Chapter 5 Asepsis and infection control
After reading this chapter, you should be able to:
Introduction
This chapter presents fundamental aspects of infection control and the application of the principles of asepsis, which are the cornerstone of perioperative nursing practice. The infective process is discussed, along with modes of transmission and how the body combats pathogenic microorganisms. Environmental controls enacted to reduce the spread of infection, along with standard and additional precautions, are described and their practical application discussed. The principles of asepsis, the practical application of aseptic technique and the concept of surgical conscience are examined; also addressed is the surgical scrub, and the methods of prepping and draping the surgical patient and creating a sterile field. Infection control as an adverse event and bioterrorism are briefly explored. Methods of sterilisation and disinfection complete this chapter.
Classification and types of microorganisms
In order to understand the infective process and the measures taken to prevent transmission of microorganisms, it is necessary to review aspects of microbiology. It is beyond the scope of this text to explore microbiology in depth but a brief examination of the particular organisms of concern, in relation to the care of surgical patients, is presented. Two main classifications of microorganisms are described by Burton and Engelkirk (2000):
Microorganisms of special interest to perioperative nurses include several types of bacteria, fungi, viruses and prions, which are outlined below; this does not include all microorganisms that may be found in the hospital setting.
Bacteria
Bacteria are simple, unicellular organisms containing internal structures, such as a nucleus, cytoplasm, plasmids and ribosomes (Lee & Bishop, 2006). Even though there are thousands of bacteria, very few cause disease/infection. Bacteria are extremely adaptable and survive and grow in various environments, often multiplying rapidly. For example, a single Escherichia coli bacterium can reproduce itself in 20 minutes and give rise to over a million bacterial cells in about 10 hours (Lee & Bishop, 2006).
Bacteria are the commonest cause of surgical site infections (SSIs), with staphylococci and streptococci being responsible for many of these (Lee & Bishop, 2006; Nicolette, 2007). Most bacteria found in the perioperative environment are shed from the skin of personnel (Nicolette, 2007); hand washing is the most efficacious way of countering their spread.
Gram-positive cocci
Staphylococci
Staphylococci (e.g. Staphylococcus aureus and Staphylococcus epidermidis) are round or spherical-shaped Gram-positive bacteria, and are part of the normal flora found on the skin and mucous membrane of the nasopharynx, urethra and vagina. They can exist in these areas without any adverse effect on the host and those that live on the skin are termed ‘transient’ organisms. Staphylococci can survive for long periods in the air, dust, bedding and clothing, making cleanliness of the perioperative environment paramount (Phillips, 2007). These bacteria are transmitted from the hands of the host to another person, where they can subsequently have significant negative effects. For example, they can enter the wound of a surgical patient and cause a wound infection or worse, because of their ability to develop resistance to antibiotics quickly (Lee & Bishop, 2006). Exotoxins secreted by S. aureus can cause toxic shock syndrome which, if left untreated, can be fatal (Lee & Bishop, 2006; Phillips, 2007). Staphylococci are strongly associated with healthcare-associated (nosocomial) infection (HAI).
Streptococci
Streptococci are responsible for a wide range of diseases and infections. These include throat and wound infections, pneumonia, septicaemia and necrotising fasciitis. Streptococcus pyogenes is frequently implicated in SSIs. Streptococci tend to be more virulent than staphylococci; however, they are much more likely than the latter to be sensitive to penicillin (Nicolette, 2007). Streptococci can be a normal resident of the upper airway, vagina and anus (Lee & Bishop, 2006; Nicolette, 2007) and are spread via direct and indirect contact, causing infection and illness in susceptible populations.
Enterococci
Enterococci are bacteria normally found in the gastrointestinal tract and female genital tract. They cause infections, such as SSI and septicaemia, when they are transmitted via the hands or contaminated equipment to susceptible, high-risk patients, including surgical patients (Nicolette, 2007). They are becoming an increasingly significant hospital pathogen because strains of enterococci have developed resistance to the antimicrobial drug vancomycin, which is the last resort treatment for methicillin-resistant staphylococcal infections (MRSA) (Lee & Bishop, 2006).
Gram-positive rods
Clostridia are Gram-positive anaerobic bacteria that produce toxins that cause serious illness, such as tetanus (Clostridium tetani) and gangrene (Clostridium perfringens). They have the ability to produce endospores, which enables them to encapsulate themselves in a special protein coat, giving them the ability to survive under adverse conditions (Lee & Bishop, 2006). Endospores can survive for many years and are highly resistant to drying and heat (Phillips, 2007). When conditions improve, the endospore germinates into a new bacterial cell. Sterilisation techniques must be able to destroy bacterial spores; these are discussed later in the chapter. Clostridium difficile, another example of this genus, can cause serious infection within the large intestine, especially in patients on long-term antibiotic therapy (Lee & Bishop, 2006; NZ Ministry of Health, 2007).
Fungi
There are two major types of fungi—yeast and moulds—and many are beneficial to humans; for example, moulds are a source of antibiotics (Lee & Bishop, 2007). They are often termed ‘nature’s original recyclers’ because they secrete enzymes that decompose dead plant and animal matter, turning them into absorbable nutrients. Although of less significance within the perioperative setting, some fungal strains, such as Candida albicans, cause localised infections in the mouth and reproductive tract, which have the potential to become systemic infections. Fungi have been isolated in the nail beds of nurses who wear acrylic nails, even following normal surgical scrub techniques (NSW Health, 2007b). This has led to policies prohibiting acrylic nails within the operating suite due to the danger of transmitting fungal infections to patients (Australian Department of Health and Ageing, 2004).
Viruses
Unlike bacteria, viruses do not have a cellular structure and do not fit the classification for a living cell because they cannot reproduce or carry out any metabolic reactions. However, like some bacteria, they cause significant infections. Viruses replicate by invading a host cell and using the host cell’s DNA, protein and other nutrients to survive and reproduce. In the process, they damage or destroy the host cell. The reproductive process concludes when the host cell bursts (cell lysis), spreading new viruses to nearby cells, where the process is repeated (Lee & Bishop, 2006). This process stimulates an antibody response in the infected person.
Hepatitis is one of the most common viruses and there are five identified strains (hepatitis A through to hepatitis E). The strains of most concern to perioperative nurses are hepatitis B and C as these, together with human immunodeficiency virus (HIV), can be transmitted through contact with blood and body fluids during invasive procedures. This may be through exposure to a sharps injury or via splashes into unprotected eyes or mucous membranes.
Prions
Prions are small infectious particles consisting of protein only with no nucleic acid. They are implicated in unusual neurodegenerative disorders, including bovine spongiform encephalopathy (BSE) or ‘mad cow disease’ and, in humans, Creutzfeldt-Jakob disease (CJD) (Lee & Bishop, 2006). The mechanism of infection that causes CJD is still unclear, although it is thought that prions have the ability to convert normal protein molecules into dangerous ones (Burton & Engelkirk, 2000). Prions are unusually resistant to conventional chemical and physical sterilising methods, and special protocols for managing instruments that have been used on infected or potentially infected patients are discussed later in this chapter (Nicolette, 2007). Table 5-1 summaries the common microorganisms found in the perioperative environment.
Table 5-1 Common microorganisms found in the perioperative environment
| Microorganism | Usual environment | Mode of transmission |
| Staphylococci | Skin, hair | Direct contact |
| Upper respiratory tract | Airborne | |
| Escherichia coli | Intestinal tract | Faeces, urine |
| Urinary tract | Direct contact | |
| Streptococci | Oronasopharynx | Airborne |
| Skin, perianal area | Direct contact | |
| Mycobacterium tuberculosis | Respiratory tract | Airborne, droplet |
| Urinary tract | Direct contact | |
| Pseudomonas | Urinary tract | Direct contact |
| Intestinal tract | Urine, faeces | |
| Water | Water | |
| Serratia marcescens | Urinary tract | Direct contact |
| Respiratory tract | Water | |
| Clostridium | Intestinal tract | Direct contact |
| Fungi | Dust, soil | Airborne |
| Inanimate objects | Direct contact | |
| Hepatitis virus | Blood | Blood-borne |
| Body fluids | Direct contact |
Development of resistance to antimicrobial drugs
The emergence of strains of pathogens that are resistant to currently available antimicrobial drugs represents a significant threat to surgical patients. Those of concern in the perioperative environment include methicillin-resistant S. aureus (MRSA), which is also resistant to other categories of antimicrobials. MRSA has become a serious concern among hospitalised patients and can be fatal in those who are susceptible (Phillips, 2007). S. aureus is frequently implicated in SSIs (Lee & Bishop, 2006). Other microorganisms that have become resistant to antimicrobial drugs include vancomycin-resistant enterococci (VRE) and multi-drug resistant tuberculosis (TB), which is transmitted by droplets from infected individuals or improperly cleaned bronchoscopes and anaesthetic equipment. The prohibitive costs of developing new antimicrobial drugs have led to a greater emphasis on appropriate prescribing practices and more stringent infection control measures to limit the spread of resistant organisms in hospitals (Lee & Bishop, 2006; NZ Ministry of Health, 2007).
Process of Infection
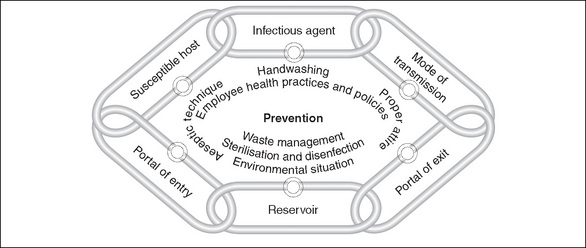
The process of infection can be likened to the links in a chain—break any of the links and infection can be prevented (Nicolette, 2007). There are six links in the chain of infection:
Infectious agent
An infection results from microorganisms invading and multiplying in the host. Pathogenic microorganisms in the form of bacteria, viruses and fungi are the causative agents in wound and systemic infections suffered by patients.
Reservoir
The microorganisms responsible for the majority of HAI (nosocomial infections) originate either from the patient’s own body flora (endogenous infections) or exogenous (external) sources, such as other patients, staff or equipment. Some microorganisms exist harmlessly on patients’ skin, in hair follicles, sweat glands (staphylococci) or within the bowel as normal flora (E. coli). However, when these microorganisms enter another area of the body, they can cause infection (e.g. E. coli can cause bladder infections and S. aureus causes SSIs). Both transient and resident microorganisms are found on the skin (Tanner et al., 2007) and these can be transferred by direct contact between patients, health care workers, visitors and equipment, or by transfer to other body sites within the same patient, where infection can subsequently develop. Transient microorganisms are easily removed by good hand hygiene.
Portal of entry
The body has natural barriers to prevent the entry of microorganisms, including the skin, mucous membranes and their various secretions, such as tears, mucous and acid produced by the stomach. However, these defences can be breached in a number of ways:
Transmission
The transmission of microorganisms cannot occur unassisted. In the hospital setting, the most common mode of transmission is through people; this is mainly via the hands of health care workers, other patients or visitors directly touching the patient or through the use of contaminated objects (Gilmour, 2005). Vigilance in hand hygiene and the use of aseptic technique are the most efficient methods of preventing the transmission of microorganisms. Understanding the routes and sources of transmission is vital if this link in the chain is to be broken.
Portal of exit
For microorganisms to continue infecting other hosts, they must have a means of leaving the body. This may be via blood or other body fluids, faeces or droplets from the respiratory tract (Gilmour, 2005).
Susceptible host
Patients undergoing surgery become susceptible hosts when their skin barrier is breached by a surgical incision. Their immune system is also compromised, further increasing their susceptibility to infection. Other risk factors that increase susceptibility include those:
Figure 5-1 demonstrates how implementing prevention strategies can break a link in the chain of transmission and safeguard the patient.
Normal body defences
Whether or not a person develops an infection as a result of invasion by microorganisms will depend on the susceptibility of that person (the host) and the virulence of the microorganism. It will also depend on the body’s ability to defend itself against the invading pathogens.
External barriers
External barriers include the skin, mucous membranes and their respective secretions; these are the body’s first line of defence in preventing infection. The epidermal layer of the skin contains a protein, keratin, which provides substantial resistance to bacterial enzymes and toxins. The dermal layer of skin contains sebum-secreting sebaceous glands, which lower the pH of the skin, inhibiting the growth of some bacteria and fungi (Lee & Bishop, 2006). Mucous membranes heal quickly despite much wear and tear, and their sticky, mucous secretions trap foreign particles and microorganisms.
Breaching the skin with a planned surgical incision bypasses this defence, increasing the risk of invasion by pathogenic organisms.
Inflammatory response
The onset of inflammation is a non-specific defence. It is the body’s response to tissue damage and is evoked following any injury (e.g. physical, chemical, radiation) or invasion by microorganisms. The function of inflammation is to clear the injured site of cellular debris and any pathogens present, and to enable tissue repair to commence (Phillips, 2007). Once the inflammatory response is evoked, several biochemical mediators are released, localised vasodilation occurs and plasma fluid (containing leucocytes and proteins) moves into the injured area. This causes the four outward signs of inflammation, namely, redness, heat, swelling and pain (Lee & Bishop, 2006). If the inflammatory response does not eliminate all organisms or foreign material, healing of the injury is delayed and chronic inflammation can be result, which can persist for weeks or even months (Lee & Bishop, 2006).
Immune response
The immune response, the third line of protection, is a specific body defence. Immunity is the capacity of the body’s immune system to defend itself successfully against potentially infectious agents. Immunity is acquired in two ways. Firstly, active immunity is acquired when the body has been exposed to or suffered an infection; this is ‘naturally acquired’ immunity. Artificially acquired active immunity results from immunisation, such as with vaccines (e.g. diphtheria) given in childhood. Passive immunity may be natural and occurs when antibodies are transferred from a person with immunity to another who does not have immunity (e.g. from mother to fetus across the placental barrier) (Lee & Bishop, 2006), or artificial and can be conferred with injections of immune globulins. For example, hepatitis B immunoglobulin injections may be given to a health care worker following a sharps injury and potential exposure to hepatitis B virus. Unlike active immunity, passive immunity is relatively short-lived (Lee & Bishop, 2006).
Infection as an adverse event
Infection is one of the most frequent adverse events associated with surgical procedures and/or interventions (Wicker & O’Neill, 2006). The cost of HAIs can be measured in terms of increased morbidity and mortality, increased length of stay in hospital and an increase in both human and clinical resources (Pittet, 2005). Worldwide, HAIs and the present threat from multiresistant organisms (MROs) are said to be responsible for the death of up to 1400 people daily; this constitutes one of medicine’s greatest challenges (Best & Neuhauser, 2004). Such is the significance of MROs that health departments are now developing and implementing MRO-specific policies (NSW Health, 2007a; NZ Ministry of Health, 2007). Surgical patients have a three-fold greater risk of HAI compared to other patients (Australian Department of Health and Ageing, 2004). Despite compelling evidence about the effectiveness of hand washing in reducing the spread of infection within health care facilities, compliance remains problematic. Increasingly, attention must be paid to all of the practices described here because they are either effective or they reduce reliance on antibiotic therapies.
Bioterrorism
Finally, microorganisms are a key component of biological warfare, which has become a very real threat (Nicolette, 2007). The US Centers for Disease Control (CDC) has identified anthrax and smallpox as the two most likely biological weapons with the ability to be spread quickly and easily within large populations. The resultant panic and disruption to the social fabric requires all health professionals to be aware of local procedures when dealing with a potential pandemic (Nicolette, 2007). Although it is unlikely that perioperative nurses will care for surgical patients with these diseases, in the event of a national crisis, they may well be called upon as part of the emergency preparedness plan.
Infection control
Successful infection control practices focus on prevention; this involves identifying hazards and classifying associated risks (Australian Department of Health and Ageing, 2004). In turn, this requires health care facilities to develop infection control risk management plans, ideally within a clinical governance framework, to minimise the risk of preventable nosocomial infections (NSW Health, 2007a). Elements of successful infection control include quality and risk management policies, effective work practices and procedures, and adequate physical facilities and operational controls (Australian Department of Health and Ageing, 2004; Nicolette, 2007; NZ Ministry of Health, 2007). Major risk factors can be found within the perioperative setting, so additional and specific requirements to prevent infection are needed (Australian Department of Health and Ageing, 2004). These are addressed below.
Environmental controls
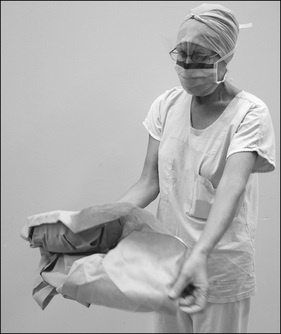
Chapter 3 looked at all aspects of the perioperative environment, noting that many operating suite design features are necessary for good infection control. These include the concept of the four zones of the perioperative environment. Personnel entering the semi-restricted and restricted zones of the operating suite must be correctly dressed in perioperative attire in order to minimise the entry of microorganisms found on the outside (street) clothing of personnel.
Attire
Correct perioperative attire (Fig 5-2) includes the following:
Face masks
The wearing of fluid-resistant face masks is an important part of perioperative attire. The tradition of wearing masks was based on the unfounded assumption that their use protected patients, although there is limited evidence to support this (Lipp & Edwards, 2002). However, there is now sufficient evidence to warrant the use of face masks to prevent the droplet spread of oropharyngeal flora during insertion of spinal or epidural anaesthesia (Siegel et al., 2007). Their continued use is also predicated on the need to protect health care workers (Australian Department of Health and Ageing, 2004; Phillips, 2007; Siegel et al., 2007). Within the perioperative setting, the following practices are recommended.
Moving around the operating room
Too much activity within the operating room provides opportunities for the transmission of microorganisms (Lee, 2005). In order to reduce unnecessary movement, forward planning is needed; for example, place all the requirements for a case in the operating room prior to commencement. Other considerations include the following:
Infection control practices
Consistent with international standards and practices, a two-tiered approach to infection control is endorsed in Australia and New Zealand. Standard precautions are used for all patients regardless of their diagnosis or presumed infection status. Additional (or transmission-based) precautions are applicable only to the care of specified patients (Australian Department of Health and Ageing, 2004; NSW Health, 2007a).
Standard precautions
Standard precautions are designed to reduce the transmission of microorganisms from both recognised and unrecognised sources. They involve safe work practices and protective barriers (Siegel et al., 2007). Standard precautions protect patients and health care workers, and they apply to:
Hand hygiene is the single most important practice to reduce transmission of infectious agents in health care settings (NSW Health, 2007b). Hands must be washed after contact with the patient, after removing gloves and between tasks that involve contact with potentially contaminated equipment. Antimicrobial agents, such as chlorhexidine and waterless alcohol-based hand rubs, are available for routine hand hygiene.
Other facets of standard precautions include the use of personal protective equipment (PPE), which must be worn during activities when there is a risk of contact with blood or body fluids. PPE consists of the following items:
Standard precautions not only rely on the use of PPE, they involve techniques that minimise the risk of transmission of blood-borne diseases. Within the operating room, these include:
Box 5-1 The father of infection control
In May 1847 in Vienna, Ignaz Phillip Semmelweis (1818–65) provided evidence of the significance of hand washing to prevent the spread of puerperal sepsis. Semmelweis, an obstetrician, observed that the maternal mortality rate in women attended by doctors was 20%, which was four to five times greater than in women attended only by midwives.
Semmelweis identified that midwives did not attend the anatomical laboratories where autopsies were carried out. Following the death of a colleague who accidentally cut his finger during an autopsy and died a few days later, it was discovered at autopsy that he had died from the same causative microorganism responsible for puerperal sepsis. This finding moved Semmelweis to immediately implement a rigorous hand washing policy using 4% chlorinated lime solution prior to the examination of women in labour. The results almost immediately lowered maternal mortality rates and a full year after the implementation of Semmelweis’ hand washing policy the mortality rate from puerperal sepsis had dropped to 1.2%.
However, these results were not published for another 14 years and, although Semmelweis had many who supported his findings, there were those who opposed the idea of the doctor being the cause of the spread of puerperal sepsis. Semmelweis was not recognised for his findings until after his death.
Although the antiseptic practices of Semmelweis were ultimately adopted by the medical community throughout the world, he was never given the recognition during his lifetime that he so richly deserved (Grant et al., 2005).
Additional precautions
Additional (or transmission-based) precautions are the second line of approach to infection control. These precautions are applied when the mode of transmission of pathogenic microorganisms is airborne, via droplet or contact, or a combination of these routes. They are applied in conjunction with standard precautions.
Airborne precautions
Airborne transmission of microorganisms involves their spread via minute particles that can remain suspended in the air for extended periods or disseminated in dust particles. Examples of airborne-transmitted diseases are TB and chickenpox. Precautions against these infections require the use of close-fitting, particulate filter, personal respiratory protection devices or a P2 mask capable of filtering particles as small as 0.3 μm (Australian Department of Health and Ageing, 2004; Nicolette, 2007).
Droplet precautions
Droplet transmission involves larger particles generated when a person sneezes or coughs. Droplets are not as robust or long-lived as airborne particles. Examples of diseases transmitted by droplet are influenza and meningococcal infection. The P2 masks are effective in preventing transmission of droplet infections. It is recommended that patients with airborne or droplet infections wear a P2 or equivalent mask when being transported within the hospital to reduce the risk of infecting other people (Australian Department of Health and Ageing, 2004).
Contact precautions
Contact precautions are intended to prevent the transmission of infectious agents, including epidemiologically important microorganisms, which are spread by direct or indirect contact with the patient or a patient’s environment (Siegel et al., 2007). They are applied to patients known to be infected with various epidemiologically significant organisms, including multiresistant organisms (e.g. MRSA, VRE), and when caring for patients with C. difficile infections (Siegel et al., 2007). As these organisms may be present on the patient’s skin, clothing, bed clothes and equipment, health care workers must wear aprons or gowns, gloves, protective eye wear and masks when participating in patient care activities. All items of protective equipment must be disposed of once contact with the patient is completed and hands thoroughly washed. Whenever feasible, these patients should be isolated during their period of hospitalisation (Nicolette, 2007).
Adherence with these precautions decreases the transmission of infectious agents in health care settings (Siegel et al., 2007). However, several observational studies have shown limited adherence to recommended practices by health care workers (Castella et al., 2006; Siegel et al., 2007). Adherence to good infection control practices requires a work environment where there is commitment to safety on the part of management, which includes education, worker participation in safety programs and the provision of appropriate protective equipment (Griffin, 2005; Siegel et al., 2007).
Asepsis
Asepsis can be defined as the absence of pathogenic microorganisms on living tissue (Gilmour, 2005; Nicolette, 2007). The aim of aseptic practices is to eliminate infectious organisms in an effort to minimise contamination of the wound and prevent other infections, and thus aid in an uneventful postoperative recovery (Nicolette, 2007). Aseptic principles and practice are based on available scientific evidence and logical thought, although some are ritualistic and lack scientific rigour to support their use. However, infection control statistics generally support the application of aseptic principles and, until empiric evidence demonstrates otherwise, their use is widely supported (Nicolette, 2007; Siegel et al., 2007). Aseptic practices guide perioperative nurses’ actions, for example, when opening sterile supplies, moving in and around the sterile field or setting up sterile instrument trolleys. In some instances, the principles provide arbitrary boundaries only, but they do assist the perioperative nurse to determine where sterile areas start and end, thus contributing to safe practice.
The sterile field
The patient is the centre of the sterile field, which comprises personnel wearing sterile attire, and those areas of the patient, operating table, instrument trolleys and other furniture that are covered in sterile drapes (Hamlin, 2008). Application of the principles and practices of aseptic technique, which are necessary to create and maintain a sterile field, rely on the perioperative nurse and other members of the surgical team exercising a surgical conscience (Phillips, 2007).
Surgical conscience
A surgical conscience is defined as an individual’s professional honesty and inner morality system, which allows no compromise in practice. When there is a breach in accepted behaviours or aseptic technique, the incident must be corrected immediately, regardless of personal consequences or embarrassment. The placing of the patients’ well-being above personal/professional embarrassment demonstrates good surgical conscience (Phillips, 2007).
Principles of asepsis
The principles of asepsis include the following:
Putting these principles into practice can be daunting for the beginning perioperative nurse. However, practice under supervision will ensure that skills and dexterity are developed. Some practical hints for opening and presenting sterile items to the sterile field are:
Microorganisms do not move around by themselves—they are transported by people, or air currents which contain dust. Discipline in controlling and monitoring one’s own behaviours is an essential component of perioperative practice.
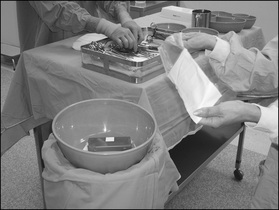
Opening sterile supplies
The circulating nurse provides a link between the sterile field and the sterile supplies required for a surgical procedure.This requires the ability to open and transfer sterile items safely onto the sterile field. Prior to opening sterile items, circulating nurses must wash their hands and carry out the following:
If any doubt exists about the sterility or integrity of the package, it should be discarded. Items that have a peel-back seal (e.g. swabs or sutures) may be suitable for ‘flipping’ onto the sterile field (depending on individual hospital policy). This practice involves using a sterile bowl, on a separate stand away from the sterile instrument trolley(s), into which small sterile items can be ‘flipped’ (Hamlin, 2008). Flipping items directly onto sterile instrument trolleys should be avoided as it may hinder the instrument nurse in setting up the instruments. Worse still, if an item is contaminated during the flipping process, the whole sterile field is contaminated, not just the bowl, which is easily replaced. Whether a package can be safely transferred by flipping will depend on the item and the experience of the circulating nurse. If in doubt, the item should be opened and presented to the instrument nurse, who can use a sponge-holding forceps to take the item safely.
Pouring liquids onto a sterile field
Any fluids added to the sterile field must first be checked by the instrument nurse to ensure that they are the correct fluid and not out of date. Points to remember are:
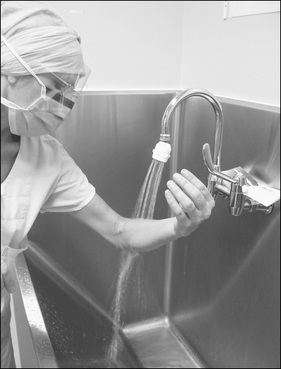
The surgical scrub
Before surgeons and instrument nurse(s) can prepare or enter a sterile field, they must perform a surgical scrub, followed by the donning of a sterile gown and gloves according to local hospital policy.
The practice of surgical scrubbing is an attempt to reduce SSIs. Although skin cannot be sterilised, it can be made surgically clean by undertaking a surgical scrub (Gruendemann & Mangum, 2001; Tanner et al., 2007). The scrubbing of the nails, hands and forearms with antiseptic solution reduces dirt and contamination, and transient and resident microorganisms, and minimises regrowth of the latter throughout the surgery.
Before the surgical scrub personnel should:
The antimicrobial scrub solution used may well be dictated by hospital policy and/or personal choice; however, alcohol-based products are generally considered the most efficacious (NSW Health, 2007b; Siegel et al., 2007; Tanner et al., 2007). These solutions are in common usage in Europe and are likely to be in widespread use in Australasia in the near future. Currently, aqueous solutions, such as povidone-iodine and chlorhexidine, are the most commonly used antimicrobial scrub solutions in use. The evidence from comparisons of aqueous scrub solutions with alcohol rubs, which contain additional active ingredients, is mixed and a systematic review by Tanner et al. (2008) revealed no difference between alcohol rubs that contain additional active ingredients and aqueous scrubs in reducing SSIs. There is evidence from studies in favour of both forms of hand antisepsis.
Both Australian and New Zealand professional perioperative organisations provide detailed descriptions of the surgical scrub and each operating suite should display the technique for all staff to follow. Although the actual technique may differ between countries, the basic principles for surgical scrubbing are:
Gowning
Sterile gowns are worn to provide a barrier to prevent the transfer of microorganisms to the patient during the surgical procedure. Their manufacture must meet relevant Australian and New Zealand standards for both disposable, resposable (i.e. limited reuse gowns) and reusable gowns to ensure barrier qualities. Reusable and disposable sterile gowns are folded differently and, therefore, the technique for donning may vary.
The main principles for gowning are (Fig 5-8):
Gloving
Closed gloving is the recommended method to don gloves as it reduces the risk of contamination of the sterile gloves by the bare hands. Double gloving is a recommended practice as it reduces the risk of sharps penetration (Australian Department of Health and Ageing, 2004; Tanner & Parkinson, 2006).
With the hands remaining inside the cuffs, remove one glove from the packet with one hand and with the palm of the other hand uppermost, the glove is placed onto the cuff with the fingers of the glove pointing towards the wrist, the thumb down and with the folded edge of the glove flush against the edge of the cuff of the gown.
With both hands working inside the gown sleeves, the thumbs hold onto the edge of the glove cuffs. Using one motion, the glove is stretched out and over the hand and the gown cuff is inserted into the glove. Both the glove and the gown are then grasped, manoeuvring the hand through the cuff into the glove (Fig 5-9). The procedure is repeated for the other hand.
The second pair of gloves are then donned by sliding each gloved hand into the second pair.
Once both pairs of gloves are donned, there is one final action taken to complete gowning. On the front of the gown are two ties, which require assistance to be tied to complete the gowning process. Disposable gowns have a paper tag attached to the ties, which can be handed to either a sterile or unsterile colleague. Having handed over the tag, the scrubbed person turns around and reclaims the tie, subsequently tying both ties together. If an unsterile colleague has assisted, care must be taken not to touch the paper tag she or he has handled. Reusable gowns do not usually have a sterile tag and the safest method of turning the gown is to hand the right hand tie to another scrubbed person and then complete the turn. The effect of this manoeuvre is to close the back panel of the sterile gown. It does not mean the back is sterile, but provides all-round protection to the wearer. Once gowned and gloved, the areas considered sterile are from the tips of the fingers to the elbows and from nipple to waist. It is acknowledged that these are arbitrary boundaries (Hamlin, 2008).
Assisted (open) gloving
Assisted gloving is the method whereby one scrubbed person gloves another scrubbed person; this may occur should the wearer contaminate a glove intraoperatively. Closed gloving cannot be achieved because the cuffs should not be pulled back over the hands as they are now unsterile. Therefore, the safest method of donning a replacement glove is for another sterile member of the team to assist with the gloving.
If both gown and gloves become contaminated, both must be removed and the donning procedure is carried out as previously described. Once gowned and gloved (Fig 5-10), the scrubbed person must stay close to the sterile field and not move out of the operating room into semi-restricted or unrestricted areas as this will increase the risk of cross-infection.
At the conclusion of the procedure, the surgical team must discard gowns, gloves and masks in contaminated waste containers within the operating room. This is done in a manner that ‘contains and confines’ contaminated items. For example, to avoid contamination of bare hands, the gown is removed first, rolled up so the inner surface is on the outside, and placed in the contaminated linen basket (if a reusable gown) or the contaminated waste bin (if a disposable gown). This is followed by the removal of the gloves. The hands should be washed following removal of the gown and gloves.
Skin preparation of the patient
The aim of preoperative skin preparation is to remove soil and transient and resident microorganisms from the patient’s skin using an antimicrobial agent. Preoperative skin cleansing mechanically removes, chemically kills and inhibits contaminating and colonising skin flora (Lipp, 2005). This is believed to contribute to reducing the risk of wound contamination and SSIs (ACORN, 2006; Lipp, 2005). However, a systematic review of the use of skin antiseptics has failed to establish conclusively the effectiveness of preoperative skin preparations and which type of solution is most effective (Edwards et al., 2004). Thus, until evidence becomes available it is wise to continue to use skin antiseptics preoperatively (Lipp, 2005). Commonly used antimicrobial agents include povidone-iodine 10% (with or without alcohol), chlorhexidine 0.5% with alcohol, and chlorhexidine and cetrimide.
Although there is no evidence that hair removal reduces SSIs among patients who have had hair removed prior to surgery (Tanner et al., 2006), it is still practised. If hair removal is required on clinical grounds, then it is recommended that clippers are used, rather than shaving with a razor, to prepare the operative site (Australian Department of Health and Ageing, 2004). Clipping has been found to be safer and less likely to produce nicks in the skin, which could lead to wound infections.
Following positioning of the patient, cleansing (often referred to as ‘prepping’) of the patient’s skin with antimicrobial solution is completed, taking into account any allergies that the patient may have, the type of procedure to be carried out and the surgeon’s preference. Preparation of the operative site is carried out either by the surgeon or instrument nurse using a broad-spectrum antimicrobial solution and gauze swabs, observing aseptic technique. Antiseptic skin cleansing commences from the cleanest area, usually the proposed operative site, and proceeds in concentric circles or squares outwards to the least clean areas. The prepared area should be wide enough to allow extension of the incision if required. Areas that have a high microbial count (e.g. groin, umbilicus, body orifices, open wounds or stomas) should be prepared last using a separate swab. The preparation of these areas is carried out in reverse, that is, the cleaner, peripheral areas are cleansed first prior to cleansing the more heavily contaminated areas, even though these may be the operative site. The surgical principle is to work from the cleanest to the least clean area (Nicolette, 2007; Phillips, 2007).
The antimicrobial solution should not be allowed to pool under the patient as this can cause skin maceration. Adequate time should be allowed for the antiseptic solution to dry before the drapes are applied. This is particularly important when using alcohol-based preparation solutions as the vapours from the alcohol must be allowed to evaporate before drapes are applied in order to reduce the risk of ignition and fire when electrosurgery is used (ACORN, 2006; Nicolette, 2007).
Draping the patient
To create a sterile field within which surgery can be carried out, sterile drapes are strategically placed on the patient in a manner that exposes only the operative site and isolates it from surrounding areas. Within this defined sterile field, the surgical procedure takes place and all those involved must be dressed in sterile gowns, gloves and personal protective attire. The drapes covering the patient’s body provide an area on which instruments and equipment, such as suction tubing and the active diathermy electrode (hand piece), can be placed.
Reusable linen or synthetic single-use drapes used in the creation of the sterile field should be made of materials that inhibit the migration of microbial particles and moisture. Drapes may be available as single items or they may be packaged in predetermined configurations for specific surgical procedures. They are folded in such a manner as to facilitate easy opening and placement on the patient. Reusable drapes are held in place with towel clips or sutures, whereas single-use drapes have an adhesive section to secure them in place without slippage. An additional, transparent adhesive drape may be placed over the operative site to further isolate surrounding skin from the incision. The adhesive drape may also be impregnated with antimicrobial solution to minimise wound contamination.
Points to consider when handling drapes are as follows:
Strikethrough
Gowns and drapes act as barriers to prevent the transmission of microorganisms from non-sterile to sterile areas. If moisture penetrates the gowns or drapes, it permits the passage of microorganisms from a non-sterile surface to a sterile surface; this is termed ‘strikethrough’. The ability to prevent strikethrough is a critical factor in maintaining a sterile field and is achieved by the use of waterproof drapes and gowns (or by the use of plastic aprons under gowns made of permeable material). However, if strikethrough occurs on either gown or drapes, they must be replaced (NSW Health, 2007b).
On completion of the surgical procedure and following the application of a wound dressing, the drapes are removed immediately using a ‘contain and confine’ approach as described for the removal of gowns and gloves.
Instrument cleaning, decontamination and sterilisation
All reusable instruments and equipment used on a patient during a surgical procedure or investigative process must be decontaminated, cleaned, inspected, packaged and sterilised or disinfected before reuse to reduce the risk of cross-infection. Spaulding first proposed a system of classifying infection risk and appropriate processing methods in 1968 (Phillips, 2007), as shown in Table 5-2.
Table 5-2 Spaulding’s classification of medical devices by infectious risk
| Classification | Type of procedure | Level of decontamination required |
| Critical | Invasive device that enters tissue that is usually sterile or enters the vascular system (e.g. surgical instruments, biopsy forceps) | Requires sterilisation |
| Semi-critical | Device contacts intact mucous membrane but does not penetrate sterile tissue (e.g. flexible endoscopes) | Requires high-level disinfection (sterilisation preferred where practicable) |
| Non-critical | Device only contacts intact skin (e.g. stethoscope, sphygmomanometer cuff) | Can be processed by cleaning (and low-level disinfection where necessary) |
The standards for decontamination, cleaning and sterilising reusable instruments and equipment are detailed in the relevant Australian and New Zealand standards (Standards Australia, 2003, AS/NZS 4187). This Standard provides a detailed description to assist staff in sterilising departments and the operating suite.
Decontamination and cleaning
The first step in the process of instrument/equipment reuse is that of decontamination. Decontamination is a process by which physical or chemical agents are used to clean inanimate objects or surfaces (Phillips, 2007). Before items can be sterilised, they must be thoroughly cleaned of all organic material; they must also have the least microbial load (bioburden) achievable. Failure to remove this material prevents the sterilising agent coming into contact with all surfaces of the item and results in failure to achieve sterilisation.
The cleaning process begins in the operating room during the procedure with the instrument nurse wiping used instruments with a sterile sponge dampened with sterile water to keep them free of blood and tissue debris. Particular attention should be given to the tips and the joints of the instruments to prevent the build-up of blood and tissue on the instruments, which may hinder their effective use by the surgeon. This also helps reduce the bioburden. Instruments with lumens should be flushed through with sterile water to prevent blockage. Heavily contaminated instruments can be cleaned in a splash bowl containing sterile water (Dunscombe, 2007). Sterile normal saline should not be used for cleaning as it is corrosive and can damage the instruments. At the conclusion of the procedure, reusable instruments and equipment are returned to the sterilising department, where manual and mechanical cleaning takes place (Gruendemann & Mangum, 2001).
One of the initial steps in cleaning metal instruments is immersing them in a water bath containing enzymatic cleaning detergent through which high-frequency ultrasound vibrations are transmitted. The vibrations produce pressure changes in the water, creating tiny air bubbles, which implode around the instruments, dislodging the organic material in a process called cavitation (Hurrell, 2005). This method is especially effective in cleaning instruments that have grooved jaws, where organic material can be difficult to remove by manual cleaning. Therefore, instruments such as scissors and clamps must be fully opened to allow the cavitation process to reach all areas of the instrument. Delicate instruments should not be placed in the ultrasonic cleaner with larger, heavier items as they may be damaged by the latter during processing. Following ultrasonic cleaning, instruments are placed in automated mechanical washers, which use detergents and jet sprays of water to further thoroughly clean, rinse and dry the instruments. Drying the instruments is an important part of the decontamination process as wet instruments will quickly become colonised with microorganisms.
Specialised instruments and equipment such as endoscopes, drills and delicate instruments are processed separately as they have individual cleaning requirements. Endoscopes have air, water and biopsy channels, which require special brushes to be inserted along the length of the channel to ensure thorough cleaning. Most drills cannot be immersed in water and require cleaning with special brushes and air jets to dry the component parts. Fine instruments, such as those used in ophthalmic surgery, generally require manual cleaning following ultrasonic decontamination (Hurrell, 2005).
Inspection, assembly and packaging
Inspection
Following mechanical washing and drying, all instruments are visually inspected by the trained sterilising department staff to ensure that there is no damage or defects and that the item is functioning correctly (i.e. scissors are sharp, the jaws of clamps are properly aligned). To sterilise an item that is not functioning correctly could place the patient at risk should it fail during surgery (Hurrell, 2005).
Assembly
Surgical instruments can be assembled in trays or be individually packaged. All operating suites will have a range of instrument trays to cater for the procedures they commonly perform (e.g. laparotomy, hysterectomy, orthopaedic trays). There will also be a general tray of instruments to which individual items may be added, depending on the procedure. All trays are standardised and will contain a specified number and type of instruments identified on a tray list, which is packaged and sterilised with the tray. The tray list is used for checking the contents preoperatively and postoperatively by the instrument and circulating nurses to ensure no items are inadvertently left inside the patient.
Packaging
The aim of packaging the instruments and equipment is to protect the sterilised items against contamination until they are opened ready for use in a surgical procedure. A variety of packaging materials are available and their choice depends on the item to be packaged and the sterilising process to be used. The packaging material must be:
Packaging materials can be reusable textiles (e.g. linen) or they can be specialised synthetic, single-use, spun bond polymer (Gruendemann & Mangum, 2001). Single-use, self-sealing pouches made of specialised paper or plastic have also been designed for individual items. Metal instruments, endoscopes and drills can be packaged in trays or containers that are made of moulded plastic or metal with perforations to allow penetration by the sterilising agent.
Once assembled, the tray/container is wrapped, usually in two layers of packaging material (this may vary depending on the material and local policy), and folded in a manner that allows subsequent opening using aseptic technique. A label identifying the tray is placed on the external surface. Depending on the sterilising agent to be used, the wrapping material will be sealed using a specialised indicator tape, which will change colour during the sterilising process. This becomes a visual check that the item has undergone the sterilisation process and is checked by the circulating nurse prior to opening the item. The change in colour of the external indicator tape alone does not guarantee sterility of the item. Other sterilising parameters must be met before sterility is assured and these are discussed below. However, if the indicator tape is found not to have changed colour, the item must be considered unsterile and not used.
Sterilisation
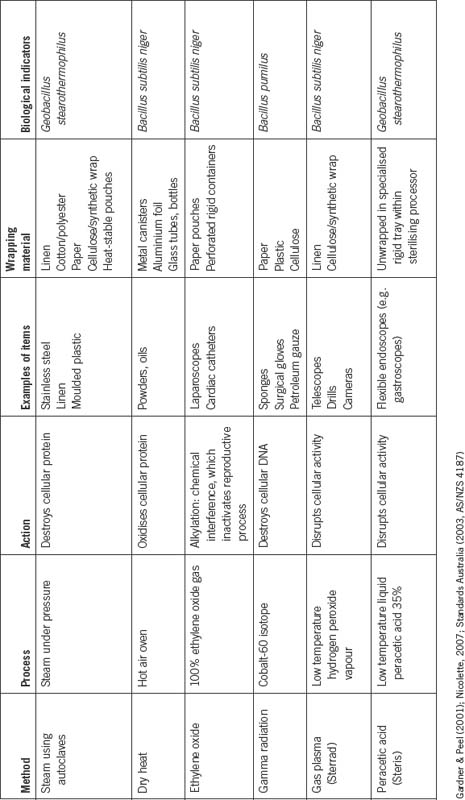
Sterilisation is defined as ‘the complete elimination or destruction of all forms of microbial life’ (Nicolette, 2007). All items introduced into the sterile field must be sterile in order to minimise the risk of surgical site (or other) infection and to promote an uneventful recovery. A number of different methods are used to sterilise items used during the surgical procedure. Their use is based on the physical properties of the item to be sterilised. For example, metal instruments, plastic disposable items, cotton swabs and linen drapes all require a different sterilisation method. Table 5-3 outlines the methods of sterilisation.
Methods of sterilisation
For the purposes of sterilisation, instruments and equipment can be categorised into two groups:
Steam
Saturated steam under pressure is one of the most effective and commonly used methods of sterilisation. It is an inexpensive method and can be used on items capable of withstanding high temperatures (121–134°C), such as metal instruments and bowls. Steam sterilisation takes place in specially designed sterilisers, often called ‘autoclaves’, into which trays and individual items are carefully arranged to allow all surfaces to come into contact with the saturated steam. The autoclaves are preprogrammed to reach and maintain a specific temperature, pressure and time ratio depending on the type of load being sterilised (i.e. settings will differ for metal ware compared to linen or mixed loads).
The autoclave chamber is sealed and the sterilisation process begins with the creation of a vacuum inside the autoclave chamber, which is achieved by sucking out all the air. Following removal of all the air, steam under pressure is introduced into the chamber and the temperature will rise to the preprogrammed setting. This is known as the ‘penetration time’. When the required temperature and pressure is reached, the actual sterilisation of the items will begin. This is known as the ‘holding time’, which is approximately 15 minutes. When the holding time is completed, the steam is withdrawn and the load will be left to dry, using the residual heat in the metal walls. In the final stage of the process, filtered air is introduced, which returns the chamber to normal atmospheric pressure.
The steam sterilising process is designed to sterilise large loads of instruments within a central sterilising department, the end result of which is a wrapped, sterile item.
‘Flash’ sterilisation
A ‘flash’ steriliser is a smaller unit that uses steam but without the drying cycle of the models used in the central sterilising department. The cycle is rapid (hence the term ‘flash’), usually only several minutes, and the item emerges wet.
Flash sterilisers are designed for the management of single items. Operating rooms may have one or two flash sterilisers, permitting the sterilisation of reusable items for immediate use (Standards Australia, 2003, AS/NZS 4187). Their use is not endorsed for regular sterilisation due to their inability to dry items, which can easily become contaminated during subsequent transport to the operative field, which may be some distance away. Flash sterilisation is generally limited to use in emergencies for single items that may have been accidentally contaminated during surgery and for which a replacement cannot be located. The use of flash sterilisation demands adequate decontamination/cleaning of instruments, and daily performance monitoring of steriliser cycles to ensure efficacy of the sterilisation process (Standards Australia, 2003, AS/NZS 4187).
Dry heat
Dry heat sterilisation in the form of hot air ovens was once the only method by which powders, oils and glass could be sterilised. Today, these items are more likely to be supplied by commercial companies who use gamma radiation as a sterilisation method. This method is more economical as large volumes of the items can be sterilised. Dry heat is a slow, expensive method of sterilisation using a temperature range of 160–180°C, depending on the item and exposure time required (Gardner & Peel, 2001).
Ethylene oxide gas
Ethylene oxide (ETO) gas is a low-temperature (36–60°C) chemical method of sterilisation that is suitable for items that cannot be exposed to the high temperatures associated with steam or to dry heat. ETO is a highly toxic agent and exposure can cause severe toxic reactions, such as nausea, vomiting and respiratory difficulties, in health care workers. Therefore, strict occupational health and safety controls exist to protect operators of ETO sterilisers.
ETO is very effective in sterilising items such as rubber, silicone and polyethylene products, especially those with narrow lumens, and telescopes and drills. Items for sterilising are wrapped in single-use packaging or pouches and placed in an ETO steriliser, where the temperature and humidity are controlled before the ETO gas is introduced. The time taken to sterilise items can be up to 2 hours and, as ETO is absorbed into the items, there must be a further 2–12 hours’ aeration time to ensure that the ETO has been completely eliminated from the items before it can be used (Gruendemann & Mangum, 2001; Nicolette, 2007; Standards Australia, 2003, AS/NZS 4187).
Gas plasma
The gas plasma method uses hydrogen peroxide vapour and plasma to create a state under which items such as cameras, telescopes and drills can be sterilised under low-temperature conditions. This method has, in many instances, superseded ETO because it is a less toxic and more rapid form of sterilisation. Gas plasma involves the introduction of hydrogen peroxide vapour into a closed vacuum chamber through which radiofrequency waves are introduced, creating an electromagnetic field and conditions that kill microorganisms. The cycle time is approximately 30–60 minutes, depending on the model of steriliser, and the by-products are oxygen and water, thus making it substantially more environmentally sound than ETO (Nicolette, 2007).
Peracetic acid
Peracetic acid is a low-temperature, liquid chemical method of sterilisation that is suited for use with endoscopes that cannot be sterilised using high temperatures. Commercially produced processing systems are increasingly used within operating rooms where flexible endoscopes (e.g. gastroscopes) are used frequently and require sterilisation close to the time of use. The active ingredient is peracetic acid 35%, which is highly corrosive and, therefore, is used in combination with an anticorrosive agent. The processing unit is fully computerised and is the size of a small suitcase, with a lid and an internal tray especially configured to fit a flexible endoscope. A connection kit fits onto the exposed ends of the internal lumen system to ensure the sterilising agent passes through each lumen. Once the lid is closed and the processor activated, the active peracetic acid mixes with water and is fed over and through the endoscope for a period of 12 minutes, followed by four rinse cycles, which use filtered water to flush the peracetic acid away. The whole cycle takes approximately 30 minutes and the result is a wet, sterile scope ready for immediate use.
As with all items, thorough cleaning of the endoscope must occur prior to sterilisation, using specialised brushes and enzymatic cleaning agents to ensure that all lumens are free of debris and bioburden (Nicolette, 2007). The end product of peracetic acid is environmentally safe acetic acid and water.
Gamma radiation
Many commercially packaged products that are unsuitable for sterilisation by chemical or heat processes are sterilised by irradiation using the isotope cobalt-60, which produces gamma rays. Gamma rays can penetrate large cartons of items, making this an economical method for large medical companies to sterilise items such as ointments, sponges, plastic drapes and surgical gloves. This method is suitable for commercial application only.
Creutzfeldt-Jakob disease
The causative prions of CJD are highly resistant to conventional decontamination and sterilisation methods. Special protocols are required to manage instruments if they have been used on patients known or thought to be at risk of carrying the disease. Ideally, disposable instruments should be used, but this may not prove to be practical. Many institutions quarantine reusable instruments used on suspected CJD patients until test results from the patients have been obtained. Negative results will mean that routine decontamination and sterilisation processes can be followed. Positive results will require instruments to be reprocessed using combinations of germicidal solutions for mechanical cleaning followed by steam sterilisation at temperatures and pressures in excess of those routinely required for steam sterilisation (Nicolette, 2007). The management of equipment exposed to CJD prions is still evolving as further research is carried out on this highly infective, but fortunately rare, disease.
Monitoring sterilisation processes
All sterilising methods must undergo validation processes to ensure that items are sterile and may be safely used on patients. The validation process refers to documented procedures for obtaining, recording and interpreting results needing to show that a process will consistently produce a sterile item. This commences with the commissioning of new sterilising equipment and ongoing performance qualifications comprising microbiological and physical parameters (Gardner & Peel, 2001).
Physical
Regular maintenance, monitoring and testing takes place of all sterilisers to ensure that they are functioning correctly in accordance with Standards Australia (2003, AS/NZS 4187). All sterilisers have external gauges, thermometers, timers and computer printouts to monitor their functions. Internal sensors can provide information about temperature, pressure and humidity for every load. Documentation of these parameters is performed to provide permanent records so as to provide retrospective proof that all loads have passed through the sterilisation process satisfactorily.
Chemical
External indicators appear on the outside of each package and these change colour when the item has passed through a sterilisation process. These may be incorporated into the wrapping material, as in pouches, or as a separate tape or spots attached to the outside of the wrapped item. Different types of external indicators are required for each type of sterilisation method. The external indicators do not demonstrate that the item is sterile but do provide important visual indicators that the item has undergone a sterilising process.
An internal chemical indicator strip, known as an integrator, is placed inside with the items to assess the complete penetration of steam or chemical vapours. If the sterilisation parameters have been met, the strip will change colour, and this will be verified at the point of use by the instrument nurse prior to using the instruments. If the integrator strip has not changed colour, the items must not be used as sterilisation may not have occurred.
Biological
Biological indicators are standardised preparations of bacterial spores that are included as part of the routine testing processes of sterilisers to demonstrate whether sterilisation conditions have been met. Biological indicators are inoculated with a known concentration of spore preparations, which will be different, depending on the sterilisation process. For example, the spores used to test steam sterilisation conditions are those of Geobacillus stearothermophilis. Following exposure to the sterilisation process, the indicators will be examined to ascertain if the spores have been destroyed, which indicates that this testing parameter has been met.
No one testing parameter alone will verify that sterilisation conditions have been met. Sterilisation is achieved when all the physical, chemical and biological parameters have been met (Gardner & Peel, 2001; Standards Australia, 2003, AS/NZS 4187).
Tracking and traceability
The ability to track instruments and trace patients is an integral component of safety and risk management processes. Written and computerised records are maintained within the sterilising department to provide retrospective proof that an item has satisfactorily passed through a sterilisation process. These records are important should an outbreak of infection occur where investigations demonstrate that there may have been a breakdown in the sterilisation process. Patients who have undergone procedures during the period in question and may have been infected need to be traced and their infectious status investigated. Tracking systems rely on trays of instruments having barcodes and being scanned during decontamination and reprocessing, with the data stored on computer for future reference (Hurrell, 2005).
However, a more effective tracking system is one that is able to track an individual instrument back to a specific patient. Currently few such systems exist; however, with advances in technology, sophisticated instrument tracking systems will become more widespread in the future.
Disinfection
Disinfection is described as a process of destroying all pathogenic organisms except spores from inanimate objects (Nicolette, 2007). The process can be used on items identified as semi-critical or non-critical (see Table 5-2). Disinfection involves the use of liquid chemical disinfectants, which can be classified as high, intermediate or low level depending on their killing capabilities.
As with items for sterilisation, effective decontamination of the items or surfaces using enzymatic cleaners, detergents and water must occur prior to disinfection (Nicolette, 2007). Staff should wear personal protective attire when using disinfecting agents as contact can produce adverse skin or respiratory effects. Material Safety Data Sheets (MSDS) containing information on each chemical, its use, possible adverse effects and first aid and spill management should be available within the operating suite.
Conclusion
In conclusion, it is important to note that HAIs are considered an accurate indicator of the quality of patient care. The focus of this chapter has been on assisting perioperative nurses to understand and implement a range of practices and strategies that are aimed at preventing or minimising infection in the surgical patient. Perioperative nurses must work in collaboration with other members of the health care team to monitor infection control practices constantly to ensure quality care for patients.
Critical thinking exercises
1 Perioperative attire
It is your first day in the operating suite and you are directed to the change room to change into perioperative attire.
Agents of bioterrorism http://www.usamriid.army.mil/publicationspage.html www.bt.cdc.gov.
Commonwealth of Australia Infection Control Policy http://www.health.gov.au/internet/wcms/publishing.nsf/Content/icg-guidelines-index.htm
Creutzfeldt-Jakob disease (CJD) http://www.cdc.gov/ncidod/dvrd/cjd/ http://www.cdc.gov/ncidod/dvrd/vcjd/index.htm.http://www.hpa.org.uk/infections/topics_az/cjd/information_documents.htm.
Multi-drug resistant organisms (MDRO) http://www.cdc.gov/ncidod/dhqp/pdf/ar/mdroGuideline2006.pd
Perioperative Nurses College of New Zealand Nurses Organisation http://www.pnc.org.nz/Site/Sections/Colleges/Perioperative/
Severe acute respiratory syndrome (SARS) www.who.int/csr/sarsarchive/2003_05_07a/en/ www.cdc.gov/ncidod/sars.
The Cochrane Collaboration http://www.cochrane.org/index.htm
ACORN. Standards for perioperative nursing, including nursing roles, guidelines, position statements, competency standards. S11. Perioperative attire. Adelaide: Australian College of Operating Room Nurses; 2006.
Australian Department of Health and Ageing. (2004). Infection control guidelines for the prevention of transmission of infectious diseases in the health care setting. Retrieved September 14, 2007, from http://www.icg.health.gov.au.
Best M., Newhauser D. Ignaz Semmelweis and the birth of infection control. Quality Safety Health Care. 2004;13:233-234.
Burton G., Engelkirk P. Microbiology for the health sciences. Sydney: Lippincott Williams & Wilkins; 2000.
Castella A., Charrier L., Di Legami V., et al. Surgical site infection surveillance: analysis of adherence to recommendations for routine infection control practices. Infection Control and Hospital Epidemiology. 2006;27(8):835-840.
Dunscombe A. Sutures, needles and instruments. In Rothrock J., McEwen D., editors: Alexander’s care of the patient in surgery, 13th ed., Louis: St Mosby, 2007. (pp. 158–182)
Edwards, P., Lipp, A., Holmes, A. (2004). Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database of Systematic Reviews, 3, CD003949.
Gardner J.F., Peel M.M. Sterilization, disinfection and infection control, 3rd ed. Sydney: Elsevier; 2001.
Gilmour D. Infection control principles. In: Woodhead K., Wicker P., editors. Textbook of perioperative care. Edinburgh: Churchill Livingstone, 2005. (pp. 81–96)
Goldman M. Pocket guide to the operating room, 3rd ed. Philadelphia: F A Davis; 2008.
Grant G., Grant A., Lockwood C., Simpson C.J. Semmelweis, and transformational change. American College of Obstetricians and Gynecologists. 2005;106(2):384-387.
Griffin F. Best-practice protocols: preventing surgical site infection. Nursing Management. 2005;36(11):22-26.
Gruendemann B., Mangum S. Infection prevention in surgical settings. Louis: St Mosby; 2001.
Hamlin L. Operating theatre practice. In: Hughes S., Mardell A., editors. Handbook of perioperative nursing. Oxford: Oxford University Press, 2008.
Hurrell D. Decontamination of reusable medical devices. In: Woodhead K., Wicker P., editors. Textbook of perioperative care. Edinburgh: Churchill Livingstone, 2005. (pp. 97–118)
Lee G., Bishop P. Microbiology and infection control for health professionals, 3rd ed. Sydney: Pearson Prentice Hall; 2006.
Lee J. Air, hair, knees, nose. Infection Control and Hospital Epidemiology. 2005;26(12):900-902.
Lipp A. An evaluation of preoperative skin antiseptics. British Journal of Perioperative Nursing. 2005;15(1):12-19.
Lipp A., Edwards P. Disposable surgical face masks: a systematic review. Journal of Advanced Perioperative Care. 2002;1(2):41-46.
NSW Health. (2007a). Infection control policy: prevention and management of multi-resistant organisms (MRO). PD2007_084. Sydney: NSW Health.
NSW Health. (2007b). Infection control policy. Policy directives. PD2007_036. Sydney: NSW Health.
NZ Ministry of Health. (2007). Guidelines for the control of multi-drug resistant organisms in New Zealand. Wellington: NZMOH. Retrieved February 13, 2008, from http://www.moh.govt.nz/moh.nsf/pagesmh/3345.
Nicolette L. Infection prevention and control in the perioperative setting. In Rothrock J., McEwen D., editors: Alexander’s care of the patient in surgery, 13th ed., Louis: St Mosby, 2007. (pp. 44–99)
Phillips N. Berry & Kohn’s operating theatre technique, 11th ed. St Louis: Mosby; 2007.
Pittet D. Infection control and quality health care in the new millennium. American Journal of Infection Control. 2005;33(5):258-267.
Santos A., Lacerda R., Graziano K. Evidence of control and prevention of surgical site infection by shoe covers and private shoes: a systematic literature review. Revista Latino—Americana de Enfermagen. 2005;13(1):86-92.
Siegel, J., Rhinehart, E., Jackson, M., et al. (2007). Guidelines for isolation precautions: preventing transmission of infectious agents in healthcare settings 2007. Retrieved March 14, 2008, from http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/Isolation2007.pdf.
Standards Australia. AS/NZS 4187 Cleaning, disinfecting and sterilizing reusable medical and surgical instruments and equipment, and maintenance of associated environments in health care facilities. Sydney: Standards Australia; 2003.
Tanner J., Blunsden C., Fakis A. National survey of hand antisepsis practices. Journal of Perioperative Practice. 2007;17(1):27-37.
Tanner J., Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database of Systematic Reviews. 3, 2006. CD003087
Tanner J., Swarbrook S., Stuart J. Surgical hand antisepsis to reduce surgical site infection (review). Cochrane Database of Systematic Reviews. 1, 2008. CD004288
Tanner J., Woodings D., Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database of Systematic Reviews. 2, 2006. CD004122
Wicker P., O’Neil J. Caring for the perioperative patient. Edinburgh: Churchill Livingstone; 2006.
Woodhead K., Wicker P. Textbook of perioperative care. Edinburgh: Churchill Livingstone; 2005.
Botwinick L., Bisognanom M., Haraden C. (2006). Leadership guide to patient safety. Institute for Healthcare Improvement. Retrieved April 3, 2008, from www.ihi.org.
Gillespie S., Bamford K. Medical microbiology and infection at a glance, 2nd ed. Malden, MA: Blackwell Publishing; 2003.
Haydon, D., Cleaveland, S., Taylor, L., Laurenson, M. (2002). Identifying reservoirs of infection: a conceptual and practical challenge. Emerging Infections Diseases, 8(12). Retrieved April 3, 2008, from http://www.cdc.gov/ncidod/eid/vol8no12/01-0317.htm.
Health Protection Surveillance Centre. (2001). Strategy for the control of antimicrobial resistance in Ireland (SARI). Retrieved April 8, 2008, from http://www.ndsc.ie/hpsc/A-Z/MicrobiologyAntimicrobialResistance/StrategyforthecontrolofAntimicrobialResistanceinIrelandSARI.
Laine J., Aaarnio P. Glove perforation in orthopaedic and trauma surgery. Journal of Bone and Joint Surgery. 2004;86(6):898-900.
NSW Therapeutics Advisory Group. (2003). Drug utilisation. Retrieved April 8, 2008, from http://www.ciap.health.nsw.gov.au/nswtag/drug_utilisation.html 8.
NSW Therapeutic Advisory Group. (2007). Indicators for quality use of medicines in Australian hospitals. Retrieved April 8, 2008, from http://www.ciap.health.nsw.gov.au/nswtag.
Roxburgh M., Gall P., Leek K. A cover up? Potential risks of wearing theatre clothing outside theatre. Journal of Perioperative Practice. 2006;16(1):30-41.
Stringer B., Haines T. Hands free technique: preventing occupational exposure during surgery. Journal of Perioperative Practice. 2006;16(10):495-500.
Tanner J. Surgical glove: perforation and protection. Journal of Perioperative Practice. 2006;16(3):148-152.