Chapter 10 Imaging Abdominal and Flank Trauma
Abdominal trauma presents challenges in diagnostic imaging. Patients with abdominal trauma may have multiple, concomitant life-threatening injuries to the head, spine, chest, and extremities. For the unstable patient, the greatest challenge may be prioritizing injuries for diagnostic and therapeutic interventions. Often, emergency department imaging of these patients must be kept to a minimum before operative intervention. For the stable patient, the challenge is often to avoid unnecessary imaging, which may lead to undesirable radiation exposure and costs of care.
In this chapter, we discuss imaging modalities useful in blunt and penetrating abdominal trauma and imaging of the unstable and the stable patient. For computed tomography (CT) in trauma, we discuss the evidence and indications for contrast agents. We review the interpretation of ultrasound, plain radiographs, and CT images, with a focus on common and serious injury patterns. We also discuss clinical decision rules for blunt abdominal trauma, which may identify patients at low risk for injury who may safely forgo imaging. We consider the safety of discharge from the emergency department following a negative imaging evaluation in blunt and penetrating trauma. Finally, we review trauma imaging in the pregnant patient and radiation exposure in trauma imaging.
Imaging Modalities in Trauma
Plain Radiographs
Plain x-ray plays a limited role in the evaluation of blunt abdominal trauma. X-rays of the chest and pelvis are often obtained to evaluate for concurrent thoracic or pelvic injuries (discussed in dedicated chapters), but normal x-rays do not rule out injuries to solid or hollow abdominal organs. Abnormal chest x-ray findings of pneumothorax and rib fractures are associated with intraabdominal injuries and are indications for abdominal imaging if a mechanism for multisystem trauma is present.1 Chest x-ray may reveal free air, suggesting hollow organ injury, although this finding is both rare and nonspecific in blunt trauma. Pneumoperitoneum can also result from thoracic injuries, with air tracking into the abdomen, and is thus not an absolute indication for laparotomy.2-4 Diaphragmatic rupture may also be seen, although Smithers et al.5 reported a sensitivity of chest x-ray of around 60% for diaphragm rupture within 48 hours after injury. X-rays of the abdomen are not useful in blunt trauma because they do not assess for solid organ injuries, which are the most commonly injured organs with blunt mechanisms.
In high-velocity, penetrating abdominal trauma such as gunshot wounds, plain radiographs are commonly obtained, including anterior–posterior (AP) and lateral x-rays of the abdomen, upright AP chest radiograph, and pelvis x-ray. In the case of gunshot wounds, x-rays identify the location and number of retained projectiles. Chest and pelvis x-rays are obtained because of the erratic path of tumbling missiles, which can lead to extra-abdominal injuries. Common findings include free abdominal air (pneumoperitoneum), pneumothorax, and hemothorax. The upright chest radiograph is most sensitive for free air. In penetrating trauma, pneumoperitoneum is generally considered an indication for laparotomy, because of the likelihood of bowel perforation as the source. Plain radiographs may also reveal important bony injuries. Plain radiographic findings of hemoperitoneum and solid organ injury are unreliable, and other imaging modalities are usually relied upon to assess for these.
In abdominal stab wounds, plain radiographs of the chest and abdomen are usually obtained, again to assess for pneumoperitoneum, pneumothorax, or hemothorax, any of which can occur from high abdominal or flank stab wounds.
Ultrasound
Ultrasound has become a common part of the initial assessment of blunt abdominal trauma. Ultrasound serves a screening function because it assesses for the presence of free fluid in the abdomen or pericardium but does not explicitly identify the source. The focused assessment with sonography in trauma (FAST) (also sometimes called focused abdominal sonography in trauma or focused abdominal sonography for trauma) examination has been a standard part of the diagnostic algorithm since the 1990s in most U.S. trauma centers. In the FAST exam, the right upper quadrant (Morison’s pouch, or hepatorenal recess), the left upper quadrant (splenorenal recess), the suprapubic space (pelvis), and the pericardium (usually via a subxiphoid approach) are inspected for anechoic material representing fluid—which in the setting of trauma is assumed to be blood. Advantages of ultrasound include portability (allowing it to be used in the trauma bay during resuscitation), lack of ionizing radiation exposure, repeatability (allowing evaluation of changes in patient condition), and rapidity of the exam. Disadvantages include significant operator dependence, meaning that equal results are not achieved with ultrasonographers of varying experience and ability. Ultrasound is considered most useful in detection of solid organ injuries with associated hemoperitoneum. It is considered insensitive for the detection of bowel or retroperitoneal injuries. More recent studies have focused on the ability of ultrasound to detect pneumoperitoneum6 and hemopneumothorax (discussed in Chapter 6 on chest trauma). Moriwaki et al.6 found ultrasound was 85% sensitive and 100% specific for detection of free air in a prospective study of 484 patients. Some small studies have also investigated the utility of ultrasound contrast agents to detect active bleeding.7-8
Ultrasound is relatively sensitive for free abdominal fluid. In one study, in which fluid was instilled into the abdomen in 100-mL aliquots during diagnostic peritoneal lavage (DPL), the median amount of fluid required for identification of an anechoic stripe in Morison’s pouch was 700 mL with the patient in a supine position. Positioning the patient in Tredelenburg (head down) position reduced the detection limit to 400 mL.9 In a second study, continuous scanning of Morison’s pouch during infusion of DPL fluid revealed a mean detection limit of 619 mL. Only 10% of ultrasonographers (attending physicians and residents in emergency medicine, radiology, and surgery) detected volumes less than 400 mL. The sensitivity at 1 L was 97%.10
Ultrasound has been extensively studied in the hands of both emergency physicians and surgeons. In one prospective study of 55 patients, the sensitivity and specificity of ultrasound were 91.7% and 94.7%, respectively, for detection of intraabdominal injury.11 Another prospective study of 200 blunt trauma patients found ultrasound to be 83% sensitive and 100% specific—although the authors concluded that only a single missed injury was significant.12 Ma et al.13 reported sensitivity of 90% and specificity of 99%. A review in 1996 found a paucity of prospective trials of ultrasound and numerous methodologic flaws in those studies.14 A systematic review and meta-analysis found 123 trials, of which 30 trials met methodologic criteria for inclusion. These trials included 9047 patients and gave a composite negative likelihood ratio of 0.23. Recall that a negative likelihood ratio of 0.1 is generally considered to provide strong evidence of the absence of pathology. In a patient with a pretest probability of abdominal injury of 50%, a negative ultrasound reduces the probability of injury only to around 25%. Ultrasound is not sufficiently sensitive to exclude intraabdominal injury, limiting its utility as a definitive test for abdominal trauma.15
What is the role of ultrasound in blunt trauma evaluation? When first introduced, ultrasound was viewed as a potential replacement for DPL.12 DPL was extremely sensitive for abdominal injuries (100% in many series), but its lack of specificity led to many nontherapeutic laparotomies. Because ultrasound requires the accumulation of greater amounts of peritoneal fluid for detection, it may fail to detect subtle injuries—leading to fewer laparotomies for minor solid organ injuries but also risking missed bowel injuries. With the growth of CT use in evaluation of blunt abdominal injury from the mid-1980s, nonoperative management of most blunt solid organ injuries became the norm, and the information provided by ultrasound became less useful in this setting. Given that a negative ultrasound does not exclude abdominal injury, CT is usually performed in stable patients with possible injury, even following negative ultrasound. CT is also usually performed in stable patients with positive ultrasound findings because further characterization of the source and degree of bleeding can allow nonoperative management, sometimes with angiographic embolization (see Chapter 16).
Ultrasound remains useful in the unstable patient. Following blunt trauma, hemodynamic instability is most often because of hemorrhage, with six potential sources: chest, abdomen, pelvis, retroperitoneum, large extremity compartments, and external bleeding sources (Table 10-1). Ultrasound can be used to exclude or confirm substantial abdominal and pelvic free fluid. A negative FAST suggests other sources of hemorrhage. A positive FAST suggests that abdominal bleeding is a significant contributor, potentially leading to immediate laparotomy. In a retrospective study, Farahmand et al.16 evaluated the performance of ultrasound in hypotensive blunt abdominal trauma patients. Whereas ultrasound was only 85% sensitive for detection of any injury, it was 97% sensitive for surgical injuries with 82% specificity in these patients, suggesting utility in patients too unstable for CT. The treatment priorities for the patient depend on the overall scenario, including the results of chest and pelvis radiographs and the presence of head injury. Patients with significant pelvic bleeding may require angiographic embolization, either before or after laparotomy. Patients with concurrent chest injuries may require thoracotomy or endovascular repair.
TABLE 10-1 Sources of Hemorrhage in Blunt Trauma, With Imaging Modality Used to Evaluate
| Bleeding Source | Diagnostic Modality | Comments |
|---|---|---|
| Chest | Chest x-ray | A negative chest x-ray (normal mediastinum and no hemo- or pneumothorax) substantially reduces the risk of thoracic hemorrhage as the cause for hemorrhagic shock. |
| Abdomen | Ultrasound (FAST examination) | A negative FAST (ultrasound) examination excludes abdominal bleeding as a likely source of shock, though it does not rule out abdominal injuries. A positive FAST examination identifies significant abdominal bleeding sources. |
| Pelvis | Pelvis x-ray | A normal pelvis x-ray excludes significant pelvic bleeding in most patients. Fractures that destabilize the pelvic ring may be associated with substantial pelvic bleeding (see Chapter 13). |
| Retroperitoneum | CT | Retroperitoneal injury is not readily evident from examination, though ecchymosis of the flanks or abdominal wall may be present. X-ray and ultrasound do not screen for this injury well, although by excluding hemorrhage sources in the chest, abdomen, and pelvis, they may identify retroperitoneal injury as a likely source of hemorrhage by process of elimination. CT scan is usually necessary to confirm or exclude retroperitoneal injury. |
| Extremities | Physical examination, x-ray | Examination may reveal significant limb swelling and possible unstable fractures. X-rays confirm fracture. |
| External sources | Physical examination | External bleeding is usually readily visible on examination. |
The role of ultrasound in patients with penetrating abdominal trauma is limited. As described later, CT is increasingly used to evaluate stable patients with penetrating abdominal injury, avoiding laparotomy in some. Because ultrasound does not distinguish between free fluid from penetrating solid organ injury (often managed nonoperatively) and that from hollow viscus injury (managed operatively), it is of little use in stable patients. Unstable patients with penetrating abdominal injury typically are taken rapidly to the operating suite, regardless of ultrasound findings.
Ultrasound can be useful in penetrating abdominal trauma in assessing for hemopericardium. Penetrating abdominal wounds can enter the chest, causing this injury pattern.
Does ultrasound improve clinical outcomes in patients with blunt abdominal trauma? Melniker et al.17 conducted a prospective randomized controlled trial of ultrasound in the initial evaluation of blunt abdominal trauma. The measured outcomes were time from emergency department arrival to transfer for operative care, CT use, hospital length of stay, complications, and charges. They enrolled 262 patients, and all measured outcomes favored patients undergoing ultrasound and reached statistical significance. Time to operative care was 64% less for ultrasound patients compared to control patients, and ultrasound patients underwent fewer CTs (odds ratio = 0.16), spent 27% fewer days in the hospital, and had fewer complications (odds ratio = 0.16). Charges were 35% less compared to control patients.17 Although this study appears at first glance to demonstrate a resounding advantage, the study was conducted in centers in which operative management of blunt solid organ injuries was common—not the case in most U.S. medical centers today. As a consequence, the reductions in time to operating room entry, CT use, and cost are unlikely to be reproduced. The study included penetrating and blunt trauma patients, two populations treated with different diagnostic and treatment algorithms in most centers. Other methodologic flaws limit the study, including lack of blinding, failure to enroll consecutive patients, and significant dropout rate (17%) after enrollment. The study did not control for the availability of operative care, which may have been the largest factor in the measured difference in time to operating room entry.
Computed Tomography
CT is discussed in detail later, including sensitivity for solid and hollow organ injuries in the setting of both blunt and penetrating trauma. For most stable trauma patients, CT has become the definitive imaging modality of choice when intraabdominal injury is suspected. CT is rapid and highly sensitive and specific for many important injury types. The information provided by CT allows prognosis of injury and selective nonoperative management in both blunt and penetrating trauma. CT is less sensitive for some important injuries, including bowel and diaphragmatic trauma, a limitation that must be recognized to prevent clinical errors following a negative CT.
Imaging Algorithms in the Unstable and Stable Abdominal Trauma Patient
Patients with hemodynamic instability should be assumed to have hemorrhagic shock as the most likely source, with obstructive shock (tension pneumothorax or pericardial tamponade) or distributive shock (spinal cord injuries) being exceedingly rare by comparison. Imaging in the emergency department should be performed quickly, with the goal of triage to the operating room or angiography suite if necessary. Sources of hemorrhage fall into six categories (see Table 10-1), which can be evaluated in the trauma bay using ultrasound and portable plain radiographs. Pericardial tamponade can be evaluated with bedside ultrasound as described in Chapter 6. Although tension pneumothorax is often described as a clinical diagnosis never to be made radiographically, rapid chest x-ray or bedside ultrasound can be used to evaluate for this condition (see Chapter 6).
No further emergency department imaging may be needed in a hemodynamically unstable patient with a single identified source of hemorrhagic shock. Patients with shock and abdominal or chest injuries typically are taken to the operating suite for surgical intervention. Patients with pelvic injuries resulting in shock usually receive external pelvic fixation and pelvic angiographic embolization of actively bleeding vessels. Preperitoneal packing is also sometimes used to tamponade bleeding. Patients with extremity hemorrhage or active external bleeding are usually treated with limb splinting and local compression to limit bleeding. If these interventions plus fluid resuscitation succeed in stabilizing the patient, then additional imaging with CT can be performed to evaluate more comprehensively for injuries.
Hemodynamically stable patients can be evaluated with plain radiographs followed by CT in patients with suspicion of injury, based on clinical judgment or published clinical decision rules (described later). Some patients with minimal trauma mechanism and no signs or symptoms of injury require no imaging. Stable blunt trauma patients with suspected abdominal injury usually undergo CT. In some centers, stable penetrating trauma patients without pneumoperitoneum on x-ray also are evaluated with CT, allowing nonoperative management for those without CT evidence of peritoneal violation and in those with isolated solid organ injury. In other centers, laparotomy or laparoscopy is performed rather than diagnostic imaging with CT.
Computed Tomography in Trauma: Indications for Contrast Agents
Blunt Trauma: Indications for CT Contrast Agents
For the evaluation of blunt abdominal or flank trauma, intravenous (IV) contrast should be routinely used, but oral contrast should not. The American College of Radiology recommends the use of 150 mL of intravenous contrast, infused at 2-4 mL per second, with CT being performed after a 60 second delay.17a Without any contrast agents, CT can reveal several important imaging findings. Free air, while rare following blunt trauma, is readily seen without any contrast agents.2-4 Air appears black on all window settings and is even more readily visible on lung or bone windows than on soft-tissue (abdominal) window settings (see Chapter 9). Free abdominal fluid representing hemoperitoneum is also readily seen on noncontrast CT. Simple fluid such as simple ascites or urine has a Hounsfield density on CT of 0 Hounsfield units (HU), whereas blood has a density of around 45 HU (though the exact value likely varies with time since hemorrhage and hematocrit).18 Using a digital radiology display called a picture archiving and communication system (PACS), the density of organs and fluid collections can be measured. On a noncontrast CT, blood appears slightly darker gray on soft-tissue windows than do solid organs such as the liver and spleen because these organs are slightly denser (around 100 HU).
The liver, spleen, kidneys, vascular structures, bones, bladder, and peritoneal fat are also visible on noncontrast CT. Use of bone windows and reconstructions to evaluate the spine and bony pelvis is discussed in Chapters 3 and 13. With the addition of IV contrast, several additional important features can be seen. First, solid organs such as the liver, spleen, and kidneys are highly vascular and intensely enhance with injected contrast on arterial phase imaging (CT images acquired at a moment coinciding with the injected contrast bolus reaching arterial beds). Enhancement is visible as a brightening or whitening of the organ because of the distribution of high-density injected contrast throughout the organ parenchyma. A normal solid organ enhances relatively uniformly. Injured areas of these solid organs fail to enhance with IV contrast because they no longer receive a normal blood supply. These regions appear darker than (“hypodense” relative to) the surrounding normally perfused organ. Areas of injury are variously described as contusions, lacerations, and contained hematomas—but these terms are essentially synonyms for regions of solid organs that do not enhance with IV contrast, indicating poor perfusion of that organ segment. Without the addition of IV contrast, these regions may be less conspicuous because the density of normal solid organs is similar to that of an injured region without IV contrast. A large area of injury may be visible on noncontrast CT if the density is sufficiently decreased by accumulated blood.
IV contrast serves a second important function: revealing active hemorrhage from injuries. With the injection of contrast, a blush of contrast on arterial phase imaging within a hypodense injured solid organ indicates active bleeding. This must be distinguished from normal enhancement of vessels within solid organs, such as portal and hepatic vessels within the liver. These normal vessels are surrounded by enhanced normal tissue on CT images. On the other hand, active bleeding within an area of injured organ appears as a bright white spot of contrast, surrounded by the hypoattenuated (darker gray) region of injury. Active bleeding into the peritoneal cavity can also be seen with injection of IV contrast. In this case, blood that had escaped into the peritoneal cavity before the moment of contrast injection and CT acquisition appears dark (around 45 HU) on soft-tissue windows. Active bleeding at the moment of CT acquisition appears as a bright white spot or amorphous patch within this hypoattenuated region of injury.
If noncontrast CT can reveal hemoperitoneum and even large areas of solid organ injury, why is IV contrast of value? In a stable patient, hemoperitoneum and even large regions of solid organ injury are often managed nonoperatively. On the other hand, active bleeding revealed by injected IV contrast predicts the need for either operative management or, more commonly today, angiographic embolization to stop hemorrhage. Angiographic embolization is discussed in more detail in Chapter 16.
IV contrast can also reveal hypoperfused organs resulting from vascular pedicle injuries. In this case, the organ appears hypodense (dark gray), failing to enhance normally following IV contrast administration. This is an indication for laparotomy, either for vascular repair to salvage the organ or for organ removal to prevent hemorrhage. Active bleeding from an injured vessel may be visible but may be limited acutely by vasospasm. Bowel injuries are sometimes recognized with the addition of IV contrast. A regional bowel injury may result in hypoattenuation of the bowel wall because IV contrast does not perfuse the injured segment.
Other injuries can be revealed by injected IV contrast. Although contrast is injected intravenously, not intra-arterially, for CT, the CT image acquisition is usually timed to coincide with arterial perfusion, so arterial bleeding may be seen directly. The timing of the CT may be intentionally selected to represent an intermediate phase of perfusion, when both arterial and venous structures are filled with contrast. Sometimes, additional “delayed” CT images are acquired following the initial CT. These additional images are obtained seconds to minutes after the injected contrast bolus to allow visualization of venous structures, venous phases of solid organ perfusion, and even renal perfusion and excretion of contrast. The use of delayed images to evaluate renal and ureteral injuries is discussed in detail in Chapter 12.
Oral contrast is not indicated for the evaluation of blunt trauma patients. Intuitively, it seems logical that addition of oral contrast might assist in recognition of bowel injuries. A bowel injury might result in bowel perforation, characterized by leak of contrast from the bowel lumen into the peritoneal cavity. Alternative, a contused and thickened bowel wall might be more visible if the bowel lumen were opacified with contrast. These theoretical benefits have not been demonstrated in clinical trials. In multiple large studies, CT without oral contrast performed comparably to CT with oral contrast. In its appropriateness criteria for blunt abdominal trauma, the American College of Radiology states “CT evaluation of the abdomen and pelvis for blunt trauma does not require the use of oral contrast.”17a
Clancy et al.19 reviewed 492 abdominal CTs for blunt trauma and compared CT interpretation with operative findings and clinical follow-up. Oral contrast was used in only 8 patients. Only 1 of 372 patients with a negative non–oral contrast CT required surgery. Five bowel injuries were found in 42 patients who underwent operation, and the authors claimed that oral contrast would not have ensured preoperative diagnosis, although their retrospective methods cannot prove this assertion.
Stafford et al.20 randomized 594 adult blunt trauma patients to receive oral contrast or no oral contrast before CT with IV contrast. Six bowel injuries were identified at laparotomy in the oral contrast group, and three were identified in the non–oral contrast group. One small-bowel injury was missed by CT in the 199 patients receiving oral contrast. No small-bowel injuries were missed by CT in the 195 patients receiving no oral contrast.
Allen et al.21 prospectively enrolled 500 consecutive adult blunt trauma patients and performed CT with IV contrast only to determine the sensitivity for blunt bowel and mesenteric injury. They compared the initial clinical CT interpretation to a composite criterion standard of laparotomy results, autopsy results, a research study–only CT reading, and clinical follow-up to determine the presence or absence of injury. CT without oral contrast was 95% sensitive and 99.6% specific, comparing favorably with the reported sensitivity of oral contrast–enhanced CT. In a separate report from the same study, the authors reported the sensitivity and specificity of CT without oral contrast for blunt diaphragmatic injury (66.7% and 100%, respectively).22
Stuhlfaut et al.23 retrospectively reviewed records of 1082 patients undergoing multidetector CT without oral contrast to determine the sensitivity of CT for bowel and mesenteric injury requiring surgical repair. The criterion standard was a composite of laparotomy report and hospital course. CT was considered positive for bowel or mesenteric injury if findings included pneumoperitoneum with other secondary findings (n = 4), mesenteric hematoma and bowel wall abnormality (n = 2), mesenteric hematoma only (n = 4), or bowel wall thickening only (n = 4). CT was considered negative for bowel or mesenteric injury if findings included no intraabdominal injury, solid organ injury only, or free fluid only. Sensitivity was 82%, with a 95% confidence interval (CI) of 52% to 95%, and specificity was 99% (95% CI = 98%-99%), similar to prior studies of CT with oral contrast.
Holmes et al.24 retrospectively reviewed records of 6052 consecutive blunt trauma patients undergoing CT with IV but without oral contrast to determine the sensitivity of CT for gastrointestinal injury. Injuries were confirmed by laparotomy, autopsy, or additional (non-CT) imaging studies. Out of 106 patients with confirmed gastrointestinal injuries, CT was abnormal in 91 patients (86%, 95% CI = 78%-92%). CT specifically suggested gastrointestinal injury in 81 patients (76%, 95% CI = 67%-84%). CT specifically suggested gastrointestinal injury in 58 of 64 patients (91%, 95% CI = 81%-96%) with major gastrointestinal injuries, defined as gastrointestinal perforation, active mesenteric hemorrhage, or mesenteric devascularization.
Why did these trials fail to demonstrate an anticipated benefit of oral contrast? Several factors may contribute. First, some bowel injuries are seen on CT without oral contrast. Bowel injuries may be suggested from free air, hypoperfusion of a segment of bowel following IV contrast administration, thickening of the bowel wall, infiltration of the mesenteric fat surrounding the bowel, or free peritoneal fluid without an evident solid organ injury. None of these findings requires oral contrast for detection. Although these findings are nonspecific, they may be sufficient to prompt appropriate management (observation or surgical exploration). Second, many bowel injuries do not result in frank perforation, at least initially. Instead, the bowel wall may be contused but not violated. As a result, oral contrast may not leak into the peritoneal cavity. Other injuries may be microperforations that seal spontaneously or do not lie in dependent loops of bowel where contrast could escape. Third, if CT is performed rapidly following oral contrast administration, or if injuries or drugs slow gastrointestinal transit of contrast, orally administered contrast may not reach an injured segment of bowel to aid in diagnosis.
Finally, bowel injuries are quite rare, occurring in only about 1% of blunt abdominal trauma. Consequently, even large studies may fail to demonstrate a moderate benefit of oral contrast. For example, in a population of 1000 blunt trauma patients, only about 10 bowel injuries would be expected. If oral contrast enabled detection of 8 of 10 injuries, whereas 5 of 10 were detected without oral contrast, a real difference might exist, but the difference would be too small to be statistically significant. A very large study of thousands of patients would be required to confirm a difference of this magnitude. From a practical standpoint, however, the number needed to treat (by administering oral contrast) to detect a single additional bowel injury would be several hundred. While bowel injuries are important and might seem to justify the treatment of many patients to prevent a single missed injury, oral contrast has potential harms. Oral contrast takes time to ingest and transit through bowel before CT, potentially wasting precious time in a patient with other life-threatening injuries. In addition, many blunt abdominal trauma patients are intoxicated, have head injuries, or are immobilized supine because of suspected spine trauma—making ingestion of oral contrast a potential aspiration hazard, though likely a rare event. In one retrospective review of 506 consecutive trauma patients undergoing CT with oral and IV contrast, one patient was noted to have instillation of enteral contrast through a nasogastric tube inadvertently placed into the right main bronchus rather than the stomach.25
Penetrating Trauma: Can “Triple-Contrast CT” Guide Nonoperative Management?
Historically, penetrating trauma of the abdomen or flank was evaluated with surgical exploration (laparotomy), laparoscopy to inspect for peritoneal violation, or local wound exploration. More recently, selected stable patients have been evaluated with so-called triple-contrast CT (CT with oral, IV, and rectal contrast). IV contrast in this setting serves the same functions as in blunt trauma, discussed in detail earlier. Oral and rectal contrast agents are used for the theoretical benefit of demonstrating leak of contrast from the bowel lumen into the peritoneum or retroperitoneum.
As we discussed earlier, oral contrast has not been shown to be diagnostically beneficial in blunt abdominal trauma. Theoretically, oral and rectal contrast might be more useful in penetrating injuries, in which bowel penetration is a more common injury, and in which larger defects in the bowel wall might be expected. However, multiple studies have failed to show contrast leak from the bowel lumen to be a sensitive indicator of hollow viscus injury. In one prospective study of 200 patients, only 19% of patients with proven bowel injury had contrast leak from bowel observed on CT. In comparison, a wound track near the bowel was 77% sensitive.26 Given this, are oral and rectal contrast agents truly essential in the setting of penetrating trauma? A single study has evaluated the sensitivity and specificity of “single-contrast CT” (CT with IV contrast and no oral or rectal contrast) for penetrating abdominal or flank injuries.27 Ramirez et al.27 retrospectively reviewed 10 years experience with IV contrast–only CT for evaluation of penetrating torso trauma in 306 hemodynamically stable patients. CT predicted “need for laparotomy” with 98% sensitivity and 90% specificity, a positive predictive value of 84%, and a negative predictive value of 99%. In 222 patients with gunshot wounds, the authors reported 100% sensitivity and a 100% negative predictive value, whereas they found 92% sensitivity and a 97% negative predictive value in patients with stab wounds. Although the authors did not compare the sensitivity and specificity of CT with IV contrast to triple-contrast CT, their results are comparable to those reported in studies of triple-contrast CT, described later. Like many retrospective studies, this study suffers from a mixed criterion standard that includes laparotomy and clinical course. The authors did not report the duration of clinical follow-up and losses to follow-up, raising the possibility that some injuries might have been missed by CT. Unlike some other studies, this study focused on prediction of “need for laparotomy” rather than sensitivity for peritoneal violation. Unfortunately, the authors did not categorize the laparotomy results (therapeutic, nontherapeutic, and negative). Because the surgeons in this study were not blinded to the CT results, they assuredly were influenced by the CT findings in their decision to operate. As a consequence, the reported performance of the CT is affected by incorporation bias. Nevertheless, the authors pointed to significant time savings by avoiding oral and rectal contrast agents.
Demetriades et al.37 prospectively studied alert hemodynamically stable patients without findings of peritonitis on examination following penetrating abdominal injury. Unstable patients, patients with peritonitis, and those who could not be evaluated clinically underwent immediate laparotomy. Of 152 patients, 61 (40.1%) met criteria for CT evaluation with IV but without oral or rectal contrast. Patients without CT findings of hollow viscus injury were observed with serial exams and laboratory evaluation for falling hematocrit or developing leukocytosis. The authors found that 43 patients with 47 solid organ injuries (liver, spleen, and kidney) had no CT evidence of hollow viscus injury by CT. Because of CT evidence of active bleeding, 4 patients underwent angiographic embolization of the liver. Whereas 41 patients (27% of total) were managed nonoperatively, 3 patients failed nonoperative management but recovered without complications after laparotomy, and 2 patients underwent laparoscopy to evaluate for possible diaphragm injury, given the relatively low reported sensitivity of CT for this injury pattern.
Inaba and Demetriades38 reviewed the indications for laparotomy in penetrating abdominal injury. Hemodynamically stable patients without examination findings of peritonitis can be managed nonoperatively with careful observation. Gunshot wounds to the abdomen can be imaged with CT to determine projectile trajectory and likely injuries. Peritoneal violation on CT without evidence of organ injury can be managed nonoperatively with careful serial examinations. Solid organ injuries can be managed with endovascular therapies, primarily embolization for control of hemorrhage. Evidence of hollow viscus injury on CT warrants surgical exploration.
Mitra et al.39 retrospectively reviewed all patients with penetrating abdominal injury over a 5-year period and found 36 hemodynamically stable patients undergoing CT for evaluation. The authors did not identify the CT protocol used, although they specified that triple contrast was not used. They reported a 77.8% combined sensitivity of CT and ultrasound in these patients.
Beekley et al.40 retrospectively reported on 145 stable patients in a military combat support hospital without peritonitis following penetrating abdominal shrapnel wounds. Patients were routinely evaluated with CT with IV contrast; oral contrast was used “rarely” and rectal contrast was used in 2 cases. Patients with no CT findings of intraperitoneal or retroperitoneal penetration were managed nonoperatively. Based on CT, 85 (59%) of patients were managed nonoperatively, and 60 (41%) underwent laparotomy. The authors reported that 75% of laparotomies were therapeutic. Negative CT missed 1 patient with an intraabdominal injury requiring laparotomy (sensitivity = 97.8%, specificity = 84.8%, negative predictive value = 99%).
A number of studies have evaluated the safety and sensitivity of the triple-contrast CT approach. Multiple large series and a metaanalysis report good outcomes with conservative nonoperative management following a CT showing no evidence of bowel injury. We discuss these shortly, but first consider some limitations. First, publication bias may skew the reported literature on this topic, with authors not reporting failures of this technique. Second, many of the published studies suffer from limited clinical follow-up, raising the possibility of missed injuries not recognized by the researchers. Third, the indications for conservative management with CT include a reassuring physical exam. Because different physicians may interpret the physical examination differently, it is unclear how safe the practice would be outside of the major trauma centers that are the source of much of the data.
Hauser et al.28 retrospectively reviewed 40 cases of posterior abdominal penetrating injuries evaluated with CT using IV, oral, and rectal contrast. They found 6 of the 40 cases to have abdominal injury by CT, confirmed by laparotomy. Although 34 patients without CT evidence of peritoneal or retroperitoneal organ injury were observed for 72 hours and discharged without laparotomy, lack of long-term follow-up prevents certainty that no injuries were missed.
Himmelman et al.29 prospectively enrolled 88 hemodynamically stable patients with penetrating back or flank wounds. In 9 patients, CT suggested surgical injuries; 5 of these underwent laparotomy, with 2 having significant injuries. The other 79 patients had no CT findings suggesting need for surgery, and 77 of them were observed for 48 hours without complications. Because of positive DPL, the remaining 2 patients underwent laparotomy, negative in both cases. The authors concluded that a reassuring CT had a 100% negative predictive value for injuries requiring surgical repair.
McAllister et al.30 reported on 53 hemodynamically stable patients with stab wounds to the back, evaluated with triple-contrast CT if local wound exploration showed muscle fascia penetration. Of the 53 patients, 51 had negative CT or injuries not requiring surgery and did well with nonoperative management. Two CTs demonstrated injuries requiring therapeutic celiotomy. The authors concluded that CT was accurate in detection of occult injury but was costly and did not contribute to clinical management decisions—a peculiar conclusion given that the authors did not assess the planned management from clinicians blinded to the CT results.
Soto et al. reported on 32 stable patients examined with triple-contrast CT and serial ultrasound following penetrating abdominal stab wounds. One patient had CT findings of extensive hepatic laceration and massive hemoperitoneum and was managed operatively. The other 31 patients were managed nonoperatively based on CT and ultrasound findings and were asymptomatic at 28-day follow-up. Of these patients, 21 had some form of intraperitoneal injury but without evidence of bowel injury.31 The small number of patients in this series, and the limited number of significant injuries, limit the external validity of this study.
Chiu et al.32 retrospectively reviewed 75 hemodynamically stable patients undergoing triple-contrast CT following penetrating torso trauma. Patients with negative CT or isolated solid organ injury were observed, whereas those with any other evidence of peritoneal violation (free air or fluid, contrast leak, or nonsolid visceral injury) underwent laparotomy. In patients with negative CT, 47 of 49 (96%) had successful nonoperative management, 1 had negative laparotomy, and 1 had a left diaphragmatic injury. In patients with positive CT, 15 of 18 had therapeutic laparotomy. Whereas 5 patients had isolated hepatic injuries, 2 had hepatic and diaphragmatic injuries, managed with angiographic embolization and thorascopic diaphragmatic repair. The authors concluded that CT is useful in selecting patients for nonoperative management, although solid organ injuries and diaphragmatic injuries may require invasive therapies.
Shanmuganathan et al.33 prospectively studied triple-contrast helical CT in predicting peritoneal violation and need for laparotomy following penetrating torso trauma in 104 hemodynamically stable patients (54 gunshot wounds and 50 stab wounds). A positive CT was defined as evidence of peritoneal violation or injury to the retroperitoneal colon, a major vessel, or the urinary tract. Laparotomy was performed in patients with a positive CT except in those patients with isolated liver injury or free fluid. Patients with negative CT were initially observed. Among those with positive CT, 19 (86%) had therapeutic laparotomy. Whereas 9 patients with isolated hepatic injury on CT were successfully managed nonoperatively, 67 of 69 patients (97%) with negative CT had successful nonoperative management. The authors reported 100% sensitivity and 96% specificity of CT, although they failed to account for the operative management in 2 patients with negative CT.
In a separate report, Shanmuganathan et al.26 reported on 200 hemodynamically stable penetrating trauma patients without peritoneal signs or radiographic (x-ray) findings of pneumoperitoneum. The group included 86 patients with gunshot wounds, 111 with stab wounds, and 3 sharp impalements. Triple-contrast CT was prospectively evaluated by three radiologists for peritoneal violation and injury to intra or retroperitoneal solid organs, bowel, mesentery, vascular structures, diaphragm, and urinary tract. CT was 97% sensitive for peritoneal violation requiring laparotomy and diagnosed 28 hepatic, 34 bowel or mesenteric, 7 splenic, and 6 renal injuries. Laparotomy based on CT findings was therapeutic in 87% of patients. Two patients with negative CT required therapeutic laparotomy.
Conrad et al.34 retrospectively reviewed the charts of 81 patients who had emergency department workup for penetrating truncal trauma using triple-contrast CT. Following negative CT, 62 patients were discharged from the emergency department. Two missed injuries were identified but had no complications according to the authors. The authors concluded that emergency department discharge is a safe disposition; unfortunately, nearly one third of those discharged had no follow-up, rendering uncertain the outcomes of this strategy. If a significant percentage of the patients lost to follow-up had missed injuries, emergency department discharge following a negative CT would be unacceptable.
Loberant and Goldfeld35 provided a case report of a patient undergoing a negative laparotomy because of a bony fragment mistakenly identified as contrast extravasation on triple-contrast CT.
Dissanaike et al.36 reported a series of 9 patients evaluated with triple-contrast CT, with no missed injuries following nonoperative management of patients with negative CT. Clearly, the small numbers of patients in this series limit the value of the study.
Goodman et al.41 performed a metaanalysis to determine the predictive value of CT for laparotomy in hemodynamically stable patients with penetrating abdominal trauma. They identified 180 studies but included only 5 because of methodologic concerns. The pooled sensitivity, specificity, negative predictive value, positive predictive value, and accuracy were 94.90%, 95.38%, 98.62%, 84.51%, and 94.70%, respectively. This meta-analysis included studies using both single-contrast and triple-contrast techniques and thus does not clarify the necessity for contrast agents.
Overall, triple-contrast CT appears to be a safe management strategy in highly selected stable patients without peritonitis on examination. Losses to follow-up, variable inclusion criteria, and the likelihood of publication bias favoring positive outcomes in the published studies all contribute to a need for caution with a negative CT. Multiple studies have demonstrated the possibility of missed diaphragmatic injuries and, rarely, missed operative bowel injuries. Following a negative triple-contrast CT, observation or close follow-up should be ensured. Further study is needed to determine the true diagnostic value of oral and rectal contrast in this setting.
Interpretation of Images
Interpretation of X-Ray
Interpretation of chest and pelvis x-rays for trauma is described in detail in Chapters 6 and 13, respectively. In blunt abdominal trauma, abdominal radiographs are not useful and should not be obtained. The chest x-ray should be evaluated for signs of free air (see Figures 6-11 and 9-7), although the trauma chest x-ray is usually obtained in a supine position and is insensitive for pneumoperitoneum. The chest x-ray can reveal diaphragmatic rupture (see Figures 10-30 and 10-33 later in the chapter), more commonly on the left than on the right. Signs of diaphragm rupture include an elevated hemidiaphragm and herniation of abdominal contents into the thorax. If a nasogastric tube has been placed, it may be seen curling in the thorax. The gastric air bubble may be seen in an abnormally elevated position.
In penetrating abdominal trauma, an upright chest x-ray should be obtained to increase the sensitivity for free air. The subdiaphragmatic region should be inspected for lucencies suggesting pneumoperitoneum (see Figures 6-11 and 9-7). The abdominal, chest, and pelvis x-rays should be inspected for retained missile fragments (see Figure 10-9) in the case of gunshot wounds. Bony injuries, particularly spine and pelvic fractures, should be sought. Hemothorax (see Figures 10-9 and 10-35) and pneumothorax are other common findings in this setting.
Interpretation of Ultrasound
The key radiographic finding in ultrasound of blunt and penetrating abdominal trauma is free fluid, which is assumed to be hemoperitoneum in the setting of trauma. Patients with medical conditions causing preexisting ascites can have false-positive ultrasound exams in this setting. Fluid is anechoic (black) on ultrasound. In the case of small amounts of hemoperitoneum, fluid may be visible only as an anechoic stripe separating the liver and the kidney on the right (Figure 10-1), or the spleen and the kidney on the left (Figure 10-2). Fluid may also accumulate between the spleen and diaphragm. Free fluid pooled in the pelvis is visible as anechoic collections lateral to the bladder on a transverse view (Figure 10-3). Free fluid may also be visible in the rectouterine recess (pouch of Douglas) in females using a sagittal view (see Figure 10-3). In cases of gross hemoperitoneum, loops of bowel may be seen floating in blood (Figure 10-4). In some cases, coagulated blood may take on a more echogenic appearance or fibrinous strands may be seen suspended in anechoic collections. Abdominal trauma can also cause hemopericardium, visible as an anechoic layer surrounding the heart on a subxiphoid view (Figure 10-5).
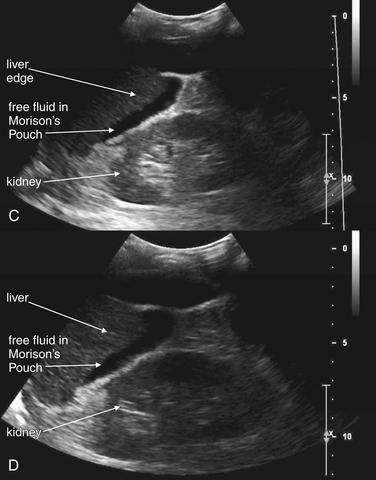
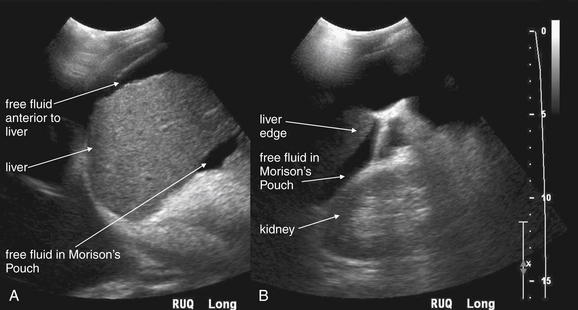
Figure 10-1 Free fluid in right upper quadrant: Ultrasound.
In these ultrasound images obtained in the right upper quadrant, free fluid in the abdomen is visible as a black (anechoic) collection separating the liver and the right kidney in Morison’s pouch (A-D). Fluid is also seen anterior to the liver (A). It is important to realize that the location of free fluid may not correlate with its source because fluid pools in dependent regions of the abdomen when the patient lies supine. Around 700 mL of fluid is required for detection with the patient supine. Fluid should be assumed to be hemoperitoneum in the setting of trauma, although preexisting ascites may have a similar appearance. Solid organ injury with hemoperitoneum is the most common cause of free fluid on ultrasound following trauma. Other causes of abdominal free fluid include bowel, mesenteric, and urinary tract injuries.
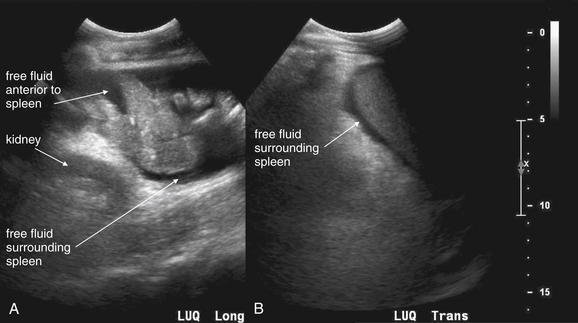
Figure 10-2 Free fluid in left upper quadrant: Ultrasound.
In these ultrasound images obtained in the left upper quadrant, free fluid in the abdomen is visible as a black (anechoic) collection separating the spleen and the left kidney (A, B).
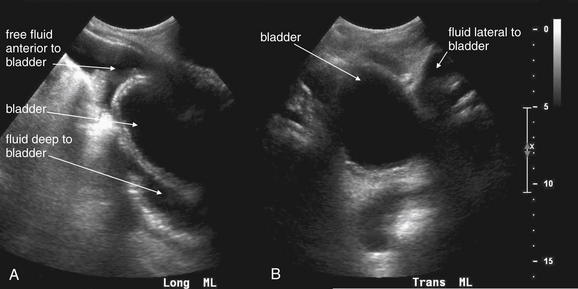
Figure 10-3 Free fluid in pelvis: Ultrasound.
The suprapubic view of the FAST examination assesses for free fluid in the pelvis. A full bladder appears as a round, well-circumscribed, midline anechoic structure. A, A midsagittal (long-axis midline) view. Fluid is visible anterior and deep to the bladder. B, A transverse view. Fluid is visible lateral to the bladder.
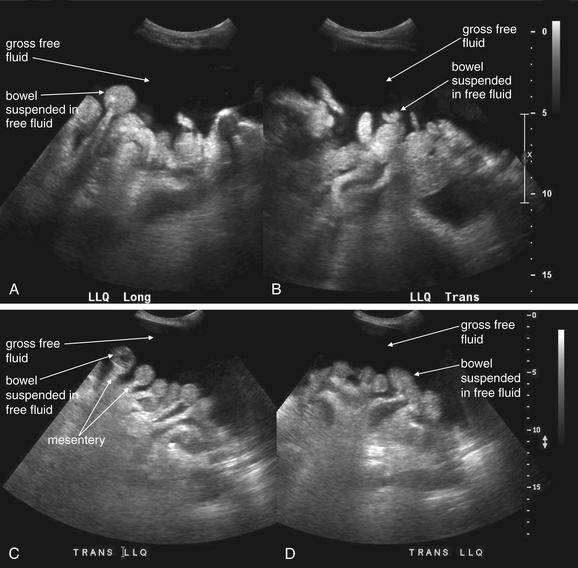
Figure 10-4 Gross free peritoneal fluid: Ultrasound.
Free fluid in the abdomen is common following blunt or penetrating abdominal trauma. After trauma, fluid in the abdomen should be considered to be a sign of hemorrhage until proven otherwise—although preexisting fluid such as ascites would have a similar appearance. A rarer source of fluid following trauma is bowel injury. Free fluid is anechoic (black) on ultrasound. When gross free fluid is present, loops of bowel may be seen suspended within it (A-D). The mesentery is also visible as “stalks” tethering the bowel (C, D).
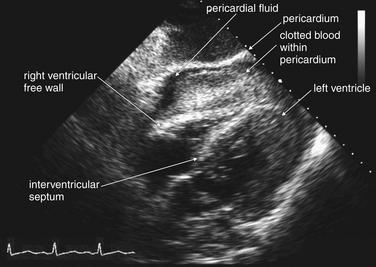
Figure 10-5 Hemopericardium: Ultrasound.
The FAST examination for blunt trauma includes inspection of the pericardium for blood. Usually a subxiphoid approach is used (as in this case), although in some patients long-axis parasternal or four-chamber apical views may be necessary. In this 57-year-old male who struck a telephone pole while riding a bicycle, a large pericardial effusion is visible (black, or anechoic). The pericardium appears bright, or hyperechoic. Clotted blood within the pericardium but outside the right ventricle is evident. The right ventricular free wall is bowed into the ventricular chamber. In the context of the hypotensive patient, these findings indicate pericardial tamponade.
Pneumoperitoneum is also sometimes visible on ultrasound. Air is hyperechoic and disrupts the ultrasound beam, preventing visualization of deeper structures. Because bowel gas is normally present in the anterior midline abdomen, free air should be sought overlying the liver, where air is not normally present.6
Interpretation of Computed Tomography
Approach to Abdominal CT
For the emergency physician, a systematic and targeted approach allows rapid and accurate identification of important injuries on abdominal CT. Because multiple injuries are common following abdominal trauma, it is extremely important to inspect all major abdominal structures. A common pitfall is to end the search after detection of a single injury. Three window settings are useful in evaluating abdominal structures: soft-tissue (sometimes called abdominal or vascular) windows, lung windows, and bone windows. Soft-tissue windows are used to assess solid organs and hollow organs, to detect free fluid, and to detect free air. Lung windows are used to assess for pneumothorax (recall that the lower lungs and pleural spaces are visible on the cephalad-most abdominal CT slices) and pneumoperitoneum, as described later. Bone windows are used to assess for fractures but can also reveal pneumoperitoneum. We recommend evaluating soft-tissue windows first, followed by lung windows, followed by bone windows. Window settings are discussed in more detail in Chapter 9. In the descriptions of traumatic injuries that follow, soft-tissue windows are the appropriate setting for evaluation unless stated otherwise.
On soft-tissue windows, evaluate each organ in turn, rather than attempting to evaluate multiple structures on each slice. For example, follow the spleen through all CT slices in which it appears, evaluating for splenic injury. Do not attempt to evaluate the liver, kidneys, or pancreas during this process, even though they are visible, because this may distract you from a splenic injury. After completing evaluation of the spleen, repeat this process with other organs.
Common and Serious Injury Patterns on Computed Tomography
Imaging Findings of Shock: Inferior Vena Cava and Bowel
In cases of severe volume depletion (generally from hemorrhagic shock following trauma), the infrahepatic inferior vena cava (IVC) appears flattened (Figure 10-6; see also Figure 10-20).42 This appearance can occur in patients before the development of clinical hypotension or hemodynamic collapse and demands immediate volume resuscitation. Rarely, it may be a normal variant. In a series of 100 trauma patients, a flattened IVC occurred in 7, 6 of whom had other evidence of severe blood loss. In 100 patients without trauma, a flattened IVC was not seen.42
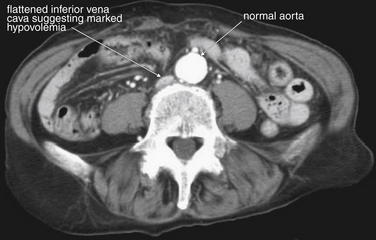
Figure 10-6 Inferior vena cava (IVC) flattening, a sign of hypovolemia and shock.
CT with IV contrast, soft-tissue window. The size of the IVC has been suggested as a surrogate for intravascular volume status. A flattened IVC can indicate impending hemodynamic collapse and should prompt immediate volume resuscitation. In this 77-year-old trauma patient, who had been traveling in a car struck by a train, the IVC is extremely flat, suggested marked hypovolemia. Compare with the aorta, which has a normal caliber (2 cm). The patient had multiple injuries noted on CT, including pulmonary contusions, a periaortic hematoma, free fluid around the liver, and pelvic superior rami fractures. She also suffered a closed head injury with subarachnoid and subdural hemorrhage. Her initial lactate was elevated. Three hours after CT, the patient suffered a pulseless electrical activity arrest and could not be resuscitated. Autopsy found more than 500 ml of hemoperitoneum, as well as mediastinal hemorrhage.
Shock bowel is a term for secondary bowel injury resulting from sustained systemic hypotension. The CT appearance includes diffuse bowel wall thickening, visible on CT without oral contrast (Figures 10-7 and 10-8). This is in contrast to primary small-bowel traumatic injuries, which usually show focal regions of bowel wall thickening. Shock bowel is variably associated with IVC and aortic flattening, abnormal pancreatic enhancement and peripancreatic fluid, and poor enhancement of the spleen and liver because of hypotension. This constellation of findings is sometimes called the CT hypotension complex and has been reported in other conditions, including head and spine injury, post–cardiac arrest, septic shock, and diabetic ketoacidosis.43
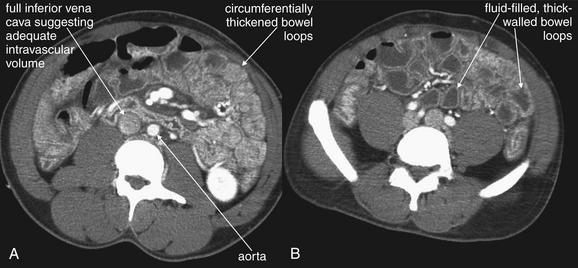
Figure 10-7 Shock bowel, a sign of severe sustained hypotension.
A and B, CT with IV contrast viewed on soft-tissue windows, demonstrating shock bowel in a trauma patient. Shock bowel refers to the appearance of bowel that has been hypoperfused for an extended period. Under these conditions, the bowel ceases peristalsis and assumes an appearance similar in some respects to small-bowel obstruction. In other respects, the bowel appears similar to a case of mesenteric ischemic—but the process is diffuse, rather than being restricted to a single vascular territory. As bowel becomes ischemic, it develops edema of the bowel wall, and the bowel wall develops a hyperenhancing pattern following administration of intravenous contrast, resulting from microvascular leak of the injected contrast. The bowel wall thus looks thick and bright, unlike its normal inconspicuously thin appearance. Loops of fluid-filled bowel are common, and the internal structure of the bowel becomes exaggerated. Sometimes plicae circularis become visible; at other times, the degree of bowel edema is so great that the lumen becomes effaced. This patient developed hemorrhagic shock from a traumatic amputation of his right arm, leading to shock bowel. By the time of the CT, the patient had been volume resuscitated, as indicated by the prominent inferior vena cava (compare with Figure 10-6).
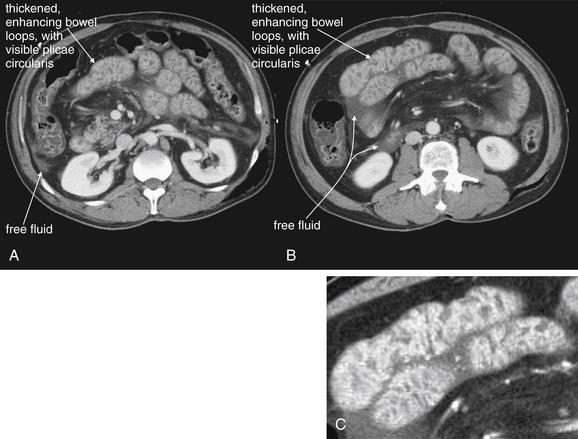
CT with IV contrast, soft-tissue window. No oral contrast has been given. A-C, Another example of shock bowel. This patient underwent exploratory laparotomy to assess for traumatic bowel injury because some free fluid was noted in the abdomen on CT. No abdominal injuries were found at laparotomy. C, Close-up from B.
Pneumoperitoneum
Pneumoperitoneum is rare following blunt abdominal injury but can indicate bowel perforation. When detected on CT, it is not specific for bowel injury because air tracking from thoracic injuries can collect in the abdomen.2-3 Following penetrating abdominal injury, pneumoperitoneum detected on CT is likely to indicate bowel perforation and prompts laparotomy in most cases.
On soft-tissue windows, air including pneumoperitoneum appears black (Figures 10-9 and 10-10; see also Figure 10-24). Large amounts of pneumoperitoneum are usually visible on this window setting, but smaller collections are more difficult to recognize because intraperitoneal and retroperitoneal fat appears quite dark on soft-tissue windows. On lung windows, all soft tissues and bones appear white, leaving only air black—thus emphasizing both pneumoperitoneum and bowel gas (see Figures 10-10 and 10-24, B). On bone windows, bone appears white or light gray, and all soft tissues are an intermediate gray, making black air readily visible. During trauma CT, the patient is generally supine, so pneumoperitoneum collects in the anterior midline and perihepatic spaces preferentially (60% in one study) rather than in a subdiaphragmatic location, as occurs on upright chest x-ray.44 Air can collect in other locations, including the pelvis and mesentery. CT can detect air collections as small as 1 mm.45
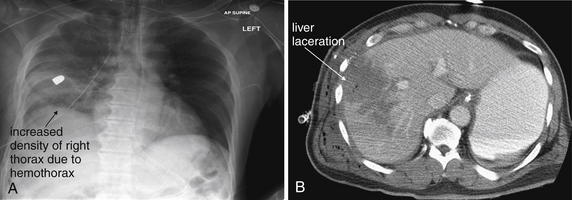
Figure 10-9 Penetrating abdominal injury with hemothorax.
This patient sustained a right upper quadrant gunshot wound. The trajectory of the bullet crossed the liver and diaphragm, entering the thorax. A, Chest x-ray shows a retained bullet and increased density of the right thorax, consistent with hemothorax. No meniscus is seen because this is a supine chest x-ray. B, CT with IV and oral contrast (soft-tissue window) shows a large hypodense area indicating hepatic laceration. Always consider chest injury with abdominal or flank gunshot wounds because the trajectory of the missile is unpredictable.
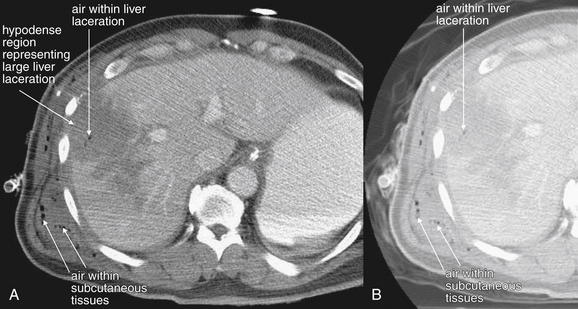
Figure 10-10 Intraperitoneal gas.
Same patient as the prior figure. Penetrating abdominal injuries can introduce exogenous air into the peritoneum and retroperitoneum. In addition, if perforation of a viscus occurs from a penetrating injury, air may escape into the peritoneum as a result. This patient sustained a gunshot wound to the right upper quadrant. CT shows air in the subcutaneous tissues, as well as air within the large hepatic laceration that resulted from the injury. Air appears black on all CT window settings, regardless of the administration of contrast. However, sometimes small collections of air may be difficult to identify on soft-tissue windows (A) because peritoneal fat is dark, nearly black, on this setting. Two other settings are useful to highlight air: bone and lung windows. On bone windows, bone is white, air is black, and all other tissues are intermediate gray. B, The same slice as A viewed on a lung window. Air is nearly black, whereas all other tissues appear white.
Hemoperitoneum and Free Abdominal Fluid
CT is believed to be highly sensitive for abdominal hemorrhage and free fluid. Hemoperitoneum is approximately 30 to 45 Hounsfield units (HU) in density and appears dark gray on CT soft-tissue windows (Figures 10-11 to 10-19 and 10-21 to 10-24).18 The density of fluid can be measured using the PACS cursor. Simple fluids such as urine or ascites have densities around 0 HU. Free fluid may also accumulate because of bowel injury and may have an intermediate density between the density of water and that of blood. Free fluid or hemoperitoneum generally collects by gravity in dependent regions of the abdomen and pelvis, so the location does not necessarily correspond to the source of bleeding. Inspect the perihepatic and perisplenic regions, including the hepatorenal recess (Morison’s pouch) and splenorenal recess. In addition, inspect the pelvic, paracolic gutters, and central abdomen because these regions may contain free fluid. Free fluid may be seen outlining bowel loops (see Figures 10-21 and 10-22).
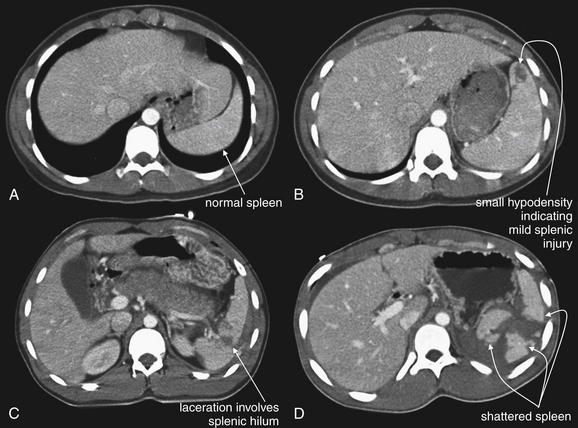
Figure 10-11 Spectrum of splenic injuries.
Injuries to the spleen are generally easy to recognize as hypoattenuated (dark) regions on CT with IV contrast. With the administration of intravenous (IV) contrast, the normal spleen enhances quite homogeneously to a fairly bright gray. Injured areas within the spleen (variously referred to as lacerations, contained hematomas, or contusions) fail to enhance with IV contrast, because they are avascular. Depending on the timing of the CT relative to the injection of contrast, normal vessels within the spleen may appear brighter than surrounding parenchyma and should not be confused with active bleeding. Active bleeding (demonstrated in Figure 10-12) appears as a blush of bright contrast but is typically surrounded by hypodense areas of injury. A, Uninjured spleen. B, Minimally injured spleen (grade I). This injury is unlikely to cause hemodynamic compromise acutely because it is small, contained within the spleen, shows no active hemorrhage, and does not involve the hilum. C, Moderately injured spleen (grade III or IV). This injury, though relative small, involves the splenic hilum and may become life-threatening. This injury type is often treated with angiographic embolization to prevent further bleeding. D, Severely injured spleen (grade V). This spleen is shattered and may require embolization or splenectomy.
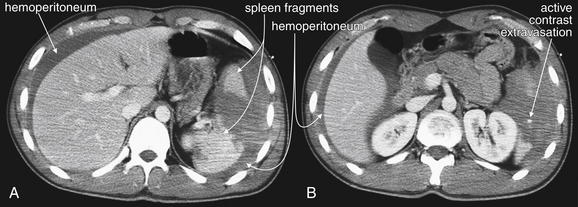
Figure 10-12 Grade IV spleen injury with hemoperitoneum and active extravasation of contrast material.
A, B, This 20-year-old male was ejected 15 feet from a vehicle and was found to have a high-grade splenic injury on CT with IV contrast. This injury was called “large” by the radiologist, “grade IV” by the surgeons. In this text, we focus on identifying injuries and accurately estimating severity, but specific scores are given less importance. CT findings include marked fragmentation of the spleen, gross hemoperitoneum, and active extravasation of injected contrast. Several points bear emphasis. First, note the quantity of fluid (blood) surrounding the liver even though the spleen is the injured organ. The location of free fluid is determined by gravity and tissue planes, not necessarily by the location of the injured organ. Free fluid appears dark gray compared with the brighter solid organs after administration of IV contrast. This is because of enhancement of the solid organ, which is receiving blood flow carrying contrast material. The free fluid accumulated before the administration of IV contrast and thus does not enhance. An exception is a region of active bleeding, which shows a bright blush of injected contrast at the moment of CT acquisition. This patient has such as blush, indicating active bleeding, in the bed of the spleen. How can active extravasation be discriminated from the normal appearance of vessels within the liver and spleen? These are, after all, extremely vascular organs. Depending on the timing of the CT scan relative to the injection of contrast material, the liver may show enhancement of hepatic arteries or veins, as well as portal veins. The liver parenchyma may remain somewhat enhancing, even after the major vessels have cleared of contrast. However, in each case, normal contrast enhancement of vessels can be recognized and discriminated from active extravasation. Active extravasation occurs in a location with an injury, which is hypodense (darker than surrounding tissue). In general, this means that the blush of active extravasation is surrounded by a darker tissue density of unenhancing fluid (blood) or poorly perfused injured tissue. Look for the bright spot with a dark background. The patient underwent exploratory laparotomy that confirmed a splenic injury with bleeding from the short gastric arteries. No other injuries were detected, and the patient underwent splenectomy. Grade IV and V splenic injuries are occasionally treated with embolization rather than splenectomy, even in the presence of active contrast extravasation, although the evidence for this approach is limited (see Chapter 16). Figures 10-13 to 10-15 explore this patient’s injury in more detail.
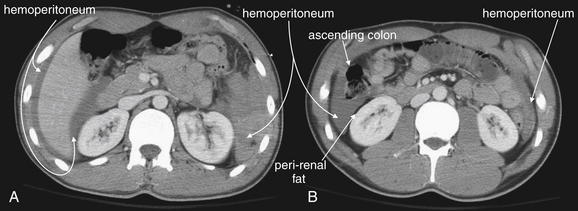
Figure 10-13 Hemoperitoneum in the setting of a grade IV spleen injury.
CT with IV contrast. These images are more caudad than those in Figure 10-12 for the same patient. A, Hemoperitoneum is present in a perihepatic location, including Morison’s pouch between the liver and the right kidney. This patient would have a positive FAST examination with fluid detected in the right upper quadrant. The liver itself was uninjured in this patient; the location of hemoperitoneum does not indicate its source. On the patient’s left, this slice is now below the level of the spleen, but a large amount of hemoperitoneum is present in this location as well. B, Still farther caudad, free fluid (hemoperitoneum) is seen in both paracolic gutters. Again, this would be seen on a FAST examination. A thin strip of fat separates the free fluid from the abdominal wall and from the kidneys, which are retroperitoneal. A common point of confusion is the appearance of fluid on CT, perhaps because emergency physicians use ultrasound so frequently. Whereas fluid appears black on ultrasound, on CT fluid appears an intermediate to dark gray (using soft-tissue windows). Fat appears nearly black on this window setting.
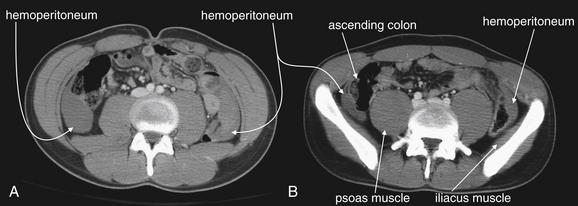
Figure 10-14 Hemoperitoneum in the setting of a grade IV spleen injury.
CT with IV contrast, soft-tissue window. These images are more caudad than those in Figure 10-13 for the same patient. Free fluid (hemoperitoneum) is seen in both paracolic gutters (A, B), extending into the pelvis (B). Again, this would be seen on a FAST examination. A thin strip of fat separates the free fluid from the iliacus muscle, which hugs the internal surface of the iliac bone of the pelvis, and the psoas muscle, which is paravertebral. Fat plays an important role as a contrast medium here, because without the interposed fat it would be difficult or impossible to identify the limits of the hemoperitoneum.
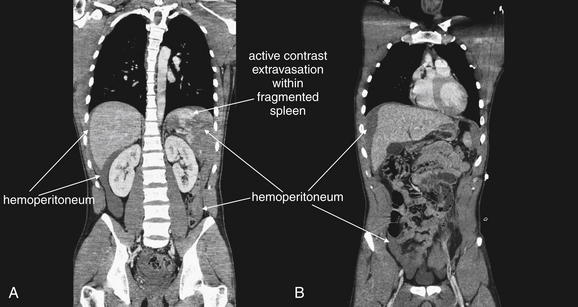
Figure 10-15 Grade IV spleen injury with hemoperitoneum and active extravasation of contrast material.
CT with IV contrast, soft-tissue window. Same patient as Figures 10-12 through 10-14. A, B, Coronal CT reconstructions are shown. Although these were not needed to make the diagnosis, they help illustrate the degree of hemoperitoneum, the fragmentation of the spleen, and the active extravasation of contrast indicating ongoing bleeding.
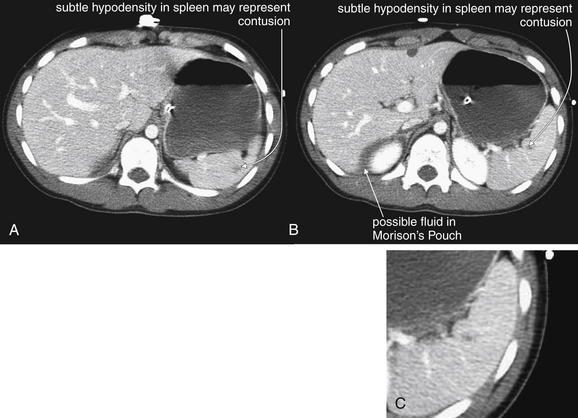
Figure 10-16 Grade I spleen injury.
CT with IV contrast, soft-tissue window. A-C, This 14-year-old male fell 15 feet from a tree. CT showed a subtle spleen injury. This injury would be characterized as grade I. Is free fluid present in Morison’s pouch? The patient did have pelvis fractures that could be a source. The patient was observed and did well without intervention. C, Close-up from B.
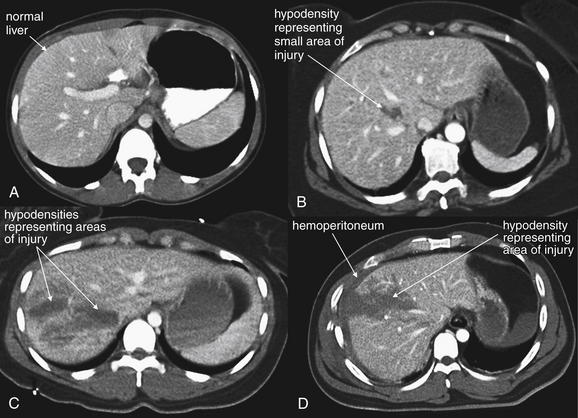
Figure 10-17 Spectrum of liver injuries.
CT with IV contrast, soft-tissue windows. In A, oral contrast is also present in the stomach. The normal liver enhances relatively uniformly with administration of intravenous contrast. Depending on the timing of the CT acquisition relative to the injection of contrast, portal and hepatic vessels are visible as bright linear markings. Injuries to the liver are generally easy to recognize as hypoattenuated (dark) regions because these regions have lost their blood supply and do not enhance. Areas of injury may be referred to by terms including hematoma, contusion, and laceration, which are essentially synonymous when describing CT findings. Hepatic injuries are graded from I to VI (see Table 10-3), but for the emergency physician, the key discriminatory categories are normal, low-grade injuries not requiring invasive therapy, and moderate- to high-grade injuries likely to require intervention (angiographic embolization or laparotomy). A, Uninjured liver. Dark areas here represent the normal anatomic divisions of the liver. B, Minimally injured liver (grade I). This injury is unlikely to cause hemodynamic compromise acutely because it is small, contained within the liver, shows no active hemorrhage, and does not involve the major vascular structures. C, D, Moderately to severely injured liver (grade III). C, The hematoma appears contained. D, The liver surface appears torn, and there is associated hemoperitoneum (dark gray crescent surrounding the liver). These injuries may progress, requiring embolization or laparotomy.
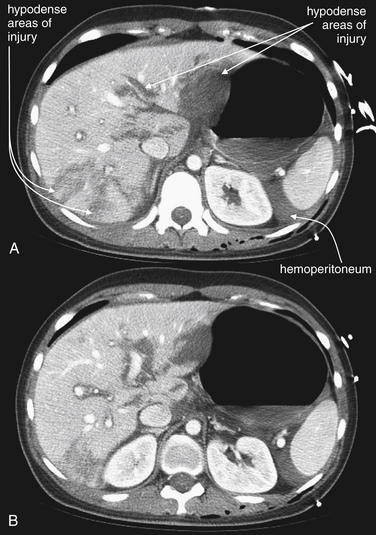
Figure 10-18 Hilar liver laceration.
CT with IV contrast, soft-tissue window. This patient sustained multiple life-threatening injuries in a motor vehicle collision, including a multifocal liver laceration extending to the liver hilum (A, B). Hemoperitoneum is present, particularly around the spleen. The patient was treated with angiographic embolization. Patients with higher grades of liver injury are sometimes too hemodynamically unstable even to undergo CT. The FAST examination may be positive, and the liver injury may be diagnosed during laparotomy or angiography.
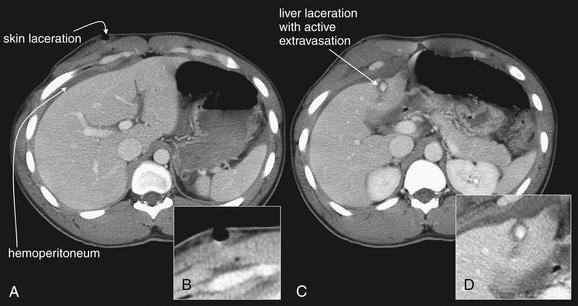
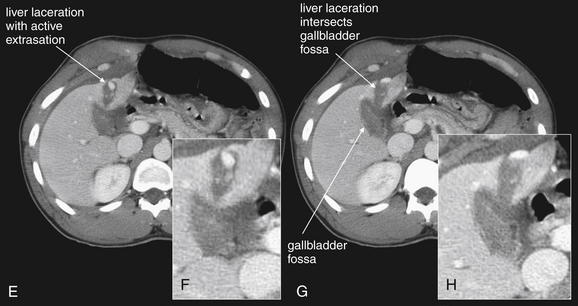
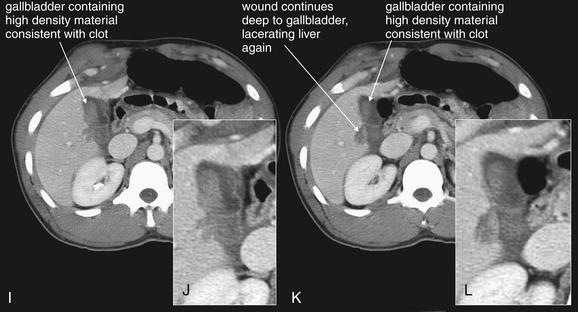
Figure 10-19 Gallbladder and biliary injuries.
A-L, Series of computed tomography (CT) slices, with close-ups from each slice. This patient sustained a single stab wound to the right upper quadrant. CT with intravenous contrast demonstrates a wound track through the liver into the gallbladder. The gallbladder has high-density material within it consistent with clot—higher in density than normal bile. The liver laceration, though small, shows evidence of active contrast extravasation. The patient underwent laparotomy, confirming a through-and-through gallbladder laceration that was treated with cholecystectomy.
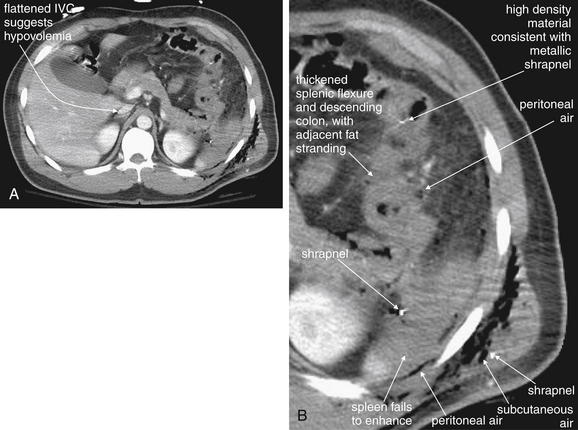
Figure 10-20 Bowel injuries or perforation from penetrating trauma.
A, This patient sustained a gunshot wound to the left chest, which entered the left upper abdomen, injuring the splenic flexure and descending colon. B, The area of injury has been enlarged to allow inspection for small foci of air and metal shrapnel. On soft-tissue windows, metallic material is bright white (remember, calcium in bone is technically a metal). Air is black. Air is visible in the subcutaneous tissues, in the abdomen around the region of the spleen, and scattered outside the colon. The splenic flexure and descending colon appear ill-defined, with a hazy appearance of the surrounding fat—inflammatory stranding. Without a history of trauma, a case of colitis or diverticulitis could have a similar appearance. One of the metal fragments appears to lie within the colon. Laparotomy confirmed perforation of the distal transverse colon, which was resected. The spleen also shows a lack of enhancement (compare with the liver and kidneys)—suggesting a splenic arterial injury resulting in hypoperfusion. The inferior vena cava (IVC) is flattened, suggesting hypovolemia.
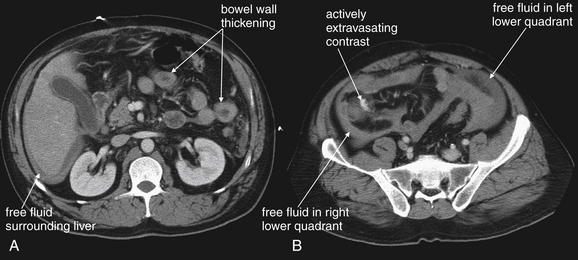
Figure 10-21 Penetrating bowel and mesentery injuries.
A, B, This male sustained a gunshot wound to the right hip. CT with intravenous contrast reveals free fluid throughout the abdomen, as well as extravasation of intravenously injected contrast in the right lower quadrant in the region of the small-bowel mesentery. Several areas of bowel wall thickening are visible. These findings indicated the need for laparotomy, which confirmed both mesentery and distal ileal injuries. The CT findings are explored in more detail in Figures 10-22 and 10-23.
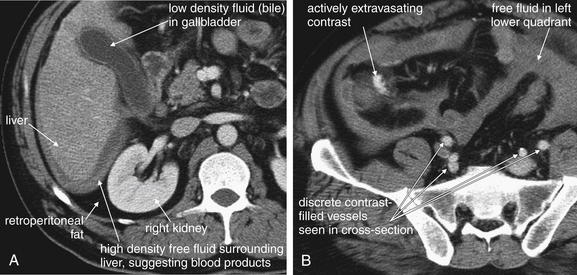
Figure 10-22 Penetrating bowel and mesentery injuries with active bleeding.
Same patient as in Figures 10-21 and 10-23. Several clues on this CT with IV contrast suggest active bleeding. A, Fluid surrounding the liver and bowel is high density, more suggestive of blood than of serous fluid such as ascites. Compare the appearance of the fluid surrounding the liver to the appearance of lower-density fluid (bile) in the gallbladder. On CT scan, regardless of window setting, denser substances are brighter (whiter) and less dense substances are darker. Fluid is identifiable around the liver because it is less dense than the solid liver but denser than retroperitoneal fat, which is nearly black on this soft-tissue window setting. Dark retroperitoneal fat surrounds the right kidney. B, Injected contrast is seen extravasating in the right lower quadrant. How can this be identified as active bleeding? This patient received no oral contrast, so high-density material in this region must be injected contrast. The contrast does not have the typical well-circumscribed, rounded shape of contrast within vessels (compare with other vessels on this image). On the digital PACS, the blush of contrast does not communicate with a well-defined vessel as we search cephalad and caudad through the stack of images.
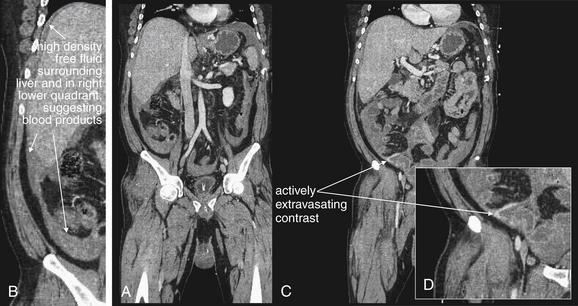
Figure 10-23 Penetrating bowel and mesentery injuries with active bleeding.
CT with IV contrast, soft-tissue windows. Coronal reconstructions from the same patient as in Figures 10-21 and 10-22. help illustrate the extent of intraabdominal hemorrhage and the location of the active bleeding. B, Close-up from A. D, Close-up from C.
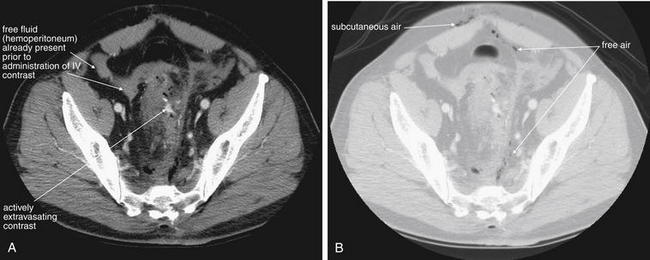
Figure 10-24 Penetrating mesenteric injuries.
CT with IV contrast. This patient sustained a gunshot wound below the umbilicus. On a soft-tissue window (A), intravenous contrast extravasation is seen just left of the midline, indicating active bleeding. Free air is seen well on the same slice using a lung window (B). Air is present just deep to the rectus muscles in the peritoneal cavity, as well as just anterior to the sacrum. This air does not appear to be contained in bowel.
When free fluid is seen, a careful search for accompanying solid organ, bowel, and mesenteric injury must be performed. Although solid organ injury is the most common source of free fluid following trauma, the presence of a solid organ injury does not rule out concomitant hollow viscus or mesenteric injury. When free fluid is observed in the absence of solid organ injury, bowel or mesenteric injury must be strongly suspected. Unfortunately, the finding is nonspecific. In a study of 259 pediatric blunt trauma patients, 24 patients (9%) had free fluid as the only abnormal finding. Among the 16 patients with a small amount of free fluid, only 2 (12%) required laparotomy. Among the 8 patients with a moderate amount of free fluid, including free fluid in multiple areas on CT, 50% required operation.46 In a study of 2299 adult blunt trauma patients, 90 patients had free abdominal fluid without solid organ injury, but only 7 (8%) had documented intestinal injuries.47
Active Bleeding
As described earlier in the section on indications for contrast agents, active bleeding is revealed by IV contrast (see Figures 10-12, 10-15, 10-19, and 10-21 through 10-24). On soft-tissue windows, parenteral contrast leaking into regions of solid organ injury or into existing hemoperitoneum is readily visible as a bright white “blush” or amorphous collection (see Figures 10-12, 10-15, 10-19, and 10-21 through 10-24). The surrounding area of hypoperfused solid organ, contained hematoma, or hemoperitoneum appears darker gray, corresponding to around 45 Hounsfield units. Normally perfused, uninjured solid organs appear brighter than hematoma but less bright than the area of active hemorrhage. Depending on the timing of CT image acquisition relative to contrast injection, normal vascular structures within solid organs may be visible as bright white linear or circular markings. These should not be confused with areas of active hemorrhage. Normal vascular structures are surrounded by moderately enhancing normal solid organ parenchyma (intermediate or bright gray) (see examples of normal livers in Figures 10-11 and 10-12;). Areas of active hemorrhage usually are surrounded by hypoattenuating or nonenhancing areas of hematoma or injured solid organ parenchyma, which appear dark gray on soft-tissue windows (see Figure 10-12). Pooling of extravasated parenteral contrast is an indication for angiographic embolization (described in more detail in Chapter 16) or laparotomy.48
Spleen Injuries
CT with IV contrast is highly sensitive for spleen injuries. Spleen injuries are evaluated using soft-tissue windows (see Figures 10-11 through 10-16). On this window setting, a normal spleen enhances with IV contrast and appears bright gray (see Figure 10-11). As described earlier in the section on indications for IV contrast, areas of splenic parenchymal injury or contained hematoma fail to enhance and appear dark gray, with a density around 45 HU (see Figure 10-11). Areas of hemoperitoneum (see Figures 10-11 and 10-12) may surround the spleen, or may settle in dependent regions of the abdomen or pelvis, remote from the bleeding source, as described earlier. Active hemorrhage is visible as a bright blush, as described earlier. If an area of blush increases in size on delayed phase images acquired 60 to 70 seconds and again 5 minutes after contrast injection, this likely represents active bleeding rather than contained vascular injury.49
Spleen injuries are graded in severity based on CT appearance using a five-point scale (Table 10-2) (see Figure 10-11).50-51 Grading schemes can be used to select patients for nonoperative therapy including angiographic embolization, described in Chapter 16. Even high-grade injuries can sometimes be managed nonoperatively with embolization.52 For the emergency physician interpreting CT, recognition of normal mild, moderate, and severe splenic injuries is probably sufficient in most cases, with formal grading being unnecessary.
TABLE 10-2 American Association for the Surgery of Trauma Splenic Injury Scale
| Grade∗ | Type | Description of Injury |
|---|---|---|
| I | Hematoma | |
| Laceration | ||
| II | Hematoma | |
| Laceration | ||
| III | Hematoma | |
| Laceration | ||
| IV | Laceration | |
| V | Laceration | |
| Vascular |
∗ Advance one grade for multiple injuries up to grade III.
From Tinkoff G, Esposito TJ, Reed J, et al. American Association for the Surgery of Trauma Organ Injury Scale I: Spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg 207:646-655, 2008.
Liver Injuries
CT with IV contrast is highly sensitive for liver injuries. Liver injuries follow a similar pattern to spleen injuries. The liver is a highly vascular solid organ and enhances with IV contrast, giving its normal parenchyma a bright appearance on soft-tissue windows (see Figure 10-17). Areas of injury are typically hypoperfused and do not enhance with IV contrast, making them darker gray (hypoattenuated) (see Figures 10-17 through 10-19 and 10-36). Areas of active hemorrhage may be visible as a blush of contrast (see the earlier section on active bleeding). Liver injuries are graded on a six-point scale that guides nonoperative management (Table 10-3).51 As with spleen injuries, specific grading of liver injuries may be unnecessary for the emergency physician interpreting CT. Differentiating normal, mild, moderate, and severe injuries is essential to management decisions. Mild injuries rarely require intervention, whereas severe injuries often require angiographic embolization or laparotomy.
TABLE 10-3 American Association for the Surgery of Trauma Liver Injury Scale
| Grade | Type | Description of Injury |
|---|---|---|
| I | Hematoma | |
| Laceration | ||
| II | Hematoma | |
| Laceration | ||
| III | Hematoma | |
| Laceration | ||
| IV | Laceration | |
| V | Vascular | |
| VI | Vascular |
∗ The liver is divided into eight Couinaud segments. Each is functionally independent, with its own vascular inflow, outflow, and biliary drainage. The center of each segment contains a branch of the portal vein, hepatic artery, and bile duct. The periphery of each segment contains vascular outflow through hepatic veins.
From Tinkoff G, Esposito TJ, Reed J, et al. American Association for the Surgery of Trauma Organ Injury Scale I: Spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg 207:646-655, 2008.
Renal Injuries
Renal injuries are discussed in detail in Chapter 12. They follow a pattern similar to that of other solid organs. The normal kidneys are well perfused and enhance brightly with IV contrast. Areas of hypoperfusion resulting from injury appear dark gray. Perinephric fluid collections appear dark gray and may represent blood or urine. Chapter 12 discusses the use of delayed CT images to differentiate urine leaks from hemorrhage. In general, images acquired during an arterial phase of contrast enhancement may show active extravasation of contrast, indicating bleeding. If contrast extravasation is seen on delayed images obtained minutes later during renal excretion of contrast, a urine leak is present (see Chapter 12).
Biliary Tract Injuries
Biliary tract injuries are rare following blunt trauma, occurring in only around 2% to 3% of patients undergoing laparotomy, a select group in today’s era of nonoperative management of many injuries.56 Gallbladder injuries are difficult to recognize because of the common occurrence of adjacent organ injury. A collapsed gallbladder or thickening or disruption of the gallbladder wall suggests injury (see Figure 10-19). Pericholecystic fluid is nonspecific because it may accumulate from other sources. Layering of dense fluid within the gallbladder can suggest hemorrhage in the gallbladder. Bile duct injury can result in free fluid.
Small Bowel, Mesentery, Stomach, or Colon Injuries
Injuries to the bowel (including stomach, small bowel, and large bowel) and mesentery are difficult to detect with CT. The reported sensitivity of CT varies from around 60% to as high as 95% in some series.20,21,24,53 Findings suggesting bowel or mesenteric injury include free peritoneal fluid without evidence of solid organ injury, mesenteric infiltration (an increased density, brightening, or smoky appearance of the normally nearly black mesenteric fat), thick-walled bowel, and free air (see Figures 10-20 through 10-24).54 As discussed earlier in the section on indications for contrast agents, oral contrast is not recommended for blunt trauma but is commonly used for penetrating trauma. Even when enteral contrast is used, leak of enteral contrast is not routinely seen with bowel injuries; however, when present, it is proof of bowel perforation.55 Other abdominal injuries are commonly present when bowel injuries occur.
Pancreatic Injuries
Pancreatic injuries are rare, occurring in around 2% of blunt trauma patients, and can be difficult to recognize on CT.56 Single-detector CT was reportedly only about 80% sensitive for pancreatic injuries, and reports of the sensitivity of multidetector CT with narrow slice thickness are lacking.56 A common mechanism for pancreatic injury is a direct blow to the epigastrium as in a handlebar injury or crush injury against the underlying spine. Pancreatic injuries are rarely isolated (<10%) and can accompany bowel injury. CT findings include abdominal free fluid with or without other solid organ injury, peripancreatic stranding or fluid, and direct evidence of pancreatic parenchymal injury (Figure 10-25). Like other solid organs, the normal pancreas is well perfused and enhances with IV contrast. Injured regions are hypoperfused and appear hypoattenuated or dark gray on soft-tissue windows. The body of the pancreas may appear completely transected or comminuted. Active hemorrhage is sometimes seen. Fluid between the splenic vein and the pancreas also suggests pancreatic injury. Injury to the pancreatic duct, which requires repair, can be difficult to recognize but is strongly suggested by deep lacerations of the pancreas (>50% of the pancreatic thickness).56-57
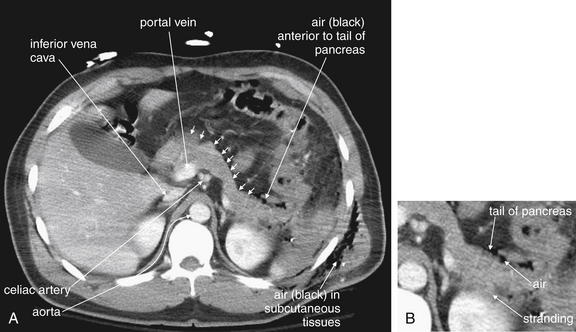
Figure 10-25 Pancreatic injuries.
A, This patient sustained multiple gunshot wounds to the left thorax, resulting in injuries to the diaphragm, transverse colon, and pancreas that were confirmed at laparotomy. CT with intravenous contrast (soft-tissue window) shows air in the subcutaneous tissues of the left chest wall. The pancreas (small arrows) can be seen in its usual position, draped over the portal vein and celiac artery and then crossing just anterior to the left kidney. Air is present along the anterior border of the pancreatic tail, perhaps having been introduced by the gunshot wound or having escaped from the injured colon. The fat plane that normally separates the pancreas and kidney is obscured by stranding. The inferior vena cava also is flattened, consistent with blood loss. B, Close-up from A.
Adrenal Injuries
Injury to the adrenal glands can occur in blunt trauma. In addition, adrenal hemorrhage is thought to occur spontaneously in some patients with shock and may subsequently lead to adrenal insufficiency. The normal adrenal glands are thin, lambda-shaped structures capping the kidneys bilaterally (Figure 10-26). Adrenal hemorrhage usually results in an enlarged, rounded appearance (see Figure 10-26). The normal adrenal glands enhance with IV contrast, whereas contained adrenal hematoma is usually hypoattenuating. Stranding around the adrenal glands may also occur.58-60
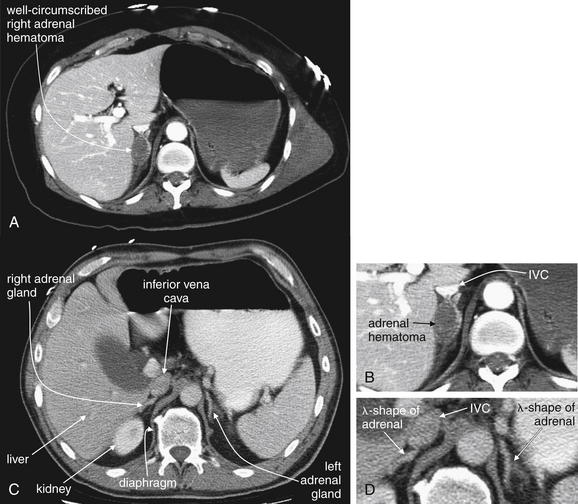
Figure 10-26 Adrenal injuries.
A, CT with IV contrast, soft-tissue window. This patient sustained an adrenal hematoma in the setting of severe blunt trauma that also resulted in multiple rib fractures, thoracic spine fractures, and a pneumothorax. Adrenal hematomas may result from the stress of severe injury, rather than from direct trauma to the gland. No injury to the surrounding liver, kidney, or adjacent ribs or vertebrae occurred. The inferior vena cava is anterior to the adrenal hematoma and filled with contrast. B, Close-up from A. C, CT with IV and oral contrast in a different patient. Normal adrenal glands for comparison. The adrenal glands are normally inconspicuous and may be difficult to locate. They usually lie just cephalad of the kidneys and have a lambda (λ) shape. D, Close-up from C.
Retroperitoneal Injuries
Retroperitoneal injuries such as psoas muscle hemorrhage are readily seen on CT. The presence of retroperitoneal fat assists in recognition of these injuries, acting as a natural contrast agent. Fat appears nearly black on soft-tissue windows, whereas the normal psoas muscle appears an intermediate gray. Blood in the retroperitoneum has a similar density to psoas muscle and can obscure fat planes (Figure 10-27).
Figure 10-27 Retroperitoneal hemorrhage (iliopsoas hematoma).
This patient fell 12 feet from a ladder, sustaining transverse process spinal fractures and a pneumothorax. In addition, this CT with IV contrast (soft-tissue window) demonstrates a right iliopsoas muscle hematoma. The source of the hemorrhage is evident—a transverse process fracture of the adjacent L2-5 lumbar vertebrae. Hemorrhage can be subtle because fresh blood and muscle have similar density on CT—both appear medium gray on soft-tissue window settings. In this case, comparison with the normal left side reveals asymmetry of the right iliopsoas resulting from an adjacent hematoma. The retroperitoneal fat separating the right kidney from the right iliopsoas is also obscured. A, A slice through the level of the kidneys. B, Two lumbar levels lower.
Abdominal Wall or Flank Injuries
Muscles of the anterior abdominal wall or flank can be damaged, resulting in herniation of abdominal contents. On CT, abdominal contents may be seen in subcutaneous fat (Figures 10-28 and 10-29).
Figure 10-28 Abdominal wall injuries.
This 6-year-old boy was injured falling off a bicycle while riding down a hill and presented with right lower abdominal pain and a protuberant abdomen. CT shows a large hernia defect in the rectus muscle of the right abdominal wall, through which the bowel has herniated into the subcutaneous tissues. Laparotomy was performed, and the hernia was found to contain both the small and the large bowel, with a contusion of the terminal ileum and a serosal tear of the cecum, which was repaired primarily. Compare the injured side with the normal muscles of the left abdominal wall. The patient is tilted slightly in the CT scanner, accounting for the apparent asymmetry of the pelvis.
Figure 10-29 Penetrating abdominal trauma with abdominal wall injury.
This patient presented with a self-inflicted stab wound to the lower abdomen. A, C, CT with oral and intravenous contrast was performed. A, B, axial CT slices through the pelvis, viewed on soft-tissue windows. B, Close-up from A. D, Close-up from C. The CT shows fat stranding consistent with blood in the subcutaneous tissues deep to the stab wound. Compare with the normal appearance of uninjured subcutaneous fat (dark gray). Of greater concern, the bowel lies just deep to the rectus muscle. Though no definite bowel injury is seen, a second slice at a more caudad level (B) shows stranding of intraperitoneal fat just deep to the rectus muscle. This suggests the stab has penetrated the peritoneum, possibly injuring the bowel. In addition, a tiny focus of contrast may represent active bleeding. The patient was observed without laparotomy despite these findings and did well.
Diaphragm Injuries
Overt diaphragm injuries with herniation of abdominal contents into the thorax are readily detected by CT (Figures 10-30 through 10-34). However, when the chest x-ray is normal and the diaphragmatic injury is subtler, CT is less sensitive. Improvements in CT technology, particularly the use of helical CT and coronal and sagittal reconstructions, appear to have improved sensitivity. Nonetheless, studies of diaphragm rupture are limited by the rarity of this injury, with wide confidence intervals because of small studies (described later). Overall, CT likely has a sensitivity of around 60% to 80%, with a specificity of 80% to 100%.
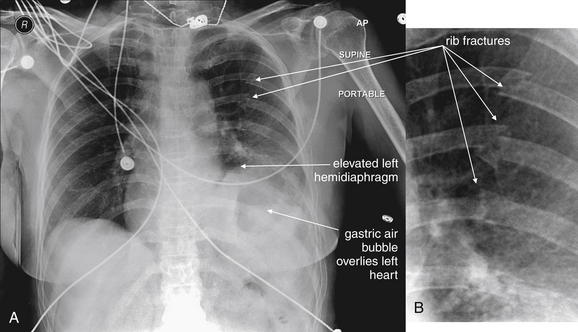
Figure 10-30 Left-sided diaphragmatic rupture from blunt trauma.
A, supine portable AP chest x-ray. Several features of this x-ray strongly suggest traumatic diaphragm rupture. First, the left hemidiaphragm is elevated relative to the right. Normally, the right hemidiaphragm is slightly higher than the left because the liver is larger than the spleen. Second, the apparent gastric air bubble overlies the heart in the left chest. Third, the costovertebral angle is not clear on the left, though this is a nonspecific finding. The patient has multiple left posterior rib fractures, suggesting a high-energy trauma mechanism that might also produce diaphragm rupture. The patient’s CT is explored in Figures 10-31 and 10-32. B, Close-up from A.
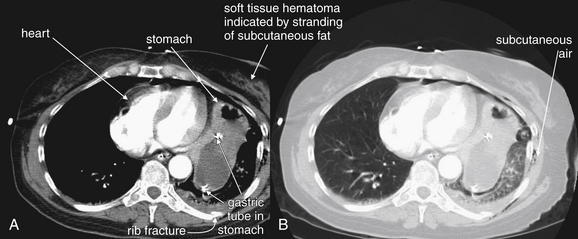
Figure 10-31 Left-sided diaphragmatic rupture from blunt trauma.
Same patient as in Figures 10-30 and 10-32. CT with IV contrast, axial images. A, Soft-tissue window of a slice through the T6 level. The stomach is seen in the left chest abutting the heart. A gastric tube is seen passing through the stomach. These findings confirm diaphragm rupture, even without direct visualization of the diaphragm injury. These findings could be recognized without the use of any contrast agents. This patient received intravenous but not oral contrast. A soft-tissue contusion is seen in the left chest wall. A posterior fracture of the sixth rib is visible—this could be inspected in more detail on a bone window. B, Lung window showing subcutaneous air but no large pneumothorax.
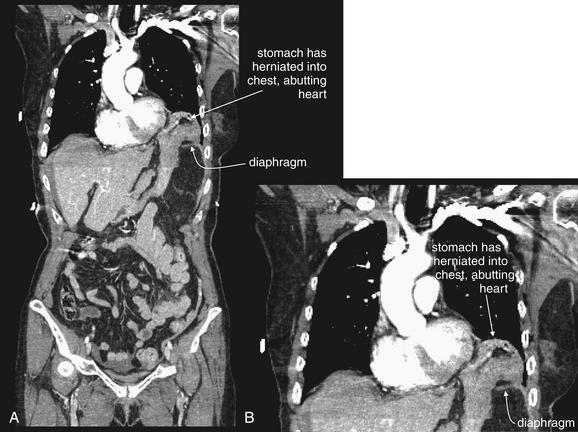
Figure 10-32 Left-sided diaphragmatic rupture from blunt trauma.
Same patient as in Figures 10-30 and 10-31. A, In this coronal reconstruction, the stomach abuts the heart, having herniated through a diaphragmatic injury. B, Close-up from A.
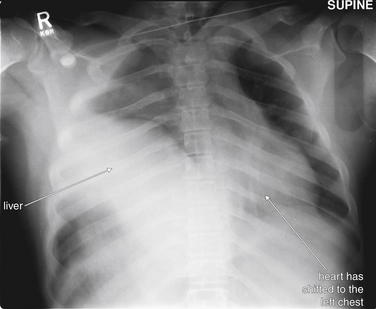
Figure 10-33 Right-sided diaphragmatic rupture from blunt trauma.
AP supine chest x-ray. Though rarer than left-sided ruptures, right diaphragm ruptures can occur and must be recognized. This 17-year-old male presented in respiratory distress after a motor vehicle collision. He has herniated the entire liver and segments of colon into the right chest, resulting in mediastinal shift to the left. His respiratory distress is likely because of complete compressive atelectasis of the right lung, as well as compression of the left lung by the shifted mediastinum. The patient’s CT is explored in Figure 10-34.
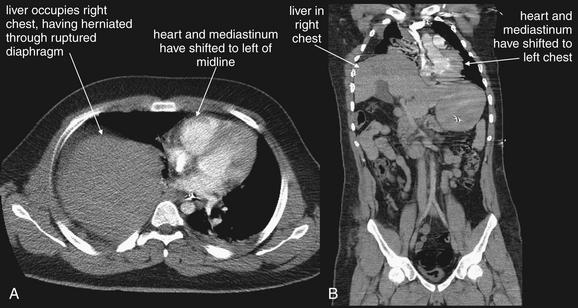
Figure 10-34 Right-sided diaphragmatic rupture from blunt trauma.
Same patient as in Figure 10-33. CT with IV contrast, soft-tissue windows. A right diaphragm rupture has occurred. A, Axial image. At the level of the scapula, the liver is seen in the right thorax adjacent to the heart, which has been displaced into the left chest. Kinking of the superior mediastinum results, similar to the case in a tension pneumothorax. B, A coronal reconstruction highlights the mediastinal shift.
CT findings of subtle diaphragmatic injury include a discontinuous diaphragm and waistlike constriction of abdominal viscera (sometimes called the “collar sign”), the most common finding in one study.61 An additional finding, the “dependent viscera sign,” may also be sensitive for diaphragm injury and is described here. Normally, an intact diaphragm prevents the upper one third of the liver from contacting the posterior ribs on the right and the stomach and bowel from contacting the posterior ribs on the left. When the diaphragm is ruptured, these organs become dependent (hence the term dependent viscera sign) and contact posterior ribs in a patient positioned supine during CT.61 The diaphragm itself may be obscured by hemothorax or hemoperitoneum.62 In some cases, the presence of a diaphragm injury can be inferred from injuries on both sides of the diaphragm, though the diaphragmatic defect itself may not be seen (Figures 10-35 and 10-36).
Figure 10-35 Transhepatic gunshot wound with hemothorax and diaphragm injury.
This patient sustained a gunshot wound to the abdomen but was hemodynamically stable on arrival. Chest x-ray shows veiling of the right thorax, suggesting a hemothorax. This appearance is typical on a supine chest x-ray, whereas an upright chest x-ray usually would show a meniscus because of dependent thoracic fluid. The patient underwent abdominal CT, shown in Figure 10-36. Always consider chest injury with any penetrating abdominal or flank wound because the course of a penetrating injury may cross the diaphragm.
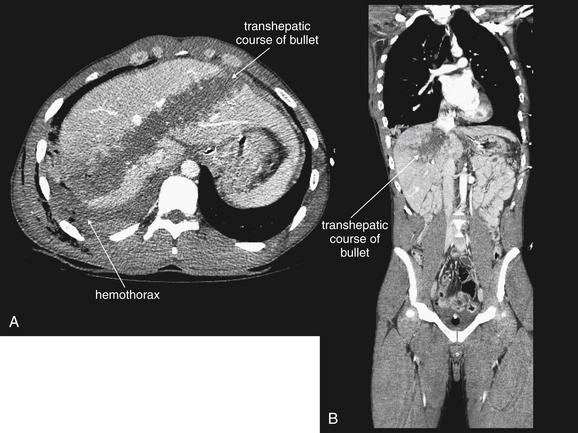
Figure 10-36 Transhepatic gunshot wound with diaphragm injury.
Same patient as in Figure 10-35. A, Axial view. B, Coronal view. CT with IV contrast (soft-tissue windows) shows a transhepatic gunshot wound with an associated hemothorax. The presence of simultaneous chest and abdominal injuries suggests a diaphragmatic perforation, which was confirmed at surgery.
The sensitivity of CT for diaphragmatic injury has improved over the past 20 years due to technological improvements, while chest x-ray sensitivity has remained static. Gelman et al.63 reported the sensitivity of chest x-ray as 46% for left-sided diaphragmatic rupture but only 17% for right-sided rupture. An additional 18% of left-sided ruptures had abnormalities not diagnostic of diaphragm rupture but prompting additional imaging. At that time (1991), CT was only 14% sensitive.
Smithers et al.5 (1991) reported a sensitivity of chest x-ray around 60% for diaphragm rupture within 48 hours after injury.
Murray et al.62 (1996) reported the performance of three radiologists for detection of diaphragm rupture on CT in a case-control study (11 cases, 21 controls). Sensitivity for detecting diaphragmatic rupture was 54% to 73%, and specificity was 86% to 90%. Average sensitivity for the three observers was 61% (95% CI = 41%-81%), and average specificity was 87% (95% CI = 76%-99%).
Killeen et al.61 (1999) reported on helical CT with sagittal and coronal reconstructions in 41 patients, 23 with proven diaphragm injury. Sensitivity for detecting left-sided diaphragmatic rupture was 78%, and specificity was 100%. Sensitivity for the detection of right-sided diaphragmatic rupture was 50%, and specificity was 100%.
Bergin et al.64 (2001) described the dependent viscera sign (discussed earlier). In their sample of 28 patients, 10 with diaphragm injury, the finding was 100% sensitive for left-sided rupture and 83% sensitive for right-sided rupture. The authors claimed a consecutive sample of patients undergoing CT and laparotomy was studied, but the high incidence of diaphragm injury in this series (36%) far exceeds other reports and strongly suggests selection bias.
Allen et al.22 (2005) reported CT without oral contrast to have a sensitivity and specificity for diaphragm injury of 66.7% (95% CI = 29.9%-92.5%) and 100% (95% CI = 99.2%-100%), respectively, although their study included only nine injuries.
Vascular (Aorta and Inferior Vena Cava) Injuries
The abdominal aorta is relatively rarely injured with blunt mechanisms of trauma. However, severe trauma (e.g., falls from great height or extremely high-speed collisions) can disrupt the abdominal aorta. The normal aorta is circular in cross section, does not exceed 3 cm in diameter, and has a smooth contour. It has a uniform location anterior to the spine, making it easy to inspect. With IV contrast, the aorta should fill uniformly with contrast, with no filling defects or intimal flaps visible. In older patients, aortic aneurysms and mural thrombus may be incidentally detected, unrelated to trauma (see Chapter 11). In cases of trauma, the aortic contour may be irregular, and active extravasation of contrast may be seen as a bright blush (Figure 10-37). Any periaortic hematoma that accumulated before the administration of IV contrast appears dark gray, approximately 45 Hounsfield units. Aortic injuries from penetrating trauma have a similar appearance to those seen with blunt trauma, if the patient is stable enough to undergo CT.
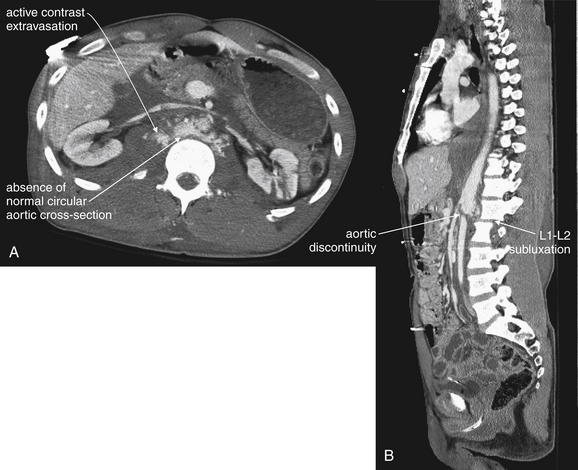
Figure 10-37 Abdominal aortic injury from blunt trauma.
This patient sustained a traumatic L1-2 spinal subluxation resulting from a fall from great height. The abdominal aorta was torn at the site of spinal trauma, an unusual injury following a blunt mechanism. CT with IV contrast, soft-tissue windows. A, Axial view shows an absence of the normal circular cross section of the aorta, as well as the presence of abnormal contrast extravasation. B, Sagittal view shows the aortic discontinuity at the level of spinal injury. Following an emergent aortic repair, the patient survived with relatively normal neurologic and vascular function.
Injuries to the IVC are more common in penetrating trauma than in blunt trauma. The IVC has a uniform location to the right of the patient’s aorta. Its size is generally smaller than that of the aorta but varies with the patient’s intravascular volume status. The IVC may be circular or elliptical in cross section. In hemorrhagic shock, the IVC appears flattened (see Figures 10-6 and 10-20).42 Active extravasation of contrast can be seen with IVC injuries.
Spine and Pelvis Injuries
Body CT performed for the evaluation of blunt or penetrating abdominal trauma also yields detailed information about bony structures. Axial, sagittal, and coronal reformations help characterize injuries in three dimensions (see Figure 10-37). Although injuries can be evaluated directly from the abdominal images by switching to bone windows, finer detail can be seen using special bone reconstruction algorithms on the original image dataset. Evaluation of these injuries on CT is discussed in Chapters 3 and 13.
Clinical Decision Rules: Which Stable Blunt Trauma Patients Require Abdominal CT?
Adult Blunt Trauma Decision Rules
Following blunt trauma, stable adult patients can be evaluated safely using CT, which has been shown to have an excellent negative predictive value for serious injuries requiring treatment (see the section on safety of discharge following a negative CT). However, CT imaging of all stable blunt trauma patients would be costly, would be time consuming, and would result in large radiation exposures (see the later section on radiation) with little clinical benefit. Studies show that around 80% of adults and 84% of pediatric patients undergoing CT for blunt abdominal injury have completely normal CT.47,65 A clinical decision rule to identify patients at low risk for serious injury could spare many stable patients unnecessary CT.
Some studies have suggested that adults with no clinical findings of blunt torso trauma may have intraabdominal injuries, a result that (if true) would suggest that CT is required in apparently uninjured patients, based on trauma mechanism. However, methodologic problems cast doubt on the findings. Salim et al.66 studied 1000 patients without obvious signs of torso trauma and reported that 8.3% (83 patients) had an abnormal abdominal CT suggesting or confirming injury. However, nearly half of patients with injuries detected by CT had abnormally depressed mental status, preventing reliable abdominal examination. The authors admitted to not examining the patients with normal mental status for signs of injury such as costal tenderness.66 It is possible that simple physical examination and history could exclude abdominal injuries in awake and alert patients.
Other studies have suggested that physical examination and complaints correlate poorly with injury. In a prospective study of more than 2000 adult blunt trauma patients, 19% of patients with CT demonstrating abdominal injury had no abdominal tenderness—although the rate of intoxication and altered mental status in this group was not documented. Among those with abdominal tenderness or bruising, only 22% had a positive CT.47 Abdominal tenderness and bruising alone appear insufficient to select patients for CT.
Another clinical scenario for which abdominal CT is often obtained is “clearance” of the abdomen in patients requiring urgent extraabdominal surgery. Often, patients with no abdominal signs or symptoms undergo CT, with the rationale for CT in this instance being the inability to observe for worsening signs or symptoms in a patient under general anesthesia for operative treatment of other injuries. Do patients of this type require abdominal CT? In a prospective observational study, Schauer et al.67 enrolled 201 patients scheduled to undergo extra-abdominal surgery for orthopedic (91%), facial (8%), soft-tissue (3%), vascular (2%), neurosurgical (1%), urologic (1%), and ophthalmologic (0.4%) injuries. Patients were excluded if they had isolated extremity injuries (with no mechanism for blunt abdominal injury), signs or symptoms of abdominal injury (including systolic blood pressure <90 mm Hg; abdominal, flank, or costal margin tenderness; abdominal wall contusion or abrasion; pelvic fracture; and gross hematuria), or unreliable findings on abdominal examination as a consequence of a Glasgow Coma Scale (GCS) score below 14, paralysis, or mental retardation. Three patients had abdominal injuries detected at CT (1.2%; 95% CI = 0.2%-3.4%), including two splenic injuries managed with simple observation. One patient (0.4%; 95% CI = 0%-2.2%) underwent splenectomy; this patient had multiple abdominal injuries, including kidney and pancreatic injuries sustained in a motorcycle collision.67 The authors concluded that intraabdominal injuries are rare (occurring in fewer than 2%) in patients with normal mental status and no examination evidence of abdominal injury.
A large prospective study to further characterize the criteria for abdominal CT in blunt trauma patients was needed. Holmes et al.1 prospectively derived and then internally validated two clinical decision rules for stable adult patients with blunt abdominal trauma in a cohort of more than 5000 patients (Box 10-1). The first rule, intended to identify injuries requiring intervention (laparotomy or angiographic embolization) consisted of hypotension, a GCS score less than 14, costal margin tenderness, abdominal tenderness, a hematuria level greater than or equal to 25 red blood cells per high-power field, and a hematocrit level less than 30%. This rule was 100% sensitive (95% CI = 93.4%-100%).1
Box 10-1 Clinical Decision Rule for Abdominal/Pelvic CT in Adults Following Blunt Trauma, detects injuries requiring intervention
Adapted from Holmes JF, Wisner DH, McGahan JP, et al. Clinical prediction rules for identifying adults at very low risk for intraabdominal injuries after blunt trauma. Ann Emerg Med 54:575-584, 2009.
This clinical decision rule identifies adult patients who require laparotomy or angiographic embolization following blunt abdominal trauma. Patients meeting any single criterion are at risk. Patients meeting none of the criteria are at low risk and are unlikely to benefit from abdominal CT.
The second (more conservative) rule, intended to identify all intraabdominal injuries, consisted of a GCS score less than 14, costal margin tenderness, abdominal tenderness, femur fracture, a hematuria level greater than or equal to 25 red blood cells per high-power field, a hematocrit level less than 30%, and an abnormal chest radiograph result (pneumothorax or rib fracture) (Box 10-2). This rule was 95.8% sensitive (95% CI = 91.1%-98.4%), was 29.9% specific (95% CI = 27.5%-32.3%), and had a negative predictive value of 98.6% (95% CI = 97.1%-99.5%) in a high-risk population (1595 patients, in whom 9% had abdominal injury).1 The authors advocated use of the combined set of criteria to identify patients at low risk for injury, not requiring CT. Use of these rules would have safely eliminated CT in around 30% of study patients. These rules have not been externally validated.
Box 10-2 Clinical Decision Rule for Abdominal/Pelvic CT in Adults Following Blunt Trauma, detects any injury
Adapted from Holmes JF, Wisner DH, McGahan JP, et al. Clinical prediction rules for identifying adults at very low risk for intraabdominal injuries after blunt trauma. Ann Emerg Med 54:575-584, 2009.
This clinical decision rule identifies adult patients with any intraabdominal injury following blunt abdominal trauma. Patients meeting any single criterion are at risk. Patients meeting none of the criteria are at low risk and are unlikely to benefit from abdominal CT.
Pediatric Blunt Trauma Decision Rules
The same imperatives for a clinical decision rule for blunt trauma take on even greater significance in pediatrics (Box 10-3). Here, concerns about radiation exposure are heightened, with models suggesting a higher risk for radiation-induced cancers from CT exposures in younger patients.68-70 The estimated attributable mortality risk from a single abdominal CT in a 1-year-old is 0.18%.68 Moreover, young patients may require sedation to undergo CT, with attendant procedural risks. Holmes et al.71-73 derived and validated a clinical decision rule to identify pediatric patients with any abdominal injury after blunt abdominal trauma. The rule consisted of six variables: low age-adjusted systolic blood pressure, abdominal tenderness, femur fracture, increased liver enzyme levels (serum aspartate aminotransferase concentration >200 U/L or serum alanine aminotransferase concentration >125 U/L), microscopic hematuria (urinalysis >5 red blood cells per high-power field), or an initial hematocrit level less than 30%. Patients meeting none of these criteria were at very low risk of injury. In a validation cohort of 1119 patients, the rule was 94.9% sensitive (95% CI = 90.2%-97.7%) and 37.1% specific (95% CI = 34%-40.3%). Use of the rule would have reduced CT use by 33%. The rule missed 8 patients, only 1 of whom underwent laparotomy, which was nontherapeutic (identifying a serosal tear and mesenteric hematoma not requiring intervention). This rule requires external validation.
Box 10-3 Clinical Decision Rule for Abdominal/Pelvic CT in Pediatric Patients Following Blunt Trauma, detects any injury
Adapted from Holmes JF, Mao A, Awasthi S, et al. Validation of a prediction rule for the identification of children with intraabdominal injuries after blunt torso trauma. Ann Emerg Med 54(4):528-533, 2009.
This clinical decision rule identifies pediatric patients with any intraabdominal injury following blunt abdominal trauma. Patients meeting any single criterion are at risk. Patients meeting none of the criteria are at low risk and are unlikely to benefit from abdominal CT.
Is There a Role for Pan-Scan CT in Patients With Blunt Abdominal Trauma?
In contrast to the admonition for limited CT based on clinical decision rules, other authors have cautioned that significant injuries are often present in patients with no signs of symptoms of abdominal trauma. Several studies have concluded that so-called pan-scan CT (CT of the head, neck, chest, abdomen, and pelvis) is required following all blunt trauma—though these studies have serious methodologic flaws and have been widely criticized. We discuss some of these flawed studies here. Many of these studies suffer from both selection and spectrum bias. The studies are typically conducted in large, high-acuity, high-volume centers, and application of techniques developed in these settings is unlikely to yield similar results when used in centers with lower patient acuity and volume. In addition, studies of this type frequently suffer from poor collection of history and examination data. In one case, the authors titled their study “Whole Body Imaging in Blunt Multisystem Trauma Patients Without Obvious Signs of Injury,” but in questions following their oral presentation, the investigators admitted failing to assess for obvious physical findings such as chest and spine tenderness.66 Some studies also included both patients with abnormal mental status and those with normal mental status—two populations whose examination findings may be different in their reliability.66
Sampson et al.74 reported a retrospective series of 296 patients undergoing comprehensive CT, including noncontrast CT of the head and cervical spine with contrast-enhanced CT of the chest, abdomen, and pelvis. The authors reported the detection of “unsuspected injuries,” including 19 cervical spine injuries, 97 pneumothoraces, and 19 splenic injuries. Whether these injuries were truly unsuspected based on history and examination is not clear from the study design and manuscript. Moreover, the clinical importance of some injuries, including small pneumothoraces detected by CT, is unknown and not measured in this study. It is possible that detection of subtle injuries resulted in worsened patient outcomes by prompting unnecessary interventions such as thoracostomy. Despite this, the authors advocated comprehensive CT in blunt trauma patients, with little evidence to guide their selection of patients. The authors stated that all hemodynamically stable blunt trauma patients with more than two body systems involved were imaged. However, this study is highly selection biased because only 296 patients in a 7-year period were imaged in this manner, with an annual emergency department census of 85,000 visits. It is likely that only relatively sick patients were selected to undergo comprehensive CT. Application of this imaging strategy to all blunt trauma patients in other practice settings would likely result in imaging of less injured patients, with less potential benefit from intensive imaging. The cost and radiation exposure of this strategy would likely not be warranted.
Huber-Wagner et al.75 retrospectively reported survival in 1494 patients undergoing whole-body CT for evaluation of blunt trauma, compared with 3127 not undergoing whole-body CT. They reported a 25% reduction in mortality risk in patients undergoing whole-body CT, compared with the predicted mortality using previously published trauma scores. The authors concluded “whole-body CT… significantly increased the probability of survival in patients with polytrauma.” Unfortunately, retrospective studies such as this can demonstrate only association, not causation, as the authors suggested. Many confounding factors may cause studies such as this to reach precisely the wrong conclusions. Although the authors attempted to control for factors such as trauma-center type and year, this is not a randomized controlled trial and bias likely played an important part in outcomes in the two groups. Patients not undergoing whole-body CT may have differed in important but unmeasured ways from patients undergoing whole-body CT. For example, more stable patients may have been recognized by the treating physicians and selected for CT imaging, giving the false appearance that CT caused improved survival.75 The type of physicians caring for the patients may have been the biggest determinant of outcome, with the use of CT as simply a marker of physician training. The local infrastructure at hospitals practicing whole-body CT may have differed from those not performing whole-body CT. The authors skipped an important step in demonstrating any cause–effect relationship when they failed to demonstrate injuries found on CT that plausibly could have been treated to reduce mortality. In commentaries upon this study, other authors pointed out that early deaths, before the completion of whole-body CT, could contribute to the worsened outcomes in the group not receiving whole-body CT.76-79 In the first hour, 58 patients died in the non–whole-body CT group, compared with only 8 in the whole-body CT group. Although the authors of this study proposed a prospective randomized controlled trial, others have criticized them for aggressively suggesting whole-body CT based on their study.
Can Patients Be Safely Discharged Following a Normal CT for Blunt Abdominal Trauma? What Injuries Can Be Missed?
Historically, patients with blunt abdominal trauma were typically observed to allow repeat clinical examination and detection of evolving abdominal injuries. The introduction of CT allowed earlier detection of injury, but the safety of discharge following a normal abdominal CT was unknown. Reports of missed injuries such as bowel, mesenteric, and diaphragmatic injury prompted frequent admission even following a normal abdominal–pelvic CT.
Livingston et al.47 (1998) prospectively studied 2299 adult blunt abdominal trauma patients using a standardized protocol with abdominal CT, followed by admission and observation. CT was negative in 1809 patients. Among these patients, 9 underwent celiotomy, including 6 therapeutic laparotomies for 3 bowel, 1 bladder, 1 kidney, and 1 diaphragmatic injury. Another 2 patients had nontherapeutic laparotomy, and 1 had a negative laparotomy. The negative predictive value of abdominal CT for celiotomy was 99.63% (lower 95% CI = 99.31%, lower 99% CI = 99.16%). The authors concluded that admission and observation are not required in patients following negative abdominal CT. This study benefits from its prospective design, protocol-driven follow-up, and high rate of injury (17% with abnormal CT). However, several important limits should be emphasized. Bowel injuries are rare following blunt abdominal injury, occurring in only 25 patients in this study (1% of patients). As a result, even a poor test will have an excellent negative predictive value for this injury. A fixed guess of “no bowel injury” will be correct in 99% of cases. However, in patients with a high pretest probability of bowel injury, a negative CT should be treated with caution. For example, a patient with increasing abdominal pain and tenderness and abdominal bruising should likely be observed despite normal CT. In this study, CT was only 88% sensitive for bowel injury, meaning that 1 in 8 patients with a bowel injury could be missed. The same principle applies to diaphragm injuries, which are also rare and difficult to detect with CT, with sensitivity as low as 66% in some studies.22
In pediatric patients, the safety of discharge following a negative abdominal CT has also been investigated. Awasthi et al.65 prospectively enrolled 1295 patients younger than 18 years undergoing CT with IV contrast for evaluation of blunt abdominal trauma. Of these patients, 210 had injuries detected on CT. The authors then followed the 1085 patients with normal abdominal CT. Only two intraabdominal injuries were noted in patients with normal CT: a mesenteric hematoma and serosal tear discovered during nontherapeutic laparotomy and a perinephric hematoma on repeat CT not requiring therapy. The negative predictive value of a normal abdominal CT was 99.8% (95% CI = 99.3%-100%). This study is strengthened by excellent follow-up, large numbers, and a high-risk population, with 16% of the initial cohort having an abnormal CT indicating injury. The authors concluded that the probability of an injury requiring intervention is low in pediatric patients with normal abdominal CT and that routine admission is not necessary. As with the adult population, observation should be considered for patients with a high suspicion for injury based on examination and history because of the limited sensitivity of CT for bowel, mesentery, and diaphragm injuries.
Can Patients Be Safely Discharged Following a Normal CT for Penetrating Abdominal Trauma? What Injuries Can Be Missed?
The sensitivity of CT for penetrating torso injuries was described in detail earlier in this chapter. Based on a meta-analysis, the sensitivity, specificity, negative predictive value, positive predictive value, and accuracy were 94.90%, 95.38%, 98.62%, 84.51%, and 94.70%, respectively.41 However, multiple studies document missed bowel and diaphragm injuries, suggesting that observation should be strongly considered following a negative CT. Studies that claim good outcomes with emergency department discharge following a negative CT suffer from significant losses to follow-up that may invalidate their results.34
Radiation Exposure in Trauma Imaging
Radiation exposures in trauma patients have received increasing attention in recent years. Winslow et al.80 reported substantial radiation exposures during the first 24 hours of trauma care. In a sample of 86 patients meeting “less acute major trauma criteria,” they noted a median effective dose of 40.2 mSv, equivalent to approximately 1005 chest radiographs. Hubert-Wagner et al.75 also noted significant radiation exposures in patients undergoing whole-body CT but defended the exposure based on a calculated mortality risk reduction from CT of nearly 25%, compared with a cancer mortality risk in the range of 1 in 1250. This mortality benefit of CT has been strongly questioned (see the earlier discussion on pan-scan CT). Even the most vocal hawks regarding radiation risk for abdominal CT concede that “the risk–benefit balance is still strongly tilted toward benefit.”68 However, as the threshold to image patients with relatively minor mechanisms of trauma becomes lower, the risk–benefit ratio becomes less favorable. Because the cancer risk from CT-radiation exposure in an individual is small, this ratio always appears to favor use of CT, but on a population basis increased cancer rates result from unnecessary imaging. Radiation exposures can be reduced in low risk patients by use of clinical decision rules, clinical observation without CT, and imaging with ultrasound, which does not expose patients to ionizing radiation.
Can CT Be Performed in Pregnant Patients With Abdominal Trauma? What Is the Radiation Risk?
In the pregnant female, radiation to the abdomen and pelvis should be minimized because of concerns for fetal effects. However, unrecognized abdominal or pelvic injuries pose a threat to both the mother and the fetus. Contrary to common physician belief, a single abdominal or pelvic CT delivers a radiation dose below the threshold known to cause teratogenic or neurologic effects.81-82 If significant concern exists for maternal injury, CT should be performed. An increase in pediatric cancers, particularly leukemias, is a theoretical risk for in utero irradiation.82-83 If possible, a well-considered imaging plan should be develop to avoid redundant radiation exposures. For example, if CT is required, plain radiographs of the pelvis and lumbar–sacral spine should be avoided because these deliver fairly high radiation doses and provide no information that could not be gleaned from CT. When the suspicion for maternal injury is lower, alternative strategies such as observation with serial physical examination, serial ultrasound, and trending of laboratory data can take the place of immediate CT.
What Is the Risk From Intravenous Contrast?
IV contrast crosses the placenta in small quantities. Animal studies do not show fetal teratogenic or mutagenic effects or effects on fertility.83a
Are Special Findings Seen on CT During Pregnancy?
Pregnancy results in special findings on CT.84 Hydronephrosis (resulting from compression of the ureters by the enlarged uterus) and enlarged ovarian veins are normal findings in later pregnancy. Diastasis of rectus abdominus muscles and widening of sacroiliac joints and pubic symphysis are normal in the third trimester. Nonenhancing regions of the placenta on IV contrast–enhanced CT (dark gray or hypoattenuating in appearance) can indicate placental infarction or abruption. These should not be confused with normal placental cotyledons that have central areas of low attenuation with surrounding high-attenuation rings. CT can demonstrate uterine rupture, characterized by extrauterine fetal parts or by oligohydramnios with free maternal abdominal and pelvic fluid. Fetal parts are not usually seen until late first trimester on CT, and direct detection of fetal injuries is rare. Hemorrhage in the amniotic cavity may be seen. Fetal loss is most often because of placental abruption or maternal hypotension.
Although MRI can be used to evaluate traumatic injuries, in most centers it is not rapidly available, making it impractical for patients with potentially serious and time-sensitive injuries.84
Can Imaging Rule Out Placental Abruption?
Placental abruption can occur with even minor trauma and should be considered in every patient with blunt abdominal trauma beyond 22 to 24 weeks.85 Ultrasound is considered insensitive for detection of placental abruption, with sensitivity, specificity, and positive and negative predictive values of 24%, 96%, 88%, and 53%, respectively.86 Retroplacental hematomas can be isoechoic with the placenta and uterus and may not be recognized.87 Consequently, even if ultrasound appears reassuring, patients should undergo fetal heart rate monitoring for several hours for signs of fetal distress, such as bradycardia or tachycardia, that may the earliest signs of abruption.88
Summary
Modern imaging techniques allow sensitive detection of many important traumatic abdominal injuries. Bowel and diaphragm injuries remain difficult to detect with CT. Ultrasound and plain radiography play important roles in patients who are too unstable to undergo CT. Clinical decision rules are being developed to identify patients at low risk for injury who can forgo CT.
1. Holmes J.F., Wisner D.H., McGahan J.P., et al. Clinical prediction rules for identifying adults at very low risk for intraabdominal injuries after blunt trauma. Ann Emerg Med. 2009;54:575-584.
2. Kane N.M., Francis I.R., Burney R.E., et al. Traumatic pneumoperitoneum: Implications of computed tomography diagnosis. Invest Radiol. 1991;26:574-578.
3. Hamilton P., Rizoli S., McLellan B., et al. Significance of intraabdominal extraluminal air detected by CT scan in blunt abdominal trauma. J Trauma. 1995;39:331-333.
4. Saku M., Yoshimitsu K., Murakami J., et al. Small bowel perforation resulting from blunt abdominal trauma: Interval change of radiological characteristics. Radiat Med. 2006;24:358-364.
5. Smithers B.M., O’Loughlin B., Strong R.W. Diagnosis of ruptured diaphragm following blunt trauma: Results from 85 cases. Aust N Z J Surg. 1991;61:737-741.
6. Moriwaki Y., Sugiyama M., Toyoda H., et al. Ultrasonography for the diagnosis of intraperitoneal free air in chest–abdominal–pelvic blunt trauma and critical acute abdominal pain. Arch Surg. 2009;144:137-141. discussion 142
7. Valentino M., Ansaloni L., Catena F., et al. Contrast-enhanced ultrasonography in blunt abdominal trauma: Considerations after 5 years of experience. Radiol Med. 2009;144(7):1080-1093.
8. Catalano O., Aiani L., Barozzi L., et al. CEUS in abdominal trauma: Multi-center study. Abdom Imaging. 2009;34:225-234.
9. Abrams B.J., Sukumvanich P., Seibel R., et al. Ultrasound for the detection of intraperitoneal fluid: the role of Trendelenburg positioning. Am J Emerg Med. 1999;17:117-120.
10. Branney S.W., Wolfe R.E., Moore E.E., et al. Quantitative sensitivity of ultrasound in detecting free intraperitoneal fluid. J Trauma. 1995;39:375-380.
11. Liu M., Lee C.H., P’Eng F.K. Prospective comparison of diagnostic peritoneal lavage, computed tomographic scanning, and ultrasonography for the diagnosis of blunt abdominal trauma. J Trauma. 1993;35:267-270.
12. McKenney M., Lentz K., Nunez D., et al. Can ultrasound replace diagnostic peritoneal lavage in the assessment of blunt trauma? J Trauma. 1994;37:439-441.
13. Ma O.J., Mateer J.R., Ogata M., et al. Prospective analysis of a rapid trauma ultrasound examination performed by emergency physicians. J Trauma. 1995;38:879-885.
14. Pearl W.S., Todd K.H. Ultrasonography for the initial evaluation of blunt abdominal trauma: A review of prospective trials. Ann Emerg Med. 1996;27:353-361.
15. Stengel D., Bauwens K., Sehouli J., et al. Systematic review and meta-analysis of emergency ultrasonography for blunt abdominal trauma. Br J Surg. 2001;88:901-912.
16. Farahmand N., Sirlin C.B., Brown M.A., et al. Hypotensive patients with blunt abdominal trauma: Performance of screening US. Radiology. 2005;235:436-443.
17. Melniker L.A., Leibner E., McKenney M.G., et al. Randomized controlled clinical trial of point-of-care, limited ultrasonography for trauma in the emergency department: The first sonography outcomes assessment program trial. Ann Emerg Med. 2006;48:227-235.
17a. ACR appropriateness criteria: blunt abdominal trauma. American College of Radiology, 2008. (Accessed 2011, at http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/BluntAbdominalTraumaDoc3.aspx.
18. Federle M.P., Jeffrey R.B.Jr. Hemoperitoneum studied by computed tomography. Radiology. 1983;148:187-192.
19. Clancy T.V., Ragozzino M.W., Ramshaw D., et al. Oral contrast is not necessary in the evaluation of blunt abdominal trauma by computed tomography. Am J Surg. 1993;166:680-684. discussion 684-5
20. Stafford R.E., McGonigal M.D., Weigelt J.A., et al. Oral contrast solution and computed tomography for blunt abdominal trauma: A randomized study. Arch Surg. 1999;134:622-626. discussion 626-627
21. Allen T.L., Mueller M.T., Bonk R.T., et al. Computed tomographic scanning without oral contrast solution for blunt bowel and mesenteric injuries in abdominal trauma. J Trauma. 2004;56:314-322.
22. Allen T.L., Cummins B.F., Bonk R.T., et al. Computed tomography without oral contrast solution for blunt diaphragmatic injuries in abdominal trauma. Am J Emerg Med. 2005;23:253-258.
23. Stuhlfaut J.W., Soto J.A., Lucey B.C., et al. Blunt abdominal trauma: Performance of CT without oral contrast material. Radiology. 2004;233:689-694.
24. Holmes J.F., Offerman S.R., Chang C.H., et al. Performance of helical computed tomography without oral contrast for the detection of gastrointestinal injuries. Ann Emerg Med. 2004;43:120-128.
25. Federle M.P., Peitzman A., Krugh J. Use of oral contrast material in abdominal trauma CT scans: Is it dangerous? J Trauma. 1995;38:51-53.
26. Shanmuganathan K., Mirvis S.E., Chiu W.C., et al. Penetrating torso trauma: Triple-contrast helical CT in peritoneal violation and organ injury—A prospective study in 200 patients. Radiology. 2004;231:775-784.
27. Ramirez R.M., Cureton E.L., Ereso A.Q., et al. Single-contrast computed tomography for the triage of patients with penetrating torso trauma. J Trauma. 2009;67:583-588.
28. Hauser C.J., Huprich J.E., Bosco P., et al. Triple-contrast computed tomography in the evaluation of penetrating posterior abdominal injuries. Arch Surg. 1987;122:1112-1115.
29. Himmelman R.G., Martin M., Gilkey S., et al. Triple-contrast CT scans in penetrating back and flank trauma. J Trauma. 1991;31:852-855.
30. McAllister E., Perez M., Albrink M.H., et al. Is triple contrast computed tomographic scanning useful in the selective management of stab wounds to the back? J Trauma. 1994;37:401-403.
31. Soto J.A., Morales C., Munera F., et al. Penetrating stab wounds to the abdomen: Use of serial U.S. and contrast-enhanced CT in stable patients. Radiology. 2001;220:365-371.
32. Chiu W.C., Shanmuganathan K., Mirvis S.E., et al. Determining the need for laparotomy in penetrating torso trauma: A prospective study using triple-contrast enhanced abdominopelvic computed tomography. J Trauma. 2001;51:860-868. discussion 868-869
33. Shanmuganathan K., Mirvis S.E., Chiu W.C., et al. Triple-contrast helical CT in penetrating torso trauma: A prospective study to determine peritoneal violation and the need for laparotomy. AJR Am J Roentgenol. 2001;177:1247-1256.
34. Conrad M.F., Patton J.H.Jr., Parikshak M., et al. Selective management of penetrating truncal injuries: Is emergency department discharge a reasonable goal? Am Surg. 2003;69:266-272. discussion 673
35. Loberant N., Goldfeld M. A pitfall in triple contrast CT of penetrating trauma of the flank. Clin Imaging. 2003;27:351-352.
36. Dissanaike S., Griswold J.A., Frezza E.E. Treatment of isolated penetrating flank trauma. Am Surg. 2005;71:493-496.
37. Demetriades D., Hadjizacharia P., Constantinou C., et al. Selective nonoperative management of penetrating abdominal solid organ injuries. Ann Surg. 2006;244:620-628.
38. Inaba K., Demetriades D. The nonoperative management of penetrating abdominal trauma. Adv Surg. 2007;41:51-62.
39. Mitra B., Gocentas R., O’Reilly G., et al. Management of haemodynamically stable patients with abdominal stab wounds. Emerg Med Australas. 2007;19:269-275.
40. Beekley A.C., Blackbourne L.H., Sebesta J.A., et al. Selective nonoperative management of penetrating torso injury from combat fragmentation wounds. J Trauma. 2008;64:S108-S116. discussion S116-S117
41. Goodman C.S., Hur J.Y., Adajar M.A., et al. How well does CT predict the need for laparotomy in hemodynamically stable patients with penetrating abdominal injury? A review and meta-analysis. AJR Am J Roentgenol. 2009;193:432-437.
42. Jeffrey R.B.Jr., Federle M.P. The collapsed inferior vena cava: CT evidence of hypovolemia. AJR Am J Roentgenol. 1988;150:431-432.
43. Ames J.T., Federle M.P. CT hypotension complex (shock bowel) is not always due to traumatic hypovolemic shock. AJR Am J Roentgenol. 2009;192:W230-W235.
44. Earls J.P., Dachman A.H., Colon E., et al. Prevalence and duration of postoperative pneumoperitoneum: Sensitivity of CT vs left lateral decubitus radiography. AJR Am J Roentgenol. 1993;161:781-785.
45. Stapakis J.C., Thickman D. Diagnosis of pneumoperitoneum: Abdominal CT vs. upright chest film. J Comput Assist Tomogr. 1992;16:713-716.
46. Hulka F., Mullins R.J., Leonardo V., et al. Significance of peritoneal fluid as an isolated finding on abdominal computed tomographic scans in pediatric trauma patients. J Trauma. 1998;44:1069-1072.
47. Livingston D.H., Lavery R.F., Passannante M.R., et al. Admission or observation is not necessary after a negative abdominal computed tomographic scan in patients with suspected blunt abdominal trauma: Results of a prospective, multi-institutional trial. J Trauma. 1998;44:273-280. discussion 280-282
48. Fang J.F., Chen R.J., Wong Y.C., et al. Pooling of contrast material on computed tomography mandates aggressive management of blunt hepatic injury. Am J Surg. 1998;176:315-319.
49. Anderson S.W., Varghese J.C., Lucey B.C., et al. Blunt splenic trauma: Delayed-phase CT for differentiation of active hemorrhage from contained vascular injury in patients. Radiology. 2007;243:88-95.
50. Shanmuganathan K., Mirvis S.E., Boyd-Kranis R., et al. Nonsurgical management of blunt splenic injury: Use of CT criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology. 2000;217:75-82.
51. Tinkoff G., Esposito T.J., Reed J., et al. American Association for the Surgery of Trauma Organ Injury Scale I: Spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg. 2008;207:646-655.
52. Rhodes C.A., Dinan D., Jafri S.Z., et al. Clinical outcome of active extravasation in splenic trauma. Emerg Radiol. 2005;11:348-352.
53. Butela S.T., Federle M.P., Chang P.J., et al. Performance of CT in detection of bowel injury. AJR Am J Roentgenol. 2001;176:129-135.
54. Rizzo M.J., Federle M.P., Griffiths B.G. Bowel and mesenteric injury following blunt abdominal trauma: Evaluation with CT. Radiology. 1989;173:143-148.
55. Donohue J.H., Federle M.P., Griffiths B.G., et al. Computed tomography in the diagnosis of blunt intestinal and mesenteric injuries. J Trauma. 1987;27:11-17.
56. Gupta A., Stuhlfaut J.W., Fleming K.W., et al. Blunt trauma of the pancreas and biliary tract: A multimodality imaging approach to diagnosis. Radiographics. 2004;24:1381-1395.
57. Lane M.J., Mindelzun R.E., Jeffrey R.B. Diagnosis of pancreatic injury after blunt abdominal trauma. Semin Ultrasound CT MR. 1996;17:177-182.
58. Ikeda O., Urata J., Araki Y., et al. Acute adrenal hemorrhage after blunt trauma. Abdom Imaging. 2007;32:248-252.
59. Pinto A., Scaglione M., Guidi G., et al. Role of multidetector row computed tomography in the assessment of adrenal gland injuries. Eur J Radiol. 2006;59:355-358.
60. Burks D.W., Mirvis S.E., Shanmuganathan K. Acute adrenal injury after blunt abdominal trauma: CT findings. AJR Am J Roentgenol. 1992;158:503-507.
61. Killeen K.L., Mirvis S.E., Shanmuganathan K. Helical CT of diaphragmatic rupture caused by blunt trauma. AJR Am J Roentgenol. 1999;173:1611-1616.
62. Murray J.G., Caoili E., Gruden J.F., et al. Acute rupture of the diaphragm due to blunt trauma: Diagnostic sensitivity and specificity of CT. AJR Am J Roentgenol. 1996;166:1035-1039.
63. Gelman R., Mirvis S.E., Gens D. Diaphragmatic rupture due to blunt trauma: Sensitivity of plain chest radiographs. AJR Am J Roentgenol. 1991;156:51-57.
64. Bergin D., Ennis R., Keogh C., et al. The “dependent viscera” sign in CT diagnosis of blunt traumatic diaphragmatic rupture. AJR Am J Roentgenol. 2001;177:1137-1140.
65. Awasthi S., Mao A., Wooton-Gorges S.L., et al. Is hospital admission and observation required after a normal abdominal computed tomography scan in children with blunt abdominal trauma? Acad Emerg Med. 2008;15:895-899.
66. Salim A., Sangthong B., Martin M., et al. Whole body imaging in blunt multisystem trauma patients without obvious signs of injury: Results of a prospective study. Arch Surg. 2006;141:468-473. discussion 73-75
67. Schauer B.A., Nguyen H., Wisner D.H., et al. Is definitive abdominal evaluation required in blunt trauma victims undergoing urgent extra-abdominal surgery? Acad Emerg Med. 2005;12:707-711.
68. Brenner D., Elliston C., Hall E., et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289-296.
69. Brenner D.J. Estimating cancer risks from pediatric CT: Going from the qualitative to the quantitative. Pediatr Radiol. 2002;32:228-231.
70. Brenner D.J., Hall E.J. Computed tomography: An increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284.
71. Holmes J.F., Mao A., Awasthi S., et al. Validation of a prediction rule for the identification of children with intraabdominal injuries after blunt torso trauma. Ann Emerg Med. 2009;54(4):528-533.
72. Holmes J.F., Sokolove P.E., Brant W.E., et al. Identification of children with intraabdominal injuries after blunt trauma. Ann Emerg Med. 2002;39:500-509.
73. Holmes J.F., Sokolove P.E., Land C., et al. Identification of intraabdominal injuries in children hospitalized following blunt torso trauma. Acad Emerg Med. 1999;6:799-806.
74. Sampson M.A., Colquhoun K.B., Hennessy N.L. Computed tomography whole body imaging in multi-trauma: 7 years experience. Clin Radiol. 2006;61:365-369.
75. Huber-Wagner S., Lefering R., Qvick L.M., et al. Effect of whole-body CT during trauma resuscitation on survival: A retrospective, multicentre study. Lancet. 2009;373:1455-1461.
76. Saltzherr T.P., Goslings J.C. Effect on survival of whole-body CT during trauma resuscitation. Lancet. 2009;374:198. author reply 199
77. Giannoudis P.V. Effect on survival of whole-body CT during trauma resuscitation. Lancet. 2009;374:198-199. author reply 197
78. Strohm P.C., Hauschild O., Sudkamp N.P. Effect on survival of whole-body CT during trauma resuscitation. Lancet. 2009;374:197-198. author reply 198-199
79. Andersohn F. Effect on survival of whole-body CT during trauma resuscitation. Lancet. 2009;374:198-199. author reply 197
80. Winslow J.E., Hinshaw J.W., Hughes M.J., et al. Quantitative assessment of diagnostic radiation doses in adult blunt trauma patients. Ann Emerg Med. 2008;52:93-97.
81. Ratnapalan S., Bona N., Chandra K., et al. Physicians’ perceptions of teratogenic risk associated with radiography and CT during early pregnancy. AJR Am J Roentgenol. 2004;182:1107-1109.
82. Hurwitz L.M., Yoshizumi T., Reiman R.E., et al. Radiation dose to the fetus from body MDCT during early gestation. AJR Am J Roentgenol. 2006;186:871-876.
83. Ratnapalan S., Bentur Y., Koren G. Doctor, will that x-ray harm my unborn child? CMAJ. 2008;179:1293-1296.
83a. ACR Manual on Contrast Media. Version 7 ed: American College of Radiology; 2010.
84. Lowdermilk C., Gavant M.L., Qaisi W., et al. Screening helical CT for evaluation of blunt traumatic injury in the pregnant patient. Radiographics. 1999;19:S243-S255. discussion S256-S258
85. Warner M.W., Salfinger S.G., Rao S., et al. Management of trauma during pregnancy. ANZ J Surg. 2004;74:125-128.
86. Glantz C., Purnell L. Clinical utility of sonography in the diagnosis and treatment of placental abruption. J Ultrasound Med. 2002;21:837-840.
87. Nyberg D.A., Cyr D.R., Mack L.A., et al. Sonographic spectrum of placental abruption. AJR Am J Roentgenol. 1987;148:161-164.
88. Oyelese Y., Ananth C.V. Placental abruption. Obstet Gynecol. 2006;108:1005-1016.