Chapter 8 Cardiac Computed Tomography
In the previous three chapters, we discussed chest imaging for a variety of indications, ignoring one of the most obvious concerns—cardiac ischemia. Among chief complaints to the emergency department, chest pain is the second most common, according to the National Hospital Ambulatory Medical Care Survey,1 and computed tomography (CT) has diagnostic value in ischemic chest pain evaluation and exclusion of pulmonary embolism and aortic dissection (discussed in detail in Chapter 7). CT scanners meeting the technical requirements for cardiac CT are widely available even in remote locations.2
In this chapter, we begin with some definitions of “cardiac CT” to provide a basis for understanding its diagnostic capabilities and limitations. We describe the major functions of cardiac CT, including calcium scoring, CT coronary angiography, CT assessment of left ventricular function, and assessment of noncardiac causes of chest pain. We explore controversial areas of relative risk and benefit, comparing CT to other diagnostic modalities and strategies for evaluating ischemia and cardiac function. We specifically consider issues of emergency department length of stay, crowding, and cost for which CT has putative benefits. We weigh these potential advantages against radiation exposure, potential for unnecessary testing (and increased costs), and risk for false-positive test results that may ensue from widespread use of cardiac CT.
What Is Cardiac CT?
Cardiac CT includes a range of protocols with the ability to provide important structural and functional information (Table 8-1). More information comes at a price, not only monetary but also in the form of increased radiation exposure or injected contrast dose, as we describe in detail later. As a consequence, not all information should be acquired in every patient. The emergency physician must carefully consider what information is clinically relevant to determine whether CT is the best test and to guide the choice of CT protocol for a given patient. Before discussing the diagnostic capabilities of CT, let’s consider the evolution of modern cardiac CT, because this sheds light on the choice of CT technique, determined by the clinical information needed.
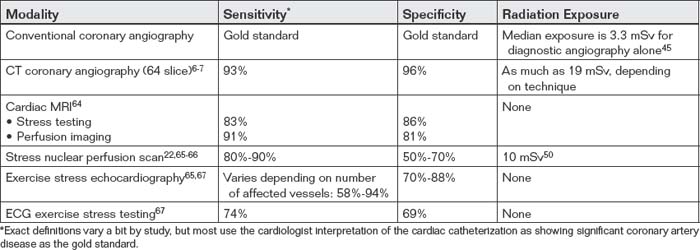
TABLE 8-1 Comparison of Cardiac Diagnostic Information Provided by Computed Tomography and Other Modalities
| Diagnostic Information | Provided by CT? | Also Supplied By |
|---|---|---|
| Presence of coronary artery disease with information on affected heart region | Yes, if ECG gated | |
| Specific coronary anatomy | Yes, if ECG gated | |
| Myocardial perfusion | Not yet routine, although experimental techniques exist for both rest and stress perfusion | |
| Ventricular function or ejection fraction | Yes, if retrospectively ECG gated | |
| Valvular stenosis or insufficiency | Yes, if retrospectively ECG gated | |
| Pericardial disease | Yes | |
| Pulmonary embolism | Yes, if triple rule-out scan | |
| Aortic dissection | Yes, if triple rule-out scan | |
| Anomalous coronary artery anatomy | Yes, if ECG gated | Coronary angiography, although with limited sensitivity |
Evolution of Cardiac Computed Tomography
Single-Slice, Nonhelical Computed Tomography
Cardiac CT has evolved dramatically since its inception in the 1970s, broadening the spectrum of clinical conditions that can be evaluated and improving the diagnostic accuracy. Early CT scanners relied on a “single slice” (also called “step and shoot”) technique to acquire images. A patient was positioned on an examination table that moved through the CT gantry, which was equipped with a single x-ray source and detector. The patient was moved into the gantry, table motion was stopped, and a single image slice was acquired with a single rotation of the gantry around the patient. The patient table was then incremented by a small distance and again stopped, and an additional image slice was acquired by another gantry rotation. The process was repeated until a complete set of images spanning the region of interest was acquired.
Limitations of this technique were numerous:
Several key technological innovations occurred by the late 1990s, surmounting many of the limitations of early CT.
Helical (“Spiral”) Computed Tomography and Volume Imaging
First, a new technique was introduced that allowed continuous gantry rotation and image data acquisition while the patient table moved through the CT scanner. This so-called spiral or helical technique allowed acquisition of three-dimensional volumes of image data (volume acquisition), without the data gaps created by discontinuous step-and-shoot single-slice scanners. Although many emergency physicians refer to CT pulmonary angiography as “spiral CT,” as if spiral technique were unique to this application, helical acquisitions are now routine for nearly every CT application in most body regions. Nonhelical, step-and-shoot acquisition is occasionally used in special circumstances described later, specifically in prospectively electrocardiogram (ECG)–gated CT for evaluation of coronary arteries.
Volume acquisition presented unique opportunities for complex computer modeling. The previous single-slice axial image acquisition technique offered reasonable image resolution in the axial plane (x- and y-axes). However, if axial image slices were “stacked” in the z-axis (cephalad-to-caudad direction) to create a three-dimensional volume and then “resliced” into coronal or sagittal planes, the resulting reconstructed images offered low quality. This was a result of both the missing data between the original axially acquired images and the relatively thick original slice thickness, which suffered from volume averaging. Coronal and sagittal reconstructions could not evaluate fine detail in the z-axis. In contrast, the new, helically acquired, three-dimensional volumes of data were isotropic—meaning that the pixel resolution in the x-, y-, and z-axes was equal. As a consequence, data could be manipulated extensively after acquisition, allowing detailed multiplanar reconstructions in axial, sagittal, coronal, and oblique planes. In addition, high-resolution three-dimensional reconstructions could be performed. Advances in computing speed from the 1970s to the 1990s were also instrumental in allowing these complex postprocessing algorithms to be performed.
Multidetector (Multislice) CT versus Single Detector (Single-Slice) CT
Another revolutionary diagnostic advance was the introduction of multidetector (sometimes called multislice) CT. The single x-ray detector of early CT scanners was replaced by an array of detectors, with 4, 16, 64, and even 256 detectors routinely available in modern scanners from multiple manufacturers. With multiple detectors simultaneously collecting image data during a helical acquisition, the speed of CT dramatically increased while image resolution was preserved or improved.
Here, it is important to clarify that the number of detectors alone does not determine resolution. Instead, the resolution is determined by the acquired slice thickness, which can be set as low as 0.625 mm even for many older-generation CT scanners with four or fewer detectors.5 However, acquiring 0.625-mm slices was impractical using single-slice scanners, as the time required to image a body region was prohibitive at this resolution. Consider our earlier example of a 35-cm (350 mm) thorax. A single-slice scanner acquiring contiguous slices 0.625 mm in thickness would require 560 gantry rotations to span the area of interest—for the earliest slow scanners operating at 3 seconds per slice, this would have required 28 minutes.
Slice number for modern scanners refers to the number of detectors, and thus the number of image slices, that can be simultaneously acquired during a single gantry rotation in the course of a helical image acquisition. A 4-slice scanner has 4 detectors and can acquire 4 slices per rotation; a 64-slice scanner has 64 detectors and can acquire 64 slices. The slice thickness to be acquired for each detector can be programmed, and this thickness multiplied by the number of detectors determines the z-axis span of patient anatomy that can be imaged during a single gantry rotation. For example, a 64-slice scanner, operating at maximum resolution (using the minimum slice thickness of 0.625 mm) can image 64 × 0.625 mm—or 40 mm—per rotation. Modern scanners have gantry rotation times under 1 second, so our 35-cm thorax could be completely imaged at maximum resolution in less than 9 seconds. If faster imaging is required, the slice thickness can be increased at the cost of some image resolution. For example, if the slice thickness is increased to 1 mm, a 64-slice scanner can cover 64 mm of z-axis per gantry rotation, and a 35-cm thorax would be imaged in fewer than 5.5 seconds. Such a fast acquisition means that the scanner can readily keep pace with the movement of the injected contrast bolus while acquiring detailed images of small thoracic structures. The extreme speed of 64-slice multidetector helical CT also eliminates many of the motion artifacts that plagued slower CT scanners, including motion artifacts from cardiac and respiratory motion and patient body movement. This, in turn, dramatically improves the diagnostic ability of CT for subtle pulmonary embolism, aortic dissection, and cardiac abnormalities including coronary artery disease. Because of their high spatial resolution and high image acquisition speed, 64-slice CT scanners allow more coronary artery segments to be assessed accurately, according to meta-analyses comparing 4-, 16-, and 64-slice CT.6-7 Klass et al.8 reported that 256-slice CT scanners provide assessment of 98.9% of coronary artery segments, compared with 95.6% for 64-slice CT.
We have simplified the preceding discussion slightly for clarity. Image quality is not determined solely by slice thickness. The CT x-ray tube voltage and current can also be modulated. Higher voltage and current result in more energy, improving the signal-to-noise ratio. This improves image quality, but it does so at the expense of increased patient radiation exposure—a topic that we explore in more detail later in this chapter. The potential benefits of an improved image must be weighed against this risk.
Electrocardiogram Gating
Fast CT imaging with multidetector CT can be modified further with the use of ECG gating. Not all CT scanners have this capability, although most modern scanners do. Without ECG gating, the CT scanner acquires images in a rapid and continuous helical motion, without regard to the phase of the cardiac cycle. Respiratory and cardiac motion occurs, but the speed of the CT acquisition is relied upon to minimize motion artifact. In ECG gating, the acquisition of CT images is timed to coincide with a particular phase of the cardiac cycle. For evaluation of coronary artery patency and course, diastole is the preferred moment of the cardiac cycle for several reasons. First, during late diastole, cardiac motion is minimized. Second, coronary artery perfusion is maximized during diastole, when the aortic valve is closed. Third, like cardiac motion, external compression of coronary arteries by the contracting myocardium is minimized during diastole. This reduces the likelihood of overestimating the degree of coronary artery stenosis. With ECG gating, CT images are typically acquired at a point 75% from the origin of the R–R interval.9
ECG gating can be performed prospectively or retrospectively (Figure 8-1), each technique with its advantages and disadvantages. In prospective ECG gating, CT images are acquired solely during an end-diastole, as described earlier. The method requires the software to recognize the R wave and to predict the occurrence of the next QRS complex; consequently, a regular cardiac rhythm is desirable, although software advances allow gating of even moderately irregular rhythms. The technique acquires a frozen snapshot of the heart with minimal motion artifact and is useful for evaluation of coronary arteries and static features of cardiac anatomy. The method requires reverting to the original step-and-shoot acquisition described earlier, rather than using helical acquisition. Multiple detectors are employed to capture numerous image slices (or data volumes) during each end-diastolic period. An advantage of this technique is a significant reduction in radiation exposure, compared with retrospective gating, which is described later. The CT x-ray source is only turned on during the brief moment of an end-diastolic period, so the total time of radiation exposure is substantially shortened, proportionally reducing the radiation dose. A disadvantage of this technique is that images of the heart during other phases of cardiac contraction are not recorded. Consequently, functional evaluation of cardiac contractility (ejection fraction or valvular motion) cannot be performed, and movie images of cardiac contraction cannot be generated.10
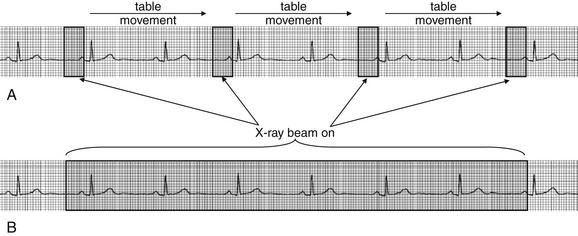
Figure 8-1 Electrocardiogram (ECG) gating of CT.
In prospective ECG gating (A), the CT x-ray beam is turned on only during late diastole. Images acquired with this technique provide a motionless view of the heart during a single phase of cardiac contraction, ideal for viewing coronary arteries but not useful for inspecting cardiac wall and valve motion or for calculation of ejection fraction. This technique reduces total radiation exposures. In retrospective ECG gating (B), the CT x-ray beam is turned on throughout the cardiac cycle. Images from late diastole can be selected and used to build a static image of the heart for inspection of the coronary arteries. The entire series of images can be sequenced as a movie to allow determination of ejection fraction and inspection of ventricular wall and valve motion. This technique results in higher patient radiation exposures because of the longer duration of x-ray exposure.
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
In retrospective ECG gating, images are acquired throughout the cardiac cycle. Acquired image data is encoded with the phase of cardiac contractility based on the ECG. After image acquisition is completed, images representing a particular phase of the cardiac cycle can be viewed as a static study. This allows images representing an end-diastolic period to be isolated, allowing evaluation of coronary arteries. In addition, images throughout the cardiac cycle can be viewed as a video, allowing assessment of cardiac motion and function (so-called four-dimensional imaging because of the ability to observe images through time). Regional wall motion abnormalities representing areas of ischemia or infarction can be identified, just as with echocardiographic images. Valvular abnormalities can be recognized. While the additional information provided by retrospective ECG gating makes it an attractive option, this information comes at the price of a significant increase in radiation exposure relative to prospective ECG gating.10 In older patients, this radiation exposure may be of limited consequence, but it is a significant concern in younger patients, as discussed later in this chapter.
Prospectively and retrospectively ECG-gated CT provide comparable coronary artery image quality, so the choice of one strategy over the other should weigh radiation dose against the need for functional information, remembering that ejection fraction, wall motion, and valvular mobility can be assessed without ionizing radiation exposure using echocardiography.10 Retrospective ECG-gated CT remains attractive because it can provide this information, along with coronary artery imaging, in a single diagnostic examination. However, in one study, prospectively ECG-gated CT reduced the radiation exposure by 77% compared with retrospective gating, from 18.1 to 4.2 mSv.10 New protocols can reduce the radiation exposure from CT further using a technique called dose modulation, which combines features of prospective and retrospective ECG gating. The x-ray source remains on during the entire scan, allowing time-resolved information about cardiac function to be gleaned throughout the cardiac cycle. However, the x-ray tube current is reduced except during the key phase of an end-diastolic period, when high signal-to-noise ratio is critical to imaging of coronary arteries.11
Cardiac Computed Tomography Clinical Applications
With this technical background let’s consider the clinical applications of cardiac CT. We review the information provided by various cardiac CT protocols, comparing this information to that provided by other test modalities. In some cases, CT provides the same information as that afforded by one or even multiple alternative tests. In other instances, CT may provide information unavailable from other modalities, even the “gold standard” of cardiac catheterization.
As we briefly mentioned at the beginning of this chapter, CT can be performed for several critical diagnostic functions:
Not every CT necessarily addresses all of these functions—the protocol can be varied to provide more or less information, with additional data requiring a higher radiation dose and vascular contrast exposure. Table 8-1 summarizes the diagnostic information provided by cardiac CT in comparison with other modalities.
Let’s consider each of these CT functions in detail.
Coronary Artery Calcium Scoring
CT can quantify coronary artery calcifications (Figure 8-2). Coronary artery calcium scores gained popularity in the late 1990s and early 2000s as a result of the observation that calcium deposits in coronary arteries correlated with the risk for obstructive coronary artery disease. Calcium deposits are visible without the use of injected contrast and appear bright white on CT. Scores are rated from 0 (no calcifications) to 1000 and greater, with scores greater than 400 denoting significant coronary artery calcium.12-13 An advantage of this technique is that it can be used in patients with contraindications to iodinated contrast, such as allergy or renal insufficiency. When coronary artery calcium scores are compared with the presence of coronary artery luminal stenoses detected by CT coronary angiography, a rising coronary artery calcium score is correlated positively with the presence of significant luminal stenosis (greater than 60%). The absence of coronary calcifications is strongly correlated with the absence of significant coronary artery disease.14
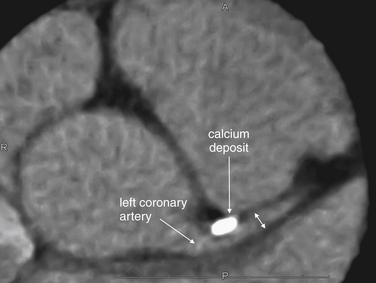
Figure 8-2 Calcium scoring or CT coronary angiography?
Older CT technology relied on the presence of calcium deposits in vessel walls to predict clinically significant coronary artery disease. Modern CT coronary angiography adds the ability to measure actual coronary artery luminal diameter, similar to measurements made during catheter angiography. In this image, a calcium deposit in the proximal left main coronary artery is visible (bright white); in addition, the vessel lumen can be visualized (double arrow).
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
Unfortunately, coronary artery calcifications are relatively nonspecific and may occur in the absence of luminal stenosis; therefore, they do not readily guide decisions about the need for coronary artery interventions without additional imaging. For example, Ho et al.14 found that in patients with the highest coronary calcium scores (greater than 1000), the prevalence of significant coronary artery stenosis was only 27%. While the absence of coronary artery calcifications may signify a low risk for significant coronary artery disease, the presence of calcifications is so nonspecific that the use of calcium scores to evaluate a low-risk chest pain population for coronary artery disease would be expected to result in numerous false positives, with the potential for additional expensive and potentially invasive diagnostic and therapeutic procedures. As the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Expert Consensus Document on Coronary Artery Calcium Scoring notes,12,13 “The relation of arterial calcification, like that of angiographic coronary artery stenosis, to the probability of plaque rupture is unknown. There is no known relationship between vulnerable plaque and coronary artery calcification.” Nonetheless, the ACCF/AHA12,13 concluded that in patients with atypical chest pain and a low risk for coronary artery disease, a calcium score of zero can be used to “help in ruling out the presence of obstructive coronary disease,” citing negative predictive values of 96% to 100% in studies of more than 7600 symptomatic patients. The ACC/AHA also noted that coronary artery calcium scores have not been compared extensively head to head with other diagnostic strategies, so the choice of calcium scoring over other strategies must be made with little evidence.12-13
CT Coronary Angiography
CT coronary angiography with intravenous (IV) contrast improves upon CT coronary artery calcium scoring by allowing measurement of coronary artery luminal diameter in the same way as conventional digital subtraction coronary angiography. The precise location and degree of coronary artery stenosis can be graded, with greater than 50% stenosis being considered clinically significant. CT images are acquired with either prospective or retrospective ECG gating, as described earlier. The images are typically viewed using specialized software, allowing synchronous viewing of axial, coronal, sagittal, and three-dimensional images (Figure 8-3). Software allows the user to plot the course of a coronary artery, select a normal and a stenotic arterial segment for comparison, and perform calculations of the degree of stenosis. Tortuous coronary arteries can be reconstructed in a “straightened” or “curved planar” view to improve visual assessment of the degree of stenosis. Thin-slab maximum intensity projection images (MIPSs) (Figure 8-4) also allow improved assessment of vessels that would be incompletely visualized on a single axial, coronal, or sagittal image because of their oblique or tortuous course.10 The AHA has defined a standardized 15-segment system of evaluation of coronary arteries, with CT allowing inspection of the main coronary arteries and their major branches from their origin down to a diameter of 1.5 mm.10 Figures 8-5 through 8-7 depict CT evaluation of coronary artery stenosis.
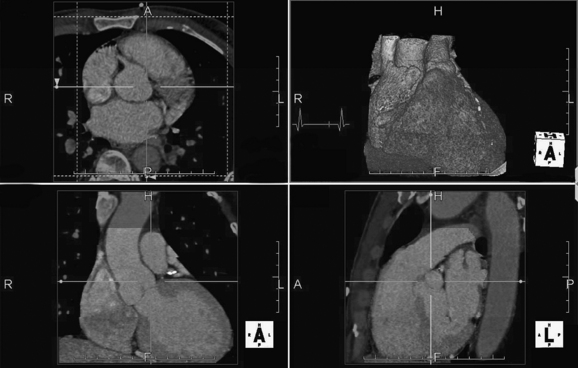
Figure 8-3 Workstation view of a prospectively gated cardiac CT study.
The dataset can be displayed in many formats, including (counterclockwise from upper left) axial, coronal, sagittal, and three-dimensional formats. These images were acquired late in the R–R interval (vertical mark on electrocardiogram tracing, upper right image). Automated software has been used to remove the bony thorax to facilitate inspection of the heart. The four windows are synchronized, so changes in view in one window are mirrored in the other windows.
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
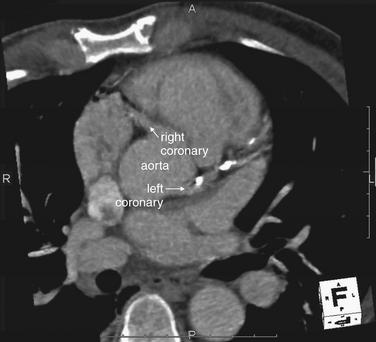
Figure 8-4 Maximum-intensity projection (MIP) image.
Although three-dimensional views are visually arresting, some of the most useful views are two-dimensional. One powerful image format is a MIP. This is an image composed of a slab of image data, the thickness of which can be adjusted depending on the structures of interest. In the image at left, the aortic root is visible with the origins of the left and right coronary arteries. The left coronary artery is heavily calcified (white deposits).
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
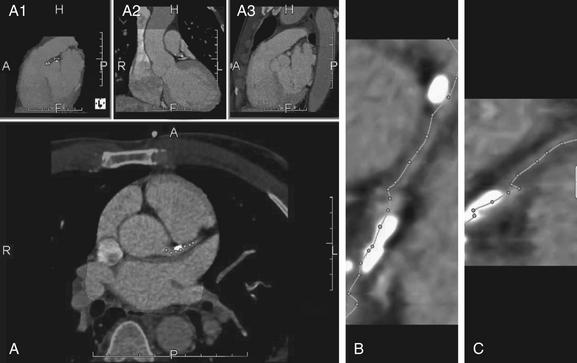
Figure 8-5 Using software to identify the center of a coronary artery and to determine the percentage of stenosis.
Several software packages are available. In this program, the user can visually inspect the coronary artery and manually deposit “seed points” (dots) with a mouse click (A). The user can move through the stack of images in any of the reformations, dropping seeds along the course of the vessel. The software then automatically connects these dots to form a planar reconstruction of the vessel, bringing a tortuous vessel into view as a single straightened output image (B, C). The user can edit this line to follow the vessel more closely. Above panel A are 3 additional small views. These images are automatically generated by the software and remain synchronized with the main image in A. The letters embedded in these images indicate anterior (A), posterior (P), right (R), left (L), head (H), and foot (F). These 3 panels demonstrate (from left to right) a midsagittal view of the heart with other thoracic structures removed (A1); a coronal view showing the right atrium, superior vena cava, left ventricle, and aorta (A2); and a midsagittal view with other thoracic structures visible, including the anterior chest wall and aorta (A3).
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
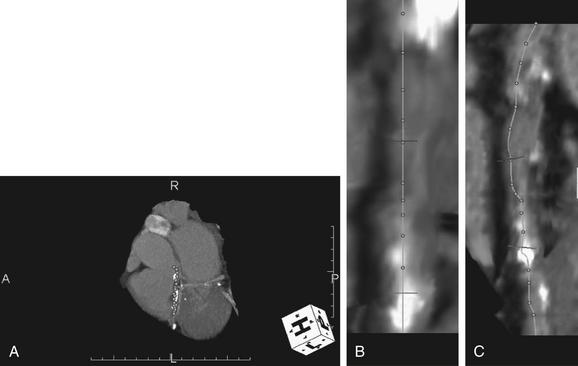
Figure 8-6 Is CT “user dependent” in calculation of stenosis?
Although sophisticated software automates many functions, the path of the coronary vessel marked by the technician can have a significant impact on calculations. This software package automatically created straightened versions of the arterial segments marked by the author in A (dots). These straightened reformations are shown in B and C. The author then manually placed a marker at a normal coronary diameter (B, C, top transverse line) and at an area of stenosis (B, C, lower transverse line). The software then automatically calculated the maximum stenosis. In this case, the maximum stenosis calculated using the author’s annotation was 75%—closely matching that found by the trained radiologist reading the CT and the cardiologist’s findings at cardiac catheterization.
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
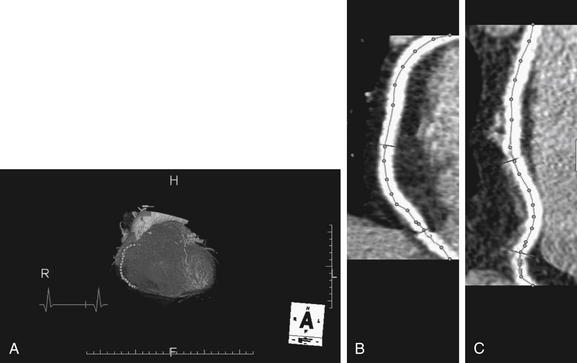
Figure 8-7 Another example of identifying the center of a coronary artery to determine the percentage of stenosis.
The author manually deposited seed points (dots) with a mouse click along the course of a coronary artery on the three-dimensional view (A). The software then automatically connected these dots to form a planar reconstruction of the vessel, bringing a tortuous vessel into view as a straightened output image (B, C). The user can edit this line to follow the vessel more closely. This vessel shows a 37% stenosis at its narrowest point (B, C, bottom transverse line).
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
Mechanism of CT Coronary Angiography in Evaluating Coronary Arteries for Stenosis
Like conventional coronary angiography, CT coronary angiography is a nonstress test. The patency of the coronary arteries is directly assessed, and the patient need not be placed under physiological stress to induce ischemia, unlike modalities such as stress echocardiography, stress nuclear perfusion scanning, stress cardiac magnetic resonance imaging (MRI), and ECG stress testing. Because CT does not risk inducing or worsening existing ischemia or infarction, a patient can undergo CT coronary angiography immediately, even with ongoing symptoms, and without an observation period for serial cardiac biomarker measurement and serial ECGs. Like conventional coronary angiography, the method should be safe even in patients with active ischemia or infarction—though emergency physicians should strive to recognize those patients from factors such as ECG findings without use of CT, because these patients may benefit from rapid conventional coronary angiography with its therapeutic revascularization potential. Many of the putative benefits of CT coronary angiography are based upon its nonstress mechanism, the ability to perform the test immediately, and the consequent ability to discharge patients with normal CT findings without prolonged observation or admission. We discuss some research findings supporting the cost- and resource-effectiveness of CT later. The nonstress capability of CT is not always an advantage. CT may identify stenotic arteries that are not responsible for inducible ischemia, because current CT coronary angiography does not test for abnormal myocardial perfusion during physiological stress. A new but not yet widely available technique called CT myocardial perfusion scanning is discussed later; this can be performed as a nonstress test or with the use of pharmacologically induced stress.15 Because current CT coronary angiography does not assess the functional perfusion deficit resulting from a stenotic region, it might result in detection of asymptomatic coronary artery disease and lead to unnecessary invasive revascularization procedures—a problem similar to that encountered with conventional coronary angiography. In a study of asymptomatic patients undergoing CT coronary angiography, significant stenoses were found in 16% of nondiabetics and 22% of diabetics.16 Whether detection and stenting of these asymptomatic lesions would improve mortality is uncertain.
Accuracy of CT
With submillimeter spatial resolution, ECG-gated 64-slice CT can visualize coronary arteries in exquisite detail (Figures 8-5 through 8-7) along much of their course, including 93% to 98% of proximal segments, 86% to 88% of proximal and middle segments, and 65% to 75% of all coronary artery segments.17-18 A systematic review found that 64-slice CT was more than 90% sensitive and 88% specific for the presence of significant coronary artery disease in patient-based evaluations.19 Patient-based refers to studies determining whether a given patient does or does not have coronary artery disease, rather than assessing for the presence of disease in a given vessel or vessel segment. This is a somewhat less stringent standard than a vessel-based comparison, because a CT scan that detects disease in one vessel when multivessel disease is found by conventional coronary angiography (or vice versa) is still credited as reaching the correct diagnosis. However, this is an acceptable standard from an emergency medicine perspective, where the clinically relevant decision point is the presence or absence of significant coronary artery disease in a given patient. The negative predictive value of 64-slice CT coronary angiography is consistently 96% to 100%, whereas positive predictive values range as low as 69% in some studies. The positive likelihood ratio of CT is 8.0, with a negative likelihood ratio below 0.1. Recall that negative likelihood ratios less than or equal to 0.1 are considered strong evidence of the absence of a disease and positive likelihood ratios of 10 or greater are useful to confirm a disease process. Therefore a negative CT coronary angiogram is a useful tool to rule out the presence of clinically important coronary artery disease, whereas a positive CT may require further diagnostic confirmation resulting from lack of specificity.19
Measurement of Coronary Artery Stenosis
CT allows quantitative measurement of luminal cross section to determine the percentage of stenosis. This may be more accurate than visual estimates of stenosis that are commonly performed with conventional coronary angiography, a method that has been shown to be inaccurate in determining the physiologic impact of moderate stenoses (<60% stenosis), with both over-estimation and underestimation of severity.20-21 CT is nonetheless likely “operator dependent.” In many CT software applications, software calculates the percentage of stenosis automatically—but only after a technician selects the location of the stenotic lesion and a normal coronary segment for comparison. As a consequence, the computer calculations are precise, but their accuracy depends partly on the accuracy of the human technician in selecting the normal and abnormal arterial segments. Sato et al.22 found that quantitative measures of stenosis from CT coronary angiography were only 79% sensitive but 92% specific for ischemia when a threshold of 70% stenosis was used. If stenosis less than 60% was observed by CT angiography, ischemia was rare. However, the criterion standard in this study was stress 201Tl single photon emission CT, a nuclear perfusion imaging technique that itself is not 100% sensitive or specific. This highlights the difficulty in evaluating new tests for cardiac ischemia, when the new test modality might theoretically be superior to existing standard tests.
How Does CT Coronary Angiography Compare with Noninvasive Stress Testing Modalities in Identifying Ischemia?
Earlier, we described the diagnostic accuracy of CT compared with the criterion standard of conventional coronary angiography. However, in the emergency department, the choice of diagnostic testing generally is not between CT and conventional angiography but between CT and a variety of stress testing modalities, such as stress echocardiography, stress nuclear perfusion testing, and stress cardiac MRI. The accuracy of CT compared with these diagnostic modalities remains in debate (Table 8-2). All are subject to false-positive and false-negative results, resulting in unnecessary invasive procedures and undiagnosed significant disease, respectively. CT provides precise anatomic visualization of the ischemia-related artery, whereas echocardiography, MRI, nuclear medicine studies, and ECG stress testing identify the heart region with inducible ischemia—which generally predicts the stenotic artery. For example, in an ECG stress test, ST segment depression in inferior ECG leads suggests ischemia of the inferior wall of the left ventricle, which is most often supplied by the right coronary artery. In a stress echocardiogram, inferior wall hypocontractility during stress testing would also suggest ischemia and disease of the right coronary artery. CT would directly demonstrate stenosis of this vessel.
What Are the Clinical Outcomes After “Negative” CT Coronary Angiography? Does a Negative CT Coronary Angiogram Allow Rapid and Safe Discharge From the Emergency Department?
We reviewed the sensitivity, specificity, and negative likelihood ratios for CT coronary angiography earlier. These characteristics suggest that CT coronary angiography could be safely used to risk-stratify emergency department patients, with a negative CT coronary angiogram allowing discharge with a low risk for adverse cardiovascular events. Naturally, other important causes of chest pain would require consideration before discharge. Next, let’s examine the evidence for the practice of emergency department use of CT coronary angiography for rapid discharge of chest pain patients without observation.
Several studies suggest that a CT coronary angiogram without significant coronary artery disease predicts a low risk for adverse cardiovascular events in the next 30 days in emergency department patients presenting with chest pain. Hollander et al.23 reported that among 54 consecutive emergency department chest pain patients undergoing CT coronary angiography, 46 (85%) were discharged immediately following a negative CT, with no adverse events at 30-day follow-up.
Gaemperli et al.24 reported clinical outcomes in 220 patients undergoing 64-slice CT coronary angiography for evaluation of chest pain, syncope, follow-up of abnormal stress testing, or preoperative evaluation. In those with normal coronary arteries by CT, none experienced death, nonfatal myocardial infarction (MI), unstable angina, or coronary revascularization at 1-year follow-up. However, this population may have differed in important ways from symptomatic emergency department chest pain patients.
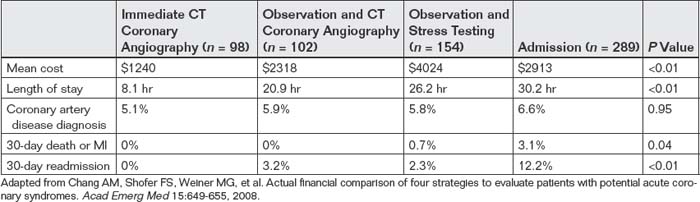
Chang et al.25 retrospectively compared four evaluation strategies for potential cardiac ischemia in low-risk patients who have a thrombolysis in myocardial infarction (TIMI) score of 0 to 2 (Box 8-1): (1) immediate CT coronary angiography in the emergency department without serial cardiac markers, (2) observation unit admission with serial cardiac markers followed by CT angiography, (3) observation unit admission with serial cardiac markers and stress testing, and (4) hospital admission with serial biomarkers and diagnostic workup directed by the hospitalist. Because this study was performed retrospectively and therefore did not involve randomization, the investigators attempted to adjust for selection biases that might have placed higher-risk patients in one diagnostic strategy by matching patients for age, race, gender, TIMI score, and initial ECG. The analysis favored the immediate CT coronary angiography strategy, with no cases of 30-day death, MI, or 30-day readmission. Coronary artery disease was diagnosed with similar frequency using all four diagnostic strategies (Table 8-3).
TABLE 8-3 Outcomes and Costs of Chest Pain Evaluations Involving CT Coronary Angiography and Other Common Care Pathways
Box 8-1 Thrombolysis in Myocardial Infarction (TIMI) Risk Score for Unstable Angina and Non-ST-Segment Elevation Myocardial Infarction
Adapted from Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 284(7):835-842, 2000.
Retrospective studies such as this can suggest but not prove that immediate CT coronary angiography is equally safe as observation and stress testing; prospective randomized controlled trials are needed to confirm the findings. Although the investigators attempted to account for selection bias by matching the various diagnostic groups for initial variables, the admitted group had a higher rate of death, MI, and readmission—suggesting that they were likely sicker at baseline. The absence of dangerous outcomes after discharge in the immediate CT group might simply indicate that this was a very-low-risk group, resulting from selection bias. Perhaps this group could have been discharged safely without any testing for coronary artery disease. That coronary artery disease was diagnosed in the immediate CT group with a similar frequency to that found in the other groups could mean that the risk profile in the various groups was similar. However, another interpretation may be that false-positive CT coronary angiography diagnosed insignificant coronary artery disease in this low-risk group—a problem that could lead to unnecessary, expensive, and invasive coronary interventions. The absence of readmissions for chest pain in the immediate CT group proves little about the safety or accuracy of CT coronary angiography; instead, it may simply indicate that physicians “believed” the results of CT coronary angiography and therefore, rightly or wrongly, did not choose to evaluate patients further if recent CT coronary angiography results were reassuring.
Hollander et al.26 also reported a prospective cohort study of 568 consecutive low- to intermediate-risk emergency department patients (TIMI score = 0 to 2, nonischemic ECG) presenting with potential acute coronary syndrome. Subjects underwent CT coronary angiography either immediately or after 9 to 12 hours of observation, including cardiac biomarker assessment. Patients were not randomized to diagnostic strategy; rather, strategy was determined by time of day. CT coronary angiography was performed immediately for 285 patients and after observation for 283 patients. 476 patients (84%) were found to have a negative CT coronary angiogram and were discharged, with no cardiovascular deaths or nonfatal MIs at 30-day follow-up.
The same investigators subsequently published results of 1-year follow-up after negative emergency department CT coronary angiography. Hollander et al.27 prospectively evaluated 588 low- to intermediate-risk patients (TIMI score = 0 to 2) undergoing emergency department CT coronary angiography for evaluation of potential acute coronary syndrome. Less than 50% stenosis by CT coronary angiography was found for 481 patients, who did not meet other exclusion criteria, including cocaine use, cancer, ejection fraction below 30%, or significant comorbid conditions reducing life expectancy. At 1-year follow-up, one death (uncertain cause), no acute MIs, and no revascularization procedures occurred in these 481 patients. The authors concluded that emergency department patients evaluated for acute coronary syndrome with a negative CT coronary angiogram have a very low risk for adverse cardiovascular outcomes at 1 year. In summary, a growing body of evidence suggests that CT coronary angiography can allow rapid and safe discharge of low- to intermediate-risk emergency department patients with chest pain and negative CT results. Critics fear that wide adoption of ED CT coronary angiography would ultimate raise costs because the ease and speed of the strategy might lead it to be misapplied to patients with essentially no risk of coronary artery disease.
Unique Diagnostic Information Provided by Computed Tomography Coronary Angiography
In addition to evaluating for the presence of significant coronary artery disease, cardiac CT can provide unique diagnostic information unavailable from other cardiac diagnostic imaging modalities, including the gold standard of conventional coronary angiography. Rarely, CT may reveal unusual cardiac pathology accounting for patient symptoms and requiring therapeutic action. Two important examples are anomalous coronary artery course28 (Figure 8-8) and myocardial bridging (Figure 8-9).
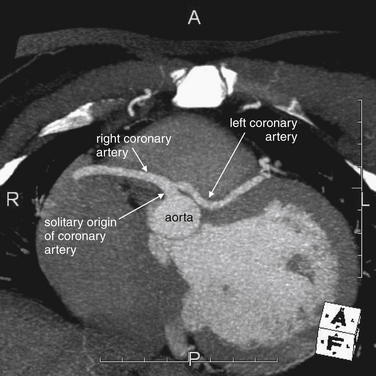
Figure 8-8 Anomalous coronary artery.
On this window setting, contrast is white and ventricular myocardium is an intermediate gray. The aorta gives off a single origin of a coronary artery, from which emerge the left and right coronary arteries. Anomalous coronary arteries such as these can be diagnosed on CT coronary angiography.
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
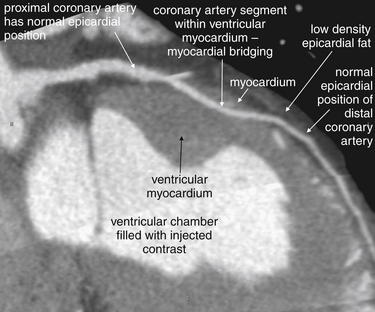
Figure 8-9 Myocardial bridging.
On this window setting, contrast is white, ventricular myocardium is an intermediate gray, and low-density epicardial fat is a dark gray. In this case, a normally epicardial coronary artery dives into the myocardium before reemerging in an epicardial position distally. This finding, called myocardial bridging, is detected by cardiac computed tomography but can be missed even by coronary angiography performed with cardiac catheterization. This may be a cause of cardiac ischemia and even sudden death after a “clean cath.”
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
Anomalous course of a coronary artery refers to the abnormal positioning of a coronary artery with respect to its origin from the aorta and its course relative to the aorta and pulmonary artery. An anomalous coronary artery may create symptoms, even when the artery is free of atherosclerotic disease. For example, an anomalous left main coronary artery positioned between the aorta and the main pulmonary artery may be compressed during high-output cardiac states such as exercise, leading to exertional ischemia or infarction and chest pain, syncope, or ventricular dysrhythmia.28 Anomalous coronary arteries may account for sudden cardiac death in young patients with “clean” or “negative” cardiac catheterizations. The rate of symptomatic anomalous coronary arteries is not well studied because of a lack of high-quality population-based studies. Life-threatening complications have been reported in as many as 20% of cases, though this may be an overestimate.29 Anomalous coronary arteries are likely a rare phenomenon, seen in only 11 of 1495 patients (0.7%) during a 2-year period in one study.28 However, Shi et al.30 found that 16-slice CT angiography detected twice as many anomalous coronary vessels as conventional angiography.
The ability of CT to simultaneously visualize the aorta, pulmonary artery, coronary arteries, and atria and ventricles accounts for its ability to make this diagnosis when conventional angiography cannot. During a conventional cardiac catheterization, either the left or the right heart is typically inspected, not both. A left heart cardiac catheterization reveals the aorta, coronary arteries, and left ventricle—but not all three structures simultaneously. The catheter tip must be positioned within each structure and contrast must be injected for the structure to be visible, and the three-dimensional relationship of structures to one another may not be recognized. The pulmonary artery is never seen during a typical left heart catheterization unless a septal defect with left to right shunting exists; a separate right heart catheterization is required. In contrast, during CT coronary angiography, the left and right heart and the coronary arteries, pulmonary artery, and aorta are seen simultaneously, revealing their three-dimensional relationship.
CT coronary angiography may also reveal myocardial bridging and the intramyocardial course of a coronary artery—findings not readily seen with conventional coronary angiography. The coronary arteries are normally epicardial vessels, located on the surface of the myocardium for most of their course. In myocardial bridging, a coronary artery enters the ventricular wall myocardium and then surfaces to an epicardial position again before terminating. A coronary artery with an intramyocardial course enters and terminates within the myocardium.31 Coronary arteries with myocardial bridging or an intramyocardial course may be compressed by the contracting myocardium during systole. Particularly during exercise or other high-output cardiac states, this compression may become flow limiting, resulting in myocardial ischemia and chest pain, syncope, or ventricular dysrhythmia. Compression during exercise may result in a “positive” stress test indicating regional ischemia in the distribution of the coronary artery. However, during conventional coronary angiography, the position of the vessel relative to the myocardium is not readily evident. Conventional coronary angiography is typically performed without pharmacologically induced stress, and the vessel may not appear compressed when the heart is beating at a resting rate and stroke volume. If the vessel lacks atherosclerosis, it may be called normal during conventional angiography, which can be thought of as “coronary artery lumenography” because it depicts only the patent interior of the vessel, not its wall or location relative to myocardium. Because coronary angiography is considered the diagnostic gold standard in most cases, the positive stress test in this scenario might be dismissed as a false positive.
Because CT scan depicts not only the vessel lumen but also the location of a vessel relative to the ventricular myocardium, it readily detects myocardial bridging and intramyocardial course of a coronary artery. These conditions may be surprisingly common—Cademartiri et al.32 found 59 cases (10.8%) of myocardial bridging in 543 consecutive patients undergoing 64-slice CT coronary angiography. De Rosa et al.31 found almost 19% of 242 consecutive patients admitted for chest pain and evaluated with CT coronary angiography had myocardial bridging. The contribution of these conditions to symptomatic cardiac ischemia is unknown, so routine use of CT to assess for these is probably not yet justified. Only 15% of coronary perfusion is thought to occur during systole, and compression of intramyocardial vessels is thought to occur only during systole, meaning that flow reductions from myocardial compression of coronary arteries should have little effect on cardiac oxygen delivery. A second possible mechanism accounting for ischemia and infarction might be injury to vessels because of compression, resulting in thrombus formation and coronary artery occlusion.31 When an intramyocardial course of a coronary artery is identified, the vessel can be surgically repositioned to an epicardial position to limit compression. CT might play a selective role in patients who remain symptomatic despite negative assessments for ischemia using other modalities.32
CT Perfusion Scanning
Data from CT coronary angiography can be reconstructed to produce maps of myocardial perfusion over time.15 Compared with measurements of regional blood flow performed during conventional coronary angiography, CT provides similar performance in small-animal and human pilot studies.33-35 Groves et al. reported a pilot study in which image data obtained routinely during contrast test bolus injection was used to construct myocardial perfusion maps.36 As described in more detail in Chapter 7, most modern CT scanners use automated tracking software to calculate the optimal timing of the initiation of the CT scan relative to the injection of contrast. This requires the administration of a small test bolus of contrast and the acquisition of a series of images to measure the opacification of the blood vessels of interest. Normally, these images are not used for diagnostic purposes but only to determine contrast timing, so the ability to use this data for evaluation of myocardial perfusion is an exciting prospect, because it would not require additional radiation or contrast exposure. CT measurements of myocardial perfusion can be performed either without induction of stress or with the use of pharmacological stress agents such as adenosine.37 More study is required before CT myocardial perfusion scanning becomes a routine practice.
Contraindications and Limitations
Contraindications to CT coronary angiography are listed in Table 8-4. A number of factors should be considered when selecting patients for CT coronary angiography.
TABLE 8-4 Contraindications and Limitations to CT Coronary Angiography
| Contraindication or Limitation | Reason |
|---|---|
| Irregular cardiac rhythm | Irregular rhythms prevent ECG gating, required for coronary artery visualization. Mildly irregular rhythms are not a contraindication. |
| Tachycardia with contraindications to beta-blockade | Tachycardia creates motion artifact, which limits coronary artery visualization. Beta-blockade in cases of unrecognized pulmonary embolism or pericardial effusion may lead to hemodynamic instability. |
| High respiratory rate | Tachypnea may create motion artifact during a scan, though this is less of an issue with 64- and 256-slice CT. It may create technical problems with adequacy of contrast bolus in patients undergoing triple rule-out studies.∗ |
| Metal in chest | Streak artifact may render image uninterpretable. |
| Obesity | A poor signal-to-noise ratio leads to poor image quality. |
| Poor vascular access | Rate of contrast injection is inadequate to provide good opacification of coronary arteries |
| Allergy to iodinated contrast | Iodinated contrast is needed for true CT coronary angiography, as well as CT aortography and pulmonary angiography—although coronary calcium scoring can be performed without contrast. |
| Renal insufficiency (calculated GFR < 60 mL/min for every 1.73 m2) | A high-contrast dose increases the risk of contrast-induced nephropathy. |
| Heavily calcified coronary arteries | Bloom artifact obscures the lumen. |
∗ From Takakuwa KM, Halpern EJ. Evaluation of a “triple rule-out” coronary CT angiography protocol: Use of 64-section CT in low-to-moderate risk emergency department patients suspected of having acute coronary syndrome. Radiology 248:438-446, 2008.
A regular cardiac rhythm is desirable for ECG gating of CT coronary angiography, described in detail earlier. Extremely irregular cardiac rhythms (including atrial fibrillation, atrial flutter with variable conduction, multifocal atrial tachycardia, or very frequent ectopic beats such as premature ventricular contractions) thwart ECG gating, although the use of fast slice (64 slices and higher) CT can mitigate motion artifacts. Mild irregularity of cardiac rhythm does not usually negate the ability to perform cardiac CT, and some investigators have performed CT coronary angiography in patients with atrial fibrillation, with moderate diagnostic success. Atrial fibrillation may result in uninterpretable coronary artery segments, meaning that coronary artery disease cannot be ruled out.38 ECG editing, a postprocessing technique that allows correction for image data acquired during irregular beats, is a technological solution for moderate rhythm irregularity. Generally, 256-slice CT is not subject to this problem, because the entire heart can be imaged in a single heart beat.
Significant tachycardia is a relative contraindication to CT coronary angiography, because the resulting images may be uninterpretable because of motion artifact.10 The use of 64-slice CT with ECG gating can significantly reduce motion artifact, even at relatively high heart rates. For example, adequate coronary artery assessment can be performed in patients with heart rates below 80 beats per minute.11,17-18 Patients with faster heart rates may require preprocedural rate control, typically achieved with oral beta-blockers (metoprolol at 50 to 100 mg PO) administered 45 to 60 minutes before CT.10 Esmolol may also be used for rapid rate control before CT, and it has the advantage of rapid onset and short duration of chronotropic action.39 Nitroglycerin (sublingual, 2 to 4 minutes before CT) is used in some protocols to dilate the coronary arteries before CT imaging, with some studies suggesting improved diagnostic accuracy.10,40 As 256-slice CT can image the entire heart during a single heart beat, concerns are eliminated about elevated heart rate or irregular rhythm.41
In patients with tachycardia and chest pain, special attention should be paid to the differential diagnosis before administration of beta-blockers to facilitate CT. Reduction in heart rate can substantially decrease cardiac output in patients who are unable to compensate by increases in cardiac stroke volume. Remember that cardiac output equals heart rate multiplied by stroke volume, so any decrease in heart rate results in a linear decrease in cardiac output when stroke volume is fixed. Conditions in which hemodynamic collapse could occur because of injudicious use of beta-blockers (or other rate-control agents) include unrecognized pulmonary embolism or large pericardial effusion with incipient pericardial tamponade. Such events may be rare but are likely underreported in the medical literature.
Patients with metallic thoracic implants experience significant metal streak artifact, which can render CT images uninterpretable. Examples including pacemaker wires and surgical clips and wires.10 Existing coronary artery stents usually can be evaluated for patency effectively with CT and do not cause prohibitive artifact. Very heavily calcified coronary arteries can cause “ bloom” artifact that makes evaluation of the vessel lumen difficult. Patients with morbid obesity (greater than 250 pounds) are difficult to evaluate with CT coronary angiography because of high noise in the CT image, although x-ray tube parameters can be adjusted to compensate.42
Contrast administration and the timing of image acquisition for cardiac CT must be synchronized so that contrast is present in the proximal aorta and coronary arteries during image acquisition. If contrast is administered too slowly or too late, the contrast bolus may have reached only the pulmonary arteries rather than the aorta and coronary arteries by the time of CT image acquisition. Ironically, faster scanners have exacerbated this problem because the scanner can outrun the contrast bolus. Radiologists have modified protocols in an attempt to give a small contrast bolus, perfectly timed with the rate of the CT scanner—a practice sometimes called “surfing the contrast bolus.” Issues of contrast timing are also critical to diagnosis of pulmonary embolism and aortic pathology and are discussed in detail in Chapter 7. Most CT coronary angiography protocols require a large-bore relatively proximal (e.g., antecubital fossa) IV catheter. A contrast injection rate of 3 to 5 mL per second is typical.10 A typical 20-gauge catheter (48 mm in length) allows a flow rate around 40 mL per minute—too low for a best-quality study, although flow rates with pressure injectors may be higher. Flow rates essentially double with each increase in gauge (e.g., 18 to 16 gauge) if catheter length is constant. Therefore a large-bore IV catheter (18 gauge or higher) usually allows adequate contrast infusion rate. Patients with poor IV access are poor candidates for CT coronary angiography because they are subject to poor contrast boluses resulting in nondiagnostic results.
Patients with allergy to iodinated contrast should not undergo coronary artery CT angiography since other methods of evaluation without use of iodinated contrast are available.
The quantity of IV contrast administered for CT coronary angiography is approximately 100 to 125 mL, including a 20-mL test bolus used to calibrate the timing of the CT, 50 to 70 mL of undiluted contrast for the principal CT coronary angiography examination, and 35 mL of contrast diluted with saline.10 This dose is significant and similar to that for conventional coronary angiography: 80 to 120 mL.43-44 Contrast doses in this range pose a risk for contrast-induced nephropathy in patients with renal insufficiency, with a 25% decrease in glomerular filtration rate (GFR) observed in around 14% of patients undergoing cardiac catheterization with similar contrast doses.44 Because CT coronary angiography has no therapeutic potential and is nonspecific relative to conventional coronary angiography, when CT reveals a lesion, a conventional coronary angiogram is generally performed to confirm stenoses and allow treatment. Thus patients with positive CT findings are subjected to a second dose of iodinated contrast, usually within a short period. Patients who have a high pretest probability of significant coronary artery disease should generally not undergo CT and instead undergo primary conventional coronary angiography to avoid this repeated contrast exposure.
The radiation exposure from CT coronary angiography can exceed the radiation dose from conventional coronary angiography (discussed in detail later),45 leading some to argue that CT should not be called “noninvasive”—although CT coronary angiography does not share the risks of bleeding, vascular injury, or embolic phenomena that can occur with conventional coronary angiography. The radiation exposure is of particular concern in young patients, an unfortunate fact because this population consists largely of low-risk patients who would otherwise be good candidates for CT risk stratification.
Structural and Functional Heart Evaluation
Another major role of cardiac CT is to assess the structure and function of the heart. Structural assessment such as measurement of atrial and ventricular size or the size of a pericardial effusion requires only static image data and thus can be performed from either retrospectively or prospectively ECG-gated studies. Functional assessment such as ejection fraction or mechanical effect of a pericardial effusion on cardiac filling requires data acquired throughout the cardiac contraction cycle because the motion of the ventricular walls and valves must be viewed over time. Consequently, such assessment requires retrospective ECG gating, with its higher radiation exposure (described earlier), rather than prospective ECG gating, which acquires images only during late diastole. A video of cardiac motion is created from ECG-gated images, allowing visual inspection of cardiac contraction, calculation of ejection fraction, recognition of regional wall motion abnormalities, and assessment of valvular motion. The information obtained is similar to that available using stress echocardiography, nuclear perfusion scanning, cardiac MRI, and ventriculography performed during cardiac catheterization. Rarely, cardiac CT can provide information that is unavailable from other common cardiac imaging modalities, including the gold standard, coronary angiography.
Computed Tomography Measurements of Left Ventricular Ejection Fraction
Automated software allows rapid and precise calculation of left ventricular ejection fraction (LVEF) from CT images.46 Measurements of LVEF from CT are highly reproducible, allowing comparison between new and prior CT studies in the same patient. They correlate moderately well with measurements made with invasive ventriculography and echocardiography, allowing comparison of LVEF over time between CT findings and echocardiographic and angiographic measurements.47-48
The three-dimensional capability of CT also makes it a powerful tool for characterizing pediatric congenital heart disease—although utility of CT is limited because of concern about radiation exposures in this young age group.49 This application of cardiac CT plays a minimal role in most emergency departments. Figures 8-10 through 8-12 show additional unsuspected cardiac and aortic structural abnormalities found on CT.
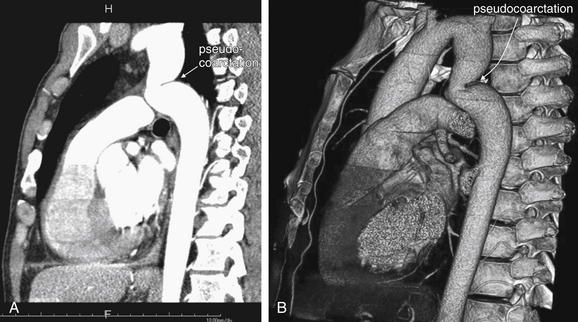
Figure 8-10 Cardiac CT may reveal unexpected findings—pseudocoarctation.
This 36-year-old male was found to have a bicuspid aortic valve and pseudocoarctation (arrow), seen on this sagittal reconstruction (A) and this gorgeous three-dimensional model (B).
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
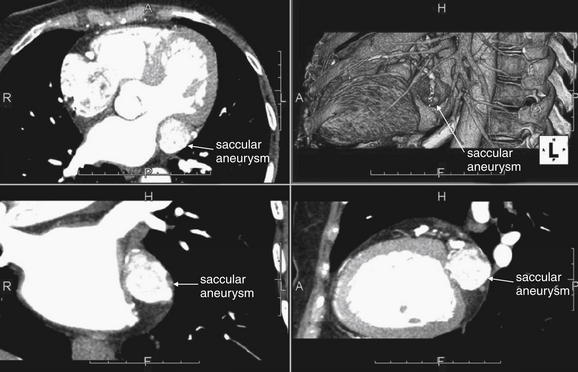
Figure 8-11 Cardiac CT may reveal an unexpected finding—a saccular aneurysm.
This 63-year-old female was found to have a large (2.5 cm) saccular aneurysm of the circumflex artery. The ability of CT to display multiplanar cross sections and three-dimensional models is particular helpful in this case. Counterclockwise from upper left are axial, coronal, sagittal, and three-dimensional views. The patient’s left and right coronal arteries also had aneurysmal sections, suggesting vasculitis.
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
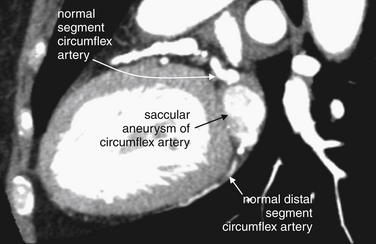
Figure 8-12 Closer view of coronary artery aneurysm.
Same patient as Figure 8-11. In this sagittal view, the circumflex artery becomes massively dilated before resuming a more typical size distally.
(From Broder J. Cardiac CT in the emergency department. Accessed at http://emedhome.com/.)
Should CT Be Used to Acquire Structural and Functional Cardiac Data When Such Information Is Available From Modalities That Do Not Require Ionizing Radiation?
Table 8-1 compares the structural and functional diagnostic information available from CT and other cardiac imaging modalities. Because it is used frequently for assessment of many conditions affecting the head, spine, chest, abdomen, and extremities, CT is widely available in most U.S. emergency departments at all hours, whereas echocardiography, MRI, and nuclear medicine studies are not. However, the expertise to interpret CT coronary angiographic images is more limited, with only a relatively small number of radiologists and cardiologists currently performing this interpretation. This limits the advantage of ubiquitous CT equipment. Much information offered by CT is similar to that from other imaging modalities. Valvular motion and ejection fraction can be obtained from CT but also from echocardiography and MRI, with no ionizing radiation exposure. Nuclear perfusion scanning provides ejection fraction but not valvular motion data and has a radiation exposure similar to that from CT, around 10 mSv.50 Echocardiography, MRI, and nuclear perfusion scanning do not require exposure to iodinated contrast, with its risk for inducing contrast nephropathy or allergic reaction. Iodinated contrast is generally required for CT, although calcium scores and pericardial effusions can be assessed without contrast. Cardiac MRI requires administration of gadolinium contrast, which has been linked to fatal nephrogenic systemic fibrosis. This condition is discussed in detail in Chapter 15. Nephrogenic systemic fibrosis occurs rarely following gadolinium administration and appears restricted to patients with severe renal disease (GFR <15 to 30 mL/min). Even in this high-risk population, it is a rare event, occurring in only 0.17% of patients receiving high doses of gadolinium—around 0.2 mmol/kg. The typical contrast dose for cardiac MRI is 0.05 to 0.2 mmol/kg, possibly posing some risk.51
CT for Assessment of Adjacent Thoracic Structures: The “Triple Rule Out”
CT techniques for evaluation of pulmonary embolism and aortic pathology are discussed in detail in Chapter 7. A potential benefit of CT is the ability to assess simultaneously for these life-threatening conditions and for significant coronary artery disease with a single immediately available test: the so-called triple rule-out CT scan. However, protocols for isolated CT coronary angiography and those for triple rule-out differ, with higher radiation exposures and injected contrast doses required for the additional assessment of structures surrounding the heart, as described here. Triple rule-out CT combines features of CT coronary angiography, CT pulmonary angiography, and CT aortography. CT pulmonary angiography requires that CT image acquisition coincide with the arrival of an injected contrast bolus in the pulmonary arteries. CT coronary angiography requires later image acquisition, after the contrast bolus has traversed the pulmonary arteries, returned to the left heart, and been ejected into the aorta and coronary arteries. CT aortography requires this same late timing but also demands that the CT scan follow the contrast bolus as it continues along the descending aorta because aortic dissections may extend into the abdomen, pelvis, and even extremities. A triple rule-out CT scan must satisfy all of these contrast timing requirements and expand the cephalad–caudad field of view beyond the heart to include much of the chest, as well as the abdomen and pelvis if visualization of the entire extent of aortic dissection is desired. The risk for additional iodinated contrast exposure (with the possibility of contrast nephropathy or allergy) and additional radiation exposure must be weighed against the probability of each of the three life-threatening disease processes in an individual patient.
Protocols for triple rule-out CT vary from institution to institution, but a common protocol is described here. An ECG-gated CT scan is performed as described earlier. ECG gating allows coronary artery assessment and minimizes motion artifact at the aortic root, which can simulate the appearance of aortic dissection. To minimize radiation exposure, prospective gating is used in patients with heart rates slower than 65 bpm. Patients with faster heart rates or moderately irregular rhythms may be scanned with retrospective gating because this allows the optimal moment of the cardiac cycle for image analysis to be determined after image acquisition. IV metoprolol is administered when necessary to achieve a heart rate of 50 to 60 bpm, unless systolic blood pressure is less than 100 mm Hg. Sublingual nitroglycerin may be given 2 minutes before CT to dilate coronary arteries and improve their visualization, unless systolic blood pressure is below 100 mm Hg. The cephalad–caudad extent of the CT scan varies. Some protocols include the entire chest, whereas others include only the region from the lower clavicles to the lower heart border, as measured on the CT scout image. A safety margin of 2 cm around this region may be added to account for cardiac, respiratory, and patient motion. The scanned region typically is 17 to 24 cm and excludes the lung apices. The duration of the scan is typically from 11 to 16 seconds with a 64-slice scanner. To allow visualization of pulmonary arteries, coronary arteries, and the aorta as described earlier, a biphasic contrast injection is performed, with an initial bolus of 70 mL of undiluted contrast, followed a 50:50 mixture of 25 mL of contrast and 25 mL of saline. Effective radiation doses range from 8.7 mSv for prospectively gated studies to 18 mSv for retrospectively gated studies.52
Risk Stratification: Is Triple Rule-Out CT Warranted in All Chest Pain Patients?
While the security of evaluating simultaneously for three life threats with a single test may be tantalizing, the risks of contrast and radiation exposure should be weighed against the pretest probability of disease in each patient. Validated clinical risk stratification systems for pulmonary embolism exist (discussed in Chapter 7), meaning that many patients with chest pain do not require imaging to rule out this disease process. Clinical assessment may exclude cardiac ischemia as a likely cause of symptoms in other patients. Aortic dissection can be a challenging diagnosis (see Chapter 7) but is relatively rare, meaning that ubiquitous aortic imaging in all chest pain patients would needlessly expose many low-risk patients to radiation and contrast.
Do real-world studies bear out this risk stratification concept, or do they suggest that triple rule-out CT should be broadly applied in emergency department chest pain patients? Takakuwa et al.52 prospectively enrolled 201 consecutive emergency department patients characterized as low to moderate risk for acute coronary syndrome based on TIMI score (see Box 8-1). Of these patients, 197 (mean age = 49 years) underwent a triple rule-out protocol CT. Takakuwa et al. found that 10 patients (5%) had severe coronary artery disease (>70% stenosis), 12 (6%) had moderate disease (50% to 70% stenosis), 46 (23%) had mild disease (<50% stenosis), and 129 (65%) had no coronary artery disease.
CT identified 22 patients (11%) with disease processes other than coronary artery disease that explained the patients’ symptoms. An additional 27 patients (14%) had clinically important disease processes that appeared unrelated to the clinical symptoms and were presumably incidental but important findings. In 133 patients (76%) with no more than mild coronary artery disease, no additional testing was performed following CT, which the authors attribute to the diagnostic information provided by CT. No adverse outcomes occurred at 30-day follow-up in this group, leading the authors to suggest that this strategy is a safe and effective means of evaluating multiple important forms of chest pathology.52 Promising though this outcome may be, the primary goal of the triple rule-out CT is to detect aortic dissection and pulmonary embolism, which occurred in only 1 patient (0.5%) and 3 patients (1.5%), respectively. Because the emergency physicians were not asked to stratify their risk assessment of these disease processes before CT, it is unclear whether these 4 patients would have been recognized as being at risk for important noncardiac diseases and evaluated appropriately had they not undergone triple rule-out CT for study purposes. It is possible that triple rule-out CT prevented misdiagnosis but also possible that these patients would have been correctly diagnosed based on other clinical factors. The authors suggest that the yield of other diagnoses justified the use of CT in this group, but the importance of the other non–coronary artery disease findings in this study is uncertain. The remaining non–coronary artery disease diagnoses included such questionably important findings as esophagitis, hiatal hernia, and chronic obstructive pulmonary disease.52
Several other studies suggest a low rate of pulmonary embolism and aortic dissection in patients undergoing chest CT for coronary artery disease evaluation.53-56 Onuma et al.53 found only one aortic dissection and no pulmonary emboli in 503 older adults (mean age = 65 years) undergoing chest CT for suspected coronary artery disease—although the protocol was not specifically tailored to detect all three disease processes. Gil et al.54 found extracardiac pathology in 56.2% of asymptomatic patients undergoing 16-slice multidetector computed tomography of the coronary arteries—including lung nodules, lung bullae or emphysema, lung masses, pericardial and liver disease, bone abnormalities, and adrenal masses. No aortic dissections or pulmonary emboli were detected, perhaps because of a CT protocol not optimized to evaluate these. The study population was also asymptomatic and thus differs substantially from symptomatic emergency department patients. White et al.56 found one pericardial effusion, one pneumonia, one pulmonary embolism, and no aortic dissections in 69 emergency department chest pain patients undergoing triple rule-out CT. Before CT, the emergency physicians caring for the patients completed a survey indicating their planned diagnostic workup, and 45 of 69 patients (65%) would not have undergone chest CT for evaluation of noncardiac etiologies of chest pain. However, it is not clear from the study manuscript whether the pulmonary embolism and other abnormalities were detected in this group or in the 35% of subjects in whom a CT was planned for evaluation of a suspected noncardiac cause.
From these studies, the diagnostic yield of triple rule-out CT for the critical conditions of pulmonary embolism and aortic dissection appears low. Whether the yield justifies broad exposure of a large patient population to a relatively high radiation dose remains in debate. Older patients may suffer little risk from the added radiation exposure, because of lower radiation sensitivity and fewer years of life remaining to “incubate” an incipient cancer. Selected patients with a high pretest probability of all three disease processes may benefit from this rapid and comprehensive test. 57
Cost- and Time-Effectiveness of Cardiac Computed Tomography
Controversy exists about the cost-effectiveness of CT coronary angiography relative to other emergency department diagnostic strategies for significant coronary artery disease. Cost-effectiveness analyses are complicated by the range of variables to be considered, including the costs of hospitalization and multiple diagnostic tests, opportunity costs of patients leaving without being seen from the emergency department when emergency department and hospital crowding occurs, and lost work productivity from patients hospitalized for diagnostic evaluation.
Chang et al.25 retrospectively compared the actual financial costs of four evaluation strategies for potential cardiac ischemia in low-risk patients (TIMI score = 0 to 2; see Box 8-1): (1) immediate emergency department CT coronary angiography without serial cardiac markers, (2) CT coronary angiography after emergency department observation and serial biomarkers, (3) stress testing after observation with serial biomarkers, and (4) hospital admission with serial biomarkers and additional diagnostic workup at the discretion of the admitting physician. This study is described in detail in the earlier section on clinical outcomes after a negative CT coronary angiogram. The analysis favored the immediate CT coronary angiography strategy in cost and length of stay, with similar 30-day clinical outcomes (see Table 8-3). Although this study suggests that immediate CT coronary angiography is faster and cheaper than other strategies yet equally safe, because this study was not a randomized controlled trial, it is possible that the patient groups differed in important ways at baseline. The patients who were selected by emergency physicians for immediate CT coronary angiography and discharge may have been at extremely low risk and possibly could have been discharged safely without any testing for coronary artery disease—undoubtedly the least expensive option. The study showed a substantial time savings for evaluating patients using an immediate CT coronary angiography approach without observation (see Table 8-3)—although again, an even more time-efficient approach would be discharge without CT coronary angiography or observation in very-low-risk patients.
The wide availability and speed of CT coronary angiography could make it the path of least resistance for disposition, with ever-lower-risk patients being evaluated using this modality. False-positive CT coronary angiography results in low-risk patients might increase costs by provoking expensive cardiac catheterizations and admission in patients who in the past have not undergone extensive cardiac evaluation. Mowatt et al.58 performed a meta-analysis of 64-slice CT comparing cost analyses. The cost benefit of CT varied, depending on the prevalence of coronary artery disease in the population. In populations with very low rates of coronary artery disease, false-positive findings on CT could raise costs by requiring follow-up studies. In very-high-risk patient populations, where most patients have abnormal findings on CT coronary angiography requiring follow-up conventional angiography, CT likely should be avoided and conventional coronary angiography should be performed as the first diagnostic test. CT coronary angiography may therefore be most cost-effective in patients with a low to moderate risk for coronary artery disease.
Radiation Exposure and Cancer Risks of Cardiac Computed Tomography
Radiation exposures from cardiac CT protocols vary widely, depending on a number of technical factors described earlier in the chapter. Prospective ECG gating decreases radiation exposure compared with retrospective ECG gating. CT protocols for simultaneous evaluation of the coronary arteries, aorta, and pulmonary arteries require a longer duration of radiation exposure and a wider field of view, increasing radiation doses. Modulation of x-ray tube current and voltage can be performed to reduce radiation exposure, though with some decrease in signal-to-noise ratio and consequent decrease in image quality. The radiation dose must be weighed carefully against the need for clinical information. Radiologists have made important concessions in image quality, aiming for the lowest radiation dose that can provide interpretable images. Radiation doses for common cardiac CT protocols are listed in Table 8-5.
TABLE 8-5 Radiation Doses From Computed Tomography Coronary Angiography Protocols
| Study | Radiation Exposure |
|---|---|
| Low-dose ECG-gated 64-slice helical CT8,11,61 | 3.40-4.95 mSv |
| 40- to 64-slice ECG-gated CT68 | 17-19 mSv |
| 64-slice CT coronary angiography performed during preoperative CT of the chest for other lesions, such as lung cancers17 | 12.06 ± 3.25 mSv for CT coronary angiography, 13.88 ± 3.49 mSV for complete chest CT |
Measures of Radiation Dose
The English measures radiation absorbed dose (rad) and Roentgen equivalent in man (rem) have been replaced by the International System units gray and sievert. A gray is defined as the absorption of 1 joule of ionizing radiation energy by 1 kilogram of matter. A sievert expresses the absorbed energy (gray), modified by a coefficient to account for the varying biological susceptibilities of different body tissues, giving an effective biologically important radiation dose or dose equivalent. Because the coefficients used to convert from gray to sievert are dimensionless (have no units), both gray and sievert measurements are expressed as joules per kilogram. For emergency physicians, there is little need to think in terms of the old English measures—this is the equivalent of discussing distance in leagues or weight in stone. For that matter, the precise physical meaning of sievert is relatively unimportant; instead, it is essential to understand this measurement in the context of common radiation exposures and patient risk.
The sievert (abbreviated Sv) is a large unit of radiation dose—acute exposures in this range would lead to radiation poisoning, with 3 Sv causing death in 50% of cases in humans within 30 days. Most medical diagnostic imaging involves exposures in the range of fractions of a millisievert (mSv) and up to several millisieverts for some CT examinations. To put these doses into perspective, the average annual background radiation on Earth is around 2.5 mSv. A chest x-ray exposes a patient to about 0.02 mSv. Radiation sickness does not occur below acute doses of 1 to 2 Sv (1000 to 2000 mSv). Survivors of atomic bomb exposures at Hiroshima were noted to experience radiation doses of around 50 mSv, and data from studies of these survivors form the basis of modern models of cancer risk from CT.59-60
Radiation exposures from CT coronary angiography commonly range from approximately 8 to 20 mSv (see Table 8-5), though lower-dose protocols have also been published. d’Agostino et al.11 studied 105 consecutive patients, using a protocol for low-dose ECG-gated 64-slice CT of the entire thorax with retrospective ECG-gated coronary angiography. The average effective dose was only 4.95 mSv, and image quality was judged to be diagnostic by two radiologists in 89.5% of patients. Klass et al.8,61 reported excellent image quality with low-dose 64- and 256-slice CT protocols, with an average dose of 3.4 to 3.7 mSv.
Extrapolating From Radiation Dose to Cancer Risk
Because CT scan has been in clinical use only since the 1970s, with explosive increases in utilization over the past 20 years, our understanding of the effect of CT-related radiation on cancer induction and cancer deaths relies largely on comparisons with similar levels of radiation exposure in very different contexts—Hiroshima survivors and workers in the U.S. nuclear power and arms industries, for example. All studies of cancer risk are limited by the relatively recent introduction of CT—currently, there are no published prospective human cohort studies on the long-term cancer risk associated with diagnostic CT.
Einstein et al.62 estimated attributable cancer risk from 64-slice CT coronary angiography using a computer model incorporating variables such as patient age and gender. Lifetime cancer induction risk (not risk for cancer death) was estimated at 1 in 143 (0.7%) for a 20-year-old female and 1 in 3261 (0.03%) for an 80-year-old man. These estimates must be considered in the context of the actual rate of cancer and cancer death in the population, occurring from all causes. Centers for Disease Control data shows that 22% of deaths in the United States in 2005 were attributable to cancer.63 Nonetheless, on a population basis, increasing use of CT in young patients likely contributes to an increase in cancer rates, an earlier onset of cancer, and increased morbidity and economic costs even when death does not occur.
The choice of diagnostic approach must balance the patient’s age at exposure, the need for rapid diagnosis, and the range of clinical conditions under consideration. In some cases, though CT may rapidly provide all necessary data, alternative imaging may provide similar data with a lower total exposure to ionizing radiation. If myocardial ischemia is the sole or primary concern, stress echocardiography or cardiac MRI can be performed with no radiation exposure. If pulmonary embolism is seriously considered, ventilation–perfusion scan may offer some radiation savings compared with chest CT and can be followed by other imaging for cardiac ischemia without radiation exposure. Aortic pathology can be evaluated by a tailored CT (rather than triple rule-out CT), or with MRI or transesophageal echocardiography in some cases, when radiation exposure is of significant concern. In a limited subset of patients in whom myocardial ischemia, pulmonary embolism, and aortic pathology are all serious concerns, triple rule-out CT may be the most radiation- and time-efficient test. For example, the total radiation exposure from a ventilation–perfusion scan for pulmonary embolism, a CT aortogram, and a cardiac nuclear perfusion imaging study exceeds that for triple rule-out CT protocols.
Summary
Cardiac CT encompasses a range of protocols that can rapidly and accurately evaluate coronary artery stenosis, ventricular and valvular function, anomalous coronary arteries, pericardial disease, pulmonary embolism, and aortic dissection. Because additional information comes at the price of increased radiation and contrast exposure, the emergency physician should consider the differential diagnosis carefully to determine whether CT coronary angiography, triple rule-out CT, or other diagnostic tests represent the best balance of diagnostic accuracy, speed, cost, and risk for adverse effects of testing.
1. McCaig L.F., Nawar E.W. National Hospital Ambulatory Medical Care Survey: 2004 emergency department summary. Adv Data. 2006:1-29.
2. Karki D.B., Neopane A., Regmi S., Acharya S. 64-slice CT scan in Kathmandu Medical College Teaching Hospital. Kathmandu Univ Med J (KUMJ). 2008;6:257-261.
3. Guthaner D.F., Wexler L., Harell G. CT demonstration of cardiac structures. AJR Am J Roentgenol. 1979;133:75-81.
4. Zindrou D., Taylor K.M., Bagger J.P. Coronary artery size and disease in UK South Asian and Caucasian men. Eur J Cardiothorac Surg. 2006;29:492-495.
5. RT Image. CT data sheet index: 2007. (Accessed at http://www.rt-image.com/datasheet/index.cfm?chartID=63&action=1.)
6. Sun Z., Jiang W. Diagnostic value of multislice computed tomography angiography in coronary artery disease: A meta-analysis. Eur J Radiol. 2006;60:279-286.
7. Vanhoenacker P.K., Heijenbrok-Kal M.H., Van Heste R., et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007;244:419-428.
8. Klass O., Walker M., Siebach A., et al. Prospectively gated axial CT coronary angiography: Comparison of image quality and effective radiation dose between 64- and 256-slice CT. Eur Radiol. 2010;20:1124-1131.
9. Mayurasakorn S., Siriapisith T., Wasinrat J. Influence of heart rate on image quality to identify the best cardiac phase in 16-slice coronary CT angiography. J Med Assoc Thai. 2008;91:1076-1081.
10. Shuman W.P., Branch K.R., May J.M., et al. Prospective versus retrospective ECG gating for 64-detector CT of the coronary arteries: Comparison of image quality and patient radiation dose. Radiology. 2008;248:431-437.
11. d’Agostino AG, Remy-Jardin M, Khalil C, et al. Low-dose ECG-gated 64-slices helical CT angiography of the chest: Evaluation of image quality in 105 patients. Eur Radiol. 2006;16:2137-2146.
12. Greenland P., Bonow R.O., Brundage B.H., et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378-402.
13. Greenland P., Bonow R.O., Brundage B.H., et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). Circulation. 2007;115:402-426.
14. Ho J.S., Fitzgerald S.J., Stolfus L.L., et al. Relation of a coronary artery calcium score higher than 400 to coronary stenoses detected using multidetector computed tomography and to traditional cardiovascular risk factors. Am J Cardiol. 2008;101:1444-1447.
15. Cury R.C., Nieman K., Shapiro M.D., et al. Comprehensive cardiac CT study: Evaluation of coronary arteries, left ventricular function, and myocardial perfusion—Is it possible? J Nucl Cardiol. 2007;14:229-243.
16. Iwasaki K., Matsumoto T., Aono H., et al. Prevalence of subclinical atherosclerosis in asymptomatic diabetic patients by 64-slice computed tomography. Coron Artery Dis. 2008;19:195-201.
17. Delhaye D., Remy-Jardin M., Rozel C., et al. Coronary artery imaging during preoperative CT staging: Preliminary experience with 64-slice multidetector CT in 99 consecutive patients. Eur Radiol. 2007;17:591-602.
18. Delhaye D., Remy-Jardin M., Salem R., et al. Coronary imaging quality in routine ECG-gated multidetector CT examinations of the entire thorax: Preliminary experience with a 64-slice CT system in 133 patients. Eur Radiol. 2007;17:902-910.
19. Stein P.D., Yaekoub A.Y., Matta F., Sostman H.D. 64-slice CT for diagnosis of coronary artery disease: a systematic review. Am J Med. 2008;121:715-725.
20. White C.W., Wright C.B., Doty D.B., et al. Does visual interpretation of the coronary arteriogram predict the physiologic importance of a coronary stenosis? N Engl J Med. 1984;310:819-824.
21. Wong J.T., Ducote J.L., Xu T., et al. Automated technique for angiographic determination of coronary blood flow and lumen volume. Acad Radiol. 2006;13:186-194.
22. Sato A., Hiroe M., Tamura M., et al. Quantitative measures of coronary stenosis severity by 64-slice CT angiography and relation to physiologic significance of perfusion in nonobese patients: Comparison with stress myocardial perfusion imaging. J Nucl Med. 2008;49:564-572.
23. Hollander J.E., Litt H.I., Chase M., et al. Computed tomography coronary angiography for rapid disposition of low-risk emergency department patients with chest pain syndromes. Acad Emerg Med. 2007;14:112-116.
24. Gaemperli O., Valenta I., Schepis T., et al. Coronary 64-slice CT angiography predicts outcome in patients with known or suspected coronary artery disease. Eur Radiol. 2008;18:1162-1173.
25. Chang A.M., Shofer F.S., Weiner M.G., et al. Actual financial comparison of four strategies to evaluate patients with potential acute coronary syndromes. Acad Emerg Med. 2008;15:649-655.
26. Hollander J.E., Chang A.M., Shofer F.S., et al. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med. 2009;53:295-304.
27. Hollander J.E., Chang A.M., Shofer F.S., et al. One-year outcomes following coronary computerized tomographic angiography for evaluation of emergency department patients with potential acute coronary syndrome. Acad Emerg Med. 2009;16:693-698.
28. Srinivasan K.G., Gaikwad A., Kannan B.R., et al. Congenital coronary artery anomalies: Diagnosis with 64 slice multidetector row computed tomography coronary angiography—A single-centre study. J Med Imaging Radiat Oncol. 2008;52:148-154.
29. Singh Nijjar P., Parameswaran A., Amanullah A.M. Evaluation of anomalous aortic origins of the coronaries by 64-slice cardiac computed tomography. Rev Cardiovasc Med. 2007;8:175-181.
30. Shi H., Aschoff A.J., Brambs H.J., Hoffmann M.H. Multislice CT imaging of anomalous coronary arteries. Eur Radiol. 2004;14:2172-2181.
31. De Rosa R., Sacco M., Tedeschi C., et al. Prevalence of coronary artery intramyocardial course in a large population of clinical patients detected by multislice computed tomography coronary angiography. Acta Radiol. 2008;49:895-901.
32. Cademartiri F., La Grutta L., Malago R., et al. Prevalence of anatomical variants and coronary anomalies in 543 consecutive patients studied with 64-slice CT coronary angiography. Eur Radiol. 2008;18:781-791.
33. Bamberg F., Klotz E., Flohr T., et al. Dynamic myocardial stress perfusion imaging using fast dual-source CT with alternating table positions: Initial experience. Eur Radiol. 2010;20:1168-1173.
34. de Roos A. Myocardial perfusion imaging with multidetector CT: Beyond lumenography. Radiology. 2010;254:321-323.
35. George R.T., Ichihara T., Lima J.A., Lardo A.C. A method for reconstructing the arterial input function during helical CT: Implications for myocardial perfusion distribution imaging. Radiology. 2010;255:396-404.
36. Groves A.M., Goh V., Rajasekharan S., et al. CT coronary angiography: Quantitative assessment of myocardial perfusion using test bolus data—Initial experience. Eur Radiol. 2008;18:2155-2163.
37. Okada D.R., Ghoshhajra B.B., Blankstein R., et al. Direct comparison of rest and adenosine stress myocardial perfusion CT with rest and stress SPECT. J Nucl Cardiol. 2010;17:27-37.
38. Oncel D., Oncel G., Tastan A. Effectiveness of dual-source CT coronary angiography for the evaluation of coronary artery disease in patients with atrial fibrillation: Initial experience. Radiology. 2007;245:703-711.
39. Degertekin M., Gemici G., Kaya Z., et al. Safety and efficacy of patient preparation with intravenous esmolol before 64-slice computed tomography coronary angiography. Coron Artery Dis. 2008;19:33-36.
40. Chun E.J., Lee W., Choi Y.H., et al. Effects of nitroglycerin on the diagnostic accuracy of electrocardiogram-gated coronary computed tomography angiography. J Comput Assist Tomogr. 2008;32:86-92.
41. Dewey M., Hamm B. [CT coronary angiography: Examination technique, clinical results, and outlook on future developments]. Rofo. 2007;179:246-260.
42. Herzog B.A., Husmann L., Valenta I., et al. Determinants of vessel contrast in BMI-adapted low dose CT coronary angiography with prospective ECG-triggering. Int J Cardiovasc Imaging. 2009;25:625-630.
43. Nakaura T., Awai K., Yauaga Y., et al. Contrast injection protocols for coronary computed tomography angiography using a 64-detector scanner: Comparison between patient weight-adjusted- and fixed iodine-dose protocols. Invest Radiol. 2008;43:512-519.
44. Brar S.S., Shen A.Y., Jorgensen M.B., et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: A randomized trial. JAMA. 2008;300:1038-1046.
45. Smith I.R., Rivers J.T. Measures of radiation exposure in cardiac imaging and the impact of case complexity. Heart Lung Circ. 2008;17:224-231.
46. Joemai R.M., Geleijns J., Veldkamp W.J., Kroft L.J. Clinical evaluation of 64-slice CT assessment of global left ventricular function using automated cardiac phase selection. Circ J. 2008;72:641-646.
47. Spoeck A., Bonatti J., Friedrich G.J., et al. Evaluation of left ventricular function by 64-multidetector computed tomography in patients undergoing totally endoscopic coronary artery bypass grafting. Heart Surg Forum. 2008;11:E218-E224.
48. Krishnam M.S., Tomasian A., Iv M., et al. Left ventricular ejection fraction using 64-slice CT coronary angiography and new evaluation software: Initial experience. Br J Radiol. 2008;81:450-455.
49. Herzog C., Mulvihill D.M., Nguyen S.A., et al. Pediatric cardiovascular CT angiography: Radiation dose reduction using automatic anatomic tube current modulation. AJR Am J Roentgenol. 2008;190:1232-1240.
50. Bedetti G., Pizzi C., Gavaruzzi G., et al. Suboptimal awareness of radiologic dose among patients undergoing cardiac stress scintigraphy. J Am Coll Radiol. 2008;5:126-131.
51. Prince M.R., Zhang H., Morris M., et al. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology. 2008;248:807-816.
52. Takakuwa K.M., Halpern E.J. Evaluation of a “triple rule-out” coronary CT angiography protocol: Use of 64-section CT in low-to-moderate risk emergency department patients suspected of having acute coronary syndrome. Radiology. 2008;248:438-446.
53. Onuma Y., Tanabe K., Nakazawa G., et al. Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol. 2006;48:402-406.
54. Gil B.N., Ran K., Tamar G., et al. Prevalence of significant noncardiac findings on coronary multidetector computed tomography angiography in asymptomatic patients. J Comput Assist Tomogr. 2007;31:1-4.
55. Savino G., Herzog C., Costello P., Schoepf U.J. 64 slice cardiovascular CT in the emergency department: Concepts and first experiences. Radiol Med (Torino). 2006;111:481-496.
56. White C.S., Kuo D., Kelemen M., et al. Chest pain evaluation in the emergency department: Can MDCT provide a comprehensive evaluation? AJR Am J Roentgenol. 2005;185:533-540.
57. Lee H.Y., Yoo S.M., White C.S. Coronary CT angiography in emergency department patients with acute chest pain: triple rule-out protocol versus dedicated coronary CT angiography. Int J Cardiovasc Imaging. 2009;25:319-326.
58. Mowatt G., Cummins E., Waugh N., et al. Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess. 2008;12:1-71.
59. Brenner D.J., Hall E.J. Computed tomography: An increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284.
60. Hall E.J., Brenner D.J. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362-378.
61. Klass O., Jeltsch M., Feuerlein S., et al. Prospectively gated axial CT coronary angiography: Preliminary experiences with a novel low-dose technique. Eur Radiol. 2009;19:829-836.
62. Einstein A.J., Henzlova M.J., Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317-323.
63. Kung H.-C., Hoyert D.L., Xu J., Murphy S.L. Deaths: final data for 2005. National Vital Statistics Reports. 2008;56(10):1-121.
64. Nandalur K.R., Dwamena B.A., Choudhri A.F., et al. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: A meta-analysis. J Am Coll Cardiol. 2007;50:1343-1353.
65. Metz L.D., Beattie M., Hom R., et al. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: A meta-analysis. J Am Coll Cardiol. 2007;49:227-237.
66. Fallahi B., Beiki D., Gholamrezanezhad A., et al. Single Tc99m Sestamibi injection, double acquisition gated SPECT after stress and during low-dose dobutamine infusion: A new suggested protocol for evaluation of myocardial perfusion. Int J Cardiovasc Imaging. 2008;24:825-835.
67. Kertai M.D., Boersma E., Bax J.J., et al. A meta-analysis comparing the prognostic accuracy of six diagnostic tests for predicting perioperative cardiac risk in patients undergoing major vascular surgery. Heart. 2003;89:1327-1334.
68. Coche E. Acute chest pain in emergency room: Preliminary findings with 40-64-slice CT ECG-gated of the whole chest. JBR-BTR. 2007;90:89-91.