Chapter 16 “Therapeutic Imaging”: Image-Guided Therapies in Emergency Medicine
Medical imaging serves first to diagnose disease or injury, but imaging techniques are increasingly used to guide temporizing or definitive therapy for a range of conditions. Depending on the practice setting, medical treatments guided by imaging techniques may be performed by interventional radiologists, cardiologists, and surgeons. In many cases, emergency physicians perform image-guided procedures, using ultrasound to guide procedures as diverse as peripheral and central venous catheter placement, abdominal paracentesis, thoracentesis, lumbar puncture, and joint aspiration. Fluoroscopic guidance of fracture reduction is a standard practice for orthopedists and many emergency physicians.
In this chapter, we discuss some selected applications of image-guided procedures, as well as the evidence supporting their use. At their best, image-guided procedures are definitive treatment, with strong evidence suggesting equivalent or superior clinical outcomes compared with other medical and surgical approaches. In other cases, the evidence disappoints the medical community, showing no benefit to a therapy with great initial expectations. Many applications of image-guided therapies have surprisingly little evidence to support them, despite having been widely accepted by the medical community. Other applications are rarely used today but offer “heroic” options in moribund patients with few treatment options for their life-threatening conditions. The emergency physician must be familiar with the available interventions, their indications and contraindications, and the class of supporting evidence.
We begin with a brief overview of imaging modalities used to guide procedures, with their advantages and disadvantages. We then discuss some important methodologic issues to consider when reviewing the evidence for any treatment, including image-guided therapies. Although most of us may never design or conduct research, we all must be capable of understanding research and recognizing when research is so seriously flawed that we can no longer trust the conclusions drawn by investigators. We provide some basic background so that we can draw appropriately limited conclusions from flawed research when possible. Finally, with this background, we grapple with some important image-guided procedures, comparing those with excellent evidence to those with more modest support.
Imaging Modalities for Procedures
Common imaging modalities used to guide procedures are listed in Table 16-1. Ultrasound is commonly used to guide procedures because it has the benefit of allowing real-time visualization with no radiation exposure to the patient or medical provider. Plain x-ray can be used to guide a procedure but has the disadvantage of radiation exposure to the patient and a lack of real-time guidance. Instead, a series of x-ray images taken at intervals is used to assess the progress of the procedure. An example is the use of repeated x-rays to assess the success of a fracture reduction. Fluoroscopy (a video x-ray technique) is frequently used for procedural guidance, with the advantage of real-time guidance (providing the opportunity for both “live video” and “stop-action snapshots” of the procedure). Fluoroscopy is the imaging modality employed in common catheter angiography procedures, and it allows visualization of bone and radiopaque contrast agents. Unfortunately, fluoroscopy provides a relatively high radiation dose to the patient, proportional to the amount of time that the fluoroscope tube is turned on. The exposure to the medical provider (and particularly the cumulative dose for physicians who routinely perform fluoroscopic exams) can also be relatively high if care is not taken to use shielding devices and to keep the practitioner’s body outside of the field of the fluoroscope. The radiation dose to the patient is relatively unimportant when the body part imaged is radiation insensitive (e.g., extremities, as during a fracture reduction). However, when the body region imaged is more radiosensitive (e.g., chest or abdomen), the total time of the imaging should be limited to reduce the radiation dose. CT provides an excellent imaging tool for procedural guidance, allowing a target structure such as an intraabdominal abscess to be precisely localized. Measurements of distance to the structure of interest are easily made with CT, and the locations of instruments such as trocars, guidewires, and catheters can be accurately determined. Continuous CT fluoroscopy allows near real-time guidance. Alternatively, in the “quick-check” technique, several images may be acquired over time, first to localize the lesion of interest and then to assess the progress of the procedure intermittently. This repeated imaging with CT results in a moderately high radiation dose in a focused area, though the cephalad–caudad extent of the repeated images is usually restricted only to the area of interest. The exposure to patients and radiologists from CT fluoroscopy can be minimized by low-milliampere CT technique and minimizing CT fluoroscopic time with the quick-check method.1a
Table 16-1 Imaging Modalities for Procedures
| Imaging Modality | Radiation Dose | Real-Time Procedure Guidance? |
|---|---|---|
| Ultrasound | None | Yes |
| X-ray | Low | No ; intermittent images are obtained at intervals during the procedure |
| Fluoroscopy | Moderate to high, depending on total exposure time (during which the fluoroscope tube is on) | Yes |
| CT | Moderate to high, depending on number of CT images obtained* | Yes; CT fluoroscopy techniques allow near real-time imaging |
* In some cases, multiple CT images must be obtained to check the progress of the procedure, though the cephalad–caudad axis of the CT scan is usually limited.
Assessing the Quality of Scientific Evidence
In the sections that follow, we describe indications for image-guided procedures and briefly review the scientific evidence supporting the use of these procedures. In some cases, the evidence is quite limited, with case series describing the use of an intervention with no control group to allow comparisons with other treatments. In other scenarios, large prospective randomized-controlled studies allow more fair assessment of the clinical benefit of image-guided procedures, compared with other standard therapies, including medical treatments and surgical interventions.
Classes of medical evidence are summarized in Table 16-2. We refer to this hierarchy later in the chapter as we consider each application of an image-guided procedure. Here, we describe study design features that strongly influence the validity of results.
Study Design Measures to Maximize Precision of Results
Studies that provide class I evidence include large populations of patients, providing the statistical power to answer the clinical question with narrow confidence intervals (CIs). This is in contrast to many initial studies, which are often small and may suggest an outcome benefit but with a broad range in the estimate of the benefit, sometimes including the possibility of no benefit or even harm because of the procedure. Small studies with broad CIs provide an imprecise estimate of the magnitude and direction of an effect. Large studies with narrow CIs provide precise estimates of magnitude and direction of effects. Whether these precise estimates reflect “truth” depends on the appropriate use of other design features to minimize bias, discussed later.
Study Design Measures to Allow Cause–Effect Determination
A second important feature of a study is the use of a comparison or control group. Without such a group, a cause–effect relationship cannot be determined for the intervention and the outcome of interest. We cannot know what the outcomes would have been if the intervention in question had not been performed. Instead, we can only state whether patients receiving the intervention did well or badly. This can overestimate or underestimate the value of intervention. For example, an intervention may have no effect, yet patients receiving it may do well clinically. The intervention might then be incorrectly credited with the good outcome, when other factors such as the severity of the original illness or the influence of other treatments may have been responsible.
Cause–effect determination also requires that the treatment be performed before the effect occurs. Although this may appear obvious, the lack of such a temporal relationship can be obscured in retrospective studies. For example, in a retrospective study, it may be that patients receiving a given intervention ultimately are seen to have better outcomes. Hidden from view may be some clinical feature that predicts better outcome and was already at play in those patients who were chosen for treatment; thus the intervention is actually a marker of that other unmeasured clinical factor rather than the cause of the clinical improvement. This effect is discussed in more detail later in our consideration of randomization. Prospective randomization is required to transform the relationship between intervention and outcome from a simple association or correlation to a likely cause–effect relationship. Because randomization is not possible in retrospective studies, prospective designs are necessary to prove causation. Retrospective studies can provide evidence of an association between an intervention and an outcome, suggesting the need for a prospective study to determine causation.
Study Design Measures to Minimize Bias and Increase the Credibility of Results
A third feature of reliable research is the use of measures to minimize bias. These include randomization, blinding, and representative study populations.
Randomization
Randomization is extremely important to the validity of a study. Randomization creates two (or more) equal groups before the intervention is performed. When randomization is performed and is ideal, the two groups start with similar conditions. This allows differences that are measured after the intervention to be attributed to the intervention, rather than to preexisting differences between the groups. Randomization would be less important if medical research involved identical subjects, sharing the same genetic composition and environmental background. Instead, human medical research subjects differ in a multitude of factors. The use of appropriate randomization attempts to balance these differences between the groups. Consider a clinical trial to be a bit like a race, with the measured outcome being to determine which of two race vehicles is faster or better. Randomization attempts to ensure that, on average, the drivers of the two race vehicles start at the same point and travel a racecourse of similar difficulty. Without this, it would be impossible to determine which vehicle is faster; one might travel a shorter or a longer distance along a straighter or a more tortuous course, with a different uphill or downhill grade.
The success of randomization in a trial can be loosely assessed by comparing the baseline characteristics of the two groups, usually reported in the first table of a published study. It cannot be assumed that randomization always works as intended. In large studies, randomization usually does distribute characteristics evenly between groups. However, in small studies, randomization often fails to achieve this goal. Consider the example of a coin flip with a fair coin. If the coin is flipped 1 million times, approximately the same number of heads and tails will occur. However, if the coin is flipped only 10 times, the number of heads and tails may differ—resulting in a random but unequal distribution of the study subjects before the intervention. By reviewing the distribution of characteristics of the study subjects, you can determine whether they differed at study inception in important measured ways, such as age, gender, race, the presence of medical conditions such as diabetes, or measures of disease severity (e.g., Acute Physiology and Chronic Health Evaluation scores). When the two study groups differ in one or more measured characteristics, beware of the possibility that any differences after the study intervention are the result of this starting difference, rather than because of the benefit or harm of the intervention. Apparent benefit may simply reflect that the subjects were healthier before the intervention. Apparent harm of the intervention may reflect that subjects receiving the intervention were sicker. Uneven distribution before the intervention can also hide a real effect. For example, consider an intervention group that was sicker at study inception despite randomization. If the two groups have similar results, it may appear that the intervention had no effect, when in reality it allowed the intervention group to overcome an initial disadvantage to achieve the same outcome as the control group. As you can see, randomizing patients to achieve two matched groups before any study intervention is essential to the credibility of the outcome. If groups differ at the beginning of the study, attempts to understand the cause–effect relationship of the intervention may be confounded.
When two groups appear similar on the basis of similar measured baseline characteristics after randomization, it remains possible that the two groups are not similar in other important but unmeasured variables. In large studies with good randomization, this is unlikely, but this possibility becomes important in small studies and in a common scenario in medical research—the statistical matching of groups. Researchers often perform statistical “corrections” to overcome either the lack of initial randomization, or the failure of randomization to perform as intended. Let’s consider both of these scenarios, because both can deceive us in our interpretation of research studies. In some studies, patients are not randomized before the study intervention is performed. This is common in retrospective studies, in which patients receive an intervention based on the decisions of the treating physician and their clinical status. As a consequence, patients in the treatment group often differ substantially from their counterparts who were treated differently. Because researchers would like to compare the outcomes in the two groups, they attempt to “correct” for these baseline differences, in effect trying to place the two groups at the same “starting line” in the “race.”
Often in observational studies without randomization, researchers match patients in both groups who share similar age, gender, and disease severity and then compare outcomes in these two “matched” groups. Although this may appear to serve the same function as prospective randomization, it differs in a key way. Using this “matching” strategy, the researchers sort patients into equal groups using measured characteristics, hoping that important unmeasured characteristics also sort in an equal way. This assumption may fail but be impossible to discern from the reported data. The most important baseline difference in the patients may not be a readily measured feature such as age or heart rate but, rather, the clinician’s judgment that one patient deserves aggressive therapy whereas another does not.
Consider this slightly absurd example: Two groups of patients are identified retrospectively, those who were treated with treatment A and those who received treatment B. Because these patients were not randomized to their treatment, they likely had some key difference that drove the physicians caring for them to choose treatment A or B. The researchers try to correct for this possible starting difference by matching patients in each group who are similar in age, height, gender, and initial disease or injury severity—perhaps measured by triage blood pressure. But the key characteristic that ultimately had the largest influence on patient outcome might have been some genetic factor, which in turn might have been manifested clinically as hair color. If the patient hair color was not recorded in the medical chart, patients cannot be matched on this variable in retrospect. It is possible that despite matching the patients for the other characteristics, the two groups continue to differ markedly in (unmeasured) hair color and the related key genetic factor. Although the comparison appears fair based on the measured and matched characteristics, an unfair comparison is occurring.
In contrast, randomization assorts patients on all characteristics, measured and unmeasured. Our ability to measure the success of randomization in creating equal groups depends (by definition) on comparing measured characteristics, but with randomization we can be relatively more assured that the assortment of measured characteristics reflects the assortment of unmeasured characteristics. Even with randomization, equal distribution of characteristics between the two groups is not assured. Sometimes, when randomization fails to achieve two groups with similar initial features, researchers then perform statistical “matching” in an attempt to correct for these baseline differences. However, such efforts are no guarantee that the compared groups are indeed similar, as described earlier.
When randomization is not performed, whether the study design is prospective or retrospective, the resulting nonrandom assortment of patients to two treatment strategies creates selection bias. Although the term selection bias sounds nefarious, as if researchers are trying to skew patient treatment in a particular way to influence outcomes, it may be an unconscious practice or a conscious but well-meaning decision. For example, in nonrandomized studies of interventional radiologic techniques, patients may be selected for treatment based on poor clinical status because clinicians or researchers may feel that these patients would benefit most from aggressive therapy. Clinicians and researchers may feel that less-sick patients may not require invasive therapy to do well. The sickest patients of all may not be selected for interventional radiologic therapies because clinicians and researchers may feel that these patients are too unstable or have no hope of survival. If the patients receiving image-guided therapy are subsequently compared to those who did not receive such therapies, outcome differences may be related more to the initial characteristics of the patients than to the therapy used. Sometimes this type of selection bias is evident based on the reported data, but even when such differences are not apparent, selection bias should be suspected whenever randomization is not used.
Some disease processes and injury patterns are not readily subject to prospective study or randomization, often because of the rarity of the condition in question or because the diagnosis is evident only in retrospect. For example, an adequately powered prospective randomized study of an intervention for bowel ischemia would require enrollment of a large population, infeasible at a single medical center. For such conditions, retrospective case-control studies are common. Matching of cases (patients with the condition treated with an intervention of interest) with appropriate control groups (patients with the condition treated with a different standard intervention) to ensure that the groups are as similar as possible at outset is critical, as described earlier. Unfortunately, even with stringent attempts at matching cases and controls, the possibility of unmeasured differences between groups remains, potentially confounding the study results. Although case-control studies sometimes provide the best feasible evidence, we must remain dubious of their results for this reason. A case-control study with statistical matching is not a true substitute for a randomized, controlled trial, no matter how ardently authors may try to convince us.
Let’s consider this in more detail. In some cases, randomization is not performed, and the intervention group is composed of an observed cohort of patients undergoing the therapy in question. A control group is constructed using historical (retrospective) data or contemporaneous patients in another location who are presumed to be similar. Although such studies often are the only source of information about the outcomes of an intervention, they are subject to many biases. For historical controls, unmeasured differences in the types of patients presenting, or in the types of other treatments (other than the measured intervention), may be responsible for outcome differences. For example, if the survival in a modern group of trauma patients treated with a new intervention for splenic trauma is compared with a historical group and found to be superior, it is possible that patients in the past were more severely injured (e.g., because of differences in speed limits or airbag design). Thus the apparent association of improved survival with the new intervention may not reflect causation. Moreover, other aspects of care may be different today from in the past. For example, it is possible that deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis practices were less effective in the past than today. If PE was a major cause of death in trauma patients in the past, mortality rates in the current group might be lower, due not to the study intervention (e. g., splenic embolization) but to behind-the-scenes improvements in other prophylactic measures.
When a contemporaneous but nonrandomized control group is used, differences in patient severity and other treatments, rather than the study intervention, may be responsible for outcomes. For example, imagine that a new intervention is available but only on weekdays during business hours. Researchers may compare patients receiving the treatment with those not receiving it, using the patient’s time of arrival as a “pseudorandomization.” Unfortunately, other factors may be associated nonrandomly with time of arrival and thus with treatment. For example, patients arriving late at night on weekends may be more likely to be intoxicated, and hospital staffing may be different at these times, translating into differences in the quality of care. If an outcome difference is noted between those receiving and those not receiving the therapy, it cannot be assumed that the outcome difference is a consequence of the therapy. As described earlier, researchers often attempt to “correct” statistically for the differences in disease or injury severity, but statistical measures cannot fully account for all potential differences. Beware of such study designs when evaluating the strength of evidence for medical interventions.
Although the limitations described earlier may appear unlikely to influence results of observational studies in important ways, such limitations have famously misled the medical community in the past, with potential harm to patients. In the 1990s, observational studies without randomization demonstrated rates of adverse cardiovascular events that were lower in patients who received estrogen replacement therapy than in those who did not—an association that was widely assumed to be causative. This in turn led to national guidelines recommending the broad implementation of estrogen supplementation in perimenopausal and postmenopausal women, before the completion of randomized, controlled trials to confirm a beneficial causative effect of estrogen therapy. When these randomized, controlled trials (heart and estrogen/progestin replacement studies HERS and HERS II) revealed no reduction in cardiovascular deaths from estrogen use and an increased risk for thromboembolic disease, routine estrogen replacement was largely abandoned.1-2
In cardiology research, observations and theoretical considerations suggested a potential benefit to “facilitated” percutaneous coronary intervention (PCI). This technique uses full- or partial-dose systemic thrombolytic therapy before PCI, which is simply a specialized form of interventional radiologic techniques applied to the coronary arteries. It “made sense” that using thrombolysis (known to have a benefit) in addition to PCI (known to have a benefit) would result in a bigger combined benefit. However, the randomized, controlled Assessment of the Safety and Efficacy of a New Treatment Strategy 4 with PCI showed doubled mortality, increased stroke, and more recurrence of cardiac ischemia from this approach.3 Experience shows that observational studies without appropriate randomized control groups can lead to mistaken beliefs in the benefit of medical interventions, sometimes with serious patient outcomes resulting.
Blinding
The measures described earlier assist in ensuring a fair “starting line” for the “race,” which is essential to the fair measurement of study outcomes. Blinding techniques are essential to the remainder of the conduct of the study—the equivalent of ensuring a fair referee in a race. Blinding helps ensure that, other than the intervention in question, other treatments of the two groups are similar. In addition, blinding increases the chance that the measurement of outcomes is not influenced by researcher or subject biases about the treatment. Without blinding, even the most honest researcher may be predisposed to see greater benefit in patients receiving a therapy. Without blinding, patients may feel better because of placebo effects of treatment. Even with blinding, this is possible, but the effect should be equal in both the treatment and control group, since patients will not know to which group they are assigned.
Study Populations
Study populations should be representative of the type of patient to which the therapy in question would be applied in clinical practice. Many published studies of interventional radiologic therapies are not representative of the typical emergency department patient but, rather, reflect highly spectrum–biased populations, sometimes including unusually sick patients. Spectrum bias is discussed in more detail in Chapter 15. Although spectrum bias may not invalidate the study findings if the therapy were applied to similar patients, it does greatly limit the applicability to a more general and diverse patient population—reducing the external validity of the study. Factors that suggest spectrum bias include nonconsecutive patient enrollment, the enrollment of patients after application of many exclusion criteria, and the use of non–emergency department patients, such as surgical intensive care unit patients. For emergency physicians practicing in community hospitals, studies published on tertiary-care medical center patients may be relatively spectrum-biased, and more relevant studies would be those that include both referral centers and community hospital patients.
Publication Bias
A pervasive problem in studies of therapy, as well as diagnosis, is the issue of publication bias—the possibility that studies with negative outcomes may fail to be published. Attempts to limit this include the recent development of study registries, which require that all clinical trials be listed publicly before study initiation (e.g., ClinicalTrials.gov). Most large journals now require study registration for publication of prospective trials and also attempt to obligate publication of negative results, but this does not solve the potential problem of publication bias in retrospective studies, which form the bulk of evidence for many interventional radiologic therapies. Case series may be particularly subject to publication bias with resulting distortion of the apparent effect of treatments. Authors may be more likely to submit series of “successful” cases for publication, and nonconsecutive case series may systematically omit cases with bad outcomes. Publication bias is thought to lead to major overestimation of treatment efficacy.4
Classes of Evidence and Clinical Recommendations
Professional societies such as the American College of Emergency Physicians publish guidelines for practicing physicians, reporting the class of medical evidence summarily and incorporating many of these and other methodologic factors. Clinical recommendations are usually linked to the class of evidence, with level A recommendations based on class I evidence, level B recommendations based on class II evidence, and level C recommendations based on class III evidence (see Table 16-2). Class I evidence provides a high level of certainty that the outcomes reported represent the true effects of interventions. Class I evidence usually comes from large prospective trials with adequate statistical power, representative populations of patients, appropriate randomization and blinding, and other measures to limit bias. Metaanalyses are another source of class I evidence. Class II evidence usually comes from weaker studies. These studies may be limited in one or more ways—for example, by limited study size and statistical power; by use of nonrepresentative study populations; by problems of selection bias, such as lack of randomization; or by problems of comparison groups, such as use of historical controls. Despite these limits, the weight of class II evidence may lead the professional organization to make a guarded clinical recommendation in the absence of stronger data. Future studies can specifically target the limitations of these studies, providing class I evidence. Class III evidence is the weakest scientific evidence, often based on single cases, very small case series, highly selection biased samples, nonrandomized studies, and studies with no comparison group. Although such studies provide the starting point for future research, they should be considered as the source of research hypotheses, not the source of precise and unbiased data to guide clinical decisions. Class III evidence, including the eyewitness accounts of interventional radiologists observing dramatic improvements in patient clinical status following treatment, lack many of the key requirements for proof of causation, including a control group, randomization, blinding, and statistical power. Publication bias also likely plays a major role in favoring publication of favorable outcomes in case reports because clinicians may be less likely to report procedural complications and failures.
With this background, we consider some major applications of image-guided procedures. We begin with angioplasty for myocardial infarction because it is common, familiar to emergency physicians, and supported by rigorous research data, forming a strong standard against which we can compare other interventional radiologic techniques. Following this discussion, we take a head-to-foot approach to additional interventions (Table 16-3).
Angiographically Guided Angioplasty and Stenting of Coronary Arteries for Treatment of ST Segment Elevation Myocardial Infarction
Coronary angiography with the potential for coronary artery intervention such as stenting has become the standard of care for treatment of acute ST elevation myocardial infarction (STEMI). The procedure is usually performed by a cardiologist in the United States, although the instruments and methods are similar to those used by radiologists in other angiographic procedures. A guidewire is introduced into the femoral artery and passed retrograde through the aorta to the coronary arteries. Catheters and stents can be directed to the coronary arteries over the guidewire. The instruments and vessel lumen are visualized using fluoroscopy and a technique called digital subtraction angiography. In this technique, images of the bones and soft tissues (sometimes called a “mask”) acquired before the administration of arterial iodinated contrast agents are digitally removed from the images acquired after contrast administration. This results in images of vascular structures without obstruction by superimposed radiodense anatomy.
Introduced in 1976, percutaneous transluminal coronary angioplasty (PTCA) for STEMI saw broad clinical adoption by the early 1980s.5 Large randomized, controlled, multinational trials in the mid-1990s confirmed the benefits of the procedure. The Global Use of Strategies to Open Occluded Coronary Arteries IIb trial randomized 1138 STEMI patients to PTCA or thrombolytic therapy with recombinant tissue plasminogen activator (t-PA) within 12 hours of STEMI and demonstrated a statistically significant reduction in 30-day mortality in patients treated with PTCA (5.7%) compared to patients treated with t-PA (7.0%).6 Six-month outcomes of death, nonfatal reinfarction, and nonfatal disabling stroke were not significantly different between the two groups. Both PTCA and t-PA outcomes were improved compared with the historical mortality among STEMI patients receiving aspirin, shown to be 9.4% in the second International Study of Infarct Survival (ISIS-2) study.7 This, in turn, was a substantial improvement in comparison with patients in ISIS-2 randomized to no aspirin, who had a mortality of 11.8%. Cucherat et al. performed a Cochrane meta-analysis in which only randomized, controlled trials with no confounding were included. Ten trials totaling 2573 patients showed early primary angioplasty to be superior to systemic intravenous (IV) thrombolysis in experienced centers for short-term mortality.8 Due to class I evidence for its use, PTCA has become the standard of care for STEMI when available within 3 hours.
Image-Guided Therapy for Ischemic Stroke
Ischemic stroke remains a morbid condition, with limited evidence for treatment safety and efficacy. Controversy has continued around the use of systemic thrombolysis because of conflicting interpretation of data, methodologic concerns, and the possibility that t-PA might harm patients through increased intracranial hemorrhage. The National Institute of Neurological Disorders and Stroke (NINDS) trial randomized patients to IV t-PA or placebo and demonstrated a 30% increase in the likelihood of having no or minimal neurologic deficit at 3-month follow-up in patients treated with t-PA, although the results have been contested because of concerns that the two treatment groups differed in stroke severity before treatment, confounding results.9-10 Katzan et al. reported the Cleveland community experience with systemic IV t-PA. In-hospital mortality was 15.7% among patients receiving t-PA, compared with only 5.1% among those not receiving t-PA. 15.7% of patients receiving t-PA also had symptomatic intracranial hemorrhage, including six fatalities. Of the treated patients, 50% suffered protocol violations. This study is a retrospective review; patients were not randomized to receive t-PA, so it is possible that differences in mortality between treated and untreated patients resulted from their preexisting clinical status, not from their treatment. Nonetheless, studies such as this suggest that the positive risk–benefit ratio reported in closely controlled clinical trials such as NINDS might not be achieved if t-PA were widely adopted in the community.11
Even if IV t-PA is assumed to be beneficial, systemic thrombolysis remains a possibility for a small number of patients because of tight time constraints from symptom onset until treatment initiation (3 hours based on the NINDS trial9 and as much as 4.5 hours based on data from the third European Cooperative Acute Stroke Study12-14) and numerous contraindications. Only about 15% of patients arrive within 3 hours after symptom onset.15 Schumacher et al.16 analyzed the National Inpatient Sample and found that 70% of hospitals did not use t-PA. Only approximately 1% of 366,194 ischemic stroke patients received t-PA, an average of only about 3 patients annually per hospital using t-PA.
With questions about benefit or harm of systemic t-PA therapy and limited populations meeting treatment criteria, interest has focused on catheter-based treatment techniques that might be more effective in relieving neurologic symptoms, extend the treatment time window and thus allow therapy for more patients, and have fewer contraindications because of a lower dose of t-PA and lower bleeding risks. These studies examine mechanical clot retrieval, intraarterial t-PA administration, and intracranial artery stenting, all performed under fluoroscopic angiographic guidance. Let’s examine some relevant studies and assess the quality of evidence.
Smith et al.17 reported the results of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial. This study enrolled 141 patients with ischemic stroke who were ineligible for t-PA and performed mechanical embolectomy within 8 hours of symptom onset using an intraarterial device, the MERCI Retriever. This prospective multicenter study was not randomized and therefore used a historical control group, with the methodologic limitations described earlier in this chapter. Vessel recanalization was achieved by 46% of enrolled patients, compared with only 18% in the historical control group. Good neurologic outcomes occurred in 46% of patients who achieved recanalization, compared with only 10% of those who did not. Mortality was lower in patients who achieved recanalization (32%) compared with those who did not (54%). Significant complications of the procedure occurred in 7.1%, and symptomatic intracranial hemorrhage occurred in 7.8%. Although the authors concluded that the device was useful within 8 hours of symptom onset in patients ineligible for t-PA, the study cannot prove that catheter-based mechanical embolectomy improves outcomes, given the absence of randomization or a contemporaneous control group.
Smith18 also reported the early results of the Multi-MERCI trial, which enrolled patients who had received systemic IV t-PA but failed to recanalize, as well as patients who were ineligible to receive t-PA. The prospective multicenter trial had no randomization and treated all patients within 8 hours of symptom onset with mechanical catheter-based clot retrieval. The trial enrolled 111 patients, and 27% had received IV t-PA before enrollment. Vessel recanalization was achieved by 54% of patients with the mechanical device alone, and an additional 15% achieved this when further treatment with intraarterial t-PA was performed. Significant procedural complications occurred in 4.5% of patients. Symptomatic intracranial hemorrhage occurred in 6.7% of those who had been treated with IV t-PA before mechanical embolectomy and in 9.9% of those who had not been so treated. Again, this study cannot prove improved neurologic outcomes without the use of randomization and in the absence of a control group.
Kim et al.19 retrospectively reviewed 24 patients treated with the MERCI Retriever outside of the MERCI trial. Of these, 9 patients were ineligible for the MERCI trial because 4 patients had received prior IV t-PA, 1 patient had received IV t-PA and was younger than 18 years, and 4 patients had exceeded 8 hours since symptom onset. Recanalization was achieved in 63%, using a combination of mechanical clot retrieval and (in some cases) intraarterial t-PA and abciximab (a glycoprotein IIb–IIIa inhibitor of platelet activity). Asymptomatic hemorrhage occurred in 38%, and symptomatic hemorrhage occurred in 8%. In-hospital mortality was 17%, 90-day mortality was 29%, and good 90-day neurologic outcomes occurred in 25%. Although the authors concluded that mechanical embolectomy is an effective means of achieving revascularization for ischemic stroke, this study cannot prove any outcome benefit to patients without randomization or use of a control group. We cannot even know whether patients might have been harmed by the device because outcomes for a control group are not available.
Layton et al.20 reported a case of left internal carotid artery occlusion treated 8 hours after symptom onset using the MERCI Retriever, with significant improvement in neurologic symptoms. The authors concluded that “the final infarct area, as demonstrated on magnetic resonance imaging, was probably much smaller than it would have been if the vessel had not been recanalized.” Such statements, while reflecting the optimism of researchers and interventional radiologists that they may improve patient outcomes, do not constitute proof of efficacy and reveal the bias inherent in such reports. Randomized, controlled trials, with blinded assessment of neurologic outcomes by independent observers, are needed.
Choi et al.21 examined the frequency of endovascular recanalization therapies using the National Inpatient Sample from 1999 to 2002 and found that 0.17% of ischemic strokes were treated with these therapies. The authors also examined outcomes in patients treated with these therapies at Columbia University Medical Center from 2001 to 2004. From the Columbia University sample, 32% of patients treated with endovascular therapies achieved modified Rankin scales of 0 to 2 (indicating slight disability or better, see Table 16-4), and 29% died—one fifth from intracranial hemorrhage. In the national sample, 15% died and 50% were discharged to home or a rehabilitation facility. Despite the lack of a control group for comparison, the authors concluded that the technique is safe and effective.
Table 16-4 Modified Rankin Sale
| Modified Rankin Score | Description |
|---|---|
| 0 | No symptoms at all |
| 1 | No significant disability despite symptoms; able to carry out all usual duties and activities |
| 2 | Slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance |
| 3 | Moderate disability; requiring some help, but able to walk without assistance |
| 4 | Moderately severe disability; unable to walk without assistance and unable to attend to own bodily needs without assistance |
| 5 | Severe disability; bedridden, incontinent and requiring constant nursing care and attention |
| 6 | Dead |
Adapted from Bonita R, Beaglehole R. Modification of Rankin Scale: Recovery of motor function after stroke. Stroke 19(12):1497-1500, 1988.
Shaltoni et al.22 retrospectively assessed data from a prospectively collected database and reported outcomes in 69 patients treated with a variety of intraarterial thrombolytic agents via a catheter after failure to recanalize with IV t-PA. Symptomatic intracranial hemorrhage occurred in 5.8%, including three fatal cases. Recanalization was reported in 72.5%, and a favorable neurologic outcome (defined as discharge to home or inpatient rehabilitation) in 55%. Like the prior studies, this case series suggests possible safety and efficacy but lacks randomization, a consistent therapeutic intervention, or any control group.
Sauvageau et al.23 retrospectively reviewed 10 cases in which middle cerebral artery stenting was performed after failure of recanalization with the MERCI Retriever and (in some cases) intraarterial t-PA. In this review, 9 of 10 patients achieved recanalization, although the rate of complications was high: it included 6 cases of intracranial hematoma or subarachnoid hemorrhage, 1 case of extradural perforation with arteriovenous fistula formation, and 4 deaths. This small case series without randomization or a control group raises the possibility of this technique but does not prove any benefit.
Gonzalez et al.24 retrospectively reviewed a series of nine patients with ischemic stroke treated with catheter-based mechanical thrombectomy with a microsnare and low-dose intraarterial t-PA or angioplasty. They report 77.8% clot removal. At 3 months, two patients had a normal modified Rankin scale, three patients had significant neurologic impairment (modified Rankin scale of 3 to 4), and two patients had died. The authors concluded that the device is safe, but larger randomized, controlled trials would be required to prove benefit.
Smith et al.25 reported final results of the Multi-MERCI trial, including patients treated with a newer generation MERCI Retriever (the L5 Retriever). Although higher rates of recanalization, lower mortality, and better neurologic outcomes were associated with the newer device compared with the older device, these did not achieve statistical significance. Moreover, because patients were not randomized to one device or the other and no third control arm existed (untreated with either device), we cannot conclude that either device is superior to no treatment.
Class II evidence including nonrandomized trials and case series suggests that catheter-based endovascular therapies, including mechanical clot retrieval and intraarterial thrombolysis, are feasible. However, in the absence of large randomized, controlled trials, it is premature to conclude that neurologic outcomes and mortality are improved by the use of any of these techniques. Suggested indications for interventional neuroradiologic therapies are shown in Box 16-1.
Angiographic Embolization for Aneurismal Subarachnoid Hemorrhage
Catheter-based therapy with endovascular embolization has become the accepted method of treatment for most aneurismal subarachnoid hemorrhage. Before the introduction of this technique, craniotomy and surgical clipping of the aneurysm neck was required, a highly invasive procedure. To perform endovascular embolization, an arterial catheter is inserted and advanced into the internal carotid artery and then to the location of the aneurysm, which is usually positioned along the circle of Willis or arterial branches emanating from this vascular ring. The position of the catheter is identified by fluoroscopy using digital subtraction of bony structures as described earlier in the section on coronary artery interventions. Intraarterial iodinated contrast is injected to reveal the intracranial vasculature, including the aneurysm sac. A thin metal wire is advanced into the aneurysm and coiled within the sac, slowing blood flow and leading to internal thrombosis of the aneurysm (Figure 16-1). This excludes the aneurysm from the arterial circulation, dramatically decreasing the risk for rupture.
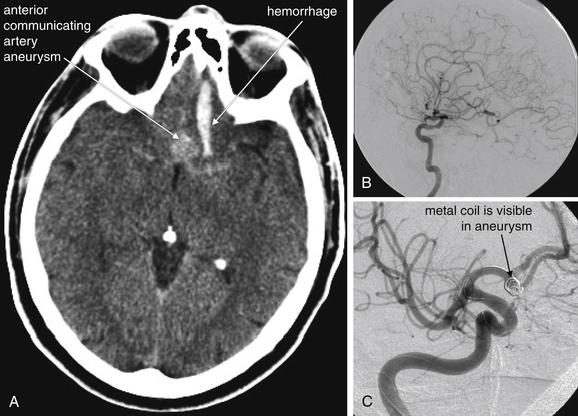
Figure 16-1 Coiling of cerebral artery aneurysms allows nonsurgical treatment.
Angiographic embolization of cerebral arterial aneurysms is now a standard treatment, based on a large international trial.A, Noncontrast head CT, showing a large anterior communicating artery aneurysm with associated subarachnoid hemorrhage. It is unusual to visualize the aneurysm itself on noncontrast head CT. Noncontrast CT and CT angiography have largely supplanted conventional angiography for diagnosis of aneurysm, with conventional angiography reserved for diagnosis of equivocal cases and treatment of cases confirmed by other modalities. B, Cerebral angiogram in the same patient. C, A metal coil is placed by an interventional neuroradiologist to obliterate the cerebral aneurysm. No contrast is seen in the aneurysm, indicating successful embolization.
(From Broder J, Kuo WT. Therapeutic applications of interventional radiological techniques in emergency medicine. 2007 Accessed at http://emedhome.com.)
Molyneux et al.26 conducted a prospective controlled trial of patients with subarachnoid hemorrhage from ruptured intracranial aneurysms, randomized to treatment with either endovascular embolization (“coiling”) or neurosurgical clipping. The International Subarachnoid Aneurysm Trial (ISAT) was planned to include 2143 patients but was stopped early after a planned interim analysis showed better outcomes in patients undergoing endovascular therapy. At 1-year follow-up, 190 of 801 (23.7%) patients randomized to endovascular therapy were dependent or dead, compared with 243 of 793 (30.6%) treated surgically (p = 0.0019). The relative and absolute risk reductions for dependency or death using endovascular therapy were 22.6% (95% CI = 8.9-34.2) and 6.9% (95% CI = 2.5-11.3), respectively. Re-bleeding risks at 1 year were minimal in both groups. In a second publication, the same authors reported the survival advantage of endovascular coiling to persist up to 7 years.27
Van der Schaaf et al.28 performed a Cochrane meta-analysis, identifying three randomized trials comparing endovascular embolization (coiling) with surgical clipping for treatment of aneurismal subarachnoid hemorrhage. One of the included trials was ISAT, discussed earlier, which dominates the overall effect because it includes by far the most enrolled patients. The trials included 2272 patients, most with anterior cerebral circulation aneurysms and in good clinical condition before their procedure. At 1-year follow-up, poor neurologic outcomes were less common in patients randomized to coiling, with a relative risk of 0.76 (95% CI = 0.67-0.88). The absolute risk reduction for bad neurologic outcomes was 7% (95% CI = 4%-11%) for patients treated with coiling compared with patients treated surgically. For patients with a posterior circulation aneurysm, the relative risk for poor neurologic outcome was 0.41 (95% CI = 0.19-0.92) with an absolute decrease of 27% (95% CI = 6%-48%).
As a consequence of these trials, which provide class I evidence of benefit from endovascular coiling of cerebral aneurysms with subarachnoid hemorrhage, endovascular therapy has become the standard approach. Not all aneurysms are amenable to endovascular coiling. The neck of the aneurysm must be relatively narrow to allow the coil to be trapped within the aneurysm sac, and the aneurysm cannot include the origins of branch vessels; otherwise, they could be thrombosed by the coiling. These features of the aneurysm sac are often evaluated today by CT angiography (CTA) with three-dimensional reconstruction, before conventional angiography. For aneurysms 5 mm or larger, CTA is approximately 94% sensitive and magnetic resonance angiography is approximately 86% sensitive.29
Interventional Radiologic Therapies for Pulmonary Embolism
Neither standard therapy with systemic anticoagulation nor aggressive therapy with systemic thrombolysis eliminates the morbidity and mortality of PE, leading to interest in image-guided interventions. Naess et al.30 found that the 30-day case-fatality rate in PE was 9.7% using population-based data from 1995 to 2001. Nijkeuter et al.31 reported 3-month mortality of 8.2% in patients treated for PE with anticoagulation. Thabut et al.32 performed a metaanalysis of nine randomized, controlled trials (461 patients) comparing heparin with thrombolysis. The studies varied considerably in methodology: eight used systemic drug administration, and only one used intrapulmonary drug administration. Thrombolysis had no statistically significant effect on mortality. The relative risk for mortality was 0.63 for patients treated with thrombolysis, with 95% CIs from 0.32 to 1.23. When 95% CIs for relative risk span the value 1.0, this indicates a lack of statistical significance because the true relative risk may be decreased (<1) or increased (>1). However, because the mean value was 0.63, which would suggest a potential mortality risk reduction of more than 30%, one interpretation is that a very large trial might yet prove a statistically significant mortality reduction. Metaanalyses such as this demonstrate the difficulty of attempting to determine the potential benefit or harm of a therapy from multiple small trials with varying methodology—a large multicenter trial following a standardized protocol is needed. Similar problems plague the existing literature on image-guided, catheter-based therapies, discussed later.
Konstantinides et al.33 conducted just such a trial in 256 hemodynamically stable patients with submassive PE, characterized by pulmonary hypertension or right ventricular dysfunction without systemic arterial hypotension or shock. They randomized 118 patients to heparin plus systemic IV t-PA and 138 patients to heparin plus placebo. The trial showed a statistically significant decreased requirement for escalated therapy (catecholamine infusion, secondary thrombolysis, endotracheal intubation, cardiopulmonary resuscitation, or emergency surgical or catheter thrombectomy) in patients treated with systemic t-PA (10.2%) compared with patients receiving placebo (24.6%). However, no statistical difference in mortality was observed. Mortality was 3.4% in patients treated with heparin and t-PA and 2.2% in those receiving heparin and placebo (p = 0.71).
Mortality from PE may not tell the complete tale; significant morbidity from PE may occur, with secondary pulmonary hypertension developing in as many as 8.8% and symptomatic pulmonary hypertension in 4.4%.34 Tapson and Humbert35 estimated chronic thromboembolic pulmonary hypertension to be 1% 6 months after PE, 3.1% 1 year after PE, and 3.8% 2 years after PE. Piovella et al.36 concluded that 1% to 4% of patients with PE develop chronic thromboembolic pulmonary hypertension.
Given the high mortality and morbidity of PE, numerous investigators have attempted catheter-based therapies for PE, including thrombectomy with mechanical clot fragmentation and aspiration, as well as intrapulmonary artery administration of thrombolytic agents (Figure 16-2). Goals of these therapies include reducing acute mortality and long-term sequelae of pulmonary hypertension. Large obstructing pulmonary emboli might create less hemodynamic compromise when fragmented and either mechanically removed or allowed to embolize distally. Clot fragmentation might increase the exposed surface area of clot, improving the action of thrombolytic agents when these are used with mechanical clot disruption. An additional potential benefit of catheter-based therapies is that mechanical clot disruption and removal can be used in patients with contraindications to systemic anticoagulation or thrombolysis. Tumor emboli (such as tumor fragments from invasive renal cell carcinomas) that do not benefit from anticoagulation or thrombolysis can be treated using image-guided, catheter-based therapies. Let’s consider the evidence for these therapeutic approaches, keeping in mind that strong evidence would require consecutive subject enrollment, adequate study size to provide statistical power, use of a concurrent control group, randomization to treatment arm, and blinded measurement of clinically important patient outcomes. As we demonstrate, the existing studies fulfill few of these criteria, and even the inclusion criteria and indications for image-guided therapies are not uniform.
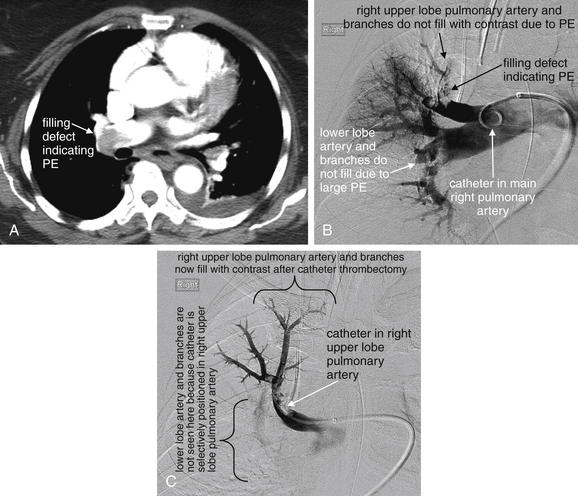
Figure 16-2 Catheter angiography can be used to mechanically fragment and remove pulmonary embolus (PE) or to introduce thrombolytic agents directly into the clot.
In this patient diagnosed with massive PE by CT (A), hypoxia and hypotension persisted after heparin treatment.B, Pulmonary angiogram shows a large filling defect in the right upper lobe pulmonary artery, as well as defects in the right lower lobe and interlobar arteries. Mechanical thrombectomy with a 6-French pigtail catheter was performed, followed by infusion of 5 mg of tissue plasminogen activator (t-PA) into the right upper lobe pulmonary artery filling defect.C, Repeat angiogram following the catheter thrombectomy and t-PA administration shows improved flow through the right upper lobe pulmonary artery, which has been selectively cannulated.
(From Broder J, Kuo WT. Therapeutic applications of interventional radiological techniques in emergency medicine. 2007. Accessed at http://emedhome.com.)
Tibbutt et al.37 randomized 30 patients to intrapulmonary artery infusion of either heparin or streptokinase. The study lacked blinding and did not follow an intention-to-treat analysis as is recommended today. Instead, 7 patients who failed to complete the protocol were excluded from analysis, leaving only 12 patients in the heparin group and 11 in the streptokinase group. The angiographic appearance of clot and pulmonary arterial pressures were improved to a greater extent in the streptokinase group initially and at 6 months, but meaningful clinical outcomes were not measured. The small study size and exclusion of nearly one third of patients from analysis limits the value of the study. Streptokinase is also no longer the thrombolytic agent typically in use today.
Verstraete et al.38 compared systemic IV t-PA to intrapulmonary artery administration (performed during fluoroscopic pulmonary angiography) in 34 patients with massive PE. Both therapies improved pulmonary artery pressures and angiographically measured severity of PE, with no apparent advantage of intrapulmonary artery therapy. However, this study was not powered to determine superiority of one approach over the other and did not measure important clinical endpoints. In addition, techniques and devices for pulmonary angiography have evolved subsequently, leading some interventional radiologists to discount this study.
Timsit et al.39 reported catheter pulmonary embolectomy in 18 patients with massive PE, with 72% survival. Greenfield et al.40 reported a series of 46 patients with massive PE undergoing vacuum extraction of clot by catheter between 1970 and 1992, with a 30-day survival of 70%. Tajima et al.41 described three cases of superselective t-PA infusion through a pulmonary artery catheter, with immediate angiographic improvement. However, no clinical outcomes or comparison group were reported. Uflacker et al.42 described five patients with massive PE treated with a catheter device that pulverized thrombus using a vortex. One patient died shortly after the procedure; the four remaining patients survived to discharge. Stock et al.43 reported five patients with massive PE treated by thrombus fragmentation and intrapulmonary artery injection of t-PA. All five showed angiographic improvement and improved pulmonary artery pressures, but two required transfusion for retroperitoneal hematomas.
Schmitz-Rode et al.44 described mechanical fragmentation of massive pulmonary emboli in 10 patients using a rotating pigtail catheter and supplemented in 8 patients with thrombolysis. Clot fragmentation was successful in 7 cases. Improvements in shock index were observed, and 48-hour pulmonary pressure measurements also decreased significantly. Despite this, the mortality was 20%. The authors reported that no procedural complications occurred, though safety cannot be established by such a small study. This study cannot prove a clinical benefit to patients, given its small size and lack of control group. Nonetheless, it demonstrated the feasibility of mechanical fragmentation.
Rocek et al.45 reported a single case of mechanical thrombectomy using a percutaneous catheter. Murphy et al.46 reported four patients with massive PE treated with percutaneous catheter clot fragmentation using standard angiography catheters and guidewires, followed by local t-PA administration. All four survived to hospital discharge and had improved pulmonary angiography findings.
Fava et al.47 described 11 patients with massive PE undergoing mechanical thrombectomy with a catheter device. Only 1 patient died during the procedure—the authors concluded the death was because of PE, not procedural complications. The other 10 patients had improved pulmonary artery pressures and arterial oxygen partial pressures and survived to discharge.
Schmitz-Rode et al.48 reported 20 patients with massive PE treated by mechanical fragmentation with a rotating pigtail catheter, some with additional thrombolysis. Shock index and pulmonary artery pressures improved, and mortality was 20%. It is unclear whether the patient group reported included some of the same patients described in the 1998 publication by the same group44 (described earlier).
De Gregorio et al.49 treated 59 patients with massive PE using mechanical clot fragmentation and intrapulmonary thrombolysis. The authors reported 6% mortality, with clinical improvements and improved pulmonary artery pressures in all survivors at 3 to 6 months.
Zeni et al.50 described fragmentation and aspiration of massive pulmonary emboli using high-velocity saline jets from a specialized catheter. They treated 17 patients, and 10 received additional thrombolytic therapy via the catheter. Angiographic improvement and initial improvement in dyspnea and oxygen saturation were seen in 16 of 17 patients. Although 2 patients died, the remaining 15 survived to hospital discharge, though long-term follow-up was not reported. Again, no control group was used in this study, preventing comparative assessment of the effect of therapy.
Reekers et al.51 described eight patients treated with both mechanical catheter thrombectomy and systemic thrombolysis. Normalization of partial pressure of oxygen values occurred in all patients, with minimal change in pulmonary arterial pressures. The authors were only moderately successful in removing clot using their technique, with a mean of 50% removed. The authors noted immediate symptomatic improvement in all patients; however, one patient died shortly after the procedure, reportedly from heart failure. Despite this, the authors asserted no procedural complications. Small studies such as this highlight the need for blinding and a control group in establishing not only benefit but also harm. It is possible that the procedure contributed to the death of one patient, but the unblinded authors did not draw this conclusion. Without randomization or a control group, we cannot establish any causal relationships or estimate the benefit or injury related to this technique.
Tajima et al.52-53 reported treatment of 25 patients with massive PE with mechanical fragmentation using a rotating pigtail catheter, with local thrombolytic therapy and clot aspiration, followed by systemic thrombolysis. All patients survived the procedure, with improvements in angiographic appearance and pulmonary artery pressures. However, long-term clinical outcomes were not reported. The authors attributed two deaths before discharge to ovarian and lung cancers—not to PE or procedural complications. Several problems exist with attempts to interpret this data. The authors did not describe their inclusion criteria, so the clinical status of the patients undergoing this therapy is uncertain. No control group, randomization, or blinding was present, so the potential benefit or harm of the intervention cannot be determined. Tajima et al.54 also reported 15 patients with massive PE treated by manual aspiration of clot through a standard large lumen catheter designed for PTCA. All patients survived the procedure.
Despite the multitude of small studies describing clinical experience with a variety of image-guided, catheter-based therapies, we cannot draw any firm conclusions about the benefit, harm, or indications for these interventions because of the lack of appropriate methodology. A large randomized, controlled trial with blinding is needed to establish evidence of benefit. In the absence of such evidence, the 2004 guidelines of the Seventh American College of Chest Physicians Conference on Antithrombotic and Thrombolytic Therapy recommended against the use of catheter-based therapies except in patients unable to receive thrombolytic therapy or whose critical status does not allow the 2-hour infusion time for systemic t-PA.55 Future studies will require more standardized inclusion criteria with stratification of patients with similar mortality risk to allow fair comparisons of outcome. Let’s briefly examine some recent studies on PE prognosis.
Pulmonary Embolism Prognosis: Role of Imaging and Biomarkers
One basic point of disagreement is the definition of massive PE, which is a vital starting point for studies purporting to examine the effect of catheter-based therapies. Some studies characterize massive PE by patient clinical response, including hypotension requiring inotropic or vasopressor support or respiratory failure requiring mechanical ventilation. Others use clot burden determined by imaging criteria, even in the absence of current severe hemodynamic or respiratory compromise. Still others characterize massive PE by biomarker surrogates of heart failure. The tantalizing promise of imaging or biomarker criteria is that these might be shown to predict risk for subsequent deterioration or death in currently stable patients. This in turn might allow selection of patients for invasive therapy while the patients are still able to tolerate the procedure and before such severe clinical deterioration that no intervention would likely improve mortality.
Engelke et al.56 described a CT scoring system to predict cor pulmonale and short-term survival in massive PE. In a retrospective study of 89 consecutive patients with acute PE diagnosed by CTA, a CT severity score was more predictive of elevated pulmonary artery pressures, cor pulmonale, and death within 30 days than were two severity scores based on conventional angiography.
Ghaye et al.57-58 reviewed the literature on CT clot burden scores and reported their limited prognostic value. In a retrospective study of 82 consecutive patients admitted to an intensive care unit for PE and evaluated with CT, the same authors measured a variety of cardiac and great vessel diameters and pulmonary artery clot burden and correlated these with patient outcome. Clot burden measured by CT did not correlate with inpatient mortality, whereas the right ventricle–left ventricle ratio and azygos vein dimensions (both thought to be surrogates of right heart failure) did correlate strongly with survival.
He et al.59 compared CT measurements and echocardiographic findings of right heart failure. In their retrospective study of 74 consecutive adults with PE diagnosed by non-cardiac-gated CT, right heart dysfunction was diagnosed if the right ventricle appeared dilated on visual inspection (no specific diameter or ratio defined) or if the interventricular septum appeared straightened or bowed into the left ventricle. Somewhat counterintuitively, the authors concluded that CT findings were more sensitive (81% CT, 56% echo) and specific (47% CT, 42% echo) than echocardiography findings in demonstrating right heart dysfunction. The reference standard for right heart dysfunction in this study is questionable: pulmonary vascular obstruction of at least 30% as measured on CT. It is unclear whether this measure should be considered more indicative of right heart dysfunction than echocardiographic findings. The CT findings of right heart failure were not compared with patient outcomes, the more clinically relevant endpoint. In addition, the reported sensitivity and specificity values for CT are likely too low to be clinically useful because one in five cases of right heart dysfunction would be missed and half of CT-diagnosed cases of right heart failure would be false positive.
In a larger study comparing CT findings to clinical outcomes, Araoz et al.60 retrospectively reviewed CT scans in 1193 patients with CT scans positive for PE and concluded that embolic burden and the ratio of right ventricular to left ventricular diameter were not predictive of short-term mortality. Ventricular septal bowing into the left ventricle was insensitive (around 20%) but specific (87%) for short-term mortality, defined as in-hospital death or death within 30 days of CT.
Ryu et al.61 retrospectively reviewed 546 consecutive patients with acute PE diagnosed by CTA and found 14 patients (2.6%) with saddle PE, none with preexisting cardiopulmonary disease. Saddle PE had previously been described as a likely marker of PE mortality. The authors noted that none of the patients died during their initial hospitalization and thus concluded that saddle PE may not require aggressive medical management in the absence of comorbid cardiopulmonary disease. However, this conclusion may be limited by several factors. One patient was treated with thrombolytic therapy, and 4 others received inferior vena cava (IVC) filter placement, interventions that may have limited their mortality. Also, 4 patients (29%) died within 1 year. Finally, the authors did not assess for morbidity such as pulmonary hypertension in these patients.
More work remains to be done in identifying CT findings that strongly predict mortality or morbidity from PE and thus might be indications for invasive therapy. Suggested indications are listed in Box 16-2.
Box 16-2 Indications for Interventional Radiologic Therapy in Massive Pulmonary Embolism
From Uflacker R. Interventional therapy for pulmonary embolism. J Vasc Interv Radiol 12:147-164, 2001.
Several studies have investigated the association of echocardiographic evidence of right heart failure and elevated cardiac troponin I with mortality in PE. Right heart dysfunction by echocardiography presumably occurs because of high pulmonary arterial pressures and obstruction of right ventricular output in massive PE. Elevated troponin I may indicate right ventricular injury produced by these same factors.
Grifoni et al.62 reported a prospective cohort study of 209 consecutive patients with acute PE and found that among patients with initial normotension the presence of right ventricular dysfunction demonstrated by echocardiography predicted an increased risk for shock (10%) and in-hospital mortality (5%), compared with patients without right ventricular dysfunction (0% shock, 0% mortality). The authors suggested that echocardiographic right ventricular dysfunction be considered a criterion for aggressive therapy—although as we saw earlier, the evidence for benefit from invasive therapies is limited.
Meyer et al.63 prospectively studied 36 patients with acute PE and found that elevated troponin I was significantly associated with right ventricular dilatation on echocardiography—a potentially clinically useful correlation because measurement of troponin I levels is more widely available and more available outside of weekday hours than is echocardiography.
In a case-control study, Yalamanchili et al.63a reported that 33% of PE patients with elevated cardiac troponin I died during hospitalization, compared with only 7% with normal cardiac troponin I, suggesting that increased troponin levels may be an indication for aggressive treatment. Again, the leap from increased mortality to indication for intervention seems logical but is not truly evidence-based without randomized, controlled trials.
Gallotta et al.64 prospectively studied 90 consecutive hemodynamically stable emergency department patients with acute PE and found that elevated troponin I predicted the development of hemodynamic instability, with a nearly 10-fold risk for instability in patients with high troponin values compared with patients without elevated levels.
In a retrospective chart review of 77 patients with acute PE, Aksay et al.64a found that elevated troponin I levels were associated with in-hospital mortality, hypotension, need for inotropic support, and need for mechanical ventilation. Again, the implication may be that troponin I elevations should prompt invasive therapy, but randomized, controlled trials to determine the clinical benefit are needed.
Despite the absence of class I evidence supporting image-guided, catheter-based therapies for PE, some experts strongly favor these therapies. Among interventional radiologists, catheter-directed embolectomy or thrombolysis for massive PE has been nicknamed the “Lazarus maneuver,” reflecting the conviction among experts that these procedures can sometimes literally bring back the dead. Advocates of catheter-based treatment cite the lower dose of t-PA typically used (20-mg average at Stanford University,65 compared with 100 mg of systemic IV t-PA), which would be predicted to have lower bleeding complications than the 1% to 5% reported with systemic thrombolysis.33 Historically, the reported mortality of pulmonary angiography was as high as 1%, but this figure may not reflect modern angiography techniques. In the past, power injection may have contributed to fatal right heart failure in patients with high pulmonary artery pressures, but modern protocols use low-pressure hand injection for patients with pulmonary hypertension.65 Hoeper et al.66 reviewed 7218 right heart catheterizations in patients with pulmonary hypertension at 15 U.S. and European medical centers from 2000 to 2005. The procedural mortality rate was 0.055%. This should be interpreted cautiously because these included diagnostic catheterizations, not therapeutic interventions in unstable patients with acute massive PE. Perhaps more aggressive use of interventional radiologic techniques could improve mortality in some patients with massive PE, although decompensation following treatment is a theoretical possibility if fragments embolize to previously unaffected lung zones.67 Until further research with appropriate comparison groups determines the clinical effect of image-guided therapies, catheter-based clot fragmentation or thrombolysis should be considered an unproven treatment option in patients with massive PE, particularly those with contraindications to t-PA, hemodynamic instability, or failure of systemic thrombolysis. Discussions with patients should include an assessment of the risk for the intervention, as well as mortality risks without the intervention.
Fluoroscopically Guided Vena Cava Filter Placement for Patients at High Risk for Venous Thromboembolism
IVC filters to prevent migration of lower extremity DVT to the lungs have been in use since the 1970s. The devices include a range of spoked and mesh wire configurations, deployed in the IVC through a catheter under fluoroscopic guidance. Controversy about their use persists. The devices offer a treatment alternative in patients with DVT or PE who cannot be anticoagulated, and an adjunct in DVT patients at high risk for death from PE, despite anticoagulation. They can be used for PE prophylaxis in patients without DVT but with high risk for developing thromboembolism because of trauma or surgery. However, the devices do not eliminate the risk for PE and have some associated risks. Small emboli may pass through the filter, and a clot trapped by the filter may propagate beyond the filter and travel to the lungs. Filters themselves can migrate or embolize to the heart. Filters may lead to IVC thrombosis, and slow IVC blood flow related to the presence of a filter can increase the subsequent risk for DVT. We briefly examine some evidence for their use.
The routine use of IVC filters to prevent PE in patients with DVT has been investigated with good study methodology. The Prevention du Risque d’Embolie Pulmonaire par Interruption Cave trial prospectively randomized 400 patients with proximal DVT to permanent IVC filter placement or no placement, in addition to standard anticoagulation. IVC filtration reduced PE incidence at 2 years, with an increase in DVT during the same time period. At 8-year follow-up, the cumulative rate of PE was 6.2% in patients with filters and 15.1% in the no-filter group (p = 0.008). DVT occurred in 35.7% of the filter group and 27.5% of the no filter group (p = 0.042). The rate of death was equivalent in the two groups at both 2- and 8-year follow-up.68-69 The investigators concluded that routine IVC filtration was not indicated in patients with DVT, although they acknowledged that high-risk patients might benefit. For example, this study did not investigate the potential benefit in patients not eligible for systemic anticoagulation or in patients whose risk for death from PE might be elevated because of significant comorbid cardiovascular disease.
Jaff et al.70 reviewed a prospective multicenter U.S. registry of patients with acute DVT diagnosed by ultrasound and found that 14% underwent IVC filter placement for a variety of indications: contraindications to anticoagulation (42%), primary prophylaxis of PE (33%), major bleeding while anticoagulated (13%), and anticoagulation failure (11%). The authors concluded that such a high rate might not be warranted—particularly use of a filter as a primary means of PE prophylaxis.
Giannoudis et al.71 reviewed the use of vena cava filters in trauma patients, a population with high short-term risk for PE and frequent contraindications to systemic anticoagulation. Historically, approximately 60% of admitted trauma patients developed DVT and 20% developed PE, although these numbers may not accurately reflect rates today with less invasive therapies for abdominal injuries and earlier mobilization of patients. Wojcik et al.72 contacted trauma patients who had undergone IVC filtration and found no pulmonary emboli had been diagnosed at a mean follow-up of 28.9 months. However, of 191 patients contacted, only 105 responded—with the possibility that many nonrespondents had developed PE or even died of PE. Of patients who had undergone prophylactic filter placement, 44% developed a subsequent DVT. Imberti et al.73 reported 30 patients undergoing retrievable IVC filter placement for a variety of indications, including acute thromboembolic disease with contraindications to anticoagulation and prophylaxis following trauma or before surgery. Of these patients, 10% had emboli trapped in the filter and 7% had DVT. No PEs were reported, although patients did not systematically undergo evaluation for PE. It is possible that physicians may have been deterred from investigating PE by the presence of the IVC filter.
Large randomized trials in trauma patients do not exist, but the Eastern Association for the Surgery of Trauma has issued guidelines for prophylactic vena caval filter insertion (Box 16-3).74 The American College of Physicians and American Academy of Family Physicians performed a systematic review and concluded that current evidence is too weak to justify treatment recommendations for IVC filters.75 The Society of Interventional Radiology multidisciplinary consensus conference developed relative indications for IVC filter placement, based on the limited available evidence (Box 16-4).76
Box 16-3 Eastern Association for the Surgery of Trauma Guidelines for Prophylactic Vena Cava Filter Insertion in Trauma Patients
From Pasquale M, Fabian TC. Practice management guidelines for trauma from the Eastern Association for the Surgery of Trauma. J Trauma 44:941-956, 1998; discussion 56-7.
Consider vena cava filter insertion if the patient meets all three of the following major criteria:
Box 16-4 Society of Interventional Radiology Indications for IVC Filter Placement
From Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol 17:449-459, 2006.
Image-Guided Therapy for Traumatic Hemorrhage: Angiographic Embolization
Before the advent of CT for diagnosis of trauma, angiography was an important diagnostic tool to detect the presence of thoracic aortic, abdominal solid organ, or pelvic vascular injury. Today, CT fills this primary diagnostic role, and angiography plays a predominantly therapeutic role. Pryor et al. compared the 1993 to 1995 period with the 2000 to 2002 period and found a decrease in the use of angiography from 7.1% to 4.0% of all trauma patients at the University of Pennsylvania, whereas the percentage of therapeutic angiograms rose from 10% to 22%.77
Angiographic embolization of abdominal solid organ injuries and hemorrhage from pelvic vascular injuries involves arterial catheterization, usually through the femoral artery. The catheter is advanced into the blood vessel of interest, usually a point of hemorrhage identified by a preceding CT scan with IV contrast. As described in Chapter 10, active hemorrhage at the moment of CT is usually visible as a bright white amorphous blush of injected contrast escaping from an injured blood vessel. The angiography catheter is advanced as far as possible into the vessel, just proximal to the point of injury. The vessel is then embolized using one of several techniques, including metal coils or Gelfoam particles. By selectively occluding just the bleeding branch vessel, the interventional radiologist seeks to terminate bleeding while avoiding ischemic infarction of large watershed areas supplied by the injured artery. The procedure is visualized using digital subtraction angiography, as described earlier in this chapter. In this technique, precontrast images are used as a “mask,” allowing the digital removal from the image of radiodense objects such as bone that would interfere with views of contrast-filled blood vessels.
Angiographic embolization can be used as definitive therapy for some injuries or as a temporizing or stabilizing maneuver in patients with multiple injuries. In some cases, embolization slows bleeding, allowing resuscitation and stabilization before an operative intervention for the same injury. In other cases, embolization stabilizes an abdominal or pelvic injury, allowing a critical head, spine, thoracic, or extremity injury to be addressed.
Determining which injuries require angiographic embolization has been an area of significant research, as we discuss later in detail. The goal of such research is to identify patients who are at high risk for ongoing or recurrent hemorrhage, allowing these patients to be treated angiographically, whereas patients at lower risk are observed and spared this invasive therapy. Ideally, patients who will ultimately fail angiographic embolization and require surgical therapy would be prospectively identified as well so that operative treatment might be selected as the primary treatment strategy. Injury grading scales have emerged from this research, providing guidance to clinicians (see Tables 10-2 and 10-3).78-84
Which Patients Are Candidates for Angiographic Embolization?
Some general principles govern the use of angiography for blunt trauma:
Angiographic embolization has become a standard part of nonoperative management of blunt traumatic solid organ injury, but surprisingly, a PubMed search reveals no randomized, controlled trials to study the effect of this therapy. The evidence for embolization therapy of various injury types is described later. As we have already discussed in this chapter, good research methodology for determining the effect of a treatment requires a large study (to allow precise measurement of treatment effects with narrow CIs), recruitment of a representative consecutive sample (to avoid selection biases and to improve both internal and external validity), prospective randomization to treatment or control arms, and blinded measurement of clinically important patient outcomes. For most therapeutic applications of angiography in blunt trauma, these criteria are not met. Instead, retrospective or prospective cohorts of patients undergoing angiographic embolization are studied. The comparison groups are concurrent or historical patient groups who were not treated with embolization. Outcomes in the two groups are then compared. Although this is the best available evidence, it falls short of proof of a causal relationship. Nonrandomized studies such as this are quite susceptible to selection bias, and the results should be recognized as demonstrating associations, not causation. We point out these limitations again as we review the major applications of angiography.
Angiographic Embolization of Blunt Splenic Injuries
Nonoperative management of blunt splenic injuries has gained favor over the past 30 years with the ability to grade splenic injuries and recognize active bleeding with CT. Nonoperative management typically consists of patient observation, with serial physical examination, measurement of hematocrit, trending of vital signs, and sometimes repeated CT scan to assess for continuing hemorrhage. Angiographic embolization is used in stable patients with active hemorrhage on initial CT, and in those with high-grade injuries, because of an increased risk for recurrent hemorrhage. Benefits of nonoperative therapy include reduction in morbidity from laparotomy and preservation of the spleen, which is believed to serve important immune functions. Initial nonoperative therapy is now widespread, applied in 60% of splenic injuries in some published series and successful in up to 80%.85 Despite the wide adoption of splenic artery embolization, the practice has not been studied with strong comparative methodology. A PubMed search (April 9, 2010) for “spleen trauma embolization” found 155 matches, with no randomized, controlled trials. Instead, most studies of this therapy compare two or more concurrent or historical cohorts and correlate the frequency of splenic artery embolization therapy with the success rate of nonoperative therapy or with mortality. This study design can suggest but not prove a causal relationship because other changes in therapy over time or a variety of biases may influence the results.
Peitzman et al.85 retrospectively studied 1488 splenic injuries using a registry from 27 trauma centers. Grade I and II injuries were unlikely to fail nonoperative management (4.8% and 9.5%, respectively), even without embolization, which was rarely used in this study. The Eastern Association for the Surgery of Trauma guidelines conclude that class III evidence indicates angiography should be used as an adjunct to nonoperative management in hemodynamically stable patients with evidence of ongoing bleeding from splenic injuries (Figure 16-3). They also conclude that class II evidence indicates that a high CT grade of splenic injury and large hemoperitoneum are not absolute contraindications to nonoperative therapy.86
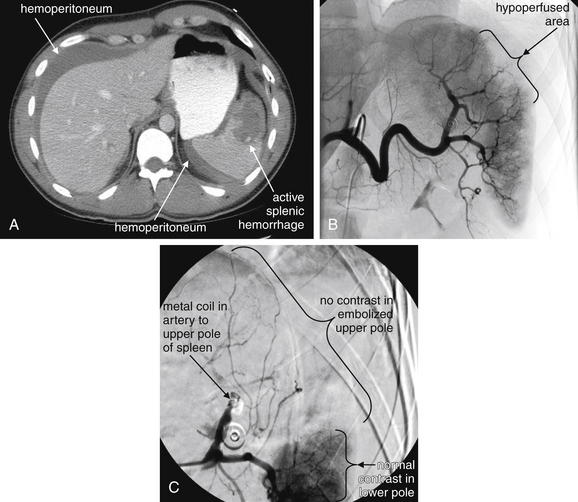
Figure 16-3 Angiography is routinely used for treatment of solid organ injuries in blunt trauma.
Patients with significant liver and spleen injury detected on CT following blunt trauma are frequently treated with angiographic embolization. Indications include active vascular contrast extravasation on CT, large hemoperitoneum, and high-grade solid organ parenchymal injury.
This 25-year-old male has active splenic hemorrhage and hemoperitoneum.A, Active extravasation within the spleen and hemoperitoneum on CT, indications for splenic artery embolization.B, Angiography shows an area of hypoperfusion in the region of injury, rather than the expected hemorrhage seen on CT. Perhaps vasospasm has halted bleeding. This is not an uncommon scenario, with angiography showing less bleeding than that detected by CT.C, A metal coil has been placed in the arterial branch to the upper pole of the spleen to prevent recurrent bleeding.
(From Broder J, Kuo WT. Therapeutic applications of interventional radiological techniques in emergency medicine. 2007. Accessed at http://emedhome.com.)
Haan et al.87 described the results of a protocol for selective angiography and embolization in patients identified by CT as having splenic injury and minimal associated injuries. The authors reported a high rate of successful nonoperative management and decreased length of stay compared with their historical practice of admission angiography for all patients with CT-identified splenic injuries. In their protocol, which excluded patients with hemodynamic instability, patients with grade I splenic injuries did not undergo routine angiography. Hemodynamically stable patients with higher grades of injury, including patients with grade IV and V injuries, were managed with routine angiography and embolization if bleeding was identified.
Rajani et al.88 performed a retrospective study comparing 403 patients from 1998 to 2005 with 222 patients from 1991 to 1998. The authors found improvements in a range of outcomes correlated with an increase in frequency of splenic artery embolization (Table 16-5)—although again, causation cannot be established.
Table 16-5 Nonoperative Management of Blunt Splenic Injuries, 1991-2005*
| Initial Cohort (1991-1998) | Later Cohort (1998-2005) | |
|---|---|---|
| Nonoperative therapy | 61% | 85% |
| Success of nonoperative therapy | 77% | 96% |
| Splenic salvage rate | 57% | 88% |
| Hospital mortality rate | 12% | 6% |
| Mean hospital length of stay | 15 days | 9 days |
| Splenic artery embolization | 2.7% | 22.6% |
* All comparisons reached statistical significance.
Data from Rajani RR, Claridge JA, Yowler CJ, et al. Improved outcome of adult blunt splenic injury: A cohort analysis. Surgery 140:625-631, 2006; discussion 631-63.
Harbrecht et al.89 retrospectively compared patients with splenic injuries managed with splenic artery embolization with a contemporaneous group treated without angiography. No difference in splenic salvage rates was observed, but only 46 patients in this study underwent splenic artery embolization. The authors attempted to account for potential confounding by comparing baseline characteristics of subjects, which appeared similar. However, this was not a randomized, controlled trial, and presumably some unmeasured difference among patients drove clinicians to perform splenic artery embolization in some patients but not in others. It is possible that some patients would have required splenectomy if they had not undergone embolization; a randomized, controlled trial is needed to answer this question.
Duchesne et al.90 retrospectively compared hemodynamically stable patients with isolated splenic injury identified by CT scan and evidence of active contrast extravasation. They divided the cohort into two groups in two consecutive 30-month periods, before and after introduction of an institutional splenic artery embolization protocol. All patients in group 1 were treated with primary splenectomy, whereas patients in group 2 underwent primary splenic artery embolization. Mortality was statistically equivalent in the two groups (18% for splenectomy and 15% for embolization). Acute respiratory distress syndrome was more common in patients undergoing embolization (22% vs. 5%, p = 0.002). In addition, 22 patients (29%) failed nonoperative therapy with splenic artery embolization and required subsequent splenectomy. The two study groups differed in the severity of their splenic injuries. In group 1, 58% had grade IV or V injuries, whereas in group 2, only 33% had such severe injuries. Despite this, the authors stated that the organ injury severity was similar between the groups. When the baseline characteristics of subjects are dissimilar, a fair comparison of outcomes following an intervention cannot be made. The authors did attempt to stratify outcomes based on the initial severity of splenic injury, but here, the analysis loses statistical power because of the small number of patients in each subgroup, limiting its ability to show real differences. The authors cautioned against embolization in high-grade injuries because of both treatment failures and risk for acute respiratory distress syndrome. However, they do not acknowledge the potential superiority of splenic artery embolization in low-grade injuries, in which neither treatment failures nor mortality was seen. The authors concluded in their title that use of splenic artery embolization did not “improve outcomes,” ignoring the potential morbidity of laparotomy.
Sabe et al.91 retrospectively compared three historical cohorts with splenic injury: an initial group in which splenic artery embolization was not routinely used (n = 222), an intermediate group in which splenic artery embolization became available and was used at physician discretion (n = 195), and a later group in which splenic artery embolization was routinely used for patients with certain high-risk splenic injuries (contrast extravasation or pseudoaneurysm on CT, grade III injury with large hemoperitoneum, and grade IV injuries) (n = 398). Nonoperative management became more common (61% initially, compared with 82% and 88% in subsequent periods) and more successful (77%, 94%, and 97%, respectively). The authors concluded that the increased use of splenic artery embolization in high-risk patients increased the success of nonoperative management. Although this association appears suggestive, it is impossible to determine a cause–effect relationship from this study. The study spans a period from 1991 to 2007, and it is possible that other changes in patient management played important roles in altering outcomes.
Many additional similar retrospective cohort studies exist, but because of similar methodologic flaws, they do not shed any additional light on the potential benefits and indications for angiographic embolization. A large prospective, randomized, controlled multicenter trial is needed to determine the rate of successful nonoperative management in patients treated with and without splenic artery embolization. A very large study would be required to further stratify patients by outcome, according to degree of initial injury. Making the equation even more difficult is that many patients with splenic injuries may have other concurrent injuries, which contribute to the patients’ overall instability and need for therapies. Although study of patients with isolated splenic injuries creates the purest experimental model to determine the effect of splenic artery embolization, it may not answer the more complex question of the role of embolization in patients with multiple injuries. As a consequence, we may never know which patients truly require embolization and which do not. Existing studies suggest that when patients are managed with embolization, many do not require laparotomy or splenectomy. Whether these patients could have been managed successfully without embolization is less certain.
Should High-Grade Injuries or Hemodynamic Instability Exclude the Possibility of Splenic Artery Embolization?
Patients with hemodynamic instability unresponsive to fluid resuscitation are generally considered inappropriate for nonoperative management and should undergo laparotomy, not primary splenic artery embolization.92 Stable patients with active extravasation from splenic injuries, large hemoperitoneum, and grade V injuries can sometimes be successfully managed nonoperatively with embolization.93-94 Haan et al.95 reported splenic salvage rates as high as 83% using embolization, even in grade IV and V splenic injuries. Given the lack of randomized, controlled trials, and the possibility of failed embolization, preparation for emergency laparotomy should be made concurrently. In rare cases, patients who development unstable vital signs in the angiography suite can continue nonoperative management with embolization. Bessoud et al.94 demonstrated a low rate of secondary splenectomy (2.7%) after initial nonoperative management in patients undergoing proximal splenic artery embolization, despite higher injury grades in the embolized group than in those selected for management without embolization. Three patients in their series became hemodynamically unstable in the angiography suite, with subsequent stabilization of vital signs immediately after successful embolization of the proximal splenic artery. The authors found minimal impact on subsequent immune function in patients undergoing proximal splenic artery embolization—which is surprising because infarction of the entire spleen could result from proximal occlusion of its blood supply.96
Based on existing data, hemodynamically stable patients with CT evidence of active splenic hemorrhage should undergo angiographic embolization. In some centers, patients with grade V injuries undergo laparotomy, regardless of the presence of active hemorrhage, though some evidence supports the use of nonoperative therapy with splenic artery embolization in this group. Patients with grade I and II splenic injuries and no evidence of active bleeding on CT scan can be managed nonoperatively and should not routinely undergo angiographic embolization because they have a low risk for rebleeding or failure of nonoperative management. Patients with grade III or IV injuries can be managed nonoperatively but generally should undergo angiographic embolization, regardless of the presence of active hemorrhage on CT, because rebleeding is a frequent occurrence. A protocol for selecting patients with splenic injuries for embolization is shown in Figure 16-4.97
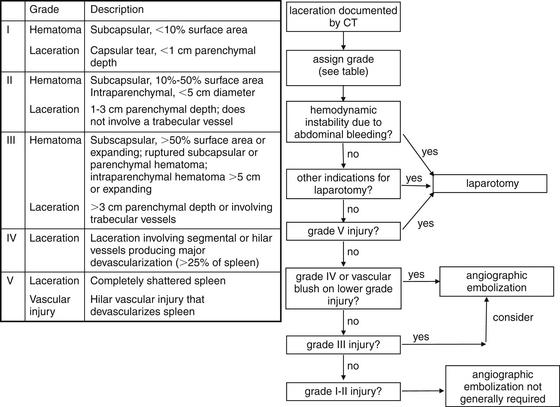
Figure 16-4 Protocol for identifying patients with splenic injuries amenable to embolization.
(From Broder J, Kuo WT. Therapeutic applications of interventional radiological techniques in emergency medicine. 2007. Accessed at http://emedhome.com.)
Angiographic Embolization of Blunt Liver Injuries
Angiographic embolization of blunt traumatic liver injuries follows many of the same principles as those we discussed for splenic injury—with the same methodologic issues limiting the quality of the available evidence. A PubMed search for “liver trauma embolization” found 408 matches but no randomized, controlled trials. Nonoperative management of hemodynamically stable blunt hepatic injuries is successful in approximately 80% of cases,92,98-99 with a combination of observation and angiographic embolization being used. A trial of nonoperative management is preferred in stable patients without other indications for immediate laparotomy, such as bowel injury.86,100-101 Among hepatic injuries, 85% are grades I through III and have low failure rates with nonoperative management. Grade IV and V hepatic injuries are relatively rare, constituting 10% and 4% of hepatic injuries, respectively, but are associated with extreme mortality (37% and 77%, respectively). These injuries are principally managed with emergency laparotomy because of the high frequency of initial hemodynamic instability. Angiographic embolization postoperatively can decrease rebleeding and is associated with improved survival.80 In these cases, the initial surgical intervention is a “damage control” procedure, intended to address the most immediately life-threatening injuries while minimizing operative time, and some degree of ongoing hemorrhage often persists after the operative intervention. A single case report describes the successful fully nonoperative management of a hemodynamically unstable patient with a grade IV liver injury using angioembolization.81 Rare grade VI injuries are uniformly fatal hepatic avulsions.80,92
As is the case with low-grade splenic injuries without active extravasation, it is unclear whether low-grade hepatic injuries without CT evidence of active bleeding benefit from routine angiography. In hemodynamically stable patients with active contrast extravasation on CT, the choice of angiographic embolization versus laparotomy has been studied, though not with rigorous methodology. Fang et al. retrospectively studied 276 patients with hepatic injuries, identifying 15 hemodynamically stable patients with contrast extravasation. The location of active hemorrhage on CT was noted and compared with later hemodynamic instability. Active peritoneal extravasation and intraparenchymal hemorrhage with hemoperitoneum predicted a high rate of subsequent hemodynamic instability. Intraparenchymal hemorrhage without hemoperitoneum was not followed by hemodynamic instability. The authors suggested that management could be determined by the location of active bleeding on CT and the presence or absence of hemoperitoneum; however, the small number of patients in this study and the lack of any intervention or control group leave this simply a hypothesis. The authors suggested that patients with free peritoneal bleeding on CT undergo laparotomy, whereas patients with hemoperitoneum with intraparenchymal active bleeding on CT should undergo hepatic artery embolization.102 In a later publication, the same authors reported a 10- to 20-fold increase in risk for operative management in initially hemodynamically stable patients with hepatic injury and intraperitoneal contrast extravasation or diffuse hemoperitoneum. They suggested that such patients should undergo operative therapy rather than angioembolization, though this suggestion has not been studied.99 Perhaps such patients could be managed by rapid embolization. Early hepatic artery embolization is associated with decreased blood transfusion.103
Overall, class II and III evidence suggest benefit of angiography and embolization in hemodynamically stable patients with high grades of hepatic injury or with active intraparenchymal contrast extravasation on CT. Patients with intraperitoneal contrast extravasation may be at higher risk for need for operative intervention.
Angiographic Embolization of Pelvic Arterial Injuries
As with splenic and hepatic blunt trauma, numerous retrospective cohort studies have investigated the role of angiographic embolization of blunt traumatic pelvic vascular injuries. These studies can illustrate correlations and raise hypotheses but cannot prove causation. At best, these provide class II and III evidence for benefit from embolization, although the sheer number of studies renders these a persuasive argument. A randomized, controlled trial of embolization would be required to prove a benefit, but such a trial is unlikely at this point because angiography has become a standard practice in the management of blunt traumatic pelvic hemorrhage. A PubMed search of “pelvis trauma embolization” finds 145 matches, with no randomized, controlled trials.
Hemorrhage associated with pelvic fractures is a prime candidate for embolization because some fracture patterns frequently are associated with life-threatening hemorrhage from vessels that are difficult to access surgically. It is thought that 15% of traumatic pelvic bleeding results from arterial injuries that can be treated with embolization. Fracture patterns were once thought to predict the risk for bleeding, but a recent retrospective review found no consistent relationship between embolization requirements and fracture patterns.104 CT with IV contrast provides a better measure of the need for angioembolization. Active contrast extravasation on CT accurately determines the site of ongoing hemorrhage, directing angiographic therapy.105 Several published reports claim 100% success of angiographic embolization in terminating pelvic arterial hemorrhage.106-108 These reports may overestimate success because of publication bias. In addition, even with control of arterial bleeding, patients may still die from venous hemorrhage, associated traumatic injuries, and sepsis. Embolization should be performed judiciously because aggressive pelvic artery embolization may result in ischemic necrosis of the gluteal muscles.109-110
Although embolization appears to be highly effective in addressing arterial hemorrhage, 85% of pelvic bleeding is attributed to venous injuries, which are not routinely treated angiographically. Instead, these injuries are usually addressed with external fixation of pelvic fractures, which is thought to facilitate tamponade of venous hemorrhage. Despite external fixation and arterial embolization, mortality of up to 40% has been reported for pelvic fractures because of ongoing venous hemorrhage and associated multisystem injuries.111 Rare injuries to the iliac veins can contribute to hemodynamic instability despite embolization therapy of arterial injuries and external fixation of fractures. Although these injuries are more often treated surgically, embolization or stenting across areas of venous injury can be performed, requiring a separate cannulation of the femoral vein.112 When external fixation and angiographic techniques fail, preperitoneal pelvic packing can be used to tamponade venous bleeding.111 A protocol for embolization in pelvic fractures is shown in Figure 16-5.113 Figure 16-6 demonstrates a pelvic arterial injury diagnosed by CT and treated with embolization. Figure 16-7 shows a lumbar arterial injury detected by CT and treated with angiographic embolization.
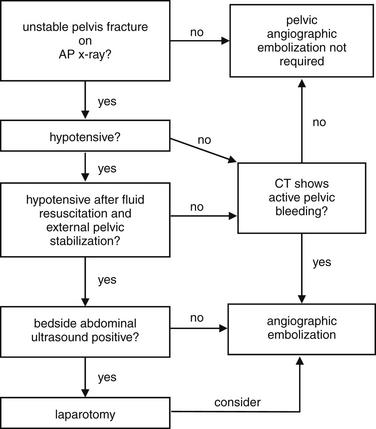
Figure 16-5 Protocol for identifying patients with pelvic injuries amenable to angiographic embolization.
In patients with unstable pelvic fractures, persistent hypotension should prompt screening for abdominal injury using focused assessment with sonography in trauma (FAST). If ultrasound suggests an abdominal bleeding source as the cause for hypotension, laparotomy is usually indicated. If significant pelvic bleeding is suspected, angiography can be performed following laparotomy.Hypotension from a pelvic source (without an abdominal source) should prompt angiographic embolization, possibly before CT.Stable patients with pelvic fractures can undergo CT first, with angiography reserved for those with active bleeding identified on CT. (AP = anterior–posterior.)
(From Broder J, Kuo WT. Therapeutic applications of interventional radiological techniques in emergency medicine. 2007. Accessed at http://emedhome.com.)
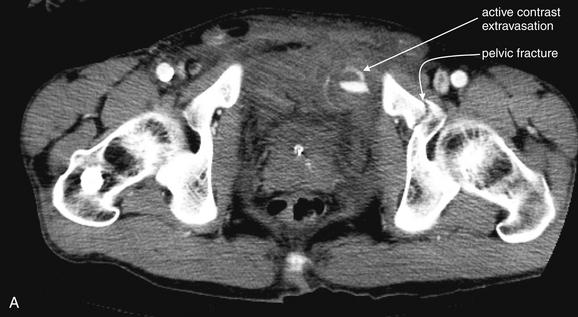
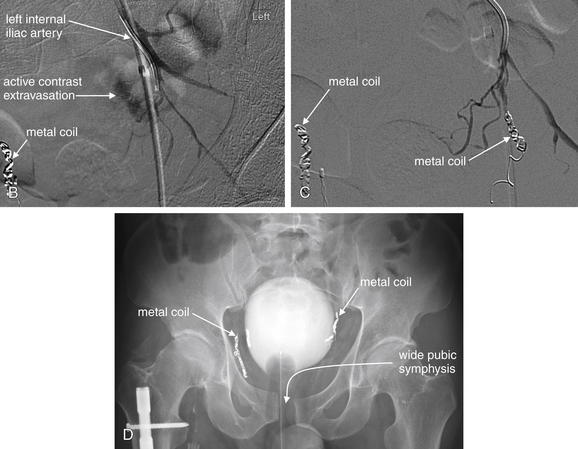
Figure 16-6 Selective arterial embolization for treatment of pelvic arterial hemorrhage.Unstable pelvic fractures may be associated with serious pelvic arterial injuries. Angiographic embolization can be used to stop hemorrhage from these surgically inaccessible vessels.A, CT with IV contrast shows an area of arterial hemorrhage, as well as a pelvic fracture.B, Angiography shows a blush of contrast from branches of the internal iliac artery in the area of active bleeding. Metal coils have already been placed on the contralateral side.C, A coil has been placed in the region of active bleeding, and the blush of contrast has disappeared, indicating hemostasis.D, An x-ray after angiography shows the metal coils. In addition, the widened pubic symphysis of this unstable pelvic fracture is visible.
(From Broder J, Kuo WT. Therapeutic applications of interventional radiological techniques in emergency medicine. 2007. Accessed at http://emedhome.com.)
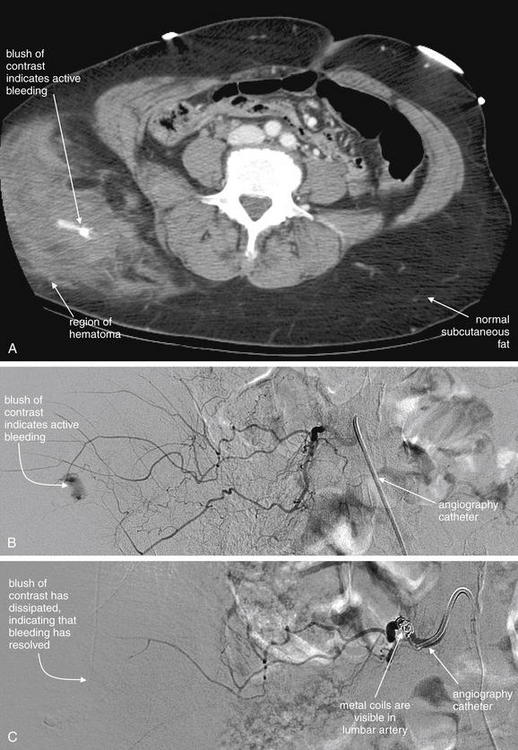
Figure 16-7 Angiography allows selective arterial embolization for treatment of traumatic hemorrhage.
This 60-year-old pedestrian was struck by a truck.A, CT with IV contrast showed an area of active hemorrhage in the right flank.
B, Angiography confirmed bleeding from a right lumbar artery.C, Embolization of the bleeding vessel was performed using a combination of small particles (250 × 355 μm) and placement of metal coils. Following the procedure, blood flow in the vessel was markedly diminished and hemostasis was achieved.
(From Broder J, Kuo WT. Therapeutic applications of interventional radiological techniques in emergency medicine. Accessed at http://emedhome.com.)
Endovascular Stenting Under Fluoroscopic Guidance for Aortic Pathology
CTA has nearly supplanted aortography for diagnosis of abdominal and thoracic aortic pathology. However, angiography has seen a renewed role as a primary therapeutic modality. Endovascular repair of aortic aneurysm showed initial promise with an apparent mortality benefit in early nonrandomized trials. However, subsequent well-designed, randomized, controlled trials have been less impressive. The first Endovascular Aneurysm Repair trial114-115 was a randomized, controlled trial comparing 543 patients undergoing endovascular repair to 539 patients undergoing open surgical repair. The 30-day mortality was 1.7% in the endovascular repair group and 4.7% in the open repair group (p = 0.009).114 At 4-year follow-up, all cause mortality was 28% in both groups, although aneurysm-related deaths remained lower in the endovascular repair group (4%, compared with 7% for open repair; p = 0.04). Postoperative complications were 41% in the endovascular repair group and 9% in the open repair group. Health-related quality of life was equivalent between the two therapies, and costs of endovascular repair were higher.
The Dutch Randomized Endovascular Aneurysm Management trial16a randomized 345 patients to open or endovascular repair and found operative mortality was 4.6% with open repair and 1.2% with endovascular therapy. However, at 2-year follow-up, cumulative survival in the two groups was equivalent (89.6% vs. 89.7%).116
Sicard et al.117 compared outcomes after endovascular repair in patients considered to be at high risk for open aortic aneurysm repair because of age of at least 60 years, aneurysm size of at least 5.5 cm, and an additional cardiac, pulmonary, or renal comorbidity. The study followed a retrospective case-control design and showed a 30-day mortality risk for 2.9% for endovascular repair and 5.1% for open repair. Endovascular repair was statistically equivalent to open repair at 1 and 4 years.
Greco et al.118 reported frequency and outcomes of endovascular repair of ruptured aortic aneurysms using a discharge database from New York, California, Florida, and New Jersey, four states accounting for nearly one third of the U.S. population. Endovascular repair accounted for 6.2% of treated cases of ruptured abdominal aortic aneurysm in 2003. Endovascular repair had significantly better mortality than open repair (39.3% vs. 47.7%, p = 0.005). However, this was not a randomized study. As one explanation for improved outcomes, patients selected for endovascular repair may have been more stable than those undergoing open repair. Nonetheless, this study demonstrates that endovascular repair is feasible in patients with abdominal aortic rupture.
Hinchliffe et al.119 performed a randomized trial of endovascular and open repair of ruptured aortic aneurysm. From 2002 to 2004, they recruited a total of 32 patients, and 30-day mortality was equivalent between the two groups (53%), with similar rates of moderate and severe complications between the groups. A larger study would be required to have adequate power to discern subtle outcomes differences, but the study again shows the feasibility of endovascular repair in patients with aortic rupture.
Kaya et al.120 examined endovascular repair outcomes in 28 patients with acute thoracic aortic syndromes, including Stanford type B aortic dissections, ruptured aneurysms of the descending aorta, and traumatic aortic injuries. The authors reported a mortality rate of 21.4% and concluded that endovascular thoracic aortic repair is feasible even in critically ill patients. The authors attributed four of the deaths to preoperative problems, rather than effects of the procedure or failure to treat the aortic pathology adequately. Although this may be true, it also illustrates the need for blinded assessment of outcomes because clinicians involved in patient treatment may be emotionally invested and reluctant to blame poor outcomes on the treatment under investigation.
Milas et al.121 reported endovascular repair of blunt traumatic thoracic aortic injuries in two adolescents, pointing out the advantage that endovascular repair avoids the need for cardiopulmonary bypass seen with open repair. Randomized, controlled trials in the trauma population are needed to compare outcomes of endovascular and open repair.
Xenos et al.122 performed a meta-analysis of endovascular and open repair of traumatic descending thoracic aortic injuries. They found 17 retrospective cohort studies from 2003 to 2007, with no randomized trials. The study included 589 patients (369 open repair and 220 endovascular thoracic stent graft). Patients undergoing endovascular repair had higher injury severity scores, perhaps reflecting surgeon preference to avoid thoracotomy in these patients. Despite this, procedure-related mortality and 30-day mortality were lower with endovascular repair, with odds ratios for death of 0.31 and 0.44, respectively. Spinal cord ischemia with resulting paraplegia, a serious potential complication of thoracic aortic repair because of possible occlusion of spinal perforating arteries, was lower in patients undergoing endovascular repair (odds ratio = 0.32). Although these results appear encouraging, true randomized, controlled trials are required to confirm the apparent benefit. It is important to remember that nonrandomized studies of endovascular abdominal aortic repair appeared to show benefit, which was not confirmed in subsequent randomized trials.
Figure 16-8 shows endovascular stent placement to treat aortic pseudoaneurysm.
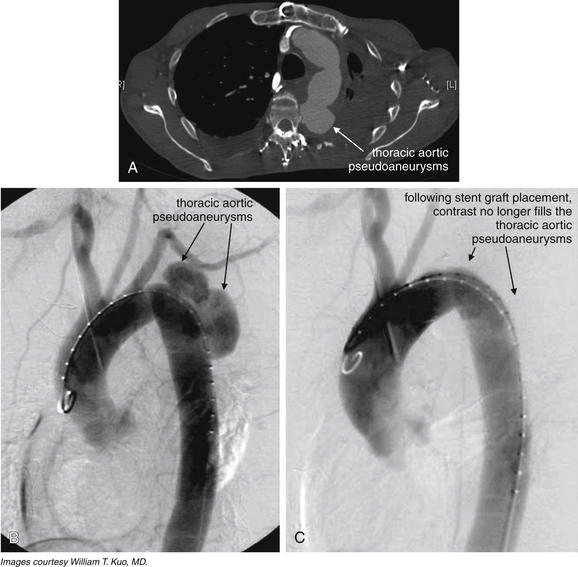
Figure 16-8 Thoracic aortic pathology can be treated using interventional radiologic techniques.A, A pair of pseudoaneurysms from the descending thoracic aorta, detected with CT.B, Angiography confirms the pseudoaneurysms.C, The patient was a poor surgical candidate and underwent endovascular stent graft placement by an interventional radiologist. After graft placement, the pseudoaneurysms have been excluded from the aortic circulation, as demonstrated by the absence of contrast within them.
(From Broder J, Kuo WT. Therapeutic applications of interventional radiological techniques in emergency medicine. 2007. Accessed at http://emedhome.com.)
Class I evidence shows the short-term mortality benefit of endovascular repair of abdominal aortic aneurysm, but this survival benefit is not sustained long term. Endovascular repair is more expensive and has more complications than open repair based on large randomized, controlled trials. Class II evidence suggests that endovascular repair of traumatic thoracic aortic aneurysm may be superior to open repair—but randomized, controlled trials are needed to confirm or refute this finding.
Angiographic Embolization Therapy for Lower Gastrointestinal Hemorrhage
Lower gastrointestinal hemorrhage poses a diagnostic and therapeutic dilemma in the emergency department. Significant upper gastrointestinal hemorrhage most often results from ulcer or esophageal varices, both of which can be diagnosed and treated effectively with endoscopy. In contrast, serious lower gastrointestinal hemorrhage may result from a variety of lesions, including polyps, cancers, angioectasia, colitis, and diverticular disease, all of which can be difficult to diagnose endoscopically in the emergency department patient who has not undergone bowel preparation. Even when the cause of lower gastrointestinal hemorrhage is identified, endoscopic therapy may be ineffective. Patients who fail conservative therapies for lower gastrointestinal bleeding may require emergency colectomy, which has high operative mortality. As a consequence, a less invasive therapeutic option is desirable. Angiographic embolization (Figure 16-9) of lower intestinal hemorrhage offers such a therapy, as we review later.
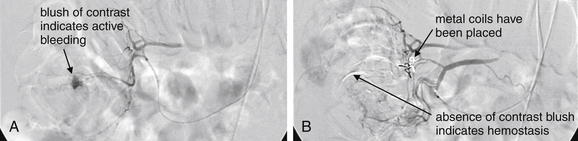
Figure 16-9 Mesenteric angiography allows selective embolization for diagnosis and treatment of lower gastrointestinal bleeding.Mesenteric angiography can be performed for evaluation and treatment of undifferentiated lower gastrointestinal hemorrhage. During the procedure, all major mesenteric branches of the aorta are cannulated to assess for bleeding or stenosis. If bleeding is found from a branch vessel, that vessel can be selectively embolized. This may stop bleeding while preserving regional blood flow to prevent iatrogenic mesenteric ischemia.A, Superior mesenteric angiography in a 38-year-old man with diverticular disease and acute lower gastrointestinal hemorrhage showed contrast extravasation in the hepatic flexure.B, Superselective embolization with two microcoils was performed within the vasa recta achieving successful hemostasis, indicated by absence of contrast extravasation.
(From Kuo WT, Lee DE, Saad WE, et al. Superselective microcoil embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol 2003;14, 1503-9.)
Diagnostic imaging to evaluate the location of intestinal hemorrhage before angiography is discussed in Chapter 9. Angiography can be used as the primary diagnostic, as well as a therapeutic, tool in a patient with obvious continued significant intestinal bleeding. During angiographic embolization of lower gastrointestinal hemorrhage, the aorta is catheterized under fluoroscopic guidance, usually via the femoral artery. The catheter is then advanced into the major mesenteric branches of the aorta, including the celiac, superior mesenteric, and inferior mesenteric arteries. Contrast is injected, and extravasation of contrast marks the site of ongoing hemorrhage. The catheter is then advanced as far as possible into the bleeding branch vessel, and embolization of that vessel is performed using metal coils, Gelfoam, or other agents. “Superselective” embolization of just the distal bleeding vessel is performed when possible because this reduces the risk for inducing mesenteric ischemia, which can occur if larger, more proximal vessels are embolized. An advantage of angiographic embolization is that the cause of the hemorrhage need not be determined before therapy is performed. In addition, because the site of bleeding is approached intravascularly, rather than from the bowel lumen, no bowel preparation is required to cleanse stool and allow visualization.123-124
Embolization may be a better option for diagnosis and treatment in the emergency department, compared with colonoscopy. No trials have directly compared the two therapies, so the evidence to date is from independent studies of the two therapies. Green et al.125 randomized 100 patients to standard care or urgent colonoscopy. Although the rate of definitive diagnosis was higher in patients in the urgent colonoscopy group, no differences were seen in other important outcomes, including mortality, hospital length of stay, intensive care unit stay, transfusion, early and late rebleeding, and need for surgery. Of the 50 patients in the urgent colonoscopy group, 17 (34%) underwent endoscopic hemostasis (using epinephrine injection and electrocautery coagulation). Despite this, the rate of early rebleeding was 22%. In this study, 14% of patients in the colonoscopy group and 12% in the standard care group required surgery (subtotal colectomy, hemicolectomy, or segmental colectomy) because of continued bleeding—highlighting the need for other therapies.
The evidence for angiographic embolization therapy of lower gastrointestinal bleeding comes from small nonrandomized case series. Outcomes are difficult to compare across studies, given different inclusion criteria and the diversity of embolization techniques, including metal coils, Gelfoam, and sclerosing agents. These studies lack a contemporaneous control group for comparison, and outcome comparisons with historical controls must be taken with skepticism, given other potential differences in degree of illness and adjuvant medical therapies. Nonetheless, meta-analyses of these published studies show a success rate of approximately 60% to 80%.124,126 Patients may fail angiographic embolization in two manners. First, embolization may fail to stop bleeding, requiring emergent colectomy or segmental colectomy for hemostasis. Second, aggressive embolization may terminate bleeding—but at the expense of inducing mesenteric ischemia in the watershed region supplied by the embolized vessel. This, too, can require surgical intervention. Because emergency colectomy has a reported mortality rate as high as 30% in ill patients with acute gastrointestinal hemorrhage, avoidance of surgery is highly desirable.
Kuo et al.126 retrospectively reviewed 10 years of superselective microcoil embolization of lower intestinal hemorrhage and identified 22 patients at the University of Rochester, New York. Of these patients, 86% were successfully treated with embolization. An ischemic complication was identified in 1 patient (4.5%), although not all patients underwent objective investigation for ischemia, and 4 patients died, with the deaths unrelated to embolization or hemorrhage, according to the authors. Results from these patients were combined with 122 additional patients in a metaanalysis of the published literature. The authors reported minor complications in 13 of 144 patients (9%), with no major ischemic complications.
Brackman et al.127 retrospectively reviewed the management of 420 patients admitted with melena or hematochezia between 1998 and 2001 and found that 3% of those admitted to surgical services and 1% admitted to medical services were treated angiographically.
D’Othee et al.128 reviewed 19 consecutive patients treated for lower gastrointestinal bleeding with microcoil embolization during an 88-month period. In 68%, hemorrhage was stopped by the procedure without complication. An additional 16% had decreased hemorrhage, which ultimately stopped without further intervention. Two patients (11%) had failure of hemostasis, requiring further intervention. Two patients (11%) developed colonic ischemia, presumably because of embolization, and underwent colectomy.
Kickuth et al.129 reported 20 patients undergoing superselective microcatheter embolization for lower gastrointestinal bleeding. According to the authors, 100% of patients had technical success of hemostasis, with 90% of these attributed to the embolization. One patient had an apparently procedure-related colonic infarction.
Angiographic embolization of lower gastrointestinal bleeding requires further study to determine its safety, efficacy, and indications. Like many other areas of therapeutic angiography, it has not been subjected to randomized, controlled trials before entering clinical use. It remains a potential therapy in patients with evidence of ongoing lower intestinal hemorrhage because it may prevent surgical intervention.
Angiographic Techniques for Foreign Body Retrieval
Intravascular foreign bodies can lead to serious complications including infection, vascular perforation, and arterial ischemia from foreign body embolization or vessel thrombosis. The rate of dire complications of unretrieved intravascular devices is reported to be as high as 70%, with mortality rates of 24% to 60%.130-132 Complication rates may be even higher because of underreporting of bad outcomes. As a consequence, rapid and minimally invasive removal of such foreign bodies is a major clinical priority. Percutaneous retrieval of an intravascular foreign body under fluoroscopic guidance was first described in 1964.133 Retrieval rates of 90% to 95% with few complications have been reported using a range of devices, including baskets, snares, loops, and graspers.134-135 Randomized trials have not compared surgical extraction with percutaneous retrieval, and such trials are unlikely given the morbidity associated with vascular surgery.
Percutaneous Drainage of Abdominal Abscesses and Fluid Collections
Percutaneous, radiographically guided drainage of abdominal fluid collections has become a common practice, though relatively little rigorous study has been devoted to outcomes. Abdominal fluid collections can be drained using ultrasound, CT, or fluoroscopic guidance. Common procedures include percutaneous nephrostomy, percutaneous drainage of the biliary system, and percutaneous drainage of abdominal abscesses. Percutaneous drainage of intraabdominal abscesses under CT guidance was described by the mid-1970s, shortly after clinical introduction of CT for diagnostic purposes.136-138 Before this time, intraabdominal abscesses had high mortality, so the ability to address these nonoperatively was a major therapeutic advance. Let’s briefly examine the evidence for image-guided percutaneous drainage of appendiceal abscesses.
Kaminski et al.139 retrospectively reviewed a Kaiser Permanente database of 32,938 patients with acute appendicitis and observed that 1012 patients (3%) were initially managed without appendectomy. Of these, 783 patients were not treated with percutaneous drainage, and 90 patients (11%) underwent interval appendectomy. Meanwhile, 229 patients did have initial percutaneous drainage, and 58 patients (25%) underwent interval appendectomy. Although this study was not randomized or blinded, and the indications for interval appendectomy were not clear, percutaneous drainage was not associated with a lower rate of interval appendectomy. In patients who were treated nonoperatively and did not undergo interval appendectomy, low rates of recurrent appendicitis were seen (<5%), with or without percutaneous drainage.
Zerem et al.140 randomized 50 patients with acute appendicitis and periappendiceal abscess to antibiotics alone or antibiotics plus percutaneous drainage under ultrasound guidance. The rate of appendectomy in the two groups was then measured. The rate of appendectomy was 36% in patients undergoing percutaneous drainage and 92% in patients treated only with antibiotics (p < 0.05). Clinical improvement occurred more quickly in patients undergoing ultrasound-guided drainage. Although this study provides an important estimation of the success rate of percutaneous drainage, it does not compare percutaneous drainage with primary appendectomy in terms of recovery time and rate of complications such as systemic sepsis. In addition, whereas the authors stated that the criteria for appendectomy were fever, pain in the right lower quadrant, and persistence of abdominal fluid collection 2 weeks after therapy, surgeons and patients were not blinded to the treatment or to other clinical parameters. Perhaps surgeons were uncomfortable treating patients with antibiotics alone and thus were more likely to perform appendectomy in patients who had not undergone percutaneous treatment, regardless of their clinical response.
Keckler et al.141 retrospectively reviewed 52 pediatric patients treated initially with percutaneous drainage of an appendiceal abscess. They noted that a recurrent abscess developed in 17.3% of patients, and 4 patients developed significant complications of drain placement, including ileal perforation, colon perforation, bladder perforation, and necrotizing thigh abscess. However, without a comparison group, it is impossible to compare this complication rate fairly with that expected from initial surgical therapy.
St. Peter et al.142 randomized 40 children with periappendiceal abscess to laparoscopic appendectomy or antibiotics and percutaneous drainage. They found no difference in hospital length of stay, recurrent abscess, or charges, though this small study is underpowered to detect an important difference.
Although nonrandomized cohorts and small randomized trials suggest that nonoperative therapy for appendicitis using percutaneous drainage can be successful with a low rate of recurrent appendicitis, larger randomized, controlled trials are needed to define which patients should undergo surgery, which require percutaneous drainage, and which can receive antibiotics alone.
Figure 16-10 demonstrates CT-guided drainage of an appendiceal abscess.
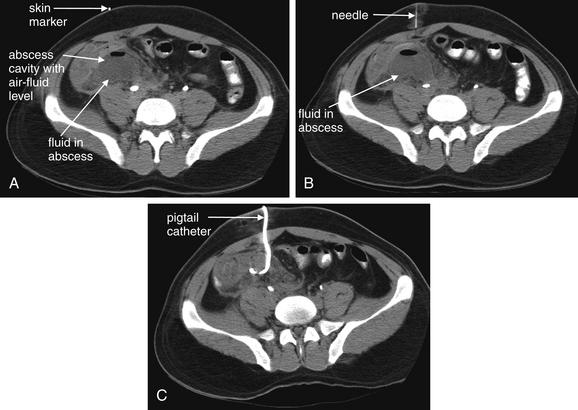
Figure 16-10 Computed tomography (CT)–guided drainage is now used for treatment of abdominal abscesses, including ruptured appendicitis.This 26-year-old male presented with right lower quadrant pain. CT demonstrated perforated appendicitis with abscess. CT-guided percutaneous drain placement was performed. After a course of antibiotics, the patient underwent laparoscopic appendectomy 4 months later.A, Skin markers are placed, and CT is performed through the region of the right lower quadrant.
B, A needle is placed, and its position is checked relative to the abscess by repeated CT of the region.C, A 10-French pigtail catheter is placed into the abscess after initial placement of a trocar needle and dilators. The catheter position is confirmed by CT. Already, a large amount of fluid has drained from the abscess.
(From Broder J, Kuo WT. Therapeutic applications of interventional radiological techniques in emergency medicine. 2007. Accessed at http://emedhome.com.)
Summary
Image-guided procedures can provide less invasive and sometimes more effective treatment alternatives for conditions that would ordinarily require surgical intervention or systemic medical therapies. Some indications have been extensively studied using excellent research methodology. In the case of acute STEMI, percutaneous coronary angioplasty has been proven to be superior to systemic thrombolytic therapy. For aneurismal subarachnoid hemorrhage, angiographic aneurysm coiling has been proven to be superior to surgical clipping of aneurysms. For abdominal aortic aneurysm repair, well-conducted randomized trials have demonstrated endovascular repair has no long-term mortality advantage and higher costs compared with open surgical repair. We should learn from these examples that the outcome benefits anticipated from observational trials do not always materialize when appropriate research methods are applied. Indications such as massive PE, traumatic abdominal and pelvic hemorrhage, and abdominal abscesses deserve the same degree of rigorous study to determine which patient populations, if any, truly benefit from image-guided interventions.
1. Hulley S., Grady D., Bush T., et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women: Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
1a. Paulson EK., Sheafor DH., Enterline DS., McAdams HP., Yoshizumi TT. CT fluoroscopy—guided interventional procedures: techniques and radiation dose to radiologists. Radiology. 2001;220:161-167.
2. Grady D., Herrington D., Bittner V., et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49-57.
3. ASSENT-4 PCI investigators. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): Randomised trial. Lancet. 2006;367:569-578.
4. Sena E.S., van der Worp H.B., Bath P.M., et al. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 8, 2010. e1000344
5. Kennedy J.W., Stewart D.K. Interventional coronary arteriography. Annu Rev Med. 1984;35:513-534.
6. A Clinical Trial Comparing Primary Coronary Angioplasty with Tissue Plasminogen Activator for Acute Myocardial Infarction. The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. N Engl J Med. 1997;336:1621-1628.
7. Second International Study of Infarct Survival Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;2:349-360.
8. Cucherat M., Bonnefoy E., Tremeau G. Primary angioplasty versus intravenous thrombolysis for acute myocardial infarction. Cochrane Database Syst Rev. 2003. CD001560
9. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-1587.
10. Hoffman J.R., Schriger D.L. A graphic reanalysis of the NINDS trial. Ann Emerg Med. 2009;54:329-336. 36 e1-35
11. Katzan I.L., Furlan A.J., Lloyd L.E., et al. Use of tissue-type plasminogen activator for acute ischemic stroke: The Cleveland area experience. JAMA. 2000;283:1151-1158.
12. Hacke W., Kaste M., Bluhmki E., et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
13. Saver J.L., Gornbein J., Grotta J., et al. Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3- to 4.5-hour window: Joint outcome table analysis of the ECASS 3 trial. Stroke. 2009;40:2433-2437.
14. Lansberg M.G., Bluhmki E., Thijs V.N. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: A metaanalysis. Stroke. 2009;40:2438-2441.
15. Katzan I.L., Hammer M.D., Hixson E.D., et al. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2004;61:346-350.
16. Schumacher H.C., Bateman B.T., Boden-Albala B., et al. Use of thrombolysis in acute ischemic stroke: Analysis of the Nationwide Inpatient Sample 1999 to 2004. Ann Emerg Med. 2007;50:99-107.
16a. Prinssen M., Verhoeven E.L., Buth J., et al. A randomized trial comparing conventional and endovascular repoaire of abdominal aortic aneurysms. N Engl J Med. 2004;351(16):1607-1618.
17. Smith W.S., Sung G., Starkman S., et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the MERCI trial. Stroke. 2005;36:1432-1438.
18. Smith W.S. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke: Results of the Multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol. 2006;27:1177-1182.
19. Kim D., Jahan R., Starkman S., et al. Endovascular mechanical clot retrieval in a broad ischemic stroke cohort. AJNR Am J Neuroradiol. 2006;27:2048-2052.
20. Layton K.F., White J.B., Cloft H.J., et al. Expanding the treatment window with mechanical thrombectomy in acute ischemic stroke. Neuroradiology. 2006;48:402-404.
21. Choi J.H., Bateman B.T., Mangla S., et al. Endovascular recanalization therapy in acute ischemic stroke. Stroke. 2006;37:419-424.
22. Shaltoni H.M., Albright K.C., Gonzales N.R., et al. Is intraarterial thrombolysis safe after full-dose intravenous recombinant tissue plasminogen activator for acute ischemic stroke? Stroke. 2007;38:80-84.
23. Sauvageau E., Samuelson R.M., Levy E.I., et al. Middle cerebral artery stenting for acute ischemic stroke after unsuccessful MERCI retrieval. Neurosurgery. 2007;60:701-706. discussion 706
24. Gonzalez A., Mayol A., Martinez E., et al. Mechanical thrombectomy with snare in patients with acute ischemic stroke. Neuroradiology. 2007;49:365-372.
25. Smith W.S., Sung G., Saver J., et al. Mechanical thrombectomy for acute ischemic stroke: Final results of the Multi MERCI trial. Stroke. 2008;39:1205-1212.
26. Molyneux A., Kerr R., Stratton I., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet. 2002;360:1267-1274.
27. Molyneux A.J., Kerr R.S., Yu L.M., et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
28. van der Schaaf I., Algra A., Wermer M., et al. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2005. CD003085
29. White P.M., Teasdale E.M., Wardlaw J.M., Easton V. Intracranial aneurysms: CT angiography and MR angiography for detection prospective blinded comparison in a large patient cohort. Radiology. 2001;219:739-749.
30. Naess I.A., Christiansen S.C., Romundstad P., et al. Incidence and mortality of venous thrombosis: A population-based study. J Thromb Haemost. 2007;5:692-699.
31. Nijkeuter M., Sohne M., Tick L.W., et al. The natural course of hemodynamically stable pulmonary embolism: Clinical outcome and risk factors in a large prospective cohort study. Chest. 2007;131:517-523.
32. Thabut G., Thabut D., Myers R.P., et al. Thrombolytic therapy of pulmonary embolism: A meta-analysis. J Am Coll Cardiol. 2002;40:1660-1667.
33. Konstantinides S., Geibel A., Heusel G., et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143-1150.
34. Dentali F., Donadini M., Gianni M., et al. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res. 2009;124:256-258.
35. Tapson V.F., Humbert M. Incidence and prevalence of chronic thromboembolic pulmonary hypertension: From acute to chronic pulmonary embolism. Proc Am Thorac Soc. 2006;3:564-567.
36. Piovella F., D’Armini A.M., Barone M., Tapson V.F. Chronic thromboembolic pulmonary hypertension. Semin Thromb Hemost. 2006;32:848-855.
37. Tibbutt D.A., Davies J.A., Anderson J.A., et al. Comparison by controlled clinical trial of streptokinase and heparin in treatment of life-threatening pulmonay embolism. BMJ. 1974;1:343-347.
38. Verstraete M., Miller G.A., Bounameaux H., et al. Intravenous and intrapulmonary recombinant tissue-type plasminogen activator in the treatment of acute massive pulmonary embolism. Circulation. 1988;77:353-360.
39. Timsit J.F., Reynaud P., Meyer G., Sors H. Pulmonary embolectomy by catheter device in massive pulmonary embolism. Chest. 1991;100:655-658.
40. Greenfield L.J., Proctor M.C., Williams D.M., Wakefield T.W. Long-term experience with transvenous catheter pulmonary embolectomy. J Vasc Surg. 1993;18:450-457. discussion 7-8
41. Tajima H., Murakami R., Kawamata H., et al. [Superselective local infusion therapy with tissue-plasminogen activator for acute massive pulmonary thromboembolism: preliminary clinical experience]. Nippon Igaku Hoshasen Gakkai Zasshi. 1995;55:423-424.
42. Uflacker R., Strange C., Vujic I. Massive pulmonary embolism: Preliminary results of treatment with the Amplatz thrombectomy device. J Vasc Interv Radiol. 1996;7:519-528.
43. Stock K.W., Jacob A.L., Schnabel K.J., et al. Massive pulmonary embolism: Treatment with thrombus fragmentation and local fibrinolysis with recombinant human-tissue plasminogen activator. Cardiovasc Intervent Radiol. 1997;20:364-368.
44. Schmitz-Rode T., Janssens U., Schild H.H., et al. Fragmentation of massive pulmonary embolism using a pigtail rotation catheter. Chest. 1998;114:1427-1436.
45. Rocek M., Peregrin J., Velimsky T. Mechanical thrombectomy of massive pulmonary embolism using an Arrow-Trerotola percutaneous thrombolytic device. Eur Radiol. 1998;8:1683-1685.
46. Murphy J.M., Mulvihill N., Mulcahy D., et al. Percutaneous catheter and guidewire fragmentation with local administration of recombinant tissue plasminogen activator as a treatment for massive pulmonary embolism. Eur Radiol. 1999;9:959-964.
47. Fava M., Loyola S., Huete I. Massive pulmonary embolism: Treatment with the hydrolyser thrombectomy catheter. J Vasc Interv Radiol. 2000;11:1159-1164.
48. Schmitz-Rode T., Janssens U., Duda S.H., et al. Massive pulmonary embolism: Percutaneous emergency treatment by pigtail rotation catheter. J Am Coll Cardiol. 2000;36:375-380.
49. De Gregorio M.A., Gimeno M.J., Mainar A., et al. Mechanical and enzymatic thrombolysis for massive pulmonary embolism. J Vasc Interv Radiol. 2002;13:163-169.
50. Zeni P.T.Jr., Blank B.G., Peeler D.W. Use of rheolytic thrombectomy in treatment of acute massive pulmonary embolism. J Vasc Interv Radiol. 2003;14:1511-1515.
51. Reekers J.A., Baarslag H.J., Koolen M.G., et al. Mechanical thrombectomy for early treatment of massive pulmonary embolism. Cardiovasc Intervent Radiol. 2003;26:246-250.
52. Tajima H., Murata S., Kumazaki T., et al. Hybrid treatment of acute massive pulmonary thromboembolism: Mechanical fragmentation with a modified rotating pigtail catheter, local fibrinolytic therapy, and clot aspiration followed by systemic fibrinolytic therapy. AJR Am J Roentgenol. 2004;183:589-595.
53. Tajima H., Murata S., Kumazaki T., et al. Recent advances in interventional radiology for acute massive pulmonary thromboembolism. J Nippon Med Sch. 2005;72:74-84.
54. Tajima H., Murata S., Kumazaki T., et al. Manual aspiration thrombectomy with a standard PTCA guiding catheter for treatment of acute massive pulmonary thromboembolism. Radiat Med. 2004;22:168-172.
55. Buller H.R., Agnelli G., Hull R.D., et al. Antithrombotic therapy for venous thromboembolic disease: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:S401-S428.
56. Engelke C., Rummeny E.J., Marten K. Acute pulmonary embolism on MDCT of the chest: Prediction of cor pulmonale and short-term patient survival from morphologic embolus burden. AJR Am J Roentgenol. 2006;186:1265-1271.
57. Ghaye B., Ghuysen A., Bruyere P.J., et al. Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics. 2006;26:23-39. discussion 40
58. Ghaye B., Ghuysen A., Willems V., et al. Severe pulmonary embolism: Pulmonary artery clot load scores and cardiovascular parameters as predictors of mortality. Radiology. 2006;239:884-891.
59. He H., Stein M.W., Zalta B., Haramati L.B. Computed tomography evaluation of right heart dysfunction in patients with acute pulmonary embolism. J Comput Assist Tomogr. 2006;30:262-266.
60. Araoz P.A., Gotway M.B., Harrington J.R., et al. Pulmonary embolism: Prognostic CT findings. Radiology. 2007;242:889-897.
61. Ryu J.H., Pellikka P.A., Froehling D.A., et al. Saddle pulmonary embolism diagnosed by CT angiography: Frequency, clinical features and outcome. Respir Med. 2007;101:1537-1542.
62. Grifoni S., Olivotto I., Cecchini P., et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101:2817-2822.
63. Meyer T., Binder L., Hruska N., et al. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36:1632-1636.
63a. Yalamanchili K., Sukhija R., Aronow SW., et al. Prevalence of increased cardiac troponin I levels in patients with and without acute pulmonary embolism and relation of increased cardiac troponin I levels with in-hospital mortality in patients with acute pulmonary embolism. Am J Cardiol. 2004;93(2):263-264.
64. Gallotta G., Palmieri V., Piedimonte V., et al. Increased troponin I predicts in-hospital occurrence of hemodynamic instability in patients with sub-massive or non-massive pulmonary embolism independent to clinical, echocardiographic and laboratory information. Int J Cardiol. 2008;124(3):351-357.
64a. Aksay E., Yanturali S., Kiyan S. Can elevated troponin I levels predict complicated clinical course and inhospital mortality in patients with acute pulmonary embolism? Am J Emerg Med. 2007;25(2):138-143.
65. Kuo WT: personal correspondence with author. 2007.
66. Hoeper M.M., Lee S.H., Voswinckel R., et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48:2546-2552.
67. Goldhaber S.Z. Integration of catheter thrombectomy into our armamentarium to treat acute pulmonary embolism. Chest. 1998;114:1237-1238.
68. Decousus H., Leizorovicz A., Parent F., et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338:409-415.
69. PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112:416-422.
70. Jaff M.R., Goldhaber S.Z., Tapson V.F. High utilization rate of vena cava filters in deep vein thrombosis. Thromb Haemost. 2005;93:1117-1119.
71. Giannoudis P.V., Pountos I., Pape H.C., Patel J.V. Safety and efficacy of vena cava filters in trauma patients. Injury. 2007;38:7-18.
72. Wojcik R., Cipolle M.D., Fearen I., et al. Long-term follow-up of trauma patients with a vena caval filter. J Trauma. 2000;49:839-843.
73. Imberti D., Bianchi M., Farina A., et al. Clinical experience with retrievable vena cava filters: Results of a prospective observational multicenter study. J Thromb Haemost. 2005;3:1370-1375.
74. Pasquale M., Fabian T.C. Practice management guidelines for trauma from the Eastern Association for the Surgery of Trauma. J Trauma. 1998;44:941-956. discussion 56-7
75. Snow V., Qaseem A., Barry P., et al. Management of venous thromboembolism: A clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007;146:204-210.
76. Kaufman J.A., Kinney T.B., Streiff M.B., et al. Guidelines for the use of retrievable and convertible vena cava filters: Report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol. 2006;17:449-459.
77. Pryor J.P., Braslow B., Reilly P.M., et al. The evolving role of interventional radiology in trauma care. J Trauma. 2005;59:102-104.
78. Madoff D.C., Denys A., Wallace M.J., et al. Splenic arterial interventions: Anatomy, indications, technical considerations, and potential complications. Radiographics. 2005;25(Suppl 1):S191-S211.
79. Velmahos G.C., Toutouzas K.G., Radin R., et al. Nonoperative treatment of blunt injury to solid abdominal organs: A prospective study. Arch Surg. 2003;138:844-851.
80. Asensio J.A., Roldan G., Petrone P., et al. Operative management and outcomes in 103 AAST-OIS grades IV and V complex hepatic injuries: Trauma surgeons still need to operate, but angioembolization helps. J Trauma. 2003;54:647-653. discussion 653-4
81. Nijhof H.W., Willemssen F.E., Jukema G.N. Transcatheter arterial embolization in a hemodynamically unstable patient with grade IV blunt liver injury: Is nonsurgical management an option? Emerg Radiol. 2006;12:111-115.
82. Hagiwara A., Sakaki S., Goto H., et al. The role of interventional radiology in the management of blunt renal injury: a practical protocol. J Trauma. 2001;51:526-531.
83. Bozeman C., Carver B., Zabari G., et al. Selective operative management of major blunt renal trauma. J Trauma. 2004;57:305-309.
84. Miller P.R., Moore P.S., Mansell E., et al. External fixation or arteriogram in bleeding pelvic fracture: initial therapy guided by markers of arterial hemorrhage. J Trauma. 2003;54:437-443.
85. Peitzman A.B., Heil B., Rivera L., et al. Blunt splenic injury in adults: Multi-institutional Study of the Eastern Association for the Surgery of Trauma. J Trauma. 2000;49:177-187. discussion 187-9
86. Maria Alonso M, Collin Brathwaite M, Victor Garcia M, et al. Eastern Association for the Surgery of Trauma Practice Management Guidelines for the Nonoperative Management of Blunt Injury to the Liver and Spleen. 2003. (Accessed 2011 at http://www.east.org/tpg.asp.)
87. Haan J., Ilahi O.N., Kramer M., et al. Protocol-driven nonoperative management in patients with blunt splenic trauma and minimal associated injury decreases length of stay. J Trauma. 2003;55:317-321. discussion 321-2
88. Rajani R.R., Claridge J.A., Yowler C.J., et al. Improved outcome of adult blunt splenic injury: A cohort analysis. Surgery. 2006;140:625-631. discussion 631-2
89. Harbrecht B.G., Ko S.H., Watson G.A., et al. Angiography for blunt splenic trauma does not improve the success rate of nonoperative management. J Trauma. 2007;63:44-49.
90. Duchesne J.C., Simmons J.D., Schmieg R.E.Jr., et al. Proximal splenic angioembolization does not improve outcomes in treating blunt splenic injuries compared with splenectomy: A cohort analysis. J Trauma. 2008;65:1346-1351. discussion 1351-3
91. Sabe A.A., Claridge J.A., Rosenblum D.I., et al. The effects of splenic artery embolization on nonoperative management of blunt splenic injury: A 16-year experience. J Trauma. 2009;67:565-572. discussion 571-2
92. Stein D.M., Scalea T.M. Nonoperative management of spleen and liver injuries. J Intensive Care Med. 2006;21:296-304.
93. Rhodes C.A., Dinan D., Jafri S.Z., et al. Clinical outcome of active extravasation in splenic trauma. Emerg Radiol. 2005;11:348-352.
94. Bessoud B., Denys A., Calmes J.M., et al. Nonoperative management of traumatic splenic injuries: Is there a role for proximal splenic artery embolization? AJR Am J Roentgenol. 2006;186:779-785.
95. Haan J.M., Bochicchio G.V., Kramer N., Scalea T.M. Nonoperative management of blunt splenic injury: A 5-year experience. J Trauma. 2005;58:492-498.
96. Bessoud B., Duchosal M.A., Siegrist C.A., et al. Proximal splenic artery embolization for blunt splenic injury: Clinical, immunologic, and ultrasound-Doppler follow-up. J Trauma. 2007;62:1481-1486.
97. Duke University Medical Center. Protocol for Management of Splenic Trauma, 2004.
98. Pachter H.L., Knudson M.M., Esrig B., et al. Status of nonoperative management of blunt hepatic injuries in 1995: A multicenter experience with 404 patients. J Trauma. 1996;40:31-38.
99. Fang J.F., Wong Y.C., Lin B.C., et al. The CT risk factors for the need of operative treatment in initially hemodynamically stable patients after blunt hepatic trauma. J Trauma. 2006;61:547-553. discussion 553-4
100. Croce M.A., Fabian T.C., Menke P.G., et al. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Results of a prospective trial. Ann Surg. 1995;221:744-753. discussion 753-5
101. Mohr A.M., Lavery R.F., Barone A., et al. Angiographic embolization for liver injuries: low mortality, high morbidity. J Trauma. 2003;55:1077-1081. discussion 1081-2
102. Fang J.F., Chen R.J., Wong Y.C., et al. Classification and treatment of pooling of contrast material on computed tomographic scan of blunt hepatic trauma. J Trauma. 2000;49:1083-1088.
103. Wahl W.L., Ahrns K.S., Brandt M.M., et al. The need for early angiographic embolization in blunt liver injuries. J Trauma. 2002;52:1097-1101.
104. Sarin E.L., Moore J.B., Moore E.E., et al. Pelvic fracture pattern does not always predict the need for urgent embolization. J Trauma. 2005;58:973-977.
105. Yoon W., Kim J.K., Jeong Y.Y., et al. Pelvic arterial hemorrhage in patients with pelvic fractures: detection with contrast-enhanced CT. Radiographics. 2004;24:1591-1605. discussion 1605-6
106. Agolini S.F., Shah K., Jaffe J., et al. Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. J Trauma. 1997;43:395-399.
107. Hagiwara A., Minakawa K., Fukushima H., et al. Predictors of death in patients with life-threatening pelvic hemorrhage after successful transcatheter arterial embolization. J Trauma. 2003;55:696-703.
108. Wong Y.C., Wang L.J., Ng C.J., et al. Mortality after successful transcatheter arterial embolization in patients with unstable pelvic fractures: Rate of blood transfusion as a predictive factor. J Trauma. 2000;49:71-75.
109. Silberzweig J.E., Khorsandi A.S. Occurrence of massive gluteal muscle necrosis following transcatheter angiographic embolization (TAE) to control pelvic hemorrhage in 6 patients. J Trauma. 2006;60:686-687.
110. Yasumura K., Ikegami K., Kamohara T., Nohara Y. High incidence of ischemic necrosis of the gluteal muscle after transcatheter angiographic embolization for severe pelvic fracture. J Trauma. 2005;58:985-990.
111. Cothren C.C., Osborn P.M., Moore E.E., et al. Preperitonal pelvic packing for hemodynamically unstable pelvic fractures: A paradigm shift. J Trauma. 2007;62:834-839. discussion 839-42
112. Kataoka Y., Maekawa K., Nishimaki H., et al. Iliac vein injuries in hemodynamically unstable patients with pelvic fracture caused by blunt trauma. J Trauma. 2005;58:704-708. discussion 708-10
113. Duke University Medical Center. Protocol for Management of Pelvic Fractures, 2004.
114. Greenhalgh R.M., Brown L.C., Kwong G.P., et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trial. Lancet. 2004;364:843-848.
115. EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): Randomised controlled trial. Lancet. 2005;365:2179-2186.
116. Blankensteijn J.D., de Jong S.E., Prinssen M., et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352:2398-2405.
117. Sicard G.A., Zwolak R.M., Sidawy A.N., et al. Endovascular abdominal aortic aneurysm repair: Long-term outcome measures in patients at high-risk for open surgery. J Vasc Surg. 2006;44:229-236.
118. Greco G., Egorova N., Anderson P.L., et al. Outcomes of endovascular treatment of ruptured abdominal aortic aneurysms. J Vasc Surg. 2006;43:453-459.
119. Hinchliffe R.J., Bruijstens L., MacSweeney S.T., Braithwaite B.D. A randomised trial of endovascular and open surgery for ruptured abdominal aortic aneurysm: Results of a pilot study and lessons learned for future studies. Eur J Vasc Endovasc Surg. 2006;32:506-513. discussion 514-515
120. Kaya A., Heijmen R.H., Overtoom T.T., et al. Thoracic stent grafting for acute aortic pathology. Ann Thorac Surg. 2006;82:560-565.
121. Milas Z.L., Milner R., Chaikoff E., et al. Endograft stenting in the adolescent population for traumatic aortic injuries. J Pediatr Surg. 2006;41:E27-E30.
122. Xenos E.S., Abedi N.N., Davenport D.L., et al. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48:1343-1351.
123. Kuo W.T. Transcatheter treatment for lower gastrointestinal hemorrhage. Tech Vasc Interv Radiol. 2004;7:143-150.
124. Darcy M. Treatment of lower gastrointestinal bleeding: Vasopressin infusion versus embolization. J Vasc Interv Radiol. 2003;14:535-543.
125. Green B.T., Rockey D.C., Portwood G., et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: A randomized controlled trial. Am J Gastroenterol. 2005;100:2395-2402.
126. Kuo W.T., Lee D.E., Saad W.E., et al. Superselective microcoil embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2003;14:1503-1509.
127. Brackman M.R., Gushchin V.V., Smith L., et al. Acute lower gastroenteric bleeding retrospective analysis (the ALGEBRA study): An analysis of the triage, management and outcomes of patients with acute lower gastrointestinal bleeding. Am Surg. 2003;69:145-149.
128. d’Othee B.J., Surapaneni P., Rabkin D., et al. Microcoil embolization for acute lower gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2006;29:49-58.
129. Kickuth R., Rattunde H., Gschossmann J., et al. Acute lower gastrointestinal hemorrhage: minimally invasive management with microcatheter embolization. J Vasc Interv Radiol. 2008;19:1289-1296. e2
130. Fisher R.G., Ferreyro R. Evaluation of current techniques for nonsurgical removal of intravascular iatrogenic foreign bodies. AJR Am J Roentgenol. 1978;130:541-548.
131. Bernhardt L.C., Wegner G.P., Mendenhall J.T. Intravenous catheter embolization to the pulmonary artery. Chest. 1970;57:329-332.
132. Richardson J.D., Grover F.L., Trinkle J.K. Intravenous catheter emboli: Experience with twenty cases and collective review. Am J Surg. 1974;128:722-727.
133. Thomas J., Sinclair-Smith B., Bloomfield D., Davachi A. Non-surgical retrieval of a broken segment of steel spring guide from the right atrium and inferior vena cava. Circulation. 1964;30:106-108.
134. Sheth R., Someshwar V., Warawdekar G. Percutaneous retrieval of misplaced intravascular foreign objects with the Dormia basket: An effective solution. Cardiovasc Intervent Radiol. 2007;30:48-53.
135. Gabelmann A., Kramer S., Gorich J. Percutaneous retrieval of lost or misplaced intravascular objects. AJR Am J Roentgenol. 2001;176:1509-1513.
136. Haaga J.R., Alfidi R.J., Havrilla T.R., et al. CT detection and aspiration of abdominal abscesses. AJR Am J Roentgenol. 1977;128:465-474.
137. Haaga J.R., Zelch M.G., Alfidi R.J., et al. CT-guided antegrade pyelography and percutaneous nephrostomy. AJR Am J Roentgenol. 1977;128:621-624.
138. Haaga R.J., Alfidi R.J., Cooperman A.M., et al. Definitive treatment of a large pyogenic liver abscess with CT guidance. Cleve Clin Q. 1976;43:85-88.
139. Kaminski A., Liu I.L., Applebaum H, et al. Routine interval appendectomy is not justified after initial nonoperative treatment of acute appendicitis. Arch Surg. 2005;140:897-901.
140. Zerem E., Salkic N., Imamovic G., Terzic I. Comparison of therapeutic effectiveness of percutaneous drainage with antibiotics versus antibiotics alone in the treatment of periappendiceal abscess: Is appendectomy always necessary after perforation of appendix? Surg Endosc. 2007;21:461-466.
141. Keckler S.J., Tsao K., Sharp S.W., et al. Resource utilization and outcomes from percutaneous drainage and interval appendectomy for perforated appendicitis with abscess. J Pediatr Surg. 2008;43:977-980.
142. St. Peter S.D., Aguayo P., Fraser J.D., et al. Initial laparoscopic appendectomy versus initial nonoperative management and interval appendectomy for perforated appendicitis with abscess: A prospective, randomized trial. J Pediatr Surg. 2010;45:236-240.
143. Uflacker R. Interventional therapy for pulmonary embolism. J Vasc Interv Radiol. 2001;12:147-164.