Chapter 12 Imaging the Genitourinary Tract
Emergency conditions of the genitourinary tract include the life threatening, such as ruptured ectopic pregnancy and renal avulsion, and threats to organ function, such as testicular and ovarian torsion. The diversity of potential clinical scenarios and differential diagnoses means that our discussion is best presented in the context of the chief complaint or specific pathologic entities, rather than in a single algorithm to evaluate the entire genitourinary tract. We begin with nontraumatic urinary tract conditions. Next, we address conditions of the male and female reproductive organs, including conditions in the pregnant patient. Finally, we discuss imaging of genitourinary trauma.
Clinical Presentations and Differential Diagnosis
A variety of chief complaints may suggest genitourinary pathology. In some cases, no imaging is required. In other cases (such as back pain with suspected aortic aneurysm or renal colic), the nongenitourinary differential diagnosis is most important and drives imaging decisions. In still others, a targeted genitourinary differential requires emergency imaging. Table 12-1 outlines chief complaints, selected differential diagnoses, and appropriate imaging modalities.
TABLE 12-1 Chief Complaints, Differential Diagnosis, and Imaging Modalities for Genitourinary Pathology
| Chief Complaint | Differential Diagnosis | Imaging Modalities |
|---|---|---|
| Nontraumatic Urinary Complaints | ||
| Flank pain with or without abdominal pain | ||
| Painless hematuria | ||
| Dysuria | ||
| Urinary retention | ||
| Abdominal pain | ||
| Fever | ||
| Perineal pain | ||
| Male Genitourinary | ||
| Testicular or scrotal pain | ||
| Testicular or scrotal mass | ||
| Urethral discharge | ||
| Prostatic pain or suspected prostatic hypertrophy | ||
| Female Genitourinary | ||
| Pregnant Patient | ||
| Vaginal bleeding | ||
| Abdominal or pelvic pain | ||
| Fluid leak or vaginal discharge | ||
| Recently Pregnant Patient | ||
| Abdominal pain, vaginal bleeding or discharge, or fever | ||
| Nonpregnant Patient | ||
| Vaginal bleeding | ||
| Abdominal or pelvic pain | ||
| Vaginal discharge | ||
| Trauma | ||
| Blunt abdominal or torso trauma | ||
| Traumatic hematuria (gross)∗ | ||
| Traumatic vaginal bleeding | ||
| Direct genitourinary trauma | ||
| Abdominal or pelvic trauma in the pregnant patient |
• Ultrasound is insensitive for placental abruption and cannot rule out the diagnosis; fetal heart monitoring is essential
|
|
| Penetrating abdominal or torso trauma | ||
∗ Microscopic hematuria generally does not require imaging in adults but does require it in pediatric patients.
Imaging Nontraumatic Genitourinary Complaints
Abdominal or Flank Pain With or Without Hematuria
Abdominal and flank pain with or without hematuria can suggest renal colic or pyelonephritis, although an array of other conditions can present with similar symptoms. Conditions such as abdominal aortic aneurysm (AAA), renal cell carcinoma, renal infarction, and perinephric abscess can cause flank and abdominal pain with hematuria. A variety of imaging options are available for evaluation of these signs and symptoms. The choice of imaging study should reflect the differential diagnosis under consideration, the clinical certainty of the diagnosis, radiation exposure concerns, contrast nephrotoxicity or allergy, time, and cost. In some cases, particularly in patients with recurrent urolithiasis, the diagnosis is virtually certain and no imaging may be required. Moreover, in patients with recent prior imaging, other diagnostic concerns such as AAA can often be ruled out based on measurements made from existing images. Later, we discuss the imaging options with strengths and weaknesses of each. Noncontrast computed tomography (CT), CT with intravenous (IV) contrast, CT with IV and oral contrast, IV urography (IVU), x-ray, renal ultrasound, and rare tests such as Lasix renal scan all have roles in the evaluation of potential renal colic.
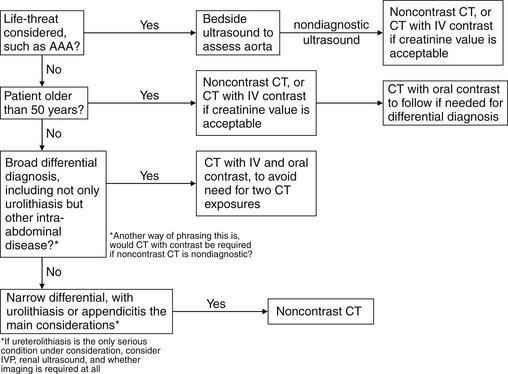
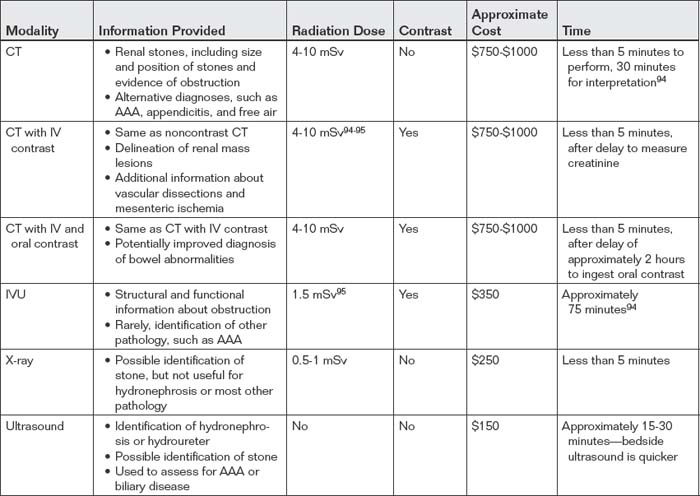
To select the best imaging test, the emergency physician should generate a differential diagnosis that is comprehensive yet tailored to the patient. If the differential diagnosis seriously includes entities other than renal colic, CT is likely the best test. If vascular abnormalities are considered, CT with IV contrast is useful, assuming the patient is stable and has an acceptable creatinine (because of concerns about nephrotoxicity of IV contrast). When the differential diagnosis is particularly broad, including renal, reproductive, vascular, bowel, and other abdominal pathology, CT with IV and oral contrast provides the most information. The decision to perform immediate noncontrast CT, CT with IV contrast, or oral and IV–contrasted CT should be based on the most dangerous pathology suspected. In young patients, in whom vascular disasters such as AAA are rare, radiation concerns may outweigh the impetus for rapid imaging. In these patients, the delay for oral contrast may be acceptable to avoid repeated radiation exposure if noncontrast CT were negative. In older patients with concern for vascular catastrophes, immediate CT without any contrast may be wise, with enhanced CT performed later if more information is needed. The danger of repeated radiation exposure in patients over the age of 50 pales in comparison with the risk for delayed diagnosis of AAA rupture. Vascular catastrophes are discussed in more detail in Chapter 11. If urolithiasis is the only suspected diagnosis, IVU can be performed. In pregnant patients, renal ultrasound can be used to assess for obstruction complicating urolithiasis, avoiding radiation exposure. This strategy may also be useful in patients of either gender with recurrent episodes of renal colic to avoid high cumulative radiation exposures from CT. Figure 12-1 shows an algorithm for CT imaging in acute flank and abdominal pain. Table 12-2 lists the information provided by the various imaging modalities, along with cost, time, and radiation information. We begin with a discussion of CT scan, followed by descriptions of other imaging modalities.
Imaging Options for Suspected Renal Colic
Computed Tomography Scan
CT scan (Figures 12-2 to 12-15) is sensitive for many types of spontaneous disease of the kidneys, ureters, and bladder, including renal tumors and urolithiasis. It is less helpful in delineating disease such as transitional cell carcinoma within the ureters or bladder. For suspected renal colic, no IV or oral contrast is needed. A calcified stone within the kidney, ureters, or bladder usually is evident as a high-density (white) lesion on abdominal windows (see Figures 12-2 to 12-6). These lesions are easily seen without contrast because few other white structures should be present in the vicinity of the kidneys, ureters, or bladder. Calcified phleboliths in the pelvis may occasionally be confused with intraureteral stones.
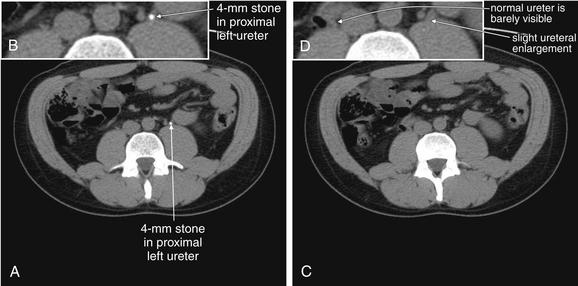
Figure 12-2 Urinary stones: Noncontrast CT on soft-tissue windows.
Noncontrast CT has become the standard imaging modality for diagnosis of urinary tract stones and related complications. The study is useful because it can identify the size and location of stones, as well as complications such as hydronephrosis, hydroureter, or rupture of the renal collecting system as a consequence of obstruction. The size and location of stones have some prognostic power and can aid in planning of procedures to remove stones or relieve obstruction. Perhaps most importantly, noncontrast CT may reveal important alternative diagnoses such as aortic pathology and appendicitis. Noncontrast CT has the advantage of being available for rapid diagnosis, because it requires no preprocedural measurement of renal function and no preparation time for ingestion of oral contrast. In some institutions, the examination is performed with the patient in the prone position to allow bowel and other peritoneal organs to fall away from retroperitoneal urinary tract structures. In other institutions, the patient is scanned in a supine position. Thin slices (3 mm) are often obtained to allow detection of small stones. Because the abdomen does not usually contain calcified structures, calcified stones are readily visible against the background of soft tissues and fat, which are nearly black or dark gray on CT soft-tissue windows. Stones are bright white, as is calcified bone. Occasionally, other calcified structures may be found in the abdomen, including vascular calcifications in the aorta and its branches or pelvic vein calcifications called phleboliths. The latter can be difficult to distinguish from urinary stones. Some urinary stones are not visible on CT because they are not calcified. The classic example is indinavir stones—this relatively insoluble protease inhibitor used to treat human immunodeficiency virus infection can precipitate from solution, forming radiolucent stones. However, the sequela of obstruction, such as hydroureter or hydronephrosis, remains visible. Noncontrast CT can be viewed on soft-tissue or bone window settings to detect stones. Soft-tissue windows are appropriate for evaluating complications such as hydroureter and hydronephrosis, as well as perinephric stranding. In this 37-year-old man with left flank pain, a 4-mm stone is seen in the proximal left ureter (A). The ureter proximal to this is slightly dilated, consistent with hydronephrosis (C, one slice cephalad to A). B, Close-up from A. D, Close-up from C.
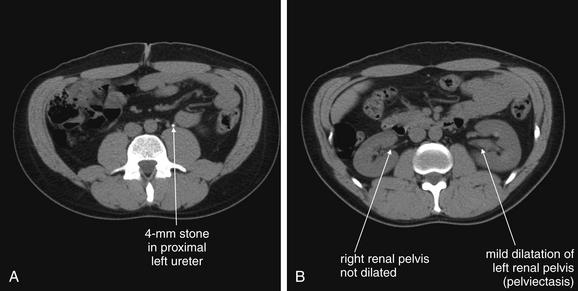
Figure 12-3 Urinary stones: Noncontrast CT on soft-tissue windows.
Same patient as in the previous figure. A, a small stone is present in the left mid ureter. B, a slice cephalad to A, The left kidney is shows mild pelviectasis (enlargement of the renal pelvis) but no frank hydronephrosis.
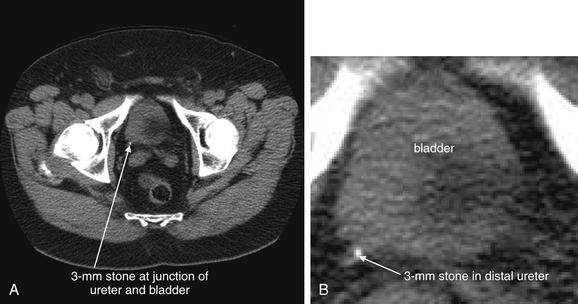
Figure 12-4 Urinary stone (small stone at ureterovesical junction): Noncontrast CT on soft-tissue windows.
This patient presented with flank pain radiating to the right lower abdomen. A, A 3-mm stone is present at the right ureterovesical junction. B, Close-up. Stones of this size and location pass spontaneously in nearly all cases.
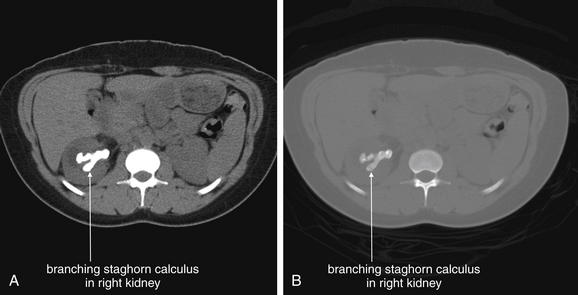
Figure 12-5 Urinary stones (staghorn calculus) noncontrast CT.
Staghorn calculi are extensive branching urinary stones that may completely fill the intrarenal collecting system. Although small stones within the kidneys are not generally thought to cause pain, staghorn calculi are usually formed in the presence of chronic colonization or infection with the gram-negative rod Proteus and may cause pain. Occasionally, surgery is performed to remove these large stones. In this patient, a noncontrast computed tomography revealed a right staghorn calculus. Urine cultures grew Proteus. A, Soft-tissue window setting. B, Same slice viewed on a bone window.
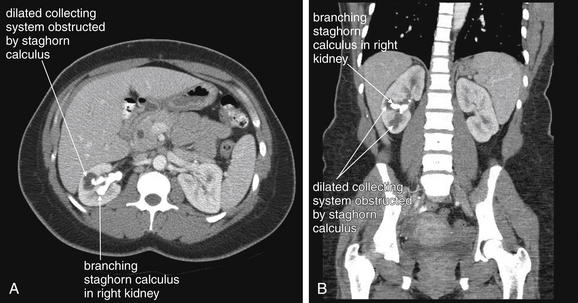
Figure 12-6 Urinary stones (staghorn calculus): Intravenous-contrasted CT soft-tissue windows.
Same patient as in the previous figure. The patient received IV contrast. Now, the right kidney enhances, revealing a hypodense region of intrarenal collecting system that is obstructed by a branch of the staghorn. A, Axial image, B, Coronal image.
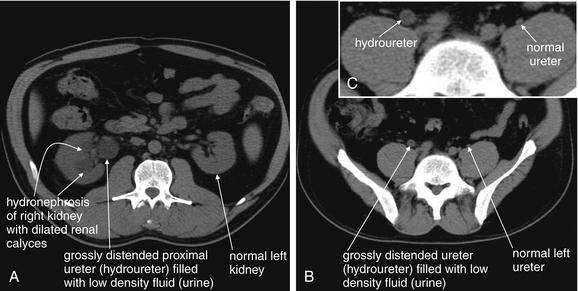
Figure 12-7 Hydronephrosis and hydroureter without urinary stones.
This 32-year-old man has a history of ureteral obstruction resulting from past ureteral stones. He now presents with flank pain and hydronephrosis, but no stones were seen on noncontrast CT. A “dual renal scan”—noncontrast CT (this figure), followed by CT with intravenous (IV) contrast (see Figure 12-8)—was performed. This provides functional information, much like standard IV pyelography. A through C, In this noncontrast CT, the right kidney appears to have marked hydronephrosis whereas the left kidney is normal. High in the pelvis, the right ureter is readily recognized and has hydroureter, whereas the left ureter is barely visible in its normal position hugging the psoas muscle. When trying to locate the ureter using a digital picture archiving and communication system, start at the kidney and follow the ureter slice by slice to the bladder. Attempting to locate a normal ureter in midcourse is often difficult because of the small size of the normal ureter.
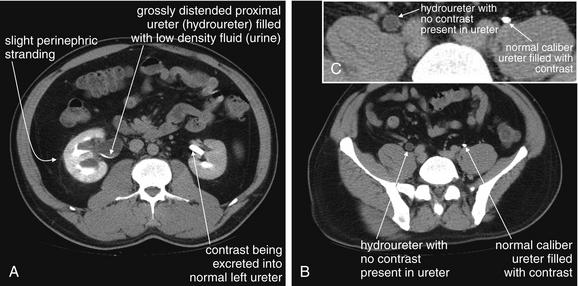
Figure 12-8 Hydronephrosis and hydroureter without urinary stones: Intravenous-contrasted CT.
Same patient as in the previous figure. Now, the patient has received IV contrast. A through C, The right kidney is enhancing, but no contrast has been excreted into the ureter, which remains distended with low-density urine. The left kidney is enhancing, and contrast has been excreted from the kidney into the ureter and is present along its entire course, visible as a bright white dot in transverse cross section or as a linear bright structure in coronal section (see Figure 12-4).
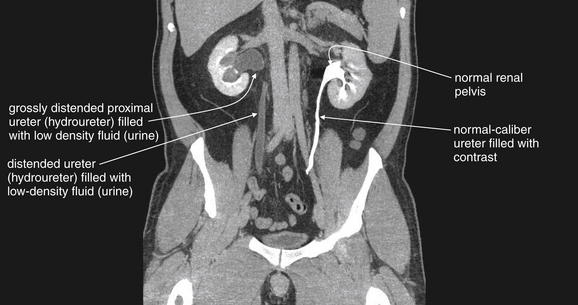
Figure 12-9 Hydronephrosis and hydroureter without urinary stones: Intravenous-contrasted computed tomography.
Same patient as in the previous figures. A coronal image shows the kidneys and ureters. The right kidney has obvious hydronephrosis and hydroureter with no contrast in the ureter. The left kidney is excreting contrast normally, and the ureter is filled with contrast. Note the difference in caliber of the right and left ureters. The distal ureters and bladder are not seen because they lie in a more anterior plane. No stones were seen in the right ureter, and no extrinsic compression was witnessed. A stricture of the ureterovesical junction on the right was suspected, likely because of prior stones.
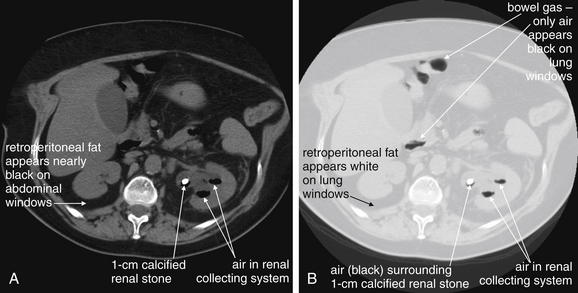
Figure 12-10 Emphysematous pyelonephritis noncontrast CT.
A, B, Emphysematous pyelonephritis is a urologic emergency in which infection with gas-forming organisms affects the kidney. This patient has a large stone in the left renal pelvis. Notably, air is filling the renal collecting system. On typical soft-tissue windows, the black appearance of air may be difficult to distinguish from dark retroperitoneal and peritoneal fat. Lung windows make every tissue type except air white, leaving black air in stark relief.
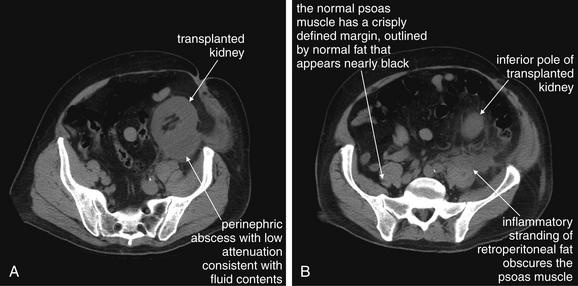
Figure 12-11 Renal or perinephric abscess: CT with no oral or IV.
A, B, In this renal transplant patient presenting with fever and pain overlying his transplanted kidney, a noncontrast CT was performed because of concerns about potential contrast nephropathy. CT revealed perinephric abscess—although the patient’s transplant had been performed more than 3 years prior. This collection was subsequently drained under CT guidance, and cultures of the fluid grew Staphylococcus aureus. In this case, CT findings suggesting perinephric abscess include regional fat stranding near the inferior pole of the kidney, extending to the left psoas muscle—which may also be involved in the abscess. The normally well-defined margins of the psoas are obscured by this fat stranding. Note that inflammatory fat stranding is visible without administration of any contrast. If IV contrast had been administered, rim-enhancement of the abscess would be expected.
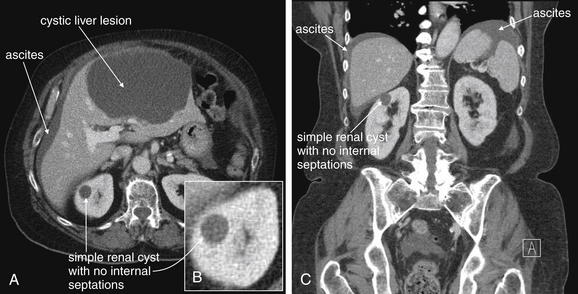
Figure 12-12 Simple renal cyst: CT with IV contrast on soft-tissue windows.
A through C, Simple renal cysts have a rounded appearance without internal septations. They do not enhance with IV contrast because they are not vascular infectious or inflammatory lesions. This patient has a simple cyst in the right kidney, as well as an enormous cystic liver lesion and ascites. These share the same fluid density and thus have the same grayscale appearance on abdominal soft-tissue windows. On the Hounsfield scale used to measure density on CT, water is assigned a value of 0 Hounsfield units (HU), air is assigned a value of 0 HU, and the densest materials such as bone have values as high as +1000 HU. The density of the fluid in the simple renal cyst is around 30 Hounsfield units, similar to that of water.
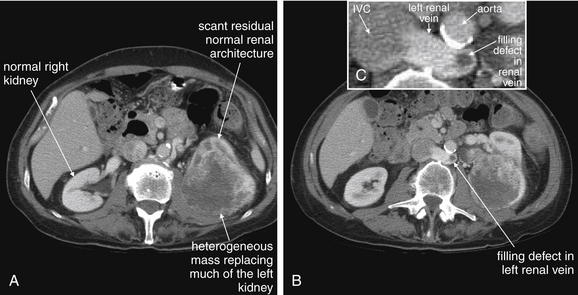
Figure 12-13 Renal masses: Renal cell carcinoma.
Noncontrast CT performed for assessment of urinary stone disease sometimes reveals underlying renal malignancies. When a malignancy is suspected, intravenous contrast should be administered to further characterize the lesion. Flank pain and painless hematuria are both sometimes presenting symptoms of renal cell carcinomas (hypernephromas). A through C, In this patient, an aggressive renal cell carcinoma has nearly replaced the left kidney. A small amount of relatively normal renal architecture can be seen anteriorly, whereas the bulk of the tumor is heterogeneously enhancing, likely because of a degree of necrosis. Incidentally, the patient has a retroaortic left renal vein (the normal course being anterior to the aorta). This vein deserves attention because renal cell carcinomas are known to invade the renal vein, enter the inferior vena cava (IVC), and embolize to the lungs. In this patient, a filling defect is seen in the left renal vein, likely representing tumor invasion. The IVC is just beginning to fill with contrast and has a heterogeneous appearance due to mixing of contrast and normal blood, so it cannot be assessed for tumor invasion. Delayed images could be obtained to identify filling defects in the IVC once the contrast appearance of the IVC has become more uniform.
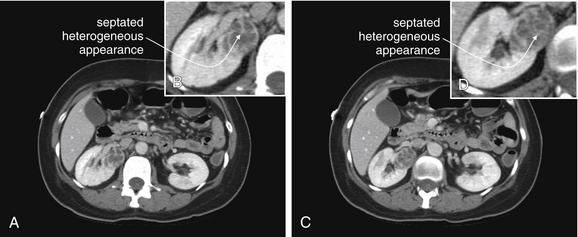
Figure 12-14 Renal masses: Smaller renal cell carcinoma identified on CT with IV contrast.
A through D, A renal cell carcinoma is seen arising from the right kidney. Typical features of this malignancy include a septated or heterogeneous appearance with the administration of intravenous contrast. In comparison, benign simple renal cysts are homogeneous and have a single cyst cavity. Small renal cell carcinomas can be missed on CT without IV contrast, so IV contrast should be administered when malignancy is suspected, such as in adults with painless hematuria.
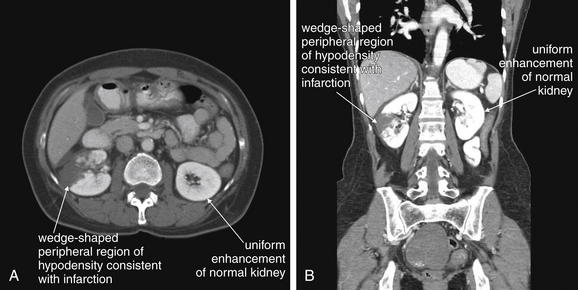
Figure 12-15 Renal infarcts: Contrast-enhanced CT on soft-tissue windows.
A, B, CT with intravenous (IV) contrast gives important information on renal perfusion. This patient presented with flank pain and hematuria and was noted to be in new-onset atrial fibrillation. Renal infarction was suspected, and both oral and IV contrast were given because of a broad differential, including possible mesenteric ischemia. The renal findings could have been delineated using IV contrast alone. The left kidney enhances uniformly. The right kidney has a peripheral wedge-shaped area of hypodensity and nonenhancement, consistent with renal infarction. This appearance is similar to what might be seen with a traumatic renal contusion, but here no history of trauma exists. Severe focal pyelonephritis has been reported to have a similar appearance, but here the history of atrial fibrillation makes infarction the leading diagnosis. In the coronal image (B), the window level has been adjusted slightly, accounting for the brighter appearance of bone and solid organs.
Noncontrast CT has become a test of choice because it can be performed immediately and provides several key pieces of information:
Compared with IV urography, CT is also advantageous because it does not require contrast administration, which can cause nephrotoxicity in patients with already obstructed urinary systems. In addition, IVU does not routinely reveal nonurinary pathology. Studies comparing emergency department length of stay show an advantage to CT over IVU, the traditional standard.2
Is Intravenous or Oral Contrast Needed for Detection of Stones? Does Contrast Interfere With the Diagnosis of Urinary Stones?
For detection of intrarenal and intraureteral stones, no contrast of any form is needed. Administration of oral and IV contrast can interfere with diagnosis of urologic stone disease; for this reason, noncontrast CT is often performed immediately before an IV-contrasted study. This also allows comparison of the noncontrast and contrasted CT for identification of lesions that enhance with IV contrast. The addition of contrast makes identification of stones more difficult by providing an array of white (contrast-filled) structures among which a white urolith must be sought. The kidneys enhance intensely after administration of IV contrast, so stones within the kidneys may be difficult or impossible to discern. Soon after IV contrast is administered, contrast is filtered and excreted by the kidneys and begins to fill the ureters, again potentially disguising stones within the ureters (see Figures 12-8 and 12-9). In cases of suspected high-grade obstruction, additional delayed images of the kidneys and ureters can be performed following IV contrast administration to assess for normal filling of the ureters, or pathologic nonfilling. This information is similar to that obtained from a standard intravenous pyelography (IVP) using conventional radiography. Oral contrast agents are not needed to detect urologic stone disease. Some CT protocols place the patient in a prone position, rather than the supine position commonly used for abdominal CT. This position leaves bowel and other intraabdominal organs in a dependent position, whereas ureters and kidneys are held tightly in their retroperitoneal position. Calcifications in the ureters may be more easily discriminated from nonurinary calcifications by this technique.
When Should Noncontrast CT Be Performed? When Should Noncontrast CT Be Followed by Contrasted CT?
This is a clinical decision, and it should be driven by the differential diagnosis. Several factors warrant consideration. First, is a life-threatening diagnosis, such as ruptured AAA or aortic dissection, under serious consideration? If so, immediate CT should be considered, perhaps without any contrast agents, depending on the stability of the patient (bedside ultrasound and surgery consultation may be appropriate in an unstable patient). AAA can be diagnosed without any contrast agents, whereas aortic dissection requires IV contrast for diagnosis. Second, is the patient old enough that the radiation exposure from multiple CT scans is not an important consideration? In patients older than 50 years, the radiation exposure from CT is unlikely to cause a clinically important cancer, and rapid diagnosis of an immediate life threat such as AAA easily trumps radiation risk. In younger patients, especially those in whom an imminent life threat is not suspected, it may be more reasonable to perform a single CT scan with IV and oral contrast to maximize the possibility of detecting nongenitourinary pathology. This strategy may be more beneficial to the patient in the long run than scanning without contrast and then scanning with contrast if no pathology is detected. At the same time, studies suggest that CT without contrast has good sensitivity for many conditions traditionally examined with contrast, including appendicitis. These studies are discussed in more detail in Chapter 9, Imaging of Nontraumatic Abdominal Conditions. Depending on the specific differential diagnosis being considered, noncontrast CT may be the only imaging needed—for example, contrasted CT may not be required if the appendix is well visualized on noncontrast CT. An additional factor to be considered is the patient’s renal function and allergies—IV-contrasted CT should be avoided in patients with renal insufficiency or dye allergies, and noncontrast CT may be adequate to evaluate fully the differential diagnosis under consideration. Noncontrast CT generally is not performed after contrasted CT because orally administered agents may remain present for a day or more and IV contrast agents are visible for minutes to hours (in the case of urinary obstruction) within the renal collecting system, ureters, and bladder as they are excreted.
Besides the Presence of Stones, What Genitourinary Abnormalities Can Noncontrast CT Identify? What Genitourinary Abnormalities Can Be Seen With the Addition of IV Contrast?
Noncontrast CT can reveal the presence of ureteral obstruction.3 Hydronephrosis is recognized by the presence of a dilated renal collecting system. The unobstructed contralateral side serves as a useful comparison (see Figures 12-7 to 12-9). Variation in size of the proximal ureter can occur because of the presence of a normal variant extrarenal pelvis, which can simulate significant proximal hydroureter. Hydroureter (see Figures 12-7 to 12-9) proximal to an obstructing stone can be detected on noncontrast CT. The upper limit of normal diameter for an unobstructed ureter is 3 mm.4 Stranding of perirenal fat (see Figures 12-8 and 12-11) can be seen without any contrast agents and can occur in the setting of obstruction or infection. Stranding is caused by lymphatic capillary leak, resulting in infiltration of fluid into the perirenal fat. This increases the density of the perirenal fat relative to normal fat. Increased density on CT results in a whiter appearance, compared with the usual dark gray appearance of fat. This finding may also occur in pyelonephritis, so the urinalysis should be examined for infection. Importantly, stranding alone does not indicate infection, unless other clinical indicators of infection (such as a positive urinalysis) are present. Emphysematous pyelonephritis (see Figure 12-10) can be observed on noncontrast CT because air appears black and does not require contrast for visualization. Perinephric abscess (see Figure 12-11) can be seen as a low-density (dark gray) fluid collection adjacent to the kidney, often with stranding. Addition of IV contrast enhances an abscess. Urine collections (urinomas) surrounding a kidney because of a leaking renal pelvis or ureter have a similar appearance to abscess on noncontrast CT but may lack stranding and do not enhance with IV contrast. Isolated simple renal cysts (see Figure 12-12) are visible on noncontrast CT. These structures have a low Hounsfield unit density near zero because they contain fluid similar in density to water. The surrounding renal parenchyma is slightly denser and thus slightly brighter without contrast. When IV contrast is administered, renal cysts become more conspicuous because the surrounding normal kidney enhances dramatically but cysts do not. Solitary simple cysts are not usually diagnostically important because they are not typically a cause of acute pain. Polycystic kidneys may be the cause of acute or chronic pain and are readily seen on noncontrast CT. Unilateral, horseshoe, or pelvic kidneys are recognized on noncontrast CT, though these are not associated with acute abdominal or flank pain. Solid renal tumors such as renal cell carcinoma (see Figures 12-13 and 12-14) can be difficult to identify on noncontrast CT when small. They may be exophytic and can invade adjacent structures (see Figure 7-56). Addition of IV contrast helps in detection of small lesions by allowing enhancement. Renal infarction is not easily identified on noncontrast CT but is readily apparent with IV contrast, as the infarcted region fails to enhance while normal renal parenchyma vividly enhances (see Figure 12-15). Retroperitoneal hemorrhage (Figure 12-16), whether spontaneous or resulting from trauma, can be seen on noncontrast CT. Addition of IV contrast is important in these cases as it can reveal active bleeding.
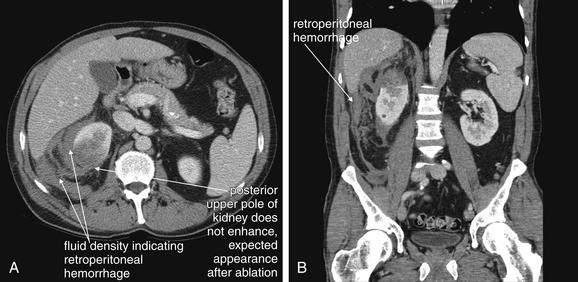
Figure 12-16 Retroperitoneal hemorrhage.
CT with IV contrast gives important information on retroperitoneal hemorrhage. Blood that has escaped before the CT scan appears dark gray and can be seen on noncontrast CT. Addition of IV contrast allows recognition of active bleeding and demonstrates normal and abnormal renal perfusion. Oral contrast is not needed. This 50-year-old man on warfarin and enoxaparin for a Saint Jude aortic valve presented with flank pain after undergoing a CT-guided percutaneous ablation of a renal mass. The CT shows no enhancement in the upper pole of the right kidney, as expected following the ablation. Fluid density (blood) is seen in the retroperitoneum surrounding the right kidney. No blush of contrast is seen within this collection, indicating that no active bleeding is present. A, Axial view. B, Coronal view.
IV contrast results in intense enhancement of the normal kidney because of the enormous blood flow to this organ. An abnormal kidney may fail to enhance compared with the normal kidney. Examples include aortic dissection (see Figure 7-83) or renal artery dissection. In addition, hypoperfused renal segments may not enhance normally. Examples include renal infarcts in the context of atrial fibrillation (see Figure 12-15). Hypoperfusion of renal segments also is seen in some cases of pyelonephritis, although no clinical significance is known and contrasted CT is not recommended for this diagnosis.5 As mentioned earlier, renal abscesses with rim enhancement may also be demonstrated on CT with IV contrast, though noncontrast CT or ultrasound may show a fluid collection around the kidney (see Figure 12-11). Perirenal fluid collections may also indicate urinomas because of a leaking urinary tract—these do not demonstrate rim enhancement with the addition of IV contrast and can be seen on noncontrast CT. Renal masses such as renal cell carcinoma (hypernephroma; see Figures 12-13 and 12-14) are also detected as enhancing masses on contrasted CT (these lesions may be detected, though not as perfectly delineated, on noncontrast CT). Exophytic bladder lesions such as transitional cell carcinomas may become visible as IV contrast excreted by the kidneys fills the bladder. The tumor mass may be visible as a void or filling defect in the contrast-filled bladder. Clot may have a similar effect and appearance. Dense contrast settles in the dependent portion of the bladder. As a consequence, if the bladder is not completely filled with contrast, lesions in the nondependent portion of the bladder may not be visible.
How Sensitive Is Noncontrast CT for Renal Stones?
CT is highly sensitive for most calcified ureteral stones. Its sensitivity and specificity approach 100%, with better positive and negative likelihood ratios than IVU.6-7 In some cases, no stone is seen because the patient passed the stone just before CT scan.3 Stones formed from indinavir, a poorly soluble protease inhibitor used in the treatment of human immunodeficiency virus infection, are not visible on CT because they are isodense with urine. However, findings of obstruction from these stones, such as hydroureter and hydronephrosis, are still readily recognized on CT. These stones can be seen by modalities such as ultrasound, and on IVU they create an obstruction picture identical to that seen with other stones.
What Is the Prognostic Value of CT for Renal Stones? What Size of Stone Will Pass? How Quickly can Stones Develop? What If the Patient Had a Scan Without Stones 1 Week Ago?
Stone size as measured on CT scan is predictive of the rate of spontaneous passage or need for surgical or procedural intervention. Only 2.2% of patients with stones smaller than 6 mm require a procedure, whereas 80% of patients with stones of at least 6 mm require a procedure. The rapidity with which stones can develop is not well studied, but in general, the absence of stones on recent CT imaging likely indicates that a large obstructing stone is unlikely.
Ultrasound
Ultrasound is a valuable tool for the assessment of obstruction of the ureters. Ultrasound has the advantage of being noninvasive, with no radiation exposure and no need for IV contrast. It is relatively inexpensive compared with CT8 and can be repeated over time without harm to the patient. Ultrasound is portable, and it can be used to assess for important alternative causes of symptoms, from aortic aneurysm to appendicitis to complications of pregnancy. It is the standard test for suspected renal colic in the pregnant patient because it does not expose the fetus to ionizing radiation. Renal ultrasound assesses for hydronephrosis and hydroureter (Figures 12-17 and 12-18). It is limited to some extent by body habitus and operator experience, and it does not always allow visualization of the cause of the obstruction, whether that is an intraureteral stone or extrinsic compression of the ureter by a mass or retroperitoneal fibrosis. On ultrasound, calcified renal stones are hyperechoic (bright white) (see Figure 12-17), reflecting the ultrasound beam and preventing its transmission deep to the stone. As a consequence, they also create an acoustic shadow deep to the stone. Ultrasound can be used to detect other renal disease including atrophic kidneys, renal cysts including polycystic kidney disease, and renal masses. With the addition of Doppler ultrasound, flow in the renal arteries and renal artery stenosis can be diagnosed.
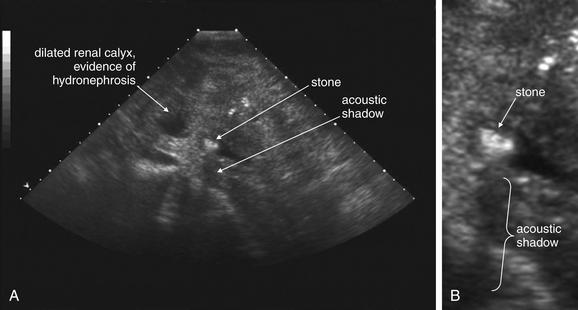
Figure 12-17 Ultrasound of renal stone.
Ultrasound can be used to assess for renal stones and complications such as hydronephrosis. Stones can be difficult to detect, whereas hydronephrosis is usually readily observed. Because stones are dense, they reflect sound and prevent its through transmission. As a result, stones are echogenic (bright) on ultrasound, and cast an acoustic shadow (black). A, Short-axis view of kidney. B, Close-up.
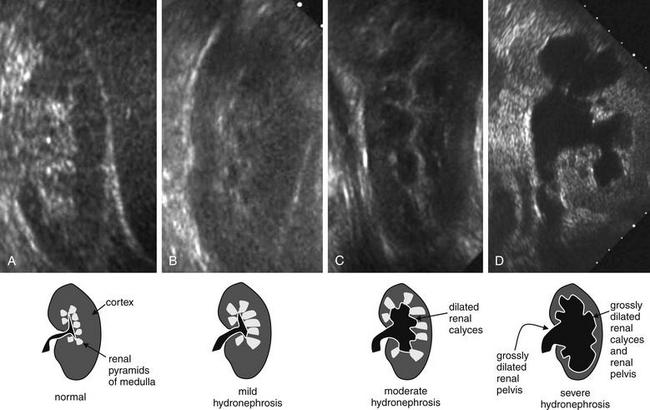
Figure 12-18 Hydronephrosis: Ultrasound.
Ultrasound can detect hydronephrosis readily, whereas renal stones are not as easily observed. The normal kidney has a hypoechoic (dark gray) outer cortex, whereas the renal pyramids of the inner medulla are hyperechoic (bright). The renal calyces of a normal kidney are barely visible because urine drains from them readily in the absence of obstruction. As obstruction develops, the calyces fill with urine, which is hypoechoic (black). Normal kidney (A) and mild (B), moderate (C), and severe (D) grades of hydronephrosis. Kidneys are shown in the long-axis view.
Studies comparing ultrasound and CT for the diagnosis of ureterolithiasis show comparable sensitivity and specificity (91% and 95%, respectively, for CT and 93% and 95%, respectively, for ultrasound).8-9 Some studies have shown lower sensitivity of ultrasound (61%) compared with CT (96%).9 Overall, ultrasound is a reasonable alternative to CT when the differential diagnosis is limited to ureteral stones and radiation reduction is a priority, as in pregnancy or young patients with multiple episodes of renal colic.
Plain Film X-ray
Plain film x-ray plays a limited role in evaluation of nontraumatic urologic emergencies. In the setting of suspected renal colic, a single plain film of the abdomen was commonly performed before the era of CT. This x-ray is often called a KUB (for kidneys, ureters, bladder)—ironically, because none of these structures is usually visible, though they may be included in the field of view. X-ray is likely insensitive for detection of renal stones: 18.6% in one study compared with a gold standard of unenhanced CT. The specificity is high, around 95%.10 This method may have some limited value when other imaging techniques are unavailable. It can demonstrate likely ureteroliths, although it may fail to detect noncalcified stones. In addition, extraurinary calcifications such as phleboliths may be misidentified as urinary stones (Figures 12-19 and 12-20A). Although the size of the stones may be measured, plain film does not allow assessment for obstruction. In addition, sensitivity for a broader differential diagnosis, including aortic aneurysm, appendicitis, and bowel obstruction, is quite limited. Perhaps the greatest benefit of a single plain film in this setting is that the radiation exposure to the patient is low—though the information gleaned from the study is so limited that no imaging is nearly as useful. Sometimes a single x-ray to evaluate for radiodense stones is combined with ultrasound to evaluate for hydronephrosis and hydroureter. When a stone has been visualized on a prior x-ray, x-ray can be used to monitor its progression. X-ray is also used to assess the position of medical devices such as ureteral stents.
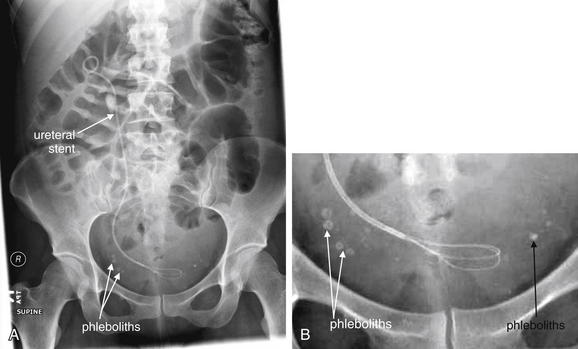
Figure 12-19 X-ray of renal stones.
X-rays of the abdomen have been used historically to diagnose and monitor urinary stones. Unfortunately, x-rays are neither sensitive nor specific. Some renal stones are relatively radiolucent and not visible on x-ray. In addition, other calcifications in the abdomen and pelvis may be indistinguishable from ureteral stones. X-ray does not show complications of urinary stones, such as ureteral obstruction and hydronephrosis. In this patient, the right ureter has been stented to relieve obstruction. Calcifications are visible in the pelvis, but these are likely phleboliths (venous calcifications) rather than ureteral stones. Notice how they lie lateral to the position of the stent in the ureter. Some also have a radiolucent (dark) center, which is a common but not pathognomonic feature of phleboliths. A, X-ray, often called a KUB (for kidneys, ureters, and bladder) because these structures lie within the field of view. Ironically, none of these structures is well seen with plain x-ray. B, Close-up.
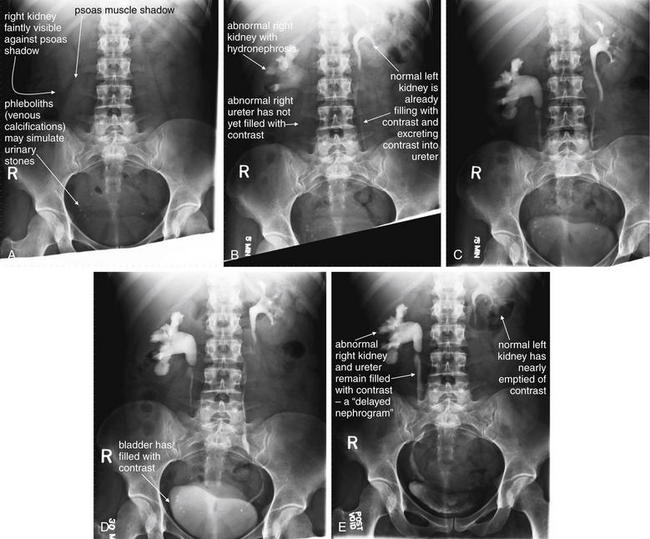
Figure 12-20 Intravenous urography, also called pyelography.
In intravenous pyelography (IVP), contrast is injected intravenously and then excreted by the kidneys, providing a cast image of the renal calyces, pelvis, ureters, and bladder. This provides structural information because hydronephrosis, hydroureter, and points of ureteral stricture or obstruction are revealed. This also provides functional information because a nonfunctioning kidney does not excrete contrast. Together, delayed appearance of contrast in a renal collecting system and slow excretion from the collecting system is called a delayed nephrogram and is a sign of partial obstruction. An IVP consists of a preinjection plain x-ray of the abdomen and pelvis, followed by a series of x-rays of the same region conducted at intervals of 5, 15, and 30 minutes, as well as a final image after voiding of urine or contrast from the bladder. Additional delayed images may be obtained. Sometimes exophytic lesions in the bladder (neoplasms) are seen as filling defects. A through E, In this IVP series, the patient has a normal left kidney, with a collecting system that briskly fills with contrast and then empties. Structurally, this collecting system also appears normal, with no dilated calyces and a thin normal ureter. In contrast, the right renal calyces are slow to fill (because of high pressure in the collecting system from distal obstruction). The right kidney not only fills more slowly than the left but also is slower to empty, remaining markedly visible even on postvoid images. The right renal calyces and pelvis are extremely dilated consistent with hydronephrosis, and the proximal right ureter is dilated, consistent with hydroureter. There is relatively little contrast in the distal right ureter at the level of L3-L4, consistent with an obstruction proximal to this level. The patient had a ureteral stone at this level previously and likely has a stricture at this location. Phleboliths are also visible in the pelvis and may be mistaken for urinary stones.
Intravenous Urography
IVU (also called IVP) is a series of plain film images taken over time (see Figure 12-20). In addition to providing information about the structure of the urinary system, including the presence of stones or obstruction, this is a “functional study,” because it provides a view of renal excretion of injected contrast. First, a plain film image of the abdomen and pelvis is obtained, before the administration of contrast. This image can be examined for radiopaque stones and medical devices such as ureteral stents, as described earlier. Occasionally, an alternative diagnosis such as appendicitis or AAA may be recognized, although this modality should never be relied on to exclude these diagnoses because it is insensitive. Next, around 50 mL of iodinated contrast is injected intravenously, and the plain film (KUB) is repeated after a delay of 5 minutes. Additional images are repeated at approximately 15-minute intervals until both kidneys have been observed to fill and then empty of contrast, allowing excretion of the contrast material to be observed over time. Depending on the speed of excretion, the examination usually takes less than 1 hour, though several hours may be required in cases of severe obstruction. A normal IVU demonstrates rapid and symmetrical enhancement of both kidneys, followed by complete filling of the ureters and bladder. Several abnormalities may be observed. First, congenital anomalies may be observed, including duplications of the collecting system, and unilateral, horseshoe, or abnormally positioned (e.g., pelvic) kidneys. In the setting of ureteral obstruction from any cause, the kidney on the affected side may show delayed enhancement and delayed emptying, called a “delayed nephrogram” (see Figure 12-20E). This is because of diminished renal filtration and excretion of contrast in the setting of obstruction. In addition, the affected ureter may fail to fill with contrast beyond the point of a complete obstruction, or the contrast may appear less intense beyond the point of a partial obstruction. IVU provides anatomic and functional information about the location and degree of obstruction, which may be important for planning urologic intervention.
Is Intravenous Urography Preferable to CT for Evaluation of Renal Colic or Ureteral Obstruction?
IVU and CT provide similar but not identical information. CT is superior in delineating a range of emergency alternative diagnoses, explaining its rapid incorporation into the imaging algorithm for suspected renal colic in the United States. CT also has the advantage of not requiring the administration of any contrast material, with the obvious benefit of not posing a threat of contrast nephropathy or dye allergy. In addition, CT can be performed immediately and diagnostic results are available within minutes, in comparison to IVU, which takes around 1 hour in most cases and longer in cases of severe obstruction. A number of studies have compared the time, expense, and radiation exposure of CT and IVU. CT generally results in slightly shorter emergency department length of stay, similar costs (depending on whether institutional or patient costs are compared), and somewhat higher radiation exposure, though new protocols have reduced radiation exposures.
Lasix Nuclear Medicine Scan: An Alternative Test for Ureteral Obstruction
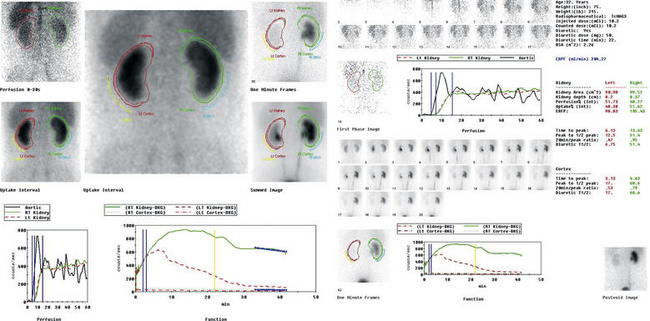
A patient with recurrent renal calculi may develop an appearance of chronic obstruction with a dilated renal pelvis and ureter by ultrasound, CT scan, or IVP. A Lasix nuclear medicine scan (Figures 12-21 and 12-22) can discriminate obstruction from a flaccidly dilated but currently unobstructed collecting system. Though rarely used in the emergency department, this test may be requested by a urologic consultant. In this test, a radiopharmaceutical, technetium-99m mercaptoacetyltriglycine, is administered intravenously. Dynamic blood flow phase images of the kidneys are obtained during bolus injection of the radiotracer. Sequential 2-minute acquisitions posteriorly over the abdomen are obtained through 30 to 60 minutes, allowing generation of graphs of radioactivity over time for the renal cortex and entire kidney. Lasix (50 mg IV) is administered after around 20 minutes. In a normal scan, rapid uniform excretion occurs after administration of Lasix. This is consistent with a flaccid but unobstructed collecting system. Lack of excretion of the tracer following Lasix administration suggests obstruction.
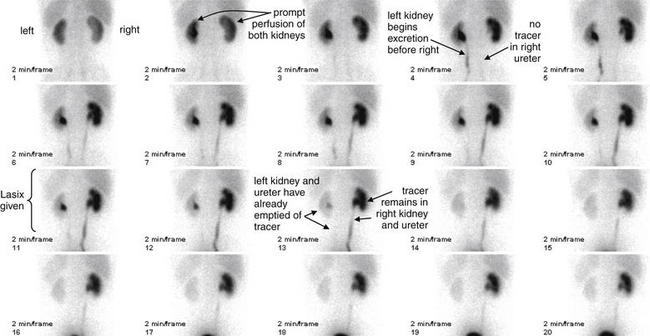
Figure 12-21 Lasix renal scan.
Same patient as in the CT scan in Figures 12-7 to 12-9. A Lasix renal scan is a nuclear medicine study that provides quantitative information about renal perfusion and excretion. Though rarely used in the emergency department, this study may occasionally be requested by consulting urologists when uncertainty exists about the functional effect of a chronically dilated urinary system. Note that the right–left convention for this study is reversed from that used for CT. The frames are numbered sequentially and occur at 2-minute intervals. In this scan, 10.2 mCi of a radiopharmaceutical, technetium-99m mercaptoacetyltriglycine, are administered intravenously. Dynamic blood flow phase images of the kidneys are obtained during bolus injection of the radiotracer. Sequential 2-minute acquisitions posteriorly over the abdomen are obtained through 42 minutes. In addition to anatomic images, radioactivity can be graphed over time for the renal cortex and whole kidney, providing quantitative measurements of renal parenchymal perfusion. Lasix (50 mg intravenously) is administered at 22 minutes. In this patient, the dynamic blood flow images above demonstrate relatively prompt and symmetrical perfusion to the bilateral kidneys. On the initial renogram images, the right kidney appears slightly larger than the left kidney, and there is central photopenia in the right kidney that suggests hydronephrosis. On subsequent renogram images over 42 minutes, the left kidney demonstrates relatively prompt uptake, excretion, and clearance of radiotracer with no evidence of obstruction. The right kidney demonstrates mildly delayed uptake and excretion of radiotracer into a prominent collecting system, from which there is only minimal clearance of tracer activity until the administration of Lasix. After Lasix administration, there is somewhat better, though incomplete, clearance of tracer activity over the duration of the study, with residual tracer activity seen in the right collecting system and the right ureter to level of the urinary bladder on the postvoid image. These findings suggest partial obstruction of the right ureter.
Is Any Imaging Needed for Suspected Renal Colic?
When a broad differential diagnosis is being considered, nongenitourinary diagnoses may drive the imaging strategy. Important diagnoses such as suspected AAA rupture or appendicitis may require CT or other imaging for diagnosis. In effect, though CT may confirm urolithiasis, the most important function of CT may be to exclude other diagnoses. When renal colic is the only serious diagnosis under consideration, the emergency physician should carefully consider whether any imaging is required. CT carries a moderately high radiation exposure, and patients with renal colic often have recurrent symptoms, resulting in multiple CT exposures over time. When complications such as infection or renal insufficiency are not present, deferring CT imaging may be reasonable. In first-time episodes of suspected renal colic, clinical judgment is incorrect in as many as 20% of cases, even when suspicion of stone is 90% or greater. Imaging of first episodes is likely warranted.11 Alternative or additional diagnoses are reported in approximately 10% of patients undergoing CT for suspected renal colic, although not all of these diagnoses require specific emergency treatment.1 In patients with recurrent symptoms, another reasonable strategy may be ultrasound to evaluate for hydronephrosis or hydroureter. Ultrasound may show a stone, though with lower sensitivity than CT. In patients older than 50 years, the risk of repeat CT radiation exposures may be relatively unimportant, and the risk of alternative causes of symptoms rises. CT may be a prudent strategy in this age group.
Painless Hematuria
Painless hematuria generally is not associated with urolithiasis because the latter condition is usually painful. Painless hematuria can indicate urinary infection, renal infarction, systemic illness such as glomerulonephritis or thrombocytopenia, or masses of the genitourinary tract, including renal cell carcinoma (see Figures 12-13 and 12-14) and transitional cell carcinoma of the ureters or bladder. Painless hematuria is an important complaint for this reason, but emergency imaging is only required in selected cases, depending on the specific differential diagnosis being entertained. For example, in the patient with atrial fibrillation, renal infarction (see Figure 12-15) should be considered. Imaging can alter patient management because confirmation of renal infarction can suggest a cardiac embolic source. When important but less urgent pathology such as renal mass is suspected, imaging can be deferred to an outpatient setting. Patients with poor follow-up may benefit from definitive imaging in the emergency department to allow appropriate referral. When the decision is made to perform imaging, CT without IV contrast followed by CT with IV contrast is most useful because it identifies enhancing renal masses such as renal cell carcinoma. CT with IV contrast can also demonstrate areas of abnormal renal parenchymal enhancement consistent with infarction. Noncontrast CT alone may fail to fully delineate these lesions. Oral contrast is not needed for these indications. CT scan does not evaluate intraluminal pathology of the bladder or ureters well, so additional follow-up for cystoscopy is indicated if transitional cell carcinoma is suspected. Exophytic bladder lesions such as transitional cell carcinomas may become visible as IV contrast excreted by the kidneys fills the bladder. Delayed CT images are often acquired to allow detection of such pathology. The tumor mass may be visible as a void or filling defect in the contrast-filled bladder. Clotted blood within the bladder may have a similar effect and appearance and may sometimes confuse the diagnosis. Renally excreted contrast within the ureters on CT or IVP may occasionally show a filling defect suggesting a ureteral mass.
Dysuria
Isolated dysuria can suggest urinary tract infection, infectious urethritis, or sterile urethritis such as Reiter’s syndrome. This symptom generally does not require emergency imaging.
Urinary Retention
Obstruction of the bladder outlet and inability to urinate is a common emergency department complaint, particularly in men. Usual causes are benign prostatic hypertrophy and hematuria with clot formation leading to urethral obstruction. Ureteral obstruction rarely causes urine retention because bilateral ureteral obstruction would be required. Retroperitoneal causes such as retroperitoneal fibrosis or tumors may occasionally obstruct both ureters. Urinary retention from a neurologic cause should be considered, but in the absence of other neurologic signs or symptoms, back pain, or other red flags for spinal pathology, mechanical bladder outlet or urethral obstruction is the most common cause. Prostatic malignancies and bladder masses may also lead to obstruction, but emergency imaging is not generally required when symptoms are relieved by placement of a urinary catheter. Urologic follow-up is necessary. If a neurologic cause is suspected, spine magnetic resonance imaging (MRI) is usually required. Ultrasound of the bladder can be performed at bedside and reveals a full bladder in cases of urethral obstruction or neurogenic bladder. Renal ultrasound commonly reveals bilateral hydronephrosis but is not necessary if the obstruction is relieved by urinary catheter placement.
Nontraumatic Abdominal Pain
Nontraumatic abdominal pain can result from a host of serious and benign conditions. The chapters on abdominal imaging explore this in detail. Some causes of abdominal pain include genitourinary abnormalities, as described later in the sections on male and female genitourinary complaints. Flank pain with or without abdominal pain was discussed earlier.
Fever
Isolated fever without other genitourinary signs or symptoms rarely requires genitourinary imaging. Fever associated with abdominal, flank, or pelvic pain requires imaging as indicated for the evaluation of the associated pain. Serious genitourinary infections such as tuboovarian abscess, perinephric abscess (see Figure 12-11), or Fournier’s gangrene (Figures 12-23 and 12-24), described in the next section, may have associated fever but are usually accompanied by localizing pain and examination findings that direct imaging. One exception is the obtunded septic patient with fever. Complicated urinary infections such as perinephric abscess and infected obstructing ureteral stone should be considered and may require imaging.
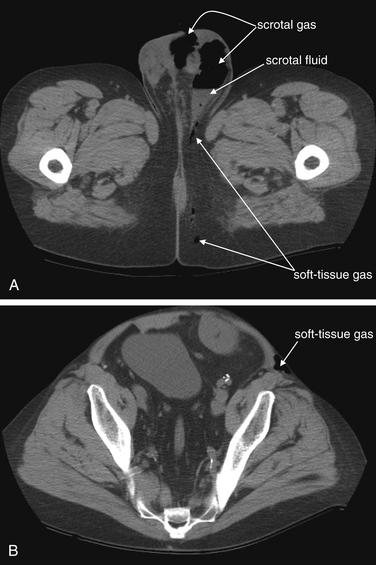
Figure 12-23 Fournier’s gangrene.
This 41-year-old man with a history of renal transplantation presented with diffuse scrotal pain and crepitus. He had first noted a “boil” in his perineal region several days prior. CT of the pelvis was performed without intravenous or oral contrast. A, A slice at the level of the scrotum shows an gas–fluid level in the left scrotum, as well as gas in the midline of the scrotum. Gas tracks posteriorly from this into gluteal subcutaneous fat. B, A slice from much higher in the pelvis and lower abdomen reveals that the patient does not have an isolated scrotal abscess. Here, gas remains visible in the subcutaneous space anterior to the left iliac wing. These findings are consistent with Fournier’s gangrene. Coronal and sagittal images from the same patient illustrate the cephalad–caudad extent of the infection in the next figure. The patient was taken emergently to the operating room, and necrotic tissue was debrided from the scrotum, abdominal wall, and gluteal region. Two important points are illustrated by this case. First, arguably the clinical findings should have been acted upon without requiring any imaging. Fournier’s gangrene requires emergent operative debridement. However, in this case, the CT findings prompted involvement of both urologists and general surgeons, whereas an isolated scrotal abscess may have been handled by a urologist alone. Second, CT without any contrast agents is exquisitely sensitive for the presence of soft-tissue gas. When necrotizing soft-tissue infections are suspected, noncontrast CT offers rapid assessment. Surgical consultation should not be delayed for imaging.
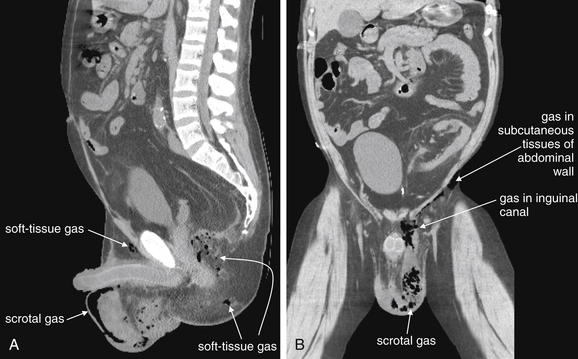
Figure 12-24 Fournier’s gangrene.
Same patient as in the previous figure. A, Sagittal view. B, Coronal view. These views demonstrate the cephalad–caudad and anterior–posterior extent of the infection. A, Gas is visible in the scrotum, as well as in soft tissues of the buttocks. B, Gas, and presumably infection, has tracked up the left inguinal canal from the scrotum and follows the muscles of the lower abdomen.
Perineal Pain
Perineal pain can result from a variety of conditions, including some serious and life-threatening causes. Local abscess formation may be difficult to detect by physical examination and can require emergency imaging. Most fearsome is Fournier’s gangrene (see Figures 12-23 and 12-24), a polymicrobial infection resulting in soft-tissue necrosis and sepsis. When Fournier’s gangrene is suspected clinically, empiric antibiotics, surgical consultation, and fluid resuscitation take precedence over imaging. CT without IV contrast can delineate the extent of infection and can show soft-tissue air and fluid collections. Addition of IV contrast can demonstrate enhancement typical of abscesses. When perirectal abscess is suspected, rectal contrast may assist in differentiating the bowel lumen from adjacent soft-tissue infectious fluid collections. MRI and ultrasound can also demonstrate local soft-tissue fluid and air collections but are rarely indicated in the emergency department. They can be used in the pregnant patient to avoid radiation exposure.
Genitourinary Imaging in the Male Patient
Several important genitourinary chief complaints may require imaging in male patients. The imaging approach varies with age and suspected differential diagnosis.
Testicular or Scrotal Pain
Testicular and scrotal pain can suggest testicular torsion, epididymitis, orchitis, local abscess, Fournier’s gangrene, hernia, hydrocele, varicocele, and malignancy. Among these, testicular torsion, Fournier’s gangrene, and incarcerated hernia are surgical emergencies. When these diagnoses are clinically suspected, immediate surgical or urologic consultation should be obtained, before or concurrent with imaging studies.
Testicular torsion is best evaluated with either ultrasound (Figure 12-25, normal ultrasound) (Figures 12-26 and 12-27, testicular torsion) or nuclear scintigraphy. Physical examination is relatively unreliable, with significant overlap in findings such as cremasteric reflex abnormalities between patients with torsion and epididymitis.12 Ultrasound is moderately sensitive and highly specific for persistent torsion (nearly 100% sensitive and specific in some studies,13-14 though others report sensitivity of only 60% to 80% and specificity of only 80% to 90%15-19). Intermittent torsion may be one explanation for negative ultrasound results. Delayed presentations of torsion beyond 8 hours have been reported to lead to false-negative ultrasound interpretations.20 Decreased blood flow demonstrated by Doppler ultrasound suggests or confirms torsion, whereas normal blood flow is reassuring. Complete loss of blood flow may not occur. Venous flow is often compromised first, followed by arterial flow. Emergency physician–performed ultrasound shows high agreement with studies performed by sonographers and interpreted by radiologists—reported as 95% sensitive and 94% specific in one study, though wide confidence intervals in this study may mean lower true sensitivity and specificity.21 Nuclear scintigraphy detects abnormal testicular perfusion and has a sensitivity and specificity similar to that of ultrasound in studies directly comparing the two modalities, around 80% sensitive and 90% specific.16,22 As stated earlier, consult a urologist as soon as torsion is suspected, before imaging. Because false-negative imaging results are possible, when torsion is strongly suspected clinically, surgery may be appropriate despite normal ultrasound or scintigraphy.
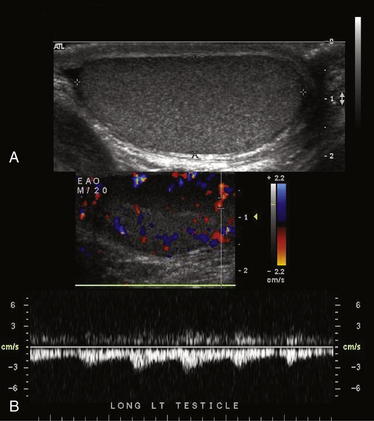
Figure 12-25 Normal testicular ultrasound.
This 20-year-old male complained of a palpable nodule on the right testicle. These images of the left testicle are normal. A, The normal echo texture of the testicle is seen. No significant fluid surrounds the testicle. B, Arterial flow is normal, with a pulsatile waveform.
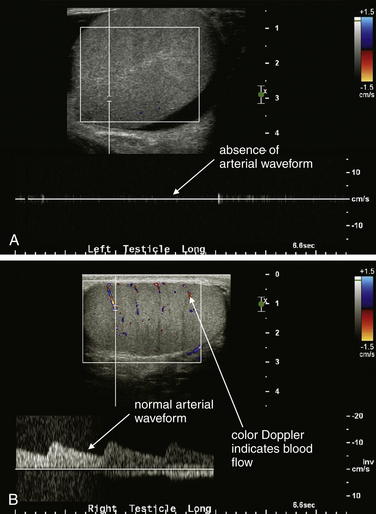
Figure 12-26 Testicular torsion ultrasound.
A, B, Ultrasound has become the standard modality to assess for testicular torsion. In this 20-year-old male with left testicular pain, the left testicle has no blood flow, whereas the right testicle has normal arterial and venous blood flow by color Doppler assessment and a normal arterial waveform. The patient was taken to the operating suite, where left testicular torsion was confirmed. After rapid detorsion and orchiopexy, the left testicle reperfused and was viable.
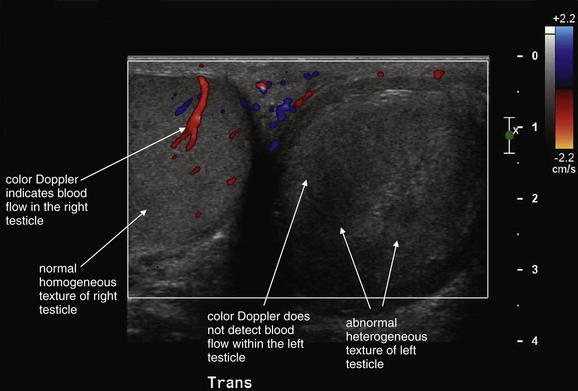
Figure 12-27 Testicular infarct.
This patient had been treated for epididymitis without improvement. Ultrasound then revealed apparent testicular torsion, confirmed operatively. The patient underwent orchiectomy, and surgical pathology was consistent with hemorrhagic infarction. This case highlights the importance of imaging for testicular pain. Epididymitis, orchitis, and testicular torsion may have similar presentations. The ultrasound findings of note are an absence of blood flow in the left testicle by color Doppler, and a heterogeneous echotexture of the left testicle compared with the right, which is consistent with infarction.
Epididymitis and orchitis (Figures 12-28 to 12-30) can be diagnosed by ultrasound. The usual indication for imaging is to rule out the diagnosis of testicular torsion. Increased Doppler blood flow signal in the epididymis or testicle suggests epididymitis or orchitis, respectively. The sensitivity and specificity are not well delineated, so other clinical factors should be incorporated into treatment decisions, rather than relying exclusively on imaging findings. Nuclear scintigraphy can also suggest increased blood flow consistent with epididymitis or orchitis.
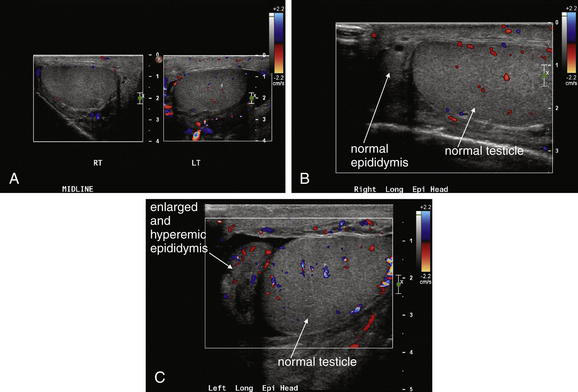
A through C, In addition to evaluating the more concerning diagnosis of testicular torsion, ultrasound can identify epididymitis and orchitis. In epididymitis, blood flow to the affected epididymis is increased. A, A hydrocele is visible (black) adjacent to the left testicle. B and C, The left epididymis shows increased blood flow compared with the normal right epididymis, based on color Doppler, consistent with epididymitis. In addition, the left epididymis is enlarged compared with the normal right epididymis. Blood flow to the testicles was normal.
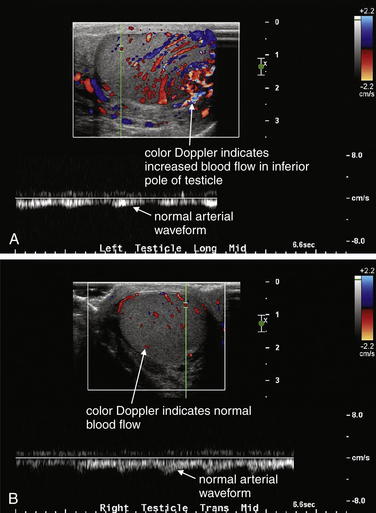
Figure 12-29 Focal orchitis on testicular ultrasound.
A and B, This 45-year-old male was treated with antibiotics for epididymitis but had recurrent pain after completing treatment. Ultrasound was performed to rule out testicular torsion. Instead, the ultrasound suggested focal orchitis. Prominent flow is seen in the inferior portion of the left testicle, consistent with focal orchitis in that location. The right testicle shows normal blood flow.
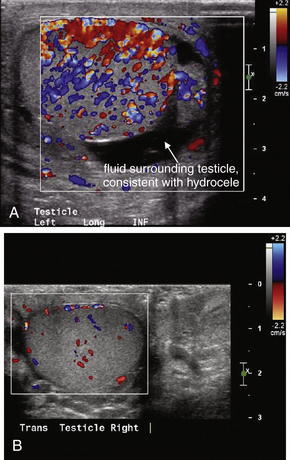
Figure 12-30 Orchitis, color Doppler ultrasound.
A and B, This patient with left testicular pain shows markedly increased blood flow on color Doppler throughout the left testicle compared with the right, consistent with orchitis of the left testicle. A hydrocele is also seen surrounding the left testicle (black collection).
Ultrasound of the scrotum can demonstrate loculated fluid collections consistent with abscess (Figures 12-31 to 12-33), although other fluids such as blood can have a similar appearance. Air in the scrotum from necrotizing infection causes dispersion of the ultrasound beam, disrupting the ultrasound image. Although this prevents a high-quality image, it can be diagnostic because air should never be found in the normal scrotum. As outlined earlier, Fournier’s gangrene and local abscesses of the scrotum can be identified by CT without IV contrast. Air, fluid, and inflammatory soft-tissue stranding are evident without contrast. IV contrast improves diagnostic sensitivity and specificity by demonstrating rim enhancement of abscesses. Rectal contrast can delineate abscesses adjacent to the distal bowel, which may otherwise be difficult to distinguish from fluid-filled bowel.
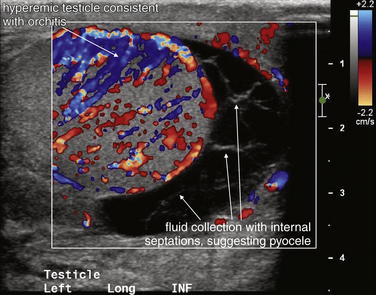
Figure 12-31 Orchitis with pyocele: Testicular ultrasound.
The left testicle is markedly hyperemic on color Doppler ultrasound compared with the right testicle (not shown), consistent with orchitis. There is complex fluid collection with internal septations and internal echoes that is predominantly located inferior to the testicle and is concerning for a pyocele. Simple hydroceles are homogeneously black without internal echoes. Fluid collections with internal structure are more likely to represent infection or blood products, whereas simple fluid is more likely serous.
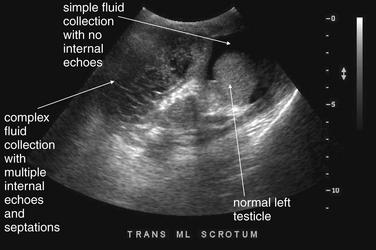
Figure 12-32 Scrotal hematocele after hernia repair.
This patient developed increased right scrotal swelling after a hernia repair. Ultrasound was performed to evaluate the cause. This image shows the midline scrotum. The right scrotum is filled with a complex collection with multiple septa—no testicle is seen because the patient is known to have an undescended testicle on that side. The left scrotum shows a normal testicle surrounded by simple fluid collection, consistent with hydrocele. These findings are examined in more detail in the next figure. No bowel loops are seen to suggest a recurrent hernia.
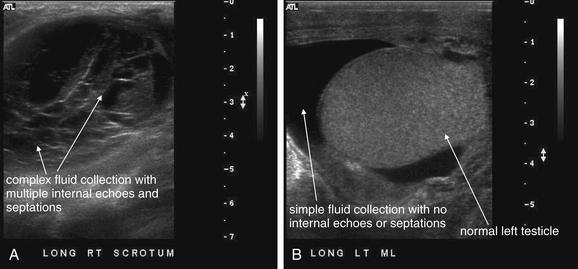
Figure 12-33 Scrotal hematocele after hernia repair.
This patient developed increased right scrotal swelling after a hernia repair. Ultrasound was performed to evaluate the cause. A, The right scrotum with complex fluid collection with multiple internal septa. This is concerning for hematocele or pyocele—the two cannot be distinguished on ultrasound. B, The left scrotum with a normal testicle and simple fluid collection consistent with hydrocele. Note the absence of any internal septa or echoes within the fluid collection.
Hydrocele (Figure 12-34), or fluid within the scrotum and outside of the testicle, is readily diagnosed by ultrasound. Because hydrocele can accompany a variety of other conditions, including infection, testicular torsion, and malignancy, its presence should not be used to exclude other diagnoses. Hydrocele can be simple fluid elaborated from an adjacent inflammatory process or communicating with abdominal ascites. Loculated or heterogeneous hydrocele can suggest infection or organizing hematoma, so the differential diagnosis should be considered carefully. Isolated hydrocele with otherwise normal imaging findings, including normal testicular blood flow, may not require additional emergency imaging, with the caveat that hydrocele can accompany both harmless and concerning conditions such as testicular torsion. Spermatocele (Figure 12-35) has a similar ultrasound appearance. CT without IV or oral contrast can also demonstrate hydrocele, although it generally should not be ordered for this indication because ultrasound conveys the same information without radiation exposure.
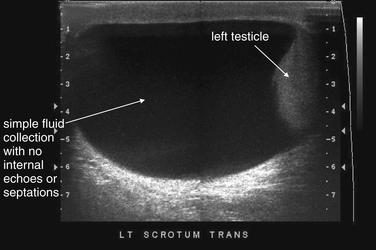
This patient has a large left hydrocele. The fluid collection has no internal echoes or septations and likely represents serous fluid. Complex fluid collection with internal echoes and septations would be more concerning for infection or blood products.
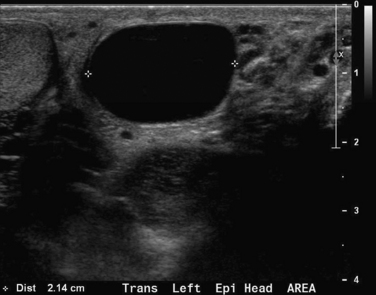
This 41-year-old man noted a painless mass in his left scrotum. On examination, a firm nodular nontender mass was noted adjacent to but distinct from the left testicle. Ultrasound showed a cystic fluid–filled structure in the region of the left epididymis. Although this isolated image resembles a hydrocele, the structure is well circumscribed and does not surround the left testicle. The important feature here is the cystic quality, which makes this a likely benign lesion. A solid structure would be more suspicious for malignancy.
Varicocele (Figure 12-36) is a benign venous abnormality characterized by a palpable mass on examination, often described as a “bag of worms.” Some patients may present with associated pain, usually not severe. Ultrasound readily confirms the diagnosis, demonstrating a series of low-flow channels using Doppler. Ultrasound is usually ordered to evaluate for other, more dangerous conditions, such as testicular torsion. CT can demonstrate this finding but is not generally indicated for its evaluation.
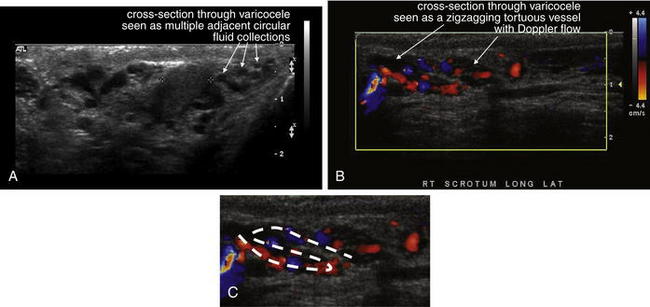
This 35-year-old man underwent ultrasound for evaluation of azoospermia. An incidental finding on this ultrasound was bilateral varicoceles. These tortuous veins may present as scrotal masses. A through C, Ultrasound confirms varicocele by demonstrating tortuous fluid-filled vessels with color Doppler flow consistent with venous blood flow. A, In the left scrotum, the vein passes in and out of the plane of the ultrasound image, giving the appearance of multiple adjacent circular cross sections. B and C, In the images from the right scrotum using color Doppler, a zigzagging vessel with flow is visible.
Malignancy can result in testicular or scrotal pain. Ultrasound can demonstrate solid testicular masses, which can include not only germ cell tumors but also metastatic and hematogenous malignancies such as lymphoma. Hydrocele may accompany these. CT may also demonstrate these abnormalities but is not the first-line imaging test because ultrasound provides accurate information without radiation. CT can be used to assess for metastatic involvement once an abnormality is confirmed with ultrasound.
Incarcerated hernia can be diagnosed using ultrasound and CT (see the chapter on imaging of nontraumatic abdominal conditions). Incarceration is largely a clinical diagnosis, based on pain, signs of bowel obstruction such as vomiting and absence of flatus, and inability to reduce the hernia on examination. However, both ultrasound and CT can confirm the presence of bowel within the scrotum. CT without IV contrast can demonstrate herniated bowel. Addition of IV contrast can illustrate bowel wall abnormalities such as abnormal enhancement suggesting ischemia. Oral contrast is rarely needed and not necessarily helpful because it may not reach the segment of herniated bowel. Ultrasound can demonstrate fluid- and air-filled bowel loops within the scrotum. Peristalsis may be visible, a reassuring finding suggesting preservation of bowel perfusion and viability. Air in the herniated bowel may scatter the ultrasound beam, disrupting the image. However, the presence of air in the scrotum is abnormal and can be recognized as suggestive of hernia.
Testicular or Scrotal Mass
Painless scrotal masses may include malignancy, hydrocele, varicocele, and nonincarcerated hernia. Infection (such as orchitis or abscess), testicular torsion, and incarcerated hernia usually result in a painful mass. The evaluation is with ultrasound as described earlier for evaluation of scrotal pain. Painless scrotal masses generally are not emergencies, although definitive diagnosis to exclude malignancy should be timely.
Urethral Discharge
As described earlier for dysuria, urethral discharge in men is a finding of urethritis, including infection with Neisseria gonorrhea and Chlamydia trachomatis. Inflammatory conditions such as Reiter’s syndrome can also result in urethral discharge. Imaging is not generally required for this complaint.
Prostatic Pain or Urinary Retention From Suspected Prostatic Hypertrophy
Urinary retention from bladder outlet obstruction in male patients usually requires no emergency imaging. Bedside transabdominal ultrasound can demonstrate a distended bladder and an enlarged prostate. The prostate is visible on noncontrast CT, and abnormalities of the prostate are sometimes noted—although CT generally need not be performed in the emergency department for evaluation of suspected prostatic hypertrophy, prostate cancer, or bladder outlet obstruction from an enlarged prostate. If perineal pain is present, serious causes should be carefully considered, including perirectal abscess or Fournier’s gangrene. CT with IV contrast and possible rectal contrast can be performed if these conditions are suspected. In patients who have undergone recent transrectal prostate biopsy, abscess may occur, best detected by CT with IV contrast, which can display abscess rim enhancement. Prostate calcifications may be noted on CT but do not distinguish benign from malignant disease.23 Lytic lesions in the pelvis and spine may be indications of metastatic prostate cancer, although they are nonspecific and may be associated with other metastatic cancers, multiple myeloma, or other disease states.
Genitourinary Imaging in the Female Patient
Genitourinary complaints in the female patient mandate careful attention because they may indicate threats to life and fertility. The differential diagnosis and imaging approach hinge on pregnancy status and trimester of pregnancy, which form the framework for our discussion. Many presenting signs and symptoms of obstetric and gynecologic conditions, including abdominal pain, fever, and vomiting, overlap with abdominal and urinary conditions. In the patient with vaginal bleeding or discharge, and even in the known pregnant patient, a broad differential diagnosis must be entertained because pregnancy may be incidental to the patient’s symptoms. Conditions such as appendicitis are common in pregnancy. In this section, we focus on the imaging diagnosis of several important obstetric and gynecologic emergencies, counting on your differential diagnostic skills to consider other conditions. We frame these as common chief complaints. In many cases, the question is whether a single imaging approach, such as abdominal CT or ultrasound, is adequate to evaluate both abdominal and gynecologic complaints or whether a negative CT must be augmented with pelvic ultrasound. In other cases, the issue is whether any imaging test can fully rule out an important diagnosis, such as ectopic pregnancy, placental abruption, or ovarian torsion.
Imaging in the Pregnant Patient
Urine pregnancy testing is the routine standard for determining pregnancy status. It is considered 99% sensitive for detection of intrauterine pregnancy. Rarely (less than 1% of cases), urine pregnancy tests may be false negative or ectopic pregnancy may occur with human chorionic gonadotropin (hCG) values below the detection limit of the urine test. Bearing this in mind, when a high suspicion of pregnancy exists (e.g., in the patient with a missed period, abdominal pain, vaginal bleeding, and hypotension), serum hCG testing is advisable.24 Confirmation of pregnancy mandates imaging for several of the complaints that follow.
Perhaps the most common and important reason for abdominal and pelvic imaging in the pregnant patient is evaluation for potential ectopic pregnancy. Ultrasound has become the key diagnostic modality because of its safety in pregnancy. Ultrasound uses no ionizing radiation and can be repeated as often as is clinically indicated to monitor the patient’s condition, response to treatment, and developmental phase of pregnancy.
Vaginal Bleeding in the Pregnant Patient
First-trimester vaginal bleeding or abdominal pain demands immediate imaging to evaluate for possible ectopic pregnancy, which occurs in about 1 in 100 pregnancies.25 Once ectopic pregnancy is excluded, the differential diagnosis for vaginal bleeding can be explored in more detail.
Hemodynamically Unstable First-Trimester Pregnant Patient
Transabdominal ultrasound is the starting point for imaging to exclude ectopic pregnancy in a hemodynamically unstable patient. In an unstable female patient with hypotension, tachycardia, altered mental status, or other findings of shock, ectopic pregnancy should be assumed and emergency obstetrical consultation should occur before or simultaneously with ultrasound. Fluid resuscitation, preoperative evaluation such as type and screen, and transfusion should be considered before imaging confirms ectopic pregnancy. Bedside transabdominal ultrasound in the emergency department can be used to search for free abdominal or pelvic fluid, which is concerning for ruptured ectopic pregnancy and hemoperitoneum in this setting (Figures 12-37 to 12-42). As little as 100 mL of pelvic free fluid and as little as 200 to 600 mL in the hepatorenal space can be seen with transabdominal ultrasound.26 In patients with suspected ectopic pregnancy, free fluid detected in Morison’s pouch by emergency-physician-performed bedside ultrasound predicts the need for operative intervention. Ultrasound may show gross evidence of an adnexal mass (see Figures 12-40 and 12-41), which may show high blood flow using Doppler (the so-called ring of fire of ectopic pregnancy). The uterus should be assessed for the presence of an intrauterine pregnancy (as described later), which markedly decreases the likelihood of a concurrent ectopic pregnancy.
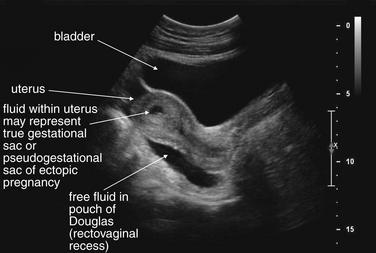
Figure 12-37 Suspected ruptured ectopic pregnancy: Transabdominal ultrasound.
This 25-year-old pregnant patient presents with abdominal pain. According to the patient, she had visited Planned Parenthood the previous day and was told she might have an ectopic or molar pregnancy based on ultrasound at that time. Her human chorionic gonadotropin value is now 28,372 mIU/mL. The ultrasound shows a large amount of free fluid in the midline lower abdomen. In the midsagittal view, the bladder and uterus are seen with a large amount of free fluid in the pouch of Douglas (rectovaginal recess). Fluid within the uterus may represent a pseudogestational sac associated with an ectopic pregnancy or a true gestational sac—but in the absence of visible yolk sac or fetal pole, ruptured ectopic pregnancy must be assumed based on the other ultrasound findings and clinical presentation. An ectopic pregnancy need not be visualized to make the presumptive diagnosis. In the operating room, the patient was found to have a ruptured ovarian cyst with a large quantity of hemoperitoneum, although ectopic pregnancy was not confirmed.
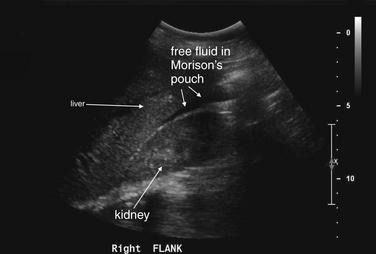
Figure 12-38 Suspected ruptured ectopic pregnancy: Transabdominal ultrasound.
Same patient as in the previous figure. This right upper quadrant view shows fluid in Morison’s pouch, indicating a large amount of hemoperitoneum. Given the clinical presentation of pregnancy, acute abdominal pain, and no visualized intrauterine pregnancy, ruptured ectopic pregnancy must be assumed.
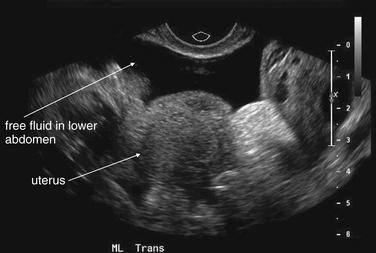
Figure 12-39 Suspected ruptured ectopic pregnancy: Transabdominal ultrasound.
Same patient as in the previous figures. The ultrasound shows a large amount of free fluid in the midline lower abdomen. Given the absence of a visualized intrauterine pregnancy, ruptured ectopic pregnancy must be assumed.
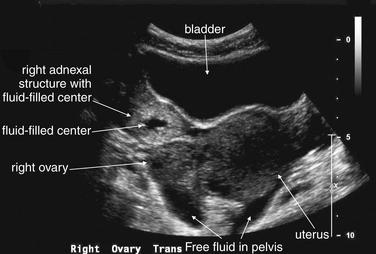
Figure 12-40 Ectopic pregnancy confirmed in operating room: Transabdominal ultrasound.
This 23-year-old female presented with vaginal bleeding and a human chorionic gonadotropin value of 4680 mIU/mL. This transverse transabdominal view shows the uterus and right ovary—and an additional right adnexal structure with a fluid-filled cavity, concerning for ectopic pregnancy. Free fluid is also visible in the pelvis, suggesting possible ruptured ectopic pregnancy. No intrauterine gestational sac or fetal pole is seen on this or any other views.
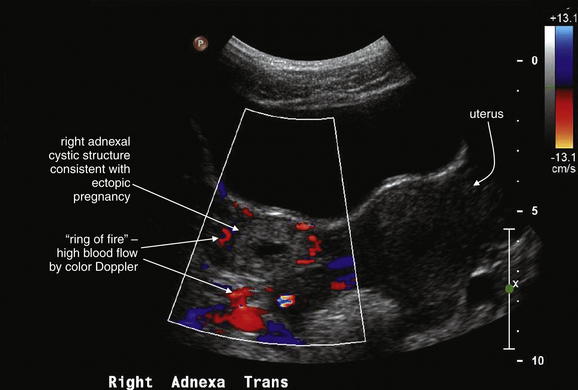
Figure 12-41 Ectopic pregnancy confirmed in operating room: Transabdominal ultrasound.
Same patient as in the previous figure. This transverse transabdominal view shows the classic adnexal “ring of fire” sign—an adnexal cystic structure with high blood flow demonstrated by color Doppler.
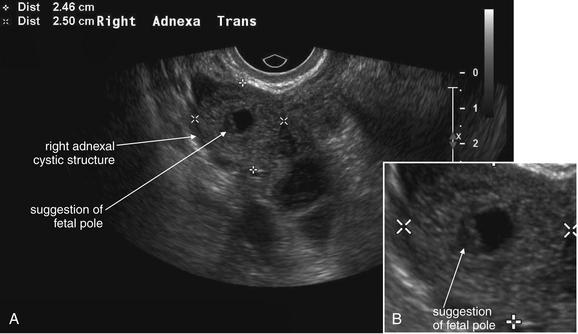
Figure 12-42 Ectopic pregnancy confirmed in operating room: Transvaginal ultrasound.
A, B, This 23-year-old woman presented with vaginal bleeding and a human chorionic gonadotropin value of 4680 mIU/mL. An adnexal cystic structure was visualized, including a likely fetal pole. This is highly suggestive of ectopic pregnancy.
Ultrasound Diagnosis of Ectopic Pregnancy
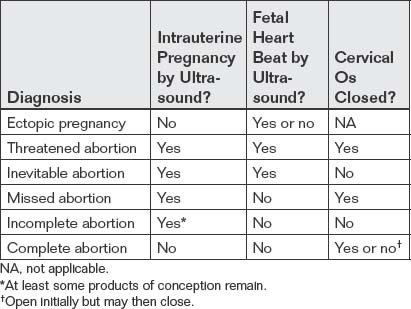
Two categories of ultrasound findings are used to diagnose ectopic pregnancy. The first category is failure to identify a normal intrauterine pregnancy. The second category is direct visualization of the ectopic pregnancy or related findings. We discuss both of these categories in detail later.
Ultrasound Findings of Normal Intrauterine Pregnancy
By about 8 weeks of pregnancy, an intrauterine pregnancy is usually readily recognized by transabdominal ultrasound as an early fetal pole with a visible heart beat. Earlier in pregnancy, an intrauterine pregnancy requires transvaginal ultrasound for detection and appears first as a gestational sac (4 to 5 weeks) (Figure 12-43), then as a gestational sac containing a yolk sac (5 weeks) (Figure 12-44), and then as a gestational sac with a fetal pole (6 weeks) (Figure 12-45). The combination of a gestational sac and yolk sac is considered sufficient to diagnose intrauterine pregnancy. On ultrasound, the gestational sac is a simple ovoid hypoechoic intrauterine structure. The yolk sac appears as a small hyperechoic ring with hypoechoic contents, contained within the larger gestational sac. Without the yolk sac, a single ring gestational sac is not sufficient to diagnose normal intrauterine pregnancy and to exclude ectopic pregnancy, unless a double-decidual sign is seen (see Figure 12-43). Although a single intrauterine ring may indicate the normal gestational sac of a very early pregnancy, it may also indicate the pseudogestational sac sometimes seen with ectopic pregnancy. A true gestational sac should have two decidual layers (the double-decidual sign) while a pseudogestational sac has only one decidual layer.27 Identification of later pregnancy features such as the fetal pole, fetal cardiac activity, or fetal movement is not considered necessary to prove intrauterine pregnancy and exclude ectopic pregnancy.28
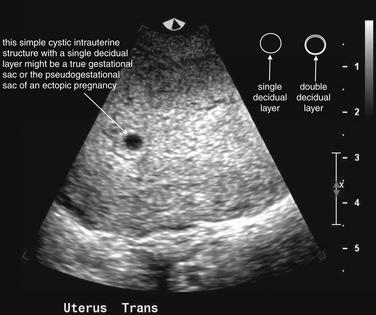
Figure 12-43 Early gestational sac versus pseudogestational sac.
This young woman presented with vaginal bleeding in early pregnancy. Her human chorionic gonadotropin value was 3600 mIU/mL. An anechoic ovoid (simple cystic) structure is seen within the uterus. Is this an intrauterine pregnancy? We cannot be certain from this image. Because concurrent intrauterine and ectopic pregnancies are a rare event except in patients undergoing fertility therapy, the presence of an intrauterine pregnancy is used as a surrogate to exclude ectopic pregnancy. However, a simple cystic structure can be the gestational sac of a normal pregnancy or the pseudogestational sac of an ectopic pregnancy. A true gestational sac can sometimes be recognized by the presence of a two-layer deciduum (the double-decidual sign). Unless this double layer sac is seen, ectopic pregnancy remains possible. The sac in this image has only one visible layer. A definitive indication of intrauterine pregnancy is a gestational sac with a yolk sac (Figure 12-44).
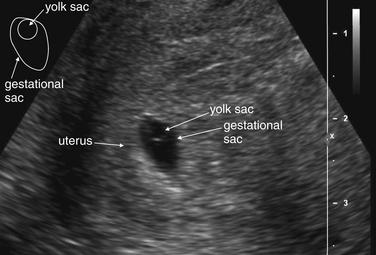
Figure 12-44 Normal intrauterine pregnancy—gestational sac with yolk sac: Transvaginal ultrasound.
This 24-year-old woman presented with right adnexal pain and vaginal spotting. Transvaginal ultrasound identified an intrauterine gestational sac containing a yolk sac. The combination of these findings confirms intrauterine pregnancy. Because heterotopic pregnancy is rare, occurring in approximately 1 in 10,000 patients, confirmation of intrauterine pregnancy effectively excludes ectopic pregnancy in all but the highest-risk patients. The presence of a gestational sac alone does not confirm intrauterine pregnancy and does not exclude ectopic pregnancy. The reason for this is simple: an intrauterine pseudogestational sac occurs with some ectopic pregnancies.
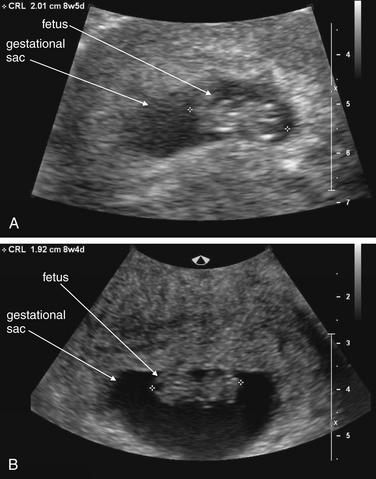
Figure 12-45 Normal intrauterine pregnancy comparing transabdominal and transvaginal ultrasound appearance.
A, A transabdominal ultrasound performed in a young woman with abdominal pain. An intrauterine pregnancy is readily seen with both a gestational sac and a fetus. Crown–rump length measurement is consistent with 8 weeks, 5 days of gestation. B, A transvaginal ultrasound performed the same day shows higher resolution. Crown–rump length measurements closely match those made with transabdominal ultrasound in this case. In earlier pregnancy, transvaginal ultrasound can reveal a gestational sac, yolk sac, or even fetal pole when none is seen transabdominally.
Human Chorionic Gonadotropin Values and Ultrasound: The “Discriminatory Zone.”
In the stable pregnant patient, transabdominal ultrasound should be performed to assess for an intrauterine pregnancy, as described earlier. If an intrauterine pregnancy is confirmed by transabdominal ultrasound, additional imaging is usually not required, depending on the clinical scenario. When transabdominal ultrasound fails to demonstrate an intrauterine pregnancy, measurement of quantitative serum hCG is used in conjunction with transvaginal ultrasound to interpret imaging results, as described here.29 Serum hCG rises rapidly during early pregnancy. Above a quantitative hCG value of 6000 mIU/mL, transabdominal ultrasound should show a normal intrauterine pregnancy, so failure to identify an intrauterine pregnancy with transabdominal ultrasound suggests abnormal pregnancy, including possible ectopic pregnancy or missed abortion.30 At an hCG value between 1500 and 2000 mIU/mL, a normal intrauterine pregnancy should be visible with transvaginal ultrasound.31 Failure to identify an intrauterine pregnancy using transvaginal ultrasound strongly suggests ectopic pregnancy when hCG values exceed 2000 mIU/mL, although multiple gestations may lead to hCG values above 2000 mIU/mL with no visible intrauterine pregnancy.32 An empty uterus with an hCG greater than 2000 mIU/mL is associated with a fivefold-higher relative risk for ectopic pregnancy.33 The hCG values of 2000 and 6000 mIU/mL have been titled the “discriminatory zone,” indicating the levels at which failure to visualize an intrauterine pregnancy with ultrasound becomes highly suspicious for ectopic pregnancy.30 Normal intrauterine pregnancy is unlikely if no gestational sac is seen by transvaginal ultrasound in a patient with an hCG greater than 3000 mIU/mL.34 Unfortunately, this has also led to misunderstanding of the utility of hCG and ultrasound in diagnosis of ectopic pregnancy. One misconception is that ultrasound need not be performed at hCG values less than 1500 to 2000 mIU/mL because a normal pregnancy would not be expected to be seen. Several studies have demonstrated that intrauterine pregnancy may be recognized by transvaginal ultrasound with hCG values less than 1000 mIU/mL.32,35-36 In addition, as many as 60% of ectopic pregnancies have hCG values lower than 1500 mIU/mL,37 and ruptured ectopic pregnancies can be seen at values below 100 mIU/mL. As many as one third of ectopic pregnancies presenting with hCG values below 1000 mIU/mL will have a diagnostic initial transvaginal ultrasound.38 Consequently, ultrasound is indicated to evaluate for ectopic pregnancy regardless of hCG value. Failure to demonstrate an intrauterine pregnancy with transvaginal ultrasound suggests ectopic pregnancy when hCG exceeds 2000 mIU/mL.
Ultrasound Findings of Ectopic Pregnancy
When ultrasound does not demonstrate an intrauterine gestational sac and yolk sac, proving intrauterine pregnancy, a diligent search must be conducted for other features of ectopic pregnancy, summarized in Table 12-3. A range of abnormal findings of ectopic pregnancy are shown in Figures 12-42 and 12-46 to 12-53 (see also Figures 12-37 to 12-41). Diagnostic findings include direct visualization of an extrauterine fetal pole with a heart beat or an extrauterine gestational sac with a yolk sac or fetal pole. These findings have low sensitivity, around 20% to 60%, but nearly 100% specificity for ectopic pregnancy. Any adnexal mass other than a simple cyst is highly suspicious for ectopic pregnancy in the absence of an intrauterine pregnancy, with a sensitivity of 84% and specificity of nearly 99%.39 When none of these findings is present, pelvic free fluid is suspicious for ectopic pregnancy. Isolated free fluid in the rectovaginal cul-de-sac suggests ectopic pregnancy; a moderate amount of anechoic fluid has a positive predictive value of 22%, whereas a large amount of anechoic fluid or any echogenic fluid has a positive predictive value of 73%.40 Moderate free fluid is defined as fluid tracking one third to two thirds up the posterior wall of the uterus without free-flowing fluid in the pelvis or abdomen. Large free fluid exceeds this amount, whereas small free fluid is less than this amount.
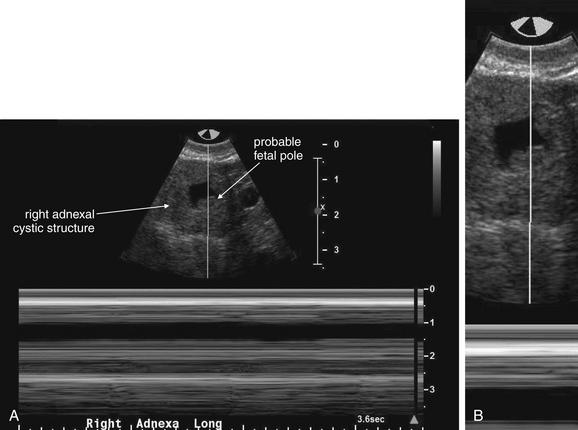
Figure 12-46 Ectopic pregnancy confirmed in operating room: Transvaginal ultrasound.
A, B, Same patient as in the previous figure. Motion (M) mode ultrasound was used to assess for fetal cardiac activity. The familiar two-dimensional grayscale images of ultrasound use brightness (B) mode. In M mode, a line is placed through the two-dimensional B mode image (top). A graph of motion along that line over time is generated along the bottom of the screen. If the line crosses through a beating fetal heart, a sinusoidal line will appear, and peak-to-peak or trough-to-trough measurements can be used to calculate fetal heart rate. In this case, no fetal cardiac activity was found.
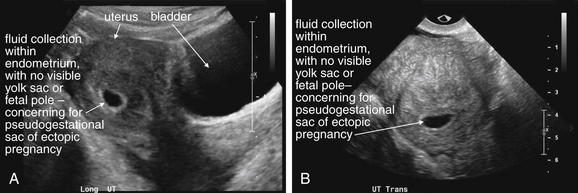
Figure 12-47 Possible ectopic pregnancy (found in the operating room to have a failed intrauterine pregnancy and two right ovarian cysts).
This woman presented with lower abdominal pain and a menstrual period 4 to 5 weeks earlier. Her human chorionic gonadotropin value was 16,368 mIU/mL. An ultrasound in clinic was interpreted as suspicious for ectopic pregnancy. In these images of the uterus (A, long axis, and, B, transverse) a fluid collection is seen within the endometrium, with no visible yolk sac or fetal pole. This is concerning for a pseudogestational sac associated with ectopic pregnancy, given the hCG value greater than 16,000 mIU/mL. A normal intrauterine pregnancy (with a yolk sac or fetal pole) should be seen at an hCG value of around 6000 mIU/mL by transabdominal ultrasound and at an hCG value of around 2000 mIU/mL by transvaginal ultrasound. At laparotomy, the patient was found to have two right ovarian cysts. The absence of an intrauterine pregnancy in this case was felt to be because of failed intrauterine pregnancy, not ectopic pregnancy, although this is a retrospective determination. The described findings should be interpreted as concerning for ectopic pregnancy.
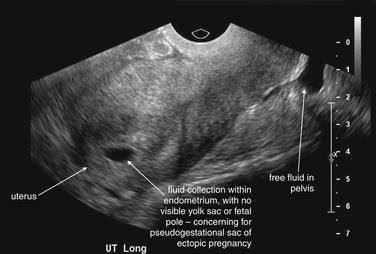
Figure 12-48 Possible ectopic pregnancy.
Same patient as in the previous figure. Free fluid is seen in the pelvis, which could represent blood from a ruptured ectopic pregnancy.
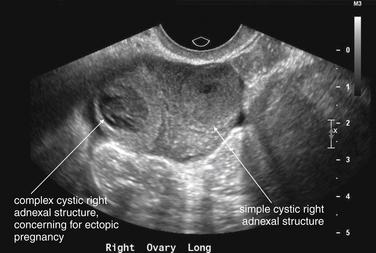
Figure 12-49 Possible ectopic pregnancy.
Same patient as in previous figures. Two cystic structures are seen in the right adnexa. Given the absence of an intrauterine pregnancy, these are concerning for ectopic pregnancy.
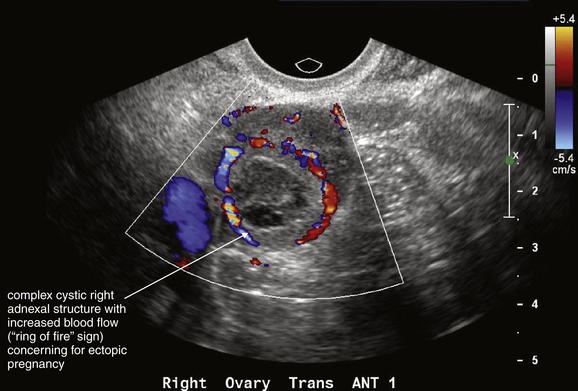
Figure 12-50 Possible ectopic pregnancy.
Same patient as in the previous figures. A right ovarian complex cystic structure is seen, with increased blood flow consistent with the “ring of fire” sign—a sign described as concerning for ectopic pregnancy.
Figure 12-51 Ectopic pregnancy.
This 23-year-old female with abdominal pain had a human chorionic gonadotropin value of 27,000 mIU/mL. A and B, These images of the uterus show no intrauterine gestational sac, yolk sac, or fetal pole—very concerning absences given the hCG value. A definite intrauterine pregnancy with a gestational sac and yolk sac should be seen at an hCG value of 6000 mIU/mL by transabdominal ultrasound and at 2000 mIU/mL by transvaginal ultrasound.
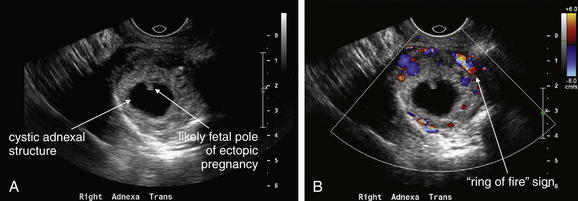
Figure 12-52 Ectopic pregnancy.
Same patient as in the previous figure. A and B, These images of the right adnexa show an ovoid echogenic structure measuring approximately 4.6 × 4.0 × 3.3 cm with a central cystic region and an eccentric hyperechoic focus concerning for an ectopic pregnancy. Color Doppler shows increased blood flow around the structure, consistent with the “ring of fire” sign.At laparotomy, right adnexal ectopic pregnancy was confirmed.
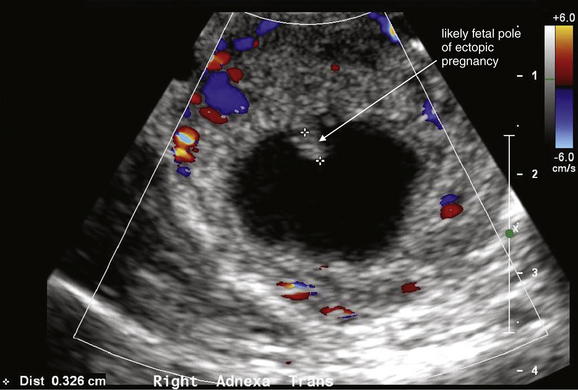
Figure 12-53 Ectopic pregnancy.
Same patient as in previous figures. No fetal heart rate was documented within the echogenic focus using motion mode. The echogenic focus measures approximately 3.3 mm.
Even when no abnormal findings are noted, the absence of an intrauterine pregnancy on transabdominal ultrasound with an hCG above 2000 mIU/mL is concerning for ectopic pregnancy. The differential diagnosis includes failed pregnancy such as blighted ovum, complete abortion not recognized by the patient, multiple gestation (with the total hCG being elevated but each gestation being too small to be seen), and ectopic pregnancy. The patient should be carefully followed for possible ectopic pregnancy, with repeat imaging and exams scheduled. In some cases, empiric therapy (methotrexate or laparoscopy) for possible ectopic pregnancy is initiated.
Heterotopic Pregnancy
In general, confirmation of intrauterine pregnancy is considered synonymous with exclusion of ectopic pregnancy, with one notable exception. Heterotopic pregnancy, the simultaneous occurrence of an intrauterine and an ectopic pregnancy, was once thought to be an extremely rare event, complicating 1 in 30,000 pregnancies. More recently, it is thought to occur in 1 in 2600 pregnancies in the United States. The risk is greatly increased by ovarian stimulation therapy, occurring in as many as 3% of these pregnancies. High-risk patients for heterotopic pregnancy require more stringent imaging in an attempt to identify an ectopic pregnancy even when intrauterine pregnancy is confirmed.41 Heterotopic pregnancy with concurrent gallstones is demonstrated in Figures 12-54 to 12-58, illustrating how caution must be taken to avoid ascribing symptoms in a pregnant patient to simultaneous abdominal disease or normal pregnancy.
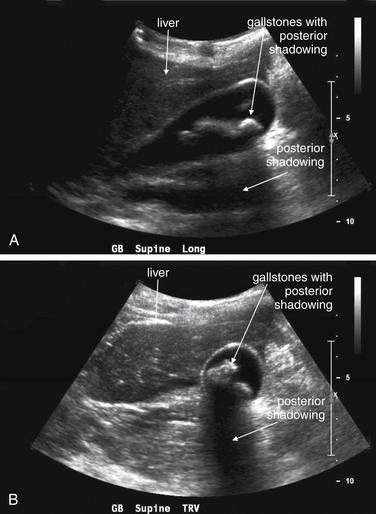
Figure 12-54 Heterotopic pregnancy with gallstones.
This 34-year-old woman presented with acute diffuse abdominal pain and vomiting, beginning 3 hours after eating dinner. She was 8 weeks pregnant after undergoing gonadotropin ovarian hyperstimulation therapy. Her white blood cell count was 24,000 with 90% neutrophils. A and B, Bedside ultrasound shows gallstones. Additional ultrasound images were obtained confirming intrauterine pregnancy (see Figure 12-56)—and a right adnexal structure concerning for ectopic pregnancy. Heterotopic pregnancy, the simultaneous occurrence of an intrauterine and an ectopic pregnancy, was once thought to be an extremely rare event, complicating 1 in 30,000 pregnancies. It now is thought to occur in 1 in 2600 pregnancies in the United States. The risk is greatly increased by ovarian stimulation therapy, occurring in as many as 3% of pregnancies. Normally, the presence of an intrauterine pregnancy nearly “rules out” ectopic pregnancy. However, in a high-risk patient, heterotopic pregnancy must be considered, and the adnexa should be carefully inspected for evidence of ectopic pregnancy. Gallstones are a common incidental finding and may not be the cause of acute abdominal pain. Care must always be taken to consider other causes of acute abdominal pain when gallstones are found. The patient underwent a right salpingectomy and continued her intrauterine pregnancy to deliver a healthy infant 7 months later.
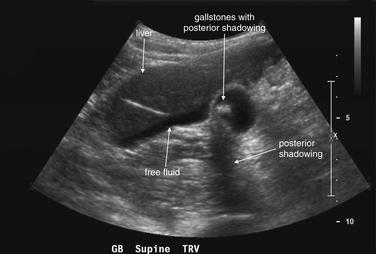
Figure 12-55 Heterotopic pregnancy with gallstones.
Same patient as in the previous figure. A clue that more may be occurring in this patient than symptomatic cholelithiasis is the presence of free fluid in the right upper quadrant.
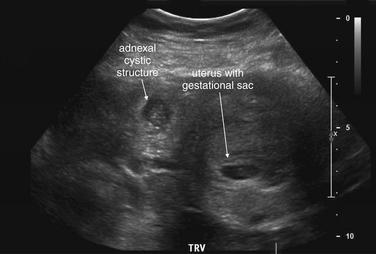
Figure 12-56 Heterotopic pregnancy with gallstones.
Same patient as in previous figures. This transverse view of the uterus shows an intrauterine pregnancy, as well as a cystic right adnexal structure, concerning for ectopic pregnancy.
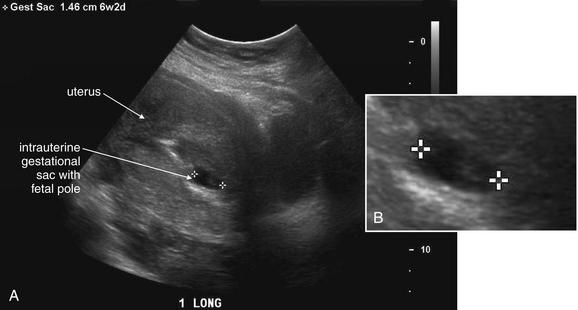
Figure 12-57 Heterotopic pregnancy with gallstones.
Same patient as in previous figures. A, This sagittal view of the uterus shows gestational sac and fetal pole consistent with an intrauterine gestation of 6 weeks, 2 days. B, Close-up.
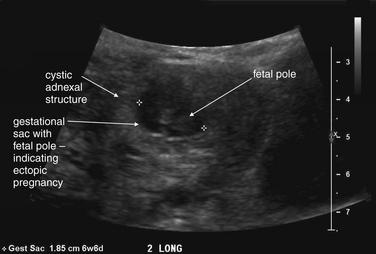
Figure 12-58 Heterotopic pregnancy with gallstones.
Same patient as in previous figures. This adnexal cystic structure contains a gestational sac with a fetal pole, consistent with an ectopic pregnancy when the fetus is approximately 6 weeks in size. Fetal cardiac activity was detected in this ectopic pregnancy.
Computed Tomography and Ectopic Pregnancy
CT is not the study of choice for ectopic pregnancy because of radiation exposure in the pregnant female. Occasionally, false-negative pregnancy testing or failure to obtain a pregnancy test before abdominal CT may lead to detection of ectopic pregnancy on CT scan. Findings may include an adnexal cystic structure, free pelvic and abdominal fluid consistent with hemoperitoneum, and active extravasation of IV contrast indicating active bleeding in the case of ruptured ectopic pregnancy (Figure 12-59).
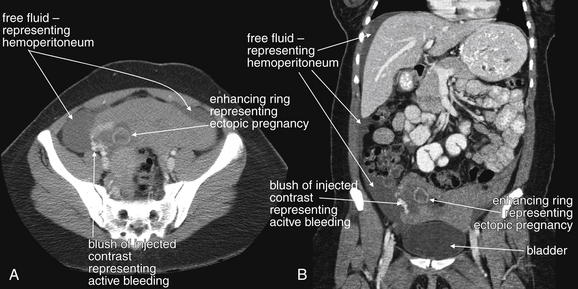
Figure 12-59 Ruptured ectopic pregnancy: CT with intravenous and oral contrast viewed on soft-tissue windows.
This 20-year-old woman underwent abdominal CT for suspected appendicitis, given a history of right lower abdominal pain. A urine pregnancy test was not obtained before CT scan. A and B, Free fluid representing hemoperitoneum is seen in the pelvis and extending to a perihepatic subdiaphragmatic location (compare this dark fluid density with the urine-filled bladder). A ringlike enhancing structure is seen in the right pelvis. A bright blush of contrast is seen that is not contained within vessels or bowel—this represents active bleeding from a ruptured ectopic pregnancy. It is bright because of active extravasation of contrast, whereas blood that accumulated before contrast injection is dark. At laparotomy, 2 L of hemoperitoneum were evacuated and active bleeding from the right Fallopian tube was noted. A 4-cm structure that looked like a gestational sac with a possible tiny embryo within it was also found free in the abdomen.
Ultrasound and Spontaneous Abortion (Miscarriage)
With first-trimester vaginal bleeding, once ectopic pregnancy has been excluded, ultrasound and examination findings are correlated to diagnose the patient with some form of spontaneous abortion (miscarriage). We briefly review some basic definitions here. A spontaneous miscarriage can be divided into five types, summarized in Table 12-4. First, a threatened abortion describes a pregnancy that may spontaneously terminate but has the potential to continue to a live birth. By definition, a live intrauterine pregnancy must be present, and the cervical os must be closed. Depending on the gestational age ultrasound can be used to measure and document fetal heart rate (Figure 12-60) in this case. An inevitable abortion describes a live intrauterine pregnancy with an open cervical os, which uniformly results in miscarriage when this occurs in the first trimester. Again, ultrasound can document fetal heart rate. A missed abortion indicates an intrauterine fetal demise (nonviable intrauterine pregnancy, with no fetal cardiac activity) with a closed cervical os. Incomplete abortion (Figure 12-61) indicates an intrauterine demise with an open cervical os and some products of conception remaining in the uterus—a miscarriage in progress. As the name suggests, complete abortion indicates that the uterus is now empty and the cervical os is closed or preparing to close. Combined with physical examination to assess the cervical os, ultrasound can distinguish these entities, with a few important caveats. A complete abortion may be impossible to distinguish from an ectopic pregnancy because both may have an elevated hCG and an empty uterus. At very low hCG values (below 2000 mIU/mL), an intrauterine pregnancy may be so small that it is not seen on ultrasound, so spontaneous miscarriage and ectopic pregnancy cannot be reliably differentiated in some cases. These patients must be carefully followed with repeat examinations and ultrasound.
TABLE 12-3 Ultrasound Findings and Ectopic Pregnancy
| Ultrasound Finding | Implications for Ectopic Pregnancy |
|---|---|
| Intrauterine Findings | |
| Gestational sac | Does not exclude ectopic pregnancy unless double-decidual sign is definitely seen |
| Gestational sac with yolk sac | Excludes ectopic pregnancy (by confirming intrauterine pregnancy) |
| Gestational sac with fetal pole | Excludes ectopic pregnancy (by confirming intrauterine pregnancy) |
| Extrauterine Findings With Empty Uterus | |
| Fetal pole with heartbeat | Confirms ectopic pregnancy (100% specific) |
| Extrauterine gestational sac and yolk sac or fetal pole | Confirms ectopic pregnancy (100% specific) |
| Any adnexal mass other than a simple cyst | Highly suspicious for ectopic pregnancy (99% specific, 84% sensitive) |
| Pelvic free fluid | Suspicious for ectopic pregnancy |
| hCG > 6000 mIU/mL, no IUP on TAUS | Suspicious for ectopic pregnancy |
| hCG > 2000 mIU/mL, no IUP on TVUS | Suspicious for ectopic pregnancy |
IUP, Intrauterine pregnancy; TAUS, transabdominal ultrasound; TVUS, transvaginal ultrasound.
Adapted from Brown DL, Doubilet PM. Transvaginal sonography for diagnosing ectopic pregnancy: Positivity criteria and performance characteristics. J Ultrasound Med 13:259-66,1994.
Figure 12-60 Measurement of fetal heart rate: Motion (M) mode ultrasound.
The familiar two-dimensional grayscale images of ultrasound are called brightness (B) mode. M mode images represent motion along a line and thus can be used to characterize moving structures such as the fetal heart. In the B mode image (top), a line is placed by the user through the position of the fetal heart. M mode is then selected, and the bottom image is generated over a period of several seconds. Fetal heart motion creates the sine wave seen in the M mode image (bottom). The user then places two markers at adjacent peaks or troughs in the waveform, and software calculates the fetal heart rate.
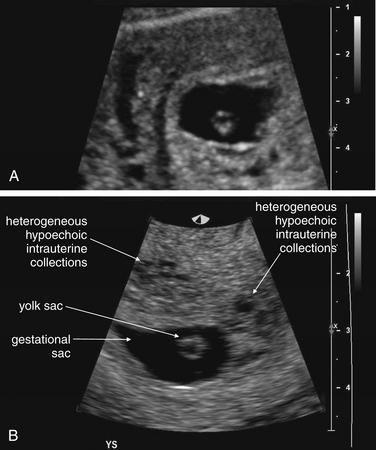
Figure 12-61 Intrauterine fetal demise: Pelvic ultrasound.
This 22-year-old woman presented with heavy vaginal bleeding and a human chorionic gonadotropin value of 13,660 mIU/mL, with a last menstrual period 2 months prior. A and B, An intrauterine gestational sac and yolk sac were noted, with no definite fetal pole found. Hypoechoic regions found within the uterus surrounding the gestational sac could indicate hemorrhage associated with a failed intrauterine pregnancy. Another consideration would be partial mole—though the patient ultimately miscarried and pathology did not show a molar pregnancy.
When an intrauterine pregnancy is confirmed in the first and second trimester, other common sonographic findings include subchorionic hemorrhage, which is associated with negative pregnancy outcomes.42-43
Imaging of Vaginal Bleeding in Second and Third Trimesters of Pregnancy
Ectopic pregnancy is an event of the first trimester, so vaginal bleeding in the second and third trimesters suggests potential pregnancy failure. Throughout pregnancy, vaginal bleeding may threaten not only the fetus but also the mother. Ultrasound is used to assess for fetal viability and for conditions that may lead to maternal morbidity and mortality: placenta previa and placental abruption. Other causes of vaginal bleeding outside of pregnancy may also rarely be the cause of bleeding in pregnancy, including fibroids and uterine or cervical malignancy. Domestic violence is common during pregnancy, so inflicted trauma including vaginal injury should be considered.
Placenta previa indicates positioning of the placenta over the cervical os. A placenta in this location, may lead to pathologic bleeding. Vasa previa refers to a placenta vessel running directly over the cervical os. Both conditions can be diagnosed by ultrasound. Because the position of the placenta moves away from the cervical os during the late third trimester, some placentas previa detected during the second trimester resolve during the third trimester. Imaging with ultrasound serves an important role. First, identification of placenta previa should lead to planned surgical rather than vaginal delivery because vaginal delivery is not possible without severe hemorrhage. Second, placenta previa and particularly vasa previa are contraindications to digital vaginal examination because manipulation of the cervix can result in hemorrhage from exposed vessels. The ultrasound findings are straightforward: a placenta and vessels overlying the cervix.
How Sensitive Is Ultrasound for Placental Abruption?
Placental abruption complicates 1% of pregnancies and can occur in the absence of significant abdominal trauma.44 Abruption after minor trauma such as falls from standing or even apparently spontaneous abruption may occur. Beyond 25 weeks of gestation, when the fetus is potentially viable, any suspicion for abruption warrants immediate investigation. The chief complaint may be vaginal bleeding, abdominal pain, or diminished fetal movement. Ultrasound is insensitive for the diagnosis of placental abruption (reported as low as 15% to 25% sensitivity) and cannot be used to rule out the diagnosis.45-46 A variety of sonographic appearances may occur depending on the time from symptom onset, from hyperechoic to isoechoic to hypoechoic—thus a hematoma from abruption may be indistinguishable from the adjacent placenta.47-49 Abruption may be an ongoing dynamic process, and a single normal ultrasound should not be reassuring. However, an abnormal ultrasound showing abruption, fetal bradycardia or tachycardia, or decreased fetal movement is extremely concerning. Diagnostic imaging thus can increase but not decrease concern for abruption. The specificity of ultrasound for abruption has been reported to be as high as 96%.45 Continuous fetal monitoring for 6 hours or more is typically advised because the most sensitive signs of placental abruption may be uterine irritability and contractions and fetal tachycardia or bradycardia.
Fetal viability can be assessed with ultrasound. Fetal movements can be directly visualized, and fetal heart rate can be recorded. The typical two-dimensional ultrasound images are generated using brightness (B) mode. Motion (M) mode can be selected to allow measurement of fetal heart rate. Most modern ultrasound machines allow a split-screen mode with both B and M modes shown. On the B mode image, a line is positioned through the fetal heart. The M mode image then displays a waveform corresponding to both the frequency and the amplitude of motion along that line. Calipers positioned at two consecutive peaks or troughs allow automatic calculation of heart rate (see Figure 12-60). The American College of Obstetricians and Gynecologists defines a normal fetal heart rate as 110 to 160 bpm. Fetal bradycardia or tachycardia outside of these limits is concerning.
Abdominal Pain in the Pregnant Patient
Abdominal pain in the pregnant patient requires a broad differential diagnosis, as with the nonpregnant patient. Complications of pregnancy, including ectopic pregnancy and spontaneous abortion, must be considered, with ultrasound used for assessment as described earlier. Endometritis should be considered, with imaging playing a limited role in its diagnosis, other than for assessment for intrauterine fetal demise, a risk factor for endometritis.
Once pregnancy-related considerations have been evaluated, conditions such as appendicitis and cholecystitis must be considered. Ultrasound can be used to assess for biliary disease with high sensitivity and specificity, as described in the chapter on abdominal imaging. Ultrasound and MRI can also be used to evaluate for appendicitis, as described in detail in the same chapter.
In general, ionizing radiation should be avoided when possible in the pregnant patient. However, many misconceptions about radiation and pregnancy exist. Regardless of the trimester, low levels of ionizing radiation likely contribute imperceptibly to the background rate of birth defects. While a truly safe threshold below which no effect occurs may not exist, the small contribution of medical radiation at low levels may pale in comparison to baseline levels of defects. Surveys of family physicians and obstetricians indicate that both groups overestimate the teratogenic effect of radiation from diagnostic imaging, with both groups believing in a significant risk from a single abdominal x-ray or CT scan. In reality, neither a single x-ray nor a single abdominal or pelvic CT scan is thought to convey sufficient radiation to increase teratogenic effects appreciably.50 This is discussed in more detail at the end of this chapter.
This is not to say that radiation should be used with impunity in pregnancy. Radiation risks are based largely on theoretical models, and the precise effects are not well delineated in humans. Moreover, other more subtle but important effects may occur, including effects on cognition and rates of childhood malignancies. Finally, although only a single diagnostic imaging test may be planned during a patient’s current medical evaluation, unforeseen events may lead to additional tests during the patient’s pregnancy, so limiting ionizing radiation is prudent. A good guideline is that whenever possible, diagnostic strategies that do not use ionizing radiation should be employed. These can include ultrasound, MRI, and observation with serial physical examinations. Rarely, laparoscopy may be a better choice than diagnostic imaging. If the mother’s health appears to be at significant risk, ionizing radiation should be used as needed.
Vaginal Discharge or Fluid Leak in the Pregnant Patient
In the first trimester, vaginal discharge or fluid leak may be normal or may be associated with vaginal or cervical infection. By the second and third trimesters, amniotic fluid leak must also be considered because this can lead to infection with fetal demise and maternal sepsis. Physical examination plays the largest role in this evaluation, but ultrasound can be used as an adjunct. Fetal viability assessment with ultrasound can be performed as described earlier. In addition, the quantity of amniotic fluid can be assessed with ultrasound. Formal measurements can be obtained and compared with standard definitions for polyhydramnios (>2000 mL, or single fluid pocket ≥8 cm in depth) and oligohydramnios (<500 mL, or no single fluid pocket ≥2 to 3 cm in depth) at 32 to 36 weeks of gestation. Bedside ultrasound by emergency physicians can provide a rough estimate of amniotic fluid levels. Low levels can be associated with intrauterine growth retardation. Oligohydramnios (Figure 12-62) may be a result of fetal anomalies including urinary obstruction or renal agenesis or, more emergently, of poor fetal perfusion or rupture of amniotic membranes with amniotic fluid leak. Polyhydramnios may result from multiple gestations, fetal gastrointestinal obstruction such as esophageal atresia, or fetal hydrops.51
Recently Pregnant Patient
Abdominal pain, vaginal bleeding, or vaginal discharge in the recently pregnancy female should prompt consideration of retained products of conception and endometritis. Ultrasound can be used to assess for this condition. Fluid and solid debris within the uterus can support this diagnosis.
In the patient who has undergone pregnancy-related surgical procedures, peritonitis and abscess should be considered. Dilatation and curettage can rarely result in uterine perforation and peritonitis. Caesarean section can be complicated by abscess, bowel perforation, or injuries to the bladder and ureters. The litany of other causes of abdominal pain should also be considered. Ultrasound can be used to assess for free pelvic fluid. CT scan may be a better choice because of the wide differential diagnosis. CT can identify abnormalities of bowel, bladder, and ureters, as well as abscesses. If time allows, oral and IV contrast should be used because of the broad differential diagnosis. IV contrast alone can be used to allow enhancement of inflammatory or infectious processes.
Septic pelvic thrombophlebitis can complicate third-trimester pregnancy. It frequently presents following delivery, with fever, pelvic pain, and vaginal discharge. The diagnosis can be difficult with CT and ultrasound. MRI with gadolinium contrast can be used to confirm the diagnosis.
Diagnostic Imaging of Genitourinary Complaints in the Nonpregnant Female
Many of the same principles of imaging described earlier apply to the nonpregnant female. Always reconfirm pregnancy status because failure to recognize pregnancy can lead to missed ectopic pregnancy, as well as use of ionizing radiation in pregnancy.
Vaginal Bleeding in the Nonpregnant Female
Once pregnancy has been excluded, vaginal bleeding can be a sign of normal menses, dysfunctional uterine bleeding, ovarian cysts including polycystic ovarian syndrome, uterine leiomyomas (fibroids), uterine or cervical malignancy, or pelvic infection. In many cases, diagnostic imaging is not needed. Painless bleeding generally does not require emergency imaging in the premenopausal patient. In the postmenopausal patient, vaginal bleeding is always abnormal and may indicate malignancy. The workup usually includes endometrial biopsy. Imaging may be performed but is not generally required on an emergency basis.
Ultrasound and CT can both be used for assessment of pelvic pathology. Ultrasound is a reasonable first choice in many cases because it avoids radiation exposure and does not require administration of IV contrast, with its potential for allergy and nephrotoxicity. Ultrasound is the preferred diagnostic modality for some conditions because it provides dynamic information about blood flow. CT of the pelvis can be diagnostically ambiguous without contrast agents because many pelvic soft tissues have similar density. In thin patients, this problem can actually be worsened by the paucity of fat, which normally separates soft tissues and has a lower density, allowing fat to act as a form of intrinsic contrast.
The ovaries are generally readily imaged by transvaginal ultrasound. Ovarian follicles and cysts (Figures 12-63 to 12-66) are discussed later in the section on abdominal pain. Ovarian mass lesions and malignant ascites may be seen.
Figure 12-63 Normal follicles in ovary.
This 37-year-old female presented with negative pregnancy test and right lower abdominal pain. A, B, Her left ovary shows normal peripheral follicles. Characteristics include the absence of internal septations and small size (roughly 0.5 cm each).
Figure 12-64 Ovarian cystic disease.
Same patient as in the previous figure. A, B, Her ultrasound images are consistent with a hemorrhagic ovarian cyst. Characteristic features include internal septations and echoes. These suggest blood products within the ovary that have coagulated. Simple cysts typically have no internal echoes or septa. No calcifications are seen—these would produce shadowing deep to the ovary.
Figure 12-65 Ovarian cystic disease: Computed tomography (CT) with intravenous contrast on soft-tissue windows.
Same patient as in the previous figures. She has a history of hysterectomy and bilateral salpingectomy. A, B, A peripherally enhancing cystic lesion is seen in the right pelvis, likely a corpus luteum. There is also a small amount of hyperdense fluid in the right and mid lower pelvis, likely hemoperitoneum. Ultrasound of the patient showed a hemorrhagic ovarian cyst. Other images from the patient’s CT showed a normal appendix.
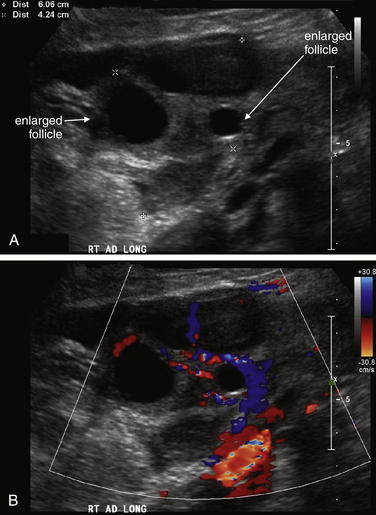
Figure 12-66 Hyperstimulated ovary.
This 34-year-old female presented with abdominal pain and vomiting. The patient was 8 weeks pregnant after undergoing gonadotropin ovarian hyperstimulation therapy. A and B, The right ovary is enlarged, measuring at least 7.7 × 4.6 cm with hypoechoic structures consistent with follicles. Both arterial and venous blood flow were documented in the ovary. This is consistent with ovarian hyperstimulation. The left ovary had a similar appearance.
Uterine leiomyomas (fibroids) and malignant lesions are usually seen well on ultrasound. Fibroids may be exophytic or intramural, or it may project into the uterine cavity. Fibroids are usually well circumscribed, whereas malignant lesions may invade or adhere to surrounding structures. CT with IV contrast is useful in investigating extent of disease when malignancy is suspected. Metastatic lesions of the liver and malignant ascites may be seen. IV contrast leads to enhancement of metastatic lesions. Ultrasound may also demonstrate ascites, raising the possibility of malignancy.
Abdominal or Pelvic Pain in the Nonpregnant Female
In the nonpregnant patient with abdominal pain, an extensive differential diagnosis exists. Imaging of abdominal pain is described in detail in the dedicated chapter on abdominal imaging. Here, we discuss specific gynecologic pathology, although in reality an individual patient often requires consideration of a diverse range of pathology. The female patient with right lower-quadrant pain is a classic example, where appendicitis, large- and small-bowel pathology, ovarian pathology, and urinary pathology may all require evaluation. Again, after assessing clinical stability the next step in diagnostic planning is to reassess pregnancy status to avoid missing ectopic pregnancy. Once pregnancy has been excluded, two important forms of pelvic pathology deserve investigation: ovarian torsion and tuboovarian abscess. Other forms of gynecologic pathology may require imaging for diagnosis but are generally less emergent, including ovarian cysts, endometriosis, uterine fibroids, and pelvic malignancies.
Ovarian Torsion
Ovarian torsion (Figures 12-67 and 12-68) should be strongly considered in any patient with acute onset of lower abdominal pain, particularly when it is severe and accompanied by other signs and symptoms, such as diaphoresis or vomiting. Ultrasound is the preferred diagnostic test, but as we discuss later, the presence of blood flow detected by ultrasound does not rule out ovarian torsion. Like testicular torsion, ovarian torsion is time dependent, and delay in diagnosis and treatment can result in ovarian necrosis and loss. When ovarian torsion is strongly considered, gynecologic consultation should occur before or concurrent with diagnostic imaging.
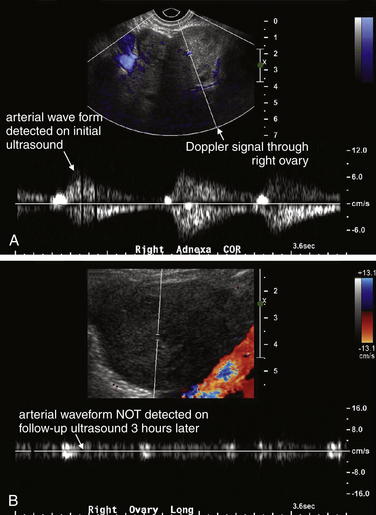
Figure 12-67 Ovarian torsion: Ultrasound.
A and B, This patient was initially found to have normal arterial and venous flow in the right ovary, but the emergency physician remained concerned for ovarian torsion given the abrupt onset of severe lateralizing pelvic pain. Repeat ultrasound showed only dampened venous flow. The patient was confirmed to have ovarian torsion at laparotomy, with the ovary twisted four complete turns on its vascular pedicle. This case demonstrates an essential point for the emergency physician. Ultrasound has been reported to have a sensitivity for ovarian torsion of only approximately 60%. When torsion is strongly suspected, a normal ultrasound should not be interpreted as ruling out torsion.
Figure 12-68 Ovarian torsion confirmed in the operating room: CT with IV contrast on soft-tissue windows.
A, There is a heterogeneous structure within the right lower quadrant that measures 6.0 × 5.0 cm, which is concerning for an enlarged right ovary. Ovarian torsion or mass are two important considerations given this appearance. An abnormally enlarged ovary is at increased risk for torsion. In addition, ovarian torsion of an initially normal ovary results in engorgement of the ovary and an enlarged appearance on imaging. B, Close-up.
Transvaginal ultrasound assesses for arterial and venous blood flow, as well as nonspecific ovarian abnormalities such as cystic and solid ovarian lesions. Complete absence of arterial and venous blood flow by Doppler ultrasound is highly specific for the diagnosis of ovarian torsion. Conversely because normal ovaries without asymmetry or enlargement relatively rarely undergo torsion, a normal ovary (lacking cysts or masses) with normal arterial and venous blood flow nearly rules out ovarian torsion. Unfortunately, intermediate ultrasound results can be nondiagnostic and may fail to diagnosis ovarian torsion. Early in the course of torsion, low-pressure venous blood flow may be lost or diminished by twisting of the vascular pedicle, whereas higher-pressure arterial blood flow may be preserved. Later, arterial blood flow may diminish or be lost. In addition, the hypoperfused ovary may swell and appear enlarged on ultrasound. Preexisting ovarian cysts or masses may also be seen.
Ultrasound with Doppler evaluation of venous and arterial blood flow is the standard imaging modality for evaluation of suspected ovarian torsion (see Figure 12-67). However, multiple small studies suggest that the sensitivity of ultrasound is only moderate and that either arterial or venous flow may be present in a torsed ovary. Dane et al.52 reported blood flow was present in 6 of 21 cases (28%). Shadinger et al.53 reviewed 39 cases of pathologically proven ovarian torsion and found that 13 (33%) had venous flow and 21 (54%) had arterial flow. Pena et al.54 reported normal Doppler findings in 6 of 10 patients with proven ovarian torsion. Pena54 also noted delays in diagnosis when flow was present, likely reflecting undue confidence by physicians in use of ultrasound to rule out ovarian torsion. Although no large studies have examined ultrasound for the diagnosis of ovarian torsion, it appears that the sensitivity of abnormal blood flow by ultrasound is probably between 50% and 70%, quite inadequate to rule out the diagnosis.
Are Other Ultrasound Findings Useful in Evaluation for Ovarian Torsion?
Ovarian enlargement is reportedly a sensitive, though nonspecific, finding in ovarian torsion.53 Kiechl-Kohlendorfer et al.55 reported fluid–debris levels in 11 of 13 (85%) ovarian torsion cases, though the specificity of this finding is uncertain.
When ovarian torsion is strongly suspected, gynecologic consultation should be pursued despite negative ultrasound findings. Although diagnostic strategies such as repeat ultrasound or CT scan can be employed, diagnostic delay can result in ovarian loss. In some cases, laparoscopy to visually inspect the ovary is preferable.
CT in ovarian torsion
The sensitivity and specificity of CT scan for ovarian torsion (see Figure 12-68) are not well studied. CT scan can demonstrate abnormal patterns of enhancement with IV contrast, as well as cystic and solid masses of the ovary. Case reports of ovarian torsion detected on unenhanced and enhanced CT describe an enlarged ovary, often displaced from the normal position, hyperdense compared with the normal ovary, and containing hypodense cystic regions. In a torsed cystic ovary, the cyst wall may exceed 10 mm in thickness. If IV contrast is given, the ovary may fail to enhance.56 The uterus may be deviated toward the torsed ovary. Free fluid may also be seen.57-61 Rarely, gas within the vessels of a torsed ovarian tumor may be seen.62
Tuboovarian Abscess
Tuboovarian abscess is an important cause of pelvic pain and can be evaluated with ultrasound or CT (Figures 12-69 and 12-70). Transvaginal ultrasound is more sensitive than is transabdominal ultrasound for this indication.63 Ultrasound findings include an enlarged ovary and fallopian tube, often with multiple loculated, fluid-filled regions. Echogenic debris with a fluid–fluid level may be visible within the structures. High blood flow may be seen using Doppler ultrasound. These findings are not specific for tuboovarian abscess.64 Because ultrasound cannot distinguish among blood, pus, and serous fluids, ultrasound does not prove the diagnosis of tuboovarian abscess. Sterile hydrosalpinx, hemosalpinx, and complex ovarian cysts could have similar appearances. A torsed ovary could have a similar ultrasound appearance, although confirmation of normal blood flow decreases the likelihood of this diagnosis. Overall, ultrasound findings can support the diagnosis of tuboovarian abscess, with clinical correlation required. Positive ultrasound findings in the context of some combination of adnexal pain and tenderness, fever, leukocytosis, and culture confirmation of pelvic infection confirm the diagnosis. A normal ultrasound is a reassuring sign that tuboovarian abscess is unlikely.
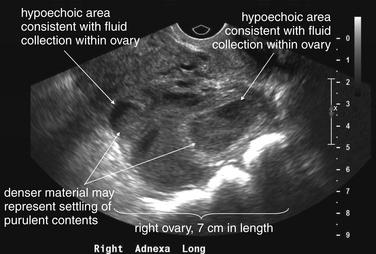
Figure 12-69 Tuboovarian abscess: Ultrasound.
This 30-year-old woman presented with exquisite right lower abdominal pain. She later tested positive for Trichomonas and Neisseria gonorrhea. Ultrasound shows a 7 × 6.5 × 5.6 cm right ovarian mass with multiple heterogeneous hypoechoic areas, suggesting internal fluid collections of varying density. Both arterial and venous flow were normal. These findings are suggestive of tuboovarian abscess.
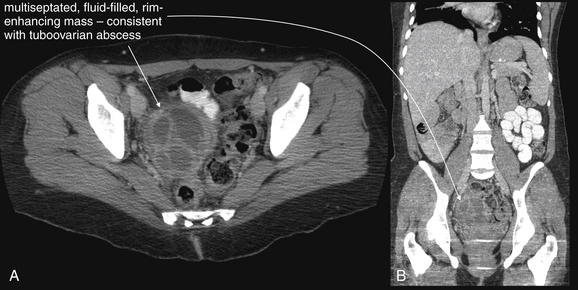
Figure 12-70 Tuboovarian abscess: CT with oral and IV contrast.
Same patient as in the previous figure. CT with oral and IV contrast demonstrates a 7.4 × 6.4 cm right adnexal mass with multiple septations and fluid-filled cavities, concerning for tuboovarian abscess. The rim enhances with IV contrast, which is typical of infectious abscess. The abscess was treated with CT-guided percutaneous drainage and IV antibiotics. A, Axial image. B, Coronal reconstruction.
CT scan demonstrates similar findings of loculated ovarian and fallopian tube fluid collections.65-67 In addition, hydroureter, increased presacral fat density (fat stranding), and increased presacral ligament density may be seen, though these are not specific for tuboovarian abscess.65,67-68 These can be recognized without any form of contrast. IV contrast leads to rim enhancement of a tuboovarian abscess, helping to confirm the diagnosis, whereas fluid collections without infection or inflammation would not be expected to enhance. Oral contrast is not necessary, although it is frequently given to assist in the differential diagnosis of lower-quadrant abdominal pain, which can include appendicitis, diverticulitis, or inflammatory bowel disease. The role of oral contrast in abdominal CT is discussed in more detail in the chapter on imaging of nontraumatic abdominal complaints.
More than one diagnostic strategy for suspected tubo-ovarian abscess may be appropriate, depending on the differential diagnosis, the age of the patient, and the most important consideration for the specific patient, such as speed of diagnosis, accuracy, cost, or limitation of radiation exposure. For example, in an older patient where radiation exposure is of limited importance and conditions such as appendicitis, diverticulitis, or vascular pathology might cause lower abdominal pain, CT may be the most appropriate initial test. In a younger, sexually active patient, where radiation exposure is a significant concern, it may be appropriate to begin the diagnostic workup using ultrasound, proceeding to CT scan if ultrasound is nondiagnostic. No formal decision rules exist for imaging patients for suspected tuboovarian abscess. One study suggests that an erythrocyte sedimentation rate greater than 50 mm per hour may predict tuboovarian abscess, but large and rigorous studies of this condition are lacking.69
Pelvic inflammatory disease (PID) may be difficult to distinguish clinically from tuboovarian abscess or ovarian torsion. Imaging is typically directed at ruling out these alternative diagnoses. Ultrasound or CT may show free fluid in the pelvis. CT may show inflammatory changes of fat surrounding pelvic structures, without focal or loculated fluid collections. Imaging may also be normal in the setting of PID, so treatment should be based on the overall clinical presentation. Normal imaging does not rule out PID because the sensitivity of ultrasound and CT are not known.
Ovarian cysts and masses such as dermoids (teratomas) (Figures 12-71 to 12-73) or ovarian cancer can cause abdominal or pelvic pain. Imaging in the emergency department is again aimed at detecting conditions such as tuboovarian abscess, ovarian torsion, or nongynecologic conditions such as appendicitis requiring emergency surgery, percutaneous drainage, or antibiotics. The choice of initial imaging study (ultrasound, CT, and rarely, MRI) depends on the most urgent potential surgical concern, with ovarian cyst being a diagnosis of exclusion in most cases. Rarely, ovarian cyst rupture can lead to significant hemoperitoneum, which can be detected by ultrasound or CT. Both modalities may demonstrate important mass lesions requiring follow-up. Dermoid tumors (see Figures 12-71 to 12-73) classically contain multiple tissue types derived from ectoderm, including skin, fat, hair, and even calcifications resembling teeth. These are visible on ultrasound as often-complex solid lesions. Calcifications or teeth are dense and cast acoustic shadows on ultrasound. On CT scan, low-density fat within dermoids is readily seen without any form of contrast; calcifications also are visible without contrast. Dermoids require follow-up and typically are electively removed because of a small risk for malignant transformation (1% to 2%). They may bleed internally, causing pain, and torsion of a dermoid may occur.70 Simple ovarian cysts are usually recognized on ultrasound or CT as simple fluid collections without loculations. On ultrasound, these appear hypoechoic. On CT, they are low-attenuation fluid density, with Hounsfield units around or slightly greater than zero (water density). Polycystic ovaries can be seen on ultrasound or CT scan. Ovarian malignancy can be detected by ultrasound and CT, but differentiation from benign lesions is difficult except in advanced cases in which local invasion or metastasis has occurred. Solid ovarian masses seen by ultrasound or CT require follow-up because malignancy is always a possibility.
Figure 12-71 Dermoid with calcifications: Noncontrast CT on soft-tissue windows.
This 46-year-old woman presented with right lower-quadrant pain and a negative pregnancy test. A CT scan was performed to evaluate for possible renal colic. A, B, Within the pelvis, a lobulated mixed attenuation mass (predominately fat attenuation, with soft-tissue components and punctate calcific foci) measures at least 8.1 × 5.2 cm. This is consistent with a dermoid cyst (teratoma). There is a small to moderate amount of free fluid within the pelvis.
Same patient as in the previous figure. In the expected location of the right adnexa, a large heterogeneous hyperechoic bi-lobed structure measures at least 8.3 × 4.3 × 6.7 cm. This demonstrates posterior shadowing because of internal structures (calcifications), seen best on CT scan in the previous figure. Free fluid is seen surrounding the structure, as on the prior CT.
Figure 12-73 Dermoid: CT with IV contrast, soft-tissue windows.
This 25-year-old woman presented with right lower abdominal pain and underwent CT, which showed a normal appendix. A, B, Incidentally, a 5.9 × 4.1 cm fat-containing lesion was identified in the left adnexa, most compatible with a dermoid. Dermoid cysts are neoplasms, usually benign but with rare potential for malignant transformation. Because they are derived from tissues with the ability to differentiate into multiple ectoplasm tissues, they frequently contain fat, skin, hair, or even teethlike structures. Typical features in this patient include a well-circumscribed appearance and low density. Because the structure is largely composed of fat, it is less dense than the adjacent bladder, which is filled with urine. Compare with normal subcutaneous fat. The window level has been adjusted from a typical soft tissue setting to make fat appear brighter.
Fibroids (Figures 12-74 and 12-75) and uterine malignancy may cause pelvic pain. Imaging is key to diagnosing these conditions, but in most cases emergency imaging is directed at ruling out ovarian torsion or tubo-ovarian abscess. Ultrasound and CT can demonstrate fibroids or uterine malignancy. Imaging does not reliably distinguish a fibroid from an early malignancy—for this reason, patients should always be advised to follow up, and the possibility of malignancy must be considered. Doppler ultrasound is somewhat more specific for malignancy than is conventional ultrasound, but it cannot rule out malignancy.71-73 MRI can provide additional differentiation but is not completely specific.74 Advanced malignancy may demonstrate invasion or entrapment of adjacent structures, as well as ascites or metastasis. In contrast, fibroids remain confined to the uterus and do not invade locally or remotely. Fibroids can be exophytic but remain bounded by the uterine serosa.
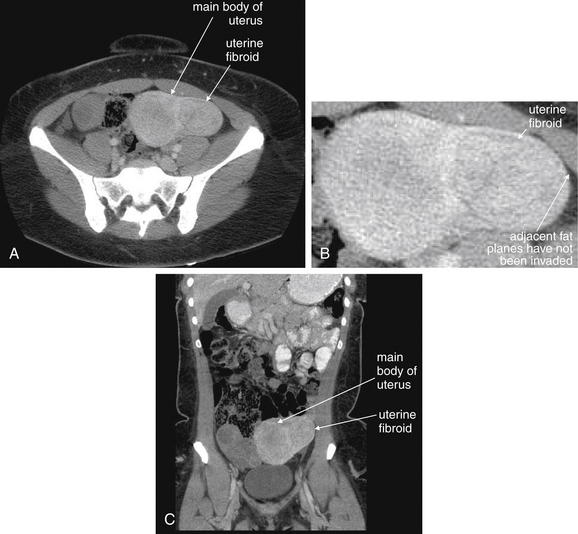
Figure 12-74 Fibroid uterus: CT with IV contrast on soft-tissue windows.
A through C, Although ultrasound is the primary modality used for evaluation of uterine fibroids (leiomyomas), these benign neoplasms are often detected on CT scan performed for evaluation of abdominal pain. Typical features are a well-circumscribed appearance and enhancement with contrast. Because uterine malignancies can also occur, a careful search of the abdomen for lymphadenopathy is required. Malignant uterine tumors may invade adjacent structures, whereas a fibroid is contained within the uterine serosa. Uterine tumors, both benign and malignant, can show central necrosis, which has a nonenhancing, dark appearance. A, axial image. B, close-up from A. C, coronal image.
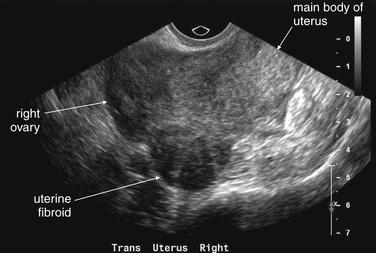
Figure 12-75 Fibroid uterus: Endovaginal ultrasound.
Ultrasound is the primary modality used for evaluation of uterine fibroids (leiomyomas). Typical features include a well-circumscribed appearance. Fibroids may be hypoechoic or hyperechoic relative to the uterus. They may be exophytic or intramural, or they may project into the uterine cavity. Malignant uterine tumors may invade adjacent structures, whereas a fibroid is contained within the uterine serosa. Uterine tumors, both benign and malignant, can show central necrosis, which usually appears hypoechoic with ultrasound. In this 38-year-old woman, the fibroid is exophytic. The right ovary lies adjacent and is difficult to distinguish in this case.
Endometriosis is not easily detected by imaging. Findings are nonspecific and can include pelvic free fluid, which has a broad differential diagnosis, including normal physiologic free fluid, blood, ascites, and infection. Ectopic endometrial tissue itself may be seen by either ultrasound or CT, but the appearance is nonspecific, with a broad radiographic differential diagnosis.75 The diagnosis is usually confirmed laparoscopically. Imaging in the emergency department is usually aimed at evaluating for other acute surgical disease, rather than detecting or excluding endometriosis.
Vaginal Discharge in the Nonpregnant Patient
In the nonpregnant patient, vaginal discharge can be a finding of vaginal or pelvic infection or, rarely, malignancy. In the absence of adnexal pain or tenderness, focal infection such as PID or tuboovarian abscess is unlikely. Retained vaginal foreign bodies are possible but are usually detected by physical examination, not imaging. Imaging is usually not required for isolated vaginal discharge in the nonpregnant female.
Imaging of Genitourinary Trauma
Following blunt trauma, injuries to the kidneys, ureters, bladder, urethra, scrotum, and uterus and vagina are possible. The imaging evaluation depends on the presentation. Common presentations are blunt or penetrating abdominal trauma with or without flank pain, traumatic hematuria, traumatic vaginal bleeding, direct perineum or scrotal injury, and abdominal or pelvic trauma in pregnancy.
Blunt Abdominal or Pelvic Trauma With Suspected Genitourinary Injury
Blunt torso trauma can result in injury to the kidneys, ureters, and bladder, best diagnosed with CT (described in more detail later). In the patient with blunt trauma, genitourinary injuries are rarely immediately life threatening, so patient stabilization and diagnosis of more imminently dangerous injuries should take priority. Significant traumatic injuries to the kidneys usually do not occur from low-energy trauma—even from direct blows, as may occur in an assault. Instead, major renal lacerations are usually found in the setting of high-energy trauma, such as falls from great height or deceleration injuries in motor vehicle collisions. Renal injuries are often noted incidentally, accompanying major injuries to the liver, spleen, pelvis, and spine. Many renal injuries require no specific treatment, exceptions being those involving the renal pedicle because these may threaten renal blood supply, result in major hemorrhage, or disrupt the ureter. The decision to image a patient to identify a traumatic renal injury should be made largely on the basis of other suspected injuries. Traumatic ureteral injuries occur in the same setting of major trauma. CT with IV contrast can identify these injuries, with additional delayed images obtained to allow time for renal excretion of injected contrast. Contrast leak from the ureters confirms injury. Bladder injuries are discussed later but usually occur in the setting of pelvic fracture. In patients receiving anticoagulation, retroperitoneal hematomas may occur with relatively minor trauma and are diagnosed by CT.
Traumatic Hematuria and Other Indications for Genitourinary Imaging
Gross traumatic hematuria may indicate injury to the kidneys, ureters, bladder, or urethra. The likely source of bleeding can be deduced from a combination of history, physical examination, and simple imaging studies. Additional imaging is then performed to evaluate selected portions of the urinary system, as described later.
Isolated traumatic microscopic hematuria (without significant abdominal, flank, or pelvic pain) in adults generally does not require specific imaging evaluation. If the patient does not require torso imaging for considerations such as spleen, liver, or bowel injury, microscopic hematuria alone should not drive renal imaging for trauma in adults because the injuries identified are generally low-grade renal contusions that do not require therapy. An example would be a stable patient struck in the flank by a baseball and noted to have microscopic hematuria on urinalysis. The patient would require imaging only if concerns existed for significant injuries to other organs, such as the spleen. In children, microscopic hematuria is considered an independent indication for imaging evaluation, as discussed later.
Miller and McAninch76 found only three significant renal injuries in 1588 adult blunt trauma patients with microscopic hematuria and no hemodynamic shock; consequently, they recommend against imaging for renal injury. The authors recommend imaging only for gross hematuria, shock, or other suspected abdominal injury. The case in pediatric patients is less clear. Abou-Jaoude et al. found that a threshold of more than 20 red blood cells per high-power field would have missed 28% of patients with a spectrum of renal anomalies, including minor and major trauma and congenital anomalies. However, this threshold would have missed only 2 of 8 patients with major injuries, out of 100 patients evaluated. Despite the investigators’ warnings, microscopic hematuria with less than 20 red blood cells per high-powered field had a negative predictive value of 98% for significant renal injury.77 Morey et al.78 found a low incidence of significant renal injuries in children (2%) with fewer than 50 red blood cells per high-power field.
Which Patients Require Imaging for Bladder and Urethral Injury?
Traditionally, microscopic hematuria greater than 50 red blood cells per high-power field was considered an indication for genitourinary imaging for renal, ureteral, bladder, and urethral injury.79 More recent studies in adults suggest that gross hematuria, not microscopic hematuria, is the indication for renal,76 bladder, and urethral imaging. In children, microscopic hematuria (>20 red blood cells per high-power field) is still cited as an indication for genitourinary imaging for trauma. Although pelvic fractures are associated with bladder injuries, when gross hematuria is absent, pelvic fractures alone do not appear to predict bladder and urethral injuries.
Morey et al.80 investigated risk factors for bladder injury to establish guidelines for imaging. The authors point out that imaging studies for suspected genitourinary injury add time to the patient workup, which could adversely affect patients requiring rapid operative repair of other injuries. The authors performed a retrospective chart review spanning a 4-year period using the trauma registries from four level-I trauma centers. They identified 53 patients (37 male and 16 female) with bladder rupture from blunt trauma. All presented with gross hematuria. Blood at the urethral meatus was identified in 6 patients (11%), all with coexisting urethral injuries. In addition, 45 patients (85%) had pelvic fractures. The authors suggest revision of older guidelines that called for cystography in all patients with either gross or microscopic hematuria. They propose mandatory cystography in all patients with gross hematuria with pelvic fracture. They argue that gross hematuria without pelvic fracture and microscopic hematuria with or without pelvic fracture are only relative indications, requiring imaging only in the presence of other findings, such as suprapubic pain and tenderness, inability to void, perineal hematoma, blood at the urethral meatus, free fluid on CT, or altered mental status.
Fuhrman et al.81 also found gross hematuria to be the only indication for lower genitourinary imaging because they found no bladder injuries in 120 patients without gross hematuria, whether or not pelvic fractures were present. Bladder injuries were found only in the 31 patients with gross hematuria, one quarter of whom had pelvic fractures. The authors argue against cystography in the absence of gross hematuria, even when pelvic fractures are present.81 Pao et al.82 found that the absence of pelvic free fluid on routine trauma CT (without CT cystography) made bladder rupture unlikely, though its presence was not specific for bladder injury.
CT Scan Evaluation of Suspected Renal and Ureteral Injury
Renal and ureteral injuries are possible whenever major blunt abdominal or torso trauma has occurred. In the setting of gross hematuria, a renal and ureteral injury is the likely source in the absence of a pelvic fracture, which is usually required for bladder or urethral injury to occur. CT for evaluation of renal and ureteral trauma is performed with IV contrast, just as for other blunt traumatic abdominal injuries (Figure 12-76). Extravasation of contrast during the initial CT indicates active bleeding from a renal parenchymal or an arterial or venous injury. Extravasated contrast appears as an amorphous bright white blush, often surrounded by a darker collection representing retroperitoneal blood that has already escaped from the kidney before administration of contrast (see Figure 12-16). This dark fluid collection may be subcapsular, extrarenal, or contained within the renal parenchyma as a contusion or renal laceration. A kidney that does not enhance with injected contrast during this initial phase is poorly perfused and may represent a complete renal pedicle avulsion requiring emergency repair or nephrectomy (see Figure 7-84 for example of poor renal perfusion resulting from aortic dissection).
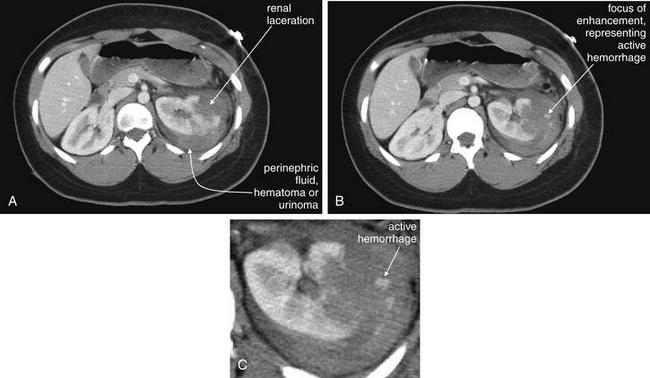
Figure 12-76 Renal laceration: CT with IV contrast on soft-tissue windows.
A, B, CT through the level of the kidneys. C, Close-up from B. Renal injuries often occur in the context of other significant torso trauma. In this CT scan with IV contrast, the majority of the left kidney is seen to enhance normally with contrast, excluding a significant vascular injury to the pedicle. The lateral left kidney has a deep laceration with surrounding perinephric hypoattenuated fluid. This represents blood that had already accumulated before the moment of CT scan acquisition—although urine from a renal pelvis or ureteral injury would have a similar appearance. Small foci of enhancement are seen beyond the apparent margin of the kidney within the perinephric hematoma. These represent small areas of continuing hemorrhage at the moment of CT scan. Delayed images were performed to assess for possible urine leak, but none was noted. Urine leaks are discussed in the next figure.
Hypoattenuated (dark) perinephric fluid may also represent urine that has leaked from an injury to the renal pelvis or ureter. To evaluate this, additional CT images are obtained following a short delay, allowing time for injected contrast to be filtered and excreted by the kidneys. These CT nephrograms and ureterograms provide important information by demonstrating renal perfusion and by allowing contrast to fill the ureters. Contrast extravasation from the ureter into the retroperitoneum indicates ureteral injury (Figures 12-77 and 12-78), which is then investigated with retrograde urography using a cystoscope (Figure 12-79). In this procedure, a cystoscope is inserted into the bladder, the affected ureter is cannulated, and contrast is injected through the ureter to the kidney to identify the location of a discontinuity or leak. Ureteral injuries may be treated by placement of a stent or with surgery.
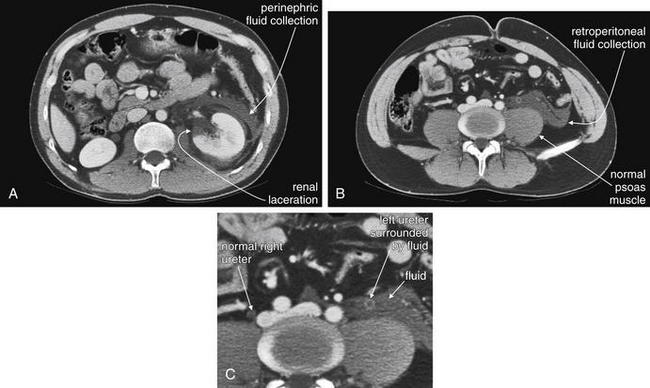
Figure 12-77 Ureteral or renal pelvis injury.
A, CT through the level of the kidneys. B, CT just above the iliac crest. C, Close-up from B. This 30-year-old man was ejected from a vehicle. Initial CT images with IV contrast show a left perinephric fluid collection and evidence of renal contusion. Is the perinephric fluid blood, urine, or both? These initial images (obtained when the injected contrast was within arteries) do not show extravasation of contrast, suggesting that active bleeding was not occurring. Delayed CT images (obtained several minutes later, when contrast was being excreted by the kidneys) were obtained to evaluate for urine leak (next figure).
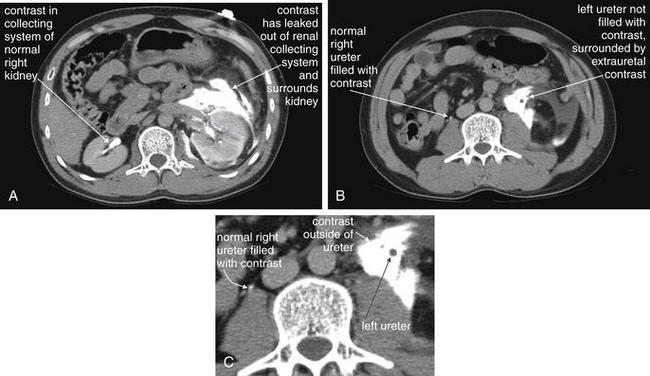
Figure 12-78 Ureteral or renal pelvis injury.
A, CT through the level of the kidneys. B, CT just above the iliac crest. C, Close-up from B. Same patient as in the previous figure. These delayed CT images (obtained several minutes later, when intravenously injected contrast is being excreted by the kidneys) now show contrast collecting around the kidney and in the retroperitoneal space anterior to the psoas. The right ureter is filling normally with or contrast. The left ureter is not filled with contrast and is seen as a filling void (dark), surrounded by retroperitoneal contrast (bright white). A leaking renal pelvis or ureter is the likely culprit. Retrograde urography was performed by the urology service, demonstrating leaking contrast from the renal pelvis (next figure).
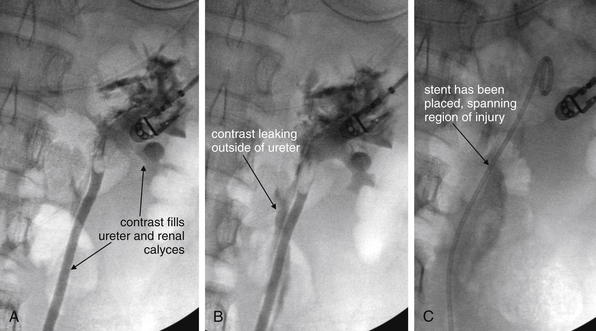
Figure 12-79 Ureteral or renal pelvis injury: Retrograde urography.
Same patient as in the previous figures. CT images had demonstrated an apparent urine leak. A, Retrograde urography (injection of contrast material into the ureter from the ureteral orifice in the bladder using a cystoscope) shows filling of the ureter and renal pelvis. B, A moment later, contrast is seen accumulating outside of the renal pelvis and ureter, indicating a urine leak. C, A stent has been placed to treat the apparent injury to the proximal ureter and renal pelvis.
Suspected Bladder Injury
Hematuria may also indicate bladder injury, which usually occurs in the setting of pelvic fracture. Standard CT with IV contrast yields several clues to this diagnosis. First, pelvic fractures are detected with great sensitivity using bone windows from standard abdominal-pelvic CT or reconstructions using specialized bone algorithms. Second, a pelvic hematoma often accompanies pelvic fracture. This hematoma may exert mass effect on the bladder, compressing and deforming the bladder from its usual symmetrical shape and midline position. Extravasation of urine from the bladder into the peritoneum (intraperitoneal rupture) or outside of the peritoneum (extraperitoneal rupture) may be suggested by the presence of low-density fluid in the pelvis or abdomen, but it may be difficult or impossible using the standard CT images to be certain that this fluid is urine. Measurement of the density of the fluid in Hounsfield units (HU) may demonstrate the fluid to be less dense than blood. Urine should be water density, or 0 Hounsfield units. Blood varies in density but is typically between 30 and 50 HU. Preexisting ascitic fluid can be indistinguishable from urine on CT, with a density near 0 HU. Additional CT images can confirm bladder rupture. This study, a CT cystogram (Figures 12-80 and 12-81), takes the place of the conventional cystogram used in the past to detect bladder injury. CT cystogram is best explained by comparison with a conventional cystogram.
Figure 12-80 Bladder rupture: Extraperitoneal.
This 18-year-old man was an unrestrained driver in a car that struck a tree. He had multiple pelvic fractures. A through D, His intravenous-contrasted abdominal and pelvic CT scan (A, B) showed free fluid and air in the extraperitoneal pelvic space, suggesting extraperitoneal bladder rupture. However, blood or other fluid in this space could have a similar appearance, particularly given the presence of pelvic fractures that could have associated bleeding. CT cystogram was performed after contrast was instilled into the bladder through a urinary catheter. This confirmed extravasation of contrast from the bladder through an anterior bladder wall defect.
Figure 12-81 Bladder rupture: Intraperitoneal and extraperitoneal.
Same patient as in the previous figure. Her intravenous-contrasted CT scan showed pelvic fractures, as well as free fluid in the abdomen and pelvis (A, B). Although theoretically this fluid could be from multiple sources, no solid organ injuries were seen. The anterior bladder appeared possibly ruptured. C, CT cystogram was performed, confirming that contrast instilled into the bladder through a urinary catheter entered the peritoneal cavity and outlined bowel loops—an intraperitoneal bladder rupture. Some extraperitoneal extravasation was also seen. This patient also had a vaginal laceration. Consider vaginal-penetrating injuries when complex pelvic fractures or bladder injuries are present. The patient underwent operative repair of her bladder and vaginal injuries.
Conventional Cystogram
In a conventional (plain film) cystogram (Figures 12-82 and 12-83), an x-ray of the pelvis is performed before the administration of contrast material. This x-ray can demonstrate the presence of pelvic fractures (see Figure 12-84). Next, a Foley or urinary catheter is placed, and contrast material is instilled into the bladder, usually under gravity pressure only. An x-ray of the pelvis is repeated, and an uninjured bladder appears symmetrically distended, with no contrast material present beyond its sharply demarcated but smooth borders. An injured bladder may have several abnormalities noted. First, a pelvic hematoma adjacent to the bladder may deform the bladder and displace it from the midline. Second, in the instance of bladder rupture, contrast instilled through the Foley catheter may be seen extending outside the confines of the bladder. The conventional cystogram is then completed by evacuating the contrast material from the bladder through the Foley catheter and repeating a plain x-ray of the pelvis. This step allows recognition of subtle contrast extravasation deep or posterior to the bladder. Extravasation in this location can be masked on the x-ray performed with the bladder distended with contrast because the full bladder may be directly in front of the contrast leak. A normal x-ray at this point should show no or little residual contrast. Linear or irregular streaks of residual contrast may indicate rupture.
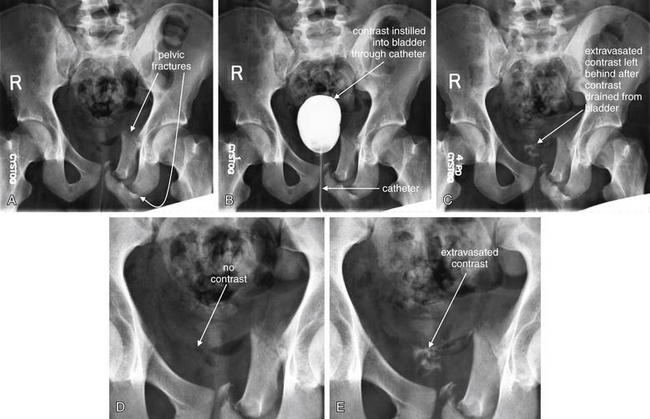
Figure 12-82 Conventional cystogram: Extraperitoneal extravasation of contrast.
Same patient as in the previous figure. The patient was treated nonoperatively with Foley catheter drainage for an extraperitoneal bladder rupture. This conventional cystogram was obtained 2 weeks after the original injury and computed tomography scan. A, First, an x-ray of the pelvis is obtained. B, Contrast is instilled into the bladder through a catheter. C, Contrast is drained from the bladder, and an x-ray is again obtained—this shows abnormal irregular collections of contrast, indicating persistent bladder leak. D, Close-up from A. E, Close-up from C, emphasizing the extravasated contrast.
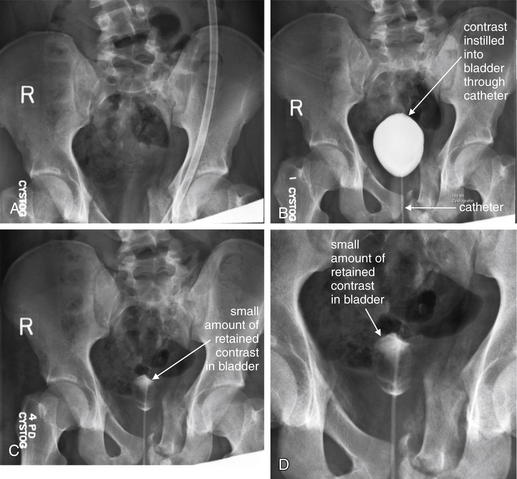
Figure 12-83 Conventional cystogram: No extravasation of contrast.
Same patient as in the previous figures. This conventional cystogram was obtained 4 weeks after the original injury and computed tomography scan. A, First, an x-ray of the pelvis is obtained. B, Contrast is instilled into the bladder through a catheter. C, Contrast is drained from the bladder, and an x-ray is again obtained. A small amount of contrast appears to be retained within the bladder. Notice the regular rounded appearance, unlike in the previous figure, where extravasated contrast had an irregular appearance. This indicated complete healing of the bladder rupture. D, Close-up from C. If doubt remained, the bladder could be gently irrigated to wash out retained contrast, and x-ray could be repeated.
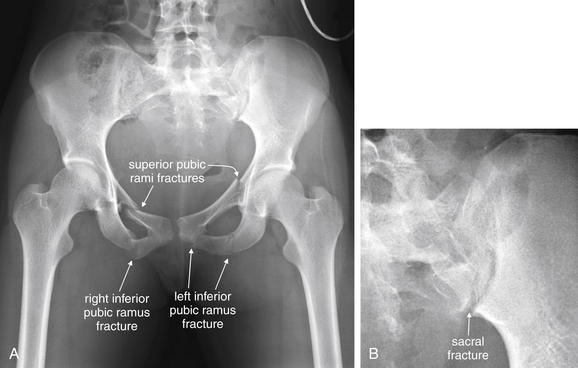
Figure 12-84 Bladder rupture: Intraperitoneal and extraperitoneal.
This 19-year-old woman was an unrestrained back seat passenger in a motor vehicle collision. A, Her pelvis x-ray shows displaced fractures of the right and left superior and inferior pubic rami fractures, as well as left pubic symphysis. B, Careful inspection reveals a left sacral fracture. Associated genitourinary injuries are discussed in the figure that follows.
Computed Tomography Cystogram
In a CT cystogram (see Figures 12-80 and 12-81), the bladder is filled with contrast through a Foley catheter under gravity pressure, just as with a conventional cystogram.80 In addition, previously administered IV contrast begins to fill the bladder as it is filtered and excreted by the kidneys. As with conventional cystogram, extravasation of contrast from the bladder can then be detected on CT images taken of the bladder and pelvis. Unlike conventional cystogram, additional images of the empty bladder are not needed because CT can detect contrast leak deep to the bladder. Contrast outlining bowel or other intraperitoneal structures indicates intraperitoneal bladder rupture. CT cystography has been found to be highly accurate when the bladder is distended with at least 300 mL of dilute contrast (usually 50 mL of Hypaque or similar contrast, mixed with 450 mL of saline) and can replace traditional cystography in patients undergoing CT.81-82
Suspected Urethral Injury
Following blunt trauma, several clinical factors may predict urethral injury, and retrograde urethrogram before bladder catheterization is indicated when urethral injury is suspected. Blood at the urethral meatus or high-riding prostate on rectal examination are clinical predictors of this injury. Urethral injuries are rare in the absence of pelvic fracture, although straddle injuries can damage the urethra without fracture. Fractures through the inferior and superior pubic rami may suggest urethral injury, particularly when the symphysis pubis is disrupted.76 Remember that similar findings and mechanisms of injury may result in perforation of the vagina, so speculum pelvic examination may also be important when pelvic fractures are suspected or detected on imaging.
Retrograde Urethrogram
Urethrogram (Figures 12-85 and 12-86) is performed by placement of the tip of a Foley catheter into the entrance of the urethra, followed by instillation of 50 mL of contrast under low pressure (usually gentle hand injection or gravity).86 A plain x-ray of the region is then performed. An uninjured urethra should show a smooth linear contour and filling of the bladder. An injured urethra may have an irregular contour, end abruptly without filling of the bladder, or show extravasation of contrast into surrounding tissues. In the case of a normal study, the Foley catheter can be advanced as usual. In the case of an abnormal study, suprapubic catheterization may be performed.
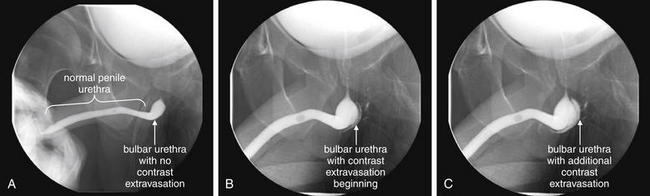
Figure 12-85 Urethral tear of the bulbar urethra: Retrograde urethrogram with contrast material.
When blood is detected at the urethral meatus after blunt torso trauma, injury to the urethra, bladder, ureters, or kidneys may be the source. If a urethral injury is present, insertion of a urinary catheter may create a false passage or complete a partial urethral tear. A retrograde urethrogram can be performed to evaluate for urethral injury before catheter placement. This study is performed by sterilely cannulating the urethral meatus with a small-caliber urinary catheter and slowly instilling 15 to 20 mL of Renografin or similar contrast into the urethra while a series of fluoroscopic images are acquired over several minutes. In this patient, the bladder is already filled with contrast because the patient had undergone computed tomography scan with intravenous contrast. This injected contrast material was filtered by the kidneys and filled the bladder normally, excluding kidney, ureteral, and bladder injury. The penile urethra appears normal in caliber, with no tears present. The bulbar urethra shows slight ballooning, a nonspecific finding. Over approximately 5 minutes (from A to C), contrast is seen extravasating from the bulbar urethra. A small amount of contrast is seen in the membranous and prostatic urethra, with no evidence of extravasation above the urogenital diaphragm.
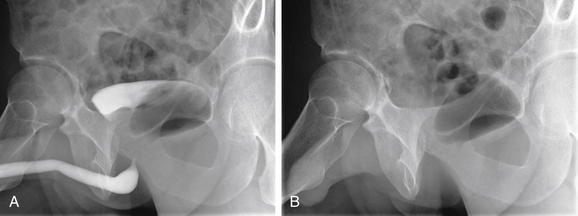
Figure 12-86 Normal retrograde urethrogram.
This retrograde urethrogram was performed in the patient from the previous figure, 2 weeks after the initial injury. The image is an oblique lateral projection of the pelvis. It now shows normal contours of the penile and bulbar urethra with no extravasation. A, The urethra is filled with contrast and has a normal contour. Some contrast is seen filling the bladder. B, Contrast has been allowed to drain out of the bladder and urethra after dwelling there for several minutes. No residual contrast is seen after this, consistent with the absence of any residual tear. If a tear were present, contrast would be expected to have leaked into adjacent tissues and be visible on the delayed images.
Traumatic Vaginal Bleeding
Traumatic vaginal bleeding can indicate injury to the uterus or vagina. In the pregnant patient, vaginal bleeding can indicate uterine rupture, placental abruption, and fetal distress—so pregnancy status should be immediately assessed in all female patients. In the pregnant patient fetal monitoring should be initiated as soon as possible.
Uterine injuries are rare except in pregnant patients because the uterus is usually small and protected within the bony pelvis, deep to the bladder. As pregnancy advances, the uterus rises out of the pelvis and is more subject to blunt and penetrating injury. Uterine injury and placental abruption are real possibilities in second- and third-trimester patients with abdominal and pelvic trauma. Concurrent with maternal stabilization, immediate abdominal ultrasound can be performed to assess fetal heart rate. Fetal bradycardia and tachycardia are ominous signs that may accompany maternal blood loss and shock or may indicate placental abruption. Abruption itself is not detected with high sensitivity by ultrasound as described earlier, so false reassurance should not be taken from a normal ultrasound. Continuous fetal monitoring should be initiated as soon as possible, regardless of normal ultrasound findings. Abnormal ultrasound should prompt consideration of immediate surgical delivery, as well as further assessment of the mother for sources of hemorrhage and shock.
Initial evaluation for uterine rupture should be conducted with ultrasound and external fetal monitoring, with the focus being on fetal viability and assessing the mother for other potentially life-threatening injuries. CT is quite sensitive for findings of uterine trauma or even uterine rupture and can be performed if other trauma considerations warrant abdominal or pelvis CT in the pregnant patent. However, uterus rupture is almost always associated with placental abruption and fetal distress, and CT diagnosis of uterine rupture likely would occur with too large a delay to allow emergency delivery of a live infant. Again, early ultrasound and fetal monitoring are imperative. Radiation and fetal safety considerations for CT in pregnant patients are discussed later. Descriptions of CT findings in critically injured pregnant patients include avascular (nonenhancing) regions of the placenta indicating placental abruption and infarction. Findings of uterine rupture include free abdominal fluid and extrauterine fetal parts. Normal changes in pregnancy, including heterogeneous placental enhancement, hydronephrosis from uterine compression of ureters, and enlarged ovarian veins, may be mistaken for pathological abnormalities.87
X-ray is of little utility in evaluating uterine rupture, although it may detect important findings such as pelvic fracture that may coexist with uterine injury. In some cases, x-ray may show the fetal skeleton in an abnormal location, indicating uterine rupture—but x-ray should not be relied upon for this diagnosis for a multitude of reasons, including radiation exposure, poor sensitivity, and diagnostic delay that would likely result in fetal demise.
What Are the Best Tests for Cervical–Vaginal Trauma? What Other Imaging Findings Should Raise Concern for These Injuries?
Cervical and vaginal trauma is not easily detected by diagnostic imaging modalities. Instead, these injuries should be suspected when bony injuries to the pelvis are present. When pelvic fractures are detected by x-ray or CT scan, vaginal perforation should be considered and speculum pelvic examination should be performed. This is particularly the case for anterior pelvic fractures such as injuries to the superior or inferior pubic rami. Pelvic examination may prove these to be open fractures whose management differs from closed pelvic injuries.
Direct Genitourinary Trauma
Direct blunt trauma to the scrotum can result in testicular injury, including vascular injury and scrotal hematoma. Ultrasound can assess for these injuries, much as in the setting of testicular torsion. CT may demonstrate scrotal soft-tissue swelling and fluid, as well as testicular dislocation.88
Abdominal-Pelvic Trauma in the Pregnant Patient: Balancing Radiation Risk With Risk for Injury
The pregnant patient with abdominal or pelvic trauma requires dual evaluation of the mother and the fetus. In the first trimester, uterine and fetal injuries are rare; moreover, the fetus is not yet viable, so maternal evaluation should proceed much as in the nonpregnant female. In the late second and the third trimesters, the enlarged uterus rises out of the pelvis and is more subject to injury. In addition, beyond 25 weeks of gestation, the fetus may be viable; therefore, if fetal distress occurs, emergency delivery may be indicated. Consequently, assessment and stabilization of the fetus should occur concurrently with stabilization of the mother. Maternal safety takes precedence over that of the fetus, so imaging studies should be performed as needed for maternal evaluation, with appropriate clinical risk stratification.
No single x-ray or CT is thought to exceed the threshold to increase birth defects measurably—regardless of trimester (Tables 12-596 and 12-697). However, trauma patients often undergo multiple imaging tests with significant cumulative radiation exposure. Therefore, whenever reasonable, ultrasound or clinical examination and observation should be substituted for ionizing radiation imaging studies. In unstable patients, fetal radiation concerns are overridden by maternal safety concerns. In stable patients, minimizing radiation exposure to the fetus should be a relative priority. Ionizing radiation exposure can be limited by using clinical parameters to determine the need for imaging. As discussed in the chapter on imaging the bony pelvis, clinical decision rules exist that can identify patients who do not require imaging of the bony pelvis. The pelvis x-ray can be avoided in patients without altered level of consciousness, complaint of pelvic pain, pelvic tenderness on examination, distracting injury, or clinical intoxication.89 In the stable patient with no significant abdominal pain or tenderness, observation with serial physical exams can be performed to avoid abdominal or pelvic irradiation with CT. Serial ultrasound of the abdomen can assess for free fluid or development of hemoperitoneum. Monitoring of vital signs for tachycardia and serial measurement of hematocrit can be used to observe for blood loss, rather than using immediate CT. This strategy of maternal observation is particularly reasonable because patients in the late second and the third trimesters require admission for extended fetal monitoring due to the risk of placental abruption, whether or not other maternal injuries are suspected.
TABLE 12-6 Estimated Radiation Doses From Common Diagnostic Imaging Procedures
| Imaging Test and Body Region | Fetal Dose |
|---|---|
| Radiographs | |
| Upper extremity | 0.01 mSv |
| Lower extremity | 0.01 mSv |
| Upper gastrointestinal series (barium) | 0.48-3.60 mSv |
| Cholecystography | 0.05-0.60 mSv |
| Chest (two views) | <0.1 mSv |
| Lumbar spine | 3.46-6.20 mSv |
| Pelvis | 0.40-2.38 mSv |
| Hip and femur series | 0.51-3.70 mSv |
| Retrograde Pyelogram | 8.00 mSv |
| Abdomen (kidneys, ureter, and bladder) | 2.00-2.45 mSv |
| Urography (IVP) | 3.58-13.98 mSv |
| Barium enema | 7.00-39.86 mSv |
| CT Scan | |
| Head | <0.5 mSv |
| Chest | 1.0-4.5 mSv |
| Lumbar spine | 35.0 mSv |
| Abdomen (10 slices) | 2.4-26.0 mSv |
| Pelvis | 7.3 mSv |
| Abdomen and pelvis | 6.4 mSv |
| Ventilation–Perfusion Scan | 0.6-10.0 mSv |
| Potentially Teratogenic Dose | 50 mSv |
Adapted from Ratnapalan S, Bentur Y, Koren G. “Doctor, will that x-ray harm my unborn child?” CMAJ 179:1293-6, 2008.
Penetrating Trauma
Penetrating genitourinary trauma has a more straightforward imaging approach than blunt trauma. In many cases, laparotomy may be needed to assess for other abdominal and pelvic injuries. In these cases, additional imaging studies to assess for genitourinary injury occur later, outside of the emergency department.
High-energy penetrating abdominal or pelvic trauma, such as gunshot wounds, traditionally requires laparotomy for assessment. Injuries to retroperitoneal and pelvic vascular structures and bowel are major concerns. Although most often the approach is surgical exploration, limited experience with “triple contrast” CT (IV, oral, and rectal contrast) has been described and is discussed in more detail in the chapter on torso trauma.90-92 As in blunt trauma, CT can demonstrate renal hemorrhage, extravasation of contrast from the renal collecting system or ureters, and bladder perforation, as well as associated abdominal injuries. In hemodynamically stable patients, CT imaging may be a reasonable approach if operative care is being deferred for reasons such as operating room availability. In most cases, simple x-rays of the chest, abdomen, and pelvis are performed in the trauma bay, although these provide little information about genitourinary injuries. Later, specific studies such as CT, cystogram, and IVP or retrograde urography can be performed to assess the kidneys, ureters, and bladder. In unstable patients requiring immediate laparotomy, IV urography can be performed in the operating room to assess for renal injuries. overall, the imaging plan for patients with penetrating trauma should be a team decision involving the emergency physician and surgical consultants.
Lower-energy-penetrating trauma, such as stab wounds to the flank, is increasingly investigated with triple-contrast CT.91 Initial studies suggest high sensitivity for injuries, but extreme caution should be used following a negative CT scan. Although CT may accurately identify genitourinary injuries requiring therapy, such as significant renal or ureteral injury, concurrent bowel injuries such as penetration of the retroperitoneal colon may be missed, with significant associated morbidity. Patients may develop sepsis from missed bowel injuries. Imaging in this setting is discussed in more detail in the chapter on abdominal trauma.
Radiation Exposures From Diagnostic Imaging in Pregnancy
Diagnostic imaging in the pregnant patient raises particular concerns related to radiation and fetal safety. Approximate fetal radiation doses associated with harmful clinical effects are shown in Table 12-5. The fetal radiation doses from individual diagnostic imaging exams are shown in Table 12-6. Note that no single common examination exceeds the threshold known to cause teratogenic effects, although the cumulative dose of multiple imaging studies should be considered. High levels of radiation exposure may lead to fetal demise. Lower levels of radiation exposure may lead to fetal malformation. Although very low levels of radiation exposure, such as those encountered in most emergency diagnostic imaging, are not thought to pose any specific fetal risk, some researchers have raised concerns that fetal irradiation may lead to increases in childhood leukemia or other subtle abnormalities, such as developmental delay or cognitive defects. Certainly radiation exposures in pregnancy contribute to maternal anxiety. However, as emergency physicians, we must balance the risk for immediate danger to fetus and mother from an ongoing and undiagnosed emergency medical condition against small and delayed risks posed by medical radiation. How accurately do physicians and patients characterize these risks? Physicians likely overestimate and underestimate risk.
In 2004, Lee et al.93 conducted a survey of radiologists, emergency physicians, and patients regarding radiation exposure and cancer risk. At that time, only about 50% of radiologists, 10% of emergency physicians, and 3% of patients recognized that radiation exposure from CT scan might pose any future risk for cancer development. In a survey of family physicians and obstetricians, Ratnapalan et al.96 asked each subject group to estimate the risk for fetal malformation resulting from a single exposure during pregnancy to abdominal x-ray or abdominal CT scan (neither of which is thought to pose a radiation exposure high enough to result in fetal harm after a single exposure, regardless of gestational age). As a follow-up question, the researchers asked each subject group to indicate their practice of advising a patient about elective termination of pregnancy following such exposure, based on fetal risk from radiation. 44% of family physicians and 11% of obstetricians believed that the fetal malformation risk from x-ray exceeded 5%. In addition, 61% of family physicians and 34% of obstetricians believed a single abdominal CT exceeded a 5% fetal malformation risk. Finally, 6% of family physicians and 5% of obstetricians recommended elective abortion following a single abdominal CT scan—a recommendation unjustified by current risk estimates.50
It is often said that maternal health must take precedence over any concerns for fetal risk from medical diagnostic radiation exposures. Although this is certainly true, this statement is sometimes used as carte blanche to perform whatever diagnostic imaging tests might be used in a nonpregnant female. Instead, consider the following approach. First, determine what life-threatening conditions are strongly considered in the mother. Truly life-threatening considerations probably warrant the same diagnostic imaging approach that would be used in a nonpregnant patient. Second, determine what other important diagnoses must be evaluated but could be diagnosed in a more measured fashion because they do not pose an immediate risk. Examples could include such diagnoses as appendicitis or even intraabdominal solid organ injury in a stable patient, which is often managed nonoperatively. Many diagnoses that might initially seem to fit into the first category actually fall into the second on further consideration. For these diagnoses, consider alternative diagnostic imaging tests that do not involve ionizing radiation. Ultrasound or MRI for appendicitis diagnosis may be appropriate; these are discussed in more detail in the chapter on abdominal imaging. Strategies for the pregnant trauma patient are discussed earlier. Finally, a third category of diagnostic considerations should be entertained. These diagnoses likely pose no significant threat to the mother at any time but are often worked up in the nonpregnant patient for purposes of closure or for exclusion of other, more dangerous diagnoses. Examples might include renal colic or simple ovarian cysts. In the pregnant patient, exclusion of dangerous causes of symptoms may be sufficient, and careful explanation to the patient of those diagnoses that are not being evaluated may take the place of diagnostic imaging. Perhaps we can learn a lesson from pregnant patients, resulting in less indiscriminate use of diagnostic imaging in nonpregnant patients for these relatively nonemergent diagnoses.
Summary
Genitourinary complaints can include life- and organ-threatening conditions. A variety of imaging modalities are available for diagnosis. For patients with significant trauma, and in the nontrauma patient with a broad differential diagnosis including abdominal pathology, CT is the most valuable modality for most complaints. Ultrasound is the key modality for assessment of complications in the pregnant patient. Ultrasound is also useful in the diagnosis of testicular and ovarian torsion but is not 100% sensitive—thus a negative imaging result does not rule out these conditions when clinical suspicion is high. No imaging modality is sensitive for placental abruption, so fetal monitoring is essential. In the pregnant patient, reasonable steps should be considered to reduce radiation exposures through clinical observation and use of modalities such as ultrasound that do not use ionizing radiation. When the mother’s life is at risk, imaging should be performed as for the nonpregnant patient. No single imaging test exceeds the threshold known to induce fetal malformation.
1. Katz D.S., Scheer M., Lumerman J.H., et al. Alternative or additional diagnoses on unenhanced helical computed tomography for suspected renal colic: Experience with 1000 consecutive examinations. Urology. 2000;56:53-57.
2. Rekant E.M., Gibert C.L., Counselman F.L. Emergency department time for evaluation of patients discharged with a diagnosis of renal colic: Unenhanced helical computed tomography versus intravenous urography. J Emerg Med. 2001;21:371-374.
3. Dalrymple N.C., Casford B., Raiken D.P., et al. Pearls and pitfalls in the diagnosis of ureterolithiasis with unenhanced helical CT. Radiographics. 2000;20:439-447. quiz 527-8, 532
4. Zelenko N., Coll D., Rosenfeld A.T., Smith R.C. Normal ureter size on unenhanced helical CT. AJR Am J Roentgenol. 2004;182:1039-1041.
5. Ha S.K., Seo J.K., Kim S.J., et al. Acute pyelonephritis focusing on perfusion defects on contrast enhanced computerized tomography (CT) scans and its clinical outcome. Korean J Intern Med. 1997;12:122-127.
6. Worster A., Preyra I., Weaver B., Haines T. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: A meta-analysis. Ann Emerg Med. 2002;40:280-286.
7. Pfister S.A., Deckart A., Laschke S., et al. Unenhanced helical computed tomography vs intravenous urography in patients with acute flank pain: Accuracy and economic impact in a randomized prospective trial. Eur Radiol. 2003;13:2513-2520.
8. Patlas M., Farkas A., Fisher D., et al. Ultrasound vs CT for the detection of ureteric stones in patients with renal colic. Br J Radiol. 2001;74:901-904.
9. Sheafor D.H., Hertzberg B.S., Freed K.S., et al. Nonenhanced helical CT and US in the emergency evaluation of patients with renal colic: Prospective comparison. Radiology. 2000;217:792-797.
10. Chan V.O., Buckley O., Persaud T., Torreggiani W.C. Urolithiasis: How accurate are plain radiographs? Can Assoc Radiol J. 2008;59:131-134.
11. Ha M., MacDonald R.D. Impact of CT scan in patients with first episode of suspected nephrolithiasis. J Emerg Med. 2004;27:225-231.
12. Kadish H.A., Bolte R.G. A retrospective review of pediatric patients with epididymitis, testicular torsion, and torsion of testicular appendages. Pediatrics. 1998;102:73-76.
13. Pepe P., Panella P., Pennisi M., Aragona F. Does color Doppler sonography improve the clinical assessment of patients with acute scrotum? Eur J Radiol. 2006;60:120-124.
14. Gunther P., Schenk J.P., Wunsch R., et al. Acute testicular torsion in children: The role of sonography in the diagnostic workup. Eur Radiol. 2006;16:2527-2532.
15. Dell’Atti L., Fabiani A., Marconi A., et al. Reliability of echo-color-Doppler in the differential diagnosis of the “acute scrotum”: Our experience. Arch Ital Urol Androl. 2005;77:66-68.
16. Nussbaum Blask A.R., Bulas D., Shalaby-Rana E., et al. Color Doppler sonography and scintigraphy of the testis: A prospective, comparative analysis in children with acute scrotal pain. Pediatr Emerg Care. 2002;18:67-71.
17. Lam W.W., Yap T.L., Jacobsen A.S., Teo H.J. Colour Doppler ultrasonography replacing surgical exploration for acute scrotum: Myth or reality? Pediatr Radiol. 2005;35:597-600.
18. Kravchick S., Cytron S., Leibovici O., et al. Color Doppler sonography: Its real role in the evaluation of children with highly suspected testicular torsion. Eur Radiol. 2001;11:1000-1005.
19. Karmazyn B., Steinberg R., Kornreich L., et al. Clinical and sonographic criteria of acute scrotum in children: A retrospective study of 172 boys. Pediatr Radiol. 2005;35:302-310.
20. Pryor J.L., Watson L.R., Day D.L., et al. Scrotal ultrasound for evaluation of subacute testicular torsion: Sonographic findings and adverse clinical implications. J Urol. 1994;151:693-697.
21. Blaivas M., Sierzenski P., Lambert M. Emergency evaluation of patients presenting with acute scrotum using bedside ultrasonography. Acad Emerg Med. 2001;8:90-93.
22. Rodriguez D.D., Rodriguez W.C., Rivera J.J., et al. Doppler ultrasound versus testicular scanning in the evaluation of the acute scrotum. J Urol. 1981;125:343-346.
23. Suh J.H., Gardner J.M., Kee K.H., et al. Calcifications in prostate and ejaculatory system: A study on 298 consecutive whole mount sections of prostate from radical prostatectomy or cystoprostatectomy specimens. Ann Diagn Pathol. 2008;12:165-170.
24. Kalinski M.A., Guss D.A. Hemorrhagic shock from a ruptured ectopic pregnancy in a patient with a negative urine pregnancy test result. Ann Emerg Med. 2002;40:102-105.
25. Salman G., Irvine L.M. Ectopic pregnancy, the need for standardisation of rate. J Obstet Gynaecol. 2008;28:32-35.
26. Von Kuenssberg Jehle D., Stiller G., Wagner D. Sensitivity in detecting free intraperitoneal fluid with the pelvic views of the FAST exam. Am J Emerg Med. 2003;21:476-478.
27. Chandrasekhar C. Ectopic pregnancy: A pictorial review. Clin Imaging. 2008;32:468-473.
28. Cacciatore B., Tiitinen A., Stenman U.H., Ylostalo P. Normal early pregnancy: Serum hCG levels and vaginal ultrasonography findings. Br J Obstet Gynaecol. 1990;97:899-903.
29. Kadar N., Taylor K.J., Rosenfield A.T., Romero R. Combined use of serum hCG and sonography in the diagnosis of ectopic pregnancy. AJR Am J Roentgenol. 1983;141:609-615.
30. Kadar N., DeVore G., Romero R. Discriminatory hCG zone: Its use in the sonographic evaluation for ectopic pregnancy. Obstet Gynecol. 1981;58:156-161.
31. Barnhart K.T., Simhan H., Kamelle S.A. Diagnostic accuracy of ultrasound above and below the beta-hCG discriminatory zone. Obstet Gynecol. 1999;94:583-587.
32. Kadar N., Bohrer M., Kemmann E., Shelden R. The discriminatory human chorionic gonadotropin zone for endovaginal sonography: A prospective, randomized study. Fertil Steril. 1994;61:1016-1020.
33. Dart R.G., Burke G., Dart L. Subclassification of indeterminate pelvic ultrasonography: Prospective evaluation of the risk for ectopic pregnancy. Ann Emerg Med. 2002;39:382-388.
34. Dart R., Kaplan B., Ortiz L., et al. Normal intrauterine pregnancy is unlikely in emergency department patients with either menstrual days > 38 days or beta-hCG > 3,000 mIU/mL, but without a gestational sac on ultrasonography. Acad Emerg Med. 1997;4:967-971.
35. Nyberg D.A., Mack L.A., Laing F.C., Jeffrey R.B. Early pregnancy complications: Endovaginal sonographic findings correlated with human chorionic gonadotropin levels. Radiology. 1988;167:619-622.
36. Shapiro B.S., Escobar M., Makuch R., et al. A model-based prediction for transvaginal ultrasonographic identification of early intrauterine pregnancy. Am J Obstet Gynecol. 1992;166:1495-1500.
37. Barnhart K., Mennuti M.T., Benjamin I., et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
38. Dart R.G., Kaplan B., Cox C. Transvaginal ultrasound in patients with low beta-human chorionic gonadotropin values: How often is the study diagnostic? Ann Emerg Med. 1997;30:135-140.
39. Brown D.L., Doubilet P.M. Transvaginal sonography for diagnosing ectopic pregnancy: Positivity criteria and performance characteristics. J Ultrasound Med. 1994;13:259-266.
40. Dart R., McLean S.A., Dart L. Isolated fluid in the cul-de-sac: How well does it predict ectopic pregnancy? Am J Emerg Med. 2002;20:1-4.
41. Bright D.A., Gaupp F.B. Heterotopic pregnancy: A reevaluation. J Am Board Fam Pract. 1990;3:125-128.
42. Abu-Yousef M.M., Bleicher J.J., Williamson R.A., Weiner C.P. Subchorionic hemorrhage: Sonographic diagnosis and clinical significance. AJR Am J Roentgenol. 1987;149:737-740.
43. Nagy S., Bush M., Stone J., et al. Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet Gynecol. 2003;102:94-100.
44. Oyelese Y., Ananth C.V. Placental abruption. Obstet Gynecol. 2006;108:1005-1016.
45. Glantz C., Purnell L. Clinical utility of sonography in the diagnosis and treatment of placental abruption. J Ultrasound Med. 2002;21:837-840.
46. Tikkanen M., Nuutila M., Hiilesmaa V., et al. Clinical presentation and risk factors of placental abruption. Acta Obstet Gynecol Scand. 2006;85:700-705.
47. Nyberg D.A., Mack L.A., Benedetti T.J., et al. Placental abruption and placental hemorrhage: Correlation of sonographic findings with fetal outcome. Radiology. 1987;164:357-361.
48. Nyberg D.A., Cyr D.R., Mack L.A., et al. Sonographic spectrum of placental abruption. AJR Am J Roentgenol. 1987;148:161-164.
49. Shukunami K., Kawakami Y., Kotsuji F. Sonolucency of a fresh hematoma in a case of marginal placental abruption. Eur J Obstet Gynecol Reprod Biol. 2001;97:249.
50. Ratnapalan S., Bona N., Chandra K., Koren G. Physicians’ perceptions of teratogenic risk associated with radiography and CT during early pregnancy. AJR Am J Roentgenol. 2004;182:1107-1109.
51. Boyd RL, Carter BS: Polyhydramnios and oligohydramnios. (Accessed at http://emedicine.medscape.com/article/975821-overview).
52. Dane B., Dane C., Kiray M., et al. Sonographic findings in adnexal torsion: A report of 34 cases. Arch Gynecol Obstet. 2009;279(6):841-844.
53. Shadinger L.L., Andreotti R.F., Kurian R.L. Preoperative sonographic and clinical characteristics as predictors of ovarian torsion. J Ultrasound Med. 2008;27:7-13.
54. Pena J.E., Ufberg D., Cooney N., Denis A.L. Usefulness of Doppler sonography in the diagnosis of ovarian torsion. Fertil Steril. 2000;73:1047-1050.
55. Kiechl-Kohlendorfer U., Maurer K., Unsinn K.M., Gassner I. Fluid-debris level in follicular cysts: a pathognomonic sign of ovarian torsion. Pediatr Radiol. 2006;36:421-425.
56. Kimura I., Togashi K., Kawakami S., et al. Ovarian torsion: CT and MR imaging appearances. Radiology. 1994;190:337-341.
57. Zissin R. Torsion of a normal ovary in a post-pubertal female: Unenhanced helical CT appearance. Br J Radiol. 2001;74:762-763.
58. Chang H.C., Bhatt S., Dogra V.S. Pearls and pitfalls in diagnosis of ovarian torsion. Radiographics. 2008;28:1355-1368.
59. Kalish G.M., Patel M.D., Gunn M.L., Dubinsky T.J. Computed tomographic and magnetic resonance features of gynecologic abnormalities in women presenting with acute or chronic abdominal pain. Ultrasound Q. 2007;23:167-175.
60. Gittleman A.M., Price A.P., Goffner L., Katz D.S. Ovarian torsion: CT findings in a child. J Pediatr Surg. 2004;39:1270-1272.
61. Rha S.E., Byun J.Y., Jung S.E., et al. CT and MR imaging features of adnexal torsion. Radiographics. 2002;22:283-294.
62. Kawahara Y., Fukuda T., Futagawa S., et al. Intravascular gas within an ovarian tumor: A CT sign of ovarian torsion. J Comput Assist Tomogr. 1996;20:154-156.
63. Lande I.M., Hill M.C., Cosco F.E., Kator N.N. Adnexal and cul-de-sac abnormalities: Transvaginal sonography. Radiology. 1988;166:325-332.
64. Varras M., Polyzos D., Perouli E., et al. Tubo-ovarian abscesses: spectrum of sonographic findings with surgical and pathological correlations. Clin Exp Obstet Gynecol. 2003;30:117-121.
65. Wilbur A.C., Aizenstein R.I., Napp T.E. CT findings in tuboovarian abscess. AJR Am J Roentgenol. 1992;158:575-579.
66. Hiller N., Sella T., Lev-Sagi A., et al. Computed tomographic features of tuboovarian abscess. J Reprod Med. 2005;50:203-208.
67. Wilbur A. Computed tomography of tuboovarian abscesses. J Comput Assist Tomogr. 1990;14:625-628.
68. Ellis J.H., Francis I.R., Rhodes M., et al. CT findings in tuboovarian abscess. J Comput Assist Tomogr. 1991;15:589-592.
69. Halperin R., Svirsky R., Vaknin Z., et al. Predictors of tuboovarian abscess in acute pelvic inflammatory disease. J Reprod Med. 2008;53:40-44.
70. Schlaff W.D., Lund K.J., McAleese K.A., Hurst B.S. Diagnosing ovarian torsion with computed tomography: A case report. J Reprod Med. 1998;43:827-830.
71. Fleischer A.C., Cullinan J.A., Kepple D.M., Williams L.L. Conventional and color Doppler transvaginal sonography of pelvic masses: A comparison of relative histologic specificities. J Ultrasound Med. 1993;12:705-712.
72. Guerriero S., Alcazar J.L., Coccia M.E., et al. Complex pelvic mass as a target of evaluation of vessel distribution by color Doppler sonography for the diagnosis of adnexal malignancies: Results of a multicenter European study. J Ultrasound Med. 2002;21:1105-1111.
73. Varras M. Benefits and limitations of ultrasonographic evaluation of uterine adnexal lesions in early detection of ovarian cancer. Clin Exp Obstet Gynecol. 2004;31:85-98.
74. Yoshitake T., Asayama Y., Yoshimitsu K., et al. Bilateral ovarian leiomyomas: CT and MRI features. Abdom Imaging. 2005;30:117-119.
75. Asch E., Levine D. Variations in appearance of endometriomas. J Ultrasound Med. 2007;26:993-1002.
76. Miller K.S., McAninch J.W. Radiographic assessment of renal trauma: Our 15-year experience. J Urol. 1995;154:352-355.
77. Abou-Jaoude W.A., Sugarman J.M., Fallat M.E., Casale A.J. Indicators of genitourinary tract injury or anomaly in cases of pediatric blunt trauma. J Pediatr Surg. 1996;31:86-89. discussion 90
78. Morey A.F., Bruce J.E., McAninch J.W. Efficacy of radiographic imaging in pediatric blunt renal trauma. J Urol. 1996;156:2014-2018.
79. Rehm C.G., Mure A.J., O’Malley K.F., Ross S.E. Blunt traumatic bladder rupture: The role of retrograde cystogram. Ann Emerg Med. 1991;20:845-847.
80. Morey A.F., Iverson A.J., Swan A., et al. Bladder rupture after blunt trauma: Guidelines for diagnostic imaging. J Trauma. 2001;51:683-686.
81. Fuhrman G.M., Simmons G.T., Davidson B.S., Buerk C.A. The single indication for cystography in blunt trauma. Am Surg. 1993;59:335-337.
82. Pao D.M., Ellis J.H., Cohan R.H., Korobkin M. Utility of routine trauma CT in the detection of bladder rupture. Acad Radiol. 2000;7:317-324.
83. Vaccaro J.P., Brody J.M. CT cystography in the evaluation of major bladder trauma. Radiographics. 2000;20:1373-1381.
84. Peng M.Y., Parisky Y.R., Cornwell E.E.3rd, et al. CT cystography versus conventional cystography in evaluation of bladder injury. AJR Am J Roentgenol. 1999;173:1269-1272.
85. Horstman W.G., McClennan B.L., Heiken J.P. Comparison of computed tomography and conventional cystography for detection of traumatic bladder rupture. Urol Radiol. 1991;12:188-193.
86. Berna-Mestre J.D., Berna-Serna J.D., Aparicio-Meson M., Canteras-Jordana M. Urethrography in men: Conventional technique versus clamp method. Radiology. 2009;252:240-246.
87. Lowdermilk C., Gavant M.L., Qaisi W., et al. Screening helical CT for evaluation of blunt traumatic injury in the pregnant patient. Radiographics. 1999;19:S243-S255. discussion S256-258
88. Ezra N., Afari A., Wong J. Pelvic and scrotal trauma: CT and triage of patients. Abdom Imaging. 2009;34:541-544.
89. Gross E.A., Niedens B.A. Validation of a decision instrument to limit pelvic radiography in blunt trauma. J Emerg Med. 2005;28:263-266.
90. Goldman S.M., Sandler C.M. Urogenital trauma: imaging upper GU trauma. Eur J Radiol. 2004;50:84-95.
91. Shanmuganathan K., Mirvis S.E., Chiu W.C., et al. Penetrating torso trauma: Triple-contrast helical CT in peritoneal violation and organ injury—A prospective study in 200 patients. Radiology. 2004;231:775-784.
92. Smith J.K., Kenney P.J. Imaging of renal trauma. Radiol Clin North Am. 2003;41:1019-1035.
93. Lee C.I., Haims A.H., Monico E.P., et al. Diagnostic CT scans: Assessment of patient, physician, and radiologist awareness of radiation dose and possible risks. Radiology. 2004;231:393-398.
94. Thomson J.M., Glocer J., Abbott C., et al. Computed tomography versus intravenous urography in diagnosis of acute flank pain from urolithiasis: A randomized study comparing imaging costs and radiation dose. Australas Radiol. 2001;45:291-297.
95. Denton E.R., Mackenzie A., Greenwell T., et al. Unenhanced helical CT for renal colic: Is the radiation dose justifiable? Clin Radiol. 1999;54:444-447.
96. Ratnapalan S., Bentur Y., Koren G. Doctor, will that x-ray harm my unborn child? CMAJ. 2008;179:1293-1296.
97. Bentur Y., Horlatsch N., Koren G. Exposure to ionizing radiation during pregnancy: Perception of teratogenic risk and outcome. Teratology. 1991;43:109-112.