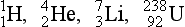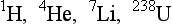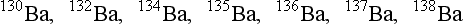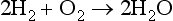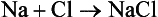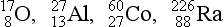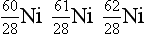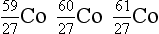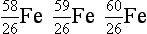The Structure of Matter
At the completion of this chapter, the student should be able to do the following:
1 Relate the history of the atom.
2 Identify the structure of the atom.
3 Describe electron shells and instability within atomic structure.
4 Discuss radioactivity and the characteristics of alpha and beta particles.
5 Explain the difference between two forms of ionizing radiation: particulate and electromagnetic.
THIS CHAPTER moves from the study of energy and force to return to the basis of matter itself. What composes matter? What is the magnitude of matter?
From the inner space of the atom to the outer space of the universe, there is an enormous range in the size of matter. More than 40 orders of magnitude are needed to identify objects as small as the atom and as large as the universe. Because matter spans such a large magnitude, exponential form is used to express the measurements of objects. Figure 2-1 shows the orders of magnitude and illustrates how matter in our surroundings varies in size.
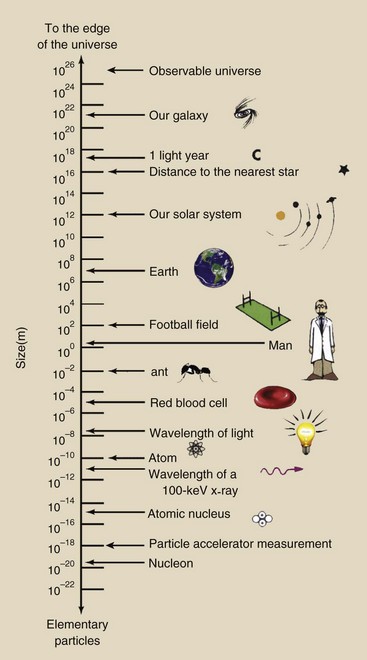
FIGURE 2-1 The size of objects varies enormously. The range of sizes in nature requires that scientific notation be used because more than 40 orders of magnitude are necessary.
The atom is the building block of the radiographer’s understanding of the interaction between ionizing radiation and matter. This chapter explains what happens when energy in the form of an x-ray interacts with tissue. Although tissue has an extremely complex structure, it is made up of atoms and combinations of atoms. By examining the structure of atoms, we can learn what happens when the structure is changed.
Centuries of Discovery
One of civilization’s most pronounced continuing scientific investigations has sought to determine precisely the structure of matter. The earliest recorded reference to this investigation comes from the Greeks several hundred years bc. Scientists at that time thought that all matter was composed of four substances: earth, water, air, and fire. According to them, all matter could be described as combinations of these four basic substances in various proportions, modified by four basic essences: wet, dry, hot, and cold. Figure 2-2 shows how this theory of matter was represented at that time.
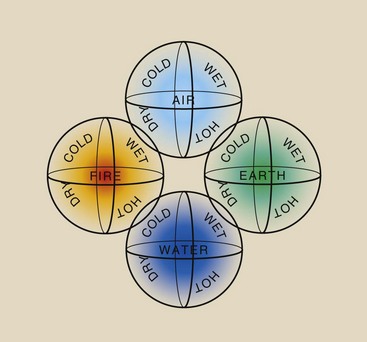
FIGURE 2-2 Symbolic representation of the substances and essences of matter as viewed by the ancient Greeks.
The Greeks used the term atom, meaning “indivisible” [a (not) + temon (cut)] to describe the smallest part of the four substances of matter. Each type of atom was represented by a symbol (Figure 2-3, A). Today, 118 substances or elements have been identified; 92 are naturally occurring, and the additional 26 have been artificially produced in high-energy particle accelerators. We now know that the atom is the smallest particle of matter that has the properties of an element. Many particles are much smaller than the atom; these are called subatomic particles.
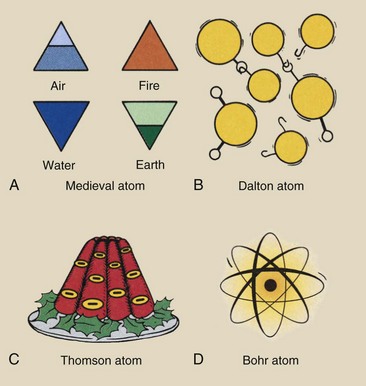
FIGURE 2-3 Through the years, the atom has been represented by many symbols. A, The Greeks envisioned four different atoms, representing air, fire, earth, and water. These triangular symbols were adopted by medieval alchemists. B, Dalton’s atoms had hooks and eyes to account for chemical combination. C, Thomson’s model of the atom has been described as a plum pudding, with the plums representing the electrons. D, The Bohr atom has a small, dense, positively charged nucleus surrounded by electrons at precise energy levels.
Dalton Atom
The Greek description of the structure of matter persisted for hundreds of years. In fact, it formed the theoretical basis for the vain efforts by medieval alchemists to transform lead into gold. It was not until the 19th century that the foundation for modern atomic theory was laid. In 1808, John Dalton, an English schoolteacher, published a book summarizing his experiments, which showed that the elements could be classified according to integral values of atomic mass.
According to Dalton, an element was composed of identical atoms that reacted the same way chemically. For example, all oxygen atoms were alike. They looked alike, they were constructed alike, and they reacted alike. They were, however, very different from atoms of any other element. The physical combination of one type of atom with another was visualized as being an eye-and-hook affair (see Figure 2-3, B). The size and number of the eyes and hooks were different for each element.
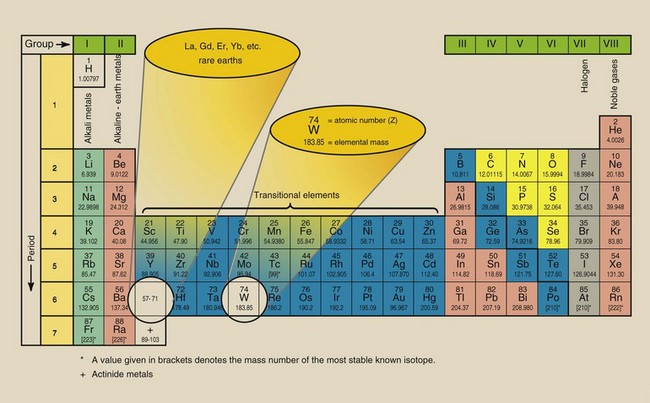
Some 50 years after Dalton’s work, a Russian scholar, Dmitri Mendeleev, showed that if the elements were arranged in order of increasing atomic mass, a periodic repetition of similar chemical properties occurred. At that time, about 65 elements had been identified. Mendeleev’s work resulted in the first periodic table of the elements. Although there were many holes in Mendeleev’s table, it showed that all the then-known elements could be placed in one of eight groups.
Figure 2-4 is a rendering of the periodic table of elements. Each block represents an element. The superscript is the atomic number. The subscript is the elemental mass.
All elements in the same group (i.e., column) react chemically in a similar fashion and have similar physical properties. Except for hydrogen, the elements of group I, called the alkali metals, are all soft metals that combine readily with oxygen and react violently with water. The elements of group VII, called halogens, are easily vaporized and combine with metals to form water-soluble salts. Group VIII elements, called the noble gases, are highly resistant to reaction with other elements.
These elemental groupings are determined by the placement of electrons in each atom. This is considered more fully later.
Thomson Atom
After the publication of Mendeleev’s periodic table, additional elements were separated and identified, and the periodic table slowly became filled. Knowledge of the structure of atoms, however, remained scanty.
Before the turn of the 20th century, atoms were considered indivisible. The only difference between the atoms of one element and the atoms of another was their mass. Through the efforts of many scientists, it slowly became apparent that there was an electrical nature to the structure of an atom.
In the late 1890s, while investigating the physical properties of cathode rays (electrons), J.J. Thomson concluded that electrons were an integral part of all atoms. He described the atom as looking something like a plum pudding, in which the plums represented negative electric charges (electrons) and the pudding was a shapeless mass of uniform positive electrification (see Figure 2-3, C). The number of electrons was thought to equal the quantity of positive electrification because the atom was known to be electrically neutral.
Through a series of ingenious experiments, Ernest Rutherford in 1911 disproved Thomson’s model of the atom. Rutherford introduced the nuclear model, which described the atom as containing a small, dense, positively charged center surrounded by a negative cloud of electrons. He called the center of the atom the nucleus.
Bohr Atom
In 1913, Niels Bohr improved Rutherford’s description of the atom. Bohr’s model was a miniature solar system in which the electrons revolved about the nucleus in prescribed orbits or energy levels. For our purposes, the Bohr atom (Figure 2-3, D) represents the best way to picture the atom, although the details of atomic structure are more accurately described by a newer model, called quantum chromodynamics (QCD).
Simply put, the Bohr atom contains a small, dense, positively charged nucleus surrounded by negatively charged electrons that revolve in fixed, well-defined orbits about the nucleus. In the normal atom, the number of electrons is equal to the number of positive charges in the nucleus.
Fundamental Particles
Our understanding of the atom today is essentially that which Bohr presented a century ago. With the development of high-energy particle accelerators, or “atom smashers,” as some call them, the structure of the atomic nucleus is slowly being mapped and identified. More than 100 subatomic particles have been detected and described by physicists working with particle accelerators.
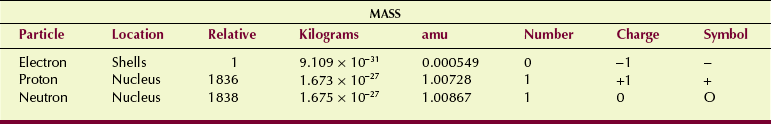
Nuclear structure is now well defined (Figure 2-5). Nucleons—protons and neutrons—are composed of quarks that are held together by gluons. These particles, however, are of little consequence to radiologic science. Only the three primary constituents of an atom, the electron, the proton, and the neutron, are considered here. They are the fundamental particles (Table 2-1).
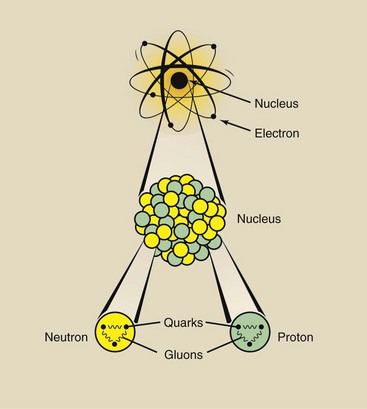
FIGURE 2-5 The nucleus consists of protons and neutrons, which are made of quarks bound together by gluons.
The atom can be viewed as a miniature solar system whose sun is the nucleus and whose planets are the electrons. The arrangement of electrons around the nucleus determines the manner in which atoms interact.
Electrons are very small particles that carry one unit of negative electric charge. Their mass is only 9.1 × 10−31 kg. They can be pictured as revolving about the nucleus in precisely fixed orbits, just as the planets in our solar system revolve around the sun.
Because an atomic particle is extremely small, its mass is expressed in atomic mass units (amu) for convenience. One atomic mass unit is equal to one twelfth the mass of a carbon-12 atom. The electron mass is 0.000549 amu. When precision is not necessary, a system of whole numbers called atomic mass numbers is used. The atomic mass number of an electron is zero.
The nucleus contains particles called nucleons, of which there are two types: protons and neutrons. Both have nearly 2000 times the mass of an electron. The mass of a proton is 1.673 × 10−27 kg; the neutron is just slightly heavier at 1.675 × 10−27 kg. The atomic mass number of each is one. The primary difference between a proton and a neutron is electric charge. The proton carries one unit of positive electric charge. The neutron carries no charge; it is electrically neutral.
Atomic Structure
You might be tempted to visualize the atom as a beehive of subatomic activity because classical representations of it usually appear like that shown in Figure 2-3, D. Because of the space limitations of the printed page, Figure 2-3, D is greatly oversimplified. In fact, the atom is mostly empty space, similar to our solar system. The nucleus of an atom is very small but contains nearly all the mass of the atom.
If a basketball, whose diameter is 0.23 m (9.6 in), represented the size of the uranium nucleus, the largest naturally occurring atom, the path of the orbital electrons would take it more than 12 km (7.2 mi) away. Because it contains all the neutrons and protons, the nucleus of the atom contains most of its mass. For example, the nucleus of a uranium atom contains 99.998% of the entire mass of the atom.
Possible electron orbits are grouped into different “shells.” The arrangement of these shells helps reveal how an atom reacts chemically, that is, how it combines with other atoms to form molecules. Because a neutral atom has the same number of electrons in orbit as protons in the nucleus, the number of protons ultimately determines the chemical behavior of an atom.
The number of protons determines the chemical element. Atoms that have the same number of protons but differ in the number of neutrons are isotopes; they behave in the same way during chemical reactions.
The periodic table of the elements (see Figure 2-4) lists matter in order of increasing complexity, beginning with hydrogen (H). An atom of hydrogen contains one proton in its nucleus and one electron outside the nucleus. Helium (He), the second atom in the table, contains two protons, two neutrons, and two electrons.
The third atom, lithium (Li), contains three protons, four neutrons, and three electrons. Two of these electrons are in the same orbital shell, the K shell, as are the electrons of hydrogen and helium. The third electron is in the next farther orbital shell from the nucleus, the L shell.
Electrons can exist only in certain shells, which represent different electron binding energies or energy levels. For identification purposes, electron orbital shells are given the codes K, L, M, N, and so forth, to represent the relative binding energies of electrons from closest to the nucleus to farthest from the nucleus. The closer an electron is to the nucleus, the greater is its binding energy.
The next atom on the periodic table, beryllium (Be), has four protons and five neutrons in the nucleus. Two electrons are in the K shell, and two are in the L shell.
The complexity of the electron configuration of atoms increases as one progresses through the periodic table to the most complex naturally occurring element, uranium (U). Uranium has 92 protons and 146 neutrons. The electron distribution is as follows: 2 in the K shell, 8 in the L shell, 18 in the M shell, 32 in the N shell, 21 in the O shell, 9 in the P shell, and 2 in the Q shell.
Figure 2-6 is a schematic representation of four atoms. Although these atoms are mostly empty space, they have been diagrammed on one page. If the actual size of the helium nucleus were that in Figure 2-6, the K-shell electrons would be several city blocks away.
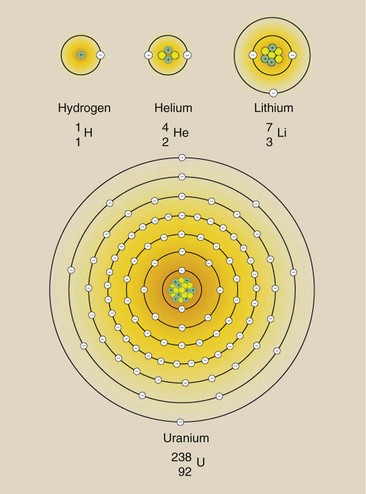
FIGURE 2-6 Atoms are composed of neutrons and protons in the nucleus and electrons in specific orbits surrounding the nucleus. Shown here are the three smaller atoms and the largest naturally occurring atom, uranium.
The total number of electrons in the orbital shells is exactly equal to the number of protons in the nucleus. If an atom has an extra electron or has had an electron removed, it is said to be ionized. An ionized atom is not electrically neutral but carries a charge equal in magnitude to the difference between the numbers of electrons and protons.
You might assume that atoms can be ionized by changing the number of positive charges as well as the number of negative charges. Atoms, however, cannot be ionized by the addition or subtraction of protons because they are bound very strongly together, and that action would change the type of atom. An alteration in the number of neutrons does not ionize an atom because the neutron is electrically neutral.
Figure 2-7 represents the interaction between an x-ray and a carbon atom, a primary constituent of tissue. The x-ray transfers its energy to an orbital electron and ejects that electron from the atom. This process requires approximately 34 eV of energy. The x-ray may cease to exist, and an ion pair is formed. The remaining atom is now a positive ion because it contains one more positive charge than negative charge.
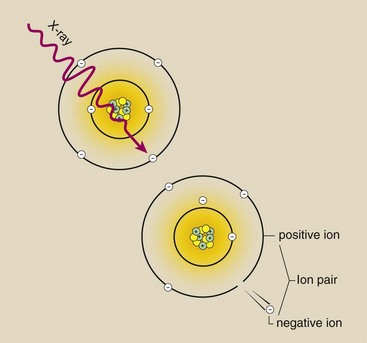
FIGURE 2-7 Ionization of a carbon atom by an x-ray leaves the atom with a net electric charge of +1. The ionized atom and the released electron are called an ion pair.
In all except the lightest atoms, the number of neutrons is always greater than the number of protons. The larger the atom, the greater the abundance of neutrons over protons.
Electron Arrangement
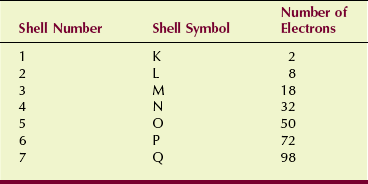
The maximum number of electrons that can exist in each shell (Table 2-2) increases with the distance of the shell from the nucleus. These numbers need not be memorized because the electron limit per shell can be calculated from the expression:

This answer, 50 electrons, is a theoretical value. Even the largest atom does not completely fill shell O or higher.
Physicists call the shell number n the principal quantum number. Every electron in every atom can be precisely identified by four quantum numbers, the most important of which is the principal quantum number. The other three quantum numbers represent the existence of subshells, which are not important to radiologic science.
The observant reader may have noticed a relationship between the number of shells in an atom and its position in the periodic table of the elements. Oxygen has eight electrons; two occupy the K shell, and six occupy the L shell. Oxygen is in the second period (row) and the sixth group (column) of the periodic table (see Figure 2-4).
Aluminum has the following electron configuration: K shell, two electrons; L shell, eight electrons; M shell, three electrons. Therefore, aluminum is in the third period (M shell) and third group (three electrons) of the periodic table.
| Question: | What are the period and group for the gastrointestinal contrast agent, barium (refer to Figure 2-4)? |
| Answer: | Period 6 and group II. |
Why does the periodic table show elements repeating similar chemical properties in groups of eight? In addition to the limitation on the maximum number of electrons allowed in any shell, the outer shell is always limited to eight electrons.
All atoms that have one electron in the outer shell lie in group I of the periodic table; atoms with two electrons in the outer shell fall in group II, and so forth. When eight electrons are in the outer shell, the shell is filled. Atoms with filled outer shells lie in group VIII, the noble gases, and are very chemically stable.
The orderly scheme of atomic progression from smallest to largest atom is interrupted in the fourth period. Instead of simply adding electrons to the next outer shell, electrons are added to an inner shell.
The atoms associated with this phenomenon are called the transitional elements. Even in these elements, no outer shell ever contains more than eight electrons. The chemical properties of the transitional elements depend on the number of electrons in the two outermost shells.
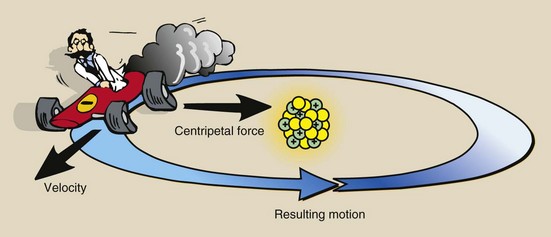
The shell notation of the electron arrangement of an atom not only identifies the relative distance of an electron from the nucleus but also indicates the relative energy by which the electron is attached to the nucleus. You might expect that an electron would spontaneously fly off from the nucleus, just as a ball twirling on the end of a string would do if the string were cut. The type of force that prevents this from happening is called centripetal force or “center-seeking” force, which results from a basic law of electricity that states that opposite charges attract one another and like charges repel.
You might therefore expect that the electrons would drop into the nucleus because of the strong electrostatic attraction. In the normal atom, the centripetal force just balances the force created by the electron velocity, the centrifugal force or flying-out-from-the-center force, so that electrons maintain their distance from the nucleus while traveling in a circular or elliptical path.
Figure 2-8 is a representation of this state of affairs for a small atom. In more complex atoms, the same balance of force exists and each electron can be considered separately.
Electron Binding Energy
The strength of attachment of an electron to the nucleus is called the electron binding energy, designated Eb. The closer an electron is to the nucleus, the more tightly it is bound. K-shell electrons have higher binding energies than L-shell electrons, L-shell electrons are more tightly bound to the nucleus than M-shell electrons, and so forth.
Not all K-shell electrons of all atoms are bound with the same binding energy. The greater the total number of electrons in an atom, the more tightly each is bound.
To put it differently, the larger and more complex the atom, the higher is the Eb for electrons in any given shell. Because electrons of atoms with many protons are more tightly bound to the nucleus than those of small atoms, it generally takes more energy to ionize a large atom than a small atom.
Figure 2-9 represents the binding energy of electrons of several atoms of radiologic importance. The metals tungsten (W) and molybdenum (Mo) are used as targets in an x-ray tube. Barium (Ba) and iodine (I) are used as radiographic and fluoroscopic contrast agents.
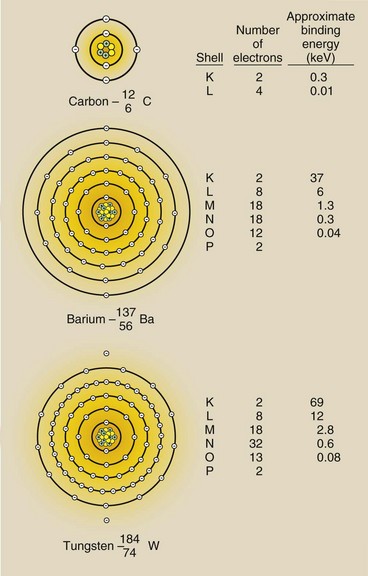
FIGURE 2-9 Atomic configurations and approximate electron binding energies for three radiologically important atoms. As atoms get bigger, electrons in a given shell become more tightly bound.
| Question: | How much energy is required to ionize tungsten through removal of a K-shell electron? |
| Answer: | The minimum energy must equal Eb or 69 keV—with less than that, the atom cannot be ionized. |
Carbon (C) is an important element in human tissue. As with other tissue atoms, Eb for the outer shell electrons is only approximately 10 eV. Yet approximately 34 eV is necessary to ionize tissue atoms. The value 34 eV is called the ionization potential. The difference, 24 eV, causes multiple electron excitations, which ultimately result in heat. The concept of ionization potential is important to the description of linear energy transfer (LET), which is discussed in Chapter 30.

Atomic Nomenclature
Often an element is indicated by an alphabetic abbreviation. Such abbreviations are called chemical symbols. Table 2-3 lists some of the important elements and their chemical symbols.
TABLE 2-3
Characteristics of Some Elements Important to Radiologic Science
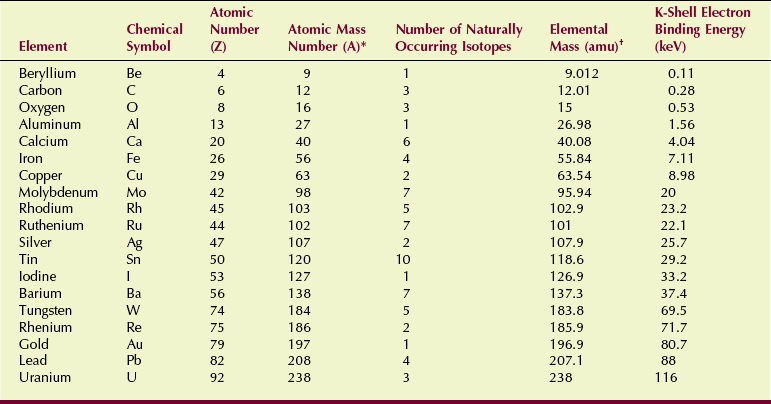
amu, atomic mass units; keV, electron kilovolt.
*Most abundant isotope.
†Average of naturally occurring isotopes.
The chemical properties of an element are determined by the number and arrangement of electrons. In the neutral atom, the number of electrons equals the number of protons. The number of protons is called the atomic number, represented by Z. Table 2-3 shows that the atomic number of barium is 56, thus indicating that 56 protons are in the barium nucleus.
The number of protons plus the number of neutrons in the nucleus of an atom is called the atomic mass number, symbolized by A. The atomic mass number is always a whole number. The use of atomic mass numbers is helpful in many areas of radiologic science.
An atom’s atomic mass number is a whole number that is equal to the number of nucleons in the atom. The actual atomic mass of an atom is determined by measurement and rarely is a whole number. 135Ba has A = 135 because its nucleus contains 56 protons and 79 neutrons. The atomic mass of 135Ba is 134.91 amu.
Only one atom, 12C, has an atomic mass equal to its atomic mass number. This occurs because the 12C atom is the arbitrary standard for atomic measure.
Many elements in their natural state are composed of atoms with different atomic mass numbers and different atomic masses but identical atomic numbers. The characteristic mass of an element, the elemental mass, is determined by the relative abundance of isotopes and their respective atomic masses.
Barium, for example, has an atomic number of 56. The atomic mass number of its most abundant isotope is 138. Natural barium, however, consists of seven different isotopes with atomic mass numbers of 130, 132, 134, 135, 136, 137, and 138; the elemental mass is determined by calculating the average of all these isotopes.
With the protocol described in Figure 2-10, the atoms of Figure 2-6 would have the following symbolic representation:
Because the chemical symbol also indicates the atomic number, the subscript is often omitted.
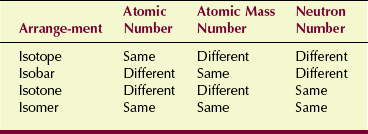
Isotopes of a given element contain the same number of protons but varying numbers of neutrons. Most elements have more than one stable isotope. The seven natural isotopes of barium are as follows:
The term isotope describes all atoms of a given element. Such atoms have different nuclear configurations but nevertheless react the same way chemically.

Isobars are atoms that have different numbers of protons and different numbers of neutrons but the same total number of nucleons. Isobaric radioactive transitions from parent atom to daughter atom result from the release of a beta particle or a positron. The parent and the daughter are atoms of different elements.
Isotones are atoms with different atomic numbers and different mass numbers but a constant value for the quantity A−Z. Consequently, isotones are atoms with the same number of neutrons in the nucleus.
The final category of atomic configuration is the isomer.
In fact, isomers are identical atoms except that they exist at different energy states because of differences in nucleon arrangement. Technetium-99m decays to technetium-99 with the emission of a 140-keV gamma ray, which is very useful in nuclear medicine. Table 2-4 presents a summary of the characteristics of these nuclear arrangements.
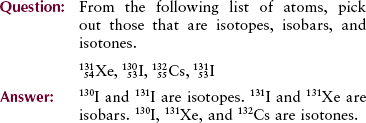
One method of association to help with these iso- definitions is: isotope, same proton; isobar, same A; isotone, same neutron; and isomer, metastable.
Combinations of ATOMS
Four atoms of hydrogen (H2) and two atoms of oxygen (O2) can combine to form two molecules of water (2 H2O). The following equation represents this atomic combination:
An atom of sodium (Na) can combine with an atom of chlorine (Cl) to form a molecule of sodium chloride (NaCl), which is common table salt:
Both of these molecules are common in the human body. Molecules, in turn, may combine to form even larger structures: cells and tissues.
Although more than 100 different elements are known, most elements are rare. Approximately 95% of the Earth and its atmosphere consists of only a dozen elements. Similarly, hydrogen, oxygen, carbon, and nitrogen compose more than 95% of the human body. Water molecules make up approximately 80% of the human body.
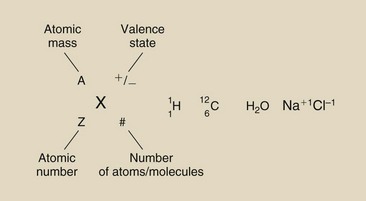
There is an organized scheme for representing elements in a molecule (see Figure 2-10). The shorthand notation that incorporates the chemical symbol with subscripts and superscripts is used to identify atoms.
The chemical symbol (X) is positioned between two subscripts and two superscripts. The subscript and superscript to the left of the chemical symbol represent atomic number and atomic mass number, respectively. The subscript and superscript to the right are values for the number of atoms per molecule and the valence state of the atom, respectively.
The formula NaCl represents one molecule of the compound sodium chloride. Sodium chloride has properties that are different from those of sodium or chlorine. Atoms combine with each other to form compounds (chemical bonding) in two main ways. The examples of H2O and NaCl can be used to describe these two types of chemical bonds.
Oxygen and hydrogen combine into water through covalent bonds. Oxygen has six electrons in its outermost shell. It has room for two more electrons, so in a water molecule, two hydrogen atoms share their single electrons with the oxygen. The hydrogen electrons orbit the H and the O, thus binding the atoms together. This covalent bonding is characterized by the sharing of electrons.
Sodium and chlorine combine into salt through ionic bonds. Sodium has one electron in its outermost shell. Chlorine has space for one more electron in its outermost shell. The sodium atom will give up its electron to the chlorine. When it does, it becomes ionized because it has lost an electron and now has an imbalance of electric charges.
The chlorine atom also becomes ionized because it has gained an electron and now has more electrons than protons. The two atoms are attracted to each other, resulting in an ionic bond because they have opposite electrostatic charges.
Sodium, hydrogen, carbon, and oxygen atoms can combine to form a molecule of sodium bicarbonate (NaHCO3). A measurable quantity of sodium bicarbonate constitutes a chemical compound commonly called baking soda.
The interrelations between atoms, elements, molecules, and compounds are orderly. This organizational scheme is what the ancient Greeks were trying to describe by their substances and essences. Figure 2-11 is a diagram of this current scheme of matter.
Radioactivity
Some atoms exist in an abnormally excited state characterized by an unstable nucleus. To reach stability, the nucleus spontaneously emits particles and energy and transforms itself into another atom. This process is called radioactive disintegration or radioactive decay. The atoms involved are radionuclides. Any nuclear arrangement is called a nuclide; only nuclei that undergo radioactive decay are radionuclides.
Radioisotopes
Many factors affect nuclear stability. Perhaps the most important is the number of neutrons. When a nucleus contains too few or too many neutrons, the atom can disintegrate radioactively, bringing the number of neutrons and protons into a stable and proper ratio.
In addition to stable isotopes, many elements have radioactive isotopes or radioisotopes. These may be artificially produced in machines such as particle accelerators or nuclear reactors. Seven radioisotopes of barium have been discovered, all of which are artificially produced. In the following list of barium isotopes, the radioisotopes are boldface:
127Ba, 128Ba, 129Ba, 130Ba, 131Ba, 132Ba, 133Ba,
134Ba, 135Ba, 136Ba, 137Ba, 138Ba, 139Ba, 140Ba
Artificially produced radioisotopes have been identified for nearly all elements. A few elements have naturally occurring radioisotopes as well.
There are two primary sources of naturally occurring radioisotopes. Some originated at the time of the Earth’s formation and are still decaying very slowly. An example is uranium, which ultimately decays to radium, which in turn decays to radon. These and other decay products of uranium are radioactive. Others, such as 14C, are continuously produced in the upper atmosphere through the action of cosmic radiation.
Radioisotopes can decay to stability in many ways, but two, beta emission and alpha emission, are important here for descriptive purposes. Radioactive decay by positron emission is important for some nuclear medicine imaging.
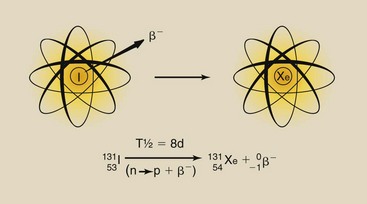
During beta emission, an electron created in the nucleus is ejected from the nucleus with considerable kinetic energy and escapes from the atom. The result is the loss of a small quantity of mass and one unit of negative electric charge from the nucleus of the atom. Simultaneously, a neutron undergoes conversion to a proton.
The result of beta emission therefore is to increase the atomic number by one (Z → Z + 1), while the atomic mass number remains the same (A = constant). This nuclear transformation results in the changing of an atom from one type of element to another (Figure 2-12).
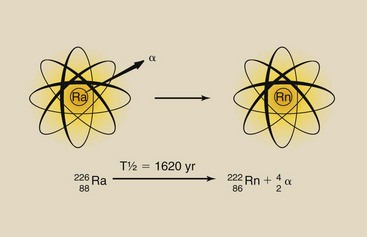
Radioactive decay by alpha emission is a much more violent process. The alpha particle consists of two protons and two neutrons bound together; its atomic mass number is 4. A nucleus must be extremely unstable to emit an alpha particle, but when it does, it loses two units of positive charge and four units of mass. The transformation is significant because the resulting atom is not only chemically different but is also lighter by 4 amu (Figure 2-13).
Beta emission occurs much more frequently than alpha emission. Virtually all radioisotopes are capable of transformation by beta emission, but only heavy radioisotopes are capable of alpha emission. Some radioisotopes are pure beta emitters or pure alpha emitters, but most emit gamma rays simultaneously with the particle emission.

Radioactive Half-life
Radioactive matter is not here one day and gone the next. Rather, radioisotopes disintegrate into stable isotopes of different elements at a decreasing rate so that the quantity of radioactive material never quite reaches zero. Remember from Chapter 1 that radioactive material is measured in becquerels and that 1 Bq is equal to disintegration of 1 atom each second.
The rate of radioactive decay and the quantity of material present at any given time are described mathematically by a formula known as the radioactive decay law. From this formula, we obtain a quantity known as half-life ( ). Half-lives of radioisotopes vary from less than a second to many years. Each radioisotope has a unique, characteristic half-life.
). Half-lives of radioisotopes vary from less than a second to many years. Each radioisotope has a unique, characteristic half-life.
The half-life of 131I is 8 days (Figure 2-14). If 10 MBq of 131I was present on January 1 at noon, then at noon on January 9, only 5 MBq would remain. On January 17, 2.5 MBq would remain, and on January 25, 1.25 MBq would remain. A plot of the radioactive decay of 131I allows one to determine the amount of radioactivity remaining after any given length of time (see Figure 2-14).
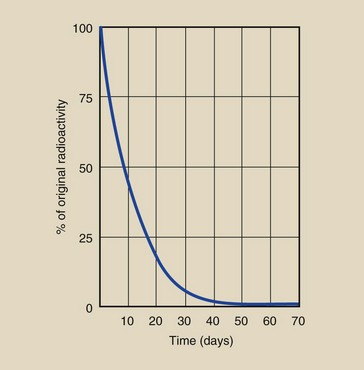
FIGURE 2-14 131I decays with a half-life of 8 days. This linear graph allows estimation of radioactivity only for a short time.
After approximately 24 days, or three half-lives, the linear-linear plot of the decay of 131I becomes very difficult to read and interpret. Consequently, such graphs are usually presented in semilogarithmic form (Figure 2-15). With a presentation such as this, one can estimate radioactivity after a very long time.
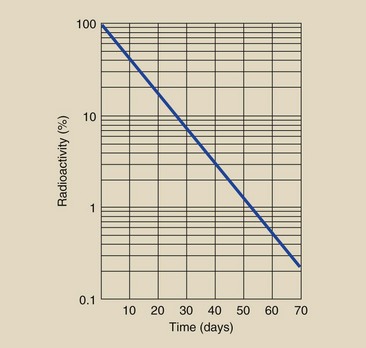
FIGURE 2-15 This semilog graph is useful for estimating the radioactivity of 131I at any given time.
| Question: | On Monday at 8 am, 10 MBq of 131I is present. How much will remain on Friday at 5 pm? |
| Answer: | The time of decay is 4 days. According to Figure 2-15, at 4 days, approximately 63% of the original activity will remain. Therefore, 6.3 MBq will be present on Friday at 5 pm. |
Theoretically, all the radioactivity of a radioisotope never disappears. After each period of time equivalent to one half-life, one-half the activity present at the beginning of that time will remain. Therefore, although the quantity of a radioisotope progressively decreases, it never quite reaches zero.
Figure 2-16 shows two similar graphs used to estimate the quantity of any radioisotope remaining after any length of time. In these graphs, the percentage of original radioactivity remaining is plotted against time, measured in units of half-life. To use these graphs, one must express the initial radioactivity as 100% and convert the time of interest into units of half-life. For decay times exceeding three half-lives, the semilog form is easier to use.
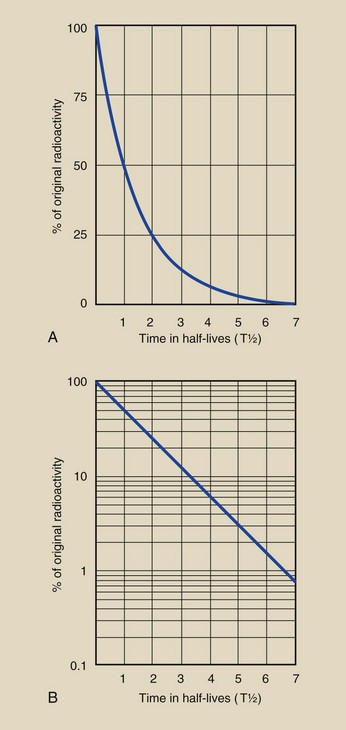
FIGURE 2-16 The radioactivity after any period can be estimated from the linear (A) or the semilog (B) graph. The original quantity is assigned a value of 100%, and the time of decay is expressed in units of half-life.

14C is a naturally occurring radioisotope with 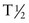 = 5730 years. The concentration of 14C in the environment is constant, and 14C is incorporated into living material at a constant rate. Trees of the Petrified Forest contain less 14C than living trees because the 14C of living trees is in equilibrium with the atmosphere; the carbon in a petrified tree was fixed many thousands of years ago, and the fixed 14C is reduced over time by radioactive decay (Figure 2-17).
= 5730 years. The concentration of 14C in the environment is constant, and 14C is incorporated into living material at a constant rate. Trees of the Petrified Forest contain less 14C than living trees because the 14C of living trees is in equilibrium with the atmosphere; the carbon in a petrified tree was fixed many thousands of years ago, and the fixed 14C is reduced over time by radioactive decay (Figure 2-17).
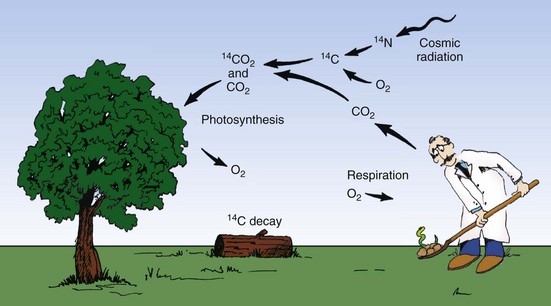
FIGURE 2-17 Carbon is a biologically active element. A small fraction of all carbon is the radioisotope 14C. As a tree grows, 14C is incorporated into the wood in proportion to the amount of 14C in the atmosphere. When the tree dies, further exchange of 14C with the atmosphere does not take place. If the dead wood is preserved by petrification, the 14C content diminishes as it radioactively decays. This phenomenon serves as the basis for radiocarbon dating.

A simpler approach finds the answer more precisely on Figure 2-16: 6.5 half-lives. Another approach is to use the following relationship:
The concept of half-life is essential to radiologic science. It is used daily in nuclear medicine and has an exact parallel in x-ray terminology, the half-value layer. The better you understand half-life now, the better you will understand the meaning of half-value layer later.
Types of Ionizing Radiation
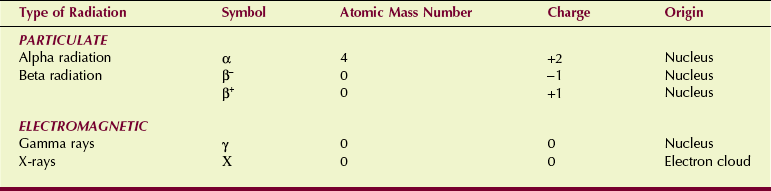
All ionizing radiation can be conveniently classified into two categories: particulate radiation and electromagnetic radiation (Table 2-5). The types of radiation used in diagnostic ultrasonography and in magnetic resonance imaging are nonionizing radiation.
Although all ionizing radiation acts on biologic tissue in the same manner, there are fundamental differences between various types of radiation. These differences can be analyzed according to five physical characteristics: mass, energy, velocity, charge, and origin.
Particulate Radiation
Many subatomic particles are capable of causing ionization. Consequently, electrons, protons, and even rare nuclear fragments all can be classified as particulate ionizing radiation if they are in motion and possess sufficient kinetic energy. At rest, they cannot cause ionization.
There are two main types of particulate radiation: alpha particles and beta particles. Both are associated with radioactive decay.
The alpha particle is equivalent to a helium nucleus. It contains two protons and two neutrons. Its mass is approximately 4 amu, and it carries two units of positive electric charge. Compared with an electron, the alpha particle is large and exerts great electrostatic force. Alpha particles are emitted only from the nuclei of heavy elements. Light elements cannot emit alpha particles because they do not have enough excess mass (excess energy).
After being emitted from a radioactive atom, the alpha particle travels with high velocity through matter. Because of its great mass and charge, however, it easily transfers this kinetic energy to orbital electrons of other atoms.
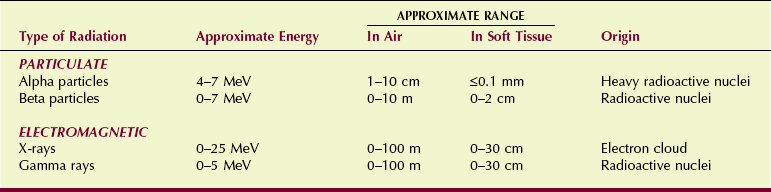
Ionization accompanies alpha radiation. The average alpha particle possesses 4 to 7 MeV of kinetic energy and ionizes approximately 40,000 atoms for every centimeter of travel through air.
Because of this amount of ionization, the energy of an alpha particle is quickly lost. It has a very short range in matter. Whereas in air, alpha particles can travel approximately 5 cm; in soft tissue, the range may be less than 100 µm. Consequently, alpha radiation from an external source is nearly harmless because the radiation energy is deposited in the superficial layers of the skin.
With an internal source of radiation, just the opposite is true. If an alpha-emitting radioisotope is deposited in the body, it can intensely irradiate the local tissue. Radon irradiating lung tissue is an important example.
Beta particles differ from alpha particles in terms of mass and charge. They are light particles with an atomic mass number of 0 and carry one unit of negative or positive charge. The only difference between electrons and negative beta particles is their origin. Beta particles originate in the nuclei of radioactive atoms and electrons exist in shells outside the nuclei of all atoms.
Positive beta particles are positrons. They have the same mass as electrons and are considered to be antimatter. We will see positrons again when we discuss pair production.
After being emitted from a radioisotope, beta particles traverse air, ionizing several hundred atoms per centimeter. The beta particle range is longer than that for the alpha particle. Depending on its energy, a beta particle may traverse 10 to 100 cm of air and approximately 1 to 2 cm of soft tissue.
Electromagnetic Radiation
X-rays and gamma rays are forms of electromagnetic ionizing radiation. This type of radiation is covered more completely in the next chapter; the discussion here is necessarily brief.
X-rays and gamma rays are often called photons. Photons have no mass and no charge. They travel at the speed of light (c = 3 × 108 m/s) and are considered energy disturbances in space.
Just as the only difference between beta particles and electrons is their origin, so the only difference between x-rays and gamma rays is their origin. Gamma rays are emitted from the nucleus of a radioisotope and are usually associated with alpha or beta emission. X-rays are produced outside the nucleus in the electron shells.
X-rays and gamma rays exist at the speed of light or not at all. After being emitted, they have an ionization rate in air of approximately 100 ion pairs/cm, about equal to that for beta particles. In contrast to beta particles, however, x-rays and gamma rays have an unlimited range in matter.
Photon radiation loses intensity with distance but theoretically never reaches zero. Particulate radiation, on the other hand, has a finite range in matter, and that range depends on the particle’s energy.
Table 2-6 summarizes the more important characteristics of each of these types of ionizing radiation. In nuclear medicine, beta and gamma radiation are most important. In radiography, only x-rays are important. The penetrability and low ionization rate of x-rays make them particularly useful for medical imaging (Figure 2-18).
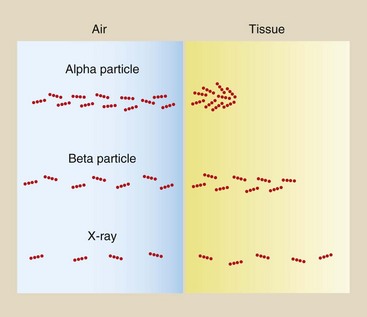
FIGURE 2-18 Different types of radiation ionize matter with different degrees of efficiency. Alpha particles represent highly ionizing radiation with a very short range in matter. Beta particles do not ionize so readily and have a longer range. X-rays have a low ionization rate and a very long range.
Summary
As a miniature solar system, the Bohr atom set the stage for the modern interpretation of the structure of matter. An atom is the smallest part of an element, and a molecule is the smallest part of a compound.
The three fundamental particles of the atom are the electron, proton, and neutron. Electrons are negatively charged particles that orbit the nucleus in configurations or shells held in place by electrostatic forces. Chemical reactions occur when outermost orbital electrons are shared or given up to other atoms. Nucleons, neutrons, and protons each have nearly 2000 times the mass of electrons. Protons are positively charged, and neutrons have no charge.
Elements are grouped in a periodic table in order of increasing complexity. The groups on the table indicate the number of electrons in the outermost shell. The elements in the periods on the periodic table have the same number of orbital shells.
Some atoms have the same number of protons and electrons as other elements but a different number of neutrons, giving the element a different atomic mass. These are isotopes.
Some atoms, which contain too many or too few neutrons in the nucleus, can disintegrate. This is called radioactivity. Two types of particulate emission that occur after radioactive disintegration are alpha and beta particles. The half-life of a radioisotope is the time required for the quantity of radioactivity to be reduced to one-half its original value.
Ionizing radiation consists of particulate and electromagnetic radiation. Alpha and beta particles produce particulate radiation. Alpha particles have four atomic mass units, are positive in charge, and originate from the nucleus of heavy elements. Beta particles have an atomic mass number of zero and have one unit of negative charge. Beta particles originate in the nucleus of radioactive atoms.
X-rays and gamma rays are forms of electromagnetic radiation called photons. These rays have no mass and no charge. X-rays are produced in the electron shells, and gamma rays are emitted from the nucleus of a radioisotope.
1. Define or otherwise identify the following:
2. Figure 2-1 shows the following approximate sizes: an atom, 10−10 m; the Earth, 107 m. By how many orders of magnitude do these objects differ?
3. How many protons, neutrons, electrons, and nucleons are found in the following?
4. Using the data in Table 2-1, determine the mass of 99Tc in atomic mass units and in kilograms.
5. Diagram the expected electron configuration of 40Ca.
6. If atoms large enough to have electrons in the T shell existed, what would be the maximum number allowed in that shell?
7. How much more tightly bound are K-shell electrons in tungsten than (a) L-shell electrons, (b) M-shell electrons, and (c) free electrons? (Refer to Figure 2-9.)
8. From the following list of nuclides, identify sets of isotopes, isobars, and isotones.
9.  Sr has a half-life of 29 years. If 10 MBq were present in 1950, approximately how much would remain in 2010?
Sr has a half-life of 29 years. If 10 MBq were present in 1950, approximately how much would remain in 2010?
10. Complete the following table with relative values.
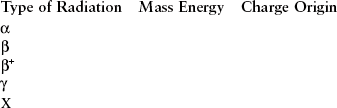
11. For what is Mendeleev remembered?
12. Who developed the concept of the atom as a miniature solar system?
13. List the fundamental particles within an atom.
14. What property of an atom does binding energy describe?
15. Can atoms be ionized by changing the number of positive charges?
16. Describe how ion pairs are formed.
17. What determines the chemical properties of an element?
18. Why doesn’t an electron spontaneously fly away from the nucleus of an atom?
19. Describe the difference between alpha and beta emission.
20. How does carbon-14 dating determine the age of petrified wood?
The answers to the Challenge Questions can be found by logging on to our website at http://evolve.elsevier.com.