Chapter 9 Postanaesthesia recovery unit
Introduction
This chapter describes the role and function of the postanaesthesia recovery unit (PARU), the assessment and ongoing observations of the patient during the immediate postoperative period, and discharge criteria. Commonly experienced post-surgical, post-procedural and postanaesthesia complications (hereafter referred to collectively as postoperative) and their management are examined, as well as the management of pain and postoperative nausea and vomiting.
Role and function of PARU
The PARU is a specialised area designed to care for patients in the immediate postoperative period. As discussed in Chapter 6, most anaesthetic agents have properties that have depressant effects on a number of body systems; the respiratory and cardiovascular systems are particularly vulnerable. Therefore, all patients who have received general or regional anaesthesia, or sedation, must be closely observed during the immediate postoperative period, and their condition evaluated and stabilised, with emphasis on anticipating and preventing complications resulting from anaesthesia and surgery (ACORN, 2006a).
The area is staffed by nurses and medical practitioners who are specially trained to manage and stabilise patients prior to their return to the ward or discharge home via the day surgery department. Many patients have a history of comorbidities which, when combined with the stress of anaesthesia and surgery, can affect their immediate postoperative management. The postoperative patient in the PARU is vulnerable because of altered physiological function, along with psychological and cognitive impairment. This places patients in a state of ultimate reliance on the nursing and medical staff to ensure their safety, privacy, dignity and comfort during a phase when they are unable (or inadequately able) to advocate or care for themselves (ACORN, 2006b; 2006c).
PARUs are mainly located within operating suites. However, patients may also undergo procedures that require sedation or anaesthesia in other departments, such as endoscopy, radiology, cardiac investigation laboratories and free-standing day surgery settings. These departments also require an area in which patients can be monitored post-procedure.
In some hospitals, particularly day surgery facilities, PARUs are often divided into stage 1 and stage 2 areas. The stage 1 area is where patients are admitted directly from the operating or procedural room and closely observed until they are haemodynamically stable and meet appropriate discharge criteria. Patients are then transferred to the stage 2 area, which consists of reclining lounge chairs, rather than beds or trolleys, and which has a more home-like environment to encourage a sense of wellness and normalcy, but where the patients can still be observed until they are ready to be discharged home (Burden, 2008). The history of the development of PARUs, which were originally called recovery rooms, is outlined in Box 9-1.
Florence Nightingale developed the first known PARU for soldiers following battlefield surgery during the Crimean War. However, PARUs did not become formally established until the 1940s. ‘Recovery’ wards evolved in Australia in response to their development in America and from recommendations arising out of studies into preventable postoperative deaths. The first known PARU in Australia, which was specifically designed to care for patients in the period immediately after surgery, was built in 1901 at Royal Adelaide Hospital. Prior to this, patients were returned to their ward immediately following surgery, most often to be cared for by a student nurse with limited experience. The nurses and medical staff in these PARU areas were seen to have advanced skills, which eventually led to larger areas being built and the admission of other longer stay, critically ill patients, such as polio victims requiring iron lung management.
During the 1950s and 1960s, intensive care units were established to provide specialised care of these critical patients and recovery wards began to be purpose-built to assist in the recovery of the increasing numbers of postoperative and postanaesthesia patients. Then, as now, these areas assisted in reducing preventable deaths and morbidity, particularly from airway complications (Hughes, 1982).
PARU design features
The majority of PARUs, particularly in larger, tertiary (or referral) hospitals, function as independent areas with their own staff within the operating suite environment, providing care for patients who have undergone a range of surgical procedures under various anaesthesia techniques. In smaller hospitals, with only one or two operating rooms, and which generally cater for less complex operative procedures, staff may be multiskilled in all the perioperative roles. Regardless of the size, such areas are still required to have monitoring and resuscitation equipment to manage postoperative patients (ACORN, 2006b; Australian and New Zealand College of Anaesthetists [ANZCA], 2006a).Figure 9-1 shows a typical PARU that could be found in Australia or New Zealand.
The location and design of the operating suite should provide for quick and easy access between each operating room and the PARU to enable an immediate response by surgeons, anaesthetists and others to assist in the management of postoperative patients who develop complications, should they arise. It should be an area that promotes comfort and reduces anxiety for the postoperative patient; therefore, design features, such as indirect lighting, soft colours, good ventilation and soundproofing to reduce noise, should be considered (Hamlin, 2005). The three most critical design features of the PARU are:
The PARU is a semi-restricted area within the operating suite, although it is important that health care workers in street clothes can access the PARU directly to consult on or provide patient care if required. However, external access into the PARU should be limited as much as practical to reduce the transfer of microorganisms into this semi-restricted area. Hospital policy will dictate whether PARU staff should wear operating suite attire (ACORN, 2006b).
The space allocated to a patient bay, which will accommodate a patient’s bed/trolley, should be at least 9 square metres, with easy access to the patient’s head (ANZCA, 2006a), at least 1.2 metres between each patient’s bed/trolley, and a specially designated area for the isolation of infectious patients when the need arises (Centre for Health Assets Australasia [CHAA], 2006). To accommodate PARU patients during peak periods, the number of allocated beds/trolley spaces within the unit should be at least 1.5 spaces per operating room; in other words, a four-room operating suite should have six PARU bays available (ANZCA, 2006a).
Equipment requirements
The set-up of each PARU bay should be standardised, with devices that are used regularly available in each bay, and emergency and other essential equipment centrally located within the PARU for easy access. Additional equipment that is used less frequently should be accessible from the operating suite environment at short notice. The essential equipment for each bay includes:
Patient transfer from operating room to PARU
Patients transported to the PARU from the operating room or procedure area should always be accompanied by an anaesthetist, a member of the nursing team and an orderly. This ensures the safe monitoring and transfer of the patient and minimises the manual handling risks to the staff. The patient must be continuously observed during transfer as complications, including apnoea, respiratory obstruction, hypoxia and/or vomiting, can often occur during this critical period. The patient will be receiving oxygen therapy during transfer, either by Hudson mask or nasal prongs. The transferred patient’s conscious state will vary from fully anaesthetised, to semi-conscious (with the possibility of an unprotected airway), to awake and alert.
Although the anaesthetist will determine the patient’s position for transfer, the supine position with the head slightly raised is a favoured position for the transportation of unconscious patients as this allows maximum observation of the patient’s airway and conscious state. If there is a risk of vomiting, the patient may be transported in a lateral position with the trolley/bed in a head-down tilt. Portable emergency equipment (e.g. oxygen, suction and ventilation devices such as ventilating bag and mask) is essential and must be present/available during transfer to the PARU, regardless of the procedure or anaesthetic (Ball, 2008).
Handover of care
Members of the team transporting the patient from the operating room to the PARU must have knowledge of the patient’s condition, history and interventions occurring during anaesthesia and surgery in order to provide a comprehensive handover of care to the PARU nurse, who will commence the patient’s postoperative care. The handover will be carried out by the anaesthetist and a member of the nursing team.
The nursing handover should include, but not be limited to:
Additional specific patient care details, which are extremely useful to the PARU nurses, may include
The anaesthetist handover should include, but not be limited to:
All documentation and follow-up information related to the patient’s anaesthetic, surgical intervention and ongoing management must be present at handover and clarification sought by the PARU nurse when required. Postoperative surgical requirements are usually documented by the surgeon as part of the operation record and should be examined to ascertain any special orders pertaining to drains, dressings or catheters, and any special observations or other postoperative actions required (Ball, 2008).
Patient management in PARU
Initial PARU patient management
A member of the anaesthetic team is required to remain with the patient until the PARU nurse assigned to the patient is available to receive handover, take over care of the patient and is satisfied that the patient’s condition is stable (Hegedus, 2003).
On taking over a patient’s care, PARU nurses will immediately position themselves at the head of the patient’s bed, preferably behind it and the patient, to allow quick and easy access to the patient’s airway and also to emergency equipment, such as oxygen and ventilating equipment, which is usually wall mounted. An initial assessment is made of the patient’s airway, breathing and colour (ABC) and if it is apparent that the patient is unable to maintain her or his own airway, then the nurse must remain with the patient and provide airway support. A second nurse may assist by assessing and documenting blood pressure, pulse, oxygen saturation level and the patient’s level of consciousness and pain status, all of which are priorities at this time (ACORN, 2006a; Ball, 2008)
Patient observations and monitoring
Once these initial patient care priorities are met and the patient is able to maintain his or her own airway, a more thorough patient assessment can be undertaken following a ‘head to toe’ approach, which includes wound status and type of dressing, location of drains and nature of any drainage, presence of catheters, any IV fluids being given and temperature measurement. If necessary, comfort measures, such as the use of warm blankets, can be initiated. Assessment of pain status and the presence of postoperative nausea and vomiting can be made and interventions commenced where necessary (see pp 226 and 233).
Regular patient observations will be carried out throughout the patient’s stay and accurate documentation is vital. Haemodynamic status is determined via assessment and documentation of vital signs, including:
Other observations include the patient’s consciousness level and, where indicated, the ECG rhythm is monitored and displayed continuously. Assessment of ABC and vital signs continue at appropriate intervals in a stable patient according to standard requirements and individual hospital policy. Unstable patients will require more frequent and prolonged assessment and documentation according to individual patient condition (ACORN, 2006a; ANZCA, 2006a, 2006b).
Assessment of airway and breathing
Airway and breathing management are a priority in the initial patient assessment and continue to be monitored throughout the patient’s stay in the PARU. Oxygen therapy is continued on arrival into the PARU via a Hudson face mask or nasal prongs at a rate of 4 litres per minute, unless otherwise ordered.
A systematic approach to assessing the patient’s airway follows a look, listen and feel approach; any untoward findings require immediate action.
Look
Assessment of the patient’s airway includes looking at the following:
Monitoring airway and respiratory function
Apart from visual observation of the patient, non-invasive monitoring of the arterial oxygen saturation level is carried out using a pulse oximeter. A detailed description of pulse oximetry is given in Chapter 6. Determination of oxygen saturation via the use of pulse oximetry is a mandatory observation carried out in the PARU and a reading of 98% is normally anticipated in the postanaesthetic patient (O’Brien, 2008).
Circulation
The effects of surgery and the side-effects of anaesthetic agents are both powerful stimulators of the stress response, which can have dramatic effects on a patient’s circulation (Desborough, 2000). Circulatory assessment includes:
Heart rate
Heart rate can be monitored using pulse oximetry or ECG, although it is preferable to palpate the patient’s pulse as this provides an assessment of the strength of the pulse, and determination of any irregularities. Touching the patient to palpate the pulse also allows the opportunity to assess skin temperature and determine peripheral perfusion, and provides reassurance to the patient.
Blood pressure
Blood pressure is usually measured using non-invasive blood pressure equipment, either manually or using an automated device.The patient’s preoperative blood pressure readings should be noted to provide a baseline and assess whether postoperative readings are within normal limits. A blood pressure reading 20% above or below the patient’s normal reading may be considered abnormal and can be attributed to a number of factors (e.g. haemorrhage and resultant hypovolaemic shock) (O’Brien, 2008).
Level of consciousness
Assessing a patient’s emergence from general anaesthesia and awareness of self and surroundings requires close monitoring; it is also an excellent indicator of ABC sufficiency and neurological function. Consciousness is usually assessed concurrently with ABC using verbal or gentle tactile stimulation and includes determining a patient’s:
Temperature control
While it is common practice to monitor vital signs such as blood pressure and pulse, monitoring the patient’s temperature is often overlooked. It is important to take active measures to assess the patient’s temperature and maintain normothermia. The issues related to the importance of monitoring for hypothermia and hyperthermia during the anaesthetic phase were discussed in Chapters 4 and 6. This must continue while the patient is in the PARU.
Assessment of temperature
Assessment of the patient’s temperature can be made using tympanic, axillary or oral thermometers, although the latter may be impractical due to airway equipment and may render a lower reading than other routes (Drain & Odom-Forren, 2008). In many PARUs the tympanic route is the route of choice to monitor temperature as it is easy to use, non-invasive, non-traumatic and provides an accurate assessment of core temperature. The aim is to maintain the patient’s temperature within the range 36.5–37.5°C and to provide comfort warming measures for a patient with a normal temperature but who feels cold. Patients who have a temperature below 36.5°C may require active warming measures, which include forced air warming devices, warm cotton body blankets and head caps or foil thermal blankets (Good et al., 2006).
It is also important to assess the patient with a temperature above 37.5°C for the possibility of malignant hyperthermia, and seek advice and assistance immediately if this condition develops (O’Brien, 2008).
General comfort measures
Although assessing and monitoring the patient’s physiological status is paramount in the immediate postoperative period, providing comfort and reassurance is also important during this time. As part of the ’head to toe’ assessment, any soiled sheets or gown should be removed and the patient made clean and as comfortable as possible. Psychological care is important as the patient may be anxious about the outcome of surgery, and the presence of a nurse speaking gently, reassuring and reorientating the patient to time and place can be very comforting. Mouth care and providing ice chips to suck will be appreciated by the patient. Some PARUs allow family members to visit, particularly for paediatric patients, where the presence of a family member may reduce patient anxiety and make the child feel more secure (Hamlin, 2005; O’Brien, 2008).
Postanaesthesia complications
Airway and breathing complications
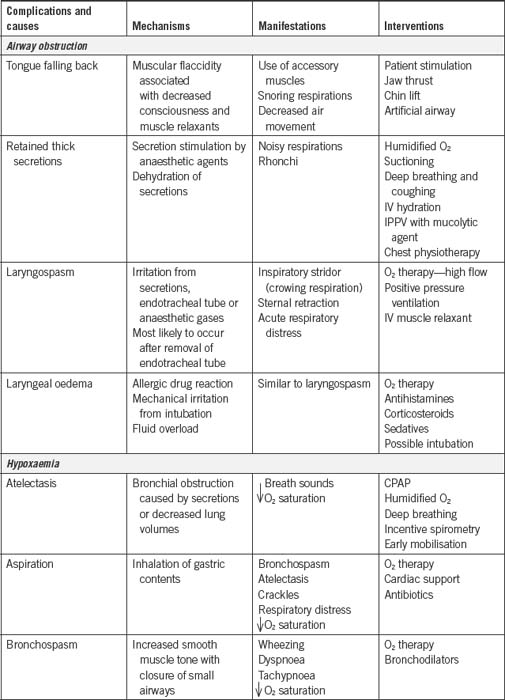
The effect of anaesthetic agents and relaxant drugs is to depress the central nervous system, resulting in potentially life-threatening postanaesthesia complications. These drugs are the main cause of airway obstruction in the PARU (Younker, 2008). Therefore, the initial patient assessment on admission to the PARU is to determine airway patency. If obstruction has occurred, immediate action is required to identify the cause, remove it if possible and maintain the patient’s airway (Hegedus, 2003). The most common causes of airway obstruction are the tongue falling backwards into the oropharynx and the presence of secretions, such as mucus, blood or vomitus (Hamlin, 2005).
Table 9-1 summarises the common postoperative respiratory complications seen in the PARU.
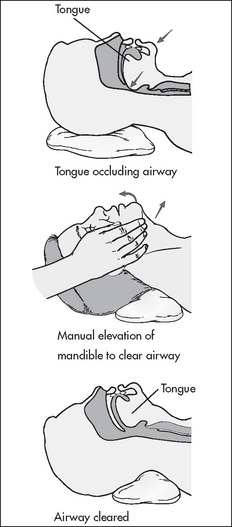
Obstruction by the tongue
Muscle relaxant drugs, if they have not been fully reversed, can affect the muscles of the pharynx and the tongue, causing the latter to fall back into the oropharynx and obstruct the upper airway, especially in patients who are lying supine.
The signs and symptoms of tongue obstruction are noisy, gurgling, choking sounds, irregular respirations, decreased arterial oxygen saturation readings and rapid onset of cyanosis.
Management is chin support and/or the instigation of the jaw thrust manoeuvre (Fig 9-2); the jaw thrust manoeuvre lifts the soft palate away from the pharyngeal wall, opening the airway (Younker, 2008). Additionally, the insertion of an artificial airway (oropharyngeal or nasopharyngeal) can be used to maintain an open airway. The patient must not be left unattended during this period until the PARU nurse is confident that the patient is able to maintain his or her own airway. Patients can also be repositioned on their side in the recovery position as this action will assist in moving the tongue forward and relieving the obstruction (Drain & Odom-Forren, 2008).
Obstruction by secretions
The upper airway can also be obstructed by the presence of secretions such as mucus, blood or vomitus. The signs and symptoms include noisy, gurgling, choking sounds, coughing, irregular respirations, decreased oxygen saturation readings and rapid onset of cyanosis.
Laryngospasm
Laryngospasm is one of the most serious life- threatening airway complications. It is the partial or complete closure of the vocal cords in response to stimulation by secretions such as mucus, vomitus or blood, vigorous suctioning of the airway or inappropriate placement of an artificial airway, which touches the vocal cords (Mahajan, 2007).
The closure of the cords in response to these stimuli is a protective reflex but can become a life-threatening event as little or no air enters the lungs. Immediate action is required to restore a patent airway.
Partial closure of the vocal cords results in a ‘crowing’-like noise on inspiration. This is known as stridor and is caused by air passing through partially closed vocal cords. The patient may be awake when this occurs and will show signs of panic and distress as this is a very frightening experience.
The above actions may be sufficient to overcome the partial spasm and the patient’s airway will be restored. Patients will require close monitoring to prevent a recurrence of the laryngospasm.
The patient may also suffer a total laryngospasm when the vocal cords close completely. In this situation, there is no sound and no air entry into the lungs, although the patient will still be making efforts to breath characterised by exaggerated chest movements. These patients will rapidly become hypoxic and then unconscious (Drain & Odom-Forren, 2008).
Management of total laryngospasm includes:
If a patent airway is not restored using the above manoeuvre, then sedation and reintubation may be carried out by the anaesthetist. This will occur following administration of a sedative drug, such as midazolam and the short-acting muscle relaxant succinylcholine (Hegedus, 2003). The use of a sedative may also prevent laryngospasm from recurring following subsequent extubation as the endotracheal tube touches the vocal cords.
Bronchospasm
Bronchospasm is a lower airway obstruction caused by the bronchial tubes constricting in response to aspiration of stomach contents or secretions, pharyngeal suctioning, or a histamine release secondary to an allergic response to the drugs used during or post anaesthesia (Odom-Forren, 2007). Bronchospasm is characterised by an expiratory wheezing and use of the accessory respiratory muscles.
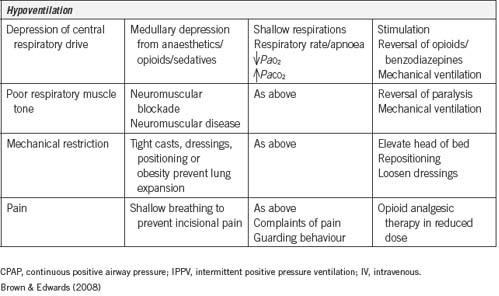
Inadequate reversal of muscle relaxants
The initial assessment of some postanaesthesia patients reveals that they are breathing weakly and have very shallow respirations. This can be an indication that the muscle relaxants used during anaesthesia have not been adequately reversed or have not been totally eliminated from the body. As well as shallow respirations, these patients may display restlessness, and their limbs may lack tone and appear ‘floppy’. The shallow respirations are not effective for adequate gas exchange and the patient can become hypoxic; this can quickly progress to respiratory arrest.
Cardiovascular complications
Hypotension
Hypotension in the immediate postoperative period is a common occurrence and may be due to a number of factors:
Most commonly, hypotension is due to hypovolaemic shock, which is caused by blood and fluid loss either intraoperatively or in the immediate postoperative period. It is characterised by a fall in venous pressure, peripheral vasoconstriction and tachycardia (Hamlin, 2005). Other signs of shock include:
The primary goal in managing hypovolaemic shock is increasing the fluid volume to a level that is able to generate adequate cardiac output and perfusion to the tissues (Drain & Odom-Forren, 2008). The fluid is usually given as a bolus and may be a crystalloid or colloid solution, or a blood product. The hypotensive patient should always receive oxygen via a face mask to aid cellular oxygenation at a tissue level (Drain & Odom-Forren, 2008). Positioning the patient flat or slightly head down will assist in raising the blood pressure in a patient who is compromised. Close observation of the patient’s vital signs must be made until his or her condition stabilises, and the cause found and treated. Accurate documentation of fluid balance must also be kept to ensure appropriate management of fluid replacement and assessment for adequate urine output.
Haemorrhage
One cause of hypotension is haemorrhage, which can occur at any time during the perioperative period and can be life-threatening if not managed quickly and effectively. As the patient recovers from the effects of the anaesthetic and surgery, the patient’s blood pressure returns to normal limits; this rise may cause blood to ooze from vessels that have been tied or resected during surgery. Often the patient’s natural haemostatic mechanisms will control the bleeding, but occasionally the haemorrhage will be profuse. Active bleeding may be seen through a wound dressing or in drains, or it may occur insidiously, resulting in the patient exhibiting the range of signs and symptoms of hypovolaemic shock. Careful assessment of the patient’s wound, drains and catheters for excessive bleeding should be made during the immediate postoperative period. The surgeon and anaesthetist should be informed and the cause of haemorrhage investigated at the same time as resuscitation of the patient is commenced using the measures described above. Unresolved haemorrhage may require that the patient is returned to the operating room to find the cause of the bleeding and initiation of surgical haemostasis (Hamlin, 2005).
Management of pain in the postoperative period
Management of pain is a vital component of a successful outcome for the surgical patient. Achieving absolute and complete pain relief postoperatively is usually unattainable and the term ‘optimal’ pain relief best describes the PARU goals when providing analgesia to patients (Macintyre & Ready, 2001). Most patients will be anxious about the pain that they are likely to experience and will seek reassurance about the pain management techniques available.
Pain assessment and management
Pain assessment should occur early and often in the PARU and is considered one of the primary functions of the PARU nurse (ANZCA, 2007; Australian and New Zealand College of Anaesthetists & Faculty of Pain Medicine [ANZCA & FPM], 2005; Iacono,2004). It is well documented that assessing pain intensity is considered the fifth vital sign and pain should be assessed when routine observations are undertaken (ANZCA & FPM, 2005). Importantly, pain is what the person experiencing it says it is, and patient self-report is the most reliable guide about the type and nature of pain being experienced (Bryant & Knights, 2007).
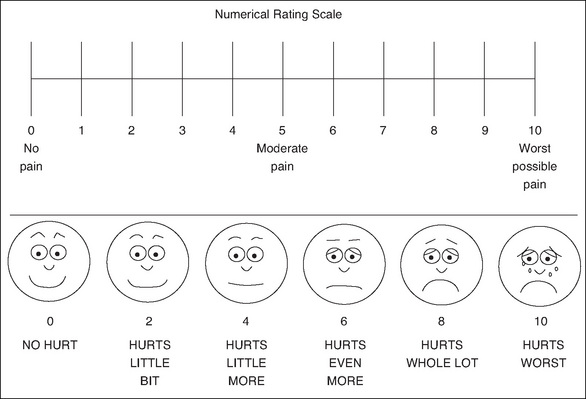
The accurate assessment of pain is of utmost importance and consistent use of a validated pain assessment tool should be used across the facility to promote awareness and correct use (Mitchell et al., 2007). Pain assessment tools that are quick and easy to use are the most suited to pain assessment in the PARU (Macintyre & Ready, 2001). Figure 9-3 shows the commonly used pain assessment scales.
Pain assessment and management in the emergence phase of anaesthesia can be difficult because the patient may be unconscious or semi-conscious, and unable to verbalise their pain adequately. In addition, normal haemodynamic responses to pain, such as tachycardia and hypertension, may be depressed in the immediate postoperative period due to the ongoing effect of anaesthetic agents (Pasero, 2003b). Observing for agitation and restlessness or changes in facial expressions, such as grimacing, evidence of clenched teeth or grinding of teeth, are all highly reliable indicators of pain (Rodriguez et al., 2004).
As well as scoring pain intensity using a validated tool, pain location should also be assessed (ANZCA & FPM, 2005). The surgical incision site is the most obvious source of discomfort or pain for the postoperative patient. However, less obvious sources of pain and discomfort, which can be equally distressing, include a sore throat from intubation, pressure points from prolonged positioning in the operating room or a distended bladder (or kinked urinary drainage tubing). Patients may also experience referred pain (e.g. following a limb amputation).
Pharmacological interventions
Drugs that may be used for pain management in the immediate postoperative period include opioids, non-steroidal anti-inflammatory drugs (NSAIDS) and local anaesthetics, as well as a range of adjuvant therapies (e.g. antidepressants) and other drugs to treat analgesia-related side-effects (ANZCA, 2007; Bryant & Knights, 2007; Windle, 2004). Drugs can be administered by oral, subcutaneous, intramuscular, IV, epidural, intrathecal, rectal, transdermal, inhaled/intranasal or transmucosal routes (ANZCA, 2007; Power & Atcheson, 2007). The methods and routes commonly used in the PARU include IV, epidural and spinal analgesia (central nerve blocks), regional blocks (e.g. of limbs) or local anaesthesia, and finally, the oral route.
Opioid drugs
IV opioids are the ‘gold standard’ for the relief of severe acute pain in the immediate postoperative period due to their rapid onset and efficacy (ANZCA & FPM, 2005). IV opioids can be given in the form of a continuous infusion or as intermittent bolus doses, which may be nurse-initiated or patient managed. To maximise the effect of the opioid and limit the side-effects, vital signs, pain and sedation scores must be assessed prior to the commencement and throughout the administration of any IV analgesia, whether given continuously or intermittently (Macintyre & Ready, 2001).
The dose administered is titrated to the individual patient (ANZCA, 2007) and is dependent on the patient’s age, pain and sedation scores and response to previous doses (Macintyre & Ready, 2001). However, titrating IV opioids in the immediate postoperative phase is a delicate balance between relieving acute pain promptly and avoiding over-sedation (Pasero, 2003b). Many PARUs use pain protocols in the form of algorithms (flow charts) to allow the nurse to play an autonomous role in assessing pain and administering prescribed analgesics by means of intermittent IV bolus doses of opioids (e.g. morphine) (Macintyre & Ready, 2001). Standard pain protocols in adul patients require the elderly to receive smaller opioid doses due to their reduced tolerance and increased susceptibility to the side-effects, particularly respiratory depression and sedation (ANZCA & FPM, 2005).
Pain protocols are for use by PARU staff who are experienced and knowledgeable in administering IV opioids, including giving loading doses, and they are only implemented where nursing goals are focused on providing rapid relief of acute pain in a monitored environment. They are not recommended for use in a general ward environment with reduced nurse-to-patient ratios (Macintyre & Ready, 2001).
Contraindications to the commencement or continuation of a pain protocol are patients who are over-sedated, hypotensive or who have poor respiratory effort (Macintyre & Ready, 2001). These patients will require review by an anaesthetist prior to the opioid loading dose being administered/continued to avoid exacerbating these conditions.
Patient-controlled analgesia
Patient-controlled analgesia (PCA) is an analgesic delivery system that allows patients to administer their own IV analgesia (usually opioids) intermittently and on demand (ANZCA & FPM, 2005). The PCA device is pre-programmed to deliver a prescribed amount of opioid or ‘bolus dose’ to patients when they press a hand-held button (Macintyre & Ready,2001). After a bolus dose is received, a ‘lockout system’, which is incorporated into a programmable device, disables the system for a prescribed amount of time, commonly 5–8 minutes (Macintyre & Ready, 2001). This prevents over-sedation and results in the patient continuing to experience the effect of the previous dose before being able to successfully initiate another dose (ANZCA & FPM, 2005). Postoperative patients in the PARU may initially require IV loading to bring pain scores to acceptable levels before commencing this analgesia maintenance therapy (Macintyre & Ready, 2001).
The use of PCA should be discussed with patients preoperatively and education provided in the operation of the device, including reassurance that they will not be able to overdose on the drugs used.
Nursing assessment and monitoring includes regular assessment and documentation of respiratory rate, amount of opioid used, sedation and pain scores, vital signs and treatment of any side-effects (Macintyre & Ready, 2001).
The advantages of PCA include the following:
The disadvantages of PCA include the following:
Continuous opioid infusions
Continuous opioid infusions describe a system where a prescribed amount of opioid is delivered continuously to the patient via an automated delivery system. This modality is beneficial for patients who are unable to use PCA. The goal of the continuous infusion is directed at keeping consistent blood levels of analgesia, thus avoiding peaks and troughs (ANZCA & FPM, 2005). In addition to the continuous infusion, the prescription usually includes a loading dose, which allows the nurse to give a bolus dose of opioid if the analgesic effect is unsatisfactory or prior to performing potentially painful activities, such as repositioning for pressure area care (Macintyre & Ready, 2001). As a result of patients receiving continuous delivery of opioid regardless of the pain intensity or sedation levels, this modality is associated with a higher incidence of respiratory depression; therefore, patients must be closely monitored.Table 6-3 provides a summary of the commonly used agents for opioid analgesia.
Patient management following local anaesthesia infiltration
A common technique used by surgeons when a short-duration local anaesthetic is required to facilitate minor surgery is infiltration of a local anaesthetic (e.g. bupivacaine) in and around the surgical site, targeting the nerve endings (Pasero, 2003b). The same technique may also be used following major surgery to provide localised postoperative pain relief, often in conjunction with other drugs. Local anaesthetics act on nerve endings to block the transmission of pain and are used to reduce the overall opioid requirements (and thus the undesired side-effects of continuously administered opioids) (Pasero, 2003b). Local anaesthetics can be given as a single dose, often on completion of the surgery and prior to the patient leaving the operating room. Alternatively, they may be continuously infused directly into and around the operative area via a catheter placed directly in the wound site, which is attached to a disposable pump device (Liu & Wu, 2007). Patients may also require oral or IV analgesia concurrently and/or prior to the locally infiltrated anaesthetic wearing off to provide ongoing multimodal pain relief (Macintyre & Ready, 2001).
Patient management following regional anaesthesia
Regional anaesthetic techniques involve injecting local anaesthetic drugs anywhere along a nerve pathway, resulting in anaesthesia to a region of the body served by that nerve without a loss of consciousness. They are usually administered by the anaesthetist preor intraoperatively (DeLamar, 2007). They are often termed ‘peripheral nerve blocks’ as they are used to facilitate surgery on peripheral areas of the body (i.e. arms or legs). They have the advantage of providing better analgesia with fewer associated side-effects compared to parentally administered opioids (Liu & Wu, 2007). The local anaesthetic is usually administered as a single bolus, which not only provides pain-free surgery, but can also provide pain relief for several hours postoperatively. It is important that other analgesic drugs are prescribed to provide ongoing pain relief when the effect of the local anaesthetic drug wears off (Macintyre & Ready, 2001).
The anaesthetist may inject local anaesthetic into the brachial plexus, which will anaesthetise the arm, or inject the sciatic nerve block, which will affect the leg (see Ch 6) (Klein et al., 2005). In the PARU, care of patients following regional anaesthesia involves prevention of injury to the affected limb that has reduced or no motor or sensory function, and monitoring the neurovascular status of the anaesthetised limb (Banks, 2007). The affected limb also requires supporting on a pillow.
Complications associated with peripheral nerve blocks include damage as a result of malpositioned needles or the side-effects of the drugs administered. Potential insertion complications detected in the PARU include, for example, pneumothorax following incorrect placement of the needle during a brachial plexus block procedure (Klein et al., 2005).
Excessive doses of local anaesthetic can cause toxicity, with signs and symptoms including dizziness, ear ringing, tingling or numbness around the lips, mouth and tongue area, which, if left untreated, can progress to seizures and cardiac instability (Pasero, 2003b).
Peripheral nerve block infusion
A method devised for prolonging the benefits of regional (peripheral) nerve block is to use a continuous peripheral nerve block infusion. This involves a fine catheter being inserted against the nerve sheath and left in situ; it is subsequently attached to a drug delivery system (Banks, 2007). Patients undergoing knee replacement or shoulder stabilisation surgery can benefit from continuous peripheral nerve block infusions. Postoperatively, the patient receives an hourly rate of local anaesthetic, with a prescription of bolus doses available in the event of unsatisfactory pain management. Peripheral nerve block infusions are commonly used in combination with other pain management strategies, such as PCA, with the benefit of reducing opioid requirements and the associated side- effects of continuous opioid administration (Pasero, 2003b).
Nursing care of patients with continuous peripheral nerve block infusions involves:
Patient management following central nerve blocks
Central nerve blocks refer to the administration of local anaesthetic and other drugs into the subarachnoid or epidural space, blocking the transmission of impulses along the nerves as they exit the spinal cord, and causing large areas of the lower body to lose sensation (hence the commonly used term ‘block’). These techniques are particularly useful during and following surgery of the abdomen and lower limbs (DeLamar, 2007). See Chapter 6 for further details.
Epidural analgesia
The use of epidural anaesthesia to facilitate surgery has been described in Chapter 6. As well as facilitating surgery, the epidural route is well recognised as one of the most effective methods for providing postoperative analgesia as part of a multimodal approach. It is particularly effective following orthopaedic, abdominal and obstetric surgery (Pasero, 2003a), and thoracic surgery (Faber & Klein, 2008). The most common selection of drugs are low concentrations of local anaesthetics (e.g. bupivacaine) and opioids (e.g. morphine or fentanyl) (ANZCA & FPM, 2005). Used together, these drugs have a synergistic effect, with a requirement for lower doses (Power & Atcheson, 2007). Opioids administered into the epidural space work via two different pathways:
The use of epidural analgesia has a number of advantages when compared to parentally administered opioids:
However, a number of side-effects and complications can occur, for which the patient must be closely monitored. Those associated with the opioids include:
Those associated with the use of local anaesthetics include:
Those associated with placement of the epidural catheter include:
Spinal block
The use of spinal anaesthesia (block) to facilitate surgery has been described in Chapter 6. The direct injection of a single dose of local anaesthetic (e.g. bupivacaine) into the subarachnoid space causes a complete loss of motor function to the lower limbs and loss of sensation to those areas below the site of the injection and the lower abdomen, thus facilitating surgery (Lee et al., 2007). The effects of the spinal anaesthesia may last well into the immediate postoperative period and the patient is unlikely to require additional postoperative analgesia while in the PARU. The possible side-effects associated with local anaesthetics are:
Nursing care for patients with spinal anaesthesia involves:
Other forms of pain management
Oral analgesia
Oral analgesics are generally impractical for use in the immediate postoperative period when patients are recovering from general anaesthesia because they are unable to take oral medications. However, oral analgesia is useful in day surgery settings or if patients have had surgery using local anaesthesia infiltration (Boss et al., 2003). Examples of oral medications include paracetamol, tramadol and NSAIDs (e.g. ibuprofen) (Power & Atcheson, 2007).
Multimodal analgesia
Multimodal analgesia refers to the concurrent use of different classes of analgesics, each working at different sites and in different ways to relieve pain (Windle, 2004). Combining different drugs results in superior pain management and the requirement for smaller doses of each of them, thus reducing the incidence of side-effects (ANZCA, 2007; Pasero, 2003a; Power & Atcheson, 2007). The combined use of local anaesthetics, NSAIDs and opioids are the main components of multimodal analgesia and their administration should commence prior to surgery to optimise the effect (Pasero, 2003b). It is well documented that as increased amounts of opioids are given, the incidence of undesirable side-effects (e.g. respiratory depression) also increases, and thus to decrease these and the subsequent additional time spent in the PARU and hospital, a multimodal analgesic approach is recommended (Pasero, 2003b).
Non-pharmacological and comfort measures
In addition to the pharmacological measures, non-pharmacological and comfort measures can be initiated by the PARU nurse. Measures that can aid in relieving pain include:
Additional measures that can be considered in conjunction with the anaesthetist and other members of the surgical team include:
These measures generally complement rather than supplant pharmacological interventions in the PARU (ANZCA, 2007). However, they can be useful adjuncts, given that patients’ attitude and beliefs about pain modify their perceptions of it and can alter their requirements for relief. Box 9-2 discusses some non-pharmacological methods of pain management.
Box 9-2 Non-pharmacological methods of pain management
A pilot study to determine the effects of guided imagery and music therapy on postoperative pain, postoperative nausea and vomiting (PONV), and length of stay for patients undergoing gynaecological laparoscopy was carried out by Laurion and Fetzer in 2003. Guided imagery audiotapes, music audiotapes or standard care were used on patients, who were randomly assigned to one of these interventions, and the outcomes evaluated. Patients who received guided imagery and music therapy reported significantly less pain on discharge from PARU to home than patients who received standard care in the control group. No significant difference was found with respect to PONV or length of stay; however, these findings suggest that guided imagery and music are effective strategies in improving pain management.
Other considerations after surgery
Postoperative nausea and vomiting
Postoperative nausea and vomiting (PONV) are anticipated postanaesthetic events that can be very distressing and potentially harmful to patients. PONV has multiple causes, which include patient movement, the side-effects of anaesthetic drugs such as opioids and nitrous oxide, surgical or vagal nerve stimulation, pain and hypotension. In many instances, nausea and vomiting are often independent phenomena. However, several factors place some patients at higher risk of developing PONV, including:
A susceptible patient may suffer from PONV for several days after surgery, producing extreme discomfort. Patients with three or more risk factors should receive prophylactic antiemetic medication as part of their anaesthesia. Several antiemetics reliably prevent nausea and vomiting (Carlisle & Stephenson, 2006). The use of P6 acupuncture stimulation to prevent nausea should be considered, as it reliably reduces the incidence of nausea, with minimal side-effects, but not vomiting (Lee & Done, 2004).
The most serious medical complications associated with PONV are regurgitation, airway obstruction and aspiration of gastric contents. Once aspiration has occurred, 50% of patients suffer a major morbidity, and there is a 4% mortality rate (Australian Patient Safety Foundation, 2006).
Patients suffering PONV will be pale, clammy, tachycardic, restless, distressed, possibly in pain, dry reaching or actively vomiting and often have a decreased blood pressure.
Persistent PONV requires medical review to assess and manage the other causes mentioned above.
Considerations for specialty surgery
As well as the routine observations carried out on every patient in the PARU, special observations will be carried out depending on the type of surgery the patient has undergone. Even though it is beyond the scope of this chapter to discuss in detail every type of surgery requiring special observations, some examples of observations that may be carried out for specialty surgery include:
Management of patients with diabetes mellitus
Patients suffering from diabetes mellitus are commonly encountered in the perioperative environment. These patients require regular checks of blood sugar levels, careful monitoring of fluid and electrolytes, and regular assessment of urine for evidence of glycosuria. This is because the stresses imposed by surgery and anaesthesia cause imbalances that may require active management to return blood sugar levels to within the normal range. Patients may require insulin given on a sliding scale during the perioperative period depending on the blood sugar results, and IV fluids to treat dehydration. Hyperglycaemia and coma may be difficult to ascertain in an unconscious patient, making regular blood sugar level monitoring a vital component of the overall monitoring of a diabetic patient (Drain & Odom-Forren, 2008).
Paediatric patients
Even though it is not within the scope of this text to provide a detailed discussion on the immediate postanaesthetic care of the paediatric patient, the following section provides an overview. PARU nursing of the paediatric patient has a number of similarities to that of the adult patient in relation to the close observation that is required. However, there are some specific areas to consider.
Patient safety, as with all patients, is paramount and the following must be taken into account with paediatric patients:
A family-centred care approach is recommended to encourage parental contact as soon as practical to provide emotional support.
Equipment must be specific for children of all ages. For example, the resuscitation trolley must be equipped with paediatric-sized airways.
Paediatric discharge criteria differs from adult discharge criteria in the parameters that are measured. Paediatric scoring systems include:
Several paediatric discharge scoring systems are available; however, as with adult patients, clinical judgement must take precedence when deciding if a child is ready for discharge from the PARU (Landriscina, 2008; Sullivan, 2001; J. Wilkinson, RN, personal communication, 2008).
Documentation and discharge
Accurate and timely documentation of the condition of patients during their stay in the PARU is vital in order to monitor their progress, provide early detection of complications, assess their readiness for discharge from the PARU and facilitate continuation of care on discharge from the PARU (Fig 9-4).
There are several numerical scoring systems that provide a set of predetermined criteria against which the patient is scored each time a set of observations are taken. These systems provide an objective means to determine patient stability and suitability for discharge to a ward or the step-down (stage 2) recovery area. One system in common use is the Aldrete scoring system, which was developed in 1970 by Drs Aldrete and Kroulik. A score is allocated to a range of discharge criteria, including:
Table 9-2 shows both the Aldrete scoring system for patients in the PARU and also the Post Anaesthetic Discharge Scoring System (PADSS) used in ambulatory surgery to assess patients prior to discharge home.
Table 9-2 Postanaesthetic scoring systems
| Aldrete Scoring System | Postanaesthetic Discharge Scoring System (PADSS) |
| Respiration | Vital signs |
| • Ability to take deep breaths and cough = 2 | • BP and pulse within 20% preoperative value = 2 |
| • Dyspnoea/shallow breathing = 1 | • BP and pulse within 20–40% preoperative value = 1 |
| • Apnoea = 0 | • BP and pulse within >40% preoperative value = 0 |
| Oxygen saturation | Activity |
| • Maintenance of >92% on room air = 2 | • Steady gait, no dizziness, or preoperative level met = 2 |
| • Oxygen inhalation needed to maintain oxygen saturation >90% = 1 | • Assistance needed = 1 |
| • Oxygen saturation <90% even with supplemental oxygen = 0 | • Inability to ambulate = 0 |
| Consciousness | Nausea and vomiting |
| • Fully awake = 2 | • Minimal or treated with oral medication = 2 |
| • Arousable on calling = 1 | • Moderate or treated with parenteral medication = 1 |
| • Not responding = 0 | • Severe or continues despite treatment = 0 |
| Circulation | Pain |
| • Blood pressure (BP) ± 20 mmHg preoperative value = 2 | • Controlled with oral analgesics and acceptable to patient: |
| • BP ± 20–50 mmHg preoperative value = 1 | Surgical bleeding |
| • BP ± 50 mmHg preoperative value = 0 | • Minimal or no dressing changes = 2 |
| Activity | • Moderate or up to 2 dressing changes needed = 1 |
| • Ability to move 4 extremities = 2 | • Severe or more than 3 dressing changes needed = 0 |
| • Ability to move 3 extremities = 1 | |
| • Ability to move 0 extremities = 0 |
Local policy will determine the total score that the patient must reach for discharge. Usually, a total score of less than 8 would require re-evaluation of the patient’s condition by the anaesthetist and surgeon, whereas a total score of 10 would indicate readiness for discharge (DeFazio Quinn, 2008).
Although the scoring system is helpful in determining the patient’s condition, it does not include detailed observations such as urinary output, pain management, temperature, wound stability or other observations required for specific types of surgery (e.g. extremity observations). These are important considerations when making decisions about patient discharge to less well staffed areas/wards. Clinicians’ judgement always takes precedence over achievement of set criteria or guidelines and, wherever doubt exists about readiness for discharge and/or patient safety, discharge should be delayed and, if necessary, advice sought from appropriate medical personnel. In some PARUs, the anaesthetist will be required to review the patient prior to discharge, whereas in other PARUs, nurse-initiated discharge is permitted using collaboratively developed protocols.
Handover to ward/step-down staff
Once the patient’s condition is stable and the discharge criteria have been met, local protocol will determine whether ward staff or PARU staff accompany the patient to the ward or stage 2 recovery area. Regardless of the local protocol, a full handover of the patient’s care must take place between the nursing staff so that continuity of care is maintained. A final written report summarising the patient’s progress in PARU will be made and included in the patient’s medical record. This will form the basis of the handover report and it should include the following pertinent facts:
The patient should be transferred on a trolley or bed accompanied by two people, one of whom should be a nurse, together with any equipment required to monitor the patient during transfer (e.g. ECG or pulse oximeter monitor).
Conclusion
The establishment of PARUs was predicated on the need to care for surgical patients in the immediate postoperative period, a time when they are high susceptible to adverse events. Decades of monitoring adverse events in anaesthesia, 50% of which were preventable, led to the creation of recovery areas within the perioperative suite. PARUs draw together the expertise of highly qualified, specialist nursing and medical staff, along with sophisticated technology, particularly equipment to monitor and manage patients with respiratory and haemodynamic instability. This chapter has provided an overview of the main features of a PARU, and explored the common complications of anaesthesia and surgery, along with their management. PARUs are high-dependency units that continue to evolve, and many now regularly care for patients who require mechanical ventilation.
Critical thinking exercises
1 Case study
Mr Morgan has undergone an open radical prostatectomy under a general anaesthetic and has just arrived in the PARU accompanied by an anaesthetist and a nurse. Mr Morgan is unconscious and pale in colour, his breathing is shallow with an obstructed airway, and he has a moderate amount of blood in his urinary catheter.
During handover you are told your patient’s medical history, indicating that Mr Morgan is a fit 60-year-old man who has oral medication for his diabetes, and has had a minor myocardial infarction 3 months prior to surgery, with some occasional angina following this event.
You are also informed that the surgery was uneventful and PCA has been commenced with morphine. Mr Morgan is receiving IV fluids of Hartmann’s solution and an irrigating urinary catheter is in situ. His wife is waiting on the surgical ward.
American Association of Nurse Anesthetists www.aana.com
American Society of PeriAnesthesia Nurses www.aspan.org
Association for Perioperative Practice www.afpp.org.uk
Australian College of Operating Room Nurses www.acorn.org.au
Australia & New Zealand College of Anaesthetists www.anzca.edu.au
British Anaesthetic & Recovery Nurses Association www.barna.co.uk
International Association for the Study of Pain www.iasp-pain.org
International Federation of Nurse Anesthetists www.aana.com
Perioperative Nursing College of the New Zealand Nurses Organisation www.pnc.org.nz
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards. NR6. Postanaesthesia recovery. Adelaide: ACORN; 2006.
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards. G4. Management of postanaesthesia recovery (PAR) Unit. Adelaide: ACORN; 2006.
ACORN. ACORN standards for perioperative nursing including nursing roles, guidelines, position statements and competency standards. S19. Staffing requirements, standard statement 5. Adelaide: ACORN; 2006.
Aitkenhead A., Smith G., Rowbotham D. Textbook of anaesthesia, (5th ed.). Edinburgh: Churchill Livingstone; 2007.
Australian and New Zealand College of Anaesthetists. (2006a). Professional documents of the Australian and New Zealand College of Anaesthetists. PS4. Recommendations for the post-anaesthesia recovery room. Retrieved April 11, 2008, from http://www.anzca.edu.au/resources/professional-documents/professional-standards/ps4.html.
Australian and New Zealand College of Anaesthetists. (2006b). Professional documents of the Australian and New Zealand College of Anaesthetists. PS20. Recommendations on the responsibilities of an anaesthetist in the post-anaesthesia period. Retrieved April 11, 2008, from http://www.anzca.edu.au/resources/professional-documents/professional-standards/ps20.html.
Australian and New Zealand College of Anaesthetists. (2007). Professional documents of the Australian and New Zealand College of Anaesthetists. PS41. Guidelines on acute pain management. Retrieved April 11, 2008, from http://www.anzca.edu.au/resources/professional-documents/professional-standards/ps41.html.
Australian and New Zealand College of Anaesthetists & Faculty of Pain Medicine. Acute pain management: scientific evidence, (2nd ed.). Melbourne: ANZCA; 2005.
Australian Patient Safety Foundation. Crisis management manual. Sydney: APSF; 2006.
Ball K. Transition from the operating room to the PACU. In Drain C., Odom-Forren J., editors: Perianesthesia nursing: acritical care approach, (5th ed.), St Louis: Saunders, 2008. (pp. 354–359).
Banks A. Innovations in post operative pain management: continuous infusion of local anaesthetics. AORN Journal. 2007;85(5):904-914.
Board T., Board R. The role of 5-HT3 receptor antagonists in preventing postoperative nausea and vomiting. AORN Journal. 83(1), 2006. 209–211, 213–216, 219–220.
Boss M.J., Ewing P.H., Long S.P. Pain management in the PACU. In Drain C., Odom-Forren J., editors: Perianesthesia nursing: a critical care approach, (5th ed.), St Louis: Saunders, 2003. (pp. 437–457)
Brown D., Edwards H. Lewis’s medical-surgical nursing. Sydney: Elsevier; 2008.
Bryant B., Knights K. Pharmacology for health professionals, (2nd ed.). Sydney: Elsevier; 2007.
Burden N. Care of the ambulatory surgical patient. In Drain C., Odom-Forren J., editors: Perianesthesia nursing: a critical care approach, (5th ed.), St Louis: Saunders, 2008. (pp. 652–664)
Carlisle J.B., Stephenson C.A. Drugs for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews. 3, 2006. CD004125
Centre for Health Assets Australasia. (2006). Australasian healthcare facility guidelines. Retrieved October 10, 2007, from www.healthfacilityguidelines.com.au.
Davis T. Regional anesthesia. In Drain C., Odom-Forren J., editors: Perianesthesia nursing: a critical care approach, (5th ed.), St Louis: Saunders, 2008. (pp. 344–351)
Defazio Quinn D.M. Management and policies. In Drain C., Odom-Forren J., editors: Perianesthesia nursing: a critical care approach, (5th ed.), St Louis: Saunders, 2008. (pp. 32–42)
DeLamar L. Anesthesia. In Rothrock J.C., editor: Alexander’s care of the patient in surgery, (13th ed.), St Louis: Mosby, 2007. (pp. 103–125)
Desborough J.P. The stress response to trauma and surgery. British Journal of Anaesthesia. 2000;85(1):109-117.
Drain C.D., Odom-Forren J., editors. Perianesthesia nursing: a critical care approach, (5th ed.), St Louis: Saunders, 2008.
Faber P., Klein A. Theoretical and practical aspects of anaesthesia for thoracic surgery. Journal of Perioperative Practice. 2008;18(3):121-129.
Good K., Verble A., Secrest J., Norwood B. Post operative hypothermia—the chilling consequences. AORN Journal. 2006;83(5):1055-1066.
Hamlin L. Perioperative concepts and nursing management. In: Farrell M., editor. Smeltzer and Bare’s textbook of medical-surgical nursing. Sydney: Lippincott, Williams & Wilkins, 2005. (pp. 400–463)
Hegedus M.B. Taking the fear out of postanesthesia care in the intensive care unit. Dimensions of Critical Care Nursing. 2003;2(6):237-244.
Hockenberry M., Wilson D. Wong’s nursing care of infants and children, (8 ed.). St Louis: Mosby; 2007.
Hughes J.E. A history of The Royal Adelaide Hospital, (2nd ed.). Adelaide: Griffin Press; 1982.
Iacono M. Managing pain: an individual responsibility. Journal of PeriAnesthesia Nursing. 2004;19(3):217-219.
Klein S., Evans H., Nielsen K., et al. Peripheral nerve block techniques for ambulatory surgery. Anesthesia and Analgesia. 2005;101(6):1663-1676.
Landriscina D. Care of the paediatric patient. In Drain C., Odom-Forren J., editors: Perianesthesia nursing: a critical care approach, (5th ed.), St Louis: Saunders, 2008. (pp. 697–716)
Laurion S., Fetzer S.J. The effect of two nursing interventions on the postoperative outcomes of gynaecologic laparoscopic patients. Journal of PeriAnesthesia Nursing. 2003;18(4):254-261.
Lee, A., & Done, M. (2004). Stimulation of the wrist acupuncture point P6 for preventing postoperative nausea and vomiting. Retrieved April 11, 2008, from http://www.cochrane.org/reviews/en/ab003281.html.
Lee Y., Ngan Kee W., Chang H., So C., Gin T. Spinal ropivicaine for lower limb surgery: a dose–response study. Anesthesia and Analgesia. 2007;105(2):520-523.
Lewis S., Heitkemper M., Dirksen, et al. Medical-surgical nursing: assessment and management of clinical problems, (7th ed.), St Louis: Elsevier, 2007.
Liu W., Wu C. The effect of analgesia technique on postoperative patient-reported outcomes including analgesia: z systematic review. Anesthesia and Analgesia. 2007;105(3):789-808.
Macintyre P.E., Ready L.B. Acute pain management: a practical guide, (2nd ed.). St Louis: Saunders; 2001.
Mahajan R. Postoperative care. In: Aitkenhead A., Smith G., Rowbotham D., editors. Textbook of anaesthesia. (5th ed.). Edinburgh: Churchill Livingstone; 2007:484-509.
Mitchell M., Wilson D., Wade V. Psychosocial and cultural care of the critically ill patient. In: Elliot D., Aitken L., Chaboyer W., editors. ACCCN’s critical care nursing. Sydney: Elsevier, 2007. (pp. 153–185)
O’Brien D. Care of the perianesthesia patient. In Drain C., Odom-Forren J., editors: Perianesthesia nursing: a critical care approach, (5th ed.), St Louis: Saunders, 2008. (pp. 390–402)
Odom-Forren J. Postoperative patient care and pain management. In Rothrock J., McEwen D., editors: Alexander’s care of the patient in surgery, (13th ed.), St Louis: Mosby, 2007. (pp. 246–270)
Pasero C. Epidural analgesia for postoperative pain, Part 2. Multimodal recovery programs improve patient outcomes. American Journal of Nursing. 2003;103(11):43-45.
Pasero C. Multimodal balanced analgesia in the PACU. Journal of PeriAnesthesia Nursing. 2003;18(4):265-268.
Power I., Atcheson R. Postoperative pain. In Aitkenhead A., Smith G., Rowbotham D., editors: Textbook of anaesthesia, (5th ed.), Edinburgh: Churchill Livingstone, 2007. (pp. 510–525)
Rathmell J., Lair T., Nauman B. The role of intrathecal drugs in the treatment of acute pain. Anaesthesia and Analgesia. 2005;101(5S):S30-S43.
Rodriguez C., McMillan S., Yarandi H. Pain measurement in older adults with head and neck cancer and communication impairments. Cancer Nursing. 2004;27(6):425-433.
Rothrock J. Alexanders care of the patient in surgery, (13th ed.). St Louis: Mosby; 2007.
Sullivan E.E. Family visitation in PACU. Journal of PeriAnesthesia Nursing. 2001;16(1):29-30.
Tramèr M.R. A rational approach to the control of postoperative nausea and vomiting: evidence from systematic reviews. Part 1. Efficacy and harm of antiemetic interventions, and methodological issues. Acta Anaesthesiologica Scandinavica. 2001;45(1):4-13.
Windle P. The challenges of pain management: adverse effects of analgesics. Journal of PeriAnesthesia Nursing. 2004;19(3):212-216.
Younker J. Care of the intubated patient in the PACU: the ‘ABCDE’ approach. Journal of Perioperative Practice. 2008;18(3):116-120.
T.J. Gan, T. Meyer, C.C. Apfel, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesthesia & Analgesia, 2003;97(1):62-71.
P. Gazarian. Identifying risk factors for postoperative pulmonary complications. AORN Journal, 2006;84(4):616,618-625.
A. Hatfield, M. Tronson. The complete recovery book, 3rd ed. Oxford University Press, Oxford, 2003.
Macintyre P.E., Schug S. Acute pain management: a practical guide, 3rd ed. St Louis: Saunders; 2007.
C.B. Maloney, J. Odom. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA Journal, 1999;67(2):155-164.
National Health & Medical Research Council. Management of severe pain: report of the working party on the management of severe pain. NHMRC, Canberra, 1989.
Oakley M. Immediate postanaesthetic recovery: recommendations from the Association of Anaesthetists. British Journal of Anaesthetic & Recovery Nursing. 2003;4(1):17-19.
S. Osborne, G. Gardner, A. Gardner, et al. Using a monitored sip test to assess risk of aspiration in post operative patients. AORN Journal, 2006;83(4)., 908–912, 915–922, 925–928
Pedersen, T., Dyrlund Pedersen, B., Moller, A. (2003). Pulse oximetry for perioperative monitoring. Retrieved April 11, 2008, from http://www.cochrane.org/reviews/en/ab002013.html.
D.J. Rowbotham. Recent advances in the non-pharmacological management of postoperative nausea and vomiting. British Journal of Anaesthesia, 2005;95(1):77-81.
S. Swatton. A discharge protocol for the post anaesthetic recovery unit. British Journal of Perioperative Nursing, 2004;14(2):74-80.
Tramèr M.R. A rational approach to the control of postoperative nausea and vomiting: evidence from systematic reviews. Part 2. Recommendations for prevention and treatment and research agenda. Acta Anaesthesiologica Scandinavica. 2001;45(1):4-13.