Chapter 3 The perioperative environment
After reading this chapter, you should be able to:
Introduction
The perioperative environment is a specially designed and regulated area that plays a significant role in patient safety during the surgical procedure. Incorporating special design features, such as positive pressure air conditioning, traffic patterns to restrict entry of contaminants from external sources of the hospital, easy-to-clean floor and wall surfaces, and electrical safety controls, all contribute to reduce the risk of infection to the surgical patient (Queensland Health, 2002). Safety features related to electrical equipment, radiation and laser further protect both patients and staff from hazards inherent in a highly technical environment.
This chapter explores the structural components of the operating suite and the impact that design features, including designated traffic patterns, control of temperature, humidity and ventilation systems, have on the safety of the patient. Occupational health and safety issues are explored, including those related to the use of electrosurgery, surgical plume, prevention of fire and explosion, latex allergy, occupational exposure, personal protective equipment and perioperative attire, radiation safety and safe chemical handling.
Operating suite design and layout
The operating suite design and layout must accommodate the day-to-day workload and the corresponding fluctuations in staff and patient numbers (Centre for Health Assets Australasia [CHAA], 2006). The operating suite is an environmentally controlled unit consisting of many distinct functional areas. It may be adjacent to a preadmission area (or perioperative unit), through which patients for day surgery and those who will be admitted for longer stays are admitted.
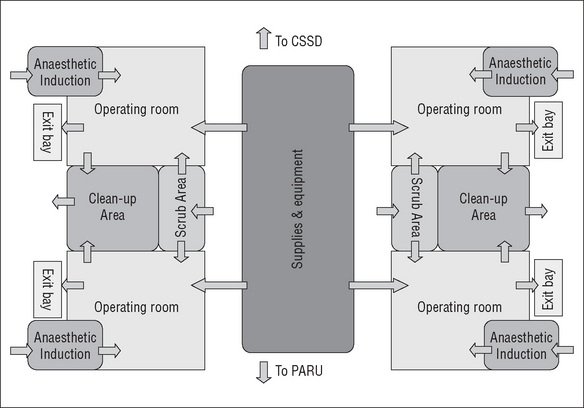
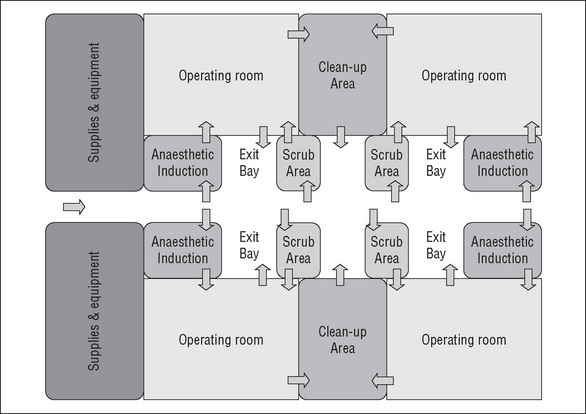
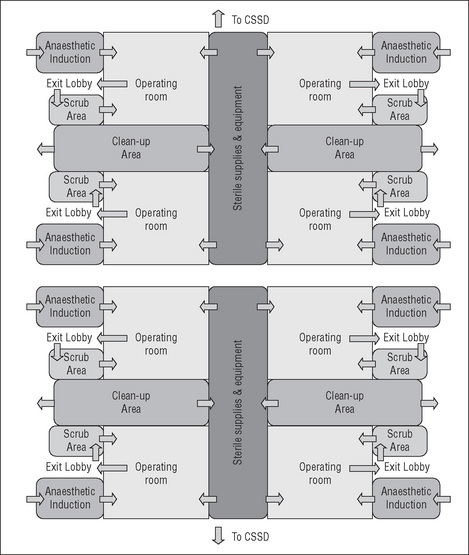
When designing the operating suite, the decision regarding the number of operating rooms and recovery spaces is governed by many factors, including the number, type and complexity of surgical procedures to be undertaken and the number of postoperative beds available. There are several design models for the operating suite layout that achieve a balance between the environmental needs of the staff, infection control, operational flow and functional requirements (CHAA, 2006).
Every Australian state and territory and New Zealand have their own set of building codes, infection control guidelines and capital works guidelines to assist with hospital design when new hospitals are being planned or refurbishments are being undertaken (Carthey, 2006). Australasian Health Facility guidelines were released in 2006 to assist Australian and New Zealand health departments undertaking health facility projects to achieve the standards for building, space, equipment, fit out and furnishings, and are the minimum standard for design (Carthey, 2007).
Some of the planning models for the operating suite include the following:
The operating suite should have close or direct links with other units for convenience, patient safety and practicality (CHAA, 2006). These units include the:
Traffic patterns
Traffic patterns are established to define movement throughout the operating suite for personnel, equipment, supplies and instrumentation, and to prevent contact from sources
of potential contamination. Ideally, waste, contaminated supplies and soiled instruments should not travel down the same corridor as clean and sterile supplies. However, if this is necessary due to the suite’s design, then measures must be undertaken to minimise any potential contamination of clean with dirty supplies (CHAA, 2006). Such a method could include using a sealed ‘closed cart’ system for transporting soiled and contaminated items to the sterilising department for decontamination.
Operating suite layout
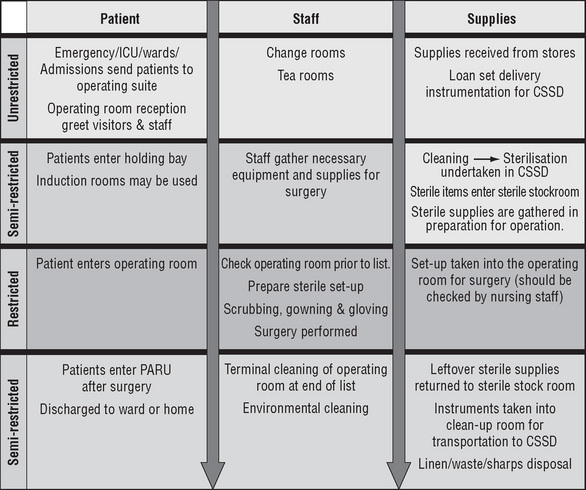
The Australian College of Operating Room Nurses (ACORN) classifies the perioperative environment into four zones—transition, unrestricted, semi-restricted and restricted— which can be defined by the activities performed.
Transition area
Change rooms
Secure designated male and female change rooms with lockers and a daily supply of laundered perioperative attire must be provided for the authorised staff (ACORN, 2006b).
Unrestricted areas
Reception
The reception area is an unrestricted area that does not require those accessing it to wear perioperative attire. The reception area is a place for patients, families and staff to access information, such as operating suite schedules, location of waiting areas and case bookings.
Preoperative holding bay
In the unrestricted areas there is unlimited access to all personnel, who may wear either perioperative attire or street clothes. The preoperative holding bay is a waiting area for patients prior to surgery where admission procedures, such as patient identification checks, are carried out, all documentation is confirmed and the responsibility for the patient is handed over by the ward or perioperative unit staff to perioperative nurses. A nurse is usually assigned to work in this area to monitor the patients’ condition and to help coordinate each operating room’s schedule by calling for patients from the wards/ perioperative unit in a timely manner to minimise delays (ACORN, 2006a).
Semi-restricted areas
The semi-restricted areas are limited to authorised personnel who are required to wear perioperative attire.
Anaesthetic rooms
Anaesthetic rooms are located directly next to the operating room and patients are transferred here from the holding bay when the anaesthetist is ready to prepare the patient for surgery. The anaesthetic nurse assigned to this area will take over the patient’s care and assist the anaesthetist in preparing the patient for surgery by commencing intravenous infusions or inserting local or regional anaesthesia. General anaesthesia may be induced in the anaesthetic room or the patient may be transferred onto the operating table for induction, depending on protocol and the anaesthetist’s preference.
Storage areas
Areas should be set aside within the operating suite to receive and decant bulk supplies before they are distributed to specialty storage areas within the suite. Consumables and specialised equipment, such as microscopes and laser machinery, must be stored in areas that are easily accessible from the operating rooms and anaesthetic bays.
Sterile stock room
Areas for storing wrapped, sterile supplies must be easily accessible from all operating rooms and may be contained in a central location. Sterile packages and trays are ideally stored on open wire shelving at least 250 mm above the floor and 440 mm from the ceiling. The open shelving allows dust to fall to the floor and permits cleaning to take place more effectively (ACORN, 2006f; Standards Australia, 2003, AS/NZS 4187).
To maximise storage space, many operating suites use mobile compactors. The sterile stock room must be kept cool and dry, with a temperature of 22–24°C and a relative humidity of 35–68% to prevent compromising the integrity of the sterile packages (ACORN 2006f). Sterile supplies need to be kept away from direct sunlight and, therefore, windows within this sterile stock area are not considered ideal (Standards Australia, 2003, AS/NZS 4187). The sterile stock room needs to be cleaned regularly and kept free of dust, vermin and insects (ACORN, 2006f).
Staff rooms
Because operating room staff wear perioperative attire, a staff room should be located within the operating suite where refreshments can be taken and staff can relax between procedures. Another area should also be designated a meeting/education room where staff meetings and in-service education can be conducted, and in which journals and textbooks, as well as access to the organisation’s intranet and to the internet, are available to staff for ongoing education purposes.
Postanaesthesia recovery unit
The PARU can also be classified as a semi-restricted area; however, the wearing of perioperative attire is at the discretion of each hospital or facility (ACORN, 2006c). As well as perioperative staff, it needs to be accessible to medical and other staff wearing street clothes in the event of an emergency (Australian and New Zealand College of Anaesthetists, 2006).
In the PARU, patients who have undergone anaesthesia and/or surgery are provided care, so the PARU needs to be located close to the operating/procedure rooms. The layout and design of the PARU needs to allow for good observation of all patients simultaneously and especially from the staff station when required. Curtained cubicles may allow privacy for patients while still maintaining a wide space, as private rooms are inappropriate in the PARU (CHAA, 2006).
Sterilising department
The sterilising department’s largest client will always be the operating suite and, therefore, it must be located within easy access to facilitate the passage of clean and dirty instruments between the two departments. Enclosed carts or containers with lids should be used to transport contaminated instruments to the sterilising department, and separate clean and dirty elevators should be used to transport instruments to and from the sterilising department if it is located on a different floor to the operating suite (CHAA, 2006).
The sterilising department layout must be clearly defined to distinguish the decontamination (dirty) and packaging/sterilising (clean) areas. Dirty instruments should be delivered directly into the cleaning or decontamination room for processing. All personnel must wear personal protective equipment while handling contaminated equipment, including protective ear wear as many instrument washers are noisy. After the instruments have been processed, they enter the clean side of the sterilising department, where specially trained staff check instruments, mark tray checklists, and package trays and instruments for sterilisation.
Restricted areas
Usually accessed from a semi-restricted area, the restricted areas include procedure and operating rooms, where staff must wear surgical attire and personal protective equipment (ACORN, 2006b).
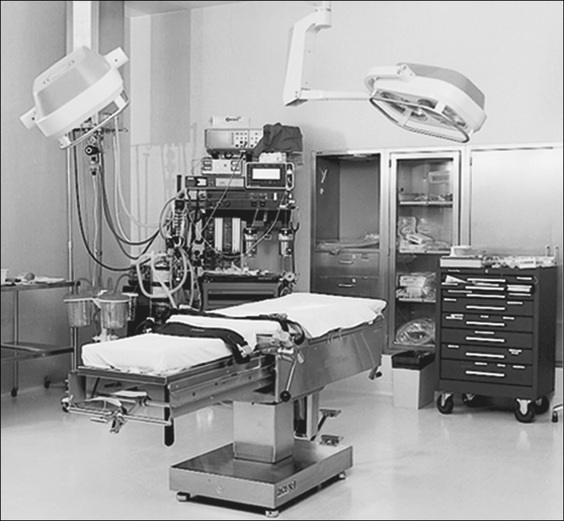
Operating rooms
The minimum size recommended for a general operating room is 42 square metres, whereas a large operating room (usually designated for cardiac, neurosurgery, orthopaedic joint or transplantation surgery) is 52 square metres (CHAA, 2006).
Dedicated operating rooms
Some hospitals, particularly larger tertiary hospitals, dedicate operating rooms to specialty surgery (e.g. neurosurgery, orthopaedic surgery). This is beneficial as it allows specialized equipment, such as microscopes and special operating room tables, to remain within the operating room, reducing the potential for damage due to movement, although fixed equipment may limit the flexibility of the operating room in smaller suites (ACORN, 2006b; CHAA 2006).
Integrated operating rooms
Highly specialised, integrated operating rooms have been built in some tertiary level hospitals. These are referred to as integrated or interventional operating rooms, where all the highly technical equipment is connected together (integrated) for easy use and where the surgeon has complete control of much of it from within the sterile field. Such equipment can include the operating room lights, camera, light source and insufflator.
Interventional operating rooms (also called endovascular operating rooms) are a cross between the cardiac catheter unit (angioplasty suite) and a regular operating room. This provides a safe environment for the radiologist or surgeon to perform angioplasty procedures, with the additional security of an operating room in the event that an operation becomes necessary. The interventional operating room has many television screens strategically placed for surgeon comfort or to facilitate the viewing of multiple X-ray images simultaneously. An intraoperative X-ray machine (‘C-arm’) can be either mobile or table-mounted to facilitate angioplasty procedures. The C-arm can slide up
and down the table to capture X-ray images anywhere along the length of the body. To accommodate the extra equipment and technology, the integrated operating room is usually double the size of a standard operating room, at about 80 square metres. With the increase in minimally invasive surgery, these highly specialised settings are likely to become the operating room of the future (Phillips, 2007).
Scrub areas
Scrub areas (or bays) provide sinks with running water and access to antiseptic solutions to accommodate several members of the surgical team undertaking the surgical scrub procedure simultaneously. To facilitate adherence to aseptic technique, these sinks need to be located directly outside the operating room to allow easy access to sterile gown and glove trolleys, which may be located in the scrub bay itself or within the operating room. To prevent slip injuries, the floor coverings in the scrub areas should be of a non-slip texture (CHAA, 2006).
Design features of the operating suite
Design considerations exist for the windows, ceilings, doors, floors and walls for the perioperative environment; they are necessitated by infection control practices and to accommodate environmental cleaning requirements.
Windows
Staff morale is always boosted by natural light, and having windows within the operating suite is recommended (ACORN, 2006b). However, it is not always practical to have windows in the operating room itself, where procedures such as minimally invasive surgery may require a darkened theatre to maximise the visual image on the screen. Also, when lasers are used, appropriate protective window coverings within the operating room must be provided (Queensland Health, 2002).
Ceilings, doors, floors and walls
Ceilings within the perioperative environment should be made of a non-reflective, non-porous material without cracks or open joins that permit the accumulation of dirt and are difficult to clean. Light fittings must be flush fitting and sealed to inhibit any dust or dirt entering the environment (CHAA, 2006). Swing-type doors for the operating room allow easy access for hands-free entry (Abreu & Potter, 2001). Consideration should be given to fitting the lower section of the door (from the floor edge up to 150 mm) with a thin layer of aluminium to protect it from constant battering and wetness due to frequent floor mopping. Floors are generally made of seamless vinyl that is impervious to moisture, easily cleaned, stain resistant, comfortable for long periods of standing and suitable for wheeled traffic (CHAA, 2006). Walls are also made of seamless vinyl and curved where they meet the floor to assist in effective cleaning. The colour should be neutral with a matt or satin finish to reduce glare, which can be disturbing to the surgical team (Gruendemann & Mangum, 2001). Tiles, once a popular wall covering, are no longer considered a suitable finish within an operating room due to the potential for cracking, which poses infection control risks (CHAA, 2006). Due to the high traffic of trolleys in the unit, damage to walls and doors is inevitable and, therefore, consideration should be given for wall and corner protection (CHAA, 2006).
Other features of the operating suite
The operating suite utilises many features to create and maintain a safe, clean environment for patients. These measures include the ability to set parameters for temperature, humidity and ventilation systems.
Temperature
The temperature range within the operating suite should be 18–24°C. Individual operating rooms should have a temperature range of 20–22°C; this temperature range inhibits bacterial growth and is tolerated well by both staff and patients (Gruendemann & Mangum, 2001). However, the temperature may require adjusting to accommodate some types of surgery and/or the condition of individual patients. For example, burns or paediatric patients require higher ambient temperatures as these patients are highly susceptible to hypothermia. In contrast, some types of neurosurgical patients require a much cooler environment.
Humidity
The humidity in the operating room should be maintained at 50–60% to both inhibit bacterial growth and decrease risks associated with static electricity. Additionally, high humidity can increase fatigue among the surgical team and is thus best avoided (ACORN, 2006b; Rothrock, 2007).
Ventilation
The three types of air-conditioning systems used within the perioperative environment are listed below.
Air-conditioning systems within the operating suite use positive pressure, which pushes air down from within the operating suite and out into the external environment. Positive air pressure is greater in the operating room than the surrounding corridors and scrub areas, with the exception of the sterile stock room, which has the same air pressure as inside the operating room (Queensland Health, 2002). This positive pressure forces air from the operating room out into the corridors, resulting in the air in the outer corridors having a higher microbial count. For this reason, operating room doors must be kept closed at all times (other than the necessary passage of staff, supplies and the patient) to reduce the risk of airborne contaminants entering the surgical field. The microbial count within the individual operating room is usually at its peak during the time of the skin incision because this follows a period of maximum air disturbance created by staff gloving and gowning, patient draping, the movement of operating room staff and the frequent opening and closing of the operating room doors. Because the microbial count rises every time the operating room doors are opened, it is necessary to ensure all required supplies are readily at hand (within the operating room, generally) during the course of any surgical procedure (Phillips, 2007; Woodhead & Wicker, 2005).
Electrical safety
The large amount of electrical equipment used in an operating suite places both staff and patients at potential risk of electrocution should equipment become faulty or be mishandled by staff. A range of safety features are incorporated into the design of operating suites to reduce the risk of electrocution. These include devices such as line isolation monitoring (LIM) panels and, within each operating room, residual current devices (RCD), which indicate faulty equipment or leakage of electrical current by initiating an audible alarm, along with activating warning lights on the LIM panel; associated circuit breakers subsequently interrupt supply for the RCD.
All electrical equipment must be checked by the hospital’s biomedical engineering department prior to installation and, thereafter, at regular intervals to ensure they are functioning safely and meet national standards. Staff should check each piece of electrical equipment prior to use to ensure correct functioning, including inspection of electrical cords for any damage. Extension cords are not used within operating rooms due to the danger they pose to patients—of accidental dislodgement—and the occupational health and safety risk for staff, who may trip over the cords (Standards Australia, 2004a, AS/NZS 2500).
There are two main types of electrical shock against which staff and patients must be protected.
Electrosurgical equipment
The electrosurgical unit (ESU), generally referred to as the diathermy machine, is a commonly used electrical device within the operating room. The ESU generates an electrical current at extremely high frequency, which cuts or coagulates tissue (as well as variations of the latter, such as tissue desiccation or fulguration) or ‘diathermies it’, thus generating a bloodless field.
There are two main types of diathermy—monopolar and bipolar—both of which have unique applications.
Monopolar diathermy
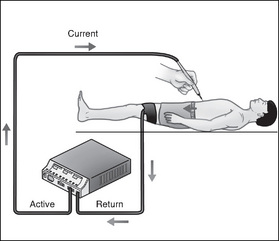
In monopolar diathermy, the current is usually applied to the tissue through the use of a small hand-held electrode, termed the ‘active’ electrode (Fig 3-7). This may be in the form of a ‘diathermy’ pencil, blade, forceps, needlepoint, loop or a ball depending on the surgery being performed. The surgeon activates the current by means of a switch on the ‘pencil’ to cut or coagulate tissue. The current then flows through the patient to the dispersive electrode pad, previously applied to the patient’s body, and back to the ESU, thus completing the electrical circuit. The dispersive electrode is usually an adhesive pad that is covered with conductive gel, which is attached to a muscular area of the patient (e.g. thigh or buttocks); it is used to safely disperse the electrical current (Standards Australia, 2004a, AS/NZS 2500). This is further discussed below.
Bipolar diathermy
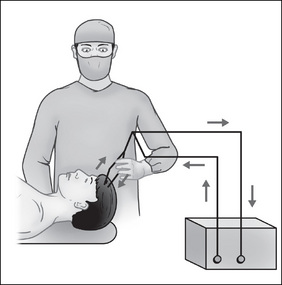
Bipolar diathermy uses a fine pair of forceps controlled by the surgeon via a foot pedal. It is mainly used for coagulation in plastic or paediatric surgery, where the vessels to be coagulated are minor (Standards Australia, 2004a, AS/NZS 2500). In this mode, the current from the ESU flows down one tyne of the forceps across the tissue that is grasped between the forceps and returns to the generator through the opposing tyne (Fig 3-8). This mode does not require a dispersive electrode pad (’patient plate’).
Hazards of electrosurgery
In addition to the risk of electrocution, burns to the patient may result if the dispersive electrode pad has insufficient contact with the patient’s skin. Depending on local policy, the dispersive electrode pad is applied to the patient either by a nurse or an orderly. If it is the latter person’s responsibility, the circulating or instrument nurse must check the placement and also the condition of the patient’s skin when the pad is removed at the conclusion of the procedure. Any damage to the patient’s skin must be reported, documented and appropriate treatment instigated. When applying the dispersive electrode pad, the following safety points must be followed:
If the current finds an alternative pathway back to earth through wet drapes, via faulty leads, electrodes or cables, or via other contact of the patient’s skin with metal (e.g. a hand touching the frame of the operating table), the patient may suffer burns. Burns to surgical or nursing staff may also occur if the current used finds an alternate pathway back to the ESU (e.g. through a hole in the glove of the person applying the active electrode, or in cases where the insulation on an endoscopic forceps is faulty). All ESUs have inbuilt alarm systems to disable faulty equipment and alert staff to situations where the dispersive electrode has become detached from the patient (Standards Australia, 2004a, AS/NZS 2500).
Another hazard of electrosurgery is the generation of smoke during tissue coagulation. The smoke plume has been shown to contain a number of dangerous toxins, carcinogens and viruses (Anderson, 2004). The surgical team in the immediate vicinity of the plume are at risk of inhaling surgical smoke for which the surgical mask provides little protection. Consideration should be given to the use of special smoke evacuation units, which suck the smoke via devices attached to the active electrode into an evacuation unit that is external to the sterile field. These units contain charcoal filters, which remove the toxic gases and odours (Ball, 2007). The wearing of well-fitting, high-filtration face masks by surgical personnel is also recommended during procedures where there is the likelihood of smoke being generated (e.g. orthopaedic or breast surgery) (Standards Australia, 2004b, AS/NZS 4173).
Related equipment
In recent years, ESUs have been developed that incorporate enhanced haemostatic features. Argon-enhanced electrosurgery combines argon gas with electrosurgery to improve the effectiveness of the coagulation mode by clearing blood more quickly from the surgical site and allowing the surgeon greater visibility. There is also less surgical smoke generated, making it a safer alternative to electrosurgery (Ball, 2007).
Another advancement in haemostasis is the use of ultrasonic technology, which uses high-frequency sound waves to cut and coagulate tissue (Johnson & Johnson Gateway, 2007). A transducer held by the surgeon converts electrical energy into mechanical vibration, which vaporises tissue in a very precise manner, resulting in less damage to surrounding tissue, no smoke, and none of the dangers associated with electrical currents as seen with electrosurgery (Ball, 2007).
Laser
The term laser is an acronym for ‘light amplification by stimulated emission of radiation’. In simple terms, the type of light emitted by a laser differs from natural light because the latter is made up of many different colours, as can be demonstrated by the use of a prism, all colours being bent at different angles. Laser light, on the other hand, is all one colour, being all one wavelength, all bent at the same angle because of the way it is produced and given off. Depending upon the wavelength, different laser beams are absorbed by different media. The wavelength of many of these beams of laser light is not visible, which adds to the potential for injury (Standards Australia, 2004b, AS/NZS 4173).
Laser energy can be delivered in variations of either continuous or pulsed modes. The effects on tissue at which the laser beam is aimed include thermal, photodisruptive, electromechanical and photochemical effects. These effects may be deep or shallow, narrow or widespread, depending upon the way the beam is delivered, the wavelength of the beam and the qualities (including the colour) of the tissue onto which it is directed (Standards Australia, 2004b, AS/NZS 4173). The uses for laser are many and varied. For example, in ophthalmology, the neodymium:YAG (Nd:YAG) laser beam can rupture a clouded membrane in the eye; cosmetic surgeons may use the erbium:YAG laser to resurface the skin; an ear, nose and throat surgeon may use a carbon dioxide laser to remove a tumour from the vocal cords; and a urologist may use a holmium:YAG laser to ablate a ureteric calculi (Ball, 2007).
Hazards of laser
Lasers used in the perioperative environment are referred to as class 3b or class 4 medical lasers. These lasers have the potential to cause eye or skin damage if appropriate protection is not employed, and should therefore be used with extreme caution. Accidental deflection of the laser beam by polished instrumentation may cause injury, and broken fibres from fibreoptic cables can cause damage where they escape from protective casing. In every health care facility it is vital that only personnel who have undergone recognised training and are familiar with the features, operation and specific safety requirements of the machine in use operate the laser (Standards Australia, 2004b, AS/NZS 4173).
Because lasers are potentially so dangerous, strict adherence to prescribed practices and protocols are essential. For each laser there is a ‘nominal ocular hazard area’ (NOHA), which is the area in which the laser beam can potentially cause damage to the cornea, including through windows (when the wavelength is not absorbed by the window itself) and when the beam is accidentally misdirected. All personnel in the NOHA must wear the appropriate eye protection (Figure 3-9), and there must be warning signs on doors and windows, both of which are screened where necessary for the protection of other personnel.
Where laser procedures are performed with the use of fibreoptic cables, these should be carefully inspected prior to use and during handling to ensure individual fibres are not broken and therefore not emitting stray laser beams (Standards Australia, 2004b, AS/NZS 4173).
Laser surgery also generates smoke plume and similar safety precautions, such as those described for electrosurgery, should be taken (Ball, 2007).
Technology and the operating suite
The worldwide boom in technology that has been witnessed over the past several decades has revolutionised the way surgical procedures are being carried out. Minimally invasive or ‘keyhole’ surgery was first introduced in the late 1980s and has now become the preferred option for many common operations that were once routinely performed as ‘open’ procedures (e.g. cholecystectomy, appendicectomy). Endoscopic technology is ever increasing, with most organs of the body accessible via a minimally invasive route. Advantages to the patient include reduced length of surgery and anaesthesia, shorter hospital stay and quicker return to normal life (Ball, 2007). While it is beyond the scope of this text to discuss all the latest technology, an overview is essential as this type of surgery presents new challenges, both for surgeons and perioperative nurses, to learn new skills and become involved in advancing technologies.
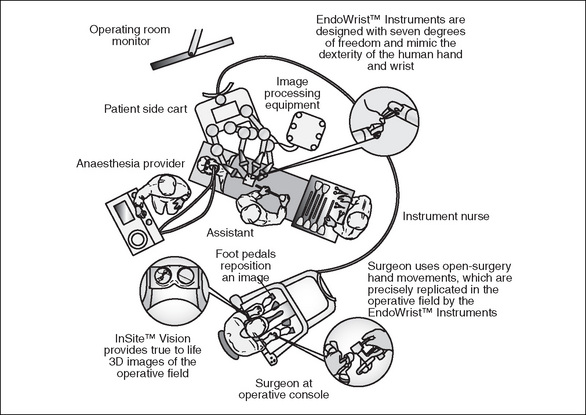
Robotics is perhaps the most advanced of the latest technologies and, even though it is only available in larger hospitals, has potential to further revolutionise minimally invasive surgery. Devices such as the da Vinci robot (Figure 3-10) use mechanical ‘arms’ to hold and manoeuvre endoscopic instruments and cameras more steadily than the human hand. The robotic devices are controlled by a surgeon sitting at a console away from the operating table, viewing the operative site through a three-dimensional (3D) viewer and manipulating the robotic instrumentation via precision hand-held controls. The advantages of undertaking surgery via this method are greater concentration levels for the surgeon, enhanced 3D visualisation of the surgical anatomy and greater precision as hand tremor is eliminated; in addition, the robotic arms have greater manoeuvrability than the human wrist (Ball, 2007).
Performing surgery using this modality has enormous potential, as a surgeon sitting at the console in one hospital can perform surgery on a patient in another hospital. It is thought that in the future this could have application for space exploration—the ultimate in remote surgery (Ball, 2007).
Preparation of the operating room
Prior to commencing work within the operating suite each day, the nurses assigned to the operating room must carry out a full check of the equipment and, by visual inspection, ensure items such as room and operating lights, trolleys, the operating table, ESU, suction equipment, linen skips and rubbish bags are present, in working order and are clean. All tables, trolleys and horizontal surfaces within the operating room are wiped over with neutral detergent to remove any dust that has settled overnight. The equipment within the theatre should be checked and, if any item is missing or faulty, corrective action should be taken before the patient enters the operating room.
The anaesthetic, circulating and instrument nurses should discuss any specific requirements for the patients on the operating list. Any additional equipment required for specific procedures (e.g. positioning equipment, microscopes) must be acquired and checked prior to commencement of surgery (ACORN, 2006a).
Room cleaning
In between each case, all rubbish and linen must be removed from the operating room and spot cleaning of any blood and body fluid spills undertaken with an approved hospital cleaning agent. Wiping trolleys and tables and cleaning contaminated furniture, equipment, floors, walls and lights also needs to be undertaken between each case. Any equipment that has been used during an operation and not required for subsequent procedures should be cleaned and returned to its storage area. These activities should be carried out by orderlies or other assistive personnel under the direction of the nursing staff.
Terminal cleaning
After the completion of the surgical procedures for the day, the operating room should be terminally cleaned. This includes a thorough cleaning of the equipment within the operating room itself, scrub areas and corridors in the immediate area that have been used during the day. All cleaning should be undertaken with an approved hospital cleaning agent, using lint-free cloths and mechanical friction to remove contamination and debris. Depending on individual local operational protocols, this cleaning is usually carried out by designated cleaning staff; however, it remains the responsibility of perioperative nurses to ensure that appropriate cleaning has taken place.
After the operating room and surrounding areas have been cleaned and tidied, the necessary restocking should be undertaken and all furniture and equipment placed in its usual position in anticipation for the next patient. This is particularly relevant for operating room readiness for emergency surgery (or disaster preparedness). In addition to cleaning between procedures and terminal cleaning, a strict schedule of periodic environmental cleaning is carried out according to hospital and infection control protocols (ACORN, 2006d).
Waste management
Correct segregation of waste is essential for infection control purposes and also in managing the volume and expense caused by the generation of clinical waste within the hospital. Staff can assist by being educated in waste management policies and consistent in placing waste in the most appropriate receptacle. Waste can be divided into:
Occupational health and safety
In addition to the hazards associated with the use of electricity, diathermy and laser, there are other hazards that are particular to the perioperative environment. Perioperative nurses must be aware of these risks for the protection of self, patients and other personnel.
Manual handling
The transfer and positioning of patients and the movement of equipment are activities with which all staff working within the operating suite will be involved and there is a need for awareness of the potential hazards that manual handling may pose. Mandatory education must be provided in manual handling techniques and injury prevention, for both patients and staff. Keeping a level of fitness and suppleness is recommended for all staff to prevent back and shoulder injuries, which are the most commonly suffered injuries by staff (Meijsen & Knibbe, 2007). Transferring and positioning patients are activities that must involve a team of people working in a coordinated manner to ensure the safety of all involved (NSW Health, 2005a). Devices to assist with the transfer of patients include slide sheets and boards (pat slides or rollers). In some hospitals, devices such as hover mats are also available, which are inflatable mattresses that are specially designed to transfer patients easily to and from the operating table with minimal manual handling requirements.
Prevention of fire or explosion
The risks of fire and explosion are high in the perioperative environment because oxygen and other flammable gases are in abundant supply due to anaesthesia requirements. There are many flammable materials available to fuel any fire, such as drapes, sponges and packaging, and the main ignition sources are electrosurgical or laser equipment. When alcoholic skin preparations are used, special care must be taken as they add an additional dangerous element, that of a known fire accelerant. This includes ensuring that the operative area is allowed to dry following application of the skin preparation solution and before the drapes are applied; and preventing solution soaking into hair or under pads (NSW Health, 2007). Although rare, the potential also exists for explosion when diathermy is used on hollow organs containing flammable gases, such as the bowel (SA Health Department, 2007; Standards Australia, 2004a, AS/NZS 2500).
Some strategies to reduce the risks to patients and staff are the use of moistened sponges around the operative site, venting the drapes during head and neck surgery or ophthalmic surgery to allow gases to escape the head area, and keeping the ‘diathermy pencil’ in a quiver/holster to avoid accidental activation (Rothrock, 2007). Knowledge of fire evacuation policies is also vital and many operating suites conduct annual fire evacuation exercises.
Box 3-1 Fire in the operating room
Although fires in the operating room are a rare event, they do still happen. In 2002 in New Zealand, a fire occurred during a caesarean section. The cause of the fire was directly related to the alcohol vapours that were released from the pooled skin preparation solution, and which had accumulated under the fire-retardant drapes. The correctly functioning ESU was the source of ignition. The patient received full thickness burns to 16% of her body. The severity of her injury was worsened by the intensity of the fire due to pooling of the alcoholic solution and its accelerant properties. No injury occurred to the staff present, whose quick actions prevented further injury to all present. The fetus was also unharmed.
Latex allergy
Natural rubber latex gloves are made from the milky sap from the Hevea brasiliensis tree. Because of the qualities of natural latex, it is used extensively in many industries, particularly health care, where it is most widely used as latex gloves. This is because the product is inexpensive, provides an excellent barrier that protects the patient and the wearer from exposure to potentially harmful infective agents, and provides good tactile properties for the wearer. When natural rubber latex is processed into gloves, many of the latex proteins are lost, and the remaining extractable proteins are those that are implicated in latex allergy.
In the 1980s and earlier, the protein content in the gloves was higher than today, and powder was applied to prevent the sides from sticking together and to facilitate easier donning. The proteins in the gloves adhered to the powder, which became airborne when the gloves were donned or removed. The proteins were then inhaled and spread through the air. The exposure of health care workers to latex proteins in this environment was, therefore, very high, and evidence suggests that there is a correlation between the use of atex products and latex allergy among health care professionals.
Powdered gloves are now rarely worn in health care environments, and advances are onstantly being made to lower the protein levels and the subsequent allergen content of atex gloves and to find ways to make them easier to don without reducing the tactility of the product (NSW Health, 2005b; Reed, 2003; Yip, 2003). Latex allergy among patients and health care workers has also increased markedly since the introduction of (initially) universal, now standard precautions, which have resulted in the increased requirement to wear gloves for every patient contact.
Types of latex allergy
Allergic reactions to latex can be wide-ranging. There are three types of reactions:
Box 3-2 Risk of developing a latex allergy
Have you experienced a severe reaction to avocado, bananas, kiwi fruit, or potatoes? If so, you are at greater risk of developing latex allergy. You are also more likely to suffer from a reaction if you suffer from conditions such as hay fever, hives or asthma or general atopia. You are more likely to develop a type I reaction if you have had repeated exposure of mucous membrane tissue to latex, such as frequent surgery, and as a health care worker if you frequently wear latex gloves or are exposed to the glove powder from powdered latex gloves.
Prevention of latex allergy
There is no known cure for latex allergy and so prevention is the key. The most effective way to prevent latex allergy is to ensure that only powder-free or latex-free gloves are used to prevent further sensitisation (ASCIA, 2005). In addition, it may be helpful to ensure hands are washed after wearing latex gloves. Hands should be kept free of abrasions and sores wherever possible, and barrier protection should be employed using a water-based hand care product rather than oil-based hand care products. If symptoms of latex allergy do occur, then synthetic gloves must be used and all contact with items containing latex should be avoided (ASCIA, 2005). This is difficult for perioperative nurses and there have been instances where those with latex allergies have been required to find work in an alternative environment. Box 3-3 provides a case study of a rather disturbing and extreme latex reaction which has changed one Australian nurse’s life dramatically.
One nurse’s experience with latex allergy began soon after she commenced practice in the perioperative environment. Within 6 months she had developed allergic dermatitis on her hands. At first she did not think too much of it and wore non-latex gloves only when she had a rash. When she began to develop generalised itching and wheals over her body, she presumed she had developed an allergy to hospital-laundered clothes. She accepted a position that involved less clinical work, but even so she began to get severe headaches and extreme fatigue at work and also became quite irritable. The symptoms suddenly escalated one day, about 7 years after commencing work in the perioperative environment. A rash began on her hands and soon developed into generalised itching, her head ached and she became extremely irritable. Then she began to experience a tight, crushing feeling in her chest, a hoarse voice and then a very hot flush. Her colleagues reacted quickly and administered treatment and she was able to go home that night, although her life was never the same.
While the organisation for which she works and its senior nursing executive have been very supportive, she has experienced financial, professional, personal and emotional ramifications. In addition:
Anonymous personal communication (2007)
Latex-sensitive patient precautions
Patients with latex sensitivity must be cared for in an environment free from contact with latex products. All operating suites should have designated ‘latex-free’ kits containing all the equipment necessary to care for a latex-sensitive patient. Requirements for caring for the latex-sensitive patient include:
Occupational exposure
Protection against exposure to infectious agents is discussed in Chapter 5. This section discusses protection against sharps injuries and exposure to chemical and radiation hazards.
Sharps injury
During perioperative procedures, potentially dangerous items are constantly being handled and passed between members of the surgical team. They include obvious items such as intravenous needles, suture needles and scalpel blades, but also less obvious items, such as retractors (e.g. cats paws and skin hooks) and even sharp body pieces, such as splintered bone.
The likelihood of sharps injury increases during highly invasive, long procedures, with team member fatigue and fast pace also adding to the risk. Procedures identified as high risk also include those where the surgical team are operating in poorly visualised or confined spaces, such as the mouth or deep body cavities, and during some orthopaedic procedures (ACORN, 2006e; Girard, 2004; Silēn-Lipponen et al., 2005).
Some points to reduce the risk of injury include:
Radiation safety
There are two types of radiation—non-ionising and ionising. Non-ionising radiation is electromagnetic radiation and is emitted from electrical sources, such as power lines, from radiofrequency sources, such as mobile phones, and from the major ultraviolet source, the sun. Ionising radiation can be divided into two subgroups: non-harmful alpha, beta and neutron particles, with exposure through activities of normal living; and gamma rays and X-rays, which can penetrate deeper into tissue and can be harmful to humans. Metals such as lead are used to protect against these harmful rays (Australian Radiation Protection And Nuclear Safety Agency [ARPANSA], 2003).
Exposure to mechanical sources of ionising radiation that emit X-rays in the perioperative environment is often unavoidable, such as when image intensification (fluoroscopy) is used intraoperatively or when mobile radiography units are used to check the placement of internally placed appliances. Because the effects of ionising radiation are cumulative but at the same time are not seen or felt, constant vigilance is required to ensure occupational exposure is kept to a minimum. The single most effective protection against exposure is avoidance (i.e. leaving the vicinity of the radiation source). If this is not possible, then standing behind mobile lead shields or using the equation—double the distance away from the source of radiation equals one-quarter of the exposure—applies (Phillips, 2007).
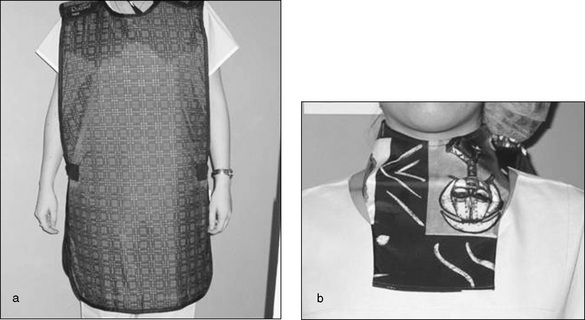
This may not be possible for some members of the surgical team and, when it is necessary to remain in the room, appropriate X-ray protective apparel that meets national standards, as described in Standards Australia AS/NZS 4543 (2000), must be worn, including protective gowns, thyroid shields, goggles and gloves. Whenever the gown is removed, it should be hung on special gown hangers or laid out flat, as any folds can crack the protective barrier and render it ineffective. Protective equipment must undergo regular checks to ensure its integrity has been maintained (Phillips, 2007). Staff regularly involved in patient screening may wear a dose meter badge that is regularly checked to identify if occupational exposure limits are exceeded (ARPANSA, 2003).
Where a member of staff is pregnant, the fetus should not be exposed to any more radiation than a member of the general public would be and the staff member should not be required to be in the operating room wherever possible (NOHSC, 2002).
Chemical safety
Many chemicals are used in the operating suite, including the detergents used to clean between cases, enzymatic cleaners used on instruments, high-grade disinfectants or chemical sterilising products, such as peracetic acid, and drugs, such as cytotoxic agents. Some basic principles always apply when handling chemical agents:
Conclusion
The perioperative environment is a complex and challenging one, where a variety of activities and design measures aid in maintaining a clean and safe environment for the patient undergoing surgery and the staff who work in this area. These measures include a well-planned operating suite with designated traffic patterns, environmentally controlled conditions and cleaning schedules. Advancing technological and electrosurgical equipment and many hazardous substances that staff are exposed to in the operating suite can pose risks to patients and staff. Safety is paramount with the use of equipment, and staff require an understanding of how, when and why equipment is used to ensure safe practice. Finally, occupational health and safety issues for both the patient and the health care worker are essential elements for consideration in the perioperative environment.
Critical thinking exercises
2 Electrical safety
Australasian Healthcare Facility Guidelines www.healthfacilityguidelines.com.au.
Australasian Society of Clinical Immunology and Allergy www.allergy.org.au.
Australian Department of Health and Ageing www.health.gov.au
Australian Radiation Protection and Nuclear Safety Agency www.arpansa.gov.au
NZNO National Division of Infection Control Nurses www.infectioncontrol.co.nz
Royal Children’s Hospital, Melbourne, Biomedical Engineering www.rch.org.au/bme_rch/safety.cfm
Standards Australia www.standards.org.au
Abreu E., Potter D. Recommendations for renovating an operating theater at an emergency obstetric care facility. International Journal of Gynecology & Obstetrics. 2001;75:287-294.
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards. G2. Management of the perioperative environment. Adelaide: Australian College of Operating Room Nurses; 2006.
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards. G3. Planning and design of the perioperative environment. Adelaide: Australian College of Operating Room Nurses; 2006.
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards G4. Management of post anaesthesia recovery (PAR) unit. Adelaide: Australian College of Operating Room Nurses; 2006.
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards. S6. Environmental management. Adelaide: Australian College of Operating Room Nurses; 2006.
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards. S7. Infection prevention. Adelaide: Australian College of Operating Room Nurses; 2006.
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards. S16. Reprocessing of reusable items: cleaning, packaging, sterilisation and storage of sterile supplies. Adelaide: Australian College of Operating Room Nurses; 2006.
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards. S22. Use of high level disinfectant in the perioperative environment. Adelaide: Australian College of Operating Room Nurses; 2006.
Anderson K. Safe use of lasers in the operating room—what perioperative nurses should know. AORN Journal. 79(1), 2004. 171–172, 174, 176–182
Australian and New Zealand College of Anaesthetists. (2006). Professional documents of the Australian and New Zealand College of Anaesthetists. PS4. Recommendations for the post anaesthesia recovery room. Retrieved October 10, 2007, from http://www.anzca.edu.au/infocentres/pdfdocs/PS4-2006.pdf10.
Australian Department of Health and Ageing. (2004). Infection control guidelines for the prevention of transmission of infectious diseases in the health care setting. Retrieved October 27, 2007, from http://www.health.gov.au/internet/wcms/Publishing.nsf/Content/icg-guidelines-index.htm/$FILE/howto.pdf.
Australian Radiation Protection and Nuclear Safety Agency. (2003). Ionising radiation and health. Retrieved October 27, 2007, from http://www.arpansa.gov.au/radiationprotection/FactSheets/is_rad.cfm.
Australian Society of Clinical Immunology and Allergy. (2005). Guidelines for the hospital management of latex-allergic patients. Retrieved October 10, 2007, from http://www.health.gov.au/internet/wcms/Publishing.nsf/Content/icg-guidelines-index.htm/$FILE/howto.pdf.
Australian Radiation Protection and Nuclear Safety Agency. (2003). Ionising radiation and health. Retrieved October 27, 2007, from http://www.arpansa.gov.au/radiationprotection/FactSheets/is_rad.cfm.
Australian Society of Clinical Immunology and Allergy. (2005). Guidelines for the hospital management of latex-allergic patients. Retrieved October 10, 2007, from www.allergy.org.au.
Ball K. Surgical modalities. In Rothrock J.C., editor: Alexander’s care of the patient in surgery, 13th ed., Louis: St Mosby, 2007. (pp. 183–227)
Carthey, J. (2006). Australian e-guidelines for health facility design. In A. Dilani (Ed.). Design and health IV. Future trends in healthcare design (pp. 227–34). Stockholm: International Academy for Design & Health. Retrieved October 29, 2007, from http://www.fbe.unsw.edu.au/chaa/downloads/Papers_Presentations/2006_Carthey_AustralianE-Guidelines.pdf.
Carthey, J. (2007). Guiding quality design. Hospital & Healthcare, March, 21–3. Retrieved October 14, 2007, from http://www.fbe.unsw.edu.au/chaa/downloads/Papers_Presentations/2007_Carthey_GuidingQualityDesign.pdf.
Centre for Health Assets Australasia. (2006). Australasian healthcare facility guidelines. Retrieved October 10, 2007, from www.healthfacilityguidelines.com.au.
Davis B. Perioperative care of patients with latex allergy. AORN Journal. 2000;72:47-53.
Girard N. Countdown to safety. AORN Journal. 2004;79(3):575-576.
Gruendemann B., Mangum S. Infection control in the surgical settings. Philadelphia: Elsevier; 2001.
Hauff, W. (2002). OR suite planning concepts. Retrieved October 15, 2007, from http://www.ordesignandconstruction.com/dp/concepts.htm.
Johnson & Johnson Gateway. (2007). Harmonic scalpel—technology overview. Retrieved October 26, 2007, from www.jnjgateway.com.
Lewis S., Heitkemper M., Dirksen S., et al, editors. Medical-surgical nursing: assessment and management of clinical problems, (7th ed.), St Louis: Elsevier, 2007.
Meijsen P., Knibbe H. Work-related musculoskeletal disorders of perioperative personnel in the Netherlands. AORN Journal. 86(2), 2007. 193–194, 196–198, 200–208
NOHSC. (1995; republished March 2002). Recommendations for limiting exposure to ionizing radiation: 3022. Retrieved October 25, 2007, from www.ascc.gov.au.
NSW Health. (2005a). Manual handling incidents—NSW Public Health Services—policy/best practice guidelines prevention. Sydney: NSW Government, PD2005_224.
NSW Health. (2005b). Latex allergy—policy framework and guidelines for the prevention and management. Sydney: NSW Government, PD2005_490.
NSW Health. (2007). Alcohol-based skin preparations and fire in the operating theatre safety information. Sydney: NSW Government, SI:001/07.
Phillips N. Berry & Kohn’s operating room technique, (11th ed.). St Louis: Mosby; 2007.
Queensland Health. (2002). Capital works guidelines building and refurbishment: infection control guidelines. Retrieved October 10, 2007, from http://www.health.qld.gov.au/cwamb/cwguide/InfectionGuide.pdf.
Queensland Health. (2007). Waste management: waste segregation procedure. Retrieved October 24, 2007, from http://qheps.health.qld.gov.au/PHS/Documents/ehu/manual/31631.pdf.
Reed D. Update on latex allergy among health care personnel. AORN Journal. 78(3), 2003. 409–412, 416–420, 422–426
Rothrock J.C. Alexander’s care of the patient in surgery, (13th ed.). St Louis: Mosby; 2007.
Royal Children’s Hospital, Melbourne. (2008). Biomedical engineering—electrical safety. Retrieved January 2, 2007, from http://www.rch.org.au/bme_rch/safety.cfm.
SA Health Department. (2007). Surgical fire prevention and management. Guidance Notice no. GI 02/07. Adelaide: SA Health Department.
Shoup A., Reilly M., Kless J. Nursing management: intraoperative care. In Brown D., Edwards H., editors: Lewis’s medical–surgical nursing. Assessment and management of clinical problems, (2nd ed.), Sydney: Elsevier, 2008. (p. 394)
Silēn-Lipponen M., Tossavainen K., Turunen H., Smith A. Potential errors and their prevention in operating room teamwork as expressed by Finnish, British and American Nurses. International Journal of Nursing Practice. 2005;11:21-32.
Standards Australia. (2000). AS/NZS 4543 Protective devices against diagnostic medical X-radiation. Sydney: Standards Australia.
Standards Australia. (2003). AS/NZS 4187 Cleaning, disinfecting and sterilizing reusable medical and surgical instruments and equipment, and maintenance of associated environments in health care facilities. Sydney: Standards Australia.
Standards Australia. (2004a). AS/NZS 2500 Guide to the safe use of electricity in patient care. Sydney: Standards Australia.
Standards Australia. (2004b). AS/NZS 4173 Guide to the safe use of lasers in health care. Sydney: Standards Australia.
Waitemata District Health Board. (2002). Report into the operating fire incident. Retrieved October 25, 2007, from http://www.medsafe.govt.nz/downloads/alertWaitemata.pdf.
Woodhead K., Wicker P. A textbook of perioperative care. Sydney: Elsevier; 2005.
Yip E. Accommodating latex allergy concerns in surgical settings. AORN Journal. 2003;78(4):595-596.
Bayley G., McIndoe A. Fires and explosion. Anaesthesia & Intensive Care Medicine. 2004;5(11):364-366.
Beesley J., Taylor L. Reducing the risk of surgical fires: are you assessing the risk? Journal of Perioperative Practice. 2006;16(12):591-597.
Cunnington J. Waste responsibility: or wasted opportunity. Journal of Perioperative Practice. 2006;16(10):476-480.
Francis P. Evolution of robotics and implementing a perioperative robotics nurse specialist role. AORN Journal. 2006;83(3):630-632. 634–642, 644–646, 649–650
Heath J., Johanson W., Blake N. Healthy work environments: a validation of the literature. Journal of Nursing Administration. 2004;34:524-530.
Nelson J., Biven A., Shinn A., et al. Microbial flora on operating room telephones. AORN Journal. 2006;83(3):607-608. 610–611, 613–617, 619–620, 622–626
Scott E., Beswick K. Hazards of diathermy plume. Part 1. A literature review. British Journal of Perioperative Nursing. 2004;14(9):409-414.
Scott E., Beswick K. Hazards of diathermy plume. Part 2. Producing quantified data. British Journal of Perioperative Nursing. 2004;14(10):452-456.