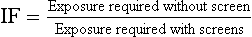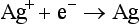Screen-Film Radiography
At the completion of this chapter, the student should be able to do the following:
1 Discuss the construction of radiographic film.
2 Describe the formation of the latent image.
3 Describe the construction of a radiographic intensifying screen.
4 Explain luminescence and its relationship to phosphorescence and fluorescence.
5 Explain detective quantum efficiency and conversion efficiency and how they affect image receptor speed and image noise.
IMAGE-FORMING x-rays exit the patient and expose the radiographic intensifying screen placed in the protective radiographic cassette. The radiographic intensifying screen emits visible light, which exposes the radiographic film placed between the two screens. Although some x-rays reach the film emulsion, it is primarily light from the radiographic intensifying screens that expose the radiographic film.
Processing the invisible latent image creates the visible image. Processing causes the silver ions in the silver halide crystal that have been exposes to light to be converted into microscopic grains of silver. The film processing sequence–wetting, developing, rinsing, fixing, washing, and drying–is completed in 90 seconds in an automatic processor.
This chapter covers the information required for an understanding of the radiographic screen-film receptor and the production of the visibile image.
The primary purpose of radiographic imaging is to transfer information from an x-ray beam to the eye–brain complex of the radiologist. The x-ray beam that emerges from the x-ray tube is nearly uniformly distributed in space. After interaction with the patient, the beam of image-forming x-rays is not uniformly distributed in space but varies in intensity according to the characteristics of the tissue through which it has passed.
The exit x-ray beam refers to the x-rays that remain as the useful beam exits the patient. It consists of x-rays scattered away from the image receptor and image-forming x-rays.
The diagnostically useful information in this exit x-ray beam must be transferred to a form that is intelligible to the radiologist. X-ray film is one such medium. Other media include the fluoroscopic image intensifier, the television or flat panel monitor, the laser imaging system, and solid-state detectors, all of which are discussed later. The medium that converts the x-ray beam into a visible image is called the image receptor (IR). The classical IR is photographic film, although solid-state digital IRs are replacing film.
Photography has its origins in the early 19th century. By the time of the American Civil War (1860–1865), photography was professionally used. Amateur photography surfaced early in the 20th century.
The construction and characteristics of radiographic film are similar to those of regular photographic film. Radiographic film is manufactured with rigorous quality control and has a spectral response different from that of photographic film; however, its mechanism of operation is much the same. The following discussion concerns radiographic film, but with very few modifications, it could be applied to photographic film.
Radiographic Film
The manufacture of radiographic film is a precise procedure that requires tight quality control. Manufacturing facilities are extremely clean because the slightest bit of contaminant in the film limits the film’s ability to reproduce information from the x-ray beam.
During the early 1960s, at the height of nuclear weapons testing, x-ray film manufacturers took extraordinary precautions to ensure that contamination from radioactive fallout did not invade their manufacturing environment. Such contamination could seriously fog the film.
Radiographic film has two parts: the base and the emulsion (Figure 12-1). In most x-ray film, the emulsion is coated on both sides; therefore, it is called double-emulsion film. Between the emulsion and the base is a thin coating of material called the adhesive layer, which ensures uniform adhesion of the emulsion to the base. This adhesive layer allows the emulsion and the base to maintain proper contact and integrity during use and processing.
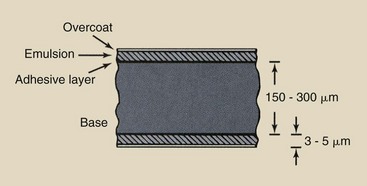
FIGURE 12-1 Cross section of radiographic film. The bulk of the film is the base. The emulsion contains the latent image, which becomes visible when processed.
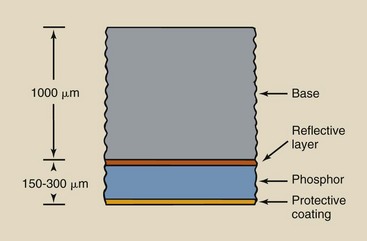
The emulsion is enclosed by a protective covering of gelatin called the overcoat. This overcoat protects the emulsion from scratches, pressure, and contamination during handling, processing, and storage. The thickness of radiographic film is approximately 150 to 300 µm.
Base
The base is the foundation of radiographic film. Its primary purpose is to provide a rigid structure onto which the emulsion can be coated. The base is flexible and fracture resistant to allow easy handling but is rigid enough to be snapped into a viewbox.
Conventional photographic film has a much thinner base than radiographic film and therefore is not as rigid. Can you imagine attempting to snap a 35 × 43 cm photographic negative into a viewbox?
The base of radiographic film maintains its size and shape during use and processing so that it does not contribute to image distortion. This property of the base is known as dimensional stability. The base is of uniform lucency and is nearly transparent to light.
During manufacturing, however, dye is added to the base of most radiographic film to slightly tint the film blue. Compared with untinted film, this coloring reduces eyestrain and fatigue, enhancing radiologists’ diagnostic efficiency and accuracy.
The original radiographic film base was a glass plate. Radiologists used to refer to radiographs as x-ray plates. During World War I, high-quality glass became largely unavailable while medical applications of x-rays, particularly by the military, were increasing rapidly.
A substitute material, cellulose nitrate, soon became the standard base. Cellulose nitrate, however, had one serious deficiency: It was flammable. Improper storage and handling of some x-ray film files resulted in severe hospital fires during the 1920s and early 1930s.
By the mid-1920s, film with a “safety base,” cellulose triacetate, was introduced. Cellulose triacetate has properties similar to those of cellulose nitrate but is not as flammable.
In the early 1960s, a polyester base was introduced. Polyester has taken the place of cellulose triacetate as the film base of choice. Polyester is more resistant to warping from age and is stronger than cellulose triacetate, permitting easier transport through automatic processors. Its dimensional stability is superior. Polyester bases are thinner than triacetate bases (≈175 µm) but are just as strong.
Emulsion
The emulsion is the heart of the radiographic film. It is the material with which x-rays or light photons from radiographic intensifying screens interact. The emulsion consists of a homogeneous mixture of gelatin and silver halide crystals. It is coated evenly with a layer that is 3 to 5 µm thick.
The gelatin is similar to that used in salads and desserts but is of much higher quality. It is clear, so it transmits light, and it is sufficiently porous for processing chemicals to penetrate to the crystals of silver halide. Its principal function is to provide mechanical support for silver halide crystals by holding them uniformly dispersed in place.
The silver halide crystal is the active ingredient of the radiographic emulsion. In the typical emulsion, 98% of the silver halide is silver bromide; the remainder is usually silver iodide. These atoms have relatively high atomic numbers (ZBr = 35; ZAg = 47; ZI = 53) compared with the gelatin and the base (for both, Z ≈ 7). The interaction of x-ray and light photons with these high-Z atoms ultimately results in the formation of a latent image on the radiograph.
Depending on the intended imaging application, silver halide crystals may have tabular, cubic, octahedral, polyhedral, or irregular shapes. Tabular grains are used in most radiographic films.
Tabular silver halide crystals are flat and typically 0.1 µm thick, with a triangular, hexagonal, or higher-order polygonal cross section. The crystals are approximately 1 µm in diameter. The arrangement of atoms in a crystal is cubic, as shown in Figure 12-2.
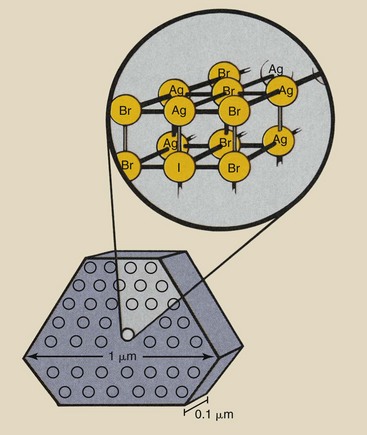
FIGURE 12-2 An example of a tabular silver halide crystal. The arrangement of atoms in the crystal is cubic.
The crystals are made by dissolving metallic silver (Ag) in nitric acid (HNO3) to form silver nitrate (AgNO3). Light-sensitive silver bromide (AgBr) crystals are formed by mixing silver nitrate with potassium bromide (KBr) in the following reaction:
The entire process takes place in the presence of gelatin and with precise control of temperature, pressure, and the rate at which ingredients are mixed.
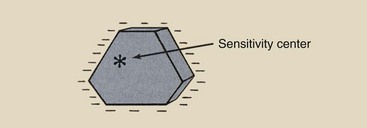
The shape and lattice structure of silver halide crystals are not perfect, and some of the imperfections result in the imaging property of the crystals. The type of imperfection thought to be responsible is a chemical contaminant, usually silver sulfide, which is introduced by chemical sensitization into the crystal lattice, usually at or near the surface.
This contaminant has been given the name sensitivity center. During exposure, photoelectrons and silver ions are attracted to these sensitivity centers, where they combine to form a latent image center of metallic silver.
Differences in speed, contrast, and spatial resolution among various radiographic films are determined by the process by which silver halide crystals are manufactured and by the mixture of these crystals into the gelatin. The number of sensitivity centers per crystal, the concentration of crystals in the emulsion, and the size and distribution of the crystals affect the performance characteristics of radiographic film.
Direct-exposure film contains a thicker emulsion with more silver halide crystals than screen film. The size and concentration of silver halide crystals primarily affect film speed. The composition of the radiographic emulsion is a proprietary secret that is closely guarded by each manufacturer.
Radiographic film is manufactured in total darkness. From the moment the emulsion ingredients are brought together until final packaging, no light is present.
Types of Film
Medical imaging is becoming extremely technical and sophisticated, and this is reflected in the number and variety of films that are now available. Each major film manufacturer produces many different films for medical imaging. When combined with the various film formats offered, more than 500 selections are possible (Table 12-1).
TABLE 12-1
Types of Film Used in Medical Imaging
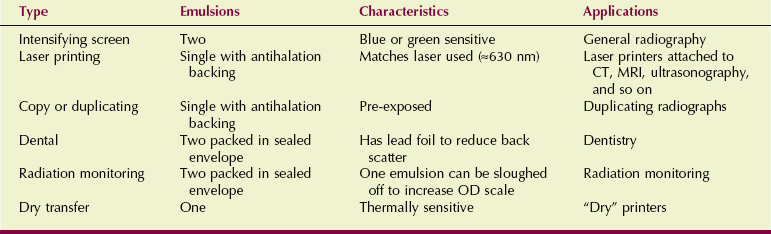
CT, computed tomography; OD, optical density; MRI, magnetic resonance imaging.
In addition to screen-film, direct-exposure film, sometimes called nonscreen film and special application film (such as that used in mammography, video recording, duplication, subtraction, cineradiography, and dental radiology), are available. Each has particular characteristics that become more familiar to radiologic technologists with use.
Table 12-2 shows standard film sizes in English and SI (Le Système International d’Unités) units. In most cases, the sizes are not exactly equivalent, but they are usually interchangeable. By far, the most commonly used film is that customarily called screen film.
TABLE 12-2
| English Units (in) | SI Units (cm) |
| 7 × 7 | 18 × 18 |
| 8 × 10 | 20 × 25 |
| 10 × 12 | 24 × 30 |
| 14 × 14 | 35 × 35 |
| 14 × 17 | 35 × 43 |
Screen-Film
Screen film is the type of film that is used with radiographic intensifying screens. Several characteristics must be considered when one is selecting screen-film: contrast, speed, spectral matching, anticrossover or antihalation dyes, and requirement for a safelight.
Contrast
Most manufacturers offer screen film with multiple contrast levels. The contrast of an IR is inversely proportional to its exposure latitude, that is, the range of exposure techniques that produce an acceptable image. Usually, the manufacturer identifies the contrast of these films as medium, high, or higher.
The difference depends on the size and distribution of the silver halide crystals. A high-contrast emulsion contains smaller silver halide grains with a relatively uniform grain size. Low-contrast films, on the other hand, contain larger grains that have a wider range of sizes.
Speed
Screen-film IRs are available with different speeds. Speed is the sensitivity of the screen-film combination to x-rays and light. Usually, a manufacturer offers several different IRs of different speeds that result from different film emulsions and different intensifying screen phosphors.
For direct-exposure film, speed is principally a function of the concentration and the total number of silver halide crystals. For screen film, silver halide grain size, shape, and concentration are the principal determinants of film speed.
To optimize speed, screen films are usually double emulsion, that is, an emulsion is layered on either side of the base. This double layering is attributable primarily to the efficiency conferred by the use of two screens to expose the film from both sides. This produces twice the speed that could be attained with a single-emulsion film even if the single emulsion were made twice as thick.
Compared with earlier technology, current emulsions contain less silver yet produce the same optical density (OD) per unit exposure. This more efficient use of silver in the emulsion is called the covering power of the emulsion.
The reported speed of a film is nearly always that for the IR: the film and two radiographic intensifying screens. When radiographic intensifying screens and film are properly matched, the reported speed is accurate. Mismatch can cause significant exposure error.
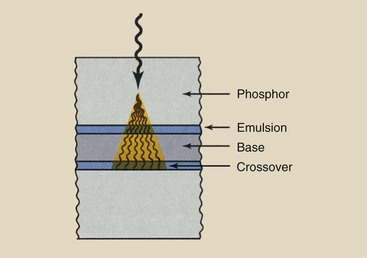
Crossover
Until recently, silver halide crystals were usually fat and three dimensional (Figure 12-3, A). Most emulsions now (Figure 12-3, B) contain tabular grains, which are flat silver halide crystals, and provide a large surface area–to-volume ratio. The result is improved covering power and significantly lower crossover.
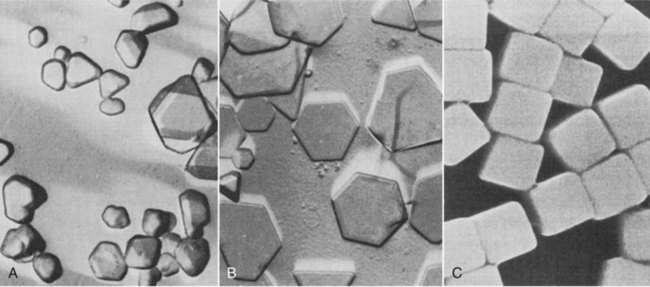
FIGURE 12-3 A, Conventional silver halide crystals are irregular in size. B, New technology produces flat, tablet-like grains. C, Cubic grains. (Courtesy Carestream Health.)
When light is emitted by a radiographic intensifying screen, it not only exposes the adjacent emulsion, it can also expose the emulsion on the other side of the base. When light crosses over the base, it causes increased blurring of the image (Figure 12-4).
Tabular grain emulsions reduce crossover because the covering power is increased, which relates not only to light absorption from the screen (which is increased) but also to light transmitted through the emulsion to cause crossover (which is reduced).
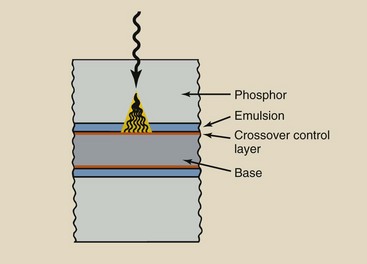
The addition of a light-absorbing dye in a crossover control layer reduces crossover to near zero (Figure 12-5). The crossover control layer has three critical characteristics: (1) It absorbs most of the crossover light, (2) it does not diffuse into the emulsion but remains as a separate layer, and (3) it is completely removed during processing.
Spectral Matching
Perhaps the most important consideration in the selection of modern screen film is its spectral absorption characteristics. Since the introduction of rare Earth screens in the early 1970s, radiologic technologists must be particularly careful to use a film whose sensitivity to various colors of light—its spectral response—is properly matched to the spectrum of light emitted by the screen.
Calcium tungstate screens, which emit blue and blue-violet light, have been largely replaced with rare Earth screens, which are faster. Now, many rare Earth phosphors emit ultraviolet, blue, green, and red. All silver halide films respond to violet and blue light but not to green, yellow, or red unless they are spectrally sensitized with dyes.
If green-emitting screens are used, they should be matched with a film that is sensitive not only to blue light but also to green light. Such film is orthochromatic and is called green-sensitive film. This is distinct from panchromatic film, which is used in photography and is sensitive to the entire visible light spectrum.
Figure 12-6 shows the spectral response of blue-sensitive and green-sensitive films. Blue-sensitive film should be used only with blue- or ultraviolet-emitting screens. Green-sensitive film usually is exposed with green-emitting screens.
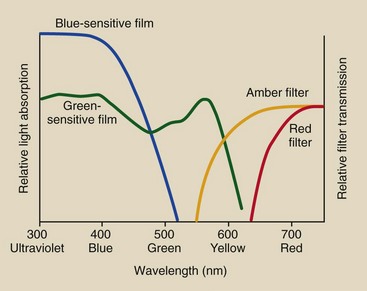
FIGURE 12-6 Radiographic films are blue sensitive or green sensitive, and they require amber- and red-filtered safelights, respectively.
If films with sensitivity only in the ultraviolet and blue regions of the spectrum are used with green-emitting screens, then the IR speed is greatly reduced, and the patient dose increases. Proper spectral matching results in selection of the correct screen-film combination.
Reciprocity Law
One would expect that the total exposure of a film would not depend on the time taken to expose it. That is the definition of the reciprocity law, which also can be stated as follows:
The reciprocity law is true for film exposed directly to x-rays. Industrial radiographers do not have to compensate for this effect. The reciprocity law fails when film is exposed to light from radiographic intensifying screens.
Very long or very short exposure times produce a lower OD than that predicted by the reciprocity law. Radiographers must be aware of this.
Reciprocity law failure is important when exposure times are long (as in mammography) or short (as in interventional radiography). The result of long or short exposures is reduced speed. An increase in radiographic technique may be required. Table 12-3 shows approximate speed loss as a function of exposure time.
Safelights
The use of radiographic film requires certain precautions in the darkroom. Most safelights are incandescent lamps with a color filter; safelights provide enough light to illuminate the darkroom while ensuring that the film remains unexposed.
Proper darkroom illumination depends not only on the color of the filter but also on the wattage of the bulb and the distance between the lamp and the work surface. A 15-W bulb should be no closer than 1.5 m (5 ft) from the work surface.
With blue-sensitive film, an amber filter is used. The amber filter transmits light that has wavelengths longer than approximately 550 nm, which is above the spectral response of blue-sensitive film.
The use of an amber filter would fog green-sensitive film; therefore, a red filter, which transmits only light above approximately 600 nm, must be used in this case. A red filter is suitable for both green- and blue-sensitive film. Figure 12-6 shows the approximate transmission characteristics for amber and red safelight filters.
Direct-Exposure Film
The use of radiographic intensifying screens with film allows reduced technique and therefore reduced patient radiation dose. However, the image is more blurred than it would be after exposure without screens. In the past, certain films were manufactured for use without screens; they were used to image thin body parts, such as hands and feet, that have high subject contrast and present a low radiation risk.
Most extremity examinations now use fine-grain, high-detail screens and double-emulsion film as the IR. The emulsion of a direct-exposure film is thicker than that of screen film, and it contains higher concentrations of silver halide crystals to improve direct x-ray interaction.
Mammography Film
Mammography was originally performed with an industrial-grade, double-emulsion, direct-exposure film. The radiation doses associated with such a technique were much too high; consequently, specialty films were developed.
Mammography film is single-emulsion film that is designed to be exposed with a single radiographic intensifying screen. All currently available mammography screen-film systems use green-emitting terbium-doped gadolinium oxysulfide screens with green-sensitive film.
The surface of the base opposite the screen is coated with a special light-absorbing dye to reduce reflection of screen light, which is transmitted through the emulsion and base. This effect is called halation, and the absorbing dye is an antihalation coating. Such an antihalation coating is used on all single-emulsion screen film, not just mammography film. The coating is removed during processing for better viewing.
Handling and Storage of Film
Radiographic film is a sensitive radiation detector and must be handled accordingly. Improper handling and storage result in poor radiographs with artifacts that interfere with diagnosis. For this reason, it is essential that anyone who handles radiographic film should be careful not to bend, crease, or otherwise subject it to rough handling. Clean hands are a must, and hand lotions should be avoided.
Improper handling or processing can cause artifacts, the marks or spurious images that sometimes appear on processed radiographs. Radiographic film is pressure sensitive, so rough handling or the imprint of any sharp object, such as a fingernail, is reproduced as an artifact on the processed radiograph.
Heat and Humidity
Radiographic film is sensitive to the effects of elevated temperature and humidity, especially for long periods. Heat increases the fog of a radiograph and therefore reduces contrast. Radiographic film should be stored at temperatures lower than approximately 20°C (68°F).
Storage under conditions of elevated humidity (e.g., >60%) also reduces contrast because of increased fog. Consequently, before use, radiographic film should be stored in a cool, dry place, ideally in a climate-controlled environment. Storage in an area that is too dry can be equally objectionable. Static artifacts are possible when the relative humidity dips to below about 40%.
Light
Radiographic film must be stored and handled in the dark. Any light at all can expose the emulsion before processing. If low-level, diffuse light exposes the film, fog is increased. If bright light exposes or partially exposes the film, a gross, obvious artifact is produced.
Control of light is ensured by a well-sealed darkroom and a light-proof storage bin for film that has been opened but not clinically exposed. The storage bin should have an electrical interlock that prevents it from being opened while the door to the darkroom is ajar or open.
Radiation
Ionizing radiation, other than the useful beam, creates an image artifact by increasing fog and reducing contrast. Film fog is the dull, uniform OD that appears if the film has been inadvertently exposed to light, x-rays, heat, or humidity.
Darkrooms usually are located next to radiographic imaging rooms and are lined with lead. However, this is not always necessary. It is usually acceptable to lead-line only the storage shelf and the film bin.
Radiographic film is far more sensitive to x-ray exposure than are people; therefore, more lead is required to protect film than people. The thickness of the lead barrier is designed to keep the total exposure of unprocessed film below 2 µGya. This, of course, requires some assumptions about the storage time of the film.
Care should be taken to ensure that the receiving area for radiographic film is not the same as that for the radioactive material used in nuclear medicine. Even though the packaging of radioactive material ensures the safety of those who handle it, the low-level radiation emitted can fog radiographic film if the radioactive material and film are stored together for even a short time.
Formation of the Latent Image
The image-forming x-rays exiting the patient and incident on the radiographic intensifying screen-film deposit visible light energy in the emulsion primarily by interaction with atoms of the silver halide crystal. This energy is deposited in a pattern that is representative of the anatomical part that is being radiographed.
Immediately after exposure, no image can be observed on the film. An invisible image is present, however, and is called a latent image. With proper chemical processing, the latent image becomes a visible image.
The interaction between photons and silver halide crystals is fairly well understood, as is the processing of the latent image into the visible image. However, the formation of the latent image, sometimes called the photographic effect, is not well understood and continues to be the subject of considerable research. The following discussion is an extraction of the Gurney-Mott theory, the accepted, although incomplete, explanation of latent image formation.
Silver Halide Crystal
The silver, bromine, and iodine atoms are fixed in the crystal lattice in ion form (Figure 12-7). Silver is a positive ion, and bromide and iodide are negative ions. When a silver halide crystal is formed, each silver atom releases an outer-shell electron, which becomes attached to a halide atom (either bromine or iodine).
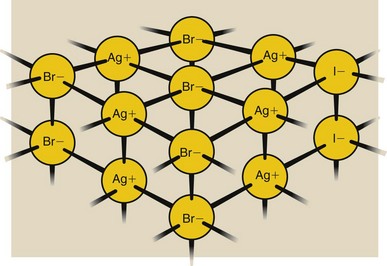
FIGURE 12-7 Silver halide crystal lattice contains ions. Electrons from Ag atoms have been loaned to Br and I atoms.
The silver atom is missing an electron and therefore is a positively charged ion, identified as Ag+. The bromine and iodine atoms each have one extra electron and therefore are negatively charged ions, identified as bromide and iodide (Br− and I−), respectively.
The silver halide crystal is not as rigid as some crystals such as diamonds. Under certain conditions, atoms and electrons are free to migrate within the silver halide crystal.
The halide ions, bromide and iodide, are generally found in greatest concentration along the surface of the crystal. Therefore, the crystal takes on a negative surface charge, which is matched by the positive charge of the interstitial silver ions, the silver ions inside the crystal. An inherent defect in the structure of silver halide crystals, the Frankel defect, consists of interstitial silver ions and silver ion vacancies. Figure 12-8 presents a model of the silver halide crystal.
Photon Interaction with Silver Halide Crystal
When light photons from the radiographic intensifying screen interact with film, it is the interaction with the silver and halide atoms (Ag, Br, I) that forms the latent image. This interaction releases electrons in the crystal (Figure 12-9, A).
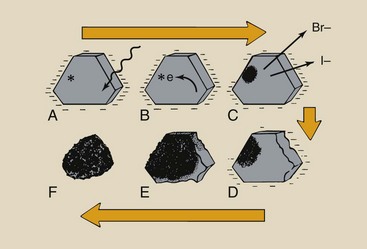
FIGURE 12-9 Production of the latent image and conversion of the latent image into a visible image require several steps. A, Light photon interaction releases electrons. B, These electrons migrate to the sensitivity center. C, At the sensitivity center, atomic silver is formed by attraction of an interstitial silver ion. D, This process is repeated many times, resulting in the buildup of silver atoms. E, The remaining silver halide is converted to silver during processing. F, The silver grain results.
These electrons are released with sufficient energy to travel a large distance within the crystal (Figure 12-9, B). While crossing the crystal, the electrons may have sufficient energy to dislodge additional electrons from the crystal lattice.
Secondary electrons liberated by the absorption event migrate to the sensitivity center and are trapped. After a sensitivity center captures an electron and becomes more negatively charged, the center is attractive to mobile interstitial silver ions (Figure 12-9, C). The interstitial silver ion combines with the electrons trapped at the sensitivity center to form metallic silver atoms.
Most of these electrons come from the bromide and iodide ions because these negative ions have one extra electron. These negative ions therefore are converted to neutral atoms, and the loss of ionic charge results in disruption of the crystal lattice.
The bromine and iodine atoms are now free to migrate because they no longer are bound by ionic forces. They migrate out of the crystal into the gelatin portion of the emulsion.
Latent Image
The concentration of electrons at the sensitivity center produces a region of negative electrification. As halide atoms are removed from the crystal, the positive silver ions are electrostatically attracted to the sensitivity center. After migrating to the sensitivity center, the silver ions are neutralized by electrons and are converted to metallic silver.
In an optimally exposed film, most developable silver halide crystals have collected 4 to 10 silver atoms at a sensitivity center (Figure 12-9, D). Consequently, this silver deposition is not observable, even microscopically.
This group of silver atoms is called a latent image center. It is here that visible quantities of silver form during processing to create the radiographic image (Figure 12-9, E).
Crystals with silver deposited at the sensitivity center are developed into black grains (Figure 12-9, F). Crystals that have not been irradiated remain crystalline and inactive. The unobservable information contained in radiation-activated and -inactivated silver halide crystals constitutes the latent image.
Radiographic Intensifying Screen Construction
Use of film to detect x-rays and to image anatomical structures is inefficient. In fact, fewer than 1% of the x-rays incident on radiographic film interact with the film and contribute to the latent image.
Most radiographs are made with the film in contact with a radiographic intensifying screen because the use of film alone requires a high patient radiation dose. A radiographic intensifying screen is a device that converts the energy of the x-ray beam into visible light. This visible light then interacts with the radiographic film, forming the latent image.
On the one hand, use of a radiographic intensifying screen lowers the patient dose considerably; on the other hand, the image is slightly blurred. With modern screens, however, such image blur is not serious.
Usually, the radiographic film is sandwiched between two screens. The film used is called double-emulsion film because it has an emulsion coating on both sides of the base. Most screens have four distinct layers; these are shown in cross section in Figure 12-10.
Protective Coating
The layer of the radiographic intensifying screen closest to the radiographic film is the protective coating. It is 10 to 20 µm thick and is applied to the face of the screen to make the screen resistant to the abrasion and damage caused by handling. This layer also helps to eliminate the buildup of static electricity and provides a surface for routine cleaning without disturbing the active phosphor. The protective layer is transparent to light.
Phosphor
The active layer of the radiographic intensifying screen is the phosphor. The phosphor emits light during stimulation by x-rays. Phosphor layers vary in thickness from 50 to 300 µm, depending on the type of screen. The active substance of most phosphors before about 1980 was crystalline calcium tungstate embedded in a polymer matrix. The rare Earth elements gadolinium, lanthanum, and yttrium are the phosphor material in newer, faster screens.
The action of the phosphor can be seen by viewing an opened cassette in a darkened room through the protective window of the control booth. The radiographic intensifying screen glows brightly when exposed to x-rays.
Many materials react in this way, but radiography requires that materials possess the characteristics given in Box 12-1. Through the years, several materials have been used as phosphors because they exhibit these characteristics. These materials include calcium tungstate, zinc sulfide, and barium lead sulfate, as well as oxysulfides of the rare Earths gadolinium, lanthanum, and yttrium.
Roentgen discovered x-rays quite by accident. He observed the luminescence of barium platinocyanide, a phosphor that was never successfully applied to diagnostic radiology. Within 1 year of Roentgen’s discovery of x-rays, the American inventor Thomas A. Edison developed calcium tungstate. Although Edison demonstrated the use of radiographic intensifying screens before the beginning of the 20th century, screen-film combinations did not come into general use until about the time of World War I. With improved manufacturing techniques and quality control procedures, calcium tungstate proved superior for nearly all radiographic techniques and, until the 1970s, was used almost exclusively as the phosphor.
Since then, rare Earth screens have been used in diagnostic radiology. These screens are faster than those made of calcium tungstate, rendering them more useful for most types of radiographic imaging. Use of rare Earth screens results in a lower patient dose, less thermal stress on the x-ray tube, and reduced shielding for x-ray rooms.
Reflective Layer
Between the phosphor and the base is a reflective layer, approximately 25 µm thick, that is made of a shiny substance such as magnesium oxide or titanium dioxide (Figure 12-11). When x-rays interact with the phosphor, light is emitted isotropically.
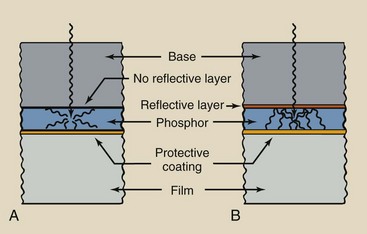
FIGURE 12-11 A, Screen without reflective layer. B, Screen with reflective layer. Screens without reflective layers are not as efficient as those with reflective layers because fewer light photons reach the film.
Less than half of this light is emitted in the direction of the film. The reflective layer intercepts light headed in other directions and redirects it to the film. The reflective layer enhances the efficiency of the radiographic intensifying screen, nearly doubling the number of light photons that reach the film.
Screen Characteristics
Any material that emits light in response to some outside stimulation is called a luminescent material, or a phosphor, and the emitted visible light is called luminescence. A number of stimuli, including electric current (the fluorescent light), biochemical reactions (a lightning bug), visible light (a watch dial), and x-rays (a radiographic intensifying screen), cause luminescence in materials.
Luminescence is similar to characteristic x-ray emission. However, luminescence involves outer-shell electrons (Figure 12-12). In a radiographic intensifying screen, absorption of a single x-ray causes emission of thousands of light photons.
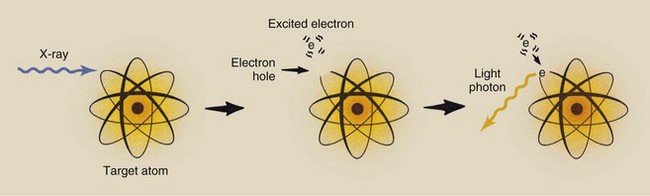
FIGURE 12-12 Luminescence occurs when an outer-shell electron is raised to an excited state and returns to its normal state with the emission of a light photon.
When a luminescent material is stimulated, the outer-shell electrons are raised to excited energy levels. This effectively creates a hole in the outer-shell electron, which is an unstable condition for the atom. The hole is filled when the excited electron returns to its normal state. This transition is accompanied by the emission of a visible light photon.
The range of excited energy states for an outer-shell electron is narrow, and these states depend on the structure of the phosphor. The wavelength of emitted light is determined by the level of excitation to which the electron was raised and is characteristic of a given phosphor. In other words, luminescent materials emit light of a characteristic color.
Two types of luminescence have been identified. If visible light is emitted only while the phosphor is stimulated, the process is called fluorescence. If, on the other hand, the phosphor continues to emit light after stimulation, the process is called phosphorescence.
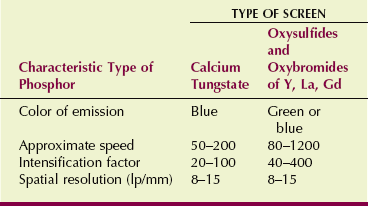
Radiologic technologists are concerned with three primary characteristics of radiographic intensifying screens: screen speed, image noise, and spatial resolution.
Because screens are used to reduce patient dose, one characteristic is the magnitude of dose reduction. This property is called the intensification factor (IF) and is a measure of the speed of the screen.
Screen Speed
Many types of radiographic intensifying screens are available, and each manufacturer uses different names to identify them. Collectively, however, screens usually are identified by their relative speed expressed numerically. Screen speeds range from 50 (slow, detail) to 1200 (very fast).
Screen speed is a relative number that describes how efficiently x-rays are converted into light. Par-speed calcium tungstate screens are assigned a value of 100 and serve as the basis for comparison of all other screens. High-speed rare Earth screens have speeds up to 1200; detail screens have speeds of approximately 50 to 80. These and other characteristics are summarized in Table 12-4.
The speed of a radiographic intensifying screen conveys no information regarding patient dose. This information is related by the IF. The IF is defined as the ratio of the exposure required to produce the same OD with a screen to the exposure required to produce an OD without a screen.
The OD chosen for comparison of one radiographic intensifying screen versus another is usually 1.0. The value of the IF can be used to determine the dose reduction accompanying the use of a screen.
| Question: | A pelvic examination performed with a 100 speed radiographic intensifying screen is taken at 75 kVp, 50 mAs and results in an entrance skin exposure (ESE) of 2 mGya (200 mR). A similar examination taken without screens would result in an ESE of 64 mGya (6400 mR). What is the approximate IF of the screen-film combination? |
| Answer: | IF = 64/2 = 32 |
Several factors influence radiographic intensifying screen speed; some of these are controlled by the radiologic technologist. Ultimately, the screen speed is determined by the relative number of x-rays that interact with the phosphor and how efficiently x-ray energy is converted into the visible light that interacts with the film.
Box 12-2 gives the properties of radiographic intensifying screens that affect screen speed and cannot be controlled by the radiologic technologist. They are listed in their relative order of importance.
Several conditions that affect radiographic intensifying screen speed are controlled by the radiologic technologist. These include radiation quality, image processing, and temperature.
Radiation Quality
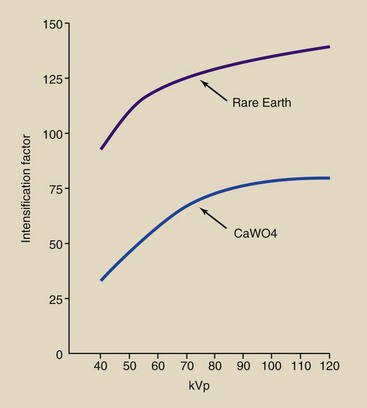
As x-ray tube potential is increased, the IF also increases (Figure 12-13). Although this may seem contrary to the discussion of x-ray absorption in Chapter 9, it is not.
In Chapter 9, x-ray absorption was shown to decrease with increasing kVp. Remember, however, that the IF is the ratio of x-ray absorption in a radiographic intensifying screen to that in radiographic film alone.
Screens have higher effective atomic numbers than films; therefore, although true absorption in the screen decreases with increasing kVp, relative absorption compared with that in film increases. At 70 kVp, whereas the IF for a typical par-speed screen is 60, that for a rare Earth screen is 150.
Image Processing
Only the superficial layers of the emulsion are affected when radiographic film is exposed to light. However, the emulsion is affected uniformly throughout when the film is exposed to x-rays.
Therefore, excessive developing time for screen film results in lowering of the IF because the emulsion nearest the base contains no latent image, yet it can be reduced to silver if the developer is allowed sufficient time to penetrate the emulsion to the depth. This too is relatively unimportant because films manufactured for use with screens have thinner emulsion layers than those produced for direct exposure.
Temperature
Radiographic intensifying screens emit more light per x-ray interaction at low temperatures than at high temperatures. Consequently, the IF is lower at higher temperatures. This characteristic, although it is relatively unimportant in a clinic with a controlled environment, can be significant in field work in hot or cold climates.
Image Noise
Image noise appears on a radiograph as a speckled background. It occurs most often when fast screens and high-kVp techniques are used. Noise reduces image contrast.
Rare Earth radiographic intensifying screens have increased speed because of two important characteristics, both of which are higher compared with other types of screens. The percentage of x-rays absorbed by the screen is higher. This is detective quantum efficiency (DQE). The amount of light emitted for each x-ray absorbed also is higher. This is conversion efficiency (CE).
Figure 12-14 illustrates why an increase in CE increases image noise but an increase in DQE does not. In Figure 12-14, A, a calcium tungstate screen has a DQE of 20% and a CE of 5%. A radiographic technique of 10 mAs results in 1000 x-rays incident on the screen, 200 of which are absorbed, resulting in light photons equivalent to 10 x-rays. We could say that this system has a speed of 100.
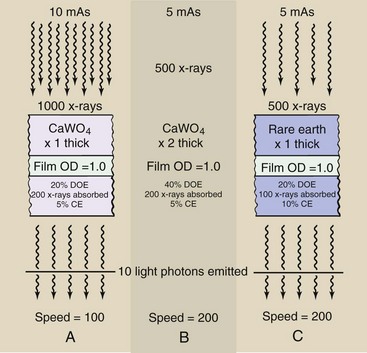
FIGURE 12-14 Image noise increases with higher conversion efficiency (CE) but not with higher detective quantum efficiency (DQE).
If phosphor thickness is doubled as in Figure 12-14, B, the DQE increases to 40%, so the mAs can be reduced to 5 mAs. The speed is now 200, but there is no increase in noise because the same number of x-rays is absorbed.
However, if the phosphor is changed to one with a CE of 10%, the speed is doubled at the expense of increased noise (Figure 12-14, C,). A 200-speed screen is attained because twice as much light is emitted per x-ray absorption. Only half as many x-rays are required, and this results in increased quantum mottle, a principal component of image noise.
Spatial Resolution
Radiographers often use the term image detail or visibility of detail when describing image quality. These qualitative terms combine the quantitative measures of spatial resolution and contrast resolution. Spatial resolution refers to how small an object can be imaged. Contrast resolution refers to the ability to image similar tissues, such as the liver and pancreas or gray matter and white matter.
The use of radiographic intensifying screens adds one more step to the process of imaging with x-rays. Radiographic intensifying screens have the disadvantage of lower spatial resolution compared with direct-exposure radiographs.
Spatial resolution is measured in a number of ways and can be assigned a numeric value. Spatial resolution in screen-film radiography is limited principally by effective focal spot size. For our purposes, a general description should be sufficient.
A photograph in focus shows good spatial resolution; one that is out of focus shows poor spatial resolution and therefore much image blur. Figure 12-15 shows the differences in spatial resolution between a direct-exposure film and a par-speed screen-film combination obtained when an x-ray test pattern is imaged.
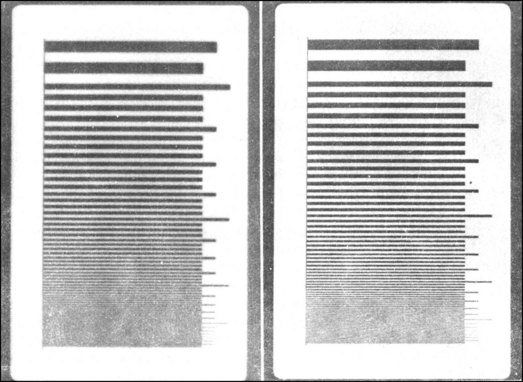
FIGURE 12-15 Radiographs of an x-ray test pattern made with direct-exposure film (right) and a par-speed screen-film combination (left). The difference in image blur is obvious.
Such a test pattern is called a line-pair test pattern. It consists of lead lines separated by interspaces of equal size. Spatial resolution is expressed by the number of line pairs per millimeter (lp/mm) that are imaged. The higher this number, the smaller is the object that can be imaged and the better is the spatial resolution.
Very fast screens can resolve 7 lp/mm, and fine-detail screens can resolve 15 lp/mm (see Table 12-4). Direct-exposure film can resolve 50 lp/mm. The unaided eye can resolve about 10 lp/mm.
When x-rays interact with the screen’s phosphor, the area of the film emulsion that is activated by the emitted light is larger than it would be with direct x-ray exposure. This situation results in reduced spatial resolution or increased image blur.
High-speed screens have low spatial resolution, and fine-detail screens have high spatial resolution. Spatial resolution improves with smaller phosphor crystals and thinner phosphor layers. Figure 12-16 shows how these factors affect image resolution. Unfortunately, these factors are not controlled by the radiologic technologist.
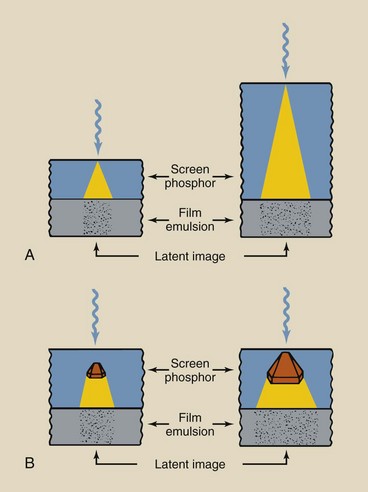
FIGURE 12-16 A, Reduction in spatial resolution is greater when the phosphor layers are thick. B, Reduction also is greater when the crystal size is large. These same conditions increase screen speed and reduce patient dose by producing a greater number of light photons per incident x-ray.
In both parts of Figure 12-16, the x-ray is shown to interact with the phosphor soon after entry; this results in screen blur. Screen blur is reduced in thinner screens.
In mammography, spatial resolution is improved by placing the single-emulsion film on the tube side of the cassette.
Screen-Film Combinations
Screens and films are manufactured for compatibility; this helps to ensure quality images at acceptable patient radiation dose.
Radiographic intensifying screens are nearly always used in pairs. Figure 12-17 is a cross section of a properly loaded cassette that contains front and back screens with a double-emulsion film. Production of the latent image is nearly evenly divided between front and back screens, with less than 1% being contributed directly by x-ray interaction. Each screen exposes the emulsion it contacts.
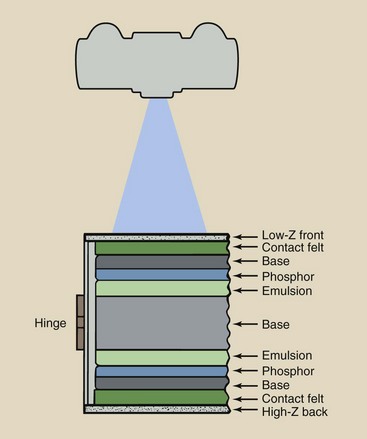
FIGURE 12-17 Cross-sectional view of cassette containing front and back screens and loaded with double-emulsion film.
In addition to reduced patient dose, use of radiographic intensifying screens in an IR offer several advantages (Box 12-3). Attaining these advantages requires proper selection, handling, and use of a screen-film combination.
Cassette
The cassette is the rigid holder that contains the film and radiographic intensifying screens. The front cover, the side facing the x-ray source, is made of material with a low atomic number such as plastic. It is thin yet sturdy. The front cover of the cassette is designed for minimum attenuation of the x-ray beam.
Attached to the inside of the front cover is the front screen, and attached to the back cover is the back screen. The radiographic film is sandwiched between the two screens.
Between each screen and the cassette cover is some sort of compression device, such as radiolucent plastic foam, which maintains close screen-film contact when the cassette is closed and latched.
The back cover is usually made of heavy metal to minimize backscatter. The x-rays transmitted through the screen-film combination to the back cover more readily undergo photoelectric effect in a high-Z material than in a low-Z material.
Carbon Fiber
One of the materials developed early in the space exploration program was carbon fiber. This material was developed for nose cone applications because of its superior strength and heat resistance. It consists principally of graphite fibers (ZC = 6) in a plastic matrix that can be formed to any shape or thickness.
In radiology, this material now is used widely in devices designed to reduce patient exposure. A cassette with a front that consists of carbon fiber material absorbs only approximately half the number of x-rays that an aluminum or plastic cassette does.
Carbon fiber also is used as pallet material for fluoroscopic examination couches and computed tomography beds.
Carbon fiber not only reduces patient exposure; it also may produce longer x-ray tube life because of the lower demand radiographic techniques required.
Screen-Film Radiographic Exposure
From its introduction in 1896 by Thomas Edison until the 1970s, calcium tungstate (CaWO4) was used almost exclusively as the phosphor for radiographic intensifying screens. Such screens, however, exhibit only 5% CE.
One reason why calcium tungstate is a useful screen phosphor is that it emits light in the violet-to-blue region. The sensitivity of conventional radiographic film is highest in the violet-to-blue region of the spectrum. Consequently, the light emitted by calcium tungstate screens is readily absorbed in radiographic film (Figure 12-18).
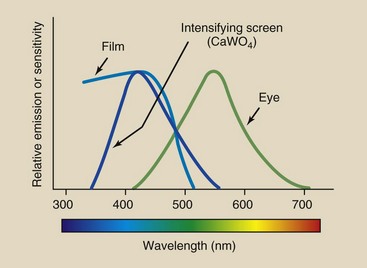
FIGURE 12-18 Importance of spectral matching is demonstrated by showing the relative emission spectrum for a radiographic intensifying screen and the relative sensitivity of radiograph film to light from that screen.
If the screen phosphor emitted green or red light, its IF would be greatly reduced because it would require a greater number of light photons to produce a latent image. The light of the screen emission would be mismatched to the light sensitivity of the film.
Rare Earth Screens
Newer phosphor materials have become the material of choice for most radiographic applications. Table 12-5 lists these phosphors and the general identification of screens into which they have been incorporated. Except for barium- and zinc-based phosphors, the other new phosphors are identified as rare Earth; therefore, all of these screens have come to be known as rare Earth screens.
TABLE 12-5
Composition and Emulsion of Radiographic Intensifying Screens
| Phosphor | Activator | Emission |
| Barium fluorochloride | Europium | Ultraviolet |
| Barium strontium sulfate | Europium | Ultraviolet |
| Barium sulfate | Lead | Ultraviolet |
| Zinc sulfide | Silver | Blue-ultraviolet |
| Calcium tungstate | Lead | Blue |
| Lanthanum oxybromide | Thulium | Blue |
| Yttrium oxysulfide | Terbium | Blue |
| Gadolinium oxysulfide | Terbium | Green |
| Lanthanum oxysulfide | Terbium | Green |
| Zinc cadmium sulfide | Silver | Yellow-green |
The term rare Earth describes those elements of group IIIa in the periodic table (see Figure 2-4) that have atomic numbers of 57 to 71. These elements are transitional metals that are scarce in nature. Those used in rare Earth screens are principally gadolinium, lanthanum, and yttrium. The compositions of the four principal rare Earth phosphors are terbium-activated gadolinium oxysulfide (Gd2O2S: Tb), terbium-activated lanthanum oxysulfide (La2O2S: Tb), terbium-activated yttrium oxysulfide (Y2O2S: Tb), and lanthanum oxybromide (LaOBr).
Rare Earth radiographic intensifying screens are manufactured to perform at several speed levels, up to 1200. This increase in speed is attained without loss of spatial or contrast resolution; however, with the fastest rare Earth screens, the effects of quantum mottle (image noise) are noticeable and can become bothersome.
Rare Earth radiographic intensifying screens obtain their increased sensitivity through higher x-ray absorption (DQE) and more efficient conversion of x-ray energy into light (CE). The light emitted by these screens, however, differs from that emitted by other screens; therefore, rare Earth screens require specially matched film.
Higher X-ray Absorption
When diagnostic x-rays interact with a calcium tungstate screen, approximately 30% of the x-rays are absorbed. The mechanism of absorption is almost entirely the photoelectric effect. Recall that photoelectric absorption occurs readily with the inner electrons of atoms of high atomic number.
The tungsten atom determines the absorption properties of a calcium tungstate screen. Tungsten has an atomic number of 74 and a K-shell electron binding energy of 69 keV. In the diagnostic range, x-ray absorption in tungsten follows the relationship shown in Figure 12-19.
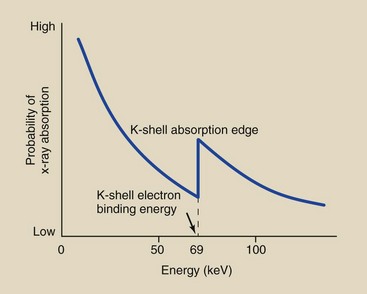
FIGURE 12-19 Probability of x-ray absorption in a calcium tungstate screen as a function of the incident x-ray energy.
At very low energies, photoelectric absorption is very high, but as the x-ray energy increases, the probability of absorption decreases rapidly until the x-ray energy is equal to the binding energy of the K-shell electrons. At x-ray energies below the K-shell electron binding energy, the incident x-ray has too little energy to ionize K-shell electrons.
When the x-ray energy equals the K-shell electron binding energy, the two K-shell electrons become available for photoelectric interaction. Consequently, at this energy, the probability of photoelectric absorption increases abruptly.
This abrupt increase in absorption at this energy level is called the K-shell absorption edge, and it is followed by another rapid reduction in photoelectric absorption with increasing x-ray energy.
The rare Earth materials used for radiographic intensifying screens all have atomic numbers less than that for tungsten. Consequently, each has lower K-shell electron binding energy. Table 12-6 lists the important physical characteristics of the elements included in radiographic intensifying screens.
TABLE 12-6
Atomic Number and K-Shell Electron Binding Energy of High-Z Elements in Radiographic Intensifying Screen Phosphors
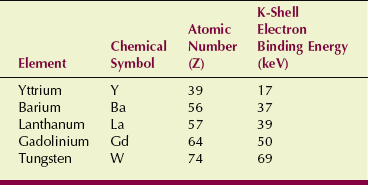
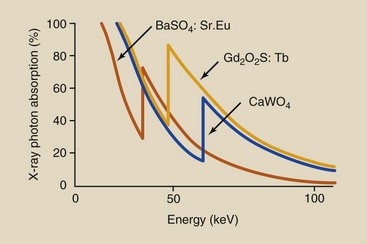
Figure 12-20 shows that the probability of x-ray absorption in rare Earth screens is lower than that in calcium tungstate screens at all x-ray energies except those between respective K-shell electron binding energies.
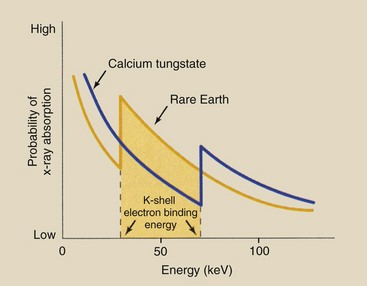
FIGURE 12-20 X-ray absorption probability in a rare earth screen compared with that in a calcium tungstate screen. In the energy interval between respective K-shell electron binding energies, absorption in a rare earth screen is greater.
Below the K-shell absorption edge for the rare Earth elements, x-ray absorption is higher in tungsten. At an x-ray energy equal to the K-shell electron binding energy of the rare Earth elements, however, the probability of photoelectric absorption is considerably higher than that for tungsten.
As with tungsten, the absorption probability of the rare Earth elements decreases with increasing x-ray energy. At x-ray energies above the K-shell absorption edge for tungsten, the rare Earth elements again exhibit lower absorption than that for tungsten.
Each of the rare Earth radiographic intensifying screens has an absorption curve characteristic of the phosphor that determines the speed of the screen and how it changes with kVp. Figure 12-21 shows the x-ray absorption in two phosphors relative to calcium tungstate. For instance, barium strontium sulfate has a higher DQE at a lower kVp than is the case with gadolinium oxysulfide.
The result of this complex interaction process is that in the x-ray energy range between the K-shell absorption edge for the rare Earth elements and that for tungsten, a rare Earth screen absorbs approximately five times more x-rays than a calcium tungstate screen. Furthermore, for each x-ray absorbed, more light is emitted by the rare Earth screens.
Rare Earth radiographic intensifying screens exhibit better absorption properties than calcium tungstate screens only in the energy range between the respective K-shell absorption edges. This energy range extends from approximately 35 to 70 keV and corresponds to most of the useful x-rays emitted during routine x-ray examinations. Outside this energy range, calcium tungstate radiographic intensifying screens absorb more x-rays than rare Earth screens.
Higher Conversion Efficiency
An additional property of the rare Earth phosphors, the CE, contributes to their higher speed. The CE is defined as the ratio of visible light energy emitted to the x-ray energy absorbed.
When an x-ray interacts photoelectrically with a phosphor and is absorbed, its energy reappears as heat or light through a rearrangement of electrons in the crystal lattice of the phosphor. If all of the energy reappeared as heat, the phosphor would be worthless as an intensifying screen. In calcium tungstate, approximately 5% of the absorbed x-ray energy reappears as light. The CE of rare Earth phosphors is approximately 20%.
Spectrum Matching
To be fully effective, rare earth radiographic intensifying screens must be used only in conjunction with film emulsions whose light absorption characteristics are matched to the light emission of the screen. This is called spectrum matching. Calcium tungstate screens emit light in a rather broad continuous spectrum centered in the violet-to-blue region, with a maximum intensity at approximately 430 nm (Figure 12-22).
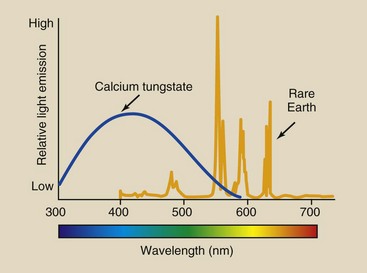
FIGURE 12-22 Calcium tungstate emits a broad spectrum of light centered in the blue region. With rare earth screens, discrete emissions are centered near the green-yellow region.
The spectral emission of rare Earth phosphors is more discrete, as indicated by the many peaks in the spectrum (see Figure 12-22). The spectral emission is centered in the green region of the visible spectrum at approximately 540 mm. Terbium activation is responsible for the shape and intensity of this emission spectrum.
The emission spectrum can be altered somewhat by various concentrations of terbium atoms in the phosphor, by the addition of activators, and by the use of light-absorbing dyes. Phosphors are available that emit ultraviolet, blue, green, and red light.
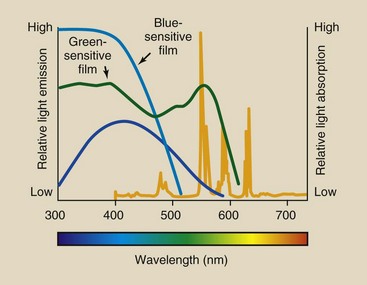
Conventional x-ray film is sensitive to blue and blue-violet light and is rather insensitive to light of longer wavelengths. Such blue-sensitive films are used with calcium tungstate screens because their absorption spectrum matches the emission spectrum of calcium tungstate.
Specially designed green-sensitive film must be used with rare earth screens (Figure 12-23). If a green-emitting screen were used with blue-sensitive film, the strong emission in the green region would go undetected, and system speed would be sharply reduced. To obtain maximum advantage and speed from rare Earth screens, the film must be sensitized for emission of the screen.
Safelights
Green-sensitive film creates problems in the darkroom. Safelight filters that are satisfactory for regular x-ray film fog film manufactured for use with rare earth screens. Rare earth screen-film requires the use of safelights that are colored even more toward the red portion of the spectrum.
Care of Screens
High-quality radiographs require that radiographic intensifying screens receive proper care. Screen handling requires the utmost care because even a small fingernail scratch can produce artifacts and degrade the radiographic image. Screens should be handled only when they are new and are being installed in cassettes and when they are being cleaned. When screens are mounted in a cassette, the manufacturer’s instructions must be followed carefully.
When loading cassettes, do not slide in the film. A sharp corner or the edge can scratch the screen. Place the film inside the cassette. Remove the film by rocking the cassette on the hinged edge and letting it fall to your fingers. Do not dig the film out of the cassette with your fingernails. Do not leave cassettes open because the screens can be damaged by whatever might fall on them, be it dust or darkroom chemicals.
Radiographic intensifying screens must be cleaned periodically. The frequency of cleaning is determined primarily by two factors: the amount of use and the level of dust in the work environment. In a busy radiology department, it may be necessary to clean screens once each month or even more often. Under other circumstances, the cleaning frequency may be extended safely to 2 to 3 months.
Special screen cleaning materials are used, and the manufacturer’s instructions should be followed carefully. One advantage of the use of these commercial preparations is that they often contain antistatic compounds, which can be helpful.
An equally important requirement in caring for radiographic intensifying screens is maintaining good screen-film contact. Screen-film contact can be checked by radiographing a wire mesh (Figure 12-24, A). If darker areas of blurring are seen, as in Figure 12-24, B, then screen-film contact is poor and should be corrected, or the cassette should be replaced.


FIGURE 12-24 Radiographs of wire mesh are used to check for screen-film contact. A, Good contact is evident. (Courtesy Cardinal Health.) B, A warped cassette cover leads to a region of poor contact. (Courtesy Barbara Smith Pruner, Portland Community College.)
Properly maintained radiographic intensifying screens will last indefinitely. X-ray interaction with the phosphor does not cause them to wear out. There is no such thing as radiation fatigue. The only way these screens become useless and need replacement is through improper handling and maintenance.
Film Processing
The latent image is invisible because only a few silver ions have been changed to metallic silver and deposited at the sensitivity center. Processing the film magnifies this action many times until all of the silver ions in an exposed crystal are converted to atomic silver, thus converting the latent image into a visible radiographic image.
The exposed crystal becomes a black grain that is visible microscopically. The silver contained in fine jewelry and tableware would also appear black except that it has been highly polished, which smoothes the surface and makes it reflective.
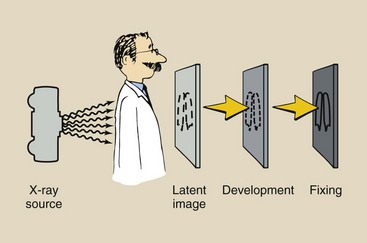
Processing is as important as technique and positioning in preparing a quality radiograph. Before the introduction of automatic film processing, x-ray films were processed manually. It took approximately 1 hour to prepare a completely dry and ready-to-read radiograph.
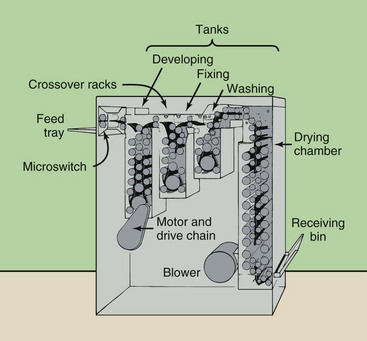
The first automatic processor was introduced by Pako in 1942 (Figure 12-25) and could process 120 films per hour with the use of special film hangers. These film hangers were dunked from one tank to another. The total cycle time for processing one film was approximately 40 minutes.
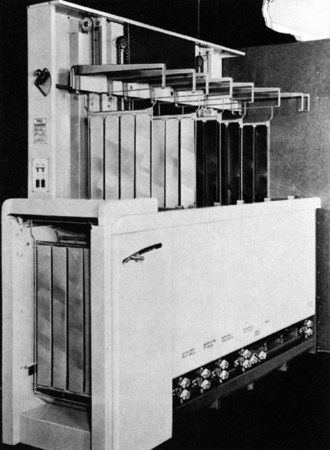
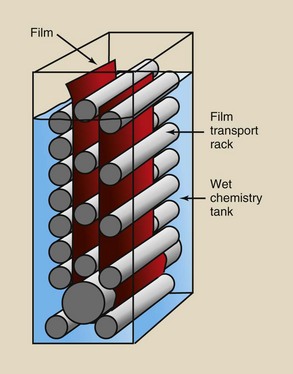
Automatic processing advanced significantly in 1956, when the Eastman Kodak Company introduced the first roller transport system for processing medical radiographs. The roller transport automatic processor shown in Figure 12-26 was about 10 feet long, weighed nearly three quarters of a ton, and sold for approximately $350,000 in today’s dollars.

FIGURE 12-26 The first roller transport automatic processor, circa 1956. (Courtesy Eastman Kodak Company.)
Another significant breakthrough was Eastman Kodak’s introduction of 90-second rapid processing in 1965. Rapid processing was possible because of the development of new chemistry and emulsions, as well as the faster drying permitted by a polyester film base. With this processor, the dry-to-drop time is 90 seconds. This type of automatic film processing system remains the standard.
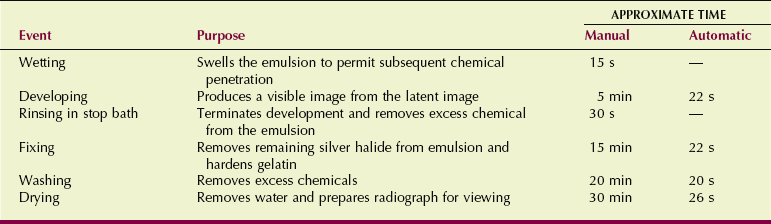
Radiographic film processing involves several steps; these are summarized in Table 12-7.
All radiographic processing is automatic today; therefore, the following discussion does not cover manual processing. The chemicals involved in both are basically the same. In automatic processing, the time for each step is shorter, and the chemical concentration and temperature are higher.
The first step in the processing sequence involves wetting the film to swell the emulsion, so that subsequent chemical baths can reach all parts of the emulsion uniformly. In automatic processing, this step is omitted, and the wetting agent is incorporated into the second step, developing.
The developing stage is very short and highly critical. After developing, the film is rinsed in an acid solution designed to stop the developing process and remove excess developer chemicals from the emulsion. Photographers call this step the stop bath. In radiographic processing, the stop bath is included in the next step, fixing.
The gelatin portion of the emulsion is hardened at the same time to increase its structural soundness. Fixing is followed by vigorous washing of the film to remove any remaining chemicals from the previous processing steps.
Finally, the film is dried to remove the water used to wash it and to make the film acceptable for handling and viewing.
Developing, fixing, and washing are important steps in the processing of radiographic film. The precise chemical reactions involved in these steps are not completely understood. However, a review of the general action is in order because of the importance of processing in a high-quality radiograph.
Processing Chemistry
The chemicals used to process films are designed to penetrate an emulsion and cause an effect. Those used in automatic processors do this very efficiently in the very short time the film is immersed.
Thus, when one is mixing solutions, cleaning a processor, or participating in any activity with or near processing solutions, these steps should be followed:
• Wear a proper mask that reduces inhalation of fumes—not the standard surgical mask that only guards against particles and bugs.
• Wear nitrile gloves. Do not use surgical gloves; they only protect against biologic matter. Remember that photographic chemicals are designed to penetrate, and thin rubber gloves provide no guarantee of safety.
• Wear protective glasses. Chemical splashes in the eyes are painful.
A solvent is a liquid into which various solids and powders can be dissolved. The universal solvent is water, which is the solvent for all the chemicals used in processing a radiograph.
Wetting
For these chemicals to penetrate the emulsion, the radiograph must first be treated by a wetting agent. The wetting agent is water, and it penetrates the gelatin of the emulsion, causing it to swell. In automatic processing, the wetting agent is in the developer.
Development
The principal action of the developer is to change the silver ions of exposed crystals into metallic silver. The developer provides electrons to the sensitivity center of the crystal to change the silver ions to silver.
In addition to the solvent, the developer contains a number of other ingredients. The composition of the developer and the function of each ingredient are outlined in Table 12-8.
TABLE 12-8
Components of the Developer and Their Functions
| Component | Chemical | Function |
| Developing agent | Phenidone | Reducing agent; produces shades of gray rapidly |
| Hydroquinone | Reducing agent; produces black tones slowly | |
| Activator | Sodium carbonate | Helps swell gelatin; produces alkalinity; controls pH |
| Restrainer | Potassium bromide | Antifog agent; protects unexposed crystals from chemical “attack” |
| Preservative | Sodium sulfite | Controls oxidation; maintains balance among developer components |
| Hardener | Glutaraldehyde | Controls emulsion swelling and enhances archival quality |
| Sequestering agent | Chelates | Removes metallic impurities; stabilizes developing agent |
| Solvent | Water | Dissolves chemicals for use |
For the ionic silver to be changed to metallic silver, an electron must be supplied to the silver ion. Chemically, the reaction is described as follows:
When an electron is given up by a chemical, in this case the developer, to neutralize a positive ion, the process is called reduction. The silver ion is said to be reduced to metallic silver, and the chemical responsible for this is called a reducing agent.
The opposite of reduction is oxidation, a reaction that produces an electron. Oxidation and reduction occur simultaneously and are called redox reactions. To help recall the proper association, think of EUR/OPE: electrons used in reduction/oxidation produce electrons.
The principal component of the developer is hydroquinone. Secondary constituents are Phenidone and Metol.
Usually, hydroquinone and Phenidone are combined for rapid processing. As reducing agents, each of these molecules has an abundance of electrons that can be easily released to reduce silver ions.
The optical density of a processed radiograph results from the development of crystals that contain a latent image (Figure 12-27).
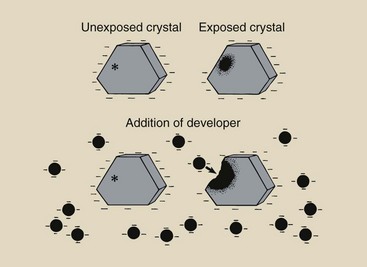
FIGURE 12-27 Development is the chemical process that amplifies the latent image. Only crystals that contain a latent image are reduced to metallic silver by the addition of developing agents.
The characteristic curve of a radiograph is shaped by the synergistic action of developing agents. Hydroquinone acts rather slowly but is responsible for the very blackest shades. Phenidone acts rapidly and influences the lighter shades of gray. Phenidone controls the toe of the characteristic curve, and hydroquinone controls the shoulder (Figure 12-28).
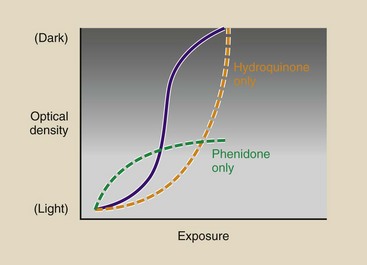
FIGURE 12-28 The shape of the characteristic curve is controlled by the developing agents. Phenidone controls the toe, and hydroquinone controls the shoulder.
An unexposed silver halide crystal has a negative electrostatic charge distributed over its entire surface. An exposed silver halide crystal, on the other hand, has a negative electrostatic charge distributed over its surface except at the sensitivity center. The similar electrostatic charges on the developing agent and the silver halide crystal make it difficult for the developing agent to penetrate the crystal surface except in the region of the sensitivity center in an exposed crystal.
In such an exposed crystal, the developing agent penetrates the crystal through the sensitivity center and reduces the remaining silver ions to atomic silver. The sensitivity center can be considered a metallic conducting electrode through which electrons are transferred from the developing agent into the crystal. Development of exposed and unexposed crystals results in the types of differences illustrated in Figure 12-29.

FIGURE 12-29 Underdevelopment results in a dull radiograph because the crystals that contain a latent image have not been completely reduced. Overdevelopment produces a similar radiograph because of the partial reduction of unexposed crystals. Proper development results in maximum contrast.
The reduction of a silver ion is accompanied by the liberation of a bromide ion. The bromide ion migrates through the remnant of the crystal into the gelatin portion of the emulsion. From there, the ion is dissolved into the developer and is removed from the film.
The developer contains alkali compounds, such as sodium carbonate and sodium hydroxide. These buffering agents enhance the action of the developing agent by controlling the concentration of hydrogen ions: the pH.
These alkali compounds are caustic, that is, they are very corrosive and can cause a skin burn. Sodium hydroxide, the strongest alkali, is commonly called lye. Be very cautious if you mix a developer solution that contains sodium hydroxide. You should wear rubber gloves and, of course, never let it get near your mouth or eyes.
Potassium bromide and potassium iodide are added to the developer as restrainers. Restrainers restrict the action of the developing agent to only those silver halide crystals that have been irradiated. Without the restrainer, even those crystals that have not been exposed are reduced to metallic silver. This results in an increased fog that is called development fog.
A preservative is also included in the developer to control the oxidation of the developing agent by air. Air is introduced into the chemistry when it is mixed, handled, and stored; such oxidation is called aerial oxidation. By controlling aerial oxidation, the preservative helps maintain the proper development rate.
Mixed chemicals last only a couple of weeks; thus, replenishment tanks require close-fitting floating lids for the control of aerial oxidation. Hydroquinone is particularly sensitive to aerial oxidation. It is easy to tell when the developing agent has been oxidized because it turns brownish. The addition of a preservative, usually sodium sulfite, causes the developer to remain clear.
Developers used in automatic processors contain a hardener, usually glutaraldehyde. If the emulsion swells too much or becomes too soft, the film will not be transported properly through the system because of the very close tolerances of the transport system.
The hardener controls swelling and softening of the emulsion. When films that drop from the processor are damp, the usual cause is depletion of the hardener.
The developer may contain metal impurities and soluble salts. Such impurities can accelerate the oxidation of hydroquinone, rendering the developer unstable. Chelates are introduced as sequestering agents that form stable complexes with these metallic ions and salts.
With proper development, all exposed crystals that contain a latent image are reduced to metallic silver, and unexposed crystals are unaffected. The development process, however, is not perfect: Some crystals that contain a latent image remain undeveloped (unreduced), but other crystals that are unexposed may be developed. Both of these actions reduce the quality of the radiograph.
Film development is basically a chemical reaction. Similar to all chemical reactions, it is governed by three physical characteristics: time, temperature, and concentration (of the developer). Long development time increases reduction of the silver in each grain and promotes the development of the total number of grains. High developer temperature has the same effect.
Similarly, silver reduction is controlled by the concentrations of developing chemicals. With increased developer concentrations, the reducing agent becomes more powerful and can more readily penetrate both exposed and unexposed silver halide crystals.
Manufacturers of x-ray film and of developing chemicals have very carefully determined the optimal conditions of time, temperature, and concentration for proper development. Optimal conditions of contrast, speed, and fog can be expected if the manufacturer’s recommendations for development are followed.
The image on a fogged film is gray and lacks proper contrast. The causes of fog are many, but perhaps the most important are those just mentioned—time, temperature, and developer concentration. An increase in any of these factors beyond manufacturer recommendations results in increased development fog.
Fog also can be produced by chemical contamination of the developer (chemical fog), unintentional exposure to radiation (radiation fog), or improper storage at an elevated temperature and humidity.
Fixing
When development is complete, the film must be treated so that the image will not fade. This stage of processing is fixing. The image is said to be fixed on the film, and this produces film of archival quality.
When the film is removed from the developer, some developer is trapped in the emulsion and continues its reducing action. If developing is not stopped, development fog results. As discussed earlier, the step in manual processing that follows development is called stop bath, and its function is just that—to neutralize the residual developer in the emulsion and stop its action. The chemical used in the stop bath is acetic acid.
In automatic processing, a stop bath is not used because the rollers of the transport system squeeze the film clean. Furthermore, the fixer contains acetic acid that behaves as a stop bath. This acetic acid, however, is called an activator. An activator neutralizes the pH of the emulsion and stops developer action. Table 12-9 lists the chemical components of the fixer.
TABLE 12-9
Components of the Fixer and Their Functions
| Component | Chemical | Function |
| Activator | Acetic acid | Neutralizes the developer and stops its action |
| Fixing agent | Ammonium thiosulfate | Removes undeveloped silver bromine from emulsion |
| Hardener | Potassium alum | Stiffens and shrinks emulsion |
| Preservative | Sodium sulfite | Maintains chemical balance |
| Buffer | Acetate | Maintains proper pH |
| Sequestering agent | Boric acids and salts | Removes aluminum ions |
| Solvent | Water | Dissolves other components |
The terms clearing agent, hypo, and thiosulfate often are used interchangeably in reference to the fixing agent. Fixing agents remove unexposed and undeveloped silver halide crystals from the emulsion. Sodium thiosulfate is the agent classically known as hypo, but ammonium thiosulfate is the fixing agent that is used in most fixer chemistries.
Hypo retention is the term used to describe the undesirable retention of the fixer in the emulsion. Excess hypo slowly oxidizes and causes the image to discolor to brown over a long time. Fixing agents retained in the emulsion combine with silver to form silver sulfide, which appears yellow-brown.
The fixer also contains a chemical called a hardener. As the developed and unreduced silver bromide is removed from the emulsion during fixation, the emulsion shrinks. The hardener accelerates this shrinking process and causes the emulsion to become more rigid or hardened.
The purpose of hardeners is to ensure that the film is transported properly through the wash-and-dry section and that rapid and complete drying occurs. The chemicals commonly used as hardeners are potassium alum, aluminum chloride, and chromium alum. Normally, only one is used in a given formulation.
The fixer also contains a preservative that is of the same composition and that serves the same purpose as the preservative in the developer. The preservative is sodium sulfite, and it is needed to maintain the chemical balance because of the carryover of developer and fixer from one tank to another.
The alkalinity and acidity—the pH—of the fixer must remain constant. This is helped by adding a buffer, usually acetate, to the fixer.
In the same way that metallic ions are sequestered in the developer, so must they be sequestered in the fixer. Aluminum ions represent the principal impurity at this stage. Boric acids and boric salts are used for sequestering.
Finally, the fixer contains water as the solvent. Other chemicals might be applicable as a solvent, but they are thicker and are more likely to gum up the transport mechanism of the automatic processor.
Washing
The next stage in processing is to wash away any residual chemicals remaining in the emulsion, particularly hypo that clings to the surface of the film. Water is used as the wash agent. In automatic processing, the temperature of the wash water should be maintained at approximately 3°C (5°F) below the developer temperature.
In this way, the wash bath also serves to stabilize developer temperature. Inadequate washing leads to excessive hypo retention and the production of an image that will fade, turn brown with time, and be of generally poor archival quality.
Drying
For the final step in processing, drying the radiograph, warm dry air is blown over both surfaces of the film as it is transported through the drying chamber.
The total sequence of events involved in manual processing takes longer than 1 hour to be completed. Most automatic processors are 90-second processors and require a total time from start to finish—the dry-to-drop time—of just that, 90 seconds.
The process of converting the latent image to a visible image can be summarized as a three-step process within the emulsion (Figure 12-30). First, the latent image is formed by x-ray exposure of silver halide grains. Next, the exposed grains and only the exposed grains are made visible by development. Finally, fixing removes the unexposed grains from the emulsion and makes the image permanent.
Automatic Processing
With the introduction of roller transport automatic processing in 1956, the efficiency of radiologic services was increased considerably. Additionally, automatic processing has resulted in better image quality because each radiograph is processed in exactly the same way. The opportunity for human variation and error is nearly absent.
The principal components of an automatic processor are the transport system, the temperature control system, the circulation system, the replenishment system, and the dryer system (Table 12-10). Figure 12-31 is a cutaway view of an automatic processor.
TABLE 12-10
Principal Components of an Automatic Processor
| System | Subsystem | Purpose |
| Transport | Transports film through various stages at precise intervals | |
| Roller | Supports film movement | |
| Transport rack | Moves and changes direction of film via rollers and guide shoes | |
| Drive | Provides power to turn rollers at a precise rate | |
| Temperature | Monitors and adjusts temperature at each stage | |
| Circulation | Agitates fluids | |
| Developer | Continuously mixes, filters | |
| Fixer | Continuously mixes | |
| Wash | Single-pass water flows at constant rate | |
| Replenishment | Developer | Meters and replaces |
| Fixer | Meters and replaces | |
| Dryer | Removes moisture, vents exhaust |
Transport System
The transport system begins at the feed tray, where the film to be processed is inserted into the automatic processor in the darkroom. There, entrance rollers grip the film to begin its trip through the processor. A microswitch is engaged to control the replenishment rate of the processing chemicals.
Always feed the film evenly using the side rails of the feed tray and alternate sides from film to film (Figure 12-32). This ensures even wear of the transport system components. From the entrance rollers, the film is transported by rollers and racks through the wet chemistry tanks and the drying chamber and is finally deposited in the receiving bin.
FIGURE 12-32 Place the short side of the film against the side rail of the feed tray and alternate films from one side to another.
The transport system not only transports the film; it also controls processing by controlling the time the film is immersed in each wet chemical. Timing for each step in processing is governed by careful control of the rate of film movement through each stage. The transport system consists of the following three principal subsystems: rollers, transport racks, and drive motor.
Three types of rollers are used in the transport system. Transport rollers, with a diameter of 1 inch, convey the film along its path. They are positioned opposite one another in pairs or are offset from one another (Figure 12-33).
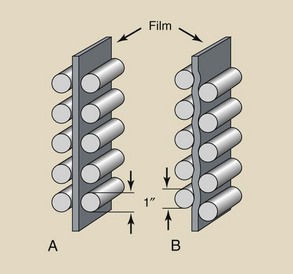
FIGURE 12-33 A, Transport rollers positioned opposite each other. B, Transport rollers positioned offset from one another.
A master roller, with a diameter of 3 inches, is used when the film makes a turn in the processor (Figure 12-34). A number of planetary rollers and metal or plastic guide shoes are usually positioned around the master roller.
FIGURE 12-34 A master roller with planetary rollers and guide shoes is used to reverse the direction of film in a processor.
Except for the entering rollers at the feed tray, most of the rollers in the transport system are positioned on a rack assembly (Figure 12-35). These racks are easily removable and provide for convenient maintenance and efficient cleaning of the processor.
When the film is transported in one direction along the rack assembly, only 25-mm (1-inch) rollers are required to guide and propel it. At each bend, however, a curved metal lip with smooth grooves guides the film around the bend. These are called guide shoes. For a 180-degree bend, the film is positioned for the turn by the leading guide shoe, is propelled around the curve by the master roller and its planetary rollers, and leaves the curve by entering the next straight run of rollers through the trailing guide shoe.
When the film exits the top of the rack assembly, it is guided to the adjacent rack assembly through a crossover rack. The crossover rack is a smaller rack assembly that is composed of rollers and guide shoes.
Power for the transport system is provided by a fractional horsepower drive motor. The shaft of the drive motor is usually reduced to 10 to 20 rpm through a gear reduction assembly.
Temperature Control System
The developer, fixer, and wash require precise temperature control. The developer temperature is most critical, and it is usually maintained at 35°C (95°F). Wash water is maintained at 3°C (5°F) lower. Temperature is monitored at each stage by a thermocouple or thermistor and is controlled thermostatically by a controlled heating element in each tank.
Circulation System
Agitation is necessary to continually mix the processing chemicals, maintain a constant temperature throughout the processing tank, and aid exposure of the emulsion to the chemicals. In automatic processing, a circulation system continuously pumps the developer and the fixer, thus maintaining constant agitation within each tank.
The developer circulation system requires a filter that traps particles as small as approximately 100 µm to trap flecks of gelatin that are dislodged from the emulsion. The particles thus have less chance of becoming attached to the rollers, where they can produce artifacts. These filters are not 100% efficient; therefore, sludge can build up on the rollers.
Filtration in the fixer circulation system is normally unnecessary because the fixer hardens and shrinks the gelatin so that the rollers are not coated. Furthermore, the fixer neutralizes the developer; therefore, the products of this reaction do not affect the final radiograph.
Water must be circulated through the wash tank to remove all of the processing chemicals from the surface of the film before drying; this ensures archival quality. An open system, rather than a closed circulation system, usually is used. Fresh tap water is piped into the tank at the bottom and overflows out the top, where it is collected and discharged directly to the sewer system. The minimum flow rate for the wash tank in most processors is 12 L/min (3 gal/min).
Replenishment System
Each time a film makes its way through the processor, it uses some of the processing chemicals. Some developer is absorbed into the emulsion and then is neutralized during fixing. The fixer, likewise, is absorbed during that stage of processing, and some is carried over into the wash tank.
The replenishment system meters the proper quantities of chemicals into each tank to maintain volume and chemical activity. Although replenishment of the developer is more important, the fixer also has to be replenished. Wash water is not recirculated and therefore is continuously and completely replenished.
When a film is inserted onto the feed tray with its widest dimension gripped by the leading rollers and its narrow side against the side rail, a microswitch is activated and turns on the replenishment for as long as film travels through the microswitch. Replenishment rates are approximately 60 to 70 mL of developer and 100 to 110 mL of fixer for every 35 cm (14 in) of film.
Dryer System
A wet or damp finished radiograph easily picks up dust particles that can result in artifacts. Furthermore, a wet or damp film is difficult to handle in a viewbox. When stored, it can become sticky and may be destroyed.
The dryer system consists of a blower, ventilation ducts, drying tubes, and an exhaust system. The dryer system extracts all residual moisture from the processed radiograph, so it drops into the receiving bin dry.
The blower is a fan that sucks in room air and blows it across heating coils through ductwork to the drying tubes. Therefore, room air should be low in humidity and free of dust. Sometimes as many as three heating coils of approximately 2500 W capacity are used. The temperature of the air entering the drying chamber is thermostatically regulated.
The hot, moist air is vented from the drying chamber to the outside, in much the same way as the air in a clothes dryer is vented. Some fraction of the exhaust air may be recirculated within the dryer system.
When damp films drop into the receiving bin, the radiologic technologist should immediately suspect a malfunction of the dryer system, although developer and fixer replenishment also should be checked. Underreplenishment reduces the concentration of hardener and is a common cause of damp films.
Summary
Image-forming x-radiation is that part of the x-ray beam that exits a patient and exposes the IR. The conventional image radiographic IR is a cassette that contains radiographic film sandwiched between two radiographic intensifying screens. Radiographic film is made up of a polyester base that is covered on both sides with a film emulsion.
The film emulsion contains light-sensitive silver bromide crystals that are made from the mixture of silver nitrate and potassium bromide. During manufacture, the emulsion is spread onto the base in darkness or under red lights because the AgBr molecule is sensitive to light.
The invisible latent image is formed in the film emulsion when light photons interact with the silver halide crystals. Processing of radiographic film converts the latent image to a visible image.
Following are some important characteristics of the radiographic screen-film IR:
• Contrast. High-contrast film produces black-and-white images. Low-contrast film produces images with shades of gray.
• Latitude. Latitude is the range of exposure techniques (kVp and mAs) that produce an acceptable image.
• Speed. Speed is the sensitivity of the screen-film combination to x-rays and light. Fast screen-film combinations need fewer x-rays to produce a diagnostic image.
• Crossover. When light is emitted from a radiographic intensifying screen, it exposes not only the adjacent film emulsion but also the emulsion on the other side of the base. The light crosses over the base and blurs the radiographic image.
• Spectral matching. The x-ray beam does not directly expose the x-ray film. Radiographic intensifying screens emit light when exposed to x-rays and the emitted light then exposes the radiographic film. The color of light emitted must match the response of the film.
• Reciprocity law. When exposed to the light of radiographic intensifying screens, radiographic film speed is less if the exposure time is very short or very long.
Film should be handled carefully and stored at specific temperatures and humidities to reduce artifacts. Artifacts on radiographic film can also be caused by rough handling.
1. Define or otherwise identify the following:
2. Diagram the cross-sectional view of a radiographic film designed for use with a pair of radiographic intensifying screens.
3. What does the term dimensional stability mean when applied to radiographic film? Which part of the film is responsible for this characteristic?
4. Identify the steps involved in the automatic processing of a radiograph and the time required for each step when a 90-second processor is used.
5. Discuss the two types of luminescence and how they are associated with radiographic intensifying screens and fluoroscopic screens.
6. Describe the process whereby a latent image is created in one crystal of the film emulsion.
7. Why are gloves and goggles recommended for persons who mix or handle developer solutions?
8. What determines proper darkroom safelight selection?
9. Describe a technique designed to test for good screen-film contact.
10. What precautions are necessary when radiographic film is used and stored?
11. If a film is damp or wet when it drops into the receiving bin, what are the problem and probable cause?
12. Write the silver halide crystal reaction. What does the arrow pointing down represent?
13. What determines the speed of radiographic film?
14. Define or describe DQE and CE.
15. Explain the Gurney-Mott theory of latent image formation.
16. What is the importance of spectral matching in selection of screen-film combinations?
17. Why do radiographers need to be aware of reciprocity law failure?
18. An amber filter on a safelight is used under what conditions? A red filter on a safelight is used under what conditions?
19. Discuss the difference between regular screen film and mammography screen film.
The answers to the Challenge Questions can be found by logging on to our website at http://evolve.elsevier.com.