| Palpate symmetrical chest expansion. Place your hands on the anterolateral wall with the thumbs along the costal margins and pointing towards the xiphoid process (Fig 14.17). | Abnormally wide costal angle with little inspiratory variation occurs with emphysema. |
| Ask the person to take a deep breath. Watch your thumbs move apart symmetrically, and note smooth chest expansion with your fingers. Any limitation in thoracic expansion is easier to detect on the anterior chest because greater range of motion exists with breathing here. | A lag in expansion occurs with atelectasis, pneumonia and postoperative guarding. A palpable grating sensation with breathing indicates pleural friction fremitus (see Table 14.5). |
| Palpate the anterior chest wall to note any tenderness (normally none is present) and to detect any superficial lumps or masses (again, normally none are present). Note skin mobility and turgor, and note skin temperature and moisture. | |
| AUSCULTATE THE ANTERIOR CHEST | |
| Breath sounds | |
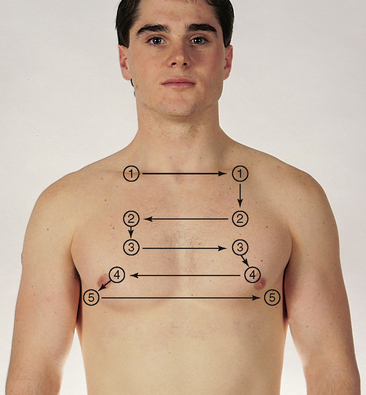
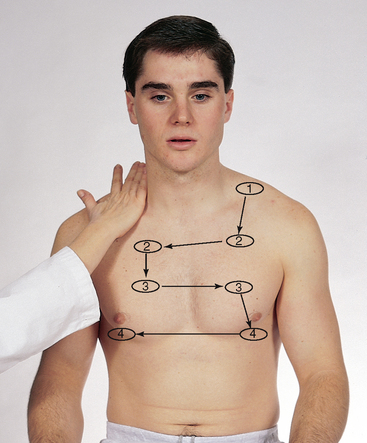
| Auscultate the lung fields over the anterior chest from the apices in the supraclavicular areas down to the sixth rib. Progress from side to side as you move downwards, and listen to one full respiration in each location. Use the sequence indicated for percussion. Do not place your stethoscope directly over the female breast. Displace the breast and listen directly over the chest wall. Use the sequence illustrated in Fig 14.18. | |
| Evaluate normal breath sounds, noting any abnormal breath sounds and any adventitious sounds. If the situation warrants, assess the voice sounds on the anterior chest. | Study Table 14.8 for a complete des cription of abnormal respiratory conditions. |
| Measurement of pulmonary function status | |
| The forced expiratory time is the number of seconds it takes for the person to exhale from total lung capacity to residual volume. It is a screening measure of air flow obstruction. Although the test is not usually performed in the respiratory assessment, it is useful when you wish to screen for pulmonary function. | |
| Ask the person to inhale the deepest breath possible and then to blow it all out hard, as quickly as possible, with the mouth open. Listen with your stethoscope over the sternum. The normal time for full expiration is 4 seconds or less. | A forced expiration of 6 seconds or more occurs with obstructive lung disease. Refer this person for more precise pul monary function studies. |
| The pulse oximeter is a noninvasive method to assess arterial oxygen saturation (Spo2) and is described in Chapter 9. A healthy person with no lung disease and no anaemia normally has an Spo2 of 97% to 98%. However, every Spo2 result must be evaluated in the context of the person’s haemoglobin level, acid–base balance and ventilatory status. | |
| The 6-minute distance (6MD) walk is a safe, simple, inexpensive, clinical measure of functional status in ageing adults (Enright, 2003). The 6MD is used as an outcome measure for people in pulmonary rehabilitation because it mirrors conditions that are used in everyday life. Locate a flat-surfaced corridor that has little foot traffic, is wide enough to permit comfortable turns and has a controlled environment. Ensure that the person is wearing comfortable shoes, and equip them with a pulse oximeter to monitor oxygen saturation. Ask the person to set their own pace to cover as much ground as possible in 6 minutes, and assure the person it is all right to slow down or to stop to rest at any time. Use a stopwatch to time the walk. A person who walks >300 m in 6 minutes is more likely to engage in activities of daily living. | Ask the person to stop the walk if you measure an SpO2 below 85% to 88% or if extreme breathlessness occurs. |
| DEVELOPMENTAL CONSIDERATIONS | |
| Infants and children | |
| To prepare, let the parent hold an infant supported against the chest or shoulder. Do not let the usual sequence of the physical examination restrain you; seize the opportunity with a sleeping infant to inspect and then to listen to lung sounds next. This way you can concentrate on the breath sounds before the baby wakes up and possibly cries. Infant crying does not have to be a problem for you though, because it actually enhances palpation of tactile fremitus and auscultation of breath sounds. | |
| A child may sit upright on the parent’s lap. Offer the stethoscope and let the child handle it. This reduces any fear of the equipment. Promote the child’s participation; school-age children usually are delighted to hear their own breath sounds when you place the stethoscope properly. While listening to breath sounds, ask the young child to take a deep breath and ‘blow out’ your penlight while you hold the stethoscope with your other hand. Time your letting go of the penlight button so the light goes off after the child blows. Or, ask the child to ‘pant like a dog’ while you auscultate. | |
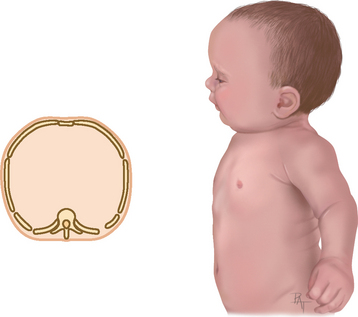
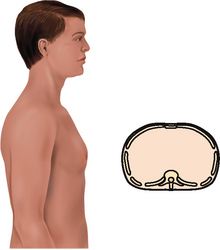
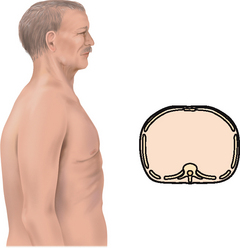
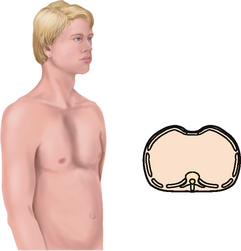
| Inspection. The infant has a rounded thorax with an equal anteroposterior-to-transverse chest diameter (Fig 14.19). By age 6 years, the thorax reaches the adult ratio of 1:2 (anteroposterior-to-transverse diameter). The newborn’s chest circumference is 30 to 36 cm and is 2 cm smaller than the head circumference until 2 years of age. The chest wall is thin with little musculature. The ribs and the xiphoid are prominent; you can see as well as feel the sharp tip of the xiphoid process. The thoracic cage is soft and flexible. | Note a barrel shape persisting after age 6 years, which may develop with chronic asthma or cystic fibrosis. |
| In newborn males and females, the breasts may look enlarged by the second or third day from maternal oestrogen. Occasionally a white fluid, sometimes referred to by the slang expression ‘witch’s milk’, can be expressed. This resolves within a week. | |
| In some children, ‘Harrison groove’ occurs normally. This is a horizontal groove in the rib cage at the level of the insertion of the diaphragm, extending from the sternum to the midaxillary line. | Harrison groove also occurs with rickets from the pull of the diaphragm on weakened ribs. |
| The newborn’s first respiratory assessment is part of the Apgar scoring system to measure the successful transition to extrauterine life (Table 14.2). The five standard parameters are scored at 1 minute and at 5 minutes after birth. A 1-minute Apgar with a total score of 7 to 10 indicates a newborn in good condition, needing only suctioning of the nose and mouth and otherwise routine care. | In the immediate newborn period, depressed respirations are due to maternal drugs, interruption of the uterine blood supply or obstruction of the tracheobronchial tree with mucus or fluid. |
| A 1-minute Apgar score with a total score of 3 to 6 indicates a moderately depressed newborn needing more resuscitation and subsequent close observation. A score of 0 to 2 indicates a severely depressed newborn needing full resuscitation, ventilatory assistance and subsequent intensive care. | |
| The infant breathes through the nose rather than the mouth and is an obligate nose breather until 3 months. Slight flaring of the lower costal margins may occur with respirations, but normally no flaring of the nostrils and no sternal retractions or intercostal retractions occur. The diaphragm is the newborn’s major respiratory muscle. Intercostal muscles are not well developed. Thus you observe the abdomen bulge with each inspiration but see little thoracic expansion. | Marked retractions of sternum and intercostal muscles indicate increased inspiratory effort, as in atelectasis, pneumonia, asthma and acute airway obstruction. |
| Count the respiratory rate for 1 full minute. Normal rates for the newborn are 30 to 40 breaths per minute but may spike up to 60 per minute. Obtain the most accurate respiratory rate by counting when the infant is asleep because infants reach rapid rates with very little excitation when awake. The respiratory pattern may be irregular when extremes in room temperature occur or with feeding or sleeping. Brief periods of apnoea less than 10 or 15 seconds are common. This periodic breathing is more common in premature infants. | |
| Palpation. Palpate symmetrical chest expansion by encircling the infant’s thorax with both hands. Further palpation should yield no lumps, masses or crepitus, although you may feel the costochondral junctions in some normal infants | |
| Rachitic rosary–prominent round knobs at costochondral junctions–is seen in infants with rickets or scurvy. | |
| Percussion. Percussion is of limited usefulness in the newborn and especially in the premature newborn because the adult’s fingers are too large in relation to the tiny chest. The percussion note of hyperresonance occurs normally in the infant and young child because of the relatively thin chest wall. Anything less than hyperresonance would have the same clinical significance as dullness in the adult. If measured, diaphragmatic excursion measures about one to two rib interspaces in children. | |
| Auscultation. Auscultation normally yields bronchovesicular breath sounds in the peripheral lung fields of the infant and young child up to age 5 to 6 years. Their relatively thin chest walls with underdeveloped musculature do not damp off the sound as do the thicker walls of adults, so breath sounds are louder and harsher. | Diminished breath sounds occur with pneumonia, atelectasis, pleural effusion or pneumothorax. |
| Fine crackles are the adventitious sounds commonly heard in the immediate newborn period from opening of the airways and clearing of fluid. Because the newborn’s chest wall is so thin, transmission of sounds is enhanced and sound is heard easily all over the chest, making localisation of breath sounds a problem. Even bowel sounds are easily heard in the chest. Try using the smaller paediatric diaphragm endpiece, or place the bell over the infant’s interspaces and not over the ribs. Use the paediatric diaphragm on an older infant or toddler. | Persistent fine crackles that are scattered over the chest occur with pneumonia, bronchiolitis or atelectasis. Crackles only in upper lung fields occur with cystic fibrosis; crackles only in lower lung fields occur with heart failure. Expiratory wheezing occurs with lower airway obstruction (e.g. asthma or bron-chiolitis). When unilateral, it may be foreign body aspiration. |
| The pregnant female | |
| The thoracic cage may appear wider, and the costal angle may feel wider than in the nonpregnant state. Respirations may be deeper, although this can be quantified only with pulmonary function tests. | |
| The adult over 65 years | |
| The chest cage commonly shows an increased anteroposterior diameter, giving a round barrel shape, and kyphosis or an outward curvature of the thoracic spine (see Table 14.3). The person compensates by holding the head extended and tilted back. You may palpate marked bony prominences because of decreased subcutaneous fat. Chest expansion may be somewhat decreased with the older person, although it should still be symmetrical. The costal cartilages become calcified with ageing, resulting in a less mobile thorax. | |
| The older person may fatigue easily, especially during auscultation when deep mouth breathing is required. Take care that this person does not hyperventilate and become dizzy. Allow brief rest periods or quiet breathing. If the person does feel faint, holding the breath for a few seconds will restore equilibrium. | |
| FURTHER SUBJECTIVE AND OBJECTIVE ASSESSMENT FOR ADVANCED PRACTICE | |
| The assessments described in the following section require advanced skill and scope of practice. Nurses working in specialist respiratory units and nurses working in community centres may need to develop these skills. | |
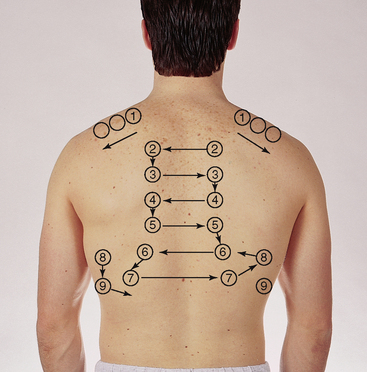
Assess tactile (or vocal) fremitus. Fremitus is a palpable vibration. Sounds generated from the larynx are transmitted through patent bronchi and through the lung parenchyma to the chest wall, where you feel them as vibrations. Use either the palmar base (the ball) of the fingers or the ulnar edge of one hand, and touch the person’s chest while they repeat the words ‘ninety-nine’ or ‘blue balloon’. These are resonant phrases that generate strong vibrations. Start over the lung apices and palpate from one side to another (Fig 14.20). |
Decreased fremitus occurs when anything obstructs transmission of vibrations (e.g. obstructed bronchus, pleural effusion or thickening, pneumothorax or emphysema). Any barrier that comes between the sound and your palpating hand will decrease fremitus. |
Increased fremitus occurs with compression or consolidation of lung tissue (e.g. lobar pneumonia). This is present only when the bronchus is patent and when the consolidation extends to the lung surface. Note that only gross changes increase fremitus. Small areas of early pneumonia do not significantly affect fremitus. Rhonchal fremitus is palpable with thick bronchial secretions. Pleural friction fremitus is palpable with inflammation of the pleura (see Table 14.5). |
|
Fremitus varies among persons but symmetry is most important; the vibrations should feel the same in the corresponding area on each side. However, just between the scapulae, fremitus may feel stronger on the right side than on the left side because the right side is closer to the bronchial bifurcation. Avoid palpating over the scapulae because bone damps out sound transmission. The following factors affect the normal intensity of tactile fremitus: • Relative location of bronchi to the chest wall. Normally, fremitus is most prominent between the scapulae and around the sternum, sites where the major bronchi are closest to the chest wall. Fremitus normally decreases as you progress down because more and more tissue impedes sound transmission. • Thickness of the chest wall. Fremitus feels greater over a thin chest wall than over an obese or heavily muscular one where thick tissue damps the vibration. A loud, low-pitched voice generates more fremitus than a soft, high-pitched one. |
|
| Note any areas of abnormal fremitus. Sound is conducted better through a uniformly dense structure than through a porous one, which changes in shape and solidity (as does the lung tissue during normal respiration). Thus conditions that increase the density of lung tissue make a better conducting medium for sound vibrations and increase tactile fremitus. | |
| Palpate the anterior chest | |
| Assess tactile (vocal) fremitus. Begin palpating over the lung apices in the supraclavicular areas (Fig 14.21). Compare vibrations from one side to the other as the person repeats ‘ninety-nine’. Avoid palpating over female breast tissue because breast tissue normally damps the sound. | |
| Percuss lung fields | |
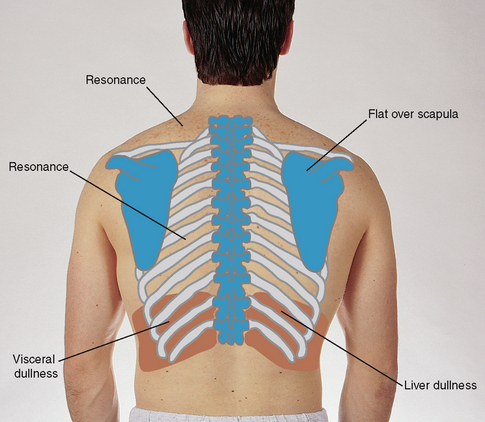
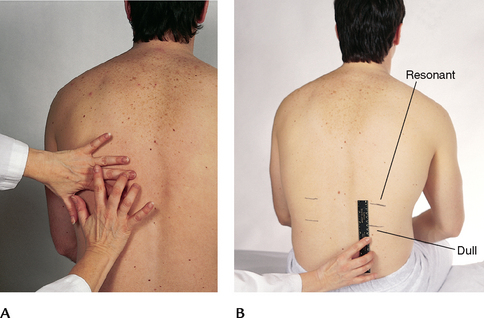
| Determine the predominant note over the lung fields. Start at the apices and percuss the band of normally resonant tissue across the tops of both shoulders (Fig 14.22). Then, percussing in the interspaces, make a side-to-side comparison all the way down the lung region. Percuss at 5-cm intervals. Avoid the damping effect of the scapulae and ribs. | |
| Resonance is the low-pitched, clear, hollow sound that predominates in healthy lung tissue in the adult (Fig 14.23). However, resonance is a relative term and has no constant standard. The resonant note may be modified somewhat in the athlete with a heavily muscular chest wall and in the heavily obese adult in whom subcutaneous fat produces scattered dullness. | Hyperresonance is a lower-pitched, booming sound found when too much air is present, as in emphysema or pneumothorax. |
| A dull note (soft, muffled thud) signals abnormal density in the lungs, as with pneumonia, pleural effusion, atelectasis or tumour. | |
| The depth of penetration of percussion has limits. Percussion sets into motion only the outer 5 to 7 cm of tissue. It will not penetrate to reveal any change in density deeper than that. Also, an abnormal finding must be 2 to 3 cm wide to yield an abnormal percussion note. Lesions smaller than that are not detectable by percussion. | |
| Diaphragmatic excursion | |
| Determine diaphragmatic excursion (Fig 14.24, A). Percuss to map out the lower lung border, both in expiration and in inspiration. First, ask the person to ‘exhale and hold it’ briefly while you percuss down the scapular line until the sound changes from resonant to dull on each side. This estimates the level of the diaphragm separating the lungs from the abdominal viscera. It may be somewhat higher on the right side (about 1 to 2 cm) because of the presence of the liver. Mark the spot. | |
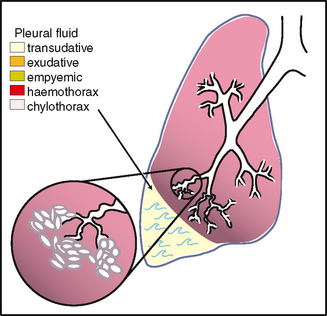
| Now ask the person to ‘take a deep breath and hold it’. Continue percussing down from your first mark and mark the level where the sound changes to dull on this deep inspiration. Measure the difference. This diaphragmatic excursion should be equal bilaterally and measure about 3 to 5 cm in adults, although it may be up to 7 to 8 cm in well-conditioned people (Fig 14.24, B). | Note an abnormally high level of dullness and absence of excursion. These occur with pleural effusion (fluid in the space between the visceral and parietal pleura) or atelectasis of the lower lobes. |
| Often, the beginning examiner becomes so involved in the subtle differences of percussion notes that they extend the patient’s limits of breathholding. Always hold your own breath when you ask your patient to. When you run out of air, the other person surely has too, especially if that person has a respiratory problem. | |
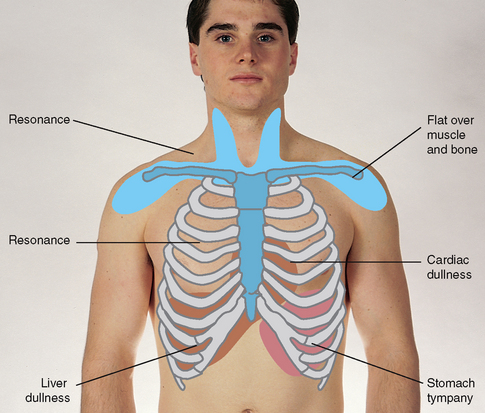
| Percuss the anterior chest | |
| Begin percussing the apices in the supraclavicular areas. Then, percussing the interspaces and comparing one side to the other, move down the anterior chest. | |
| Interspaces are easier to palpate on the anterior chest than on the back. Do not percuss directly over female breast tissue because this would produce a dull note. Shift the breast tissue over slightly using the edge of your stationary hand. In females with large breasts, percussion may yield little useful data. With all people, use the sequence illustrated in Figure 14.18. | |
| Sequence for percussion of anterior chest | |
| Note the borders of cardiac dullness normally found on the anterior chest and do not confuse these with suspected lung pathology (Fig 14.25). In the right hemithorax the upper border of liver dullness is located in the fifth intercostal space in the right midclavicular line. On the left, tympany is evident over the gastric space. | Lungs are hyperinflated with chronic emphysema, resulting in hyperresonance where you would expect cardiac dullness. |
| DEVELOPMENTAL CONSIDERATIONS | |
| Infants and children | |
| Percussion. Percussion is of limited usefulness in the newborn and especially in the premature newborn because the adult’s fingers are too large in relation to the tiny chest. The percussion note of hyperresonance occurs normally in the infant and young child because of the relatively thin chest wall. Anything less than hyperresonance would have the same clinical significance as dullness in the adult. If measured, diaphragmatic excursion measures about one to two rib interspaces in children. |
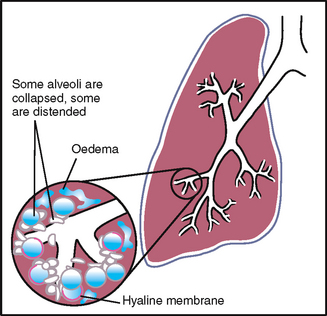
TABLE 14.3 Configurations of the thorax
TABLE 14.4 Respiration patterns*
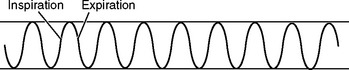 |
| Normal adult (for comparison) |
 |
| Sigh |
| Occasional sighs punctuate the normal breathing pattern and are purposeful to expand alveoli. Frequent sighs may indicate emotional dysfunction. Frequent sighs also may lead to hyperventilation and dizziness. |
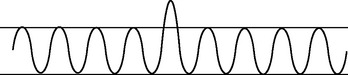
 |
| Tachypnoea |
| Rapid shallow breathing. Increased rate >24 per minute. This is a normal response to fever, fear or exercise. Rate also increases with respiratory insufficiency, pneumonia, alkalosis, pleurisy and lesions in the pons. |
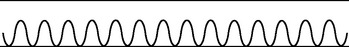
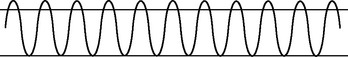 |
| Hyperventilation |
| Increase in both rate and depth. Normally occurs with extreme exertion, fear or anxiety. Also occurs with diabetic ketoacidosis (Kussmaul’s respirations), hepatic coma, salicylate overdose (producing a respiratory alkalosis to compensate for the metabolic acidosis), lesions of the midbrain and alteration in blood gas concentration (either an increase in carbon dioxide or decrease in oxygen). Hyperventilation blows off carbon dioxide, causing a decreased level in the blood (alkalosis). |
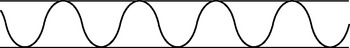 |
| Bradypnoea |
| Slow breathing. A decreased but regular rate (less than 10 per minute), as in drug-induced depression of the respiratory centre in the medulla, increased intracranial pressure and diabetic coma. |
 |
| Hypoventilation |
| An irregular shallow pattern caused by an overdose of narcotics or anaesthetics. May also occur with prolonged bed rest or conscious splinting of the chest to avoid respiratory pain. |
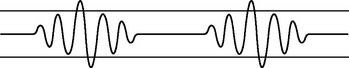 |
| Cheyne-Stokes respiration |
| A cycle in which respirations gradually wax and wane in a regular pattern, increasing in rate and depth and then decreasing. The breathing periods last 30 to 45 seconds, with periods of apnoea (20 seconds) alternating the cycle. The most common cause is severe heart failure; other causes are renal failure, meningitis, drug overdose and increased intracranial pressure. Occurs normally in infants and ageing persons during sleep. |
 |
| Biot’s respiration |
| Similar to Cheyne-Stokes respiration, except that the pattern is irregular. A series of normal respirations (three to four) is followed by a period of apnoea. The cycle length is variable, lasting anywhere from 10 seconds to 1 minute. Seen with head trauma, brain abscess, heat stroke, spinal meningitis and encephalitis. |
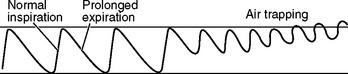 |
| Chronic obstructive breathing |
| Normal inspiration and prolonged expiration to overcome increased airway resistance. In a person with chronic obstructive lung disease, any situation calling for increased heart rate (exercise) may lead to dyspnoeic episode (air trapping), because then the person does not have enough time for full expiration. |
* Assess the (1) rate, (2) depth (tidal volume) and (3) pattern.
TABLE 14.5 Abnormal tactile fremitus
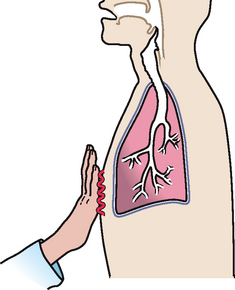 |
| Increased tactile fremitus |
| Occurs with conditions that increase the density of lung tissue, thereby making a better conducting medium for vibrations (e.g. compression or consolidation (pneumonia)). There must be a patent bronchus and consolidation must extend to lung surface for increased fremitus to be apparent. |
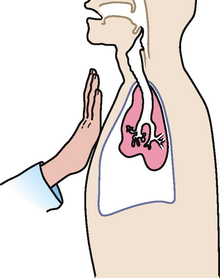 |
| Decreased tactile fremitus |
| Occurs when anything obstructs transmission of vibrations (e.g. an obstructed bronchus, pleural effusion or thickening, pneumothorax and emphysema). Any barrier that gets in the way of the sound and your palpating hand decreases fremitus. |
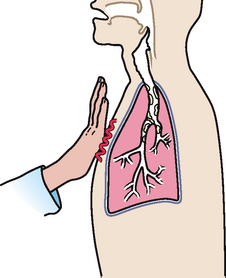 |
| Rhonchal fremitus |
| Vibration felt when inhaled air passes through thick secretions in the larger bronchi. This may decrease somewhat by coughing. |
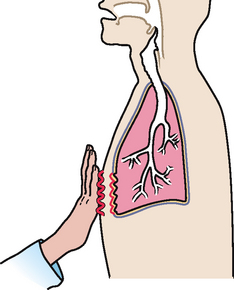 |
| Pleural friction fremitus |
| Produced when inflammation of the parietal or visceral pleura causes a decrease in the normal lubricating fluid. Then the opposing surfaces make a coarse grating sound when rubbed together during breathing. Although this sound is best detected by auscultation, it may sometimes be palpable and feels like two pieces of leather grating together. It is synchronous with respiratory excursion. Also called a palpable friction rub. |
| Technique | Normal finding | Abnormal finding |
|---|---|---|
| Normal voice transmission is soft, muffled e and indistinct; you can hear sound through the stethoscope but cannot distinguish exactly what is being said | ||
 |
||
| Normally, you should hear ‘eeeeeeee’ through your stethoscope | Over area of consolidation or compression, the spoken ‘eeee’ sound changes to a bleating long ‘aaaaa’ sound | |
| The normal response is faint, muffled and almost inaudible | With only small amounts of consolidation, the whispered voice is transmitted very clearly and distinctly, although still somewhat faint; it sounds as if the person is whispering right into your stethoscope, ‘one-two-three’ | |
TABLE 14.8 Assessment of common respiratory conditions
Promoting a healthy lifestyle Environmental tobacco smoke (ETS)
Secondhand smoke–there is no risk-free level of exposure!
Secondhand smoke, also referred to as environmental tobacco smoke (ETS), is a mixture of sidestream smoke, the smoke from the burning end of a cigarette, pipe or cigar, and mainstream smoke, the smoke exhaled from the lungs of the individual smoking. The evidence indicates that there is no risk-free level of exposure to secondhand smoke. Exposure to secondhand smoke, which is primarily involuntary, increases our risk for adverse health effects. Further, the general public’s exposure to secondhand smoke, regardless of whether they are smokers, is much higher than most people realise.
Secondhand smoke is especially harmful to young children, increasing respiratory infection rates, inner ear infections and aggravation of asthma. There is a causal relationship between maternal smoking during pregnancy and:
Following birth, infants and children exposed to ETS face an increased risk of:
According to the Australian Bureau of Statistics in 2004–05 37% of children aged 0–14 years lived in households with one or more regular smokers, while 10% lived with one regular smoker who smoked indoors. Secondhand smoke contains hundreds of chemicals known to be toxic or carcinogenic, including formaldehyde, benzene, vinyl chloride, arsenic and cyanide. Involuntarily inhaled by nonsmokers, it can linger in the air for hours long after the cigarette, cigar or pipe has been extinguished.
Exposure to secondhand smoke places nonsmokers at risk for the same diseases as active smoking does. Nonsmokers exposed to secondhand smoke are 25% more likely to have heart disease and 20% more likely to have lung cancer than are those nonsmokers who are not exposed to smoke. Separating smokers from nonsmokers, cleaning the air indoors and ventilating buildings do not eliminate the exposure risk to nonsmokers. However, eliminating smoking from indoor spaces does fully protect the nonsmoker.
Where do you start?
Exposure to secondhand smoke is a common public health hazard that is completely preventable. Everyone should be able to go about their daily lives without exposure to other people’s cigarette smoke. Therefore, all workplaces, homes, cars, enclosed indoor public places and outdoor restricted public places, such as sporting venues, should be smoke free. While there has been significant progress over the last decade to achieving this in public places in Australia, more action needs to be taken.
In your home and car
If you, family members or visitors to your home do smoke, smoke the cigarettes outside. Blowing smoke away from people, going into another room to smoke or opening a window will not protect family and friends from the dangers of secondhand smoking.
If you don’t smoke but family members do, be sympathetic and understanding but encourage them to quit. There are many publicly available and online support groups for smokers trying to kick the habit.
Secondhand smoking and your health
Don’t smoke in your car or allow others to do so. Children and babies have no choice about exposure to secondhand smoke in confined spaces and it damages their health. Be a good role model for your children: don’t smoke. There is good evidence to suggest that children whose parents don’t smoke are much less likely to take up smoking.
In public places
Know the law. Food preparation areas, public transport, lifts, airports and aircraft, theatres, schools, childcare centres and cinemas in Australia are all smoke free. All states and one territory have introduced legislation for smoke-free workplaces and public places, including restaurants, hotels and nightclubs.
DOCUMENTATION AND CRITICAL THINKING
FOCUSED ASSESSMENT: CLINICAL CASE STUDY
Thomas G is a 58-year-old, thin, male retired police officer who appears older than stated age. Face is anxious and tense, although in no acute distress at this time. Seeks care for ‘increasing shortness of breath and fatigue in last couple of months’.
Subjective
1 year prior to the assessment noticed feeling more ‘winded’ than usual when walking > 3–4 blocks. Early morning cough present daily × 10 years, but now increased sputum production to 2 teaspoons per morning, frothy white.
6 months prior to the assessment had a ‘cold’ with severe harsh coughing, productive of ½ cup thick white sputum/day. Noted midsternal chest pain (mild) with cough. Lasted 2 weeks. Treated self with humidifier and cough syrup–minimal relief.
3 months prior to the assessment noticed increasing SOB with less activity. Fatigue and SOB when walking outside during peak hour traffic. Unable to take evening walks (usually 2–3 blocks) due to SOB and fatigue. Has two-pillow orthopnoea. Wakes 3–4 times during night.
Now–feels he is ‘worse and needs some help’. Continues with 2-pillow orthopnoea. Unable to walk >2 blocks or climb >1 flight stairs without resting. Unable to blow out birthday candles on cake last week. Morning cough productive of ¼ cup thin white sputum, cough continues sporadically during day.
No chest pain, haemoptysis, night sweats or paroxysmal nocturnal dyspnoea. No history of allergies, hospitalisations or injuries to chest. No family history of TB, allergies, asthma or cancer. Smokes cigarettes 2 packs per day × 30 years. Alcohol < one 6-pack beer/week summer months only.
Objective
Inspection: Sitting on side of bed with arms propped on bedside table. Resp. resting 24/min, regular, shallow with prolonged expiration; resp. 34/min ambulating. Increased use of accessory muscles, AP = transverse diameter with widening of costal angle, slightly flushed face, tense expression.
Palpation: Minimal but symmetrical chest expansion. No lumps, masses or tenderness on palpation.
Auscultation: Breath sounds diminished. Expiratory wheeze throughout posterior chest, R > L. No crackles.
Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115:1254–1260.
Australian Bureau of Statistics, Tobacco smoking in Australia: a snapshot, 2004–05. ABS, Canberra, 2006. Available at http://www.abs.gov.au/AUSSTATS/abs@.nsf/mf/4831.0.55.001?OpenDocument.
Centers for Disease Control and Prevention. Trends in tuberculosis, 1998–2003. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5310a2.
Centers for Disease Control and Prevention. MMWR Wkly Rep 55z:305–308. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5511a3.htm#top.
Charlebois D. Early recognition of pulmonary embolism. Cardiovasc Nurs. 2005;20:254–259.
Chen E, Herman C, Rodgers D. Symptom perception in childhood asthma: The role of anxiety and asthma severity. Health Psychol. 2006;25:389–395.
Communicable Diseases Intelligence, Tuberculosis in Australia: bacteriologically confirmed cases and drug resistance, 2004: a report of the Australian Mycobacterium Reference Laboratory Network 2006;3(1). Available at http://www.health.gov.au/internet/main/publishing.nsf/content/cda-cdi3001d.htm.
Conboy-Ellis K. Asthma pathogenesis and management. Nurse Pract. 2006;31:24–39.
Cunningham FG, et al. Williams’ obstetrics, 22nd edn. New York: McGraw-Hill Professional, 2005.
Deguchi F, et al. Prognostic significance of posturally induced crackles: Long term follow-up of patients after recovery from acute myocardial infarction. Chest. 1993;103:1457–1462.
DiFranza JR, Aligne A, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015.
Enright PL. The six-minute walk test. Respir Care. 2003;48:783–785.
Forgacs P. Lung sounds. London: Baillière Tindall, 1978.
Forgacs P. The functional basis of pulmonary sounds. Chest. 1978;73:399–405.
Goldberg H, Peters J. Life-threatening asthma, part 1: identifying the risk factors. Consultant. 2006;46:609–612.
Goldrick BA. Respiratory syncytial virus. Am J Nurs. 2004;104:55–56.
Goldrick BA. Update: tuberculosis in the United States. Am J Nurs. 2005;105:85–86.
Holm K, Foreman M. Analysis of measures of functional and cognitive ability for aging adults with cardiac and vascular disease. Cardiovasc Nurs. 2006;21:40–47.
Holman M. Obstructive sleep apnea syndrome: Implications for primary care. Nurse Pract. 2005;30:39–43.
Kalesan B, Stine J, Alberg A. The joint influence of parental modeling and positive parent concern on cigarette smoking in middle and high school students. J Sch Health. 2006;76:402–407.
Koschel MJ. Pulmonary embolism. Am J Nurs. 2004;104:46–50.
Kum-Nji P, Meloy L, Herrod HG. Environmental tobacco smoke exposure: prevalence and mechanisms of causation of infection in children. Pediatrics. 2006;117:1745–1754.
Law M, Wald N. Environmental tobacco smoke and ischemic heart disease. Progress in Cardiovascular Diseases. 2003;46:31–38.
Loudon RG. The lung exam. Clin Chest Med. 1987;8:265–272.
Miskovich-Riddle L, Keresztes PA. CAP management guidelines. Nurse Pract. 2006;31:43–55.
Mody L, Rongjun S, Bradley SF. Assessment of pneumonia in older adults: effect of functional status. J Am Geriatr Soc. 2006;54:1062–1067.
Moolchan E, Ernst M, Henningfield J. A review of tobacco smoking in adolescents: treatment implications. J Am Acad Child Adolesc Psychiatry. 2000;39:682–693.
Moore BA, Augustson EM, Moser RP. Respiratory effects of marijuana and tobacco use in a U.S. sample. Gen Intern Med. 2004;20:33–37.
Murphy KR, Cecil B, Server NL. Helping patients breathe easier. Nurse Pract. 2004;29:39–57.
National Health and Medical Research Council (NHMRC). The health effects of passive smoking—a scientific information paper. Canberra: Commonwealth of Australia, 1997.
Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease. Lancet. 2004;364:613–620.
Rosenthal LD. Carbon monoxide poisoning. Am J Nurs. 2006;106:40–47.
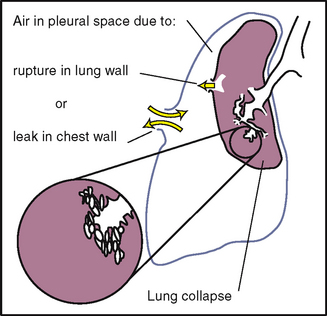
Ryan B. Pneumothorax. Cardiovasc Nurs. 2005;20:251–253.
Schneiderman H, Kagan JM. Resumption of cigarette smoking manifested by “tar” and nicotine staining of hair: the Kagan sign. Consultant. 2006;46:1489–1496.
Simon BM. Lung cancer diagnosis in primary care. Nurse Pract. 2007;32:43–49.
Steinbis S. What you should know about pulmonary hypertension. Nurse Pract. 2004;29:8–19.
Taylor MM. ARDS diagnosis and management. Dimensions Crit Care Nurs. 2005;24:197–207.
Turner L, Mermelstein R, Flay B. Individual and contextual influences on adolescent smoking. Ann NY Acad Sci. 2004;1021:175–197.
US Department of Health and Human Services. Reducing tobacco use. a report of the Surgeon General. Atlanta, Georgia: US Department of Health and Human Services, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2000.
Wright WL. Viral or acute bacterial rhinosinusitis? Determining the difference. Nurse Pract. 2005;30:31–43.